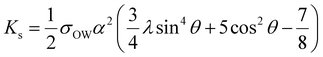Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Pickering multiphase materials using plant-based colloidal lignin nanoparticles
Jian Gan†
 ad,
Yifei Zhan†b,
Jing Fan†b,
Jifu Wang
ad,
Yifei Zhan†b,
Jing Fan†b,
Jifu Wang c,
Qi Gaod,
Caoxing Huang*a,
Wenji Yu*ad and
Kai Zhang
c,
Qi Gaod,
Caoxing Huang*a,
Wenji Yu*ad and
Kai Zhang *b
*b
aCo-Innovation Center of Efficient Processing and Utilization of Forest Resources, Nanjing Forestry University, Nanjing 210037, China. E-mail: hcx@njfu.edu.cn; chinayuwj@126.com
bSustainable Materials and Chemistry, Dept. Wood Technology and Wood-based Composites, University of Göttingen, Büsgenweg 4, 37077 Göttingen, Germany. E-mail: kai.zhang@uni-goettingen.de
cInstitute of Chemical Industry of Forest Products, Chinese Academy of Forestry, Nanjing, 210042, China
dResearch Institute of Wood Industry, Chinese Academy of Forestry, Beijing 100091, China
First published on 3rd December 2024
Abstract
A Pickering emulsion, stabilized by amphiphilic solid particles, is a highly functional and stable system that has attracted significant research interest. Lignin, an amphiphilic biomacromolecule found widely in nature, can be transformed into nanoparticles using modern nanotechnology with great potential for use in Pickering emulsions. Despite numerous studies exploring the function of colloidal lignin particles (CLPs) in producing Pickering emulsions, there are few systematic reviews on the state-of-the-art works related to CLP-stabilized Pickering emulsions. In this review, we summarize recent advances in synthesis processes, formation mechanisms, structural characteristics and surface properties of CLPs on the stability and functionality of Pickering emulsion systems. We also highlight advanced applications of CLP-stabilized Pickering emulsions at present and propose future development directions for improving their synthesis technology using lignin as a stabilizer to enhance their properties. Our hope is that this review will serve as a roadmap for scientists engaged in research on CLP-Pickering emulsions across different scientific fields to achieve optimal material performance goals.
1. Introduction
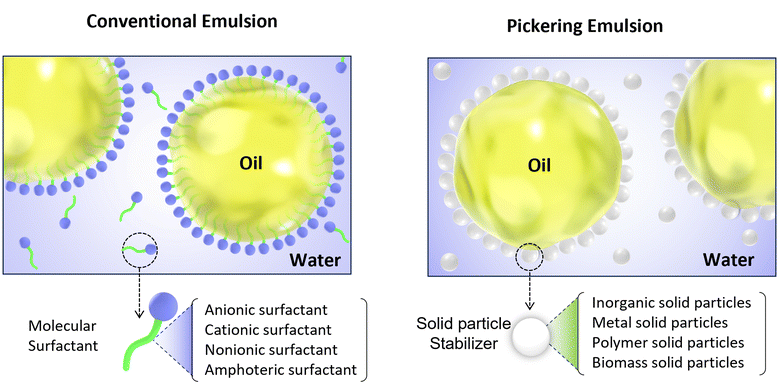
Emulsions, which are inherently thermodynamically unstable systems, consist of two phases that are incompatible with each other. Surfactants are commonly used to effectively reduce the interfacial tension between these phases. This reduction in interfacial tension serves to decrease the overall free energy of the mixing system, thereby maintaining the dispersion stability of the constituent phases of the emulsion.1 In contrast to conventional emulsions that rely on surfactants for stabilization, Pickering emulsions represent a distinct class of emulsion systems anchored in the utilization of solid particles (see Fig. 1). The earliest recorded instance of a Pickering emulsion can be traced back to 1904 when Ramsden made a pioneering discovery. He found that solid powder could form a dense particle coating on the surface of paraffin droplets, enabling even dispersion in water and subsequent formation of stable emulsions.2 In 1907, following extensive research, Pickering offered a preliminary elucidation of the stabilization mechanism associated with this distinctive type of emulsion.3 Subsequently, as researchers gained deeper insights, the stabilization mechanism of Pickering emulsions was gradually unveiled and clarified.4,5However, the emulsion known as “Pickering emulsion” in honor of its inventor initially failed to garner widespread attention and did not incite international research fervor until the 21st century.6,7 In comparison to conventional emulsions, Pickering emulsions stabilized by solid particles offer significant advantages such as low toxicity, versatility, high stability, and reduced emulsifier content,8,9 and have been known and used in industrial polymerization.10–12 Initially, research primarily focused on inorganic particles with silica particles designated as the “gold standard” Pickering stabilize, providing fundamental insights into the principles of Pickering emulsion stabilization.13 Recently, colloidal science has entered a new era of Pickering stabilization by expanding its scope from simple inorganic materials to a diverse range of functional nanoparticles and micro-particles including magnetic nanoparticles, conductive particles, pH-responsive particles, and carbon nanotubes.6,14 Notably, there has been an increasing emphasis on sustainability which has driven exploration into clean-label natural biopolymer particles specifically addressing the rising global demand for eco-friendly materials.15 Consequently, extensive investigations have been conducted on various biomass nanoparticles such as cellulose nanofibers,16–18 chitosan nanoparticles, plant protein particles, and starch nanoparticles.19–21 This transition aligns with the broader realm of colloidal science that encompasses a wide array of particle types aimed at satisfying the growing requirement for environmentally conscious and naturally sourced materials.22
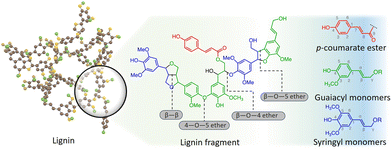
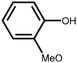
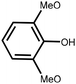
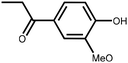
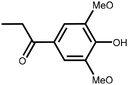
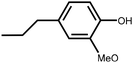
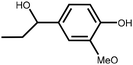
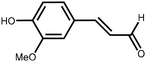
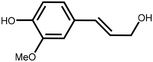
Lignin is the second most abundant natural polymer derived from plants, following cellulose.23 It possesses an aromatic composition with a three-dimensional spatial structure, consisting of numerous syringyl (S), guaiacyl (G), and p-hydroxyphenyl (H) units linked together by both ether and carbon–carbon bonds (Fig. 2).24,25 This unique lignin structure contributes to several desirable properties, including sustainability, biodegradability, multiple active functional groups, cost-effective production, as well as antioxidant and UV-absorbent capabilities.25,26 Consequently, lignin can be utilized as a functional material in the fabrication of composites such as thermoset resins, thermoplastics, foams, and adhesives through physical blending or cross-linking processes.27
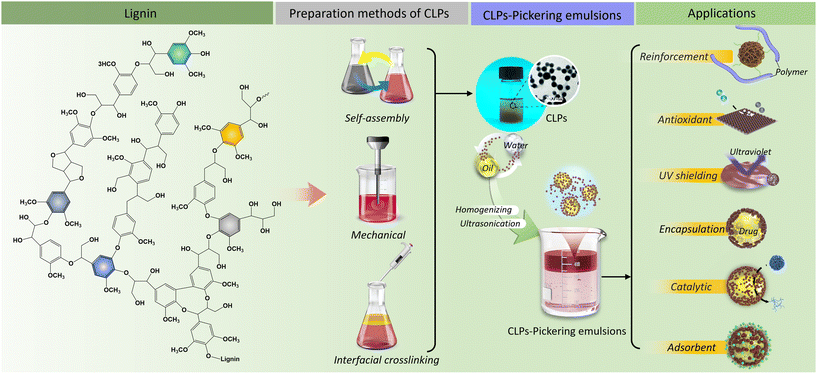
However, the potential of lignin-containing composites as high-value materials is often limited by their rigid structure and the presence of a heterogeneous, polydisperse, and irregular morphology. In recent years, modern nanotechnology has emerged as a novel approach for efficient utilization of lignin. Lignin can be processed into spherical colloidal lignin particles (CLPs) through strong π–π interactions and hydrogen bonding.28 Moreover, surface functionalization modifications can significantly expand its diverse range of applications.29,30 Unlike macromolecular lignin, CLPs possess controllable particle sizes, uniform structures, and excellent dispersion properties.31 These attributes have generated significant research interest in various fields such as catalysis, drug delivery, sterilization, hydrogel development etc. Particularly noteworthy are the nonpolar aromatic and polar hydroxy groups in lignin macromolecules that endow natural amphiphilicity to their particulate counterparts. Extensive investigations have been carried out regarding the functionality of CLPs as stabilizers within oil-in-water (O/W) Pickering emulsions. These emulsions exhibit remarkable dispersion stability, coupled with thermal resilience, salt resistance, and noteworthy pH-responsive characteristics. These attributes collectively present substantial promise for diverse applications, encompassing UV shielding, antioxidant formulations, and drug packaging among others.32,33
However, there is currently a lack of a systematic review on the research related to Pickering emulsions stabilized by CLPs, particularly in terms of the preparation process of CLPs, the distinct types and forms of lignin, and their impacts on the properties and applications of Pickering emulsions. It is crucial to gain a comprehensive understanding of the formation mechanism of CLPs based Pickering emulsions. This knowledge will enable researchers to optimize synthesis conditions and parameters for tailoring the properties of CLPs-Pickering emulsions. Such a concerted effort has the potential to enhance efficiency, cost-effectiveness, and accelerate the exploration of novel applications for CLPs-Pickering emulsions.
In this review, our focus is on the methods, formation mechanisms, properties, and applications of CLPs-Pickering emulsions. Firstly, we delve into the structure and properties of CLPs utilized in the preparation of Pickering emulsions, considering their preparation and modification processes. Subsequently, we provide a comprehensive overview of the characteristics, properties, and applications of various functional materials derived from CLPs-Pickering emulsions. Lastly, we present an outlook on the current challenges and opportunities in the field of CLPs-Pickering emulsions. The primary objective of this review is to consolidate and analyze research findings and advancements related to CLPs in Pickering emulsions. The main purpose of this review is to offer valuable insights for future innovative designs and applications involving CLPs-Pickering emulsions (Scheme 1).
2. Preparation process and formation mechanism of CLPs
Lignin exhibits an interconnected network structure composed of aromatic units, formed through the radical coupling of phenylpropanoid monomers—precursor hydroxy-cinnamic alcohols, specifically p-coumaryl, coniferyl, and synapyl.34 Typically binding to hemicelluloses, lignin envelops cellulose in the primary and secondary cell walls of plant cells, establishing covalent bonds with cellulose.35,36 This primary structure manifests as a highly branched and amorphous biomacromolecule with substantial molecular weight and polydispersity. The proportions of various lignin monomers and chemical bonds vary across sources, intensifying the challenge of precisely determining lignin's chemical structure.37 Currently, the precise molecular structure of natural lignin remains unknown, and the predominant form of lignin is a byproduct of the pulping process in the paper industry, primarily harnessed for its thermal energy generation through combustion processes.38,39Industrial lignin can be categorized into four types (Kraft, lignosulfonate, soda, and enzymatic hydrolysis lignin) based on various sulfur or sulfur-free processes in pulp manufacturing.34,40 These lignins retain numerous active chemical sites, ensuring reactivity in chemical reactions.41 However, the hydrophobic properties and complex physicochemical structure of lignin, including inter- and intra-molecular hydrogen bonding, impact its compatibility with host matrices. The functional properties of blends containing lignin depend significantly on the compatibility between lignin and the host matrices.42–45
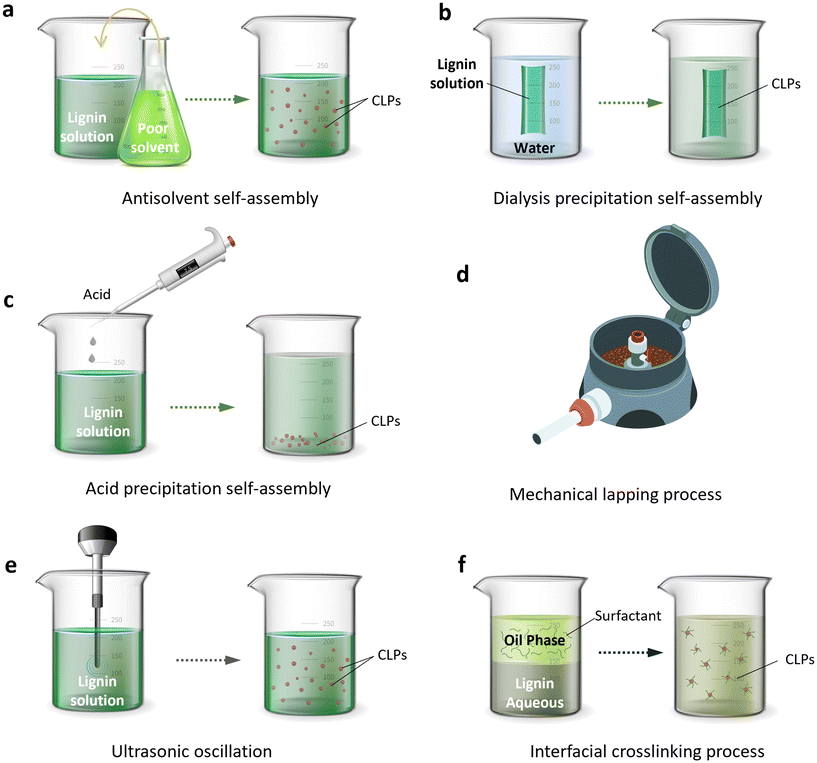
Lignin's amphiphilic characteristic enables the facile formation of nanoparticles, thereby enhancing material homogeneity and reducing the impact of its structural intricacies on material properties. Additionally, it imparts nano-specific attributes, such as a substantial specific surface area, which broadens the applicability of lignin nanoparticles.29,46 As shown in Fig. 3, several nano strategies have been developed for transforming lignin which include self-assembly, mechanical crushing, and interfacial crosslinking techniques.47,48 The resulting nano-sized lignin exhibits diverse forms such as nanospheres, nanotubes, nano micelles, and nano capsules due to different shaping processes employed.49–51 More detailed information regarding these methods for producing CLPs is provided below.
2.1. Self-assembly method
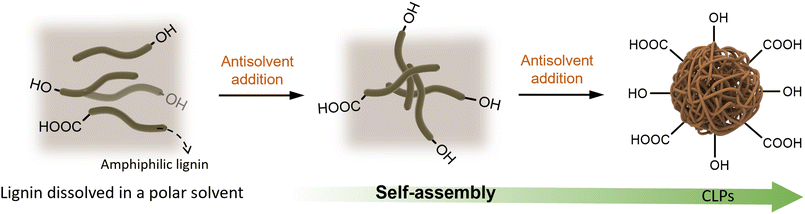
Self-assembly refers to the spontaneous formation of an ordered or organized structure in the absence of external influences, driven by specific non-covalent intermolecular interactions such as hydrophobic forces, electrostatic interactions, hydrogen bonding, and van der Waals forces.52 However, in the case of CLPs, the concept of “self-assembly” cannot be precisely defined as a standalone method since it is always accompanied by other indirect methods. In other words, various techniques can induce the self-assembly process of CLPs.In polar solvents, the hydrogen bond network of lignin, both inter-molecular and intra-molecular, undergoes disruption. This results in the self-assembly of hydrophobic regions of lignin into the core of CLPs through hydrophobic interactions, while exposing the hydrophilic regions on the surface. Upon introduction to water, non-covalent interactions such as hydrogen bonding between surface hydroxyl groups facilitate the formation of hydrophilic aggregates. Consequently, lignin tends to aggregate into spherical nanoparticles in order to minimize contact with the non-solvent phase and maximize its surface area.55
However, relying solely on hydrophobic interactions proves insufficient to overcome repulsive forces between charged lignin molecules. Hence, it has been proposed that non-bonding orbital interactions among aromatic components of lignin molecules drive their association.56 The strong π-stacking interaction leads to a reduction in energy levels of electrons in CLPs as evidenced by ultraviolet absorption spectra analysis.57,58 There is a shift in absorption peak from 280 nm (lignin nanospheres in THF) to 284 nm (lignin nanospheres in water).59 This shift is attributed to π–π transitions within guaiacyl groups present in CLPs. During the self-assembly process, the strong hydrophobic nature of the lignin phenylpropane structure prompts the movement of lignin molecules as water molecules are introduced. This movement results in the formation of a film at the interface between the organic phase and the water phase. As the proportion of water phase increases, the pressure gradient inside and outside the lignin molecule membrane intensifies, leading to the gradual penetration of water molecules into the membrane.53 Lignin molecules with pronounced hydrophilicity progressively accumulate on the inner surface of the membrane through π–π interactions.60 Simultaneously, the internal and external pressure gradient continues to grow until the organic phase is entirely removed, ultimately yielding stable lignin nanospheres.33,57 CLPs prepared using the reverse addition sequence typically exhibit larger particle sizes than those produced when the water phase is added to the organic phase. The size difference can be attributed to the increased precipitation rate resulting from the abrupt change in internal and external pressure during the addition of the organic phase to the water phase.61,62 Various solvent systems, such as tetrahydrofuran/water, ethanol/water, acetone/water,63,64 and dioxane/cyclohexane, have been extensively developed and applied in nano-lignin preparation. In these studies, the primary driving force behind CLPs self-assembly is attributed to π–π interaction.55
In addition, a more comprehensive understanding of the distinctions between different solvent systems is still necessary.61 In the study conducted by Chen et al.,65 an extensive investigation was carried out to elucidate the influence mechanism of solvent diversity on CLPs formation. Their findings suggest that the solvation of various organic solvents plays a crucial role in CLPs formation. This research focused on lignin model compounds and revealed three distinct configurations resulting from three interactions (π–π, CH–π, and no interaction) within the molecule: accumulation, lateral movement, and stretching behaviors. The study indicated that lignin molecules adopting a biased stack conformation in the solvent were more prone to self-assemble through intermolecular π–π interactions while being surrounded by solvent molecules forming stacked gum clusters. On the other hand, lignin molecules exhibiting lateral moving and stretching conformations may face difficulties in tightly clustering due to occupying molecular space already occupied by solvent molecules. Solvents such as DMF and DMSO disrupt lignin's intramolecular π–π packing behaviours, hindering the self-assembly process due to strong solvation effects. Additionally, methanol or acetone partially disrupts π–π accumulation leading to only a small number of nanoparticles being formed. In contrast, GVL and THF effectively maintain π–π packing within the molecule resulting in high-yield nanospheres.65
In summary, the reversed-solvent precipitation method emerges as the predominant technique for CLPs preparation, deeply rooted in the fundamental composition and properties of lignin. The hydrophobicity of lignin molecules is intricately associated with van der Waals forces and π–π interactions.56 The driving force behind the self-assembly process of lignin is believed to vary in different environments, with the potent π–π interaction recognized as the primary force shaping CLPs formation. The hollow structure of CLPs is attributed to the presence of phenol hydroxyl groups. A higher content of phenol hydroxyl groups strengthens intermolecular hydrogen bonding and electrostatic forces, thereby weakening the compact structure formed by π–π interaction of CLPs and ultimately leading to a hollow structure.55 Furthermore, CLPs prepared through traditional solvent exchange methods exhibit an initial negative charge of approximately 30 mV and remain stable in the short term. However, over time, ionic species present in media neutralize the surface charge of nanoparticles, resulting in CLPs aggregation or even precipitation. This poses a critical challenge that needs to be addressed for their sustained retention and widespread large-scale application.66
However, chemical modification of lignin is often necessary to enhance its solubility in solvents during the dialysis self-assembly process. In a study conducted by Miikka Lievonen et al.,68 it was observed that higher concentrations of lignin solution led to larger pressure differences between the inside and outside of the semi-permeable membrane, resulting in faster nucleation and growth rates during the self-assembly process. This increased availability of lignin within the system for nanoparticle growth made it more susceptible to variations in particle size during lignin particle formation. Therefore, strict control over production processes is often required when using dialysis precipitation methods to prepare lignin nanoparticle, ensuring nanoparticles with regular morphology and uniform size.
However, numerous studies have indicated that hydrogen bonding is not considered the primary driving force behind the association of lignin in high-concentration solutions. The morphology and size changes of nano lignin are directly influenced by pH value.73 Research demonstrates that as the pH value decreases, the π–π interaction and hydrophobicity of lignin molecules are enhanced, facilitating lignin accumulation. However, excessive acidity can reduce the surface negative charge of lignin particles, resulting in decreased stability. Ma et al. utilized the acid precipitation method to obtain CLPs with uniformity, excellent dispersion,66 controllable size, and long-term stability (no apparent size change or particle precipitation observed in aqueous solution for 90 days) directly from black liquor. The improved stability of CLPs is primarily attributed to their hydrophilicity and high content of negatively charged hemicellulose. The study also observed a gradual decrease in particle size and theta potential on the surface of CLPs with decreasing pH value.
2.2. Mechanical method
The preparation of CLPs through mechanical methods primarily involves the application of external forces, such as ultrasonic waves and mechanical grinding, resulting in their size reaching or approaching the nanometre scale. In comparison to the self-assembly method, the mechanical approach eliminates the need for organic solvents, thereby offering advantages in terms of cost-effectiveness and environmental friendliness.Leonidas Matsakas et al. utilized steam explosion fractionation with mixed organic solvents to separate birch flakes from lignin at varying ethanol contents.75 They employed homogeneous shear method in ethanol solutions with different volume fractions for preparing CLPs. Fourier infrared spectroscopy demonstrated that during the homogeneous shear process, there was no chemical modification or decomposition occurring in the composition of CLPs. The only influence observed was on their size and uniformity due to variations in ethanol content. In the study conducted by Yaqoob et al.,76 the homogenization method was employed to reduce lignin into nanoparticles, with four different shear speeds (i.e., 6400, 8400, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400, and 12
400, and 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 rpm) investigated for controlling the particle size of lignin. Notably, the highest speed of homogenizer yielded nanoparticles with superior size distribution and favourable zeta potential value.
400 rpm) investigated for controlling the particle size of lignin. Notably, the highest speed of homogenizer yielded nanoparticles with superior size distribution and favourable zeta potential value.
In summary, this environmentally friendly mechanical method eliminates the need for organic solvents making it suitable for large-scale applications. This promotes research on applying lignin nanoparticle in various fields such as food industry, medicine, and healthcare sectors. However, it should be noted that CLPs prepared using this method currently exhibit poor structural and dimensional stability which limits their mature application in industrial production.
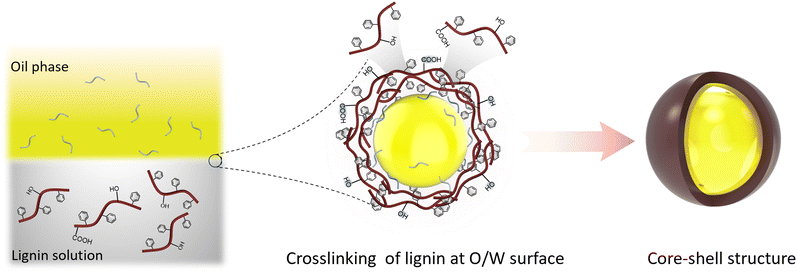
2.3. Interfacial crosslinking process
The interfacial crosslinking process involves dissolving lignin and other monomers or polymers with diverse functional groups in two immiscible solvents. As shown in Fig. 3f and 5, this process typically entails the emulsion inversion reaction at the interface of the two solutions when one solution is dispersed into the other. The method serves to stabilize, generate particles, and form capsules.80In this process, the lignin chain must undergo crosslinking induction to render it amphiphilic, enabling its dissolution in an alkaline solution to form a dispersed phase and in a solvent with surfactants. In the second step, a water–oil microemulsion is prepared, and the dissolved lignin is emulsified to facilitate lignin crosslinking at the oil–water interface. The addition of crosslinking agents during the emulsification process, often mediated by ultrasound, can enhance the cross-linking reaction of lignin.80 The interfacial crosslinking method allows for the production of spherical micro and nano lignin particles as well as spherical micro/nano lignin capsules, modified lignin particles with high porosity, and composites with CLPs.
Wang et al. conducted a study where they synthesized carboxymethylated lignin in aqueous solution using nucleophilic substitution methods. They combined this with a small amount of alkyl polyglycoside as a macromolecular surfactant to stabilize a high internal phase emulsion loaded with 87% curcumin under neutral conditions. The capsule shell formed by carboxymethyl enzymatic hydrolysis lignin exhibited excellent anti-ultraviolet properties in their study. In another study by Wang et al., sodium lignosulfonate rich in sulfonic and carboxyl groups was dissolved in water. Mixed surfactants with opposite charges were introduced into the system while adjusting the action of lignin and mixed surfactants through non-ionic surfactants tritonX-100 and NaCl. This resulted in successful construction of CLPs consisting of rigid solid microspheres or hollow packages using interfacial crosslinking method. The interfacial crosslinking method demonstrated its applicability for rapidly preparing lignin spherical micro/nano capsules which showcased potential applications in biomedicine for encapsulating hydrophobic compounds and drug protection.
2.4. Other methods
As research progresses in the field of lignin nanotechnology, various methods have been explored for the preparation of CLPs, including the aerosol method. The aerosol method is a high-flux technique for preparing nano-sized lignin particles, which involves circulating a gas carrier containing a solution of lignin through a high-temperature heat pipe. This facilitates rapid drying and aggregation of lignin, followed by downstream collection of CLPs. By adjusting parameters such as the source of lignin, precursor concentration, atomization, and drying, the aerosol flow reactor method allows for one-step production of CLPs with uniform size and controllable properties. Ago et al. have successfully utilized aerosol spray technology to synthesize lignin particles with adjustable sizes and hydrophilicity, thereby demonstrating their ability to create stable Pickering emulsions.84The advantages offered by aerosol technology include absence of liquid by-products, simple particle collection process, low-cost implementation, reduced number of processing steps required, continuous operation capability, and high product yield potential. Other synthesis methods that have been explored include controlled evaporation of atomized droplets containing lignin solution-circulating flash precipitation,92 microbial degradation,93 enzyme decomposition,94 ice separation,95 and CO2 anti-solvent techniques.96 However, these alternative approaches are yet to overcome challenges related to cost, stability and large-scale production.
Table 1 summarizes the particle sizes and morphologies of CLPs prepared from different lignin precursors under different processes. While the antisolvent method remains the primary process for preparing lignin nanoparticle, it faces challenges concerning solvent consumption and recovery, environmental safety, nanoparticle stability, and yield.50 The selection of a suitable solvent is crucial in determining the production cost of cellulose CLPs. Green and simplified processes are increasingly advocated, with dialysis precipitation emerging as a notable method for producing CLPs with adjustable sizes; however, its scalability is limited due to expensive dialysis equipment. Mechanical methods such as ultrasonic crushing or mechanical grinding offer high efficiency, yield, and cost-effectiveness but present difficulties in controlling the quality of CLPs.77 Biological methods encounter issues related to CLPs yield, enzyme production costs, and solvent usage. Consequently, further research is needed to promote and develop environmentally friendly large-scale processes for CLPs preparation.
| Lignin species | Preparation methodology | Particle dimension/nm | Morphology | Ref. |
|---|---|---|---|---|
| Enzymatic hydrolysis lignin | Acid precipitation self-assembly | 48.9 ± 16.4 | Spherical | 81 |
| Lignosulfonate | Antisolvent self-assembly | 55 ± 26 and 86 ± 29 | Spherical and hollow nanospheres | 82 |
| Alkali lignin | Acid precipitation self-assembly | 20–100 | Surface collapse | 83 |
| Kraft lignin and alkali lignin | Aerosol spray technology | >30 | Spherical | 84 |
| Sodium lignosulfonate | Interfacial crosslinking process | 100–400 | Nanocapsules | 85 |
| Acid precipitation self-assembly | Antisolvent self-assembly | 200 | Spherical | 54 |
| Kraft lignin | Dialysis precipitation self-assembly | >300 | Spherical | 68 |
| Kraft lignin | Antisolvent self-assembly | 97 | Spherical | 86 |
| Alkaline lignin | Acid precipitation self-assembly | 182 | Spherical | 87 |
| Kraft lignin | Antisolvent self-assembly | 100 | Spherical | 88 |
| Alkaline lignin | Antisolvent self-assembly | 120–150 | Spherical | 65 |
| Kraft lignin | Ultrasonic oscillation | 39–185 | Hollow and solid particle | 55 |
| Depolymerized lignin | Ultrasonic oscillation | 237–539 | Spherical | 89 |
| Kraft lignin | Ultrasonic oscillation | 10–50 | Sheet structure | 78 |
| Lignin | Acid precipitation self-assembly | >500 | Irregular shape | 71 |
| Kraft lignin | Dialysis precipitation self-assembly | 259 ± 4–274 ± 5.3 | Spherical | 90 |
| Kraft lignin | Interfacial crosslinking process | 27.4 ± 12.8–127.8 ± 43.6 μm | Spherical | 91 |
| Kraft lignin | Homogenization processes | 250–1000 | Spherical | 75 |
| Softwood kraft lignin | Antisolvent self-assembly, acid precipitation self-assembly with ultrasonic oscillation | 87–125 | Sheet structure | 61 |
| Hardwood birch lignin | 112.5–187 | Irregular spherical | ||
| Soda lignin | 90–150 | Spherical |
3. Effect of CLPs properties on Pickering emulsions
At the two-phase interface, oil and water are typically immiscible due to the presence of strong interfacial tension. In Pickering emulsions, CLPs function as emulsifiers, reducing the tension between the two interfaces and promoting homogeneous mixing of the two phases. In emulsion technology, the stability of an emulsion is a critical factor for any application, which can be determined by observing a longer time required for phase separation (Scheme 2).97 The study of Pickering emulsion stability encompasses essential physical properties such as nanoparticle type, droplet size, particle size distribution, and level of emulsion formation.73 Among these factors, CLPs play a pivotal role in directly influencing the quality of the emulsion and its other physical properties including particle size and distribution. The impact of CLPs on the emulsion can be summarized by examining particle wettability, morphology, surface chemical properties, and concentration.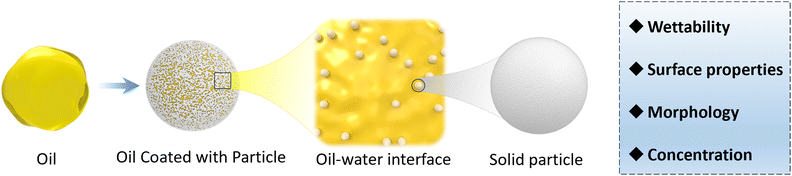 | ||
| Scheme 2 The existence state and properties of nanoparticles at the interface of Pickering emulsion. | ||
3.1. Wettability of CLPs
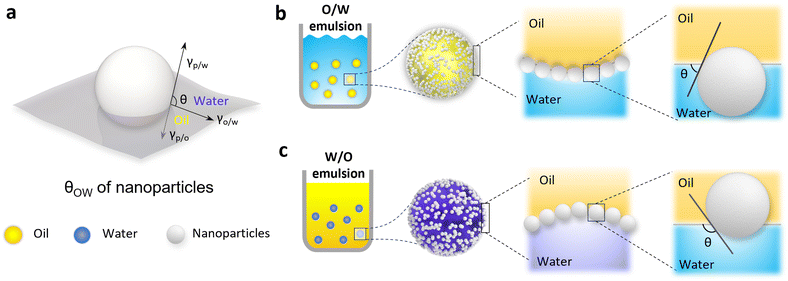
The assessment of CLPs’ surface wettability is a critical factor in evaluating the stability of lignin particles at the oil–water interface. Within the nature lignin structure, a stable and intricate three-dimensional network carbon skeleton is formed by C6 and C3, establishing a hydrophobic nature. Concurrently, hydrophilic groups such as hydroxyl and carboxyl are distributed within the interior and at the ends of the skeleton. This dual distribution imparts a two-phase affinity to natural lignin, with a stronger hydrophobicity compared to hydrophilicity.The surface wettability of CLPs can be characterized by the three-phase contact angle (θOW) at the oil–water interface, as illustrated in Fig. 6a. When θOW < 90°, it indicates a stronger hydrophilicity, facilitating the formation of an “oil-in-water” (O/W) emulsion. Conversely, if θOW > 90°, it signifies stronger lipophilicity, promoting the formation of a water-in-oil (W/O) emulsion (Fig. 6b and c). When θOW approaches 90°, it suggests a balanced hydrophilic–lipophilic character, contributing to superior interfacial stability and fostering the creation of a highly stable Pickering emulsion. To achieve a balanced two-phase affinity of lignin, it is essential to decompose the carbon skeleton, thereby releasing its polar groups. This process plays a crucial role in harmonizing the interaction between lignin particles and the interface, ensuring the stability of lignin particles in diverse conditions.
Lignin is known to be partially soluble in good solvents with high hydrogen-bonding capacity, such as ethanol, or moderately high polarity, such as acetone, THF and DMF.98 The deconstruction and recombination of lignin can be effectively achieved through the selective dissolution of lignin in different solvents, leading to a reduction in the surface heterogeneity of macromolecular lignin.99 Tian et al. utilized a binary mixed solvent composed of water and THF to fractionate CLPs with diverse surface wettability. The differentiation in surface properties was determined by considering the interaction of molecular weight, particle size, and hydrophilic groups.55 As a result, the θOW of these CLPs exhibited distinct hydrophilic characteristics at 13°, 136°, and 109°, respectively.55 In Gao et al.'s study,63 CLPs were synthesized from enzymatically hydrolyzed lignin powder using the acetone and water co-solvent method. In comparison with the original lignin, the θOW of CLPs at the oil–water interface decreased from 141° to 89°. The change in wettability primarily was attributed to the roughness variation, which is also related to the variation of the sizes and size distributions of the constituting particles (Fig. 7).
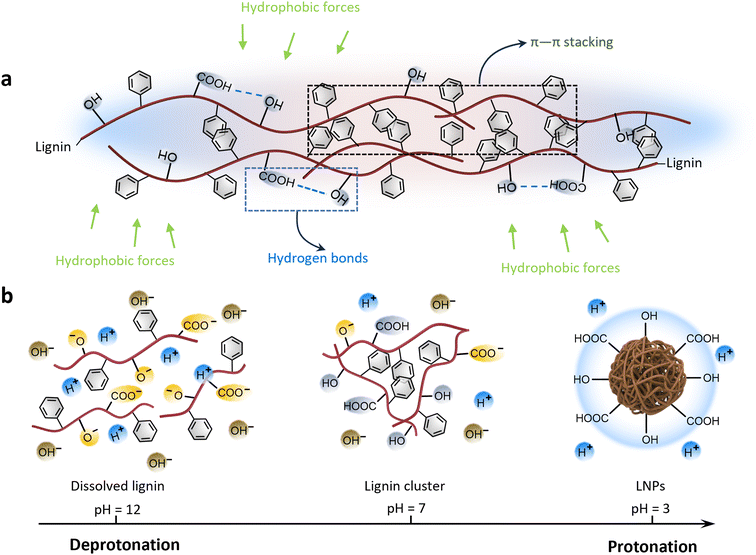 | ||
| Fig. 7 The driving force of lignin in the self-assembly process (a); protonation of lignin as pH decreases during acid precipitation self-assembly (b). | ||
During the self-assembly process of lignin to form nanoparticles, alterations in solution polarity serves as a trigger for the self-assembly process of lignin. In response to strong polar bonding, the hydrophilic groups of lignin tend to preferentially orient themselves towards the polar environment, while the hydrophobic carbon skeleton congregates in the interior of the formed CLPs. As illustrated in Fig. 8a, this selective arrangement of lignin components within the nanoparticles is driven by the interplay of non-covalent bonds among lignin molecules, including hydrogen bonds, hydrophobic forces, and π–π stacking, leading to a distinct organization of hydrophilic and hydrophobic elements during the self-assembly process. Additionally, lignin molecules with higher hydrophilicity have a propensity to aggregate, leading to the formation of smaller-sized nanoparticles under the influence of hydrophobic forces. As CLPs undergo assembly and growth, the particle size gradually increases, accompanied by a reduction in the uniform distribution of functional groups on the particle surface. This phenomenon influences the surface wettability of CLPs, as the evolving structure and size of the particles modify the interaction between the surface and the surrounding environment.
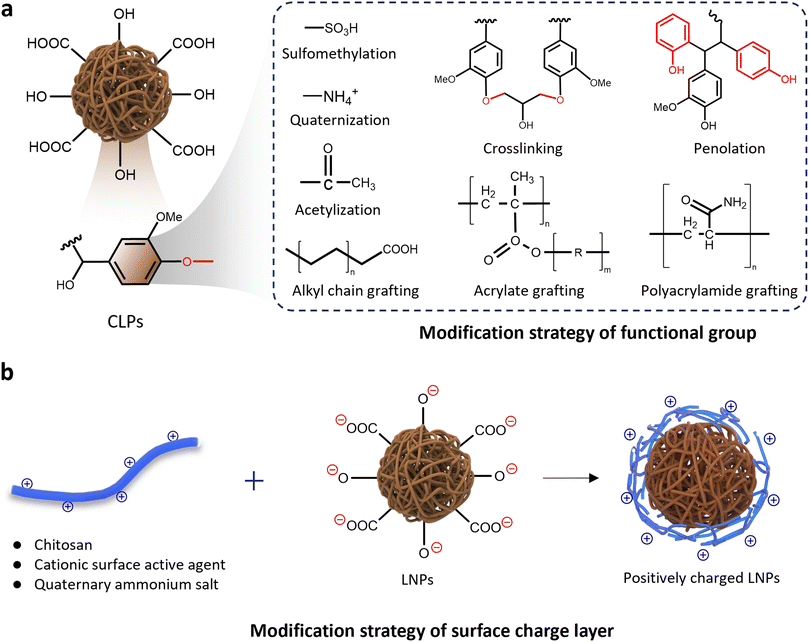 | ||
| Fig. 8 Surface chemical modification strategy (a) and surface charge modification strategy (b) of lignin. | ||
Moreover, the protonation and deprotonation of lignin represents a classical method for regulating the surface wettability of CLPs through surface charge (Fig. 8b). Nasim Ghavidel observed that at pH 11, the functional groups on the surface of CLPs undergo deprotonation, resulting in a θOW at the oil–water interface of less than 20°, indicating superhydrophilicity.100 Conversely, at pH 3, the θOW increases to 50°. Comparable results were obtained in the study of Tian et al., who investigated the surface wettability of CLPs derived from dissolving Kraft lignin under varying pH conditions. The three-phase contact angles were measured at 36°, 75°, and 118° for pH values of 11, 7, and 2, respectively. These results suggest that Kraft CLPs (KLP) could serve as an effective emulsifier for Pickering emulsions. The change in pH value leads to the protonation and deprotonation of functional groups of the CLPs. Studies indicate that the pKa of the carboxylic and phenolic groups is about 4.7 and 9–10, respectively; these anionic groups are mostly deprotonated in alkaline media. At a pH > 11, lignin molecules are present mainly as hydrocolloids. As the pH decreases, the electrostatic repulsion between lignin molecules diminishes, leading to lignin molecules being predominantly present as both particles and hydrocolloids. When the environment is highly acidic (pH < 3), CLPs are formed due to the groups are dominantly protonated and the strong association strength of the lignin macromolecules. The absence of electrostatic repulsion between CLPs usually results in a compact and dense structure.
Caroline Hadjiefstathiou et al. investigated the interfacial tension generated by lignin micelles and colloidal particles at varying pH levels.101 As the pH decreases, the quantity of soluble lignin micelles in the solution diminishes, resulting in a gradual increase in interfacial tension. In the established emulsion system, the competitive adsorption of different forms of lignin in the continuous aqueous phase can alter the emulsion's stability, fostering diverse rates and degrees of aggregation. This process gives rise to two states: Pickering stabilization and non-Pickering stabilization in the emulsion. The synergistic mechanism, characterized by a balanced presence of lignin particles and lignin micelles, was considered to contributes to the heightened stability of O/W emulsions.
Based on above, the self-polymerization of traditional lignin molecules into CLPs is triggered by the solvent environment, with the surface functional groups playing a pivotal role in influencing the surface wettability of these particles. Despite these studies, inconsistent or insufficient properties of the structurally heterogeneous lignin's compared to synthetic surfactants have obstructed commercialization. Consequently, aggregation of lignin in composites and phase separation in blends are common hurdles, especially with non-polar systems. To overcome this limitation, chemical functionalization of lignin by grafting polymer chains has classically been conducted. Consequently, the ongoing exploration of surface modification techniques for lignin, with a specific focus on adjusting the hydrophilicity and hydrophobicity of functional groups, has garnered significant attention. Furthermore, as the self-assembly progresses, the gradual homogenization of surface wettability in lignin particles becomes a key factor influencing the changing driving forces for self-assembly. This dynamic process directly governs the final state of CLPs, a critical aspect closely tied to their stability at the oil–water interface. This stability, in turn, is intricately linked to the particle size and morphology, forming a complex interplay in the overall behavior and applicability of CLPs in various fields.
3.2. Surface properties of CLPs
Chemical modification of lignin is sometimes necessary to facilitate the formation of nanoparticles due to its ability to enhance the hydrophobicity of the initial lignin material, thereby resulting in a more homogeneous particle size distribution. Surface engineering plays a crucial role in modifying CLPs by recognizing the pivotal influence of the self-assembly process on their growth and distribution. The inherent stochasticity in this process presents challenges for controlling the surface wettability of CLPs, thereby necessitating the implementation of surface engineering strategies. Chemical modifications, such as amination,102 carboxymethylation,103 grafting,85,104,105 or condensation polymerization of lignin surface functional groups,88,106 provide precise approaches to reprocess and regulate surface wettability.107,108 As demonstrated in Fig. 8, these strategies are designed to strike a balance in the number and distribution of amphiphilic functional groups on nanoparticle surfaces or adjust the surface charge of lignin particles,90,109 effectively regulating their hydrophilicity. Through strategic chemical modifications, the surface characteristics of lignin can be tailored with precision, presenting a versatile approach to influencing how lignin interacts with its surroundings.105The implementation of surface engineering strategies significantly enhances the independent controllability of CLPs surface properties. Pang et al.'s work demonstrates the versatility of this approach, obtaining six distinct types of CLPs by modifying the surface of alkali lignin with varying hydrophilic and hydrophobic properties.110 The study reveals that alkyl-bridged lignin particles, exhibiting stronger hydrophobicity, display improved emulsification effects on less polar cyclohexane, while carboxymethylated lignin particles, with enhanced hydrophilicity, exhibit superior emulsification effects on more polar n-heptanol. Within general strategies, additive modifications such as alkylation, esterification, and chain grafting prove effective in enhancing the hydrophobic properties of CLPs.105,111 Conversely, methods like sulfonation, phenolation, and amination are tailored to augment the hydrophilicity of CLPs,83 offering a comprehensive toolkit for precise surface property adjustments.
Liu et al. applied the photoinitiated reversible addition–fragmentation chain transfer (RAFT) strategy to graft various monomers onto the lignin surface, resulting in the creation of amphiphilic CLPs with diverse structures.89 The choice of the grafted monomer exerted a substantial influence on both the particle size and surface wettability of the CLPs. The study reveals a dynamic interplay between monomer chain length and chargeability. Shorter, electronegative monomers heighten the negative charge on the particle surface, while larger-volume monomers facilitate extensive aggregates and elongate hydrophilic chains on the particle surface. The proposed fundamental relationship between charge amount and chain length serves as a guiding principle with broad applicability across a spectrum of graft modification strategies.
Tang et al. demonstrated the general modification principle based on charge changes by modifying the surface of CLPs with chitosan (Fig. 8b). This resulted in the formation of lignin/chitosan nanoparticles (Lig/Chi NPs) with heightened hydrophobicity compared to CLPs alone, evidenced by an increase in the water contact angle from 41.2° to 89.7°. During Lig/Chi NP formation, the positively charged groups of chitosan interacted with the negatively charged groups of lignin. Consequently, chitosan coated the hydrophilic groups of lignin, distributing itself in the outermost layer of Lig/Chi NPs. The addition of cationic chitosan led to CLPs and Lig/Chi NPs with a soft chitosan shell structure, exhibiting opposite surface charges. Notably, the dispersion of Lig/Chi NPs was observed to be more stable due to their enhanced surface charges, highlighting a fundamental modification principle with broad applicability.86,112
During the construction of a new surface charge layer, the condensation of charged molecules has the potential to generate novel surface structures. Wang et al., leveraging the properties of polyelectrolytes in aqueous environments, condensed single chain/geminin cationic surfactants onto the negatively charged surface of sodium lignosulfonate to construct CLPs with a cationic gel layer.109 Driven by the hydrophobicity and π–π interactions between the aromatic groups of lignin, the weakly charged cationic active agent associated with the mixed aggregates of lignin anions, forming a coacervate with a nanoscale network microstructure. This interaction resulted in changes to the surface properties of the CLPs. Mika Henrikki Sipponen et al. proposed the adsorption of cationic lignin onto the CLPs surface to form dense CLPs with a positive surface charge. Studies have proved that cationic CLPs with increased cationic net charge exhibit superior performance in stabilizing Pickering emulsions in acidic environments compared to irregular solid lignin particles or unmodified CLPs. This modification underscores the potential to fine-tune the surface properties of CLPs by introducing heterogeneous charges, thereby influencing their hydrophobicity and stability at the oil–water interface.
In summary, the modification of CLPs through surface engineering involves refining the self-assembly process, specifically targeting lignin surface functional groups. This can be achieved through various chemical modifications to enhance control over surface wettability, supplying a more precise and effective approach to nanoparticle engineering. The impact of these surface property changes extends beyond individual modifications, influencing dynamic changes in the driving forces during assembly and growth.112 These alterations, particularly in surface properties, play a key role in deciding the particle size range of CLPs. Furthermore, the self-reactivity of lignin derived from various sources exhibits significant variability, which in turn influences the efficiency of surface modification processes. Consequently, during practical operations, it is advisable to select lignin with a high density of active sites—such as alkali lignin—or to pre-treat the precursor to enhance the exposure of additional active sites. In the upcoming discussion, the intricate relationship between particle size, distribution, and the behavior of CLPs at the oil–water interface will be explored in detail, shedding light on the multifaceted impact of surface engineering.
3.3. Morphology of CLPs
The geometry and morphology of the CLPs are to be considered as additional, critical parameter that affects their interaction at oil–water interface. It mainly includes the balance of the interaction energy of CLPs with different particle size and oil–water interface and the relative magnitude of interfacial area covered by the CLPs relative to their size.64The particle size of CLPs can be adjusted using the anti-solvent precipitation property of lignin. Earlier research suggests that CLPs formation involves two concurrent processes: the surface orientation of hydrophilic segments and the adsorption of small polar lignin fragments. Controlling the nucleation size and growth rate in the self-assembly process is achievable by changing the initial concentration of lignin,64 the rate of anti-solvent addition, the proportion of anti-solvent, and the system's pH value. Additionally, although CLPs exhibit similar particle size behavior in various anti-solvent systems, the nucleation mechanism was different. Chen et al. fabricated CLPs using the solvent exchange method.65 During the experimental procedure, they investigated different solvents with mild solvating ability, such as GVL or THF. It was seen that lignin molecules maintained intramolecular stacking, where the intramolecular aromatic interaction of lignin fragment closely approximated (−5.59 kJ mol−1) that observed in water, resulting in the formation of a spherical seed during the heterogeneous nucleation process (Fig. 9b).65 When a highly concentrated solution of lignin was introduced into water, these nuclei form clusters. Subsequently, these clusters undergo further aggregation to give rise to CLPs by diffusion-limited cluster clustering, which aligned with classical nucleation theory.113 As the solvation capacity increases, solvent molecules such as DMSO exert their influence by displacing the aromatic ring of lignin molecules from intramolecular accumulation. Consequently, this impedes the formation of spherical nuclei and subsequent aggregation into CLPs by lignin molecules.65 Therefore, to achieve consistent and stable CLPs with a high yield using the solvent exchange method, it is crucial to employ a solvent capable of dissolving lignin while preserving its spherical core. Moreover, as shown in Fig. 9a, a solvent possessing weak solvation capacities would be more advantageous in facilitating the formation of CLPs characterized by smaller particle sizes.
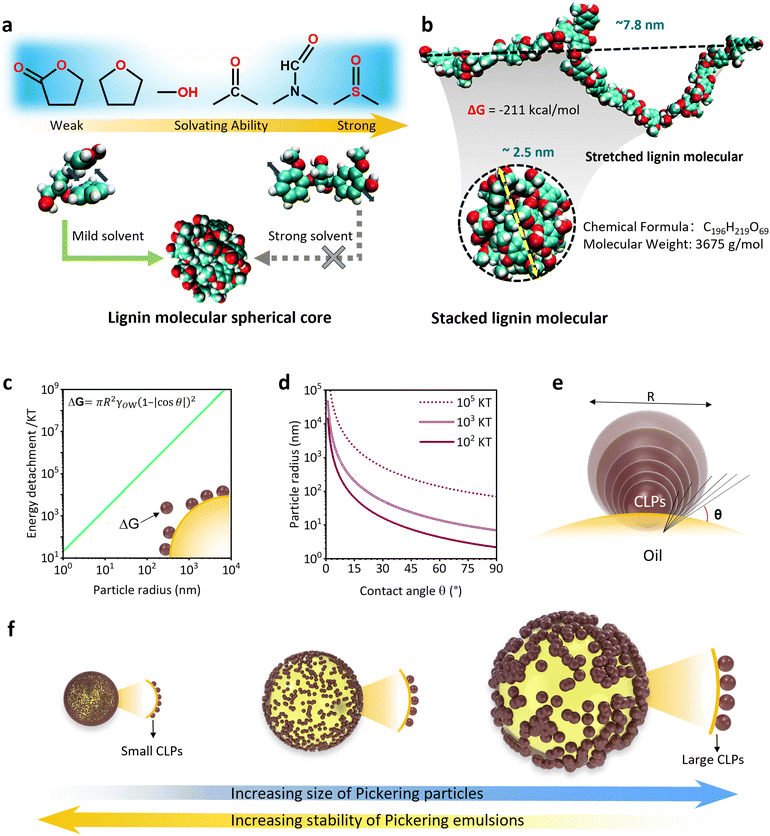 | ||
| Fig. 9 (a) Formation mechanism of LNPs from different solvents.65 Reproduced from ref. 65 with permission from Royal Society of Chemistry copyright 2022. (b) Stacked and stretched configurations of a large softwood lignin molecule.65 Reproduced from ref. 65 with permission from Royal Society of Chemistry copyright 2022. (c) Plot illustrating the increase in energy of detachment of a single spherical particle as a function of its size (contact angle 90°, oil–water interfacial tension 27 m N m−1).114 Reproduced from ref. 114 with permission from Elsevier copyright 2014. (d) Contour plot of equal detachment energy (ΔG/kT) for various particle radii and contact angles combinations.114 Reproduced from ref. 114 with permission from Elsevier copyright 2014. (e) The contact angle of nanoparticles with different sizes at the droplet interface. (f) Different sizes of Pickering droplets stabilized by LNPs of various sizes. | ||
The remarkable stability of Pickering emulsions primarily stems from the substantial interfacial energy of solid particles, which undergo nearly irreversible adsorption onto the oil–water interface. This adsorption process ensures the enduring maintenance of a stable state where oil and water are effectively mixed. The particle's adsorption capacity at the oil–water interface can be quantified by the desorption energy, denoted as ΔG (J):
ΔG = πR2γOW(|1 ± cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ|)2 θ|)2 |
According to the calculation formula of emulsion droplet size:
| D = 4CρpdpVd/mp |
In the formula: C is the particle interface coverage; ρp is the concentration of particles in the emulsion (kg m−3); dp is the diameter of the particles (m); Vd is the volume of the dispersed phase (m3); mp is the mass of the particles (kg). In emulsion systems, the size of the droplets is directly proportional to the particle size. A large number of studies have shown that the smaller the particle size of the emulsion droplets in the emulsion system, the higher its stability.76
Yu et al. introduced a nanomanufacturing technique involving electrospray to generate a range of CLPs characterized by distinct particle sizes and regular shapes.116 Their investigation revealed that Olive O/W Pickering emulsions stabilized by various CLPs exhibited conventional system behavior: as the particle size increased, the size of the stable droplets also increased, while the stability of these droplets increased with smaller particle sizes. Similar observations were made in the research conducted by Ago et al.84 Their findings strongly support the conclusion that when there is a higher concentration of larger particles and smaller particle sizes, the resulting droplets tend to be smaller and exhibit improved stability.
These research findings can be summarized as follows: the size of solid CLPs has a significant impact on their flow efficiency within the continuous phase of the emulsion system and the curvature they create when adsorbed at the oil–water interface. The particle size of the emulsion droplets increases as the particle size of CLPs grows larger (Fig. 9f). This is attributed to the fact that larger particles cannot firmly adhere to the surface of smaller droplets, which have greater curvature (see Fig. 9e). This phenomenon can be elucidated through the bending elasticity of solid particles at the oil–water interface, as described by the following formula:
In this equation, Ks represents the surface bending elasticity of the milk droplet, σOW is the interfacial tension between oil and water, α denotes the particle radius, and λ (λ = π/2√3) is a constant. Additionally, θ signifies the particle interface contact angle. It's evident that the bending resistance of tightly packed monolayer particles at the interface is directly proportional to the square of their radius. Therefore, as the particle size of CLPs increases, the bending resistance that occurs when CLPs attach to the droplet's surface increases as well. This ultimately results in a reduction in curvature and an increase in the size of the droplet (Fig. 10a).
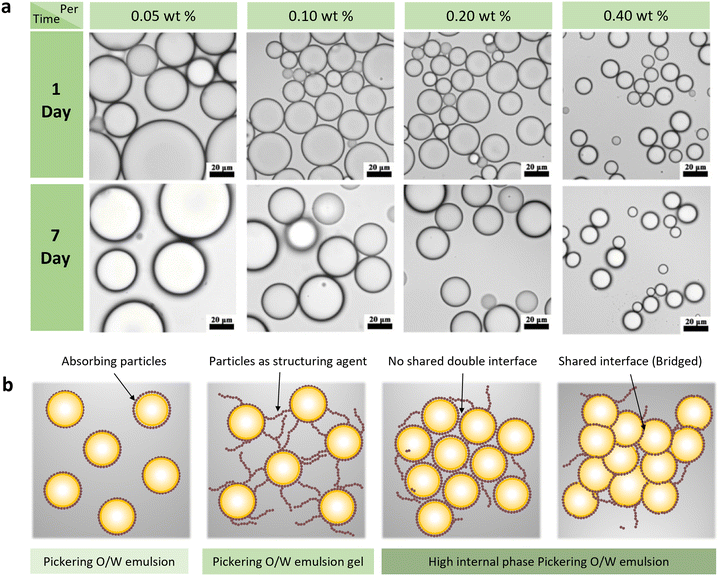 | ||
Fig. 10 (a) O.M. images of mean diameter of a lignin-stabilized Pickering emulsion using 40.0 g L−1-lignin (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 v/v) with various lignin concentrations for 7 day of storage.64 Reproduced from ref. 64 with permission from American Chemical Society copyright 2021. (b) Schematic representations of Pickering emulsions and emulsion gels incorporating particles as structuring agents in the continuous phase, along with high internal phase Pickering emulsions (HIPPEs) featuring either a single shared interface between neighboring droplets or a nonshared double interface.127 Reproduced from ref. 127 with permission from Elsevier copyright 2020. 7 v/v) with various lignin concentrations for 7 day of storage.64 Reproduced from ref. 64 with permission from American Chemical Society copyright 2021. (b) Schematic representations of Pickering emulsions and emulsion gels incorporating particles as structuring agents in the continuous phase, along with high internal phase Pickering emulsions (HIPPEs) featuring either a single shared interface between neighboring droplets or a nonshared double interface.127 Reproduced from ref. 127 with permission from Elsevier copyright 2020. | ||
Moreover, the relationship between the stability characteristics of Pickering emulsions and the colloidal particle size can be understood through the Brownian motion formula:
| D = kT/6πμα |
In this equation, D stands for the diffusion coefficient, k is the Boltzmann constant, T stands for the absolute temperature, μ is the viscosity of the liquid, and α is the radius of the particle. In comparison to larger particles, nano-sized CLPs exhibit a larger diffusion coefficient, allowing them can rapidly adsorb to the oil–water interface, facilitating the creation of smaller emulsion droplets. Subsequently, they can establish a dense physical barrier of CLPs particles on the surface of these droplets, preventing contact between droplets. However, it is not advisable to blindly optimize for small particle sizes or contact angles approaching 90° in many cases. Candidate CLPs offer more flexibility for design purposes. In Fig. 9d, contour plots depicting equal detachment energies are calculated within a specific range of particle sizes and contact angles. The enhanced stability of the Pickering emulsion can be attributed to the separation energy exhibited by particles at the oil–water interface, there exist various combinations of contact angles and particle sizes that fulfill this criterion.114
While solid particles do adsorb at the oil–water interface, they do not spread or arrange themselves in the same way surfactants do.117 Their ability to reduce interfacial tension depends on factors like size, surface charge, wettability, and concentration, and their influence on interfacial tension is much less direct than that of surfactants.118,119 Solid particles are much larger than surfactant molecules and cannot form a continuous monolayer at the interface. Instead of fluidly spreading across the surface to reduce tension, they lock into place, creating a mechanical barrier.118 This makes the interfacial tension reduction a secondary or minimal effect compared to the primary stabilization mechanism (physical adsorption).120
3.4. Concentration of CLPs
The concentration of CLPs holds a significant sway over both particle coverage at the oil–water interface and the rheological properties of the emulsion system.121 When the particle concentration is low in an emulsion, it fails to adequately cover the dispersed phase, resulting in flocculation and coalescence during migration, ultimately leading to the separation of the oil and water phases.To shed light on the nuanced effects of CLPs concentration, Gordobil et al.122 conducted a pivotal study systematically examining the impact of lignin concentration, oil type, and oil–water ratio on Pickering emulsion properties. Across numerous studies, a consistent conclusion emerges higher concentrations of CLPs tend to produce emulsion droplets of uniform size and stable dispersion. This conclusion gains additional support from Li et al.,64 who used CLPs of varying particle sizes to stabilize Pickering emulsions of thyme oil in water. Notably, despite different CLPs sizes, all three emulsions exhibited a similar average droplet size. Significantly, the study revealed a negative correlation between the amount of lignin loaded on the oil–water interface and the particle size of the emulsion droplets-higher particle count resulted in smaller droplet sizes and improved storage stability (Fig. 10a).
Elevated lignin concentration not only influences particle interactions but also plays a crucial role in promoting increased interactions at the droplet interface. This phenomenon is driven by the presence of aromatic hydrophobic and hydrophilic fragments, inducing self-aggregation and self-organization of lignin at the interface layers. Consequently, a robust solid-like film forms around the oil droplets, effectively inhibiting flocculation, and coalescence of the dispersed phase, thereby enhancing emulsion stability.123 As particle concentration increases, there is a corresponding rise in coverage at the oil–water interface, leading to the formation of an interface film with a specific thickness. This film plays a crucial role in inhibiting the flocculation and coalescence of the dispersed phase, ultimately enhancing the overall stability of the emulsion.73 With continued concentration increases, particles accumulate in the continuous phase, resulting in the development of a dense bridging particle layer and a three-dimensional mesh particle layer.
These structural formations typically involve some degree of particle aggregation or droplet flocculation. Such as, in a single dense bridged particle layer, each particle, though still in the water continuous phase, becomes partially wetted by two dispersed phases and binds with adjacent particles due to attractive forces. This process leads to an elevated viscosity of the emulsion system, obstructing the migration of the dispersed phase, and consequently, reinforcing the stability of the emulsion. In high internal phase emulsion systems (volume fraction >0.74),124 a minimal quantity of particle emulsifier is adequate to stabilize elevated concentrations of oil droplets, owing to the combined effect of adsorbed particles forming a protective layer and an aggregation network between particle-coated droplets in small gaps.125,126 Fig. 10b illustrates two stable models for particle-stabilized high internal phase emulsions: one with continuous Pickering-stable monolayers formed by particles around each dispersed droplet, and another where multiple adsorbed particles are shared among adjacent droplets in a single-particle bridging structure. Notably, these two idealized structural arrangements often coexist in real HIPPEs systems.
This intricate interplay between particle concentration, interface film formation, and structural developments underscores the multifaceted mechanisms influencing the stability of emulsions.128 These combined factors further reinforce the stability of the emulsion, creating a comprehensive understanding of how CLPs concentration intricately shapes the properties of Pickering emulsions.
In addition to the decisive influence brought by colloidal particles, the stability of Pickering emulsions is also affected by several factors. Factors such as the size of colloidal particles, interaction between colloidal particles, interface structure, interfacial rheology of Pickering emulsion, oil/water phase volume ratio, energy input, and emulsification process play crucial roles. These factors are interconnected and complementary, for instance, changing the pH value of the system may alter the salt ion strength, and the strong mutual attraction between colloidal particles can lead to flocculation, impacting particle size and emulsion stability. From a fundamental research perspective, plant-based materials still present various challenges in terms of optimization of functionality through the manipulation of particle size, wettability, shape anisotropy, aggregation and surface chemistry. Understanding at a greater depth how to control these factors will surely keep experimental and theoretical colloid scientists occupied over the coming years.
4. Functional applications of CLPs-Pickering materials
The global green movement has promoted widespread research and application of bio-based emulsions. The chemical composition of the lignin, as well as its size, shape, surface charge, and hydrophobicity, determine its potential application in Pickering emulsions. Many properties of lignin, such as resistance to decay and biological attack, ultraviolet absorption, high hardness,129,130 and the ability to delay and inhibit oxidation reactions, have enhanced its application value in composite materials. Therefore, the introduction of lignin nanoparticles into Pickering emulsions and composites based on Pickering emulsions template method can improve the properties of the materials and provide new special effects to enhance their application value.114,131 In this section, the application fields, production methods and properties of lignin Pickering emulsions are reviewed (Scheme 3).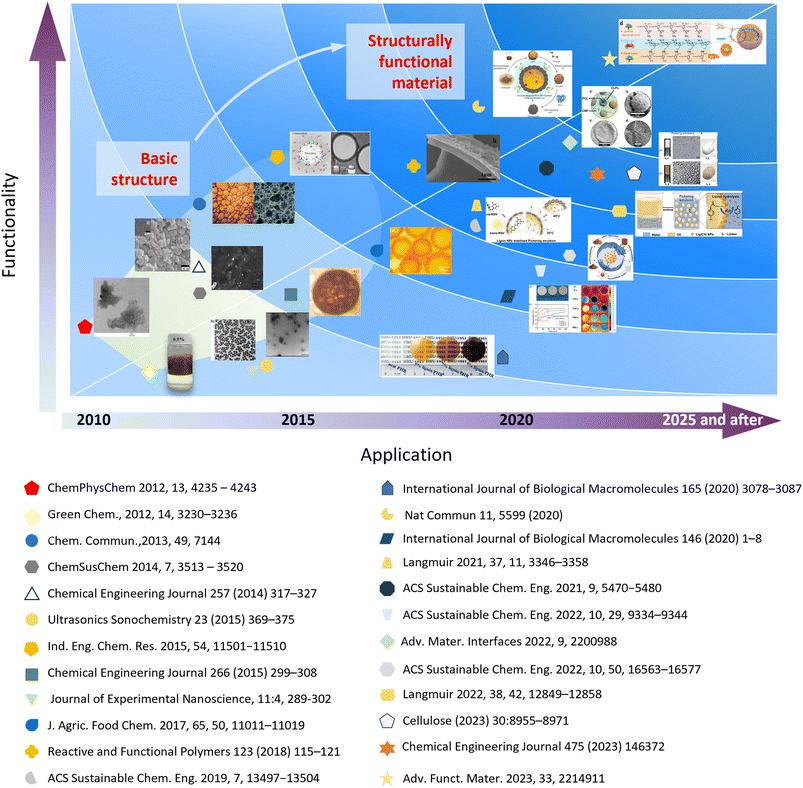 | ||
| Scheme 3 The functional application development of CLPs-Pickering emulsion from 2010 to the present. | ||
4.1. Application of CLPs as reinforcement in Pickering emulsions
A significant emphasis is often placed on the mechanical properties of composites. By employing rational design, diverse interactions between CLPs and matrix materials can be established, encompassing covalent bonds, hydrogen bonds, or ionic bonds (Fig. 11).78 The incorporation of CLPs as reinforcing agents in polymer matrices and nanocomposite materials enhances crucial mechanical properties such as rigidity, strength, toughness, along with thermal stability and barrier properties of the composites for their intended applications.74,86,132 The even dispersion of nanoparticles within a matrix poses a significant challenge for their use as reinforcing materials. The utilization of the Pickering emulsion system has gained popularity in preparing composite materials due to its ability to achieve uniform distribution of nanoparticles within the composite phase.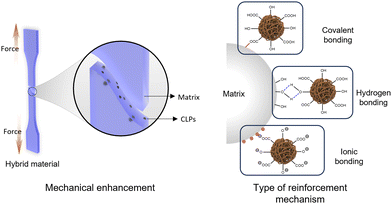 | ||
| Fig. 11 Schematic illustration of lignin reinforcement and the underlying mechanisms for enhancing lignin augmentation. | ||
In the study of Lugoloobi et al.,132 the CLPs were dispersed homogeneously in the poly(3-hydroxybutyrate) (PHB) matrix to form an improved PHB composite film by employing W/O Pickering emulsion template method. Due to the uniform dispersion of CLPs and the strong interfacial adhesion between filler and matrix formed by hydrogen bond, the tensile strength and Young's modulus of the composite film increased by 13.2% and 43.9% respectively when the addition of CLPs was 7%. Li et al. introduced CLPs into polylactic acid in Pickering emulsion and prepared lignin/polylactic acid composite films by compression molding method.133 The results showed that the introduction of CLPs improved the crystallinity and thermal stability of the polymer (the decomposition temperature was also increased by 10 °C). Due to the rigid structure of lignin, the Young's modulus of the composite is increased, but the pull-up property and elongation at break are decreased. This study suggests that the enhancement of CLPs depends on the properties of the substrate. It is smaller in stiff and larger in soft matrices because of the different relative load capacity of the components. Given the inherent rigidity of CLPs as a nanoparticle, in numerous studies on reinforcement, the mechanical properties of composite materials have been comprehensively enhanced through synergistic reinforcement involving multiple nanoparticles or surface grafting of CLPs.
Moreno et al. employed chitosan (Chi) and glucose oxidase (GOx) for surface modification of lignin nanoparticle (Fig. 12b), resulting in the synthesis of polymerized latex dispersion through free radical polymerization within a Pickering emulsion system stabilized by hybrid lignin.88 Subsequently, nanocomposites comprising polystyrene (PS) and polybutyl methylbenenoate (PBMA) embedded with lignin nanoparticles (PS/PBMA–GOx–Chi–CLPs) were prepared via a melting process. The incorporation of modified CLPs significantly enhances the tensile properties of the polymer nanocomposites compared to pure PS and PBMA, without compromising elasticity. Moreover, composites reinforced with 15% hybrid nanoparticles exhibit an increase in toughness by factors of 2.5 and 15 respectively.
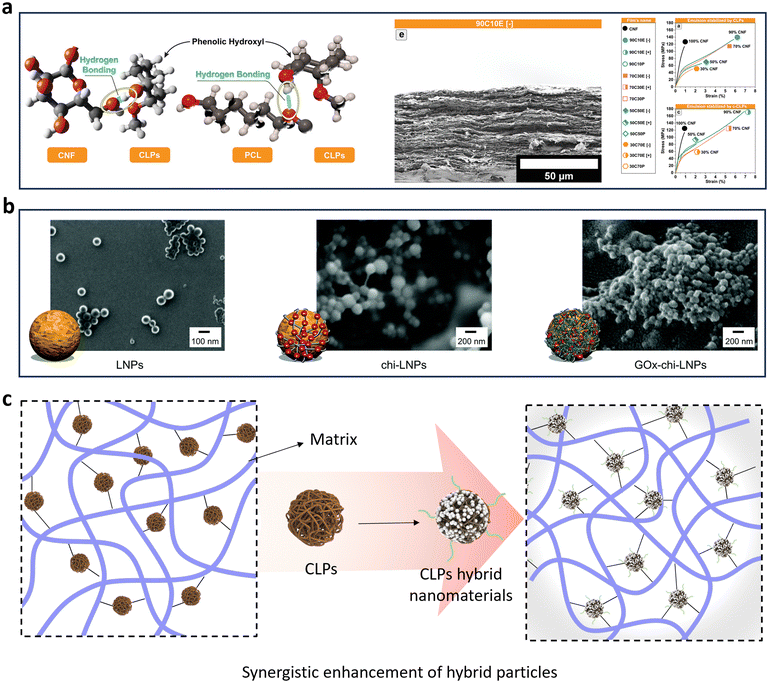 | ||
| Fig. 12 (a) Schematic illustration of possible interactions between nanocomposite's components, SEM image from cross-section view and mechanical and morphological properties of neat CNF and nanocomposite films in dry condition.129 Reproduced from ref. 129 with permission from John Wiley and Sons copyright 2022. (b) SEM images of LNPs, the adsorption of chitosan onto LNPs and GOx–Chi–LNPs produced by the deposition of GOx onto Chi–LNPs.88 Reproduced from ref. 88 with permission from Royal Society of Chemistry copyright 2022. (c) Reinforcement mechanism of lignin hybrid particles. | ||
Similarly, the inherent properties of cellulose, lignin, and polycaprolactone were harnessed by Kimiaei et al. to propose a Pickering emulsion approach for the production of multifunctional CLPs hybird cellulose nanofibril (CNF) composite.129 An aqueous dispersion of CNF was combined with hydrophobic polycaprolactone (PCL), utilizing CLPs as the emulsion stabilizer (Fig. 12a). Fabrication of CNF–PCL nanocomposite resulted in over a 134% increase in dry strength compared to nanocomposites without CLPs. The mechanism underlying the excellent dry and wet strength as well as water resistance achieved was investigated, suggesting that it is attributed to the amphiphilic nature of CLPs which can form non-covalent bonds with both cellulose and PCL, effectively binding them together.
Although CLPs can serve as nano-fillers to enhance the mechanical properties of polymers, the focus lies in improving specific tensile properties of matrix polymers, such as tensile strength, modulus, and elongation at break. It should be noted that CLPs cannot universally enhance all mechanical properties within any given matrix; rather, this synergistic effect is more likely to occur when there is good compatibility between the particles and the matrix.18,134 Merely controlling the concentration and relative content of CLPs in composite materials often fails to achieve optimal performance improvement.135 Therefore, surface modification strategies or collaborative enhancement involving multiple nanomaterials have been extensively emphasized with a primary objective of establishing various types of forces within copolymers including covalent bonds and hydrogen bond systems (Fig. 12c).19,123,136 These efforts aim to construct multiple network structures capable of meeting the strengthening and toughening requirements dictated by the target application.
4.2. Application of CLPs as antioxidant in Pickering emulsions
Lignin, in its natural state, is rich in methoxy aromatic ring groups, phenolic hydroxyl groups, aliphatic hydroxyl groups, carboxyl groups and carbonyl groups, and is a natural antioxidant.137 As demonstrated in Fig. 13a, these functional groups stop the oxidation reaction by reacting with hydrogen.138,139 In addition, studies involving lignin nanoparticles have shown that when lignin is converted to nanoparticles, its antioxidant activity increases. This can be explained by the fact that nano-sized lignin particles have a higher surface area to volume ratio, which brings more surface antioxidant active functional sites. However, the extraction method and preparation process of lignin significantly impact the antioxidant capacity of its nanoparticles.67 This can be attributed to variations in surface functional groups resulting from different sources of lignin raw materials and nanoparticle preparation techniques. Table 2 provides a summary of the antioxidant capacity exhibited by various lignin model compounds. Interestingly, due to the amphiphilic nature of CLPs, the antioxidant activity of the Pickering emulsion template can be directly applied to pharmaceutical, cosmetic and food processing applications.104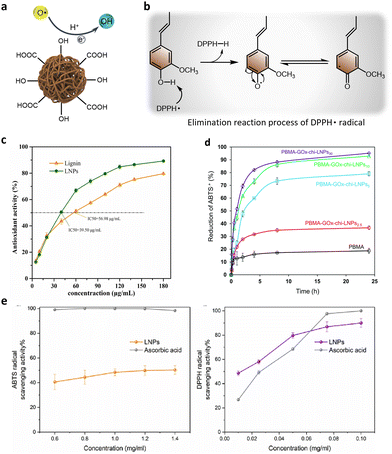 | ||
| Fig. 13 (a) Schematic diagram of antioxidant mechanism of CLPs. (b) The elimination reaction process of DPPH˙ radical by lignin. (c) DPPH˙ radical scavenging activity test against lignin and LNPs.33 Reproduced from ref. 33 with permission from Elsevier copyright 2022. (d) Antioxidant activity kinetics of pure PBMA and PBMA–GOx–Chi–LNP composite films.88 Reproduced from ref. 88 with permission from John Wiley and Sons copyright 2021. (e) ABTS and DPPH free radical scavenging activity (%) of LNPs, ascorbic acid was used as positive control.116 Reproduced from ref. 116 with permission from Elsevier copyright 2023. | ||
Zhang et al. reported a simple green method for the preparation of CLPs by water droplet induced self-assembly using deep eutectic solvent and the preparation of lignin-based Pickering emulsions with antioxidant activity.33 They studied and compared the antioxidant activities of native lignin and CLPs based on the elimination of the π-radical (DPPH˙). Fig. 13b illustrated the possible elimination reaction process of DPPH˙ radical by lignin. The results showed that the antioxidant activities of both samples increased with the increased of the concentration. Thanks to the increase of phenolic hydroxyl group and alcohol hidden inside CLPs, it showed higher free radical scavenging ability (up to 10%) at high concentrations (Fig. 13c). Similarly, in the study of Moreno et al.,88 they evaluated the antioxidant activity of composite membranes after the combination of chitosan and glucose oxidase hybrid CLPs with PRMA through ABTS+˙ free radical scavenging experiments. The results showed that the antioxidant activity of PBMA supplemented with 2.5% and 5% GOx–Chi–CLPs was 30% and 70%, respectively, compared with pure PBMA (18%). However, when the content of hybrid CLPs in the composite film exceeded the concentration threshold of 10%, its antioxidant activity did not increase significantly (Fig. 13d). This result may be related to the layer-by-layer particle deposition of CLPs at the internal interface of the non-wettable polymer matrix, since the antioxidant activity is postulated to occur primarily at the liquid–solid interface. Yu et al. employed an electrospray technique to synthesize lignin nanoparticles with uniform size and regular shape, which were utilized for the stabilization of oil-in-water Pickering emulsions.116 The antioxidant capacity of the Pickering emulsion was assessed by evaluating its free radical scavenging activity against ABTS and DPPH radicals. The results were quantified in terms of the concentration required to inhibit 50% of free radicals (IC50 value). Fig. 13e illustrates the investigation on the antioxidant activity of these lignin nanoparticles, revealing IC50 values of 1.3 mg ml−1 for ABTS quenching and 0.018 mg ml−1 for DPPH activity.
The study revealed that the presence of a substantial quantity of phenolic hydroxyl radicals is essential, and the abundance of methoxyl groups in lignin can stabilize the formation of phenolic hydroxyl radicals. The scavenging activity against free radicals relies on the rate at which these radicals capture hydrogen atoms from phenol molecules.138,139 These functional groups play a crucial role in providing hydrogen to terminate oxidative propagation reactions, with free phenolic hydroxyls being indispensable for antioxidant activity.140
In summary, the antioxidant activity of CLPs, as a green natural antioxidant, can be directly extended to various fields including pharmaceuticals, cosmetics, and food processing by utilizing the Pickering emulsion template.
4.3. Application of CLPs as anti-ultraviolet in Pickering emulsions
Unsaturated double bonds in the molecular structure of lignin (such as carbonyl and vinyl groups) can form conjugated structures with benzene rings. Active π electrons and chromophores (such as hydroxyl, amino, ether bonds, and carboxyl groups) in conjugated systems give lignin bandwidth absorption from ultraviolet to visible light.141 Although the various extraction processes of lignin result in different chemical structures, the ultraviolet (UV) absorption of lignin is generally similar.142,143 The UV shielding performance of three lignin groups is illustrated in Fig. 14a. The S phenolic group containing more methoxyl groups has a stronger UV shielding property over other phenolic groups.144,145 Because CLPs has a larger specific surface area than the original lignin at the same mass, more functional groups are distributed on the surface of the lignin particles, which has better UV resistance. In addition, the improved performance depends on the effective interfacial adhesion and uniform dispersion of CLPs within the polymer body, which is a problem that cannot be ignored, and Pickering emulsions not only avoid the agglomeration problem of CLPs, but also allow their introduction into the hydrophobic polymer.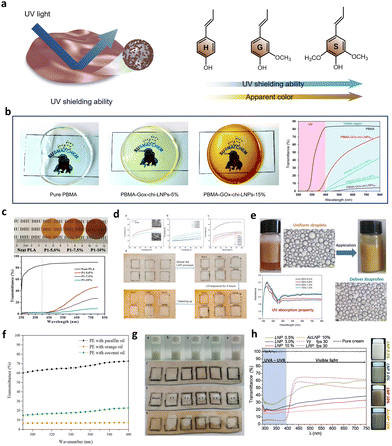 | ||
| Fig. 14 (a) Schematic diagram of anti-ultraviolet properties of lignin composites and comparison of anti-oxidation properties of each unit. (b) Digital images and UV-vis light transmittance spectra of pure PBMA and PBMA–GOx–Chi–CLP composite films.88 Reproduced from ref. 88 with permission from John Wiley and Sons copyright 2021. (c) Photographs of thin films as a function of lignin concentration (up) and UV–Vis transmission spectra of these films (down).132 Reproduced from ref. 132 with permission from Elsevier copyright 2020. (d) UV transmittance of Pickering emulsions stabilized by CLPs and schematic illustration of pig skin to simulate human skin under strong UV-light.116 Reproduced from ref. 116 with permission from Elsevier copyright 2023. (e) Photograph of Pickering emulsions and UV absorption spectra of the Pickering emulsion with CLPs as the stabilizer.106 Reproduced from ref. 106 with permission from American Chemical Society copyright 2022. (f) UV transmittance of Pickering emulsions prepared with 3.50 wt% of CLPs and 40% of oil.122 Reproduced from ref. 122 with permission from Elsevier copyright 2023. (g) Schematic illustration of pig skin to simulate human skin under strong UV-light.142 Reproduced from ref. 142 with permission from Springer Nature copyright 2024. (h) UV-vis transmittance curves and appearance color of creams with different amounts of CLPs.146 Reproduced from ref. 145 with permission from Elsevier copyright 2020. | ||
Moreno et al. evaluated the UV shielding potential of PBMA–GOx–Chi–CLPs composite films prepared by them by using UV-Vis colorimetric spectroscopy.88 The results show that the composite films have strong absorption in UV-A (315–400 nm) and UV-B (280–315 nm) bands compared with the original PBMA. They also highlight that even a concentration of only 5 wt% of GOx–Chi–CLPs in the composite can reduce UV light transmission to 3% with a reduction of only 40% in visible light transmission (Fig. 14b). In the study of Lugoloobi et al. CLP–PHB composite films with yellowish brown color and good UV isolation performance were prepared by using Pickering emulsion method.132 With the increase of the proportion of CLPs in the composite film, the UV shielding performance of the film is gradually enhanced, and the light transmittance is gradually decreased (Fig. 14c). This is not only due to the increased absorption of ultraviolet and visible light by more CLPs, but also due to the increased reflection and refraction of incident light by high content of nanoparticles. A similar conclusion was also reached in a study by Li et al.133
Yu et al. found that lignin nanoparticles with smaller particle size had better anti-ultraviolet performance.116 In addition, they emphasized that the methoxyl functional groups in S-type and G-type lignin, which were easy to absorb UV light, could also significantly promote the conjugated system of lignin, and thus have stronger UV absorption (Fig. 14d).145 Hong used sulfur methylation and alkyl chain bridging modification of lignin to prepare Pickering emulsions. As shown in Fig. 14e, the introduction of higher molecular weight alkyl chains effectively enhanced the stability of the emulsion, allowing it to maintain strong UV absorption capacity even at low lignin loading (0.2%) and oil–water ratio (50%).106
Cosmetics with natural light protection properties are an ideal application direction for lignin's anti-UV performance.143 The CLPs were prepared by Gordobil et al. using softwood sulfate lignin as the raw material, and three O/W Pickering emulsion systems of orange oil, coconut oil, and paraffin oil were formulated.122 In vitro sun protection factor (SPF) testing demonstrated that these Pickering emulsions exhibited UVA/UVB values ranging from 0.74 to 0.90 with C > 380, confirming their broad-spectrum sunscreen efficacy. Furthermore, the incorporation of natural oils containing phenolic compounds into the emulsion system enhanced its SPF value (Fig. 14f), whereas paraffin oils did not show such improvement due to the synergistic effect of π–π stacking between lignin particles and phenolic compounds. The CLPs were extracted from coffee beans through acid precipitation by Xin et al.142 Subsequently, the obtained coffee CLPs was utilized to formulate a stable Pickering emulsion, which underwent UV resistance testing. Notably, the results demonstrated that even after 4 hours of exposure to 254 and 365 nm UV light, the emulsion effectively preserved pigskin color (Fig. 14g). Trevisan et al. isolated nano-lignin from elephant leaf grass, which can be stably dispersed in commercial neutral creams and significantly improve its UV-visible shielding properties.146 Although the general fact is that the higher the concentration of lignin, the stronger the protection against UV rays. However, the undesirable dark color appearance of CLPs based sunscreen creams and composite and will be its limitations in some applications, such as cosmetics (Fig. 14h). Thus, light-colored lignin with anti-ultraviolet activity is important, which can be achieved through the acetylation of the lignin and subsequent conversion into nanoparticles. In addition, the improved performance depends on the effective interfacial adhesion and uniform dispersion of CLPs within the polymer body, which is a problem that cannot be ignored, and Pickering emulsions not only avoid the agglomeration problem of CLPs, but also allow their introduction into the hydrophobic polymer. In addition, the UV-resistant properties of lignin particles can also be used for protection against photosensitive drugs, which will be discussed in the next section.104,147,148
4.4. Application of CLPs as encapsulation in Pickering emulsions
Contributed to its amphiphilicity and good biocompatibility, lignin is considered as a promising drug delivery material.149 In the Pickering emulsion system, CLPs can be used to achieve the loading and controlled release of various drug molecules, such as drug activity, sunscreen activity, model drugs, agricultural chemicals and even enzymes,80,86,109,147,148,150 through embedding and encapsulation. Based on the characteristics of CLPs, the use of CLPs for drug encapsulation has the following obvious advantages: 1. Protect sensitive chemicals from decomposition, evaporation and degradation caused by external environmental factors.104 2. Prolonging the duration of the drug effect by controlled release;85 3. Reduce the toxicity and stimulation of the drug to non-targeted organisms.151According to the type of encapsulated drug and application field, two different encapsulation ideas are proposed (Fig. 15). One is to prepare solid CLPs and use Pickering template method to achieve encapsulation of photosensitive drugs.109 The other is to use interfacial emulsion polymerization method to prepare lignin microcapsules and realize the encapsulation of drugs through cross-linking reaction in microemulsion system.
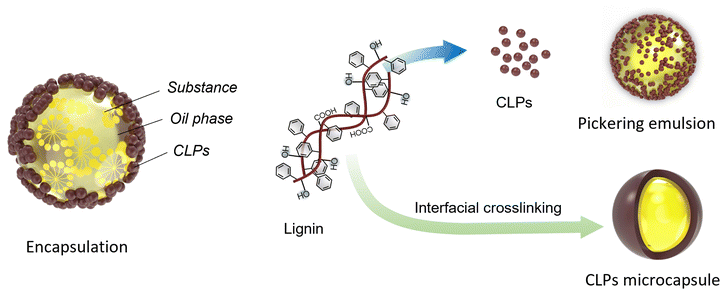 | ||
| Fig. 15 Schematic representation of CLPs as a encapsulation material and two encapsulation processes. | ||
For example, Wang et al. used single-chain/gemini surfactants and sodium lignosulfonate to prepare water-based agglutinates for stabilizing O/W emulsions.109 Attributed to the nanoscale network structure of a wide range of hydrophilic and hydrophobic microdomains in the condensate (Fig. 16a), it can achieve encapsulation and anti-ultraviolet protection against photosensitive pesticides (photosensitive abscisic acid). Chen et al. prepared an O/W high internal phase emulsions (HIPEs) with an internal phase fraction of up to 80 vol% using 5 wt% lignin and 1 wt% sodium dodecyl sulfate (SDS) as emulsifiers.148 The photosensitive bio-anti-inflammatory agent curcumin was dispersed in the organic phase and effectively encapsulated in HIPEs. As shown in Fig. 16b, the CLPs aggregation on the surface of HIPEs could be adjusted by using PBS buffer to control the release of the reagent. As shown in Fig. 16b down, after 72 hours of ultraviolet irradiation, curcumin encapsulated with 3 wt% CLPs-stabilized HIPEs was able to maintain 60.3% of its original concentration.
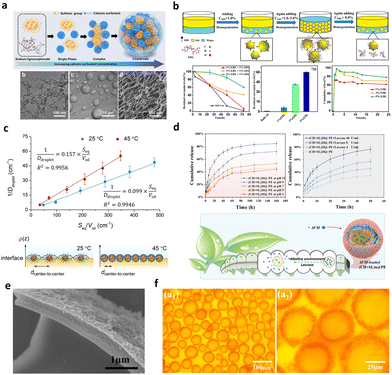 | ||
| Fig. 16 (a) Formation of lignin-based coacervates and TEM image CLPs and Bright field microscopy and cryo-SEM image of coacervate droplets in mixed solution.109 Reproduced from ref. 109 with permission from Elsevier copyright 2022. (b) Mechanism diagram of the HIPEs stabilized by CLPs and SDS (up) and residual curcumin levels in HIPEs-cur prepared with various CLPs concentrations after UV radiation and release profiles of curcumin from HIPEs stabilized with various CLPs concentrations (down).148 Reproduced from ref. 148 with permission from American Chemical Society copyright 2018. (c) Influence of the amount of AL-g-PNIPAM NPs on emulsions droplet size at 25 and 45 °C (up), schematic representation of the adsorbed AL-g-PNIPAM NPs at 25 and 45 °C (down).104 Reproduced from ref. 104 with permission from American Chemical Society copyright 2019. (d) Effects of pH and laccase on the release performance of AVM from (Chi + SL)20@PE Pickering emulsion and schematic diagram of pH/laccase-responsive release of AVM-loaded microcapsules (Chi + SL)n@PE in pests.112 Reproduced from ref. 112 with permission from John Wiley and Sons copyright 2023. (e) SEM images of wall layer structure of lignin–polyurea shell microcapsule.152 Reproduced from ref. 152 with permission from Elsevier copyright 2018. (f) Optical microscopy images of the lignin/polyurea composite microcapsules.153 Reproduced from ref. 153 with permission from American Chemical Society copyright 2017. | ||
Due to the differences in drug electronegativity, release speed, release environment and efficacy mechanism, encapsulation materials with corresponding different stimuli have been widely emphasized.80,85,104,154,155 Consequently, stricter requirements are placed on the charge properties, wall thickness, polydispersity, and stability of CLPs as carriers for drug transport.26,31 To address this need, researchers have developed a series of packaging systems that exhibit both stability and environmentally induced response through hybrid modification of lignin or layer assembly.83,102,106,112
Dai et al. grafted poly(N-isopropylacrylamide) (PNIPAM) onto lignin to prepare thermoresponsive lignin copolymers.104 The hybrid CLPs were formed by self-assembly to stabilize trans-resveratrol (trans-RSV)-containing palm oil emulsion droplets in water. Contributed to the excellent UV resistance of lignin, t the light stability of trans-RSV was significantly improved by the protecting of the CLPs layer. Moreover, the emulsion properties and release behavior strongly depend on the temperature and nanoparticles size: decreasing temperature induced deformation of hybrid CLPs at the interface, an increase in droplet size, and the accelerated release of trans-RSV (Fig. 16c). Similar strategies have been applied in Wen et al.'s study.155
Yu et al. deposited chitosan (Chi) and sodium lignosulfonate (SL) alternately on the interface of CLPs-based Pickering emulsion by electrostatic interaction, formed a simple and effective Pickering emulsion strengthening strategy.112 The prepared Pickering emulsion has adjustable interface thickness with strong stability and forms a microcarrier suitable for the coating of hydrophobic and photosensitive pesticide Avermectin (AVM). As shown in Fig. 16d, the drug release behavior of CLPs-Pickering emulsion can be carefully controlled by controlling the number of sediment layers.86 In addition, the encapsulation system has special stimuli response behaviors for the alkaline environment and laccase stimuli in the insect digestive tract to realize the targeted release of drugs.
Pang et al. realized the encapsulation protection of AVM by using interfacial emulsion polymerization in lignin-stabilized O/W emulsions.152 In this study, diphenyl methane diisocyanate (MDI) and AVM were dispersed in toluene to form an oil phase. During the preparation of Pickering emulsion, MDI in the oil phase would react with water and phenolic hydroxyl of lignin at the oil–water interface to form a thin polyurea layer and lignin–polyurethane shell (Fig. 16e). Ethylenediamine (EDA) is introduced into the system to accelerate shell formation and increase wall thickness. The study of drug release kinetics showed that controlled release of drugs could be achieved by controlling lignin content in emulsion system. Further observation of the wall layer of the formed microcapsules showed that the wall structure was composed of a dense polyurea inner layer and a loose and porous lignin–polyurethane outer layer. The increase of lignin content would consume more MDI, thus reducing the thickness of the polyurea layer and accelerating the release of drugs. Similarly, Pang et al. applied hybrid CLPs to a stable Pickering emulsion and prepared microcapsules containing lignin.153 In this study, a “physical sulfonation” strategy was proposed for lignin. SDS was used to doping acidified alkaline lignin to obtain negatively charged SDS–CLPs. At the oil–water interface of Pickering emulsion, isophorone diisocyanate (IPDI) in the emulsion droplets reacts with ethylenediamine to form a strong polyuurea shell (Fig. 16f), while SDA–CLPs can cross-link with positively charged thylenediamine hydrochloride ion (EH) to form an outer gel shell, thereby improving the stability of the system and controlling the release of encapsulated drugs.
The preparation of environmentally appropriate lignin microcapsules is also the development direction of interfacial crosslinking method. Chen et al. synthesized pH-responsive lignin microcapsules and encapsulated coumarin-6 in a microemulsion system by heat-induced interfacial crosslinking.85 In this study, the allyl group was grafted onto the surface of water-soluble lignosulfonate by etherification reaction, and then the oil–water microemulsion system was prepared with the help of SDS. At the oil–water interface of microemulsion system, amphiphilic lignin and the organic phase crosslinker trimethylolpropane tris(3-mercaptopro-pionate) through the free radical induced thiol–ene reaction lignin nanocapsules were formed by cross-linking. The study found that the acid-labile β-thiopropionate cross-linkages in microcapsule wall layer induced an acid-triggered quick release in low pH environment. In acidic (pH = 4) environments, the cumulative 48 hour release of coumarin-6 reached 60%, compared to 40% in neutral (pH = 7.4) environments.
In summary, the use of CLPs as a carrier for drug or chemical appears to be the most widely application, including biomedicine, agricultural chemicals and so on.29,148,150,154 The types of encapsulated drugs involved in these applications, the use environment, the target and the release cycle, etc., need to be considered as the criteria for the preparation of lignin microcapsules. The selection of the packaging process of lignin should be more differentiated from different fields, in the field of pesticide-controlled release grade plant protection, the selection of hybrid lignin with lower purity, and in the field of biomedicine, purified or graded lignin should be considered.34,154 In particular, the introduction of the concept of composite materials requires CLPs-based microcapsule systems to have more versatile properties, such as magnetic lignin-based Pickering emulsion and highly absorbent lignin-based gel systems.115,156 Overall, the CLPs can serve well as an effective Pickering stabilizer for emulsion, not only due to its excellent partial wettability but also its stimuli-tunable surface properties, which allowed the facile and rapid reversible emulsification/demulsification of Pickering emulsion.26 This poses a challenge to the researchers’ knowledge background and to lignin, whose macromolecular structure is not yet fully understood. However, CLPs has been widely accepted as a green, bio-friendly, multi-functional packaging material.
4.5. Application of CLPs as catalysts in Pickering emulsions
Due to its diverse hydrophilic and hydrophobic functional groups, along with acidic and basic active sites on the surface, lignin has emerged as a catalyst with enhanced reactivity.157,158 In numerous studies, catalysts derived from lignin have proven to be a highly promising alternative to traditional catalysts, offering advantages such as eco-friendliness, reusability, and efficiency. The use of lignin as a catalyst contributes to a greener environment by utilizing renewable sources, in contrast to conventional heavy metal catalysts like titanium and palladium, which can have detrimental effects on the environment during disposal.159The various benefits of CLPs over their macro-sized counterparts suggest the potential for improved catalytic activity. In catalytic reactions, a critical parameter is the surface area, which can enhance conversion rates and ultimately improve yields. Therefore, employing CLPs with a higher surface area-to-volume ratio, in comparison to conventional lignin, holds promise for the development of novel and environmentally friendly catalysts with superior catalytic performance.
Tang et al. developed positively charged chitosan hybrid lignin nanoparticles (Lig/Chi NPs) for stabilizing O/W Pickering emulsions.160 The modification with chitosan enhanced the lignin-emulsion's stability, emulsification properties, and resistance to salt and alkali. Furthermore, they investigated the catalytic impact of lipase on the hydrolysis of p-nitrophenol palmitate within this system. The results indicated that the Pickering emulsion system increased the interface area for significant oil–water reactions. The negatively charged lipase was effectively adsorbed onto the surface of positively charged hybrid lignin through electrostatic adsorption and enrichment, thereby reducing mass transfer distances and resistance (Fig. 17a). In this system, the reaction conversion rate can reach nearly 100% within 30 minutes, which is almost three times higher than that of the conventional two-phase system (Fig. 17b). Additionally, lipases stabilized in Pickering emulsion exhibit strong recyclability, thanks to the protective effect of Lig/Chi NPs.
 | ||
| Fig. 17 (a) Schematic of lipase-catalyzed hydrolysis of p-NPP in the Pickering emulsion system stabilized by Lig/Chi NPs and the conventional two-phase system.160 Reproduced from ref. 160 with permission from American Chemical Society copyright 2022. (b) The effects of reaction time, p-NPP concentration, lipase content and recycle times on catalytic performances of lipase in the conventional two-phase system and Pickering emulsion system stabilized by Lig/Chi NPs.160 Reproduced from ref. 160 with permission from American Chemical Society copyright 2022. (c) Proposed emulsion reactor and W/O interface. Reproduced from ref. 162 with permission from American Chemical Society copyright 2015. (d) Evaluation of the enzyme-degassed controlled radical polymerization of BMA using GOx–Chi–LNPs as stabilizers.161 Reproduced from ref. 161 with permission from Springer Nature copyright 2020. | ||
In addition to using CLPs at the emulsion interface to enhance catalytic efficiency, the Pickering emulsion system can also facilitate the catalytic cracking of lignin itself. For instance, Cai et al. pioneered a novel and highly efficient lignin depolymerization W/O Pickering emulsion, employing lignin as a self-surfactant.162 The Pickering emulsion was prepared using n-butanol and n-hexane as the oil phase and lignin as the emulsifier, achieved by dissolving an ionic liquid in the water phase. In the emulsion system, lignin serves a dual purpose: it not only stabilizes the emulsion but also degrades at the water–liquid interface (Fig. 17c). This results in the exposure of hydrophilic α-O-4 and β-O-4 connection points in lignin to the oil–water interface, facilitating enhanced contact with the IL catalyst and significantly promoting lignin depolymerization.162
Moreover, the system offers advantages in the demulsification process, with automatic phase division occurring at the end of the reaction due to lignin degradation and condensation. Following the reaction, two distinct phases emerge: the depolymerization product dissolves in the upper phase of the n-butanol dispersion, while the IL catalyst is present in the lower phase of the water. This enables efficient product separation and catalyst recovery through a one-step separation and filtration process. The results indicate that lignin depolymerization at the oil–water interface has a significant impact. Under optimized conditions, the conversion rate of bagasse lignin exceeded 89.1%, the selectivity of 4-ethylphenol was 53.9%, and the yield of 4-ethylphenol was 29.60 mg g−1 (3.3 times higher than that without emulsion).
In the study of Moreno et al., they show that lignin nanoparticles (LNPs) coated with chitosan and glucose oxidase (GOx) enable efficient stabilization of Pickering emulsion and in situ enzymatic degassing of single electron transfer-living radical polymerization (SET-LRP) without extraneous hydrogen peroxide scavengers.161 The feasibility of GOx–Chi–LNPs to provide an oxygen-tolerant and controlled polymerization process was evaluated by the polymerization of butyl methacrylate (BMA) as a model monomer in closed vials to avoid the loss of volatile BMA from the reaction mixture. These results (shown in Fig. 17d) not only validate our original hypothesis and confirm GOx–Chi–LNPs as efficient degassing stabilizers for Pickering emulsions polymerizations, but also demonstrate that GOx–Chi–LNPs can provide even a faster polymerization rate than Chi–LNPs after applying nitrogen purging, which could be of potential interest for industrial applications.
4.6. Application of CLPs as adsorbent in Pickering emulsions
Leveraging the structural characteristics of lignin and the functional groups present within its molecular framework, surface engineering or nanotechnology can be employed to modify its spatial network architecture. This approach facilitates the development of lignin-based adsorbent materials exhibiting superior adsorption capabilities. Such materials serve as viable alternatives to costly activated carbon and ion exchange resins for the efficient removal of heavy metal ions, dyes, and organic pollutants. In comparison to conventional lignin powders, CLPs exhibits enhanced affinity towards the oil/water interface due to its larger contact area between oil and water phases. This unique characteristic renders CLPs a promising template for the development of novel adsorbents in Pickering emulsion systems. The optimized interaction between oil and water phases within the Pickering emulsion system ensures superior adsorption performance.In the study conducted by Padilha et al., corncob (CC) and green coconut fiber (GCF) were utilized for the preparation of two CLPs.121 The Pickering emulsion template method was employed to compare the adsorption capacity of these CLPs towards emulsified oil in a water environment. The results indicate that at an CLPs concentration of 2 g L−1, CC–CLPs and GCF–CLPs exhibited maximum removal values of 90.6% and 94.4%, respectively, in emulsified oil experiments (Fig. 18a). Due to its higher lignin content, GCF–CLPs demonstrated enhanced hydrophobicity, resulting in superior ability to remove emulsified oil. This stronger hydrophobic interaction led to gradual adhesion between CLPs layers near the droplets, thereby increasing aggregate size and enhancing their oil adsorption capacity.
 | ||
Fig. 18 (a) Effect of the CC-LNP (white bar) and GCF-LNP (black bar) for separation of emulsified oil by coagulation.121 Reproduced from ref. 121 with permission from Elsevier copy 2020. (b) The appearance and droplet morphology of the Pickering emulsions stabilized by CLPs-CNF and the subsequent foams, with respective SEM images.134 Reproduced from ref. 134 with permission from Springer Nature copyright 2023. (c) Schematic diagram of imprinted molecular. (d) Equilibrium data and modelling for the adsorption of LC onto MIPs-LC-0.11 and NIPs-0.11 at different temperature (left) and kinetic data and modelling for the adsorption of LC onto MIPs-LC-0.11 and NIPs-0.11(right).163 Reproduced from ref. 163 with permission from Elsevier copyright 2014. (e) Optical microscope images of the primary W/O emulsions with W![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O of 1 O of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and the corresponding double W1/O/W2 emulsion and SEM images of multi-hollow microsphere.164 Reproduced from ref. 164 with permission from Elsevier copyright 2015. (f) Construction strategy and mechanism of magnetic CLPs stabilized Pickering emulsion.156 Reproduced from ref. 156 with permission from Royal Society of Chemistry copyright 2023. (g) Optical photos, infrared camera images and the surface temperature of pure gypsum and GS@TDA/PMMA/MPC. Reproduced from ref. 166 with permission from American Chemical Society copyright 2020. 1 and the corresponding double W1/O/W2 emulsion and SEM images of multi-hollow microsphere.164 Reproduced from ref. 164 with permission from Elsevier copyright 2015. (f) Construction strategy and mechanism of magnetic CLPs stabilized Pickering emulsion.156 Reproduced from ref. 156 with permission from Royal Society of Chemistry copyright 2023. (g) Optical photos, infrared camera images and the surface temperature of pure gypsum and GS@TDA/PMMA/MPC. Reproduced from ref. 166 with permission from American Chemical Society copyright 2020. | ||
Agustin et al. harnessed the synergistic effect of nanocellulose on CLPs to achieve stabilization of polyethylene in O/W Pickering emulsion.134 By adjusting the content of CLPs in the emulsion, the interaction with the oil phase can be finely tuned to produce a stable emulsion with varying viscosity. The resulting freeze-dried cellular foam exhibits promising potential as an adsorbent for pharmaceutical contaminants, while the incorporation of CLPs facilitates efficient removal of aromatic drugs possessing diverse ionic properties (Fig. 18b). Additionally, due to lignin's various active functional groups (such as hydroxyl, carbonyl, carboxyl, methyl), chemical modifications can be made to obtain efficient lignin-based adsorption materials. This not only enhances the commercial value of lignin but also opens up new avenues for its high-value utilization. The research conducted by Yang et al. demonstrates a versatile and straightforward approach for the formation of interconnected foams templated from Pickering-HIPE using a coactive system consisting of CLPs and monomers.125 It has been observed that the foam structure transitions from closed-cell to open-cell as the concentration of monomers increases. Furthermore, these interconnected foams exhibit exceptional adsorption capacity for Cu2+ ions.
In Gan et al.'s study,163 CLPs were employed as stabilizers to establish a stable Pickering emulsion. By incorporating functional and cross-linked monomers along with Lambda-cyhalothrin (LC) as template molecules, into the oil phase, a molecularly imprinted polymer (MIPs-LC) was prepared via interfacial polymerization method for selective adsorption of LC (Fig. 18c). The surface hydrophobicity of MIPs-LC obtained after emulsion polymerization was enhanced, facilitating the adsorption of hydrophobic organic pollutants with low water solubility such as LC molecules. Preliminary results demonstrated that MIPs exhibited excellent recognition towards LC, with an adsorption equilibrium time of approximately 3.0 h and an equilibrium adsorption capacity of 72.25 lmol g−1 compared to nonimprinted polymers (NIPs) which showed an adsorption capacity of 43.10 lmol g−1. Isothermal curve analysis, adsorption kinetics studies, and selectivity tests collectively indicated that LC had the highest cavity matching with the imprinted polymer due to its structural characteristics and hydrogen bonding interactions formed within the imprinted cavities (Fig. 18d). Furthermore, the synthesized MIPs could be effectively regenerated and recycled for at least three cycles without significant loss in their adsorption capacity (8.73%).
Similarly, Pan et al. conducted a facile chemical modification of lignin using oleic acid, followed by stabilizing the original and modified lignin at two interfaces to form a surfactant-free water-in-oil-in-water (W1/O/W2) Pickering double emulsion.164 Molecular-imprinted hollow spheres (MIMMs) with adjustable pore structures were prepared by manipulating the volume ratio of the oil phase to the water phase in the emulsion and investigated as adsorbents for selective and efficient removal of LC (Fig. 18e). The static adsorption experiment revealed that MIMMs with increased and larger pore structures exhibited enhanced selective adsorption capacity towards LC. The equilibrium adsorption capacity of MIMMs for LC was determined to be 24.79 mg g−1, with an adsorption equilibrium time of approximately 3.0 hours. Furthermore, MIMMs demonstrated excellent regeneration capability through seven effective regeneration and recirculation cycles, making this approach applicable for the adsorption of various pharmaceutical compounds.
4.7. Other application of CLPs in Pickering emulsions
Additionally, the potential applications of CLPs-Pickering emulsions can be further expanded through their combination with other novel technologies and materials.165 Hasan et al. demonstrated the adsorption and magnetic properties of lignin@Fe3O4 at the castor oil–water interface as an environmentally friendly magnetically controllable Pickering emulsion and polymer oil stabilizer for oil spill collection using external permanent magnets with varying magnetic fields (Fig. 18f).156 Wang et al. investigated microencapsulation using a CLPs-stabilized Pickering emulsion, which when combined with gypsum resulted in an effective heat-storage composite material that holds promising prospects for building thermal management (Fig. 18g).166 Yi et al., fabricated multilayered composite microcapsules coated with isophorone diisocyanate (IPDI) healing agent using an oil-in-water Pickering emulsion stabilized by lignin nanoparticles as a template.91 This technology was applied to anticorrosive polymer coatings by incorporating these microcapsules into epoxy coatings. Results from salt immersion accelerated corrosion tests demonstrated that the self-healing coatings containing IPDI capsules exhibited a remarkable anticorrosive effect on steel plates. After immersing the samples in a 10% wt% NaCl solution for 120 hours, no visible signs of corrosion were observed on the coating matrix due to the controlled release and subsequent polymerization of active substances from the encapsulated microcapsules, resulting in the formation of solid materials for coating repair. Qian et al. doped submicron particles of poly (3,4-ethylenedioxythiophene)/lignosulfonate (PEDOT/LS) into a mixture of 3,4-ethylenedioxythiophene (EDOT) and water to form a solid-stabilized Pickering emulsion.167 The conductivity of the resulting PEDOT/LS complex prepared via Pickering emulsion polymerization was increased by two orders of magnitude. As a result, the surface resistance of glass coated with PEDOT/LS-PEP decreased from 1012 to 106 Ω sq−1. These novel PEDOT/LS-PEP complexes effectively fulfill the requirements for industrial antistatic materials.Yan et al. proposed a hybrid of Ni(OH)2 and lignin sulfonate to synthesize micron-sized particles, which were utilized for the synergistic emulsification in oil spill adsorption at sea.168 The co-emulsification process was facilitated by the electrostatic attraction between Ni(OH)2 particles and oppositely charged SL, effectively mitigating the flocculation of Ni(OH)2 in seawater. In comparison with individual emulsifiers such as Ni(OH)2 particles or SL alone, the hybrid particles exhibited superior performance in producing smaller, more uniform, and highly stable oil–water emulsion droplets, enabling efficient adsorption of highly viscous crude oi. The utilization of Pickering emulsion systems for the development of lignin composites with other materials has emerged as a ubiquitous strategy in material design, thereby enhancing the functional attributes of emulsions. The study conducted by Li et al. presents a novel and facile approach for synthesizing antibacterial phase change microcapsules (micro-PCMs) decorated with silver particles, wherein CLPs served as both the Pickering stabilizer and the reducing agent for silver ions.165 The results demonstrated that the shell of the micro-PCMs incorporated embedded lignin particles, which effectively reduced silver ions to form Ag/lignin micro-PCMs. These resulting Ag/lignin micro-PCMs exhibited a well-defined core–shell spherical morphology with a high phase-transition enthalpy of 177.6 J g−1, excellent encapsulation efficiency of 69.0%, and remarkable thermal durability.
5. Challenges and prospects
The Pickering emulsion is a distinctive dispersion system stabilized by solid particles. The heterogeneous nature of lignin, characterized by various chemical bonds formed by aromatic and phenolic units, enhances the amphiphilic structure of lignin and facilitates its enrichment into nanoparticles for application in emulsion systems. As an emerging emulsion stabilized by lignin nanoparticles, the CLPs-Pickering emulsion exhibits significant potential across multiple fields owing to its unique physicochemical properties; however, there remain several challenges that need to be addressed.21 In terms of the dispersion mechanism of the emulsion, a single theory cannot comprehensively define all studies at present due to the diverse modification strategies employed for lignin nanoparticles resulting in different interface combinations. Before fully understanding the mechanism of interface interaction in this two-phase system, it is crucial to harness the stabilizing role of CLPs as polymer blend stabilizers and emphasize the functional characteristics of lignin such as antioxidant activity, antibacterial properties, and stability performance. This will enable biopolymers like lignin to realize their full potential for practical applications.The first and foremost challenge faced by this technology lies in gaining a more detailed understanding of CLPs formation process. However, due to the varied chemical structures of lignin along with different extraction methods and sources used, development and utilization across a wider range of applications pose certain challenges. Ensuring consistent product properties especially regarding controlled formation of spherical nanoparticles becomes imperative for standardized production. From a fundamental research perspective, optimizing function of lignin nanomaterials through manipulation of particle size, wettability, shape anisotropy aggregation behavior, and surface chemistry presents various challenges that future researchers must focus on addressing. A more comprehensive understanding of how to effectively control these factors is a primary focus for future researchers. In order to achieve sustainable production of CLP-Pickering emulsions on a commercial scale, it is crucial to gain deeper insights into the underlying mechanisms. Specifically, it is important to determine the relative significance of interface stabilization by adsorption particles as true Pickering stabilizers compared to their contribution as functional modulators.
Furthermore, the field of CLPs faces additional challenges in terms of achieving faster, simpler, and more environmentally friendly production methods. Additionally, there is a need to explore new and/or combined applications that align with the concept of transforming waste into wealth. While CLPs offer numerous opportunities for high-value applications utilizing Pickering emulsion systems, there is still potential for further exploration. Consequently, various chemical modifications have been proposed to expand the potential uses of lignin in developing advanced lignin-based polymers and nanostructures. For example, esterification or alkylation can be employed to enhance compatibility between the lignin polymer and non-polar polymer matrices by targeting hydroxyl groups on the lignin structure. Another challenge lies in addressing the high polydispersity of lignin which complicates controlling its macromolecular structure after undergoing chemical modification. The emergence of nano-lignin based materials has enabled better control over the macromolecular structure of lignin, thereby enhancing properties of polymer blends and nanocomposites along with their biomedical applications.
However, it is important to acknowledge that while lignin itself demonstrates limited ecotoxicity, the modification and utilization of chemicals may lead to unintended consequences, such as undesired interactions with various molecules.149 Moreover, due to the hindrance of phenolic hydroxyl groups, the biodegradability of modified lignin is also compromised, potentially impacting the environment. Extensive research is crucial for comprehending the potential toxicity, interactions, degradability, accumulation, and localization of nanoparticles in living organisms. Additionally, enhancing its photostability poses a significant challenge in harnessing lignin as an effective component in anti-ultraviolet agents. It can be argued that advancing Pickering emulsions for encapsulating and delivering bioactive compounds will continue to be a pivotal area of investigation; thus, envisioning breakthroughs through developing functionalized CLPs-Pickering emulsions with responsive stimulus capabilities.
The industrialization of CLPs-Pickering products is experiencing a significant upsurge in terms of industrial development. It is imperative to promptly initiate pilot trials and cultivate more sophisticated, application-oriented industrial production processes. The expeditious implementation of structural optimization for operational units and comprehensive economic evaluation throughout the entire cycle should be prioritized.
Author contributions
Conceptualization, C. H., W. Y., K. Z.; Writing – original draft, J. G.; Writing – review & editing, all authors; investigation, J. G.; Visualization, J. G.; Supervision, W. Y., C. H.; Funding acquisition, W. Y.Data availability
Data availability is not applicable to this article as no new data were created or analyzed in this study.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Fundamental Research Funds of CAF (CAFYBB2021ZX001).References
- M. Zembyla, B. S. Murray and A. Sarkar, Trends Food Sci. Technol., 2020, 104, 49–59 CrossRef CAS.
- W. Ramsden and F. Gotch, Proc. R. Soc. London, 1904, 72, 156–164 Search PubMed.
- S. U. Pickering, J. Chem. Soc., Trans., 1907, 91, 2001–2021 RSC.
- L. E. Low, S. P. Siva, Y. K. Ho, E. S. Chan and B. T. Tey, Adv. Colloid Interface Sci., 2020, 277, 102117 CrossRef CAS.
- Y. Chevalier and M.-A. Bolzinger, Colloids Surf., A, 2013, 439, 23–34 CrossRef CAS.
- Z. Wang and Y. P. Wang, Materials, 2016, 9, 903 CrossRef.
- C. Miesch, E. Pentzer and T. Emrick, in Polymer Science: A Comprehensive Reference, ed. K. Matyjaszewski and M. Möller, Elsevier, Amsterdam, 2012, ch. 7.15, vol. 7, pp. 287–311 Search PubMed.
- H. Jiang, Y. Sheng and T. Ngai, Curr. Opin. Colloid Interface Sci., 2020, 49, 1–15 CrossRef CAS PubMed.
- J. Texter, Colloid Polym. Sci., 2022, 300, 587–592 CrossRef CAS.
- V. Chaudhary and S. Sharma, J. Polym. Res., 2019, 26, 102 CrossRef.
- G. J. Marshall, US Pat, 25949131947, 1947 Search PubMed.
- K.-D. Hungenberg, Macromol. Chem. Phys., 2007, 208, 1020–1020 CrossRef CAS.
- D. G. Ortiz, C. Pochat-Bohatier, J. Cambedouzou, M. Bechelany and P. Miele, Engineering, 2020, 6, 468–482 CrossRef CAS.
- S. A. F. Bon, in Particle-Stabilized Emulsions and Colloids: Formation and Applications, ed. T. Ngai and S. A. F. Bon, The Royal Society of Chemistry, 2014, pp. 1–7 Search PubMed.
- V. Calabrese, J. C. Courtenay, K. J. Edler and J. L. Scott, Curr. Opin. Green Sustain. Chem., 2018, 12, 83–90 CrossRef.
- C. Ji and Y. Wang, Adv. Colloid Interface Sci., 2023, 318, 102970 CrossRef CAS PubMed.
- C. Miao, S. Atifi and W. Y. Hamad, Carbohydr. Polym., 2020, 248, 116775 CrossRef CAS.
- S. Guo, X. Li, Y. Kuang, J. Liao, K. Liu, J. Li, L. Mo, S. He, W. Zhu, J. Song, T. Song and O. J. Rojas, Carbohydr. Polym., 2021, 253, 117223 CrossRef CAS.
- B. L. Pelegrini, F. M. B. Fernandes, T. Fernandes, J. H. de Oliveira, H. C. Rosseto, A. G. O. Junior, A. V. Reis, E. V. Castelani, F. N. C. Sobral, W. V. I. Shirabayashi, L. Benyahia, C. Chassenieux and M. M. de Souza Lima, Cellulose, 2021, 28, 6201–6238 CrossRef CAS.
- X. Liu, M.-C. Li, B. Liao, S. Liu, K. Lu, K. Lv, J. Sun, C. Liu, C. Mei and Q. Wu, Chem. Eng. J., 2023, 475, 146372 CrossRef CAS.
- H. Dupont, V. Maingret, V. Schmitt and V. Héroguez, Macromolecules, 2021, 54, 4945–4970 CrossRef CAS.
- N. Ghavidel and P. Fatehi, ChemSusChem, 2021, 14, 4850–4877 CrossRef CAS PubMed.
- R. C. Sun, ChemSusChem, 2020, 13, 4385–4393 CrossRef CAS PubMed.
- L. Bai, L. G. Greca, W. Xiang, J. Lehtonen, S. Huan, R. W. N. Nugroho, B. L. Tardy and O. J. Rojas, Langmuir, 2019, 35, 571–588 CrossRef CAS.
- R. Rinaldi, R. Jastrzebski, M. T. Clough, J. Ralph, M. Kennema, P. C. A. Bruijnincx and B. M. Weckhuysen, Angew. Chem., Int. Ed., 2016, 55, 8164–8215 CrossRef CAS PubMed.
- S. Beisl, A. Friedl and A. Miltner, Int. J. Mol. Sci., 2017, 18, 2367 CrossRef.
- K. Chen, S. Wang, Y. Qi, H. Guo, Y. Guo and H. Li, ChemSusChem, 2021, 14, 1284–1294 CrossRef CAS.
- E. Lizundia, M. H. Sipponen, L. G. Greca, M. Balakshin, B. L. Tardy, O. J. Rojas and D. Puglia, Green Chem., 2021, 23, 6698–6760 RSC.
- W. D. H. Schneider, A. J. P. Dillon and M. Camassola, Biotechnol. Adv., 2021, 47, 107685 CrossRef CAS PubMed.
- W. D. H. Schneider, A. J. P. Dillon and M. Camassola, Biotechnol. Adv., 2021, 47, 107685 CrossRef CAS PubMed.
- L. E. Low, K. C. Teh, S. P. Siva, I. M. L. Chew, W. W. Mwangi, C. L. Chew, B.-H. Goh, E. S. Chan and B. T. Tey, Environ. Nanotechnol., Monit. Manage., 2021, 15, 100398 CAS.
- Y. Pang, Y. Sun, Y. Luo, M. Zhou, X. Qiu, C. Yi and H. Lou, Ind. Crops Prod., 2021, 167, 113468 CrossRef CAS.
- W. Zhang, J. Shen, P. Gao, Q. Jiang and W. Xia, Ind. Crops Prod., 2022, 188, 115651 CrossRef CAS.
- M. Stanisz, Ł. Klapiszewski, M. N. Collins and T. Jesionowski, Mater. Today Chem., 2022, 26, 101198 CrossRef CAS.
- J. Y. Xu, C. Y. Li, L. Dai, C. L. Xu, Y. D. Zhong, F. X. Yu and C. L. Si, ChemSusChem, 2020, 13, 4284–4295 CrossRef CAS PubMed.
- Y. H. P. Zhang, J. Ind. Microbiol. Biotechnol., 2008, 35, 367–375 CrossRef CAS PubMed.
- L. Hu, T. Li and Y. Jing, J. Phys. Chem. C, 2024, 128, 3832–3838 CrossRef CAS.
- P. F. O. Ferreira, A. L. S. Pereira, M. F. Rosa and R. S. de Santiago-Aguiar, Ind. Crops Prod., 2022, 186, 115119 CrossRef CAS.
- T. Yuan, J. Zeng, D. Guo, Q. Sun, B. Wang, L. Sha and K. Chen, Int. J. Biol. Macromol., 2023, 224, 1142–1151 CrossRef CAS.
- J. Gould, G. Garcia-Garcia and B. Wolf, Materials, 2016, 9, 791 CrossRef PubMed.
- H. Guo, X. Lu, Y. Yang, J. Wei, L. Wu, L. Tan, Y. Tang and X. Gu, Mol. Catal., 2023, 540, 113041 CrossRef CAS.
- M. R. Ridho, E. A. Agustiany, M. Rahmi Dn, E. W. Madyaratri, M. Ghozali, W. K. Restu, F. Falah, M. A. Rahandi Lubis, F. A. Syamani, Y. Nurhamiyah, S. Hidayati, A. Sohail, P. Karungamye, D. S. Nawawi, A. H. Iswanto, N. Othman, N. A. Mohamad Aini, M. H. Hussin, K. Sahakaro, N. Hayeemasae, M. Q. Ali and W. Fatriasari, Adv. Mater. Sci. Eng., 2022, 2022, 1363481 Search PubMed.
- V. K. Thakur, M. K. Thakur, P. Raghavan and M. R. Kessler, ACS Sustainable Chem. Eng., 2014, 2, 1072–1092 CrossRef CAS.
- X. Lu and X. Gu, Int. J. Biol. Macromol., 2022, 205, 539–552 CrossRef CAS.
- X. Shi, S. Gao, C. Jin, D. Zhang, C. Lai, C. Wang, F. Chu, A. J. Ragauskas and M. Li, Green Chem., 2023, 25, 5907–5915 RSC.
- C. Frangville, M. Rutkevicius, A. P. Richter, O. D. Velev, S. D. Stoyanov and V. N. Paunov, ChemPhysChem, 2012, 13, 4235–4243 CrossRef CAS.
- P. K. Mishra and A. Ekielski, Nanomaterials, 2019, 9, 243 CrossRef.
- S. Behera, S. Mohapatra, B. C. Behera and H. Thatoi, Crit. Rev. Biotechnol., 2024, 44, 774–794 CrossRef CAS.
- T. R. Pang, G. H. Wang, H. Sun, L. L. Wang, Q. M. Liu, W. J. Sui, A. M. Parvez and C. L. Si, ACS Sustainable Chem. Eng., 2020, 8, 9174–9183 CrossRef CAS.
- H. Li, Y. H. Deng, B. Liu, Y. Ren, J. Q. Liang, Y. Qian, X. Q. Qiu, C. L. Li and D. F. Zheng, ACS Sustainable Chem. Eng., 2016, 4, 1946–1953 CrossRef CAS.
- Y. Zhou, Y. M. Han, G. Y. Li, F. Q. Xiong and F. X. Chu, Int. J. Biol. Macromol., 2020, 165, 2136–2142 CrossRef CAS PubMed.
- F. Wang, J. Tang, H. Liu, G. Yu and Y. Zou, Mater. Chem. Front., 2019, 3, 356–364 RSC.
- G. Colucci, A. Santamaria-Echart, S. C. Silva, L. G. Teixeira, A. Ribeiro, A. E. Rodrigues and M. F. Barreiro, Colloids Surf., A, 2023, 666, 131287 CrossRef CAS.
- H. Cuthill, C. Elleman, T. Curwen and B. Wolf, Foods, 2021, 10, 371 CrossRef CAS PubMed.
- J. Tian, J. Chen, P. Wang, J. Guo, W. Zhu, M. R. Khan, Y. Jin, J. Song and O. J. Rojas, Green Chem., 2023, 25, 3671–3679 RSC.
- W. Zhao, B. Simmons, S. Singh, A. Ragauskas and G. Cheng, Green Chem., 2016, 18, 5693–5700 RSC.
- R. Li, D. Huang, S. Chen, L. Lei, Y. Chen, J. Tao, W. Zhou and G. Wang, Nanoscale, 2022, 14, 10299–10320 RSC.
- J. Chen, Z. Pang, Y. Zhang, J. Chu, D. Zhang, X. Lu and C. Dong, Bioresour. Technol., 2022, 343, 126130 CrossRef CAS PubMed.
- F. Xiong, Y. Han, S. Wang, G. Li, T. Qin, Y. Chen and F. Chu, Ind. Crops Prod., 2017, 100, 146–152 CrossRef CAS.
- X. Zhao, J. Wang, K. Lan, Z. Zhao, C. Lai, C. Huang and Q. Yong, ACS Sustainable Chem. Eng., 2024, 12, 2871–2880 CrossRef CAS.
- P. Figueiredo, M. H. Lahtinen, M. B. Agustin, D. M. de Carvalho, S. P. Hirvonen, P. A. Penttila and K. S. Mikkonen, ChemSusChem, 2021, 14, 4718–4730 CrossRef CAS PubMed.
- A. Czaikoski, A. Gomes, K. C. Kaufmann, R. B. Liszbinski, M. B. de Jesus and R. L. D. Cunha, Ind. Crops Prod., 2020, 154, 112762 CrossRef CAS.
- K. Gao, J. Liu, X. Li, H. Gojzewski, X. Sui and G. J. Vancso, ACS Sustainable Chem. Eng., 2022, 10, 9334–9344 CrossRef CAS.
- X. Li, J. Shen, B. Wang, X. Feng, Z. Mao and X. Sui, ACS Sustainable Chem. Eng., 2021, 9, 5470–5480 CrossRef CAS.
- L. Chen, S.-M. Luo, C.-M. Huo, Y.-F. Shi, J. Feng, J.-Y. Zhu, W. Xue and X. Qiu, Green Chem., 2022, 24, 285–294 RSC.
- M. Ma, L. Dai, C. Si, L. Hui, Z. Liu and Y. Ni, ChemSusChem, 2019, 12, 5239–5245 CrossRef CAS PubMed.
- M. R. V. Bertolo, L. B. Brenelli de Paiva, V. M. Nascimento, C. A. Gandin, M. O. Neto, C. E. Driemeier and S. C. Rabelo, Ind. Crops Prod., 2019, 140, 111591 CrossRef CAS.
- M. Lievonen, J. J. Valle-Delgado, M.-L. Mattinen, E.-L. Hult, K. Lintinen, M. A. Kostiainen, A. Paananen, G. R. Szilvay, H. Setälä and M. Österberg, Green Chem., 2016, 18, 1416–1422 RSC.
- M. B. Agustin, P. A. Penttilä, M. Lahtinen and K. S. Mikkonen, ACS Sustainable Chem. Eng., 2019, 7, 19925–19934 CrossRef CAS.
- Z. Wei, Y. Yang, R. Yang and C. Wang, Green Chem., 2012, 14, 3230–3236 RSC.
- R. Yin, L. Chen and L. Ma, J. Sep. Sci., 2019, 42, 3563–3570 CrossRef CAS PubMed.
- T. Lindströmn, Colloid Polym. Sci., 1979, 257, 277–285 CrossRef.
- J. Tian, J. Chen, J. Guo, W. Zhu, M. R. Khan, Q. Fu, Y. Jin, H. Xiao, J. Song and O. J. Rojas, Colloids Surf., A, 2023, 670, 131503 CrossRef CAS.
- S. S. Nair, S. Sharma, Y. Pu, Q. Sun, S. Pan, J. Y. Zhu, Y. Deng and A. J. Ragauskas, ChemSusChem, 2014, 7, 3513–3520 CrossRef CAS.
- L. Matsakas, A. Karnaouri, A. Cwirzen, U. Rova and P. Christakopoulos, Molecules, 2018, 23, 1822 CrossRef PubMed.
- A. A. Yaqoob, S. H. Sekeri, M. B. H. Othman, M. N. M. Ibrahim and Z. H. Feizi, Arabian J. Chem., 2021, 14, 103182 CrossRef CAS.
- I. A. Gilca, V. I. Popa and C. Crestini, Ultrason. Sonochem., 2015, 23, 369–375 CrossRef CAS PubMed.
- M. N. Garcia Gonzalez, M. Levi, S. Turri and G. Griffini, J. Appl. Polym. Sci., 2017, 134, 45318 CrossRef.
- H. Yin, L. Liu, X. Wang, T. Wang, Y. Zhou, B. Liu, Y. Shan, L. Wang and X. Lü, Colloids Surf., A, 2018, 545, 51–59 CrossRef CAS.
- Y. Pang, Z. Qin, S. Wang, C. Yi, M. Zhou, H. Lou and X. Qiu, Colloid Polym. Sci., 2020, 298, 1001–1012 CrossRef CAS.
- W. Yang, F. Dominici, E. Fortunati, J. M. Kenny and D. Puglia, Ind. Crops Prod., 2015, 77, 833–844 CrossRef CAS.
- H. Trevisan and C. A. Rezende, Ind. Crops Prod., 2020, 145, 112105 CrossRef CAS.
- X. Yu, S. Chen, W. Wang, T. Deng and H. Wang, J. Cleaner Prod., 2022, 339, 130769 CrossRef CAS.
- M. Ago, S. Huan, M. Borghei, J. Raula, E. I. Kauppinen and O. J. Rojas, ACS Appl. Mater. Interfaces, 2016, 8, 23302–23310 CrossRef CAS.
- N. Chen, L. A. Dempere and Z. Tong, ACS Sustainable Chem. Eng., 2016, 4, 5204–5211 CrossRef CAS.
- T. Zou, M. H. Sipponen and M. Osterberg, Front. Chem., 2019, 7, 370 CrossRef CAS.
- Z. Wei, Y. Yang, R. Yang and C. Wang, Green Chem., 2012, 14, 3230–3236 RSC.
- A. Moreno, M. Morsali, J. Liu and M. H. Sipponen, Green Chem., 2021, 23, 3001–3014 RSC.
- J. Liu, X. Shi, L. Ma, D. Zhang, C. Lai, C. Wang, M. Li, A. J. Ragauskas, F. Chu and Y. Xu, Green Chem., 2023, 25, 5428–5437 RSC.
- M. H. Sipponen, M. Smyth, T. Leskinen, L.-S. Johansson and M. Österberg, Green Chem., 2017, 19, 5831–5840 RSC.
- H. Yi, Y. Yang, X. Gu, J. Huang and C. Wang, J. Mater. Chem. A, 2015, 3, 13749–13757 RSC.
- C. G. Conner, A. N. Veleva, V. N. Paunov, S. D. Stoyanov and O. D. Velev, Part. Part. Syst. Charact., 2020, 37, 2000122 CrossRef CAS.
- W. Zhang, C. Y. Diao and L. Wang, Biotechnol. Biofuels Bioprod., 2023, 16, 55 CrossRef CAS PubMed.
- D. Tian, J. Hu, R. P. Chandra, J. N. Saddler and C. Lu, ACS Sustainable Chem. Eng., 2017, 5, 2702–2710 CrossRef CAS.
- S. Beisl, A. Miltner and A. Friedl, Int. J. Mol. Sci., 2017, 18, 1244 CrossRef CAS PubMed.
- A. A. Myint, H. W. Lee, B. Seo, W.-S. Son, J. Yoon, T. J. Yoon, H. J. Park, J. Yu, J. Yoon and Y.-W. Lee, Green Chem., 2016, 18, 2129–2146 CAS.
- Z. Sun, X. X. Yan, Y. Xiao, L. J. Hu, M. Eggersdorfer, D. Chen, Z. Z. Yang and D. A. Weitz, Particuology, 2022, 64, 153–163 CAS.
- W. W. Zhao, B. Simmons, S. Singh, A. Ragauskas and G. Cheng, Green Chem., 2016, 18, 5693–5700 CAS.
- Y. Z. Zhu and Q. Shen, Abstr. Pap. Am. Chem. Soc., 2005, 230, U1227–U1227 Search PubMed.
- N. Ghavidel and P. Fatehi, ChemSusChem, 2020, 13, 4567–4578 CAS.
- C. Hadjiefstathiou, A. Manière, J. Attia, F. Pion, P.-H. Ducrot, E. Gore and M. Grisel, J. Mol. Liq., 2023, 390, 123030 CrossRef CAS.
- C. Cai, X. Zhan, M. Zeng, H. Lou, Y. Pang, J. Yang, D. Yang and X. Qiu, Green Chem., 2017, 19, 5479–5487 RSC.
- S. Li, J. A. Willoughby and O. J. Rojas, ChemSusChem, 2016, 9, 2460–2469 CrossRef CAS.
- L. Dai, Y. Li, F. Kong, K. Liu, C. Si and Y. Ni, ACS Sustainable Chem. Eng., 2019, 7, 13497–13504 CrossRef CAS.
- Z. Shomali and P. Fatehi, ACS Sustainable Chem. Eng., 2022, 10, 16563–16577 CrossRef CAS.
- N. Hong, J. Agric. Food Chem., 2022, 70, 1196–1202 CrossRef CAS.
- A. M. Borrero-Lopez, L. Wang, H. Li, T. V. Lourencon, C. Valencia, J. M. Franco and O. J. Rojas, Int. J. Biol. Macromol., 2023, 242, 124941 CrossRef CAS PubMed.
- X. Shi, K. Wang, S. Gao, D. Zhang, C. Lai, C. Jin, M. Li, C. Wang, Q. Yong and F. Chu, Ind. Eng. Chem. Res., 2023, 62, 17765–17775 CrossRef.
- J. Wang, Y. Fan, H. Wang, J. Yin, W. Tan, X. Li, Y. Shen and Y. Wang, Chem. Eng. J., 2022, 430, 132920 CrossRef CAS.
- Y. Pang, Y. Cheng, Y. Luo, C. Yi, M. Zhou and H. Lou, J. Dispersion Sci. Technol., 2024, 45, 1972–1980 CrossRef CAS.
- K. S. Silmore, C. Gupta and N. R. Washburn, J. Colloid Interface Sci., 2016, 466, 91–100 CrossRef CAS PubMed.
- X. Yu, X. Li, S. Ma, Y. Wang, W. Zhu and H. Wang, Adv. Funct. Mater., 2023, 33, 2214911 CrossRef CAS.
- S. A. Gundersen and J. Sjöblom, Colloid Polym. Sci., 1999, 277, 462–468 CrossRef CAS.
- M. Rayner, D. Marku, M. Eriksson, M. Sjöö, P. Dejmek and M. Wahlgren, Colloids Surf., A, 2014, 458, 48–62 CrossRef CAS.
- T. E. Nypelo, C. A. Carrillo and O. J. Rojas, Soft Matter, 2015, 11, 2046–2054 RSC.
- M. Yu, H. Xin, D. He, C. Zhu, Q. Li, X. Wang and J. Zhou, Int. J. Biol. Macromol., 2023, 238, 123938 CrossRef CAS PubMed.
- M. Derakhshandeh, B. K. Pilapil, B. Workman, M. Trifkovic and S. L. Bryant, Soft Matter, 2018, 14, 4268–4277 RSC.
- M. S. Manga, T. N. Hunter, O. J. Cayre, D. W. York, M. D. Reichert, S. L. Anna, L. M. Walker, R. A. Williams and S. R. Biggs, Langmuir, 2016, 32, 4125–4133 CrossRef CAS PubMed.
- B. K. Pilapil, H. Jahandideh, S. L. Bryant and M. Trifkovic, Langmuir, 2016, 32, 7109–7116 CrossRef CAS PubMed.
- E. Vignati, R. Piazza and T. P. Lockhart, Langmuir, 2003, 19, 6650–6656 CrossRef CAS.
- C. E. D. A. Padilha, C. D. C. Nogueira, S. C. B. Matias, J. D. B. D. Costa Filho, D. F. D. S. Souza, J. A. D. Oliveira and E. S. D. Santos, Colloids Surf., A, 2020, 603, 125260 CrossRef CAS.
- O. Gordobil, N. Blazevic, M. Simonic and A. Sandak, Int. J. Biol. Macromol., 2023, 233, 123561 CrossRef CAS.
- S. Lu, D. Yang, M. Wang, M. Yan, Y. Qian, D. Zheng and X. Qiu, Colloids Surf., A, 2020, 585, 124158 CrossRef CAS.
- A. M. Bago Rodriguez and B. P. Binks, Curr. Opin. Colloid Interface Sci., 2022, 57, 101556 CrossRef CAS.
- Y. Yang, Z. Wei, C. Wang and Z. Tong, Chem. Commun., 2013, 49, 7144–7146 RSC.
- Z. Zhang, A. Mulyadi, X. Kuang, W. Liu, V. Li, P. Gogoi, X. Liu and Y. Deng, Polym. Eng. Sci., 2018, 59, 964–972 CrossRef.
- A. Sarkar and E. Dickinson, Curr. Opin. Colloid Interface Sci., 2020, 49, 69–81 CrossRef CAS.
- N. Ghavidel and P. Fatehi, Langmuir, 2021, 37, 3346–3358 CrossRef CAS PubMed.
- E. Kimiaei, M. Farooq, R. Grande, K. Meinander and M. Österberg, Adv. Mater. Interfaces, 2022, 9, 2200988 CrossRef CAS.
- Q. Dai, Y. Bai, B. Fu and F. Yang, ACS Omega, 2023, 8, 7430–7437 CrossRef CAS.
- Q. Zhao, L. Fan, J. Li and S. Zhong, Food Hydrocolloids, 2024, 146, 109185 CrossRef CAS.
- I. Lugoloobi, X. Li, Y. Zhang, Z. Mao, B. Wang, X. Sui and X. Feng, Int. J. Biol. Macromol., 2020, 165, 3078–3087 CrossRef CAS PubMed.
- X. Li, N. Hegyesi, Y. Zhang, Z. Mao, X. Feng, B. Wang, B. Pukánszky and X. Sui, Eur. Polym. J., 2019, 110, 378–384 CrossRef CAS.
- M. B. Agustin, N. Nematollahi, M. Bhattarai, E. Oliaei, M. Lehtonen, O. J. Rojas and K. S. Mikkonen, Cellulose, 2023, 30, 8955–8971 CrossRef CAS PubMed.
- K. Wang, M. Zhu, Z. Yang, L. Bai, S. Huan and C. Wang, ACS Sustainable Chem. Eng., 2023, 11, 9132–9142 CrossRef CAS.
- D. M. D. Carvalho, M. H. Lahtinen, M. Bhattarai, M. Lawoko and K. S. Mikkonen, Green Chem., 2021, 23, 9084–9098 RSC.
- V. Ugartondo, M. Mitjans and M. P. Vinardell, Bioresour. Technol., 2008, 99, 6683–6687 CrossRef CAS.
- J. Ponomarenko, T. Dizhbite, M. Lauberts, A. Volperts, G. Dobele and G. Telysheva, J. Anal. Appl. Pyrolysis, 2015, 113, 360–369 CrossRef CAS.
- T. Dizhbite, G. Telysheva, V. Jurkjane and U. Viesturs, Bioresour. Technol., 2004, 95, 309–317 CrossRef CAS PubMed.
- M. Azadfar, A. H. Gao, M. V. Bule and S. Chen, Int. J. Biol. Macromol., 2015, 75, 58–66 CrossRef CAS.
- S. Lv, S. Liang, J. Zuo, S. Zhang, J. Wang and D. Wei, Iran. Polym. J., 2023, 32, 1477–1497 CrossRef CAS.
- H. Xin, J. Xu, J. Zhou and X. Wang, Waste Biomass Valorization, 2024, 15, 3651–3658 CrossRef CAS.
- Y. Bai, X. Li, X. Wang, X. Wang, X. Yang, H. Xin, D. Sun, J. Zhou and M. Chai, Langmuir, 2024, 40, 554–560 CrossRef CAS PubMed.
- M. Lin, L. Yang, H. Zhang, Y. Xia, Y. He, W. Lan, J. Ren, F. Yue and F. Lu, Ind. Crops Prod., 2021, 174, 114212 CrossRef CAS.
- S. R. Yearla and K. Padmasree, J. Exp. Nanosci., 2015, 11, 289–302 CrossRef.
- H. Trevisan and C. A. Rezende, Ind. Crops Prod., 2020, 145, 112105 CrossRef CAS.
- K. Chen, L. Lei, H. Lou, J. Niu, D. Yang, X. Qiu and Y. Qian, Int. J. Biol. Macromol., 2020, 158, 430–442 CrossRef CAS.
- K. Chen, L. Lei, Y. Qian, A. Xie and X. Qiu, ACS Sustainable Chem. Eng., 2018, 7, 810–818 CrossRef.
- S. H. Sekeri, M. N. M. Ibrahim, K. Umar, A. A. Yaqoob, M. N. Azmi, M. H. Hussin, M. B. H. Othman and M. Malik, Int. J. Biol. Macromol., 2020, 164, 3114–3124 CrossRef CAS PubMed.
- K. Chen, Y. Qian, C. Wang, D. Yang, X. Qiu and B. P. Binks, J. Colloid Interface Sci., 2021, 591, 352–362 CrossRef CAS PubMed.
- B. Yan, G. Lu, R. Wang, S. Kang, C. Huang, H. Wu and Q. Yong, Front. Chem. Sci. Eng., 2023, 17, 976–989 CrossRef CAS.
- Y. Pang, X. Li, S. Wang, X. Qiu, D. Yang and H. Lou, React. Funct. Polym., 2018, 123, 115–121 CrossRef CAS.
- Y. Pang, S. Wang, X. Qiu, Y. Luo, H. Lou and J. Huang, J. Agric. Food Chem., 2017, 65, 11011–11019 CrossRef CAS PubMed.
- A. D. E. S. Pereira, J. Luiz de Oliveira, S. Maira Savassa, C. Barbara Rogério, G. Araujo de Medeiros and L. F. Fraceto, J. Cleaner Prod., 2022, 345, 131145 CrossRef CAS.
- R. Wen and S. Fu, J. Wood Chem. Technol., 2022, 42, 456–466 CrossRef CAS.
- M. J. Hasan, E. Westphal, P. Chen, A. Saini, I. W. Chu, S. J. Watzman, E. Urena-Benavides and E. S. Vasquez, RSC Adv., 2023, 13, 2768–2779 RSC.
- H. Fan, Z. Zhang, M. Hou, J. Song, G. Yang and B. Han, ACS Appl. Mater. Interfaces, 2021, 13, 25234–25240 CrossRef CAS.
- J. Liu, P. Xiu, Y. Zhu, K. Wang, S. Tong, Y. Ma, H. Guo and X. Gu, Catal. Commun., 2024, 187, 106868 CrossRef CAS.
- H. Guo, J. Zhao, Y. Chen, X. Lu, Y. Yang, C. Ding, L. Wu, L. Tan, J. Long, G. Yang, Y. Tang, N. Tsubaki and X. Gu, ACS Catal., 2024, 14, 703–717 CrossRef CAS.
- C. Tang, Y. Chai, C. Wang, Z. Wang, J. Min, Y. Wang, W. Qi, R. Su and Z. He, Langmuir, 2022, 38, 12849–12858 CrossRef CAS PubMed.
- A. Moreno and M. H. Sipponen, Nat. Commun., 2020, 11, 5599 CrossRef CAS.
- Z. Cai, Y. Li, H. He, Q. Zeng, J. Long, L. Wang and X. Li, Ind. Eng. Chem. Res., 2015, 54, 11501–11510 CrossRef CAS.
- M. Gan, J. Pan, Y. Zhang, X. Dai, Y. Yin, Q. Qu and Y. Yan, Chem. Eng. J., 2014, 257, 317–327 CrossRef CAS.
- J. Pan, Y. Yin, M. Gan, M. Meng, X. Dai, R. Wu, W. Shi and Y. Yan, Chem. Eng. J., 2015, 266, 299–308 CrossRef CAS.
- X. Li, Y. Wang, B. Wang, X. Feng, Z. Mao and X. Sui, Int. J. Biol. Macromol., 2020, 144, 624–631 CrossRef CAS.
- Y. Wang, X. Li, C. Shen, Z. Mao, H. Xu, Y. Zhong, X. Sui, X. Feng and B. Wang, Int. J. Biol. Macromol., 2020, 146, 1–8 CAS.
- Y. Qian, T. Wang, X. Qiu, D. Zhao, D. Liu and Y. Deng, ACS Sustainable Chem. Eng., 2016, 4, 7193–7199 CAS.
- B. Yan, X. Wang, X. Zhang, S. Liu, M. Li and R. Ran, J. Environ. Chem. Eng., 2021, 9, 106607 CAS.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |