Two nickel-added poly(polyoxometalate)s built of Keggin-type {Ni6PW9} and Anderson-type NiW6O24via WO4/Sb2O bridges and Ni–O–W linkages with efficient hydrogen evolution activity†
Peng-Yun
Zhang
 ,
Chen
Lian
,
Zhen-Wen
Wang
,
Juan
Chen
,
Hongjin
Lv
,
Chen
Lian
,
Zhen-Wen
Wang
,
Juan
Chen
,
Hongjin
Lv
 * and
Guo-Yu
Yang
* and
Guo-Yu
Yang
 *
*
MOE Key Laboratory of Cluster Science, School of Chemistry and Chemical Engineering, Beijing Institute of Technology, Beijing 102488, China. E-mail: ygy@bit.edu.cn; hlv@bit.edu.cn
First published on 29th July 2024
Abstract
Two Ni-added poly(polyoxometalate)s built of Keggin-type {Ni6PW9} and Anderson-type NiW6O24 units via WO4/Sb2O bridges and Ni–O–W linkages, Na4H8[Ni(enMe)2][(Sb2O)2(NiW6O24)-{Ni12O2(OH)4(enMe)4(H2O)3(WO4)2(B-α-PW9O34)2}2]·39H2O (1) and H9[Ni(en)2(H2O)][Ni0.5(en)2(H2O)][Ni-(enMe)2(H2O)][(Sb2O)2(NiW6O24){Ni12O2(OH)4(en)2(enMe)2(H2O)3(WO4)2}-{Ni12O2(OH)4(en)4(H2O)3(WO4)2}(B-α-PW9O34)4]·45H2O (2), have been hydrothermally synthesized and characterized, in which the {Ni12(WO4)2(PW9)2} subunit was obtained by the synergistic directing effect of 2 lacunary PW9O34 (PW9) fragments and further linked by a central Anderson-type (Sb2O)2(NiW6O24) bridge. Both compounds represent the first example of Ni-added polyoxometalates (POMs) simultaneously based on Keggin-type and Anderson-type POM components. Photocatalytic studies revealed that 2 can work as an efficient heterogeneous catalyst towards a light-driven H2 evolution reaction, achieving a hydrogen evolution rate of as high as 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 214 μmol g−1 h−1 (TON = 1500), which is superior to most of the reported POM-based heterogeneous catalysts.
214 μmol g−1 h−1 (TON = 1500), which is superior to most of the reported POM-based heterogeneous catalysts.
Introduction
To alleviate the energy crisis and environmental pollution, green and high-energy density hydrogen (H2) will be an important energy source for future development.1 Solar-driven water splitting has been considered a promising way to produce H2. In particular, efforts should be concentrated on the pursuit of efficient, environmentally friendly, and cost-effective catalysts capable of splitting water into H2. In addition to a number of nanomaterial-based photocatalysts,2,3 an emerging type of POM-based hydrogen-evolving catalyst has been attracting tremendous attention in recent years.4,5 POMs are typical anionic metal–oxygen clusters built of MOx (M = Mo, W, V, Nb, Ta, etc.) polyhedra with diverse architectures and promising applications in catalysis, biomedicine, materials science, etc.6–15 Lacunary POMs can be used as structural directing agents to induce the aggregation of transition metal (TM) ions to form TM-added POMs (TMAPs).16–18 The introduction of TM ions imparts the TMAPs extensive tunability in structural diversity, electronic structure, and multi-electron/proton-transfer redox properties, thus benefiting photocatalytic water reduction.19–23 Therefore, the fabrication of gigantic high-nuclearity TMAPs, such as Ni25-,24 Cu20-,25 Fe48-,26 Co21-,27 and Zr24-28 added POMs, has been continuously explored as an attractive research hotspot for several decades. Our research group has been focusing on making novel high-nuclearity Ni-added POMs (NiAPs) by the reaction of lacunary POM fragments with Ni2+ ions under hydrothermal conditions and achieving a series of NiAPs built by using {Ni6PW9} as structural building units (SBUs).29,30 One representative example is the 20-Ni-added POM,31 in which the terminal and bridging O atoms of {Ni4PW6} and W4O16 groups substitute the water molecules and hydroxyl groups of {Ni6PW9} units to build a large NiAP. Hence, the utilization of hexanuclear Ni-oxo cluster-based {Ni6PW9} units as SBUs may represent a promising synthetic strategy for efficiently making high-nuclearity NiAPs.Along with the interest in exploring high-nuclearity TM-oxo clusters, intense attention has also been focused on the combination of diverse types of POMs with different geometries and compositions to construct TMAP clusters.32 The design and synthesis of mixed-type POM SBUs in one molecular structure not only enriches the structural diversity of POM chemistry, but also results in distinctive physicochemical properties.33–35 Although the past several decades witnessed the prosperity and development of TMAPs with novel configurations, most of them are assembled by only one type of POM fragment.36–40 TMAPs containing mixed-type POM fragments with diverse electronic configurations and molecular sizes have rarely been reported, and most of the TMAPs have been made from lacunary Keggin and Dawson-type POM units, such as [Mn6(PW6O26)(α-P2W15O56)2(H2O)2]23−,41 [Zr3(μ2-OH)2(μ2-O)(A-α-GeW9O34)(1,4,9-α-P2W15O56)]14−,42etc. In contrast, the coexistence of Keggin and Anderson-type POMs in one TMAP framework is extremely uncommon. Additionally, the so-called Anderson-type fragment of one representative compound [As6Fe7Mo22O98]25− is different from the classic Anderson-type XM6O24 unit.43 Therefore, it is of high significance but a challenging goal to make structurally-new TMAPs with mixed Keggin- and Anderson-type POM fragments.
Herein, we report two Ni-added POMs, Na4H8[Ni(enMe)2]-[(Sb2O)2(NiW6O24){Ni12O2(OH)4(enMe)4(H2O)3(WO4)2(B-α-PW9-O34)2}2]·39H2O (1) and H9[Ni(en)2(H2O)][Ni0.5(en)2(H2O)][Ni-(enMe)2(H2O)][(Sb2O)2(NiW6O24){Ni12O2(OH)4(en)2(enMe)2(H2O)3(WO4)2}{Ni12O2(OH)4(en)4(H2O)3(WO4)2}(B-α-PW9O34)4]·45H2O (2). Both clusters can be simplified as four Keggin-type {Ni6PW9} units linked by one central Anderson-type NiW6O24 subunit and further stabilized by WO4/Sb2O bridges and Ni–O–W linkages. 1 is a one-dimensional (1D) chain linked by [Ni-(enMe)2]2+ complexes, while 2 is a discrete cluster. In addition, 1 and 2 can work as multi-electron-transfer catalysts to drive photocatalytic H2 evolution reactions. 2 exhibited high efficiency in heterogeneous photocatalytic H2 production with the highest H2 evolution rate of up to 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 214 μmol g−1 h−1 (TON = 1500).
214 μmol g−1 h−1 (TON = 1500).
Results and discussion
Structural description
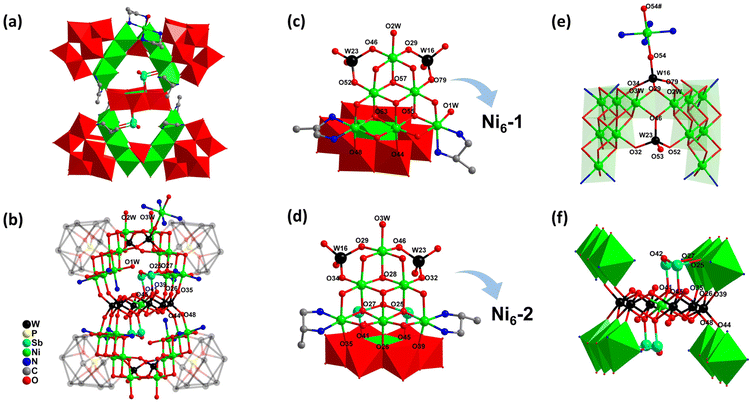
Single crystal X-ray diffraction shows that 1 (Fig. 1a and b, S1†) crystallizes in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (Table 1) and consists of a [(Sb2O)2(NiW6O24){Ni12O2(OH)4(enMe)4(H2O)3(WO4)2(PW9O34)2}2]14− polyoxoanion (1a), one bridging complex [Ni(enMe)2]2+, 4 Na+, 8 protons, and 39 lattice water molecules. The bond lengths of Ni–O and Ni–N range from 1.99 to 2.26 Å, and the valence states of Ni atoms are all +2 according to the bond valence sum (BVS) calculations (Table S1†). The hourglass-like polyoxoanion 1a comprises two different {Ni6} clusters, namely [Ni6O6(OH)3(enMe)2(H2O)2]3− (Ni6-1, Fig. S2a†) and [Ni6O9(OH)(enMe)2-(H2O)]7− (Ni6-2, Fig. S2b†), added Keggin subunits, one central Anderson [NiW6O24]10− unit, and two kinds of linkers WO4 and Sb2O. The Ni6-1 subunit not only connects with 2 WO4 units, but also has 2 terminal water molecules which are further substituted by 2 terminal O atoms (O44, O48) of the [NiW6O24]10− unit (Fig. 1c). In contrast, the Ni6-2 subunit not only links with 2 WO4 units, but also has 3 terminal water molecules which are substituted by 2 terminal O(35, 39) atoms and 1 bridging O26 atom of the [NiW6O24]10− unit (Fig. 1d). Additionally, 2 μ3-O(27, 25) atoms of Ni6-2 form a linkage with 2 μ3-O(41, 45) atoms of the [NiW6O24]10− unit through a Sb2O moiety (Fig. 1d). The BVS results of O55, O63, and O57 in Ni6-1 are 1.08, 1.11, and 1.12 (Table S1†), respectively, indicating the monoprotonated nature of these μ3-O atoms. However, the BVS results of 3 homologous O(25, 27, 28) atoms in Ni6-2 are 2.01, 2.02, and 1.17 (Table S1†), respectively, illustrating that only O28 is monoprotonated. Different from μ3-O28, the μ4-O25 and μ4-O27 atoms in Ni6-2 not only act as bridges for 3 Ni2+ ions but also connect with an Sb3+ ion.
(Table 1) and consists of a [(Sb2O)2(NiW6O24){Ni12O2(OH)4(enMe)4(H2O)3(WO4)2(PW9O34)2}2]14− polyoxoanion (1a), one bridging complex [Ni(enMe)2]2+, 4 Na+, 8 protons, and 39 lattice water molecules. The bond lengths of Ni–O and Ni–N range from 1.99 to 2.26 Å, and the valence states of Ni atoms are all +2 according to the bond valence sum (BVS) calculations (Table S1†). The hourglass-like polyoxoanion 1a comprises two different {Ni6} clusters, namely [Ni6O6(OH)3(enMe)2(H2O)2]3− (Ni6-1, Fig. S2a†) and [Ni6O9(OH)(enMe)2-(H2O)]7− (Ni6-2, Fig. S2b†), added Keggin subunits, one central Anderson [NiW6O24]10− unit, and two kinds of linkers WO4 and Sb2O. The Ni6-1 subunit not only connects with 2 WO4 units, but also has 2 terminal water molecules which are further substituted by 2 terminal O atoms (O44, O48) of the [NiW6O24]10− unit (Fig. 1c). In contrast, the Ni6-2 subunit not only links with 2 WO4 units, but also has 3 terminal water molecules which are substituted by 2 terminal O(35, 39) atoms and 1 bridging O26 atom of the [NiW6O24]10− unit (Fig. 1d). Additionally, 2 μ3-O(27, 25) atoms of Ni6-2 form a linkage with 2 μ3-O(41, 45) atoms of the [NiW6O24]10− unit through a Sb2O moiety (Fig. 1d). The BVS results of O55, O63, and O57 in Ni6-1 are 1.08, 1.11, and 1.12 (Table S1†), respectively, indicating the monoprotonated nature of these μ3-O atoms. However, the BVS results of 3 homologous O(25, 27, 28) atoms in Ni6-2 are 2.01, 2.02, and 1.17 (Table S1†), respectively, illustrating that only O28 is monoprotonated. Different from μ3-O28, the μ4-O25 and μ4-O27 atoms in Ni6-2 not only act as bridges for 3 Ni2+ ions but also connect with an Sb3+ ion.
| 1 | 2 | |
|---|---|---|
| a R = ∑||Fo| − |Fc||/∑|Fo|, wR2 = [∑w(Fo2 − Fc2)2/∑w(Fo2)2]1/2. | ||
| CCDC | 2240812 | 2240815 |
| Formula | C30H206N20Na4Ni26-O235P4Sb4W46 | C32H245N28Ni27.5O244-P4Sb4W46 |
| M r | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 294.54 294.54 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 610.05 610.05 |
| Crystal system | Triclinic | Monoclinic |
| Space group |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
Cc |
| a/Å | 14.1447(7) | 36.8164(10) |
| b/Å | 19.4035(9) | 29.8064(8) |
| c/Å | 22.8651(10) | 25.4292(7) |
| α/° | 90.1970(10) | 90 |
| β/° | 106.1290(10) | 105.4530(10) |
| γ/° | 98.5440(10) | 90 |
| V/Å3 | 5955.0(5) | 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 896.3(13) 896.3(13) |
| Z | 1 | 4 |
| D c/g cm−3 | 4.265 | 3.855 |
| μ/mm−1 | 24.711 | 21.987 |
| F(000) | 6846.0 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 092.0 092.0 |
| GOF on F2 | 1.034 | 1.037 |
| Final R indexes [I ≥ 2σ(I)]a | R 1 = 0.0456, wR2 = 0.0951 | R 1 = 0.0463, wR2 = 0.0978 |
| Final R indexes [all data] | R 1 = 0.0624, wR2 = 0.1009 | R 1 = 0.0638, wR2 = 0.1024 |
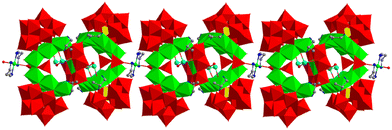
The Ni6-1 and Ni6-2 subunits are integrated to form a [Ni12O7(OH)4(enMe)4(H2O)3(WO4)2]2+ cluster (1b) through 2 Ni2+ ions located at the vertices of the {Ni6} clusters in an edge-sharing manner (O29 and O46 are shared), which were further stabilized by 2 WO4 groups (Fig. 1e). The connection mode of 1b is different from the previously reported Ni12-based POMs formed by 2 {Ni6SiW9} units via Ni–O–W bonds.44 Each WO4 group substitutes 2 terminal coordination water molecules (O34 and O79 for W16; O32 and O52 for W23) of 2 Ni2+ ions in 2 {Ni6} clusters, respectively, and further connects with the edge-sharing Ni2+ ions (Fig. 1e). Different from the W23 center, the W16 center of the WO4 group is further supported by a bridging complex [Ni(enMe)2]2+via the O54 atom, which links adjacent polyoxoanions to form a AAA 1D chain (Fig. 2). The formation of the 1D chain through Ni-amine complexes that are supported on the WO4 ligand is rarely reported. In most instances, the complexes are supported on the WO6 of XW9 (X = P, Si, Ge) units.45 Significantly, the 57° angle between Ni6-1 and Ni6-2 units results in ca. 8.1 Å between the Ni2+ ions on both sides of the opening position (Fig. S3a†), which matches approximately with the diameter of the Anderson-type cluster (ca. 7.6 Å, Fig. S3b†), providing a possibility of confined growth.
The central Ni-containing Anderson-type unit consists of 6 WO6 octahedra linked in edge-sharing mode, which could be derived from the transformation of [PW9O34]9− units. To date, the Anderson-type precursors have been commonly functionalized by triol organic ligands or tri-dentate inorganic groups.46–49 However, in this polyoxoanion, the terminal and bridging oxygen atoms of the Anderson [NiW6O24]10− unit substitute 2 water molecules of Ni6-1 units and 3 water molecules of Ni6-2 units on two sides through 10 Ni–O–W bonds (Fig. 1f). Moreover, as previously mentioned, 2 μ3-O(41,45) atoms of the [NiW6O24]10− unit connect with 2 μ3-O(27,25) atoms of Ni6-2 through a Sb2O moiety on both sides (Fig. 1f). In POM chemistry, Sb atoms are usually present as heteroatoms in precursor species.50,51 However, they function as bridging entities within the framework of 1 in this work.
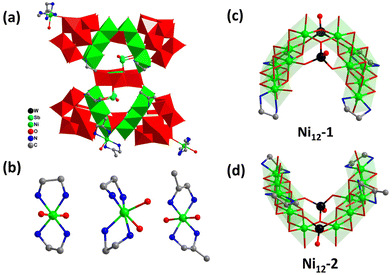
By following the synthetic strategy of 1, the introduction of mixed organic amines (en and enMe) led to the formation of an isolated cluster H9[Ni(en)2(H2O)][Ni0.5(en)2(H2O)][Ni(enMe)2(H2O)][(Sb2O)2(NiW6O24) {Ni12O2(OH)4(en)2(enMe)2(H2O)3(WO4)2}{Ni12O2(OH)4(en)4(H2O)3(WO4)2}(B-α-PW9O34)4]·45H2O (2, Fig. 3a and S4†). 2 crystallized in the monoclinic space group Cc (Table 1) and can be viewed as the assembly of a polyoxoanion cluster [(Sb2O)2(NiW6O24){Ni12O2(OH)4(en)2(enMe)2(H2O)3(WO4)2}-{Ni12O2(OH)4(en)4(H2O)3(WO4)2}(B-α-PW9O34)4]14− (2a) similar to 1a except for the different coordination amine molecules to Ni2+ ions, three counter cations of [Ni(en)2(H2O)]2+, [Ni0.5(en)2(H2O)]+, and [Ni-(enMe)2(H2O)]2+ complexes (Fig. 3b), 9 protons, and 45 lattice water molecules. 2a is built of one [(Sb2O)2NiW6O24]2− unit integrating two different Ni12-clusters, [Ni12O7(OH)4(en)4(H2O)3(WO4)2]2+ (Ni12-1, Fig. 3c) and [Ni12O7(OH)4(en)2(enMe)2(H2O)3(WO4)2]2+ (Ni12-2, Fig. 3d), added POM moieties. The difference between such two Ni12 clusters is caused by the different types and orientations of organic amines. Considering the acentric Cc space group of 2, a second-harmonic-generation (SHG) test of the sieved crystals was conducted by employing a Q-switched Nd:YAG laser (1064 nm). As illustrated in Fig. S5,† sample 2 manifested an SHG response approximately 0.6 times that of KDP, revealing its potential utilization as a nonlinear optical material.52
Photocatalytic properties
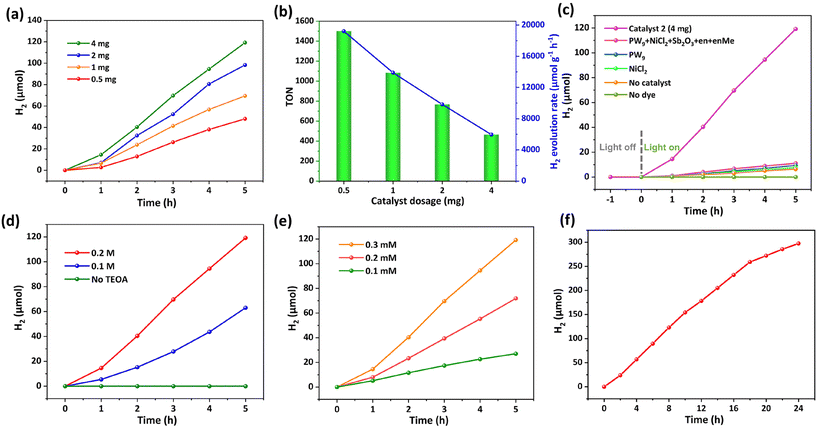
Inspired by the properties of TMAPs synthesized using transition metals as active sites and POM ligands as electron sponges, we expected that 1 and 2 should be potential catalysts for the H2 evolution reaction. To evaluate their visible-light-driven catalytic activity, a well-established three-component catalytic system was employed.5,53 The mixture containing 1 or 2 as catalysts, 0.3 mM [Ir(coumarin)2(dtbbpy)]+ as a photosensitizer, 0.2 M triethanolamine (TEOA) as a sacrificial electron donor, and 2.5 M H2O as a proton source in DMF/CH3CN (6 ml, v/v, 3/1) was degassed with Ar/CH4 (v/v, 4/1) and irradiated under 10 W white light (λ = 400–800 nm) at 25 °C. The evolved H2 was detected by gas chromatography and quantified based on the internal CH4 standard.Under otherwise identical conditions, the amount of H2 generated reached 55 μmol and 69 μmol after 5 hours of irradiation while using 1 mg of 1 and 2 as catalysts, respectively (Fig. S6†). Therefore, 2 was used for the following studies unless otherwise noted. Upon illumination of white LED light, the continuous generation of H2 gas was detected over time. As shown in Fig. 4a, varying the catalyst dosage from 0.5 mg to 4 mg resulted in an increasing H2 evolution from 48 μmol to 119 μmol, respectively. However, the corresponding H2 evolution rate decreased from 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 214 μmol g−1 h−1 to 5959 μmol g−1 h−1 and the calculated turnover number (TON) also decreased from 1500 to 465 (Fig. 4b), which is probably attributed to the strong light scattering effect of the heterogeneous photocatalytic system at the high amount of catalyst, thereby leading to a decreased incident photon absorption. The present H2 evolution rate of 19
214 μmol g−1 h−1 to 5959 μmol g−1 h−1 and the calculated turnover number (TON) also decreased from 1500 to 465 (Fig. 4b), which is probably attributed to the strong light scattering effect of the heterogeneous photocatalytic system at the high amount of catalyst, thereby leading to a decreased incident photon absorption. The present H2 evolution rate of 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 214 μmol g−1 h−1 (TON = 1500) revealed the outstanding catalytic activity of 2 which was superior to that of most heterogeneous POM-based catalysts (Table S2†).
214 μmol g−1 h−1 (TON = 1500) revealed the outstanding catalytic activity of 2 which was superior to that of most heterogeneous POM-based catalysts (Table S2†).
Various control experiments were further conducted to better understand the catalytic system. Running the reaction in the dark environment resulted in no production of H2, indicating the light-driven nature of the catalytic reaction (Fig. 4c). Moreover, the absence of [Ir(coumarin)2(dtbbpy)]+, TEOA, or catalyst 2 in these blank control tests yielded no or negligible H2, respectively (Fig. 4c and d). Such a phenomenon proved that any one of these three components is indispensable for efficient photocatalysis. Further parallel tests by replacing catalyst 2 with the molar equivalents of PW9, NiCl2 or mixed reactants including PW9, NiCl2, Sb2O3, en and enMe also produced a small amount of H2 (Fig. 4c). In addition, the increment of both TEOA concentration from 0 to 0.2 M (Fig. 4d) and [Ir(coumarin)2-(dtbbpy)]+ concentration from 0.1 mM to 0.3 mM (Fig. 4e) positively contributed to the production of H2.
Then, we studied the stability of catalyst 2 under simulated photo-catalytic conditions by immersing the fresh samples into different solutions including acidic HCl aqueous solution (pH = 3), alkaline NaOH aqueous solution (pH = 13), and organic solvents (DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3CN = v/v, 3
CH3CN = v/v, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) for 24 h. The IR spectra and PXRD patterns of the soaked samples are consistent with those of the freshly synthesized samples, demonstrating the chemical stability of catalyst 2 in acids, bases, and organic solvents (Fig. S7†). The long-term stability of catalyst 2 was further evaluated under turnover conditions by performing the catalytic reaction for 24 h in the presence of 1 mg of catalyst 2. The H2 evolution yield can achieve 297 μmol with an H2 production rate of 12
1) for 24 h. The IR spectra and PXRD patterns of the soaked samples are consistent with those of the freshly synthesized samples, demonstrating the chemical stability of catalyst 2 in acids, bases, and organic solvents (Fig. S7†). The long-term stability of catalyst 2 was further evaluated under turnover conditions by performing the catalytic reaction for 24 h in the presence of 1 mg of catalyst 2. The H2 evolution yield can achieve 297 μmol with an H2 production rate of 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 390 μmol g−1 h−1 at a 24 hour time scale, proving the robustness of the light-driven catalytic system (Fig. 4f). The easy recovery and reusability of catalysts are important attributes of heterogeneous catalytic systems. To better evaluate the performance of catalyst 2, three successive recycling tests were carried out using 2 mg of catalyst 2 (Fig. S8†). After completing one catalytic test, the catalyst was isolated by centrifugation, washed with CH3CN, and reused in a fresh reaction solution for subsequent cycles. It is noted that the slight decrease of H2 yield after each cycle could be attributed to the loss of catalyst during isolation treatment. The IR spectra and PXRD patterns before and after catalysis remained largely unchanged, illustrating the stability of catalyst 2 (Fig. S9†).
390 μmol g−1 h−1 at a 24 hour time scale, proving the robustness of the light-driven catalytic system (Fig. 4f). The easy recovery and reusability of catalysts are important attributes of heterogeneous catalytic systems. To better evaluate the performance of catalyst 2, three successive recycling tests were carried out using 2 mg of catalyst 2 (Fig. S8†). After completing one catalytic test, the catalyst was isolated by centrifugation, washed with CH3CN, and reused in a fresh reaction solution for subsequent cycles. It is noted that the slight decrease of H2 yield after each cycle could be attributed to the loss of catalyst during isolation treatment. The IR spectra and PXRD patterns before and after catalysis remained largely unchanged, illustrating the stability of catalyst 2 (Fig. S9†).
Generally, POM-based catalysts can function as multi-electron-storing/transferring mediators in the photocatalytic process, accompanied by the reduction of Mo6+ to Mo5+ or W6+ to W5+ to form heteropoly blue species.54 In this work, since no obvious color change of the catalyst was observed by the naked eye, we speculated that the stored electrons in catalyst 2 can be quickly utilized for H2 generation. To deeply elucidate this process, X-ray photoelectron spectroscopy (XPS) measurements were further performed to reveal the chemical environment and oxidation changes of catalyst 2 before and after photocatalysis. The full survey XPS spectra show well-matched elemental signals of catalyst 2 before and after catalysis, implying good catalyst stability (Fig. S10†). The Ni 2p3/2 and Ni 2p1/2 signals of the fresh catalyst 2 before catalysis were observed at 855.9 eV and 873.6 eV (Fig. 5a), respectively, indicating a +2 state of Ni ions. The oxidation state of W atoms was assigned as +6 as revealed by binding energies of W 4f7/2 and W 4f5/2 at 35.3 eV and 37.5 eV (Fig. 5b). In contrast, slight negative shifts of 0.1 eV and 0.2 eV were observed for Ni 2p and W 4f signals after photocatalysis (Fig. 5c and d), respectively, which could be caused by partial reduction of catalyst 2 during catalysis.55 It is worth mentioning that the reduction of W is not as obvious as that observed in a reported polyoxomolybdate-based catalytic system,49 where the reduction of Mo6+ to Mo5+ was detected in the XPS spectra after catalysis. Such discrepancy should be ascribed to the oxidation potential difference between Mo6+/Mo5+ and W6+/W5+,56 and Mo5+ can stably exist under ambient conditions while W5+ tends to be oxidized back to W6+ upon exposure to air.
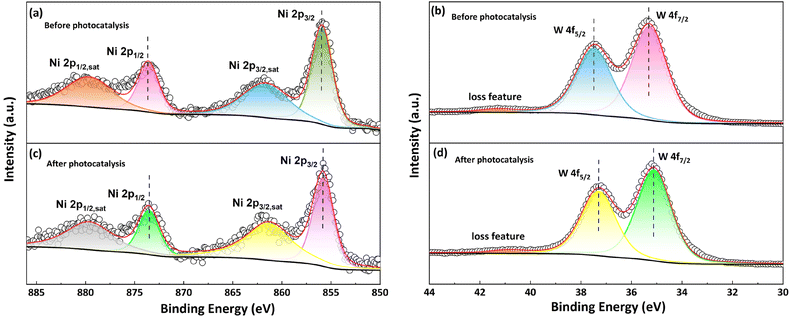 | ||
| Fig. 5 The XPS spectra of fresh compound 2 before catalysis: (a) Ni 2p and (b) W 4f; and the spectra of recovered catalyst 2 after catalysis: (c) Ni 2p and (d) W 4f. | ||
Conclusions
In summary, we have synthesized two unprecedented mixed-type Keggin and Anderson POM-based NiAPs, [(Sb2O)2(NiW6O24)-{Ni12O2(OH)4(enMe)4(H2O)3(WO4)2(PW9O34)2}2]14− and [(Sb2O)2-(NiW6O24){Ni12O2(OH)4(en)2(enMe)2(H2O)3(WO4)2}{Ni12O2(OH)4-(en)4(H2O)3(WO4)2}(PW9O34)4]14−, under hydrothermal conditions. The resulting two polyoxoanions could be structurally viewed as one central {(Sb2O)2(NiW6O24)} fragment linking two {Ni12(WO4)2(PW9)2} subunits through Sb2O moieties and Ni–O–W bonds. In addition, 1 is the first 1D chain linked by [Ni(enMe)2]2+ complexes and 25-Ni incorporated POMs. Upon light irradiation, 2 exhibited effective H2 evolution activity in a three-component photocatalytic system, which is superior to most of the reported POM-based heterogeneous catalysts. The present work not only enriches the structural diversity of POM chemistry, but also encourages the future exploration of POM-based catalysts for solar energy conversion.Experimental
Synthesis of 1
A mixture of NiCl2·6H2O (0.802 g, 3.374 mmol), Na9[A-α-PW9O34]·7H2O (1.882 g, 0.734 mmol), K2CO3 (0.205 g, 1.483 mmol), NH4B5O8·4H2O (0.503 g, 1.848 mmol) and Sb2O3 (0.125 g, 0.429 mmol) was dissolved in 15 ml distilled water under continuous stirring. Then 0.20 ml of 1,2-enMe (1,2-diaminopropane) was dropwise added to the cloudy solution and the pH was adjusted to 6.8 with 4 M HCl solution. After two hours of stirring, the solution was sealed and placed in an oven at 170 °C for 10 days. After cooling down to room temperature naturally, green crystals were obtained. Yield: 41% based on Na9[A-α-PW9O34]·7H2O. Elemental analysis calc. for 1 (%): C 2.36, H 1.36, N 1.83, Ni 9.98, Sb 3.18, W 55.30; found: C 2.47, H 1.54, N 1.98, Ni 10.12, Sb 3.31, W 55.49. IR (KBr, cm−1, Fig. S11a†): 3446 (vs), 2925 (w), 1622 (s), 1464 (w), 1403 (w), 1046 (s), 949 (s), 845 (w), 793 (m) and 722 (s). As shown in Fig. S12a,† the experimental powder X-ray diffraction (PXRD) of 1 matches well with the simulated results derived from the single crystal diffraction data. The thermogravimetric analysis (TGA) was conducted from 25 to 700 °C (Fig. S13a†), and a weight loss of 14.27% was detected for 1 (see the ESI† for details). The band gap value of 3.41 eV for 1 demonstrated the properties of a semiconductor (see the ESI for details, Fig. S14a†).Synthesis of 2
The synthetic process of 2 is similar to that of 1 except for the employment of mixed organic amines en (ethylenediamine, 0.10 ml) and 1,2-enMe (0.10 ml) as a substitute for 1,2-enMe (0.20 ml) and reducing the reaction time from 10 days to 5 days. Green crystals were obtained in a yield of 39% based on Na9[A-α-PW9O34]·7 H2O. Elemental analysis calc. for 2 (%): C 2.46, H 1.58, N 2.51, Ni 10.34, Sb 3.12, W 54.18; found: C 2.59, H 1.64, N 2.67, Ni 10.41, Sb 3.27, W 55.36. IR (KBr, cm−1, Fig. S11b†): 3450 (vs), 2950 (w), 1636 (s), 1458 (w), 1391 (w), 1040 (s), 943 (s), 835 (m), 792 (m) and 725 (s). The experimental PXRD of 2 coincides well with the simulated patterns from the single crystal diffraction data (Fig. S12b†). The TGA curve showed a weight loss of 12.84% for compound 2 from 25 to 700 °C (see the ESI for details, Fig. S13b†). The band gap value of 2 was 3.32 eV, revealing that 2 is a potential semiconductor material (see the ESI for details, Fig. S14b†).Author contributions
Peng-Yun Zhang: methodology, investigation, data curation, formal analysis, and writing – original draft. Chen Lian: writing – review & editing. Zhen-Wen Wang: data curation. Juan Chen: investigation. Hongjin Lv: writing – review & editing. Guo-Yu Yang: validation, writing – review & editing, and funding acquisition.Data availability
The data supporting this article have been included as part of the ESI.† Crystallographic data for compounds 1 and 2 have been deposited at the CCDC under 2240812 and 2240815.Electronic supplementary information (ESI) available: experimental procedures, IR spectra, thermogravimetric analyses, optical band gaps, and supporting figures and tables. CCDC 2240812 (1) and 2240815 (2).
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors sincerely acknowledge the financial support from the National Natural Science Foundation of China (no. 21831001, 21571016, 91122028, and 21871025) and the National Natural Science Foundation of China for Distinguished Young Scholars (No. 20725101).References
- Q. Shen, C. J. Gómez-García, W. Sun, X. Lai, H. Pang and H. Ma, Green Chem., 2021, 23, 3104 RSC.
- D. Zeng, P. Wu, W. J. Ong, B. Tang, M. Wu, H. Zheng, Y. Chen and D. L. Peng, Appl. Catal., B, 2018, 233, 26 CrossRef CAS.
- S. H. Chen, W. T. Wang, Y. Hou, Y. C. Hao, Y. C. Zhao, S. Y. Wang, J. F. Meng and H. Y. Xu, ACS Appl. Nano Mater., 2023, 6, 8717 CrossRef CAS.
- N. Li, J. Liu, B. X. Dong and Y. Q. Lan, Angew. Chem., Int. Ed., 2020, 59, 20779 CrossRef CAS PubMed.
- H. J. Lv, W. W. Guo, K. F. Wu, Z. Y. Chen, J. Bacsa, D. G. Musaev, Y. V. Geletii, S. M. Lauinger, T. Q. Lian and C. L. Hill, J. Am. Chem. Soc., 2014, 136, 14015 CrossRef CAS PubMed.
- C. F. Li, K. Yamaguchi and K. Suzuki, Angew. Chem., Int. Ed., 2021, 60, 6960 CrossRef CAS PubMed.
- L. Yang, Z. Zhang, C. N. Zhang, S. Li, G. C. Liu and X. L. Wang, Inorg. Chem. Front., 2022, 9, 4824 RSC.
- Z. M. Zhang, S. Yao, Y. G. Li, H. H. Wu, Y. H. Wang, M. Rouzières, R. Clérac, Z. M. Su and E. B. Wang, Chem. Commun., 2013, 49, 2515 RSC.
- Z. Zhang, Y. L. Wang, H. L. Li, K. N. Sun and G. Y. Yang, CrystEngComm, 2019, 21, 2641 RSC.
- X. K. Fang and M. Luban, Chem. Commun., 2011, 47, 3066 RSC.
- Y. L. Wu, X. X. Li, Y. J. Qi, H. Yu, L. Jin and S. T. Zheng, Angew. Chem., Int. Ed., 2018, 57, 8572 CrossRef CAS PubMed.
- W. H. Fang, W. D. Wang and G. Y. Yang, Dalton Trans., 2015, 44, 12546 RSC.
- M. Ibrahim, Y. Xiang, B. S. Bassil, Y. Lan, A. K. Powell, P. de Oliveira, B. Keita and U. Kortz, Inorg. Chem., 2013, 52, 8399 CrossRef CAS PubMed.
- P. E. Car, M. Guttentag, K. K. Baldridge, R. Alberto and G. R. Patzke, Green Chem., 2012, 14, 1680 RSC.
- P. Y. Zhang, Z. Zhang, X. Y. Li and G. Y. Yang, Inorg. Chem. Commun., 2020, 113, 107753 CrossRef CAS.
- S. T. Zheng, D. Q. Yuan, H. P. Jia, J. Zhang and G. Y. Yang, Chem. Commun., 2007, 18, 1858 RSC.
- C. Lian, H. L. Li and G. Y. Yang, Sci. China: Chem., 2023, 66, 1394 CrossRef CAS.
- P. Y. Zhang, Y. Wang, L. Y. Yao and G. Y. Yang, Inorg. Chem., 2022, 61, 10410 CrossRef CAS PubMed.
- X. J. Kong, Z. Lin, Z. M. Zhang, T. Zhang and W. B. Lin, Angew. Chem., Int. Ed., 2016, 55, 6411 CrossRef CAS PubMed.
- X. B. Han, C. Qin, X. L. Wang, Y. Z. Tan, X. J. Zhao and E. B. Wang, Appl. Catal., B, 2017, 211, 349 CrossRef CAS.
- Y. H. Zhu, Z. Y. Du, J. L. Wang, J. B. Yang, H. Mei and Y. Xu, Inorg. Chem., 2022, 61, 20397 CrossRef CAS PubMed.
- X. Q. Du, J. L. Zhao, J. Q. Mi, Y. Ding, P. P. Zhou, B. C. Ma, J. W. Zhao and J. Song, Nano Energy, 2015, 16, 247 CrossRef CAS.
- H. L. Li, M. Zhang, C. Lian, Z. L. Lang, H. J. Lv and G. Y. Yang, CCS Chem., 2021, 3, 2095 CrossRef CAS.
- X. B. Han, Y. G. Li, Z. M. Zhang, H. Q. Tan, Y. Lu and E. B. Wang, J. Am. Chem. Soc., 2015, 137, 5486 CrossRef CAS PubMed.
- S. S. Mal and U. Kortz, Angew. Chem., Int. Ed., 2005, 44, 3777 CrossRef CAS PubMed.
- J. Goura, B. S. Bassil, J. K. Bindra, I. A. Rutkowska, P. J. Kulesza, N. S. Dalal and U. Kortz, Chem. – Eur. J., 2020, 26, 15821 CrossRef CAS PubMed.
- D. Y. Shi, C. J. Cui, C. X. Sun, J. P. Du and C. S. Liu, New J. Chem., 2020, 44, 11336 RSC.
- L. Huang, S. S. Wang, J. W. Zhao, L. Cheng and G. Y. Yang, J. Am. Chem. Soc., 2014, 136, 7637 CrossRef CAS PubMed.
- S. T. Zheng, J. Zhang, X. X. Li, W. H. Fang and G. Y. Yang, J. Am. Chem. Soc., 2010, 132, 15102 CrossRef CAS PubMed.
- L. Huang, J. Zhang, L. Cheng and G. Y. Yang, Chem. Commun., 2012, 48, 9658 RSC.
- S. T. Zheng, J. Zhang, J. M. Clemente-Juan, D. Q. Yuan and G. Y. Yang, Angew. Chem., Int. Ed., 2009, 48, 7176 CrossRef CAS PubMed.
- H. L. Li, C. Lian, L. J. Chen, J. W. Zhao and G. Y. Yang, Nanoscale, 2020, 12, 16091 RSC.
- D. E. S. Marcano, M. A. Moussawi, A. V. Anyushin, S. Lentink, L. V. Meervelt, L. Ivanović-Burmazović and T. N. ParacVogt, Chem. Sci., 2022, 13, 2891 RSC.
- H. L. Li, C. Lian and G. Y. Yang, Inorg. Chem., 2023, 62, 9014 CrossRef CAS PubMed.
- M. Y. Cheng, H. Y. Wang, Y. F. Liu, J. W. Shi, M. Q. Zhou, W. X. Du, D. D. Zhang and G. P. Yang, Chin. Chem. Lett., 2023, 34, 107209 CrossRef CAS.
- X. X. Li, W. H. Fang, J. W. Zhao and G. Y. Yang, Chem. – Eur. J., 2015, 21, 2315 CrossRef CAS PubMed.
- S. T. Zheng, J. Zhang and G. Y. Yang, Angew. Chem., Int. Ed., 2008, 47, 3909 CrossRef CAS PubMed.
- X. X. Li, X. Ma, W. X. Zheng, Y. J. Qi, S. T. Zheng and G. Y. Yang, Inorg. Chem., 2016, 55, 8257 CrossRef CAS PubMed.
- H. J. Lv, Y. Chi, J. van Leusen, P. Kögerler, Z. Chen, J. Bacsa, Y. V. Geletii, W. W. Guo, T. Lian and C. L. Hill, Chem. – Eur. J., 2015, 21, 17363 CrossRef CAS PubMed.
- J. Goura, B. S. Bassil, X. Ma, A. Rajan, E. Moreno-Pineda, J. Schnack, M. Ibrahim, A. K. Powell, M. Ruben, J. P. Wang, L. Ruhlmann and U. Kortz, Chem. – Eur. J., 2021, 27, 15081 CrossRef PubMed.
- M. Martin-Sabi, R. S. Winter, C. Lydon, J. M. Cameron, D. L. Long and L. Cronin, Chem. Commun., 2016, 52, 919 RSC.
- Z. Zhang, J. W. Zhao and G. Y. Yang, Eur. J. Inorg. Chem., 2017, 3244 CrossRef CAS.
- P. F. Wu, Y. P. Zhang, C. T. Feng, B. Liu, H. M. Hu and G. L. Xue, Dalton Trans., 2018, 47, 15661 RSC.
- L. Yang, Y. Huo and J. Y. Niu, Dalton Trans., 2013, 42, 364 RSC.
- X. X. Li, S. T. Zheng, J. Zhang, W. H. Fang, G. Y. Yang and J. M. Clemente-Juan, Chem. – Eur. J., 2011, 17, 13032 CrossRef CAS PubMed.
- Q. H. Zhuang, Z. Q. Sun, C. G. Lin, B. Qi and Y. F. Song, Inorg. Chem. Front., 2023, 10, 1695 RSC.
- A. Macdonell, N. A. B. Johnson, A. J. Surman and L. Cronin, J. Am. Chem. Soc., 2015, 137, 5662 CrossRef CAS PubMed.
- X. X. Li, Y. X. Wang, R. H. Wang, C. Y. Cui, C. B. Tian and G. Y. Yang, Angew. Chem., Int. Ed., 2016, 55, 6462 CrossRef CAS PubMed.
- M. Z. Chi, H. J. Li, X. Xin, L. Qin, H. J. Lv and G. Y. Yang, Inorg. Chem., 2022, 61, 8467 CrossRef CAS PubMed.
- X. Du, Y. Ding, F. Song, B. Ma, J. Zhao and J. Song, Chem. Commun., 2015, 51, 13925 RSC.
- H. L. Li, C. Lian and G. Y. Yang, Inorg. Chem., 2021, 60, 14622 CrossRef CAS PubMed.
- C. A. Chen and G. Y. Yang, Inorg. Chem., 2023, 62, 14163 CrossRef CAS PubMed.
- T. T. Cui, L. Qin, F. Y. Fu, X. Xin, H. J. Li, X. K. Fang and H. J. Lv, Inorg. Chem., 2021, 60, 4124 CrossRef CAS PubMed.
- Z. W. Wang, Q. Zhao, C. A. Chen, J. J. Sun, H. J. Lv and G. Y. Yang, Inorg. Chem., 2022, 61, 7477 CrossRef CAS PubMed.
- L. Jiao, Y. Y. Dong, X. Xin, L. Qin and H. J. Lv, Appl. Catal., B, 2021, 291, 120091 CrossRef CAS.
- X. López, C. Bo and J. M. Poblet, J. Am. Chem. Soc., 2002, 124, 12574 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2240812 and 2240815. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4dt00928b |
| This journal is © The Royal Society of Chemistry 2024 |