Open Access Article
Open Access ArticleChallenges in the design and synthesis of self-assembling molecules as selective contacts in perovskite solar cells
Carlos E.
Puerto Galvis
 *a,
Dora A.
González Ruiz
*a,
Dora A.
González Ruiz
 ab,
Eugenia
Martínez-Ferrero
ab,
Eugenia
Martínez-Ferrero
 a and
Emilio
Palomares
a and
Emilio
Palomares
 *ac
*ac
aInstitute of Chemical Research of Catalonia (ICIQ), Avda. Països Catalans, 16, Tarragona, Spain. E-mail: epalomares@iciq.es
bDepartament d'Enginyeria Electrònica, Elèctrica i Automàtica., Universitat Rovira i Virgili, Avda. Països Catalans, 26, Tarragona, Spain
cCatalan Institution for Research and Advanced Studies (ICREA), Passeig Lluïs Companys, 23, Barcelona, Spain
First published on 10th November 2023
Abstract
Self-assembling molecules (SAMs), as selective contacts, play an important role in perovskite solar cells (PSCs), determining the performance and stability of these photovoltaic devices. These materials offer many advantages over other traditional materials used as hole-selective contacts, as they can be easily deposited on a large area of metal oxides, can modify the work function of these substrates, and reduce optical and electric losses with low material consumption. However, the most interesting thing about SAMs is that by modifying the chemical structure of the small molecules used, the energy levels, molecular dipoles, and surface properties of this assembled monolayer can be modulated to fine-tune the desired interactions between the substrate and the active layer. Due to the important role of organic chemistry in the field of photovoltaics, in this review, we will cover the current challenges for the design and synthesis of SAMs PSCs. Discussing, the structural features that define a SAM, (ii) disclosing how commercial molecules inspired the synthesis of new SAMs; and (iii) detailing the pros- and cons- of the reported synthetic protocols that have been employed for the synthesis of molecules for SAMs, helping synthetic chemists to develop novel structures and promoting the fast industrialization of PSCs.
Introduction
Since their discovery in 2009, perovskite solar cells (PSCs) are considered nowadays amongst the most promising next-generation photovoltaic technologies.1 PSCs have achieved a power conversion efficiency (PCE) of 25.8% (vs. 3.8% in 2009), comparable to the current record certificated for traditional crystalline silicon solar cells (26–27%).2 Among the advantages that have caught the attention and interest of many researchers are their high efficiency, low-cost manufacturing, versatility (shapes, colors, and flexibility) or their potential to combine with either c-Si, CIGS, organic or other existing solar cells in multijunction devices (tandem cells). In addition, their high performance in low light conditions and compatibility with other renewable energy sources have pushed the further industrialization of PSCs.3–7Such as other photovoltaic devices, PSCs are made of a multilayer device structure in which the active and light-absorbing layer (perovskite layer) is sandwiched between two charge transporting layers (CTLs), i.e., hole transporting layer (HTL) and electron transporting layer (ETL).8–10 According to the direction of the current flow, perovskite devices can be divided into two well-known architectures: regular (n-i-p) and inverted (p-i-n) configurations (Fig. 1). Although slightly higher PCE are currently achieved with the regular nip-structures,11 the pin-type PSCs exhibit several attractive advantages, such as low-temperature fabrication process, high device stability, negligible hysteresis, and excellent compatibility with flexible and tandem devices.12
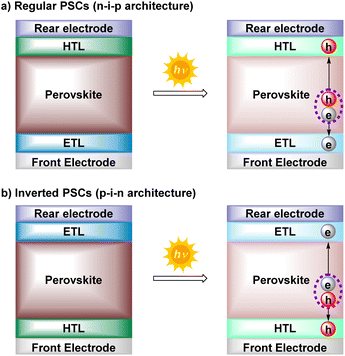 | ||
| Fig. 1 Common architectures of PSCs (regular and inverted) and their working mechanism. h: holes, e: electrons. | ||
To design stable and efficient PSCs, the selection of the electron and hole transport layers, as well as the perovskite material, results are crucial. The light-absorbing perovskite material is represented with the general formula ABX3, and its composition, morphology, and crystallinity are well optimized to boost the performance of PSC devices toward higher efficiency and stability.3,13 The ETL promotes the collection of photogenerated electrons from the perovskite layer to the corresponding electrode, consisting of metal oxides (TiO2, ZnO, SnO) for regular PSCs and hydrophobic [6,6]-phenyl-C61-butyric acid methyl ester (PCBM) or its derivatives for inverted PSCs.14–16 On the other hand, the 2,2′,7,7′-tetrakis[N,N-di(4-methoxyphenyl)amino]-9,9′-spirobifluorene (Spiro-MeOTAD) has been the most employed hole transport material (HTM) for regular PSCs. In the case of inverted PSCs, different HTLs have evolved from copper (CuI, CuCrO2), nickel (NiOx), and polymers such as (poly(3,4-ethylene dioxythiophene)polystyrene sulfonate (PEDOT:PSS) and poly[bis(4-phenyl)(2,5,6-trimethylphenyl)amine](PTAA)), to diverse organic small molecules (SMs), including the emerging self-assembled monolayers (SAMs).17–20
Regardless of its composition, the role of these hole transport materials (HTMs) is (i) the extraction and transport of holes from the perovskite layer to the electrode;21 (ii) establishing an energy barrier to block the flow of electrons, and thus, reducing recombination and current leakage;22 and (iii) protecting the perovskite layer from moisture and oxygen, improving the device stability by reducing corrosion.23 Although several works focused on the development of different HTMs are available in the literature,24–28 it is worth mentioning that the design principles and the desired properties of the currently used HTMs differ substantially between regular (n-i-p) and inverted (p-i-n) architectures.
Among all the HTLs, during the last 5 years, special attention has been put to the development of self-assembled molecules as HTMs. These ordered arrays of organic molecules with a thickness of one or a few molecules have promoted the fabrication and study of efficient perovskite devices, the so-called SAM-based PSCs. Considering the accelerated growth, and the importance of organic chemistry in the design of these organic molecules, in this review we will cover the current challenges for the design and synthesis of SAMs for perovskite solar cells. The present review will be divided into three main topics: (i) the structural features that define a SAM, and how each part of the molecule has a counterpart and interacts with other layers; (ii) a quick overview of all commercial SAMs that have been used from the beginning, and how they have inspired the synthesis of new and complex SAMs to pursuit better efficiencies; and (iii) we will discuss all the synthetic protocols, mostly reported in the Supporting information of already published articles, to discuss their pros- and cons- and enhance the role of organic chemistry in this field, guiding the synthetic chemists to develop novel structures and benign procedures that promote the industrialization of PSCs.
Self-assembled molecules (SAMs)
Low molecular weight organic molecules (so-called “small molecules”) can be used as HTMs under two different approaches: first, as a randomly deposited non-ordered array of planar and extended π-conjugated organic molecules deposited as a layer, or, as an ordered array of organic molecules with a very low thickness creating a layer that is well-known as a self-assembled molecule (SAM) (Fig. 2). The concept of SAMs was first described in the 1940s when a method to adsorb surfactants on different metal surfaces was reported. Then, in 2014, it was first applied in the fabrication of PSCs,29 when benzoic acid and a fullerene derivative were used in inverted (p-i-n) configurations,30 inspired by the results obtained in polymer solar cells.31–33The formation of SAMs is a thermodynamically favorable process depending on two main aspects: (i) the adsorption of each molecule to a specific surface, and (ii) the intermolecular interactions that form the unidirectional monolayer.34,35 Nevertheless, the most attractive feature of SAMs is that each molecule that conforms to the monolayer can have a specific functional group, resulting in a highly functionalized, packed, and ordered unidirectional monolayer with different properties deposited on a desired surface. The combination and control of these three remarkable aspects of SAMs can be easily tuned varying the chemical structure of the molecules that form the SAMs. Therefore, it is in this field that organic chemistry plays an important role in the design and synthesis of the molecules that will be an important part of perovskite devices.
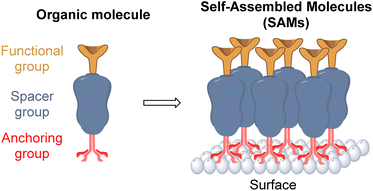
Thus, the structural requirements needed for a molecule to be able to form a SAM are an anchoring group, that allows the chemical adsorption onto the surface, a linker or spacer moiety, to assist and promote the self-assembly process, and a functional group, to modify the nature of the monolayer and trigger all the possible interactions with the overlayer in the PSCs device (Fig. 3).
The anchoring group is involved in the bonding interactions of each molecule to the surface through chemical reactions such as hydrolysis and condensation.36 For PSCs the most used and common anchoring groups are Brønsted–Lowry acids such as the carboxylic acids (–COOH) and phosphonic acids (–PO(OH)2) that can allow mono-, bi-, and tridentate binding modes, depending on the surface type.37
The spacer group, besides being the bridge between the anchoring and the functional groups, is in charge of developing a well-organized molecular packing during the self-assembly process via van der Waals forces. Thus, inactive aliphatic and conjugated benzene rings, of different sizes and lengths, are often used as spacer groups.38
Finally, the functional group will define the nature of the new monolayer. Inspired by other HTMs, these groups are responsible for all the physical and chemical interactions at the interface, which in most cases is in contact with the perovskite layer, so these functional groups create a template for the deposition of the next layer, influencing in its structure and morphology. Different nitrogen, sulfur, and oxygen heterocycles and some organic cations that possess strong interaction with the perovskite frameworks are the most common groups used in PSCs.39
The deposition techniques (vapor deposition, dip-coating, and spin-coating methods), characterization, and role in the electronic function and performance of PSCs of SAMs are well documented in the literature.38–41 The presence of an anchoring group and a functional group, with different electron densities and separated by the spacer group, in the chemical structure of the molecules that form SAMs, results in a monolayer with a specific dipole that could decrease or increase the work function of the metal surface containing the SAM.42 The spacer group governs the charge transport at the interface, determining the electronic conductivity and optical properties of the PSCs.43 While the functional groups, beyond interacting with the overlayer, contribute to the energy level alignment (HOMO–LUMO) between the SAM and the perovskite, and add additional surface dipoles that contribute to the changes in the work function.44 Other important effects of SAMs in the performance of PSCs are: improving the crystal quality of the perovskite layer reducing the degree of defects, protecting the perovskite layer isolating it from the metal contact, and reducing the undesired charge recombination. Noteworthy, the low cost of the material and low material consumption during the fabrication of large-area perovskite devices, makes the design and synthesis of organic molecules, for the preparation of SAMs, an interesting and promising field to contribute to the study and industrialization of PSCs.
Commercial self-assembled molecules (SAMs)
Before organic chemists became interested in the design and preparation of molecules to form SAMs, several research groups recognized the potential of SAMs for the development of efficient photovoltaic devices. By that time, those studies were based on different simple and easily commercially available compounds as a platform to make progress in the formation (deposition techniques), characterization (IR, X-ray, SEM, contact angle, etc.), role (conductivity, passivation, template, work function, etc) of SAMs for the fabrication of perovskite solar cells.These pioneer works not only established the structural parameters that a molecule must have to be considered as a promising building block for SAMs, but also, inspired the organic chemists to actively participate in the molecular design of novel chemical entities, proposing and exploring different anchoring, spacer and functional groups, creating interesting combinations between them, and reaching outstanding PCEs and stable PSCs.
Although nowadays one of the main topics in the field is the design and synthesis of small molecules to form SAMs for PSC devices, some interesting works continue exploring commercial compounds with remarkable results. The library of available molecules used for SAMs is quite vast, and it has been well described in the literature,38,39 however in this section we selected some of the relevant molecules, that in our opinion, have inspired most of the synthesis of novel derivatives, according to their chemical structure (Fig. 4).
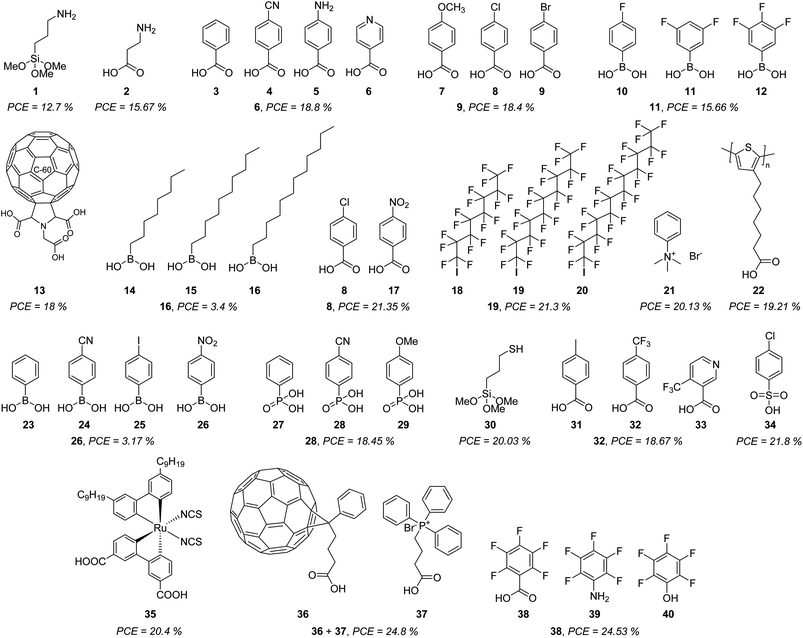 | ||
| Fig. 4 Commercial molecules are used for the formation of SAMs in PSCs, indicating the highest PCE obtained in each case. | ||
The first report based on the use of a commercial SAM in PSCs backs to 2015, where aminopropyltrimethoxysilane 1 was used to incorporate a SAM between the TiO2 and CH3NH3PbI3 perovskite, obtaining a PCE of 12.7% with a SAM composed of silane groups as an anchoring group, a propyl chain as linker and a primary amine as a functional group.45 At the same time, ZnO-coated glass/ITO substrates were modified with a SAM formed by 3-aminopropanioc acid 2, and the PCE was improved by 15.6% by using carboxylic acids as anchoring groups and reducing the length of the linker group.46 This study inspired the use of some benzoic acid derivatives 3–9 where the most efficient result was 4-pyridine carboxylic acid 6 with a PCE = 18.8%, demonstrating that ternary amines or aromatic N-heterocycles were better functional groups than primary amines,47 while 4-bromobenzoic acid 9 demonstrated to have a positive effect passivating the surface, reducing the recombination and minimizing the energy offset between NiOx nanoparticles and the perovskite in both, regular and inverted devices.48
The acidity of carboxylic acid function as an anchoring group results in a problem for the stability of the PSCs, due to the corrosion that induces. To reduce this negative impact, boronic acids were tested as an anchoring group where the best results were obtained with the 3,5-difluoro derivative 11 (PCE = 15.66%).49
The localization of positive ions caused by hydroxyl groups present at the metal surface (SnO2, TiO2, etc.) increases hysteresis and degradation of the device. Thus, to prevent this event, the respective C60 pyrrolidine 13, with 3-carboxylic acid functions, was used as a SAM for the fabrication of a PSCs device that resulted stable after 1000 h of continuous illumination without any significative decrease in the PCE.50
Aliphatic boronic acids 14–16 of different chain lengths, and without any functional group, demonstrated that the charge transport properties were enhanced from C8 to C12, although very low PCE was achieved (3.4%).51
Exploring other benzoic acid derivatives, but using TiO2 as a substrate and Cs8FAMA as a perovskite layer, afforded efficient PSCs devices when 4-chlorobenzoic acid 8 was employed as a SAM (PCE = 21.35%), while derivatives with electron-withdrawing groups such as nitro group (-NO2) 17 resulted in very inefficient devices (PCE = 1.01%) in both, regular and inverted configurations.52
Usually, the SAM layer is deposited below the perovskite layer. However, a self-assembled monolayer could also be formed on top of the perovskite layer to encapsulate and protect the active material. This was achieved with perfluorinated aliphatic carbon chains of different lengths 18–20 and terminated with an iodine atom as the anchoring group, finding that a C12-carbon chain exhibited a PCE of 21.3%.53 Another example of the formation of SAMs on top of the perovskite layer was reported with the phenyltrimethylammonium bromide 21, which successfully passivated the PbI3− defects and enhanced the photovoltaic properties and stability of the device with a PCE of 20.13%, exhibiting a PCE = 17.31% after 60 days.54
A monolayer of self-assembled molecules can be formed as well by polymers as reported for poly[3-(6-carboxyhexyl)thiophene-2,5-diyl] 22 that formed an ordered and homogeneous monolayer promoting the growth of high-quality perovskite film in a device that exhibited a PCE of 19.21%, being stable for 5000 h.55
Despite their low PCE, boronic acids continue to captivate attention as a precursor of SAMs. Although the nitro group demonstrated to be less efficient when carboxylic acids were used as an anchoring group 17, when boronic acids were employed instead, the changes in the dipole and the work function induced by (4-nitrophenyl)boronic acid 26 resulted in a slight improvement of the PCE for this kind of PSCs (PCE = 3.17%).56
Phosphonic acids are one of the best anchoring groups that can be used in the formation of SAMs for PSCs, since they strongly attach, through one-, two-, or three binding sites, to different metal surfaces. This was demonstrated by a series of p-substituted phenylphosphonic acids 27–29 on NiOx-ITO substrates, where the best results were obtained from the cyano derivative 28 with a PCE of 18.45%.57
Sulphur is one of the atoms that have shown great affinity for lead-based perovskites, because of the S⋯Pb interactions, many crystallization defects are covered by sulphur. For example, 3-mercaptopropyltrimethoxysilane (MPTMS) 30 formed a SAM in regular PSCs devices on SnO2 reaching a PCE of 20.03%, enhancing the extraction efficiency of photogenerated electrons and restraining recombination.58
Due to its small size and strong electron-withdrawing property, fluorine influences the perovskite crystallization kinetics and regulates the properties of the interface more than other functional groups. This was observed in the series of compounds 31–33 where 4-(trifluoromethyl)benzoic acid 32 improved the PCE to 18.67% and a high open-circuit voltage (VOC) of 1.160 V was achieved with a significant decrease of the defects and non-radiative recombination.59
Although less common, sulphonic acids can also be promising anchoring groups, as in the case of p-chlorobenzenesulfonic acid 34 which mitigates two of the most adverse problems in NiOx-based inverted perovskites. On one side, the chlorine functional group of 34 promotes perovskite growth, while the sulfonic acid group passivates the surface defects detected in NiOx, reaching a PCE of 21.8%.60
One of the benchmark sensitizers widely employed in dye-sensitized solar cells (DSSCs) has been recently used to promote the formation of SAM in PSCs. The Z907 dye 35 is an amphiphilic ruthenium dye that is chemically and spontaneously absorbed on the TiO2 forming a monomolecular layer that allows the fabrication of a perovskite device with low energy loss and higher interface coupling with the perovskite. In addition, the obtained SAM built a back surface field through a p-type doping effect that promoted the hole extraction and suppressed the carrier recombination, resulting in a PCE of 20.4%, a high value for a transition metal-SAM.61
But SAMs cannot only be formed by one single molecule, in 2023. It was demonstrated the synergic effect of combining [6,6]-phenyl-C61-butyric acid 36 and 3-carboxypropyl-triphenyl phosphonium bromide 37 to form a monolayer where the strong fullerene 36 interactions promoted the ordered array of 37. The resulting monolayer simultaneously influences defect passivation and improves carrier transportation in a PSC device to reach a PCE of 20.4%.62
Finally, and more recently, a series of simple polar molecules 38–40, with permanent dipole moments and different anchoring groups, were used to modify the perovskite surface through a SAM approach. Once again, the role of the fluorine atom becomes relevant, especially the pentafluoride benzene moiety, because of its hydrophobicity that protects the perovskite layer from water. On the other hand, the dipoles at the interface produced an offset in the vacuum level, leading to PSCs with a PCE of 24.53% for molecule 38, which possessed the carboxylic acid function as an anchoring group (Fig. 4).63
The chemical structures depicted in Fig. 4 are just correlated with the power conversion efficiency (PCE) achieved by each molecule in different PSC devices. Nonetheless, we want to state that other important parameters are needed to take into account for evaluating the performance of those devices, such as the work function (WF), open circuit voltage (VOC), current density (JSC), and fill factor (FF), so we encourage the readers to examine these excellent works and also get inspired to design new molecules that can be used as SAM.
The success of small organic molecules and SAMs is not exclusively for PSCs, indeed some commercial molecules such as fluorobenzoic phosphonic acids have been deposited on ITO surfaces for the fabrication of organic light-emitting diodes (OLEDs).64 While techniques such as atomic layer deposition (ALD) have been employed and explored for the formation of high-quality self-assembled molecules (SAMs) using octadecylphosphonic acid in other photovoltaic applications.65
In the following sections, we will discuss in detail the protocols developed for the preparation of the most promising synthetic molecules, according to their main structural features, reported so far for the formation of SAMs in perovskite solar cells, including some interesting examples that are already patented and commercialized.
C60-SAMs
To the best of our knowledge, 2014 was reported as the first SAM for PSCs inspired by previous works on polymer solar cells, where fullerene derivatives proved to be efficient.66 Thus, the C60-substituted benzoic acid 44 (Scheme 1) was used for regular PSC devices reaching a 17.3% PCE with significantly reduced hysteresis. Compound 44 was synthesized through a 1,3-dipolar cycloaddition reaction, known as a Prato reaction, between C6041, 4-carboxybenzaldehyde 42, and N-methylglycine 43 using chlorobenzene as a solvent under reflux for 12 h. This reaction yielded the desired fullerene 44 functionalized with the carboxylic acid function as an anchoring group in 95% (Scheme 1).67 Derivative 44 is now commercialized under the name C60-SAM (CAS No. 631918-72-4) and was recently used for the fabrication of regular (n-i-p) perovskite/silicon tandem solar cells with a PCE of 27.1%.68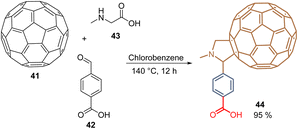 | ||
| Scheme 1 Synthesis of C60-SAM 44 through Prato reaction. (Red) Anchoring group; (Blue) spacer group; (Brown) functional group. | ||
Using a molecular hybridization strategy, where two molecular entities are bonded through a specific linker. Derivative 49 was prepared to employ a click chemistry strategy, where the commercially available C60-carboxylic acid 45 was subjected to an esterification process with the corresponding 10-undecyn-1-ol 46 to give the functionalized C60 intermediate 47 in 75% yield. Then, it reacted with the respective dihydroxy azide 48 under the traditional reaction conditions for the azide–alkyne Huisgen cycloaddition, furnishing the fullerene diol 49 in 56% yield (Scheme 2). SAMs were formed on tin oxide (SnO2) surfaces, using the diol fragments as an anchoring group, for the fabrication of planar heterojunction perovskite solar cells with PCE = 21.3%, that resulted in stable and displayed a negligible hysteresis.69 Employing the Prato reaction for the functionalization of fullerenes, compound 52 was designed and prepared based on C60-SAM 44, selecting the cyano group as an anchoring group to enhance the solubility of these derivatives and adding a thiophene ring into the pyrrolidine core to promote the interaction of the perovskite material and the sulfur atom and π–π interactions. Thus, 52 was isolated in 44% yield from C6041, N-(2-cyanoethyl)glycine 50, and 2-thiophenecarboxaldehyde 51 after 22 h at 135 °C (Scheme 3). Derivative 52 was used as an ETM in PSCs to achieve a PCE of 20.58%, which is one of the highest values reached for fulleropyrrolidine analogs reported to date in PSCs.70
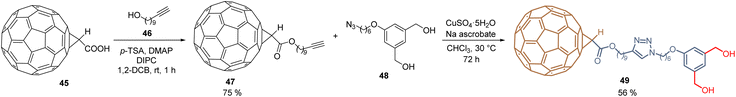 | ||
| Scheme 2 Click chemistry approach for the functionalization of fullerene core and synthesis of fullerene diol 49. | ||
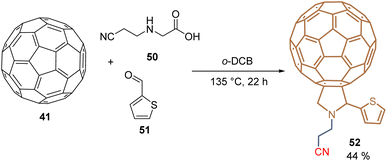 | ||
| Scheme 3 Prato reaction for the synthesis of fulleropyrrolidine 52 substituted with the thiophene moiety. | ||
Fullerenes are widely used in the construction and study of efficient PSCs, improving stability, lowering the hysteresis, and increasing the PCE.71 Although phenyl-C61-butyric acid methyl ester (PCBM) is one of the most common fullerene-based materials used in PSCs, it is mainly employed nowadays as an ETM instead of a SAM. In this sense, the Prato reaction stands out as one of the best approaches for the synthesis of fullerene-based molecules that can be used as SAM in PSCs, however, two main challenges need to be addressed: (i) explore the structural diversity of the starting materials, and (ii) study the reaction conditions, to decrease the reaction temperatures and use alternative solvents to replace traditional halogenated aromatic hydrocarbons.
Carbazole-SAMs
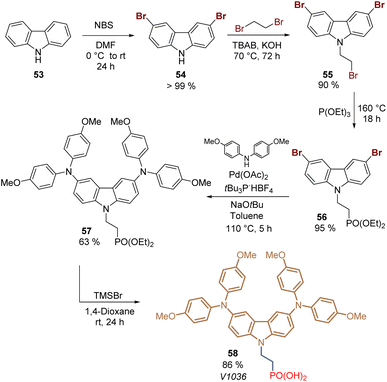
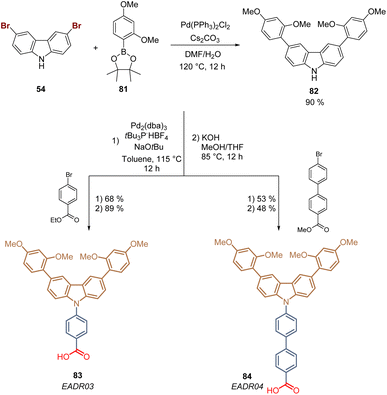
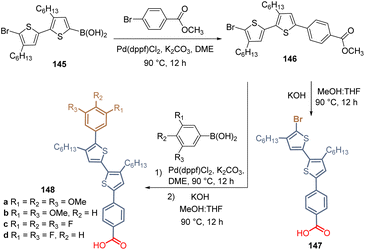
Carbazole is a tricyclic ring system based on a pyrrole ring fused with two benzene rings, and it is the presence of the electron-rich pyrrole ring that one responsible for the hole-transporting properties of this inexpensive starting material. Due to its substitution pattern, carbazoles can be easily functionalized at the nitrogen and the “benzene” rings, furnishing a huge library of small molecules and polymers with applications in photovoltaics.72 Carbazole exhibits good charge transporting ability, chemical stability, and electronic and optical properties that can be tuned by well-known organic chemistry processes, which has attracted researchers to design molecules as SAMs for PSCs.73,74The first example of a carbazole-based HTM modified with a phosphonic acid as an anchoring group used as a SAM was prepared in 2018 from carbazole 53.75 The synthesis of 58 involved 5 steps starting with the bromination of carbazole 53 to furnish 3,6-dibromocarbazole 54 (>99%), that was then alkylated with 1,2-dibromoethane to give the intermediate 55 with yield over 90%. The subsequent transformation of the aliphatic bromide into the corresponding phosphonic acid ethyl ester 56 was performed through the Arbuzov reaction with excellent yield. In the next step, the dimethoxydiphenylamine fragments were introduced via palladium-catalyzed Buchwald–Hartwig amination reaction to give the derivative 57 in moderate yield. Finally, bromotrimethylsilane (TMSBr) was used to cleavage the ester functions and obtain the desired carbazole derivative 58 in 86% (Scheme 4). Compound 58, known as a V1036, was obtained in 46% global yield and formed a layer of SAMs onto indium tin oxide (ITO) surfaces through the phosphonic acid group that acted as an anchoring group, giving a PCE of 17.8% in p-i-n PSCs devices.
Certainly, the later application in 2019 of [2-(9H-carbazol-9-yl)ethyl]phosphonic acid (2PACz) constituted a breakthrough in the development of PSCs, since the prepared devices outperformed the traditional polymer PTAA. In comparison to V1036 58, the structure of 2PACz 61 is quite simple, and this compound and its derivatives have allowed us to prepare devices with excellent efficiencies and stability. The synthesis of 2PACz 61 and its derivatives 65–71 follows the same approach as for V1036 58, involving the N-alkylation with 1,2-dibromoethane, then the Arbuzov reaction and finally the cleavage of the ethyl groups to obtain the anchoring group (Scheme 5). In the case of 2PACz 61,76 it was prepared from carbazole 53 in a 3-step lineal synthesis in 40% global yield, and although the first PCE reported was 20.9% for a SAM formed in Pb–PSCs, recently, 2PACz 61 was absorbed with methyl phosphonic acid to fabricate a Sn–Pb PSC with a PCE of 23.3% (Scheme 5).77
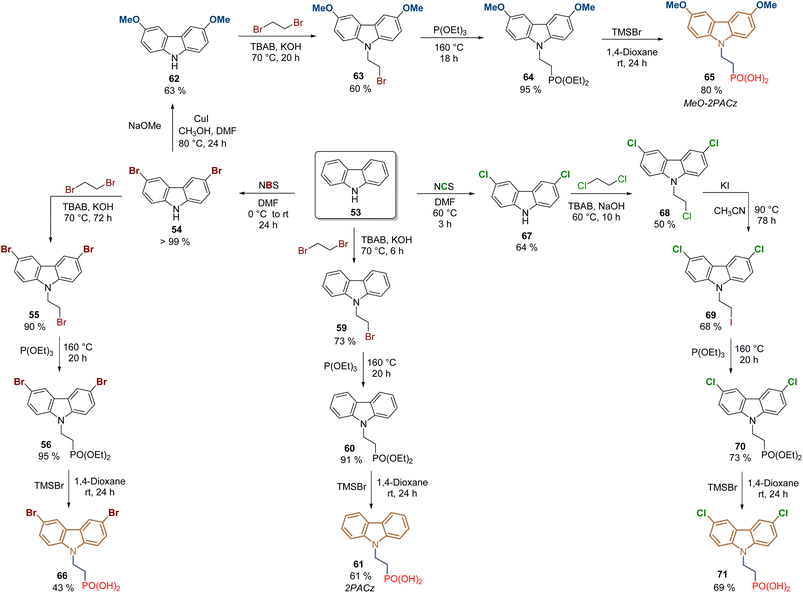 | ||
| Scheme 5 Synthesis of 2PACz 61 and its derivatives under the reactions sequence: halogenation/N-alkylation/Arbuzov reaction. | ||
For the insertion of the methoxy groups, intermediate 54 was transformed into the corresponding 3,6-dimethoxy-9H-carbazole 62 through a Cu-catalyzed methoxylation reaction in 63% yield,78 then the same approach was performed to obtain the MeO-2PACz 65 after 5 steps and in 28% global yield (Scheme 5).76 The presence of methoxy donor groups in MeO-2PACz 65 resulted in PSCs with a slightly lower PCE of 20.2% in the first study. However, currently, the synergy between 2PACz 61 and MeO-2PACz 65 is successfully applied, so when they are combined, mixed layers of SAMs are formed where the uncovered areas of one molecule are filled with the other, allowing the fabrication of flexible PSCs (PCE = 24.7%)79 and perovskite-silicon tandem solar cells (PCE = 28.3%).80
To modify the electronic nature of 2PACz 61, by introducing electron-withdrawing groups such as bromine and chlorine, compounds 66 and 71 were prepared under the same approach. The dibromo derivative 66 was obtained in 36% global yield after 4 steps from carbazole 53 and a PCE = 18.4% was reported for the PSCs devices where the SAM layer was formed on ITO surfaces.81 Although 71 was prepared to form a SAM for perovskite light-emitting diodes (PeLEDs), the synthesis of this dichloride derivative has been also reported under the same protocol but using N-chlorosuccinimide (NCS), however, the use of 1,2-dichoroethane for the N-alkylation required an additional step to exchange the halogen and increase the reactivity of 69 towards the Arbuzov reaction.82
2PACz 61 (CAS No. 20999-38-6) and MeO-2PACz 65 (CAS No. 2377770-18-6) are now commercially available references. However, it is worth mentioning that, as an alternative approach, in our group the synthesis of 2PACz analogs is carried out with the commercial diethyl 2-bromoethyl phosphonate as a starting material, reducing the synthetic steps and reactions carried out at high temperatures for prolonged times.
New derivatives have been explored to study the effect of the length of the spacer group applying the synthetic protocol of 2PACz 61, as a starting point. Thus, a series of derivatives with 2C, 4C- and 6C-aliphatic chains between the anchoring and the function group, were designed, synthesized, and applied in devices, revealing an optimum value of the fill factor for 2C and 4C derivatives. On the other hand, current–voltage hysteresis was observed in the devices prepared with SAMs of the 6-carbon chain independently from the substituent at the carbazole core.83 In this study, the methylation of 3,6-dibromocarbazole 54 was performed under Ni-catalysis in moderate yield and after the halogenation/N-alkylation/Arbuzov reactions sequence, the series of derivatives 75a–e were obtained in good to excellent global yields (Scheme 6). Compound 75b proved to be the most promising derivative to form a layer of SAM on ITO surfaces for the fabrication of monolithic perovskite/silicon tandem cells with a PCE of 29.15%.
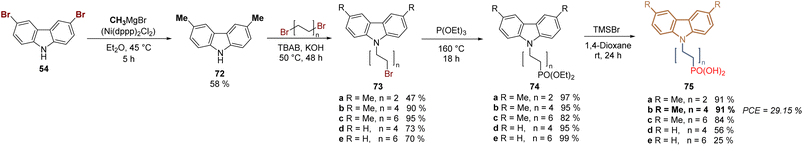 | ||
| Scheme 6 Synthesis of 2PACz-based molecules 75a–e with different spacer group lengths, n = 2, 4 and 6. | ||
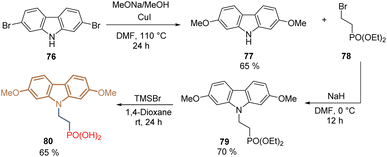
Another important study based on the structure of MeO-2PACz 65 was focused on the insertion of the methoxy groups at different positions in the carbazole core. Thus, introducing the methoxy groups at positions 2 and 7, instead of 3 and 6, allowed the preparation of compound 80 from the commercial carbazole 76 using diethyl 2-bromoethyl phosphonate 78 for the N-alkylation, furnishing the desired product in 36% global yield over 4 steps (Scheme 6). The authors found that the change in the position of the methoxy groups resulted in the reduction of the electron-donating strength in comparison to MeO-2PACz 65. This effect downshifted the highest occupied molecular orbital (HOMO) energy creating a favorable dipole moment. Thus, derivative 80 was combined with 6-(iodo-λ5-azanyl) hexanoic acid (IAHA) to form a SAM in inverted PSCs reaching a PCE = 23.59% (Scheme 7).84
In 2021, our group reported for the first time the use of aromatic rings in the spacer group in carbazole-based SAMs for PSCs. These molecules were designed inspired by our previously reported HTMs based on the triphenylamine moiety,85 and included the 1,3-dimethoxybenzene substituent at the carbazole core to align the energy levels of the molecules with the perovskite material. The synthesis started with the bromination of carbazole 53 followed by the double Suzuki coupling to give the disubstituted intermediate 82 in 90% yield. This was subsequently subjected to the Buchwald–Hartwig amination with bromo benzoic acid esters derivatives that, upon hydrolysis, furnished the derivatives 83 (89%) and 84 (48%). Each molecule had one and two aromatic rings as spacer groups, respectively, with the carboxylic acid function as an anchoring group (Scheme 8).86 Both molecules formed good quality SAM layers on ITO giving outstanding efficiencies and excellent lifetimes. The derivative 83, also known as EADR03, gave PCE of 21.2% and is currently commercialized while the derivative 84, EADR04, has demonstrated promising results in the increase of the efficiency and lifetime in PeLEDs.87
Conjugated and aromatic spacer groups exhibit effective hole-transporting capabilities in comparison with the aliphatic linkers, along with higher stability due to the stabilization of the electron-rich carbazole core through electron/charge delocalization. This was also demonstrated with a series of molecules containing the same functional group that MeO-2PACz 65, but with one and two aromatic rings as a spacer group, in addition to the cyanoacetic acid moiety as an anchoring group. Compounds 88 and 91 were prepared from 3,6-dimethoxy-9H-carbazole 62 through a Ullmann-type reaction to give the N-phenyl carbazole intermediates 87 and 88 good to excellent yields (Scheme 9). Carboxaldehyde derivative 87 was subjected to a traditional Knoevenagel condensation with cyanoacetic acid to furnish the final product 89 in 48% global yield, while the bromine analog 88 was transformed into the corresponding carboxaldehyde intermediate via Suzuki coupling in 74% yield to finally introduce the anchoring group, obtaining the compound 91 in 35% global yield. Both molecules formed SAM layers on ITO giving similar results in terms of efficiency (PCE ≈ 20%), slightly lower in comparison to analogues 83 and 84. However, it is important to highlight that the strong electron-withdrawing properties of this anchoring group resulted in beneficial charge delocalization, and the passivation of the perovskite material due to the presence of the –CN.88
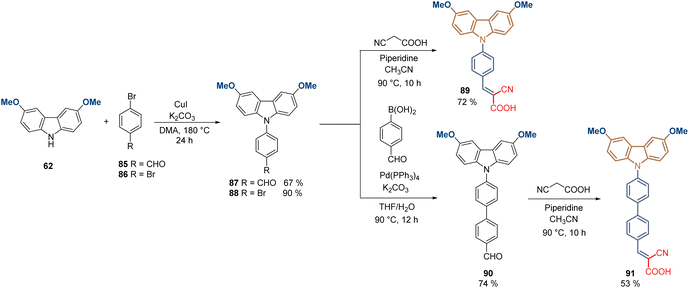 | ||
| Scheme 9 Synthesis of molecules 89 and 91 with conjugated spacer groups and cyanoacetic moiety as anchoring group. | ||
One of the latest structural modifications performed on the 2PACz 61 structure has been the asymmetric and helical π-expansion of the carbazole core and the use of a 4C aliphatic chain as a spacer group to obtain derivatives with higher dipole moment and strengthen the π–π interactions. Under this approach, commercially available 7H-benzo[c]carbazole 92 and 7H-dibenzo[c,g]carbazole 93 has been employed as starting materials to perform the reaction sequence: N-alkylation/Arbuzov reaction/deprotection, giving the corresponding (4-(7H-benzo[c]carbazol-7-yl)butyl)phosphonic acid 98 (asymmetric π-expansion) and (4-(7H-dibenzo[c,g]carbazol-7-yl)butyl)phosphonic acid 99 (helical π-expansion) in 66% and 63% global yield, respectively (Scheme 10). Both compounds exhibited excellent photo and thermal stability under sunlight and at temperatures above 366 °C. A PCE of 24.1%, with improved device stability was achieved by compound 99 in an inverted PSC. The higher dipole moment of 99 (2.4 D-calculated), down-shifted the WF to −5.15 eV and induced a more efficient hole extraction to achieve a FF of 83.39% and a VOC of 1.17 V.89
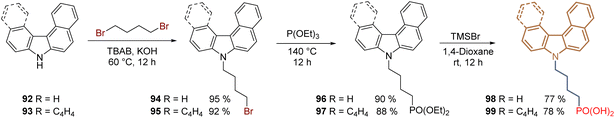 | ||
| Scheme 10 Synthesis of π-expanded carbazoles 98 and 99 as an approach for the formation of stable and efficient SAMs. | ||
The derivative 99 stands out as one of the most promising SAMs for PSCs, indeed, this compound has been also recently used to form a SAM layer in perovskite tandem solar cells with a PCE = 27.0%, FF = 82.6 and VOC 2.12 V.90
In conclusion, carbazoles have shown their potential to act as excellent cores for the synthesis of HTMs with application in perovskite solar cells, increasing the PCE from 17.8 to 24.1%. Despite its low cost, high carrier mobility, chemical stability, and easy functionalization, the commercialization of the carbazole derivatives will come with the improvement of cost-effective synthetic methods. On the other hand, the increase in the PCEs will require the exploration of other functional groups along with the study of cost-effective synthetic protocols.
TPA-SAMs
The triphenylamine (TPA) group is a strong electron donor moiety commonly employed in molecules used as an HTM in several optoelectronic applications such as organic light-emitting diodes (OLEDs), Dye-Sensitized Solar Cells (DSSCs), and organic field-effect transistors (OFETs). This fragment has a butterfly-like shape with a sp2 central nitrogen that generates a non-planar structure, in comparison with the rigid carbazole core, controlling the packing of the molecules, which is very useful during the formation of layers of SAMs.91The TPA fragments show good thermal and morphological stabilities, ionization potentials, as well as excellent charge transport properties.92 The nitrogen atom in the TPA core can be easily oxidized and stabilized by hyperconjugation electronic effects, resulting in high hole mobilities. For these reasons, the TPA fragment is the main core in two of the most important HTMs used in PSCs nowadays: the 2,2′,7,7′-tetrakis[N,N-di(4-methoxyphenyl)amino]-9–9′-spirobifluorene (spiro-MeOTAD)93 and the poly[bis(4-phenyl)(2,4,6-trimethylphenyl)amine] (PTAA).94
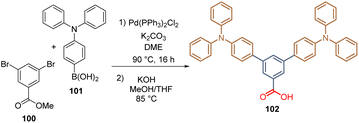
Inspired by our previous studies in DSSCs, our group reported for the first time the introduction of the TPA in two derivatives for the formation of SAMs for PSCs. The first molecule included two TPA fragments attached to a benzene ring, a spacer group, and a carboxylic acid function as an anchoring group.95 The synthesis of this derivative involved the double Suzuki cross-coupling between methyl 3,5-dibromobenzoic acid 100 and 4-(diphenylamino)phenyl boronic acid 101 to give an intermediate that was then hydrolysed to obtain the desired compound 102 (Scheme 11).
The second molecule, 109, prepared in our group was a structural analog of 84, substituting the carbazole core with a TPA moiety, which is attached to the spacer group (two aromatic rings) and possesses two electron-donating fragments substituted with dimethoxy groups. The synthesis of 109 starts with the double Suzuki coupling between 4-bromo-N-(4-bromophenyl)-N-phenyl aniline 103 and 2-(2,4-dimethoxyphenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane 104. The obtained intermediate 105 (92%) is then brominated with NBS in good yield (88%) resulting in the functionalized TPA fragment 106, which upon a Suzuki reaction with [4-(methoxycarbonyl)phenyl]boronic acid 107, gives the key intermediate 108 in 74% yield. Finally, the hydrolysis generates the corresponding anchoring group, and the desired compound 109 is obtained after 4 steps in 50% global yield (Scheme 12).96 Molecules 102 and 109 formed layers of SAMs on ITO and were used in the fabrication of p-i-n PSCs. Due to the electron-donating groups introduced in the TPA backbone, a PCE = 17.3% was achieved because of the reduction of the oxidation potential of 109 in comparison to 102. Although the PSCs devices fabricated with 84 and 109 have a different architecture, closing the TPA fragment and generating the pyrrole ring to form the carbazole derivative 109, enhances the PCE to 20%.
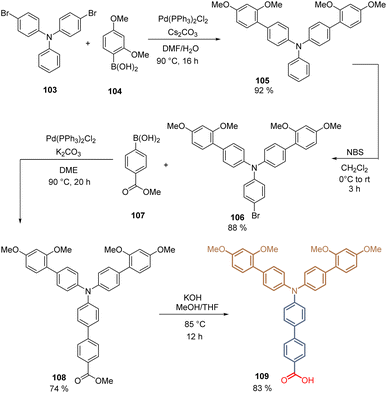 | ||
| Scheme 12 Synthesis of derivative 109 introducing the donor dimethoxyphenyl fragment on the TPA moiety. | ||
Further, we examined the role of the substitution pattern in the TPA fragment by modifying the positions of the methoxy substituents. Thus, disubstituted molecules: ortho–para113a, ortho–meta113b, and meta–para113c were prepared in a three-step synthetic route starting from 4-bromo-N-(4-bromophenyl)-N-phenyl aniline 103 that was formylated through the Vilsmeier–Haak reaction to furnish the carboxaldehyde 110. This intermediate was then subjected to the Suzuki coupling with different disubstituted boronic acids 111a–c to obtain compounds 112a–c in good to excellent yields (85–90%). Finally, the oxidation with KMnO4 furnished the desired compounds 113a–c with the carboxylic acid as an anchoring group in moderate yields (Scheme 13).97
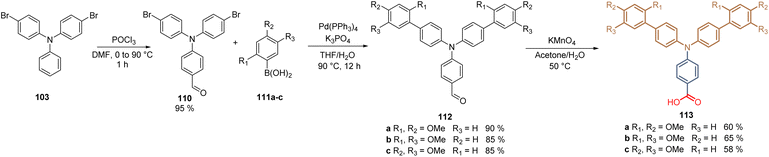 | ||
| Scheme 13 Synthesis of molecules 113a–c prepared to study the role of the substitution pattern in TPA-based SAMs in PSCs. | ||
Depending on the substitution pattern, the work function and surface properties of the electrode changed, and following theoretical calculations, the ortho–para113a derivative gave the best results with a PCE = 19.8%. This is because the methoxy groups at ortho–para positions contributed to forming a well-ordered array during the SAM deposition, enhancing its stability and bonding on the metal oxide surface as well.
In 2020, it was reported the combination of the benzothiadiazole core with the TPA in the design of HTMs and SAMs for PSCs. This combination arose from previous experience on the synthesis of several metal-free dyes used in DSSCs following the strategy of the donor–acceptor backbone, where the benzothiadiazole core acted as a spacer and the 2-cyanoacetic acid as the anchoring group. The resulting dyes enhanced the intramolecular charge transfer, the JSC values, and the solubility of the corresponding compounds in polar solvents. Thus, in the first approach, MeOTPA-boronic ester 114a reacted with benzo[c][1,2,5]-thiadiazole carbaldehyde 115via Suzuki coupling to give intermediate 116 in excellent yield (95%), the final Knoevenagel condensation with cyanoacetic acid afforded the HTM 117 in 75% yield allowing the formation of SAM on ITO to fabricate a PSCs device that displayed a PCE of 21.24% (VOC = 1.13 V, JSC = 22.25 mA cm−2, FF = 84.8%) (Scheme 14).98 The same research group explored the effect of different anchoring groups in the TPA-benzothiadiazole moiety. For that, from intermediate 116, and employing rhodanine-3-propionic acid 118 as a nucleophile, the Knoevenagel reaction furnished the derivative 119 in 71% global yield for the fabrication of PSCs that showed a PCE of 19.65% (VOC = 1.10 V, JSC = 22.03 mA cm−2, FF = 81.1%). The low performance of the rhodamine fragment as the anchoring group was attributed to the aggregation in the solid state of 119 due to the sp3 hybridization of the carbon atom on this group, affecting the assembling of the molecules. While the preparation of 122 required the previous synthesis of 120 from commercially available reagents, and after the Suzuki coupling and hydrolysis, a TPA-benzothiadiazole derivative with the carboxylic acid 122 as an anchoring group was obtained in 21% global yield (Scheme 14). The PSC devices prepared with the SAM formed by 122 displayed a PCE of 20.58% (VOC = 1.12 V, JSC = 22.76 mA cm−2, FF = 81.0%).99
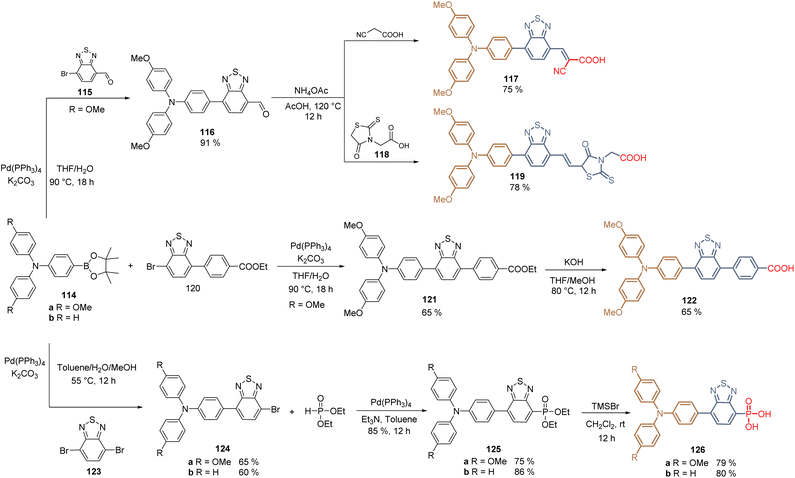 | ||
| Scheme 14 Synthesis of TPA- TPA-benzothiadiazole HTMs for the fabrication of SAMs with different anchoring groups. | ||
Finally, the introduction of the phosphonic acid function as an anchoring group in the TPA-benzothiadiazole moiety was recently achieved through the stoichiometrically controlled Suzuki reaction between MeOTPA 114a and TPA 114b and dibromobenzothiadiazole 123. The reaction gave the intermediates 124a an b in moderate yields (60–65%), which were modified by the Michaelis–Becker reaction to furnish the phosphonates 125a and b in good yields (75–86%). The corresponding ester functions were cleaved using TMSBr to obtain compounds 126a and b (79–80%) (Scheme 14). The corresponding SAMs interacted with ITO through the oxygen of the anchoring group and the sulfur of the spacer group, forming a preferred “face-on” molecular orientation that reduced the hole injection barrier, constituting a new insight for SAMs. Interestingly, the devices fabricated with 126b resulted to be more efficient (PCE of 23.24%, VOC = 1.14 V, JSC = 24.83 mA cm−2, FF = 82.0%) than the ones with the 126a (PCE of 21.52%, VOC = 1.10 V, JSC = 24.70 mA cm−2, FF = 79.2%), that contained the methoxy donor groups in the TPA core.100
As a concluding remark, the molecules based on the TPA-benzothiadiazole moiety 117, 119, 122 and 126a and b, show the beneficial effect of the benzothiadiazole core, where the planarity of this spacer group enhances the intermolecular stacking, while the sulfur atom can interact with undercoordinated Pb2+ ions via coordination bonds. Finally, passivation effects were also observed for molecule 126b containing the phosphonic acid function as an anchoring group.
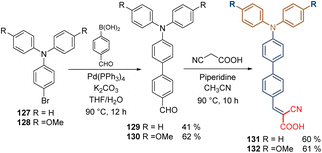
The same authors that reported the synthesis of carbazole-based molecules with conjugated spacer groups (See Scheme 9), studied the effect of the TPA moiety using two aromatic rings as a spacer group in combination with the cyanoacetic acid as an anchoring group. The synthesis of compounds 131 and 132 involved a traditional Suzuki coupling from the commercial TPA-Br derivatives 127 and 128, followed by a Knoevenagel condensation to insert the corresponding anchoring group (Scheme 15).88 Indeed, the performance of compound 132 in inverted PSCs was better than its related carbazole derivative 91 exhibiting a PCE = 22.53%, (vs. PCE = 20.66% of 91), and enhanced stability under UV light.
Similar results have been obtained recently with a TPA derivative containing just one aromatic group as a spacer group and the cyanoacetic acid moiety as an anchoring group when the SAM is formed onto a NiOx surface (PCE = 20.94%).101
Based on the synthetic concepts described in this section, we can conclude that the discovery of the next generation of molecules containing the triphenylamine core, and taking into account the excellent PCEs of TPA-based SAMs PSCs, efforts are required to achieve the facile and cost-effective synthetic routes. In addition, the commercial availability of building blocks containing TPA makes it easier to explore alternative donor moieties on the TPA core, and combinations with different spacers and anchoring groups.
Phenothiazine-SAMs
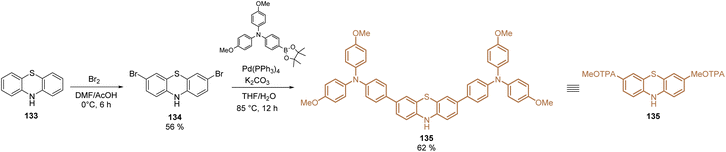
Phenothiazine derivatives have been extensively used in DSSCs as organic sensitizers.102 However, during the last year this structure has been raised as one of the most promising materials for SAMs in PSCs.103 Phenothiazine is a well-known electron-rich heterocyclic compound whose main properties are the presence of the electron-donating sulfur and nitrogen heteroatoms, and its nonplanar butterfly conformation, that prevents intermolecular aggregation, leading to efficient hole-transport capability.104The optical, electrochemical, and photovoltaic properties of HTMs based on the phenothiazine core have inspired the design and synthesis of small molecules for SAMs. The first study was published in 2019 with the synthesis of a unique phenothiazine unit functionalized with TPA groups 135. Using commercial phenothiazine 133 as the starting material, a bromination reaction was performed to selectively activate positions 3 and 7 and proceed with a Suzuki coupling to obtain the fragment 135 in good yield (65%), with both excellent hole-extraction/transportation fragments (Scheme 16).105
The same authors then reported the introduction of an alkyl chain at the 10-position of phenothiazine 135 and also decorated the terminal position of this chain with different anchoring groups: –COOH 137, –SO3H 138, and –PO(OH)2139.106 These transformations were performed via a nucleophilic substitution reaction with the respective alkyl bromides under basic conditions. In general, compounds 136–139 were obtained in moderate to good yields (47–79%) using cheap and accessible starting materials (Scheme 17).
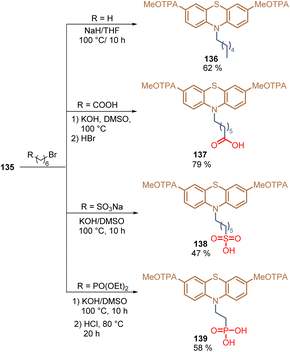 | ||
| Scheme 17 Synthesis of phenothiazines with different anchoring groups: –H 136, –COOH 137, –SO3H 138, and –PO(OH)2139. | ||
Using compound 136, without any anchoring group as a reference, the adsorption and layer formation of SAMs 137–139 on ITO substrates were studied for the fabrication of PSCs. Among these anchoring groups, the phosphonic acid functionality of 139 exhibited the strongest anchoring interaction giving the highest PCE (21.93%).
With the idea to reduce the molecular complexity compared with phenothiazine derivatives 136–139 and test the effect of electron-withdrawing groups in this core, bromide groups were included at positions 3 and 7 through a rapid and straightforward synthetic route. It started from phenothiazine 133 that was N-alkylated to give intermediate 140, whose hydroxyl function was transformed into a terminal bromide group in 141 (Scheme 18). The tribromated derivative 142 was obtained after Br2 treatment and the subsequent Arbuzov reaction followed by the cleavage of the ethyl groups furnished the dibromo phenothiazine 144 in 9% global yield.107 The tailored SAM 144 was used in p-i-n PSCs, exhibiting a PCE = 22.44%, with FF = 82%, being one of the highest efficiencies reported for p-i-n PSCs.
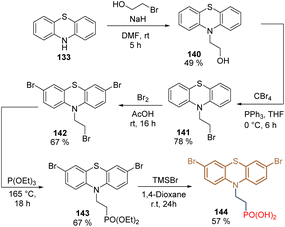 | ||
| Scheme 18 Synthesis of phenothiazine 144 substituted with electron-withdrawing groups at positions 3 and 7. | ||
The bulky sulfur atom present in the phenothiazine core helps to prevent the effective π–π stacking of molecules, facilitating their assembly and increasing the solubility of these derivatives in various organic solvents. So, based on these facts, the same research group reported the design and synthesis of two variants of 144 by replacing the S with oxygen and selenium in the head group of the phenothiazine core. To increase the global yields, another strategy was performed for the synthesis of these derivatives. The corresponding phenothiazine 133, phenoxazine 133a, and phenoselenazine 133b were subjected to an acylation reaction to obtain derivatives 145 in good yields and with the terminal bromide already installed in the alkyl chain. The following reduction of the carbonyl function promoted by the borane–tetrahydrofuran complex afforded intermediates 141 in good yields, that were finally transformed using the same protocol as for 144 (bromination/Arbuzov/deprotection) (Scheme 19).108
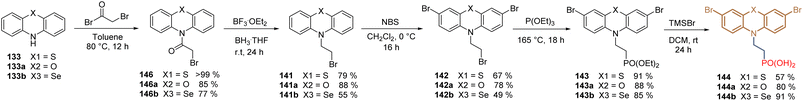 | ||
| Scheme 19 Phenothiazines derivatives incorporating sulfur 144, oxygen 144a and selenium 144b on the head group. | ||
Derivatives 144 formed a well-assembled layer providing an adequate interface with the perovskite layer to reduce the interfacial trap density and enhance the charge-carrier lifetime. Although the SAMs showed negligible differences in the device parameters, it was found that the interfacial interaction energies between the SAMs and the perovskite layer were strongly influenced by the polarizability of the heteroatoms present at the phenothiazine core, which increased in the order of Se > S > O. This was reflected on the PCE, being 144b-Se (22.73%) > 144-S (21.63%) > 144a-O (21.02%).
One of the main reasons why phenothiazine is captivating the interest of several research groups, besides the promising results obtained in PSC devices, is its low cost as a building block, which allows for the sustainable synthesis of stable HTMs with different tuneable photo- and electrochemical properties due to its well-known reactivity.
Thiophene-SAMs
Thiophene, and other related S-rich heterocyclic derivatives such as thienopyridine, thienothiophene, and bithiophene imide, have been used as π-type linkers in different HTMs. The flat, rigid, and good electron delocalized skeleton in the thiophene ring, plus the prolonged molecular conjugation and intermolecular S⋯S interactions, results in HTMs with good hole mobilities and conductivities for PSCs.109The first work in which thiophene rings were used as spacers was reported in 2020. It consisted of the synthesis of bithiophene derivative 147 after the Suzuki coupling followed by the corresponding hydrolysis to obtain the carboxylic acid function as an anchoring group (Scheme 20). Compound 147, having the bromide atom as a functional group, formed a layer of SAM on the ITO electrode and the PSC device showed a PCE = 11.89% in a p-i-n type device configuration.110
The same authors then introduced a series of aromatic rings via Suzuki coupling at the brominated position of 146, with different electron-donating (MeO–) and withdrawing (F–) characteristics, obtaining a series of derivatives 148. Unfortunately, the yields are not reported in any of their works (Scheme 20). In this study, the highest PCE (12.03%) was achieved with the compound 148b, substituted with two methoxy groups at positions 3 and 5. Later, they reported the introduction of TPA and carbazole moieties as a functional group in the structure of bithiophene 146 (Scheme 21). Thus, using the same synthetic approach, Suzuki coupling/hydrolysis, the series of derivatives 150a–c were obtained and used in the fabrication of p-i-n type PSC devices.111 Although the efficiency showed by these devices resulted to be slightly higher than for the ones prepared with compounds 147 and 148a–d, the best PCE (13.71%) was achieved by compound 150b, having the MeOTPA core as a functional group.
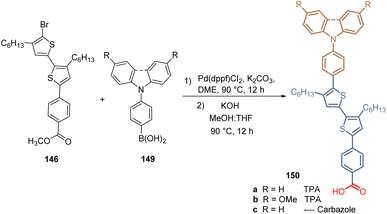 | ||
| Scheme 21 Synthesis of bithiophene derivatives 150a–c having the TPA and carbazole moieties as functional groups. | ||
Finally, the same research group continued with their work by designing two molecules 152a and b for the formation of bidentate SAMs by reacting the intermediates 146 with the TPA-diboronic acid derivative 151 under their standardized synthetic protocol (Scheme 22). Unfortunately, the efficiency did not exceed 13.12% for derivative 152a, although this concept of two anchoring groups in the same molecular architecture would be interesting to obtain better SAMs. It was found that the presence of hexyl chains could create a steric hindrance that would affect the assembled process during the SAM formation.112
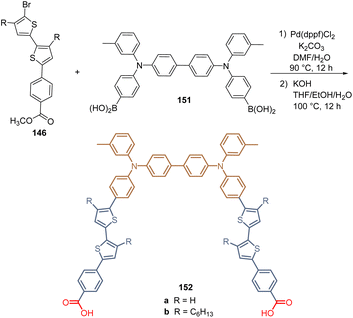 | ||
| Scheme 22 Synthesis of the thiophene derivative 152 with two anchoring groups for the formation of bidentate SAM. | ||
The layers of SAMs not only can be formed on metal oxide surfaces, as discussed in the sections above. The crystalline perovskite layer could also serve as a template for the ordered assembly of molecules containing the same elements as a SAM: anchoring, spacer, and functional groups. This approach was reported in 2021 where three molecules with one thiophene unit as a spacer group were prepared via Suzuki coupling/Knoevenagel condensation protocol starting from thiophene carboxaldehyde 153 and different boronic acids 154. The respective intermediates 155 were then condensed with cyanoacetic acid and the desired molecules 156a–c were obtained (Scheme 23). The reaction yields unfortunately were not reported although the dipole moment of these molecules was determined, being 11.33 D the highest one for the compound 156c with the dimethylaminophenyl moiety as a functional group. The dipole moments play a key role in the preferential orientation of the assembled molecules on the perovskite surface. This is due because the functional group (which has the highest electron density) interacts with the undercoordinated Pb(II) of the perovskite, promoting the growth of a layer of SAMs on the top. As a result, the PSCs device using compound 156c, with the highest dipole moment, exhibited a PCE of 21.4%.113
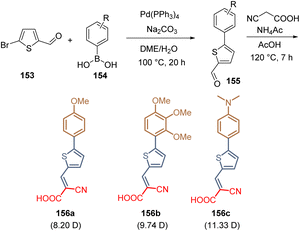 | ||
| Scheme 23 Examples of molecules with one thiophene ring are a spacer group, a similar anchoring group, and different functional groups showing different dipole moments. | ||
The works discussed in this section are focused exclusively on the use of thiophene as a spacer group. Besides compound 156c, the PSC devices fabricated with the other related molecules show poor efficiencies. However, S-rich heterocyclic compounds can be still applied in the design of SAMs, because they form part of efficient HTMs. In the case of small molecules designed as SAMs, these electron-rich systems should be used as a functional group instead of a spacer to take advantage of the well-known interaction and passivation of the Pb by the heterocyclic sulfur atoms.
Uncommon-SAMs
In the last section, we will cover the application of a series of SAMs that do not belong to any of the families of compounds already described, thus they are classified as “uncommon” SAMs. These molecules possess a unique structure keeping at the same time the requirements for SAMs, prepared through elegant synthetic approaches.In 2019, the naphthalimide core was introduced as a spacer and as a functional group. Thus, exploiting the reactivity of naphthalic anhydride 157, the electron-rich piperidine fragment was incorporated through nucleophilic aromatic substitution to give 158 and, after the condensation with β-alanine tertbutyl ester, 159 was hydrolysed into 160 with a global yield of 27% (3 steps). On the other hand, compound 163, with the 4-trifluoromethylphenyl electron withdrawing fragment, was prepared similarly but performing first the condensation reaction to furnish 161, then the Suzuki coupling and finishing with the hydrolysis to give 163 52% global yield (3 steps) (Scheme 24). In both compounds 160 and 163, the naphthalimide core acted as a spacer group, however, the performance did not exceed 6% (163, PCE = 5.66%). In contrast, when the naphthalimide core was used as a functional group, such as in 165, the PCE increased up to 14.92%. This molecule was prepared from the naphthalenetetracarboxylic acid anhydride 164 to obtain the bidentade molecule with a 90% yield (Scheme 24).
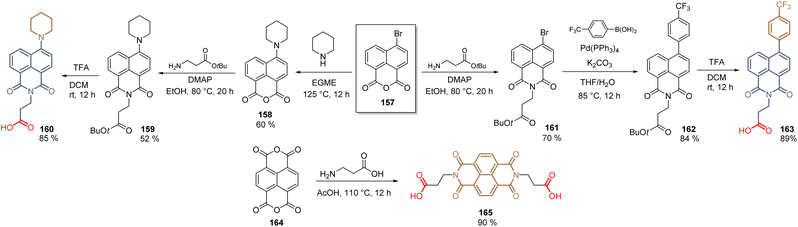 | ||
| Scheme 24 Synthesis of naphthalimide derivatives 160, 163, and 165 for the formation of SAMs in n-i-p perovskite solar cells. | ||
The increase in the PCE for SAM 165 is due to the planar interaction between the functional group and the perovskite layer that enhances the electron extraction.114
Ionic liquids have also been applied as SAMs due to the reactivity of hydroxyl groups towards metal oxide surfaces and the high conductivity, wide electrochemical window, and excellent thermal stability. The hydroxyethyl functionalized imidazolium iodide 169 was obtained as a solid at room temperature in high yield through the Ullman coupling between bromide 166 and imidazole 167, followed by the metathesis with 2-iodoethanol (Scheme 25). The layer of SAMs formed by 169 onto the FTO substrate lowered the work function of the conductive electrode and increased the interfacial electron extraction, retarding at the same time the charge recombination, resulting in a PCE = 17.31%. This work demonstrated the potential of ionic liquids as SAMs to reduce the number of layers in the devices and the associated fabrication costs.115
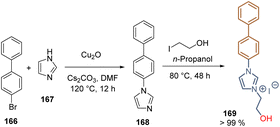 | ||
| Scheme 25 Preparation of functionalized ionic liquid 169 for the deposition of SAMs on the FTO surface. | ||
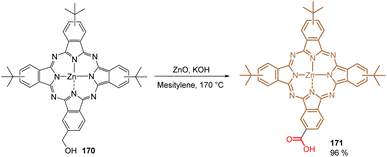
In 2020, our group reported what has been so far the only organometallic compound used to form a layer of SAMs for PSCs. Phthalocyanine 171 is efficiently attached to metal oxide surfaces such as ITO and gives promising results when applied in DSSCs. We thus reported the direct transformation of the hydroxyl methyl phthalocyanine 170 into its corresponding carboxyl derivative 171 catalysed by ZnO in high yield (96%) (Scheme 26). Traditionally, this transformation requires strong oxidants and harsh conditions that may provoke the demetallation of 170, furnishing a complex mixture of crude materials that is difficult to purify. Under our reaction conditions, 171 was obtained in a clean protocol and tested in PSCs as a SAM, exhibiting a PCE of 13.11% and establishing that the open-circuit voltage and overall efficiency are correlated to the differences in the energy levels rather than recombination kinetics.116
Besides some commercial molecules, all the synthetic molecules used to form SAMs contain tertiary amines as functional groups. However, primary amines, especially the protonated amines groups, could also passivate the perovskite layer and create a 2D perovskite sublayer between the SAM and the active 3D-based perovskite layer. Bearing this idea in mind, two molecules with protonated amino groups as functional groups were designed and prepared easily from commercial and cheap precursors. The molecules, 177a and 177b are made of the biphenyl moiety as a spacer group to promote the self-assemble process and ensure conductivity, and methylene groups to add flexibility to the SAM, facilitating the bonding to the surface and the interaction with the perovskite layer. Starting from benzonitrile 172 and boronic acid 173, intermediate 176 with the anchoring group directly connected to the spacer group was obtained and subjected to a nitrile reduction to afford the first compound 177a in good yield (Scheme 27). On the other side, exchanging the functionalities in precursors 174 and 175, product 177b having the methylene group on both sides of the molecule was prepared in good yield as well (Scheme 27). It is worth highlighting that the Suzuki couplings reported in this study were performed under mild conditions and using the more accessible and stable Pd/C catalyst instead of the traditional Pd(PPh3)4 approach, opening the door to its implementation in this kind of transformation.
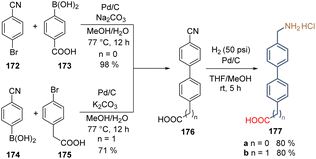 | ||
| Scheme 27 Synthesis of molecules 177a and b bearing a methylene group to induce flexibility on the layer of SAMs formed on the ZnO surface. | ||
The deposition of SAMs formed by 177a and b was done onto the ZnO surface and, despite the modest PCE of 8.8% for 177b, this work demonstrated that the free rotation of SAMs, promoted by the –CH2- groups, contributes to the spatial adaptation and bonding to the metal surface. Moreover, it also affects the interaction and adaptation of the perovskite network.117
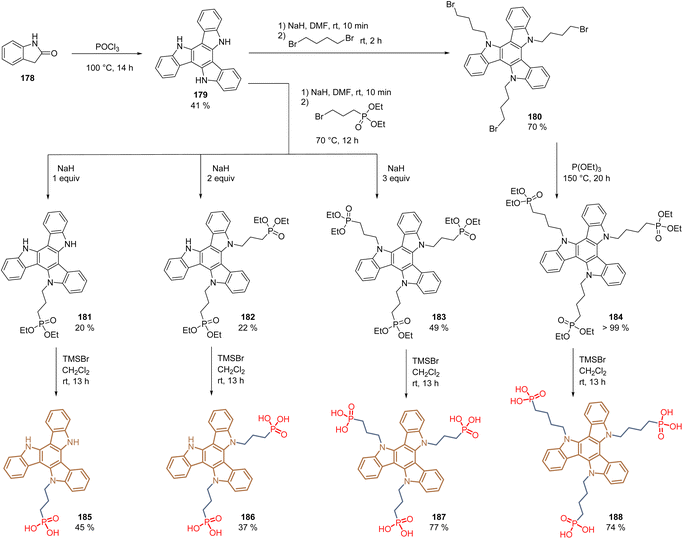
The concept of molecules having two anchoring groups resulted attractive in studies with molecules such as 49, 152, and 165. However, this approach was extended recently to molecules with multiple anchoring sites, creating tridentate SAMs. The bonding of a molecule to a metal surface through one anchoring group usually orients the π-plane of the functional group perpendicular to the metal surface and the perovskite layer. Through the multipodal strategy, the π-conjugated backbone of the functional group is face-on orientated to the substrate and the perovskite surface, resulting in an efficient orbital overlap that suppresses the interfacial recombination and enhances the hole extraction. Under this approach, the structure of triazatruxene 179, prepared from 2-oxindole 178 in moderate yield, was functionalized via N-alkylation to obtain derivatives 180, substituted with three C4-alkyl chains, and intermediates 181–183, that depending on the number of equivalents of NaH employed, generated the corresponding the mono-, di- and trialkylated products with C3-alkyl chains (Scheme 28). In the case of derivatives 181–183, simple hydrolysis using TMSBr gave the desired monopodal 185, dipodal 186, and tripodal 187 compounds with the phosphonic acid function as anchoring groups. The derivative 180 was first subjected to the Arbuzov reaction followed by the cleavage of the ethyl groups to obtain the corresponding tripodal compound 188 substituted with three C4-alkyl chains and the phosphonic acids as anchoring groups (Scheme 28).
 | ||
| Scheme 28 Synthesis of the triazatruxene derivatives 185–188 for the formation of multipodal SAMs in PSCs. | ||
In general, the reactions proceeded well, except for derivatives 181 and 182, where despite controlling the equivalents of NaH, side reaction occurred, generating by-products that hindered the purification processes.
Compounds 185–188 form a layer of SAMs on both ITO and FTO surfaces via mono-, bi- and tridentate binding modes. The PSC devices fabricated with these molecules gave the following PCEs: 185 (21.1%), 186 (22.2%), 187 (23.0%), and 188 (22,1%), demonstrating the effectiveness of the tripodal mode in comparison to mono-and dipodal modes. Moreover, it was shown that the spacer length of 3-carbon atoms is more adequate to achieve a face-on orientation, without affecting the charge transport, in comparison with the 4-carbon atom spacer group.118
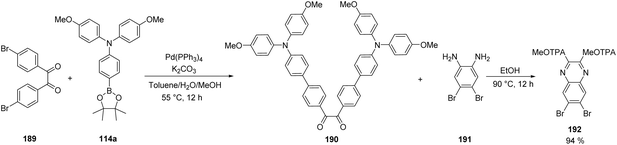
Quinoxalines are a class of heterocyclic compounds in which two nitrogen atoms replace two carbons in the ring of naphthalene. These moderate electron-withdrawing cores have a low synthetic cost, whereas the pyrazine heterocycle facilitates chemical modification giving the possibility of obtaining molecules with different shapes. Thus, a series of quinoxalines 197–200 were prepared through various synthetic steps pursuing the design of novel compounds with a quinoxaline core in the spacer and exhibiting an X-shape, where the four reactive sites are symmetrically substituted.
One of the most used protocols for the synthesis of quinoxalines is the condensation of ortho-diamines with 1,2-diketones. This strategy was implemented using the modified diketone 190, in which the donor MeOTAP fragments were introduced via Suzuki coupling between substrate 189 and boronic ester 114a. Then, the condensation of 190 with diamine 191 successfully afforded intermediate 192 in excellent yield, containing two MeOTAP fragments as functional groups and the quinoxaline core functionalized with two bromides to be transformed in the next steps (Scheme 29).
 | ||
| Scheme 29 Preparation of quinoxaline 192 containing two MeOTAP fragments as functional groups via condensation reaction. | ||
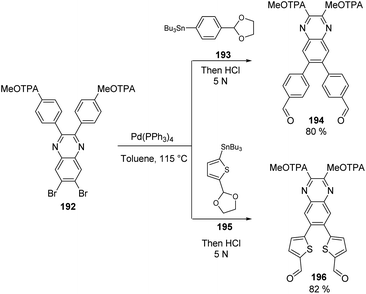
The intermediate 192 was then used as a precursor in the Stille coupling reaction with different organostannanes, 193 and 195, to obtain the derivatives 194 and 196 in good yields (Scheme 30). With this transformation, the spacer groups were completed, being the quinoxaline-phenyl core the one for 194 and the quinoxaline-thiophene core for 196. The aldehyde functions on precursors 193 and 195 were protected as acetals due to their sensitivity under the Stille reaction conditions, but a routine acidification during the work-up process restored these functional groups needed in the further steps to insert the anchoring groups.
 | ||
| Scheme 30 Synthetic route for the construction of the spacer group around the quinoxaline core in derivatives 194 and 196. | ||
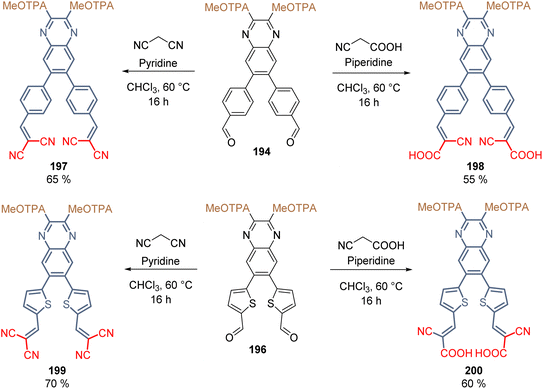
Finally, the two anchoring groups (CN/CN and CN/COOH) were introduced in each derivative 194 and 196 through the Knoevenagel condensation (Scheme 31). For the insertion of the CN/CN anchoring group, the reactions were performed using malononitrile and pyridine as a base, while the insertion of CN/COOH was achieved with cyanoacrylic acid and piperidine as a base (Scheme 31). Products 197–200 were obtained in moderate yields and, according to their substitution pattern, they exhibited a well-defined X-shape around the quinoxaline spacer group.
 | ||
| Scheme 31 Synthesis of the X-shape derivatives 197–200 with the MeOTAP donor group and nitrile functionalities as anchoring groups. | ||
The layer of SAMs was formed on ITO using molecules 197–200 and Sn-based PSCs were fabricated with these materials, obtaining acceptable efficiencies. The PCE values were 6.1% (197), 7.1% (198), 8.0% (199) and 8.3% (200), where the derivative with thiophene π-extended conjugation and the cyanoacetic moiety as anchoring group exhibited the highest hole extraction rates, greatest hole mobilities, and slowest charge recombination, resulting in the best device performance ever reported in Sn-PSCs.119
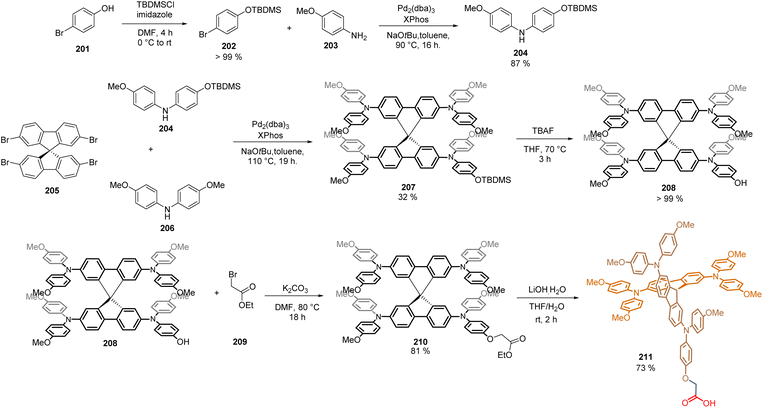
The commercially available Spiro-MeOTAD is one of the most studied HTMs in high-efficiency PSCs, achieving a record PCE = 25.8% in the n-i-p configuration. Inspired by this, our group recently reported the synthesis of a Spiro-MeOTAD derivative featuring a carboxylic acid unit to act as an anchoring group. The synthesis of this Spiro-acid compound 210 required the preparation of intermediate 208, in which one of its methoxy groups in the TAP core is replaced by a hydroxyl group. Thus, bromo phenol 201 was efficiently protected using tert-butyldimethylsilyl chloride as a protecting group. Later, 202 was coupled via Buchwald–Hartwig reaction with anisidine 203, to yield diphenylamine mono-methoxylated 204 (87%) (Scheme 32). Then, the 2,2′,7,7′-tetrabromo-9,9′-spirobifluorene 205 was subjected to another Buchwald–Hartwig tetra-amination reaction using the intermediate 204 and the commercial bis(4-methoxyphenyl)amine 206. At this point, the crucial step of the synthetic protocol was the isolation and purification of the desired product 207, since during this reaction, a complex mixture of by-products was formed. However, once the product 207 (32%) was obtained and characterized, the O-deprotection furnished the hydroxyl compound 208 in quantitative yield and was then coupled, through a Williamson ether reaction with the commercially available ethyl bromoacetate 209, to obtain the ester 210. Then, it underwent a saponification reaction with lithium hydroxide to give Spiro-acid 211 in 16% overall yield (Scheme 32).
The SAM 211 was deposited on the ITO surface in the p-i-n configuration of the PSC giving PCE = 18.15%. The devices showed ultralow energy loss and a remarkable fill factor (82%), paving the way for the application of modified structures of Spiro-MeOTAD at the hole transport layer without the need for chemical dopants.120
Conclusions
The number of “small molecules” that can be potentially synthesised and used as SAMs are endless. Yet, the understanding of the effects of such molecules on the perovskite material remains elusive and needs further investigation. The pursuit of higher efficiencies in perovskite solar cells will, definitively, make use of SAMs at the interface either in tandem solar cells with silicon films or with organic semiconductor thin films. A myriad of new molecules has been described and for sure this perspective will provide a handy tool to quickly read what has been done so far in the scientific literature about SAMs for PSC.In this respect, we have presented a comprehensive overview of the rational design and synthesis of all the molecules used as SAMs in PSCs, from the original seminal works using commercial molecules to the most complex structures prepared so far in this field. Despite the efforts made to prepare these molecules and fabricate efficient and stable perovskite devices, several key challenges remain for organic chemists, who need to focus their efforts on the design of new molecules containing the concepts described in this review, selecting the best combination of anchoring, spacer, and functional groups, also targeting facile and cost-effective protocols for the synthesis of these molecules to accelerate the commercial and industrial generation of solar energy with perovskite solar cells. A true challenge will be to design molecules that, from one side, improve charge transfer from the perovskite to the contacts and, from the other side, modify the perovskite surface to vastly enhance the perovskite stability and avoid leaking of lead from the crystalline structure. Of course, SAMs that achieve similar effects on Sn-based perovskite films are the other challenges scientists face for the next decade.
Author contributions
All authors contributed to the writing and revision of the manuscript, and have approved the final version of this review article.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge financial support from Spanish Government and AGAUR (Ministerio de Ciencia e Innovacion Severo Ochoa Grant MCIN/AEI/10.13039/501100011033 (CEX2019-000925-S), PID2019-109389RB-I00, PID2022-139866NB-I00 funded by MCIN/AEI/10.13039/501100011033/FEDER, UE and 2021 SGR 01261, respectively). C.P. thanks the European Union (Horizon 2020 Marie Skłodowska-Curie COFUND grant agreement no. 801474) D. A. G. acknowledges financial support from the MINECO predoctoral fellowship (BES-2017-082439). E. P. also acknowledges ICIQ, CERCA, and ICREA for financial support.References
- J. Y. Kim, J.-W. Lee, H. S. Jung, H. Shin and N.-G. Park, Chem. Rev., 2020, 120, 7867–7918 CrossRef CAS.
- Best Research-Cell Efficiency Chart, https://www.nrel.gov/pv/cell-efficiency.html, accessed: May 2023.
- R. Wang, M. Mujahid, Y. Duan, Z.-K. Wang, J. Xue and Y. Yang, Adv. Funct. Mater., 2019, 29, 1808843 CrossRef CAS.
- H. Li and W. Zhang, Chem. Rev., 2020, 120, 9835–9950 CrossRef CAS PubMed.
- V. Romano, A. Agresti, R. Verduci and G. D'Angelo, ACS Energy Lett., 2022, 7, 2490–2514 CrossRef CAS.
- E. Aktas, N. Rajamanickam, J. Pascual, S. Hu, M. H. Aldamasy, D.-D. Girolamo, W. Li, G. Nasti, E. Martínez-Ferrero, A. Wakamiya, E. Palomares and A. Abate, Commun. Mater., 2022, 3, 104 CrossRef CAS.
- W. Chi, S. K. Banerjee, K. G. D. I. Jayawardena, S. Ravi P. Silva and S. Seok, ACS Energy Lett., 2023, 8, 1535–1550 CrossRef CAS.
- C. McDonald, C. Ni, P. Maguire, P. Connor, J. T. S. Irvine, D. Mariotti and V. Svrcek, Nanomaterials, 2019, 9, 1481 CrossRef CAS PubMed.
- V. M. Le Corre, M. Stolterfoht, L. Perdigón Toro, M. Feuerstein, C. Wolff, L. Gil-Escrig, H. J. Bolink, D. Neher and L. J. Anton Koster, ACS Appl. Energy Mater., 2019, 2, 6280–6287 CrossRef CAS.
- S. Foo, M. Thambidurai, P. S. Kumar, R. Yuvakkumar, Y. Huang and C. Dang, Int. J. Energy Res., 2022, 46, 21441–21451 CrossRef CAS.
- J. J. Yoo, G. Seo, M. R. Chua, T. G. Park, Y. Lu, F. Rotermund, Y.-K. Kim, C. S. Moon, N. J. Jeon, J.-P. Correa-Baena, V. Bulović, S. S. Shin, M. G. Bawendi and J. Seo, Nature, 2021, 590, 587–593 CrossRef CAS PubMed.
- X. Lin, D. Cui, X. Luo, C. Zhang, Q. Han, Y. Wang and L. Han, Energy Environ. Sci., 2020, 13, 3823–3847 RSC.
- (a) X. Zheng, Y. Hou, C. Bao, J. Yin, F. Yuan, Z. Huang, K. Song, J. Liu, J. Troughton, N. Gasparini, C. Zhou, Y. Lin, D.-J. Xue, B. Chen, A. K. Johnston, N. Wei, M. N. Hedhili, M. Wei, A. Y. Alsalloum, P. Maity, B. Turedi, C. Yang, D. Baran, T. D. Anthopoulos, Y. Han, Z.-H. Lu, O. F. Mohammed, F. Gao, E. H. Sargent and O. M. Bakr, Nat. Energy, 2020, 5, 131–140 CrossRef CAS; (b) B. Chen, P. N. Rudd, S. Yang, Y. Yuanc and J. Huang, Chem. Soc. Rev., 2019, 48, 3842–3867 RSC; (c) P. Roy, A. Ghosh, F. Barclay, A. Khare and E. Cuce, Coatings, 2022, 12, 1089 CrossRef CAS; (d) Z. Guo, A. K. Jena, G. M. Kim and T. Miyasaka, Energy Environ. Sci., 2022, 15, 3171–3222 RSC.
- Y. Shao, Z. Xiao, C. Bi, Y. Yuan and J. Huang, Nat. Commun., 2014, 5, 5784 CrossRef CAS PubMed.
- M. Dkhili, G. Lucarelli, F. De Rossi, B. Taheri, K. Hammedi, H. Ezzaouia, F. Brunetti and T. M. Brown, ACS Appl. Energy Mater., 2022, 5, 4096–4107 CrossRef CAS PubMed.
- K. Mahmood, S. Sarwar and M. T. Mehran, RSC Adv., 2017, 7, 17044 RSC.
- X. Zheng, B. Chen, J. Dai, Y. Fang, Y. Bai, Y. Lin, H. Wei, X. C. Zeng and J. Huang, Nat. Energy, 2017, 2, 17102 CrossRef CAS.
- W.-Y. Chen, L.-L. Deng, S.-M. Dai, X. Wang, C.-B. Tian, X.-X. Zhan, S.-Y. Xie, R.-B. Huang and L.-S. Zheng, J. Mater. Chem. A, 2015, 3, 19353–19359 RSC.
- H. Zhang, H. Wang, H. Zhu, C.-C. Chueh, W. Chen, S. Yang and A. K.-Y. Jen, Adv. Energy Mater., 2018, 8, 1702762 CrossRef.
- K. C. Wang, J. Y. Jeng, P. S. Shen, Y. C. Chang, E. W. Diau, C. H. Tsai, T. Y. Chao, H. C. Hsu, P. Y. Lin, P. Chen, T. F. Guo and T. C. Wen, Sci. Rep., 2014, 4, 4756 CrossRef PubMed.
- M. Stolterfoht, C. M. Wolff, J. A. Márquez, S. Zhang, C. J. Hages, D. Rothhardt, S. Albrecht, P. L. Burn, P. Meredith, T. Unold and D. Neher, Nat. Energy, 2018, 3, 847 CrossRef CAS.
- J. S. Yeo, R. Kang, S. Lee, Y. J. Jeon, N. Myoung, C. L. Lee, D. Y. Kim, J. M. Yun, Y. H. Seo, S. S. Kim and S. I. Na, Nano Energy, 2015, 12, 96 CrossRef CAS.
- X. Sun, Z. Zhu and Z. Li, Front. Optoelectron., 2022, 15, 46 CrossRef PubMed.
- S. Li, Y. L. Cao, W. H. Li and Z. S. Bo, Rare Met., 2021, 40, 2712 CrossRef CAS.
- X. Yin, Z. Song, Z. Li and W. Tang, Energy Environ. Sci., 2020, 13, 4057 RSC.
- P. Mahajan, B. Padha, S. Verma, V. Gupta, R. Datt, W. C. Tsoi, S. Satapathi and S. Arya, J. Energy Chem., 2022, 68, 330 CrossRef CAS.
- Y. Wang, L. Duan, M. Zhang, Z. Hameiri, X. Liu, Y. Bai and X. Hao, Sol. RRL, 2022, 6, 2200234 CrossRef CAS.
- P. Murugan, T. Hu, X. Hu and Y. Chen, J. Mater. Chem. A, 2022, 10, 5044 RSC.
- W. C. Bigelow, D. L. Pickett and W. A. Zisman, J. Colloid Sci., 1946, 1, 513 CrossRef CAS.
- K. Wojciechowski, S. D. Stranks, A. Abate, G. Sadoughi, A. Sadhanala, N. Kopidakis, G. Rumbles, C. Z. Li, R. H. Friend, A. K. Y. Jen and H. J. Snaith, ACS Nano, 2014, 8, 12701 CrossRef CAS.
- S. K. Hau, H. L. Yip, O. Acton, N. S. Baek, H. Ma and A. K. Y. Jen, J. Mater. Chem., 2008, 18, 5113 RSC.
- S. K. Hau, Y. J. Cheng, H. L. Yip, Y. Zhang, H. Ma and A. K. Y. Jen, ACS Appl. Mater. Interfaces, 2010, 2, 1892 CrossRef CAS.
- A. Abrusci, S. D. Stranks, P. Docampo, H. L. Yip, A. K. Y. Jen and H. J. Snaith, Nano Lett., 2013, 13, 3124 CrossRef CAS PubMed.
- A. Ulman, Chem. Rev., 1996, 96, 1533 CrossRef CAS PubMed.
- M. Singh, N. Kaur and E. Comini, J. Mater. Chem. C, 2020, 8, 3938 RSC.
- Y. Wang, Q. Liao, J. Chen, W. Huang, X. Zhuang, Y. Tang, B. Li, X. Yao, X. Feng, X. Zhang, M. Su, Z. He, T. J. Marks, A. Facchetti and X. Guo, J. Am. Chem. Soc., 2020, 142, 16632 CrossRef CAS PubMed.
- T. Bauer, T. Schmaltz, T. Lenz, M. Halik, B. Meyer and T. Clark, ACS Appl. Mater. Interfaces, 2013, 5, 6073 CrossRef CAS PubMed.
- F. Ali, C. Roldán Carmona, M. Sohail and M. Khaja Nazeeruddin, Adv. Energy Mater., 2020, 10, 2002989 CrossRef CAS.
- S. Y. Kim, S. J. Cho, S. E. Byeon, X. He and H. J. Yoon, Adv. Energy Mater., 2020, 10, 2002606 CrossRef CAS.
- J. Hu, W. Fu, X. Yang and H. Chen, J. Polym. Sci., 2022, 60, 2175 CrossRef CAS.
- S. Wang, H. Guo and Y. Wu, Mater. Futures, 2023, 2, 012105 CrossRef.
- C. Ganzorig, K. J. Kwak, K. Yagi and M. Fujihira, Appl. Phys. Lett., 2001, 79, 272 CrossRef CAS.
- J. V. Passarelli, D. J. Fairfield, N. A. Sather, M. P. Hendricks, H. Sai, C. L. Stern and S. I. Stupp, J. Am. Chem. Soc., 2018, 140, 7313 CrossRef CAS.
- E. Arkan, E. Yalcin, M. Unal, M. Z. Yigit Arkan, M. Can, C. Tozlu and S. Demic, Mater. Chem. Phys., 2020, 254, 123435 CrossRef CAS.
- L. Liu, A. Mei, T. Liu, P. Jiang, Y. Sheng, L. Zhang and H. Han, J. Am. Chem. Soc., 2015, 137, 1790 CrossRef CAS.
- L. Zuo, Z. Gu, T. Ye, W. Fu, G. Wu, H. Li and H. Chen, J. Am. Chem. Soc., 2015, 137, 2674 CrossRef CAS PubMed.
- L. Liu, A. Mei, T. Liu, P. Jiang, Y. Sheng, L. Zhang and H. Han, Nano Lett., 2017, 17, 269 CrossRef.
- Q. Wang, C. C. Chueh, T. Zhao, J. Cheng, M. Eslamian, W. C. H. Choy and A. K.-Y. Jen, ChemSusChem, 2017, 10, 3794 CrossRef CAS PubMed.
- D. A. Kara, K. Kara, G. Oylumluoglu, M. Zeliha Yigit, M. Can, J. J. Kim, E. K. Burnett, L. Gonzalez Arellano, S. Buyukcelebi, F. Ozel, O. Usluer, A. L. Briseno and M. Kus, ACS Appl. Mater. Interfaces, 2018, 10, 30000 CrossRef.
- G. Tumen-Ulzii, T. Matsushima, D. Klotz, M. R. Leyden, P. Wang, C. Qin, J. W. Lee, S. J. Lee, Y. Yang and C. Adachi, Commun. Mater., 2020, 1, 31 CrossRef.
- Ç. Kırbıyık, T. Yılmaz Alıç and M. Kuş, Microelectron. Eng., 2020, 231, 111394 CrossRef.
- T. Zhu, J. Su, F. Labat, I. Ciofini and T. Pauporté, ACS Appl. Mater. Interfaces, 2020, 12, 744 CrossRef CAS PubMed.
- C. M. Wolff, L. Canil, C. Rehermann, N. Ngoc Linh, F. Zu, M. Ralaiarisoa, P. Caprioglio, L. Fiedler, M. Stolterfoht, S. Kogikoski, Jr., l. Bald, N. Koch, E. L. Unger, T. Dittrich, A. Abate and D. Neher, ACS Nano, 2020, 14, 1445 CrossRef CAS PubMed.
- H. Shu, J. Xia, H. Yang, J. Luo, Z. Wan, H. Ashraf Malik, F. Han, X. Yao and C. Jia, ACS Sustainable Chem. Eng., 2020, 8, 10859 CAS.
- C. Y. Chang, H. H. Huang, H. Tsai, S. L. Lin, P. H. Liu, W. Chen, F. C. Hsu, W. Nie, Y. F. Chen and L. Wang, Adv. Sci., 2021, 8, 2002718 CrossRef CAS PubMed.
- Ç. K. Kurukavak, T. Yılmaz, A. Büyükbekar and M. Kus, Opt. Mater., 2021, 112, 110783 CrossRef.
- N. Singh and Y. T. Tao, Nano Sel., 2021, 2, 2390 CrossRef CAS.
- Y. Shi, H. Zhang, X. Tong, X. Hou, F. Li, Y. Du, S. Wang, Q. Zhang, P. Liu and X. Zhao, Sol. RRL, 2021, 5, 2100128 CrossRef CAS.
- H. Dong, G. Shen, Z. Lin, Q. Cai, Y. Li, X. Xu, W. Zhang and C. Mu, ChemNanoMat, 2022, 8, e202100475 CrossRef CAS.
- J. Zhang, J. Yang, R. Dai, W. Sheng, Y. Su, Y. Zhong, X. Li, L. Tan and Y. Chen, Adv. Energy Mater., 2022, 12, 2103674 CrossRef CAS.
- L. Liu, Y. Yang, M. Du, Y. Cao, X. Ren, L. Zhang, H. Wang, S. Zhao, K. Wang and S. Liu, Adv. Energy Mater., 2023, 13, 2202802 CrossRef CAS.
- Z. Chen, Y. Li, Z. Liu, J. Shi, B. Yu, S. Tan, Y. Cui, C. Tan, F. Tian, H. Wu, Y. Luo, D. Li and Q. Meng, Adv. Energy Mater., 2023, 13, 2202799 CrossRef CAS.
- T. Yang, W. Zhao, X. Liu and S. Liu, Adv. Energy Mater., 2023, 13, 2204192 CrossRef CAS.
- H. Zheng, F. Zhang, N. Zhou, M. Sun, X. Li, Y. Xiao and S. Wang, Org. Electron., 2018, 56, 89 CrossRef CAS.
- D. Bobb Semple, L. Zeng, I. Cordova, D. S. Bergsman, D. Nordlund and S. F. Bent, Langmuir, 2020, 36, 12849 CrossRef CAS PubMed.
- S. K. Hau, Y. J. Cheng, H. Yip, Y. Zhang, H. Ma and A. K. Y. Jen, ACS Appl. Mater. Interfaces, 2010, 2, 1892 CrossRef CAS.
- K. Wojciechowski, S. D. Stranks, A. Abate, G. Sadoughi, A. Sadhanala, N. Kopidakis, G. Rumbles, C. Z. Li, R. H. Friend, A. K. Y. Jen and H. J. Snaith, ACS Nano, 2014, 8, 12701 CrossRef CAS PubMed.
- E. Aydin, J. Liu, E. Ugur, R. Azmi, G. T. Harrison, Y. Hou, B. Chen, S. Zhumagali, M. De Bastiani, M. Wang, W. Raja, T. G. Allen, A. ur Rehman, A. S. Subbiah, M. Babics, A. Babayigit, F. H. Isikgor, K. Wang, E. Van Kerschaver, L. Tsetseris, E. H. Sargent, F. Laquai and S. De Wolf, Energy Environ. Sci., 2021, 14, 4377 RSC.
- K. Liu, S. Chen, J. Wu, H. Zhang, M. Qin, X. Lu, Y. Tu, Q. Meng and X. Zhan, Energy Environ. Sci., 2018, 11, 3463 RSC.
- Z. Xing, F. Liu, S. H. Li, Z. C. Chen, M. W. An, S. Zheng, A. K. Y. Jen and S. Yang, Adv. Funct. Mater., 2021, 31, 2107695 CrossRef CAS.
- R. Zahran and Z. Hawash, Adv. Mater. Interfaces, 2022, 9, 2201438 CrossRef CAS.
- G. Sathiyan, E. K. T. Sivakumar, R. Ganesamoorthy, R. Thangamuthu and P. Sakthivel, Tetrahedron Lett., 2016, 57, 243 CrossRef CAS.
- A. Jegorov, M. A. Truong, R. Murdey, M. Daskeviciene, T. Malinauskas, K. Kantminiene, V. Jankauskas, V. Getautis and A. Wakamiya, Sol. RRL, 2022, 6, 2100877 CrossRef.
- K. Radhakrishna, S. Beranagodu Manjunath, D. Devadiga, R. Chetri and A. Tantri Nagaraja, ACS Appl. Energy Mater., 2023, 6, 3635 CrossRef CAS.
- A. Magomedov, A. Al-Ashouri, E. Kasparavicius, S. Strazdaite, G. Niaura, M. Jošt, T. Malinauskas, S. Albrecht and V. Getautis, Adv. Energy Mater., 2018, 8, 1801892 CrossRef.
- A. Al-Ashouri, A. Magomedov, M. Roß, M. Jošt, M. Talaikis, G. Chistiakova, T. Bertram, J. A. Márquez, E. Köhnen, E. Kasparavičius, S. Levcenco, L. Gil-Escrig, C. J. Hages, R. Schlatmann, B. Rech, T. Malinauskas, T. Unold, C. A. Kaufmann, L. Korte, G. Niaura, V. Getautis and S. Albrecht, Energy Environ. Sci., 2019, 12, 3356 RSC.
- G. Kapil, T. Bessho, Y. Sanehira, S. R. Sahamir, M. Chen, A. Kumar Baranwal, D. Liu, Y. Sono, D. Hirotani, D. Nomura, K. Nishimura, M. Akmal Kamarudin, Q. Shen, H. Segawa and S. Hayase, ACS Energy Lett., 2022, 7, 966 CrossRef CAS.
- S. H. Hsiao, S. C. Peng, Y. R. Kung, C. M. Leu and T. M. Lee, Eur. Polym. J., 2015, 73, 50 CrossRef CAS.
- L. Li, Y. Wang, X. Wang, R. Lin, X. Luo, Z. Liu, K. Zhou, S. Xiong, Q. Bao, G. Chen, Y. Tian, Y. Deng, K. Xiao, J. Wu, M. I. Saidaminov, H. Lin, C. Q. Ma, Z. Zhao, Y. Wu, L. Zhang and H. Tan, Nat. Energy, 2022, 7, 708 CrossRef CAS.
- R. Mishima, M. Hino, M. Kanematsu, K. Kishimoto, H. Ishibashi, K. Konishi, S. Okamoto, T. Irie, T. Fujimoto and W. Yoshida, Appl. Phys. Express, 2022, 15, 076503 CrossRef.
- Y. Lin, A. Magomedov, Y. Firdaus, D. Kaltsas, A. El-Labban, H. Faber, D. R. Naphade, E. Yengel, X. Zheng, E. Yarali, N. Chaturvedi, K. Loganathan, D. Gkeka, S. Alshammari, O. Bakr, F. Laquai, L. Tsetseris, V. Getautis and T. Anthopoulos, ChemSusChem, 2021, 14, 3569 CrossRef CAS PubMed.
- Y. S. Shin, S. Ameen, E. Oleiki, J. Yeop, Y. Lee, S. Javaid, C. B. Park, T. Song, D. Yuk, H. Jang, Y. J. Yoon, W. Lee, G. Lee, B. Kim and J. Y. Kim, Adv. Opt. Mater., 2022, 10, 2201313 CrossRef CAS.
- A. Al-Ashouri, E. Köhnen, B. Li, A. Magomedov, H. Hempel, P. Caprioglio, J. A. Márquez, A. B. Morales Vilches, E. Kasparavicius, J. A. Smith, N. Phung, D. Menzel, M. Grischek, L. Kegelmann, D. Skroblin, C. Gollwitzer, T. Malinauskas, M. Jošt, G. Matič, B. Rech, R. Schlatmann, M. Topič, L. Korte, A. Abate, B. Stannowski, D. Neher, M. Stolterfoht, T. Unold, V. Getautis and S. Albrecht, Science, 2020, 370, 1300 CrossRef CAS PubMed.
- X. Deng, F. Qi, F. Li, S. Wu, F. R. Lin, Z. Zhang, Z. Guan, Z. Yang, C. S. Lee and A. K.-Y. Jen, Angew. Chem., Int. Ed., 2022, 61, e202203088 CrossRef CAS PubMed.
- C. Rodríguez-Seco, M. Méndez, C. Roldán-Carmona, R. Pudi, M. K. Nazeeruddin and E. Palomares, Angew. Chem., 2020, 132, 5341 CrossRef.
- E. Aktas, N. Phung, H. Kobler, D. A. González, M. Méndez, I. Kafedjiska, S. H. Turren-Cruz, R. Wenisch, I. Lauermann, A. Abate and E. Palomares, Energy Environ. Sci., 2021, 14, 3976 RSC.
- S. Kumari, J. G. Sánchez, M. Imran, E. Aktas, D. A. González, L. Manna, E. Martínez-Ferrero and E. Palomares, J. Mater. Chem. C, 2023, 11, 3788 RSC.
- S. Zhang, R. Wu, C. Mu, Y. Wang, L. Han, Y. Wu and W. H. Zhu, ACS Mater. Lett., 2022, 4, 1976 CrossRef CAS.
- W. Jiang, F. Li, M. Li, F. Qi, F. R. Lin and A. K. Y. Jen, Angew. Chem., Int. Ed., 2022, 61, e202213560 CrossRef CAS PubMed.
- R. He, W. Wang, Z. Yi, F. Lang, C. Chen, J. Luo, J. Zhu, J. Thiesbrummel, S. Shah, K. Wei, Y. Luo, C. Wang, H. Lai, H. Huang, J. Zhou, B. Zou, X. Yin, S. Ren, X. Hao, L. Wu, J. Zhang, J. Zhang, M. Stolterfoht, F. Fu, W. Tang and D. Zhao, Nature, 2023, 618, 80 CrossRef CAS PubMed.
- L. Calio, S. Kazim, M. Graetzel and S. Ahmad, Angew. Chem., Int. Ed., 2016, 55, 14522 CrossRef CAS PubMed.
- A. Farokhi, H. Shahroosvand, G. Delle Monache, M. Pilkington and M. Khaja Nazeeruddin, Chem. Soc. Rev., 2022, 51, 5974 RSC.
- L. Nakka, Y. Cheng, A. Gerhard Aberle and F. Lin, Adv. Energy Sustainability Res., 2022, 3, 2200045 CrossRef CAS.
- Y. Wang, L. Duan, M. Zhang, Z. Hameiri, X. Liu, Y. Bai and X. Hao, Sol. RRL, 2022, 6, 2200234 CrossRef CAS.
- E. Yalcin, D. A. Kara, C. Karakaya, M. Z. Yigit, A. K. Havare, M. Can, C. Tozlu, S. Demic, M. Kus and A. Aboulouard, Opt. Mater., 2017, 69, 283 CrossRef CAS.
- E. Yalcin, M. Can, C. Rodriguez-Seco, E. Aktas, R. Pudi, W. Cambarau, S. Demic and E. Palomares, Energy Environ. Sci., 2019, 12, 230 RSC.
- E. Aktas, R. Pudi, N. Phung, R. Wenisch, L. Gregori, D. Meggiolaro, M. A. Flatken, F. De Angelis, I. Lauermann, A. Abate and E. Palomares, ACS Appl. Mater. Interfaces, 2022, 14, 17461 CrossRef CAS PubMed.
- Y. Wang, Q. Liao, J. Chen, W. Huang, X. Zhuang, Y. Tang, B. Li, X. Yao, X. Feng, X. Zhang, M. Su, Z. He, T. J. Marks, A. Facchetti and X. Guo, J. Am. Chem. Soc., 2020, 142, 16632 CrossRef CAS PubMed.
- Q. Liao, Y. Wang, Z. Zhang, K. Yang, Y. Shi, K. Feng, B. Li, J. Huang, P. Gao and X. Guo, J. Energy Chem., 2022, 68, 87 CrossRef CAS.
- R. Guo, X. Zhang, X. Zheng, L. Li, M. Li, Y. Zhao, S. Zhang, L. Luo, S. You, W. Li, Z. Gong, R. Huang, Y. Cui, Y. Rong, H. Zeng and X. Li, Adv. Funct. Mater., 2023, 33, 2211955 CrossRef CAS.
- H. Liu, K. Yan, J. Rao, Z. Chen, B. Niu, Y. Huang, H. Ju, B. Yan, J. Yao, H. Zhu, H. Chen and C. Z. Li, ACS Appl. Mater. Interfaces, 2022, 14, 6794 CrossRef CAS PubMed.
- L. Mao, Y. Wu, J. Jiang, X. Guo, P. Heng, L. Wang and J. Zhang, J. Phys. Chem. C, 2020, 124, 9233 CrossRef CAS.
- S. Thokala and S. P. Singh, ACS Omega, 2020, 5, 5608 CrossRef CAS PubMed.
- G. D. Blanco, A. J. Hiltunen, G. N. Lim, C. B. KC, K. M. Kaunisto, T. K. Vuorinen, V. N. Nesterov, H. J. Lemmetyinen and F. D'Souza, ACS Appl. Mater. Interfaces, 2016, 8, 8481 CrossRef CAS PubMed.
- E. Li, E. Bi, Y. Wu, W. Zhang, L. Li, H. Chen, L. Han, H. Tian and W. H. Zhu, Adv. Funct. Mater., 2019, 30, 1909509 CrossRef.
- E. Li, C. Liu, H. Lin, X. Xu, S. Liu, S. Zhang, M. Yu, X. M. Cao, Y. Wu and W. H. Zhu, Adv. Funct. Mater., 2021, 31, 2103847 CrossRef CAS.
- A. Ullah, K. H. Park, H. D. Nguyen, Y. Siddique, S. F. A. Shah, H. Tran, S. Park, S. I. Lee, K. K. Lee, C. H. Han, K. Kim, S. Ahn, I. Jeong, Y. S. Park and S. Hong, Adv. Energy Mater., 2021, 32, 2103175 Search PubMed.
- A. Ullah, K. H. Park, Y. W. Lee, S. Park, A. Bin Faheem, H. D. Nguyen, Y. Siddique, K. K. Lee, Y. Jo, C. H. Han, S. Ahn, I. Jeong, S. Cho, B. Kim, Y. S. Park and S. Hong, Adv. Funct. Mater., 2022, 32, 2208793 CrossRef CAS.
- S. Liu, W. Cao, D. Xia, J. Zhang, J. Fan, C. An, R. Fan, S. Hao, K. Lin and Y. Yang, Dyes Pigm., 2021, 193, 109506 CrossRef CAS.
- E. Arkan, E. Yalcin, M. Unal, M. Zeliha Yigit Arkan, M. Can, C. Tozlu and S. Demic, Mater. Chem. Phys., 2020, 254, 123435 CrossRef CAS.
- E. Arkan, M. Unal, E. Yalcin, M. Zeliha Yigit Arkan, S. Yurtdas, M. Can, C. Tozlu and S. Demic, Mater. Sci. Semicond. Process., 2021, 123, 105514 CrossRef CAS.
- E. Arkan, M. Zeliha Yigit Arkan, M. Unal, E. Yalcin, H. Aydin, C. Celebi, M. Can, C. Tozlu and S. Demic, Opt. Mater., 2020, 105, 109910 CrossRef CAS.
- C. Zhang, W. Kong, T. Wu, X. Lin, Y. Wu, J. Nakazaki, H. Segawa, X. Yang, Y. Zhang, Y. Wang and L. Han, ACS Appl. Mater. Interfaces, 2021, 13, 44321 CrossRef CAS PubMed.
- L. Li, Y. Wu, E. Li, C. Shen, H. Zhang, X. Xu, G. Wu, M. Cai and W. H. Zhu, Chem. Commun., 2019, 55, 13239 RSC.
- H. Cheng, Y. Li, M. Zhang, K. Zhao and Z. S. Wang, ChemSusChem, 2020, 13, 2779 CrossRef CAS PubMed.
- E. Aktas, J. Jiménez-López, K. Azizi, T. Torres and E. Palomares, Nanoscale Horiz., 2020, 5, 1415 RSC.
- H. Kouki, S. Pitié, A. Torkhani, F. Mamèche, P. Decorse, M. Seydou, F. Kouki and P. Lang, ACS Appl. Energy Mater., 2022, 5, 1635 CrossRef CAS.
- M. A. Truong, T. Funasaki, L. Ueberricke, W. Nojo, R. Murdey, T. Yamada, S. Hu, A. Akatsuka, N. Sekiguchi, S. Hira, L. Xie, T. Nakamura, N. Shioya, D. Kan, Y. Tsuji, S. Iikubo, H. Yoshida, Y. Shimakawa, T. Hasegawa, Y. Kanemitsu, T. Suzuki and A. Wakamiya, J. Am. Chem. Soc., 2023, 145, 7528 CrossRef CAS PubMed.
- S. N. Afraj, C. H. Kuan, J. S. Lin, J. S. Ni, A. Velusamy, M. C. Chen and E. W. G. Diau, Adv. Funct. Mater., 2023, 33, 2213939 CrossRef CAS.
- W. Li, M. Cariello, M. Méndez, G. Cooke and E. Palomares, ACS Appl. Energy Mater., 2023, 6, 1239 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |