Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Nature-inspired batteries: from biomaterials to biomimetic design strategies
Stefano
Tagliaferri
a,
Louis
Gaspard
 b,
Heather
Au
b,
Heather
Au
 c,
Cecilia
Mattevi
c,
Cecilia
Mattevi
 a,
Maria-Magdalena
Titirici
a,
Maria-Magdalena
Titirici
 *b and
Maria
Crespo-Ribadeneyra
*bc
*b and
Maria
Crespo-Ribadeneyra
*bc
aDepartment of Materials, Imperial College London, London, SW7 2AZ, UK
bSchool of Engineering and Materials Science, Queen Mary University of London, London E1 4NS, UK
cDepartment of Chemical Engineering, Imperial College London, London, SW7 2AZ, UK
First published on 8th May 2024
Abstract
Nature has vast experience in optimising systems to perform adaptively to their environment. Bio-organisms are intrinsically dynamic: as they react to stimuli, their components work synergistically to achieve the appropriate response to the range of conditions of the environment they are in. Due to this singular specificity, achieved with exquisite tailoring of function and structure from the nanoscopic to the macroscopic level, biological systems have been an inspiration not only for materials design but for many engineering-related applications, including energy storage devices, in particular batteries, which are covered in this review. Bioinspiration is explored as a tool to unlock new materials, hierarchical architectures, and chemistries to achieve specific functions that will be key to addressing the complex range of performance and sustainability requirements for future batteries. Many excellent examples of biomimicry and bioinspiration can be found in the literature, yet the conceptualization of fully biomimetic batteries has not been accomplished. In this review we analyse the possibilities by which batteries could expand beyond structure replication of individual materials, components or chemistries found in nature and provide a broader perspective on their integration. We place battery systems at the interface between green chemistry, nanotechnology, and bioengineering to analyse their basic requirements and contrast how nature has achieved these needs in biological systems.
Introduction
According to the description provided by J. R. Schramski et al.,1 the Earth is a chemical battery where the harvested energy is stored within the billions of tons of living biomass and the fossil fuels that we have, by now, almost depleted. The Earth's discharge, caused by our extended reliance on non-renewable resources, represents the greatest challenge for the balance of humanity with nature. We have reached a point of no return where all actions have accountability.Batteries are an essential driver to help mitigate climate change, as they enable the uptake of renewables by levelling their intermittency in the transport and power sectors. According to the World Economic Forum2 batteries have already resulted in a colossal 0.4 Gt decrease in global CO2 emissions from transport; they have the potential to decrease CO2 emissions by a further 2.2 Gt by 2030, 30% of the required global decrease to reach current targets. In addition to decarbonising the economy, by enabling decentralized and off-grid energy solutions, batteries also address the UN Sustainable Development Goals to provide energy to the 850 million people who currently lack access to electricity. However, for batteries to realise their full potential and impact, it is imperative to diversify our energy storage technologies towards new battery chemistries beyond Li-ion, employing non-critical elements, and to move away from current design and production practices; we must catalyse a rapid change towards a circular battery economy to create more sustainable, low-cost and safer batteries.
More compact batteries with greater power, autonomy and lower price are now a reality. However, due to their current dependence on non-earth-abundant elements (i.e. Li, Co, Mn, Ni) and the still predominant linear economy that regulates their life cycle (i.e. mining → manufacture → use → disposal) we are, once again, contributing to the “Earth's discharge” in the name of ‘clean technologies’. Cobalt, in particular, raises the greatest concerns for supply chain reliability and availability of the material reserves.3 Even considering the sustained replacement of Co with Ni in the cathode chemistry, the global demand for cobalt might grow more than twenty times by 2040, outpacing the capability of the cobalt production line.4 Additionally, current estimates indicate that only ∼40% of the available Ni reserves can be used to produce high-quality battery-grade nickel, and they are located in regions that have been already extensively depleted by mining.5 The supply of cobalt and nickel is geographically concentrated and carries environmental and ethical criticalities, such as pollution, depletion of water reserves and child labour. Although the battery supply chain is not currently limited by the availability of lithium, lithium resources are also localized, with just three countries (Australia, Chile and Argentina) collectively accounting for more than 80% of the world reserves.6 Such a concentration of resources exposes the battery supply chain to potential disruptions, arising from geopolitical risks and political instabilities. The quest now relies on finding truly sustainable ways to advance green technologies that do not compromise our nature/society balance even further. We can reduce the environmental footprint of energy materials by extending their service life (through battery second-life reuse, and recycling), by increasing their performance and efficiency (targeting higher capacity combined with greater durability) and by replacing components with more sustainable alternatives.
Batteries are multicomponent, spanning different length scales (from Å to mm) and involving dynamic processes across different timeframes (from femtoseconds to days), hence balancing performance with sustainability becomes a multi-faceted challenge. Very surprisingly, almost all batteries today are fabricated using rigid components such as metal collectors, solid electrodes and in the case of solid-state batteries, also electrolytes. However, as batteries (dis)charge, they undergo dynamic processes, and failure to comply with the associated structural changes is the major cause of premature degradation. Analogously to living systems, rechargeable batteries expand and contract repeatedly during operation and, therefore, careful examination of how nature has solved structural, mechanical, and chemical challenges in dynamic systems is of key importance to enable the development of adaptative battery technologies.
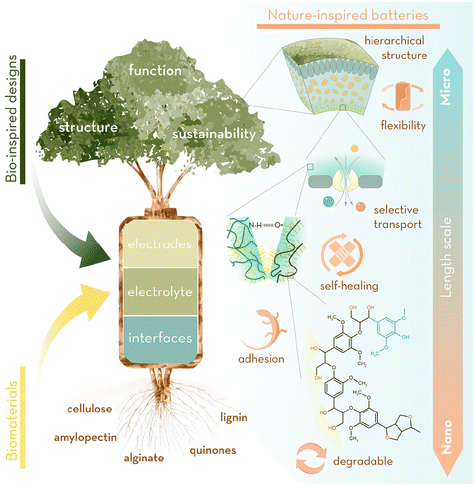
Nature has chosen a narrow assortment of available building blocks for a wide palette of multifunctional biomaterials: minerals, polysaccharides and proteins have been hierarchically structured and perfected over hundreds of millions of years to obtain specific physical properties at each length scale, from structural composites (e.g. nacre, bone) to compliant and self-repairing membranes. Structure and synthesis of these materials and chemicals has been perfected with extreme atom economy, minimising unwanted by-products and only producing substances that are unharmful to the environment where they are segregated. Natural products are biodegradable or easy to recycle into new chemicals and the energy invested to synthesise them is normally recovered with a very high turnover. The efficiency of bio-inspired solutions, combined with the availability and renewability of bio-derived materials, offers the possibility to advance batteries beyond the constraints of current technologies (hindered by the use of critical elements and a linear, non-circular, cycle life), (Fig. 1). Translating all this natural efficiency and sustainability into the energy storage manufacturing industry would undoubtedly enable us to engineer sustainable, long-lasting, high energy-density and fast charging materials for the batteries of the future. This goal has led us to formulate the open questions below from which this review stem:
(i) Is it possible to synthesise efficient materials, comparable to those currently industrialised, from renewable resources and unwanted natural by-products with a high atom economy?
(ii) How can we find structural and functional bioinspiration to reach an equilibrium between ion transport and electron conduction in electrodes, whilst accommodating dynamic mechanical strain exerted on components and interfaces during cycling?
(iii) Is it possible to copy biochemical functionalities found in natural tissues to enable self-healable electrodes?
(iv) Can we generate biomimetic interphases or membranes that can conduct a specific ion while disabling the passage of counterions and solvents towards the electrodes to passivate electrochemical interfaces?
(v) Can we control the electrochemical interfaces while controlling the elastic properties of the membrane?
(vi) Could we decrease the number of materials and elements that we rely on for energy storage systems?
(vii) Can we enable smoother recycling and reusing schemes using bioinspiration?
Thus far, there is a huge body of literature for structural bio-imitation (or inspiration) in any field of materials-related research, yet these are based on the reproduction of isolated structural patterns. While these strategies are indeed fascinating, most of the times they fail to reproduce the orchestrated functional and structural designs that nature has developed jointly across all length scales to perform, very efficiently, a particular action of interest. Such a holistic approach (biomimicry) to design batteries and battery materials is scarcely found in the literature and it is reported without drawing a line on how, through a similar rationale, nature has solved that particular challenge.
We question whether bio-derived systems can be a source of bio-inspiration and biomimicry to boost materials properties (e.g. stress/strain dissipation, ionic conductivity, structural integrity, elasticity, additional redox activity) that are crucial also in other disciplines (such as bioengineering, composite science). Such an analysis across different disciplines of how challenges in battery systems could be addressed by taking inspiration from Nature has not been reported before and we anticipate future successful strategies to solve current shortcomings based on biomimicry. This review is relevant and important in the light of the growing necessity for more efficient and eco-friendly batteries, not only at the individual material level but also to create bioinspired manufacturing methods at cell level. Throughout this review, we will critically examine how this can be accomplished by drawing inspiration from natural systems and processes. In addition, the benefits of a cross-fertilisation approach presented in this review, where ideas are borrowed from different fields, are not limited to batteries alone, but can have a much broader impact in other disciplines, for example fuel cells and electrolysers.
We have centred our analysis on bioinspired approaches applied to electrodes and electrolytes + electrode/electrolyte interfaces, subdividing each of these parts within structural or functional strategies, and analyse their figures of merit. Additionally, we review some examples where bioinsipiration has been taken one step further to emulate complete energy systems.
Bio-inspired electrodes
Biomaterials as precursors
Biomaterials are a useful and versatile feedstock of carbon, and it is largely in this capacity of carbon precursor that they have been employed as battery electrode materials. Besides carbon, biomaterials contain other useful elements, such as O-, N-, S- and P-functionalities, that can have beneficial effects on ion transport and storage. Biomass is typically converted into biochar using high temperature pyrolysis under inert atmosphere. This step is important to introduce the requisite conductivity but at the cost of heteroatom sites. Depending on the composition of the feedstock, preparation and activation methods, and carbonisation temperature or environment, the properties of the resulting material, including porosity, degree of graphitisation, surface functionality, particle size or morphology, may be carefully tuned. A wide variety of biomaterials and bio-derived chemicals have been used as precursors for battery applications, from building block molecules for fundamental studies such as glucose, fructose, polysaccharides, lignin, and chitosan, all the way up to bulk raw materials such as sugarcane bagasse, rice husk, hardwood, softwood, and nut shells.7The resultant carbons have been variously employed as anodes in lithium-ion (LIBs) and sodium-ion batteries (NIBs), current collectors to support metal anode plating in lithium–metal and sodium–metal batteries, or as cathode supports in lithium–sulfur or lithium–air batteries. These carbons show great versatility in their application due to the tunability of structure, and are often described as sustainable since they can be derived from renewable resources. However, given that usually high temperatures and stringent inert conditions are required for conversion of biomaterial to end product, it is important to evaluate the sustainability credentials in comparison to existing electrode materials. Certain studies have demonstrated that even when employing a carbonisation temperature of 1300 °C, the global warming potential (GWP) of the resulting hard carbon is still lower than that of natural graphite commonly used for LIBs.8 Moreover, employing an extra hydrothermal step before pyrolysis improves the electrochemical performance and increases carbon yield in comparison to the directly carbonised material, reducing the GWP still further.9 However, while these materials are interesting from a sustainability standpoint, with ample comprehensive reviews already on the subject, they represent only bio-derivation and not bio-inspiration in its true sense.10
Bioinspired microstructural designs for ion transport, electron conduction and mechanical strength
Electrodes are multicomponent composite materials, typically containing cathode/anode particles as the principal component, a binder to hold these together and maintain the contact to the current collector, and a conductive additive to increase electronic transport across the coated electrode layer. Satisfying a balance between ion transport, electron conduction and structural dissipation of mechanical strain, thus, becomes a multi-faceted challenge. In nature, usage of high tensile strength materials in fibres, such as spider silk (0.45–2.0 GPa), cellulose fibrils (0.2 GPa) or nonwoven fabrics found in diverse types of cocoons (i.e. silkworm or caterpillar, 0.38–0.50 GPa), is a common pattern to achieve strength. Hierarchy is not only used to address structural integrity in biomechanical tissues (e.g. nanofibril → macrofibre → bundle in cellulose or bone) but also to address irrigation of fluids across length scales (e.g. bronchi and bronchiole in lungs, xylem and phloem in plant stems and leaves, blood vessels in tissue).11–13Nanofibre-based materials with enhanced tensile strengths have been extensively reported in the literature through the use of electrospinning.14 Web-like structures or mats have been made conductive with additives (i.e. carbon nanotubes or graphene) or by the carbonisation of polymer precursors, to favour electrical connection between active particles upon expansion/contraction or pulverisation. This concept was illustrated by Nicolosi et al. for Si particles,15 which undergo a 400% volume expansion when fully lithiated.16 The connection between the particles was maintained using a small amount of high tensile strength CNTs forming segregated networks (Fig. 2a and b), resulting in electrodes that were >500 times tougher than traditionally prepared electrodes. This design enabled thick electrodes (30–310 μm) that were able to reach very high areal capacities (45 and 30 mA h cm−2 for anodes and cathodes, respectively) and specific/volumetric energies (480 W h kg−1 and 1600 W h L−1). Bhattacharya et al.17 imitated a ‘sticky’ spider of CNTs functionalised with carbonyl groups, to allow for, in addition to electrical connection, electrostatic interactions with the particles of active material, Fe2O3. The enhanced interaction between the Fe2O3 particles and the CNT web preserved the electrode structure after repeated cycling, ensuring higher capacity retention (>88% after 310 cycles) than that of the physical mixture of Fe2O3 with non-functionalized CNTs. In a similar manner, Ryu et al.18–20 used peptides to promote mineralization to produce iron phosphate nanotubes. In particular, the acidic and polar moieties on the peptide molecules acted as a nucleation site for FePO4. The morphology of the resulting nanotubes ensured enhanced capacity when used in positive electrodes for Li-ion batteries.
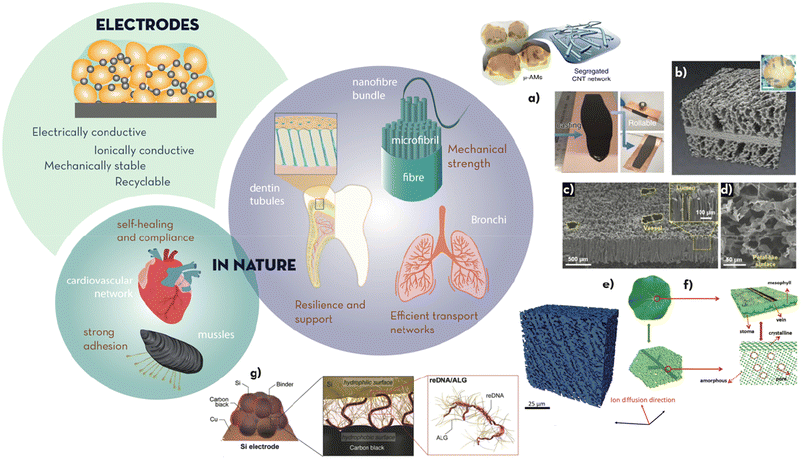 | ||
| Fig. 2 Electrodes (active material particles, conductive additive, and binder) must achieve high electrical and ionic conductivity, and mechanical stability during (dis)charge. The binder should enable strong adhesion and repair, as in nature through cardiovascular networks and coordination bonds, respectively. Some hierarchical microstructures found in high tensile strength natural materials (i.e. spider silk, nonwoven cocoon fibres, cellulose fibres, and human tendons), transport networks (i.e. bronchi) and mineral tissue (i.e. dentin tubules connecting the internal part of teeth and the external enamel) must be a source of inspiration to design better electrodes. Some examples include: (a) segregated CNT networks interlinking Si microparticles for highly manipulable coatings onto current collectors (adapted from ref. 15 with permission from Springer Nature, copyright 2019); (b) X-ray tomography reconstruction of a sintered porous/dense/porous Li7La3Zr2O12 (LLZO) solid electrolyte tri-layer scaffold to infiltrate the cathode active material (adapted from ref. 24 with permission from the ACS, copyright 2020); (c) and (d) SEM pictures of the delignified balsa wood covered with carbon nanotubes used to emulate the transport mechanism of trees (adapted from ref. 22 with permission from John Wiley and Sons, copyright 2019); (e) X-ray microtomography 3D rendering of a directionally ice-templated electrode structures (adapted from ref. 21 with permission from the RSC, copyright 2018); (f) structure of leaves presenting veins for structural support, and their artificial equivalent made of amorphous MnO2 nanosheets supported by a crystalline skeleton (adapted from ref. 25 with permission from John Wiley and Sons, copyright 2019); and (g) structure of the mucin-inspired amphiphilic binder developed by Kim et al.26 (adapted from ref. 26 with permission from John Wiley and Sons, copyright 2018). | ||
To increase resilience in brittle materials, as active particles in electrodes normally are, inspiration from the microstructure of teeth has been found to be an excellent strategy. Dentinal tubules, behaving as blood vessels, form channels for the fast transportation of ions across the thick dentin layer. The porous structure provides cushioning and shock-absorbing properties to the external and brittle enamel layer that has a higher degree of mineralisation. Grant et al.,21 for example, reported ice-templated cathodes with a dentin-like structure where ion-transport across the thickness (900 μm) of the electrode was facilitate by the longitudinal channels remaining once the ice columns were lyophilised (Fig. 2e). This specific structure enabled thick electrodes with high areal and gravimetric capacities (14 mA h cm−2 and 142 mA h g−1 at 0.1C) even at high charging rates (12 mA h cm−2 and 124 mA h g−1 at 1C), outperforming electrodes prepared by standard slurry coating (0.5 mA h cm−2 and 141 mA h g−1 at 0.1C; 0.4 mA h cm−2 and 103 mA h g−1 at 1C). Although this technique to structure electrodes beyond 2D is still relatively recent, it has the potential to be incorporated into standard roll-to-roll manufacturing techniques.
Another excellent source of inspiration for hierarchical microstructure combined with materials multifunctionality can be found in plant leaves. Their primary function is photosynthesis; however, their hierarchical combination of veins and soft tissue is also extremely well designed for bearing stress and distributing electrolytes, connecting the stomata and the xylem channels across the bark. In Li–O2 batteries, akin to plants, the challenge lies in multiphase gas transport, where oxygen diffusion competes with electrolyte transportation. Taking inspiration from stomata, Chen et al.22 used a bio-derived template (delignified balsa wood coated with carbon nanotubes, Fig. 2c and d) to fabricate electrodes with longitudinal conductive paths for fast electron transport. These channels also improved the Li-ion diffusion without hindering the transport of oxygen, thus increasing the capacity and cycling lifetime of the cell. Another leaf-inspired example is that reported by Jia et al.,23 recreating the veins supporting the soft parenchymal tissue of leaves to increase the tensile strength of electrodes; veins of crystalline MnO2 within an amorphous and soft bulk of MnO2 nanosheets (Fig. 2e and f) showed higher reversible capacities (520 mA hg−1 after 2550 cycles vs. 160 mA h g−1 after 600 cycles for purely amorphous MnO2 electrodes) and complete recovery at high current densities (1 A g−1).
Many of the above bioinspired solutions look for improved ionic conductivity whilst preserving the electrical contact through 3D microstructural motifs consisting of vertically aligned channels with low tortuosity. As such, additive manufacturing (3D printing) has been also explored to achieve 3D microstructural design in electrodes, as it allows for a high-level control on the geometry of the pores. Direct Ink Writing (DIW), Selective Laser Melting (SLM) or Fused Deposition Modeling (FDM) are amongst the techniques that have generated research interest. In particular, DIW is a good candidate for electrode manufacturing thanks to its ease of use and high resolution (<50 μm in x- or y-axis and 10 μm in z-axis). Through this technique, octet truss structures (i.e. combination of micro and macropores) which are highly mechanically stable thanks to its triangular lattice can be obtained.27 Within 3D design, interdigitated electrodes have been reported to increase the contact area between cathode and anode, thus reducing the travelling distance of ions.28,29 Werner et al. manufactured a 3D interpenetrating design with nanometer scale features from gyroidal mesoporous carbon. They reported areal capacities of 0.2 mA h cm−2, while a traditional layered design with the same composition and layer dimension would require a surface more than 4000 times larger.30 Another example was shown by M. M. Doeff et al. in an all-solid-state battery where a porous/dense/porous tri-layer of an Al-substituted Li7La3Zr2O12 (LLZO) solid electrolyte was used as the scaffold to infiltrate active materials on one of the porous layer on one side and metallic Li on the other, using an easy to scale up freeze-tape-casting method.24 The cells showed excellent discharge capacities of 125–135 mA h g−1, comparable to NMC-622 cells with liquid electrolyte.
Similarly to leaves, plant roots31 and blood vessels32 were explored as inspiration to improve ionic and electronic transfer in Li-ion and Li-air batteries, respectively. Yang et al.32 mimicked blood capillary tissue to facilitate the diffusion of ions through the in situ grown CNTs on the surface of stainless-steel mesh. With a similar rationale, Liao et al.33 investigated a root-like structure in Li–S battery cathodes, obtained through electrospun C fibres, which presented interconnected cavities for ionic transport and large voids to accommodate and stabilise the volume expansion associated with sulfur/lithium sulfide conversion, leading to high sulfur utilisation and good reversible capacity (983 mA h g−1). Ant-nest architectures can also ensure fast transport kinetics, with their large storage spaces interconnected by open channels.34 The design of ant nests was reproduced by Ai et al.35 using NaCl microparticles as a sacrificial template in sulfur electrodes. After electrode casting, the salt microparticles were dissolved in water, providing high surface area and leaving highly connected channels for ion transport and micro-chambers for the storage of polysulfides. Consequently, it was possible to achieve large sulfur utilization even at high currents, without the formation of obstructions in the ionic diffusion pathways.
Another approach for preserving the structure of electrodes and decreasing the irreversible loss of capacity during the formation of the solid electrolyte interface is to draw inspiration form the natural design of pomegranates. Several examples can be found in the literature of pomegranate-like structures in batteries, especially for active materials displaying large volume variation during cycling. Typically, the active material nanoparticles (pomegranate seeds) are embedded or encapsulated into secondary particles (pomegranate arils). This allows for tuneable internal void space that can accommodate the volume variation of the primary particles. The secondary particles aggregate to form larger clusters, resembling the internal architecture of a pomegranate fruit. Using an intermediate SiO2 template, Liu et al.36 encapsulated silicon nanoparticles into conductive carbon spheres, which served as a volume buffer and electrolyte barrier, preventing the growth of the SEI directly on the silicon nanoparticles. The hierarchical pomegranate-like electrode fabricated in this way achieved an extraordinary 97% capacity retention after 1000 cycles in LIBs, corresponding to a capacity higher than 1160 mA h g−1, more than three times that of graphite anodes.
Advanced binder systems
Binders are commonly used in rechargeable batteries to provide high tensile strength and promote adhesion between the active material particles, the conductive additives and the current collector. An appropriate binder should not deteriorate over time and, ideally, it should assist with electron transfer and ion diffusion to enable a stable electrode and electrode/electrolyte interface. Polyvinylidene fluoride (PVDF) is among the most widely used binders for its electrochemical stability, although it is reprotoxic and carcinogenic and presents relatively weak adhesion capability and mechanical properties. Promising alternatives to toxic fluorinated polymers with excellent binding capabilities can be naturally found in biological systems: carboxymethyl cellulose (CMC), carbonyl-β-cyclodextrin, polyacrylic acid, amylopectin, gelatin, and alginate are commonly employed alternatives. These are characterized by polar hydroxy and carboxy moieties, which provide strong adhesion, mediated by hydrogen bonds, to the surface of common electrode materials. In contrast, fluorinated binders such as PVDF predominantly interact though van der Waals forces, too weak to prevent disconnection and pulverisation of the electrodes while cycling.37Alginate is a natural polysaccharide rich in carboxylic moieties that can be found in brown algae. When used as a binder for silicon anodes it provided high stability and no detectable swelling in the electrolyte solvent vapours compared to PVDF – suggesting a low level of polymer/electrolyte interaction which might prevent undesirable access of the liquid electrolyte to the binder/silicon surface.38 Amylopectin, another natural polysaccharide made of α-D-glucose units, also proved to be a suitable binder for silicon electrodes, providing sufficient adhesion strength coupled with unimpaired transport of lithium ions.39 The transport of lithium ions to the silicon surface can be further enhanced by the polar hydroxyl groups present in biopolymers like guar gum, which was used as binder for stable Si anodes with a discharge capacity of 3364 mA h g−1 and a coulombic efficiency of 88.3% at the current density of 2100 mA g−1.40 A systematic study on the use of polysaccharides as binders for Si anodes identified the superstructure of the polymeric chain and the possibility to form noncovalent interactions (e.g. H-bonding and ion–dipole interactions) as the two crucial features for promoting cycling capability.41 Xanthan gum, in particular, was able to form abundant ion–dipole interactions favourably placed on the side polymeric chains, which promoted numerous points of contact with the active material, resembling the excellent adhesion of millipedes to rough surfaces permitted by their numerous small legs.41
In addition to the hydroxyl and carboxylate groups found in polysaccharides, there are many other functional moieties that actively promote adhesion in biological systems, such as the catechol groups found in mussels. Owing to its chemical structure, consisting of a benzene ring with two neighbouring hydroxy groups, catechol can strongly adhere to the surface of electrode material particles through different types of interactions, including hydrogen bonds, π–π interactions, covalent and coordination bonds.42 Catechol moieties were artificially inserted into bio-derived polysaccharides such as alginate to enhance the interaction of the polymeric chains with silicon particles. These electrodes were able to fully retain their capacity for 400 cycles, performing significantly better than the ones based on PVDF binder (60% of capacity retention after 50 cycles).43 DFT studies have demonstrated that catechol can bind to silanol groups, likely present on the surface of silicon particles, by hydrogen bonds, while also being able to form π–π interactions with the carbon additives in the electrode.44,45 Despite their advantages, the hydrophilic moieties found in polysaccharides cannot efficiently interact with the carbonaceous component of the electrodes (e.g. super P or graphite), for which a certain degree of amphiphilicity might be favourable to obtain higher mechanical integrity. Inspired by the structure of mucin, the protein that covers the walls of mucous membranes withstanding continuous mechanical strain, Kim et al. developed an amphiphilic binder from bio-derived DNA and alginate (Fig. 2g).26 The DNA–alginate binder was able to increase the bond between Si and C and improve the adhesion to the current collector, leading to Si–graphite electrodes with a capacity retention of 92.2% after 300 cycles when tested at 0.5C (1C = 1100 mA g−1).
Bio-directed synthesis can be seen as a biomimetic approach to reproduce function rather than structure. In contrast to templating processes, bio-directed synthesis is not based on reproducing natural structures; instead, the working principle behind a natural process is reverse-engineered to obtain new materials which are absent in nature. Therefore, this approach offers greater versatility in the optimization of battery performance through functions such as self-healing and enhanced binding strength. Self-healing includes a plethora of regeneration processes encountered in almost every living system, from the wound-healing of macroscopic organs and tissues to the self-repair of single cells.46 Self-healing permits regeneration of damaged structures and thus restoration of their functionalities, prolonging the life of the injured organism. Similarly, in batteries, self-healing materials have been explored with the aim of repairing structural damage and extending the use life, while improving the interfacial adhesion between components. An example of self-repairing electrodes for Li-ion batteries has been reported by Wang et al.,47 who coated silicon microparticles with a polymeric binder able to self-heal through the dynamic recreation of hydrogen bonds. The role of the polymeric layer was to restore the cracks caused by volume variation during cycling, ensuring an increase in the cycle life of one order of magnitude. Moved by a similar purpose, Jeong et al.48 developed a self-healing polymeric binder that mimicked the natural proteins found in mussel byssal thread. The capability to repair structural damage was derived from the strong Fe3+–(tris)catechol coordination bonds, presenting dynamic and healable nature, and simultaneously ensuring covalent-like elastic modulus. Mussel-inspired catecholic groups were combined with pyrene moieties by Zhao et al.49 to obtain a conductive polymer with enhanced binding capability towards silicon, which allowed the formation of a more stable SEI on the silicon particles.
Circularity, design for disassembly and recyclability
Bio-inspiration has been applied not only to materials and manufacturing, but also to facilitate disassembly of electrodes at the end of life and enable easier recycling of the individual materials found in electrodes (i.e. current collectors, active particles, binder, conductive additives). In addition to the challenges of re-conditioning active anode/cathode particles that have lost their elemental efficiency during their first service life, a critical hindrance for battery recycling is the separation of the current collector from the electrode. Here, a clean delamination would be ideal to separate active materials from the current collector. Currently, battery components are shredded altogether, and recycling is only centred on the recovery of the most expensive elements found in common cathodes (i.e. Co, Ni). Separating the coating electrode layer from the current collector is challenging since strong adhesion between the active material and the current collector is pursued for maintaining electrical contact and extending lifetime. To address this challenge, Jin et al.50 developed a microscale architecture at the interface between the current collector and the composite film in LIBs to obtain a controllable and directional adhesion. This controlled adhesion was based on the ability of geckos to attach strongly to surfaces; geckos possess controllable attachment and detachment capabilities on a wide range of smooth and rough surfaces, due to angled hierarchical micro- and nanoscale fibrillar structures on their feet. The strong adhesion arises mostly from intermolecular forces such as van der Waals forces, which exist for all surfaces and are mostly insensitive to surface chemistry. In addition, the fibrillar structures exhibit highly directional adhesion allowing the gecko to ‘stick’ and ‘unstick’ by altering the mechanical loading direction. Based on these findings, Jin et al. patterned the surface of the current collector to allow strong interactions whilst also facilitating directional delamination. While the electrochemical performance was not confirmed in this study, this approach could prove to be promising for achieving high-performance LIBs that can be easily recycled.Bio-inspired electrolytes and electrode/electrolyte interfaces
Biomaterials as precursors
As electrolytes, biomaterials have generally been explored in gel- or solid-polymer electrolytes (GPE, SPE) as sustainable alternatives to fossil-derived polyethylene oxide (PEO), polyvinylidene fluoride (PVDF), polymethyl methacrylate (PMMA) or polyacrylonitrile (PAN), amongst others.51 Biomass has a variety of microstructures and abundant functional groups that can be exploited for effective electrolytes.52 Many biopolymers, including proteins, polysaccharides, or lignin, contain a large fraction of polar groups (for example, –NH2, –OH, –CONH-, –CONH2) to dissolve metal salts and allow ion conduction along their backbone. However, polymer electrolytes must balance ionic conductivity with mechanical properties, and typically, increasing the rigidity of the polymer reduces ion mobility. To address this challenge, blends of biopolymers have been explored to decrease the crystallinity, serving to increase conductivity while maintaining cycling robustness.53 Alternatively, engineering of oriented channels by templating, freeze drying or exploiting the intrinsic natural structures of certain biomass additives can improve ion conduction. For example, cellulose nanocrystals were incorporated into a triglycoldimethyl ether (TriG) and cyclohexanedimethanol (CHDM) matrix;54 the subsequently formed structured channels resulted in a 300% improvement in ionic conductivity whilst also improving the mechanical properties of the composite. Chemical modification of pendant groups has also been explored to allow extensive crosslinking or enhance ion transport. However, while biomaterials offer an abundant pool of polymers from which to engineer GPEs and SPEs, in their widest usage, they offer examples only of bio-derivation rather than bio-inspiration.Bioinspired electrode/electrolyte interfaces
Electrochemical reactions occur at the interface between the electrode and the electrolyte, or where electric charge drives the oxidation and reduction of species. As such, interfaces are a key element of batteries, as it is where all the chemical and mechanical instabilities that lead to battery degradation start. As summarised in Fig. 3, high ionic conductivity, mechanical/chemical stability, and selective ion permeability are the required characteristics at the electrode/electrolyte interface to achieve stable performance over time. However, at the start of the first (dis)charge cycle, the SEI layer is formed, due to the irreversible electrochemical decomposition of molecules (e.g. electrolyte, additives) triggered by the working potential of the battery and the subsequent polarisation of the electrodes’ surface. The nature of this SEI (i.e. porosity; electronic/ionic conductivity; mechanical, chemical, and thermal stability; chemical composition and crystallinity) plays a pivotal role in the long-term cyclability, capacity, efficiency, rate capability and safety of batteries, yet controlling the SEI evolution over time is a multifaceted challenge.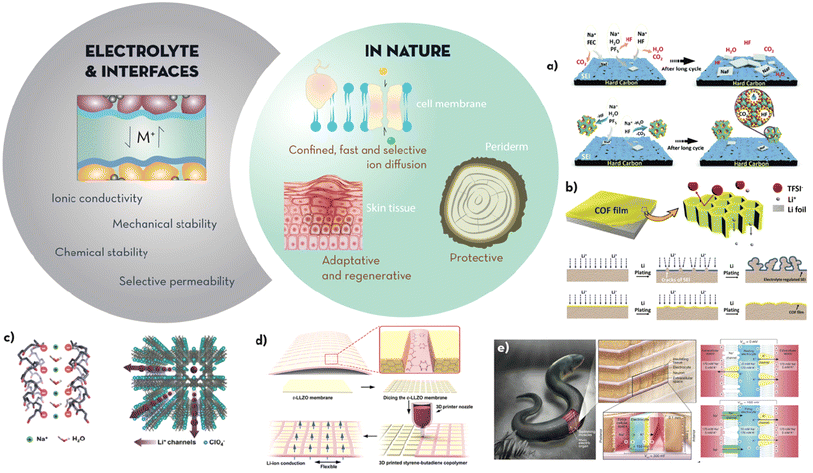 | ||
| Fig. 3 The electrolyte and interfaces must achieve high ionic conductivity, mechanical and chemical stability and be selectively permeable to the storage ions during (dis)charge. Additionally, interfaces should enable intimate contact between the electrode and the electrolyte to ensure no disruption to the electrochemical processes takes place. Microstructures found in nature that enable passage of fluids/nutrients but also serve as protective layers are found in highly elastic, adaptative and self-regenerating skin tissues, the protective external layer or periderm found in trees or the bilipid membrane of cells which enables the selective passage of ions between different compartments. Examples taking inspiration of these properties found in nature include: (a) schematic presenting the capture mechanism of the cycling products from a nanozeolite layer where water, CO2 and HF byproducts are entrapped in the pores, compared to traditional fluoroethylene carbonate (FEC) additives (adapted from ref. 57 with permission from the RSC, copyright 2020); (b) schematic showing the synthesis mechanism and the effect of the COF film proposed by Meng et al.60 on a Li anode (adapted from ref. 60 with permission from John Wiley and Sons, copyright 2019); (c) structure of MOF-based electrolyte proposed by Dunn et al.67 presenting anisotropic and nanoconfined negatively charged transport channels for fast, single ion conduction (adapted from ref. 67 with permission from John Wiley and Sons, copyright 2018); (d) tile and grout design for flexible LLZO membranes with longitudinal pillars of conduction connected through a soft material for flexibility and compliance (adapted from ref. 68 with permission from the ACS, copyright 2019); (e) schematic showing the structure and mechanism behind the generation of voltage in electric eels (i) and the artificial system developed by Mayer et al.69 that emulates it (ii) (adapted from ref. 69 with permission from Springer Nature, copyright 2017). | ||
In nature, as in batteries, many processes are controlled through the chemical and physical properties at interfacial sections (Fig. 3). Extremely well-designed membranes divide functional compartments to enable the selective passage of certain ions and trigger electrochemical processes. Cellular membranes for example, have transmembrane proteins that regulate ionic concentrations55 based on size differentiation and specific binding through charge or van der Waals interactions.56 Other membranes are also prepared to protect inner tissues from harsh environments (i.e. periderm tissue in plants), self-heal (i.e. skin tissue), regulate pressure between different environments, exchange ions very rapidly, maintain a certain temperature gradient and self-assemble in the most optimised manner to obtain mechanical reinforcement in the predominant stress direction. Some of these examples are summarised in Fig. 3.
All the above properties would be desirable at the electrode/electrolyte interface of batteries. However, mimicking the chemistry and structures of the natural world to overcome electrolyte challenges is a vastly unexplored area even for Li-ion batteries. Strategies to stabilise or control interfacial degradation processes often incorporate synthetic interfacial layers (artificial SEIs) able to isolate the surface of the cathode/anode, from molecules other than the cation to be stored, whilst increasing its interfacial cationic conductivity and enabling the dissipation of stresses of expansion/contraction during (dis)charge. For example, Kendrick et al. (Fig. 3a) reported the addition of a nano-zeolite layer on the hard carbon anode of a Na-ion cell.57 The zeolite behaved analogously to a selective transmembrane channel by selectively absorbing small molecules derived from the electrolyte decomposition (CO2, HF and H2O) into the framework. By trapping these by-products, the SEI remained stable, with no evolution of large NaF deposits, for more than 500 cycles and with a remarkable capacity retention of 62%. In a similar manner Myung et al. reported a P2-type layered cathode (Na2/3[Ni1/3Mn2/3]O2) which consisted of a nanolayer of b-NaCaPO4 that prevented the decomposition of the electrolyte salt above 4 V vs. Na+/Na.58 The nanolayer was able to scavenge the HF and the H2O generated by the decomposition of Na/LiPF6, delay the exothermic decomposition reaction of the desodiated electrodes and suppress oxygen evolution from the charged cathode.
Analogously to transmembrane proteins, polymers of intrinsic microporosity (PIMs) have been studied as ionic sieves near anode surfaces. Explored mainly for Li anodes, PIMs offer the possibility of generating SEIs with specific pore sizes to impede the passage of solvated active ions (i.e. Li+, Na+). PIM layers can therefore limit the uncontrolled degradation of solvents on the electrode surface and thus extend the lifetime of the battery.59 Although there are no actual reports on PIMs specifically designed for NIBs, it is worth highlighting the work by Meng et al.60 where a covalent organic framework (COF) was polymerised in situ by the Schiff-base reaction between 1,3,5-tris(4-aminophenyl) benzene (TAPB) and terephthaldehyde (PDA). As shown in the scheme of Fig. 3b, the COF film allowed the passage of Li + but not that of the anion TFSI−, as the pores of the 2D self-assembly were only 10 nm in size. This microcellular structure enabled a homogeneous flux of Li-ions and therefore consistent and clean plating/stripping. The high Young's modulus of the film (6.8 GPa), together with the ability to redistribute the ions on the anode surface, enabled stable Li|Li cycling for more than 400 hours at 2 mA cm−2.
Another excellent example of highly optimized interfacial interactions is found in the specialised nutrient absorption system of cells, where high surface-area brush-like membranes are used to capture essential molecules. These have been emulated in Li–S batteries, using an interlayer of ZnO nanowires grown on C nanofibers.61 The dissolution and uncontrolled deposition of soluble intermediates during conversion of sulfur to lithium sulfide62,63 is a main cause of capacity fade, but in this cell, the ZnO interlayer was able to capture migrating polysulfides, ensuring a capacity loss of only 0.05% per cycle. With a similar rationale, Shen et al.64 mimicked the hydrophobic nature of fish scales to create a scaly and polysulfiphobic stable artificial interphase layer on Li electrodes for controlling the shuttling of polysulfides. The patterned layer made of lithium decylphosphonate, effectively repelled lithium polysulfides, improving the coulombic efficiency (99% for 200 cycles).
Other approaches aiming to stabilize and strengthen the interfacial interactions in secondary batteries have drawn more abstract inspiration from natural structures, such as the protective shells of scallops, and processes, such as biomineralization. Zhang et al.65 for example, enveloped Si particles in a layer of graphene and embedded them in a reduced graphene oxide matrix, to stabilize the SEI and improve the transport of electrons and Li ions. Although the graphene envelope resembles the structure of sea scallops, it does not match the protective function of mollusc shells, providing instead paths for charge transport and voids that buffer the mechanical strain during cycling. More interestingly, the biomineralization process behind the formation of crustacean shells was also mimicked to obtain a mineral hydrogel binder for Li–S batteries, based on polyacrylic acid and calcium carbonate nanoparticles.66
The stability of the electrode–electrolyte interface and particle-to-particle interaction in the electrode crucially affects the performance of rechargeable batteries. Although more stable interfaces would extend the life of all batteries, efforts to enhance interfacial interactions with bio-inspired strategies are still scarce.
Bioinspired microstructural electrolyte designs for ion transport, electron conduction and mechanical strength
An optimal electrolyte should ideally have the same properties as those stated in the previous section on interfaces: electrically insulative, high ionic conductivity, and stability at the electrochemical potentials and temperatures at which the battery is operating. Electrolytes enable (dis)charge to take place by supporting ionic transport between the anode and cathode whilst impeding their electrical contact. In liquid electrolytes, electrically insulating porous membranes (separators) are saturated with the electrolyte solution. However, liquid electrolytes can become explosive or flammable under certain conditions (i.e. severe deformation, heat), they are prone to leak, the derived SEI is affected by the decomposition of the electrolyte, and, in metal anode batteries, dendrite growth is notoriously enhanced due to the instability of the electric field close to the highly reducing surface of the metals. Considering these shortcomings, many strategies are currently centred on developing solid or gel-type electrolytes where virtually no oxidative decomposition occurs. However, the challenges are to achieve high ionic conductivity, favour single ion conduction (i.e. high transference number), enhance interfacial interaction with the electrodes to prevent uncontrolled polarisation, and to achieve membranes able to withstand the interfacial stresses developed upon the cyclic ‘breathing’ of repeated (dis)charge. Strategies to address these demands are directed towards increasing the electrolyte/electrode contact area and increasing the mechanical compliance and ionic conduction at the interface whilst reducing the tortuosity for the ions to be transported from the anode to the cathode and vice versa. In nature, this is done using multiscale transport networks (i.e. roots or tree leaves to improve ionic conduction towards all the system parts), selective conduction channels (i.e. transport channels in cell membranes for selective and fast ion passage) and structural hierarchy (i.e. deformation tolerant and self-healable skin tissue), which have been discussed both in the electrode and interface sections. All these natural features are a source of inspiration also for electrolyte design and will be analysed in this section.An excellent example of mimicking the fast and selective ionic conduction of transmembrane proteins was reported by Dunn et al.67 for enhancing the performance of a LIB solid electrolyte. A metal–organic framework (MOFs) with anisotropic transportation channels was synthesised (Fig. 3c). Extraordinary room temperature conductivity (ca. 10−3 S cm−1) was achieved at activation energies below 0.21 eV. The boost in conductivity can be attributed to the immobilisation of the anions in the structure (Li+ single ion conduction mechanism) and to the alignment of the channels in the direction of Li+ conduction. The anisotropic channels of the MOF scaffold enabled a LiFePO4|Li battery with a specific capacity of 146 mA h g−1 at 0.2C and 106 mA h g−1 at 2C. The capacity retention was 75% at 1C for more than 500 cycles. Following a similar strategy, Biradha et al.70 reported a porous Li–MOF with high surface area (605 m2 g−1) and infiltrated with LiBF4. Through the modification of the open metal sites by coordinating the anion of the Li–salt, conductivities of 1.09 × 10−5 S cm−1 and low activation energies of 0.18 eV were achieved. Similarly, Yan et al.,71 inspired by the Na+/K+ biological transport in membranes, a (–COO–)-modified COF was used as a Na-ion quasi-solid-state electrolyte with sub-nanometre-sized Na+ transport zones (6.7–11.6 Å). Na+ transport along the nanometric and electronegative longitudinal channels, resulted in a remarkably high Na+ conductivity of 1.30 × 10−4 S cm−1 up to 5.32 V (versus Na+/Na). Na||Na3V2(PO4)3 half-cells using this electrolyte showed fast ionic transport, low polarization, and a stable cycling performance over 1000 cycles at 60 mA g−1, with excellent capacity retention (final capacity of 83.5 mA h g−1).
Another approach based on the preferential alignment of conduction paths was explored by Hu et al.,68 with the additional bio-inspired functionality of tissue flexibility and deformability for enabling stress dissipation: a tile-and-grout design prepared through additive manufacturing (Fig. 3d) were Li-conductive garnet tiles of Li6.75La3Zr1.75Ta0.25O12 (LLZO) were held together by a deformable buffer of styrene–butadiene copolymer. The geometrical factors for optimised conductivity/flexibility were obtained through fracture mechanics analysis and the membranes were prepared by additive manufacturing. Fast Li-ion conduction across the membrane was facilitated though the garnet channels (bulk ionic conductivity 1.6 × 10−4 S cm−1) while the flexibility (up to 220% extensibility) and strength (ultimate tensile strength of 5.12 MPa) achieved by the design allowed for stress dissipation upon cycling. Another example of function affected by microstructure was reported by Kim et al.72 A NASICON solid electrolyte scaffold with uniaxial porosity was pre-sintered and then infiltrated with a high tensile strength epoxy polymer; the mechanical strength of the composite was enhanced by approximately 2 times, while the conductivity (1.45 × 10−4 S cm−1) remained close to that of NASICON due to the preservation of the aligned conduction channels upon infiltrating with the epoxy resin. A half-cell using a Na3V2(PO4)3 cathode showed capacities of 120 mA h g−1, maintained for at least 20 cycles. We envisage that this concept, together with the alignment of highly conductive features within the matrix,73 will enable higher conductivities and better control of mechanical/chemical properties at the electrolyte/electrode interfaces in NIBs.
The chemical functionality of certain molecules that take part in electrochemical processes occurring in nature, such as photosynthesis and aerobic respiration, can also be mimicked or re-engineered for improving charge storage in battery materials. For example, quinones, a class of redox-active molecules responsible for multi electron-transfer processes in many biological systems, was studied by Wang et al.74 as an electrode in sodium-ion batteries due to the possibility of storing up to 2 Na+ ions in their structure whilst also forming π–π bonds with carbonaceous supports. Owing to the strong immobilization of the quinone molecules onto the carbon framework, the packing density of the electrode and the high Na+ storage capacity per quinone molecule, a full battery with a Na3V2 (PO4)3/C cathode achieved 305 mA h g−1 and retained 280 mA h g−1 after 100 cycles. Flavins are another example of biological redox-active molecules involved in respiration, able to engage in one- or two-electron transfer processes. The diazabutadiene motif in riboflavin, in particular, can undergo two consecutive one-electron transfer steps at 2.64 V and 2.4 V when tested versus a lithium anode.75 Riboflavin cathodes can achieve a capacity of ∼106 mA h g−1 (at 10 mA g−1), which correspond to the reversible exchange of 1.49 Li atoms per formula unit.75 It should be noted that in these examples a bio-derived material, produced by a natural organism, is used with no structural or functional re-design, to fulfil an analogous function as the one intended in the original biological system. However, molecules which mimic the redox centres of biological systems can also be artificially synthesised, as demonstrated by Hong et al., starting from flavin and simplifying its molecular structure to remove the inactive moieties and enhance gravimetric properties.76 Substituents with strong electron-withdrawing properties can also be inserted to increase the potential of the electron transfer processes, and control the solubility of the electroactive molecules in the electrolyte.75 This approach holds great promise to address the performance and stability issues affecting batteries with organic redox-active electrodes. These examples show how redox-active bio-based molecules can enhance the storage capacity of certain materials, however, their relatively low thermal stability might be a drawback in attaining stable performance upon the high temperature fluctuations observed in industrially relevant batteries. Strategies such as polymerisation or partial crosslinking can increase their voltage operation window and enhance their thermal stability whilst also preventing dissolution.77,78
Self-repair is a highly desired property for both interfaces and electrolytes and is an intrinsic property of living organism. However, the specific mechanisms and chemistries through which healing is achieved in nature (i.e. sealing of wounded tissues or regeneration of fractured bones) remain largely unexplored in the context of battery materials, let alone specifically for electrolytes. Some examples employing polymers able to form transient chemical bonds (similar to those self-repairing binders described in the electrodes section) can be found in the context of electrolytes too. Zhou et al.79 developed a self-healing polymer electrolyte with ureido-pyrimidinone (UPy) dimers, able to provide dynamic quadruple hydrogen bonds to repair mechanical damage. The self-healable separator was used to fabricate a cell with a capacity of 130 mA h g−1 and a coulombic efficiency of 99.1% at 0.1C. A similar approach was investigate by Jaumaux et al.,80 who also relied on UPy moieties to fabricate a separator for Li metal batteries, demonstrating ionic conductivities of 1.79 × 10−3 S cm−1 and high voltage stability (4.5 V vs. Li/Li+).
These examples show how bioinspiration has been successfully applied for the optimization of microstructure, ionic transport, and mechanical strength of solid electrolytes. However, it is the inspiration on the orchestrated combination of functionalities found in living organisms which will enable paving the way for next-generation electrolytes: inspired by the ability of electric eels (Electrophorus electricus) for example, which generate electric power from ionic gradients, Mayer et al.69 designed an electrolyte composed of polyacrylamide hydrogel compartments connected in a repeating sequence of cation- and anion-selective hydrogel membranes (Fig. 3e). The scalable and biocompatible synthetic approach combines mechanical compliance, high ionic conductivity, and optimised structure to generate multiple electrolyte stacks achieving 110 V at open circuit or 27 mW m−2 per gel cell upon the synchronised contact of the thousands of gel compartments connected in series.
Bioinspired and sustainable whole system manufacturing: a future to develop
Current battery manufacturing at cell level is highly energy demanding, involving high-temperature processing of raw materials, coating, drying and cell assembly in a moisture-controlled environment. Recent estimates for LIBs indicate that the production of 1 W h storage capacity requires an energy consumption of 328 W h and causes the emission of 110 g CO2 eq.81 Truly sustainable batteries cannot rely on such environmentally disruptive processes, and should seek inspiration from natural solutions, which are resource-efficient and renewable, ensuring the regeneration and thriving of the ecosystem. Additionally, a ubiquitous principle in natural processes is the structural and morphological optimization of both the individual constituents and the ‘whole system’. When we observe the fine and intricate designs of natural organisms, with highly integrated components tailored towards a functional purpose from the micro- to the macro-scale, it comes as no surprise that their replication is far beyond the capability of the current manufacturing methods for battery components.Advanced manufacturing processes, such as 3D printing, are starting to be considered both at the research and commercial level for battery fabrication. Several parallels can be drawn between 3D printing and how Nature creates its structures. Many hierarchical biological systems are generated through additive processes, such as biomineralization in molluscs and ossification in mammals, which minimize material and energy consumption, following meticulously programmed and optimized designs. These features find resonance in 3D printing techniques, which enable low material waste and shape control on different scales.82 On a system level, natural organisms integrate multiple materials and structures to work in synergy and provide specific functions. Many 3D printing techniques have demonstrated the unique integration of components with spatially programmed composition, structure and properties into multi-material systems. In battery research, notable examples are represented by the fabrication of integrated battery components (electrodes, electrolyte, separator and casing) in the same manufacturing process,83,84 or by the creation of electrodes for pouch cells with graded composition and porosity.85,86 These examples underscore the advantages that the bio-inspired implementation of 3D printing techniques can bring to battery manufacturing, addressing the charge transfer limitations and chemo-mechanical degradation of electrodes. 3D printed electrodes with optimized geometry perform much better than conventional structures at high rates, as shown by Hu et al. for 3D printed LMFP based batteries, retaining a capacity of 108.45 mA h g−1 at the high current of 100C.87 Additionally, 3D printing can endow batteries with advanced functionalities, towards the sustainability goal advocated in this review. McOwen et al.88 demonstrated that 3D printing can be used to tailor the interfacial surface area between the electrode and electrolyte in solid state cells, patterning complex nonplanar architectures. This enabled high interfacial contact, reducing the cell resistance. The maximization of surface area is a common motif in natural structures responsible for adsorption of nutrients, whose geometry could be conveniently mimicked with 3D printing.
Although the literature contains many examples of bio-inspired structures obtained with 3D printing, from light and mechanically flexible armours to stimuli-responsive and shape morphing composites,89 studies on additively manufactured batteries that are rationally guided by bioinspiration are still extremely rare. Tang et al. used 3D printing to create leaf-inspired electrodes, with nickel cobalt sulfide nanoparticles acting as chloroplasts hosting redox reactions, a graphene framework serving as hierarchical leaf veins for electron transport, and a 3D printed structure with interconnected macropores providing fast ionic transport.90 As exemplified by this work, existing studies on printed batteries primarily centre on the single-electrode level, and are often confined to a limited range of recurring geometries (such as interdigitated electrodes), not exploiting the versatility of 3D printing to its full potential. Here, nature can offer an endless set of structural and functional motifs as templates for the next generation of batteries and electrodes. For example, as the field of flexible and wearable electronics grows, there is an accompanying demand for batteries with flexibility and high energy density. To this end, the structure of the human spine can be mimicked to fabricate devices with flexibility and strong mechanical properties. The vertebral column consists of interlocking vertebrae that are able to move freely against each other, despite being connected. Based on this design, flexible LIBs were manufactured, consisting of stacked components in multiple branched strips; these strips were wrapped around the backbone individually to form the individual vertebrae of the structure.91 The interconnecting section maintained electrical contact between each backbone whilst also providing high flexibility to the battery. The battery demonstrated ∼242 W h L−1 power density and ∼94% retention. At 0.5C, the discharge capacity was above 125 mA h g−1 under continuous dynamic load. Meanwhile, mechanical load tests illustrated much lower strain on the interconnect joints compared to prismatic and stacked pouch cells. Although this work did not use 3D printing to fabricate the bio-inspired spine-like structure, it is an excellent example of how bio-inspiration can be applied to the system level, rather than to single components, to achieve high durability and mechanical flexibility.
Flexibility is just one of many bioinspired smart functionalities that 3D printing can facilitate at the system level in batteries. Other advanced features, such as shape responsiveness to environmental stimuli and self-healing, which are inherently present in nature, are also highly coveted for battery applications. With the successful demonstration of these features in other 3D printed systems, the prospect of their application to batteries appears readily attainable in the near future. However, greater effort is needed in the direction of system integration, recognizing the multi-material and multi-component nature of biological systems, and mimicking it on a holistic level with the capabilities offered by 3D printing and other advanced manufacturing methods.
In this context, we envisage 3D printing as a technique that has the capacity to enable the development of bioinspired whole systems. 3D printing has numerous advantages for electrode manufacturing. In addition to improving ionic diffusion, it enables the production of electrodes at the exact geometry required, thus reducing cut-off waste.28 Recently, the company Sakuu developed their own printing process for solid state battery production. Their process allows the printing of metal or polymer either via binder jetting or metal jetting. They can produce tailored battery geometry and have reported an energy density of 800 W h L−1.92 Some 3D printing technologies work on dry powders, removing the need for solvent and extensive drying. Furthermore, 3D printed resin via stereolithography (SLA) can be directly pyrolyzed to turn the polymer into a carbonaceous material. Hard carbon formed via carbonization of resin is electronically conductive and keeps its original design features from the 3D printing process, avoiding the need for additives or binder.93 Laser technologies too, might be able to offer great promise in battery manufacturing processes. Higher design flexibility can be achieved using laser cutting compared to mechanical punching, with limited equipment wear.94 However, cross contamination of metal particles and production speed are still challenging for upscaling this technology. Furthermore, drying is one of the most energy-consuming steps of current electrode manufacturing (19% for large-scale and more than 80% of small-scale factories) and one of the most critical.95 Localized laser beam can improve drying efficiency by limiting heat loss, but also adjust material crystallinity or microstructure where needed.96 Further improvements in drying can come from a reduction in the amount of solvent needed or from a switch to water-based systems to avoid toxic solvent recovery. Increasing solid content of the slurry would overall decrease the volume of solvent used. However, the viscosity of the slurry is greatly affected and can cause processing issues with the slot-die coater equipment.97 Using low molecular weight binder particles followed by Electron Beam (EB) curing is an efficient way to increase solid content. Curing will increase the molecular weight via polymerization of the particles, and the mechanical strength of the coated layer.95
Laser texturing can provide performance improvement on both thin and thick film electrode. Even though it is a subtractive manufacturing technique, cell capacity is improved at higher C-rate compared to the casted slurry.94 Structuring the current collector could also provide higher electrode adhesion, especially for Si–C anodes undergoing significant volume change during cycling. Tang et al. showed that laser drilling of the copper current collector in Si–C anodes resulted in a coulombic efficiency of 60% after 40 cycles while the non-structured current collector efficiency dropped to less than 30%.98
Recyclability could also be improved using laser technologies. After disassembly, the SEI layer formed during cycling can be removed by applying a pulsed laser with low fluency (0.313 J cm−2) without impacting the electrode microstructure. Higher energy per surface area could cause melting of the electrode particles.99
Conclusions
Materials
Examples in the literature prove that the synthesis of equally efficient energy materials can be achieved prioritising the use of renewable feedstocks. Atom economy and waste minimisation is the most important principle of biological reactions and should be a precept to produce energy materials. Approaches such as employing bio-based polymers instead of fossil-derived plastics, waste valorisation, green synthesis methodologies (i.e. microwave-assisted or ionic liquid-based synthesis of chemicals) and usage of non-precious catalysts to facilitate certain reactions are some strategies that should be preferred to enhance materials sustainability.Electrodes
Bio-inspiration is fundamentally different from bio-derivation, since it allows adaptive re-design of the materials found in nature according to specific technological needs. Multiscale transport networks, anisotropic conduction channels, porous and infiltrated structures, self-healing chemistries, and flexible/extensible electrode designs have been demonstrated in the literature by mimicking biochemical functionalities found in natural tissues. Most examples in the literature correspond to electrode preparation and corroborate that electrochemical materials can be re-designed following natural processes as a guiding principle, however, examples of their integration in bio-inspired whole systems are still scarce.Electrolytes
Must be designed to minimise the oxidative/reductive decomposition at the electrode/electrolyte interface whilst also aiming for high ionic conductivity, single-ion conduction, and effective interfacial interaction with electrodes. As in nature, anisotropic transportation channels and nanoconfinment of conduction paths has demonstrated excellent results for boosting the conductivity in solid and gel electrolytes. Self-repair mechanisms in living organisms, shows promise in improving the cycling performance of batteries.Interfaces
Play the most critical role in determining the long-term stability, capacity, efficiency, rate capability, and safety of the whole battery. As in biological interfaces, these must be designed with the ability of regulating interfacial processes, aiming to stabilise and control the SEI thorough approaches that enhance the permeability as well as the selectivity of the primary storage ion, whilst incorporating nanolayers of specific compounds that can absorb by-products and prevent electrolyte decomposition.Whole cell manufacturing
Current battery manufacturing, particularly for lithium-ion, is extremely energy-intensive, wasteful, and environmentally impactful. For a bioinspired approach with a better atom economy, techniques that can improve performance and the same time than reduce resources will be essential. Additive manufacturing and laser-based technologies present great promise as they offered a tailored approach, however, there are only few examples that follow bio-inspired or sustainability-driven manufacturing. We envisage great expansion of this fields as new battery chemistries emerge.Perspective
Bio-inspiration represents a versatile design tool that can be used to tailor sustainability of materials and functional properties of electrodes, electrolytes and interfaces. The biological structures, processes and chemistries that have served as a model for nature-inspired batteries have been reviewed, discussing how they can be combined to fabricate advanced devices with a higher level of bio-mimicry; either using natural materials, or re-engineering existing materials to emulate processes occurring in living organisms. That superior level of bio-inspiration can be achieved when the functionalities of sustainable, bio-derived materials are manipulated and re-designed following natural processes as a guide, employing a highly multidisciplinary approach. To pave the way towards this holistic view, further biomimicry exploration at all battery manufactory levels should be incorporated, to identify key principles and structures for the efficient storage, release and conduction of ions that can be extrapolated to synthetic energy storage systems. To achieve this, it will be essential to invest in cross-disciplinary collaborations between scientists, engineers, and biologists, to create teams capable of tackling the multifaceted challenges associated with biomimetic battery development. Material selection should be also taken as a critical step for battery manufacturing, exploring only those candidates scoring high in sustainability, especially focusing on bio-based materials with minimal environmental footprint, and with properties similar to those found in natural energy storage systems. Renewable feedstocks should be incentivised, aiming for a closed-loop system where the components can be easily repurposed or recycled. For achieving an efficient battery design, natural systems that exhibit long-term cyclic performance and adaptability should be considered, focusing on life cycle extension, negligible degradation over time and minimal by-product generation.Although numerous reports of nature-inspired batteries have been published in recent years, the field is still at its infancy and the systems that have been studied so far represent only a small fraction of the energy storage devices available today. In conjunction with the limited number of bio-inspired systems investigated, biomimicry has also been mostly applied to the production of single battery components, predominantly the electrodes or the electrolyte. A more holistic approach, taking into account the life cycle of the entire device, from fabrication to end-of-life, will be key to obtain efficient batteries that are truly sustainable. Combining insights from nature with advancements in materials science and engineering will enable accelerating the development of sustainable battery technologies for a net-zero future.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
C.M. would like to acknowledge the award of funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme (Grant Agreement No. 819069) and the award of a Royal Society University Research Fellowship (UF160539).References
- J. R. Schramski, D. K. Gattie and J. H. Brown, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 9511–9517 CrossRef CAS PubMed
.
- A Vision for a Sustainable Battery Value Chain in 2030: Unlocking the Full Potential to Power Sustainable Development and Climate Change Mitigation, 2019 Search PubMed.
- R. Schmuch, R. Wagner, G. Hörpel, T. Placke and M. Winter, Nat. Energy, 2018, 3, 267–278 CrossRef CAS
.
-
IEA, The Role of Critical Minerals in Clean Energy Transitions, Paris, 2021 Search PubMed
.
- A. Mayyas, D. Steward and M. Mann, Sustainable Mater. Technol., 2019, 19, e00087 CrossRef CAS
.
- B. Sayahpour, H. Hirsh, S. Parab, L. H. B. Nguyen, M. Zhang and Y. S. Meng, MRS Energy Sustainability, 2022, 9, 183–197 CrossRef
.
- Z. Zhu and Z. Xu, Renewable Sustainable Energy Rev., 2020, 134, 110308 CrossRef CAS
.
- H. Liu, Z. Xu, Z. Guo, J. Feng, H. Li, T. Qiu and M. Titirici, Phil. Trans. R. Soc. A., 2021, 379, 20200340 CrossRef CAS PubMed
.
- Z. Xu, J. Wang, Z. Guo, F. Xie, H. Liu, H. Yadegari, M. Tebyetekerwa, M. P. Ryan, Y. S. Hu and M. M. Titirici, Adv. Energy Mater., 2022, 12, 2200208 CrossRef CAS
.
- C. Liedel, ChemSusChem, 2020, 13, 2110–2141 CrossRef CAS PubMed
.
- C. Chen, S. Xu, Y. Kuang, W. Gan, J. Song, G. Chen, G. Pastel, B. Liu, Y. Li, H. Huang and L. Hu, Adv. Energy Mater., 2019, 9, 1–10 Search PubMed
.
- X. Xu, L. Heng, X. Zhao, J. Ma, L. Lin and L. Jiang, J. Mater. Chem., 2012, 22, 10883–10888 RSC
.
- Z. Tang, N. A. Kotov, S. Magonov and B. Ozturk, Nat. Mater., 2003, 2, 413–418 CrossRef CAS PubMed
.
- Y. Jin, H. Yuan, J. Le Lan, Y. Yu, Y. H. Lin and X. Yang, Nanoscale, 2017, 9, 13298–13304 RSC
.
- S. H. Park, P. J. King, R. Tian, C. S. Boland, J. Coelho, C. Zhang, P. McBean, N. McEvoy, M. P. Kremer, D. Daly, J. N. Coleman and V. Nicolosi, Nat. Energy, 2019, 4, 560–567 CrossRef CAS
.
- B. Jerliu, E. Hüger, L. Dörrer, B.-K. Seidlhofer, R. Steitz, V. Oberst, U. Geckle, M. Bruns and H. Schmidt, J. Phys. Chem. C, 2014, 118, 9395–9399 CrossRef CAS
.
- P. Bhattacharya, M. Kota, D. H. Suh, K. C. Roh and H. S. Park, Adv. Energy Mater., 2017, 7, 1–11 Search PubMed
.
- J. Ryu, S. W. Kim, K. Kang and C. B. Park, Adv. Mater., 2010, 22, 5537–5541 CrossRef CAS PubMed
.
- S. X. Wang, S. Chen, Q. Wei, X. Zhang, S. Y. Wong, S. Sun and X. Li, Chem. Mater., 2015, 27, 336–342 CrossRef CAS
.
- S. Kim and C. B. Park, Adv. Funct. Mater., 2013, 23, 10–25 CrossRef CAS
.
- C. Huang and P. S. Grant, J. Mater. Chem. A, 2018, 6, 14689–14699 RSC
.
- C. Chen, S. Xu, Y. Kuang, W. Gan, J. Song, G. Chen, G. Pastel, B. Liu, Y. Li, H. Huang and L. Hu, Adv. Energy Mater., 2019, 9, 1–10 Search PubMed
.
- B. Jia, W. Chen, J. Luo, Z. Yang, L. Li and L. Guo, Adv. Mater., 2020, 32, 1–8 Search PubMed
.
- E. Yi, H. Shen, S. Heywood, J. Alvarado, D. Y. Parkinson, G. Chen, S. W. Sofie and M. M. Doeff, ACS Appl. Energy Mater., 2020, 3, 170–175 CrossRef CAS
.
- B. Jia, W. Chen, J. Luo, Z. Yang, L. Li and L. Guo, Adv. Mater., 2020, 32, 1–8 Search PubMed
.
- S. Kim, Y. K. Jeong, Y. Wang, H. Lee and J. W. Choi, Adv. Mater., 2018, 30, 1707594 CrossRef PubMed
.
- T. Chu, S. Park and K. Fu, Carbon Energy, 2021, 3, 424–439 CrossRef
.
- C. D. Reynolds, G. Alsofi, J. Yang, M. J. H. Simmons and E. Kendrick, J. Manuf. Process., 2024, 110, 161–172 CrossRef
.
- M. Cheng, R. Deivanayagam and R. Shahbazian-Yassar, Batteries Supercaps, 2020, 3, 130–146 CrossRef CAS
.
- J. G. Werner, G. G. Rodríguez-Calero, H. D. Abruña and U. Wiesner, Energy Environ. Sci., 2018, 11, 1261–1270 RSC
.
- J. Yu, S. Chen, W. Hao and S. Zhang, ACS Nano, 2016, 10, 2500–2508 CrossRef CAS PubMed
.
- X. Y. Yang, J. J. Xu, Z. W. Chang, D. Bao, Y. Bin Yin, T. Liu, J. M. Yan, D. P. Liu, Y. Zhang and X. B. Zhang, Adv. Energy Mater., 2018, 8, 1–7 Search PubMed
.
- Y. Liao, J. Xiang, L. Yuan, Z. Hao, J. Gu, X. Chen, K. Yuan, P. K. Kalambate and Y. Huang, ACS Appl. Mater. Interfaces, 2018, 10, 37955–37962 CrossRef CAS PubMed
.
- G. Ai, Y. Dai, W. Mao, H. Zhao, Y. Fu, X. Song, Y. En, V. S. Battaglia, V. Srinivasan and G. Liu, Nano Lett., 2016, 16, 5365–5372 CrossRef CAS PubMed
.
- G. Ai, Y. Dai, W. Mao, H. Zhao, Y. Fu, X. Song, Y. En, V. S. Battaglia, V. Srinivasan and G. Liu, Nano Lett., 2016, 16, 5365–5372 CrossRef CAS PubMed
.
- N. Liu, Z. Lu, J. Zhao, M. T. McDowell, H.-W. Lee, W. Zhao and Y. Cui, Nat. Nanotechnol., 2014, 9, 187–192 CrossRef CAS PubMed
.
- F. Zou and A. Manthiram, Adv. Energy Mater., 2020, 10, 2002508 CrossRef CAS
.
- I. Kovalenko, B. Zdyrko, A. Magasinski, B. Hertzberg, Z. Milicev, R. Burtovyy, I. Luzinov and G. Yushin, Science, 2011, 334, 75–79 CrossRef CAS PubMed
.
- M. Murase, N. Yabuuchi, Z.-J. Han, J.-Y. Son, Y.-T. Cui, H. Oji and S. Komaba, ChemSusChem, 2012, 5, 2307–2311 CrossRef CAS PubMed
.
- J. Liu, Q. Zhang, T. Zhang, J.-T. Li, L. Huang and S.-G. Sun, Adv. Funct. Mater., 2015, 25, 3599–3605 CrossRef CAS
.
- Y. K. Jeong, T. W. Kwon, I. Lee, T. S. Kim, A. Coskun and J. W. Choi, Energy Environ. Sci., 2015, 8, 1224–1230 RSC
.
- J. Saiz-Poseu, J. Mancebo-Aracil, F. Nador, F. Busqué and D. Ruiz-Molina, Angew. Chem., Int. Ed., 2019, 58, 696–714 CrossRef CAS PubMed
.
- M.-H. Ryou, J. Kim, I. Lee, S. Kim, Y. K. Jeong, S. Hong, J. H. Ryu, T.-S. Kim, J.-K. Park, H. Lee and J. W. Choi, Adv. Mater., 2013, 25, 1571–1576 CrossRef CAS PubMed
.
- S. A. Mian, L. C. Saha, J. Jang, L. Wang, X. Gao and S. Nagase, J. Phys. Chem. C, 2010, 114, 20793–20800 CrossRef CAS
.
- Y. Li, M. Liao and J. Zhou, J. Phys. Chem. C, 2018, 122, 22965–22974 CrossRef CAS
.
- S. K. Y. Tang and W. F. Marshall, Science, 2017, 356, 1022–1025 CrossRef CAS PubMed
.
- C. Wang, H. Wu, Z. Chen, M. T. Mcdowell, Y. Cui and Z. Bao, Nat. Chem., 2013, 5, 1042–1048 CrossRef CAS PubMed
.
- Y. K. Jeong and J. W. Choi, ACS Nano, 2019, 13, 8364–8373 CrossRef CAS PubMed
.
- H. Zhao, Y. Wei, C. Wang, R. Qiao, W. Yang, P. B. Messersmith and G. Liu, ACS Appl. Mater. Interfaces, 2018, 10, 5440–5446 CrossRef CAS PubMed
.
- C. Jin, Z. Yang, J. Li, Y. Zheng, W. Pfleging and T. Tang, Extreme Mech. Lett., 2020, 34, 100594 CrossRef
.
- E. Lizundia and D. Kundu, Adv. Funct. Mater., 2021, 31, 2005646 CrossRef CAS
.
- O. Sheng, C. Jin, T. Yang, Z. Ju, J. Luo and X. Tao, Energy Environ. Sci., 2023, 16, 2804–2824 RSC
.
- N. N. A. Amran, N. S. A. Manan and M. F. Z. Kadir, Ionics, 2016, 22, 1647–1658 CrossRef CAS
.
- J. Choi, O. Zabihi, R. J. Varley, B. Fox and M. Naebe, ACS Appl. Mater. Interfaces, 2022, 14, 45320–45332 CrossRef CAS PubMed
.
- Y. Shen, W. Song, D. R. Barden, T. Ren, C. Lang, H. Feroz, C. B. Henderson, P. O. Saboe, D. Tsai, H. Yan, P. J. Butler, G. C. Bazan, W. A. Phillip, R. J. Hickey, P. S. Cremer, H. Vashisth and M. Kumar, Nat. Commun., 2018, 9, 2294 CrossRef PubMed
.
- E. Gouaux and R. MacKinnon, Science, 2005, 310, 1461–1465 CrossRef CAS PubMed
.
- L. Chen, B. Kishore, M. Walker, C. E. J. Dancer and E. Kendrick, Chem. Commun., 2020, 56, 11609–11612 RSC
.
- C.-H. Jo, J.-H. Jo, H. Yashiro, S.-J. Kim, Y.-K. Sun and S.-T. Myung, Adv. Energy Mater., 2018, 8, 1702942 CrossRef
.
- G. H. Moon, H. J. Kim, I. S. Chae, S. C. Park, B. S. Kim, J. Jang, H. Kim and Y. S. Kang, Chem. Commun., 2019, 55, 6313–6316 RSC
.
- D. Chen, S. Huang, L. Zhong, S. Wang, M. Xiao, D. Han and Y. Meng, Adv. Funct. Mater., 2020, 30, 1907717 CrossRef CAS
.
- T. Zhao, Y. Ye, X. Peng, G. Divitini, H.-K. Kim, C.-Y. Lao, P. R. Coxon, K. Xi, Y. Liu, C. Ducati, R. Chen and R. V. Kumar, Adv. Funct. Mater., 2016, 26, 8418–8426 CrossRef CAS
.
- C. Deng, Z. Wang, L. Feng, S. Wang and J. Yu, J. Mater. Chem. A, 2020, 8, 19704–19728 RSC
.
- J. Conder, R. Bouchet, S. Trabesinger, C. Marino, L. Gubler and C. Villevieille, Nat. Energy, 2017, 2, 17069 CrossRef CAS
.
- X. Shen, T. Qian, P. Chen, J. Liu, M. Wang and C. Yan, ACS Appl. Mater. Interfaces, 2018, 10, 30058–30064 CrossRef CAS PubMed
.
- X. Zhang, R. Guo, X. Li and L. Zhi, Small, 2018, 14, 1800752 CrossRef PubMed
.
- M. Tian, X. Chen, S. Sun, D. Yang and P. Wu, Nano Res., 2019, 12, 1121–1127 CrossRef CAS
.
- L. Shen, H. Bin Wu, F. Liu, J. L. Brosmer, G. Shen, X. Wang, J. I. Zink, Q. Xiao, M. Cai, G. Wang, Y. Lu and B. Dunn, Adv. Mater., 2018, 30, 1707476 CrossRef PubMed
.
- H. Xie, Y. Bao, J. Cheng, C. Wang, E. M. Hitz, C. Yang, Z. Liang, Y. Zhou, S. He, T. Li and L. Hu, ACS Energy Lett., 2019, 4, 2668–2674 CrossRef CAS
.
- T. B. H. Schroeder, A. Guha, A. Lamoureux, G. VanRenterghem, D. Sept, M. Shtein, J. Yang and M. Mayer, Nature, 2017, 552, 214–218 CrossRef CAS PubMed
.
- K. Nath, A. Bin Rahaman, R. Moi, K. Maity and K. Biradha, Chem. Commun., 2020, 56, 14873–14876 RSC
.
- Y. Yan, Z. Liu, T. Wan, W. Li, Z. Qiu, C. Chi, C. Huangfu, G. Wang, B. Qi, Y. Yan, T. Wei and Z. Fan, Nat. Commun., 2023, 14, 3066 CrossRef CAS PubMed
.
- Y. J. Lim, J. Han, H. W. Kim, Y. Choi, E. Lee and Y. Kim, J. Mater. Chem. A, 2020, 8, 14528–14537 RSC
.
- W. Liu, S. W. Lee, D. Lin, F. Shi, S. Wang, A. D. Sendek and Y. Cui, Nat. Energy, 2017, 2, 17035 CrossRef CAS
.
- H. Wang, P. Hu, J. Yang, G. Gong, L. Guo and X. Chen, Adv. Mater., 2015, 27, 2348–2354 CrossRef CAS PubMed
.
- M. Lee, J. Hong, D.-H. Seo, D. H. Nam, K. T. Nam, K. Kang and C. B. Park, Angew. Chem., Int. Ed., 2013, 52, 8322–8328 CrossRef CAS PubMed
.
- J. Hong, M. Lee, B. Lee, D. H. Seo, C. B. Park and K. Kang, Nat. Commun., 2014, 5, 1–9 Search PubMed
.
- T. Lap, N. Goujon, D. Mantione, F. Ruipérez and D. Mecerreyes, ACS Appl. Polym. Mater., 2023, 5, 9128–9137 CrossRef CAS PubMed
.
- P. Poizot, J. Gaubicher, S. Renault, L. Dubois, Y. Liang and Y. Yao, Chem. Rev., 2020, 120, 6490–6557 CrossRef CAS PubMed
.
- B. Zhou, M. Yang, C. Zuo, G. Chen, D. He, X. Zhou, C. Liu, X. Xie and Z. Xue, ACS Macro Lett., 2020, 9, 525–532 CrossRef CAS PubMed
.
- P. Jaumaux, Q. Liu, D. Zhou, X. Xu, T. Wang, Y. Wang, F. Kang, B. Li and G. Wang, Angew. Chem., Int. Ed., 2020, 59, 9134–9142 CrossRef CAS PubMed
.
- J. F. Peters, M. Baumann, B. Zimmermann, J. Braun and M. Weil, Renewable Sustainable Energy Rev., 2017, 67, 491–506 CrossRef CAS
.
- L. Hirt, A. Reiser, R. Spolenak and T. Zambelli, Adv. Mater., 2017, 29, 1604211 CrossRef PubMed
.
- T. S. Wei, B. Y. Ahn, J. Grotto and J. A. Lewis, Adv. Mater., 2018, 30, 1–7 Search PubMed
.
- K. Sun, T. S. Wei, B. Y. Ahn, J. Y. Seo, S. J. Dillon and J. A. Lewis, Adv. Mater., 2013, 25, 4539–4543 CrossRef CAS PubMed
.
- S. Wang, H. Shi, Y. Xia, D. Liu, C. Min, M. Zeng, S. Liang, R. Shao, X. Wu and Z. Xu, J. Mater. Chem. A, 2022, 10, 24258–24268 RSC
.
- D. Lin, S. Chandrasekaran, J.-B. Forien, X. Xue, A. Pinongcos, E. Coester, M. A. Worsley and Y. Li, Adv. Energy Mater., 2023, 13, 2300408 CrossRef CAS
.
- J. Hu, Y. Jiang, S. Cui, Y. Duan, T. Liu, H. Guo, L. Lin, Y. Lin, J. Zheng, K. Amine and F. Pan, Adv. Energy Mater., 2016, 6, 1600856 CrossRef
.
- D. W. McOwen, S. Xu, Y. Gong, Y. Wen, G. L. Godbey, J. E. Gritton, T. R. Hamann, J. Dai, G. T. Hitz, L. Hu and E. D. Wachsman, Adv. Mater., 2018, 30, 1–7 CrossRef PubMed
.
- Y. Yang, X. Song, X. Li, Z. Chen, C. Zhou, Q. Zhou and Y. Chen, Adv. Mater., 2018, 30, 1706539 CrossRef PubMed
.
- X. Tang, C. Zhu, D. Cheng, H. Zhou, X. Liu, P. Xie, Q. Zhao, D. Zhang and T. Fan, Adv. Funct. Mater., 2018, 28, 1–10 Search PubMed
.
- G. Qian, B. Zhu, X. Liao, H. Zhai, A. Srinivasan, N. J. Fritz, Q. Cheng, M. Ning, B. Qie, Y. Li, S. Yuan, J. Zhu, X. Chen and Y. Yang, Adv. Mater., 2018, 30, 1–8 Search PubMed
.
- A. Örüm Aydin, F. Zajonz, T. Günther, K. B. Dermenci, M. Berecibar and L. Urrutia, Batteries, 2023, 9, 555 CrossRef
.
- Y. Katsuyama, A. Kudo, H. Kobayashi, J. Han, M. Chen, I. Honma and R. B. Kaner, Small, 2022, 18, 2202277 CrossRef CAS PubMed
.
- W. Pfleging, Nanophotonics, 2018, 7, 549–573 CAS
.
- S. N. Bryntesen, A. H. Strømman, I. Tolstorebrov, P. R. Shearing, J. J. Lamb and O. Stokke Burheim, Energies, 2021, 14, 1406 CrossRef CAS
.
- J. Pröll, R. Kohler, M. Torge, S. Ulrich, C. Ziebert, M. Bruns, H. J. Seifert and W. Pfleging, Appl. Surf. Sci., 2011, 257, 9968–9976 CrossRef
.
- J. Li, J. Fleetwood, W. B. Hawley and W. Kays, Chem. Rev., 2022, 122, 903–956 CrossRef CAS PubMed
.
- X. X. Tang, W. Liu, B. Y. Ye and Y. Tang, Trans. Nonferrous Met. Soc. China, 2013, 23, 1723–1727 CrossRef CAS
.
- M. O. Ramoni, Y. Zhang, H. C. Zhang and T. Ghebrab, Int. J. Adv. Manuf. Technol., 2017, 88, 3067–3076 CrossRef
.
| This journal is © The Royal Society of Chemistry 2024 |