Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Controlling the diffusion of small molecules from matrices processed by all-aqueous methodologies: towards the development of green pharmaceutical products
Bárbara S.
Neves
 ,
Raquel C.
Gonçalves
,
Raquel C.
Gonçalves
 ,
João F.
Mano
,
João F.
Mano
 * and
Mariana B.
Oliveira
* and
Mariana B.
Oliveira
 *
*
Department of Chemistry, CICECO – Aveiro Institute of Materials, University of Aveiro. Campus Universitário de Santiago, 3810-193 Aveiro, Portugal. E-mail: jmano@ua.pt; mboliveira@ua.pt
First published on 28th February 2024
Abstract
Green technologies for the development of drug delivery systems (DDSs) are important to lower the environmental impact associated with drug manufacturing and may help in decreasing risks associated with common excipients. All-aqueous technologies may be plausible routes to realize the sustainable and safe development of DDSs. In general, the aqueous processing of polymeric materials culminates in the formation of structures that behave as hydrogels, which have been widely used in the fields of tissue engineering and regenerative medicine, agriculture, and food development. Although a high number of studies can be found involving hydrogels for controlled drug delivery purposes, they usually focus on the encapsulation and controlled release of medium to large sized molecules (usually proteins). Concerning the controlled release of small hydrophilic molecules (<1000 Da), few examples are available, and from the point of view of clinical translation and market approval, examples are even scarcer. Retention in the encapsulating matrix normally relies on drug–polymer interactions since the regulation of the mesh size of the network is not sufficient to provide a controlled release of such drugs or depends on steps that lead to low initial drug contents in the matrix. Here, we critically discuss the advantages of green approaches for producing DDSs and highlight the main advances in the challenging task of using matrices fabricated in all-aqueous settings for the encapsulation and release of small hydrophilic drugs.
1. Introduction
When administered in a free form, most drugs, ranging from small molecules to biopharmaceuticals (Fig. 1a), exhibit a tendency for degradation due to the contact with biological agents and environmental stimuli variations (e.g., enzymes, pH variations). Depending on their nature, mainly biopharmaceuticals are also often recognized and further cleared by the immune system.1 Therefore, to achieve and improve the therapeutic effect of drugs, the use of high dosages or repeated administrations are normally required, often leading to side effects related to systemic circulation of drugs, including toxicity, and the burden of renal and hepatic clearance mechanisms. The repeated intake of drugs has also been associated with low patient compliance with therapeutics.2,3 Short circulation times are common, limiting drug bioavailability and overall therapeutic efficacy.2 In order to overcome such inherent disadvantages associated with drug administration, drug delivery systems (DDSs) have gained momentum in the pharmaceutical industry, with a global market size rated at USD 34.70 billion in 2021 and envisioned to grow to USD 78.76 billion by 2030.4 DDSs, defined as a dosage form which comprises the active pharmaceutical ingredient (API) and excipients, often aim at tailoring drug release over time and, for some cases, in space, limiting the actuation region of the delivered drug.5 Overall, the main objective of DDSs typically relies on maximizing the timeframe in which drugs are in the therapeutic window (the region between the minimum level needed to achieve efficacy and the maximum level associated with toxicity) and, ultimately, leading to the less frequent administration of lower dosages, with less off-target effects, and expected increasing adherence of patients to therapies.2,3,6 The production of DDSs through green and all-aqueous methodologies may be of great interest to overcome the environmental concerns related to the most commonly applied strategies to obtain these drug carriers and will be later explored in this review.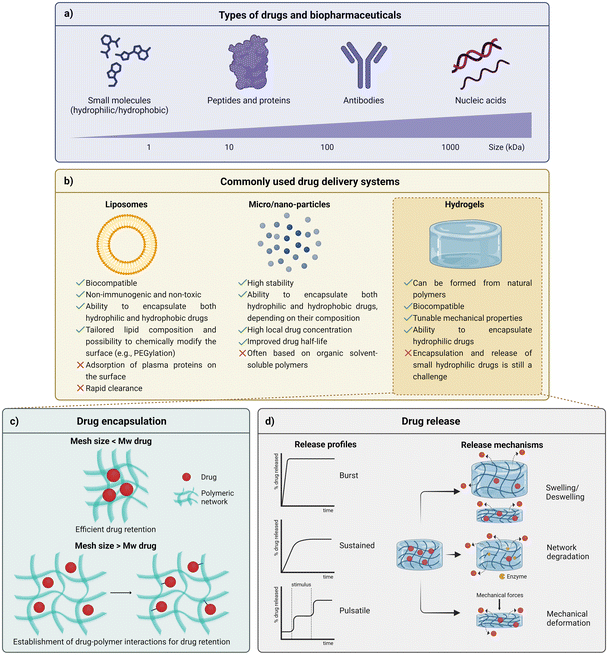 | ||
| Fig. 1 Key parameters to control the diffusion of drugs from drug delivery systems (created with BioRender.com). (a) Drugs ranging from small molecules to biopharmaceuticals. (b) Most common types of drug delivery systems and their advantages and limitations. (c) Relationship between the mesh size and the molecular weight (Mw) of the drug and its influence on the efficacy of drug retention. (d) Typical drug release profiles obtained from hydrogels with different predominant release mediating mechanisms. (d) was partially inspired by a schematic representation of ref. 2. | ||
1.1. Fundamentals of drug encapsulation, drug release, common types of drug delivery systems and current challenges
Sphere-shaped DDSs are the most common systems available both in academic and market approaches.8 Their predominance is justified not only because their processing is often considered simple, but also because the achievement of sustained drug release profiles is facilitated by their architecture.9 The high surface-to-volume ratio of spherical-shaped DDSs results in more interaction sites between the drug and the encapsulating excipient matrix, which are important for drug delivery purposes, mainly in systems where the degradation of the excipient matrix and molecular diffusion are the forces driving drug release. Therefore, water-insoluble materials susceptible to medium-term degradation under aqueous conditions – including synthetic polymers such as polylactic acid (PLA), poly(caprolactone) (PCL) and poly(lactic-co-glycolic acid) (PLGA) are often employed by the pharmaceutical industry to prepare particles that constitute oral intake tablets.10,11 Through the architectonic control of microparticles and their aggregation in tablets, it is possible to regulate their pharmacokinetics, often avoiding the occurrence of the burst release phenomenon.12
Liposomes and similar structures. Unlike continuous polymeric particles, liposomes are formed through the hydrophilic/hydrophobic interaction between lipid/water and lipid/lipid molecules, resulting in an aqueous core surrounded by a lipidic bilayer. Several formulations of self-assembled vesicles, that can be categorized based on their size and lamellarity (number of bilayer membranes), have been reported to be generally accepted as biocompatible, non-immunogenic, non-toxic, and capable of encapsulating both hydrophilic and hydrophobic drugs.6 Additionally, since they can be tailored by changing lipid composition and/or by chemically modifying their surface, liposomes are considered promising candidates to integrate versatile DDSs. However, these colloidal particles can adsorb plasma lipoproteins on their surface, which limits their stability and contributes to their rapid clearance.5 In order to overcome these limitations, it is possible to functionalize the surface of the liposomes with polymers, most commonly using poly(ethylene glycol) (PEG). In fact, this strategy – usually addressed as PEGylation – is a widely adopted strategy to provide liposomes and other nanoparticles with shielding from the action of the immune system. Thus, the use of this polymer to decorate the surface improves the stability of the nanoparticulated DDSs and, consequently, the drug delivery efficiency since it promotes a reduction of clearance while extending the circulation half-time of particles.13 Alternatively, functional amphiphilic polymers can self-assemble into structures similar to liposomes, named polymersomes, which are more stable than liposomes and may be tailored to showcase responsiveness to environmental cues (e.g., changes in the temperature or pH).5,6 However, the toxicity related to residual organic solvents and some laborious fabrication steps have limited the clinical application of both liposomes and polymersomes.13 Nevertheless, it is worth noting that, recently, lipid-based nanoparticles stabilized with PEG were applied as a DDS for the delivery of nucleic acids, namely modified mRNA, in COVID-19 vaccines.1
Hydrogels. Since most micro-objects currently applied in the clinic are based on organic solvent-soluble polymers, hydrogels have emerged as promising aqueous-based DDSs, which can be formed from natural polymers (e.g., sodium alginate (SA), gelatin). In fact, due to their usual low toxicity and, in some cases, biodegradability, as well as relatively low cost, natural polymers are appealing materials to surpass toxicity concerns related to the use of organic solvents.6,14 Owing to their high-water content (usually in the range of 70 to 99%), hydrogels also display physical similarity to tissues, with some examples showing high biocompatibility. Due to their crosslinked polymer network, which may result from non-covalent or covalent bonds, these three-dimensional (3D) networks also exhibit tuneable mechanical properties. Moreover, hydrogels can be tuned to showcase a myriad of sizes (macroscopic hydrogels, microgels and nanogels) and architectures. When effective drug entrapment is achieved, and no burst release occurs, hydrogels can ensure the protection of the therapeutic agents from degradation. However, drug release from such highly hydrated matrices is often mediated by swelling mechanisms, which condition the mesh size of these structures in the presence of solvents. Drugs and biopharmaceuticals with average sizes higher than hydrogel mesh size have been effectively retained in hydrogel structures (Fig. 1c). Nevertheless, small drugs are normally much smaller than the retentive mesh of the hydrogels, which often culminates in a rapid and burst release of such drugs whenever the hydrogel is in contact with the delivery medium (e.g., in vitro medium solutions; blood and other body fluids). Thus, most approaches seeking the retention and controlled release of small molecules from hydrogels have been based on the tailoring of chemical drug-polymer interactions (Fig. 1c).2,13
1.2. Conventional and green techniques to produce DDSs
The pharmaceutical industry adopts a wide range of techniques (common examples in Table 1) to produce DDSs, aiming for the improvement of drug solubility and bioavailability and promoting a proper encapsulation, delivery, and release.15,16Table 1 provides a summary of the main advantages and limitations associated with conventional technologies used in the pharmaceutical industry (relevant reviews about the topic can be found in ref. 17–21), along with a comparison with rising green technologies mostly explored in the literature.| Conventional Technique | Advantages | Limitations | Materials/equipment | DDSs’ carrier molecules |
|---|---|---|---|---|
| Emulsification-solvent evaporation | - Low cost10 | - Large amounts of oils and organic solvents10 | - Need for characterization (microscopy and zeta-potential)23,24 | - Microparticles25,26 |
| - High speed10 | - Takes several steps22 | - Nanoparticles27,28 | ||
| Spray drying | - Bath-free technique17 | - Requires organic solvents29 | - It is difficult to collect particles < 2 μm with cyclone16 | - Microparticles30–34 |
| - One-step continuous process16 | - Difficult to control the form of the drug16 | - Bulky equipment16 | - Nanoparticles35,36 | |
| - Medium/low energy requirements16 | ||||
| Spray congealing/spray chilling/spray cooling | - Solvent-free17,18 | - High energy input17 | - Limited number of equipment available (modified/adapted spray dryers)18 | - Microparticles37–40 |
| - Low cost17,18 | - Molten fluid influences the shape and the size of the microparticles18 | - Zmax® (azithromycin microspheres in poloxamer)41 | ||
| - One-step process17,18 | ||||
| Hot-melt extrusion (HME) | - Solvent-free19–21 | - High energy input19–21 | - Limited availability of thermally stable polymers19,21 | - Films42–45 |
| - Low cost19,21 | - Downstream processes to obtain the final product17 | - Highly complex process equipment21 | - Capsules46–48 | |
| - Continuous manufacturing19–21 | - Nanocomposites49,50 | |||
| - High reproducibility19 | - NuvaRing® (progestagen and an estrogen in polyethylene vinylacetate copolymers)51 | |||
| - Lacrisert® (hydroxypropyl cellulose)52 | ||||
| - Kaletra® (lopinavir and ritonavir)21 | ||||
| Green approach | ||||
| Supercritical fluid (SCF) technology | - Mild, environmentally friendly process conditions53,54 | - Limited solubility of polar substrates53 | - High costs of the equipment (temperature- and pressure-resistant chambers)53 | - Microparticles57–64 |
| - Does not require additional post-treatment54 | - Insufficient research on the phase behaviour of SCFs53 | - High cost of some SCFs (e.g., Xe and SF6)56 | - Nanoparticles61,65–67 | |
| - Uniform particle size distribution55 | - Requires safety rules and stringent regulations (e.g., Current-Good Manufacturing Practices (cGMP)) due to the extreme conditions (pressure and temperature)53 | - Liposomes68–72 | ||
| - Requires an experienced analyst to run the samples55 | ||||
| Ionic liquids (ILs) | - Designer solvents73 | - Eventual toxicity73,74 | - Relatively inexpensive products75 | - API-ILs*76–82 |
| - Unique tailored properties73,74 | - Lack of information about in vivo effects74 | - Low production cost75 | - Films83,84 | |
| - Microemulsions85–87 | ||||
| - Microspheres88 | ||||
| - Nanoparticles89 | ||||
| Deep eutectic solvents (DESs) | - Mild processing conditions90,91 | - Limited solubility91 | - Inexpensive compounds90 | - API-DESs*92,93 |
| - Produced from low toxic compounds90 | - Does not need complex facilities90 | - Fibers94–96 | ||
| - Ion-gel97 | ||||
| - Microemulsions98 | ||||
| - EMLA® cream (eutectic lidocaine/prilocaine cream)99 | ||||
Conventional techniques applied in the pharmaceutical industry often require high energy inputs (e.g., high temperatures) and/or the use of organic solvents – the latter is difficult to remove during the washing steps, requires treatment after discarding, and also may produce toxic and pollutant volatile organic compounds (VOCs). Therefore, there is an urge to develop alternative methods able to surpass the high environmental impact generated, and compliant with the UN Sustainable Development goals.56 Aiming to reduce the emission of VOCs in various industrial installations and processes, namely in the pharmaceutical field, several political actions, such as the EU Solvent Emissions Directive (1999/13/EC), have been decreed. The Clean Air Program for Europe also sets objectives for EU air policy up to 2030 in order to reduce the nefarious effects of air pollution on health by half compared with 2005.100–102 Thus, some alternative processing techniques have been arising (as showcased in Table 1), mainly at the laboratory scale.
RESS uses SCFs for drug encapsulation and comprises two steps: (i) dissolution of both drug and carrier in an SCF, forming a supercritical solution, and (ii) passage of such supercritical solution through a nozzle into an expansion vessel. In this last step, there is depressurization from supercritical conditions to atmospheric pressure, promoting a rapid expansion of the SCF and a reduction of the solvating power that, in turn, leads to the formation of nucleation sites and, ultimately, the creation of particles through the crystallization of the API inside the matrix. The antisolvent effect of SCFs refers to SAS, which is used to prepare DDSs, namely microparticles, and consists in a previous dissolution of the drug and the polymer in an organic solvent (e.g., acetone, dichloromethane, or dimethyl sulfoxide) which is then sprayed via a nozzle to a vessel containing a supercritical fluid which acts as an antisolvent. The SCF promotes the reduction of solubility and, therefore, the precipitation of fine particles. Conversely, PGSS is the most used organic solvent-free processing for encapsulation of small molecules and relies on the melting of the carriers followed by saturation with the SCF which acts as the plasticizing agent. Through the reduction of the melting and glass transition temperatures of the solute, small solid particles are formed during depressurization.53,55,104–106 Although both lyophilization technique and SCF technology are suitable for heat-sensitive drugs, SCF methodologies, namely PGSS, exhibit a competitive advantage since they offer better control over uniformity (size and morphology) of the particles.104,107
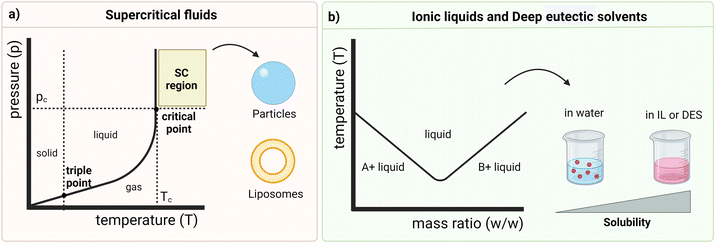
SCFs are also known for their gas-like diffusivity and viscosity (i.e., high diffusivity and low viscosity) and liquid-like density (i.e., high density) in the supercritical phase.56 Their physical properties can be adapted through temperature and pressure regulation above the critical point (as depicted in the graph of Fig. 2a), where these fluids present both liquid-like and gas-like behaviour, acting as a hybrid fluid.53,56 This high control over the critical conditions and the flow rate of the SCF leads to the crystallization of a single polymorph and determines the performance efficiency of the SCF technology in DDS design, which is also dependent on the selection of an appropriate solvent. In fact, the solubility of the drug in the SCF depends on the density of the fluid and the size of the particles is related to the pre-expansion concentration of the solute. As mentioned above, SC-CO2 (critical temperature = 31.1 °C and critical pressure = 7.38 MPa) is the most common choice since it is inert, non-toxic, non-flammable, cost effective and exists in high abundance. It is also related to relatively simple processing and manufacture of pharmaceutical products.53,54,56 To encapsulate low molecular weight (LMW) drugs in a polymeric shell, the API is solubilized in the SCF, and the polymer is then added for impregnation. This strategy, which relies on the formation of an initial amorphous form of the API, is particularly relevant for drugs with low solubility in the crystalline state because the API is firstly solubilized and then the crystallization occurs inside the polymeric matrix.106 One of the key steps in this method is the selection of the polymer, which normally also exhibits low solubility in SCF. Therefore, by increasing pressure and/or temperature, the solubility in these fluids increases and its viscosity decreases.53 The polymer plasticization upon contact with the SCF also contributes to the reduction of its viscosity, leading to the formation of smaller particles, effective entrapment of drugs, and the possible further controlled release from the hydrophilic polymer matrix.53 This green technology also increases the surface area of the particles, that, consequently, enhances the dissolution rate, culminating in higher efficacy and a decreased dosage requirement for delivery.53 Interestingly, it has been shown that the SCF technology is versatile enough to enable the preparation of liposomes, enabling surpassing the excessive use of organic solvents and the multiple steps needed for their preparation and also improving the encapsulation efficiency. The mechanism relies on the simultaneous pressurization and depressurization phenomena that cause CO2 to be released upon depressurization and dispersed in phospholipids, leading to liposomes with high encapsulation efficiency, improved drug release, high stability as well as narrow particle size distribution.53 However, the application of SCFs is still limited to the poor solubility of polar substrates, including some drugs and polymers, thus requiring large amounts of SCF and, ultimately, increasing the production costs.104 Such drawback is lightly surpassed by pre-mixing the drug, the polymer and other excipients before the SCF treatment.53 Additionally, owing to the insufficient research defining the phase behaviour of multi-component mixtures in detail, as well as due to the high costs associated with the equipment required to withstand the high pressure and temperature conditions, the extrapolation of this technology to the pharmaceutical field at an industrial scale still presents limitations.53,105 However, such high costs of equipment are counterbalanced by their ability to optimize the process, reducing the greenhouse gas emissions and, consequently, the carbon footprint which is quantified according to the life cycle assessment (LCA). The use of SCFs also presents economic benefits by reducing the disposal costs of the solvents typically used in conventional techniques. Moreover, the recovery of some SCFs (e.g., SC-CO2) further reduces the disposal costs, which contributes to the overall classification of the SCF technology as a cost-effective approach.108
Ionic liquids (ILs). Ionic Liquids (ILs) (Fig. 2b) are organic–ionic hybrid solvents able to combine numerous asymmetrical organic cations and organic or inorganic anions, and are considered “designer solvents”. These solvents also present melting points at or below 100 °C and, hence, can be liquid at room temperature (so called room-temperature ILs – RTILs).74,75 Moreover, they exhibit unique tailored properties, such as low vapor pressure under ambient conditions and tuneable solubility in both polar and non-polar solvents. In fact, the physicochemical properties of each IL are highly dependent on the combination of ions, therefore determining the biological outcomes.73,74 These features as well as their mostly hydrophilic nature contribute to the classification of ILs as greener organic solvent alternatives and make them promising candidates to be applied in the pharmaceutical industry.73,109,110 ILs have, then, a wide range of applications, namely in drug delivery (e.g., through topical, transdermal, and oral routes), synthesis and purification of pharmaceutical compounds, solubilization of hydrophobic drugs, and formulation of APIs (API-ILs). Since they can self-assemble into nanostructures when in an aqueous environment, it is possible to increase the solubility of drugs, that is mainly driven by the anion, through the formation of hydrogen bonds.73,74 These solvents can also improve pharmaceutical parameters, namely the pharmacokinetic and pharmacodynamic of drugs, and have been used to fabricate micro and nanoemulsions, to enhance their stability and drug loading.73,74
However, the application of ILs as solvents in the pharmaceutical field is still limited due to the eventual toxicity and the lack of knowledge about the microscopic interactions that occur both within the solvent and between the solvent and the drug and, ultimately, their effect in vivo (e.g., biocompatibility and biodegradability).74 The selection of the cation and the length of the alkyl side chains attached to it are crucial since they define the toxicity and biodegradability of the IL, as it has been reported that a longer alkyl side chain exhibits better biodegradability but is more toxic due to the increase of interactions with the phospholipidic layers in the cell membranes.111 In fact, the toxicity of some ILs (mainly, first-generation ILs) is a barrier to developing DDSs, which has been lightly surpassed through the adoption of second- and third-generation ILs that are formed from more biocompatible cations and anions.73,75 The adoption of precursors from biocompatible sources is an interesting strategy to develop biocompatible ILs.75 Since purified protein-derived compounds are usually considered non-toxic, biodegradable, and biocompatible, they constitute the main building blocks to synthesize cations and anions to, ultimately, form such biocompatible ILs. The selection of cholinium or, more recently, glycine betaine as cations and the use of anions derived from biological buffers (e.g., zwitterionic amino acid derivatives) or organic acids (e.g., malic acid) are also strategies to design biocompatible ILs to be used as pharmaceutical excipients due to their general safety.111–113
Besides improving the solubility of drugs and acting as permeation enhancers,114 ILs have been applied in the preparation of biomaterials used for drug delivery.112 For example, Dias et al. took advantage of the abovementioned properties of choline-based ILs and loaded choline chloride and choline dihydrogen phosphate in chitosan films, that were further used for the development of a pH-responsive DDS for dexamethasone.83 Hua et al. also studied the application of chitosan to develop stimuli-responsive DDSs but by conjugating such a polymer with a hydrophobic drug using the 1-butyl-3-methylimidazolium chloride IL and, finally, adding poly(N-isopropylacrylamide) (PNIPAAm).115
Deep eutectic solvents (DESs). Deep eutectic solvents (DESs) (Fig. 2b) comprise a different branch of green solvents to develop DDSs and consist of a combination of at least two compounds homogeneously mixed that melt at a temperature that is lower than the melting temperature of any of the constituents. Therefore, they deviate from the ideal thermodynamic solid–liquid phase behaviour, being liquid at room and human body temperature (Fig. 2b), and are also considered biocompatible.90,91 Starting from low toxic, easily available and inexpensive compounds, DESs are produced by heating, grinding, vacuum evaporation or freeze-drying methods, being considered a green approach.
Furthermore, they are characterized by their low vapor pressure and non-inflammability, can be chemically tailored, have a solvency power for several solutes and are not reactive in water.90 Thus, these solvents have emerged as a versatile method for enhancing the solubility, permeability, stability and bioavailability and, consequently, the therapeutic efficacy of drugs.91
The improvement of solubility, particularly important for hydrophobic compounds, may be due to the hydrotropic effect exhibited by some DESs. Such an effect is defined by altering the solubility, and changing the concentration of the additive. Oliveira et al. demonstrated that the solubility of gallic acid is higher using the DES composed of cholinium chloride ([Ch]Cl) and 1,2-propanediol at a concentration of around 80 wt% and using the DES composed of [Ch]Cl and ethylene glycol at a concentration around 60 wt%, compared to pure constituents.116
This improvement in the drug properties may be achieved through the dissolution of the API in DES (DES acts as a pharmaceutical solvent) or by integrating the API as one of the components of the DES (API-DES).91 Particularly, drug solubilization and stabilization can be adjusted by selecting compounds considering their physical–chemical properties as well as their ratio. Moreover, DESs avoid the thermal and light degradation of drugs.91 Regarding the API-DES approach, also called therapeutic DESs, it aims to decrease the drug melting temperature to obtain a liquid form of the drug, that is also influenced by the second DES component. The already reported API-DESs display melting temperatures near or below the temperature of the human body and are topically or orally administered. In fact, DESs are mainly used as permeation enhancers and scarcely explored as DDSs. However, owing to the ability of DESs to increase the solubility of both APIs and biopolymers in an aqueous environment, the exploration of novel administration routes and the development of stimuli-responsive systems relying on this technique is envisioned.91 A study developed by Mukesh et al. is an example of the preparation of biomaterials for drug delivery using these solvents. Owing to the abovementioned high biocompatibility and non-toxicity of choline-based solvents, they synthesized chitin nanofibers using the choline chloride-thiourea DES. Such nanofibers were, then, incorporated in calcium alginate beads, promoting a sustained release of 5-fluorouracil, under physiological conditions, for 24 h.94
2. Focus on hydrogel-based DDSs
All-aqueous processing approaches have been gaining more relevance owing to their advantages compared to conventional techniques. As mentioned above, conventional methods are typically associated with the use of organic solvents and the production of toxic and volatile by-products. All-aqueous methods are then a promising alternative which rely on the use of water, under mild processing conditions, and do not produce such compounds, so there is no need for treatment of hazardous solvents and there is a reduction in the emission of pollutant VOCs. Thus, simpler apparatus is required, ultimately leading to a reduction of costs of such technology. However, the use of water as a green approach should be discussed with caution because, despite being a cheap raw material, its global availability is reduced, and its use has become limited by the depletion of water resources. In fact, it has been estimated that millions of gallons of wastewater are produced by several industries, mainly in European countries.108,117 Aiming to reduce these numbers, several European policies (e.g., the Blueprint to Safeguard Europe's Water Resources and Environmental Quality Standards Directive) for the preservation and management of water have been applied, involving different activities such as industry. For instance, in LbL approaches applied for pharmaceutical purposes, high volumes of water are required due to the need to maintain the drug-loaded templates immersed in such solvent, which is not appealing from an environmental perspective. Thus, the adoption of all-aqueous structures as DDSs (e.g., hydrogel-based) should rely on the total utilization of the water involved in the process, in order to minimize wastewater and to be considered a green approach.2.1. Theoretical consideration of drug retention and release from hydrogels
In continuous hydrogel models, it is often considered that – in the absence of relevant chemical interactions between the drug and the matrix – the retention and release profile of the drug is mostly driven by diffusion mechanisms. This process takes over the drug release when the mesh size is bigger than the drug. The value of diffusivity (D) can be calculated according to eqn (1) (Stokes–Einstein equation), where R is the gas constant, T is the absolute temperature, η is the viscosity of the solution and rdrug represents the radius of the drug, that relates with its Mw. For small molecules (and, particularly, for hydrophilic ones), this value is high, meaning that these drugs easily diffuse through the network.2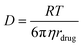 | (1) |
In order to immobilize drugs by steric hindrance, the mesh size could be reduced through the increase of either polymer or crosslinker concentration, thus defining the molecular weight cut-off (MWCO). Finally, for the release of the entrapped drugs, the value of the MWCO may be modulated over time, according to several strategies (Fig. 1d).2
2.2. Current strategies for the retention and controlled release of small hydrophilic drugs from hydrogels
Physical adsorption. Physical adsorption (Fig. 3a) is a simple process in which molecules are physically adsorbed through inter-molecular interactions (e.g., ionic interactions, hydrogen bonding, hydrophobic interactions, π–π interactions), presenting high biocompatibility. Polyelectrolytes, that contain charged functional groups, are extensively used for the retention of LMW hydrophilic drugs because they can capture and load such drugs and also form stable nanoparticles through the compression of the polymer chains. Once this strategy is typically based on electrostatic interactions, there are no toxicity concerns related to the use of chemical crosslinking agents or solvents. Moreover, it is possible to obtain entrapment by mixing the drugs and the carrier polymers at room temperature. Despite these advantages, the retention through physical adsorption is highly dependent on the bonds established between the matrix and the drug. Thus, owing to the non-covalent nature of these bonds, physical adsorption is characterized by a less controlled retention, leading to an initial rapid drug release that may also be explained by the saturation of the counter-ions of the polymers or by rapid ion exchange.13
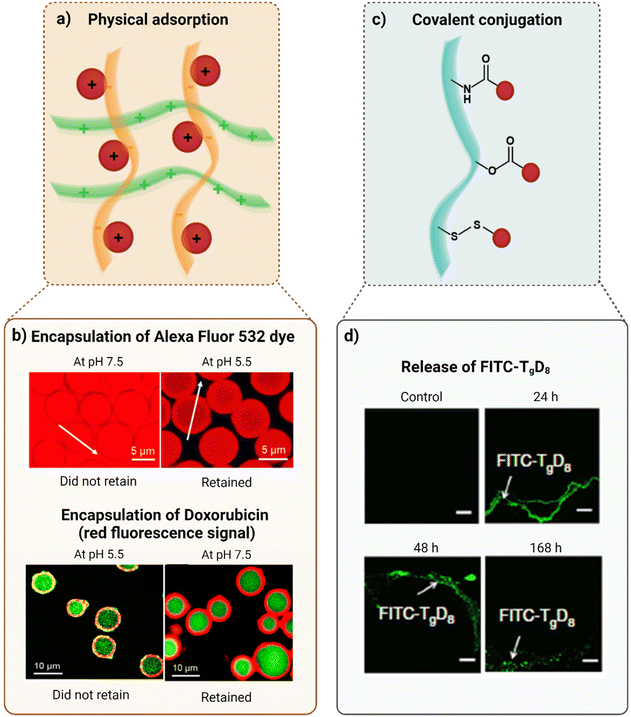 | ||
| Fig. 3 Strategies to retain small hydrophilic drugs (schematically represented by red spheres) (created with BioRender.com). (a) Physical adsorption. The orange and green polymer chains are oppositely charged. (b) Confocal laser scanning microscopy (CLSM) images of the pH-induced encapsulation of the Alexa Fluor 532 dye inside capsules, and of doxorubicin (the red fluorescence signal) into the capsule wall. (c) Covalent conjugation through an amide bond, ester bond and disulfide bond, respectively (from top to bottom). (d) CLSM images 24, 48, and 168 h after sciatic nerve injection of FITC–TgD8. Here, FITC represents the interaction that occurred with tetrodotoxin. Figures (b) and (d) were reproduced from ref. 120 and 121, respectively, with permissions from American Chemical Society, copyright 2018, and Nature Portfolio (https://creativecommons.org/licenses/by/4.0/.), copyright 2019. | ||
Studies performed by Klak et al. were focused on evaluating the release profile of small charged molecules (methylene blue, eosin, and bromothymol blue) from gelatin gels, (i) composed of gelatin only, (ii) containing additional viscous non-crosslinking alginate (a semi-interpenetrating polymer network), (iii) with an interpenetrating alginate calcium-gelled network (mixed gels) and (iv) containing pre-formed and mixed alginate beads.122 It was verified that molecule release from gelatine gels does not depend on the Mw of the molecules but relies on the ionic interactions between the loaded dyes and the protein network (that was positively charged at the pH of the experiments). Therefore, eosin (Mw = 692 Da; negatively charged) diffused slowly, whereas methylene blue (Mw = 320 Da; positively charged) was rapidly released due to the ionic repulsion with the gelatin network. Bromothymol blue (Mw = 624 Da; uncharged dye) was used as a control to evaluate the effect of ionic interactions in dye release because, owing to the absence of charges in its structure, it would not be retained in the network by electrostatic interactions.122 Through the incorporation of alginate (negatively charged polysaccharide) into the protein gel, the effect of the physical state of alginate on the diffusion of these LMW molecules was assessed, indicating that the viscosity of alginate did not exert a great influence. According to the results obtained, it was possible to conclude that ionic interactions were, then, the main players in the regulation of the diffusion of these dyes, so, increasing the concentration of alginate slowed the release of methylene blue but the same effect was not verified for the release profile of eosin. Besides, once the bromothymol blue was not retained by alginate, its release was not affected by the concentration of this polymer.122 On the other hand, the formation of mixed gelatin–alginate gels (in which alginate was gellified with calcium ions inside the gelatin gel) was also not sufficient to allow the definition of a release profile independent of ionic interactions. The networks remained too loose, with large mesh sizes, and the entrapment of small molecules by simple steric hindrance was not possible. Consequently, the negatively charged polysaccharide delayed the release of the positively charged molecules (methylene blue), through ionic interactions.122 Regarding gelatin gels containing pre-formed alginate beads with calcium ions (that, in turn, contained methylene blue), the release of molecules occurred more slowly than in the previously mentioned approaches since here the diffusion firstly occurred from the polysaccharide gel to the protein gel and, afterwards, for the external medium. Concerning eosin encapsulation in alginate beads following this method, this dye was rapidly released from alginate (due to the opposite charges) but was then retained by the gelatin gel through ionic interactions, delaying its release into the external medium. Furthermore, the effect of alginate lyase on alginate beads degradation, within the gelatin gel (in which the enzyme was incorporated), was analysed as well as the respective impact on the release kinetics of the dyes. While for methylene blue it was demonstrated that the release rate of the dye was directly related to the concentration of enzyme, this effect was not observed for the negatively charged eosin due to lack of interaction with the alginate gel.122
This drug retention approach was also tested by Kozlovskaya et al. who reported the pH-induced post-loading of hydrophilic compounds both in the inner cavity and in the shell of multilayer hydrogel capsules, that were then resealed with 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da dextran. The negatively charged Alexa Fluor 532 dye (Mw = 723.8 Da) was retained inside the positively charged capsule cavity, at pH 5.5, while the cationic doxorubicin (Mw = 543.5 Da) was encapsulated in the anionic shell, at pH 7.5, as showcased in Fig. 3b.120
000 Da dextran. The negatively charged Alexa Fluor 532 dye (Mw = 723.8 Da) was retained inside the positively charged capsule cavity, at pH 5.5, while the cationic doxorubicin (Mw = 543.5 Da) was encapsulated in the anionic shell, at pH 7.5, as showcased in Fig. 3b.120
Another study in this field refers to the one performed by Moreno-Villoslada and colleagues, in which LMW hydrophilic cationic molecules were immobilized in chitosan/poly(sodium 4-styrenesulfonate) nanoparticles through aromatic-aromatic interactions. It was demonstrated that the intensity of the binding between the entrapped molecules and the particles increased with decreasing hydrophilicity. Regardless of the abundance of negative charges, poly(sodium 4-styrenesulfonate) exhibited a lower ability to bind the LMW cationic molecules as it complexed with chitosan. Thus, aromatic-aromatic interactions dictated the association efficiency between positively charged aromatic groups of the molecules and poly (sodium 4-styrenesulfonate), that also contained aromatic groups and was negatively charged.123
In order to provide a sustained release of hydrophilic drugs for a few days, Schulze and co-workers established a polyelectrolyte-layered system composed of alginate beads (in which the drug was incorporated), formed through electrohydrodynamic atomization, that were further coated with polyelectrolyte layers. The addition of polyelectrolyte layers proved to delay the burst release, as verified with adenosine 5′-triphosphate (ATP) (water soluble; Mw = 507 Da). The application of five alternating layers of poly(allyl amine) (polycation layer) and alginate (polyanion layer) led to a more successful sustained release since ATP electrostatically interacted with the polycation layers.124
Covalent conjugation. As opposed to physical adsorption, covalent conjugation (Fig. 3c) exhibits improved stability due to the strong linkages between LMW molecules and the polymers or lipid chains, that can be highly stable (e.g., amide bonds) or cleaved in response to stimuli (e.g., ester bonds and disulfide bonds). Furthermore, by modifying polymer composition and the number of reactive sites as well as the drug-to-polymer ratio, it is possible to control the drug entrapment with high precision. However, coupling agents and solvents, that have inherent toxicity and, consequently, environmental implications and regulatory issues, are often required in this type of drug retention.2,13,123
Studies performed by Zhao et al. have proven that tetrodotoxin (Mw = 319 Da) remained retained in PEGylated and non-PEGylated polymers through hydrolysable ester bonds and its release rate could be controlled, as represented in Fig. 3d, based on the hydrophilicity of the polymers, in a proportional way.13,121
Exploration of multicompartmentalized hydrogel-in-hydrogel devices. Since multilayer hydrogels have emerged as an excellent alternative to the drug leakage phenomenon that monolayer hydrogels face, Hu et al. created a double-layer hydrogel sustained-release system (Fig. 4a) consisting of a polysaccharide (SA and carboxymethyl cellulose) inner core, formed through physical crosslinking of Ca2+, and a synthetic polymer (poly(acrylamide) or its derivatives) outer layer, added by chemical crosslinking. Considering the advantages of these systems, namely the possibility to control both layers, that can be relatively independent from each other, it was possible to control the release profile of LMW drugs (indomethacin and metformin), by changing not only the inner layer composition but also the thickness of the outer layer of the hydrogel. The inner interpenetrating network hydrogel, formed by natural polymers, exhibited an alkaline pH-sensitive behaviour, avoiding the burst release phenomenon in the stomach and providing a sustained release in the intestinal environment. This effect was reinforced by the synthetic polymer outer layer that, owing to its residual swelling capacity, could prevent the inner hydrogel expansion and further drug diffusion, controlling drug release. It was proven that the inner hydrogel layer tended to gradually erode, due to the weak physical crosslinking, while the outer hydrogel kept its integrity towards swelling. Thus, it was possible to achieve a sustained-release effect, that was also positively affected by increasing the thickness of the outer layer. When exposed to the intestinal pH, indomethacin (Mw = 358 Da, hydrophobic molecule) was gradually released due to the equilibrium between the swell of the inner layer and the diffusion resistance offered by the thickness of the outer layer. However, this behaviour was not displayed by metformin (Mw = 129 Da, hydrophilic drug), that, by contrast, exhibited a burst-release effect, being almost totally released in 2 h. These results, depicted in Fig. 4b, corroborated how challenging it is to control the release of hydrophilic LMW drugs because, owing to its water solubility, metformin easily diffused through the hydrogel, even before the occurrence of swelling.14
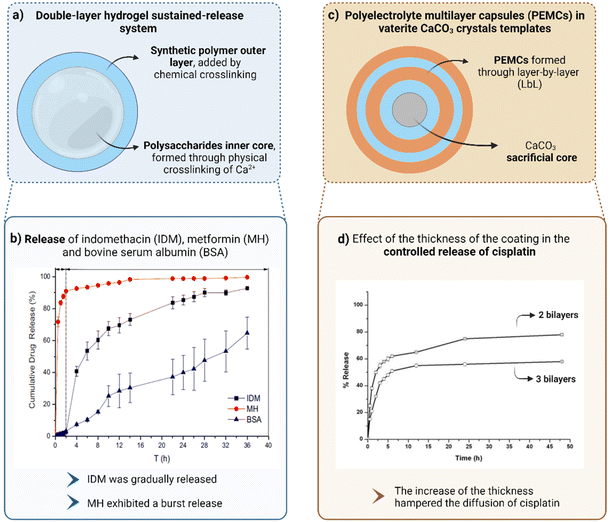 | ||
| Fig. 4 Strategies to retain low molecular weight molecules with highlight on physical barriers (created with BioRender.com). (a) Double-layer hydrogel sustained–release system. (b) Graphical representation of the cumulative release of indomethacin (IDM), metformin (MH) and bovine serum albumin (BSA) (high molecular weight molecule). BSA and IDM were gradually released while MH exhibited a burst release. (c) Polyelectrolyte multilayer capsules (PEMCs) in vaterite CaCO3 crystal templates. (d) Graphical representation of the effect of the thickness of the coating in the controlled release of cisplatin from layer-by-layer (LbL) nanocapsules. (b) and (d) were reproduced from ref. 14 and 125, respectively, with permissions from Nature Portfolio (https://creativecommons.org/licenses/by/4.0/.), copyright 2021, and Elsevier Ltd, copyright 2015. | ||
Exploration of the layer-by-layer (LbL) technology. In order to try to obtain a more controlled release of small hydrophilic drugs, recently, a strategy based on the formation of polyelectrolyte multilayer capsules (PEMCs) in vaterite CaCO3 crystals templates has been applied (Fig. 4c).126 As previously mentioned, the adoption of multilayer systems is an alternative to delay the drug release process,127 so PEMCs were produced according to layer-by-layer (LbL) technique, from which core–shell complexes were formed. Oppositely charged polyelectrolytes were alternately deposited onto degradable core templates, that were further removed, leaving behind the polymeric shell, that culminated in a PEMC.128 Although PEMCs are already used to encapsulate macromolecules, these multilayer capsules often exhibit low capacity to retain LMW drugs. Therefore, they were combined with biocompatible and readily decomposable vaterite CaCO3 crystals that acted as sacrificial cores and led to the high retention of LMW drugs (that might occur during the formation of the capsules – co-synthesis – or after this process – physisorption) as well as allowed the reduction of release rate while hindering the initial burst release. In studies performed by Trushina et al., capsules were subjected to heat-treatment, that annealed the polymer multilayers, resulting in a shrunk structure, with reduced permeability, that promoted the sustained release of LMW drugs from the capsule lumen.129 It was also proven by Vergaro et al. that the release rate was influenced by the polymer density within the capsule matrix or shell, that, in turn, could be tuned by changing the polymer deposition time or the number of deposition steps. Thus, it was verified that increasing the thickness of an (alginate/protamine sulfate)n system or raising the polymer shell density hampered the diffusion of cisplatin (Mw = 301 Da, hydrophilic drug) (Fig. 4d).125 The release profile of this drug was also studied by Mehnath et al. who encapsulated it in poly(diallyldimethylammonium chloride) (PDADMAC)/poly [di(sodium carboxyphenoxy)phosphazene] (PDCPP) coated CaCO3 nanoparticles and, through the formation of pores in the shell, the matrix swelled, causing cisplatin diffusion, following a release profile characterized by a previous burst-release phenomenon, followed by sustained release.130 However, owing to the time-consuming multistep process behind the processing of the inorganic templates, this strategy is not ideal to release LMW molecules independently of the chemical interactions with the matrix.
2.3. Aqueous two-phase systems (ATPSs)
Aqueous two-phase systems (ATPSs) are totally aqueous systems with low energy requirements, with potential to be used as DDSs (core–shell capsules and continuous hydrogel systems).132 In fact, the mild and all-aqueous environment provided by these systems is advantageous to maintain the bioactivity of hydrophilic drugs such as proteins and prevent their denaturation as well as broaden the range of drugs that can be used. Another important feature is the very low interfacial tension between the aqueous phases, which enhances their contact area and, therefore, the free diffusion and exchange of solutes.133,134 The most common ATPS relies on the use of dextran (DEX) and PEG since these two polymers are biodegradable, biocompatible, and exhibit a stabilizing effect on most biological products.133 Oppositely charged polyelectrolytes can be added to each aqueous phase, thus promoting single-step interfacial reactions and the formation of microparticles and capsules.132,135 For example, Ma et al. proved that the addition of poly(sodium-4-styrenesulfonate) (PSS) and poly(allylamine hydrochloride) (PAH) to DEX and PEG, respectively, resulted in microparticles, while the addition of these two polyelectrolytes to the opposite ATPS phases, PAH + DEX and PSS + PEG, led to the formation of stimuli-responsive microcapsules with liquid cores. An outside-to-inside encapsulation was performed with FITC-DEX, confirming the potential of such systems to encapsulate, protect, and trigger the release of several compounds.136 A system with similar potential was developed by Jiang et al. who fabricated pectin–chitosan–collagen microcapsules through their self-assembly in ATPS phases. Pectin (anionic polysaccharide) was added to the DEX phase whereas both chitosan (cationic polysaccharide) and collagen were added to the PEG phase. The addition of collagen promoted the formation of more robust capsules with anti-swelling and anti-shrinkage properties from which FITC-DEX (Mw = 70 kDa) was sustained released when exposed to certain stimuli.137 Vilabril et al. proposed the combination of PEG/DEX ATPS with the oppositely charged polyelectrolytes ε-poly-L-lysine (EPL) and alginate, respectively, to also form robust capsules with an opaque semipermeable membrane with potential to be applied as a DDS. However, it was reported that molecules with a molecular weight of 150 kDa easily diffused from the macrocapsules, emphasizing the incompatibility of their application to retain LMW molecules, mostly due to the polyelectrolyte complex hydrogel-like nature of the formed membranes.135 A similar conclusion was drawn from the study performed by Zhang et al. in which platelet-derived growth factor-BB (PDGF-BB; high Mw) was effectively encapsulated and released from polyelectrolyte microcapsules while small polyelectrolytes easily diffused from such capsules.138 All these studies proved that, although ATPSs are a promising green technology, the encapsulation of LMW molecules using this method is still a challenge.3. Conclusion and future perspectives
The development of DDSs has enabled disruptive advances for the pharmaceutical industry since these systems are broadly applied to encapsulate and release high to low molecular weight drugs. However, the conventional methods followed for their production still face some limitations, namely the use of organic solvents. In order to fulfil legislative requirements and substantially decrease the environmental impact of pharmaceutical manufacturing, there is an urge to employ greener alternatives. The use of SCFs has been one of the most explored methods to prepare DDS + API formulations in the pharmaceutical research field. However, solubility limitations and the use of specific equipment associated with SCF technologies still justify the development of alternative versatile techniques compatible with green processing of drug and drug-carrier formulations. The use of ILs and DESs has been mostly addressed to improve drug permeability in tissues, namely through skin. Additionally, they have also been used to improve or promote the concomitant solubility of drugs and DDS carrier precursors upon processing, although such applications are still scarce, namely, for DESs. The use of simpler technologies based on the processing of hydrogels made of water-soluble polymers and directly encapsulated drugs is also a growing trend for the preparation of drug delivery formulations. However, its application has been mostly limited to the encapsulation and release of biopharmaceuticals. While the latter are mostly constituted by proteins and their derivatives, with molecular weights in the range of dozens or hundreds of thousands of Daltons, the encapsulation and effective controlled delivery of small molecular weight drugs and therapeutic molecules using all-aqueous and easily processed hydrogels remains a challenge.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the Programa Operacional Competitividade e Internacionalização, in the component FEDER, and by National Funds (OE) through FCT/MCTES, in the scope of the projects “CellFi” (PTDC/BTM-ORG/3215/2020), and CICECO-Aveiro Institute of Materials, UIDB/50011/2020, UIDP/50011/2020 & LA/P/0006/2020. M.B.O acknowledges National Funds through FCT – Fundação para a Ciência e a Tecnologia, I.P., under the Scientific Employment Stimulus – Institutional Call – CEECINST/00013/2021.References
- A. M. Vargason, A. C. Anselmo and S. Mitragotri, Nat. Biomed. Eng., 2021, 5, 951 CrossRef PubMed.
- J. Li and D. J. Mooney, Nat. Rev. Mater., 2016, 1, 16071 CrossRef CAS PubMed.
- P. Trucillo, Processes, 2022, 10, 1094 CrossRef CAS.
- Fortune Business Insights, Drug Delivery Systems Market, 2022–2029, 2023 Search PubMed.
- S. Adepu and S. Ramakrishna, Molecules, 2021, 26, 5905 CrossRef CAS PubMed.
- J. F. Coelho, P. C. Ferreira, P. Alves, R. Cordeiro, A. C. Fonseca, J. R. Góis and M. H. Gil, EPMA J., 2010, 1, 164 CrossRef.
- K. Moore, J. Amos, J. Davis, R. Gourdie and J. D. Potts, Microsc. Microanal., 2013, 19, 213 CrossRef CAS PubMed.
- A. C. Lima, P. Sher and J. F. Mano, Expert Opin. Drug Delivery, 2012, 9, 231 CrossRef CAS PubMed.
- M. D. Neto, M. B. Oliveira and J. F. Mano, Trends Biotechnol., 2019, 37, 1011 CrossRef CAS PubMed.
- A. Vlachopoulos, G. Karlioti, E. Balla, V. Daniilidis, T. Kalamas, M. Stefanidou, N. D. Bikiaris, E. Christodoulou, I. Koumentakou, E. Karavas and D. N. Bikiaris, Pharmaceutics, 2022, 14, 359 CrossRef CAS PubMed.
- S. Fredenberg, M. Wahlgren, M. Reslow and A. Axelsson, Int. J. Pharm., 2011, 415, 34 CrossRef CAS PubMed.
- E. H. Nafea, A. Marson, L. A. Poole-Warren and P. J. Martens, J. Controlled Release, 2011, 154, 110 CrossRef CAS.
- Q. Li, X. Li and C. Zhao, Front. Bioeng. Biotechnol., 2020, 8, 437 CrossRef.
- Y. Hu, S. Hu, S. Zhang, S. Dong, J. Hu, L. Kang and X. Yang, Sci. Rep., 2021, 11, 9142 CrossRef CAS PubMed.
- S. Leick, A. Kemper and H. Rehage, Soft Matter, 2011, 7, 6684 RSC.
- M. P. A. Ferreira, J. P. Martins, J. Hirvonen and H. A. Santos, Nanotechnology for Oral Drug Delivery: From Concept to Applications, Elsevier, 2020, pp. 253–284 Search PubMed.
- S. Bertoni, B. Albertini and N. Passerini, Molecules, 2019, 24, 3471 CrossRef CAS PubMed.
- S. Bertoni, L. S. Dolci, B. Albertini and N. Passerini, Ther. Delivery, 2018, 9, 833 CrossRef CAS.
- S. Tambe, D. Jain, Y. Agarwal and P. Amin, J. Drug Delivery Sci. Technol., 2021, 63, 102452 CrossRef CAS.
- M. A. Repka, S. Majumdar, S. K. Battu, R. Srirangam and S. B. Upadhye, Expert Opin. Drug Delivery, 2008, 5, 1357 CrossRef CAS.
- Y. Ren, L. Mei, L. Zhou and G. Guo, AAPS PharmSciTech, 2019, 20, 92 CrossRef CAS.
- I. Akartuna, E. Tervoort, A. R. Studart and L. J. Gauckler, Langmuir, 2009, 25, 12419 CrossRef CAS PubMed.
- I. D. Rosca, F. Watari and M. Uo, J. Controlled Release, 2004, 99, 271 CrossRef CAS PubMed.
- R. H. Staff, D. Schaeffel, A. Turshatov, D. Donadio, H. J. Butt, K. Landfester, K. Koynov and D. Crespy, Small, 2013, 9, 3514 CrossRef CAS PubMed.
- B. K. Kim, S. J. Hwang, J. B. Park and H. J. Park, J. Microencapsulation, 2002, 19, 811 CrossRef CAS PubMed.
- E. Villicaña-Molina, E. Pacheco-Contreras, E. A. Aguilar-Reyes and C. A. León-Patiño, Int. J. Polym. Mater. Polym. Biomater., 2020, 69, 467 CrossRef.
- J. Jaiswal, S. K. Gupta and J. Kreuter, J. Controlled Release, 2004, 96, 169 CrossRef CAS PubMed.
- M. Nabi-Meibodi, A. Vatanara, A. R. Najafabadi, M. R. Rouini, V. Ramezani, K. Gilani, S. M. H. Etemadzadeh and K. Azadmanesh, Colloids Surf., B, 2013, 112, 408 CrossRef CAS PubMed.
- L. Mu and S. S. Feng, J. Controlled Release, 2001, 76, 239 CrossRef CAS PubMed.
- K. E. Bremmell, A. Tan, A. Martin and C. A. Prestidge, J. Pharm. Sci., 2013, 102, 684 CrossRef CAS.
- L. Mu, M. M. Teo, H. Z. Ning, C. S. Tan and S. S. Feng, J. Controlled Release, 2005, 103, 565 CrossRef CAS PubMed.
- C. Sander, K. D. Madsen, B. Hyrup, H. M. Nielsen, J. Rantanen and J. Jacobsen, Eur. J. Pharm. Biopharm., 2013, 85, 682 CrossRef CAS PubMed.
- D. X. Li, Y. K. Oh, S. J. Lim, J. O. Kim, H. J. Yang, J. H. Sung, C. S. Yong and H. G. Choi, Int. J. Pharm., 2008, 355, 277 CrossRef CAS PubMed.
- I. Aranaz, I. Paños, C. Peniche, Á. Heras and N. Acosta, Molecules, 2017, 22, 1980 CrossRef PubMed.
- T. H. Tran, B. K. Poudel, N. Marasini, S. C. Chi, H. G. Choi, C. S. Yong and J. O. Kim, Int. J. Pharm., 2013, 443, 50 CrossRef CAS PubMed.
- S. N. Harsha, B. E. Aldhubiab, A. B. Nair, I. A. Alhaider, M. Attimarad, K. N. Venugopala, S. Srinivasan, N. Gangadhar and A. H. Asif, Drug Des., Dev. Ther., 2015, 9, 273 CrossRef CAS PubMed.
- N. Passerini, B. Perissutti, B. Albertini, D. Voinovich, M. Moneghini and L. Rodriguez, J. Controlled Release, 2003, 88, 263 CrossRef CAS PubMed.
- A. Maschke, C. Becker, D. Eyrich, J. Kiermaier, T. Blunk and A. Göpferich, Eur. J. Pharm. Biopharm., 2007, 65, 175 CrossRef CAS.
- A. G. Balducci, G. Colombo, G. Corace, C. Cavallari, L. Rodriguez, F. Buttini, P. Colombo and A. Rossi, Int. J. Pharm., 2011, 421, 293 CrossRef CAS.
- P. C. H. Wong, P. W. S. Heng and L. W. Chan, Mol. Pharm., 2015, 12, 1592 CrossRef CAS.
- J. B. Lo, L. E. Appel, S. M. Herbig, S. B. McCray and A. G. Thombre, Drug Dev. Ind. Pharm., 2009, 35, 1522 CrossRef CAS PubMed.
- M. B. Pimparade, A. Vo, A. S. Maurya, J. Bae, J. T. Morott, X. Feng, D. W. Kim, V. I. Kulkarni, R. Tiwari, K. Vanaja, R. Murthy, H. N. Shivakumar, D. Neupane, S. R. Mishra, S. N. Murthy and M. A. Repka, Eur. J. Pharm. Biopharm., 2017, 119, 81 CrossRef CAS PubMed.
- C. R. Palem, S. Kumar Battu, S. Maddineni, R. Gannu, M. A. Repka and M. R. Yamsani, Pharm. Dev. Technol., 2013, 18, 186 CrossRef CAS PubMed.
- M. A. Repka, K. Gutta, S. Prodduturi, M. Munjal and S. P. Stodghill, Eur. J. Pharm. Biopharm., 2005, 59, 189 CrossRef CAS PubMed.
- E. Albarahmieh, S. Qi and D. Q. M. Craig, Int. J. Pharm., 2016, 514, 270 CrossRef CAS PubMed.
- C. M. Khor, W. K. Ng, P. Kanaujia, K. P. Chan and Y. Dong, J. Microencapsulation, 2017, 34, 29 CrossRef CAS PubMed.
- Q. Ma, C. Wang, X. Li, H. Guo, J. Meng, J. Liu and H. Xu, Sci. Rep., 2016, 6, 29348 CrossRef CAS PubMed.
- C. De Brabander, C. Vervaet, L. Van Bortel and J. P. Remon, Int. J. Pharm., 2004, 271, 77 CrossRef CAS PubMed.
- S. Y. Lee, S. Nam, Y. Choi, M. Kim, J. S. Koo, B. J. Chae, W. S. Kang and H. J. Cho, Appl. Sci., 2017, 7, 902 CrossRef.
- M. Adnan, M. O. K. Azad, H. S. Ju, J. M. Son, C. H. Park, M. H. Shin, M. Alle and D. H. Cho, Appl. Nanosci., 2020, 10, 1305 CrossRef CAS.
- J. A. H. Van Laarhoven, M. A. B. Kruft and H. Vromans, J. Controlled Release, 2002, 82, 309 CrossRef CAS PubMed.
- I.M. Katz, Shaped Ophthalmc Inserts For Treating Dry Eye Syndrome, Lansdale Pa, 4343787, 1982 Search PubMed.
- R. K. Kankala, Y. S. Zhang, S.-B. Wang, C. H. Lee and A. Z. Chen, Adv. Healthc. Mater., 2017, 6, 1700433 CrossRef PubMed.
- P. Chakravarty, A. Famili, K. Nagapudi and M. A. Al-Sayah, Pharmaceutics, 2019, 11, 629 CrossRef CAS PubMed.
- L. K. Bin, A. K. Janakiraman, F. S. A. Razak, A. B. M. H. Uddin, M. Z. I. Sarker, L. C. Ming and B. H. Goh, Indian J. Pharm. Educ. Res., 2020, 54, s1 CrossRef CAS.
- P. B. Deshpande, G. A. Kumar, A. R. Kumar, G. V. Shavi, A. Karthik, M. S. Reddy and N. Udupa, PDA J. Pharm. Sci. Technol., 2011, 65, 333 CrossRef CAS PubMed.
- P. M. Gosselin, R. Thibert, M. Preda and J. N. Mcmullen, Int. J. Pharm., 2003, 252, 225 CrossRef CAS PubMed.
- A. Montes, M. D. Gordillo, C. Pereyra and E. Martínez De La Ossa, J. Supercrit. Fluids, 2012, 63, 92 CrossRef CAS.
- C. I. Park, M. S. Shin and H. Kim, Korean J. Chem. Eng., 2008, 25, 581 CrossRef CAS.
- R. Adami, S. Liparoti and E. Reverchon, Chem. Eng. J., 2011, 173, 55 CrossRef CAS.
- P. Chattopadhyay and R. B. Gupta, Ind. Eng. Chem. Res., 2002, 41, 6049 CrossRef CAS.
- N. Elvassore, A. Bertucco and P. Caliceti, Ind. Eng. Chem. Res., 2001, 40, 795 CrossRef CAS.
- A. R. C. Duarte, M. S. Costa, A. L. Simplício, M. M. Cardoso and C. M. M. Duarte, Int. J. Pharm., 2006, 308, 168 CrossRef CAS PubMed.
- T. M. Martin, N. Bandi, R. Shulz, C. B. Roberts and U. B. Kompella, AAPS PharmSciTech, 2002, 3, 18 CrossRef PubMed.
- P. Pathak, M. J. Meziani, T. Desai and Y. P. Sun, J. Supercrit. Fluids, 2006, 37, 279 CrossRef CAS.
- M. C. Paisana, K. C. Müllers, M. A. Wahl and J. F. Pinto, J. Supercrit. Fluids, 2016, 109, 124 CrossRef CAS.
- M. S. Kim, S. J. Jin, J. S. Kim, H. J. Park, H. S. Song, R. H. H. Neubert and S. J. Hwang, Eur. J. Pharm. Biopharm., 2008, 69, 454 CrossRef CAS PubMed.
- R. Campardelli, I. Espirito Santo, E. C. Albuquerque, S. V. De Melo, G. Della Porta and E. Reverchon, J. Supercrit. Fluids, 2016, 107, 163 CrossRef CAS.
- L. Zhao and F. Temelli, J. Supercrit. Fluids, 2015, 100, 110 CrossRef CAS.
- S. Naik, D. Patel, N. Surti and A. Misra, J. Supercrit. Fluids, 2010, 54, 110 CrossRef CAS.
- K. Otake, T. Imura, H. Sakai and M. Abe, Langmuir, 2001, 17, 3898 CrossRef CAS.
- U. S. Kadimi, D. R. Balasubramanian, U. R. Ganni, M. Balaraman and V. Govindarajulu, Nanomedicine, 2007, 3, 273 CrossRef PubMed.
- N. Adawiyah, M. Moniruzzaman, S. Hawatulaila and M. Goto, MedChemComm, 2016, 7, 1881 RSC.
- A. M. Curreri, S. Mitragotri and E. E. L. Tanner, Adv. Sci., 2021, 8, 2004819 CrossRef CAS PubMed.
- R. M. Moshikur, M. R. Chowdhury, M. Moniruzzaman and M. Goto, Green Chem., 2020, 22, 8116 RSC.
- E. Janus, P. Ossowicz, J. Klebeko, A. Nowak, W. Duchnik, Ł. Kucharski and A. Klimowicz, RSC Adv., 2020, 10, 7570 RSC.
- H. Wu, F. Fang, L. Zheng, W. Ji, M. Qi, M. Hong and G. Ren, J. Mol. Liq., 2020, 300, 112308 CrossRef CAS.
- R. M. Moshikur, M. R. Chowdhury, R. Wakabayashi, Y. Tahara, N. Kamiya, M. Moniruzzaman and M. Goto, J. Mol. Liq., 2020, 299, 112166 CrossRef CAS.
- P. Maneewattanapinyo, A. Yeesamun, F. Watthana, K. Panrat, W. Pichayakorn and J. Suksaeree, AAPS PharmSciTech, 2019, 20, 322 CrossRef PubMed.
- H. Wu, Z. Deng, B. Zhou, M. Qi, M. Hong and G. Ren, J. Mol. Liq., 2019, 283, 399 CrossRef CAS.
- A. Abednejad, A. Ghaee, E. S. Morais, M. Sharma, B. M. Neves, M. G. Freire, J. Nourmohammadi and A. A. Mehrizi, Acta Biomater., 2019, 100, 142 CrossRef CAS PubMed.
- C. Wang, S. A. Chopade, Y. Guo, J. T. Early, B. Tang, E. Wang, M. A. Hillmyer, T. P. Lodge and C. C. Sun, Mol. Pharm., 2018, 15, 4190 CrossRef CAS PubMed.
- A. M. A. Dias, A. R. Cortez, M. M. Barsan, J. B. Santos, C. M. A. Brett and H. C. De Sousa, ACS Sustainable Chem. Eng., 2013, 1, 1480 CrossRef CAS.
- C. King, J. L. Shamshina, G. Gurau, P. Berton, N. F. A. F. Khan and R. D. Rogers, Green Chem., 2017, 19, 117 RSC.
- M. Moniruzzaman, Y. Tahara, M. Tamura, N. Kamiya and M. Goto, Chem. Commun., 2010, 46, 1452 RSC.
- S. Goindi, P. Arora, N. Kumar and A. Puri, AAPS PharmSciTech, 2014, 15, 810 CrossRef CAS PubMed.
- S. Goindi, R. Kaur and R. Kaur, Int. J. Pharm., 2015, 495, 913 CrossRef CAS PubMed.
- S. Y. Kim, J. Y. Hwang, J. W. Seo and U. S. Shin, J. Colloid Interface Sci., 2015, 442, 147 CrossRef CAS PubMed.
- M. Halayqa, M. Zawadzki, U. Domańska and A. Plichta, J. Mol. Struct., 2019, 1180, 573 CrossRef CAS.
- S. Emami and A. Shayanfar, Pharm. Dev. Technol., 2020, 25, 779 CrossRef CAS PubMed.
- S. N. Pedro, C. S. R. Freire, A. J. D. Silvestre and M. G. Freire, Encyclopedia, 2021, 1, 942 CrossRef.
- C. V. Pereira, J. M. Silva, L. Rodrigues, R. L. Reis, A. Paiva, A. R. C. Duarte and A. Matias, Sci. Rep., 2019, 9, 14926 CrossRef PubMed.
- J. M. Silva, R. L. Reis, A. Paiva and A. R. C. Duarte, ACS Sustainable Chem. Eng., 2018, 6, 10355 CrossRef CAS.
- C. Mukesh, D. Mondal, M. Sharma and K. Prasad, Carbohydr. Polym., 2014, 103, 466 CrossRef CAS PubMed.
- Q. Zhang, Z. Lin, W. Zhang, T. Huang, J. Jiang, Y. Ren, R. Zhang, W. Li, X. Zhang and Q. Tu, RSC Adv., 2020, 11, 1012 RSC.
- F. Mano, M. Martins, I. Sá-Nogueira, S. Barreiros, J. P. Borges, R. L. Reis, A. R. C. Duarte and A. Paiva, AAPS PharmSciTech, 2017, 18, 2579 CrossRef CAS PubMed.
- M. Mokhtarpour, H. Shekaari and A. Shayanfar, J. Drug Delivery Sci. Technol., 2020, 56, 101512 CrossRef CAS.
- W. Wang, Y. Cai, Y. Liu, Y. Zhao, J. Feng and C. Liu, Artif. Cells, Nanomed., Biotechnol., 2017, 45, 1241 CrossRef CAS PubMed.
- M. M. Buckley, P. Benfield, E. Bushell and P. Rosenberg, Drugs, 1993, 46, 126 CrossRef CAS PubMed.
- D. Guedelha and T. Friedli, Pharmaceutical Cluster in Portugal and Michael Porter Diamond Theory Business Case Study, 2018 Search PubMed.
- K. Wallenius, H. Hovi, J. Remes, S. Mahiout and T. Liukkonen, Int. J. Environ. Res. Public Health, 2022, 19, 4411 CrossRef CAS PubMed.
- European Commission, Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions a Clean air Programme for Europe, Brussels, 2013 Search PubMed.
- A. R. C. Duarte, J. F. Mano and R. L. Reis, Int. Mater. Rev., 2009, 54, 214 CrossRef CAS.
- P. Tran and J. S. Park, Int. J. Pharm., 2021, 610, 121247 CrossRef CAS PubMed.
- P. Franco and I. De Marco, Appl. Sci., 2021, 11, 1 Search PubMed.
- C. Pando, A. Cabañas and I. A. Cuadra, RSC Adv., 2016, 6, 71134 RSC.
- M. Yavuz-Düzgün, S. Kareth, B. Özçelik and E. Weidner, J. Supercrit. Fluids, 2023, 203, 106065 CrossRef.
- P. Blowers and M. Titus, Environ. Prog., 2004, 23, 284 CrossRef CAS.
- Z. Lei, B. Chen, Y. M. Koo and D. R. Macfarlane, Chem. Rev., 2017, 117, 6633 CrossRef PubMed.
- S. K. Singh and A. W. Savoy, J. Mol. Liq., 2020, 297, 112038 CrossRef CAS.
- J. M. Gomes, S. S. Silva and R. L. Reis, Chem. Soc. Rev., 2019, 48, 4317 RSC.
- D. M. Correia, L. C. Fernandes, M. M. Fernandes, B. Hermenegildo, R. M. Meira, C. Ribeiro, S. Ribeiro, J. Reguera and S. Lanceros-Méndez, Nanomaterials, 2021, 11, 2401 CrossRef CAS PubMed.
- X. Li, N. Ma, L. Zhang, G. Ling and P. Zhang, Int. J. Pharm., 2022, 612, 121366 CrossRef CAS PubMed.
- E. E. L. Tanner, A. M. Curreri, J. P. R. Balkaran, N. C. Selig-Wober, A. B. Yang, C. Kendig, M. P. Fluhr, N. Kim and S. Mitragotri, Adv. Mater., 2019, 31, 1901103 CrossRef PubMed.
- D. Hua, J. Jiang, L. Kuang, J. Jiang, W. Zheng and H. Liang, Macromolecules, 2011, 44, 1298 CrossRef CAS.
- G. Oliveira, F. O. Farias, F. H. B. Sosa, L. Igarashi-Mafra and M. R. Mafra, J. Mol. Liq., 2021, 341, 117314 CrossRef CAS.
- Y. Dai and Z. Liu, Ecol. Indic., 2023, 153, 110386 CrossRef.
- J. F. Mano, Adv. Eng. Mater., 2008, 10, 515 CrossRef CAS.
- P. R. Ninawe and S. J. Parulekar, Biotechnol. Prog., 2011, 27, 1442 CrossRef CAS PubMed.
- V. Kozlovskaya, J. Chen, O. Zavgorodnya, M. B. Hasan and E. Kharlampieva, Langmuir, 2018, 34, 11832 CrossRef CAS PubMed.
- C. Zhao, A. Liu, C. M. Santamaria, A. Shomorony, T. Ji, T. Wei, A. Gordon, H. Elofsson, M. Mehta, R. Yang and D. S. Kohane, Nat. Commun., 2019, 10, 2566 CrossRef PubMed.
- M. C. Klak, E. Lefebvre, L. Rémy, R. Agniel, J. Picard, S. Giraudier and V. Larreta-Garde, Macromol. Biosci., 2013, 13, 687 CrossRef CAS PubMed.
- J. P. Fuenzalida, M. E. Flores, I. Móniz, M. Feijoo, F. Goycoolea, H. Nishide and I. Moreno-Villoslada, J. Phys. Chem. B, 2014, 118, 9782 CrossRef CAS PubMed.
- M. Witzler, S. Vermeeren, R. O. Kolevatov, R. Haddad, M. Gericke, T. Heinze and M. Schulze, ACS Appl. Bio Mater., 2021, 4, 6719 CrossRef CAS PubMed.
- V. Vergaro, P. Papadia, S. Leporatti, S. A. De Pascali, F. P. Fanizzi and G. Ciccarella, J. Inorg. Biochem., 2015, 153, 284 CrossRef CAS PubMed.
- J. Campbell, G. Kastania and D. Volodkin, Micromachines, 2020, 11, 717 CrossRef PubMed.
- C. F. V. Sousa, L. P. G. Monteiro, J. M. M. Rodrigues, J. Borges and J. F. Mano, J. Mater. Chem. B, 2023, 11, 6671 RSC.
- R. R. Costa, M. Alatorre-Meda and J. F. Mano, Biotechnol. Adv., 2015, 33, 1310 CrossRef CAS PubMed.
- D. B. Trushina, R. A. Akasov, A. V. Khovankina, T. N. Borodina, T. V. Bukreeva and E. A. Markvicheva, J. Mol. Liq., 2019, 284, 215 CrossRef CAS.
- S. Mehnath, M. Arjama, M. Rajan, G. Annamalai and M. Jeyaraj, Biomed. Pharmacother., 2018, 104, 661 CrossRef CAS PubMed.
- J. Pan, H. Liao, G. Gong, Y. He, Q. Wang, L. Qin, Y. Zhang, H. Ejima, B. L. Tardy, J. J. Richardson, J. Shang, O. J. Rojas, Y. Zeng and J. Guo, J. Controlled Release, 2023, 360, 433 CrossRef CAS PubMed.
- R. C. Gonçalves, S. Vilabril, C. M. S. S. Neves, M. G. Freire, J. A. P. Coutinho, M. B. Oliveira and J. F. Mano, Adv. Mater., 2022, 34, 2200352 CrossRef PubMed.
- S. Daradmare and C. S. Lee, Colloids Surf., B, 2022, 219, 112795 CrossRef CAS PubMed.
- Y. Zhang, Y. Luo, J. Zhao, W. Zheng, J. Zhan, H. Zheng and L. Feng, Acta Pharm. Sin. B, 2023, 14, 110 CrossRef PubMed.
- S. Vilabril, S. Nadine, C. M. S. S. Neves, C. R. Correia, M. G. Freire, J. A. P. Coutinho, M. B. Oliveira and J. F. Mano, Adv. Healthc. Mater., 2021, 10, 2100266 CrossRef CAS PubMed.
- Q. Ma, Y. Song, J. W. Kim, H. S. Choi and H. C. Shum, ACS Macro Lett., 2016, 5, 666 CrossRef CAS PubMed.
- Z. Jiang, S. Zhao, M. Yang, M. Song, J. Li and J. Zheng, Food Hydrocolloids, 2022, 125, 107413 CrossRef CAS.
- L. Zhang, L. Cai, P. S. Lienemann, T. Rossow, I. Polenz, Q. Vallmajo-Martin, M. Ehrbar, H. Na, D. J. Mooney and D. A. Weitz, Angew. Chem., 2016, 128, 13668 CrossRef.
| This journal is © The Royal Society of Chemistry 2024 |