Green and sustainable metal–air batteries for powering flexible wearable electronics: current status and future prospects
Arpana
Agrawal
a and
Chaudhery Mustansar
Hussain
 *b
*b
aDepartment of Physics, Shri Neelkantheshwar Government Post-Graduate College, Khandwa 450001, India
bDepartment of Chemistry and Environmental Science, New Jersey Institute of Technology, USA. E-mail: chaudhery.hussain@njit.edu
First published on 10th September 2024
Abstract
The use of eco-unfriendly materials in wearable electronic devices poses a serious threat to the environment. It is therefore crucial to develop flexible, wearable devices that are environmentally friendly and safe for human skin. To power such devices, miniaturized power sources are needed, such as metal–air batteries (MABs) that have excellent power density and longevity. However, traditional MABs are built on rigid, non-flexible platforms and use a large amount of electrolyte, which is not sustainable or suitable for flexible electronics. Green wearable MABs can be created by using biocompatible and biodegradable battery components, such as electrolytes, electrodes, and flexible platforms, or by reducing the electrolyte volume. In this review, we critically examine a range of cost-effective, downsized, green, and sustainable wearable MABs that use non-toxic and abundant natural materials and can withstand bending, twisting, stretching, and folding. The various components, their green and sustainable aspects and the synthesis approaches of the key air-cathode have been demonstrated in detail. The electrochemical performance of various green MABs, as well as the obstacles to their commercialization has also been discussed.
1. Introduction
In this fast-moving world, nowadays people are highly interested in electronic devices that are highly flexible, easily wearable, and compatible with human skin such as various healthcare devices including glucometers, PH sensors, smart watches, wrist bands, etc., together with environmental friendliness.1 So far, several energy storage devices including supercapacitors, fuel cells, metal-ion batteries, etc. have been reported for powering such wearable electronic devices.2,3 Among these, metal–air batteries (MABs) such as zinc–air batteries (ZABs), aluminium–air batteries (AABs), lithium–air batteries (LABs), magnesium–air batteries (MgABs), etc. have proven themselves as potentially important candidate sources owing to their excellent electrochemical performances including remarkably high energy densities and durability.4–6 It should be noted that the main components of a conventional MAB include a metal anode, an air cathode, a cathode catalyst, a separator, and an electrolyte (Fig. 1) and its operation involves the oxygen reduction reaction (ORR) and oxygen evolution reaction (OER) on the air electrode, while the dissolution and deposition of cations on the metal electrode.4 For the various types of MABs, the overall reaction equations can be expressed differently; for example, the reaction equations for ZABs and LABs can be given as4–6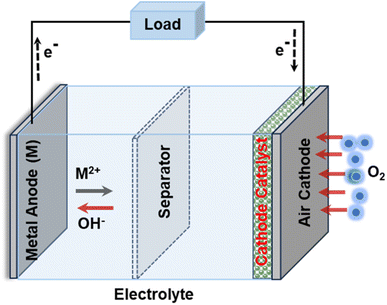 | ||
| Fig. 1 MAB's structure comprises a metal anode, an air cathode, a cathode catalyst, a separator, and an electrolyte. | ||
Zinc–air battery:
| Zn → Zn2+ + 2e− (anode reaction) | (1) |
| Zn2+ + 4OH− → Zn(OH)42− | (2) |
| Zn(OH)42− → ZnO + H2O + 2OH− | (3) |
| O2 + 2H2O + 4e− → 4OH− (cathode reaction) | (4) |
| 2Zn + O2 → 2ZnO (overall reaction) | (5) |
Lithium–air battery:
| Li → Li+ + e− (anode reaction) | (6) |
| O2 + 4Li+ + 4e− → 2Li2O (cathode reaction) | (7) |
| 4Li + 4O2 → 2Li2O4 (overall reaction) | (8) |
The various parameters such as the theoretical specific capacity and output voltage of MABs depend significantly on the choice of MAB components including the metal anode, the cathode catalyst, and the electrolyte employed.5 These components in turn rely on easy availability, cost-effectiveness, and an eco-friendly nature. MABs possess high theoretical energy density, making them suitable for applications requiring long-lasting power. They generally employ metals such as Zn and Al which are abundant and inexpensive, reducing material costs, and are generally more environmentally friendly compared to traditional batteries due to their use of non-toxic materials. However, the cycle life of MABs is often limited due to issues such as electrode degradation and electrolyte evaporation. The efficiency of MABs can be lower due to ORR and OER inefficiencies. Furthermore, controlling the air supply and managing humidity are critical challenges that impact the performance and longevity of MABs. Furthermore, conventional MABs generally bulky and employ materials (electrolytes/electrodes/substrates/platforms) that are environmentally unfriendly raising serious concerns for our environment. They are commonly fabricated in rigid configurations with flexible or non-flexible components and hence ignore the important green MABs. The term “green” refers to technologies and processes that have a reduced environmental impact and contribute to sustainability.7 Green materials are increasingly being integrated into MABs due to their environmental benefits, sustainability, safety, economic viability, and potential for improved performance. Green materials reduce the ecological footprint of MABs by minimizing the use of toxic and non-renewable resources which is crucial for mitigating the environmental challenges associated with battery production and disposal. Utilizing renewable and biodegradable materials ensures the sustainability of battery technologies which aligns well with global efforts to transition towards greener energy storage solutions.8 Their non-toxic and biocompatible nature enhances the safety of batteries, which is especially important for applications in wearable electronics and medical devices. Importantly, abundant and low-cost green materials contribute to reducing the overall cost of battery production, making these technologies more accessible and economically feasible for widespread adoption. Moreover, eco-friendly manufacturing processes such as additive manufacturing (3D printing) minimize waste and energy consumption.9–11 In the context of MABs, these materials include eco-friendly electrolytes (e.g. aqueous electrolytes, ionic liquids, etc.), biodegradable polymers (polylactic acid (PLA), chitosan, etc.), abundant, non-toxic metals (such as Zn, Mg etc.), and carbon-based catalysts.12 Water-based electrolytes such as KOH, NaOH, etc. are non-flammable, non-toxic, and environmentally benign.13 Another alternative for green electrolytes is ionic liquids, which are salts in the liquid state at room temperature and are used as electrolytes due to their high ionic conductivity, thermal stability, and non-volatility. These electrolytes minimize environmental and safety risks associated with battery leaks and disposal, enhancing the overall green profile of batteries. PLA is a biodegradable polymer, commonly used as a binder and separator and is derived from renewable resources such as corn starch. Its biodegradable and non-toxic nature makes it an eco-friendly alternative to traditional synthetic polymers.12 Similarly, chitosan, derived from chitin found in crustacean shells, can also be employed as a binder and electrolyte additive and also promotes ionic conductivity. These materials reduce the environmental impact of battery disposal, promote sustainability, and can be safely used in wearable devices due to their biocompatibility.14 For the fabrication of anodes, various nontoxic abundant metals such as Zn, Mg etc. are being employed. Zn is a widely used anode material due to its abundance, low cost, and non-toxicity, offers good electrochemical performance and is recyclable. Another abundant and non-toxic metal, Mg, provides high energy density and is safe for the environment. Using such abundant and non-toxic metals ensures that batteries are not only environmentally friendly but also economically viable for large-scale production. Another important component of MABs is catalysts, mainly carbon-based catalysts such as graphene-based materials, activated carbon, etc.15–18 These materials are used as cathode catalysts due to their high surface area, electrical conductivity, and catalytic activity. They can be derived from renewable sources such as biomass and are often cheaper and more sustainable compared to precious metal catalysts and hence facilitate the reduction of the overall environmental footprint of batteries.
It is noteworthy to mention here that apart from greenness, the flexibility of MABs is also very important and can be obtained either by utilizing flexible gel/polymer electrolytes such as poly(acrylic acid), poly(ethylene oxide), poly(vinyl alcohol), poly(vinylidene fluoride), poly(vinylidene fluoride-co-hexafluoropropene),19–26etc. or flexible electrode/substrate materials such as carbonaceous materials (graphene-based materials, carbon nanotubes (CNTs or multiwall CNTs (MWCNTs)), etc.), conductive polymers, sheets/foil of metals or their oxides,27–38etc. A nanocellulose composite gel possessing high ionic conductivity was also employed to fabricate flexible ZABs.39 However, the decisive factor for the accomplishment of green aspects in general or such flexible MABs should precisely include the implementation of green alternatives for fabricating MABs i.e., switching from toxic materials to eco-friendly, biodegradable and biocompatible materials or reducing the volume of electrolytes, downsizing, cost-effectiveness, and easy fabrication.7,40 The second major concern is their endurance to bear up with various deformable conditions such as bending, twisting, stretching, folding, etc. To simultaneously fulfil both of the mentioned requirements. e.g., environmental friendliness along with flexibility and wearability, it is highly imperative to fabricate MABs employing naturally occurring materials (e.g., bamboo) or materials obtained from nature such as paper or textile/yarn/cotton, etc. which are non-toxic, easily available, highly flexible, light weight and ultrathin. Consequently, paper-based MABs and textile/cotton/yarn-based MABs are very popular among several other MABs for wearable electronics.41–43 There exist several reports demonstrating the applicability of paper- or textile-based energy storage devices.44–48 Jost et al.49 studied flexible energy storage devices employing carbon-coated textiles. Wearable yarn/fabrics coated with CNTs and polyelectrolytes are also used for human biomonitoring.50 Cotton-based low-cost portable AABs exhibiting high specific energy were also developed.51
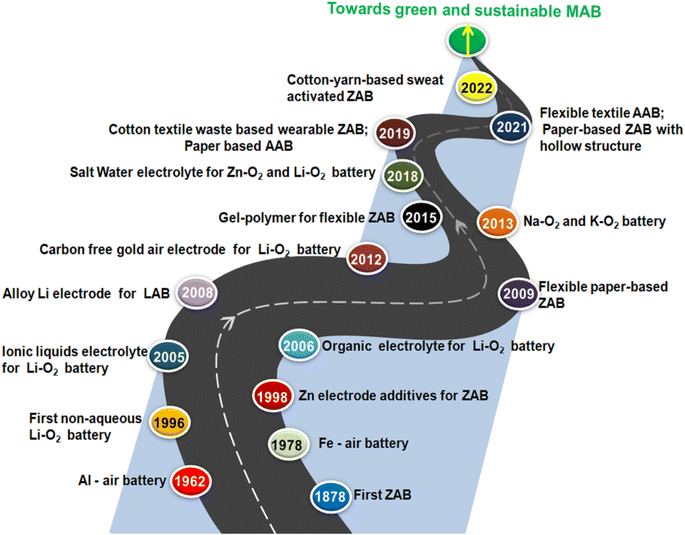
Fig. 2 schematically illustrates the various milestones from the overall journey of the progress of various MABs which clearly shows a very vast and progressive history of MABs from the very first rigid ZABs to paper-based MABs or segmented structured cotton-yarn-based sweat-activated ZABs comprising carbon-black-modified (cathode), pristine yarn (as a salt bridge) and wrapped Zn foil (anode).52Fig. 3(a)–(c) schematically illustrate the design and mechanism of a salt bridge, yarn-based sweat transport and the structure of the cotton-yarn-based sweat-activated ZAB.
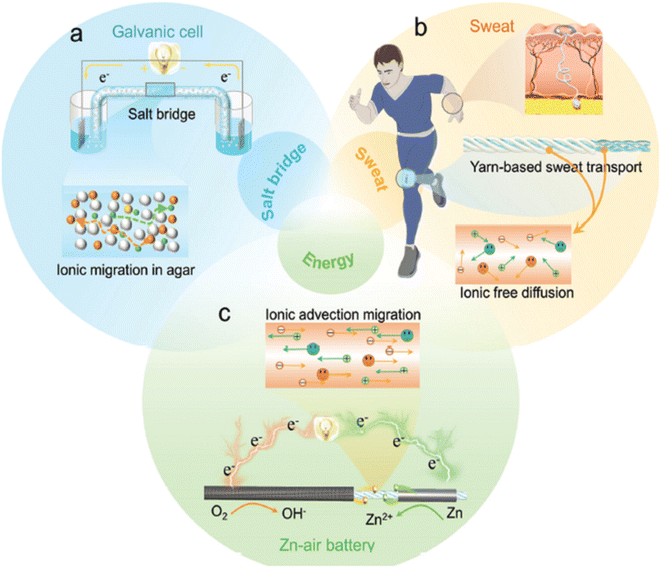 | ||
| Fig. 3 (a) Design and mechanism of a salt bridge; (b) yarn-based sweat transport; (c) structure of a cotton-yarn-based sweat-activated ZAB. Reproduced from ref. 52 with permission from [Wiley], copyright [2022]. | ||
In comparison to various other flexible materials, paper, on the one hand, via its capillary action, can automatically transport and store electrolytes that too in much reduced volume and is environmentally friendly upon disposition and incineration and hence ensures the green aspect of paper-based MABs. On another hand, it is extremely light weight, soft, and flexible, facilitating the flexibility of wearable MABs. Similarly, yarn/textile-based materials are also low cost, light weight, and extremely flexible along with their easy weaving, knitting and stitching ability and consist of interlocked structures of various long continuous strands of fibres with several micro/nanosized channels allowing the easy delivery of electrolyte via capillary force and hence can also serve as an ideal candidate for flexible wearable green MABs. It should also be noted here that a suitable balance between the greenness of MABs and their prospective functionality along with utility is highly desirable to achieve overall sustainable development.
Additionally, MABs have several green and sustainable advantages compared to conventional batteries without using paper or cotton/cloths as substrates which include lower environmental impact during raw material extraction, materials production, and cell production, as well as potential for second-life usage and more environmentally friendly disposal and recycling.7,40 They have the potential to reduce the environmental impact of raw material extraction compared to conventional batteries via utilizing abundant and low-cost metals having lower environmental impact during the extraction process such as Al and Mg as anode materials and MnO2 as an air-cathode material due to its biodegradability, low toxicity, ready availability and low environmental impact. These MABs can also have a lower environmental impact during the materials production process compared to conventional batteries owing to the utilization of green solvents such as ionic liquids and deep eutectic solvents which are less toxic and hazardous compared to the organic electrolytes used in conventional batteries.53 During cell production, such MABs have the potential to be more environmentally friendly compared to conventional batteries because they can be produced using low-cost and simple manufacturing processes such as printing and roll-to-roll processes which require less energy and produce less waste compared to the complex and energy-intensive processes used in conventional battery production. MABs also have the potential for second-life usage compared to conventional batteries owing to the ease of recyclability and reuse of anode materials such as Al or Mg in other applications which can facilitate reducing the environmental impact of battery disposal and promote a circular economy. Finally, MABs have the potential to be more environmentally friendly during disposal and recycling compared to conventional batteries due to the use of non-toxic and non-flammable materials which are easier and safer to dispose of or recycle. Additionally, the use of abundant and recyclable anode materials in MABs can reduce the demand for new raw materials and promote a more sustainable materials economy.
Accordingly, the present review article provides a comprehensive overview of the various green wearable MABs for cost-effective powering of flexible wearable electronics including the reduction of volume of electrolyte via capillary action or replacement of toxic materials (electrodes) by employing paper-based or textile-based substrates/electrodes. The synthesis and electrochemical performance of various green MABs (paper/textile-based) along with the factors hindering their large-scale commercialization have also been critically discussed.
2. Key components of flexible MABs
Flexible MABs have emerged as a promising technology for next-generation power sources in the field of portable and wearable devices due to their high energy density, long cycle life, and environmental friendliness. The key components of a flexible MAB are the flexible air cathode, cathode catalyst, flexible anode, exchange membrane, and flexible electrolyte. The air cathode is an essential component of MABs, responsible for the ORR and electron transfer to an external circuit. In a flexible MAB, a flexible air cathode is required to maintain the flexibility of the battery. The air cathode consists of a porous layer of a catalyst coated on a gas-permeable support material, such as carbon cloth or paper, which provides a high surface area for the electrochemical reaction. Some commonly used catalyst materials for the ORR in flexible MABs are carbon-based materials, such as CNTs, graphene, conductive polymers, hybrid catalysts, etc.Cathode catalysts play a crucial role in the electrochemical performance of MABs because they facilitate the ORR and OER which are critical for the battery's operation. Efficient catalysts improve the overall performance, stability, and efficiency of the battery. Cathode catalysts lower the activation energy required for the ORR and OER, increasing the reaction rates and improving the battery's efficiency, leading to higher specific energy and power densities. They can withstand the harsh electrochemical environment of the battery, contributing to longer battery life and stability. Effective catalysts can also help in enhancing the selectivity of the desired reactions, reducing side reactions that can degrade the battery's performance. Research on cathode catalysts has focused on various materials to improve their performance. They can be broadly classified as precious metal catalysts, such as Pt, Ir etc., and non-precious catalysts including transition metal oxides (MnOx, CoOx, NiOx, etc.), carbon-based catalysts (graphene-based materials, CNTs, etc.), and hybrid catalysts.54 Precious metal catalysts are highly effective for the ORR and OER due to their excellent catalytic properties. However, their high cost and scarcity limit their widespread use. However, research is ongoing to reduce the amount of precious metals required or to develop cost-effective alternatives. Contrary to this, non-precious metal catalysts have shown good catalytic activity and are more abundant and cheaper than precious metals, and nowadays, efforts are focused on enhancing the catalytic efficiency and stability of these materials through doping and nano-structuring. Compared to precious and non-precious catalysts, carbon-based catalysts offer high surface area and excellent electrical conductivity, which are beneficial for catalytic activity. Functionalizing carbon materials with various heteroatoms (e.g., nitrogen, sulfur, etc.) can further enhance their catalytic properties. It should be noted here that hybrid catalysts combine different types of materials, such as metal oxides with carbon-based materials, which can synergistically enhance the catalytic performance. They aim to balance cost, efficiency, and stability, providing a comprehensive solution for high-performance MABs.
The anode is another critical component of the MAB, which is responsible for the oxidation of the metal and electron transfer to the external circuit. In a flexible MAB, a flexible anode is required to maintain the flexibility of the battery. The anode consists of a porous layer of metal coated on a flexible substrate, such as carbon paper, cloth, or textile. The metal used as the anode material varies depending on the type of MAB, such as zinc, lithium, or aluminium. Flexible anodes can be prepared using various methods, including electrodeposition, physical vapor deposition, or chemical vapor deposition. The exchange membrane is also a critical component of the MAB, which separates the anode and cathode and allows the transport of ions between them while preventing the mixing of electrolytes. The exchange membrane should have good ionic conductivity, high mechanical strength, and chemical stability to ensure the long-term stability of the battery. In a flexible MAB, a flexible exchange membrane is required to maintain the flexibility of the battery. Flexible exchange membranes can be prepared using various methods, including electrospinning, solution casting, or layer-by-layer assembly. Apart from this, electrolyte is also a very crucial component of the MAB, which facilitates the transport of ions between the anode and cathode during the electrochemical reaction. In a flexible MAB, a flexible electrolyte is required to maintain the flexibility of the battery. The electrolyte can be either aqueous or non-aqueous, depending on the type of MAB. Flexible electrolytes can be prepared using various methods, including gel polymer electrolytes, solid polymer electrolytes, or ionic liquid electrolytes; for example, a flexible gel polymer electrolyte made of polyacrylonitrile (PAN) and lithium bis(trifluoromethanesulfonyl)imide.
It is worth mentioning here that there are several key parameters used to evaluate the flexibility of flexible MABs, including the bending radius (bending angle), bending cycles, tensile strength, flexural endurance, elastic modulus, and fatigue resistance. The bending radius is the minimum radius at which a battery can be bent without significant performance loss, with a smaller bending radius indicating better flexibility. Bending cycles define the number of times a battery can be bent to a certain radius before its performance degrades, indicating its durability under repetitive flexing. Tensile strength, the maximum stress a battery material can withstand while being stretched or pulled before breaking, directly relates to mechanical robustness. Flexural endurance is the battery's ability to endure repeated bending and flexing without structural failure or significant performance degradation. A lower elastic modulus indicates greater flexibility and fatigue resistance refers to the battery's ability to resist degradation under cyclic mechanical loading, maintaining performance after numerous flexing cycles. Each of these parameters plays a critical role in determining the overall flexibility, durability, and mechanical robustness of flexible MABs. Mao et al.55 thoroughly discussed the mechanical analyses and structural design requirements for flexible energy storage devices”.
3. Green and sustainable aspects of various components of MABs
Green and sustainable components of flexible MABs including the substrate, active materials for the anode or cathode, electrolyte or the current collector for MABs play a crucial role in reducing the environmental impact of the energy storage system. Natural and renewable substrates including cotton, textiles, paper, and bamboo offer several benefits including biodegradability, low environmental impact, and good performance and have been explored as potential flexible substrates for flexible green and sustainable MABs. These materials offer several benefits over traditional substrates, such as plastics, which are often not biodegradable and can hurt the environment.Green and sustainable anode materials should be low-cost, eco-friendly, and biodegradable and have low toxicity. Zn and Mg are a few sustainable and eco-friendly anode materials for ZABs or MgABs which are low-cost and abundant.56,57 For AABs, Al foil is used as the anode material which is also abundant, cost-effective, and eco-friendly, can be extracted with low environmental impact and is easily recycled.58–60 Additionally, Al can be easily extracted from its ores using renewable energy sources such as hydroelectricity, making the production process more sustainable. Furthermore, the use of AABs as primary cells can reduce the demand for non-renewable energy sources such as fossil fuels owing to their high energy density and long runtime, making them suitable for applications such as electric vehicles and grid energy storage.61–64
The cathode of MABs typically uses air or oxygen as the active material, making it a sustainable and green option. However, the use of certain catalysts such as platinum or other precious metals in the cathode can raise concerns about the environmental impact on the cell. To address this issue, researchers have explored the use of non-precious metal catalysts such as transition metal oxides or carbon-based materials, which are sustainable and low-cost options.65–70 Generally, transition metal oxide, particularly MnO2, is employed for air-cathode materials due to its biodegradability, low toxicity, ready availability and low environmental impact. It should also be noted here that MWCNTs are one of the potential ORR catalyst supports in MABs and are considered a costly and potentially toxic material. However, the use of MWCNTs as an ORR catalyst support in MABs can contribute to a more sustainable and eco-friendly battery. First, the high efficiency of MWCNTs as an ORR catalyst support can improve the overall performance of the battery, resulting in longer lifetimes and minimal waste and hence reducing the need for frequent battery replacements and, therefore, reducing the environmental impact of battery production and disposal. Additionally, the use of MWCNTs in battery production can contribute to the development of a circular economy, where materials are recycled and reused to minimize waste and resource consumption. MWCNTs can be recovered from used batteries and recycled for use in new battery production, reducing the need for virgin materials.
Electrolytes play a crucial role in the performance and safety of MABs. Traditional aqueous or non-aqueous electrolytes may contain toxic and hazardous chemicals, which can pose a threat to the environment during production, use, and disposal. To circumvent this issue, sustainable and eco-friendly electrolyte materials such as seawater, organic electrolytes derived from biomass, and ionic liquids can be utilized. Ionic liquid-based electrolytes or deep eutectic solvents are promising options for MABs due to their low environmental impact and non-toxic nature. They also have the potential for high ionic conductivity and thermal stability, making them a good candidate for high-performance batteries.70,71 Examples of ionic liquids that have been used in MABs include 1-ethyl-3-methylimidazolium tetrafluoroborate and 1-butyl-3-methylimidazolium trifluoromethanesulfonate.
So far, the most commonly used current collectors in MABs are metals such as copper, nickel or silver. However, the production of these metals can cause environmental issues such as pollution and resource depletion. Wang et al.72 utilized an Ag grid as a current collector for fabricating paper-based Zn–air/Ag hybrid batteries and it should be noted here that Ag is not an abundant or sustainable material due to its limited availability and high cost. However, there are ways to mitigate the environmental impact of using Ag as a current collector in MABs and hence improve its sustainability. One way to reduce the amount of Ag used is to optimize the design and manufacturing processes to minimize the thickness and surface area of the Ag grid by using advanced manufacturing techniques such as additive manufacturing or thin-film deposition. It is interesting to note here that, in contrast to metals, conductive polymer composites are a lightweight and low-cost option for current collectors in MABs and are typically made from carbon-based materials, such as carbon black or graphene oxide, and a conductive polymer such as polyaniline. These materials have been shown to have good conductivity and mechanical properties, making them suitable for flexible battery applications. Lamiel et al.73 presented a critical review of the properties, functions, and challenges of current collectors. Wu et al.74 also reviewed various carbon-based materials which can serve as current collectors and bifunctional integrated air electrode materials.
Hence, more abundant and sustainable carbon-based materials or conductive polymers should be employed for the current collector. These materials can be engineered to have similar or even better performance than Ag while being more environmentally friendly and cost-effective.
4. Synthesis methods of key air cathodes for flexible MABs
Various synthesis methods have been successfully employed for the preparation of key air cathodes for flexible MABs including chemical vapor deposition (CVD), physical vapor deposition (PVD), chemical reduction method, electrospray deposition, electrochemical deposition, electrospinning, screen printing, etc.11 However, they can vary depending on the specific materials and structures used, the desired properties of the resulting cathode, the type of catalyst material being used, and the scale of production. The CVD method involves depositing a thin layer of the catalyst onto a porous substrate using a gaseous precursor.75,76 The precursor gas is introduced into a heated reactor, where it decomposes and forms a thin layer of catalyst on the substrate. This method is commonly used to deposit catalysts such as platinum or palladium onto carbon substrates. Mi et al.77 reported the synthesis of CNTs and N-doped CNTs as key air cathodes via a floating catalyst CVD approach for LABs. Similarly, Yu et al.78 also employed the CVD technique to grow the air cathode for LABs where aligned CNTs were vertically grown on Ta foil as a substrate. In contrast to CVD, PVD involves depositing a thin layer of catalyst onto a substrate using a physical process such as sputtering or evaporation.79–82 The catalyst material is evaporated or sputtered onto the substrate, where it forms a thin film. This method is commonly used to deposit catalysts such as silver or nickel onto substrates. Pham et al.83 developed a ZAB comprising Ni@Ni3Pt, glass fibre, Zn foil and KOH as the cathode, separator, anode, and electrolyte, respectively where the pulsed laser deposition technique was employed synthesizing the key air cathode by depositing the Ni3Pt alloy on 3D nickel foam. Chemical reduction is another effective technique which involves reducing a metal precursor to form a metallic catalyst on a carbon substrate. The precursor is dissolved in a reducing agent, which reduces the metal ions to form a thin layer of the metallic catalyst on the substrate. This method is commonly used to deposit catalysts such as cobalt or iron onto carbon substrates.Another popular approach is electrochemical deposition which allows the deposition of a thin layer of catalyst onto a substrate using an electrochemical process where a solution containing metal ions is placed in contact with the substrate and an electric current is applied to reduce the metal ions and deposit a thin layer of metallic catalyst on the substrate. This method is commonly used to deposit catalysts such as manganese or copper onto carbon substrates. Qu et al.84 employed an electrochemical method to synthesize an air cathode comprising Co3O4 gown on carbon cloth for flexible and stretchable ZABs. Huang et al.85 reported an electrodeposition technique for preparing a key air cathode for cotton textile waster ZABs. The electrodeposition technique was also utilized by Hong et al.86, to develop a binder-free key cathode where Ag catalysts were deposited on carbon fiber papers for AABs. Similar to electrodeposition, in the electrospray deposition technique, an electric field is utilized to create a fine aerosol spray of charged particles, which are then deposited onto a substrate to form a thin film. In the case of flexible air cathodes, the aerosol spray consists of the catalyst material, which is then coated onto a flexible substrate such as carbon fibre cloth. This method allows for precise control over the thickness and uniformity of the catalyst layer, as well as the flexibility of the resulting cathode. Apart from this, electrospinning and screen printing are also very popular approaches to preparing air cathodes for flexible MABs. The electrospinning technique uses an electric field to create a fine, continuous fibre from a polymer solution or melt. In the case of flexible air cathodes, the polymer solution contains the catalyst material, which is then electrospun onto a flexible substrate such as carbon paper. This method allows for the formation of a highly porous, three-dimensional structure that provides a high surface area for the electrochemical reaction.
Among all the techniques, screen printing is the most popular technique to synthesize air cathodes where a stencil is used to transfer ink or other materials onto a substrate in a desired pattern.10,11 In the case of flexible air cathodes, the ink contains the catalyst material, which is then screen-printed onto a flexible substrate such as carbon cloth or paper. One of the major advantages of this technique is the formation of a uniform catalyst layer with high reproducibility and scalability. Kheawhom and Suren87 reported the screen-printing technology for fabricating the electrodes for flexible ZABs comprising nano-silver conductive ink screen printed on polymer-based substrates i.e., a polyethylene terephthalate substrate and a polypropylene membrane serving as the anode and cathode current collector, respectively.
The above-mentioned synthesis techniques employed for preparing air-cathodes have their own advantages and disadvantages in terms of material availability, cost, scalability, performance requirements, and environmental impact. In terms of material availability, chemical reduction methods may be advantageous as they often use readily available materials such as metal salts. However, methods such as CVD and PVD may require more specialized and less abundant materials. In terms of cost, chemical reduction methods and screen printing may be advantageous due to their low cost and ease of scale-up. However, methods such as CVD and PVD may be more expensive due to the equipment required. For scalability, CVD, PVD, and electrochemical deposition may have limitations due to the size of the equipment and the need for specialized substrates. On the other hand, methods such as chemical reduction and screen printing may be easier to scale up due to their simplicity and compatibility with various substrates. In terms of environmental impact, electrochemical deposition and chemical reduction may be more environmentally friendly due to their lower energy consumption and less waste generation. However, CVD and PVD due to their higher energy consumption and potential for hazardous waste generation may have a larger environmental impact. Regarding performance requirements, CVD and PVD may be advantageous due to their precise control over the structure and morphology, which can lead to improved electrochemical performance. However, methods such as electrospray deposition and electrospinning may also offer precise control over the structure and morphology, as well as high deposition rates.
Overall, the choice of the synthesis method for flexible air cathodes for MABs will depend on the application's specific requirements, including material availability, cost, scalability, performance requirements, and environmental impact. Table 1 summarizes the advantages and disadvantages of the synthesis methods for flexible air cathodes for MABs.
| Synthesis method | Advantages | Disadvantages |
|---|---|---|
| CVD | High purity, precise control over the structure and morphology, and good scalability | Expensive equipment, high energy consumption, limited substrate size, and low deposition rate |
| PVD | High purity, precise control over the structure and morphology, and good scalability | Expensive equipment, limited substrate size, and low deposition rate |
| Chemical reduction | Low cost, simple procedure, and easy to scale up | Poor control over the structure and morphology, low purity, and difficulty in achieving uniformity |
| Electrospray deposition | Precise control over the structure and morphology, a high deposition rate, and compatibility with various substrates | Limited scalability and requires high-voltage power supply and complex equipment |
| Electrochemical deposition | High deposition rate, precise control over the structure and morphology, and low cost | Limited scalability and compatibility issues with non-conductive substrates |
| Electrospinning | High deposition rate, compatibility with various substrates, and precise control over the structure and morphology | Limited scalability, complex equipment, and difficulty in achieving uniformity |
| Screen printing | Low cost, simple procedure, easy to scale up, and compatibility with various substrates | Limited control over the structure and morphology, low deposition rate, and difficulty in achieving uniformity |
Once the key air-cathode is prepared for flexible MABs, one should always analyze the same via several analytical methods including scanning electron microscopy (SEM), energy dispersive X-ray spectroscopy (EDX), X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), electrochemical characterization, etc. SEM analysis allows us to observe the morphology and structure of the air cathode, including the size and shape of the catalyst particles, the porosity of the carbon substrate, and the uniformity of the catalyst distribution while EDX helps in determining the actual elemental composition of the air cathode, including the distribution of the catalyst material and any impurities that may be present. XRD and FTIR techniques facilitate the identification of the crystal structure of the catalyst material, as well as any impurities or phases that may be present and the functional groups on the surface of the air cathode, such as carbon–carbon double bonds or oxygen-containing groups, which can affect the catalytic activity of the material, respectively. To examine the performance of the air cathode in an MAB, the air cathode is subjected to electrochemical characterization to investigate its ORR activity, its stability over time, and its efficiency in generating an electrical current.
5. Green alternatives for flexible wearable MABs
Green wearable MABs employed for the fabrication of flexible wearable MABs are inspired by naturally occurring or nature-derived/processed materials, including paper, cotton yarn/textile/cloth and bamboo. All these materials are light weight, cost-effective, naturally abundant, and encouragingly flexible and can assist in the transportation of reduced volume of electrolyte via capillary action. Herein, the paper/cotton yarn may serve as the effective electrolyte absorption substrate or the cathode material which may be polished with some catalysts. This section extensively discussed the construction and electrochemical performances of various green wearable MABs including paper/cotton yarn-based MABs under several deformable circumstances.5.1. Paper-inspired flexible wearable MABs
It is noteworthy to mention here that the eco-friendliness along with the electrochemical performance under several deformable conditions is one of the decisive factors for the flexibility of green MABs and hence their wearability. Several researchers have employed different kinds of papers including cellulose paper, chromatographic paper, filter paper, carbon paper, wipes, etc. serving as a substrate/platform and paper-based gel electrolyte or paper-based/modified electrodes for fabricating wearable MABs mainly AABs, LABs and ZABs.A downsized, cost-effective paper-inspired flexible AAB was reported by Avoundjian et al.88 hey optimized the battery parameters including the cathode material, size of the electrode and device, and the choice of the electrolyte and its concentration. Kim wipes were employed as the paper substrate and folded Al was kept on the paper substrate, acting as an anode with 1.5 M KOH as electrolyte. This optimized 9 cm2 sized AAB can provide encouraging current (17.4 mA) and power (3 mW). Wang et al.89 reported the development of flexible wearable AABs on cellulose paper where Al foil serving as an anode was enclosed within the paper substrate during the process of paper manufacturing whereas oxygen reduction ink was utilized to deposit the air-breathing cathode on the paper substrate. To prepare the Al foil embedded cellulose paper substrate, 0.01 mm thick Al foil with 98.2% purity was sandwiched between paper pulp layers which were homogeneously spread over a stainless-steel mesh. Later on, the prepared three-layered anode structure namely paper pulp/Al foil/paper pulp was extracted from the stainless-steel mesh and was pressed uniformly to remove additional water content followed by drying at 60 °C for 30 min, which facilitates structure stability. The overall thickness of the prepared anode structure was very small ∼0.57 ± 0.02 mm facilitating diminished ionic resistance and hence encouraging battery performance. On the other hand, the ORR-based air-breathing cathode was made up of MnO2 and multiwalled CNTs serving as the ORR catalyst and catalyst support, respectively, along with a binder, all dispersed and sonicated in an ethanol–water solvent. This prepared ink was then poured on an Ag grid (area: 5 × 5 cm2) and deposited on a paper substrate. Such a configuration contributes to the confinement of the cathode area. Fig. 4(a) schematically illustrates the fabrication steps of the Al foil anode and the flexible paper-based AAB and its overall structure. The corresponding cross-sectional SEM image is depicted in Fig. 4(b). The structural morphology of the MnO2/CNT catalyst and homemade paper is shown in Fig. 4(c) and (d), respectively.
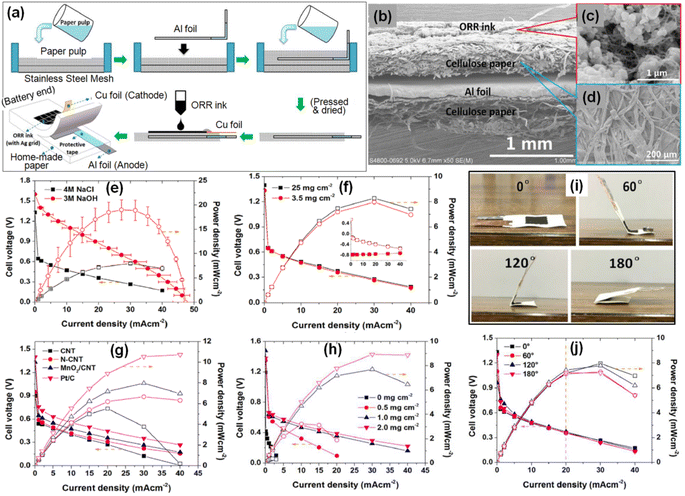 | ||
| Fig. 4 (a) Pictorial representation of the fabrication process and structure of a flexible paper-based AAB. (b) Cross-sectional SEM image of the flexible AAB. SEM images of a MnO2/CNT catalyst(c) and homemade paper (d). Polarization curves: effect of electrolyte species (e); Al loading (f); ORR catalyst species (g); and ORR catalyst MnO2/CNT loadings (h) on the battery performance. Battery performance at various bending angles ranging from 0° to 180° (images of the bent AAB) (i) and the corresponding polarization curves (j). Reproduced from ref. 89 with permission from [Elsevier], copyright [2019]. | ||
Wang et al.89 also examined the effect of electrolyte species (NaOH and NaCl), Al loading, ORR catalyst species and ORR catalyst loading on the battery performance as revealed in Fig. 4(e)–(h), respectively. A better peak power density was achieved using 3 M NaOH (19.0 ± 2.1 mW cm−2) as compared to 4 M NaCl (8.0 ± 0.3 mW cm−2) (Fig. 4(e)), whereas no notable change in the battery performance was observed with Al loading (Fig. 4(f)). Battery performance tested under various ORR catalyst species (Fig. 4(g)) reveals superior peak power density for Pt/C (10.7 mW cm−2) followed by MnO2/CNT (8.0 mW cm−2), N-CNT (6.7 mW cm−2) and CNT (5.7 mW cm−2). In the case of different ORR catalyst loadings (Fig. 4(h)), the peak power density was observed to improve and vary from 0.36 mW cm−2 to 8.9 mW cm−2 by varying the MnO2/CNT loading ranging from 0 to 2.0 mg cm−2. Finally, the AAB was examined for its flexibility at various bending angles ranging from 0° to 180° (Fig. 4(i)) and the corresponding polarization curves are represented in Fig. 4(j). It is evident from the figure that there was no significant change in the battery polarization curves as a result of bending angles; however, the peak power densities were found to reduce by 2.1–9.4% at current density varying from 20 to 40 mA cm−2.
The same group has also demonstrated the construction of a low-cost paper-based screen-printed AAB possessing high energy density which can be utilized for powering electronic devices. They printed ink made up of Al microsphere, CNT cellulose binder and oxygen reduction inks on cellulose paper.90 In another study, they also reported a green energy technology-based paper-inspired AAB for the mini-watt market.91 For this purpose, Al foil, a filter or cellulose paper and carbon paper acting as an anode, an electrolyte substrate and a cathode respectively, were sandwiched between PMMA shells. Fig. 5 schematically illustrates the working principle of the paper-inspired AAB.
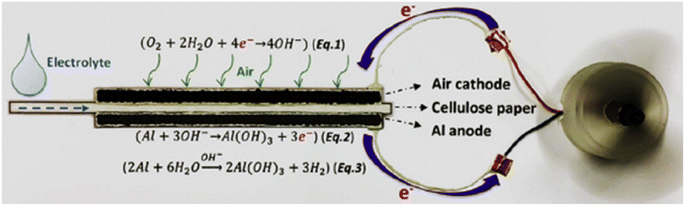 | ||
| Fig. 5 Working principle of the paper-based AAB comprising an air cathode, cellulose paper as an electrolyte substrate and an Al anode. Reproduced from ref. 91 with permission from [Elsevier], copyright [2019]. | ||
Cao et al. [9270] also fabricated highly flexible paper-inspired Al/Polyaniline (PANI) air batteries. In the proposed structure, Al, a PANI/Fe composite film grown on graphite paper and filter paper soaked with gel electrolytes of NH4Cl, TEA and NaNO3 serves as an anode, an air electrode, and a supporting substrate along with the separators of the anode and cathode, respectively. The fabricated filter paper-based Al/PANI/Fe-air battery exhibits a discharge capacity of 50 mA h cm−2. Apart from the paper-based substrate/platforms, paper-based gel electrolyte was also employed to preserve the greenness of MABs. Wang et al.92 demonstrated the development of liquid-free flexible AABs utilizing paper-based gel-electrolyte where the alkaline gel was poured inside the skeleton of a cellulose paper network.
It is worth mentioning here that AABs are primary cells and have a limited lifespan, but they still have the potential to be environmentally friendly. This is because they do not contain toxic materials and aluminium is a highly abundant and recyclable material. However, extensive research is being conducted to develop green rechargeable AABs based on various green solvents such as ionic liquids, deep eutectic solvents, etc. The very first study demonstrating the possibility of rechargeable AABs was reported by Bogolowski and Drillet.93, employing an Al foil anode, ionic liquid-based electrolytes and a Pt/C oxygen catalyst as an air cathode. Mori94 developed a rechargeable battery using electrolytes based on deep eutectic solvents.
Similar to paper-based AABs, a paper-based flexible wearable LAB was also reported by Liu et al.95 They employed Li anodes and discussed the fabrication of a paper ink cathode-based LAB which is extremely flexible and foldable. The paper ink cathode was obtained by dipping a brush into an ink made up of various active materials and drawing various-sized rectangular boxes onto a paper flowed by air drying at 120 °C. Finally, the paper ink cathode was prepared by the loading and distribution of the active materials on each rectangle and serves as both the cathode and current collector.
Apart from paper-based AABs or LABs, paper-based ZABs are also very popular. Very recently, hollow structure-based paper-inspired flexible ZABs were fabricated.41 A hollow channel structured paper-based ZAB possessing high excellent peak power density (102 mW cm−2) was demonstrated by Zhang et al.42 Herein, chromatographic paper dipped in KOH electrolyte forms a paper channel acting as an electrolyte substrate. To separate the electrolytic paper-channel layer from the electrodes, hollow channel layers are inserted between them, which helps enhance the battery performance. A novel cellulose paper-inspired zinc–air/Ag hybrid battery was proposed by Wang et al.72, where Zn foil and Ag grid-supported oxygen catalysts serve as the anode and cathode, respectively. This composite cathode facilitates battery working in switchable working modes mainly primary zinc–air mode and secondary Zn–Ag mode. In the former, oxidation of the Zn anode and reduction of ambient air via an oxygen catalyst inside the cathode occur leading to a peak power density of 17.8 mW cm−2, whereas in the latter, oxidation and reduction of the Ag grid inside the cathode occur following the reduction and periodic oxidation of the Zn anode.
Fig. 6(a) illustrates the battery structure which consists of four main parts including MnO2/carbon black, an Ag grid, a KOH gel electrolyte impregnated cellulose paper substrate and Zn foil acting as a catalyst layer, a current collector, electrolyte and an anode, respectively. This complete structure was packed within plastic sealing films consisting of an air-breathing window. The corresponding cross-section SEM image along with the element mapping is shown in Fig. 6(b). Fig. 6(c) shows the polarization curve of the zinc–air/Ag hybrid battery in primary zinc–air mode which clearly shows a peak power density and maximum current output of 17.8 mW cm−2 and 60 mA cm−2, respectively. The galvanostatic discharging curve at several current densities ranging from 1 mA cm−2 to 10 mA cm−2 (Fig. 6(d)) depicts excellent battery performance with high durability for lower current densities. Monolithic heteronanomat paper air cathodes were also employed for designing origami foldable and rechargeable ZABs.96
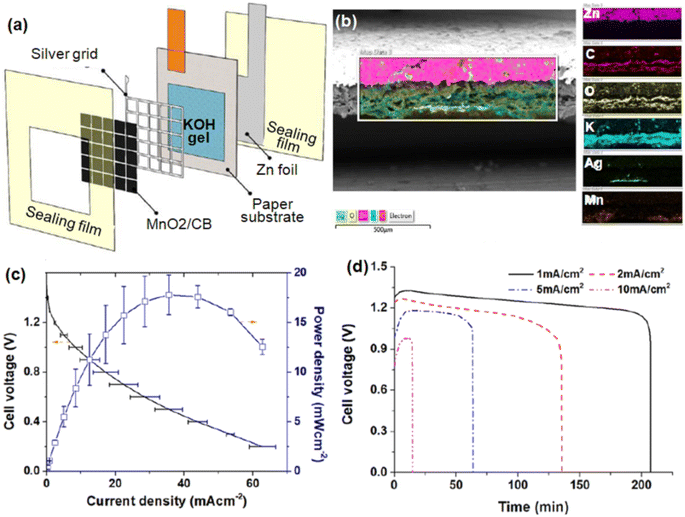 | ||
| Fig. 6 (a) Schematic illustration of the structure of a paper-based Zn–air/Ag hybrid battery. (b) Cross-sectional SEM image along with the element mapping of the hybrid battery. Polarization curve (c) and the galvanostatic discharge curve (d) at various current densities. Reproduced from ref. 72 with permission from [Elsevier], copyright [2021]. | ||
It is worth mentioning that paper-based flexible wearable MABs can endure several repeated deformations; however, their poor tensile strength hampers their practical utility. To circumvent this drawback, textile-supported MABs are introduced for powering flexible wearable electronic devices.
5.2. Textile/cotton/cloth-inspired flexible wearable MABs
Easy fabrication of textile/yarn/bamboo fabric supported durable MABs owing to their ability to weave, knit and stitch, better tensile strength along with environmental friendliness, easy availability and tolerance to numerous repeated deformations makes them extremely useful for wearable electronic applications. Textile-based supercapacitors obtained via printing several metal oxide inks on bamboo fabric and conductive cotton yarn-inspired wearable supercapacitors have already been reported.97,98 Fascinatingly, bamboo slips derived from wearable LABs with woven anodes and cathode structures also provide a remarkably very high energy density (>523 W h kg−1).99 Fabric composites can also be employed for such purposes. Hecht et al.100 demonstrated the electronic properties of CNT/fabric composites. A textile-based flexible AAB was developed by Valisevskis et al.101, where Ag-polished fabric acts as a cathode. The same group proposed a novel open design of multi-cell batteries where cells are sealed from one another facilitating easily approachable electrolytes to all cells in proper amounts and avoiding piling of excessive electrolytes.43 The individual battery design includes Al as an anode, Ag-coated polyamide as a cathode current collector and carbon granules with optional NaCl as electrolyte. The overall structure as represented in Fig. 7(a) is enclosed in a cotton enclosure and works upon activation and resets afterwards.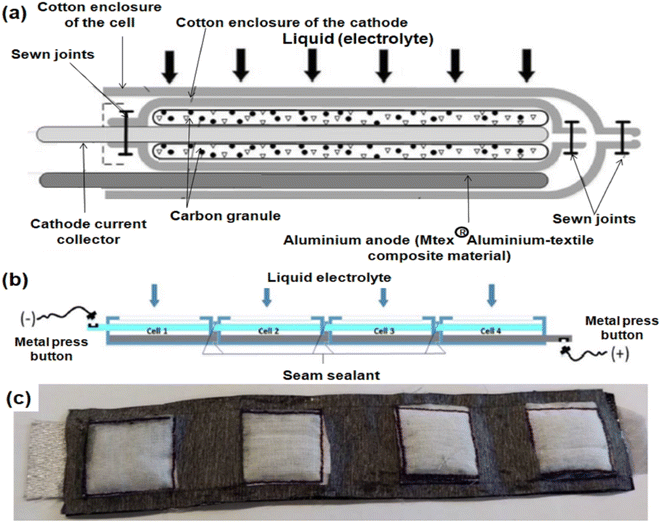 | ||
| Fig. 7 (a) Schematic design of a textile-based flexible AAB. Side view (b) and the actual photograph (c) of the multi-cell battery with cells arranged on a plane. Reproduced from ref. 43 with permission from [Springer Nature], copyright [2021]. | ||
The multicell battery consists of four cells arranged on a plane and connected in series such that the cathode of each one overlays the anode of the next one and electrodes are sewed with the textile-based material using conducting threads. Fig. 7(b) shows the side view of the multi-cell battery and the actual photograph is depicted in Fig. 7(c). Hydrophobic coatings are done on fabric to reduce electrolyte accumulation and their uniform distribution among the cells. Pan et al.102 also reported an AAB on a cotton substrate employing kitchen Al foil (1.5 cm2) coated with silicon carbide as the Al anode and carbon paper (1.5 cm2) printed with MnO2 catalyst ink as the air-breathing cathode, while for electrolyte, NaOH solution was coated onto the cotton substrate (1.5 cm2), followed by drying at 185 °C. This fabricated battery possesses an excellent peak power density and specific energy of 73 mW cm−2 and 930 mW h g−1.
Haung et al.84 developed a flexible wearable ZAB using cotton textile waste serving as the electrode material. This approach not only reduces environmental issues but also assists in the cutting of flexible electrodes. In their work, Ni metalized cotton textile acts as a flexible substrate for both the anode and cathode and is prepared via electrodeposition of Zn metal and NiFe hydroxide on Ni metalized cotton textile waste. The overall fabrication process of the electrodes is depicted in Fig. 8(a) where the textile was first coated with electroless plating with Ni which changed its color from white to grey. Later on, Zn metal and Ni hydroxide catalysts were electrodeposited over it to form Zn-coated Ni metalized cotton textile waste electrodes which are highly flexible as evident from the figure. Fig. 8(b) and (c) show the enlarged SEM image of Ni metalized cotton textile waste showing uniform dispersion of Ni particles on cotton textile waste and Zn electroplated cotton textile waste representing uniform coating of Ni with the Zn layer. Fig. 8(d) depicts the cross-section SEM image of the ZAB (Scale bar: 100 μm) which clearly shows the sandwich structure where the hydrogel electrolyte layer is sandwiched between NiFe hydroxide, the air-electrode and the zinc-coated anode.
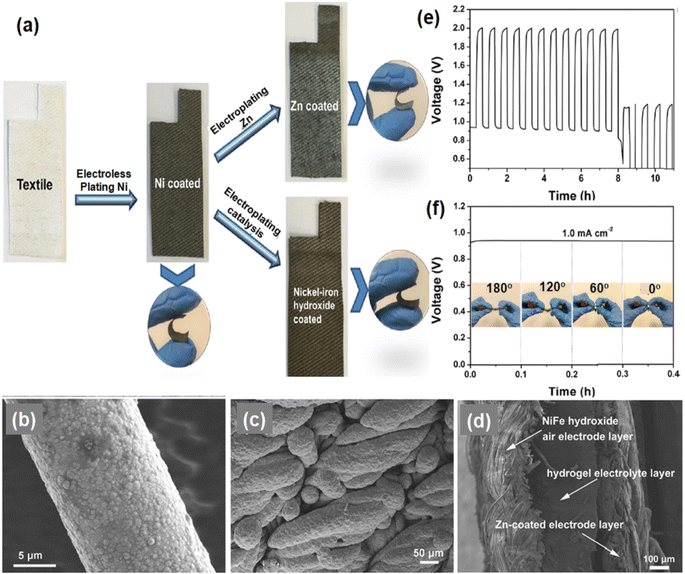 | ||
| Fig. 8 (a) Schematic representation of a strategy to fabricate a Zn-coated electrode and NiFe hydroxide electrode. Enlarged SEM images of Ni metalized cotton textile waste (b) and Zn electroplated cotton textile waste (c). (d) Cross-sectional SEM image of the assembled sandwich structured ZAB. (e) Galvanostatic charge and discharge curve of the ZAB (current density: 1 mA cm−2; cycle period: 20 min of discharge and then charge). (f) Galvanostatic discharge curve of the fabricated ZAB at several mechanical bending deformation angles. Reproduced from ref. 85 with permission from [American Chemical Society], copyright [2019]. | ||
To examine the stability and performance of charging/discharging processes, galvanostatic curves were obtained at a current density of 1 mA cm−2 for a discharging and charging cycle period of 20 min as shown in Fig. 8(e). Encouraging stability has been evident from the figure signifying the fascinating charging/discharging stability of the cotton textile waste-based ZAB. The performance of the fabricated cotton textile waste-based ZAB at various mechanical bending deformation angles varying from 0° to 180° is also shown in Fig. 8(f), demonstrating the extraordinary flexibility of the fabricated ZAB.
A novel approach to developing large-scale air electrodes employing industrially wearable metal wire/cotton fibre yarn possessing excellent conductivity as a substrate and current collector was also reported by Lin et al.103 The fabricated Li–O2 battery is highly flexible showing outstanding stability and durability even under several deformable conditions with a discharge capacity of 1981 mA h g−1 at 320 mA g−1. A flexible rechargeable LAB encompassing undoped TiO2 nanowire arrays grown on carbon textile (TiO2 NAs/CT) as a cathode and Li foil as an anode, along with a glass-fibre membrane showing encouraging electrochemical performance including recoverability and durability under several deformable conditions (bending and twisting), was also demonstrated by Liu et al.104 The overall growth process of the cathode electrode is illustrated in Fig. 9(a). The SEM images of the undoped carbon textile and the grown cathode along with its enlarged image are depicted in Fig. 9(b)–(d), respectively, indicating the vertical growth of TiO2 nanowire arrays onto the carbon textile. The complete assembly mode of the carbon textile-based LAB is presented in Fig. 8(e). Fig. 9(f) and (g) show the first discharging–charge curves (at 100 mA g−1) and rate capacity results (at different current densities) of the LAB with two different cathodes (pristine cotton textile and the developed TiO2 NAs/CT cathode), respectively. The flexibility of the fabricated LAB under bending and twisting deformable conditions (Fig. 9(h)–(j)) indicated its potential utility as a power source for flexible and wearable electronics.
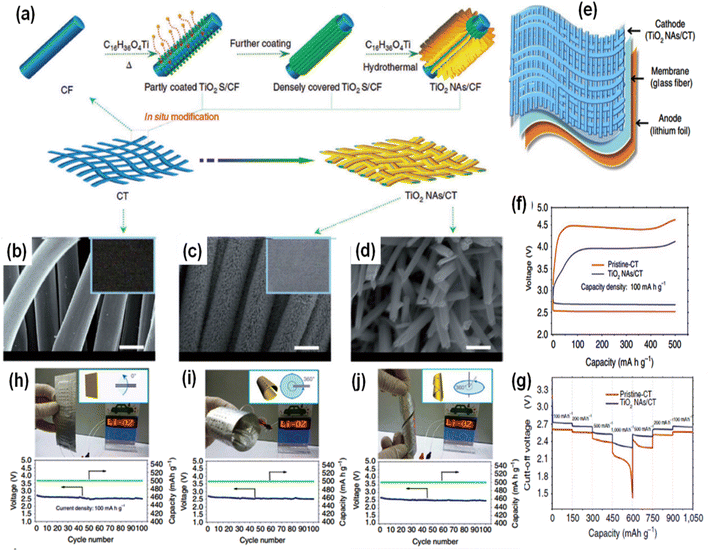 | ||
| Fig. 9 (a) Pictorial illustration of the fabrication procedure and structure of a TiO2 NAs/CT cathode. SEM images of pristine–carbon textile (scale bar: 10 mm) (b) and the fabricated TiO2 NAs/CT cathode (scale bar: 10 mm) (c), with its enlarged image (scale bar: 500 nm) (d); corresponding insets show their respective photographs. (e) Overall cell assembly mode of the carbon textile-based LAB consisting of a cathode (TiO2 NAs/CT), a separator (glass fibre) and an anode (lithium foil). First discharging–charge curves (at 100 mA g−1) (f) and rate capacity results (at different current densities) (g) of the fabricated LiO2 battery with two different cathodes e.g., pristine cotton textile and the developed TiO2 NAs/CT cathode. (h–j) Flexibility of the fabricated LiO2 battery examined under bending and twisting deformable conditions. Reproduced from ref. 104 with permission from [Nature Publishing], copyright [2015]. | ||
Carbon cloth has also been employed as an excellent substrate for the fabrication of electrodes for flexible wearable electronics. Nitrogen-doped carbon cloth can also serve as a flexible and freestanding electrocatalyst for ORR.105 A facile carbon cloth activation approach to enhance ORR performance for flexible ZAB applications was also elaborated by Manjunatha et al.106 Tan et al.107 illustrated the development and performance of a ZAB with NiO–Al–Co/carbon cloth electrodes. Herein, they have reported Al and Co-codoped NiO nanosheet electrodes developed on carbon cloth without adding any binder and such a battery leads to an encouraging peak power density of 36.3 mW cm−2. A carbon cloth-based flexible recharging ZAB was also reported where poly(vinylalcohol) (PVA) gel electrolyte was sandwiched between bifunctional catalyst-loaded carbon cloth and a zinc film serving as the cathode and anode, respectively.108 Chen et al.109 demonstrated the thermally driven phase transition of MnO2 on carbon cloth to boost the performance of flexible all-solid-state ZABs. Table 2 summarizes the electrochemical properties of a few green flexible wearable MABs including ZABs, LABs and AABs.
| Battery type | Battery components | Performance parameters | Ref. |
|---|---|---|---|
| Paper-based AAB (paint-brush technology) | Substrate: Kim wipes | Battery size: 9 cm2 | 88 |
| Electrolyte: KOH | Current: 17.4 mA | ||
| Anode: Al foil | Power: 3 mW | ||
| Cathode: activated carbon ink | |||
| Current collector: steel mesh | |||
| Paper-based AAB (screen printing technology) | Substrate: cellulose paper | Peak power density: 19.0 ± 2.1 mW cm−2 | 89 |
| Electrolyte: NaOH | |||
| Anode: Al foil into paper pulp | |||
| Cathode: MnO2/CNT ink on carbon paper | |||
| Paper-based AAB (screen printing technology) | Substrate: cellulose paper | Peak power density: 8.0 ± 0.3 mW cm−2 | 89 |
| Electrolyte: NaCl | |||
| Anode: Al foil into paper pulp | |||
| Cathode: MnO2/CNT ink on carbon paper | |||
| Paper-based AAB (screen printing technology) | Substrate: cellulose paper | Peak power density: 6.6 mW cm−2 | 90 |
| Electrolyte: NaOH | Current density: 40 mA cm−2 | ||
| Anode: Al foil into paper pulp | Battery discharge specific capacity: 951 mA h g−1 | ||
| Cathode: MnO2/CNT ink on carbon paper | |||
| Current collector: Ag grid | |||
| Paper-based AAB (screen printing technology) | Electrolyte substrate: filter or cellulose paper | Al specific capacity: 814 mA h g−1 at 1 mA cm−2 | 91 |
| Electrolyte: NaCl | Al specific capacity: 547 mA h g−1 at 2 mA cm−2 | ||
| Anode: dispersive Al microspheres | Al specific capacity: 387 mA h g−1 at 5 mA cm−2 | ||
| Cathode: MnO2/CNT ink on carbon paper | |||
| Paper-based Al/PANI-air battery | Electrolyte substrate: filter paper soaked with gel electrolyte of NH4Cl, TEA and NaNO3 | Discharge capacity: 50 mA h cm−2 | 110 |
| Anode: Al, PANI/Fe composite film on graphite paper | |||
| Cathode: air electrode | |||
| Carbon fiber paper based AAB | Substrate: carbon fibre | Discharge current densities >100 mA cm−2 | 86 |
| Electrolyte: alkaline | Peak power density: 109.5 mW cm−2 | ||
| Anode: Al metal | Specific capacity density: 2783.5 mA h g−1 | ||
| Cathode: Ag/carbon fibre paper | Energy density: 4342.3 W h kg−1 | ||
| Membrane: nylon polymer membrane | |||
| Cotton based AAB | Substrate: cotton substrate | Peak power density: 73 mW cm−2 | 102 |
| Electrolyte: NaOH | Specific energy: 930 mW h g−1 | ||
| Anode: Al foil coated with silicon carbide | |||
| Cathode: MnO2 catalyst ink on carbon paper | |||
| Textile based AAB | Substrate: textile fabric | Open circuit voltage: 690 mV | 101 |
| Electrolyte: NaCl | |||
| Cathode: Ag-polished textile fabric | |||
| Anode: Al-textile composite material | |||
| Cathode current collector: Shieldex Budapest conductive | |||
| Textile | |||
| Gas diffusion layer: carbon granules | |||
| Paper-based LAB | Substrate: paper | Capacity: 6500 mA h g−1 at 200 mA g−1 | 95 |
| Anode: Li | |||
| Cathode: paper-ink cathode | |||
| Carbon fiber-based LAB | Electrolyte: deep eutectic solvent | Capacity: 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 310 mA h g−1 at 100 mA g−1 310 mA h g−1 at 100 mA g−1 |
111 |
| Cathode: Li coating polythiophene-derived (S-doped) carbon fiber (Li/SC) | High-capacity retention: 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 896 mA h g−1 at 500 mA g−1 896 mA h g−1 at 500 mA g−1 |
||
| Anode: deep eutectic solvent coated Li/SC | Long cycling life of up to 300 cycles | ||
| Bamboo slip derived LAB | Electrolyte: gel polymer electrolyte | Energy density > 523 W h kg−1 | 99 |
| Anode: Li foil | |||
| Cathode: woven structured cathode | |||
| Paper-inspired zinc–air/Ag hybrid battery (Zn–air mode) | Electrolyte substrate: cellulose paper-impregnated gel electrolyte (KOH-PANa) | Open circuit voltage: 1.5 V | 72 |
| Anode: Zn foil | Peak power density: 17.8 mW cm−2 | ||
| Cathode: MnO2/carbon black catalyst | Maximum current output: 60 mA cm−2 | ||
| Current collector: Ag grid | |||
| Paper-inspired zinc–air/Ag hybrid battery (Zn–air mode) | Electrolyte substrate: cellulose paper-impregnated gel electrolyte (KOH-PANa) | Peak power density: 28.6 mW cm−2 | 72 |
| Anode: Zn foil | |||
| Cathode: MnO2/CNT catalyst | |||
| Current collector: Ag grid | |||
| Hollow channel structured paper-based ZAB | Electrolyte substrate: chromatographic paper dipped in KOH electrolyte | Peak power density: 102 mW cm−2 | 42 |
| Anode: Zn foil | |||
| Cathode: carbon paper coated with Pt/C powder as catalyst ink | |||
| Paper-based ZAB | Electrolyte substrate: filter paper saturated in KOH + Zn(Ac)2 | Maximum power density: 127 mW cm−2 | 112 |
| Anode: Zn foil | Specific capacity: 614 mA h g−1 | ||
| Cathode: CaCu3Ti4O12 perovskite catalyst loaded onto a Ni foam | |||
| Cotton textile waste-based ZAB (electrodeposition method) | Substrate: Ni metalized cotton textile waste (NMCTW) | Energy density: 547 W h kg−1 | 85 |
| Electrolyte: hydrogel electrolyte | |||
| Anode: Zn metal on/NMCTW | |||
| Cathode: NiFe hydroxide thin-film catalyst on NMCTW | |||
| Cloth based ZAB | Substrate: Ecoflex substrate | Discharged stably at 1 V at a high current density of 2 mA cm−2 | 113 |
| Anode: Zn foil | |||
| Cathode: Co3O4/CC air electrode | |||
| Cotton pad-derived ZAB | Electrolyte: gel polymer electrolyte | Open-circuit voltage: 1.328 V | 114 |
| Anode: Zn metal | Maximum power density: 65.1 mW cm−2 | ||
| Cathode: NO-G@CP grown on a cotton pad | |||
| Bamboo derived ZAB | Cathode: bamboo-derived carbon-based metal-free catalyst | Power density: 279.5 mW cm−2 | 115 |
| Anode: Zn foil | |||
| Electrolyte: KOH + Zn(Ac)2 | |||
| Cloth based ZAB | Electrolyte: PAA/KOH gel electrolyte | Open circuit voltage: 1.47 V | 116 |
| Anode: Zn sheet | Round-trip efficiency: 62.4% after 120 cycles | ||
| Cathode: MnOx on carbon cloth | Long cycling life: 45 h over an operating voltage of 1.2 V | ||
| High capacity: 728 mA h g−1 | |||
| Carbon cloth-based ZAB (electrodeposition method) | Substrate: polyethylene terephthalate | Discharge voltage ≈1.03 V | 117 |
| Electrolyte: Hydrogel electrolyte | Charge voltage ≈1.95 V at 2 mA cm−2 | ||
| Anode: Zn | Energy density: 546 W h kg−1 | ||
| Cathode: Co3O4/carbon cloth |
6. Challenges for commercialization
Extensive research efforts are being devoted to their commercialization in light of the scientific interest sparked by green MABs and their notable contribution to the field of flexible wearable electronics. MABs can be made using a variety of environmentally friendly alternatives for electrodes and substrates, and they have significant commercial potential. It should be noted here that each material used in MABs has its own challenges and advantages. Zinc is abundant and non-toxic, and has a high theoretical energy density, making ZABs attractive for both large-scale and portable applications. The major challenge is the formation of Zn dendrites, which may occur during charging, leading to short circuits and reduced battery life. The issues with dendrite formation must be addressed through advanced material engineering and design improvements to make these batteries commercially viable. LABs have an exceptionally high theoretical energy density, making them ideal for applications requiring lightweight and long-lasting power sources, such as electric vehicles. However, searching for a stable electrolyte that can withstand the reactive nature of lithium and the products of the ORR and OER is challenging. Also, carbon-based oxygen electrodes can degrade due to side reactions with lithium peroxide. The technical challenges associated with LABs need significant advancements in materials and safety protocols to achieve commercial viability. Al is abundant and light weight and has a high energy density, making it suitable for applications where weight and cost are critical factors. It should be noted here that Al may react with the electrolyte, causing corrosion and reducing battery life. Also, the formation of aluminium hydroxide as a by-product can clog the battery and decrease efficiency. Apart from this, currently, AABs are not easily rechargeable, which limits their use to primary (single-use) applications. Addressing the issues with corrosion and by-product management is essential to enhance the reusability and commercial appeal of AABs. Magnesium is light weight and abundant and has a good balance of energy density and cost, making it a potential candidate for various applications. The formation of a passivation layer on the magnesium anode hinders its electrochemical performance. Similar to Al, finding a compatible electrolyte that does not corrode Mg is challenging. Overcoming the passivation issue and finding compatible electrolytes is necessary to improve performance and enable commercialization. Carbon-based catalysts, such as graphene and carbon nanotubes, offer high surface area and electrical conductivity, which are beneficial for enhancing battery performance. However, the challenges associated with the use of carbon-based catalysts include durability, scalability and performance consistency. Carbon-based catalysts can degrade over time, especially under high ORR and OER activity and producing high-quality carbon catalysts at scale can be challenging and costly. Also, achieving consistent performance across different batches of carbon materials can be difficult. Addressing the durability and scalability issues is crucial for making these catalysts a reliable and cost-effective choice for commercial applications. Eco-friendly electrolytes, such as aqueous and ionic liquids, reduce environmental impact and enhance the safety profile of MABs. However, some eco-friendly electrolytes have lower ionic conductivity compared to traditional electrolytes and ensuring long-term chemical stability under battery operating conditions can also be challenging. Improving their conductivity and stability while ensuring cost-effective production is essential for commercial viability.Overall, commercializing MABs involves overcoming numerous material-specific challenges to ensure that they are viable for widespread use. While Zn and Li show high potential due to their energy density and abundance, their technical and safety challenges need to be addressed for commercial viability. Al and Mg offer cost and weight benefits but face issues with corrosion. Carbon-based catalysts and eco-friendly electrolytes provide promising paths towards greener and safer batteries, but their performance and manufacturing scalability need further improvement. Future research should focus on overcoming these challenges associated with materials employed to unlock the full commercial potential of MABs. It should be noted here that apart from the several advantages offered by green MABs over other advanced flexible MABs, including the use of environmentally friendly materials, easy recyclability because of biodegradable materials and processes, reduced the overall ecological footprint, flexible designs ideal for wearable electronics, providing comfort and durability during use, high energy density which may vary based on materials, beneficial for long-lasting power in portable devices and lightweight nature improving user comfort and device portability, there remain several other limitations. Flexible materials may have lower mechanical strength and durability compared to rigid batteries, potentially leading to shorter lifespans. They may also have lower energy efficiency compared to conventional materials, affecting overall battery performance. Manufacturing processes such as 3D printing for flexible and green batteries may still face scalability challenges, impacting mass production.118 Advanced materials and eco-friendly processes can be more expensive, increasing the cost of green flexible wearable MABs. Achieving an optimal balance between flexibility, durability, and energy density remains a significant challenge.
As of now, several researchers have been devotedly working in the area of green MABs and are aware of the difficulties in system integration and standardization as well as other issues that prevent the commercialization of green MABs. Gniotek and Krucinska discussed several basic issues in textile-based electronics.119 To simultaneously offer flexibility and wearability with reduced material volume or the use of eco-friendly materials, a proper unification of various battery components as well as the assessment of threats to the user and environment imposed by the materials employed must be properly balanced. Additionally, a variety of technologies are used to create them, such as printing- or lithography-based techniques, depending on the substrate material selected. However, there is currently no leading technological protocol or ideal material for the production of a particular MAB, which raises the concern of standardization and subsequently limits their commercialization.
In particular, for paper-based flexible wearable MABs, the poor tensile strength of paper restricts the use of green wearable MABs made of paper. Additionally, electrodes are typically printed on such substrates using screen printing technology, which heavily depends on the ink used for printing them. Additionally, the washability of such printed electrodes limits their ability to be used repeatedly, which prevents their commercialization. These issues are more likely to arise in two-dimensional paper- and textile-based MABs, but they can be partially solved by using one-dimensional structures to create green wearable MABs.
The various green and sustainable aspects to be considered for the fabrication of flexible MABs include the environmental impact of raw material extraction, material production, cell production, possibilities of life usage, cell disposal, recycling, processing cost, environmental benefits, etc. However, there should be a proper balance between the performance of the MAB and its green and sustainable aspects. One should not overlook the functionality while considering the above-mentioned aspects. Also, the reduction in the need for frequent battery replacements and hence minimizing the environmental impact of battery production and disposal, recovering from used batteries and recycling for use in new battery production, and reducing the need for virgin materials can lead to sustainable green MABs.
7. Conclusions
The present article critically reviews the synthesis and electrochemical performances of various green wearable MABs including eco-friendly paper-based and textile/yarn/fabric-based MABs for powering various electronic devices that can withstand extensive harsh deformable conditions, e.g. bending, twisting, stretching, and folding, along with the study of various green aspects such as biocompatibility and biodegradability of materials employed, cost-effectiveness, minimization of the amount of electrolyte used via the capillary action of paper/textile, and easy fabrication and operation. Through rapid technological and scientific advancements, including novel and environmentally friendly methods of fabrication, improved designs, and green materials, green flexible and wearable MABs are continuously being advanced. Furthermore, research is required to be conducted to explore the use of alternative, more sustainable catalyst support materials, such as CNTs derived from biomass, which could further improve the sustainability of MABs. Therefore, in the near future, additional efforts on sustainability and greenness will be required by considering green principles as the driving force and making ethical environmental commitments. Additionally, complications with green wearable MABs for wearable electronics that hinder their commercialization have also been thoroughly examined and discussed.Overall, green flexible MABs offer a more sustainable and environmentally friendly alternative to conventional batteries, while also providing improved performance and durability. By using sustainable materials such as paper or cotton/cloths as substrates, these batteries can help reduce the environmental impact of battery production and disposal, while also conserving resources and energy.
Data availability
“No primary research results, software or code have been included and no new data were generated or analysed as part of this review”.Conflicts of interest
There are no conflicts to declare.References
- P. Lukowicz, T. Kirstein and G. Troster, Wearable systems for health care applications, Methods Inf. Med., 2004, 43, 232–238 CrossRef CAS.
- D. H. Kim and J. A. Rogers, Stretchable Electronics: Materials Strategies and Devices, Adv. Mater., 2008, 20, 4887–4892 CrossRef CAS.
- N. Nishide and K. Oyaizu, Toward flexible batteries, Science, 2008, 319, 737–738 CrossRef.
- H. F. Wang and Q. Xu, Materials design for rechargeable metal-air batteries, Matter, 2019, 1, 565–595 CrossRef CAS.
- P. Tan, B. Chen, H. Xu, H. Zhang, W. Cai, M. Ni, M. Liu and Z. Shao, Flexible Zn–and Li–air batteries: recent advances, challenges, and future perspectives, Energy Environ. Sci., 2017, 10, 2056–2080 RSC.
- L. Wang, Y. Zhang, J. Pan and H. Peng, Stretchable lithium-air batteries for wearable electronics, J. Mater. Chem. A, 2016, 4, 13419–13424 RSC.
- A. Agrawal, R. Keçili, F. Ghorbani-Bidkorbeh and C. M. Hussain, Green miniaturized technologies in analytical and bioanalytical chemistry, Trends Anal. Chem., 2021, 143, 116383 CrossRef CAS.
- A. Agrawal, C. G. Hussain, R. Keçili and C. M. Hussain, Carbonaceous materials in green sample preparation, in Handbook of Green Sample Preparation Techniques, Green Chemistry Series, Royal Society of Chemistry, 2023 Search PubMed.
- A. Agrawal and C. M. Hussain, Metal–air batteries for wearable electronics: a case study for modern society, in Next-Generation of Human Resources and Technologies, ed. C. M. Hussain, A. Petrillo and S. U. Islam, CRC Press, 2023 Search PubMed.
- A. Agrawal and C. M. Hussain, Wearable metal–air batteries, in Handbook of Metal–Air Batteries: Principles, Progresses and Perspective, ed. R. Gupta, CRC Press, 2023 Search PubMed.
- A. Agrawal, Materials and technologies for flexible and wearable sensors, in Handbook of Flexible and Wearable Sensors: Materials, Technologies, and Challenges, ed. R. Gupta, CRC Press, 2023 Search PubMed.
- A. Agrawal, Polymer materials for metal–air battery, in Springer Book – Recent Advancements in Polymeric Materials for Electrochemical Energy Storage, ed. R. Gupta, Springer Nature, 2023 Search PubMed.
- S. Khan, T. Noor, N. Iqbal and E. Pervaiz, Recent Advancement in Metal-Organic Framework for Water Electrolysis: A Review, ChemNanoMat, 2022, 8, e202200115 CrossRef CAS.
- S. Ejaz, A. Ihsan, T. Noor, S. Shabbir and M. Imran, Mannose functionalized chitosan nanosystems for enhanced antimicrobial activity against multidrug-resistant pathogens, Polym. Test., 2020, 91, 106814 CrossRef CAS.
- T. Noor, L. Yaqoob and N. Iqbal, Recent advances in electrocatalysis of oxygen evolution reaction using noble-metal, transition-metal, and carbon-based materials, ChemElectroChem, 2021, 8, 447–483 CrossRef CAS.
- M. D. Haider, N. Iqbal, S. A. M. Rizvi, T. Noor, S. Hanif and R. Anwar, ZIF-derived Cu-doped electrocatalyst for oxygen reduction reaction, J. Electrochem. Energy Convers. Storage, 2021, 18, 021001 CrossRef CAS.
- N. Zaman, N. Iqbal and T. Noor, Comparative study of Mn-ZIF-67 derived carbon (Mn-Co/C) and its rGO-based composites for the methanol oxidation, J. Environ. Chem. Eng., 2022, 10, 108351 CrossRef CAS.
- A. S. Lodhi, N. Iqbal, T. Noor, N. Zaman and J. Gao, ZIF-67 derived ternary NiMnCo-based nanoporous carbon material for methanol oxidation reaction, Int. J. Energy Res., 2022, 46, 16736–16750 CrossRef CAS.
- Z. Zhang, C. Zuo, Z. Liu, Y. Yu, Y. Zuo and Y. Song, All-solid-state Al–air batteries with polymer alkaline gel electrolyte, J. Power Sources, 2014, 251, 470–475 CrossRef CAS.
- N. Vassal, E. Salmon and J. F. Fauvarque, Electrochemical properties of an alkaline solid polymer electrolyte based on P (ECH-co-EO), Electrochim. Acta, 2000, 45, 1527–1532 CrossRef CAS.
- C. C. Yang and S. J. Lin, Preparation of alkaline PVA-based polymer electrolytes for Ni–MH and Zn–air batteries, J. Appl. Electrochem., 2003, 33, 777–784 CrossRef CAS.
- G. M. Wu, S. J. Lin and C. C. Yang, Preparation and characterization of PVA/PAA membranes for solid polymer electrolytes, J. Membr. Sci., 2006, 275, 127–133 CrossRef CAS.
- X. Lei, X. Liu, W. Ma, Z. Cao, Y. Wang and Y. Ding, Flexible lithium–air battery in ambient air with an in situ formed gel electrolyte, Angew. Chem., Int. Ed., 2018, 57, 16131–16135 CrossRef CAS.
- X. Fan, J. Liu, Z. Song, X. Han, Y. Deng, C. Zhong and W. Hu, Porous nanocomposite gel polymer electrolyte with high ionic conductivity and superior electrolyte retention capability for long-cycle-life flexible zinc–air batteries, Nano Energy, 2019, 56, 454–462 CrossRef CAS.
- J. Fu, D. U. Lee, F. M. Hassan, L. Yang, Z. Bai, M. G. Park and Z. Chen, Flexible high-energy polymer-electrolyte-based rechargeable zinc–air batteries, Adv. Mater., 2015, 27, 5617–5622 CrossRef CAS.
- J. Li, Z. Wang, L. Yang, Y. Liu, Y. Xing, S. Zhang and H. Xu, A Flexible Li–Air Battery Workable under Harsh Conditions Based on an Integrated Structure: A Composite Lithium Anode Encased in a Gel Electrolyte, ACS Appl. Mater. Interfaces, 2021, 13, 18627–18637 CrossRef CAS PubMed.
- D. Ji, L. Fan, L. Li, S. Peng, D. Yu, J. Song, S. Ramakrishna and S. Guo, Atomically transition metals on self-supported porous carbon flake arrays as binder-free air cathode for wearable zinc− air batteries, Adv. Mater., 2019, 31, 1808267 CrossRef.
- J. Zhang, J. Fu, X. Song, G. Jiang, H. Zarrin, P. Xu, K. Li, A. Yu and Z. Chen, Laminated cross-linked nanocellulose/graphene oxide electrolyte for flexible rechargeable zinc–air batteries, Adv. Energy Mater., 2016, 6, 1600476 CrossRef.
- Z. Wu, Y. Wang, X. Liu, C. Lv, Y. Li, D. Wei and Z. Liu, Carbon-nanomaterial-based flexible batteries for wearable electronics, Adv. Mater., 2019, 31, 1800716 CrossRef PubMed.
- S. Ozcan, M. Tokur, T. Cetinkaya, A. Guler, M. Uysal, M. O. Guler and H. Akbulut, Free standing flexible graphene oxide+ α-MnO2 composite cathodes for Li–Air batteries, Solid State Ionics, 2016, 286, 34–39 CrossRef CAS.
- G. Fu, Y. Tang and J. M. Lee, Recent Advances in Carbon-Based Bifunctional Oxygen Electrocatalysts for Zn− Air Batteries, ChemElectroChem, 2018, 5, 1424–1434 CrossRef CAS.
- W. Zhang, Z. Li, J. Chen, X. Wang, X. Li, K. Yang and L. Li, Three-dimensional CoNi alloy nanoparticle and carbon nanotube decorated N-doped carbon nanosheet arrays for use as bifunctional electrocatalysts in wearable and flexible Zn-air batteries, Nanotechnol, 2020, 31, 185703 CrossRef CAS PubMed.
- M. M. OttakamThotiyl, S. A. Freunberger, Z. Peng and P. G. Bruce, The carbon electrode in nonaqueous Li–O2 cells, J. Am. Chem. Soc., 2013, 135, 494–500 CrossRef CAS.
- C. Dai, G. Sun, L. Hu, Y. Xiao, Z. Zhang and L. Qu, Recent progress in graphene-based electrodes for flexible batteries, InfoMat, 2020, 2, 509–526 CrossRef CAS.
- Y. Qin, Z. Ou, C. Xu, Z. Zhang, J. Yi, Y. Jiang, J. Wu, C. Guo, Y. Si and T. Zhao, Progress of carbon-based electrocatalysts for flexible zinc-air batteries in the past 5 years: recent strategies for design, synthesis and performance optimization, Nanoscale Res. Lett., 2021, 16, 1–3 CrossRef PubMed.
- J. Y. Lee, S. T. Connor, Y. Cui and P. Peumans, Solution-processed metal nanowire mesh transparent electrodes, Nano Lett., 2008, 8, 689–692 CrossRef CAS.
- A. Agrawal, Graphene based 2D nanomaterials for battery applications, in Handbook of Energy Applications of 2D Nanomaterials, ed. R. Gupta, CRC Press, 2022 Search PubMed.
- A. Agrawal, 3D graphene for flexible electronics, in Springer Book – 3D Graphene: Fundamentals, Synthesis, and Emerging Applications, ed. R. Gupta, Springer Nature, 2023 Search PubMed.
- Z. Li, X. Li, Y. Jiang, Q. Ding and W. Han, Nanocellulose composite gel with high ionic conductivity and long service life for flexible zinc-air battery, Polym. Test., 2021, 104, 107380 CrossRef CAS.
- A. Agrawal, Ü. Y. Yıldız, C. G. Hussain, S. K. Kailasa, R. Keçili and C. M. Hussain, Greenness of lab-on-a-chip devices for analytical processes: advances & future prospects, J. Pharm. Biomed. Anal., 2022, 219, 114914 CrossRef CAS.
- Y. Yang, H. Zhang, X. Zhu, D. Ye, R. Chen and Q. Liao, A hollow structure for flow and bendable paper-based zinc-air battery, Int. J. Energy Res., 2022, 46, 16075–16081 CrossRef CAS.
- H. Zhang, B. Zhang, Y. Yang, D. Ye, R. Chen, Q. Liao and X. Zhu, A high power density paper-based zinc–air battery with a hollow channel structure, Chem. Commun., 2021, 57, 1258–1261 RSC.
- A. Vališevskis, U. Briedis, M. Carvalho and F. Ferreira, Development of flexible textile aluminium-air battery prototype, Mater. Renew. Sustain. Energy, 2021, 10, 1–6 CrossRef.
- K. Jost, K. Dion and Y. Gogotsi, Textile energy storage in perspective, J. Mater. Chem. A, 2014, 2, 10776–10787 RSC.
- L. Hu, M. Pasta, F. La Mantia, L. Cui, S. Jeong, H. D. Deshazer, J. W. Choi, S. M. Han and Y. Cui, Stretchable, porous, and conductive energy textiles, Nano Lett., 2010, 10, 708–714 CrossRef CAS PubMed.
- S. Park and S. Jayaraman, Smart textiles: wearable electronic systems, MRS Bull., 2003, 8, 585–591 CrossRef.
- L. Hu, J. W. Choi, Y. Yang, S. Jeong, F. La Mantia, L. F. Cui and Y. Cui, Highly conductive paper for energy-storage devices, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 21490–21494 CrossRef CAS.
- V. L. Pushparaj, M. M. Shaijumon, A. Kumar, S. Murugesan, L. Ci, R. Vajtai, R. J. Linhardt, O. Nalamasu and P. M. Ajayan, Flexible energy storage devices based on nanocomposite paper, Proc. Natl. Acad. Sci. U.S.A., 2007, 104, 13574–13577 CrossRef CAS.
- K. Jost, C. R. Perez, J. K. McDonough, V. Presser, M. Heon, G. Dion and Y. Gogotsi, Carbon coated textiles for flexible energy storage, Energy Environ. Sci., 2011, 4, 5060–5067 RSC.
- B. S. Shim, W. Chen, C. Doty, C. Xu and N. A. Kotov, Smart electronic yarns and wearable fabrics for human biomonitoring made by carbon nanotube coating with polyelectrolytes, Nano Lett., 2008, 8, 4151–4157 CrossRef CAS PubMed.
- W. Pan, Y. Wang, H. Y. Kwok and D. Y. Leung, A low-cost portable cotton-based aluminum-air battery with high specific energy, Energy Procedia, 2019, 158, 179–185 CrossRef CAS.
- G. Xiao, J. Ju, H. Lu, X. Shi, X. Wang, W. Wang, Q. Xia, G. Zhou, W. Sun, C. M. Li and Y. Qiao, A weavable and scalable cotton-yarn-based battery activated by human sweat for textile electronics, Adv. Sci., 2022, 9, 2103822 CrossRef CAS.
- A. Agrawal and G.-C. Yi, Sample pretreatment with graphene materials, in Comprehensive Analytical Chemistry: Analytical Applications of Graphene for Comprehensive Analytical Chemistry, ed. C. M. Hussain, Elsevier, 2020, vol. 91, pp. 21–47 Search PubMed.
- A. Agrawal, Multi-atom catalysts for metal–air batteries, in Atomically Precise Electrocatalysts for Electrochemical Energy Applications, ed. R. Gupta, Springer Nature Search PubMed.
- L. Mao, Q. Meng, A. Ahmad and Z. Wei, Mechanical analyses and structural design requirements for flexible energy storage devices, Adv. Energy Mater., 2017, 7, 1700535 CrossRef.
- X. Xiao, Z. Zheng, X. Zhong, R. Gao, Z. Piao, M. Jiao and G. Zhou, Rational design of flexible Zn-based batteries for wearable electronic devices, ACS Nano, 2023, 17, 1764–1802 CrossRef CAS.
- X. Yu and A. Manthiram, Sustainable battery materials for next-generation electrical energy storage, Adv. Energy Sustainability Res., 2021, 2, 2000102 CrossRef CAS.
- R. Revel, T. Audichon and S. Gonzalez, Non-aqueous aluminium–air battery based on ionic liquid electrolyte, J. Power Sources, 2014, 272, 415–421 CrossRef CAS.
- D. R. Egan, C. P. De León, R. J. K. Wood, R. L. Jones, K. R. Stokes and F. C. Walsh, Developments in electrode materials and electrolytes for aluminium–air batteries, J. Power Sources, 2013, 236, 293–310 CrossRef CAS.
- F. Tong, W. Zhuang, M. Song, J. Kim, W. Gao and S. Wei, Micro-alloyed aluminium alloys as anodes for aluminium-air batteries with a neutral electrolyte, Mater. Today Commun., 2024, 39, 108518 CrossRef CAS.
- R. Apte, Ecosystem Feasibility and Sustainability of Aluminium–Air Battery Powered Electric Vehicle, 2019;26-0115, SAE Technical Paper Search PubMed.
- X. Liu, H. Jiao, M. Wang, W. L. Song, J. Xue and S. Jiao, Current progresses and future prospects on aluminium–air batteries, Int. Mater. Rev., 2022, 67, 734–764 CrossRef CAS.
- J. F. Cooper, Aluminum-air battery development: toward an electric car, Int. Mater. Rev., 1983, 20–33 Search PubMed.
- S. Yang and H. Knickle, Design and analysis of aluminum/air battery system for electric vehicles, J. Power Sources, 2002, 112, 162–173 CrossRef CAS.
- R. Cao, J. S. Lee, M. Liu and J. Cho, Recent progress in non-precious catalysts for metal-air batteries, Adv. Energy Mater., 2012, 2816–2829 Search PubMed.
- J. Liu, H. Zhang, M. Qiu, Z. Peng, M. K. Leung, W. F. Lin and J. Xuan, A review of non-precious metal single atom confined nanomaterials in different structural dimensions (1D–3D) as highly active oxygen redox reaction electrocatalysts, J. Mater. Chem. A, 2020, 8, 2222–2245 RSC.
- S. Haller, V. Gridin, K. Hofmann, R. W. Stark, B. Albert and U. I. Kramm, Application of Non-Precious Bifunctional Catalysts for Metal-Air Batteries, Energy Technol., 2021, 9, 2001106 CrossRef CAS.
- E. Davari and D. G. Ivey, Bifunctional electrocatalysts for Zn–air batteries, Sustainable Energy Fuels, 2018, 2, 39–67 RSC.
- C. Han, W. Li, H. K. Liu, S. Dou and J. Wang, Design strategies for developing non-precious metal based bi-functional catalysts for alkaline electrolyte based zinc–air batteries, Mater. Horiz., 2019, 6, 1812–1827 RSC.
- M. Xu, H. Dou, Z. Zhang, Y. Zheng, B. Ren, Q. Ma, G. Wen, D. Luo, A. Yu, L. Zhang and X. Wang, Hierarchically Nanostructured Solid-State Electrolyte for Flexible Rechargeable Zinc–Air Batteries, Angew. Chem., Int. Ed., 2022, 61, e202117703 CrossRef CAS.
- R. Puttaswamy, C. Mondal, D. Mondal and D. Ghosh, An account on the deep eutectic solvents-based electrolytes for rechargeable batteries and supercapacitors, Sustainable Mater. Technol., 2022, 33, e00477 CrossRef CAS.
- Y. Wang, X. Zhao, W. Pan, K. W. Leong, S. Luo and D. Y. Leung, A printed paper-based Zn-air/Ag hybrid battery with switchable working modes, Electrochim. Acta, 2021, 396, 139237 CrossRef CAS.
- C. Lamiel, I. Hussain, X. Ma and K. Zhang, Properties, functions, and challenges: current collectors, Mater. Today Chem., 2022, 26, 101152 CrossRef.
- J. Wu, B. Liu, X. Fan, J. Ding, X. Han, Y. Deng, W. Hu and C. Zhong, Carbon-based cathode materials for rechargeable zinc-air batteries: from current collectors to bifunctional integrated air electrodes, Carbon Energy, 2020, 2, 370–386 CrossRef CAS.
- A. Agrawal, Y. Tchoe, J. Y. Park, P. Sen and G. C. Yi, Unravelling absorptive and refractive optical nonlinearities in CVD grown graphene layers transferred onto foreign quartz substrate, Appl. Surf. Sci., 2020, 505, 144392 CrossRef CAS.
- A. Agrawal, R. K. Saroj, T. A. Dar, P. Baraskar, P. Sen and S. Dhar, Insight into the effect of dislocations and oxygen vacancy defects on the optical nonlinearity in chemically grown ZnO/Al2O3 films, J. Appl. Phys., 2018, 122, 195303 CrossRef.
- R. Mi, H. Liu, H. Wang, K. W. Wong, J. Mei, Y. Chen, W. M. Lau and H. Yan, Effects of nitrogen-doped carbon nanotubes on the discharge performance of Li-air batteries, Carbon, 2014, 67, 744–752 CrossRef CAS.
- R. Yu, W. Fan, X. Guo and S. Dong, Highly ordered and ultra-long carbon nanotube arrays as air cathodes for high-energy-efficiency Li-oxygen batteries, J. Power Sources, 2016, 306, 402–407 CrossRef CAS.
- F. A. Tantray, A. Agrawal, M. Gupta, J. T. Andrews and P. Sen, Effect of oxygen partial pressure on the structural and optical properties of Ion beam sputtered TiO2 thin films, Thin Solid Films, 2016, 619, 86–90 CrossRef CAS.
- R. Chouhan, P. Baraskar, A. Agrawal, M. Gupta and P. Sen, Effects of oxygen partial pressure and annealing on dispersive optical nonlinearity in NiO thin films, J. Appl. Phys., 2017, 122, 025301 CrossRef.
- A. Agrawal, T. A. Dar, P. Sen and D. M. Phase, Anomalous band bowing in pulsed laser deposited MgxZn1-xO films, J. Cryst. Growth, 2013, 384, 9–12 CrossRef CAS.
- T. A. Dar, A. Agrawal and P. Sen, Pulsed laser deposited nickel doped zinc oxide thin films: structural and optical investigations, J. Nano- Electron. Phys., 2013, 5, 02024–02027 CAS.
- T. V. Pham, Y. Li, W. B. Luo, H. P. Guo, X. W. Gao, J. Z. Wang and H. K. Liu, Binder-Free 3D Integrated Ni@ Ni3Pt Air Electrode for Zn–Air Batteries, Global Chall., 2019, 3, 1900027 CrossRef.
- S. Qu, Z. Song, J. Liu, Y. Li, Y. Kou, C. Ma, X. Han, Y. Deng, N. Zhao, W. Hu and C. Zhong, Electrochemical approach to prepare integrated air electrodes for highly stretchable zinc-air battery array with tunable output voltage and current for wearable electronics, Nano Energy, 2017, 39, 101–110 CrossRef CAS.
- X. Huang, J. Liu, J. Ding, Y. Deng, W. Hu and C. Zhong, Toward Flexible and Wearable Zn–Air Batteries from Cotton Textile Waste, ACS Omega, 2019, 4, 19341–19349 CrossRef CAS.
- Q. Hong and H. Lu, In-situ Electrodeposition of Highly Active Silver Catalyst on Carbon Fiber Papers as Binder Free Cathodes for Aluminum-air Battery, Sci. Rep., 2017, 7, 3378 CrossRef.
- S. Kheawhom and S. Suren, Printed air cathode for flexible and high energy density zinc-air battery, MRS Adv., 2016, 1, 3585–3591 CrossRef CAS.
- A. Avoundjian, V. Galvan and F. A. Gomez, An inexpensive paper-based aluminum-air battery, Micromachines, 2017, 8, 222 CrossRef PubMed.
- Y. Wang, H. Y. Kwok, W. Pan, Y. Zhang, H. Zhang, X. Lu and D. Y. Leung, Combining Al-air battery with paper-making industry, a novel type of flexible primary battery technology, Electrochim. Acta, 2019, 319, 947–957 CrossRef CAS.
- Y. Wang, H. Y. Kwok, W. Pan, Y. Zhang, H. Zhang, X. Lu and D. Y. Leung, Printing Al-air batteries on paper for powering disposable printed electronics, J. Power Sources, 2020, 450, 227685 CrossRef CAS.
- Y. Wang, H. Kwok, W. Pan, H. Zhang and D. Y. Leung, Innovative paper-based Al-air batteries as a low-cost and green energy technology for the miniwatt market, J. Power Sources, 2019, 414, 278–282 CrossRef CAS.
- Y. Wang, W. Pan, H. Y. Kwok, H. Zhang, X. Lu and D. Y. Leung, Liquid-free Al-air batteries with paper-based gel electrolyte: a green energy technology for portable electronics, J. Power Sources, 2019, 437, 226896 CrossRef CAS.
- N. Bogolowski and J. F. Drillet, An electrically rechargeable Al-air battery with aprotic ionic liquid electrolyte, ECS Trans., 2017, 75, 85 CrossRef CAS.
- R. Mori, All solid-state rechargeable aluminum–air battery with deep eutectic solvent based electrolyte and suppression of byproducts formation, RSC Adv., 2019, 9, 22220–22226 RSC.
- Q. C. Liu, L. Li, J. J. Xu, Z. W. Chang, D. Xu, Y. B. Yin, X. Y. Yang, T. Liu, Y. S. Jiang, J. M. Yan and X. B. Zhang, Flexible and foldable Li–O2 battery based on paper-ink cathode, Adv. Mater., 2015, 27, 8095–8101 CrossRef CAS.
- D. Lee, H. Lee, O. Gwon, O. Kwon, H. Y. Jeong, G. Kim and S. Y. Lee, Monolithic heteronanomat paper air cathodes toward origami-foldable/rechargeable Zn–air batteries, J. Mater. Chem. A, 2019, 7, 24231–24238 RSC.
- P. Sundriyal and S. Bhattacharya, Textile-based supercapacitors for flexible and wearable electronic applications, Sci. Rep., 2020, 10, 1–5 CrossRef.
- Y. Ma, Q. Wang, X. Liang, D. Zhang and M. Miao, Wearable supercapacitors based on conductive cotton yarns, J. Mater. Sci., 2018, 53, 14586–14597 CrossRef CAS.
- Q. C. Liu, T. Liu, D. P. Liu, Z. J. Li, X. B. Zhang and Y. Zhang, A flexible and wearable lithium–oxygen battery with record energy density achieved by the interlaced architecture inspired by bamboo slips, Adv. Mater., 2016, 28, 8413–8418 CrossRef CAS.
- D. S. Hecht, L. Hu and G. Grüner, Electronic properties of carbon nanotube/fabric composites, Curr. Appl. Phys., 2007, 7, 60–63 CrossRef.
- A. Vališevskis, U. Briedis, Ž. Juchnevičienė, M. Jucienė and M. Carvalho, Design improvement of flexible textile aluminium-air battery, J. Text. Inst., 2020, 111, 985–990 CrossRef.
- W. Pan, Y. Wang, H. Y. Kwok and D. Y. Leung, Aluminum-air battery with cotton substrate: controlling the discharge capacity by electrolyte pre-deposition, Green Energy Environ., 2023, 78, 757–766 CrossRef.
- X. Lin, Q. Kang, Z. Zhang, R. Liu, Y. Li, Z. Huang, X. Feng, Y. Ma and W. Huang, Industrially weavable metal/cotton yarn air electrodes for highly flexible and stable wire-shaped Li–O2 batteries, J. Mater. Chem. A, 2017, 5, 3638–3644 RSC.
- Q. C. Liu, J. J. Xu, D. Xu and X. B. Zhang, Flexible lithium–oxygen battery based on a recoverable cathode, Nat. Commun., 2015, 6, 1–8 Search PubMed.
- Y. Xiang, T. Yang, K. Tong, T. Fu, Y. Tang, F. Liu, Z. Xiong, Y. Si and C. Guo, Constructing flexible and self-standing electrocatalyst for oxygen reduction reaction by in situ doping nitrogen atoms into carbon cloth, Appl. Surf. Sci., 2020, 523, 146424 CrossRef CAS.
- R. Manjunatha, J. Yuan, L. Hongwei, S. Q. Deng, E. R. Ezeigwe, Y. Zuo, L. Dong, A. Li, W. Yan, F. Zhang and J. Zhang, Facile carbon cloth activation strategy to boost oxygen reduction reaction performance for flexible zinc-air battery application, Carbon Energy, 2022, 4, 762–775 CrossRef CAS.
- P. Tan, B. Chen, H. Xu, W. Cai, W. He and M. Ni, Growth of Al and Co co-doped NiO nanosheets on carbon cloth as the air electrode for Zn-air batteries with high cycling stability, Electrochim. Acta, 2018, 290, 21–29 CrossRef CAS.
- J. Fu, D. U. Lee, F. M. Hassan, L. Yang, Z. Bai, M. G. Park and Z. Chen, Flexible high-energy polymer-electrolyte-based rechargeable zinc–air batteries, Adv. Mater., 2015, 27, 5617–5622 CrossRef CAS PubMed.
- S. Chen, X. Shu, H. Wang and J. Zhang, Thermally driven phase transition of manganese oxide on carbon cloth for enhancing the performance of flexible all-solid-state zinc–air batteries, J. Mater. Chem. A, 2019, 7, 19719–19727 RSC.
- H. Cao, S. Si, X. Xu, J. Li and C. Lan, A novel flexible aluminum//polyaniline air battery, Int. J. Electrochem. Sci., 2019, 14, 9796–9804 CrossRef CAS.
- C. Wang, Z. Guo, S. Zhang, G. Chen, S. Dong and G. Cui, Constructing in-situ polymerized electrolyte on lithiophilic anode for high-performance lithium–air batteries operating in ambient conditions, Energy Storage Mater., 2021, 43, 221–228 CrossRef.
- U. Bhardwaj, A. Sharma, V. Gupta, K. M. Batoo, S. Hussain and H. S. Kushwaha, High energy storage capabilities of CaCu3Ti4O12 for paper-based zinc–air battery, Sci. Rep., 2022, 12, 3999 CrossRef CAS PubMed.
- S. Qu, Z. Song, J. Liu, Y. Li, Y. Kou, C. Ma, X. Han, Y. Deng, N. Zhao, W. Hu and C. Zhong, Electrochemical approach to prepare integrated air electrodes for highly stretchable zinc-air battery array with tunable output voltage and current for wearable electronics, Nano Energy, 2017, 39, 101–110 CrossRef CAS.
- X. Zheng, X. Cao, K. Zeng, Z. Sun, J. Yan, X. Li, C. Jin, X. Chen and R. Yang, Cotton pad-derived large-area 3D N-doped graphene-like full carbon cathode with an O-rich functional group for flexible all solid Zn–air batteries, J. Mater. Chem. A, 2020, 8, 11202–11209 RSC.
- J. Yu, Z. Chen, L. Zhong, W. Yang, T. Li, Y. Huang, Y. Wei and X. Peng, Bamboo fiber-derived bifunctional electrocatalyst for rechargeable Zn-Air batteries, ChemElectroChem, 2023, 10, 716–721 Search PubMed.
- Y. Xue, H. Zhou, K. Wang, H. Zhu, L. Zhuang, Z. Xu and H. He, Tailoring of an ultralow temperature adaptive cellulose nanofiber-based flexible zinc-air battery with long cycle life, J. Mater. Chem. A, 2022, 10, 22730–22741 RSC.
- X. Chen, B. Liu, C. Zhong, Z. Liu, J. Liu, L. Ma, Y. Deng, X. Han, T. Wu, W. Hu and J. Lu, Ultrathin Co3O4 layers with large contact area on carbon fibers as high-performance electrode for flexible zinc-air battery integrated with flexible display, Adv. Energy Mater., 2017, 7, 1700779 CrossRef.
- A. Agrawal and C. M. Hussain, 3D Printed Hydrogel for Diverse Applications: A review, Gels, 2023, 9, 960 CAS.
- K. Gniotek and I. Krucinska, The basic problems of textronics, Fibres Text. East. Eur., 2004, 12, 13–16 CAS.
| This journal is © The Royal Society of Chemistry 2024 |