Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Asymmetric cyclopropanation via an electro-organocatalytic cascade†
Anastasiya
Krech‡
 ,
Marharyta
Laktsevich-Iskryk‡
,
Marharyta
Laktsevich-Iskryk‡
 ,
Nora
Deil
,
Nora
Deil
 ,
Mihhail
Fokin
,
Mihhail
Fokin
 ,
Mariliis
Kimm
,
Mariliis
Kimm
 and
Maksim
Ošeka
and
Maksim
Ošeka
 *
*
Department of Chemistry and Biotechnology, Tallinn University of Technology, Akadeemia tee 15, Tallinn 12618, Estonia. E-mail: maksim.oseka@taltech.ee
First published on 22nd October 2024
Abstract
We report an iminium ion-promoted, asymmetric synthesis of cyclopropanes via an electrocatalytic, iodine-mediated ring closure. The mild, controlled electrochemical generation of electrophilic iodine species in catalytic quantities prevents organocatalyst deactivation, while also eliminating the need for halogenating reagents, thus simplifying traditional synthetic approaches.
Over the past two decades, amino-organocatalysis has been extensively employed in the development of asymmetric reactions, exploiting both polar and radical pathways, while combining organocatalysis with metal- and photocatalysis has enabled unconventional transformations.1 Electrochemistry, in turn, offers a greener approach to synthesizing simple molecules and performing late-stage functionalization of complex targets.2 Thus, merging aminocatalysis with electrochemistry presents a highly attractive strategy that could unlock novel enantioselective reactivities in a more sustainable manner.3 However, electrochemical aminocatalysis has been demonstrated feasible only via asymmetric enamine-mediated pathways, which can be categorized into two main strategies (Scheme 1a). The first involves the in situ electrochemical generation of electrophilic partners, which subsequently react with enamines,4 while the second is based on the single-electron oxidation of the enamine intermediate, leading to the formation of a radical cation.5 A major limitation of such transformations stems from the oxidative degradation of chiral organocatalysts or their intermediates under electrochemical conditions. To address this, Mazzarella and Dell’Amico recently employed redox shuttles for a milder, indirect single-electron transfer (SET) oxidation of the enamine intermediate, thereby protecting the catalyst from oxidative damage.5c In another approach, the Hao Xu group designed a bifunctional, diarylprolinol-based chiral electrocatalyst, which acts both as a redox mediator for substrate electrooxidation and a promoter for asymmetric induction through the enamine formation.4f
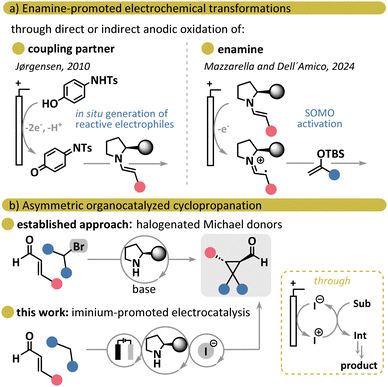 | ||
| Scheme 1 (a) Asymmetric organocatalytic electrochemical strategies. (b) Asymmetric cyclopropanation via MIRC. | ||
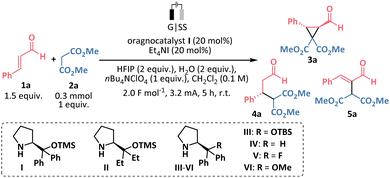
Despite these advances, electrochemical aminocatalysis involving the formation of an iminium ion remains underexplored to the best of our knowledge. Inspired by the concept of constructing cyclopropane rings electrochemically,6 particularly via halogen-mediated reactions,6c–i we sought to develop an asymmetric cyclopropanation under electrochemical conditions to demonstrate the proof-of-principle that electrochemistry is compatible with iminium ion organocatalysis. Considering our group's expertise in Michael-initiated ring closure (MIRC) reactions7 and electrochemical transformations,8 we selected an established protocol for asymmetric cyclopropanation via iminium ion catalysis that previously utilized a cascade process involving α,β-unsaturated aldehydes and halogenated Michael donors as a benchmark reaction (Scheme 1b).9 Importantly, streamlining this protocol to employ simple malonates, while performing the ring-closure cascade using electrocatalytically generated electrophilic halogen species, would improve atom economy for the overall process by eliminating the additional step for the synthesis of halogenated Michael donors that involves stoichiometric amounts of electrophilic halogen sources.
We initiated our investigation using cinnamic aldehyde 1a and dimethyl malonate 2a as model substrates, with organocatalyst I (20 mol%) in a simple undivided cell setup, featuring a graphite (G) anode and a stainless-steel (SS) cathode, under galvanostatic conditions (Table 1). Following the work of the Nikishin group,10 preliminary experiments were conducted in ethanol with catalytic amounts of tetraethylammonium iodide as a halogen source and acid additives (see Table S1 in ESI†). The reproducibility of the reaction improved significantly when ethanol was replaced by dichloromethane as the solvent, with hexafluoroisopropanol (HFIP) and water as additives, and tetrabutylammonium perchlorate as the electrolyte. HFIP serves as a protons source for the cathodic reaction and was found to stabilize iminium ions,11 while water most likely plays a role in the formation and hydrolysis of the iminium ion. The optimal reaction time for our transformation was determined to be 5 hours (Table 1, entry 1). Upon completion of the reaction, we observed the intermediate 4a (up to 32%) in some instances, which results from the nucleophilic addition of malonate 2a to the iminium ion, along with the byproduct 5a (7–24% yield). The formation of 5a has previously been reported in the aminocatalyzed synthesis of 3a, arising from a base-induced retro-Michael reaction of the cyclopropane adduct.9b Prolonging the reaction time to 16 hours led to further conversion of the product 3a into 5a (Table 1, entry 2). Lowering the amount of tetraethylammonium iodide or substituting it with other halogen sources reduced the yield (entries 3–6). To address the partial catalyst decomposition caused by oxidation,5c,12 we explored the use of presumably more stable aminocatalysts II–VI.13 Unfortunately, none of these catalysts improved the yield of the desired product and all showed some degree of decomposition, as confirmed by GC-MS and NMR analyses of the crude reaction mixtures (Table 1, entry 7). Substituting the cathode material with graphite or platinum significantly suppressed cyclopropane 3a formation (Table 1, entry 8), and using acetonitrile as the solvent also lowered the yield (entry 9). Without a halogen source, the reaction yielded only the Michael adduct 4a with excellent enantioselectivity (96% ee, Table 1, entry 10), while no reaction occurred in the absence of the organocatalyst (entry 11). Lastly, a control experiment confirmed that electricity is essential for cyclopropane ring formation (entry 12).
| # | Deviation from reaction conditions | Yield,a % | ee 3a,b % | ||
|---|---|---|---|---|---|
| 3a | 4a | 5a | |||
a Yields were determined by 1H NMR analysis of the crude reaction mixture using trimethoxybenzene as an internal standard.
b Enantiomeric excess of 3a was determined by chiral HPLC analysis.
c Diastereomeric ratio of 3a was determined by 1H NMR analysis of the crude reaction mixture and was for all entries >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. n.d.: not determined. 1. n.d.: not determined.
|
|||||
| 1 | None | 59 | — | 12 | 96 |
| 2 | 1.0 mA, 16 h | 48 | — | 24 | 96 |
| 3 | nBu4NI instead of Et4NI | 47 | 4 | 7 | 97 |
| 4 | I2 instead of Et4NI | 29 | — | — | 94 |
| 5 | nBu4NBr instead of Et4NI | 6 | 21 | — | nd |
| 6 | 0.1 equiv. of Et4NI | 39 | 14 | 7 | 98 |
| 7 | II–VI as organocatalyst | 38–46 | — | Traces | 70–92 |
| 8 | Pt or G as cathode | 9 | 29–32 | — | nd |
| 9 | CH3CN instead of CH2Cl2 | 30 | 9 | — | 94 |
| 10 | No halogen source | — | 33 | — | |
| 11 | No organocatalyst | — | — | — | |
| 12 | No electricity | — | 49 | — | |
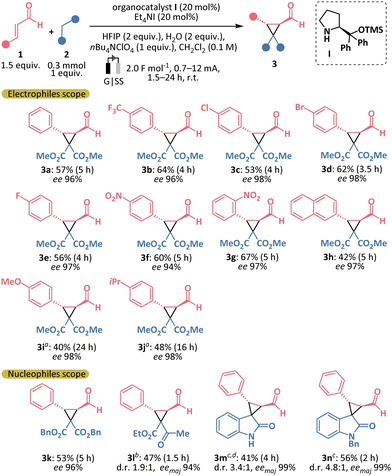
With the optimal conditions established, we investigated the substrate scope for the cascade electro-organocatalytic cyclopropanation (Scheme 2). Aromatic α,β-unsaturated aldehydes (1) reacted efficiently with malonate (2a) under the optimized conditions, yielding the corresponding cyclopropanes (3a–j) with excellent stereoselectivity (94–98% ee and >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 d.r. in all cases). Notably, cyclopropanes 3b–3g, containing electron-deficient substituents in the aromatic ring, were isolated in moderate to good yields. Potentially sensitive to electrochemical conditions nitro- or methoxy- groups were tolerated in the reaction (3f, 3g, 3i). However, in the case of 4-methoxycinnamaldehyde, rapid formation of the byproduct 5i was observed at room temperature (Scheme 4a). To suppress the side product formation, the reaction was performed at 0 °C, which improved the yield of cyclopropane 3i, with 24 hours identified as the optimal reaction time. Similarly, cyclopropane 3j, bearing an electron-donating alkyl group, was obtained at a lower temperature. We then explored different nucleophilic reaction partners. Dibenzyl malonate reacted smoothly, yielding product 3k with results comparable to those obtained with dimethyl malonate. When 1,3-ketoester was used as the nucleophile, cyclopropane 3l was obtained in only 1.5 hours, though with moderate yield and diastereoselectivity. Furthermore, we were pleased to obtain biologically relevant spirooxindoles 3m and 3n with good diastereoselectivity.14
1 d.r. in all cases). Notably, cyclopropanes 3b–3g, containing electron-deficient substituents in the aromatic ring, were isolated in moderate to good yields. Potentially sensitive to electrochemical conditions nitro- or methoxy- groups were tolerated in the reaction (3f, 3g, 3i). However, in the case of 4-methoxycinnamaldehyde, rapid formation of the byproduct 5i was observed at room temperature (Scheme 4a). To suppress the side product formation, the reaction was performed at 0 °C, which improved the yield of cyclopropane 3i, with 24 hours identified as the optimal reaction time. Similarly, cyclopropane 3j, bearing an electron-donating alkyl group, was obtained at a lower temperature. We then explored different nucleophilic reaction partners. Dibenzyl malonate reacted smoothly, yielding product 3k with results comparable to those obtained with dimethyl malonate. When 1,3-ketoester was used as the nucleophile, cyclopropane 3l was obtained in only 1.5 hours, though with moderate yield and diastereoselectivity. Furthermore, we were pleased to obtain biologically relevant spirooxindoles 3m and 3n with good diastereoselectivity.14
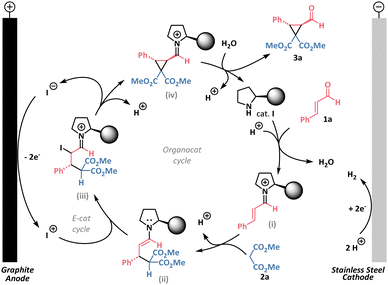
Based on our observations and previous studies, we propose a plausible mechanism, as depicted in Scheme 3. First, in the organocatalytic cycle, condensation of the chiral aminocatalyst I with cinnamic aldehyde 1a occurs forming an iminium ion (i). Upon Michael addition of malonate 2a to the iminium ion (i), an enamine (ii) is generated, which may hydrolyze to intermediate 4a. Electrooxidation of iodide occurs at the anode, forming electrophilic iodine species, which are captured by the enamine (ii).15 The iodinated iminium ion (iii) then undergoes intramolecular alkylation, releasing iodide anion back into the electrocatalytic cycle and forming an iminium ion (iv). Subsequent hydrolysis of the iminium ion (iv) yields the desired product 3a and regenerates the organocatalyst I. Under certain conditions, the intermediate (iv) can undergo a retro-Michael reaction, leading to the formation of the byproduct 5a. Meanwhile, the evolution of hydrogen occurs at the cathode as the counter half-reaction. Thus, the reaction mechanism involves two catalytic cycles, and balancing their rates is crucial for efficient product formation. The electrochemical cycle is controlled by current density, while the organocatalytic cycle can be influenced by temperature (see kinetic studies in Fig. S4 in ESI†).
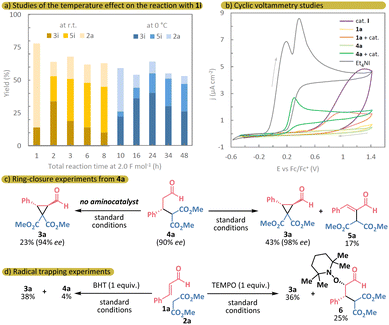
To better understand the reaction mechanism, we first measured the oxidation potentials of the main reaction components using cyclic voltammetry (Scheme 4b). Iodide exhibits two distinct oxidation peaks at potentials of +0.19 V and +0.38 V, with the first oxidation occurring at the lowest potential in the system. However, the enamine (II), formed from the intermediate 4a and organocatalyst I, shows an oxidation peak at +0.27 V, which is lower than iodine's second oxidation peak, suggesting that it may undergo SET oxidation under the reaction conditions. The other reaction components have higher oxidation potentials than iodide and are unlikely to undergo anodic oxidation. Nevertheless, decomposition of the free catalyst through reaction with electrophilic iodine species via Grob-type fragmentation is also possible.6d,11a
We then conducted several control experiments. In the reaction with stoichiometric amounts of molecular iodine or N-iodosuccinimide under the standard conditions, but without applying electricity, no conversion of the starting materials was detected, and organocatalyst I began to decompose. This suggests that controlled electrocatalytic generation of electrophilic iodine likely protects the catalyst from Grob-type fragmentation. Next, we attempted to form the cyclopropane ring under our electrochemical conditions using the pre-synthesized intermediate 4a.16 After the addition of 2.0 F mol−1 over 5 hours, 43% of the desired product 3a was formed, along with 17% of the byproduct 5a (Scheme 4c). Interestingly, after 1 hour of reaction, the intermediate 4a partially fragmented back to cinnamic aldehyde 1a and dimethyl malonate 2a (40% NMR yield). This demonstrates the reversibility of the Michael addition step and highlights the complex kinetics of the overall process (see Fig. S5 in ESI†). In the absence of the organocatalyst, the product 3a was formed less efficiently, with a yield of 23%, and no formation of 1a or 2a was observed.
To determine whether the reaction mechanism has a polar or radical nature, we conducted control experiments with radical scavengers (Scheme 4d). The reaction with 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) under our electrochemical conditions resulted in only minor suppression of the product 3a formation (36% NMR yield), and a TEMPO-adduct 6 was observed (25% NMR yield) (see Scheme S2 in ESI† for the alternative radical pathway).6i However, electrochemical experiments using TEMPO as a radical trap can produce ambiguous results, as TEMPO can be oxidized to its N-oxoammonium salt under electrochemical conditions and react with the enamine (ii) in a polar manner.17 We then switched to dibutylhydroxytoluene (BHT) as a more reliable radical scavenger in electrochemistry and still observed the formation of cyclopropane 3a (38% NMR yield), with only 4% of 4a detected in the crude NMR, indicating that no quenching of the potential enamine radical cation occurred. These results, combined with the earlier experiment demonstrating ring closure in the absence of the aminocatalyst directly from the intermediate 4a, suggest a predominantly polar mechanistic pathway in the electrochemical catalytic cycle.
In conclusion, we demonstrated the successful integration of electrochemistry with iminium ion organocatalysis to achieve highly enantioselective cyclopropanation. This streamlined process enhances atom economy by eliminating the need for pre-synthesized halogenated Michael donors. Mechanistic studies, supported by cyclic voltammetry and control experiments, suggest a predominantly polar pathway in the electrochemical catalytic cycle, driven by anodic iodide oxidation.
This work was supported by the Estonian Research Council grant (PSG828). The authors would also like to thank Tatsiana Jarg for the HRMS analysis.
Data availability
The data supporting this article are included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Notes and references
- Selected reviews on asymmetric organocatalysis with metal- and photocatalysis, see: (a) D.-F. Chen, Z.-Y. Han, X.-L. Zhou and L.-Z. Gong, Acc. Chem. Res., 2014, 47, 2365–2377 CrossRef CAS PubMed; (b) Q. Zhou, Angew. Chem., Int. Ed., 2016, 55, 5352–5353 CrossRef CAS PubMed; (c) M. Silvi and P. Melchiorre, Nature, 2018, 554, 41–49 CrossRef CAS PubMed; (d) D. A. Nagib, Chem. Rev., 2022, 122, 15989–15992 CrossRef CAS PubMed; (e) T. Bortolato, S. Cuadros, G. Simionato and L. Dell’Amico, Chem. Commun., 2022, 58, 1263–1283 RSC.
- (a) Y. Wang, S. Dana, H. Long, Y. Xu, Y. Li, N. Kaplaneris and L. Ackermann, Chem. Rev., 2023, 123, 11269–11335 CrossRef CAS PubMed; (b) L. F. T. Novaes, J. S. K. Ho, K. Mao, E. Villemure, J. A. Terrett and S. Lin, J. Am. Chem. Soc., 2024, 146, 22982–22992 CrossRef CAS PubMed; (c) B. P. Smith, N. J. Truax, A. S. Pollatos, M. Meanwell, P. Bedekar, A. F. Garrido-Castro and P. S. Baran, Angew. Chem., Int. Ed., 2024, 63, e202401107 CrossRef CAS PubMed.
- For recent reviews on asymmetric electrochemistry, see: (a) K. A. Ogawa and A. J. Boydston, Chem. Lett., 2015, 44, 10–16 CrossRef; (b) C. Margarita and H. Lundberg, Catalysts, 2020, 10, 982 CrossRef CAS; (c) J. Rein, S. B. Zacate, K. Mao and S. Lin, Chem. Soc. Rev., 2023, 52, 8106–8125 RSC; (d) D. Mazzarella, J. Flow Chem., 2024, 14, 357–366 CrossRef CAS.
- (a) K. L. Jensen, P. T. Franke, L. T. Nielsen, K. Daasbjerg and K. A. Jørgensen, Angew. Chem., Int. Ed., 2010, 49, 129–133 CrossRef CAS PubMed; (b) N. Fu, L. Li, Q. Yang and S. Luo, Org. Lett., 2017, 19, 2122–2125 CrossRef CAS PubMed; (c) L. Li, Y. Li, N. Fu, L. Zhang and S. Luo, Angew. Chem., Int. Ed., 2020, 59, 14347–14351 CrossRef CAS PubMed; (d) F.-Y. Lu, Y.-J. Chen, Y. Chen, X. Ding, Z. Guan and Y.-H. He, Chem. Commun., 2020, 56, 623–626 RSC; (e) Z.-H. Wang, P.-S. Gao, X. Wang, J.-Q. Gao, X.-T. Xu, Z. He, C. Ma and T.-S. Mei, J. Am. Chem. Soc., 2021, 143, 15599–15605 CrossRef CAS PubMed; (f) J.-Y. He, C. Zhu, W.-X. Duan, L.-X. Kong, N.-N. Wang, Y.-Z. Wang, Z.-Y. Fan, X.-Y. Qiao and H. Xu, Angew. Chem., Int. Ed., 2024, e202401355 CAS.
- (a) X. Ho, S. Mho, H. Kang and H. Jang, Eur. J. Org. Chem., 2010, 4436–444s1 CrossRef CAS; (b) N. Bui, X. Ho, S. Mho and H. Jang, Eur. J. Org. Chem., 2009, 5309–5312 CrossRef CAS; (c) D. Mazzarella, C. Qi, M. Vanzella, A. Sartorel, G. Pelosi and L. Dell’Amico, Angew. Chem., Int. Ed., 2024, 63, e202401361 CrossRef CAS PubMed.
- (a) X. Han, N. Zhang, Q. Li, Y. Zhang and S. Das, Chem. Sci., 2024, 15, 13576–13604 RSC; (b) L.-H. Jie and H.-C. Xu, J. Electrochem., 2024, 30, 2313001 Search PubMed For the reviews and examples of halogen-mediated transformations, see: ; (c) B. V. Lyalin and V. A. Petrosyan, Russ. J. Electrochem., 2013, 49, 497–529 CrossRef CAS; (d) C. Luo, Z. Wang and Y. Huang, Nat. Commun., 2015, 6, 10041 CrossRef PubMed; (e) M. N. Elinson, E. O. Dorofeeva, A. N. Vereshchagin and G. I. Nikishin, Russ. Chem. Rev., 2015, 84, 485–497 CrossRef CAS; (f) K. Liu, C. Song and A. Lei, Org. Biomol. Chem., 2018, 16, 2375–2387 RSC; (g) H.-T. Tang, J.-S. Jia and Y.-M. Pan, Org. Biomol. Chem., 2020, 18, 5315–5333 RSC; (h) Z. Wang, M. Gausmann, J.-H. Dickoff and M. Christmann, Green Chem., 2024, 26, 2546–2551 RSC; (i) P. Zhou, W. Li, J. Lan and T. Zhu, Nat Commun., 2022, 13, 3827 CrossRef CAS PubMed.
- (a) A. Noole, M. Ošeka, T. Pehk, M. Öeren, I. Järving, M. R. J. Elsegood, A. V. Malkov, M. Lopp and T. Kanger, Adv. Synth. Catal., 2013, 355, 829–835 CrossRef CAS; (b) M. Ošeka, A. Noole, S. Žari, M. Öeren, I. Järving, M. Lopp and T. Kanger, Eur. J. Org. Chem., 2014, 3599–3606 CrossRef.
- (a) M. Ošeka, G. Laudadio, N. P. Van Leest, M. Dyga, A. D. A. Bartolomeu, L. J. Gooßen, B. De Bruin, K. T. De Oliveira and T. Noël, Chem, 2021, 7, 255–266 CrossRef; (b) A. Kooli, L. Wesenberg, M. Beslać, A. Krech, M. Lopp, T. Noël and M. Ošeka, Eur. J. Org. Chem., 2022, e202200011 CrossRef CAS; (c) M. Laktsevich-Iskryk, A. Krech, M. Fokin, M. Kimm, T. Jarg, T. Noël and M. Ošeka, J. Flow Chem., 2024, 14, 139–147 CrossRef CAS.
- Previous reports on the reaction: (a) R. Rios, H. Sundén, J. Vesely, G. Zhao, P. Dziedzic and A. Córdova, Adv. Synth. Catal., 2007, 349, 1028–1032 CrossRef CAS; (b) H. Xie, L. Zu, H. Li, J. Wang and W. Wang, J. Am. Chem. Soc., 2007, 129, 10886–10894 CrossRef CAS PubMed; (c) I. Ibrahem, G. Zhao, R. Rios, J. Vesely, H. Sundén, P. Dziedzic and A. Córdova, Chem. Eur. J., 2008, 14, 7867–7879 CrossRef CAS PubMed; (d) P. Llanes, C. Rodríguez-Escrich, S. Sayalero and M. A. Pericàs, Org. Lett., 2016, 18, 6292–6295 CrossRef CAS PubMed; (e) A. Kunzendorf, G. Xu, M. Saifuddin, T. Saravanan and G. J. Poelarends, Angew. Chem., Int. Ed., 2021, 60, 24059–24063 CrossRef CAS PubMed.
- M. N. Elinson, S. K. Feducovich, A. N. Vereshchagin, S. V. Gorbunov, P. A. Belyakov and G. I. Nikishin, Tetrahedron Lett., 2006, 47, 9129–9133 CrossRef CAS.
- (a) G. Hutchinson, C. Alamillo-Ferrer and J. Burés, J. Am. Chem. Soc., 2021, 143, 6805–6809 CrossRef CAS PubMed; (b) N. Zeidan, S. Bicic, R. J. Mayer, D. Lebœuf and J. Moran, Chem. Sci., 2022, 13, 8436–8443 RSC.
- M. Silvi, C. Verrier, Y. P. Rey, L. Buzzetti and P. Melchiorre, Nat. Chem., 2017, 9, 868–873 CrossRef CAS PubMed.
- (a) M. H. Haindl, M. B. Schmid, K. Zeitler and R. M. Gschwind, RSC Adv., 2012, 2, 5941 RSC; (b) X. Companyó and J. Burés, J. Am. Chem. Soc., 2017, 139, 8432–8435 CrossRef PubMed.
- L.-M. Zhou, R.-Y. Qu and G.-F. Yang, Expert Opin. Drug Discovery, 2020, 15, 603–625 CrossRef CAS PubMed.
- T. Kano, M. Ueda and K. Maruoka, J. Am. Chem. Soc., 2008, 130, 3728–3729 CrossRef CAS PubMed.
- S. Brandau, A. Landa, J. Franzén, M. Marigo and K. A. Jørgensen, Angew. Chem., Int. Ed., 2006, 45, 4305–4309 CrossRef CAS PubMed.
- M. Schämann and H. J. Schäfer, Electrochim. Acta, 2005, 50, 4956–4972 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4cc05092d |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2024 |