Open Access Article
Open Access ArticleRecent advances in porous organic cages for energy applications
Chao
Liu
 a,
Zhixuan
Wang
a,
Hailong
Wang
a,
Zhixuan
Wang
a,
Hailong
Wang
 *b and
Jianzhuang
Jiang
*b and
Jianzhuang
Jiang
 *b
*b
aState Key Laboratory of New Pharmaceutical Preparations and Excipients, College of Chemistry and Materials Science, Hebei University, Baoding 071002, China
bBeijing Advanced Innovation Center for Materials Genome Engineering, Beijing Key Laboratory for Science and Application of Functional Molecular and Crystalline Materials, Department of Chemistry, School of Chemistry and Biological Engineering, University of Science and Technology Beijing, Beijing 100083, China. E-mail: hlwang@ustb.edu.cn; jianzhuang@ustb.edu.cn
First published on 23rd October 2024
Abstract
In recent years, the energy and environmental crises have attracted more and more attention. It is very important to develop new materials and technologies for energy storage and conversion. In particular, it is crucial to develop carriers that store energy or promote mass and electron transport. Emerging porous organic cages (POCs) are very suitable for this purpose because they have inherent advantages including structural designability, porosity, multifunction and post-synthetic modification. POC-based materials, such as pristine POCs, POC composites and POC derivatives also exhibit excellent energy-related properties. This latest perspective provides an overview of the progress of POC-based materials in energy storage and conversion applications, including photocatalysis, electrocatalysis (CO2RR, NO3RR, ORR, HER and OER), separation (gas separation and liquid separation), batteries (lithium–sulfur, lithium-ion and perovskite solar batteries) and proton conductivity, highlighting the unique advantages of POC-based materials in various forms. Finally, we summarize the current advances, challenges and further perspectives of POC-based materials in energy applications. This perspective will promote the design and synthesis of next-generation POC-based materials for energy applications.
1 Introduction
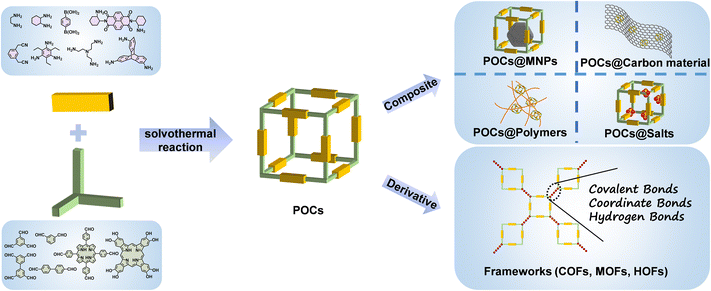
In order to achieve the goal of sustainable energy development, exploring and innovating renewable energy sources is critical to reducing the current serious dependence on fossil fuels. However, the region limitations of renewable energy such as tidal energy and solar energy are still a serious bottleneck for its large-scale practical application.1 In addition, some energy-intensive industries have also urgently prompted researchers to develop advanced and sustainable energy-related technologies towards separation, rechargeable batteries, electrocatalysis and photocatalysis.2 In order to further realize these advanced applications, it is imperative to develop novel materials with appropriate structures and functions. Recently, emerging materials based on porous organic cages (POCs), including pristine POCs, POC composites and POC derivatives, have attracted great attention due to their significant advantages compared to traditional inorganic materials in energy applications.3–5POCs, which are defined as porous organic compounds with cage-like structures, are usually self-assembled by two or more different structural organic units through covalent bonds. This makes the original POCs show the advantages of high porosity, diverse structures, rich functionality and easy tailorability. At the same time, the parent POCs can be adjusted by the post-modification strategy to further broaden their application range. POCs can be combined with other auxiliary components as functional carriers to create POC composites with predictable structures for a variety of applications. There are many cases in which ultrafine and stable metal nanoparticles (MNPs) are encapsulated by POCs.6–8 In comparison with reticular frameworks such as covalent organic frameworks (COFs), metal–organic frameworks (MOFs) and hydrogen-bonded organic frameworks (HOFs),9–14 the extended networks of POCs are composed of discrete porous molecules stacked by van der Waals forces, and their porous structures contain intercrossed molecule inner and external pores. POCs are able to be classified as supramolecular organic frameworks, being well characterized by mass spectrometry, nuclear magnetic spectroscopy, single crystal X-ray diffraction techniques, etc. The good solubility of POCs in solvents also makes it easy to process them into devices. On the other hand, POCs obtained by using irreversible bonds can be used as reaction precursors to derive different types of materials, such as COFs, MOFs, HOFs, etc.15–20 POC derivatives have more exposed active sites and higher specific surface areas while retaining the functional advantages of POCs, which further improves their performances.
Although the fascinating structures and various novel applications of POC-based materials have attracted the in-depth exploration of researchers, and the applications of POC-based materials in the energy field have also been widely reported, there are still challenges in their practical applications. For example, due to the low sun light energy utilization of POC-based materials in photocatalysis and the low conductivity of electrocatalysis, it is still difficult to meet the actual industrial needs. In this perspective, we summarize the recent advances in the applications of POC-based materials in various energy-related fields. First, we introduce the special structural advantages of POC-based materials, and then comprehensively evaluate their applications in a wide range of energy technologies, including photo/electrocatalysis, separation, batteries and proton conductivity. We list the latest breakthroughs in the design, synthesis and application of POC-based materials. We hope that this perspective can summarize the latest and most effective information to help researchers understand and master the design strategies of POC-based materials more comprehensively. This is also true for the advantages and challenges when POCs are applied in different energy scenarios, helpful in increasing their economic value and broadening their application fields.
2 Porous organic cages (POCs)
2.1 Pristine POCs
As a new class of crystalline porous molecular-based materials, POCs are assembled from cage-like molecules formed by connecting different organic precursors by covalent bonds.21–23 Because of the diversity of organic precursors, three-dimensional (3D) organic cage molecules have a variety of topological structures due to the incorporation of two or three components. Herein, the bicomponent topology is constructed by the number (m, n) of two organic building blocks, leading to [m + n] topology. The adjacent cage molecules are stacked together to form cage exterior-pores, which are connected with the cavity inside the cage to form 3D intercrossing channels throughout the materials. The characteristics of organic precursors (such as bond angle, length, geometry, etc.) play a key role in adjusting the structures and functions of POCs, so that POCs have the advantage of more structural tunability than traditional porous inorganic materials. On one hand, the flexible integration of a wide range of selectable functional organic connectors enables POCs to be precisely designed for targeted applications such as catalysis, adsorption and separation.24–27 On the other hand, the original POCs have well-defined pores and large specific surface areas. Combined with the weak forces provided by the specific groups attached on POCs, POCs can be used as special containers to encapsulate diverse guest species (molecules, clusters, nanoparticles, etc.), or POCs can be directly used as nanoreactors to achieve functional properties.28–31 The unique properties of POCs combine readily available structures to further obtain performance–structure relationships to guide the rational design of POC based materials for energy applications.So far, the vast majority of POCs are mainly obtained by the reactions involved in dynamic covalent chemistry (DvCC), such as imine condensation, boric acid ester condensation, and olefin/alkyne metathesis,32–38 as shown in Table 1. The most thermodynamically stable molecular structure is fabricated by using the natural ‘proof reading’ and ‘error checking’ mechanisms due to the highly reversible covalent bonds. Usually, the synthesis process is simple, and the yield is high. Among the DvCC reactions, the imine condensation reaction is one of the most commonly used methods to synthesize well-defined POCs by selecting the appropriate preparation conditions. The reaction conditions include monomer concentration, reaction solvent type, catalyst and temperature. It is worth noting that reversible reactions are not a necessary prerequisite for constructing POCs. In 2023, the Yuan group synthesized new stable [2 + 3] type sp2 conjugated C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond-linked POCs by the Knoevenagel condensation reaction of bowl-shaped trialdehyde with two V type diacetonitriles, which provided more reaction ideas for constructing POCs.39 POCs can be also connected by irreversible bonds, including C–C bonds, amide bonds, azide–alkyne cycloaddition and nucleophilic substitutions.40–45 This type of chemical bond formation is rapid, and the obtained POCs have higher chemical stability. However, there are a large number of by-products, the yield is low, and the separation and purification are difficult. Therefore, there are relatively few reports on POCs based on these kinds of irreversible bonds. Importantly, in addition to considering their functionalities before designing POCs, the original POCs can be simply adjusted and designed by post-modification strategies such as modifying functional groups on the cage and inserting metal ions to generate/optimize specific functions. Therefore, the flexibility of POC structures makes them show great potential in various energy applications.
C bond-linked POCs by the Knoevenagel condensation reaction of bowl-shaped trialdehyde with two V type diacetonitriles, which provided more reaction ideas for constructing POCs.39 POCs can be also connected by irreversible bonds, including C–C bonds, amide bonds, azide–alkyne cycloaddition and nucleophilic substitutions.40–45 This type of chemical bond formation is rapid, and the obtained POCs have higher chemical stability. However, there are a large number of by-products, the yield is low, and the separation and purification are difficult. Therefore, there are relatively few reports on POCs based on these kinds of irreversible bonds. Importantly, in addition to considering their functionalities before designing POCs, the original POCs can be simply adjusted and designed by post-modification strategies such as modifying functional groups on the cage and inserting metal ions to generate/optimize specific functions. Therefore, the flexibility of POC structures makes them show great potential in various energy applications.
| Ligand 1 | Ligand 2 | Formed bond | Reaction type | Ref. |
|---|---|---|---|---|
| a Reversible reactions. b Irreversible reactions. | ||||
| –CHO | –NH2 | –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– N– |
Imine condensationa | 32 |
| –B(OH)2 | –OH | –BO2– | Boronic ester condensationa | 38 |
| –CHO | –CH2CN | –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CC CC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N– N– |
Knoevenagel condensationa | 39 |
| –CHO | –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ONHNH2 ONHNH2 |
–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ONHN ONHN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH– CH– |
Hydrazone condensationa | 33 |
| –SH | –SH | –S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S– S– |
Disulfide bond exchangea | 34 |
–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CR CR |
–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CR CR |
–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C– C– |
Alkyne metathesisa | 35 |
–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CR CR |
–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CR CR |
–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– C– |
Olefin metathesisa | 37 |
–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C C |
–N3 |
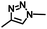
|
Azide–alkyne cycloadditionb | 40 |
| –OH | –CI/Br/I | –O– | Nucleophilic substitutionb | 41 |
–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH CH |
–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH CH |
–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–C C–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C– C– |
Carbon–carbon couplingb | 42 |
| –CI/Br/I |
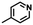
|
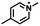
|
Nucleophilic substitutionb | 43 |
The crystallization process of POCs under controlled conditions is also very important. The Cooper group placed the desolvated organic cages in different organic solvent vapors (ethyl acetate, o-xylene, and dichloromethane), and obtained three non-porous, selective porous, and non-selective porous polymorphs, respectively.46 The ‘open’ and ‘close’ of the micropores in POCs are realized by controlling conditions. In addition, the topological structure can be changed during the recrystallization of POCs. For example, the [3 + 6] triangular prism organic cage obtained by the reaction of tetraaldehyde and diamine can be transformed into a [6 + 12] tetrahedral organic cage after crystallization in dichloromethane and methanol.47 Furthermore, the individual cages form interlocking organic cages during the crystallization process through the cleavage and reorganization of reversible bonds.48
POCs not only have porosity in the crystalline state, but also in the amorphous state. Common strategies for obtaining amorphous cage materials include interrupting crystallization during processing.49 The Cooper group used the freeze-drying method to obtain a nearly double increase in Brunauer–Emmett–Teller (BET) surface area of amorphous solids, which is due to the increase in external pores available in disordered solids. Nevertheless, covalent interference reactions can also be used to hinder the crystallization of POCs.50 By using different vertex diamines (ethylenediamine and cyclohexanediamine) to form a series of POCs with different vertices, the Cooper group obtained disordered stacking solids with a BET surface area of up to 818 m2 g−1.
2.2 POC composites
With the increasing demand for efficient energy storage and conversion devices, it is sometimes difficult for these single-component materials to achieve better performance. Therefore, by combining POCs with various materials (carbon materials, polymers, MNPs, inorganic salts, etc.), the functionalities of POCs are further expanded to obtain a new generation of materials with improved performance. POC composites are a new type of material that combines POCs with other auxiliary materials. It can incorporate the advantages of two different materials and expand the functionalities of POCs through the synergy of the two species. In principle, POC-based composites can generally be divided into two categories according to the main functional components. For the first type of composite, POCs play a major role, and the auxiliary components will not directly participate in energy storage and conversion applications, but simply adjust the related properties of POCs. As an example, the combination of POCs and carbon materials in electrocatalysis improves the conductivity of the material, which makes POCs exhibit higher electrocatalytic performances. In the other type of composite, both POCs and auxiliary components play a key role in energy conversion and storage applications. The combination of POCs and MNPs is the most reported.6–8 In this type of composite, POCs can be used as a new kind of host template to encapsulate and prepare ultrafine MNPs. The metal anchoring sites in the cage cavity can induce metal ion binding, nucleation and further growth of fine nanoparticles in the cage. The cage itself also acts as a protective shell for the generated MNPs to prevent their aggregation. In this direction, the tubular POC, MTC1, was prepared by the condensation of benzo[c][1,2,5]thiadiazole derivatives with cyclohexanediamine.51 Ultrafine and well dispersed PdNPs (1.9 ± 0.4 nm) were prepared by using the interaction between nitrogen-containing sites and palladium ions in MTC1. The synergistic effect of PdNPs and the photocatalyticactive POC in Pd@MTC1 composites achieved a two-step sequential reaction from 4-nitrophenylboronic acid to 4-aminophenol. So far, gold, silver, palladium, ruthenium, rhodium, platinum, iridium and other precious MNPs based on POCs have been reported, which are widely used in thermal catalysis and photocatalysis.2.3 POC-derived materials
POCs are also used as precursors to construct other forms of materials through a variety of ways, and cage-to-framework strategies have been studied for higher performance applications. Generally, the preparation of POC derivatives is mainly based on the post-synthetic modification strategy of the original POCs. In essence, most POCs have low conductivity and poor stability under extreme environmental conditions, in particular for photo/electrocatalytic reactions in strongly acidic or alkaline electrolytes. The good dissolution of POCs in some organic solvents limits their heterogeneous applications, hindering their practical applications to a certain extent. Fortunately, the original POCs can be directly used as special building blocks to derive various materials, including COFs, MOFs and HOFs (Scheme 1). The POC derivation method is an effective synthesis strategy to expand and optimize their functions. In particular, compared to the weak van der Waals force between POC molecules, the covalent bonds, coordination bonds and hydrogen bonds endow the derived materials with stronger stability. The obtained POC derivatives not only inherit the structural advantages of the original POCs, but also further improve their carrier mobility and specific surface area. The uniform dispersion of the active centers in the POC building blocks in the derivatives exposes more active sites and improves the utilization of intrinsic active sites, which leads to impressive performance in energy applications, including adsorption separation and catalysis. Recently, the Chen group prepared cage-based MOFs (mcm-MOF-1) by using a 6-connected [2 + 3] trigonal prismatic cage and Cu2+. Mcm-MOF-1 has high specific surface area and can adsorb aniline efficiently in the liquid phase.15 A porous HOF was prepared based on non-porous organic cages. The specific surface area of HOF material obtained by cage transformation is as high as 685 m2 g−1, and an efficient catalytic Suzuki reaction was achieved after loading divalent Pd ions.52Not only can the POCs be used as a building block directly, but they also can be converted into other materials by DvCC-induced dissociation and reconstruction. The converted materials often retain some of the characteristics of the parent POCs. The Hou group used chiral POCs to obtain COF materials without chiral groups through joint exchange, but COFs can retain the chiral information of the original organic cage, and the prepared materials show excellent chiral separation performance.53 There is another example where the materials prepared by the cage-to-COF conversion strategy have a higher specific surface area. In 2020, for the first time, the Zhang group realized the transfer of POCs to COFs through DvCC.54 Later, the Patra group transformed POC molecules into 2D COF films with higher crystallinity and specific surface area through DvCC at room temperature.55 The film can be used for size-selective molecular separation. In the same year, the COFs prepared based on POCs showed a higher specific surface area and iodine adsorption capacity than the directly synthesized COFs.56
3 The energy applications of POCs
The rapid consumption of non-renewable fossil fuels has caused a serious energy crisis and environmental problems. Newly developed POC-based materials are used to deal with these problems. In the past decade, topological design and functional unit synthesis strategies have been rapidly developed as two basic aspects of the POC field, greatly supplementing molecular design and functional exploration. Thus far, in the process of constructing functional POCs, the most common strategy is to design and develop corresponding POC materials based on specific functional units. In addition, functional optimization can also be achieved by combining POCs with other materials (MNPs, organic polymers, carbon materials, salts, etc.) or constructing other types of derivatives based on POCs. Using POCs to synthesize POC-derived materials is also an optional strategy to obtain higher performance materials. The energy applications of POC-based materials mainly focus on photocatalysis, electrocatalysis, separation, batteries and proton conductivity.3.1 Photocatalysts
 | ||
| Fig. 1 Porphyrin-based POCs with different topological structures generate reactive oxygen species (ROS) under visible light for multiple photocatalytic organic conversion reactions. | ||
Another example of a porphyrin organic cage for visible light photocatalysis was reported in 2020.59 The [3 + 6] tubular organic cage, PTC-1(2H), is composed of three porphyrin segments and six diaminocyclohexane groups linked by 12 imine bonds. The long triplet lifetime and microporous supramolecular framework of PTC-1(2H) ensure high-efficiency singlet oxygen evolution. Based on this, PTC-1(2H) can realize heterogeneous visible light photocatalysis of various primary amines, and the conversion rate is more than 99%. Its photocatalytic efficiency exceeds that of the typical mesoporous MOF material, PCN-222. In addition to porphyrin as a photoactive group, POCs based on conjugated groups such as pyrene60 and thiazole61,62 also exhibit high photocatalytic activity.
The structure modification of POCs is also an effective method to improve their photocatalytic performances. To date, ionization of POCs is an effective means to improve their photocatalytic performance. In 2022, the Chang group attempted to introduce additional second-sphere functionalities into the organic cage supramolecular framework, and the synergy between multiple interactions improves the photocatalytic activity (Fig. 2a–c).63 Based on the previously reported topological structure of Zn-PB, an alkynyl-functionalized porphyrin box (FePB-3(N)) was designed, and then 24 positively charged ammonium groups were added to the organic cage molecule by a synthetic post-modification strategy to obtain FePB-2(P). The synergistic effect of porosity and charge enhances the photocatalytic CO2RR performance. Through the comparison in a series of experiments, the CO2RR photocatalytic activity of the bifunctional FePB-2(P) is more than 40 times higher than that of the FeTPP reference materials. In contrast, CO2RR photocatalytic activity of materials with only pores or only charge interactions are increased by 4 times or 6 times, respectively. The selectivity of FePB-2(P) for photocatalytic reduction of CO2 to CO is 97%, and its turnover frequency (TON) is more than 1100. Similarly, the Sun group designed another porphyrin cage, TPPCage·8I.65 This ionization strategy effectively avoids the spontaneous aggregation of porphyrins by utilizing the naturally occurring electrostatic repulsion between adjacent cage hosts. At the same time, hydrophilic iodine ions also provide additional heavy atom effects. These modifications significantly enhance the photophysical properties and lead to extraordinary catalytic activity in the photocatalytic oxidative coupling reaction of benzylamine.
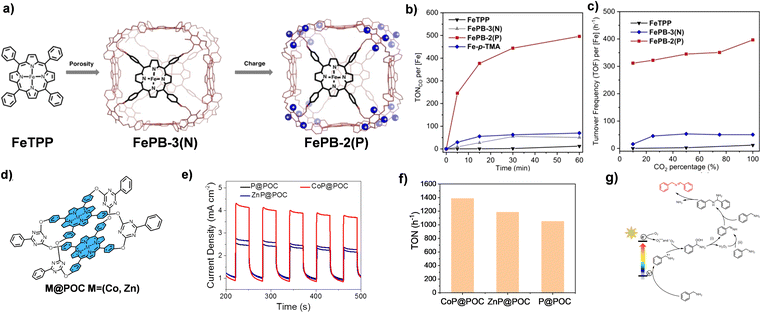 | ||
| Fig. 2 (a) The design of FePB-3(N) and FePB-3(P) catalysts; (b) photocatalytic CO2RR activity of FePB-2(P), FePB-3(N), FeTPP and Fe-p-TMA in CO2-saturated DMF; (c) photochemical CO2RR activity of FePB-2(P), FePB-3(N) and FeTPP in DMF at different CO2 concentrations. Reproduced from ref. 63. Copyright 2022, Wiley-VCH. (d) The molecular structures of P@POC and M@POC; (e) photocurrent response of P@POC, CoP@POC and ZnP@POC; (f) comparison of photocatalytic activity of P@POC, CoP@POC and ZnP@POC; (g) photocatalytic mechanism of oxidative coupling of benzylamines. Reproduced from ref. 64. Copyright 2022, The American Chemical Society. | ||
The optimization of photocatalytic performance is achieved by regulating the metal ions of N4 coordination in the porphyrin center. In 2022, the Li group used 5,10,15,20-tetrakis(4-hydroxyphenyl)porphyrin as the surface and 2,4-dichloro-6-phenyl-1,3,5-triazine as the pillar to prepare a metal-free porphyrin organic cage, P@POC, by a stepwise nucleophilic substitution reaction (Fig. 2d–g).64 Then, by refluxing the mixture of P@POC and CoCl2 or ZnCl2, a single Co or Zn atom is embedded in the porphyrin ring by a post-synthetic modification strategy. The introduction of metal ions into the N4 cavity of porphyrin significantly improves the light absorption and promotes the separation and transfer of photogenerated electrons. The Co atom-anchored organic cage (CoP@POC) has a more prominent photocatalytic efficiency in oxidizing amines into imines under visible light. The reaction conversion and selectivity of CoP@POC are as high as 99%, and the TOF is 1389 h−1, superior to those of most reported photocatalysts.
Later, a light-controlled active group is introduced into POCs to encapsulate NPs. In 2021, the Maji group designed a photochromic organic cage (TAE-DTE) based on a dithienylethene (DTE) unit (Fig. 3a–e).68 Subsequently, stable ultra-small (<2 nm) AuNPs (Au@TAE-DTE) were prepared by a unique reverse double solvent method. Au@TAE-DTE exhibits photochromic properties similar to those of TAE-DTE. Based on the opening and closing of the DTE ring, two metastable photoisomers are transformed between Au@TAE-DTE-O and Au@TAE-DTE-C. The Au@TAE-DTE composite can realize the catalytic reduction of CO2 under visible light (λ = 400–750 nm). In addition, Au@TAE-DTE-O and Au@TAE-DTE-C coexist under full-range (λ = 250–750 nm) light irradiation, showing wider spectral absorption and significantly enhanced photocatalytic CO2 reduction performance. This case is based on the encapsulation of MNPs by light-controlled organic cages, which improves its catalytic activity and selectivity.
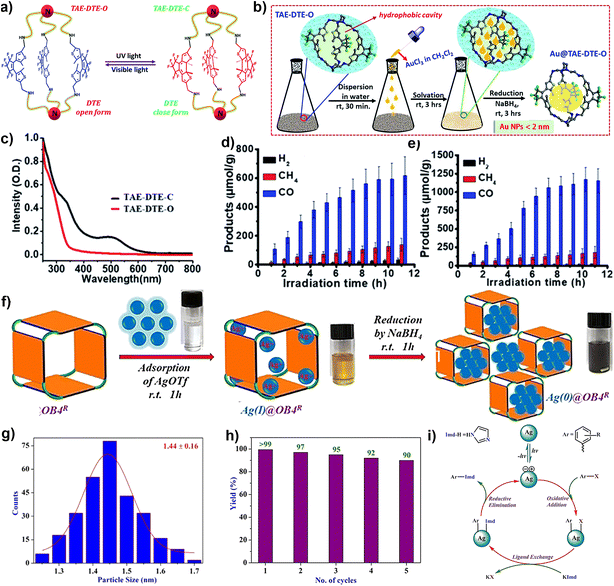 | ||
| Fig. 3 (a) The molecular structures and structure interconversion of TEA-DTE-O and TEA-DTE-S; (b) the electronic absorption spectra of TEA-DTE-O and TEA-DTE-S; (c) schematic diagram for in situ growth and stabilization of Au NPs inside the organic cage; CO2 reduction using Au@TAE-DTE-O under (d) visible light (400–750 nm) and (e) full-range light (250–750 nm). Reproduced from ref. 68. Copyright 2021, The Royal Society of Chemistry. (f) Pictorial representation of the formation of Ag(0)@OB4R; (g) particle size distribution of Ag(0)@OB4R; (h) reusability plot of Ag(0)@OB4R; (i) reaction pathway for Ullmann-type aryl-amination coupling by using photocatalyst Ag(0)@OB4R. Reproduced from ref. 69. Copyright 2020, Wiley-VCH. | ||
The composite material Ag0@OB4R represents the first example of discrete POC encapsulated Ag NPs, which were used as a photocatalyst in an Ullmann-type coupling reaction at room temperature (Fig. 3f–i).69 A discrete tetragonal organic cage (OB4R) is reported by the Mukherjee group, which is formed by the condensation of imine bonds between rigid tetraaldehyde and flexible diamine, and then the reduction of imine bonds. OB4R can be used as a molecular container to control the nucleation of AgNPs, and the size can be finely adjusted by binding with Ag+ in a closed cavity, and then reduced to obtain ultrafine AgNPs. The average particle size of Ag0@OB4R was 1.44 ± 0.16 nm, and the silver content was about 52 wt%. With an efficient photocatalyst, the Ullmann-type arylamine coupling of halogenated aromatic hydrocarbons can be easily achieved at ambient temperature.
In addition to AuNPs and AgNPs, PdNPs also can be encapsulated in POCs. The Wei group reported a highly catalytically active nanocomposite based on PdNPs and POCs for the photoreduction of CO2 to CH4 in the presence of a photosensitizer, tris(2,2′-bipyridyl) ruthenium chloride(II).70 The PdNPs with a diameter of 4.7 nm can achieve a high selectivity of 97% for CO2 to CH4 photoreduction at a rate of 10.2 μmol g−1 h−1. When these PdNPs are co-assembled with POCs (CC3), the catalytic efficiency of CO2 photoreduction is significantly enhanced (78.5 μmol g−1 h−1), which is mainly due to the increase in CO2 absorption by these cage compounds. In addition, the effect of the size of PdNPs on the photoreduction was also discussed. The smaller size of PdNPs leads to a higher reaction rate but lower selectivity, while the larger size of PdNPs results in a lower reaction rate but higher selectivity. These self-assembled nanocomposites also exhibit high CO2 photoreduction performance under a low CO2 atmosphere with an evolution rate of 34.5 μmol g−1 h−1.
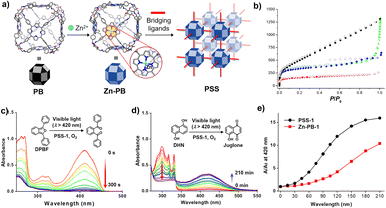 | ||
| Fig. 4 (a) Construction of cage-based MOFs by using a porphyrin box; (b) N2 sorption isotherm at 77 K for ‘PB’ (red), ‘PSS1’ (black), ‘PSS3’ (green) and ‘PSS4’ (blue); electronic absorption spectra of DPBF (c) and DHN (d) in the presence of PSS-1, O2 and visible-light irradiation; (e) comparative catalytic performance of PSS-1 (black) and Zn-PB (red) for oxidation of DHN. Reproduced from ref. 57. Copyright 2018, The American Chemical Society. | ||
3.2 Electrocatalysis
POCs, POC-based composites and POC-derived materials play an important role in converting light energy into chemical energy. They are not only excellent in the field of photocatalysis, but also represent a kind of star material in the field of electrocatalysis, and have aroused strong research interest of scientists. The electrocatalytic applications of POC-based materials are mainly focused on the CO2 reduction reaction (CO2RR), oxygen reduction reaction (ORR), hydrogen evolution reaction (HER), nitrate reduction reaction (NO3RR), oxygen evolution reaction (OER) and so on.The chloroiron(III) porphyrin box (Fe-PB) was designed by reacting the previously reported porphyrin organic cage PB-1 with FeBr2.75 Compared with the monomer catalyst, the large cavity of PB-1 facilitates the exposure of active sites and substrate diffusion, thus generating carbon monoxide with a higher rate, Faraday efficiency, current density and TON in water at pH 7.3. Fe-PB did not significantly change the reaction mechanism compared with 1, indicating that the POC structure could enhance the electrocatalytic efficiency. These results provide a new idea for the design of supramolecular catalysts. It is also the first case of electrocatalytic reduction of carbon dioxide to carbon monoxide by POC molecular materials. In 2023, a series of cofacial porphyrin POCs (CPOC-M, M = H2, Co(II), Ni(II), Cu(II) and Zn(II)) were constructed from 5,10,15,20-tetrakis(4-formylphenyl) porphyrin (TFPP) and chiral(2-aminocyclohexyl)-1,4,5,8-naphthalimide (ANDI) via covalent template self-assembly and subsequent metallization,76 as shown in Fig. 5a–e. The photophysical behavior and electrocatalytic performance of CO2 reduction were studied comprehensively. According to the results of femtosecond transient absorption spectroscopy, the photoexcitation of CPOC-H2 and its subsequently synthesized Zn and Co counterparts leads to a rapid energy transfer from the triplet porphyrin to the NDI unit. In addition, compared with other metals CPOC-M (M = Ni(II), Cu(II), Zn(II)) and monomeric porphyrin cobalt, CPOC-Co has better electrocatalytic activity, providing a partial current density of 18.0 mA cm−2 at −0.90 V, and the Faraday efficiency of CO is 90%.
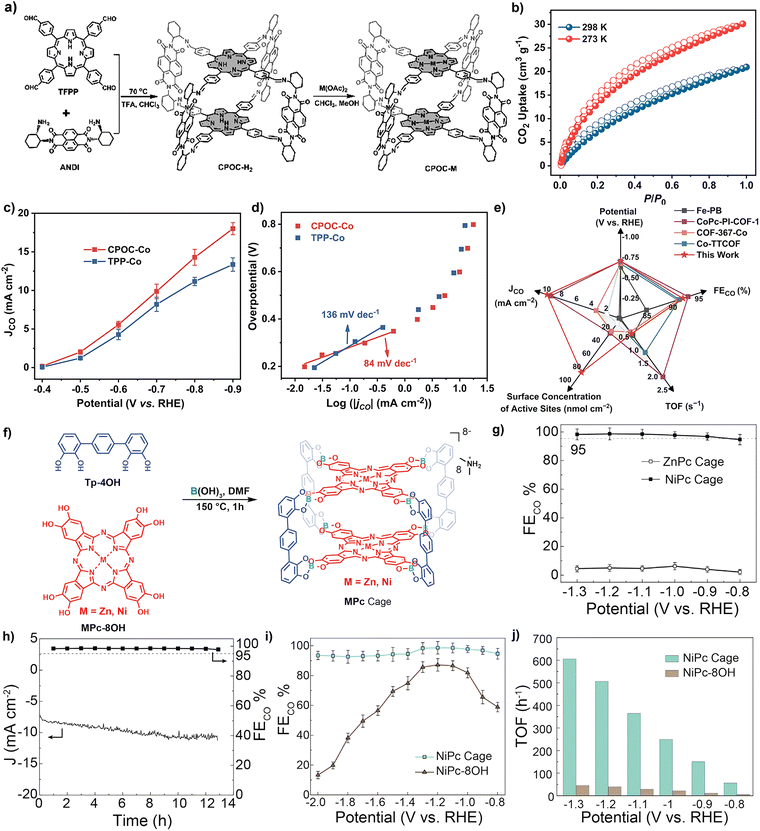 | ||
| Fig. 5 (a) Schematic synthesis of CPOC-M (M = H2, Co(II), Ni(II)); (b) CO2 adsorption (solid) and desorption (hollow) curves of POC-H2; (c) comparison of the partial CO current density between CPOC-Co and TPP-Co; (d) Tafel plots of CPOC-Co and TPP-Co; (e) comparison of the performance to that of other materials. Reproduced from ref. 76. Copyright 2023, The Royal Society of Chemistry. (f) Pictorial representation of the formation of an MPc cage; (g) CO faradaic efficiency of ZnPc and NiPc cages; (h) stability test of the NiPc cage at 1.2 V for 13 h; (i) CO faradaic efficiency of the NiPc cage and NiPc-8OH; (j) TOF comparison of the NiPc cage and NiPc-8OH. Reproduced from ref. 77. Copyright 2023, The Cell Press. | ||
Similar to the porphyrin molecular structure, planar conjugated macrocyclic phthalocyanine (Pc) is also an excellent electrocatalytic active group. Due to the large aromatic system and modifiable structure, it can act as a catalyst in the electrochemical reaction to achieve the CO2 reduction reaction. However, the synthetic challenges associated with geometric requirements and poor solubility of phthalocyanines lead to only one discrete shape-persistent POC composed of Pc parts. The Zhang group reported the synthesis of Zn and Ni metallized phthalocyanine molecular cages by one-step connection from dynamic borates, and revealed the cage-like structure of ZnPc by single crystal X-ray diffraction (Fig. 5f–j).77 In addition, the isolated redox active metal sites, easily accessible cavities and stable skeletons ensure that nickel-metallized phthalocyanine (NiPc) cages exhibit high catalytic efficiency, selectivity and stability, and are superior to other molecules in the electrocatalytic CO2RR.
The electrocatalytically active sites of the POCs described above are located in the cage. In addition, with the help of the cavity of POCs and the special adsorption capacity of CO2, it can also be combined with other materials to improve its electrocatalytic performance. The Han group reported a strategy to enhance the electrocatalytic reduction of CO2 to multi-carbon products by using CC3 as an additive to improve the diffusion of CO2 to the Cu-nanorod catalyst surface.78 In this work, nanocatalysts were combined with CC3 and used as catalysts in a gas-diffusion electrode. Studies have shown that the addition of CC3 can significantly improve the efficiency of various nanocatalysts (Fig. 6). Among them, the Faraday efficiency of the Cu-nanorod/CC3 composite catalyst can reach 76.1%, and the current density is 1.7 A cm−2. Studies have shown that the pores of CC3 can absorb the CO2 accumulated during the reaction, and the CO2 in CC3 is easier to diffuse into the catalytic layer than the CO2 in the liquid electrolyte. This work opens up a new way to design and construct an efficient CO2RR catalyst.
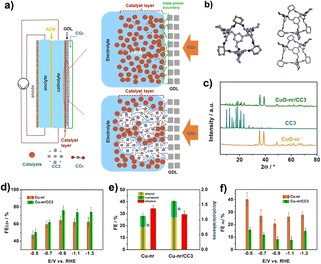 | ||
| Fig. 6 (a) Schematic diagram of the flow-cell; (b) the crystal structure of CC3; (c) the XRD patterns of CC3, CuO-nr and CuO-nr/CC3; (d and e) the FE of C2+ products and H2 at different potentials of Cu-nr/CC3 and Cu-nr; (f) FE of C2+ alcohols and ethylene over different catalysts at a potential of 0.9 V versus RHE. Reproduced from ref. 78. Copyright 2022, Wiley-VCH. | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 56 s−1, respectively. In contrast to CoTPP, the catalytic efficiency of CoPB-1(8) increased by 15 times, which can be attributed to the increase in electrochemically active cobalt centers and the supramolecular adsorption of NO3− on the CoPB-1(8) catalyst. Further modification of peripheral alkyl substituents reveals the important influence of cage porosity and cavity size on the electrochemical NO3RR. This research group also assembled a permanently porous supramolecular cage Co-PB-1 for a selective ORR catalyst,80 which contains 6 Co-TPP subunits and is connected by 24 imine bonds (Fig. 7a and c). These imine connectors were reduced to amines to obtain a more flexible cage-like Co-rPB-1. Co-PB-1 and Co-rPB-1 produced 90–100% H2O2 by the electrocatalytic ORR in neutral pH water, while the Co-TPP monomer produces a mixture of 50% H2O2 and H2O. The high H2O2 selectivity is attributed to the site isolation of discrete molecular units in a supramolecular solid. The same material, Co-PB-1,81 was used for the electrochemical HER (Fig. 7a and d). Compared with CoTPP, the electrochemically active cobalt atoms of the Co-PB-1 film are increased by five times. The Tafel slope of Co-PB-1 is 119 mV per decade, and the HER activity reached 19
000 and 56 s−1, respectively. In contrast to CoTPP, the catalytic efficiency of CoPB-1(8) increased by 15 times, which can be attributed to the increase in electrochemically active cobalt centers and the supramolecular adsorption of NO3− on the CoPB-1(8) catalyst. Further modification of peripheral alkyl substituents reveals the important influence of cage porosity and cavity size on the electrochemical NO3RR. This research group also assembled a permanently porous supramolecular cage Co-PB-1 for a selective ORR catalyst,80 which contains 6 Co-TPP subunits and is connected by 24 imine bonds (Fig. 7a and c). These imine connectors were reduced to amines to obtain a more flexible cage-like Co-rPB-1. Co-PB-1 and Co-rPB-1 produced 90–100% H2O2 by the electrocatalytic ORR in neutral pH water, while the Co-TPP monomer produces a mixture of 50% H2O2 and H2O. The high H2O2 selectivity is attributed to the site isolation of discrete molecular units in a supramolecular solid. The same material, Co-PB-1,81 was used for the electrochemical HER (Fig. 7a and d). Compared with CoTPP, the electrochemically active cobalt atoms of the Co-PB-1 film are increased by five times. The Tafel slope of Co-PB-1 is 119 mV per decade, and the HER activity reached 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 turnovers with 100% Faraday efficiency in over 24 hours.
000 turnovers with 100% Faraday efficiency in over 24 hours.
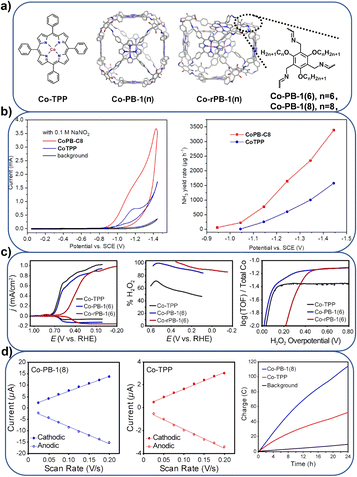 | ||
| Fig. 7 (a) Pictorial representation of different porphyrin boxes; (b) the NO3RR performance comparison of CoPB-1(8) and Co-TPP; Reproduced from ref. 79. Copyright 2023, Wiley-VCH. (c) The ORR performance comparison of Co-PB-1(6), Co-rPB-1(6) and Co-TPP; Reproduced from ref. 80. Copyright 2020, Wiley-VCH. (d) The HER performance comparison of Co-PB-1(8) and CoTPP. Reproduced from ref. 81. Copyright 2021, Wiley-VCH. | ||
In addition, the cage-to-COF strategy has also been explored to develop high-performance electrocatalysts.82 The Chen group used bicyclocalix[2]arene[2]triazines tri-aldehyde as a building block to design and synthesize POC derivatives, Cage-COF-1 (Fig. 8). The cage-like structure results in a weak interaction between adjacent layers in the COF structure, which can exfoliate the original bulk COF into thin COF nanosheets (Cage-COF-1-Ns). Subsequently, Cage-COF-1-Ns/Co was obtained by post-modification of Co2+. The material has a low overpotential (330 mV) and Tafel slope (56 mV dec−1) in the OER, showing good catalytic activity and stability.
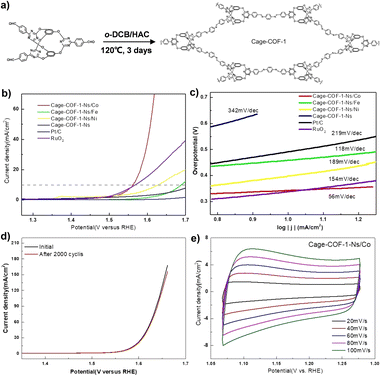 | ||
| Fig. 8 (a) The synthesis and structure of a POC-derived material, Cage-COF-1; (b) OER polarization curves for different Cage-COF-1-Ns; (c) Tafel slopes of Cage-COF-1-Ns in the OER; (d) OER polarization curves of Cage-COF-1-Ns/Co before and after 2000 potential cycles; (e) the CV curves of Cage-COF-1-Ns/Co. Reproduced from ref. 82. Copyright 2022, Wiley-VCH. | ||
So far, there are only limited electrocatalytic reactions based on the POC-based materials described above, and more types of electrocatalytic reactions still need to be further explored. These cases have shown the important role of POCs with discrete catalytically active sites in electrocatalytic reactions. The versatility of POCs as supramolecular platforms for the development of adjustable and efficient electrocatalysts will continue to be expanded.
3.3 Separation
Light hydrocarbon (LH) separation is a key process in the petrochemical industry. At present, the separation is achieved mainly by low temperature distillation, which consumes a lot of energy in the separation process. The use of porous solids as adsorption and separation materials has received extensive attention due to its lower energy consumption and higher efficiency. POCs have excellent stability, an adjustable pore structure and abundant adsorption active sites, POCs can be used as a new type of adsorbent to separate light hydrocarbons. The Yuan group proposed a new strategy to adjust the gas adsorption and separation performance by using different supramolecular isomers of the same POC molecule.83 A lantern-like calix [4] resorcinol calixarene-based POC, CPOC-101, was prepared, and eight different solvatomorphs can be obtained by crystallization in different solvents. Those solvatomorph materials show significant differences in gas adsorption capacity and separation ability. The Brunauer–Emmett–Teller (BET) surface area of CPOC-101α crystallized in toluene/chloroform was as high as 406 m2 g−1 measured by nitrogen adsorption at 77 K, which was much higher than that of other CPOC-101 solvatomorphs. More interestingly, the C2H2 and CO2 adsorption capacity of CPOC-101α and the C2H2/CO2 separation selectivity at room temperature are superior to those of the representative of POC solvatomorphs. Later, two novel nitrogen-rich POCs, CPOC-107 and CPOC-203, were constructed from bowl-shaped tetraformylcalix[4]resorcinol calixarene and different nitrogen-rich imidazolyl diamines.84 CPOC-107 has a [2 + 4] lantern-like structure, while CPOC-203 has a [3 + 6] triangular prism structure (Fig. 9). In addition, CPOC-107 has a large cavity volume of up to 787 Å3 and a high BET specific surface area of up to 1202 m2 g−1. Their high specific surface area and high nitrogen content show that both cages showed good C2H2 adsorption capacity. Specifically, CPOC-107 achieved a C2H2 adsorption value of up to 146 cm3 g−1 at 298 K and 1 atm, which is the highest among all porous organic materials. The experimental breakthrough test confirms the effective separation of the C2H2/CO2 mixture by the CPOC-107 adsorbent, and the breakthrough time is 13 min g−1. This study suggests that nitrogen-rich POCs may be more promising materials for C2H2 storage and purification.
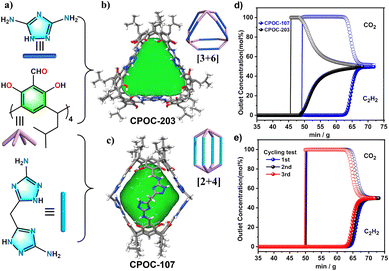 | ||
| Fig. 9 (a–c) The chemical and crystal structures of CPOC-203 and CPOC-107; (d) experimental breakthrough curves for an equimolar mixture of C2H2/CO2 at 298 K; (e) the recyclability of CPOC-107 under multiple mixed gas column breakthrough tests. Reproduced from ref. 84. Copyright 2023, The Royal Society of Chemistry. | ||
The Zhang group reported another flexible and stable POC crystal (NKPOC-1-α), which can be used for effective acetylene separation (Fig. 10).85 The large-amplitude continuous breathing behavior of the C2H2 adsorption experiment proves that the intra-cage pores and inter-cage pores of the organic cage are the reasons for its high adsorption capacity and selectivity. However, for C2H4, its breathing behavior only comes from the inter-cage pores, which has been proved by adsorption experiments and molecular dynamics simulations. In addition, at 253 and 273 K, NKPOC-1-α adsorbs a large amount of C2H2 through the opening of a gate, while the amount of C2H4 adsorbed is negligible. Breakthrough experiments reveal that this flexible and robust material can effectively separate C2H2 from the C2H2/C2H4 mixture. In addition, NKPOC-1-α is the first POC with this function, and is expected to be used for industrial gas separation.
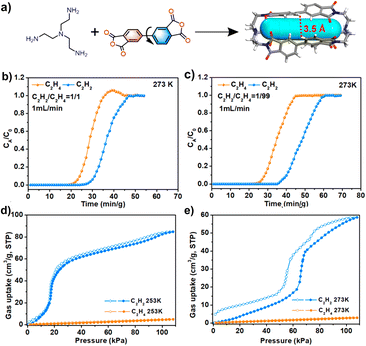 | ||
| Fig. 10 (a) Synthesis of NKPOC-1; (b and c) adsorption–desorption isotherms of NKPOC-1-α for C2H2 and C2H4 at 253 and 273 K; (d and e) breakthrough curves of NKPOC-1-α for the C2H2/C2H4 mixture (1/99, v/v) and (50/50, v/v) with a gas flow of 1 mL min−1 at 273 K. Reproduced from ref. 85. Copyright 2023, The American Chemical Society. | ||
The removal of C2H6 from its analogue C2H4 is also crucial in the petrochemical industry, and their similar physical and chemical properties make their separation extremely challenging. The emerging POC materials for C2H6/C2H4 separation are still in their infancy. An example of a POC adsorbent (CPOC-301) based on octahedral calix[4]resorcinarene is reported,86 which can be used as a robust adsorbent to directly separate high-purity C2H4 from the C2H6/C2H4 mixture. Molecular simulation studies have shown that the excellent C2H6 selectivity is due to the suitable cage cavity in CPOC-301, which forms more multiple C–H⋯π and hydrogen bonds with C2H6 than C2H4. This work provides another new way for the highly selective separation of industrially important hydrocarbons using POCs.
Another design strategy for light hydrocarbon separation materials is to introduce POCs into the polymers to form a composite membrane. Because POCs are soluble in organic solvents, they can be mixed with polymers at the molecular level, which is more advantageous than traditional solid fillers. Based on this, the CC3 particles or CC3 molecules were uniformly distributed in the polymer to form a hierarchical transport channel membrane.87 The membrane displays very fast C3H6 permeability (390 Barrer) and a good C3H6/C3H8 separation factor (12.1) through the C3H6 and C3H8 mixture (1/1, v/v) penetration test. More importantly, it also shows a stable separation effect in long-term tests, and has practical application potential in light hydrocarbon separation. Similarly, ionic liquids (ILs) were introduced into CC3 to construct an IL@CC3/PIM-1 membrane with the help of polymers of intrinsic microporosity (PIM), achieving effective separation of CO2 and CH4.88 Doping ILs in the membrane can regulate the cavity size of POCs, enhance the solubility of CO2, improve the compatibility of POCs and PIM, and further improve the diffusion selectivity, permeability and stability of the membrane. Compared with the CC3/PIM-1-10% membrane, the CO2 permeability coefficient and CO2/CH4 selectivity of the membrane with ILs were increased by 15.9% and 106.2%, respectively. This provides a new idea for the separation of light hydrocarbons by POC-based membranes. In 2023, a composite membrane formed by POC (RCC3) cross-linked with terephthaloyl chloride (TPC) was proposed and constructed for the first time.89 The pore size of RCC3 is about 5.4 Å with a high specific surface area of 442.3 m2 g−1, and imine-rich nanochannels are responsible for the rapid penetration of CO2. In addition, the interfacial cross-linking reaction between RCC3 and TPC enables the TPC-RCC3 ultrathin film to be assembled on the surface of the modified polysulfone substrate. In addition, trace piperazine was further used to regulate the cross-linking degree of the ultra-thin film for improving the CO2/N2 selectivity. The prepared composite membrane has a high CO2 permeance of 4303 GPU with a CO2/N2 selectivity of 30, and CH4 permeance of 1216 GPU with a CH4/N2 selectivity of 3.0 at 1 bar, and can maintain permeation selectivity under long-term operation. Its excellent separation performance provides a more economical solution for CO2 capture in flue gas or natural gas purification.
The cage-to-COF strategy can also be used to design new materials for the separation of light hydrocarbons. The Yuan group introduced different substituents on the linker to fine-tune the network conformation of the material to obtain three isostructural POC-based COF materials (3D-OC-COF-H, 3D-OC-COF-OH, and 3D-OC-COF-Cl),90 as shown in Fig. 11. All three COFs exhibit high specific surface area compared to the precursor cage. Due to the unique pore environment and different functional groups, 3D-OC-COF-OH exhibits excellent CO2/CH4 separation performance.
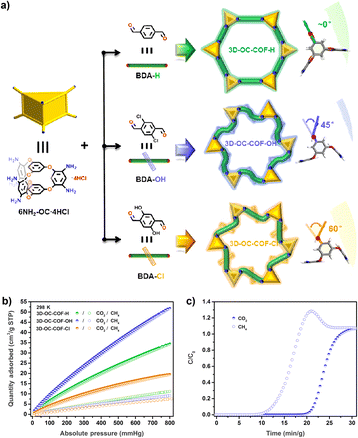 | ||
Fig. 11 (a) POC-based COFs containing different substituents; (b) N2, CO2, and CH4 adsorptions of the three COFs at 298 K; (c) experimental column breakthrough curves for CO2/CH4 (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, v/v) of 3D-OC-COF-OH at room temperature. Reproduced from ref. 90. Copyright 2021, The Chinese Chemical Society. 50, v/v) of 3D-OC-COF-OH at room temperature. Reproduced from ref. 90. Copyright 2021, The Chinese Chemical Society. | ||
Rare gases are often used as protective gas or chemical raw materials in industrial production. The separation and purification of rare gases is a complex and high energy consumption process. POCs also have unique advantages in the separation of rare gases. In 2014, the separation of rare gases was realized using CC3.91 The diameter of the internal cavity of the CC3 molecule is 4.4 Å, which is very similar to the diameter of the xenon (Xe) gas (4.1 Å). The narrowest diameter of the 3D channel formed by the stacking of adjacent cage molecules is 3.6 Å, slightly smaller than the diameter of krypton (Kr) (3.69 Å), which seems to hinder the diffusion of Xe or radon (Rn) gas. However, molecular dynamics simulations show that the flexible structure of CC3 molecules can lead to dynamic fluctuations in the pore size, which makes it possible to adsorb large-sized rare gases (including Kr, Xe and Rn) in a limited time range. Breakthrough experiments also confirm that CC3 can separate Kr, Xe and Rn from air at very low concentrations.
In 2020, the Carreon group successfully grew continuous CC3 composite membranes on alumina porous tubes and evaluated the performance of the membrane in the separation of Xe from light gases (He, CO2, Kr and CH4).92 As a part of the membrane, CC3 is suitable for screening He, CO2, Kr and CH4 (kinetic pore size 2.6–3.8 Å). The dynamic diameter of Xe is 4.1 Å, which is larger than the maximum pore size of CC3, so the membrane can effectively separate Xe from light gas. In addition, the CC3 membrane has high gas permeability for He, CO2, Kr and CH4, reaching 2114, 1705, 773 and 1962 GPU, respectively.
In the present case, the separation of D2 and H2 is mainly achieved by electrolysis of heavy water or low temperature distillation at 24 K, and the separation process is cumbersome, costly and energy-consuming. It is difficult for organic framework materials to screen D2 and H2 by finely adjusting the pore size. Small changes in the molecular structure of discrete POCs will have a profound impact on its solid accumulation. From the realization of the regulation of pore structure, POCs are expected to become the sorbent candidates for D2 and H2.93 The strategy of organic synthesis was employed to regulate the internal cavity of the cage molecule, with “tying” of the diamine group with formaldehyde, acetone or acetaldehyde to realize ultrafine control over the cage cavity size. Combining small pore and large pore cages into a porous solid makes the material have high D2/H2 selectivity (8.0) and high D2 absorption capacity (4.7 mmol g−1).
The Vogel group elucidated the mechanism of different functionalizations in POCs to control the separation of D2 and H2 from the perspective of theoretical simulation.94 By using ab initio molecular dynamics simulations, the specific mechanism of the interaction between RCC3, CC3-S and 6ET-RCC3 and hydrogen isotopes was clarified. Through the evaluation of multiple CC3-type POCs, the effects of temperature and functionalization on the movement of hydrogen isotopes were confirmed. This is expected to help realize the directional design of new materials that can be used for hydrogen isotope separation.
As early as 2013,95 the Cooper group used CC3 to separate C8 (such as xylene isomers) and C9 hydrocarbons (such as mesitylene and ethyltoluene isomers). Molecular simulation shows that linear molecules (p-xylene and 4-ethyltoluene) preferentially bind to CC3 molecules rather than other C8 and C9 isomers. Gas chromatography (GC) analysis showed that mesitylene passed through the CC3 column quickly, while 4-ethyltoluene was selectively retained in the column. This study reveals the unique advantages of POC materials in performing specific separation tasks. In 2016,96 this group ultilized CC3 coated in a standard capillary column as a chromatographic stationary phase for GC separation of a series of mixtures, including aromatic compounds, racemic mixtures and hexane isomers. For the separation of hexane isomers, the separation effect of the CC3-based column was better than that of other commercially available GC columns. In 2020,97 an azobenzene-based organic cage (AZO-Cage) was reported to selectively separate steam and liquid state xylene isomers, with the highest selectivity among organic adsorbents. Although the crystal structure did not show a porous structure, the selective adsorption of p-xylene was achieved through the C–H⋯π interaction between the azo bonds in the AZO-Cage and the methyl hydrogens of the xylene. The material can also be regenerated under vacuum and reused. The Khashab group designed a triamine crystal molecule for the separation of styrene and ethylbenzene, and the selectivity of styrene was as high as 99%.98 It was confirmed by DFT calculation that the planar styrene can be easily embedded in the cavity of triamine compared to ethylbenzene, thus showing excellent separation for styrene. A thienothiophene cage (ThT-cages) with adaptive porosity has been reported for the separation of cyclohexane from benzene in 2021.99 The purity of cyclohexane selectively captured by ThT-cages from an equimolar benzene/cyclohexane mixture was 94%. This high selectivity is due to the multiple interactions between cyclohexane and thienothiophene ligands. In 2022, this group synthesized a cylindrical triamine macrocycle (P-TA) with a deeper cavity,100 which can selectively capture 1-hexene from trans-3-hexene (selectivity > 84%), as shown in Fig. 12. This selective adsorption is produced by the formation of a thermodynamically stable host–guest complex between 1-hexene and P-TA. After that, this group designed a highly flexible 4,4′-oxybisbenzaldehyde-based cage (Oba-cage), which can selectively adsorb ortho-isomers from monohalotoluene isomers (X-toluene, X = F, Cl, Br), and the adsorption rate of ortho-bromotoluene is as high as 93%.101 In addition, the adsorption rate of Oba-cage for these ortho-isomers increases with the increase in halogen substituents.
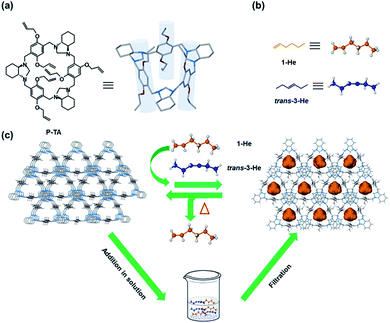 | ||
| Fig. 12 (a) Chemical and crystal structures of P-TA; (b) linear hexene isomers; (c) schematic representation of selective hexene isomer separation by T-PA. Reproduced from ref. 100. Copyright 2023, The Royal Society of Chemistry. | ||
In practical applications, more industrial product separation scenarios involve more complex mixtures of ternary systems. For example, alkanes of different components are separated from crude oil. Graded molecular sieving, that is, using the same membrane to separate molecules of different sizes from a multivariate mixture, greatly reduces cost and energy consumption compared to using different techniques to screen different molecules. The POCs are prepared as a thin film material to regulate the selectivity of the membrane to different molecules to achieve graded molecular sieving.
The Cooper group prepared composite membranes by growing crystalline CC3 on a polyacrylonitrile carrier.102 The membrane has ultrafast solvent permeability and a high rejection rate for organic dyes with a molecular weight greater than 600 g mol−1. The pore size of the composite membrane can be switched to larger pores in methanol, and the molecular weight cut-off reaches 1400 g mol−1. By changing the content of methanol, the composite membrane can be switched between two phases with different selectivities, which makes it possible to use a single membrane to screen the three organic dyes from the mixture. In 2022, the Sun group chemically crosslinked a series of cage-like molecules into flexible nanofilms with a thickness of 8 nm.103 The composite membrane exhibits nanofluidic channels with excellent water permeability, and is superior to commercial nanofiltration membranes by 1–2 orders of magnitude. In addition, the membrane shows a photoresponsive gating effect, which can realize the separation of different organic dyes. In 2023, the Celebi group reported a hierarchically networked POC (CC3) membrane with dynamic pore control.104 The cage membrane can reversibly adjust the pore size and selectivity through different solvents and ratios (water, methanol and DMF). The membrane can be effectively used for the separation of complex organic mixtures. A series of crystalline self-supporting POC films were obtained by interfacial polymerization design.105 During the condensation of aldehydes and amines, intramolecular hydrogen bonds ensure the formation of a defect-free POC film. Powdery products are more easily obtained in organic cages without intramolecular hydrogen bonds. In addition, the obtained membranes showed high rejection for organic dyes in water and organic solvents. The membrane also has accurate screening ability in organic solvents.
3.4 Batteries
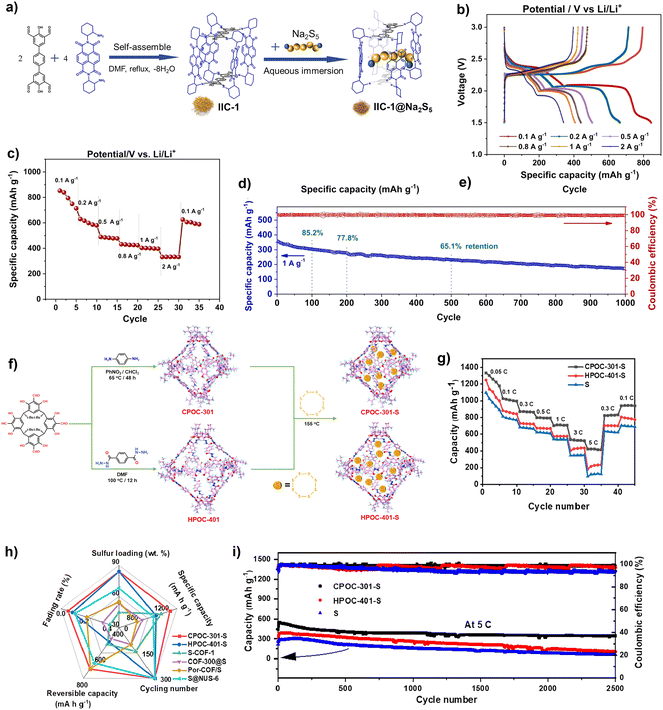 | ||
| Fig. 13 (a) Synthesis of IIC-1 and the encapsulation of Na2S5; (b) galvanostatic charge/discharge profiles at different current densities; (c) rate performance; (d) cycling performance and CE. Reproduced from ref. 106. Copyright 2022, Elsevier. (e) Synthesis of CPOC-301, HPOC-401, CPOC-301-S and HPOC-401-S; (f) rate performance of S, CPOC-301-S, and HPOC-401-S; (g) cycling performance of S, CPOC-301-S, and HPOC-401-S; (h) comparison of the performance to that of other cathodes in LSBs; (i) cycling performance of S, CPOC-301-S and HPOC-401-S. Reproduced from ref. 107. Copyright 2023, The American Chemical Society. | ||
Compared with other reported MOFs and COFs, as shown in Table 2, the cathode based POC materials show comparable stability performance in LSBs. Although the specific capacity of IIC-1 and CE is relatively low, their cycle stabilities are not reduced due to the nature of the molecular material with a high percentage of the inert component. Even some POC materials have a cycle stability of more than MOFs and COFs, such as CPOC-301 can still maintain 63% of its initial capacity after 2500 cycles, and the specific capacity also reached 1302 mA h g−1. Unfortunately, so far, there are only a few cases of the application of POCs in LSBs. The POC-based LSB materials, including the electrode, electrolyte and separation membrane, need to be further developed toward enhancing the corresponding performance.
| Materials | Category | Specific capacity | Capacity retention | Ref. |
|---|---|---|---|---|
| IIC-1 | POC | 852 mA h g−1 | 50% (1000 cycles) | 106 |
| CPOC-301 | POC | 1302 mA h g−1 | 63% (2500 cycles) | 108 |
| CE | POC | 907 mA h g−1 | 52% (200 cycles) | 107 |
| HZ-DMTD | COF | 642 mA h g−1 | 79% (200 cycles) | 109 |
| 3D-flu-COF | COF | 1249 mA h g−1 | 75% (1000 cycles) | 110 |
| TFPB-TAA | COF | 1288 mA h g−1 | 69% (400 cycles) | 111 |
| PI-COF | COF | 1330 mA h g−1 | 96% (100 cycles) | 112 |
| Ni3(HITP)2 | MOF | 1303 mA h g−1 | 65% (100 cycles) | 113 |
| Cu-TDPAT | MOF | 1675 mA h g−1 | 56% (500 cycles) | 114 |
| Mn-CCs | MOF | 1460 mA h g−1 | 68% (200 cycles) | 115 |
| ZIF-8 | MOF | 1055 mA h g−1 | 76% (300 cycles) | 116 |
Thin films with effective ion screening ability are in great demand in energy storage batteries. Another application of POCs is as a separator for LSBs. Solution processing into various devices is one of the advantages of POC materials. With this in mind, the Xie group reported a functional separator based on a POC (CC3) for fast and selective ion transport (Fig. 14).117 The ion separation effect can be adjusted by using the design and preparation of the controllable thickness and porosity of the film. In this case, the functional separator assembled by using CC3 can selectively screen Li ions and effectively inhibit the production of unexpected polysulfides. The separator modified by using a POC film can lead to a battery with good cycling performance and rate performance. This new composite separator made of soluble POCs and the PP shows broad prospects in high-performance energy storage devices.
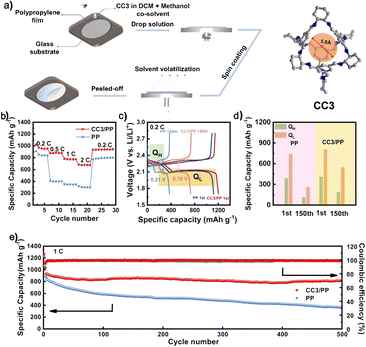 | ||
| Fig. 14 (a) The fabrication process to produce CC3/PP separators; (b and c) rate performance and charge/discharge profiles of the batteries with different separators; (d) specific capacities of high-voltage plateaus (QH) and low-voltage plateaus (QL) for batteries with different separators; (e) cycling performance of batteries with different separators. Reproduced from ref. 117. Copyright 2022, The American Chemical Society. | ||
Compared with liquid LIBs, all solid-state lithium batteries (SSLBs) have higher energy density and better safety, but the entire battery needs to have an effective ion conduction path. A POC-based ionic conductor, Li-RCC1-ClO4, has been developed as a solid cathode for SSLBs (Fig. 15).120 Due to the solution processability of POCs, Li-RCC1-ClO4 can be dissolved in the cathode slurry, which allows the use of traditional slurry coating methods to manufacture solid cathodes. This is often not possible for other insoluble porous solids, such as MOFs and COFs. The functional groups in the cavity of POC were ionized to provide an environment for efficient dielectric screening. Lithium ions and added lithium salts (such as LiClO4) dissociated into mobile ions. These cage materials can uniformly dissolve in the cathode slurry, and grow and crystallize on the cathode surface during the coating process, and thereby an effective ion conduction network was formed inside the cathode. This employment of organic cages in SSLBs (the SSLBs containing 20% of Li-RCC1-ClO4) can minimize ionic additives for the cathode, producing excellent cycling performance (capacity retention rate of 88.2% after 750 cycles) at room temperature.
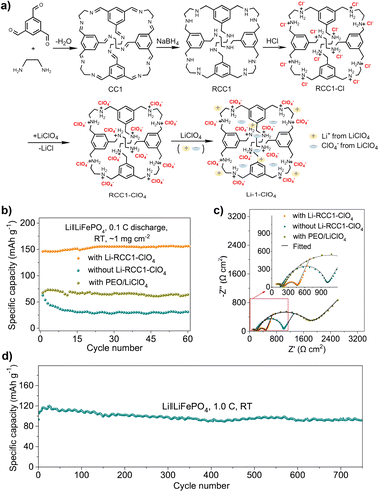 | ||
| Fig. 15 (a) Synthetic procedure for the porous cage electrolyte, Li-RCC1-ClO4; (b) cycling performance and (c) electrochemical impedance spectra profiles of the SSLBs at room temperature using LiFePO4 solid-state cathodes with different catholytes; (d) cycle performance of the all-solid-state cell with Li-RCC1-ClO4. Reproduced from ref. 120. Copyright 2022, Springer Nature. | ||
Rechargeable Li–Cl2 batteries are high energy density and safe energy storage batteries. Although the theoretical specific capacity is very high, the cycle life of the Li–Cl2 battery is short and the CE is low due to the slow and insufficient supply of Cl2 during the redox reaction. In order to solve this problem, an imine-functionalized POC was utilized as the cathode material of the Li–Cl2 battery to capture Cl2 molecules for achieving high discharge capacity and CE.121 Density functional theory calculations show that the imine group sites in the host cage have a strong interaction with Cl2, which is conducive to the rapid capture of Cl2. As a result, the capacity of the Li–Cl2 battery using the POC (Li–Cl2@POC) is significantly improved, and an ultra-high discharge capacity of 4000 mA h g−1 is achieved with 100% CE. The Li–Cl2@POC battery exhibits excellent electrochemical performance even at low temperature. It can be stably cycled for 200 cycles at a capacity of 2000 m Ah g−1 at −20 °C, and the average CE is 99.7%.
Light-assisted LIBs are considered to be a promising technology that can simultaneously convert and store sunlight into electrochemical energy. However, there are still limitations in the design and synthesis strategies of photo-assisted organic cathodes for lithium-organic batteries. The Wang group reported a material composed of C60 and POCs, C60@POC, which can be used as a positive electrode for photo-assisted lithium-organic batteries to achieve efficient solar energy capture, conversion and storage (Fig. 16).122 The structural characterization shows that the thermodynamic C60 and POC are conducive to the formation of a complex with a host–guest stoichiometric ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. Optical and electrochemical analyses showed that C60@POC not only enhanced charge generation/separation, but also accelerated the kinetics of the electrochemical lithium storage/release reaction compared with pristine POC. Under illumination, the battery based on the C60@POC cathode shows excellent improved performance including increasing output power, reducing input power, enhancing light conversion efficiency, and promoting efficient solar energy conversion and storage during charging and discharging. This example integrates the capture and conversion of light energy with the storage of chemical energy in POCs, which is helpful in expanding the application of more POCs in energy conversion and storage.
2. Optical and electrochemical analyses showed that C60@POC not only enhanced charge generation/separation, but also accelerated the kinetics of the electrochemical lithium storage/release reaction compared with pristine POC. Under illumination, the battery based on the C60@POC cathode shows excellent improved performance including increasing output power, reducing input power, enhancing light conversion efficiency, and promoting efficient solar energy conversion and storage during charging and discharging. This example integrates the capture and conversion of light energy with the storage of chemical energy in POCs, which is helpful in expanding the application of more POCs in energy conversion and storage.
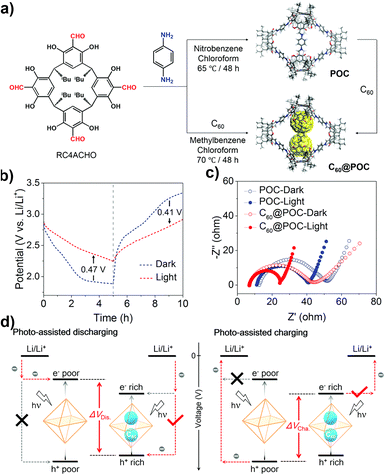 | ||
| Fig. 16 (a) Schematic representation of the synthesis of POC and C60@POC; (b) charge and discharge curves of C60@POC with or without illumination; (c) Nyquist plots of POC and C60@POC with or without illumination; (d) schematic diagram of the photo-assisted Li-organic battery with POC and C60@POC cathodes. Reproduced from ref. 122. Copyright 2022, The Royal Society of Chemistry. | ||
3.5 Proton conductivity
Solid electrolytes for ion conduction have attracted extensive interest of researchers due to their wide application in energy fields such as fuel cells, flow batteries and LIBs. In recent years, crystalline porous framework materials such as MOFs and COFs have been widely studied due to their high porosity, high specific surface area and chemical functional groups. Unlike amorphous polymers, crystalline materials have tunable pore networks and regular frameworks, which make them potential candidates for proton conductivity. However, due to their poor machinability, it is still challenging to fabricate crystalline porous materials into mechanically stable films, which limits their proton conductivity properties. POCs are an emerging kind of porous molecular material. Due to the inherent channel structures and good machinability, POCs show great potential for proton conductivity.A bottom-up strategy was proposed to obtain two different supramolecular conductors by self-assembly of Cage-1 (larger cavity size and more hydrogen bond anchors) and Cage-2 (small cavity size and fewer hydrogen bond anchors) with the same chemical composition but different structural characteristics.124 Cage-1 is self-assembled to form a crystalline phase with a proton hopping path. The proton conductivity is based on the Grotthuss mechanism, and the proton conductivity is 1.59 × 10−4 S cm−1. The proton conductivity of Cage-2 is based on the vehicle mechanism. Its proton conductivity is about 1/200 of the former. Theoretical simulation reveals the different hydrogen bond dynamics in Cage-1 and Cage-2, which provides further insights into the potential proton diffusion mechanism.
A soluble POC (cage 3) was fabricated into a proton exchange membrane by in situ crystallization, obtaining a composite proton exchange membrane.125 The crystalline cage 3 dispersed in the Nafion matrix has an inherent three-dimensional interconnected proton pathway, which promotes the proton conductivity of the proton-exchange membrane (Fig. 17). Due to the multiple hydrogen bond interactions between cage 3 and Nafion, more continuous proton pathways are constructed. In addition, cage 3 has high water absorption, and the retention of membrane moisture ensures proton transfer at low humidity. The prepared membranes show higher proton conductivity than cast Nafion under both low and high humidity conditions. The proton conductivity of the Nafion–Cage 3 membrane reaches 0.27 S cm−1 at 90 °C and 95% relative humidity, which is much higher than that of the recast Nafion (0.08 S cm−1) under the same conditions.
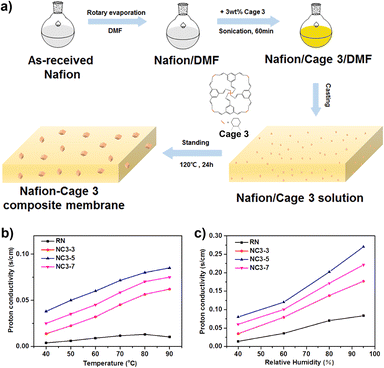 | ||
| Fig. 17 (a) Schematic illustration of preparation of the Nafion−Cage 3 composite membrane; (b) temperature-dependent (40% RH) and (c) humidity-dependent (90 °C) proton conductivity of recast Nafion and the Nafion−Cage 3 composite membrane. Reproduced from ref. 125. Copyright 2018, The American Chemical Society. | ||
A POC-based salt was developed to construct proton-transporting crystalline porous molecular solids with 3D channel structures (Fig. 18).126 For the neutral imine cage CC3, there is a hydrated 3D pore network in the material, but the proton conductivity is relatively low under wet conditions. RCC1 is obtained by reducing CC3 and then transforming it into a crystalline hydrated salt, (H12RCC1)12+·12Cl−·4H2O, the proton conductivity is significantly increased by more than 150 times, and its proton conductivity is even comparable to that of proton-conducting MOFs. For (H12RCC1)12+·6(SO4)2−·27.25(H2O), the larger radius of (SO4)2− introduced limits the diffusion of water, making the effect of RH on its proton conductivity more significant.
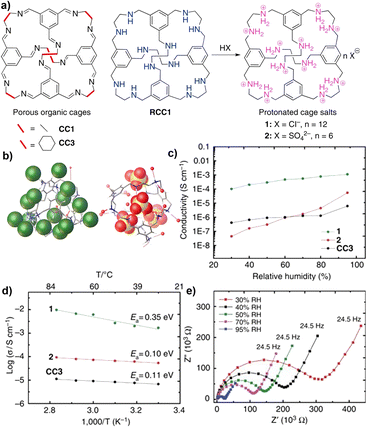 | ||
| Fig. 18 (a) Molecular proton conductors based on neutral organic cage molecules and protonated cage salts; (b) the single-crystal structures of cage salts 1 and 2; (c) proton conductivities for salts 1 and 2, and neutral CC3 at 303 K as a function of relative humidity; (d) Arrhenius plots showing the activation energies of the cage materials; (e) Nyquist plots showing the impedance of CC3 at 303 K. Reproduced from ref. 126. Copyright 2016, Springer Nature. | ||
4 Conclusions and outlook
The continuous development of advanced materials with new structures and functions is crucial for energy applications. In this perspective, we comprehensively review the latest advances in the applications of pristine POCs, POC composites and POC derivatives in energy applications, including photocatalysis, electrocatalysis (NO3RR, OER, HER, ORR and CO2RR), separation (gas separation and liquid separation), batteries (LSBs, other lithium batteries and perovskite solar batteries) and proton conductivity, as shown in Table 3. (1) Pristine POCs with microporous structures and abundant active sites have been used in separation, solar energy capture, batteries, etc., and achieved good performance. (2) POC composites integrated with POCs and functional components have been explored and efficiently utilized in photocatalysis, electrocatalysis, and batteries. POC composites can not only make up for the shortcomings of poor conductivity of original POCs, but also realize the synergistic effect between POCs and functional units, resulting in customized performance. (3) POC derivative materials, including COFs, MOFs, HOFs and other materials, have more abundant pores, higher stability and more exposed functional active sites, showing enhanced performance in many fields such as adsorption separation and catalysis. In summary, more energy-related applications can be achieved by using POCs, further strengthening and expanding their functionality.| POC | Category | Applications | Performance | Ref. |
|---|---|---|---|---|
| Zn-PB | Pristine POCs | Photocatalysts | Photooxidation of 1,5-dihydroxynaphthalene | 57 |
| P12L24 | Pristine POCs | Photocatalysts | Photooxidation of 1,5-dihydroxynaphthalene | 58 |
| PTC-1(2H) | Pristine POCs | Photocatalysts | Photooxidation of amines to imines (99% conversion, TOF > 33 h−1) | 59 |
| PyTC1 | Pristine POCs | Photocatalysts | Photooxidation of benzeneboronic acid to phenols (99% conversion) | 60 |
| Pd@MTC1-1/5 | POC composites | Photocatalysts | Photooxidation of 4-nitrophenylboronic acid to 4-aminophenol | 51 |
| Green box 5 | Pristine POCs | Photocatalysts | Photocatalytic hydrogen production (34 μmol h−1) | 62 |
| FePB-3(N) | Pristine POCs | Photocatalysts | Photocatalytic reduction of CO2 (97% selectivity; TON > 1100 h−1) | 63 |
| TPPCage·8I | Pristine POCs | Photocatalysts | Photooxidation of amines to imines (99% conversion; TOF > 1980 h−1) | 65 |
| CoP@POC | Pristine POCs | Photocatalysts | Photooxidation of amines to imines (99% conversion; TOF > 1389 h−1) | 64 |
| Au@OC1R | POC composites | Photocatalysts | Nitrobenzene to the azo compound (92% yield; TOF > 9200 h−1) | 66 |
| Ag@CC3-OH | POC composites | Photocatalysts | Nitrobenzene to the azo compound (99% conversion; TOF > 9200 h−1) | 67 |
| Au@TAE-DTE | POC composites | Photocatalysts | Photocatalytic reduction of CO2 (56.4 μmol g−1 h−1 under visible light) | 68 |
| Ag0@OB4R | POC composites | Photocatalysts | Ullmann-type coupling reaction (63–95% yield) | 69 |
| Pd5-PS | POC composites | Photocatalysts | Photocatalytic reduction of CO2 (98% CH4 selectivity; 78.5 μmol g−1 h−1) | 70 |
| PSS-1 | POC derivatives | Photocatalysts | Photooxidation of 1,5-dihydroxynaphthalene | 57 |
| Fe-PB | Pristine POCs | Electrocatalysis | Electrochemical CO2 to CO (TOF of 0.64 s−1 at −0.63 V, 100% FE) | 75 |
| CPOC-Co | Pristine POCs | Electrocatalysis | Electrochemical CO2 to CO (current density of 18.0 mA cm−2 at −0.90 V; 90% FE) | 76 |
| ZnPc | Pristine POCs | Electrocatalysis | Electrochemical CO2 to CO (TOF of 606 h−1 at −1.3 V; >95% FE) | 77 |
| Cu-nr/CC3 | POC composites | Electrocatalysis | Electrochemical CO2 to C2+ (current density of 1.7 A cm−2 at −0.90 V; 76.1% FE) | 78 |
| CoPB-1(8) | Pristine POCs | Electrocatalysis | Electrochemical NO3− to ammonia (TOF of 56 s−1 at −1.54 V; 90% FE) | 79 |
| Co-rPB-1(6) | Pristine POCs | Electrocatalysis | Electrochemical ORR (Ecat/2 of 0.48 V; 90% FE) | 80 |
| Co-PB-1 | Pristine POCs | Electrocatalysis | Electrochemical HER (Tafel slope of 119 mV per decade; 100% FE; TOF of 0.22 s−1) | 81 |
| Cage-COF-1-Ns/Co | POC derivatives | Electrocatalysis | Electrochemical OER (Tafel slope of 56 mV per decade; overpotential of 330 mV) | 82 |
| CPOC-101α | Pristine POCs | C2H2/CO2 separation | Selectivity of 11.9 and separation factor of 2.2 | 83 |
| CPOC-107 | Pristine POCs | C2H2/CO2 separation | C2H2 adsorption value of 146 cm3 g−1 at 298 K; breakthrough time of 13 min g−1 | 84 |
| NKPOC-1-α | Pristine POCs | C2H2/C2H4 separation | C2H2 adsorption value of 58 cm3 g−1 at 273 K; breakthrough time of 16 min g−1 | 85 |
| CPOC-301 | Pristine POCs | C2H6/C2H4 separation | C3H6 adsorption value of 87 cm3 g−1 at 293 K; separation factor of 1.3 | 86 |
| CC3@MMM | POC composites | C3H6/C3H8 separation | C3H6 permeability of 390 Barrer; separation factor of 12.1 | 87 |
| IL@CC3/PIM-1-10% | POC composites | CO2/CH4 separation | CO2 permeability of 7868 Barrer; CO2/CH4 selectivity of 73.4 | 88 |
| TPC-RCC3 | POC composites | CO2/N2 separation | CO2 permeance of 4303 GPU; CO2/N2 selectivity of 30 | 89 |
| 3D-OC-COF-OH | POC derivatives | CO2/CH4 separation | CO2 capacity of 89.2 cm3 g−1 at 273 K; CO2Qst of 22.4 kJ mol−1 | 90 |
| CC3 | POC composites | Xe/Kr/Rn separation | Xenon retained time > 15 min; flow rate of 40 cm3 STP per min | 91 |
| CC3 membranes | Pristine POCs | He/CO2/Kr/CH4/Xe separation | He permeability of 2114 GPU; He/Xe ideal selectivity of 13 | 92 |
| 6FT-RCC3 | Pristine POCs | D2/H2 separation | D2/H2 selectivity of 8.0; high D2 absorption capacity of 4.7 mmol g−1 | 94 |
| CC3 | Pristine POCs | C8/C9 separation | Mesitylene from C8/C9 isomers | 95 |
| CC3-R | Pristine POCs | Hexane separation | Reusability > 300 injections | 96 |
| AZO-cage | Pristine POCs | C8 separation | p-Xylene from C8 isomers | 97 |
| Macrocycle 1 | Pristine POCs | Styrene/ethylbenzene separation | Styrene selectivity of 99% | 98 |
| ThT-cages | Pristine POCs | Benzene/cyclohexane separation | Cyclohexane selectivity of 94% | 99 |
| P-TA | Pristine POCs | 1-Hexene/trans-3-hexene separation | 1-Hexene selectivity of selectivity > 84% | 100 |
| Oba-cage | Pristine POCs | Monohalotoluene separation | Ortho-bromotoluene selectivity of 93% | 101 |
| CC3 composite membranes | POC composites | Organic dye separation | Molecular weight cut-off of 1400 g mol−1 | 102 |
| IIC-1 | Pristine POCs | Cathode of LSBs | Discharge capacity of 852 mA h g−1; capacity retention of 50% (1000 cycles) | 106 |
| CE | Pristine POCs | Cathode of LSBs | Discharge capacity of 970 mA h g−1; capacity retention of 52% (100 cycles) | 108 |
| CPOC-301 | Pristine POCs | Cathode of LSBs | Discharge capacity of 1302 mA h g−1; capacity retention of 32% (2500 cycles) | 107 |
| CC3/PP | POC composites | Separator of LSBs | Discharge capacity of 956 mA h g−1; capacity retention of 80% (500 cycles) | 117 |
| SLEN | POC composites | Ionic conductivity | Ionic conductivity of 1 × 10−3 S cm−1; low activation energy of 16 kJ mol−1 | 118 |
| Cage 1 | Pristine POCs | Cathode of LIBs | Discharge capacity of 83 mA h g−1; capacity retention of 34% (100 cycles) | 119 |
| Li-RCC1-ClO4 | Pristine POCs | Cathode of SSLBs | Discharge capacity of 122–135 mA h g−1; capacity retention of 75% (882 cycles) | 120 |
| Li–Cl2@POC | POC composites | Cathode of Li–Cl2 batteries | Discharge capacity of 2000 mA h g−1 at −20 °C; CE of 99.7% | 121 |
| C60@POC | POC composites | Cathodes of lithium-organic batteries | Charge separation efficiency (tCS/CR = 20.83/171.17); solar energy conversion efficiency of ∼1% | 122 |
| POC@PbI2 | POC composites | Perovskite solar cells | Power conversion efficiency of 24.13%; efficiency retention of 93% (5000 cycles) | 123 |
| Cage-1-TREN | Pristine POCs | Proton conductivity | Proton conductivity of 1.59 × 10−4 S cm−1 | 124 |
| Nafion–Cage 3 | POC composites | Proton conductivity | Proton conductivity of 0.27 S cm−1 | 125 |
| (H12RCC1)12+·12Cl−·4H2O | POC composites | Proton conductivity | Proton conductivities of 10−3 S cm−1 | 126 |
Although great progress has been made in energy applications, POC-based materials are in their infancy and more efforts should be invested in the following areas. (1) The stability of pristine POCs is the main problem in the application of photoelectric and electrochemical energy due to the metastable imine bonds for most cages, and its performance needs to be further improved towards the practical application. For example, some reasonable design strategies can be used to develop more stable POCs, such as converting reversible bonds into irreversible bonds through post-modification, and adjusting the hydrophilicity and hydrophobicity of materials. In addition, the structure of the original POCs can also be optimized, such as the introduction of new groups, heteroatom doping, and pore size regulation. (2) The development of simpler and greener POC synthesis methods for scalable production, such as mechanical grinding synthesis and aqueous synthesis strategies, to achieve universal synthesis of POC-based materials, thereby reducing costs and reducing environmental pollution. (3) POC-based composites should be further developed, not only limited to the combination with MNPs, but also to explore more functional components, such as organic small molecules, enzymes, and even inorganic semiconductors. Through new synthesis methods, the functional diversity of POC composites materials is enriched. (4) Compared with powder materials, the corresponding devices are closer to practical applications. For example, POCs are converted into a film to achieve efficient gas separation or proton conductivity, so more efforts should be devoted to device manufacturing. (5) How to further improve the performance of POCs in energy applications? For electrocatalysis, although the catalytic performance of POCs has reached a high level in the lab, it still needs to be further improved for reaching the standard of commercial products. In addition, increasing the specific surface area and porosity of POCs may also improve the pore-related performance of POCs in the energy field. From the perspective of POCs' structure design and transformation into POC derivatives, the synthesis of nanoscale POC solid materials may be beneficial to improve their performance. (6) Advanced characterization techniques (e.g., crystal structure characterization is an important basis for the structure of POCs, and in situ characterization is crucial for mechanism research) and theoretical calculations are essential as powerful tools to systematically study the transformation process and related mechanisms in POC-based materials, including micro-component changes, kinetic and thermodynamic processes, and possible synergy between multiple components.
Indeed, opportunities and challenges coexist in developing POCs for energy applications, and pioneering studies are very urgently desired. In the present case, most of the research studies are still in the laboratory stage, and large-scale practical promotion is encouraged. There is still a long way for practical applications of POC-based materials in energy applications. POC-based materials have made great progress in structural design and application, which is indeed exciting. Therefore, we believe that the application of POC-based materials in the energy field will have bright prospects through the mutual promotion of structural design, structural regulation and theoretical calculation.
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Author contributions
All the authors wrote the perspective together.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 22235001, 22175020, 22131005, and 22261132512), the Xiaomi Young Scholar Program, the Fundamental Research Funds for the Central Universities (FRF-EYIT-23-02 and QNXM2023), the Natural Science Foundation of Hebei Province (No. B2024201002), the Excellent Youth Research Innovation Team of Hebei University (QNTD202410) and the Hebei University High-level Talent Research Program (No. 521100223007). Hebei University and the University of Science & Technology Beijing are gratefully acknowledged.Notes and references
- X. Yang, Z. Ullah, J. F. Stoddart and C. T. Yavuz, Chem. Rev., 2023, 123, 4602–4634 CrossRef CAS.
- G. Zhang and M. Mastalerz, Chem. Soc. Rev., 2014, 43, 1934–1947 RSC.
- H. Wang, Y. Jin, N. Sun, W. Zhang and J. Jiang, Chem. Soc. Rev., 2021, 50, 8874–8886 RSC.
- G. Montà-González, F. Sancenón, R. Martínez-Máñez and V. Martí-Centelles, Chem. Rev., 2022, 122, 13636–13708 CrossRef PubMed.
- R. A. Borse, Y. Tan, D. Yuan and Y. Wang, Energy Environ. Sci., 2024, 17, 1307–1329 RSC.
- M. Wilms, L. V. Melendez, R. J. Hudson, C. R. Hall, S. P. Ratnayake, T. Smith, E. D. Gaspera, G. Bryant, T. U. Connell and D. E. Gómez, Angew. Chem., Int. Ed., 2023, 62, e202303501 CrossRef CAS.
- P. Bhandari and P. S. Mukherjee, ACS Catal., 2023, 13, 6126–6143 CrossRef CAS.
- H. Guo, Y. Liu, H. Dong, W. Zong, K. Chu, W. Li, Z. Fan, G. He, Y. Miao, I. P. Parkin, F. Lai and T. Liu, Sci. Bull., 2022, 67, 2428–2437 CrossRef.
- A. P. Côté, A. I. Benin, N. W. Ockwig, M. O'Keeffe, A. J. Matzger and O. M. Yaghi, Science, 2005, 310, 1166–1170 CrossRef.
- W. Zhao, Q. Zhu, X. Wu and D. Zhao, Chem. Soc. Rev., 2024, 53, 7531–7565 RSC.
- R. Liu, K. T. Tan, Y. Gong, Y. Chen, Z. Li, S. Xie, T. He, Z. Lu, H. Yang and D. Jiang, Chem. Soc. Rev., 2021, 50, 120–242 RSC.
- H. Lin, Y. Yang, Y. Hsu, J. Zhang, C. Welton, I. Afolabi, M. Loo and H. Zhou, Adv. Mater., 2024, 36, 2209073 CrossRef.
- H. Jin, P. Zhao, Y. Qian, J. Xiao, Z. Chao and H. Jiang, Chem. Soc. Rev., 2024, 53, 9378–9418 RSC.
- R. Lin, Y. He, P. Li, H. Wang, W. Zhou and B. Chen, Chem. Soc. Rev., 2019, 48, 1362–1389 RSC.
- L. Shi, Z. Xiong, H. Wang, H. Cao and Z. Chen, Chem, 2024, 10, 2464–2472 CAS.
- Q. Zhu, L. Wei, C. Zhao, H. Qu, B. Liu, T. Fellowes, S. Yang, A. Longcake, M. J. Hall, M. R. Probert, Y. Zhao, A. I. Cooper and M. A. Little, J. Am. Chem. Soc., 2023, 145, 23352–23360 CrossRef CAS PubMed.
- K. Cheng, H. Li, Z. Li, P. Li and Y. Zhao, ACS Mater. Lett., 2023, 5, 1546–1555 CrossRef CAS.
- Q. Zhu, X. Wang, R. Clowes, P. Cui, L. Chen, M. A. Little and A. I. Cooper, J. Am. Chem. Soc., 2020, 142, 16842–16848 CrossRef CAS.
- J. Ma, J. Li, Y. Chen, R. Ning, Y. Ao, J. Liu, J. Sun, D. Wang and Q. Wang, J. Am. Chem. Soc., 2019, 141, 3843–3848 CrossRef CAS PubMed.
- X. Ding, B. Han, B. Yu, H. Wang and J. Jiang, Sci. China: Chem., 2023, 66, 2019–2027 CrossRef CAS.
- Y. Jin, Q. Wang, P. Taynton and W. Zhang, Acc. Chem. Res., 2014, 47, 1575–1586 CrossRef CAS.
- T. Tozawa, J. T. A. Jones, S. I. Swamy, S. Jiang, D. J. Adams, S. Shakespeare, R. Clowes, D. Bradshaw, T. Hasell, S. Y. Chong, C. Tang, S. Thompson, J. Parker, A. Trewin, J. Bacsa, A. M. Z. Slawin, A. Steiner and A. I. Cooper, Nat. Mater., 2009, 8, 973–978 CrossRef CAS PubMed.
- K. Acharyya and P. S. Mukherjee, Angew. Chem., Int. Ed., 2019, 58, 8640–8653 CrossRef CAS.
- X. Dong, H. Qu, A. C. H. Sue, X. Wang and X. Cao, Acc. Chem. Res., 2024, 57, 1111–1122 CrossRef CAS.
- J. Kou, W. D. Wang, J. Fang, F. Li, H. Zhao, J. Li, H. Zhu, B. Li and Z. Dong, Appl. Catal., B, 2022, 315, 121487 CrossRef CAS.
- C. Liu, W. Li, Y. Liu, H. Wang, B. Yu, Z. Bao and J. Jiang, Chem. Eng. J., 2022, 428, 131129 CrossRef CAS.
- H. Ren, C. Liu, X. Ding, X. Fu, H. Wang and J. Jiang, Chin. J. Chem., 2022, 40, 385–391 CrossRef CAS.
- W. Liu and J. F. Stoddart, Chem, 2021, 7, 919–947 CAS.
- K. G. Andrews, T. K. Piskorz, P. N. Horton and S. J. Coles, J. Am. Chem. Soc., 2024, 146, 17887–17897 CrossRef PubMed.
- M. Zhang, Z. He, L. Wang, X. Zhang and G. Li, Small, 2024, 20, 2308400 CrossRef.
- S. Zhang, Z. Kochovski, H. Lee, Y. Lu, H. Zhang, J. Zhang, J. Sun and J. Yuan, Chem. Sci., 2019, 10, 1450–1456 RSC.
- C. Liu, Y. Jin, D. Qi, X. Ding, H. Ren, H. Wang and J. Jiang, Chem. Sci., 2022, 13, 7014–7020 RSC.
- H. Li, H. Zhang, A. D. Lammer, M. Wang, X. Li, V. M. Lynch and J. L. Sessler, Nat. Chem., 2015, 7, 1003–1008 CrossRef PubMed.
- Y. Horng, T. Lin, C. Tu, T. Sung, C. Hsieh, C. Hu, H. M. Lee and T. Kuo, Eur. J. Org Chem., 2009, 2009, 1511–1514 CrossRef.
- C. Zhang, Q. Wang, H. Long and W. Zhang, J. Am. Chem. Soc., 2011, 133, 20995–21001 CrossRef.
- Q. Wang, C. Zhang, B. C. Noll, H. Long, Y. Jin and W. Zhang, Angew. Chem., Int. Ed., 2014, 53, 10663–10667 CrossRef PubMed.
- J. Taesch, V. Heitz, F. Topić and K. Rissanen, Chem. Commun., 2012, 48, 5118–5120 RSC.
- S. Ivanova, E. Köster, J. J. Holstein, N. Keller, G. H. Clever, T. Bein and F. Beuerle, Angew. Chem., Int. Ed., 2021, 60, 17455–17463 CrossRef.
- F. Qiu, X. Chen, W. Wang, K. Su and D. Yuan, CCS Chem., 2023, 6, 149–156 CrossRef.
- J. Zhang, Y. Li, W. Yang, S. Lai, C. Zhou, H. Liu, C. Che and Y. Li, Chem. Commun., 2012, 48, 3602–3604 RSC.
- Z. Wang, H. Ma, T. Zhai, G. Cheng, Q. Xu, J. Liu, J. Yang, Q. Zhang, Q. Zhang, Y. Zheng, B. Tan and C. Zhang, Adv. Sci., 2018, 5, 1800141 CrossRef.
- R. Kaur, S. Sen, M. C. Larsen, L. Tavares, J. Kjelstrup-Hansen, M. Ishida, A. Zieleniewska, V. M. Lynch, S. Bähring, D. M. Guldi, J. L. Sessler and A. Jana, J. Am. Chem. Soc., 2020, 142, 11497–11505 CrossRef.
- W. Liu, C. Lin, J. A. Weber, C. L. Stern, R. M. Young, M. R. Wasielewski and J. F. Stoddart, J. Am. Chem. Soc., 2020, 142, 8938–8945 CrossRef PubMed.
- A. Avellaneda, P. Valente, A. Burgun, J. D. Evans, A. W. Markwell-Heys, D. Rankine, D. J. Nielsen, M. R. Hill, C. J. Sumby and C. J. Doonan, Angew. Chem., Int. Ed., 2013, 52, 3746–3749 CrossRef.
- A. S. Bhat, S. M. Elbert, W. Zhang, F. Rominger, M. Dieckmann, R. R. Schröder and M. Mastalerz, Angew. Chem., Int. Ed., 2019, 58, 8819–8823 CrossRef PubMed.
- J. T. A. Jones, D. Holden, T. Mitra, T. Hasell, D. J. Adams, K. E. Jelfs, A. Trewin, D. J. Willock, G. M. Day, J. Bacsa, A. Steiner and A. I. Cooper, Angew. Chem., Int. Ed., 2011, 50, 749–753 CrossRef CAS.
- C. J. Pugh, V. Santolini, R. L. Greenaway, M. A. Little, M. E. Briggs, K. E. Jelfs and A. I. Cooper, Cryst. Growth Des., 2018, 18, 2759–2764 CrossRef.
- T. Hasell, X. Wu, J. T. A. Jones, J. Bacsa, A. Steiner, T. Mitra, A. Trewin, D. J. Adams and A. I. Cooper, Nat. Chem., 2010, 2, 750–755 CrossRef PubMed.
- S. Jiang, K. E. Jelfs, D. Holden, T. Hasell, S. Y. Chong, M. Haranczyk, A. Trewin and A. I. Cooper, J. Am. Chem. Soc., 2013, 135, 17818–17830 CrossRef.
- S. Jiang, J. T. A. Jones, T. Hasell, C. E. Blythe, D. J. Adams, A. Trewin and A. I. Cooper, Nat. Commun., 2011, 2, 207 CrossRef.
- N. Sun, C. Wang, H. Wang, L. Yang, P. Jin, W. Zhang and J. Jiang, Angew. Chem., Int. Ed., 2019, 58, 18011–18016 CrossRef.
- B. Han, H. Wang, C. Wang, H. Wu, W. Zhou, B. Chen and J. Jiang, J. Am. Chem. Soc., 2019, 141, 8737–8740 CrossRef.
- Q. Song, J. Yang, K. Zheng, T. Zhang, C. Yuan, L. Yuan and X. Hou, J. Am. Chem. Soc., 2024, 146, 7594–7604 CrossRef PubMed.
- Z. Shan, X. Wu, B. Xu, Y. Hong, M. Wu, Y. Wang, Y. Nishiyama, J. Zhu, S. Horike, S. Kitagawa and G. Zhang, J. Am. Chem. Soc., 2020, 142, 21279–21284 CrossRef.
- A. Giri, G. Shreeraj, T. K. Dutta and A. Patra, Angew. Chem., Int. Ed., 2023, 62, e202219083 CrossRef PubMed.
- C. Liu, Y. Jin, Z. Yu, L. Gong, H. Wang, B. Yu, W. Zhang and J. Jiang, J. Am. Chem. Soc., 2022, 144, 12390–12399 CrossRef PubMed.
- Y. Kim, J. Koo, I. Hwang, R. D. Mukhopadhyay, S. Hong, J. Yoo, A. A. Dar, I. Kim, D. Moon, T. J. Shin, Y. H. Ko and K. Kim, J. Am. Chem. Soc., 2018, 140, 14547–14551 CrossRef PubMed.
- J. Koo, I. Kim, Y. Kim, D. Cho, I. Hwang, R. D. Mukhopadhyay, H. Song, Y. H. Ko, A. Dhamija, H. Lee, W. Hwang, S. Kim, M. Baik and K. Kim, Chem, 2020, 6, 3374–3384 Search PubMed.
- C. Liu, K. Liu, C. Wang, H. Liu, H. Wang, H. Su, X. Li, B. Chen and J. Jiang, Nat. Commun., 2020, 11, 1047 CrossRef.
- N. Sun, D. Qi, Y. Jin, H. Wang, C. Wang, C. Qu, J. Liu, Y. Jin, W. Zhang and J. Jiang, CCS Chem., 2021, 4, 2588–2596 CrossRef.
- H. Song, T. Y. Li, Y. Pan, X. Han, Y. Guo, L. Shi and M. Song, Dyes Pigm., 2023, 219, 111584 CrossRef.
- Y. Li, N. Li, G. Li, Y. Qiao, M. Zhang, L. Zhang, Q. Guo and G. He, J. Am. Chem. Soc., 2023, 145, 9118–9128 CrossRef PubMed.
- L. An, P. De La Torre, P. T. Smith, M. R. Narouz and C. J. Chang, Angew. Chem., Int. Ed., 2023, 62, e202209396 CrossRef PubMed.
- F. Zhang, J. Ma, Y. Tan, G. Yu, H. Qin, L. Zheng, H. Liu and R. Li, ACS Catal., 2022, 12, 5827–5833 CrossRef.
- C. Li, J. Cui, J. Zhou, Y. Xu and J. Sun, Catal. Sci. Technol., 2024, 14, 1558–1567 RSC.
- B. Mondal and P. S. Mukherjee, J. Am. Chem. Soc., 2018, 140, 12592–12601 CrossRef CAS PubMed.
- G. Chen, W. Xin, J. Wang, J. Cheng and Y. Dong, Chem. Commun., 2019, 55, 3586–3589 RSC.
- A. Singh, P. Verma, D. Samanta, A. Dey, J. Dey and T. K. Maji, J. Mater. Chem. A, 2021, 9, 5780–5786 RSC.
- B. Mondal, P. Bhandari and P. S. Mukherjee, Chem.–Eur. J., 2020, 26, 15007–15015 CrossRef CAS PubMed.
- H. Li, L. Geng, S. Si, H. Cheng, Z. Yang and J. Wei, Chem. Eng. J., 2023, 474, 145431 CrossRef CAS.
- Q. Fu, T. Zhang, M. An, X. Sun, Y. Li, B. Zhang, S. Zhang, G. I. N. Waterhouse, X. Liu, H. Li and S. Ai, Chem. Eng. J., 2024, 492, 152293 CrossRef CAS.
- S. Zhang, S. Wang, L. Guo, H. Chen, B. Tan and S. Jin, J. Mater. Chem. C, 2020, 8, 192–200 RSC.
- S. Fu, S. Yao, S. Guo, G. Guo, W. Yuan, T. Lu and Z. Zhang, J. Am. Chem. Soc., 2021, 143, 20792–20801 CrossRef CAS PubMed.
- L. Chen, Y. Wang, F. Yu, X. Shen and C. Duan, J. Mater. Chem. A, 2019, 7, 11355–11361 RSC.
- P. T. Smith, B. P. Benke, Z. Cao, Y. Kim, E. M. Nichols, K. Kim and C. J. Chang, Angew. Chem., Int. Ed., 2018, 57, 9684–9688 CrossRef CAS PubMed.
- X. Liu, C. Liu, X. Song, X. Ding, H. Wang, B. Yu, H. Liu, B. Han, X. Li and J. Jiang, Chem. Sci., 2023, 14, 9086–9094 RSC.
- Y. Hu, S. Huang, L. J. Wayment, J. Wu, Q. Xu, T. Chang, Y. Chen, X. Li, B. Andi, H. Chen, Y. Jin, H. Zhu, M. Du, S. Lu and W. Zhang, Cell Rep. Phys. Sci., 2023, 4, 101285 CrossRef CAS.
- C. Chen, X. Yan, Y. Wu, S. Liu, X. Zhang, X. Sun, Q. Zhu, H. Wu and B. Han, Angew. Chem., Int. Ed., 2022, 61, e202202607 CrossRef CAS.
- L. An, M. R. Narouz, P. T. Smith, P. De La Torre and C. J. Chang, Angew. Chem., Int. Ed., 2023, 62, e202305719 CrossRef CAS PubMed.
- P. T. Smith, Y. Kim, B. P. Benke, K. Kim and C. J. Chang, Angew. Chem., Int. Ed., 2020, 59, 4902–4907 CrossRef CAS PubMed.
- P. T. Smith, B. P. Benke, L. An, Y. Kim, K. Kim and C. J. Chang, Chemelectrochem, 2021, 8, 1653–1657 CrossRef CAS.
- Y. Yan, H. Qin, D. Ding, P. Yang, Q. Hou, J. Basset and Y. Chen, Chemnanomat, 2022, 8, e202200305 CrossRef CAS.
- W. Wang, K. Su, E. M. El-Sayed, M. Yang and D. Yuan, ACS Appl. Mater. Interfaces, 2021, 13, 24042–24050 CrossRef CAS.
- L. Feng, Y. Xie, W. Wang, K. Su and D. Yuan, J. Mater. Chem. A, 2023, 11, 25316–25321 RSC.
- Z. Wang, Y. Zhang, J. Liu, T. Wang, J. Wang, K. Yu, Y. Chen, P. Cheng and Z. Zhang, ACS Mater. Lett., 2023, 5, 2754–2759 CrossRef CAS.
- K. Su, W. Wang, S. Du, C. Ji and D. Yuan, Nat. Commun., 2021, 12, 3703 CrossRef CAS PubMed.
- Q. Zhang, H. Li, S. Chen, J. Duan and W. Jin, J. Membr. Sci., 2020, 611, 118288 CrossRef CAS.
- L. Yu, L. Hao, Y. Feng, J. Pang, M. Guo, L. Li, W. Fan, L. Fan, R. Wang, Z. Kang and D. Sun, Nano Res., 2024, 17, 4535–4543 CrossRef CAS.
- Z. Jiang, Y. Wang, M. Sheng, Z. Zha, J. Wang, Z. Wang and S. Zhao, J. Mater. Chem. A, 2023, 11, 6831–6841 RSC.
- C. Ji, K. Su, W. Wang, J. Chang, E. M. El-Sayed, L. Zhang and D. Yuan, CCS Chem., 2021, 4, 3095–3105 CrossRef.
- L. Chen, P. S. Reiss, S. Y. Chong, D. Holden, K. E. Jelfs, T. Hasell, M. A. Little, A. Kewley, M. E. Briggs, A. Stephenson, K. M. Thomas, J. A. Armstrong, J. Bell, J. Busto, R. Noel, J. Liu, D. M. Strachan, P. K. Thallapally and A. I. Cooper, Nat. Mater., 2014, 13, 954–960 CrossRef CAS PubMed.
- J. M. Lucero and M. A. Carreon, ACS Appl. Mater. Interfaces, 2020, 12, 32182–32188 CrossRef CAS.
- M. Liu, L. Zhang, M. A. Little, V. Kapil, M. Ceriotti, S. Yang, L. Ding, D. L. Holden, R. Balderas-Xicohténcatl, D. He, R. Clowes, S. Y. Chong, G. Schütz, L. Chen, M. Hirscher and A. I. Cooper, Science, 2019, 366, 613–620 CrossRef CAS PubMed.
- D. J. Vogel, T. M. Nenoff and J. M. Rimsza, ACS Omega, 2022, 7, 7963–7972 CrossRef CAS.
- T. Mitra, K. E. Jelfs, M. Schmidtmann, A. Ahmed, S. Y. Chong, D. J. Adams and A. I. Cooper, Nat. Chem., 2013, 5, 276–281 CrossRef CAS PubMed.
- A. Kewley, A. Stephenson, L. Chen, M. E. Briggs, T. Hasell and A. I. Cooper, Chem. Mater., 2015, 27, 3207–3210 CrossRef CAS.
- B. Moosa, L. O. Alimi, A. Shkurenko, A. Fakim, P. M. Bhatt, G. Zhang, M. Eddaoudi and N. M. Khashab, Angew. Chem., Int. Ed., 2020, 59, 21367–21371 CrossRef CAS PubMed.
- A. Dey, S. Chand, B. Maity, P. M. Bhatt, M. Ghosh, L. Cavallo, M. Eddaoudi and N. M. Khashab, J. Am. Chem. Soc., 2021, 143, 4090–4094 CrossRef CAS PubMed.
- Y. Ding, L. O. Alimi, B. Moosa, C. Maaliki, J. Jacquemin, F. Huang and N. M. Khashab, Chem. Sci., 2021, 12, 5315–5318 RSC.
- Y. Ding, L. O. Alimi, J. Du, B. Hua, A. Dey, P. Yu and N. M. Khashab, Chem. Sci., 2022, 13, 3244–3248 RSC.
- L. O. Alimi, B. Moosa, W. Lin and N. M. Khashab, ACS Mater. Lett., 2024, 6, 1467–1473 CrossRef CAS.
- A. He, Z. Jiang, Y. Wu, H. Hussain, J. Rawle, M. E. Briggs, M. A. Little, A. G. Livingston and A. I. Cooper, Nat. Mater., 2022, 21, 463–470 CrossRef CAS.
- S. Liu, J. Zhou, C. Wu, P. Zhang, X. Cao and J. Sun, Nat. Commun., 2024, 15, 2478 CrossRef CAS PubMed.
- A. Ghaffar, M. Hassan, O. V. Penkov, C. T. Yavuz and K. Celebi, Environ. Sci. Technol., 2023, 57, 20380–20391 CrossRef CAS PubMed.
- Z. Song, L. Liu, Q. Sun, J. Du, J. Guan, P. Dou, R. Zhang, Z. Jiang and J. Liu, Angew. Chem., Int. Ed., 2024, 63, e202409296 CrossRef CAS.
- L. Zhang, Y. Jia, F. Meng, L. Sun, F. Cheng, Z. Shi, R. Jiang and X. Song, J. Alloys Compd., 2022, 923, 166488 CrossRef CAS.
- Y. Xie, M. Yang, E. M. El-Sayed, K. Su, Z. Li and D. Yuan, ACS Appl. Nano Mater., 2023, 6, 7910–7919 CrossRef CAS.
- Y. Yen, T. Chen, Y. Wang, A. Robles, M. Đerić, O. Š. Miljanić, W. Kaveevivitchai and S. Chung, J. Power Sources, 2023, 565, 232891 CrossRef CAS.
- R. Yan, B. Mishra, M. Traxler, J. Roeser, N. Chaoui, B. Kumbhakar, J. Schmidt, S. Li, A. Thomas and P. Pachfule, Angew. Chem., Int. Ed., 2023, 62, e202302276 CrossRef CAS PubMed.
- W. Liu, K. Wang, X. Zhan, Z. Liu, X. Yang, Y. Jin, B. Yu, L. Gong, H. Wang, D. Qi, D. Yuan and J. Jiang, J. Am. Chem. Soc., 2023, 145, 8141–8149 CrossRef CAS PubMed.
- Z. Wang, X. Wu, S. Wei, Y. Xie and C. Lu, Chem. Mater., 2024, 36, 2412–2419 CrossRef CAS.
- H. Duan, K. Li, M. Xie, J. Chen, H. Zhou, X. Wu, G. Ning, A. I. Cooper and D. Li, J. Am. Chem. Soc., 2021, 143, 19446–19453 CrossRef CAS PubMed.
- D. Cai, M. Lu, L. Li, J. Cao, D. Chen, H. Tu, J. Li and W. Han, Small, 2019, 15, 1902605 CrossRef CAS.
- X. Hong, T. Tan, Y. Guo, X. Tang, J. Wang, W. Qin and Y. Cai, Nanoscale, 2018, 10, 2774–2780 RSC.
- X. Liu, X. Guo, R. Wang, Q. Liu, Z. Li, S. Zang and T. C. W. Mak, J. Mater. Chem. A, 2019, 7, 2838–2844 RSC.
- J. Zhou, R. Li, X. Fan, Y. Chen, R. Han, W. Li, J. Zheng, B. Wang and X. Li, Energy Environ. Sci., 2014, 7, 2715–2724 RSC.
- H. Li, Y. Huang, Y. Zhang, X. Zhang, L. Zhao, W. Bao, X. Cai, K. Zhang, H. Zhao, B. Yi, L. Su, A. K. Cheetham, S. Jiang and J. Xie, Nano Lett., 2022, 22, 2030–2037 CrossRef CAS.
- A. Petronico, T. P. I. Moneypenny, B. G. Nicolau, J. S. Moore, R. G. Nuzzo and A. A. Gewirth, J. Am. Chem. Soc., 2018, 140, 7504–7509 CrossRef CAS.
- S. Bera, N. Goujon, M. Melle-Franco, D. Mecerreyes and A. Mateo-Alonso, Chem. Sci., 2024, 15, 14872–14879 RSC.
- J. Li, J. Qi, F. Jin, F. Zhang, L. Zheng, L. Tang, R. Huang, J. Xu, H. Chen, M. Liu, Y. Qiu, A. I. Cooper, Y. Shen and L. Chen, Nat. Commun., 2022, 13, 2031 CrossRef CAS.
- Y. Xu, S. Zhang, M. Wang, Y. Meng, Z. Xie, L. Sun, C. Huang and W. Chen, J. Am. Chem. Soc., 2023, 145, 27877–27885 CrossRef CAS.
- X. Zhang, K. Su, A. G. A. Mohamed, C. Liu, Q. Sun, D. Yuan, Y. Wang, W. Xue and Y. Wang, Energy Environ. Sci., 2022, 15, 780–785 RSC.
- F. Gao, C. Luo, X. Wang, C. Zhan, Y. Li, Y. Li, Q. Meng, M. Yang, K. Su, D. Yuan, R. Zhu and Q. Zhao, Adv. Funct. Mater., 2023, 33, 2211900 CrossRef CAS.
- Z. Yang, N. Zhang, L. Lei, C. Yu, J. Ding, P. Li, J. Chen, M. Li, S. Ling, X. Zhuang and S. Zhang, JACS Au, 2022, 2, 819–826 CrossRef CAS PubMed.
- R. Han and P. Wu, ACS Appl. Mater. Interfaces, 2018, 10, 18351–18358 CrossRef CAS PubMed.
- M. Liu, L. Chen, S. Lewis, S. Y. Chong, M. A. Little, T. Hasell, I. M. Aldous, C. M. Brown, M. W. Smith, C. A. Morrison, L. J. Hardwick and A. I. Cooper, Nat. Commun., 2016, 7, 12750 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |