Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The need to integrate mass- and energy-based metrics with life cycle impacts for sustainable chemicals manufacture†
Elysia
Lucas
 ,
Antonio J.
Martín
,
Antonio J.
Martín
 ,
Sharon
Mitchell
,
Sharon
Mitchell
 ,
Abhinandan
Nabera
,
Abhinandan
Nabera
 ,
Lucas F.
Santos
,
Lucas F.
Santos
 ,
Javier
Pérez-Ramírez
,
Javier
Pérez-Ramírez
 * and
Gonzalo
Guillén-Gosálbez
* and
Gonzalo
Guillén-Gosálbez
 *
*
Institute for Chemical and Bioengineering, Department of Chemistry and Applied Biosciences, ETH Zürich, Vladimir-Prelog-Weg 1, 8093 Zürich, Switzerland. E-mail: jpr@chem.ethz.ch; gonzalo.guillen.gosalbez@chem.ethz.ch
First published on 3rd July 2024
Abstract
Effective use of quantitative metrics is fundamental to guiding innovation toward more sustainable chemicals. At present, metrics employed in Green Chemistry, such as the E-factor, Process Mass Intensity, or Energy Intensity, focus on mass or energy efficiency at specific levels – reaction, process, or plant. However, a more holistic approach is needed, especially at early stages of research and development, utilising more complex impact-based indicators from Life Cycle Assessments (LCAs) to gain a deeper understanding of the environmental footprint of chemical systems. To date, the need to couple mass- and energy-based process metrics with life cycle impacts for more comprehensive assessments has been qualitatively discussed but not quantitatively demonstrated. Therefore, this study quantifies the level of correlation and linkages between five mass- and energy-based metrics and 16 LCA indicator scores by leveraging data for over 700 chemical manufacturing processes. The primary finding is the weak correlations between process metrics and life cycle impacts, as the former approach lacks appropriate weights for each input and output to account for their life cycle environmental implications. While improving process efficiency can lead to lower overall environmental impact, enhanced granularity for comparing alternative chemical routes provides insights into the relative impact levels throughout the supply chain, particularly concerning raw materials as they are major contributors to life cycle environmental impacts. This study also provides practical insights for expanding the application of LCA by making it more accessible to the research community through simplified approaches and working collaboratively with LCA practitioners.
Introduction
Amidst global environmental degradation, there is a growing urgency to minimise the environmental consequences of how chemicals are produced considering their essential role in everyday materials and products.1,2 To help ensure the selection and development of truly more environmentally sustainable pathways, early-stage decisions should be guided by quantitative metrics which are practical to compute, fit for purpose, and provide an accurate indication of environmental performance levels.In line with the Twelve Principles of Green Chemistry,3 the earliest set of environmental indicators for chemical synthesis routes includes quantifiable metrics such as the E-factor, Atom Economy, Process Mass Intensity (PMI), Reaction Mass Efficiency, and Energy Intensity.4–7 These metrics, addressing aspects like waste prevention, resource, and energy efficiency, have found varying degrees of adoption within academia and industry.8,9 For example, the well-established E-factor measures the mass ratio of waste to the desired product as an indicator of the negative environmental impact of a process.10–12 Alternatively, resource intensity,4,7 evaluated by metrics such as the PMI13 (mass ratio of input materials to final product) and energy intensity7 (total energy input per mass of product), respectively, can also offer an indication of the environmental footprint of chemical manufacture.6
Quantitative performance metrics gain traction when they are easily understandable, computable, and offer relevant insights. Due to these characteristics, mass-based metrics focusing on resource efficiency have gained prominence in the fine chemical and pharmaceutical industries. The PMI, specifically endorsed by the American Chemical Society Green Chemistry Institute Pharmaceutical Roundtable,13,14 stands out in pharmaceutical process development for examining environmental friendliness due to its simplicity.15 Furthermore, its direct alignment with economic sustainability enhances its appeal, as improving resource efficiency leads to reduced costs.
While mass- and energy-based metrics serve as robust tools for initial estimation of environmental performance,16,17 certain decisions could benefit from the integration of impact-based metrics.18–25 Unlike mass- and energy-based metrics, which focus solely on quantities (e.g., kilograms of input material), impact-based metrics calculated using Life Cycle Assessments (LCAs) explicitly estimate the environmental impacts associated with a chemical process over its life cycle.26,27 Using LCA can therefore account for the impacts beyond the chemical process itself – from resource extraction, transport, the production of procured material and energy, and waste treatment. Recently, life cycle impact indicators have been applied to low Technology Readiness Level (TRL) chemical production pathways to guide research and development of these technologies – e.g., as a component of the Safe and Sustainable by Design framework proposed by the European Commission.28 Renewable methanol29 and ammonia synthesis,30 or plastic production,31 and recycling,32 are highly relevant examples, though LCA has signalled clear pathways on other emerging areas such as the production of fine chemicals using heterogeneous catalysts.33,34 Moreover, there is growing consensus that a more comprehensive assessment framework for sustainable chemistry can be achieved by combining both mass- and energy-based process metrics and life cycle impact-based indicators.35–37
Despite qualitative discussion in the scientific literature,38,39 there remains a lack of quantitative demonstrations of the alignment and potential synergies between process metrics and life cycle impacts. Existing studies typically concentrate on a single case study20 or a small number of representative chemical processes13,35 focusing on the PMI alone13,20 or, if several metrics are considered, often examining them at a highly simplified level.40 Statistical approaches can be applied as done in previous LCA studies41–43 to explore whether simpler indicators (e.g., PMI or E-factor) can serve as predictors for more complex impact-based metrics.
In this work, we aim to bridge this gap by characterising the differences between mass- and energy-based metrics and life cycle indicators, offering quantitative evidence on why they should be integrated into environmental assessments. To this end, we analyse data for over 700 chemical production routes, evaluating the extent to which mass- and energy-based metrics and life cycle impacts correlate and whether the former can be used independently to guide innovation toward more sustainable chemical production routes at early stages. In addition, we identify the major contributors to the life cycle impacts of a chemical process to highlight key areas for improvement across the supply chain. With these results, we aim to promote awareness and understanding among researchers of suitable quantitative metrics, providing recommendations on how these can be calculated and used to guide decisions towards truly more environmentally sustainable pathways, especially for low TRL technologies.
Methods
To investigate the relationship between mass- or energy-based process metrics and the life cycle impacts of chemical production processes, we used a three-stage approach (Fig. 1). We began with data compilation, in which chemical processes with available data were selected and filtered out based on some exclusion criteria thoroughly explained in section S1 of the ESI.† This preprocessing step ensures that the quality of the data suffices to perform the correlation analysis later presented. While the filtered data employed in the analysis might still rely on some assumptions and simplifications,44 the latter will affect all metrics in a similar manner. Hence, they are not expected to affect the outcome of the analysis significantly. The next step is the computation of a range of mass-, energy-, and life cycle impact-based metrics for each chemical process. The former task is elaborated and formulated in section S1,† while the latter is reviewed in detail in section S2 of the ESI.† We then examined patterns and trends in metric and impact values using correlation testing to study the existence of any association between metric types, as well as contribution analysis to uncover insights into the main drivers of life cycle impacts of chemical synthesis. We utilised Spearman's Rank correlation analysis to measure the strength and direction of association between each mass- or energy-based metric and the life cycle impact score of all chemical processes in our dataset. A justification of why Spearman's coefficient was selected for this analysis is provided in section S1 of the ESI.† The definitions of terminology used in this work are briefly provided in Table S1 of the ESI.†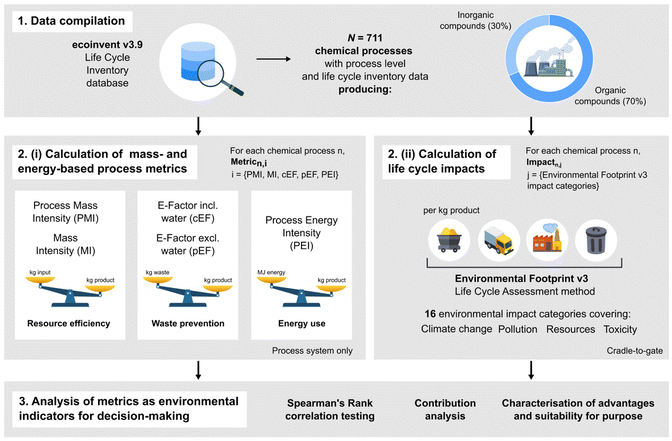 | ||
| Fig. 1 Framework of the three-phase approach taken in this study: dataset compilation, computation of metrics and impacts, and analysis of results to uncover patterns and trends. | ||
Results and discussion
Do mass- and energy-based metrics correlate with life cycle impacts?
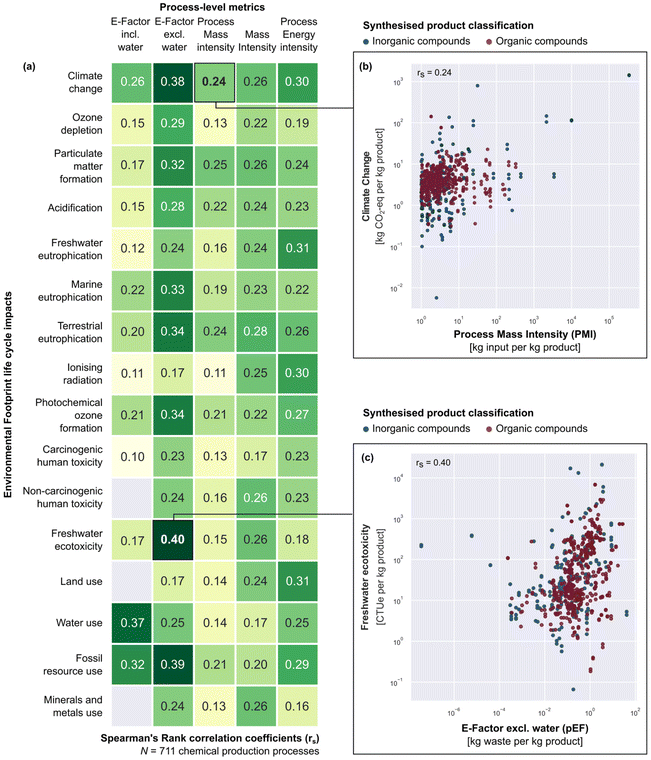
We used Spearman's Rank correlation testing to assess whether a process with a higher E-factor, PMI, or PEI implies higher associated life cycle impacts of chemicals production. Overall, our results indicate a lack of strong correlations between mass- or energy-based metrics (focusing on input material, waste, or energy intensity) and life cycle impacts on environmental and human areas of protection such as climate change, pollution, toxicity, and resource use (Fig. 2a). Statistically significant correlations (significance level set at a p-value of 0.05) were found between most mass- or energy-based metric and life cycle impact pairings, but they are only weakly to moderately positive – with correlation coefficients (rs) in the range of 0.10 to 0.40. Here, we regard a correlation to be ‘strong’ if the coefficient value is 0.70 or above.45Fig. 2b and c provide an overview of correlation strengths, taking climate change and PMI as an example of a weaker correlation (rs = 0.24) compared to the correlation found between freshwater ecotoxicity and E-factor excluding water (rs = 0.40). Scatter plots for all mass- and energy-based and EF impact category pairings can be found in the ESI (Fig. S1–S16†).
In practical terms, the mixed correlation testing results we found indicate that when comparing different chemicals, those with higher mass- or energy-based metric scores may correspond to having a higher overall environmental footprint, but this is not always the case. This overarching trend aligns with the pattern uncovered in a previous study for 12 polymers, where a higher-ranked material based on its ‘adherence to green design’ did not necessarily correspond to a higher rank based on its life cycle impact levels.40
This result can be explained by how mass- or energy-based metrics penalise equally all kilograms of input or waste, or megajoules of energy, regardless of their nature and provenance. In contrast, LCA employs distinct weights, i.e., eco-vectors assigned to each input considering its life cycle emissions and the corresponding characterisation factors to translate them into impacts (see a detailed explanation of LCA calculation in section S2 of the ESI†). The PMI, for instance, penalises one kilogram of all materials equally, and such general treatment masks how different materials are, in reality, associated with widely varying life cycle impacts due to distinct emissions (type and amount) over their life cycle. Specifically, the environmental impact of one input material can be drastically different from another due to differences in initial feedstocks (fossil- or bio-based), the number of stages of transformation to produce that material, complexity of separation processes, transport, distribution, etc. For instance, the range of carbon footprints (kg CO2-eq per kg) of the chemicals analysed in this study spans six orders of magnitude. Similarly, all kilograms of waste are treated the same by the E-factor but, in reality, the environmental implications of their treatment or disposal can be very different – e.g., disposal of inert material to landfill vs. incineration of spent solvent mixture. Lastly, all energy inputs are also accounted for in the same manner in the PEI without consideration of their source and form of generation. However, the variation in environmental impacts of energy depends on its type and source, e.g., electricity from the grid vs. heat from natural gas. Additionally, there is an intrinsic scope difference between mass- and energy-based metrics and LCA, as the former takes into consideration the gate-to-gate process efficiency and the latter naturally considers the life-cycle implications of a process.
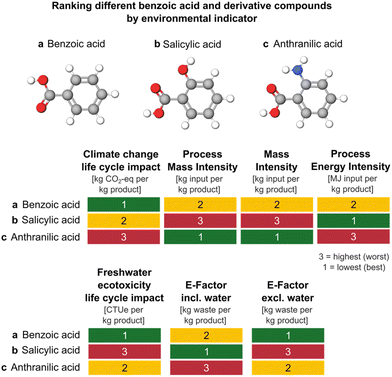
To illustrate the extent of alignment identified from our correlation analysis, we use the environmental metric scores for production of three similar compounds (benzoic acid vs. two of its derivatives, salicylic acid and anthranilic acid). In Fig. 3, we rank their environmental performances based on different metric types (1 in green indicates the best performing and 3 in red indicates the worst). For ease of demonstration, we focus on one environmental impact type at a time. As depicted in Fig. 3, when comparing ranks according to climate change life cycle impact vs. resource intensity process metrics (PMI, MI, and PEI), we observe that ranking orders do not match, reflecting the relatively weak correlation coefficients we find between climate change vs. PMI (rs = 0.24), MI (rs = 0.26), or PEI (rs = 0.30). Alternatively, we also examine freshwater ecotoxicity life cycle impacts vs. E-factor including and excluding water. In this case, we observe that ranking by E-factor excluding water would lead to the same order (rs = 0.40, Fig. 2) as freshwater ecotoxicity life cycle impacts, while this is not the case for E-factor including water (rs = 0.17). This is, in fact, a general result of our analysis. The inclusion of process water in mass-based metrics results largely affects their value4 and result in weaker correlations with life cycle impacts.
Overall, our results provide evidence that the E-factor, PMI, or MI cannot robustly quantify the overall environmental impact of a chemical production process. Some mass-based and life cycle indicator pairs do have appreciable correlation strengths (i.e., 0.35 to 0.40) but are not consistent across all pairings and vary widely across different impact categories. For instance, PMI is more weakly correlated with freshwater eutrophication (rs = 0.16) compared to its alignment with particulate matter formation (rs = 0.25). Resource, waste, or energy efficiency metrics can provide a reasonable first gauge of how environmentally impactful a chemical process is, but decision-makers should be aware that process-level metrics measure environmental friendliness through the lens of a single dimension and may potentially mask important trade-offs or burden shifting between different environmental impact types.
What are the main contributors to the life cycle impacts of chemical production?
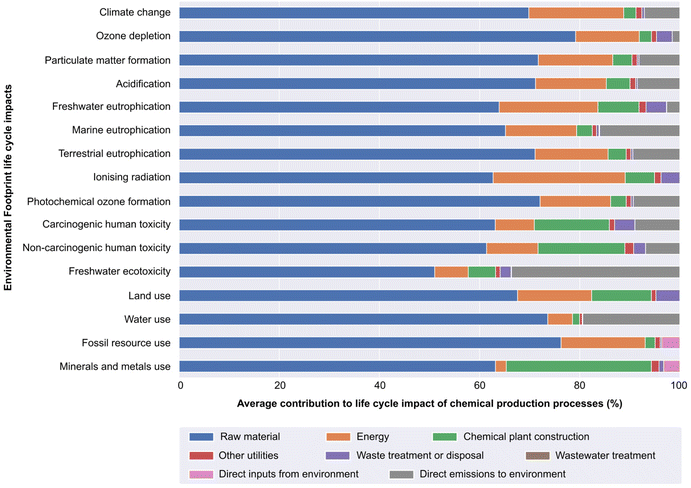
We examined the contributions from different process aspects (procured materials, energy, waste treatment, etc.) to the total life cycle impact, highlighting the major hotspots of chemical production processes across various environmental impact categories to help decision-makers and shaping focus in improvement strategies.46,47Contributions from raw materials (blue, in Fig. 4) refer to the life cycle impacts associated with the activities to produce and distribute each input material until entering the chemical process. In LCA calculations, only the amount of consumed material per kilogram of product is considered. Therefore, the activities in the ecoinvent database already account for any recycling and regeneration of, e.g., catalysts and solvents in the process. Similarly, contributions from energy (orange) correspond to the impacts associated with the generation and distribution of electricity, heating from steam or natural gas, etc. Contributions from other utilities (red) in the Fig. 4 refer to impacts from cooling water, liquid nitrogen, and compressed air. Chemical plant contributions (green) are the impacts from the construction of the chemical plant, including required materials (e.g., steel and concrete), energy, and water. Waste treatment or disposal (purple) refers to the impacts from incinerating spent solvent or hazardous waste from the production process, while wastewater (brown) contributions cover impacts from treating the amount of wastewater generated by the process. Lastly, impacts due to inputs from (pink) and emissions to (grey) the environment are caused by the natural resources consumed (e.g., water from natural bodies such as rivers) or emissions released directly (e.g., carbon dioxide) by the chemical process, respectively. The latter two represent exchanges taking place directly between the chemical system and the ecosphere.
When considering the total impact per kilogram of the final product, averaged across all 711 processes, input raw materials generally account for the majority (51–80%) of life cycle impacts of all chemical production processes analysed in Fig. 4. Average contributions from energy (2–26%) and chemical plant materials and construction (1–29%) are also notable, in contrast with modest impact shares from utilities (1–2%), waste treatment or disposal (up to 5%), and wastewater treatment (<1%).
This analysis thus suggests that environmental strategies should include a focus on actions related to input materials. Reducing the environmental impacts of input materials can be achieved by (i) improving efficiency or (ii) switching to alternatives with lower embodied impacts. Regarding the former, reducing the MI or PMI will indeed lead to a reduction in life cycle impacts. We however underscore that discerning materials for potential substitution, as well as identifying truly less impactful alternatives rely on understanding relative life cycle impact levels of current feedstock and candidate materials. When planning measures to improve environmental performance, particularly for early-stage low TRL technologies, considering the life cycle impacts of input materials would increase the likelihood that such measures are indeed effective.
Results in Fig. 4 also underline the importance of considering different types of environmental impacts. Consequently, courses of action to improve environmental sustainability may appear different depending on the effect being addressed. For instance, contrasting the contribution profile of freshwater ecotoxicity impacts with other categories clearly shows that hotspots could vary widely depending on the type of impact being considered. If strategies intend to address freshwater ecotoxicity mitigation, our hotspot analysis indicates that measures should focus on tackling impacts associated with direct emissions (grey bar in Fig. 4) alongside material inputs. Factoring in the multi-dimensional nature of environmental effects through various impact-based metrics could, therefore, greatly strengthen and deepen the level of insight from quantitative performance assessments when seeking to understand and act on the environmental footprint of a chemical process.
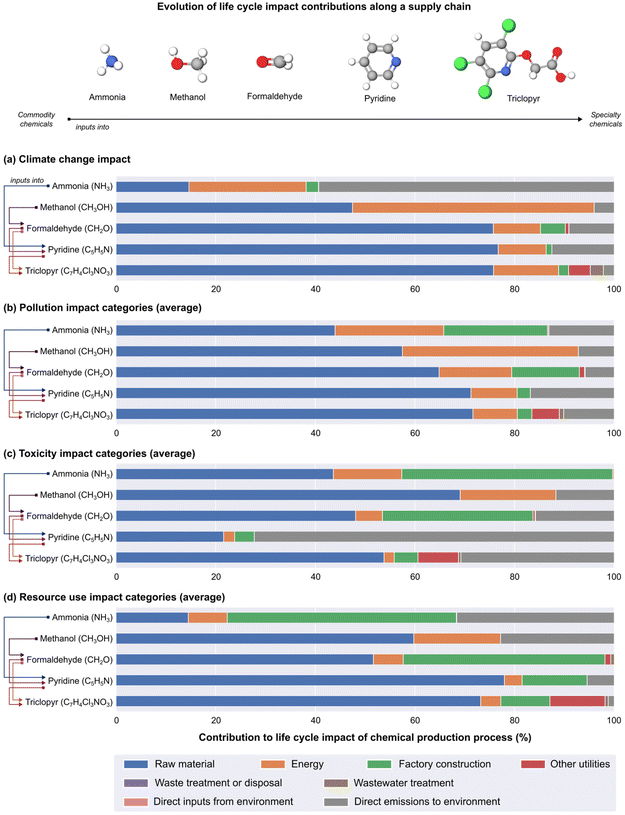
In addition to variation across different environmental impact categories, we can also conclude that the main drivers of impact, and in turn, areas to focus on for action, depend on how far down the process sits on the chemical value chain life cycle. To quantify contributions across all chemical production processes, we evaluate the mean and coefficient of variation (calculated as the ratio of the standard deviation to the mean) as shown in Table S3.† As an illustrative example, Fig. 5 shows how impact contribution shares vary at different points of the triclopyr (a speciality agrochemical product) supply chain. Ammonia and methanol are inputs into the production of formaldehyde and pyridine, respectively, which in turn are used to produce triclopyr. We display the percentage contributions in Fig. 5 rather than absolute values to emphasise the relative significance of each stage in the supply chain, considering the diverse applications and impacts of chemicals. The climate change impacts are shown in Fig. 5a, whereas the contributions of all the pollution-based impact categories (i.e., ozone depletion, particulate matter formation, ionising radiation, photochemical ozone formation, acidification, and freshwater, marine and terrestrial eutrophication) are averaged and shown in Fig. 5b. Similarly, Fig. 5c displays the averaged contributions for toxicity-related impact categories (i.e., freshwater ecotoxicity, non-carcinogenic, and carcinogenic human toxicity), and Fig. 5d shows the averaged contributions for the resource use-based impact categories (i.e., land, water, minerals and metals, and fossil resource use). They show that for climate change, pollution, and resource use-related impact categories, shares from input materials typically increase when traversing from commodity-type chemicals to larger specialty chemicals (Fig. 5a, b, and d).
The high number of transformation stages needed to produce chemicals further down the supply chain implies that the initial raw materials for these chemical products carry significant environmental impacts of their own. That is the case because, for each synthesis step, energy in the form of heat and electricity is required in the chemical reaction and in the separation units (such as distillation and absorption columns, evaporators, etc.) required to separate and recycle unreacted raw materials and to purify final products. Similarly, direct emissions from upstream processes contribute to the higher environmental impacts of downstream chemicals as they are part of the scope 3 emissions (i.e., emissions that occur beyond the gates of an organisation and are not related to energy) that accumulate over the life cycle of chemicals across the synthesis tree. Furthermore, Fig. S17† depicts that the environmental impacts along the chemical value chain aggregated by reference products mainly come from electricity and heat that build up along the chemical's life cycle. It also reveals a diminishing environmental impact contribution downstream from primary feedstock (e.g., natural gas and coal), as they are mostly employed in the early stages of the chemical value chain for synthesising building block chemicals.
Starting with building block chemicals in the Fig. 5 example (ammonia and methanol in this case), the embodied impacts of material inputs represent relatively minor shares while more critical impact hotspots involve energy, direct emissions, and infrastructure/construction. Enhancements in the environmental performance of these hotspots in the upstream production will propagate to downstream processes. In general, strategies for the production of building block chemicals will likely need to have an equal focus on energy efficiency, cleaner energy sources, and direct emissions management, alongside sustainable feedstock selection. On the other hand, strategies for routes producing more complex compounds (pyridine and triclopyr in this case) would generally benefit from reducing the quantity and/or life cycle impacts of input materials. Toxicity-related impact categories (Fig. 5c) are an exception as life cycle impact contributions are not found to be a function of position in the value chain, suggesting that toxicity effects vary on a case-by-case basis.
While life cycle impacts provide additional insights over mass and energy-based metrics, we must also notice limitations affecting LCA studies. Data availability and quality pose constraints to the analysed set of chemical processes, particularly for multi-stage processes typically linked to synthesis of complex products. A more detailed summary of the limitations and assumptions adopted in this study is listed in section S6 of the ESI.† Despite these limitations, the main conclusions of our study are expected to hold in view of the large dataset analysed after applying a thorough data quality filter explained in more detail in the section S1 of the ESI.†
Recommendations for integrating mass- and energy-based process metrics and life cycle impacts
Our results suggest that process metrics focusing on mass or energy, such as E-factor, PMI and PEI, and metrics derived from life cycle impact approaches can act as complementary sets of indicators. Our hotspot analysis of life cycle impact contributions indicates that a reasonable understanding of a process's life cycle impacts can be achieved by initially estimating the embodied life cycle impacts of its input raw materials. By accurately estimating these raw material impacts, we could potentially estimate up to 70% of the overall life cycle impacts.As also shown by our contribution analysis, the PMI, MI, E-factor and PEI all relate to at least one driver of a chemical production process’ life cycle impact (raw materials, waste, and energy, respectively). When improving or optimising a particular synthesis route or process, improving these metrics – through efficiency gains alone, without changing provenance or the type of material/energy source/waste treatment method – will reduce the overall life cycle environmental impact of the process. Understanding the extent to which this happens can be achieved by integrating a simplified LCA of input materials. Another advantage of this strategy is safeguarding against increasing environmental impacts when trying to improve or optimise a process. For instance, selecting an alternative reactant could lead to a reduction in PMI, but if the newly substituted reactant has significantly higher embodied life cycle impacts, then the benefit of a PMI improvement could be overpowered and result in a net increase in environmental impact. Similarly, when selecting a synthesis route, complementing mass- and energy-based metrics with life cycle impact estimates of at least the main input materials would provide decision makers with more robust tools toward the optimal choice.
At present, challenges for the widespread application of LCA mainly relate to time and resource intensiveness,18 difficulties with data acquisition and availability,7,20,35,48 uncertainty and reliability of data and methodologies, and expertise requirements. Additionally, the availability of LCI for chemical production is heavily constrained to high-production volume processes.49 This limitation leaves substantial gaps in the chemicals value chain, given over 350![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 registered chemicals and chemical mixtures (of which 100
000 registered chemicals and chemical mixtures (of which 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 are in significant market quantities)50 have been found recently in a compilation of 22 chemical inventories.51 In contrast, only 711 inventories were analysed in this study from the ecoinvent database, which provided sufficient data. While existing databases can help assess the gate-to-gate sustainability of chemicals;52 understanding the broad environmental implications of a product along its supply chain for well-informed sustainable decision-making is critical, which comes naturally from LCA. To help fill these data gaps, a number of predictive53,54 and streamlined55 LCA tools and approaches have been developed, particularly for complex intermediate inputs for fine chemicals synthesis. However, a common issue in predicting the life-cycle environmental impacts of chemicals is dealing with overfitting (i.e., accurate model predictions in the training dataset but low performance on unseen chemicals), which can happen when using a large number of molecular descriptors (e.g., number of atoms, total number of bonds, molecular weight, etc.).56 Nonetheless, improving data availability remains an outstanding area for further work. In order for LCA to become a common procedure when assessing the sustainability of processes, particularly in earlier stages of research and development, a shift towards more life cycle and systems thinking,57 standardisation of metrics, and partnering with LCA practitioners must increase.58
000 are in significant market quantities)50 have been found recently in a compilation of 22 chemical inventories.51 In contrast, only 711 inventories were analysed in this study from the ecoinvent database, which provided sufficient data. While existing databases can help assess the gate-to-gate sustainability of chemicals;52 understanding the broad environmental implications of a product along its supply chain for well-informed sustainable decision-making is critical, which comes naturally from LCA. To help fill these data gaps, a number of predictive53,54 and streamlined55 LCA tools and approaches have been developed, particularly for complex intermediate inputs for fine chemicals synthesis. However, a common issue in predicting the life-cycle environmental impacts of chemicals is dealing with overfitting (i.e., accurate model predictions in the training dataset but low performance on unseen chemicals), which can happen when using a large number of molecular descriptors (e.g., number of atoms, total number of bonds, molecular weight, etc.).56 Nonetheless, improving data availability remains an outstanding area for further work. In order for LCA to become a common procedure when assessing the sustainability of processes, particularly in earlier stages of research and development, a shift towards more life cycle and systems thinking,57 standardisation of metrics, and partnering with LCA practitioners must increase.58
As stated before, a good initial approach is to estimate the embodied impacts of main input materials as a proxy. This requires only data on the amounts of input materials to be recorded. Research and development chemists are thus recommended to first calculate simpler process efficiency metrics such as the PMI, E-factor and PEI, as the data required for these process metrics is enough for a simplified LCA calculation. More specifically, calculating the PMI, E-factor and PEI of a process entails recording data (for all steps until the final purified product is reached) on the mass amount of product formed, mass amounts of all resource inputs, estimated amounts of electricity and heating energy, and estimated amount of waste generated. This data can then be used to estimate the life cycle impacts of the process (per kilogram of product, or any other mass or functionality basis) by multiplying, e.g., grams of reactant by its estimated life cycle impact intensity (impact per gram of reactant). Beyond the analysis of a concrete process, systematically feeding this data to database of simplified life cycle impacts could give birth to a centralised database shared among all working in sustainable chemistry research and development.
Conclusions and outlook
In this study, we have characterised the differences between mass- and energy-based process metrics and life cycle impacts and described their scope to guide decision-making. Supporting previous qualitative arguments and evidence, we have presented the largest quantitative analysis to date demonstrating the need and value in considering simple mass- and energy-based metrics alongside simplified impact-based indicators estimated via LCA approaches.In addition to highlighting the lack of correlations between mass- and energy-based metrics and life cycle impacts, this study also lays some conceptual groundwork for reaching consensus on the minimum set of metrics to sufficiently capture the environmental sustainability of a chemical process, concluding that life cycle impact approaches should be incorporated at earlier stages of process development.59 On the practical side, we encourage the research community to start from a standardised baseline of using process metrics such as the PMI, MI, E-factor, and energy intensity, allowing future work to explore middle ground solutions to bridge these simpler metrics towards full LCA. Only when multidimensional life cycle impact-based approaches are guiding early-stage decisions, especially when screening feedstock alternatives, can claims of identifying a greener or more sustainable pathway be well substantiated.
Ultimately, we hope that this work stands as a launching point toward further action within both the chemistry and LCA communities and promote collaboration between them, advocating for the widespread adoption of integrated mass, energy, and impact-based quantitative environmental assessments, especially in academic research.
Data availability
The python code used to compute metrics and undertake correlation and contribution analyses can be found in Zenodo under the https://doi.org/10.5281/zenodo.10551697. Life cycle inventory data for the calculation of chemical process life cycle impacts can be accessed in the commercially available database ecoinvent (https://ecoinvent.org/the-ecoinvent-database/).Conflicts of interest
There are no conflicts to declare.Acknowledgements
This publication was created as part of NCCR Catalysis (grant number 180544), a National Centre of Competence in Research funded by the Swiss National Science Foundation.References
- I. T. Horváth, Chem. Rev., 2018, 118, 369–371 CrossRef PubMed.
- S. A. Matlin, S. E. Cornell, A. Krief, H. Hopf and G. Mehta, Chem. Sci., 2022, 13, 11710–11720 RSC.
- P. T. Anastas and J. C. Warner, Green Chemistry: Theory and Practice, Oxford University Press, 1998 Search PubMed.
- R. A. Sheldon, ACS Sustainable Chem. Eng., 2018, 6, 32–48 CrossRef CAS.
- F. Roschangar, R. A. Sheldon and C. H. Senanayake, Green Chem., 2015, 17, 752–768 RSC.
- H. C. Erythropel, J. B. Zimmerman, T. M. De Winter, L. Petitjean, F. Melnikov, C. H. Lam, A. W. Lounsbury, K. E. Mellor, N. Z. Janković, Q. Tu, L. N. Pincus, M. M. Falinski, W. Shi, P. Coish, D. L. Plata and P. T. Anastas, Green Chem., 2018, 20, 1929–1961 RSC.
- C. “Conchita” Jimenez-Gonzalez and C. Lund, Curr. Opin. Green Sustainable Chem., 2022, 33, 100564 CrossRef.
- W. J. W. Watson, Green Chem., 2012, 14, 251–259 RSC.
- J. L. Tucker and M. M. Faul, Nature, 2016, 534, 27–29 CrossRef CAS.
- R. A. Sheldon, Green Chem., 2017, 19, 18–43 RSC.
- R. A. Sheldon, Green Chem., 2023, 25, 1704–1728 RSC.
- R. A. Sheldon, M. L. Bode and S. G. Akakios, Curr. Opin. Green Sustainable Chem., 2022, 33, 100569 CrossRef CAS.
- C. Jimenez-Gonzalez, C. S. Ponder, Q. B. Broxterman and J. B. Manley, Org. Process Res. Dev., 2011, 15, 912–917 CrossRef CAS.
- L. J. Diorazio, P. Richardson, H. F. Sneddon, A. Moores, C. Briddell and I. Martinez, ACS Sustainable Chem. Eng., 2021, 9, 16862–16864 CrossRef CAS.
- A. Borovika, J. Albrecht, J. Li, A. S. Wells, C. Briddell, B. R. Dillon, L. J. Diorazio, J. R. Gage, F. Gallou, S. G. Koenig, M. E. Kopach, D. K. Leahy, I. Martinez, M. Olbrich, J. L. Piper, F. Roschangar, E. C. Sherer and M. D. Eastgate, Nat. Sustain., 2019, 2, 1034–1040 CrossRef.
- M. J. Eckelman, J. B. Zimmerman and P. T. Anastas, J. Ind. Ecol., 2008, 12, 316–328 CrossRef CAS.
- R. Grisorio, L. De Marco, C. Baldisserri, F. Martina, M. Serantoni, G. Gigli and G. P. Suranna, ACS Sustainable Chem. Eng., 2015, 3, 770–777 CrossRef.
- L. Summerton, H. F. Sneddon, L. C. Jones and J. H. Clark, Green and Sustainable Medicinal Chemistry: Methods, Tools and Strategies for the 21st Century Pharmaceutical Industry, Royal Society of Chemistry, 2016 Search PubMed.
- J. Li, E. M. Simmons and M. D. Eastgate, Green Chem., 2017, 19, 127–139 RSC.
- D. Cespi, E. S. Beach, T. E. Swarr, F. Passarini, I. Vassura, P. J. Dunn and P. T. Anastas, Green Chem., 2015, 17, 3390–3400 RSC.
- S. M. Mercer, J. Andraos and P. G. Jessop, J. Chem. Educ., 2012, 89, 215–220 CrossRef CAS.
- J. Becker, C. Manske and S. Randl, Curr. Opin. Green Sustainable Chem., 2022, 33, 100562 CrossRef CAS.
- M. G. T. C. Ribeiro and A. A. S. C. Machado, Green Chem. Lett. Rev., 2013, 6, 1–18 CrossRef CAS.
- R. Rosa, M. Pini, G. M. Cappucci and A. M. Ferrari, Curr. Opin. Green Sustainable Chem., 2022, 37, 100654 CrossRef CAS.
- P. Jessop, Green Chem., 2020, 22, 13–15 RSC.
- International Standards Organization, in ISO 14040:2006 Environmental Management–Life Cycle Assessment–Principles and Framework, 2006 Search PubMed.
- International StandardsOrganization, in ISO 14044:2006 Environmental Management–Life Cycle Assessment–Requirements and Guidelines, 2006 Search PubMed.
- E. Commission, J. R. Centre, C. Caldeira, L. Farcal, I. Garmendia Aguirre, L. Mancini, D. Tosches, A. Amelio, K. Rasmussen, H. Rauscher, J. Riego Sintes and S. Sala, Safe and sustainable by design chemicals and materials – Framework for the definition of criteria and evaluation procedure for chemicals and materials, Publications Office of the European Union, 2022 Search PubMed.
- A. González-Garay, M. S. Frei, A. Al-Qahtani, C. Mondelli, G. Guillén-Gosálbez and J. Pérez-Ramírez, Energy Environ. Sci., 2019, 12, 3425–3436 RSC.
- S. C. D'Angelo, A. J. Martín, S. Cobo, D. F. Ordóñez, G. Guillén-Gosálbez and J. Pérez-Ramírez, Energy Environ. Sci., 2023, 16, 3314–3330 RSC.
- D. Faust Akl, G. Giannakakis, A. Ruiz-Ferrando, M. Agrachev, J. D. Medrano-García, G. Guillén-Gosálbez, G. Jeschke, A. H. Clark, O. V. Safonova, S. Mitchell, N. López and J. Pérez-Ramírez, Adv. Mater., 2023, 35, 2211464 CrossRef PubMed.
- M. Bachmann, C. Zibunas, J. Hartmann, V. Tulus, S. Suh, G. Guillén-Gosálbez and A. Bardow, Nat. Sustain., 2023, 6, 599–610 CrossRef.
- X. Hai, Y. Zheng, Q. Yu, N. Guo, S. Xi, X. Zhao, S. Mitchell, X. Luo, V. Tulus, M. Wang, X. Sheng, L. Ren, X. Long, J. Li, P. He, H. Lin, Y. Cui, X. Peng, J. Shi, J. Wu, C. Zhang, R. Zou, G. Guillén-Gosálbez, J. Pérez-Ramírez, M. J. Koh, Y. Zhu, J. Li and J. Lu, Nature, 2023, 622, 754–760 CrossRef PubMed.
- D. Faust Akl, D. Poier, S. C. D'Angelo, T. P. Araújo, V. Tulus, O. V. Safonova, S. Mitchell, R. Marti, G. Guillén-Gosálbez and J. Pérez-Ramírez, Green Chem., 2022, 24, 6879–6888 RSC.
- C. R. McElroy, A. Constantinou, L. C. Jones, L. Summerton and J. H. Clark, Green Chem., 2015, 17, 3111–3121 RSC.
- V. Hessel, M. Escribà-Gelonch, J. Bricout, N. N. Tran, A. Anastasopoulou, F. Ferlin, F. Valentini, D. Lanari and L. Vaccaro, ACS Sustainable Chem. Eng., 2021, 9, 9508–9540 CrossRef CAS.
- P. T. Anastas and R. L. Lankey, Green Chem., 2000, 2, 289–295 RSC.
- Life Cycle Assessment in the Chemical Product Chain: Challenges, Methodological Approaches and Applications, ed. S. Maranghi and C. Brondi, Springer International Publishing, Cham, 2020 Search PubMed.
- R. L. Lankey and P. T. Anastas, Ind. Eng. Chem. Res., 2002, 41, 4498–4502 CrossRef CAS.
- M. D. Tabone, J. J. Cregg, E. J. Beckman and A. E. Landis, Environ. Sci. Technol., 2010, 44, 8264–8269 CrossRef CAS PubMed.
- M. A. J. Huijbregts, L. J. A. Rombouts, S. Hellweg, R. Frischknecht, A. J. Hendriks, D. Van De Meent, A. M. J. Ragas, L. Reijnders and J. Struijs, Environ. Sci. Technol., 2006, 40, 641–648 CrossRef CAS.
- M. A. J. Huijbregts, S. Hellweg, R. Frischknecht, H. W. M. Hendriks, K. Hungerbühler and A. J. Hendriks, Environ. Sci. Technol., 2010, 44, 2189–2196 CrossRef CAS PubMed.
- R. Heijungs and E. Dekker, Int. J. Life Cycle Assess., 2022, 27, 993–1015 CrossRef.
- C. Oberschelp, S. Hellweg, E. Bradford, S. Pfister, J. Huo and Z. Wang, Poor data and outdated methods sabotage the decarbonization efforts of the chemical industry, 2023 Search PubMed.
- P. Schober, C. Boer and L. A. Schwarte, Anesth. Analg., 2018, 126, 1763–1768 CrossRef.
- P. G. Jessop and A. R. MacDonald, Green Chem., 2023, 25, 9457–9462 RSC.
- Life Cycle Management, ed. G. Sonnemann and M. Margni, Springer Netherlands, Dordrecht, 2015 Search PubMed.
- A. G. Parvatker, H. Tunceroglu, J. D. Sherman, P. Coish, P. Anastas, J. B. Zimmerman and M. J. Eckelman, ACS Sustainable Chem. Eng., 2019, 7, 6580–6591 CrossRef.
- D. Zhang, Z. Wang, C. Oberschelp, E. Bradford and S. Hellweg, ACS Sustainable Chem. Eng., 2024, 12, 2700–2708 CrossRef.
- M. Overcash, Green Chem., 2016, 18, 3600–3606 RSC.
- Z. Wang, G. W. Walker, D. C. G. Muir and K. Nagatani-Yoshida, Environ. Sci. Technol., 2020, 54, 2575–2584 CrossRef CAS.
- A. J. Williams, C. M. Grulke, J. Edwards, A. D. McEachran, K. Mansouri, N. C. Baker, G. Patlewicz, I. Shah, J. F. Wambaugh, R. S. Judson and A. M. Richard, J. Cheminf., 2017, 9, 61 Search PubMed.
- G. Wernet, S. Hellweg and K. Hungerbühler, Int. J. Life Cycle Assess., 2012, 17, 720–728 CrossRef CAS.
- R. Calvo-Serrano, M. González-Miquel, S. Papadokonstantakis and G. Guillén-Gosálbez, Comput. Chem. Eng., 2018, 108, 179–193 CrossRef CAS.
- A. D. Curzons, C. Jiménez-González, A. L. Duncan, D. J. C. Constable and V. L. Cunningham, Int. J. Life Cycle Assess., 2007, 12, 272–280 CrossRef CAS.
- Y. Sun, X. Wang, N. Ren, Y. Liu and S. You, Environ. Sci. Technol., 2023, 57, 3434–3444 CrossRef CAS.
- D. J. C. Constable, iScience, 2021, 24, 103489 CrossRef CAS.
- S. Mitchell, A. J. Martín, G. Guillén-Gosálbez and J. Pérez-Ramírez, Angew. Chem., Int. Ed., 2024, e202318676 CAS.
- J. M. Weber, Z. Guo, C. Zhang, A. M. Schweidtmann and A. A. Lapkin, Chem. Soc. Rev., 2021, 50, 12013–12036 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4gc00394b |
| This journal is © The Royal Society of Chemistry 2024 |