 Open Access Article
Open Access ArticlePotential of Brazilian berries in developing innovative, healthy, and sustainable food products
Nayara Macêdo
Peixoto Araujo
*a,
Paulo
Berni
b,
Lais Ramalho
Zandoná
b,
Nataly Maria Viva de
Toledo
c,
Paula Porrelli Moreira da
Silva
d,
Angélica Aparecida de
Toledo
b and
Mário Roberto
Maróstica Junior
b
aSchool of Food Engineering, Institute of Technology, Federal University of Pará, Belém, Pará, Brazil. E-mail: nayarapeixoto@ufpa.br
bFood Science and Nutrition Department, Faculty of Food Engineering, The State University of Campinas, Campinas, São Paulo, Brazil
cUniversity Center of Araras Dr. Edmundo Ulson, Araras, São Paulo, Brazil
dDepartment of Agroindustry, Food and Nutrition, Laboratory of Fruits and Vegetables. University of São Paulo, Superior School of Agriculture “Luiz de Queiroz” (ESALQ/USP), São Paulo, Brazil
First published on 13th December 2023
Abstract
There is a considerable diversity of Brazilian berries, purple in color, and potentially rich in anthocyanins, which are an unexplored source of new foods, products, extracts, and compounds of economic and social interest. Nevertheless, none of these berries are significantly marketed or invested in for increasing the production and extraction of target compounds. Therefore, this review combined scientific data regarding the use of Brazilian berries as an innovative approach to the healthy and sustainable development of food products. The current review provides an overview of the main Brazilian berries (e.g., camu-camu, nhamburi, pitanga preta, cherry of Rio Grande, grumixama, açaí, jabuticaba, juçara, capinuriba and guabiju) and their nutritional and mineral profile, content of bioactive compounds and their biological activities. In addition, we report Brazilian berries used in the development of healthy products using emergent strategies and the use of Brazilian berry by-products in food innovation.
Sustainability spotlightThe United Nations Sustainable Development Goals (SDGs) are a set of 17 interconnected goals adopted by the United Nations General Assembly in 2015 as part of the 2030 Agenda for Sustainable Development. In our review, we showed how the exploitation of Brazilian berries as raw materials for the development of new food and nutraceutical products is based on the SDGs, especially sustainability, including social, economic, and environmental aspects, to achieve a more sustainable future for all. |
1 Introduction
Sustainability is well understood as the ability to meet present needs without compromising the ability of future generations to meet their own needs. It involves balancing social, economic, and environmental factors. Sustainable practices aim to conserve resources, protect ecosystems, and promote long-term well-being for both humans and the planet. The United Nations Sustainable Development Goals (SDGs) are a set of 17 interconnected goals adopted by the United Nations General Assembly in 2015 as part of the 2030 Agenda for Sustainable Development.1These goals aim to address various dimensions of sustainability, including social, economic, and environmental aspects, to achieve a more sustainable future for everyone. Biodiversity refers to the variety of living organisms in a habitat or on Earth.2 It includes species diversity, genetic variation, and ecosystem diversity. Biodiversity is essential for ecosystem health and provides numerous benefits to humanity. According to the World Health Organization, “sustainable diets are those diets with low environmental impacts, which contribute to food and nutrition security and a healthy life for present and future generations. Sustainable diets are protective and respectful of biodiversity and ecosystems; culturally acceptable; accessible; economically fair and affordable; nutritionally adequate, safe, and healthy, while optimizing natural and human resources”.3
The UN Decade of Action on Nutrition 2016–2025 recommends that health systems should consider the long-term consequences associated with excess weight and obesity by promoting prevention and control through diet. One of its pillars is the causal relationship between sustainable food systems and the promotion of healthy eating, emphasizing the importance of investments and public policies that integrate nutrition, food, and agriculture, and strengthening local food production and processing.4,5
The World Health Organization (WHO) tirelessly advocates that a healthy and efficient food system, linked to healthy lifestyles, can significantly reduce the costs of treating Non-Communicable Diseases (NCDs), along with improving people's quality of life, thus promoting productivity and job creation.4–6
Therefore, countries should adopt policies encouraging the development, production, and promotion of food products that contribute to a healthier diet. It is necessary to offer healthy and affordable options that can effectively prevent and control overweight and obesity.5,6 In this sense, expanding dietary diversity through the utilization of biodiversity emerges as a strategic option, being intensely supported by scientific evidence, as we intend to show in this review.
Non-Communicable Chronic Diseases (NCDs) drive much morbidity, mortality, and national healthcare costs, which impacts the economy of many countries independently of the development situation. Currently, chronic diseases account for 41 million deaths worldwide with cardiovascular diseases and diabetes leading the charge. In 2016, 67.5% of US adults were overweight, 63.5% in Egypt, 53.8% in Brazil, and 32% in China. The overall prevalence of obesity in Brazil increased from 11.7% to 18.1% in men, and from 12.1% to 18.8% in women between 2006–2016.7,8
These alarming data are probably underestimated nowadays due to a lack of actualized epidemiological research and since obesity rates have risen markedly in the past few decades maintaining this tendency. This rise in obesity worldwide affects all age groups and contributes to the prevalence of NCDs. The recent COVID-19 pandemic has brought new concerns about obesity from an epidemiological point of view. Obesity is identified as both a risk factor for the manifestation of a severe form of the disease and a serious nutritional consequence of prolonged confinement.7
Regular consumption of common commercial berries, like blueberries, has proved to be helpful in the prevention of chronic diseases, based on epidemiological data, whose benefits are attributed to their phytochemical's composition.9 In contrast, the review by Aguilera and Toledo (2022) highlights the global significance of wild berries in the nutrition of rural communities. These berries are rich in bioactive compounds that can combat chronic diseases linked to oxidative stress and inflammation.10 While chemical analyses confirm that wild berries, have high bioactive content and antioxidant activity, and in vivo studies demonstrate their health effects, however, the exact mechanisms of their therapeutic action remain elusive. In essence, wild berries show promise fighting NCDs, but further research is essential to reinforce their health benefits.
Thus far, some Brazilian native Myrtaceae fruits, like Jabuticaba (Myrciaria cauliflora) and camu-camu (Myrciaria dubia), have already been proved as potent sources of biologically active polyphenols, primarily tannins like ellagitannins and proanthocyanidins. There is extensive research, including in vitro, in vivo, and clinical studies, that consistently demonstrates their unique ability to regulate glucose levels, improve glucose homeostasis, mitigate dyslipidemia, combat inflammation, reduce oxidative stress, and prevent excessive weight gain.11 Furthermore, there is evidence that Brazilian native fruits hold significant promise as potential candidates for innovative cancer treatments since several studies show their unique ability to selectively target cancer cells while sparing healthy ones.12 Incorporating these fruits into one's diet may help reduce the risk and improve the management of chronic diseases, offering a natural and delicious approach to better health.
Brazil harbors the greatest plant biodiversity in the world.2 A variety of species in Brazilian ecosystems represents available and unexplored sources of food and compounds with the potential to improve people's quality of life and health. The chemical characterization (e.g., anthocyanin profile) and potential health benefits (e.g., anti-inflammatory, anti-obesity, and prebiotic effects) of numerous native fruits remain unknown and are disregarded by farmers, consumers, traders, and industries.2,13
Despite Brazil's exceptional biodiversity, most cultivated commodities such as soybeans and sugarcane are not originally from South American ecosystems. The expansion of agriculture and urbanization promote deforestation and the destruction of vast areas of typical biomes such as the Atlantic Forest and the Amazon Rainforest in an unnecessary and uncontrolled manner. Therefore, monocultures and livestock expansion result in immeasurable losses of biodiversity that may take centuries to recover.14 Consequently, many plants may disappear even before being catalogued or studied. To mitigate this waste of biodiversity and protect this natural heritage, it is urgent to investigate and rediscover the richness of native fruits in Brazil. For the construction of a sustainable future, it is essential to understand the incalculable value of biodiversity for generating employment and income.
The fruits of Brazilian biodiversity emerge as strategic foods and raw materials for functional foods and nutraceutical products. Brazilian native fruits, are rich sources of opportunities for innovation, generating jobs and income, improving public health, and significantly contributing to sustainable development, mainly SDG 2 (Zero Hunger), in the sense of new food sources, SDG 3 (Good health and well-being), in the sense of new functional products for health purposes, SDG 9 (Industry, Innovation and Infrastructure), in the sense of innovative technologies to generate new products and SDG 12 (Responsible Consumption and Production), in the sense of generating new food products that attend to new sustainability needs.
Natural foods and ingredients from plant biodiversity represent a value-creation strategy in the transition to a sustainable biobased economy, especially in biodiversity-rich countries.15 In this regard, integrating Brazilian berries into food markets will promote responsible consumption and production, especially if they are cultivated by local and traditional communities. Consequently, adding value to the sustainable production of Brazilian berries will stimulate local industry and infrastructure. Eating Brazilian berries fruits and products diversify our diets and reduce the demand for monoculture agriculture, which often leads to soil degradation and water pollution.
Additionally, Brazilian berries are rich in antioxidants, vitamins, and other essential nutrients. Thus promoting these berries can induce healthier habits which are more accessible and available for low-income people, thus ensuring food security. Moreover, sustainable food sources, such as Brazilian berries, plays a crucial part in biodiversity preservation, thus protecting invaluable ecosystems from deforestation.16 Deforestation in tropical forests, like Atlantic Forest and Amazon rainforest has reached unprecedented rates, requiring effective and achievable conservation. Preventing deforestation is imperative, but restoration is also required. Areas of Brazilian tropical forest losses greatly affected fruit availability, such as the ones from Sapatoceae, Myrtaceae, and Lecythidaceae families. It also affected fruit quality, in terms of lipids and proteins. These alterations in the quantity and quality of fruits can trigger severe impacts for plant–animal interactions, likely interfering in the seed dispersal process, thus harming forests' resilience.17 Then, protecting Brazilian forest areas for fruits production, and additionally, reforestation of areas with theses commercially Brazilian native berries, can help mitigate damagedue to deforestation.
The functional properties of Brazilian native fruits can stimulate the interest of producers and consumers, which in turn opens perspectives for the application of advanced techniques for sustainable processing, and full use of plant materials, spreading the consumption of unique fruits from Brazilian biodiversity in a sustainable fashion. This expansion of exploitation of Brazilian berries can benefit the local producers, protect biodiversity and its environment, generate economic development, and improve health and well-being.
In this sense, this review addresses Brazilian native berries as a strategy to generate new food products using innovative techniques as strategies against NCDs worldwide. Additionally, we propose investing resources, research, and efforts in Brazilian berries production, innovation, and consumption for helping reach the mentioned SDGs.
2 New raw materials found in Brazilian biodiversity
Berry is defined in botanical terms as a simple fruit that develops from a single flower with one ovary, typically having a soft and fleshy pericarp, and often containing multiple seeds. Examples of true berries include blueberries, cranberries, and grapes.18In the context of gastronomy and consumer sciences, berries present small size, balanced sweetness and acidity, vibrant color, and are often juicy fruits that are typically consumed fresh or used as an ingredient in various dishes, desserts, and beverages. Berries are often sought-after for their pleasant taste, appealing appearance, and versatility in various food preparations and products.16
In food science, a berry is a fruit characterized by its small size, high water content, red to dark-purple colours, and high concentration of bioactive compounds such as antioxidants, vitamins, and dietary fibers.19 Despite the similarities of definitions, the berry industry which expanded its global presence, is based exclusively in strawberries, blackberries, raspberries, and blueberries.20 Then, most people worldwide recognize berries as only the widely popular and expensive four fruits mentioned above. Moreover, it can wrongly transmit an image of these fruits as sustainable, while they are provided mostly by monocultures, needs vast application of agrochemicals, and are mostly traded internationally, creating lots of environmental impact. This misconception can prejudice local and wild berry demand, market value, and production.
In this review, berries are considered as red to purple, eventually black, coloured small fruits, since this is the definition which is closest to consumer perception. Then, all fruits presented can be easily included in the ‘berry’ category, presenting them as a more healthy and sustainable alternatives for commercial and imported berries.
Brazil is renowned for its rich biodiversity, and within its diverse ecosystems, a wide variety of berries have naturally emerged. Most of these fruits have contributed in the past to indigenous nutrition and medicine. Nowadays, only a few species still contribute to locals' diet and traditional ethnicities in national indigenous lands. Some Brazilian berries resist the country's culinary traditions, like açai, jaboticaba and pitanga.14,16
Years ago, several public parks and squares in big cities were planted with native fruits, like cherry of Rio Grande (Eugenia involucrata), grumixama (Eugenia brasiliensis), and pitanga (Eugenia uniflora), but they remain unnoticed by most of the population. Brazilian berries are perfectly adapted for tropical climates, however, they are not commercially grown, while food markets are dealing with an increased demand for imported berries (blueberry, cranberry and cherries).2,21
There is a considerable diversity of fruits native to Brazil, purple in color, and potentially rich in anthocyanins, which are an unexplored source of new foods, products, extracts, and compounds of economic and social interest.22 For example, grumixama is a dark-purple fruit with a sweet and sour taste. Pitanga is widely consumed both fresh and in juices and has a sweet and tangy flavor. Jabuticaba, often referred to as the Brazilian grape tree, produces unique fruits that grow directly on the trunk. These Brazilian berries are used in diverse culinary applications. Mostly are consumed fresh, mixed in juices, or turned into homemade jams. However, none of these berries are significantly marketed, or invested in for increasing production, while the traditional berries which are sold and consumed in Brazil (e.g., blackberries, blueberries, and cherries) are imported and expensive. There is a lot of effort and investment to increase the production of imported berries in Brazil, but with many challenges due to the little or no adaptation of these plants to our climate, as is the case of blueberries which are only profitable due to their high market value.23
Brazilian berries possess unique botanical characteristics, since their botanical families have evolved within the biomes they inhabit, and most of them are endemic, i.e., only naturally occurring in Brazilian biomes. Brazilian berries belong to various botanical families, each with its own distinct characteristics. Besides huge scientific efforts, there is still a shortage of reliable primary taxonomic data and description of biodiversity patterns of Brazilian plants, including edible fruits. However, it is well documented that Brazil is a megadiverse country, home to more plant species than any other country. 46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 975 native species of algae, fungi, and plants have been catalogued, of which 19
975 native species of algae, fungi, and plants have been catalogued, of which 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 669 are endemic to the country. The Atlantic Rainforest is, to date, the most diverse Brazilian domain, especially in the group of angiosperms – the group of plants which produce flowers and fruits.2
669 are endemic to the country. The Atlantic Rainforest is, to date, the most diverse Brazilian domain, especially in the group of angiosperms – the group of plants which produce flowers and fruits.2
There are several berries which have been studied in past decades as food and sources of bioactive compounds.24–28 Most of them are included in the Myrtaceae family, followed by Rubus and Arecaceae.27
There is also a representantive of the Myrciaria family, called camu-camu (Myrciaria dubia), which has great market potential due to its taste and appearance.10 The berries from the Myrtaceae family are all endemic to Brazil, since their original biome is the Atlantic Rainforest, which grows across the Brazilian coast. The ones from the Amazon rainforest are also found in neighbouring countries, like Peru, Bolivia, and Colombia.18,29
The açaí berry (Euterpe oleracea) sets an important precedent on how the media's appreciation of the beneficial properties of a fruit, validated by science, can expand the sustainable production and commercialization of a native fruit, thus promoting the protection of national biodiversity. It illustrates the potential results that can be obtained from investigating native biodiversity. Açaí was traditionally consumed only by indigenous, riverside and local populations in the northern region of the country. From the 60's, many industries intensified the extractive production, often illegal, of canned heart of palm, almost putting the açaí tree at risk of extinction. This scenario was transformed when açaí began to be publicized as an energetic and healthy food for youth in the television media, conquering an important market in the Brazilian metropolises.30–32 The beneficial health effects of the composition of bioactives (anthocyanins and healthy fat acids) in açaí have gradually been proven.33
Açaí has emerged as a noteworthy economic force with remarkable growth trends. Brazilian açaí production increased from approximately 116![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons in 2009 to an impressive 227
000 tons in 2009 to an impressive 227![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons by 2021. This booming industry substantially elevated açaí’s economic significance. It is worth noting that in 2020, açaí claimed the second-largest share of plant-based food products extracted in Brazil. The economic implications of açaí have reached international borders, as evidenced by the burgeoning export market with the United States being the largest importer, followed by Germany, Belgium, and the Netherlands. This escalating demand is indicative of açaí's growing role in the global market for functional foods, nutraceuticals, and ‘superfoods’. The economic viability of açaí has extended beyond traditional consumption, with companies recognizing its potential as a natural, health-conscious offering.34 Other Brazilian berries carry the same appeal, thus having the same potential for gaining the world's attention. Further, the açaí industry has incorporated social and environmental responsibility into their production and market strategies,34 which represents the most important lesson taken, and must be applied in the development of other Brazilian berries' food markets. In summary, açaí's economic journey, from a traditional staple to global commodity, is replete with remarkable growth, especially due to the sustainable practices implemented and scientific efforts which clearly indicate the best path for innovation within Brazilian berries.
000 tons by 2021. This booming industry substantially elevated açaí’s economic significance. It is worth noting that in 2020, açaí claimed the second-largest share of plant-based food products extracted in Brazil. The economic implications of açaí have reached international borders, as evidenced by the burgeoning export market with the United States being the largest importer, followed by Germany, Belgium, and the Netherlands. This escalating demand is indicative of açaí's growing role in the global market for functional foods, nutraceuticals, and ‘superfoods’. The economic viability of açaí has extended beyond traditional consumption, with companies recognizing its potential as a natural, health-conscious offering.34 Other Brazilian berries carry the same appeal, thus having the same potential for gaining the world's attention. Further, the açaí industry has incorporated social and environmental responsibility into their production and market strategies,34 which represents the most important lesson taken, and must be applied in the development of other Brazilian berries' food markets. In summary, açaí's economic journey, from a traditional staple to global commodity, is replete with remarkable growth, especially due to the sustainable practices implemented and scientific efforts which clearly indicate the best path for innovation within Brazilian berries.
The global value chain of berries (strawberries, blackberries, raspberries, and blueberries) is undergoing intense expansion. The United States of America (USA) and China are the central players which command international trade, despite most of the plant breeding to obtain fruits with better sensory characteristics, higher productivity, and longer post-harvest life being mainly undertaken in Northern Europe. Only in the last decade, due to expansion of demand for ‘superfoods', were Chile and Mexico could integrated into the global chain with minor share, providing mostly to USA.20 The main barrier to entry in the berry industry historically has been agroclimatic conditions and funding of technologies for plant breeding and fruit preservation. The negative impacts faced by countries which entered this international market includes water and soil contamination due to intensified use of chemical fertilizers and pesticides, deforestation, land use conversion, and invasion into wildlife habitats, contributing to global climate change.16,20
Therefore, the environmental and socioeconomic benefits expected from investing in the development of Brazilian berries' production instead of the commercially available ones are vast. Brazilian berries are already adapted to agroclimatic conditions in the areas they naturally occur. This is reinforced since most Brazilian berries are endemic (Table 1), and there is a great genetic diversity available which can be used for plant breeding strategies. Moreover, starting Brazilian berry production may require less initial investment in infrastructure formation, land areas and agricultural traits. Like açaí, the extractivism is a simple start for obtaining the fruits as the raw material for commercialization in the domestic market. The development of Brazilian berries value chain will support job creation and potentialize income increase. However, best practices in production, respect for local communities and culture, providing good conditions of work, and forest protection are essential for the success of Brazilian berries.16
| Common namea | Botanical name | Family | Native biomeb | Natural occurrencec | Appearanced |
|---|---|---|---|---|---|
| a Common names are dependent on location and ethnic groups; thus, the same fruit can receive different names. b Native biome refers the ecologic characteristics where these fruits emerged. c Natural occurrence indicates the regions of Brazil where it is confirmed they naturally grow. Maps are provided by the Brazilian Flora Data Bank – REFLORA, different colors in the map indicate different Brazilian regions. d All pictures were downloaded from Wikimedia commons and have Creative Commons Licenses (CC), unless referred differently. All pictures are allowed to be shared and adapted for any purpose. Pictures were only re-shaped to fit the table. | |||||
| Pitanga | Eugenia uniflora | Myrtaceae | Atlantic Forest, Cerrado, Pampa, Caatinga |
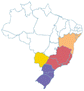 -171451206500 -171451206500 |

|
| Jabuticaba | Plinia cauliflora | Myrtaceae | Atlantic Forest, Cerrado, Pampa |
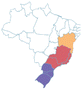 centercenter00 centercenter00 |

|
| Grumixama | Eugenia brasiliensis | Myrtaceae | Atlantic Forest |
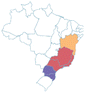 centercenter00 centercenter00 |

|
| Cherry of Rio Grande | Eugenia involucrata | Myrtaceae | Atlantic Forest, Cerrado, Pampa |
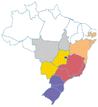 centercenter00 centercenter00 |

|
| Araçaúna | Psidium myrtoides | Myrtaceae | Atlantic Forest, Cerrado, Caatinga |
 centercenter00 centercenter00 |

|
| Camu-camu | Myrciaria dúbia | Myrtaceae | Amazon, Cerrado |
 centercenter00 centercenter00 |

|
| Guabiju | Myrcyanthes pungens | Myrtaceae | Atlantic Forest, Cerrado |
 centercenter00 centercenter00 |

|
| Capinuriba | Rubus sellowii | Rosaceae | Atlantic Forest, Pampa |
 centercenter00 centercenter00 |
 lefttop00 lefttop00 |
| Red amora | Rubus rosaefolius | Rosaceae | Atlantic Forest, Cerrado |
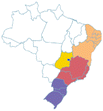 -67945000 -67945000 |

|
| Nhamburi | Rubus urticaefolius | Rosaceae | Atlantic Forest |
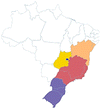 centercenter00 centercenter00 |

|
| Açaí | Euterpe oleracea | Arecaceae | Amazon |
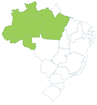 -406403619500 -406403619500 |

|
| Jussara | Euterpe edullis | Arecaceae | Atlantic Forest, Cerrado |
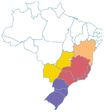
|

|
3 Brazilian berries – biodiversity, nutritive value, and bioactive compounds
3.1 Camu-camu (Myrciaria dubia)
Camu-camu is an Amazonian fruit that contains several nutrients such as carbohydrates, proteins, lipids, fibers, minerals, vitamins, essential amino acids (phenylalanine, threonine, valine and leucine) and essential fatty acids (α-linolenic acid and linoleic acid) (Table 2, 3 and 5).Furthermore, the fruit demonstrates high content of phytochemicals, such as phenolic compounds and vitamin C.35 It is estimated that camu-camu has 60 times more vitamin C than orange juice (15 mg/100 g) (Table 2).36 In relation to phenolics, more than 20 compounds have already been identified in camu-camu, specially catechin, epicatechin, quercetin, luteolin, rutin, p-coumaric, gallic acid, ellagic acid and cyanidin 3-glucoside (Table 3).37,38
| Species | Moisture | Ash | PTN | CHO | TS | LIP | Fiber | TSS | TTA | pH | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| a PTN: protein; CHO: carbohydrate; LIP: lipids; TSS: total soluble solids; TA: total titratable acidity (% citric acid). b Total carbohydrates. c Crude dietary fiber. d The data expressed in dry basis and wet basis. All the data were expressed in grams (g), except TSS (°Brix) and TTA (%). | |||||||||||
| Myrciaria dubia | 84.00–94.40 | 0.20–0.30 | 0.40–0.80 | 3.5–4.7 | 1.28–1.48 | 0.20–0.30 | 0.10–0.60 | 5.50–7.20 | 2.63–2.86 | 2.51–3.24 | 39 and 40 |
| Myrciaria dubia | — | 3.67 | 6.65 | 47.00 | — | — | 19.23 | — | — | 2.61 | 40 |
| Myrcianthes pungens | 77.11–84.29 | 0.38 | 2.14 | 11.34 | — | 0.38 | 1.46c | 11.00–17.03 | 0.02–0.22 | 4.63–5.37 | 26, 28 and 41 |
| Myrcianthes pungens | — | 2.25–2.32 | 2.47–3.23 | 52.88–81.22 | 0.82–0.84 | — | — | — | — | 26 and 28 | |
| Rubus sellowii | 82.56–83.02 | 0.63–1.02 | 0.90–0.93 | 15.10–15.31 | — | — | — | — | 2.99–3.28 | 3.06–3.11 | 42 |
| Eugenia uniflora | — | 1.47 | 4.46 | 52.00b | 33.40 | 0.30 | — | — | — | — | 43 |
| Eugenia involucrata | 91.6 | — | — | — | 4.64 | — | — | 5.66–9.53 | 0.98–15.93 | 3.42 | 44–46 |
| Eugenia involucrata | — | 5.67 | 9.97 | 62.38 | — | 3.36 | 18.61 | — | — | — | 46 |
| Eugenia brasiliensis | 82.96–90.15 | 0.36–0.60 | 0.39–0.71 | 9.07 | 7.94–9.45 | 0.02–0.72 | 1.25–5.94 | 5.00 | — | — | 44, 47–49 |
| Eugenia brasiliensis | — | 3.65 | 3.96 | 92.18 | — | 0.20 | 12.69 | — | — | — | 47 |
| Euterpe oleracea | 82.74–88.64 | 0.36–0.71 | 0.2–1.51 | — | 21.43 | 4.28–10.67 | — | 3.9 | 0.29–0.53 | 5.11–5.2 | 50–52 |
| Euterpe oleracea | — | 3.77–4.41 | 8.79–9.47 | 18.73–25.35 | — | 47.59–61–75 | 8.03–19.8 | — | — | — | 51, 53 and 54 |
| Euterpe edulis | 63.62–92 | 0.39–1.03 | 0.82–4.49 | — | 4.62 | 19.59 | — | 2.6–10.15 | 0.13–0.36 | 4.9–5.13 | 42, 55–59 |
| Euterpe edulis | — | 2.4–4.0 | 6.3–7.87 | 16.94–54.75 | 43.08 | 3.33–33.36 | 34.25–63.8 | — | — | — | 55–57, 60 and 61 |
| Eugenia cauliflora | 80.87–86.16 | — | — | — | — | — | — | 7.99–14 | 0.19–22.33 | 3.44–3.8 | 26, 46 and 62 |
| Eugenia cauliflora | — | 2.97–3.85 | 6.02–14.88 | 63.34–76.14 | — | 0.79–5.11 | 26.87 | — | 14.2 | — | 26, 46, 63 and 64 |
| Species | Ca | K | P | Mg | Na | Mn | Fe | Cu | Zn | Vit. C | Nia | Ribo | Thi | Vit. A | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a Ca: calcium; K: potassium; Mg: magnesium; Na: sodium; Mn: manganese; Fe: iron; Cu: cuprum; Zn: zinc; Vit: vitamin; Nia: niacin; Rib: riboflavin; Thi: thiamine. b The data expressed in dry basis and wet basis. All the data were expressed in milligrams (mg), except Nia, Rib, and Thi, which were expressed in micrograms (μg) and vit. A (RE 100 g−1). | |||||||||||||||
| Myrciaria dubia | 6.2–15.7 | 60–144.1 | 25.6–29.5 | 4.7–12.4 | 2.7–11.1 | 0.14–0.21 | 0.18–0.66 | 0.1 | ns | 960–2990 | 62 | 40 | 10 | 14.2–24.5 | 40 |
| Myrciaria dubia | 22.12 | 796.99 | — | 33.47 | — | — | — | — | — | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 310 310 |
— | — | — | — | 40 |
| Myrcianthes pungens | 47.46 | — | — | 8.18 | 13.56 | 0.05 | 0.44 | 0.04 | 0.23 | 117.3 | — | — | — | — | 41 |
| Myrcianthes pungens | 357.17–2276.48 | 697.37–9603.64 | — | 120.58–585.78 | 100.43–309.94 | 0.97–1.01 | 0.92–1.69 | 0.24 | 0.61–0.67 | — | — | — | — | — | 26 and 28 |
| Eugenia involucrata | 157.09 | 2102.92 | 96.39 | 110.27 | 10.13 | 1.47 | 17.65 | 0.92 | 1.45 | 125.87 | 10 | 173.33 | 20 | 623.64 | 46 and 65 |
| Eugenia brasiliensis | 29.86–323 | 84–361.01 | 1.93 | 22.57–44.64 | 1.93–117.51 | 5 | 0.32–154 | — | 0.22–21 | — | — | — | — | — | 47–49 |
| Euterpe oleracea | 46.88 | — | — | 23.32 | — | 6.4 | 0.3 | 0.18 | 0.25 | — | — | — | — | — | 66 |
| Euterpe oleracea | 524.2 | — | — | 77.54 | — | 0.065 | 8.12 | 2.71 | 1.94 | 126 | — | — | — | — | 59 and 67 |
| Euterpe edulis | — | — | — | — | — | — | — | — | — | 0.031 | — | — | — | 58 | |
| Euterpe edulis | 271.97–541.23 | 1067.70–1160.70 | 106.77 | 165.17–170.29 | 9.39–24.49 | 7.77–10.8 | 2.87–6.54 | 0.89–1.11 | 2.72–2.99 | — | — | — | — | — | 56 and 68 |
| Eugenia cauliflora | 110.18 | 4059.22 | 153.38 | 86.98 | — | 0.87 | 01.10 | 02.06 | 0.68 | 1.18 | — | — | — | — | 60 and 63 |
| Eugenia cauliflora | 80.53–330.13 | 1291.93–4533.83 | 149.99 | 107.79–455.60 | 359.80 | 3.67 | 2.37 | 0.75 | 1.57 | 0.251 | — | 23.33 | 363 | 59.47 | 26, 46 and 65 |
3.2 Nhamburi (Rubus urticaefolius)
Nhamburi is considered an acidic fruit (pH = 2.18), with a humidity of 82.60% and total soluble solids close to 8.8 °Brix (which can vary according to the maturation of the fruit) (Table 2).This fruit has significant antioxidant activity (157.54 mg Eq. Trolox/100 g fresh fruit), which is mainly due to the presence of some phenolic compounds (1452.07 mg GAE/100 g fresh fruit) in its composition (Table 5).69 Anthocyanins are compounds found in high amount in nhamburi (concentrations of total anthocyanins between 35.37 and 111.24 mg of cyanidin 3-glucoside/L of sample) (Table 5).70
Considering that information about the fruit is still limited in the literature, it would be interesting for other studies to investigate its bioactive compounds and health benefits, in addition to exploring its sensory attributes, as the fruit is well known for its acidic and sweet flavor, which is quite characteristic.
3.3 Pitanga preta (Eugenia uniflora)
Depending on the biotype and maturation it is possible to find pitanga of different colors, such as yellow, red, purple and black, which present differences in their nutritional and sensory aspects. Therefore, this study included nutritional information on the purple and black pitanga's biotypes. The fruit pulp demonstrates significant contents of carbohydrates, with emphasis on reducing sugars and total sugars, proteins and minerals (Tables 2 and 3). Furthermore, in relation to their pigments, there is a prevalence of anthocyanins (34.90 ± 1.02 mg/100 mL) compared to yellow flavonoids (4.48 ± 0.66 mg/100 mL) (Table 5),71 which is due to the fact that during pitanga's maturation, anthocyanin content increased, while flavonoid content decreased.Studies have also already demonstrated that the biotype and maturation of pitanga influence the biosynthesis of phytochemicals. Ramalho et al.72 identified hydrolysable tannins in samples of pitanga of different colors, and oenothein B (23.59–33.10 mg g−1) was the major compound presented in the purple pitanga, followed by eugeniflorin D2, O-galloyl-D-glucose and Tri-O-galloyl-β-D-glucose (Table 5).
3.4 Cherry of Rio Grande (Eugenia involucrata)
The cherry of Rio Grande presents an attractive appearance, with an intense and shiny red color, similar to the traditional cherry, with a sweet and slightly acidic flavor and succulent pulp. It is a good source of fiber, which aids in digestion and promotes satiety, and it also has a high amount of carbohydrates. The fruit stands out for its high content of potassium and calcium (Table 3).65Studies have shown that cherry of Rio Grande has a high content of phenolic compounds, such as anthocyanins, which are responsible for the intense red color of the fruit. Among the anthocyanins found in red/purple fruits, cherry of Rio Grande contains cyanidin 3-glucoside (194.82 mg/100 g DB), delphinidin 3-glucoside (577.13 mg/100 g DB), pelargonidin 3-glucoside (23.3 mg/100 g DB), and pelargonidin 3,5-diglucoside (70.3 mg/100 g DB) (Table 5). The antioxidant activity (0.163 mol Trolox equiv./kg DB) of the fruit have been associated to its phenolic and flavonoid composition, in which the most abundant compounds are rutin (48.48 mg/100 g DB) and epicatechin (24.32 mg/100 g DB).65
3.5 Grumixama (Eugenia brasiliensis)
Grumixama shows an attractive appearance with its small size and deep purplish-black color when ripe. Also, it showcases appealing sensory characteristics, valuable nutrition, and a rich content of phenolic compounds and antioxidants. Nutritionally, grumixama offers several benefits. It is a good source of dietary fiber (Table 2). Among the minerals present in the edible portion of fresh fruit, calcium, potassium, and iron stand out (Table 3).47Grumixama is rich in anthocyanins, which are responsible for its deep purple color and contribute to its antioxidant capacity.48 Studies have shown that grumixama contains a significant amount of total phenolics (up to 338.22 mg GAE/100 g) and flavonoids (0.364 mg equivalent of catechin/g WB), which contribute to its antioxidant activity. It has been found to have high levels of specific phenolic compounds, such as ellagic acid (11.59 mg/100 g WB), quercetin (3.78 mg/100 g WB), and gallic acid (41.14 mg/100 g WB) (Table 5), which are associated with health benefits, including anti-inflammatory effects.
3.6 Açai (Euterpe oleracea)
Açai is a fruit native to the Amazon region (Brazil) that exhibits distinct sensory characteristics due to its dark purple color, significant nutritional value, and high content of phenolic compounds, anthocyanins, and antioxidants. It is known for its slightly acidic and sweet flavor, with hints of red fruits and an earthy touch. From a nutritional standpoint, acai has a nutrient-rich composition. It is an excellent source of energy, primarily due to its carbohydrate content and lipids (up to 61.75 g/100 g DB) (Table 2). Additionally, it contains a considerable amount of healthy fats, such as linoleic and alpha-linolenic acids, which are beneficial for cardiovascular health.73It also contains vitamin C67 and is considered a good source of calcium and magnesium74 (Table 3). One of the most notable characteristics of acai is its high content of phenolic compounds (up to 708.22 mg GAE/100 g WB),75 anthocyanins (up to 383.23 mg of cyanidin 3-glucoside/100 g WB) (Table 5), and antioxidants (820.0 μmol Trolox equiv. g−1).76
The predominant anthocyanins in acai are cyanidin 3-glucoside and cyanidin 3-rutinoside (51.5 and 96.7 mg/100 g DB, respectively). Studies have shown that acai contains a significant amount of phenolic compounds, such as rutin (3.4 mg/100 g DB) and syringic acid (4.8 mg/100 g DB),77 which contribute to its antioxidant capacity (Table 5).
3.7 Juçara (Euterpe edulis)
The juçara is a palm tree native to the Atlantic Forest in Brazil. This species is widely appreciated for its fruit, which has a dark purple to black color, small and round, with approximately 1 cm in diameter, and a sweet to earthy flavor. Additionally, it presents nutritional properties and phenolic compounds that provide a remarkable antioxidant capacity.55Regarding its nutritional properties, juçara is an energy source due to its carbohydrate content and also has a moderate amount of lipids and fiber (Table 2). Furthermore, juçara contains interesting levels of potassium, magnesium, calcium, phosphorus, and iron (Table 3).56,68
Juçara is recognized for its high concentration of phenolic compounds, including anthocyanins (214.3–1570.6 mg of cyanidin 3-glucoside/100 g WB), flavonoids (1313.8 mg of quercetin equiv./g DB), and phenolic acids such as quercetin (0.42–102 mg/100 g DB), ellagic acid (7.13–140 mg/100 g DB), and chlorogenic acid (128 mg/100 g DB), which are known for their antioxidant properties (Table 5). The most abundant anthocyanins in the edible part of juçara fruits are cyanidin 3-glucoside (281–623 mg/100 g DB) and cyanidin-3-rutinoside (532–2307 mg/100 g DB).60
3.8 Jabuticaba (Eugenia cauliflora)
Jabuticaba is a fruit tree native to the Brazilian Atlantic Forest. This species is appreciated for its small and round fruits, with a purple skin, enclosing a gelatinous and sweet pulp that contains one or more seeds. The taste of jabuticaba is generally sweet and slightly acidic, with a pH ranging from 3.44 to 3.8. The fruit is a source of carbohydrates and fibers (Table 2).Jabuticaba is a source of vitamins, including vitamin C,60 vitamin A, and some from the B complex, such as thiamine and riboflavin (Table 3).65 Regarding minerals, jabuticaba contains high levels of potassium, phosphorus, calcium, and magnesium.63
Jabuticaba is known for its richness in phenolic compounds and its antioxidant capacity (Table 5).78 Anthocyanins are mainly present in the fruit skin, with the main compounds being cyanidin 3-glucoside, delphinidin 3-glucoside, and pelargonidin 3,5-diglucoside.39,78,79
3.9 Capinuriba (Rubus sellowii)
Little information is available regarding the nutritional composition of capinuriba. However, it is known that this blackberry has significant amounts of carbohydrates, proteins and minerals (Tables 2 and 3), in addition to demonstrating a high acidity.80Given the limited information available in the literature, it is suggested that studies be carried out to investigate the nutritional composition of the fruit, in addition to identifying possible compounds of interest to human health (e.g., phenolic compounds, anthocyanins, and vitamins). Furthermore, it would be relevant to highlight the use of fruit in the preparation of new food products, suggesting new ways of consuming it.
3.10 Guabiju (Myrcianthes pungens)
Guabiju is a fruit with a pleasant taste (ratio = 77.74–83.99), which has relevant contents of carbohydrates, proteins, lipids, vitamin C and minerals (Tables 2 and 3). The fruit contains significant amounts of total anthocyanins, with higher levels than other berries such as red pitanga and acerola.28 An expressive bioactive potential was also identified in the fruit due to its bioactive compounds. It was observed that guabiju's fruits in the intermediate stage of maturation present higher antioxidant capacity compared to the ripe stage.Phenolic compounds are one of the most prominent antioxidant substances present in guabiju (Table 5), and they have already been identified in fruit mainly gallic acid, quercetin, and isoquercitrin, followed by 3,4-dihydroxybenzoic acid, kaempferol, ferulic acid, p-coumaric acid and syringic acid.26
4 Emergent strategies in the development of healthy products from berries
As introduced, Brazilian berries are recognized as a bioactive compounds-rich food source with potential health benefits. Several parameters related to the use of Brazilian berries, such as high nutritional value, phytochemical composition, pleasant taste, and fruit perishability, have encouraged the industrial processing of these fruits in the development of new products. Brazilian berries' extracts can also be used for new formulations or to replace synthetic components.However, the use of Brazilian berries for the development of new products by the food industry is still limited due to the low supply of berries and the lack of scientific knowledge about sustainable processing strategies for using these fruits. In this way, new alternatives have been directed toward the development of food supplements, functional foods, nutraceuticals, and functional components from Brazilian berries.
In this context, emerging technologies have been presented as an option for the sustainable development of new products from Brazilian berries. Among the main emerging technologies applied, we can mention ultrasound, high hydrostatic pressure (HPP), supercritical fluid extraction (SFE), pressurized liquid extraction (PLE), dielectric barrier atmospheric cold plasma, microwave, and ohmic heating. Emerging technologies are an innovative and efficient technique for achieving a high yield of extraction beyond sustainable eco-friendly chemistry.81 However, some of these green technologies can show some disadvantages, such as the problem of separation, hot spots, need of know-how and difficulty of operation.82
Several studies on emerging technologies have been carried out for the development of new products from different berries, such as jabuticaba (Myrciaria jabuticaba), açaí (Euterpe oleracea), camu-camu (Myrciaria dubia), and juçara (Euterpe edulis Mart.), among others, as you can see below.
The effect of high-intensity ultrasound (HIUS) technology on jabuticaba peels instead of the conventional extraction process resulted in the higher recovery and antioxidant potential of bioactive compounds. In addition, the exhaustion of bioactive compounds in the dried jabuticaba (Myrciaria jabuticaba (Vell.) Berg) peel after the HIUS processing at an ultrasound intensity of 3.7 W cm−2 and 50 g water/100 g proved the high recovery of target compounds.83
Anthocyanins and total phenolic compounds from açai obtained by pressurized liquid were applied to commercial products: freeze-dried açai, açai juice, an açai tablet, and an açai jam.84 After the application, high concentrations of total phenolic compounds were found in the lyophilized açai samples (12.25 mg total phenolics/g açai), while considerable amounts were found in açai juice (2.25 mg g−1), followed by juices (2.15 mg g−1), tablets (2.02 mg g−1), and jam (1.25 mg g−1).
Dielectric barrier atmospheric cold plasma is another newly emerging technology with a positive effect on food application studies.85 An interesting outcome from the use of this new technology was reported in a study with camu-camu juice, in which it improved the nutritional quality due to the increase of phenolic compound content, antioxidant activity, and inactivation of endogenous enzymes (peroxidase and polyphenol oxidase). The increase in excitation frequencies did not degrade the phenolic compounds due to the use of cold plasma and also increased the bioaccessibility and bioavailability of these compounds, thus improving consumer health.
The combination of emerging technologies has also been shown as an option to improve the quality and nutritional composition of food products. For instance, the effect of high hydrostatic pressure and thermal pasteurization on anthocyanins and non-anthocyanin phenolic compounds content of açaí juice (Euterpe oleracea) has been investigated. The HPP at 500 MPa/5 min/20 °C was 40% more effective for the preservation of target compounds than thermal pasteurization (85 °C/1 min) may be due to its thermal sensitivity.86 The high temperature of the pasteurization processing may have caused the degradation of compounds in açaí juice and decreased the compound's recovery. HPP stands out as a widespread non-thermal process for food preservation providing additive-free products and foods with minimal loss of nutritional, functional, and organoleptic properties, and maintenance of quality of the final product.87
The combined use of conventional techniques (conventional heating) and emerging technologies (ultrasound-assisted) was applied to camu-camu nectar processing to evaluate quality parameters, the nutritional value of the nectars, and the inactivation of enzymatic activity.88 The thermosonication resulted in a nectar rich in bioactive compounds and reduced action of peroxidase and polyphenol oxidase enzymes on the end product, improving the quality of food.
Along with the use as an extraction technology, the combination of ultrasound-assisted extraction (UAE) followed by the concentration of samples by reverse osmosis was used in order to obtain a functional camu-camu (Myrciaria dubia) product.37 As result, a concentrated camu-camu with 3.2 times higher phenolic compounds and 6.5 times higher anthocyanins was obtained. Furthermore, it provided high antioxidant activity and vitamin C content. The synergism of these techniques turned out to be a promising alternative in order to apply bioactive compounds in food and in the possible development of nutraceutical products from berries.
An anthocyanin-rich extract from the juçara pulp (Euterpe edulis Mart.), microencapsulated with maltodextrin was used in the preparation of formulations of fermented milk. The authors showed that microencapsulation with maltodextrin provided greater stability of the product's red color and better encapsulation efficiency, especially when compared to food without microencapsulated extract. In addition, the sensory analysis of the fermented milk showed high scores for all evaluated attributes, such as taste, flavor, texture, and buy intention, suggesting to the evaluated consumers that the anthocyanin microencapsulated from juçara pulp can be successfully used to formulate fermented milk. This can contribute to the development of innovative food products with more stability and the possibility of good commercialization.89
In another approach to new product development, there is a high demand for food and pharmaceutical products with healthier formulations enriched with natural compounds to replace synthetic additives through the use of sustainable eco-friendly processing. In this sense, a recent study evaluated the stability of the microencapsulated jabuticaba Myrciaria cauliflora extracts and their application in manioc starch fermented biscuits processed at 180 °C for 20 min.90 In this work, the microencapsulation provided greater stability and higher content of polyphenols and antioxidant potential in the fermented cassava starch, thus protecting their compounds after thermal processing. Therefore, jabuticaba extracts are good alternatives for microencapsulation by softening the effects of heat applied in biscuit baking.
From a general point of view, the benefits of emerging technologies presented above can be summarized in (1) high extraction yield, (2) improving process time and control extraction (3) enhancement of safe and food quality (high antioxidant activity, phenolic content, and improved nutritional/sensory properties), (4) use of mild process condition – improvement of environmental sustainability of the entire process (lower energy input, low consumption and use of eco-friendly solvent) and/or (5) reduction of costs. Therefore, our study showed that emerging technologies turned out to be a promising way to the development of new products from berries as simple, eco-friendly, and relatively fast technology that may be used by the food industry to produce new berries-based formulations with high market value.
5 Use of Brazilian berry by-products in food innovation
Food production is the main driver of global environmental change, as agriculture occupies about 40% of the world's land area and food production is responsible for up to 30% of global greenhouse gas emissions and 70% of freshwater use.91 However, many studies point to the opportunity to increase company profits by using berries as an ingredient, thus contributing to food innovation.The use of waste can improve economic performance, optimize the use of resources, and is associated with a lower environmental impact, and 90% of an organization's environmental expenditures can be caused by waste-related costs.92
South America is one of the largest agricultural producers, with Brazil and Argentina as the main producers.93 For this, there is a large amount of food by-products generated by the industry during processing, and this indicates an important environmental problem. However, it is already known that the waste generated, such as peels and seeds, can be functional food ingredients or used for pharmaceutical applications, for the prevention or treatment of human diseases due to the action of different active compounds.94,95 Recent studies have shown that berries have antioxidant compounds, which are mostly concentrated in the peels and seeds.96
Jabuticaba has been used to produce juice, jam, syrup, liqueur, and fermented beverages, among others (Table 4). To produce jabuticaba-based products, the fruit peels and seeds are discarded, which represent 40% to 50% of the fruit weight. This residue has a high content of phenolic compounds, suggesting, therefore, their potential use for the development of natural colorants, functional ingredients, and food supplements, which could be used in the food and pharmaceutical industries.97,98
| Myrciaria dubia | Myrcianthes pungens | E. uniflora | Eugenia involucrata | Eugenia brasiliensis | Euterpe oleracea | Euterpe edulis | Eugenia cauliflora | |
|---|---|---|---|---|---|---|---|---|
| a Wet basis. b Dry basis; TMA: total monomeric anthocyanins; TPC: total phenolic compounds; TF: total flavonoids. c mg of cyanidin-3-glucoside/100 g. d mg of gallic acid equiv. (GAE) g−1. e mg tannic acid equiv. g−1. f mg of quercetin equiv. (QE) g−1. g mg rutin equiv. g−1. h mg equiv. of catechin per g. i mg of gallic acid equiv. (GAE)/100 g. The data of compounds were expressed in mg/100 g. | ||||||||
| TMA | 10.22–66.16a,c | 64.57–245.31b,c | 0.49–7.03b,c | 50.97–158.4a,c | 1.54–518.64a,c | 46.12–383.23a,c | 214.3–1570.6a,c | 28.9–109.23a,c |
| 669.8b,c | 159.03–1020b,c | 33.03–1154.0b,c | 930.56–2400b,c | |||||
| TPC | 7.79–25.79a,d | 28.41–59.34b,d | 25.02–27.87b,e | 136.83–757.6a,i | 207.83–338.22a,i | 44.31–708.22a,i | 415.1–4918.5a,i | 395.88–1696.13a,i |
| 250.89b,i | 2558.37–5434.3b,i | 1237.73–8840b,i | 13.55–1323.8b,i | |||||
| Tannins | — | — | 10.78–13.94b,e | — | 7.97a,e | — | — | 0.025a,f |
| 46.78b,e | ||||||||
| TF | 6.90–28.37a,f | 22.78–33.84b,f | 0.14–0.31b,g | 0.27–206.06a,f | 0.364a,h | 1.97a,f | 1313.8b,f | 7.984a,f |
| 95.48b,f | 0.21–6.39b,f | |||||||
| Cyanidin-3-glucoside | 0.62–2.78a | — | — | 194.82b | 51.5b | 71.4a | 107.9–725b | |
| 281–623b | ||||||||
| Delphinidin-3-glucoside | — | — | — | 577.13b | — | — | — | 41.9–1364b |
| Pelargonidin-3-glucoside | — | — | — | 23.3b | — | — | 0.6b | — |
| Pelargonidin-3,5-diglucoside | — | — | — | 70.3b | — | — | — | 109b |
| Cyanidin-3-rutinoside | — | — | — | — | 96.7b | 191.0a | — | |
| 532–2307b | ||||||||
| p-coumaric acid | 0.06–0.15a | 0.11–0.17b | — | — | 0.078a | — | 0.81b | 0.36b |
| Rutin | 2.44–9.78a | — | — | 39.4a | — | 0.4a | 0.15–31.72b | — |
| 48.48b | 3.4b | |||||||
| Gallic acid | 22.72–97.29a | 4.37–7.78b | — | — | 41.14a | — | 0.25b | 17.47a |
| 4.16–127b | ||||||||
| Caffeic acid | — | 0.02–0.04b | — | — | — | — | — | 0.26b |
| Chlorogenic acid | — | 0.01b | — | — | — | — | 128b | 0.194b |
| 3,4-Dihydroxybenzoic acid | — | 0.08–0.12b | — | — | — | — | — | — |
| Ferulic acid | — | 0.12b | — | — | — | — | 4.1b | 0.199b |
| Salicylic acid | — | 0.02b | — | — | — | — | — | — |
| Sinapic acid | — | — | 0.01–0.02b | — | — | — | — | — |
| Syringic acid | — | 0.34–0.50b | — | — | 0.016a | 0.6a | 2.96b | 0.097b |
| 0.74–4.8b | ||||||||
| Myricetin | 0.20–0.30a | — | — | 6.06a | 3.99a | — | 66b | 0.9–1.2b |
| Kaempferol | — | 0.09–1.07b | — | — | 0.19a | — | 0.62–44b | 0.033b |
| Luteolin | 77.22b | — | — | — | — | 0.1a | 37.6a | 0.005–5b |
| 0.9b | ||||||||
| Pinobanksin | — | 0.33–0.47 | — | — | — | — | — | — |
| Quercetin | 8.55–26.45b | 7.88–8.24b | — | 18.18a | 3.78a | — | 0.42–102b | 1.1–5.21b |
| Isoquercitrin | — | 1.41–2.16b | — | — | 7.96a | — | — | — |
| Isorhamnetin | — | 0.08–0.10b | — | — | — | — | — | — |
| Epicatechin | 6.53–292.83b | 0.01–0.03b | — | 15.76a | 0.012a | — | 0.06–0.36b | 5.3b |
| 24.32b | ||||||||
| Ellagic acid | — | — | — | 9.09a | 11.59a | — | 7.13–140b | 0.987a |
| 15.4–229b | ||||||||
| Epigallocatechin gallate | — | 0.97–1.57b | — | — | — | — | — | |
| Catechin | 1108.49–2269.30b | 0.19–0.48b | — | 6.06a | 0.83a | — | 0.35–1.78b | 0.82a |
| 16.985b | 9.3b | |||||||
| Vanillic acid | — | — | — | — | — | — | 9.43a | — |
| Protocatechuic | — | — | — | — | — | — | 0.066–14.58b | — |
| Aromadendrin | — | — | — | — | — | — | 15.09b | — |
| 4-Hydroxybenzoic acid | — | — | — | — | — | — | — | 1.29–15.4b |
| Ref. | 38 and 39 | 26 and 41 | 72 | 45, 46, 65, 103–105 | 47–49 and 75 | 52, 53, 66, 67, 75, 77 and 106 | 57, 58, 60, 68, 107–112 | 26, 39, 60, 64, 65, 75, 78 and 113 |
The investment in these products incentivizes research on sustainable technological processes that maintain not only the sensory characteristics but also the phenolic compounds of the fruit.114–116 Therefore, it is important to analyze new alternatives to reduce the environmental impact and add value to the by-products, such as direct incorporation into foods (yogurt, cookies).115
Besides jabuticaba, açai has great economic, nutritional, and cultural value for the region of Manaus and Pará in Brazil. This fruit has been exploited in many ways and generated products such as juices, ice cream, sweets, and food supplements (Table 4). Several studies have been showing that açai has important biological activities, such as antioxidant activity, anti-inflammatory, protection against hepatic steatosis and fibrosis, and neuroprotectors, among other benefits. The products generated are sold both in national and international markets. This productive growth has generated income, employment, and new business, and stimulated research and innovation.117
However, the large production of this fruit has also led to some negative environmental and social consequences related to the increase in the accumulation of waste as seeds, after the pulping of the fruit, thus affecting the environment and society in urban areas. The accumulation of waste can cause damage to the advancement of a sustainable economy in the Amazon region, generating environmental fines, and economic instability.117
There are attempts to use waste, but they are not yet solutions with a logistical and operational capacity capable of having efficient results. Some applications that have been explored are the use of waste to generate energy, products with added value, the development of catalysts, the production of activated charcoal, and products for civil construction, among others.117
In the Amazon rainforest, it is very common to find the fruit camu-camu, also known as caçari and araça d'água. This fruit is known to be a rich source of different active compounds which is related to their biological activities (see in Section 3.1).118 Due to its acidic taste, camu-camu is not consumed in nature, so it is used in the form of juices, purees and especially pulp, applied in the production of beverages and as a food ingredient (Table 4). As with other fruits, industrial processing generates by-products that can harm the environment. About 40% of the weight of the fruit is composed of peel and seed, so in recent years, research has been demonstrating ways to reduce the environmental impact and economically exploit these by-products.118
In the search for new sources of bioactive compounds, some researchers have focused on juçara because it is a fruit rich in bioactive compounds, mainly anthocyanins.119 In the industry, the pulp is separated from the peel through processing, generating residues of the peel and seed, but the anthocyanins, one of the main compounds of this fruit are in the peel. Therefore, the by-products generated from these fruits are rich sources of bioactive compounds which can be used as raw materials of low cost and high added value.120
The residues of grumixama have high contents of dietary fiber, magnesium, phenolic compounds, tannins and carotenoids, in addition to antioxidant potential; therefore, they have also been reported as raw materials for industrial use, such as natural dye extraction.121
Among the berries that are processed by the national agribusiness is the blackberry (Rubus sp.), which is a source of phenolic compounds, mainly anthocyanins and ellagic acid, and a powerful antioxidant, being beneficial for preventing degenerative diseases, and among the minerals are mainly calcium and potassium, besides vitamin C. Despite this, due to its fragile structure and seasonality, its consumption in nature is not very common. Therefore, this fruit is used to make products such as juice, jam, pulp, and others. However, with the processing of pulp, 30 to 40% is discarded, and this waste is a source of bioactive compounds important for physiological functions and the prevention of chronic diseases.96
Therefore, due to the high added value of by-products and to reduce the negative environmental impact they cause when they are discarded irregularly, more research is needed on the sustainable use of by-products rich in bioactive compounds by the food and pharmaceutical industries.
6 Potential health benefits of products obtained from Brazilian berries
Berries native to Brazil have already gained space in the literature works related to chronic non-communicable diseases (CNCDs) due to the fact that they are fruits that have compounds in their composition that contribute both to the management and prevention of these diseases. The following section will present recent works that investigate the potential of these berries in CNCDs. Table 6 presents the main in vitro, in vivo, and preclinical studies with different Brazilian native berries and their respective potential against CNCDs.| Berries | CNCDs | Assay/dose | Main results | Ref. |
|---|---|---|---|---|
| a ROS – reactive oxygen species. b AuNPs – synthesis of cdcgold nanoparticles. c bw – body weight. d LJE – Lyophilized Jabuticaba Extract. e YE – yoghurt extract. f BAT – brown adipose tissue. g BWG – body weight gain. h WAT – white adipose tissue. i LDL – low density lipoprotein. j LDL – total cholesterol. k TAG – triacylglycerol. l HDL – high density lipoprotein. m FFA – free fatty acid. n GAE – gallic acid equiv. o PPE – purple pitanga fruit. | ||||
| Açaí (Euterpe oleracea) | Cancer | In vitro açaí seed extract at concentrations of 0.25 μg mL−1–250 μg mL−1 | Cytotoxic effects against MCF-7 | 44 |
| Morphological changes in cell lineage by autophagy | ||||
| Increase in the production of ROSa | ||||
| Açaí (Euterpe oleracea) | Cancer | In vitro açaí powder diluted in PBS buffer at concentrations of 100, 40, and 10 mg mL−1 | Potent antitumor for the proliferation of head and neck tumor lines (SCC9) | 122 |
| Açaí (Euterpe oleracea) | Cancer | In vitro açaí dry extract (0–200 mg mL−1) and AuNPsb (0–0.4 mg mL−1) | AuNPs effective in killing prostate and pancreas cancer cells | 123 |
| High concentrations of açaí dry extract showed cytotoxic effect on PC-3 cells | ||||
| Açaí (Euterpe oleracea) | Cancer | In vitro açaí oil at concentrations of 30 mg kg−1, 100 mg kg−1 or 300 mg kg−1 bwc | Liver: disorganization of liver tissue. Proliferation of Kupffer cells | 124 |
| Thyroid: alterations in the size of the follicular lumen and in the connective tissue between the follicles | ||||
| Açaí (Euterpe oleracea) | Obesity, dyslipidemia | In vivo 150 mg kg−1 | ↓ Body weight and lipid accumulation in the adipose tissue | 125 |
| ↓ Lipogenesis with a ↑ on fatty acid oxidation | ||||
| Change the structure of the gut microbiota (↑ Akkermansia muciniphila) | ||||
| Açaí (Euterpe oleracea Mart.) | Obesity, diabetes, dyslipidemia | In vivo 2.5 mL of the reconstituted concentrated extract in 50 mL water | ↓ Of ghrelin hormone (satiety effects) | 126 |
| ↓ in weight and glycemic index | ||||
| Significant improvement in the triglycerides levels | ||||
| Açaí (Euterpe oleracea Mart.) | Diabetes | In vitro: 500, 100, and 50 μg mL−1 | In vitro: suppressing inflammatory mediator signaling and, consequently, ↓ proliferation and oxidative stress induced by high glucose | 127 |
| In vivo: 200 mg kg−1 per day | In vivo: ↓ oxidative stress, improvement in kidney function, with less glycosidic degeneration in renal tissue | |||
| Açaí (Euterpe oleracea Mart.) | Cardiovascular | In vivo 2% and 5% of açaí pulp | Attenuated cardiac remodeling after acute myocardial infarction due to morphological changes and functional alterations | 128 |
| Açaí (Euterpe oleracea Mart.) | Cardiovascular | In vivo 300 mg kg−1 per day of açaí seed extract | Reduced oxidative damage in the aorta | 129 |
| Antioxidant effect with increased expression of Nrf2 (aorta and heart) and SIRT-1 (heart) | ||||
| Jabuticaba (Myrciaria jaboticaba (Vell.) O. Berg.) | Cancer | In vitro: LJEd (177 μg mL−1) | ↓ Aberrant crypt foci | 130 |
| In vivo: YEe (4% JEe) (1, 5, and 10 mL kg−1 bwc) | ↓ Proinflammatory parameters. ↓ RNA expression of anti-apoptotic cytokines | |||
| ↑ Expression of pro-apoptotic cytokines | ||||
| Modulate gut microbiota | ||||
| Jabuticaba (Myrciaria jaboticaba (Vell.) O. Berg.) | Cancer | In vitro: LJEd (10–100 μg mL−1) 0, 0.10, 0.20, 0.30, and 0.40 g/100 g of YEe | In vitro: cytotoxic effects against cancer cells | 130 |
| In vivo: 10 mL kg−1 bw | Anti-hemolytic effects | |||
| In vivo: modulation of the intestinal bacterial microbiota | ||||
| 5 varieties of jabuticaba | Cancer | In vitro peel extract (2, 5, 25, 50, and 250 μg mL−1) | All varieties showed a decrease in the cell proliferation of tumor cells | 131 |
| Jabuticaba (Myrciaria jaboticaba) | Obesity | In vivo 5%, 10%, or 15% (w/w) of peel and seed powder | Improved pro-inflammatory response associated with obesity | 114 |
| Prevented the progression of weight gain and fat accumulation | ||||
| Jabuticaba (Myrciaria jaboticaba) | Obesity | In vivo 5%, 10%, or 15% (w/w) of peel and seed powder | Increased OPA1 expression related to the process of lipolysis and thermogenesis | 132 |
| Increased capacity and maintenance of BATf functionality | ||||
| Reduction in the size of adipocytes | ||||
| Jabuticaba-sabará (Plinia jabuticaba (Vell.) Berg) | Obesity; diabetes; dyslipidemia | In vivo 50 and 100 mg GAE2/kg bw | Prevented BWGg and excessive gain of WATh | 133 |
| ↓ Adipocyte hyperplasia and inflammation | ||||
| ↓ GLUT4 expression and modulation of the Akt/mTORC pathway | ||||
| ↓ LDLi and TCj (plasma levels)/TCj and TAGk (hepatic levels) | ||||
| Jabuticaba (Plinia cauliflora (Mart.) Kausel) | Diabetes; dyslipidemia | In vivo freeze-dried peel: 200 mg kg−1 per day and 400 mg kg−1 per day | Extract administration showed decrease in blood glucose levels in a dose-dependent manner (↑ dose = ↑ effect) | 134 |
| ↓ Concentration in cholesterol and ↑ concentration in HDLl-c levels | ||||
| Juçara (Euterpe edulis) | Cancer | In vivo 0.3% solution of fruit pulp | ↓ Number of lesions in the colorectal mucosa | 135 |
| ↑ Production of antioxidant enzymes | ||||
| Juçara (Euterpe edulis) | Obesity | Double-blind randomized controlled trial 5 g of juçara pulp powder | Metabolism modulation. (↓ body fat and ↑ A/H ratio, HDLl-c, and adiponectin concentrations) | 136 |
| Juçara (Euterpe edulis) | Obesity; diabetes | In vivo 5 g of juçara freeze-dried powder in 1 kg of diet | ↓ In body weight gain and ↑ energy expenditure | 137 |
| Improves insulin resistance and glucose intolerance | ||||
| Camu-camu (Myrciaria dubia) | Obesity | In vivo 1 g kg−1 per day of hydroalcoholic extract | ↓ Glycemia, cholesterol, and triglycerides | 138 |
| Regulated injury and inflammatory response induced by excess adipose cells | ||||
| Camu-camu (Myrciaria dubia) | Diabetes; obesity | In vivo 200 mg kg−1 | ↓ Fat accumulation | 139 |
| ↓ Metabolic inflammation and endotoxemia | ||||
| Changes in the gut microbiota (↑ Akkermansia muciniphila and ↓ Lactobacillus) | ||||
| Improved glucose homeostasis and insulin sensitivity | ||||
| Camu-camu (Myrciaria dubia) | Obesity; dyslipidemia; diabetes | In vivo 62.5 mg kg−1 per day and 200 mg kg−1 per day | Low dose: ↓ non-HDLl cholesterol and FFAm | 140 |
| ↑ Akkermansia mucinphila | ||||
| High dose: prevented excessive body weight gain and fat mass gain. ↓ peak blood glucose after T + 15 min | ||||
| Camu-camu (Myrciaria dubia (Kunth) McVaugh) | Diabetes | In vivo 0.5 mL to 2000 mg kg−1 of aqueous extract | Aqueous extract at doses of 100 and 500 mg kg−1 showed antidiabetic effects | 141 |
| At a dose of 1000 mg kg−1, the effect of the extract is similar to the negative control (healthy) | ||||
| Camu-camu (Myrciaria dubia) | Diabetes | In vitro 1 g of freeze-dried powder to 100 mL of distilled water | The freeze-dried fruit showed α-amylase and α-glucosidase inhibitory activity, indicating antidiabetic properties | 142 |
| Camu-camu (Myrciaria dubia) | Cardiovascular; hypertension | In vivo 75 mg encapsulated lyophilized powder (1 capsule = 25 mg) | Flow-mediated vasodilation showed a change in arterial diameter in participants who received camu-camu | 143 |
| An improvement in blood pressure was observed in the participants after the administration of the fruit | ||||
| Pitanga (Eugenia uniflora L.) | Dyslipidemia | In vivo 0.76, 1.52, 2.28, and 3.8 mg mL−1 of GAEn for liposomes containing PPEo | Liposomes containing extract of purple pitanga attenuated the levels of total lipids and triacylglycerides | 144 |
| Pitanga (Eugenia uniflora L.) | Diabetes | In vivo 0.06 g of the leaf/100 mL of water | Chronic treatment with the aqueous extract decreased the incidence of diabetes | 145 |
| Decrease infiltration of immune response cells in the pancreatic islets | ||||
| Cereja do Rio Grande (Eugenia involucrate) | Cancer | In vitro raw seed extract or raw leaf extract | Cytotoxic activity against tumor cells | 146 |
| Inhibition of cell migration (antimetastatic activity) | ||||
6.1 Açaí (Euterpe oleracea)
Açaí has gained great space in the literature through works that use it to improve CNCDs. Cancer is one of the most researched diseases in the world and this fruit has already demonstrated benefits against various mechanisms of this disease, such as a positive cytoprotective action for estrogen receptors (MCF-7), chemopreventive potential, effective for cytotoxic effects in breast cancer cell lines, induces autophagy and morphological changes in treated cells, increase mitochondria production after 24 hours of treatment and reduction of tumor cells (Table 6).147–150Besides cancer, açaí has also been effective in managing obesity, through a positive influence against pro-inflammatory biomarkers, in addition to also being effective against metabolic diseases, such as diabetes, dyslipidemias, inflammation, oxidative stress and hypertension.33
Some of the benefits found from açaí against metabolic disorders are the ability of glucose tolerance, insulin resistance, control of lipid metabolism, decreased protein expression of genes involved in lipogenesis and increased expression of transport proteins involved in the excretion of lipids. Extracts with part of the fruit were also effective in preventing the development of hypertension and endothelial dysfunction.74,151
Since açaí presents a rich composition of flavonoids, highlighting the anthocyanins, a work conducted by151 through a clinical trial identified that the fruit improved vascular function in overweight individuals, with the hypothesis that its compounds can reduce the activity of NADP(H) oxidase which is linked to an increase in the concentration of nitric oxide through inhibition of superoxide.
6.2 Jabuticaba (Myrciaria cauliflora Berg., Plinia cauliflora, Myrciaria jabuticaba)
Several species of jabuticaba (e.g., Myrciaria cauliflora Berg., Plinia cauliflora and Myrciaria jabuticaba) have also been identified as potential berries against CNCDs. In works carried out on cancer, it has already been identified that this fruit contributes to antitumor effects in different cell lines of cancer cells, as well as in the inhibition of proteins involved in cancer proliferation, cell survival, adhesion, angiogenesis and chemopreventive effect (Table 6).115,150,152It also has positive advantages against obesity by preventing weight gain and adiposity, and in diabetes by being able to modulate the intestinal microbiota of animals and prevent insulin resistance via inflammatory signaling by LPS/TLR4.132,133,153,154
6.3 Juçara (Euterpe edulis) and camu-camu (Myrciaria dubia)
Juçara are berries that can have some benefits for CNCDs, despite presenting few works that use this fruit. The compounds present in juçara showed a potential antioxidant capacity related to cancer, in addition to being significantly effective in reducing body fat and modulating metabolism. Studies also show the importance of juçara in the control of systemic arterial hypertension, showing significant effects in improving baroreflex sensitivity and reducing blood pressure (Table 6).155–157Camu-camu (Myrciaria dubia) presented compounds in its composition that were effective in reducing inflammatory markers, oxidative stress and efficacy in modulating enzymatic activity, and inhibiting α-amylase and α-glucosidase.11,158,159
6.4 Grumixama cherry of Rio Grande and pitanga
On the other hand, while some native Brazilian berries already have well-established works in the literature, others still need to be studied for their effectiveness in CNCDs, for example, grumixama, cherry of Rio Grande, pitanga and some varieties of amora (blackberry) that are native to Brazil.Work on cherry of Rio Grande demonstrated its effectiveness as an antitumor agent involving a heterogeneous and complex mechanism related to proliferation, oxidative stress and cytoskeletal architecture of tumor cells and has also presented promising activity for the management of diabetes through the inhibitory effect of the enzymes α-glucosidase and acetylcholinesterase (Table 6).103,160
Pitanga also presented compounds in its composition that are effective in inhibiting α-amylase and extracted nanoparticles from this fruit have already been identified as potential cholesterol reducers.161,162
7 Final considerations and future research directions
The berries and their residues, such as peels and seeds, are a rich source of bioactive compounds with several proven bioactivities. Bioactive compounds from both berries and their by-products can be extracted using emerging technologies that have a low negative impact on the environment. The compounds of interest can be used as dietary supplements, natural dyes, and functional ingredients by the food industry as well as by the pharmaceutical industry in the development of medicines, foodstuffs, and other pharmaceutical products.Despite the idea that exploring biodiversity from remaining forests in the food industry can be harmful to the environment, the overwhelming pressure for lands from large-scale monoculture and livestock is literally destroying forests and their biodiversity. There is no scientific evidence that this kind of large-scale food system model can help to save the world from hunger, malnutrition, and NCDs, in fact, it leads to several damages, including the increase in NCDs. In contrast, we showed that taking a profit from Brazilian berries in the food industry has the potential to develop income locally while protecting biodiversity.
The emergent sustainable strategies for processing Brazilian berries can provide safe and healthy new products bringing new flavors to the market. Moreover, regularly including intake of Brazilian berries and their products has the potential to improve quality of life, helping to fight the burden of NCDs. Therefore, it is fundamental to promote the shift of mindset from ‘untouched nature is protected biodiversity’ to the idea that sustainable usage of biodiversity, in this case Brazilian berries, can help protect both people and the environment. In this way, promoting Brazilian berry consumption and investing in food science and technology for developing innovative, healthy, and sustainable products are smart strategies for reaching SDGs. Also, these actions will increase the demand for growing Brazilian berries, which requires much less efforts than adapting foreign berries to a tropical climate.
The evidence shown in this review indicates the potential products, process, health benefits and sustainable practices which can be applied as strategy actions for meeting SDGs, mainly SDG 2 (Zero Hunger), SDG 3 (Good health and well-being), SDG 9 (Industry, Innovation and Infrastructure) and SDG 12 (Responsible Consumption and Production). On this matter, much has been done with açai fruit, which is now in the phase of increasing exports and innovation of products. The key for the success of açai fruit and its products is the combination of investment on discovering its health benefits and the wide disclosure of the benefits for a healthier and sustainable life.
The future perspectives for other Brazilian berries following the same path – research, technology, and scientific dissemination – can be remarkable. However, it is important to point that the population connected within the forest and the local farmers will not start commercially cultivating these fruits before increasing the demand and the reliable indications of a fair trade. Additionally, sustainable practices should be adopted in the whole production chain to guarantee the farmers and consumers that the Brazilian biodiversity is being protected. Also, the health benefits which are being discovered about Brazilian berries need further investment, mainly for clinical studies and process innovation, to confirm health claims and guarantee that they are safe and effective products for consumers.
Conflicts of interest
The authors in this manuscript have no conflicts of interest.Acknowledgements
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001; CNPq (403976/2021-9; 301496/2019-6) and FAPESP [2022/09493-9]. The author Paulo Berni thank FAPESP [2021/02271-8] and [2022/09493-9] for the financial support. The author Lais Ramalho Zandoná (140880/2022-4) thanks CNPq for the financial support. The author Angélica Aparecida de Toledo (88887.828742/2023-00) thanks Capes for the financial support.References
- UN, Transforming Our World: the 2030 Agenda for Sustainable Development Transforming Our World: the 2030 Agenda for Sustainable Development Preamble, United Nations, 2015, 70(1) Search PubMed.
- J. Gomes-da-Silva, F. L. R. Filardi, M. R. V. Barbosa, J. F. A. Baumgratz, C. E. M. Bicudo, T. B. Cavalcanti, M. A. N. Coelho, A. F. Costa, D. P. Costa, E. C. Dalcin, P. Labiak, H. C. Lima, L. G. Lohmann, L. C. Maia, V. F. Mansano and A. R. Menezes, Brazilian Flora 2020: Leveraging the power of a collaborative scientific network, Taxon, 2022, 71(1), 178–198 CrossRef.
- WHO, Connecting Global Priorities: Biodiversity and Human Health A State of Knowledge Review, Who/UNEP/Convention on Biological Diversity, 2015, vol. 1 Search PubMed.
- C. P. Bocchi, É. de Souza Magalhães, L. Rahal, P. Gentil and R. de Sá Gonçalves, The nutrition decade, the public policy for food security, and public purchases from family farming in Brazil, Pan Am. J. Public Health, 2019, 43, 1–5 CrossRef.
- FAO/WHO, Proceedings of the FAO/WHO International Symposium on Sustainable Food Systems for Healthy Diets and Improved Nutrition, 2018 Search PubMed.
- D. Hunter, B. Burlingame and R. Remans, Chapter 6: Biodiversity and nutrition, in Connecting Global Priorities: Biodiversity and Human Health A State of Knowledge Review, WHO/UNEP/Convention on biological diversity, 2015, Vol. 1, p. 364 Search PubMed.
- A. M. Jackson-Morris, R. Nugent, J. Ralston, O. Barata Cavalcante and J. Wilding, Strengthening resistance to the COVID-19 pandemic and fostering future resilience requires concerted action on obesity, Global Health Action, 2020, 13(1), 1–4 CrossRef PubMed.
- R. Flores-Ortiz, D. C. Malta and G. Velasquez-Melendez, Adult body weight trends in 27 urban populations of Brazil from 2006 to 2016: A population-based study, PLoS One, 2019, 14(3), 1–18 CrossRef PubMed.
- I. Koss-Mikołajczyk, B. Kusznierewicz and A. Bartoszek, The Relationship between Phytochemical Composition and Biological Activities of Differently Pigmented Varieties of Berry Fruits; Comparison between Embedded in Food Matrix and Isolated Anthocyanins, Foods, 2019, 8, 646 CrossRef PubMed.
- C. A. B. Freitas, R. C. S. Müller, W. M. O. Nascimento, M. O. Lima, K. C. F. do Faial and A. S. Lopes, Multivariate Analysis of Mineral Composition of Fruits of Camu-camu (Myrciaria dubia), Rev. Virtual Quim., 2019, 11(3), 741–753 CrossRef.
- C. M. Donado-Pestana, M. H. C. Moura, R. L. de Araujo, G. de L. Santiago, H. R. de M. Barros and M. I. Genovese, Polyphenols from Brazilian native Myrtaceae fruits and their potential health benefits against obesity and its associated complications, Curr. Opin. Food Sci., 2018, 19, 42–49, DOI:10.1016/j.cofs.2018.01.001.
- A. P. A. de Carvalho and C. A. Conte-Junior, Health benefits of phytochemicals from Brazilian native foods and plants: Antioxidant, antimicrobial, anti-cancer, and risk factors of metabolic/endocrine disorders control, Trends Food Sci. Technol., 2021, 111, 534–548 CrossRef.
- K. R. Biazotto, L. M. De Souza Mesquita, B. V. Neves, A. R. C. Braga, M. M. P. Tangerina, W. Vilegas, A. Z. Mercadante and V. V. De Rosso, Brazilian Biodiversity Fruits: Discovering Bioactive Compounds from Underexplored Sources, J. Agric. Food Chem., 2019, 67(7), 1860–1876 CrossRef CAS PubMed.
- D. C. da Cruz, J. M. R. Benayas, G. C. Ferreira, S. R. Santos and G. Schwartz, An overview of forest loss and restoration in the Brazilian Amazon, New For., 2021, 52, 1–16 CrossRef.
- R. Vargas-Carpintero, F. Romero-Perdomo, J. F Martínez and I. Lewandowski, A review of the knowledge base for the development of natural ingredients value chains for a sustainable biobased economy in Colombia, Discovery Sustainability, 2023, 33(4) DOI:10.1007/s43621-023-00150-w.
- J. M. Aguilera and T. Toledo, Wild berries and related wild small fruits as traditional healthy foods, Crit. Rev. Food Sci. Nutr., 2022 Search PubMed.
- D. Faria, J. C Morante-Filho, J. Baumgarten, R. S. Bovendorp, E. Cazetta, F. A. Gaiotto, E. Mariano-Neto, M. S. Mielke, M. S. Pessoa, L. Rocha-Santos, A. S. Santos, L. A. S. S. Soares, D. C. Talora, E. M. Vieira and M. Benchimol, The breakdown of ecosystem functionality driven by deforestation in a global biodiversity hotspot, Biol. Conserv., 2023, 283, 110126 CrossRef.
- A. Vega-Galvez, A. Rodríguez and K. Stucken, Antioxidant, functional properties and health-promoting potential of native South American berries: a review, J. Sci. Food Agric., 2021, 101(2), 364–378 CrossRef CAS PubMed.
- H. M. Salo, N. Nguyen, E. Alakärppä, L. Klavins, A. L. Hykkerud, K. Karppinen, L. Jaakola, M. Klavins and H. Häggman, Authentication of berries and berry-based food products, Compr. Rev. Food Sci. Food Saf., 2021, 20(5), 5197–5225 CrossRef CAS PubMed.
- M. G. González-Ramírez, V. H. Santoyo-Cortés, J. J. Arana-Coronado, M. Muñoz-RodrígueZ and N. Albis-Salas, Global traders and the integration of Chile and Mexico into the configuration of the global value chain of berries, Int. Food Agribus. Manag. Rev., 2023, 26(2), 225–241 CrossRef.
- R. S. Da Motta, Caracterização e Quadros de Análise Comparativa da Governança Metropolitana no Brasil: Análise comparativa das funções públicas de interesse comum, Instituto de Pesquisa Econômica Aplicada (Ipea), 2015, pp. 1–21 Search PubMed.
- F. F. de Araújo, I. A. Neri-Numa, D. de Paulo Farias, G. R. M. C. da Cunha and G. M. Pastore, Wild Brazilian species of Eugenia genera (Myrtaceae) as an innovation hotspot for food and pharmacological purposes, Food Res. Int., 2019, 121, 57–72 CrossRef.
- R. B. Medina, T. E. Cantuarias-Avilés, S. F. Angolini and S. R. Da Silva, Performance of “Emerald” and “Jewel” blueberry cultivars under no-chill incidence, Pesqui. Agropecu. Trop., 2018, 48(2), 147–152 CrossRef.
- J. Infante, P. L. Rosalen, J. G. Lazarini, M. Franchin and S. M. de. Alencar, Antioxidant and Anti-Inflammatory Activities of Unexplored Brazilian Native Fruits, PLoS One, 2016, 11(4), e0152974 CrossRef PubMed.
- C. A. Lamas, L. A. Kido, T. A. Hermes, E. Nogueira-Lima, E. Minatel, C. B. Collares-Buzato, M. R. Maróstica and V. H. A. Cagnon, Cagnon, Brazilian berry extract (Myrciaria jaboticaba): A promising therapy to minimize prostatic inflammation and oxidative stress, The Prostate, 2020, 80(11), 859–871 CrossRef CAS PubMed.
- S. K. T. Seraglio, M. Schulz, P. Nehring, F. Della Betta, A. C. Valese, H. Daguer, L. V. Gonzaga, R. Fett and A. C. O. Costa, Nutritional and bioactive potential of Myrtaceae fruits during ripening, Food Chem., 2018, 239, 649–656, DOI:10.1016/J.FOODCHEM.2017.06.118.
- M. da S. Vasconcelos, E. F. Mota, N. F. Gomes-Rochette, D. C. S. Nunes-Pinheiro, S. M. Nabavi and D. F. de Melo, Açai or Brazilian Berry (Euterpe oleracea), in Nonvitamin and Nonmineral Nutritional Supplements, 2019, pp. 131–133 Search PubMed.
- E. Detoni, D. L. Kalschne, A. Bendendo, N. K. Silva, O. D. Leite and A. C. Rodrigues, Guabijú (Myrcianthes pungens): Characterization of in natura and lyophilized Brazilian berry, Res., Soc. Dev., 2021, 10(3), e37810313337, DOI:10.33448/rsd-v10i3.13337.
- F. L. Ranzato Filardi, F. De Barros, J. F. A. Baumgratz, C. E. M. Bicudo, T. B. Cavalcanti, M. A. Nadruz Coelho, A. F. Costa, D. P. Costa, R. Goldenberg, P. H. Labiak, J. M. Lanna, P. Leitman, L. G. Lohmann, L. Costa Maia, V. F. Mansano, M. P. Morim, D. F. Peralta, J. R. Pirani, J. Prado and A. R. Zuntini, Brazilian Flora 2020: Innovation and collaboration to meet Target 1 of the Global Strategy for Plant Conservation (GSPC), Rodriguésia, 2018, 69(4), 1513–1527 CrossRef.
- J. D. A. S. Diniz and N. Cialdella, Informal markets, marginal populations, and the bioeconomy – the success story of açaí (Euterpe oleracea Mart.) in the Guiana Shield, in The Bioeconomy and Non-timber Forest Products, 2022, pp. 76–91 Search PubMed.
- F. W. Maciel-Silva, L. S. Buller, M. L. M B B Gonçalves, M. A. Rostagno and T. Forster-Carneiro, Sustainable development in the Legal Amazon: energy recovery from açaí seeds, Biofuels, Bioprod. Biorefin., 2021, 15(4), 1174–1189 CrossRef CAS.
- L. Mourão, Vista do História e natureza: Do açaí ao palmito, Revista Territórios e Fronteiras, 2010, 3(2), 74–96 CrossRef.
- L. F. Laurindo, S. M. Barbalho, A. C. Araújo, E. L. Guiguer, A. Mondal, G. Bachtel and A. Bishayee, Açaí (Euterpe oleracea Mart.) in Health and Disease: A Critical Review, Nutrients, 2023, 15(4), 989 CrossRef CAS , https://www.mdpi.com/2072-6643/15/4/989.
- J. T. da. Silveira, A. P. C. da. Rosa, M. G. de Morais, F. N. Victoria and J. A. V. Costa, An integrative review of Açaí (Euterpe oleracea and Euterpe precatoria): Traditional uses, phytochemical composition, market trends, and emerging applications, Food Res. Int., 2023, 173, 113304 CrossRef.
- J. C. Castro, J. D. Maddox and S. A. Imán, Camu-camu – Myrciaria dubia (Kunth) McVaugh, in Exotic Fruits Reference Guide, 2018, pp. 97–105 Search PubMed.
- USDA, FoodData Central. Orange Juice, 2023 Search PubMed.
- L. M. Rodrigues, E. B. Romanini, E. Silva, E. J. Pilau, S. C. da Costa and G. S. Madrona, Camu-camu bioactive compounds extraction by ecofriendly sequential processes (ultrasound assisted extraction and reverse osmosis), Ultrason. Sonochem., 2020, 64, 105017, DOI:10.1016/J.ULTSONCH.2020.105017.
- E. C. E. Cunha-Santos, C. Rodrigues-Silva, T. F. F. da Silveira and H. T. Godoy, Optimization of Phenolic Compounds Extraction of Different Parts of Camu-camu Fruit from Different Geographic Regions, Plant Foods Hum. Nutr., 2022, 77(3), 340–344 CrossRef CAS.
- C. E. R. Senes, A. E. Nicácio, C. A. Rodrigues, L. P. Manin, L. Maldaner and J. V. Visentainer, Evaluation of Dispersive Solid-Phase Extraction (d-SPE) as a Clean-up Step for Phenolic Compound Determination of Myrciaria cauliflora Peel, Food Anal. Methods, 2020, 13(1), 155–165, DOI:10.1007/s12161-019-01566-9.
- J. C. Castro, J. D. Maddox and S. A. Imán, Camu-camu—Myrciaria dubia (Kunth) McVaugh, Em Exotic Fruits, Elsevier, 2018, pp. 97–105, DOI:10.1016/B978-0-12-803138-4.00014-9.
- V. B. Bombana, C. E. D. Oro, D. Rigo, C. C. Polina, A. F. Denti, B. P. Tres, M. S. W. Wisniewski, J. Steffens, N. Paroul, R. M. Dallago, G. T. Backes and R. L. Cansian, Influence of drying on bioactive compounds and antioxidant activity of fruits of guabiju (Myrcianthes pungens), Res., Soc. Dev., 2021, 10(8), e5510817024, DOI:10.33448/rsd-v10i8.17024.
- M. Teixeira, T. Altmayer, F. Bruxel, C. R. Orlandi, N. F. de Moura, C. N. Afonso, E. M. Ethur, L. Hoehne and E. M. de. Freitas, Rubus sellowii Cham. & Schlitdl. (Rosaceae) fruit nutritional potential characterization, Braz. J. Biol., 2019, 79(3), 510–515, DOI:10.1590/1519-6984.186435.
- D. M. Pereira, K. Á. R. de Oliveira, L. Chantelle, A. M. da S. Sant'Ana, J. P. de S. Guedes, C. T. de Carvalho, G. A. Azerêdo and I. de L. Brito, Caracterização Da Composição Nutricional E Do Teor De Pigmentos De Pitanga (Eugenia Uniflora L.) Nas Variedades Vermelha E Roxa, Braz. J. Dev., 2020, 6(8), 58026–58038, DOI:10.34117/bjdv6n8-276.
- M. da Silva, J. H. Costa, T. Pacheco-Fill, A. Ruiz, F. C. B. Vidal, K. R. A. Borges, S. J. A. Guimaraes, A. P. S. de Azevedo-Santos, K. E. Buglio, M. A. Foglio, M. D. L. Barbosa, M. Nascimento and J. E. de Carvalho, Acai (Euterpe oleracea Mart.) Seed Extract Induces ROS Production and Cell Death in MCF-7 Breast Cancer Cell Line, Molecules, 2021, 26(3546), 1–23 Search PubMed.
- B. D Antonia, Master Thesis in Science and Phytotechnics, Qualidade pós-colheita de cereja-do-rio-grande (Eugenia involucrata DC.): Caracterização de acessos e estádios de maturação, 2020 Search PubMed.
- H. O. S. de Schmidt, Master Thesis in Food Science and Technology, Caracterização físico-química, nutricional e de compostos bioativos de sete espécies da família Myrtaceae nativas da Região Sul do Brasil, 2018 Search PubMed.
- F. G. Zola, A. C. Rodrigues, B. D. Oliveira, N. T. B. Sacramento, J. G. Taylor, U. M. Pinto and M. C. Bertoldi, Mineral and centesimal contents, antioxidant activity and antimicrobial action of phenolic compounds from Eugenia Brasiliensis Lam. Pulp, Food Sci. Technol., 2019, 39, 378–385, DOI:10.1590/fst.18518.
- P. Nehring, S. Katia Tischer Seraglio, M. Schulz, F. Della Betta, L. Valdemiro Gonzaga, L. Vitali, M. da Silva, G. Amadeu Micke, A. Carolina Oliveira Costa and R. Fett, Grumixama (Eugenia brasiliensis Lamarck) functional phytochemicals: Effect of environmental conditions and ripening process, Food Res. Int., 2022, 157, 111460, DOI:10.1016/j.foodres.2022.111460.
- K. Xu, A. M. Alves-Santos, T. Dias and M. M. V. Naves, Grumixama (Eugenia brasiliensis Lam.) cultivated in the Cerrado has high content of bioactive compounds and great antioxidant potential, Ciência Rural. Food Technology, 2020, 50(4) DOI:10.1590/0103-8478cr20190630.
- M. V. Dias-Souza, R. M. dos Santos, I. P. Cerávolo, G. Cosenza, P. H. Ferreira Marçal and F. J. B. Figueiredo, Euterpe oleracea pulp extract: Chemical analyses, antibiofilm activity against Staphylococcus aureus, cytotoxicity and interference on the activity of antimicrobial drugs, Microb. Pathog., 2018, 114, 29–35, DOI:10.1016/j.micpath.2017.11.006.
- E. C. Minighin, L. R. Anastácio, J. O. F. Melo and R. A. Labanca, Açai (Euterpe oleracea) e suas contribuições para alcance da ingestão diária aceitável de ácidos graxos essenciais, Res., Soc. Dev., 2020, 9(8), 1–26 Search PubMed.
- S. M. Miranda Affonso, D. C. Dos Santos, D. M. De Oliveira, S. C. P. Uchoa, A. V. S. Cardoso and L. O. De Sousa, Nutritional composition and antioxidant compounds of coconut candy with added açaí pulp, Brazilian journal of agrarian and environmental science, 2022, 16 DOI:10.18227/1982-8470ragro.v16i0.7191.
- A. V. Carvalho, T. Ferreira Ferreira da Silveira, R. de A. Mattietto, M. do S. Padilha de Oliveira and H. T. Godoy, Chemical composition and antioxidant capacity of açaí (Euterpe oleracea) genotypes and commercial pulps, J. Sci. Food Agric., 2017, 97(5), 1467–1474, DOI:10.1002/jsfa.7886.
- R. C. da Silva, A. Batista, D. C. F. da Costa, N. Moura-Nunes, J. C. Koury, C. A. da Costa, Â. C. Resende and J. B. Daleprane, Açai (Euterpe oleracea Mart.) seed flour prevents obesity-induced hepatic steatosis regulating lipid metabolism by increasing cholesterol excretion in high-fat diet-fed mice, Food Res. Int., 2018, 111, 408–415, DOI:10.1016/j.foodres.2018.05.043.
- R. C. de A. B. Campos, E. M. F. Martins, B. de Andrade Pires, M. do Carmo Gouveia Peluzio, A. N. da Rocha Campos, A. M. Ramos, B. R. de Castro Leite Júnior, A. D. de Oliveira Martins, R. R. da Silva and M. L. Martins, In vitro and in vivo resistance of Lactobacillus rhamnosus GG carried by a mixed pineapple (Ananas comosus L. Merril) and jussara (Euterpe edulis Martius) juice to the gastrointestinal tract, Food Res. Int., 2019, 116, 1247–1257, DOI:10.1016/j.foodres.2018.10.012.
- F. C. Rockett, H. de O. Schmidt, C. H. Pagno, É. S. Fochezatto, V. R. de Oliveira, V. L. da Silva, S. H. Flôres and A. de O. Rios, Native fruits from southern Brazil: Physico-chemical characterization, centesimal composition, and mineral content, J. Food Process. Preserv., 2020, 44(8), e14582, DOI:10.1111/jfpp.14582.
- M. C. M. Madalão, E. M. F. Lima, D. B. Benincá, S. H. Saraiva, R. V. de Carvalho and P. I. Silva, Extraction of bioactive compounds from juçara pulp (Euterpe edulis M.) is affected by ultrasonic power and temperature, Ciencia e Agrotecnologia. Food Science and Technology, 2021, 45(1981–1829), 1–11 Search PubMed.
- L. M. O. da Silva, G. A. S. Santos, A. A. Rocha, A. K. da S. Raposo, L. C. Paixão and A. A. Santana, Physicochemical and nutritional properties of jussara pulp powder (Euterpe edulis M.) by spray-drying, Res., Soc. Dev., 2021, 10(1), e44110111256, DOI:10.33448/rsd-v10i1.11256.
- M. P. Silva, S. H. B. Sousa, V. M. B. Cunha, A. R. Salazar, M. de los, C. B. Amarante, M. E. Araujo and R. N. Carvalho Junior, Avaliação da estrutura morfológica, química elementar, parâmetros de cor e composição em minerais da polpa de açaí (Euterpe oleracea mart.) De três diferentes localidades da região Amazônica, Braz. J. Dev., 2020, 6(4), 18793–18803, DOI:10.34117/bjdv6n4-157.
- J. Garcia, R. C. G. Corrêa, L. Barros, C. Pereira, R. M. V. Abreu, M. J. Alves, R. C. Calhelha, A. Bracht, R. M. Peralta and I. C. F. R. J. Ferreira, Chemical composition and biological activities of Juçara (Euterpe edulis Martius) fruit by-products, a promising underexploited source of high-added value compounds, J. Funct. Foods, 2019, 55, 325–332, DOI:10.1016/j.jff.2019.02.037.
- A. G. da Silva Carvalho, M. T. da Costa Machado, H. D. de Freitas Queiroz Barros, C. B. B. Cazarin, M. R. Maróstica Junior and M. D. Hubinger, Anthocyanins from jussara (Euterpe edulis Martius) extract carried by calcium alginate beads pre-prepared using ionic gelation, Powder Technol., 2019, 345, 283–291, DOI:10.1016/j.powtec.2019.01.016.
- T. P. Freitas, I. B. Taver, P. C. Spricigo, L. B. do Amaral, E. Purgatto and A. P. Jacomino, Volatile Compounds and Physicochemical Quality of Four Jabuticabas (Plinia sp.), Molecules, 2020, 25(19), 4543, DOI:10.3390/molecules25194543.
- L. G. C. Garcia, F. A. Da Solva, E. R. Asquoero, E. V. D. B. Volas Bdas, F. O. B. Dgandd, C. L. De Aguoar and C. Damoano, Proximate composition, minerals profile, and predominant sugars by ion chromatograph along the physiological development of jabuticaba var. Pingo de mel, Food Sci. Technol., 2018, 38, 16–21, DOI:10.1590/fst.08117.
- L. D. Pereira, J. M. G. Barbosa, A. J. Ribeiro da Silva, P. H. Ferri and S. C. Santos, Polyphenol and Ellagitannin Constituents of Jabuticaba (Myrciaria cauliflora) and Chemical Variability at Different Stages of Fruit Development, J. Agric. Food Chem., 2017, 65(6), 1209–1219, DOI:10.1021/acs.jafc.6b02929.
- H. de O. Schmidt, F. C. Rockett, C. H. Pagno, J. Possa, R. Q. Assis, V. R. de Oliveira, V. L. da Silva, S. H. Flôres and A. de O. Rios, Vitamin and bioactive compound diversity of seven fruit species from south Brazil, J. Sci. Food Agric., 2019, 99(7), 3307–3317, DOI:10.1002/jsfa.9544.
- F. V. Matta, J. Xiong, M. A. Lila, N. I. Ward, M. Felipe-Sotelo and D. Esposito, Chemical Composition and Bioactive Properties of Commercial and Non-Commercial Purple and White Açaí Berries, Foods, 2020, 9(10), 1481, DOI:10.3390/foods9101481.
- A. P. de G. Carneiro, A. L. L. de Aguiar, M. L. da C. Gonzaga, D. J. Soares, E. A. T. de Figueiredo, P. H. M. de Sousa and R. W. de. Figueiredo, Stability of bioactive compounds, antioxidant and microbiological activity of açaí powder (Euterpe oleracea Mart.), Res., Soc. Dev., 2020, 9(7), e229973810, DOI:10.33448/rsd-v9i7.3810.
- M. Schulz, F. C. Biluca, L. V. Gonzaga, G. S. C. Borges da, L. Vitali, G. A. Micke, J. S. de Gois, T. S. de Almeida, D. L. G. Borges, P. R. M. Miller, A. C. O. Costa and R. Fett, Bioaccessibility of bioactive compounds and antioxidant potential of juçara fruits (Euterpe edulis Martius) subjected to in vitro gastrointestinal digestion, Food Chem., 2017, 228, 447–454, DOI:10.1016/j.foodchem.2017.02.038.
- A. Yanahuillca Vargas, Determinación de capacidad antioxidante y compuestos bioactivos (fenólicos) de los frutos silvestres: siraca roja (Rubus urticifolius poir), siraca negra (Rubus sparciflorus j. f Macbr) y pacra (Hesperomeles palsensis c. Scheneider), Universidad Nacional José María Arguedas Facultad De Ingeniería Escuela Profesional De Ingeniería Agroindustrial, 2019 Search PubMed.
- C. E. Pacheco Llamocca, Influencia de la temperatura y el pH en la estabilidad de las antocianinas de los frutos de zarzamora (Rubus urticifolius poir), 2019 Search PubMed.
- D. M. Pereira, K. Á. R. De Oliveira, L. Chantelle, A. M. da S. Sant'Ana, J. P. de S. Guedes, C. T. De Carvalho, G. A. Azerêdo and I. de L. Britos, Characterization of nutritional composition and content of pitanga pigments (Eugenia uniflora L.) in red and purple varieties, Braz. J. Dev., 2020, 6(8), 58026–58038 CrossRef.
- R. R. F. Ramalho, J. M. G. Barbosa, P. H. Ferri and S. C. Santos da, Variability of polyphenols and volatiles during fruit development of three pitanga (Eugenia uniflora L.) biotypes, Food Res. Int., 2019, 119, 850–858, DOI:10.1016/j.foodres.2018.10.068.
- E. C. Minighin, L. R. Anastácio, J. O. F. Melo and R. A. Labanca, Açai (Euterpe oleracea) and its contributions to achieve acceptable daily intake of essential fatty acids, Res., Soc. Dev., 2020, 9(8), e760986116 Search PubMed.
- A. de S. da Silva, D. V. Q. Nunes, L. C. Carvalho, R. M. de. dos, I. B. Santos, M. P. de Menezes, G. F. de Bem, C. A. da Costa, R. S. de Moura, A. C. Resende and D. T. Ognibene, Açaí (Euterpe oleracea Mart) seed extract protects against maternal vascular dysfunction, hypertension, and fetal growth restriction in experimental preeclampsia, Hypertens. Pregnancy, 2020, 39, 211–219 CrossRef CAS PubMed.
- A. P. Stafussa, G. M. Maciel, V. Rampazzo, E. Bona, C. N. Makara, B. Demczuk Junior and C. W. I. Haminiuk, Bioactive compounds of 44 traditional and exotic Brazilian fruit pulps: phenolic compounds and antioxidant activity, Int. J. Food Prop., 2018, 21(1), 106–118, DOI:10.1080/10942912.2017.1409761.
- A. P. de Souza Silva, A. C. de Camargo, J. G. Lazarini, M. Franchin, J. de C. O. Sardi, P. L. Rosalen and S. M. de Alencar, Phenolic Profile and the Antioxidant, Anti-Inflammatory, and Antimicrobial Properties of Açaí (Euterpe oleracea) Meal: A Prospective Study, Foods, 2023, 12(1), 86 CrossRef CAS.
- G. A. Garzón, C. E. Narváez-Cuenca, J. P. Vincken and H. Gruppen, Polyphenolic composition and antioxidant activity of açai (Euterpe oleracea Mart.) from Colombia, Food Chem., 2017, 217, 364–372 CrossRef.
- K. O. P. Inada, S. Nunes, J. A. Martínez-Blázquez, F. A. Tomás-Barberán, D. Perrone and M. Monteiro, Effect of high hydrostatic pressure and drying methods on phenolic compounds profile of jabuticaba (Myrciaria jaboticaba) peel and seed, Food Chem., 2020, 309(125794), 1–10 Search PubMed.
- L. D. Pereira, J. M. G. Barbosa, A. J. R. Silva, P. H. Ferri and S. C. Santos, Polyphenol and ellagitannin constituents of jabuticaba (Myrciaria cauliflora) and chemical variability at different stages of fruit development, J. Agric. Food Chem., 2017, 65(6), 1209–1219 CrossRef CAS PubMed.
- M. Teixeira, T. Altmayer, F. Bruxel, C. R. Orlandi, N. F. de. Moura, C. N. Afonso, E. M. Ethur, L. Hoehne and E. M. Freitas, Rubus sellowii Cham. & Schlitdl. (Rosaceae) fruit nutritional potential characterization, Braz. J. Biol., 2019, 79(3), 1678–4375 Search PubMed.
- N. M. Peixoto Araujo, E. K. Silva, H. S. Arruda, D. Rodrigues de Morais, A. Angela, M. Meireles, G. A. Pereira and G. M. Pastore, Recovering phenolic compounds from Eugenia calycina Cambess employing high-intensity ultrasound treatments: A comparison among its leaves, fruit pulp, and seed as promising sources of bioactive compounds, Sep. Purif. Technol., 2021, 272, 118920 CrossRef CAS.
- F. Chemat, N. Rombaut, A. G. Sicaire, A. Meullemiestre, A. A. S. Fabiano-Tixier and M. Abert-Vian, Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review, Ultrason. Sonochem., 2017, 34, 540–560 CrossRef CAS PubMed.
- A. Gadioli Tarone, E. Keven Silva, H. Dias de Freitas Queiroz Barros, C. Baú Betim Cazarin and M. Roberto Marostica Junior, High-intensity ultrasound-assisted recovery of anthocyanins from jabuticaba by-products using green solvents: Effects of ultrasound intensity and solvent composition on the extraction of phenolic compounds, Food Res. Int., 2021, 140, 110048 CrossRef CAS.
- M. J. Aliaño-González, M. Ferreiro-González, E. Espada-Bellido, C. Carrera, M. Palma, J. A. Álvarez, J. Ayuso and G. F. Barbero, Extraction of Anthocyanins and Total Phenolic Compounds from Açai (Euterpe oleracea Mart.) Using an Experimental Design Methodology. Part 1: Pressurized Liquid Extraction, Agronomy, 2020, 10(2), 183 CrossRef.
- D. R. G. de Castro, J. M. Mar, L. S. da Silva, K. A. da Silva, E. A. Sanches, J. de Araújo Bezerra, S. Rodrigues, F. A. N. Fernandes and P. H. Campelo, Dielectric barrier atmospheric cold plasma applied on camu-camu juice processing: Effect of the excitation frequency, Food Res. Int., 2020, 131, 109044 CrossRef PubMed.
- T. F. F. da Silveira, M. Cristianini, G. G. Kuhnle, A. B. Ribeiro, J. T. Filho and H. T. Godoy, Anthocyanins, non-anthocyanin phenolics, tocopherols and antioxidant capacity of açaí juice (Euterpe oleracea) as affected by high pressure processing and thermal pasteurization, Innovative Food Sci. Emerging Technol., 2019, 55, 88–96 CrossRef CAS.
- U. Trych, M. Buniowska, S. Skąpska, Z. Zhu, J. Bi, X. Liu, F. J. Barba and K. Marszałek, Impact of HPP on the bioaccessibility/bioavailability of nutrients and bioactive compounds as a key factor in the development of food processing, Present and Future of High-Pressure Processing: A Tool for Developing Innovative, Sustainable, Safe and Healthy Foods, 2020, pp. 87–109 Search PubMed.
- F. das C. do Amaral Souza, L. Gomes Sanders Moura, K. de Oliveira Bezerra, J. Paiva Lopes Aguiar, J. Moreira Mar, E. A. Sanches, F. F. dos Santos, A. M. Bakry, B. Nicolau Paulino and P. H. Campelo, Thermosonication applied on camu-camu nectars processing: Effect on bioactive compounds and quality parameters, Food Bioprod. Process., 2019, 116, 212–218 CrossRef CAS.
- T. T. da Silva Crozatti, C. S. Mangolim, P. V. Larentis, J. C. P. de Mello and G. Matioli, Extraction, microencapsulation, and application of anthocyanins from juçara palm fruit (Euterpe edulis Mart.): enhancement of natural pigment, J. Food Sci. Technol., 2023, 60(1), 361–371 CrossRef CAS PubMed.
- D. de Cássia Sousa Mendes, E. R. Asquieri, R. D. Batista, C. C. de Morais, D. P. Ramirez Ascheri, I. Y. L. de Macêdo and E. de Souza Gil, Microencapsulation of jabuticaba extracts (Myrciaria cauliflora): Evaluation of their bioactive and thermal properties in cassava starch biscuits, LWT, 2021, 137, 110460 CrossRef.
- W. Steffen, K. Richardson, J. Rockström, S. E. Cornell, I. Fetzer, E. M. Bennett, R. Biggs, S. R. Carpenter, W. de Vries, C. A. de Wit, C. Folke, D. Gerten, J. Heinke, G. M. Mace, L. M. Persson, V. Ramanathan, B. Reyers and S. Sörlin, Sustainability. Planetary boundaries: guiding human development on a changing planet, Science, 2015, 347, 6223 CrossRef PubMed.
- N. May and E. Guenther, Shared benefit by Material Flow Cost Accounting in the food supply chain – The case of berry pomace as upcycled by-product of a black currant juice production, J. Cleaner Prod., 2020, 245(1), 118946 CrossRef.
- P. Teluguntla. P. S. Thenkabail, J. Xiong, M. K. Gumma, C. Giri, C. Milesi, M. Ozdogan, R. G. Congalton, J. Tilton, T. T. Sankey, R. Massey, A. Phalke and K. Yadav, Global Food Security Support Analysis Data at Nominal 1 km (GFSAD1km) Derived from Remote Sensing in Support of Food Security in the Twenty-First Century: Current Achievements and Future Possibilities, Remote Sensing Handbook – Three Volume Set, 2018, pp. 865–894 Search PubMed.
- A. P. da F. Machado, M. V. Geraldi, R. de P. do Nascimento, A. M. T. M. Moya, T. Vezza, P. Diez-Echave, J. J. Gálvez, C. B. B. Cazarin and M. R. Maróstica Júnior, Polyphenols from food by-products: An alternative or complementary therapy to IBD conventional treatments, Food Res. Int., 2021, 140, 110018 CrossRef CAS PubMed.
- M. A. C. de Albuquerque, R. Levit, C. Beres, R. Bedani, A. de Moreno de LeBlanc, S. M. I. Saad and J. G. LeBlanc, Tropical fruit by-products water extracts as sources of soluble fibres and phenolic compounds with potential antioxidant, anti-inflammatory, and functional properties, J. Funct. Foods, 2019, 52, 724–733 CrossRef.
- A. V. G. Mazallli, Flour processing waste pulp of blackberry (Rubus sp.) and evaluation of biocompounds, Dissertation (Master in Biosciences). – Faculdade de Ciências e Letras, Universidade Estadual Paulista “Júlio de Mesquita Filho, 2014, pp. 1–77 Search PubMed.
- K. O. P. Inada, A. A. Oliveira, T. B. Revorêdo, A. B. N. Martins, E. C. Q. Lacerda, A. S. Freire, B. F. Braz, R. E. Santelli, A. G. Torres, D. Perrone and M. C. Monteiro, Screening of the chemical composition and occurring antioxidants in jabuticaba (Myrciaria jaboticaba) and jussara (Euterpe edulis) fruits and their fractions, J. Funct. Foods, 2015, 17, 422–433 CrossRef CAS.
- P. D. Gurak, G. S. De Bona, I. C. Tessaro and L. D. F. Marczak, Jaboticaba Pomace Powder Obtained as a Co-product of Juice Extraction: A Comparative Study of Powder Obtained from Peel and Whole Fruit, Food Res. Int., 2014, 62, 786–792 CrossRef CAS.
- R. L. Almeida, N. C. Santos, T. dos S. Pereira, V. M. de A. Silva, M. B. Cabral, E. R. Barros, N. C. Souza, M. R. Luiz, F. V. Amorim and L. R. I. da Silva, Determination of bioactive compounds and physicochemical composition of jabuticaba bark flour obtained by convective drying and lyophilization, Res., Soc. Dev., 2020, 9(1), e157911876 CrossRef.
- C. Marquetti, Master Thesis in Food Science and Technology, Desenvolvimento e obtenção de farinha de casca de jabuticaba (Plinia cauliflora) para adição em biscoito tipo cookie, 2014 Search PubMed.
- E. G. L. das. P Chagas, Master Thesis in Food Science and Technology, Caracterização e aplicação de farinhas obtidas a partir do resíduo agroindustrial do processamento do camu-camu, 2019 Search PubMed.
- G. C. Murta, Manaus: Instituto Nacional de Pesquisas da Amazônia, Desenvolvimento e efeito de bebida tipo shake à base de pó de camu-camu (Myrciaria Dubia [HBK] McVaugh) no controle da diabetes, in vivo, 2017 Search PubMed.
- J. R. Girardelo, E. L. Munari, J. C. S. Dallorsoleta, G. Cechinel, A. L. F. Goetten, L. R. Sales, F. H. Reginatto, V. C. Chaves, F. A. Smaniotto, S. Somacal, T. Emanuelli, J. C. Benech, C. Soldi, E. Winter and G. M. M. Conterato, Bioactive compounds, antioxidant capacity and antitumoral activity of ethanolic extracts from fruits and seeds of Eugenia involucrata DC, Food Res. Int., 2020, 137, 109615, DOI:10.1016/j.foodres.2020.109615.
- J. O. S. V. de Navarro, Master Thesis in Bromatology of the Faculty of Pharmaceutical Sciences, Caracterização de compostos fenólicos de frutas nativas brasileiras e atividade inibitória das enzimas digestórias α-amilase e α-glicosidase, 2022 Search PubMed.
- G. Mannino, G. Serio, A. Asteggiano, N. Gatti, C. M. Bertea, C. Medana and C. Gentile, Bioactive Compounds and Antioxidant Properties with Involved Mechanisms of Eugenia involucrata DC Fruits., Antioxidants, 2022, 11(9), 1769 CrossRef CAS PubMed.
- A. P. de Souza Silva, A. C. de Camargo, J. G. Lazarini, M. Franchin, J. de C. O. Sardi, P. L. Rosalen and S. M. de Alencar, Phenolic Profile and the Antioxidant, Anti-Inflammatory, and Antimicrobial Properties of Açaí (Euterpe oleracea) Meal: A Prospective Study, Foods, 2022, 12(1), 86, DOI:10.3390/foods12010086.
- P. P. Argentato, C. A. Morais, A. B. Santamarina, H. de C. César, D. Estadella, V. V. de Rosso and L. P. Pisani, Jussara (Euterpe edulis Mart.) supplementation during pregnancy and lactation modulates UCP-1 and inflammation biomarkers induced by trans-fatty acids in the brown adipose tissue of offspring, Clin. Nutr. Exp., 2017, 12, 50–65, DOI:10.1016/j.yclnex.2016.12.002.
- R. D. do Nascimento, L. M. Reguengo, A. P. D. Machado and M. R. Marostica, The preventive and therapeutic potential of native Brazilian fruits on colorectal cancer, Food Biosci., 2022, 46 DOI:10.1016/j.fbio.2021.101539.
- M. Schulz, L. V. Gonzaga, V. de Souza, M. Farina, L. Vitali, G. A. Micke, A. C. O. Costa and R. Fett, Neuroprotective effect of juçara (Euterpe edulis Martius) fruits extracts against glutamate-induced oxytosis in HT22 hippocampal cells, Food Res. Int., 2019, 120, 114–123, DOI:10.1016/j.foodres.2019.02.030.
- M. Schulz, S. K. T. Seraglio, P. Brugnerotto, L. V. Gonzaga, A. C. O. Costa and R. Fett, Composition and potential health effects of dark-colored underutilized Brazilian fruits – A review, Food Res. Int., 2020, 137 DOI:10.1016/j.foodres.2020.109744.
- G. S. Vieira, A. S. F. Marques, M. T. C. Machado, V. M. Silva and M. D. Hubinger, Determination of anthocyanins and non-anthocyanin polyphenols by ultra performance liquid chromatography/electrospray ionization mass spectrometry (UPLC/ESI–MS) in jussara (Euterpe edulis) extracts, J. Food Sci. Technol., 2017, 54(7), 2135–2144, DOI:10.1007/s13197-017-2653-1.
- J. C. G. Rocha, F. R. Procopio, A. C. Mendonça, L. M. Vieira, I. T. Perrone, F. A. R. Barros and P. C. Stringheta, Optimization of ultrasound-assisted extraction of phenolic compounds from jussara (Euterpe edulis M.) and blueberry (Vaccinium myrtillus) fruits., Food Sci. Technol., 2018, 38(1) DOI:10.1590/1678-457x.36316.
- N. de Andrade Neves, P. César Stringheta, I. Ferreira da Silva, E. García-Romero, S. Gómez-Alonso and I. Hermosín-Gutiérrez, Identification and quantification of phenolic composition from different species of Jabuticaba (Plinia spp.) by HPLC-DAD-ESI/MSn, Food Chem., 2021, 355, 129605, DOI:10.1016/j.foodchem.2021.129605.
- P. L. Trindade, E. D. R. Soares, K. O. P. Inada, F. F. Martins, M. Rudnicki, D. Perrone, M. Monteiro, V. Souza-Mello and J. B. Daleprane, Consumption of phenolic-rich jabuticaba (Myrciaria jaboticaba) powder ameliorates obesity-related disorders in mice, Br. J. Nutr., 2022, 127(3), 344–352, DOI:10.1017/S0007114521001136.
- K. O. P. Inada, I. B. Leite, A. B. N. Martins, E. Fialho, F. A. Tomás-Barberán, D. Perrone and M. Monteiro, Jaboticaba berry: A comprehensive review on its polyphenol composition, health effects, metabolism, and the development of food products, Food Res. Int., 2021, 147, 10518 CrossRef.
- L. Benvenutti, A. A. F. Zielinski and S. R. S. Ferreira, Jaboticaba (Myrtaceae cauliflora) fruit and its by-products: Alternative sources for new foods and functional components, Trends Food Sci. Technol., 2021, 112, 118–136, DOI:10.1016/j.tifs.2021.03.044.
- J. R. Barbosa and R. N. de. Carvalho Junior, Food sustainability trends – How to value the açaí production chain for the development of food inputs from its main bioactive ingredients?, Trends Food Sci. Technol., 2022, 124, 86–95 CrossRef CAS.
- N. Conceição, B. R. Albuquerque, C. Pereira, R. C. G. Corrêa, C. B. Lopes, R. C. Calhelha, M. J. Alves, L. Barros and C. F. R. I. Ferreira, By-Products of Camu-Camu [Myrciaria dubia (Kunth) McVaugh] as Promising Sources of Bioactive High Added-Value Food Ingredients: Functionalization of Yogurts, Molecules, 2020, 25(1), 70 CrossRef PubMed.
- E. S. De Brito, M. C. P. de Araújo, R. E. Alves, C. Carkeet, B. A. Clevidence and J. A. Novotny, Anthocyanins present in selected tropical fruits: acerola, jambolão, jussara, and guajiru, J. Agric. Food Chem., 2007, 55, 9389–9394 CrossRef PubMed.
- M. del P. Garcia-Mendoza, F. A. Espinosa-Pardo, A. M. Baseggio, G. F. Barbero, M. R. Maróstica Junior, M. A. Rostagno and J. Martínez, Extraction of phenolic compounds and anthocyanins from juçara (Euterpe edulis Mart.) residues using pressurized liquids and supercritical fluids, J. Supercrit. Fluids, 2017, 119, 9–16 CrossRef CAS.
- Xu. KaWai, A. M. Alves-Santos, T. Dias and M. M. V. Naves, Grumixama (Eugenia brasiliensis Lam.) cultivada no Cerrado possui altos teores de compostos bioativos e elevado potencial antioxidante, Food Technol., 2020, 50(4), 1–7 Search PubMed.
- Z. S. Silva, L. A. D. dos Santos, M. L. L. Goncalves, J. Gallo, T. da Silva, L. J. Motta, E. M. Santos, A. Horliana, K. P. S. Fernandes, R. A. Mesquita-Ferrari and S. K. Bussadori, Photodynamic therapy with acai (Euterpe oleracea) and blue light in oral cells: A spectroscopic and cytotoxicity analysis, J. Biophotonics, 2022, 16(3) DOI:10.1002/jbio.202200259.
- N. R. S. Sibuyi, V. C. Thipe, K. Panjtan-Amiri, M. Meyer and K. V. Katti, Green Synthesis of Gold Nanoparticles Using Acai Berry and Elderberry Extracts and Investigation of Their Effect on Prostate and Pancreatic Cancer Cells, BJGP Open, 2021, 8, 1–8, DOI:10.1177/1849543521995310.
- E. D. Marques, J. G. Froder, P. R. de Oliveira, F. F. Perazzo, P. C. P. Rosa, I. O. D. Gaivao, M. I. C. Mathias and E. L. Maistro, Cytotoxic effects of Euterpe oleraceae fruit oil (acai) in rat liver and thyroid tissues, Rev. Bras. Farmacogn., 2019, 29(1), 54–61, DOI:10.1016/j.bjp.2018.12.001.
- H. Song, X. Shen, R. Deng, Y. Zhang and X. Zheng, Dietary anthocyanin-rich extract of açai protects from diet-induced obesity, liver steatosis, and insulin resistance with modulation of gut microbiota in mice, Nutrition, 2021, 86, 111176, DOI:10.1016/j.nut.2021.111176.
- V. B. Bezerra, C. E. D. Aguiar, D. da Silva and C. C. das Chagas do Amaral Souza, Efeitos do extrato hidrossolúvel de açaí (Euterpe oleracea Mart.) em ratos Wistar com obesidade induzida, diabetes e colesterol, Res., Soc. Dev., 2021, 10(13), e5510817024 Search PubMed.
- D. Yorgos De Lima, M. Adelson Rodrigues, M. G. Mouro, J. M. Elias Seif, R. Giovana Punaro, E. Mieko and S. Higa, Açai (Euterpe oleracea Mart) modulates oxidative stress and inflammation by NF-κB inactivation and Nrf2 up-regulation in experimental diabetes, Cold Spring Harbor Protocols, biorxiv, 2022, preprint, DOI:10.1101/2022.02.14.480447.
- A. M. Figueiredo, A. C. Cardoso, B. L. B. Pereira, R. A. C. Silva, A. F. G. Della Ripa, T. F. B. Pinelli, B. C. Oliveira, B. P. M. Rafacho, L. L. W. Ishikawa, P. S. Azevedo, K. Okoshi, A. A. H. Fernandes, L. A. M. Zornoff, M. F. Minicucci, B. F. Polegato and S. A. R. Paiva, Acai supplementation (Euterpe oleracea Mart.) attenuates cardiac remodeling after myocardial infarction in rats through different mechanistic pathways, PLoS One, 2022, 17, 3, DOI:10.1371/journal.pone.0264854.
- B. J. D. Arnoso, F. M. Magliaccio, C. A. De Araujo, R. D. Soares, I. B. Santos, G. F. De Bem, C. Fernandes-Santos, D. T. Ognibene, R. S. De Moura, A. C. Resende, J. B. Daleprane and C. A. Da Costa, Acai seed extract (ASE) rich in proanthocyanidins improves cardiovascular remodeling by increasing antioxidant response in obese high-fat diet-fed mice, Chem.-Biol. Interact., 2022, 351, 109721 CrossRef PubMed.
- M. A. V. do Carmo, M. Fidelis, P. F. de Oliveira, L. Q. Feitoza, M. J. Marques, E. B. Ferreira, W. Y. Oh, F. Shahidi, J. Hellström, L. A. Almeida, R. D. Novaes, D. Granato and L. Azevedo, Ellagitannins from jabuticaba (Myrciaria jaboticaba) seeds attenuated inflammation, oxidative stress, aberrant crypt foci, and modulated gut microbiota in rats with 1,2 dimethyl hydrazine-induced colon carcinogenesis, Food Chem. Toxicol., 2021, 154, 112287, DOI:10.1016/j.fct.2021.112287.
- M. C. Paludo, S. B. P. de Oliveira, L. F. de Oliveira, R. C. Colombo, S. Gómez-Alonso, I. Hermosín-Gutiérrez, R. Prata, A. F. Lima, J. T. Filho, C. A. Ballus and H. T. Godoy, Phenolic composition of peels from different Jaboticaba species determined by HPLC-DAD-ESI/MSn and antiproliferative activity in tumor cell lines, Curr. Plant Biol., 2022, 29, 100233 CrossRef CAS.
- P. L. Trindade, F. F. Martins, E. dos Ramos Soares, E. M. Bernardes, F. Vardiero, A. de Castro Resende, V. Souza-Mello and J. B. Daleprane, Polyphenol-rich jaboticaba (Myrciaria jaboticaba) peel and seed powder induces browning of subcutaneous white adipose tissue and improves metabolic status in high-fat-fed mice, J. Funct. Foods, 2022, 97, 105238 CrossRef CAS .https://www.sciencedirect.com/science/article/pii/S1756464622003085.
- M. H. C. Moura, C. M. Donado-Pestana, L. Rodrigues, E. V. M. Pessoa, R. Rossi e Silva, W. T. Festuccia and M. I. Genovese, Long-term supplementation with phenolic compounds from jaboticaba (Plinia jaboticaba (Vell.) Berg) reduces adiposophaty and improves glucose, lipid, and energy metabolism, Food Res. Int., 2021, 143, 110302 CrossRef CAS PubMed , https://www.sciencedirect.com/science/article/pii/S0963996921002015.
- T. G. da S. Brito, A. P. S. A. da Silva, R. X. Cunha, C. S. M. da da Fonseca, T. F. da S. Araújo, J. K. de L. Campos, W. M. Nascimento, H. D. A. Araújo, J. P. R. e. de Silva, J. F. Tavares, B. S. dos Santos and V. L. de M. Lima, Anti-inflammatory, hypoglycemic, hypolipidemic, and analgesic activities of Plinia cauliflora (Mart.) Kausel (Brazilian grape) epicarp, J. Ethnopharmacol., 2021, 268(113611), 1–11 Search PubMed.
- S. O. dos Reis, T. C. da Luz, C. Couto, J. Dalbo, L. D. Nunes, M. C. Martins, P. I. Silva, A. M. A. da Silva and L. O. Trivilin, Jucara (Euterpe edulis Mart.) Supplementation Reduces Aberrant Crypt Foci and Increases SOD1 Expression in the Colorectal Mucosa of Carcinogenesis-Induced Rats, Nutr. Cancer, 2020, 72(4), 610–619, DOI:10.1080/01635581.2019.1649437.
- G. Jamar, A. B. Santamarina, A. C. Flygare, A. Gagliardi, V. V. de Rosso, V. Z. Dourado and L. P. Pisani, Effects of the juçara fruit supplementation on metabolic parameters in individuals with obesity: a double-blind randomized controlled trial, J. Nutr. Biochem., 2020, 83(108430), 1–8 Search PubMed.
- M. Barthichoto, T. L. Moretto, F. P. de Carvalho, I. D. Benfato, V. V. de Rosso, D. A. Ribeiro, D. Estadella, L. Le Sueur-Maluf, L. P. Pisani and C. A. M. de Oliveira, Juçara (Euterpe edulis Mart.) supplementation reduces body weight gain and protects mice from metabolic complications induced by high-fat diet, Nutrire, 2021, 46(6), 1–11 Search PubMed.
- H. M. O. do Carmo, F. M. A. de Souza, A. C. L. Soares, J. A. M. Munhoz, F. G. de A. Santos, N. G. de Siqueira and R. P. M. Silva, Análise dos efeitos da suplementação dietética com Camu-Camu comparada à gastrectomia vertical no controle de peso de ratos Wistar, Revista do Colégio Brasileiro de Cirurgiões, 2019, 46(4), 1–8 CrossRef PubMed.
- F. F. Anhê, R. T. Nachbar, T. V. Varin, J. Trottier, S. Dudonné, M. Le Barz, P. Feutry, G. Pilon, O. Barbier, Y. Desjardins, D. Roy and A. Marette, Treatment with camu camu (Myrciaria dubia) prevents obesity by altering the gut microbiota and increasing energy expenditure in diet-induced obese mice, Gut, 2019, 68(3), 453–464, DOI:10.1136/gutjnl-2017-315565.
- A. Abot, A. Brochot, N. Pomié, E. Wemelle, C. Druart, M. Régnier, N. M. Delzenne, W. M. De Vos and P. D. Cani, Camu-camu reduces obesity and improves diabetic profiles of obese and diabetic mice: a dose-ranging study, Metabolities, 2022, 12(3), 301 CrossRef CAS PubMed.
- M. Peña Hidalgo, F. Orlando, E. Campos, M. Donayre Ramirez, J. Villacrés-Vallejo and D. V. Torres, Efecto tóxico y antidiabético de tres plantas amazónicas en ratones balb/c inducidas con estreptozotocina, Ciencia Amazónica (Iquitos), 2021, 9(2), 21–32 CrossRef.
- A. Fujita, D. Sarkar, S. Wu, E. Kennelly, K. Shetty and M. I. Genovese, Evaluation of phenolic-linked bioactives of camu-camu (Myrciaria dubia Mc. Vaugh) for antihyperglycemia, antihypertension, antimicrobial properties and cellular rejuvenation, Food Res. Int., 2015, 77, 194–203, DOI:10.1016/j.foodres.2015.07.009.
- T. Miyashita, R. Koizumi, T. Myoda, Y. Sagane, K. Niwa, T. Watanabe and K. Minami, Data on a single oral dose of camu camu (Myrciaria dubia) pericarp extract on flow-mediated vasodilation and blood pressure in young adult humans, Data Brief, 2018, 16, 993–999, DOI:10.1016/j.dib.2017.12.009.
- J. F. F. Roncato, D. Camara, T. C. Brussulo Pereira, C. B. Quines, L. M. Colomé, C. Denardin, S. Haas and D. S. Ávila, Lipid reducing potential of liposomes loaded with ethanolic extract of purple pitanga (Eugenia uniflora) administered to Caenorhabditis elegans, J. Liposome Res., 2019, 29(3), 274–282, DOI:10.1080/08982104.2018.1552705.
- N. S. G. Schumacher Caracterização da propriedade antiinflamátoria dos componentes do extrato aquoso das folhas de Eugenia uniflora sobre a expressão do diabetes, no modelo experimental de diabetes espontâneo Tipo 1 (Camundongos Nod), Master Thesis Medical Clinic, 2015, https://repositorio.unicamp.br/Busca/Download?codigoArquivo=450703 Search PubMed.
- E. L. Munari, J. L. Girardelo, G. G. Cechinel, C. Soldi, G. M. M. Conterato, J. C. Benech and E. Winter, Potential antitumoral de extratos de Eugenia involucrata DC: uso de produtos regionais para tratar um problema mundial, Repositório Institucional UFSC, 2021, Vol. 18 Search PubMed.
- L. M. Reguengo, R. de P. do Nascimento, A. P. da F. Machado and M. R. Marostica Junior, Signaling pathways and the potential anticarcinogenic effect of native Brazilian fruits on breast cancer, Food Res. Int., 2022, 155, 111117, DOI:10.1016/j.foodres.2022.111117.
- M. A. C. N. Da Silva, J. H. Costa, T. Pacheco-Fill, A. L. T. G. Ruiz, F. C. B. Vidal, K. R. A. Borges, S. J. A. Guimarães, A. P. S. de Azevedo-Santos, K. E. Buglio, M. A. Foglio, M. D. C. L. Barbosa, M. D. D. S. B. Nascimento and J. E. de Carvalho, Açai (Euterpe oleracea Mart.) Seed Extract Induces ROS Production and Cell Death in MCF-7 Breast Cancer Cell Line, Molecules, 2021, 26(12), 1–23 CrossRef PubMed.
- A. M. N. Da Silva, C. S. Soares, K. R. A. Borges, L. A. S. Wolff, M. D. C. L. Barbosa, D. S. B. Nascimento, M. do and J. E. De. Carvalho, Ultrastructural changes induced by açaí (Euterpe oleracea Mart) in MCF-7 breast cancer cell line, Ultrastruct. Pathol., 2022, 46, 511–518 CrossRef PubMed.
- A. P. da F. Machado, M. da R. Alves, R. de P. do Nascimento, L. M. Reguengo and M. R. Marostica Junior, Antiproliferative effects and main molecular mechanisms of Brazilian native fruits and their by-products on lung cancer, Food Res. Int., 2022, 162, 111953, DOI:10.1016/j.foodres.2022.111953.
- H. Song, X. Shen, R. Deng, Y. Zhang and X. Zheng, Dietary anthocyanin-rich extract of açai protects from diet-induced obesity, liver steatosis, and insulin resistance with modulation of gut microbiota in mice, Nutrition, 2021, 86, 111176, DOI:10.1016/j.nut.2021.111176.
- A. T. Holkem, V. Robichaud, C. S. Favaro-Trindade and M. Lacroix, Chemopreventive Properties of Extracts Obtained from Blueberry (Vaccinium myrtillus L.) and Jabuticaba (Myrciaria cauliflora Berg.) in Combination with Probiotics, Nutr. Cancer, 2020, 73, 671–685, DOI:10.1080/01635581.2020.1761986.
- S. A. Lenquiste, C. de Almeida Lamas, R. da Silva Marineli, É. A. Moraes, P. C. Borck, R. L. Camargo, V. H. A. C. Quitete, E. M. Carneiro and M. R. M. Junior, Jaboticaba peel powder and jaboticaba peel aqueous extract reduces obesity, insulin resistance and hepatic fat accumulation in rats, Food Res. Int., 2019, 120, 880–887 CrossRef CAS PubMed .https://www.sciencedirect.com/science/article/pii/S0963996918309384.
- P. S. Loubet Filho, A. M. Baseggio, M. M. Vuolo, L. M. Reguengo, A. C. Telles Biasoto, L. C. Correa, S. B. Junior, V. H. Alves Cagnon, C. B. Betim Cazarin and M. R. Maróstica Júnior, Gut microbiota modulation by jabuticaba peel and its effect on glucose metabolism via inflammatory signaling, Curr. Res. Food Sci., 2022, 5, 382–391, DOI:10.1016/j.crfs.2022.02.001.
- G. Jamar, A. B. Santamarina, A. C. Flygare, A. Gagliardi, V. V. de Rosso, V. Z. Dourado and L. P. Pisani, Effects of the juçara fruit supplementation on metabolic parameters in individuals with obesity: a double-blind randomized controlled trial, J. Nutr. Biochem., 2020, 83, 108430, DOI:10.1016/j.jnutbio.2020.108430.
- R. P. Nascimento, L. M. Reguengo, A. P. F. Machado da and M. R. Marostica Junior, Signaling pathways and the potential anticarcinogenic effect of native Brazilian fruits on breast cancer, Food Biosci., 2022, 46, 1–18 Search PubMed.
- A. A. Puppin, C. L. da Silva, S. N. Ronchi, M. de A. Silva, E. M. de Lima, B. G. de Oliveira, W. Romão, N. S. Bissoli, T. U. de Andrade, D. C. Endringer and G. A. Brasil, Chronic treatment with juçara (Euterpe edulis) fruit pulp produces antihypertensive effect and improve on baroreflex sensitivity in Spontaneous Hypertensive Rats (SHR), Res., Soc. Dev., 2022, 11, 1–11 Search PubMed.
- M. Fidelis, M. A. V. do Carmo, T. M. da Cruz, L. Azevedo, T. Myoda, M. Miranda Furtado, M. Boscacci Marques, A. S. Sant'Ana, M. Inês Genovese, W. Young Oh, M. Wen, F. Shahidi, L. Zhang, M. Franchin, S. M. de Alencar, P. Luiz Rosalen and D. Granato, Camu-camu seed (Myrciaria dubia) – From side stream to an antioxidant, antihyperglycemic, antiproliferative, antimicrobial, antihemolytic, anti-inflammatory, and antihypertensive ingredient, Food Chem., 2020, 310, 125909 CrossRef CAS PubMed.
- J. M. García-Chacón, J. C. Marín-Loaiza and C. Osorio, Camu Camu (Myrciaria dubia (Kunth) McVaugh): An Amazonian Fruit with Biofunctional Properties-A Review, ACS Omega, 2023, 8, 5169–5183 CrossRef PubMed .https://pubs.acs.org/doi/abs/10.1021/acsomega.2c07245.
- A. Cipriani, A. L. de Sousa, A. Tenfen, D. A. Siebert, A. L. Gasper, L. de Vitali, G. A. Micke and M. D. Alberton, Phenolic compounds of Eugenia involucrata (Myrtaceae) extracts and associated antioxidant and inhibitory effects on acetylcholinesterase and α-glucosidase, Nat. Prod. Res., 2020, 36, 1134–1137, DOI:10.1080/14786419.2020.1855640.
- K. Lutfiyah, K. Lutfiyah, S. Pujiyanto and S. N. Jannah, Production of α-Amylase Inhibitors of Aspergillus RD2 from Dewandaru (Eugenia uniflora L.) as Diabetes Drug, Biosaintifika, 2022, 14, 340–347, DOI:10.15294/biosaintifika.v14i3.39334.
- O. T. W. Lamdho, M. Angelina, I. D. Dewijanti and R. T. Dewi, Effect of nanoparticles dewandaru fruits extract to reduce total cholesterol on hyperlipidemic mice, AIP Conf. Proc., 2022, 2493(1) DOI:10.1063/5.0110178.
| This journal is © The Royal Society of Chemistry 2024 |
