Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
“Advanced materials” and the challenges on the horizon for testing their (eco)toxicity and assessing their hazard
Fazel
Abdolahpur Monikh
 *ab,
Willie
Peijnenburg
*ab,
Willie
Peijnenburg
 cd,
Agnes G.
Oomen
de,
Eugenia
Valsami-Jones
cd,
Agnes G.
Oomen
de,
Eugenia
Valsami-Jones
 f,
Vicki
Stone
g,
Raine
Kortet
f,
Vicki
Stone
g,
Raine
Kortet
 a,
Jarkko
Akkanen
a,
Peng
Zhang
a,
Jarkko
Akkanen
a,
Peng
Zhang
 e,
Jukka
Kekäläinen
a,
Alena
Sevcu
e,
Jukka
Kekäläinen
a,
Alena
Sevcu
 h and
Jussi V. K.
Kukkonen
a
h and
Jussi V. K.
Kukkonen
a
aDepartment of Environmental & Biological Sciences, University of Eastern Finland, P.O. Box 111, FI-80101 Joensuu, Finland. E-mail: fazel.monikh@uef.fi; f.a.monikh@gmail.com
bDepartment of Chemical Sciences, University of Padua, Via Marzolo 1, 35131 Padova, Italy
cInstitute of Environmental Sciences (CML), Leiden University, Einsteinweg 2, 2333 CC Leiden, The Netherlands
dNational Institute for Public Health and the Environment (RIVM), Center for Safety of Substances and Products, Bilthoven, The Netherlands
eInstitute for Biodiversity and Ecosystem Dynamics (IBED), University of Amsterdam, Amsterdam, The Netherlands
fSchool of Geography, Earth and Environmental Sciences, University of Birmingham, Edgbaston, B15 2TT Birmingham, UK
gInstitute of Biological Chemistry, Biophysics and Bioengineering, School of Engineering and Physical Sciences, Heriot-Watt University, Edinburgh, UK
hInstitute for Nanomaterials, Advanced Technologies and Innovation, Technical University of Liberec, Bendlova 1409/7, 460 01, Liberec, Czech Republic
First published on 21st December 2022
Abstract
“Advanced Materials” (AdMas) represent the next technology frontier. According to the European Union, AdMas are materials that feature a series of exceptional properties or functionalities compared to conventional materials. Considering the progress made in the design and application of AdMas, their adverse effects are still largely unknown whilst this is critical for assessing their environmental and human health risk. In this perspective, we first summarize the available definitions/descriptions and categorizations that cover AdMas and evaluate their adequacy from a toxicological point of view. We further describe the challenges and outlook on the toxicology of AdMas and propose solutions to tackle some of the challenges. Criteria related to which AdMas might induce hazards are discussed and used to propose a starting point of how to address AdMas in legal frameworks that consider human and environmental risks. Finally, we highlight the benefit of classification, e.g., enabling differentiation between AdMas based on their properties that might induce specific hazards and facilitate a faster pathway to identify the hazards of new AdMas, which is particularly relevant for safe-by-design.
Environmental significanceAdvanced materials (AdMas) are evolving to offer new materials for different applications ranging from food to medicine and electronics. Addressing the safety and the sustainability of materials, in general, at an early stage of their design requires adequate methods for risk and sustainability assessment. The current risk assessment framework for chemicals and nanomaterials cannot cover AdMas. In this perspective we highlight the challenges the toxicology community might face in optimal design and efficient use of the frameworks for AdMas and predicting their (environmental) risk. We performed an analysis of the existing knowledge pertaining to AdMas and their physicochemical properties to propose some criteria for the classification of AdMas to facilitate generating toxicological data for risk assessment. |
1. Introduction
The technology of Advanced Materials (AdMas) is still maturing as a discipline, with many new material discoveries expanding its realm. AdMas are more complex than conventional materials and cover a wide variety of materials, material combinations and material scales (including the nano-scale). They are of interest due to their novel or enhanced properties1 that potentially enable applications such as digital innovations and health advancement and aid in increasing energy conversion and storage,2 and advanced environmental remediation potential. For example, some AdMas can function dynamically which means they have passive and active states, leading to the performance of specific tasks upon activation, e.g., catalytically active AdMas known as nanozymes. AdMas are evolving to offer seemingly endless possibilities by elaborating molecular architectures for different applications ranging from food to medicine and electronics.Assessing the potential risks associated with use of different types of AdMas is critical. This is important not only for technological development but also to reach some of the transitions and goals of the European Green Deal.3 Indeed, the introduction of new and evolving technologies to the market to benefit society and the economy requires balancing the risks and benefits for humans and the environment. Addressing the safety and the sustainability of materials, in general, at an early stage of their design may benefit (risk) governance, but requires adequate methods for risk and sustainability assessment.4
The considerations of balancing risks and benefits are therefore an important question to address if AdMas are to reach their full commercial and societal potential. However, such considerations do not start from zero. For example, risk is calculated from exposure (the dose delivered) and hazard (how toxic the substance is). The relationships between physicochemical properties and both exposure and hazard have been widely studied for nanomaterials (NMs), which can provide information relevant to AdMas. NMs are defined based on their size (1–100 nm) (EU Commission, 2011), where the size-dependent unique properties distinguish them from their bulk counterparts. NMs could be considered as AdMas, but not all AdMas are NMs. For example, some AdMas have a size larger than the dimension proposed by the European Commission (EC) for defining NMs, such as artificial bacterial flagellum (200 nm) and two-armed nanoswimmer (200 nm). Various studies have assessed the human health and environmental risks of NMs, which facilitated some political actions, e.g. in the EU.5 Now, similar concerns arise for AdMas, noting that some of these materials are already used in products with biomedical, cosmetic and electronic applications.6
The current risk assessment frameworks for NMs are based on the data generated for the so-called “first generation” of NMs,7 where the materials are made of one main substance7 (such as TiO2, ZnO, CeO2 and Ag), sometimes with an additional substance coating used to provide surface functionalization or colloidal stability. The question is whether such frameworks can be utilised (or adapted) for AdMas. This review focuses on hazards (rather than exposure and risk assessment), for which it is critical to understand whether the AdMas can be assessed either
• on the basis of the known hazards of their constituents,
• on a more complex consideration of the possible toxic effects of AdMas resulting from new or enhanced function, or
• by addressing the potential for different components to interact and exacerbate the toxicological response.
Toxicological data can serve to provide an early warning of risk, while a lack of such toxicological data can cause risk governance to lag behind innovation. However, the hazard assessment of AdMas might face challenges due to uncertainty on the adequacy of current test methods. The Organisation for Economic Cooperation and Development (OECD) has conducted analyses of a number of guidance documents and test guidelines for their relevance to adequately assess the toxicity of NMs.8 These considerations could be adopted for some AdMas. Such considerations would need to incorporate the complexity of the materials, their properties, and their dynamic functions, which can make comparisons between AdMas and other substances difficult. Such uncertainties can also lead to a lack of clarity with respect to their consideration in legal frameworks, e.g., as NMs, substances, or as an article.
It is therefore now opportune to develop a perspective on the applicability of existing frameworks for the hazard assessment of AdMas, to see where it applies, where modifications or new approaches might be needed. Such considerations will highlight the challenges the toxicology community might face in optimal adaptation and therefore efficient use of existing frameworks for assessing AdMa hazards. We performed an analysis of the existing knowledge pertaining to AdMas and their physicochemical properties to propose some criteria for the classification of AdMas to facilitate generating toxicological data for risk assessment. We propose potential solutions that may be applicable to tackle some of the challenges anticipated for AdMas and help to assess the hazard associated with these materials by using some of the knowledge generated on NMs. We identify the knowledge gaps to be further studied and scrutinized, and we provide some recommendations for future toxicological studies of AdMas.
2. What are AdMas?
Several definitions or working descriptions have been proposed for AdMas (Table 1). Recently, the German Environment Agency has provided a description for AdMas for regulatory purposes.9 The EC uses a broad definition of “AdMas”, which includes any material that features a series of exceptional properties (mechanical, electrical, optical, magnetic, etc.) or functionalities (self-repairing, shape change, decontamination, the transformation of energy, etc.) which can be new or enhanced compared to the conventional materials.10 This definition covers almost all materials, including all NMs and their future generations. It is worth mentioning that the OECD has launched a Steering Group on AdMas that addresses the suitability of existing safety regulatory systems. The OECD does not aim to develop an exact definition for AdMas, instead a “working description” for AdMas that falls within the scope of OECD is elaborated. Meanwhile, a technical committee of the International Organization for Standardization (ISO) is independently working on a formal definition for AdMas.| Proposed definition/description of AdMas | References |
|---|---|
| Any material that, through the precise control of its composition and internal structure, features a series of exceptional properties (mechanical, electric, optic, magnetic, etc.) or functionalities (self-repairing, shape change, decontamination, transformation of energy, etc.) that differentiate it from the rest of the universe of materials, or one that, when transformed through advanced manufacturing techniques, features these properties or functionalities | European Commission10 |
| Materials that are rationally designed to have new or enhanced properties, and/or targeted or enhanced structural features with the objective to achieve specific or improved functional performance | OECD11 |
| Materials that are rationally designed through the precise control of their composition and internal or external structure in order to fulfil new functional requirements | The German Environment Agency9 |
| Materials, and their associated process technologies, with the potential to be exploited in high value-added products | UK Technology Strategy Board1 |
| Materials that have been developed to the point that unique functionalities have been identified and these materials now need to be made available in quantities large enough for innovators and manufacturers to test and validate in order to develop new products | 12 |
| Materials that are specifically engineered to exhibit novel or enhanced properties that confer superior performance relative to conventional materials | 1 |
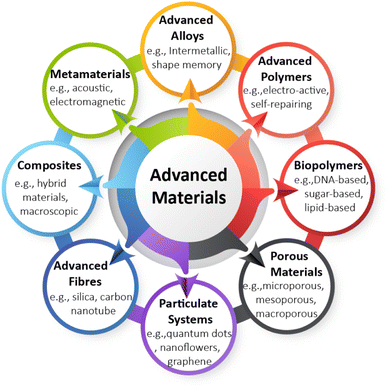
The German Environment Agency identified eight clusters of AdMas10 (Fig. 1) based upon their structures that demonstrate their breadth of chemistry and applications, which clearly indicates the wide variety of AdMas available and under development. Here we provide some specific examples of AdMas in order to exemplify this diversity and furthermore their usefulness. The first example includes multi-layered nickel–cobalt organic framework nanosheets (based on the scheme in Fig. 1, this AdMa can be categorized as a composite), developed as electrode materials for energy storage.13 Some of these materials can be switched off and on or controlled remotely, which defines them as smart AdMas or smart NMs.6 As another example, nanoscale bending-sensitive and optically transparent pressure sensors have been fabricated using composite nanofibers.14 Many other NMs, e.g. ionic polymer–metal composites, carbon nanotube composites, deformable polymer-based systems and biological molecular motors, have been fabricated so that these can be activated with a specific stimulus, such as pH, light, or temperature.7,15 Interesting examples are NMs consisting of an elastic polymer network and a molecular switch, which can change their structure from ribbon to a tight coil, then back to a ribbon when activated by light,16 and nanorobots which are currently being extensively researched and developed for medical applications.4 Using advanced polymers or hybrid advanced materials, we might also encounter advanced plastics in the future that can release smart microplastics and nanoplastics into the environment.
When considering the hazards of AdMas, the mode of action through which AdMas induce toxicity is not yet understood. Furthermore, we cannot assume that the hazards of AdMas across different clusters or within each cluster (of Fig. 1) will be the same. Therefore, the clustering based on the hazard might look different to what is proposed in Fig. 1. This Perspective briefly describes the possible challenges associated with the hazard investigation of AdMas before considering possible categorisation strategies.
3. Challenges surrounding the hazard assessment of AdMas
The hazards associated with different AdMas will vary, and even for an individual AdMa they may vary during their life cycle, e.g., from the development phase to production, use, recycling and disposal. Two decades of research on NM hazard assessment show that materials in general can induce toxicity through other modes of action compared to their chemical counterpart. Here we use this wealth of knowledge to anticipate the possible modes of action of AdMas (including a comparison to NMs), and the challenges such hazard assessments might pose. We also evaluate the challenges for extrapolation of hazard assessment of NMs to other AdMas.3.1. Chemical composition is not the only influential factor
One of the main advantages of AdMas, in general, is the possibility to design them with different physicochemical properties such as size, shape, aspect ratio, hydrophobicity, etc. Systematic studies have to some extent been performed to test the influence of the physicochemical properties of NMs on their toxicity.17,18 The findings have clearly confirmed that chemical composition is not the only factor influencing the toxicity of NMs, but other physicochemical properties can play important roles as well.19 This most likely is also true for other AdMas, where there is further complexity, for example because of multi-elemental and functional properties which may change their uptake pathways, interactions with cells and subsequently their toxicity to organisms.Comprehensive characterization of NMs has therefore been required both for research publications and for legislative frameworks. Such characterisation includes size distributions, surface charge, shape, surface area, impurities, etc. Such requirements are likely to be required for AdMas. This information will be useful to understand the toxicity of AdMas and link their properties to the hazards. Although publications are improving, many toxicological studies still do not report a detailed characterization of the tested material, even for single element NMs, partly due to the limitations in analytical capability and availability. Without this information, accurate comparisons between datasets from different toxicological studies, laboratories, or even comparisons between species exposed to the same materials would be impossible. Hence, the scientific community is urged to include information on these characteristics both for NMs and AdMas.
3.2. Dynamic behaviour of AdMas
Characterization of AdMas should not be restricted to the pristine material, because of the potential complexity of transformations that influence the fate and hazard. Moreover, applications of AdMas in consumer products such as food, beverage, pharmaceuticals, cosmetics, and feed should be considered, where they might enter organisms and move through food webs. The transformations of materials in the body and environment are well documented. For example, studies have shown that some NMs such as Ag20,21 and Cu22 may dissolve relatively quickly upon entering the environment or the human body, whereas others such as TiO2 (ref. 23) and carbon nanotubes (CNTs)24 may last longer. Considerable effort has been made to understand the toxicity of slowly and quickly dissolving NMs and to differentiate between the toxicity of particulate and ionic or molecular forms of NMs.21 The dissolution rate of a NM determines whether exposure to biological cells is to the intact NM, the dissolved ions/molecules or a combination of the two.25 For example, toxicity of CdTe/CdS quantum dots (QD) in algae was largely accounted for by dissolved Cd, while the QD NMs were however also taken up by the cells and induced unique impacts in the cells compared to Cd.26 Studer et al. (2010)27 tested the cytotoxicity of Cu NMs stabilized with a carbon layer and CuO to HeLa cells. They concluded that the toxicity of the Cu NMs is related to the Cu ion released from the particles rather than to the particle itself. For an AdMa composed of both quickly and slowly dissolving components, linking the biological and ecological effects to the physical form during exposure or uptake might be complicated. Azevedo et al. (2017)28 investigated the toxicity of a nanostructure composed of ZnO with Ag NMs on its surface (designated as the ZnO/Ag nanostructure) to Daphnia magna. The toxicity of ZnO and Ag NMs as single components, along with their nanostructure (ZnO/Ag) was tested. The authors concluded that neither the toxicity of the prepared mixture of ZnO and Ag nor of the ZnO/Ag nanostructure can be predicted based on the toxicity of their components alone. The toxicity of the nanostructure showed higher toxicity than predicted on the basis of the toxicity of the individual NMs. The stability of the intact AdMa, and its propensity to break down is thus expected to cause a challenge for hazard assessment of the AdMa and it is an important piece of information for further risk assessment of these materials.To facilitate the description or understanding of which components of an AdMa drive the hazards, we use Ag NMs which are coated with graphene-sheets containing Quantum Dots (QDs) as an existing example of a multi-elemental AdMa used as an antibacterial material29 (Fig. 2a). These AdMas consist of stable NMs (graphene) as well as quickly soluble (Ag NMs) and slowly soluble (QDs) fractions. From a toxicological perspective, the challenge is to use existing information on the single components as much as possible and complement this with additional issues, e.g., related to the multicomponent nature or new or enhanced functionalities (synergistic or antagonistic between any possible combinations: Fig. 2a).
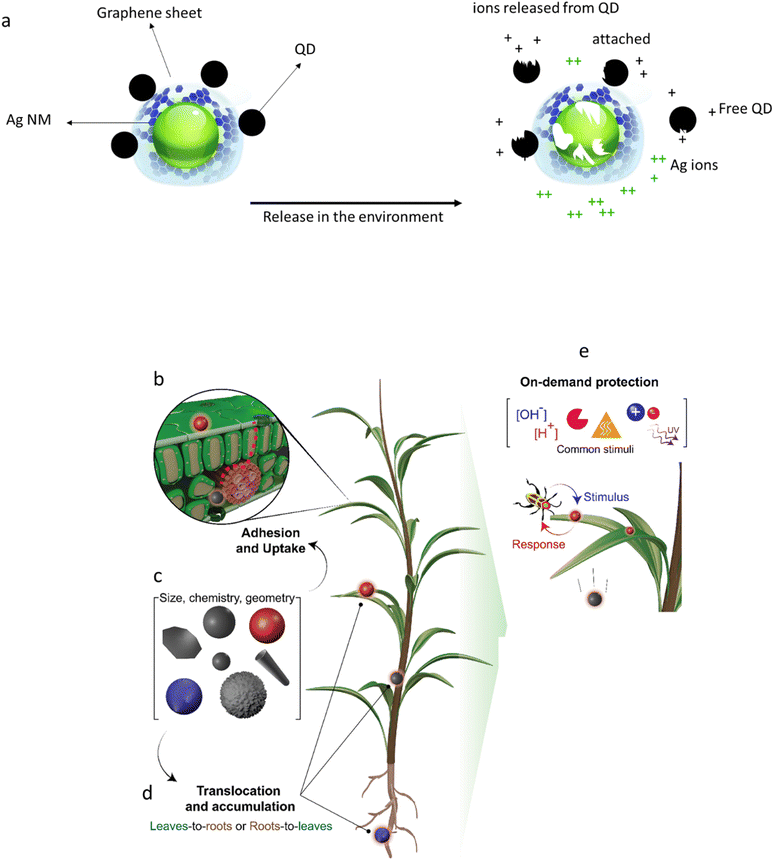 | ||
| Fig. 2 (a) Examples of AdMas that are released in the environment. The illustration shows Ag NMs stabilized with graphene-QD NMs. In the environment, the Ag NMs and some elements of the QDs will dissolve (at different rates), leading to the release of Ag ions and QD-related metals, whereas the graphene is stable. (b–e) Smart nano-pesticides. (b) Particle attachment to the surface of the plant. (c) The uptake of the NMs is influenced by the physicochemical properties of the NMs. (d) The NMs translocate in different tissues in the plant. (e) After targeting a specific tissue, the NMs respond to specific stimuli, such as pH, light, enzymes, ionic strength, and temperature (modified after Grillo et al. 2021 (ref. 15)). | ||
The considerations of dissolution are further complicated by the fact that AdMas may undergo homoaggregation and heteroaggregation in the environment. There is a wealth of knowledge on NMs showing that particles might immediately homoaggregate with themselves or heteroaggregate with background colloids in the environment.30 This could dramatically influence the behavior and fate of AdMas in nature. For example, upon heteroaggregation with naturally occurring iron oxide, AdMas can sediment and be removed from the aquatic phase. Moreover, aggregation might change the solubility of AdMas as was reported for NMs.22
Transformation of materials in the environment or the human body goes beyond dissolution and agglomeration, to include (but not limited to) processes such as accumulation of other molecules onto the surface, modification of the surface chemistry and dissociation of components. All of these considerations are relevant to both NMs and AdMas.
For example, it is well known that when NMs enter the body of an organism, the surfaces of the particles are rapidly covered by biomolecules such as proteins, forming the so-called “protein corona”.31 The same phenomenon can happen when NMs enter the environment, where they can be covered by natural organic matter (NOM). Little information is available on the formation of a NOM corona and the composition of the NOM corona on NMs in the environment due to limitations in analytical techniques. The protein corona consists of proteins which get absorbed to the NM during a time span of a few minutes up to several hours.32 The formation of a protein or NOM corona is dictated by the physicochemical properties of NMs such as size, aspect ratio, surface charge and chemical composition,33 as well as by the presence of the NOM or proteins in the surroundings and other conditions of the surroundings (pH, temperature, etc.).
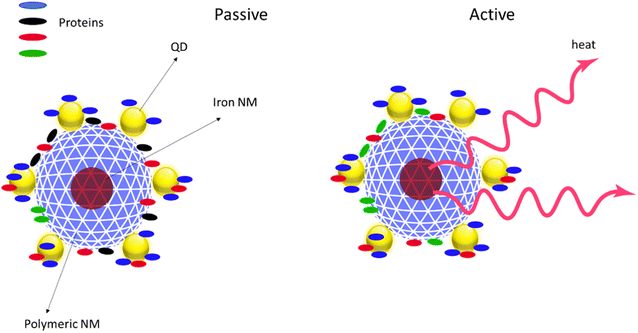
For AdMas, the formation and evolution of a protein or NOM corona is probably also controlled by the physicochemical properties of the materials. We describe our expectation of protein corona formation on AdMas by using smart NMs as a model of AdMas in a hypothetical example of a polymeric particle with an iron NM core and a QD doped surface (Fig. 3). In this illustration, we expect that different proteins adsorbed to the surface of the QD NM compare to the case of exposure of solely the polymeric NM in the same medium. It is also likely that activation of the iron NM with a magnetic field would generate heat34 that can influence the formation of the protein corona (Fig. 3).
Understanding the formation and evolution of protein or NOM corona is useful for hazard assessment. Sorption of biomolecules on the surface of AdMas confers a new biological identity to the materials, which influences the biological fate and biodistribution of the particles in various organs, tissues, and cells in organisms. For example, it is known that adsorption of proteins facilitates the recognition and uptake of particles by immune cells, which are involved in the uptake and metabolism of foreign particulates.35 We believe that while the existing data on protein corona formation on NMs can help to understand the corona formation on AdMas, alone it will not be sufficient. Some physicochemical properties of AdMas such as the multi-elemental composition and implementation of switchable properties in some AdMas, which imparts dynamic properties to the NMs, may add another dimension to the biological fate of AdMas, consequently complicating the prediction of their biodistribution and hazard.
Most of the available information on the elimination of NMs from the body is medically oriented and it is indicated that >6 nm particles cannot be eliminated via renal excretion.36 Few (eco)toxicological studies on fish showed that NMs might be excreted from the gills.37,38 Many promising AdMas have a size large than 6 nm.39 More studies are still needed to understand the uptake and elimination pathway of AdMas from different model organisms with (eco)toxicological purposes.
However, extensive characterization of pristine materials, in products or in various life cycle stages is not always practically feasible for many laboratories due to the extensive instrumentation and the skills required to perform the comprehensive characterization. These challenges have already been recognized for first generation of NMs, which apply to AdMas. Any characterisation should be attempted in a media that best represents the biological or environmental compartment relevant to the life cycle stage under consideration. There are some limitations that can further challenge the characterization of AdMas (including smart NMs). For example, the characterization of a multi-element AdMa consisting of inorganic and organic components requires combinations of techniques suitable for the characterization of each component (by assuming that the sample preparation for the target component does not influence the other component of the AdMa). Moreover, it is yet unknown how to characterize the activity of smart AdMas upon stimulation for toxicological purposes, e.g., in nontarget organisms.
3.3. Being smart further challenges the toxicity testing of smart AdMas
Some AdMas are designed to undergo changes in their physicochemical properties in response to a specific stimulus. These are referred to as smart AdMas or smart NMs (although most of them have size larger than 100 nm). Examples of such AdMas include light-driven molecular motors and smart nano-pesticides. For example, it is possible to design nano-pesticides (Fig. 2b) that minimize biocidal leaching. They thus reduce bioaccumulation in non-targeted organisms,40 which is a drawback of traditional pesticides. The nano-pesticides can be designed to target specific tissues in plants and remain passive (Fig. 2c and d). They could be activated by stimuli such as pH or a specific enzyme,15 leading to, e.g., triggered and controlled cargo release in the target organisms. Similar AdMas have been developed for medical applications and for environmental remediation. These smart NMs might find their way to the market within a few years. Regardless of their terminologies, e.g., nanomachines, nanobiodevices, actuators, nanomotors and nanostructures, from a toxicological perspective they are AdMas interacting with cells and biomolecules in organisms.41 The question is what drives these interactions and what are the consequences for risk assessment.There are few toxicological studies in which the effect of activated smart NMs has been investigated, although such tests are currently uncommon.42,43 The question is whether and to what extent the smart or enhanced properties must be considered in toxicological studies. Hazard assessment based on the passive form of smart NMs is unlikely to be sufficient to assess their risk. The controlled functionality of smart NMs, therefore, adds another level of complexity to the toxicological studies of AdMas. Testing of the different forms of a material – passive and active – can be considered, but could be difficult to generate. It will also be difficult to assess and simulate the location of the bioavailability of active forms within the body or within cells. Also, assessing the hazards of different forms would lead to higher costs for toxicity testing as well as a greater animal use. While guidelines and protocols exist for assessing hazards of dissolved chemicals, and in some cases for NMs, further work will be required to determine their suitability for the assessment of smart AdMa-induced toxicity, and to make modifications if required.
3.4. Not all AdMas are NMs
For regulatory purposes, the EC recommended a definition for NMs in 2011 and the revision will be released soon.44 To enable safety assessment and management and implementation of regulation, the size range of 1–100 nm was proposed and adopted. Toxicity of materials is however not limited to a specific size, e.g. <100 nm, and each organism and cell might respond differently to different sizes.45 It has for instance been shown that a particle with size larger than 100 nm might be more toxic than smaller counterparts of the same material.46 AdMas often exceed the threshold limit of 100 nm.7 For example, many AdMas such as nanomotors and nano-composites have sizes larger than 100 nm. This excludes them from being NMs according to the EC recommended definition despite having the term “nano” in their terminologies. It is also likely that AdMas with sizes larger than 100 nm will be made of NMs with sizes smaller than 100 nm. Since not all AdMas are considered to be NMs and, thus, the nano-specific requirements are not applicable to all AdMas, these materials will not be regulated as NMs whereas physicochemical properties rather than chemical properties alone determine their fate/toxicokinetics and hazards. This may require new adaptations in regulations.3.5. Can current (eco)toxicological guidelines cover AdMas?
The OECD and the ISO are working extensively on developing toxicological test guidelines (TGs) or Guidance Documents (GDs).47 From a regulatory perspective, these TGs and GDs are critical for current risk assessment procedures and support the international acceptance of data as well as harmonization across different labs and the generation of reliable data.48 Examples of published TGs for NMs include TG 125 on particle size and particle size distribution, TG 413 of subchronic inhalation tests, and GD 317 on aquatic and sediment toxicological testing. These TGs or GDs have been newly developed for NMs or adapted from existing ones for chemicals.49,50 Further work is ongoing for a number of other TGs and GDs. The increased complexity of AdMas, such as for the smart and multi-elemental AdMas, might further challenge the adequateness and fit-for-purpose of the TGs and GDs for NMs, which have been developed for mono-element NMs.4. Recommendation for classification of AdMas for toxicological and risk assessment purposes
Given the anticipated developments of AdMas in the near future, it is important to consider how to address AdMas in legal frameworks, and if and what adaptations are needed to adequately gather information on safety to accommodate AdMas. Some AdMas will fall under the definition of NMs whereas others will not. Due to the large diversity in AdMas, it is useful to streamline assessing potential consequences of AdMas for physicochemical and (eco)toxicity testing by classification of AdMas into different groups. These groups should be based on their physicochemical properties that may involve similar mode-of-action of toxicity, e.g., the mode-of-action could include release of ions and particles of different types, surface area, etc. For example, in the clusters proposed by the German Environment Agency, advanced alloys and QDs are classified into two different clusters. From a toxicological perspective, advanced alloys and QDs could be particles consisting of more than one metal, where each metal within one particle might induce toxicity through similar pathways, e.g., generating oxidative stress or cell apoptosis. The benefits of classification are that it: (1) enables differentiation between AdMas based on their properties that might induce specific hazards, (2) provides measurable criteria that can be integrated into toxicological concepts, (3) provides clearer insight into what is needed to address AdMas in legal frameworks and (4) facilitates a faster pathway to identify the hazards of new AdMas.Further refinements required to address the safety assessment of AdMas include:
– Investigate mode-of-action for each class of AdMas.
– Consider when and how the existing information for single components can be used.
– Consider how information on new or enhanced functionality can be used.
– Consider how to address mixture effects of multicomponent AdMas.
Currently, the Horizon Europe project SUNSHINE is developing approaches to address the toxicity of some AdMas and how existing information on single components or similar AdMas can be used in safety assessment (https://www.h2020sunshine.eu/). Unlike NMs, where strict adherence to a 100 nm size threshold has been proposed, we recommend not to limit the investigations on the toxicity of AdMas to particle sizes smaller than 100 nm. As we described earlier, it is highly likely that the size of AdMas will not be limited to the 100 nm threshold, some components of an AdMa may be smaller than 100 nm whereas the entire structure is not, and there is no scientific rationale related to safety for a strict cut-off at 100 nm.51
5. Summary and recommendations
The identified challenges in toxicity and risk assessment of AdMas might go beyond the problems recognized for the first generation of NMs and even question the future of NM risk assessment. The European Chemicals Agency published an inventory of new AdMas.6 There is no doubt that the number of AdMas on the market will increase in the future. An important step to support technological development, in general, is the implementation of a safe-and-sustainable-by-design (SSbD) strategy. The AdMa-based technologies will benefit from the identification of potential risks and sustainability issues associated with AdMas as of the early stages of innovation to support the SSbD development, and the production, use, and end-of-life treatment of the materials.3 A systematic early warning system has been proposed and established jointly by the Dutch National Institute of Public Health and the Environment (RIVM) and German agencies (UBA, BfR, BAUA) to identify potential risks and sustainability issues of advanced NMs which can also be applied to AdMas in general. Moreover, within OECD, an early warning system is under development.It is critical now to support (eco)toxicological studies of AdMas and to advance toxicology to tackle the challenges associated with the development of innovative AdMas. The generated data are important in order to be able to assess the risk of AdMas and to gather information to support the early warning systems, grouping and SSbD. The hazard assessment of a variety of AdMas may need to expand because new toxicological phenomena, which are not covered by the classical (apical) endpoints, might be induced by AdMas. We finally recommend the development of an efficient network between innovators, researchers and policy-makers, where up-to-date findings are transferred to facilitate the development of regulations for AdMas.
Author contributions
F. A. M. conceptualized, supervised, wrote, and reviewed the Perspective. W. J. G., V. S., E. V. J., A. O., and J. V. K. K. contributed to the conceptualization, writing and editing of the Perspective. R. K., J. K., J. A., P. Z., and A. S. contributed to the editing of the Perspective.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was funded by the University of Eastern Finland Water Research Program funded by the Saastamoinen Foundation, the Wihuri Foundation, and the Olvi Foundation. The study was also partially funded by the European Union's Horizon 2020 Research and Innovation Program, via the SUNSHINE (Safe and Sustainable Design for Advanced Materials) project (G.A. No. 952924) and Academy of Finland (No. 308485).References
- A. Kennedy, J. Brame, T. Rycroft, M. Wood, V. Zemba, C. Weiss, M. Hull, C. Hill, C. Geraci and I. Linkov, Risk Anal., 2019, 39, 1783–1795 CrossRef PubMed
.
- W.-J. Ong, N. Zheng and M. Antonietti, Nanoscale, 2021, 13, 9904–9907 RSC
.
- W. Peijnenburg, A. G. Oomen, L. G. Soeteman-Hernández, M. Groenewold, A. J. A. M. Sips, C. W. Noorlander, J. A. B. Kettelarij and E. A. J. Bleeker, NanoImpact, 2021, 23, 100342 CrossRef CAS PubMed
.
- R. Arvidsson and S. F. Hansen, Environ. Sci.: Nano, 2020, 7, 2875–2886 RSC
.
- H. Rauscher, K. Rasmussen and B. Sokull-Klüttgen, Chem.-Ing.-Tech., 2017, 89, 224–231 CrossRef CAS
.
- S. Gottardo, A. Mech, J. Drbohlavová, A. Małyska, S. Bøwadt, J. Riego Sintes and H. Rauscher, NanoImpact, 2021, 21, 100297 CrossRef CAS PubMed
.
-
M. Camboni, J. Hanlon, R. P. García and P. Floyd, A State of Play Study of the market for so called ‘Next Generation’ Nanomaterials, 2019 Search PubMed
.
- K. Rasmussen, M. González, P. Kearns, J. R. Sintes, F. Rossi and P. Sayre, Regul. Toxicol. Pharmacol., 2016, 74, 147–160 CrossRef CAS PubMed
.
-
K. Schwirn, D. Völker, A. Haase, J. Tentschert, U. Bernauer, R. Packroff and V. Bachmann, Risk Governance of Advanced Materials, Umweltbundesamt, Wörlitzer Platz 1, 2021 Search PubMed
.
-
B. Giese, M. Drapalik, L. Zajicek, D. Jepsen, A. Reihlen and T. Zimmermann, Advanced Materials: Overview of the Field and Screening Criteria for Relevance Assessment, Umweltbundesamt, 2020 Search PubMed
.
-
OECD Environment, Health and Safety Publications Series on the Safety of Manufactured Nanomaterials No. 104: Advanced Materials: Working Description, 2022, pp. 1–26 Search PubMed
.
-
National Institute of Standards and Technology (NIST), Manufacturing and biomanufacturing: Materials advances and critical processes, Department of Commerce, Gaithersburg, MD, 2011, pp. 1–23 Search PubMed
.
- Y. Chi, W. Yang, Y. Xing, Y. Li, H. Pang and Q. Xu, Nanoscale (NIST), 2020, 12, 10685–10692 RSC
.
- S. Lee, A. Reuveny, J. Reeder, S. Lee, H. Jin, Q. Liu, T. Yokota, T. Sekitani, T. Isoyama, Y. Abe, Z. Suo and T. Someya, Nat. Nanotechnol., 2016, 11, 472–478 CrossRef CAS PubMed
.
- R. Grillo, B. D. Mattos, D. R. Antunes, M. M. L. Forini, F. A. Monikh and O. J. Rojas, Nano Today, 2021, 37, 101078 CrossRef CAS
.
- J. Osicka, M. Ilčiková, A. Popelka, J. Filip, T. Bertok, J. Tkac and P. Kasak, Langmuir, 2016, 32, 5491–5499 CrossRef CAS PubMed
.
- F. Abdolahpur Monikh, D. Arenas-lago, P. Porcal, R. Grillo, P. Zhang, Z. Guo, M. G. Vijver, W. J. G. M. Peijnenburg, F. Abdolahpur, D. Arenas-lago, P. Porcal and R. Grillo, Nanotoxicology, 2019, 1–16 Search PubMed
.
- F. Abdolahpur Monikh, L. Chupani, Z. Guo, P. Zhang, G. K. Darbha, M. G. Vijver, E. Valsami-Jones and W. J. G. M. Peijnenburg, Ecotoxicol. Environ. Saf., 2021, 218, 112280 CrossRef CAS PubMed
.
- X. Li, W. Liu, L. Sun, K. E. Aifantis, B. Yu, Y. Fan, Q. Feng, F. Cui and F. Watari, J. Biomed. Mater. Res., Part A, 2015, 103, 2499–2507 CrossRef CAS PubMed
.
- J. M. Unrine, B. P. Colman, A. J. Bone, A. P. Gondikas and C. W. Matson, Environ. Sci. Technol., 2012, 46, 6915–6924 CrossRef CAS PubMed
.
- J. E. Rajala, E. R. Vehniäinen, A. Väisänen and J. V. K. Kukkonen, Environ. Toxicol. Chem., 2018, 37, 1889–1897 CrossRef CAS PubMed
.
- D. Arenas-Lago, F. Abdolahpur Monikh, M. G. Vijver and W. J. G. M. Peijnenburg, Chemosphere, 2019, 226, 841–850 CrossRef CAS PubMed
.
- F. Abdolahpur Monikh, L. Chupani, E. Zusková, R. Peters, M. Vancová, M. Vijver, P. Porcal and W. Peijnenburg, Environ. Sci. Technol., 2019, 53, 946–953 CrossRef CAS PubMed
.
- E. Heister, C. Lamprecht, V. Neves, C. Tîlmaciu, L. Datas, E. Flahaut, B. Soula, P. Hinterdorfer, H. M. Coley, S. R. P. Silva and J. McFadden, ACS Nano, 2010, 4, 2615–2626 CrossRef CAS PubMed
.
- V. Stone, S. Gottardo, E. A. J. Bleeker, H. Braakhuis, S. Dekkers, T. Fernandes, A. Haase, N. Hunt, D. Hristozov, P. Jantunen, N. Jeliazkova, H. Johnston, L. Lamon, F. Murphy, K. Rasmussen, H. Rauscher, A. S. Jiménez, C. Svendsen, D. Spurgeon, S. Vázquez-Campos, W. Wohlleben and A. G. Oomen, Nano Today, 2020, 35, 100941 CrossRef CAS
.
- R. F. Domingos, D. F. Simon, C. Hauser and K. J. Wilkinson, Environ. Sci. Technol., 2011, 45, 7664–7669 CrossRef CAS PubMed
.
- A. M. Studer, L. K. Limbach, L. Van Duc, F. Krumeich, E. K. Athanassiou, L. C. Gerber, H. Moch and W. J. Stark, Toxicol. Lett., 2010, 197, 169–174 CrossRef CAS PubMed
.
- S. L. Azevedo, T. Holz, J. Rodrigues, T. Monteiro, F. M. Costa, A. M. V. M. Soares and S. Loureiro, Sci. Total Environ., 2017, 579, 337–344 CrossRef CAS PubMed
.
- X. Zhong, C. Tong, T. Liu, L. Li, X. Liu, Y. Yang, R. Liu and B. Liu, Biomater. Sci., 2020, 8, 6670–6682 RSC
.
- J. Labille, C. Harns, J. Y. Bottero and J. Brant, Environ. Sci. Technol., 2015, 49, 6608–6616 CrossRef CAS PubMed
.
- M. Mahmoudi, M. P. Monopoli, M. Rezaei, I. Lynch, F. Bertoli, J. J. Mcmanus and K. A. Dawson, ChemBioChem, 2013, 14, 568–572 CrossRef CAS PubMed
.
- M. P. Monopoli, A. S. Pitek, I. Lynch and K. A. Dawson, Methods Mol. Biol., 2013, 1025, 137–155 CrossRef CAS PubMed
.
- D. Baimanov, R. Cai and C. Chen, Bioconjugate Chem., 2019, 30, 1923–1937 CrossRef CAS PubMed
.
- R. R. Shah, T. P. Davis, A. L. Glover, D. E. Nikles and C. S. Brazel, J. Magn. Magn. Mater., 2015, 387, 96–106 CrossRef CAS PubMed
.
- R. Cai, J. Ren, Y. Ji, Y. Wang, Y. Liu, Z. Chen, Z. Farhadi Sabet, X. Wu, I. Lynch and C. Chen, ACS Appl. Mater. Interfaces, 2020, 12, 1997–2008 CrossRef CAS PubMed
.
- W. Poon, Y. N. Zhang, B. Ouyang, B. R. Kingston, J. L. Y. Wu, S. Wilhelm and W. C. W. Chan, ACS Nano, 2019, 13(5), 5785–5798 CrossRef CAS PubMed
.
- F. Abdolahpur Monikh, L. Chupani, D. Arenas-Lago, Z. Guo, P. Zhang, G. K. Darbha, E. Valsami-Jones, I. Lynch, M. G. Vijver, P. M. van Bodegom and W. J. G. M. Peijnenburg, Nat. Commun., 2021, 12, 899 CrossRef CAS PubMed
.
- Z. J. Zhu, R. Carboni, M. J. Quercio, B. Yan, O. R. Miranda, D. L. Anderton, K. F. Arcaro, V. M. Rotello and R. W. Vachet, Small, 2010, 6, 2261–2265 CrossRef CAS PubMed
.
- M. Yoshida and J. Lahann, ACS Nano, 2008, 2, 1101–1107 CrossRef CAS PubMed
.
- Y. Zhai, F. Abdolahpur Monikh, J. Wu, R. Grillo, D. Arenas-Lago, G. K. Darbha, M. G. Vijver and W. J. G. M. Peijnenburg, Environ. Sci.: Nano, 2020, 7, 3372–3384 RSC
.
- V. Subramanian, J. Youtie, A. L. Porter and P. Shapira, J. Nanopart. Res., 2010, 12, 1–10 CrossRef PubMed
.
- C. Xu, M. Ojeda, R. A. D. Arancon, A. A. Romero, J. L. Domingo, M. Gómez, J. Blanco and R. Luque, ACS Sustainable Chem. Eng., 2015, 3, 2716–2725 CrossRef CAS
.
- M. J. Bessa, C. Costa, J. Reinosa, C. Pereira, S. Fraga, J. Fernández, M. A. Bañares and J. P. Teixeira, Toxicol. Appl. Pharmacol., 2017, 316, 114–122 CrossRef CAS PubMed
.
- F. Abdolahpur Monikh, S. F. Hansen, M. G. Vijver, E. Kentin, M. B. Nielsen, A. Baun, K. Syberg, I. Lynch, E. Valsami-Jones and W. J. G. M. Peijnenburg, Environ. Sci. Technol., 2022, 56(7), 3836–3839 CrossRef CAS PubMed
.
- T. Silva, L. R. Pokhrel, B. Dubey, T. M. Tolaymat, K. J. Maier and X. Liu, Sci. Total Environ., 2014, 468–469, 968–976 CrossRef CAS PubMed
.
- L. Yildirimer, N. T. K. Thanh, M. Loizidou and A. M. Seifalian, Nano Today, 2011, 6, 585–607 CrossRef CAS PubMed
.
- L. P. W. Clausen and S. F. Hansen, Nat. Nanotechnol., 2018, 13, 766–768 CrossRef CAS PubMed
.
- M. B. Nielsen, A. Baun, A. Mackevica, A. Thit, I. Odnevall Wallinder, J. A. Gallego, L. P. Westergaard Clausen, J. Rissler, L. Skjolding, A. Castro Nilsson, T. Cedervall and S. Foss Hansen, Environ. Sci.: Nano, 2021, 8, 731–747 RSC
.
- S. I. L. Gomes, J. J. Scott-Fordsmand and M. J. B. Amorim, Nano Today, 2021, 40, 101242 CrossRef CAS
.
- K. Rasmussen, H. Rauscher, P. Kearns, M. González and J. Riego Sintes, Regul. Toxicol. Pharmacol., 2019, 104, 74–83 CrossRef PubMed
.
- E. A. J. Bleeker, W. H. de Jong, R. E. Geertsma, M. Groenewold, E. H. W. Heugens, M. Koers-Jacquemijns, D. van de Meent, J. R. Popma, A. G. Rietveld, S. W. P. Wijnhoven, F. R. Cassee and A. G. Oomen, Regul. Toxicol. Pharmacol., 2013, 65, 119–125 CrossRef PubMed
.
| This journal is © The Royal Society of Chemistry 2023 |