Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The influence of Michael acceptors on the structural reactivity of renewable fuels
Timothy
Deehan
 *,
Paul
Hellier
*,
Paul
Hellier
 and
Nicos
Ladommatos
and
Nicos
Ladommatos

University College London's Mechanical Engineering Department, Roberts Engineering Building, University College London, Torrington Place, London WC1E 7JE, UK. E-mail: timothy.deehan.17@ucl.ac.uk
First published on 22nd July 2024
Abstract
Expanding the use of sustainable fuels in hard to decarbonise transport vehicles utilising heavy-duty engines is urgently required to reduce greenhouse gas emissions from sectors reliant on these engines. As biofuel production turns to alternative sources of biomass with a differing chemical composition to fossil fuel, it is increasingly important to understand how chemical functional groups which may be present in biomass influence the process of combustion. Biofuels are more homogeneous in chemical composition than fossil diesel or gasoline, which are a variety of compounds with a common range of boiling points. As the transport industry progresses in replacing fossil volumes with renewable liquid fuels it also moves towards fuels which are more homogeneous in chemical composition and therefore reactivity. Esters and carbon–carbon double bonds are two common functional groups found in biodiesel and many other classes of bioderived molecules. When adjacent to each other in a specific conformation, they are classed as a Michael acceptor functional group which has a unique reactivity with free radicals separate to either the ester or alkene alone and may play a key role in the low temperature reactions preceding auto-ignition in the combustion process. In this study, the combustion and emissions characteristics of a series of saturated and unsaturated fatty acid esters were tested as single component test fuels, to observe how the inclusion of a Michael acceptor group in esters influences reactivity in a heavy-duty compression ignition engine. Under constant injection duration and timing conditions, it was found that the inclusion of the Michael acceptor in methyl non-2-enoate reduced the duration of ignition delay and increased the IMEP relative to methyl nonanoate and methyl non-3-enoate. Conversely, the inclusion of the Michael acceptor in C8 and C10 ethyl esters resulted in a longer duration of ignition delay and similar observed IMEPs.
Introduction
Recognition of the devastating potential impacts of global climate change has resulted in ambitious targets for the urgent reduction of carbon emissions from the use of fossil fuels. However, industries serviced by large internal combustion engines (ICEs), such as road and marine freight, are considered particularly difficult sectors of the economy to decarbonise.1 The European Union is pushing for advancement in replacement propulsion systems for ICEs in these sectors; however, electrification has not yet progressed meaningfully in the heavy goods transport sector, and is still not considered a viable technology for the marine sector which instead is incentivised to consider biofuels.2,3 The incentivisation for expanding biofuel consumption to decarbonise HGVs is lacking in Europe, with the commission redirecting attention to RFNBOs and RCFs.4 However, outside of Europe, for example in Indonesia, there remains a focus on biofuels, not electrification for the freight and road transport sector.5The development of renewable future fuels (whether RFNBOs or biofuels) presents an opportunity to incorporate molecular structure functionality to improve ignition quality and the efficiency of energy release during combustion, especially as liquid products resulting from biomass conversion or the Fischer–Tropsch process are inherently more homogeneous in chemical structure than fractions of crude oil.6,7 The focus of research investigating the influence of fuel composition on combustion in heavy-duty engines has so far been dominated by the differences between the major vegetable oils used in biodiesel production,8 or seeking to replicate the physical properties of fossil diesel.9
The pathways through which fuel molecular structure influences combustion in larger compression ignition engines remain an important research topic.10 The higher compression ratios,11 greater in-cylinder pressures and slower speeds12 appreciably alter conditions relative to high-speed light-duty automotive diesel engines and therefore there is a need to explore the effects of fuel composition in the specific context of heavy duty engines.13,14
While fuel development and production often focuses on the realisation of specific physicochemical properties defined by standards such as ASTM 6751,15 for biofuels differences in molecular structure must be considered to fully explain the performance of certain fuels.16 For example, dimethyl ether has received widespread attention for use in compression ignition engines owing to its high cetane number and low particulate emissions,17 despite DME having a lower density and significantly lower boiling point than fossil diesel.18 In this instance, the ignition quality and potential of DME can instead be attributed to the presence of ether linkages which accelerate the rate of low temperature reaction kinetics towards auto-ignition.19,20
Fatty acid methyl esters were quickly adopted as drop-in diesel fuels for their high energy density,21 miscibility with fossil diesel,22 and similar physical properties.23 The chemical difference between biodiesels and fossil diesel is the presence of the ester functional group, and as this is more easily available for fuel radical interactions than the aliphatic hydrocarbon chain, B100 blends can exhibit higher cetane numbers than fossil diesel, resulting in shorter durations of ignition delay.24
Further to hydrocarbon fuels, kinetic modelling and shock tube experiments have often focused on esters as these, alongside ethanol, are the most widely available liquid biofuels. Early studies often included methyl crotonate as a simple surrogate for the unsaturated esters found in biodiesel (albeit with a considerably shorter alkyl moiety). Sarathy et al. (2007)25 and Gaïl et al. (2008)26 conducted two such studies into the effect of unsaturation on combustion chemistry, and by coincidence of choosing methyl crotonate (methyl but-2-enoate) and methyl butanoate as test fuels for their investigations, the studies also compared the combustion chemistry of saturated esters to α,β-unsaturated esters, a specific functional group which could exhibit unique reactivity compared to a saturated ester.25 These models improved upon previous schemes where the underlying assumptions had been extrapolated from simple ideal hydrocarbon models, and as further experimental data became available these models were updated to reflect new results. For example, Fisher et al. (2000)27 presented an early model of reaction kinetics of methyl butanoate and methyl formate combustion, based on existing hydrocarbon models. This was compared against available experimental data, setting the basis of further computational kinetic studies,28 supported by gas phase kinetic studies29 and ignition studies in shock tubes.30
Sarathy et al. (2007) studied methyl butanoate (MB) and methyl but-2-enoate (M2B) in flame burners and jet stirred reactors, and developed a radical reaction pathway; however, the study did not consider radical addition to the α,β-unsaturated ester (M2B).25 Gaïl et al. (2008) updated the models for MB and M2B to include additional reaction pathways and potential addition reactions between M2B and H, OH and HO2 radicals were considered; however, the authors focused on discussing the more dominant pathways of fuel consumption.26
Kinetic models continued to be updated frequently and dependent on the reaction conditions employed, the radical addition to α,β-unsaturated carbonyls would either be considered a dominant pathway or alternatively unimportant in ester decomposition. This changed when Johnson et al. (2021) and Li et al. (2022) reviewed these models and focused on including several potential reaction routes which were previously not considered, specifically updating the models to better reflect the radical additions which are available to alkenes.31,32 Johnson et al.'s main finding was that their new methyl but-2-enoate model came significantly closer to matching experimental observations than the earlier work of Sarathy and Gaïl, which was attributable to the new reactions included. Johnson et al.'s updated model found H or O addition products to M2B to account for 31% of the initial fuel consumption during combustion. Li et al. comparing two structural isomers, methyl acrylate (an α,β-unsaturated ester) and vinyl acetate, in a shock tube found that methyl acrylate exhibited a shorter duration of ignition delay than vinyl acetate and was significantly more active at lower temperatures prior to autoignition. The authors attributed this higher reactivity to hydrogen addition reactions to the double bond consuming the majority of fuel. Liu et al. (2022) conducted a comprehensive investigation into the reaction kinetics of abstraction and addition of methyl hex-3-enoate by H and OH radicals.33 The authors concluded that H-addition reactions to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond are the dominating reaction below 1200 K, with H abstraction dominating above 1250 K. The OH addition to methyl hex-3-enoate was less kinetically favoured than H addition, but remained the dominating reaction over H abstraction below 900 K.33
C double bond are the dominating reaction below 1200 K, with H abstraction dominating above 1250 K. The OH addition to methyl hex-3-enoate was less kinetically favoured than H addition, but remained the dominating reaction over H abstraction below 900 K.33
The literature discussed so far on α,β-unsaturated esters has focused on experiments in shock tubes, jet stirred reactors and burners which were used to validate kinetic mechanisms; however, only one previous study investigating the structure of these esters in an actual engine has been reported. Zhang et al. (2009) investigated how premixed ignition reactivity changes with the double bond position in C9 unsaturated esters which included methyl non-2-enoate in the test set, an α,β-unsaturated ester.34 The experiments were performed in a Cooperative Fuel Research (CFR) engine using a gasoline direct injector in the air intake manifold to provide a homogeneous mixture to the combustion cylinder. The fuels were tested at variable compression ratios, starting at CR 4.43 and increasing until significant high temperature heat release was observed. It was found that the fully saturated methyl nonanoate exhibited significantly higher reactivity in the low temperature region than both methyl nonenoate isomers and that the C2 unsaturated isomer was more reactive than the C3 isomer. Methyl nonanoate exceeded a threshold of 80% of the maximum possible fuel carbon conversion to CO2 at a compression ratio of 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, whilst methyl non-2-enoate (α,β-unsaturated ester) reached this CO2 value at a CR of 8.5
1, whilst methyl non-2-enoate (α,β-unsaturated ester) reached this CO2 value at a CR of 8.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and finally methyl non-3-enoate reached this CO2 value at CR 9.5
1 and finally methyl non-3-enoate reached this CO2 value at CR 9.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.34 The authors concluded that regardless of any additional reaction pathways available, the addition of an alkene in methyl nonanoate reduced its reactivity. However, it is clear that methyl non-2-enoate exhibited appreciably improved combustion relative to methyl non-3-enoate, which the authors attributed to the double bond at the more central C3 position within the alkyl moiety reducing the number of 5 and 6 membered transition rings which can be formed in the low-temperature branching reactions.
1.34 The authors concluded that regardless of any additional reaction pathways available, the addition of an alkene in methyl nonanoate reduced its reactivity. However, it is clear that methyl non-2-enoate exhibited appreciably improved combustion relative to methyl non-3-enoate, which the authors attributed to the double bond at the more central C3 position within the alkyl moiety reducing the number of 5 and 6 membered transition rings which can be formed in the low-temperature branching reactions.
Across all literature investigating unsaturated esters where an α,β-unsaturated ester was used in the comparative set, the authors were unable to fully resolve the behaviour of this fuel relative to structural isomers of the molecule. This is potentially due to the oversight of a unique chemical reactivity exhibited by α,β-unsaturated esters, known as the Michael reaction.35
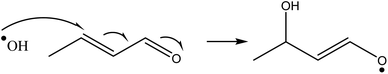
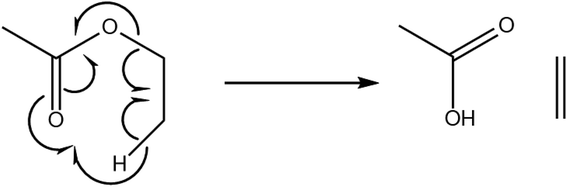
The Michael addition reaction is the nucleophilic addition of a carbanion (or another nucleophile) to an α,β-unsaturated carbonyl compound which was first classed by Arthur Michael in 1897.36 These two different reagents are commonly referred to as a Michael donor (nucleophile) and Michael acceptor (unsaturated carbonyl). Fig. 1 shows a textbook example of the reaction. The Michael acceptor is a specific functional structure, but the Michael donor can be any negatively charged species and does not require a specific structural motif.38
 | ||
| Fig. 1 The Michael addition reaction, a conjugated addition onto an α,β-unsaturated ketone.37 | ||
It is important to consider known reactions in the field of organic chemistry as organic radicals are an important species in many synthetic routes and research into fuel chemistry is inherently radical dominated organic chemistry.39
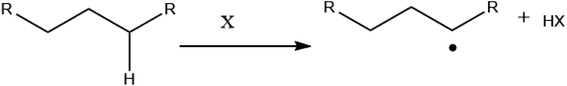
The general consensus is that the low temperature reactions which initiate combustion proceed following a hydrogen abstraction from an alkyl chain:11
In the case of oxygenated fuels, the initiated abstraction is often from an adjacent carbon to the oxygenate, owing to the resulting lower bond dissociation energies.40–42 In the case of unsaturated molecules there is also the potential abstraction from the alkene carbon or additional reactions of other radical species.43,44
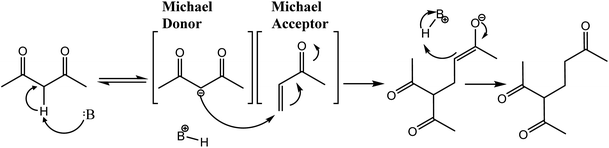
Atkinson et al. (1983) in a study on atmospheric pollutant formation investigated the reaction rates of hydroxyl radicals with 1,4 α,β-unsaturated carbonyl compounds (which can all act as Michael acceptors) and cyclohexane as a comparative molecule.45 When these compounds react with OH radicals, they can either undergo hydrogen abstraction of any hydrogen present in the molecule forming water and a carbon centred radical, or it can undergo 1,4-addition to the molecule, similar to a Michael donor as shown in Fig. 2.46
Hydrogen abstraction forming water and a carbon centred radical is the only reaction available when a hydroxyl radical encounters cyclohexane, as this is a fully saturated cyclo-alkane. Atkinson et al.'s investigation showed that the addition reaction to the alkene portion at the 4′ position as is typical of a Michael addition reaction had an ∼2 to 10 times higher reaction rate constant than hydrogen abstraction of cyclohexane. In the cases of reaction with the 1,4 α,β-unsaturated ketones it was stated with certainty that the reaction proceeds with the addition reaction, but in the case of aldehydes hydrogen abstraction from the aldehyde carbon can also proceed, with the ratio of these two reactions not quantified.46
Considering the potential for inclusion of the Michael acceptor functional group in the development of renewable fuels, one example is that the 1,4 α,β-unsaturated carbonyl could be produced by engineering the fatty acid biosynthesis pathway in microbes.47,48 This pathway is controlled enzymatically and can potentially be modified such that triglycerides or fatty acids containing the Michael acceptor functional group can be isolated and then converted into suitable biofuels.49 Many chemical conversions from C5 and C6 sugars have been found which result in Michael acceptors, such as the formation of certain acrylates, aspartic acid derivatives, itaconic acid derivatives and levulinic acid derivatives, all of which are considered conventional platform chemicals in lignocellulosic biomass conversion.50
This work presents the results of engine experiments in which the combustion and gaseous exhaust emissions of three series of fatty acid ester molecules were investigated. The series were chosen to each include a molecule which contained a Michael acceptor functional group and could be compared to similar molecules which did not contain this group. Relative to previous studies of Michael acceptors, this is the first to utilise a modern direct injection heavy-duty compression ignition engine with a significantly higher compression ratio than previous tests. These experiments were undertaken to test the hypothesis that the inclusion of a double bond on the alpha and beta carbons adjacent to the carbonyl in an ester group, thereby forming a Michael acceptor, may reduce the duration of ignition delay in a slow-speed compression ignition engine.
Heavy-duty engine test facility
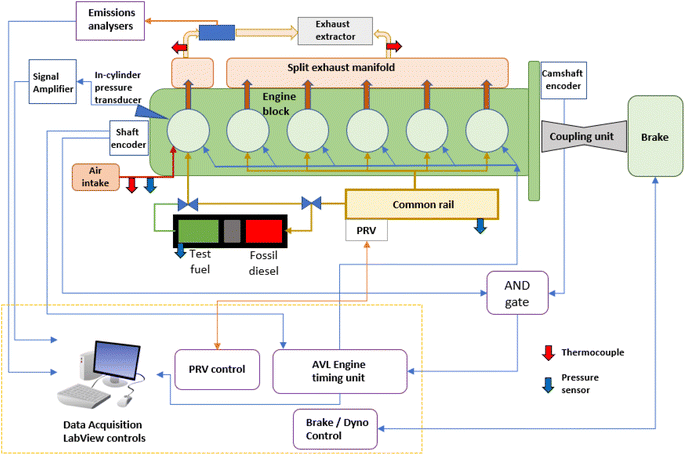
All of the combustion experiments presented were performed in a heavy-duty compression ignition engine which was appropriately modified for supplying test fuels to one of the six cylinders while the remaining were supplied with fossil diesel to ensure continuous engine operation. The volume of diesel supplied per cylinder to the back cylinders (two to six) was a much smaller volume than the amount of test fuel supplied to cylinder one, thereby keeping the total engine load below the maximum limit for the water-cooled Schenck Eddy Current Dynamometer W130 which controlled engine speed.In-cylinder pressure data was read with a Kistler type 6052C pressure transducer installed adjacent to the cylinder one exhaust valves. Injection timing, injection duration and cylinder firing order were controlled by an AVL engine timing unit, timed according to an AND signal from an Turck proximity sensor on the camshaft and a Baumer EIL580-T Optopulse crankshaft encoder with a 0.1 CAD resolution. Experimental data was recorded in a custom LabView environment directly from the timing unit and pressure transducer signals. Pressure data was averaged over 100 continuous combustion cycles during each experiment, and used to calculate the net apparent heat release rate and in-cylinder temperatures exhibited during combustion, using standard heat capacities and values of gamma for in-cylinder gasses presented in Heywood (2018).51
Fig. 3 depicts the testing facility, and the divided common rail system and low-volume fuel system used to supply the single component test fuels to the D8k, while Table 1 provides details of the engine specification and operating conditions. The operation of the high-volume test fuel system remains unchanged from that previously reported in detail by Hellier et al. (2011)52 & Schönborn et al. (2007).53 Gaseous emissions were measured with a Horiba MEXA 9100 HEGR, incorporating individual instruments to analyse concentrations of each of the CO, CO2, O2, NO and NOx. THC was measured by a Horiba FIA Ov-04 flame ionisation detector.
