Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A π-extended β-diketiminate ligand via a templated Scholl approach†
Lars
Killian
a,
Martin
Lutz
 b and
Arnaud
Thevenon
b and
Arnaud
Thevenon
 *a
*a
aOrganic Chemistry and Catalysis, Institute for Sustainable and Circular Chemistry, Faculty of Science, Utrecht University, Universiteitsweg 99, 3584 CG, Utrecht, The Netherlands. E-mail: a.a.thevenon-kozub@uu.nl
bStructural Biochemistry, Bijvoet Centre for Biomolecular Research, Faculty of Science, Utrecht University, Universiteitsweg 99, 3584 CG, Utrecht, The Netherlands
First published on 6th June 2024
Abstract
We report a templated Scholl oxidation strategy for the preparation of the first β-diketiminate (BDI) ligands embedded within a 24-electron π-system backbone. The resulting benzo[f,g]tetracene BDI ligand was coordinated to a zinc centre and electrochemical studies showed the redox active nature of the ligand.
Polycylic (hetero-)aromatic hydrocarbons (PAHs) play a pivotal role in the field of organic optoelectronic materials due to their unique electronic, optical and self-assembly properties.1–3 Their interesting properties have also received attention from the field of coordination chemistry, notably with the investigations of “superbenzene”-like ligands,4 perylene-based ligands5 and extended π-conjugated porphyrins.6
From the perspective of ligand design in homogeneous catalysis, it is expected that a ligand embedded in a PAH could act as an electron reservoir to store and provide multiple electrons, on demand, to the active metal centre.7 However, the lack of suitable and easily accessible ligands hinder further development in the field. One of the reasons is the challenging “bottom-up” synthesis of the extended conjugated π-system, often involving cyclo-dehydrogenation methods such as the Scholl reaction.8 Another reason is the large number of steps often required to install solubilizing groups to prevent strong π–π stacking. In many cases, the final product is obtained only on a milligram scale. Even then, the properties of the resulting material often hinder solution state characterization and reactivity studies.
β-Diketiminates (BDIs) are a well-known class of ligands widely used in homogeneous catalysis and coordination chemistry.9 Despite being typically seen as spectator ligands, their non-innocent behaviour has been widely investigated, with examples such as chemical reactivity at the γ-position and redox non-innocence.10 Using a phenyl-substituted BDI, it was shown that the ligand system is capable of acting as an electron reservoir through conjugation to aromatic substituents (Fig. 1a).10d Despite this, the incorporation of large, conjugated π-systems within BDI-type ligands has been largely limited to 2,2′-dipyrromethene-type ligands (Fig. 1b). Interestingly, few studies used the smallest polycyclic odd alternant hydrocarbon found in graphene sheets, phenalenyl,11 incorporated into BDI ligands.12 Complexes of these ligands were used as molecular conductor,13 and for various catalytic transformations.12a,14 The ligand's stability across three different oxidation states was further shown to modulate the activity of aluminium complexes in the hydroamination reaction.15
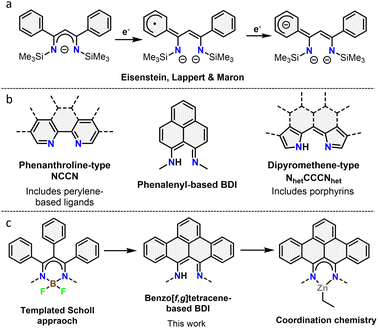 | ||
| Fig. 1 (a) Redox non-innocence on a phenyl substituted BDI ligand. (b) Overview of different binding pockets reported in nanographene ligands. (c) Overview of the work described herein. | ||
In this context, we report the synthesis and characterization of the first example of BDI ligands embedded within a benzo[f,g]tetracene backbone. The ligand is isolated in relatively few steps and successfully coordinated to Zn demonstrating its potential as ligand for transition metals (Fig. 1c). Cyclic voltammetry was used to reveal the redox active nature of the ligand.
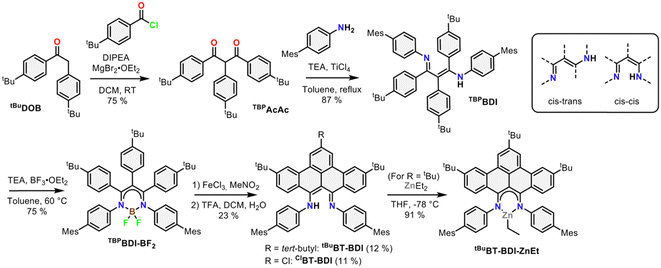
The 4-step synthesis of the β-,γ-tBu-phenyl substituted BDI pre-ligand (TBPBDI, Scheme 1) starts with the synthesis of the corresponding 1,3-diketone TBPAcAc. TBPAcAc was prepared in 75% yield via soft-enolization of 4-tBu-desoxybenzoin (tBuDOB)16 with Hünig's base/MgBr2·Et2O and reaction with 4-tBu-benzoyl chloride.17 Inspired by a reported procedure for the synthesis of bulky BDI ligands,18 double imine condensation between TBPAcAc and 4-mesityl aniline was achieved using TiCl4 as a dehydration agent, giving pre-ligand TBPBDI in 87% yield. The number of signals in both 1H and 13C NMR spectra of TBPBDI suggests a cis–trans conformation of the diimine motif in the solution state, as opposed to a C2v symmetric cis–cis or trans–trans conformation (Scheme 1).
With pre-ligand TBPBDI in hand, several conditions were screened for the cyclodehydrogenation reaction to form a large, extended π-system (see ESI†). Interestingly, when using DDQ and methanesulfonic acid (MSA), full conversion was observed within an hour at room temperature by TLC. Dark crystals suitable for single-crystal XRD were grown from a toluene solution of the crude product at 5 °C and show the formation of a C–N bond as opposed to a C–C bond, leading to an indole-type framework (TBPIndole·HMeSO3, Scheme 2 and Fig. S70, ESI†). Similar oxidative C–N bond formation reactions are known in literature, for example using hypervalent iodide reagents as oxidants.19 We reason that the formation of a C–N bond over the desired C–C bonds is due to the solution-state cis–trans conformation of the diimine motive discussed above. This prompted us to investigate a novel templating approach to aid the favourable positioning of the aromatic rings for C–C bond formation.
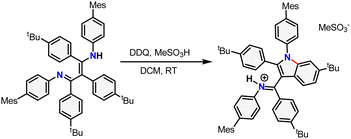 | ||
| Scheme 2 Synthesis of TBPIndole·HMeSO3 from TBPBDI by Scholl oxidation using DDQ and MSA. The newly formed bond is drawn in red. | ||
Inspired by the synthesis of BODIPY-type compounds, as well as several examples of successful π-extension of such compounds using Scholl oxidation,20 we set out to explore the use of a BF2 template for the synthesis of a π-extended BDI from TBPBDI. Reaction of pre-ligand TBPBDI with BF3·OEt2 in the presence of NEt320b gave a boron complex in a 75% yield, showing C2v symmetry in NMR spectroscopy as well as the incorporation of BF2 by 19F and 11B NMR spectroscopy. The structure of TBPBDI-BF2 was further confirmed by single crystal X-ray structure determination (Fig. S71, ESI†).
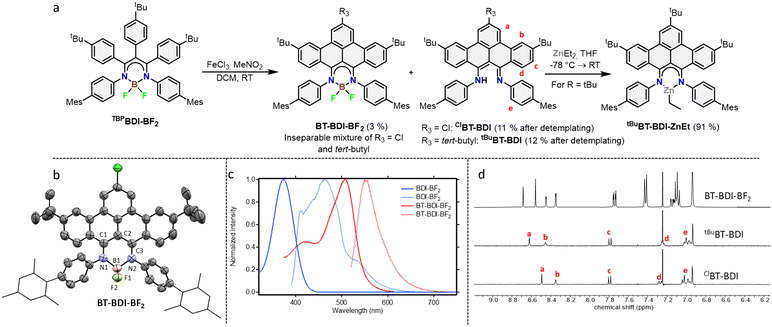
With the boron complex in hand, Scholl oxidation conditions were investigated. Reaction with FeCl3/MeNO2 showed the best results with good conversion towards four main products (Fig. 2a). Two of them were isolated by two consecutive column chromatographic separations. Their 1H NMR spectra reveal highly similar patterns with multiple signals overlapping, pointing towards the formation of two closely related, yet distinct, C2v symmetric compounds. The HRMS of the first compound showed a difference of 4 m/z compared to pre-ligand TBPBDI, suggesting two oxidative dehydrogenations. This, combined with a more detailed analysis of the 1H NMR spectrum (Fig. 2d) confirmed the formation of a benzo[f,g]tetracene backbone on the BDI (tBuBT-BDI). In addition, concomitant loss of BF2 is observed, as evidenced by the 1H-signal at 13.2 ppm corresponding to the amine proton (Fig. S28, ESI†). The aliphatic region of the 1H NMR spectrum of the other compound shows the absence of 9H, i.e. one of the tBu-signals of the benzo[f,g]tetracene backbone (Fig. S33, ESI†). This, combined with HRMS data suggests the substitution of tBu for a chloride (ClBT-BDI). Although chlorination is commonly observed as a side-reaction in FeCl3 mediated Scholl reactions,21 this is, to the best of our knowledge, the first example of regioselective chloride-for-tBu substitution.
The two other products, isolated as one fraction in a 3% total yield, co-crystalized via slow evaporation of a solution in MTBE or Et2O to form orange blocks and were analysed by single-crystal X-ray structure determination. Interestingly, the crystal structure consists of the two previously described compounds – with chloride substitution and without – but coordinated to BF2 (Fig. 2b). This suggests partial deprotection of the BT-BDIs takes place during the reaction. The benzo[f,g]tetracene backbone is found to be curved (Fig. S74, ESI†) and the p-mesityl aniline arms bend out of the BDI plane (Fig. S75, ESI†), likely due to steric repulsion with the benzo[f,g]tetracene unit. The boron centre is nearly tetrahedral with angles around boron all close to 109° (Table S3, ESI†). The delocalization over the N–C–C–C–N binding pocket is clearly seen in the solid-state structure, with both C–N bonds and C–C bonds being of similar length (Table S3, ESI†). The solution state data is in agreement with the solid-state structure, with the 1H NMR spectrum indicating C2v symmetric compounds. Efficient deprotection of BT-BDI-BF2 was achieved by treating the crude reaction mixture with 90/5/5 TFA/DCM/H2O,22 giving a total yield of 12% for tBuBT-BDI and 11% for ClBT-BDI.
The extension of the conjugated system leads to a bathochromic shift in the absorption and emission spectra (Fig. 2c). Comparing TBPBDI-BF2 with BT-BDI-BF2, the highest wavelength absorption maximum – corresponding to the π → π* transition – shifts from 374 nm to 507 nm, a shift of 133 nm. The corresponding emission peaks shifts by 85 nm upon extension of the conjugated system. The smaller Stokes shift observed for BT-BDI-BF2 compared to TBPBDI-BF2 is in agreement with the more rigid structure of the former. It is worth noting, however, that the BT-BDI-BF2 is a mixture of the chloride substituted and tBu-substituted compounds.
To further probe the coordination chemistry behaviour of the BT-BDI framework, we reacted the new ligand tBuBT-BDI with 1.2 equivalents of diethylzinc in THF at −78 °C. Upon reaction, the colour of the solution gradually changed from dark red to dark blue/purple. After evaporation of the volatiles, tBuBT-BDI-ZnEt was obtained as a dark purple powder in 91% yield. The 1H NMR spectrum shows the absence of the –NH proton, as well as the presence of an aliphatic triplet (1.50 ppm) which is part of the same spin system of an aliphatic quartet (0.92 ppm), together corresponding to the ethyl ligand (Fig. S45, ESI†). Although a small amount of THF is still present in the sample according to 1H NMR, this is far below 1 equivalent, which suggests the absence of an additional THF ligand and a trigonal geometry around the zinc centre. Further spectroscopic evidence for the structure of tBuBT-BDI-ZnEt is given in the ESI† (Fig. S51 and S52, ESI†). The UV-vis spectrum of tBuBT-BDI-ZnEt shows a large bathochromic shift of 103 nm compared to the free ligand, shifting from 475 to 578 nm (ε = 9.9 × 103 M−1 cm−1, Fig. S59, ESI†).
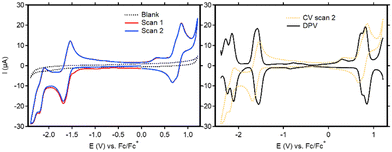
Cyclic voltammetry (CV) was used to study the redox activity of the new ligands and complexes. For BT-BDI-BF2, the first event when scanning cathodically (E1/2 = −1.61 V vs. Fc/Fc+) is fully reversible (Fig. 3, left and Fig. S60, ESI†). The following reversible reduction at approximately −2.2 V vs. Fc/Fc+, as well as the reversible oxidation event on the return scan at approximately 0.8 V vs. Fc/Fc+, each seem to be consisting of two overlapping events. This is confirmed by the DPV (Fig. 3, right), which shows two peaks in the regions of both these events. We attribute these overlapping events to the presence of both the chloride- and tBu-substituted compounds. The observed redox non-innocence shows the relatively facile introduction of two electrons into the conjugated ligand, which is largely in line with what has been observed for phenalenyl-based BDIs.15 The electrochemical HOMO–LUMO gap, taken as the potential difference between the peak potentials in the anodic DPV at −1.58 V vs. Fc/Fc+ and the average peak potential of the two oxidative peaks at 0.77 V vs. Fc/Fc+ is 2.35 eV. This corresponds well with the optical HOMO–LUMO gap, taken from the onset of the absorbance maximum at 475 nm (553 nm), of 2.24 eV. The cyclic voltammetry of the free ligands (Fig. S62–S67, ESI†) is markedly different than that of BT-BDI-BF2. Interestingly, none of the observed electrochemical events are reversible. Furthermore, two extra reduction peaks are observed in the second scan, and additional measurements revealed that these events are both coupled to a single oxidation event at 0.35 V vs. Fc/Fc+ (Fig. S67, ESI†). The irreversibility of the oxidation events, combined with the fact that the oxidations are coupled to two reductive events implies the occurrence of (a) chemical reaction(s) at oxidative potentials for BT-BDI. We hypothesize that through further oxidative dehydrogenation steps, it is possible to form a larger extended π-system, with C–C bond formation between the benzo[f,g]tetracene unit and the rings originating from the 4-mesityl aniline. We are currently investigating this possibility.
In conclusion, we have reported the facile synthesis of a BDI ligand bearing a π-extended benzo[f,g]tetracene (BT) backbone. Using a novel boron-templated strategy, this ligand is obtained together with a second BT-BDI compound, in which one tBu-group has been selectively substituted for a chloride, which is uncommonly observed in Scholl oxidations. This strategy is, to the best of our knowledge, the first reported case in which coordination templating steers reactivity in Scholl oxidation and paves the way for the synthesis of other nanographene ligands. Electrochemical characterization shows the aptitude of the ligand framework to undergo multiple reduction steps, confirming the high redox activity of the ligand. Coordination to a Zn centre further shows that this new ligand framework can be used to explore the coordination chemistry of BDI ligands bearing large extended π-systems.
A. T. thanks the Dutch Research Council (NWO) for funding via Veni grant (Veni.212.039) and Utrecht University for financial support. The X-ray diffractometer has been financed by NWO.
Conflicts of interest
There are no conflicts to declare.References
- J. Wu, W. Pisula and K. Müllen, Chem. Rev., 2007, 107, 718–747 CrossRef CAS PubMed.
- A. Narita, X.-Y. Wang, X. Feng and K. Müllen, Chem. Soc. Rev., 2015, 44, 6616–6643 RSC.
- A. Borissov, Y. K. Maurya, L. Moshniaha, W.-S. Wong, M. Żyła-Karwowska and M. Stępień, Chem. Rev., 2022, 122, 565–788 CrossRef CAS PubMed.
- S. M. Draper, D. J. Gregg, E. R. Schofield, W. R. Browne, M. Duati, J. G. Vos and P. Passaniti, J. Am. Chem. Soc., 2004, 126, 8694–8701 CrossRef CAS PubMed.
- (a) A. Chouai, S. E. Wicke, C. Turro, J. Bacsa, K. R. Dunbar, D. Wang and R. P. Thummel, Inorg. Chem., 2005, 44, 5996–6003 CrossRef CAS PubMed; (b) S. Kammer, H. Müller, N. Grunwald, A. Bellin, A. Kelling, U. Schilde, W. Mickler, C. Dosche and H.-J. Holdt, Eur. J. Inorg. Chem., 2006, 1547–1551 CrossRef CAS; (c) S. Kammer, A. Kelling, H. Baier, W. Mickler, C. Dosche, K. Rurack, A. Kapp, F. Lisdat and H.-J. Holdt, Eur. J. Inorg. Chem., 2009, 4648–4659 CrossRef CAS; (d) M. Schulze, A. Steffen and F. Würthner, Angew. Chem., Int. Ed., 2015, 54, 1570–1573 CrossRef CAS PubMed; (e) J. Shi, J. Fan, Z. Qu, S. Wang and Y. Wang, Dyes Pigm., 2018, 154, 263–268 CrossRef CAS; (f) S. Mardanya, S. Karmakar, M. Bar and S. Baitalik, Dalton Trans., 2015, 44, 21053–21072 RSC; (g) S. Mardanya, S. Karmakar, D. Mondal and S. Baitalik, Inorg. Chem., 2016, 55, 3475–3489 CrossRef CAS PubMed; (h) S. Mardanya, D. Mondal and S. Baitalik, Dalton Trans., 2017, 46, 17010–17024 RSC.
- J. P. Lewtak and D. T. Gryko, Chem. Commun., 2012, 48, 10069–10086 RSC.
- (a) W. Nie, D. E. Tarnopol and C. C. L. McCrory, Curr. Opin. Electrochem., 2021, 28, 100716 CrossRef CAS; (b) X. Qiao, Q. Li, R. N. Schaugaard, B. W. Noffke, Y. Liu, D. Li, L. Liu, K. Raghavachari and L. Li, J. Am. Chem. Soc., 2017, 139, 3934–3937 CrossRef CAS PubMed.
- (a) R. S. Jassas, E. U. Mughal, A. Sadiq, R. I. Alsantali, M. M. Al-Rooqi, N. Naeem, Z. Moussa and S. A. Ahmed, RSC Adv., 2021, 11, 32158–32202 RSC; (b) M. Grzybowski, K. Skonieczny, H. Butenschön and D. T. Gryko, Angew. Chem., Int. Ed., 2013, 52, 9900–9930 CrossRef CAS PubMed.
- (a) L. Bourget-Merle, M. F. Lappert and J. R. Severn, Chem. Rev., 2002, 102, 3031–3066 CrossRef CAS PubMed; (b) C. Chen, S. M. Bellows and P. L. Holland, Dalton Trans., 2015, 44, 16654–16670 RSC.
- (a) C. Camp and J. Arnold, Dalton Trans., 2016, 45, 14462–14498 RSC; (b) P. Ghosh, R. Naastepad, C. F. Riemersma, M. Lutz, M.-E. Moret and R. J. M. Klein Gebbink, Chem. – Eur. J., 2017, 23, 10732–10737 CrossRef CAS PubMed; (c) A. G. Avent, A. V. Khvostov, P. B. Hitchcock and M. F. Lappert, Chem. Commun., 2002, 1410–1411 RSC; (d) O. Eisenstein, P. B. Hitchcock, A. V. Khvostov, M. F. Lappert, L. Maron, L. Perrin and A. V. Protchenko, J. Am. Chem. Soc., 2003, 125, 10790–10791 CrossRef CAS PubMed; (e) S. K. Ibrahim, A. V. Khvostov, M. F. Lappert, L. Maron, L. Perrin, C. J. Pickett and A. V. Protchenko, Dalton Trans., 2006, 2591–2596 RSC.
- J. Ahmed and S. K. Mandal, Chem. Rev., 2022, 122, 11369–11431 CrossRef CAS PubMed.
- (a) A. Mukherjee, T. K. Sen, S. K. Mandal, D. Kratzert, D. Stalke, A. Döring and C. Schulzke, J. Chem. Sci., 2011, 123, 139–144 CrossRef CAS; (b) A. Mukherjee, S. C. Sau and S. K. Mandal, Acc. Chem. Res., 2017, 50, 1679–1691 CrossRef CAS PubMed.
- S. K. Mandal, M. E. Itkis, X. Chi, S. Samanta, D. Lidsky, R. W. Reed, R. T. Oakley, F. S. Tham and R. C. Haddon, J. Am. Chem. Soc., 2005, 127, 8185–8196 CrossRef CAS PubMed.
- A. Mukherjee, T. K. Sen, P. Kr. Ghorai, P. P. Samuel, C. Schulzke and S. K. Mandal, Chem. – Eur. J., 2012, 18, 10530–10545 CrossRef CAS PubMed.
- A. Mukherjee, T. K. Sen, P. K. Ghorai and S. K. Mandal, Sci. Rep., 2013, 3, 2821 CrossRef PubMed.
- X. Ren and H. Du, J. Am. Chem. Soc., 2016, 138, 810–813 CrossRef CAS PubMed.
- S. O. Aderibigbe and D. M. Coltart, J. Org. Chem., 2019, 84, 9770–9777 CrossRef CAS PubMed.
- L. M. R. Hill, B. F. Gherman, N. W. Aboelella, C. J. Cramer and W. B. Tolman, Dalton Trans., 2006, 4944–4953 RSC.
- (a) X. Ban, Y. Pan, Y. Lin, S. Wang, Y. Du and K. Zhao, Org. Biomol. Chem., 2012, 10, 3606–3609 RSC; (b) Y. He, J. Huang, D. Liang, L. Liu and Q. Zhu, Chem. Commun., 2013, 49, 7352–7354 RSC.
- (a) Y. Hayashi, N. Obata, M. Tamaru, S. Yamaguchi, Y. Matsuo, A. Saeki, S. Seki, Y. Kureishi, S. Saito, S. Yamaguchi and H. Shinokubo, Org. Lett., 2012, 14, 866–869 CrossRef CAS PubMed; (b) W. Sheng, Y.-Q. Zheng, Q. Wu, K. Chen, M. Li, L. Jiao, E. Hao, J.-Y. Wang and J. Pei, Sci. China Chem., 2020, 63, 1240–1245 CrossRef CAS.
- (a) J. P. Lewtak, D. Gryko, D. Bao, E. Sebai, O. Vakuliuk, M. Ścigaj and D. T. Gryko, Org. Biomol. Chem., 2011, 9, 8178 RSC; (b) X. Yang, F. Rominger and M. Mastalerz, Angew. Chem., Int. Ed., 2019, 58, 17577–17582 CrossRef CAS PubMed; (c) N. K. S. Davis, A. L. Thompson and H. L. Anderson, Org. Lett., 2010, 12, 2124–2127 CrossRef CAS PubMed.
- M. Yu, J. K.-H. Wong, C. Tang, P. Turner, M. H. Todd and P. J. Rutledge, Beilstein J. Org. Chem., 2015, 11, 37–41 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, NMR/IR, absorption/emission and HRMS spectra, voltammograms. CCDC 2340032–2340035. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4cc01627k |
| This journal is © The Royal Society of Chemistry 2024 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
: