Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The impact of surfaces on indoor air chemistry following cooking and cleaning†
Ellen
Harding-Smith‡
 ab,
Helen L.
Davies‡
ab,
Helen L.
Davies‡
 a,
Catherine
O'Leary
a,
Catherine
O'Leary
 b,
Ruth
Winkless
b,
Marvin
Shaw
bc,
Terry
Dillon
b,
Benjamin
Jones
d and
Nicola
Carslaw
b,
Ruth
Winkless
b,
Marvin
Shaw
bc,
Terry
Dillon
b,
Benjamin
Jones
d and
Nicola
Carslaw
 *a
*a
aDepartment of Environment and Geography, University of York, UK. E-mail: nicola.carslaw@york.ac.uk
bWolfson Atmospheric Chemistry Laboratory, Department of Chemistry, University of York, UK
cNational Centre for Atmospheric Science, University of York, UK
dDepartment of Architecture and Built Environment, University of Nottingham, UK
First published on 13th September 2024
Abstract
Cooking and cleaning are common sources of indoor air pollutants, including volatile organic compounds (VOCs). The chemical fate of VOCs indoors is determined by both gas-phase and multi-phase chemistry, and can result in the formation of potentially hazardous secondary pollutants. Chemical interactions at the gas-surface boundary play an important role in indoor environments due to the characteristically high surface area to volume ratios (SAVs). This study first characterises the VOC emissions from a typical cooking and cleaning activity in a semi-realistic domestic kitchen, using real-time measurements. While cooking emitted a larger amount of VOCs overall, both cooking and cleaning were sources of chemically reactive monoterpenes (peak mixing ratios 7 ppb and 2 ppb, respectively). Chemical processing of the VOC emissions from sequential cooking and cleaning activities was then simulated in a kitchen using a detailed chemical model. Results showed that ozone (O3) deposition was most effective onto plastic and soft furnishings, while wooden surfaces were the most effective at producing formaldehyde following multi-phase chemistry. Subsequent modelling of cooking and cleaning emissions using a range of measured kitchen SAVs revealed that indoor oxidant levels and the subsequent chemistry, are strongly influenced by the total and material-specific SAV of the room. O3 mixing ratios ranged from 1.3–7.8 ppb across 9 simulated kitchens, with higher concentrations of secondary pollutants observed at higher O3 concentration. Increased room volume, decreased total SAV, decreased SAVs of plastic and soft furnishings, and increased wood SAV contributed to elevated formaldehyde and total peroxyacetyl nitrates (PANs) mixing ratios, of up to 1548 ppt and 643 ppt, respectively, following cooking and cleaning. Therefore, the size and material composition of indoor environments has the potential to impact the chemical processing of VOC emissions from common occupant activities.
Environmental significanceDomestic cooking and cleaning result in emissions of a large number of volatile organic compounds, which, through gas-phase chemistry and surface interactions, can form a wide range of potentially harmful secondary products. This study uses experimental cooking and cleaning emission data to simulate the impact of building design and surface materials on the secondary chemistry following these activities. It is shown that the concentrations of oxidants and secondary products are strongly influenced by the total surface area to volume ratio of a room and the specific surface material composition. Data from this study provides indications of how building design and surface materials could be altered in order to reduce the effects of indoor air pollution resulting from domestic activities. |
1 Introduction
Many emission sources contribute to indoor air pollution, including building materials and furnishings, combustion sources such as stoves, candles and log burners, and occupant activities such as cooking and cleaning.1–5 The numerous and highly variable indoor emission sources often result in pollution levels greater indoors compared to outdoors.6 Together with the considerable proportion of time spent in built environments, indoor air quality is a significant factor in determining human exposure to air pollutants.7A growing number of studies have emerged, aiming to characterise the impacts of occupant activities on indoor air pollution.8,9 Cooking and cleaning are frequent occupant activities which serve as potentially large, intermittent sources of indoor air pollution in domestic and commercial environments.1,10 Cooking emits a diverse range of indoor air pollutants, including VOCs, particulate matter (PM), and inorganic gases such as oxides of carbon and nitrogen.11 The composition and quantity of emissions is highly dependent on ingredients, type of oil used, cooking method (e.g. boiling, frying, etc.), and temperature.12 For example, Klein et al. identified that vegetables were a dominant source of alcohol and sulphur-containing VOCs, oils emitted predominantly aldehyde species, and herbs and pepper emitted large quantities of terpenes and terpenoids.13,14
Cleaning activities similarly result in large emissions of VOCs and PM indoors. The composition and quantity of emissions from cleaning is highly dependent on a range of factors, including the chemical composition of the product formulation, and the application mode (spraying, diluting, wiping, mopping etc.).15 Fragranced household cleaners have been identified as a significant source of terpene species indoors, while chlorine-based bleach products emit hazardous chlorinated VOCs.16
Many VOCs emitted from cooking and cleaning activities readily react with oxidants present indoors (O3, OH, NO3) to generate secondary pollutants, some of which are more hazardous than the parent compound.17 In particular, monoterpenes, which are emitted both from cooking and cleaning activities, are susceptible to rapid ozonolysis due to the presence of unsaturated C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds in their chemical structure. Some products of this chemistry, for example formaldehyde, organic nitrates, and peroxyacetyl nitrate-type species (PANs), are known or suspected to have adverse health effects.18–22 Therefore, it is important to characterise the fate of VOC emissions from occupant activities to determine the potential implications on occupant health.
C bonds in their chemical structure. Some products of this chemistry, for example formaldehyde, organic nitrates, and peroxyacetyl nitrate-type species (PANs), are known or suspected to have adverse health effects.18–22 Therefore, it is important to characterise the fate of VOC emissions from occupant activities to determine the potential implications on occupant health.
The chemical fate of VOCs indoors differs from outdoors. The surface area to volume ratio (SAV) is notably greater within buildings compared to outdoor spaces.23 Consequently, the relative importance of surface emissions, multi-phase reactions, and surface deposition for determining the composition and concentrations of gas-phase species is greater for indoor environments compared to outdoors.24 Deposition of VOCs onto indoor surfaces may have a significant influence on the peak concentration and temporal profiles of pollutants during transient emission events. Indeed, Singer et al. demonstrated for a range of compounds that surface deposition may compete with, or exceed, ventilation as the most important removal process following an emission event, depending on the intrinsic vapour pressure of the depositing compound.25
Indoor surfaces influence the concentration of potentially hazardous secondary pollutants indoors (e.g. formaldehyde and longer chain aldehydes26) by facilitating VOC oxidation chemistry. Sorption of VOCs and oxidants onto indoor surfaces removes the constraint of ventilation on residence time, thus increasing the potential for chemical transformations to occur via gas-phase surface interactions.24 Interactions of O3 with surface-sorbed VOCs result in the production of oxidised products, which are often volatile enough to be emitted from indoor surfaces, thus affecting indoor air quality.27,28
In realistic indoor settings, the ongoing deposition of VOCs onto indoor surfaces results in the creation of organic films. These films serve as a reservoir for reactive contaminants, which further influence indoor air quality via the emission of secondary pollutants.29 In kitchen environments, where VOCs such as cooking oils and terpenes from cleaning deposit on indoor surfaces, surfaces are likely to have high film coverage. Deming et al. reported surface films containing up to 65% alkenes from painted walls and glass windows following cooking, cleaning, and occupancy experiments.30 In a study of four homes, Wang et al. showed that kitchen countertops exhibited consistently high secondary emission rates following exposure to O3 between new and old homes.31 In contrast to carpets, which age over time, the reactive surface films on kitchen countertops are continually replenished by occupant activities, thus suggesting that kitchen surfaces may be a dominant source of secondary pollutants.31
The deposition and subsequent multi-phase chemistry that occurs on indoor surfaces is dependent on the surface material. For instance, Won et al. demonstrated through a series of chamber experiments that carpet was the most significant sink for non-polar VOCs, while gypsum board was a significant sink for highly polar VOCs.32 There are an increasing number of studies which investigate the uptake of pollutants onto indoor surfaces and the products of multi-phase surface interactions. Of particular interest is O3, on account of its ubiquitous presence indoors via infiltration from outdoors, and its importance in the oxidative processing of surface film constituents.24,33 The literature on O3-surface interactions was reviewed and summarised by Carter et al., in addition to that of a less-studied oxidant, hydrogen peroxide (H2O2).28 The reported deposition velocities and secondary pollutant production yields were used to represent oxidant deposition and heterogeneous chemistry in a detailed chemical model, INCHEM-Py, which will be used in this work.34
Domestic kitchens vary widely in their designs, with consequent impacts on surface-mediated indoor air chemistry. The physical characteristics of the room, including the total SAV and surface materials, impact the processing of VOCs which are emitted from activities frequently carried out in kitchens, i.e. cooking and cleaning. The room volume determines the dilution of pollutants emitted into the room, while the surface area and surface materials control the extent of surface deposition and heterogeneous chemistry. Weschler et al. highlighted the evolving changes in indoor surfaces over time, for example the replacement of natural products with synthetic products for building materials and furnishings.35 This shift in the composition and complexity of indoor surfaces is likely to impact indoor air quality as a result of differing emissions, deposition, and multi-phase chemistry.
To our knowledge, the impacts of variations in realistic kitchen SAVs and surface materials on the resulting chemical fate of VOC emissions from typical occupant activities such as cooking and cleaning have not been evaluated in detail. This study first characterises the VOC emissions from typical cooking and cleaning activities in a semi-realistic domestic kitchen, using real-time mass spectrometry for time-resolved measurements. We then use a detailed chemical model to simulate the measured VOC emissions, and investigate the impacts of varying kitchen designs with respect to material-specific surface areas and total SAV, on the resulting indoor air chemistry. This study aims to identify building design factors relating to the SAV and material composition of indoor surfaces, which impact the indoor air quality and chemistry following high emission events.
2 Methods
2.1 The test pod facility and diagnostic equipment
A 4 week experimental campaign was conducted during February/March 2022 at the Department of Architecture & Built Environment, University of Nottingham, UK. The purpose of this campaign was to investigate the impacts of cooking and cleaning on indoor air chemistry under semi-realistic conditions. The experiments were performed at the Test Pod facility, which is comprised of two buildings: one meeting current UK Building Regulations Part L,36 and the other meeting the Passivhaus Standard.37 All experiments were performed in the Part L (test) pod to ensure that the building ventilation was representative of typical houses in the UK. The test pod had a volume of 22.2 m3 (3.53 m × 2.62 m × 2.40 m), with a single external door and a north-east facing window which was partially covered with an MDF board (Fig. S1†). The room consisted of linoleum tile flooring, painted plasterboard walls and ceiling, and minimal furnishings (total surface area 53.6 m2).A Voice200 ultra selected-ion flow-tube mass spectrometer (SIFT-MS, Syft Technologies, Christchurch, New Zealand) was used to quantify air concentrations of targeted VOCs in the experimental container, and outside, throughout the campaign. The SIFT-MS principles of operation are described elsewhere,38,39 and the instrument was operated using the same conditions as described by Davies et al.4 Indoor air was sampled from the centre of the room at 2 m above the floor (blue circle, Fig. S1†). Outdoor air was sampled from directly outside the facility at a similar height. The sample lines were connected to the SIFT-MS housed in the Wolfson Atmospheric Chemistry Laboratory (WACL) Air Sampling Platform (WASP),40 which was positioned adjacent to the test pod.
The specific ions measured by SIFT-MS during the cooking and cleaning experiments are shown in Table S1,† along with the species molecular weights, product ions, rate coefficients and branching ratios. Overall, 40 and 18 VOCs were measured by the SIFT-MS during cooking and cleaning experiments, respectively, with a time resolution of less than 10 seconds. The SIFT-MS was externally calibrated six times during the experimental campaign. The calibration factors applied to the data are summarised in Table S3.†
The instrument background was assessed by sampling zero air from an in-house heated palladium alumina-based zero air generator. Background VOC mixing ratios, defined as the 3 minutes average of the zero air measurements, were subtracted from the data where available. The limits of detection were calculated as 3.2 times the standard deviation of the zero air measurements, as shown in Table S2.†
2.2 Experimental design
The experimental campaign involved three, day-long experiment types (background, cooking, cleaning). The purpose of the background days was to characterise the unoccupied test pod, including background gas and particle concentrations relating to the building and furnishing materials, stationary furnishings, and indoor/outdoor exchange. Background days involved minimal perturbation to the room, with experimentalists only briefly present periodically to take passive air samples and perform air change rate (ACR) assessments. Background days were assigned to one day before and after the cooking/cleaning experiments to assess the impact of recent occupant activities on background room emissions.Each experiment was conducted on a separate day to minimise complexity and to allow determination of the indoor air pollution over approximately 20 hours following individual occupant activities. Prior to each experiment, the test pod was well ventilated for 1 hour by opening the external door. The room was left unperturbed for a minimum of 2 hours following the high ventilation period to allow indoor conditions to equilibrate before a scripted cooking or cleaning activity was performed at approximately 13:00 UTC. Each experiment was repeated several times throughout the campaign to assess reproducibility.
The scripted cooking activity involved the preparation of a chicken stir-fry, based on a published recipe.4 The scripted cleaning activity involved the use of a UK market-leading lemon-scented surface spray cleaner (‘SR1’ from Harding-Smith et al.41). The cleaner was applied to a tabletop (2 m2) and wiped using a damp cloth after 1 minute. After each activity, all cooking/cleaning apparatus were removed from the room to ensure that all measured perturbations in indoor air quality derived only from the activity. The tabletop was rinsed with water between experiments to remove product residue and minimise carryover between experiments.
Throughout the campaign, the indoor temperature was manually controlled at (17 ± 1) °C using a plug-in oil heater in the centre of the room. The measured relative humidity was (47 ± 4)%. Natural ventilation only was used throughout all experiments to emulate the ventilation of a typical UK dwelling. The ACR of the test pod was measured using methane tracer releases on 6 days. The methane concentration decay was monitored by UGGA, and log-linear regression analysis of the background-subtracted data over two hours following the release resulted in an average ACR of (0.33 ± 0.06) h−1 (Fig. S2†).
2.3 Experimental reproducibility
Cooking and cleaning experiments were conducted in triplicate over three consecutive days to assess reproducibility and improve reliability in results. This was made possible because each activity was scripted, meaning that the timings of emissions remained consistent between repeats. In general, the timings of VOC emission peaks measured by SIFT-MS were reproducible between replicate experiments. Whilst the relative change in VOC concentration during the emission periods were similar between repeat experiments, there was variation in the absolute concentrations of some VOCs measured during background and emission periods. The mixing ratios of total monoterpenes measured during each repeat of the cooking and cleaning experiments is shown in Fig. 1a and b, respectively. For each measured VOC, the data from the three repeats were averaged to determine species concentration from average cooking and cleaning activities, shown as the black lines in Fig. 1. The average cooking and cleaning data were used for all further analyses.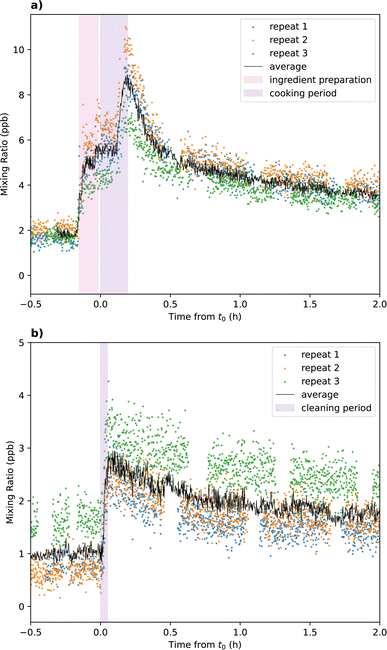 | ||
| Fig. 1 Total monoterpene mixing ratio measured by SIFT-MS during three repeat (a) cooking and (b) cleaning experiments. The average of the three repeats is shown as the black line. | ||
2.4 Modelling
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 different reactions and over 6000 species.42,45–48 INCHEM-Py also includes terms for indoor photolysis,49 indoor/outdoor exchange, and particle formation for three terpene species.45,50
000 different reactions and over 6000 species.42,45–48 INCHEM-Py also includes terms for indoor photolysis,49 indoor/outdoor exchange, and particle formation for three terpene species.45,50
The general equation for the ODEs created and solved by INCHEM-Py to calculate the concentration C of species i through time is as follows:
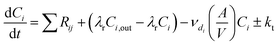 | (1) |
The rate of irreversible deposition of species onto indoor surfaces is defined in INCHEM-Py for 3371 species as the product of species deposition velocities (cm s−1) and the total SAV (cm−1). Specific deposition velocities are provided for 22 species, however all other species which are assumed to deposit onto indoor surfaces in the model have deposition velocities which are estimated based on their chemical functionality.34 The deposition of these species to indoor surfaces is independent of the surface material and does not consider subsequent emission of secondary pollutants from surface chemistry. However, for O3 and H2O2, surface-specific deposition mechanisms have been developed which consider the rates of deposition and secondary pollutant emissions from multiple indoor surface materials.28 Loss rates of O3 and H2O2 to indoor surfaces and subsequent emission of aldehydes is calculated from the specific deposition velocities and SAVs of the following materials: metal, glass, wood, plastic, linoleum, paint, paper, concrete, soft furnishings, and skin.
INCHEM-Py v1.2 was used for this study. Further details of this version of the model, including all of the assumptions, are available from ref. 34.
The outdoor concentrations of 110 VOCs were defined as static concentrations using representative data sourced from published literature and measurement databases, while trace gases (O3, NO, and NO2) were defined using diurnally varying concentrations based on measurements taken in a suburban London location.34 The indoor background VOC concentrations were determined by the ingress and egress of species, which was controlled by an ACR typical of residential dwellings (0.5 h−1).53 Background emissions of acetone, ethanol, methanol, isopropanol, and isoprene were also present at emission rates corresponding to the breath emissions of one adult.54,55
The indoor light levels in the average kitchen were determined based on an assumed latitude of 51.45 °N, date 20/06/2020, and low emissivity glass glazed windows (transmission 330–800 nm (ref. 56)). It was also assumed that artificial incandescent lighting was on between 07:00 and 19:00, although having these lights on makes negligible difference to the results.
The VOC emissions from average cooking and cleaning activities were simulated at 12:00 and 13:00 hours, respectively. Emission rates (molecule cm−3 s−1) were calculated from the averaged SIFT-MS data of the three repeats for each cooking and cleaning experiment by calculating the rate of increase in species concentrations during the cooking/cleaning activity. These emission rates were then applied to the model as timed emissions, with a correction factor to account for differences in room volume between the test pod and simulated kitchen. Emissions from the cleaning experiment included acetaldehyde, methanol, ethanol, monoterpenes (limonene, carene, camphene, terpinolene, α-phellandrene, α-terpinene, α-pinene), butyl pyruvate, and dihydromyrcenol. Emissions from the cooking experiment included acetaldehyde, methanol, ethanol, acrolein, monoterpenes (limonene, α-pinene, camphene), hexanal, heptanal, octanal, nonanal, n-octane, n-nonane, 1,2,4-trimethyl benzene and dimethyl sulphide. The total monoterpene emissions from the cleaning experiment were speciated using data from Harding-Smith et al.41 for cleaner ‘SR1’, while those from the cooking experiment were speciated using data from Davies et al.4 Model emissions of butyl pyruvate were used as a proxy for measured emissions of 2-tert-butylcyclohexyl acetate, with mass correction. Overall, the VOC emissions from the cooking activity lasted 23 minutes (including 10 minutes of ingredient preparation and 13 minutes of cooking), while those from the cleaning activity lasted 5 minutes. Full details of the VOC emission rates input to the model are shown in Tables S4 and S5.†
For the first study, simulations were performed using a ‘basic kitchen’ scenario. The nominal volume (height × length × width) of the basic kitchen was 29 m3, based on the average nominal volume reported by Manuja et al.51 The room volume minus contents was 23.84 m3 and the total surface area was 72.06 m2, resulting in a total SAV of 3.02 m−1. The basic kitchen consisted of an L-shaped layout, with lower and upper kitchen cabinets spanning two of the walls. There was an internal and external door, one window, and basic kitchen amenities (sink, tap, refrigerator, oven, extractor fan, bin). The individual components of the basic kitchen, their respective surface areas, and the surface materials considered for each component are shown in Table 1. For the purpose of this study, tile and stone materials were classified as concrete in the model.
| Component | Surface area (m2) | Soft fabric | Paint | Wood | Metal | Concrete | Linoleum | Plastic | Glass | Human |
|---|---|---|---|---|---|---|---|---|---|---|
| Walls | 15.74 | ✓ | ||||||||
| External door | 1.51 | ✓ | ✓ | ✓ | ||||||
| Internal door | 1.51 | ✓ | ||||||||
| Window | 0.76 | ✓ | ||||||||
| Floor | 8.32 | ✓ | ✓ | ✓ | ✓ | |||||
| Ceiling | 12.10 | ✓ | ||||||||
| Cupboards and kickboards | 11.68 | ✓ | ✓ | ✓ | ||||||
| Backsplash | 3.21 | ✓ | ✓ | |||||||
| Worktop | 2.62 | ✓ | ✓ | ✓ | ||||||
| Sink | 0.59 | ✓ | ✓ | |||||||
| Tap | 0.12 | ✓ | ||||||||
| Oven | 1.82 | ✓ | ||||||||
| Oven doors | 0.52 | ✓ | ||||||||
| Extractor fan | 1.62 | ✓ | ||||||||
| Refrigerator | 7.27 | ✓ | ||||||||
| Bin | 0.68 | ✓ | ||||||||
| Occupant | 2.00 | ✓ |
Based on the likely surface materials of each component defined in Table 1, 20 permutations of the basic kitchen were defined by randomly selecting the material of each component using the Python random.choice() method. The sum of each material SAV used to initialise the model for the 20 basic kitchen simulations is shown in Fig. 2a. In all simulations the SAV of human skin remained constant, corresponding to the presence of one occupant. All other SAVs varied depending on the defined material of specific kitchen components, resulting in 20 unique combinations of material-specific SAVs.
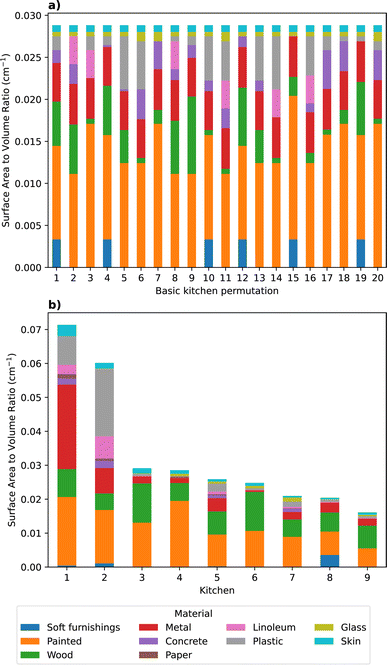 | ||
| Fig. 2 The material-specific SAVs (cm−1) used to initialise the model for the (a) basic kitchen scenario, and (b) real-life kitchen modelling studies. | ||
The second modelling study involved simulating the cooking and cleaning activities using SAVs based on the 1 cm resolution measurements of kitchens in nine residences in Blacksburg, Virginia, that were built between 1941 and 2003.51 The purpose of this study was to initialise the model using room volumes, surface areas, and surface materials which represented real domestic kitchens. The individual kitchen volumes ranged from 6 to 46 m3 and the total surface areas ranged from 38 to 96 m2, resulting in total SAVs ranging from 1.61 to 7.14 m−1. For the purposes of these simulations, kitchens 1–9 were defined based on descending order of the ratio of surface area to volume including contents (S*/V*, as defined in Manuja et al.51). Generally, kitchens with a larger room volume resulted in a smaller SAV, however, SAV was also influenced by the contents in the room. The surface area of materials categorised by Manuja et al.51 as ‘other’ were not accounted for in our simulations, and the total surface area of 5 kitchens was therefore underestimated by 0.6–32%. Materials categorised as cardboard and paper by Manuja et al.51 were summed and classified as ‘paper’ in our simulations. The surface area of plastic reported by Manuja et al.51 in each kitchen was assumed to be 75% plastic and 25% linoleum. A summary of the material-specific SAVs considered in the model for each of the nine kitchens is shown in Fig. 2b.
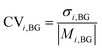 | (2) |
To compare the change in concentrations of species i as a result of activities across different kitchens, the average change in concentration for each species in each kitchen (μi,ΔCi,kn) were calculated as μi,Act,kn − μi,BG,kn. The coefficient of variation for activity-induced concentration change (CVi,ΔCi) was then calculated by obtaining the overall mean (Mi,ΔCi) and standard deviation (σi,ΔCi) of μi,ΔCi,k1–20 and using the following equation:
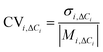 | (3) |
3 Results and discussion
3.1 Typical measured cooking and cleaning VOC emissions
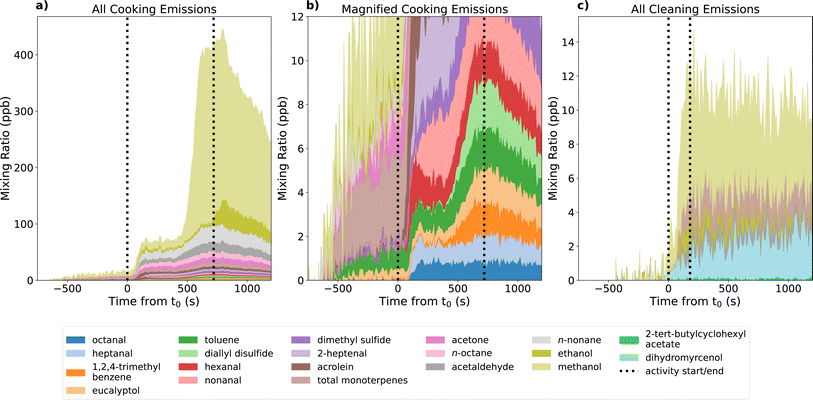
The average VOC emissions measured during the experimental cooking and cleaning activities are shown in Fig. 3. As the focus of this study is on gas-phase pollutants, particulate matter emissions were not considered at this time. The cooking activity emitted a larger concentration of VOCs compared to the cleaning activity, with a total maximum increase in emitted VOCs of 434 ppb, compared to the 15 ppb increase observed from the cleaning emissions. For both activities, the largest VOC emission was of methanol, which constituted 69% and 53% of the total VOC emissions for cooking and cleaning, respectively. Other VOC emissions measured from both activities included ethanol, acetaldehyde and monoterpenes. In all cases, cooking was a larger source of these VOCs compared to cleaning. The maximum concentration of monoterpenes during the cooking activity amounted to 7 ppb (1.6% of total maximum VOC), whereas cleaning emitted 2 ppb monoterpenes (13% of total maximum VOC). Monoterpenes are potentially important for indoor air chemistry because they are chemically reactive towards oxidants present indoors, thus have the potential to generate harmful secondary pollutants. These results indicate that while cleaning is a smaller source of VOC emissions compared to cooking, a larger proportion of the emitted species are chemically reactive species, which contribute to secondary pollutant formation.Cooking emitted a range of species not observed from cleaning, including acrolein, trimethylbenzene, dimethyl sulphide, and a range of long chain aldehydes and alkanes. Different species peaked at different times during the activity, corresponding to the different stages in the cooking progress. For example, an increase in monoterpenes of 4 ppb was observed several minutes prior to the start of the cooking period, corresponding with the preparation of spices in the room, followed by a second increase to a total of 7 ppb resulting from adding the spices to the pan at 360 s. These results clearly indicate that one or more of the spices (garlic, ginger, chilli) were a source of monoterpene emissions, which is in agreement with previous studies.14 A similar pattern was observed for eucalyptol and dimethyl disulfide to a lesser extent, the latter of which is a constituent of garlic.57
Other notable emissions were observed during the oil heating stages (0 s, 300 s) and the addition of chicken, vegetables, and sauce to the pan (60 s, 380 s, and 660 s, respectively). The heating of oil resulted in emissions of a range of alkane and aldehyde species, the most notable being acetaldehyde (+18 ppb), nonane (+31 ppb), and propanal (+16 ppb). Alkane and aldehyde emissions from cooking oils have been well characterised in previous studies, highlighting the potential health risks of these emissions.58 Alcohol emissions, particularly methanol and ethanol, were attributed to the addition of various cooking ingredients to the pan. These emissions formed the largest contribution to the total VOC emissions during the cooking process, with an increase of approximately 300 ppb of methanol observed following the addition of vegetables, and an increase of approximately 7 ppb and 43 ppb of ethanol observed following the addition of chicken and sauce, respectively.
These observations are largely consistent with a previous study reported by Davies et al.,4 which is based on the same scripted cooking experiment. The magnitude of emissions observed in this study were less than those reported by Davies et al.,4 particularly for methanol, which reached a maximum mixing ratio of approximately 5 times less. However, it was concluded that there were large background emissions of methanol in the experimental facility used by Davies et al.,4 likely from the relatively new building materials. This highlights the potentially large impact of various experimental factors, which could contribute to the differences observed between experimental studies. Other factors which are likely to have impacted the results include variations in ingredient sourcing and freshness, differences in cooking temperatures, and human variability in the cooking process. However, the timings of VOC emissions observed during the cooking activity showed good agreement with those reported by Davies et al.,4 illustrating repeatability in the types of VOCs emitted during various aspects of the cooking processes.
In addition to alcohols, acetaldehyde, monoterpenes, and eucalyptol, VOC emissions unique to the cleaning activity included dihydromyrcenol and 2-tert-butylcyclohexyl acetate. In contrast to the cooking activity, the cleaning protocol was not a multi-step process, thus all VOC emissions were observed simultaneously. Overall, the emitted VOC species measured from the cleaning activity were consistent with the VOC composition of the same cleaning product (SR1) reported by Harding-Smith et al.,41 evidencing the relationship between cleaning product formulation composition and the observed VOC emissions resulting from product use. The relative contribution of alcohols and acetaldehyde to the maximum total VOC mixing ratio observed in this study were consistent with the relative mass concentrations reported by Harding-Smith et al.41 However, we observed a larger relative emission of monoterpenes and lower relative emissions of dihydromyrcenol and 2-tert-butylcyclohexyl acetate in this study compared to that reported by Harding-Smith et al.41 Furthermore, citral was reported to constitute 4.2% of the product formulation by mass, however emissions of this species were not observed in the current study.
These differences in the relative proportions of VOC measured from headspace analysis of the cleaning product and from a realistic usage scenario may indicate that cleaning product components demonstrate complex emission dynamics, resulting in a non-linear relationship between the cleaning product chemical composition and the emissions resulting from use. Indeed, Angulo Milhem et al.59 reported that the liquid-to-gas transfer of terpenes from essential oil-based cleaners is driven by molecular properties such as volatility and interactions with the bulk solution, and the liquid content of individual terpenes.
3.2 Modelling typical cooking and cleaning events
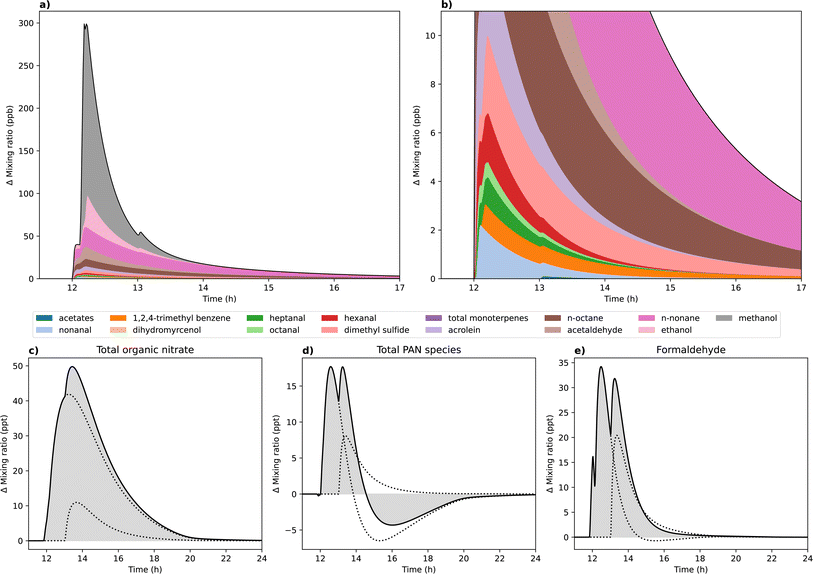
Using the experimental data, emission rates for each of the VOCs emitted during the average cooking and cleaning activity were calculated. These emission rates were applied to the INCHEM-Py model to simulate the emission events and investigate the secondary chemical processing of VOC emissions further. For the purposes of this study, it was assumed that the cooking activity occurred at midday and the cleaning activity commenced one hour later, representing a real-life scenario, in which cooking is followed by cleaning. Background model runs (which did not include activity-induced emissions) and activity model runs (which did include emissions) were performed to show the effects of activities.The activity-induced change in species concentrations were determined by subtracting the background simulation (no timed emissions) from the activity simulation (including timed emissions). The activity-induced change in emitted VOC mixing ratio is shown in Fig. 4a. The y-axis is magnified in Fig. 4b, focussing on VOCs emitted at lower concentrations. The maximum simulated increase in total emitted VOC concentrations was approximately 300 ppb, with methanol and ethanol emissions from the cooking event at 12 h contributing the most to the overall increase relative to the baseline simulation. Elevated VOC concentrations persisted for several hours following the cooking and cleaning activities. The straight-chain alkanes, nonane and octane, persisted for over 5 hours following cooking, whereas alcohol species quickly decayed in concentration following the emission event due to differences in species loss pathways.
The simulated activity-induced change in concentration of three key classes of secondary pollutant are also shown in Fig. 4c–e. Formaldehyde, organic nitrates and PAN species are products of VOC oxidation chemistry, and are known or suspected to have adverse health effects.18–22,60Fig. 4 shows that the concentrations of these secondary pollutants increase relative to the baseline simulation by 18 to 50 ppt, orders of magnitude smaller than the increase in primary VOC concentrations. Secondary PM can also form following cooking and cleaning,12,45 however, this study focused on gas-phase secondaries so PM is not considered here.
Both modelled emission events contributed to the formation of secondary pollutants, thus consecutive activities had a compound effect on the total species concentrations. The increase in total organic nitrates compared to the baseline simulation persisted for longer compared to the other secondary products, resulting in the cleaning event elevating the maximum concentration above that achieved following cooking. Formaldehyde and PAN species were both shorter lived, hence the consecutive emission events prolonged the elevated concentrations of these species, but the cleaning event did not elevate the maximum concentration above that which was achieved from prior cooking. The dashed lines illustrate that the total PANs concentration dropped by about 6 ppt a few hours following cooking. However, the additional VOC emissions from cleaning resulted in an increase in total PANs, thus reducing the overall decline in PAN species concentrations observed. Surface deposition dominates the loss processes for organic nitrates, PANs and formaldehyde. However, the surface deposition rates for organic nitrates are lower than for PANs or formaldehyde, meaning organic nitrates have a longer lifetime compared to the other species.
These simulation results illustrate that the occurrence of several occupant activities in sequence, as would be expected in a realistic scenario, can result in prolonged and exacerbated secondary pollutant concentrations resulting from the chemical processing of VOC emissions from these activities. While the concentrations observed here are below what would be expected to cause adverse health effects in occupants, it highlights the importance of considering the potential concentrations of secondary pollutants which could be achieved in occupational settings, where high emission occupant activities such as cooking and cleaning occur regularly throughout the day.
3.3 Impact of kitchen designs on indoor air chemistry
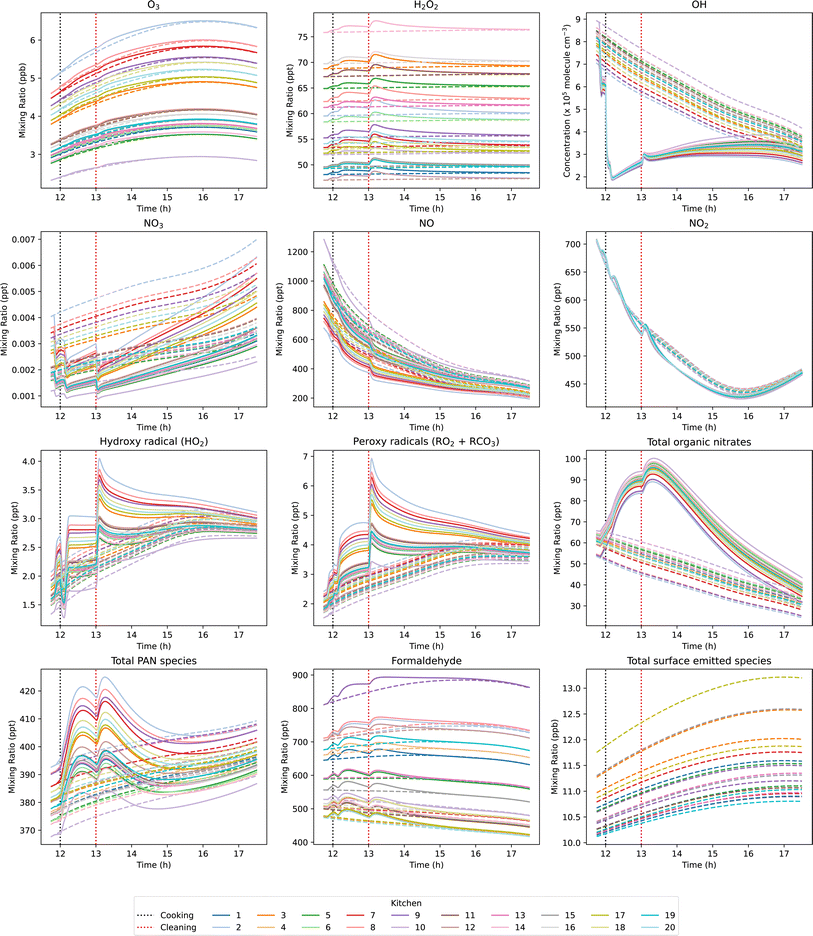
For each kitchen, a background (excluding cooking and cleaning emissions) and activity (including cooking and cleaning emissions) simulation were performed. The resulting concentrations of key oxidants, radical species, and secondary pollutants in the background (dashed lines) and activity (solid lines) simulations are shown in Fig. 5. Concentrations are shown for oxidants (O3, OH), intermediate species (formed from initial VOC oxidation pathways; peroxy radicals (RO2), NO, HO2, NO2) and secondary species, formed as a result of downstream gas-phase secondary chemistry (formaldehyde, total PANs, and total organic nitrates). In addition, products formed following O3 and H2O2 deposition to different surface materials are also shown as a summed “total surface emitted species”. This summation comprises acetaldehyde, propanal, butanal, pentanal, hexanal, heptanal, octanal, nonanal, decanal, 2-nonenal, acrolein, methacrolein, crotonaldehyde, benzaldehyde, m-tolualdehyde, 4-oxopentanal, acetone, formic acid, acetic acid, isopentanal and 2,5-dimethylbenzaldehyde, as given in Carter et al.28 Formaldehyde is also emitted from surfaces, but is shown separately for clarity. Several of these species were also measured as primary emissions from the cooking and cleaning activities. Therefore, background concentrations only are shown to highlight the effects of indoor surface materials on their concentrations. The background concentrations of most species change over the time period shown, despite there being no activities taking place. This is because INCHEM-Py models a diurnal variation in outdoor O3, NO and NO2 concentrations, meaning that different concentrations of these species are entering the simulated room over the simulated time course, and thus, affecting the background concentrations and chemistry.
Fig. 5 shows that there are two processes affecting species concentrations: (i) the kitchen surface material composition, which impacts the background concentrations even in the absence of occupant activities, and (ii) the VOC emissions from cooking and cleaning, which may or may not also be influenced by the surface material composition. These processes will be explored further in the following sections.
3.3.1.1 Material-dependent background concentration variation. To compare the influence of indoor surface material composition on the background concentration of oxidants, intermediates, and secondary pollutants of interest, the variation in concentrations was compared for each species across the 20 basic kitchen permutations by calculating the coefficient of variation (CVi,BG, see methods section for details). The CVi,BG values are presented in Table 2 for a range of oxidants, intermediate species, and secondary pollutants from gas-phase and multi-phase chemistry. O3, formaldehyde, and a number of surface-emitted secondary aldehydes showed CV > 0.2 (i.e. standard deviation more than 20% of mean), indicating a considerable degree of variability. Therefore, the dependencies of these species on indoor surface materials were investigated further. Of particular interest are O3 and formaldehyde, as they are both contaminants of concern that should be prioritised for removal in homes,61 and O3 is also fundamental in initiating VOC oxidation and subsequent formation of secondary products.
| Species | CVi,BG |
|---|---|
| Butanal | 0.749 |
| Acrolein | 0.710 |
| Benzaldehyde | 0.689 |
| Crotonaldehyde | 0.677 |
| m-Tolualdehyde | 0.619 |
| Pentanal | 0.514 |
| Hexanal | 0.456 |
| Propanal | 0.387 |
| Heptanal | 0.264 |
| Acetaldehyde | 0.220 |
| 4-Oxopentanal | 0.211 |
| Formaldehyde | 0.210 |
| O3 | 0.210 |
| Methacrolein | 0.177 |
| 2-Nonenal | 0.170 |
| H2O2 | 0.141 |
| NO | 0.138 |
| Nonanal | 0.111 |
| Decanal | 0.090 |
| OH | 0.087 |
| Total organic nitrates | 0.082 |
| RO2 | 0.071 |
| Octanal | 0.058 |
| HO2 | 0.055 |
| Acetone | 0.016 |
| Total PANs | 0.015 |
| 2,5-Dimethylbenzaldehyde | 0.007 |
| Isopentanal | 0.003 |
| Formic acid | 0.002 |
| Acetic acid | 0.001 |
The influence of indoor surfaces on gas-phase pollutant concentrations is two-fold. Firstly, species may deposit onto indoor surfaces, thus removing them from the system. In INCHEM-Py, surface-specific deposition velocities of O3 and H2O2 only are considered, while the deposition of other species is represented by constant deposition velocities, independent of surface material.28,34 Secondly, the emission of pollutants from indoor surfaces resulting from O3 and H2O2 surface interactions contributes to the gas-phase concentrations of secondary pollutants such as formaldehyde and larger straight-chain aldehydes.28 Therefore, gas-phase concentrations may be affected by the removal of key oxidants, thus limiting gas-phase oxidation chemistry, or by the production of secondary pollutants from multi-phase chemical transformations. As shown in Fig. 5, the concentrations of various oxidants, radical intermediates, and secondary pollutants were affected by variations in surface materials between permutations. Therefore, indoor surfaces influence all stages of indoor air chemistry.
In the background simulations, (78 ± 3)% of O3 was deposited onto indoor surfaces. This result is comparable to previous studies, which observed 85% deposited in a simulated apartment with an ACR of 0.76 h−1, and 91% in a simulated kitchen with an ACR of 0.5 h−1.28,52 The dependence of O3 concentration on indoor surface materials (CV ≈ 0.21) resulted from variations in O3 deposition rates and O3 formation from the chemical processing of surface-emitted secondary aldehydes. O3 is a major determinant of indoor air chemistry due to its ubiquitous presence indoors at chemically relevant concentrations and its reactivity towards unsaturated VOCs. Therefore, the variation in O3 observed between simulations had consequent effects on the concentrations of intermediate and secondary products, such as formaldehyde.
To investigate the surface-dependence of O3 and formaldehyde, their average background concentrations in each kitchen were plotted against the SAV of each material in the kitchen scenario. Then, linear regression was used to determine the degree of correlation between the species and material SAV.
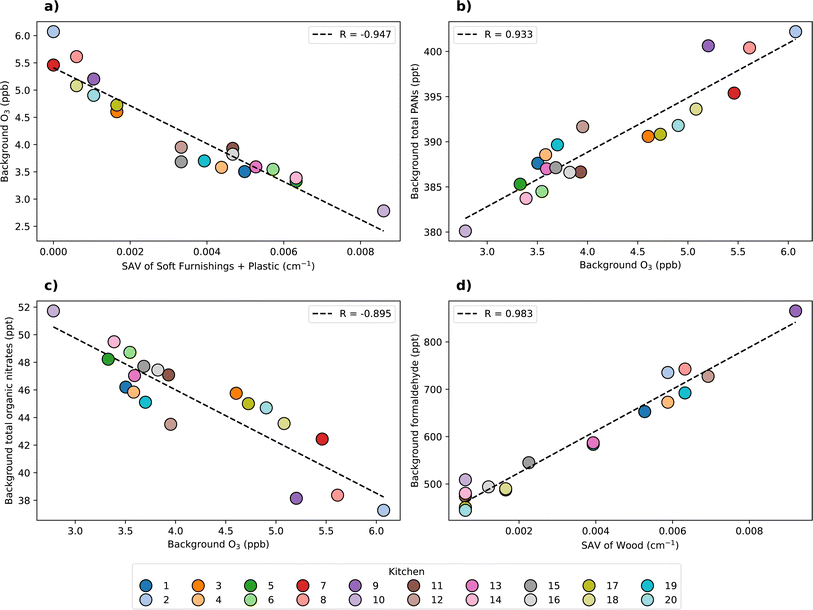
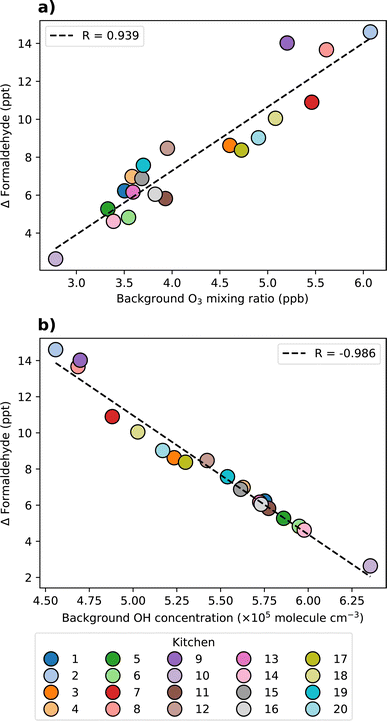
A strong negative correlation was observed between the background concentration of O3 and plastic SAV. For example, the lowest O3 concentrations were observed from kitchens 5, 6, and 14, which had the highest proportion of plastic surfaces. This suggests that plastic surfaces are a major determinant of indoor O3 concentration, as evidenced by the high O3 deposition velocity onto plastic compared to other surface materials (0.12 cm s−1). Soft furnishings were present in some of the basic kitchen permutations due to the inclusion of carpeted flooring. Soft furnishings were also efficient at removing O3 from the system, with a deposition velocity of 0.15 cm s−1. The observed correlation between background O3 and the sum of plastic and soft furnishings SAVs showed a strong negative correlation (R = −0.95), illustrating the influence of these surface materials on O3 deposition (Fig. 6a). Kitchen 10 had the highest combined proportion of plastic surfaces and soft furnishings, resulting in the lowest observed background O3 concentration of approximately 2.8 ppb.
The observed variation in background O3 concentration was expected to affect the concentration of key secondary pollutants from gas-phase VOC oxidation chemistry. Fig. 6b and c show the correlation of background O3 with total PANs and organic nitrates, respectively. A strong positive correlation was observed between O3 and total PANs background concentrations (R = 0.933), while a negative correlation was observed between O3 and total organic nitrates (R = −0.895). The reason for the differing correlations between O3 and PANs, and O3 and organic nitrates is due to the differences in their formation pathways, and the relationship between O3, NO and NO2. At greater O3 concentrations, VOC oxidation to generate RO2 and RCO3 (peroxyacetyl radical, the precursor to PANs formation) is more efficient. These radicals subsequently react with NO (forming organic nitrates, RNO3) and NO2 (forming PANs), respectively. Meanwhile, O3, NO and NO2 are linked via reaction (4), as follows:
| O3 + NO → NO2 + O2. | (4) |
This reaction means that as O3 increases, NO is consumed while NO2 is produced. Therefore, increasing O3 differentially affects the formation of PANs and organic nitrates, as summarised below:
| RO2 + NO → RNO3, ↓rate with ↑[O3] | (5) |
| RCO3 + NO2 → PANs, ↑rate with ↑[O3] | (6) |
Formaldehyde is also a secondary pollutant of concern due to its irritant and carcinogenic properties.18 Formaldehyde is an important product of both gas-phase and multi-phase chemistry. This species showed a strong correlation with wood SAV (R = 0.983, Fig. 6d). The average background concentration of formaldehyde varied from 444 to 865 ppt, with the highest concentration observed from kitchen 9 which had the highest proportion of wood surfaces. Wood has an O3 deposition velocity of approximately 10 times lower than soft furnishings, however, the formaldehyde production yield from wood is the highest of all surface materials (over 21 times greater than soft furnishings). Therefore, wood surfaces may have a strong influence on formaldehyde concentrations. These results indicate that keeping the total SAV of a room consistent, but changing the surface material composition can strongly impact the background formaldehyde concentration.
Similarly to formaldehyde, of the surface-emitted secondary carbonyls with CV > 0.2, all species except for heptanal were strongly positively correlated with wood surfaces due to the high production yields of these species from wood compared to all other surface materials.26,28 Soft furnishings also showed a positive correlation with the concentration of hexanal. The production yield of hexanal from soft furnishings is approximately 0.2 times that of wood, however, due to the high O3 deposition velocity of soft furnishings compared to wood, the resulting impact of these surface materials on the emission of secondary hexanal was of a similar magnitude. Moderate correlations of heptanal with soft furnishings and plastic surfaces were observed, as these were the only surface materials which emitted heptanal as a product of multi-phase chemistry. An inverse correlation was observed between the concentrations of surface-emitted secondary products and the SAV of materials which did not emit them due to the substitution of emitting surfaces with non-emitting surfaces in the permutations. Painted surfaces generally showed a negative correlation with most surface-emitted secondary carbonyls as this material only emitted C8–C10 straight chain aldehydes, and at low yields compared to other materials. Fig. S3 and S4† show the correlations between material surface areas and background concentrations of surface-emitted species.
In contrast to the other surface-emitted secondary products, 4-oxopentanal was only emitted from skin, which remained at a constant SAV between the different kitchens, corresponding to a single occupant. Therefore, the observed variation in this species concentration was due to changes in gas-phase oxidant concentration and/or changes in the rate of production from gas-phase chemistry induced by the variation in surface material SAVs. A perfect correlation was observed between the background concentrations of 4-oxopentanal and O3. Thus, the availability of O3 to deposit onto the skin surface was the only influencing factor, and differences in gas-phase chemistry had minimal effect on 4-oxopentanal formation between simulations.
These results suggest that room material composition has a complex effect on indoor air chemistry. For example, reducing the indoor concentration of O3 by increasing the SAV of plastic surfaces and soft furnishings may be beneficial for minimising the production of PANs, but comes at the cost of increasing the concentrations of organic nitrates and some surface-emitted carbonyl species. More comprehensive toxicological information will be essential to drive our understanding of the relative importance of these air pollutants on occupant health. This information would allow more informed decisions to be made on which material choices are most beneficial for improved indoor air quality.
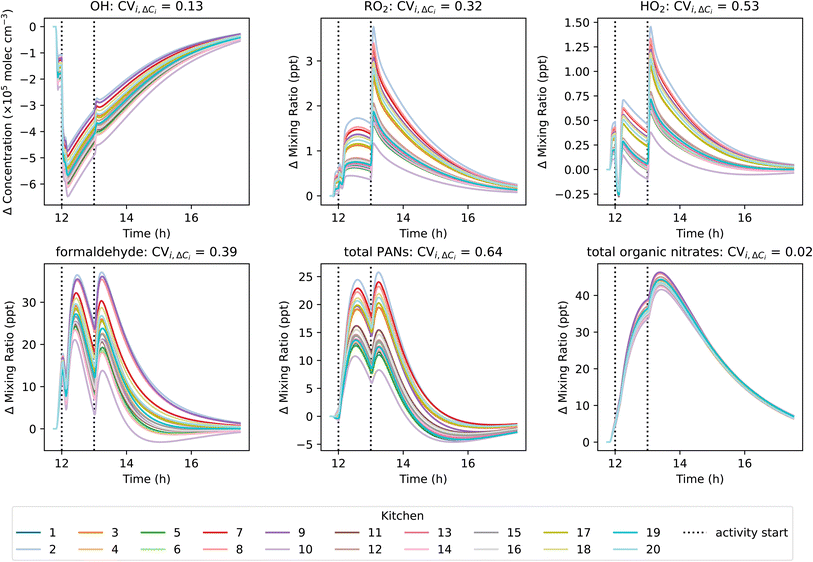
3.3.1.2 Effects of materials on secondaries from cooking and cleaning. The combination of surface materials not only affected the background species concentrations, but they also influenced the chemical processing of VOC emissions from cooking and cleaning activities. The activity-induced change in concentration of OH, HO2, RO2, formaldehyde, total PANs, and total organic nitrates for each basic kitchen permutation is shown in Fig. 7. To quantify the effect of room surface composition on activity-induced chemistry, CVi,ΔCi values were calculated and are shown in Fig. 7.
The activity-induced change in OH concentration was not strongly impacted by variation in surface materials (CVi,ΔCi = 0.13). Most of the observed change in OH concentration was due to reaction with the VOCs emitted from cooking and cleaning, which remained constant for all simulations. Conversely, HO2 and RO2 were generated as products of oxidation reactions initiated by both OH and O3. Therefore, the difference in background O3 discussed earlier due to variations in O3 surface deposition between the different kitchens impacted the overall efficiency at which the emitted VOCs were oxidised to generate HO2 and RO2, resulting in high CVi,ΔCi of 0.53 and 0.32, respectively.
Of the secondary pollutants, high CVi,ΔCi values were observed for formaldehyde and total PANs of 0.39 and 0.64, respectively. This indicates that the activity-induced formation of these secondary pollutants were strongly influenced by variations in material-specific SAVs between simulations. However, total organic nitrates showed very little variation between simulations (CVi,ΔCi = 0.02), suggesting that these species were not significantly influenced by variations in the kitchen surface materials.
The effects of surface materials on the formation of secondary pollutants following cooking and cleaning are both direct (emissions of secondary pollutants from multi-phase chemistry) and indirect (removal of oxidants by surface deposition, which would otherwise contribute to secondary pollutant formation via gas-phase indoor air chemistry). The influence of key indoor oxidants (O3 and OH) on the observed variation in activity-induced change in formaldehyde concentrations was investigated further in Fig. 8. Here, linear regression analysis was performed between oxidant concentration and activity-induced change in formaldehyde concentration.
The variability in activity-induced changes in formaldehyde concentration was observed due to the influence of indoor surfaces on both the direct emissions from multi-phase chemistry and the concentrations of oxidant species contributing to the formation and destruction of formaldehyde via gas-phase reactions. Linear regression analysis indicated a positive correlation between activity-induced changes in formaldehyde concentration and O3 concentration (R = 0.939, Fig. 8a), and a negative correlation with OH concentration (R = −0.986, Fig. 8b).
As Fig. 8 shows, indoor oxidant concentrations were a strong mediator of secondary pollutant formation following cooking and cleaning activities. Therefore, it follows that the surface materials which influenced secondary pollutant formation the most were also those that had the greatest influence on indoor O3 concentrations (i.e. plastic and soft furnishings). Elevated O3 concentrations led to more efficient ozonolysis of unsaturated VOC emissions from cooking and cleaning activities, thereby resulting in the production of formaldehyde as a secondary product of gas-phase chemistry. Formaldehyde is not effectively destroyed by O3 due to its lack of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds, thus elevated O3 concentrations yielded higher concentrations of formaldehyde (although these are small in an absolute sense). During the cooking and cleaning activities, O3 concentrations increased slightly as a result of VOC oxidation, resulting in more O3 deposition onto surfaces. Therefore, additional formaldehyde was produced during activities as a result of multi-phase chemistry, particularly on wooden surfaces, as well as from gas-phase oxidation of VOC emissions. A negative correlation was observed between the activity-induced change in formaldehyde and OH concentration, because elevated formaldehyde (and other VOC) concentrations were associated with the cooking and cleaning activities, and these enhanced concentrations suppressed the OH radical concentration.
C bonds, thus elevated O3 concentrations yielded higher concentrations of formaldehyde (although these are small in an absolute sense). During the cooking and cleaning activities, O3 concentrations increased slightly as a result of VOC oxidation, resulting in more O3 deposition onto surfaces. Therefore, additional formaldehyde was produced during activities as a result of multi-phase chemistry, particularly on wooden surfaces, as well as from gas-phase oxidation of VOC emissions. A negative correlation was observed between the activity-induced change in formaldehyde and OH concentration, because elevated formaldehyde (and other VOC) concentrations were associated with the cooking and cleaning activities, and these enhanced concentrations suppressed the OH radical concentration.
Considering both the direct and indirect effects of surface materials on formaldehyde concentration, decreasing the SAV of plastic and soft furnishings (thereby decreasing O3 deposition) and increasing the SAV of wood (thus increasing formaldehyde emissions from multi-phase chemistry) generally increased formaldehyde formation. For example, kitchens 2, 8 and 9 had low plastic SAVs, no soft furnishings and high wood SAVs, and these were the kitchens where the most formaldehyde (≈35 ppt) was formed following activities. However, compared to the background simulations where the average formaldehyde concentration varied by ≈3 ppb as a result of room surface composition, the difference in activity-induced formaldehyde concentration was only ≈20 ppt (over 100 × smaller). Overall, this suggests that room material composition is more important to consider in the context of ambient background pollution indoors, rather than for influencing secondary chemistry of sporadic activities like cooking and cleaning.
Fig. 9 shows the average concentrations of key oxidants, intermediate species, and secondary pollutants in the background and activity simulations of the 9 realistic kitchen scenarios. These results show that as total SAV decreased, the average concentration of oxidants, intermediates, and secondary pollutants generally increased. The average O3 mixing ratio in the 9 kitchens ranged from 1.3 ppb to 7.8 ppb, with (91 ± 2)% O3 deposited onto indoor surfaces. The relationship between kitchen SAV and average O3 mixing ratio was not linear, illustrating the influence of surface materials on oxidant deposition. For example, kitchens 7 and 8 had similar total SAVs of 2.10 and 2.04 m−1, respectively, however the average background O3 concentration in kitchen 8 was 2.0 ppb lower than in kitchen 7. Kitchen 8 had the largest SAV of soft furnishings (0.00355 cm−1, surface area = 15.6 m2), which has a relatively high O3 deposition velocity. Furthermore, the average background O3 concentration in kitchen 2 was lower than that of kitchen 1, despite kitchen 1 having a larger SAV. Over 30% of kitchen 2 SAV constituted plastic, which is another surface material which has a high O3 deposition velocity. Therefore, the presence of soft furnishings and plastic indoor surfaces has a clear impact on background O3 concentrations as a result of increased removal by surface deposition.
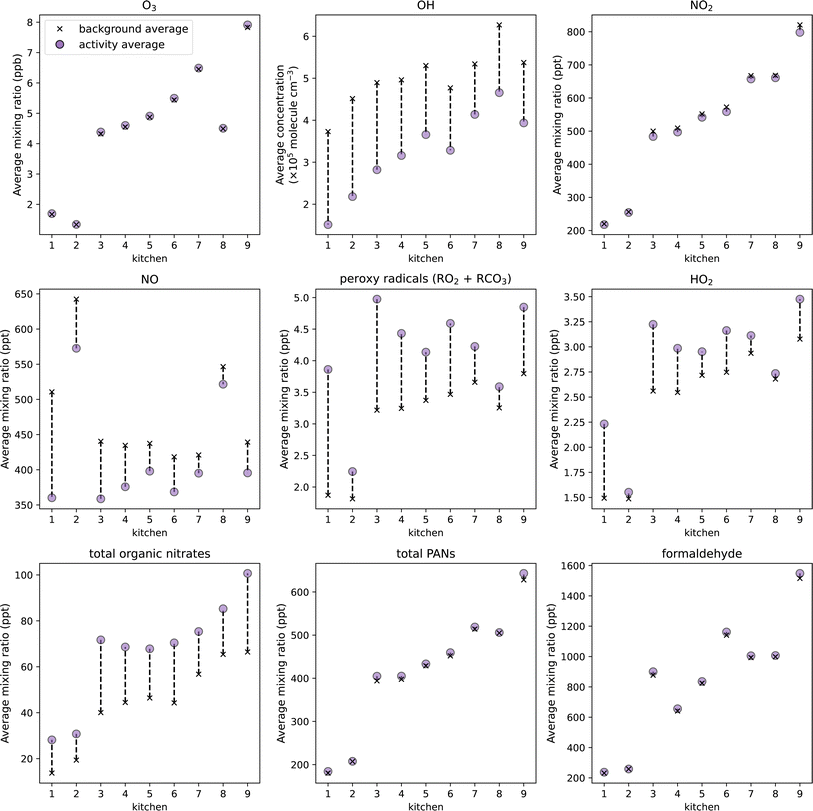 | ||
| Fig. 9 Change in average mixing ratios or concentrations of oxidants, intermediate species, and secondary pollutants between baseline and activity simulations when cooking and cleaning are simulated in the 9 kitchens described in Manuja et al.,51 in order of decreasing total SAV. Averages calculated from 15 min before t0 to 5.5 h after t0. | ||
The average background and activity OH concentrations showed less variability between kitchens compared to O3, as OH is short lived, and not directly influenced by surface deposition in the model. Kitchens 6 and 9 showed slightly lower OH concentrations than expected based on their SAVs. Kitchen 9 had a lower room volume than kitchens 7 and 8, but a lower overall SAV. The background and activity concentrations of VOCs and radical intermediates were higher in kitchen 9 owing to the lower room volume, resulting in greater consumption of OH radicals by more efficient OH chemistry. This effect also caused the lower OH concentration in kitchen 6 compared to kitchen 5 with a higher total SAV.
The background concentrations of intermediate species and secondary pollutants followed a similar trend to O3, illustrating the significance of O3 in driving indoor air chemistry. Higher O3 concentrations resulted in more efficient removal of NO and production of NO2, as a result of reaction (4). Additionally, higher O3 concentrations resulted in more efficient VOC oxidation chemistry, resulting in the formation of RO2 and HO2 radical intermediates. However, kitchens 2 and 8 showed high background NO concentrations due to the removal of O3 by surface deposition. Therefore, the peroxy radicals and HO2 formed from the oxidation of cooking and cleaning VOC emissions are suppressed by the NO, resulting in a relatively small increase in average peroxy radical and HO2 concentrations between the background and activity simulations of kitchens 2 and 8.
The production of secondary pollutants following cooking and cleaning was dependent on (i) the dilution of VOC emissions into the room volume, (ii) the availability of indoor oxidants for gas-phase VOC oxidation, and (iii) secondary emissions from indoor surfaces following multi-phase chemistry. As demonstrated in the previous section, indoor surfaces do not have a strong influence on the production of organic nitrates following VOC emission events. Fig. 9 shows that the average background concentration of organic nitrates ranged from 14 to 66 ppt, as a result of differences in background NO and RO2 concentrations. The increase in concentration observed between the background and post-activity simulations ranged from 11 to 34 ppt, and followed a similar trend to OH radicals. This suggests that the dilution of VOCs due to room volume were the major influencing factor on organic nitrate concentration.
The background and activity concentrations of PANs showed higher variability between kitchens compared to organic nitrates, following a similar pattern to O3. The formation of PANs was strongly dependent on the concentration of O3 available for gas-phase VOC oxidation chemistry, thus the influence of material-specific SAVs on O3 deposition also impacted the concentration of PANs. Total PANs concentration in kitchens 2 and 8 were greater than expected given the relatively low O3 concentration in these kitchens relative to kitchens 1 and 7 with similar SAVs, respectively. This is because with increased O3 deposition, there was also an increase in aldehyde emissions from multi-phase ozonolysis reactions. Elevated aldehyde concentrations in kitchens 2 and 8 from increased O3 surface interactions contributed to the formation of PANs through the production of RCO3 radicals from surface-derived aldehyde oxidation.
Finally, the average background concentration of formaldehyde varied from 232 to 1515 ppt between kitchens. The total SAV had a significant influence on formaldehyde concentrations. Formaldehyde deposition velocity is approximately three times higher compared to total PANs and organic nitrates. Therefore, variations in total SAV had a more pronounced effect on formaldehyde concentrations. Generally, as the total SAV decreased, average formaldehyde concentrations tended to increase due to reduced surface deposition. There was greater variability in formaldehyde concentration observed between kitchens compared to PANs. This was a result of wood surfaces being a strong source of formaldehyde emissions from multi-phase chemistry. For example, kitchens 3 and 6 had the largest wood SAVs of 0.0116 and 0.0115 cm−1, respectively, and showed higher average formaldehyde concentrations compared to kitchens 4 and 5, which had comparable total SAVs but lower wood SAVs of 0.0053 and 0.0068 cm−1, respectively.
The largest increase in secondary pollutants between the background and activity simulations, and highest absolute concentrations, was observed for kitchen 9, owing to its high O3 concentration. These results suggest that the effects of kitchen SAVs on the gas-phase concentration of O3 are most important in determining the production of secondary pollutants following cooking and cleaning activities indoors. Therefore, larger room volume, smaller surface area, and less soft furnishings and plastic surfaces may contribute to higher secondary pollutant concentrations. However, it is also important to consider the primary emissions from these surface materials to get a more holistic view of how surfaces may impact indoor air pollution. For example, while increasing the surface area of plastic in a room may effectively remove O3 by deposition, and subsequently thwart gas-phase oxidation chemistry, it may also introduce hazardous pollutants as direct emissions from the surface material. Beel et al. identified plastics as a potentially large source of hazardous VOC emissions, including styrene, toluene, and phenol.62 Primary VOC emissions from surface materials have not been considered in this study. It will be important to improve our understanding of the primary and secondary emissions from indoor surfaces, as well as their differential health impacts, to gain insight into how building designs may impact indoor air quality and the resulting impacts on occupant health. This comprehensive understanding is crucial for informing decisions about building design and management practices aimed at promoting healthier indoor environments.
4 Conclusions
This study has highlighted cooking and cleaning activities as large sources of VOC emissions, which have the potential to produce hazardous secondary pollutants as products of indoor air chemistry. Using an indoor air chemistry model, INCHEM-Py, indoor surfaces have been shown to impact the gas-phase concentration of oxidants indoors, with consequent effects on the chemical processing of VOC emissions from cooking and cleaning. Simulations revealed that plastic surfaces and soft furnishings were the most efficient at removing O3 by surface deposition, resulting in secondary emissions of aldehyde species. Furthermore, indoor surfaces contributed to the production of hazardous secondary pollutants via multi-phase oxidant interactions. Wooden surfaces were most efficient at producing formaldehyde as a secondary pollutant from multi-phase ozonolysis reactions. Simulations performed under a range of realistic kitchen SAVs highlighted that higher secondary pollutant concentrations were achieved at lower total SAVs, however, the specific combinations of surface materials also impacted results. This study has focused on domestic kitchens. Commercial kitchens may show quite different results, as they typically have a higher proportion of metal surfaces, and higher air change rates. The latter means that surface chemistry would likely be much less important in commercial kitchens, and this topic could be worthy of further investigation.These results illustrate the influence of kitchen surface materials, in addition to the total SAV of the room, on the secondary production of formaldehyde and other potentially hazardous secondary pollutants following cooking and cleaning activities. Therefore, when aiming to enhance indoor air quality and minimise occupant exposure to hazardous pollutants, considerations should be given to room volume, total surface area of contents, and the specific materials used. However, it is worth noting that ventilation remains one of the most effective methods for improving indoor air quality following high-emission occupant activities such as cooking and cleaning. Changing trends in building designs, such as the adoption of open-plan living arrangements likely resulting in increased room volume and the incorporation of more wood and soft furnishings, are expected to influence the chemical processing of VOC emissions from typical occupant activities within these spaces.
Further research aimed at elucidating the kinetics of VOC and oxidant interactions across a broader range of indoor surface materials would be highly advantageous. Furthermore, it will be important to explore how external factors such as temperature and relative humidity affect heterogeneous chemical reactions, given their variability from room to room and across different climates. It will also be important to consider the contributions of primary VOC emissions from surface materials towards indoor air chemistry, as building materials have been identified as a significant source of VOCs which vary with material age. Developments in our understanding of these aspects of surface effects on indoor air chemistry would facilitate future model developments, thereby enabling more comprehensive modelling investigations.
Data availability
All data for this article, including raw simulation data and analysis codes, are available from the ‘Research Data York’ repository at the University of York, doi: https://doi.org/10.15124/f6598a1a-2f7e-4235-aa2c-98cb9a9607f3.Author contributions
Ellen Harding-Smith: conceptualisation, methodology, data curation, investigation, formal analysis, visualisation, writing – original draft, writing – review and editing. Helen Davies: conceptualisation, methodology, investigation, formal analysis, writing – original draft, writing – review and editing, visualisation. Catherine O'Leary: methodology, investigation, formal analysis, data curation. Ruth Winkess: methodology, investigation, formal analysis, data curation. Marvin Shaw: methodology, investigation, data curation. Benjamin Jones: methodology, investigation. Terry Dillon: methodology, investigation, writing – review and editing. Nicola Carslaw: conceptualisation, methodology, writing – review and editing, project administration, funding acquisition.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors wish to thank Chris Anthony and Athina Ruangkanit for their assistance during the experimental campaign. The authors acknowledge EPSRC who funded this work under grant numbers EP/T014474/1 and EP/T518025/1. The development of INCHEM-Py has been funded by grant from the Alfred P. Sloan Foundation COMMODIAC (G-2018-10083). Conclusions reached or positions taken by researchers or other grantees represent the views of the grantees themselves and not those of the Alfred P. Sloan Foundation or its trustees, officers, or staff.Notes and references
- Y. Liu, P. K. Misztal, J. Xiong, Y. Tian, C. Arata and R. J. Weber, et al., Characterizing sources and emissions of volatile organic compounds in a northern California residence using space- and time-resolved measurements, Indoor Air, 2019, 29(4), 630–644 CAS.
- H. Wang, J. Xiong and W. Wei, Measurement methods and impact factors for the key parameters of VOC/SVOC emissions from materials in indoor and vehicular environments: A review, Environ. Int., 2022, 168, 107451 CrossRef CAS PubMed.
- H. Destaillats, R. L. Maddalena, B. C. Singer, A. T. Hodgson and T. E. McKone, Indoor pollutants emitted by office equipment: A review of reported data and information needs, Atmos. Environ., 2008, 42(7), 1371–1388 CrossRef CAS.
- H. L. Davies, C. O'Leary, T. Dillon, D. R. Shaw, M. Shaw and A. Mehra, et al., A measurement and modelling investigation of the indoor air chemistry following cooking activities, Environ. Sci.: Processes Impacts, 2023, 25(9), 1532–1548 RSC.
- W. W. Nazaroff and C. J. Weschler, Cleaning products and air fresheners: Exposure to primary and secondary air pollutants, Atmos. Environ., 2004, 38(18), 2841–2865 CrossRef CAS.
- S. K. Brown, Volatile organic pollutants in new and established buildings in Melbourne, Australia, Indoor Air, 2002, 12(1), 55–63 CrossRef CAS.
- N. E. Klepeis, W. C. Nelson, W. R. Ott, J. P. Robinson, A. M. Tsang and P. Switzer, et al., The National Human Activity Pattern Survey (NHAPS): a resource for assessing exposure to environmental pollutants, J. Exposure Anal. Environ. Epidemiol., 2001, 11(3), 231–252 CrossRef CAS.
- S. Vardoulakis, E. Giagloglou, S. Steinle, A. Davis, A. Sleeuwenhoek and K. S. Galea, et al., Indoor exposure to selected air pollutants in the home environment: A systematic review, Int. J. Environ. Res. Public Health, 2020, 17, 1–24 Search PubMed.
- S. Audignon-Durand, O. Ramalho, C. Mandin, A. Roudil, O. L. Bihan and F. Delva, et al., Indoor exposure to ultrafine particles related to domestic activities: A systematic review and meta-analysis, Sci. Total Environ., 2023, 904, 166947 CrossRef CAS.
- J. C. Ditto, L. R. Crilley, M. Lao, T. C. VandenBoer, J. P. D. Abbatt and A. W. H. Chan, Indoor and outdoor air quality impacts of cooking and cleaning emissions from a commercial kitchen, Environ. Sci.: Processes Impacts, 2023, 25, 964–979 RSC.
- D. K. Farmer, M. E. Vance, J. P. D. Abbatt, A. Abeleira, M. R. Alves and C. Arata, et al., Overview of HOMEChem: House Observations of Microbial and Environmental Chemistry, Environ. Sci.: Processes Impacts, 2019, 21(8), 1280–1300 RSC.
- K. L. Abdullahi, J. M. Delgado-Saborit and R. M. Harrison, Emissions and indoor concentrations of particulate matter and its specific chemical components from cooking: A review, Atmos. Environ., 2013, 71, 260–294 CrossRef CAS.
- F. Klein, U. Baltensperger, A. S. H. Prévôt and I. E. Haddad, Quantification of the impact of cooking processes on indoor concentrations of volatile organic species and primary and secondary organic aerosols, Indoor Air, 2019, 29, 926–942 CrossRef CAS.
- F. Klein, N. J. Farren, C. Bozzetti, K. R. Daellenbach, D. Kilic and N. K. Kumar, et al., Indoor terpene emissions from cooking with herbs and pepper and their secondary organic aerosol production potential, Sci. Rep., 2016, 6, 36623 CrossRef CAS.
- B. C. Singer, H. Destaillats, A. T. Hodgson and W. W. Nazaroff, Cleaning products and air fresheners: Emissions and resulting concentrations of glycol ethers and terpenoids, Indoor Air, 2006, 16(3), 179–191 CrossRef CAS.
- C. Arata, P. K. Misztal, Y. Tian, D. M. Lunderberg, K. Kristensen and A. Novoselac, et al., Volatile organic compound emissions during HOMEChem, Indoor Air, 2021, 31(6), 2099–2117 CrossRef CAS PubMed.
- A. W. Nørgaard, J. D. Kudal, V. Kofoed-Sørensen, I. K. Koponen and P. Wolkoff, Ozone-initiated VOC and particle emissions from a cleaning agent and an air freshener: Risk assessment of acute airway effects, Environ. Int., 2014, 68, 209–218 CrossRef.
- World Health Organization, WHO Guidelines for Indoor Air Quality: Selected Pollutants, World Health Organization, Geneva, 2010 Search PubMed.
- G. D. Nielsen and P. Wolkoff, Cancer effects of formaldehyde: a proposal for an indoor air guideline value, Arch. Toxicol., 2010, 84(6), 423–446 CrossRef CAS.
- J. Q. Koenig, D. S. Covert and W. E. Pierson, Effects of Inhalation of Acidic Compounds on Pulmonary Function in Allergic Adolescent Subjects, Environ. Health Perspect., 1989, 79, 173–178 CrossRef CAS.
- T. Berkemeier, M. Ammann, T. F. Mentel, U. Pöschl and M. Shiraiwa, Organic Nitrate Contribution to New Particle Formation and Growth in Secondary Organic Aerosols from α-Pinene Ozonolysis, Environ. Sci. Technol., 2016, 50(12), 6334–6342 CrossRef CAS.
- G. Zhang, Y. Mu, L. Zhou, C. Zhang, Y. Zhang and J. Liu, et al., Summertime distributions of peroxyacetyl nitrate (PAN) and peroxypropionyl nitrate (PPN) in Beijing: Understanding the sources and major sink of PAN, Atmos. Environ., 2015, 103, 289–296 CrossRef CAS.
- A. P. Ault, V. H. Grassian, N. Carslaw, D. B. Collins, H. Destaillats and D. J. Donaldson, et al., Indoor Surface Chemistry: Developing a Molecular Picture of Reactions on Indoor Interfaces, Chem, 2020, 6(12), 3203–3218 CAS.
- J. P. D. Abbatt and C. Wang, The atmospheric chemistry of indoor environments, Environ. Sci.: Processes Impacts, 2020, 22, 25–48 RSC.
- B. C. Singer, A. T. Hodgson, T. Hotchi, K. Y. Ming, R. G. Sextro and E. E. Wood, et al., Sorption of organic gases in residential rooms, Atmos. Environ., 2007, 41, 3251–3265 CrossRef CAS.
- Y. H. Cheng, C. C. Lin and S. C. Hsu, Comparison of conventional and green building materials in respect of VOC emissions and ozone impact on secondary carbonyl emissions, Build. Environ., 2015, 87, 274–282 CrossRef.
- M. Nicolas, O. Ramalho and F. Maupetit, Reactions between ozone and building products: Impact on primary and secondary emissions, Atmos. Environ., 2007, 41, 3129–3138 CrossRef CAS.
- T. J. Carter, D. G. Poppendieck, D. Shaw and N. Carslaw, A Modelling Study of Indoor Air Chemistry: The Surface Interactions of Ozone and Hydrogen Peroxide, Atmos. Environ., 2023, 297, 119598 CrossRef CAS.
- C. Y. Lim and J. P. Abbatt, Chemical Composition, Spatial Homogeneity, and Growth of Indoor Surface Films, Environ. Sci. Technol., 2020, 54, 14372–14379 CrossRef CAS.
- B. L. Deming and P. J. Ziemann, Quantification of alkenes on indoor surfaces and implications for chemical sources and sinks, Indoor Air, 2020, 30, 914–924 CrossRef CAS.
- H. Wang and G. C. Morrison, Ozone-Initiated Secondary Emission Rates of Aldehydes from Indoor Surfaces in Four Homes, Environ. Sci. Technol., 2006, 40, 5263–5268 CrossRef CAS.
- D. Won, R. L. Corsi and M. Rynes, Sorptive Interactions between VOCs and Indoor Materials, Indoor Air, 2001, 11, 246–256 CrossRef CAS PubMed.
- G. Pei, Y. Xuan, G. Morrison and D. Rim, Understanding Ozone Transport and Deposition within Indoor Surface Boundary Layers, Environ. Sci. Technol., 2022, 56, 7820–7829 CrossRef CAS.
- D. R. Shaw, T. J. Carter, H. L. Davies, E. Harding-Smith, E. C. Crocker, G. Beel and Z. Wang, Carslaw, INCHEM-Py v1.2: a community box model for indoor air chemistry, Geosci. Model Dev., 2023, 16, 7411–7431 CrossRef CAS.
- C. J. Weschler, Changes in indoor pollutants since the 1950s, Atmos. Environ., 2009, 43(1), 153–169 CrossRef CAS.
- HM Government, The Building Regulations 2010, 2010, available from: https://www.legislation.gov.uk/uksi/2010/2214/contents, accessed: 2024-04.
- A. Moreno-Rangel, Passivhaus, Encyclopedia, 2020, 1, 20–29 CrossRef.
- D. Smith and P. Španěl, Selected ion flow tube mass spectrometry (SIFT-MS) for on-line trace gas analysis, Mass Spectrom. Rev., 2005, 24(5), 661–700 CrossRef CAS.
- P. Spanel and D. Smith, Selected ion flow tube: a technique for quantitative trace gas analysis of air and breath, Med. Biol. Eng. Comput., 1996, 34, 409–419 CrossRef CAS.
- R. L. Wagner, N. J. Farren, J. Davison, S. Young, J. R. Hopkins and A. C. Lewis, et al., Application of a mobile laboratory using a selected-ion flow-tube mass spectrometer (SIFT-MS) for characterisation of volatile organic compounds and atmospheric trace gases, Atmos. Meas. Tech., 2021, 14(9), 6083–6100 CrossRef CAS.
- E. Harding-Smith, D. R. Shaw, M. Shaw, T. J. Dillon and N. Carslaw, Does green mean clean? Volatile organic emissions from regular versus green cleaning products, Environ. Sci.: Processes Impacts, 2024, 26(2), 436–450 RSC.
- N. Carslaw, A new detailed chemical model for indoor air pollution, Atmos. Environ., 2007, 41(6), 1164–1179 CrossRef CAS.
- M. E. Jenkin, S. M. Saunders and M. J. Pilling, The tropospheric degradation of volatile organic compounds: A protocol for mechanism development, Atmos. Environ., 1997, 31(1), 81–104 CrossRef CAS.
- S. M. Saunders, M. E. Jenkin, R. G. Derwent and M. J. Pilling, Protocol for the development of the Master Chemical Mechanism, MCM v3 (Part A): Tropospheric degradation of non-aromatic volatile organic compounds, Atmos. Chem. Phys., 2003, 3(1), 161–180 CrossRef CAS.
- N. Carslaw, T. Mota, M. E. Jenkin, M. H. Barley and G. McFiggans, A Significant role for nitrate and peroxide groups on indoor secondary organic aerosol, Environ. Sci. Technol., 2012, 46(17), 9290–9298 CrossRef CAS.
- N. Carslaw, A mechanistic study of limonene oxidation products and pathways following cleaning activities, Atmos. Environ., 2013, 80, 507–513 CrossRef CAS.
- N. Carslaw, L. Fletcher, D. Heard, T. Ingham and H. Walker, Significant OH production under surface cleaning and air cleaning conditions: Impact on indoor air quality, Indoor Air, 2017, 27(6), 1091–1100 CrossRef CAS.
- A. C. Terry, N. Carslaw, M. Ashmore, S. Dimitroulopoulou and D. C. Carslaw, Occupant exposure to indoor air pollutants in modern European offices: An integrated modelling approach, Atmos. Environ., 2014, 82, 9–16 CrossRef CAS.
- Z. Wang, D. Shaw, T. Kahan, C. Schoemaecker and N. Carslaw, A modeling study of the impact of photolysis on indoor air quality, Indoor Air, 2022, 32(6), e13054 CAS.
- M. Kruza, G. McFiggans, M. S. Waring, J. R. Wells and N. Carslaw, Indoor secondary organic aerosols: Towards an improved representation of their formation and composition in models, Atmos. Environ., 2020, 240, 117784 CrossRef CAS.
- A. Manuja, J. Ritchie, K. Buch, Y. Wu, C. M. A. Eichler and J. C. Little, et al., Total surface area in indoor environments, Environ. Sci.: Processes Impacts, 2019, 21(8), 1384–1392 RSC.
- M. Kruza, A. C. Lewis, G. C. Morrison and N. Carslaw, Impact of surface ozone interactions on indoor air chemistry: A modeling study, Indoor Air, 2017, 27(5), 1001–1011 CrossRef CAS.
- W. W. Nazaroff, Residential air-change rates: A critical review, Indoor Air, 2021, 31(2), 282–313 CrossRef PubMed.
- M. Kruza and N. Carslaw, How do breath and skin emissions impact indoor air chemistry?, Indoor Air, 2019, 29(3), 369–379 CrossRef CAS.
- C. J. Weschler, A. Wisthaler, S. Cowlin, G. Tamás, P. Strøm-Tejsen and A. T. Hodgson, et al., Ozone-initiated chemistry in an occupied simulated aircraft cabin, Environ. Sci. Technol., 2007, 41(17), 6177–6184 CrossRef CAS PubMed.
- M. Blocquet, F. Guo, M. Mendez, M. Ward, S. Coudert and S. Batut, et al., Impact of the spectral and spatial properties of natural light on indoor gas-phase chemistry: Experimental and modeling study, Indoor Air, 2018, 28(3), 426–440 CrossRef CAS.
- K. Abe, Y. Hori and T. Myoda, Volatile compounds of fresh and processed garlic (Review), Exp. Ther. Med., 2019, 19, 1585–1593 Search PubMed.
- D. C. Zhang, J. J. Liu, L. Z. Jia, P. Wang and X. Han, Speciation of VOCs in the cooking fumes from five edible oils and their corresponding health risk assessments, Atmos. Environ., 2019, 211, 6–17 CrossRef CAS.
- M. S. Angulo, M. Verriele, M. Nicolas and F. Thevenet, Indoor use of essential oil-based cleaning products: Emission rate and indoor air quality impact assessment based on a realistic application methodology, Atmos. Environ., 2021, 246, 118060 CrossRef.
- P. Wolkoff and G. D. Nielsen, Non-cancer effects of formaldehyde and relevance for setting an indoor air guideline, Environ. Int., 2010, 36(7), 788–799 CrossRef CAS PubMed.
- G. Morantes, B. Jones, C. Molina and M. H. Sherman, Harm from Residential Indoor Air Contaminants, Environ. Sci. Technol., 2024, 58, 242–257 CrossRef CAS.
- G. Beel, B. Langford, N. Carslaw, D. Shaw and N. Cowan, Temperature driven variations in VOC emissions from plastic products and their fate indoors: A chamber experiment and modelling study, Sci. Total Environ., 2023, 881, 163497 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4em00410h |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |