Yeast bio-batteries†
Frank N.
Crespilho
 *abd,
Ricardo
Brito-Pereira
bc,
Rita
Policia
bc,
Nelson
Pereira
bc,
Graziela C.
Sedenho
*abd,
Ricardo
Brito-Pereira
bc,
Rita
Policia
bc,
Nelson
Pereira
bc,
Graziela C.
Sedenho
 a,
Carlos M.
Costa
a,
Carlos M.
Costa
 bc and
Senentxu
Lanceros-Méndez
bcde
bc and
Senentxu
Lanceros-Méndez
bcde
aSão Carlos Institute of Chemistry, University of São Paulo (USP), 13560-970, São Carlos, Brazil. E-mail: frankcrespilho@iqsc.usp.br
bPhysics Centre of Minho and Porto Universities (CF-UM-UP), Laboratory of Physics for Materials and Emergent Technologies, LapMET, University of Minho, 4710-057 Braga, Portugal
cInstitute of Science and Innovation for Bio-Sustainability (IB-S), University of Minho, 4710-053, Braga, Portugal
dBCMaterials, Basque Center for Materials, Applications and Nanostructures, UPV/EHU Science Park, 48940 Leioa, Spain
eIKERBASQUE, Basque Foundation for Science, Bilbao, 48009, Spain
First published on 1st October 2024
Abstract
In this work, we present the development of a fully rechargeable bio-battery, powered by Saccharomyces cerevisiae and utilizing recyclable PET carbon-based electrodes. Through the integration of yeast with the iota-carrageenan hydrogel and potassium ferricyanide as a redox mediator, the bio-battery consistently delivers 450 mV with excellent cyclability. This eco-friendly approach demonstrates great potential for advancing sustainable energy solutions, particularly in powering low-energy applications such as biomedical devices. Ongoing advancements in membrane design are expected to significantly boost the long-term performance and operational stability of this system, further solidifying its applicability in real-world scenarios.
Catalysis by microorganisms to decompose biomass is an exergonic process that plays an important role in bioenergy production. Some microorganisms, particularly Saccharomyces cerevisiae, have demonstrated promising electrically conductive properties, making them viable candidates for bio-batteries.1 Although Saccharomyces cerevisiae has been successfully utilized in biofuel cells,2–5 its potential in fully chargeable batteries has yet to be explored. In contrast to biofuel cells, which directly oxidize organic substrates using microorganisms to generate electrical energy from fuels like glucose, Saccharomyces cerevisiae-based bio-batteries harness the yeast's metabolic activity to produce extracellular polymeric substances (EPSs),6 serving as the anode material at low potentials for exergonic energy delivery. Many components of EPSs, such as proteins, polysaccharides, and certain redox-active molecules, can exhibit conductive properties.7 However, the electrical conductivity of EPSs remains largely unexplored in traditional biofilm studies.8 Recent research has shown that EPSs contain electrochemically active compounds, such as flavins and c-type cytochromes, which act as mediators for electron transfer.7 These compounds allow EPS-encased cells to transport extracellular electrons to acceptors or from donors through an electron hopping mechanism. The conductive properties of EPSs, along with their role in biofilm formation and stability on the anode surface, are critical factors for the enhanced performance observed in bio-batteries.9
The transition from microbial MFCs to bio-batteries marks a significant advancement, as bio-batteries not only “convert” electricity but also store it, providing a more reliable and efficient power source for a variety of applications. What distinguishes bio-batteries is their capacity to store and release energy, making them far more suitable for practical applications requiring a stable power supply, such as small biomedical devices.10 Bio-batteries, like the one introduced in this study, target energy densities in the range of 10–20 W h kg−1 and power densities of approximately 10–15 mW kg−1, making them ideal for low-power devices such as implantable sensors and environmental monitoring systems.10 Despite the feasibility of MFCs at the laboratory scale, they face challenges such as low power output and limited long-term stability. The transition to bio-batteries, particularly those utilizing Saccharomyces cerevisiae, represents a significant advancement in addressing these limitations by combining both energy generation and storage within a single system.
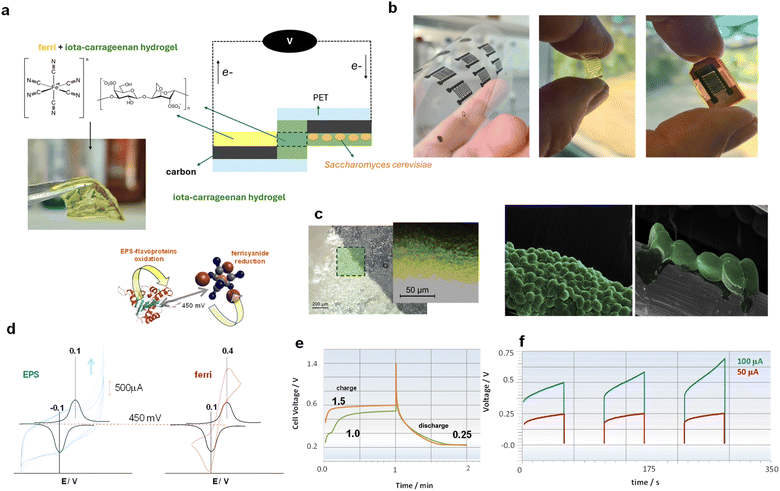
In the present study, we demonstrate the transition from a purely biological system to a functional battery configuration, a task that demands a combination of microbial metabolic pathways and precise engineering of electrochemical materials and interfaces to optimize electron transfer efficiency (Fig. 1a). Our approach features the utilization of Saccharomyces cerevisiae on screen printed carbon-based electrodes, using a recyclable polyethylene terephthalate (PET) substrate (Fig. 1b), to stimulate the production of EPSs (Fig. 1c). These EPSs serve as the anode material alongside an iota-carrageenan hydrogel (which, in previous studies, typically exhibit ionic conductivities around 10−6 S cm−1, depending on the preparation method and hydration level11). Moreover, by integrating potassium ferricyanide within the iota-carrageenan hydrogel in the cathode, we engineered a bio-battery capable of yielding a voltage output of 450 mV.
For the preparation of the bio-batteries, iota-carrageenan hydrogel membranes were initially prepared by soaking them in a 0.1 mol L−1 phosphate buffer pH 7.2 for 10 minutes. The anodic and cathodic compartments consisted of two hydrogel membranes each. The cathodic hydrogel was immersed in a saturated solution of potassium ferricyanide. For the anode, a bioelectrode was obtained by incubating the electrode in a bioreactor containing Saccharomyces cerevisiae previously activated with glucose. The membranes made of iota-carrageenan hydrogel containing EPSs produced by the yeast cells or ferricyanide were then separated by a separator. Three types of membrane separators were tested: a home-made membrane of 210 micrometer thickness made of carrageenan, a home-made fibroin membrane obtained by electrospinning, and a sandwich-shaped membrane consisting of two commercial Whatman membranes filled with iota-carrageenan membrane (410 microns). In all three cases, the battery operated adequately, but when a carrageenan membrane conjugated with Whatman was used, a comparatively low crossover rate was observed. The current collectors were prepared by silk-screening carbon ink onto recycled PET. Initial studies of crossover showed that the battery could be used for between 2 and 3 hours, and due to this, all our experiments were limited to 2 hours. After this time, a new bio-battery was produced and used in reproducibility studies. Since our objective is to demonstrate for the first time that it is possible to charge and discharge a battery using EPSs, we are not concerned with crossover occurring after hours of use. We anticipate that other membranes and configurations will need to be incorporated to allow for a greater number of cycles.
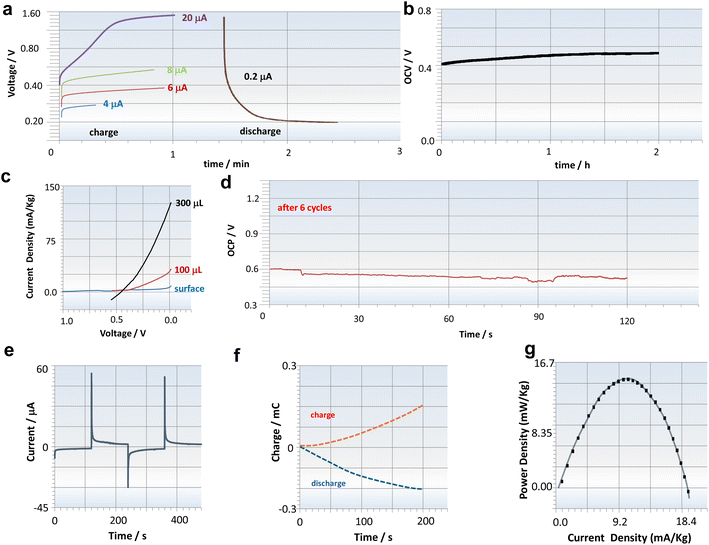
Fig. 1d illustrates the two half-cell reactions, with the onset-wave oxidation potential of EPSs at −0.100 V and reduction potential of ferricyanide at +0.350 V, resulting in a theoretical open-circuit voltage (OCV) expectation of 450 mV. Although these results are consistent with the literature for each semi-reaction,1 this is the first reported experiment of pairing these compounds for application in a battery. For the initial charge–discharge tests, currents of 1 μA (green) and 1.5 μA (orange) were employed, and their corresponding discharge curves, which overlap, are illustrated in Fig. 1e. After 1 minute of charging, the battery was discharged, and the same voltage profile was observed in both cases, indicating the susceptibility for battery cycling beyond its use as a primary bio-battery. Additionally, the possibility of using higher currents for charging was tested, such as 50 and 100 μA, approximately 100 times higher than the previous current. While it was feasible to charge the battery with these currents, a distinctive profile began to emerge in the voltage elevation. Since the anodic compartment is obtained through EPS formation on the electrode surface, the charging capacity likely reaches its maximum. Upon discharging the battery with the same current values, a rapid decay is observed, confirming the charging limit provided by the anode compartment (Fig. 1f). To verify this hypothesis, intermediate current values were utilized, enabling the observation of a perfect alignment of results. Values between 4 and 20 mA maintained proportionalities in the obtained voltage. When low currents were used to discharge the bio-battery, for example, 0.2 μA, a diffusion profile was observed, confirming that the reaction is limited by the EPS (Fig. 2a). It's worth noting that even after the final cycles of charge and discharge, the bio-battery maintains its OCV relatively stable. The OCV value achieved was at 100% of the theoretical value when the hydrogels were paired (Fig. 2b).
Considering that EPSs can be optimized regarding their quantity in the bio-battery, it was also observed that the amount of EPSs influences the current. Adding EPSs near the anodic compartment increased the current density (Fig. 2c). These findings highlight the dynamic nature of the influence of EPSs on bio-battery performance, offering insights into strategies for enhancing the overall efficiency and stability in long-term operation. Experiments conducted after increasing the quantity of EPSs in the compartment also show that the battery maintains a stable OCV (Fig. 2d). A charge and discharge of 0.3mC were achieved, demonstrating a state of charge of 99%, indicating excellent stability (Fig. 1e and f). The transient mode density obtained from the polarization curve shows a maximum power density of approximately 15 mW kg−1 (Fig. 1g), which is notably higher, for example, when compared to a microbial fuel cell.4,5,12–14
The preparation process, utilizing recyclable materials such as PET carbon-based electrodes and hydrogel membranes, shows the feasibility of sustainable manufacturing practices in bio-battery fabrication. With the theoretical open-circuit voltage expectation of 450 mV closely matching experimental results, our findings validate the efficacy of the engineered system.
The transient mode density analysis revealing a maximum power density of approximately 15 mW kg−1 highlights the superior performance of our bio-battery compared to traditional microbial fuel cells. A fundamental aspect of the bio-battery design lies in the structure of the hydrogel electrolyte containing carrageenan. Carrageenan, a polysaccharide derived from seaweed,15 offers unique properties that make it an ideal candidate for use in hydrogel electrolytes. The structure of the hydrogel is important for facilitating ion transport within the battery while maintaining structural integrity and stability. Carrageenan hydrogels exhibit a porous network structure,15 providing ample space for the diffusion of ions necessary for the electrochemical reactions occurring within the battery. This porous structure allows for efficient ion transport, facilitating the movement of ions between the anode and cathode compartments of the bio-battery.16,17 Furthermore, carrageenan hydrogels possess excellent mechanical properties, offering the necessary support and stability to the overall battery structure. The hydrogel can be readily manipulated using forceps and repositioned within each battery compartment to achieve optimal placement (see Fig. 1a). This ensures that the hydrogel electrolyte can withstand the mechanical stresses experienced during bio-battery operation without compromising its functionality. Integrating ferricyanide within the carrageenan hydrogel further enhances the electrochemical performance of the bio-battery. The presence of ferricyanide ions within the hydrogel facilitates redox reactions at the electrode–electrolyte interface. A long-term stability assessment conducted over a period of 2 hours revealed that the system maintained itself without significant losses during this time (Fig. 2b). This timeframe is necessary, for instance, to meet the energy demand of bio-medical devices such as endoscopic pills.18,19
To date, two bio-batteries have employed bacteria as biological redox agents. One study developed a micro-scale bio-battery using Shewanella oneidensis MR-1, featuring horizontally aligned anodic and cathodic chambers connected by a salt bridge.20 This system achieved a power density of 0.33 mW cm−3 and a current density of 45 μA cm−2 after 96 hours. Another design utilized a combination of three microbial species (Shewanella oneidensis MR-1, Bacillus subtilis, and Synechocystis sp. PCC 6803) in a typical micro fuel cell configuration, yielding a maximum power density of 42 μW cm−2 and a current density of 220 μA cm−2.21 While these bacterial bio-batteries present valuable findings, the yeast-based bio-battery developed in this work advances the concept of sustainability by leveraging Saccharomyces cerevisiae and its EPS. Unlike many systems that rely on synthetic or non-renewable materials, this approach prioritizes biocompatibility and environmental stewardship, aligning with broader goals of sustainability.
However, achieving long-term stability poses significant challenges. Issues such as biofilm detachment from electrodes, loss of EPSs, and hydrogel degradation can undermine the battery's efficiency over time. Addressing these challenges could involve reinforcing the hydrogel's physical properties and employing membrane separators—particularly advanced microporous membranes—to prevent cross-linking between the anode and cathode. Additionally, exploring more durable electrode materials and maintaining steady EPS production could extend the battery's operational life, making it more viable for practical applications.
Conclusions
Saccharomyces cerevisiae demonstrates promising electrically conductive properties, making it a viable candidate for bio-batteries in low-power applications such as biomedical devices, environmental sensors, and portable electronics. The successful implementation of S. cerevisiae-based bio-batteries, however, requires a comprehensive understanding of its microbial metabolic pathways, along with precise engineering of electrochemical materials and interfaces to optimize electron transfer efficiency.Utilizing Saccharomyces cerevisiae on recyclable 3D-printed PET carbon-based electrodes to produce EPSs represents a novel approach to bio-battery development. Integration of potassium ferricyanide within the iota-carrageenan hydrogel, alongside Saccharomyces cerevisiae on PET carbon-based electrodes, yielded a bio-battery with a voltage output of 450 mV and demonstrated high-performance cyclability. With future implementations of membranes more resistant to crossover, it will be possible to increase long-term stability and broaden its applications. The use of environmentally friendly materials such as iota-carrageenan and Saccharomyces cerevisiae, combined with recyclable PET platforms, highlights the potential for sustainable and eco-friendly bio-batteries.
Future research should focus on integrating this bio-battery into existing energy systems, either independently or in hybrid setups with traditional power sources. Scaling up the design, improving materials, and enhancing the stability could enable the transition from the lab prototype to real-world applications. Additionally, exploring advanced cathode materials like organic compounds, quinones, or organic frameworks with customizable redox properties could further enhance the performance, paving the way for a new generation of sustainable energy storage and low-power devices.
Data availability
The datasets generated and/or analyzed during the current study are available from the authors upon request.Author contributions
F. N. C. and S. L. M.: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, supervision, validation, visualization, writing – original draft, review, and editing. R. B. P., R. P., N. P., G. C. S., and C. M. C.: data curation, formal analysis, investigation, methodology, validation, writing – original draft, review, and editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank the São Paulo Research Foundation (FAPESP) grant numbers 20-0904796-8, 22/09164-5, 8/22214-6, and 23/13288-4 and Fundação para a Ciência e Tecnologia (FCT) for financial support under the framework of Strategic Funding UID/FIS/04650/2020 and UID/QUI/00686/2020 and project 10.54499/2022.03931.PTDC. The authors also thank the FCT for financial support under the FCT investigator contract 2020.04028.CEECIND (DOI: 10.54499/2020.04028.CEECIND/CP1600/CT0018) (C.M.C.). The authors are thankful for the funding under the project NGS-New Generation Storage, C644936001-00000045, supported by IAPMEI (Portugal) with funding from the European Union NextGenerationEU (PRR). This study forms part of the Advanced Materials program and was supported by MCIN with funding from European Union NextGenerationEU (PRTR-C17.I1) and by the Basque Government under the IKUR program.References
- G. Sedenho, I. Modenez, G. Mendes and F. Crespilho, The role of extracellular polymeric substance matrix on Saccharomyces cerevisiae bioelectricity, Electrochim. Acta, 2021, 393, 139080 CrossRef CAS.
- K. Rabaey and R. A. Rozendal, Microbial electrosynthesis — revisiting the electrical route for microbial production, Nat. Rev. Microbiol., 2010, 8(10), 706–716 CrossRef CAS PubMed.
- R. Santomartino, N. J. H. Averesch, M. Bhuiyan, C. S. Cockell, J. Colangelo and Y. Gumulya, et al., Toward sustainable space exploration: a roadmap for harnessing the power of microorganisms, Nat. Commun., 2023, 14(1), 1391 CrossRef CAS PubMed.
- K. C. Pagnoncelli, A. R. Pereira, G. C. Sedenho, T. Bertaglia and F. N. Crespilho, Ethanol generation, oxidation and energy production in a cooperative bioelectrochemical system, Bioelectrochemistry, 2018, 122, 11–25 CrossRef CAS PubMed.
- M. Verma and V. Mishra, Recent trends in upgrading the performance of yeast as electrode biocatalyst in microbial fuel cells, Chemosphere, 2021, 284, 131383 CrossRef CAS PubMed.
- T. B. Reynolds and G. R. Fink, Bakers' Yeast, a Model for Fungal Biofilm Formation, Science, 2001, 291(5505), 878–881 CrossRef CAS PubMed.
- Y. Xiao, E. Zhang, J. Zhang, Y. Dai, Z. Yang and H. E. M. Christensen, et al., Extracellular polymeric substances are transient media for microbial extracellular electron transfer, Sci. Adv., 2017, 3(7), e1700623 CrossRef PubMed.
- A. Borole, G. Reguera, B. Ringeisen, Z. Wang, Y. Feng and B. Kim, Electroactive Biofilms: Current Status and Future Research Needs, Energy Environ. Sci., 2011, 4, 4813–4834 RSC.
- H.-C. Flemming, J. Wingender, U. Szewzyk, P. Steinberg, S. A. Rice and S. Kjelleberg, Biofilms: an emergent form of bacterial life, Nat. Rev. Microbiol., 2016, 14(9), 563–575 CrossRef CAS PubMed.
- Y. Gao, J. H. Cho, J. Ryu and S. Choi, A scalable yarn-based biobattery for biochemical energy harvesting in smart textiles, Nano Energy, 2020, 74, 104897 CrossRef CAS.
- J. W. Y. Liew, K. S. Loh, A. Ahmad, K. L. Lim and W. R. Wan Daud, Synthesis and characterization of modified κ-carrageenan for enhanced proton conductivity as polymer electrolyte membrane, PLoS One, 2017, 12, 1–15 CrossRef PubMed.
- D. Nath and M. M. Ghangrekar, Plant secondary metabolites induced electron flux in microbial fuel cell: investigation from laboratory-to-field scale, Sci. Rep., 2020, 10(1), 17185 CrossRef CAS PubMed.
- P. Pushkar and A. Kumar Mungray, Electrochemical evaluation of lab-scale chamber benthic microbial fuel cell, Sustain. Energy Technol. Assessments, 2021, 48, 101655 CrossRef.
- S. Babanova, J. Jones, S. Phadke, M. Lu, C. Angulo and J. Garcia, et al., Continuous flow, large-scale, microbial fuel cell system for the sustained treatment of swine waste, Water Environ. Res., 2020, 92(1), 60–72 CrossRef CAS PubMed.
- Y.-Q. Wang, Y.-T. Han, J.-N. Yan, Y.-N. Du, X.-Y. Jiang and H.-T. Wu, Gel properties and network structure of the hydrogel constructed by iota-carrageenan and Ala-Lys dipeptide, Int. J. Biol. Macromol., 2021, 182, 244–251 CrossRef CAS PubMed.
- F. N. Crespilho, G. C. Sedenho, D. De Porcellinis, E. Kerr, S. Granados-Focil and R. G. Gordon, et al., Non-corrosive, low-toxicity gel-based microbattery from organic and organometallic molecules, J. Mater. Chem. A, 2019, 7(43), 24784–24787 RSC.
- R. M. Iost, F. N. Crespilho, K. Kern and K. Balasubramanian, A primary battery-on-a-chip using monolayer graphene, Nanotechnology, 2016, 27(29), 29LT01 CrossRef PubMed.
- G. Iddan, G. Meron, A. Glukhovsky and P. Swain, Wireless capsule endoscopy, Nature, 2000, 405(6785), 417 CrossRef CAS PubMed.
- S. Sharma, K. B. Ramadi, N. H. Poole, S. S. Srinivasan, K. Ishida and J. Kuosmanen, et al., Location-aware ingestible microdevices for wireless monitoring of gastrointestinal dynamics, Nat. Electron., 2023, 6(3), 242–256 CrossRef PubMed.
- M. Mohammadifar and S. Choi, A solid phase bacteria-powered biobattery for low-power, low-cost, internet of Disposable Things, J. Power Sources, 2019, 429, 105–110 CrossRef CAS.
- A. Elhadad, L. Liu and S. Choi, Plug-and-play modular biobatteries with microbial consortia, J. Power Sources, 2022, 535, 231487 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4se00903g |
| This journal is © The Royal Society of Chemistry 2024 |