Open Access Article
Open Access ArticleSynthesis of biobased polyacetals: a review
Anna C. Renner
 ,
Sagar S. Thorat
,
Sagar S. Thorat
 and
Mukund P. Sibi
and
Mukund P. Sibi
 *
*
Department of Chemistry and Biochemistry, North Dakota State University, Fargo, North Dakota 58108-6050, USA. E-mail: mukund.sibi@ndsu.edu
First published on 18th October 2024
Abstract
The molecular structure of a polymer is a key determinant of the properties and thus potential applications of the bulk material. The presence of acetal functional groups in a polymer can impart advantageous characteristics such as reprocessability, degradability, and recyclability. Many biobased monomers contain functional groups amenable to acetal formation, and a variety of polyacetals have been synthesized from renewable starting materials. These polymers range from elastomers to rigid materials, with their diverse mechanical properties depending on both the acetal units and other structural features of the polymers. A partially biobased poly(acetal-ester) with the trade name Akestra is commercially available and can be used in food packaging applications. In this review, the synthesis and properties of biobased polyacetals are surveyed with an emphasis on the sustainability advantages offered by these materials. A brief overview of polythioacetals, the sulfur analogs of polyacetals, is also included.
Sustainability spotlightThe vast majority of plastics and other synthetic polymers are derived from petroleum, a nonrenewable resource. Moreover, many cannot be readily recycled or degraded, and their use results in large amounts of waste. The development of new, more sustainable polymers holds promise for addressing these sustainability challenges. Polyacetals are a class of polymers that can undergo recycling and degradation, which may be leveraged to reduce plastic waste. Numerous polyacetals have been synthesized from renewable, biomass-derived monomers. This review provides an up-to-date overview of biobased polyacetals, supporting the further development and use of these polymers. In this way, this work aligns with the United Nations' Sustainable Development Goals, especially Goal 12, Responsible Consumption and Production. |
1. Introduction
As a class of materials, synthetic polymers are ubiquitous in industrial and consumer products, offering a wide range of material properties that can be tailored for different applications. However, it remains challenging to produce and use these materials in a sustainable and environmentally benign manner. Most synthetic polymers currently produced are derived from petroleum, a nonrenewable resource.1 Not all polymers can be recycled, and only a portion of recyclable plastics are actually recycled in practice.2–4 Plastic waste has become a major global problem: vast quantities have been released into the environment,5,6 and most synthetic polymers do not degrade readily in water and soil.Numerous approaches can be taken to address the sustainability challenges associated with synthetic polymers. These include the use of biomass instead of petrochemicals for polymer synthesis7 as well as the development and use of polymers that can be more readily recycled or degraded.8 In both of these areas, chemical innovations are necessary to access new polymers with desirable properties and greater sustainability.
The bulk properties, recyclability, and degradability of a polymer depend on the chemical structure of the material, including the structures of repeating units and the presence or absence of crosslinks between polymer chains. In this review, we cover polyacetals, polymers with repeating units that contain the acetal functional group. Due to the presence of acetal linkages in their structures, polyacetals offer not only potentially valuable material properties but also sustainability advantages including recyclability and degradability. Like other classes of polymers—polyurethanes, polyesters, polyamides, etc.—polyacetals can be derived either from petroleum or biomass. We highlight biomass-derived polyacetals in this review (Fig. 1).
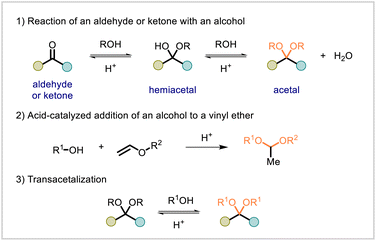
The acetal functional group can be formed via the condensation of a carbonyl compound (aldehyde or ketone) with an alcohol, typically catalyzed by a Brønsted or Lewis acid. Another synthetic approach to acetals is the acid-catalyzed addition of an alcohol to a vinyl ether. Acetals can undergo transacetalization reactions with alcohols, resulting in a net exchange of the alkoxy substituents of the acetal group. All of these synthetic approaches to acetals (Scheme 1) find use in polymer synthesis. Polyacetalization or polytransacetalization of difunctional or polyfunctional monomers yields a polyacetal.
As alternatives to polyacetalization or polytransacetalization, other polymerization reactions can be used to construct polyacetals. Monomers that already contain acetal units can undergo polymerizations involving reactions of other functional groups present in the monomer (for example, transesterifications, thiol–ene reactions, etc.). The resulting polymer features new linkages formed in the polymerization reaction but retains the acetal groups from the constituent monomers.
Acetal formation is a reversible process. While acetals are generally stable under alkaline conditions, they are susceptible to hydrolysis or alcoholysis under acidic conditions. In the presence of water, an acetal can be cleaved to afford the corresponding carbonyl compound and alcohol; in the presence of an alcohol, transacetalization with the alcohol can occur. The susceptibility of the acetal unit to hydrolysis or alcoholysis can allow acetal-based polymers to be degraded to small-molecule units. Degradation of the polymer can potentially be leveraged to reduce polymeric waste. Moreover, if the small-molecule degradation products can be recovered and reused, chemical recycling of the polymer may be possible.9–11
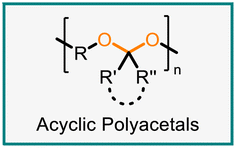
Polyacetals can contain linear (acyclic) or cyclic acetal units (Fig. 2A). Cyclic acetals, in which the acetal is formally derived from a carbonyl compound and a diol, can be more stable to hydrolysis than their acyclic counterparts, in particular if the cyclic acetal is a five- or six-membered ring.12,13 The stability of an acetal structure has potential implications for the hydrolytic stability of polymers containing the acetal linkage. Additionally, the incorporation of rigid, cyclic units may affect the mechanical or thermal properties of the material.
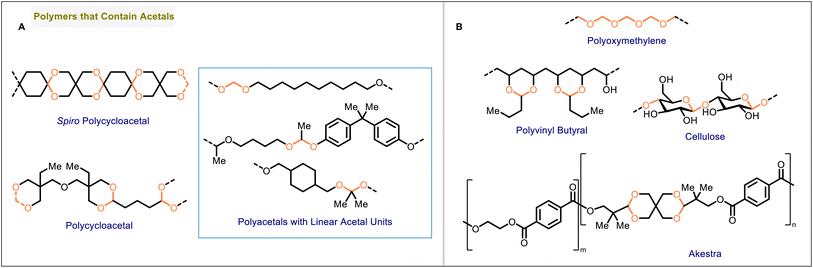 | ||
| Fig. 2 (A) General structures of polyacetals with cyclic and linear acetal units. (B) Structures of cellulose, polyoxymethylene, polyvinyl butyral, and Akestra. | ||
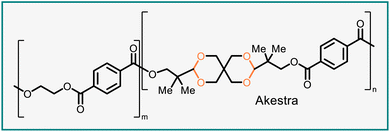
The suitability of a polymer for potential applications is determined by the properties of the material, encompassing chemical, physical, thermal, mechanical, and optical properties. These properties vary greatly among polyacetals, depending on functional groups present in the polymer, and can be potentially tuned by modifying the structure of the polymer.14,15 Cellulose (Fig. 2B) is a naturally occurring polyacetal that is utilized in many different products.16 Two synthetic, acetal-containing polymers are currently widely established in commercial markets: polyoxymethylene and polyvinyl butyral (Fig. 2B). Polyoxymethylene is a semicrystalline thermoplastic that offers high strength, rigidity, and dimensional stability as well as low friction.17 Polyvinyl butyral, produced through the acetalization of polyvinyl alcohol with butyraldehyde, is a transparent thermoplastic with suitable properties for the formation of adhesives and films; a major application is in the construction of safety glass.18 In addition to polyoxymethylene and polyvinyl butyral, another polyacetal has become available under the trade name Akestra. Akestra19–22 (Fig. 2B) is structurally analogous to poly(ethylene terephthalate) (PET)23 and can be subjected to similar processing methods, but it exhibits higher glass transition temperatures than PET due to the presence of spirocyclic acetal units in the polymer backbone.
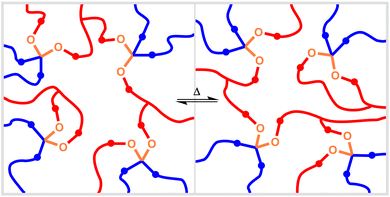
The acetal functional group has been incorporated into a wide range of polymer types encompassing both thermoplastics and thermosets. Notably, the reversibility of acetal formation has enabled the development of acetal-based covalent adaptable networks (CANs). Like thermosets, CANs (also known as dynamic covalent networks) are crosslinked polymer networks. However, unlike conventional thermosets, these crosslinks can form reversibly and undergo bond exchanges (Fig. 3), allowing the material to respond to stress and potentially undergo reprocessing, similar to a thermoplastic.24,25 Due to their intermediate properties relative to traditional thermosets and thermoplastics, CANs may offer performance characteristics like those of thermosets but enable reprocessing like thermoplastics.24,25
In general, the incorporation of acetal functional groups into a material can provide advantageous properties including reprocessability, degradability, and recyclability, which are not always attainable through the sole use of other structural units. Polyacetals may be mechanically recycled and/or chemically degraded, and it may be possible to recover and recycle monomers following degradation of the polymer. With respect to sustainability, these are attractive prospects for the end-of-life stage in the lifecycle of a material. At the production stage in their lifecycle, on the other hand, polyacetals can be constructed in a more sustainable manner through the use of renewable chemical building blocks.
Renewable monomers can be derived from a variety of biomass sources including cellulose, hemicellulose, lignin, and plant oils.26–28 Compared with petroleum, biomass sources tend to have higher oxygen-to-carbon ratios,29 and many feedstock chemicals from biomass feature oxygen-containing functionalities, including alcohol and aldehyde groups potentially useful for acetal synthesis.30 Depending on their structure, biobased monomers may serve as replacements for petroleum-derived counterparts or as alternatives offering complementary or superior properties.31
Cellulose, hemicellulose, and lignin are natural polymers from plants. Importantly, they are highly abundant and can be obtained from nonedible biomass such as wood and agricultural waste.32 Cellulose and hemicellulose can be broken down into their monomeric constituents—monosaccharides—and converted to other carbohydrate derivatives including sugar alcohols, isohexides, levoglucosenone, levulinic acid, and biobased furans (e.g., 5-hydroxymethylfurfural, furfural, etc.).26,30,33 Depolymerization of lignin can provide aromatic compounds such as vanillin and syringaldehyde.34
Plant oils primarily consist of triglycerides, esters of glycerol and fatty acids. Both glycerol35 and fatty acid derivatives27,28,36 can be employed as monomers. Apart from lignocellulosic biomass and plant oils, other biobased starting materials include terpenes37–39 and organic acids such as lactic acid,40–42 tartaric acid,43 and succinic acid.30,44
In this review, we discuss polyacetals that are derived at least in part from biobased precursors. Polyacetals lacking crosslinks are covered first, followed by crosslinked polyacetals including thermoset- and CAN-type polymers (for a previous review on thermoplastic—non-crosslinked—polyacetals, we direct the reader to a 2019 review by Hufendiek et al.45). We focus on polymers that consist of a main chain containing acetal linkages. However, we also survey selected examples of polymers that lack acetal units in the polymer backbone but feature acetal-containing sidechains. A brief discussion of polythioacetals, the sulfur-based analogs of polyacetals, is also included. We highlight sustainability aspects of acetal-based materials and conclude with a summary and outlook on the development of sustainable acetal-based polymers.
2. Non-crosslinked polyacetals
Polymer properties are greatly affected by the presence or absence of crosslinks between polymer chains in the material. Non-crosslinked polymers are thermoplastics and can typically be melted and reprocessed. In contrast, many crosslinked polymers are thermosets and contain irreversible crosslinks that prevent changes in the topology of the polymer, making reprocessing difficult or impossible. Some polymers contain reversible crosslinks, but these also modify the properties of the material significantly with implications for the polymer's potential applications. Following this general division of polymers based on the existence of crosslinking, we separately discuss crosslinked and non-crosslinked polyacetals, beginning with those that lack crosslinking.2.1. Polymers with acyclic acetal units
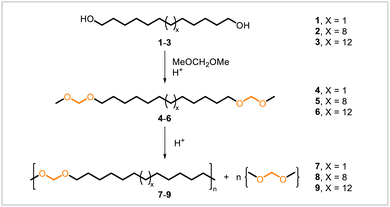
The simplest polyacetals are polymers consisting of a main chain containing only a series of repeating, linear acetal units (Fig. 4). The commercially available polymer polyoxymethylene (see Fig. 2) is one member of this class. Other, related polyacetals have been synthesized with longer alkyl chains between methylene acetal units. The synthesis of these polymers has been accomplished through the transacetalization of diacetal monomers derived from linear aliphatic diols.In 2012, Mecking and coworkers synthesized polyacetals via polytransacetalization of renewable diacetal monomers (Scheme 2).46 Diacetals 5 and 6 were prepared from the plant-oil-derived diols 2 and 3, respectively; for comparison, diacetal 4 was prepared from commercially available 1,12-dodecanediol (1). Monomers 4–6 were each converted to the corresponding polyacetals 7–9 through acid-catalyzed transacetalization at 80–100 °C, under reduced pressure to remove dimethoxymethane and favor formation of longer-chain polyacetals. Polyacetals 7–9 had molecular weights of 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–32
000–32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 by NMR (end-group analysis). The polyacetals were semicrystalline and had melting temperatures of 68–88 °C and crystallization temperatures of 43–68 °C. The melting and crystallization temperatures increased with increasing length of the methylene spacers in the polymers. The more crystalline and hydrophobic polyacetals with longer methylene sequences also exhibited greater stability to aqueous acid. All three polyacetals degraded in acidic solutions, but 8 and 9 did so at much slower rates than 7.
000 by NMR (end-group analysis). The polyacetals were semicrystalline and had melting temperatures of 68–88 °C and crystallization temperatures of 43–68 °C. The melting and crystallization temperatures increased with increasing length of the methylene spacers in the polymers. The more crystalline and hydrophobic polyacetals with longer methylene sequences also exhibited greater stability to aqueous acid. All three polyacetals degraded in acidic solutions, but 8 and 9 did so at much slower rates than 7.
The Miller group reported the synthesis of linear polyacetals through transacetalization and named the method “acetal metathesis polymerization” for its resemblance to acyclic diene metathesis polymerization.28 In initial work, 1,10-decanediol 10 (derivable from ricinoleic acid, a component of castor oil) was converted to diacetal 11 (Scheme 3). From diacetal 11, polydecylene acetal 12 was synthesized via polytransacetalization, which was conducted at 125–200 °C in the presence of p-toluenesulfonic acid as catalyst and with the application of vacuum to remove the volatile byproducts ethanol and diethoxymethane. Subsequently, a one-pot procedure was developed for the polymerization, in which a polyacetal (16, general structure) could be synthesized directly from a diol (13, general structure) and diethoxymethane. The obtained polyacetals could be resubjected to the reaction conditions to yield samples with greater degrees of polymerization. Using the developed polymerization conditions, a series of polyacetals was prepared from C5–C12 linear aliphatic diols. The polyacetals from the longer, C7–C12 diols had higher molecular weights (Mn = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700–38
700–38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700) than those from the shorter, C5 and C6 diols (Mn = 9700 and 8800 for the respective polyacetals), which tended to cyclize and polymerized less readily. Increasing alkyl chain length resulted in a linear increase in melting temperature of the corresponding polyacetals. Degradation studies on the polydecylene acetal 12 showed that the polymer degraded in acidic tetrahydrofuran solution.
700) than those from the shorter, C5 and C6 diols (Mn = 9700 and 8800 for the respective polyacetals), which tended to cyclize and polymerized less readily. Increasing alkyl chain length resulted in a linear increase in melting temperature of the corresponding polyacetals. Degradation studies on the polydecylene acetal 12 showed that the polymer degraded in acidic tetrahydrofuran solution.
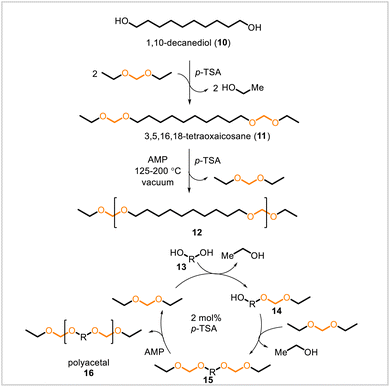 | ||
| Scheme 3 Acetal metathesis polymerization of an isolated diacetal (11) or in a one-pot process from diol (13). | ||
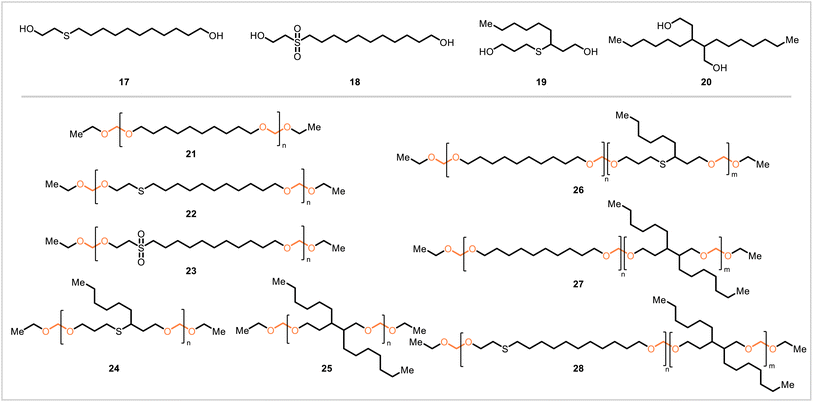
In work by Moreno et al., other polyacetals and copolyacetals were synthesized through acetal metathesis polymerization (Scheme 4).27 Linear diol monomers 17 and 18 were synthesized from 10-undecenoic acid, and branched diol monomers 19 and 20 were prepared from heptanal (both 10-undecenoic acid and heptanal are derivable from the castor oil component ricinoleic acid47). Drawing from this collection of monomers as well as 1,10-decanediol (10, Scheme 3), several polymers (Mn = 8800–27,500) were prepared: linear polyacetals 21, 22, and 23; branched polyacetals 24 and 25; and branched copolyacetals 26, 27, and 28. With the exception of 27, the branched polymers were liquids at room temperature; the linear polymers and 27 had low melting points and were semicrystalline. Most of the polymers were exposed to acidic solution to evaluate their degradability, and all underwent degradation at comparable rates.
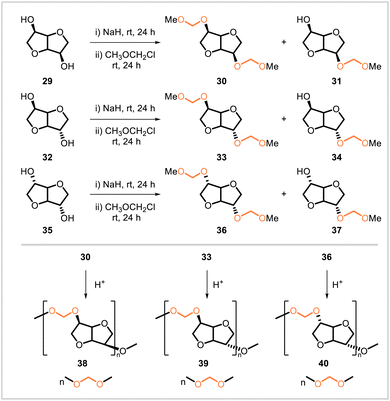
Other polyacetals containing methylene acetal units have been synthesized from isohexide monomers. Chikkali and coworkers synthesized diacetal monomer 30 from isomannide (29), 33 from isosorbide (32), and 36 from isoidide (35) (Scheme 5).48 These monomers were then converted to the corresponding polyacetals 38, 39, and 40 through acetal metathesis polymerization. Molecular weights (Mn, values obtained by gel permeation chromatography) up to 2300 for 38, 4400 for 39, and 2100 for 40 were obtained. The glass transition temperatures of the highest-molecular-weight samples of 38 and 40 were 66 °C and 52 °C, respectively. The melting temperatures of 38 (130 °C), 39 (117 °C), and 40 (156 °C) were over 70 °C higher than those of the corresponding linear C5 and C6 polyacetal analogs that had been previously reported.28
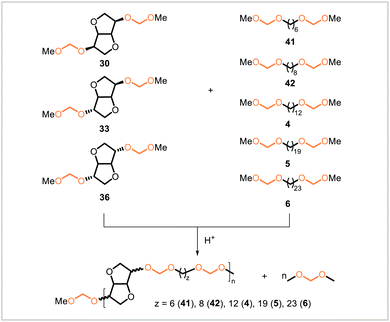
Chikkali and coworkers later reported acetal metathesis copolymerizations of isohexide- and diol-derived diacetal monomers (Scheme 6).49 Isohexide-derived diacetals 30, 33, and 36 were each polymerized under acidic conditions with the linear diol-derived diacetals 41, 42, 4, 5, and 6 to yield a total of 15 different copolymers, each containing units from one of the isohexide-derived monomers and one of the diol-derived monomers. Under acidic conditions, the polyacetals degraded, and faster degradation rates were observed for the polyacetals derived from shorter-chain diols.
Hammami et al. prepared isosorbide-derived polymer 45 via the reaction of isosorbide (32) with methylene chloride under basic conditions (Scheme 7).50 The molecular weight of the product was fairly low (Mn = 8630). In subsequent work, a sample of the primarily linear polyacetal (45) with Mn = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 was converted to a higher-molecular-weight, cyclic polyacetal (46) with Mn = 21
800 was converted to a higher-molecular-weight, cyclic polyacetal (46) with Mn = 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100.51 The glass transition temperature for product 46 was 65 °C, 10 °C higher than that of its lower-molecular-weight precursor.
100.51 The glass transition temperature for product 46 was 65 °C, 10 °C higher than that of its lower-molecular-weight precursor.
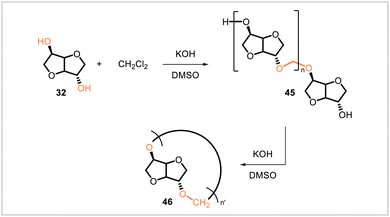 | ||
| Scheme 7 Synthesis of polyacetal 45 directly from isosorbide (32) and subsequent conversion to a higher-molecular-weight polymer (46). | ||
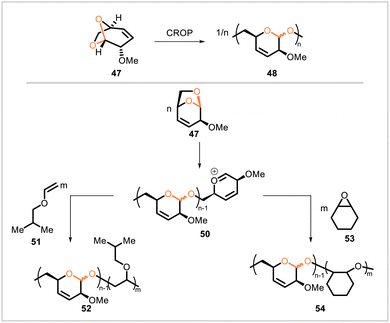
Other strategies for the synthesis of polyacetals involve ring-opening reactions of acetal-containing monomers. In the presence of boron trifluoride diethyl etherate in dichloromethane, the levoglucosenone-derived monomer 47 underwent cationic ring-opening polymerization to yield polyacetal 48 (Mn = 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800, Đ = 1.4) (Scheme 8).52 This polymer had a glass transition temperature of 35 °C and was thermally stable below 220 °C. The material was semicrystalline and had melting transitions at 40–120 °C. The double bonds underwent radical addition by methyl 3-mercaptopropionate as well as palladium-catalyzed hydrogenation. Monomer 47 also underwent photoinitiated cationic ring-opening polymerization under several different conditions, and through photopolymerization it was possible to synthesize block copolymers of 47 with cyclohexene oxide (53) or isobutyl vinyl ether (51).53
800, Đ = 1.4) (Scheme 8).52 This polymer had a glass transition temperature of 35 °C and was thermally stable below 220 °C. The material was semicrystalline and had melting transitions at 40–120 °C. The double bonds underwent radical addition by methyl 3-mercaptopropionate as well as palladium-catalyzed hydrogenation. Monomer 47 also underwent photoinitiated cationic ring-opening polymerization under several different conditions, and through photopolymerization it was possible to synthesize block copolymers of 47 with cyclohexene oxide (53) or isobutyl vinyl ether (51).53
Aoshima and coworkers studied cationic ring-opening copolymerizations of 2-methyl-1,3-dioxepane (55, formed in situ from 4-hydroxybutyl vinyl ether) with lactones (Scheme 9). Copolymerization of 55 with δ-valerolactone (56),54 ε-caprolactone (58),55 or γ-butyrolactone (60)56 in the presence of a sulfonic acid catalyst afforded the corresponding poly(acetal-ester) 57, 59, or 61, respectively. A polymerization–depolymerization equilibrium existed due to transacetalization under the reaction conditions, and the copolymer compositions could be altered by changing the monomer concentrations, increasing the reaction temperature, or applying vacuum. Upon an increase in temperature from 30 °C to 90 °C, copolymer 57 containing a 2.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of 55 to 56 underwent a depolymerization and scrambling process to yield a pseudo-alternating copolymer (with some homosequences of 56) containing a 1.1
1 ratio of 55 to 56 underwent a depolymerization and scrambling process to yield a pseudo-alternating copolymer (with some homosequences of 56) containing a 1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of 55 to 56.54 In the copolymerization of acetal 55 with lactone 58, the application of vacuum (which selectively removed the more volatile monomer 55) converted copolymers with a 3.7
1 ratio of 55 to 56.54 In the copolymerization of acetal 55 with lactone 58, the application of vacuum (which selectively removed the more volatile monomer 55) converted copolymers with a 3.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 ratio of 55 to 58 to alternating copolymer–like sequences with approximately equal numbers of acetal- and lactone-derived units.55 A similar phenomenon was also observed initially upon the application of vacuum in the copolymerization of acetal 55 with lactone 60; however, prolonged vacuuming or a temperature increase (from 30 °C to 90 °C) induced more extensive depolymerization, resulting in the formation of oligomers.56
1.1 ratio of 55 to 58 to alternating copolymer–like sequences with approximately equal numbers of acetal- and lactone-derived units.55 A similar phenomenon was also observed initially upon the application of vacuum in the copolymerization of acetal 55 with lactone 60; however, prolonged vacuuming or a temperature increase (from 30 °C to 90 °C) induced more extensive depolymerization, resulting in the formation of oligomers.56
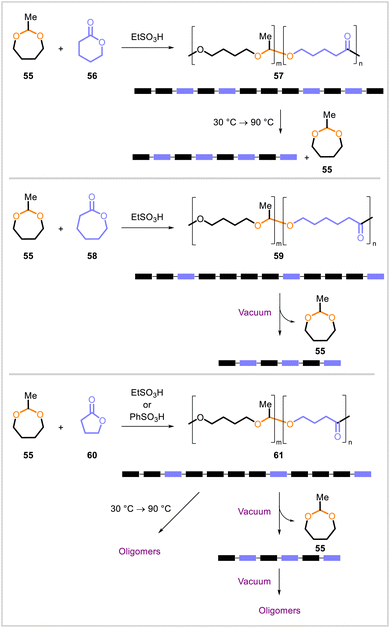 | ||
| Scheme 9 Cationic ring-opening copolymerizations of 2-methyl-1,3-dioxepane (55) with δ-valerolactone (56), ε-caprolactone (58), and γ-butyrolactone (60). | ||
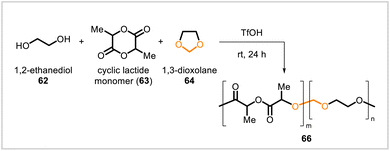
Poly(lactic acid), or PLA, is a renewable and biodegradable polymer with numerous applications.42 Copolymers of lactide 63 with 1,3-dioxolane (64) were synthesized by cationic ring-opening copolymerization in the presence of triflic acid and 1,2-ethanediol (62) (Scheme 10).40 The copolymers (66) had 7–27 mol% 1,3-dioxolane content, depending on the composition of the reaction mixture, and exhibited glass transition temperatures of 8–32 °C. Consistent with the presence of acetal linkages in the structures, the copolymers were hydrolyzed more rapidly than PLA under acidic conditions.
In related work, ABA-triblock copolymers were synthesized from lactide 63 and 1,3-dioxolane (64).57 First, a homopolymer of 64 with hydroxyl end groups was prepared. Subsequently, this homopolymer was used as a macroinitiator in the ring-opening polymerization of lactide 63, affording an ABA-triblock copolymer with polylactide A-blocks and an acid-degradable poly(1,3-dioxolane) B-block. The copolymers' composition and properties (including thermal properties and hydrophilicity) could be tuned by modifying the composition of the reaction mixture. An analogous copolymerization approach was also used to prepare ABA-triblock copolymers with A-blocks consisting of polycaprolactone instead of polylactide.
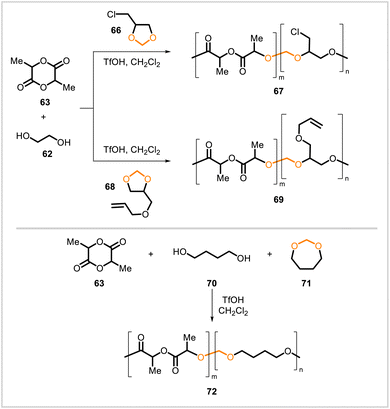
Degradable polylactides with pendent functional groups were obtained through cationic ring-opening copolymerizations of cyclic lactides with substituted cyclic acetals.41 Polymerization of lactide 63 with chloro-substituted acetal 66 and allyl-substituted acetal 68 afforded the corresponding polymers 67 and 69 (Scheme 11). For comparison purposes, polyacetal 72 was also synthesized in a similar manner from the unsubstituted cyclic acetal 71. Polymers 67 and 72 degraded under acidic conditions. Further functionalization of polyacetals 67 and 69 was shown to be feasible through reactions of the chloro- and allyl-substituted sidechains.
Monomers 73, each prepared in five steps from furfuryl alcohol, were used to synthesize polyacetals through cascade enyne metathesis polymerization (Scheme 12).58 The rate of polymerization and dispersity of the resulting polymer depended on the structure of the monomer employed. The polymerization of 76 was shown to proceed in a living manner, and a block copolymer was synthesized from monomers 76 and 78. Homopolymer 80, derived from 76, was functionalized by Diels–Alder reactions with the dienophile 4-phenyl-1,2,4-triazole-3,5-dione (PTAD, 81), affording derivative 82. Both 80 and 82 degraded in acidic media, but 82 was substantially more stable, exhibiting a significantly lower degradation rate.
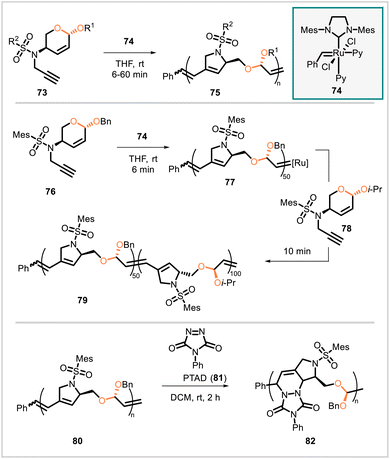 | ||
| Scheme 12 Synthesis of bio-based polymers via cascade enyne metathesis polymerization and subsequent functionalization via a Diels–Alder reaction. | ||
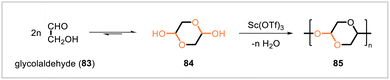
Luebben and Raebiger disclosed methods for the synthesis of a polymer derived from the biobased compound glycolaldehyde (83) (Scheme 13).59 The glycolaldehyde dimer 2,5-dihydroxy-1,4-dioxane (84) formed in concentrated aqueous solutions of 83 and crystallized out of solution. Polymerization of dimer 84 afforded polyacetal 85. Different polymerization conditions were evaluated, but all resulted in fairly low molecular weights of 85. The highest molecular weight (Mn = 5967) was obtained by performing the polymerization in the ionic liquid 1-butyl-3-methylimidazolium triflate with scandium triflate as the catalyst. The hydrolytic stability of the polymer was evaluated in buffer solutions over 21 days at 37 °C. After this time, the mass loss from the polymer was 4% at pH 4 and 3% at pH 7.
2.2. Poly(cycloacetals)
Many biobased polyacetals contain cyclic rather than linear acetal units (Fig. 5). Cyclic acetals, especially those in which the acetal unit is part of a spirocyclic ring system, can potentially contribute greater rigidity to the structure of a polymer. Typically, five- or six-membered cyclic acetals have been employed, and these tend to be more resistant to hydrolysis than linear acetals.12,13 Poly(cycloacetals) often contain other functional groups such as esters, carbonates, and carbamates (urethane linkages). Here, biobased, non-crosslinked poly(cycloacetals) are discussed in subsections according to the other functional groups present in the repeat units of the polymers.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–100
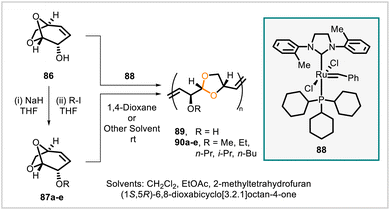
000–100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, Đ = 1.8–2.9 depending on catalyst loading) in the presence of Grubbs catalyst 88.60 In subsequent work, the alkoxy-substituted monomers 87a–e were likewise polymerized using the same catalyst to provide polymers 90a–e.61 The polymerization of 86 was performed in 1,4-dioxane. Compared with polymer 89 (derived from 86), polymers 90a–e were more soluble in a greater range of solvents compatible with the metathesis reaction, and the polymerizations of the corresponding monomers 87a–e were successfully carried out in dichloromethane, 2-methyltetrahydrofuran, Cyrene (dihydrolevoglucosenone), and ethyl acetate as well as 1,4-dioxane. Polymers 89 and 90a–e were thermally stable up to 220–285 °C. The glass transition temperature of hydroxy-substituted 89 was much higher, at 100 °C, than the glass transition temperatures of the alkoxy-substituted polymers 90a–e, which ranged from approximately 43 °C (for 90a) to 0 °C (for 90e). Greater alkyl chain lengths on the alkoxy substituents of 90a–e were correlated with lower glass transition temperatures.
000, Đ = 1.8–2.9 depending on catalyst loading) in the presence of Grubbs catalyst 88.60 In subsequent work, the alkoxy-substituted monomers 87a–e were likewise polymerized using the same catalyst to provide polymers 90a–e.61 The polymerization of 86 was performed in 1,4-dioxane. Compared with polymer 89 (derived from 86), polymers 90a–e were more soluble in a greater range of solvents compatible with the metathesis reaction, and the polymerizations of the corresponding monomers 87a–e were successfully carried out in dichloromethane, 2-methyltetrahydrofuran, Cyrene (dihydrolevoglucosenone), and ethyl acetate as well as 1,4-dioxane. Polymers 89 and 90a–e were thermally stable up to 220–285 °C. The glass transition temperature of hydroxy-substituted 89 was much higher, at 100 °C, than the glass transition temperatures of the alkoxy-substituted polymers 90a–e, which ranged from approximately 43 °C (for 90a) to 0 °C (for 90e). Greater alkyl chain lengths on the alkoxy substituents of 90a–e were correlated with lower glass transition temperatures.
Four different poly(cycloacetals) were synthesized from the vanillin-derived monomer 91 and syringaldehyde-derived monomer 92 (Scheme 15).62 Step-growth polymerization of 91 with the di(fluoroaryl) monomers 93 and 94 yielded poly(cycloacetals) 95 and 96, respectively. Likewise, polymerization of 92 with monomers 93 and 94 yielded 97 and 98. Polymers 95–98 (Mn = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700–16
700–16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100) had high glass transition temperatures (179–243 °C) and melting temperatures (≥244 °C) as well as high thermal degradation temperatures (5% mass loss occurred in the range 343–370 °C). The polymers degraded in the presence of aqueous hydrochloric acid, with higher rates of acetal hydrolysis in acetone and dimethyl sulfoxide than in aqueous media without organic solvent.
100) had high glass transition temperatures (179–243 °C) and melting temperatures (≥244 °C) as well as high thermal degradation temperatures (5% mass loss occurred in the range 343–370 °C). The polymers degraded in the presence of aqueous hydrochloric acid, with higher rates of acetal hydrolysis in acetone and dimethyl sulfoxide than in aqueous media without organic solvent.
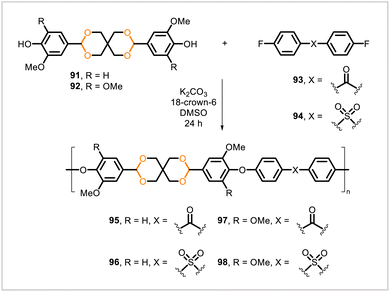 | ||
| Scheme 15 Synthesis of poly(cycloacetals) from vanillin- and syringaldehyde-derived monomers 91 and 92. | ||
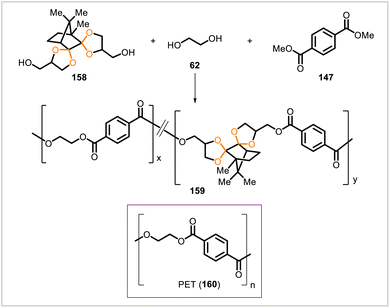
Miller and coworkers synthesized a series of poly(acetal-ether)s through polyacetalization of dialdehyde and tetrol monomers (Scheme 16).63 Dialdehyde monomers 99, 100, 101, and 102 were prepared from 4-hydroxybenzaldehyde, vanillin, syringaldehyde, and ethylvanillin, respectively. (With the exception of ethylvanillin, these starting materials can be obtained as degradation products of lignin.34) Pentaerythritol (103) was polymerized with the dialdehydes 99–102 to afford the corresponding polyacetals 104–107. Likewise, polymerization of tetrol 108 with dialdehydes 99–102 yielded the polyacetals 109–112. The polymers derived from tetrol 103 had higher glass transition temperatures than their analogs derived from tetrol 108. The glass transition temperatures of polyacetals 105–107 ranged from 108 °C to 152 °C whereas those of polyacetals 110–112 ranged from 68 °C to 98 °C; no glass transition was observed for 104 or 109. In acidic solution, polyacetals 105 and 110 degraded into smaller particles, and greater degradation rates were observed for 110.
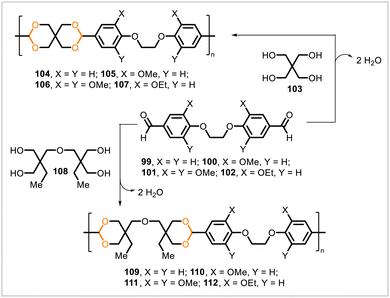 | ||
| Scheme 16 Synthesis of poly(cycloacetals) via polycondensations of dialdehyde monomers with tetrols. | ||
In related work, the Miller group polymerized dialdehydes 100 and 101 with erythritol, a biobased tetrol.14 Four series of copolymers were prepared by including varying amounts of another tetrol—either 103 or 108—in the polymerizations of dialdehyde 100 and 101 with erythritol. Erythritol was less reactive than tetrols 103 and 108, and generally lower molecular weights were obtained in the polymerizations utilizing more erythritol relative to the other tetrols.
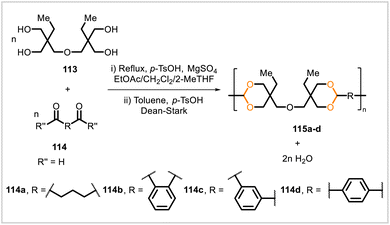
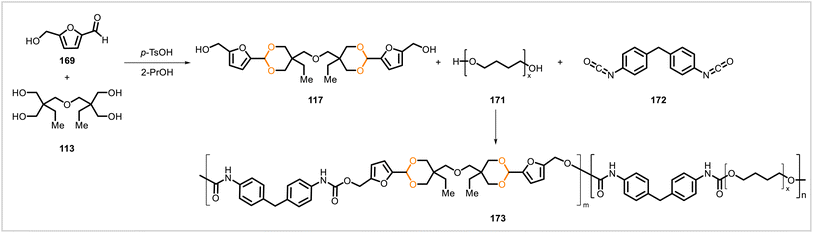
Poly(cycloacetals) 115a–d were synthesized through polyacetalization of di-trimethylolpropane (113) with glutaraldehyde (114a), o-phthalaldehyde (114b), m-phthalaldehyde (114c), and p-phthalaldehyde (114d) (Scheme 17).64 The reaction of 113 with glutaraldehyde was faster than the reactions of 113 with the phthalaldehydes, requiring less time to reach full conversion of the aldehyde. Samples of polymers 115a, 115b, 115c, and 115c prepared under similar reaction conditions had glass transition temperatures of 30 °C, 102 °C, 86 °C, and 115 °C, respectively. The glutaraldehyde-derived polymer 115a showed no significant molecular weight decrease after one month in solutions at pH 3, 7, and 10. The stability of the poly(cycloacetal) to acid was attributed to the material's hydrophobicity, disfavoring hydrolysis of the acetal groups in an aqueous medium.
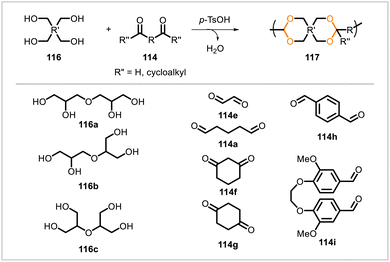
Poly(cycloacetals) 117 were synthesized through polyacetalization reactions of diglycerol (116) with aldehydes and ketones (114) (Scheme 18).65 Diglycerol was used as a mixture of isomers 116a, 116b, and 116c. Glyoxal (114e), glutaraldehyde (114a), 1,3-cyclohexanedione (114f), 1,4-cyclohexanedione (114g), terephthalaldehyde (114h), and the vanillin-based monomer 114i were each polymerized with diglycerol. Brittle polymers were obtained from 1,3-cyclohexanedione, 1,4-cyclohexanedione, and 114i. The polymer from glyoxal was flexible and strong but insoluble. The polymers from terephthalaldehyde and glutaraldehyde were both flexible, but that from glutaraldehyde was stronger. The glutaraldehyde-based polymer exhibited elastomeric behavior. Copolymers of glutaraldehyde with meso-erythritol and pentaerythritol as well as diglycerol were also synthesized. Incorporation of meso-erythritol or pentaerythritol imparted greater strength to the glutaraldehyde-based polymers.
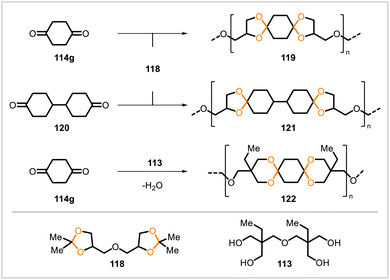
Du Prez and coworkers66 synthesized diglycerol bisacetonide (118) from glycerol and polymerized this monomer with 1,4-cyclohexanedione (114g) and 4,4′-bicyclohexanone (120) (Scheme 19). (1,4-Cyclohexanedione is biobased as it can be derived from succinic acid, a naturally occurring compound.) The resulting polyacetals 119 (from 114g) and 121 (from 120) had number-average molecular weights between 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 30
000 and 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, were semicrystalline, and had glass transition temperatures of 48 °C and 65 °C, respectively. The monomers 114g and 118 were also copolymerized with the tetrol 113, and a series of copolymers was prepared by including varying amounts of 113 in the polymerization. Higher levels of 113 resulted in higher glass transition temperatures of the polymeric products. The polyacetals were stable to aqueous acid in the pH range 3–7.
000, were semicrystalline, and had glass transition temperatures of 48 °C and 65 °C, respectively. The monomers 114g and 118 were also copolymerized with the tetrol 113, and a series of copolymers was prepared by including varying amounts of 113 in the polymerization. Higher levels of 113 resulted in higher glass transition temperatures of the polymeric products. The polyacetals were stable to aqueous acid in the pH range 3–7.
Other polyacetals from 1,4-cyclohexanedione have been reported. Sudo et al. prepared oligo(spiroacetal)s (Mn = 1400–2100) via reactions of 1,4-cyclohexanedione with tetrols derived from myo-inositol.67 Alder and Reddy polymerized monomeric acetal derivatives of 1,4-cyclohexanedione and pentaerythritol through transacetalization, but their studies of the polymerization reaction were hampered by the insolubility of the polymer.68
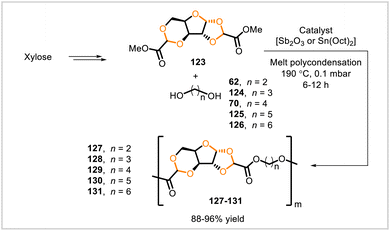
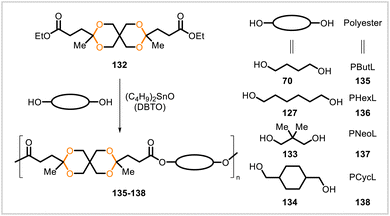
Manker et al. reported a series of poly(acetal-ester)s, poly(alkylene xylosediglyoxylates) 127–131, synthesized from monomer 123 (Scheme 20).69 Compound 123 was derived in a one-pot process from xylose, a major monomeric component of hemicellulose,70 and could be prepared from commercial xylose or from hemicellulosic fractions obtained from lignocellulosic biomass through a fractionation process. Diester 123 was polymerized with linear aliphatic diols 62, 70, and 124–126 by melt polycondensation. Polyesters 129, 130, and 131, derived in this manner from the corresponding longer diols (70, 125, and 126), had number-average molecular weights (Mn) of approximately 35 kDa whereas polyesters 127 and 128 from ethylene glycol (62) and propylene glycol (124) had Mn = 3 kDa and Mn = 8 kDa, respectively. Polyesters 129, 130, and 131 had glass transition temperatures of 100 °C, 84 °C, and 72 °C, respectively, and exhibited toughness, hardness, and strength in mechanical testing. These three polymers could be processed by compression and injection molding, vacuum-forming, twin-screw extrusion, and 3D printing. To demonstrate the potential recyclability of the polymers, 131 was depolymerized by methanolysis under acidic conditions, and the monomers (123 and 126) were recovered and used to resynthesize 123. The polymers also underwent degradation over time in water.
Polyesters 135–138 (Mn = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–18
000–18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300) were synthesized from the levulinic acid-derived monomer 132 and diols 70, 127, 133, 134 (Scheme 21).15 Additionally, a copolyester containing 20 mol% of 132 was prepared from a mixture of 70, diethyl adipate, and 132. Polymers 135 and 136 were soft and sticky with lower glass transition temperatures while 137 and 138 had higher glass transition temperatures (41 °C and 49 °C, respectively) and were formed into mechanically strong, transparent films.
300) were synthesized from the levulinic acid-derived monomer 132 and diols 70, 127, 133, 134 (Scheme 21).15 Additionally, a copolyester containing 20 mol% of 132 was prepared from a mixture of 70, diethyl adipate, and 132. Polymers 135 and 136 were soft and sticky with lower glass transition temperatures while 137 and 138 had higher glass transition temperatures (41 °C and 49 °C, respectively) and were formed into mechanically strong, transparent films.
 | ||
| Scheme 21 Polymerizations of levulinic acid-derived diester 132 with aliphatic diols to afford polyesters. | ||
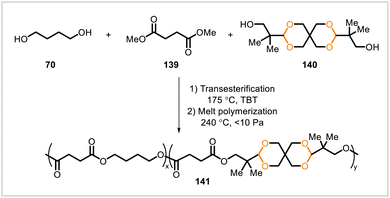
Bulky acetal-containing units were incorporated into poly(butylene succinate) copolyesters.71 The new copolyesters were synthesized via transesterification of dimethyl succinate (139) with 1,4-butanediol (70) and bioderivable diol 140, followed by melt polymerization (Scheme 22). While poly(butylene succinate) is biodegradable, a copolymer with a relatively high content of 140 underwent little degradation in the presence of a lipase (Candida antarctica lipase B), presumably due to the steric hindrance imposed by the bulky units of 140 in the polymer. After pretreatment with acidic solution to cleave acetal groups in the polymer, enzymatic hydrolysis proceeded to a much greater extent.
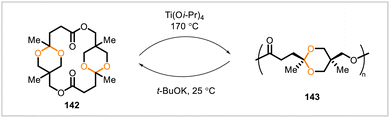
Meng et al. described poly(acetal-ester) 143, prepared from the biobased macrocyclic lactone monomer 142 (Scheme 23).9 The polymerization–depolymerization equilibrium of this system was exploited to enable chemical recycling of the polymer back to the monomer. Ring-opening polymerization of monomer 142 occurred at 170 °C in the presence of the catalyst titanium(IV) isopropoxide. Depolymerization of the resulting polymer 143 back to monomer 142 proceeded at 25 °C with potassium tert-butoxide as catalyst. During the depolymerization, monomer 142 precipitated from solution, and >99% conversion of the polymer was achieved.
 | ||
| Scheme 23 Interconversion of macrocyclic monomer 142 and poly(acetal-ester) 143 under different conditions effecting polymerization and depolymerization. | ||
A range of other aliphatic, biobased polyesters with cyclic acetal units have been reported.72–78 In general, glycerol has been a popular biobased building block for these polymers. Other renewable precursors include levulinic acid,72 citric acid,73 and biobased lactones.74
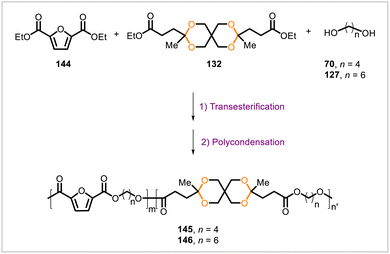
Apart from aliphatic polyesters, a variety of aromatic and heteroaromatic polyesters containing cyclic acetal units have been synthesized from biobased starting materials. Two series of biobased poly(cycloacetal)s were synthesized from diethyl 2,5-furandicarboxylate (144) and the acetal-containing diester 132 through a two-step melt polycondensation (Scheme 24).79 Transesterification of the diester monomers with 1,4-butanediol (70) or 1,6-hexanediol (127) followed by polycondensation afforded the corresponding copolyesters 145 and 146. Polymers 145 and 146 were prepared with varying amounts of the spirocyclic acetal-containing monomer 132. Samples with relatively greater amounts of 132 relative to 144 had lower crystallinity and melting points. Among the polyesters prepared from 1,6-hexanediol, the copolyesters containing acetal units from 132 degraded more rapidly in aqueous hydrochloric acid than did a homopolyester that was prepared only from diester 144 and 1,6-hexanediol and thus contained no acetal linkages.
Other furanic poly(acetal-ester)s were reported by Hayashi et al.80 A mixture of isomeric diol monomers derived from furfural and glycerol was polymerized with succinic anhydride and phthalic anhydride. The polymerizations were conducted using a condensation reagent (N,N′-diisopropylcarbodiimide) with 4-(dimethylamino)pyridine as a catalyst and resulted in number-average molecular weights up to 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 and 7
200 and 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 for the polymerizations with succinic anhydride and phthalic anhydride, respectively.
500 for the polymerizations with succinic anhydride and phthalic anhydride, respectively.
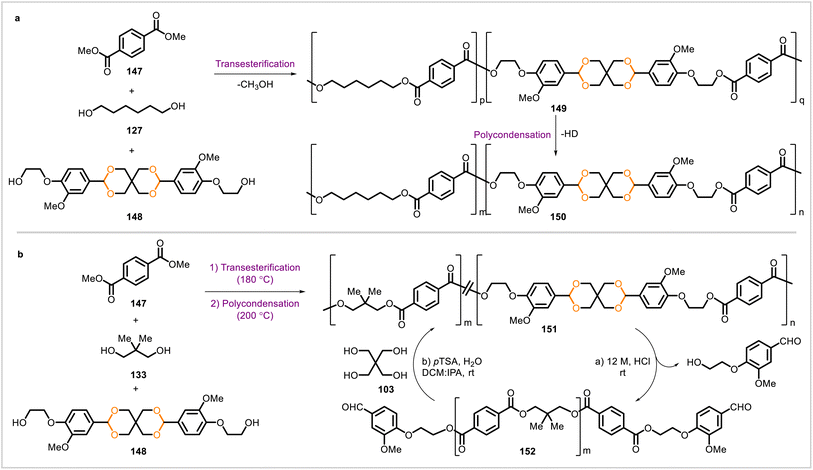
Mankar et al. synthesized polyesters from the vanillin-derived diol 148 (Scheme 25).81,82 Diol 148 and 1,6-hexanediol (127) were polymerized with dimethyl terephthalate (147) to yield polyester 150 (entry a, Scheme 25). Similarly, polymerization of diol 148 and neopentyl glycol (133) with dimethyl terephthalate (147) afforded polyester 151 (entry b, Scheme 25). Higher contents of 148 in each polyester resulted in higher glass transition temperatures. Polyesters 150 containing 20–30 mol% of 148 had similar storage moduli to that of the commercial high-performance polyester Akestra 90. The polyester 151 could be hydrolyzed under acidic conditions to telechelic polyesters with aldehyde end groups (152), and the telechelic polyesters were repolymerized with pentaerythritol (103) to reconstruct the acetal groups present in 151.
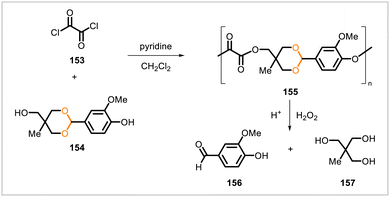
Vanillin has been shown to have antioxidant and anti-inflammatory activities.83–86 As a strategy to release vanillin to cells experiencing oxidative stress, Kwon et al.87 constructed a polymeric prodrug designed to release vanillin in the presence of hydrogen peroxide (a biomarker and effector of oxidative stress) and in low pH conditions (such as at pH ∼5.5, the pH of endosomes). An acetal derivative of vanillin (154) reacted with oxalyl chloride (153) to yield polymer 155 (Scheme 26). In aqueous solutions, polymer 155 underwent hydrolysis and released vanillin (156), with a faster rate of hydrolysis at pH 5.5 than at 7.4. The presence of hydrogen peroxide slightly accelerated hydrolysis as well. Nanoparticles formed from 155 scavenged hydrogen peroxide and exhibited antioxidant and anti-inflammatory effects in subsequent experiments.
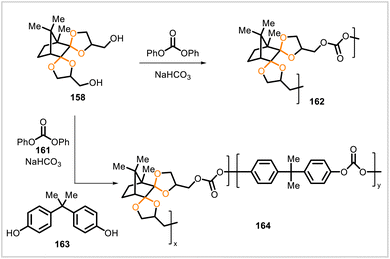
Poly(ethylene terephthalate) (160, PET) (Scheme 27), is a widely used polyester that can be prepared via the polycondensation of ethylene glycol (62) and dimethyl terephthalate (147).88 Related, partially biobased polyesters have been synthesized from dimethyl terephthalate (147), camphor-derived diol 158, and ethylene glycol (62) (Scheme 27).37 Varying proportions of diols 158 and 62 were polymerized with diester 147 in a melt polymerization process comprising transesterification and polycondensation steps. The glass transition temperatures of the resulting copolyesters ranged from 78 °C to 129 °C, rising as the ratio of 158 to 62 in the material increased. The copolymers degraded in acidic (pH 2.0) solution, and the hydrolysis rates were higher for copolymers with greater contents of the acetal-containing monomer 158. A copolymer containing 158 and 62 in a 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90 ratio was, like PET, semicrystalline but exhibited a lower melting temperature (209 °C) than that of PET (230 °C).
90 ratio was, like PET, semicrystalline but exhibited a lower melting temperature (209 °C) than that of PET (230 °C).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 283 to 17
283 to 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 963. Their glass transition temperatures (Tg = 128.3–151.2 °C) compared favorably with those of polycarbonates of bisphenol A (A homopolycarbonate of bisphenol A obtained under the same reaction conditions had Tg = 120.6 °C while a commercial bisphenol A-based polycarbonate had Tg = 150 °C).
963. Their glass transition temperatures (Tg = 128.3–151.2 °C) compared favorably with those of polycarbonates of bisphenol A (A homopolycarbonate of bisphenol A obtained under the same reaction conditions had Tg = 120.6 °C while a commercial bisphenol A-based polycarbonate had Tg = 150 °C).
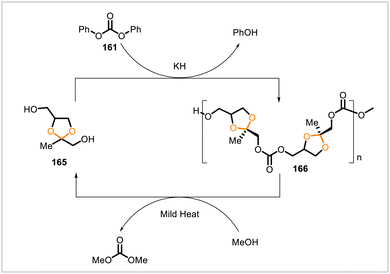
Diol monomer 165 (Scheme 29) was synthesized using the commercially available starting materials epichlorohydrin (bio-derivable), hydroxyacetone (derivable from glycerol), and benzyl alcohol (petroleum-derived).10 Monomer 165 underwent polycondensation with diphenyl carbonate at 220 °C in the presence of catalytic potassium hydride; vacuum was applied to remove the phenol produced over the course of the reaction. Number-averaged molecular weights (Mn) up to 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and glass transition temperatures of 25–35 °C were observed for the resulting polycarbonate (166) depending on the polymerization conditions. The polymer was thermally stable up to 200 °C, at which point decomposition began to occur. The material was stable in acidic (pH 0–7) solutions at 50 °C and in methanol or ethanol at room temperature. However, complete depolymerization occurred in methanol at 50 °C within 24 hours. The depolymerization involved cleavage of the carbonate units and release of monomer 165 rather than transacetalization. Monomer 165 could be recovered after depolymerization of the pure polycarbonate 166 (>95% recovery of 165) or after depolymerization of 166 in a mixture with other plastics (60% recovery of 165).
000 and glass transition temperatures of 25–35 °C were observed for the resulting polycarbonate (166) depending on the polymerization conditions. The polymer was thermally stable up to 200 °C, at which point decomposition began to occur. The material was stable in acidic (pH 0–7) solutions at 50 °C and in methanol or ethanol at room temperature. However, complete depolymerization occurred in methanol at 50 °C within 24 hours. The depolymerization involved cleavage of the carbonate units and release of monomer 165 rather than transacetalization. Monomer 165 could be recovered after depolymerization of the pure polycarbonate 166 (>95% recovery of 165) or after depolymerization of 166 in a mixture with other plastics (60% recovery of 165).
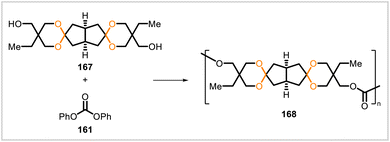
A diol monomer containing spirocyclic acetal groups (167) was synthesized from a citric acid-derived diketone.89 The monomer, obtained as a mixture of stereoisomers, was subjected to melt polycondensation with diphenyl carbonate (161), yielding a rigid, amorphous polycarbonate (168) (Scheme 30). Samples of the polycarbonate were formed into strong, flexible films with high transparency and little coloration.
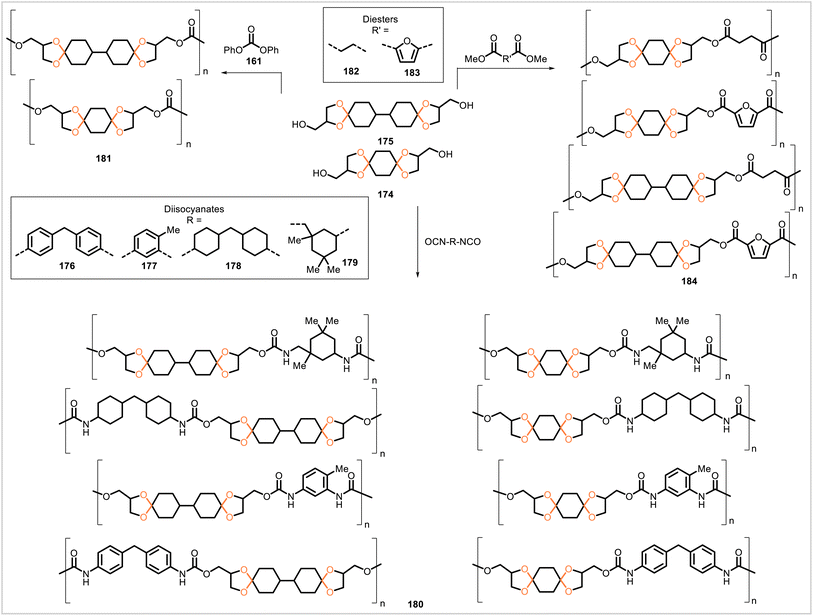
Du Prez and coworkers prepared acetal monomers 174 and 175 by acetalization of the corresponding diketone (1,4-cyclohexanedione and 4,4′-bicyclohexanone, respectively) with glycerol (Scheme 32).91 These monomers were polymerized with diisocyanates 176–179 to yield polyurethanes 180, with diphenyl carbonate to yield polycarbonates 181, and with diesters 182 and 183 to yield polyesters 184. The polyurethanes had glass transition temperatures of 95–150 °C and were mechanically strong but brittle. Of the polycarbonates, the polycarbonate derived from 175 exhibited more promising properties when compared with an analogous polycarbonate derived from bisphenol A (BPA). However, relative to the BPA-derived polycarbonate, the polycarbonate from 175 had a lower glass transition temperature of 100 °C (Tg = 150 °C for the BPA-derived polycarbonate) and exhibited lower modulus, yield stress, and ductility. The polyesters were obtained with lower molecular weights (Mn = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–19
000–19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) than the polyurethanes and polycarbonates and had glass transition temperatures ranging from 40 °C to 96 °C. In aqueous acid at 50 °C, the polyurethane derived from 175 and 178, the polycarbonate from 174 and 161, and the polyester from 174 and 182 did not undergo any significant degradation over two weeks even at pH 1.
000) than the polyurethanes and polycarbonates and had glass transition temperatures ranging from 40 °C to 96 °C. In aqueous acid at 50 °C, the polyurethane derived from 175 and 178, the polycarbonate from 174 and 161, and the polyester from 174 and 182 did not undergo any significant degradation over two weeks even at pH 1.
 | ||
| Scheme 32 Polymerizations of acetal-containing diol monomers 175 and 174 yielding polycarbonates, polyesters, and polyurethanes. | ||
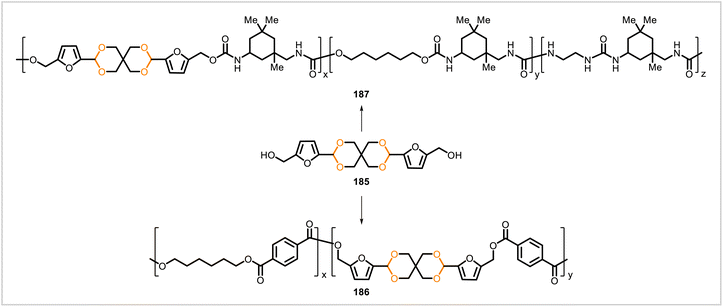
Polyester 186 and poly(urethane-urea) 187 were synthesized from the acetal-containing monomer 185, derived from 5-(hydroxymethyl)furfural (Scheme 33).92 For the synthesis of 186, monomer 185 was polymerized with dimethyl terephthalate and 1,6-hexanediol in the presence of dibutyltin oxide as the catalyst. To prepare poly(urethane-urea) 187, monomer 185 was polymerized with 1,6-hexanediol and isophorone diisocyanate. Samples of each polymer were prepared with varying amounts of 185. The content of 185 in the polyester samples (Mn = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100–15
100–15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400) ranged from 3 to 19 mol% while the content of 185 in the poly(urethane-urea) samples (Mn = 16
400) ranged from 3 to 19 mol% while the content of 185 in the poly(urethane-urea) samples (Mn = 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–53
000–53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) ranged from 5.4 to 62 mol%. Glass transition temperatures (measured by DSC) were 25–47 °C for the polyesters and 90–131 °C for the poly(urethane-urea)s. In each series of polymers, glass transition temperatures increased with increasing content of 185.
000) ranged from 5.4 to 62 mol%. Glass transition temperatures (measured by DSC) were 25–47 °C for the polyesters and 90–131 °C for the poly(urethane-urea)s. In each series of polymers, glass transition temperatures increased with increasing content of 185.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500–20
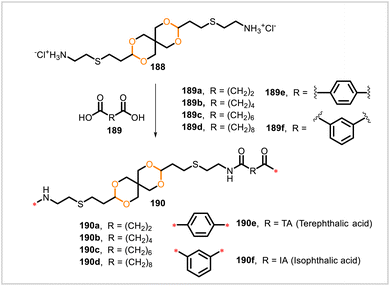
500–20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) than polymers 190a, 190e, and 190f. The higher-molecular-weight poly(acetal-amide)s 190b–d were semicrystalline and had glass transition temperatures of 36–48 °C and melting temperatures of 132–144 °C. These materials were thermally stable, with 10% weight loss occurring at 334–350 °C. Polymers 190b–d were processed by compression molding and exhibited moduli in the range 1.0–1.5 GPa during mechanical testing. In aqueous solutions at 50 °C, polymer 189c was found to be stable over two weeks at pH 3 but degraded at pH 1.
000) than polymers 190a, 190e, and 190f. The higher-molecular-weight poly(acetal-amide)s 190b–d were semicrystalline and had glass transition temperatures of 36–48 °C and melting temperatures of 132–144 °C. These materials were thermally stable, with 10% weight loss occurring at 334–350 °C. Polymers 190b–d were processed by compression molding and exhibited moduli in the range 1.0–1.5 GPa during mechanical testing. In aqueous solutions at 50 °C, polymer 189c was found to be stable over two weeks at pH 3 but degraded at pH 1.
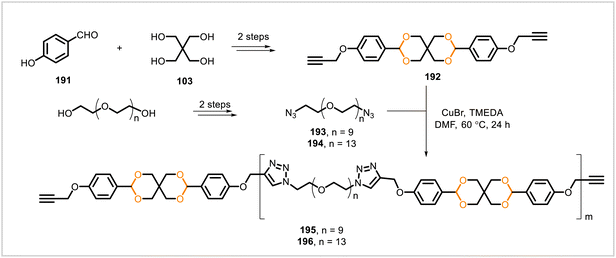
Dialkyne monomer 192, derived from 4-hydroxybenzaldehyde (191), was polymerized with diazides 193 and 194 through copper-catalyzed alkyne–azide cycloadditions (Scheme 35).94 The resulting polymers 195 (from diazide 193) and 196 (from diazide 194) contained repeating triazole and acetal units and were evaluated for their ability to encapsulate hydrophobic cargo as a potential drug delivery system. Polymers 195 and 196 self-assembled into spherical particles that were capable of encapsulating the hydrophobic dye Nile red, a model drug. The dye was gradually released from the particles at similar rates at pH 5.5 and 7.4; however, the particles underwent chemical degradation only at pH 5.5, not 7.4.
3. Crosslinked polyacetals
The incorporation of crosslinks into polymers can be used to modulate thermal and mechanical properties, impart dimensional stability, or increase solvent resistance.95 Commonly used crosslinked polymers include epoxy, polyurethane, alkyd, unsaturated polyester, melamine, urea, and phenolic resins.95 These and other crosslinked polymers currently employed in commercial applications are typically thermosets, in which crosslinks are formed irreversibly in a curing process. Although these crosslinks are integral to the dimensional stability and other properties of the material, they also prevent the thermoset from being reversibly softened and reshaped in fabrication processes (e.g., molding or extrusion) as is possible with thermoplastic polymers, which lack crosslinks. This imposes limitations on the materials' range of applications and the potential for recycling the polymer by mechanical means. Furthermore, the crosslinked structures of thermosets are often resistant to chemical degradation—a potential benefit during the polymers' useful lifespans but an obstacle for efforts to minimize polymeric waste.Other crosslinked polymers have been designed to overcome the reprocessing, recycling, and degradation barriers associated with conventional thermosets. Thermosets with chemically labile linkages may be more readily degraded in response to acids, bases, high temperatures, or other conditions in comparison with their conventional counterparts that do not break down readily.96 Covalent adaptable networks (CANs), also called dynamic covalent networks (DCNs), may offer mechanical properties similar to those of conventional thermosets but can be more readily reprocessed and mechanically recycled.24,25 Degradable thermosets and CANs are not mutually exclusive categories: some polymers (including biobased polyacetals) have structures endowing them with enhanced degradability as well as the characteristic behavior and reprocessability of CANs.97–99
CANs derive their unique properties from the presence of chemical linkages that can undergo reversible reactions involving bond exchanges within the material. The types of reversible reactions employed in these materials have included transacetalization as well as numerous other chemistries including transesterification, Diels–Alder (and retro-Diels–Alder) reactions, imine metathesis, and disulfide bond exchanges.24 CANs can be further classified based on the mechanism of bond exchange: associative or dissociative. In associative CANs, bond formation occurs before bond breaking; this is the case, for example, during transesterification reactions.100 In dissociative CANs, bond breaking occurs first and is followed by bond formation—for example, a retro-Diels–Alder reaction followed by a Diels–Alder reaction involving a different reactive group within the material.100 The term “vitrimer”101 commonly refers to associative CANs, although it has been suggested that the term is applicable to both associative and dissociative CANs due to the similarity in behavior of these CAN subclasses.100 The reversible reactions occur in response to specific stimuli such as heating, photoactivation, or catalysis at elevated temperature.24 The resulting bond exchanges allow the network to change its form to an extent that would be impossible in a conventional thermoset lacking dynamic bonds.
The acetal functional group has been employed for the development of reprocessable and degradable crosslinked polymers. The reversibility of acetal formation can allow bond exchanges to occur within a polymer at high temperatures, conferring dynamic behavior and reprocessability on the material. Because acetals undergo relatively facile hydrolysis and alcoholysis in the presence of acid, polymeric networks containing these functional groups may be degraded in acidic media. With these advantages, acetal-based linkages have been incorporated into a variety of crosslinked polymers, including epoxy resins, crosslinked polyurethanes, and other structures. In the following sections, we survey the structures and properties of such polymers, covering examples that are derived from biobased precursors.
3.1. Acetal-containing epoxy resins
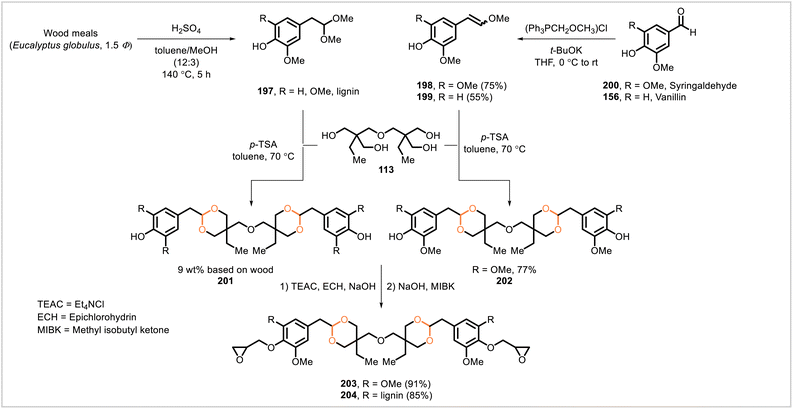
A variety of acetal-containing epoxy resins have been reported. Most epoxy resins used for commercial applications are derived from the diglycidyl ether of bisphenol A.102 Bisphenol A is a non-biobased compound that has shown endocrine-disrupting effects.103 Due to their popularity and utility, bisphenol A-derived epoxy resins have been commonly employed as a standard for comparison in the development of alternative, biobased epoxy resins, including the acetal-containing polymers discussed in this section.Kaiho et al. synthesized epoxy resins from acetal-containing derivatives of lignin and syringaldehyde (Scheme 36).104 A low-molecular-mass lignin fraction was obtained by heating wood particles with a solution of sulfuric acid in a methanol/toluene mixture at 140 °C. NMR data for the resulting depolymerized material were consistent with the presence of dimethyl acetal groups in the structure (197). A transacetalization reaction with the tetrol 113 was then performed, affording a modified lignin-based structure (201). This product was then treated with epichlorohydrin to yield a glycidated derivative (204). A series of structurally related compounds were also prepared from syringaldehyde (200) and served as a comparison to the lignin-derived materials because lignin contains syringyl substructures. After conversion of syringaldehyde (200) to enol methyl ether 198, reaction with tetrol 113 afforded diacetal 202. The NMR spectra of intermediate 202 were compared with the analogous lignin-derived material 201 and provided evidence for the presence of the cyclic acetal units in 201. Glycidation of the syringaldehyde-derived compound 202 yielded diglycidyl ether 203. Using a phenol novolac hardener and triphenylphosphine as catalyst, epoxy resins were synthesized from the lignin-derived compound 204 and the syringaldehyde-derived compound 203. The epoxy resins from 204 and 203 had glass transition temperatures of 94 °C and 67 °C, respectively. Notably, the glass transition temperature of 204 (94 °C) was approximately equal to that of a bisphenol A-derived epoxy resin (95 °C).
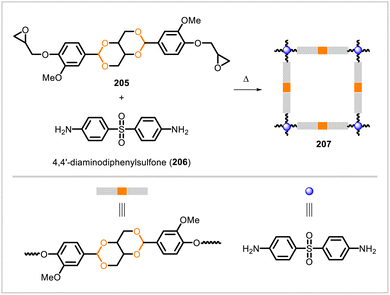
The acetal-containing epoxy monomer 205 was prepared from vanillin and cured with 4,4′-diaminodiphenylsulfone (206) to yield a crosslinked network (207) (Scheme 37).105 The properties of this polymer were compared with bisphenol A epoxy resin cured with 206. Polymer 207 had a lower crosslink density yet exhibited greater hardness and higher tensile modulus than the BPA-derived thermoset, with the difference in these mechanical properties presumably arising from structural differences between the epoxy monomers. The vanillin-derived polymer 207 also had a somewhat lower glass transition temperature (184 °C) than that of its BPA-derived counterpart (208 °C) and lower thermal stability. In acidic media, degradation of the acetal-containing network occurred. However, in a hot, humid environment (60 °C and 95% humidity for 15 days), the glass transition temperature of 207 did not change significantly, indicating that the material was stable under these conditions.
In another study, the vanillin-derived monomer 91 was converted to two different epoxy derivatives: derivative 208 via reaction of 91 with epichlorohydrin and derivative 210 via reaction of 91 with the bisphenol A epoxy resin 209 (Scheme 38).106 Samples of 208, 209, and 210 were each cured with isophorondiamine or 4,4′-diaminodiphenylmethane. Additionally, coatings and carbon fiber-reinforced composites were prepared from 208 and 209 in combination with amine curing agents. Compared with cured 209, cured 208 had a slightly higher glass transition temperature, approximately equal tensile strength and elongation at break, and greater tensile modulus. The coatings and composites based on 208 also exhibited favorable properties. In acidic solution, cured 208 degraded, dissolving completely, while cured 210 underwent partial degradation into smaller pieces of material. In the carbon-fiber composite based on 208, the resin was degraded through treatment with hydrochloric acid solution (1 M in 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 acetone/water), and the carbon fiber was recovered with retained mechanical properties and morphology.
1 acetone/water), and the carbon fiber was recovered with retained mechanical properties and morphology.
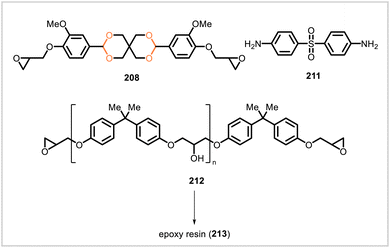
In work by Yan et al., a partially biobased epoxy resin (213) was synthesized from a mixture of vanillin-derived monomer 208 and bisphenol A-derived epoxy 212 cured with 4,4′-diaminodiphenyl sulfone (211) (Scheme 39).107 Resin samples containing more 208 relative to 212 had higher glass transition temperatures as well as greater impact strength, flexural strength, and flexural modulus. Using a resin mixture containing a 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 ratio (by mass) of 208 to 212, a carbon fiber-reinforced composite was prepared. The resin could be degraded under acidic conditions, allowing the carbon fiber to be recovered. The mechanical performance of the recovered carbon fiber was similar to that of virgin carbon fiber.
30 ratio (by mass) of 208 to 212, a carbon fiber-reinforced composite was prepared. The resin could be degraded under acidic conditions, allowing the carbon fiber to be recovered. The mechanical performance of the recovered carbon fiber was similar to that of virgin carbon fiber.
The same vanillin-derived, diepoxy monomer 208 used in the studies described above106,107 has also been incorporated into cellulose nanofiber/epoxy composites.108 The resulting composites were entirely derived from biobased starting materials and could be hydrolyzed to small-molecule degradation products in acidic media.
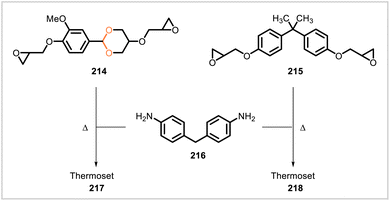
The vanillin-derived epoxy monomer 214 was cured with 4,4′-diaminodiphenylmethane (216) to form a crosslinked material (217) (Scheme 40).109 An analogous thermoset (218) was prepared from bisphenol A diglycidyl ether 215 for comparison. In mechanical testing, material 217 had higher Young's modulus, tensile strength, elongation at break, and fracture energy than the bisphenol A-derived thermoset 218. Slightly lower glass transition temperature (Tg) and initial degradation temperature (Td5%, corresponding to 5% weight loss) were observed for 217 (Tg = 164 °C, Td5% = 330 °C) relative to 218 (Tg = 169 °C, Td5% = 356 °C). Polymer 217 degraded under acidic conditions at varying rates depending on the acid and solvent system employed. However, 217 and 218 exhibited similar resistance to hygrothermal aging under conditions of 60 °C and 70% humidity.
 | ||
| Scheme 40 Construction of thermosets from biobased epoxy monomer 214 and bisphenol A diglycidyl ether (215). | ||
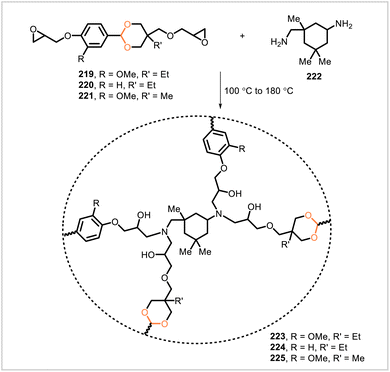
Epoxy networks 223, 224, and 225 were prepared from monomers 219, 220, and 221, respectively, each cured with isophorone diamine (222) (Scheme 41).110 Monomers 219 and 221 were derived from vanillin whereas 220 was derived from p-hydroxybenzaldehyde. The viscosities of the monomers and resins were studied since viscosity can have important implications for the processability of resins. Monomer 220 demonstrated the lowest viscosity, and the corresponding resin system consisting of 220 and 222 showed temperature-dependent viscosities compatible with vacuum-assisted resin transfer molding (VARTM). Subsequently, the VARTM process was used to prepare a carbon fiber composite from the resin system of 220 and 222 and carbon fiber woven fabric. The cured resin in the composite degraded under acidic conditions, and the carbon fiber was recovered, showing nearly identical properties to those of the original carbon fiber.
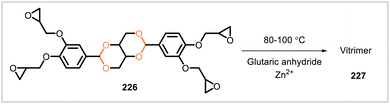
An epoxy vitrimer (227) was synthesized from the vanillin-derived monomer 226 (Scheme 42).111 Monomer 226 was cured with glutaric anhydride at 80–100 °C with zinc(II) acetylacetonate as catalyst. The mechanical properties of the resulting vitrimer varied with the amount of zinc(II) acetylacetonate used. For a vitrimer sample prepared with a 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 weight ratio of zinc(II) acetylacetonate to 226, tensile strength was 60 MPa, flexural strength was 119 MPa, and impact strength was 20 kJ m−2. Glass transition temperatures of the vitrimer samples ranged from 111 °C to 117 °C. The samples exhibited stress relaxation behavior consistent with the ability to undergo topological rearrangements through bond exchange reactions. The material showed self-healing behavior upon heating to 200 °C. Additionally, the polymer degraded in solutions of hydrochloric acid in acetone/water (9
100 weight ratio of zinc(II) acetylacetonate to 226, tensile strength was 60 MPa, flexural strength was 119 MPa, and impact strength was 20 kJ m−2. Glass transition temperatures of the vitrimer samples ranged from 111 °C to 117 °C. The samples exhibited stress relaxation behavior consistent with the ability to undergo topological rearrangements through bond exchange reactions. The material showed self-healing behavior upon heating to 200 °C. Additionally, the polymer degraded in solutions of hydrochloric acid in acetone/water (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at 25 °C.
1) at 25 °C.
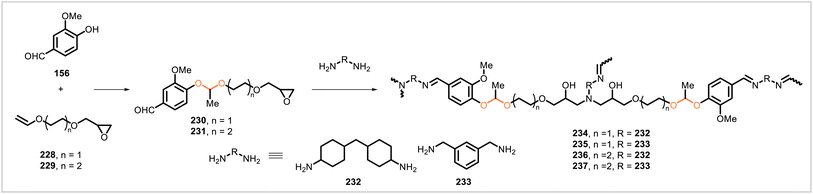
Reaction of vanillin (156) with vinyl ethers 228 and 229 afforded the epoxy-functionalized, acetal-containing monomers 230 and 231 (Scheme 43).112 Each of these monomers was cured with diamines 232 and 233 to afford the corresponding networks 234–237 containing imine as well as acetal linkages. Notably, the monomers were liquid, and the curing step could be conducted without added solvent. The epoxy networks had glass transition temperatures of 36–63 °C. Mechanical testing showed that the networks had Young's moduli of 918.7–1249.6 MPa, tensile strengths of 49.0–72.7 MPa, and 12.3–21.5% elongations at break. Further results demonstrated the reprocessability, degradability, and recyclability achievable with these polymer systems. A sample of network 234 was chopped into pieces and successfully remolded by hot-pressing, affording a reprocessed sample with no significant change in mechanical properties. Materials 236 and 237 were depolymerized at 65 °C in a mixture of tetrahydrofuran and 1 M aqueous HCl. Vanillin was recovered in >90% yield, and a polyol/diamine mixture was separately obtained from the depolymerized mixtures. The polyol mixture from 237 underwent reaction with an isocyanate prepolymer, yielding a polyurethane.
Zhang et al. synthesized covalent adaptable networks from epoxidized soybean oil (241) and the diacetal monomers 238, 239, and 240 (Scheme 44), derived from syringaldehyde, vanillin, and ethylvanillin, respectively.113 In the presence of the catalyst dimethylimidazole, each monomer reacted with epoxidized soybean oil to yield the corresponding product (242, 243, or 244) as a polymer film. Network 242 had the highest crosslink density, followed by 243 and then 244. This trend of decreasing crosslink densities corresponded to decreasing glass transition temperatures (59 °C down to 41 °C), decreasing Young's moduli (449 MPa down to 9.7 MPa), and increasing elongation at break (6.0–117.7%) in the series 242–244. Each polymer was reprocessable: pieces of each polymer were reformed into a film by hot-pressing, and the new film showed only small variations in mechanical performance relative to the material before reprocessing. The materials degraded in solutions of HCl in 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 acetone/water, and the rates of hydrolysis corresponded to the crosslink densities, with 242 degrading the slowest and 244 the fastest.
1 acetone/water, and the rates of hydrolysis corresponded to the crosslink densities, with 242 degrading the slowest and 244 the fastest.
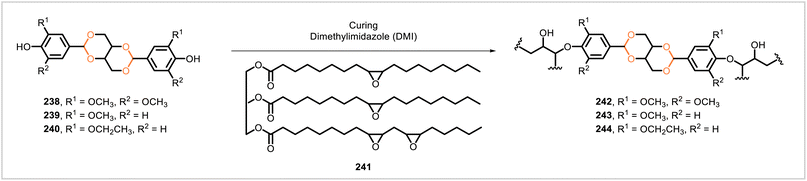 | ||
| Scheme 44 Formation of acetal-based covalent adaptable networks via reactions of phenolic monomers 238–240 with epoxidized soybean oil (241). | ||
3.2. Crosslinked polyurethanes with acetal linkages
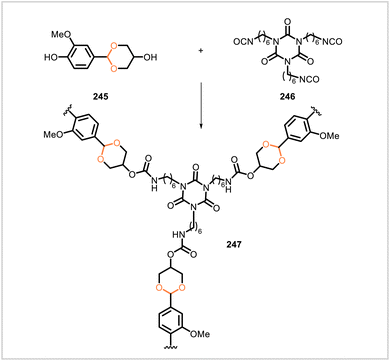
Wang et al. prepared a crosslinked polyurethane (247) through the reaction of vanillin-derived monomer 245 and hexamethylene diisocyanate trimer (246) (Scheme 45).114 The glass transition temperature, tensile strength, tensile modulus, and elongation at break of 247 were similar but slightly lower than the same properties of an analogous polyurethane derived from bisphenol A in place of monomer 245. Carbon fiber-reinforced composites based on the polyurethanes were also prepared. Polyurethane 247 degraded under acidic conditions and could be removed from the carbon fiber in the composite without damage to the carbon fiber.Li et al. synthesized four different covalent adaptable networks containing acetal and urethane linkages (Scheme 46).115 The furfural-derived, acetal-containing diol 248 reacted with triisocyanate 246, forming the crosslinked network 249. The analogous benzaldehyde-derived monomer 252 likewise reacted with 246 to yield a similar network (253). An additional network (251) was synthesized from bismaleimide 250, furfural-derived diol 248, and triisocyanate 246; Diels–Alder reactions between the furan and maleimide moieties were employed to form a more highly crosslinked structure containing rigid oxanorbornene units. Furfural-derived polymer 249 had a glass transition temperature of 29 °C, tensile modulus of 7.2 MPa, tensile strength of 5.4 MPa, and elongation at break of 101%. The corresponding properties of the analogous benzaldehyde-derived polymer 253 were similar. The more highly crosslinked polymer 251 exhibited significantly different thermal and mechanical properties with a glass transition temperature of 100 °C, tensile modulus of 1599 MPa, tensile strength of 106 MPa, and elongation at break of 11%. The acetal-containing materials 249, 253, and 251 exhibited stress relaxation upon heating, and they could be reprocessed and reformed into films with little loss of mechanical performance. In 0.1 M solutions of HCl in acetone/water, network 249 degraded, with greater degradation rates observed as the ratio of acetone to water was increased.
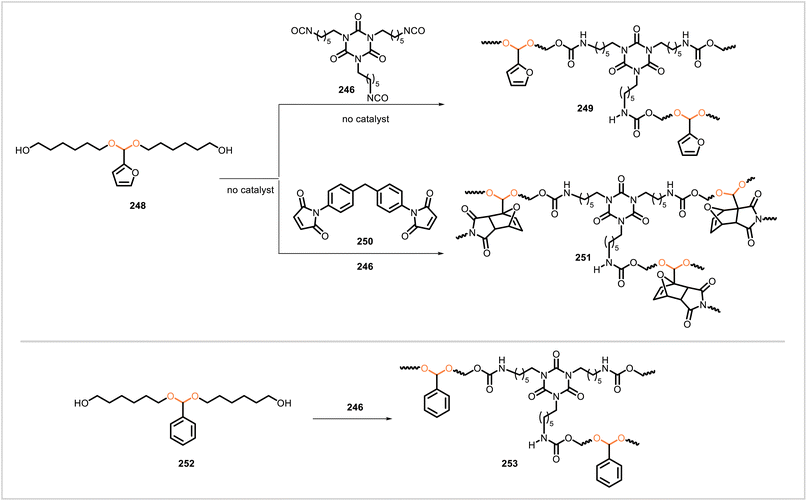 | ||
| Scheme 46 Construction of urethane-based covalent adaptable networks from acetal-containing monomers. | ||
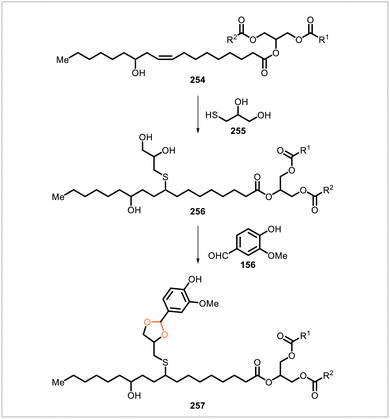
Taher et al. chemically modified castor oil (depicted by 254, a representative triglyceride component), first by a thiol–ene reaction with 1-thioglycerol (255) to afford derivative 256 and then by an acetalization reaction with vanillin (156) to yield derivative 257 (Scheme 47).116 Derivatives 256 and 257 were each converted to three different polyurethanes through reaction with isophorone diisocyanate, 1,6-hexamethylene diisocyanate, and 4,4′-methylenedicyclohexyl diisocyanate. The acetal-containing polyurethanes prepared from 257 had glass transition temperatures of 22.6–37.4 °C and exhibited tensile moduli of 150–257 MPa, tensile strengths of 17.4–46.4 MPa, and elongations at break of 5.2–49.7%. The polyurethane network prepared from 257 and isophorone diisocyanate could be reprocessed by hot-pressing, although the tensile modulus, tensile strength, and elongation at break of the material were somewhat lower after one reprocessing cycle. This polyurethane was also shown to undergo degradation under acidic conditions.
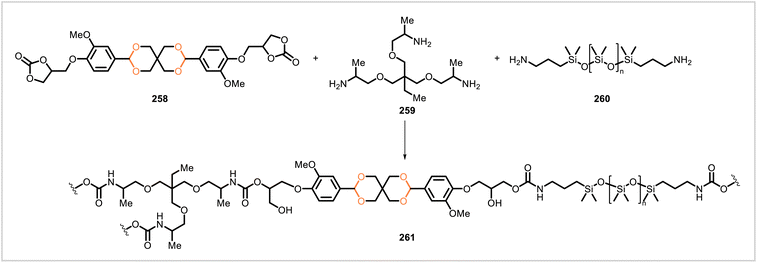
Monomer 258, prepared in three steps from vanillin, was used for the synthesis of non-isocyanate polyurethanes (Scheme 48).117 The cyclic carbonate groups of 258 underwent reaction with diamine 260 and triamine 259, affording crosslinked polyurethane networks. A series of these polymers with different ratios of 260 to 259, and thus different crosslink densities, were synthesized. Stress relaxation experiments indicated that the materials could undergo dynamic bond exchanges. The materials could be remolded by hot-pressing with little loss in mechanical properties. The polymers also degraded in acidic solutions. The degradation products from one of the polymers were recovered and used to resynthesize the same polymer with nearly identical mechanical and thermal properties.
3.3. Other crosslinked polyacetals
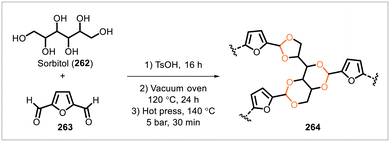
In addition to acetal-containing epoxy resins and crosslinked polyurethanes, a variety of other crosslinked polyacetals have been reported. While the structures of the monomers and resulting polymer networks vary widely, these crosslinked polymers share the qualities of degradability and/or reprocessability thanks to the presence of acetal linkages in the materials.Png et al.118 synthesized an acetal-based covalent adaptable network from the sugar derivatives furan-2,5-dicarbaldehyde (263) and sorbitol (262) (Scheme 49). Polymer 264 was prepared via the acid-catalyzed condensation of 263 and 262 in a 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 molar ratio, performed by stirring the reactants and p-toluenesulfonic acid in DMSO under a stream of nitrogen, then drying the resulting gel-like solid in a vacuum oven and finally hot-pressing the polymer. The rigid, hard product had a glass transition temperature of 120 °C (as measured by thermogravimetric analysis), a storage modulus of 3300 MPa, and tensile strength of 13 MPa. The material could be cut into pieces and hot-pressed into films, whose tensile strength (9 MPa) was only somewhat decreased relative to that of the pristine polymer. Alternatively, the polymer could be chemically recycled: most of the polymer was dissolved by heating in water, the resulting solution was concentrated in vacuo, and the polymer was reformed under the same conditions used for the synthesis of the pristine polymer. The tensile strength of the chemically recycled polymer was close to that of the pristine polymer. The monomers 263 and 262 could also be separated and recovered (in 86% and 90% recovery, respectively).
3 molar ratio, performed by stirring the reactants and p-toluenesulfonic acid in DMSO under a stream of nitrogen, then drying the resulting gel-like solid in a vacuum oven and finally hot-pressing the polymer. The rigid, hard product had a glass transition temperature of 120 °C (as measured by thermogravimetric analysis), a storage modulus of 3300 MPa, and tensile strength of 13 MPa. The material could be cut into pieces and hot-pressed into films, whose tensile strength (9 MPa) was only somewhat decreased relative to that of the pristine polymer. Alternatively, the polymer could be chemically recycled: most of the polymer was dissolved by heating in water, the resulting solution was concentrated in vacuo, and the polymer was reformed under the same conditions used for the synthesis of the pristine polymer. The tensile strength of the chemically recycled polymer was close to that of the pristine polymer. The monomers 263 and 262 could also be separated and recovered (in 86% and 90% recovery, respectively).
Benzoxazine 265 (Scheme 50) was prepared from paraformaldehyde and the biobased compounds erythritol, vanillin, and stearylamine.119 Upon heating at 180–200 °C, 265 was converted to an acid-degradable benzoxazine (266). The benzoxazine network underwent fast stress relaxation and was mechanically reprocessable: a sample was crushed into a powder and subsequently was formed into a film by hot-pressing.
Starch is a natural polyacetal consisting of glucose units joined through α-1,4-glycosidic bonds. Oxidation of starch with sodium periodate yields dialdehyde starch (267).120 In work by Zhang et al., biobased plastics were synthesized by crosslinking 267 with the C6–C12 diamines 268–271, which reacted with the aldehyde groups of 267 to form imine linkages (Scheme 51).121 The crosslinked polymers 272–275 had glass transition temperatures ranging from 107 °C to 191 °C, with higher values for the materials crosslinked with shorter diamines. The polymers exhibited greater mechanical strength than polypropylene, low-density polyethylene, poly(methyl methacrylate), and polyhydroxybutyrate. The materials could be heat-pressed into films and showed high thermal stability. Polymer 273, prepared from 267 and the C8 diamine 269, could be remolded by heat-pressing and showed self-healing behavior. All of the materials exhibited significantly higher water resistance than thermoplastic starch, with much lower water uptake rates. However, a sample of polymer 273 underwent chemical degradation in 5% acetic acid solution at room temperature or in 1,8-octanediamine at 80 °C.
Tomović and coworkers synthesized crosslinked poly(imine-acetal)s 279a–d from vanillin-derived dialdehyde 276, triamine 278, and a series of diamines (277a–d) (Scheme 52).122 The polymers showed varying properties, from glassy to rubbery, depending on the diamine structure. The materials derived from the cyclic diamines 277a and 277b were more rigid while those from the linear and flexible diamines 277c and 277d were more elastomer-like. An analogous poly(imine-acetal) (279e) was synthesized from dialdehyde 276 and triamine 278 without the inclusion of a diamine. The acetal and imine groups in this polymer (279e) were shown to undergo hydrolysis in 0.1 M HCl solution. From the mixture of hydrolysis products, vanillin and triamine 278 were recovered and used to resynthesize polymer 279e. The thermal and mechanical properties of the resulting 279e synthesized from recycled monomers were very close to those measured for the original sample of 279e.
Lignin-based vitrimers 282 were synthesized from softwood kraft lignin (280) and poly(ethylene glycol) divinyl ether (281), using a thermally induced reaction of the lignin hydroxyl groups with the vinyl ether functionalities to construct a network with acetal linkages (Scheme 53).123 Four different vitrimers containing 28–50% lignin by weight were prepared. The mechanical properties varied with lignin content: for example, tensile strength increased from 3.3 MPa to 50.9 MPa with increasing lignin content while elongation at break concomitantly decreased from 35% to 1.0%. In stress relaxation analysis, faster relaxation rates and lower activation energies were observed for the materials with higher lignin contents, potentially due to the greater concentrations of acetal bonds and hydroxyl groups available to participate in transacetalization. A vitrimer sample was successfully reprocessed by compression molding and exhibited approximately equivalent mechanical performance as the sample prior to reprocessing. The adhesive behavior of the vitrimer containing 50% lignin by weight was also investigated. An adhesion sample consisting of aluminum sheets bonded with the vitrimer was found to have a 6.0 MPa lap shear strength, comparable to the lap shear strengths of previously reported lignin-based vitrimers (6.5 MPa)124 and a bisphenol A-based epoxy resin (6 MPa).125 After separation, the two aluminum sheets were re-glued to each other by hot-pressing and then showed a lap shear strength of 5.6 MPa, demonstrating that the adhesion performance was largely retained.
Acetal-based covalent adaptable networks (285) designed for fast reprocessability were synthesized from epigallocatechin gallate (283) and varying proportions of tri(ethylene glycol) divinyl ether (284) (Scheme 54).11 A model study was performed to investigate the effect of a nearby phenolic hydroxyl group on the rate of acetal exchange reactions. Compound 286, featuring a phenolic hydroxyl ortho to the acetal substituent, underwent faster acetal exchange with p-cresol (287) than the analogous acetal 290 that contained no adjacent phenolic hydroxyl. This result indicated that the reaction could be accelerated by the neighbouring-group effect of phenolic hydroxyl groups, which were also present in polymer network 285. In elevated-temperature stress-relaxation analysis, complete stress relaxation was observed for CANs 285, confirming the dynamic nature of the covalent networks. The CANs exhibited high rigidity and cross-link density, and they could be reprocessed by hot-pressing, welding, and extrusion. Demonstrating the chemical recyclability of the CAN system, one of the polymer networks was degraded under acidic conditions, 283 was recovered (82% recovery), and the recovered 283 was again cross-linked with 284 to yield a polymer network with nearly equivalent mechanical performance as the pristine material.
 | ||
| Scheme 54 Preparation of an acetal-based covalent adaptable network and model reactions demonstrating acceleration of acetal exchange by an ortho phenolic hydroxyl. | ||
In a study by Qi and coworkers, vitrimers with elastomeric properties were synthesized from glycerol and biobased δ-lactones.35 In the presence of the catalyst diphenyl phosphate, δ-lactones (292, 293, or 294) reacted with glycerol to form the corresponding glycerol esters (296–298) with alcohol functionalities (Scheme 55). These triols were then directly crosslinked with 1,4-cyclohexanedimethanol divinyl ether (299), forming networks 300–302 with acetal linkages. The cross-linked polymers 300–302 exhibited elastomeric properties including Young's moduli in the range 0.27–1.24 MPa. The presence of longer alkyl sidechains from the δ-lactone component imparted greater stretchability and also resulted in lower glass transition temperatures. A broken sample of 300 was repeatedly reprocessed by hot-pressing; its modulus was maintained well after one reprocessing cycle but diminished significantly in subsequent reprocessing cycles. A cut sample of 300 was successfully healed by remolding at 130 °C. Depolymerization of the polymers 300–302 was also possible at 200 °C, which was attributed to relatively low ceiling temperatures for the lactone monomers 292–294. After depolymerization, the lactone monomers were recovered in good yield and high purity. Overall, the materials resembled polydimethylsiloxane in their physical properties but had the advantages of being reprocessable, healable, and depolymerizable.
 | ||
| Scheme 55 Ring-opening transesterification polymerization (ROTP) of lactones with glycerol and subsequent crosslinking through “click” reactions with divinyl ether 299. | ||
Diallyl-substituted monomer 303 (Scheme 56) was synthesized in two steps from vanillin.126 Through thiol–ene “click” photopolymerization, three different polymer networks were prepared: one from 303 and trithiol 304, a second from 303 and tetrathiol 305, and a third from 303 and a mixture of thiols 304 and 305. The resulting cross-linked materials had glass transition temperatures ranging from 20 °C to 49 °C and exhibited a range of mechanical properties depending on the polymer structure. The polymer prepared from 303 and 305 was flexible and was considered to have the best mechanical properties on the basis of its tensile strength (18.2 MPa), elongation at break (103.5%), and toughness (14.5 MPa). All three polymer networks exhibited high transparency, with over 87.7% transmittance of visible light (425–800 nm). The materials also gradually degraded in acidic solution (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 acetic acid/water).
1 acetic acid/water).
 | ||
| Scheme 56 Crosslinked networks derived from monomer 303 and multifunctional thiol monomers 304 and 305. | ||
The diacrylate monomer 310 (derived from 1,4-cyclohexanedione, glycerol, and acryloyl chloride) was used as a crosslinker for UV-curing nanoimprint lithography (Scheme 57).127 Monomer 310 underwent UV curing with an acrylated polysiloxane (309) to afford a nanoimprint resist for the lithography process. Due to the presence of hydrolyzable acetal groups in the crosslinker, residual polymer in the nanoimprint mold could be degraded and removed by heating in acidic solution followed by sonication in acetone.
 | ||
| Scheme 57 Combination of diacetal crosslinker 310 and acrylated polysiloxane 309 employed for UV-curing nanoimprint lithography. | ||
4. Polymers with acetal-containing sidechains
In the previous sections, we have covered biobased polyacetals in which the main chain or backbone of the polymer contains acetal linkages. However, a variety of polymers have been reported in which the acetal functionality is present as part of a sidechain or branch. The latter category of acetal-containing polymers includes the commercial polymer polyvinyl butyral, formed by acetalizing polyvinyl alcohol with butyraldehyde.128 A range of other polymers with pendent acetal groups have been synthesized from biobased starting materials.The Miller group synthesized biobased polyvinyl acetals (312, general structure) by acetalizing polyvinyl alcohol (311) with thirteen different aldehydes129,130 and eleven different ketones130 that are naturally occurring or biobased (entry a, Scheme 58). The acetal groups underwent hydrolysis in acidic aqueous solutions, releasing polyvinyl alcohol. Because polyvinyl alcohol is biodegradable under appropriate conditions, the polyvinyl acetals were potentially entirely degradable.
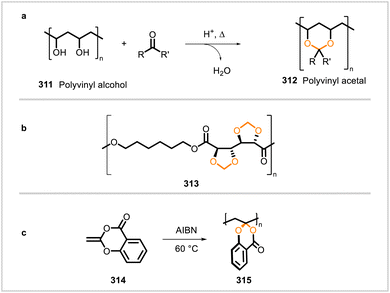 | ||
| Scheme 58 Examples of polymers with pendent acetal groups: (a) polyvinyl acetals, (b) galactose-based polyester, (c) acetylsalicylic acid derived polymer. | ||
A variety of condensation polymers with acetal-containing sidechains have been synthesized. Muñoz-Guerra and coworkers reported several polyesters based on glucose,131,132 galactose,133–135 mannitol,136,137 and tartaric acid43 derivatives containing cyclic methylene acetal units. Scheme 58 (entry b) shows a galactose-based polyester (313)133 from this work. In a study by Zhang et al., furan-derived monomers containing methylene acetal groups were converted to polyesters.138 Diot-Néant et al. prepared an acetal-containing diol monomer from levoglucosenone and synthesized polyesters from this monomer.139 Kolender and coworkers synthesized poly(amide-triazole)s from a galactose derivative featuring isopropylidene acetals.140
Polymerization of cyclic ketene acetal monomers can afford polymers with pendent acetal groups. Kazama and Kohsaka synthesized acetal-containing polymers via radical polymerization and cationic ring-opening polymerization of a cyclic ketene acetal monomer (314) derived from acetylsalicylic acid.141,142 Radical polymerization of 314 affording polymer 315 (ref. 141) is depicted in Scheme 58 (entry c). Polymers prepared through both polymerization methods—radical or cationic ring-opening—underwent hydrolysis under alkaline conditions, resulting in degradation of the polymer and the release of salicylic acid and acetic acid. In work by Buchard and coworkers, another cyclic ketene acetal, prepared from D-glucal, was also employed for radical polymerization.143
The Luterbacher group has used acetalization chemistry to develop processes for the depolymerization and fractionation of lignocellulosic biomass. Upon treatment with aldehydes under acidic conditions, the hydroxyl groups present in lignin and hemicellulose underwent acetalization with the aldehydes to afford acetal-modified derivatives of the biopolymers.69,144–146 A fractionation process based on this strategy yielded a variety of monomers including an acetal-containing monomer (123, Scheme 20) that was subsequently used for the synthesis of poly(acetal-esters).69 In another application of this approach, acetalized xylan and lignin were obtained and converted to amphiphilic surfactants.146
5. Polythioacetals
As discussed in the preceding sections, the acetal functional group has been incorporated into a wide range of polymeric materials. Less commonly, thioacetals—the sulfur-based analogs of acetals—have been utilized in polymer synthesis. A comparison of thioacetals and polythioacetals (Fig. 7) with their oxygen-based counterparts serves to highlight the properties and potential applications of each functional group in polymeric materials.Thioacetals have found extensive application in organic synthesis, and a variety of methods exist for the formation of the functional group and subsequent transformations.147,148 Thioacetal-containing polymers can be prepared either by polymerizing thioacetal-containing monomers149–155 or by constructing the thioacetal units in the polymerization process. Polymerization methods affording thioacetal linkages include polycondensations of thiols with aldehydes,150,156–158 reactions of O,O-acetals with thiols,159–161 and thiol-yne addition reactions.162 Cationic ring-opening polymerizations of cyclic O,S-acetal monomers gave products containing S,S- and O,O-acetal linkages.163,164
Like their oxygen-based counterparts, thioacetal linkages can undergo bond exchanges at high temperatures.152 Thus, polymers with thioacetal crosslinks have functioned as covalent adaptable networks (CANs), undergoing dynamic stress relaxation and allowing for mechanical reprocessing of the network.152 In other thioacetal-based CANs, the thioacetal linkages were reversibly formed through thiol-yne crosslinking.162 A CAN based on O,S-acetal groups has also been reported.165
Compared with oxygen, sulfur is larger and less electronegative, has greater atomic refraction, and exhibits additional modes of reactivity. The differences between these two chalcogenides may have manifold consequences for the structures and properties of polymers with carbon–oxygen or carbon–sulfur bonds.166–171 One important distinction between polyacetals and polythioacetals is the conditions under which they undergo degradation.
In the presence of protic acids, thioacetals tend to undergo hydrolysis less rapidly than acetals.12 Thioacetal hydrolysis can be catalyzed by soft Lewis acids such as Hg2+ and Ag+ ions,12,172 and thioacetal-based materials have been investigated as tools for Hg2+ sensing.157,158 Thioacetals are also susceptible to oxidative cleavage, and thioacetal-containing polymers can degrade in the presence of reactive oxygen species (ROS).149,151,153,161,173,174 Consequently, polythioacetals have been used as investigational ROS-responsive materials for biomedical applications, including nanoparticles for drug delivery153,161,174 and polymeric foam dressings for wound healing.151
The degradation rates of polythioacetals and the conditions under which a polythioacetal degrades depend on other structural features and functional groups in the polymer. The rate of hydrogen peroxide-mediated oxidative cleavage of thioacetals varies according to the substituents on the thioacetal group.175 Additionally, polymeric nanoparticles containing photoreactive 2-nitrophenyl-substituted thioacetal linkages underwent light-triggered degradation but were stable in solutions containing the oxidizing agents H2O2 and KO2 at 100 mM.176
Like oxygen-based polyacetals, polythioacetals may be prepared from biobased starting materials. For example, cinnamaldehyde-based polythioacetals have been reported.153,174 Continued investigations of polythioacetals and their unique properties may lead to the development of new materials with greater sustainability.
6. Conclusion
Acetal-containing polymers exhibit diverse structures and mechanical properties, but common characteristics within this group of materials frequently include degradability and reprocessability. Degradation of polyacetals under acidic conditions could potentially be leveraged to decrease polymeric waste and may enable chemical recycling of the materials. The reversible nature of acetal linkages has particularly striking implications for polymers with acetal-based crosslinks. Unlike conventional thermosets with irreversible crosslinks, these crosslinked polyacetals can be chemically degraded, and acetal-based covalent adaptable networks can undergo reprocessing, allowing for mechanical recycling of the material. In addition to our main discussion of polyacetals, we also note polythioacetals as a less-explored group of polymers that share some characteristics with polyacetals while also offering different properties including alternative degradation behavior.As highlighted in this review, a variety of biobased starting materials have been used for the synthesis of polyacetals. The commercially available polyacetal known as Akestra is derived in part from biobased pentaerythritol. Numerous polyacetals have been prepared from vanillin (derivable from lignin), and some vanillin-based, acetal-containing epoxy resins have shown mechanical properties that compare favorably with those of epoxy resins based on the commercial, widely used monomer bisphenol A.104,106,109 Other polyacetals have been prepared using monomers from carbohydrates, lignin, and plant oils as well as other natural compounds such as the terpenoid camphor. Given the advantageous characteristics of these and other polyacetals, the development of new biobased polyacetals holds promise for a more sustainable future.
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Research was sponsored by the Army Research Laboratory and was accomplished under Cooperative Agreement Number W911NF-19-2-0138. The views and conclusions contained in this document are those of the authors and should not be interpreted as representing the official policies, either expressed or implied, of the Army Research Laboratory or the U.S. Government. The U.S. Government is authorized to reproduce and distribute reprints for Government purposes notwithstanding any copyright notation herein.Notes and references
- L. Shen, E. Worrell and M. Patel, Present and future development in plastics from biomass, Biofuels, Bioprod. Biorefin., 2010, 4, 25–40, DOI:10.1002/bbb.189.
- A. Schade, M. Melzer, S. Zimmermann, T. Schwarz, K. Stoewe and H. Kuhn, Plastic Waste Recycling-A Chemical Recycling Perspective, ACS Sustainable Chem. Eng., 2024, 4c02551, DOI:10.1021/acssuschemeng.4c02551.
- R. L. Smith, S. Takkellapati and R. C. Riegerix, Recycling of Plastics in the United States: Plastic Material Flows and Polyethylene Terephthalate (PET) Recycling Processes, ACS Sustainable Chem. Eng., 2022, 10, 2084–2096, DOI:10.1021/acssuschemeng.1c06845.
- B. D. Vogt, K. K. Stokes and S. K. Kumar, Why is Recycling of Postconsumer Plastics so Challenging?, ACS Appl. Polym. Mater., 2021, 3, 4325–4346, DOI:10.1021/acsapm.1c00648.
- J. R. Jambeck, R. Geyer, C. Wilcox, T. R. Siegler, M. Perryman, A. Andrady, R. Narayan and K. L. Law, Plastic waste inputs from land into the ocean, Science, 2015, 347, 768–771, DOI:10.1126/science.1260352.
- J. N. Hahladakis, Delineating and preventing plastic waste leakage in the marine and terrestrial environment, Environ. Sci. Pollut. Res., 2020, 27, 12830–12837, DOI:10.1007/s11356-020-08139-y.
- G. Fiorentino, M. Ripa and S. Ulgiati, Chemicals from biomass: technological versus environmental feasibility. A review, Biofuels, Bioprod. Biorefin., 2017, 11, 195–214, DOI:10.1002/bbb.1729.
- H. Nishida, Development of materials and technologies for control of polymer recycling, Polym. J., 2011, 43, 435–447, DOI:10.1038/pj.2011.16.
- X.-B. Meng, T. Zhou, C. Yang, X.-Y. Cheng, X.-T. Wu, C. Shi, F.-S. Du and Z.-C. Li, Thermally Stable and Chemically Recyclable Poly(ketal-ester)s Regulated by Floor Temperature, J. Am. Chem. Soc., 2024, 146, 15428–15437, DOI:10.1021/jacs.4c03523.
- C. D. Roland, C. M. Moore, J. H. Leal, T. A. Semelsberger, C. Snyder, J. Kostal and A. D. Sutton, Fully Recyclable Polycarbonates from Simple, Bio-Derived Building Blocks, ACS Appl. Polym. Mater., 2021, 3, 730–736, DOI:10.1021/acsapm.0c01028.
- Q. Li, S. Ma, P. Li, B. Wang, Z. Yu, H. Feng, Y. Liu and J. Zhu, Fast Reprocessing of Acetal Covalent Adaptable Networks with High Performance Enabled by Neighboring Group Participation, Macromolecules, 2021, 54, 8423–8434, DOI:10.1021/acs.macromol.1c01046.
- D. P. N. Satchell and R. S. Satchell, Mechanisms of hydrolysis of thioacetals, Chem. Soc. Rev., 1990, 19, 55–81, 10.1039/cs9901900055.
- P. Deslongchamps, Y. L. Dory and S. Li, The Relative Rate of Hydrolysis of a Series of Acyclic and Six-Membered Cyclic Acetals, Ketals, Orthoesters, and Orthocarbonates, Tetrahedron, 2000, 56, 3533–3537, DOI:10.1016/S0040-4020(00)00270-2.
- M. Rostagno, E. J. Price, A. G. Pemba, I. Ghiriviga, K. A. Abboud and S. A. Miller, Sustainable polyacetals from erythritol and bioaromatics, J. Appl. Polym. Sci., 2016, 133, 44089, DOI:10.1002/app.44089.
- N. G. Valsange, M. N. Garcia Gonzalez, N. Warlin, S. V. Mankar, N. Rehnberg, S. Lundmark, B. Zhang and P. Jannasch, Biobased aliphatic polyesters from a spirocyclic dicarboxylate monomer derived from levulinic acid, Green Chem., 2021, 23, 5706–5723, 10.1039/D1GC00724F.
- J. Wang, L. Wang, D. J. Gardner, S. M. Shaler and Z. Cai, Towards a cellulose-based society: opportunities and challenges, Cellulose, 2021, 28, 4511–4543, DOI:10.1007/s10570-021-03771-4.
- Polyoxymethylene Handbook: Structure, Properties, Applications and Their Nanocomposites, ed. S. Lüftl, P. M. Visakh and S. Chandran, Scrivener Publishing LLC, Beverly, MA, 2014 Search PubMed.
- C. Carrot, A. Bendaoud and C. Pillon, in Handbook of Thermoplastics, ed. O. Olabisi and K. Adewale, CRC Press, Boca Raton, FL, 2nd edn, 2016, ch. 3, pp. 89–138 Search PubMed.
- L. Aristizábal-Lanza, S. V. Mankar, C. Tullberg, B. Zhang and J. A. Linares-Pastén, Comparison of the enzymatic depolymerization of polyethylene terephthalate and Akestra™ using Humicola insolens cutinase, Front. Chem. Eng., 2022, 4, 1048744, DOI:10.3389/fceng.2022.1048744.
- Product data sheet for Akestra™ 90 (CAS number: 102070-64-4) from Perstorp AB, https://www.perstorp.com/en/products/akestra_90, accessed July 15, 2024 Search PubMed.
- Product data sheet for Akestra™ 100 (CAS number: 102070-64-4) from Perstorp AB, https://www.perstorp.com/en/products/akestra_100, accessed July 15, 2024 Search PubMed.
- Product data sheet for Akestra™ 110 (CAS number: 102070-64-4) from Perstorp AB, https://www.perstorp.com/en/products/akestra_110, accessed July 15, 2024 Search PubMed.
- T. N. Tsironi, S. M. Chatzidakis and N. G. Stoforos, The future of polyethylene terephthalate bottles: Challenges and sustainability, Packag. Technol. Sci., 2022, 35, 317–325, DOI:10.1002/pts.2632.
- N. Zheng, Y. Xu, Q. Zhao and T. Xie, Dynamic Covalent Polymer Networks: A Molecular Platform for Designing Functions beyond Chemical Recycling and Self-Healing, Chem. Rev., 2021, 121, 1716–1745, DOI:10.1021/acs.chemrev.0c00938.
- Y. Zhang, L. Zhang, G. Yang, Y. Yao, X. Wei, T. Pan, J. Wu, M. Tian and P. Yin, Recent advances in recyclable thermosets and thermoset composites based on covalent adaptable networks, J. Mater. Sci. Technol., 2021, 92, 75–87, DOI:10.1016/j.jmst.2021.03.043.
- I. Delidovich, P. J. C. Hausoul, L. Deng, R. Pfützenreuter, M. Rose and R. Palkovits, Alternative Monomers Based on Lignocellulose and Their Use for Polymer Production, Chem. Rev., 2016, 116, 1540–1599, DOI:10.1021/acs.chemrev.5b00354.
- A. Moreno, G. Lligadas, J. C. Ronda, M. Galià and V. Cádiz, Linear and branched acetal polymers from castor oil via acetal metathesis polymerization, Eur. Polym. J., 2018, 108, 348–356, DOI:10.1016/j.eurpolymj.2018.09.013.
- A. G. Pemba, J. A. Flores and S. A. Miller, Acetal metathesis polymerization (AMP): A method for synthesizing biorenewable polyacetals, Green Chem., 2013, 15, 325–329, 10.1039/c2gc36588j.
- C. Wang, Z. Du, J. Pan, J. Li and Z. Yang, Direct conversion of biomass to bio-petroleum at low temperature, J. Anal. Appl. Pyrolysis, 2007, 78, 438–444, DOI:10.1016/j.jaap.2006.10.016.
- F. H. Isikgor and C. R. Becer, Lignocellulosic biomass: a sustainable platform for the production of bio-based chemicals and polymers, Polym. Chem., 2015, 6, 4497–4559, 10.1039/C5PY00263J.
- R. M. Cywar, N. A. Rorrer, C. B. Hoyt, G. T. Beckham and E. Y.-X. Chen, Bio-based polymers with performance-advantaged properties, Nat. Rev. Mater., 2022, 7, 83–103, DOI:10.1038/s41578-021-00363-3.
- S. Nanda, R. Azargohar, A. K. Dalai and J. A. Kozinski, An assessment on the sustainability of lignocellulosic biomass for biorefining, Renewable Sustainable Energy Rev., 2015, 50, 925–941, DOI:10.1016/j.rser.2015.05.058.
- M. B. Comba, Y. Tsai, A. M. Sarotti, M. I. Mangione, A. G. Suárez and R. A. Spanevello, Levoglucosenone and Its New Applications: Valorization of Cellulose Residues, Eur. J. Org Chem., 2018, 2018, 590–604, DOI:10.1002/ejoc.201701227.
- M. I. F. Mota, P. C. Rodrigues Pinto, J. M. Loureiro and A. E. Rodrigues, Recovery of Vanillin and Syringaldehyde from Lignin Oxidation: A Review of Separation and Purification Processes, Sep. Purif. Rev., 2016, 45, 227–259, DOI:10.1080/15422119.2015.1070178.
- L. Yue, Y.-L. Su, M. Li, L. Yu, S. M. Montgomery, X. Sun, M. G. Finn, W. R. Gutekunst, R. Ramprasad and H. J. Qi, One-Pot Synthesis of Depolymerizable δ-Lactone Based Vitrimers, Adv. Mater., 2023, 35, 2300954, DOI:10.1002/adma.202300954.
- J. J. La Scala, J. M. Sands, J. A. Orlicki, E. J. Robinette and G. R. Palmese, Fatty acid-based monomers as styrene replacements for liquid molding resins, Polymer, 2004, 45, 7729–7737, DOI:10.1016/j.polymer.2004.08.056.
- J. E. Park, D. Y. Hwang, G. H. Choi, K. H. Choi and D. H. Suh, Fast Hydrolysis Polyesters with a Rigid Cyclic Diol from Camphor, Biomacromolecules, 2017, 18, 2633–2639, DOI:10.1021/acs.biomac.7b00761.
- G.-H. Choi, D. Y. Hwang and D. H. Suh, High Thermal Stability of Bio-Based Polycarbonates Containing Cyclic Ketal Moieties, Macromolecules, 2015, 48, 6839–6845, DOI:10.1021/acs.macromol.5b01112.
- F. Della Monica and A. W. Kleij, From terpenes to sustainable and functional polymers, Polym. Chem., 2020, 11, 5109–5127, 10.1039/D0PY00817F.
- B. Kost and M. Basko, Synthesis and properties of l-lactide/1,3-dioxolane copolymers: preparation of polyesters with enhanced acid sensitivity, Polym. Chem., 2021, 12, 2551–2562, 10.1039/D1PY00358E.
- K. Cichoń, I. I. Bak-Sypien, M. Basko and B. Kost, Synthesis and Characterization of Functionalized Polylactides Containing Acetal Units, Macromolecules, 2023, 56, 6951–6967, DOI:10.1021/acs.macromol.3c01343.
- H. Ramezani Dana and F. Ebrahimi, Synthesis, properties, and applications of polylactic acid-based polymers, Polym. Eng. Sci., 2023, 63, 22–43, DOI:10.1002/pen.26193.
- C. Japu, A. Martínez de Ilarduya, A. Alla and S. Muñoz-Guerra, Bio-based poly(ethylene terephthalate) copolyesters made from cyclic monomers derived from tartaric acid, Polymer, 2014, 55, 2294–2304, DOI:10.1016/j.polymer.2014.03.018.
- Y. Tachibana, T. Masuda, M. Funabashi and M. Kunioka, Chemical Synthesis of Fully Biomass-Based Poly(butylene succinate) from Inedible-Biomass-Based Furfural and Evaluation of Its Biomass Carbon Ratio, Biomacromolecules, 2010, 11, 2760–2765, DOI:10.1021/bm100820y.
- A. Hufendiek, S. Lingier and F. E. Du Prez, Thermoplastic polyacetals: chemistry from the past for a sustainable future?, Polym. Chem., 2019, 10, 9–33, 10.1039/C8PY01219A.
- S. Chikkali, F. Stempfle and S. Mecking, Long-Chain Polyacetals From Plant Oils, Macromol. Rapid Commun., 2012, 33, 1126–1129, DOI:10.1002/marc.201200226.
- M. Konig, L. M. Chiarello, L. Curbani, V. Botton, V. R. Wiggers and L. Ender, Heptaldehyde and undecylenic acid production from thermal and thermo-catalytic cracking of castor oil, Ind. Crops Prod., 2023, 201, 116931, DOI:10.1016/j.indcrop.2023.116931.
- B. S. Rajput, S. R. Gaikwad, S. K. Menon and S. H. Chikkali, Sustainable polyacetals from isohexides, Green Chem., 2014, 16, 3810–3818, 10.1039/C4GC00543K.
- B. S. Rajput, U. Chander, K. Arole, F. Stempfle, S. Menon, S. Mecking and S. H. Chikkali, Synthesis of Renewable Copolyacetals with Tunable Degradation, Macromol. Chem. Phys., 2016, 217, 1396–1410, DOI:10.1002/macp.201600071.
- N. Hammami, N. Jarroux, M. Robitzer, M. Majdoub and J.-P. Habas, Optimized Synthesis According to One-Step Process of a Biobased Thermoplastic Polyacetal Derived from Isosorbide, Polymers, 2016, 8, 294, DOI:10.3390/polym8080294.
- N. Hammami, M. Majdoub and J.-P. Habas, Structure-properties relationships in isosorbide-based polyacetals: Influence of linear or cyclic architecture on polymer physicochemical properties, Eur. Polym. J., 2017, 93, 795–804, DOI:10.1016/j.eurpolymj.2017.03.050.
- T. Debsharma, Y. Yagci and H. Schlaad, Cellulose-Derived Functional Polyacetal by Cationic Ring-Opening Polymerization of Levoglucosenyl Methyl Ether, Angew. Chem., Int. Ed., 2019, 58, 18492–18495, DOI:10.1002/anie.201908458.
- K. Kaya, T. Debsharma, H. Schlaad and Y. Yagci, Cellulose-based polyacetals by direct and sensitized photocationic ring-opening polymerization of levoglucosenyl methyl ether, Polym. Chem., 2020, 11, 6884–6889, 10.1039/D0PY01307B.
- M. Higuchi, A. Kanazawa and S. Aoshima, Unzipping and scrambling reaction-induced sequence control of copolymer chains via temperature changes during cationic ring-opening copolymerization of cyclic acetals and cyclic esters, J. Polym. Sci., 2021, 59, 2730–2741, DOI:10.1002/pol.20210197.
- M. Higuchi, A. Kanazawa and S. Aoshima, Tandem Unzipping and Scrambling Reactions for the Synthesis of Alternating Copolymers by the Cationic Ring-Opening Copolymerization of a Cyclic Acetal and a Cyclic Ester, ACS Macro Lett., 2020, 9, 77–83, DOI:10.1021/acsmacrolett.9b00874.
- K. Takebayashi, A. Kanazawa and S. Aoshima, Cationic ring-opening copolymerization of a cyclic acetal and γ-butyrolactone: monomer sequence transformation and polymerization–depolymerization control by vacuuming or temperature changes, Polym. J., 2024, 56, 309–317, DOI:10.1038/s41428-023-00847-9.
- K. Cichoń, B. Kost and M. Basko, Synthesis and properties of ABA-triblock copolymers from polyester A-blocks and easily degradable polyacetal B-blocks, Polym. Chem., 2022, 13, 5243–5255, 10.1039/D2PY00620K.
- L. Fu, X. Sui, A. E. Crolais and W. R. Gutekunst, Modular Approach to Degradable Acetal Polymers Using Cascade Enyne Metathesis Polymerization, Angew. Chem., Int. Ed., 2019, 58, 15726–15730, DOI:10.1002/anie.201909172.
- S. D. Luebben and J. W. Raebiger, in Green Polymer Chemistry: Biobased Materials and Biocatalysis, ACS Symposium Series, ed. H. N. Cheng, R. A. Gross and P. B. Smith, American Chemical Society, Washington, DC, 2015, ch. 19, vol. 1192, pp. 305–328 Search PubMed.
- T. Debsharma, F. N. Behrendt, A. Laschewsky and H. Schlaad, Ring-Opening Metathesis Polymerization of Biomass-Derived Levoglucosenol, Angew. Chem., Int. Ed., 2019, 58, 6718–6721, DOI:10.1002/anie.201814501.
- T. Debsharma, B. Schmidt, A. Laschewsky and H. Schlaad, Ring-Opening Metathesis Polymerization of Unsaturated Carbohydrate Derivatives: Levoglucosenyl Alkyl Ethers, Macromolecules, 2021, 54, 2720–2728, DOI:10.1021/acs.macromol.0c02821.
- M. Shen, S. Vijjamarri, H. Cao, K. Solis and M. L. Robertson, Degradability, thermal stability, and high thermal properties in spiro polycycloacetals partially derived from lignin, Polym. Chem., 2021, 12, 5986–5998, 10.1039/D1PY01017D.
- A. G. Pemba, M. Rostagno, T. A. Lee and S. A. Miller, Cyclic and spirocyclic polyacetal ethers from lignin-based aromatics, Polym. Chem., 2014, 5, 3214–3221, 10.1039/C4PY00178H.
- S. Lingier, S. Nevejans, P. Espeel, S. De Wildeman and F. E. Du Prez, High molecular weight poly(cycloacetals) towards processable polymer materials, Polymer, 2016, 103, 98–103, DOI:10.1016/j.polymer.2016.09.040.
- A. C. Law, D. S. Stankowski, B. H. Bomann, S. Suhail, K. H. Salmon, S. W. Paulson, M. J. Carney and N. J. Robertson, Synthesis and material properties of elastomeric high molecular weight polycycloacetals derived from diglycerol and meso-erythritol, J. Appl. Polym. Sci., 2020, 137, 48780, DOI:10.1002/app.48780.
- A. Hufendiek, S. Lingier, P. Espeel, S. De Wildeman and F. E. Du Prez, Polycycloacetals via polytransacetalization of diglycerol bisacetonide, Polym. Chem., 2018, 9, 4789–4797, 10.1039/C8PY01191E.
- A. Sudo, T. Sano, M. Harada and D. Ishida, Synthesis of Oligo(spiroketal)s from Naturally Occurring myo-Inositol, ACS Macro Lett., 2014, 3, 808–812, DOI:10.1021/mz500353y.
- R. W. Alder and B. S. R. Reddy, Attempted equilibration of an insoluble spiran polymer with monomers and oligomers through reversible chemical reactions: transketalization route to spiropolymers from 1,4-cyclohexanedione and pentaerythritol, Polymer, 1994, 35, 5765–5772, DOI:10.1016/S0032-3861(05)80054-0.
- L. P. Manker, G. R. Dick, A. Demongeot, M. A. Hedou, C. Rayroud, T. Rambert, M. J. Jones, I. Sulaeva, M. Vieli, Y. Leterrier, A. Potthast, F. Maréchal, V. Michaud, H.-A. Klok and J. S. Luterbacher, Sustainable polyesters via direct functionalization of lignocellulosic sugars, Nat. Chem., 2022, 14, 976–984, DOI:10.1038/s41557-022-00974-5.
- C. Schädel, A. Blöchl, A. Richter and G. Hoch, Quantification and monosaccharide composition of hemicelluloses from different plant functional types, Plant Physiol. Biochem., 2010, 48, 1–8, DOI:10.1016/j.plaphy.2009.09.008.
- H. Hu, Y. Tian, Z. Kong, W. B. Ying, C. Chen, F. Li, R. Zhang and J. Zhu, A High Performance Copolyester with “Locked” Biodegradability: Solid Stability and Controlled Degradation Enabled by Acid-Labile Acetal, ACS Sustainable Chem. Eng., 2021, 9, 2280–2290, DOI:10.1021/acssuschemeng.0c08274.
- T. Zhou, X. Meng, F. Du and Z. Li, Fully Bio-based Poly(ketal-ester)s by Ring-opening Polymerization of a Bicylcic Lactone from Glycerol and Levulinic Acid, Chem.–Asian J., 2023, 18, e202201238, DOI:10.1002/asia.202201238.
- R. Sedrik, O. Bonjour, S. Laanesoo, I. Liblikas, T. Pehk, P. Jannasch and L. Vares, Chemically Recyclable Poly(β-thioether ester)s Based on Rigid Spirocyclic Ketal Diols Derived from Citric Acid, Biomacromolecules, 2022, 23, 2685–2696, DOI:10.1021/acs.biomac.2c00452.
- P. Verdugo, G. Lligadas, J. C. Ronda, M. Galià and V. Cádiz, Bio-based ABA triblock copolymers with central degradable moieties, Eur. Polym. J., 2021, 147, 110321, DOI:10.1016/j.eurpolymj.2021.110321.
- A. S. Amarasekara and S. A. Hawkins, Synthesis of levulinic acid–glycerol ketal–ester oligomers and structural characterization using NMR spectroscopy, Eur. Polym. J., 2011, 47, 2451–2457, DOI:10.1016/j.eurpolymj.2011.09.007.
- F. Socha, I. Dobáš and J. Šňupárek, Cyclic acetals: Synthesis and polymerization, J. Appl. Polym. Sci., 2001, 81, 2875–2880, DOI:10.1002/app.1737.
- W. R. Miller, R. A. Awl, E. H. Pryde and J. C. Cowan, Poly(ester-acetals) from Azelaaldehydic Acid-Glycerol Compounds, J. Polym. Sci., Part A-1: Polym. Chem., 1970, 8, 415–427, DOI:10.1002/pol.1970.150080213.
- R. W. Lenz, Structure, Properties, and Cross-linking Reactions of Poly(ester acetals), Macromolecules, 1969, 2, 129–136, DOI:10.1021/ma60008a004.
- N. G. Valsange, N. Warlin, S. V. Mankar, N. Rehnberg, B. Zhang and P. Jannasch, Improved chemical recyclability of 2,5-furandicarboxylate polyesters enabled by acid-sensitive spirocyclic ketal units, Green Chem., 2024, 26, 2858–2873, 10.1039/D3GC03099G.
- S. Hayashi, Y. Tachibana, N. Tabata and K. Kasuya, Chemically recyclable bio-based polyester composed of bifuran and glycerol acetal, Eur. Polym. J., 2021, 145, 110242, DOI:10.1016/j.eurpolymj.2020.110242.
- S. V. Mankar, M. N. Garcia Gonzalez, N. Warlin, N. G. Valsange, N. Rehnberg, S. Lundmark, P. Jannasch and B. Zhang, Synthesis, Life Cycle Assessment, and Polymerization of a Vanillin-Based Spirocyclic Diol toward Polyesters with Increased Glass-Transition Temperature, ACS Sustainable Chem. Eng., 2019, 7, 19090–19103, DOI:10.1021/acssuschemeng.9b04930.
- S. V. Mankar, J. Wahlberg, N. Warlin, N. G. Valsange, N. Rehnberg, S. Lundmark, P. Jannasch and B. Zhang, Short-Loop Chemical Recycling via Telechelic Polymers for Biobased Polyesters with Spiroacetal Units, ACS Sustainable Chem. Eng., 2023, 11, 5135–5146, DOI:10.1021/acssuschemeng.2c07176.
- J. P. Kamat, A. Ghosh and T. P. A. Devasagayam, Vanillin as an antioxidant in rat liver mitochondria: Inhibition of protein oxidation and lipid peroxidation induced by photosensitization, Mol. Cell. Biochem., 2000, 209, 47–53, DOI:10.1023/a:1007048313556.
- M. Makni, Y. Chtourou, H. Fetoui, E. M. Garoui, T. Boudawara and N. Zeghal, Evaluation of the antioxidant, anti-inflammatory and hepatoprotective properties of vanillin in carbon tetrachloride-treated rats, Eur. J. Pharmacol., 2011, 668, 133–139, DOI:10.1016/j.ejphar.2011.07.001.
- Y. Murakami, A. Hirata, S. Ito, M. Shoji, S. Tanaka, T. Yasui, M. Machino and S. Fujisawa, Re-evaluation of Cyclooxygenase-2-inhibiting Activity of Vanillin and Guaiacol in Macrophages Stimulated with Lipopolysaccharide, Anticancer Res., 2007, 27, 801–807 CAS.
- S.-L. Wu, J.-C. Chen, C.-C. Li, H.-Y. Lo, T.-Y. Ho and C.-Y. Hsiang, Vanillin Improves and Prevents Trinitrobenzene Sulfonic Acid-Induced Colitis in Mice, J. Pharmacol. Exp. Ther., 2009, 330, 370–376, DOI:10.1124/jpet.109.152835.
- J. Kwon, J. Kim, S. Park, G. Khang, P. M. Kang and D. Lee, Inflammation-Responsive Antioxidant Nanoparticles Based on a Polymeric Prodrug of Vanillin, Biomacromolecules, 2013, 14, 1618–1626, DOI:10.1021/bm400256h.
- L. De Vos, B. Van de Voorde, L. Van Daele, P. Dubruel and S. Van Vlierberghe, Poly(alkylene terephthalate)s: From current developments in synthetic strategies towards applications, Eur. Polym. J., 2021, 161, 110840, DOI:10.1016/j.eurpolymj.2021.110840.
- O. Bonjour, I. Liblikas, T. Pehk, T. Khai-Nghi, K. Rissanen, L. Vares and P. Jannasch, Rigid biobased polycarbonates with good processability based on a spirocyclic diol derived from citric acid, Green Chem., 2020, 22, 3940–3951, 10.1039/D0GC00849D.
- N. Warlin, E. Nilsson, Z. Guo, S. V. Mankar, N. G. Valsange, N. Rehnberg, S. Lundmark, P. Jannasch and B. Zhang, Synthesis and melt-spinning of partly bio-based thermoplastic poly(cycloacetal-urethane)s toward sustainable textiles, Polym. Chem., 2021, 12, 4942–4953, 10.1039/D1PY00450F.
- S. Lingier, Y. Spiesschaert, B. Dhanis, S. De Wildeman and F. E. Du Prez, Rigid Polyurethanes, Polyesters, and Polycarbonates from Renewable Ketal Monomers, Macromolecules, 2017, 50, 5346–5352, DOI:10.1021/acs.macromol.7b00899.
- N. Warlin, M. N. Garcia Gonzalez, S. Mankar, N. G. Valsange, M. Sayed, S.-H. Pyo, N. Rehnberg, S. Lundmark, R. Hatti-Kaul, P. Jannasch and B. Zhang, A rigid spirocyclic diol from fructose-based 5-hydroxymethylfurfural: synthesis, life-cycle assessment, and polymerization for renewable polyesters and poly(urethane-urea)s, Green Chem., 2019, 21, 6667–6684, 10.1039/C9GC03055G.
- A. A. Wróblewska, S. Lingier, J. Noordijk, F. E. Du Prez, S. M. A. De Wildeman and K. V. Bernaerts, Polyamides based on a partially bio-based spirodiamine, Eur. Polym. J., 2017, 96, 221–231, DOI:10.1016/j.eurpolymj.2017.08.056.
- B. Andrade-Gagnon, M. Bélanger-Bouliga, P. T. Nguyen, T. H. D. Nguyen, S. Bourgault and A. Nazemi, Degradable Spirocyclic Polyacetal-Based Core-Amphiphilic Assemblies for Encapsulation and Release of Hydrophobic Cargo, Nanomaterials, 2021, 11, 161, DOI:10.3390/nano11010161.
- D. Ratna, Recent Advances and Applications of Thermoset Resins, Elsevier, Amsterdam, 2nd edn, 2022 Search PubMed.
- S. Ma and D. C. Webster, Degradable thermosets based on labile bonds or linkages: A review, Prog. Polym. Sci., 2018, 76, 65–110, DOI:10.1016/j.progpolymsci.2017.07.008.
- S. Wang, S. Ma, Q. Li, X. Xu, B. Wang, W. Yuan, S. Zhou, S. You and J. Zhu, Facile in situ preparation of high-performance epoxy vitrimer from renewable resources and its application in nondestructive recyclable carbon fiber composite, Green Chem., 2019, 21, 1484–1497, 10.1039/C8GC03477J.
- G. Choi, Y. Oh, S. Jeong, M. Chang and H. Kim, Synthesis of Renewable, Recyclable, Degradable Thermosets Endowed with Highly Branched Polymeric Structures and Reinforced with Carbon Fibers, Macromolecules, 2023, 56, 2526–2535, DOI:10.1021/acs.macromol.2c02479.
- Z. Ma, Y. Wang, J. Zhu, J. Yu and Z. Hu, Bio-based epoxy vitrimers: Reprocessibility, controllable shape memory, and degradability, J. Polym. Sci., Part A: Polym. Chem., 2017, 55, 1790–1799, DOI:10.1002/pola.28544.
- B. R. Elling and W. R. Dichtel, Reprocessable Cross-Linked Polymer Networks: Are Associative Exchange Mechanisms Desirable?, ACS Cent. Sci., 2020, 6, 1488–1496, DOI:10.1021/acscentsci.0c00567.
- M. Capelot, D. Montarnal, F. Tournilhac and L. Leibler, Metal-Catalyzed Transesterification for Healing and Assembling of Thermosets, J. Am. Chem. Soc., 2012, 134, 7664–7667, DOI:10.1021/ja302894k.
- R. Auvergne, S. Caillol, G. David, B. Boutevin and J.-P. Pascault, Biobased Thermosetting Epoxy: Present and Future, Chem. Rev., 2014, 114, 1082–1115, DOI:10.1021/cr3001274.
- T. T. Schug, A. F. Johnson, L. S. Birnbaum, T. Colborn, L. J. Guillette Jr, D. P. Crews, T. Collins, A. M. Soto, F. S. Vom Saal, J. A. McLachlan, C. Sonnenschein and J. J. Heindel, Minireview: Endocrine Disruptors: Past Lessons and Future Directions, Mol. Endocrinol., 2016, 30, 833–847, DOI:10.1210/me.2016-1096.
- A. Kaiho, D. Mazzarella, M. Satake, M. Kogo, R. Sakai and T. Watanabe, Construction of the di(trimethylolpropane) cross linkage and the phenylnaphthalene structure coupled with selective β-O-4 bond cleavage for synthesizing lignin-based epoxy resins with a controlled glass transition temperature, Green Chem., 2016, 18, 6526–6535, 10.1039/C6GC02211A.
- W. Yuan, S. Ma, S. Wang, Q. Li, B. Wang, X. Xu, K. Huang, J. Chen, S. You and J. Zhu, Synthesis of fully bio-based diepoxy monomer with dicyclo diacetal for high-performance, readily degradable thermosets, Eur. Polym. J., 2019, 117, 200–207, DOI:10.1016/j.eurpolymj.2019.05.028.
- S. Ma, J. Wei, Z. Jia, T. Yu, W. Yuan, Q. Li, S. Wang, S. You, R. Liu and J. Zhu, Readily recyclable, high-performance thermosetting materials based on a lignin-derived spiro diacetal trigger, J. Mater. Chem. A, 2019, 7, 1233–1243, 10.1039/C8TA07140C.
- C. Yan, J. Wei, Y. Zhu, H. Xu, D. Liu, G. Chen, X. Liu, J. Dai and D. Lv, Preparation and properties of carbon fiber reinforced bio-based degradable acetal-linkage-containing epoxy resin composites by RTM process, Polym. Compos., 2023, 44, 4081–4094, DOI:10.1002/pc.27381.
- E. Subbotina, C. Montanari, P. Olsén and L. A. Berglund, Fully bio-based cellulose nanofiber/epoxy composites with both sustainable production and selective matrix deconstruction towards infinite fiber recycling systems, J. Mater. Chem. A, 2022, 10, 570–576, 10.1039/D1TA07758A.
- B. Wang, S. Ma, Q. Li, H. Zhang, J. Liu, R. Wang, Z. Chen, X. Xu, S. Wang, N. Lu, Y. Liu, S. Yan and J. Zhu, Facile synthesis of “digestible”, rigid-and-flexible, bio-based building block for high-performance degradable thermosetting plastics, Green Chem., 2020, 22, 1275–1290, 10.1039/C9GC04020J.
- P. Li, S. Ma, B. Wang, X. Xu, H. Feng, Z. Yu, T. Yu, Y. Liu and J. Zhu, Degradable benzyl cyclic acetal epoxy monomers with low viscosity: Synthesis, structure-property relationships, application in recyclable carbon fiber composite, Compos. Sci. Technol., 2022, 219, 109243, DOI:10.1016/j.compscitech.2021.109243.
- Y. Zhang, L. Yuan, G. Liang and A. Gu, Bio-based Tetrafunctional Epoxy Monomer Containing a Dicyclo Diacetal Structure and Its Medium-Temperature-Cured Epoxy Vitrimer System with Excellent Mechanical and Multifunctional Properties, ACS Sustainable Chem. Eng., 2023, 11, 9264–9280, DOI:10.1021/acssuschemeng.3c02568.
- T. Türel and Ž. Tomović, Chemically Recyclable and Upcyclable Epoxy Resins Derived from Vanillin, ACS Sustainable Chem. Eng., 2023, 11, 8308–8316, DOI:10.1021/acssuschemeng.3c00761.
- W. Zhang, F. Gao, X. Chen, L. Shen, Y. Chen and Y. Lin, Recyclable, Degradable, and Fully Bio-Based Covalent Adaptable Polymer Networks Enabled by a Dynamic Diacetal Motif, ACS Sustainable Chem. Eng., 2023, 11, 3065–3073, DOI:10.1021/acssuschemeng.2c06870.
- B. Wang, S. Ma, X. Xu, Q. Li, T. Yu, S. Wang, S. Yan, Y. Liu and J. Zhu, High-Performance, Biobased, Degradable Polyurethane Thermoset and Its Application in Readily Recyclable Carbon Fiber Composites, ACS Sustainable Chem. Eng., 2020, 8, 11162–11170, DOI:10.1021/acssuschemeng.0c02330.
- Q. Li, S. Ma, P. Li, B. Wang, H. Feng, N. Lu, S. Wang, Y. Liu, X. Xu and J. Zhu, Biosourced Acetal and Diels–Alder Adduct Concurrent Polyurethane Covalent Adaptable Network, Macromolecules, 2021, 54, 1742–1753, DOI:10.1021/acs.macromol.0c02699.
- M. A. Taher, Y. Su, X. Wang, X. Xu, M. A. Habib, J. Zhu and J. Chen, Castor oil-derived polyurethane networks multiple recyclability based on reversible dynamic acetal bond, Adv. Mater., 2024, 5, 199–208, 10.1039/D3MA00464C.
- P. Miao, J. Liu, M. He, X. Leng and Y. Li, Bio-based non-isocyanate polyurethane with closed-loop recyclability and its potential application, Chem. Eng. J., 2023, 475, 146398, DOI:10.1016/j.cej.2023.146398.
- Z. M. Png, J. Zheng, S. Kamarulzaman, S. Wang, Z. Li and S. S. Goh, Fully biomass-derived vitrimeric material with water-mediated recyclability and monomer recovery, Green Chem., 2022, 24, 5978–5986, 10.1039/D2GC01556K.
- J. Chen, X. Lu and Z. Xin, A bio-based reprocessable and degradable polybenzoxazine with acetal structures, React. Funct. Polym., 2023, 191, 105653, DOI:10.1016/j.reactfunctpolym.2023.105653.
- W. Lu, Y. Shen, A. Xie and W. Zhang, Preparation and Protein Immobilization of Magnetic Dialdehyde Starch Nanoparticles, J. Phys. Chem. B, 2013, 117, 3720–3725, DOI:10.1021/jp3110908.
- H. Zhang, Z. Su and X. Wang, Starch-Based Rehealable and Degradable Bioplastic Enabled by Dynamic Imine Chemistry, ACS Sustainable Chem. Eng., 2022, 10, 8650–8657, DOI:10.1021/acssuschemeng.2c02537.
- K. Saito, T. Türel, F. Eisenreich and Ž. Tomović, Closed-Loop Recyclable Poly(imine-acetal)s with Dual-Cleavable Bonds for Primary Building Block Recovery, ChemSusChem, 2023, 16, e202301017, DOI:10.1002/cssc.202301017.
- A. Moreno, M. Morsali and M. H. Sipponen, Catalyst-Free Synthesis of Lignin Vitrimers with Tunable Mechanical Properties: Circular Polymers and Recoverable Adhesives, ACS Appl. Mater. Interfaces, 2021, 13, 57952–57961, DOI:10.1021/acsami.1c17412.
- S. Zhang, T. Liu, C. Hao, L. Wang, J. Han, H. Liu and J. Zhang, Preparation of a lignin-based vitrimer material and its potential use for recoverable adhesives, Green Chem., 2018, 20, 2995–3000, 10.1039/C8GC01299G.
- S. G. Prolongo, G. del Rosario and A. Ureña, Comparative study on the adhesive properties of different epoxy resins, Int. J. Adhes. Adhes., 2006, 26, 125–132, DOI:10.1016/j.ijadhadh.2005.02.004.
- M. Ge, J.-T. Miao, K. Zhang, Y. Wu, L. Zheng and L. Wu, Building biobased, degradable, flexible polymer networks from vanillin via thiol–ene “click” photopolymerization, Polym. Chem., 2021, 12, 564–571, 10.1039/D0PY01407A.
- X. Hu, T. Yang, R. Gu, Y. Cui, C. Yuan, H. Ge, W. Wu, W. Li and Y. Chen, A degradable polycyclic cross-linker for UV-curing nanoimprint lithography, J. Mater. Chem. C, 2014, 2, 1836–1843, 10.1039/c3tc32048k.
- H. D. Hermann, J. Ebigt and U. M. Hutten, US Pat., US4205146, 1980 Search PubMed.
- M. Rostagno, S. Shen, I. Ghiviriga and S. A. Miller, Sustainable polyvinyl acetals from bioaromatic aldehydes, Polym. Chem., 2017, 8, 5049–5059, 10.1039/C7PY00205J.
- Y.-K. Su, C. M. Coxwell, S. Shen and S. A. Miller, Polyvinyl alcohol modification with sustainable ketones, Polym. Chem., 2021, 12, 4961–4973, 10.1039/D1PY00656H.
- C. Japu, A. Alla, A. Martínez de Ilarduya, M. G. García-Martín, E. Benito, J. A. Galbis and S. Muñoz-Guerra, Bio-based aromatic copolyesters made from 1,6-hexanediol and bicyclic diacetalized d-glucitol, Polym. Chem., 2012, 3, 2092–2101, 10.1039/c2py20145c.
- C. Japu, A. Martínez de Ilarduya, A. Alla, M. G. García-Martín, J. A. Galbis and S. Muñoz-Guerra, Bio-based PBT copolyesters derived from d-glucose: influence of composition on properties, Polym. Chem., 2014, 5, 3190–3202, 10.1039/C3PY01425H.
- C. Lavilla, A. Alla, A. Martínez de Ilarduya, E. Benito, M. G. García-Martín, J. A. Galbis and S. Muñoz-Guerra, Carbohydrate-Based Polyesters Made from Bicyclic Acetalized Galactaric Acid, Biomacromolecules, 2011, 12, 2642–2652, DOI:10.1021/bm200445w.
- C. Lavilla, A. Alla, A. Martínez de Ilarduya, E. Benito, M. G. García-Martín, J. A. Galbis and S. Muñoz-Guerra, Carbohydrate-based copolyesters made from bicyclic acetalized galactaric acid, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 1591–1604, DOI:10.1002/pola.25930.
- C. Lavilla, A. Alla, A. Martínez de Ilarduya, E. Benito, M. G. García-Martín, J. A. Galbis and S. Muñoz-Guerra, Bio-based poly(butylene terephthalate) copolyesters containing bicyclic diacetalized galactitol and galactaric acid: Influence of composition on properties, Polymer, 2012, 53, 3432–3445, DOI:10.1016/j.polymer.2012.05.048.
- C. Lavilla, A. Martínez de Ilarduya, A. Alla, M. G. García-Martín, J. A. Galbis and S. Muñoz-Guerra, Bio-Based Aromatic Polyesters from a Novel Bicyclic Diol Derived from d-Mannitol, Macromolecules, 2012, 45, 8257–8266, DOI:10.1021/ma3013288.
- C. Lavilla, A. Martínez de Ilarduya, A. Alla and S. Muñoz-Guerra, PET copolyesters made from a d-mannitol-derived bicyclic diol, Polym. Chem., 2013, 4, 282–289, 10.1039/C2PY20531A.
- H. Zhang, G. Zhou, M. Jiang, H. Zhang, H. Wang, Y. Wu and R. Wang, Bio-Based Polyesters with High Glass-Transition Temperatures and Gas Barrier Properties Derived from Renewable Rigid Tricyclic Diacid or Tetracyclic Anhydride, Macromolecules, 2020, 53, 5475–5486, DOI:10.1021/acs.macromol.0c00344.
- F. Diot-Néant, L. M. M. Mouterde, C. Veith, J. Couvreur, S. A. Miller and F. Allais, Sustainable One-Pot Synthesis and Polycondensation of a Levoglucosenone-Derived Cyclic Acetal Diol, ACS Sustainable Chem. Eng., 2022, 10, 10132–10143, DOI:10.1021/acssuschemeng.2c01362.
- M. V. Rivas, O. Varela and A. A. Kolender, Galactose-derived poly(amide-triazole)s. Degradation, deprotection and derivatization studies, Eur. Polym. J., 2020, 130, 109653, DOI:10.1016/j.eurpolymj.2020.109653.
- A. Kazama and Y. Kohsaka, Radical polymerization of ‘dehydroaspirin’ with the formation of a hemiacetal ester skeleton: a hint for recyclable vinyl polymers, Polym. Chem., 2019, 10, 2764–2768, 10.1039/C9PY00474B.
- A. Kazama and Y. Kohsaka, Diverse chemically recyclable polymers obtained by cationic vinyl and ring-opening polymerizations of the cyclic ketene acetal ester “dehydroaspirin”, Polym. Chem., 2022, 13, 6484–6491, 10.1039/D2PY01181F.
- C. Hardy, M. E. Levere, G. Kociok-Köhn and A. Buchard, Radical Ring Opening Polymerization of Cyclic Ketene Acetals Derived From d-Glucal, ACS Macro Lett., 2023, 12, 1443–1449, DOI:10.1021/acsmacrolett.3c00397.
- L. Shuai, M. T. Amiri, Y. M. Questell-Santiago, F. Héroguel, Y. Li, H. Kim, R. Meilan, C. Chapple, J. Ralph and J. S. Luterbacher, Formaldehyde stabilization facilitates lignin monomer production during biomass depolymerization, Science, 2016, 354, 329–333, DOI:10.1126/science.aaf7810.
- W. Lan, M. T. Amiri, C. M. Hunston and J. S. Luterbacher, Protection Group Effects During α,γ-Diol Lignin Stabilization Promote High-Selectivity Monomer Production, Angew. Chem., Int. Ed., 2018, 57, 1356–1360, DOI:10.1002/anie.201710838.
- S. Sun, G. De Angelis, S. Bertella, M. J. Jones, G. R. Dick, E. Amstad and J. S. Luterbacher, Integrated Conversion of Lignocellulosic Biomass to Bio-Based Amphiphiles using a Functionalization-Defunctionalization Approach, Angew. Chem., Int. Ed., 2024, 63, e202312823, DOI:10.1002/anie.202312823.
- P. G. M. Wuts, Greene's Protective Groups in Organic Synthesis, John Wiley & Sons, Inc., Hoboken, NJ, 5th edn, 2014 Search PubMed.
- B.-T. Gröbel and D. Seebach, Umpolung of the Reactivity of Carbonyl Compounds Through Sulfur-Containing Reagents, Synthesis, 1977, 1977, 357–402, DOI:10.1055/s-1977-24412.
- S. Kim, H. Park, F. Fuß and Y. Lee, Synthesis of ROS-responsive poly(thioacetal)s with narrow molecular weight distributions via lactone ring-opening polymerization, Polym. Chem., 2023, 14, 2610–2616, 10.1039/D3PY00239J.
- L. S. Kariyawasam, J. F. Highmoore and Y. Yang, Chemically Recyclable Dithioacetal Polymers via Reversible Entropy-Driven Ring-Opening Polymerization, Angew. Chem., Int. Ed., 2023, 62, e202303039, DOI:10.1002/anie.202303039.
- P. Patil, K. A. Russo, J. T. McCune, A. C. Pollins, M. A. Cottam, B. R. Dollinger, C. R. DeJulius, M. K. Gupta, R. D'Arcy, J. M. Colazo, F. Yu, M. G. Bezold, J. R. Martin, N. L. Cardwell, J. M. Davidson, C. M. Thompson, A. Barbul, A. H. Hasty, S. A. Guelcher and C. L. Duvall, Reactive oxygen species–degradable polythioketal urethane foam dressings to promote porcine skin wound repair, Sci. Transl. Med., 2022, 14, eabm6586, DOI:10.1126/scitranslmed.abm6586.
- H. Zeng, Z. Tang, Y. Duan, S. Wu and B. Guo, Recyclable crosslinked elastomer based on dynamic dithioacetals, Polymer, 2021, 229, 124007, DOI:10.1016/j.polymer.2021.124007.
- L. Xu, M. Zhao, H. Zhang, W. Gao, Z. Guo, X. Zhang, J. Zhang, J. Cao, Y. Pu and B. He, Cinnamaldehyde-Based Poly(ester-thioacetal) To Generate Reactive Oxygen Species for Fabricating Reactive Oxygen Species-Responsive Nanoparticles, Biomacromolecules, 2018, 19, 4658–4667, DOI:10.1021/acs.biomac.8b01423.
- S. Hilf and A. F. M. Kilbinger, Thiol-functionalized ROMP polymers via Sacrificial Synthesis, Macromolecules, 2009, 42, 4127–4133, DOI:10.1021/ma900036c.
- T. Okazaki, F. Sanda and T. Endo, Synthesis and Radical Ring-Opening Polymerization Behavior of Vinylcyclopropanes Bearing Cyclic Thioacetal Moieties, Polym. J., 1998, 30, 365–371, DOI:10.1295/polymj.30.365.
- Y. Jin, C. Hu, J. Wang, Y. Ding, J. Shi, Z. Wang, S. Xu and L. Yuan, Thiol-Aldehyde Polycondensation for Bio-based Adaptable and Degradable Phenolic Polymers, Angew. Chem., 2023, 62, e202305677, DOI:10.1002/ange.202305677.
- Y. Gu, R. Jia, Y. Yu, S. Li, J. Zhu, X. Feng and Y. Lu, Triphenylamine-Based Polythioacetal for Selective Sensing of Mercury(II) with High Specificity and Sensitivity, ACS Appl. Mater. Interfaces, 2024, 16, 10805–10812, DOI:10.1021/acsami.3c19521.
- Y. Gu, S. Li, Y. Yu, J. Zhu, X. Yuan, X. Feng and Y. Lu, Pyrene-Based “Turn-On” Fluorescent Polymeric Probe with Thioacetal Units in the Main Chain for Mercury(II) Detection in Aqueous Solutions and Living Cells, Macromol. Rapid Commun., 2024, 45, 2300631, DOI:10.1002/marc.202300631.
- S. Du, S. Yang, B. Wang, P. Li, J. Zhu and S. Ma, Acetal-thiol Click-like Reaction: Facile and Efficient Synthesis of Dynamic Dithioacetals and Recyclable Polydithioacetals, Angew. Chem., Int. Ed., 2024, 63, e202405653, DOI:10.1002/anie.202405653.
- J. S. Kim, S. D. Jo, G. L. Seah, I. Kim and Y. S. Nam, ROS-induced biodegradable polythioketal nanoparticles for intracellular delivery of anti-cancer therapeutics, J. Ind. Eng. Chem., 2015, 21, 1137–1142, DOI:10.1016/j.jiec.2014.05.026.
- D. S. Wilson, G. Dalmasso, L. Wang, S. V. Sitaraman, D. Merlin and N. Murthy, Orally delivered thioketal nanoparticles loaded with TNF-α–siRNA target inflammation and inhibit gene expression in the intestines, Nat. Mater., 2010, 9, 923–928, DOI:10.1038/nmat2859.
- N. Van Herck, D. Maes, K. Unal, M. Guerre, J. M. Winne and F. E. Du Prez, Covalent Adaptable Networks with Tunable Exchange Rates Based on Reversible Thiol–yne Cross-Linking, Angew. Chem., Int. Ed., 2020, 59, 3609–3617, DOI:10.1002/anie.201912902.
- J. Guzmán and E. Riande, Polymerization of 1-Oxa-3-thiacyclopentane. Structure of the Polymer, Macromolecules, 1980, 13, 1715–1717, DOI:10.1021/ma60078a065.
- M. Uchiyama, Y. Murakami, K. Satoh and M. Kamigaito, Synthesis and Degradation of Vinyl Polymers with Evenly Distributed Thioacetal Bonds in Main Chains: Cationic DT Copolymerization of Vinyl Ethers and Cyclic Thioacetals, Angew. Chem., Int. Ed., 2023, 62, e202215021, DOI:10.1002/anie.202215021.
- Q. Li, S. Ma, N. Lu, J. Qiu, J. Ye, Y. Liu, S. Wang, Y. Han, B. Wang, X. Xu, H. Feng and J. Zhu, Concurrent thiol–ene competitive reactions provide reprocessable, degradable and creep-resistant dynamic–permanent hybrid covalent networks, Green Chem., 2020, 22, 7769–7777, 10.1039/D0GC02823A.
- G. Maier and R. Hecht, Poly(aryl ether thiazole)s with Pendent Trifluoromethyl Groups, Macromolecules, 1995, 28, 7558–7565, DOI:10.1021/ma00126a038.
- H. Mutlu, E. B. Ceper, X. Li, J. Yang, W. Dong, M. M. Ozmen and P. Theato, Sulfur Chemistry in Polymer and Materials Science, Macromol. Rapid Commun., 2019, 40, 1800650, DOI:10.1002/marc.201800650.
- A. Kausar, S. Zulfiqar and M. I. Sarwar, Recent Developments in Sulfur-Containing Polymers, Polym. Rev., 2014, 54, 185–267, DOI:10.1080/15583724.2013.863209.
- H. R. Kricheldorf and G. Schwarz, Poly(thioester)s, J. Macromol. Sci., Part A: Pure Appl.Chem., 2007, 44, 625–649, DOI:10.1080/10601320701285094.
- K. Mazumder, B. Voit and S. Banerjee, Recent Progress in Sulfur-Containing High Refractive Index Polymers for Optical Applications, ACS Omega, 2024, 9, 6253–6279, DOI:10.1021/acsomega.3c08571.
- J. Liu and M. Ueda, High refractive index polymers: fundamental research and practical applications, J. Mater. Chem., 2009, 19, 8907–8919, 10.1039/b909690f.
- T. E. Burghardt, Developments in the deprotection of thioacetals, J. Sulfur Chem., 2005, 26, 411–427, DOI:10.1080/17415990500195198.
- C. Sun, Y. Liang, N. Hao, L. Xu, F. Cheng, T. Su, J. Cao, W. Gao, Y. Pu and B. He, A ROS-responsive polymeric micelle with a π-conjugated thioketal moiety for enhanced drug loading and efficient drug delivery, Org. Biomol. Chem., 2017, 15, 9176–9185, 10.1039/C7OB01975K.
- Y. Tu, X. Xiao, Y. Dong, J. Li, Y. Liu, Q. Zong and Y. Yuan, Cinnamaldehyde-based poly(thioacetal): A ROS-awakened self-amplifying degradable polymer for enhanced cancer immunotherapy, Biomaterials, 2022, 289, 121795, DOI:10.1016/j.biomaterials.2022.121795.
- B. Liu and S. Thayumanavan, Mechanistic Investigation on Oxidative Degradation of ROS-Responsive Thioacetal/Thioketal Moieties and Their Implications, Cell Rep. Phys. Sci., 2020, 1, 100271, DOI:10.1016/j.xcrp.2020.100271.
- Y. Men, T. G. Brevé, H. Liu, A. G. Denkova and R. Eelkema, Photo cleavable thioacetal block copolymers for controlled release, Polym. Chem., 2021, 12, 3612–3618, 10.1039/D1PY00514F.
| This journal is © The Royal Society of Chemistry 2024 |