Open Access Article
Open Access Article1-Azahomocubane†
Tyler
Fahrenhorst-Jones
 a,
David L.
Marshall
a,
David L.
Marshall
 b,
Jed. M.
Burns
b,
Jed. M.
Burns
 a,
Gregory K.
Pierens
a,
Gregory K.
Pierens
 c,
Robert E.
Hormann
d,
Allison M.
Fisher
d,
Paul V.
Bernhardt
c,
Robert E.
Hormann
d,
Allison M.
Fisher
d,
Paul V.
Bernhardt
 a,
Stephen J.
Blanksby
a,
Stephen J.
Blanksby
 b,
G. Paul
Savage
e,
Philip E.
Eaton
d and
Craig M.
Williams
b,
G. Paul
Savage
e,
Philip E.
Eaton
d and
Craig M.
Williams
 *a
*a
aSchool of Chemistry and Molecular Biosciences, University of Queensland, Brisbane, 4072, Queensland, Australia. E-mail: c.williams3@uq.edu.au
bCentral Analytical Research Facility and School of Chemistry and Physics, Queensland University of Technology, Brisbane, 4000, Queensland, Australia
cCentre for Advanced Imaging, University of Queensland, Brisbane, 4072, Queensland, Australia
dDepartment of Chemistry, University of Chicago, Chicago, Illinois 60637, USA
eCSIRO Manufacturing, Ian Wark Laboratory, Melbourne, 3168, Victoria, Australia
First published on 22nd February 2023
Abstract
Highly strained cage hydrocarbons have long stood as fundamental molecules to explore the limits of chemical stability and reactivity, probe physical properties, and more recently as bioactive molecules and in materials discovery. Interestingly, the nitrogenous congeners have attracted much less attention. Previously absent from the literature, azahomocubanes, offer an opportunity to investigate the effects of a nitrogen atom when incorporated into a highly constrained polycyclic environment. Herein disclosed is the synthesis of 1-azahomocubane, accompanied by comprehensive structural characterization, physical property analysis and chemical reactivity. These data support the conclusion that nitrogen is remarkably well tolerated in a highly strained environment.
Introduction
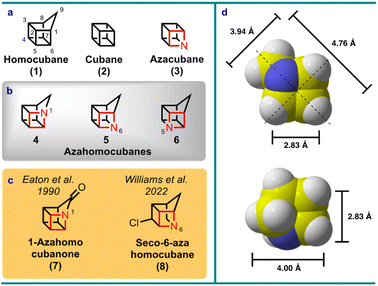
Homocubane (1) was synthesized by Dunn et al.1 and then Dauben et al.2 as simultaneous contributions in 1966, just two years after the Platonic solid mimic cubane (2) by Eaton and Cole in 1964.3 Some 25 years later the nitrogen (aza) analogs of cubane became the subject of considerable theoretical and synthetic interest (i.e., azacubane, 3),4–6 but no member of the azacubanes have to-date succumbed to synthesis due to the considerable synthetic challenge these molecules pose.7 In addition, the corresponding homologues, azahomocubanes 4–6, are unprecedented in the literature, although 1-azahomocuban-9-one (7) was suggested as a probable reactive intermediate in related studies by Eaton et al.5,8 The closest relative is a seco-6-azahomocubane derivative (i.e., 8), which was only recently synthesized by Williams et al.9 (Fig. 1). Therefore, with the prospect of gaining additional physical property insight on uniquely strained aza hydrocarbons,10 combined with the formidable synthetic challenge, and the contemporary interest in exploring fundamental cage hydrocarbon scaffolds for bioactive molecule11,12 and materials13 discovery, avenues to accessing and isolating an azahomocubane were pursued as reported herein.Results and discussion
Synthesis of 1-azahomocubane
1-Azahomocubane (4, Fig. 1) was the pinnacle target chosen for this endeavour given the parent status of this molecule class (i.e., first in the series). From a retrosynthetic perspective, adopting a synthetic approach to 1-azahomocubane (4) via a cubyl azide (e.g., 9) was logical, because the majority of the target skeleton could be imported from a commercial cubane derivative via nitrene insertion chemistry. Although, the route presented potential hazards inherent of high energy systems, early inspiration towards this goal was achieved with 4-methylcubyl azide (10). Even though azide 10 had previously been reported to display promiscuous rearrangement behaviour,14 it was discovered that on thermolysis in the presence of dimethylbutadiene the methylazahomocubanes 11 and 12 could be obtained (Scheme 1). However, the yield was observed to be very low (i.e. 2–4%), presumably due to the high energy conditions required to drive the nitrene (13) to carbene (14) rearrangement,15 which also led to the formation of other isolated products (see ESI†).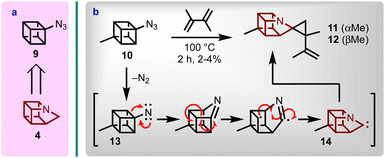 | ||
| Scheme 1 (a) Retrosynthesis of 4 from 9. (b) Synthesis of spirocyclopropyl methyl azahomocubane (11 and 12; diastereomers tentatively assigned) via nitrene 14. | ||
In addition to the findings above, it had also been reported that exposure of cubyl azide (9) to strongly acidic conditions affords seco-azacubane ester (15, Scheme 2).8 However, experimental details were scant, and furthermore adopting 15 as a viable precursor would necessitate working with sensitive azides on synthetic scale.16 Conversely, the core strength of this approach was the programmed installation of the nitrogen atom, via an “aza-Favorskii” type transformation, initiated by ring-expansion of 9 to 16 through a Schmidt rearrangement, and then followed by ring-contraction of N-chloro acetate 17 to 15 (likely via7) (Scheme 2).
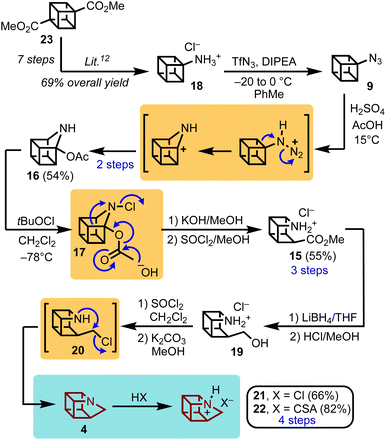 | ||
| Scheme 2 Synthesis of 1-azahomocubane (4), and the corresponding salts (21 and 22), via the seco-azacubane ester (15) arising from an acid promoted azide rearrangement. | ||
Therefore, in pursuit of this synthetic direction, a modified route was developed by treating aminocubane 18 with triflyl azide under conditions that obviated the need to handle cubyl azide (9) neat. With safety hazards mitigated, and reliable and reproducible access to 15 achieved, the remaining aspects of the route were within reach. Reduction of 15 with lithium borohydride proceeded smoothly to give the primary alcohol 19 as the hydrochloride salt, which was subjected to chlorination using thionyl chloride. This was quickly followed by treatment with base to give the neutral chloride 20, which facilitated intramolecular alkylation (i.e., cyclization) and delivery of 1-azahomocubane (4) (Scheme 2). The free amine 4 was immediately trapped as either the hydrochloride (21) or camphorsulfonate salt (22) to avoid evaporation. Overall, the synthesis was achieved in 16 steps in 17% yield from commercial dimethyl 1,4-cubanedicarboxylate (23).
Chemical stability of 1-azahomocubane
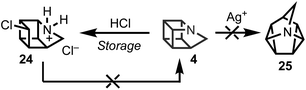
1-Azahomocubane (4) could be liberated from either ammonium salt with potassium carbonate, which enabled full NMR characterization of both the free base and salts. Treatment of 4 with excess hydrochloric acid at room temperature (RT) facilitated ring opening and lead to seco-azahomocubane 24 (Scheme 3). Ring opening could be significantly reduced by careful addition of 1 equiv. hydrochloric acid (1.0 M) at low temperatures (i.e., <0 °C). This decomposition pathway was not observed during routine handling of the hydrochloride salt at room temperature, but was noted on prolonged storage in the refrigerator over several months. Considering the high ring strain inherent in the homocubane structure, all attempts to reconstitute 1-azahomocubane (4) from base treatment of 24 understandably failed. That said, both the hydrogen camphorsulfonate 22 (and triflate) salts of 4 demonstrated remarkable stability. In the case of the free amine it could be stored in solution (e.g., C6D6) for over a month with no discernible evidence of decomposition (stored on the bench at room temp. in the absence of light). This level of stability was reinforced through multiple unsuccessful attempts to promote valence bond isomerization to 25 (Scheme 3),17 which consistently returned starting material (i.e., 4) – even at elevated temperatures (e.g., 70 °C) in the presence of Ag+.Structural analyses and physical properties of 1-azahomocubane
From Density Functional Theory (DFT) calculations, the sum of the bond angles around the nitrogen atom in azahomocubane (4) was found to be 299.6°, indicating significant deviation from ideal sp3 hybridization as expected (328.5°, Fig. 2a). Comparison to the conjugate acid (Fig. S14a†) predicted that protonation induces planarization of the nitrogen atom (e.g., C2–N–C9 increases from 105.8° to 108.3°), in addition to lengthening of the adjacent bonds: supporting the proposed mechanism for ring-opening of protonated azahomocubane (21) by chloride via SN2 displacement to give 24. X-ray crystallographic data were obtained for 4 as a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 silver perchlorate complex (Fig. 2b), which confirmed the structure (Cs point group). Gratifyingly, the solid state geometries for the silver complex were close to the predicted bond lengths and angles computed for the free amine (e.g., C2–N–C9: 105.7°).
1 silver perchlorate complex (Fig. 2b), which confirmed the structure (Cs point group). Gratifyingly, the solid state geometries for the silver complex were close to the predicted bond lengths and angles computed for the free amine (e.g., C2–N–C9: 105.7°).
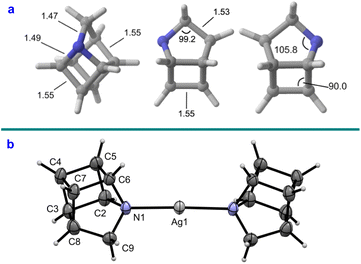 | ||
Fig. 2 (a) Calculated geometries of 4 in Ångstroms. (b) X-ray crystal structure analysis of 4 as a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex with silver perchlorate – ellipsoids shown at 50% probability. 1 complex with silver perchlorate – ellipsoids shown at 50% probability. | ||
To gain wider physical property perspective, 1-azahomocubane was compared against the most relevant bridgehead azabicyclic systems available, 1-azabicyclo[2.2.1]heptane (26) and 1-azabicyclo[2.2.2]octane (i.e., quinuclidine, 27) (Table 1). Firstly, aqueous acidity measurements (pKa) were undertaken (via the 1H NMR titration method),18 which revealed a clear trend whereby increasing acidity of the conjugate acid (i.e., pKa 11.5 to 9.7) correlated with increasing geometrical strain (i.e., 27 to 4). This trend was supported by gas-phase proton affinity (PA) measurements,19 which demonstrated that, even in the absence of solvent, the highly strained nitrogen bonding arrangement decreases the intrinsic basicity of 4 (PA = 975 kJ mol−1) relative to other bridgehead amines (Table 1).20
| Amine | pKaa | Proton affinityb,c (calcdd) | 15N NMRf (C6D6) | Natural electron configurationg s (p) orbitals | Bridgehead 1JC–Hh,i | ∑bond angles for nitrogenl | Strain energyl | |
|---|---|---|---|---|---|---|---|---|
a pKa values were determined using a H2O/D2O ratio of 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 [note: The Ka (acidity constant) values are the reciprocals of the protonation constants and converted to pKa].
b Gas-phase proton affinity determinations in kJ mol−1.
c Absolute uncertainty in PA values is considered to be ±8 kJ mol−1 due to uncertainty in reference value while relative PA values have much lower uncertainties.
d Calculated proton affinity (and ionization potential energies, Table S4) determined at the M06-2X(GD)/6-311++G(d,p) level of theory.
e Empirical correction to calculated value, see Fig. S12.
f Measured in ppm.
g Number of electrons.
h Measured in Hertz against the corresponding (all carbon) cage hydrocarbon.
i For all bridgehead position 13C–1H coupling constants see Table S8 in the ESI.
j Position 8.
k Position 4.
l Sum of all bond angles around the nitrogen atom expressed in degrees and strain energies expressed in kcal mol−1 as calculated at the M062X-D3/Def2TZVPP level of theory – corresponding hydrocarbon value in parentheses. 1 [note: The Ka (acidity constant) values are the reciprocals of the protonation constants and converted to pKa].
b Gas-phase proton affinity determinations in kJ mol−1.
c Absolute uncertainty in PA values is considered to be ±8 kJ mol−1 due to uncertainty in reference value while relative PA values have much lower uncertainties.
d Calculated proton affinity (and ionization potential energies, Table S4) determined at the M06-2X(GD)/6-311++G(d,p) level of theory.
e Empirical correction to calculated value, see Fig. S12.
f Measured in ppm.
g Number of electrons.
h Measured in Hertz against the corresponding (all carbon) cage hydrocarbon.
i For all bridgehead position 13C–1H coupling constants see Table S8 in the ESI.
j Position 8.
k Position 4.
l Sum of all bond angles around the nitrogen atom expressed in degrees and strain energies expressed in kcal mol−1 as calculated at the M062X-D3/Def2TZVPP level of theory – corresponding hydrocarbon value in parentheses.
|
||||||||
| 1-Azacubane (3) |

|
— | — [975e (968)] | — | 1.47 (3.89) | — | 267.9 (270) | 155.5 (162.8) |
| 1-Azahomocubane (4) |

|
9.7 (±0.1) | 975 (968) | 78.7 | 1.38 (3.99) | 146.7 (146) | 299.6 (300.6) | 110.4 (117.6) |
| 1-Azabicyclo[2.2.1]heptane (26) |

|
11.1 (±0.1) | 980 (973) | 58.7 | 1.35 (4.03) | 143.0 (140.1)k | 309.5 (—) | 15.7 (—) |
| 1-Azabicyclo[2.2.2]octane (27) |

|
11.5 (±0.1) 11.3 (ref. 22) | 983 (ref. 23) (978) | 15.0 | 1.30 (4.09) | 137.5 (134.3)k | 327.6 (—) | 10.6 (—) |
Potential differences in skeletal hybridization were then assessed by measuring the 13C–1H coupling constants (1JC–H)21 at the bridgehead positions opposite to the aza-bridgehead. Comparing these values with the corresponding hydrocarbons (Tables 1 and S8†) indicated little change had occurred within the aza series. This was reinforced by the calculated geometries for homocubane (1) (Fig. S14c†), which demonstrated that nitrogen substitution leads to slight shortening of the adjacent bonds (as expected due to the increased electronegativity), and enhanced pyramidalization around the nitrogen centre (e.g., C–N–C: 105.8° vs. 106.7°). To explore these effects further, natural abundance 15N NMR measurements (Table 1) were performed using long-range 1H–15N heteronuclear shift correlation methods (HMBC).24 These measurements revealed that 1-azahomocubane (4) (δN 78.7 ppm) is less electron rich at the nitrogen centre (deshielding the nucleus) compared to the related azabicyclic systems, 26 (δN 58.7 ppm) and 27 (δN 15.0 ppm). Combined with the 13C–1H coupling constant data suggested an upward trend in lone pair s-character, as supported by natural electronic configuration calculations, which is in line with the increasing pKa and PA acidity trend i.e., decreasing basicity (Table 1).
In order to obtain a deeper appreciation for the ring strain present in these systems, hypothetical atom exchange and hypohomodesmotic reactions25 were conducted. For the conversion of cubane (2) to azacubane (3), and homocubane (1) into azahomocubane (4) (Fig. S13†), it was determined that 3 is more stable than 2 by 7.3 kcal mol−1, and 4 is similarly 7.2 kcal mol−1 (strain energy, Table 1) more stable than 1. Overall, homologation of azacubane to azahomocubane lowers the strain energy by ∼45 kcal mol−1, which can be rationalized by the inclusion of a more stable 5-membered ring. Interestingly, the azabicyclo[2.2.1] (26) and [2.2.2] (27) systems are approximately an order of magnitude less strained. To rationalize the unexpected stabilization observed for 1-azahomocubane, Second Order Perturbation Theory (SOPT, Table S6†)26 analysis was also performed. Nitrogen lone pair – sigma* interactions with the antiperiplanar carbon atoms were thus determined to be 5.8 kcal mol−1 (“cubic” C–C bonds) and 4.6 kcal mol−1 (“basket handle” C–C bond) respectively. A weaker interaction (2.9 kcal mol−1) was found for the nitrogen lone pair into the adjacent cyclobutyl C–H antibonding orbitals. This analysis suggested that the modest stabilization on introducing a nitrogen atom at the bridgehead of homocubane was due to hyperconjugative effects, though the minimal structural deformation (see above) indicated that this effect is minor. Orbital rehybridization effects, are also likely to be an additional contributor to the observed stabilization [i.e., Bent's rule and associated theories27] as evidenced by the observable change in s-character of the nitrogen lone pair orbital (e.g., 13C–1H coupling constants and pKa).28
Conclusions
The synthesis of 1-azahomocubane (4) has been achieved 56 years after the corresponding parent hydrocarbon was reported [i.e., homocubane (1)]. Schmidt and aza-Favorskii type rearrangements were adopted, as the key steps, to achieve the synthesis. Access to 4 enabled a comprehensive experimental and computational analysis of a nitrogen atom positioned within a highly strained environment. In consideration of the compiled data set, this ring system not only displayed unexpected and remarkable stability as the free amine (and protonated CSA and triflate salts), but incorporation of a tertiary nitrogen atom into a small ring strained polycyclic framework yielded surprisingly small geometric changes relative to the all-carbon system. Finally, future investigations involving this ring system will aim to determine potential viability as a novel scaffold.Data availability
All experimental data are provided in the ESI.†Author contributions
T. F. J., G. P. S., P. E. E. and C. M. W. conceived the project. T. F. J., R. E. H. and A. M. F performed chemical synthesis. D. L. M. undertook proton affinity determinations. J. M. B. and S. J. B. carried out in silico calculations. G. K. P. obtained heteroatom coupling constants and nitrogen NMR data. T. F. J. and P. V. B. performed the acidity measures and analysis. P. V. B. acquired the X-ray crystallographic data and performed the analysis. C. M. W. coordinated the study, assisted in analysing and interpreting the data, and wrote the manuscript. All authors read and agreed on the content of the paper.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the University of Queensland (UQ), the Commonwealth Scientific and Industrial Research Organisation (CSIRO), Queensland University of Technology and the University of Chicago for financial support. The Australian Research Council is also gratefully acknowledged; DP180103004 (C. M. W.), DP170101596 (S. J. B.), FT110100851 (C. M. W.). T. F.-J. gratefully acknowledges the Australian Government for a Research Training Program (RTP) Scholarship. J. M. B. gratefully acknowledges computational resources provided by the NCI NF and UQ RCC. Em. Prof. Curt Wentrup (UQ) is sincerely thanked for in-depth physical organic chemistry discussions.Notes and references
- G. L. Dunn, V. J. DiPasquo and J. R. E. Hoover, Synthesis of pentacyclo[4.3.0.02,5.03,8.04,7]nonane and some 4-substituted derivatives, Tetrahedron Lett., 1966, 7, 3737–3742 CrossRef
.
- W. G. Dauben and D. L. Whalen, Pentacyclo[4.4.0.02,5.03,8.04,7]decane and pentacyclo[4.3.0.02,5.03,8.04,7]nonane, Tetrahedron Lett., 1966, 7, 3743–3750 CrossRef
.
- P. E. Eaton and T. W. Cole, CubaneJ. Am. Chem. Soc., 1964, 86, 3157–3158 CrossRef CAS
.
- I. Alkorta, J. Elguero, I. Rozas and A. T. Balaban, Theoretical studies of aza analogues of platonic hydrocarbons: Part 1. Cubane and its aza derivatives, J. Mol. Struct.: THEOCHEM, 1990, 206, 67–75 CrossRef
.
- J. S. Murray, J. M. Seminario, P. Lane and P. Politzer, Anomalous energy effects associated with the presence of aza nitrogens and nitro substituents in some strained systems, J. Mol. Struct.: THEOCHEM, 1990, 207, 193–200 CrossRef
.
- P. E. Eaton, A. M. Fisher and R. E. Hormann, Azidocubanes; 2. Acid-Induced Rearrangement: Formation of 9-Azahomocubanes, Synlett, 1990, 737–738 CrossRef CAS
.
- K. S. A. Arachchige, T. Fahrenhorst-Jones, J. M. Burns, H. A. AL-Fayaad, J. N. Behera, C. N. R. Rao, J. K. Clegg and C. M. Williams, 1,4-Diazacubane crystal structure rectified as piperazinium, Chem. Commun., 2019, 55, 11751–11753 RSC
.
- R. Gilardi, C. George, J. Karle, P. E. Eaton and A. M. Fisher, Aza-analogues of cage compounds: Potential precursors to azacubanes and 1-azahomocubanes, J. Heterocycl. Chem., 1993, 30, 1385–1388 CrossRef CAS
.
- T. Fahrenhorst-Jones, P. V. Bernhardt, G. P. Savage and C. M. Williams, The (±)-6-Aza[1.0]triblattane Skeleton: Contraction beyond the Wilder–Culberson Ring System, Org. Lett., 2022, 24, 903–906 CrossRef CAS PubMed
.
- D. H. Aue, H. M. Webb and M. T. Bowers, Photoelectron spectrum and gas-phase basicity of manxine. Evidence for a planar bridgehead nitrogen, J. Am. Chem. Soc., 1975, 97, 4136–4137 CrossRef CAS
.
-
(a) T. P. Stockdale and C. M. Williams, Pharmaceuticals that contain polycyclic hydrocarbon scaffolds, Chem. Soc. Rev., 2015, 44, 7737–7763 RSC
; (b) B. A. Chalmers, H. Xing, S. Houston, C. Clark, S. Ghassabian, A. Kuo, B. Cao, A. Reitsma, C.-E. P. Murray, J. E. Stok, G. M. Boyle, C. J. Pierce, S. W. Littler, D. A. Winkler, P. V. Bernhardt, C. Pasay, J. J. De Voss, J. McCarthy, P. G. Parsons, G. H. Walter, M. T. Smith, H. M. Cooper, S. K. Nilsson, J. Tsanaktsidis, G. P. Savage and C. M. Williams, Validating Eaton's Hypothesis: Cubane as a Benzene Bioisostere, Angew. Chem., Int. Ed., 2016, 55, 3580–3585 CrossRef CAS PubMed
.
-
(a) M. A. M. Subbaiah and N. A. Meanwell, Bioisosteres of the Phenyl Ring: Recent Strategic Applications in Lead Optimization and Drug Design, J. Med. Chem., 2021, 64, 14046–14128 CrossRef CAS PubMed
, see also strain release entry to cage systems.; (b) J. M. Lopchuk, K. Fjelbye, Y. Kawamata, L. R. Malins, C.-M. Pan, R. Gianatassio, J. Wang, L. Prieto, J. Bradow, T. A. Brandt, M. R. Collins, J. Elleraas, J. Ewanicki, W. Farrell, O. O. Fadeyi, G. M. Gallego, J. J. Mousseau, R. Oliver, N. W. Sach, J. K. Smith, J. E. Spangler, H. Zhu, J. Zhu and P. S. Baran, Strain-Release Heteroatom Functionalization: Development, Scope, and Stereospecificity, J. Am. Chem. Soc., 2017, 139, 3209–3226 CrossRef CAS PubMed
; (c) J. Turkowska, J. Durka and D. Gryko, Strain release – an old tool for new transformations, Chem. Commun., 2020, 56, 5718–5734 RSC
; (d) P. K. Mykhailiuk, Saturated bioisosteres of benzene: where to go next?, Org. Biomol. Chem., 2019, 17, 2839–2849 RSC
.
- M. Sugiyama, M. Akiyama, Y. Yonezawa, K. Komaguchi, M. Higashi, K. Nozaki and T. Okazoe, Electron in a cube: Synthesis and characterization of perfluorocubane as an electron acceptor, Science, 2022, 377, 756–759 CrossRef CAS PubMed
.
- P. E. Eaton and R. E. Hormann, Azidocubanes. 1. Photolysis: formation of a homoprismyl nitrile, J. Am. Chem. Soc., 1987, 109, 1268–1269 CrossRef CAS
.
- C. Wentrup, Flash Vacuum Pyrolysis of Azides, Triazoles, and Tetrazoles, Chem. Rev., 2017, 117, 4562–4623 CrossRef CAS PubMed
.
- M. A. Dallaston, S. D. Houston and C. M. Williams, Cubane, Bicyclo[1.1.1]pentane and Bicyclo[2.2.2]octane: Impact and Thermal Sensitiveness of Carboxyl-, Hydroxymethyl- and Iodo-substituents, Chem.–Eur. J., 2020, 26, 11966–11970 CrossRef CAS PubMed
.
- L. A. Paquette, J. S. Ward, R. A. Boggs and W. B. Farnham, Valence isomerization of homocubanes. Reversible complex formation and kinetic substituent effects operating during silver(I)-induced bond reorganization, J. Am. Chem. Soc., 1975, 97, 1101–1112 CrossRef CAS
.
- Z. Szakács and G. Hägele, Accurate determination of low pK values by 1H NMR titration, Talanta, 2004, 62, 819–825 CrossRef PubMed
.
- R. G. Cooks and P. S. H. Wong, Kinetic Method of Making Thermochemical Determinations: Advances and Applications, Acc. Chem. Res., 1998, 31, 379–386 CrossRef CAS
.
- Applying an empirical correction to computed PA values based on the experimentally determined basicities, including 4, predicted an identical proton affinity of 975 kJ/mol for 1-azacubane (3) (Figure S12†).
- E. W. Della, P. T. Hine and H. K. Patney, Carbon-13 spectral parameters of some polycyclic hydrocarbons, J. Org. Chem., 1977, 42, 2940–2941 CrossRef CAS
.
- C. A. Grob, Inductive Charge Dispersal in Quinuclidinium Ions. Polar Effects, Part 13, Helv. Chim. Acta, 1985, 68, 882–886 CrossRef CAS
.
- E. P. L. Hunter and S. G. Lias, Evaluated Gas Phase Basicities and Proton Affinities of Molecules: An Update, J. Phys. Chem. Ref. Data, 2009, 27, 413 CrossRef
.
-
(a) S. E. Wheeler, K. N. Houk, P. v. R. Schleyer and W. D. Allen, A Hierarchy of Homodesmotic Reactions for Thermochemistry, J. Am. Chem. Soc., 2009, 131, 2547–2560 CrossRef CAS PubMed
; (b) For an example that correlates to 15N NMR shifts and proton affinity for non-basic caged amines, see I. A. Yaremenko, Y. Yu. Belyakova, P. S. Radulov, R. A. Novikov, M. G. Medvedev, N. V. Krivoshchapov, A. A. Korlyukov, I. V. Alabugin and A. O. Terenťev, Inverse α-Effect as the Ariadne's Thread on the Way to Tricyclic Aminoperoxides: Avoiding Thermodynamic Traps in the Labyrinth of Possibilities, J. Am. Chem. Soc., 2022, 144, 7264–7282 CrossRef CAS PubMed
.
- S. E. Wheeler, K. N. Houk, P. v. R. Schleyer and W. D. Allen, A Hierarchy of Homodesmotic Reactions for Thermochemistry, J. Am. Chem. Soc., 2009, 131, 2547–2560 CrossRef CAS PubMed
.
- A. E. Reed, L. A. Curtiss and F. Weinhold, Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint, Chem. Rev., 1988, 88, 899–926 CrossRef CAS
.
- I. V. Alabugin, S. Bresch and M. Manoharan, Hybridization Trends for Main Group Elements and Expanding the Bent's Rule Beyond Carbon: More than Electronegativity, J. Phys. Chem. A, 2014, 118, 3663–3677 CrossRef CAS PubMed
.
- H. G. Raubenheimer and S. F. Mapolie, Acid and base strength variations: rationalization for cyclic amine bases and acidic aqua cations, Dalton Trans., 2021, 50, 17864–17878 RSC
.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2177501. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3sc00001j |
| This journal is © The Royal Society of Chemistry 2023 |