| Engine model | Volvo D8k 320 |
| No. of cylinders | 6 |
| Displacement | 7.7 dm3 |
| Stroke | 135 mm |
| Bore | 110 mm |
| Compression ratio | 17.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| Firing order (where cylinder 1 is that furthest from the flywheel) | 1, 4, 2, 6, 3, 5 |
| Engine dimensions h × w × l (mm) | 1004.06 × 830 × 1252.17 |
| Combustion chamber geometry (mm) | |
| Piston diameter | 108.195 ± 0.012 |
| Con rod length | 213.5 |
| Cylinder bore | 110 |
| Crank radius | 67.5 |
| Clearance volume (mm3) | 775.7 |
| Piston bowl geometry | Re-entrant design |
| Injection pressure | 700 ± 5 bar |
| Shaft encoder | 0.1 CAD resolution |
| Engine speed | 820 ± 20 rpm |
Test fuels
Seven fatty acid esters were tested as pure components to investigate the effect of including the Michael acceptor structure on compression ignition combustion and emissions. Table 2 provides an overview of these single component fuels with a clear indication of their chemical structure and which are considered potentially able to undergo the Michael reaction.| Fuel (Abbreviations) | Skeletal structure | Michael acceptor? | Boiling point (oC) | Density (g cm−3) | Viscositya at 50 °C (Cp) | Gross calorific valueb (J g−1) |
|---|---|---|---|---|---|---|
| a Measured using a Brookfield III-Ultra rheometer. b Measured using an IKA C1 bomb calorimeter. c Technical data for the reference diesel provided by Haltermann Carless. | ||||||
| Ethyl octanoate (EtO) |
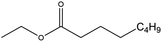
|
✗ | 206–208 | 0.867 | 1.225 ± 0.015 | 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 539.3 ± 136.9 539.3 ± 136.9 |
| Ethyl oct-2-enoate (EtO2) |
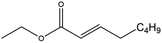
|
✓ | 222 | 0.883 | 1.540 ± 0.030 | 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 975.3 ± 16.2 975.3 ± 16.2 |
| Methyl nonanoate (MN) |
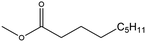
|
✗ | 213 | 0.875 | 1.44 (ref. 54) | N/A |
| Methyl non-2-enoate (MN2) |
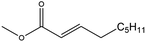
|
✓ | 215 | 0.895 | 1.580 ± 0.020 | 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 568.7 ± 108.9 568.7 ± 108.9 |
| Methyl non-3-enoate (MN3) |
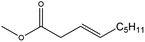
|
✗ | 213 | 0.885 | 1.425 ± 0.025 | 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 370.7 ± 169.0 370.7 ± 169.0 |
| Ethyl decanoate (ED) |
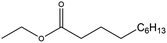
|
✗ | 245 | 0.865 | 1.555 ± 0.035 | 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 230.7 ± 46.0 230.7 ± 46.0 |
| Ethyl dec-2-enoate (ED2) |
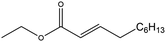
|
✓ | 251 | 0.880 | 1.890 ± 0.030 | 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 732.0 ± 30.8 732.0 ± 30.8 |
| Reference diesel (ref. D) | N/A | ✗ | 189.9–355.5c | 0.832 c | 2.245 ± 0.035 | 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800c 800c |
Three sets of similar molecules were used, two ethyl C8 esters, three methyl C9 esters and two ethyl C10 esters. As shown in Table 2, each of these sets contains the Michael acceptor equivalent molecule of the fatty acid ester (FAE) single component fuel. The methyl C9 set further contains methyl non-3-enoate, which is a structural isomer of the Michael acceptor fuel, but the change in double bond position from 2 to 3 prevents it from undergoing the Michael reaction.
These molecules were selected so as to test the hypothesis that inclusion of the Michael acceptor within fatty acid esters would have an appreciable impact on combustion. The differences between the ethyl C8 and C10 fuel sets allowed determination of the effects of fatty acid chain length on Michael acceptor combustion behaviour. The methyl C9 fuels provide insight into the significance of Michael acceptor structure compared to similar unsaturated esters.
Table 2 shows results of the viscosity and calorific tests of the esters, both of which are key values for the understanding of combustion and were unavailable from previous literature. Due to limited fuel availability, the viscosity and calorific values for methyl nonanoate were not measured.
Experimental procedure
Each of the single component ester fuels, and reference fossil diesel, were tested in the Volvo D8k engine under identical conditions, including a constant engine speed of 820 rpm, constant injection pressure of 700 bar, constant injection timing of 5.3 CAD BTDC and constant injection duration of 6.5 CAD. The injection timing of 5.3 CAD BTDC was chosen as the reference diesel consistently ignited at TDC in preliminary tests verifying the final D8k operating conditions. Table 3 summarises the experimental conditions and key combustion parameters obtained.| Fuel | Michael acceptor? | IMEP (bar) | Ignition delay (CAD) | Duration of combustion (CAD) | APHRR (J deg−1) | Timing of APHRR (CAD) | Maximum global calculated in-cylinder temperatures (K) |
|---|---|---|---|---|---|---|---|
| Ethyl octanoate (EtO) | ✗ | 5.05 | 6.4 | 24.9 | 541.7 | 363.5 | 1749.7 |
| Ethyl oct-2-enoate (EtO2) | ✓ | 5.10 | 8.8 | 14.8 | 727.3 | 367.1 | 1805.3 |
| Methyl nonanoate (MN) | ✗ | 5.06 | 6 | 25.9 | 417.7 | 362.7 | 1706.4 |
| Methyl non-2-enoate (MN2) | ✓ | 5.31 | 5.9 | 25.7 | 450.0 | 362.6 | 1776.8 |
| Methyl non-3-enoate (MN3) | ✗ | 5.14 | 8.4 | 17.1 | 800.6 | 366.4 | 1826.9 |
| Ethyl decanoate (EtD) | ✗ | 5.59 | 5.5 | 27.1 | 348.2 | 362 | 1774.5 |
| Ethyl dec-2-enoate (EtD2) | ✓ | 5.39 | 7.1 | 26.1 | 687.9 | 364 | 1790.9 |
| Reference diesel | ✗ | 7.12 ± 0.09 | 5.2 ± 0.09 | 32.4 | 312.2 | 361.6 | 1896.5 |
Results
In-cylinder pressure and heat release rate
Fig. 4 shows the in-cylinder pressure and apparent heat release rate of methyl nonanoate (MN), the methyl nonenoate isomers (MN2 & MN3) and reference fossil diesel. Immediately apparent is the significantly longer duration of ignition delay and higher peak heat release rate displayed by MN3 relative to the other test fuels. Also readily visible is that while MN, MN2 and the reference diesel exhibit appreciable heat release in both the premixed and diffusion-controlled combustion phases (albeit less less diffusion-controlled combustion in the case of the esters), that of MN3 is dominated by the premixed burn phase. The longer duration of ignition delay has a direct influence on combustion phasing, allowing for greater air fuel mixing prior to the start of combustion and thus a more combustible mixture available on autoignition, resulting in higher rates of heat release as the fuel is consumed more rapidly.52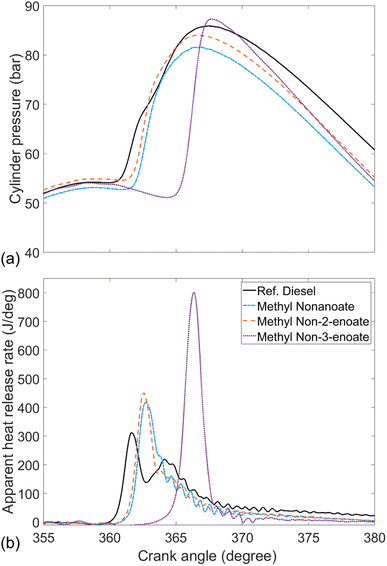 | ||
| Fig. 4 (a) In-cylinder pressure and (b) apparent heat release rates of methyl nonanoate, methyl nonenoate isomers and reference fossil diesel. | ||
Visible from the heat release rates (Fig. 4) is that MN exhibited a higher amount of diffusion-controlled combustion relative to the unsaturated esters.
Finally, from Fig. 4 we can observe that moving the double bond from the second to third carbon of the alkyl moiety of the unsaturated methyl esters, from MN2 to MN3, further reduces the extent of diffusion-controlled combustion observed.
Fig. 5 shows the in-cylinder pressure and apparent heat release rate of ethyl octanoate (EtO), ethyl oct-2-enoate (EtO2) and reference fossil diesel. Apparent is the larger period of premixed combustion and lower levels of diffusion-controlled combustion exhibited by both esters compared to reference diesel. It can also be seen that in EtO2 inclusion of a double bond at the 2-position led to a longer ignition delay and higher peak heat release rate relative to the fully saturated EtO.
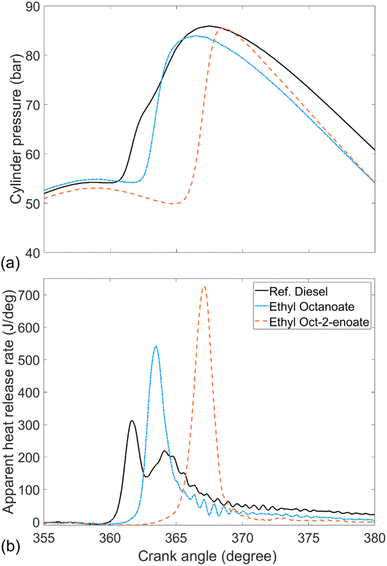 | ||
| Fig. 5 (a) In-cylinder pressure and (b) apparent heat release rates of ethyl octanoate, ethyl oct-2-enoate and reference fossil diesel. | ||
Fig. 6 shows the in-cylinder pressure and apparent heat release rate of ethyl decanoate (EtD), ethyl dec-2-enoate (EtD2) and reference fossil diesel. Readily apparent is that the majority of heat release occurred during the premixed burn fraction in the case of EtD2. Inclusion of a double bond at the 2-position led to a longer ignition delay and higher peak heat release rate relative to EtD. However, unlike the shorter saturated esters MN and EtO (Fig. 4b and 5b), EtD displayed a similar pattern of heat release to that exhibited by the reference diesel with a larger diffusion-controlled phase (Fig. 4 and 5). This can be attributed to the longer alkyl chain length and subsequently shorter ignition delay allowing less time for the fuel and air to mix before the start of combustion, further leading to a lower premixed burn faction.
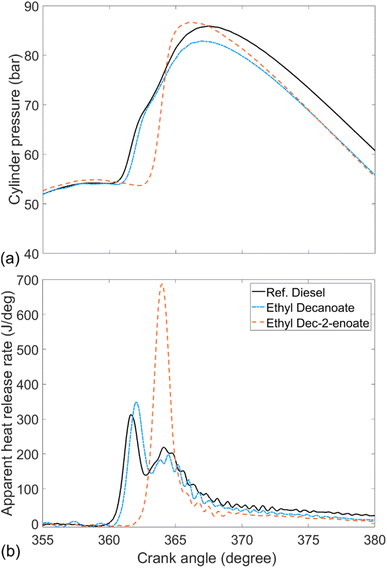 | ||
| Fig. 6 (a) In-cylinder pressure and (b) apparent heat release rates of ethyl decanoate, ethyl dec-2-enoate and reference fossil diesel. | ||
Combustion characteristics
Fig. 7 shows the impact of the Michael acceptor chemical structure on the duration of ignition delay in ethyl C8, methyl C9 and ethyl C10 saturated and unsaturated esters. In this figure and all subsequent references, ignition delay is defined as the interval between the start of the fuel injection signal supply to the injector control system and the start of combustion as determined from the apparent heat release rate. Additionally, in Fig. 7 and all subsequent figures, the error bars show the standard deviation about the mean of repeat tests of the reference diesel performed alongside the Michael acceptor and other fatty acid ester fuel tests. The ignition delay of MN3 and EtD2 showed a slight instance of variability within the 100 consecutive combustion cycles recorded and the coefficient of variance was found to be 0.009 and 0.101 respectively. For the remaining test fuels and reference diesel the coefficient of variance was 0.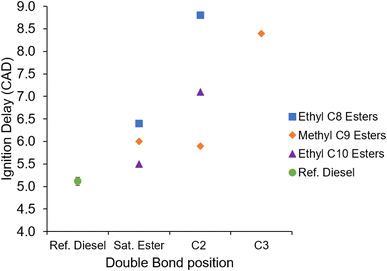 | ||
| Fig. 7 Ignition delays of the saturated and unsaturated esters, and reference diesel at constant injection timing and duration. | ||
Under the constant injection timing conditions, it is apparent from Fig. 7 that all of the fatty acid esters tested exhibited an ignition delay longer than that of the reference diesel.
Fig. 7 also shows a general trend of increasing ignition delay where a carbon-to-carbon double bond is included in the ester alkyl moiety. However, while inclusion of the double bond at the 2-position, creating the Michael acceptor chemical structure, increases ignition delay in both EtO2 and EtD2, MN2 displays a reduction in ignition delay relative to the saturated ester of equivalent chain length (MN). Moving the double bond to the 3-position in MN3 also leads to an increased ignition delay relative to the equivalent Michael acceptor (MN2).
Of the saturated esters, EtD exhibited the shortest duration of ignition delay and EtO the longest; this trend is expected and in agreement with the literature.55,56 With inclusion of the double bond at the 2-position, this trend is still observed for the ethyl C8 and C10 esters (Fig. 7); however, with the double bond addition the methyl C9 ester exhibited a shorter ignition delay than the C10 ester, an unexpected result as this is contrary to the known trend of hydrocarbon cetane numbers increasing with the number of –CH2 groups in aliphatic chains.57
Finally, from Fig. 7 it is clear that the fuel boiling points and viscosities shown in Table 2 had no systematic effect on the wide range of ignition delays observed. MN2 and MN3 present with similar values for both physical properties, but the two fuels exhibited ignition delays 2.5 CAD apart. This was larger than the difference in ignition delay of 0.7 CAD exhibited between EtO, which has the lowest boiling point and viscosity, and EtD2, which has the highest boiling point and viscosity. It is therefore reasonable to assume that the 40 °C difference in boiling point between the two was an insignificant driver of the ignition delays observed.
Fig. 8 shows the IMEP of the saturated and unsaturated esters and reference diesel during combustion with a constant duration of injection. It can be seen in Fig. 8 that all of the ester fuels exhibited an IMEP lower than that of the reference diesel, which is to be expected given the constant injection duration and lower calorific value of the oxygenated fuels (Table 3).
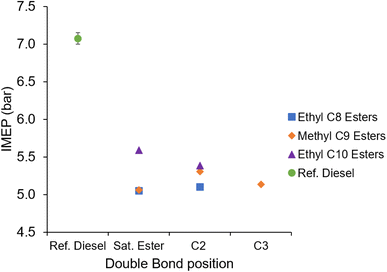 | ||
| Fig. 8 Indicated mean effective pressure (IMEP) of the saturated and unsaturated esters, and reference diesel at constant injection timing and duration. | ||
MN and EtO are structural isomers of each other, both having the chemical formula of C10H20O2. These esters both produced approximately 5.1 bar IMEP, whilst the larger EtD produced a 10% increase in IMEP (Fig. 8), attributed to its higher calorific value (Table 3).
Fig. 8 also shows the impact on IMEP of introducing a double to the ester alkyl moiety. Apparent is the slight reduction in IMEP exhibited by EtD2 relative to EtD, likely due to the lower calorific value of the unsaturated ester (Table 3). Also apparent is the increase in IMEP displayed by MN2 relative to MN, in contrast to the lower calorific value of MN2. Of further note is that the IMEP produced by MN2 is appreciably higher than that of other esters with the same carbon count; MN, EtO, MN3 and EtO2 all exhibit an IMEP value of 5.10 ± 0.05 bar, while an IMEP of 5.31 bar was recorded in the case of MN2. MN2 notably has the highest density of the fatty acid esters tested (Table 2), which under the test conditions of constant injection duration and timing may result in a higher mass of fuel entering the combustion chamber relative to the other fuels.58 However, the relative increase in both viscosity and density between MN and MN2 is similar to the change in both properties between EtO and Et2O, and there was no observed difference in IMEP between both fuels. Additionally, the properties of viscosity and density for all seven fatty acid esters were found to be within a tight range relative to the reference fossil diesel (Table 2) and so any changes in IMEP are attributable to improved combustion efficiency.
Fig. 9 shows the peak apparent heat release rate of the saturated and unsaturated esters tested. Is it readily apparent that for all esters, the inclusion of an unsaturated carbon bond results in a higher apparent peak heat release rate (APHRR) relative to their saturated counterparts. In the case of MN2, the observed increase in APHRR was far less pronounced than those of Et2O, Et2D, and MN3 relative to the saturated esters. The direct influence ignition delay has on the energy release during the premixed combustion phase causes the trends observed in PHRR to closely align with the trends seen in ignition delay, previously shown in Fig. 7. The higher APHRR of MN2 is also noteworthy as, despite exhibiting a shorter duration of ignition delay relative to MN, it exhibited a higher APHRR which is counterintuitive to the well-established relationship between increasing the duration of ignition delay and observing higher APHRRs.51,59,60
Fig. 10a and b show the calculated maximum in-cylinder global temperature and time at which it occurs respectively for the fatty acid esters tested and reference diesel. It can be seen in Fig. 10 that the maximum average cylinder temperature increases for the fatty acid esters with the inclusion of the double bond and increases further as this double bond is moved from the 2-position to 3-position in methyl C9 esters. Furthermore, the timing of the maximum in-cylinder temperature occurs earlier in the fatty acid esters with the inclusion of the double bond and earlier still as the double bond moves from the 2-position to the 3-position in the methyl C9 esters. Also apparent in Fig. 10a and b is that the reference diesel reached a significantly higher average temperature and reaches this temperature at a later stage in combustion than the fatty acid esters. This can be attributed to the smaller premixed burn fraction of combustion and larger mixing-controlled phase than the ester fuels (Fig. 4–6).
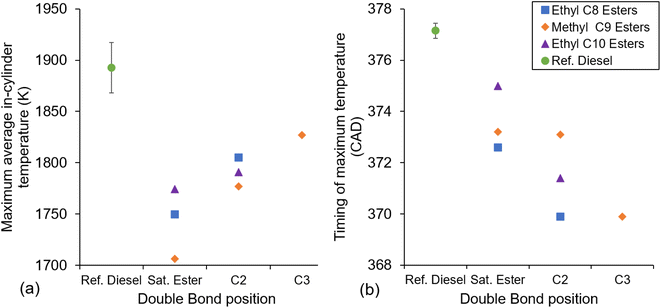 | ||
| Fig. 10 Maximum average cylinder temperature (a) and timing of maximum temperature (b) of the saturated and unsaturated esters, and reference diesel at constant injection timing and duration. | ||
Fig. 10b shows a reverse trend in timing of maximum temperature to that observed in the duration of ignition delay shown in Fig. 7 and to the level of PHRR shown in Fig. 9. As discussed in the context of Fig. 9, a longer ignition delay resulted in a higher PHRR, which in turn would be expected to lead to an earlier timing of the maximum average in-cylinder temperature.61,62
Two exceptions to trends within the fatty acid esters should be noted; the first is that the inclusion of the double bond at the 2-position reverses the relative order of the ethyl esters when considering the calculated maximum in-cylinder temperatures (Fig. 10). The fully saturated decanoate ester, EtD, exhibits a higher maximum average in-cylinder temperature than EtO; however, following the addition of the unsaturated double bond Et2D instead displays a lower maximum temperature than with Et2O. For both the saturated and C2 unsaturated esters, MN and MN2 exhibit lower maximum temperatures relative to the ethyl esters.
The second notable exception is that the methyl esters do not follow the trend of an earlier maximum average in-cylinder temperature with the unsaturated ester at the 2-position; instead, MN and MN2 reach their maximum average in-cylinder temperature at almost the same time (±0.1 CAD).
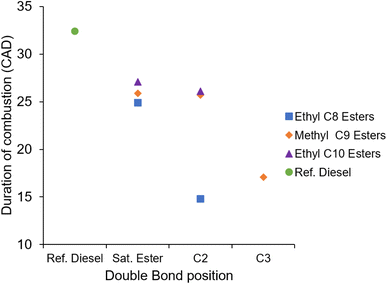
Fig. 11 shows the duration of combustion exhibited by the ethyl C8, ethyl C10, and methyl C9 esters and the reference diesel. The initial trend to note is that inclusion of a double bond at the 2-position significantly shortened the duration of combustion in Et2O relative to EtO, whilst MN2 and EtD2 remain close to their saturated counterparts. Moving the double bond to the 3-position further leads to a significantly reduced duration of combustion in MN3. It can further be seen that the three saturated esters exhibit a similar duration of combustion and finally it can be seen that all the ester test fuels exhibit a shorter duration of combustion than the reference diesel.
Previously shown in Fig. 7 was the long ignition delay of MN3 and EtO2 with both autoigniting 2.4 CAD after the respective saturated esters. A long duration of ignition delay allows for more of the air and fuel to be well mixed prior to autoignition. Premixed combustion has a short duration and, therefore, the overall combustion period reduces as the amount of premixed combustion increases.61,63
Exhaust emissions
Fig. 12 shows the measured NOx emissions during combustion of the three groups of fatty acid esters and reference diesel. Readily apparent is that the NOx emissions of EtO2 and MN3 are significantly higher than those of the other esters and of the reference diesel. This is to an extent an unexpected result, as NOx emissions would typically correlate with IMEP, and MN, MN3, EtO and EtO2 all exhibited similar IMEPs, approximately 5.1 ± 0.1 bar (Table 2 and Fig. 8). Fig. 13 shows the calculated in-cylinder temperature curves for the investigated fuels, in which EtO2 and MN3 exhibit the sharpest gradient relative to the remaining test fuels. NOx is produced either by fuel bound nitrogen, by prompt NOx, or by thermal NOx. Under the high temperature conditions of ignition compression, it is most likely the NOx formed is through oxidation of atmospheric nitrogen and thermal in source, requiring in-cylinder temperatures above 1800 K, known as the Zeldovich mechanism.64 As shown in Fig. 13, the calculated in-cylinder global temperatures for the reference diesel, EtO2 and MN3 surpass this threshold of 1800 K, and as the remaining fuels all surpass 1600 K, it is likely hotspots within the cylinder formed at suitable temperatures for thermal NOx production.65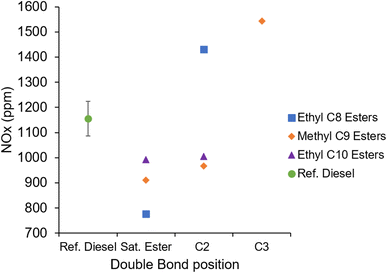 | ||
| Fig. 12 Exhaust gas NOx emissions of the saturated and unsaturated esters, and reference diesel at constant injection timing and duration. | ||
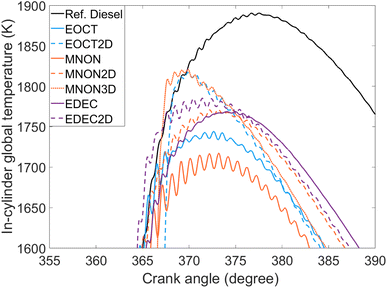 | ||
| Fig. 13 Calculated in-cylinder global temperatures near TDC of the saturated and unsaturated esters, and reference diesel at constant injection timing and duration. | ||
NOx production relies on the residence time and stoichiometry of the hot gaseous products of combustion. NOx production is highly time dependent and the thermochemical kinetics ‘freeze’ during the expansion stroke when the in-cylinder temperature and pressure decrease.65
The longer duration of combustion (Fig. 11) exhibited by the reference diesel, large mixing-controlled burn phase (Fig. 4–6) and higher in-cylinder temperatures (Fig. 13) relative to the esters therefore accounts for the higher NOx emitted by the reference diesel in comparison to the ester fuels except EtO2 and MN3.65,66
The significantly higher NOx emissions of EtO2 and MN3 (Fig. 12) can instead be attributed to these two esters exhibiting the longest durations of ignition delay (Fig. 7) which allowed for greater air fuel mixing prior to autoignition. With most of the fuel well-mixed prior to autoignition, the premixed burn phase dominated the combustion process and led to a shorter duration of combustion relative to the other test fuels (Fig. 11). Reaching a higher maximum in-cylinder temperature and completing combustion faster than the ester fuels allows more time for the hot gaseous combustion products to remain at high pressure prior to freezing of NOx kinetics.67
Fig. 14 shows the unburnt hydrocarbon emissions of the fatty acid esters and reference diesel. Apparent are the high levels of THC emitted by EtO2 and MN3, which is attributable to the long ignition delay and large premixed burn phase of both esters. A long ignition delay allows for greater air fuel mixing, over-diluting the fuel spray fringe, and creating areas in the chamber which are too lean to auto-ignite or support a self-propagating flame, increasing the amount of unreacted and partially reacted fuel.68
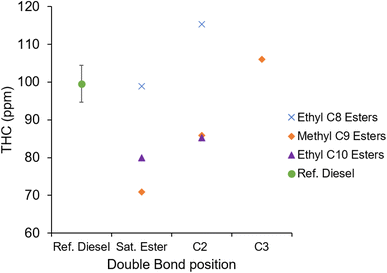 | ||
| Fig. 14 Exhaust gas unburnt hydrocarbon emissions of the saturated and unsaturated esters, and reference diesel at constant injection timing and duration. | ||
Also apparent is the effect of including a double bond in the alkyl chain which resulted in higher observed THC emissions for each of these esters; this is in agreement with higher THC emissions observed from esters with increased olefinic content or a longer duration of ignition delay which both can increase the amount of unreacted fuel.62,69 Higher THC emissions were also measured from MN2 relative to MN, despite MN2 not exhibiting a longer duration of ignition delay (Fig. 7).
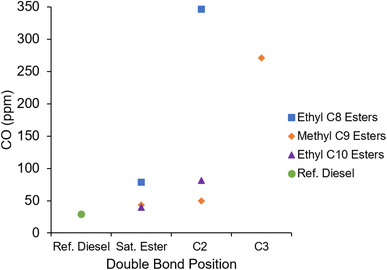
Fig. 15 shows the CO emissions of the fatty acid esters and reference diesel. Apparent is the significantly higher emissions of EtO2 and MN3, both of which also exhibited increased unburnt hydrocarbon emissions (Fig. 14). As discussed previously, the long ignition delay can lead to areas of air fuel mixture which are too lean to support a self-propagating flame. As with THC emissions, the inclusion of a double bond in the ethyl C8, ethyl C10 and methyl C9 esters led to increased CO emissions. Also apparent is the lower CO emitted by the reference diesel relative to the esters.
Fig. 16 shows the CO2 emissions of the ethyl octanoate, ethyl decanoate, and methyl nonanoate esters and reference diesel. Apparent is the lower CO2 emitted by the esters investigated relative to the reference diesel. All of the test fuels were injected for the same duration (Table 3), therefore the lower CO2 produced by the esters can be attributed to the lower carbon content by mass resulting from the esters' oxygen content. By molecular weight, the reference fossil diesel has a carbon content of 86.7%, while the carbon content of the esters is approximately 70%, e.g. EtO and MN contain 69.7% and EtD2 contains 72.7% carbon. The esters therefore contain 16–20% less carbon by mass, which closely aligns with the observation of the esters emitting 18–24% less CO2.
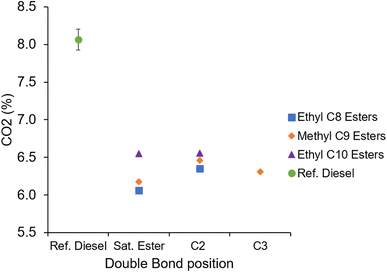 | ||
| Fig. 16 Exhaust gas CO2 emissions of the saturated and unsaturated esters, and reference diesel at constant injection timing and duration. | ||
Impact of the Michael acceptor group on combustion
It is clear from the results discussed in Sections 2.1 to 2.3 that MN2, a Michael acceptor molecule, exhibits similar combustion phasing (Fig. 4) and levels of exhaust emission species to its saturated analogue, MN. In particular, it has a slightly reduced ignition delay (Fig. 7), which correlates with the expectation of radical addition to the double bond outpacing H-abstraction. To further investigate the unique potential of the Michael acceptor functional group within target fuel molecules, a structural isomer of MN2 was also investigated, MN3, which is unable to undergo Michael addition. As a single component fuel MN3 exhibits a significantly longer ignition delay than MN2, which supports the hypothesis that radical addition to the Michael acceptor in MN2 is taking place and is important during low temperature kinetics.Previous work by Zhang et al.34 investigated the ratio between the low temperature and high temperature heat release for the same methyl C9 esters investigated within this work. The test fuels were tested in a CFR engine, at increasing compression ratios until significant high temperature heat release was observed, starting at a CR of 4.43 up to a CR of 9.5. They found that MN was far more reactive than MN2 in the low temperature region. In the work presented here, MN and MN2 are observed to exhibit similar heat release rates (Fig. 4) and MN2 exhibits a shorter ignition delay than MN (Fig. 7) indicating higher reactivity. These results observed in the D8k engine can be attributed to the significantly different experimental set up, and higher compression ratio of 17.5, which is more applicable to current engine designs, indicating that the Michael acceptor radical addition route is important to consider in practical diesel engines.
Also clear from the engine tests is that the ethyl C8 and C10 ester test fuels do not follow the same trend with structure as seen in the methyl C9 esters. In both cases the Michael acceptor molecules EtO2 and EtD2 do not exhibit clear similarities relative to the equivalent structural esters as shown between MN and MN2. Of particular note is that EtO and MN are structural isomers, as are EtO2 and MN2, therefore differences observed in combustion characteristics must result from structural isomerism. It is suggested that this difference in reactivity can be attributed to a unimolecular decomposition reaction unique to fatty acid esters with alcohol moieties longer than one carbon. Shown in Fig. 17, this reaction has a low activation energy. Studies by Li et al.70 and Bennadji et al.29 have shown that during the combustion of fatty acid ethyl esters, 98% of fuel molecule decomposition is initiated by this reaction.
In the case of the unsaturated ethyl esters, it is expected that they will undergo unimolecular decomposition before any potential Michael addition can occur, as intramolecular reactions generally occur faster than intermolecular reactions. Following unimolecular decomposition, the remaining molecule is an unsaturated carboxylic acid. The carboxylic acid group is significantly less electron withdrawing than an ester group and reduces the reactivity of the unsaturated carbonyl, making the Michael reaction unlikely to occur.71
Further to the lower Michael acceptor reactivity, Namysl et al.72 have shown that H-abstraction in carboxylic acids preferably proceeds at the C2 position, which in an alpha beta unsaturated carboxylic acid comprises the unsaturated hydrogens which have a higher bond dissociation enthalpy than saturated positions. As an unsaturated carboxylic acid, oct-2-enoic acid would now be expected to exhibit poor combustion.57
As with EtO2, the breakdown of EtD2 will initiate via the unimolecular decomposition reaction. In the observed rates of heat release and exhaust emissions EtD2 compared to EtD exhibits poorer performance, with a longer ignition delay, higher PHRR, higher CO and lower IMEP than its saturated counterpart. However, the differences observed between EtD and EtD2 are not as significant as with EtO and EtO2, which can be attributed to the longer carbon chain in the fatty acid moiety of the fatty acid ester. Following unimolecular decomposition EtD2 produces dec-2-enoic acid which allows an increased number of six- or seven-member transition state rings to form relative to oct-2-enoic acid. These transition state rings are a key feature of the low temperature reactions leading to autoignition and their ability to form directly correlates with fuel performance. Increasing alkyl chain length is known to reduce ignition delay, so it is expected that the unsaturated carboxylic acid with a longer alkyl chain would ignite more easily despite the poor reactivity of carboxylic acids.57,73
Conclusions
This study investigated the potential differences in combustion caused by the presence of α,β-unsaturated carbonyls (Michael acceptors) within an ester fuel as this chemical structure could provide a unique reaction pathway and shorten the ignition delay.• In methyl nonanoate inclusion of an alpha beta unsaturated carbon bond, a Michael acceptor, to form methyl non-2-enoate leads to a reduced ignition delay and higher IMEP relative to methyl nonanoate. This agrees with the proposed hypothesis that structures which facilitate radical addition to fuel molecules could reduce ignition delay.
• Movement of the double bond to form methyl non-3-enote from methyl non-2-enoate leads to a longer ignition delay, lower IMEP and an increase in undesirable NOx and CO emissions, indicating that the Michael acceptor present in methyl non-2-enoate improved low temperature reactivity relative to unsaturated esters without Michael acceptor reactivity.
• In ethyl octanoate inclusion of an alpha beta unsaturated carbon bond to form ethyl oct-2-enoate leads to a significant increase in ignition delay and emissions of NOx and CO. Likewise, the inclusion of the alpha beta unsaturated carbon bond in ethyl decanoate to form ethyl dec-2-enoate led to a longer ignition delay and increased pollutant emissions, but to a lesser extent than observed for ethyl octanoate esters.
• The increased ignition delay and poorer performance as a single component fuel of the ethyl ester Michael acceptors can be attributed to the rapid decomposition of the fuel from an unsaturated ester to a less reactive unsaturated carboxylic acid which is unable to undergo Michael acceptor reactions.
In this study of Michael acceptor fuel reactivity, it has been demonstrated clearly that minor changes in chemical structure can result in significant changes in fuel reactivity and combustion profiles in heavy-duty compression ignition engines. Such internal combustion engines as the D8k, or larger, will continue to service the road freight and marine sector for the foreseeable future, necessitating a better understanding of fuel molecular structure. This work also demonstrated the potential detailed chemical reactivity when choosing specific features in fuel. This has the potential to aid in the design of biofuels and renewable fuel with improved fuel properties such as higher ignition quality.
A limitation of the work is the small sample size, as the Michael acceptor could not be tested in an additional set of methyl esters. It is likely further ethyl esters would reflect the same result found here, that the unimolecular decomposition will continue to outpace any available radical intermolecular radical reactions. However, by studying the ethyl esters it has been made clear how intramolecular synergies can influence combustion in complex real world fuel molecules.
Abbreviations
| AHRR | Apparent heat release rate |
| ATDC | After top dead centre |
| BTDC | Before top dead centre |
| CAD | Crank angle degree |
| CFR engine | Cooperative fuel research engine |
| CO | Carbon monoxide |
| CO2 | Carbon dioxide |
| CR | Common rail |
| C-SOC | Constant start of combustion |
| CX (X = any number) | The carbon at position ‘X’ according to IUPAC convention |
| D8k | Volvo D8k engine |
| DAQ | Data acquisition |
| Dyno | Dynamometer |
| EtD | Ethyl decanoate |
| EtD2 | Ethyl dec-2-enoate |
| EtO | Ethyl octanoate |
| EtO2 | Ethyl oct-2-enoate |
| FAE | Faty acid ester |
| FAME | Fatty acid methyl ester |
| GHG | Greenhouse gas |
| ID | Ignition delay |
| IMEP | Indicated mean effective pressure |
| MN | Methyl nonanoate |
| MN2 | Methyl non-2-enoate |
| MN3 | Methyl non-3-enoate |
| NO | Nitrogen monoxide |
| NO2 | Nitrogen dioxide |
| NOx | Nitrogen oxides |
| O2 | Oxygen |
| PHRR | Peak heat release rate |
| PRV | Pressure relief valve |
| PS | Pressure sensor |
| Ref. D | Reference diesel |
| rpm | Revolutions per minute |
| SOC | Start of combustion |
| THC/UHC | Unburnt hydrocarbons |
Data availability
Supporting the publication of “The influence of Michael acceptors on the structural reactivity of renewable fuels” with RSC Sustainable Energy & Fuels, the following data has been uploaded and made publicly available with the Open Science Framework: raw in-cylinder pressure data, raw temperature and emissions data, processed temperature and emissions data, and a summary file containing the results from processing the raw in-cylinder pressure data. Data may be cited with the following information: T. Deehan, P. Hellier and N. Ladommatos, 2024, Open Science Framework data repository, DOI https://doi.org/10.17605/OSF.IO/MUNVS.Conflicts of interest
There are no conflicts to declare.References
- SMMT P. P., Charging and Refuelling Requirements of the Heavy Goods Vehicle Sector, 2023 Search PubMed.
- European Commission, European Commission-Press Release European Green Deal: EU Agrees Stronger Legislation to Accelerate the Rollout of Renewable Energy, 2023 Search PubMed.
- European Commission, What Is the European Geen Deal, Retrieved Oct. 11, 2021 from http://https//ec.europa.eu/info/strategy/priorities-2019-2024/european-green-deal_en, 2019 Search PubMed.
- The European Commission, Commission Delegated Regulation (Eu) 2023/1185, 2023, vol. 1185, pp. 20–33 Search PubMed.
- M. L. Yeoh and C. S. Goh, Hydrotreated vegetable oil production from palm oil mill effluents: Status, opportunities and challenges, Biofuels, Bioprod. Biorefin., 2022, 16, 1153–1158 CrossRef CAS.
- M. E. Dry, The Fischer-Tropsch process: 1950–2000, Catal. Today, 2002, 71, 227–241 CrossRef CAS.
- A. E. Atabani, A. S. Silitonga, H. C. Ong, T. M. I. Mahlia, H. H. Masjuki and I. A. Badruddin, et al. , Non-edible vegetable oils: A critical evaluation of oil extraction, fatty acid compositions, biodiesel production, characteristics, engine performance and emissions production, Renewable Sustainable Energy Rev., 2013, 18, 211–245 CrossRef CAS.
- H. Baker, R. Cornwell, E. Koehler and J. Patterson, Review of Low Carbon Technologies for Heavy Goods Vehicles, Ricardo, 2010, pp. 1–206, DOI:10.1038/430006a.
- T. D. Durbin, D. R. Cocker, A. A. Sawant, K. Johnson, J. W. Miller and B. B. Holden, et al. , Regulated emissions from biodiesel fuels from on/off-road applications, Atmos. Environ., 2007, 41, 5647–5658 CrossRef CAS.
- N. Mizushima, D. Kawano, H. Ishii, Y. Takada and S. Sato, Evaluation of Real- World Emissions from Heavy-Duty Diesel Vehicle Fueled with FAME, HVO and BTL using PEMS, SAE Tech. Pap., 2014 DOI:10.4271/2014-01-2823.
- C. K. Westbrook, H. J. Curran, W. J. Pitz, J. F. Griffiths, C. Mohamed and S. K. Wo, The effects of pressure, temperature, and concentration on the reactivity of alkanes: Experiments and modeling in a rapid compression machine, Symp. Combust., 1998, 27, 371–378 CrossRef.
- W. T. Lyn, Study of burning rate and nature of combustion in diesel engines, Symp. Combust., 1963, 9, 1069–1082 CrossRef.
- P. D. Patel, A. Lakdawala, S. Chourasia and R. N. Patel, Bio fuels for compression ignition engine: A review on engine performance, emission and life cycle analysis, Renewable Sustainable Energy Rev., 2016, 65, 24–43 CrossRef CAS.
- P. R. Ganji, R. N. Singh, V. R. K. Raju and S. Srinivasa Rao, Design of piston bowl geometry for better combustion in direct-injection compression ignition engine, Sadhana Acad. Proc. Eng. Sci., 2018, 43, 1–9 Search PubMed.
- M. E. Baumgardner, T. L. Vaughn, A. Lakshminarayanan, D. Olsen, M. A. Ratcliff and R. L. McCormick, et al. , Combustion of Lignocellulosic Biomass Based Oxygenated Components in a Compression Ignition Engine, Energy Fuels, 2015, 29, 7317–7326 CrossRef CAS.
- F. Jafarihaghighi, M. Ardjmand, M. Salar Hassani, M. Mirzajanzadeh and H. Bahrami, Effect of Fatty Acid Profiles and Molecular Structures of Nine New Source of Biodiesel on Combustion and Emission, ACS Omega, 2020, 5, 16053–16063 CrossRef CAS PubMed.
- D. Stepanenko and Z. Kneba, DME as alternative fuel for compression ignition engines – a review, Combust. Engines, 2019, 177, 172–179 CrossRef.
- J. P. Szybist, S. Mclauglin and S. Iyer, Emission and Performance Benchmarking of a Prototype Dimethy Ether Fueled Heavy-Duty Truck, Oak Ridge Nation Labaratory, 2014 Search PubMed.
- L. S. Tran, O. Herbinet, Y. Li, J. Wullenkord, M. Zeng and E. Bräuer, et al. , Low-temperature gas-phase oxidation of diethyl ether: Fuel reactivity and fuel-specific products, Proc. Combust. Inst., 2019, 37, 511–519 CrossRef CAS.
- C. Arcoumanis, C. Bae, R. Crookes and E. Kinoshita, The potential of di-methyl ether (DME) as an alternative fuel for compression-ignition engines: A review, Fuel, 2008, 87, 1014–1030 CrossRef CAS.
- R. L. McCormick, M. S. Graboski, T. L. Alleman, A. M. Herring and K. S. Tyson, Impact of biodiesel source material and chemical structure on emissions of criteria pollutants from a heavy-duty engine, Environ. Sci. Technol., 2001, 35, 1742–1747 CrossRef CAS PubMed.
- G. Anastopoulos, Y. Zannikou, S. Stournas and S. Kalligeros, Transesterification of vegetable oils with ethanol and characterization of the key fuel properties of ethyl esters, Energies, 2009, 2, 362–376 CrossRef CAS.
- S. A. Basha, K. R. Gopal and S. Jebaraj, A review on biodiesel production, combustion, emissions and performance, Renewable Sustainable Energy Rev., 2009, 13, 1628–1634 CrossRef CAS.
- A. Schönborn, N. Ladommatos, J. Williams, R. Allan and J. Rogerson, The influence of molecular structure of fatty acid monoalkyl esters on diesel combustion, Combust. Flame, 2009, 156, 1396–1412 CrossRef.
- S. M. Sarathy, S. Gaïl, S. A. Syed, M. J. Thomson and P. Dagaut, A comparison of saturated and unsaturated C4 fatty acid methyl esters in an opposed flow diffusion flame and a jet stirred reactor, Proc. Combust. Inst., 2007, 31, 1015–1022 CrossRef.
- S. Gaïl, S. M. Sarathy, M. J. Thomson, P. Diévart and P. Dagaut, Experimental and chemical kinetic modeling study of small methyl esters oxidation: Methyl (E)-2-butenoate and methyl butanoate, Combust. Flame, 2008, 155, 635–650 CrossRef.
- E. M. Fisher, W. J. Pitz, H. J. Curran and C. K. Westbrook, Detailed Chemical Kinetic Mechanisms, Proc. Combust. Inst., 2000, 28, 1579–1586 CrossRef CAS.
- S. M. Sarathy, S. Vranckx, K. Yasunaga, M. Mehl, P. Oßwald and W. K. Metcalfe, et al. , A comprehensive chemical kinetic combustion model for the four butanol isomers, Combust. Flame, 2012, 159, 2028–2055 CrossRef CAS.
- H. Bennadji, P. A. Glaude, L. Coniglio and F. Billaud, Experimental and kinetic modeling study of ethyl butanoate oxidation in a laminar tubular plug flow reactor, Fuel, 2011, 90, 3237–3253 CrossRef CAS.
- L. Coniglio, H. Bennadji, P. A. Glaude, O. Herbinet and F. Billaud, Combustion chemical kinetics of biodiesel and related compounds (methyl and ethyl esters): Experiments and modeling-Advances and future refinements, Prog. Energy Combust. Sci., 2013, 39, 340–382 CrossRef.
- P. N. Johnson, M. L. Lavadera, A. A. Konnov and K. Narayanaswamy, Oxidation kinetics of methyl crotonate: A comprehensive modeling and experimental study, Combust. Flame, 2021, 229, 111409 CrossRef CAS.
- C. Li, Z. Zhang, L. He, M. Ye, H. Ning and Y. Shang, et al. , Experimental and kinetic modeling study on the ignition characteristics of methyl acrylate and vinyl acetate: Effect of C[dbnd]C double bond, Energy, 2022, 245, 123257 CrossRef CAS.
- B. Liu, Z. Zhou, Z. Zhang and H. Ning, Theoretical Study on Abstraction and Addition Reaction Kinetics for a Medium-Size Unsaturated Methyl Ester: Methyl-3-hexenoate + H/OH Radicals, J. Phys. Chem. A, 2022, 126, 9461–9474 CrossRef CAS PubMed.
- Y. Zhang, Y. Yang and A. L. Boehman, Premixed ignition behavior of C9 fatty acid esters: A motored engine study, Combust. Flame, 2009, 156, 1202–1213 CrossRef CAS.
- B. E. P. Kohler and H. Potter, The properties of unsaturated sulfur compounds. I. Alpha beta unsaturated sulfones, J. Am. Chem. Soc., 1935, 57(7), 1316–1321 CrossRef.
- A. Michael and O. Schulthess, Ueber die Addition von Natriumacetessig- und Natriummalonsäureäthern zu den Aethern ungesättigter Säuren, J. Prakt. Chem., 1892, 45, 55–63 CrossRef.
- F. Wu, H. Li, R. Hong and L. Deng, Construction of quaternary stereocenters by efficient and practical conjugate additions to α,β-unsaturated ketones with a chiral organic catalyst, Angew. Chem., Int. Ed., 2006, 45, 947–950 CrossRef CAS PubMed.
- B. D. Mather, K. Viswanathan, K. M. Miller and T. E. Long, Michael addition reactions in macromolecular design for emerging technologies, Prog. Polym. Sci., 2006, 31, 487–531 CrossRef CAS.
- L. Feray, N. Kuznetsov and P. Renaud, Hydrogen Atom Abstraction, Radicals Org. Synth., 2001, 246–278, DOI:10.1002/9783527618293.ch39.
- S. J. Blanksby and G. B. Ellison, Bond dissociation energies of organic molecules, Acc. Chem. Res., 2003, 36, 255–263 CrossRef CAS PubMed.
- A. Sudholt, L. Cai, J. Heyne, F. M. Haas, H. Pitsch and F. L. Dryer, Ignition characteristics of a bio-derived class of saturated and unsaturated furans for engine applications, Proc. Combust. Inst., 2015, 35, 2957–2965 CrossRef CAS.
- X. You, Y. Chi and T. He, Theoretical Analysis of the Effect of C=C Double Bonds on the Low-Temperature Reactivity of Alkenylperoxy Radicals, J. Phys. Chem. A, 2016, 120, 5969–5978 CrossRef CAS PubMed.
- S. Cagnina, A. Nicolle, T. De Bruin, Y. Georgievskii and S. J. Klippenstein, First-Principles Chemical Kinetic Modeling of Methyl trans-3-Hexenoate Epoxidation by HO2, J. Phys. Chem. A, 2017, 121, 1909–1915 CrossRef CAS PubMed.
- C. W. Zhou, Y. Li, U. Burke, C. Banyon, K. P. Somers and S. Ding, et al. , An experimental and chemical kinetic modeling study of 1,3-butadiene combustion: Ignition delay time and laminar flame speed measurements, Combust. Flame, 2018, 197, 423–438 CrossRef CAS.
- R. Atkinson, S. M. Aschmann and J. N. Pitts, Kinetics of the gas-phase reactions of OH radicals with a series of α,β-unsaturated carbonyls at 299 ± 2 K, Int. J. Chem. Kinet., 1983, 15, 75–81 CrossRef CAS.
- R. Atkinson, S. M. Aschmann and J. N. Pitts, Kinetics of the gas-phase reactions of OH radicals with a series of α,β-unsaturated carbonyls at 299 ± 2 K, Int. J. Chem. Kinet., 1983, 15, 75–81 CrossRef CAS.
- Z. Shang, Z. Khalil, L. Li, A. A. Salim, M. Quezada and P. Kalansuriya, et al. , Roseopurpurins: Chemical Diversity Enhanced by Convergent Biosynthesis and Forward and Reverse Michael Additions, Org. Lett., 2016, 18, 4340–4343 CrossRef CAS PubMed.
- A. Miyanaga, Michael additions in polyketide biosynthesis, Nat. Prod. Rep., 2019, 36, 531–547 RSC.
- S. Vollenweider, H. Weber, S. Stolz, A. Chételat and E. E. Farmer, Fatty acid ketodienes and fatty acid ketotrienes: Michael addition acceptors that accumulate in wounded and diseased Arabidopsis leaves, Plant J., 2000, 24, 467–476 CrossRef CAS PubMed.
- F. H. Isikgor and C. R. Becer, Lignocellulosic biomass: a sustainable platform for the production of bio-based chemicals and polymers, Polym. Chem., 2015, 6, 4497–4559 RSC.
- J. B. Heywood, Heat-Release-Rate analysis, in Internal Combustion Engine Fundamentals, McGraw-Hill Education, 2nd edn, 2018, pp. 858–860 Search PubMed.
- P. Hellier, N. Ladommatos, R. Allan, M. Payne and J. Rogerson, The impact of saturated and unsaturated fuel molecules on diesel combustion and exhaust emissions, SAE Tech. Pap., 2011, 5, 106–122 Search PubMed.
- A. Schönborn, N. Ladommatos, J. Williams, R. Allan and J. Rogerson, Effects on diesel combustion of the molecular structure of potential synthetic bio-fuel molecules, SAE Tech. Pap., 2007 DOI:10.4271/2007-24-0125.
- C. Wedler and J. P. M. Trusler, Review of density and viscosity data of pure fatty acid methyl ester, ethyl ester and butyl ester, Fuel, 2023, 339, 127466 CrossRef CAS.
- C. K. Westbrook, C. V. Naik, O. Herbinet, W. Pitz, M. Mehl and S. M. Sarathy, et al. , Detailed chemical kinetic reaction mechanisms for soy and rapeseed biodiesel fuels, Combust. Flame, 2011, 158, 742–755 CrossRef CAS.
- B. Yang, C. K. Westbrook, T. A. Cool, N. Hansen and K. Kohse-Höinghaus, The effect of carbon-carbon double bonds on the combustion chemistry of small fatty acid esters, Z. fur Phys. Chem., 2011, 225, 1293–1314 CrossRef CAS.
- M. A. Ratcliff, R. L. Mccormick and J. D. Taylor, Compendium of Experimental Cetane Numbers Compendium of Experimental Cetane Numbers, 2017 Search PubMed.
- R. L. Mccormick, J. D. Ross and M. S. Graboski, Effect of several oxygenates on regulated emissions from heavy-duty diesel engines, Environ. Sci. Technol., 1997, 31, 1144–1150 CrossRef CAS.
- K. Thakkar, S. S. Kachhwaha, P. Kodgire and S. Srinivasan, Combustion investigation of ternary blend mixture of biodiesel/n-butanol/diesel: CI engine performance and emission control, Renewable Sustainable Energy Rev., 2021, 137, 110468 CrossRef CAS.
- M. Talibi, P. Hellier and N. Ladommatos, Impact of increasing methyl branches in aromatic hydrocarbons on diesel engine combustion and emissions, Fuel, 2018, 216, 579–588 CrossRef CAS.
- J. B. Heywood, Ignition Delay. in Internal Combustion Engine Fundamentals, McGraw-Hill Education, 2nd edn, 2018, 916–940 Search PubMed.
- E. Koivisto, N. Ladommatos and M. Gold, The influence of various oxygenated functional groups in carbonyl and ether compounds on compression ignition and exhaust gas emissions, Fuel, 2015, 159, 697–711 CrossRef CAS.
- J. B. Heywood, Combustion in Compression-Ignition Engines, in Internal Combustion Engine Fundamentals, McGraw-Hill Education, 2nd edn, 2018, pp. 836–839 Search PubMed.
- Y. B. Zeldvich, The Oxidation of Nitrogen in Combustion and Explosions, J. Acta Physicochim., 1946, 21, 577 Search PubMed.
- J. B. Heywood, Nitrogen Oxides, in Internal Combustion Engine Fundamentals, McGraw-Hill Education, 2nd edn, 2018, pp. 978–1005 Search PubMed.
- S. K. Hoekman and C. Robbins, Review of the effects of biodiesel on NOx emissions, Fuel Process. Technol., 2012, 96, 237–249 CrossRef CAS.
- Y. Nada, Y. Komatsubara, T. Pham, F. Yoshii and Y. Kidoguchi, Evaluation of NOx Production Rate in Diesel Combustion Based on Measurement of Time Histories of NOx Concentrations and Flame Temperature, SAE Int. J. Engines, 2014, 8, 303–313 CrossRef.
- J. B. Heywood, Hydrocarbon Emissions, in Internal Combustion Engine Fundamentals, McGraw-Hill Education, 2nd edn, 2018, pp. 1010–1059 Search PubMed.
- S. Pinzi, P. Rounce, J. M. Herreros, A. Tsolakis and M. Pilar Dorado, The effect of biodiesel fatty acid composition on combustion and diesel engine exhaust emissions, Fuel, 2013, 104, 170–182 CrossRef CAS.
- W. Li, C. Cao, X. Zhang, Y. Li, J. Yang and J. Zou, et al. , Exploring combustion chemistry of ethyl valerate at various pressures: Pyrolysis, laminar burning velocity and kinetic modeling, Combust. Flame, 2021, 227, 27–38 CrossRef CAS.
- F. E. Critchfield and J. B. Johnson, Determination of Alpha, Beta-Unsaturated Compounds by Reaction with Sodium Sulfite, Anal. Chem., 1956, 28, 73–75 CrossRef CAS.
- S. Namysl, M. Pelucchi, O. Herbinet, A. Frassoldati, T. Faravelli and F. Battin-Leclerc, A first evaluation of butanoic and pentanoic acid oxidation kinetics, Chem. Eng. J., 2019, 373, 973–984 CrossRef CAS.
- P. Hellier and N. Ladommatos, The influence of biodiesel composition on compression ignition combustion and emissions, Proc. Inst. Mech. Eng., Part A, 2015, 229, 714–726 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |