Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Ratiometric sensing of β-galactosidase based on excited-state intramolecular proton transfer (ESIPT) and solid-state luminescence enhancement†
He
Tian
Jr.
 a,
Wei
Lin
a,
Wei
Lin
 a,
Xi-Le
Hu
a,
Xi-Le
Hu
 a,
Jing-Bo
Wang
a,
Jing-Bo
Wang
 a,
Min-Yu
Zhang
a,
Min-Yu
Zhang
 a,
Yi
Zang
a,
Yi
Zang
 bfg,
Xin-Yan
Wu
bfg,
Xin-Yan
Wu
 a,
Jia
Li
a,
Jia
Li
 *bf,
Tony D.
James
*bf,
Tony D.
James
 *de and
Xiao-Peng
He
*de and
Xiao-Peng
He
 *ac
*ac
aKey Laboratory for Advanced Materials and Joint International Research Laboratory of Precision Chemistry and Molecular Engineering, Feringa Nobel Prize Scientist Joint Research Center, School of Chemistry and Molecular Engineering, East China University of Science and Technology, 130 Meilong Rd, Shanghai 200237, China. E-mail: xphe@ecust.edu.cn
bNational Center for Drug Screening, State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai 201203, China. E-mail: jli@simm.ac.cn
cNational Center for Liver Cancer, The International Cooperation Laboratory on Signal Transduction, Eastern Hepatobiliary Surgery Hospital, Shanghai 200438, China
dDepartment of Chemistry, University of Bath, Bath, BA2 7AY, UK. E-mail: t.d.james@bath.ac.uk
eSchool of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang 453007, China
fSchool of Pharmaceutical Science and Technology, Hangzhou Institute for Advanced Study, University of Chinese Academy of Sciences, Hangzhou 310024, China
gLingang Laboratory, Shanghai 201203, China
First published on 12th May 2023
Abstract
Glycosidases play important roles in modulating the structural and functional integrity of glycoproteins and glycolipids, and thus are promising biomarkers for disease diagnosis. While current approaches for glycosidase detection mainly rely on an enhancement of the UV-vis absorbance or fluorescence emission of glycosyl indicators, here we develop a ratiometric fluorescent probe for the sensitive and selective detection of glycosidase activity based on the combined mechanisms of excited-state intramolecular proton transfer (ESIPT) and solid-state luminescence enhancement (SSLE). The probe behaves like a typical SSLE when glycosylated, and exhibits a ∼140 nm red-shift in fluorescence owing to activation of ESIPT after deglycosylation. Such a large Stokes shift may facilitate the unbiased analysis of glycosidase activities when used in diagnostic and drug-screening assays.
Glycosylation and deglycosylation reactions of biomacromolecules including proteins, peptides and lipids are implicated in a myriad of biological and pathological processes.1–3 Deglycosylation is the removal of a glycosyl residue from a glycoconjugate as mediated by glycosidases, which are conserved in almost all eukaryotes. In human cells, they mainly distribute in the endoplasmic reticulum (ER), Golgi apparatus and lysosomes.4,5 Glycosidases localized in the ER and Golgi apparatus are responsible for tailoring the N-glycans on proteins after translation, and lysosomal glycosidases are known to hydrolyze the glycosyl residues on glycoconjugates endocytosed by cells.
However, the abnormal expression of glycosidases is associated with human diseases. For example, during cell senescence, β-galactosidase (β-Gal) and α-fucosidase are overly expressed,6,7 and the abnormally high expression of β-Gal is closely related to the tumorigenesis and metastasis of ovarian cancer.8 In addition, a recent proteomics study suggests that the expression level of cytoplasmic β-glucocerehrosidase in liver cancer tissues is significantly lower than that in para-carcinoma tissues.9 As a consequence, the effective detection of glycosidase activities is important for glycobiological studies and disease diagnosis.
The current approaches for analysis of glycosidase activity mainly rely on colorimetric assays, which use glycosylated indicators such as 4-nitrophenol as the colorimetric substrate. However, assays that are dependent on color changes are easily compromised by the intrinsic color of the sample itself and are generally of low sensitivity. To overcome these issues, activatable fluorescent probes that exhibit a “turn-on” fluorescence upon enzymatic hydrolysis have been developed.10–17 Based on a variety of fluorescent dyes, molecular probes capable of sensing glycosidases in cells and in vivo with emission wavelengths that range from the visible to the near-infrared region, have been synthesized in recent years.18–27
Here, we report the construction of a ratiometric fluorescent probe that exhibits a large Stokes shift of the fluorescence emission wavelength upon hydrolysis by glycosidases based on the combination of excited-state intramolecular proton transfer (ESIPT) and solid-state luminescence enhancement (SSLE).28–30 The probe is a typical SSLE system when glycosylated, and after deglycosylation, the ESIPT process is activated, thereby achieving a ∼140 nm red-shift in fluorescence emission.
To construct the ratiometric glycosidase probe, we first synthesized a fluorescent reporter with dual photophysical mechanisms. In the ortho position relative to the phenol group of tetraphenylethylene (TPE), benzothiazole (BT) was introduced. The resulting conjugate (TPE-BT) formed by the cycloaddition of 2-hydroxy-5-(1,2,2-triphenylethenyl)-benzaldehyde and 2-aminothiophenol exhibits ESIPT due to the intramolecular hydrogen bonding interaction between the nitrogen atom and the phenolic proton.31,32 Then, galactose was introduced to the phenolic site to inhibit ESIPT by removing the hydrogen-bond donor. A benzyl group was used to connect the TPE-BT and galactose (producing the Gal-TPE-BT probe) in order to enhance the sensitivity for glycosidases.33 After deglycosylation, the benzyl moiety undergoes a “self-immolation” process resulting cleavage (Fig. 1), thereby recovering the ESIPT nature of TPE-BT. The synthetic details for the probe are shown in Scheme S1.†
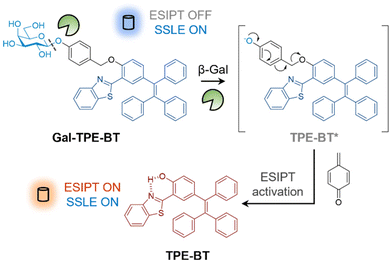 | ||
| Fig. 1 Schematic illustration of the ratiometric detection of β-galactosidase based on excited-state intramolecular proton transfer (ESIPT) and solid-state luminescence enhancement (SSLE). | ||
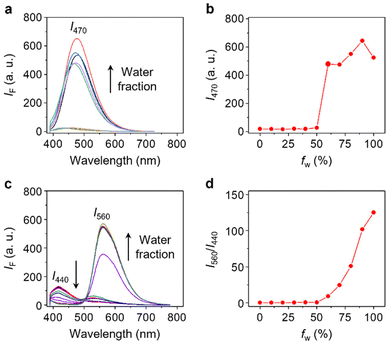
With the probe in hand, we evaluated its photophysical properties in the absence and presence of β-Gal. β-Gal isolated from Escherichia coli was used as a model enzyme for the analysis. We first determined that Gal-TPE-BT exhibited typical SSLE properties. The fluorescence of the probe (excited at 360 nm) was negligible in dimethyl sulfoxide (DMSO) as the good solvent, whereas a gradual increase in the H2O fraction of the solvent system led to a gradual fluorescence enhancement at λmax = 470 nm, which is characteristic of TPE (Fig. 2a and b). The fluorescence of the probe dropped slightly in pure water, which is common for TPE-based fluorogens.34 This suggests the successful suppression of the ESIPT process in Gal-TPE-BT through the masking of the phenolic proton.
In the presence of β-Gal, the fluorescence emission spectra of Gal-TPE-BT in mixed H2O/DMSO solvents changed substantially (Fig. 2c). We observed a new red-shifted emission band with λmax at 560 nm that was sharply enhanced as the water fraction increased, and the original emission band with λmax at 440 nm gradually decreased. The newly emerged emission band is assignable to the keto-state emission of TPE-BT,31 suggesting the recovery of the ESIPT mechanism of the probe.35,36 More interestingly, the gradually enhanced fluorescence at λmax = 560 nm with increasing water fraction suggests the maintenance of the SSLE mechanism in TPE-BT, which favours sensing applications in an aqueous phase.
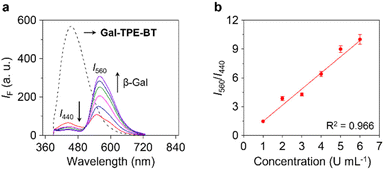
To confirm the enzymatic hydrolysis, mass spectroscopic (MS) analysis generated a MS peak assignable to TPE-BT detected after treatment of Gal-TPE-BT with β-Gal (Fig. S1†), corroborating that the galactosyl substrate can be deglycosylated by the enzyme. We also determined that the ratiometric fluorescence changes (I560/I440) of the probe were dependent on the concentration of β-Gal (Fig. 3a), and a good linearity from 1–6 U mL−1 was determined (Fig. 3b). The limit of detection of the probe for β-Gal was determined to be 0.03 U mL−1 (3σ/k, where σ is the standard deviation of ten blank samples, and k is the linear slope of the ratiometric changes of the probe as a function of β-Gal concentration). In addition, a kinetic study indicated a 13.5-fold increase in the I560/I440 ratio after the reaction between the probe and the enzyme for 300 min (Fig. S2a and b†), and the Km and Vmax were determined to be 27 μM and 0.04 μM s−1, respectively, from the Lineweaver–Burk plots (Fig. S2c and d†). We also measured the Km and Vmax of a commercial fluorescent β-Gal probe, 4-methylumbelliferyl-β-D-galactoside (4-MU-β-Gal) (Fig. S3†). The ∼twofold smaller Km of Gal-TPE-BT (27 μM) than that of 4-MU-β-Gal (48 μM) suggests a higher affinity of our probe for the enzyme.
Next, we studied the morphological changes of Gal-TPE-BT before and after the enzymatic reaction by high-resolution transmission electron microscopy (HRTEM). In its representative TEM images (Fig. S4†), we observed tube-like structures of Gal-TPE-BT, and after addition of β-Gal, aggregated particles began to emerge. In addition, DLS (dynamic light scattering) used showed that the hydrodynamic parameter of the probe after reaction with β-Gal was much larger than that of Gal-TPE-BT without the treatment of β-Gal (Fig. S5a†). These results corroborate the SSLE property of the probe before and after treatment of the enzyme. The critical micelle concentration (CMC) of the probe was determined to be 5.2 μM (Fig. S5b†), and subsequent DLS and fluorescence analyses by continuously incubating the probe in PBS for 60 h suggest the good colloidal stability of Gal-TPE-BT (Fig. S5c and d†).
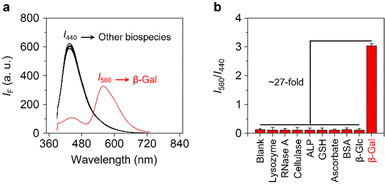
Finally, we evaluated the selectivity of Gal-TPE-BT with a range of different biological species (Fig. 4a and b). We determined that the presence of unselective enzymes including lysozyme, ribonuclease A (RNase A), cellulase and alkaline phosphatase (ALP), and biologically relevant species including γ-glutathione (GSH), vitamin C (VC) and bovine serum albumin (BSA) did not cause the fluorescence emission of the probe to change. More importantly, the treatment of Gal-TPE-BT with a β-glucosidase (β-Glc) that hydrolyzes glucose, which is the C4-epimer of galactose similarly caused minimal fluctuation in fluorescence of the probe. These results suggest the good selectivity of Gal-TPE-BT for β-Gal sensing.
To conclude, the incorporation of both ESIPT and SSLE mechanisms into a single molecular probe led us to achieve the ratiometric detection of β-Gal activity over a range of other enzymes. The large Stokes shift associated with the probe makes it a promising tool for the unbiased analysis of glycosidase activities in diagnostic and drug-screening assays. This research also paves the way for the design of sensitive fluorescent probes for the detection of other enzymatic activities based on mixed photophysical mechanisms.37–41
Conflicts of interest
The authors have no conflicts of interest to declare.Acknowledgements
The authors thank the Natural National Science Foundation of China (no. 91853201, 92253306 and 82151219), the Shanghai Municipal Science and Technology Major Project (no. 2018SHZDZX03), the Fundamental Research Funds for the Central Universities (222201717003), the Programme of Introducing Talents of Discipline to Universities (B16017) and the Open Funding Project of the State Key Laboratory of Bioreactor Engineering. TDJ wishes to thank the Open Research Fund of the School of Chemistry and Chemical Engineering, Henan Normal University for support (2020ZD01). The Research Center of Analysis and Test of East China University of Science and Technology is gratefully acknowledged for assistance in analytical experiments.Notes and references
- C. Reily, T. J. Stewart, M. B. Renfrow and J. Novak, Glycosylation in health and disease, Nat. Rev. Nephrol., 2019, 15, 346 CrossRef PubMed.
- M. R. Pratt and C. R. Bertozzi, Synthetic glycopeptides and glycoproteins as tools for biology, Chem. Soc. Rev., 2005, 34, 58 RSC.
- B. A. H. Smith and C. R. Bertozzi, The clinical impact of glycobiology: targeting selectins, Siglecs and mammalian glycans, Nat. Rev. Drug Discovery, 2021, 20, 217 CrossRef CAS PubMed.
- B. Winchester, Lysosomal metabolism of glycoproteins, Glycobiology, 2005, 15, 1R CrossRef CAS PubMed.
- K. Ohtsubo and J. D. Marth, Glycosylation in Cellular Mechanisms of Health and Disease, Cell, 2006, 126, 855 CrossRef CAS PubMed.
- B. Y. Lee, J. A. Han, J. S. Im, A. Morrone, K. Johung, E. C. Goodwin, W. J. Kleijer, D. DiMaio and E. S. Hwang, Senescence-associated β-galactosidase is lysosomal β-galactosidase, Aging Cell, 2006, 5, 187 CrossRef CAS PubMed.
- D. G. Hildebrand, S. Lehle, A. Borst, S. Haferkamp, F. Essmann and K. Schulze-Osthoff, α-Fucosidase as a novel convenient biomarker for cellular senescence, Cell Cycle, 2013, 12, 1922 CrossRef CAS PubMed.
- S. K. Chatterjee, M. Bhattacharya and J. J. Barlow, Glycosyltransferase and glycosidase activities in ovarian cancer patients, Cancer Res., 1979, 39, 1943 CAS.
- Y. Jiang, A. Sun, Y. Zhao, W. Ying, H. Sun, X. Yang, B. Xing, W. Sun, L. Ren, B. Hu, C. Li, L. Zhang, G. Qin, M. Zhang, N. Chen, M. Zhang, Y. Huang, J. Zhou, Y. Zhao, M. Liu, X. Zhu, Y. Qiu, Y. Sun, C. Huang, M. Yan, M. Wang, W. Liu, F. Tian, H. Xu, J. Zhou, Z. Wu, T. Shi, W. Zhu, J. Qin, L. Xie, J. Fan, X. Qian, F. He and Chinese Human Proteome Project (CNHPP) Consortium, Proteomics identifies new therapeutic targets of early-stage hepatocellular carcinoma, Nature, 2019, 567, 257 CrossRef CAS PubMed.
- J.-J. Zhang, X.-Z. Chai, X.-P. He, H.-J. Kim, J. Y. Yoon and H. Tian, Fluorogenic probes for disease-relevant enzymes, Chem. Soc. Rev., 2019, 48, 683 RSC.
- X.-L. Hu, H.-Q. Gan, F.-D. Meng, H.-H. Han, D.-T. Shi, S. Zhang, L. Zou, X.-P. He and T. D. James, Fluorescent probes and functional materials for biomedical applications, Front. Chem. Sci. Eng., 2022, 16, 1425 CrossRef CAS.
- W.-T. Dou, H.-H. Han, A. C. Sedgwick, G.-B. Zhu, Y. Zang, X.-R. Yang, J. Y. Yoon, T. D. James, J. Li and X.-P. He, Fluorescent probes for the detection of disease-associated biomarkers, Sci. Bull., 2022, 67, 853 CrossRef CAS PubMed.
- H. Singh, K. Tiwari, R. Tiwari, S. K. Pramanik and A. Das, Small Molecule as Fluorescent Probes for Monitoring Intracellular Enzymatic Transformations, Chem. Rev., 2019, 119, 11718 CrossRef CAS PubMed.
- X.-H. Li, X.-H. Gao, W. Shi and H.-M. Ma, Design Strategies for Water-Soluble Small Molecular Chromogenic and Fluorogenic Probes, Chem. Rev., 2014, 114, 590 CrossRef CAS PubMed.
- H.-W. Liu, L.-L. Chen, C.-Y. Xu, Z. Li, H.-Y. Zhang, X.-B. Zhang and W.-H. Tan, Recent progresses in small-molecule enzymatic fluorescent probes for cancer imaging, Chem. Soc. Rev., 2018, 47, 7140 RSC.
- X.-F. Wu, R. Wang, N. Kwon, M.-H. Ma and J.-Y. Yoon, Activatable fluorescent probes for in situ imaging of enzymes, Chem. Soc. Rev., 2022, 51, 450 RSC.
- H.-D. Li, D. Kim, Q.-C. Yao, H.-Y. Ge, J. Chung, J.-L. Fan, J.-Y. Wang, X.-J. Peng and J.-Y. Yoon, Activity-Based NIR Enzyme Fluorescent Probes for the Diagnosis of Tumors and Image-Guided Surgery, Angew. Chem., Int. Ed., 2021, 60, 17268 CrossRef CAS PubMed.
- Y. Urano, M. Kamiya, K. Kanda, T. Ueno, K. Hirose and T. Nagano, Evolution of Fluorescein as a Platform for Finely Tunable Fluorescence Probes, J. Am. Chem. Soc., 2005, 127, 4888 CrossRef CAS PubMed.
- G.-Y. Jiang, G.-J. Zeng, W.-P. Zhu, Y.-D. Li, X.-B. Dong, G.-X. Zhang, X.-L. Fan, J.-G. Wang, Y.-Q. Wu and B.-Z. Tang, A selective and light-up fluorescent probe for β-galactosidase activity detection and imaging in living cells based on an AIE tetraphenylethylene derivative, Chem. Commun., 2017, 53, 4505 RSC.
- K.-Z. Gu, Y.-S. Xu, H. Li, Z.-Q. Guo, S.-J. Zhu, S.-Q. Zhu, P. Shi, T. D. James, H. Tian and W.-H. Zhu, Real-Time Tracking and In Vivo Visualization of β-Galactosidase Activity in Colorectal Tumor with a Ratiometric Near-Infrared Fluorescent Probe, J. Am. Chem. Soc., 2016, 138, 5334 CrossRef CAS PubMed.
- X.-K. Li, W.-J. Qiu, J.-W. Li, X. Chen, Y.-L. Hu, Y. Gao, D.-L. Shi, X.-M. Li, H.-L. Lin, Z.-L. Hu, G.-Q. Dong, C.-Q. Sheng, B. Jiang, C.-L. Xia, C.-Y. Kim, Y. Guo and J. Li, First-generation species-selective chemical probes for fluorescence imaging of human senescence-associated β-galactosidase, Chem. Sci., 2020, 11, 7292 RSC.
- K.-Z. Gu, W.-S. Qiu, Z.-Q. Guo, C.-X. Yan, S.-Q. Zhu, D.-F. Yao, P. Shi, H. Tian and W.-H. Zhu, An enzyme-activatable probe liberating AIEgens: on-site sensing and long-term tracking of β-galactosidase in ovarian cancer cells, Chem. Sci., 2019, 10, 398 RSC.
- D. Asanuma, M. Sakabe, M. Kamiya, K. Yamamoto, J. Hiratake, M. Ogawa, N. Kosaka, P. L. Choyke, T. Nagano, H. Kobayashi and Y. Urano, Sensitive β-galactosidase-targeting fluorescence probe for visualizing small peritoneal metastatic tumours in vivo, Nat. Commun., 2015, 6, 6463 CrossRef CAS PubMed.
- S. Cecioni, R. A. Ashmus, P.-A. Gilormini, S. Zhu, X. Chen, X.-Y. Shan, C. Gros, M. C. Deen, Y. Wang, R. Britton and D. J. Vocadlo, Quantifying lysosomal glycosidase activity within cells using bis-acetal substrates, Nat. Chem. Biol., 2022, 18, 332 CrossRef CAS PubMed.
- A. K. Yadav, D. L. Shen, X.-Y. Shan, X. He, A. R. Kermode and D. J. Vocadlo, Fluorescence-Quenched Substrates for Live Cell Imaging of Human Glucocerebrosidase Activity, J. Am. Chem. Soc., 2015, 137, 1181 CrossRef CAS PubMed.
- S.-J. Chen, X.-D. Ma, L. Wang, Y.-Y. Wu, Y.-P. Wang, W.-K. Fan and S.-C. Hou, Design and application of lysosomal targeting pH-sensitive β-galactosidase fluorescent probe, Sens. Actuators, B, 2023, 379, 133272 CrossRef CAS.
- L. Dong, M.-Y. Zhang, H.-H. Han, Y. Zang, G.-R. Chen, J. Li, X.-P. He and S. Vidal, A General Strategy to the Intracellular Sensing of Glycosidases using AIE-Based Glycoclusters, Chem. Sci., 2022, 13, 247 RSC.
- Z.-G. Song, R. T. K. Kwok, E.-G. Zhao, Z.-K. He, Y.-N. Hong, J. W. Y. Lam, B. Liu and B.-Z. Tang, A Ratiometric Fluorescent Probe Based on ESIPT and AIE Processes for Alkaline Phosphatase Activity Assay and Visualization in Living Cells, ACS Appl. Mater. Interfaces, 2014, 6, 17245 CrossRef CAS PubMed.
- Y. Liu, J. Nie, J. Niu, W.-S. Wang and W.-Y. Lin, An AIE + ESIPT ratiometric fluorescent probe for monitoring sulfur dioxide with distinct ratiometric fluorescence signals in mammalian cells, mouse embryonic fibroblast and zebrafish, J. Mater. Chem. B, 2018, 6, 1973 RSC.
- G.-L. Zeng, Z.-H. Liang, X. Jiang, T.-T. Quan and T.-S. Chen, An ESIPT-Dependent AIE Fluorophore Based on HBT Derivative: Substituent Positional Impact on Aggregated Luminescence and its Application for Hydrogen Peroxide Detection, Chem. – Eur. J., 2022, 28, e202103241 CAS.
- Q. Chen, C.-M. Jia, Y.-F. Zhang, W. Du, Y. Wang, Y. Huang, Q.-Y. Yanga and Q. Zhang, A novel fluorophore based on the coupling of AIE and ESIPT mechanisms and its application in biothiol imaging, J. Mater. Chem. B, 2017, 5, 7736 RSC.
- A. C. Sedgwick, L.-L. Wu, H.-H. Han, S. D. Bull, X.-P. He, T. D. James, J. L. Sessler, B.-Z. Tang, H. Tian and J. Yoon, Excited-state intramolecular proton-transfer (ESIPT) based fluorescence sensors and imaging agents, Chem. Soc. Rev., 2018, 47, 8842 RSC.
- C. Rivasa, M. Kamiya and Y. Urano, A novel sialidase-activatable fluorescence probe with improved stability for the sensitive detection of sialidase, Bioorg. Med. Chem., 2020, 30, 126860 CrossRef PubMed.
- X. Feng, C.-X. Qi, H.-T. Feng, Z. Zhao, H. H. Y. Sung, L. D. Williams, R. T. K. Kwok, J. W. Y. Lam, A. Qin and B.-Z. Tang, Dual fluorescence of tetraphenylethylene-substituted pyrenes with aggregation-induced emission characteristics for white-light emission, Chem. Sci., 2018, 9, 5679 RSC.
- N. A. Kukhta and M. R. Bryce, Dual emission in purely organic materials for optoelectronic applications, Mater. Horiz., 2021, 8, 33 RSC.
- S. K. Behera, S. Y. Park and J. Gierschner, Dual Emission: Classes, Mechanisms, and Conditions, Angew. Chem., Int. Ed., 2021, 60, 22624 CrossRef CAS PubMed.
- W.-T. Dou, X. Wang, T. Liu, S.-W. Zhao, J.-J. Liu, Y. Yan, J. Li, C.-Y. Zhang, A. C. Sedgwick, H. Tian, J. L. Sessler, D.-M. Zhou and X.-P. He, A homogeneous high-throughput array for the detection and discrimination of influenza A viruses, Chem, 2022, 8, 1750 CAS.
- W.-T. Dou, Z.-Y. Qin, J. Li, D.-M. Zhou and X.-P. He, Self-assembled sialyllactosyl probes with aggregation-enhanced properties for ratiometric detection and blocking of influenza viruses, Sci. Bull., 2019, 64, 1902 CrossRef CAS PubMed.
- W.-T. Dou, W. Chen, X.-P. He, J. Su and H. Tian, Vibration-Induced-Emission (VIE) for imaging amyloid β fibrils, Faraday Discuss., 2017, 196, 395 RSC.
- A. C. Sedgwick, K.-C. Yan, D. N. Mangel, Y. Shang, A. Steinbrueck, H.-H. Han, J. T. Brewster, X.-L. Hu, D. W. Snelson, V. M. Lynch, H. Tian, X.-P. He and J. L. Sessler, Deferasirox (ExJade): An FDA-Approved AIEgen Platform with Unique Photophysical Properties, J. Am. Chem. Soc., 2020, 142, 1925 CrossRef PubMed.
- X.-L. Hu, A. C. Sedgwick, D. N. Mangel, Y. Shang, A. Steinbrueck, K.-C. Yan, L. Zhu, D. W. Snelson, S. Sen, C. V. Chau, G. Juarez, V. M. Lynch, X.-P. He and J. L. Sessler, Tuning the Solid- and Solution-State Fluorescence of the Iron Chelator Deferasirox, J. Am. Chem. Soc., 2022, 144, 7382 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Additional figures, experimental section and original spectral copies of new compounds. See DOI: https://doi.org/10.1039/d3qo00605k |
| This journal is © the Partner Organisations 2023 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
: