Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Endohedral metallofullerene molecular nanomagnets
Ziqi
Hu
 * and
Shangfeng
Yang
* and
Shangfeng
Yang
 *
*
Key Laboratory of Precision and Intelligent Chemistry, Collaborative Innovation Center of Chemistry for Energy Materials (iChEM), Department of Materials Science and Engineering, Anhui Laboratory of Advanced Photon Science and Technology, University of Science and Technology of China, Hefei 230026, China. E-mail: huziqi@ustc.edu.cn; sfyang@ustc.edu.cn
First published on 7th February 2024
Abstract
Magnetic lanthanide (Ln) metal complexes exhibiting magnetic bistability can behave as molecular nanomagnets, also known as single-molecule magnets (SMMs), suitable for storing magnetic information at the molecular level, thus attracting extensive interest in the quest for high-density information storage and quantum information technologies. Upon encapsulating Ln ion(s) into fullerene cages, endohedral metallofullerenes (EMFs) have been proven as a promising and versatile platform to realize chemically robust SMMs, in which the magnetic properties are able to be readily tailored by altering the configurations of the encapsulated species and the host cages. In this review, we present critical discussions on the molecular structures and magnetic characterizations of EMF-SMMs, with the focus on their peculiar molecular and electronic structures and on the intriguing molecular magnetism arising from such structural uniqueness. In this context, different families of magnetic EMFs are summarized, including mononuclear EMF-SMMs wherein single-ion anisotropy is decisive, dinuclear clusterfullerenes whose magnetism is governed by intramolecular magnetic interaction, and radical-bridged dimetallic EMFs with high-spin ground states that arise from the strong ferromagnetic coupling. We then discuss how molecular assemblies of SMMs can be constructed, in a way that the original SMM behavior is either retained or altered in a controlled manner, thanks to the chemical robustness of EMFs. Finally, on the basis of understanding the structure–magnetic property correlation, we propose design strategies for high-performance EMF-SMMs by engineering ligand fields, electronic structures, magnetic interactions, and molecular vibrations that can couple to the spin states.
1. Introduction
Molecular nanomagnets represented by single-molecule magnets (SMMs) are magnetic molecules that possess bistable magnetic states depending on the alignment of the unpaired electrons and are able to maintain magnetization in the absence of an applied magnetic field.1 This magnetic bistability at the molecular level and not related to a cooperative effect, concomitant with the intriguing quantum phenomena in the dynamics of their magnetization as well as the feasibility of rational design, atomically precise synthesis and multidimensional organizations, enables SMMs to be promising candidates for applications in high-density information storage,1 spintronic devices2 and quantum information technologies.3,4 Since the discovery of the high-spin manganese cluster Mn12O12(acetate)16 (Mn12Ac) as the first SMM in 1993,5 the major challenge for SMMs is their fragile magnetic bistability that is present so far only at cryogenic temperatures, and thus a central topic in the field has always been the exploration of methods to increase the blocking temperature (TB), up to which the SMM behavior can be observed.The advent of SMMs initially aroused research interests in multinuclear transition metal cluster compounds with the aim to increase the effective energy barrier to magnetization reversal (Ueff) by increasing the spin ground state and ligand field splitting exerted by the coordinated ligands. Later, it was found that metal complexes containing a single lanthanide (Ln) ion may serve as SMMs with large Ueff in the presence of ligand fields, which stems from the larger magnetic moment and single-ion anisotropy of Ln ions as a result of much stronger spin–orbit coupling (SOC) compared to the transition metal ions.6–8 This category of Ln-based single-ion magnets (SIMs) constitutes a key part of the whole SMM family and has been receiving much research attention. The fine control of the single-ion anisotropy in SIMs thus becomes feasible through coordination chemistry, for which several design criteria were proposed mainly focusing on the enhancement of ligand fields.9,10 These synthetic strategies are exemplified, for instance, by introducing cyclopentadienyl (Cp) ligands to ensure an axial anisotropy of a dysprosium metallocene cation [Dy(Cpttt)2]+ (Cpttt = 1,2,4-tri-tert-butyl Cp), enabling a remarkably high TB of 60 K at which magnetic hysteresis still persists. Such an extraordinary SMM property has been later improved up to 80 K, exceeding the liquid nitrogen temperature (77 K), by engineering ligand structures to protect spin states from the phonon relaxation bath which is mediated by molecular vibrations.11,12
Besides the low TB that hampers the applications of SMMs, another major obstacle to their practical application in information storage is the prevailing chemical instability of organometallic compounds, which precludes their sublimation in order to obtain organized molecular assemblies on surfaces. Thus, inert ligands that are able to protect magnetic Ln ions are necessary. In this context, encapsulating Ln ion(s) into a chemically stable fullerene cage offers an alternative solution. The as-formed endohedral metallofullerenes (EMFs) feature a unique intramolecular host–guest molecular structure, in which specific metal clusters with previously unseen bonding and electronic configurations are stabilized in a way that could not be fulfilled by conventional synthetic routes.13,14 Since the discovery of the first EMF-SMMs in a mixed trimetallic nitride clusterfullerene (NCF) DySc2N@C80,15 EMFs have become a fertile territory in the exploration of high-performance SMMs by providing a rich structural diversity of both the encapsulated species and the host fullerene cages. Apart from metal clusterfullerenes bearing metal clusters in the cage, conventional EMFs based on the encapsulation of single or multiple metal ions only are also found to behave as decent SMMs.16,17 A recent manifestation demonstrates that a low-coordination monometallic azafullerene Dy@C81N displays a high standardized blocking temperature (TB,100s, defined as the temperature of 100 s relaxation time T(τ100s)) of 45 K despite a weak ligand field, highlighting the uniqueness of EMFs in which the outstanding SMM performance is ascribed to the quenching of the extra radical on the cage by nitrogen substitution and the very weak coupling of the molecular vibrations to the spin states.16 Another notable example lies in the discovery of several dimetallic EMF-SMMs M2@C79N (M = Dy and Tb) and M2@C80-R (R = CF3 and PhCH2) exhibiting giant coercive field and a high TB,100s of up to 25.2 K for Tb2@C80(CH2Ph), where a single-electron metal–metal bond (SEMB) is stabilized to evoke strong magnetic coupling through quenching the unpaired electron on the cage by nitrogen substitution or exohedral derivatization.18–22 Remarkably, this structural peculiarity is not an exclusive case in EMFs, yet offers a general synthetic insight and can be extended to strongly coupled mixed-valence dilanthanide SMMs (CpiPr5)2Ln2I3 (Ln = Dy and Tb) that hold the current record TB,100s value of 72 K.23
Exploiting the high chemical and thermal stability of fullerene structures, neutral EMF-SMMs can be readily processed into organized molecular assemblies, such as sub-monolayer deposition on substrates through sublimation or self-assembly.24–26 This is in striking contrast to most ionic organometallic Ln-SMMs, which are air- and moisture-sensitive and would decompose upon heating or interacting with the substrates. This merit of EMFs allows a step forward in the field of SMMs by facilitating their further integration into spintronic devices, which would be eventually utilized to write in and read out magnetic information. Thus, EMFs are expected to continue to play a crucial role in the future development of SMMs. Moreover, the structural diversity of EMFs shall lead to further synthetic advancements by presenting new structures that in turn contribute to new physical phenomena in molecular magnetism.
In this review, we present an exhaustive review on all types of EMF-SMMs reported to date, including their syntheses, molecular structures, magnetic properties and organizations into molecular assemblies. We pay special attention to the versatile molecular structures of EMFs to unveil their unique structural features in comparison with conventional organometallic compounds, and manage to disclose the correlation between their structures with the magnetic properties. At the end, we propose design strategies for EMF-SMMs towards improved performance by engineering underlying factors such as ligand fields, electronic structures, magnetic interactions, and molecular vibrations that can couple to the spin states.
2. Syntheses and molecular structures of EMFs
EMFs are typically synthesized by using the Krätschmer–Huffman DC-arc discharge method,27 in which a metal precursor, in the form of metal alloy or metal oxide, is evaporated together with the graphite anode under a helium atmosphere as a cooling gas. Other gases or solid compounds, such as N2, NH3, CH4 and Prussian blue (Fe4[Fe(CN)6]3), can be added as reactive gases and nitrogen sources for the synthesis of a desired EMF.28–30 The EMF-containing raw soot produced by the DC-arc discharge approach is then extracted using organic solvents, such as CS2 and ortho-dichlorobenzene (o-DCB), using a Soxhlet extraction or reflux method to separate soluble fullerene-related compounds from other carbon materials. The final step is to isolate isomerically pure EMFs using multi-stage high-performance liquid chromatography (HPLC). Since the production process takes place at very high temperatures (3000–5000 K), the extracted products include a rich variety of empty fullerenes and endohedral metallofullerenes with different structures of the host cages and the encapsulated species. This makes the HPLC isolation a demanding and time-consuming process, but it is still feasible by employing a combination of different modified HPLC columns. Overall, the yields of EMFs in the purified forms are usually very low (in the order of milligram). Efforts have been devoted to increasing their production yields, resulting in some success in the selective synthesis of particular types of EMFs.20,29–31The accurate structural determination of the isolated EMFs is achieved by single-crystal X-ray crystallography, for which co-crystals are obtained in the presence of nickel octaethylporphyrin (NiII(OEP))32 or decapyrrylcorannulene (DPC)33 hosts to restrict the rotation of the fullerene cages. For additional structural characterization various spectroscopic techniques including 13C NMR, IR and Raman spectroscopies are utilized in aid of DFT calculations. Note that for EMFs containing paramagnetic Ln ions that are of interest for magnetic characterization, it is difficult to record 13C NMR spectra due to spectral broadening caused by such magnetic centers. The detailed discussions on the synthesis, separation and structural characterization of EMFs have been summarized in several recent reviews.13,14,34–36
EMFs have versatile molecular structures dependent on the encapsulated species and the outer fullerene cages, and thus the corresponding magnetic properties are tunable upon changing their structures.13 For the conventional EMFs based on encapsulation of single or multiple metal ions only, mono-, di- and tri-metallofullerenes can be formed by encaging different numbers (one to three) of metal atoms. In this context, mono- and di-EMFs are of particular interest for the magnetic studies in this review due to their structural diversity, whereas examples of tri-EMFs are very limited.16,17,37 In fact, mono-EMF La@C82 dates back to a very early period just after the discovery of the empty fullerene C60.38,39 It should be noted that the encapsulated metal atoms transfer their valence electrons to the host fullerene cage, which is distinct from the situation of endohedral fullerenes encapsulating only nonmetal elements as exemplified by N@C60.40 This feature provides another aspect for fine-tuning the magnetic properties of EMFs by altering their electronic structures through nitrogen substitution41–43 and exohedral functionalization18,20,44 on the cages.
Alternatively, metal atoms can bond with nonmetal ligands inside fullerene cages to form clusterfullerenes.14 Starting from a coincidental discovery of the nitride clusterfullerene (NCF) Sc3N@C80,28 which has a relatively high yield during the DC-arc discharging process that is inferior to only C60 and C70, various types of clusterfullerenes have been synthesized including carbide,45 oxide,46 sulfide,29 cyanide47 clusterfullerenes, etc., where the nonmetal ligands formally possess negative charges such as N3−, C22−, O2−, S2−, and CN−, respectively. From the point of view of magnetic properties, these clusterfullerenes allow the manipulation of the ligand fields exerted on the Ln ions via coordination chemistry, thus adding another degree of freedom that can be used for the design of EMF-SMMs.
On the other hand, the structures of fullerene cages hosting encapsulated metal species are diverse not only in cage size but also in cage isomerism. The reported EMFs to date have a cage size ranging very broadly from C60 in Li@C60 cations to C108 in Y2C2@C108.48,49 The large cage family of EMFs, considering numerous isomers for the same cage size involving different connection ways of pentagons and hexagons, is further expanded by breaking the famous isolated-pentagon rule (IPR).50 Among them, the most notable example is the highly symmetric icosahedral Ih(7)-C80 due to its exceptional stability after accepting six electrons from the encapsulated species, based on which various EMF-SMMs are constructed.17,51,52 These rich cage structural features provide the feasibility of engineering ligand fields around metal ions by the control of metal–cage interaction. Furthermore, the configuration of the encapsulated species may be influenced by the structural variation of the cage, in a way that the cluster geometry or the metal–metal distance is changed, which enables the manipulation of ligand fields or magnetic exchange interactions. Molecular structures of representative EMFs of interest for magnetic studies in this review are illustrated in Fig. 1.
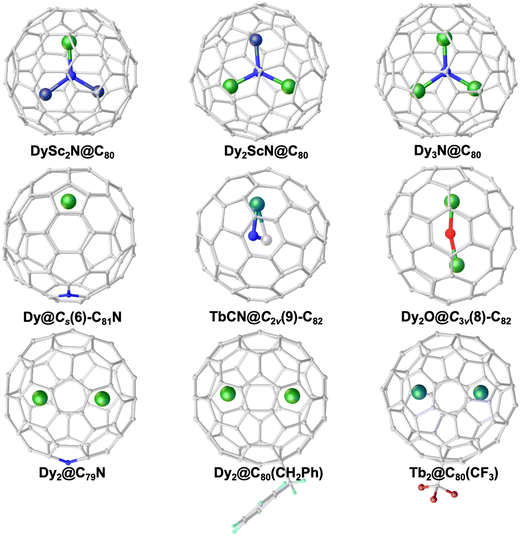 | ||
| Fig. 1 Molecular structures of representative EMF-SMMs. Green: Dy, cyan: Tb, dark blue: Sc, blue: N, red: O, magenta: F, and gray: C. | ||
3. Magnetic characterization of EMF-SMMs
The majority of SMMs are characterized using the conventional superconducting quantum interference device (SQUID) magnetometers. There are several metrics to evaluate the performance of an SMM. Here we summarize and explain these different metrics, and unify common characterization of the static magnetism and the dynamic behavior. When it comes to the magnetic characterization of EMFs, special care should be undertaken mainly owing to their low yields, which may lead to various challenges in sample preparation, measurement and data analysis. We try to clarify some practical concerns in these steps and discuss detailed issues that need to be taken into account.The low sample amount of EMFs (usually around 1 mg) makes the precise determination of the sample mass difficult, which normally requires a sample preparation method by drop-casting from CS2 solution of an EMF into a diamagnetic propylene capsule.18 Another method utilizes paramagnetic Al foil as the sample holder with its well-characterized magnetic behaviors.22,53 In any case, background correction is necessary to accurately record the magnetization signal of the measured EMF. Since the EMF samples are mainly prepared in powder rather than in crystalline forms for magnetic characterization studies, the fullerene molecules are strongly disordered such that the measured values of magnetization should be doubled due to an isotropic distribution of easy axes of the molecules.18,54 It becomes impossible to precisely determine the sample mass when it is less than 0.1 mg. Under this circumstance, a standard quartz sample holder can be used for a vibrating sample magnetometer (VSM) SQUID, which has a negligible diamagnetic background signal at helium temperatures.55 Valuable information can still be obtained in this context by relying on the shapes, instead of the absolute values, of the variable-field magnetic susceptibility and variable-temperature magnetization data.
One of the most straightforward ways to identify an SMM is to record a magnetic hysteresis loop at a given temperature, which presents a magnetic memory effect that comes from the magnetic bistability. The opening of the hysteresis loops up to a certain temperature has been well-used as one definition of TB. However, this hysteretic behavior is highly dependent on the sweep rate of the applied magnetic field during the measurement, such that the temperature at which hysteresis persists is not parameter-free. Another definition of TB is derived from the bifurcation point of the zero-field cooling/field cooling (ZFC/FC) magnetic susceptibility curves. Similarly, this bifurcation temperature relies on the experimental temperature sweep rate. Thus, the comparison of the TB values among different SMMs, determined either as hysteresis temperature or ZFC/FC bifurcation temperature, should be done with caution. Apart from TB, the width of the hysteresis loop (i.e. coercive field, Hc) is equally important, which is the measure of the ability of an SMM to withstand while being demagnetized, and thus it would directly affect the stability and reliability of magnetic information that can be stored. The coercive field is mainly governed by the quantum tunneling effect that takes place close to zero magnetic field. This again is related to the sweep rate of the applied magnetic field.
A more reliable definition of TB, at which 100 s magnetic relaxation time (τ) is determined from the decay of magnetization, is free of experimental parameters and thus serves as a golden standard for SMMs in current state-of-the-art research.1 This demagnetization at a given temperature follows an exponential decay of magnetization M over time
| M(t) = Meq + (M0 − Meq)exp[−(t/τ)β] | (1) |
τ−1 = τQTM−1 + AHT + CTn + τ0−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−Ueff/T) exp(−Ueff/T) | (2) |
In addition to demagnetization measurements which normally determine τ > 10 s, the full characterization of the magnetic relaxation with τ spanning over a large range from 10−3 s to 1 s requires the dynamic studies by alternating current (AC) magnetometry. These AC measurements could be done in the absence of a static magnetic field with the variable frequency of the AC field in the range of 1–1000 Hz, and give rise to frequency-dependent in-phase (χ′) and out-of-phase (χ′′) susceptibilities, from which τ can be determined at different temperatures by fitting the data using a generalized Debye model. The exceptionally low yields of EMFs, however, often impede the accurate characterization using AC susceptibility to record the Orbach process for the determination of Ueff. This problem becomes worse when the slow magnetic relaxation is extended to high temperatures (>50 K), at which the sensitivity becomes lower since the out-of-phase signal χ′′ almost vanishes based on the small population difference between spin states separated by a weak (∼5 Oe) driving AC field.16,64
Alternatively, magnetic characterization of an SMM can also be performed via X-ray circular magnetic dichroism (XMCD) experiments on the atomic level. This advanced element-specific technique relies on X-ray absorption spectroscopy (XAS) for an SMM at the Ln M4,5 edge, allowing for the unambiguous determination of the magnetic properties at its Ln origin, from which field-dependent XMCD hysteresis loops are extracted. Moreover, detailed atomic information, such as the expected values of the spin quantum number, angular momentum and the exact magnetic moment, can be precisely obtained by applying XMCD sum rules at the Ln edge, offering an in-depth insight into the magnetic behaviors of an SMM. It is noteworthy that much less sample is required for XMCD measurements compared to the amount used for commercial SQUID magnetometers, which is suitable for EMF-SMMs with low yields. However, the much noisier magnetic signal from XMCD measurements hampers the characterization of SMMs with weak coercive fields and low remanence at relatively high temperatures.
4. Categories of EMF-SMMs
4.1 Clusterfullerenes
4.1.1.1 NCFs as single-ion magnets. The advent of trimetallic nitride clusterfullerenes (NCFs) showing the composition of (M13+)x(M23+)3−x(N3−)@C2n6− (M1/M2 = rare earth metals, Ti, V; x = 1–3),31,65–71 with Sc3N@Ih(7)-C80 as an elegant example,28 has opened up a new avenue for EMF research by offering a possibility to construct metal clusters inside the carbon cages. It is noteworthy that in this family of compounds, the most stable configuration of the encapsulated nitride cluster is a planar one, thus inducing a strong cluster size-dependent selection of preferential fullerene cages, which move from the most prevalent C80 to C88 and even to C96 as the radius of the metal ion increases.32,72–74 From the magnetic point of view, the single-ion anisotropy of a magnetic Ln ion can thus be tailored by the encapsulated N3− ligand and the outer cage coordination. To understand this feature in NCFs employing the trimetallic nitride template, the simplest model is a single-ion magnet (SIM) with only one magnetic center. This calls for a mixed trimetallic nitride through substituting one diamagnetic Sc3+ ion in Sc3N@C80 by a magnetic Ln3+ ion. In this regard, Dy3+ and Tb3+ with the oblate 4f electron density strongly facilitate axial single-ion anisotropy and thus are the most prevalent building blocks to design SMMs.75 Specifically, Dy3+ has an odd number of f electrons (f9) with the ground multiplet of 6H15/2. This follows the Kramers theorem which indicates that the degeneracy of the ground doublet (mJ = ±15/2) cannot be lifted in the absence of a magnetic field. In contrast, the non-Kramers ion Tb3+ is an even-number system without fundamental degeneracy. This leads to a tunnel splitting between the two lowest spin states and often gives rise to fast magnetic relaxation when the strict axiality is broken by low-symmetry ligand fields. As a result, majority of SIMs are based on Dy3+.76,77
Following this strategy, the first EMF-SMM was reported by Gerber et al. in 2012 for DySc2N@C80, in which the nitrogen ligand (N3−) imposes a strong ligand field around Dy3+ to achieve axial magnetic anisotropy.15 This results in a magnetic hysteresis as shown in Fig. 2a that persists up to 5 K using a SQUID magnetometer at a slow magnetic field sweep rate of 1.3 mT s−1. The hysteresis loop shows a clear drop in the low-field region with a butterfly shape, which indicates a fast QTM process typical of SIMs. This magnetic behavior is also confirmed by element-specific XMCD measurement, which shows hysteresis of the 4f spin and orbital moment in Dy3+ (Fig. 2b). Despite this, slow magnetic relaxation with τ exceeding 2000 s has been evidenced at zero field. The QTM effect can be effectively quenched by applying a magnetic field of 0.3 T, which facilitates the determination of TB,100s = 4.6 K. Such an effect is also suppressed by diluting DySc2N@C80 within a diamagnetic C60 matrix to greatly reduce intermolecular interactions. As a result, τ > 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 s is determined at zero field.
000 s is determined at zero field.
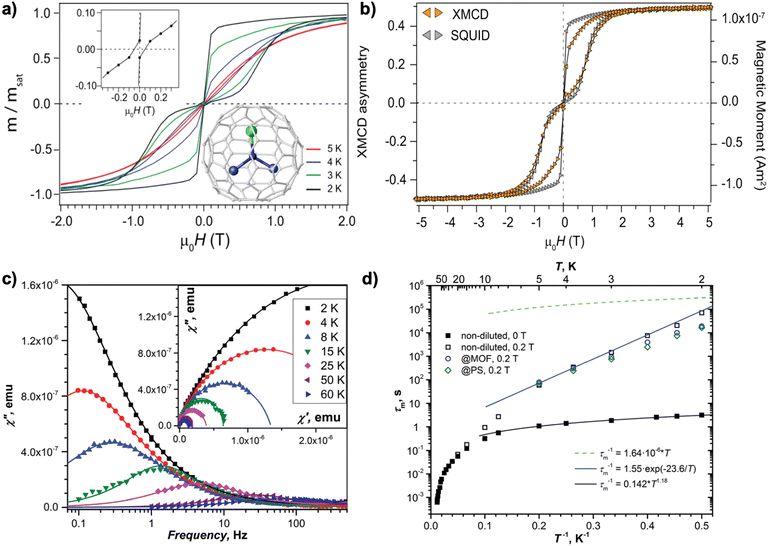 | ||
| Fig. 2 (a) Magnetic hysteresis loops for DySc2N@C80 (the molecular structure is shown in the inset) recorded using SQUID magnetometry at 2–5 K with a field sweep rate of 1.3 mT s−1. (b) Comparison of the magnetic hysteresis loops for DySc2N@C80 recorded by SQUID and XMCD magnetometry at 2 K. Reproduced with permission from ref. 15 (Copyright 2012, American Chemical Society). (c) Out-of-phase magnetic susceptibility χ′′ of DySc2N@C80 measured in zero field in the 2–60 K range. The inset shows Cole–Cole plots. The solid lines are fits by the generalized Debye model. (d) Magnetization relaxation times of DySc2N@C80 at temperatures of 2–87 K. Zero-field values are shown as full dots and in-field (0.2 T) values are denoted as open dots. Relaxation times for non-diluted DySc2N@C80 are shown in black, and the values for diluted samples are shown in blue (diluted with MOFs) and green (diluted with polystyrene, PS). Reproduced from ref. 64. | ||
Since this first discovery, the magnetic behaviors of DySc2N@C80 have been extensively studied. Using the XMCD technique, the same group reported that X-rays lead to an increased relaxation rate of magnetization, which is induced by the resonant absorption of the X-ray irradiation and is dependent on its dose.80 This additional demagnetization upon X-ray irradiation is helpful in understanding the thinner XMCD magnetic hysteresis compared to that recorded by using conventional SQUID magnetometry. An in-depth research of DySc2N@C80, focusing on its powder and single-crystal samples, as well as with solid dilutions in three different diamagnetic matrices, demonstrates that the dilution enlarges the hysteresis loops in the low-field region.64 In this context, the use of a polystyrene diamagnetic matrix is the most effective approach, which steers the QTM resonance from 150 mT in an undiluted sample to <1 mT. This change of the zero-field tunneling resonance width upon dilution significantly affects the relaxation rates and can be understood with the aid of an analysis of dipolar field distributions. Surprisingly, magnetic relaxation of DySc2N@C80 can be accessed by AC magnetometry in a very broad temperature range from 2 to 87 K (Fig. 2c and d). Magnetic relaxation up to such a high temperature is caused by the absence of a fast and linear Orbach process usually observed for other SMMs.
The single-ion anisotropy of Dy3+ can be manipulated in this type of NCF when Sc3+ is replaced by other diamagnetic rare earth metal ions such as Y3+ and Lu3+. This is essentially due to the inner strain of the trimetallic cluster that is controlled by the sizes of different metals. Note that DyLa2N@C80 cannot be synthesized since the encapsulated cluster is too large to maintain a planar configuration. It has been shown that introducing a larger Lu3+ ion leads to a shorter Dy–N bond and hence a stronger ligand field than in DySc2N@C80.78 Consequently, improved SMM properties are observed for DyLu2N@C80 with a TB,ZFC of 9.5 K in comparison with that of its traditional scandium congener (7 K). This study also points out that the mass of the diamagnetic metal ion is another important factor governing the SMM properties of NCFs by influencing cluster-based vibrations, which is achieved via a strong mixing of the local cluster vibrations with acoustic phonons, thus eventually affecting the magnetic relaxation. This observation has further been analyzed systematically in DyM2N@C80 (M = Sc, Y and Lu), which reveals that a larger size of M leads to stronger ligand fields in the order of Y > Lu > Sc. Moreover, magnetic behaviors are found to be correlated with the mass of M. This results in the improved SMM properties, both in terms of hysteresis at higher temperature and longer relaxation times, in the order of Lu > Y > Sc (Fig. 3a and c, Table 1).79 A similar conclusion was also obtained by Wang et al. showing a larger opening of hysteresis in DyY2N@C80 compared to that of DySc2N@C80.81
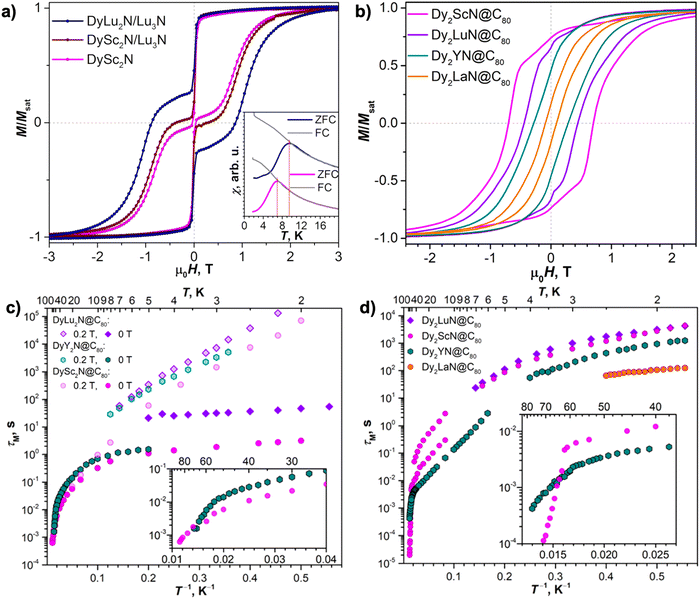 | ||
Fig. 3 (a) Magnetic hysteresis loops of DyLu2N@C80/Lu3N@C80 measured at 2 K and compared with those of DySc2N@C80 and DySc2N@C80 diluted with Lu3N@C80 in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. Sweep rate: 2.9 mT s−1. The inset shows the determination of the bifurcation temperature, TB,ZFC, from the temperature dependence of the magnetic susceptibility with a temperature sweep rate of 5 K min−1. (b) Magnetic hysteresis loops for Dy2MN@C80 (M = Sc, Y, La, Lu) at 2 K (2.1 K for the La case) with a field sweep rate of 2.9 mT s−1. (c) and (d) Magnetization relaxation times of DyM2N@C80 (M = Sc, Y, Lu) (c) and of Dy2MN@C80 (M = Sc, Y, La, Lu) (d). The insets in (c) and (d) show enlargement of high-temperature parts. Reproduced from (a) ref. 78 and (b)–(d) ref. 79. 1 ratio. Sweep rate: 2.9 mT s−1. The inset shows the determination of the bifurcation temperature, TB,ZFC, from the temperature dependence of the magnetic susceptibility with a temperature sweep rate of 5 K min−1. (b) Magnetic hysteresis loops for Dy2MN@C80 (M = Sc, Y, La, Lu) at 2 K (2.1 K for the La case) with a field sweep rate of 2.9 mT s−1. (c) and (d) Magnetization relaxation times of DyM2N@C80 (M = Sc, Y, Lu) (c) and of Dy2MN@C80 (M = Sc, Y, La, Lu) (d). The insets in (c) and (d) show enlargement of high-temperature parts. Reproduced from (a) ref. 78 and (b)–(d) ref. 79. | ||
| Type | EMF | T B,100s [K] | T B,hys [K](dH/dt) [mT s−1]c | T B,ZFC [K](dT/dt)d [K min−1] | U eff [K] | ΔEAFM–FM [K] | Ref. |
|---|---|---|---|---|---|---|---|
| a The value of TB,100s is determined under an applied magnetic field of 0.2 T. b Field-induced SMMs. c The value in the bracket indicates the field sweep rate (mT s−1) used to record hysteresis loops. d The value in the bracket indicates the temperature sweep rate (K min−1) used to record ZFC/FC curves. | |||||||
| NCF-SIMs | DySc2N@D3(6140)-C68 | 2.3a | 5 (2.9) | 3.8 (5) | 23.6 | — | 84 |
| DySc2N@D5h(6)-C80 | 3.6a | 7 (2.9) | 5.9 (5) | 17.7 | — | 84 | |
| DySc2N@Ih(7)-C80 | 4.6a | 7 (2.9) | 6.9 (5) | 23.6 | — | 15 and 64 | |
| DyY2N@Ih(7)-C80 | ∼6a | 8 (2.9) | 8.4 (5) | 929 | — | 79 | |
| DyLu2N@Ih(7)-C80 | ∼6.5a | 9 (2.9) | 9.5 (5) | 24.2 | — | 78 and 79 | |
| HoSc2N@Ih(7)-C80b | — | — | — | 16.5 | — | 85 | |
| NCFs with multiple magnetic metals | Dy2ScN@Ih(7)-C80 | 5.0 | 7 (2.9) | 8 (5) | 10.7/1735 | 8.1 | 95 |
| Dy2YN@Ih(7)-C80 | ∼3.5 | 5 (2.9) | 4.7 (5) | 43.8/680 | 1.4 | 79 | |
| Dy2LaN@Ih(7)-C80 | ∼2 | 4 (2.9) | 3.3 (5) | — | −1.2 | 79 | |
| Dy2LuN@Ih(7)-C80 | 5.2 | 8 (2.9) | 8 (5) | 4.3 | 4.3 | 78 and 79 | |
| Dy2GdN@Ih(7)-C80 | ∼1.5 | ∼1.8 (5.3) | — | 15.1 | — | 96 | |
| DyErScN@Ih(7)-C80 | ∼4.5 | 9 (33) | ∼8 (3) | 12.5 | 7.0 | 97 | |
| Tb2ScN@Ih(7)-C80 | 0.4 | ∼0.4 (3.3) | — | 1/10.5/56.4 | 9.4 | 98 | |
| Dy2ScN@D5h(6)-C80 | 2.6 | 7 (2.9) | 5.3 (5) | 8.4 | — | 84 | |
Dy2ScN@Ds(51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 365)-C84 365)-C84 |
∼1.8 | 5 (2.9) | 3.3 (5) | — | — | 84 | |
| Dy3N@Ih(7)-C80 | — | ∼2 (0.8) | — | — | 3.5 | 93 | |
| CYCFs | TbCN@C2v(19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 138)-C76b 138)-C76b |
— | — | — | 12 | — | 99 |
| TbCN@C2(5)-C82b | — | — | — | 10–20 | — | 100 | |
| TbCN@Cs(6)-C82b | — | — | — | 10–20 | — | 100 | |
| TbCN@C2v(9)-C82b | — | — | — | 10–20 | — | 100 | |
| OCFs | Dy2O@Cs(10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 528)-C72 528)-C72 |
3.4 | 7 (2.9) | 8 (5) | — | 2.2 | 55 |
Dy2O@C2(13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 333)-C74 333)-C74 |
5.0a | 14 (2.9) | 14 (5) | — | ∼0.1 | 55 | |
| Dy2O@C2v(5)-C80 | 3.2 | 6 (2.9) | 11 (5) | 25.9 | −26.6 | 101 | |
| Dy2O@Cs(6)-C82 | 2.8 | 6 (2.9) | 10 (5) | 10.8 | −10.8 | 102 | |
| Dy2O@C3v(8)-C82 | 5.9 | 7 (2.9) | 9 (5) | 7.8 | −7.8 | 102 | |
| Dy2O@C2v(9)-C82 | 3.7 | 7 (2.9) | 8 (5) | 18.6 | −18.6 | 102 | |
| Dy2O@C1(26)-C88 | 6.0 | 8 (2.9) | 10.5 (5) | 20.4 | −23.7 | 103 | |
| Dy2O@Cs(32)-C88 | 4.6a | 8 (2.9) | 8.5 (5) | — | −0.6 | 103 | |
| Dy2O@D2(35)-C88 | 3.9 | 8 (2.9) | 8.5 (5) | — | 5.2 | 103 | |
| SCFs | Dy2S@Cs(10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 528)-C72 528)-C72 |
— | 3.0 (8.33) | — | — | — | 29 |
| Dy2S@Cs(6)-C82 | — | 3.0 (8.33) | — | 17.8 | 15.8 | 29 and 104 | |
| Dy2S@C3v(8)-C82 | 2.0 | 5 (8.33) | 4.0 (5) | 6.0 | 9.2 | 29 and 104 | |
| DyScS@Cs(6)-C82 | ∼4 | 9 (10) | 7.3 (5) | 15.2 | — | 105 | |
| DyScS@C3v(8)-C82 | ∼2 | 9 (10) | 7.3 (5) | 6.5 | — | 105 | |
| CCFs | DyYTiC@Ih(7)-C80 | ∼5a | 7 (2.9) | 8 (5) | 14.9 | — | 106 |
| Dy2TiC@Ih(7)-C80 | 1.7 | 3 (5) | — | 9.5 | 12.2 | 107 | |
| Dy2TiC2@Ih(7)-C80 | — | 1.8 (5) | — | — | — | 107 | |
| Dy2TiC@D5h(6)-C80 | — | 1.8 (5) | — | — | — | 107 | |
| Dy2C2@Cs(6)-C82 | — | 3.0 (8.33) | — | 17.4 | 17.4 | 29 | |
| Dy2C2@Cs(32)-C88 | — | 2.1 (2.9) | — | — | 9.1 | 103 | |
| Dy2C2@D2(35)-C88 | — | 2.1 (2.9) | — | — | 4.7 | 103 | |
In addition to the encapsulated diamagnetic metal that plays an important role in determining the single-ion anisotropy and thus the SMM properties of NCFs, the cage structure is expected to be another degree of freedom that can be used for chemical design towards such a goal. In this line, it is the carbon ring motifs coordinating to the metal inside the cage that are of significance to the single-ion anisotropy and ligand fields, which include various binding sites such as a hexagon, pyracylene, and a fused-pentagon in a cage violating the isolated pentagon rule (IPR).82,83 However, the number of mixed-metal NCFs based on fullerene cages instead of Ih(7)-C80 is rather limited, partly due to the exceptional stability and high yield of C806− and also associated with the synthetic difficulty in HPLC separation of NCFs with different metallic clusters inside the same cage. Popov et al. reported the synthesis of DySc2N nitride clusterfullerenes with different carbon cages, including D3(6140)-C68 and D5h(6)-C80 as well as the well-studied Ih(7)-C80.84 Magnetic characterization of this series of NCFs shown in Fig. 4a and c indicates cage-dependent SMM properties, with the Ih(7)-C80 case as the best SMM featuring the broadest hysteresis loop at 2 K, the longest relaxation times and the highest bifurcation temperature (TB,ZFC) of 7 K in ZFC/FC curves, the D5h(6)-C80 fullerene as the intermediate one with TB,ZFC = 5.9 K followed by the D3(6140)-C68 non-IPR cage with only 3.8 K.
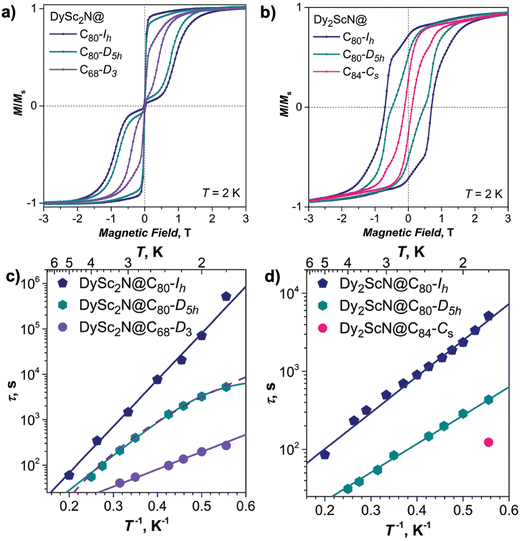 | ||
Fig. 4 (a) Magnetic hysteresis loops for different Dy-Sc NCFs, with the DySc2N cluster encapsulated inside D3(6140)-C68, D5h(6)-C80 and Ih(7)-C80 cages, measured at 2 K with a field sweep rate of 2.9 mT s−1. (b) Magnetic hysteresis loops for NCFs, with the Dy2ScN cluster encapsulated inside D5h(6)-C80, Ih(7)-C80 and Cs(51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 365)-C84 cages, measured at 2 K with a field sweep rate of 2.9 mT s−1. (c) and (d) Magnetization relaxation times of DySc2N@C2n (2n = 68, 80 (two isomers)) (c) and of Dy2ScN@C2n (2n = 84, 80 (two isomers)) (d). Reproduced from ref. 84. 365)-C84 cages, measured at 2 K with a field sweep rate of 2.9 mT s−1. (c) and (d) Magnetization relaxation times of DySc2N@C2n (2n = 68, 80 (two isomers)) (c) and of Dy2ScN@C2n (2n = 84, 80 (two isomers)) (d). Reproduced from ref. 84. | ||
The possibility to construct NCF-based SIMs using other anisotropic Ln3+ ions, instead of Dy3+, has also been explored. AC measurements of HoSc2N@Ih(7)-C80 reveal that it is a field-induced SMM, that is, slow relaxation of magnetization is only shown in the presence of an applied DC field.85 Although it is not a “genuine” SMM that functions at zero field, relaxation times in the order of milliseconds are determined below 6 K. The much faster relaxation rate of HoSc2N@C80 in comparison with that of DySc2N@C80 is attributed to its low-symmetry ligand field imposed by the cage which efficiently promotes the magnetic relaxation in the non-Kramers SIM. Similar to Dy-NCFs discussed above, the metal-dependent single-ion anisotropy of HoM2N@C80 (M = Sc, Lu, Y) was studied by paramagnetic 13C NMR studies, XMCD analysis and ligand field calculations using a point-charge model.86 The results indicate that despite the same magnetic ground state of Ho3+ with |mJ| = 8 in these molecules, chemical shifts and ligand fields are strongly dependent on the size of the M3+ ion, which is consistent with the conclusion extracted from DyM2N@C80.79 Furthermore, an interesting behavior was revealed for HoLu2N@C80, in which the encapsulated cluster can be oriented in a magnetic field since the Ho magnetic moment aligns along the Ho-N axis due to the strong ligand field.87 Thus, a hopping motion of the cluster is possible upon applying external magnetic fields, and the activation energy associated with such a process can be determined for HoLu2N@C80 as well as for TbSc2N@C80, providing insights into the cluster–cage binding interaction.
Popov et al. systematically studied MSc2N@C80, using paramagnetic NMR combined with point-charge calculations, with M including all lanthanides (M = La, Ce, Pr, Nd, Tb, Dy, Ho, Er, Tm, Lu) that can be introduced into NCFs.88 The 45Sc NMR spectra are especially useful for the study of the magnetic properties of these NCF-based SIMs. The interpretation of these spectra accomplished by the point-charge model shows a strong axial ligand field generated by the short M–N bond length in each case. This leads to an easy-axis magnetic anisotropy for the lanthanide ions with an oblate shape of the 4f density (Ce3+, Pr3+, Nd3+, Tb3+, Dy3+, and Ho3+), and an easy-plane anisotropy for prolate lanthanide ions Er3+ and Tm3+.
4.1.1.2 NCFs with multiple magnetic metal ions. Soon after the discovery of DySc2N@C80 as the first EMF-SMM, its single-ion anisotropy was investigated by Chibotaru et al. through ab initio calculations, revealing a large effective barrier exceeding 1000 K.89 This high height of the barrier, however, does not simply guarantee a high blocking temperature due to the fast QTM in the ground doublet as discussed above, which is further facilitated by hyperfine coupling to nuclear spins.1,90 How to inhibit QTM has been central to the studies of SMMs directed towards magnetic bistability at high temperatures. To this end, the local symmetry of the coordination environment is found to be crucial to suppress QTM by minimizing the transverse ligand fields and reducing the mixing between different mJ states.9 Another synthetic strategy is to design a di-nuclear SMM in which simultaneous reversal of magnetic moments on both lanthanide ions becomes difficult due to magnetic exchange interaction (or magnetic coupling) between them.91,92 However, it is challenging in conventional organometallic compounds to achieve strong magnetic exchange between lanthanide ions having well-shielded 4f electrons. The confined sub-nanospace provided by a rigid fullerene cage, on the other hand, offers a possibility to enforce close proximity of Ln ions in an encapsulated metal cluster so as to enhance their magnetic coupling.
An ab initio investigation of the dinuclear Dy2ScN@C80 shows an exchange splitting with an energy barrier of ∼8 cm−1, which is expected to efficiently block the QTM process at low temperatures, and thus may give rise to a high-performance SMM with a long magnetic relaxation time and large magnetic remanence.89 Indeed, this prediction was later confirmed by magnetic characterization showing a much wider magnetic hysteresis at 2 K in contrast to the butterfly-shaped hysteresis of mononuclear DySc2N@C80.93 The suppressed QTM in Dy2ScN@C80, as predicted by Chibotaru et al.,89 is ascribed to a ferromagnetic coupling between two Dy3+ ions in the molecule. As a result, a 100 s blocking temperature TB,100s in the absence of a magnetic field is determined to be about 5.5 K. The angle between the magnetic moments on the two Dy sites can be precisely determined from temperature-dependent magnetization curves, in excellent agreement with the theoretical result showing that the quantization axes of the two Dy ions are collinear with the two Dy–N bonds.94 Slow magnetic relaxation of DySc2N@C80 at higher temperatures can be probed by AC magnetometry up to 76 K.95 A remarkably high effective barrier Ueff of 1735 K is extracted from the temperature dependence of τ, which exhibits an Orbach process functioning above 60 K. This thermally activated relaxation process is understood by ab initio calculations, and takes place via the fifth-excited Kramers doublet of the single-ion ligand field splitting in Dy2ScN@C80.
Similar to monodysprosium NCFs, the role that diamagnetic metals play in the SMM properties can be studied in didysprosium NCFs, where intramolecular magnetic coupling between two Dy3+ ions is of particular importance. Substituting Sc with Lu in Dy2ScN@C80, a similar bifurcation temperature (TB,ZFC = 8 K) is obtained for Dy2LuN@C80, while they differ in the interaction between the magnetic moments of Dy3+, leading to different temperature and field dependence of the relaxation times.78 In the series of Dy2MN@C80 (M = Sc, Y, La, and Lu), Dy⋯Dy coupling and magnetic relaxation is mainly controlled by the size of M3+.79 The hysteresis becomes narrower when the ionic radius of M3+ is larger, and the relaxation rate at low temperatures is similar for NCFs entrapping Dy2ScN and Dy2LuN, but becomes progressively faster for Dy2YN and Dy2LaN with much larger clusters (Fig. 3b and d). These observations are correlated with the strength of Dy⋯Dy coupling, and the corresponding energy difference between ferromagnetic and antiferromagnetic states (ΔEAFM–FM) changes gradually from 8.1 K to −1.2 K as the size of M3+ increases. The mass of M, on the other hand, is less critical for the magnetic properties, meaning that the phonon degrees of freedom do not contribute heavily to the relaxation pathways in didysprosium NCFs compared to the situation in monodysprosium SIMs. Substitution of a diamagnetic Sc3+ ion in Dy2ScN@C80 with Gd3+ having half-filled f orbitals was also investigated.96 It is demonstrated that the energy of the excited exchange state ΔEAFM–FM decreases in Dy2GdN@C80 relative to that in Dy2ScN@C80. This results in a much faster QTM in Dy2GdN@C80, as evidenced by its very narrow hysteresis at 1.8 K; this acceleration of magnetic relaxation is due to the fact that the isotropic Gd3+ can act as a single-atom catalyst.
The interplay between cage structures and intramolecular magnetic coupling in didysprosium NCFs has been studied by encapsulating Dy2ScN clusters into Ih(7)-C80, D5h(6)-C80 and a non-IPR Cs(51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 365)-C84 (Fig. 4b and d).84 Similar to the result of monodysprosium NCFs employing different host cages, the best SMM properties of the didysprosium compounds are found in Ih(7)-C80, which exhibits the broadest hysteresis and the highest bifurcation temperature TB,ZFC determined from ZFC/FC curves. The NCF with the non-IPR cage has the worst SMM performance in the series of compounds. This is also verified by the larger Ueff of the Orbach relaxation in Ih(7)-C80via the exchange excited state compared to that in D5h(6)-C80, whereas shorter relaxation time of Dy2ScN@C84 precludes the study of its temperature dependence of relaxation time.
365)-C84 (Fig. 4b and d).84 Similar to the result of monodysprosium NCFs employing different host cages, the best SMM properties of the didysprosium compounds are found in Ih(7)-C80, which exhibits the broadest hysteresis and the highest bifurcation temperature TB,ZFC determined from ZFC/FC curves. The NCF with the non-IPR cage has the worst SMM performance in the series of compounds. This is also verified by the larger Ueff of the Orbach relaxation in Ih(7)-C80via the exchange excited state compared to that in D5h(6)-C80, whereas shorter relaxation time of Dy2ScN@C84 precludes the study of its temperature dependence of relaxation time.
Apart from Dy3+, the possibility to incorporate another Ln3+ into an NCF-based dinuclear SMM has also been explored. A heteronuclear NCF DyErScN@Ih(7)-C80 was reported as an SMM with a broadened hysteresis close to zero field compared to that of DySc2N@C80 due to the suppressed QTM, which stems from the magnetic exchange interaction between Dy3+ and Er3+.97 The more important feature of this molecule is the luminescent Er3+ ion integrated into the cage, which provides an additional function of characteristic Er3+ near-infrared emission for the SMM, rendering a bifunctional magneto-luminescent molecule. Incorporation of two non-Kramers Tb3+ ions instead of Dy3+ leads to a Tb2ScN@C80 SMM exhibiting magnetic hysteresis at sub-Kelvin temperatures, at which a relaxation time in the order of 100 s can be determined.98 Analysis of the temperature dependence of τ indicates an Orbach process with 10 K Ueff arising from the exchange excited state due to magnetic coupling. The prefactor (τ0 in eqn (1)) is 4 orders of magnitude smaller than that of the Dy congener, which is ascribed to the lack of Kramers protection in Tb2ScN@C80. The magnetic coupling can be better studied in dinuclear systems with two isotropic Gd3+ ions instead of other anisotropic Ln3+.108 Despite the fact that strong axial anisotropy is lacking in Gd3+, which is beneficial to the design of an SMM, magnetic characterization of Gd2ScN@C80 clearly confirms a ferromagnetic coupling between two Gd3+ ions, and also suggests a non-negligible anisotropy in the molecule.
The situation gets much more complicated when three magnetic Ln3+ ions are embedded simultaneously inside a fullerene cage using the trimetallic nitride template, where various ways of coupling three magnetization moments are possible in the trimetallic triangle. This magnetic coupling was first analyzed in Ho3N@C80 and Tb3N@C80 using magnetization curves.109 The strong ligand fields are able to induce a collinear alignment of the individual moments parallel to the M–N bonds, giving rise to a ferromagnetically coupled net magnetic moment in each case. The ferromagnetic coupling was reported in experiment for Gd3N@C80,108,110 as well as in theory for Dy3N@C80.89 The latter is expected to trigger magnetic frustration that can greatly suppress magnetization blocking in the SMM regardless of the exchange barrier. Such a magnetic frustrated state is caused by the quasi-degeneracy of the three exchange multiplets in the ground state, that is, the net magnetic moment of the molecule can be aligned along each of the three Dy–N bonds. These predictions of the magnetic properties for Dy3N@C80 were later confirmed by experiments showing a very narrow magnetic hysteresis at 2 K, which is caused by the frustrated ground state as one of the simplest realizations of a frustrated and ferromagnetically coupled system.93
To this end, Tb3+ with large magnetic anisotropy was later introduced to form TbCN@C2(5)-C82.116 The same group further synthesized terbium-CYCF based on three isomers of C82, namely C2(5), Cs(6) and C2v(9), all adopting a triangular cluster geometry as illustrated in Fig. 5a–c.100 Variable-temperature magnetization measurements with the aid of a noncollinear magnetic moment model, which considers isotropic distribution of magnetic moments, reveal a magnetic ground state of |mJ| = 6 for each case. AC susceptibility studies demonstrate field-induced SIM properties for all these series of compounds (Fig. 5d–f). Remarkably, it is found that as the isomeric structure of the C82 cage varies from C2(5) to Cs(6) and to C2v(9), the Tb–N distance becomes shorter, and the relaxation time increases accordingly, which is likely attributed to the enhanced ligand fields imposed on the Tb3+ ion. Thus, this direct correlation between the magnetic properties and the cluster geometry indicates a facile approach to fine-tune single-ion anisotropy by changing the cage structure in these SIMs. Indeed, upon further varying the host cage from the popular C82 to a non-IPR C2v(19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 138)-C76, the embedded TbCN cluster becomes nearly linear instead of maintaining the triangular geometry.99 This geometric change is relevant to the strong metal–cage interaction in the presence of the fused-pentagon motif on the cage, as also evidenced by a combined experimental and theoretical study on CYCFs encapsulating a LuCN cluster into C76 and C82 cages.117 The field-induced SIM properties were verified by the frequency-dependent out-of-phase susceptibility χ′′ observed in AC measurements for TbCN@C76, from which a shorter relaxation time is extracted in comparison with that of its C82 counterparts. The reason for the faster relaxation rate in CYCFs with a linear cluster is still not clear. The QTM process governed by the local symmetry or spin-phonon coupling mediated by cluster-based vibrations may be accountable for this observation.
138)-C76, the embedded TbCN cluster becomes nearly linear instead of maintaining the triangular geometry.99 This geometric change is relevant to the strong metal–cage interaction in the presence of the fused-pentagon motif on the cage, as also evidenced by a combined experimental and theoretical study on CYCFs encapsulating a LuCN cluster into C76 and C82 cages.117 The field-induced SIM properties were verified by the frequency-dependent out-of-phase susceptibility χ′′ observed in AC measurements for TbCN@C76, from which a shorter relaxation time is extracted in comparison with that of its C82 counterparts. The reason for the faster relaxation rate in CYCFs with a linear cluster is still not clear. The QTM process governed by the local symmetry or spin-phonon coupling mediated by cluster-based vibrations may be accountable for this observation.
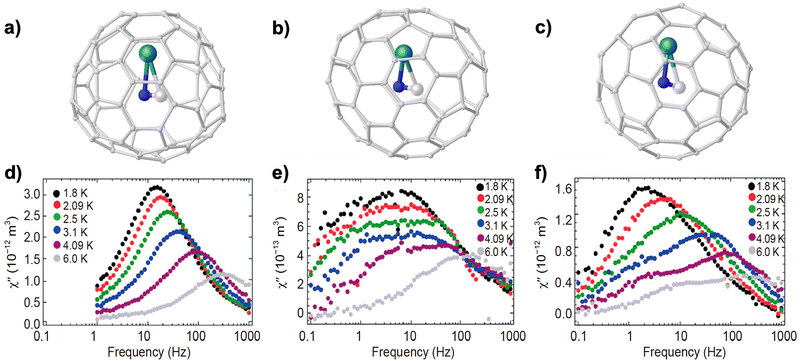 | ||
| Fig. 5 Molecular structures of TbCN@C2(5)-C82 (a), TbCN@Cs(6)-C82 (b) and TbCN@C2v(9)-C82 (c). Out-of-phase AC magnetic susceptibility of TbCN@C2(5)-C82 (d), TbCN@Cs(6)-C82 (e) and TbCN@C2v(9)-C82 (f) at 1.8–6 K. Reproduced from ref. 100. | ||
Although no further CYCF-based SIMs have been reported to date besides TbCN@C76,82, synthetic efforts have been devoted in the field to expand this family of molecules by introducing different lanthanide metals and fullerene cages. In this regard, Dy-based CYCFs are of particular interest owing to their Kramers ion nature, which competes with non-Kramers Tb3+ and is usually associated with the suppressed QTM process. By employing Prussian blue as a cheap solid cyanide/nitrogen dual-source, Dy-containing CYCFs can be readily synthesized with high yields.30 X-Ray crystallographic studies of the three isomers of DyCN@C82 indicate the triangular geometry of the embedded cluster which is tunable upon varying the isomeric structure of the cage. Recently, the host cage of CYCFs was extended to C84 with two isomers C2(13) and C2v(17), while the metals involved encompass Y, Dy and Tb.118 The missing C2v(17)-C84, which had never been reported before both in empty fullerenes and EMFs, can be interconverted from the C2(13)-C84 isomer through two steps of Stone–Wales transformation. This demonstrates the advantage of the monometallic cyanide cluster in stabilizing novel fullerene structures. The subtle difference in the ionic radii of Y3+, Dy3+, and Tb3+ gives rise to a noticeable change in the metal–cage interaction, which can also be varied within different isomeric cages. Thus, the metal–cage interactions and the related single-ion anisotropy, if a magnetic Ln ion is introduced, can be altered via both the encapsulated cluster and host cage.
An alternative strategy to modify the cluster geometry was realized by exohedral functionalization on the cage via high-temperature trifluoromethylation.119,120 This leads to the synthesis of multi-trifluoromethylated CYCFs based on C82 and C84. Different derivatized products, including YCN@C82(CF3)16/18 and YCN@C84(CF3)16/18, have been structurally characterized using X-ray crystallography. These compounds are stabilized by the formation of isolated C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds and benzenoid rings on the fullerene cages after CF3 addition, which is able to localize the metal atoms within a particular fragment of the cage. Moreover, the encapsulated YCN cluster with triangular geometry can be further altered depending on the exohedral addition pattern. This shall allow the manipulation of the single-ion anisotropy for Dy3+- or Tb3+-based CYCFs and thus would certainly stimulate further studies on their SMM properties.
C bonds and benzenoid rings on the fullerene cages after CF3 addition, which is able to localize the metal atoms within a particular fragment of the cage. Moreover, the encapsulated YCN cluster with triangular geometry can be further altered depending on the exohedral addition pattern. This shall allow the manipulation of the single-ion anisotropy for Dy3+- or Tb3+-based CYCFs and thus would certainly stimulate further studies on their SMM properties.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 333)-C74 (M = Tb, Ho, Lu) featuring a linear M2O cluster inside the non-IPR fullerene cage.133–135 Understanding the interplay between the cluster geometry and the cage structure would be crucial to explore their magnetic properties.
333)-C74 (M = Tb, Ho, Lu) featuring a linear M2O cluster inside the non-IPR fullerene cage.133–135 Understanding the interplay between the cluster geometry and the cage structure would be crucial to explore their magnetic properties.
Magnetic studies on this type of OCF started from a theoretical work focused on the single-ion anisotropy of heterometallic compounds DyMO@C2n (M = Sc, Lu; 2n = 72, 76, 82), where diamagnetic rare-earth metal ions are used in conjunction with anisotropic Dy3+ to avoid the relaxation pathway through low-lying exchange excited states that are induced by generally weak 4f–4f magnetic coupling.136 As expected from a more electronegative O2− compared to N3−, ab initio calculations show a strong splitting of the 6H15/2 ground multiplet for Dy3+ in each case. Detailed analysis of the wavefunctions of the doublets and the transition probability among them indicates that QTM can be quenched up to the third-excited doublet in the series of compounds. A very large effective barrier Ueff exceeding 2000 K is determined in DyScO@C82. This highly axial anisotropy is expected to give rise to a strong SIM. However, heteronuclear OCFs have not yet been synthesized due to the difficulties both in synthesis and in separation. It should be noted that the overall yields of OCFs are much lower than those for NCFs, and the mixture of Dy2O, Sc2O and DyScO species makes the HPLC separation process a challenging task. Thus, one has to focus on the homonuclear system at this stage, where two magnetic Dy3+ ions are involved simultaneously. In this context, enhancing magnetic coupling interaction between two Dy3+ ions becomes crucial to suppress QTM as discussed in the above section on NCFs with multiple magnetic metal ions (4.1.1.2). Indeed, oxygen-bridged dinuclear compounds in various organometallic SMMs have been demonstrated to induce strong coupling between the two Dy3+ ions via superexchange interactions.137–141
To start with the discussion of the magnetic properties for these dinuclear Dy-SMMs, their low-energy spin states can be generally described using the following spin Hamiltonian
| Ĥspin = ĤLF1 + ĤLF2 − 2j12Ĵ1Ĵ2 + ĤZEE | (3) |
![[S with combining tilde]](https://www.rsc.org/images/entities/i_char_0053_0303.gif) i = 1/2 for the i-th magnetic metal center. To avoid ambiguity, therefore, we compare the corresponding energy difference between ferromagnetic (FM) and antiferromagnetic (AFM) states (ΔEAFM–FM) throughout this review. Under the −2j12Ĵ1Ĵ2 convention illustrated in eqn (3), this energy splitting for a didysprosium system can be computed as
i = 1/2 for the i-th magnetic metal center. To avoid ambiguity, therefore, we compare the corresponding energy difference between ferromagnetic (FM) and antiferromagnetic (AFM) states (ΔEAFM–FM) throughout this review. Under the −2j12Ĵ1Ĵ2 convention illustrated in eqn (3), this energy splitting for a didysprosium system can be computed asΔEAFM–FM = 225j12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos(α) cos(α) | (4) |
The first magnetically characterized OCF-based SMMs were reported in 2019 including three isomers of Dy2O@C82 with Cs(6), C3v(8) and C2v(9) cage symmetries.102 As illustrated in Fig. 6a–c, each of these molecules possesses a slightly bent shape for the encapsulated Dy2O cluster which features unusually short Dy–O bonds of ≈2.0 Å, resulting in a very strong magnetic anisotropy of Dy ions with their magnetic moments aligning along the Dy–O bonds. Dy2O@C82 isomers show SMM behaviors with a broad magnetic hysteresis at low temperature (2 K), which persists up to 6–7 K using a field sweep rate of 2.9 mT s−1 (Fig. 6d–f). The opening of the hysteresis loop close to zero field is clear evidence of effective quenching of QTM by magnetic coupling of two Dy3+, in contrast to the butterfly-shaped hysteresis observed for NCF-based SIMs.15,81 A more interesting feature in Dy2O@C82 isomers lies in the inflection of their hysteresis loops. The abrupt drop of magnetization at zero field at 2 K, noticeable in the C2v(9) case, arises from QTM that is not completely quenched by magnetic coupling. The inflections away from the zero-field region, on the other hand, are ascribed to the Zeeman splitting of the energy of the FM and AFM states in the presence of a certain magnetic field, at which the crossing point is found between these spin states. It should be noted that this inflection phenomenon is much more obvious than that in the hysteresis of the ferromagnetically coupled Dy2ScN@C80,95 in which the FM state is more likely to be the ground state at a certain molecular orientation without level crossing due to its stronger Zeeman response. Therefore, the complex feature of hysteresis indicates an antiferromagnetically coupled system for Dy2O@C82. The particular field of inflection can then be used to determine ΔEAFM–FM as well as the coupling constant j12 combining eqn (3) and (4). Alternatively, the coupling situation can be understood, as discussed above, by the fitting of magnetization curves using eqn (3) and (4), or by the study of their relaxation mechanisms applying eqn (2) and (4). The latter gives the Orbach relaxation process via the FM excited state at low temperatures with Ueff corresponding to ΔEAFM–FM. The value of ΔEAFM–FM is thus determined as −7.8 to −18.6 K, suggesting that the Dy⋯Dy exchange coupling in Dy2O@C82 is the strongest at this time among all dinuclear Dy SMMs with diamagnetic bridges.137,138,143–145
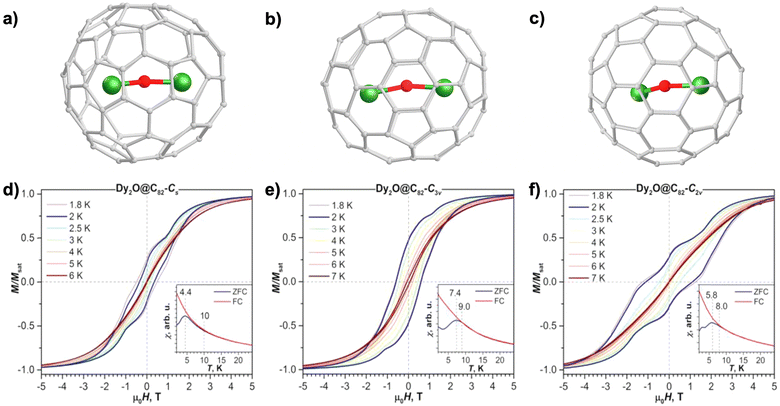 | ||
| Fig. 6 Molecular structures of Dy2O@Cs(6)-C82 (a), Dy2O@C3v(8)-C82 (b) and Dy2O@C2v(9)-C82 (c). Magnetic hysteresis curves of Dy2O@Cs(6)-C82 (d), Dy2O@C3v(8)-C82 (e) and Dy2O@C2v(9)-C82 (f) in the range of 1.8–7 K using a field sweep rate of 2.9 mT s−1. The insets represent their corresponding ZFC/FC curves at an applied magnetic field of 0.2 T with a temperature sweep rate of 5 K min−1. Reproduced from ref. 102. | ||
The possibility of putting Dy2O clusters into other fullerene cages has been explored, discovering two non-IPR cages Cs(10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 528)-C72 and C2(13
528)-C72 and C2(13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 333)-C74 as suitable hosts.55 The fused-pentagon moieties in these cages govern the positions of Dy3+ inside thanks to the strong metal–cage interaction, and the geometry of the Dy2O cluster is controlled in this way, which is bent within C72 but becomes linear in C74. The cage-dependent cluster geometry greatly affects the SMM behaviors of these two OCFs. Both molecules show open magnetic hysteresis at 1.8 K but with very different shapes. In the case of Dy2O@C72, the hysteresis loop is typical of an antiferromagnetically coupled SMM featuring quenched QTM in the absence of field and an inflection in the presence of field, similar to the situation of the abovementioned Dy2O@C82 isomers.102 In contrast, Dy2O@C74 exhibits an SIM-like hysteretic behavior which is almost closed at zero field due to the strong QTM. Detailed analysis reveals that in this dinuclear Dy-SMM, the ferromagnetic dipolar coupling is nearly completely counterbalanced by the antiferromagnetic Dy–O–Dy superexchange interaction, thus leading to decoupled Dy moments that are accountable for such a SIM-like hysteresis. Although the relaxation rate of Dy2O@C74 is fast at zero field, slow relaxation (with τ > 40 s) can be observed up to 6 K at a finite magnetic field of 0.2 T or 0.8 T. The magnetic hysteresis remains open up to 14 K in Dy2O@C74 and up to 7 K in Dy2O@C72 at a field sweep rate of 2.9 mT s−1. The former value is the highest hysteresis temperature among all clusterfullerenes reported so far.
333)-C74 as suitable hosts.55 The fused-pentagon moieties in these cages govern the positions of Dy3+ inside thanks to the strong metal–cage interaction, and the geometry of the Dy2O cluster is controlled in this way, which is bent within C72 but becomes linear in C74. The cage-dependent cluster geometry greatly affects the SMM behaviors of these two OCFs. Both molecules show open magnetic hysteresis at 1.8 K but with very different shapes. In the case of Dy2O@C72, the hysteresis loop is typical of an antiferromagnetically coupled SMM featuring quenched QTM in the absence of field and an inflection in the presence of field, similar to the situation of the abovementioned Dy2O@C82 isomers.102 In contrast, Dy2O@C74 exhibits an SIM-like hysteretic behavior which is almost closed at zero field due to the strong QTM. Detailed analysis reveals that in this dinuclear Dy-SMM, the ferromagnetic dipolar coupling is nearly completely counterbalanced by the antiferromagnetic Dy–O–Dy superexchange interaction, thus leading to decoupled Dy moments that are accountable for such a SIM-like hysteresis. Although the relaxation rate of Dy2O@C74 is fast at zero field, slow relaxation (with τ > 40 s) can be observed up to 6 K at a finite magnetic field of 0.2 T or 0.8 T. The magnetic hysteresis remains open up to 14 K in Dy2O@C74 and up to 7 K in Dy2O@C72 at a field sweep rate of 2.9 mT s−1. The former value is the highest hysteresis temperature among all clusterfullerenes reported so far.
Compared to that of Dy2O@C82, even stronger Dy⋯Dy coupling was found by Popov et al. in Dy2O@C2v(5)-C80.101 The ΔEAFM–FM determined by the fitting of magnetization data shows a very large value of −26.6 K (the dipolar and exchange contributions are 3.9 and −30.5 K, respectively), which is also consistent with the Ueff of 25.9 K extracted from the temperature dependence of magnetic relaxation time. Dy2O@C80 has a broad hysteresis at 1.8 K opening between −5 T and 5 T and with a coercive field of 0.65 T. A pronounced inflection of the hysteresis loop upon decreasing the field is observed, which is related to the level crossing between AFM and FM states. The magnetic hysteresis persists until 6 K using a field sweep rate of 2.9 mT s−1, while the bifurcation temperature TB,ZFC is determined as 11 K from ZFC/FC measurements with a temperature sweep rate of 5 K min−1.
Recently, this family of OCF-SMMs has been further extended to three C88 isomers with cage symmetries of C1(26), Cs(32), and D2(35).103 All isomers of Dy2O@C88 display slow relaxation of magnetization and magnetic hysteresis. The latter measure of SMMs can be observed up to 7–9 K at 2.9 mT s−1 sweep rate. It is worth noting that Dy2O@C88 represents EMF-SMMs possessing the largest carbon cages to date. The three isomers of Dy2O@C88 also allows for the study of their SMM properties as a function of the isomeric fullerene cage. Similar to the above analysis of other OCF-SMMs with smaller cages, it seems clear that the O2− bridge favors antiferromagnetic exchange coupling between the two Dy3+ ions. The strength of Dy⋯Dy superexchange interactions, however, differs significantly upon varying the isomeric structure of the C88 cage. The comparative analysis of all nine OCF-SMMs reported so far reveals that only Dy2O@D2(35)-C88 has a moderately positive ΔEexchAFM–FM value of 2.0–3.2 K, considering only superexchange interactions without the contribution from the dipolar terms. All the other eight molecules feature a negative exchange term, which ranges from the modest −2.2 K in the case of C72 to very large −27.3 K in C1(26)-C88 and −30.5 K in C80, with the latter two being the strongest Dy⋯Dy interactions in all didysprosium compounds reported by far. Further enhancing the magnetic coupling in Dy-OCFs is definitely a research direction aimed at increasing the blocking temperature of this type of SMM. Despite the fact that unfortunately no clear structural correlation of the exchange coupling strength is concluded in the present literature for Dy-OCFs, the structural diversity of the fullerene cage would become a rich playground to achieve such a goal in future studies.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 528)-C72 cage violating the isolated-pentagon rule.149 Following this synthetic strategy, Sc2S clusters can be entrapped in an even smaller non-IPR C2(7892)-C70 cage, which is closely connected with the previously reported Cs(10
528)-C72 cage violating the isolated-pentagon rule.149 Following this synthetic strategy, Sc2S clusters can be entrapped in an even smaller non-IPR C2(7892)-C70 cage, which is closely connected with the previously reported Cs(10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 528)-C72 by a direct insertion of a C2 unit.150 This sulfide template can be extended to Er-SCFs employing the two most stable C82 isomers (Cs(6) and C3v(8)),151 and is also able to stabilize transition metal-only clusters inside a fullerene cage, forming Ti2S@D3h(5)-C78.152 DFT calculations of the latter suggest an almost linear geometry of the Ti2S cluster which transfers six electrons to the cage due to the tetravalent oxidation state of titanium, distinct from the four-electron transfer in conventional SCFs encaging trivalent rare-earth metal ions.
528)-C72 by a direct insertion of a C2 unit.150 This sulfide template can be extended to Er-SCFs employing the two most stable C82 isomers (Cs(6) and C3v(8)),151 and is also able to stabilize transition metal-only clusters inside a fullerene cage, forming Ti2S@D3h(5)-C78.152 DFT calculations of the latter suggest an almost linear geometry of the Ti2S cluster which transfers six electrons to the cage due to the tetravalent oxidation state of titanium, distinct from the four-electron transfer in conventional SCFs encaging trivalent rare-earth metal ions.
Similar to OCF-SMMs with strong magnetic coupling between two Dy3+ ions, Dy-SCFs are of particular interest for magnetic studies. By using Dy2S3 as a solid source of metal and sulfur, and adding methane to a reactive atmosphere so as to reduce the formation of empty fullerenes in the arc-discharging process, Dy-SCFs can be selectively synthesized as the main fullerene products.29 Among them, two C82 isomers (Cs(6) and C3v(8)) and Cs(10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 528)-C72 have been successfully isolated, and the structures of the former two isomers were unambiguously determined by single-crystal XRD characterization to reveal significantly bent Dy2S clusters inside the cages. All these three molecules exhibit magnetic hysteresis at low temperatures, with the C3v(8)-C82 case being the best SMM showing open hysteresis up to 4 K using a field sweep rate of 8.33 mT s−1 and TB,ZFC = 4 K at a temperature sweep rate of 5 K min−1 in ZFC/FC measurements. Its dynamic magnetic properties can be studied by DC and AC susceptibility measurements in the temperature range from 1.6 K to 70 K. This exceptional slow magnetic relaxation up to high temperatures enables the determination of three Orbach relaxation processes with Ueff of 10.5, 48 and 1232 K, respectively. The first energy barrier is associated with the magnetic exchange excited state similar to that for Dy-OCFs; the intermediate barrier can be ascribed to the local vibrational mode of librating the Dy2S cluster within the cage, while the highest value matches the excited doublet in the single Dy3+ ligand field. On the other hand, Dy2S@Cs(6)-C82 has a faster magnetic relaxation featuring two barriers of 15.2 and 523 K, corresponding to relaxation via the exchange excited state and via the excited doublet in ligand field splitting, respectively. Comparing the magnetic behaviors of these SCFs with their OCF counterparts, it is evident that generally weaker SMM performance can be found in SCFs. This disparity originates from the lower electronegativity and the larger covalent radius of sulfur. The latter results in the bent shape of the cluster instead of nearly linear one usually observed in OCFs; and the former means that a weaker ligand field is imposed by S2− in comparison with that by O2−. Nevertheless, Dy-SCFs still have the longest relaxation times and the highest energy barriers compared to other Dy-SMMs that contain a sulfur bridge.153–155 This is because sulfur possesses a larger negative charge inside the fullerene, while the Dy–S bond length is also considerably shorter, thus giving rise to a strong ligand field.
528)-C72 have been successfully isolated, and the structures of the former two isomers were unambiguously determined by single-crystal XRD characterization to reveal significantly bent Dy2S clusters inside the cages. All these three molecules exhibit magnetic hysteresis at low temperatures, with the C3v(8)-C82 case being the best SMM showing open hysteresis up to 4 K using a field sweep rate of 8.33 mT s−1 and TB,ZFC = 4 K at a temperature sweep rate of 5 K min−1 in ZFC/FC measurements. Its dynamic magnetic properties can be studied by DC and AC susceptibility measurements in the temperature range from 1.6 K to 70 K. This exceptional slow magnetic relaxation up to high temperatures enables the determination of three Orbach relaxation processes with Ueff of 10.5, 48 and 1232 K, respectively. The first energy barrier is associated with the magnetic exchange excited state similar to that for Dy-OCFs; the intermediate barrier can be ascribed to the local vibrational mode of librating the Dy2S cluster within the cage, while the highest value matches the excited doublet in the single Dy3+ ligand field. On the other hand, Dy2S@Cs(6)-C82 has a faster magnetic relaxation featuring two barriers of 15.2 and 523 K, corresponding to relaxation via the exchange excited state and via the excited doublet in ligand field splitting, respectively. Comparing the magnetic behaviors of these SCFs with their OCF counterparts, it is evident that generally weaker SMM performance can be found in SCFs. This disparity originates from the lower electronegativity and the larger covalent radius of sulfur. The latter results in the bent shape of the cluster instead of nearly linear one usually observed in OCFs; and the former means that a weaker ligand field is imposed by S2− in comparison with that by O2−. Nevertheless, Dy-SCFs still have the longest relaxation times and the highest energy barriers compared to other Dy-SMMs that contain a sulfur bridge.153–155 This is because sulfur possesses a larger negative charge inside the fullerene, while the Dy–S bond length is also considerably shorter, thus giving rise to a strong ligand field.
The two isomers of Dy2S@C82 were further magnetically characterized at sub-Kelvin temperature, which shows broad hysteresis at 0.41 K with two clear QTM steps (one at zero field and the other at a certain field) in each case (Fig. 7c and d).104 This allows a detailed analysis of the level crossing for different orientations in the presence of an applied magnetic field, providing precise information on the strength of Dy⋯Dy interactions in Dy2S@C82. The derived ΔEAFM–FM values are 15.4 K and 7.3 K for Cs and C3v isomers, respectively, which are in line with the results extracted from the fitting of temperature dependence of relaxation times as discussed above.29 It is worthwhile to note that while the O2− bridge facilitates an antiferromagnetically coupled system in Dy-OCFs, a ferromagnetically coupled ground state is found for Dy-SCFs. The sub-Kelvin temperatures enable the determination of relaxation times by DC demagnetization measurements. The QTM regime is thus achieved, rendering long relaxation times near 900 s and 3200 s for Cs and C3v isomers. This QTM process is related to the situation where the two Dy3+ magnetic moments flip simultaneously as an entity.
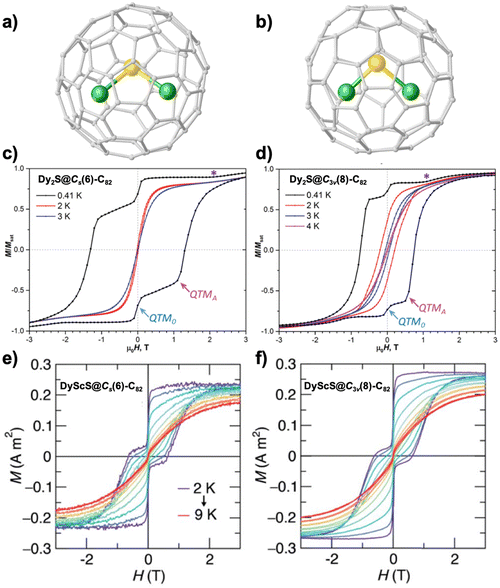 | ||
| Fig. 7 Molecular structures of Dy2S@Cs(6)-C82 (a) and Dy2S@C3v(8)-C82 (b). Magnetic hysteresis loops of Dy2S@Cs(6)-C82 (c) and Dy2S@C3v(8)-C82 (d) at 0.41 K and compared to some higher-temperature curves recorded until the hysteresis is closed. Field sweep rates 2.9 mT s−1 for T = 2 K and above, and 3.3 mT s−1 for T = 0.41 K. QTM0, QTMA, and asterisk denote the features appearing because of the level crossing in Zeeman energies. Magnetic hysteresis loops of DyScS@Cs(6)-C82 (e) and DyScS@C3v(8)-C82 (f) at 2–9 K with a sweep rate of 10 mT s−1. Reproduced from (c) and (d) ref. 104 and (e) and (f) ref. 105. | ||
Substituting one Dy3+ by a diamagnetic Sc3+ ion in these two isomers of Dy2S@C82 further leads to two SIMs.105 Magnetic characterization of these DyScS@C82 isomers shows an open magnetic hysteresis even at zero field at 2 K, indicating a quenched QTM effect compared to that of Dy-NCFs featuring a nearly closed hysteresis loop at zero field (Fig. 7e and f).76 The hysteresis of DyScS@C82 is open until 9 K in each case, where a fast field sweep rate of 10 mT s−1 is used. The bifurcation temperatures determined from ZFC/FC curves are both around 7.3 K. This better SMM performance of DyScS@C82 than Dy2S@C82 suggests potential interest in future magnetic studies of DyScO@C82 with an even stronger axial ligand field.136
In addition to the C22− bridging ligand, metal carbide containing a single C atom is also available within the fullerenes. This type of clusterfullerene was first synthesized by Popov et al. in Lu2TiC@Ih(7)-C80 having a μ3-carbide ligand and mimics the structure of M3N@C80 (M = rare earth metals) NCFs.162 The tetravalent oxidation state of Ti gives rise to a Ti![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond in the former. As a result, both of these clusterfullerenes feature a cluster-to-cage six-electron transfer. Replacing the diamagnetic Lu3+ by Dy3+ leads to two dinuclear Dy-SMMs Dy2TiC@C80 with the cage symmetries of Ih(7) and D5h(6) that are structurally characterized by spectroscopic methods.107 The two isomers display similar hysteresis loops at 1.8 K. The hysteresis of the Ih(7) isomer can be observed up to 3 K using 5 mT s−1 field sweep rate, showing a much weaker SMM performance compared to that of Dy2ScN@C80 (TB,hys = 7 K at 2.9 mT s−1 sweep rate), despite a stronger magnetic coupling found for Dy2TiC@C80.95 This may be explained by a larger deviation of the orientation of the Dy magnetic moments from the Dy–C bond axes.94 Adding one more carbon atom in the encapsulated cluster, Dy2TiC2@C80 only exhibits a narrow hysteresis at 1.8 K. An SIM can also be constructed in DyYTiC@C80 that contains three different metals, with two being diamagnetic.106 Similar to DySc2N@C80,15 a butterfly-shaped hysteresis is observed for DyYTiC@C80 at 1.8 K, which is open up to 7 K at 2.9 mT s−1 sweep rate.
C double bond in the former. As a result, both of these clusterfullerenes feature a cluster-to-cage six-electron transfer. Replacing the diamagnetic Lu3+ by Dy3+ leads to two dinuclear Dy-SMMs Dy2TiC@C80 with the cage symmetries of Ih(7) and D5h(6) that are structurally characterized by spectroscopic methods.107 The two isomers display similar hysteresis loops at 1.8 K. The hysteresis of the Ih(7) isomer can be observed up to 3 K using 5 mT s−1 field sweep rate, showing a much weaker SMM performance compared to that of Dy2ScN@C80 (TB,hys = 7 K at 2.9 mT s−1 sweep rate), despite a stronger magnetic coupling found for Dy2TiC@C80.95 This may be explained by a larger deviation of the orientation of the Dy magnetic moments from the Dy–C bond axes.94 Adding one more carbon atom in the encapsulated cluster, Dy2TiC2@C80 only exhibits a narrow hysteresis at 1.8 K. An SIM can also be constructed in DyYTiC@C80 that contains three different metals, with two being diamagnetic.106 Similar to DySc2N@C80,15 a butterfly-shaped hysteresis is observed for DyYTiC@C80 at 1.8 K, which is open up to 7 K at 2.9 mT s−1 sweep rate.
The trimetallic carbide can be further extended to a special case of Dy3C2@C80 reported recently by Yang et al., in which a three-center single-electron metal–metal bond is disclosed within the triangular Dy3 plane with very short Dy–Dy distances of ∼3.4 Å.163 A similar trimetallic single-electron bond was later unveiled in Er3C2@C80.164 This peculiar metal–metal bonding character is distinct from the situation in Sc3C2@C80 where the unpaired electron is localized on the C2 unit,165–167 and thus is expected to evoke strong magnetic coupling between three Ln ions, which has just been confirmed very recently in a trinuclear gadolinium organometallic compound with a giant S = 11 ground state.168
4.2 Conventional EMFs
It is reasonably expected that the unpaired electron on the cage of these trivalent mono-EMFs could be detrimental to their magnetic stability that intrinsically originated in the anisotropic Ln ions, such as Dy3+. This can be rationalized by considering that on the one hand, the cage spin is weakly coupled with the encapsulated magnetic metal, which is sensitive to the subtle variations in the environment, such as lattice phonons and intermolecular interaction in a powder form. This strong molecular interaction is even able to induce dimerization of a relatively unstable Y@Cs(6)-C82 isomer in its cocrystal with Ni(OEP).174 The harmful influence of the open-shell cage on the metal, on the other hand, is aggravated due to the fact that the charge and the associated spin are actually delocalized on the cage, and thus numerous cage-based molecular vibrations can easily affect the spin state of the whole molecule. Very often these vibrations are able to accelerate magnetic relaxation and need to be carefully engineered at molecular and atomic levels. In fact, mono-EMFs represent an ideal case in which the Ln ion is coordinated with only one rigid carbon cage ligand, thus resulting in an ultralow phonon density of state involving metal-based vibrations. Only three vibrational modes are present in mono-EMFs in which the metal ion oscillates along the x-, y- and z-axes.83 This extraordinarily low-coordination environment, being inaccessible via conventional synthetic strategies since Ln ions always tend to have large coordination numbers, resembles the cases where single Ln atoms (Ho and Dy) are attached on graphene or metallic surfaces.175–177 Thanks to the reduced spin-phonon coupling as a result of low coordination, these single-atom magnets display magnetic bistability at an atomic level despite the large strength difference of ligand fields imposed by distinct surfaces.
Extracted from the above analysis, mono-EMFs are a promising platform to design SMMs, yet it is crucial to quench the unpaired electron on the cage which can then be used as a diamagnetic matrix for the Ln ion, with the aim to protect the spin state against the influence from the open-shell carbon cage. Such a goal can be realized by encapsulating divalent metals, such as Eu2+, Sm2+ and Yb2+. While the latter possesses fully occupied f orbitals (f14), Eu2+ has a half-filled f7 configuration and thus is considered to be isotropic at its atomic origin but slightly anisotropic in real molecules.178 Sm2+, on the other hand, could be interesting from the magnetic point of view. 13C NMR spectra also show changes in chemical shifts due to the paramagnetic lanthanide ion.111,179,180 However, it is not anisotropic enough to induce SMM behaviors. Thus, one has to again resort to trivalent mono-EMFs employing conventional magnetic Ln ions such as Dy3+ and Tb3+. In this context, a possible solution to quench the unpaired electron on the cage is exohedral functionalization via radical addition. This strategy can be achieved either by in situ derivatization during the arc-discharging process or by chemical reaction during extraction of the raw soot using organic solvents, affording covalent modifications of the cage with trifluoromethyl,181,182 dichlorophenyl,44,183–185 benzyl,186,187 and other radical groups.188,189 Not only mono-EMF derivatives have been reported based on the most popular C82 cages, but also various unstable fullerenes can be stabilized in this way, such as La@C72-C6H3Cl2 having a non-IPR cage and “missing” mono-EMFs like M@C74 and M@C70 (M = Y, La). The successful syntheses of these otherwise unstable species evidence the stabilization effect induced by radical addition on the open-shell mono-EMFs. The closed-shell carbon cage obtained via this approach also leads to the activation of the luminescence of Er3+ in its mono-EMFs due to the enlarged optical band gap upon functionalization.184
An even more straightforward method to realize the closed-shell fullerene cage in mono-EMFs is in situ nitrogen substitution of the pristine carbon cage, thus producing an azafullerene. Indeed, the idea of azafullerene was first achieved in (C59N)2 dimers190 and was later applied in mono-EMF La@C81N together with the synthesis of the pristine La@C82.191 However, this observation of monometallic azafullerene was only suggested by mass spectroscopy. The precise confirmation of its structure had long remained an open question over decades. Recently, such an approach towards a nitrogen-substituted mono-EMF with a closed-shell fullerene cage has been accomplished in La@C81N which is derived from the pristine C3v-C82 cage.42 The closed-shell nature of the azafullerene cage is verified by EPR spectroscopy showing an EPR-silent behavior for La@C81N.
Introducing Dy3+ to design a novel SMM, an azafullerene compound Dy@C81N was reported by Coronado et al. very recently based upon Cs-C82.16 In the structure of the mono-EMF Dy@C81N, the Dy3+ ion is asymmetrically coordinated by one side to a hexagonal carbon ring on the cage, while lacking any nonmetal ligand or negative charge on the other side towards the center of the cage. This extraordinary low coordination results in a very weak ligand field causing a small overall splitting (∼230 cm−1) of the 6H15/2 ground multiplet for Dy3+, similar to that found for a single Dy atom attached on graphene with the Dy-C6 ring coordination.177Ab initio calculations not only confirm the weak ligand field, but also indicate a mixed ground state for Dy3+. This special feature of single-ion anisotropy, often associated with weak SMM properties,192 is in striking contrast to the situation in all high-performance Dy-SMMs, where a pure mJ = ±15/2 ground Kramers doublet and a strongly axial field are always required. Despite this, the DC magnetic characterization of Dy@C81N shows slow magnetic relaxation (with τ > 40 s) up to 60 K (Fig. 8c). Analysis of the temperature dependence of the relaxation times reveals a temperature-independent QTM process below 17 K and a thermally activated Raman-like mechanism at high temperatures. The 100-s blocking temperature TB,100s, extracted from the fitting of the τ(T−1) curve, is determined as 45 K. This represents a record value among all known EMF-SMMs reported to date, being only behind the cyclopentadienyl (Cp)-based lanthanide complexes.11,12,23,193–195 The fast drop of τ due to an Orbach process normally detected for high-performance SMMs is not observed in Dy@C81N even up to 60 K. The lack of the Orbach process was reported before in DySc2N@C80 as discussed above,64 which suggests a weak spin-phonon coupling in this type of EMF-SIMs. Among them, Dy@C81N is even cleaner in terms of relaxation through the phonon bath because it has a minimal number (three) of vibrational modes that can couple to the spin states.
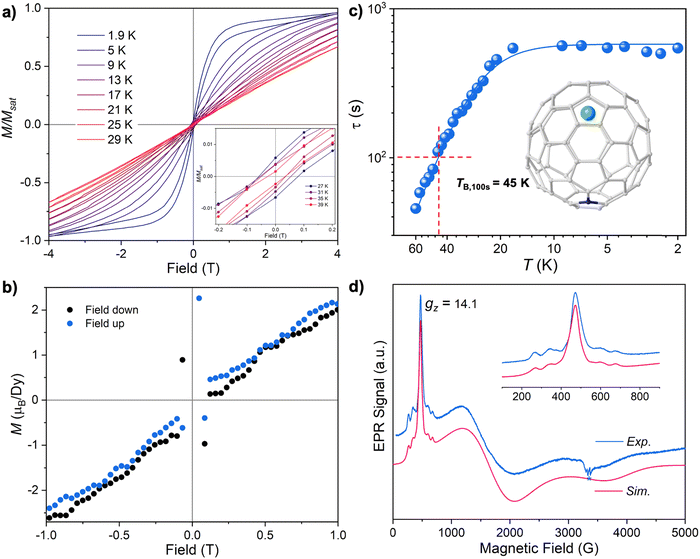 | ||
| Fig. 8 (a) Magnetic hysteresis loops for Dy@C81N at 1.9–39 K using SQUID magnetometry with a field sweep rate of 3.5 mT s−1. (b) Magnetic hysteresis for Dy@C81N at 5 K recorded by using the XMCD technique. (c) Temperature-dependence of magnetization relaxation times for Dy@C81N and the determination of its blocking temperature TB,100s = 45 K. The inset shows its molecular structure. (d) Experimental and simulated EPR spectra of Dy@C81N in a toluene solution at 10 K. The inset illustrates the hyperfine structures. Reproduced from ref. 16. | ||
As shown in Fig. 8a, magnetic hysteresis of Dy@C81N was recorded up to 39 K at a magnetic field sweep rate of 3.5 mT s−1, and further persists until 60 K using a faster sweep rate of 100 Oe s−1. Surprisingly, the hysteresis is thin even at the lowest measured temperature of 1.9 K, i.e., with weak coercive field and magnetization remanence. This very special hysteretic feature is further confirmed by element-specific XMCD measurement at the Dy M4,5 edge (Fig. 8b). The direct process in the presence of a strong applied magnetic field is likely responsible for the usual reduction of magnetic hysteresis due to the strongly mixed ground doublet of Dy3+ in the low-coordination environment, whose Zeeman energy is sensitive to the distortion caused by coupling of long-range vibrations to the spin states. The mixing nature of the ground doublet in Dy@C81N also leads to the observation of the EPR signal at 10 K using a conventional X-band spectrometer (Fig. 8d). A strong hyperfine coupling is evidenced for Dy@C81N between the electron spin and the nuclear spin within Dy3+, in which the hyperfine constant Az = 1600 MHz is determined for 163Dy. This information extracted from the EPR spectrum, in contrast, is elusive for other Dy-based SMMs due to the fast spin–lattice relaxation arising from the strong spin–orbit coupling in Dy3+, which may be ascribed to the well-protected Dy3+ spin from the destabilizing interactions with phonons in Dy@C81N at zero or finite magnetic fields where QTM dominates. Taking advantage of the hyperfine structures, it could be feasible to fine-tune magnetic relaxation upon applying magnetic fields, as already shown in SMMs based on Ho3+ and Tb3+.196,197
The goal of strong magnetic coupling can be realized by introducing a N23− radical bridge between two Ln3+ ions in an organometallic compound due to the rather diffuse unpaired electron from the N23− bridge.198 Such diffuse electron spin is able to strongly interact with the 4f electrons of Ln3+, thus giving rise to magnetic hysteresis up to 8.3 K at a sweep rate of 80 mT s−1 when the high-anisotropy Dy3+ is incorporated. Better SMM properties are obtained in Tb3+–N23−–Tb3+ three-center systems, among which a TB,100s value of 20 K is determined by employing Cp-based ligands together with the radical bridge.199,200 An advantageous consequence of the strong magnetic coupling is a broad hysteresis with a large coercive field thanks to the effective quenching of QTM at zero field. This design strategy of radical-bridged dilanthanide compounds as high-performance SMMs has been extensively studied, featuring strong antiferromagnetic or ferromagnetic coupling between the radical and Ln3+.201,202
Taking advantage of fullerene cages with confined nanospace, dimetallofullerenes (di-EMFs) are of particular interest for magnetic studies aiming at direct metal–metal interactions and thus strong magnetic coupling without any nonmetal ligand between the two metals. This ligand-unsupported metal–metal bond can be stabilized in M2@C82 (M = Sc, Y, Er, Lu), in which each metal adopts the divalent oxidation state, offering one electron to form a σ bond, and as a result the most popular tetravalent C82 cages are favored.203–205 For Ih(7)-C80 with exceptional stability in a 6-charged state, on the other hand, di-EMFs were initially expected to contain a rare-earth metal with the high oxidation state (+3), as exemplified in La2@C80. However, the valence state of the metal atom in di-EMFs is in fact determined by its ns2(n − 1)d1 to ns1(n − 1)d2 excitation energy that corresponds to the metal–metal bonding orbital.17,203 The relation of this energy to the cage molecular orbitals (MOs) is then crucial to govern the electronic structure of a di-EMF. A peculiar triplet ground state is thus found in theory for M2@C80 (M = Sc, Y, Gd, Er, Lu) resulting from the half filling of the low-lying bonding MOs of the M2 dimer to form a single-electron metal–metal bond (SEMB), while the cage possesses another unpaired electron.206,207 Under this circumstance, M2 transfers five electrons to the cage, leaving each metal with a formal charge of +2.5. This is akin to the situation of radical-bridged dilanthanide complexes as mentioned above, but with much structural simplicity in which the unpaired electron is shared solely by two metals, instead of being localized on the bridging ligand, affording an M3+–e–M3+ three-center system.
Preceding the in-depth understanding of the SEMB, such a bonding peculiarity was realized in a stable dimetallic azafullerene M2@C79N (M = Y, Tb) by Dorn et al., which is derived from the unstable M2@C80 by quenching its unpaired electron on the cage via nitrogen substitution.41 The singly-occupied metal–metal bonding molecular orbital (SOMO) is low-lying below the highest occupied MO (HOMO), thus leading to the stabilization of this open-shell molecule. Despite having a SEMB inside the cage, crystallographic study of Tb2@C79N shows a long Tb–Tb distance of 3.902 Å, even exceeding the sum of the covalent radius of two Tb atoms (3.88 Å), which is likely attributed to the strong Coulombic repulsion within the Tb25+ dimer. The open-shell nature is better understood in an S = 1/2 species, Y2@C79N, which enables the detection of single electron spin using EPR spectroscopy. The signal is split into three peaks with an intensity ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 arising from the hyperfine interaction of the single electron with the I = 1/2 89Y nuclear spin. A very large hyperfine coupling constant of 81 G (with the isotropic g factor of 1.9740 measured at room temperature) is observed for Y2@C79N. In comparison, the value is only 3.1 G in a radical-bridged diyttrium compound with a Y3+–N23−–Y3+ three-center system. This indicates the substantially stronger interaction between the unpaired electron and metals in di-EMFs containing SEMBs without any nonmetal bridging ligand, which can be evidenced by the sharing of the SOMO by the two metals in Y2@C79N whereas a SOMO mainly localized on the N2 unit is found for the radical-bridged diyttrium compound.208
1 arising from the hyperfine interaction of the single electron with the I = 1/2 89Y nuclear spin. A very large hyperfine coupling constant of 81 G (with the isotropic g factor of 1.9740 measured at room temperature) is observed for Y2@C79N. In comparison, the value is only 3.1 G in a radical-bridged diyttrium compound with a Y3+–N23−–Y3+ three-center system. This indicates the substantially stronger interaction between the unpaired electron and metals in di-EMFs containing SEMBs without any nonmetal bridging ligand, which can be evidenced by the sharing of the SOMO by the two metals in Y2@C79N whereas a SOMO mainly localized on the N2 unit is found for the radical-bridged diyttrium compound.208
The same group later also synthesized Gd2@C79N, which was characterized by X- and W-band EPR spectroscopy to show a giant spin ground state of S = 15/2, as a result of strong ferromagnetic coupling between the S =1/2 unpaired electron and two Gd3+ ions with S = 7/2.43Ab initio studies of the isoelectronic [Gd2@C80]− anion suggest that this ferromagnetic coupling arises from Hund's rule, but not from the double exchange mechanism conventionally observed in dinuclear mixed-valence transition-metal compounds.209 The exceptional stability of these SEMB-containing open-shell di-EMFs comes from the low-lying metal–metal bonding orbital as discussed before. Even for La2@C80 with a relatively high-energy La2 valence MO, SEMBs can still be stabilized by accepting one electron in such an MO upon reduction, radical addition and light-induced charge separation in an electron donor–acceptor conjugate.210–213 This is because that the La2 valence MO becomes the lowest unoccupied MO (LUMO) of the whole molecule. The existence of SEMBs in these cases can be then verified by EPR spectroscopy to show strong hyperfine splitting in the spectra due to the 139La nucleus.
The possibility of utilizing SEMB-containing di-EMFs towards high-performance SMMs was explored theoretically by Rajaraman et al.214 Broken-symmetry (BS) DFT studies of Gd2@C79N reveal a very strong ferromagnetic exchange between Gd and the unpaired electron with the coupling constant jGd–e (in the −2jGd–eSGdSe convention) in the order of 200 cm−1. Ab initio calculations performed on the Dy2@C79N molecule suggest that the exceptionally strong Dy-e magnetic coupling not only quenches QTM but can also increase the effective barrier of magnetization reversal. Thus, Dy2@C79N is anticipated to be a high-performance SMM. This idea was later realized by Popov et al. in a benzyl monoadduct, instead of substituting one carbon atom by nitrogen on the cage, of Dy2@C80 to quench the unpaired cage radical so as to obtain air-stable Dy2@C80(CH2Ph) containing a SEMB.18 In this molecule, the magnetic moments of two Dy3+ and the unpaired electron align collinearly to form a single spin unit of 21 μB. The spin Hamiltonian of this three-center system should be defined as follows derived from eqn (3) in absence of magnetic field
| Ĥspin = ĤLF1 + ĤLF2 − 2jLn–e(Ĵ1Se + Ĵ2Se) − 2j12Ĵ1Ĵ2 | (5) |
 | ||
| Fig. 9 (a)–(c) ZFC/FC curves of Dy2@C80(CH2Ph) (a), Tb2@C80(CH2Ph) (b), and Tb2@C79N (c). The magnetic field was 0.2–0.3 T, and the temperature sweep rate was 5 K min−1. (d)–(f) Magnetic hysteresis of Dy2@C80(CH2Ph) (d), Tb2@C80(CH2Ph) (e), and Tb2@C79N (f). The magnetic field sweep rate was 2.9 mT s−1 in (d) and (f) and 9.5 mT s−1 in (e). (g)–(i) Low-energy part of the spectra of Dy2@C80(CH2Ph) (g), Tb2@C80(CH2Ph) (h), and Tb2@C79N (i), using the effective spin Hamiltonian in eqn (5), with transition probabilities visualized as lines of different thickness (thicker lines correspond to higher probabilities), the x axis is the projection of magnetic moment upon the main anisotropy axis, μz. A schematic description of the spin alignment in the ground state and exchange-excited states is also shown (Dy and Tb, green arrows; single electron spin, dark blue and red arrows). Reproduced with permission from (a)–(f) ref. 17, (g) ref. 18, (h) ref. 19 and (i) ref. 21. | ||
| Type | EMF | T B,100s [K] | T B,hys [K] (dH/dt) [mT s−1] | T B,ZFC [K] (dT/dt) [K min−1] | U eff [K] | j Ln–e [cm−1] | Ref. |
|---|---|---|---|---|---|---|---|
| a Field-induced SMMs. b The value in the bracket indicates the field sweep rate (mT s−1) used to record hysteresis loops. c The value in the bracket indicates the temperature sweep rate (K min−1) used to record ZFC/FC curves. | |||||||
| Mono-EMF | Dy@Cs(6)-C81N | 45 | 39 (3.5)/60 (10) | 69 (1) | — | — | 16 |
| Di-EMFs | Dy2@C80(CH2Ph) | 18 | 22 (2.9) | 21.9 (5) | 613 | 32 | 18 |
| Dy2@C79N | 12 | 24 (20) | 21 (3) | 669 | 32 | 22 | |
| Tb2@C79N | 24 | 26 (2.9) | 28 (5) | 757 | 45 | 21 | |
| Tb2@C80(CH2Ph) | 25.2 | 27 (9.5) | 28.9 (5) | 799 | 55 | 19 | |
| Tb2@C80(CF3) | 25 | 26 (2.9) | 28.5 (5) | 801 | — | 20 | |
| Tb2@C80(CH2Ph)(pyr2) | 26.6 | 28 (2.9) | 30.3 (5) | 725 | — | 26 | |
| Ho2@C80(CH2Ph) | — | — | — | 334 | 40 | 19 | |
| Er2@C80(CH2Ph)a | — | — | — | — | 20 | 19 | |
| TbGd@C80(CH2Ph) | — | — | 14.4 (5) | — | — | 19 | |
| TbY@C80(CH2Ph) | — | 5 (2.9) | 5 (5) | — | 35 | 19 | |
| Nd2@C80(CF3)a | — | — | — | — | — | 217 | |
| Gd2@C79Na | — | — | — | 6.5 | 175/170 | 53 and 218 | |
| DyEr@C3v(8)-C82 | — | 3 (33) | 5 (3) | 6.4 | — | 226 | |
Using the cage derivatization method of benzyl radical addition, a series of air-stable M2@C80(CH2Ph) (M2 = Y2, Gd2, Tb2, Dy2, Ho2, Er2, TbY, TbGd) compounds containing SEMBs have been synthesized.19 Among them, Tb2@C80(CH2Ph) exhibits a gigantic coercive field of 8.2 T at 5 K extracted from its hysteresis loops, which can be recorded without closing up to 27 K at a field sweep rate of 9.5 mT s−1 (Fig. 9e). Analysis of the temperature-dependence of magnetic relaxation time gives a high TB,100s of 25.2 K and a large Ueff of 799 K (Fig. 9h). Meanwhile, TB,ZFC of 28.9 K can be extracted from the ZFC/FC curves (Fig. 9b). All these SMM metrics are significantly superior to those of its Dy counterpart, and are similar to the situation of N23−-radical-bridged dilanthanide compounds in which Tb is also better than Dy.198,200 In contrast to the worse Tb-SIMs often observed in organometallic compounds, the exceptional SMM behaviors for Tb2@C80(CH2Ph) lie in the Kramers Tb3+–e–Tb3+ three-center system, instead of the single non-Kramers Tb3+ ion, in such a way that QTM within the degenerate ground doublet of a strongly coupled system is essentially forbidden. Examining the magnetic properties of di-EMFs containing other anisotropic Ln ions reveals much weaker SMMs for Ho2 and Er2 cases. The former shows slow magnetization relaxation below 1 s with non-colinear alignment of the three magnetic moments, while the latter only exhibits field-induced AC signals due to the easy-plane anisotropy of the Er spin. Weaker SMM performance is also observed for the mixed-metal TbGd and TbY compounds, where the blocking temperatures (TB,ZFC) defined by ZFC/FC measurements are 14 K and 5 K, respectively. It is thus demonstrated that the coupling of the single Ln spin to the electron spin of the SEMB is not sufficiently strong, and the involvement of two anisotropic Ln ions is necessary to construct a remarkably high-performance SMM. When diamagnetic Y3+ and Sc3+ are introduced into M2@C80(CH2Ph) with an M3+–e–M3+ three-center system, strong hyperfine splitting can be observed in the presence of 89Y and 45Sc nuclear spins, giving rise to fully-resolved EPR spectra with well-defined transitions between specific electron-nuclear quantum states.215,216
The extraordinary SMM performance of Tb-based di-EMF was then further realized in Tb2@C79N using an azafullerene to obtain a closed-shell fullerene cage.21 Magnetic characterization of Tb2@C79N indicates similar results yet a slightly weaker SMM in comparison with Tb2@C80(CH2Ph). Specifically, Tb2@C79N exhibits a slightly lower TB,100s of 24 K and Ueff of 757 K (Fig. 9i). Its hysteresis, albeit broad, has a weaker coercive field of 3.8 T between 1.8 and 10 K, which then closes down up to 26 K at 2.9 mT s−1 sweep rate (Fig. 9f). Similarly, a slightly lower bifurcation temperature TB,ZFC of 28 K is determined (Fig. 9c). This difference in magnetic properties between di-EMFs with the carbon cage modified by N-substitution and by benzyl addition is consistent with the above discussion on the Dy counterparts. More importantly, ab initio calculations demonstrate that the magnetic moments of Tb ions in Tb2@C79N are tilted from the Tb–Tb bond by approximately 7°. This slight non-collinearity of the Tb quantization axes may be caused by the strong variation of the electrostatic potential distribution upon replacing one carbon atom by nitrogen, and could contribute to the change of magnetic behaviors. Apart from nitrogen substitution and exohedral benzyl addition on the cage, trifluoromethylation of di-EMF anions was then demonstrated to be a highly selective alternative route to this kind of structures featuring a ferromagnetically coupled M3+–e–M3+ three-center system.20 Through this method, Tb2@C80(CF3) can be readily synthesized as the main reaction product, which behaves as a strong SMM with a broad magnetic hysteresis, a high TB,100s of 24 K and an effective barrier Ueff of 801 K. Very recently, this method has been applied to stabilize SEMBs within an early lanthanide dimer Nd2 inside the Ih(7)-C80 and D5h(6)-C80 cages, making Nd2@C80(CF3) based on the Ih(7) isomer a field-induced SMM showing slow relaxation below 3 K.217
The magnetic coupling situation in di-EMFs containing SEMBs can be better understood in a Gd3+–e–Gd3+ system incorporating two nearly isotropic Gd3+ ions without spin–orbit coupling. Fitting the magnetic susceptibility and magnetization curves of Gd2@C79N clearly reveals a giant ferromagnetic Gd–e coupling with a coupling constant of 160–180 cm−1.53,218 Note that this value is comparable to the result predicted by BS-DFT calculations (∼200 cm−1)214 and greatly surpasses the antiferromagnetic coupling constant (−27 cm−1) obtained in the N23−-radical-bridged digadolinium compound.198 Such a strong magnetic coupling enables a giant total spin of S = 15/2 for the whole molecule. This high-spin ground state is well separated from the excited states and can be retained even at room temperature. Surprisingly, low temperature AC measurements also disclose that Gd2@C79N is a field-induced SMM showing slow relaxation of magnetization with the τ values at the millisecond scale at low temperatures below 3 K.218
The isotropic nature of the half-filled Gd3+ ion precludes the possibility to design a high-performance SMM based on it. This isotropy in the free Gd3+ ion is lifted in a coordination compound in the presence of ligand fields, which is ascribed to the mixing of the excited state with the ground multiplet 8S7/2. Note that the splitting of the ground multiplet in this case is very small (in the order of 1 cm−1) compared to that for other anisotropic Ln-based compounds in the same coordination environment (normally 100–1000 cm−1), but matches with the energy scale of conventional X-band EPR (∼0.3 cm−1), which can then be used as a powerful tool to probe the spin states of compounds having Gd3+. Despite the fragile magnetic bistability in a slightly anisotropic Gd-SMM, superposition between their two spin states could be created utilizing the pulsed EPR technique, giving rise to a spin qubit candidate showing a quantum coherence effect that could pave a way for quantum information processing.219–222 Indeed, Gd2@C79N was found to be an excellent high-spin qubit with microsecond-scale long coherence time at 5 K.53 A more intriguing feature of this molecule lies in its well-defined 16-fold spin sublevels derived from an S = 15/2 high-spin ground state. This allows coherent manipulation of the arbitrary superposition state between each adjacent level pair in a periodic manner, resulting in diverse Rabi oscillations performed at different applied magnetic fields. Gd2@C79N thus represents the largest-spin molecule that possesses Rabi oscillations reported so far. Aiming at a step forward using SMMs in quantum information technologies, the non-equidistant energy levels and long coherence times in the high-spin Gd2@C79N fulfill the requirements of Grover's searching algorithm exploiting multi-levels of the anisotropic SMMs,223 which has been realized on the nuclear spin of a Tb-SMM.224
The appealing magnetic properties in these SEMB-containing di-EMFs further stimulated the synthesis of strongly coupled dilanthanide organometallic compounds (CpiPr5)2Ln2I3 (Ln = Dy, Tb, Gd) directed at high-performance SMMs.23 By employing two Cp-based ligands as a source of strong axial ligand fields and three I− anions as bridging ligands, very strong SEMBs are constructed, giving rise to a ferromagnetically coupled high-spin ground state. These features jointly result in an enormous coercive field with a lower bound of 14 T in (CpiPr5)2Dy2I3 at 60 K, which surpasses even commercial magnets. Analysis of its relaxation times show a record-high TB,100s of 72 K, while this value is 65 K for the Tb congener. As a result of strong coupling, their effective barriers Ueff are determined to be as high as 2347 K and 1990 K, respectively. The better SMM performance, compared to that of di-EMFs with the same Ln3+–e–Ln3+ three-center system (Ln = Tb, Dy) derived from the pristine Ln2@C80, originates in the stronger magnetic coupling in (CpiPr5)2Ln2I3. Fitting the variable-temperature magnetic susceptibility curve of the Gd case using the three-center model gives a giant Gd–e coupling constant of jGd–e = 389 cm−1, more than twice the value of 175 cm−1 determined for Gd2@C79N.53 This strong SEMB can also be evidenced from the short Ln–Ln distances in (CpiPr5)2Ln2I3 that are within the sum of covalent radii for each metal atom. In contrast, substantially longer Ln–Ln distances are found for di-EMFs in the presence of strong Coulombic repulsion and metal–cage interactions that tend to separate the two Ln ions.
Extracted from the above comparative analysis, enhancing magnetic coupling in SEMB-containing di-EMFs is key to further improve their SMM properties. Since the previously discussed species are all based on Ih(7)-C80, it seems inevitable to alter the cage structure to achieve this goal. In this context, finding a much smaller cage is a logical next step to enforce close contact of the two encapsulated metals and overcome Coulombic repulsion by the confinement effect. This idea was confirmed theoretically in an actinide di-EMF that direct overlap of 5f orbitals may be realized to form U![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) U triple bonds in U2@C60,227 while the short U–U distance is not observed in U2@C80 due to the strong metal–cage interactions.228 Similar to Ln2@C79N (Ln = Tb, Dy, Gd) that are of particular interest to molecular magnetism, Ln2@C59N has been computed to show strong SEMBs.229Ab initio calculations yield a large Gd–e coupling constant of jGd–e = 434.8 cm−1 that is two times larger than the value determined for Gd2@C79N. Very high effective barriers Ueff are also predicted with values of 1702 and 2160 K for Dy and Tb cases, respectively, comparable to the current record determined in (CpiPr5)2Gd2I3.23
U triple bonds in U2@C60,227 while the short U–U distance is not observed in U2@C80 due to the strong metal–cage interactions.228 Similar to Ln2@C79N (Ln = Tb, Dy, Gd) that are of particular interest to molecular magnetism, Ln2@C59N has been computed to show strong SEMBs.229Ab initio calculations yield a large Gd–e coupling constant of jGd–e = 434.8 cm−1 that is two times larger than the value determined for Gd2@C79N. Very high effective barriers Ueff are also predicted with values of 1702 and 2160 K for Dy and Tb cases, respectively, comparable to the current record determined in (CpiPr5)2Gd2I3.23
Although these theoretical studies point out a promising future direction, synthesizing di-EMFs with such a small cage based on C60 is not feasible at the current stage of research. By slightly varying the cage size from C80, it has been shown that a SEMB may also be formed in Er2@C3v(8)-C82 by electrochemically manipulating the Er–Er bonding orbital.151 Similarly, two isomers of DyEr@C82 with C3v(8) and Cs(6) cage symmetries were reported, exhibiting cage-dependent magnetic and photoluminescent properties.226 Magnetic characterization studies indicate that DyEr@C3v(8)-C82 is an SMM with a thin hysteresis which closes below 3 K at a sweep rate of 33 mT s−1, while DyEr@Cs(6)-C82 is a paramagnet. Theoretical studies suggest the likely presence of SEMBs in these cases and consequently open-shell cage structures.
Very recently, Yang et al. have made a step forward towards the stabilization of SEMBs inside various pristine cages without the need for nitrogen substitution or chemical derivatization.225 This is accomplished by introducing a transition metal Ti in conjunction with La to form a heterometallic LaTi dimer inside the D3h(5)-C78, Ih(7)-C80, D5h(6)-C80 and C2v(9)-C82 carbon cages, each of which has a formal 6-charged state. After transferring six electrons to the cage, there is one unpaired electron left within the LaTi dimer, creating a SEMB. The La–Ti distances can be largely varied from 4.31 to 3.97 Å upon increasing the cage size from C78 to C82 (Fig. 10a). This uncommon anticorrelation between the cage size and the La–Ti distance suggests that the metal–cage interaction is instead the dominating effect in determining the strength of a SEMB inside medium-size fullerene cages. As shown in Fig. 10b, DFT calculations confirm the existence of SEMBs in each case featuring a metal–metal bonding SOMO, despite the long La–Ti separation due to the much smaller Ti atom, and its increased strength is verified by the gradual shift in the accumulation of electron density towards the center of the LaTi dimer. Interestingly, EPR spectroscopy shows a consecutive enhancement of hyperfine coupling between the unpaired electron and La following the decrease of La–Ti distance (Fig. 10c and d). The strongest coupling is thus found in LaTi@C2v(9)-C82. By changing metal–metal interactions and magnetic coupling, therefore, this proof-of-concept study offers a possibility to design SMMs, based on other anisotropic lanthanide metals such as Dy and Tb, which show improved performance in comparison with di-EMFs employing a modified Ih(7)-C80 cage.
 | ||
| Fig. 10 (a) Molecular structures of LaTi@D3h(5)-C78, LaTi@Ih(7)-C80, LaTi@D5h(6)-C80, and LaTi@C2v(9)-C82 (from left to right). La: cyan, Ti: violet. Their La-Ti distances are indicated. (b) SOMOs of LaTi@D3h(5)-C78, LaTi@Ih(7)-C80, LaTi@D5h(6)-C80, and LaTi@C2v(9)-C82 (from left to right). (c) Experimental and simulated EPR spectra of LaTi@C2n (2n = 78, 80 (two isomers), 82) measured in toluene solutions at 243 K. (d) Average hyperfine coupling constant (Aav in MHz) and La-Ti Mayer bond order of LaTi@C2n (2n = 78, 80 (two isomers), 82) as a function of the La-Ti distance. Reproduced with permission from ref. 225 (Copy right 2023, American Chemical Society). | ||
5. Assemblies of EMF-SMMs
The practical use of SMMs in information storage requires a well-defined molecular array that allows single molecules of interest to be precisely addressed. To this end, SMMs are required at first to be processed into a molecular assembly, such as a 2D monolayer on a substrate, in which sublimation is needed. This means that SMMs should be both chemically and thermally stable. In fact, these features constitute the major advantage of using EMFs as SMMs thanks to their highly robust fullerene structures. In this section, we move from the magnetic properties obtained from molecular ensembles in powder EMF samples towards the feasibility and prospect of their assembled molecular arrangements into different dimensions.5.1 1D SMM assemblies inside single walled carbon nanotubes (SWCNTs)
With cylindrical empty space, single walled carbon nanotubes (SWCNTs) with a typical diameter range of 1–2 nm are intuitively considered as a suitable container for atoms or molecules to construct a 1D assembly. Spherical EMF molecules, such as Gd@C82 and La2@C80, usually possess diameters less than 1 nm and can be readily encapsulated into SWCNTs to form a peapod-like hybrid structure, thus enabling single atom imaging of lanthanides that may be transformed into lanthanide atomic wires upon heating.230–232 Regarding SMMs, nevertheless, very few examples have been demonstrated combining them and SWCNTs, one of which is achieved through attaching bis-phthalocyaninato terbium (TbPc2) onto the surfaces of SWCNTs, generating supramolecular spin valves with giant magnetoresistance.233 On the other hand, encapsulation of SMMs into SWCNTs is expected to give rise to a quasi 1D arrangement in which their SMM properties should be enhanced.234 A non-1D manifestation is using multi-walled carbon nanotubes (MWCNTs) with large diameters of ∼6.5 nm to accommodate [Mn12O12(O2CCH3)16(H2O)4] (Mn12Ac, with a diameter of 1.6 nm), whose SMM behaviors are significantly altered inside MWCNTs compared to its bulk properties.235The first example to encapsulate EMF-SMMs into SWCNTs was reported by Yamashita et al. focusing on a well-studied NCF-SMM DySc2N@C80via a sublimation method.236 Transmission electron microscopy clearly showcases peapod structures wherein the EMF molecules line up in a quasi 1D chain as depicted in Fig. 11a and b. Magnetic characterization of this peapod structure [DySc2N@C80]@SWCNT by using SQUID magnetometers confirms that its characteristic SMM properties are retained inside SWCNTs with stepwise hysteresis at 1.8 K (Fig. 11c). Remarkably, the coercive field is greatly increased from 0.5 Oe in the bulk measurement to 0.4 T in the peapod structures, and correspondingly a slow relaxation of magnetization is observed in the absence of an external magnetic field with τ of 5650 s determined at 2 K, which is longer than that of DySc2N@C80 measured as a powder sample.15 Suppression of QTM by diluting the EMF upon encapsulation into SWCNTs is likely accountable for these improvements in the SMM performance, demonstrating that SWCNTs are able to protect SMMs from the environment in this 1D hybrid system.
 | ||
| Fig. 11 (a) and (b) TEM images and structural model of [DySc2N@C80]@SWCNT. (c) Magnetic hysteresis loops for DySc2N@C80 (open circles) and [DySc2N@C80]@SWCNT (filled circles) at 1.8 K. Arrows indicate the direction of the measurements. Reproduced with permission from ref. 236 (Copy right 2018, American Chemical Society). | ||
At the same time, another NCF bearing two Dy3+ ions, Dy2ScN@C80, was also successfully packed inside SWCNTs.237 Element-specific XMCD was performed for this hybrid material at the Dy M5-edge. The results show a deviation in its X-ray absorption spectra from that of an isotropic bulk sample, indicating a preferential orientation of the encapsulated Dy2ScN cluster that partially aligns the magnetization easy axis of the SMM in a 1D chain. This partial ordering of the clusters is also verified by ab initio calculations considering the diamagnetic counterpart Y2ScN@C80 inside the SWCNTs. Surprisingly and in striking contrast to the situation in mononuclear DySc2N@C80 packed peapod structures as described above, the broad hysteresis of the bulk Dy2ScN@C80 sample is not preserved when being filled inside the SWCNTs. Instead, magnetization curves for the Dy2ScN@C80/SWCNTs hybrid recorded at ∼2 K reveal a reduced magnetic bistability and vanishing hysteretic behavior. Various possible reasons, such as a higher local temperature in XMCD measurements, interaction with conducting electrons in metallic SWCNTs, and dipole–dipole interactions with residue metallic catalyst nanoparticles, were proposed to explain the observed reduction of SMM properties, yet no conclusive rationale has been reached.
5.2 2D SMM assemblies on surfaces
Attaching SMMs onto conducting surfaces to form 2D molecular assemblies is a crucial step forward in the field, in which the inherent magnetic properties of the SMMs should be maintained or precisely controlled.238–240 Among the plethora of examples of SMMs prepared so far, only a handful of candidates have been studied in surface science, such as Mn12Ac, TbPc2, and Fe4, where the XMCD technique is greatly needed.241–246 Various reasons can be ascribed to the lack of research in this area, majorly including the chemical instability that is often associated with the SMMs which precludes their processability onto surfaces under ultrahigh vacuum conditions at room temperature by sublimation and facilitates the loss of their magnetic bistability due to the detrimental interaction with the surfaces.2 EMFs can thus be a suitable candidate in this context.A submonolayer of a magnetic EMF Gd3N@C80 was prepared by depositing molecules onto metallic surfaces by thermal evaporation at room temperature.247 Field-, temperature- and angle-dependent XMCD measurements of Gd3N@C80 on Cu(001) reveal that the magnetic moments of the individual 4f7 Gd3+ ion couple ferromagnetically to each other. On the other hand, changing the surface to ferromagnetic Ni(001) induces a spin polarization of the Gd3+ magnetic moments, enabling the detection of two different Gd species that couple ferromagnetically and antiferromagnetically to the Ni substrate. An indirect exchange mechanism mediated by the fullerene cage is accountable for the magnetic coupling to the substrate, and the different orientations of the cage are likely responsible for the observation of different coupling interactions within the same submonolayer.
The first EMF-SMM for surface science studies was reported by Greber et al. via depositing Dy2ScN@C80 onto a Rh(111) substrate using the same sublimation method under vacuum.250 A submonolayer is generated in this way, in which the Dy3+ magnetic moments are aligned because of the interaction between the SMM and the metallic substrate. The angular anisotropy of X-ray absorption spectra and multiplet calculations indicate that the Dy2ScN cluster is orientated parallel to the surface. Remarkably, magnetic hysteresis is observed in this submonolayer at ∼ 4 K. The relaxation time is estimated to be about 30 s at zero field, much faster than that measured for powder samples. Despite this reduced magnetic bistability after deposition, which should be partly due to the demagnetization effect caused by X-ray irradiation as mentioned before,80 the surface-aligned magnetic ordering and the SMM behaviors in this EMF/Rh submonolayer are observed at one order of magnitude higher sample temperature than that for the transition-metal cluster SMMs.242–244
This result demonstrates the merit of using EMF-SMMs in surface science and thus has sparked further studies. For instance, employing the same preparation method, the same EMF-SMM Dy2ScN@C80 was evaporated onto a different surface, h-BN/Rh(111) nanomesh, to form 1.3 monolayers.251 Similarly, partial orientations of the encapsulated units and of the magnetic moments are determined by angle-dependent XMCD measurements. It is demonstrated that the monolayers exhibit a larger hysteresis with a coercive field of 0.4 T at 2 K in comparison with the result of Dy2ScN@C80 on a pure Rh(111) surface. The magnetic behaviors of Dy2ScN@C80 monolayers on different substrates were then studied, where not only metals like Au(111) and Ag(100) but also insulators such as MgO|Ag(100) were chosen for SMM deposition.248 A strong influence of the surface on the structural ordering of Dy2ScN@C80 monolayers is revealed. Despite this, their SMM properties are very similar in XMCD studies, all exhibiting broad magnetic hysteresis, with a coercive field of ca. 0.4 T at 2 K (Fig. 12e), that is independent of the substrate. DFT calculations indicate that the charge state of the encapsulated cluster remains intact, which is due to the protection of its electronic and magnetic properties provided by the fullerene, acting as a Faraday cage, against interactions with conducting substrates.
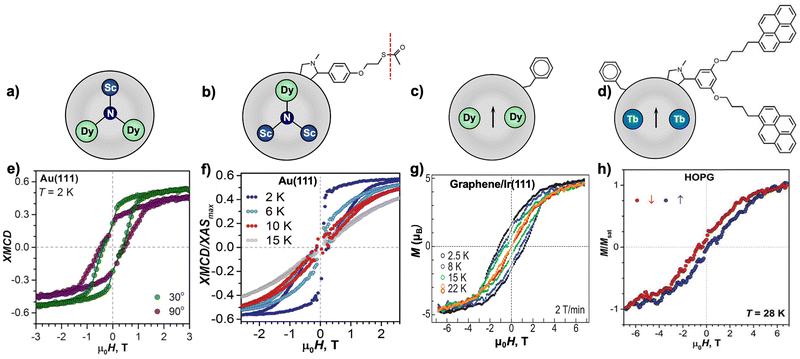 | ||
| Fig. 12 (a)–(d) Illustrations of Dy2ScN@C80 (a), functionalized DySc2N@C80 with a surface-anchoring thioacetate group (b), Dy2@C80(CH2Ph) (c), and pyrene-functionalized Tb2@C80(CH2Ph). (e)–(h) Magnetic hysteretic behaviors of submonolayers for Dy2ScN@C80 on Au(111) (e), functionalized DySc2N@C80 on Au(111) (f), Dy2@C80(CH2Ph) on graphene/Ir(111) (g), and pyrene-functionalized Tb2@C80(CH2Ph) on HOPG (h). Reproduced with permission from (e) ref. 248, (f) ref. 249, (g) ref. 24 and (h) ref. 26. | ||
Apart from evaporating the pristine EMFs to construct a 2D molecular assembly on surfaces, the chemical possibility to achieve the same goal, using functionalized Dy2ScN@C80 and DySc2N@C80 SMMs, was explored by Popov et al.249 This is realized by 1,3-dipolar cycloaddition with surface-anchoring thioether groups (–S–CH3). Compared to the pristine EMFs, the two functionalized molecules display different magnetic behaviors. In the case of DySc2N@C80, functionalization increases the bifurcation temperature TB,ZFC by 1 K, while the shape of hysteresis remains largely unchanged. For Dy2ScN@C80, the value of TB,ZFC becomes 4 K smaller and the hysteresis is noticeably thinner after functionalization. The thioether groups make it feasible to link these two functionalized EMF-SMMs to an Au(111) surface by simple physisorption, so as to generate low-coverage self-assembled monolayers (SAMs). XMCD measurements evidence that the magnetic bistability of these EMF-SMMs is retained in SAMs, featuring magnetic hysteresis at 2 K for each case. However, close contact of the fullerene cage with the metallic surface is favored in the preferential horizontal configurations, which is a result of the strong interaction of the fullerene and the linker with the substrate. This feature is expected to facilitate magnetic relaxation in SAMs. Furthermore, calculations indicate a random orientation of the Dy3+ magnetic moments at room temperature because of the highly mobile nature of these structures, whereas an orientation parallel to the surface is expected at low temperatures. A similar observation was also found recently for DySc2N@C80 adsorbed on a Pt(111) surface, which is directly inferred from the X-ray absorption spectra with in-plane polarization and indicates a weak but non-negligible interaction between the encapsulated cluster and the metallic surface.252
Changing the thioether linker –S–CH3 to a thioacetate group –S–Ac as the surface-anchoring group (Fig. 12b), cycloadducts of Dy2ScN@C80 and DySc2N@C80 SMMs can be grafted on gold via chemisorption, rather than a pure physisorption process, to form self-assembled films with submonolayer coverage.253 This is realized by dissolving functionalized EMFs in o-dichlorobenzene (o-DCB)/ethanol (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) upon adding H2SO4. The obtained SAMs from solution demonstrate that in both cases the magnetic bistability of bulk samples is maintained and the magnetic moments of the Dy ions are preferentially aligned parallel to the surface. Comparative magnetic studies were performed by XMCD measurements of the grafted SMMs using this facile solution-based procedure and of the pristine EMFs on the substrate prepared by sublimation. It is shown that while this chemical functionalization tends to reduce the SMM properties of Dy2ScN@C80, magnetic bistability in the on-surface DySc2N@C80, as can be seen in Fig. 12f, is significantly enhanced with observable magnetic hysteresis up to 10 K. The shielding effect of the carbon cage on the encapsulated magnetic centers also enables robust SMM behaviors that are not hinged upon the metallic substrate.
1) upon adding H2SO4. The obtained SAMs from solution demonstrate that in both cases the magnetic bistability of bulk samples is maintained and the magnetic moments of the Dy ions are preferentially aligned parallel to the surface. Comparative magnetic studies were performed by XMCD measurements of the grafted SMMs using this facile solution-based procedure and of the pristine EMFs on the substrate prepared by sublimation. It is shown that while this chemical functionalization tends to reduce the SMM properties of Dy2ScN@C80, magnetic bistability in the on-surface DySc2N@C80, as can be seen in Fig. 12f, is significantly enhanced with observable magnetic hysteresis up to 10 K. The shielding effect of the carbon cage on the encapsulated magnetic centers also enables robust SMM behaviors that are not hinged upon the metallic substrate.
Moving from the NCFs to SEMB-containing di-EMFs with better SMM performance, the possibility of generating a 2D molecular assembly based on Dy2@C80(CH2Ph) was first explored.24 It should be noted that in contrast to the non-derivatized EMFs with the pristine fullerene cages, lower thermal stability of Dy2@C80(CH2Ph) precludes its direct deposition on a surface by sublimation. Meanwhile, this functionalized EMF also lacks a surface-anchoring group that is necessary for the formation of self-assembled submonolayers. To this end, a gentle deposition method of electrospray technique was used in the preparation of Dy2@C80(CH2Ph) on graphene/Ir(111) as a suitable substrate. Remarkably, the as-formed submonolayer exhibits SMM behaviors that are fully comparable to those observed for the bulk sample. A broad magnetic hysteresis can be recorded by XMCD measurements at 2 K, which persists until around 20 K (Fig. 12g). Moreover, time-dependent XMCD relaxation measurements allow disentanglement of the contribution of the X-ray photon flux to the demagnetization effect. The analysis of its temperature-dependent relaxation times thus gives rise to a high 100-s blocking temperature TB of 17 K in this surface-supported SMM. In this 2D assembly, scanning tunneling microscopy was applied to directly image the unoccupied single-electron Dy–Dy bonding orbital (LUMO) with the aid of ab initio calculations.254 This result is further validated by changing the encapsulated metal from Dy to Er, which leads to the observation of an energy shift of the metal-based LUMO with respect to the neighboring cage-based orbitals. The energetically and spatially resolved SEMB orbital thus offers a direct access to this exchange-coupled magnetic system in transport experiments.
The bulk studies of di-EMFs indicate the better SMM performance of the Tb3+–e–Tb3+ three-center system, with Tb2@C80(CH2Ph) as an example, than that of its Dy counterpart. In order to obtain its self-assembled monolayers, further exohedral functionalization is necessary. This was done by introducing pyrene groups which show high affinity to graphitic substrates.26 Interestingly, pyrene-functionalized Tb2@C80(CH2Ph)(pyr2) (Fig. 12d) features similar but slightly better SMM performance than Tb2@C80(CH2Ph). Higher TB,100s of 26.6 K and TB,ZFC of 30.3 K are obtained after functionalization compared to those of Tb2@C80(CH2Ph) (TB,100s = 25.2 K and TB,ZFC = 28.9 K) determined using the same experimental conditions. Tb2@C80(CH2Ph)(pyr2) SAMs can then be simply constructed by the solution-based preparation procedure on graphitic substrates (graphene and highly oriented pyrolytic graphite HOPG) in an ambient environment. The magnetic bistability of this EMF-SMM is preserved on substrates, showing a broad magnetic hysteresis that closes down up to a very high temperature of 28 K (Fig. 12h), which is a major step forward in the field of on-surface SMMs.
In the case of Tb2@C79N with a similar three-center system but without the derivatized “branch” outside the cage, the growth of thin films by sublimation under ultra-high vacuum conditions becomes feasible thanks to its sufficient thermal stability. Thus, monolayers of Tb2@C79N were prepared on Cu(111) and Au(111) substrates.25 While the bulk sample of Tb2@C79N is a high-blocking temperature (TB,100s = 24 K) SMM featuring a strongly ferromagnetic coupling between two Tb magnetic moments and the unpaired electron spin, a distinct antiferromagnetically coupled ground state is identified for its monolayers in XMCD studied at the Tb-M4,5 edge. This results in a non-magnetic state in which magnetization is completely lost, and can be assigned to anionic Tb2@C79N− species with a doubly-occupied Tb–Tb bonding orbital, likely due to charge transfer from the substrate or trapping of secondary electrons. Since the antiferromagnetic coupling in this non-magnetic state is not strong enough, a metamagnetic transition can be found in the field of 2.5–4 T, where the ferromagnetically coupled state dominates. By depositing Tb2@C79N on other substrates, such as h-BN|Rh(111) and MgO|Ag(100), the coexistence of neutral and anionic species can be observed, leading to a narrow magnetic hysteresis that persists up to 25 K.
5.3 3D SMM assemblies within metal–organic frameworks (MOFs)
A more complex network of SMMs is a 3D molecular assembly, for which porous metal–organic frameworks can be naturally considered as a suitable host.255,256 Regarding EMFs, paramagnetic Y2@C79N was first introduced by Wang et al. inside the pores of a MOF crystal (MOF-177) to show axisymmetric paramagnetic properties.257 These different magnetic properties in the host–guest complex, in comparison with the isotropic system observed for Y2@C79N both in solution and in powder, suggest that the electron spin can be steered inside the pores of the MOF. The same group later reported that by combining the same MOF-177 and a different paramagnetic EMF Sc3C2@C80, the latter is able to act as a spin probe to sensitively detect the pore environment of the MOF.258 On the other hand, gas adsorption can be detected when a pyrene-based covalent organic framework (Py-COF) is introduced as the host.259By embedding DySc2N@C80 SMM into the pores of MOF-177, it is demonstrated that the QTM effect at zero field can be effectively suppressed upon encapsulation.260 A similar observation was also found for the same EMF incorporated into a different MOF structure, which is due to the dilution effect that weakens intermolecular interactions.64 By incarcerating DySc2N@C80 into a photo-switchable azobenzene functionalized MOF (AzoMOF), magnetic hysteresis opens up at zero field at 2 K by suppressing the QTM effect.261 More importantly, the hysteresis becomes broader after ultraviolet (365 nm) irradiation. The isomerization of azobenzene groups is expected to change the host–guest interaction between the EMF molecule and the MOF structure, and should be responsible for the enhanced SMM properties.
6. Conclusions and outlook
Since the discovery of DySc2N@C80 as the first EMF-SMM in 2012, EMFs have constituted an important branch of lanthanide-based SMMs during the last decade as elaborately summarized in this article. For the SMMs that are hinged upon only one magnetic lanthanide ion (SIMs), single-ion anisotropy plays a pivotal role. This factor can be precisely manipulated by engineering the structures of the encapsulated cluster and of the host fullerene cage in various types of clusterfullerene, in which the confinement effect imposed by the carbon cage facilitates strong axial anisotropy towards the nonmetal ligand with a short metal–nonmetal distance. Removing this nonmetal ligand and changing the electronic structure of the fullerene give rise to a low-coordination mono-EMF with a closed-shell cage, where vibrational degrees of freedom are minimized, thus reducing another equally important factor of spin–vibration coupling that is detrimental to magnetic bistability. In these EMF-SIMs, how to effectively suppress QTM in non-diluted bulk samples? To address this critical issue, judicious design of the cluster and cage structures is necessary. Heterometallic oxide clusterfullerenes with the largest possible effective barrier may be a target because their extremely axial ligand field may be able to quench QTM within the highly pure ground doublet mJ = ±15/2 for the encapsulated Dy3+ ion. Fine control of the local symmetry of the coordination environment is another possible solution, which nevertheless remains largely unexplored in EMFs. The design strategy for the special case of mono-EMFs with closed-shell fullerene cages, on the other hand, is more straightforward since the cage structure is the only variable factor given the same encapsulated Ln. Here, a fullerene cage with a more rigid metal–cage binding interaction is expected to reduce spin–vibration coupling so as to slow down the magnetic relaxation. To this end, mono-EMFs with non-IPR cages are of particular relevance.For the SMMs consisting of two magnetic lanthanide ions, magnetic coupling between them becomes a decisive factor governing their magnetic properties by blocking quantum tunneling of magnetization. This factor can be applied in clusterfullerenes by designing different bridging ligands that are able to transfer superexchange interactions as well as by altering the cage structures which in turn fine-tune the cluster geometry. Alternatively, such a goal can be realized in di-EMFs by engineering the electronic structure of the cage to generate single-electron metal–metal bonds (SEMBs), in which the unpaired electron directly couples to the two metals in the absence of any nonmetal bridging ligand. In these dinuclear EMFs, how to enhance magnetic coupling is the key to realize better SMMs. Whether it is feasible to generate an efficient direct Heisenberg exchange, rather than superexchange interaction, in a Ln–Ln bonding system becomes an intriguing aspect. On the other hand, although single-electron Ln–Ln bonds are an eye-catching case, the problem lies in the fact that the Ln–Ln distance is not short enough to induce strong magnetic coupling comparable to that of the best-performing SMM. Changing the host cage to compress the Ln2 dimer appears to be a promising direction despite its synthetic challenge given that available cage structures are very limited so far.
Another more general consideration is focused on the lanthanide metals. Since now the magnetic bistability of all reported EMF-SMMs comes from Dy3+ and Tb3+ only, the possibility of exploiting other Ln metals, especially early lanthanides, is of great interest to researchers. These candidates are in fact being widely used in permanent magnets, such as Nd2Fe14B and SmCo5. The similarity of single-ion anisotropy between early and late lanthanide ions that differ by seven f electrons may provide a general guidance herein. Beyond single molecular behaviors in bulk, several EMF-SMMs have been further processed into molecular assemblies via sublimation or self-assembly thanks to their rich structural diversity, exceptional magnetic properties and high chemical and thermal robustness. Their intrinsic SMM properties can be largely maintained in these organized structures, showcasing their great potential in information storage where single-molecule addressing is needed. With these exciting advancements, a next step forward would be to construct molecular spintronic devices based on SMMs towards their practical use as magnetic memory units to store information, yet very little is known about the role SMMs can play in this context. To fulfill future advancement directed at high-temperature, processable EMF-SMMs and beyond, there are many open questions remaining to be addressed. These endeavors, from the point of view of synthetic chemistry, shall be undertaken with an emphasis on the new structures, new related physical phenomena as well as the understanding of their interplay at an atomic level.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (51925206, U1932214, and 52302052), the Anhui Provincial Natural Science Foundation (2308085MB33), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB0450301), the Fundamental Research Funds for the Central Universities (20720220009), and the National Synchrotron Radiation Laboratory (KY2060000240)References
- D. Gatteschi, R. Sessoli and J. Villain, Molecular Nanomagnets, OUP Oxford, 2006 Search PubMed.
- E. Coronado, Nat. Rev. Mater., 2020, 5, 87–104 CrossRef.
- A. Gaita-Ariño, F. Luis, S. Hill and E. Coronado, Nat. Chem., 2019, 11, 301–309 CrossRef PubMed.
- M. Atzori and R. Sessoli, J. Am. Chem. Soc., 2019, 141, 11339–11352 CrossRef CAS PubMed.
- R. Sessoli, D. Gatteschi, A. Caneschi and M. Novak, Nature, 1993, 365, 141–143 CrossRef CAS.
- N. Ishikawa, M. Sugita, T. Ishikawa, S.-Y. Koshihara and Y. Kaizu, J. Am. Chem. Soc., 2003, 125, 8694–8695 CrossRef CAS PubMed.
- S.-D. Jiang, B.-W. Wang, H.-L. Sun, Z.-M. Wang and S. Gao, J. Am. Chem. Soc., 2011, 133, 4730–4733 CrossRef CAS PubMed.
- S. Cotton, Lanthanide and Actinide Chemistry, John Wiley & Sons, 2013 Search PubMed.
- J.-L. Liu, Y.-C. Chen and M.-L. Tong, Chem. Soc. Rev., 2018, 47, 2431–2453 RSC.
- D. N. Woodruff, R. E. P. Winpenny and R. A. Layfield, Chem. Rev., 2013, 113, 5110–5148 CrossRef CAS PubMed.
- K. Randall McClain, C. A. Gould, K. Chakarawet, S. J. Teat, T. J. Groshens, J. R. Long and B. G. Harvey, Chem. Sci., 2018, 9, 8492–8503 RSC.
- F.-S. Guo, B. M. Day, Y.-C. Chen, M.-L. Tong, A. Mansikkamäki and R. A. Layfield, Science, 2018, 362, 1400–1403 CrossRef CAS PubMed.
- A. A. Popov, S. Yang and L. Dunsch, Chem. Rev., 2013, 113, 5989–6113 CrossRef CAS PubMed.
- S. Yang, T. Wei and F. Jin, Chem. Soc. Rev., 2017, 46, 5005–5058 RSC.
- R. Westerström, J. Dreiser, C. Piamonteze, M. Muntwiler, S. Weyeneth, H. Brune, S. Rusponi, F. Nolting, A. Popov, S. Yang, L. Dunsch and T. Greber, J. Am. Chem. Soc., 2012, 134, 9840–9843 CrossRef PubMed.
- Z. Hu, Y. Wang, A. Ullah, G. M. Gutiérrez-Finol, A. Bedoya-Pinto, P. Gargiani, D. Shi, S. Yang, Z. Shi and A. Gaita-Ariño, Chem, 2023, 9, 3613–3622 CAS.
- F. Liu, L. Spree, D. S. Krylov, G. Velkos, S. M. Avdoshenko and A. A. Popov, Acc. Chem. Res., 2019, 52, 2981–2993 CrossRef CAS PubMed.
- F. Liu, D. S. Krylov, L. Spree, S. M. Avdoshenko, N. A. Samoylova, M. Rosenkranz, A. Kostanyan, T. Greber, A. U. B. Wolter, B. Büchner and A. A. Popov, Nat. Commun., 2017, 8, 16098 CrossRef CAS PubMed.
- F. Liu, G. Velkos, D. S. Krylov, L. Spree, M. Zalibera, R. Ray, N. A. Samoylova, C.-H. Chen, M. Rosenkranz, S. Schiemenz, F. Ziegs, K. Nenkov, A. Kostanyan, T. Greber, A. U. B. Wolter, M. Richter, B. Büchner, S. M. Avdoshenko and A. A. Popov, Nat. Commun., 2019, 10, 571 CrossRef CAS PubMed.
- Y. Wang, G. Velkos, N. J. Israel, M. Rosenkranz, B. Büchner, F. Liu and A. A. Popov, J. Am. Chem. Soc., 2021, 143, 18139–18149 CrossRef CAS PubMed.
- G. Velkos, D. S. Krylov, K. Kirkpatrick, L. Spree, V. Dubrovin, B. Büchner, S. M. Avdoshenko, V. Bezmelnitsyn, S. Davis, P. Faust, J. Duchamp, H. C. Dorn and A. A. Popov, Angew. Chem., Int. Ed., 2019, 58, 5891–5896 CrossRef CAS PubMed.
- Y. Wang, J. Xiong, J. Su, Z. Hu, F. Ma, R. Sun, X. Tan, H.-L. Sun, B.-W. Wang, Z. Shi and S. Gao, Nanoscale, 2020, 12, 11130–11135 RSC.
- C. A. Gould, K. R. McClain, D. Reta, J. G. C. Kragskow, D. A. Marchiori, E. Lachman, E.-S. Choi, J. G. Analytis, R. D. Britt, N. F. Chilton, B. G. Harvey and J. R. Long, Science, 2022, 375, 198–202 CrossRef CAS PubMed.
- F. Paschke, T. Birk, V. Enenkel, F. Liu, V. Romankov, J. Dreiser, A. A. Popov and M. Fonin, Adv. Mater., 2021, 33, e2102844 CrossRef PubMed.
- E. Koutsouflakis, D. Krylov, N. Bachellier, D. Sostina, V. Dubrovin, F. Liu, L. Spree, G. Velkos, S. Schimmel, Y. Wang, B. Büchner, R. Westerström, C. Bulbucan, K. Kirkpatrick, M. Muntwiler, J. Dreiser, T. Greber, S. M. Avdoshenko, H. Dorn and A. A. Popov, Nanoscale, 2022, 14, 9877–9892 RSC.
- L. Spree, F. Liu, V. Neu, M. Rosenkranz, G. Velkos, Y. Wang, S. Schiemenz, J. Dreiser, P. Gargiani, M. Valvidares, C.-H. Chen, B. Büchner, S. M. Avdoshenko and A. A. Popov, Adv. Funct. Mater., 2021, 31, 2105516 CrossRef CAS.
- W. Krätschmer, L. D. Lamb, K. Fostiropoulos and D. R. Huffman, Nature, 1990, 347, 354–358 CrossRef.
- S. Stevenson, G. Rice, T. Glass, K. Harich, F. Cromer, M. Jordan, J. Craft, E. Hadju, R. Bible and M. Olmstead, Nature, 1999, 401, 55–57 CrossRef CAS.
- C.-H. Chen, D. S. Krylov, S. M. Avdoshenko, F. Liu, L. Spree, R. Yadav, A. Alvertis, L. Hozoi, K. Nenkov, A. Kostanyan, T. Greber, A. U. B. Wolter and A. A. Popov, Chem. Sci., 2017, 8, 6451–6465 RSC.
- J. Xin, F. Jin, R. Guan, M. Chen, X.-M. Xie, Q. Zhang, S.-Y. Xie and S. Yang, Inorg. Chem. Front., 2021, 8, 1719–1726 RSC.
- J. Zhang, S. Stevenson and H. C. Dorn, Acc. Chem. Res., 2013, 46, 1548–1557 CrossRef CAS PubMed.
- S. Yang, S. I. Troyanov, A. A. Popov, M. Krause and L. Dunsch, J. Am. Chem. Soc., 2006, 128, 16733–16739 CrossRef CAS PubMed.
- Y.-Y. Xu, H.-R. Tian, S.-H. Li, Z.-C. Chen, Y.-R. Yao, S.-S. Wang, X. Zhang, Z.-Z. Zhu, S.-L. Deng and Q. Zhang, Nat. Commun., 2019, 10, 485 CrossRef CAS PubMed.
- S. Yang, F. Liu, C. Chen, M. Jiao and T. Wei, Chem. Commun., 2011, 47, 11822–11839 RSC.
- X. Lu, L. Feng, T. Akasaka and S. Nagase, Chem. Soc. Rev., 2012, 41, 7723–7760 RSC.
- A. Rodríguez-Fortea, A. L. Balch and J. M. Poblet, Chem. Soc. Rev., 2011, 40, 3551–3563 RSC.
- W. Xu, L. Feng, M. Calvaresi, J. Liu, Y. Liu, B. Niu, Z. Shi, Y. Lian and F. Zerbetto, J. Am. Chem. Soc., 2013, 135, 4187–4190 CrossRef CAS PubMed.
- H. W. Kroto, J. R. Heath, S. C. Obrien, R. F. Curl and R. E. Smalley, Nature, 1985, 318, 162–163 CrossRef CAS.
- J. Heath, S. O'brien, Q. Zhang, Y. Liu, R. Curl, F. Tittel and R. Smalley, J. Am. Chem. Soc., 1985, 107, 7779–7780 CrossRef CAS.
- T. A. Murphy, T. Pawlik, A. Weidinger, M. Höhne, R. Alcala and J.-M. Spaeth, Phys. Rev. Lett., 1996, 77, 1075 CrossRef CAS PubMed.
- T. Zuo, L. Xu, C. M. Beavers, M. M. Olmstead, W. Fu, T. D. Crawford, A. L. Balch and H. C. Dorn, J. Am. Chem. Soc., 2008, 130, 12992–12997 CrossRef CAS PubMed.
- W. Xiang, X. Jiang, Y.-R. Yao, J. Xin, H. Jin, R. Guan, Q. Zhang, M. Chen, S.-Y. Xie and A. A. Popov, J. Am. Chem. Soc., 2022, 144, 21587–21595 CrossRef CAS PubMed.
- W. Fu, J. Zhang, T. Fuhrer, H. Champion, K. Furukawa, T. Kato, J. E. Mahaney, B. G. Burke, K. A. Williams, K. Walker, C. Dixon, J. Ge, C. Shu, K. Harich and H. C. Dorn, J. Am. Chem. Soc., 2011, 133, 9741–9750 CrossRef CAS PubMed.
- T. Wakahara, H. Nikawa, T. Kikuchi, T. Nakahodo, G. A. Rahman, T. Tsuchiya, Y. Maeda, T. Akasaka, K. Yoza and E. Horn, J. Am. Chem. Soc., 2006, 128, 14228–14229 CrossRef CAS PubMed.
- H. Yang, C. X. Lu, Z. Y. Liu, H. X. Jin, Y. L. Che, M. M. Olmstead and A. L. Balch, J. Am. Chem. Soc., 2008, 130, 17296–17300 CrossRef CAS PubMed.
- B. Q. Mercado, M. A. Stuart, M. A. Mackey, J. E. Pickens, B. S. Confait, S. Stevenson, M. L. Easterling, R. Valencia, A. Rodriguez-Fortea, J. M. Poblet, M. M. Olmstead and A. L. Balch, J. Am. Chem. Soc., 2010, 132, 12098–12105 CrossRef CAS PubMed.
- S. Yang, C. Chen, F. Liu, Y. Xie, F. Li, M. Jiao, M. Suzuki, T. Wei, S. Wang, Z. Chen, X. Lu and T. Akasaka, Sci. Rep., 2013, 3, 1487 CrossRef PubMed.
- C. Pan, L. Bao, X. Yu, H. Fang, Y. Xie, T. Akasaka and X. Lu, ACS Nano, 2018, 12, 2065–2069 CrossRef CAS PubMed.
- S. Aoyagi, E. Nishibori, H. Sawa, K. Sugimoto, M. Takata, Y. Miyata, R. Kitaura, H. Shinohara, H. Okada, T. Sakai, Y. Ono, K. Kawachi, K. Yokoo, S. Ono, K. Omote, Y. Kasama, S. Ishikawa, T. Komuro and H. Tobita, Nat. Chem., 2010, 2, 678–683 CrossRef CAS PubMed.
- R. Guan, M. Chen, F. Jin and S. Yang, Angew. Chem., Int. Ed., 2020, 59, 1048–1073 CrossRef CAS PubMed.
- Y. Wang, S. Diaz-Tendero, F. Martin and M. Alcami, J. Am. Chem. Soc., 2016, 138, 1551–1560 CrossRef CAS PubMed.
- T. Wang and C. Wang, Acc. Chem. Res., 2014, 47, 450–458 CrossRef CAS PubMed.
- Z. Hu, B. W. Dong, Z. Liu, J. J. Liu, J. Su, C. Yu, J. Xiong, D. E. Shi, Y. Wang, B. W. Wang, A. Ardavan, Z. Shi, S. D. Jiang and S. Gao, J. Am. Chem. Soc., 2018, 140, 1123–1130 CrossRef CAS PubMed.
- J. Dreiser, K. S. Pedersen, C. Piamonteze, S. Rusponi, Z. Salman, M. E. Ali, M. Schau-Magnussen, C. A. Thuesen, S. Piligkos and H. Weihe, Chem. Sci., 2012, 3, 1024–1032 RSC.
- G. Velkos, W. Yang, Y.-R. Yao, S. M. Sudarkova, X. Liu, B. Büchner, S. M. Avdoshenko, N. Chen and A. A. Popov, Chem. Sci., 2020, 11, 4766–4772 RSC.
- A. Lunghi, F. Totti, R. Sessoli and S. Sanvito, Nat. Commun., 2017, 8, 14620 CrossRef PubMed.
- D. Reta, J. G. C. Kragskow and N. F. Chilton, J. Am. Chem. Soc., 2021, 143, 5943–5950 CrossRef CAS PubMed.
- A. Ullah, J. Cerdá, J. J. Baldoví, S. A. Varganov, J. Aragó and A. Gaita-Ariño, J. Phys. Chem. Lett., 2019, 10, 7678–7683 CrossRef CAS PubMed.
- L. Escalera-Moreno, J. J. Baldoví, A. Gaita-Ariño and E. Coronado, Chem. Sci., 2018, 9, 3265–3275 RSC.
- E. Garlatti, A. Chiesa, P. Bonfà, E. Macaluso, I. J. Onuorah, V. S. Parmar, Y.-S. Ding, Y.-Z. Zheng, M. J. Giansiracusa, D. Reta, E. Pavarini, T. Guidi, D. P. Mills, N. F. Chilton, R. E. P. Winpenny, P. Santini and S. Carretta, J. Phys. Chem. Lett., 2021, 12, 8826–8832 CrossRef CAS PubMed.
- J. G. C. Kragskow, J. Marbey, C. D. Buch, J. Nehrkorn, M. Ozerov, S. Piligkos, S. Hill and N. F. Chilton, Nat. Commun., 2022, 13, 825 CrossRef CAS PubMed.
- A. L. Blockmon, A. Ullah, K. D. Hughey and Y. Duan, et al. , Inorg. Chem., 2021, 60, 14096–14104 CrossRef CAS PubMed.
- A. Chiesa, F. Cugini, R. Hussain, E. Macaluso, G. Allodi, E. Garlatti, M. Giansiracusa, C. A. P. Goodwin, F. Ortu, D. Reta, J. M. Skelton, T. Guidi, P. Santini, M. Solzi, R. De Renzi, D. P. Mills, N. F. Chilton and S. Carretta, Phys. Rev. B, 2020, 101, 174402 CrossRef CAS.
- D. S. Krylov, F. Liu, A. Brandenburg, L. Spree, V. Bon, S. Kaskel, A. U. B. Wolter, B. Buchner, S. M. Avdoshenko and A. A. Popov, Phys. Chem. Chem. Phys., 2018, 20, 11656–11672 RSC.
- A. A. Popov, C. Chen, S. Yang, F. Lipps and L. Dunsch, ACS Nano, 2010, 4, 4857–4871 CrossRef CAS PubMed.
- T. Wei, S. Wang, X. Lu, Y. Tan, J. Huang, F. Liu, Q. Li, S. Xie and S. Yang, J. Am. Chem. Soc., 2016, 138, 207–214 CrossRef CAS PubMed.
- T. Wei, F. Jin, R. Guan, J. Huang, M. Chen, Q. Li and S. Yang, Angew. Chem., Int. Ed., 2018, 57, 10273–10277 CrossRef CAS PubMed.
- S. Yang, C. Chen, A. A. Popov, W. Zhang, F. Liu and L. Dunsch, Chem. Commun., 2009, 6391–6393, 10.1039/b911267g.
- C. Chen, F. Liu, S. Li, N. Wang, A. A. Popov, M. Jiao, T. Wei, Q. Li, L. Dunsch and S. Yang, Inorg. Chem., 2012, 51, 3039–3045 CrossRef CAS PubMed.
- S. Stevenson, P. Fowler, T. Heine, J. Duchamp, G. Rice, T. Glass, K. Harich, E. Hajdu, R. Bible and H. Dorn, Nature, 2000, 408, 427–428 CrossRef CAS PubMed.
- M. M. Olmstead, H. M. Lee, J. C. Duchamp, S. Stevenson, D. Marciu, H. C. Dorn and A. L. Balch, Angew. Chem., Int. Ed., 2003, 42, 900–903 CrossRef CAS PubMed.
- M. N. Chaur, R. Valencia, A. Rodriguez-Fortea, J. M. Poblet and L. Echegoyen, Angew. Chem., Int. Ed., 2009, 48, 1425–1428 CrossRef CAS PubMed.
- M. N. Chaur, F. Melin, J. Ashby, B. Elliott, A. Kumbhar, A. M. Rao and L. Echegoyen, Chem. – Eur. J., 2008, 14, 8213–8219 CrossRef CAS PubMed.
- M. N. Chaur, F. Melin, B. Elliott, A. Kumbhar, A. J. Athans and L. Echegoyen, Chem. – Eur. J., 2008, 14, 4594–4599 CrossRef CAS PubMed.
- J. D. Rinehart and J. R. Long, Chem. Sci., 2011, 2, 2078–2085 RSC.
- L. Spree and A. A. Popov, Dalton Trans., 2019, 48, 2861–2871 RSC.
- S. T. Liddle and J. van Slageren, Chem. Soc. Rev., 2015, 44, 6655–6669 RSC.
- L. Spree, C. Schlesier, A. Kostanyan, R. Westerstrom, T. Greber, B. Buchner, S. M. Avdoshenko and A. A. Popov, Chem. – Eur. J., 2020, 26, 2436–2449 CrossRef CAS PubMed.
- Y. Hao, G. Velkos, S. Schiemenz, M. Rosenkranz, Y. Wang, B. Büchner, S. M. Avdoshenko, A. A. Popov and F. Liu, Inorg. Chem. Front., 2023, 10, 468–484 RSC.
- J. Dreiser, R. Westerström, C. Piamonteze, F. Nolting, S. Rusponi, H. Brune, S. Yang, A. Popov, L. Dunsch and T. Greber, Appl. Phys. Lett., 2014, 105, 032411 CrossRef.
- M. Nie, J. Liang, C. Zhao, Y. Lu, J. Zhang, W. Li, C. Wang and T. Wang, ACS Nano, 2021, 15, 19080–19088 CrossRef CAS PubMed.
- M. Garcia-Borras, S. Osuna, J. M. Luis, M. Swart and M. Sola, Chem. Soc. Rev., 2014, 43, 5089–5105 RSC.
- Z. Hu, A. Ullah, H. Prima-Garcia, S.-H. Chin, Y. Wang, J. Aragó, Z. Shi, A. Gaita-Ariño and E. Coronado, Chem. – Eur. J., 2021, 27, 13242–13248 CrossRef CAS PubMed.
- C. Schlesier, L. Spree, A. Kostanyan, R. Westerstrom, A. Brandenburg, A. U. B. Wolter, S. Yang, T. Greber and A. A. Popov, Chem. Commun., 2018, 54, 9730–9733 RSC.
- J. Dreiser, R. Westerstrom, Y. Zhang, A. A. Popov, L. Dunsch, K. Kramer, S. X. Liu, S. Decurtins and T. Greber, Chem. – Eur. J., 2014, 20, 13536–13540 CrossRef CAS PubMed.
- Y. Zhang, D. Krylov, S. Schiemenz, M. Rosenkranz, R. Westerstrom, J. Dreiser, T. Greber, B. Buchner and A. A. Popov, Nanoscale, 2014, 6, 11431–11438 RSC.
- A. Kostanyan, R. Westerstrom, Y. Zhang, D. Kunhardt, R. Stania, B. Buchner, A. A. Popov and T. Greber, Phys. Rev. Lett., 2017, 119, 237202 CrossRef PubMed.
- Y. Zhang, D. Krylov, M. Rosenkranz, S. Schiemenz and A. A. Popov, Chem. Sci., 2015, 6, 2328–2341 RSC.
- V. Vieru, L. Ungur and L. F. Chibotaru, J. Phys. Chem. Lett., 2013, 4, 3565–3569 CrossRef CAS.
- N. Ishikawa, M. Sugita and W. Wernsdorfer, Angew. Chem., Int. Ed., 2005, 44, 2931–2935 CrossRef CAS PubMed.
- Y.-C. Chen and M.-L. Tong, Chem. Sci., 2022, 13, 8716–8726 RSC.
- B.-K. Ling, Y.-Q. Zhai, P.-B. Jin, H.-F. Ding, X.-F. Zhang, Y. Lv, Z. Fu, J. Deng, M. Schulze and W. Wernsdorfer, Matter, 2022, 5, 3485–3498 CrossRef CAS.
- R. Westerström, J. Dreiser, C. Piamonteze, M. Muntwiler, S. Weyeneth, K. Krämer, S.-X. Liu, S. Decurtins, A. Popov, S. Yang, L. Dunsch and T. Greber, Phys. Rev. B: Condens. Matter Mater. Phys., 2014, 89, 060406(R) CrossRef.
- R. Westerström, V. Dubrovin, K. Junghans, C. Schlesier, B. Büchner, S. M. Avdoshenko, A. A. Popov, A. Kostanyan, J. Dreiser and T. Greber, Phys. Rev. B, 2021, 104, 224401 CrossRef.
- D. S. Krylov, F. Liu, S. M. Avdoshenko, L. Spree, B. Weise, A. Waske, A. U. B. Wolter, B. Buchner and A. A. Popov, Chem. Commun., 2017, 53, 7901–7904 RSC.
- A. Kostanyan, C. Schlesier, R. Westerström, J. Dreiser, F. Fritz, B. Büchner, A. A. Popov, C. Piamonteze and T. Greber, Phys. Rev. B, 2021, 103, 014404 CrossRef CAS.
- M. Nie, J. Xiong, C. Zhao, H. Meng, K. Zhang, Y. Han, J. Li, B. Wang, L. Feng, C. Wang and T. Wang, Nano Res., 2019, 12, 1727–1731 CrossRef CAS.
- A. Kostanyan, R. Westerström, D. Kunhardt, B. Büchner, A. A. Popov and T. Greber, Phys. Rev. B, 2020, 101, 134429 CrossRef CAS.
- F. Liu, S. Wang, C. L. Gao, Q. Deng, X. Zhu, A. Kostanyan, R. Westerstrom, F. Jin, S. Y. Xie, A. A. Popov, T. Greber and S. Yang, Angew. Chem., Int. Ed., 2017, 56, 1830–1834 CrossRef CAS PubMed.
- F. Liu, C.-L. Gao, Q. Deng, X. Zhu, A. Kostanyan, R. Westerström, S. Wang, Y.-Z. Tan, J. Tao, S.-Y. Xie, A. A. Popov, T. Greber and S. Yang, J. Am. Chem. Soc., 2016, 138, 14764–14771 CrossRef CAS PubMed.
- G. Velkos, W. Yang, Y. R. Yao, S. M. Sudarkova, F. Liu, S. M. Avdoshenko, N. Chen and A. A. Popov, Chem. Commun., 2022, 58, 7164–7167 RSC.
- W. Yang, G. Velkos, F. Liu, S. M. Sudarkova, Y. Wang, J. Zhuang, H. Zhang, X. Li, X. Zhang, B. Buchner, S. M. Avdoshenko, A. A. Popov and N. Chen, Adv. Sci., 2019, 6, 1901352 CrossRef CAS PubMed.
- W. Yang, G. Velkos, S. Sudarkova, B. Büchner, S. M. Avdoshenko, F. Liu, A. A. Popov and N. Chen, Inorg. Chem. Front., 2022, 9, 5805–5819 RSC.
- D. Krylov, G. Velkos, C. H. Chen, B. Buchner, A. Kostanyan, T. Greber, S. M. Avdoshenko and A. A. Popov, Inorg. Chem. Front., 2020, 7, 3521–3532 RSC.
- W. Cai, J. D. Bocarsly, A. Gomez, R. J. L. Lee, A. Metta-Magana, R. Seshadri and L. Echegoyen, Chem. Sci., 2020, 11, 13129–13136 RSC.
- A. Brandenburg, D. S. Krylov, A. Beger, A. U. B. Wolter, B. Buchner and A. A. Popov, Chem. Commun., 2018, 54, 10683–10686 RSC.
- K. Junghans, C. Schlesier, A. Kostanyan, N. A. Samoylova, Q. Deng, M. Rosenkranz, S. Schiemenz, R. Westerstrom, T. Greber, B. Buchner and A. A. Popov, Angew. Chem., Int. Ed., 2015, 54, 13411–13415 CrossRef CAS PubMed.
- A. L. Svitova, Y. Krupskaya, N. Samoylova, R. Kraus, J. Geck, L. Dunsch and A. A. Popov, Dalton Trans., 2014, 43, 7387–7390 RSC.
- M. Wolf, K. H. Muller, Y. Skourski, D. Eckert, P. Georgi, M. Krause and L. Dunsch, Angew. Chem., Int. Ed., 2005, 44, 3306–3309 CrossRef CAS PubMed.
- B. Náfrádi, Á. Antal, Á. Pásztor, L. Forró, L. F. Kiss, T. Fehér, É. Kováts, S. Pekker and A. Jánossy, J. Phys. Chem. Lett., 2012, 3, 3291–3296 CrossRef.
- Z. Hu, Y. Hao, Z. Slanina, Z. Gu, Z. Shi, F. Uhlik, Y. Zhao and L. Feng, Inorg. Chem., 2015, 54, 2103–2108 CrossRef CAS PubMed.
- L. Bao, P. Yu, Y. Li, C. Pan, W. Shen, P. Jin, S. Liang and X. Lu, Chem. Sci., 2019, 10, 4945–4950 RSC.
- L. Bao, Y. Li, P. Yu, W. Shen, P. Jin and X. Lu, Angew. Chem., Int. Ed., 2020, 59, 5259–5262 CrossRef CAS PubMed.
- X. Lu, Z. Slanina, T. Akasaka, T. Tsuchiya, N. Mizorogi and S. Nagase, J. Am. Chem. Soc., 2010, 132, 5896–5905 CrossRef CAS PubMed.
- H. Yang, H. Jin, X. Wang, Z. Liu, M. Yu, F. Zhao, B. Q. Mercado, M. M. Olmstead and A. L. Balch, J. Am. Chem. Soc., 2012, 134, 14127–14136 CrossRef CAS PubMed.
- F. Liu, S. Wang, J. Guan, T. Wei, M. Zeng and S. Yang, Inorg. Chem., 2014, 53, 5201–5205 CrossRef CAS PubMed.
- W. Shen, Z. Hu, P. Yu, Z. Wei, P. Jin, Z. Shi and X. Lu, Inorg. Chem. Front., 2020, 7, 4563–4571 RSC.
- R. Guan, M. Chen, J. Xin, X.-M. Xie, F. Jin, Q. Zhang, S.-Y. Xie and S. Yang, J. Am. Chem. Soc., 2021, 143, 8078–8085 CrossRef CAS PubMed.
- F. Jin, S. Wang, S. Yang, N. B. Tamm, I. N. Ioffe and S. I. Troyanov, Inorg. Chem., 2016, 55, 12523–12526 CrossRef CAS PubMed.
- F. Jin, S. Wang, N. B. Tamm, S. Yang and S. I. Troyanov, Angew. Chem., Int. Ed., 2017, 129, 12152–12156 CrossRef.
- S. Stevenson, M. A. Mackey, M. A. Stuart, J. P. Phillips, M. L. Easterling, C. J. Chancellor, M. M. Olmstead and A. L. Balch, J. Am. Chem. Soc., 2008, 130, 11844–11845 CrossRef CAS PubMed.
- B. Q. Mercado, M. M. Olmstead, C. M. Beavers, M. L. Easterling, S. Stevenson, M. A. Mackey, C. E. Coumbe, J. D. Phillips, J. P. Phillips, J. M. Poblet and A. L. Balch, Chem. Commun., 2010, 46, 279–281 RSC.
- L. Feng, Y. Hao, A. Liu and Z. Slanina, Acc. Chem. Res., 2019, 52, 1802–1811 CrossRef CAS PubMed.
- M. Zhang, Y. Hao, X. Li, L. Feng, T. Yang, Y. Wan, N. Chen, Z. Slanina, F. Uhlík and H. Cong, J. Phys. Chem. C, 2014, 118, 28883–28889 CrossRef CAS.
- Q. Tang, L. Abella, Y. Hao, X. Li, Y. Wan, A. Rodriguez-Fortea, J. M. Poblet, L. Feng and N. Chen, Inorg. Chem., 2015, 54, 9845–9852 CrossRef CAS PubMed.
- T. Yang, Y. Hao, L. Abella, Q. Tang, X. Li, Y. Wan, A. Rodríguez-Fortea, J. M. Poblet, L. Feng and N. Chen, Chem. – Eur. J., 2015, 21, 11110–11117 CrossRef CAS PubMed.
- Y. Hao, Q. Tang, X. Li, M. Zhang, Y. Wan, L. Feng, N. Chen, Z. Slanina, L. Adamowicz and F. Uhlík, Inorg. Chem., 2016, 55, 11354–11361 CrossRef CAS PubMed.
- L. Feng, M. Zhang, Y. Hao, Q. Tang, N. Chen, Z. Slanina and F. Uhlík, Dalton Trans., 2016, 45, 8142–8148 RSC.
- Q. Tang, L. Abella, Y. Hao, X. Li, Y. Wan, A. Rodriguez-Fortea, J. M. Poblet, L. Feng and N. Chen, Inorg. Chem., 2016, 55, 1926–1933 CrossRef CAS PubMed.
- P. Yu, M. Li, W. Shen, S. Hu, P.-Y. Yu, X. Tian, X. Zhao, L. Bao and X. Lu, Inorg. Chem. Front., 2022, 9, 2264–2270 RSC.
- P. Yu, H. Mei, S. Hu, C. Pan, W. Shen, P. Yu, K. Guo, Y. Xie, T. Akasaka and L. Bao, Chin. J. Chem., 2023, 41, 1915–1920 CrossRef CAS.
- H. Cong, A. Liu, Y. Hao, L. Feng, Z. Slanina and F. Uhlik, Inorg. Chem., 2019, 58, 10905–10911 CrossRef CAS PubMed.
- J. C. Duchamp, H. C. Dorn, A. L. Wysocki, K. Park, M. M. Olmstead, M. Roy and A. L. Balch, Inorg. Chem., 2023, 62, 5114–5122 CrossRef CAS PubMed.
- A. Liu, M. Nie, Y. Hao, Y. Yang, T. Wang, Z. Slanina, H. Cong, L. Feng, C. Wang and F. Uhlik, Inorg. Chem., 2019, 58, 4774–4781 CrossRef CAS PubMed.
- P. Yu, M. Li, S. Hu, C. Pan, W. Shen, G. Kun, Y.-P. Xie, L. Bao, R. Zhang and X. Lu, Chem. Commun., 2023, 59, 12990–12993 RSC.
- M. K. Singh and G. Rajaraman, Chem. Commun., 2016, 52, 14047–14050 RSC.
- J. Xiong, H.-Y. Ding, Y.-S. Meng, C. Gao, X.-J. Zhang, Z.-S. Meng, Y.-Q. Zhang, W. Shi, B.-W. Wang and S. Gao, Chem. Sci., 2017, 8, 1288–1294 RSC.
- J. Wang, Q. W. Li, S. G. Wu, Y. C. Chen, R. C. Wan, G. Z. Huang, Y. Liu, J. L. Liu, D. Reta and M. J. Giansiracusa, Angew. Chem., Int. Ed., 2021, 60, 5299–5306 CrossRef CAS PubMed.
- P. H. Lin, T. J. Burchell, R. Clérac and M. Murugesu, Angew. Chem., Int. Ed., 2008, 47, 8848–8851 CrossRef CAS PubMed.
- Y.-N. Guo, G.-F. Xu, W. Wernsdorfer, L. Ungur, Y. Guo, J. Tang, H.-J. Zhang, L. F. Chibotaru and A. K. Powell, J. Am. Chem. Soc., 2011, 133, 11948–11951 CrossRef CAS PubMed.
- J. Long, F. Habib, P.-H. Lin, I. Korobkov, G. Enright, L. Ungur, W. Wernsdorfer, L. F. Chibotaru and M. Murugesu, J. Am. Chem. Soc., 2011, 133, 5319–5328 CrossRef CAS PubMed.
- E. Moreno Pineda, N. F. Chilton, R. Marx, M. Dorfel, D. O. Sells, P. Neugebauer, S. D. Jiang, D. Collison, J. van Slageren, E. J. McInnes and R. E. Winpenny, Nat. Commun., 2014, 5, 5243 CrossRef CAS PubMed.
- X.-Q. Ji, F. Ma, J. Xiong, J. Yang, H.-L. Sun, Y.-Q. Zhang and S. Gao, Inorg. Chem. Front., 2019, 6, 786–790 RSC.
- C. Y. Chow, H. Bolvin, V. E. Campbell, R. Guillot, J. W. Kampf, W. Wernsdorfer, F. Gendron, J. Autschbach, V. L. Pecoraro and T. Mallah, Chem. Sci., 2015, 6, 4148–4159 RSC.
- P. Zhang, F. Benner, N. F. Chilton and S. Demir, Chem, 2022, 8, 717–730 CAS.
- L. Dunsch, S. F. Yang, L. Zhang, A. Svitova, S. Oswald and A. A. Popov, J. Am. Chem. Soc., 2010, 132, 5413–5421 CrossRef CAS PubMed.
- N. Chen, M. N. Chaur, C. Moore, J. R. Pinzon, R. Valencia, A. Rodriguez-Fortea, J. M. Poblet and L. Echegoyen, Chem. Commun., 2010, 46, 4818–4820 RSC.
- B. Q. Mercado, N. Chen, A. Rodríguez-Fortea, M. A. Mackey, S. Stevenson, L. Echegoyen, J. M. Poblet, M. M. Olmstead and A. L. Balch, J. Am. Chem. Soc., 2011, 133, 6752–6760 CrossRef CAS PubMed.
- N. Chen, C. M. Beavers, M. Mulet-Gas, A. Rodriguez-Fortea, E. J. Munoz, Y. Y. Li, M. M. Olmstead, A. L. Balch, J. M. Poblet and L. Echegoyen, J. Am. Chem. Soc., 2012, 134, 7851–7860 CrossRef CAS PubMed.
- N. Chen, M. Mulet-Gas, Y.-Y. Li, R. E. Stene, C. W. Atherton, A. Rodríguez-Fortea, J. M. Poblet and L. Echegoyen, Chem. Sci., 2013, 4, 180–186 RSC.
- N. A. Samoylova, S. M. Avdoshenko, D. S. Krylov, H. R. Thompson, A. C. Kirkhorn, M. Rosenkranz, S. Schiemenz, F. Ziegs, A. U. B. Wolter, S. Yang, S. Stevenson and A. A. Popov, Nanoscale, 2017, 9, 7977–7990 RSC.
- F.-F. Li, N. Chen, M. Mulet-Gas, V. Triana, J. Murillo, A. Rodríguez-Fortea, J. M. Poblet and L. Echegoyen, Chem. Sci., 2013, 4, 3404 RSC.
- W. Cao, C. Gao, Y.-Q. Zhang, D. Qi, T. Liu, K. Wang, C. Duan, S. Gao and J. Jiang, Chem. Sci., 2015, 6, 5947–5954 RSC.
- F. Tuna, C. A. Smith, M. Bodensteiner, L. Ungur, L. F. Chibotaru, E. J. McInnes, R. E. Winpenny, D. Collison and R. A. Layfield, Angew. Chem., Int. Ed., 2012, 51, 6976–6980 CrossRef CAS PubMed.
- S.-S. Liu, K. Lang, Y.-Q. Zhang, Q. Yang, B.-W. Wang and S. Gao, Dalton Trans., 2016, 45, 8149–8153 RSC.
- X. Lu, T. Akasaka and S. Nagase, Acc. Chem. Res., 2013, 46, 1627–1635 CrossRef CAS PubMed.
- Z. Q. Shi, X. Wu, C. R. Wang, X. Lu and H. Shinohara, Angew. Chem., Int. Ed., 2006, 45, 2107–2111 CrossRef CAS PubMed.
- J. Zhang, F. L. Bowles, D. W. Bearden, W. K. Ray, T. Fuhrer, Y. Ye, C. Dixon, K. Harich, R. F. Helm, M. M. Olmstead, A. L. Balch and H. C. Dorn, Nat. Chem., 2013, 5, 880–885 CrossRef CAS PubMed.
- S. Zhao, P. Zhao, W. Cai, L. Bao, M. Chen, Y. Xie, X. Zhao and X. Lu, J. Am. Chem. Soc., 2017, 139, 4724–4728 CrossRef CAS PubMed.
- C. R. Wang, T. Kai, T. Tomiyama, T. Yoshida, Y. Kobayashi, E. Nishibori, M. Takata, M. Sakata and H. Shinohara, Angew. Chem., Int. Ed., 2001, 40, 397–399 CrossRef CAS PubMed.
- J. Zhang, T. Fuhrer, W. Fu, J. Ge, D. W. Bearden, J. Dallas, J. Duchamp, K. Walker, H. Champion, H. Azurmendi, K. Harich and H. C. Dorn, J. Am. Chem. Soc., 2012, 134, 8487–8493 CrossRef CAS PubMed.
- A. L. Svitova, K. B. Ghiassi, C. Schlesier, K. Junghans, Y. Zhang, M. M. Olmstead, A. L. Balch, L. Dunsch and A. A. Popov, Nat. Commun., 2014, 5, 3568 CrossRef CAS PubMed.
- F. Jin, J. Xin, R. Guan, X.-M. Xie, M. Chen, Q. Zhang, A. A. Popov, S.-Y. Xie and S. Yang, Chem. Sci., 2021, 12, 6890–6895 RSC.
- S. Hu, P. Zhao, B. Li, P. Yu, L. Yang, M. Ehara, P. Jin, T. Akasaka and X. Lu, Inorg. Chem., 2022, 61, 11277–11283 CrossRef CAS PubMed.
- H. Fang, H. Cong, M. Suzuki, L. Bao, B. Yu, Y. Xie, N. Mizorogi, M. M. Olmstead, A. L. Balch and S. Nagase, J. Am. Chem. Soc., 2014, 136, 10534–10540 CrossRef CAS PubMed.
- K. Tan and X. Lu, J. Phys. Chem. A, 2006, 110, 1171–1176 CrossRef CAS PubMed.
- Y. Iiduka, T. Wakahara, T. Nakahodo, T. Tsuchiya, A. Sakuraba, Y. Maeda, T. Akasaka, K. Yoza, E. Horn and T. Kato, J. Am. Chem. Soc., 2005, 127, 12500–12501 CrossRef CAS PubMed.
- K. R. McClain, H. Kwon, K. Chakarawet, R. Nabi, J. G. Kragskow, N. F. Chilton, R. D. Britt, J. R. Long and B. G. Harvey, J. Am. Chem. Soc., 2023, 145, 8996–9002 CrossRef CAS PubMed.
- H. Funasaka, K. Sakurai, Y. Oda, K. Yamamoto and T. Takahashi, Chem. Phys. Lett., 1995, 232, 273–277 CrossRef CAS.
- H. Funasaka, K. Sugiyama, K. Yamamoto and T. Takahashi, J. Phys. Chem., 1995, 99, 1826–1830 CrossRef CAS.
- K. Furukawa, S. Okubo, H. Kato, H. Shinohara and T. Kato, J. Phys. Chem. A, 2003, 107, 10933–10937 CrossRef CAS.
- H. Huang, S. Yang and X. Zhang, J. Phys. Chem. B, 1999, 103, 5928–5932 CrossRef CAS.
- H. Huang, S. Yang and X. Zhang, J. Phys. Chem. B, 2000, 104, 1473–1482 CrossRef CAS.
- L. Bao, C. Pan, Z. Slanina, F. Uhlik, T. Akasaka and X. Lu, Angew. Chem., Int. Ed., 2016, 55, 9234–9238 CrossRef CAS PubMed.
- F. Donati, S. Rusponi, S. Stepanow, C. Wäckerlin, A. Singha, L. Persichetti, R. Baltic, K. Diller, F. Patthey, E. Fernandes, J. Dreiser, Ž. Šljivančanin, K. Kummer, C. Nistor, P. Gambardella and H. Brune, Science, 2016, 352, 318–321 CrossRef CAS PubMed.
- F. Donati, S. Rusponi, S. Stepanow, L. Persichetti, A. Singha, D. M. Juraschek, C. Wäckerlin, R. Baltic, M. Pivetta, K. Diller, C. Nistor, J. Dreiser, K. Kummer, E. Velez-Fort, N. A. Spaldin, H. Brune and P. Gambardella, Phys. Rev. Lett., 2020, 124, 077204 CrossRef CAS PubMed.
- R. Baltic, M. Pivetta, F. Donati, C. Wäckerlin, A. Singha, J. Dreiser, S. Rusponi and H. Brune, Nano Lett., 2016, 16, 7610–7615 CrossRef CAS PubMed.
- H. Matsuoka, N. Ozawa, T. Kodama, H. Nishikawa, I. Ikemoto, K. Kikuchi, K. Furukawa, K. Sato, D. Shiomi, T. Takui and T. Kato, J. Phys. Chem. B, 2004, 108, 13972–13976 CrossRef CAS.
- W. Xu, B. Niu, L. Feng, Z. Shi and Y. Lian, Chem. – Eur. J., 2012, 18, 14246–14249 CrossRef CAS PubMed.
- W. Xu, B. Niu, Z. Shi, Y. Lian and L. Feng, Nanoscale, 2012, 4, 6876–6879 RSC.
- Z. Wang, Y. Nakanishi, S. Noda, H. Niwa, J. Zhang, R. Kitaura and H. Shinohara, Angew. Chem., Int. Ed., 2013, 52, 11770–11774 CrossRef CAS PubMed.
- Z. Wang, S. Aoyagi, H. Omachi, R. Kitaura and H. Shinohara, Angew. Chem., Int. Ed., 2016, 55, 199–202 CrossRef CAS PubMed.
- H. Nikawa, T. Kikuchi, T. Wakahara, T. Nakahodo, T. Tsuchiya, G. A. Rahman, T. Akasaka, Y. Maeda, K. Yoza and E. Horn, J. Am. Chem. Soc., 2005, 127, 9684–9685 CrossRef CAS PubMed.
- D. Xu, Y. Jiang, Y. Wang, T. Zhou, Z. Shi, H. Omachi, H. Shinohara, B. Sun and Z. Wang, Inorg. Chem., 2019, 58, 14325–14330 CrossRef CAS PubMed.
- H. Nikawa, T. Yamada, B. Cao, N. Mizorogi, Z. Slanina, T. Tsuchiya, T. Akasaka, K. Yoza and S. Nagase, J. Am. Chem. Soc., 2009, 131, 10950–10954 CrossRef CAS PubMed.
- X. Han, J. Xin, Y. Yao, Z. Liang, Y. Qiu, M. Chen and S. Yang, Nanomaterials, 2022, 12, 3291 CrossRef CAS PubMed.
- Y. Takano, A. Yomogida, H. Nikawa, M. Yamada, T. Wakahara, T. Tsuchiya, M. O. Ishitsuka, Y. Maeda, T. Akasaka and T. Kato, J. Am. Chem. Soc., 2008, 130, 16224–16230 CrossRef CAS PubMed.
- L. Feng, T. Nakahodo, T. Wakahara, T. Tsuchiya, Y. Maeda, T. Akasaka, T. Kato, E. Horn, K. Yoza and N. Mizorogi, J. Am. Chem. Soc., 2005, 127, 17136–17137 CrossRef CAS PubMed.
- H. Huang, L. Zhang, X. J. Gao, X. Guo, R. Cui, B. Xu, J. Dong, Y. Li, L. Gan and F. Chang, Chem. Mater., 2018, 30, 64–68 CrossRef CAS.
- J. C. Hummelen, B. Knight, J. Pavlovich, R. González and F. Wudl, Science, 1995, 269, 1554–1556 CrossRef CAS PubMed.
- T. Akasaka, S. Okubo, T. Wakahara, K. Yamamoto, K. Kobayashi, S. Nagase, T. Kato, M. Kako, Y. Nakadaira and Y. Kitayama, Chem. Lett., 1999, 945–946 CrossRef CAS.
- M. Briganti, E. Lucaccini, L. Chelazzi, S. Ciattini, L. Sorace, R. Sessoli, F. Totti and M. Perfetti, J. Am. Chem. Soc., 2021, 143, 8108–8115 CrossRef CAS PubMed.
- C. A. Gould, K. R. McClain, J. M. Yu, T. J. Groshens, F. Furche, B. G. Harvey and J. R. Long, J. Am. Chem. Soc., 2019, 141, 12967–12973 CrossRef CAS PubMed.
- J. C. Vanjak, B. O. Wilkins, V. Vieru, N. S. Bhuvanesh, J. H. Reibenspies, C. D. Martin, L. F. Chibotaru and M. Nippe, J. Am. Chem. Soc., 2022, 144, 17743–17747 CrossRef CAS PubMed.
- C. A. P. Goodwin, F. Ortu, D. Reta, N. F. Chilton and D. P. Mills, Nature, 2017, 548, 439–442 CrossRef CAS PubMed.
- E. Moreno-Pineda, M. Damjanović, O. Fuhr, W. Wernsdorfer and M. Ruben, Angew. Chem., Int. Ed., 2017, 56, 9915–9919 CrossRef CAS PubMed.
- S.-G. Wu, Z.-Y. Ruan, G.-Z. Huang, J.-Y. Zheng, V. Vieru, G. Taran, J. Wang, Y.-C. Chen, J.-L. Liu and L. F. Chibotaru, Chem, 2021, 7, 982–992 CAS.
- J. D. Rinehart, M. Fang, W. J. Evans and J. R. Long, Nat. Chem., 2011, 3, 538–542 CrossRef CAS PubMed.
- S. Demir, M. I. Gonzalez, L. E. Darago, W. J. Evans and J. R. Long, Nat. Commun., 2017, 8, 2144 CrossRef PubMed.
- J. D. Rinehart, M. Fang, W. J. Evans and J. R. Long, J. Am. Chem. Soc., 2011, 133, 14236–14239 CrossRef CAS PubMed.
- L. E. Darago, M. D. Boshart, B. D. Nguyen, E. Perlt, J. W. Ziller, W. W. Lukens, F. Furche, W. J. Evans and J. R. Long, J. Am. Chem. Soc., 2021, 143, 8465–8475 CrossRef CAS PubMed.
- C. A. Gould, E. Mu, V. Vieru, L. E. Darago, K. Chakarawet, M. I. Gonzalez, S. Demir and J. R. Long, J. Am. Chem. Soc., 2020, 142, 21197–21209 CrossRef CAS PubMed.
- A. A. Popov, S. M. Avdoshenko, A. M. Pendas and L. Dunsch, Chem. Commun., 2012, 48, 8031–8050 RSC.
- W. Shen, L. Bao, Y. Wu, C. Pan, S. Zhao, H. Fang, Y. Xie, P. Jin, P. Peng, F.-F. Li and X. Lu, J. Am. Chem. Soc., 2017, 139, 9979–9984 CrossRef CAS PubMed.
- S. Hu, W. Shen, L. Yang, G. Duan, P. Jin, Y. Xie, T. Akasaka and X. Lu, Chem. – Eur. J., 2019, 25, 11538–11544 CrossRef CAS PubMed.
- Z. Wang, R. Kitaura and H. Shinohara, J. Phys. Chem. C, 2014, 118, 13953–13958 CrossRef CAS.
- Y. Jiang, Z. Li, J. Zhang and Z. Wang, J. Phys. Chem. C, 2019, 124, 2131–2136 CrossRef.
- W. J. Evans, M. Fang, G. Zucchi, F. Furche, J. W. Ziller, R. M. Hoekstra and J. I. Zink, J. Am. Chem. Soc., 2009, 131, 11195–11202 CrossRef CAS PubMed.
- A. Mansikkamäki, A. A. Popov, Q. Deng, N. Iwahara and L. F. Chibotaru, J. Chem. Phys., 2017, 147, 124305 CrossRef PubMed.
- T. Tsuchiya, M. Wielopolski, N. Sakuma, N. Mizorogi, T. Akasaka, T. Kato, D. M. Guldi and S. Nagase, J. Am. Chem. Soc., 2011, 133, 13280–13283 CrossRef CAS PubMed.
- L. Feng, S. G. Radhakrishnan, N. Mizorogi, Z. Slanina, H. Nikawa, T. Tsuchiya, T. Akasaka, S. Nagase, N. Martin and D. M. Guldi, J. Am. Chem. Soc., 2011, 133, 7608–7618 CrossRef CAS PubMed.
- M. Yamada, H. Kurihara, M. Suzuki, M. Saito, Z. Slanina, F. Uhlik, T. Aizawa, T. Kato, M. M. Olmstead, A. L. Balch, Y. Maeda, S. Nagase, X. Lu and T. Akasaka, J. Am. Chem. Soc., 2015, 137, 232–238 CrossRef CAS PubMed.
- L. Bao, M. Chen, C. Pan, T. Yamaguchi, T. Kato, M. M. Olmstead, A. L. Balch, T. Akasaka and X. Lu, Angew. Chem., Int. Ed., 2016, 55, 4242–4246 CrossRef CAS PubMed.
- M. K. Singh, N. Yadav and G. Rajaraman, Chem. Commun., 2015, 51, 17732–17735 RSC.
- R. B. Zaripov, Y. E. Kandrashkin, K. M. Salikhov, B. Büchner, F. Liu, M. Rosenkranz, A. A. Popov and V. Kataev, Nanoscale, 2020, 12, 20513–20521 RSC.
- Y. E. Kandrashkin, R. B. Zaripov, F. Liu, B. Büchner, V. Kataev and A. A. Popov, Phys. Chem. Chem. Phys., 2021, 23, 18206–18220 RSC.
- W. Yang, G. Velkos, M. Rosenkranz, S. Schiemenz, F. Liu and A. A. Popov, Adv. Sci., 2023, 2305190 Search PubMed.
- G. Velkos, D. S. Krylov, K. Kirkpatrick, X. Liu, L. Spree, A. U. B. Wolter, B. Buchner, H. C. Dorn and A. A. Popov, Chem. Commun., 2018, 54, 2902–2905 RSC.
- M. J. Martinez-Perez, S. Cardona-Serra, C. Schlegel, F. Moro, P. J. Alonso, H. Prima-Garcia, J. M. Clemente-Juan, M. Evangelisti, A. Gaita-Arino, J. Sese, J. van Slageren, E. Coronado and F. Luis, Phys. Rev. Lett., 2012, 108, 247213 CrossRef CAS PubMed.
- M. D. Jenkins, Y. Duan, B. Diosdado, J. J. García-Ripoll, A. Gaita-Ariño, C. Giménez-Saiz, P. J. Alonso, E. Coronado and F. Luis, Phys. Rev. B, 2017, 95, 064423 CrossRef.
- J. Lopez-Cabrelles, L. Escalera-Moreno, Z. Hu, H. Prima-Garcia, G. M. Espallargas, A. Gaita-Arino and E. Coronado, Inorg. Chem., 2021, 60, 8575–8580 CrossRef CAS PubMed.
- F. Luis, P. J. Alonso, O. Roubeau, V. Velasco, D. Zueco, D. Aguilà, J. I. Martínez, L. A. Barrios and G. Aromí, Commun. Chem., 2020, 3, 176 CrossRef CAS PubMed.
- M. N. Leuenberger and D. Loss, Nature, 2001, 410, 789 CrossRef CAS PubMed.
- C. Godfrin, A. Ferhat, R. Ballou, S. Klyatskaya, M. Ruben, W. Wernsdorfer and F. Balestro, Phys. Rev. Lett., 2017, 119, 187702 CrossRef CAS PubMed.
- W. Xiang, Z. Hu, J. Xin, H. Jin, Z. Jiang, X. Han, M. Chen, Y.-R. Yao and S. Yang, J. Am. Chem. Soc., 2023, 145, 22599–22608 CrossRef CAS PubMed.
- M. Nie, L. Yang, C. Zhao, H. Meng, L. Feng, P. Jin, C. Wang and T. Wang, Nanoscale, 2019, 11, 18612–18618 RSC.
- A. Moreno-Vicente, Y. Roselló, N. Chen, L. Echegoyen, P. W. Dunk, A. Rodríguez-Fortea, C. de Graaf and J. M. Poblet, J. Am. Chem. Soc., 2023, 145, 6710–6718 CrossRef PubMed.
- X. Zhang, Y. Wang, R. Morales-Martinez, J. Zhong, C. de Graaf, A. Rodriguez-Fortea, J. M. Poblet, L. Echegoyen, L. Feng and N. Chen, J. Am. Chem. Soc., 2018, 140, 3907–3915 CrossRef CAS PubMed.
- S. Dey and G. Rajaraman, Chem. Sci., 2021, 12, 14207–14216 RSC.
- K. Suenaga, M. Tence, C. Mory, C. Colliex, H. Kato, T. Okazaki, H. Shinohara, K. Hirahara, S. Bandow and S. Iijima, Science, 2000, 290, 2280–2282 CrossRef CAS PubMed.
- K. Hirahara, K. Suenaga, S. Bandow, H. Kato, T. Okazaki, H. Shinohara and S. Iijima, Phys. Rev. Lett., 2000, 85, 5384 CrossRef CAS PubMed.
- L. Guan, K. Suenaga, S. Okubo, T. Okazaki and S. Iijima, J. Am. Chem. Soc., 2008, 130, 2162–2163 CrossRef CAS PubMed.
- M. Urdampilleta, S. Klyatskaya, J.-P. Cleuziou, M. Ruben and W. Wernsdorfer, Nat. Mater., 2011, 10, 502–506 CrossRef CAS PubMed.
- K. Katoh, S. Yamashita, N. Yasuda, Y. Kitagawa, B. K. Breedlove, Y. Nakazawa and M. Yamashita, Angew. Chem., Int. Ed., 2018, 57, 9262–9267 CrossRef CAS PubMed.
- C. Gimenez-Lopez Mdel, F. Moro, A. La Torre, C. J. Gomez-Garcia, P. D. Brown, J. van Slageren and A. N. Khlobystov, Nat. Commun., 2011, 2, 407 CrossRef PubMed.
- R. Nakanishi, J. Satoh, K. Katoh, H. Zhang, B. K. Breedlove, M. Nishijima, Y. Nakanishi, H. Omachi, H. Shinohara and M. Yamashita, J. Am. Chem. Soc., 2018, 140, 10955–10959 CrossRef CAS PubMed.
- S. M. Avdoshenko, F. Fritz, C. Schlesier, A. Kostanyan, J. Dreiser, M. Luysberg, A. A. Popov, C. Meyer and R. Westerström, Nanoscale, 2018, 10, 18153–18160 RSC.
- N. Domingo, E. Bellido and D. Ruiz-Molina, Chem. Soc. Rev., 2012, 41, 258–302 RSC.
- A. Cornia, M. Mannini, P. Sainctavit and R. Sessoli, Chem. Soc. Rev., 2011, 40, 3076–3091 RSC.
- R. J. Holmberg and M. Murugesu, J. Mater. Chem. C, 2015, 3, 11986–11998 RSC.
- M. Clemente-León, H. Soyer, E. Coronado, C. Mingotaud, C. J. Gómez-García and P. Delhaès, Angew. Chem., Int. Ed., 1998, 37, 2842–2845 CrossRef.
- M. Mannini, F. Pineider, P. Sainctavit, C. Danieli, E. Otero, C. Sciancalepore, A. M. Talarico, M.-A. Arrio, A. Cornia and D. Gatteschi, Nat. Mater., 2009, 8, 194–197 CrossRef CAS PubMed.
- G. Serrano, L. Poggini, M. Briganti, A. L. Sorrentino, G. Cucinotta, L. Malavolti, B. Cortigiani, E. Otero, P. Sainctavit and S. Loth, Nat. Mater., 2020, 19, 546–551 CrossRef CAS PubMed.
- M. Mannini, F. Pineider, C. Danieli, F. Totti, L. Sorace, P. Sainctavit, M.-A. Arrio, E. Otero, L. Joly and J. C. Cezar, Nature, 2010, 468, 417–421 CrossRef CAS PubMed.
- L. Margheriti, D. Chiappe, M. Mannini, P. E. Car, P. Sainctavit, M. A. Arrio, F. B. de Mongeot, J. C. Cezar, F. M. Piras and A. Magnani, Adv. Mater., 2010, 22, 5488–5493 CrossRef CAS PubMed.
- C. Wäckerlin, F. Donati, A. Singha, R. Baltic, S. Rusponi, K. Diller, F. Patthey, M. Pivetta, Y. Lan and S. Klyatskaya, Adv. Mater., 2016, 28, 5195–5199 CrossRef PubMed.
- C. F. Hermanns, M. Bernien, A. Krüger, C. Schmidt, S. T. Waßerroth, G. Ahmadi, B. W. Heinrich, M. Schneider, P. W. Brouwer and K. J. Franke, Phys. Rev. Lett., 2013, 111, 167203 CrossRef PubMed.
- D. S. Krylov, S. Schimmel, V. Dubrovin, F. Liu, T. T. N. Nguyen, L. Spree, C. H. Chen, G. Velkos, C. Bulbucan, R. Westerstrom, M. Studniarek, J. Dreiser, C. Hess, B. Buchner, S. M. Avdoshenko and A. A. Popov, Angew. Chem., Int. Ed., 2020, 59, 5756–5764 CrossRef CAS PubMed.
- C. H. Chen, D. S. Krylov, S. M. Avdoshenko, F. Liu, L. Spree, R. Westerstrom, C. Bulbucan, M. Studniarek, J. Dreiser, A. U. B. Wolter, B. Buchner and A. A. Popov, Nanoscale, 2018, 10, 11287–11292 RSC.
- R. Westerstrom, A. C. Uldry, R. Stania, J. Dreiser, C. Piamonteze, M. Muntwiler, F. Matsui, S. Rusponi, H. Brune, S. Yang, A. Popov, B. Buchner, B. Delley and T. Greber, Phys. Rev. Lett., 2015, 114, 087201 CrossRef PubMed.
- T. Greber, A. P. Seitsonen, A. Hemmi, J. Dreiser, R. Stania, F. Matsui, M. Muntwiler, A. Popov and R. Westerström, Phys. Rev. Mater., 2019, 3, 014409 CrossRef CAS.
- R. Sagehashi, W. C. Lee, F. Liu, A. A. Popov, M. Muntwiler, B. Delley, P. Krüger and T. Greber, Phys. Rev. Mater., 2023, 7, 086001 CrossRef CAS.
- C. H. Chen, L. Spree, E. Koutsouflakis, D. S. Krylov, F. Liu, A. Brandenburg, G. Velkos, S. Schimmel, S. M. Avdoshenko and A. Fedorov, Adv. Sci., 2021, 8, 2000777 CrossRef CAS PubMed.
- F. Paschke, T. Birk, S. M. Avdoshenko, F. Liu, A. A. Popov and M. Fonin, Small, 2022, 18, 2105667 CrossRef CAS PubMed.
- G. M. Espallargas and E. Coronado, Chem. Soc. Rev., 2018, 47, 533–557 RSC.
- D. Aulakh, J. B. Pyser, X. Zhang, A. A. Yakovenko, K. R. Dunbar and M. Wriedt, J. Am. Chem. Soc., 2015, 137, 9254–9257 CrossRef CAS PubMed.
- Y. Feng, T. Wang, Y. Li, J. Li, J. Wu, B. Wu, L. Jiang and C. Wang, J. Am. Chem. Soc., 2015, 137, 15055–15060 CrossRef CAS PubMed.
- H. Meng, C. Zhao, M. Nie, C. Wang and T. Wang, J. Phys. Chem. C, 2019, 123, 6265–6269 CrossRef CAS.
- J. Zhang, L. Liu, C. Zheng, W. Li, C. Wang and T. Wang, Nat. Commun., 2023, 14, 4922 CrossRef CAS PubMed.
- Y. Li, T. Wang, H. Meng, C. Zhao, M. Nie, L. Jiang and C. Wang, Dalton Trans., 2016, 45, 19226–19229 RSC.
- H. Meng, C. Zhao, M. Nie, C. Wang and T. Wang, ACS Appl. Mater. Interfaces, 2018, 10, 32607–32612 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |