Open Access Article
Open Access ArticleSolvent processing for improved separation of hydrothermal liquefaction products†
Uriah
Kilgore
 *,
Emily
Diaz
*,
Emily
Diaz
 ,
Ben
Spry
,
Yuan
Jiang
,
Shuyun
Li
,
Andrew
Schmidt
and
Michael R.
Thorson
,
Ben
Spry
,
Yuan
Jiang
,
Shuyun
Li
,
Andrew
Schmidt
and
Michael R.
Thorson

Pacific Northwest National Laboratory, 902 Battelle Blvd, Richland, WA 99354, USA. E-mail: uriah.kilgore@pnnl.gov
First published on 19th June 2024
Abstract
Hydrothermal liquefaction (HTL) is a technology capable of producing sustainable hydrocarbon fuels from wet waste, reducing volumes of that waste as an added benefit. However, sustainable fuel production through HTL has yet to reach commercial scale and opportunities for improvements to process safety remain. This work describes low-pressure, low-temperature, two-stage solvent extraction and separation of HTL products utilizing naphtha range hydrocarbons. The similar qualitative solubility behavior of bitumen and biocrude (BC) with respect to paraffin versus naphthene or aromatic solvent composition allows us to examine a process comparable to solvent processing of bitumen. Lab-scale experiments were carried out to demonstrate the basic process and evaluate key parameters. The laboratory work indicates that using aliphatic/aromatic solvent mixtures at 80 °C results in a recovery of nearly 100% of the biocrude from the product mixture with reduced carbon content on the hydro-char. The findings illustrate the potential of solvent extraction for HTL biocrude processing. On a commercial scale, such a process may de-risk HTL, improving prospects for commercialization, opening the door to widespread conversion of wet-waste and waste biomass to sustainable fuels by HTL.
Introduction
As the global drive toward sustainable energy solutions intensifies, the demand for renewable diesel and sustainable aviation fuels is increasing. However, current supplies of these fuels are limited, necessitating exploration of alternative feedstocks and innovative processing technologies.1,2 Hydrothermal liquefaction (HTL) emerges as a promising pathway, offering a transformative approach to converting a wide range of wet-waste, such as sewage sludge, food waste, and agricultural waste, into a biocrude which can be refined into transportation fuels.3–6 The potential benefits of HTL extend beyond production of sustainable fuels, including reducing the volume and mass of waste that is disposed of in landfills, generating renewable energy, and producing other sustainable chemicals from HTL co-products.In HTL, biomass feedstocks are converted to biocrude (BC) using subcritical water at high temperature (250–350 °C) and high pressure (1450–3600 PSI). These reaction conditions produce an environment in which the ion product of water (KW) may be elevated by three orders of magnitude over those encountered under ambient conditions.7,8 At the same time, dielectric permittivity is significantly reduced, affecting the solvating properties of water.9 These combined conditions enable biomass hydrolysis.7,10,11 The resulting BC is a complex mixture of hydrocarbons, oxygenates, and nitrogen-containing compounds resulting from breakdown of lipids, proteins, and carbohydrates contained in biomass feedstocks. While biocrudes typically contain higher oxygen and nitrogen contents than petroleum crudes,12–15 upgrading allows production of transportation fuels, chemicals, and other value-added products.
Current efforts to reduce reliance on fossil fuels, reduce greenhouse gas emissions, and manage waste more sustainably have positioned HTL to play an important role in our future energy mix. There have been numerous attempts to commercialize HTL dating back to the second half of the 20th century, and significant investments in small demonstration units were made by various organizations following the 1970s oil embargo and high energy prices in the early 1980s. However, widespread commercial-scale implementation has yet to be realized. Some challenges to commercialization of HTL remain, including management of the aqueous phase co-product (HTL-AP), a dilute but complex mixture of organic compounds, and HTL product solids (hydro-char).16,17 HTL requires high pressures and temperatures, which makes cost and safety a primary consideration when scaling up for commercial deployment. Minimizing the number of operations performed at HTL reactor temperature and pressure, product separation, for example, will help mitigate these issues. The solvent extraction approach described in this paper for separating biocrude from HTL product mixtures provides lower-risk operating conditions and a scalable process design. As will be described in more detail, the method and solvent choice was designed to accommodate downstream upgrading of BC in existing refining infrastructure (co-processing).
HTL product separation
Separating biocrude from aqueous and solid products in the HTL process is a critical step in producing a high-quality BC product. The HTL reaction produces a mixture of biocrude oil, fine solids, gas-phase products, and an aqueous stream containing a high concentration of soluble organic molecules. The organic (BC), aqueous, and solid phases form stable emulsions that, in some cases, may not separate even after several days. Interactions between the solids and interfacially active chemical species in the organic and aqueous phases are known to be the cause of the emulsion stability and after HTL solids are removed the oil and water phases separate by gravity.At the laboratory scale, especially for HTL reactions conducted in batch reactors, separation of biocrude from HTL aqueous and solid products has often relied on solvent extraction methods along with centrifugation and filtration.18,19 A general method for small-scale batch HTL solvent extraction from the product mixture involves cooling the mixture to room temperature, adding a solvent to the mixture, and allowing the mixture to phase separate. The organic phase containing the BC is removed, and the BC can be isolated by evaporating the solvent. Others have also attempted to use only gravity settling.20 Some researchers have included co-solvents such as glycerol with feed to improve downstream product separation intending to eliminate the need for solvent extraction of biocrude.21
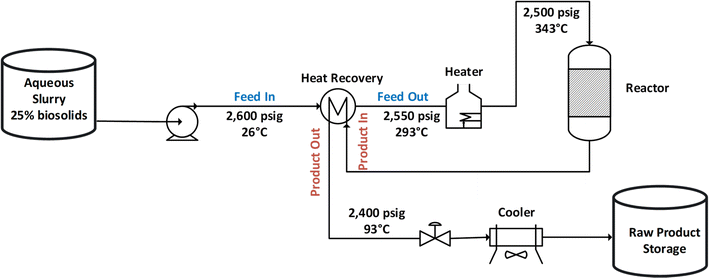
As currently practiced at Pacific Northwest National Laboratory (PNNL), HTL liquids (aqueous and biocrude) are separated from solids at high temperatures and pressures (blowdown of solids following the reactor). Following high pressure, high temperature blowdown, BC is separated from the aqueous phase at a lower temperature and pressure in the absence of added extractive solvents (general process diagram for HTL in Fig. 1).22,23 While this approach to product separation has worked well at laboratory and engineering scales, the demanding conditions of the separation process may be challenging at the commercial scale. For example, using high pressure and temperature blowdown solid separation, at the HTL commercial scale (i.e., design case: 110 dry tons per day), the separation section might be as high as 60 feet tall requiring stainless-steel vessels with walls >7.5 inches thick at a design pressure of 3000 PSI and temperature of 370 °C. It is important to consider that operability and safety factors change with the process scale and HTL unit operations need to be practical and safe to operate at the commercial scale. A low-pressure, low temperature product separation process could significantly reduce safety concerns and maintenance requirements that arise from high pressure, high temperature product separation including erosion from high velocities, material stress and vessel fatigue due to thermal cycling, and filter plugging.
Low pressure, low temperature solvent processing of HTL product mixtures provides a potential alternative to the current PNNL approach. In addition to being safer, such a process may ultimately be lower in cost and more reliable to operate. In this work, we describe a hypothetical two-stage solvent processing approach to HTL product separation and lab-scale experimental efforts supporting the design of that process.
Biocrude extraction methods
The selection of solvent for BC extraction depends on the properties of the BC and the desired separation efficiency. There is a significant body of research describing the fractionation of BC through solvent extraction methods including the use of supercritical fluid extraction (SFE).24–36 Previous attention has been given to the impact of extractive solvent choice on oil yields and its important consequences with respect to demetallation of biocrude.19,37 Solvents commonly used for HTL biocrude at the laboratory scale include polar solvents such as methanol, ethanol, and acetone which are effective at dissolving oxygenated compounds such as carboxylic acids, alcohols, and ketones. Nonpolar solvents including hexane, heptane, decane, cyclohexane, and hexadecane have also been studied for biocrude recovery.30Solvent cost and compatibility with downstream processing have been concerns for BC extraction. It is typically not feasible to completely remove all solvent from the BC product, which creates several issues. The first issue is economic because the solvent is often worth more than the biocrude. The second is compatibility with downstream BC upgrading facilities, as many popular solvents used in research studies are detrimental to refinery operations.
Relevance of bitumen solvent processing
When considering a possible alternative process for processing the HTL product mix, we noted the mixture of solids and biocrude and considered the relevance of processing heavy, viscous crude bitumen extracted from oil sands or oil shale deposits. Bitumen comprises a complex mixture of hydrocarbons, including high-molecular-weight asphaltenes, which results in several physical properties similar to those of BC. For example, both are viscous, have densities close to the density of water, and contain some resin and surfactant molecules. Consequently, the bitumen/solids/aqueous phase emulsion encountered in oil sand processing has many similarities to the emulsion produced by HTL.Excellent summaries of the tar sand extraction process are available,38,39 but only the elements directly relevant to the proposed BC extraction process are covered here. There are two main approaches to mining bitumen: surface mining and in situ steam injection. Which method is selected depends on local geology, but both techniques ultimately use hot water to provide rough separation of bitumen and earth. The resulting slurry (bitumen, earth, and water) then undergoes a froth treatment step, where additional earth and water is removed to produce an emulsion in the range of 50–60% bitumen, 30–40% water, and 10–15% solids. The solids at this stage are extremely fine, with a significant portion of particles smaller than 10 microns. The emulsion is very stable and cannot be gravity separated. Solvent extraction is used to further purify the bitumen. A hydrocarbon solvent in the naphtha boiling range is mixed with the emulsion and the bitumen partitions into the hydrocarbon phase, which separates by gravity. The remaining aqueous and solvent phase is typically washed a second time with hydrocarbon solvent to extract any remaining bitumen. The bitumen is separated from the hydrocarbon solvent through distillation and the solvent is recycled back to the extraction stage. Any remaining solvent in the aqueous phase is steam stripped and recovered to be recycled. This process is shown in Fig. 2.
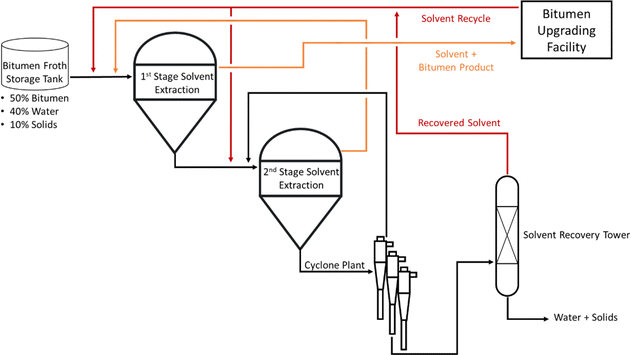 | ||
| Fig. 2 Process flow diagram of a generic solvent extraction process used in oil sand bitumen processing production. | ||
Some naphtha is intentionally left in the bitumen to be transported to downstream upgrading facilities, saving energy but also improving the pipeline transportation properties by reducing viscosity. The naphtha is recovered in the upgrading facility and recycled back to the bitumen solvent extraction process.
Bitumen solvent extraction is a well understood process used for many decades and has been optimized for economics, reliability, and safety. The most important operating and design parameters are as follows:
Solvent to bitumen (S/B) ratio: this is the primary energy impact and operational expense of the process. The solvent must be boiled off the bitumen for recovery; therefore lowering S/B reduces energy consumption. However, S/B ratios that are too low result in poor emulsion separation and a lower-quality bitumen product.
Settler temperature: a higher operating temperature results in faster emulsion settling time and allows for a smaller settling vessel. However, there is an economic trade-off because the solvent is relatively volatile, and the vessel design pressure must increase with operating temperature.
Solvent composition: solvents range from paraffins (pentane/hexane) to naphthenes. Naphthenes have increased recoveries because they better solubilize the asphaltenes in bitumen, but this results in a lower-quality product relative to paraffin solvents. In addition to more asphaltenes, more water and solids remain in the bitumen when using naphthene solvents. The solvent choice depends on the downstream customers' specifications.
Two-stage solvent extraction concept
As discussed above, solvent extraction of HTL BC is well studied in a lab setting but has not been practiced at commercial or demonstration scales. There are a few reasons why this is likely the case: first, use of chemical-grade solvents to recover BC is typically not economically attractive even if only small amounts of the solvent are lost in the process; second, supplying most solvents in large volumes can be logistically difficult because chemical manufacturing is often confined to certain geographical areas; third, some solvents carry potential environmental risks, especially at large scales—this is partially due to additional contamination of the aqueous product stream with the solvent and the required treatment; and finally, contamination of the BC with the organic solvent is inevitable—therefore, the downstream processing infrastructure (oil refineries) and final customers (in many cases, the public) must accept the solvent. The process concept discussed here addresses these problems through process configuration, mild process conditions, and selection of widely available solvents that can be handled safely and are compatible with existing infrastructure.The sections that follow describe our initial research efforts to define the proper solvents and processing conditions that will be used to guide future studies and potential scale-up of a two-stage solvent extraction process for low temperature, low pressure HTL product separation.
Fig. 3 shows the conceptual flow for continuous solvent extraction integrated with the HTL process. In brief, a solvent extraction step can be carried out directly downstream from the HTL reactor. In solvent extraction, the HTL product emulsion is thoroughly mixed with the solvent and then allowed to phase separate. The BC will preferentially be drawn into the solvent. Because the solvent has a lower density than the aqueous and solid materials, phase separation by gravity should occur. In the simplest case, the organic phase (solvent and BC) will float to the top of the extraction vessel, allowing it to be preferentially drawn from a high-elevation take-off point such that the aqueous and solid phases are excluded. Solvent is then removed from the BC by distillation and recycled for reuse in the extraction step. Aqueous and solid materials are drawn from the bottom of the liquid extraction vessel and sent for further downstream treatment.
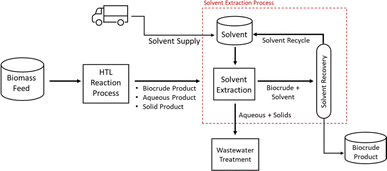 | ||
| Fig. 3 Simplified conceptual flow diagram of the BC solvent extraction process integrated into the HTL process. | ||
Materials and methods
Inductively coupled plasma (ICP) analyses were conducted using a PerkinElmer Optima 7300-DV ICP-OES equipped with a cyclonic spray chamber and a Meinhard nebulizer. Elements selected for detection are Ag, Al, As, Ba, Ca, Cd, Co, Cr, Cu, Fe, K, Mn, Na, Ni, P, Pb, Re, Sr, V, Y, Zn, Mo, Si, Ti, W, Au, Pd, Pt, Rh, S, Sn, Zr, and Ru. Calibration was performed with certified reference standards. Gas chromatography-mass spectrometry (GC-MS) analyses of aqueous samples were carried out using an Agilent 5795C GC-MS. The column was an Agilent HP-5MS with 30 m × 0.25 mm × 0.25 μm film thickness with a carrier gas of helium at 1.0 mL min−1. Oven temperature was initially held for 0.1 min at 35 °C and ramped at 6 °C min−1, with a final temperature of 325 °C. A final oven hold of 1 minute was used. The inlet was heated at 270 °C, and 1 μL of sample was injected using a splitless injection. Ion chromatography (IC) samples were analysed using a Dionex ICS-3000 equipped with a Dionex AS11-AC (4.0 × 250 mm) column and a conductivity detector. Aqueous samples were diluted as necessary to fall within the calibration range. Detection limits range from 1.0 to 100 ppm. Analytes include fluoride, bromide, nitrate, chloride, sulphate, and phosphate. Nitrogen, ammonia (NH3–N), and chemical oxygen demand (COD) analyses were carried out using a HACH DR2800 following the manufacturer's recommended methods. CHNS combustion analysis was carried out using an Elementar Vario Macro Cube. Combustion and reduction tubes were packed accordingly to analyse carbon, nitrogen, sulphur, and hydrogen. The combustion tube was heated to 1150 °C and the reduction tube to 850 °C. Helium was used as the carrier gas. Infrared spectra were collected on a Nicolet iS50 Fourier transform infrared spectrometer.BC miscibility experiments were carried out using a BC derived from food waste obtained from Joint Base Lewis-McChord. Toluene (Honeywell), ethyl acetate (Thermo Scientific), methylcyclohexane (Sigma-Aldrich), and decane (Alfa Aesar) were all used as received. Road-ready cetane gasoline (EtOH free) was purchased from a local gas station. Solvents were combined with the BC in the ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, 1
4, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 4
1, and 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The mixtures were heated to 38 °C, 40 °C, 60 °C, and 82 °C. Solvents with lower boiling points were not heated to a maximum temperature of 82 °C. The mixtures were heated and stirred for 40 minutes and then left to cool at room temperature. The first set of miscibility experiments was performed to determine miscibility of BC with each solvent at each temperature (except for temperatures above the solvent boiling point). These experiments were qualitative and helped determine which solvents to use in the following experiments.
1. The mixtures were heated to 38 °C, 40 °C, 60 °C, and 82 °C. Solvents with lower boiling points were not heated to a maximum temperature of 82 °C. The mixtures were heated and stirred for 40 minutes and then left to cool at room temperature. The first set of miscibility experiments was performed to determine miscibility of BC with each solvent at each temperature (except for temperatures above the solvent boiling point). These experiments were qualitative and helped determine which solvents to use in the following experiments.
Solvent extraction and separation experiments were carried out using a reconstituted HTL product emulsion, composed of 5% BC, 80% HTL aqueous, and 15% hydro-char slurry (∼39% solids, 61% HTL liquids). The HTL product mixtures were combined in glass jars and vigorously stirred with a stir bar on an electric stir plate for several hours. The HTL products including HTL-AP, BC, and hydro-char slurry used in these experiments were derived from sewage sludge provided by the Great Lakes Water Authority (GLWA) wastewater treatment plant in Detroit, Michigan.
Solvent extraction and separation were performed using 200 g or 300 g of reconstituted HTL product mixtures. The product mixtures were heated to 40 °C, 60 °C, or 80 °C in a 500 mL jacketed separation funnel (Ace Glass). Temperature was maintained using a fluid circulating heater with process temperature monitored using a thermocouple immersed in the product mixture. For stage 1 separation, the designated quantity of solvent was added to the reconstituted emulsions in the funnel only when it reached the desired temperature. The mixture was stirred thoroughly and then heated for the designated time. Pictures were taken every 10–15 minutes to document the settling rate of the mixture. A sample of the organic phase of ∼30–50% of the total solvent mass was taken off the top after the designated time for stage 1 was reached. Only the top portion was sampled to avoid uptake of the rag layer at the liquid–liquid phase interface. This sampling method would also simulate a possible commercial operation, where the organic phase would be drawn off (or spill over) near the top of the organic phase liquid.
In stage 2, a mass of solvent approximately equal to the organic mass removed in stage 1 was added back to the mixture in the funnel. The mixture was stirred thoroughly and heated for the designated time. After the completion of stage 2, 30–50% of the total solvent mass was sampled from the top. Preliminary studies were carried out using toluene as the solvent, while later evaluations used a mixture of toluene and heptane in an 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 mass ratio as the solvent.
20 mass ratio as the solvent.
At the conclusion of stage 2, approximately 30% of the volume of the aqueous and solid product mixture was drained from the bottom of the funnel into a glass jar. The sample taken from the bottom was filtered using filter paper and a Büchner funnel. The aqueous filtrate was analysed by ICP, CHNS combustion analysis, GC-MS, NH3N, COD, IC, and HPLC. After filtration, solvent processed hydro-char (HTL solids) samples were oven dried overnight at 105 °C. Dried hydro-char was analysed by CHNS and ICP (with HF digestion) techniques.
Solvent from stage 1 and 2 organic samples was removed by rotary evaporation followed by heating at 105 °C for 18 hours. The BC remaining after solvent extraction was sampled and analysed by ICP, Karl Fischer titration, and CHNS techniques. The solvent was sampled and analysed with CHNS, ICP, GC-MS, and Karl Fischer titration. The remainder of the BC after sampling was heated to 1000 °C for 20 hours under an atmosphere of air. The remaining solids were considered “ash.”
Results and discussion
BC solubility
The BC extraction process has high potential for fouling due to precipitation of low solubility species. The chemical properties of the solvent significantly influence how much material precipitates during the extraction process. Therefore, it is critical that solubility be assessed.Solubility evaluation was first carried out at room temperature with a solvent mix of toluene and heptane (80/20 mass/mass meant to roughly simulate reformate) and two separate batches of BC. When mixed at a 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio (solvent: BC mass) at room temperature, the BC derived from sewage sludge (used in most of the experiments in this work) left 1.18% of the residual mass undissolved, while the BC derived from food waste resulted in 0.63% undissolved material. When mixed with solvent at room temperature in a 15
1 ratio (solvent: BC mass) at room temperature, the BC derived from sewage sludge (used in most of the experiments in this work) left 1.18% of the residual mass undissolved, while the BC derived from food waste resulted in 0.63% undissolved material. When mixed with solvent at room temperature in a 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 solvent to BC mixture ratio, 0.77% of the residual mass was left undissolved. The residual mass was collected and dried at 105 °C for 15 hours in air, resulting in brown solids. Similarly, solvent was removed from soluble portions of BC and then dried overnight at 105 °C. The resulting dried residual solids and BCs were analysed by CHNS combustion analysis (Table 1) and infrared spectroscopy (see the ESI, Fig. S18†). Dried residual solids contain higher relative quantities of nitrogen and lower H/C ratios compared to the solvent soluble portion of the BC. These values appear to be consistent with the bio-derived asphaltenes (“bio-asphaltenes”) studied by Robertson, et al.40 The infrared spectra of the insoluble fractions differ from those of the dried solvent soluble portion of the BC in the relatively greater intensity of the broad O–H region compared to the C–H alkane stretching region.
1 solvent to BC mixture ratio, 0.77% of the residual mass was left undissolved. The residual mass was collected and dried at 105 °C for 15 hours in air, resulting in brown solids. Similarly, solvent was removed from soluble portions of BC and then dried overnight at 105 °C. The resulting dried residual solids and BCs were analysed by CHNS combustion analysis (Table 1) and infrared spectroscopy (see the ESI, Fig. S18†). Dried residual solids contain higher relative quantities of nitrogen and lower H/C ratios compared to the solvent soluble portion of the BC. These values appear to be consistent with the bio-derived asphaltenes (“bio-asphaltenes”) studied by Robertson, et al.40 The infrared spectra of the insoluble fractions differ from those of the dried solvent soluble portion of the BC in the relatively greater intensity of the broad O–H region compared to the C–H alkane stretching region.
| Sample | Carbon (mass%) (C) | Hydrogen (mass%) (H) | Nitrogen (mass%) (N) |
|---|---|---|---|
| a HTL BC derived from sewage sludge dried at 105 °C for 15 hours. b Residual solids from solvent: BC mixture, dried at 105 °C for 15 hours. c Soluble BC, rotary evaporation, and then dried at 105 °C for 15 hours. | |||
| Dry BCa | 76.33 | 9.64 | 4.96 |
Residual solids (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)b 1)b |
73.57 | 7.17 | 7.42 |
Residual solids (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)b 1)b |
72.74 | 7.26 | 7.40 |
Soluble BC (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)c 1)c |
76.70 | 10.81 | 4.29 |
Soluble BC (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)c 1)c |
76.88 | 10.24 | 4.53 |
Following the room temperature studies, BC solubility was evaluated at elevated temperatures (40–82 °C) with a variety of solvents including decane, ethyl acetate, toluene, methylcyclohexane, and gasoline at three different solvent-to-BC ratios (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, 1
4, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 4
1, and 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The selection of solvents provides a wide range in polarity (e ∼2.0–6.0 @ 25 °C) and boiling points (77–174 °C). The results from select experiments are shown in Table 2. Qualitative testing indicated that the use of aliphatic solvents would result in significant quantities of undissolved BC, while toluene and ethyl acetate left very little residual BC.
1). The selection of solvents provides a wide range in polarity (e ∼2.0–6.0 @ 25 °C) and boiling points (77–174 °C). The results from select experiments are shown in Table 2. Qualitative testing indicated that the use of aliphatic solvents would result in significant quantities of undissolved BC, while toluene and ethyl acetate left very little residual BC.
| Solvent | Temperature (°C) | Ratio (solvent![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : BC) : BC) |
% undissolved residual mass |
|---|---|---|---|
| Decane | 60 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
16% |
| Decane | 80 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
14% |
| Methylcyclohexane | 60 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
8% |
| Methylcyclohexane | 80 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
9% |
| Toluene | 60, 80 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, 1 4, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 4 1, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
<1% |
| Ethyl acetate | 60, 80 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, 1 4, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 4 1, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
<1% |
Two-stage processing
Two-stage solvent processing of the HTL product mixtures was carried out on a “reconstituted HTL product emulsion.” These reconstituted mixtures were made by recombining previously separated HTL product streams (solids hydro-char slurry, aqueous phase, and BC) resulting from HTL processing of sewage sludge (see Fig. 4 and Materials and methods). The composition of the reconstituted HTL product emulsion is intended to closely match the wet product yields isolated after HTL processing. In the extraction experiments, reconstituted HTL product emulsions were mixed with solvent at three different temperatures (40, 60, and 80 °C) and three different mass ratios (solvent: HTL product emulsion = 0.7, 0.5, and 0.25). After agitation, the mixtures were allowed to settle at the designated temperature, and the progress was recorded through photographs at 15-minute intervals. The initial product separation was carried out using toluene as the diluent/solvent. Later experiments were carried out using a mixture of toluene (80% mass) and heptane (20% mass). The procedure is described in the Materials and Methods section of this report. Images of the equipment used are available in the ESI.†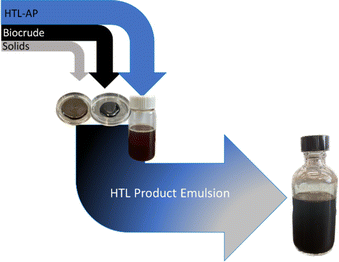 | ||
| Fig. 4 Reconstitution of HTL product emulsions (right) from previously isolated HTL products (left). | ||
We consistently observed that solvent/HTL product mixtures held at higher temperatures tended to settle more quickly with both toluene and toluene/heptane mixtures as the solvent. This behaviour was generally expected due to the dependence of settling on fluid density (and/or viscosity) and dependence of fluid density on temperature.41–43 The settling of the product mixture at 40 °C in 90 minutes is comparable to 15 minutes of settling at 80 °C as shown in 5-minute interval settling images at different temperatures and solvent ratios (Fig. S15†).
