Open Access Article
Open Access ArticleRecovery of metals and valuable chemicals from waste electric and electronic materials: a critical review of existing technologies
Sahil
Gulliani
a,
Maurizio
Volpe
 *b,
Antonio
Messineo
*b,
Antonio
Messineo
 b and
Roberto
Volpe
b and
Roberto
Volpe
 *a
*a
aSchool of Engineering and Materials Science, Queen Mary University of London, Mile End Road, London E1 4NS, UK. E-mail: r.volpe@qmul.ac.uk
bFaculty of Engineering and Architecture, University of Enna Kore, Cittadella Universitaria, 94100, Enna, Italy. E-mail: maurizio.volpe@unikore.it
First published on 22nd May 2023
Abstract
The growing development of technology has increased the amount of waste generated by electrical and electronic equipment (WEEE) every year. WEEE contains valuable metals and hazardous materials which, if not properly recovered, may drastically contribute to the depletion of natural resources while posing threat to the environment. The recent escalation of geopolitical tensions has fueled a growing spike in commodity and energy prices. In today's world, the recycling technologies have already evolved from primitive methods to more sophisticated techniques such as automatic disassembly, chemical leaching, electrolysis and so on. It is mandatory that researchers will develop novel technologies to tackle the complexity of WEEE treatment and material recovery. This analysis critically reviews the accomplishments in the field of e-waste recycling and further assesses the principles of recycling, separation, and optimized parameters of different technologies. The application of conventional techniques like pyrometallurgy and chemical leaching (non-cyanide, reduced wastewater) results in an active recovery of various materials. Compared to cyanide and strong acid leading, thiourea and thiosulphate have achieved significant advancements in environmental protection. Additionally, novel technologies like bio-metallurgy cryo-milling, siderophores and supercritical extraction technology also resulted in enhanced recovery efficiencies for base and precious metals, along with metal recovery techniques using recyclable lixiviates. However, the application of these technologies is restricted due to the heterogeneous nature of WEEE. Therefore, this review focuses on the deficiencies of each of them and further discusses the interpretation of future urgent developments in the WEEE recycling sector.
Sustainability spotlightThe ever-increasing growth of technology generates large amounts of waste, in particular by electrical and electronic equipment (WEEE), which contain metals and hazardous materials that, if not properly recovered, drastically contribute to the depletion of natural resources and pose a threat to the environment. Researchers and industry have developed novel technologies to tackle the complex WEEE treatment and the recovery of the materials it is made of. Our analysis reviews the accomplishments in e-waste recycling and assesses the principles of recycling, separation, and the parameters of different technologies, by looking at both traditional and novel technologies. Overall this work is timely and relevant to the UN goal for sustainable development number 12: ensure sustainable consumption and production patterns. |
1. Introduction
Current developments in technology have led to the consumption of an increased number of electrical and electronic equipment (EEE) worldwide.1 Appliances like mobile phones, laptops, and tablets have pierced into our everyday lives, and with frequent advancements, the waste of electrical and electronic equipment (WEEE) has grown exponentially. Global E-waste Monitor in 2020 reported that an estimated 53.6 million metric tonnes (Mt) of WEEE was generated in 2019, representing an increase of 21% since 2014, with e-waste on a predicted course to 74 Mt by 2030. Therefore, global e-waste generation is growing annually by 2 Mt, a problem attributed to higher consumption rates of electronics (increasing 3% annually), shorter product lifecycles, and limited repair options (“International E-Waste Day: 57.4 M Tonnes Expected in 2021,” 2021). The WEEE contains a diverse range of products, making it a very complex stream to recycle. Between these products, the most commonly found are printed circuit boards (PCBs), Li-ion batteries (LIBs) and other types of batteries, cathode ray tubes (CRTs), liquid crystal displays (LCDs), cell phones, and televisions (TVs), and these products contain on average by weight up to 60% of metals, 12% of CRTs and LED screens, 15% of plastics, and 5% of metal-plastic mixtures. WEEE has several valuable materials like metals, but also organics and glass fibers.2 WEEE mainly contains metals like copper, silver, gold, palladium, iridium, and rare earth materials in much smaller quantities, representing a valuable source of critical and strategical raw materials that otherwise should be recovered mainly by mining the corresponding originating ores. Efficient recovery of those materials from WEEE (Urban mining) not only would contribute to the depletion of natural resources but also in fact make their supply dependent on producing countries with significant problems linked to the increasingly common geopolitical tensions.Table 1 shows the quantities of base and precious metals found in different WEEE components like PCBs, TVs, mobiles, and computers.
| Metal content of various WEEE | ||||||||
|---|---|---|---|---|---|---|---|---|
| Type of WEE | Fe (wt%) | Cu (wt%) | Al (wt%) | Pb (wt%) | Ni (wt%) | Ag (ppm) | Au (ppm) | Pd (ppm) |
| Mobile phone | 50 | 12 | 1 | 0.3 | 0.1 | 1380 | 350 | 210 |
| DVD player | 62 | 5 | 2 | 0.3 | 0.05 | 115 | 15 | 10 |
| TV board | 28 | 10 | 10 | 1 | 0.2 | 280 | 20 | 4 |
| PC board | 7 | 20 | 85 | 1.5 | 1 | 1000 | 250 | 110 |
| PC main board | 4.5 | 14.3 | 2.8 | 2.2 | 1.1 | 566 | 566 | 124 |
| Conventional electronic device | 8 | 20 | 2 | 2 | 2 | 2000 | 1000 | 50 |
| PCB | 12 | 10 | 7 | 1.2 | 0.85 | 280 | 110 | — |
However, large volumes of WEEE create problems as they contain toxic and hazardous compounds like heavy metals, brominated flame retardants (BFRs) and epoxy resins which are harmful to humans and the environment. As the current infrastructure of the recycling industry is not equipped to handle such large volumes of hazardous and complex waste streams at low cost, the job is taken up by the informal sector employing primitive methods for the recovery of economically valuable materials in developing countries like China, India, Pakistan, and Nigeria, where environmental regulations and control are less strict thus exposing the operators to toxic substances.4 The majority of WEEE generated is either incinerated or landfilled after its end-of-life.5 The heavy metals and toxic compounds present in WEEE leech over time and reach and contaminate the groundwater streams and soil. Table 2 shows some of the harmful and beneficial materials found in WEEE.
| Metal content of various WEEE | ||
|---|---|---|
| Component | Beneficial materials | Harmful materials |
| PCBs | Glass fiber, epoxy resin, Cu | BFRs, heavy metals |
| Li-ion batteries | Cu, Al, Li, Co, Mn, steel | LiPF6 |
| Cathode ray tube | Glass | Pb (PbO) |
| LCD | Glass, In2O3 | TCA, PVA, liq. crystal |
| Refrigerators | Cu, Al, glass | Freon |
The EU produced more than 12 million tons of WEEE in 2020, and just approximately 40% by mass was reported to be recycled (adapted from Eurostat – European Commission, 2022) (Fig. 1).
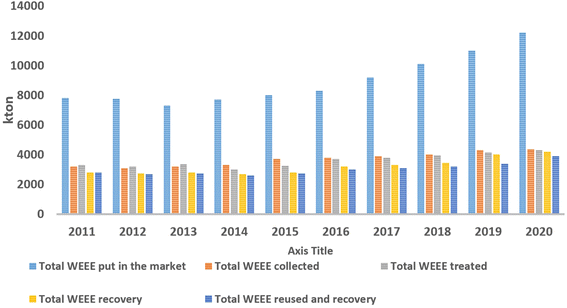 | ||
| Fig. 1 EEE put every year in the EU market and amount of WEEE treated, collected, recovered, recycled and/or reused between 2011 and 2020. | ||
Moreover, some developed countries like the USA, along with others, export the majority of their e-waste produced to developing nations like China, India, Pakistan and Nigeria.7 The USA is the main world's exporter with 7.1 million tonnes a year, exporting to Mexico, Venezuela, Paraguay, Ghana, Nigeria and China (Danciu et al., 2018). The world per capita yearly generation spans from the highest generating country, Norway (28.3 kg per capita) to African countries (10.8 kg per capita) and China (4.4 kg per capita).8,9
However, to tackle the problem, solutions that target processing the e-waste at its source are required, for a possible sustainable future. To discard complex WEEE scrap in an environmentally sustainable and economical way, various advanced metallurgical technologies are proposed along with mechanical pre-treatment by many researchers. Therefore, this critical review presents various improvements that have been achieved in the recycling industry, to recover valuable materials from WEEE using mechanical–physical separation, pyrometallurgy, hydrometallurgy, bio-metallurgy and supercritical fluid extraction. Meanwhile, opinions have been provided on the enhancement of recycling efficiency for future developments to recover metals and non-metals from WEEE. The pre-treatment of WEEE is vital for freeing the metals and non-metal components and their easy separation and recovery. The pretreatment is followed by a pyrometallurgical process of the pulverized materials, in smelters to remove non-metals. Subsequently, valuable metals can be extracted by chemical leaching. Although hydrometallurgy steps are included in this study as a sustainable approach, they produce large amounts of wastewater, due to leaching treatments, thus hampering the development of an environmentally friend process. Bio-metallurgy processes that employ organisms like fungi and bacteria to carry out the leaching also suffer from several problems and between them the long times of leaching. A better, environmentally speaking, approach is offered by supercritical CO2 or water extraction technologies that suffer from high investment and maintenance costs.
2. WEEE recycling process pre-treatments: physical–mechanical procedures
The pre-treatment of WEEE is employed to release the metals and non-metals present in them, using physical methods such as crushing, milling, and screening. Lately, the extraction of valuable metals like Au, Ag, Pd, and Cu and valuable elements like rare earth compounds has attracted attention for using physical, chemical and hydro/pyro-metallurgical techniques.Physical separation is the most practiced method due to its low operating and capital costs; however, it is responsible for significant metal losses (15–30%). The physical and mechanical separation step has reached nowadays its maximum industrialization.10 The pre-treatment of WEEE involves four main processes: disassembly and de-soldering; physical processing (size reduction to a fine powder); material screening (rotary or vibratory); physical separation of metallic fraction (MF) & non-metallic fraction (NMF). Physical and mechanic pre-treatments are also needed to improve the recovery of valuable chemical compounds like those containing rare earth elements (REEs).11
2.1 Disassembly and de-soldering
Disassembly aims to remove hazardous and reusable components from WEEE to reduce the toxicity of the main feed before further processing.12,13 There are two forms of disassembly: first, selective disassembly which is subdivided into mechanical and manual or physical methods, and the latter is extensively used in developing countries due to its competitive costs whereas the former is employed in developed countries; and the second is simultaneous disassembly.4Semi-automatic disassembly is performed by a synergistic application of heat, above the melting point of solder, and external forces like vibrating, shearing or impact to dismount the electrical components in a part removal unit. The heat produced using infrared heaters reaches temperatures of 250 °C. These processes do not require any gas controlling, solder removal or bare board collection units. Park and Fray developed an apparatus that could achieve a 94% disassembly ratio, at a temperature of 250 °C and a feed rate of 0.33 cm s−1.19 An automatic disassembly is an intellectual approach where mechanical robots disassemble e-waste components by a recognition system. Optical means like 1-D, 2-D, or 3-D images, infrared imaging and radio frequency identification20 are being used for contemporary recognition systems. Object recognition is possible using sensors and IR lasers21 and involves three steps: identification of objects, providing knowledge for identification, and categorizing objects by comparing their characteristics stored in the database or knowledge-base. Nowakowski20 suggested a procedure emphasizing the use of 2D-codes and radio frequency identification for disassembly (RFID) of components from WEEE at their end of life. Laboratory scale simulations stipulated easy reading of 2D-code labels and RFID tags for medium-sized equipment. An automatic disassembly system developed by Feldmann and Scheller22 utilized manual dismantling of reusable or hazardous parts followed by heating to desolder the joints and provided selective disassembly by procuring information from 3D images. The components of interest were isolated from other parts by image processing algorithms. Subsequently, the components of interest recovered were sent to an identification unit which sorted out components into different categories using pattern recognition based on defined parameters.
Furthermore, an automatic reverse manufacturing system involves recognition systems, automatic de-soldering units and a robot unit. An automated recognition system determined the class, size, and position of the components. Moreover, a knowledge-base was developed according to the component layout, which assisted in efficiently locating resourceful components. Components were de-soldered by applying hot gas and then cherry-picked by the robotic unit. The system was also equipped with an automatic optical inspection system coupled with an image processing program to monitor the size and appropriate geometric specifications in 3D.23
Another concept for selective dismantling PCBs and mobile phones in brief cycle times suggested employing an image processing unit, robotic handling units, 3D laser computation, and material recognition through laser spectroscopy along with laser treatment to unsolder and cut the components. The knowledge base required in-depth information regarding the physical and chemical attributes related to the components to be handled.24
2.2 Physical processing techniques (pulverizing)
Upon the removal of hazardous and reusable elements, the waste PCB board goes through physical processing. Effective liberation of abundant metals, plastics, and other materials by employing various mechanical tools and equipment like hammer crushers and crushing and cutting mills is a critical step. Crushers equipped with a bottom sieve, rotary crushers, and shredders have also been reported in various studies. Comminution and high-efficiency liberation are vital for better recovery rates of materials.25 Shredding or grinding operations can produce hazardous fine metal particles, dioxins, and BFRs in the dust and reduce the number of precious metals by around 40%.26Ghosh and co-workers12 stated that disc and ball milling can effectively pulverize the feed after processing PCB boards through cutters. Two-step crushing is an efficient way to liberate materials. Firstly, for ideal output, primary crushing is carried out at low speed and high torque shearing in shredders (10 mm) followed by finer comminution through various milling processes.27,28 A shift has been seen in recent years with the increased use of swing hammer mills for the second step.29–31
2.3 Material screening (sieving)
In this process, particles are segregated into different categories depending on their size, using methods like shape separation, screening or sieving. This procedure helps in producing a homogeneously sized feed and also enhances the metal concentration. Screening is a necessary process because metals compared to plastics and ceramics differ in their physical properties (shape/size). The most widely practiced screening method in metal recovery, using rotary screens (trammel), is employed by the automobile and solid municipal waste sectors. For non-metallic recovery, the most common apparatus is vibrating screens.32 Shape separation methods are essential as particle size defines the characteristic properties of the materials. Shape separation techniques like rotary horizontal sieve drums and inclined plates are used to recover materials from waste electrical wires, PCBs, and TVs.33,342.4 Physical separation of metals and non-metals
The effectiveness of the physical separation of materials is controlled by their physical properties like geometry, shape and size. Some major physical separation techniques used in the WEEE recycling industry are provided in Table 3 below.| Technology | Material separated | Merits and demerits |
|---|---|---|
| Magnetic separation (MS) | Ferrous metals | MS was most suitable for separating steel or iron but not suitable for separating non-ferrous metals |
| Electron-conductivity separation (ECS) | Ferrous and non-ferrous metals | ECS was encouraged to recover non-ferrous metallic particles and hard to separate ferrous metals/other metals |
| Air current separation (ACS) | Separation of light particles from heavy particles | Wind velocity, particle size, particle density, etc. were the critical influences for ACS |
| Corona electrostatic separation (CES) | Separation of metallic particles (size from 0.1 to 2 mm) from non-metallic particles | The movement trajectory and collection position of metallic particles in CES were hard to predict and compute |
Density separation processes like gravity separation work on the principle that differently sized particles have different densities, whereas magnetic separation is used to segregate ferrous materials from non-ferrous materials. Eddy current, triboelectric35 and corona electrostatic separators, working on the principle of electric conductivity, segregate MF from NMF. A schematic scheme of these techniques for physical separation is given in Fig. 2.
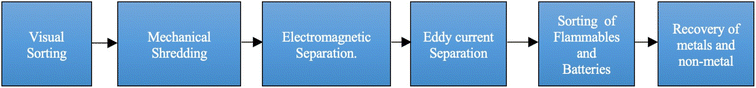 | ||
| Fig. 2 Schematic representation of physical separation techniques.37 | ||
| Material | Specific gravity (g cm−3) |
|---|---|
| Non-metallics | 1.8–2.0 |
| Aluminium, titanium, magnesium | 1.7–4.5 |
| Copper, nickel, zinc, iron | 7.0–9.0 |
| Silver, lead, molybdenum | 10.2–11.3 |
| Gold and platinum | 19.3–21.4 |
Moreover, the external force exerted by a viscous fluid (e.g. air or water) acts as the separation medium. Some dense fluids used in the separation of metals from organics and ceramics are tetrabromoethane and acetone.32,38 The most widely used density separators in WEEE recycling are heavy fluid separators, water-flow beds, and air-flow beds. Concentrating tables or air tables can be used to further distribute the metals present in various sizes. As the inclined concentrating table shakes, the difference in the specific gravity and size of the particles helps attain the required segregation. In air-tables, particles are suspended in an air stream and get separated due to the difference in their densities. Therefore, for ideal operation, the feedstock needs to be continuously monitored for optimum particle size, to encounter the size effect and help in the relative motion of particles based on their specific gravity. WEEE contains plastics, glass, light (i.e. Al) and heavy metals (i.e., Cu, Fe, and Pb) having densities of <2 g cm−3, 2.7 g cm−3, and >7 g cm−3, respectively. Zhang and Forssberg,39,40 reported that by applying the sink-float technique it was possible to recover 50 wt% of the primary plastic by flotation at a specific density of 2.0 g cm−3. Several other research reports have been published which show improvements in recent years. Peng et al.28 achieved 95 wt% metal recovery by implementing inclined separation troughs, and Eswaraiah et al. developed an air classifier that separated particles based on their settling velocity.41 It was also reported that some specific metals allow efficient separation only at an optimum particle size, which was found to be approximately 150 μm for Cu.42,43 Sarvar and co-workers reported a jig apparatus for the separation of 0.59 mm and 1.68 mm particles.44
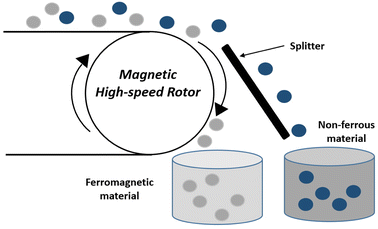
Magnetic separators operate by transporting the comminuted waste towards the magnetic drum by a conveyer belt. As the waste enters the high-intensity magnetic field, ferrous metals are attracted to the conveyer belt whereas non-ferrous materials freely fall into a vessel placed below due to the gravitational force. Another vessel placed at a position where the magnetic field ends collects particles stuck to the conveyer belt.36
The main drawback of high-intensity magnetic separators is an agglomeration of fine fragments, leading to the accumulation of non-ferrous metals on the surface of ferrous metals, resulting in reduced efficiencies. The magnetic and non-magnetic metals recovered include Fe, Ni, and Cu, respectively.47,48 A study carried out by Veit et al.49 successfully segregated 15.2% Ni and 43% Fe, by applying a magnetic field in the range of 6000 to 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Gauss (G). However, the Cu content in magnetic metals was significant. A modified two-step process was reported by Yoo et al.50 for magnetic separation. They applied gravity separation to segregate heavy particles (>5.0 mm) of milled PCBs from small-sized particles (<5.0 mm). Subsequently, in the first and second steps they applied a magnetic field intensity of 700 and 3000 G respectively. The results indicated a recovery of 83% Ni, Fe and 92% Cu following the first step. After the second step, the Cu concentration increased while the Ni and Fe concentration decreased.
000 Gauss (G). However, the Cu content in magnetic metals was significant. A modified two-step process was reported by Yoo et al.50 for magnetic separation. They applied gravity separation to segregate heavy particles (>5.0 mm) of milled PCBs from small-sized particles (<5.0 mm). Subsequently, in the first and second steps they applied a magnetic field intensity of 700 and 3000 G respectively. The results indicated a recovery of 83% Ni, Fe and 92% Cu following the first step. After the second step, the Cu concentration increased while the Ni and Fe concentration decreased.
The most common eddy current separator used today is the belt-driven rotary drum design.52 This technology is based on the principle that when a ferrous material passes through a changing magnetic field, an eddy current is induced in it, which deflects the particles to a higher degree due to repulsive forces. In the separation section, other external forces like gravity, centrifugal, and friction are also present except the magnetic force. Therefore, to achieve efficient separation, the magnetic forces acting on the ferrous particles must overcome other forces.43 Ruan and co-workers recovered Al by applying eddy current separators and varying parameters such as the feeding speed to improve the output of the system.56 Excluding Al, eddy current separators have also been used to recover Cu, non-ferrous metals, glass and other materials. Moreover, the aluminum recovered is sent for further processing like density separation to be of economic value.32 Corona electrostatic separation is regarded as the most effective technology in mechanical separation as it doesn't emit any gas or wastewater and for this reason is considered environmentally friendly. The rotor speed, particle size, moisture content, and electrode used are the factors that influence the separation efficiency. A vibrating feeder transports the pulverized particles (<0.6 mm) towards a rotating drum where a high electrostatic field is generated using the corona electrode and an electrostatic electrode.57,58 The charged NMF is attracted towards the drum, eventually falling into the collection tank below, whereas MF gets discharged in the direction of the earthed electrode instantaneously.
3. Metallurgy processes
Metallurgy is the science that studies the production metals that begins with the processing of ores to extract them, and includes the mixture of metals to make alloys. Metallurgy thus involves chemical and physical processes which are used to produce different kinds of metal alloys using both metal and non-metal compounds.3.1 Pyrometallurgy
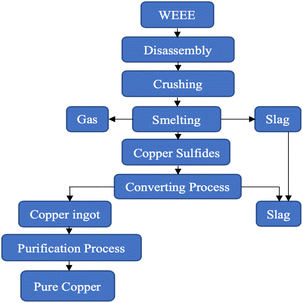
Pyrometallurgy has been performed using smelting, sintering, melting, plasma arc furnaces or blast furnaces involving gaseous reactions at elevated temperatures.Pyrometallurgy is one of the techniques that has achieved successful industrialisation, helping in the separation of metals, using thermo-physical properties.59 Also rare earth elements have been successfully recovered via pyrometallurgical processes.60 Physical–mechanical pretreatment of WEEE is a prerequisite for large scale pyrometallurgical processes like smelting to improve the extraction efficiency of metals. Two extensively used methods for smelting are flash smelting, the addition of oxygen-rich air to remove impurities, and bath smelting where reactions take place in a molten pool containing both molten metal and slag.61 The pulverized feed containing metals is combusted in a furnace, volatilizing the metals due to chemical reactions at high temperatures, and the impurities get transformed into slag. In the conversion process, copper converters are used to produce copper matte by blowing in air from the nozzles. The iron sulphides get oxidised whereas the copper sulphides transform into metal copper. Subsequent refining procedures are applied to procure pure copper in a rotary furnace. The final compounds obtained are metal ingots containing copper with some other precious metals.47,62,63Fig. 4 provides a flow chart of this process.
Many researchers focused on reviewing current pyrometallurgical technologies and industrial methods being utilised in the recycling sector.3,55 Pyrometallurgy can be regarded as a primary step to incinerate organic material from the metal phases as the former gets converted to slag. Therefore, to further refine and isolate the metals, a synergistic treatment by hydro- and bio-metallurgical methods are commonly utilised. An excellent example of application is the Norada smelter in Canada, having a capacity to treat 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tonnes of waste yearly to produce 99.1% pure Cu. Moreover, the Rönnskar smelting facility operating in Sweden is one of the world's largest recyclers boasting a capacity to treat 120
000 tonnes of waste yearly to produce 99.1% pure Cu. Moreover, the Rönnskar smelting facility operating in Sweden is one of the world's largest recyclers boasting a capacity to treat 120![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tonnes of WEEE annually via two distinct furnaces, and one of those is a kaldo furnace. The pulverized scrap is introduced into the system at different intervals of the process, and the Cu rich WPCBs are introduced directly into the system, whereas Cu deficient WPCBs are added to the kaldo furnace. Cu blisters along with Zn and Ni and precious metals like Ag, Au, and Pd were recovered after products obtained from the kaldo furnace were refined. Dust and gas collection systems were installed to recover Pb, Sn, In and Cd from the off-gasses and to harness the waste thermal energy.64 Furthermore, Umicore facility situated in Belgium can process 25
000 tonnes of WEEE annually via two distinct furnaces, and one of those is a kaldo furnace. The pulverized scrap is introduced into the system at different intervals of the process, and the Cu rich WPCBs are introduced directly into the system, whereas Cu deficient WPCBs are added to the kaldo furnace. Cu blisters along with Zn and Ni and precious metals like Ag, Au, and Pd were recovered after products obtained from the kaldo furnace were refined. Dust and gas collection systems were installed to recover Pb, Sn, In and Cd from the off-gasses and to harness the waste thermal energy.64 Furthermore, Umicore facility situated in Belgium can process 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tonnes of waste yearly with the amount of WEEE being 10%.
000 tonnes of waste yearly with the amount of WEEE being 10%.
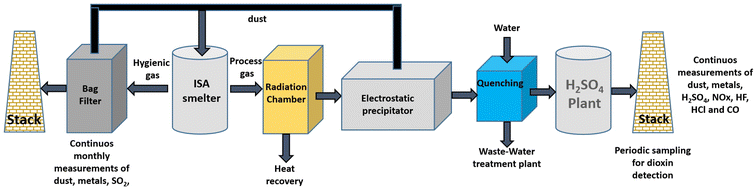
The process is carried out in two stages, first, recovering precious metals followed by base metal recovery using electrolytic refining to obtain highly pure Cu. The facility uses Isa Smelt furnaces (Fig. 5) based on the submerged lance technique. The final products obtained by smelting are Cu, Au, Ag, Pt, Pd, Rh, Ir and Ru. The Umicore facility can produce 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 2
000, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400, 100 and 50 t per year of Cu, Ag, Au and Pt group metals, respectively. Precious metals are recovered with more than 95% efficiency.65 Pyrometallurgy operations utilise the organic matter that remains in pre-treated WEEE partly as the reducing agent and partly as fuel, which in turn helps reduce the energy costs.66 The Umicore plant uses 4.5% coke, or 6% WEEE combined with 1% coke, as the reducing agent to supply comparable metal recovery and operational efficiency. Correspondingly, the Rönnskar smelter uses fossil fuels for the aforementioned purposes, and therefore, pulverized WEEE was assessed as a source of alternate energy from the metal extraction process.55,64
400, 100 and 50 t per year of Cu, Ag, Au and Pt group metals, respectively. Precious metals are recovered with more than 95% efficiency.65 Pyrometallurgy operations utilise the organic matter that remains in pre-treated WEEE partly as the reducing agent and partly as fuel, which in turn helps reduce the energy costs.66 The Umicore plant uses 4.5% coke, or 6% WEEE combined with 1% coke, as the reducing agent to supply comparable metal recovery and operational efficiency. Correspondingly, the Rönnskar smelter uses fossil fuels for the aforementioned purposes, and therefore, pulverized WEEE was assessed as a source of alternate energy from the metal extraction process.55,64
3.2 Hydrometallurgy (leaching and metal recovery)
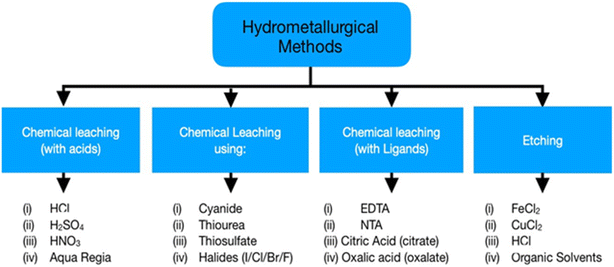
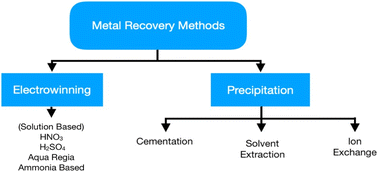
The primary focus of hydrometallurgy is recovering metals from pre-treated WEEE by controlled and predictable chemical reactions. Moreover, it is an eco-friendly approach compared to pyrometallurgy. Base and precious metal recovery is the main focus of hydrometallurgy as they affect the economic viability of the system. Hydrometallurgy includes two distinct steps, chemical leaching of metals and their subsequent extraction from the leachate via precipitation/cementation, solvent extraction, and ion exchange.38 Chemical leaching has been an extensive field of study as researchers are trying to shift towards sustainable techniques for metal recovery. Initial treatment for the recovery of base metals like Cu helps increase the concentration of precious metals in the solid remains to make the leaching process easier.12 However, these processes produce a great deal of wastewater which needs proper handling before its disposal to the environment. Moreover, this method suffers from slow reaction kinetics and increased acid consumption. Fig. 6 shows the various processes used in hydrometallurgy, acid leaching being the most widely used while other processes have not yet achieved industrialisation. Cyanide leaching is hazardous due to its toxicity; thus, thiourea and thiosulphate are preferable for precious metal recovery.The least developed branches are leaching with ligands like EDTA and DTPA, and etching, presenting low economic value.
Therefore, leaching of precious metals is carried out after the base metals have been stripped, enhancing the selectivity while reducing contamination. Sheng and Etsell68 employed a multi-step approach to recover Au and Cu. The first step used HNO3 for the dissolution of Cu, followed by leaching of Au from the remaining leachate using aqua regia in the second step. Finally, Au was precipitated using ferrous sulphate. However, due to the corrosive nature of HNO3 and aqua regia, the construction of an industrial-scale reactor is not practical and/or economically viable.
Acid leaching is the most prevalent route of hydrometallurgy. Reagents like are H2SO4, HCl and HNO3 have been extensively studied in the past due to their low cost and highly predictable and easily controllable behavior.69 Weak dilute acids successfully leach metals like Zn and Fe, but fail to leach Cu and other precious metals (like Ag and Au) because of their higher electrochemical potentials. For the liberation of a considerable portion of these metals, the application of strong acids (e.g., concentrated HNO3) or weak acids complimented with oxidizing agents (e.g., O2, H2O2, Cl2, Cu2+, and Fe3+) is necessary. Table 5 shows a summary of the studies performed on chemical leaching. Insoluble salt formation such as those of Ag and Pb also leads to reduced leaching efficiency. Therefore, acid leaching can be categorized as (a) using acids only, (b) using oxidizing acids/combination of an acid and oxidant, and (c) multi-step leaching.
| Source | Reagent | Metal extracted (%) | Conditions | Particle size | Reference |
|---|---|---|---|---|---|
| Computer PCBs | 0.5H2SO4 + 0.5 g per L Cu2+ + 25.6 g per L Cl− + O2 | Cu: 100 | 80 °C – 2 h | <0.25 mm | (Yazici and Deveci, 2013)75 |
| WPCBs | 0.5 M H2SO4 + 35% H2O2 | Cu: 86 | 25 °C – 3 h | <0.3 mm | (Behnamfard et al., 2013)73 |
| WPCBs | HNO3 + organic swelling | Pb: 99.9 | 90 °C – 2 h (Sn), 45 min (Pb) | <5 mm | (Jha et al., 2012)79 |
| Sn: 98.7 | |||||
| WPCBs | 2–5 M HNO3 | Ag: 68 | 30–70 °C – 2 h | n.a. | (Bas et al., 2013)77 |
| Cu: 99.9 | |||||
| WPCBs | 4.5 M HCl (Sn), 0.1 M HNO3 (Pb) | Pb: 99.9 | 90 °C – 1 h | <5 mm | (Jha et al., 2012)79 |
| Sn: 97.8% |
Using H2SO4, HNO3, HCl and Aqua regia, Bas et al.70 reported that when the concentration of HNO3 increased from 1 to 5 M, the leaching rate of Cu and Ag also increased. Higher leaching efficiency of Cu (88.5–99.9%) was achieved with 2 M (or more) HNO3, at 6 w/v% pulp density and 70 °C. With 1–5 M HNO3, leaching yielded only 68% Ag because of its high reduction potential (0.80 V for Ag+/Ag vs. 0.34 V for Cu2+/Cu) suggesting that a higher concentration of HNO3 might lead to a higher leaching rate of precious metals. Under ambient conditions, dilute H2SO4 failed to leach relevant amounts of Cu; with 1 M H2SO4 solution only 8.8% Cu was leached in 96 h.69 However, the leaching rate of 1 M H2SO4 can be enhanced by applying pressure (2 MPa) and high temperature (120 °C) resulting also in a complete recovery of Cu, Ni, Zn and Fe.71,72
Sulphuric acid has advantages of low corrosion activity, spontaneous regeneration, and reduced cost compared to aqua regia, making it a better product for industrial applications. Aqua regia, a universal solvent, is not a very selective leaching agent towards many base and precious metals (e.g. Cu, Pb, Zn, Ni and Au).63 Park and Fray19 effectively leached 97% Au using aqua regia from WPCBs. Their results demonstrated high durability of Ag in aqua regia, leaching only 2% Ag in 3 h at 20 °C with a liquid to solid ratio of 20 mL g−1. Moreover, Pd present in the solution precipitated as Pd(NH4)2Cl6. Therefore, due to its highly corrosive and oxidising nature towards the reactor, the industrial-scale application of aqua regia remains restricted.73
The application of acids along with an oxidant provides faster reaction kinetics of the process. H2SO4 combined with H2O2, as an oxidising agent, known as piranha acid, is very efficient and results in complete leaching of Cu from WPCBs.74 In some studies, Cu2+ and Fe3+ ions and O2 have also been utilised as oxidising agents. Yazici and Deveci75 successfully leached more than 90% Cu, Fe, Ni and Ag along with 58% Pd from WPCBs by using Cu2+ as the oxidizing agent in a chloride solution. Moreover, a superior leaching efficiency of 82% Pd was achieved when O2 was used to enhance the in situ regeneration of Cu2+.
However, it is reported that the formation of Cl− should be sustained below an adequate level to prevent the precipitation of Cu+ as CuCl compounds, which can hinder leaching. In another study, Yazici and Deveci76 used HCl–CuCl2–NaCl, a chloride solution, to extract Cu. The cupric ion (Cu2+) formed the stable cuprous ion (Cu+) when an appropriate ligand (Cl−) was present in the sulphate solution. The formation of CuCl compounds (CuCln2−n) was also reported due to the presence of Cu2+ ions.77 Increasing the concentration of Cu2+ ions to 79 mmol l−1 efficiently leached 98% Cu in 2 h. The following equations (eqn (1)–(4)) show the copper leaching mechanism:63
| Cu0 + Cu2+ → 2Cu+ | (1) |
| Cu+ + nCl− → CuCln1−n, (1 n 4) | (2) |
| Cu0 + Cu2+ + 2Cl− → 2CuCl(s), (ΔG20°C = −41 kJ mol−1) | (3) |
| Cu0 + Cu2+ + 4Cl− → 2CuCl2−, (ΔG20°C = −25 kJ mol−1) | (4) |
Yazici and Deveci76,77 also studied the effects of various characteristics like the primary concentration of Cu2+ and Cl− and the temperature on the leaching process. As the quantity of Cu2+ ions and temperature increased, enhanced leaching of metals was observed. The molar ratio of Cl−/Cu2+ is of prime importance in the process due to the resilient interaction of Cu2+ and Cl− ions during leaching. Some studies have reported using multi-step leaching to enhance the selectivity of the leaching process. Somasundaram and co-workers78 used a two-step approach to selectively leach 92% Sn along with trace amounts of Cu, Ni and Pb from WPCBs using 3 M HCl and 0.1 M CuCl2 at 35 °C. In the subsequent step, the temperature and concentration of CuCl2 were increased to 50 °C and 0.5 M, respectively, to leach the remaining Cu, Ni and Pb. Another study conducted by Jha and co-workers79 focused on leaching Sn and Pb from solder of WPCBs. In the first step, treatment with 0.2 M HNO3 leached 99.99% Pb while no leaching of Sn was observed as it was transformed into SnO2. In the second step, the SnO2 formed previously was treated with 3.5 M HCl to leach the remaining Sn. For optimum recovery of Cu, Sn and Pb, the most suitable reagents were found to be H2SO4 + H2O2 + NH3, HNO3 and HCl, respectively.
Studies have suggested that base metals can be easily leached under mild conditions by an inorganic acid from WEEE scrap, but for leaching precious metals these lixiviants remain uneconomical; a higher concentration of acid, increased pressure and longer leaching time are some reasons limiting their use. Aqua regia, HCl and HNO3 are highly reactive and volatile compounds which present risk to the workers handling them as well as the environment. Moreover, the corrosive nature of strong acids requires the equipment to be anti-corrosive and thus more expensive. Harmful gases like NOx are emitted during leaching along with the formation of undesired by-products which requires proper management of the wastewater (acidic) before its release. Consequently, a large number of studies suggest a multi-step approach: leaching of base metals using inorganic acids as the first step followed by precious metal recovery in the second step by applying alternative lixiviants like cyanide, thiourea, and thiosulphate. Acid leaching may be a favoured method for leaching because of its fast kinetics and high leaching rate but suffers from being highly caustic.80
| 4Au + 8CN− + O2 + 2H2O → 4Au(CN)2− + 4OH− | (5) |
Montero and co-workers67 recovered 62.3% Cu, 51.3% Ag, 46.6% Au and 47.2% Nb from crushed WPCB using NaCN solution by the column leaching method. Another study by Quinet and co-workers82 inspected a two-step approach to recover precious metals from WPCBs. The first step involved the dissolution of Cu and Ag with oxidative sulphuric acid and subsequent treatment by oxidative chloride for the dissolution of Cu and Pd, and eventually recovered 99%, 95% and 93% of Pd, Au and Ag, respectively, by cyanidation. However, cyanidation processes to recover metals from WEEE occur near urban areas, and the use of large quantities of cyanide is restricted in urban areas due to environmental issues. Thus, alternate lixiviant substitutes are necessary for Au leaching. Over the past few years, thiourea has attracted attention from researchers as a primary reagent to leach Au due to its low toxicity and higher leaching rate.83 It was found that its performance of leaching depends on the redox potential (ORP), concentration of thiourea and pH. Thiourea can achieve 99% dissolution of Au in an acidic medium by forming cationic complexes as shown in eqn (6):63
| Au + 2CS(NH2)2 → Au(CS(NH2)2+ + e− | (6) |
Ferric sulphate as an oxidant along with acidic thiourea can also be employed to leach Au.84 The addition of Fe3+ ions helped raise the ORP of the solution resulting in enhanced Au recovery83 (Gurung et al., 2013). The reaction taking place is reported in eqn (7):
| Au + 2SC(NH2)2 + Fe3+ → Au(SC[NH2]2)2+ + Fe2+ | (7) |
The presence of a trace amount of other base metals in the acidic thiourea medium can also be leached, resulting in excess thiourea consumption. It was reported that the optimal pH value for thiourea leaching is between 1 and 2.85 Gurung and co-workers83 leached Cu and trace amounts of Fe, Ni, Pb and Zn along with Au and Ag using thiourea from WPCBs. They reported that Ag leaching was not affected by the addition of Fe3+ ions due to the faster kinetics of Ag leaching compared to Au. Moreover, Birloaga and Vegliò80 employed two-stage leaching from WPBS. Initial treatment with H2SO4 and H2O2 resulted in base metal recover. Subsequently, 90% Au and 75% Ag were recovered using thiourea leaching.
Thiosulphate (S2O32−) can substitute cyanide for leaching precious metals. Thiosulphate presents lower environmental toxicity, low corrosively, low cost and high selectivity, making it beneficial for leaching application.86 Thiosulphate leaching is carried out in pH ranging from 9 to 10.5 as it quickly decays in an acidic medium.87 The leaching rate using thiosulphate is very low even with oxidants like O2. However, the addition of Cu2+ and ammonia can increase the leaching efficiency as they form the cupric ammonia complex, which acts as a catalyst. The leaching reaction mechanism is shown in eqn (8) and (9):3
| Au + 5S2O32− + Cu(NH3)42+ → Au(S2O3)23− + 4NH3 + Cu(S2O3)35− | (8) |
| 2Cu(S2O3)35− + 8NH3 + 1/2O2 + H2O → 2Cu(NH3)42+ + 2OH− + 6S2O32− | (9) |
Most of the recovery rates reported in research papers suggested leaching rates below 16% for Au and Ag from intact WPCBs. Reagents like CuSO4 or H2O2, NH3·H2O, and Na2S2O3 or (NH4)2S2O3 failed to leach a considerable amount of precious metals.88,89 Similarly, Petter et al.90 employed 0.015–0.03 M CuSO4, 0.2 M NH3·H2O, and 0.1 M Na2S2O3 to recover Au from pulverized WPCBs of phones (∼1 mm). They reported only 15% Au leaching from the solution. Other systems involving the application of H2O2 also did not seem to enhance the leaching efficiency of Au and Ag but using pulverised WPCBs for longer leaching times did increase recover rates (>95%) for Au and Ag.88 Tesfaye et al.91 suggested that issues related to thiosulphate leaching are high consumption rates and low reaction kinetics, making the process inefficient and expensive regardless of the eco-friendly nature.
Au leaching with halogens (Cl/Br/I/F) has been used in the mining industry long before cyanide was introduced. However, a lack of research has been noted in halogen leaching of WEEE but the only halide used on an industrial scale is chloride/chlorine.3,12 The mechanism uses gold present in two oxidation states, Au+ and Au3+, undergoing complexation with chloride/iodide/bromide as shown in eqn (10) and (11) (ref. 92) where M and L represent a precious metal and halide element, respectively.
| 2M + L2 + 2L → 2ML2 | (10) |
| 2M + 3L2 + 2L → 2ML4 | (11) |
The conventional medium for leaching gold is aqua regia and the reaction mechanism are shown in eqn (12) and (13).68
| 2HNO3 + 6HCl → 2NO + 4H2O + 3Cl2 | (12) |
| 2Au + 11HCl + 3HNO3 → 2HAuCl4 + 3NOCl + 6H2O | (13) |
The industrial-scale adaptation of this method is limited because chlorine is highly toxic, and the conditions required for the reaction to take place are highly corrosive requiring expensive equipment like special stainless steel and rubber lined reactors. New studies on using iodine as a leaching agent under alkali conditions have been conducted in recent years. This method is usually employed to leach precious metals in the second step after leaching of base metals from WPCBs. Iodine/iodide is a better alternative for chlorine/chloride as it is non-toxic, non-corrosive, and highly selective towards precious metals and has fast kinetics as well.87 In addition to this, the most stable compound formed by Au and halogens is the Au-iodide complex.12 Altansukh et al.71 reported leaching >99% Au from WPCBs pretreated with acid leaching using 2 g per L I2 and 12 g per L KI. On the other hand, disadvantages like high iodine consumption and higher cost of iodine hinder successful industrialisation of this method.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HNO3 (1
HNO3 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at 60 °C for 1 h to recover 99.9% Au. Kim et al.108 employed Cl2 as an oxidant for leaching Au from PCBs. The first stage was extracting Cu along with 5% Au. Further treatment by the ion-exchange method resulted in 93% extraction of Au. Moreover, heavy metals like Cu, Fe, Ni, Al, Au and Ag have also been extracted by indulging organic solvents for leaching. The solvent extraction method has been used by other researchers to extract Cu, Co, In, Pt, Rh, Ni and V.109–112
1) at 60 °C for 1 h to recover 99.9% Au. Kim et al.108 employed Cl2 as an oxidant for leaching Au from PCBs. The first stage was extracting Cu along with 5% Au. Further treatment by the ion-exchange method resulted in 93% extraction of Au. Moreover, heavy metals like Cu, Fe, Ni, Al, Au and Ag have also been extracted by indulging organic solvents for leaching. The solvent extraction method has been used by other researchers to extract Cu, Co, In, Pt, Rh, Ni and V.109–112
In cementation, metals with higher reduction potential like Fe, Zn, and Cu readily replace metals that have lower reduction potential such as precious metals.92 The most commercialized process to recover precious metals globally is called the Merrill–Crowe process.
The mechanism includes Au deposition on the cathode and corrosion of Zn at the anode (surface of Zn). Furthermore, the Zn cementation is constant for pH 8–11. The anodic and cathodic reactions are as follows (eqn (14) and (15):
| 2Au(CN)2− + 2e− → 2Au + 4CN− | (14) |
| Zn + 4CN− → Zn(CN)42− + 2e− | (15) |
The demerits of this process include passivation of the zinc particle surface by the formation of zinc hydroxide or oxides, due to low cyanide quantity, resulting in inhibition of the redox reaction, H2 production, and the damaging effect of impurities like Cu, Ni, and S in gold cementation.113 Moreover, high reagent consumption and co-precipitation of metals are some other problems of this process. Behnamfard et al.73 reported using 8 g per L sodium borohydride (NaBH4) solution at room temperature for 15 min to extract 100% Ag and Au in thiourea and thiosulphate media through reduction precipitation. Moreover, Awadalla and Ritcey114 used an aqueous solution of 12% NaBH4 and 40% NaOH to reduce Au ions to metallic gold at moderate temperatures. Thiourea works as both a leaching agent and a stripping agent for Au and therefore thiourea is the best alternative to cyanide for Au recovery. Additionally, Joda and Rashchi115 precipitated 87% Ag+ from HNO3 using NaCl whereas other advanced methods like precipitation using oxalate116 and solvent displacement117 from WPCB leachate have also been put forward.
Solvent extraction is applied to extract metals from the aqueous leachates. This method provides benefits like improved selectivity of metals and moderate reaction conditions, while the consumption rate for reagents remains high for WPCBs. Moreover, after the extraction of metals is concluded, a back extraction needs to be performed to recover the reagent for further usage.92 Reagents like the LIX series including LIX26, LIX841C, LIX84, and LIX984N have been successfully used to extract Cu in acidic media with recovery rates above 99%. Oishi et al.118 reported that they successfully recovered above 95% of impurities such as Fe, Zn, Ni, Pb and Mn from an ammonia–ammonium medium which in turn improved the Cu extraction efficiency. Other research efforts have also been conducted on the recovery of precious metals, but studies based on WEEE remain to be limited. Various systems have applied organophosphorus based media, guanidine-based media and a combination of amine and organophosphorus-based media for solvent exchange recovery.92 The most relevant lixiviant for the recovery of aurocyanide complexes (Au) from an alkaline cyanide medium was found to be LIX79.3 Therefore, LIX79 can be utilized to recover Au from WEEE treated with cyanide for leaching.
Moreover, Tanda and co-workers119 demonstrated efficient recovery of Cu using oximes and diketones as lixiviants from an alkali glycinate medium. Ion exchange (IE) methods work on the principle of adsorption and can provide better precious metal recovery rates. Moreover, the resins involved in adsorption have excellent recyclability and can conduct desorption under ambient conditions. The characteristics of resins employed in IE include superior mechanical strength, wear resistance, and breakage rate.92 Gurung and co-workers113 conducted a study to selectively adsorb Au and Ag using a cross-linked persimmon tannin gel (CPT) from WPCBs. They reported that Ag could be recovered with higher selectivity from the thiourea leach liquor using the unique bio-sorbent.
Furthermore, Zhang and Dreisinger120 experimented on three gel-based resins, namely Dowex G51, Dowex 21K, and ambulate IRA-410 to recover the Au present in ammoniacal thiosulphate leachate. They concluded that high Au recovery rates could be achieved at higher pulp densities without the presence of Cu whereas, in the presence of Cu, lower pulp destinies are required due to the unstable thiosulphate solution which can lead to the formation of polythionates. However, the effect of Cu can be eliminated by using Amberlite XAD-7HP to recover Au from chlorine-based leach liquor. Kim and co-workers108 achieved this by using 0.1 mol per L HCl and 1 mol per L HCl in acetone to extract Cu and Ag, respectively. In another study,83 a combination of bisthiourea modified persimmon tannin (BTU-PT) and CTP gel was used for recovering 100% Au3+ from chloride leachate of WPCBs. They reported that the thiocarbonyl group was the primary cheating agent in the process and that CPT has better selectivity for Au over other bases and precious metals. After Au recovery, BTU-PT gel was employed to adsorb Pd from the remaining solution. In another attempt, Dhiman and Gupta121 used Cyphos IL102, for the first time, to recover 98.6% Co, 99.9% Mn, and 99.6% Li from spent LIBs as Co3O4, MnO2, and Li2CO3, respectively. It was reported that the IE exchange mechanism used CoCl42− anions as the cheating agent to extract Co. As the leachate of WEEE includes both precious and base metals, inventions of new resin materials with adaptive functional groups are required.
Chu and co-workers122 studied the effect of various parameters such as current density, electrolysis time, and NaCl, H2SO4, and CuSO4·5H2O concentrations on the extracted Cu powder size and current efficiency. More recently citrate-based solutions have been proposed because citric acid is considered to be an environmentally friendly component. A high deposition rate and current efficiency of various heavy metals have been achieved at a deposition potential −0.85 V at 60 °C.123 The use of electrocatalysts has also been recently successfully implemented for highly efficient Cu recovery by electrowinning.124 Highly pure Cu recovery was recently achieved by integrating solid–liquid extraction and electrowinning of bottom ash (rich in Cu, Zn, Al, Ca, Mg, and P) derived from municipal solid waste incineration.125 Selective recovery of Sn and Cu from printed circuit board waste via selective leaching combined with cyclone electrowinning was reported by Guo and co-workers.126 A recent critical review on the recovery of copper from e-waste underlined the challenges and opportunity of the electrowinning technology.127 The use of electrocatalysts improved Cu electrowinning as they reduced the cathodic reduction overpotentials, enhanced surface reaction kinetics and increased current efficiency.127
Here, S2O82− salts are usually added batchwise to the solution which has the disadvantage that S2O82− has a limited stability in strongly acidic solutions, that are typically required for leaching unreactive metals. To overcome this problem, S2O82− can be produced electrochemically in situ with high efficiency on boron-doped diamond electrodes. These electrodes possess a high overpotential for O2-evolution and thus allow the generation of strongly oxidizing species which have been proven to be kinetically effective for leaching Ag, Cu and Sn from WEEE (Liu et al., 2019; Modrzynski et al., 2022).128,130
The adsorption mechanism is defined by the physicochemical properties of the adsorbent and heavy metals and operating conditions (i.e., temperature, adsorbent amount, pH value, adsorption time, and initial concentration of metal ions). This method was reported to have low operating costs, high removal capacity, easy implementation, and simple treatment by regenerating the adsorbed heavy metal ions.124 Heavy metal adsorption processes can be distinguished by the nature of the adsorbent like carbon-based, mineral, magnetic, and bio-sorbent.131
Carbon-based nanoporous adsorbents, especially activated carbon (AC), carbon nanotubes (CNTs), and graphene (GN), are extensively used in the applications of heavy metal removal owing to their tremendous surface area (500–1500 m2 g−1).132 More recently carbon based adsorbents showing high adsorption capacities have also been produced via thermochemical treatments of waste biomass.133–135 Mineral adsorbents such as zeolites, silica, and clay show high efficiency in heavy metal water purification with low operating costs.136 Clay has extraordinary cation exchange capacity (CEC), selectivity, surface hydrophilicity, and surface electronegativity.137 Magnetic adsorbents, which show low cost, easy-synthesis, extraordinary surface charge, and reusability, are a specific material matrix that hosts iron particles (usually magnetic nanoparticles, such as Fe3O4). Magnetic nanoparticles could be supported on carbon, polymers, starch, or biomass. The adsorption process is affected by the magnetic field, surface charge, and redox activity characteristics.131 Bio-sorbent materials show high heavy metal removal efficiency thanks to the presence of numerous functional groups (i.e., carboxyl, amino, hydroxyl, phosphate, and thiol) on the surface.138 Generally, the interaction between pollutants and the surface of the biosorbent can occur through electrostatic interaction, aggregation, complexation/coordination, microprecipitation, ion exchange, reduction, or oxidation. Biosorption can be defined as a type of adsorption in which the adsorbent is a material of biological origin, either natural or waste from an agro-industrial process. Biosorbents should preferably be industrial waste with no added value, high bio-availability and a fast production/growth cycle. The combination of these factors reduces the cost of the bio-sorption process, which is the main advantage of this approach. High bio-sorption capacity, rapid removal and resistance to friction are highly desirable features for the material to be considered as a potential bio-sorbent.139 In general, the most commonly used bio-sorbents for the uptake of toxic metals can be divided into three groups: microorganisms, algae and agro-industrial waste.131
3.3 Biometallurgy
Biometallurgy employs micro-organisms to leach metals from their primary and secondary metal sources like ores and WEEE, respectively. Biometallurgy is preferable over typical pyrometallurgy and hydrometallurgy techniques because of its reduced energy consumption, simple mechanism, reduced chemical reagent consumption, lower capital requirements, and environmentally benign nature.140,141 Most of the research conducted in the WEEE sector is still on a laboratory scale and has not achieved commercialization because of prolonged leaching times. Micro-organisms can utilize metal ions for structural and catalytic functions while their interaction mechanism is subjective to their type, prokaryotes or eukaryotes. These organisms either bind with the metal ions or are consumed by the cell to perform intracellular activities, thus providing selective metal extractions.3 Therefore, bioleaching uses sulfur- and iron-oxidizing acidophilic microbes to recover base (e.g., Cu, Zn, Al, Mn, Co, and In) and precious (e.g., Au and Ag) metals. Fungal strains like Aspergillus niger and Penicillium simplicissimum thermophilic bacterial strains like Acidithiobacillus ferrooxidans, Acidithiobacillus thiooxidans, Leptospirillum ferrooxidans, Pseudomonas putida, and Cyanobacterium violaceum are among the most extensively researched micro-organisms for the recovery of heavy metals from WPCBs, LIBs, and LCDs.142–148 These mesophilic chemolithotrophic microbes use Fe2+ and S° as an energy source, e.g. A. ferrooxidans, which are perfect candidates to carry out leaching from WEEE. Metal dissolution can place in two ways: first, direct leaching mechanisms where bacteria are directly employed, and second, indirect mechanisms where bacterial oxidation of Fe2+ to Fe3+ is used. The mechanism involves oxidation of ferrous ions (Fe2+) to ferric ions (Fe3+) and oxidation of elemental sulfur to produce biogenic sulphuric acid. The ferric ions produced by the bacteria are capable of leaching metal sulfides like CuS, NiS, ZnS, or FeS2 from acidic media. This reaction mechanism is given in eqn (16)–(20).77,143| 2Fe2+ + 0.5O2 + 2H+ → 2Fe3+ + H2O | (16) |
| MS + 2Fe3+ → M2+ + 2Fe2+ + S° | (17) |
| S° + 1.5O2 + H2O → H2SO4 | (18) |
| 4FeS2 + 15O2 + 2H2O → 4Fe3+ + 8SO42− + 4H+ | (19) |
| FeS2 + 14Fe3+ + 8H2O → 15Fe2+ + 2SO42− + 16H+ | (20) |
The ferric ion oxidizes the metal while the microbes assist in the circular regeneration of Fe3+ to Fe2+. Moreover, heterotrophic micro-organisms (e.g., Fungi; A niger) produce organic acids like citric acid, malic acid, and oxalic acid, which assist in dissolving metals like Cu in WEEE by acidification and complexation.145 For example leaching of Cu in an acidic medium is due to a reduction couple, i.e. reduction of hydrogen at the cathode and oxidation of Cu at the anode as shown in eqn (21) and (22):
| 2H3O+ + 2e− → H2 + 2H2O | (21) |
| Cu → Cu2+ + 2e− | (22) |
Bahaloo-Horeh and Mousavi149 used A. niger to recover Cu, Co, Mn, Al, Li, and Ni from spent mobile LIBs. They concluded that maximum recovery could be attained at 1% PD and out of the various acids produced by A. niger, citric acid dominated the leaching process. The ligands present in organic acids like citrate (Ct) can form stable complexes with metals which elevates their solubility in the solution as shown in eqn (23)–(25).
| Cu2+ + CtH3 ↔ CuCt− + 3H+ | (23) |
| Cu2+ + CtH2 ↔ CuCt + 2H+ | (24) |
| Cu2+ + CtH ↔ CuCt+ + H+ | (25) |
In a similar study, Faraji and co-workers recovered 100% of Zn, 85.88% of Cu, and 80.39% of Ni in 30 days using A. niger and performed kinetic studies on the mechanism.150 The Zn, Cu, and Ni dissolution was found to be controlled by diffusion through the liquid and chemical reaction in multistage and spent medium approaches, respectively.150 Initially, Brandl et al.142 utilized A. ferrooxidans, A. thiooxidans, P. simplicissimum, and A. niger to successfully leach Cu, Zn, Al, Ni, Pb, and Sn from WEEE scrap in a modified two-step approach. They suggested that the bacteria or fungi should be inoculated on a culture medium to grow separately in the primary step, followed by the introduction of WEEE into the grown culture in the subsequent step. This pre-growth method is required as WEEE is highly alkaline and contains other toxic organic compounds which inhibit the growth of microbes in a direct contact mechanism. Therefore, by providing microbes the time to adapt and grow, they can significantly leach increased amounts of metals in the second step. Maximum leaching efficiency was achieved for concentrations lower than 10 g L−1 WEEE or PD of 1%.
Furthermore, the two-step, indirect mechanism seems to have become the standard practice in bioleaching these days because it is highly predictable and controllable. Akbari and Ahmadi investigated the bioleaching of Cu and Zn from a mixture of WPCBs and sulphidic tailing containing Cu–Ni–Co by employing Fe and S oxidizing bacteria.143 The two-step approach resulted in bioleaching of 92% Cu, 67% Zn, and 45% Al in batch mode whereas 96.8% Cu recovery was attained in continuous mode. Moreover, Zn and Ni start to co-precipitate at a pH value of 2.5, which hinders the Cu leaching. The metals were recovered using solvent extraction with LIX984N diluted in kerosene.
Exhausted batteries carrying heavy metals require proper management not only because of the environmental hazard but also to recover metals such as Li (5–20%) and Co (5–7%) as they are scarce natural resources.151 Presently, recycling attempts are being made to leach Co and Li from the cathodes of LIB containing LiCoO2.152 Mishra and co-workers reported that with an increase in Fe2+ concentration, the dissolution rate of Co decreases due to the precipitation of Fe3+ as jarosite whereas greater pulp densities inhibit the dissolution of metals because of the excess metals present in the sample.152 Another study by153 attempted to recover Li, Co, Ni, and Mn from spent electric vehicle LIBS cathodes made up of LiMn2O4, LiFePO4, and LiNix CoyMn1−x−yO2 at 1% PD. The results demonstrated that the best Li recovery could be achieved using A. thiooxidans. The pH optimization significantly enhanced the bioleaching performance resulting in leaching more than 95% of all four metals on average.
Moreover, the indirect mechanism is best suited for high yields of Li, whereas a direct contact mechanism is essential for the dissolution of Co, Ni, and Mn.153 Furthermore, WEEE components like LCDs contain minute amounts of rare metal indium. Xie and his research group reported that 100% of indium could be leached from LCDs using Acidithiobacillus genus.146 The study investigated three systems, namely Fe-based, S-based, and mixed systems. Maximum leaching was achieved in the sulfur-based system with leaching times reduced by half. They concluded that A. thiooxidans acts as a catalyst, accelerating the bioleaching rate of indium.
The selectivity of metal dissolution depends on the ORP of metals. Therefore, metals with higher E° like Cu, Ag, and Au are more challenging to oxidize compared to metals with lower E° like Zn and Al.154 A multi-step bioleaching approach can be applied to increase the Au mobilization.148 Complete leaching of base metals is essential in the first step so that precious metals can be leached with greater efficiencies subsequently. Marra and co-workers recovered Au using cyanide producing bacteria P. putida combined with pre-treatment of WEEE dust with A. thiooxidans to extract base metals, followed by the recovery of precious and rare earth metals: (>95%) Ce, Eu, Nd and (>80%) La.155 They reported complete leaching of Cu in 8 days whereas it only took 3 h to mobilize 48% Au. Moreover, Au recovery can be performed by other cyanogenic bacteria like chromobacterium violaceum but P. fluorescens exhibits a better growth rate and metal resistance, thus achieving better leaching kinetics compared to C. violaceum.156
The industrial application of bioleaching is restricted due to the excessive sensitivity of the microbes, limiting the processing amount of WEEE in a particular batch. It is also worth noting that bioleaching efficiency decreases significantly as target metals precipitate to form jarosite, further reducing the efficiency of metal recovery. Consequently, the ferric (Fe3+) ions produced by the microbial activity increase the pH of the solution as the H+ ions get depleted. If the pH value goes over 2.0, then hydrolysis of Fe3+ ions takes place, resulting in further production of H+ ions and a drop in the pH. This provides ideal conditions for the formation of jarosite as cations like K+, Na+, Ag+, and Pb2+ readily react with Fe(OH)3. Jarosite formation is influenced by conditions like high pH, temperature, or concentration of Fe2+ ions.152 Moreover, jarosite passivates the surface of WEEE scrap, stopping the leaching to take place any further, increasing the number of Fe3+ ions. Mishra and his research group also conducted bioleaching of spent LIBs containing LiCoO2 and reported slow dissolution rates of Li and Co as the Fe3+ ions combined with other metal ions to form metal complexes.152Eqn (26) shows the jarosite formation reaction, where M stands for metal.
| M+ + 5OH− + 3Fe3+ + 2SO42− + H2O → MFe3(SO4)2(OH)6 + H+ | (26) |
To mitigate the problems like slow dissolution kinetics and low metal yields, some researchers focused on employing different materials like Ag+ ions, Cu2+ ions, graphene, citric acid, and nitrogen-based carbon nanotubes as catalysts in an attempt to reduce leaching times drastically.157–159
Zeng and co-workers suggested the addition of Cu2+ ions as catalysts to recover Li and Co from spent LIB cathodes made of LiCoO2. The Cu2+ ion forms an intermediate compound CuCo2O4 on the surface of the cathode material due to the cationic exchange reaction between the Cu2+ ions and LiCoO2. The Fe3+ ions present in the solution can effortlessly dissolve the intermediate compound CuCo2O4 followed by oxidation of CuCo2O4 to Cu2+ and regeneration of Fe3+ ions from Fe2+ ions by microbial activity. The mechanism is shown in eqn (27)–(29).16
| Cu2+ + 2LiCoO2 → CuCo2O4 + 2Li+ | (27) |
| CuCo2O4 + 6Fe3+ → 6Fe2+ + Cu2+ + 2O2 + 2Co2+ | (28) |
| 4Fe2+ + O2 + 4H+ → 4Fe3+ + 2H2O | (29) |
In a similar study by Zeng and coworkers, Ag+ ions were demonstrated to act as a catalyst for better leaching kinetics and metal recovery from cathode material LiCoO2 of LIBs. The Ag+ ions substituted the Li+ ions to form an intermediate AgCoO2 which subsequently gets oxidized by the presence of Fe3+ ions and results in the discharge of Ag+ ions. The oxidation of Fe2+ ions by bacteria converts them back into Fe3+ again.17 Sliver sulfate, silver nitrate, and silver chloride can be utilized as sources of Ag+ ions in this process.151 The reaction mechanism is given in eqn (30) and (31).
| Ag+ + LiCoO2 → AgCoO2 + Li+ | (30) |
| AgCoO2 + 3Fe3+ → 3Fe2+ + Ag+ + O2 + Co2+ | (31) |
Although these methods enhance the leaching kinetics of the bioleaching process significantly, the high cost of metal catalysts can prove to be a challenge in the industrialization of the process. Therefore, other non-metallic catalysts like citric acid (lime juice), graphene, NCNTs, and activated carbon should be looked into as well. Moreover, a combination of metallic and non-metallic catalysts can also have a synergistic effect on the leaching efficiency (e.g. Ag+ ions and activated carbon).151Table 6 shows a summary of bioleaching studies.
| Source | Lixiviant | Metal recovery (%) | Conditions | Reference |
|---|---|---|---|---|
| Crushed PCBs | Cyanide 65.12 g per mol CN− + O2 (air) | Au: 46.4 | E°: −0.67 V | (Montero et al., 2012)67 |
| Ag: 51.3 | pH > 10 | |||
| Nb: 47.2 | 25 °C | |||
| Cu: 62.3 | ||||
| WPCBs | Thiourea 20 g per mol CS(NH2)2 + 6 g per L Fe3+ + 10 g per L H2O2 | Au: 85.76 | E°: 0.38 V | (Behnamfard et al., 2013)73 |
| Ag: 71.36 | pH: 1–2 | |||
| 25 °C, 3 h | ||||
| WPCBs | Thiosulphate 0.2 M (NH4)2S2O3 + 0.02 M Cu(SO4) + 0.4 M NH4OH | Au: 95 | E°: 0.274–0.038 V | (Oh et al., 2003)178 |
| Ag: 100 | pH > 8–11 | |||
| 25 °C | ||||
| 400 °C, 48 h | ||||
| WPCBs | 1–1.2 iodide + 1–2% H2O2 | Pb: 99.9 | E°: 1 V | (Altansukh et al., 2019)177 |
| Sn: 97.8 | pH: 7 | |||
| 25 °C | ||||
| 1 h |
Priya and Hait159 suggested a hybrid hydro-biometallurgy approach using lime juice (citric acid), an organic tetradentate chelating agent, improved the base metal recovery from PCBs. They reported that the addition of 0.2 M citric acid improved the production of exopolymeric substances (lipopolysaccharides) by A. ferrooxidans and decreased the formation of jarosite which provided enhanced dissolution of metals. The results demonstrated 94% Cu, 92% Zn, 81% Ni and 64% Pb leaching in 18 days at a PD of 7.5 g L−1. Subsequently, using chemical precipitation, 99% Cu was extracted from the leachate. Gu et al. suggested the application of graphene to leach Cu using A. ferrooxidans from WPCBs, resulting in enhanced Cu leaching. Graphene is a nanomaterial that can provide greater surface areas and stability while providing good conduction properties. The microbial growth decreased as the quantity of graphene was added beyond a limit.160 The enhanced leaching of Cu could be a result of adsorption of bacteria on the graphene surface providing increased reaction time between the waste and the bacteria. Leached metals could also be adsorbed on the graphene surface, decreasing the amount of the metal ions present in the solution and a rise in metal mobilization could be achieved from the waste to the solution.
They reported that the optimum graphene dosage was 0.04 g for 50 mL of culture medium at 1% PD. Moreover, after the bioleaching process was complete, graphene particles were analyzed, and it was found that Fe3+ precipitates along with some other cations were surrounding the graphene particles.160
Similarly, in another test Gu and co-workers used an A. ferrooxidans and nitrogen-doped carbon nanotube (NCNT) modified electrode as a catalyst to leach 99% Cu from WPCBs.160 The reported optimal conditions were and initial pH of 2.0, modified electrode 2.5 mg cm−2, temperature 28 °C, solid/liquid ration of 1/50 and 1 g WPCB powder. Complete copper leaching could be achieved in 9 days using these conditions. They concluded that the formation of jarosite wasn't affected by the NCNT electrode. Table 7 shows the summary of studies based on catalyst bioleaching.
| Source | Lixiviant | Metal recovery (%) | Reference |
|---|---|---|---|
| WPCBs | A. ferrooxidans + graphene (catalyst) | Cu: 91.8 | (Gu et al., 2017a)160 |
| WPCBs | A. ferrooxidans + nitrogen based carbon nanotube modified electrode (catalyst) | Cu: 99 | (Gu et al., 2017a)160 |
| High grade WPCBs from computers | A. ferrooxidans + Cu2+ (catalyst) | Cu: 94 | (Priya and Hait, 2018)159 |
| Zn: 92 | |||
| Ni: 91 | |||
| Pb: 64 | |||
| Spent LiB cathode | A. ferrooxidans + Ag+ (catalyst) | Co: 99.9 | (Zeng et al., 2012)16 |
| Spent LiB cathode | 1–1.2 iodide + 1–2% H2O2 | Co: 98.4 | (Zeng et al., 2013)17 |
4. Emerging technologies for WEEE treatment and material recovery
4.1 Supercritical fluid technology
Lately, supercritical fluid (SCF) extraction (SFE) technology has gained much recognition because of its beneficial physicochemical properties like high selectivity, low viscosity, high mass transfer rate, high diffusion rate, and high solubility.161,162 It holds enormous potential to enable eco-friendly extraction of WEEE from solvents using reagents like water and carbon dioxide, with the addition of some modifiers or co-solvents like methanol. Generally, an SCF is any fluid above its critical temperature (Tc) and critical pressure (Pc). In this phase, the fluid's physiochemical properties intermediate between the gaseous phase (low diffusivity and viscosity) and the liquid phase (high dissolution).163 Researchers have employed SCFs for the recovery of organic materials like halogenated epoxy resins, BFRs and base and precious metals in a simultaneous process.162,164–168 The most extensively used supercritical fluids are supercritical water (SCW) and supercritical CO2 (SC–CO2). SCW (Tc = 374 °C and Pc = 22.1 MPa) is a non-polar compound, which can easily oxidise organic material. However, SC–CO2 is preferred over SCW due to its lower critical point (Tc = 31.1 °C; Pc = 7.38 MPa), lower costs, highly efficient mass transfer, easy separation, recyclability, and ability to completely dissolution with gaseous reagents.161,166,169 Moreover, for metal extraction applications, SC–CO2 is frequently used with co-solvents because of its non-polar characteristics which form weak interactions with polar metal cations.168Wang and Zhang165 reported complete decomposition of BFRs using SC–water, methanol, and isopropanol. They achieved maximum extraction (>95%) of bromine at 420 °C for all three fluids. Moreover, the organic bromine was successfully transformed into an inorganic compound through the application of SCE. Some researchers attempted de-bromination of BERs by employing SCW along with other co-solvents. Xing and Zhang carried out an experiment, indicating the rapid decomposition of BER from WPCB using SCW as solvent.170 The de-bromination process conducted at 400 °C, 20 MPa pressure, and 120 minutes retention time resulted in 97% bromine, which was converted to HBr, along with by-products, phenol, and 4-(1-methyl ethyl)-phenol.170 In another study, SCW was used to decompose BER from memory cards in a semi-batch type reactor. They achieved 90% decomposition of BER into phenols at 495 °C and 33 MPa pressure and a retention time of 305 minutes.161 To treat WPCBs, Xiu and co-workers164 used SCW along with alcohol as a co-solvent. They reported that the introduction of alcohol reduced the critical temperature and pressure in the system resulting in decreased corrosion of the system. Moreover, the reaction could be controlled by altering the reaction conditions to form different compounds at different temperatures. At 300 °C, the majority of the products in the oil phase were found to be 4-(1-methyl ethyl)-phenol, whereas at 400 °C they were p-xylene and methoxybenzene.
Liu and Zhang recovered 95% Co and 98% Li from spent LIBs and PVC (polyvinyl chloride) simultaneously using sub/supercritical water. In addition to this, they successfully de-chlorinated PVCs with no toxic by-products at 350 °C temperature, 30 min retention time, a PVC/LiCoO2 ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and a PD of 16 g L−1. The de-chlorination of PVC resulted in the formation of HCl, which readily solubilized the metals from LiCoO2.171
1, and a PD of 16 g L−1. The de-chlorination of PVC resulted in the formation of HCl, which readily solubilized the metals from LiCoO2.171
Calgaro and co-workers utilized SC–CO2 along with H2SO4 and H2O2 as modifiers to extract base metal Cu from WPCBs. The addition of 20% H2O2 in 2.5 M H2SO4 resulted in the extraction of 88.79% Cu at 7.5 MPa and 35 °C in just 20 min.166 Xiu et al. recovered base metals from WPCBs using a combination of pre-treatment with SCW and dilute hydrochloric acid leaching. The pre-treatment process, including SCW oxidation and SCW de-polymerization, resulted in enhanced base metal extraction of 99.8% Cu (with H2O2, in 60 min) and 90% Zn, Cd, Sn, Mn, and Cr (in 60 min). However, high temperatures (420–440 °C) make it an energy intensive process.172 Recently it has been reported that a reaction time of 20 min was needed to recover 90% Cu using a combination of SC–CO2, H2SO4, and H2O2 from comminuted WPCBs.173 An application of SC-methanol (Tc = 240 °C and Pc = 8.09 MPa) was proposed by Xiu and co-workers.167 The leachate produced by the chemical leaching of WPCB, rich in HNO3 and other metals, was used to produce highly fine Cu particles. During the SCE process, the SC-methanol played the role of a solvent as well as a reducing agent, reducing the Cu2+ ions to Cu°. SC-methanol has the ability to form intermediately charged compounds that act like positive (CH3+) and negative (OH−) charge centers, the latter acting as the electron donors. The reaction is represented by eqn (32).169
| 2HO− + Cu2+ → Cu0–(HO)2 | (32) |
Parameters like reaction temperature and initial Cu concentration affect the production of uniform and smaller-sized particles. Low initial Cu concentration and elevated reaction temperatures favor the formation of more uniform-sized particles. The optimum temperature, pressure, and retention time of the reaction were found to be 360 °C, 28 MPa, and 10 min, respectively.
Xiu and his research group employed a two-step approach to recover precious metals Au, Ag, and Pd from WPCBs of mobile phones. The first step included pre-treatment of the waste using SCW oxidation followed by iodine/iodide leaching of precious metals in the subsequent step. The results demonstrated recovery of 98.5% Au, 99% Ag, and 97.2% Pd at 410 °C and 30 min for Ag, and 420 °C and 60 min for Au and Pd. For the maximum dissolution rate the optimum conditions like leaching time, PD, pH, and iodine mole ratio for iodine/iodide leaching were found to be 120 min (60 min for Ag), 1/10 g mL−1 (1/8 g mL−1 for Ag), 9.0, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (1
5 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 for Ag), respectively.174 No inorganic acids or cyanide were used to recover precious metals in the process. Therefore, it can be concluded that SCW treatment with an iodine/iodide leaching system is an environmentally benign process to recover precious metals. Liu et al. carried out a comparative study to recover Ag and Pd from WPCBs using SC oxidation and extraction in a multi-step process. In the first step, SCW oxidation and HCl leaching were used to decompose any organic material present to enrich the precious metal concentration. Subsequently, in the second step, SC–CO2 extraction was carried out using acetone and KI–I2 as co-solvents, to recover 96.4% Ag and 93.7% Pd at 50 °C, 30 MPa, and a leaching time of just 10 min.168 Moreover, He and Xu5 reported that SCW oxidation produces a significant effect to enhance the extraction rates of Cu and Au (99%). Even though various research papers have suggested SCF technology as a feasible option to recover metals from WEEE, the high capital and operating costs of high pressure and corrosion resistant reactors must be considered for its large-scale application. Although SCF technology can be regarded as an eco-friendly approach due to its various advantages like significantly lower leaching times, zero toxicity, non-hazardous nature, an abundance of green reagents like CO2 and water, it also suffers from challenges like corrosion, salt precipitation, high energy consumption, and high operational and maintenance costs along with the construction of reactors from expensive corrosion-resistant alloys.161 Moreover, the research database for the economic viability of supercritical systems is scarce at the moment. Therefore, more research and development are required before an industrial scale plan can be achieved.
6 for Ag), respectively.174 No inorganic acids or cyanide were used to recover precious metals in the process. Therefore, it can be concluded that SCW treatment with an iodine/iodide leaching system is an environmentally benign process to recover precious metals. Liu et al. carried out a comparative study to recover Ag and Pd from WPCBs using SC oxidation and extraction in a multi-step process. In the first step, SCW oxidation and HCl leaching were used to decompose any organic material present to enrich the precious metal concentration. Subsequently, in the second step, SC–CO2 extraction was carried out using acetone and KI–I2 as co-solvents, to recover 96.4% Ag and 93.7% Pd at 50 °C, 30 MPa, and a leaching time of just 10 min.168 Moreover, He and Xu5 reported that SCW oxidation produces a significant effect to enhance the extraction rates of Cu and Au (99%). Even though various research papers have suggested SCF technology as a feasible option to recover metals from WEEE, the high capital and operating costs of high pressure and corrosion resistant reactors must be considered for its large-scale application. Although SCF technology can be regarded as an eco-friendly approach due to its various advantages like significantly lower leaching times, zero toxicity, non-hazardous nature, an abundance of green reagents like CO2 and water, it also suffers from challenges like corrosion, salt precipitation, high energy consumption, and high operational and maintenance costs along with the construction of reactors from expensive corrosion-resistant alloys.161 Moreover, the research database for the economic viability of supercritical systems is scarce at the moment. Therefore, more research and development are required before an industrial scale plan can be achieved.
4.2 Siderophores
Siderophores are small, high-affinity iron-chelating compounds secreted by microorganisms such as bacteria, fungi, and grasses. In the most recent years siderophores have been shown to be an interesting and environmentally sustainable alternative for the biological removal of metals and metalloids.175 The chelating molecules are produced to trap ferric ions from the environment to satisfy the microorganism's metabolic needs. They are known as the best ligands for ferric ions but may also have a strong affinity towards other metals, allowing a new understanding of an interesting biological and eco-friendly method that can be used for the recovery of REEs.176 Many researchers have compared the REEs' recovery efficiency and chemical leaching of siderophores, and they have found higher recovery efficiency for lithium and molybdenum, and lower recovery efficiency for cerium, mainly due to the cerium complex formation with siderophores, which was inhabited by the competing cations present in the solution, such as Fe3+. The recovery of REEs using siderophores is cost-effective, rapid, reversible, and eco-friendly technology compared with conventional methods for the recovery of REEs which are available in small concentration from different end-of-life electronic wastes.1764.3 Cryo-milling
Cryo-milling is an alternative physical treatment approach, which is a green and novel technology for the recovery of metals and recycling of REEs from WEEE. In this process, WEEE is degraded into nanoparticle sizes using a ball mill operated at low temperatures. This leads to an increase in the efficiency of the separation of oxides, polymers, and meal constituents.173 Moreover, this eco-friendly process, due to the generation of small volumes of waste, is scaled up on a large industrial scale. As a counterpart cryo-milling needs more energy when compared to hydrometallurgical methods. Moreover, further research is still necessary to improve the capacity of the cryo-milling process, by developing a mechanical process, using liquid nitrogen as a cooling, which could be economically viable for the treatment of electronic waste.176 Milling at low temperature was shown to be very effective in separating different kinds of materials (polymers, oxides, and metals) due to their different toughness and textile strengths. This method revealed higher recovery rates and shorter recovery times when compared to conventional milling methods.1735. Conclusions
Out of all the other municipal waste produced, WEEE has shown the most rapid growth lately. WEEE contains toxic and hazardous materials that can be harmful to the environment as well as human beings. Therefore, we should resort to the recycling of WEEE and up-cycling of the re-useable parts of the electrical components as the best-suited alternative to disposal. When the WEEE gets transferred from a developed nation to developing nations, the unskilled labor available there uses informal methods of recycling to recover valuable material from them, resulting in the production of harmful and non-eco-friendly compounds. Moreover, the exponential growth of WEEE will keep up in the future as we move towards a more technology-driven world. If a greater amount of WEEE is landfilled, it can cause an imbalance in the natural mineral resource cycle as a whole, which can have vast implications. Thus, it is important to develop a methodology to set up a circular economy of these natural resources. Considering the complex nature of WEEE, developing an exact approach for recycling is cumbersome. The main objective of a recycling method should be to increase metal extraction efficiency and reduce environmental pollution. Several studies have provided various recycling routes to reduce environmental pollution and recover 30% metal content in it, simultaneously.This review shows that a synergistic approach of mechanical–physical pre-treatment, pyrometallurgy, hydrometallurgy and biometallurgy would help achieve these goals. Moreover, the review reports some of the emerging technologies such as supercritical fluid extraction, siderophores extraction and cryo-milling, which if correctly implemented could represent a more environmentally sustainable approach for WEEE material recovery and recycling. Each of the processes mentioned in the review plays a crucial role in the recycling process as a whole, starting from the collection of WEEE until the extraction of metals and recoverable organics.
Therefore, it can be concluded that in the last two decades, WEEE recycling has seen various advancements in the field of recycling, but further research attempts are required to find an economically feasible method for the large-scale application of this process. Moreover, the more environmentally friendly processes also have higher energy consumption which is eventually mainly generated from fossil fuels. Thus, a balance needs to be maintained between recovery efficiency and energy consumption to develop an economically feasible recycling system. Furthermore, a 3R policy should be implemented to reuse, recycle, and reduce WEEE, preferentially in their sequential order. Having said this, the manufacturers of these products have EPR for developing equipment with longer life, higher modularity (providing the possibility of systems upgrade), less complex designs (for easier disassembly), lighter weight, easy access, and low cost of spare parts, promoting repair, reuse, and recovery of products at the end of their life.
Conflicts of interest
There are no conflicts to declare.References
- International E-Waste Day: 57.4M Tonnes Expected in 2021, 2021, pp. 1–7, https://weee-forum.org/ws_news/international-e-waste-day-2021/.
- J. Wang and Z. Xu, Disposing and Recycling Waste Printed Circuit Boards: Disconnecting , Resource Recovery , and Pollution Control, Environ. Sci. Technol., 2015, 49, 721–733 CrossRef CAS PubMed
.
- J. Cui and L. Zhang, Metallurgical recovery of metals from electronic waste: A review, J. Hazard. Mater., 2008, 158(2–3), 228–256 CrossRef CAS PubMed
.
- X. Chi, M. Streicher-Porte, M. Y. L. Wang and M. A. Reuter, Informal electronic waste recycling: A sector review with special focus on China, Waste Manage., 2011, 31(4), 731–742, DOI:10.1016/j.wasman.2010.11.006
.
-
Eurostat - European Commission, Waste statistics - electrical and electronic equipment, Eurostat Stat Explain, 2022, pp. 1–10, https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Waste_statistics_-_electrical_and_electronic_equipment#Electronic_equipment_.28EEE.29_put_on_the_market_and_WEEE_processed_in_the_EU Search PubMed
.
- Y. He and Z. Xu, Recycling gold and copper from waste printed circuit boards using chlorination process, RSC Adv., 2015, 5(12), 8957–8964 RSC
.
- M. Kaya, Recovery of metals and nonmetals from electronic waste by physical and chemical recycling processes, Waste Manage., 2016, 57, 64–90, DOI:10.1016/j.wasman.2016.08.004
.
- A. M. Danciu, M. Greenley and A. P. Cobuz, An overview of global e-waste, its effects on developing countries and possible solutions, Rev. Appl. Socio-Economic Res., 2018, 15(1), 20–28 Search PubMed
.
- K. Liu, Q. Tan, J. Yu and M. Wang, A global perspective on e-waste recycling, Circ. Econ., 2023, 2(1), 100028 Search PubMed
.
-
N.-E. Menad, in Physical Separation Processes in Waste Electrical and Electronic Equipment Recycling, ed. Chagnes A., Cote G., Ekberg C., Nilsson M. and Retegan T. B. T.-W. R., Elsevier, 2016, pp. 53–74, https://www.sciencedirect.com/science/article/pii/B9780128033630000031 Search PubMed
.
- Y. E. Ouardi, S. Virolainen, E. Salomon, M. Mouele, M. Laatikainen and E. Repo,
et al., The recent progress of ion exchange for the separation of rare earths from secondary resources – A review, Hydrometallurgy, 2023, 218, 106047 CrossRef
.
- B. Ghosh, M. K. Ghosh, P. Parhi, P. S. Mukherjee and B. K. Mishra, Waste Printed Circuit Boards recycling: An extensive assessment of current status, J. Cleaner Prod., 2015, 94, 5–19, DOI:10.1016/j.jclepro.2015.02.024
.
- J. Li, P. Shrivastava, Z. Gao and H. C. Zhang, Printed circuit board recycling: A state-of-the-art survey, IEEE Trans. Electron. Packag. Manuf., 2004, 27(1), 33–42 CrossRef CAS
.
- K. Huang, J. Guo and Z. Xu, Recycling of waste printed circuit boards: A review of current technologies and treatment status in China, J. Hazard. Mater., 2009, 164(2–3), 399–408 CrossRef CAS PubMed
.
- L. Chen, X. Tang, Y. Zhang, L. Li, Z. Zeng and Y. Zhang, Process for the recovery of cobalt oxalate from spent lithium-ion batteries, Hydrometallurgy, 2011, 108(1–2), 80–86, DOI:10.1016/j.hydromet.2011.02.010
.
- X. Zeng, L. Zheng, H. Xie, B. Lu, K. Xia and K. Chao,
et al., Current Status and Future Perspective of Waste Printed Circuit Boards Recycling, Procedia Environ. Sci., 2012, 16, 590–597, DOI:10.1016/j.proenv.2012.10.081
.
- G. Zeng, S. Luo, X. Deng, L. Li and C. Au, Influence of silver ions on bioleaching of cobalt from spent lithium batteries, Miner. Eng., 2013, 49, 40–44, DOI:10.1016/j.mineng.2013.04.021
.
- P. Zhu, Y. Chen, L. Y. Wang and M. Zhou, Treatment of waste printed circuit board by green solvent using ionic liquid, Waste Manage., 2012, 32(10), 1914–1918, DOI:10.1016/j.wasman.2012.05.025
.
- Y. J. Park and D. J. Fray, Recovery of high purity precious metals from printed circuit boards, J. Hazard. Mater., 2009, 164(2–3), 1152–1158 CrossRef CAS PubMed
.
- P. Nowakowski, A novel, cost efficient identification method for disassembly planning of waste electrical and electronic equipment, J. Cleaner Prod., 2018, 172, 2695–2707, DOI:10.1016/j.jclepro.2017.11.142
.
- J. Beigbeder, D. Perrin, J. F. Mascaro and J. M. Lopez-Cuesta, Study of the physico-chemical properties of recycled polymers from waste electrical and electronic equipment (WEEE) sorted by high resolution near infrared devices, Resour., Conserv. Recycl., 2013, 78, 105–114 CrossRef
.
- K. Feldmann and H. Scheller, Disassembly of electronic products, IEEE Int. Symp. Electron. Environ., 1994, 9, 81–86 Search PubMed
.
- I. Stobbe, H. Griese, H. Pötter, H. Reichl and L. Stobbe, Quality assured disassembly of electronic components for reuse, IEEE Int. Symp. Electron. Environ., 2002, 299–305 Search PubMed
.
-
R. Noll, R. Ambrosch, K. Bergmann, S. W. Britten, H. Brumm, A. Chmielarz, et al., Automated disassembly, separation and recovery of valuable materials from electronic equipment: overview of R\&D approaches and first results of the European project ADIR, Next Generation Urban Mining, 2017 Search PubMed
.
-
M. Kaya, Current WEEE recycling solutions, Waste Electrical and Electronic Equipment Recycling: Aqueous Recovery Methods, Elsevier Ltd, 2018, pp. 33–93, DOI:10.1016/B978-0-08-102057-9.00003-2
.
- P. Jiang, M. Harney, B. Chen, Y. Song, Q. Chen and T. Chen,
et al., Improving the end-of-life for electronic materials via sustainable recycling methods, Procedia Environ. Sci., 2012, 16, 712–715, DOI:10.1016/j.proenv.2012.10.066
.
- M. Iji and S. Yokoyama, Recycling of Printed Wiring Boards with Mounted Electronic Components, Circuit World, 1997, 23(3), 10–15, DOI:10.1108/03056129710370196
.
- P. Mou, L. Wa, D. Xiang, J. Gao and G. Duan, A physical process for recycling and reusing waste printed circuit boards, IEEE Int. Symp. Electron. Environ., 2004, 237–242 Search PubMed
.
- M. Goosey and R. Kellner, Recycling technologies for the treatment of end of life printed circuit boards (PCBs), Circuit World, 2003, 29(3), 33–37 CrossRef CAS
.
- S. Sander, G. Schubert and H. G. Jäckel, The fundamentals of the comminution of metals in shredders of the swing-hammer type, Int. J. Miner. Process., 2004, 74, 385–393 CrossRef
.
- G. Schubert and S. Bernotat, Comminution of non-brittle materials, Int. J. Miner. Process., 2004, 74, 19–30 CrossRef
.
- J. Cui and E. Forssberg, Mechanical recycling of waste electric and electronic equipment: A review, J. Hazard. Mater., 2003, 99(3), 243–263 CrossRef CAS PubMed
.
- M. Furuuchi and K. Gotoh, Shape separation of particles, Powder Technol., 1992, 73(1), 1–9 CrossRef CAS
.
- A. Gungor and S. M. Gupta, Disassembly sequence planning for products with defective parts in product recovery, Comput. Ind. Eng., 1998, 35(1), 161–164 CrossRef
. https://www.sciencedirect.com/science/article/pii/S0360835298000473.
- J. Yang, H. Wang, G. Zhang, X. Bai, X. Zhao and Y. He, Recycling organics from non-metallic fraction of waste printed circuit boards by a novel conical surface triboelectric separator, Resour., Conserv. Recycl., 2019, 146, 264–269 CrossRef
. https://www.sciencedirect.com/science/article/pii/S0921344919301132.
- J. Ruan and Z. Xu, Constructing environment-friendly return road of metals from e-waste: Combination of physical separation technologies, Renewable Sustainable Energy Rev., 2016, 54, 745–760 CrossRef
.
- N. Shirodkar and R. Terkar, Stepped Recycling: The Solution for E-waste Management and Sustainable Manufacturing in India, Mater. Today: Proc., 2017, 4(8), 8911–8917, DOI:10.1016/j.matpr.2017.07.242
.
- E. Hsu, K. Barmak, C. A. West and A.-H. Park, Advancements
in the Treatment and Processing of Electronic Waste with Sustainability: A Review of Metal Extraction and Recovery Technologies, Green Chem., 2019, 21(5), 919–936 RSC
.
- S. Zhang, E. Forssberg, B. Arvidson and W. Moss, Aluminum recovery from electronic scrap by High-Force® eddy-current separators, Resour., Conserv. Recycl., 1998, 23(4), 225–241 CrossRef
. https://www.sciencedirect.com/science/article/pii/S0921344998000226.
- S. Zhang and E. Forssberg, Intelligent Liberation and classification of electronic scrap, Powder Technol., 1999, 105(1), 295–301 CrossRef CAS
. https://www.sciencedirect.com/science/article/pii/S0032591099001515.
- C. Eswaraiah, T. Kavitha, S. Vidyasagar and S. S. Narayanan, Classification of metals and plastics from printed circuit boards (PCB) using air classifier, Chem. Eng. Process., 2008, 47(4), 565–576 CrossRef CAS
.
- J. Hanafi, E. Jobiliong, A. Christiani, D. C. Soenarta, J. Kurniawan and J. Irawan, Material Recovery and Characterization of PCB from Electronic Waste, Procedia - Soc. Behav. Sci., 2012, 57, 331–338 CrossRef
. https://www.sciencedirect.com/science/article/pii/S1877042812046563.
- N. Menad, S. Guignot and J. A. van Houwelingen, New characterisation method of electrical and electronic equipment wastes (WEEE), Waste Manage., 2013, 33(3), 706–713 CrossRef CAS PubMed
.
- M. Sarvar, M. M. Salarirad and M. A. Shabani, Characterization and mechanical separation of metals from computer Printed Circuit Boards (PCBs) based on mineral processing methods, Waste Manage., 2015, 45, 246–257 CrossRef CAS PubMed
. https://www.sciencedirect.com/science/article/pii/S0956053X15004341.
-
L. Dascalescu, T. Zeghloul and A. Iuga, Electrostatic Separation of Metals and Plastics from Waste Electrical and Electronic Equipment, ed. Chagnes A., Cote G., Ekberg C., Nilsson M. and Retegan T. B. T.-W. R., Elsevier, 2016, ch. 4, pp. 75–106. https://www.sciencedirect.com/science/article/pii/B9780128033630000043 Search PubMed
.
-
T. Veasey, R. J. Wilson and D. M. Squires, The physical separation and recovery of metals from wastes, Process engineering for the chemical, metals and minerals industries, 1993, vol. 1 Search PubMed
.
- H. M. Veit, A. M. Bernardes, J. Z. Ferreira, J. A. S. Tenório and C. de Fraga Malfatti, Recovery of copper from printed circuit boards scraps by mechanical processing and electrometallurgy, J. Hazard. Mater., 2006, 137(3), 1704–1709 CrossRef CAS PubMed
.
- L. H. Yamane, V. T. de Moraes, D. C. R. Espinosa and J. A. S. Tenório, Recycling of WEEE: characterization of spent printed circuit boards from mobile phones and computers, Waste Manage., 2011, 31(12), 2553–2558 CrossRef CAS PubMed
.
- H. M. Veit, T. Diehl, A. P. Salami, J. da Silva Rodrigues, A. M. Bernardes and J. A. S. Tenório, Utilization of magnetic and electrostatic separation in the recycling of printed circuit boards scrap, Waste Manage., 2005, 25(1), 67–74 CrossRef CAS PubMed
.
- J.-M. Yoo, J. Jeong, K. Yoo, J. Lee and W. Kim, Enrichment of the metallic components from waste printed circuit boards by a mechanical separation process using a stamp mill, Waste Manage., 2009, 29(3), 1132–1137 CrossRef CAS PubMed
.
- R. Jujun, Q. Yiming and X. Zhenming, Environment-friendly technology for recovering nonferrous metals from e-waste: Eddy current separation, Resour., Conserv. Recycl., 2014, 87, 109–116 CrossRef
.
- Y. R. Smith, J. R. Nagel and R. K. Rajamani, Eddy current separation for recovery of non-ferrous metallic particles: A comprehensive review, Miner. Eng., 2019, 133, 149–159 CrossRef CAS
. https://www.sciencedirect.com/science/article/pii/S089268751830582X.
- A. Q. Mairizal, A. Y. Sembada, K. M. Tse and M. A. Rhamdhani, Electronic waste generation, economic values, distribution map, and possible recycling system in Indonesia, J. Cleaner Prod., 2021, 293, 126096 CrossRef
. https://www.sciencedirect.com/science/article/pii/S0959652621003164.
- F. Hamerski, A. Krummenauer, A. M. Bernardes and H. M. Veit, Improved settings of a corona-electrostatic separator for copper concentration from waste printed circuit boards, J. Environ. Chem. Eng., 2019, 7(1), 102896 CrossRef CAS
.
- A. Khaliq, M. A. Rhamdhani, G. Brooks and S. H. Masood, Metal Extraction Processes for Electronic Waste and Existing Industrial Routes: A Review and Australian Perspective, Resources, 2014, 3, 152–179 CrossRef
.
- J. Ruan, L. Dong, J. Zheng, T. Zhang, M. Huang and Z. Xu, Key factors of eddy current separation for recovering aluminum from crushed e-waste, Waste Manage., 2017, 60, 84–90 CrossRef CAS PubMed
.
- H. Lu, J. Li, J. Guo and Z. Xu, Movement behavior in electrostatic separation: Recycling of metal materials from waste printed circuit board, J. Mater. Process. Technol., 2008, 197, 101–108 CrossRef CAS
.
- P. Hadi, M. Xu, C. S. K. Lin, C.-W. Hui and G. McKay, Waste printed circuit board recycling techniques and product utilization, J. Hazard. Mater., 2015, 283, 234–243 CrossRef CAS PubMed
. https://www.sciencedirect.com/science/article/pii/S0304389414007705.
- J. Lee, H. T. Song and J.-M. Yoo, Present status of the recycling of waste electrical and electronic equipment in Korea, Resour., Conserv. Recycl., 2007, 50(4), 380–397 CrossRef
. https://www.sciencedirect.com/science/article/pii/S0921344907000250.
- A. Canal Marques, J. M. Cabrera and C. De Fraga Malfatti, Printed circuit boards: A review on the perspective of sustainability, J. Environ. Manage., 2013, 131, 298–306, DOI:10.1016/j.jenvman.2013.10.003
.
- V. Montenegro, H. Sano and T. Fujisawa, Recirculation of high arsenic content copper smelting dust to smelting and converting processes, Miner. Eng., 2013, 49, 184–189 CrossRef CAS
.
- H. Cui and C. G. Anderson, Literature Review of Hydrometallurgical Recycling of Printed Circuit Boards (PCBs), J. Adv. Chem. Eng., 2016, 6(1), 1000142 Search PubMed
.
- L. Zhang and Z. Xu, A review of current progress of recycling technologies for metals from waste electrical and electronic equipment, J. Cleaner Prod., 2016, 127, 19–36, DOI:10.1016/j.jclepro.2016.04.004
.
-
F. E. Mark and T. M. Lehner, Plastics Recovery from Waste Electrical \& Electronic Equipment in Non-ferrous Metal Processes, 2000 Search PubMed
.
- C. Hagelüken, Recycling of electronic scrap at Umicore's integrated metals smelter and refinery, World Metall.–Erzmet., 2006, 59, 1–16 Search PubMed
.
- B. Kim, J. Lee, J. Jeong, D.-H. Yang, D. Shin and K.-I. Lee, A Novel Process for Extracting Precious Metals from Spent Mobile Phone PCBs and Automobile Catalysts, Mater. Trans., 2013, 54, 1045–1048 CrossRef CAS
.
-
R. Montero, A. Guevara and E. de la Torre, Recovery of Gold, Silver, Copper and Niobium from Printed Circuit Boards Using Leaching Column Technique, 2012 Search PubMed
.
-
P. P. Sheng and T. H. Etsell, Recovery of Gold from Computer Circuit, 2007 Search PubMed
.
- U. U. Jadhav and H. Hocheng, Hydrometallurgical Recovery of Metals from Large Printed Circuit Board Pieces, Sci. Rep., 2015, 5, 14574 CrossRef CAS PubMed
.
- A. D. Bas, H. Deveci and E. Y. Yazici, Treatment of manufacturing scrap TV boards by nitric acid leaching, Sep. Purif. Technol., 2014, 130(2), 151–159, DOI:10.1016/j.seppur.2014.04.008
.
- B. Altansukh, K. Haga, N. Ariunbolor, K. Shigeru and A. Shibayama, Leaching and Adsorption of Gold from Waste Printed Circuit Boards Using Iodine-Iodide Solution and Activated Carbon, Eng. J., 2016, 20, 29–40 CrossRef CAS
.
- A. Kumari, M. K. Jha, J. Lee and R. P. Singh, Clean process for recovery of metals and recycling of acid from the leach liquor of PCBs, J. Cleaner Prod., 2016, 112, 4826–4834 CrossRef CAS
.
- A. Behnamfard, M. Mehdi and F. Veglio, Process development for recovery of copper and precious metals from waste printed circuit boards with emphasize
on palladium and gold leaching and precipitation, Waste Manage., 2013, 33(11), 2354–2363, DOI:10.1016/j.wasman.2013.07.017
.
- I. Birloaga, V. Coman, B. Kopacek and F. Vegliò, An advanced study on the hydrometallurgical processing of waste computer printed circuit boards to extract their valuable content of metals, Waste Manage., 2014, 34(12), 2581–2586, DOI:10.1016/j.wasman.2014.08.028
.
- E. Y. Yazici and H. A. Deveci, Extraction of metals from waste printed circuit boards (WPCBs) in H2SO4 CuSO4 NaCl solutions, Hydrometallurgy, 2013, 139, 30–38 CrossRef CAS
.
- E. Y. Yazici and H. A. Deveci, Cupric chloride leaching (HCl–CuCl2–NaCl) of metals from waste printed circuit boards (WPCBs), Int. J. Miner. Process., 2015, 134, 89–96 CrossRef CAS
.
- A. D. Bas, H. Deveci and E. Y. Yazici, Hydrometallurgy Bioleaching of copper from low grade scrap TV circuit boards using mesophilic bacteria, Hydrometallurgy, 2013, 138, 65–70, DOI:10.1016/j.hydromet.2013.06.015
.
- M. Somasundaram, R. Saravanathamizhan, C. A. Basha, V. Nandakumar, S. N. Begum and T. Kannadasan, Recovery of copper from scrap printed circuit board: modelling and optimization using response surface methodology, Powder Technol., 2014, 266, 1–6 CrossRef CAS
.
- M. K. Jha, A. Kumari, P. K. Choubey, J. Lee, V. Kumar and J. Jeong, Leaching of lead from solder material of waste printed circuit boards (PCBs), Hydrometallurgy, 2012, 121–124, 28–34 CrossRef CAS
. https://www.sciencedirect.com/science/article/pii/S0304386X1200093X.
- I. Birloaga and F. Vegliò, Journal of Environmental Chemical Engineering Study of multi-step hydrometallurgical methods to extract the valuable content of gold , silver and copper from waste printed circuit boards, Biochem. Pharmacol., 2016, 4(1), 20–29, DOI:10.1016/j.jece.2015.11.021
.
- A. Akcil, C. Erust, C. Sekhar, M. Ozgun, M. Sahin and A. Tuncuk, Precious metal recovery from waste printed circuit boards using cyanide and non-cyanide lixiviants – A review, Waste Manage., 2015, 45, 258–271, DOI:10.1016/j.wasman.2015.01.017
.
- P. Quinet, J. Proost and A. Van Lierde, Recovery of precious metals from electronic scrap by hydrometallurgical processing routes, Min., Metall., Explor., 2005, 22(1), 17–22, DOI:10.1007/BF03403191
.
- M. Gurung, B. Adhikari, H. Kawakita, K. Ohto, K. Inoue and S. Alam, Selective Recovery of Precious Metals from Acidic Leach Liquor of Circuit Boards of Spent Mobile Phones Using Chemically Modified Persimmon Tannin Gel, Ind. Eng. Chem. Res., 2012, 51, 11901–11913 CrossRef CAS
.
- J. Li and J. D. Miller, Reaction kinetics of gold dissolution in acid thiourea solution using ferric sulfate as oxidant, Hydrometallurgy, 2007, 89(3), 279–288 CrossRef CAS
. https://www.sciencedirect.com/science/article/pii/S0304386X07001697.
- G. Hilson and A. J. Monhemius, Alternatives to cyanide in the gold mining industry: what prospects for the future?, J. Cleaner Prod., 2006, 14(12), 1158–1167 CrossRef
. https://www.sciencedirect.com/science/article/pii/S0959652605000636.
- B. Xu, W. Kong, Q. Li, Y. Yang, T. Jiang and X. Liu, A review of thiosulfate leaching of gold: Focus on thiosulfate consumption and gold recovery from pregnant solution, Metals, 2017, 7(6), 222 CrossRef
.
- Z. Yanhua, S. Liu, H. Xie, X. Zeng and J. Li, Current Status on Leaching Precious Metals from Waste Printed Circuit Boards, Procedia Environ. Sci., 2012, 16, 560–568 CrossRef
.
- J. Ficeriová, P. Balá\vz and E. P. D. Gock, Leaching of gold, silver and accompanying metals from circuit boards (PCBs) waste, Acta Montan. Slovaca, 2011, 16, 128–131 Search PubMed
.
- P. M. H. Petter, H. M. Veit and A. M. Bernardes, Leaching of gold and silver from printed circuit board of mobile phones, REM, Rev. Esc. Minas, 2015, 68, 61–68 CrossRef
.
- P. M. H. Petter, H. M. Veit and A. M. Bernardes, Evaluation of gold and silver leaching from printed circuit board of cellphones, Waste Manage., 2014, 34 2, 475–482 CrossRef PubMed
.
-
F. Tesfaye, D. Lindberg and J. Hamuyuni, Valuable Metals and Energy Recovery from Electronic Waste Streams, 2017 Search PubMed
.
- Z. Wu, W. Yuan, J. Li, X. Wang, L. Liu and J. Wang, A critical review on the recycling of copper and precious metals from waste printed circuit boards using hydrometallurgy, Front. Environ. Sci. Eng., 2017, 11, 1–14 CAS
.
- G. Chauhan, K. K. Pant and K. D. P. Nigam, Environmental Science Processes & Impacts Chelation technology: a promising green approach for resource management and waste minimization, Environ. Sci. Technol., 2015, 17, 12–40 CAS
.
- R. W. Peters, Chelant extraction of heavy metals from contaminated soils, J. Hazard. Mater., 1999, 66(1), 151–210 CrossRef CAS PubMed
. https://www.sciencedirect.com/science/article/pii/S0304389499000102.
- K.-J. Hong, S. Tokunaga and T. Kajiuchi, Extraction of heavy metals from MSW incinerator fly ashes by chelating agents, J. Hazard. Mater., 2000, 75, 57–73 CrossRef CAS PubMed
.
- M. Cheikh, J. P. Magnin, N. Gondrexon and J. Willison, Zinc and lead leaching from contaminated industrial waste sludges using coupled processes, Environ. Technol., 2010, 31(14), 1577–1585 CrossRef CAS
.
- P. Jadhao, G. Chauhan, K. K. Pant and K. D. P. Nigam, Greener approach for the extraction of copper metal from electronic waste, Waste Manage., 2016, 57, 102–112 CrossRef CAS PubMed
. https://europepmc.org/abstract/MED/26597372.
- M. Pociecha and D. Lestan, Recycling of EDTA solution after soil washing of Pb, Zn, Cd and As contaminated soil, Chemosphere, 2012, 86(8), 843–846 CrossRef CAS PubMed
. https://www.sciencedirect.com/science/article/pii/S0045653511012562.
- D. Pant, P. Singh and M. K. Upreti, Metal leaching from cathode ray tube waste using combination of Serratia plymuthica and EDTA, Hydrometallurgy, 2014, 146, 89–95 CrossRef CAS
.
- Z. A. Begum, I. M. M. Rahman, Y. Tate, H. Sawai, T. Maki and H. Hasegawa, Remediation of toxic metal contaminated soil by washing with biodegradable aminopolycarboxylate chelants, Chemosphere, 2012, 87(10), 1161–1170, DOI:10.1016/j.chemosphere.2012.02.032
.
- J. Yoo, C.-D. Lee, J.-S. Yang and K. Baek, Extraction characteristics of heavy metals from marine sediments, Chem. Eng. J., 2013, 228, 688–699 CrossRef CAS
.
- L. Di Palma and R. Mecozzi, Heavy metals mobilization from harbour sediments using EDTA and citric acid as chelating agents, J. Hazard. Mater., 2007, 147(3), 768–775 CrossRef CAS PubMed
.
- H. A. Elliott and N. L. Shastri, Extractive Decontamination of Metal-Polluted Soils Using Oxalate, Water, Air, Soil Pollut., 1999, 110(3), 335–346, DOI:10.1023/A:1005067404259
.
- D.-Y. Chung, E. Kim, E.-H. Lee and J.-H. Yoo, Solubility of Rare Earth Oxalate in Oxalic and Nitric Acid Media, J. Ind. Eng. Chem., 1998, 4, 277–284 CAS
.
- L. Barbieri, R. Giovanardi, I. Lancellotti and M. Michelazzi, A new environmentally friendly process for the recovery of gold from electronic waste, Environ. Chem. Lett., 2010, 8(2), 171–178, DOI:10.1007/s10311-009-0205-2
.
- A. K. Awasthi and J. Li, An overview of the potential of eco-friendly hybrid strategy for metal recycling from WEEE, Resour., Conserv. Recycl., 2017, 126, 228–239, DOI:10.1016/j.resconrec.2017.07.014
.
- A. M. H. Ghasem and S. Khoramnejadian, The extraction of gold from e-waste by hydrometallurgy, Orient. J. Chem., 2015, 31(1), 113–120 CrossRef
.
- E. Kim, M. Kim, J. Lee and B. D. Pandey, Selective recovery of gold from waste mobile phone PCBs by hydrometallurgical process, J. Hazard. Mater., 2011, 198, 206–215 CrossRef CAS PubMed
. https://www.sciencedirect.com/science/article/pii/S030438941101274X.
- K. Zhang, Y. Wu, W. X. Wang, B. Li, Y.-N. Zhang and T. Zuo, Recycling indium from waste LCDs: A review, Resour., Conserv. Recycl., 2015, 104, 276–290 CrossRef
.
- J. Yang, T. Retegan and C. Ekberg, Indium recovery from discarded LCD panel glass by solvent extraction, Hydrometallurgy, 2013, 137, 68–77 CrossRef CAS
.
- B. Raju, J. R. Kumar, J. Lee, H.-S. Kwonc, M. L. Kantam and B. R. Reddy, Separation of platinum and rhodium from chloride solutions containing aluminum, magnesium and iron using solvent extraction and precipitation methods, J. Hazard. Mater., 2012, 227–228, 142–147 CrossRef CAS PubMed
.
- S. P. Barik, K. H. Park and C. W. Nam, Process development for recovery of vanadium and nickel from an industrial solid waste by a leaching-solvent extraction technique, J. Environ. Manage., 2014, 146, 22–28, DOI:10.1016/j.jenvman.2014.06.032
.
- M. Gurung, B. Adhikari, H. Kawakita, K. Ohto, K. Inoue and S. Alam, Recovery of gold and silver from spent mobile phones by means of acidothiourea leaching followed by adsorption using biosorbent prepared from persimmon tannin, Hydrometallurgy, 2013, 133, 84–93 CrossRef CAS
.
- F. T. Awadalla and G. M. Ritcey, Recovery of Gold from Thiourea, Thiocyanate, or Thiosulfate Solutions by Reduction-Precipitation with a Stabilized Form of Sodium Borohydride, Sep. Sci. Technol., 1991, 26(9), 1207–1228, DOI:10.1080/01496399108050525
.
- N. N. Joda and F. Rashchi, Recovery of ultra fine grained silver and copper from PC board scraps, Sep. Purif. Technol., 2012, 92, 36–42 CrossRef
.
-
J.-W. Ahn, S.-H. Ryu and T. Kim, Recovery of Tin and Nitric Acid from Spent Solder Stripping Solutions, 2015, pp. 426–31 Search PubMed
.
-
E. Yazıcı and H. Deveci, Recovery of Copper Sulphate from Sulphuric Acid Leach Solutions via Solvent Displacement Crystallisation Using Acetone, 2016 Search PubMed
.
- T. Oishi, K. Koyama, M. Tanaka and J. Lee, Influence of Electrolyte on an Energy-Saving Copper Recycling Process Using Ammoniacal Alkaline Solutions, Mater. Trans., 2006, 47, 2871–2876 CrossRef CAS
.
- B. C. Tanda, E. A. Oraby and J. J. Eksteen, Recovery of copper from alkaline glycine leach solution using solvent extraction, Sep. Purif. Technol., 2017, 187, 389–396 CrossRef CAS
. https://www.sciencedirect.com/science/article/pii/S1383586617303854.
- H. Zhang and D. B. Dreisinger, The recovery of gold from ammoniacal thiosulfate solutions containing copper using ion exchange resin columns, Hydrometallurgy, 2004, 72(3), 225–234 CrossRef CAS
. https://www.sciencedirect.com/science/article/pii/S0304386X0300183X.
- S. Dhiman and B. Gupta, Partition studies on cobalt and recycling of valuable metals from waste Li-ion batteries via solvent extraction and chemical precipitation, J. Cleaner Prod., 2019, 225, 820–832, DOI:10.1016/j.jclepro.2019.04.004
.
- Y. Chu, M. Chen, S. Chen, B. Wang, K. Fu and H. Chen, Micro-copper powders recovered from waste printed circuit boards by electrolysis, Hydrometallurgy, 2015, 156, 152–157, DOI:10.1016/j.hydromet.2015.06.006
.
- H. Cesiulis and N. Tsyntsaru, Eco-Friendly Electrowinning for Metals Recovery from Waste Electrical and Electronic Equipment (WEEE), Coatings, 2023, 13(3), 574 CrossRef CAS
.
- A. Tripathi and M. Rawat Ranjan, Heavy Metal Removal from Wastewater Using Low Cost Adsorbents, J. Biorem. Biodegrad., 2015, 6, 315 Search PubMed
.
- M. Reig, X. Vecino, C. Valderrama, I. Sirés and J. Luis Cortina, Waste-to-energy bottom ash management: Copper recovery by electrowinning, Sep. Purif. Technol., 2023, 311, 123256 CrossRef CAS
.
- X. Guo, H. Qin, Q. Tian and D. Li, Recovery of metals from waste printed circuit boards by selective leaching combined with cyclone electrowinning process, J. Hazard. Mater., 2020, 384, 121355, DOI:10.1016/j.jhazmat.2019.121355
.
- A. Fathima, J. Y. B. Tang, A. Giannis, I. M. S. K. Ilankoon and M. N. Chong, Catalysing electrowinning of copper from E-waste: A critical review, Chemosphere, 2022, 298, 134340 CrossRef CAS PubMed
. https://www.sciencedirect.com/science/article/pii/S0045653522008335.
- C. Modrzynski, J. Z. Bloh and C. Weidlich, Kinetic Investigations of the Electrochemically Assisted Leaching of Metals for the Recycling of Photovoltaics, J. Electrochem. Soc., 2022, 169(7), 073513 CrossRef CAS
.
- J. Lee, U. Von Gunten and J. H. Kim, Persulfate-Based Advanced Oxidation: Critical Assessment of Opportunities and Roadblocks, Environ. Sci. Technol., 2020, 54(6), 3064–3081 CrossRef CAS PubMed
.
- Z. Liu, H. Ding, C. Zhao, T. Wang, P. Wang and D. D. Dionysiou, Electrochemical activation of peroxymonosulfate with ACF cathode: Kinetics, influencing factors, mechanism, and application potential, Water Res., 2019, 159, 111–121, DOI:10.1016/j.watres.2019.04.052
.
- N. A. A. Qasem, R. H. Mohammed and D. U. Lawal, Removal of heavy metal ions from wastewater: a comprehensive and critical review, npj Clean Water, 2021, 4(1), 36 CrossRef CAS
.
- M. Karnib, A. Kabbani, H. Holail and Z. Olama, Heavy metals removal using activated carbon, silica and silica activated carbon composite, Energy Procedia, 2014, 50, 113–120 CrossRef CAS
.
- X. Zhang, Y. Wang, J. Cai, K. Wilson and A. F. Lee, Bio/hydrochar Sorbents for Environmental Remediation, Energy Environ. Mater., 2020, 3(4), 453–468 CrossRef CAS
.
- M. A. Islam, M. J. Ahmed, W. A. Khanday, M. Asif and B. H. Hameed, Mesoporous activated coconut shell-derived hydrochar prepared via hydrothermal carbonization-NaOH activation for methylene blue adsorption, J. Environ. Manage., 2017, 203, 237–244, DOI:10.1016/j.jenvman.2017.07.029
.
- N. Saha, M. Volpe, L. Fiori, R. Volpe, A. Messineo and M. T. Reza, Cationic dye adsorption on hydrochars of winery and citrus juice industries residues: Performance, mechanism, and thermodynamics, Energies, 2020, 13(18), 1–16 CrossRef
.
- Y. Li, L. Li and J. Yu, Applications of Zeolites in Sustainable Chemistry, Chem, 2017, 3(6), 928–949 CAS
.
- T. Zhang, W. Wang, Y. Zhao, H. Bai, T. Wen and S. Kang,
et al., Removal of heavy metals and dyes by clay-based adsorbents: From natural clays to 1D and 2D nano-composites, Chem. Eng. J., 2021, 420, 127574, DOI:10.1016/j.cej.2020.127574
.
- H. P. d. S. Costa, M. G. C. da Silva and M. G. A. Vieira, Biosorption of aluminum ions from aqueous solutions using non-conventional low-cost materials: A review, J. Water Process Eng., 2021, 40, 101925, DOI:10.1016/j.jwpe.2021.101925
.
- M. I. A. Abdel Maksoud, A. M. Elgarahy, C. Farrell, A. H. Al-Muhtaseb, D. W. Rooney and A. I. Osman, Insight on water remediation application using magnetic nanomaterials and biosorbents, Coord. Chem. Rev., 2020, 403, 213096, DOI:10.1016/j.ccr.2019.213096
.
- N. K. Singh and J. Li, Bio-Extraction of Metals as Secondary Resources from E-Waste, Appl. Mech. Mater., 2015, 768, 602–611 Search PubMed
.
- J. Li, T. Xu, J. Liu, J. Wen and S. Gong, A review of current progress of supercritical fluid technologies for e-waste treatment, Environ. Sci. Pollut. Res., 2021, 28(33), 44622–44637, DOI:10.1007/s11356-021-15074-z
.
- H. Brandl, R. Bosshard and M. Wegmann, Computer-munching microbes: metal leaching from electronic scrap by bacteria and fungi, Hydrometallurgy, 2001, 59, 319–326 CrossRef CAS
.
- S. Akbari and A. Ahmadi, Chemical Engineering & Processing : Process Intensification Recovery of copper from a mixture of printed circuit boards ( PCBs ) and sulphidic tailings using bioleaching and solvent extraction processes, Chem. Eng. Process., 2019, 142, 107584, DOI:10.1016/j.cep.2019.107584
.
- B. Mikoda, A. Potysz and E. Kmiecik, Bacterial leaching of critical metal values from Polish copper metallurgical slags using Acidithiobacillus thiooxidans, J. Environ. Manage., 2019, 236, 436–445 CrossRef CAS PubMed
. https://www.sciencedirect.com/science/article/pii/S0301479719301835.
- M. Saidan, B. Brown and M. Valix, Leaching of Electronic Waste Using Biometabolised Acids, Chin. J. Chem. Eng., 2012, 20(3), 530–534 CrossRef CAS
. https://www.sciencedirect.com/science/article/pii/S1004954111602152.
- Y. Xie, S. Wang, X. Tian, L. Che, X. Wu and F. Zhao, Leaching of indium from end-of-life LCD panels via catalysis by synergistic microbial communities, Sci. Total Environ., 2019, 655, 781–786 CrossRef CAS PubMed
. https://www.sciencedirect.com/science/article/pii/S0048969718344978.
- M. Xia, Y.-P. Wang, T. Peng, L. Shen, R. Yu and Y. Liu,
et al., Recycling of metals from pretreated waste printed circuit boards effectively in stirred tank reactor by a moderately thermophilic culture, J. Biosci. Bioeng., 2017, 123 6, 714–721 CrossRef PubMed
.
- A. I`1ldar, J. van de Vossenberg, E. R. Rene, E. D. van Hullebusch and P. N. L. Lens, Two-step bioleaching of copper and gold from discarded printed circuit boards (PCB), Waste Manage., 2016, 57, 149–157 CrossRef PubMed
.
- N. Bahaloo-horeh and S. M. Mousavi, Enhanced recovery of valuable metals from spent lithium-ion batteries through optimization of organic acids produced by Aspergillus niger, Waste Manage., 2017, 60, 666–679, DOI:10.1016/j.wasman.2016.10.034
.
- F. Faraji, R. Golmohammadzadeh and F. Rashchi, Fungal bioleaching of WPCBs using Aspergillus niger: Observation, optimization and kinetics, J. Environ. Manage., 2018, 217, 775–787, DOI:10.1016/j.jenvman.2018.04.043
.
- M. Baniasadi, F. Vakilchap, N. Bahaloo-Horeh, S. M. Mousavi and S. Farnaud, Advances in bioleaching as a sustainable method for metal recovery from e-waste: A review, J. Ind. Eng. Chem., 2019, 76, 75–90, DOI:10.1016/j.jiec.2019.03.047
.
- D. Mishra, D.-J. Kim, D. E. Ralph, J. Ahn and Y. H. Rhee, Bioleaching of metals from spent lithium ion secondary batteries using Acidithiobacillus ferrooxidans, Waste Manage., 2008, 28 2, 333–338 CrossRef PubMed
.
- Y. Xin, X. Guo, S. Chen, J. Wang, F. Wu and B. Xin, Bioleaching of valuable metals Li, Co, Ni and Mn from spent electric vehicle Li-ion batteries for the purpose of recovery, J. Cleaner Prod., 2016, 116, 249–258 CrossRef CAS
. https://www.sciencedirect.com/science/article/pii/S0959652616000056.
- Y. Hong and M. Valix, Bioleaching of electronic waste using acidophilic sulfur oxidising bacteria, J. Cleaner Prod., 2014, 65, 465–472 CrossRef CAS
.
- A. Marra, A. Cesaro, E. R. Rene, V. Belgiorno and P. N. L. Lens, Bioleaching of metals from WEEE shredding dust, J. Environ. Manage., 2018, 210, 180–190 CrossRef CAS PubMed
.
- V. A. Pham and Y. P. Ting, Gold Bioleaching of Electronic Waste by Cyanogenic Bacteria and its Enhancement with Bio-Oxidation, Adv. Mater. Res., 2009, 71–73, 661–664 CAS
.
- A. R. K. Gollakota, N. Kishore and S. Gu, A review on hydrothermal liquefaction of biomass, Renewable Sustainable Energy Rev., 2018, 81, 1378–1392 CrossRef
.
- W. Gu, J. Bai, B. Dong, X. Zhuang, J. Zhao and C.-L. Zhang,
et al., Enhanced bioleaching efficiency of copper from waste printed circuit board driven by nitrogen-doped carbon nanotubes modified electrode, Chem. Eng. J., 2017, 324, 122–129 CrossRef CAS
.
- A. Priya and S. Hait, Extraction of metals from high grade waste printed circuit board by conventional and hybrid bioleaching using Acidithiobacillus ferrooxidans, Hydrometallurgy, 2018, 177, 132–139, DOI:10.1016/j.hydromet.2018.03.005
.
- W. Gu, J. Bai, B. Dong, X. Zhuang, J. Zhao and C. Zhang,
et al., Catalytic effect of graphene in bioleaching copper from waste printed circuit boards
by Acidithiobacillus ferrooxidans, Hydrometallurgy, 2017, 171, 172–178 CrossRef CAS
. https://www.sciencedirect.com/science/article/pii/S0304386X16308982.
- K. Li and Z. Xu, Application of supercritical water to decompose brominated epoxy resin and environmental friendly recovery of metals from waste memory module, Environ. Sci. Technol., 2015, 49(3), 1761–1767 CrossRef CAS PubMed
.
- E. Hsu, C. J. Durning, A. C. West and A.-H. A. Park, Enhanced extraction of copper from electronic waste via induced morphological changes using supercritical CO2, Resour., Conserv. Recycl., 2021, 168, 105296 CrossRef CAS
. https://www.sciencedirect.com/science/article/pii/S092134492030611X.
- S. Sanyal, Q. Ke, Y. Zhang, T. H. Ngo, J. Carrell and H. Zhang,
et al., Understanding and optimizing delamination/recycling of printed circuit boards using a supercritical carbon dioxide process, J. Cleaner Prod., 2013, 41, 174–178 CrossRef CAS
.
- F. Xiu, Y. Qi, S. Wang, W. Nie, H. Weng and M. Chen, Application of critical water-alcohol composite medium to treat waste printed circuit boards: Oil phase products characteristic and debromination, J. Hazard. Mater., 2018, 344, 333–342 CrossRef CAS PubMed
.
- Y. Wang and F.-S. Zhang, Degradation of brominated flame retardant in computer housing plastic by supercritical fluids, J. Hazard. Mater., 2012, 205–206, 156–163 CrossRef CAS PubMed
.
- C. O. Calgaro, D. F. Schlemmer, M. D. C. R. Silva, E. V. Maziero, E. H. Tanabe and D. A. Bertuol, Fast copper extraction from printed circuit boards using supercritical carbon dioxide, Waste Manage., 2015, 45, 289–297, DOI:10.1016/j.wasman.2015.05.017
.
- F. Xiu, H. Weng, Y. Qi, G. Yu, Z. Zhang and F.-S. Zhang,
et al., A novel recovery method of copper from waste printed circuit boards by supercritical methanol process: Preparation of ultrafine copper materials, Waste Manage., 2017, 60, 643–651 CrossRef CAS PubMed
.
- K.-X. Liu, Z. Zhang and F.-S. Zhang, Direct extraction of palladium and silver from waste printed circuit boards powder by supercritical fluids oxidation-extraction process, J. Hazard. Mater., 2016, 318, 216–223 CrossRef CAS PubMed
.
- K.-Y. Li and Z. Xu, A review of current progress of supercritical fluid technologies for e-waste treatment, J. Cleaner Prod., 2019, 227, 794–809 CrossRef CAS
.
- M. Xing and F.-S. Zhang, Degradation of brominated epoxy resin and metal recovery from waste printed circuit boards through batch sub/supercritical water treatments, Chem. Eng. J., 2013, 219, 131–136 CrossRef CAS
.
- K. Liu and F.-S. Zhang, Innovative leaching of cobalt and lithium from spent lithium-ion batteries and simultaneous dechlorination of polyvinyl chloride in subcritical water, J. Hazard. Mater., 2016, 316, 19–25 CrossRef CAS PubMed
. https://www.sciencedirect.com/science/article/pii/S0304389416304435.
- F.-R. Xiu, Y. Qi and F.-S. Zhang, Recovery of metals from waste printed circuit boards by supercritical water pre-treatment combined with acid leaching process, Waste Manage., 2013, 33(5), 1251–1257 CrossRef CAS PubMed
. https://www.sciencedirect.com/science/article/pii/S0956053X13000536.
- C. S. Tiwary, S. Kishore, R. Vasireddi, D. R. Mahapatra, P. M. Ajayan and K. Chattopadhyay, Electronic waste recycling via cryo-milling and nanoparticle beneficiation, Mater. Today, 2017, 20(2), 67–73 CrossRef CAS
. https://www.sciencedirect.com/science/article/pii/S1369702116303972.
- F.-R. Xiu, Y. Qi and F.-S. Zhang, Leaching of Au, Ag, and Pd from waste printed circuit boards of mobile phone by iodide lixiviant after supercritical water pre-treatment, Waste Manage., 2015, 41, 134–141 CrossRef CAS PubMed
. https://www.sciencedirect.com/science/article/pii/S0956053X15001038.
- Z. Roskova, R. Skarohlid and L. McGachy, Siderophores: an alternative bioremediation strategy?, Sci. Total Environ., 2022, 819, 153144 CrossRef CAS PubMed
. https://www.sciencedirect.com/science/article/pii/S0048969722002340.
- T. G. Ambaye, M. Vaccari, F. D. Castro, S. Prasad and S. Rtimi, Emerging technologies for the recovery of rare earth elements (REEs) from the end-of-life electronic wastes: a review on progress, challenges, and perspectives, Environ. Sci. Pollut. Res., 2020, 27(29), 36052–36074 CrossRef CAS PubMed
.
- A. Batnasan,
et al., Gold recovery from Waste Printed Circuit Boards by Advanced Hydrometallurgical Processes, Mater. Trans., 2019, 60, 287–296 CrossRef
.
- C. J. Oh,
et al., Selective leaching of valuable metals from waste printed circuit boards, J. Air Waste Manage. Assoc., 2003, 53(7), 897–902 CrossRef CAS PubMed
.
| This journal is © The Royal Society of Chemistry 2023 |