Biomass-derived metal-free heteroatom doped nanostructured carbon electrocatalysts for high-performance rechargeable lithium–air batteries
Molla Asmare
Alemu
 *a,
Muluken
Zegeye Getie
ab,
Hailemariam
Mulugeta Wassie
b,
Mulat
Shitye Alem
b,
Addisu Alemayehu
Assegie
c,
Mustafa
llbaş
d and
Rafat
Al Afif
e
*a,
Muluken
Zegeye Getie
ab,
Hailemariam
Mulugeta Wassie
b,
Mulat
Shitye Alem
b,
Addisu Alemayehu
Assegie
c,
Mustafa
llbaş
d and
Rafat
Al Afif
e
aBahir Dar Energy Center, Bahir Dar Institute of Technology, Bahir Dar University, Ethiopia. E-mail: mollaasmare98@gmail.com
bFaculty of Mechanical and Industrial Engineering, Bahir Dar Institute of Technology, Bahir Dar University, Ethiopia
cSchool of Material Science and Engineering, Bahir Dar Institute of Technology, Bahir Dar University, Ethiopia
dDepartment of Energy Systems Engineering, Gazi University, Turkey
eInstitute of Chemical and Energy Engineering, University of Natural Resources and Life Sciences, Vienna, Austria
First published on 24th October 2024
Abstract
Renewable energy sources are crucial for addressing the energy crisis and global warming, but their intermittent nature necessitates storage. Metal–air batteries, such as Li–air batteries, offer high specific capacity and environmental friendliness but face issues like poor reaction kinetics and high overpotential during charging and discharging. To address these issues, noble metal-based catalysts have been utilized, which require the replacement of such precious and scarce resources with affordable and commercially accessible materials. Biomass, a renewable resource, plays a critical role in preparing carbon-based electrocatalysts and porous cathodes with excellent performance due to its rich heteroatom and pore structure, and potential doping and co-doping with transition metals and their oxides. Metal-free biomass carbon nanostructured bifunctional electrocatalysts have been identified as potential alternatives for the next generation of oxygen reduction and evolution reactions. These catalysts have comparable catalytic activity and improved stability compared to the current state-of-the-art Pt-based catalysts, making them essential for the commercialization of lithium–air batteries. Thus, this paper reviews the most recent advances in biomass-derived metal-free heteroatom-doped nanostructured carbon electrocatalysts for rechargeable lithium–air batteries and discusses how different biomass sources affect the cathode's composition, morphology, and structure–activity relationship. It gives a reasonable approach to doping methodologies, which may guide non-noble electrocatalyst and electrode designs.
1. Introduction
The global economy's swift growth, increasing population, and excessive fossil fuel consumption have necessitated the development of renewable energy technologies to combat environmental degradation and the energy crisis. However, renewable energy sources are often unreliable and intermittent, limiting their large-scale applications. Electrochemical energy storage and conversion systems, including solar cells, batteries, fuel cells, and supercapacitors, are viable solutions. Among them, lithium-ion batteries (LIBs) have been extensively studied, but their shortcomings like low energy density, material scarcity, and poor safety have not been effectively addressed,1–3 highlighting the need for alternative energy storage solutions.Biomass, as a renewable resource, plays a critical role in the preparation of carbon-based catalysts and porous cathodes for metal–air batteries.4,5 This is due to the inherent rich heteroatom and pore structure of biomass. Metal–air batteries (MABs) have broad prospects in the field of electrochemical energy storage due to their simple structure, lower cost, higher energy efficiency, high security, environmental friendliness, high specific capacity, and high energy density. They use an anode made from pure metal and an external cathode of ambient air, typically with an aqueous or aprotic electrolyte. The specific capacity and energy density of metal–air electrochemical cells are higher than those of LIBs, making them a prime candidate for use in electric vehicles (EVs). During the discharging of a metal–air electrochemical cell, a reduction reaction occurs in the ambient air cathode while the metal anode is oxidized. However, complications associated with the metal anodes, catalysts, electrolytes, poor reaction kinetics, and high overpotential during the charging–discharging process have hindered the development and implementation of MABs.5–8 These issues can be alleviated by the application of an electrochemical catalyst and a porous cathode. Biomass carbon materials have emerged as an important alternative for the development of high-performance cathode and electrocatalyst materials in MABs, owing to their remarkable electrochemical characteristics, environmental friendliness, and cost-effectiveness.5,7–12 They offer a sustainable alternative to LIBs, with a specific energy density nearly 10 times greater. Mainly, zinc–air batteries (ZABs) and lithium–air batteries (LABs) are popular due to their large application potential, with ZABs being used in hearing aids due to their superior security and energy density. However, utilizing their high specific capacity remains challenging.3,13–19 They face challenges in widespread application due to insufficient mass transport of oxygen electrodes, slow reaction kinetics in oxygen evolution and oxygen reduction reactions, low electrical conductivity, and a deprived nano–micro structure.7,10,13 Their performance is primarily limited by the characteristic of the air cathode, which can lead to high overpotential and poor round-trip energy efficiency.
State-of-the-art Pt-based catalysts show superior oxygen reduction reaction (ORR) performance, while IrO2 and RuO2-based catalysts exhibit higher oxygen evolution reaction (OER) performance. The catalysts, including Pt/C, Pt-based alloy catalysts, Pt-based intermetallic compounds, and Pt-based single-atom catalysts, enhance the reaction rate of the ORR due to their high catalytic activity.20–22 RuO2 is considered a promising OER catalyst due to its high pH adaptability and activity, while IrO2 is valued for its durability.23–25 The performance of these catalysts is crucial for energy conversion devices and sustainable energy technologies. However, their high costs are major limitations for large-scale applications. Rechargeable zinc–air batteries (RZABs) also face challenges due to the alkaline nature of the electrolyte, passivation, and corrosion of the Zn anode.6,10,11,26,27 The cathode electrocatalyst and electrode, made of heteroatom (N, P, S, B)-doped carbon-based materials, are crucial for enhancing the performance of ZABs, offering low prices, stability, and high conductivity. Doping heteroatoms with nitrogen (N) is a popular method for enhancing the electrochemical properties of carbon materials, resulting in advanced metal-free carbon catalysts for RMABs. Nitrogen, a promising heteroatom, has the potential to improve the ORR and OER catalytic activity, a larger electronegativity than carbon, a comparable atomic size, and five valence electrons for bonding with carbon atoms. N-doped carbon materials possess structural defects that can enhance conductivity and improve battery performance by withdrawing electrons from carbon atoms. Other heteroatoms, such as sulfur, boron, fluorine, and phosphorus, can also cooperate with N atoms to enhance ORR performance.4,28–32 This method has the potential to improve the performance of green electrocatalysts in the fight against pollution and energy shortages.
This review explores recent advancements in synthesizing metal-free heteroatom-doped biomass-derived carbon nanostructured electrocatalysts for sustainable energy solutions. The review also discusses the need to understand the kinetics and reaction barriers at the electrolyte–electrode interface and the rational design and functionalization of biomass-derived carbons. It discusses the catalytic applications and the importance of superior biomass-derived electrocatalysts for the ORR and OER in metal–air batteries, particularly in LABs.
2. Metal-free heteroatom-doped carbon nanomaterials for lithium–air batteries
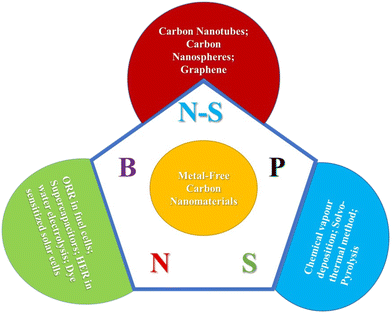
Carbon nanomaterials, with high electrical conductivities, surface areas, porosity, and stability, can improve MAB electrochemical performance by facilitating charge transfer and providing channels for electrolyte and oxygen diffusion.4,28,32–35 The introduction of dopants and co-dopants significantly alters the chemical properties of these materials, affecting their nanostructure and electrochemical performance.4,29–31,36–38 These nanomaterials facilitate charge transfer, provide channels for electrolyte and oxygen diffusion, and enable rapid electrolyte diffusion and charge transfer. The chemical properties of these nanomaterials can be varied by introducing chemical dopants, and the incorporation of heteroatoms can significantly alter their nanostructure and electrochemical performance. Biomass-derived metal-free heteroatom-doped nanostructured carbon electrocatalysts have been gaining attention in recent years due to their potential to enhance the performance of RLABs. Heteroatoms such as nitrogen, sulfur, boron, and phosphorus are incorporated into specially structured metal-free carbon nanomaterials to enhance their electrocatalytic performance.39–41 These heteroatom-doped nanomaterials have shown potential as electrocatalysts in various applications, including water splitting, supercapacitors, and dye-sensitized solar cells. This doping process can be achieved through various synthetic strategies like chemical vapor deposition, pyrolysis, and thermal processes. In summary, the use of biomass-derived carbon materials in the electrodes of metal–air batteries is a promising approach for enhancing their performance and making them more sustainable. These studies demonstrate the potential of using biomass-derived, metal-free bifunctional electrocatalysts in RLABs.Metal-free heteroatom-doped carbon materials have emerged as promising electrocatalysts for LABs.40,42–44 These materials, including heteroatom-doped carbons and carbon-encapsulated metal materials, are attractive due to their excellent electrocatalytic performance, long-term stability, and cost-effectiveness. Doping heteroatoms into carbon structures can modify the electronic and physicochemical properties of carbon materials, generating active sites for the ORR. This approach has been used to enhance the performance of the batteries. For instance, N–S- and N–P-doped nanocarbon (SCNP and PCNP) electrocatalysts have been prepared through sustainable microwave-assisted synthesis. These heteroatom-doped nanocarbon materials are active catalysts for the two-electron ORR, which is a critical process in lithium–air batteries. The development of these metal-free heteroatom-doped carbon materials could lead to significant advancements in the performance and efficiency of LABs. This represents an exciting direction for research and development in the field of energy storage technologies.
A study of a highly oriented pyrolytic graphite catalyst with distinct pentagon carbon faulty patterns (D-HOPG) demonstrates that nitrogen doping may selectively induce certain carbon defect types. D-HOPG pentagon defects provide more active sites for the acidic oxygen reduction process than nitrogen-doped graphite.45 Metal-free carbon materials are very attractive because of their excellent conductivity, better electrical and mechanical capabilities, and tolerance for corrosive liquids. Despite their low electrocatalytic activity, they can be improved by heteroatom doping strategies such as N and S doping to increase ORR and OER activities.40,46 This has resulted in the creation of improved multifunctional carbon-based electrocatalysts that have been modified or doped with heteroatoms and are seen as viable replacements for precious metal-based oxygen electrocatalysts. For example, N-doped hierarchically porous carbon skeletons were created by enzyme treatment and pyrolysis with NH4Cl, enhancing catalytically active site exposure and mass transfer throughout the ORR and OER processes. A new N-doped and edge-rich 3D graphene mesh exhibited multifunctional electrocatalytic activity for the ORR and OER, with topological defects being more beneficial for both processes. Recently, a variety of heteroatom-doped carbon compounds displayed significant ORR and OER activity. However, their low activity, instability, and durability restrict their commercial applicability.
Hu et al. (2019) presented the preparation of phosphorus-doped carbon materials with abundant active functional groups and stable structural characteristics for eco-friendly metal-free catalysis.40,47 The materials, which have a highly porous structure and high surface area (>1600 m2 g−1), were prepared using a convenient and scalable strategy with soluble starch and phosphoric acid. The catalyst prepared using this strategy demonstrated remarkable catalytic performance in aerobic oxidation of benzyl alcohol with a higher TOF value than those of other previously reported catalysts. It also showed great tolerance for various substrates, including aromatic, alicyclic, heterocyclic, and aliphatic alcohols. The incorporation of heteroatoms into carbon nanomaterials has been found to enhance their electrocatalytic performance. This enhancement is particularly beneficial for promoting the catalytic process of the oxygen reduction reaction (ORR), a key process in lithium–air batteries (LABs).39,42,47 The use of biomass-derived materials and the absence of metals in these electrocatalysts align with the push toward greener and more sustainable energy storage solutions.
Lithium-air batteries are gaining global attention due to their high theoretical energy density and potential to replace traditional LIBs in longer driving ranges. However, the irreversible decomposition of Li2O2 during charge can deteriorate cycling performance, making it challenging to maximize capacity while maintaining good cycling stability and hindering its practical applications.48 Despite recent achievements, many unsolved issues remain in developing practical LABs. Li–air batteries are promising energy storage devices due to their high energy density and large specific capacity. However, their practical applications are limited by high overpotential, slow oxygen reduction and evolution reaction kinetics, and poor cycling stability. To address these issues, various catalysts and electrolyte mediators have been studied.46 However, these catalysts still face challenges in capacity, overpotential, and cycle life. Solid-phase catalysts and liquid-phase redox mediators have their advantages and disadvantages in performance and reaction mechanisms. Findings also highlight the importance of geometrical orientations batteries, showcasing how design influences their overall performance and efficiency. Cell design parameters such as adding carbon paste to the air side of the battery can increase cell power density under forced air convection. Increasing the anode/cathode ratio and decreasing the anode-cathode distance can also improve their performance.49–52 Thus, optimizing cell design parameters and geometrical configuration and developing efficient electrocatalysts can significantly impact ZABs' performance, making them more viable for practical use. Furthermore, the rational design of carbon-based electrocatalytic materials with design principles includes morphology, crystal structure, interface strategies, atomic engineering and atomic dispersion of active sites in metal-air batteries facilitates fast oxygen electrocatalytic reactions, improving overall specific capacity and energy density of the devices.53–56
The ORR and OER are crucial in electrochemical devices like metal–air batteries, but their overpotential requires Pt-based catalysts. Low-cost and Earth-abundant materials such as biomass carbon nanomaterials are being explored for electrocatalysts due to their excellent electronic conductivity and high specific surface area. However, the low reactivity of carbon is a major limiting factor. Indeed, the ORR and OER are key processes in various energy conversion devices, including fuel cells, electrolyzers, and MABs.57,58 The ORR and OER have high activation barriers, limiting the effectiveness of energy conversion devices that use these processes. Typically, noble-metal-based catalysts are required to improve the slow kinetics of the ORR and OER, but their high cost limits their practical commercial utilization. As a result, it is critical to produce more active and reliable low-cost electrocatalysts, particularly for extreme settings. Transition metal oxides not belonging to the platinum group metal-based oxides are low-cost substances that could give a d orbital for oxygen species binding. As a result, transition metal oxides are regarded as a substitute for typical precious metal oxygen electrocatalysts.58 However, the development of oxide catalysts for oxygen reduction and oxygen evolution reactions still faces significant challenges, e.g., catalytic activity, stability, cost, and reaction mechanism.
Recent studies have shown that chemical modification of the carbon network can alter the reactivity of carbon nanomaterials. Doping strategies, including single-atom doping, co-doping, and multi-atom doping, have been proposed. Yisilamu et al. (2023) have fabricated biomass-derived carbon materials, specifically nitrogen and phosphorus co-doped porous carbon (CSAN10P-2), using a simple and green approach.59 These materials exhibit high electrocatalytic activity for the oxygen reduction reaction (ORR) in alkaline solution. These heteroatom-doped carbon materials can serve as excellent oxygen-reduction electrocatalysts for fuel cells and metal–air batteries, including lithium–air batteries.14 They offer a promising alternative to traditional precious metal-based catalysts, contributing to the cost-effectiveness and sustainability of energy storage devices.42 Kim et al. (2020)60 also suggested a redox-mediated polymer catalyst (RPC) as a cathode catalyst made of Li and poly(vinylidene fluoride-co-hexafluoropropylene) (PVDF-HFP), with multi-wall carbon nanotubes (MWCNTs) serving as the cathode material. The RPC adds iodine molecules to the PVDF-HFP chain, improving the cycling performance by 194% and lowering the overpotential by 21.1% at 0.1 mA cm−2. This shows that RPCs including a polymer chain and redox mediators might be widely applied as extremely effective LAB catalysts.60
Hong et al. (2024)61 aimed to create composites for the Li–air battery cathode using NiCo2O4 and CNTs. The composites have a 3D needle-like structure, providing extensive transport channels for electrolyte infiltration and numerous charge transfer reactions. The synergistic effect of highly electrocatalytic NiCo2O4 and highly conductive CNTs results in active catalytic performance for both the OER and ORR. The composites were successfully fabricated using a hydrothermal method and sequential annealing treatment, demonstrating their potential use as a cathode in Li–air batteries. They also performed over 120 cycles at current densities of 200 mA g−1 and 500 mA g−1 under a capacity-limiting condition of 500 mA h g−1.61
Carbon-based metal-free electrocatalysts (C-MFECs) are gaining attention due to their attractive physicochemical characteristics, cost-effectiveness, and efficient energy conversion and storage. The performance of electrocatalysis is determined by efficient intramolecular and intermolecular charge transfer. The rational design of metal-free carbon nanomaterials, coupled with appropriate intramolecular charge transfer through heteroatom doping, Stone–Wales defects, and/or intermolecular charge transfer through adsorption of molecules/moieties, can promote efficient electrocatalysis.43 Biomass-derived carbon aerogels have shown significant potential for use in rechargeable lithium–air batteries. These aerogels are prepared using biomass as precursors, making them ecologically beneficial, affordable, and abundant.57 Pham et al. (2024) have developed a carbon aerogel from rice straw cellulose for high-performance lithium-ion battery anodes, demonstrating an impressive initial specific charge capacity of 348.6 mA h g−1 and maintaining stable performance with nearly 257.5 mA h g−1 after over 100 cycles.62 Another study also revealed that carbon aerogels also demonstrated remarkable performance in oxygen reduction and oxygen evolution reactions, crucial electrochemical reactions in energy conversion, and storage technologies like fuel cells and metal–air batteries.57 These studies highlight the potential of biomass-derived carbon aerogels in improving rechargeable lithium–air batteries. However, more research is needed to further optimize these materials and assess their commercial viability.
Metal–air batteries and reversible fuel cells rely on the ORR and OER on air cathodes. However, these processes require expensive and scarce precious metal catalysts, making them difficult to use on a large scale. Platinum is the most effective catalyst for the ORR, while oxides of iridium and ruthenium are active electrocatalysts for the OER.63 Therefore, developing non-precious metal electrocatalysts as precious metal alternatives is crucial. Bifunctional oxygen catalysts can significantly reduce costs at both the catalyst and device levels. Lv et al. (2017)63 have synthesized a new non-precious-metal catalyst, N, F-co-doped porous carbon, using a composite of inexpensive polyaniline and superfine polytetrafluoroethylene powder. The catalyst exhibits similar onset (0.97 V vs. RHE) and half-wave potential (0.84 V vs. RHE), better durability, and higher crossover resistance in an alkaline medium compared to commercial 20% Pt/C. This is due to the good dispersion of fluorine and nitrogen atoms in the carbon matrix, high specific surface area, and synergistic effects of fluorine and nitrogen on carbon atom polarization. This new strategy offers a highly efficient metal-free electrocatalyst for the ORR in fuel cells.63
Orange peels were used to create activated carbon (AC-OP) using a low-temperature carbonization process with a nickel mesh.64 The electrode's electrochemical behavior was studied, revealing a honeycomb form with an energy gap of 2.06 eV. The AC-OP powder, placed on the nickel mesh, functions as a current collector. Electrochemical impedance spectroscopy and cyclic voltammetry were used to analyze the electrodes. Galvanostatic charge–discharge experiments validated the AC-OP electrode's extraordinary capacitive potential, making it suitable for supercapacitor manufacturing. These findings could be applied to power storage using low-cost carbon resources in various energy storage applications. Jiang et al. (2023)65 have developed low-cost and high-performance electrocatalysts using hetero-atom-doped carbon nanomaterials to overcome their poor hydrophilicity and few active sites. Three nitrogen-doped carbon microspheres at temperatures of 800, 900, and 1000 °C were obtained by carbonizing cuttlefish juice. The produced samples have nanoscale, high specific surface areas, and hydrophilicity, increasing the reaction area and promoting vanadium ion adsorption. Cyclic voltammetry and AC impedance test results show that the modified electrode has the highest electrocatalytic performance, with an energy efficiency of 62% higher than that of the blank battery at 150 mA cm−2.65 These high-performance electrocatalysts with low cost and simple preparation processes are worthy of further development.
J. Li et al. (2017)66 studied nitrogen and phosphorus co-doped porous carbon (NP-PC) catalysts as cost-effective oxygen reduction reaction (ORR) catalysts. The optimized precursors’ mass ratios, pyrolysis temperatures, and heating rates are investigated. NP-PC beats commercial Pt/C catalysts at 20 wt% when it comes to ORR performance, stability, and methanol crossover resistance. Its exceptional electrocatalytic performance is ascribed to its well-developed porous structure, huge Brunauer–Emmett–Teller surface area, and strong nitrogen–phosphorus synergy.66 This work provides fresh information for investigating cost-effective non-precious metal electrocatalysts for sustainable energy conversion systems.
Recent advances in aqueous LABs, notably in the solid electrolyte separator, have resulted in a potential energy density of more than 1700 W h kg−1 while avoiding the essential pure oxygen atmosphere concerns seen in non-aqueous systems. However, issues persist with the solid electrolyte separator, lithium stripping/plating at the lithium anode, and catholyte design.67 Recently, single-atom catalysts (SACs) with high atom usage and cost-effectiveness have demonstrated good ORR/OER kinetics in various catalytic systems.68 Biomass-derived porous carbon has also been applied in lithium–sulfur batteries.69 These biomass carbons have their characteristic structure, composition, and design. These advancements are promising for the future of lithium–air batteries, but further research and development is needed to overcome the remaining challenges and bring these technologies to market.
Cotton, silk, wood, and fibers are being explored as biomass carbon precursors for the synthesis of carbon fibers (CFs). This is due to their various advantages such as availability, sustainability, and biocompatibility. These natural lignocellulosic materials are a more sustainable and cost-effective alternative to typical petroleum-based precursors. These biomass sources are converted into CFs by procedures including spinning, stabilization, and carbonization.70,71 Their strong chemical activity allows for the production of flexible carbon membranes and monolithic free-standing structures. These materials have a large specific surface area, many functional groups, and a three-dimensional mesh structure. Their exceptional folding and bending properties, combined with excellent electrical conductivity, make them promising candidates for use as flexible electrode materials in energy storage devices such as metal-ion batteries, supercapacitors, and metal–air batteries, which will meet the needs of next-generation flexible electronics (Fig. 1).
 | ||
| Fig. 1 Heteroatoms dopants.39 | ||
Room-temperature lithium–sulfur (Li/S8) and lithium–oxygen (Li/O2) batteries have received a lot of attention over the last decade due to their high theoretical energy density and availability of sulfur and oxygen.72 However, cell chemistry is difficult, and progress toward practical device development is slowed by basic critical challenges (Fig. 2).
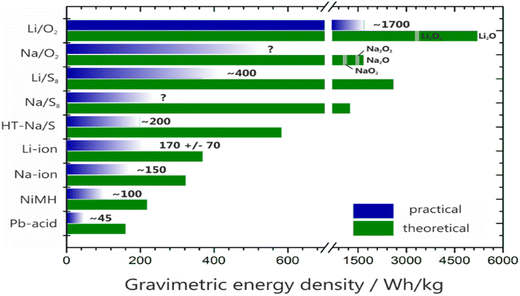 | ||
| Fig. 2 The performance examination of theoretical and practical energy densities of several rechargeable batteries.72 | ||
3. Methods for preparing biomass-based carbon for lithium–air batteries
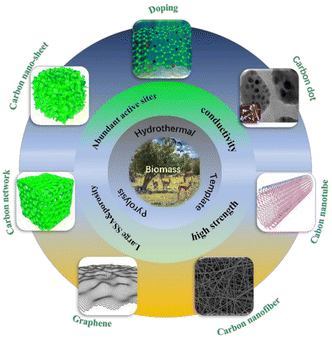
Carbon materials are crucial for human civilization's progress, with classic materials like coal, graphite, and activated carbon driving social and technological advancements. However, newer materials like fullerene, carbon nanotube, graphene, and graphdiyne have been explored for their potential in energy, sensing, catalysis, and environmental applications.73–75 These materials typically perform better than traditional ones due to their unique structures. However, most of these materials are synthesized using non-renewable petroleum and coal products, requiring harsh and energy-intensive processes. This can exacerbate social issues like energy shortages, climate warming, and environmental pollution. Biomass-based natural resources have been used as renewable precursors for the controllable preparation of carbon materials (BCMs), which could reduce fossil reserve consumption and accelerate human society's sustainable development.73,76–79 BCMs can be categorized into four classes based on their structures and morphologies: 0D carbon spheres and dots,80–85 1D carbon fibers and tubes,86–88,89 2D carbon sheets and nanosheets,90–94 3D carbon aerogels, and hierarchical carbon materials.95–99 These structures enable applications in fields like fluorescence bio-imaging, energy storage, and pollutant removal. Therefore, developing environmentally friendly, low-cost, scalable, and renewable carbon sources and processes is highly desired.Carbonization is a crucial step in the preparation of biomass-carbon materials for superior energy storage devices. Biomass-derived materials are used to create porous carbons with high specific surface areas and unique structures.107,108 Through carbonization processes, biomass materials are converted into porous carbon with high surface area and tailored porosity, which are essential for efficient electrocatalysis.114,115 Carbonization also introduces heteroatoms like nitrogen, improving electrocatalytic performance by increasing defect degree and creating active sites for reactions116 and can lead to the formation of carbon supports that enhance the activity of catalysts for the ORR, showcasing improved stability and performance.117 These carbons have excellent electrochemical performance, delivering high reversible capacities and maintaining capacity retention even after hundreds of cycles in batteries.106,108 The utilization of biomass-derived carbon in the production of energy storage materials, particularly for LABs, has garnered significant attention due to its renewable nature and potential for high performance.4,102 Biomass-based carbon materials, such as carbon nanofibers (CNFs) and hierarchical N-rich 3D-carbon frameworks (NCFs), have demonstrated excellent electrochemical properties for energy storage applications.79 Additionally, the conversion of bio-char into highly pure and crystalline flake graphite agglomerates has shown promise for tailored lithium battery energy storage materials, offering customizable shapes, sizes, and porosities for enhanced performance.79 Furthermore, the synthesis of tubular carbon from cattail fiber and its composite with T–WS2 have exhibited improved physicochemical adsorption capacities and electrochemical performance, showcasing the great potential for lithium–sulfur (Li–S) batteries.118 These findings mutually highlight the versatility and efficacy of biomass carbon in advancing energy storage technologies, particularly in the realm of lithium-based batteries.
Researchers have turned to biomass-derived carbon materials as electrocatalysts and porous cathodes.100,101 These materials offer abundant renewable resources, high specific capacity, low cost, durability, environmental friendliness, hierarchical porous structures, and heteroatom-doping sites, enhancing battery performance.4,102–105 The use of biomass to produce highly porous carbon (HPC) electrocatalysts, further doped with nitrogen and sulfur, has shown excellent bifunctional catalytic activity for the ORR and OER.104,105 They exhibit a unique porous architecture with meso/micropores, allowing effective anchoring, leading to high reversible capacity and excellent cycling stability.106 In electrocatalysis, these materials can create porous cathodes with high specific surface area, improving battery performance by improving oxygen reduction and evolution reactions.107,108 The utilization of biomass-derived carbon materials in both electrode preparation and electrocatalysis showcases their versatility and potential for advancing energy storage technologies. However, the composition, morphology, and structure–activity relationship of cathodes are influenced by biomass sources.4,107 Techniques like thermal decomposition (pyrolysis), hydrothermal carbonization (HTC), molten salt carbonization (MSC), and templates are customarily deployed for biomass carbon material production for higher battery performance.107,108 However, these methods affect the microstructure of carbon materials.109 Optimizing the ratio and mixing method of the precursor material and the activation agent, final activation temperature, heating rate, and activation atmosphere can regulate the microstructure and surface chemical properties of the obtained material.109,110–112 This can increase specific surface area, improve surface chemistry, and build different nanostructures, resulting in better electrochemical performance. The nanostructure and electrochemical performance of biomass carbon materials also depend on the physical/chemical properties and microstructure of the biomass precursor (Fig. 3).
 | ||
| Fig. 3 Biomass precursors for carbon materials for lithium–air batteries.4,107 | ||
Biomass-derived carbon electrocatalysts, electrodes, and electrolytes have been developed to improve the performance of LABs.107,108 One method involves using waste biomass nitrogen-doped carbon catalysts through simple pyrolysis, acid dissolution, and high-temperature heteroatom doping.104,105,107 This results in a catalyst with high electrocatalytic activity and faster kinetics (Fig. 4).
Hydrothermal carbonization (HTC) is a promising method for producing biomass-based carbon materials for LABs, offering large surface area, functionalization, cost-effectiveness, and their structural and morphological properties improve electrochemical behavior in various batteries.102,119 Carbon spheres can be produced using a template-free hydrothermal technique, resulting in oxygen-doped graphitic carbon spheres with increased surface area.120 These spheres are suitable for electrode applications in lithium batteries due to their fibrous morphology, high specific capacity, and environmentally friendly properties. Biomass carbon fibers can also be used as negative electrode materials for various batteries, including lithium-, sodium–, and zinc–air batteries.102,121 The use of biomass carbon electrocatalysis enhances battery performance by increasing structural and morphological properties, contributing to the advancement of energy storage technologies.4
Molten salt carbonization is a viable thermo-chemical process for converting biomass into functional carbon materials in a molten salt environment with applications in energy storage and catalysis, addressing both energy and environmental problems.122 This process involves a single thermal treatment in a molten salt reaction medium, leading to high-efficiency conversion, rapid production, and low costs. Additionally, the use of molten salts creates an ionic environment at high temperatures, enabling in situ activation of the biocarbon and the introduction of desired ions, leading to the creation of unique functionalities and a well-balanced pore structure, specific surface area, and oxygen-containing groups, enhancing its versatility for different uses.122 The resulting biocarbon offers several advantages, including a large specific surface area, environmental friendliness, balanced pore structure, specific surface oxygen-containing groups, lower energy consumption, high thermal stability, improved heat transfer properties, and good dissolution ability, making it versatile for various applications.123–125 Furthermore, the use of molten salts in carbonization processes, such as in the synthesis of graphite materials, allows for operations at moderate temperatures, reducing energy consumption and costs while maintaining high-quality product outcomes suitable for energy storage applications like batteries124,126 but some disadvantages limit the widespread application, including high operating temperatures required for the process (850 °C), although lower than traditional methods124 (Table 1). However, further study and optimization may lead to industrial-scale deployment of a circular economy.122
| Methods | Advantages | Disadvantages |
|---|---|---|
| Thermal decomposition | ○ Environmentally friendly production89,137 | ○ Requires efficient carbonization methods due to the variability in biomass composition and properties62 |
| ○ Biomass carbon is cost-effective compared to synthetic carbon materials137 | ○ May require additional purification steps137 | |
| ○ Biomass-derived carbon can be tailored for specific applications | ○ Structural heterogeneity affects electrochemical performance | |
| ○ High specific surface area and porosity enhance lithium-ion diffusion | ○ Limited stability during cycling | |
| ○ Good electrical conductivity | ○ Thermal decomposition can lead to complex reactions, affecting the overall process | |
| ○ Gasification processes may have a long start-up time | ||
| Hydrothermal carbonization | ○ High-value utilization that allows efficient use of biomass, lowering costs, forming porous structures, and augmenting electrocatalysis via heteroatom doping | ○ The generated surface may have unsatisfactory surface properties for pollutant removal |
| ○ Allowing a wet feedstock process, eliminating the need for prior dewatering | Limited Functionality that might not be ideal for certain applications without further improvement | |
| ○ Integration Challenges as HTC uses only solid products; integrating other processes is essential | ||
| Molten salt carbonization | ○ Single Thermal Treatment, simplifying production | ○ Intolerance to sulfur, which limits its applicability |
| ○ High Efficiency | ○ Have slow initiation/startup times | |
| ○ Rapid Production, which is faster than some other methods | ○ Lack of integration with other components of the batteries | |
| ○ Low Resource and Processing Costs, which is Economical and sustainable | ○ Limited Industrial Implementation as it needs further research and optimization work | |
| ○ Environmental Impact that helps reduce or neutralize carbon impact on the environment | ||
| ○ Properties of functional biocarbon that can produce fairly large specific surface area, well-developed and balanced pore structures for various applications, and surface oxygen-containing groups that enable diverse functionalities | ||
| ○ Effective for removing pollutants from the environment | ||
| Template | ○ Controlled process that allows precise control over carbonization conditions, such as temperature, time, and atmosphere138 | ○ Complexity requires careful design and setup of templates, which can be intricate and time-consuming138 |
| ○ Consistent Product that produces uniform carbon materials with predictable properties | ○ The availability of suitable templates may be restricted | |
| ○ Versatility that can be applied to various precursor materials, including biomass, polymers, and other organic compounds | ○ Energy-intensive as it requires a high-temperature treatment process | |
| ○ Tailored pore structures as templates allow control over pore size and distribution in the resulting carbon material | ○ Efficient removal of templates without damaging the carbon structure can be challenging | |
| Physically activated | ○ Relatively it is cost-effective121,139–141 | ○ Lower yields compared to other methods121,139 |
| ○ Ecologically Friendly139–141 | ○ Limited Porosity than chemically activated carbon139 | |
| ○ It is simple and straightforward to implement142 | ○ Less control over the resulting porous structures139 |
Template carbonization technology has been extensively explored in various contexts for enhancing the performance of different types of batteries. It offers a balance between precision and versatility, but it requires careful planning and consideration of template-related factors. For instance, research has focused on utilizing template carbonization to prepare hollow carbon nanoballs (HCNBs) as buffering supports for tin-based anodes in LIBs.127 Additionally, the synthesis of carbon hollow spheres (CHS) from separators and reduced graphene oxide (rGO) from spent LIB components showcases a “Waste-to-Wealth” approach, demonstrating the versatility of template carbonization in battery applications.128 Furthermore, the use of sacrificial templates has been highlighted in the synthesis of sulfur/nitrogen-codoped carbon-supported manganese single-atom catalysts for high-performance Li–S batteries, emphasizing the prominence of efficient electrocatalysts in battery technology.129 These studies collectively underscore the significance of template carbonization in advancing the development of next-generation battery systems, including lithium–air batteries.
Furthermore, biomass-derived carbon can be prepared using physical and chemical activation processes.114,130 Physical activation involves the use of gases like CO2 or steam to create porosity, while chemical activation uses chemicals like KOH or ZnCl2 to enhance the pore structure.131 Physical activation involves carbonization and activation under high temperatures and oxidizing atmospheres, resulting in activated carbon with a porous structure. Physically activated biomass carbon offers several advantages, including renewability, low cost, and high availability, making it an environmentally friendly and sustainable option for energy storage devices.131–134 These materials exhibit excellent electrochemical performance as physically activated biomass carbon can possess high surface areas, hierarchical pore structures, and good stability, enhancing their efficiency as electrode materials for energy storage and conversion systems.135,136 However, challenges such as the potential for cracking in electrodes with higher mass loadings, which can lead to reduced flexibility and device stability,136 optimizing activation methods, controlling pore size distribution, and improving mass activity effects need to be addressed to further enhance the performance of these materials in practical applications.94–99,109–112 Chemical activation, divided into alkaline and salt activation, is commonly used in biochar synthesis due to its lower activation temperature and higher pore generation rate.113 The activation mechanism involves reduction reactions with carbon atoms in a carbon framework, resulting in a nanoporous structure. The activator is then decomposed to generate steam under high temperatures. Despite these drawbacks, activated biomass carbon is a promising material for applications in energy storage devices (Fig. 5).
To summarize, the advantages of biomass carbon preparation methods include cost-effectiveness, environmentally friendly processes, and the ability to tailor the material's properties for specific applications.117,131–133 However, these methods may have disadvantages such as longer processing times, the need for specific activation agents, and potential challenges in scaling up production (Table 1). Understanding these methods and their pros and cons is crucial for optimizing biomass carbon preparation for various uses.132,133 The kind of biomass used and the carbonization procedure can have a considerable influence on the performance of biomass-derived carbon materials in lithium–air batteries as presented in Table 2.
| Advantages | Disadvantages |
|---|---|
| Renewable resource: biomass is abundant and renewable, making it an eco-friendly choice | Heterogeneity: biomass carbon can have varying compositions and structures, affecting performance |
| Rich heteroatom content: biomass-derived carbon contains heteroatoms (such as nitrogen, sulfur, and oxygen), enhancing catalytic activity | Pore structure variability: pore size distribution in biomass carbon can be inconsistent, impacting electrode performance |
| Porous structure: biomass-based carbon materials often have a porous structure, providing a high surface area for catalysis | Cost and processing: harvesting, transporting, and processing biomass can be expensive |
| Synergistic effects: biomass carbon can synergize with other components, improve overall electrocatalytic performance | Limited energy density: lithium–air batteries face challenges in achieving high energy density |
| Environmental friendliness: biomass utilization aligns with sustainability goals | Stability and durability: biomass-based materials may degrade over extended cycling |
4. Principles of oxygen reduction and evolution reactions in lithium–air batteries
Lithium–air batteries are metal–air electrochemical cells that use the oxidation of lithium at the anode and the reduction of oxygen at the cathode to induce a current flow. The theoretical specific energy of a non-aqueous Li–air battery is comparable to gasoline. However, practical power and cycle life need significant improvements before they can find a market niche. Aligned with this, new LAB designs are being developed, offering a longer driving range compared to LIBs. These designs use a solid electrolyte instead of a liquid one, potentially increasing energy density up to four times.1Lithium–air batteries undergo two processes:8,57 the Oxygen Reduction Reaction (ORR) and the Oxygen Evolution Reaction (OER) as shown in Fig. 6. During discharging (ORR process), molecular oxygen is bound to the catalyst surface through oxygen vacancies, reduced and results in a superoxide species. This superoxide combines with solvated lithium ions to regenerate active sites. The intermediate superoxide is converted to lithium peroxide (Li2O2) through a dismutase reaction or direct reduction. During charging (the OER process), lithium peroxide is bound to vacant sites with lithium-ion cleavage, forming peroxide ions (O22−). These peroxide ions are oxidized stepwise to superoxide ions (O2−) and then to molecular oxygen (O2). The ORR and OER processes are crucial for the energy efficiency and reversibility of LABs due to their large polarization and high overpotential. Efficient catalysts are essential to promote reversible ORR/OER and overcome the sluggish kinetics associated with these reactions.
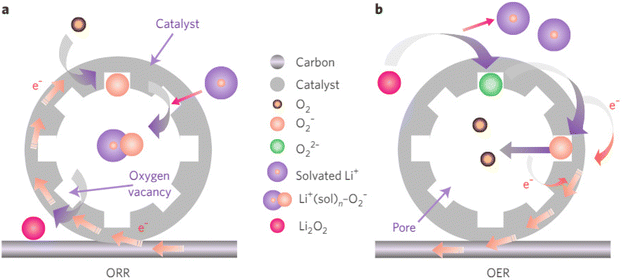 | ||
| Fig. 6 The mechanism of a porous oxygen-deficient oxide catalyst for Li–air batteries8 (a) discharging (ORR) and (b) charging (OER). | ||
Lithium–air batteries, which exchange oxygen with ambient air, face challenges from oxidative agents like moisture and carbon dioxide, which can degrade the metal lithium anode, and affect their performance.143 Research has been conducted on rechargeable lithium–air batteries using biomass-derived, metal-free bifunctional electrocatalysts. L. Wang et al. (2020) have developed a biological enzyme, Laccase from Trametes versicolor (LacTv), as a highly efficient bifunctional catalyst in a rechargeable lithium–air battery system.144 The intrinsic pH change during discharge/charge regulates enzyme recovery, allowing the battery to provide a flat discharge voltage of ∼3.75 V for 120 hours and exceptional longevity (cycling consistently for 1100 hours) with a tiny voltage gap of ∼0.24 V. These studies demonstrate the potential of using biomass-derived, metal-free bifunctional electrocatalysts in RLABs. However, more research is needed to further improve the performance and commercial viability of these batteries.
5. Electrochemical reactions in lithium–air batteries
Biomass-derived carbon materials are being used in lithium–air batteries for their high specific surface area and hierarchical pore structures.4,29 These materials enhance the ORR and OER kinetics, improving battery performance. To enhance their electrochemical properties, these materials can be tailored by increasing the number of functional groups on their surfaces and introducing heteroatoms like nitrogen, oxygen, fluorine, hydrogen, sulfur, and boron into the carbon structure.7 Overall, biomass-derived carbon materials offer promising prospects for improving the electrochemical reactions and performance of lithium–air batteries.MABs typically involve two fundamental electrochemical reactions: oxidation at the anode and reduction at the cathode.72 The metal (M) at the anode gets oxidized to produce metal ions (M+), which move through the electrolyte. The general anode reaction can be represented as:
| M(s) ⇔ M+ + e− | (1) |
At the cathode, oxygen from the air is reduced, and the oxygen molecules react with the metal ions to form metal oxides (MO2x). The general cathode reaction is:
| M+ + xO2 + e− ⇔ MO2x | (2) |
| Net reaction: M + xO2 ⇔ MO2x | (3) |
For instance, in an LAB, Li metal at the anode is oxidized to Li+, which migrates from the anode to the cathode to participate in the electrochemical reaction of the battery process. At the cathode, O2 is reduced to O2−, which combines with Li+ to make LiO2. Li2O2 is subsequently stored in the cathode by a succession of disproportionation and solvation growth processes. The efficiency and reversibility of these reactions are crucial for the performance and cycle life of lithium–air batteries. The electrochemical reaction process is expressed as follows:
During discharging:
• Lithium at the anode is oxidized, releasing electrons into the external circuit and lithium ions into the electrolyte:
| 4Li → 4Li+ + 4e− | (4) |
• At the cathode, oxygen from the air is reduced by the electrons from the external circuit to form oxide ions:
| O2 + 4e− → 2O2− | (5) |
The lithium ions then react with the oxide ions to form lithium peroxide:
| 2Li+ + 2O2− → Li2O2 | (6) |
The overall reaction for the discharging process is as follows:
| 4Li + O2 → 2Li2O2 | (7) |
During charging:
• The process is reversed; lithium peroxide decomposes at the cathode, releasing oxygen and forming lithium ions:
| Li2O2 → 2Li+ + 2O2− + 4e− | (8) |
• Lithium ions move back to the anode, where they receive electrons and are deposited as lithium metal:
| 4Li+ + 4e− → 4Li | (9) |
The overall reaction for the charging process is as follows:
| 2Li2O2 → 4Li + O2 | (10) |
6. Challenges and future directions of lithium-battery development
Lithium–air batteries have garnered significant attention due to their extremely high theoretical energy density, making them strong contenders to replace traditional lithium-ion batteries. However, maximizing capacity while maintaining good cycling stability has hindered their practical applications. Despite recent achievements, several challenges impede their practical applications including high overpotential, slow kinetics, poor cycling stability, environmental sensitivity, and material challenges. High overpotential refers to the extra energy needed to drive the electrochemical reaction, which can affect battery efficiency. Slow kinetics in the oxygen reduction and oxygen evolution reactions limit performance. Poor cycling stability to maintain its capacity over multiple charge–discharge cycles can lead to a rapid decrease in battery capacity over time. Environmental sensitivity is another issue, as additional oxidative agents can degrade the metal lithium anode. There are still many unsolved issues for developing practical Li–air batteries, including the design of cathodes, anodes, and electrolytes. Despite these challenges, lithium–air batteries remain an exciting area of research due to their high energy density. Hence, future studies aim to address these issues including exploring the use of solid electrolytes, durable non-noble metal bifunctional electrocatalysts, which are safer and can boost energy density compared to liquid batteries, and the development of catalysts and redox mediators from eco- and cos-friendly biomass carbon to improve battery electrochemical performance. The development of lithium–air batteries is a dynamic field, and these future directions could overcome current challenges and make them a viable option for various applications. Thus, anode protection and air purification systems are being combined to achieve large-scale, long-cycle applications of LABs. The development of lithium–air batteries is a dynamic field. The future directions include further exploration of solid electrolytes, catalysts, redox mediators, and anode protection and air purification systems. These could potentially overcome the current challenges and make lithium–air batteries a viable option for various applications. Improving Electrochemical Performance: future research should focus on exploiting the advantages of catalysts and redox mediators in terms of stability and overpotential to improve the electrochemical performance of the battery.7. Conclusion
High energy density batteries are crucial for vehicle electrification and storing energy from renewable sources to meet peak demands. MABs, including LABs, have the highest energy density (about 3458 W h kg−1) due to their high charge-to-mass ratio. However, they face several challenges, including the formation of a passivation layer called the solid electrolyte interphase film by metal anodes, internal short circuits leading to explosions due to dendrite growth, and finding an electrolyte with desired properties such as high stability, low volatility, non-toxicity, high oxygen solubility, and a wide electrochemical window. The stability of materials where cathodic reactions occur is another main challenge, leading to side reactions that cause performance loss and reduced cyclability of the batteries. These limitations must be resolved before replacing Li-ion batteries, which have about ten times less theoretical energy density than LABs.Biomass-derived metal-free heteroatom-doped nanostructured carbon electrocatalysts are a promising alternative to traditional metal-based catalysts in the development of high-performance RLABs. These electrocatalysts, often derived from sustainable sources, offer environmental compatibility and high catalytic efficiency. The incorporation of heteroatoms like nitrogen, sulfur, or phosphorus enhances electrocatalytic activity for the OER and ORR, a critical process of battery operation. The high surface area and porosity of these materials facilitate better electron transfer, improving ORR kinetics and overall battery performance. Biomass-derived electrocatalysts align with green chemistry principles, offering a low-cost, abundant, and renewable source of materials.
In summary, biomass-derived metal-free heteroatom-doped nanostructured carbon electrocatalysts hold great promise for enhancing the performance of lithium–air batteries. Their development is a step towards more sustainable and efficient energy storage solutions, which is crucial for meeting the growing demand for renewable energy technologies. Thus, the use of biomass-derived metal-free heteroatom-doped carbon electrocatalysts presents a promising pathway toward the development of high-performance, sustainable, and cost-effective lithium–air batteries. However, further research is needed to optimize these materials for commercial application, focusing on long-term stability, scalability, and integration into existing battery systems.
Author contributions
1. Dr. Molla Asmare Alemu: Original drafting of the whole document.2. Dr. Muluken Zegeye Getie: conceptualization and drafting mainly Introduction and Challenges and future directions of lithium-battery development.
3. Mr. Hailemariam Mulugeta Wassie and Mulat Shitye Alem: Developing figures and Tables, collecting references, and correcting the manuscript as per the guidelines of the journal in guiding Molla Asmare Alemu.
4. Dr. Addisu Alemayehu Assegie: Editing and proofreading of the whole document.
5. Profesor Dr. Mustafa Ilbaş and Rafat Al Afif: Supervision, Reviewing and updating the whole document.
Consent for publication
I, the undersigned, give my approval on behalf of all contributors to the publishing of identifying details, such as figures and review submissions, in green chemistry.Data availability
No primary data were used for the research described in this article review work. This means no primary research results, software or codes have been included and no new data were generated or analysed as part of this review.Conflicts of interest
The authors state that they have no known conflicting financial interests or personal ties that might have influenced the work presented in this study.Acknowledgements
The authors would like to thank the Bahir Dar Energy Center, Bahir Dar Institute of Technology of Bahir Dar University for their assistance in completing the endeavour effectively. The authors credit QuillBot, which was utilized to edit the language used in the text.References
- J. E. Harmon, New design for lithium-air battery could offer much longer driving range compared with the lithium-ion battery, 2023. https://www.anl.gov/article/new-design-for-lithiumair-battery-could-offer-much-longer-driving-range-compared-with-the-lithiumion Search PubMed.
- Y. Chen, Y. Kang, Y. Zhao, L. Wang, J. Liu and Y. Li, et al., A review of lithium-ion battery safety concerns: The issues, strategies, and testing standards, J. Energy Chem., 2021, 59, 83–99, DOI:10.1016/j.jechem.2020.10.017.
- M. Asmare Alemu, A. Ketema Worku and M. Zegeye Getie, Recent advancement of electrically rechargeable alkaline Metal-Air batteries for future mobility, Results Chem., 2023, 6, 101048 CrossRef CAS.
- X. Lv, M. Chen, H. Kimura, W. Du and X. Yang, Biomass-Derived Carbon Materials for the Electrode of Metal–Air Batteries, Int. J. Mol. Sci., 2023, 24(4), 1–20, DOI:10.3390/ijms24043713.
- M. Wang, S. Wang, H. Yang, W. Ku, S. Yang and Z. Liu, et al., Carbon-Based Electrocatalysts Derived From Biomass for Oxygen Reduction Reaction: A Minireview, Front. Chem., 2020, 8, 1–5 CrossRef PubMed.
- D. Zhang, H. Zhao, F. Liang, W. Ma and Y. Lei, Nanostructured arrays for metal–ion battery and metal–air battery applications, J. Power Sources, 2021, 493, 229722 CrossRef CAS.
- G. S. D. Reis, S. Petnikota, C. M. Subramaniyam, H. P. de Oliveira, S. Larsson and M. Thyrel, et al., Sustainable Biomass-Derived Carbon Electrodes for Potassium and Aluminum Batteries: Conceptualizing the Key Parameters for Improved Performance, Nanomaterials, 2023, 13(4), 2–19, DOI:10.3390/nano13040765.
- F. Cheng and J. Chen, Something from nothing, Nat. Chem., 2012, 4(12), 962–963 CrossRef CAS PubMed.
- L. L. Lu, Q. S. Li, Y. N. Sun, K. B. Kuang, Z. Li and T. Wang, et al., Research progress on biomass carbon as the cathode of a metal-air battery, New Carbon Mater., 2023, 38(6), 1018–1034 CrossRef CAS.
- M. Asadi, B. Sayahpour, P. Abbasi, A. T. Ngo, K. Karis and J. R. Jokisaari, et al., Lithium-Oxygen Batteries with Long Cycle Life in a Realistic Air Atmosphere---Supplemental, Nature, 2018, 555, 502–506 CrossRef CAS PubMed.
- R. Mori, All solid state rechargeable aluminum-air battery with deep eutectic solvent based electrolyte and suppression of byproducts formation, RSC Adv., 2019, 9(39), 22220–22226 RSC.
- S. Boyd , Batteries and Electrification R&D Overview. Batter Electrif R&D Overview, in US Dep Energy Veh Technol Off Annu Merit Rev Meet, 2018, vol. 20.
- C. Pei, D. Zhang, J. Kim, X. Yu, U. Sim and H. S. Park, et al., Bifunctional 2D structured catalysts for air electrodes in rechargeable metal-air batteries, Energy Mater., 2024, 4(1), 1–53, DOI:10.20517/energymater.2023.66.
- M. Hao, R. Dun, Y. Su, L. He, F. Ning and X. Zhou, et al., In situ self-doped biomass-derived porous carbon as an excellent oxygen reduction electrocatalyst for fuel cells and metal–air batteries, J. Mater. Chem. A, 2021, 9(25), 14331–14343 RSC.
- M. A. Alemu, A. K. Worku and M. Z. Getie, Recent advances in electrically rechargeable transition metal-based-air batteries for electric mobility, Inorg. Chem. Commun., 2023, 159, 111742 CrossRef.
- M. Asmare Alemu, A. Ketema Worku and M. Zegeye Getie, Recent Advancement of Electrically Rechargeable Di-Trivalent Metal-Air Batteries for Future Mobility, Results Chem., 2023, 6, 101048 CrossRef CAS.
- M. A. Alemu, M. Z. Getie and A. K. Worku, Advancement of electrically rechargeable multivalent metal-air batteries for future mobility, Ionics, 2023, 29(9), 3421–3435 CrossRef CAS.
- M. Asmare, M. Zegeye and A. Ketema, Advancement of electrically rechargeable metal-air batteries for future mobility, Energy Rep., 2024, 11, 1199–1211 CrossRef.
- Y. Li and J. Lu, Metal-Air Batteries: Future Electrochemical Energy Storage of Choice?, ACS Energy Lett., 2017, 2(6), 1370–1377 CrossRef CAS.
- Z. Li, P. Zhou, Y. Zhao, W. Jiang, B. Zhao and X. Chen, et al., Ultradurable Pt-Based Catalysts for Oxygen Reduction Electrocatalysis, Catalysts, 2024, 14(57), 1–34, DOI:10.3390/catal14010057.
- F. Zhan, K.-S. Hu, J.-H. Mai, L.-S. Zhang, Z.-G. Zhang and H. He, et al., Recent progress of Pt-based oxygen reduction reaction catalysts for proton exchange membrane fuel cells, Rare Met., 2024, 43(6), 2444–2468 CrossRef CAS.
- H. Li, H. Zhao, B. Tao, G. Xu, S. Gu and G. Wang, et al., Pt-Based Oxygen Reduction Reaction Catalysts in Proton Exchange Membrane Fuel Cells: Controllable Preparation and Structural Design of Catalytic Layer, Nanomaterials, 2022, 12(23), 2079–4991, DOI:10.3390/nano12234173.
- Z. Ma, Y. Zhang, S. Liu, W. Xu, L. Wu and Y. C. Hsieh, et al., Reaction mechanism for oxygen evolution on RuO2, IrO2, and RuO2@IrO2 core-shell nanocatalysts, J. Electroanal. Chem., 2018, 819, 296–305 CrossRef CAS.
- Y. Qin, T. Yu, S. Deng, X. Y. Zhou, D. Lin and Q. Zhang, et al., RuO2 electronic structure and lattice strain dual engineering for enhanced acidic oxygen evolution reaction performance, Nat. Commun., 2022, 13(1), 1–8, DOI:10.1038/s41467-022-31468-0.
- J. Bai, W. Zhou, J. Xu, P. Zhou, Y. Deng and M. Xiang, et al., RuO2 Catalysts for Electrocatalytic Oxygen Evolution in Acidic Media: Mechanism, Activity Promotion Strategy and Research Progress, Molecules, 2024, 29, 1420–3049, DOI:10.3390/molecules29020537.
- M. Cheiky, L. Danczyk and M. Wehrey, Rechargeable Zinc-Air Batteries in Electric Vehicle Applications.
- J. Huang, Z. Guo, Y. Ma, D. Bin, Y. Wang and Y. Xia, Recent Progress of Rechargeable Batteries Using Mild Aqueous Electrolytes, Small Methods, 2019, 3(1), 1–20 CrossRef.
- O. Nurhilal, S. Hidayat, D. Sumiarsa and R. Risdiana, Natural Biomass-Derived Porous Carbon from Water Hyacinth Used as Composite Cathode for Lithium Sulfur Batteries, Sustainability, 2023, 15(2), 1–9, DOI:10.3390/su15021039.
- Y. J. Wang, B. Fang, D. Zhang, A. Li, D. P. Wilkinson and A. Ignaszak, et al., A Review of Carbon-Composited Materials as Air-Electrode Bifunctional Electrocatalysts for Metal–Air Batteries, Electrochem. Energy Rev., 2018, 1, 1–34 CrossRef CAS.
- C. Wang, S. Ran, W. Sun and Z. Zhu, Biomass-derived carbon materials with controllable preparation and their applications in zinc-air batteries: A mini review, Electrochem. Commun., 2023, 154, 107557 CrossRef CAS.
- Q. Li, T. He, Y. Q. Zhang, H. Wu, J. Liu and Y. Qi, et al., Biomass Waste-Derived 3D Metal-Free Porous Carbon as a Bifunctional Electrocatalyst for Rechargeable Zinc-Air Batteries, ACS Sustainable Chem. Eng., 2019, 7(20), 17039–17046 CrossRef CAS.
- G. Hristea, M. Iordoc, E. M. Lungulescu, I. Bejenari and I. Volf, A sustainable bio-based char as emerging electrode material for energy storage applications, Sci. Rep., 2024, 14(1), 1–19 CrossRef PubMed.
- W. Wan, Q. Wang, L. Zhang, H. W. Liang, P. Chen and S. H. Yu, N-, P- and Fe-tridoped nanoporous carbon derived from plant biomass: An excellent oxygen reduction electrocatalyst for zinc-air batteries, J. Mater. Chem. A, 2016, 4(22), 8602–8609 RSC.
- S. Sun, Q. Yan, M. Wu and X. Zhao, Carbon aerogel based materials for secondary batteries, Sustainable Mater. Technol., 2021, 30, e00342 CrossRef CAS.
- T. He, Y. Zhang, Y. Chen, Z. Zhang, H. Wang and Y. Hu, et al., Single iron atoms stabilized by microporous defects of biomass-derived carbon aerogels as high-performance cathode electrocatalysts for aluminum–air batteries, J. Mater. Chem. A, 2019, 7(36), 20840–20846 RSC.
- M. Zhang and L. Peng, Research progress of biomass-derived carbon for the supercapacitors, Mater. Res. Express, 2024, 11(1), 1328–1339, DOI:10.1088/2053-1591/ad1013.
- M. Gong, Electrodes Investigation on Zinc-Air Battery, 2021.
- G. Fu, Y. Liu, Y. Chen, Y. Tang, J. B. Goodenough and J. M. Lee, Robust N-doped carbon aerogels strongly coupled with iron-cobalt particles as efficient bifunctional catalysts for rechargeable Zn-air batteries, Nanoscale, 2018, 10(42), 19937–19944 RSC.
- J. Chattopadhyay, T. S. Pathak and D. Pak, Heteroatom-Doped Metal-Free Carbon Nanomaterials as Potential Electrocatalysts, Molecules, 2022, 27(3), 1–26, DOI:10.3390/molecules27030670.
- Y. Rangraz and M. M. Heravi, Recent advances in metal-free heteroatom-doped carbon heterogonous catalysts, RSC Adv., 2021, 11(38), 23725–23778 RSC.
- C. Hu and L. Dai, Multifunctional Carbon-Based Metal-Free Electrocatalysts for Simultaneous Oxygen Reduction, Oxygen Evolution, and Hydrogen Evolution, Adv. Mater., 2017, 29(9), 1–9, DOI:10.1002/adma.201604942.
- Y. Deng, H. Zhang, Y. Lin, Q. Ying, F. Yu and Y. Yang, Heteroatom-doped carbon sheets as metal-free electrocatalysts for promoting the oxygen reduction reaction in Zn–air batteries, Dalton Trans., 2022, 51(47), 18152–18158 RSC.
- R. Paul, Q. Zhai, A. K. Roy and L. Dai, Charge transfer of carbon nanomaterials for efficient metal–free electrocatalysis, Interdiscip. Mater., 2022, 1(1), 28–50 CAS.
- Y. Meng, X. Huang, H. Lin, P. Zhang, Q. Gao and W. Li, Carbon-Based Nanomaterials as Sustainable Noble-Metal-Free Electrocatalysts, Front. Chem., 2019, 7, 1–9 CrossRef PubMed.
- Y. Jia, L. Zhang, L. Zhuang, H. Liu, X. Yan and X. Wang, et al., Identification of active sites for acidic oxygen reduction on carbon catalysts with and without nitrogen doping, Nat. Catal., 2019, 2(8), 688–695 CrossRef CAS.
- J. Wang, J. Zheng and X. Liu, The key to improving the performance of Li–air batteries: recent progress and challenges of the catalysts, Phys. Chem. Chem. Phys., 2022, 24(30), 17920–17940 RSC.
- X. Hu, M. Fan, Y. Zhu, Q. Zhu, Q. Song and Z. Dong, Biomass-derived phosphorus-doped carbon materials as efficient metal-free catalysts for selective aerobic oxidation of alcohols, Green Chem., 2019, 21(19), 5274–5283 RSC.
- C. Wang, Z. Xie and Z. Zhou, Lithium-air batteries: Challenges coexist with opportunities, APL Mater., 2019, 7(4), 1–12, DOI:10.1063/1.5091444.
- Y. Gao, L. Liu, Y. Jiang, D. Yu, X. Zheng, J. Wang, J. Liu, D. Luo, Y. Zhang, Z. Shi, X. Wang, Y. P. Deng and Z. Chen, Design Principles and Mechanistic Understandings of Non-Noble-Metal Bifunctional Electrocatalysts for Zinc–Air Batteries, Springer Nature Singapore, 2024. DOI:10.1007/s40820-024-01366-9.
- M. E. Berry, E. Malone and H. Lipson, Freeform fabrication of zinc air batteries with tailored geometry and performance, 16th Solid Free. Fabr. Symp. SFF, 2005, vol. 2005, pp. 295–307.
- C. T. Lu, Z. Y. Zhu, S. W. Chen, Y. L. Chang and K. L. Hsueh, Effects of Cell Design Parameters on Zinc-Air Battery Performance, Batteries, 2022, 8, DOI:10.3390/batteries8080092.
- J. Wang, Y. Zhang, S. Liao, D. Chen, A. Mensah and Q. Wei, MoNP-doped Defective Carbon Fibers with Bark-like Nanosurface as Effective Bifunctional Electrocatalysts for Zn-air Batteries, ChemSusChem, 2024, 17, e202301510, DOI:10.1002/cssc.202301510.
- W. Lao-atiman, Modeling of zinc-air batteries using theoretical and empirical approach Modeling of Zinc-Air Batteries Using Theoretical and Empirical Approach, 2020 Search PubMed.
- J. Wang, D. Deng, Y. Wang, H. Zheng, M. Liu, Y. Chen, Y. Bai, J. Jiang, X. Zheng, P. Yang, Q. Wu, X. Xiong and Y. Lei, Long-cycle Zn–air batteries at high depth of discharge enabled by a robust Zn|electrolyte interface, Chem. Commun., 2023, 59, 13034–13037, 10.1039/D3CC04372J.
- X. Zhang, X. Wu, Y. Lv, J. Guo, N. Liang, R. Guo, Y. Zhu, H. Liu and D. Jia, Fabrication of Zn–Air Battery with High Output Capacity Under Ultra-Large Current, Small, 2024, 20, 1–9, DOI:10.1002/smll.202307999.
- M. A. Alemu, D. T. Ebissa, M. Z. Getie, A. K. Worku, H. M. Wassie and M. S. Alem, Recent development of rechargeable solid-state metal-air batteries for electric mobility, Energy Reports, 2024, 12, 517–528, DOI:10.1016/j.egyr.2024.06.007.
- Y. Jiao, K. Xu, H. Xiao, C. Mei and J. Li, Biomass-Derived Carbon Aerogels for ORR/OER Bifunctional Oxygen Electrodes, Nanomaterials, 2023, 13(17), 1–30, DOI:10.3390/nano13172397.
- N. Han, W. Zhang, W. Guo, H. Pan, B. Jiang and L. Xing, et al., Designing Oxide Catalysts for Oxygen Electrocatalysis: Insights from Mechanism to Application, Nano-Micro Lett., 2023, 15, 1–33 CrossRef PubMed.
- Z. Yisilamu, X. Zhao, X. Maimaitiyiming and A. Liu, Preparation of Biomass-Based Heteroatom-Doped Porous Carbon and Its Electrochemical Properties, Fibers Polym., 2023, 24(12), 4129–4141 CrossRef CAS.
- M. C. Kim, J. H. Song, Y. W. Lee and J. I. Sohn, Redox-mediated polymer catalyst for lithium-air batteries with high round-trip efficiency, Catalysts, 2020, 10(12), 1–9 Search PubMed.
- D. S. Hong, Y. J. Choi, C. S. Jin, K. H. Shin, W. J. Song and S. H. Yeon, Enhanced Cycle Performance of NiCo2O4/CNTs Composites in Lithium-Air Batteries, Energies, 2024, 17(1), 1–14, DOI:10.3390/en17010058.
- C. D. Pham, K. D. Tran, T. M. Truong and P. K. Le, Cellulose-derived carbon aerogel from rice straw for high-performance lithium-ion battery anodes, Biomass Convers. Biorefin., 2024, 5, 05319 Search PubMed.
- Y. Lv, L. Yang and D. Cao, Nitrogen and Fluorine-Codoped Porous Carbons as Efficient Metal-Free Electrocatalysts for Oxygen Reduction Reaction in Fuel Cells, ACS Appl. Mater. Interfaces, 2017, 9(38), 32859–32867 CrossRef CAS PubMed.
- B. V. Raghu Vamshi Krishna, T. Nageswara Rao and H. C. A. Murthy, Orange peel-derived activated carbon as an electrode material for supercapacitor application using nickel mesh, Chem. Pap., 2023, 77(9), 4953–4962 CrossRef CAS.
- Q. Jiang, J. Li, J. Gao, W. Zhu, H. Liu and Y. Yang, et al., Marine biomass–derived nitrogen-doped carbon microsphere electrocatalyst for vanadium redox flow battery, Ionics, 2023, 29(1), 259–269 CrossRef CAS.
- J. Li, W. Wang, Y. Kang, S. Xue, S. Liu and Z. Lei, Tuning the performance of nitrogen, phosphorus co-doped nanoporous carbon for oxygen reduction reaction, J. Taiwan Inst. Chem. Eng., 2017, 80, 728–737 CrossRef CAS.
- P. Chen, F. Bai, J. w. Deng, B. Liu and T. Zhang, Recent progresses and challenges in aqueous lithium–air batteries relating to the solid electrolyte separator: A mini-review, Front. Chem., 2022, 10, 1–7 Search PubMed.
- T. Bai, D. Li, S. Xiao, F. Ji, S. Zhang and C. Wang, et al., Recent progress on single-atom catalysts for lithium–air battery applications, Energy Environ. Sci., 2023, 16(4), 1431–1465 RSC.
- Y. Ding, Y. Li and Z. S. Wu, Recent advances and challenges in the design of Li–air batteries oriented solid-state electrolytes, Battery Energy, 2023, 2(2), 1–20 CrossRef.
- Z. Jiao, J. Hu, M. Ma, X. Han, Y. Ma and A. Ma, et al., Research progress of cellulose-derived carbon-based composites for microwave absorption, J. Mater. Sci.: Mater. Electron., 2023, 34(6), 536 CrossRef CAS.
- Y. Wu, X. Gao, T. T. Nguyen, J. Wu, M. Guo and W. Liu, et al., Green and Low-Cost Natural Lignocellulosic Biomass-Based Carbon Fibers—Processing, Properties, and Applications in Sports Equipment: A Review, Polymers, 2022, 14(13), 2591, DOI:10.3390/polym14132591.
- P. Adelhelm, P. Hartmann, C. L. Bender, M. Busche, C. Eufinger and J. Janek, From lithium to sodium: Cell chemistry of room temperature sodium-air and sodium-sulfur batteries, Beilstein J. Nanotechnol., 2015, 6(1), 1016–1055 CrossRef CAS PubMed.
- G. V. Dubacheva, C.-K. Liang and D. M. Bassani, Functional monolayers from carbon nanostructures – fullerenes, carbon nanotubes, and graphene – as novel materials for solar energy conversion, Coord. Chem. Rev., 2012, 256(21), 2628–2639 CrossRef CAS . https://www.sciencedirect.com/science/article/pii/S0010854512000896.
- S. Bakhsh, Carbon Nanotubes and Gr ene, Carbon Nanotubes and Graphene: A compraphene: A compr aphene: A comprehen-siv ehen-sive review, Karbala International Journal of Modern Science, 2023, 9, 341–356 CrossRef.
- A. Bianco, Y. Chen, E. Frackowiak, M. Holzinger, V. Meunier and S. Mikhailovsky, et al., Carbon Science Perspective in 2020: Current Research and Future Challenges, Carbon, 2020, 161, 373–391 CrossRef CAS.
- J. Narayan and B. Kangkana, Recent advances in the functionalization, substitutional doping and applications of graphene/graphene composite nanomaterials, RSC Adv., 2024, 1(19), 13413–13444 RSC.
- S. Teixeira, A Review of Biomass Thermal Analysis, Kinetics and Product Distribution for Combustion Modeling: From the Micro to Macro Perspective, Energies, 2023, 16(18), 6705, DOI:10.3390/en16186705.
- N. A. Banek, K. R. McKenzie Jr., D. T. Abele and M. J. Wagner, Sustainable conversion of biomass to rationally designed lithium ion battery graphite, Sci. Rep., 2022, 1–11, DOI:10.1038/s41598-022-11853-x.
- M. Nazhipkyzy, A. B. Maltay, K. Askaruly, D. D. Assylkhanova, A. R. Seitkazinova and Z. A. Mansurov, Biomass-Derived Porous Carbon Materials for Li-Ion Battery, Nanomaterials, 2022, 12(20), 3710, DOI:10.3390/nano12203710.
- S. Balou, P. Shandilya and A. Priye, Carbon dots for photothermal applications, Front. Chem., 2022, 10, 1023602, DOI:10.3389/fchem.2022.1023602.
- https://s10853-023-08408-4.pdf.crdownload .
- S. Dinç, M. Kara and E. Yavuz, Chapter 4 - Synthesis of carbon dots from biomass resources, in Carbon Dots in Agricultural Systems, ed. R. Khan, S. Murali and S. B. T. Gogoi, Academic Press, 2022, pp. 69–116. https://www.sciencedirect.com/science/article/pii/B9780323902601000012 Search PubMed.
- R. Pathak, Multifunctional role of carbon dot-based polymer nanocomposites in biomedical applications: a review, J. Mater. Sci., 2023, 58(15), 6419–6443, DOI:10.1007/s10853-023-08408-4.
- R. P. Forbes and N. J. Coville, The Transformation of 0-D Carbon Dots into 1-, 2- and 3-D Carbon Allotropes: A Minireview, Nanomaterials, 2022, 12(15), 2515, DOI:10.3390/nano12152515.
- W. Shi, J. Gao, H. Sun, Z. Liu, F. Guo and L. Wang, Highly efficient visible/near-infrared light photocatalytic degradation of antibiotic wastewater over 3D yolk-shell ZnFe2O4 supported 0D carbon dots with up-conversion property, Chin. J. Chem. Eng., 2022, 49, 213–223 CrossRef CAS https://www.sciencedirect.com/science/article/pii/S1004954122000143 .
- X. H. Zhou, A one-dimensional continuous model for carbon nanotubes, Eur. Phys. J. B, 2012, 85(2), 81, DOI:10.1140/epjb/e2012-21061-0.
- N. Maley, P. Patel, F. M. de Souza and R. K. Gupta, One-Dimensional Carbon for Electrocatalytic Activities, in NanoCarbon: A Wonder Material for Energy Applications, ed. R.K Gupta, Springer Nature Singapore, Singapore, 2024, vol. 1, pp. 81–98. DOI:10.1007/978-981-99-9935-4_5.
- L. Kou, Y. Liu, C. Zhang, L. Shao, Z. Tian and Z. Deng, et al., A Mini Review on Nanocarbon-Based 1D Macroscopic Fibers: Assembly Strategies and Mechanical Properties, Nano-Micro Lett., 2017, 9(4), 1–18 Search PubMed.
- A. S. Jamaludin, A. M. Rosli, M. Z. Mohd Zawawi, I. Ishak and R. Hamidon, Advancements in 1D Nanostructure-Enhanced Carbon/carbon Composites for Aerospace Structures, in Intelligent Manufacturing and Mechatronics, ed. R. Abd. Aziz, Z. Ismail, A. K. M. A. Iqbal and I. Ahmed, Springer Nature Singapore, Singapore, 2024, pp. 487–496 Search PubMed.
- Z. Lai, Y. Chen, C. Tan, X. Zhang and H. Zhang, Self-Assembly of Two-Dimensional Nanosheets into One-Dimensional Nanostructures, Chem, 2016, 1(1), 59–77, DOI:10.1016/j.chempr.2016.06.011.
- K. Li, M. Zhang, X. Ou, R. Li, Q. Li and J. Fan, et al., Strategies for the Fabrication of 2D Carbon Nitride Nanosheets, Acta Phys. -Chim. Sin, 2020, 37(8), 2008010, DOI:10.3866/PKU.WHXB202008010.
- Y. Fan, J. Zhang, Y. Shen, B. Zheng, W. Zhang and F. Huo, Emerging porous nanosheets: From fundamental synthesis to promising applications, Nano Res., 2021, 14(1), 1–28, DOI:10.1007/s12274-020-3082-4.
- D. Rodríguez-San-Miguel, C. Montoro and F. Zamora, Covalent organic framework nanosheets: preparation, properties and applications, Chem. Soc. Rev., 2020, 49(8), 2291–2302, 10.1039/C9CS00890J.
- E. Kirchner, T. Hirsch and T. Hirsch, Recent developments in carbon-based two-dimensional materials: synthesis and modification aspects for electrochemical sensors become one of the dominating materials in many sensor, Microchimica Acta, 2020, 187, 441, DOI:10.1007/s00604-020-04415-3.
- Y. Zhao, X. Zuo, Y. Guo, H. Huang, H. Zhang and T. Wang, et al., Structural Engineering of Hierarchical Aerogels Comprised of Multi dimensional Gradient Carbon Nanoarchitectures for Highly Efficient Microwave Absorption, Nano-Micro Lett., 2021, 13(1), 1–20, DOI:10.1007/s40820-021-00667-7.
- K. Yang, Q. Fan, Y. Zhang, G. Ren, X. Huang and P. Fu, Hierarchical porous carbon aerogels as a versatile electrode material for high-stability supercapacitors, RSC Adv., 2024, 14(2), 1123–1133, 10.1039/D3RA07014J.
- Z. Xu, W. Deng and X. Wang, 3D Hierarchical Carbon-Rich Micro-/Nanomaterials for Energy Storage and Catalysis, Electrochem. Energy Rev., 2021, 4(2), 269–335, DOI:10.1007/s41918-021-00094-7.
- S. Chandrasekaran, P. G. Campbell, T. F. Baumann and M. A. Worsley, Carbon aerogel evolution: Allotrope, graphene-inspired, and 3D-printed aerogels, J. Mater. Res., 2017, 4166–4185, DOI:10.1557/jmr.2017.411.
- X. Gao, Z. Xing, Z. Li, X. Dong, Z. Ju and C. Guo, A review on recent advances in carbon aerogels: their preparation and use in alkali-metal ion batteries, New Carbon Mater., 2020, 35(5), 486–507 CrossRef CAS https://www.sciencedirect.com/science/article/pii/S1872580520605042 .
- M. Jiang, X. Yu, H. Yang and S. Chen, Optimization strategies of preparation of biomass-derived carbon electrocatalyst for boosting oxygen reduction reaction: A minireview, Catalysts, 2020, 10(12), 1–17 Search PubMed.
- T. F. Abraham, E. Marcel and K. Alexis, Intermittent Drying of Mango Slices (Mangifera indica L.) “Amelie”: A New Model, Am. J. Food Sci. Technol., 2020, 8(3), 81–86 Search PubMed.
- V. Panneerselvam, K. Kumar, T. S. Shyju and M. Paulraj, Biomass-derived Carbon as Electrode Materials for Batteries, in Biomass–Derived Carbon Materials, John Wiley & Sons, Ltd, 2022, pp. 171–213 Search PubMed.
- C. Ma, M. Zhang, Y. Ding, Y. Xue, H. Wang and P. Li, et al., Green Production of Biomass-Derived Carbon Materials for High-Performance Lithium–Sulfur Batteries, Nanomaterials, 2023, 13(11), 1768, DOI:10.3390/nano13111768.
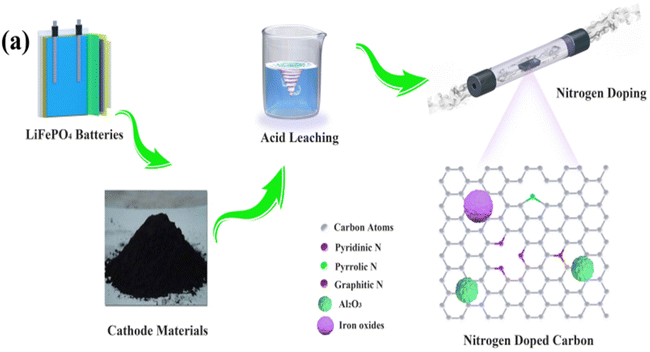
- Y. Shen, G. Zhang, R. Wang, L. Shen, Q. Li and F. Zheng, et al., Waste Lithium Ion Battery Evolves into Heteroatom Doped Carbon as Oxygen Reduction Electrocatalyst for Aqueous Al-Air Batteries, ChemPlusChem, 2022, 87(12), e202200328 CrossRef CAS PubMed.
- C. Murugesan, B. Senthilkumar and P. Barpanda, Biowaste-Derived Highly Porous N-Doped Carbon as a Low-Cost Bifunctional Electrocatalyst for Hybrid Sodium–Air Batteries, ACS Sustainable Chem. Eng., 2022, 10(28), 9077–9086 CrossRef CAS.
- J. Yang, Z. Hansen, M. Jamal, K. Mathew, G. Wang and J. Xiong, et al., Biomass-Derived Carbon for High-Performance Lithium-Sulfur Batteries, ECS Meet. Abstr., 2022, MA2022-02(4), 543 CrossRef.
- J. Niu, Y. Liu, X. Wang, J. Liu, Z. Zhao and X. Liu, et al., Biomass-Derived Bifunctional Cathode Electrocatalyst and Multiadaptive Gel Electrolyte for High-Performance Flexible Zn–Air Batteries in Wide Temperature Range, Small, 2023, 19(38), 1–11 Search PubMed.
- F. Ahmed, G. Almutairi, P. M. Z. Hasan, S. Rehman, S. Kumar and N. M. Shaalan, et al., Fabrication of a Biomass-Derived Activated Carbon-Based Anode for High-Performance Li-Ion Batteries, Micromachines, 2023, 14(1), 1–12 CrossRef PubMed.
- S. Rani, P. Kumar, K. Kumari, S. R. Dhakate and S. Kumari, Effect of carbonization conditions on microstructural properties of isotropic coal tar pitch-based general-purpose carbon fibers, Diamond Relat. Mater., 2023, 140, 110525 CrossRef CAS https://www.sciencedirect.com/science/article/pii/S0925963523008506 .
- W. Zhao, L. Luo, H. Wang and M. Fan, Synthesis of Bamboo-Based Activated Carbons with Super-High Specific Surface Area for Hydrogen Storage, BioResources, 2017, 12(1), 1246–1262 CAS.
- N. Isoda, R. Rodrigues, A. Silva, M. Gonçalves, D. Mandelli and F. C. A. Figueiredo, et al., Optimization of preparation conditions of activated carbon from agriculture waste utilizing factorial design, Powder Technol., 2014, 256, 175–181 CrossRef CAS https://www.sciencedirect.com/science/article/pii/S0032591014001454 .
- J. Serafin, B. Dziejarski, O. F. Cruz Junior and J. Sreńscek-Nazzal, Design of highly microporous activated carbons based on walnut shell biomass for H2 and CO2 storage, Carbon, 2023, 201, 633–647 CrossRef CAS https://www.sciencedirect.com/science/article/pii/S0008622322007370 .
- H. Zhang, Y. Zhang, L. Bai, Y. Zhang and L. Sun, Effect of physiochemical properties in biomass-derived materials caused by different synthesis methods and their electrochemical properties in supercapacitors, J. Mater. Chem. A, 2021, 9(21), 12521–12552 RSC.
- Y. Wu, Q. Hou, F. Li, Y. Sang, M. Hao and X. Tang, et al., Mitigating Co Metal Particle Agglomeration and Enhancing ORR Catalytic Activity through Nitrogen-Enriched Porous Carbon Derived from Biomass, Catalysts, 2023, 13(7), 1118, DOI:10.3390/catal13071118.
- T. H. Le, V. H. Ngo, M. T. Nguyen, V. C. Nguyen, D. N. Vu and T. D. Pham, et al., Enhanced Electrochemical Performance of Porous Carbon Derived from Cornstalks for Supercapacitor Applications, J. Electron. Mater., 2021, 50(12), 6854–6861 CrossRef CAS.
- T. Zhang, Y. Zhu, Y. Lv, Q. Yu, S. Yao and W. Zhu, et al., N-doped biomass carbon materials as superior catalyst to improve electrochemical performance of vanadium redox flow battery, Ionics, 2021, 27(11), 4771–4781 CrossRef CAS.
- H. O. Panganoron, J. D. A. Pascasio, E. A. Esparcia Jr., J. Anne, D. Rosario and J. D. Ocon, Hydrothermally Carbonized Waste Biomass as Reduction Reaction, Catalysts, 2020, 10(177), 1–18 Search PubMed.
- T. Zeng, M. Tang, L. Luo, L. Wu, M. Chen and S. Fu, et al., 1T-WS2 Nanosheet-Modified Biomass Carbon as Sulfur Host for High-Performance Lithium–Sulfur Batteries, Adv. Eng. Mater., 2023, 25(20), 2300542 CrossRef CAS https://onlinelibrary.wiley.com/doi/abs/10.1002/adem.202300542 .
- K. I. John and M. O. Omorogie, Biomass-based hydrothermal carbons for catalysis and environmental cleanup: a review, Green Chem. Lett. Rev., 2022, 15(1), 162–186, DOI:10.1080/17518253.2022.2028017.
- P. Baruah, B. K. Das, M. Bora, B. K. Saikia and D. Mahanta, Hydrothermally prepared sugar-derived carbon spheres for all-solid-state symmetric electrochemical capacitors, Mater. Today Commun., 2022, 33, 104219 CrossRef CAS https://www.sciencedirect.com/science/article/pii/S2352492822010698 .
- K. Yu, J. Wang, K. Song, X. Wang, C. Liang and Y. Dou, Hydrothermal Synthesis of Cellulose-Derived Carbon Nanospheres from Corn Straw as Anode Materials for Lithium ion Batteries, Nanomaterials, 2019, 9(1), 1–13, DOI:10.3390/nano9010093.
- I. L. Egun, H. He, D. Hu and G. Z. Chen, Molten Salt Carbonization and Activation of Biomass to Functional Biocarbon, Adv. Sustainable Syst., 2022, 2200294, DOI:10.1002/adsu.202200294.
- B. Lu, L. Hu, H. Yin, W. Xiao and D. Wang, One-step molten salt carbonization (MSC) of fi rwood biomass for capacitive carbon, RSC Adv., 2016, 106485–106490, 10.1039/C6RA22191B.
- F. Zhu, J. Ge, Y. Gao, S. Li, Y. Chen and J. Tu, et al., Molten salt electro-preparation of graphitic carbons, Exploration, 2023, 3(1), 20210186 CrossRef PubMed https://onlinelibrary.wiley.com/doi/abs/10.1002/EXP.20210186 .
- Y. Ren, S. Li, Z. Lv, Y. Fan, J. He and J. Song, Electrolysis Synthesis of Carbides and Carbon Dioxide Capture in Molten Salts, Small, 2023, 19(23), 2207863 CrossRef CAS PubMed https://onlinelibrary.wiley.com/doi/abs/10.1002/smll.202207863 .
- Z. Ma, Y. Huang, M. Gu, L. Wang, K. Bao and X. Cheng, et al., In situ generation of carbon-doped modified KCl–LiCl high-efficiency thermal storage materials with 3D network structure, Mater. Today Sustain., 2023, 23, 100436 Search PubMed https://www.sciencedirect.com/science/article/pii/S2589234723001227 .
- H. Wang, J. Wang, S. Xie, W. Liu and C. Niu, Template synthesis of graphitic hollow carbon nanoballs as supports for SnOx nanoparticles towards enhanced lithium storage performance, Nanoscale, 2018, 10(13), 6159–6167, 10.1039/C8NR00405F.
- S. Natarajan, H. C. Bajaj and V. Aravindan, Template-free synthesis of carbon hollow spheres and reduced graphene oxide from spent lithium-ion batteries towards efficient gas storage, J. Mater. Chem. A, 2019, 7(7), 3244–3252, 10.1039/C8TA11521D.
- S. Qiao, Q. Wang, Q. Zhang, C. Huang, G. He and F. Zhang, Sacrificial Template Method to Synthesize Atomically Dispersed Mn Atoms on S, N-Codoped Carbon as a Separator Modifier for Advanced Li–S Batteries, ACS Appl. Mater. Interfaces, 2022, 14(37), 42123–42133, DOI:10.1021/acsami.2c12114.
- https://dx.doi.org/10.1039/pericles_9783527832903 .
- A. Shetty, V. Molahalli, A. Sharma and G. Hegde, Biomass-Derived Carbon Materials in Heterogeneous Catalysis: A Step towards Sustainable Future, Catalysts, 2023, 13(1), 1–36, DOI:10.3390/catal13010020.
- Y. Wang, M. Zhang, X. Shen, H. Wang, H. Wang and K. Xia, et al., Biomass-Derived Carbon Materials: Controllable Preparation and Versatile Applications, Small, 2021, 17(40), 2008079 CrossRef CAS PubMed.
- Y. Xia, Y. Jin, J. Qi, H. Chen, G. Chen and S. Tang, Preparation of biomass carbon material based on Fomes fomentarius via alkali activation and its application for the removal of brilliant green in wastewater, Environ. Technol. Innovation, 2021, 23, 101659 CrossRef CAS.
- A. Ahmad, M. A. Gondal, M. Hassan, R. Iqbal, S. Ullah and A. S. Alzahrani, et al., Preparation and Characterization of Physically Activated Carbon and Its Energetic Application for All-Solid-State Supercapacitors: A Case Study, ACS Omega, 2023, 8(24), 21653–21663 CrossRef CAS PubMed.
- S. K. Guchhait, D. Sutradhar, R. Nandi and A. K. Sarma, Biomass derived metal free hierarchical porous activated carbon for efficient oxygen evolution reaction, Energy Sources, Part A, 2023, 45(2), 5957–5969 CrossRef CAS.
- E. Taer, A. Agustino, E. S. Gultom and R. Taslim, Sustainable development of biomass-derived activated carbon through chemical and physical activations and its effect on the physicochemical and electrochemical activity, Energy Sources, Part A, 2023, 45(1), 319–330 CrossRef CAS.
- Q. Li, Y. Liu, Y. Wang, Y. Chen, X. Guo and Z. Wu, et al., Review of the application of biomass-derived porous carbon in lithium-sulfur batteries, Ionics, 2020, 26(10), 4765–4781, DOI:10.1007/s11581-020-03694-3.
- B. Kong, H. Xu, L. Xie and S. Zhou, Synthesis Methods of Mesoporous Carbon-Based Materials, in Functional Mesoporous Carbon-Based Film Devices for Energy Systems, ed. B. Kong, H. Xu, L. Xie and S. Zhou, Springer Nature Singapore, Singapore, 2024, pp. 17–79. DOI:10.1007/978-981-99-7498-6_3.
- D. Bergna, T. Varila, H. Romar and U. Lassi, Comparison of the Properties of Activated Carbons Produced in One-Stage and Two-Stage Processes, C, 2018, 4(3), 41 Search PubMed.
- G. Zhang, X. Liu, L. Wang and H. Fu, Recent advances of biomass derived carbon-based materials for efficient electrochemical energy devices, J. Mater. Chem. A, 2022, 10(17), 9277–9307 RSC.
- S. Das, S. Ghosh, T. Kuila, N. C. Murmu and A. Kundu, Biomass-Derived Advanced Carbon-Based Electrocatalysts for Oxygen Reduction Reaction, Biomass, 2022, 155–177 CrossRef CAS.
- R. K. Azega, M. M. Haque, A. Vyas, P. L. Tam, A. D. Smith and P. Lundgren, et al., Durable Activated Carbon Electrodes with a Green Binder, Phys. Status Solidi B, 2022, 259(2), 2100311 CrossRef.
- D. Geng, N. Ding, T. S. A. Hor, S. W. Chien, Z. Liu and D. Wuu, et al., From Lithium-Oxygen to Lithium-Air Batteries: Challenges and Opportunities, Adv. Energy Mater., 2016, 6(9), 1–14 Search PubMed.
- L. Wang, Y. Wang, Y. Qiao, S. Wu, X. Lu and J. J. Zhu, et al., Superior efficient rechargeable lithium-air batteries using a bifunctional biological enzyme catalyst, Energy Environ. Sci., 2020, 13(1), 144–151 RSC.
| This journal is © The Royal Society of Chemistry 2024 |