Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Convergent synthesis of bicyclic boronates via a cascade regioselective Suzuki–Miyaura/cyclisation protocol†
Alessandro
Marotta
ab,
Hannah M.
Kortman
ab,
Chiara
Interdonato
a,
Peter H.
Seeberger
 ab and
John J.
Molloy
ab and
John J.
Molloy
 *a
*a
aDepartment of Biomolecular Systems, Max-Planck-Institute of Colloids and Interfaces, 14476 Potsdam, Germany. E-mail: john.molloy@mpikg.mpg.de
bDepartment of Chemistry and Biochemistry, Freie Universität Berlin, 14195 Berlin, Germany
First published on 18th October 2024
Abstract
Bicyclic boronates have recently emerged as promising candidates to invoke targeted biomolecular interactions, given their selectivity for specific functionalities. Despite this, the general stability of the C–B bond in vivo, for such heterocycles, remains an intractable challenge that can often preclude their utility in drug discovery. To address this challenge, de novo strategies that allow expedient access to strategically substituted boronates, that enable modulation of the C–B bond are urgently required. Herein we disclose an operationally simple, regioselective cross-coupling/cyclisation reaction of easily accessible vicinal boronic esters with 2-halophenols to rapidly forge 3-substituted bicyclic boronates. The utility of the platform was demonstrated via expedient access to Xeruborbactam derivatives, chemoselective manipulation of formed products and the convergent approach to bicyclic boronates with a pendent biomolecular probe.
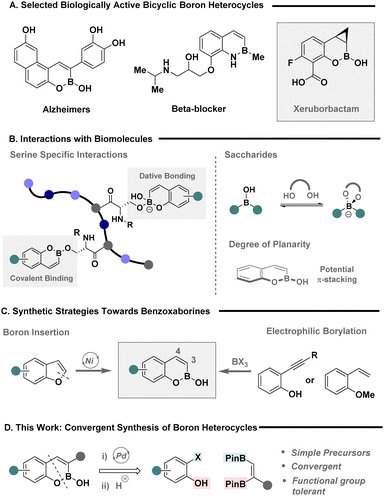
Boron heterocycles are privileged scaffolds with applications that transcend the chemical sciences, including chemical sensing and their use as conducting materials.1–3 Their unique properties have, more recently, been translated to drug discovery,4–6 where bicyclic systems such as benzoxaborines (BOBs) and benzazaborines (BABs) among others, are frequently leveraged to tune stability of the boron handle and elicit a desired target inhibition (Fig. 1A).7–11 The versatility of these motifs is exemplified with reactivity against neurological, oncological and bacterial targets,5 with Xeruborbactam, a potent beta-lactamase inhibitor, currently in phase III clinical trials.11 Their utility stems from the dexterity of boron to form various covalent and non-covalent interactions with biomolecules (Fig. 1B), enabling the practitioner to design therapeutics with site-specific interactions in mind. For example, benzoxaborines offer the ability to selectivity bind serine amino acids, form chelation with sugar targets and elicit π-stacking interactions, underpinning their potential as a powerful pharmacophore.12 However, the unpredictable metabolic stability of heterocyclic C–B bonds remains an intractable challenge in the design of state-of-the-art therapeutics,13,14 and as such, novel platforms that allow expedient access to boron heterocycles to assess their biological activity and stability are urgently required.
Whilst the wide spread utility of boron heterocycles has culminated in a plenum of strategies for their chemical synthesis,15–18 the construction of BOB scaffolds is predominantly achieved via two main synthetic strategies (Fig. 1C); nickel catalysed boron insertion,19,20 or the activation of unsaturated bonds using electrophilic boron reagents.21 The former was elegantly achieved by the Yorimitsu group using benzofuran precursors to provide expedient access to BOB scaffolds in a single step,19 while the latter is typically achieved using ortho-substituted, styrenes or phenyl acetylenes, in the presence of a highly electrophilic boron reagent, such as BBr3 that compromise functional group compatibility. Despite these notable advances, strategies that enable the strategic incorporation of substituents in either the 3-, or 4-position remain a persistent challenge. The Ingleson group recently made prominent strides in addressing this deficiency through the advent of a halo-borylation protocol that facilitates the incorporation of a chloride handle on the 4-position.22 However, a general strategy to achieve 3-substitution is conspicuously underdeveloped, yet desirable given its spatial proximity and potential ability to modulate steric and electronic properties of the adjacent boron motif.
Given the vast array of approaches to construct unsaturated vicinal boron systems,23–25 in combination with Miyaura's venerable, sterically driven regioselective activation of the terminal boron,26,27 we envisaged a simple disconnection that would facilitate a convergent approach to 3-substituted benzoxaborines via a cascade annulation with easily accessible 2-halophenols (Fig. 1D).28–30 Here, the terminal boron serves as a traceless handle for Suzuki–Miyaura cross-coupling, while the latter is trapped under basic media with the pendent phenolate. Subsequent removal of the pinacol ligand via a switch to acidic media would grant direct access to 3-substituted boron heterocycles. Herein we describe the convergent synthesis of highly coveted 3-substituted boron heterocycles via palladium-catalysed annulation of ortho-halo phenols with vicinal BPins. The strategy demonstrates high functional group tolerance and is leveraged to, provide expedient access to Xeruborbactam derivatives, facilitate subsequent chemoselective activation of boron heterocycles, and provide a construct for the chemical synthesis of BOB containing biomolecular probes.
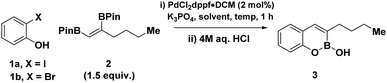
We initiated our reaction optimisation probing the efficiency of 2-iodophenol 1a and vicinal BPin 2 to undergo a cascade cross-coupling/cyclization protocol (Table 1). Utilizing PdCl2dppf·DCM as a catalyst, at 60 °C, provided our initial hit forming the target cyclised BOB 3, as a single regioisomer in low yields after acidic work up (entry 1). Regulating reaction media was critical, as biphasic mixtures with increasing water content proved to be more effective for cross-coupling for both THF and acetonitrile (entries 2 to 4). Increasing reaction concentration afforded the target BOB in quantitative yield (entry 5), while the use of less equivalents of vicinal BPin was detrimental to the reaction (entry 6). Translation of optimal conditions to 2-bromophenol was ineffective (entry 7). Although increasing temperature to 80 °C and reaction time to 16 hours with acetonitrile only resulted in a moderate increase in yield (entry 8), a concomitant solvent switch enabled a substantial increase to 73% yield (entry 9). Finally increasing the temperature to 100 °C afforded the target BOB in 83% yield.
| Entry | X | Solvent (ratio) | Temp. (°C) | Yieldb (%) |
|---|---|---|---|---|
| a Standard conditions: 1 (0.1 mmol), 2 (1.5 equiv.), K3PO4 (3 equiv.), solvent (0.1 M), temp., 1 h. b Determined by 1H NMR spectroscopy against a known internal standard (1,3,5-trimethoxybenzene). c 0.2 M reaction concentration used. d 1.1 equiv. of 2 used. e Reactions were run for 16 h. | ||||
| 1 | I | THF/H2O (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
60 | 16 |
| 2 | I | THF/H2O (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
60 | 68 |
| 3 | I | THF/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
60 | 91 |
| 4 | I | MeCN/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
60 | 92 |
| 5 | I |
MeCN/H
2
O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) 1)
|
60 | Quant. |
| 6cd | I | MeCN/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
60 | 74 |
| 7c | Br | MeCN/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
60 | Trace |
| 8ce | Br | MeCN/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
80 | 27 |
| 9ce | Br | THF/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
80 | 73 |
| 10 | Br |
THF/H
2
O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) 1)
|
100 | 83 |
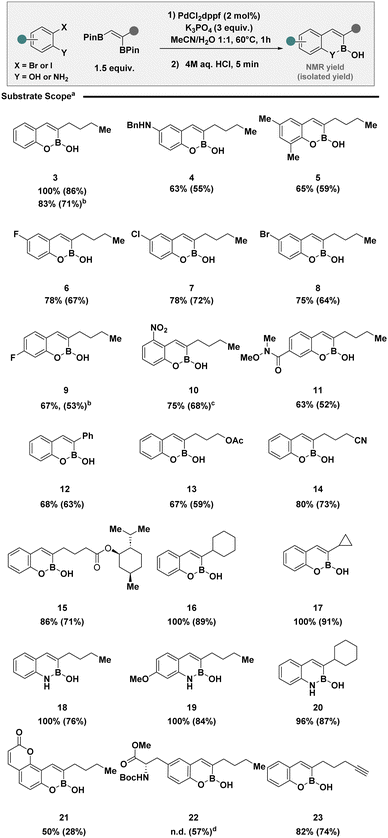
Having developed a set of general reaction conditions for both 2-iodo- and 2-bromophenols, the scope and limitations were established (Scheme 1). Pleasingly, the protocol was amenable to a range of 2-halophenols with varying substitution patterns including electron neutral (3) and electron rich examples (4 and 5). Electron poor systems, often problematic for activation with electrophilic reagents,21 were compatible (6 to 11). It is pertinent to note, chemoselective activation of 2-iodophenols in the presence of competing halides was well tolerated, providing a synthetic handle for subsequent transformations (7 and 8), which would be otherwise challenging using nickel catalysis technologies.19 Modifications of the vicinal boronic ester was also effective including styrenes (12), long chain aliphatics containing modifiable functionalities (13–15), and adjacent alicyclic rings (16 and 17). The use of 2-iodoanilines, enabled translation to benzazaborines (18 to 20). The exploration of substrate scope culminated in the synthesis of coumarin derivative (21), tyrosine analogue (22) and BOB (23) with a strategically placed alkyne motif for application in click chemistry to access biomolecular probes.
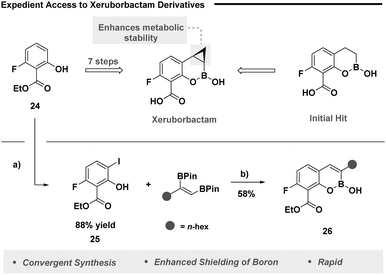
Cognizant of the therapeutic effects of Xeruborbactam, we next set out to probe our designed method on a similar framework (Fig. 2). Xeruborbactam is readily accessed from intermediate 24 in a seven-step large-scale process (Fig. 2, top).31 It was envisaged the strategic pairing of a site selective iodination strategy with our developed method would grant expedient access to analogues of this nature. Selective iodination was readily achieved using an established platform (Fig. 2, bottom),32 while our annulation process enabled the synthesis of analogue 26, underpinning the ability of the developed protocol to access biologically relevant heterocyclic frameworks.
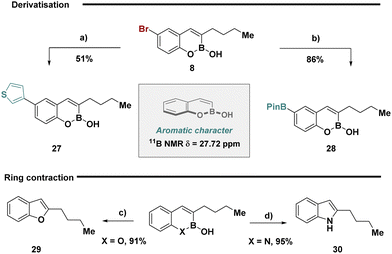
Inspired by the proposed aromatic character of bicyclic boronates and given their ability to be effectively retained during developed reaction conditions, we anticipated that substrate 8 could be leveraged in chemoselective cross-coupling enabling exploration of chemical space with the retention of the core heterocycle scaffold (Fig. 3, top). Selective Suzuki–Miyaura cross-coupling of thiophene boronic acid was achieved to forge 27, while a Miyaura borylation enabled the inversion of reactivity generating the nucleophilic boronic ester 28. Motivated by recent advances in skeletal editing deletion sequences,33 substrates 3 and 18 could be easily transformed to the benzofuran and indole respectively in high yields (Fig. 3, bottom).
Given their unique ability to selectively bind serine amino acid residues,12 bicyclic boronates have been frequently employed as chemical probes for target identification in order to unravel the intricacies of biomolecular mechanisms.5 To further demonstrate the utility of our convergent approach we utilized substrate 23 in copper catalysed “click” reactions to efficiently connect biomolecular probes (Fig. 4). The reaction was efficient in incorporating biotin (31), an efficient tool for targeted delivery,34 and warhead 32, a potent ubiquitin recruiter.35
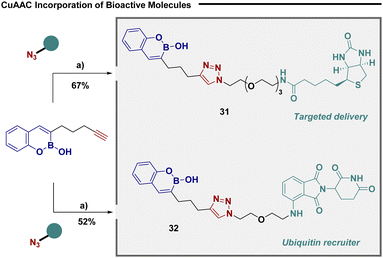 | ||
| Fig. 4 Click enabled synthesis of biochemical probes. (a) 23 (1 equiv.), azide (1 equiv.), Cu(MeCN)4BF4 (30 mol%), DCM, rt. | ||
In summary, we have developed an operationally simple convergent synthesis of 3-substituted bicyclic boronates via a cascade regioselective cross-coupling/annulation protocol using easily accessible vicinal boronic esters and 2-halophenols. The transformation demonstrates high functional group tolerance enabling late stage functionalisation of targets and expedient access to Xeruborbactam derivatives. The stability of the bicyclic boronates under aqueaous basic media inspired chemoselective cross-coupling strategies, while a convergent approach could be strategically aligned with copper-based “click” strategies to attach pendent biochemical probes. It is envisaged the enclosed platform will enable users to efficiently access a prominent set of boron containing heterocycles finding future applications in the chemical sciences, most notably medicinal chemistry.
We gratefully acknowledge financial support from the Max- Planck Society. A. M. and J. J. M. thank the Fonds der Chemischen Industrie, FCI for funding. We thank the Mass Spec facility in the Organic Chemistry Department of Freie Universität Berlin. We thank Kane Bastick for preliminary experiments. Open Access funding provided by the Max Planck Society.
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Notes and references
- A. Pal, M. Bérubé and D. G. Hall, Angew. Chem., Int. Ed., 2010, 49, 1492–1495 CrossRef CAS PubMed
.
- C. R. Wade, A. E. J. Broomsgrove, S. Aldridge and F. P. Gabbaï, Chem. Rev., 2010, 110, 3958–3984 CrossRef CAS PubMed
.
- C. D. Entwistle and T. B. Marder, Angew. Chem., Int. Ed., 2002, 41, 2927–2931 CrossRef CAS PubMed
.
- S. Chatterjee, N. M. Tripathi and A. Bandyopadhyay, Chem. Commun., 2021, 57, 13629–13640 RSC
.
- R. J. Grams, W. L. Santos, I. R. Scorei, A. Abad-García, C. A. Rosenblum, A. Bita, H. Cerecetto, C. Viñas and M. A. Soriano-Ursúa, Chem. Rev., 2024, 124, 2441–2511 CrossRef CAS PubMed
.
- M. Z. H. Kazmi, O. M. Schneider and D. G. Hall, J. Med. Chem., 2023, 66, 13768–13787 CrossRef CAS PubMed
.
- C.-J. Lu, J. Hu, Z. Wang, S. Xie, T. Pan, L. Huang and X. Li, MedChemComm, 2018, 9, 1862–1870 RSC
.
- A. Vlasceanu, M. Jessing and J. P. Kilburn, Bioorg. Med. Chem., 2015, 23, 4453–4461 CrossRef CAS PubMed
.
- F. J. R. Rombouts, F. Tovar, N. Austin, G. Tresadern and A. A. Trabanco, J. Med. Chem., 2015, 58, 9287–9295 CrossRef CAS PubMed
.
- A. Krajnc, J. Brem, P. Hinchliffe, K. Calvopiña, T. D. Panduwawala, P. A. Lang, J. J. A. G. Kamps, J. M. Tyrrell, E. Widlake, B. G. Saward, T. R. Walsh, J. Spencer and C. J. Schofield, J. Med. Chem., 2019, 62, 8544–8556 CrossRef CAS PubMed
.
- S. J. Hecker, K. R. Reddy, O. Lomovskaya, D. C. Griffith, D. Rubio-Aparicio, K. Nelson, R. Tsivkovski, D. Sun, M. Sabet, Z. Tarazi, J. Parkinson, M. Totrov, S. H. Boyer, T. W. Glinka, O. A. Pemberton, Y. Chen and M. N. Dudley, J. Med. Chem., 2020, 63, 7491–7507 CrossRef CAS PubMed
.
- D. B. Diaz and A. K. Yudin, Nat. Chem., 2017, 9, 731–742 CrossRef CAS PubMed
.
- B. J. Graham, I. W. Windsor, B. Gold and R. T. Raines, Proc. Natl. Acad. Sci. U. S. A., 2021, 118, e2013691118 CrossRef CAS PubMed
.
- B. J. Graham, I. W. Windsor and R. T. Raines, ACS Med. Chem. Lett., 2023, 14, 171–175 CrossRef CAS PubMed
.
- C. Körner, P. Starkov and T. D. Sheppard, J. Am. Chem. Soc., 2010, 132, 5968–5969 CrossRef PubMed
.
- J. M. Halford-McGuff, M. Varga, D. B. Cordes, A. P. McKay and A. J. B. Watson, ACS Catal., 2024, 14, 1846–1854 CrossRef CAS PubMed
.
- J. J. Blackner, O. M. Schneider, W. O. Wong and D. G. Hall, J. Am. Chem. Soc., 2024, 146, 19499–19508 CrossRef CAS PubMed
.
- Y. Sumida, R. Harada, T. Kato-Sumida, K. Johmoto, H. Uekusa and T. Hosoya, Org. Lett., 2014, 16, 6240–6243 CrossRef CAS PubMed
.
- H. Saito, S. Otsuka, K. Nogi and H. Yorimitsu, J. Am. Chem. Soc., 2016, 138, 15315–15318 CrossRef CAS PubMed
.
- H. Lyu, I. Kevlishvili, X. Yu, P. Liu and G. Dong, Science, 2021, 372, 175–182 CrossRef CAS PubMed
.
- P.-Y. Peng, G.-S. Zhang, M.-L. Gong, J.-W. Zhang, X.-L. Liu, D. Gao, G.-Q. Lin, Q.-H. Li and P. Tian, Commun. Chem., 2023, 6, 176 CrossRef CAS PubMed
.
- K. Yuan and M. J. Ingleson, Angew. Chem., Int. Ed., 2023, 62, e202301463 CrossRef CAS PubMed
.
- T. Ishiyama, N. Matsuda, N. Miyaura and A. Suzuki, J. Am. Chem. Soc., 1993, 115, 11018–11019 CrossRef CAS
.
- R. L. Thomas, F. E. S. Souza and T. B. Marder, J. Chem. Soc., Dalton Trans., 2001, 1650–1656 RSC
.
- J. B. Morgan and J. P. Morken, J. Am. Chem. Soc., 2004, 126, 15338–15339 CrossRef CAS PubMed
.
- T. Ishiyama, M. Yamamoto and N. Miyaura, Chem. Lett., 1996, 1117–1118 CrossRef CAS
.
- S. N. Mlynarski, C. H. Schuster and J. P. Morken, Nature, 2014, 505, 386–390 CrossRef CAS PubMed
.
- M. Wienhold, J. J. Molloy, C. G. Daniliuc and R. Gilmour, Angew. Chem., Int. Ed., 2021, 60, 685–689 CrossRef CAS PubMed
.
- F. H. Vaillancourt, E. Yeh, D. A. Vosburg, S. Garneau-Tsodikova and C. T. Walsh, Chem. Rev., 2006, 106, 3364–3378 CrossRef CAS PubMed
.
- T. P. Pathak and S. J. Miller, J. Am. Chem. Soc., 2012, 134, 6120–6123 CrossRef CAS PubMed
.
- S. H. Boyer, A. Gonzalez-de-Castro, J. A. H. Dielemans, L. Lefort, Z. Zhu, M. Gnahn, J. Schörghuber, S. Steinhofer, A. H. M. de Vries and S. J. Hecker, Org. Process Res. Dev., 2022, 26, 925–935 CrossRef CAS
.
- R. C. Cambie, P. S. Rutledge, T. Smith-Palmer and P. D. Woodgate, J. Chem. Soc. Perkin Trans. 1, 1976, 1161–1164 RSC
.
- S. H. Kennedy, B. D. Dherange, K. J. Berger and M. D. Levin, Nature, 2021, 593, 223–227 CrossRef CAS PubMed
.
- J. B. Geri, J. V. Oakley, T. Reyes-Robles, T. Wang, S. J. McCarver, C. H. White, F. P. Rodriguez-Rivera, D. L. Parker, E. C. Hett, O. O. Fadeyi, R. C. Oslund and D. W. C. MacMillan, Science, 2020, 367, 1091–1097 CrossRef CAS PubMed
.
- G. Lu, R. E. Middleton, H. Sun, M. Naniong, C. J. Ott, C. S. Mitsiades, K.-K. Wong, J. E. Bradner and W. G. Kaelin, Science, 2014, 343, 305–309 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4cc04653f |
| This journal is © The Royal Society of Chemistry 2024 |