Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Europium(II/III) coordination chemistry toward applications
Elizabeth C.
Lewandowski
 ,
Colin B.
Arban
,
Colin B.
Arban
 ,
Morgan P.
Deal
,
Morgan P.
Deal
 ,
Andrea L.
Batchev
,
Andrea L.
Batchev
 and
Matthew J.
Allen
and
Matthew J.
Allen
 *
*
Department of Chemistry, Wayne State University, 5101 Cass Avenue, Detroit, Michigan 48202, USA. E-mail: mallen@chem.wayne.edu
First published on 4th September 2024
Abstract
Europium is an f-block metal with two easily accessible oxidation states (+2 and +3) that have vastly different magnetic and optical properties from each other. These properties are tunable using coordination chemistry and are useful in a variety of applications, including magnetic resonance imaging, luminescence, and catalysis. This review describes important aspects of coordination chemistry of Eu from the Allen Research Group and others, how ligand design has tuned the properties of Eu ions, and how those properties are relevant to specific applications. The review begins with an introduction to the coordination chemistry of divalent and trivalent Eu followed by examples of how the coordination chemistry of Eu has made contributions to magnetic resonance imaging, luminescence, catalysis, and separations. The article concludes with a brief outlook on future opportunities in the field.
Introduction
Europium is most stable in the +3 oxidation state but has the most accessible +2 oxidation state of all the lanthanides. Eu prefers to bind to donors like O or N and usually has a coordination number between eight and ten. The valence 4f-orbitals of Eu are shielded from the environment by fully occupied 5s and 5p orbitals, preventing participation of the 4f orbital in bonding. EuIII ions typically give rise to 4f–4f transitions but 4f–5d transitions can occur within the far UV region and are minimally impacted by choice of ligand. However, the 4f-to-5d transitions typically exhibited by EuII are heavily influenced by ligands. The dramatic differences in electron configurations between ground and excited states of the Eu ions can be thought of as a switch to turn on or off useful magnetic and electronic properties that arise from interactions with the environment. This review describes contributions of the Allen Research Group, and others, regarding the coordination chemistry of EuII and EuIII, how ligands alter the electronic and magnetic properties of the two ions, and the ability to controllably switch between the two ions. These points of discussion are contextualized within the framework of applications in magnetic resonance imaging (MRI), luminescence, catalysis, and the isolation of Eu from other elements.EuII and EuIII coordination chemistry
One of the coarsest ways to control properties of europium is through the oxidation state (+2 versus +3). Despite only one-electron difference between EuII and EuIII, their electronic energy states are drastically different from each other (Fig. 1).1,2 EuIII has an electron configuration of 4f6 with ground-state term symbols of 7F0–6 and excited states that tend to nonradiatively decay to a long-lived 5D0 state. Most transitions of EuIII occur between these two energy states,3 but these f–f transitions are Laporte forbidden and, consequently, tend to be weak. To increase emission from these transitions, energy transfers to Eu from light-absorbing compounds are used. This process is called the photoinduced electron transfer effect.4 EuII has an electron configuration of 4f7 with a ground-state term symbol of 8S7/2.5 Interestingly, EuII has two common excited state possibilities that result from 4f–4f or 4f–5d transitions. Because the 4f–5d transitions are lower in energy than the 4f–4f transitions, they are more common. Additionally, because 4f–5d transitions are Laporte allowed, they result in intense emissions without the need for photoinduced electron transfer. The dramatic differences in electronic states between EuII and EuIII influence how the ions interact with ligands and, consequently, the electronic and magnetic properties of Eu-containing complexes.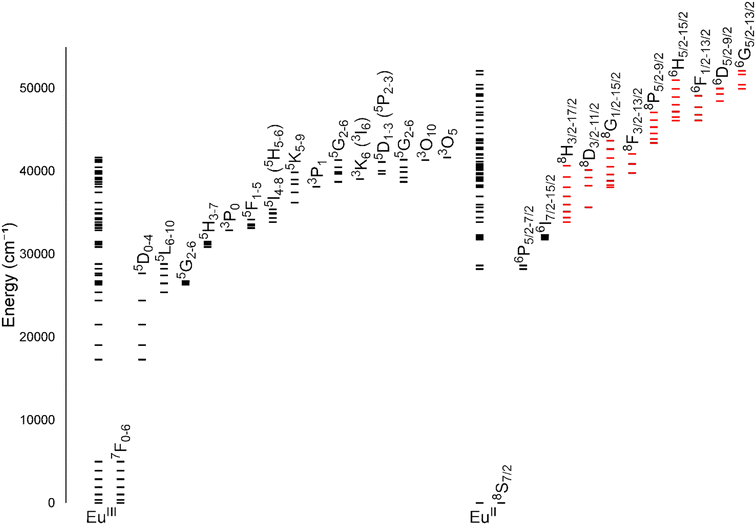 | ||
| Fig. 1 Energy levels for EuIII and EuII. All levels for each ion are shown directly over the ion label. For clarity, subsets of levels are projected to the right of the full diagram and labeled with term symbols. Projected subsets with black lines represent 4f states, and red lines represent 4f5d states. Values for the levels were obtained from previous reports.1,2 | ||
With respect to divalent europium, the ion largely adopts three coordination numbers: eight, nine, and ten, with eight being the most common. Trivalent europium typically adopts coordination numbers of eight and nine because of the smaller atomic radius of EuIII, 1.09 Å, compared to divalent europium, 1.31 Å.6 With lanthanides, coordination number and ligand bulk tend to drive geometry. Readers are referred elsewhere to learn about the possible geometry of Eu ions with coordination numbers 8–10.7–9 EuII complexes adopt geometries like bicapped trigonal antiprisms, hula hoops, and tetracapped trigonal prisms.10–18 Within the hula hoop and tetracapped trigonal prism geometries, there is often a combination of a multidentate ligand and one or more monodentate coordinated solvent molecules. Overall, the shape of the europium complex depends on the ligand and the space available on the metal coordination sphere.
Beyond coordination number and geometry, hard–soft acid–base properties of ligands are another way to tune the properties of europium. Hard–soft acid–base theory is a way to predict and explain the general types of atoms that bond together. In essence, classifying an atom as either hard or soft is dependent on its polarizability, or its response to an electric field.19 Trivalent europium, and other trivalent lanthanides, tend to be thought of as hard acids, but divalent europium is a much softer ion. Consequently, the types of donor atoms used for the two ions tend to be harder donors like oxygen, used more for the trivalent ion, and softer donors like nitrogen and sulfur, used for the divalent ion. The hard–soft properties of donor atoms affect the stability of Eu-containing complexes. For example, sulfur donor atoms shift the oxidation potentials of EuII-containing complexes more positive.20 The ability to tune the electrochemical properties of Eu-containing complexes is an important aspect of ligand design for applications in MRI and separation science. However, substantial overlap exists in the identities of ligand donor atoms that will bind to divalent and trivalent ions.
Solvent selection is another important factor to consider when studying Eu-containing complexes. As previously mentioned, solvent molecules coordinate with the Eu ion, influencing electrochemical potentials. For example, the formal potential of EuII/III is −0.26 V versus the calomel electrode in acetonitrile, a weakly coordinating solvent, but the formal potential of EuII/III is −0.95 V versus the calomel electrode in strongly coordinating solvents like hexamethylphosphoramide.21 Therefore, when performing studies in solution, selection of solvent is an important consideration.
Finally, when working with complexes of EuII, it is critical to prevent inadvertent oxidation to EuIII by atmospheric O2. This prevention involves standard inert atmosphere methods including glovebox and Schlenk techniques and the use of degassed solvents. For applications relevant to MRI, wet gloveboxes are often used for sample preparation in which water is allowed but not molecular oxygen. For many other applications, dry gloveboxes are often used. When preparing samples for solution-phase characterization, including MRI, NMR spectroscopy, and fluorescence or UV-visible spectroscopy, samples need to be sealed to prevent oxygen from entering the sample. Depending on the longevity of the study the samples can be sealed with tape or wax or placed in flame-sealed tubes. For a thorough review of methods to handle divalent europium, readers are referred elsewhere.22
EuII-based contrast agents for MRI
The different electronic and magnetic properties of EuII and EuIII show potential for responsive contrast agent design in MRI. MRI is an imaging modality that noninvasively generates images based on the relaxation times and chemical shifts of nuclei, most often 1H, in a magnetic field. These times and shifts are influenced by paramagnetic molecules called contrast agents, that alter the contrast of images. Clinically, contrast agents are largely used to provide anatomical information, but there is an abundance of research focused on developing contrast agents that provide functional information.23–28 Contrast agents for MRI are often metal-containing complexes that consist of a ligand and a paramagnetic ion. The ligand chelates the ion to prevent dissociation but enables interaction with the environment, for example with water molecules. Current clinically approved contrast agents include a paramagnetic ion, like GdIII, to enhance image contrast by decreasing 1H nuclear relaxation times. The ability to enhance contrast is measured in terms of relaxivity that has units of mM−1 s−1. Properties like electronic relaxation time, magnetic moment, and unpaired electrons are well suited for relaxing 1H nuclear spins, which are important for consideration in the design of contrast agents for MRI.29,30 Aside from GdIII-containing complexes, contrast agents using other paramagnetic metals such as MnII, FeIII, and EuII have also been explored.31–33 Interest in EuII-based contrast agents has arisen because EuII and GdIII are isoelectronic and have similar magnetic properties that are useful in MRI.34 The use of Eu is well suited for inclusion in responsive imaging probes because of the orthogonal imaging properties of EuII and EuIII, enabling complexes of the ions to function as imaging reporters of oxygen concentration.Studying aqueous coordination chemistry is critically important for applications involving aqueous solutions of europium-containing complexes. An important factor of a contrast agent is that it needs to be water soluble and coordinate with rapidly exchanging water molecules. For divalent europium, short metal–water distances, multiple coordinated water molecules, and optimal water exchange rates increase contrast enhancement. However, slow dissociation rates are also important to avoid europium dissociation from complexes. Ultimately, a balance of properties is required to tune all of these interconnected aspects of coordination chemistry. Ligands described in this section that influence coordination chemistry relevant to MRI are depicted in Fig. 2. This focus is shared by the Allen Research Group in the study of europium complexes.
Ligand characteristics and electrochemical potentials
When thinking about ligands to bind EuII in potential contrast agents, the size of the ligand binding pocket has a critical role. This concept was explored by observing differences between a 2.2.2-cryptand, 1, and a 2.2.1-cryptand, 2, with differences in the size of the cryptand cavity.35,36 Ligand 2 is smaller than 1 by the difference of two carbon atoms and an oxygen atom, reducing the cavity size. The stability of Eu-containing complexes of 1 and 2 differs depending on the oxidation state of the Eu ion. The dissociation of EuII-containing cryptates are faster than those of the corresponding EuIII-containing cryptates.35,36 These trends related to cavity size and oxidation state are translatable to other ligands. Overall, study of the kinetic rates and thermodynamic stability of Eu-containing complexes is relevant to MRI because ligands need to possess the ability to coordinate Eu both before and after oxidation from EuII to EuIII.One useful technique for determining dissociation constants is UV-visible spectroscopy; however, the absorption spectra of EuII-containing complexes and the EuII aqua ions often overlap, limiting the ability to monitor dissociation. Inspired by UV-visible studies of murexide with other metals,37,38 murexide was studied as an indicator to aid in the measurement of dissociation rates of Eu. Using EuII1 and murexide, absorption measurements at 485 nm were recorded as a function of time, and the absorption values were used to determine the concentration of EuII.15 The natural log of the concentration versus time indicated a first order relationship with the slope of the plot revealing the dissociation constant. Results from the absorption spectra analysis were consistent with the results determined from electrochemical techniques. When comparing EuII1 and EuII3, dissociation rates increased upon addition of a benzo group to the complex. Differentiation between functional group additions to benzo group complexes is seen when comparing EuII4 and EuII5. The results show that the addition of a variety of electron-withdrawing groups can be differentiated when using murexide with UV-visible spectroscopy to determine dissociation rates. Overall, this study demonstrated that murexide can be used to measure dissociation rates of EuII-complexes. The murexide indicator adds to the toolbox of methods for determining dissociation rates of EuII-containing complexes. Additionally, the inertness of EuII-containing cryptands was studied with respect to transmetallation by endogenous ions such as CaII, MgII, and ZnII based on previous studies.39 Along these lines, EuII1, EuII3, EuII6, and EuII7 were studied in the presence of CaII, MgII, and ZnII and found that EuII-containing cryptates without amide groups are kinetically stable in the presence of the ions.40
Another important aspect of Eu chemistry relevant to contrast agents is the electrochemical potential, which needs to be more positive than the electrochemical potential of water to avoid reduction of water by EuII. EuII-containing complexes in aqueous solutions were studied to report the complexes that are most oxidatively stable.20 Results demonstrated that ligand modifications to the hard–soft acid–base properties of donor atoms shifted the oxidative potential of EuII. Additionally, the electrochemical behavior of Eu-cyclen-based complexes was studied with various ratios of glycinamide to acetate arms.41 It was found that the substitution of an amide for a carboxylate donor in the coordination environment led to more positive oxidation potentials of EuII. The results from this investigation showed the significance of the ligand design in the tuning of the electrochemical potentials of the Eu-containing complexes that are used for redox-responsive contrast agents.
Understanding the thermodynamics and kinetics of oxidation is important to aid in the rational design of new complexes for the potential use of oxidatively responsive EuII-containing complexes in vivo. Toward this goal, the oxidation of EuII-containing complexes, EuII4, EuII8 and EuCl2 was studied.42 The oxidation of EuII by molecular oxygen was observed in varying pH conditions because more acidic media results in slowed oxidation rate.34 Interestingly, the largest oxidant studied, glutathione disulfide, was unable to oxidize EuII, hinting that it is possible to kinetically control oxidation. This kinetic control would later be demonstrated by the Allen Research Group with ligand selection instead of oxidant selection.43
Relaxivity
A key measure of whether a complex will serve as an effective contrast agent is relaxivity. Large relaxivities are desirable because they enable small limits of detection. Variations in structural characteristics of ligands can tune EuII properties that influence relaxivity. As previously mentioned, some of these properties include water-exchange rate, water-coordination number, and molecular reorientation time.Other groups have demonstrated that the EuII aqua ion and cryptate have outstanding water-exchange properties for potential use in contrast agents for MRI.34,44,45 One report illustrates the relationship between relaxivity and the molecular weight of EuII-containing cryptates, EuII1, EuII3, and EuII9.46 The relaxivity of EuII1, EuII3, and EuII9 increases as a function of molecular weight at all field strengths, consistent with complexes of GdIII.47 Further, the correlation between slowing molecular tumbling rates and relaxivity through covalent and noncovalent interactions with macromolecules were investigated.15,48 In this study, cryptate EuII9 was expected to form noncovalent inclusion complexes with cyclodextrins and albumin to increase relaxivity by slowing the molecular tumbling rate. It was found that the relaxivity of EuII9 in the presence of the macromolecules increased relaxivity as a function of the molecular weight of the macromolecules; however, the increase in relaxivity was smaller than expected based purely on molecular weight due to internal rotations.
To address the disparity between molecular weight and rotation, a rigid linker was incorporated to bridge two ions of EuII to study the relationship between rotational dynamics and relaxivity.18 Comparison of the rotational dynamics with the relaxivity of the contrast agents was done by comparing EuII10, a rigid complex, with EuII11, a EuII-containing complex that can rotate freely around the single bonds of the linker between two EuII ions. The nuclear magnetic relaxation dispersion data shows local rotation of EuII11 stemming from the rotation of about the flexible linker between the two cryptates. The more rigid linker in EuII10 correlated to longer global rotational correlation time, resulting in an increase in relaxivity. These results demonstrate that rigidity in linking EuII to other molecules is beneficial for increasing the relaxivity of redox-active, EuII-based contrast agents.
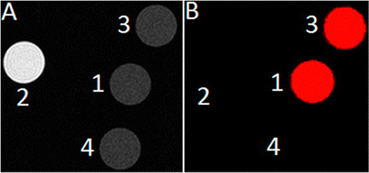
The knowledge of EuII relaxivity and oxidation kinetics was integrated with coordination chemistry to enable screening of new ligands.49 The rational design of ligands to complex both oxidation states of Eu is important to prevent dissociation upon oxidation of the metal; this design requires a method to detect ligands that can bind both the soft, large EuII ion, as well as the harder and smaller EuIII ion. The method involves complexation of ligands to both EuII and GdIII, in which GdIII is used as an MRI-active surrogate for EuIII. In the method, metals are mixed with ligands, and uncomplexed metal is precipitated with phosphate buffer. The resulting mixtures are filtered into a multiwell plate that is imaged using MRI, with bright spots in the image indicating that a contrast-enhancing complex was formed. Additionally, for EuII-containing complexes, a bright spot indicates that a complex was formed that does not reduce water. Dark spots in the images indicate lack of coordination with the ligand or the formation of a EuII-containing complex that reduced water to form EuIII. To test the screening procedure, a set of eight ligands, 1–3, 8, 12–15, were selected due to the range of ability of the ligands to coordinate EuII and GdIII. The screening procedure was able to provide binding information (Fig. 3),49 and was subsequentially used in future studies.43
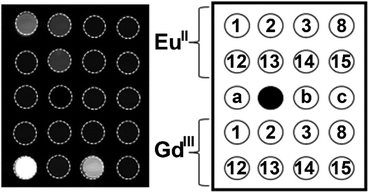 | ||
| Fig. 3 T 1-weighted MR image of a multiwell plate of solutions of EuII1–3, EuII8, EuII12–15 and GdIII1–3, GdIII8, GdIII12–15 at ambient temperature. Label a represents phosphate-buffered saline, b denotes GdCl3, and c represents EuCl2. Adapted from Corbin et al.,49 copyright 2018, with permission from Elsevier. | ||
In vivo studies
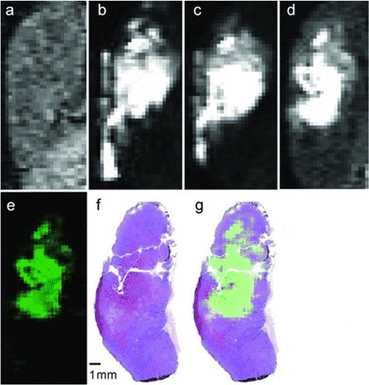
Although EuII was proposed as a redox-active contrast agent around the turn of the century,34 no studies of its use in vivo had been reported before 2015.13 Based upon in vitro findings, in vivo imaging using EuII-containing complexes was pursued. The first report of a EuII-based complex demonstrating an ability to serve as an oxygen-responsive contrast agent in vivo was reported using tumor-bearing and healthy mice.13,50 These initial reports involved two studies: one studying direct injection into hypoxic tumors (Fig. 4)13 and one looking at different injection sites to understand rates of oxygen diffusion.50 Across both studies, areas of less oxygen diffusion corresponded to longer imaging times (up to several hours for hypoxic tumors) and areas of more oxygen diffusion corresponded to nearly instantaneous oxidation (intravenous injections). The ability to differentiate between hypoxic and normoxic tissues has the potential to aid in the study of many diseases. Despite the benefits of having an oxygen-responsive contrast agent based on EuII, these studies are limited by lack of EuIII imaging after EuII is oxidized and by the need for direct injection into sites of interest.Kinetic approaches for slowing EuII oxidation
One of the limitations preventing systemic delivery of EuII-containing contrast agents is their lack of persistence in oxygenated solutions. This limitation confines the use of EuII to injection directly into sites of interest, which is not always feasible. For example, the locations of potential hypoxic regions of interest are not always known prior to imaging. To overcome this limitation, persistence of EuII in oxygenated environments needs to occur. Previous studies of EuII-containing complexes for MRI of hypoxia focused on increasing relaxivity and thermodynamic stability by altering the coordination chemistry of EuII; however, to enable delivery followed by imaging, slowing the oxidation rate of EuII became a target of research.The first example of persistence of EuII in oxygenated solution stemmed from the study of 21 ligands with varying denticities, sizes, donor atom identities, and cavity sizes that were screened for ability to coordinate both EuII and EuIII.43 From this screening process, a tetraamide phosphonate-containing ligand, 16, was identified as being able to coordinate to both EuII and EuIII,43 and was further investigated. Interestingly, previous studies with 16 demonstrated pH-dependent relaxivity with GdIII.51–54 The pH-dependency was of interest to study based on other EuII studies of tetraamine ligands showing temperature-dependent cage formation, contrast enhancement, and slowed oxidation of EuII.55 If similar cages are formed with the phosphonate groups to trap coordinated water molecules, those cages could sterically interfere with the approach of oxygen to slow oxidation, similar to how glutathione could not oxidize EuII8 despite being thermodynamically possible.42 Studies of EuII16 were performed to understand if the phosphonate groups could slow oxidation of EuII as a function of pH.43 UV-visible measurements were performed from pH 7 to 10 to understand EuII persistence within EuII16. As pH increased, the persistence of EuII increased, with EuII half-lives of 6.4 and 12.4 minutes at pH 7 and 10, respectively. Those pH values surround one of the pKa values of phosphonates.56,57 Consequently, it was hypothesized that the longer persistence of EuII in basic solution is due to a cage-like formation of 16, trapping an innersphere molecule of water as a function of the protonation state of the phosphonates. Because of the oxygen resistance observed in the UV-visible measurements, EuII16 was used for systemic delivery studies in healthy mice that were monitored using dynamic contrast-enhanced MRI.43 EuII16 was injected into the tail vein of mice, and contrast-enhanced signal was monitored over time using MRI. The half-life of the contrast enhanced signal is roughly seven minutes, which is consistent with in vitro studies at neutral pH. Overall, this study introduces a new EuII-containing complex that showed persistence in oxygenated solutions during in vitro and in vivo measurements, which is an important step toward systemic delivery of EuII contrast agents for detection of hypoxia.
In addition to increasing persistence using coordination chemistry of phosphonate-containing ligands, an outersphere approach toward increasing persistence was pursued using highly fluorinated ligands in perfluorocarbon nanoemulsions.58 Within this study, EuII was complexed to a perfluorinated, cyclen-based ligand, 17, and subsequently dispersed in an N2-saturated perfluorocarbon/lecithin nanoemulsion to slow oxygen diffusion toward EuII (Fig. 5). The multiple interfaces between water and lecithin and lecithin and N2 surrounding perfluorocarbons were hypothesized to slow oxygen diffusion to EuII based on reports of gas diffusion across interfaces.59,60 Additionally, saturation of the perfluorocarbon emulsion with N2 was also hypothesized to slow diffusion of oxygen in the vicinity of Eu. The perfluorinated ligand and nanoemulsion are useful for detection of EuII concentration via19F-MRI measurements because 19F-signal increases as EuII concentration decreases due to EuII line broadening effects.58 To understand the relationship between 19F-signal and EuII, 19F-NMR measurements of EuII17 and EuIII17 in equimolar concentrations were acquired in degassed and oxygenated perfluorocarbon mixtures. These studies revealed that the oxidation state of EuII is responsible for almost all the signal change in 19F-NMR measurements. Contrast enhanced signal of solutions of EuII17 within a perfluorinated nanoemulsion and a solution of EuII15 were measured within thigh muscles of healthy mice using MRI measurements. The perfluorinated nanoemulsions of EuII17 revealed persistence of EuII in detectable amounts for at least 30 minutes (compared to <5 minutes for a control complex), indicating that the nanoemulsion is able to slow the diffusion of oxygen to EuII. This study represents an important step toward increasing the persistence of EuII signal through an outersphere approach. Overall, these studies show how the surrounding environment of EuII can influence EuII persistence in oxygenated solutions, which is an important step toward hypoxia detection using MRI via systemic delivery of contrast agents.
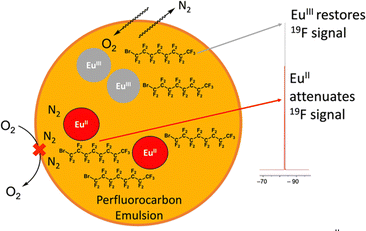 | ||
| Fig. 5 Cartoon representation of EuII17 in lecithin/perfluorocarbon emulsion. The orange represents a perfluorocarbon mixture surrounding EuIII17, the grey circle, and EuII17, the red circle. | ||
Multimodality
With the ability of EuII-based contrast agents to generate images in vivo, limitations arise from the lack of the ability to image the EuIII ion after oxidation of EuII. As mentioned previously, contrast enhancement of Eu is not observed in the oxidized EuIII state, so methodology for designing a contrast agent that can functionally image the trivalent oxidation state is important. The strategy that the Allen Research Group has taken is to study modalities that have demonstrated the ability to image EuIII with other systems and then try to combine those modalities with MRI of EuII. Additionally, the use of multimodal probes can overcome the concentration dependency of responsive contrast agents.61 The remainder of this section highlights some of those attempts.One study involved encapsulation of a EuII-containing complex within liposomes to create a dual-mode contrast agent that is oxidation-responsive, and upon oxidation of EuII to EuIII would result in a probe for chemical exchange saturation transfer (CEST) MRI.62 CEST is a method of using exchangeable protons to create images using MRI.63,64 Liposomes were selected because the inner cavity can hold water-soluble contrast agents, increasing the ratio of water protons associated with the liposome to bulk water protons. The designed liposome system uses T1 enhancement that visualizes the presence of the EuII oxidation state along with CEST that visualizes both EuII and EuIII.61 From observation of the potential outcomes from the CEST and T1 measurements, the oxidation state of the Eu ion can be determined without knowledge of the concentration of Eu. The liposome encapsulated EuII-containing complex enabled the observation of the first oxidation-responsive dual-mode contrast agent designed around the oxidation state of Eu. Moving from liposomes to small molecules as CEST probes, the use of EuII/III-cyclen based derivatives as oxygen-sensitive MRI contrast agents was explored.65 In this study, EuII8 provides T1-weighted contrast enhancement but no CEST signal, and EuIII8 produces CEST signal but no T1-weighted signal enhancement (Fig. 6). The small molecule probes were characterized by a variety of methods to characterize both oxidation states of the complex. Ultimately, the ability to image before and after oxidation of EuII was demonstrated.
Another system studied as potential multimodal contrast agent integrated MRI and photoacoustic imaging techniques.66 In this system, MRI has the ability to scan large areas and photoacoustic imaging has the ability to create images of areas that are accessible by light. To determine the potential of having a redox-responsive multimodal contrast agent for both MRI and photoacoustic imaging, Eu8 was selected based on previous studies demonstrating that the divalent complex is yellow in color, to enable absorption for photoacoustic imaging, and that it is an effective contrast agent for MRI.65 Eu8 was effectively able to function as a multimodal contrast agent; however, because both modalities could only be detected with EuII and not EuIII, the system was not ideal for overcoming the challenge of detecting EuIII.
In addition to CEST MRI, the use of 19F signals can be combined with 1H MRI in multimodal probes, because 19F MRI has been used in vivo in various applications.67–70 A major concern of using 19F signal is finding the amount of fluorine that enables solubility of EuII-containing complexes in water and results in detectable signal. One study aimed to address this concern by studying changes based on the number and placement of fluorine atoms in a set of ligands.55,71 One of the ligands, 15, contains four p-trifluoromethylbenzyl groups that when complexed with Eu form a pocket where a coordinated water molecule is caged in the solid state.55 The trapping of the water molecule is unique compared to other tetraamide complexes that have arms that point away from each other instead of toward each other.72–74 Oxidation of EuII to EuIII enables the presence of an observable 19F signal and the detection of the EuIII-containing complex.55 The ratio of 19F and 1H signals could also be potentially used for ratiometric imaging beyond just observing EuIII after oxidation of EuII.71 Ratiometric imaging is important because of its ability to lead to quantification of medically relevant biomarkers like oxygen concentration in hypoxic environments. Quantification of hypoxia is difficult because the MRI signal produced using EuII-based contrast agents is dependent on the concentrations of oxygen and EuII. Based on previous studies involving multimodal imaging and the relationship of 19F signal and EuII signal, levels of hypoxia can be determined ratiometrically using a fluorinated EuII-based contrast agent.71 and comparing the 1H and 19F-MRI signals produced from a dual-mode redox responsive contrast agent.64 In this study, hypoxia was detected by plotting the T1-weighted 1H-MRI signal with 19F-MRI signal. These results enable quantification without the knowledge of the concentration of Eu.
Eu in luminescence
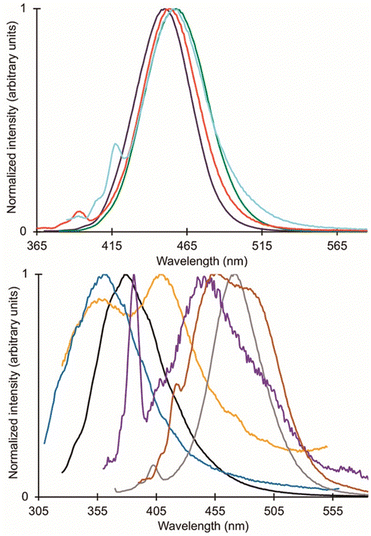
Within the Allen Research Group, interest in EuII luminescence stemmed from the discovery of EuII-aza-[2.2.2]-cryptate,75 EuII12, that occurred during the search for new contrast agents for MRI. Luminescence refers to the emission of light after a molecule has been excited by a specific source like heat, light, or energy transfer. The luminescence of Eu ions is relevant to lighting, electronic screens, and imaging. Further, the luminescence of both EuII and EuIII can be tuned with ligand design and coordination chemistry. Within this section, general information about the orbital transitions relevant to EuII luminescence involving ligand design studies for EuII and EuIII are described. Ligands described in this section that influence coordination chemistry relevant to luminescence are depicted in Fig. 7. For detailed descriptions of the luminescence of EuII and EuIII, readers are referred elsewhere.3,76–784d–5f transitions of EuII-containing complexes
The broad electromagnetic spectra of EuII-containing complexes in the UV-visible range arise from 4f6–5d1 transitions that are tunable using coordination chemistry. Before understanding how ligands affect these transitions, it is helpful to describe some general information about 4f7–4f7 and 4f6–5d1 transitions of EuII. EuII undergoes 4f7–4f7 transitions, manifested as narrow, low-intensity peaks in luminescence spectra and 4f6–5d1 transitions that give rise to broad, intense peaks. Often because of the difference in peak intensities, the 4f6–5d1 transitions obscure 4f7–4f7 transitions.78 Although 4f6–5d1 transitions are broad, the intensity of these peaks can be quenched by nonradiative decay when OH and NH oscillators are within the EuII coordination sphere. Because OH and NH oscillators decrease the intensity of EuII luminescence, it is useful to avoid solvents and ligands containing OH and NH groups. Overall, the diffuse nature of the 5d orbitals relative to the 4f orbitals, which are shielded from the environment, enable tuning of the 4f6–5d1 transitions using coordination chemistry. For more information describing the EuII orbital transitions and energy splitting, readers are referred elsewhere.5,79–82Divalent Eu complexes used in luminescence studies
As mentioned earlier, EuII12, was initially pursued as a potential contrast agent for MRI. Ligand 12 was synthesized to be structurally similar to [2.2.2]-cryptand, 1, but with N donors instead of O donors. Using N donors instead of O donors was targeted to tune the hard–soft acid–base matching between EuII and the ligand to tune the EuII/III redox couple. Crystal structures of EuII1 and EuII12 both reveal nine-coordinate geometries in which all donor atoms and one counterion coordinate to EuII.14,75 However, despite the similarities in the solid state, EuII12 shows extremely different emission wavelengths and quantum yields in solution compared to EuII1. The excitation and emission maxima of EuII12 are 415 and 580 nm, respectively, and EuII1 has excitation and emission maxima of 259 and 471 nm, respectively.81 The emission wavelength of EuII12 is shifted relative to EuII1, likely due to lower energy 4f–5d transitions. The quantum yield of EuII12 is 26% in basic aqueous solution and 37% in methanol.82 The crystal structure of EuII12 reveals a chloride ion in the inner coordination sphere that is in a position to form hydrogen bonds with NH groups of the ligand.75 Conductivity studies to understand the coordination of Cl− to EuII12 in solution showed that Cl− is likely coordinated to EuII in aqueous solution. The chloride ion enables high quantum yield because it blocks coordination of nonradiatively quenching OH oscillators in the innersphere of EuII12 in basic aqueous solution. The aqueous coordination of Cl− to EuII12 is explained using hard–soft acid–base theory because EuII is a soft ion and coordinates better to Cl− than OH−.19,83 The differences in luminescence between the two complexes is explained through computational studies comparing the differences of EuII1 and EuII12.81 The computational calculations indicate that EuII1 and EuII12 have similar excitation spectra but different emission spectra because EuII12 undergoes a geometry change to a more stable conformation. The geometry change in EuII12 increases the splitting of EuII d-orbitals and results in a more red-shifted emission spectra by bringing the 4f and 4d orbital energies closer together than without the geometry change in EuII1 (Fig. 8). The initial report of EuII12 opened several studies in the Allen Research Group focused on exploring the luminescence of EuII12 and other selected complexes.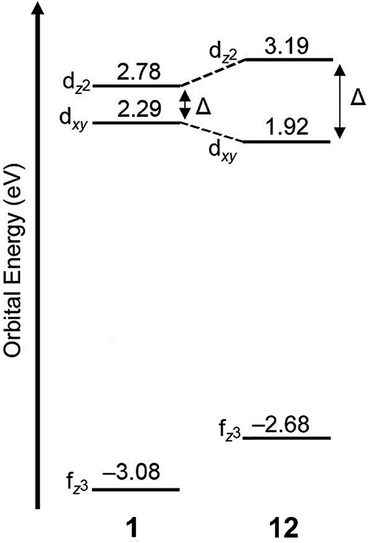 | ||
| Fig. 8 Calculated d-orbital splitting of EuII1 and EuII12. Adapted from Corbin et al.,81 copyright 2018, with permission from Elsevier. | ||
Because of the seemingly important role of the chloride ligand in the luminescence of EuII12, one subsequent study of EuII12 investigated how the identity of counterions influences excitation and emission peaks.10 Ligand 12 was used to occupy eight coordination sites of EuII so only one coordination site was left for the following ions: Cl−, Br−, I−, and PF6−. Initial luminescence measurements were performed in methanol and showed similar absorbance and emission values for each counterion in solution. Acetonitrile was also used because it is a weaker ligand than methanol, decreasing competition between solvent and counterion for binding to EuII.84 In acetonitrile, a blue shift in emission spectra is observed with Cl− and Br− counterions in EuII12 relative to the same complexes in methanol, whereas I− and PF6− counterions do not result in changes in emission from methanolic solutions.10 The blue shift arising from Cl− and Br− is attributed to those ions binding more preferentially to EuII than acetonitrile. The I− and PF6− ions are less likely to coordinate,84 in which case acetonitrile coordinates to EuII12 to complete a nine-coordinate geometry or no monodentate ligand coordinates, resulting in an eight-coordinate geometry. This study demonstrates the importance of counterions and solvent selection for EuII luminescence measurements.
Another avenue of study of the luminescence of EuII explored the effect of changing the secondary amines in 12 to tertiary amines.11 In this study, the secondary amines of 12 were methylated to yield 18 that has increased steric bulk and slightly stronger donors compared to 12. The initial expectation with 18 was that the tertiary amine donors would split the d-orbitals of the EuII complex more than the secondary amines within 12, resulting in more red-shifted emissions with EuII18 than with EuII12. Additionally, the lack of NH oscillators on the ligand and steric bulk was expected to increase luminescence quantum yield by minimizing pathways for nonradiative quenching. The crystal structure of EuII18 revealed that the methyl groups blocked all inner sphere coordination sites for monodentate ligands, resulting in EuII adopting an eight-coordinate geometry in the ligand. Upon studying the luminescence of EuII18, emission wavelengths are more shifted toward the UV region compared to the emission wavelengths of EuII12. A computational study suggested that the change in geometry was the dominant factor that regulated the emission wavelength. Despite the unexpected luminescence wavelength, the quantum yield of the EuII18 was 47%. This quantum yield was the largest reported for EuII in aqueous solution at the time and is due to the methyl groups on the tertiary amines shielding EuII from luminescence quenchers in solution. This study highlights the importance of geometry and the effect of quenchers on the luminescence of EuII.
In an attempt to understand what components of 12 were needed to control the luminescence of EuII, several ligands of smaller denticity that represent parts of 12 were studied with EuII.85 Within this study, absorbance measurements were compared of EuII in the presence of nineteen ligands of varying degree of functionalization, denticity, and structure. When comparing degree of functionalization of amines between ligands 19–25, the secondary amines on linear and macrocyclic ligands bathochromically shift the absorbance of EuII more than primary and tertiary amines. The absorbance of EuII did not shift greatly when comparing linear and macrocyclic ligands 20 and 23–25, except for an additional shoulder reaching into the blue-light region for 23. Finally, increasing denticity of both macrocyclic and linear ligands further blue-shifted absorbance spectra of EuII, likely due to the chelating effect. Overall, secondary amines, macrocyclic ligands, and increasing denticity shifted the emission maxima from the UV toward the blue region. This study compared ligand characteristics necessary to understand and control the luminescence of EuII, and it is an important contribution toward rational ligand design.
Outside of the Allen Research Group, others have investigated how varying donor atoms within ligands affect emission maxima, luminescence lifetime, and redox potential of EuII. Liu, Bian and coworkers, compared 1 against ligands with fewer oxygen donors, resulting in ligands 26 and 27.86 The photophysical properties in the solid state and in solution were studied of each EuII complex. Absorption and emission maxima of EuII-complexes in methanol shifted toward the blue-light region when ligands had fewer oxygen donors and more nitrogen donors. Specifically for emission maxima, there was a shift of 19 nm toward the blue region when the number of N donors was increased, consistent with a greater d-orbital splitting of EuII. Luminescence lifetime of EuII also increased with more N donors than O donors when in methanol. Luminescence lifetime also increased with more N donors by ∼175 ns because as the number of N donors increase, the excited state of EuII is stabilized. Conversely, emission maxima and luminescence lifetime of EuII with ligands containing more O donors decreased. Overall, an increasing, linear trend was found between the number of O and N donors and the emission maxima and luminescence lifetime of EuII complexes. Within this study, the different effects of N and O donors for EuII luminescence were compared to understand the tunability of EuII properties that are important for light-emitting diodes.
Following studies with 12 and 18, further studies to tune EuII electronic properties were undertaken using another ligand, tris[2-(2-methoxyethoxy)ethyl]amine, 28.87 Ligand 28 is structurally similar to 1, but lacks one tertiary amine, making it acyclic and more flexible than 1. EuII28 was of interest to investigate how flexible acyclic ligands influence the properties of EuII with respect to coordination number, geometry, bond length, and luminescence spectra. Within this study, 1 and 28 were complexed with EuII and studied initially in the solid state. Complexes of EuII1 contain EuII with coordination numbers of ten and square antiprism geometries, and complexes of EuII28 contain EuII with coordination numbers of nine with muffin geometries.75,87 The solid-state structures show slight differences in bond lengths and bond angles of EuII1 and EuII28, which were likely due to differences in counterion and solvent molecule binding between each complex. To further understand coordination environment with different counterions, absorption and luminescence spectra of each complex in solution were studied (Fig. 9). The UV-visible spectra for EuII1 and EuII28 show almost no dependence on counterion identity for most complexes of EuII1 and EuII28, with absorbance peaks centered around 320 nm.87 For complexes of EuII1, the emission and excitation spectra did not vary greatly between different coordinating counterions, indicating that different counterions do not influence the luminescence of EuII1 significantly in acetonitrile. Despite the lack of shifts seen in excitation and emission spectra for EuII1, complexes of EuII28 with different counterions have a wide variety of emission and excitation wavelengths, spanning an emission maxima range of 355 to 525 nm. The wide range of wavelengths recorded implies that the flexibility of 28 enables innersphere interactions of counterions with EuII, splits d-orbitals, and influences geometry in solution. This study highlights the difference between flexible, linear ligands, and macrocyclic ligands, and how that difference alters the properties of EuII. The ligands studied for luminescence with EuII can also be used with other lanthanides to elicit similar trends in luminescence properties.88,89 Further, the luminescence studies of EuII have influenced ligand design for applications in catalysis, lighting, and imaging.
Eu in catalysis
Catalytic studies in the Allen Research Group were initially inspired by luminescence-decay measurements of EuIII toward understanding catalytic mechanisms. Additionally, luminescence studies of EuII led to studies of photoredox catalysis with the ion. Ligands described in this section relevant to catalysis influence are depicted in Fig. 10. Within this section, an overview of studies about the role of EuII and EuIII in catalytic mechanisms and ligand design are described.EuIII precatalyst studies
One of the first studies of catalysis in the Allen Research Group involved understanding the role of EuIII in the Mukaiyama aldol reaction.90 The Mukaiyama aldol reaction uses Lewis acids, including EuIII(OTf)3 (OTf = trifluoromethanesulfonate), as catalysts to form carbon–carbon bonds in aqueous solution.91,92 Importantly, Eu(OTf)3 is able to catalyze this reaction in aqueous conditions.93 The mechanism of how OTf dissociates from EuIII was studied by monitoring luminescence decay during the Mukaiyama aldol reaction to understand water coordination to EuIII. Luminescence decay is an analytical technique to measure water-coordination numbers of EuIII and TbIII. The technique was pioneered by Horrocks and others,94–97 and it is commonly used in the study of contrast agents for MRI to measure water-coordination number. In applying the technique to aqueous catalysis, information about the behavior of EuIII(OTf)3 as a precatalyst in aqueous solutions can be obtained.90 In 100% water solutions, luminescence measurements showed that EuIII coordinates to about eight water molecules. In 20% or less water in tetrahydrofuran solutions, roughly five water molecules were coordinated to EuIII, indicating that OTf almost completely dissociates. To understand how benzaldehyde, a reactant in the Mukaiyama aldol reaction, interacts with the EuIII catalyst, benzaldehyde was spiked into solutions of EuIII(OTf)3 at various ratios of water to tetrahydrofuran. In solutions of less than 1% water in tetrahydrofuran, benzaldehyde partially displaced water, indicating that benzaldehyde interacts with EuIII during the reaction. The maximum overall yield for the Mukaiyama aldol reaction using EuIII(OTf)3 was at 20% water in tetrahydrofuran, which is likely because there is the most interaction with benzaldehyde in that solvent due to few OTf ions coordinating to EuIII.90,98 Overall, this study demonstrated how luminescence measurements of EuIII can be useful in elucidating catalytic mechanisms.Ligand design for EuIII-containing precatalysts
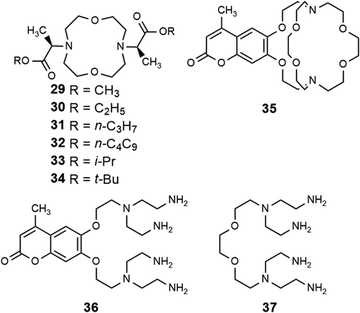
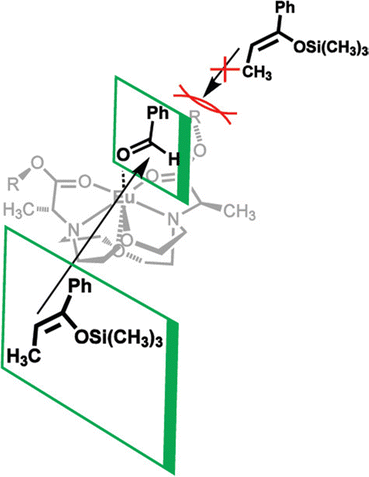
In addition to adapting analytical techniques from MRI to study catalysis in aqueous solution, ligand design from MRI was adapted to synthesize enantioselective precatalysts.99 Ligands 29–34 were inspired by contrast agents involving 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid. Modifications were incorporated to make the ligands chiral and provide open coordination sites to promote reactivity. Importantly, because of the labile nature of ligands with small denticities with trivalent lanthanides, large denticities are needed to maintain complexation in aqueous solution. The synthesized ligands had various ester groups attached with different chain lengths and bulkiness, that controlled substrate binding to EuIII to improve enantioselectivity of reactions. The ligands synthesized had six sites for coordination to EuIII, multiple stereocenters, and C2 symmetry, to promote metal–substrate binding from either side of the precatalyst. Catalytic studies demonstrated that linear ester groups enable larger yields than bulky ester groups and improve enantioselectivity. To understand the catalytic mechanism, luminescence-decay measurements were acquired for all EuIII-containing complexes during catalytic reactions. When the benzaldehyde substrate was added into the reaction, water-coordination number to EuIII decreased, implying benzaldehyde was coordinating to EuIII. Proposed transition states of benzaldehyde to EuIII-containing complexes suggests that the benzaldehyde substrate coordinates to EuIII and is blocked by the ester group on one side enabling the other silyl enol substrate to only attack from one side, resulting in enantioselectivity (Fig. 11).99To further understand the proposed transition state of EuIII-containing complexes and the aldehyde substrates, a variety of aldehyde and silyl enol ether substrates were used in different reactions to monitor enantioselectivity.97 The yields of the various substrates using EuIII-containing complexes as catalysts had some of the largest stereoselective yields reported in aqueous solution. Further support for the proposed transition state was obtained by demonstrating that as bulkiness of the aldehyde substituent increased, the yield decreased. One study focused on ligand design for EuIII-containing precatalysts, specifically how changing functional groups from esters to alcohols, carboxylic acids, and amides influences catalytic yields.100 From these studies, catalytic yields varied between 6 and 20% for ligands with alcohols, carboxylic acids, or amides due to the lack of steric bulk compared to previously studied ester groups. Overall, the results of these studies demonstrate how adapting ligand motifs from MRI enable the generation of highly enantioselective catalysts in aqueous conditions, where lanthanide complexes tend to dissociate, resulting in outstanding enantioselectivities of the aqueous Mukaiyama aldol reaction. These studies further demonstrate the utility of luminescence-decay measurements in elucidating catalytic mechanisms of EuIII-containing complexes.
Photoredox catalysis
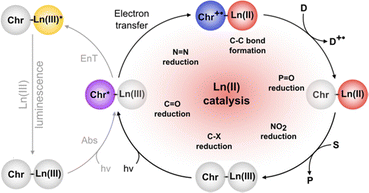
The previous section described the use of EuIII as a Lewis acid catalyst. Observations of the luminescence of EuII inspired the use of that ion in photoredox catalysis. This section describes photoredox catalysis using EuII, which uses light to excite EuII into a state where it becomes a potent reductant.Photoredox catalysis uses a redox-active catalyst to transfer an electron to a substrate to form products during organic reactions, typically using transition metals as catalysts.101,102 Previous luminescence studies of EuII12 showed that the excitation and emission maxima were blue shifted compared to EuII1, with luminescence lifetimes of 0.98 μs.75 Both of these qualities are desirable in photoredox catalysts, which led to studies using EuII12 as a potential photoredox catalyst. First, the excited-state potential was calculated using cyclic voltammetry. The excited state potential of EuII12 is −3.0 V vs. Ag/AgCl,82 which is more reducing than SmI2, a commonly used metal for photoredox catalysis.103–105 The reactivity of EuII12 was tested using reductive coupling reactions of benzyl chloride to form 1,2-diphenylethane.83 An in situ mixture of one equivalent of EuII, 12, and benzyl chloride were reacted in the presence of 460 nm blue light in methanol, which gave a 85% yield of 1,2-diphenylethane and 4.7% of toluene in 30 minutes. Control reactions revealed that no product formed when blue light, EuII, or 12, were omitted, indicating all three components are needed to form product. To investigate why there were differences in quenching and product yields for the substrates, cyclic voltammograms were acquired of each substrate to find the cathodic peak to compare to the excited-state potential. The cathodic peak, indicating reduction, of (CH3)3CCl, C6H5Cl, CH2CHCH2Cl, and C6H5CH2Cl were −3.05, −2.93, −2.35, and −2.34 V, respectively vs. silver/silver chloride. Once the electron transfer mechanism was studied, catalytic reactions of Eu and 12 were investigated using benzyl chloride reductive coupling reactions. Within these reactions, EuCl3, 12, and Zn0 were used as catalytic precursors, with Zn as a sacrificial reducing agent. UV-visible absorbance and fluorescence measurements of EuCl3, 12, and Zn0 show that Zn0 reduces EuIII to EuII but is not able to perform reductive coupling. Benzyl chloride reactions at 10 mol % of EuCl3 and 12 in blue light over six hours gave 80% yield of 1,2-diphenylethane and 11% of toluene. Catalyst loading was also studied by varying the amount of EuIII and 12. At 5, 1, and 0.5% loading of EuIII and 12, the yield of 1,2-diphenylethane was 71, 70, and 60%, respectively, with increasing catalytic turnovers at lower catalyst loading. As the catalytic loading was decreased, toluene yield increased. All reactions in this study were performed under anhydrous conditions, so to understand how small concentrations of water affected these reactions, EuCl3·6H2O was used in a wet glovebox, allowing water but no molecular oxygen within the atmosphere. The yield for the wet reaction was 80%, indicating that water does not affect the precatalyst. The proposed catalytic mechanism for this reaction is EuII12 is excited by blue light to form EuII12*. EuII12* transfers an electron to benzyl chloride that ultimately leads to a reductively coupled product and oxidation of EuII to EuIII and 12. Zn0 reduces EuIII to restart the cycle (Fig. 12).77 This study showed the first visible-light promoted EuII-based photoredox catalysis and was an important step in using Eu-containing complex for photoredox catalysis.
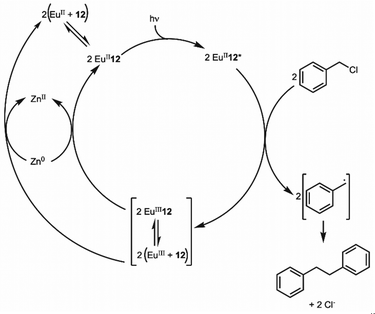 | ||
| Fig. 12 Proposed photoredox catalytic mechanism of EuII12 for reductive coupling reactions of benzyl chloride. | ||
Beyond ligand 12, previously studied complexes of EuII that shifted the absorbance spectrum of EuII into the visible light region from the UV based on various ligand structures were studied to understand their ability as photoredox catalysts.85 To understand the excited-state potentials, cyclic voltammograms of EuII19, EuII21, EuII22, and EuII23 were obtained. The excited-state potentials of the complexes were between −2.3 and −3.2 V versus natural hydrogen electrode. More negative excited-state potentials are useful because they enable the reduction of a wide range of substrates. To study the catalysis-promoting ability of each complex, benzyl chloride coupling reactions were performed in blue light with 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ligand-to-metal ratios to enable complex formation in solution. At large metal-to-ligand ratios, EuII22 produced low yields and precipitated. The primary amine complex EuII21 had the largest yields compared to secondary and tertiary amine-containing ligands. For EuII21, moderate and large ligand-to-metal ratios produced good yields, but large ligand-to-metal ratios produced multiple alkylated byproducts. Comparisons of yields of EuII12 and EuII21 show that cryptates like EuII12 are less likely to form ZnII-containing complexes due to the macrocyclic effect that improves overall product yield by increasing catalytic turnover. This study demonstrated the importance of ligand design for photoredox catalysts and how ligand structures and coordination chemistry can influence catalysis.
1 ligand-to-metal ratios to enable complex formation in solution. At large metal-to-ligand ratios, EuII22 produced low yields and precipitated. The primary amine complex EuII21 had the largest yields compared to secondary and tertiary amine-containing ligands. For EuII21, moderate and large ligand-to-metal ratios produced good yields, but large ligand-to-metal ratios produced multiple alkylated byproducts. Comparisons of yields of EuII12 and EuII21 show that cryptates like EuII12 are less likely to form ZnII-containing complexes due to the macrocyclic effect that improves overall product yield by increasing catalytic turnover. This study demonstrated the importance of ligand design for photoredox catalysts and how ligand structures and coordination chemistry can influence catalysis.
EuII-based photoredox catalysis was reported by Borbas and coworkers using chromophore-based ligands.106 They investigated how chromophores excited by blue light transfer energy to reduce EuIII to EuII, to perform a variety of reductive couplings and functional group transformations. A series of three ligands with varying denticity and donor atoms were studied, with 35 and 36 having 6,7-oxycoumarin as a chromophore, and 37 having 7-aminocarbostyril as a chromophore. The excited-state potentials of these chromophores are more negative than −1.88 V vs. ferrocene/ferrocenium. These potentials are negative enough to reduce EuIII to EuII. To test the reactivity of EuIII35–EuIII37, several reductive coupling reactions with benzyl halide-based substrates and reduction reactions for sulfoxide, diazo, imine, azo, alkyl, phosphonate, aldehyde, ketone, and nitrile groups were performed. In most of these, EuIII35–EuIII37 was successfully reduced to EuII35–EuII37 resulting in excellent yields when in the presence of blue light and a sacrificial reductant (Zn0 or diisopropylethylamine). Control experiments showed that no product formed without EuIII, 35–37, or blue light. The proposed mechanism for this reaction is a photoinduced electron transfer from the chromophore to EuIII results in the formation of EuII that transfers an electron to a substrate (Fig. 13). This mechanism was confirmed using electron paramagnetic spectroscopy. The results of these studies showed that Eu-based photoredox catalysts using a chromophore was capable of multiple reductive coupling and reduction reactions through photoinduced electron transfer.106
EuII in separations
Applications of Eu, including MRI and catalysis, are only possible with stable feedstocks of the element. With the demand for rare-earth elements, including Eu, increasing because of their use in modern society, there is a need for efficient separation, extraction, and recovery of these elements. Common approaches for extraction involve sulfuric acid to leach lanthanides from ores or hydrochloric acid and heat to process rare-earth oxides to purify the elements.107 One limitation of these processes is that they generate considerable amounts of waste and are not highly selective for isolating target elements. Many developments in this area involve the principles of coordination chemistry to enhance the separation, extraction, and enrichment of rare-earth elements while attempting to minimize waste. An example by Binnemans and coworkers is the report of liquid–liquid extraction using nitrogen- or phosphorus-containing ionic liquids to trap Eu and then precipitate Eu salts with water.108,109 Aside from using ionic liquids, liquid–liquid extraction methods have also been studied with photochemical separation methods in alcoholic solutions to selectively precipitate lanthanides.110 Although the coordination chemistry of all the rare-earth elements is similar in many aspects across the series, methods have been developed to target the chemistry of individual elements.111–113 Some coordination chemistry factors that can be tuned to increase selectivity are the pKa values of ligand donor atoms, kinetic inertness, thermodynamic stability, and ligand denticity. Ligands described in this section that influence coordination chemistry relevant to separations are depicted in Fig. 14.Solid–liquid extraction is another method that uses coordination chemistry to separate rare-earth elements from each other.114 Solid–liquid extraction involves a solid support, which is often chemically modified with ligands, that collects metals from solution. After a metal ion of interest coordinates with the ligand on the support, and other metal ions are removed by washing, the metal ion of interest is removed from the ligand by washing with a different solvent. Solid–liquid extraction is often tuned through the ligand attached at the solid support, because ligands can bind rare-earth elements with a range of affinities. The interaction between the solid support and the ligand can also be tuned through covalent or noncovalent interactions. Solid–liquid extraction has gained interest because of its specificity and the small amount of waste it produces, and will be further discussed within this section.
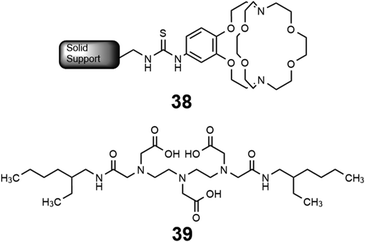
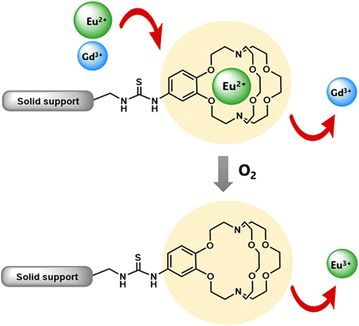
Some methods of separation specific to the isolation of Eu from other elements involve reducing EuIII to EuII,115–122 but those methods often require many steps to obtain high purity Eu. One collaboration with the Dittrich Research Group involved the hypothesis that 1 covalently bound to a solid support to form 38 would enable separation of Eu from Gd.123 This hypothesis is based on these two elements having similar coordination chemistries when they are both in the +3 oxidation state but having large differences in coordination chemistry when Eu is in the +2 oxidation state. The change in coordination chemistry between EuII and GdIII arise from differences in atomic radii and charge density. Further inspiration for this hypothesis stems from reports that show 1 selectively binds divalent lanthanides over trivalent lanthanides.36 The atomic radius of EuII enables the ion to fit within 1 and coordinate more effectively with faster rates of association and slower rates of dissociation than EuIII.36 The resulting thermodynamic and kinetic differences between EuII and trivalent lanthanides with 1 increase the efficiency of separations.123 To separate Eu and Gd using solid-supported cryptands, a mixture of EuII and GdIII was passed through solid NovaPEG resin that was covalently functionalized with 1 to form 38 (Fig. 15) which had a greater affinity for EuII than GdIII. After GdIII was eluted, the EuII-loaded support was exposed to air to oxidize EuII to EuIII, causing the atomic radius to change, releasing it from the solid support. The purity of the recovered EuII ranged from 86.7 to 99.3% after a single pass through the solid support. Solid support 38 is most effective between pH 3 and 5.5. Overall, the cryptand-modified solid support enabled isolation of high-purity Eu from a mixture of Eu and Gd.
Following studies using 38 for solid–liquid separations of EuII, another study reported noncovalently solid-associated diesters of diethylaminetriaminepenta-acetic acid, 39, to separate rare-earth elements based on the affinity of 39 for the heavy rare-earth elements at an acidic pH.124 Ligand 39 was selected for this separation because it binds strongly to lanthanides and is reusable. Ligand 13 was functionalized with ethylhexylamine to form 39 that differs from 13 by the incorporation of two hydrophobic amides. To test the separation efficiency of 39, a solution containing 16 rare-earth elements (only Pm is excluded), 5 ppm each, was prepared. The results of the separation showed that 39 elutes the elements in the same order as the affinity 13 for the elements (Fig. 16). Solid support 39 is 130 times more effective at separating rare-earth elements at pH 3.3 than unmodified media.
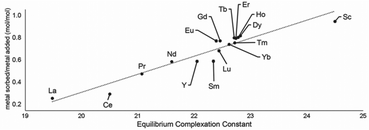 | ||
| Fig. 16 Metal sorbed onto 39 as plotted as a function of complexation constants of 13 for rare-earth elements at pH 3.3. Reprinted with permissions from Hovey et al.,124 copyright 2021, with permission from Elsevier. | ||
Solid support 39 shows a tendency to bind to heavy rare-earth elements during separations using coal-fly ash that contains many different elements. Unlike other systems, 39 has shown the ability to selectively bind heavy rare-earth elements over Ce and La (Fig. 17), two common elements that can make separations more challenging. This extraction of heavy rare-earth elements using 39 is achieved without the use of organic solvents during the separation, an issue with modern separation methods. This system has demonstrated effectiveness at recovering rare-earth elements from waste products with a preference for the rare-earth elements over Fe and Al, despite the concentration of metals. Further studies of this system showed improved enrichment of rare-earth elements in coal fly-ash from 0.024 to 10 wt%.125 The ligand involved in the system was also used to electrochemically remove Gd from samples with potential applications in hospital effluents.126
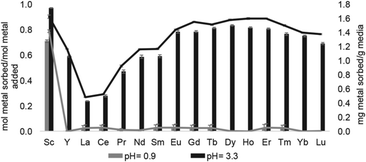 | ||
| Fig. 17 Sorption at pH 0.9 and 3.3 using 39. Reprinted with permissions from Hovey et al.,124 copyright 2021, with permission from Elsevier. | ||
Another system designed to separate Eu from Yb uses inorganic tetrathiotungstate (WS4−) anions to induce electron transfers to the metal upon heating or exposure to light.127 Within this study, WS4− reduces EuIII to EuII. When EuIII is mixed with WS4− in acetonitrile under ambient light, a EuII-containing precipitate forms. The proposed mechanism of this precipitation is that EuIII binds to WS4−, and once bound, light exposure induces an electron transfer from WS4− to reduce EuIII to EuII, with the resulting EuII-containing complex precipitating. The WS4− separation technique was used on a complex phosphor mixture, resulting in 98.9% separation efficiency of Eu from the mixture. This study is an important step toward separation of rare-earth metals, showing the ability to separate metals from complex lanthanide mixtures like phosphors.127
Overall, solid support systems designed using the principles of coordination chemistry can selectively separate europium from other lanthanides using differences in oxidation state and pH, and these techniques can be extended to other lanthanides.125,128
Conclusions
Throughout this article, information is presented regarding the differences in the coordination chemistry, magnetic properties, and optical properties of complexes of divalent and trivalent europium. Additionally, the influence of ligand design is described with respect to the tunable properties the of Eu ions and the relevance of these properties toward applications in MRI, luminescence, catalysis, and separation science. From these descriptions, several opportunities for future study arise. For example, translation of EuII-containing contrast agents that exhibit kinetically controlled oxidation to biological systems that require systemic delivery is an area ripe for advancement. Also, with the prospect of increasing demand for rare-earth elements, more studies into separations of these elements from each other and from other elements will be needed. Further, gaps in knowledge of lanthanides largely fall in oxidation states other than +3, and to address those gaps, the study of other lanthanides in addition to Eu will be needed. Overall, continuing research using divalent and trivalent Eu, and the rest of the lanthanides, will aid in better understanding of the wide range of these ions in applications such as MRI, luminescence, catalysis, and separation science.Author contributions
All authors contributed to the writing, reviewing, and editing of this manuscript.Data availability
No primary research results, software, or code have been included, and no new data were generated or analyzed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank all of the current and former members of the Allen Research Group and collaborators for their roles in the discoveries described in this review. We also acknowledge that those discoveries were made possible through the support of several agencies, including the National Institutes of Health (R00EB007129, R01EB013663, R01EB026453, R01EB027103, F30EB035471, F31CA247600, S10OD028488, T34GM140932, and T32GM142519), the National Science Foundation (CAREER CHE-0955000 and CHE-1564755), and the U.S. Army Engineer Research and Development Center (W912HZ-21-2-0048).Notes and references
- W. T. Carnall, P. R. Fields and K. Rajnak, J. Chem. Phys., 1968, 49, 4450 CrossRef CAS.
- Atomic Energy Levels–The Rare Earth Elements: The Spectra of Lanthanum, Cerium, Praseodymium, Neodynium, Promethium, Samarium, Europium, Gadolinium, Terbium, Dysprosium, Holmium, Erbium, Thulium, Ytterbium, and Lutetium, ed. W. C. Martin, R. Zalubus and L. Hagan, National Bureau of Standards, Washington D.C., 1978, p. 205 Search PubMed.
