Open Access Article
Open Access ArticleRelating norbornene composition-to-reactivity for thiol–ene photopolymerizations and 3D printing†
Yutong Liua,
Henry L. Catera,
Elizabeth A. Reckerb and
Zachariah A. Page *ab
*ab
aDepartment of Chemistry, The University of Texas at Austin, Austin, TX, USA. E-mail: zpage@utexas.edu
bMcKetta Department of Chemical Engineering, The University of Texas at Austin, Austin, TX, USA
First published on 12th February 2025
Abstract
Structure–reactivity relationships for an array of norbornene derivatives in light-initiated thiol–ene reactions were unveiled using real-time Fourier-transform infrared (FTIR) spectroscopy. Guided by these results, poly(N-hydroxyethyl acrylamide) was functionalized with exo-norborneneimide and used in digital light processing (DLP) 3D printing of biomimetic hydrogels.
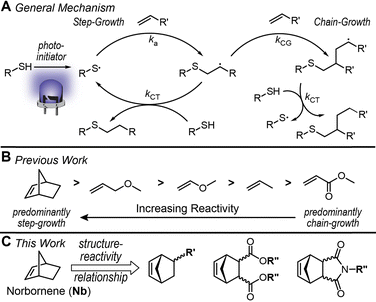
Photo-induced thiol–ene “click” chemistry has been widely used in polymer functionalization,1 coatings,2 and biomolecule patterning3 owing to its high speed, yields, and selectivity. Its metal-free nature and oxygen insensitivity make it well-suited for emerging technologies in 3D printing4 and tissue engineering.5 Contemporary light-based 3D printing techniques, such as digital light processing (DLP), predominantly use acrylic resins given their rapid curing upon exposure to light. However, these polymerizations typically proceed by an uncontrolled free-radical chain-growth mechanism, forming heterogeneous networks with compromised mechanical integrity. In contrast, thiol–ene reactions undergo a step-growth polymerization mechanism, providing more uniform polymer networks and enhanced mechanical properties as a result (e.g., higher strength and toughness).6 Yet, step-growth kinetics delay gelation relative to analogous chain-growth processes, hampering their implementation in DLP 3D printing.
In a typical thiol–ene reaction, a thiyl radical is generated upon light irradiation in the presence of a photo-initiator (Fig. 1A). The thiyl radical adds across an alkene to generate a carbon-centered radical, which can undergo either step- or chain-growth. The chemical structure of the alkene influences radical propagation kinetics, including thiyl radical addition to alkene, ka, along with carbon-centered radical chain-transfer to thiol, kCT, and addition to alkene (i.e., chain-growth), kCG.7 Bowman,8 Hoyle9 and their coworkers have conducted systematic experimental studies on thiol–ene reactivity for various alkenes, revealing that acrylates exhibit predominantly chain-growth kinetics (kCG >> kCT), whilst norbornene (Nb) and allyl ether groups display ∼pure step-growth kinetics (kCG ≪ kCT). Building on this body of work, Coffey and coworkers6 evaluated radical-initiated thiol–ene kinetics via computation, again showing how alkene structure impacts reactivity.
Based on these prior studies, Nb stands out for its high radical thiol–ene reactivity and clean step-growth mechanism,7,9,10 making it an ideal candidate for photocurable applications, such as forming hydrogels11 via 3D printing.12 However, Nb functionality is diverse,13 with examples of hydrocarbon,14 ester-,15–17 amide-,18,19 and imide20 linkages in prepolymers for light-initiated network formation. Despite this variety and the known influence of alkene composition on radical thiol–ene kinetics, a detailed study to elucidate structure–reactivity relationships in Nb derivatives is lacking.
Here, we fill this gap in knowledge by monitoring thiol–ene kinetics for ten distinct Nb derivatives via real-time Fourier transform infrared (FTIR) spectroscopy (Fig. 1C). Regio- and stereo-isomers were examined, revealing a 10-fold difference in rate between the fastest and slowest Nb substrates, which was attributed in-part to steric hindrance. Appending the most reactive Nb substrate to a hydrophilic polymer backbone showcased utility in 3D printing hydrogels.
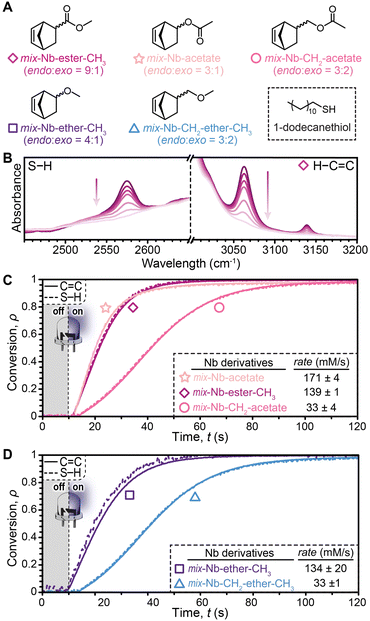
Starting from the corresponding mixed endo-/exo-carboxylic acid or -alcohol, five Nb derivatives were synthesized bearing: methyl ester (mix-Nb-ester-CH3), methylene acetate (mix-Nb-CH2-acetate), acetate (mix-Nb-acetate), methyl ether (mix-Nb-ether-CH3), or methylene methyl ether (mix-Nb-CH2-ether-CH3) (Fig. 2A). Using 1H-NMR, endo to exo ratios were confirmed to range from ∼3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 to 4
2 to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively (Fig. S2, ESI†). These monofunctional derivatives were representative of precursors used to incorporate Nb into polymer scaffolds for photoinduced thiol–ene crosslinking. They are commercially supplied as endo/exo mixtures (∼1.5×–4× more endo, Table S1, ESI†). Notably, the exo-carboxylic acid Nb derivative is also available, but cost prohibitive (∼20× the cost of the mixed isomers), limiting its utility in applications such as 3D printing.
1, respectively (Fig. S2, ESI†). These monofunctional derivatives were representative of precursors used to incorporate Nb into polymer scaffolds for photoinduced thiol–ene crosslinking. They are commercially supplied as endo/exo mixtures (∼1.5×–4× more endo, Table S1, ESI†). Notably, the exo-carboxylic acid Nb derivative is also available, but cost prohibitive (∼20× the cost of the mixed isomers), limiting its utility in applications such as 3D printing.
With the mixed Nb stereoisomers, real-time FTIR spectroscopy in a transmission configuration was used to characterize photoinduced thiol–ene reactions by monitoring the disappearance of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H stretches (3000–3100 cm−1) and S–H stretches (2525–2625 cm−1) (Fig. 2B), along with validation by monitoring the C
C–H stretches (3000–3100 cm−1) and S–H stretches (2525–2625 cm−1) (Fig. 2B), along with validation by monitoring the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretch (∼1620 cm−1) on salt plates (Fig. S3, ESI†). Nb derivatives were reacted with 1-dodecanethiol (stoichiometrically balanced, neat) using phenylbis(2,4,6-trimethylbenzoyl)phosphine oxide (BAPO, 0.01 mol%) as the photoinitiator. Samples (100 μm thick) between glass slides were irradiated with a 405 nm LED (1 mW cm−2), turned on 10 s after beginning each measurement. The Nb acetate and ester derivatives showed comparably high reaction rates (∼170 and 140 mM s−1, respectively) (Fig. 2C). Interestingly, the presence of a methylene spacer between Nb and acetate (Nb-CH2-aceate) resulted in a ∼4× slowdown (∼33 mM s−1). Similarly observed for the Nb-ether, a methylene spacer decreased its reactivity (Fig. 2D). The higher reactivity observed for the Nb derivatives with functionality directly attached (no CH2 spacer) was hypothesized to arise from reduced steric interactions, although further studies are needed to elucidate the exact roles of each substituent, including the influence of electronics (e.g., inductive effects), aggregation, and matrix viscosity.
C stretch (∼1620 cm−1) on salt plates (Fig. S3, ESI†). Nb derivatives were reacted with 1-dodecanethiol (stoichiometrically balanced, neat) using phenylbis(2,4,6-trimethylbenzoyl)phosphine oxide (BAPO, 0.01 mol%) as the photoinitiator. Samples (100 μm thick) between glass slides were irradiated with a 405 nm LED (1 mW cm−2), turned on 10 s after beginning each measurement. The Nb acetate and ester derivatives showed comparably high reaction rates (∼170 and 140 mM s−1, respectively) (Fig. 2C). Interestingly, the presence of a methylene spacer between Nb and acetate (Nb-CH2-aceate) resulted in a ∼4× slowdown (∼33 mM s−1). Similarly observed for the Nb-ether, a methylene spacer decreased its reactivity (Fig. 2D). The higher reactivity observed for the Nb derivatives with functionality directly attached (no CH2 spacer) was hypothesized to arise from reduced steric interactions, although further studies are needed to elucidate the exact roles of each substituent, including the influence of electronics (e.g., inductive effects), aggregation, and matrix viscosity.
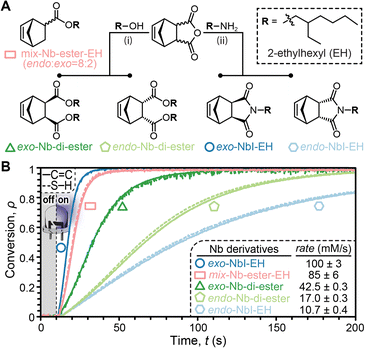
Next we examined Nb-diesters and -imides, which are widely used in ring-opening metathesis polymerization (ROMP)21,22 due to their rapid polymerization rates and ease of versatile synthesis. Four derivatives bearing 2-ethylhexyl (EH) groups were synthesized in one-step via substitution of inexpensive pure endo- or exo-carbic anhydride with the corresponding EH-alcohol or -amine (Fig. 3A and Schemes S6, S7, ESI†). The resulting Nb-EH derivatives were soluble in 1-dodecanethiol, enabling neat photoinitiated thiol–ene reactions.
For comparison, an analogous mix-Nb-ester-EH derivative was prepared and examined using real-time FTIR spectroscopy under the same conditions. Impressively, exo-NbI was found to be ∼10× faster than the corresponding endo-NbI (∼100 to 11 mM s−1, respectively) (Fig. 2B). This trend was also observed, albeit to a lesser extent, for the Nb-diester derivatives; 43 to 17 mM s−1 for exo- and endo-isomers, respectively. The faster rates for the exo-isomers were again attributed to reduced steric hinderance of thiyl radical addition. Notably, pure exo-Nb-ester-EH (and –CH3) controls deviated from this trend, with slower reactions relative to the mixed analogues (Fig. S4 and S5, ESI†). However, this was attributed to poor miscibility of the pure exo-isomers. Also of note was that the Nb-imide (NbI) derivatives required backgrounding with the NbI product to accurately monitor the thiol stretch at ∼2600 cm−1 (Fig. S6 and S7, ESI†). Overall, the exo-NbI resulted in the fastest thiol–ene reaction, followed by the mix-Nb-ester-EH control at a rate of 85 mM s−1.
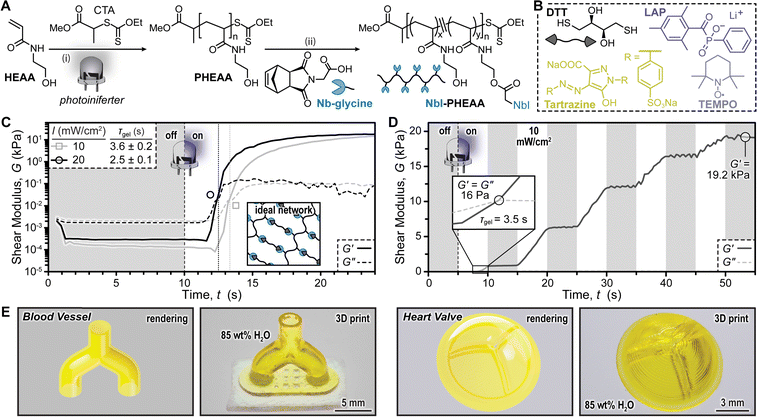
To implement photoinduced thiol–ene crosslinking for hydrogels, we appended exo-NbI to poly(N-hydroxyethyl acrylamide) (PHEAA), which was chosen for its hydrophilicity and ease of post-functionalization. PHEAA was synthesized via photoiniferter polymerization23 of HEAA in water, using a xanthate chain-transfer agent (CTA) and 8 mW cm−2 365 nm LED light exposure (Fig. 4A and Fig. S10, ESI†). Targeting a degree of polymerization of 1000, photoiniferter was rapid, reaching >95% monomer conversion in ∼10 minutes. Characterizing number average molecular weight (Mn) and dispersity (Đ) using size exclusion chromatography (SEC) revealed that the photoiniferter polymerization was moderately controlled, as Mn increased linearly with percent monomer conversion and Đ < 1.5 (Fig. S11, ESI†). After precipitating PHEAA into acetone to remove unreacted monomer, exo-NbI bearing a glycine-linker was installed onto the sidechain via Steglich esterification, with a feed ratio of 10 mol% exo-NbI-glycine relative to HEAA repeat units. After post-functionalization, Nb-PHEAA maintained a Gaussian distribution via SEC, with an Mn of ∼117 kDa relative to polystyrene standards (Fig. S12, ESI†). The percent Nb functionality was characterized using 1H-NMR spectroscopy, revealing ∼4 mol% incorporation (Fig. S13, ESI†). This synthetic protocol is versatile and scalable, providing access to “prepolymers” with tunable Nb composition and concentration (Fig. S15, ESI†).
Photoinduced crosslinking kinetics were next probed using parallel plate oscillatory shear photo-rheology. Samples contained DL-dithiothreitol (DTT) as the thiol crosslinker (∼equimolar thiol to ene functionality), lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP, 0.5 wt% ≈ 17 mM) as the water soluble photoinitiator, 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO, 0.05 wt%) as a stabilizer, and tartrazine as a passive light absorber (i.e., opaquing agent)24 to improve 3D printing resolution (discussed later) (Fig. 4B). Irradiating this mixture in 85 wt% water (100 μm thickness) with a 405 nm LED while monitoring changes in storage (G′) and loss (G′′) moduli provided an estimate for the time to gelation (τgel) when G′ = G′′ (Fig. 4C). Targeting equimolar amounts of thiol and ene functional groups led to a τgel of 2.5 ± 0.1 s and 3.6 ± 0.2 s for 20 and 10 mW cm−2 light intensities, respectively, while a plateau G′ of ∼24 kPa was observed for both conditions (Table S5, ESI†). Aside from light intensity, Nb composition, Nb concentration, and prepolymer Mn provided handles to alter gelation kinetics. For example, using a prepolymer that contained monofunctional Nb-ester or endo-NbI sidechains in-place of exo-NbI led to τgel values that were similar or 2× longer, respectively (Fig. S18 and Table S6, ESI†). Increasing [NbI] from ∼4 to 8 mol% resulted in a 1.4× decrease in τgel. Finally, increasing Mn from 8 to 105 kDa led to a 3× decrease in τgel (Fig. S18, S19 and Table S6, ESI†). Notably, the resins thickened over days and displayed a decrease in τgel indicative of a background reaction, common for reactive thiol–ene systems (Fig. S20, ESI†). Overall, these results demonstrate the control over gelation kinetics that the present platform offers.
To assess compatibility with DLP 3D printing we characterized the temporal control over photoinduced thiol–ene crosslinking using a resin comprising prepolymer with ∼4 mol% NbI-glycine in 85 wt% water (Fig. 4D). Cycling the 405 nm LED on/off in 10 second intervals at an intensity of 10 mW cm−2 revealed excellent temporal control (i.e., G′ increasing only while the LED was on). These results indicated that the present thiol-Nb resin might be compatible with DLP 3D printing.
As a final proof-of-concept, DLP 3D printing of the same resin was accomplished using a 405 nm LED at an intensity of 20 mW cm−2 (Fig. 4E). Initially, disks were printed with varying outer diameter and wall thickness under different exposure times (8–12 s/100 μm layer) to balance speed and resolution (Fig. S21 and S22, ESI†). Wall thicknesses as small as 100 μm were successfully printed and characterized with optical microscopy. Overall, the combination of speed and feature fidelity are competitive with state-of-the-art examples in thiol–ene DLP 3D printing of hydrogels (Table S7, ESI†). We then targeted several objects that contained small features and overhangs, including models of a blood vessel and heart valve (Fig. 4E), as well as a “test your printer” file (Fig. S23, ESI†) and gyroid (Fig. S24, ESI†). The bio-inspired vessel model was printed at 12 s/100 μm layer and contained hollow channels (Movie S1, ESI†). The heart valve model, printed at 6 s/100 μm, contained three leaf-like flaps to enable one-way fluid flow (Movie S2, ESI†). The successful printing of these biological models demonstrated the potential of exo-NbI functionality in preparing high resolution hydrogel scaffolds for biomedical applications, such as the investigation of blood flow dynamics in heart valve models.
In summary, a systematic examination of ten norbornene derivatives revealed that exo-NbI represents a good candidate for rapid photoinduced thiol–ene crosslinking in additive manufacturing. The influence of sterics on thiol–ene reactivity was exemplified by altering Nb stereochemistry (exo-vs. endo-) and mono- vs. di-substitution patterns, providing design principals that inform their future utility. Overall, exo-NbI stood out in its ease of synthetic derivatization from carbic anhydride, high thiyl radical addition rate and even higher thiol chain-transfer rate, along with its excellent spatiotemporal control in photoinduced thiol–ene crosslinking. Thus, exo-NbI serves as an ideal functionality to prepare polymer networks with well-defined topologies. Finally, incorporating Nb into hydrophilic polymers, such as PHEAA, was demonstrated to provide a route towards rapid light-based 3D printing of biologically relevant hydrogels that have future applications in tissue engineering.
The authors acknowledge support from the Harry S Moss Heart Trust, Bank of America, N. A., Trustee (YL, HLC, ZAP) and the Welch Foundation under Grant No. F-2007 (ZAP). Partial support was provided by the NSF Graduate Research Fellowship Program under Grant No. DGE-2137420 (EAR).
Conceptualization (YL, ZAP); methodology (YL, HLC, EAR, ZAP); investigation (YL, HLC, EAR); visualization (YL, EAR, ZAP); funding acquisition (ZAP); project administration (ZAP); supervision (ZAP); writing – original draft (YL, ZAP); writing – review & editing (YL, HLC, EAR, ZAP).
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.References
- Y. Lv, Z. Lin and F. Svec, Analyst, 2012, 137, 4114–4118 RSC.
- A. K. Tucker-Schwartz, R. A. Farrell and R. L. Garrell, J. Am. Chem. Soc., 2011, 133, 11026–11029 CrossRef CAS PubMed.
- J. C. Grim, T. E. Brown, B. A. Aguado, D. A. Chapnick, A. L. Viert, X. Liu and K. S. Anseth, ACS Cent. Sci., 2018, 4, 909–916 CrossRef CAS PubMed.
- T. J. K. Buchner, S. Rogler, S. Weirich, Y. Armati, B. G. Cangan, J. Ramos, S. T. Twiddy, D. M. Marini, A. Weber, D. Chen, G. Ellson, J. Jacob, W. Zengerle, D. Katalichenko, C. Keny, W. Matusik and R. K. Katzschmann, Nature, 2023, 623, 522–530 CrossRef CAS PubMed.
- B. D. Fairbanks, M. P. Schwartz, A. E. Halevi, C. R. Nuttelman, C. N. Bowman and K. S. Anseth, Adv. Mater., 2009, 21, 5005–5010 CrossRef CAS PubMed.
- M. Arreguín-Campos, M. Ebrahimi, A. Z. Dookhith, N. A. Lynd, G. E. Sanoja, A. A. Aldana, M. B. Baker and L. M. Pitet, Eur. Polym. J., 2024, 212, 113070 CrossRef.
- B. H. Northrop and R. N. Coffey, J. Am. Chem. Soc., 2012, 134, 13804–13817 Search PubMed.
- N. B. Cramer, S. K. Reddy, A. K. O’Brien and C. N. Bowman, Macromolecules, 2003, 36, 7964–7969 Search PubMed.
- T. M. Roper, C. A. Guymon, E. S. Jönsson and C. E. Hoyle, J. Polym. Sci., Part A:Polym. Chem., 2004, 42, 6283–6298 CrossRef CAS.
- S. K. Reddy, N. B. Cramer and C. N. Bowman, Macromolecules, 2006, 39, 3681–3687 CrossRef CAS.
- H. Shih and C.-C. Lin, Biomacromolecules, 2012, 13, 2003–2012 CrossRef CAS PubMed.
- M. H. Kim and C.-C. Lin, ACS Appl. Mater. Interfaces, 2023, 15, 2737–2746 CrossRef CAS PubMed.
- W. Ma, N. Wright and Y. Wang, ACS Macro Lett., 2024, 13, 915–920 CrossRef CAS PubMed.
- K. D. Q. Nguyen, M. Crespo-Ribadeneyra, O. Picot, B. Colak and J. E. Gautrot, ACS Appl. Polym. Mater., 2021, 3, 5373–5385 CrossRef CAS.
- F.-Y. Lin and C.-C. Lin, ACS Macro Lett., 2021, 10, 341–345 CrossRef CAS PubMed.
- J. Van Hoorick, P. Gruber, M. Markovic, M. Rollot, G.-J. Graulus, M. Vagenende, M. Tromayer, J. Van Erps, H. Thienpont, J. C. Martins, S. Baudis, A. Ovsianikov, P. Dubruel and S. Van Vlierberghe, Macromol. Rapid Commun., 2018, 39, 1800181 CrossRef PubMed.
- W. M. Gramlich, I. L. Kim and J. A. Burdick, Biomaterials, 2013, 34, 9803–9811 CrossRef CAS PubMed.
- Z. Mũnoz, H. Shih and C.-C. Lin, Biomater. Sci., 2014, 2, 1063–1072 RSC.
- V. T. G. Tan, D. H. T. Nguyen, R. H. Utama, M. Kahram, F. Ercole, J. F. Quinn, M. R. Whittaker, T. P. Davis and J. J. Gooding, Polym. Chem., 2017, 8, 6123–6133 RSC.
- Y. Liu, H. L. Cater, A. J. Arrowood, G. E. Sanoja and Z. A. Page, Macromolecules, 2024, 57, 6522–6530 CrossRef CAS.
- S. C. Radzinski, J. C. Foster, R. C. Chapleski, D. Troya and J. B. Matson, J. Am. Chem. Soc., 2016, 138, 6998–7004 CrossRef CAS PubMed.
- H. L. Cater, I. Balynska, M. J. Allen, B. D. Freeman and Z. A. Page, Macromolecules, 2022, 55, 6671–6679 CrossRef CAS.
- R. N. Carmean, T. E. Becker, M. B. Sims and B. S. Sumerlin, Chem, 2017, 2, 93–101 CAS.
- L. M. Stevens, E. A. Recker, K. A. Zhou, V. G. Garcia, K. S. Mason, C. Tagnon, N. Abdelaziz and Z. A. Page, Adv. Mater. Technol., 2023, 8, 2300052 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Materials, methods, synthesis, characterization. See DOI: https://doi.org/10.1039/d4cc6196a |
| This journal is © The Royal Society of Chemistry 2025 |