Real-time visualization of carbon quantum dot transport in homogeneous and heterogeneous porous media†
Ying
Zhao‡
ab,
Jian
Song‡
ab,
Qingchun
Yang
c,
Yuelei
Li
d,
Zhuqing
Liu
*ab and
Fan
Yang
 *ab
*ab
aSchool of Water Conservancy & Civil Engineering, Northeast Agricultural University, Harbin 150030, China
bInternational Cooperation Joint Laboratory of Health in Cold Region Black Soil Habitat of the Ministry of Education, Harbin 150030, China. E-mail: yangfan_neau@163.com
cKey Laboratory of Groundwater Resources and Environment Ministry of Education, Jilin University, Changchun 130021, China
dSchool of Environment, Harbin Institute of Technology, Harbin, 150090, China
First published on 25th September 2024
Abstract
The widespread applications of carbon quantum dots (CQDs) have attracted much attention. This study presents a novel research system to study the transport and retention of CQDs in homogeneous and heterogeneous porous media. The light transmission visualization technique was used to visualize the real-time distribution and transport of CQDs. Results showed that the increase in quartz sand particle size and increased pH significantly enhanced the transport of CQDs. Due to the negative surface charge of CQDs shielded by high IS, the agglomeration of CQDs enhanced the clogging of CQDs. Particularly, significant aggregated fluorescence quenching of CQDs occurred at IS = 100 mM and IS = 200 mM. In heterogeneous media, the layer structure alteration and preferential flow contribute significantly in the transport of CQDs. Compared to the fine sand layer, most of the CQDs outflow from the coarse sand layer. The breakthrough curves for CQD transport in porous media can be matched by a simplified double-Monod model with high accuracy (R2 > 0.92). Moreover, the DLVO theory and clogging mechanism well explain the environmental behavior of CQDs in 2D porous media. This study visualized the fate of CQDs in 2D porous media, enabling us to further assess and predict their environmental risks.
Environmental significanceCarbon quantum dots (CQDs) are widely used in chemical sensing and biological and environmental applications. In this study, we found that1 the light transmission visualization technique can visualize the real-time transport and retention behavior of CQDs under different conditions without disturbances.2 In particular, CQDs are highly mobile in alkaline environments with low IS, homogeneous and large porosity, posing a significant potential risk to the environment. This study will help to further assess and predict their environmental risks. |
1. Introduction
In recent years, carbon-based nanomaterials, such as fullerene, carbon nanotubes, carbon black, graphene oxide, and carbon quantum dots (CQDs) have been widely reported because of their wide application potential and high tunability.1–7 Among them, CQDs are a new kind of ‘zero-dimensional’ nanomaterial, which have unique physicochemical properties, including excellent light stability, thermal stability, tiny size (usually less than 10 nm), high sorption capacity, good biocompatibility, and excellent water solubility,8–13 and the discovery and synthesis of quantum dots earned their discoverers the 2023 Nobel prize in chemistry. These favorable properties have attracted much attention from researchers in catalysis, environment, energy storage, etc.14–18 Specifically, CQDs possess excellent biocompatibility, and water dispersibility, which are used to monitor toxic pollutants as nano-tracers in groundwater.19–22 However, when the concentration of CQDs reaches a certain amount, it will cause adverse effects on environmental safety. Chousidis et al. found that CQDs (>150 μg mL−1) will cause significant toxicity to zebrafish, affecting its embryonic development.23 In addition, high concentrations of CQDs may also rupture the antioxidant structure of algae.24 The widespread applications of CQDs will contribute to their inevitable release into soil or groundwater.25,26 Therefore, understanding the transport behavior of CQDs in porous media is essential to assess the environmental impact of this novel nanomaterial.The environmental behavior, stability, and mobility in porous media of CQDs have attracted the attention of many researchers. Previous studies have shown that both physical (particle size, shape) and chemical (IS, pH, natural mineral composition) conditions in the environment may affect the transport of carbon-based nanomaterials in porous media.27–29 Similarly, changes in IS and pH were shown to determine the mobility of CQDs in saturated and unsaturated porous media.30 It has been reported that the stability of CQDs is enhanced when large amounts of dissolved organic matter are present in the environment, which in turn facilitates their transport in porous media. In addition, the binding of metal ions to CQDs also plays a vital role in the aggregation of CQDs and their transport in the environment. Zhang et al. found that CQDs provide numerous adsorption sites for Cd2+.31 Meanwhile, CQDs promoted the transport of Cd2+ in porous media through electrostatic interaction and complexation.
However, most of the current research on nanomaterials in porous media has been limited to one-dimensional (1D) experiments (column experiment), which may not reflect well the complexity of the size and structure of soil and groundwater systems in natural environments.32 Flow fields used in column tests ignore the variation in flow velocity at different locations and are far from practical applications.33 Thus, this study designed a two-dimensional (2D) sand tank to visualize the transport behavior of CQDs using the light transmission visualization (LTV) technique. This non-intrusive and non-disturbing technique can accurately characterize nanoparticle transport and distribution.34–39 Numerous studies have demonstrated that the transport of chemical and colloidal pollutants (e.g., graphene oxide, DNAPL, and NZVI) in porous media can be successfully reflected by LTV.32,33,37,40
Major studies have explored the photoluminescence properties of CQDs and their effects on the biochemical environment. However, we lack an understanding of the small-scale in situ dynamics of transport, retention, and fluorescence quenching of CQDs in porous media under different physicochemical conditions. Additionally, previous studies have not investigated the effect of structural heterogeneity on the transport of CQDs in porous media, which greatly limits the prediction and monitoring of the fate of CQDs in the environment. Therefore, we revealed the real-time transport pattern and mechanism of CQDs in 2D porous media under homogeneous and heterogeneous porous media conditions using LTV. The analysis of ζ-potential, FTIR, 3D-EEM, and TEM was conducted to determine the possible mechanisms governing the transport of CQDs. In addition, the transport mechanism of CQDs in porous media was further analyzed using DLVO theory and the colloidal clogging mechanism. These results contribute to revealing and elucidating the transport mechanism of CQDs in porous media and providing a basis for research on reducing the environmental risks of CQDs.
2. Materials and methods
2.1 Materials and characterization
2.2 LTV system
The schematic diagram of the 2D porous media is shown in Fig. 1, including a sand tank, light source (visible and ultraviolet light sources), and CCD camera. The sand tank (30 cm long, 20 cm high, 1.2 cm wide) consists of a frame with two pieces of clear glass, acrylic sides, and a removable top. Three small holes were drilled equidistant from the bottom of the inner frame on each side of the inlet and outlet to allow fluid inflow and outflow. In order to distribute the water flow horizontally and uniformly in the 2D porous medium, slots were cut in the inner sides of the left and right frames, which served as an inlet well and outlet well, respectively, and covered with a sieve (200 mesh). The CQD injection hole was set in the top cover 5 cm from the inlet boundary and 10 cm from the bottom.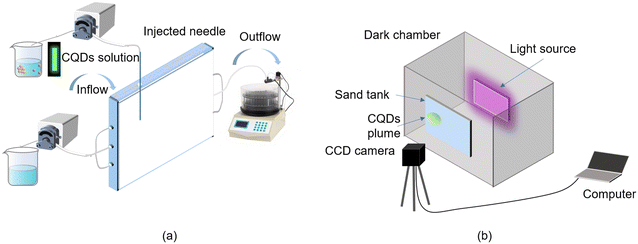 | ||
| Fig. 1 Schematic diagram of the 2D experiment device. Schematic diagram of the 2D sand tank (a) and LTV system (b). | ||
2.3 Sand tank experiment
In this study, the experiments were divided into three groups to investigate the effects of the different quartz sand particle sizes, IS, and pH on the transport and retention behavior of CQDs (Table 1).| No. | Porous media | Particle size (μm) | IS (mM) | pH | θ | K (cm min−1) | D (cm2 min−1) | k (min−1) | R 2 | TMR |
|---|---|---|---|---|---|---|---|---|---|---|
| θ: porosity; K: saturated hydraulic conductivity; D: dispersibility; k: first-order sorption coefficient; TMR: mass recovery rate. | ||||||||||
| A1 | Homogeneous porous media | 120–180 | 1 | 7 | 0.30 | 1.19 | 0.017 | 0.00250 | 0.964 | 76.84% |
| A2 | 180–380 | 1 | 7 | 0.32 | 1.79 | 0.027 | 0.00160 | 0.973 | 81.96% | |
| A3 | 380–830 | 1 | 7 | 0.37 | 5.69 | 0.384 | 0.00011 | 0.927 | 94.49% | |
| B1 | 180–380 | 1 | 7 | 0.32 | 1.79 | 0.027 | 0.00160 | 0.973 | 81.96% | |
| B2 | 180–380 | 100 | 7 | 0.32 | 1.79 | 0.027 | 0.00210 | 0.977 | 68.13% | |
| B3 | 180–380 | 200 | 7 | 0.32 | 1.79 | 0.027 | 0.00330 | 0.972 | 56.40% | |
| C1 | 180–380 | 1 | 5 | 0.32 | 1.79 | 0.027 | 0.00230 | 0.973 | 68.01% | |
| C2 | 180–380 | 1 | 7 | 0.32 | 1.79 | 0.027 | 0.00160 | 0.973 | 81.96% | |
| C3 | 180–380 | 1 | 9 | 0.32 | 1.79 | 0.027 | 0.00057 | 0.942 | 93.72% | |
| NH1 | Heterogeneous porous media | 120–180 | 0.30 | 1.19 | 0.017 | 0.00035 | ||||
| + | 1 | 7 | + | + | + | + | 0.941 | 78.27% | ||
| 380–830 | 0.37 | 5.69 | 0.384 | 0.00430 | ||||||
| NH2 | 380–830 | 11 | 7 | 0.37 | 5.69 | 0.384 | 0.00010 | |||
| + | 1 | 7 | + | + | + | + | 0.934 | 90.18% | ||
| 120–180 | 0.30 | 1.19 | 0.017 | 0.00230 | ||||||
The experimental solution was pumped at 2.0 ml min−1 into a sand tank. Before the experiment, deionized water was passed through for 24 hours and then rinsed with the background solution for 24 hours. CQD transport experiments were performed by injecting 100 mL CQD solution at 0.5 mL min−1 into the sand tank. The CCD camera captured gray-level images (Fig. S1–S4†) and fluorescence images of CQDs in the sand tank with an interval of 1 min. Among them, the fluorescence images are acquired by replacing the visible light source with the UV-light source with a wavelength of 365 nm and using the photoluminescence of CQDs to obtain its fluorescence images. After subtracting the background from the gray-level images, a MATLAB program is developed to process and analyze the image and convert gray-level images to color images based on the Beer–Lambert law.
2.4 Numerical simulations and DLVO theory
In this study, considering the vertical diffusion of CQD in the sand tank, we use a simplified double-Monod model to simulate the transport of CQD in the porous medium, which is analyzed using RT-3D code by GMS: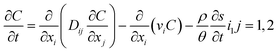 | (1) |
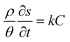 | (2) |
The model parameters were adjusted according to the experimentally derived breakthrough curves (BTCs) of the CQDs. The detailed model parameters are shown in Table 1.
The Derjaguin–Landau–Verwey–Overbeek (DLVO) theory was used to calculate the interaction energies between CQDs and the quartz sand surface. The details of the DLVO theory are shown in the ESI† S4.
3. Results and discussion
3.1 Properties of CQDs
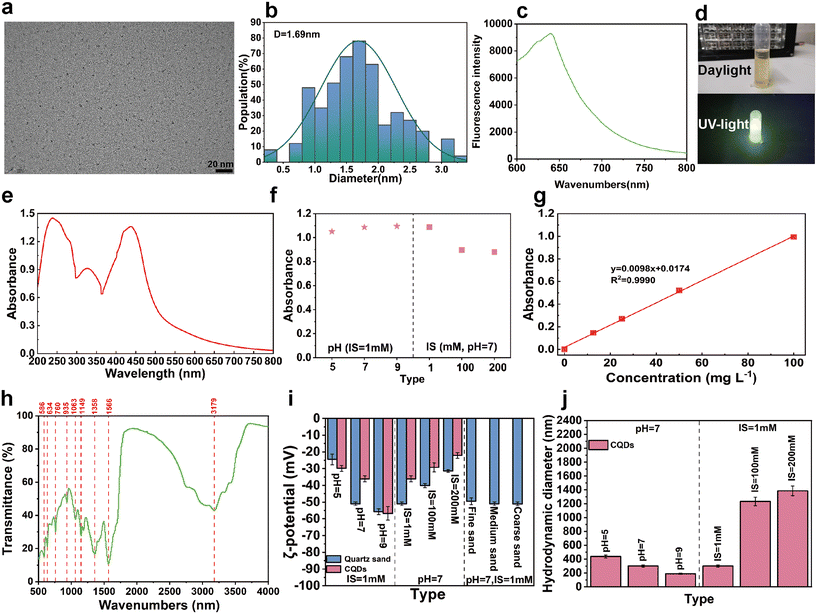
As can be seen from the TEM images of CQDs, which are mainly well-dispersed spherical nanoparticles (Fig. 2a), the average size of CQDs was 1.69 ± 0.60 nm (Fig. 2b). The fluorescence spectrum of CQDs is shown in Fig. 2c. Under visible light conditions, the solution of CQDs appeared yellowish-brown, however, under the excitation of UV light at 365 nm, its solution showed noticeable yellow-green fluorescence (Fig. 2d). The UV-vis spectrum of the CQDs in aqueous solutions is presented in Fig. 2e, which shows a peak centered on the wavelength of 438 nm. The UV absorbance at this location varied slightly. However, realistically with the IS and pH ranges used in this study (Fig. 2f), the absorbance at 438 nm was used in this study to estimate the concentration of CQDs (Fig. 2g). In addition, FT-IR results confirmed the presence of C–H (3179 cm−1), C–O (1149 cm−1, 1063 cm−1), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (1566 cm−1), O–H (1358 cm−1, 760 cm−1), C–O–C (1083 cm−1, 935 cm−1) and N–H (634 cm−1) on the surface of CQDs (Fig. 2h). These oxygen-containing functional groups alter the surface structure of CQDs, and give CQDs excellent water dispersibility. Under different chemical conditions, CQDs and quartz sand show many negative charges (Fig. 2i). Also, the negative charge density on the surface of CQDs and quartz sand tended to increase with decreasing IS and increasing pH. In contrast, the HDD of CQDs tended to decrease with decreasing IS and increasing pH (Fig. 2j).
O (1566 cm−1), O–H (1358 cm−1, 760 cm−1), C–O–C (1083 cm−1, 935 cm−1) and N–H (634 cm−1) on the surface of CQDs (Fig. 2h). These oxygen-containing functional groups alter the surface structure of CQDs, and give CQDs excellent water dispersibility. Under different chemical conditions, CQDs and quartz sand show many negative charges (Fig. 2i). Also, the negative charge density on the surface of CQDs and quartz sand tended to increase with decreasing IS and increasing pH. In contrast, the HDD of CQDs tended to decrease with decreasing IS and increasing pH (Fig. 2j).
3.2 Visualization of CQD transport and retention in 2D homogeneous porous media
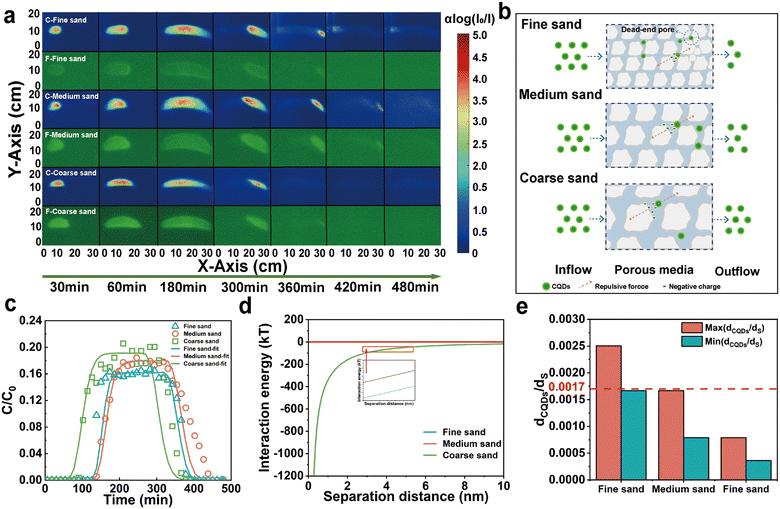
Under all experimental conditions, the ζ-potentials of CQDs and quartz sand were negative, and the average ζ-potential of CQDs decreased from −49.44 mV to −51 mV with the increase of the grain size. However, the ζ-potential increased slightly with increasing quartz sand grain size, and the change is insignificant. Correspondingly, the repulsive force between the CQDs and the quartz sand surface increases slightly with increasing particle size (Fig. 3b). In addition, DLVO theoretical calculations also show that there is no significant change in the total interaction energy between the CQDs and the quartz sand (Fig. 3d). Therefore, the change in quartz sand particle size has little effect on the repulsive force between the CQDs and the quartz sand surface. The transport of nanoparticles in porous media is often affected by Brownian motion. As the porosity increased from 0.30 to 0.37, the collision of CQD particles when the surface of quartz sand decreased, which promoted the transport of CQDs in the 2D sand tank. In addition, based on previous studies,42 collector and colloid dimensions, and the ratio of colloid diameter to collector diameter are considered to be important factors affecting pore clogging, and is especially more significant when the value of dp (colloid diameter)/dc (collector diameter) is greater than the critical value dp/dc = (min (0.0017), max (0.18)). The HDD to quartz sand ratio of CQDs ((dCQDs/dS)Min = 0.0017, (dCQDs/dS)Max = 0.0025) exceeded 0.0017 in the fine sand layer, which inhibited the transport of CQDs in the sand tank (Fig. 3e). Additionally, the colloidal filtration theory (CFT) described the transport behavior of CQDs in porous media as described in the ESI† S5 and Table S1. The collision coefficients and settling rate coefficients of the CQDs with the quartz sand surface decrease as the quartz sand particle size increases. The transport of CQDs in the quartz sand pores is affected by a variety of forces including hydrodynamic drag, uplift force from flow velocity differences, electrostatic repulsion, collision and attractive forces.43 When the hydrodynamic resistance is greater than the secondary minimum resistance, the CQDs collide less with the surface of the quartz sand and pass more easily through the pore channels of the porous medium.44 Hence, the transport of CQDs in different quartz sand grain sizes is mainly affected by the attenuation of the clogging effect, the dead-end pores, and the contact area of the quartz sand.
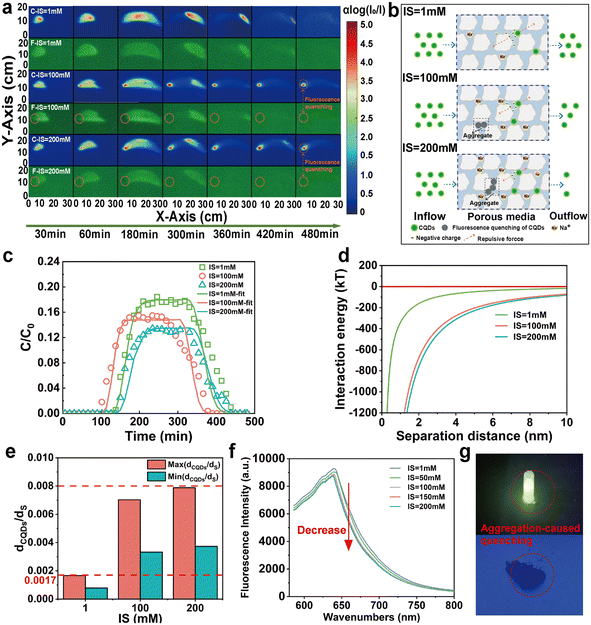
With increasing IS, the ζ-potential value of CQDs increased from −50.95 mV to −31.52 mV (Fig. 2i), decreasing the repulsive force between CQDs and quartz sand. Meanwhile, the significant increase in HDD of CQDs is also attributed to decreased repulsion and charge shielding. The agglomeration of CQDs becomes increasingly significant as the IS increased from 1 mM to 200 mM, and the HDD increases from 300.53 nm to 1419 nm. Unlike previous two-dimensional studies of colloids,41 the effect of IS on the retention of CQDs is weaker than the effect of IS on biochar colloids. On the one hand, this is mainly due to the fact that CQDs have smaller particle sizes compared to biochar colloids, and on the other hand, CQDs have good hydrophilicity. Moreover, primary minimal deposition or large aggregates can also cause CQDs to be more challenging to transport out of the quartz sand medium under high IS. The minimum value of the ratio of the average diameter of CQDs to quartz sand exceeds 0.0017 at both IS = 100 mM and IS = 200 mM (Fig. 4e). In addition, the CFT showed that agglomeration of CQDs was evident at 200 mM, and the total contact efficiency parameter increased due to an increase in the attachment parameter by gravitational settling. An increase in the α value increased the collision efficiency of the CQDs with the surface of the quartz sand, and more CQDs adhered to the surface of the quartz sand. In addition, according to the DLVO theory (Fig. 4d), the interaction barrier between the CQD and the quartz sand surface decreases with the increase of IS. It is suggested that the increase in IS was more favorable for the deposition of CQDs onto the surface of the quartz sand, thus decreasing their mobility in the quartz sand. Therefore, these results suggested that an increase in IS can lead to significant deposition of a large number of CQDs at the injection inlet (e.g., C-IS = 100 mM and C-IS = 200 mM in Fig. 4a). In particular, these agglomerated CQDs occurred with a significant aggregation-induced fluorescence quenching (ACQ) effect.45 We found that when CQDs are dispersed in the background solution, they can emit distinct fluorescence under UV light (Fig. 4g). However, when many CQDs are agglomerated together, their fluorescence disappears. In addition, the fluorescence intensity of the CQD solution decreases with the increase of IS (Fig. 4f). On the one hand, this phenomenon was attributed to the fact that the short wavelength end of the fluorescence emission spectrum of the CQDs solution partially overlaps with the long wavelength end of its absorption spectrum, and its fluorescence emission spectrum is absorbed by some other molecules, which leads to a decrease in its fluorescence intensity.46 On the other hand, the interactions between CQD molecules are enhanced, making them more prone to non-radiative transitions and decreasing fluorescence intensity.41,47 The results show that with the increase of IS, charge shielding and blocking effects greatly affect the transport of CQDs. Additionally, the agglomeration of CQDs also leads to a significant ACQ effect.
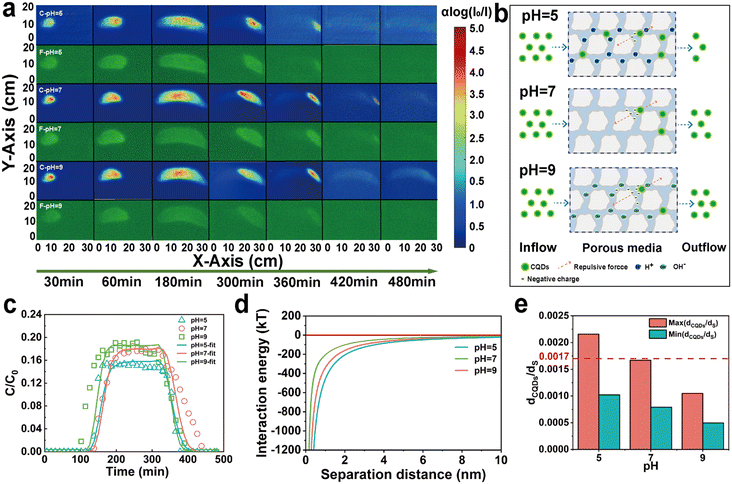
According to the FT-IR results (Fig. 2h), CQDs are rich in oxygenated functional groups. Increased pH causes the dissociation of oxygenated functional groups, which resulted in a significant increase in the negative charge on the surface of the CQDs. With the pH increased from 5 to 9, the ζ-potential of CQDs decreased from −29.85 mV to −56.77 mV (Fig. 2i). Therefore, the increased pH enhanced the repulsive force between CQD–CQD and CQD–quartz sand, which in turn reduced the aggregation probability of CQD and its adhesion to quartz sand particles. In addition, DLVO calculations showed that the repulsive interaction energy between the CQDs and the quartz sand surface increased with increasing pH (Fig. 5d). Moreover, the agglomeration effect of CQDs in a low pH environment increased their HDD. As the pH decreases from 9 to 5, the HDD increases from 188.9 nm to 388.23 nm. Correspondingly, the maximum ratio of the mean diameter of CQDs to quartz sand rose from 0.0010 to 0.0021, and the possibility of clogging of CQDs increased (Fig. 5e). In general, the clogging effect was not significant in different pH environments. Particularly in pH = 7 and pH = 9, the (dCQDs/dS)Max were both less than 0.00017, which largely enhanced the mobility of CQDs. Consistent with the similarity of other carbon-based nanoparticle transport processes in porous media,48,49 the transport process of CQDs was facilitated under alkaline conditions. In addition, the dissociation of acidic functional groups (carboxyl groups, phenolic hydroxyl groups, etc.) of CQDs was enhanced with the increase of pH, bringing more negative charges to the surface of the porous medium, which promoted the transport of CQDs in the quartz sand. Based on the CFT, it was found that the settling rate of CQDs in porous media also showed a decrease with increasing pH (Table S1†). Consequently, high pH can increase the negative charge density of CQDs, improving the mobility and stability of CQDs in the sand tank.
3.3 Visualization of CQD transport and retention in 2D heterogeneous porous media
Natural soil-groundwater systems are complex and may exhibit wide heterogeneity.50 Therefore, two typical heterogeneous porous media are considered in this experiment: NH1: the upper layer is fine sand, and the lower layer is coarse sand; NH2: the upper layer is coarse sand, and the lower layer is fine sand. In both types of filling, the proportion of layers is 50% (Fig. 6c). The transport behavior of CQDs in the sand tank changed significantly due to the altered sand layer structure (Fig. 6a). Comparison with the homogeneous sand tank revealed that most of the CQDs chose to flow out from the coarse sand layer. Interestingly, in NH1, although CQDs preferentially flowed out of the coarse sand layer, a small portion still entered the fine sand layer by convection–dispersion at the injection port. However, in NH2, almost all CQDs chose to transport in the coarse sand layer in the early stage and only entered the fine sand layer later due to the outlet. The results suggested that the sand layer structure alteration resulted in preferential channels,51 prompting the preferential choice of CQDs to enter the coarse sand layer. Furthermore, it has been inferred that a capillary barrier may exist at the interface junction of the layer structure,52 which could also impact the transport path of CQDs. Similarly, CQDs were also affected in heterogeneous fine sand layers by dead-end pores and increased contact area, inhibiting their transport in porous media. In addition, by calculating the flow rates of different sand layers in a heterogeneous sand tank, it can be seen that the flow rate of the large-size sand layer is much larger than that of the small-size sand layer. Consequently, the transport rate of CQDs was faster in the coarse sand layer, which resulted in CQDs in NH1 flowing out of the sand tank earlier than CQDs in NH2.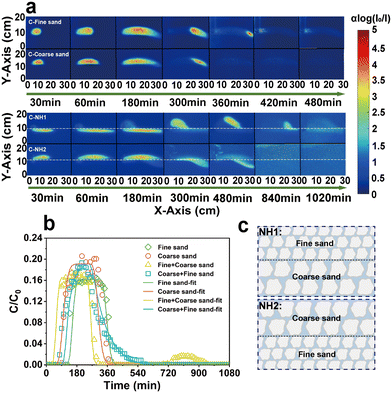 | ||
| Fig. 6 Color images of CQDs under different porous media (a), BTCs and fitted simulation of CQDs (b), and the schematic diagram of heterogeneous filling (c). | ||
Consistent with the transport trend of CQDs in the color images, the BTCs of CQDs in the heterogeneous sand tank showed two peaks due to the change in the sand layer structure (Fig. 6b). The first peak reflected that many CQDs were transported out of the sand tank through the preferred channel, and the second peak reflected that the CQDs trapped in the fine sand layer also broke through out of the sand tank under a hydrodynamic force. Correspondingly, the TMR of CQDs in the homogeneous fine sand tank, homogeneous coarse sand tank, heterogeneous NH1, and heterogeneous NH2 were 76.84%, 94.49%, 78.27%, and 90.18%, respectively. In addition, in NH1, the k value was 0.00035 min−1 for the fine sand layer and 0.00430 min−1 for the coarse sand layer. They were increased compared to the homogeneous fine and coarse sand tank. In NH2, the k value was 0.00010 min−1 for the fine sand layer and 0.00230 min−1 for the coarse sand layer. They decreased compared to homogenized fine sand tank and coarse sand layer.
Therefore, it was shown that the transport pattern of CQDs in laminated sand layers can be affected by laminated moisture conditions and sand layer structure sequencing. In addition, the time for the CQDs to flow out the sand tank is shorter in the upper coarse and lower fine structures than in the upper fine and lower coarse structures.
4. Conclusion
In this paper, the LTV technique was used to observe the concentration distribution and transport pattern of CQDs in two-dimensional porous media. Based on the photoluminescence property of CQDs, fluorescence images and color images of CQDs are compared and analyzed at the same time. Results show that at low IS, the changes of CQDs and color images are almost consistent. However, at high IS, the aggregated CQDs occurred a strong ACQ effect, and their fluorescence images were clearly distinguished from the corresponding color images. The mobility of CQDs increased with increasing particle size, decreasing IS, and increasing pH in homogeneous porous media. In heterogeneous media, the alteration of the layer structure and the presence of preferential flow significantly affected the transport of CQDs. The double-Monod model fits the CQDs well to the BTCs in the experiment. In addition, the transport and retention mechanisms of CQDs under different conditions are well explained by the DLVO theory and the clogging mechanism. Among them, the influence of the clogging effect on the transport of CQDs is more significant in high IS and fine sand media.These results suggest that the potential hazards posed by CQDs should be of greater concern for areas with high porosity and permeability, weakly alkaline or alkaline soils or aquifers. In addition, for some high IS areas, attention should be paid to the aggregated fluorescence quenching phenomenon of CQDs under high IS conditions when they are used for environmental monitoring.
Data availability
The data and code that support the findings of this study are available from the corresponding author upon reasonable request.Author contributions
Ying Zhao: supervision, writing – review & editing. Jian Song: data curation, methodology, and writing – original draft. Qingchun Yang: software. Yuelei Li: resources. Zhuqing Liu: supervision and methodology. Fan Yang: supervision and resources. All authors read and contributed to the final manuscript.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
The authors appreciate the financial support from the National Key Research and Development Program of China (2022YFD1500100), the National Natural Science Foundation of China (52279034), the International Cooperation Joint Laboratory of Health in Cold Region Black Soil Habitat of the Ministry of Education Open Subjects (HCRBSH202311-03), and the Key Laboratory of Germplasm Innovation and Physiological Ecology of Cold land Grain Crops, Ministry of Education (CXSTOP202304).References
- A. Bacanu, J. F. Pelletier, Y. Jung and N. Fakhri, Inferring scale-dependent non-equilibrium activity using carbon nanotubes, Nat. Nanotechnol., 2023, 18(8), 905, DOI:10.1038/s41565-023-01395-2.
- A. Chuvilin, U. Kaiser, E. Bichoutskaia, N. A. Besley and A. N. Khlobystov, Direct transformation of graphene to fullerene, Nat. Chem., 2010, 2(6), 450–453 CrossRef CAS PubMed.
- J. Yang, S. Kang, D. Chen, L. Zhao, Z. Ji and K. Duan, et al., South Asian black carbon is threatening the water sustainability of the Asian Water Tower, Nat. Commun., 2022, 13(1), 7360 CrossRef CAS PubMed.
- K. Zhang, Q. Fu, N. Pan, X. Yu, J. Liu and Y. Luo, et al., Direct writing of electronic devices on graphene oxide by catalytic scanning probe lithography, Nat. Commun., 2012, 3, 1194, DOI:10.1038/ncomms2200.
- L. Wang, W. T. Li, L. Q. Yin, Y. J. Liu, H. Z. Guo and J. W. Lai, et al., Full-color fluorescent carbon quantum dots, Sci. Adv., 2020, 6(40), eabb6772, DOI:10.1126/sciadv.abb6772.
- L.-L. Zhang, L. Tong, X.-H. Lv, Q.-Q. Yan, Y.-W. Ding and Y.-C. Wang, et al., A Top-Down Templating Strategy toward Functional Porous Carbons, Small, 2022, 18(26), 2201838, DOI:10.1002/smll.202201838.
- R. K. Lindsey, N. Goldman, L. E. Fried and S. Bastea, Chemistry-mediated Ostwald ripening in carbon-rich C/O systems at extreme conditions, Nat. Commun., 2022, 13(1), 1424, DOI:10.1038/s41467-022-29024-x.
- Q. Zhang, R. Wang, B. Feng, X. Zhong and K. K. Ostrikov, Photoluminescence mechanism of carbon dots: triggering high-color-purity red fluorescence emission through edge amino protonation, Nat. Commun., 2021, 12(1), 6856 CrossRef CAS PubMed.
- C. Ding, A. Zhu and Y. Tian, Functional Surface Engineering of C-Dots for Fluorescent Biosensing and in Vivo Bioimaging, Acc. Chem. Res., 2014, 47(1), 20–30 CrossRef CAS PubMed.
- S. J. Park, J. Y. Park, J. W. Chung, H. K. Yang, B. K. Moon and S. S. Yi, Color tunable carbon quantum dots from wasted paper by different solvents for anti-counterfeiting and fluorescent flexible film, Chem. Eng. J., 2020, 383, 123200, DOI:10.1016/j.cej.2019.123200.
- X. X. Shi, H. M. Meng, Y. Q. Sun, L. B. Qu, Y. H. Lin and Z. H. Li, et al., Far-Red to Near-Infrared Carbon Dots: Preparation and Applications in Biotechnology, Small, 2019, 15(48), 1901507, DOI:10.1002/smll.201901507.
- D. L. Zhao and T. S. Chung, Applications of carbon quantum dots (CQDs) in membrane technologies: A review, Water Res., 2018, 147, 43–49 CrossRef CAS PubMed.
- H. Zhang, G. Wang, Z. Zhang, J. H. Lei, T. M. Liu and G. Xing, et al., One step synthesis of efficient red emissive carbon dots and their bovine serum albumin composites with enhanced multi-photon fluorescence for in vivo bioimaging, Light: Sci. Appl., 2022, 11(1), 113 CrossRef CAS PubMed.
- G. A. M. Hutton, B. C. M. Martindale and E. Reisner, Carbon dots as photosensitisers for solar-driven catalysis, Chem. Soc. Rev., 2017, 46(20), 6111–6123 RSC.
- X. M. Li, M. C. Rui, J. Z. Song, Z. H. Shen and H. B. Zeng, Carbon and Graphene Quantum Dots for Optoelectronic and Energy Devices: A Review, Adv. Funct. Mater., 2015, 25(31), 4929–4947 CrossRef CAS.
- S. Y. Lim, W. Shen and Z. Q. Gao, Carbon quantum dots and their applications, Chem. Soc. Rev., 2015, 44(1), 362–381 RSC.
- U. Abd Rani, L. Y. Ng, C. Y. Ng and E. Mahmoudi, A review of carbon quantum dots and their applications in wastewater treatment, Adv. Colloid Interface Sci., 2020, 278, 102124, DOI:10.1016/j.cis.2020.102124.
- A. Mehta, A. Mishra, S. Basu, N. P. Shetti, K. R. Reddy and T. A. Saleh, et al., Band gap tuning and surface modification of carbon dots for sustainable environmental remediation and photocatalytic hydrogen production - A review, J. Environ. Manage., 2019, 250, 109486, DOI:10.1016/j.jenvman.2019.109486.
- X. H. Liu, Y. Yang, H. P. Li, Z. G. Yang and Y. Fang, Visible light degradation of tetracycline using oxygen-rich titanium dioxide nanosheets decorated by carbon quantum dots, Chem. Eng. J., 2021, 408, 127259, DOI:10.1016/j.cej.2020.127259.
- Z. Hu, H. Gao, S. B. Ramisetti, J. Zhao, E. Nourafkan and P. W. J. Glover, et al., Carbon quantum dots with tracer-like breakthrough ability for reservoir characterization, Sci. Total Environ., 2019, 669, 579–589 CrossRef CAS PubMed.
- R. B. Gonzalez-Gonzalez, A. Sharma, R. Parra-Saldivar, R. A. Ramirez-Mendoza, M. Bilal and H. M. N. Iqbal, Decontamination of emerging pharmaceutical pollutants using carbon-dots as robust materials, J. Hazard. Mater., 2022, 423, 127145, DOI:10.1016/j.jhazmat.2021.127145.
- S. O. Sanni, T. H. G. Moundzounga, E. O. Oseghe, N. H. Haneklaus, E. L. Viljoen and H. G. Brink, One-Step Green Synthesis of Water-Soluble Fluorescent Carbon Dots and Its Application in the Detection of Cu2+, Nanomaterials, 2022, 12(6), 958, DOI:10.3390/nano12060958.
- I. Chousidis, C. D. Stalikas and I. D. Leonardos, Induced toxicity in early-life stage zebrafish (Danio rerio) and its behavioral analysis after exposure to non-doped, nitrogen-doped and nitrogen, sulfur-co doped carbon quantum dots, Environ. Toxicol. Pharmacol., 2020, 79, 103426, DOI:10.1016/j.etap.2020.103426.
- A. Xiao, C. Wang, J. Chen, R. Guo, Z. Yan and J. Chen, Carbon and Metal Quantum Dots toxicity on the microalgae Chlorella pyrenoidosa, Ecotoxicol. Environ. Saf., 2016, 133, 211–217 CrossRef CAS PubMed.
- X. Liu, J. X. Li, X. H. Wu, Z. Zeng, X. L. Wang and T. Hayat, et al., Adsorption of carbon dots onto Al2O3 in aqueous: Experimental and theoretical studies, Environ. Pollut., 2017, 227, 31–38 CrossRef CAS PubMed.
- H. J. Zhang, T. T. Lu, J. Y. Chen, Q. Zhang, Y. X. Li and W. F. Chen, et al., Insights into the effect of citric acid on the carbon dot-mediated transport of Cd2+ through saturated porous media, Environ. Sci.: Nano, 2022, 9(6), 2061–2072 RSC.
- F. Lian, W. Yu, Z. Wang and B. Xing, New Insights into Black Carbon Nanoparticle-Induced Dispersibility of Goethite Colloids and Configuration-Dependent Sorption for Phenanthrene, Environ. Sci. Technol., 2019, 53(2), 661–670 CrossRef CAS PubMed.
- A. Serrano-Aroca, K. Takayama, A. Tunon-Molina, M. Seyran, S. S. Hassan and P. P. Choudhury, et al., Carbon-Based Nanomaterials: Promising Antiviral Agents to Combat COVID-19 in the Microbial-Resistant Era, ACS Nano, 2021, 15(5), 8069–8086 CrossRef CAS PubMed.
- X. Chen, J. Wang, R. Wang, D. Zhang, S. Chu and X. Yang, et al., Insights into growth-promoting effect of nanomaterials: Using transcriptomics and metabolomics to reveal the molecular mechanisms of MWCNTs in enhancing hyperaccumulator under heavy metal(loid)s stress, J. Hazard. Mater., 2022, 439, 129640, DOI:10.1016/j.jhazmat.2022.129640.
- S. Kamrani, M. Rezaei, M. Kord and M. Baalousha, Transport and retention of carbon dots (CDs) in saturated and unsaturated porous media: Role of ionic strength, pH, and collector grain size, Water Res., 2018, 133, 338–347 CrossRef CAS PubMed.
- H. Zhang, T. Lu, J. Chen, Q. Zhang, Y. Li and W. Chen, et al., Insights into the effect of citric acid on the carbon dot-mediated transport of Cd2+ through saturated porous media, Environ. Sci.: Nano, 2022, 9(6), 2061–2072 RSC.
- S. Dong, B. Gao, Y. Sun, H. Guo, J. Wu and S. Cao, et al., Visualization of graphene oxide transport in two-dimensional homogeneous and heterogeneous porous media, J. Hazard. Mater., 2019, 369, 334–341 CrossRef CAS PubMed.
- D. Lin, L. Hu and I. M. C. Lo, Two-Dimensional Modeling of Nano Zero-Valent Iron Transport and Retention before and after Phosphate Adsorption, Environ. Sci. Technol., 2022, 56(24), 17712–17719 CrossRef CAS PubMed.
- R. M. Niemet and J. S. Selker, A new method for quantification of liquid saturation in 2D translucent porous media systems using light transmission, Adv. Water Resour., 2001, 24, 651–666 CrossRef.
- M. M. Bob, M. C. Brooks, S. C. Mravik and A. L. Wood, A modified light transmission visualization method for DNAPL saturation measurements in 2-D models, Adv. Water Resour., 2008, 31(5), 727–742 CrossRef.
- V. Loukopoulos-Kousis and C. V. Chrysikopoulos, Use of GreenZyme® for remediation of porous media polluted with jet fuel JP-5, Environ. Technol., 2020, 41(3), 277–286 CrossRef CAS PubMed.
- S. Kashuk, S. R. Mercurio and M. Iskander, Visualization of dyed NAPL concentration in transparent porous media using color space components, J. Contam. Hydrol., 2014, 162–163, 1–16 CrossRef CAS PubMed.
- C. V. Chrysikopoulos, C. C. Plega and V. E. Katzourakis, Non-invasive in situ concentration determination of fluorescent or color tracers and pollutants in a glass pore network model, J. Hazard. Mater., 2011, 198, 299–306 CrossRef CAS PubMed.
- W. E. Huang, C. C. Smith, D. N. Lerner, S. F. Thornton and A. Oram, Physical modelling of solute transport in porous media: evaluation of an imaging technique using UV excited fluorescent dye, Water Res., 2002, 36(7), 1843–1853 CrossRef CAS PubMed.
- J. M. Thomas and C. V. Chrysikopoulos, A new method for in situ concentration measurements in packed-column transport experiments, Chem. Eng. Sci., 2010, 65(14), 4285–4292 CrossRef.
- Y. Zhao, D. Fan, S. Cao, W. Lu and F. Yang, Visualization of biochar colloids transport and retention in two-dimensional porous media, J. Hydrol., 2023, 619, 129266, DOI:10.1016/j.jhydrol.2023.129266.
- S. A. Bradford, J. Simunek, M. Bettahar, M. T. van Genuchten and S. R. Yates, Significance of straining in colloid deposition: Evidence and implications, Water Resour. Res., 2006, 42(12), W12S15, DOI:10.1029/2005WR004791.
- S. Dong, M. Zhou, X. Su, J. Xia, L. Wang and H. Wu, et al., Transport and retention patterns of fragmental microplastics in saturated and unsaturated porous media: A real-time pore-scale visualization, Water Res., 2022, 214, 118195, DOI:10.1016/j.watres.2022.118195.
- H. J. Bai, L. Lassabatere and E. Lamy, Colloid Transport in Aggregated Porous Media with Intra- and Interaggregate Porosities, Ind. Eng. Chem. Res., 2018, 57(18), 6553–6567 CrossRef CAS.
- S. Lee, J. Lee and S. Jeon, Aggregation-induced emission of matrix-free graphene quantum dots via selective edge functionalization of rotor molecules, Sci. Adv., 2023, 9(7), eade2585, DOI:10.1126/sciadv.ade2585.
- T. Arumugham, M. Alagumuthu, R. G. Amimodu, S. Munusamy and S. K. Iyer, A sustainable synthesis of green carbon quantum dot (CQD) from Catharanthus roseus (white flowering plant) leaves and investigation of its dual fluorescence responsive behavior in multi-ion detection and biological applications, Sustainable Mater. Technol., 2020, 23, e00138, DOI:10.1016/j.susmat.2019.e00138.
- T. K. Mondal, A. Kapuria, M. Miah and S. K. Saha, Solubility tuning of alkyl amine functionalized carbon quantum dots for selective detection of nitroexplosive, Carbon, 2023, 209, 117972, DOI:10.1016/j.carbon.2023.03.047.
- Y. J. Jiang, D. Guan, Y. M. Liu, X. Q. Yin, S. Zhou and G. L. Zhang, et al., The transport of graphitic carbon nitride in saturated porous media: Effect of hydrodynamic and solution chemistry, Chemosphere, 2020, 248, 125973, DOI:10.1016/j.chemosphere.2020.125973.
- D. Wang, W. Zhang, X. Hao and D. Zhou, Transport of biochar particles in saturated granular media: effects of pyrolysis temperature and particle size, Environ. Sci. Technol., 2013, 47(2), 821–828 CrossRef CAS PubMed.
- C. Chen, J. Shang, X. Zheng, K. Zhao, C. Yan and P. Sharma, et al., Effect of physicochemical factors on transport and retention of graphene oxide in saturated media, Environ. Pollut., 2018, 236, 168–176 CrossRef CAS PubMed.
- S. E. Allaire, S. Roulier and A. J. Cessna, Quantifying preferential flow in soils: A review of different techniques, J. Hydrol., 2009, 378(1–2), 179–204 CrossRef.
- K. W. Cho, K. G. Song, J. W. Cho, T. G. Kim and K. H. Ahn, Removal of nitrogen by a layered soil infiltration system during intermittent storm events, Chemosphere, 2009, 76(5), 690–696 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4en00563e |
| ‡ Ying Zhao and Jian Song contribute equally to this work and should be considered co-first authors. |
| This journal is © The Royal Society of Chemistry 2024 |