Based on the photographs captured every 15–20 minutes, rough settling curves were constructed for HTL product mixtures processed with the toluene/heptane solvent (refer to the ESI†). The images and settling curves show the significant differences in the rate of settling for processing at 40 °C and 80 °C. The initial settling rates recorded for processing at 80 °C were >3× the rate observed for processing at 40 °C. When processed at 80 °C, the settling rate of the mixture appears to slow significantly within 30 minutes, while mixtures processed at 40 °C require nearly 1.5 hours to reach the same rate with the phase interface height being significantly higher. Given the lower interface height and time to settle, solvent processing the HTL product mixture at 80 °C would allow a scaled-up process to use fewer or smaller process vessels.
Yields and quality of solvent-processed HTL products
In the following sections, we discuss the impacts of process variables on the yield and quality of the BCs, solids, and aqueous fractions collected after solvent processing. It is important for the reader to remember that in the following experiments, we have solvent-processed “reconstituted” HTL product mixtures: the solids, aqueous, and BCs collected from the current PNNL HTL process, carried out at high pressures in the absence of additional solvents, were re-mixed to simulate the original, unseparated HTL product mixture. Therefore, when discussing “BC recovery,” we are referring to the percent of BC mass recovered from an organic-phase sample after solvent processing compared to the known mass of BC added to the reconstituted HTL product mixture (see Fig. 4 above). Effectively, the concentration of BC in the organic-phase sample after processing is divided by the concentration of BC expected to be in the organic phase at the beginning of the experiment (eqn (1)). Because we expect nearly 100% of the BC added to be soluble in the organic solvent (based on elevated temperature solubility testing) in stage 1, the concentration of BC expected in the organic solvent is simply the mass of BC added to the reconstituted product emulsion divided by the mass of organic solvent used in stage 1 (eqn (1)). “BC remaining” (stage 2) is the mass of BC expected to be in the mixture at the beginning of stage 2 after removal of the organic sample at the end of stage 2 (eqn (2)).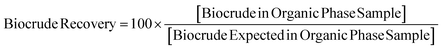 | (1) |
| Biocrude remaining = Mass of BC Added to the Reconstituted Emulsion − Mass of BC Collected in Stage 1 Solvent Processing | (2) |
In Table 3, we present temperatures of product separation and masses used in the reconstituted HTL product emulsion, along with masses and concentrations of BC recovered after solvent processing for both stage 1 and stage 2 for a set of four separate experiments (A–D). An organic sample is collected from the mixture in each stage, and the solvent is then removed to assess the yield and quality of the recovered BC. In several cases, BC recovery exceeds 100% based on the concentration of BC collected from the organic phase at the end of stage processing. In general, the recovery of BC is higher when processed at a higher temperature in both stages 1 and 2.
| Stage 1 & 2 samples | T (°C) | BC mass (g) | Mass solventc,d (g) | [BC] in organic solution (g g−1) | Mass organic sample (g) | Mass BC recovered (g) | [BC] in collected organic (g g−1) | % BC recoveryh |
|---|---|---|---|---|---|---|---|---|
a Mass of HTL BC added to “Reconstituted HTL Product.” Moisture corrected.
b BC remaining in stage 2. BC (corrected for moisture) in the reconstituted HTL product mixture – BC recovered in stage 1.
c Toluene and heptane in an 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 mass ratio.
d Solvent added to stage 2 in replacement of the removed organic sample from stage 1.
e BC remaining/organic mass in stage 2.
f Mass organic layer taken from the processed mixture. In samples A and B, 4 g was removed as an analysis aliquot.
g After solvent removal and drying the organic sample at 105 °C.
h See eqn (1).
i ∼100% after subtracting residual toluene in BC. 20 mass ratio.
d Solvent added to stage 2 in replacement of the removed organic sample from stage 1.
e BC remaining/organic mass in stage 2.
f Mass organic layer taken from the processed mixture. In samples A and B, 4 g was removed as an analysis aliquot.
g After solvent removal and drying the organic sample at 105 °C.
h See eqn (1).
i ∼100% after subtracting residual toluene in BC.
|
||||||||
| Stage 1 A | 80 | 9.37a | 100.00c | 0.086 | 40.95f | 4.39g | 0.107 | 125% |
| Stage 1 B | 40 | 9.26a | 100.00c | 0.085 | 41.16f | 3.71g | 0.090 | 106% |
| Stage 1 C | 80 | 9.10a | 100.05c | 0.083 | 50.00f | 4.28g | 0.086 | 103% |
| Stage 1 D | 40 | 9.10a | 100.42c | 0.083 | 40.08f | 3.43g | 0.086 | 103%i |
| Stage 2 A | 80 | 5.52b | 45.00d | 0.050e | 41.68 | 2.85 | 0.068 | 136% |
| Stage 2 B | 40 | 5.43b | 45.00d | 0.050e | 41.44 | 1.67 | 0.040 | 81% |
| Stage 2 C | 80 | 4.93b | 50.00d | 0.045e | 50.73 | 2.56 | 0.051 | 112% |
| Stage 2 D | 40 | 5.77b | 40.00d | 0.053e | 41.12 | 2.17 | 0.053 | 100% |
Because BC recovery from the reconstituted HTL product mixture exceeded 100% of BC mixed into the emulsion in most cases, we considered the possibility that the recovered mass of solvent-processed BC may be artificially inflated by incomplete removal of solvent. However, after processing and solvent extraction, only limited concentrations (<0.1%) to no toluene was found in most of the BCs as assessed by GC-MS (Fig. S17†). In one case, after replicate processing of BC at 40 °C, the stage 1 sample was found to contain approximately 2.5 weight% toluene (toluene in BC). Based on this analysis, we propose that the apparent recovery of additional BC is likely due to solubilization of organic components in the HTL-AP and/or hydro-char slurry.
The impact of process parameters on the quality of BC was assessed primarily by CHN elemental analysis and gravimetric analysis of ash in BC. Based on CHN analysis (Table 4), after solvent processing (extracted BC), the carbon content of the solvent-processed BCs (76.00–78.01%) is very similar to that of HTL BC collected by the current PNNL separation process (76.33%). Hydrogen contents are also quite similar, although slightly higher, in the BCs isolated from solvent processing. These compositional analyses suggest that the solvent extraction process should result in HTL BCs that are of a similar quality to BCs collected in the current PNNL extraction process.
| Sample | N (mass%) | C (mass%) | H (mass%) |
|---|---|---|---|
| a PNNL BC after heating to 105 °C for 18 hours. b BC extracted from emulsion. 0.5 solvent/BC ratio. Toluene/Heptane solvent 80/20 (by mass). | |||
| As received PNNL BC | 5.39 | 69.24 | 9.62 |
PNNL BC, dried 105![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °Ca °Ca |
4.96 | 76.33 | 9.64 |
| Extracted BC, 40 °C stage 1b | 4.55 | 77.67 | 10.11 |
| Extracted BC, 40 °C stage 2b | 4.55 | 78.01 | 10.12 |
| Extracted BC, 80 °C stage 1b | 4.61 | 76.12 | 9.94 |
| Extracted BC, 80 °C stage 2b | 4.56 | 76.00 | 10.11 |
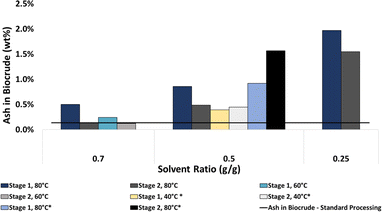
BCs processed at lower temperatures tended to have a higher carbon content than those processed at higher temperatures. For example, BC processed at 80 °C with a 0.5 solvent-to-HTL product ratio had slightly lower carbon content than BC that was not solvent processed, while BC processed at 40 °C contained higher carbon content (Fig. 5).
The processing temperature and solvent: the HTL product mass ratio also had measurable effects on the ash content in solvent-processed BCs. BC recovered after processing with lower solvent-to-HTL emulsion mass ratios tended to have higher ash content than BCs collected by the current PNNL process (PNNL HTL BC). This was true for all temperatures and stages of processing (Fig. 5, S16†). We also found that BCs processed at higher temperatures tend to have higher quantities of ash (Fig. 5). This was only studied in the case of toluene as the solvent but is expected to be true in the case of toluene/heptane mixtures. In most cases, ash in the solvent-processed/extracted BCs was higher than that in the BC that was not solvent processed.
Hydro-char collected after solvent processing at higher temperatures tended to have lower carbon content for a given solvent-to-HTL product emulsion mass ratio than those processed at lower temperatures (Table 5). This was true for both toluene and toluene/heptane solvent scenarios. This is observed in the CHNS combustion analysis and is further supported by infrared spectroscopy (Fig. 6). In the infrared spectra of the hydro-char, the absorbances of the sp3 C–H stretching region (2850–3000 cm−1) were diminished in solids that were solvent processed at 80 °C compared to the solids that were not solvent processed or even processed at lower temperatures. Additional data collected using only toluene as solvent also suggest that carbon content of solids is lower in processing scenarios using higher solvent-to-HTL product ratios. This is unsurprising given the solubility of the HTL BC in toluene. In all cases, the carbon present in the solvent-processed solids was lower than that in the HTL product solids that were not solvent processed (“PNNL process dry hydro-char” in Table 5).
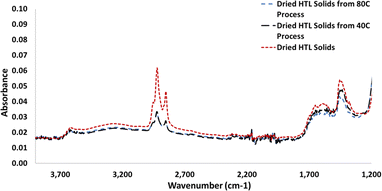 | ||
| Fig. 6 Infrared spectra of dried hydro-char. Solvent processed (blue and black) and unprocessed (red). C–H stretching bands at 2950, 2920, and 2850 cm−1. | ||
In the above experimentation, our determination of BC recovery was based on concentration of BC in the total organic samples collected in extraction stages 1 and 2. To better assess mass balance and distribution, we processed another reconstituted HTL product slurry on the 300 g scale and attempted to collect all fractions and determine recovery and carbon yield for these products. The organic portion was collected in three parts: a sample at the end of stage 1 (∼50% of the stage 1 organic mass), a sample at the end of stage 2 (∼50% of the stage 2 organic mass), and a final fraction which separated from the 3rd portion of aqueous collected at the end of the run. Solvent was removed from organic fractions by rotary evaporation followed by heating for 18 hours at 105 °C. Aqueous and solids were collected at the end of the process and separated by vacuum filtration into three fractions. A sample of the bottom portion of the aqueous plus solids fraction was collected and filtered in stage 2 (set 1). The remaining portions of organic, aqueous, and solid (set 2) were also collected and filtered followed by separation of the organic and aqueous portions. A total of three sets of solids were collected: a large sample from the bottom of the separatory funnel (set 1), a second set from the remaining aqueous portion (set 2), and a third set that later precipitated from the remaining aqueous portion (set 3)(Fig. S22†). Solids were weighed both wet and after drying at 105 °C for 24 hours. The differences in the dry and wet masses were accounted for by adding the volatilized mass to the aqueous total. All aqueous phase portions were combined for analysis.
At the conclusion of the processing, nearly 94% of mass inputs were recovered or otherwise accounted for. Total BC recovery was 105.2%, which was consistent with values obtained in previous experiments (Table 6). Total solids and aqueous portions were somewhat lower at ∼93%. Some of these losses may be attributed to evaporation or incomplete transfer of solids in the filtration step.
| Sample | Aqueous phase | Solid slurry/Solids | Solids + aqueous | BC | Total mass |
|---|---|---|---|---|---|
| a Combined mass of aqueous and solid slurry added. b Total aqueous recovered + total dried solids recovered. | |||||
| Input mass (g) | 240.24 | 45.02 | 285.26a | 15.23 | 300.49 |
| Mass recovered and/or accounted (g) | 249.88 | 15.31 | 265.19b | 16.02 | 281.21 |
| Accounted/Recovered (%) | 92.96% | 105.20% | 93.58% | ||
While ∼94% of total mass was recovered or otherwise accounted for, ∼92% of carbon from the total HTL product inputs was accounted for in the processed products. While the overall carbon balance is <100%, the balance of carbon in the BC is >100%. Before solvent processing, the HTL BC contained 69.2 wt% carbon, while after processing it contained ∼76% carbon (Table 7).
| Sample | BC | Solids slurry | Aqueous | Total |
|---|---|---|---|---|
| a Based on CHN combustion analysis. b Added as a solid-aqueous slurry (HTL blow-down slurry). c BC collected from stage 1 and stage 2 and separated from aqueous set 2. d Carbon content of dried hydro-char filtered from set 1, set 2, and set 3; liquids filtered were added to the aqueous fraction. | ||||
| Carbon contenta (%) | 69.20 | 8.80b | 1.92 | |
| Carbon mass input (g) | 10.50 | 3.96 | 4.60 | 19.10 |
| Carbon outa (%) | 76.89/75.56/45.81c | 14.40/15.81/22.61d | 1.68 | |
| Carbon mass output (g) | 10.90 | 2.48 | 4.19 | 17.60 |
| Accounted/recovered (%) | 103.20 | 62.70 | 91.0 | 91.90 |
After drying the solids at 105 °C for 24 hours, sets 1 and 2 contained approximately 14 and 16% carbon by mass. The third set of solids which was collected by filtration of the third fraction of the aqueous phase contained nearly 23% carbon. The solids (hydro-char) in the reconstituted HTL product emulsion were introduced into the mixture as part of a slurry that contains the HTL aqueous product. This slurry (solids and aqueous) contains 8.8 wt% carbon as assessed by combustion analysis. The solids that result upon drying this slurry contain 21% carbon. The first two sets of solids, which made up most of the solid mass (85%), had significantly reduced carbon content when compared to the solids isolated from the unprocessed hydro-char slurry mixture. Infrared analysis of the solids shows a reduced intensity of C–H bands (sp3 C–H stretching region 2850–3000 cm−1) in the solvent-processed solids compared to dried hydro-char that was not solvent processed (Fig. S24†). The carbon content of the aqueous component was not significantly changed by solvent processing, decreasing from 1.92 wt% to 1.68 wt% after solvent processing.
For comparison, in separate (independent) toluene extractions of the solids slurry (hydrochar slurry), we found that ∼8% of the solids slurry (hydrochar slurry) mass was extracted into the toluene solvent. The GC-MS analysis of this extract suggests that numerous compounds containing more than six carbons are dissolved in toluene, including fatty acids, long chain amides, long chain oxygenates, and low molecular mass polycyclic molecules (Fig. S25 and S26†). Based on these solvent extractions of the solids slurry, we believe that a significant portion of the excess carbon found in the BC after solvent processing was dissolved from the solid slurry mixture and later isolated as part of the BC. The contribution of toluene remaining in any fraction of the BC appears to be less than 0.5% as assessed by GC-MS.
The BCs collected after solvent processing contained elevated metals, phosphorus, and sulphur when compared to the BC processed by current PNNL methods. The stage 1 metals, phosphorus, and sulphur contents were considerably higher than those in the unprocessed BC at 1.7%. The metals, phosphorus, and sulphur content in the stage 2 solvent-processed BC was slightly elevated as assessed by ICP but comparable to that in the BC collected by the current PNNL process (dried at 105 °C) at 1.3% compared to 1.2% (Table S1†). These increased metal levels are consistent with the elevated ash observed in solvent-processed BCs from the related experiments discussed above. Some caution is due with respect to these comparisons because some portion of the inorganic content in solvent-processed BCs may be attributable to the manual method of collecting the organic solutions in each stage—it is possible that small amounts of the aqueous or range layer may have been pulled into the pipette when sampling from the organic layer. Given the detrimental effects that metals and other main group elements can have on downstream hydrotreating catalysts, mitigation steps may be required. Preliminary tests indicate that low pressure and temperature filtration of the biocrude-solvent organic phase is effective in reducing the solids and metal content.
The density of the dried solvent-processed/extracted BCs for stages 1 and 2 was measured at 40, 60, and 80 °C (refer to the ESI†). The density of the solvent-processed BCs was slightly increased from that of the unprocessed BC (0.9985 g cm−3vs. 0.9838 g cm−3 at 40 °C). This slight increase in density for the solvent-processed BCs could be a result of the removal of volatile organic compounds from the processed BC at 105 °C.
| Item | Impact |
|---|---|
| Solvent consumption | +0.05 $ per GGE BC |
| Natural gas consumption | +0.03 $ per GGE BC |
| Electricity consumption | +0.01 $ per GGE BC |
| Capital depreciation | +0.03 $ per GGE BC |
| Income tax and return on investment | +0.08 $ per GGE BC |
Conclusions
This study found the solvent extraction process for HTL BC using naphtha-range hydrocarbons to be very similar to what is observed in the analogous solvent extraction process used in tar sand bitumen production. Many operational parameters, such as the solvent-to-emulsion ratio, solvent composition, and extraction temperature behave similarly to what is documented in the tar sand process. The solubility of the BC, although chemically different from that of bitumen, shows the same qualitative behaviour with respect to the paraffin vs. naphthene or aromatic composition of the solvent. At elevated temperatures, the BC studied was completely soluble in an 80% toluene/20% heptane mixture, which was chosen to represent an unfinished naphtha stream (high-severity reformate) that could be provided by a typical refinery for use as a solvent.Quick settling times with good separation efficiency are possible using solvent-to-emulsion ratios as low as 0.5 wt/wt. Extraction temperatures in the range of 40–80 °C result in good separation efficiency, with the trade-off of faster settling time, but poorer BC quality (additional water and ash) at higher temperatures. Solvent extraction appears to yield slightly more BC than the mechanical filtration technique to separate the emulsion. Improved BC yields correspond to a reduction in the measured organic material in the solid and aqueous phases. Although TEA suggests a modest increase to the MFSP by implementation of the solvent extraction process, the potential benefits include a safer more reliable process which could lead to improved prospects for commercialization of HTL for production of sustainable fuels.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the U.S. Department of Energy (DOE), Office of Energy Efficiency and Renewable Energy, Bioenergy Technologies Office (BETO), and was performed at Pacific Northwest National Laboratory (PNNL) under contract number DE-AC05-76RL01830. The views expressed in this article do not necessarily represent the views of the U.S. Department of Energy or the United States Government. The U.S. Government retains and the publisher, by accepting the article for publication, acknowledges that the U.S. Government retains a nonexclusive, paid-up, irrevocable, worldwide license to publish or reproduce the published form of this work, or allow others to do so, for U.S. Government purposes. Neither the U.S. Government nor any agency thereof, nor any of their employees, makes any warranty, expressed, or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights.Notes and references
- N. Sönnichsen, Biodiesel Production in the U.S. 2001-2020, Jul 7, 2021 Search PubMed.
- U. S. E. I. Administration, U.S. Renewable Diesel Fuel and Other Biofuels Plant Production Capacity, accessed October 10, 2023, 2023 Search PubMed.
- D. Castello, M. S. Haider and L. A. Rosendahl, Renewable Energy, 2019, 141, 420–430 CrossRef CAS.
- S. Subramaniam, D. M. Santosa, C. Brady, M. Swita, K. K. Ramasamy and M. R. Thorson, ACS Sustainable Chem. Eng., 2021, 9, 12825–12832 CrossRef CAS.
- W. T. Chen, Y. H. Zhang, T. H. Lee, Z. W. Wu, B. C. Si, C. F. F. Lee, A. Lin and B. K. Sharma, Nat. Sustain., 2018, 1, 702–710 CrossRef.
- M. R. Thorson, D. M. Santosa, R. T. Hallen, I. Kutnyakov, M. V. Olarte, M. Flake, G. Neuenschwander, L. Middleton-Smith, A. H. Zacher, T. R. Hart, A. J. Schmidt, T. Lemmon and M. Swita, Energy Fuels, 2021, 35, 11346–11352 CrossRef CAS.
- M. Möller, P. Nilges, F. Harnisch and U. Schröder, ChemSusChem, 2011, 4, 566–579 CrossRef.
- W. L. Marshall and E. U. Franck, J. Phys. Chem. Ref. Data, 1981, 10, 295–304 CrossRef CAS.
- J. Zhang, C. Wen, H. Zhang, Y. Duan and H. Ma, Trends Food Sci. Technol., 2020, 95, 183–195 CrossRef CAS.
- N. Akiya and P. E. Savage, Chem. Rev., 2002, 102, 2725–2750 CrossRef CAS.
- S. S. Toor, L. Rosendahl and A. Rudolf, Energy, 2011, 36, 2328–2342 CrossRef CAS.
- M. H. Huang, Y. M. Li and G. W. Gu, Desalination, 2010, 262, 36–42 CrossRef CAS.
- W. T. Chen, M. A. Haque, T. Lu, A. Aierzhati and G. Reimonn, Curr. Opin. Environ. Sci. Health, 2020, 14, 63–73 CrossRef.
- J. M. Jarvis, J. M. Billing, R. T. Hallen, A. J. Schmidt and T. M. Schaub, Energy Fuels, 2017, 31, 2896–2906 CrossRef CAS.
- J. G. Speight, in Handbook of Petroleum Refining, CRC Press, 2016 Search PubMed.
- C. Gai, Y. H. Zhang, W. T. Chen, Y. Zhou, L. Schideman, P. Zhang, G. Tommaso, C. T. Kuo and Y. P. Dong, Bioresour. Technol., 2015, 184, 328–335 CrossRef CAS.
- B. Maddi, E. Panisko, T. Wietsma, T. Lemmon, M. Swita, K. Albrecht and D. Howe, ACS Sustainable Chem. Eng., 2017, 5, 2205–2214 CrossRef CAS.
- F. Cheng and C. E. Brewer, in 3rd Generation Biofuels, ed. E. Jacob-Lopes, L. Q. Zepka, I. A. Severo and M. M. Maroneze, Woodhead Publishing, 2022, pp. 1061–1119, DOI:10.1016/B978-0-323-90971-6.00003-6.
- R. F. Beims, Y. Hu, H. Shui and C. Xu, Biomass Bioenergy, 2020, 135, 105510 CrossRef CAS.
- T. H. Pedersen, I. F. Grigoras, J. Hoffmann, S. S. Toor, I. M. Daraban, C. U. Jensen, S. B. Iversen, R. B. Madsen, M. Glasius, K. R. Arturi, R. P. Nielsen, E. G. Søgaard and L. A. Rosendahl, Appl. Energy, 2016, 162, 1034–1041 CrossRef CAS.
- Z. Cui, J. M. Greene, F. Cheng, J. C. Quinn, U. Jena and C. E. Brewer, Algal Res., 2020, 51, 102077 CrossRef.
- D. C. H. Elliott, R. Todd, G. G Neuenschwander, J. R. Oyler, J. L. Rotness, J. A. Schmidt and H. A. Zacher, System and process for efficient separation of biocrudes and water in a hydrothermal liquefaction system, US9404063B2, Battelle Memorial Institute, Genifuel Corporation and Genifuel Corporation, Richland, WA (US), Salt Lake City UT (US), 2016.
- S. B. Jones, Y. Zhu, D. B. Anderson, R. T. Hallen, D. C. Elliott, A. J. Schmidt, K. O. Albrecht, T. R. Hart, M. G. Butcher, C. Drennan, L. J. Snowden-Swan, R. Davis and C. Kinchin, Process Design and Economics for the Conversion of Algal Biomass to Hydrocarbons: Whole Algae Hydrothermal Liquefaction and Upgrading, United States, 2014 Search PubMed.
- N. Montesantos, T. H. Pedersen, R. P. Nielsen, L. Rosendahl and M. Maschietti, J. Supercrit. Fluids, 2019, 149, 97–109 CrossRef CAS.
- B. Guo, B. Yang, P. Weil, S. Zhang, U. Hornung and N. Dahmen, Energy Fuels, 2022, 36, 922–931 CrossRef CAS.
- H. Jahromi, T. Rahman, P. Roy and S. Adhikari, Energy Convers. Manage., 2022, 263, 115719 CrossRef CAS.
- J. Lu, Z. Liu, Y. Zhang and P. E. Savage, Ind. Eng. Chem. Res., 2019, 58, 13971–13976 CrossRef CAS.
- A. Mathanker, S. Das, D. Pudasainee, M. Khan, A. Kumar and R. Gupta, Energies, 2021, 14, 4916 CrossRef CAS.
- N. Montesantos and M. Maschietti, Energies, 2020, 13, 1600 CrossRef CAS.
- P. J. Valdez, J. G. Dickinson and P. E. Savage, Energy Fuels, 2011, 25, 3235–3243 CrossRef CAS.
- J. Watson, J. Lu, R. de Souza, B. Si, Y. Zhang and Z. Liu, Energy, 2019, 167, 189–197 CrossRef CAS.
- W.-H. Yan, P.-G. Duan, F. Wang and Y.-P. Xu, Fuel, 2016, 185, 229–235 CrossRef CAS.
- J. Yang, Q. He, K. Corscadden and H. Niu, Fuel Process. Technol., 2018, 178, 353–361 CrossRef CAS.
- X. Yang, H. Lyu, K. Chen, X. Zhu, S. Zhang and J. Chen, Bioresources, 2014, 9, 5219–5233 Search PubMed.
- B. Zhao, H. Wang, S. Xu, L. Qian, H. Li, J. Gao, G. Zhao, M. B. Ray and C. C. Xu, Fuel, 2022, 307, 121930 CrossRef CAS.
- W. Maqbool, K. Dunn, W. Doherty, N. McKenzie, D. Cronin and P. Hobson, Ind. Eng. Chem. Res., 2019, 58, 5202–5214 CrossRef CAS.
- J. Jiang and P. E. Savage, Algal Res., 2017, 26, 131–134 CrossRef.
- F. Lin, S. R. Stoyanov and Y. Xu, Org. Process Res. Dev., 2017, 21, 492–510 CrossRef CAS.
- H. Zhang, X. Tan and Q. Liu, Fuel, 2021, 288, 119727 CrossRef CAS.
- G. Robertson, K. V. Adiningtyas, S. A. Ebrahim, L. Scoles, E. A. Baranova and D. Singh, Renewable Energy, 2021, 173, 128–140 CrossRef CAS.
- Mineral Processing Design and Operations, ed. A. Gupta and D. Yan, Elsevier, Amsterdam, 2nd edn, 2016, pp. 563–628, DOI:10.1016/B978-0-444-63589-1.00016-2.
- C. D. Robinson, Ind. Eng. Chem., 1926, 18, 869–871 CrossRef CAS.
- M. S. Shankar, M. Pandey and A. K. Shukla, Water, 2021, 13, 1987 CrossRef.
- L. Snowden-Swan, S. Li, Y. Jiang, M. Thorson, A. Schmidt, T. Seiple, J. Billing, M. Santosa, T. Hart, S. Fox, D. Cronin, K. Ramasamy, D. Anderson, R. Hallen, X. F. Almansa and J. Norton, Wet Waste Hydrothermal Liquefaction and Biocrude Upgrading to Hydrocarbon Fuels: 2021 State of Technology, Report PNNL-32731, Pacific Northwest National Laboratory, 2022 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4se00516c |
| This journal is © The Royal Society of Chemistry 2024 |