Open Access Article
Open Access ArticlePhotoswitchable TCB-2 for control of the 5-HT2A receptor and analysis of biased agonism†
Alireza Jafar
Esmaeili‡
,
Pantea
Montazeri‡
,
Jasmine Cristina
Gomez
,
Didier J.
Dumervil
,
Faezeh Safar
Nezhad
and
Rachel C.
Steinhardt
 *
*
Syracuse University, Department of Chemistry, 111 College Pl., Syracuse, NY 13244, USA. E-mail: rcsteinh@syr.edu
First published on 25th September 2024
Abstract
Therapies that target the serotonin 2A receptor (5-HT2AR) are promising. However, probes are needed to better understand the role of 5-HT2AR. Here, we design and synthesize a photoswitch and photoswitchable 5-HT2AR ligand based on highly potent agonist TCB-2 and arylazopyrazole, which also boasts photoswitchable G protein vs. β-arrestin pathway bias.
Serotonin is required for manifold processes throughout the body, ranging from homeostasis and digestion to complex central nervous function. There are 14 subtypes of serotonin receptor, and stemming their importance in biological processes such as cognition and mood, there has been intense focus on developing new drugs and probes for this receptor family.1–3 Recently, agonists of the 5-HT2AR have generated intense interest. Specifically, psychedelic compounds like psilocybin are under study for treatment of disease ranging from anxiety, depression, obsessive-compulsive disorder, to substance use disorder.4–10 However, these drugs are also active at many neurologically important monoamine receptors. The precise role of 5-HT2AR in the dramatic and potentially long-ranging benefits of these therapeutic interventions remains unclear and under intense study.10–19
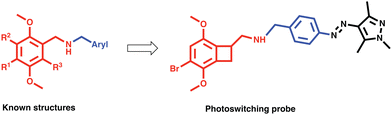
To understand 5-HT2AR's therapeutic potential, new selective probes are needed. Here we develop a photopharmacological probe for 5-HT2AR based on the subtype selective and highly potent ligand TCB-2.20,21 Such probes provide spatial and temporal precision.22,23 There only a couple of such 5-HT2AR probes known, and ours is the first to be based on such a selective ligand.24,25 TCB-2 is a high affinity ligand for 5-HT2AR, with a potency analogous to lysergic acid diethylamide (LSD).20 It is a member of a family of synthetic mescaline derivatives based on the 2,5-methoxyphenethylamine scaffold, where it is known that lipophilic substituents at the 4′ position of the aryl ring confer increased potency at 5-HT2AR (Fig. 1).26 Among this family are the class of N-benzylated derivatives known as the N-benzyl phenethylamine (NBOMe) which contains some of the most potent and selective 5-HT2AR ligands known (Fig. 1).19,26,27
With this information in mind, we designed a 5-HT2AR probe via N-arylating TCB-2 with a photoswitch (Fig. 1). We hypothesized that the change in sterics by isomerization of the extended pi system of the switch would lead to altered activity at the receptor. Additionally, we wanted to determine whether the large volume change of photoswitching would alter the signalling bias of 5-HT2AR.28 “Bias” refers to the fact that 5-HT2AR can signal via multiple pathways, and crucially for drug design, 5-HT2AR can interact with the G protein or β-arrestin2 pathways.29–31 These pathways known to induce dramatically different physiological effects for other GPCRs, and it is hypothesized that these pathways differentially contribute to the psychedelic and therapeutic effects of psychedelic drugs.10,17,32–34 This area is currently under study and more work is needed to clarify the biochemistry of these pathway contributions. There are only two biased photoswitchable ligands reported so far, Broichhagen et al. reported on a photoswitching probe “LirAzo” with biased functionality at GLP-1R, and the Decker group disclosed a biased photoswitching cannabinoid 2 receptor agonist.35,36
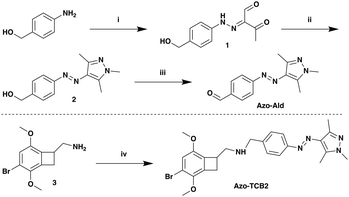
For the photoswitch identity, we chose an arylazopyrazole (AAP), due to its excellent photophysical and photochemical properties, and well-documented use in biological systems.23,37–41 For the present application we synthesized a novel AAP, Azo-Ald (Scheme 1), for ease of installation via reductive amination. We modified the electronics of the pyrazole ring based on the findings of the Fuchter group.42
The syntheses of Azo-Ald and Azo-TCB2 began with commercially available 4-aminobenzyl alcohol, which was first diazotized with sodium nitrite in acetic and HCl, followed by diazo coupling with the enolate of 2,4-pentanedione to furnish hydrazone 1.43,44 The product was then condensed with methylhydrazine to provide pyrazole 2.44,45 The benzylic alcohol on 2 was readily oxidized using activated MnO2 to provide Azo-Ald. The drug TCB-2 was incubated with Azo-Ald, followed by the addition of sodium cyanoborohydride to yield the reductive amination product Azo-TCB2.
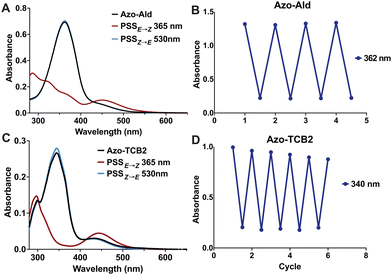
Next, we investigated the photophysical properties of Azo-Ald and Azo-TCB2 using UV/Vis spectroscopy and 1H NMR. Solutions were prepared in 22 μM solution in DMSO, and a UV transilluminator was used to provide the 365 nm irradiation for E → Z conversion, and a 530 nm LED (Thorlabs) was used for the Z → E conversion. Photostationary states (PSS) were determined by 1H NMR and the UV/Vis spectra were recorded both at the dark equilibrated and PSS states, with the dark states showing absorbance maxima at 362 nm for Azo-Ald and 340 nm for Azo-TCB2 and PSSs with absorption maxima at 446 nm and 440 nm, this well resolved wavelength shift also reveals isosbestic points at ∼400 nm for both compounds (Fig. 2a and c). Both Azo-Ald and Azo-TCB2 showed good fatigue resistance over multiple switching cycles (Fig. 2b and d).
NMR spectroscopy showed that after irradiation at 365 nm the PSSE→Z for Azo-Ald in DMSO at was 62%, this was improved to 98% for Azo-TCB2. This may be due to the presence of an electron-donating group at the para position of the benzene ring. The rate of thermal relaxation of the less stable E-state was then investigated and showed that Azo-TCB2 has good potential for in vivo use, due to its 29.14 h half-life at 25 °C (Table 1) and 21.01 h half life at 37 °C. These photophysical data showed that Azo-TCB2 was an effective photoswitch meriting investigation of its biochemical parameters.
| Ligand | PSSE–Z (%Z) | PSSZ–E (%E) | t 1/2 Z isomer | ϕ E–Z (%) | ϕ Z–E (%) |
|---|---|---|---|---|---|
| Azo-Ald | 62 | 100 | 11 minutes | 0.13 | 0.36 |
| Azo-TCB2 | 98 | 83 | 29.14 hours | 0.26 | 0.24 |
We next analysed the binding of Azo-TCB2 at the 5-HT2A receptor via assays for G-protein and β-arrestin pathway activation. G-protein mediated signalling by 5-HT2A was determined by intracellular calcium measurement. The assay functions by measurement of changes in intracellular calcium flux via imaging of a ratiometric calcium-binding fluorescent dye. To perform the assay, HEK293T cells were transfected with 5-HT2A, followed by loading with the calcium-sensing dye Cal-590™AM. Ligand binding to 5-HT2AR results in an influx of calcium in the cytoplasm, which is monitored in real time by confocal imaging of the increase in calcium sensor fluorescence.
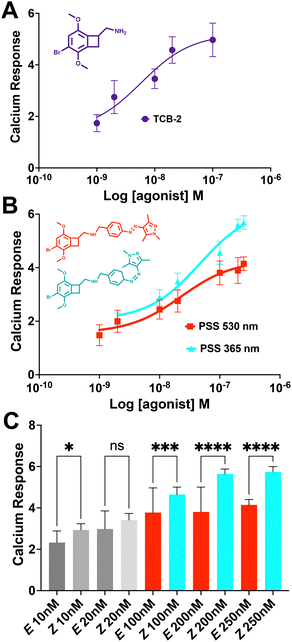
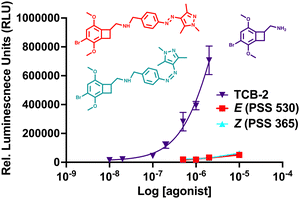
The live cell imaging calcium flux data (Fig. 3) shows that Azo-TCB2 pharmacophore activates 5-HT2A in a dose-dependent fashion in the E and Z states, with a relative increase in maximal partial agonism in the photoswiched Z state. Comparison of EC50 curves between the two states shows the Z state with ≈ 2,4-fold increase in EC50 over the E state. In the Z state, the orientation of the arylpyrazole group decreases the receptor's maximal response to Azo-TCB2 relative to TCB-2, rendering this isomer an apparent partial agonist as compared to the apparent full agonism of the E isomer. Statistical analysis of the two curves using ordinary one-way ANOVA (analysis of variance) shows statistically significant differences between the higher concentrations of the E and Z states. Both E and Z forms have good potency at the receptor, with EC50 of 22.8 nM nm and 46.7 nM respectively, compared to our measured 5.9 nM nm for the positive control, which is in agreement with the literature value of 0.75 nM.20 This indicates a rightward shift in agonism, and thus relative decrease in potency vs. the parent compound, TCB-2. Overall, these data indicate that in the context of the G-protein pathway, the E and Z states are both still excellent at initiating a Ca2+ response, and photoswitching Azo-TCB2 results in a decrease in EC50 and maximal response. With this information in hand, we next sought to determine the 5-HT2AR β-arrestin response.
The response of the β-arrestin effector pathway was characterized with the parallel-receptor-ome expression and screening via transcriptional output TANGO (PRESTO–TANGO) assay developed in the Roth lab.46,47 This assay monitors β-arrestin activity by expression of a luciferase reporter gene. The β-arrestin recruitment data showed some notable differences compared to the G-protein pathway. For the isomers, data (Fig. 4), show that both E and Z states are rendered apparent partial agonists of 5-HT2AR, with ≈14-fold difference in maximal response between the two isomers and that of the parent compound. In terms of potency, the E state retains an EC50 equivalent to the parent compound, while the Z state differed, and both are greatly decreased in maximal response.
Next, we analysed the differential initiation of G-protein vs. β-arrestin pathways for the Azo-TCB2 (Fig. 5). To quantify pathway bias for the two states, we first converted each isomer's response to percent of the reference compound's maximal response, with TCB-2 serving as the reference compound. From comparing these numbers, we can see that the predominantly Z compound has become more β-arrestin pathway biased vs. the predominantly E.
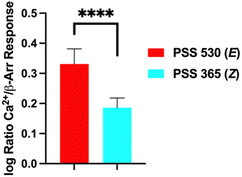 | ||
| Fig. 5 Agonism bias for Azo-TCB2 isomers. ****p < 0.0001, Student's t-test. Error bars represent mean ± standard deviation. | ||
In conclusion, here we have described one of the first photoswtiching probes for 5-HT2AR, and the first to demonstrate switchable pathway bias. There is differential potency between the two isomers in the G-protein pathway, while the potencies are similar in the β-arrestin pathway. The isomers have excellent photophysical parameters such as low rate of thermal back switching and fatigue resistance. Additionally, this work provides medicinal chemistry data on this structural class which is of intense biomedical interest, specifically on the sensitivity to substitution with extended N-aryl groups.27,33 In future steps, this probe can be used in studies towards treating diseases ranging from anxiety, depression, obsessive-compulsive disorder, to substance use disorder.4–10 This probe can also contribute to the fundamental untangling of the G-protein and β-arrestin pathway contributions to therapeutic effects.
The authors wish to thank the National Science Foundation, award # 2238400.
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Notes and references
- D. E. Nichols and C. D. Nichols, Chem. Rev., 2008, 108, 1614–1641 CrossRef CAS PubMed.
- M. Berger, J. A. Gray and B. L. Roth, Annu. Rev. Med., 2009, 60, 355–366 CrossRef CAS PubMed.
- J. D. McCorvy and B. L. Roth, Pharmacol. Ther., 2015, 150, 129–142 CrossRef CAS PubMed.
- R. L. Carhart-Harris, M. Bolstridge, C. M. J. Day, J. Rucker, R. Watts, D. E. Erritzoe, M. Kaelen, B. Giribaldi, M. Bloomfield, S. Pilling, J. A. Rickard, B. Forbes, A. Feilding, D. Taylor, H. V. Curran and D. J. Nutt, Psychopharmacology, 2018, 235, 399–408 CrossRef CAS PubMed.
- P. A. Davoudian, L.-X. Shao and A. C. Kwan, ACS Chem. Neurosci., 2023, 14, 468–480 CrossRef CAS PubMed.
- R. R. Griffiths, M. W. Johnson, M. A. Carducci, A. Umbricht, W. A. Richards, B. D. Richards, M. P. Cosimano and M. A. Klinedinst, J. Psychopharmacol., 2016, 30, 1181–1197 CrossRef CAS PubMed.
- C. L. Raison, G. Sanacora, J. Woolley, K. Heinzerling, B. W. Dunlop, R. T. Brown, R. Kakar, M. Hassman, R. P. Trivedi, R. Robison, N. Gukasyan, S. M. Nayak, X. Hu, K. C. O’Donnell, B. Kelmendi, J. Sloshower, A. D. Penn, E. Bradley, D. F. Kelly, T. Mletzko, C. R. Nicholas, P. R. Hutson, G. Tarpley, M. Utzinger, K. Lenoch, K. Warchol, T. Gapasin, M. C. Davis, C. Nelson-Douthit, S. Wilson, C. Brown, W. Linton, M. W. Johnson, S. Ross and R. R. Griffiths, JAMA, 2023, 330, 843 CrossRef CAS PubMed.
- R. L. Carhart-Harris and G. M. Goodwin, Neuropsychopharmacology, 2017, 42, 2105–2113 CrossRef CAS PubMed.
- T. Chi and J. A. Gold, J. Neurol. Sci., 2020, 411, 116715 CrossRef CAS PubMed.
- W. Duan, D. Cao, S. Wang and J. Cheng, Chem. Rev., 2024, 124, 124–163 CrossRef CAS PubMed.
- M. Hibicke, A. N. Landry, H. M. Kramer, Z. K. Talman and C. D. Nichols, ACS Chem. Neurosci., 2020, 11, 864–871 CrossRef CAS PubMed.
- L. P. Cameron, S. D. Patel, M. V. Vargas, E. V. Barragan, H. N. Saeger, H. T. Warren, W. L. Chow, J. A. Gray and D. E. Olson, ACS Chem. Neurosci., 2023, 14, 351–358 CrossRef CAS PubMed.
- D. A. Martin and C. D. Nichols, EBioMedicine, 2016, 11, 262–277 CrossRef PubMed.
- M. V. Vargas, L. E. Dunlap, C. Dong, S. J. Carter, R. J. Tombari, S. A. Jami, L. P. Cameron, S. D. Patel, J. J. Hennessey, H. N. Saeger, J. D. McCorvy, J. A. Gray, L. Tian and D. E. Olson, Science, 2023, 379, 700–706 CrossRef CAS PubMed.
- D. E. Olson, ACS Pharmacol. Transl. Sci., 2021, 4, 563–567 CrossRef CAS PubMed.
- D. B. Yaden and R. R. Griffiths, ACS Pharmacol. Transl. Sci., 2021, 4, 568–572 CrossRef CAS PubMed.
- J. F. López-Giménez and J. González-Maeso, in Behavioral Neurobiology of Psychedelic Drugs, ed. A. L. Halberstadt, F. X. Vollenweider and D. E. Nichols, Springer Berlin Heidelberg, Berlin, Heidelberg, 2017, vol. 36, pp. 45–73 Search PubMed.
- D. E. Olson, Biochemistry, 2022, 61, 127–136 CrossRef CAS PubMed.
- J. Wallach, A. B. Cao, M. M. Calkins, A. J. Heim, J. K. Lanham, E. M. Bonniwell, J. J. Hennessey, H. A. Bock, E. I. Anderson, A. M. Sherwood, H. Morris, R. De Klein, A. K. Klein, B. Cuccurazzu, J. Gamrat, T. Fannana, R. Zauhar, A. L. Halberstadt and J. D. McCorvy, Nat. Commun., 2023, 14, 8221 CrossRef CAS PubMed.
- T. H. McLean, J. C. Parrish, M. R. Braden, D. Marona-Lewicka, A. Gallardo-Godoy and D. E. Nichols, J. Med. Chem., 2006, 49, 5794–5803 CrossRef CAS PubMed.
- M. A. Fox, H. T. French, J. L. LaPorte, A. R. Blackler and D. L. Murphy, Psychopharmacology, 2010, 212, 13–23 CrossRef CAS PubMed.
- J. Broichhagen, J. A. Frank and D. Trauner, Acc. Chem. Res., 2015, 48, 1947–1960 CrossRef CAS PubMed.
- M. J. Fuchter, J. Med. Chem., 2020, 63, 11436–11447 CrossRef CAS PubMed.
- H. Gerwe, F. He, E. Pottie, C. Stove and M. Decker, Angew. Chem., Int. Ed., 2022, 61, e202203034 CrossRef CAS PubMed.
- J. Morstein, G. Romano, B. E. Hetzler, A. Plante, C. Haake, J. Levitz and D. Trauner, Angew. Chem., Int. Ed., 2022, 62, e202117094 Search PubMed.
- E. M. Rørsted, A. A. Jensen, G. Smits, K. Frydenvang and J. L. Kristensen, J. Med. Chem., 2024, 67, 7224–7244 CrossRef PubMed.
- E. Märcher Rørsted, A. A. Jensen and J. L. Kristensen, ChemMedChem, 2021, 16, 3263–3270 CrossRef PubMed.
- A. Gonzalez, E. S. Kengmana, M. V. Fonseca and G. G. D. Han, Mater. Today Adv., 2020, 6, 100058 CrossRef.
- E. Reiter, S. Ahn, A. K. Shukla and R. J. Lefkowitz, Annu. Rev. Pharmacol. Toxicol., 2012, 52, 179–197 CrossRef CAS PubMed.
- J. S. Smith, R. J. Lefkowitz and S. Rajagopal, Nat. Rev. Drug Discovery, 2018, 17, 243–260 CrossRef CAS PubMed.
- J. W. Wisler, H. A. Rockman and R. J. Lefkowitz, Circulation, 2018, 137, 2315–2317 CrossRef CAS PubMed.
- E. Pottie, C. B. M. Poulie, I. A. Simon, K. Harpsøe, L. D’Andrea, I. V. Komarov, D. E. Gloriam, A. A. Jensen, J. L. Kristensen and C. P. Stove, ACS Chem. Neurosci., 2023, 14, 2727–2742 CrossRef CAS PubMed.
- C. B. M. Poulie, E. Pottie, I. A. Simon, K. Harpsøe, L. D’Andrea, I. V. Komarov, D. E. Gloriam, A. A. Jensen, C. P. Stove and J. L. Kristensen, J. Med. Chem., 2022, 65, 12031–12043 CrossRef CAS PubMed.
- E. Pottie and C. P. Stove, J. Neurochem., 2022, 162, 39–59 CrossRef CAS PubMed.
- J. Broichhagen, T. Podewin, H. Meyer-Berg, Y. vonOhlen, N. R. Johnston, B. J. Jones, S. R. Bloom, G. A. Rutter, A. Hoffmann-Röder, D. J. Hodson and D. Trauner, Angew. Chem., Int. Ed., 2015, 54, 15565–15569 CrossRef CAS PubMed.
- S. A. M. Steinmüller, J. Fender, M. H. Deventer, A. Tutov, K. Lorenz, C. P. Stove, J. N. Hislop and M. Decker, Angew. Chem., Int. Ed., 2023, 62, e202306176 CrossRef PubMed.
- C. E. Weston, A. Krämer, F. Colin, Ö. Yildiz, M. G. J. Baud, F.-J. Meyer-Almes and M. J. Fuchter, ACS Infect. Dis., 2017, 3, 152–161 CrossRef CAS PubMed.
- L. Stricker, M. Böckmann, T. M. Kirse, N. L. Doltsinis and B. J. Ravoo, Chem. – Eur. J., 2018, 24, 8639–8647 CrossRef CAS PubMed.
- P. Kobauri, F. J. Dekker, W. Szymanski and B. L. Feringa, Angew. Chem., Int. Ed., 2023, 62, e202300681 CrossRef CAS PubMed.
- J. Volarić, W. Szymanski, N. A. Simeth and B. L. Feringa, Chem. Soc. Rev., 2021, 50, 12377–12449 RSC.
- A. K. Gaur, D. Gupta, A. Mahadevan, P. Kumar, H. Kumar, D. N. Nampoothiry, N. Kaur, S. K. Thakur, S. Singh, T. Slanina and S. Venkataramani, J. Am. Chem. Soc., 2023, 145, 10584–10594 CrossRef CAS PubMed.
- J. Calbo, A. R. Thawani, R. S. L. Gibson, A. J. P. White and M. J. Fuchter, Beilstein J. Org. Chem., 2019, 15, 2753–2764 CrossRef CAS PubMed.
- T. M. Courtney, T. J. Horst, C. P. Hankinson and A. Deiters, Org. Biomol. Chem., 2019, 17, 8348–8353 RSC.
- J. Simke, T. Bösking and B. J. Ravoo, Org. Lett., 2021, 23, 7635–7639 CrossRef CAS PubMed.
- T. M. Courtney, T. J. Horst, C. P. Hankinson and A. Deiters, Org. Biomol. Chem., 2019, 17, 8348–8353 RSC.
- W. K. Kroeze, M. F. Sassano, X.-P. Huang, K. Lansu, J. D. McCorvy, P. M. Giguère, N. Sciaky and B. L. Roth, Nat. Struct. Mol. Biol., 2015, 22, 362–369 CrossRef CAS PubMed.
- G. Barnea, W. Strapps, G. Herrada, Y. Berman, J. Ong, B. Kloss, R. Axel and K. J. Lee, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 64–69 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4cc03892d |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |