Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Recent advances in combining photo- and N-heterocyclic carbene catalysis
Xiaochen
Wang
,
Senhui
Wu
,
Rongxin
Yang
,
Hongjian
Song
 ,
Yuxiu
Liu
,
Yuxiu
Liu
 and
Qingmin
Wang
and
Qingmin
Wang
 *
*
State Key Laboratory of Elemento-Organic Chemistry, Research Institute of Elemento-Organic Chemistry, Frontiers Science Center for New Organic Matter, College of Chemistry, Nankai University, Tianjin 300071, People's Republic of China. E-mail: wangqm@nankai.edu.cn
First published on 31st October 2023
Abstract
N-Heterocyclic carbenes (NHCs) are unique Lewis basic catalysts that mediate various organic transformations by means of polarity reversal. Although the scope of research on two-electron reactions mediated by NHC catalysts has been expanding, the types of these reactions are limited by the inability of NHCs to engage sp3-electrophiles. However, the revival of photocatalysis has accelerated the development of free-radical chemistry, and combining photoredox catalysis and NHC catalysis to achieve NHC-mediated radical reactions under mild conditions could overcome the above-mentioned limitation. This review summarizes recent advances in combining photoredox and NHC catalysis, focusing on elucidation and exploration of mechanisms, with the aim of identifying challenges and opportunities to develop more types of catalytic models.
1. Introduction
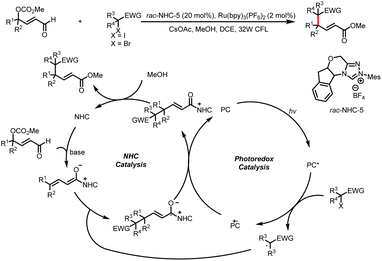
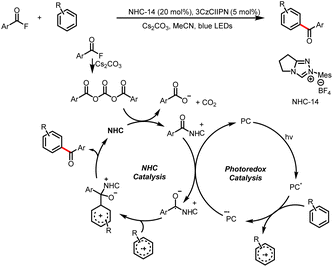
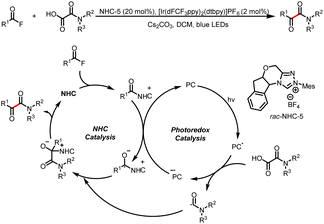
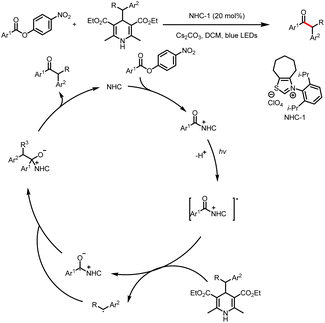
Organic small-molecule catalysts can simulate the activity of enzymes and are inexpensive, readily available, mild, and less toxic than metal catalysts. In particular, N-heterocyclic carbenes (NHCs) have been successfully used as organocatalysts over the past few decades because of their versatility and unique structure and been a research hotspot in the field of catalysis.1 On the basis of their general properties and chemical applications, NHC-bound intermediates can be broadly divided into two types: electron-rich and electron-deficient (Scheme 1a and b, respectively).1e The traditional mode of NHC catalysis typically involves direct participation of such intermediates in reactions to form chemical bonds through a two-electron process; however, the scope with respect to NHC-derived operators is restricted by their inability to engage sp3 electrophiles.2 Therefore, the discovery of new reaction modes for NHC catalysts, such as single-electron transfer (SET) radical reactions, will bring about new reaction modes to NHC catalysis and also rejuvenate its vitality and vigour.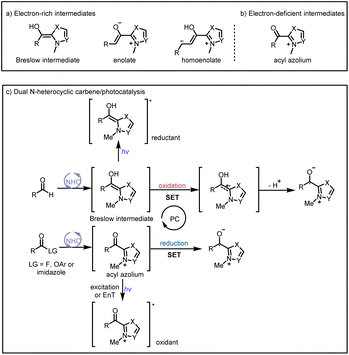 | ||
| Scheme 1 (a) Typical electron-rich NHC-bound intermediates. (b) Typical electron-deficient NHC-bound intermediates. (c) Main modes of NHC/photoredox dual catalysis. | ||
Recent advances in single-electron reactions involving photoredox catalysis3 have enabled bond connections that were previously not possible by means of traditional methods, and these advances have been used for organic synthesis.4 The revival of photocatalysis research has accelerated the development of free-radical chemistry, and visible-light catalysis has been combined with NHC catalysis to achieve NHC-mediated radical reactions under mild conditions. In recent years, the cocatalysis model has been in a state of explosion, and there are some reviews in related fields.5 Although there have some big shoes to fill, our review focuses on the summary of the newly developed catalytic mechanism, especially in the last four years. At present, there are two main modes of NHC/photoredox dual catalysis: (1) single-electron oxidation of classical electron-rich NHC intermediates to generate ketyl radicals under photocatalytic conditions and (2) single-electron reduction of novel NHC-derived electron-deficient radical intermediates under photocatalytic conditions. Among them, the electron-rich or electron-deficient intermediates can be used as oxidizing or reducing agents after direct photoexcitation (Scheme 1c, top and bottom, respectively). And the generated ketyl radicals will engage in radical/radical cross-couplings; although not employing light activation directly, the pioneering work of Ohmiya and co-workers based on a NHC-derived ketyl radical using NHPI esters as oxidative radical precursors under thermal conditions will also be discussed.
This review is divided into two main sections. The first is dedicated to production of ketyl radicals by single-electron oxidation (via Breslow intermediates), and the second focuses on generation of ketyl radicals by single-electron reduction (via acyl azolium intermediates).
2. Ketyl radicals generated via Breslow intermediates
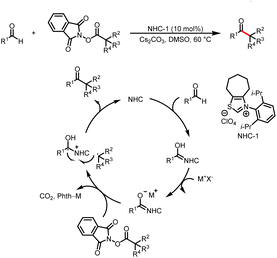
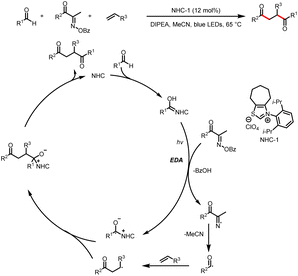
Although not employing light activation directly, as pioneering work, in 2019, Ohmiya reported the first example of radical cross-coupling reactions based on a NHC-catalyzed decarboxylative coupling reaction between aryl aldehydes and tertiary or secondary alkyl carboxylic acid-derived redox-active esters to deliver aryl alkyl ketone (Scheme 2).6 The Breslow intermediate formed from the aldehyde and NHC reduces the NHPI ester to afford a ketyl radical and an alkyl radical, and the radical–radical coupling between them followed by the elimination of NHC will afford the desired ketone product.2.1 Reactions requiring an external photocatalyst
The Du group described modification of α-amino acids and peptides with aldehydes by photoredox/NHC dual catalysis to access structurally diverse α-amino ketones (Scheme 4).8 Amino acids prepared in advance or generated in situ act as radical precursors. These reactions proceed by a mechanism similar to that described by Rovis et al.7
In 2020, Shu and a co-workers reported the direct synthesis of amides from aldehydes and imines under redox-neutral conditions at room temperature. The key to the success of this method is NHC/visible light dual catalysis, which enables photocatalytic reduction of imino esters to nitrogen-centered radicals, which react with radical cationic intermediates to form C–N bonds (Scheme 5).9
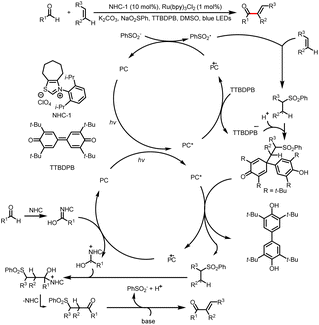
Shortly thereafter, Shu's group reported intermolecular vinylation of aldehydes, which was achieved by two sequential C–H functionalization reactions involving alkenes as vinylating reagents (Scheme 6).10 The reaction conditions are compatible with aldehydes and olefins bearing a wide range of functional groups. It is worth mentioning that TTBDPB (3,3′,5,5′-tetra-tert-butyldiphenoquinone), which is used in these reactions, acts both as an oxidant and as a reservoir for radical intermediates.
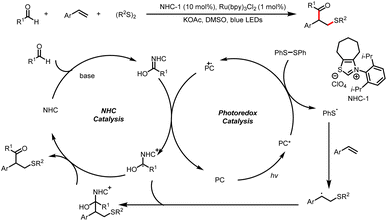
Along with this two-component coupling process, Shu and co-workers also developed a related three-component protocol for straightforward access to β-thiolated ketones from aldehydes, styrenes, and disulfides (Scheme 7).11 First, a Breslow intermediate undergoes single-electron oxidation by the excited-state photocatalyst, and then reduction of a diaryl disulfide generates a sulfur radical. The addition of the sulfur radical to a styrene derivative forms a new carbon-centered radical intermediate, which undergoes radical cross-coupling with a ketyl radical to afford the α-arylated-β-thiolated ketone product. A more stable benzyl radical was obtained by the addition with styrene analogues.
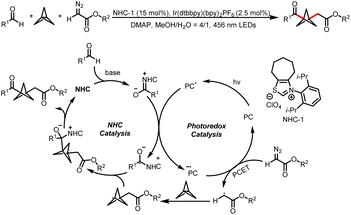
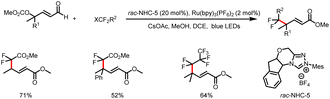
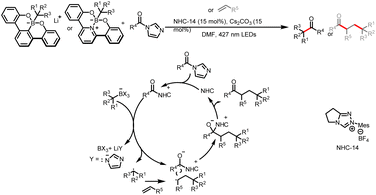
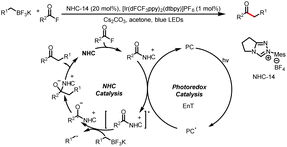
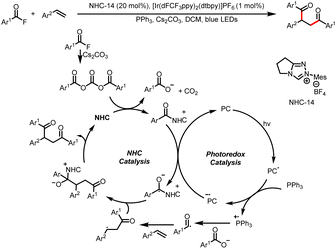
Meanwhile, the Wang group also developed a method for three-component bisfunctionalization of unactivated olefins by means of NHC/photoredox dual catalysis. Proton-coupled electron transfer (PCET) generates free radicals from a diazo ester, and the radicals add to olefins to generate new radicals that couple with ketyl radicals generated via photoredox-catalyzed oxidation of Breslow intermediates (Scheme 8a).12 In addition, acyldifluoromethylation of inert alkenes can be achieved via a similar mechanism when 2-((difluoromethyl)-sulfonyl)benzo[d]thiazole is used as a radical precursor (Scheme 8b).13
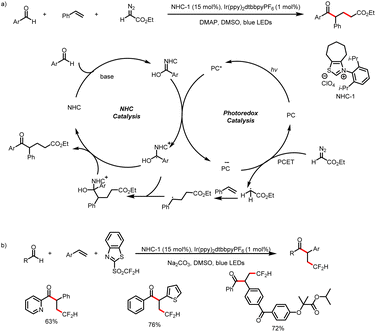 | ||
| Scheme 8 (a) Bisfunctionalization of unactivated olefins by aldehydes and a diazoester. (b) Bisfunctionalization of unactivated olefins by aldehydes and 2-((difluoromethyl)-sulfonyl)benzo[d]thiazole. | ||
Recently, the Jiang and Yu group also developed a three-component reaction to access 1,3-disubstituted bicyclo[1.1.1]pentane (BCP) ketones (Scheme 9).14 The BCP scaffold is a bioisostere for the phenyl ring and could enhance the solubility and permeability of bioactive molecules. In their work, radicals derived from diazo esters perform an addition reaction onto [1.1.1] propellane to afford BCP radicals, which are then coupled with the ketyl radicals that are generated via oxidation of the Breslow intermediates by the photoredox catalyst.
In 2019, the Ye group reported the use of γ-oxidized enals as substrates for the synthesis of γ-multisubstituted-α,β-unsaturated esters by intramolecular alkylation reactions with alkyl halides bearing an electron-withdrawing group (Scheme 11).16 An alkyl radical generated from the alkyl halide by photocatalysis reacts with a dienolate intermediate generated from the enal by NHC catalysis to give a homoenolate radical. The subsequent SET oxidation of the homoenolate radical mediated by the photocatalyst affords an α,β-unsaturated acyl azolium intermediate and completes the photocatalytic cycle. Trapping of the acyl azolium intermediate by methanol gives the final product and regenerates the NHC catalyst.
The Ye group also developed a method for γ-difluoroalkylation of γ-preoxidized enals to afford γ-difluoroalkyl-α,β-unsaturated esters (Scheme 12).17 This method allows for efficient construction of C(sp3)–CF2R bonds at the γ position of carbonyl compounds bearing an all-carbon quaternary center.
Subsequently, Ye and a colleague expanded the substrate scope of the reaction to include cyclopropane enals. Initial ring-opening by C–C bond cleavage and subsequent γ-alkylation with a halogenated compound via a radical process afford γ-alkyl-α,β-unsaturated esters (Scheme 13).18 A variety of alkyl halides work well in the reaction, providing the desired γ-alkyl-α,β-unsaturated ester products in moderate to good yields.
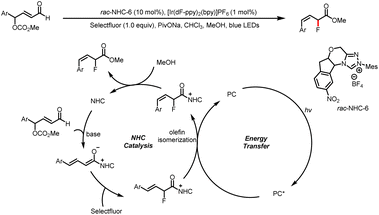
In 2021, Huang and Chen et al. reported a method for photoinduced energy-transfer reactions of NHC-bound intermediates to yield (Z)-allylic fluorides with good stereochemical control (Scheme 14).19 A homoenolate intermediate is generated by nucleophilic addition of an NHC to an enal substrate followed by a hydride shift. A dienolate formed by release of CO2 and methanol reacts with Selectfluor to give an acyl azolium intermediate. By energy transfer from the triplet excited state of the photocatalyst, the acyl azolium intermediate undergoes double bond isomerization to afford predominantly (Z)-acyl azolium, which reacts with MeOH to yield the product and regenerate the NHC catalyst.
Then Ye et al. extended the conjugate system further and realized regioselective ε-benzylation of γ-alkenyl-γ-oxidized enals to afford the corresponding ε-benzyl-α,β-γ,δ-bisunsaturated esters in moderate to good yields (Scheme 15).20
Recently, the Chauhan group developed a stereoselective strategy to access pyrrolo[1,2-d][1,4]-oxazepin-3(2H)-ones (Scheme 16).21 Imine is formed upon one-electron oxidation, hydrogen atom transfer, and proton transfer of amine. Then the imine intermediate enters into the NHC catalytic cycle, where the Breslow intermediate acts as the homoenolate equivalent, which after tautomerization undergoes nucleophilic addition to imine to forge acyl azolium species. Eventually the azolium intermediate delivers the product.
2.2 Reactions not requiring an external photocatalyst
The process without an external photocatalyst is usually to reduce the radical precursor by generating an excited state of the Breslow intermediate under light excitation, directly generate free radicals through special radical precursors under light or generate EDA complexes through the Breslow intermediates and other reactants in the system. Various reactions can be achieved by combining light activation and NHC organocatalysis without an external photocatalyst. For example, in 2020, Chen's group demonstrated that transition-metal-free decarboxylative C(sp3)–X bond formation can be accomplished with photochemically active N-(acyloxy)phthalimide ester–NaI–NHC complexes; these reactions offer a convenient way to construct C(sp3)–C(sp2), C(sp3)–S, C(sp3)–O, and C(sp3)–Cl bonds (Scheme 17).22 The key to these reactions is the electrostatic NHC–Na+ interaction, which facilitates the formation of electron donor–acceptor complexes, irradiation of which generates radicals that go on to form the products. Later, the same research group described catalytic reactions of N-alkenoxypyridinium salts and NaI to give various α-iodo ketones (Scheme 18).23In 2022, the Larionov group developed a method for visible-light-induced NHC-catalyzed regioselective 1,2-diacylation reactions of alkenes that afford 1,4-diketones via three-component C–C-bond-forming radical coupling (Scheme 19).24 Notably, in this system, the NHC catalyst plays two roles: an NHC-catalyst-derived intermediate forms an electron donor–acceptor complex, and the NHC catalyst acts as an acyl transfer reagent. Under irradiation by blue LEDs, intramolecular SET between a Breslow intermediate and an oxime generates nitrogen and ketyl radicals. The nitrogen radical fragments to release acetonitrile and an acyl radical, which adds to an olefin to generate an adduct radical. The adduct radical participates in a cross-coupling reaction with the ketyl radical to afford a 1,4-diketone in high yield.
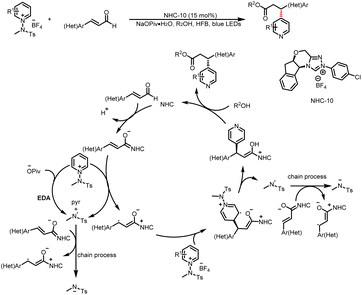
The Hong group developed a catalytic method for enantioselective, C4-selective functionalization of pyridine derivatives (Scheme 20),25 which proceeds by means of a mechanism similar to that reported by Larionov et al.24 The key to these asymmetric β-pyridylation reactions of enals is an enhanced interactions between a chiral–NHC–bound homoenolate and a pyridinium salt in the presence of a pivalate salt and hexafluorobenzene, which effectively distinguishes between the two faces of a homoenolate radical. Because light-facilitated reactivity and rate acceleration were observed, Hong et al.25 proposed an alternative mechanism involving photon absorption by a pyridine–pivalate electron donor–acceptor complex that triggers the formation of amidine radicals when the EDA complex is irradiated with visible light.
Other systems can, upon photoirradiation, directly generate radicals that can then participate in a catalytic cycle involving NHC. In 2020, the Hui group achieved stereoselective [4 + 2] cycloaddition reactions of 3-alkylenyloxindoles and α-diazoketones that proceed via this mechanism (Scheme 21).26 Initially, a ketene is formed from the α-diazoketone through a Wolff rearrangement reaction under blue light. Subsequently, the addition of NHC to the ketene generates an enolate, which participates in a [4 + 2] annulation reaction with the 3-alkylenyloxindole to give an intermediate that is transformed into a tetrahydropyrano[2,3-b]indole upon release of the NHC catalyst. Subsequently, the Yao group developed a method for asymmetric [4 + 2] annulation reactions of saccharine-derived azadienes and α-diazoketones, affording the corresponding sultam-fused dihydropyridinones in moderate to good yields with satisfactory to excellent enantio- and diastereo-selectivities (Scheme 22).27
In 2020, the Xuan group described a multicomponent reaction that relies on the different reactivities of two carbenes (Scheme 23).28 One of the N-heterocyclic carbenes, acting as an organocatalyst, mediates the formation of a hydroxamic acid in situ, whereas the other carbene, which is formed by photolysis of diazoalkane, acts as a reactant. Then the hydroxamic acid and carbene participate in a solvent-dependent four-component reaction that provides hydroxamic acid esters, which are biologically important compounds.
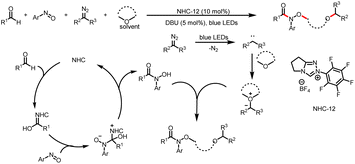 | ||
| Scheme 23 Formation of hydroxamic acid esters by a four-component reaction in a cyclic ether as a reaction medium. | ||
3. Ketyl radicals generated via acyl azolium intermediates
3.1 Acyl imidazoles as acyl sources
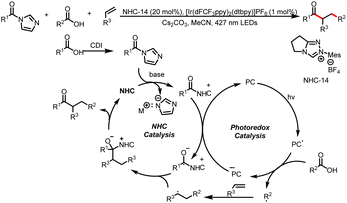
Scheidt et al. later expanded the reaction to alkyl acyl imidazoles by altering the structure of the NHC catalyst. Their findings revealed that both electronic and steric modifications of the NHC catalyst would affect the stability and accessibility of the radical intermediates, thereby controlling the reactivity of NHC catalysis (Scheme 25).30
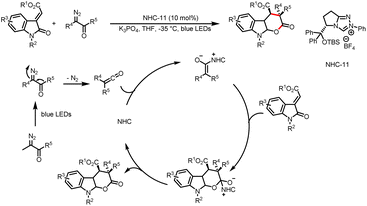
In 2022, the Scheidt group developed a strategy for the construction of two contiguous C–C bonds via a formal [5 + 1] cycloaddition for the synthesis of α,β-disubstituted cyclohexanones from HEs and alkyl acyl imidazoles (Scheme 26).31 The key to this transformation lies in the two photocatalytic cycles to achieve intramolecular cyclization. In the first cycle, the photoexcited photocatalyst oxidizes the HE to afford a benzyl radical, and single-electron reduction of an acyl azolium provides a ketyl radical. Intermolecular coupling of the two radicals and loss of the NHC give a linear ketone intermediate. In the second cycle, the corresponding enol or cesium enolate undergoes single-electron oxidation to produce a benzyl radical, which participates in an intramolecular cyclization reaction with the remote double bond. The resulting radical undergoes hydrogen atom transfer (HAT) or is reduced to the corresponding anion by the photocatalyst, and subsequent deprotonation by the solvent or bicarbonate affords the desired cyclohexanone product.
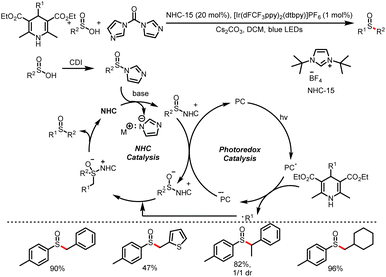
Subsequently, the Wu group reported the generation of sulfoxides from sulfinic acids and 4-substituted HEs in the presence of carbonyldiimidazole (Scheme 27).32 Acting as free-radical precursors, HEs can generate not only benzyl radicals but also alkyl radicals, and a series of sulfinyl products were obtained by this method. This method further expands the previous carboxylic acid category to sulfonic acid, and using sulfinyl imidazoles as pseudo-acyl sources.
In 2022, the Scheidt group reported a multicomponent reaction for the synthesis of γ-aryloxyketones via aryloxymethyl potassium trifluoroborate salts. An aryloxymethyl radical adds to a styrene derivative to provide a stabilized benzyl radical, and a subsequent radical–radical coupling reaction with an azolium radical affords the γ-aryloxyketone product (Scheme 29).34
In the same year, the Chi group published a method for coupling carboxylic acids and acyl imidazoles by means of a combination of NHC catalysis and photocatalysis (Scheme 30).35 The carboxylic acids are directly used as radical precursors, and late-stage modification of commercial drugs and direct coupling of fragments of two medicinally active molecules were performed to demonstrate the utility of this method.
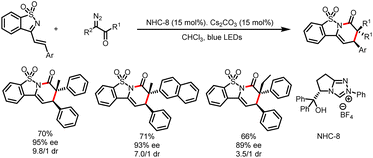
Recently, the Ye group reported the iminoacylation of alkenes via decarboxylation of α-imino-oxy acids to generate iminyl radical intermediates. The addition of the iminyl radicals to a tethered alkene in a 5-exo-trig manner gives dihydropyrrole-derived carbon radicals (most of the examples were benzyl radicals), which couple with ketyl radicals generated from acyl azolium intermediates to form substituted 3,4-dihydro-2H-pyrroles (Scheme 31).36
3.2 Acyl fluorides as acyl sources
In addition, this research group also generated benzyl radicals by means of SET oxidation of the double bonds of benzofurans (Scheme 35).40 Specifically, they reported fluoroaroylation of benzofurans by acyl fluorides, which act as bifunctional reagents to incorporate both an aroyl moiety and fluorine into the product. Upon visible-light irradiation, the benzofuran is oxidized to a radical cation by a photoexcited photocatalyst. A ketyl radical is generated from an acyl azolium intermediate, and cross-coupling of the radical cation and the ketyl radical leads to an oxocarbenium ion. Diastereoselective trapping of this ion by a F anion affords the 3-aroyl-2-fluoro-2,3-dihydrobenzofuran product.
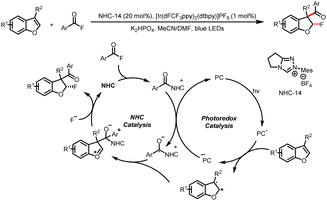 | ||
| Scheme 35 Construction of 3-aroyl-2-fluoro-2,3-dihydrobenzofurans from acyl fluorides and benzofurans. | ||
Like benzofurans, substituted styrenes can act as benzyl radical precursors by undergoing metal-mediated HAT. In 2022, the Wang group achieved Markovnikov-selective hydroacylation of alkenes by using a synergistic combination of cobalt, photoredox, and NHC catalysis (Scheme 36).41 The cobalt catalytic cycle starts with SET oxidation of CoII to CoIII, and then CoIII is captured by phenylsilane to furnish a CoIII–H intermediate. This intermediate engages in a HAT reaction with the substituted styrene to produce a benzyl radical. Meanwhile, SET reduction of an acyl azolium ion gives a ketyl radical, which undergoes radical–radical cross-coupling with the benzyl radical to generate the hydroacylation product.
Shortly thereafter, the Li group reported cross-coupling reactions of alkyl trifluoroborates with acid fluorides to generate various ketones; this method provides an alternative to the classical acylative Suzuki coupling chemistry (Scheme 37). Li et al. proposed the activation of an acyl azolium intermediate with a triplet-state photocatalyst, through an energy-transfer process, to form an excited-state acyl azolium intermediate.42 This intermediate oxidizes the alkyl trifluoroborates to give a ketyl radical and a benzyl radical. The coupling of these two radicals affords the ketone product.
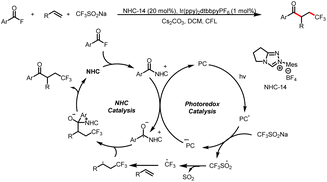
In 2020, the Studer group reported three-component coupling reactions of aroyl fluorides, styrenes, and the Langlois reagent (CF3SO2Na) to give various β-trifluoromethylated-α-substituted ketones (Scheme 39).44 Reductive quenching of the excited-state photocatalyst by the trifluoromethanesulfinate anion gives a trifluoromethylsulfonyl radical that fragments to release SO2 and a trifluoromethyl radical, which then adds to the double bond of styrene to generate a transient benzylic radical. Meanwhile, SET reduction of an acyl azolium ion gives a ketyl radical, which undergoes radical–radical cross-coupling with the benzyl radical to give the β-trifluoromethyl-α-substituted ketones.
Shortly thereafter, Studer et al. reported a ring-opening/arylcarboxylation/acylation cascade reaction for the 1,3-difunctionalization of aryl cyclopropanes (Scheme 40).45 The key to this transformation is that the aryl cyclopropane radical cation generated by SET oxidation of the aryl cyclopropane undergoes ring opening by a nucleophilic benzoate ion to give a benzylic radical. Radical–radical cross-coupling of the benzylic radical and a ketyl radical affords γ-aroyloxy ketones.
The Feng and Fan group developed a method for intermolecular 1,2-diacylation of styrenes via cooperative NHC and photoredox catalysis with mediation by PPh3 and Cs2CO3 (Scheme 41).46 The mechanism is similar to that described by Studer et al.45 An NHC-mediated reaction of a bisacyl carbonate intermediate generated from an acyl fluoride produces a benzoate anion and an acyl azolium ion. The benzoate anion combines with a triphenylphosphine cation radical to form a phosphorus-centered radical, which undergoes β-scission to generate an acyl radical. The acyl radical attacks the styrene substrate to generate the corresponding benzyl radical. Next, intermolecular cross-coupling between a ketyl radical (derived from the acyl azolium ion) and the benzyl radical produces the desired product. In a similar manner, keto acids can also be used as acyl radical precursors via single-electron oxidation, and subsequent dicarbonylation of alkenes provides direct access to 1,4-dicarbonyl compounds (Scheme 42).47
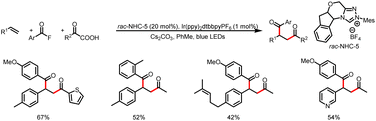 | ||
| Scheme 42 Construction of unsymmetrical 1,4-dicarbonyl compounds from alkenes, acyl fluorides, and keto acids. | ||
Unlike trifluoromethylsulfonyl radicals, aryl sulfonyl radicals do not undergo SO2 extrusion to produce the corresponding aryl radicals. Therefore, in 2022, Studer and a colleague developed an arylsulfonate-catalyzed alkene acylation reaction. In this reaction, an arylsulfonyl radical first adds to the alkene substrate to generate a carbon-centered radical, which then couples with a ketyl radical (generated from an acyl azolium), providing a three-component coupling intermediate. The subsequent base-mediated elimination of arylsulfinate forms the α-acylated olefin product. Three catalytic cycles involving a carbene are interwoven (Scheme 43).48
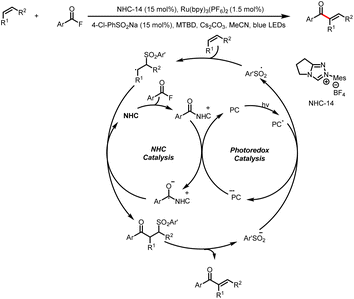
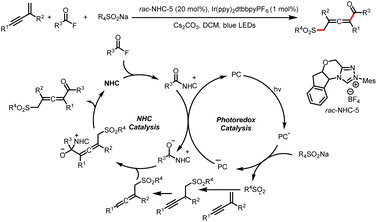
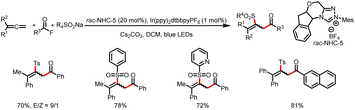
The Zhang group described a similar reaction: 1,4-sulfonylacylation of 1,3-enynes to provide valuable structurally diverse tetrasubstituted allenyl ketones (Scheme 44).49 The addition of a sulfonyl radical to a 1,3-enyne delivers a propargyl radical that can undergo reversible isomerization to generate a trisubstituted allenyl radical, which can participate in a radical–radical cross-coupling reaction with a ketyl radical generated by reduction of an acyl azolium ion. By replacing the 1,3-enyene acceptor of the sulfonyl radical with an allene, these investigators also achieved 1,2-sulfonylacylation of allenes to provide valuable sulfonyl-containing multisubstituted allyl ketones (Scheme 45).50
3.3 Esters as acyl sources
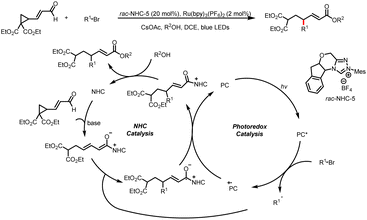
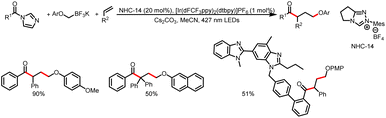
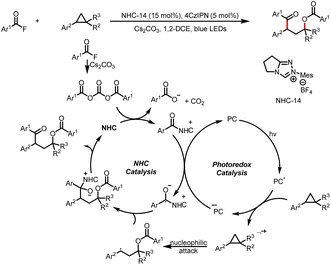
In addition to imidazoles and fluorides, esters can also act as leaving groups. For example, in 2021, the Chi group developed a method for alkylation of aryl carboxylic esters with HEs (Scheme 48).53 The reaction starts with addition of an NHC catalyst to the ester to generate an acyl azolium intermediate. The photoexcitation of this intermediate converts it to an excited state that act as a single-electron oxidant. Subsequently, SET between the HE and the excited-state acyl azolium leads to a benzyl radical and a ketyl radical, and coupling of these two radicals affords the desired ketone product. Structurally sophisticated ketones, including ketones bearing medicinal fragments, could readily be prepared.4. Conclusion and outlook
As complements to two-electron reaction modes, single-electron reaction modes give new vitality to NHC-catalyzed reactions. This review has focused on combinations of NHC catalysis with photocatalysis. The combinations have been classified on the basis of whether the ketyl radical is generated by single-electron oxidation of an electron-rich Breslow intermediate or by single-electron reduction of an electron-deficient acyl azolium intermediate. Reactions in the first category have been further categorized on the basis of their substrates and whether an external photocatalyst is required, whereas reactions in the second category have been categorized mainly on the basis of the type of leaving group and the type of radical that is generated. Through the combination of NHC catalysis and photocatalysis, many transformations that cannot be achieved by means of two-electron reactions can be realized.Although NHC-mediated single-electron reactions have made great progress and breakthroughs, there are still some problems that remain to be solved, including: (1) acyl substrate scope: since the previously activated acyl precursors are mostly carboxylic acids, it would be important to explore whether the activation process of carboxylic acid derivatives, aldehyde derivatives and imines could be realized through the co-catalysis mode of light and NHC; (2) types of free radicals: since most of the literature reported was on the direct formation of benzyl radicals or the addition of alkyl radicals to aryl olefins to achieve the indirect formation of benzyl radicals, to explore other unstable radical such as selective and efficient acylation of unactivated C–H bonds through this catalytic mode will be meaningful; (3) enantioselectivity control: how to build a quaternary or tertiary stereocenter at the α-position of a carbonyl group with high enantioselectivity should be considered; (4) catalytic mode: the development of new catalytic modes through merging NHC catalysis with otherwise established chemistry protocols such as electrochemistry or metal catalysis should be explored as well.
Author contributions
Xiaochen Wang: conceptualization, visualization, and writing – original draft. Senhui Wu and Rongxin Yang: Investigation. Hongjian Song and Yuxiu Liu: writing – review & editing. Qingmin Wang: supervision, conceptualization, writing – review & editing, and funding acquisition. All authors have given approval to the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful to the National Natural Science Foundation of China (22271166 and 22077071) and the Frontiers Science Center for New Organic Matter, Nankai University (63181206) for generous financial support for our programs.References
- (a) D. Enders, O. Niemeier and A. Henseler, Organocatalysis by N-Heterocyclic Carbenes, Chem. Rev., 2007, 107, 5606–5655 CrossRef CAS; (b) X. Y. Chen and S. Ye, Enantioselective Cycloaddition Reactions of Ketenes Catalyzed by N-Heterocyclic Carbenes, Synlett, 2013, 24, 1614–1622 CrossRef CAS; (c) X. Y. Chen and S. Ye, N-heterocyclic carbene-catalyzed reactions of C–C unsaturated bonds, Org. Biomol. Chem., 2013, 11, 7991–7998 RSC; (d) M. N. Hopkinson, C. Richter, M. Schedler and F. Glorius, An overview of N-heterocyclic carbenes, Nature, 2014, 510, 485–496 CrossRef CAS PubMed; (e) D. M. Flanigan, F. Romanov-Michailidis, N. A. White and T. Rovis, Organocatalytic Reactions Enabled by N-Heterocyclic Carbenes, Chem. Rev., 2015, 115, 9307–9387 CrossRef CAS; (f) C. Zhang, J. F. Hooper and D. W. Lupton, N-Heterocyclic Carbene Catalysis via the α,β-Unsaturated Acyl Azolium, ACS Catal., 2017, 7, 2583–2596 CrossRef CAS; (g) M. Zhao, Y. T. Zhang, J. Chen and L. Zhou, Enantioselective Reactions Catalyzed by N-Heterocyclic Carbenes, Asian J. Org. Chem., 2018, 7, 54–69 CrossRef CAS; (h) S. Mondal, S. R. Yetra, S. Mukherjee and A. T. Biju, NHC-Catalyzed Generation of α,β-Unsaturated Acylazoliums for the Enantioselective Synthesis of Heterocycles and Carbocycles, Acc. Chem. Res., 2019, 52, 425–436 CrossRef CAS PubMed; (i) X. Y. Chen, Z. H. Gao and S. Ye, Bifunctional N-Heterocyclic Carbenes Derived from l-Pyroglutamic Acid and Their Applications in Enantioselective Organocatalysis, Acc. Chem. Res., 2020, 53, 690–702 CrossRef CAS; (j) H. Ohmiya, N-Heterocyclic Carbene-Based Catalysis Enabling Cross-Coupling Reactions, ACS Catal., 2020, 10, 6862–6869 CrossRef CAS.
- (a) R. Breslow, On the Mechanism of Thiamine Action. IV.1 Evidence from Studies on Model Systems. On the Mechanism of Thiamine Action. IV.1 Evidence from Studies on Model Systems, J. Am. Chem. Soc., 1958, 80, 3719–3726 CrossRef CAS; (b) B. E. Maki, A. Chan, E. M. Phillips and K. A. Scheidt, Tandem Oxidation of Allylic and Benzylic Alcohols to Esters Catalyzed by N-Heterocyclic Carbenes, Org. Lett., 2007, 9, 371–374 CrossRef CAS PubMed; (c) T. Ukai, R. Tanaka and T. Dokawa, J. Pharm. Soc. Jpn., 1943, 63, 296–300 CrossRef CAS PubMed; (d) For selected reviews, see: T. Ishii, K. Nagao and H. Ohmiya, Recent advances in N-heterocyclic carbine-based radical catalysis, Chem. Sci., 2020, 11, 5630–5636 RSC.
- (a) N. R. Patel, C. B. Kelly, A. P. Siegenfeld and G. A. Molander, Mild, Redox-Neutral Alkylation of Imines Enabled by an Organic Photocatalyst, ACS Catal., 2017, 7, 1766–1770 CrossRef CAS PubMed; (b) J. Jia, Q. Lefebvre and M. Rueping, Reductive Coupling of Imines with Redox-active Esters by Visible Light Photoredox Organocatalysis, Org. Chem. Front., 2020, 7, 602–608 RSC; (c) P. Ji, Y. Zhang, Y. Wei, H. Huang, W. Hu, P. A. Mariano and W. Wang, Visible-Light-Mediated, Chemo- and Stereoselective Radical Process for the Synthesis of C-Glycoamino Acids, Org. Lett., 2019, 21, 3086–3092 CrossRef CAS PubMed; (d) H.-H. Zhang and S. Yu, Radical Alkylation of Imines with 4-Alkyl-1,4-dihydropyridines Enabled by Photoredox/Brønsted Acid Cocatalysis, J. Org. Chem., 2017, 82, 9995–10006 CrossRef CAS; (e) D. P. Plasko, C. J. Jordan, B. E. Ciesa, M. A. Merrill and J. M. Hanna, Visible light-promoted alkylation of imines using potassium organotrifluoroborates, Photochem. Photobiol. Sci., 2018, 17, 534–538 CrossRef CAS PubMed; (f) K. Cao, S. M. Tan, R. Lee, S. Yang, H. Jia, X. Zhao, B. Qiao and Z. Jiang, Catalytic Enantioselective Addition of Prochiral Radicals to Vinylpyridines, J. Am. Chem. Soc., 2019, 141, 5437–5443 CrossRef CAS PubMed; (g) Y. Li, K. Zhou, Z. Wen, S. Cao, X. Shen, M. Lei and L. Gong, Copper(II)-Catalyzed Asymmetric Photoredox Reactions: Enantioselective Alkylation of Imines Driven by Visible Light, J. Am. Chem. Soc., 2018, 140, 15850–15858 CrossRef CAS.
- For reviews and selected work on photochemistry (a) T. P. Yoon, M. A. Ischay and J. Du, Visible light photocatalysis as a greener approach to photochemical synthesis, Nat. Chem., 2010, 2, 527–532 CrossRef CAS; (b) J. Xuan and W.-J. Xiao, Visible-Light Photoredox Catalysis, Angew. Chem., Int. Ed., 2012, 51, 6828–6838 CrossRef CAS; (c) C. K. Prier, D. A. Rankic and D. W. C. MacMillan, Visible Light Photoredox Catalysis with Transition Metal Complexes: Applications in Organic Synthesis, Chem. Rev., 2013, 113, 5322–5363 CrossRef CAS PubMed; (d) R. Francke and R. D. Little, Redox catalysis in organic electrosynthesis: basic principles and recent developments, Chem. Soc. Rev., 2014, 43, 2492–2521 RSC; (e) N. A. Romero and D. A. Nicewicz, Organic Photoredox Catalysis, Chem. Rev., 2016, 116, 10075–10166 CrossRef CAS PubMed; (f) K. L. Skubi, T. R. Blum and T. P. Yoon, Dual Catalysis Strategies in Photochemical Synthesis, Chem. Rev., 2016, 116, 10035–10074 CrossRef CAS; (g) M. H. Shaw, J. Twilton and D. W. C. MacMillan, Photoredox Catalysis in Organic Chemistry, J. Org. Chem., 2016, 81, 6898–6926 CrossRef CAS PubMed.
- For reviews and selected work on radical NHC catalysis (a) K. Liu, M. Schwenzer and A. Studer, Radical NHC Catalysis, ACS Catal., 2022, 12, 11984–11999 CrossRef CAS; (b) R. Song, Z. Jin and R. Y. Chi, NHC-catalyzed covalent activation of heteroatoms for enantioselective reactions, Chem. Sci., 2021, 12, 5037–5043 RSC; (c) K.-Q. Chen, H. Sheng, Q. Liu, P.-L. Shao and X.-Y. Chen, N- heterocyclic carbene-catalyzed radical reactions, Sci. China: Chem., 2021, 64, 7–16 CrossRef CAS; (d) Q.-Z. Li, R. Zeng, B. Han and J.-L. Li, Single-Electron Transfer Reactions Enabled by N-Heterocyclic Carbene Organocatalysis, Chem. – Eur. J., 2021, 27, 3238–3250 CrossRef CAS PubMed; (e) T. Ishii, K. Nagao and H. Ohmiya, Recent advances in N-heterocyclic carbene-based radical catalysis, Chem. Sci., 2020, 11, 5630–5636 RSC; (f) D. Wan and H. Yang, Research Progress on N-Heterocyclic Carbene Catalyzed Reactions for Synthesizing Ketones through Radical Mechanism, Synthesis, 2022, 54, 3307–3316 CrossRef CAS; (g) Q. Liu and X. Chen, Dual N-heterocyclic carbene/photocatalysis: a new strategy for radical processes, Org. Chem. Front., 2020, 7, 2082–2087 RSC; (h) L. Dai and S. Ye, Recent advances in N-heterocyclic carbene-catalyzed radical reactions, Chin. Chem. Lett., 2021, 32, 660–667 CrossRef CAS; (i) M. N. Hopkinson and A. Mavroskoufis, Photo-NHC Catalysis: Accessing Ketone Photochemistry with Carboxylic Acid Derivatives, Synlett, 2021, 32, 95–101 CrossRef CAS; (j) A. V. Bay and K. A. Scheidt, Single-electron carbene catalysis in redox processes, Trends Chem., 2022, 4, 277–290 CrossRef CAS.
- (a) T. Ishii, Y. Kakeno, K. Nagao and H. Ohmiya, N-Heterocyclic Carbene-Catalyzed Decarboxylative Alkylation ofAldehydes, J. Am. Chem. Soc., 2019, 141, 3854–3858 CrossRef CAS PubMed; (b) Y. Kakeno, M. Kusakabe, K. Nagao and H. Ohmiya, Direct Synthesis of Dialkyl Ketones from Aliphatic Aldehydes through Radical N-Heterocyclic Carbene Catalysis, ACS Catal., 2020, 10, 8524–8529 CrossRef CAS; (c) T. Ishii, K. Ota, K. Nagao and H. Ohmiya, N-Heterocyclic Carbene-Catalyzed Radical Relay Enabling Vicinal Alkylacylation of Alkenes, J. Am. Chem. Soc., 2019, 141, 14073–14077 CrossRef CAS; (d) J.-L. Li, Y.-Q. Liu, W.-L. Zou, R. Zeng, X. Zhang, Y. Liu, B. Han, Y. He, H.-J. Leng and Q.-Z. Li, Radical Acylfluoroalkylation of Olefins through N-Heterocyclic Carbene Organocatalysis, Angew. Chem., Int. Ed., 2020, 59, 1863–1870 CrossRef CAS; (e) L. Chen, C. Lin, S. Zhang, X. Zhang, J. Zhang, L. Xing, Y. Guo, J. Feng, J. Gao and D. Du, 1,4-Alkylcarbonylation of 1,3-Enynes to Access Tetra-Substituted Allenyl Ketones via an NHC-Catalyzed Radical Relay, ACS Catal., 2021, 11, 13363–13373 CrossRef CAS; (f) Y. Matsuki, N. Ohnishi, Y. Kakeno, S. Takemoto, T. Ishii, K. Nagao and H. Ohmiya, Aryl radical-mediated N-heterocyclic carbene catalysis, Nat. Commun., 2021, 12, 3848 CrossRef CAS PubMed; (g) I. Kim, H. Im, H. Lee and S. Hong, N-Heterocyclic carbene catalyzed deaminative cross-coupling of aldehydes with Katritzky pyridinium salts, Chem. Sci., 2020, 11, 3192–3197 RSC; (h) Q.-Z. Li, R. Zeng, Y. Fan, Y.-Q. Liu, T. Qi, X. Zhang and J.-L. Li, Remote C(sp3)–H Acylation of Amides and Cascade Cyclization via N-Heterocyclic Carbene Organocatalysis, Angew. Chem., Int. Ed., 2022, 61, e202116629 CrossRef CAS PubMed.
- D. A. DiRocco and T. Rovis, Catalytic Asymmetric α-Acylation of Tertiary Amines Mediated by a Dual Catalysis Mode: N-Heterocyclic Carbene and Photoredox Catalysis, J. Am. Chem. Soc., 2012, 134, 8094–8097 CrossRef CAS PubMed.
- D. Du, K. Zhang, R. Ma, L. Chen, J. Gao, T. Lu, Z. Shi and J. Feng, Bio- and Medicinally Compatible α-Amino-Acid Modification via Merging Photoredox and N-Heterocyclic Carbene Catalysis, Org. Lett., 2020, 22, 6370–6375 CrossRef CAS PubMed.
- M. Liu and W. Shu, Catalytic, Metal-Free Amide Synthesis from Aldehydes and Imines Enabled by a Dual-Catalyzed Umpolung Strategy under Redox-Neutral Condition, ACS Catal., 2020, 10, 12960–12966 CrossRef CAS.
- M. Liu, L. Min, B. Chen and W. Shu, Dual Catalysis Relay: Coupling of Aldehydes and Alkenes Enabled by Visible-Light and NHC-Catalyzed Cross-Double C–H Functionalizations, ACS Catal., 2021, 11, 9715–9721 CrossRef CAS.
- H. Du, M. Liu and W. Shu, Synthesis of β-Thiolated-α-arylated Ketones Enabled by Photoredox and N-Heterocyclic Carbene-Catalyzed Radical Relay of Alkenes with Disulfides and Aldehydes, Org. Lett., 2022, 24, 5519–5524 CrossRef CAS.
- B. Zhang, J. Qi, Y. Liu, Z. Li and J. Wang, Visible-Light-Driven Bisfunctionalization of Unactivated Olefins via the Merger of Proton-Coupled Electron Transfer and Carbene Catalysis, Org. Lett., 2022, 24, 279–283 CrossRef CAS PubMed.
- B. Zhang and J. Wang, Acyldifluoromethylation Enabled by NHC-Photoredox Cocatalysis, Org. Lett., 2022, 24, 3721–3725 CrossRef CAS PubMed.
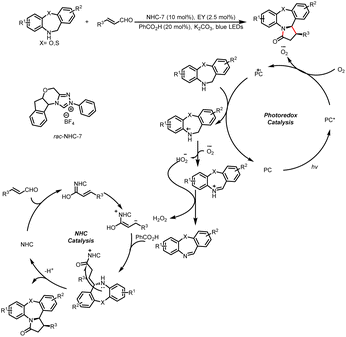
- Y. Gao, Z. Zheng, Y. Zhu, W. Xu, Y. Zhou, C. Yu and X. Jiang, Visible light-induced synthesis of 1,3-disubstituted bicyclo[1.1.1]pentane ketones via cooperative photoredox and N-heterocyclic carbene catalysis, Green Chem., 2023, 25, 3909–3915 RSC.
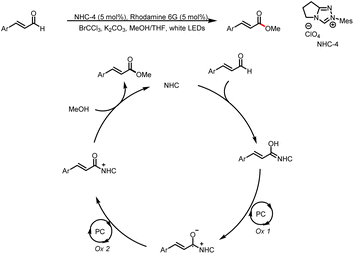
- E. Yoshioka, M. Inoue, Y. Nagoshi, A. Kobayashi, R. Mizobuchi, A. Kawashima, S. Kohtani and H. Miyabe, Oxidative Functionalization of Cinnamaldehyde Derivatives: Control of Chemoselectivity by Organophotocatalysis and Dual Organocatalysis, J. Org. Chem., 2018, 83, 8962–8970 CrossRef CAS PubMed.
- L. Dai, Z. Xia, Y. Gao, Z. Gao and S. Ye, Visible-Light-Driven N-Heterocyclic Carbene Catalyzed g- and eAlkylation with Alkyl Radicals, Angew. Chem., Int. Ed., 2019, 58, 18124–18130 CrossRef CAS PubMed.
- L. Dai, Y. Xu, Z. Xia and S. Ye, γ-Difluoroalkylation: Synthesis of γ-Difluoroalkyl-α,β-Unsaturated Esters via Photoredox NHC-Catalyzed Radical Reaction, Org. Lett., 2020, 22, 8173–8177 CrossRef CAS PubMed.
- L. Dai and S. Ye, Photo/N-Heterocyclic Carbene Co-catalyzed Ring Opening and γ-Alkylation of Cyclopropane Enal, Org. Lett., 2020, 22, 986–990 CrossRef CAS PubMed.
- X. Jiang, E. Li, J. Chen and Y. Huang, Photo-induced energy transfer relay of N-heterocyclic carbene catalysis: an asymmetric a-fluorination/isomerization cascade, Chem. Commun., 2021, 57, 729–732 RSC.
- Y. Xu, L. Dai, Z. Gao and S. Ye, ε-Benzylation via Cooperative Photoredox and N-Heterocyclic Carbene Catalysis, J. Org. Chem., 2022, 87, 14970–14974 CrossRef CAS PubMed.
- Y. Hussain, D. Sharma, Tamanna and P. Chauhan, Relay Organophotoredox/N-Heterocyclic Carbene Catalysis-Enabled Asymmetric Synthesis of Dibenzoxazepine-Fused Pyrrolidinones, Org. Lett., 2023, 25, 2520–2524 CrossRef CAS PubMed.
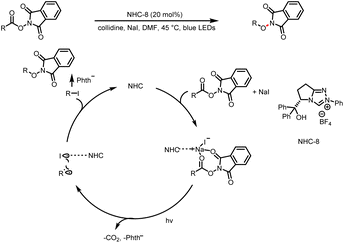
- K. Chen, Z. Wang and X. Chen, Photochemical Decarboxylative C(sp3)–X Coupling Facilitated by Weak Interaction of N-Heterocyclic Carbene, Org. Lett., 2020, 22, 8059–8064 CrossRef CAS PubMed.
- H. Sheng, Q. Liu, X. Su, Y. Lu, Z. Wang and X. Chen, Visible-Light-Triggered Iodinations Facilitated by Weak Electrostatic Interaction of N-Heterocyclic Carbenes, Org. Lett., 2020, 22, 8059–8064 CrossRef PubMed.
- S. Jin, X. Sui, G. C. Haug, V. D. Nguyen, H. T. Dang, H. D. Arman and O. V. Larionov, N-Heterocyclic Carbene-Photocatalyzed Tricomponent Regioselective 1,2-Diacylation of Alkenes Illuminates the Mechanistic Details of the Electron Donor–Acceptor Complex Mediated Radical Relay Process, ACS Catal., 2022, 12, 285–294 CrossRef CAS.
- H. Choi, G. R. Mathi, S. Hong and S. Hong, Enantioselective functionalization at the C4 position of pyridinium salts through NHC catalysis, Nat. Commun., 2022, 13, 1776–1783 CrossRef CAS PubMed.
- C. Wang, Z. Wang, J. Yang, S. Shi and X. Hui, Sequential Visible-Light and N-Heterocyclic Carbene Catalysis: Stereoselective Synthesis of Tetrahydropyrano[2,3-b]indoles, Org. Lett., 2020, 22, 4440–4443 CrossRef CAS PubMed.
- Y. Chen, B. Shi, H. Yin, Y. Liu, C. Yu, K. Zhang, T. Li and C. Yao, Stereoselective synthesis of chiral sultam-fused dihydropyridinones via photopromoted NHC catalyzed [4 + 2] annulation, Org. Chem. Front., 2022, 9, 5191–5196 RSC.
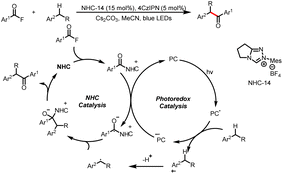
- B. Cai, Q. Li, C. Empel, L. Li, R. M. Koenigs and J. Xuan, Dark and Light Reactions of Carbenes–Merging Carbene Transfer Reactions with N-Heterocyclic Carbene Catalysis for the Synthesis of Hydroxamic Acid Esters, ACS Catal., 2022, 12, 11129–11136 CrossRef CAS.
- A. V. Bay, K. P. Fitzpatrick, R. C. Betori and K. A. Scheidt, Combined Photoredox and Carbene Catalysis for the Synthesis of Ketones from Carboxylic Acids, Angew. Chem., Int. Ed., 2020, 59, 9143–9148 CrossRef CAS PubMed.
- A. V. Bay, K. P. Fitzpatrick, G. A. González-Montiel, A. O. Farah, P. H. Cheong and K. A. Scheidt, Light-Driven Carbene Catalysis for the Synthesis of Aliphatic and a-Amino Ketones, Angew. Chem., Int. Ed., 2021, 60, 17925–17931 CrossRef CAS PubMed.
- A. V. Bay, E. J. Farnam and K. A. Scheidt, Synthesis of Cyclohexanones by a Tandem Photocatalyzed Annulation, J. Am. Chem. Soc., 2022, 144, 7030–7037 CrossRef CAS PubMed.
- X. Wang, Y. Tang, S. Ye, J. Zhang, Y. Kuang and J. Wu, Access to Sulfoxides under NHC/Photocatalysis via a Radical Pathway, Org. Lett., 2022, 24, 2059–2063 CrossRef CAS PubMed.
- Y. Sato, Y. Goto, K. Nakamura, Y. Miyamoto, Y. Sumida and H. Ohmiya, Light-Driven N-Heterocyclic Carbene Catalysis Using Alkylborates, ACS Catal., 2021, 11, 12886–12892 CrossRef CAS.
- P. Wang, K. P. Fitzpatrick and K. A. Scheidt, Combined Photoredox and Carbene Catalysis for the Synthesis of γ-Aryloxy Ketones, Adv. Synth. Catal., 2022, 364, 518–524 CrossRef CAS PubMed.
- S. Ren, X. Yang, B. Mondal, C. Mou, W. Tian, Z. Jin and Y. R. Chi, Carbene and photocatalyst-catalyzed decarboxylative radical coupling of carboxylic acids and acyl imidazoles to form ketones, Nat. Commun., 2022, 13, 2846–2855 CrossRef CAS PubMed.
- Y. Dong, C. Zhang, Z. Gao and S. Ye, Iminoacylation of Alkenes via Photoredox N-Heterocyclic Carbene Catalysis, Org. Lett., 2023, 25, 855–860 CrossRef CAS PubMed.
- X. Wang, B. Zhu, Y. Liu and Q. Wang, Combined Photoredox and Carbene Catalysis for the Synthesis of α-Amino Ketones from Carboxylic Acids, ACS Catal., 2022, 12, 2522–2531 CrossRef CAS.
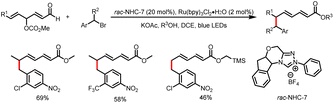
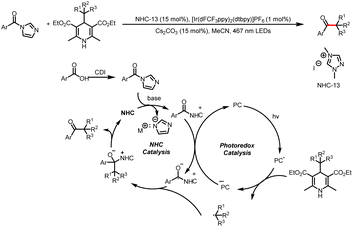
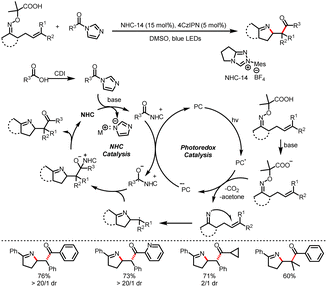
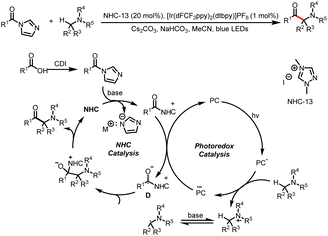
- X. Wang, R. Yang, B. Zhu, Y. Liu, H. Song, J. Dong and Q. Wang, Direct Allylic Acylation via Cross-Coupling Involving Cooperative N-Heterocyclic Carbene, Hydrogen Atom Transfer, and Photoredox Catalysis, Nat. Commun., 2023, 14, 2951–2960 CrossRef CAS PubMed.
- Q. Meng, L. Lezius and A. Studer, Benzylic C–H acylation by cooperative NHC and photoredox catalysis, Nat. Commun., 2021, 12, 2068–2075 CrossRef CAS PubMed.
- X. Yu, Q. Meng, C. G. Daniliuc and A. Studer, Aroyl Fluorides as Bifunctional Reagents for Dearomatizing Fluoroaroylation of Benzofurans, J. Am. Chem. Soc., 2022, 144, 7072–7079 CrossRef CAS PubMed.
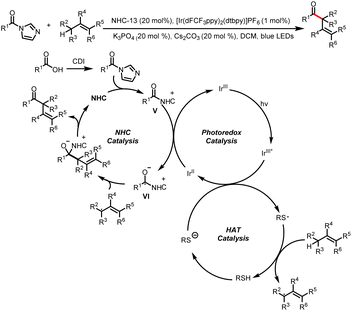
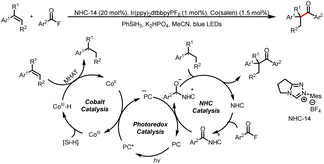
- X. Tao, Q. Wang, L. Kong, S. Ni, Y. Pan and Y. Wang, Branched-Selective Hydroacylation of Alkenes via Photoredox Cobalt and N-Heterocyclic Carbene Cooperative Triple Catalysis, ACS Catal., 2022, 12, 15241–15248 CrossRef CAS.
- H. Huang, Q. Dai, H. Leng, Q. Li, S. Yang, Y. Tao, X. Zhang, T. Qia and J. Li, Suzuki-type cross-coupling of alkyl trifluoroborates with acid fluoride enabled by NHC/photoredox dual catalysis, Chem. Sci., 2022, 13, 2584–2590 RSC.
- (a) A. Mavroskoufis, K. Rajes, P. Golz, A. Agrawal, V. Ruß, P. J. Götze and M. N. Hopkinson, N-Heterocyclic Carbene Catalyzed Photoenolization/Diels–Alder Reaction of Acid Fluorides, Angew. Chem., Int. Ed., 2020, 59, 3190–3194 CrossRef CAS; (b) A. Mavroskoufis, M. Lohani, M. Weber, M. N. Hopkinson and J. P. Götze, A. (TD-)DFT study on photo-NHC catalysis:photoenolization/Diels–Alder reaction of acid fluorides catalyzed by N-heterocyclic carbenes, Chem. Sci., 2023, 14, 4027–4037 RSC.
- Q. Meng, N. Döben and A. Studer, Cooperative NHC and Photoredox Catalysis for the Synthesis of b-Trifluoromethylated Alkyl Aryl Ketones, Angew. Chem., Int. Ed., 2020, 59, 19956–19960 CrossRef CAS.
- Z. Zuo, C. G. Daniliuc and A. Studer, Cooperative NHC/Photoredox Catalyzed Ring-Opening of Aryl Cyclopropanes to 1-Aroyloxylated-3-Acylated Alkanes, Angew. Chem., Int. Ed., 2021, 60, 25252–25257 CrossRef CAS.
- S. Li, H. Shu, S. Wang, W. Yang, F. Tang, X. Li, S. Fan and Y. Feng, Cooperative NHC and Photoredox Catalysis for the Synthesis of 1,4- Dicarbonyl Compounds via Diacylation of Alkenes, Org. Lett., 2022, 24, 5710–5714 CrossRef CAS.
- L. Wang, J. Sun, J. Xia, M. Li, L. Zhang, R. Ma, G. Zheng and Q. Zhang, Visible light-mediated NHCs and photoredox co-catalyzed radical 1,2-dicarbonylation of alkenes for 1,4-diketones, Sci. China: Chem., 2022, 65, 1938–1944 CrossRef CAS.
- K. Liu and A. Studer, Direct α-Acylation of Alkenes via N-Heterocyclic Carbene, Sulfinate, and Photoredox Cooperative Triple Catalysis, J. Am. Chem. Soc., 2021, 143, 4903–4909 CrossRef CAS PubMed.
- L. Wang, R. Ma, J. Sun, G. Zheng and Q. Zhang, NHC and visible light-mediated photoredox cocatalyzed 1,4-sulfonylacylation of 1,3-enynes for tetrasubstituted allenyl ketones, Chem. Sci., 2022, 13, 3169–3175 RSC.
- L. Wang, J. Sun, J. Xia, R. Ma, G. Zheng and Q. Zhang, Visible light-mediated NHC and photoredox co-catalyzed 1,2-sulfonylacylation of allenes via acyl and allyl radical cross-coupling, Org. Chem. Front., 2023, 10, 1047–1055 RSC.
- J. Reimler, X. Yu, N. Spreckelmeyer, C. G. Daniliuc and A. Studer, Regiodivergent C−H Acylation of Arenes by Switching from Ionic- to Radical-Type Chemistry Using NHC Catalysis, Angew. Chem., Int. Ed., 2023, 62, e202303222 CrossRef CAS.
- H. Yang, X. Jin, H. Jiang and W. Luo, Construction of C(CO)–C(CO) Bond via NHC-Catalyzed Radical Cross-Coupling Reaction, Org. Lett., 2023, 25, 1829–1833 CrossRef CAS.
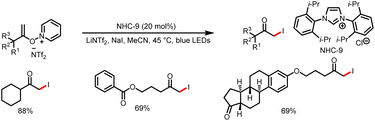
- S. Ren, W. Lv, X. Yang, J. Yan, J. Xu, F. Wang, L. Hao, H. Chai, Z. Jin and Y. R. Chi, Carbene-Catalyzed Alkylation of Carboxylic Esters via Direct Photoexcitation of Acyl Azolium Intermediates, ACS Catal., 2021, 11, 2925–2934 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2023 |