- K. Binnemans, Coord. Chem. Rev., 2015, 295, 1 CrossRef CAS.
- D. Kovacs and E. Borbas, Coord. Chem. Rev., 2018, 364, 1 CrossRef CAS.
- N. Sabbatini, M. Ciano, S. Dellonte, A. Bonazzi, F. Bolletta and V. Balzani, J. Phys. Chem., 1984, 88, 1534 CrossRef CAS.
- R. D. Shannon and C. T. Prewitt, Acta. Chim., 1969, B25, 925 Search PubMed.
- J. K. Burdett, R. Hoffmann and R. C. Fay, Inorg. Chem., 1978, 17, 2253 CrossRef.
- A. Ruiz-Martínez, D. Casanova and S. Alvarez, Chem. – Eur. J., 2008, 14, 1291 CrossRef PubMed.
- A. Ruiz-Martínez and S. Alvarez, Chem. – Eur. J., 2009, 15, 7470 CrossRef PubMed.
- L. A. Basal, A. B. Kajjam, M. D. Bailey and M. J. Allen, Inorg. Chem., 2020, 59, 9476 CrossRef CAS PubMed.
- T. C. Jenks, M. D. Bailey, B. A. Corbin, A. N. W. Kuda-Wedagedara, P. D. Martin, H. B. Schlegel, F. A. Rabuffetti and M. J. Allen, Chem. Commun., 2018, 54, 4545 RSC.
- G.-X. Jin, M. D. Bailey and M. J. Allen, Inorg. Chem., 2016, 55, 9085 CrossRef CAS PubMed.
- L. A. Ekanger, L. A. Polin, Y. Shen, E. M. Haacke, P. D. Martin and M. J. Allen, Angew. Chem., Int. Ed., 2015, 54, 14398 CrossRef CAS PubMed.
- C. U. Lenora, F. Carniato, Y. Shen, Z. Latif, E. M. Haacke, P. D. Martin, M. Botta and M. J. Allen, Chem. – Eur. J., 2017, 23, 15404 CrossRef CAS PubMed.
- C. U. Lenora, R. J. Staples and M. J. Allen, Inorg. Chem., 2020, 59, 86 CrossRef CAS PubMed.
- D. N. Huh, J. W. Ziller and W. J. Evans, Inorg. Chem., 2019, 58, 9613 CrossRef CAS PubMed.
- D. N. Huh, C. M. Kotyk, M. Gembicky, A. L. Rheingold, J. W. Ziller and W. J. Evans, Chem. Commun., 2017, 53, 8664 RSC.
- M. D. Bailey, G.-X. Jin, F. Carniato, M. Botta and M. J. Allen, Chem. – Eur. J., 2021, 27, 3114 CrossRef CAS PubMed.
- R. G. Parr and R. G. Pearson, J. Am. Chem. Soc., 1983, 105, 7512 CrossRef CAS.
- N. D. H. Gamage, Y. Mei, J. Garcia and M. J. Allen, Angew. Chem., Int. Ed., 2010, 49, 8923 CrossRef CAS PubMed.
- H. Elzanowska and Z. Galus, J. Electroanal. Chem., 1983, 157, 251 CAS.
- L. A. Basal and M. J. Allen, Front. Chem., 2018, 6, 65 CrossRef PubMed.
- D. V. Hingorani, A. S. Bernstein and M. D. Page, Contrast Media Mol. Imaging, 2015, 10, 245 CrossRef CAS PubMed.
- S. Shuvaev, E. Akam and P. Caravan, Invest. Radiol., 2021, 56, 20 CrossRef CAS PubMed.
- Q. N. Do, J. S. Ratnakar, Z. Kovács and A. D. Sherry, Chem. Med. Chem., 2014, 9, 1116 CrossRef CAS PubMed.
- J. Wahsner, E. M. Gale, A. Rodríguez-Rodríguez and P. Caravan, Chem. Rev., 2019, 119, 957 CrossRef CAS PubMed.
- C. S. Bonnet and É. Tóth, Curr. Opin. Chem. Biol., 2021, 61, 154 CrossRef CAS PubMed.
- A. L. Batchev, M. M. Rashid and M. J. Allen, Handbook on the Physics and Chemistry Rare Earths, 2023, vol. 63, p. 55 Search PubMed.
- The Chemistry of Contrast Agents in Medical Magnetic Resonance Imaging Second Edition, ed. A. Merbach, L. Helm and É. Tóth, John Wiley & Sons, Ltd., Chichester, 2013 Search PubMed.
- Contrast Agents for MRI: Experimental Methods, ed. V. C. Pierre and M. J. Allen, Royal Society of Chemistry, Cambridge, 2018 Search PubMed.
- A. Gupta, P. Caravan, W. S. Price, C. Platas-Iglesias and E. M. Gale, Inorg. Chem., 2020, 59, 6648 CrossRef CAS PubMed.
- P. B. Tsitovich, P. J. Burns, A. M. McKay and J. R. Morrow, J. Inorg. Biochem., 2014, 133, 143 CrossRef CAS PubMed.
- É. Tóth, L. Burai and A. E. Merbach, Coord. Chem. Rev., 2001, 216, 363 CrossRef.
- L. Burai, R. Scopelliti and É. Tóth, Chem. Commun., 2002, 2366 RSC.
- O. A. Gansow, A. R. Kausar, K. M. Triplett, M. J. Weaver and E. L. Yee, J. Am. Chem. Soc., 1979, 99, 7087 CrossRef.
- E. L. Yee, O. A. Gansow and M. J. Weaver, J. Am. Chem. Soc., 1980, 102, 2278 CrossRef CAS.
- R. Pizer and R. Selzer, Inorg. Chem., 1983, 22, 1359 CrossRef CAS.
- V. M. Loyola, R. Pizer and R. G. Wilkins, J. Am. Chem. Soc., 1977, 99, 7185 CrossRef CAS.
- S. Laurent, L. Vander Elst and R. N. Muller, Contrast Media Mol. Imaging, 2006, 1, 128 CrossRef CAS PubMed.
- J. Garcia, A. N. W. Kuda-Wedagedara and M. J. Allen, Eur. J. Inorg. Chem., 2012, 2135 CrossRef CAS PubMed.
- M. E. Burnett, B. Adebesin, A. M. Funk, Z. Kovacs, A. D. Sherry, L. A. Ekanger, M. J. Allen, K. N. Green and S. J. Ratnakar, Eur. J. Inorg. Chem., 2017, 5001 CrossRef CAS PubMed.
- L. A. Ekanger, L. A. Basal and M. J. Allen, Chem. – Eur. J., 2017, 23, 1145 CrossRef CAS PubMed.
- M. M. Rashid, B. A. Corbin, P. Jella, C. J. Ortiz, M. A. H. Samee, R. G. Pautler and M. J. Allen, J. Am. Chem. Soc., 2022, 144, 23053 CrossRef CAS PubMed.
- S. Seibig, É. Tóth and A. E. Merbach, J. Am. Chem. Soc., 2000, 122, 5822 CrossRef CAS.
- P. Caravan, É. Tóth, A. Rockenbauer and A. E. Merbach, J. Am. Chem. Soc., 1999, 121, 10403 CrossRef CAS.
- J. Garcia, J. Neelavalli, E. M. Haacke and M. J. Allen, Chem. Commun., 2011, 47, 12858 RSC.
- J. Costa, R. Ruloff, L. Burai, L. Helm and A. E. Merbach, J. Am. Chem. Soc., 2005, 127, 5147 CrossRef CAS PubMed.
- J. Garcia and M. J. Allen, Inorg. Chim. Acta, 2012, 393, 324 CrossRef CAS PubMed.
- B. A. Corbin, L. A. Basal, S. A. White, Y. Shen, E. M. Haacke, K. W. Fishbein and M. J. Allen, Bioorg. Med. Chem., 2018, 26, 5274 CrossRef CAS PubMed.
- L. A. Ekanger, L. A. Polin, Y. Shen, E. M. Haacke and M. J. Allen, Contrast Media Mol. Imaging, 2016, 11, 299 CrossRef CAS PubMed.
- F. K. Kálmán, M. Woods, P. Caravan, P. Jurek, M. Spiller, G. Tircsó, R. Király, E. Brücher and A. D. Sherry, Inorg. Chem., 2007, 46, 5260 CrossRef PubMed.
- N. Raghunand, S. Zhang, A. D. Sherry and R. J. Gillies, Acad. Radiol., 2002, 9, S481 CrossRef PubMed.
- S. Zhang, K. Wu and A. D. Sherry, Angew. Chem., Int. Ed., 1999, 38, 3192 CrossRef CAS PubMed.
- P. Caravan, M. T. Greenfield, X. Li and A. D. Sherry, Inorg. Chem., 2001, 40, 6580 CrossRef CAS PubMed.
- L. A. Basal, M. D. Bailey, J. Romero, M. M. Ali, L. Kurenbekova, J. Yustein, R. G. Pautler and M. J. Allen, Chem. Sci., 2017, 8, 8345 RSC.
- T. S. Elliott, A. Slowey, Y. Ye and S. J. Conway, Med. Chem. Commun., 2021, 3, 735 Search PubMed.
- G. M. Blackburn and D. E. Kent, J. Chem. Soc., Perkin Trans. 1, 1986, 913 RSC.
- J. C. Lutter, A. L. Batchev, C. J. Ortiz, A. G. Sertage, J. Romero, S. A. A. S. Subasinghe, S. E. Pedersen, M. A. H. Samee, R. G. Pautler and M. J. Allen, Adv. Healthcare Mater., 2023, 12, 2203209 CrossRef CAS PubMed.
- R. Kimmich, A. Peters and K.-H. Spohn, J. Membr. Sci., 1981, 9, 313 CrossRef CAS.
- S. Kim, S. Kim, W. Y. Oh, Y. Lee and J. Lee, Int. J. Food Sci. Technol., 2022, 57, 6082 CrossRef CAS.
- L. A. Ekanger and M. J. Allen, Metallomics, 2015, 7, 405 CrossRef CAS PubMed.
- L. A. Ekanger, M. M. Ali and M. J. Allen, Chem. Commun., 2014, 50, 14835 RSC.
- S. Aime, D. Castelli and E. Terreno, Angew. Chem., Int. Ed., 2005, 44, 5513 CrossRef CAS PubMed.
- K. M. Ward, A. H. Aletras and R. S. Balaban, J. Magn. Reson., 2000, 143, 79 CrossRef CAS PubMed.
- L. A. Ekanger, D. R. Mills, M. M. Ali, L. A. Polin, Y. Shen, E. M. Haacke and M. J. Allen, Inorg. Chem., 2016, 55, 9981 CrossRef CAS PubMed.
- L. A. Basal, Y. Yan, Y. Shen, E. M. Haacke, M. Mehrmohammadi and M. J. Allen, ACS Omega, 2017, 2, 800 CrossRef CAS PubMed.
- R. P. Mason, W. Rodbumrung and P. P. Antich, NMR Biomed., 1996, 9, 125 CrossRef CAS PubMed.
- H. Matsushita, S. Mizukami, F. Sugihara, Y. Nakanishi, Y. Yoshioka and K. Kikuchi, Angew. Chem., Int. Ed., 2014, 53, 1008 CrossRef CAS PubMed.
- M. Srinivas, P. A. Morel, L. A. Ernst, D. H. Laidlaw and E. T. Ahrens, Magn. Reson. Med., 2007, 58, 725 CrossRef CAS PubMed.
- E. T. Ahrens, R. Flores, H. Xu and P. A. Morel, Nat. Biotechnol., 2005, 23, 983 CrossRef CAS PubMed.
- S. A. A. S. Subasinghe, C. J. Ortiz, J. Romero, C. L. Ward, A. G. Sertage, L. Kurenbekova, J. T. Yustein, R. G. Pautler and M. J. Allen, Proc. Natl. Acad. Sci. U. S. A., 2023, 120, e2220891120 CrossRef CAS PubMed.
- J. R. Slack and M. Woods, J. Biol. Inorg. Chem., 2014, 19, 173 CrossRef CAS PubMed.
- T. Mani, A. C. L. Opina, P. Zhao, O. M. Evbuomwan, N. Milburn, G. Tircso, C. Kumas and A. D. Sherry, J. Biol. Inorg. Chem., 2014, 19, 161 CrossRef CAS PubMed.
- T. Mani, G. Tircsó, P. Zhao, A. D. Sherry and M. Woods, Inorg. Chem., 2009, 48, 10338 CrossRef CAS PubMed.
- A. N. W. Kuda-Wedagedara, C. Wang, P. D. Martin and M. J. Allen, J. Am. Chem. Soc., 2015, 137, 4960 CrossRef CAS PubMed.
- G. Vicentini, L. B. Zinner, J. Zukerman-Schpector and K. Zinner, Coord. Chem. Rev., 2000, 196, 353 CrossRef CAS.
- J. Jiang, N. Higashiyama, K. Machida and G. Adachi, Coord. Chem. Rev., 1998, 170, 1 CrossRef CAS.
- T. C. Jenks and M. J. Allen, Divalent Lanthanide Luminescence in Solution, in Modern Applications of Lanthanide Luminescence, ed. A. de Bettencourt-Dias, Springer, Cham, 2021, p. 67 Search PubMed.
- X. Qin, X. Liu, W. Huang, M. Bettinelli and X. Liu, Chem. Rev., 2017, 117, 4488 CrossRef CAS PubMed.
- A. de Bettencourt-Dias, Introduction to Lathanide Ion Luminescence, in Luminescence of Lanthanide Ions in Coordination Compounds and Nanomaterials, ed. A. Bettencourt-Dias, John Wiley and Sons Ltd., 2014, p. 1 Search PubMed.
- B. A. Corbin, J. L. Hovey, B. Thapa, H. B. Schlegel and M. J. Allen, J. Organomet. Chem., 2018, 857, 88 CrossRef CAS.
- T. C. Jenks, M. D. Bailey, J. L. Hovey, S. Fernando, G. Basnayake, M. E. Cross, W. Li and M. J. Allen, Chem. Sci., 2018, 9, 1273 RSC.
- R. G. Pearson, J. Am. Chem. Soc., 1963, 85, 3533 CrossRef CAS.
- S. Alvarez, Chem. – Eur. J., 2020, 26, 4350 CrossRef CAS PubMed.
- R. Barraza Jr., A. G. Sertage, A. B. Kajjam, C. L. Ward, J. C. Lutter, H. B. Schlegel and M. J. Allen, Inorg. Chem., 2022, 61, 19649 CrossRef CAS PubMed.
- W. Yan, T. Li, Z. Cai, H. Qi, R. Guo, P. Huo, Z. Liu and Z. Bian, Inorg. Chem. Front., 2022, 9, 4794 RSC.
- S. S. Bokouende, D. N. Kulasekara, S. A. Worku, C. L. Ward, A. B. Kajjam, J. C. Lutter and M. J. Allen, Inorg. Chem., 2024, 63, 9434 CrossRef CAS PubMed.
- S. S. Bokouende, T. C. Jenks, C. L. Ward and M. J. Allen, Dalton Trans., 2022, 51, 10852 RSC.
- T. C. Jenks, A. N. W. Kuda-Wedagedara, M. D. Bailey, C. L. Ward and M. J. Allen, Inorg. Chem., 2020, 59, 2613 CrossRef CAS PubMed.
- P. Dissanayake and M. J. Allen, J. Am. Chem. Soc., 2009, 131, 6342 CrossRef CAS PubMed.
- J. Matsuo and M. Murakami, Angew. Chem., Int. Ed., 2013, 52, 9019 CrossRef PubMed.
- T. Mukaiyama, K. Narasaka and K. Banno, Chem. Lett., 1973, 1011 CrossRef CAS.
- T. Kitanosono and S. Kobayashi, Adv. Synth. Catal., 2013, 355, 3095 CrossRef CAS PubMed.
- R. M. Supkowski and W. D. Horrocks, Jr., Inorg. Chim. Acta, 2002, 340, 44 CrossRef CAS.
- W. D. Horrocks Jr. and D. R. Sudnick, J. Am. Chem. Soc., 1979, 101, 334 CrossRef.
- A. Beeby, I. Clarkson, R. Dickins, S. Faulkner, D. Parker, A. S. de Sousa, J. A. G. Williams and M. Woods, J. Chem. Soc., Perkin Trans. 2, 1999, 493 RSC.
- P. Dissanayake, Y. Mei and M. J. Allen, ACS Catal., 2011, 1, 1203 CrossRef CAS.
- S. Kobayashi and I. Hachiya, J. Org. Chem., 1994, 59, 3590 CrossRef CAS.
- Y. Mei, P. Dissanayake and M. J. Allen, J. Am. Chem. Soc., 2010, 132, 12871 CrossRef CAS PubMed.
- D. J. Averill and M. J. Allen, Inorg. Chem., 2014, 53, 6257 CrossRef CAS PubMed.
- T. Yoon, Acc. Chem. Res., 2016, 49, 2307 CrossRef CAS PubMed.
- C. K. Prier, D. A. Rankic and D. W. C. MacMillan, Chem. Rev., 2013, 113, 5322 CrossRef CAS PubMed.
- T. Kondo, M. Akazome and Y. Watanabe, J. Chem. Soc., Chem. Commun., 1991, 757 RSC.
- A. Dahlén and G. Hilmersson, Eur. J. Inorg. Chem., 2004, 3393 CrossRef.
- E. Prasad, B. W. Knettle and R. A. Flowers, II, Chem. – Eur. J., 2005, 11, 3105 CrossRef CAS PubMed.
- M. Tomar, R. Bhimpuria, D. Kocsi, A. Thapper and K. E. Borbas, J. Am. Chem. Soc., 2023, 145, 22555 CrossRef CAS PubMed.
- C. K. Gupta and N. Krishnamurthy, Int. Mater. Rev., 1992, 37, 197 CrossRef CAS.
- R. Banda, F. Forte, B. Onghena and K. Binnemans, RSC Adv., 2019, 9, 4876 RSC.
- A. Rout and K. Binnemans, Dalton Trans., 2014, 43, 1862 RSC.
- B. Van de Bogaert, L. Gheeraert, M. Enis Leblebici and K. Binnemans, Phys. Chem. Chem. Phys., 2016, 18, 29961 RSC.
- M. Wehbie, G. Arrachart, C. A. Cruz, I. Karame, L. Ghannam and S. Pellet-Rostaing, Sep. Purif. Technol., 2017, 187, 311 CrossRef CAS.
- S. A. Ansari and P. K. Mohapatra, J. Chromatogr. A, 2017, 1499, 1 CrossRef CAS PubMed.
- V. V. Kurysheva, E. V. Ivanova and P. E. Prohorova, Chim. Techno Acta, 2016, 3, 97 CrossRef.
- J. L. Hovey, T. M. Dittrich and M. J. Allen, J. Rare Earths, 2023, 41, 1 CrossRef CAS.
- E.-H. Kim, S.-W. Kwon, E.-H. Lee and J.-H. Yoo, Korean J. Chem. Eng., 2002, 19, 305 CrossRef CAS.
- C. A. Morais and V. S. T. Ciminelli, Hydrometallurgy, 2001, 60, 247 CrossRef CAS.
- A. V. Val’kov, S. N. Igumnov and K. V. Ovchinnikov, Theor. Found. Chem. Eng., 2021, 55, 821 CrossRef.
- T. Hirai and I. Komasawa, Ind. Eng. Chem. Res., 1995, 34, 237 CrossRef CAS.
- S. A. Sayed, K. A. Rabie and I. E. Salama, Sep. Purif. Technol., 2005, 46, 145 CrossRef CAS.
- K. G. Myhre, J. R. Knowles and M. Du, Sep. Sci. Technol., 2020, 55, 1695 CrossRef CAS.
- N. A. Ismail and M. A. Abidin, Phys. Chem. Earth, 2021, 121, 102958 CrossRef.
- S.-C. Li, S.-C. Kim, C.-S. Kang, C.-J. Kim and C.-J. Kang, Hydrometallurgy, 2018, 178, 181 CrossRef CAS.
- D. N. Kulasekara, A. B. Kajjam, S. Praneeth, T. M. Dittrich and M. J. Allen, ACS Appl. Mater. Interfaces, 2023, 15, 42037 CrossRef CAS PubMed.
- J. L. Hovey, M. Dardona, M. J. Allen and T. M. Dittrich, Sep. Purif. Technol., 2021, 258, 118061 CrossRef CAS.
- M. Dardona, S. K. Mohanty, M. J. Allen and T. M. Dittrich, Sep. Purif. Technol., 2023, 314, 123532 CrossRef CAS.
- R. Ranaweera, S. Wijesinghe, U. Weerarathna, A. Chowdhury, A. B. Kajjam, B. Wang, T. M. Dittrich, M. J. Allen and L. Luo, ACS EST Eng., 2024, 4, 540 CrossRef CAS.
- M. A. Perrin, P. Duthiel, M. Wörle and V. Mougel, Nat. Commun., 2024, 15, 4577 CrossRef CAS PubMed.
- J. Roosen and K. Binnemans, J. Mater. Chem. A, 2014, 2, 1530 RSC.
| This journal is © The Royal Society of Chemistry 2024 |