MAX phase coatings: synthesis, protective performance, and functional characteristics
Guanshui
Ma
,
Anfeng
Zhang
,
Zhenyu
Wang
 *,
Kaihang
Wang
,
Jiayue
Zhang
,
Kaixuan
Xu
,
Yuxi
Xu
,
Shenghao
Zhou
and
Aiying
Wang
*,
Kaihang
Wang
,
Jiayue
Zhang
,
Kaixuan
Xu
,
Yuxi
Xu
,
Shenghao
Zhou
and
Aiying
Wang
 *
*
Key Laboratory of Advanced Marine Materials, Ningbo Institute of Materials Technology and Engineering, Chinese Academy of Sciences, Ningbo, 315201, China. E-mail: wangzy@nimte.ac.cn; aywang@nimte.ac.cn
First published on 18th December 2024
Abstract
Mn+1AXn (MAX) phases are a novel class of materials with a closely packed hexagonal structure that bridge the gap between metals and ceramics, garnering tremendous research interest worldwide in recent years. Benefiting from their unique layered structure and mixed covalent–ionic–metallic bonding characteristics, MAX phase coatings possess excellent oxidation resistance, and exceptional electrical and thermal conductivities, making them highly promising for applications in advanced nuclear materials, battery plate protection materials, and aero-engine functional materials. This review aims to provide a comprehensive understanding of MAX phase coatings. It presents an overview of their compositions and microstructure, highlighting well-established structures like 211, 312, and 413. Furthermore, it delves into the various synthesis methods employed in fabricating MAX phase coatings, including physical vapor deposition, chemical vapor deposition, spraying methods, and laser cladding, among others. The potential applications of MAX phase coatings, high-temperature oxidation resistance, mechanical protection, salt spray corrosion resistance, etc., are also investigated. Finally, this review discusses the future potential of MAX phase coatings and proposes areas for further research and improvement. The primary goal is to offer theoretical guidance and innovative ideas for the synthesis and development of superior MAX phase coatings for commercial applications.
Wider impactThe synthesis and application of MAX phase coatings have attracted significant attention in materials science due to their unique metallic and ceramic properties. This review on their synthesis, protective performance, and functional characteristics broadens our understanding and potential advancements in several critical areas. The theoretical insights and innovative synthesis methods discussed pave the way for future research and development. By exploring new compositions and techniques, researchers can enhance the properties of MAX phase coatings, making them more versatile and effective. MAX phase coatings offer exceptional resistance to high-temperature oxidation and corrosion, making them ideal for extreme environments such as marine and nuclear fields. Understanding the oxidation and corrosion mechanisms discussed provides valuable insights for designing high-performance coatings. This review also summarizes the functional protective capabilities of MAX phase coatings in electrical conductivity, corrosion resistance, and electromagnetic shielding, and highlights their properties as radiation-resistant nuclear cladding and interfacial layers for thermal barrier coatings. This opens new avenues for applications in electronics, advanced energy, and aerospace sectors, driving innovation and contributing to more sustainable and efficient industrial practices. |
1. Introduction
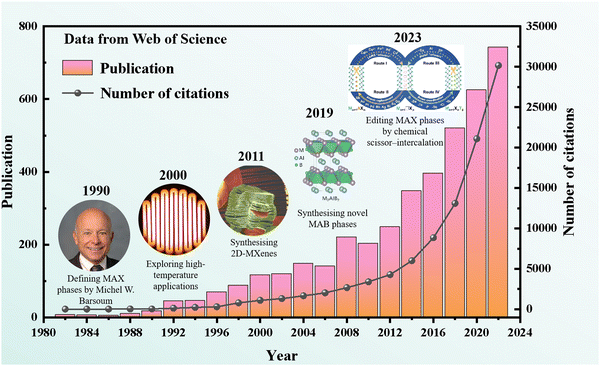
In the 1960s, Nowotny1 and his group in Vienna discovered more than 100 new carbides and nitrides. Among these, over 30 had similar layered hexagonal structures, known initially as Hagg phases, characterized by M2X layers interspersed with pure A layers. The significant breakthrough that reignited interest in these materials occurred in the mid-1990s when Barsoum et al.2 synthesized relatively phase-pure samples of Ti3SiC2. However, in 2000 the Hagg phase was renamed Mn+1AXn(MAX), where M represents a transition metal element mainly including Ti, Nb, Zr, Hf, V, Mo, Ta and Cr,3 the A sites are occupied by elements from group IIIA or IVA, such as Al, Si, and Ge, and also included post-transition metal elements like Au, Ir, and Zn, while X was C or N.4 In 2011,5 the first 2D transition metal carbide (Ti3C2), known as “MXene”, was synthesized by selectively etching Al atoms from the Ti3AlC2 MAX phase, which further stimulated the development of MAX phases.6–10 Subsequently, the structures of in-plane MAX (i-MAX) and out-of-plane (o-MAX) were studied from both experimental and theoretical simulation calculations.11–13Fig. 1 illustrates the history and research trends of MAX phases.All reported MAX phases were C-based or N-based MAX systems until 2019, when Ade et al.14 attempted to synthesize a new MAX phase by introducing B as the X element. The first stable B-based (X site) MAX phase was prepared by Wang et al.15 Recently, the X site element expanded to P and Se,16 and the M site elements have been extended from transition metals (including Mn, Fe, Co, Ni, Cu, and Zn) to rare-earth elements, including Ce, Pr, Nd, Sm, Gd, Tb, Dy, Ho, Er, Tm and Lu.17–19 Moreover, the discovery of new solid-solution MAX phases has allowed for the incorporation of more elements into ternary MAX phases, significantly expanding the MAX phase family. Meanwhile, since the positions of oxygen and carbon peaks perfectly match with each other, oxygen was identified as a potential X element in the MAX.20 Additionally, the medium-entropy MAX phases composed of 3 elements mixed at a specific site and high-entropy phases formed by correspondingly mixing equal or relatively large proportions of 4 or more elements were found, which also promoted the diversity of MAX phases.21–29 In 2023, Huang et al.30 discovered a series of layered carbonitride materials with new structure and composition characteristics by using the universal “chemical scissors” structure editing strategy, which further expanded the types of MAX phases. Recently, Dahlqvist et al. identified 182 new theoretically stable MAX phases by calculation of phase stability.31Fig. 2 provides an overview of the elements constituting the MAX phases, which encompass both solid solution and chemical order, and covers a significant portion of the periodic table. Specifically, it includes 28 different elements for the M-site, 28 elements for the A-site, and 6 elements for the X-site.
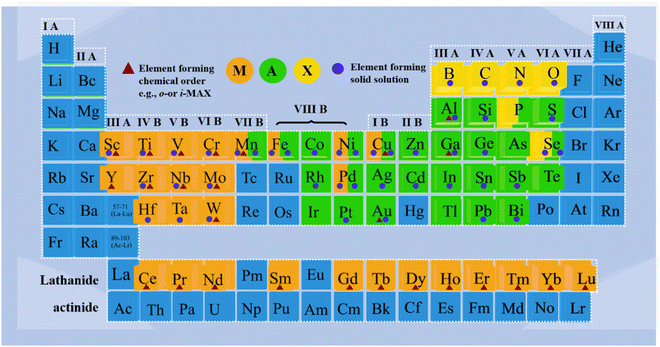 | ||
| Fig. 2 The corresponding positions of MAX phase elements in the periodic table of chemical elements. Reproduced and adapted with permission.31 Copyright 2023 Elsevier Ltd. | ||
Depending on the value of n, the Mn+1AXn phases were initially classified into 211, 312, and 413 phases.32 These MAX phases all have layered hexagonal structures with space group P63/mmc (No. 194), consisting of M6X octahedra separated by A atomic layers.33 M–X bonds in M6X octahedra are strong covalent bonds, while the M–A bonds between the M6X layers and the A atomic layer are much weaker, particularly in shear.34 The primary structural differences among the 211, 312 and 413 phases lie in the number of M6X layers separating the A layers. As illustrated in Fig. 3a, two, three and four M6X layers exist in between every two A layers in the 211, 312 and 413 phases crystal structures, respectively. Another distinction among the three crystal structures is their polymorphism: the 211 phase has one polymorph, the 312 phase has two polymorphs (α and β), and the 413 phases have three (α, β and γ), as shown in Fig. 3b. The polymorphs differ slightly in the atomic stacking sequences of the M–X blocks.35 With ongoing research into MAX phases, the family has expanded to include higher-order phases such as the 514, 615, and 716 series.36–38
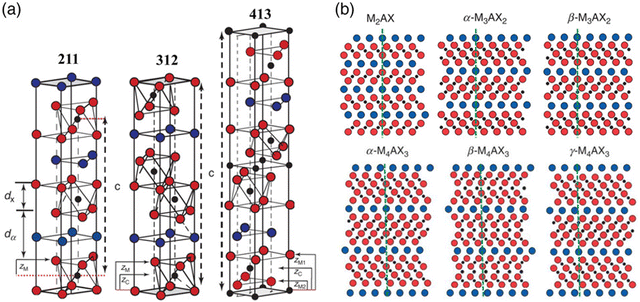 | ||
Fig. 3 Crystal structure of 211, 312, and 413 MAX phases. (a) Unit cell of the three phases. The lattice parameter c of each unit cell is outlined by vertical dashed lines. The thicknesses of the M6X layer and A layer are denoted as dX and dα, respectively. (b) Schematics of the [11![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 0] planes and the polymorphism of the MAX phases. Reproduced with permission.35 Copyright 2020 Wiley-VCH GmbH. 0] planes and the polymorphism of the MAX phases. Reproduced with permission.35 Copyright 2020 Wiley-VCH GmbH. | ||
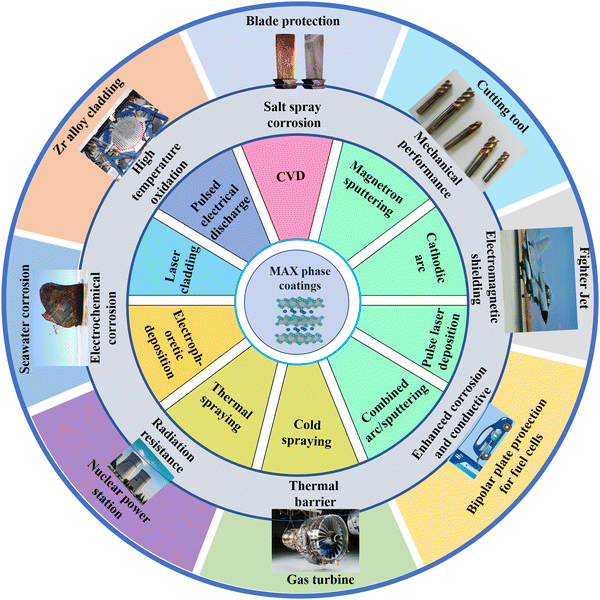
Over the last few decades, MAX phases have garnered considerable interest from researchers, due to their unique combination of both metallic and ceramic characteristics.37,39 The study of MAX phases has been systematically and extensively investigated, ranging from theoretical models to experimental validations,40–42 and from structural design to performance evaluation.17,43–45 Particularly, thanks to their unique layered structure and atomic bond characteristics, MAX phase coatings, which have been developed since 2004, exhibit outstanding damage tolerance,46–48 excellent corrosion/oxidation resistance,49,50 remarkable mechanical properties,51,52 and high electrical conductivity.53,54 These superior advantages highlight the potential applications of MAX phases as protective coatings for vital components in severe environments,55 such as advanced nuclear materials,54,56 battery plate protection materials,57,58 and aero-engine functional materials.59Fig. 4 shows the potential applications, properties, and common preparation methods of MAX phase coatings. Benefiting from the tremendous contribution of researchers worldwide, numerous innovative synthesis methodologies and novel microstructures of MAX phase coatings have been documented in recent years. Here, we summarize the synthesis techniques and prospective application domains utilized in the creation of MAX phase coatings, alongside the ongoing research endeavors to elucidate their physical properties and viable commercial utilization.
2. Preparation methods of MAX phase coatings
2.1. Physical vapor deposition
Physical vapor deposition (PVD) is a widely used method for preparing various MAX phase coating systems, known for its low operating temperature, convenient equipment operation, environmentally friendly nature, and the ability to achieve large-area preparation.60,61 In the fabrication of MAX phase coatings, popular PVD techniques mainly include magnetron sputtering, cathodic arc deposition and pulse laser deposition.Furthermore, high power impulse magnetron sputtering (HiPIMS) is a novel magnetron sputtering technique that overcomes the limitations of insufficient heat capacity in traditional magnetron sources by utilizing high peak power and low duty cycle (1–10%), enabling operation at high power levels and short pulse widths (50–200 μs).65 During the fabrication process, HiPIMS allows for adjustable plasma density (up to 1018–1019 m−3 near the target), ionization rate (≥30%) and deposition particle energy, offering significant controllability.66
Li and co-authors67 deposited Ti–Al–C coatings on a Ti–6Al–4V substrate using the HiPIMS technique. Experimental results demonstrated that HiPIMS, with its high ionization plasma flux and high kinetic energy, facilitated the formation of nanostructured TiAlx compounds. After a low-temperature annealing step at 700 °C, HiPIMS promoted the generation of dense and smooth Ti3AlC2 phase coatings. Fig. 5 shows a schematic illustration of HiPIMS synthesis of the Ti3AlC2 MAX phase coating. The sufficiently high ion energy provided by HiPIMS supported the continuous growth of the coatings, resulting in a denser microstructure and relatively stronger adhesion. Zhang et al.68 utilized HiPIMS to prepare Ti–Al–N coatings from a Ti2AlN composite target. The coatings, composed of MAX-phase Ti2AlN and tetragonal Ti2N phases, were obtained at a temperature of 450 °C, exhibiting a stable coating structure after vacuum annealing at 800 °C. Recently, Zhou et al.69 also obtained highly crystallized Cr2AlC MAX phase coatings on a Ti–6Al–4V substrate at a temperature as low as 480 °C using a hybrid DCMS/HiPIMS technique (Fig. 6a and b). The research revealed that Cr2AlC MAX phase coatings deposited within the temperature range of 480–530 °C consisted of uniform, densely packed, and randomly oriented grains (Fig. 6c and d), but started to decompose at 800 °C. Moreover, the hybrid DCMS/HiPIMS technique resulted in a calculated surface temperature increment of approximately 122 °C, with Cr and Ar ions/atoms contributing 64% and 31% of the total energy flux, respectively.
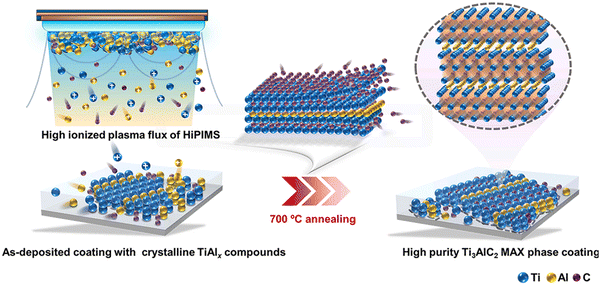 | ||
| Fig. 5 Schematic illustration for low temperature synthesis of HiPIMS induced Ti3AlC2 MAX phase coatings. Reproduced with permission.67 Copyright 2023 Elsevier Ltd. | ||
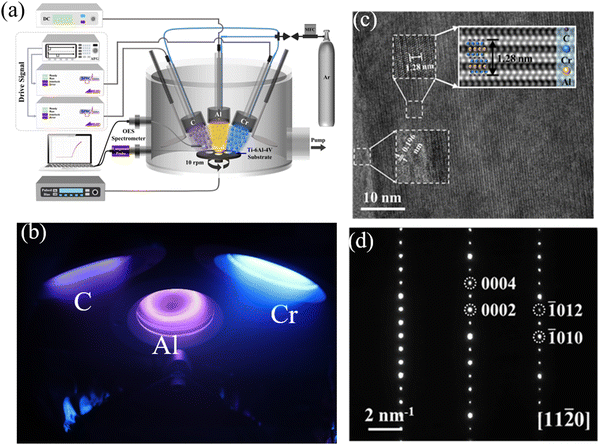 | ||
| Fig. 6 (a) Schematic diagram of the hybrid DCMS/HiPIMS deposition system used, (b) physical images of three target materials during operation, (c) HRTEM images and the Fourier filter of the Cr–Al–C coating at 480 °C, and (d) the SAED pattern of the Cr–Al–C coating at 480 °C. Reproduced and adapted with permission.69 Copyright 2024 Elsevier B.V. | ||
The HiPIMS technique significantly enhances the diffusion of adsorbed atoms on the surface of coatings, thereby improving their density and protective capabilities. This makes HiPIMS crucial for future PVD deposition coatings. However, variations in HiPIMS process parameters can substantially impact coating properties. Zhou et al.70 utilized HiPIMS to fabricate Ti–Al–C coatings at different deposition pressures, observing that as the pressure increased, the thickness and deposition rate of the coatings initially rose and then declined. Qureshi et al.71 conducted a comparative study on the influence of different frequencies (1.2–1.6 kHz) and pulse widths (20–60 μs) of average power on Cr2AlC MAX phase coatings. The results demonstrated that the pulse width had a more pronounced effect on enhancing the compactness of coatings in HiPIMS compared to the frequency. Therefore, further research is essential to investigate how deposition process parameters such as pulse width, frequency, and bias voltage affect the coating structure, performance, and other characteristics.
Magnetron sputtering is the most commonly used PVD method due to the easy processing, high flexibility, good control over phase purity and composition, and the smooth coating surface. However, the coating prepared by this method has a low rate, and the required time is relatively long when the thickness of the prepared coating is large. In addition, the coating prepared by this method is limited by the substrate material, and a transition layer is often needed to improve the bonding strength.
Rosen et al.73 fabricated Ti2AlC coatings using the high-current pulsed cathodic arc deposition method. They employed three independent cathodes to deposit epitaxial coatings under alternating plasma pulses at 900 °C, allowing for high-level control over the deposition material flux ratio. Li and co-authors74 utilized filtered cathode vacuum arc deposition to deposit Ti3AlC2 coatings at room temperature, followed by annealing at 800 °C for 1 h. The prepared coatings exhibited a smooth and dense structure with a significant reduction in surface micro-particles. Guenette et al.75 successfully produced directionally oriented Ti2AlC coatings using pulsed cathodic arc deposition through various ratios of Ti:Al:C, achieving this without the need for a TiCx seed layer. In a separate study conducted by Mahmoudi et al.,76 the arc method was employed to generate Ti2AlC and Ti3AlC2 MAX phase coatings in a mixed gas environment of C2H2/Ar following an annealing treatment. The research findings revealed that annealing the coatings at 900 °C increased the content of Ti2AlC and Ti3AlC2, thus improving the overall deposition effect. The optimal deposition effect was observed when maintaining a volume ratio of 1/4 for C2H2/Ar. It is crucial to have a sufficient volume of C2H2 during the deposition process, as excessively low levels can result in carbon deficiency, ultimately impeding the formation of Ti2AlC and Ti3AlC2 MAX phases.
Cathodic arc deposition is known for its high cathodic deposition rate and low production cost, which enables rapid preparation of coatings and enhances the adhesion between the coating and the substrate. However, the prepared coatings often have large particles on the surface of the coating, resulting in an uneven coating surface. Therefore, the advantages of cathodic arc deposition and magnetron sputtering can be combined to prepare excellent coatings.
Wang et al.77 innovated and developed the high ionization arc combined magnetron sputtering technique for the preparation of MAX phase coatings. In this method, the arc provides the M-element, the magnetron supplies the A-element, and reactive gases such as hydrocarbons are introduced. By combining this with heat treatment, high-purity and well-dense Cr2AlC MAX phase coatings were successfully obtained. The prepared Cr2AlC coatings exhibit an equiaxed grain structure without columnar crystals and possess high bonding strength with substrates such as titanium alloys. This makes MAX phase coatings promising for high-temperature protection and corrosion resistance in harsh environments.
Based on the special crystallographic relationship between the MAX phase and Mn+lXn, a two-step strategy of pre-constructing the Mn+lXn precursor for the subsequent solid-state reaction with element A can also reduce the phase formation temperature of MAX phases. Wang et al.78,79 used a cathodic arc/sputter system to deposit multilayer Cr–C/Al phases and subsequently conducted heat treatment to obtain the Cr2AlC, as shown in Fig. 7(a–c). Analysis of its formation mechanism revealed the alternating growth of (Cr, C)-rich and Al-rich layers. The multi-layer phase composition was initially CrCx, but after vacuum annealing, the internal diffusion of Al resulted in the blurring and disappearance of the multi-layer interface, and the thickness of the Al-rich layer decreased, forming the MAX phase. This revealed the lowest temperature synthesis process for the preparation of MAX coatings so far. The mechanism of the intercalation method was also found in the preparation of Ti2AlC,80 and the atom diffusion during annealing illustrated in Fig. 7(d). The strategy employs a “building blocks” approach in crystallography, where the A atomic layer diffuses and inserts into the MX (111) twin boundaries under thermal driving forces, leading to the formation of the MAX phase. Importantly, this synthesis process effectively circumvents the formation of intermediate competitive phases, offering a novel approach for the low-temperature fabrication of high-purity MAX phases.
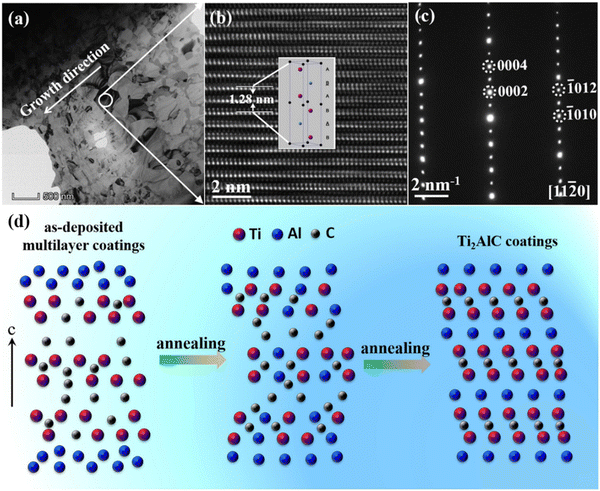 | ||
| Fig. 7 (a) TEM bright-field image of the Cr2AlC coating at low magnification. (b) HRTEM image of the grain within the round solid line in (a), and the inserted image showing the unit cell of Cr2AlC. (c) The corresponding SAED with the electron beam parallel to the [11−20] direction. (d) Schematic diagram of a MAX phase coating prepared by depositing a precursor combined with a subsequent solid-phase reaction. Reproduced and adapted with permission.79,80 Copyright 2020 Elsevier Ltd and 2019 Elsevier B.V. | ||
Hu et al.82 prepared Ti3SiC2 coatings with a friction coefficient of 0.2 in humid air and a hardness between 30 and 40 GPa using MS assisted PLD at near room temperature to 300 °C. To control the mirror system, which allowed the laser beam to randomly strike the target over the selected ablation area, beam steering was accomplished using a computer with 248 nm wavelength, 20 ns duration, 1–50 Hz rate and 200–600 mJ energy. A schematic illustration of the magnetron sputtering assisted pulsed laser deposition system is shown in Fig. 8. Lange and co-authors83 employed PLD with a pulsed Nd:YAG laser to the synthesized Cr2AlC MAX phase coating on stainless steel (SS) substrates. The method demonstrated that the ion-beam has several significant effects on coating thickness and composition, concentration gradients within the coatings as well as on conductivity and hardness.
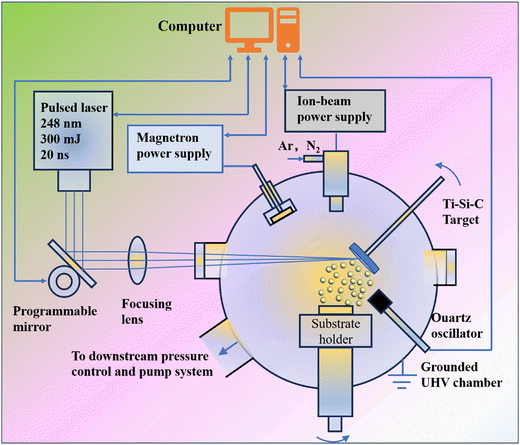 | ||
| Fig. 8 Schematic illustration of the magnetron sputtering assisted pulsed laser deposition system. Reproduced and adapted with permission.82 Copyright 2004 Plenum Publishing Corporation. | ||
Ma et al.85 fabricated amorphous Cr–Al–C coatings using a hybrid MS/cathodic arc deposition technique (Fig. 9a), and subsequently obtained the Cr2AlC MAX phase by in situ heating TEM (Fig. 9b). Increasing the temperature from 25 to 370 °C led to the structural transformation from amorphous Cr–Al–C to crystalline Cr2Al interphases, with the high-purity Cr2AlC MAX phase distinctly forming at 500 °C, accompanied by the diminished amorphous feature. Similar phase evolution was also evidenced by the ab initio molecular dynamics calculations, where the bond energy of Cr–Cr, Cr–Al, and Cr–C played the key role in the formed crystalline stability during the heating process. Furthermore, Yuan et al.59 also used sputtering/cathodic arc deposition and an in situ XRD technique to successfully prepare M2AlC (M = Ti, V, Cr) MAX phases. Compared to V2AlC and Cr2AlC MAX phase coatings, the Ti2AlC coating displayed a higher phase-forming temperature, without any intermediate phases before the appearance of the Ti2AlC MAX phase. The results of the first-principles calculations correlated with the experience in which Ti2AlC exhibited the largest formation energy and density of states.
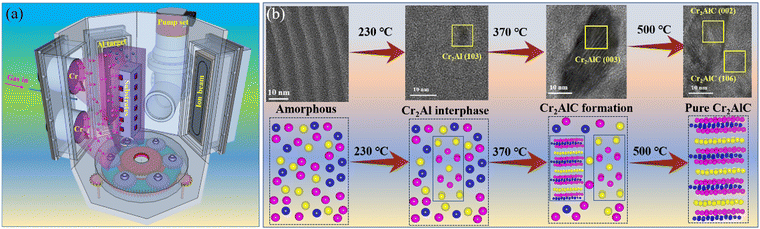 | ||
| Fig. 9 (a) A schematic of the hybrid cathodic arc/magnetron sputtering process for depositing Cr–Al–C coatings. (b) Microstructure of TEM and schematic diagram evolution of the Cr–Al–C coating during in situ TEM heating. Reproduced and adapted with permission.85 Copyright 2024 Elsevier Ltd on behalf of the editorial office of the Journal of Materials Science & Technology. | ||
Bahiraei et al.86 developed a novel approach using a combination of PVD and post-laser treatment to successfully fabricate Ti2AlC MAX phase coatings on Ti–6Al–4V alloy substrates. This method involves an initial carbon coating treatment of the substrate using PVD, followed by Nd:YAG laser and semiconductor laser irradiation of the coating. Compared to the Ti–6Al–4V substrate, the wear rate of the laser-treated samples was reduced by approximately 81%, the average friction coefficient decreased by nearly 66%, and the hardness increased by 2.5 to 4.5 times. This method enhances the wear resistance and hardness of the coatings, further expanding their range of application.
2.2. Chemical vapor deposition
Chemical vapor deposition (CVD) is a method for forming coatings on a substrate surface through chemical reactions using gas-phase elemental or compound species containing the coating elements. This process involves temperatures that facilitate the diffusion of reactants on the substrate surface, and appropriate control of the reaction temperature ensures a strong bond between the coating and the substrate.In 1972, Nickl et al.87 successfully prepared Ti3SiC2 coatings using a mixture of gases including TiCl4, SiCl4, CCl4, and H2 at 1200 °C, demonstrating the feasibility of CVD for fabricating MAX phase coatings for the first time. Subsequently, researchers replaced CCl4 with CH4 as the carbon source and successfully prepared Ti3SiC2 coatings.88 Pickering et al.89 utilized the CVD method to fabricate Ti3SiC2, obtaining a complex microstructure consisting of multiple phases such as Ti3SiC2, TiC, and TiSi2. This provided a deeper understanding of the chemical vapor deposition evolution process for Ti3SiC2.
However, the process of fabricating MAX phase coatings using CVD methods is relatively complex, requiring strict control over parameters such as gas flow rate, temperature, and composition. This increases the difficulty of operation and the equipment costs. Additionally, there may be issues with gas contaminants during the deposition process, which not only degrade coating quality or affect equipment performance, but also pollutes the environment. Moreover, controlling phase purity is challenging in the CVD fabrication of MAX phase coatings. In addition, the temperature for preparing MAX phase coatings by CVD deposition is relatively high, which can easily cause tempering of the substrate material and limit its application. Consequently, the widespread application of CVD techniques for producing MAX phase coatings is presently limited.
2.3. Spraying method
Thermal spraying is a process that involves melting the coating material using thermal energy and then spraying it onto the substrate surface through high-speed gas flow atomization. Molten or semi-melted droplets are sprayed onto the substrate surface, “splashing” to cool and form a layered microstructure.90 Depending on the heat source used, thermal spraying techniques can be classified into methods such as plasma spraying, arc spraying, and high-velocity oxy-fuel (HVOF) spraying.91 For the fabrication of MAX phase coatings, HVOF spraying is commonly employed. Frodelins et al.92 utilized the HVOF spraying method to produce dense Ti2AlC coatings. In their experiments, the coatings, over 100 μm thick, exhibited good bonding with SS substrates and had a hardness ranging from 3–5 GPa. Nevertheless, the thermal spraying process necessitates the substrate to be heated to elevated temperatures, which constrains the choice of matrix materials and frequently results in the formation of cracks and potential delamination of the coating. Moreover, the thermal spraying process is prone to the formation of pores and metal oxides, resulting in a substantial presence of interfaces and defects within the coating. These issues negatively impact electron conduction within the coating, leading to poor electrical conductivity, as well as reduced heating efficiency and low utilization of the sprayed material. Thus, achieving large-scale production of MAX coatings through thermal spraying techniques still poses challenges.Compared to thermal spraying, cold spraying technology utilizes high-speed and relatively low-temperature acceleration of micron-sized solid particles towards the substrate to form a coating through deformation and bonding mechanisms. Gutzmann et al.93 achieved Ti2AlC coatings with a thickness of approximately 110–155 μm on Cu substrates using cold spraying. The coating maintained the crystalline structure of the starting material during the cold spraying process and exhibited a relatively low porosity. Maier et al.94 utilized cold spraying to prepare Ti2AlC MAX phase coatings on Zr alloys. Experimental results demonstrated that the wear resistance of the coatings was significantly superior to that of the zirconium alloy substrate, with the depth of wear tracks reduced from 12 μm to 1 μm after coating deposition.
Cold spraying of MAX phase coatings avoids many issues such as tensile residual stress, low oxidation, adverse chemical reactions, and phase decomposition. However, cold spraying of MAX phase coatings typically requires micron-sized solid particles, and there are specific requirements for particle size and morphology. Additionally, the interface characteristics between cold-sprayed MAX phase coatings and the metal substrate are not yet well understood, including phenomena such as interface melting and interface amorphization.95 Currently, only a few MAX phases, such as Ti3AlC2, Ti3SiC2, Cr2AlC, and Ti2AlC, have been studied and reported for cold-sprayed coatings.96 To meet practical application demands, developing MAX phase coatings of different types is necessary. Although some studies have been conducted on the mechanical and wear properties of cold-sprayed MAX phase coatings, research on their high-temperature oxidation resistance, corrosion resistance, and electrical properties is still relatively limited. Further in-depth research is needed in these areas.
2.4. Other methods
A new path for the production of MAX phase coating has been developed using a laser cladding layer, due to its advantages such as high binding strength, low dilution rate, dense coating, good strength and toughness, and a small heat-affected zone. Richardson et al.97 investigated the method of laser cladding for in situ synthesis and successfully prepared a three-layer dense and well-bonded Ti2AlC MAX phase composite coating with a thickness of 1.33 ± 0.02 mm. It is the first instance of in situ synthesis of MAX phase coatings from elemental powders through laser cladding. Tian et al.98 utilized a two-step approach of laser cladding and laser post-treatment to fabricate high-content Ti2AlC MAX phase coatings on a TC4 titanium alloy substrate. During the laser cladding process, the diffusion of Al atoms resulted in the formation of a core–shell structure with TiC as the core and Ti2AlC as the shell in the coating. In the laser post-treatment process, the diffusion of C and Al atoms intensified, promoting the nucleation and growth of the Ti2AlC phase. Prior to laser post-treatment, the microhardness of the coating increased dramatically from the bonding region to the surface. After laser irradiation, with increasing laser power, the microhardness distribution in the coating became more uniform. This novel two-step laser technique opens up new prospects for the efficient fabrication of MAX phase coatings and related structures.Electrophoretic deposition (EPD) is a method that involves suspending particles in a liquid medium and applying an electric field between two electrodes to uniformly coat the particles onto complex geometries. Galvin et al.99 deposited Ti3SiC2 coatings on titanium substrates through electrophoretic deposition and then performed rapid consolidation using laser sintering. By adjusting the laser power and focal length, they were able to achieve high coverage or good adhesion with approximately 30 μm thick coatings. This method is simple to operate, can be carried out at room temperature, and provides uniform coatings on complex geometries.
Zamulaeva et al.100 prepared Cr2AlC coatings on Ti alloy substrates by applying pulsed electrical discharge. The results showed that the deposited coatings were primarily composed of the Cr2AlC phase, along with small amounts of γ-Al2O3, TiC, (Ti, Cr)C, and Cr1−xAlx phases. This technique allowed for the production of high-density coatings with good adhesion. However, the transient heating pulses and high heating and cooling rates during the pulsed electrical discharge process may result in overheating and thermal decomposition of the coating material, leading to the formation of impurity phases in the prepared coatings.
3. Protective performance of MAX phase coatings
3.1. High temperature air oxidation resistance of MAX phase coatings
The formation of an Al2O3 protective oxide coating plays an important role in the oxidation resistance of the material, which can greatly improve the service life of the material in the high-temperature air atmosphere.101 In MAX phase coatings, due to the weaker M–A bond and stronger M–X bond, the Al element in Al-containing MAX phase coatings readily diffuses to the surface and forms a dense, continuous passive coating, which exhibits excellent oxidation resistance under high temperature, and oxidation of the MAX phases occurs as follows:| Mn+1AXn + bO2 → (n + 1)MOx/n+1 + AOy + XnO2b–x–y | (1) |
In the present study, all oxidation kinetic curves of MAX-phase coatings exhibited parabolic shapes, which could be attributed to the initial rapid oxidation reaction of MAX phases upon exposure to oxygen. As the oxidation reaction progresses, a stable oxide layer formed on the surface of the MAX phase coating, effectively slowing down the oxidation process and establishing a protective layer. This results in a decrease in the rate of oxidation weight gain. Particularly noteworthy are the continuous and dense Al2O3 coating produced during oxidation, which highlight the potential of Ti2AlC and Cr2AlC coatings as promising materials for high-temperature protection. Consequently, current research on the oxidation properties of MAX phase coatings has largely focused on Cr-based Cr2AlC and Ti-based Ti2AlC coatings.102–105
 | ||
| Fig. 10 (a) TEM bright-field image of the Cr2AlC coating oxidized at 1000 °C for 20 h, where the inset shows the corresponding STEM image, (b) EDS profiles acquired from A and B points in (a), and (c) and (d) HRTEM images of points A and B in (a), respectively. Reproduced with permission.107 Copyright 2011 Elsevier Ltd. | ||
To improve the oxidation resistance of Cr2AlC coatings, a diffusion barrier layer may be applied at the interface between the coating and substrate during the preparation process. Additionally, modifying the coating's columnar crystal structure to equiaxial crystals or reducing the number of columnar crystals can hinder oxygen diffusion pathways, ultimately leading to lower inward oxygen diffusion rates. These measures are believed to slow down inward oxygen diffusion and improve the oxidation resistance of Cr2AlC coatings.
To inhibit internal nitridation and delay mutual diffusion rates at the coating/substrate interface, Li et al.108 developed a three-layer Cr2AlC coating structure, featuring a crystalline outer layer, an amorphous middle layer, and a (Cr, Al)2O3 bottom layer. Compared to single-layer Cr2AlC coatings, the three-layer structure demonstrated superior oxidation resistance at high temperatures, with lower oxidation weight gain observed. Nevertheless, multilayer coating preparation can result in cracking during high-temperature oxidation, primarily due to significant thermal expansion coefficient mismatches and thermal stress concentration. To mitigate these factors, diffusion barrier layers can be incorporated between the coating and substrate to slow the mutual diffusion of coating elements and reduce Al consumption.
Wang et al.79 conducted a study where they prepared MAX phase coatings on hastelloy substrates, consisting of Cr intermediate and Cr–Al–C layers (Fig. 11a). Following annealing at 700 °C for 5 h, the inward diffusion of coating elements resulted in the formation of a diffusion layer, transforming the Cr2AlC coating into an equiaxial crystal structure. As shown in Fig. 11b, after oxidation at 1000 °C for 40 h, a continuous Al2O3 layer formed on the coating surface, with a Cr–C layer forming at the bottom. Upon increasing the oxidation temperature to 1100 °C, the oxide coating converted to (Al, Cr)2O3, and a Cr–C–N layer formed at the bottom due to nitridation. However, Al diffused inward and aggregated in the substrate, forming a continuous Al2O3 layer. This Al2O3 layer provided protection against further oxidation of the substrate and reduced elemental interdiffusion. It is notable, however, that Cr2AlC coatings exhibit limitations in oxidation resistance due to Al and Cr depletion and the generation of gases at high temperatures, which can lead to visible pore formation in the carbide layer.
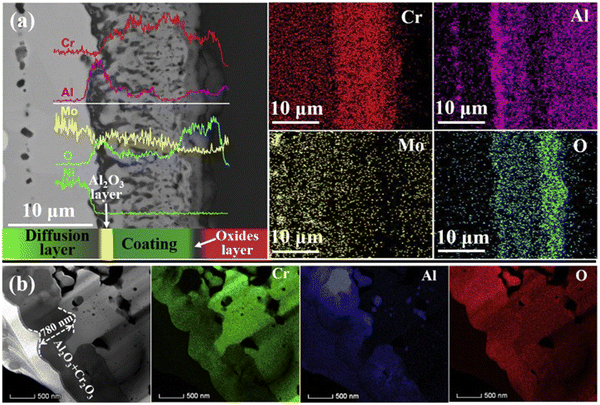 | ||
| Fig. 11 (a) Cross-sectional image of the Cr2AlC coatings at 1000 °C for 40 h, and the corresponding line-scanning results. (b) Cross-sectional STEM micrographs of the outmost oxide layer for these oxidized Cr2AlC coatings. Reproduced with permission.79 Copyright 2020 Elsevier Ltd. | ||
Chen et al.109 conducted a study comparing the high-temperature oxidation resistance of Cr2AlC coatings with columnar and equiaxial crystal structures (Fig. 12a and b). The equiaxial crystal Cr2AlC exhibited better oxidation resistance, characterized by a thinner oxide coating after oxidation. The grain boundaries of equiaxial crystals were denser than those of columnar crystals, reducing the diffusion rate of corrosive media. Intriguingly, nanopores (2–25 nm) were observed in the columnar grain boundaries, which reduced the oxidation resistance and increased the oxidation rate of Cr2AlC coatings. Therefore, reducing nanoporosity and transforming from columnar to equiaxed crystals can improve the oxidation resistance of Cr2AlC coatings.
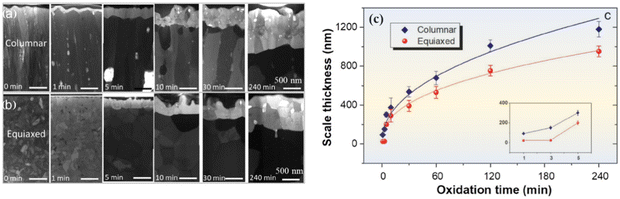 | ||
| Fig. 12 STEM bright field images of the cross-sections of (a) columnar and (b) equiaxed grained Cr2AlC coatings before oxidation and after oxidation at 1100 °C from 1 to 240 min. (c) Variation of the oxide scale thickness with oxidation time and corresponding power lawfit in the equiaxed and columnar grained samples. Reproduced and adapted with permission.109 Copyright 2020 Taylor & Francis INC. | ||
Cr2AlC coatings have shown excellent oxidation resistance in high temperature atmospheres, and have also been widely investigated in water vapor atmospheres. Tang et al.110 investigated the water vapor oxidation behavior at 1000 °C of single layer Cr2AlC and Cr/Cr2AlC multilayer coatings for a short duration. The findings revealed that both coatings outperformed the Zr alloy substrate in terms of oxidation resistance, although their mass gain was similar. In the case of the Cr/Cr2AlC multilayer oxidation, the columnar grain boundaries of the Cr overlayer served as fast diffusion paths for the oxidation medium, resulting in internal oxidation beneath the Cr2AlC layer. Notably, oxidation occurred within both the Cr layer and the Cr2AlC layer, forming a protective Al2O3 barrier that hindered the further inward diffusion of oxidizing species towards the substrate. Additionally, the high Cr content in the overlayer facilitated the formation of a passivation coating comprising Cr2O3 at high temperatures. This dual-layer passivation coating (Cr2O3/Al2O3) effectively impeded the diffusion of corrosive media, making it a promising candidate for potential applications in accident-tolerant fuel (ATF) systems.
Wang et al.111 fabricated a dense equiaxial crystal structure Cr2AlC MAX phase coating on a Zr alloy substrate, featuring a Cr/CrCx interfacial layer structure. The researchers investigated the steam oxidation resistance of the coating at temperatures ranging from 1000 to 1200 °C. The findings indicated that the incorporation of the interfacial layer played a significant role in inhibiting the rapid diffusion of Al into the substrate and preventing premature delamination of the coating during the annealing process. By selectively oxidizing Al, the coating experienced rapid growth of α-Al2O3 on the surface while inhibiting the formation of other oxides. This enhanced the high-temperature steam oxidation capability of the Cr2AlC coating. However, the surface did not exhibit an (Al, Cr)2O3 coating, most likely due to the relatively short steam oxidation duration, leading to incomplete oxidation of the Cr element.
The aforementioned findings illustrate the exceptional oxidation resistance of Cr2AlC coatings, which exhibit the ability to form passivation coatings of Cr2O3 and Al2O3 in high-temperature atmospheres. Nonetheless, it is important to note that at elevated temperatures, mutual diffusion occurs between the Cr2AlC coatings and the substrate. This diffusion process, coupled with the depletion of Al content and the generation of gases, resulting in the formation of pore defects, thus reduced the service life of the coatings. Therefore, future research efforts should prioritize the structural design of the coatings, explore variations in crystal structure, investigate alterations in Al content, and delve into the dynamics of diffusion. These aspects represent key focal points for further exploration concerning the preparation and performance of Cr2AlC coatings.
To inhibit mutual diffusion and improve adhesion between the coatings and substrate, Li et al.113 prepared an intermediate layer of TiC between the Ti2AlC coatings and investigated oxidation resistance at 1000–1200 °C. The oxidation scale exhibits a three-layer structure, with the outermost layer being a TiO2 + Al2O3 mixed oxide layer, the intermediate layer being Al2O3, and the inner layer being TiO2. However, visible pore defects appeared, attributed to the diffusion of Ti following the Kirkendall mutual diffusion mechanism and the formation of CO2 and H2 during the reaction process.114 The transformation of the oxide structure was closely related to the diffusion kinetics and thermodynamics of the elements in the M-site and A-site. Due to the fact that Al is bonded with weak bonds, outward diffusion more easily occurs during the oxidation process. However, because TiO2 has a more disordered structure than Al2O3, its growth rate is much higher than that of Al2O3. Thus, the outermost layer is a mixed oxide layer of TiO2 + Al2O3 at 1000–1200 °C. As Al continues to diffuse and accumulate at the bottom of the outermost oxide layer, an Al2O3 layer is formed. But as the Al in Ti2AlC is depleted and aggregates in the middle layer, TiC forms, which is eventually fully oxidized to TiO2 due to the inward diffusion of oxygen.
Self-healing materials are capable of repairing cracks and damage automatically, thereby prolonging their service life. Wang et al.115 achieved self-healing of cracks in Ti2AlC coatings by Sn doping at 700 °C. The primary mechanism for self-healing involved filling the cracks with SnO2 and TiO2, which demonstrated better oxidation resistance than the unhealed coatings. However, achieving effective elemental doping requires consideration of factors such as the thermal expansion coefficient, thermal conductivity, and diffusion coefficient, all of which significantly affect the formation of cracks and other defects.116 Meanwhile, the Al content also affects the formation of oxides.117 The formation of a continuous, dense Al2O3 layer consumes a large amount of Al in the coating. Insufficient Al content can hinder the formation of Al2O3 during oxidation and lead to the formation of TiO2 in the outer oxide layer, which is detrimental to the oxidation resistance of Ti2AlC coatings at high temperatures.
3.2. Corrosion of MAX phase coatings in harsh environments
To explore the corrosion mechanism of Ti2AlC MAX phase coatings in NaCl solution, Fu et al.120 compared the corrosion resistance of the amorphous Ti–Al–C coatings and Ti2AlC coatings in a 3.5 wt% NaCl aqueous solution. The Ti2AlC coatings exhibited a higher corrosion potential value (−0.301 V) and a lower corrosion current density value (5.82 × 10−8 A cm−2) compared to those of amorphous coatings (−0.618 V and 1.44 × 10−6 A cm−2). The exceptional corrosion resistance of Ti2AlC coating was attributed to the high mobility of active Al atoms, which could readily migrate to the coating surface and form a dense Al2O3 oxide layer. This Al2O3 layer acts as a passivation barrier, effectively preventing further corrosion during chemical attacks.
Wang et al.49 conducted a comprehensive investigation on the corrosion process of Ti2AlC MAX phase coatings in NaCl deposits in water vapor at 600 °C. After subjecting the Ti2AlC coatings to 75 h of corrosion, a compact and continuous corrosion scale with a thickness of 3 μm was observed on the coating surface, leading to remarkable corrosion resistance. This dense corrosion scale was composed of NaxTiyOz fine grains with a size of 11 ± 0.4 nm and amorphous Al2O3 phases, which self-healed the generated defects during corrosion, benefiting from the novel layered structure of the MAX phase.
Li et al.121 conducted a comparative study on the hot NaCl salt corrosion behavior of three kinds of MAX phase materials (Ti3SiC2, Ti2AlC and Cr2AlC) at a high temperature of 750 °C for 240 h. They found that Ti3SiC2 exhibited the best high-temperature corrosion resistance, maintaining its surface integrity well after corrosion (Fig. 13a), without layering phenomenon in the corrosion layer (Fig. 13d). The surface damage of Ti2AlC after corrosion was more significant (Fig. 13b), and its corrosion products mainly consisted of rod-shaped TiO2, bright block shaped Al2O3, and the presence of Na+ in its oxide layer (Fig. 13e). However, Cr2AlC exhibited the worst high-temperature corrosion resistance (Fig. 13c). The generated oxide layer had a three-layer structure: the upper layer was Al2O3, the middle layer was Cr2O3, and the lower layer was Cr7C3 (Fig. 13f). Its poor high-temperature corrosion resistance was primarily due to its strong reaction with NaCl, which produces volatile Na2CrO4, making it difficult to form a dense oxide layer on the surface of Cr2AlC.
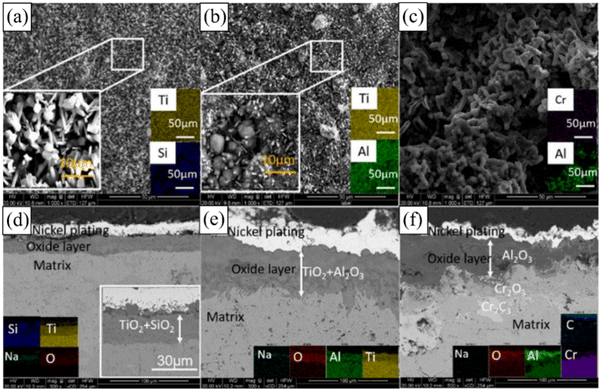 | ||
| Fig. 13 Surface and cross-sectional morphology after corrosion: (a)–(c) are the surface morphology and (d)–(f) are the cross-sectional morphology of Ti3SiC2, Ti2AlC, and Cr2AlC. Reproduced with permission.121 Copyright 2022 Elsevier Ltd and Techna Group S.r.l. | ||
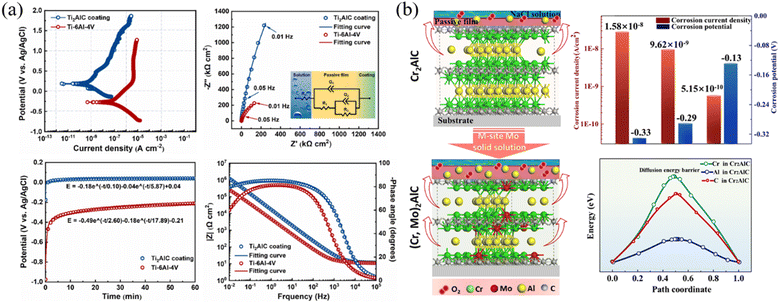 | ||
Fig. 14 (a) Electrochemical performance of the Ti2AlC coating and uncoated Ti–4Al–4V in 3.5 ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) wt% NaCl solution. (b) Schematic of the mechanism and electrochemical performance for the comparative corrosion inhibition of Cr2AlC MAX phase coating with and without an Mo solid solution in 3.5 wt% NaCl solution. Reproduced and adapted with permission.122,124 Copyright 2024 Elsevier Ltd and 2024 American Chemical Society. wt% NaCl solution. (b) Schematic of the mechanism and electrochemical performance for the comparative corrosion inhibition of Cr2AlC MAX phase coating with and without an Mo solid solution in 3.5 wt% NaCl solution. Reproduced and adapted with permission.122,124 Copyright 2024 Elsevier Ltd and 2024 American Chemical Society. | ||
Recently, Zhang et al.125 studied the corrosion behavior of the amorphous, partially crystalline, and fully crystalline Cr2AlC MAX phase coatings in 3.5 wt% NaCl aqueous solutions. It was revealed that both Cr and Al played important roles in the corrosion process. To further increase the electrochemical corrosion resistance, Zhang and co-authors124 fabricated (Cr0.9, Mo0.1)2AlC coatings based on the concept of M-site solid solutions. Results showed that the corrosion current density of (Cr, Mo)2AlC solid solutions was reduced, and the electric impedance was enhanced by about an order of magnitude in 3.5 wt% NaCl solution compared to pristine Cr2AlC coating. According to the density functional theory (DFT) simulation, this phenomenon was mainly ascribed to the partial substitution of Mo for Cr in Cr2AlC, which favored the stronger mobility of Al atoms due to the reduction in vacancy formation energy, leading to the rapid growth of passivating Al oxides substantially (Fig. 14b).
Zakeri-Shahroudi et al.127 investigated the performance of Cr2AlC MAX phase coatings in the presence of 40 wt% V2O5 + 60 wt% Na2SO4. After 5 h of cyclic hot corrosion behavior at 900 °C, it was found that the Cr2AlC coating exhibited good corrosion resistance for up to 30 h. But, after 40 h, microcracks appeared on the sample surface. With the corrosion time increased to 50 h, the sample was completely degraded due to spallation.
3.3. Mechanical performance protection of MAX phase coatings
Due to the unique nano-laminated structure and combination of covalent, ionic and metallic chemical bonds, MAX phases generally exhibit high stiffness, compressive strength, and fracture toughness.128 According to the well-known Hall–Petch relationship, MAX phase coatings are expected to be stronger and harder than their corresponding bulk materials because the grains in coatings are typically smaller than those in bulk materials. Table 1 summarizes the hardness and modulus of common MAX phases such as Cr2AlC, Ti2AlC, Ti3AlC2 and Ti3SiC2. Most MAX phase coatings exhibit a hardness of 10–20 GPa and a modulus of 200–300 GPa, which impart greater resistance to impact and wear, making them promising for applications as protective coatings.| Coatings | Hardness (GPa) | Modulus (GPa) | Ref. |
|---|---|---|---|
| Cr2AlC | 13.7 ± 0.5 | 196 ± 14 | Wear, 2018, 402–403, 187–195 |
| 19.0 ± 1.7 | 268 ± 16 | J. Alloys Compd., 2018, 753, 11–17 | |
| 13 ± 2 | 298 ± 21 | Scr. Mater., 2007, 57, 1137–1140 | |
| 16 ± 2 | — | Mater. Design, 2021, 206, 109757 | |
| 16.6 ± 0.5 | 261 ± 7 | Mater. Des., 2022, 222, 111060 | |
| 12.8 ± 0.4 | 334 ± 24 | J. Mater. Sci. Technol., 2023, 143, 140–152 | |
| 18.4 ± 0.8 | 328 ± 26 | Wear, 2024, 540–541, 205221 | |
| 15.4 | 288 | Surf. Coatings Technol., 2013, 235, 454–460 | |
| 15.1 ± 1.8 | 259 ± 20 | J. Nucl. Mater., 2019, 526, 151742 | |
| 15.3 ± 3.6 | 254 ± 22 | Intermetallics, 2023, 163, 108039 | |
| Ti2AlC | 11.6 ± 0.3 | — | Adv. Eng. Mater., 2022, 24, 1–11 |
| 11.2 ± 1.4 | 191 ± 26 | Wear, 2015, 342–343, 391–397 | |
| 17.2 ± 1.6 | 257 ± 12 | Surf. Coatings Technol., 2017, 309, 445–455 | |
| 15.8 ± 2.1 | 273 ± 20 | Surf. Coatings Technol., 2018, 334, 384–393 | |
| 17.9 ± 0.8 | 279 ± 4 | J. Eur. Ceram. Soc., 2023, 43, 4673–4683 | |
| 10.2 ± 0.5 | 221 ± 10 | Appl. Surf. Sci., 2021, 537, 147864 | |
| 16.9 ± 0.5 | 347 ± 16 | J. Mater. Sci. Technol., 2023, 143, 140–152 | |
| 14.2 ± 0.1 | 231 ± 3 | Ceram. Int., 2019, 45, 13912–13922 | |
| Ti3AlC2 | 3.2 ± 0.8 | 120 ± 21 | Appl. Surf. Sci., 2021, 537, 147864 |
| 5.3 ± 0.9 | — | Adv. Eng. Mater., 2022, 24, 1–11 | |
| 12.3 ± 1.1 | 232 ± 11 | J. Eur. Ceram. Soc., 2023, 43, 4673–4683 | |
| 19 ± 1 | 250 ± 9 | Appl. Phys. Lett., 2004, 85, 1066–1068 | |
| 20.7 ± 0.5 | 237 ± 4 | J. Eur. Ceram. Soc., 2022, 42, 2073–2083 | |
| 18.0 | 240 ± 4 | Mater. Charact., 2022, 194, 112421 | |
| Ti3SiC2 | 19 ± 1 | 282 ± 9 | J. Appl. Phys., 2004, 96, 4817–4826 |
| 11.1 ± 1.6 | 229 ± 4 | Mater. Sci. Technol., 2013, 29, 975–979 | |
Preparation parameters and methods are widely investigated to improve the mechanical properties of MAX phase coatings. Naveed et al.129 deposited Cr2AlC coatings with different sputtering powers, resulting in variations in the intermetallic AlCr2 and Cr7C3 carbide phases and the preferred orientation of the coating. Hardness values were measured in the range of 11–14 GPa, showing a slight increase with increasing sputtering powers, which was attributed to the preferred orientation. Du et al.130 studied the temperature effect on the bulk moduli of Cr2AlC within the quasiharmonic Debye model by first-principles GGA+U calculations, which revealed the origin of the deviation between the experimental and theoretical data.
Eichner et al.131 confirmed that the hardness of the Cr2AlC coating on softer IN718 substrates with a (100) preferred orientation was higher than the hardness measured in cross-sections. Yang et al.40 studied the elastic anisotropies, thermodynamic and tensile properties of Ti2AX (A = Al and Ga, X = C and N) MAX phases using first-principles calculations. The calculated anisotropy indexes indicated that the MAX phases were anisotropic in elastic modulus. Moreover, according to the tensile calculation, the tensile strength along the [110] direction is greater than that along the [001] direction, and fracture failure will occur in the [110] direction. Li et al.132 predicted the mechanical and thermodynamics of MAX phase V2SiC under different pressures based on DFT simulations. The results showed that elastic constants satisfied the Born stability criterion, ensuring mechanical stability under pressurized and unpressurized conditions. And the increase in U from 0.239 to 0.279 indicated that the compound changed from brittle to ductile, with the maximum ductility observed at 20 GPa. Liu et al.133 compared the mechanical properties of different phase compositions in Cr2AlC coatings obtained by varying the current of the Al target during the deposition process. When the Al content was maximized at an Al-target current of 1.0 A, the coating exhibited the highest hardness, elastic modulus, H/E, H3/E2 and toughness fracture. Increasing or decreasing the Al-target current led to a reduction in Cr2AlC content and an increase in intermetallic Al8Cr5, which deteriorated the hardness and fracture toughness of the coatings. This phenomenon demonstrates that reducing ductile intermetallic content in MAX phase coatings is in favor of their mechanical properties.
Rueß et al.134 investigated the mechanical properties of Cr2AlC prepared by DCMS and HiPIMS, respectively. Generally, the coating prepared by HiPIMS had superior density and larger elastic moduli compared to that prepared by DCMS, indicating that ion bombardment by ionized coating-forming species is beneficial. The findings demonstrated that there is an optimum ion energy for forming dense and high-purity MAX phase coatings. Too low energy results in the formation of under-dense coatings, while too high energy yields the formation of (Cr, Al)2Cx in addition to Cr2AlC. Similar observations were reported by Völker et al.135 and Qureshi et al.,71 where the hardness and modulus of Cr2AlC coatings prepared by HiPIMS were higher than those prepared by DCMS. The HiPIMS-deposited coating had the highest fracture toughness 2.0 ± 0.2 MPa m1/2, whereas the coating deposited by DCMS had the lowest value of 1.8 ± 0.1 MPa m1/2.135 This further explains the influence of preparation methods on the fracture toughness of Cr2AlC coatings. The equiaxed crystal coating prepared by HiPIMS has entangled grain boundaries and a longer crack propagation path compared to the columnar crystal coating deposited by DCMS, demonstrating better crack resistance.
Molina-Aldareguia et al.136 analyzed the deformation mechanisms in single-crystal Ti3SiC2 (0001) coatings by nanoindentation. Basal plane dislocation glide created dislocation walls and formed kink bands and delamination cracks at the free surface near indentations. Yuan et al.137 studied the mechanical properties of Cr2AlC coatings with a grain size of 88 nm by nanoindentation. An ultrahigh compressive strength of 5.3 GPa and plastic strain beyond 12.5% were achieved in the Cr2AlC coating according to micropillar compression tests (Fig. 15a). Several slip bands at 45° from the load direction were visible in the compressed micropillar without vertical cracks or delamination (Fig. 15b). TEM micrographs of the deformed region revealed the synchronous emergence of two dislocations under the diffraction vector g = 0001, demonstrating that these were not routine basal dislocations with Burger's vector b = 1/3〈11![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 0〉 (Fig. 15c and d). These non-basal dislocations form in nanograins under complex stress states, thereby increasing the forms of dislocation slip and ultimately enhancing the plasticity deformation ability. Additionally, deformation twins were observed in the severely-deformed region (Fig. 15e). SAED patterns (Fig. 15f) displayed close but distinctly separate spots (10
0〉 (Fig. 15c and d). These non-basal dislocations form in nanograins under complex stress states, thereby increasing the forms of dislocation slip and ultimately enhancing the plasticity deformation ability. Additionally, deformation twins were observed in the severely-deformed region (Fig. 15e). SAED patterns (Fig. 15f) displayed close but distinctly separate spots (10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) ) shared by the matrix and twin, confirming the presence of an incoherent (10
) shared by the matrix and twin, confirming the presence of an incoherent (10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) ) twin boundary. These twins, which withstand high shear stress during deformation, favor the enhancement of both strength and plasticity.
) twin boundary. These twins, which withstand high shear stress during deformation, favor the enhancement of both strength and plasticity.
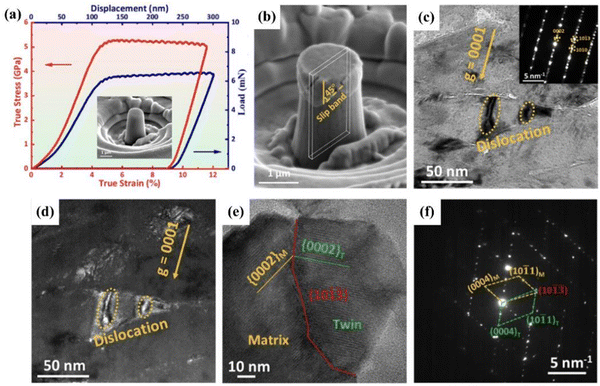 | ||
| Fig. 15 Micropillar compression test of Cr2AlC coatings: (a) load–displacement and true stress–strain curves of the Cr2AlC coating micropillar. (b) SEM image of the micropillar after compression. (c) Bright-field TEM micrograph of a deformed grain under the diffraction vector g = 0001. (d) Weak-team dark-field micrograph of (c). (e) HRTEM micrograph of the twins in the deformed region. (f) SAED patterns in (e). Reproduced with permission.137 Copyright 2023 Acta Materialia Inc, Elsevier Ltd. | ||
For MAX phase coatings prepared by the deposition-annealing two-step method, the annealing process plays a crucial role in determining the mechanical properties of the coatings. In general, the preparation requires a specific annealing temperature range. As the annealing temperature increases, the hardness of the coating typically increases because as-deposited coatings are often amorphous or partially crystalline with low hardness, and heating transforms this disordered structure into densely arranged hexagonal layered MAX phases.138–140 However, further increases in annealing temperature can led to either a decrease or an increase in hardness. The former is due to grain growth at elevated temperature, while the latter is associated with phase transitions, such as the formation of the hard TiC phase resulting from the decomposition of Ti3AlC2 and Ti2AlC coatings above 900 °C.59,141
Zhang et al.142 found that the porosity of Ti3SiC2 coatings significantly declined with the increasing annealing temperature at 500–900 °C, contributing to gradually improved hardness and fracture toughness of the coating. Wang et al.143 investigated the annealing effect on the helium-irradiated Cr2AlC coating. As the annealing temperature increased, the gradual recovery in the hardness of the MAX phase coating was observed due to the recombination of defects, suggesting that appropriately raising the annealing temperature could enhance the mechanical properties of MAX phase coatings by eliminating defects.
Moreover, alloying to form solid solution is an effective method to improve the mechanical properties of MAX phase coatings.144–146 Cr2AlC MAX phase coatings typically suffer from low hardness and toughness as well as a lack of lubrication at high temperature. Wang et al.147 demonstrated that the hardness of the Cr2AlC MAX phase coating was enhanced by 34.3% when Cr was partially substituted with V (47 at%), and the coating toughness was also improved (Fig. 16a). Simultaneously, both the friction coefficient and the wear rate of the coatings at 900 °C against Al2O3 balls were significantly reduced at 47 at% V (Fig. 16b), which was attributed to the formation of a large number of molten V2O5 that encapsulated (Cr, Al)2O3 hard crystal grains (Fig. 16c).
 | ||
| Fig. 16 Characterization of (Cr1−xVx)2AlC coatings: (a) hardness, elastic modulus, H/E and H3/E2 of the coatings obtained by nanoindentation. (b) COF and wear rate. (c) Schematic diagram of the friction mechanism. Reproduced with permission.147 Copyright 2022 Elsevier Ltd. | ||
4. New functional protection performance of MAX phase coatings
4.1. Protection on metal bipolar plates
MAX phase materials, with their unique crystal structure, combine the excellent electrical and thermal conductivity of metals and the high-temperature oxidation resistance and corrosion resistance of ceramics. This makes them an ideal protective coating material for metal bipolar plates in proton exchange membrane fuel cells (PEMFCs).148 Abbas et al.64 applied Ti2AlC MAX phase coating to metal bipolar plates for testing. Due to the formation of the Ti2AlC MAX phase, the coating exhibited excellent electrical conductivity, with an interfacial contact resistance (ICR) of only 3.27 mΩ cm2, much lower than the United States Department of Energy (DOE) standard.149 Lu et al.58 studied the conductive and corrosion-resistant protective performance of Ti3AlC2 MAX phase coating on SS304 with a TiC diffusion layer in H2SO4 solution (pH = 3) containing 2 ppm F− at 70 °C in simulated PEMFC environments. The ICR of the Ti3AlC2-coated SS304 was only 3.725 mΩ cm2 at 140 N cm−2. The outstanding electrical conductivity was attributed to the presence of an easier pathway for the transport of electrons via Ti3AlC2 channels. In the potentiostatic polarization test simulating PEMFC environments, the average current densities at 0.6 V (vs. SCE) and −0.1 V (vs. SCE) were 8.0 × 10−7 A cm−2 and 5.0 × 10−8 A cm−2, respectively, demonstrating the coating's excellent corrosion resistance and durability. Moreover, Lu, et al.57 also studied the protection performance of Ti3SiC2 on metal bipolar plates.Ma et al.77 studied the conductivity and corrosion resistance of MAX phase coatings with different degrees of crystallization. The as-deposited samples showed the best corrosion resistance, with a stable potentiostatic polarization current density of 3.7 × 10−7 A cm−2, much lower than the corrosion current density of the SS316L substrate. This improvement was attributed to the preferential formation of the passivation film on the surface of amorphous coatings. The strength of corrosion resistance is ascribed to the differences in passivation films, which can be traced back to the differences in the stacking of atoms with different orientations in the crystal. Subsequently, they fabricated Cr2AlC coatings with different crystallographic orientations on SS316L.79 The Cr2AlC coating with a (103) preferred orientation exhibited the best corrosion resistance, with an average current density of 9.0 × 10−3 μA cm−2, because the exposed Cr atom in coatings with (103) preferred orientation had the lowest binding energy and was more likely to preferentially form a passivation film by binding with oxygen. Liu et al.150 also researched the conductivity and corrosion resistance of Cr2AlC MAX phase coating, finding that the corrosion current of the coated sample was 2.43 × 10−7 A cm−2, two orders of magnitude lower than that of the uncoated SS304.
4.2. Radiation resistance property
MAX phases have emerged as a promising structural material for components requiring high-temperature durability and exposure to extreme radiation environments in upcoming nuclear reactors.151–155 Neutron or ion radiation exposure can induce the accumulation of Frenkel defects, such as voids and interstitial atoms, due to displacement cascades within materials.154,156 Assessing the radiation resilience of MAX phases is essential to identify the most suitable MAX phases for this domain.157–159 Bowden et al.160 assessed the radiation resilience of MAX phase coatings of Zr3AlC2, Nb4AlC3, and (Zr0.5, Ti0.5)3AlC2 by proton irradiation followed by post-irradiation examination based primarily on X-ray diffraction analysis. The findings indicated that Zr-based 312-MAX phase, Zr3AlC2 and (Zr0.5, Ti0.5)3AlC2, exhibited remarkable defect recovery capabilities above 400 °C, whereas Nb4AlC3 showed no significant defect recovery below 600 °C. DFT calculations suggest that the structural distinctions between the 312 and 413 MAX phases govern the variations in radiation resistance observed in these materials. Similarly, Dai et al.161 and Duan et al.162 investigated the point defect and mono-vacancy defective in the MAX phase Cr2AlC coating using first-principles calculations, which suggested the excellent anti-radiation performance in nuclear applications.4.3. Electromagnetic shielding performance
In recent years, there has been significant focus on the MAX phase coatings as exceptional electromagnetic interference (EMI) shielding materials due to their high conductivity, remarkable thermal conductivity, resistance to high temperatures and oxidation resistance.163–165 The superior EMI shielding capabilities of MAX phases stem from their high absorption and reflection of EM waves. Huang and co-authors166 studied the EMI shielding performance of MAX@ carbonised wood (CW) composites (Ti2AlC@CW, V2AlC@CW and Cr2AlC@CW) with a porous structure. The MAX@CW composite materials preserve the porous structure characteristics of CW templates, while the CW template facilitates the oriented assembly of the MAX phase by filling the porous structure of CW, creating multiple interfaces. These distinctive structures establish a pathway for terahertz wave dissipation in porous network structures, leading to a notable rise in transmission loss. Consequently, MAX@CW composite materials display significantly higher shielding effectiveness than CW.4.4. MAX phase as a bond coating for thermal barrier coatings
In order to prevent the interdiffusion of elements at the substrate alloy/coating interface, MAX phase coatings are also used as intermediate or diffusion barriers in some composites.167,168 For example, Li et al.105,169 assesses the performance of Cr2AlC coatings as a thin transition layer between the NiCrAlY coating and alloy substrate at high temperature. It was observed that the Al in Cr2AlC could diffuse outwardly to the substrate alloy and NiCrAlY coating, which strengthened the metallurgical bonds at the interfaces between the substrate alloy and Cr2AlC layer, as well as between the Cr2AlC layer and NiCrAlY coating. However, due to the rapid outward diffusion of Al, the Al content in Cr2AlC steadily decreased, leading to the phase transformation of Cr2AlC to dense Cr–C compounds (Cr7C3, Cr23C6) on both sides of the transition layer. These Cr–C compounds are typical ceramic candidates for diffusion barriers. Moreover, Mourad et al.170 demonstrated the thermodynamic, dynamic and mechanical stability of Hf2GeX (X = C, N, and B) 211 MAX phases through a first-principles investigation, which displayed that they were likely to have thermal barrier coating applications.5. Summary and outlook
To summarize, this report reviews the development, chemical compositions, and microstructures of MAX phase coatings, as well as various synthesis techniques such as PVD, CVD, spraying methods, and laser cladding. It also surveys the protective performances of MAX phase coatings and explores their potential functional applications. Thanks to the dedicated efforts of material researchers, significant progress has been made in understanding and characterizing the microstructures and properties of MAX phases, discovering new coatings and identifying potential applications. However, there are still some issues which need to be solved or more deeply investigated.To date, over 340 types of MAX phase materials have been synthesized, with most research focusing on bulk materials and powder preparation. Developing a wider range of MAX phase coating systems, including MAX phase solid solutions, is crucial for expanding the research and functional applications of MAX phase materials. Although many MAX phases have been theoretically predicted to be thermodynamically and mechanically stable, their experimental realization remains challenging due to immature synthesis techniques. Achieving high-purity MAX phases is particularly difficult, complicating accurate property characterization. Consequently, improving the purity of MAX phase coatings using existing synthesis methods, as well as developing novel synthesis techniques, remains a key priority. Moreover, further research is needed to elucidate the microstructure–property relationships of MAX phases. For instance, the stability of MAX phases with atomic vacancies requires deeper investigation, as it can provide valuable insight into their application as high temperature oxidation resistance and self-healing materials. Additionally, a wide range of chemical and physical properties of the newly developed MAX phase coatings await further characterization. In particular, the magnetic properties of MAX phases and their solid solutions demand attention, including the effects of atomic configuration, alloy composition, temperature, magnetic fields, and applied pressure. Finally, developing commercially viable applications for MAX phase materials is an urgent priority, as successful market penetration could significantly stimulate further research and development. The potential applications for this family of layered metallic ceramics are vast, and their commercialization could revolutionize multiple industries.
Author contributions
Guanshui Ma: conceptualization, investigation, writing – review & editing, project administration, and funding acquisition. Anfeng Zhang: investigation and writing – original draft. Zhenyu Wang: resources, writing – review & editing, project administration, and funding acquisition. Kaihang Wang: investigation and writing – original draft. Jiayue Zhang: investigation and writing – original draft. Kaixuan Xu: investigation and writing – original draft. Yuxi Xu: investigation and writing – review & editing. Shenghao Zhou: investigation and writing – original draft. Aiying Wang: resources, writing – review & editing, project administration, and funding acquisition.Data availability
All data sets and research materials used in this review are cited appropriately and can be accessed through the respective publishers or repositories. For any additional information or specific inquiries about the data used in this review, please contact the corresponding author. Data will be shared in compliance with the relevant institutional and ethical guidelines, ensuring they are used solely for academic and research purposes.Conflicts of interest
The authors declare no competing interests.Acknowledgements
This work was supported by the financial support from the National Science Fund for Distinguished Young Scholars of China (52025014), the National Natural Science Foundation of China (52171090, 52101109), the Zhejiang Provincial Natural Science Foundation of China (LD24E010003), and the Natural Science Foundation of Ningbo (2023QL049, 2023J410). We are also very grateful to Yingjie Wang, Yan Zhang, and Kaiwei Yang for their help in writing this manuscript.References
- V. H. Nowotny, Solid State Chem., 1971, 5, 27–70 CrossRef.
- M. W. Barsoum and T. El-Raghy, J. Am. Ceram. Soc., 1996, 79, 1953–1956 CrossRef CAS.
- J. C. Schuster, H. Nowotny and C. Vaccaro, J. Solid State Chem., 1980, 32, 213–219 CrossRef CAS.
- M. W. Barsoum, Solid State Chem., 2000, 28, 201–281 CrossRef CAS.
- M. Naguib, M. Kurtoglu, V. Presser, J. Lu, J. Niu, M. Heon, L. Hultman, Y. Gogotsi and M. W. Barsoum, Adv. Mater., 2011, 23, 4248–4253 CrossRef CAS PubMed.
- W. Eom, H. Shin, W. Jeong, R. B. Ambade, H. Lee and T. H. Han, Mater. Horiz., 2023, 10, 4892–4902 RSC.
- W. T. Cao, W. Feng, Y. Y. Jiang, C. Ma, Z. F. Zhou, M.-G. Ma, Y. Chen and F. Chen, Mater. Horiz., 2019, 6, 1057–1065 RSC.
- N. Liu, J. Yuan, X. Zhang, Y. Ren, F. Yu and J. Ma, Mater. Horiz., 2024, 11, 1223–1233 RSC.
- L. Ma, L. F. Wei, M. Hamidinejad and C. B. Park, Mater. Horiz., 2023, 10, 4423–4437 RSC.
- S. Zhang, X. Xu, X. Liu, Q. Yang, N. Shang, X. Zhao, X. Zang, C. Wang, Z. Wang, J. G. Shapter and Y. Yamauchi, Mater. Horiz., 2022, 9, 1708–1716 RSC.
- Q. Z. Tao, M. Dahlqvist, J. Lu, S. Kota, R. Meshkian, J. Halim, J. Palisaitis, L. Hultman, M. W. Barsoum, P. O. Å. Persson and J. Rosen, Nat. Commun., 2017, 8, 14949 CrossRef.
- L. Chen, M. Dahlqvist, T. Lapauw, B. Tunca, F. Wang, J. Lu, R. Meshkian, K. Lambrinou, B. Blanpain, J. Vleugels and J. Rosen, Inorg. Chem., 2018, 57, 6237–6244 CrossRef CAS.
- X. Feng, N. Li, B. Chen, C. Zeng, T. Bai, K. Wu, Y. Cheng and B. Xiao, J. Mater. Sci. Technol., 2023, 134, 81–88 CrossRef CAS.
- M. Ade and H. Hillebrecht, Inorg. Chem., 2015, 54, 6122–6135 CrossRef CAS.
- J. Wang, T. N. Ye, Y. Gong, J. Wu, N. Miao, T. Tada and H. Hosono, Nat. Commun., 2019, 10, 2284 CrossRef.
- T. Rackl, L. Eisenburger, R. Niklaus and D. Johrendt, Phys. Rev. Mater., 2019, 3, 054001 CrossRef CAS.
- M. Li, J. Lu, K. Luo, Y. Li, K. Chang, K. Chen, J. Zhou, J. Rosen, L. Hultman, P. Eklund, P. O. Å. Persson, S. Du, Z. Chai, Z. Huang and Q. Huang, J. Am. Chem. Soc., 2019, 141, 4730–4737 CrossRef CAS PubMed.
- H. M. Ding, Y. Li, J. Lu, K. Luo, K. Chen, M. Li, P. O. Å. Persson, L. Hultman, P. Eklund, S. Du, Z. Huang, Z. Chai, H. Wang, P. Huang and Q. Huang, Mater. Res. Lett., 2019, 7, 510–516 CrossRef CAS.
- M. Griseri, B. Tunca, S. G. Huang, M. Dahlqvist, J. Rosén, J. Lu, P. O. Å. Persson, L. Popescu, J. Vleugels and K. Lambrinou, Science, 2020, 40, 1829–1838 CAS.
- P. P. Michalowski, M. Anayee, T. S. Mathis, S. Kozdra, A. Wojcik, K. Hantanasirisakul, I. Jozwik, A. Piatkowska, M. Mozdzonek, A. Malinowska, R. Diduszko, E. Wierzbicka and Y. Gogotsi, Nat. Nanotechnol., 2022, 17, 1192–1197 CrossRef CAS.
- Q. Wang, L. Velasco, B. Breitung and V. Presser, Adv. Energy Mater., 2021, 11, 2102355 CrossRef CAS.
- J. Zhou, Q. Tao, B. Ahmed, J. Palisaitis, I. Persson, J. Halim, M. W. Barsoum, P. O. Å. Persson and J. Rosen, Chem. Mater., 2022, 34, 2098–2106 CrossRef CAS.
- J. B. Shen, M. Zhang, S. Lin, W. Song, H. Liu, Q. Liu, X. Zhu and Y. Sun, J. Appl. Phys., 2023, 133, 235101 CrossRef CAS.
- S. Schweidler, M. Botros, F. Strauss, Q. Wang, Y. Ma, L. Velasco, G. Cadilha Marques, A. Sarkar, C. Kübel, H. Hahn, J. Aghassi-Hagmann, T. Brezesinski and B. Breitung, Nat. Rev. Mater., 2024, 9, 266–281 CrossRef.
- Z. Wu, X. Zhu, Y. Shen, X. Zong, Y. Wu, Q. Wang, J. Tang, Z. Wang, H. Zhu, X. Yuan, Z. Zhou, X. Liu, X. Zhang, H. Wang, S. Jiang, M. J. Kim and Z. Lu, Mater. Today, 2024, 80, 61–73 CrossRef CAS.
- C. Liu, Y. Y. Yang, Z. F. Zhou, C. W. Nan and Y. H. Lin, J. Am. Ceram. Soc., 2021, 105, 2764–2771 CrossRef.
- L. Qiao, J. Bi, G. Liang, C. Liu, Z. Yin, Y. Yang, H. Wang, S. Wang, M. Shang and W. Wang, J. Mater. Sci. Technol., 2023, 137, 112–122 CrossRef CAS.
- G. He, Y. Zhang, P. Yao, X. Li, K. Ma, J. Zuo, M. Li, C. Liu and J. Xu, J. Mater. Sci. Technol., 2023, 137, 91–99 CrossRef CAS.
- W. Bao, X. G. Wang, H. Ding, P. Lu, C. Zhu, G. J. Zhang and F. Xu, Scr. Mater., 2020, 183, 33–38 CrossRef CAS.
- H. M. Ding, Y. B. Li, K. Chen, K. Liang, G. X. Chen, J. Lu, J. Palisaitis, P. O. Å. Persson, P. Eklund, L. Hultman, S. Du, Z. F. Chai, Y. Gogotsi and Q. Huang, Science, 2023, 379, 1130–1135 CrossRef CAS.
- M. Dahlqvist, M. W. Barsoum and J. Rosen, Mater. Today, 2024, 72, 1–24 CrossRef.
- Z. Zhang, X. Duan, D. Jia, Y. Zhou and S. Zwaag, J. Eur. Ceram. Soc., 2021, 41, 3851–3878 CrossRef CAS.
- N. Goossens, B. Tunca, T. Lapauw, K. Lambrinou and J. Vleugels, Encyclopedia of Materials: Technical Ceramics and Glasses, 2021, vol. 2, pp. 182–199 Search PubMed.
- Z. M. Sun, Int. Mater. Rev., 2013, 56, 143–166 CrossRef.
- L. Fu and W. Xia, Adv. Eng. Mater., 2021, 23, 2001191 CrossRef CAS.
- Z. Lin, M. Zhuo, Y. Zhou, M. Li and J. Wang, J. Am. Ceram. Soc., 2006, 89, 3765–3769 CrossRef CAS.
- J. P. Palmquist, S. Li, P. O. Å. Persson, J. Emmerlich, O. Wilhelmsson, H. Högberg, M. I. Katsnelson, B. Johansson, R. Ahuja, O. Eriksson, L. Hultman and U. Jansson, Phys. Rev. B: Condens. Matter Mater. Phys., 2004, 70, 165401 CrossRef.
- M. Uddin, Md., M. A. Ali and M. S. Ali, Structural, elastic, electronic and optical properties of metastable MAX phase Ti5SiC4 compound, Indian J. Pure Appl. Phys., 2016, 54, 386–390 Search PubMed.
- J. Lyu, E. B. Kashkarov, N. Travitzky, M. S. Syrtanov and A. M. Lider, J. Mater. Sci., 2020, 56, 1980–2015 CrossRef.
- M. J. Peng, Y. C. Guo, A. C. Yang, Y. H. Duan, H. M. Yang, Y. J. Wu and M. N. Li, Indian J. Phys., 2024, 98, 3165–3177 CrossRef CAS.
- Y. Bai, N. Srikanth, C. K. Chua and K. Zhou, Crit. Rev. Solid State Mater. Sci., 2017, 44, 56–107 CrossRef.
- E. Y. Andrei and A. H. MacDonald, Nat. Mater., 2021, 20, 571 CrossRef CAS PubMed.
- W. B. Yu, W. Z. Jia, F. Guo, Z. Y. Ma, P. C. Zhang, C. Tromas, V. Gauthier-Brunet, P. R. C. Kent, W. W. Sun and S. Dubois, J. Eur. Ceram. Soc., 2020, 40, 2279–2286 CrossRef CAS.
- A. Shamsipoor, M. Farvizi, M. Razavi, A. Keyvani, B. Mousavi and W. Pan, Ceram. Int., 2021, 47, 2347–2357 CrossRef CAS.
- C. X. Wang, T. F. Yang, C. L. Tracy, J. R. Xiao, S. S. Liu, Y. Fang, Z. F. Yan, W. Ge, J. M. Xue, J. Zhang, J. Y. Wang, Q. Huang, R. C. Ewing and Y. G. Wang, Acta Mater., 2018, 144, 432–446 CrossRef CAS.
- C. Wang, T. Yang, J. Xiao, S. Liu, J. Xue, Q. Huang, J. Zhang, J. Wang, Y. Wang and R. Koc, J. Am. Ceram. Soc., 2016, 99, 1769–1777 CrossRef CAS.
- D. W. Clark, S. J. Zinkle, M. K. Patel and C. M. Parish, Acta Mater., 2016, 105, 130–146 CrossRef CAS.
- C. Wang, T. Yang, J. Xiao, S. Liu, J. Xue, J. Wang, Q. Huang and Y. Wang, Acta Mater., 2015, 98, 197–205 CrossRef CAS.
- Z. Y. Wang, G. S. Ma, Z. Li, H. Ruan, J. H. Yuan, L. Wang, P. L. Ke and A. Y. Wang, Corros. Sci., 2021, 192, 109788 CrossRef CAS.
- C. Guo, X. Duan, Z. Fang, Y. Zhao, T. Yang, E. H. Wang and X. M. Hou, Acta Mater., 2022, 241, 118378 CrossRef CAS.
- W. B. Tian, P. L. Wang, G. J. Zhang, Y. M. Kan and Y. X. Li, J. Am. Ceram. Soc., 2007, 90, 1663–1666 CrossRef CAS.
- M. Rougab and A. Gueddouh, Comput. Theor. Chem., 2024, 1233, 114497 CrossRef CAS.
- L. O. Xiao, S. B. Li, G. Song and W. G. Sloof, J. Eur. Ceram. Soc., 2011, 31, 1497–1502 CrossRef CAS.
- D. J. Tallman, E. N. Hoffman, E. A. N. Caspi, B. L. Garcia-Diaz, G. Kohse, R. L. Sindelar and M. W. Barsoum, Acta Mater., 2015, 85, 132–143 CrossRef CAS.
- A. Biswas, V. Natu and A. B. Puthirath, Oxford Open Mater. Sci., 2021, 1, 1–11 Search PubMed.
- C. Wang, T. Yang, S. Kong, J. Xiao, J. Xue, Q. Wang, C. Hu, Q. Huang and Y. Wang, J. Nucl. Mater., 2013, 440, 606–611 CrossRef CAS.
- J. L. Lu, N. Abbas, J. Tang, R. Hu and G. M. Zhu, Electrochem. Commun., 2019, 105, 106490 CrossRef CAS.
- J. L. Lu, N. Abbas, J. N. Tang, J. Tang and G. M. Zhu, Corros. Sci., 2019, 158, 108106 CrossRef CAS.
- J. H. Yuan, Z. Y. Wang, G. S. Ma, X. J. Bai, Y. Li, X. Cheng, P. L. Ke and A. Y. Wang, J. Mater. Sci. Technol., 2023, 143, 140–152 CrossRef CAS.
- Q. M. Wang, R. Mykhaylonka, A. Flores Renteria, J. L. Zhang, C. Leyens and K. H. Kim, Corros. Sci., 2010, 52, 3793–3802 CrossRef CAS.
- Q. M. Wang, A. Flores Renteria, O. Schroeter, R. Mykhaylonka, C. Leyens, W. Garkas and M. to Baben, Surf. Coat. Technol., 2010, 204, 2343–2352 CrossRef CAS.
- R. Grieseler, B. Hähnlein, M. Stubenrauch, T. Kups, M. Wilke, M. Hopfeld, J. Pezoldt and P. Schaaf, Appl. Surf. Sci., 2014, 292, 997–1001 CrossRef CAS.
- Y. Li, G. Zhao, Y. Qian, J. Xu and M. Li, Vacuum, 2018, 153, 62–69 CrossRef CAS.
- N. Abbas, X. Qin, S. Ali, G. Zhu, J. Lu, F. E. Alam, A. G. Wattoo, X. Zeng, K. Gu and J. Tang, J. Eur. Ceram. Soc., 2020, 40, 3338–3342 CrossRef CAS.
- V. Stranak, J. Kratochvil, J. Olejnicek, P. Ksirova, P. Sezemsky, M. Cada and Z. Hubicka, J. Appl. Phys., 2017, 121, 171914 CrossRef.
- X. Zuo, D. Zhang, R. D. Chen, P. L. Ke, M. Odén and A. Y. Wang, Plasma Sources Sci. Technol., 2020, 29, 015013 CrossRef CAS.
- Z. C. Li, G. X. Zhou, Z. Y. Wang, J. H. Yuan, P. L. Ke and A. Y. Wang, J. Eur. Ceram. Soc., 2023, 43, 4673–4683 CrossRef CAS.
- T. F. Zhang, Q. M. Wang, J. Lee, P. Ke, R. Nowak and K. H. Kim, Surf. Coat. Technol., 2012, 212, 199–206 CrossRef CAS.
- G. X. Zhou, Z. Li, J. H. Yuan, R. D. Chen, Z. Y. Wang, P. L. Ke and A. Y. Wang, Appl. Surf. Sci., 2024, 666, 160371 CrossRef CAS.
- D. W. Zhou, Z. Z. Li, Z. Y. Wang, G. S. Ma, P. L. Ke, X. J. Hu and A. Y. Wang, China Surf. Eng., 2022, 35, 236–245 Search PubMed.
- M. W. Qureshi, X. Ma, G. Tang, B. Miao and J. Niu, Materials, 2021, 14, 826 CrossRef CAS PubMed.
- D. M. Sanders and A. Anders, Surf. Coat. Technol., 2000, 133, 78–90 CrossRef.
- J. Rosén, L. Ryves, P. O. Å. Persson and M. M. M. Bilek, J. Appl. Phys., 2007, 101, 056101 CrossRef.
- H. Li, H. S. Cao, F. J. Liu, Y. H. Li, F. G. Qi, X. P. Ouyang and N. Zhao, J. Eur. Ceram. Soc., 2022, 42, 2073–2083 CrossRef CAS.
- M. C. Guenette, M. D. Tucker, M. Ionescu, M. M. M. Bilek and D. R. McKenzie, Thin Solid Films, 2010, 519, 766–769 CrossRef CAS.
- Z. Mahmoudi, S. H. Tabaian, H. R. Rezaie, F. Mahboubi and M. J. Ghazali, Ceram. Int., 2020, 46, 4968–4975 CrossRef CAS.
- G. S. Ma, J. H. Yuan, R. D. Chen, H. Li, H. C. Wu, J. S. Yan, Z. Y. Wang and A. Y. Wang, Appl. Surf. Sci., 2022, 597, 153670 CrossRef CAS.
- G. S. Ma, D. Zhang, P. Guo, H. Li, Y. Xin, Z. Y. Wang and A. Y. Wang, J. Mater. Sci. Technol., 2022, 105, 36–44 CrossRef CAS.
- Z. Y. Wang, G. S. Ma, L. Liu, L. Wang, P. L. Ke, Q. Xue and A. Y. Wang, Corros. Sci., 2020, 167, 108492 CrossRef CAS.
- Z. Y. Wang, W. Li, Y. Liu, J. T. Shuai, P. L. Ke and A. Y. Wang, Appl. Surf. Sci., 2020, 502, 144130 CrossRef CAS.
- A. R. Phani, J. E. Krzanowski and J. J. Nainaparampil, J. Vac. Sci. Technol., A, 2001, 19, 2252–2258 CrossRef CAS.
- J. J. Hua, J. E. Bultman, S. Patton and J. S. Zabinski, Tribol. Lett., 2004, 16, 113–122 CrossRef.
- C. Lange, M. Hopfeld, M. Wilke, J. Schawohl, T. Kups, M. W. Barsoum and P. Schaaf, Phys. Status Solidi A, 2011, 209, 545–552 CrossRef.
- Z. J. Feng, P. L. Ke and A. Y. Wang, J. Mater. Sci. Technol., 2015, 31, 1193–1197 CrossRef CAS.
- G. S. Ma, H. C. Wu, Z. Fang, X. H. Zhou, R. D. Chen, W. Yang, J. Zhang, Z. Y. Wang and A. Y. Wang, J. Mater. Sci. Technol., 2025, 206, 176–184 CrossRef CAS.
- M. Bahiraei, Y. Mazaheri, M. Sheikhi and A. Heidarpour, Surf. Coat. Technol., 2020, 385, 125314 CrossRef CAS.
- J. J. Nickl, K. K. Schweitzer and P. Luxenberg, J. Less-Common Met., 1972, 26(3), 335–353 CrossRef CAS.
- C. Racautl, F. Langlais and R. Naslain, J. Mater. Sci. Technol., 1994, 29, 3941–3948 CrossRef.
- E. Pickering, W. J. Lackey and S. Crain, Chem. Vap. Deposition, 2000, 6, 289–295 CrossRef CAS.
- V. K. Champagne, Handbook the Cold Spray Materials Deposition Process, Woodhead Publishing and Maney Publishing on behalf of The Institute of Materials, Minerals & Mining, 2007 Search PubMed.
- X. Lei and N. M. Lin, Crit. Rev. Solid State Mater. Sci., 2022, 47, 736–771 CrossRef CAS.
- J. Frodelius, M. Sonestedt, S. Björklund, J. P. Palmquist, K. Stiller, H. Högberg and L. Hultman, Surf. Coat. Technol., 2008, 202, 5976–5981 CrossRef CAS.
- H. Gutzmann, F. Gärtner, D. Höche, C. Blawert and T. Klassen, J. Therm. Spray Technol., 2012, 22, 406–412 CrossRef.
- B. R. Maier, B. L. Garcia-Diaz, B. Hauch, L. C. Olson, R. L. Sindelar and K. Sridharan, J. Nucl. Mater., 2015, 466, 712–717 CrossRef CAS.
- H. K. H. Assadi, F. Gärtner and T. Klassen, Acta Mater., 2016, 116, 382–407 CrossRef CAS.
- W. Zhang, S. Li, X. Zhang and X. Chen, Coatings, 2023, 13, 869 CrossRef CAS.
- P. Richardson, D. Cuskelly, M. Brandt and E. Kisi, Surf. Coat. Technol., 2020, 385, 125360 CrossRef CAS.
- Y. X. Tian, H. Q. Xiao, L. R. Ren, J. Y. Feng, Y. Xiao, N. Chen and X. Zhou, Surf. Coat. Technol., 2022, 448, 128944 CrossRef CAS.
- T. Galvin, N. C. Hyatt, W. M. Rainforth, I. M. Reaney and D. Shepherd, Surf. Coat. Technol., 2019, 366, 199–203 CrossRef CAS.
- E. I. Zamulaeva, E. A. Levashov, T. A. Sviridova, N. V. Shvyndina and M. I. Petrzhik, Surf. Coat. Technol., 2013, 235, 454–460 CrossRef CAS.
- M. Haftani, M. Saeedi Heydari, H. R. Baharvandi and N. Ehsani, Int. J. Refract. Met. Hard Mater., 2016, 61, 51–60 CrossRef CAS.
- Z. Zhang, D. M. Y. Lai, S. H. Lim, H. Jin, S. Wang, J. Chai and J. Pan, J. Alloys Compd., 2019, 790, 536–546 CrossRef CAS.
- N. Laska, R. Swadźba, P. Nellessen, O. Helle and R. Anton, Surf. Coat. Technol., 2024, 480, 130601 CrossRef CAS.
- Y. Xiao, H. Xiao, T. Mo, L. Ren, Y. Tian and L. Zhu, Ceram. Int., 2023, 49, 38672–38682 CrossRef CAS.
- Y. Li, J. J. Xu, J. J. Li, Y. M. Li, K. Ma, W. T. Wang, X. T. Zhang, Y. Zhang and M. S. Li, Corros. Sci., 2023, 222, 111416 CrossRef CAS.
- L. Mengis, C. Oskay, N. Laska and M. C. Galetz, Intermetallics, 2023, 163, 108039 CrossRef CAS.
- J. J. Li, M. S. Li, H. M. Xiang, X. P. Lu and Y. C. Zhou, Corros. Sci., 2011, 53, 3813–3820 CrossRef CAS.
- J. Li, H. Xiang, J. Xu, L. Zheng and Y. Qian, Surf. Coat. Technol., 2021, 422, 127554 CrossRef CAS.
- X. Chen, B. Stelzer, M. Hans, R. Iskandar, J. Mayer and J. M. Schneider, Mater. Res. Lett., 2020, 9, 127–133 CrossRef.
- C. Tang, M. Große, S. Ulrich, M. Klimenkov, U. Jäntsch, H. J. Seifert, M. Stüber and M. Steinbrück, Surf. Coat. Technol., 2021, 419, 127263 CrossRef CAS.
- Z. C. Li, Z. Y. Wang, G. S. Ma, R. D. Chen, W. Yang, K. H. Wang, P. L. Ke and A. Y. Wang, Corros. Commun., 2024, 13, 27–36 CrossRef.
- Z. J. Feng, P. L. Ke, Q. Huang and A. Y. Wang, Surf. Coat. Technol., 2015, 272, 380–386 CrossRef CAS.
- W. T. Li, Z. Y. Wang, J. T. Shuai, B. B. Xu, A. Y. Wang and P. L. Ke, Ceram. Int., 2019, 45, 13912–13922 CrossRef CAS.
- A. Hesnawi, H. Li, Z. Zhou, S. Gong and H. Xu, Vacuum, 2007, 81, 947–952 CrossRef CAS.
- Z. Y. Wang, J. Sun, B. B. Xu, Y. R. Liu, P. L. Ke and A. Y. Wang, J. Eur. Ceram. Soc., 2020, 40, 197–201 CrossRef CAS.
- A. S. Farle, C. Kwakernaak, S. Zwaag and W. G. Sloof, J. Eur. Ceram. Soc., 2015, 35, 37–45 CrossRef CAS.
- J. Wang, X. Wang, Y. Zhang, G. Bai, J. Zhang and Y. Lei, J. Inorg. Mater., 2021, 36, 1097–1102 CrossRef.
- D. J. Tallman, B. Anasori and M. W. Barsoum, Mater. Res. Lett., 2013, 1, 115–125 CrossRef CAS.
- L. Guo, Y. Li and G. Li, J. Adv. Ceram., 2023, 12, 1712–1730 CrossRef CAS.
- J. Fu, T. F. Zhang, Q. Xia, S. H. Lim, Z. Wan, T. W. Lee and K. H. Kim, J. Nanomater., 2015, 2015, 1–12 Search PubMed.
- X. Li, S. Wang, G. Wu, D. Zhou, J. Pu, M. Yu, Q. Wang and Q. Sun, Ceram. Int., 2022, 48, 26618–26628 CrossRef CAS.
- Z. C. Li, Y. Zhang, K. H. Wang, Z. Y. Wang, G. S. Ma, P. L. Ke and A. Y. Wang, Corros. Sci., 2024, 228, 111820 CrossRef CAS.
- M. Zhu, R. Wang, C. Chen, H. B. Zhang and G. J. Zhang, Ceram. Int., 2017, 43, 5708–5714 CrossRef CAS.
- Y. Zhang, A. F. Zhang, Z. C. Li, Z. Y. Wang, P. L. Ke and A. Y. Wang, J. Phys. Chem. C, 2024, 128, 3916–3923 CrossRef CAS.
- Z. Zhang, Y. Qian, J. Xu, J. Zuo and M. Li, Corros. Sci., 2021, 178, 109062 CrossRef CAS.
- H. Shi, R. Azmi, L. Han, C. Tang, A. Weisenburger, A. Heinzel, J. Maibach, M. Stüber, K. Wang and G. Müller, Corros. Sci., 2022, 201, 110275 CrossRef CAS.
- F. Zakeri-Shahroudi, B. Ghasemi, H. Abdolahpour and M. Razavi, J. Mater. Eng. Perform., 2023, 23, 1–13 Search PubMed.
- M. W. Barsoum and M. Radovic, Annu. Rev. Mater. Res., 2011, 41, 195–227 CrossRef CAS.
- M. Naveed, A. Obrosov, A. Zak, W. Dudzinski, A. Volinsky and S. Weiß, Metals, 2016, 6, 265 CrossRef.
- Y. L. Du, Z. M. Sun, H. Hashimoto and M. W. Barsoum, J. Appl. Phys., 2011, 109, 063707 CrossRef.
- D. Eichner, A. Schlieter, C. Leyens, L. Shang, S. Shayestehaminzadeh and J. M. Schneider, Wear, 2018, 403, 187–195 CrossRef.
- X. Wei, L. Li, Y. Wu and F. Liu, Mater. Today Commun., 2023, 35, 105907 CrossRef CAS.
- J. J. Liu, X. Zuo, Z. Y. Wang, L. Wang, X. C. Wu, P. L. Ke and A. Y. Wang, J. Alloys Compd., 2018, 753, 11–17 CrossRef CAS.
- H. Rueß, J. Werner, Y. Unutulmazsoy, J. W. Gerlach, X. Chen, B. Stelzer, D. Music, S. Kolozsvari, P. Polcik, T. E. Weirich and J. M. Schneider, J. Eur. Ceram. Soc., 2021, 41, 1841–1847 CrossRef.
- B. Völker, B. Stelzer, S. Mráz, H. Rueß, R. Sahu, C. Kirchlechner, G. Dehm and J. M. Schneider, Mater. Design, 2021, 206, 109757 CrossRef.
- J. Molina-Aldareguia, Scr. Mater., 2003, 49, 155–160 CrossRef CAS.
- J. H. Yuan, S. H. Zhou, H. C. Wu, Z. Y. Wang, Y. Zhang, G. X. Zhou, G. S. Ma, P. L. Ke and A. Y. Wang, Scr. Mater., 2023, 235, 115594 CrossRef CAS.
- C. Tang, M. Klimenkov, U. Jaentsch, H. Leiste, M. Rinke, S. Ulrich, M. Steinbrück, H. J. Seifert and M. Stueber, Surf. Coat. Technol., 2017, 309, 445–455 CrossRef CAS.
- F. Y. Zhang, G. X. Yu, S. Yan, J. W. Chen, H. L. Ma, J. N. He and F. X. Yin, Ceram. Int., 2022, 48, 26063–26071 CrossRef CAS.
- F. Y. Zhang, J. Chen, S. Yan, G. X. Yu, H. L. Ma, J. J. He and F. X. Yin, Surf. Coat. Technol., 2022, 441, 128584 CrossRef CAS.
- H. Li, H. Cao, J. Yang, Y. Li, F. Qi, N. Zhao and X. Ouyang, Mater. Charact., 2022, 194, 112421 CrossRef CAS.
- F. Zhang, C. Li, S. Yan, J. He and F. Yin, Ceram. Int., 2021, 47, 3173–3184 CrossRef CAS.
- C. Wang, H. Tu, R. Su, J. Gao, B. V. King, D. J. O'Connor and L. Shi, J. Am. Ceram. Soc., 2020, 104, 593–603 CrossRef.
- C. Azina and P. Eklund, Results Mater., 2021, 9, 100159 CrossRef CAS.
- C. Azina, B. Tunca, A. Petruhins, B. Xin, M. Yildizhan, P. O. Å. Persson, J. Vleugels, K. Lambrinou, J. Rosen and P. Eklund, Appl. Surf. Sci., 2021, 551, 149370 CrossRef CAS.
- C. Azina, T. Bartsch, D. M. Holzapfel, M. Dahlqvist, J. Rosen, L. Löfler, A. S. J. Mendez, M. Hans, D. Primetzhofer and J. M. Schneider, J. Am. Ceram. Soc., 2022, 106, 2652–2665 CrossRef.
- Z. Y. Wang, C. C. Wang, Y. Zhang, A. Y. Wang and P. L. Ke, Mater. Design, 2022, 222, 111060 CrossRef CAS.
- Y. Wang, D. F. Ruiz Diaz, K. S. Chen, Z. Wang and X. C. Adroher, Mater. Today, 2020, 32, 178–203 CrossRef CAS.
- Y. S. Song and Y. J. Park, Endocrinol Metab, 2019, 34, 1–10 CrossRef CAS PubMed.
- R. Y. Liu, J. Chen, L. B. Zhou, W. Qiu, W. Y. Huang and Y. Niu, J. Chinese. Soc. Corros. Prot., 2023, 43, 62–68 CrossRef.
- C. Wang, C. L. Tracy and R. C. Ewing, Appl. Phy. Rev., 2020, 7, 041311 CrossRef CAS.
- C. Wang, Z. Han, R. Su, J. Gao and L. Shi, Nucl. Instrum. Methods Phys. Res., Sect. B, 2019, 450, 286–290 CrossRef CAS.
- R. Su, H. Zhang, L. Liu, L. Shi and H. Wen, J. Eur. Ceram. Soc., 2021, 41, 6309–6318 CrossRef CAS.
- M. Imtyazuddin, A. H. Mir, M. A. Tunes and V. M. Vishnyakov, J. Nucl. Mater., 2019, 526, 151742 CrossRef CAS.
- M. Imtyazuddin, A. H. Mir, E. Aradi and V. Vishnyakov, Nanotechnology, 2020, 31, 385602 CrossRef CAS PubMed.
- C. Tang, M. K. Grosse, P. Trtik, M. Steinbrück, M. Stüber and H. J. Seifert, Acta Polytech., 2018, 58, 69–76 CrossRef CAS.
- E. N. Hoffman, D. W. Vinson, R. L. Sindelar, D. J. Tallman, G. Kohse and M. W. Barsoum, Nucl. Eng. Des., 2012, 244, 17–24 CrossRef CAS.
- M. Bugnet, V. Mauchamp, E. Oliviero, M. Jaouen and T. Cabioc’h, J. Nucl. Mater., 2013, 441, 133–137 CrossRef CAS.
- M. A. Tunes, M. Imtyazuddin, C. Kainz, S. Pogatscher and V. M. Vishnyakov, Sci. Adv., 2021, 7, 1–12 Search PubMed.
- D. Bowden, J. Ward, S. Middleburgh, S. Moraes Shubeita, E. Zapata-Solvas, T. Lapauw, J. Vleugels, K. Lambrinou, W. E. Lee, M. Preuss and P. Frankel, Acta Mater., 2020, 183, 24–35 CrossRef CAS.
- W. Ling, K. Lai, J. Chen, F. Guo, D. Kang, Z. Zhao and J. Dai, Nucl. Mater. Energy, 2023, 36, 101486 CrossRef CAS.
- W. D. Ling, P. Wei, D. Q. Zhao, Y. P. Shao, J. Z. Duan, J. F. Han and W. S. Duan, Phys. B, 2019, 552, 178–183 CrossRef CAS.
- Y. Liu, X. Jian, X. Su, F. Luo, J. Xu, J. Wang, X. He and Y. Qu, J. Alloys Compd., 2018, 740, 68–76 CrossRef CAS.
- Y. Liu, J. Yang, J. Xu, L. Lu and X. Su, J. Alloys Compd., 2022, 899, 163327 CrossRef CAS.
- J. Su, W. Zhou, Y. Liu, Y. Qing, F. Luo and D. Zhu, Surf. Coat. Technol., 2015, 270, 39–46 CrossRef CAS.
- J. W. Huang, J. Hu, M. Z. Li, M. Yi, J. L. Zhu, W. Shui, Y. F. Li, Q. Wen and X. H. Xiao, J. Adv. Ceram., 2021, 10, 1291–1298 CrossRef CAS.
- T. Go, Y. J. Sohn, G. Mauer, R. Vaßen and J. Gonzalez-Julian, J. Eur. Ceram. Soc., 2019, 39, 860–867 CrossRef CAS.
- J. Gonzalez-Julian, G. Mauer, D. Sebold, D. E. Mack and R. Vassen, J. Am. Ceram. Soc., 2019, 103, 2362–2375 CrossRef.
- Y. Li, K. Ma, J. J. Xu, J. J. Li, Y. M. Li, Y. Zhang, J. Zuo and M. S. Li, Corros. Sci., 2024, 226, 111696 CrossRef CAS.
- M. Rougab and A. Gueddouh, J. Phys. Chem. Solids, 2023, 176, 111251 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |