Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Polyelectrolyte complex-based materials for separations: progress, challenges and opportunities
Jiaying Lia,
Lijie Li ab,
Hestie A. Brink
ab,
Hestie A. Brink b,
Giulia Allegri
b,
Giulia Allegri a and
Saskia Lindhoud
a and
Saskia Lindhoud *a
*a
aDepartment of Molecules and Materials, Faculty of Science and Technology, MESA+ Institute for Nanotechnology, University of Twente, P.O. Box 217, 7500 AE Enschede, The Netherlands. E-mail: s.lindhoud@utwente.nl
bDepartment of Membrane Science and Technology, Faculty of Science and Technology, MESA+ Institute for Nanotechnology, University of Twente, P.O. Box 217, 7500 AE Enschede, The Netherlands
First published on 21st March 2025
Abstract
Polyelectrolyte complex (PEC) based materials could provide a sustainable alternative to conventional materials, especially for separation applications. However, reproducible production remains a challenge due to the many parameters influencing the polyelectrolyte complexation process, eventually affecting the properties and performance of the final material. Here, we provide an overview of how different parameters affect polyelectrolyte complexation and discuss promising PEC-based materials for separation applications, i.e., porous membranes, functional and barrier coatings, adhesives, saloplastics, and extraction media. Additionally, we highlight the challenges and opportunities and discuss what is needed to get to the next level. We envision that collaboration between experimentalists and theoreticians can leverage experimental datasets with accurate descriptions of all the parameters for multiscale modelling, machine learning and artificial intelligence approaches that can be used to design PEC materials and predict their properties.
Wider impactModern materials based on petroleum-derived polymers are mainly processed by organic solvents and are difficult to recycle. Polyelectrolyte complex-based materials that are processable in water can be recycled and have demonstrated self-healing properties. These properties make them interesting candidates for renewable materials. Although polyelectrolyte complexes have been studied for over a century, many aspects are not fully understood, and it is challenging to make accurate theoretical descriptions for these systems. Reproducible production of polyelectrolyte complexes has been hampered by batch-to-batch variation of the starting material and the way of mixing solutions containing oppositely charged polyelectrolytes affects the properties of the polyelectrolyte complex. In addition, there are many parameters influencing polyelectrolyte complexation, e.g., the chemistry of the polymers, their length, their mixing ratio, the salt concentration, pH, and temperature resulting in a multidimensional phase space which is difficult to systematically study. To overcome these challenges and elevate our understanding of these interesting materials to a higher level, open-access datasets with metadata that accurately describe the system, i.e., how the sample was prepared, are needed. These datasets can be used to improve the theory and will allow for multiscale modelling and AI approaches to make predictions of material properties. |
1. Introduction
For more than a century, researchers have been interested in polyelectrolyte complexation, a phenomenon in which mixing aqueous solutions of oppositely charged polyelectrolytes causes the system phase to separate into a dense phase and a dilute phase. As early as 1911, Tiebackx was the first to report this phenomenon when he mixed aqueous solutions of gelatin and gum arabic.1 In the late 1920s, Bungenberg de Jong and Kruyt further described this formation of liquid–liquid phase separation. They named this type of phase separation “complex coacervation”.2 From then on, researchers have been studying biopolymer complex coacervation systematically, mainly focussing on proteins and polysaccharides.3,4 When synthetic polyelectrolytes became available, the formation of solid-like complexes was first described by Fuoss and Sadek in the 1940s.5 Later in 1957, the first theory on complex coacervation was reported by Overbeek and Voorn.6 The potential of polyelectrolyte complexes (PECs) as a material was soon recognised by Michaels in 1965.7 In this paper, he envisioned the possible applications of PECs based on their unique properties, including in membranes for medical and electrical applications, plastic composites, conductive coatings, and environmental sensors.Now, about 60 years later, single polyelectrolytes are commercially available for water treatment as flocculants, for household products as rheology modifiers, and for diapers as a superabsorbent material.8 However, the commercial use of PEC-based materials is rare. If these materials are so promising, why are PEC-based materials not on the market yet? Especially because PECs offer several advantages, including low toxicity9 and high recyclability potential,10–12 therefore these materials could play a pivotal role in the transition to more sustainable materials. Furthermore, PECs are processed using water as a solvent, which reduces the use of harmful chemicals and minimises the environmental impact of the manufacturing process. Additionally, PEC materials have self-healing properties,8,12–14 which can extend the lifespan of products and reduce the need for frequent replacements.
In this review, we address this question by examining current research works on PECs, summarising the potential and limitations of their materials, and providing possible solutions to overcome these challenges.
Polyelectrolytes are polymers with ionisable functional groups. These functional groups are mainly amine-based for polycations, and sulphate/carboxylic group based for polyanions. Bediako et al. illustrated the chemical structures of the most commonly used polyelectrolytes categorised as natural, semi-synthetic, and synthetic.15 To maintain electroneutrality in solution, the polyelectrolytes are accompanied by oppositely charged counterions. Upon mixing, the entropic gain from releasing the counterions is the main driving force for the formation of PECs, as shown in eqn (1):8,16
| Pol+A−·xH2O + Pol−M+·yH2O ⇌ Pol+Pol−·iH2O + A− + M+ + zH2O | (1) |
In addition, considering eqn (1), the mixing ratio between the polyelectrolytes is important. Depending on the salt concentration and composition of the PEC system, different phases will be encountered as shown in Fig. 1a.17 If one starts with a polycation solution at low salt concentration upon the addition of polyanions, first positively charged soluble complexes will form, and subsequently, at a certain mixing ratio, macroscopic phase separation occurs. In theory, maximal complexation is observed at charge stoichiometry, the ratio at which the number of positively and negatively charged monomers of the polyelectrolytes are equal (Pol+:Pol− = 1). Further increasing the amount of polyanions will dissolve the complex and negatively charged soluble complexes will be present in the solution. At low salt concentration in general, solid-like PECs are formed, and increasing the salt concentration may lead to the formation of liquid-like complex coacervates. At high salt concentration the PEC phase dissolves, and a one phase system is obtained. Apart from salt, pH is an important tuning parameter when the system contains weak polyelectrolytes, i.e., polyelectrolytes whose degree of ionisation is dependent on the pH.18 Weak polycations are fully charged at low pH and uncharged at high pH, and the weak polyanions are fully charged at high pH and uncharged at low pH as shown in Fig. 1b. To make it more complex, when both weak polyanions and polycations are present in the system these molecules can affect each other's dissociation behaviour.19–21
 | ||
Fig. 1 (a) Phase diagram of polyelectrolyte complexation between the oppositely charged polycation (Pol+) and polyanion (Pol−) as a function of charge stoichiometry and salt concentration (Csalt). S+ and S− represent soluble PECs.  is the critical salt concentration, beyond which liquid-like coacervate can form or at high salt concentration, there is no complexation. Reproduced with permission.17 Copyright 2002, American Chemical Society. Below is the critical salt concentration, beyond which liquid-like coacervate can form or at high salt concentration, there is no complexation. Reproduced with permission.17 Copyright 2002, American Chemical Society. Below  solid-like PECs form. (b) Charge density of polyelectrolytes and proteins versus pH as a function. Reproduced with permission.18 Copyright 2019, Elsevier. solid-like PECs form. (b) Charge density of polyelectrolytes and proteins versus pH as a function. Reproduced with permission.18 Copyright 2019, Elsevier. | ||
Focusing on sustainability, most of the discussed polyelectrolytes in this review are water-soluble and/or can be processed using water as the main solvent. These polyelectrolytes are hygroscopic and for many of their materials, their properties depend on the presence of water, i.e., many PEC-based materials become brittle when they are dried. Because of this hygroscopic nature, Schaaf and Schlenoff8 added water into eqn (1). Water as the solvent of polyelectrolytes acts as a plasticizer for PECs which strongly influences the final mechanical properties.22 This explains why most of the PEC applications are associated with an aqueous environment. Salt and water together are a powerful tool to process PECs similar to the glass transition temperature (Tg) for processing polymers.23,24
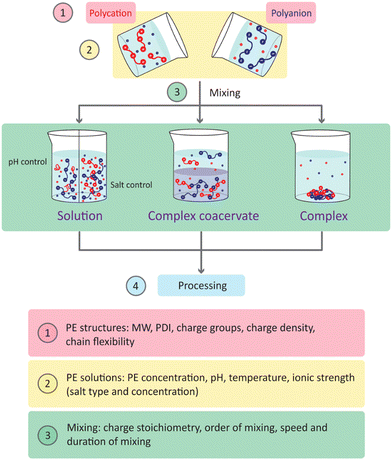
The morphology and composition of the final PECs can be influenced by many other parameters as discussed in a few reviews: choice of polyelectrolyte (synthetic/natural sources), nature of the charged groups, chain flexibility, charge density, molecular weight (MW), polydispersity (PDI), preparation of individual polyelectrolyte solutions (polyelectrolyte concentration, pH, ionic strength, temperature), and upon mixing (charge stoichiometry, mixing order, mixing speed and duration).15,25–28 These parameters and in which step of sample preparation they are important are summarised in Scheme 1, starting with the properties of the polyelectrolytes (step 1). After mixing (step 2), three forms of PEC systems can be obtained: solution, complex coacervate, and polyelectrolyte complex (step 3). Solution refers to one single homogenous liquid phase. This phase either contains dissolved polyelectrolytes e.g., at high ionic strength or pH values at which one of the polyelectrolytes is uncharged, or water-soluble PECs. The latter can form when there is a large mismatch in polyelectrolyte structures or stoichiometric ratio.27 Complex coacervate is the intermediate form between a solid complex and a liquid solution. Upon mixing, polyelectrolytes undergo a liquid–liquid phase separation resulting in a polymer-poor phase (the supernatant) and a polymer-rich phase (the complex coacervate).29 Polyelectrolyte complexes are usually solid-like aggregates/precipitates.30 Depending on the PEC systems, different processing methods can be used. For solutions, a change in pH or salt concentration can result in the formation of PECs. Here, we consider the solution as a form being purposefully designed for easy processing. This can be achieved by pH/salt control. Adjusting pH to “uncharge” weak polyelectrolytes results in a homogeneous solution after mixing with the other polyelectrolytes.31 Scheme 1 shows an example where the polycation remains uncharged thus no complexation occurs with the presence of polyanion. When oppositely charged polyelectrolytes are mixed at high salt concentration, complexation can be induced when Milli-Q water is added.16
Depending on the PEC-preparation method PECs with different rheological properties, varying from solid-like to liquid-like, can be obtained. Thus, different processing methods (step 4) are required to transform them into the final desired PEC materials. In this review, we will discuss these parameters in detail according to each specific application since they may have different procedures and requirements. At the beginning of every section, we will refer back to Scheme 1 and will discuss and summarise the common rules that all applications share. PEC materials are often responsive to changes in the environment, such as water, pH, and ionic strength. After processing post-treatments can be used to further induce a new functionality or improve the properties of PECs, such as crosslinking32–35 and thermal/salt annealing.36–41
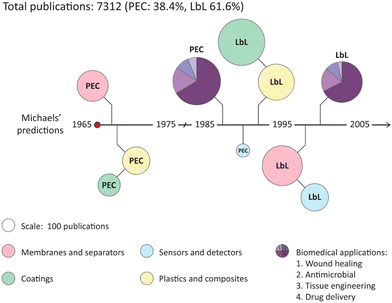
The most studied method to construct PEC materials, however, is not included in Scheme 1. One reason why PECs themselves became less popular to study is the development of layer-by-layer (LbL) assembly of polyelectrolytes. Instead of step 3 of mixing, LbL directly fabricates polyelectrolyte multilayers (PEMs) by sequential deposition of polycations and polyanions. First proposed by Decher in the 1990s,42–45 the development of LbL accelerated the development of PEMs, and many different types of polyelectrolytes and substrates can be used, and different parameters can be easily tuned to build this confined structure. Many reviews have elaborated on the development of LbL focusing on both theories and their potential applications with a particular interest in biomedical fields, such as drug delivery and biosensing,46–53 and membranes.54–58 As shown in Fig. 2, LbL has dominated the study of polyelectrolyte complexation since its discovery, in most of the applications suggested by Michaels. Although research-wise LbL has been developed in all directions and has shown great potential, commercially, there are very limited products. Some commercial PEM-based nanofiltration membranes are available by NX filtration and Pentair.59 The industrialization of PEMs is limited by the time-consuming steps and in all cases, a substrate is required, which in the case of membranes is not always produced via a sustainable process. This is why we need to rethink how to utilize bulk PECs as Michaels proposed, using water as the solvent.
Apart from layer-by-layer assemblies, in biomedical applications, PECs, especially complex coacervates, are a well-studied topic as highlighted by several reviews.60–63 These applications also make use of the responsiveness of PECs to changes in the (local) environment. In many cases, the PECs have dimensions in the nanometre range. In this review, we will focus on non-biomedical and non-LbL prepared macroscopic PEC-based materials, produced starting from (1) homogenous polyelectrolyte solutions, (2) polyelectrolyte complexes, and (3) considering both the complex phase and the dilute phase. We will focus on porous membranes, functional barrier coatings, adhesives, saloplastics, and aqueous-based extraction media. Most of these PEC-based materials were proposed by Michaels. We will discuss the state-of-the-art and what challenges e.g., reproducibility issues, need to be overcome, to bring these materials to higher technology readiness levels. The goal is to set all practical criteria and show how far we are from replacing current polymer materials. In addition, to bridge the gap between theoretical predictions and experiments, standards need to be set to build reliable datasets. These datasets could then also be used for multiscale modelling, machine learning, and artificial intelligence (AI) and could be used to make predictions of material properties.
2. From starting solution(s) to material
Depending on the material to be produced, different starting solutions need to be prepared as shown in Scheme 1. In this section, we will first focus on materials that are prepared starting with homogenous solutions containing both oppositely charged polyelectrolytes. Then we will discuss materials for which the starting point is either a homogenous solution dispersion of polyelectrolyte complexes or a complex coacervate. Followed by a discussion on materials of which a polyelectrolyte complex is the starting point. We conclude with aqueous two-phase systems in which both the complex phase and the supernatant phase play a role.Homogenous solutions containing oppositely charged polyelectrolytes can be obtained at a high salt concentration or, when one of the polyelectrolytes has a pH-dependent charge, mixing the polyelectrolytes at a pH at which the weak polyelectrolyte is uncharged. By decreasing the salt concentration or changing the pH, polyelectrolyte complexation can be induced. This principle can be used to produce porous membranes and functional coatings. Currently, for the preparation of these materials organic solvents are being used and replacing these solvents by water would make the production process more environmentally friendly.
2.1 PEC membranes for water treatment
Because of their unique transport properties and ability to selectively separate compounds polymer membranes have been widely used in agriculture,64 medicine,65 food,66 environment, and energy67 industries.68,69 Currently, non-solvent induced phase separation (NIPS) is the predominant method for polymeric membrane production because of its simplicity and efficiency, and it allows for preparing defect-free membranes for a large industrial scale with broad performance.70,71 The NIPS process includes the preparation of dope solutions and phase separation in a non-solvent bath, typically water. To get the dope or casting solutions, organic solvents that are mixable with water, such as N-methyl pyrrolidone (NMP), dimethyl formamide (DMF), or dimethyl acetamide (DMAc) are commonly used to dissolve the polymers that are water insoluble. When these polymer solutions are cast on a support plate and submerged in water, the organic solvent, water exchange, and the polymeric membranes form. NIPS relies on organic solvents which are unsustainable and harmful to the environment.72 In 2018, the European Council imposed a restriction on the use of NMP, so it is urgent to find other solutions to prepare membranes in a more sustainable way.73The unique properties of PECs such as non-solubility in common organic solvents, high compatibility, and stability under environmental conditions make PECs suitable materials for membrane preparation.74,75 Michaels et al. discussed the possibility of utilizing PECs to prepare porous membranes. However, solid PECs are difficult to process, and one research direction is to tune the composition of polyelectrolyte solutions to obtain processable PECs.7 Successful examples include acid-protection and complexation-sulfation to produce processable solid-like PECs. These solid-like PECs were first made by adding acid or sulfated groups and then dissolving them in suitable aqueous solutions to obtain PEC solutions, followed by a casting and drying process to obtain PEC membranes.76–79 The acid blending method adds excess acid into weak polyelectrolyte solution to depress the ionic complexation in low-concentration polyelectrolyte solutions.80,81 Some reviews have provided comprehensive discussions on the preparation and applications of different PEC-based membranes.74,82,83 They showed great performance for applications in pervaporation, water treatment, and electrodialysis, etc.74,82 However, these PEC-based membranes were prepared via a drying process and need a supporting substrate and there is a lack of control over the membrane structures and performance.
Recently, it was shown that by changing the solubility of solutions containing oppositely charged polyelectrolytes, free-standing PEC-based membranes can be produced via the NIPS-inspired aqueous phase separation (APS) method. The preparation process is similar to NIPS, a homogenous casting solution is prepared first, and then a solid membrane is obtained via phase separation in a coagulation bath. Unlike NIPS using non-solvents to induce phase separation, the phase separation of APS is induced via the change in pH/salinity of the bath solutions. In Scheme 1, the starting point is homogeneous polyelectrolyte solutions obtained after mixing polycation and polyanion solutions, for membrane formation the viscosity of these solutions is very important for the structure and membrane performance. In addition, the formation of PEC membranes, i.e., phase separation, can also be controlled by tuning the parameters in the coagulation bath. The development of APS opened a new way to prepare free-standing PEC-based membranes, and the APS-produced membranes demonstrated tunable structures and performance. Below we will discuss the APS approach for pH change-induced and salinity change-induced membrane formation, the advantages of this approach, and the challenges to overcome to produce membranes via this sustainable method.
The APS approach was first shown to work for the preparation of single polyelectrolyte membranes based on the pH-responsive solubility of weak polyelectrolytes.85–88 Poly(4-vinyl pyridine) (P4VP) was dissolved at a low pH where it is charged and soluble (pH < 4), then its solution was cast as a thin film and switched to a high pH where P4VP is uncharged and insoluble. This process led to the phase separation and solidification of P4VP. Both symmetric and asymmetric membranes with controlled structures were obtained by tuning the pH difference between the casting solution and coagulation bath. The APS approach was also used to prepare membranes using copolymer polystyrene-alt-maleic acid (PSaMA). Like P4VP, the solubility of this polymer depends on the pH of the casting solution, but for PSaMA the polymer is soluble in alkaline water and can be precipitated in an acidic water bath. By adding different types of weak acids in the coagulation bath, membranes with dense separation layers were obtained and possessed an average rejection >92% towards the diverse range of micropollutants. These two examples show that by changing the solubility of the polymer through switching the pH from low to high or vice versa, similar to the conventional NIPS approach, free-standing porous polymeric membranes can be obtained.
In 2020, the APS approach was successfully used to prepare PEC membranes for oppositely charged polyelectrolyte pairs. Baig et al. first prepared PEC membranes based on the complexation of strong polycation poly(sodium 4-styrene sulfonate) (PSS) and weak polyanion poly(allylamine hydrochloride) (PAH).89,90 As shown in Fig. 3a, homogenous casting solutions were prepared at a high pH (pH ∼ 14) to discharge PAH and the PEC membranes were obtained at a low pH (pH ∼ 1). Like NIPS, APS demonstrated great control over the PEC membrane structure and separation performance by tuning the composition of the casting solutions such as the polyelectrolyte molecular weight, concentration, and mixing ratio. Besides, the addition of sodium chloride in the coagulation bath also tuned the membrane structure, which is a new tuning parameter for membrane preparation compared to the NIPS process. The APS-based PAH–PSS membranes demonstrated desirable separation performance from microfiltration to nanofiltration. One disadvantage was that the formation of the PAH–PSS casting solution needed the addition of enough sodium hydroxide to make PAH uncharged. For another polyelectrolyte pair, polyethyleneimine (PEI) and PSS, a homogenous casting solution could be obtained by directly mixing PEI and PSS without further pH adjustment, and the APS was induced through a milder pH change (from pH ∼ 11 to pH ∼ 4). Besides the molecular weight of PEI, the pH and concentration of the acetate buffer coagulation bath showed a significant influence on the membrane structures.91,92 Poly(diallyldimethylammonium chloride) (PDADMAC)–poly(acrylic acid) (PAA) membranes were also prepared by immersing the low pH casting solution into a high pH coagulation bath.93 Apart from flat membranes, the pH change-induced APS has been successfully used to prepare hollow fiber membranes (Fig. 3b).94 With the addition of glycerol to the bore liquid, stable hollow fiber PSS–PEI membranes were prepared and showed desirable microfiltration and ultrafiltration performance. Furthermore, for all the membranes, chemical crosslinking can be achieved in the coagulation bath, for example, glutaraldehyde (GA) was used to crosslink amine groups.
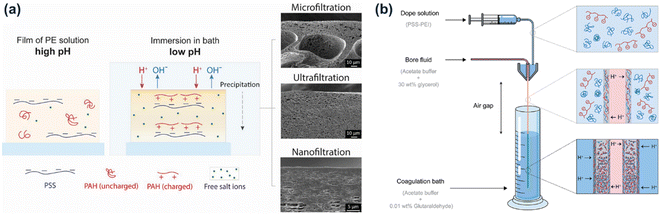 | ||
| Fig. 3 Schematic illustration of PEC-based (a) flat membranes (reproduced with permission.89 Copyright 2020, Wiley) and (b) hollow-fiber membranes (reproduced with permission.94 Copyright 2022, American Chemical Society) via pH change-induced APS. | ||
The pH change-induced APS works well on these synthetic polyelectrolyte-based PEC membranes. Polyelectrolytes derived from nature are also an important category due to their abundant sources, biocompatibility, and biodegradability, etc. As many polyelectrolytes derived from biosources are weak polyelectrolytes, it is possible to tune the solution charge by changing the pH. Therefore, the first bio-based PEC membranes produced by APS were reported using the two widely used biopolymers chitosan (CS) and sodium carboxymethyl cellulose (CMC).95 Homogeneous CS–CMC casting solution was prepared at pH ∼ 1 where CMC was uncharged, and mechanically stable membranes were obtained in an acetate buffer bath. The pH and concentration of the buffer influenced the membrane structure and membranes showed 99% retention for n-hexadecane-in-water emulsion.
Sadman et al. first reported the salt-induced phase inversion method to prepare PEC membranes with controllable porosity in 2019 (Fig. 4a). In this work, anionic PSS and cationic poly(N-ethyl-4-vinylpyridinium) (QVP-C2) were first mixed to prepare solid PECs, then potassium bromide (KBr) was used to dissolve the complex to obtain viscous coacervate. The coacervate was cast and immersed in a deionized water bath to extract the salt and induce membrane formation.98 The resultant membranes showed controllable porosity by changing the salt concentration in the initial coacervate. This work confirmed the possibility of preparing free-standing PEC membranes via salt-induced phase separation while the preparation process needs multiple steps. A simpler one-step salt change-induced APS membrane was proposed using PDADMAC and PSS.99 Instead of coacervate, here, a homogenous casting solution was obtained with high salinity to eliminate the entropic driving force for the complexation. Then the solution was cast and immersed in a low-salinity bath, to induce phase separation. The PSS molecular weight and total polymer concentration as well as the coagulation bath salinity were tuned and nanofiltration membranes with a >60% magnesium sulphate (MgSO4) retention were obtained. In this work, sodium chloride (NaCl) was used to prevent complexation, while in other APS-produced PDADMAC–PSS membranes, KBr was used and porous symmetric and asymmetric membranes with ultrafiltration and nanofiltration properties were obtained by varying the polyelectrolyte mixing ratio.100
 | ||
| Fig. 4 Schematic illustration of PEC-based (a) flat membranes (reproduced with permission.98 Copyright 2019, American Chemical Society) and (b) hollow-fiber membranes (reproduced with permission.101 Copyright 2021, Wiley) via salinity change-induced APS. | ||
Besides the flat membranes, using the salinity change-induced APS, a tubular PDADMAC–PSS membrane was successfully prepared via a dry-jet wet spinning as shown in Fig. 4b.101 Excess KBr concentration was added to suppress the polyelectrolyte complexation, and glycerol was added to the bore fluid to prevent the tubular membrane from collapsing. A ceramic support was needed to give mechanical strength in this work, but further research successfully prepared self-supporting hollow-fiber membranes with the same polyelectrolyte pairs via the salinity change-induced APS.102
It should be noticed that for salinity change-induced APS, the choice of salts is important. In the work of preparing QVP-C2–PSS membranes, it was found that the solid complex remained stable in many salt solutions such as NaCl, KCl, CaCl2, and MgCl2, and only KBr caused high swelling of the complex. For the PDADMAC–PSS system, both NaCl and KBr can be used for dissolving the complex. Therefore, the suitable types of salts are limited based on the polyelectrolyte pairs, and in our opinion a thorough understanding of polyelectrolyte counterion interactions is desirable for the production of APS membranes. Salts that have relatively high solvation-free energy are more efficient in breaking the PEC ion pairs and thus are more suitable as the salt for APS.98
| Type of PECs | Condition of APS | Studied parameters | Types of membranes | Ref. |
|---|---|---|---|---|
| PAH–PSS | pH ∼ 14 to pH ∼ 1 | PE MW, concentration, and mixing ratio; salinity of bath; crosslinker (GA) | Microfiltration | 89, 90 |
| Ultrafiltration | ||||
| Nanofiltration | ||||
| PEI–PSS | pH ∼11 to pH ∼4 | PE MW and mixing ratio; temperature of casting solution; pH and concentration of acetate buffer bath; crosslinker (GA) | Microfiltration | 91, 92 |
| Ultrafiltration | ||||
| Nanofiltration | ||||
| PEI–PSS hollow fiber membranes | pH ∼11 to pH ∼4 | Glycerol concentration; pH and concentration of acetate buffer bath | Microfiltration | 94 |
| Ultrafiltration | ||||
| PDADMAC–PAA | pH ∼1 or 3 to pH ∼4 | Casting solution pH; PE MW and mixing ratio; pH and salinity of bath | Microfiltration | 93 |
| CS–CMC | pH ∼1 to pH ∼5 | pH and concentration of acetate buffer bath | Microfiltration | 95 |
| QVP-C2–PSS | Salinity change (KBr) | Salt concentration in the coacervate | Microfiltration | 98 |
| Ultrafiltration | ||||
| PDADMAC–PSS | Salinity change (NaCl) | PE MW and concentration; salinity of bath | Nanofiltration | 99 |
| PDADMAC–PSS | Salinity change (KBr) | PE mixing ratio | Ultrafiltration | 100 |
| Nanofiltration | ||||
| PDADMAC–PSS tubular membranes | Salinity change (KBr) | Glycerol concentration | Nanofiltration | 101 |
However, there are also problems existing in the APS method. Here, we summarise the main challenges and also possible improvements from the following aspects, tracing back to Scheme 1 in the introduction:
(a) Choice of polyelectrolytes: for now, the APS approach has only been applied to limited polyelectrolyte pairs. A suitable polyelectrolyte pair is vital for the successful preparation of PEC membranes. The pH change-induced APS needs weak polyelectrolytes that are pH-responsive. The salt change-induced APS can use strong polyelectrolytes to prepare PEC membranes while suitable salt and high salinity are needed. The properties of the polyelectrolytes, such as molecular weight, acid dissociation constant (pKa) value, and film-forming property, should be considered before preparing the membranes.
(b) Preparation of polyelectrolyte solutions: the APS approach starts from a homogenous polyelectrolyte solution. The solution properties, such as polymer concentrations, viscosity, pH, and ionic strength, directly affect the final structure and performance of the membranes. To obtain membranes with high mechanical strength, it is better to use higher polymer concentrations. However, this leads to an increase in the viscosity, and high viscosity can affect the processability of the solutions. A balance between the polymer concentration and solution viscosity is very important. A possible solution is to increase the temperature to obtain a higher polyelectrolyte concentration with low viscosity. Because increasing temperature can improve the mobility of polymer chains and thus decrease the intrinsic viscosity.
(c) Preparation of membranes: the membranes are formed from the phase separation of polyelectrolyte solutions in the coagulation bath, and their structure can be tuned by the composition of the coagulation bath, such as pH and ionic strength. The driving force from the pH or salinity between the casting solution and the coagulation bath has to be high enough to make stable PEC membranes. Besides, additives/crosslinkers can be added to the bath to make membranes with denser structures.
(d) Reproducibility: this problem is especially important for the membranes prepared from natural-based polyelectrolytes.95 This is because the membranes are formed through the complexation of oppositely charged polyelectrolytes. Different batches or sources of polyelectrolytes showed differences in charge density which would therefore affect the membrane formation process. The charge density of polyelectrolytes also influences the solution's viscosity, which is an important factor that affects the phase separation process.89,103 A full characterization of the polyelectrolytes, such as molecular weight and charge density, will be important before preparing the membranes.
(e) Applications: the NIPS-produced membranes have been used in various fields, as the APS method of preparing PEC membranes is still new, APS-produced membranes have only demonstrated potential in water treatment fields, and more research needs to be done to extend the applications. For example, developing more natural polyelectrolyte membranes for biomedical applications.
The APS approach also opens new opportunities for membrane functionalisation. APS-produced membranes can be used as support membranes for coating PEMs to enhance the separation performance of the membranes.104 A film formed by the one-step APS can also act as a coating to functionalise hollow fiber membranes.105 Furthermore, since water is the solvent, it is possible to introduce biocatalysts into the APS-produced membranes. Different from the LbL or coating method, in APS, the biocatalyst can be directly added into the polyelectrolyte solutions. After the phase separation process, membranes with catalytic functions are obtained. So far, biocatalytic PAH–PSS and PEI–PSS membranes functionalised with lysozyme have been successfully prepared via the pH change-induced APS.103,106 In addition, alkaline phosphatase was immobilized on the PDADMAC–PSS hollow fiber membranes through the salinity change-induced APS.102 For appropriate characterization of the enzymatic activity in these membranes, having a methodology to determine the concentration of enzymes that are incorporated in these membranes is desirable. Nevertheless, membranes with added biocatalytic functions are not only interesting for applications in water purification, but these materials could also be interesting for biomedical purposes.
Overall, the development of the APS approach reveals that bulk PEC materials can be well used in controllable membrane preparation. The APS method shows great control of the structure and performance of membranes, just like the traditional NIPS. The APS-produced membranes demonstrated separation ability from microfiltration to nanofiltration, which are capable of separating various species in water treatment. Besides, the APS approach can be used to functionalise the membranes.
2.2 PEC functional coatings
For coating applications, one could start with a homogenous solution, with the viscous complex coacervate phase or a polyelectrolyte complex dispersion, so any starting point in Scheme 1. Coatings are defined as functional layers deposited on a substrate, serving specific purposes like protection or decoration.107 The coatings are usually a continuous film and have a small fraction of the thickness when compared to the bulk substrate.108 To reduce the use of volatile organic components (VOCs), waterborne coatings such as polyurethanes and acrylic polymers have been developed.109 Polyelectrolytes, especially the water-soluble ones, are powerful tools for surface modifications. For the deposition of PEC coatings, the most commonly used method is LbL. The electrostatic interaction between two oppositely charged polyelectrolytes is utilized to build up layers by repeatedly dipping, spraying, etc. Richardson et al. summarised a wide range of methods to prepare such LbL films.52 The LbL assembly method is straightforward and versatile since it is not limited by forms of interactions and the type of substrates. As discussed in the introduction, the boost of the LbL development has helped to realize the use of PEMs. Michaels proposed PECs as conductive/antistatic coatings according to the charged nature of polyelectrolytes.7 He suggested that these specific functionalities could be achieved by adding conductive components or playing with the stoichiometric ratio. There are a few studies regarding this specific area, including PEDOT/PSS for conductive coatings,110 copper ion-crosslinked sodium alginate–graphene oxide for antistatic and antibacterial fabric coatings,111 PEI/GO conductive coatings,112 polyelectrolytes and ITO nanoparticles for conductive paper coatings,113 chitosan/sodium phytate/TiO2–CuO nanoparticle composites for flame-retardant and antistatic wood coatings,114 carboxymethyl cellulose/surfactant complexes for antistatic paper coatings,115 and chitosan/hyaluronic acid complexes with electric responsive behaviour.116 Most of these coatings were prepared via LbL, and LbL as a powerful method can not only easily construct polyelectrolyte–polyelectrolyte/surfactant complexes but also immobilize all types of functional nanocomponents.Although LbL has shown great potential in various research fields, the major hindrance for its industrial application is its laborious preparation. With the conventional immersion method, coating one bilayer (a layer of positively charged polyelectrolyte and a layer of negatively charged polyelectrolyte) typically takes 45 min and is mostly done manually.117 To upgrade the method, researchers have tried to narrow the time for each step or by using an automated robot.118,119 Other approaches have also been developed, such as automated spraying,120 however, a true single-step deposition method is still lacking. Here, we focus on PEC coatings prepared in limited steps or one step which are usually beyond the nm thickness range.
One approach is building from PEC particles, similar to conventional film formation of coatings where a polymer dispersion is first deposited, followed by coalescence.121 Wang et al. developed a coating strategy by spraying poly(L-lysine) (PLL) and hyaluronan (HA) complex followed by humidity curing.122 By directly mixing PLL and HA, nanoparticles of PEC were formed which were stable for more than 24 h. These particles were then sprayed and cured on glass at different time lengths and relative humidity (RH) to achieve homogeneous films. These coatings showed potential to be used as extracellular matrix (ECM) membranes. Basu et al. prepared CS and CMC fiber-like particles by high-speed mixing.123 The barrier properties of coated paperboard substrates showed significant improvements against both water and oil after heat treatment. Later, Chi and Catchmark further improved the barrier properties by adding crystalline nanocellulose, showing that the method can also allow for adding other reinforcements.124 Chi et al. also prepared such complex particles utilizing cationic and anionic starches. The key finding was that high MW and charge density were essential to form a dense network for barrier properties.125 Some controlling parameters and potential applications are summarised in Table 2.
| Type of complex | Preparation methods | Functionality and performance | Ref. |
|---|---|---|---|
| PLL and HA | PE+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PE− (v/v) = 0.5 PE− (v/v) = 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 to 2.0 1.0 to 2.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0 |
Successful incorporation of 7-hydroxycoumarin (7-HC), FITC labelled bovine serum albumin (BSA-FITC), DNAs, and VEGF | 122 |
| Mixing: strong stirring | |||
| Spraying: 0–30 min using an ultrasonic spray device, glass substrates | |||
| Annealing: under 60 or 100% RH conditions at 25 °C for 0–1 h | |||
| CS and CMC | PE+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PE− (wt%) = 1 PE− (wt%) = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Oil and water barrier properties at room temperature and 80 °C, also resistance against water vapor (transmission rate: 60 g mm d−1 m−2), toluene, n-heptane, salt solutions | 123 |
Mixing: blended at 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 5–60 min at pH 3.0 and 4.5 000 rpm for 5–60 min at pH 3.0 and 4.5 |
|||
| Dip coating: only one side in contact, paperboard substrates | |||
| Annealing: dried in an oven at 140 °C for 10–15 min | |||
| Cationic and anionic starches | PE+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PE− (wt%) = 1 PE− (wt%) = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Improved mechanical properties (18% increase in tensile strength and 21% increase in Young's modulus), and excellent barrier against water vapor (40% lowered), grease (kit number of 12), and oil penetration (Cobb60: 1.85 g m−2) | 125 |
| Mixing: shear homogenization at 1500 rpm for 5 min | |||
| Dip coating: only one side in contact, paperboard substrates | |||
| Annealing: dried in an oven at 150 °C for 10 min |
Another promising deposition method of PEC coating is starting from coacervate. Grunlan's group was the first to study PEC coacervate coatings and their potential functionalities, starting with PEI and PAA (Fig. 5a).126 Both pH and salt concentration were tuned, and the thickness can be controlled by the casting rod. Stronger complexation was achieved by post-treatment in a buffer bath. This specific combination of polyelectrolytes has been proven to work as an excellent gas barrier as PEM,127 here, this single-step coating also showed excellent oxygen barrier properties. Later, the same group applied this method to different combinations of PEC:PDADMAC and PAA.128 Instead of starting from a coacervate, a solution was prepared by keeping PAA uncharged at pH 2 and using a pH switch discussed in the introduction (Scheme 1). The substrate was dip-coated with this solution, then dried, and a buffer bath was used to initiate the complexation (Fig. 5b). The resulted films again exhibited excellent oxygen barrier properties. To further enhance the barrier properties, clays, such as kaolinite, can also be investigated as an additive.129 Edible PECs of CS and pectin (PT) were also successfully prepared while this method showed a good oxygen barrier for fruit.130 The application of PDADMAC and PAA complex coating reduced bacterial adhesion preventing bacterial fouling.131 Another main focus of Grunlan's group is flame retardant coatings. Polyamine and polyphosphate are used to form the layer, following a similar method starting with deposition followed by using a buffer bath. More details are discussed in their review focusing on flame retardant functionality and some examples are given in Table 3.132
 | ||
| Fig. 5 Examples of PEC coacervate/solution-deposited coatings: (a) PEI/PAA coacervate oxygen barrier coatings. Reproduced with permission.126 Copyright 2017, Wiley-VCH. (b) PDADMAC/PAA solution oxygen barrier coatings, reproduced with permission.128 Copyright 2018, American Chemical Society. (c) Film formation mechanism of evaporation-induced complexation coatings. Green: PEI, orange: polyanion, reproduced with permission.133 Copyright 2024, American Chemical Society. | ||
| Type of complex | Preparation methods | Functionality and performance | Ref. |
|---|---|---|---|
| PEI and PAA | PE+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PE− (wt%) = 1 PE− (wt%) = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Lowest oxygen transmission rate (OTR) 0.08 cm3 (m2 d atm)−1 | 126 |
| Mixing: stirring at pH 8 and different salt concentrations (0–1.5 M NaCl), then heated for 2 h at 70 °C | |||
| Casting: using a hand-drawn rod on poly(ethylene terephthalate) (PET) | |||
| Annealing: immersed into citric acid/citrate buffer solutions at pH 2, 4, and 6 in combination with humidity and heating treatments | |||
| CS and PT | PE+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PE− (wt%) = 1 PE− (wt%) = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
Prevention of browning of apple slices for 3 h, banana was kept green for 1 week, blow-dried coating has an OTR of 0.291 cm3 m−2 day−1 atm−1 and an oxygen permeability of 1.02 × 10−18 cm3 cm cm−2 s−1 Pa−1 | 130 |
| Mixing: rolling for at least 12 h | |||
| Dip coating: PET immersed in PEC for 5 min and then dried at 70 °C for 5 min and 150 °C for 1 h in an oven or dried with blowing | |||
| Annealing: cured with pH 5 200 mM CA buffer for 5 min, followed by water rinsing and the same drying procedures | |||
| PDADMAC and PAA | PE+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PE− (wt%) = 1 PE− (wt%) = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
Removal of >95% of deposited Staphylococcus aureus after rinsing with water | 131 |
| Mixing: magnetically stirred overnight at pH 2 | |||
| Dip coating: polyester fabric immersed in PEC for 5 min and dried for 3 h at 70 °C | |||
| Annealing: immersed in 200 × 10−3 M citric acid at pH 3 or 5 for 20 min, washed and dried at 70 °C overnight | |||
| PEI and poly(phosphate sodium salt) (PSP) | PE+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PE− (wt%) = 1 PE− (wt%) = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
52.7% reduction in total heat release | 134 |
| Mixing: PEI was poured into PSP, both with a pH of 7 | |||
| Dip coating: cotton fabric immersed in PEC for 30 min then hung to dry in a 70 °C oven for 3 h | |||
| PEI and PAA | PE+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PE− = 4 PE− = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1 1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
Lowest oxygen permeability below 4 cm3 m−2 day−1 atm−1 (<0.002 barrer) | 135 |
| Mixing: NH3 was first added to PAA, then PAA-NH3 was added into PEI solution. The mixture was mixed vigorously for 30 min. | |||
| Casting: using a casting machine with different thicknesses of Meyer rods on biaxially orientated polypropylene (BOPP) substrates |
More recently, a single-step evaporation-induced film formation of PEC was first proposed by Pietsch et al.136 then developed by Li et al. As shown in Fig. 5c, a homogeneous solution with polyanions can be obtained at high pH when keeping PEI uncharged.133 The unique point is that the pH change was induced by an evaporative base ammonia, in this way, complexation can be triggered during drying. This method has proven to work for PEI and PAA or PSS.135,137 Since PEI was kept uncharged, homogeneous solutions with different charge ratios could be prepared. The viscosity was influenced by both MW and polymer concentrations. The film thickness could be controlled easily by the casting rod. Again, the combination of PEI and PAA showed excellent oxygen barrier properties.135 The experimental details and performance of some mentioned works are summarised in Table 3.
(a) Choice of polyelectrolytes: to achieve specific functionality, it could be controlled by the intrinsic properties of individual polyelectrolytes, such as polar PAA as the gas-barrier126 and PSP as the fire retardant.139 For the film formation, high MW is usually desired to have enough entanglements plus mechanical stability. This also applies to the charge density that normally high charge density is desired for good complexation. Biobased polyelectrolytes are promising, especially for edible coatings for food preservation.130 The design of one-step PEC coatings can use LbL coatings as a guide since the same functionality can be reached with a different approach.
(b) Preparation of PECs: the ratio between polycation and polyanion is crucial for the final properties of PEC coatings. As shown in Tables 2 and 3, however, it is often not studied as the charge molar ratio. Depending on the final form of PECs, different parameters should be considered. For PEC particles, to achieve uniform particle size, strong stirring is needed. In principle, there are wide choices of polyelectrolytes for forming these particles. The difficulty remains in the coalescence of the particles as defects can form during the film formation and the formation of a continuous film is not achieved. For complex coacervates, again there are many choices of polyelectrolytes. The salt and ratio between the polyelectrolytes can be tuned to optimize the viscosity of the complex coacervates. Compared to particles, complex coacervates are more processible, and compared to solutions, they are one step closer to complexation. The problem could be the unknown concentration and composition of the formed complex coacervates. The last form of starting PEC is high-concentration solutions where the complexation was avoided by keeping the weak polyelectrolyte uncharged. In this way, the ratio can be easily adjusted, and the final composition is known. However, the choices of polyelectrolytes are limited since certain pKa values of the weak polyelectrolyte are required.
(c) Deposition methods: despite the form of PECs, viscosity is the most important parameter. For conventional water-borne coatings, viscosity modifiers can be used to adjust the viscosity, which are not yet studied in PEC coating formulations. The viscosity of PECs is mostly controlled by the polyelectrolyte concentration. According to the viscosity and the desired end product, different methods can be chosen, such as spraying, dip coating, or casting. Among these methods, casting is the most convenient, and thickness can be easily controlled.
(d) Post-treatments: as summarised in Tables 2 and 3, post-treatments including a buffer bath, heating, humidity treatment, or chemical crosslinking can allow rearrangements, enhance complexation, or provide extra strength. The resulting films usually show less swelling and a smoother surface.
(e) Additives: additives could be used to improve the properties or induce functionality, for example, nanoclays were added to enhance the gas and water barrier properties.129 The compatibility between the additives and PECs is key so that no phase separation should occur during the preparation. The challenge is to achieve an even distribution within the PEC matrix.
(f) Durability/stability: for functional coatings, it is important to study the stability of the coating. With time or damage, delamination or defects that compromise the functionality could happen. In current studies, this is not covered and it should be investigated in the future.
(g) Characterization techniques: the final PEC coatings are usually examined by various techniques such as atomic force microscopy (AFM),126,128 scanning electron microscopy (SEM),135 and mechanical measurements.133 Unlike the LbL process that can be studied using refractometry or ellipsometry, they lack in situ characterizations to capture the film formation process and monitor the kinetics. One promising method is laser speckle imaging (LSI). Van der Kooij et al. developed this technique to observe and quantify the dynamic changes during film formation of paints.140,141 Recently, Li et al. applied this method to track PEC coating drying, which shows potential for future study of the kinetics of bulk PEC coatings.133
In summary, PEC-based functional coatings prepared by non-LbL methods have only been developed recently and they already show great potential in various fields. Among the different preparation methods, solution casting is the most promising since the thickness can be controlled, and the composition is known. The gas barrier properties are particularly interesting since these coatings can be used in food packaging to enhance recyclability. To gain a deeper understanding of their properties and further push them to commercialization, it is essential to investigate film formation and structural morphology using (in situ) characterization techniques.
2.3 PEC underwater adhesives
Another type of application similar to coatings is the use of PECs as underwater adhesives, for which the starting solutions are mainly complex coacervates (Scheme 1). Under these underwater conditions, the adsorption of water between the interfaces would disrupt the adhesion interactions; thus, most of the conventional glues fail under a wet condition.142 Marine organisms such as sand worms and mussels contain a special non-canonical amino acid 3,4-dihydroxy-L-phenylalanine (DOPA) which provides the wet adhesion.143–146 Inspired by nature, scientists have been developing polymers that mimic this interfacial chemistry,142,147–151 designing special patterns for the contact surface,152–154 or the commonly used approach: utilizing complex coacervation.155–162 The coacervation of proteins found in marine organisms is a vital step for initial adhesion which helps in spreading on the surface.157,163,164 Despite the conventional route which usually requires two charged components, coacervation can also be achieved via self-coacervation of zwitterionic polymers,158,160–162 gel formation of polycations and multivalent anions,155,165 or based on other intermolecular interactions.156,166,167 There are a few comprehensive reviews focusing on the theory of adhesion, design principles, and mechanisms of all types of underwater adhesives.168–173 Here, we only focus on the examples that use polyelectrolyte complexation/coacervation and electrostatic interactions between two or more components are the main interactions to form these adhesives.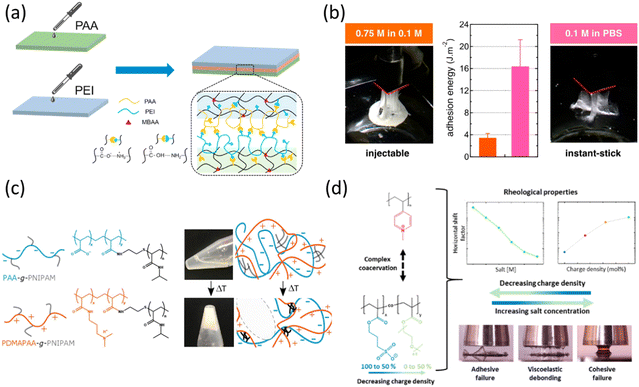 | ||
| Fig. 6 Examples of adhesive coacervates prepared by synthetic polyelectrolytes: (a) hydrogels held together using PEI and PAA, reproduced with permission.174 Copyright 2020, Elsevier. (b) Salt-induced adhesion of PAMPS and PMADAP, reproduced with permission.175 Copyright 2020, American Chemical Society. (c) Temperature-induced adhesion of PAA-g-PNIPAM and PDMAPAA-g-PNIPAM, reproduced with permission.176 Copyright 2019, Wiley-VCH. (d) Charge density-induced adhesion of QP4VP and P(SPMAx-co-OEGMAy), reproduced with permission.177 Copyright 2024, American Chemical Society. | ||
Held together by ionic crosslinking, salt naturally can be utilized as a trigger for tuning the adhesion strength. Vahdati et al. studied the coacervate formation of poly(2-acrylamido-2-methylpropanesulfonic acid) (PAMPS) and poly(N,N-[(dimethylamino)propyl]methacrylamide) (PMADAP).175 As shown in Fig. 6b, the complex coacervate remained injectable at 0.75 M NaCl, while at 0.1 M, the complex coacervate was closer to the gel-point, thus exhibited instant stickiness. One crucial parameter to achieve underwater adhesion found by this study was the low molecular weight. With the degrees of polymerization (DP) close to 100, a system with suitable water content, unentangled chains, and appropriate sol–gel transition can be obtained which fits the requirements for biomedical applications.
Other parameters can also be optimized to trigger wet adhesion, such as pH and temperature. Kamperman's group has been focusing on thermoresponsive poly(N-isopropylacrylamide) (PNIPAM) based on coacervates. Dompé et al. grafted both PAA and poly(dimethylaminopropyl acrylamide) (PDAMAPAA) with PNIPAM chains. As shown in Fig. 6c, a temperature trigger was embedded so that above the lower critical solution temperature (LCST), a liquid-to-gel transition can be achieved.176 When the temperature was raised to 50 °C, PNIPAM chains formed physical crosslinked domains which strengthened the coacervate. Later, a follow-up study focused on the salt effect on this system.178 When the salt concentration of the environment was lowered to 0.1 M NaCl, salt ions diffused out from the coacervate, initiating a stronger electrostatic interaction. As a result, better adhesion could be achieved at 20 °C when compared to using a temperature switch. Van Hees et al. synthesized PNIPAM-b-poly(acrylic acid)-b-PNIPAM and poly(2-(dimethylamino)ethyl methacrylate) (PDMAEMA) to form complex coacervates.179 A similar result was found, that increasing the temperature and lowering the salt content can both alter the viscoelastic behaviour of the complex coacervate. This study further stressed the importance of determining the optimal PNIPAM content in order to balance all properties.
Focusing on the effect of charge density on adhesion strength, van Westerveld et al. conducted two similar studies by keeping the polycation poly(N-methyl-4-vinylpyridinium iodide) (QP4VP) the same, while tuning the hydrophobicity of the polyanions: poly(3-sulfopropyl methacrylate) (PSPMA) partially substituted with oligo([ethylene glycol]methyl ether methacrylate) (OEGMA) units P(SPMAx-co-OEGMAy)177 and P(BSPMA-co-SPMA).180 Taking the P(SPMAx-co-OEGMAy) and QP4VP complex coacervate system as an example (Fig. 6d), diluting the charge of one of the polyelectrolytes can also be used as a parameter to tune the viscoelastic behaviour of the coacervate. This strategy is more feasible than the salt switch since for biomedical applications, the salt concentration is typically low.
 | ||
| Fig. 7 Examples of adhesive coacervates prepared by one or two biobased polyelectrolytes: (a) coacervate formation of polyphosphate-polyaminated gelatin–divalent cation, reproduced with permission.181 Copyright 2010, Wiley-VCH. (b) PVAm and CMC strengthened wet cellulose, reproduced with permission.182 Copyright 2007, American Chemical Society. (c) LS and PAE-Cl underwater adhesive, reproduced with permission.183 Copyright 2019, American Chemical Society. (d) CS and SA hydrogel, reproduced with permission.184 Copyright 2022, Elsevier. (e) Thermal-cured CS and TA coacervate. Reproduced with permission.185 Copyright 2024, American Chemical Society. | ||
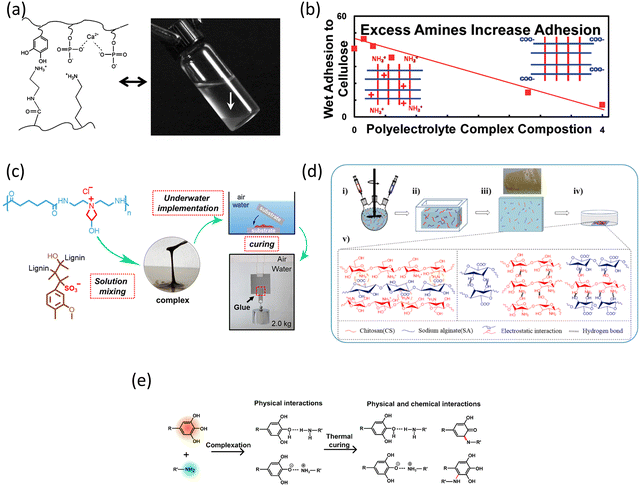
Another example is using CMC and polyvinyl amine (PVAm) colloidal complexes to improve wet paper strength where wet adhesion is important.182 As shown in Fig. 7b, the wet adhesion was enhanced when more amines were presented, which was contradictory to the results under dry conditions. The formation of the complex did not benefit the adhesion properties when compared to only the use of PVAm. The advantage of using this complex is that a higher amount could be deposited than a linear-soluble polymer. This study also emphasized that the adhesion of PVAm may be mainly from primary amines, instead of quaternary amines since PDADMAC did not show wet adhesion properties.
Some other studies have investigated lignosulfonate as the polyanion, which is a waste product derived from wood during the sulphite pulping processes.186 Sodium lignosulfonate (L-SO3Na) was used to form complexes with three different polycations: poly(allylamine) (PAH), PDADMAC, and ε-poly-L-lysine (ε-PL).187 The importance of the substrates was emphasized since these complexes showed much better adhesion on polar substrates like metal and wood than non-polar plastic polypropylene. Among the three combinations, L-SO3Na/ε-PL complexes are the most promising since both polyelectrolytes are biomass-derived. Another example is the mixing of lignosulfonate (LS) and a polyamidoamine-epichlorohydrin (PAE-Cl) solution.183 Instant underwater adhesion could be achieved and the self-curing ability of PAE further improved the wet adhesion during curing (Fig. 7c). Again, in this work, better adhesion was achieved with hydrophilic substrates than hydrophobic substrate PTFE.
Chitosan is a derivative of abundant crustacean sources, such as crab and shrimp shells, which contain positively charged amine groups.188 Waite et al. designed a coacervate system using catechol-functionalised PAA and bis(trifluoromethane)sulfonamide (Tf2N−) modified quaternised chitosan.189 Tf2N− groups were used to increase the solubility of chitosan in DMSO. These two polyelectrolytes were premixed in DMSO and then applied on different substrates in water. The phase inversion was then activated by water–DMSO solvent exchange, charging the carboxylic groups, eventually leading to wet adhesion. This is a unique work, which used solvent exchange for stimuli, however, one review has pointed out that the use of DMSO may be harmful to the bio-organisms.168 In another work, Li et al. utilized CS or quaternary ammonium salt of chitosan (QCS) to form hydrogels with sodium alginate (SA), as shown in Fig. 7d.184 High molecular weight polyelectrolytes (approx. 105 Da) were used which contributed to the high strength due to both hydrogen bonding and electrostatic interaction. The obtained CS/SA hydrogels showed excellent adhesion to various biological tissues. The addition of Ag+ further improved the strength and the antibacterial performance. Similarly, Wei et al. fabricated an injectable hydrogel based on QCS and carboxymethylcellulose sodium (CMCNa).190 The obtained hydrogel coating exhibited excellent interfacial properties such as superspreading on various substrates underwater, maintaining interfacial toughness, and improving lubrication. Another fully biobased system was proposed by Galland et al. where they used CS and hyaluronic acid (HA).191 After examining the salt-induced changes, one big advantage of this complex coacervate was that no large trigger was required to induce adhesion. The last example is using CS and tannic acid (TA) with a curing step (Fig. 7e).185 Besides the adhesion strength, this mild temperature treatment improved both water resistance and long-time durability underwater. In general, the usage of chitosan or quaternised chitosan as the polycation can also offer other functionalities such as antibacterial properties, which was also studied in some of these mentioned works.57,191
 | ||
| Fig. 8 Coacervation of Rmfp-1 and MADQUAT based on cation–π interaction. Reproduced with permission.193 Copyright 2016, National Academy of Sciences. | ||
(a) Choice of polyelectrolytes: as discussed in previous sections, the use of polyelectrolytes can be categorized in two main directions: design synthetic polyelectrolytes to mimic biological wet adhesion or utilize biobased polyelectrolytes, such as polysaccharides. For synthetic polyelectrolytes, the introduction of hydrophobic components can help the first stage of adhesion, which is removing the interfacial water layer. It is thus important to balance the ratio between hydrophilic and hydrophobic parts.168 For biobased PECs, Lu et al. pointed out that there is a trade-off between biocompatibility and mechanical strength when using natural adhesives.173 They tend to have short life spans thus it is important to study the self-healing properties.172 One important parameter is the MW. Using lower MW polyelectrolytes is easier for processing due to lower viscosities, however, sacrifices the final mechanical stability due to lack of entanglements. One suggestion was to combine small and large MW polyelectrolytes or use polyelectrolytes with large PDIs.171 In addition, other weak interactions are often combined with electrostatic interactions to work synergically towards better adhesion, such as hydrophobic interaction, hydrogen bonding, and metal–ligand coordination.171
(b) Phase inversion: the phase inversion of PECs is a powerful tool to transform from injectable/flowable liquid to strong solid-like adhesive. In Table 4, the stimuli conditions for selected examples are summarised. The most important factors studied are temperature, pH, and ionic strength. Here, Vahdati et al. concluded that high salt concentration and high-temperature switches are not practical for biomedical uses since the tolerance for both is normally low. Another factor is that a stable water content should be achieved for consistent adhesion performance. The final water content of the adhesives also determines whether they are suitable for hydrophilic or hydrophobic surfaces.171 Charge stoichiometry is also studied in this field. Around a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 charge ratio is optimal probably due to the maximum stability at neutral charge.
1 charge ratio is optimal probably due to the maximum stability at neutral charge.
| Type of complex | Type of interactions | Adhesion conditions | Stability over time | Ref. |
|---|---|---|---|---|
| PEI and PAA (with Fe3+) | Electrostatic interaction, hydrogen bonding, metal–ligand coordination | Curing time: 24 h | Intact after 3-day soaking in water | 174 |
| Temperature: 25 °C | ||||
| pH: adjusted to 3 for samples with Fe3+ | ||||
| PAMPS and PMADAP | Electrostatic interaction | PE+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PE− = 53.2–46.8 mol% PE− = 53.2–46.8 mol% |
N/A | 175 |
| Curing conditions: 1 h | ||||
| Temperature: 20–25 °C | ||||
| pH: 7 | ||||
| Salt switch: 0.75 M to 0.1 M NaCl; 0.1 M NaCl to PBS (physiological condition) | ||||
| Water content: 56 wt% (0.1 M sample) | ||||
| PDMAPAA-g-PNIPAM and PAA-g-PNIPAM | Electrostatic interaction, hydrophobic interaction | PE+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PE− = 1 PE− = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
N/A | 176, 178 |
| Curing conditions: until a fixed thickness was reached, then 1 h contact | ||||
| pH: 7 | ||||
| Temperature switch: from 20 °C to 50 °C when keeping the NaCl concentration at 0.75 M | ||||
| Salt switch: from 0.75 M to 0.1 M NaCl when keeping the temperature at 20 °C | ||||
| Combined salt and temperature switch: 0.75 M to 0.1 M NaCl and 20 °C to 50 °C | ||||
| Water content: 83.1–92.9 wt% | ||||
| P(SPMAx-co-OEGMAy) and QP4VP | Electrostatic interaction, hydrophobic interaction | PE+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PE− = 1 PE− = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
N/A | 177 |
| Curing conditions: 1 min contact | ||||
| Salt concentrations: 0.00 M vs. 0.5 M NaCl | ||||
| Water content: 51–84 wt% | ||||
| Polyphosphate–gelatin–divalent cation complexes | Electrostatic interaction | PE+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PE− = 0.6 PE− = 0.6 |
N/A | 181 |
| Curing conditions: fully submerged in water for ≈24 h | ||||
| Temperature: 10, 20, and 37 °C | ||||
| pH: 7.4 | ||||
| PVAm and CMC | Electrostatic interaction | PE+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PE− (wt%) = 0.25 to 1 PE− (wt%) = 0.25 to 1 |
N/A | 182 |
| Curing conditions: equilibrated at 23 °C and 50% humidity for 24 h | ||||
| pH: 4 to 9 | ||||
| Salt concentration: 0.01 M NaCl | ||||
| PAE-Cl and LS | Electrostatic interaction, hydrophilic stabilization | PE+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PE− (wt%) = 1.25 PE− (wt%) = 1.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Stable up to 30 days | 183 |
| Curing conditions: 48 h conditioning vs. instant in different soaking solutions | ||||
| Temperature: 50, 80, and 100 °C | ||||
| pH: 3, 5, water, 8, 9, 10, and 11 | ||||
| Salt concentrations: water, 0.2, 0.5, 0.8, and 1.0 M NaCl | ||||
| CS/QCS and SA | Electrostatic interaction, hydrogen bonding | PE+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PE− (wt%) = 1 PE− (wt%) = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4 to 1 0.4 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.6 1.6 |
N/A | 57 |
| Curing conditions: 1 min contact | ||||
| Water content: 67.6–95.3 wt% for CS/SA and 50.3–83.9 wt% for HACC/SA | ||||
| CS and HA | Electrostatic interaction, hydrogen bonding | PE+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PE− (wt%) = 1 PE− (wt%) = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 1.1 |
Maintains 80% of its adhesion strength after 24 h of immersion in PBS | 191 |
| Temperature: 37 °C | ||||
| pH: 5 | ||||
| Salt concentrations: supernatant, 0.1, 0.2, 0.3, 0.4 and 0.45 M NaCl | ||||
| Water content: >85 wt% | ||||
| CS and TA | Electrostatic interaction, hydrogen bonding, and cation–π interaction | PE+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PE− (wt%) = 1 PE− (wt%) = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25 to 1 0.25 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
Immersion in water for 1 day to 2 months (3.5 MPa maintained after 2 months) | 185 |
| Curing conditions: cured in the oven at 70 °C for 2 h | ||||
| Temperature: room temperature | ||||
| MADQUAT and Rmfp-1 | Cation–π interaction | MADQUAT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Rmfp-1 = 1 Rmfp-1 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (stoichiometry of tyrosine/trimethylammonium units) 1 (stoichiometry of tyrosine/trimethylammonium units) |
N/A | 193 |
| pH: 3 | ||||
| Curing time: at least 10 min |
For future developments, Cui et al. proposed to construct phase diagrams showing pH, ionic strength, polyelectrolyte concentration etc. as guidelines for future designs.196 Natural adhesives produced by organisms are dynamic materials that enable the adhesion process to proceed at different lengths, and time scales, thus it is vital to design and study the adhesion with changing parameters.170,196 Furthermore, the environmental impact should also be taken into account, for example, when using solvent-exchange as the trigger which may be harmful to the environment.189
(c) Stability: due to the nature of electrostatic interactions, it is difficult for PEC-based adhesives to remain stable in sea water applications.168 Also, some of the PECs are sensitive to both ionic strength and pH, which makes it hard for them to sustain dynamic flows, such as blood.169 In general, there lacks an injectable adhesive which cures at body temperature and adheres to biological tissues with specificity to the target surfaces under complex body fluidic conditions.168 The long-time adhesion was only investigated in a few studies (Table 4), which should also be considered.
(d) Characterization techniques: one general limitation emphasized by Narayanan et al. is the lack of standardized measurement methods.169 As summarised in Table 5, the adhesion strength was measured using various methods and under different experimental conditions, for example, some of them were not conducted underwater. This makes the parallel comparison difficult. Despite the difference in measurements, Vahdati et al. set a standard for robust adhesion strength which should be larger than 100 J m−2, however, most of the examples could not reach this value yet.171
| Type of complex | Tested substrate | Measurement method | Adhesion strength | Ref. |
|---|---|---|---|---|
| PEI and PAA (with Fe3+) | Two hydrogels, one hydrogel and solid substrates (glass, stainless steel plate, plexiglass, pig skin) | 180° peeling tests for hydrogels and pigskin, 90° peeling tests for hydrogel and other solid substrates | Highest 2178 J m−2 (two hydrogels), 404 J m−2 (glass), 345 J m−2 (stainless steel plate), 103 J m−2 (PMMA), 177 J m−2 (pig skin) | 174 |
| PAMPS and PMADAP | Probe and glass plate | Custom-made underwater probe tack | Highest 65 J m−2 | 175 |
| PDMAPAA-g-PNIPAM and PAA-g-PNIPAM | Probe and PAA hydrogel film, glass, polytetrafluoroethylene (PTFE) | Underwater probe tack | Temperature switch: 1.6 J m−2 (PAA), 3.8 J m−2 (glass), 3.2 J m−2 (PTFE) at 50 °C | 176, 178 |
| Salt switch: 6.5 J m−2 | ||||
| Combing temperature and salt switch: 7.2 J m−2 | ||||
| P(SPMAx-co-OEGMAy) and QP4VP | Probe and stainless-steel plate | Small amplitude oscillatory shear and probe tack tests | Highest ∼5.5 J m−2 | 177 |
| Polyphosphate–gelatin–divalent cation complexes | Aluminium | Underwater lap shear tests | Highest 765 kPa | 181 |
| PVAm and CMC | Wet laminated cellulose membranes | Delamination force measurements | Highest ∼48 N m−1 | 182 |
| Cationic polyelectrolytes and L-SO3Na | Stainless steel, aluminium, wood, and polypropylene (PP) | Shear adhesion tests | Highest ∼6.9 J m−2 (stainless steel), ∼4.7 J m−2 (aluminium), ∼6.5 J m−2 (wood), ∼0.6 J m−2 (PP) | 187 |
| PAE-Cl and LS | Glass, aluminium, stainless steel, ceramics, and PTFE | Underwater pull-off tests | Cured ∼400 kPa (glass, aluminium, and stainless steel), ∼300 kPa (ceramics), ∼51 kPa (PTFE) | 183 |
| QCS-Tf2N and PAAcat | Aluminium, metal, leaf, stone, wood, glasses, and plastics including polyethylene (PE), PP, polystyrene (PS), polymethyl methacrylate (PMMA), PET, and PTFE | Underwater surface forces apparatus | ∼2 J m−2 | 189 |
| CS/QCS and SA | Biological tissues, including porcine skin, liver, fat, bone, muscle, and myocardium | Tissue adhesion by visual observation | N/A | 57 |
By the hydrophilic nature of PECs, most of these adhesives work better on hydrophilic substrates. They also mentioned the roughness of the substrate was less studied where most of work used smooth surfaces. In real applications, surface roughness may also play a role.169 Li et al. emphasized the importance of developing characterization techniques to understand the chemical compositions down to molecular levels for biological adhesives, which also holds true for PEC adhesives.172
In summary, complex coacervation is proven to be a vital step for marine organisms to achieve wet adhesion. There are still many unknowns in fully understanding the biological and applicational processes of the wet adhesion thus it is still at the research stage. In the future, it is important to further study the coacervation mechanism and design the structures of the polyelectrolytes. Proteins and biobased polyelectrolytes should be the focus due to their biocompatibility and biodegradability. These adhesives can be promising materials for biomedical applications such as surgical use, wound dressing, and multifunctional bioelectronics.168,197,198
2.4 Saloplastics as separators and functionalised materials
In this section, we will examine the processing of PECs into dense plastic materials. As illustrated in Scheme 1, the preparation of a PEC is relatively straightforward, involving the mixing of oppositely charged polyelectrolytes at low salt concentrations, which results in the aggregation and precipitation of a solid-like complex.199 Historically, the processing of these complexes has presented significant challenges due to their inherent brittleness in the dry state and their insolubility in water and most solvents.200 This changed in 2009 when the Schlenoff group highlighted the importance of saltwater in the processing of PECs, and introduced the term “saloplastics” to describe the resulting materials. The presence of salt facilitates the doping of the complex,200,201 effectively disrupting the ionic crosslinks between the anionic and cationic groups, which allows for the rearrangement of polymer chains. Additionally, hydration of the PEC is essential, as water serves as a plasticiser for these materials.10 Furthermore, saloplastic processing typically occurs at elevated temperatures, as a temperature/doping equivalence10 exists. This relationship indicates that plasticization is enhanced at higher temperatures, thereby accelerating the processing of saloplastic materials.By controlling the PEC composition and studying their behaviour in different environments, it should be possible to functionalise the properties and morphologies of saloplastic materials for a diverse range of applications. The selection of polyelectrolyte pairs and their respective molecular weights (step 1 of Scheme 1) can have a significant impact on the properties of the resulting saloplastic materials. The interaction between the polyelectrolytes is determined by a number of factors, including charge density,202–204 chain flexibility,205,206 and molecular weight,204,207,208 which can influence the overall structure and stability of the complex. Furthermore, complexation is known to be a kinetically limited10 process, meaning that the morphology and composition (stoichiometry) of the complex can be manipulated by changes in the polyelectrolyte solutions (step 2 of Scheme 1) and the mixing conditions (step 3 of Scheme 1).201,209,210 This phenomenon is illustrated in Fig. 9, which shows the impact of salt type and concentration on the formation of PECs derived from PSS and PDADMAC. This study found that PECs with a firm and compact structure, along with a clear supernatant, were the easiest to process into saloplastic materials.211 This outcome was achieved using a concentration of 250 mM potassium bromide, suggesting a significant correlation between the strength of the dopant, as categorized by the Hofmeister series, and the formation of the complex.199 Nevertheless, the determination of these optimal conditions largely depends on a trial-and-error approach, as the effects of salt type and concentration on PEC formation are not well studied or understood.
 | ||
| Fig. 9 (a) Effect of NaCl concentration (0–400 mM) on PSS–PDADAMC PEC formation. (b) and (c) Effect of salt type and salt concentration [(b) 125 mM and (c) 250 mM] on PSS–PDADMAC PEC formation – from Krishna et al.211 | ||
In recent years, there has been a growing interest in saloplastic materials, leading researchers to identify several processing methods and potential applications. Early research focused on the fabrication of multi-shaped materials such as tapes, tubes, and rods through extrusion of solution-precipitated PECs.10 This breakthrough in PEC processing was followed by other techniques like curtain coating, mold-dialysis, injecting spinning, and dropping-dialysis to transform highly doped PEC hydrogels into films, sheets, fibers, and capsules.13 Researchers have also explored the use of 3D printing to create intricate structures with potential applications in biomedical devices and artificial tissues.212 Furthermore, the hydrophilic nature of PSS–PDADMAC PECs was exploited for the production of metal-ion adsorbents for wastewater treatment213 and desiccants.214
Thus, numerous processing techniques have been employed to functionalise saloplastic materials for a diverse range of applications, highlighting the significance of both the PEC composition and its processing in the final functionality and properties of the resulting saloplastic. The comprehensive review by Bediako et al. has already addressed the factors influencing polyelectrolyte complexation and the strategies for processing PECs into saloplastics,215 thus, these aspects will not be revisited in the current review. Instead, this review will specifically focus on the advancements made in saloplastics within separation processes. This aligns with Michaels’ prediction that there is potential for producing thin, dense films from polyelectrolyte complexes that are suitable for use as battery separators and fuel cell membranes,216 a prospect that remains highly relevant in facilitating the energy transition. The advancements made in these areas, which were not covered in Bediako's review, will be the focus of this section. We will explore both the challenges and opportunities associated with the development and application of saloplastic materials.
2.4.1.1 Hot-pressed saloplastic ion exchange membranes. To address these requirements, a hot-pressing method has been developed to produce thin, dense, and transparent films from salt-plasticised polyelectrolyte complexes.211 The hot-pressing method involves placing the solution precipitated PEC in a hot-pressing setup, where it is heated to a predetermined temperature and subjected to pressure for a specified time to achieve plasticisation. After cooling under pressure, the saloplastic film can be removed from the mould. A schematic of this process is given in Fig. 10. When pressing non-stoichiometric PECs, either the polyanion or polycation is present in excess, imparting a negative or positive charge to the film. Consequently, these saloplastic films can function as IEMs. This membrane fabrication method is unique in that it relies solely on salt water as a solvent,224 eliminating the need for harmful chemicals. The membranes are also recyclable,10–12 making this a truly sustainable IEM.
 | ||
| Fig. 10 Schematic of hot-pressing polyelectrolyte complexes into dense saloplastic membranes – from Krishna et al.211 | ||
A subsequent study demonstrated that the choice of polyelectrolyte pair as well as their stoichiometric ratios could affect the charge density and overall performance of the saloplastic IEMs.225 It was established that both anion and cation exchange membranes can be produced from the same polyelectrolyte pair by varying the molar ratio during complexation. Given the known chemical stability of PSS–PDADMAC PECs, this system was further investigated to assess its long-term pH stability by comparing the permselectivity of the PSS–PDADMAC anion exchange membrane before and after exposure to extreme pH environments (pH 1 and 14). The results indicate no significant change in permselectivity, confirming the stability of materials across a broad pH range (1–14), suggesting that saloplastic PSS–PDADMAC IEMs may be suitable for fuel cell applications.224,225 In a separate study, cation exchange membranes prepared from PSS–PVAm were investigated. Previously, PVA and PVAm were found to be monovalent-selective for K+ over Na+, which opens up the possibility of removing excess sodium from agricultural feed streams, such as greenhouses, to maintain a healthy balance of salt ions.226
While significant progress has been made in the development of sustainable, chemically stable saloplastic IEMs, further optimisation is required to improve the charge density of these membranes. This is necessary to improve their performance at higher concentrations, which is currently inferior to that of commercial membranes.224 This enhancement is critical for improving their performance at higher concentrations, which currently lags behind that of commercial membranes. Additionally, the processing of these membranes largely relies on trial and error to identify optimal hot-pressing conditions (temperature, pressure, and time) for each PEC; in our opinion, efforts are needed to transform PEC processing into a more systematic scientific approach.
2.4.1.2 Thermally compressed PECs as battery separators. A similar approach was used to produce thermally compressed PSS–PDADMAC films to be used as separators for rechargeable zinc–air batteries.227 In this investigation, PSS–PDADMAC PECs were prepared at various stoichiometric ratios (PDADMAC
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS – 2
PSS – 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1.5
1, 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.15, 1
1.15, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5, and 1
1.5, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and then thermally compressed into thin films. By adjusting the molar ratio during complexation, the study successfully produced separators with both negative (excess PSS) and positive (excess PDADMAC) charges, which were qualitatively assessed through selective absorption of anionic and cationic dyes. The performance of these separators was evaluated based on ionic conductivity, selectivity, and performance in rechargeable zinc–air battery applications.
2) and then thermally compressed into thin films. By adjusting the molar ratio during complexation, the study successfully produced separators with both negative (excess PSS) and positive (excess PDADMAC) charges, which were qualitatively assessed through selective absorption of anionic and cationic dyes. The performance of these separators was evaluated based on ionic conductivity, selectivity, and performance in rechargeable zinc–air battery applications.In this application, the separator's role is crucial as it allows the selective permeation of hydroxide ions while preventing crossover of zincate ions, thereby enhancing the battery's efficiency and longevity. The separator with the highest PDADMAC content (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) exhibited superior performance by achieving the lowest zincate permeability and the highest ionic conductivity. Notably, the performance of this separator was comparable to that of a commercially available zinc–air battery separator. Moreover, when conducting galvanostatic cycling testing using a homemade rechargeable zinc–air battery, this separator showed good stability after 150 cycles of testing.227
1) exhibited superior performance by achieving the lowest zincate permeability and the highest ionic conductivity. Notably, the performance of this separator was comparable to that of a commercially available zinc–air battery separator. Moreover, when conducting galvanostatic cycling testing using a homemade rechargeable zinc–air battery, this separator showed good stability after 150 cycles of testing.227
This study underscores the significant influence of PEC composition on the performance of the separator. However, the precise charge or exact composition of the PEC remains undetermined, complicating reproducibility. The results also indicate the complexity of achieving the desired stoichiometry, as the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio resulted in a positively charged membrane. Therefore, improved control over charge compensation and enhanced methodologies for quantifying the charge are essential for future research.
1 ratio resulted in a positively charged membrane. Therefore, improved control over charge compensation and enhanced methodologies for quantifying the charge are essential for future research.
2.4.1.3 Functionalised saloplastic films. Li et al. investigated an innovative method to enhance the antimicrobial properties of saloplastic films through lysozyme functionalization. By integrating lysozyme into hot-pressed PSS–PDADMAC saloplastic films, the authors aimed to expand the applicability of these materials in sustainable antibacterial applications, such as food packaging, biomedical devices, and microbiological environments. The functionalization process involved a post-treatment procedure that introduced lysozyme into the films through the formation of micropores. These pores were created by annealing the saloplastics in a solution with a high salt concentration, which breaks the ionic crosslinks within the material. The films were subsequently immersed in water, leading to a significant reduction in salinity, which induced pore formation. These pores served as sites for lysozyme entrapment, after which the films were immersed in a salt solution to close the pores. The resulting films demonstrated strong enzymatic activity against Micrococcus lysodeikticus and retained 72% of their activity after seven days. This work proves that it is possible to impart biological functionality to saloplastic films, thereby enhancing their potential as sustainable antimicrobial materials.103
Saloplastic materials also hold promise in energy storage applications, particularly as electrode materials for supercapacitors. Supercapacitors, known for their rapid charge–discharge rates, rely on the development of thin, flexible films that can effectively store and release energy.228 Polyelectrolyte complex composite (CPEC) membranes, formed by combining polyelectrolyte complexes (matrix materials) with electroactive fillers, offer a versatile platform for supercapacitor electrodes. While a matrix is not always essential for supercapacitor electrodes, its inclusion can provide structural support and improve the mechanical stability and flexibility of the electrode material. Luangaramvej and Dubas (2021) demonstrated the incorporation of polyaniline, a conductive filler, into a stoichiometrically prepared PSS–PDADMAC polyelectrolyte complex matrix. The resulting salt-plasticized CPECs were formed into membranes via compression molding. The inclusion of the PEC matrix allowed the membrane to withstand deformation, whereas pure polyaniline would have been prone to cracking. Furthermore, the CPECs were optimized through in situ polymerization of polyaniline, resulting in a membrane that exhibited stable specific capacitance over 2000 charge–discharge cycles. This approach underscores the potential of polyaniline-loaded CPECs as environmentally friendly materials for supercapacitor electrodes, offering a promising avenue for the development of sustainable energy storage solutions.228
This section discussed two innovative approaches to functionalizing saloplastic films, showcasing their potential in both antimicrobial and energy storage applications. Together, these studies highlight the versatility of saloplastic materials and their potential for further development and use in specialized, sustainable applications across different industries.
2.4.2.1 Challenges in PEC production and processing. (a) Complexation control: reproducibility and scalability of the complexation step is one of the biggest issues facing saloplastic production. Studies have shown that stoichiometric ratios of polyelectrolytes do not consistently yield stoichiometric PECs, with non-stoichiometric complexes often forming despite the intended molar ratios.201,225,227 This issue arises because complexation is a kinetically limited process that is influenced by various factors, including polyelectrolyte molecular weight, polyelectrolyte ratio, polyelectrolyte concentration, pH, salt type, ionic strength, solution temperature, mixing order, addition rate, and mixing time.215 Scale-up is therefore challenging as it is much more difficult to control complexation when large quantities are produced.10
(b) Polyelectrolyte variability: variability in polyelectrolytes is a significant factor affecting the reproducibility of complexation. For instance, we have observed variations in solid content (wt%) in PDADMAC across different batches and suppliers. If this variability is not accounted for, the molar ratio at which polyelectrolytes are mixed may be inaccurate, impacting PEC stoichiometry. PDADMAC is also commercially supplied with a broad molecular weight distribution (MWD) with varying PDIs.229 This could potentially lead to issues with reproducibility since it is known that molecular weight influences complexation.
(c) Processing: PEC processing for saloplastics has traditionally been a trial-and-error process. Research has shown that processing conditions, such as temperature and pressure, are PEC-specific211,225 and that the science behind these parameters is not well understood. In our experience, different polyelectrolyte pairs and molar ratios require distinct processing conditions for optimal performance, which can be a time-consuming and challenging process. Furthermore, as previously outlined, the composition of PECs is difficult to control due to the large number of variables influencing complexation. This variability makes them challenging to process into saloplastics, as each batch may have a different composition and characteristics.
2.4.2.2 Stability challenges in different environments and applications. (a) Hydration: the hydration state of saloplastic materials significantly impacts their mechanical properties. In a dry state, these materials tend to become brittle,10 which limits their use in applications where they are not maintained in a hydrated environment. If these materials are used in applications where they are not fully immersed in a solution, fluctuations in humidity may impact their mechanical stability. This phenomenon is attributed to the plasticizing effect of water on polyelectrolyte materials. We noticed that the comparatively high water uptake of saloplastic materials negatively affects their performance as ion exchange membranes and separators.224 High water uptake essentially dilutes the concentration of charges within ion exchange membranes which will allow more undesired ions to pass, thus reducing their selectivity.230
(b) Salt concentration: saloplastics can also be affected by salt type and concentration. This is because salt dopes PECs, affecting their stability by disrupting ion pairing within the complexes. Consequently, the use of saloplastic ion exchange membranes is currently restricted to processes involving lower salt concentrations to maintain good selectivities.224 Sudden fluctuations in salt concentration can also result in the formation of micropores201,231,232 within the PEC structure, which could impact its structural integrity and functionality. For ion exchange membranes, it is important to have dense films since pores would negatively impact the membranes’ selectivity and conductivity.
(c) Temperature: temperature sensitivity is another limitation of saloplastics. The glass transition temperatures of saloplastic materials are highly dependent on factors such as salt concentration and PEC stoichiometry.210 Studies have shown that the glass transition of PSS–PDADMAC PECs is relatively close to room temperature,233 which severely affects their mechanical stability and limits their use to low-temperature applications.
(d) pH: for strongly charged polyelectrolytes, pH does not affect the charge of the materials. However, exposure to extreme pH environments could affect their stability. Literature has reported that quaternary ammonium groups, which are present in PDADMAC, might be degraded at high pH through Hoffman elimination,234 nucleophilic substitution235 or ylide formation.236 However, studies have shown that PSS–PDADMAC saloplastic membranes exposed to extreme alkaline and acidic environments (pH 1–14) for 30 days did not show signs of degradation.224
2.4.2.3 Opportunities. To address the challenges associated with PEC production and processing, a deeper understanding of the variables influencing complexation is needed. This knowledge could enable the development of more controlled and reproducible methods for producing PECs at a large scale. Research by Shamoun and colleagues has made important strides in this area, by first developing a 1H-NMR method to accurately quantify the composition of PSS–PDADMAC PECs and then identifying conditions under which large quantities of stoichiometric PECs can be consistently produced.10 Their work demonstrated that dilute polyelectrolyte solutions (125 mM) and low salt concentrations (250 mM KBr) lead to the formation of stoichiometric complexes when equimolar solutions of PSS and PDADMAC are added simultaneously in a separate beaker under stirring.10 These findings provide a promising starting point for further research aimed at scaling up PEC production and optimizing the complexation process.
Addressing variability in polyelectrolyte materials presents additional challenges, particularly when using commercially available polyelectrolytes. In our opinion, simple quality control measures, such as verifying the solid content of batches, could significantly reduce variability. Furthermore, molecular weight distribution in commercial polyelectrolytes may be improved by fractionation techniques. For instance, one study successfully narrowed the molecular weight distribution of PDADMAC from 3.3 to 1.4 through acetone precipitation and centrifugation, recovering higher molecular weight chains.229 We think alternative fractionation methods, such as dialysis, could also be employed to reduce the polydispersity of polyelectrolyte solutions.
Further opportunities lie in optimizing the processing conditions of PECs. Previous studies have explored the impact of time, temperature, and salt concentration on PEC behaviour. For example, Shamoun et al. observed that PECs doped with higher salt concentrations could be extruded at lower temperatures. This observation led to further investigation into the relationships between processing time, salt doping, and temperature on the thermal behaviour of extruded PSS–PDADMAC PECs. Using dynamic mechanical thermal analysis, the authors measured the Tg of the PECs, noting significant transitions in modulus as the material shifted from a glassy to a rubbery state. The study revealed that glass transition temperature varied with the deformation rate, in accordance with the time/temperature superposition principles, and that higher salt concentrations led to a reduction in glass transition temperature, indicating a plasticizing effect due to the breakage of ion pairs within the complexes. These findings were used to derive an empirical equation that can be used to predict the thermal behaviour of PECs under varying conditions, thus offering valuable insights into how time, salt concentration, and temperature can be manipulated to optimize PEC processing.233 Such insights provide valuable guidelines for optimizing PEC processing, offering a shift from trial-and-error experimentation to more systematic, science-driven approaches.
Finally, the application of saloplastic materials is often limited by their sensitivity to environmental factors such as salt concentration, temperature, pH, and hydration. While some of these challenges may be difficult to fully overcome, modifying saloplastic materials through crosslinking presents a promising strategy to enhance their performance. Crosslinking introduces covalent bonds between polymer chains, which can reduce chain mobility, thereby improving the material's mechanical stability, thermal resistance, and reduced swelling.34,237–239
To summarise, the development of saloplastic materials offers significant potential but faces challenges related to reproducibility, scalability, and environmental stability. However, opportunities to address these challenges exist, driven by an improved understanding of material properties, processing conditions, and material modifications. By adopting a more systematic, science-driven approach, a lot of these challenges can be overcome.
2.5 PECs as aqueous two-phase systems
Polyelectrolyte complex phases and their supernatants are aqueous two-phase systems. Indicated in Scheme 1 are the complex coacervate and its supernatant and the PEC and its dilute phase. Since molecules can distribute among these phases, these systems can potentially be used to extract, separate and/or purify molecules.240–242 To effectively study the uptake of molecules in aqueous two-phase systems based on polyelectrolyte complex phases, develop new applications, or improve materials derived from the PEC phase, a clear understanding of both the dense and dilute phases is essential. Particular attention should be given to the partitioning behaviour of molecules within these systems, as this plays a crucial role in their performance. Protein partitioning, in particular, is a key factor that significantly influences the properties and functionalities of PECs, as proteins may interact differently across phases depending on their charge, structure, and environmental conditions.The partitioning of molecules plays a pivotal role in cellular life, enabling the organization and regulation of biochemical processes in response to changing conditions. Membrane-less organelles (MLOs) are prime examples of this mechanism in action, acting as dynamic, spatiotemporal hubs that coordinate and control cellular activities. Unlike traditional organelles bound by membranes, MLOs form through liquid–liquid phase separation, allowing them to compartmentalise specific biomolecules and create distinct microenvironments within the cytoplasm. The ability to assemble and disassemble in response to cellular signals enables MLOs to regulate the biochemical reactions on demand, ensuring both precise timing and spatial organization of processes critical for maintaining homeostasis and adapting to environmental changes.243,244
The unique behaviour of MLOs, their capacity to phase-separate, partition specific molecules, and dynamically reorganize, presents an exciting model for bio-inspired materials design. One promising approach for mimicking these natural systems relies on polyelectrolyte complexation. These complexes exhibit the potential to create compartmentalized environments, making them useful in applications that require the selective partitioning of molecules. By leveraging the principles underlying MLOs, polyelectrolyte complexes could be designed to achieve selective extraction, separation, and concentration of target molecules in non-biological settings.
Water and wastewater treatment is a particularly promising field for applying polyelectrolyte complexes. While polyelectrolytes have been used as additives in coagulation and flocculation processes to assist in potable water and sludge dewatering, PECs offer a more direct and potentially more efficient alternative. In fact, PECs can extract contaminants from water, bypassing the need for inorganic coagulants commonly used in traditional processes.245
To improve and develop novel applications, a deeper understanding of the underlying mechanisms governing polyelectrolyte complexation is crucial. This might include a thorough characterization of polyelectrolyte systems, such as understanding the intrinsic properties of the individual polyelectrolytes i.e. response to environmental factors such as pH and ionic strength. These properties influence how polyelectrolytes interact with each other and with other molecules. Furthermore, a detailed investigation of the complexation process is needed, such as the kinetics of complex formation, the stability of the complexes, and their ability to encapsulate and release specific molecules. Moreover, the collection of extensive data sets on these interactions will enable the development of predictive models. Such models can optimize the design of polyelectrolyte complexes for specific applications, such as targeting specific molecules or achieving desired levels of extraction efficiency. These insights can lead to the development of systems with improved performance, scalability, and environmental compatibility. We believe that by integrating the principles of MLOs’ behaviour into PECs, we open the door to innovative materials that can mimic the ability of nature to organize and partition molecules with precision.
Building on this foundation, the following section will delve into the uptake and release of molecules, specifically proteins, in polyelectrolyte complexes and complex coacervates. By examining successful systems and identifying key parameters for optimization, we aim to advance the use of PECs as aqueous two-phases for separation.
Proteins exhibit a high degree of structural organization, characterised by four levels: primary, which refers to the linear sequence of amino acids; secondary, which involves the local folding into alfa-helices and beta-sheets; tertiary, which describes the three-dimensional shape of a single polypeptide chain; and quaternary, which pertains the assembly of multiple polypeptide subunits into a functional complex.247
Due to their sensitivity, proteins are prone to denaturation, a process where they lose their functional structure when exposed to changes in pH, temperature, or chemicals. This instability outside their natural environment presents significant challenges for storage and practical applications. Therefore, developing effective strategies for both extraction and encapsulation that maintain protein stability is essential for advancing protein-based uses.248,249 Both polyelectrolytes and complex coacervates can be used to stabilise proteins in aqueous- environments, avoiding the use of solvents that can potentially destabilise these molecules.
As Scheme 1 illustrates, the phenomenon of polyelectrolyte complexation is influenced by several factors, including polyelectrolyte architecture, pH, salt concentration, ionic strength, mixing order and speed, and temperature. These factors influence the final morphology and composition of PECs, allowing for the formation of structures ranging from liquid-like to solid-like complexes.
Proteins, as a special type of polyelectrolyte, exhibit charge behaviour that is dependent on their isoelectric point (pI), i.e. the pH at which the net charge is zero (refer to Fig. 1b). At pH values above the pI, proteins carry a net-negative charge, whereas below the pI, they bear a net-positive charge. Since proteins can be considered polyelectrolytes, they can form polyelectrolyte complexes with oppositely charged (synthetic) polyelectrolytes. Here we focus on three component systems where two oppositely charged polyelectrolytes form the PEC or complex coacervate and proteins are taken up by this complex phase. This variability in the charge of the protein as a function of pH is crucial when designing protein extraction or encapsulation methods using polyelectrolyte complexation, as it directly impacts loading and release efficiency.
By controlling factors such as pH, salt concentration, and ionic strength, the extraction and release of molecules250,251 from PECs can be finely tuned. Specifically, increasing salt concentration disrupts intrinsic ion pairs, converting them into extrinsic ion pairs, which loosens the PEC matrix and promotes the release of extracted molecules.252–254 Adjusting the pH, on the other hand, modifies the charge density of the polyelectrolytes themselves (for weak polyelectrolytes), either enhancing or reducing complexation strength and can thus trigger molecule release at specific pH thresholds (see Fig. 1b). Together, these parameters allow for targeted control over PEC composition, regulating both the encapsulation and release of molecules from the complex. If the encapsulation process is selective towards one specific species in the solution, PECs could be used for extraction, and the controlled release could then be called back-extraction.
Several polyelectrolyte complex systems have been used to encapsulate proteins.255–260 Perry et al. investigated the encapsulation of a range of proteins with different charges within a poly(L-lysine) and poly(D,L-glutamate) system across different solution conditions and polymer properties. They found that proteins with clustered-like charged residues showed enhanced uptake, resulting in increased sensitivity of the system to solution conditions.255 In our group, we found that the concentration of the lysozyme inside the complex can become so high that proteins irreversibly aggregate inside the complex.258 In addition, we observed that the uptake of lysozyme and a chemically modified version of this protein strongly depends on the stoichiometry of the complex phase.258–260 This selectivity in protein uptake can be used to separate proteins from protein mixtures. A study by Van Lente et al. explored the extraction of lysozyme from a multi-protein mixture using four PEC systems,260 each composed of different weak and strong polyelectrolyte combinations. Fig. 11 shows the partitioning profiles of lysozyme across these PEC systems at pH 7. While all systems exhibited similar lysozyme uptake profiles as a function of the complex compositions, significant differences emerged during the release phase.
 | ||
| Fig. 11 The partitioning profiles of lysozyme in various PECs at pH 7. (a) Weak/weak PAH/PAA, (b) weak/strong PAH/PSS, (c) strong/weak PDADMAC/PAA, and (d) strong/strong PDADMAC/PSS, at different PEC compositions as expressed in F−. A low supernatant lysozyme content corresponds to a high PEC lysozyme content and vice versa. Values represent individual measurements; lines connect averages of duplicates. Adapted with permission.260 Copyright 2022, Wiley. | ||
The process of releasing proteins into a new supernatant is referred to as back-extraction. Lysozyme release was induced either by salt addition or pH reduction (Fig. 12). Adding salt increases charge screening within PECs, weakening the intrinsic electrostatic interactions, loosening the PEC structure, and promoting protein release (Fig. 12a). In contrast, Fig. 12b shows lysozyme release through pH reduction, which alters the charge properties of the polyelectrolytes. Systems composed of strong polyelectrolytes, like PDADMAC/PSS, remain more stable under varying conditions, whereas PECs containing weak polyelectrolytes or combinations of weak and strong polyelectrolytes disintegrate more easily under pH changes. This effect occurs due to changes in the degree of ionization of charged groups on both the polyelectrolytes and proteins, which affect the overall stability of the PEC structure. Additionally, salt and pH variations cause PECs to swell or shrink, sometimes resulting in structural transformations such as hydrogel formation in PDADMAC/PAA PECs at low pH.260
 | ||
| Fig. 12 The back-extraction of lysozyme from the different PEC systems using (a) 500 mM NaCl, and (b) 4 mM HCl (pH decrease of 7 to 4). Columns represent the average of n = 4 with error bars indicating standard deviation. Adapted with permission.260 Copyright 2022, Wiley. | ||
These findings emphasize how the properties of the polyelectrolyte complex, along with environmental conditions, can significantly affect the efficiency of protein extraction. Moreover, the back-extracted lysozyme retained its enzymatic activity, proving the capability of PEC systems to function as extraction media for proteins. These findings underscore the broad applicability of PECs for protein extraction, particularly in fields such as biotechnology and wastewater treatment, where selective separation is essential.
Overall, polyelectrolyte complexation is a versatile and tuneable method applicable to both extraction and encapsulation of proteins. While extraction through PEC focuses on the selective separation of proteins from complex mixtures based on electrostatic interactions, encapsulation offers a way to stabilize and protect proteins for further use. Both processes are essential for advancing applications in biotechnology, pharmaceuticals, and environmental science.
In addition to the extraction and encapsulation processes, the reversibility and long-term stability of PECs are critical considerations, especially for applications requiring multiple uses. PEC systems that can undergo reversible complexation, by adjusting factors like pH or ionic strength, allow for the recovery of both the extracted protein and the polyelectrolytes. Studies, such as those by Van Lente et al., have demonstrated the controlled release of proteins and other molecules by altering the composition and environmental conditions, suggesting that PEC could be recycled for multiple rounds of extraction.258,259,261 However, understanding how to optimize the balance between stability and reversibility is an important direction for future research, especially for systems designed for sustained use in industrial or environmental settings.
Apart from proteins, the extraction of small molecules250,261 and the stabilisation of viruses262,263 have been studied. Design rules for sequestering viruses in complex coacervates have been proposed.263 One interesting finding for the extraction of the small molecule butanol is that the partitioning is temperature-dependent.261 The partitioning coefficient at high temperature is significantly larger than at room temperature, opening the possibility to extract butanol at high temperature and back extract at low temperature. Using the temperature to extract and back extract molecules from e.g., wastewater might open possibilities to recycle and upcycle molecules. However, the back extraction is not yet very efficient and could be improved, but in order to improve this the mechanism of temperature-dependent partitioning needs to be understood. To achieve this goal and obtain a deeper understanding of why composition-dependent and temperature-dependent partitioning occurs we need techniques to fully characterise the PEC systems.
(a) Challenges in control: developing reliable methods for precise control over molecule partitioning and release in PECs remains challenging. Factors such as pH, salt concentration, ionic strength, and environmental conditions play critical roles, as illustrated in Scheme 1. Additionally, proteins often face denaturation when removed from their native environment, requiring PECs to maintain protein integrity during extraction and encapsulation. The design of PECs for preserving protein stability, particularly under environmental stresses or long-term storage, is an ongoing focus. To address these challenges, integrating real-time monitoring systems, such as in situ spectroscopy or microfluidic platforms, could enable dynamic tuning of PEC formation. Furthermore, developing AI-driven predictive models could help optimize molecular partitioning by learning from large datasets of PEC behaviour under varying conditions.
(b) Stability and reversibility: for applications such as water treatment, stability and reversibility in PECs are crucial but challenging to balance. Systems designed for repeated use must retain their structural integrity across variable environmental conditions, demanding advancements in stability without sacrificing functionality. A potential breakthrough could involve the use of biobased PECs that biodegrade, enhancing the sustainability of the process.
(c) Opportunities and innovations: despite these challenges, PECs offer significant opportunities. As sustainable alternatives to traditional coagulants in water treatments, PECs enable contaminant removal without relying on harsh chemicals. Additionally, they serve as water-based, organic solvent-free alternatives for protein encapsulation, protecting proteins from environmental stresses and enabling controlled release, making them well-suited for pharmaceutical and biocatalytic applications.
(d) Future directions: enhancing our understanding of PEC behaviour through robust data collection and advanced characterization will enable the development of predictive models. These models can optimize PECs for specific applications, refining their use across biotechnology and environmental technologies.
In summary, while polyelectrolyte complexation presents technical challenges in stability and molecular partitioning control, we envision it as a promising bio-inspired approach for selective extraction and encapsulation. With continued innovation in predictive modelling and characterization, PECs are poised to become versatile tools in biotechnology and environmental applications.
3. Getting PEC-based materials to the next level
In Scheme 1 we summarised the different controlling parameters per step for the production of PEC-based materials. Depending on the end material different challenges have been identified per step. For all the materials the properties of the starting polyelectrolytes should be known (step 1, Scheme 1). For the preparation of porous membranes, the viscosity of the casting solution is important for the kinetics of the phase separation and eventually affects the porosity of the membrane. Especially when biopolyelectrolytes are used, batch-to-batch variation could have an impact on the reproducibility of the membrane production process.95 The starting polyelectrolytes are either in powder form or can be purchased as solutions. In the powder form, one has full control of the concentration of the polyelectrolyte solution. When the polyelectrolytes are delivered as solutions, it is important to check whether the concentration on the label is correct. In our experience, if the label says 20 wt% this can vary between 18–22 wt%. Since the mixing ratio of the polyelectrolytes is important for the characteristics of the final material, assuming that the concentration of the polyelectrolytes is correct could result in reproducibility issues. In addition, the polydispersity of the polyelectrolytes could affect the final PEC-material, and methods to fractionate polyelectrolytes have been proposed.229For step 2 in Scheme 1, it is important to record all the parameters in sufficient detail. If weak polyelectrolytes are part of the system, measuring the pH before and after complexation (in step 3) is advisable since charge regulation is expected to occur in these systems.19–21 Ion-specific interactions between the polyelectrolytes could play a role in this step. The temperature as a parameter during polyelectrolyte complexation has not been extensively explored, but it is known that it may well affect the properties of the polyelectrolyte complex225 and the properties of the material after processing.103
The final composition of the PEC in terms of complex charge-stoichiometry is strongly affected by the mixing conditions. Some polyelectrolyte pairs might even have a preferential non-stoichiometric composition.201,224,225,227,264 To avoid human errors and enhance reproducibility flow cells could be possibly used to produce PECs. This approach could be coupled with machine learning algorithms to optimize PEC composition in real-time based on immediate feedback from characterization data. To understand how the way of mixing influences the final PEC a full characterisation of the system is required. Below we give an overview of the different characterization methods that have been used to characterise PECs and the final PEC-based materials.
3.1 Characterization techniques
To fully understand the mechanisms behind polyelectrolyte complexation and the partitioning of molecules, it is crucial to use effective characterization techniques. These methods not only allow for the detection and quantification of the overall composition of PECs in terms of polyelectrolytes and counterions but also provide valuable insights into the mechanism of the uptake of proteins and other molecules under different environmental conditions. The following section will outline the most commonly used techniques for characterizing both the overall composition of PECs and the protein content, with a particular focus on how these methods can be used to generate detailed data sets that can be used for multiscale modelling, machine learning and AI.3.1.1.1 Attenuated total reflection–Fourier transform infrared spectroscopy. Attenuated total reflection–Fourier transform infrared spectroscopy (ATR–FTIR) is an effective technique for characterizing polyelectrolyte complexes. This method allows for the assessment of the degree of ionization in polyelectrolytes or protonation levels265,266 in the case of weak polyelectrolytes, which is a key factor in complex formation. Typically, studies utilize ATR–FTIR to investigate how the degree of ionization varies with the pH of the solution.200,266–268
While ATR–FTIR is a valuable tool for characterizing polyelectrolyte complexes, it has some limitations. Compared to other techniques, ATR–FTIR offers more qualitative rather than quantitative analysis. Additionally, the technique is sensitive to experimental conditions such as temperature, humidity, and pressure, which can influence the results, given that polyelectrolytes are often affected by environmental factors. Furthermore, the presence of functional groups with similar vibrational frequencies can lead to overlapping peaks, complicating the process of peak assignments. In summary, ATR–FTIR is a useful analytical tool for polyelectrolyte complex characterisation, especially to obtain information about the degree of ionisation in the PEC, but it should be used in conjunction with other techniques to provide a more comprehensive and quantitative understanding of these systems.269
3.1.1.2 Thermal gravimetric analysis. Thermal gravimetric analysis (TGA) is another method for quantifying both polymers and salt content in polyelectrolyte complexes.30 TGA measures changes in the physical and chemical properties of a sample as a function of increasing temperature or time, making it applicable for analysing complex phases. However, TGA has several limitations. It primarily provides data on mass loss related to degradation and thermal stability but does not offer insights into molecular interactions or the degree of complexation. The thermal decomposition of the components in the complex can overlap, complicating the differentiation of contributions from individual polyelectrolytes, and counterions. Additionally, since polyelectrolyte complexes are often hygroscopic, absorbed water can cause mass loss at low temperatures, which could hide thermal events associated with the decomposition of the compound itself. Moreover, TGA is a destructive technique, meaning samples cannot be preserved post-analysis. Therefore, to achieve a more comprehensive chemical analysis, it is advisable to pair TGA with complementary techniques, such as NMR, which can provide additional specificity.
3.1.1.3 Nuclear magnetic resonance. NMR has proven to be a versatile technique for characterizing polyelectrolytes and their counterions.270 For instance, 1H-NMR has been used to determine the mole ratio of polyelectrolytes in the dense phase by redissolving dried complexes in high salt solutions when using strong polyelectrolytes264 or in a mixture of an acid or base in a high salt concentration solution if weak polyelectrolytes were used, to protonate or deprotonate one of the polyelectrolytes.267 Currently, 1H-NMR allows for the quantification of individual polyelectrolytes and their mole ratios in both the dense and supernatant phases, with the latter phase being diluted with D2O.271–277
To gain a comprehensive understanding of the entire system, it is essential to quantify the inorganic content alongside the organic one. Compared to more sophisticated techniques such as radiolabelling38,231 and neutron activation analysis,278 which involve extensive preparation steps and longer processing times, NMR offers a non-invasive, in situ, and rapid approach. It allows the analysis of various NMR-active nuclei,277,279 including Na and Cl, through 23Na and 35Cl-NMR, respectively.280,281 This makes it possible to quantify the inorganic content in polyelectrolyte systems.
Since polyelectrolyte complexation is entropically driven, the majority of counterions are expected to be in the supernatant phase, with a minor amount remaining in the dense phase. Accurate quantification of counterions provides valuable insight into the mechanisms underlying protein partitioning within polyelectrolyte complexes.282
NMR does not face the same challenges related to the physical state of the sample as other techniques. Nevertheless, it is recommended to conduct experiments at high polyelectrolyte concentrations to enhance the formation of a denser phase and facilitate phase separation. An advanced approach would involve performing complexation directly within the NMR tube, which could minimize errors associated with sample separation.
To conclude, NMR, along with the previously discussed techniques, offers valuable insights into the composition and behaviour of PECs. However, each method has its limitations, and till now, no single technique provides a complete picture. Therefore, the most comprehensive understanding of PEC systems comes from using a combination of these methods, allowing researchers to explore protein partitioning, polyelectrolyte interactions, and counterion behaviour with greater precision. As the field advances, further refinement of these characterization techniques will be crucial for optimizing PEC-based applications in protein extraction, encapsulation, and other emerging technologies.
To accurately characterize the extraction behaviour of polyelectrolyte complexes, methods are required to determine which molecules are present in each phase. For protein partitioning specifically, UV-vis spectroscopy is a common approach, as it detects the characteristic absorbance peak of tyrosine and tryptophan residues at 280 nm.258–260,283 However, while effective for high protein concentrations, this method is less sensitive at lower concentrations. Additionally, if polyelectrolytes contain aromatic or conjugated groups, their UV absorbance may interfere, complicating accurate protein quantification.284
Colorimetric assays offer another option for protein quantification, particularly useful when proteins lack distinctive signals or are present at low concentrations.285 These assays rely on the interaction between a dye and specific amino acid residues within the protein, though they cannot differentiate between multiple proteins within a sample.255,286
For more sensitive detection, especially at low protein concentrations, fluorescence spectroscopy is effective when proteins are intrinsically fluorescent or labelled with fluorescent tags, such as GFP.287–289 This approach can also apply to other molecules. For instance, Spruijt et al. used a fluorescent label on one polyelectrolyte to measure its distribution between the polymer-rich phase and the supernatant.290 However, introducing fluorescent labels can potentially influence the complexation process itself, and fluorescence measurements have additional limitations. Fluorescence is highly dependent on the local environment, which may vary significantly within PECs. Therefore, to accurately determine the composition of the phases without affecting complexation, it is preferable to use less invasive methods.
Typically, protein quantification in polyelectrolyte complexes is performed by separating the dense and the supernatant phases, either through pipetting or decantation, followed by individual analysis. The partition coefficient is then calculated as the ratio of the concentration of protein in the dense phase divided by the concentration of protein in the supernatant. Despite the straightforward nature of these techniques, they present certain challenges. For solid-like complexes, the dense phase is hard to characterize using these methods due to its physical state.
In the case of liquid-like complexes, the small volume of the coacervate, often in the range of a few microliters, complicates the quantifications and necessitates assumptions or approximations. One approach to address this issue relies on calculating the mass balance of the system, where the protein mass in the coacervate phase is inferred by subtracting the protein mass in the supernatant from the total protein mass added.255 To overcome these limitations, it is essential to explore new techniques that account for these physical constraints, thereby improving the accuracy of protein quantification in polyelectrolyte complexes.
PEC-based materials offer sustainable alternatives to conventional materials as they can be produced in water and are recyclable. To get these materials to the next level concise experimental open-access datasets are needed that can optimise polyelectrolyte complexation theory and serve as training data for machine learning approaches and AI. These datasets should contain as much information as possible about how the samples are being prepared and characterised. The parameters mentioned in Scheme 1 can be used as a guide (see Fig. 13). An accurate description of as many parameters as possible should be provided in a metafile. Ideally, these data sets should be peer-reviewed by experimentalists and theoreticians and could be shared via a trusted database.
4. Summary and outlook
In this review, we discussed how different starting points, as indicated in Scheme 1 as being homogenous solutions, complex coacervates, and polyelectrolyte complexes, can be used to make a variety of materials. Many of these materials were first proposed in 1965 by Michaels. Sixty years later, the field has advanced, but each of the discussed materials, porous membranes, coatings, underwater adhesives, dense ion-exchange membranes, and PEC-based aqueous two-phase systems, presents its own challenges and opportunities.A key challenge common to all materials is achieving reproducibility. Factors contributing to this challenge include the variability in starting materials, such as incorrect weight percentages or biological variations when biopolyelectrolytes are used. Additionally, differences in sample mixing methods or parameter adjustments can further impact reproducibility. As discussed, numerous factors influence polyelectrolyte complexation, creating a vast parameter space that is difficult to explore experimentally.
In our opinion, polyelectrolyte complex-based materials are particularly promising, as they can be processed in water and have the potential to replace current materials that either contain harmful molecules or are produced through environmentally damaging processes. PEC-based materials, such as porous membranes and aqueous two-phase systems, can be used to purify water, while ion-exchange membranes have potential applications in electrochemical cells. These materials could play a key role in achieving the sustainable development goals 6 (clean water and sanitation) and 7 (affordable and clean energy).
To unlock the full potential of PEC-based materials and advance the field, we envision that recent developments in multiscale modelling, machine learning, and AI could be highly beneficial for their design and prediction. Achieving this will require collaboration between experimentalists and theoreticians to generate detailed experimental datasets, including metadata describing the experimental conditions, varied parameters, and fixed parameters. These datasets can serve as input to validate and improve the theoretical models. To facilitate this, it would be beneficial to establish standardized, peer-reviewed open-access databases for data sharing.
List of abbreviations
Data availability
The review entitled “Polyelectrolyte complex-based materials for separations, progress, challenges and opportunities” by Jiaying Li, Lijie Li, Hestie A. Brink, Giulia Allegri and Saskia Lindhoud does not contain any new data. Only previously published work has been cited.Conflicts of interest
The authors declare that there are no conflicts of interest regarding this article.Acknowledgements
The PhD project of Hestie Brink is funded by NWO. Lijie Li appreciates the China Scholarship Council (CSC) for providing a scholarship.References
- F. W. Tiebackx, Z. Chem. Ind. Kolloide, 1911, 8, 198–201 CrossRef CAS.
- H. G. Bungenberg de Jong and H. R. Kruyt, Proc. K. Ned. Akad. Wet., 1929, 32, 849–856 Search PubMed.
- C. G. de Kruif, F. Weinbreck and R. de Vries, Curr. Opin. Colloid Interface Sci., 2004, 9, 340–349 CrossRef CAS.
- T. Moschakis and C. G. Biliaderis, Curr. Opin. Colloid Interface Sci., 2017, 28, 96–109 CrossRef CAS.
- R. M. Fuoss and H. Sadek, Science, 1949, 110, 552–554 CrossRef CAS PubMed.
- J. T. G. Overbeek and M. J. Voorn, J. Cell. Comp. Physiol., 1957, 49, 7–26 CrossRef CAS.
- A. S. Michaels, Ind. Eng. Chem., 1965, 57, 32–40 CrossRef CAS.
- P. Schaaf and J. B. Schlenoff, Adv. Mater., 2015, 27, 2420–2432 CrossRef CAS PubMed.
- R. A. Ghostine, R. F. Shamoun and J. B. Schlenoff, Macromolecules, 2013, 46, 4089–4094 CrossRef CAS.
- R. F. Shamoun, A. Reisch and J. B. Schlenoff, Adv. Funct. Mater., 2012, 22, 1923–1931 CrossRef CAS.
- J. Y. Li, G. van Ewijk, D. J. van Dijken, J. van der Gucht and W. M. de Vos, ACS Appl. Mater. Interfaces, 2021, 13, 21844–21853 CrossRef CAS PubMed.
- T. Yuan, X. M. Cui, X. K. Liu, X. X. Qu and J. Q. Sun, Macromolecules, 2019, 52, 3141–3149 CrossRef CAS.
- F. Luo, T. L. Sun, T. Nakajima, T. Kurokawa, A. Bin Ihsan, X. F. Li, H. L. Guo and J. P. Gong, ACS Macro Lett., 2015, 4, 961–964 CrossRef CAS PubMed.
- A. Reisch, E. Roger, T. Phoeung, C. Antheaume, C. Orthlieb, F. Boulmedais, P. Lavalle, J. B. Schlenoff, B. Frisch and P. Schaaf, Adv. Mater., 2014, 26, 2547–2551 CrossRef CAS PubMed.
- J. K. Bediako, E. S. M. Mouele, Y. El Ouardi and E. Repo, Chem. Eng. J., 2023, 462, 142322 CrossRef CAS.
- S. Manoj Lalwani, C. I. Eneh and J. L. Lutkenhaus, Phys. Chem. Chem. Phys., 2020, 22, 24157–24177 RSC.
- D. Kovacevic, S. van der Burgh, A. de Keizer and M. A. Cohen Stuart, Langmuir, 2002, 18, 5607–5612 CrossRef CAS.
- W. M. de Vos and S. Lindhoud, Adv. Colloid Interface Sci., 2019, 274, 102040 CrossRef CAS PubMed.
- S. Lindhoud, W. Norde and M. A. Cohen Stuart, J. Phys. Chem. B, 2009, 113, 5431–5439 CrossRef CAS PubMed.
- V. Pryamitsyn and V. Ganesan, J. Chem. Phys., 2015, 143, 164904 CrossRef PubMed.
- H. Wu, J. M. Ting and M. V. Tirrell, Macromolecules, 2020, 53, 102–111 CrossRef CAS.
- H. H. Hariri, A. M. Lehaf and J. B. Schlenoff, Macromolecules, 2012, 45, 9364–9372 CrossRef CAS.
- M. Tirrell, ACS Cent. Sci., 2018, 4, 532–533 CrossRef CAS PubMed.
- R. Zhang, Y. Zhang, H. S. Antila, J. L. Lutkenhaus and M. Sammalkorpi, J. Phys. Chem. B, 2017, 121, 322–333 CrossRef CAS PubMed.
- A. D. Kulkarni, Y. H. Vanjari, K. H. Sancheti, H. M. Patel, V. S. Belgamwar, S. J. Surana and C. V. Pardeshi, Artif. Cells, Nanomed., Biotechnol., 2016, 44, 1615–1625 CrossRef CAS PubMed.
- S. Lankalapalli and V. R. Kolapalli, Indian J. Pharm. Sci., 2009, 71, 481–487 CrossRef CAS PubMed.
- V. S. Meka, M. K. G. Sing, M. R. Pichika, S. R. Nali, V. R. M. Kolapalli and P. Kesharwani, Drug Discovery Today, 2017, 22, 1697–1706 CrossRef CAS PubMed.
- B. P. Das and M. Tsianou, Adv. Colloid Interface Sci., 2017, 244, 71–89 CrossRef CAS PubMed.
- C. E. Sing and S. L. Perry, Soft Matter, 2020, 16, 2885–2914 RSC.
- Q. Wang and J. B. Schlenoff, Macromolecules, 2014, 47, 3108–3116 CrossRef CAS.
- A. E. Neitzel, G. X. De Hoe and M. V. Tirrell, Curr. Opin. Solid State Mater. Sci., 2021, 25, 100897 CrossRef CAS.
- Y. Liu, M. Zhu, Q. Zhao, Q. An, J. Qian, K. Lee and J. Lai, J. Membr. Sci., 2011, 385–386, 132–140 CrossRef CAS.
- C.-A. Ghiorghita, F. Bucatariu and E. S. Dragan, Mater. Sci. Eng., C, 2019, 105, 110050 CrossRef CAS PubMed.
- S. H. Lee, Polym. J., 2000, 32, 716–721 CrossRef CAS.
- B. Han, T. Ma, J. H. Vergara, G. R. Palmese, J. Yin, D. Lee and L. Han, RSC Adv., 2017, 7, 53334–53345 RSC.
- E. Diamanti, N. Muzzio, D. Gregurec, J. Irigoyen, M. Pasquale, O. Azzaroni, M. Brinkmann and S. E. Moya, Colloids Surf., B, 2016, 145, 328–337 CrossRef CAS PubMed.
- T. Liu, Q.-F. An, Q. Zhao, J.-K. Wu, Y.-H. Song, B.-K. Zhu and C.-J. Gao, Sci. Rep., 2015, 5, 7782 CrossRef CAS PubMed.
- R. A. Ghostine, R. M. Jisr, A. Lehaf and J. B. Schlenoff, Langmuir, 2013, 29, 11742–11750 CrossRef CAS PubMed.
- M. Zerball, A. Laschewsky, R. Köhler and R. Von Klitzing, Polymers, 2016, 8, 120 CrossRef PubMed.
- R. Steitz, V. Leiner, K. Tauer, V. Khrenov and R. V. Klitzing, Appl. Phys. A:Mater. Sci. Process., 2002, 74, s519–s521 CrossRef CAS.
- D. M. Reurink, J. P. Haven, I. Achterhuis, S. Lindhoud, H. D. W. Roesink and W. M. de Vos, Adv. Mater. Interfaces, 2018, 5, 1800651 CrossRef.
- G. Decher and J. Schmitt, Darmstadt, 1992.
- G. Decher, Science, 1997, 277, 1232–1237 CrossRef CAS.
- G. Decher, M. Eckle, J. Schmitt and B. Struth, Curr. Opin. Colloid Interface Sci., 1998, 3, 32–39 CrossRef CAS.
- G. Decher, B. Lehr, K. Lowack, Y. Lvov and J. Schmitt, Biosens. Bioelectron., 1994, 9, 677–684 CrossRef CAS.
- E. Guzmán, R. G. Rubio and F. Ortega, Adv. Colloid Interface Sci., 2020, 282, 102197 CrossRef PubMed.
- M. M. de Villiers, D. P. Otto, S. J. Strydom and Y. M. Lvov, Adv. Drug Delivery Rev., 2011, 63, 701–715 CrossRef CAS PubMed.
- A. Mateos-Maroto, I. Abelenda-Núñez, F. Ortega, R. G. Rubio and E. Guzmán, Polymers, 2021, 13, 1221 CrossRef CAS PubMed.
- A. P. R. Johnston, C. Cortez, A. S. Angelatos and F. Caruso, Curr. Opin. Colloid Interface Sci., 2006, 11, 203–209 CrossRef CAS.
- K. Ariga, Y. M. Lvov, K. Kawakami, Q. Ji and J. P. Hill, Adv. Drug Delivery Rev., 2011, 63, 762–771 CrossRef CAS PubMed.
- R. M. Iost and F. N. Crespilho, Biosens. Bioelectron., 2012, 31, 1–10 CrossRef CAS PubMed.
- J. J. Richardson, J. Cui, M. Björnmalm, J. A. Braunger, H. Ejima and F. Caruso, Chem. Rev., 2016, 116, 14828–14867 CrossRef CAS PubMed.
- J. J. Richardson, M. Björnmalm and F. Caruso, Science, 2015, 348, aaa2491 CrossRef PubMed.
- M. Michel, V. Toniazzo, D. Ruch and V. Ball, Int. Scholarly Res. Not., 2012, 2012, 701695 Search PubMed.
- G.-R. Xu, S.-H. Wang, H.-L. Zhao, S.-B. Wu, J.-M. Xu, L. Li and X.-Y. Liu, J. Membr. Sci., 2015, 493, 428–443 CrossRef CAS.
- J. Saqib and I. H. Aljundi, J. Water Process Eng., 2016, 11, 68–87 CrossRef.
- C. Wang, M. J. Park, H. Yu, H. Matsuyama, E. Drioli and H. K. Shon, J. Membr. Sci., 2022, 661, 120926 CrossRef CAS.
- N. B. Pramanik and S. L. Regen, Chem. Rec., 2020, 20, 163–173 CrossRef CAS PubMed.
- W. A. Jonkers, E. R. Cornelissen and W. M. de Vos, J. Membr. Sci., 2023, 669, 121234 CrossRef CAS.
- P. S. Roy, Ind. Eng. Chem. Res., 2024, 63, 5414–5487 CrossRef CAS.
- L. Shu, Y. Gong, M. Lin, J. Sun and X. Chen, Appl. Phys. Rev., 2024, 11, 021333 CAS.
- S. Chen, Q. Guo and J. Yu, Aggregate, 2022, 3, e293 CrossRef CAS.
- W. C. Blocher and S. L. Perry, Wiley Interdiscip. Rev.:Nanomed. Nanobiotechnol., 2017, 9, e1442 Search PubMed.
- C. A. Quist-Jensen, F. Macedonio and E. Drioli, Desalination, 2015, 364, 17–32 CrossRef CAS.
- B. Krause, M. Storr, T. Ertl, R. Buck, H. Hildwein, R. Deppisch and H. Göhl, Chem. Ing. Tech., 2003, 75, 1725–1732 CrossRef CAS.
- C. Charcosset, Food Eng. Rev., 2021, 13, 322–343 CrossRef CAS.
- A. Basile and S. P. Nunes, Advanced Membrane Science and Technology for Sustainable Energy and Environmental Applications, 1st edn, 2011 Search PubMed.
- K. A. Leamy, S. M. Assmann, D. H. Mathews and P. C. Bevilacqua, Q. Rev. Biophys., 2016, 49, e10 CrossRef PubMed.
- M. Ulbricht, Polymer, 2006, 47, 2217–2262 CrossRef CAS.
- D.-M. Wang and J.-Y. Lai, Curr. Opin. Chem. Eng., 2013, 2, 229–237 CrossRef.
- G. R. Guillen, Y. Pan, M. Li and E. M. Hoek, Ind. Eng. Chem. Res., 2011, 50, 3798–3817 CrossRef CAS.
- D. Prat, J. Hayler and A. Wells, Green Chem., 2014, 16, 4546–4551 RSC.
- J. Sherwood, T. J. Farmer and J. H. Clark, Chem, 2018, 4, 2010–2012 CAS.
- Q. Zhao, Q. F. An, Y. Ji, J. Qian and C. Gao, J. Membr. Sci., 2011, 379, 19–45 CrossRef CAS.
- V. S. Meka, M. K. Sing, M. R. Pichika, S. R. Nali, V. R. Kolapalli and P. Kesharwani, Drug Discovery Today, 2017, 22, 1697–1706 CrossRef CAS PubMed.
- Q. Zhao, J.-W. Qian, Q.-F. An, Q. Yang and P. Zhang, J. Membr. Sci., 2008, 320, 8–12 CrossRef CAS.
- Q. Zhao, Y.-L. Ji, J.-K. Wu, L.-L. Shao, Q.-F. An and C.-J. Gao, RSC Adv., 2014, 4, 52808–52814 RSC.
- X.-S. Wang, Y.-L. Ji, P.-Y. Zheng, Q.-F. An, Q. Zhao, K.-R. Lee, J.-W. Qian and C.-J. Gao, J. Mater. Chem. A, 2015, 3, 7296–7303 RSC.
- P.-Y. Zheng, W.-H. Zhang, K.-F. Chen, N.-X. Wang and Q.-F. An, J. Taiwan Inst. Chem. Eng., 2019, 95, 627–634 CrossRef CAS.
- G. Zhumadilova, A. Gazizov, L. Bimendina and S. Kudaibergenov, Polymer, 2001, 42, 2985–2989 CrossRef CAS.
- B. Smitha, S. Sridhar and A. Khan, Macromolecules, 2004, 37, 2233–2239 CrossRef CAS.
- X. Li, C. Liu and B. Van der Bruggen, J. Mater. Chem. A, 2020, 8, 20870–20896 RSC.
- E. N. Durmaz, S. Sahin, E. Virga, S. De Beer, L. C. De Smet and W. M. De Vos, ACS Appl. Polym. Mater., 2021, 3, 4347–4374 CrossRef CAS PubMed.
- A. Panagiotopoulos, J. Phys.: Condens. Matter, 2009, 21, 424113 CrossRef CAS PubMed.
- J. D. Willott, W. M. Nielen and W. M. de Vos, ACS Appl. Polym. Mater., 2019, 2, 659–667 CrossRef PubMed.
- W. M. Nielen, J. D. Willott and W. M. de Vos, ACS Appl. Polym. Mater., 2020, 2, 1702–1710 CrossRef CAS PubMed.
- W. M. Nielen, J. D. Willott, Z. M. Esguerra and W. M. de Vos, J. Colloid Interface Sci., 2020, 576, 186–194 CrossRef CAS PubMed.
- W. M. Nielen, J. D. Willott, J. A. Galicia and W. M. de Vos, Polymers, 2021, 13, 1775 CrossRef CAS PubMed.
- M. I. Baig, E. N. Durmaz, J. D. Willott and W. M. de Vos, Adv. Funct. Mater., 2020, 30, 1907344 CrossRef CAS.
- M. I. Baig, J. D. Willott and W. M. de Vos, J. Membr. Sci., 2020, 615, 118502 CrossRef CAS.
- M. I. Baig, P. P. I. Sari, J. Li, J. D. Willott and W. M. de Vos, J. Membr. Sci., 2021, 625, 119114 CrossRef CAS.
- M. M. H. Mizan, M. Rastgar, S. A. Aktij, A. Asad, P. Karami, A. Rahimpour and M. Sadrzadeh, J. Membr. Sci., 2023, 668, 121197 CrossRef.
- E. N. Durmaz, J. D. Willott, A. Fatima and W. M. de Vos, Eur. Polym. J., 2020, 139, 110015 CrossRef CAS.
- M. I. Baig, M. Pejman, J. D. Willott, A. Tiraferri and W. M. De Vos, ACS Appl. Polym. Mater., 2022, 4, 1010–1020 CrossRef CAS PubMed.
- L. Li, M. I. Baig, W. M. de Vos and S. Lindhoud, ACS Appl. Polym. Mater., 2023, 5, 1810–1818 CrossRef CAS.
- S. Ali and V. M. Prabhu, Gels, 2018, 4, 11 CrossRef PubMed.
- S. Lindhoud and M. A. C. Stuart, Polyelectrolyte complexes in the dispersed and solid state i: Principles and theory, 2014, pp. 139–172 Search PubMed.
- K. Sadman, D. E. Delgado, Y. Won, Q. Wang, K. A. Gray and K. R. Shull, ACS Appl. Mater. Interfaces, 2019, 11, 16018–16026 CrossRef CAS PubMed.
- E. N. Durmaz, M. I. Baig, J. D. Willott and W. M. de Vos, ACS Appl. Polym. Mater., 2020, 2, 2612–2621 CrossRef CAS PubMed.
- J. Kamp, S. Emonds, J. Borowec, M. A. R. Toro and M. Wessling, J. Membr. Sci., 2021, 618, 118632 CrossRef CAS.
- S. Emonds, J. Kamp, J. Borowec, H. Roth and M. Wessling, Adv. Eng. Mater., 2021, 23, 2001401 CrossRef CAS.
- M. A. Restrepo, S. Emonds, A. Zhao, F. Karakas, J. Kamp, H. Roth and M. Wessling, J. Membr. Sci., 2024, 689, 122157 CrossRef CAS.
- L. Li, M. I. Baig, W. M. de Vos and S. Lindhoud, Soft Matter, 2024, 20, 5425–5434 RSC.
- M. I. Baig, J. D. Willott and W. M. De Vos, ACS Appl. Polym. Mater., 2021, 3, 3560–3568 CrossRef CAS PubMed.
- M. A. Restrepo, J. Kamp, L. Guericke, R. Schnichels, H. Roth and M. Wessling, J. Membr. Sci. Lett., 2023, 3, 100039 CrossRef.
- J. J. van Lente, M. I. Baig, W. M. de Vos and S. Lindhoud, J. Colloid Interface Sci., 2022, 616, 903–910 CrossRef CAS PubMed.
- Performance of Bio-based Building Materials, ed. D. Jones and C. Brischke, Woodhead Publishing, 2017, pp. 187–247 DOI:10.1016/B978-0-08-100982-6.00004-5.
- N. Kampf, Polym. Adv. Technol., 2002, 13, 895–904 CrossRef.
- V. D. Athawale and R. V. Nimbalkar, J. Am. Oil Chem. Soc., 2011, 88, 159–185 CrossRef CAS.
- R. R. Smith, A. P. Smith, J. T. Stricker, B. E. Taylor and M. F. Durstock, Macromolecules, 2006, 39, 6071–6074 CrossRef CAS.
- Y. Zhao, J. Hu, X. Hu, F. Zhu, J. Su and J. Han, Surf. Coat. Technol., 2023, 453, 129143 CrossRef CAS.
- T. Kruk, R. P. Socha, L. Szyk-Warszyńska and P. Warszyński, Appl. Surf. Sci., 2019, 484, 501–510 CrossRef CAS.
- C. Q. Peng, Y. S. Thio and R. A. Gerhardt, Nanotechnology, 2008, 19, 505603 CrossRef CAS PubMed.
- Y. Fu, Y. Zhang and Y. Wang, Wood Mater. Sci. Eng., 2023, 18, 1553–1561 CrossRef CAS.
- M. Tiitu, J. Laine, R. Serimaa and O. Ikkala, J. Colloid Interface Sci., 2006, 301, 92–97 CrossRef CAS PubMed.
- S. J. Kim, S. G. Yoon, K. B. Lee, Y. D. Park and S. I. Kim, Solid State Ionics, 2003, 164, 199–204 CrossRef CAS.
- J. A. Regenspurg, W. A. Jonkers, M. A. Junker, I. Achterhuis, E. te Brinke and W. M. de Vos, Desalination, 2024, 583, 117693 CrossRef CAS.
- L. M. Petrila, F. Bucatariu, M. Mihai and C. Teodosiu, Materials, 2021, 14, 4152 CrossRef CAS PubMed.
- C. Elosua, D. Lopez-Torres, M. Hernaez, I. R. Matias and F. J. Arregui, Nanoscale Res. Lett., 2013, 8, 539 CrossRef PubMed.
- K. C. Krogman, N. S. Zacharia, S. Schroeder and P. T. Hammond, Langmuir, 2007, 23, 3137–3141 CrossRef CAS PubMed.
- L. A. Felton, Int. J. Pharm., 2013, 457, 423–427 CrossRef CAS PubMed.
- J. Wang, Y.-F. Xue, X.-C. Chen, M. Hu, K.-F. Ren and J. Ji, Adv. Healthcare Mater., 2020, 9, 2000381 CrossRef CAS PubMed.
- S. Basu, A. Plucinski and J. M. Catchmark, Green Chem., 2017, 19, 4080–4092 RSC.
- K. Chi and J. M. Catchmark, Food Hydrocolloids, 2018, 80, 195–205 CrossRef CAS.
- K. Chi, H. Wang and J. M. Catchmark, Food Hydrocolloids, 2020, 103, 105696 CrossRef CAS.
- M. Haile, O. Sarwar, R. Henderson, R. Smith and J. C. Grunlan, Macromol. Rapid Commun., 2017, 38, 1600594 CrossRef PubMed.
- Y.-H. Yang, M. Haile, Y. T. Park, F. A. Malek and J. C. Grunlan, Macromolecules, 2011, 44, 1450–1459 CrossRef CAS.
- R. J. Smith, C. T. Long and J. C. Grunlan, Langmuir, 2018, 34, 11086–11091 CrossRef CAS PubMed.
- H. C. Chiang, T. J. Kolibaba, B. Eberle and J. C. Grunlan, Macromol. Rapid Commun., 2021, 42, e2000540 CrossRef PubMed.
- H.-C. Chiang, B. Eberle, D. Carlton, T. J. Kolibaba and J. C. Grunlan, ACS Food Sci. Technol., 2021, 1, 495–499 CrossRef CAS.
- R. J. Smith, M. G. Moule, P. A. Leon, E. T. Iverson, T. J. Kolibaba, J. D. Cirillo and J. C. Grunlan, Macromol. Mater. Eng., 2021, 306, 2100579 CrossRef CAS.
- S. T. Lazar, T. J. Kolibaba and J. C. Grunlan, Nat. Rev. Mater., 2020, 5, 259–275 CrossRef CAS.
- J. Li, M. H. P. de Heer Kloots, G. van Ewijk, D. J. van Dijken, W. M. de Vos and J. van der Gucht, Langmuir, 2024, 40, 2531–2542 CrossRef CAS PubMed.
- A. A. Cain, S. Murray, K. M. Holder, C. R. Nolen and J. C. Grunlan, Macromol. Mater. Eng., 2014, 299, 1180–1187 CrossRef CAS.
- J. Li, G. van Ewijk, D. J. van Dijken, J. van der Gucht and W. M. de Vos, ACS Appl. Mater. Interfaces, 2021, 13, 21844–21853 CrossRef CAS PubMed.
- I. Pietsch, A. Weiss, P. Preishuber-Pflügl, P. Bippus and K. Hünerfauth, Use of Aqueous Polyanion-Polyethyleneimine Solutions for Producing Polymer Films with Oxygen-Barrier Properties, Patent EP2859035B1, CN104350091A, EP2859035A1, US20150086734, WO2013182444A1, 2016 Search PubMed.
- J. Li, A. Krishna B, G. van Ewijk, D. J. van Dijken, W. M. de Vos and J. van der Gucht, Colloids Surf., A, 2022, 648, 129143 CrossRef CAS.
- M. Hubbe, BioResources, 2021, 16, 4544–4605 Search PubMed.
- M. Haile, C. Fincher, S. Fomete and J. C. Grunlan, Polym. Degrad. Stab., 2015, 114, 60–64 CrossRef CAS.
- H. M. van der Kooij, R. Fokkink, J. van der Gucht and J. Sprakel, Sci. Rep., 2016, 6, 34383 CrossRef CAS PubMed.
- H. M. van der Kooij and J. Sprakel, Soft Matter, 2015, 11, 6353–6359 RSC.
- Y. Zhao, Y. Wu, L. Wang, M. Zhang, X. Chen, M. Liu, J. Fan, J. Liu, F. Zhou and Z. Wang, Nat. Commun., 2017, 8, 2218 CrossRef PubMed.
- H. Lee, N. F. Scherer and P. B. Messersmith, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 12999–13003 CrossRef CAS PubMed.
- J. H. Waite, Integr. Comp. Biol., 2002, 42, 1172–1180 CrossRef CAS PubMed.
- B. P. Lee, P. B. Messersmith, J. N. Israelachvili and J. H. Waite, Annu. Rev. Mater. Res., 2011, 41, 99–132 CrossRef CAS PubMed.
- R. J. Stewart, T. C. Ransom and V. Hlady, J. Polym. Sci., Part B: Polym. Phys., 2011, 49, 757–771 CrossRef CAS PubMed.
- M. J. Brennan, B. F. Kilbride, J. J. Wilker and J. C. Liu, Biomaterials, 2017, 124, 116–125 CrossRef CAS PubMed.
- J. D. White and J. J. Wilker, Macromolecules, 2011, 44, 5085–5088 CrossRef CAS.
- Y. Zhou, C. Zhang, S. Gao, W. Li, J.-J. Kai and Z. Wang, ACS Appl. Mater. Interfaces, 2021, 13, 50451–50460 CrossRef CAS PubMed.
- C. Heinzmann, C. Weder and L. M. de Espinosa, Chem. Soc. Rev., 2016, 45, 342–358 RSC.
- H. Lee, S. M. Dellatore, W. M. Miller and P. B. Messersmith, Science, 2007, 318, 426–430 CrossRef CAS PubMed.
- H. Lee, B. P. Lee and P. B. Messersmith, Nature, 2007, 448, 338–341 CrossRef CAS PubMed.
- L. Xue, B. Sanz, A. Luo, K. T. Turner, X. Wang, D. Tan, R. Zhang, H. Du, M. Steinhart, C. Mijangos, M. Guttmann, M. Kappl and A. del Campo, ACS Nano, 2017, 11, 9711–9719 CrossRef CAS PubMed.
- M. Follador, F. Tramacere and B. Mazzolai, Bioinspiration Biomimetics, 2014, 9, 046002 CrossRef CAS PubMed.
- P. G. Lawrence and Y. Lapitsky, Langmuir, 2015, 31, 1564–1574 CrossRef CAS PubMed.
- Q. Peng, J. Chen, T. Wang, L. Gong, X. Peng, M. Wu, Y. Ma, F. Wu, D. Yang, H. Zhang and H. Zeng, J. Mater. Chem. A, 2021, 9, 12988–13000 RSC.
- R. J. Stewart, C. S. Wang and H. Shao, Adv. Colloid Interface Sci., 2011, 167, 85–93 CrossRef CAS PubMed.
- S. Kim, H. Y. Yoo, J. Huang, Y. Lee, S. Park, Y. Park, S. Jin, Y. M. Jung, H. Zeng, D. S. Hwang and Y. Jho, ACS Nano, 2017, 11, 6764–6772 CrossRef CAS PubMed.
- J.-S. Jhiang, T.-H. Wu, C.-J. Chou, Y. Chang and C.-J. Huang, J. Mater. Chem. B, 2019, 7, 2878–2887 RSC.
- B. K. Ahn, S. Das, R. Linstadt, Y. Kaufman, N. R. Martinez-Rodriguez, R. Mirshafian, E. Kesselman, Y. Talmon, B. H. Lipshutz, J. N. Israelachvili and J. H. Waite, Nat. Commun., 2015, 6, 8663 CrossRef CAS PubMed.
- W. Wei, Y. Tan, N. R. Martinez Rodriguez, J. Yu, J. N. Israelachvili and J. H. Waite, Acta Biomater., 2014, 10, 1663–1670 CrossRef CAS PubMed.
- I. Kaminker, W. Wei, A. M. Schrader, Y. Talmon, M. T. Valentine, J. N. Israelachvili, J. H. Waite and S. Han, Soft Matter, 2017, 13, 9122–9131 RSC.
- H. Shao, K. N. Bachus and R. J. Stewart, Macromol. Biosci., 2009, 9, 464–471 CrossRef CAS PubMed.
- D. S. Hwang, H. Zeng, A. Srivastava, D. V. Krogstad, M. Tirrell, J. N. Israelachvili and J. H. Waite, Soft Matter, 2010, 6, 3232–3236 RSC.
- Y. Huang, P. G. Lawrence and Y. Lapitsky, Langmuir, 2014, 30, 7771–7777 CrossRef CAS PubMed.
- Z. Bashir, W. Yu, Z. Xu, Y. Li, J. Lai, Y. Li, Y. Cao and B. Xue, Int. J. Mol. Sci., 2022, 23, 9987 CrossRef CAS PubMed.
- W. Sun, T. Liu, X. Zhang, X. Zhang, Q. Yan, J. Yin and S. Luan, Adv. Healthcare Mater., 2023, 12, 2300669 CrossRef CAS PubMed.
- C. Cui and W. Liu, Prog. Polym. Sci., 2021, 116, 101388 CrossRef CAS.
- A. Narayanan, A. Dhinojwala and A. Joy, Chem. Soc. Rev., 2021, 50, 13321–13345 RSC.
- H. Fan and J. P. Gong, Adv. Mater., 2021, 33, 2102983 CrossRef CAS PubMed.
- M. Vahdati, D. Hourdet and C. Creton, Prog. Polym. Sci., 2023, 139, 101649 CrossRef CAS.
- M. Li, A. Mao, Q. Guan and E. Saiz, Chem. Soc. Rev., 2024, 53, 8240–8305 RSC.
- Y. Lu, X. Xu and J. Li, J. Mater. Chem. B, 2023, 11, 3338–3355 RSC.
- J.-N. Zhang, H. Zhu, T. Liu, Y. Chen, C. Jiao, C. He and H. Wang, Polymer, 2020, 206, 122845 CrossRef CAS.
- M. Vahdati, F. J. Cedano-Serrano, C. Creton and D. Hourdet, ACS Appl. Polym. Mater., 2020, 2, 3397–3410 CrossRef CAS.
- M. Dompé, F. J. Cedano-Serrano, O. Heckert, N. van den Heuvel, J. van der Gucht, Y. Tran, D. Hourdet, C. Creton and M. Kamperman, Adv. Mater., 2019, 31, 1808179 CrossRef PubMed.
- L. van Westerveld, T. Pelras, A. H. Hofman, K. Loos, M. Kamperman and J. Es Sayed, Macromolecules, 2024, 57, 652–663 CrossRef CAS PubMed.
- M. Dompé, F. J. Cedano-Serrano, M. Vahdati, L. van Westerveld, D. Hourdet, C. Creton, J. van der Gucht, T. Kodger and M. Kamperman, Adv. Mater. Interfaces, 2020, 7, 1901785 CrossRef.
- I. A. van Hees, A. H. Hofman, M. Dompé, J. van der Gucht and M. Kamperman, Eur. Polym. J., 2020, 141, 110034 CrossRef CAS.
- L. van Westerveld, J. Es Sayed, M. de Graaf, A. H. Hofman, M. Kamperman and D. Parisi, Soft Matter, 2023, 19, 8832–8848 RSC.
- H. Shao and R. J. Stewart, Adv. Mater., 2010, 22, 729–733 CrossRef CAS PubMed.
- X. Feng, K. Pouw, V. Leung and R. Pelton, Biomacromolecules, 2007, 8, 2161–2166 CrossRef CAS PubMed.
- C. Wei, X. Zhu, H. Peng, J. Chen, F. Zhang and Q. Zhao, ACS Sustainable Chem. Eng., 2019, 7, 4508–4514 CrossRef CAS.
- X. Li, L. Shang, D. Li, W. Wang, S. Chen, H. Zhong, Y. Huang and S. Long, Polym. Test., 2022, 109, 107547 CrossRef CAS.
- R. Qie, S. Zajforoushan Moghaddam and E. Thormann, ACS Sustainable Chem. Eng., 2024, 12, 4456–4463 CrossRef CAS.
- G. Hernandez-Pérez, G. Goma and J. L. Rols, Water Res., 1999, 33, 1837–1844 CrossRef.
- K. Ushimaru, T. Morita and T. Fukuoka, J. Wood Chem. Technol., 2020, 40, 172–177 CrossRef CAS.
- I. Aranaz, A. R. Alcántara, M. C. Civera, C. Arias, B. Elorza, A. Heras Caballero and N. Acosta, Polymers, 2021, 13, 3256 CrossRef CAS PubMed.
- Q. Zhao, D. W. Lee, B. K. Ahn, S. Seo, Y. Kaufman, J. N. Israelachvili and J. H. Waite, Nat. Mater., 2016, 15, 407–412 CrossRef CAS PubMed.
- C. Wei, C. Zhao, Y. Ru, Y. Sun, T. Zhao, S. Qi, J. Zhou and M. Liu, Adv. Funct. Mater., 2024, 2400581 CrossRef CAS.
- P. Galland, M. H. Iqbal, D. Favier, M. Legros, P. Schaaf, F. Boulmedais and M. Vahdati, J. Colloid Interface Sci., 2024, 661, 196–206 CrossRef CAS PubMed.
- X. Liu, M. Haddou, I. Grillo, Z. Mana, J.-P. Chapel and C. Schatz, Soft Matter, 2016, 12, 9030–9038 RSC.
- S. Kim, J. Huang, Y. Lee, S. Dutta, H. Y. Yoo, Y. M. Jung, Y. Jho, H. Zeng and D. S. Hwang, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, E847–E853 CrossRef CAS PubMed.
- J. Zhang, H. Lei, M. Qin, W. Wang and Y. Cao, Supramol. Mater., 2022, 1, 100005 Search PubMed.
- H. Fan, J. Wang, Z. Tao, J. Huang, P. Rao, T. Kurokawa and J. P. Gong, Nat. Commun., 2019, 10, 5127 CrossRef PubMed.
- M. Cui, S. Ren, S. Wei, C. Sun and C. Zhong, APL Mater., 2017, 5, 116102 CrossRef.
- Y. Chen, J. Meng, Z. Gu, X. Wan, L. Jiang and S. Wang, Adv. Funct. Mater., 2020, 30, 1905287 CrossRef CAS.
- H. Y. Yuen, H. P. Bei and X. Zhao, Chem. Eng. J., 2022, 431, 133372 CrossRef CAS.
- Q. F. Wang and J. B. Schlenoff, Macromolecules, 2014, 47, 3108–3116 CrossRef CAS.
- C. H. Porcel and J. B. Schlenoff, Biomacromolecules, 2009, 10, 2968–2975 CrossRef CAS PubMed.
- H. H. Hariri and J. B. Schlenoff, Macromolecules, 2010, 43, 8656–8663 CrossRef CAS PubMed.
- R. Steitz, W. Jaeger and R. von Klitzing, Langmuir, 2001, 17, 4471–4474 CrossRef CAS.
- K. Glinel, A. Moussa, A. M. Jonas and A. Laschewsky, Langmuir, 2002, 18, 1408–1412 CrossRef CAS.
- B. Vishalakshi, S. Ghosh and V. Kalpagam, Polymer, 1993, 34, 3270–3275 CrossRef CAS.
- A. Shakya, M. Girard, J. T. King and M. O. de la Cruz, Macromolecules, 2020, 53, 1258–1269 CrossRef CAS.
- C. Schatz, J. M. Lucas, C. Viton, A. Domard, C. Pichot and T. Delair, Langmuir, 2004, 20, 7766–7778 CrossRef CAS PubMed.
- Z. J. Sui, D. Salloum and J. B. Schlenoff, Langmuir, 2003, 19, 2491–2495 CrossRef CAS.
- J. Yang, M. Y. Shen, T. Wu, Y. Luo, M. Y. Li, H. L. Wen and J. H. Xie, Food Chem., 2020, 333, 127493 CrossRef CAS PubMed.
- A. S. Michaels, L. Mir and N. S. Schneide, J. Phys. Chem., 1965, 69, 1447–1455 CrossRef CAS.
- Y. H. Chen, M. Yang, S. Abou Shaheen and J. B. Schlenoff, Macromolecules, 2021, 54, 7890–7899 CrossRef CAS.
- A. Krishna, J. D. Willott, S. Lindhoud and W. M. De Vos, Polymer, 2022, 242, 124583 CrossRef.
- F. B. Zhu, L. B. Cheng, J. Yin, Z. L. Wu, J. Qian, J. Z. Fu and Q. Zheng, ACS Appl. Mater. Interfaces, 2016, 8, 31304–31310 CrossRef CAS PubMed.
- J. K. Bediako, J. H. Kang, Y. S. Yun and S. H. Choi, ACS Appl. Polym. Mater., 2022, 4, 2346–2354 CrossRef CAS.
- J. Akintola, Z. A. Digby and J. B. Schlenoff, ACS Appl. Mater. Interfaces, 2023, 15, 9962–9969 CrossRef CAS PubMed.
- J. K. Bediako, E. S. M. Mouele, Y. El Ouardi and E. Repo, Chem. Eng. J., 2023, 462, 142322 CrossRef CAS.
- A. S. Michaels, Ind. Eng. Chem., 1965, 57, 32–40 CrossRef CAS.
- L. Gubler, Curr. Opin. Electrochem., 2019, 18, 31–36 CrossRef CAS.
- M. Ahmad and M. Ahmed, J. Appl. Electrochem., 2023, 53, 1537–1562 CrossRef CAS.
- H. Strathmann, A. Grabowski and G. Eigenberger, Ind. Eng. Chem. Res., 2013, 52, 10364–10379 CrossRef CAS.
- Y. X. Yang, P. Li, X. B. Zheng, W. P. Sun, S. X. Dou, T. Y. Ma and H. G. Pan, Chem. Soc. Rev., 2022, 51, 9620–9693 RSC.
- J. Ran, L. Wu, Y. B. He, Z. J. Yang, Y. M. Wang, C. X. Jiang, L. Ge, E. Bakangura and T. W. Xu, J. Membr. Sci., 2017, 522, 267–291 CrossRef CAS.
- S. A. N. Mehrabani, V. Vatanpour and I. Koyuncu, Sep. Purif. Technol., 2022, 298, 121691 CrossRef.
- J. Rozière and D. J. Jones, Annu. Rev. Mater. Res., 2003, 33, 503–555 CrossRef.
- B. A. Krishna, S. Lindhoud and W. M. de Vos, J. Colloid Interface Sci., 2021, 593, 11–20 CrossRef PubMed.
- B. A. Krishna, W. M. de Vos and S. Lindhoud, Langmuir, 2023, 39, 6874–6884 CrossRef PubMed.
- A. Krishna, H. J. Zwijnenberg, S. Lindhoud and W. M. de Vos, J. Membr. Sci., 2022, 652, 120463 CrossRef.
- M. B. Arif, S. Kheawhom and S. T. Dubas, J. Energy Storage, 2023, 74, 109425 CrossRef.
- P. Luangaramvej and S. T. Dubas, e-Polym., 2021, 21, 194–199 CrossRef CAS.
- M. Yang, Z. A. Digby, Y. Chen and J. B. Schlenoff, Sci. Adv., 2022, 8, eabm4783 CrossRef CAS PubMed.
- D. Kitto and J. Kamcev, J. Membr. Sci., 2023, 677, 121608 CrossRef CAS.
- M. Yang, Z. A. Digby and J. B. Schlenoff, Macromolecules, 2020, 53, 5465–5474 CrossRef CAS.
- H. M. Fares, Q. F. Wang, M. Yang and J. B. Schlenoff, Macromolecules, 2019, 52, 610–619 CrossRef CAS.
- R. F. Shamoun, H. H. Hariri, R. A. Ghostine and J. B. Schlenoff, Macromolecules, 2012, 45, 9759–9767 CrossRef CAS.
- Y. S. Ye and Y. A. Elabd, Macromolecules, 2011, 44, 8494–8503 CrossRef CAS.
- S. Chempath, B. R. Einsla, L. R. Pratt, C. S. Macomber, J. M. Boncella, J. A. Rau and B. S. Pivovar, J. Phys. Chem. C, 2008, 112, 3179–3182 CrossRef CAS.
- M. G. Marino and K. D. Kreuer, ChemSusChem, 2015, 8, 513–523 CrossRef CAS PubMed.
- Y. X. Liu, M. H. Zhu, Q. Zhao, Q. F. An, J. W. Qian, K. Lee and J. Y. Lai, J. Membr. Sci., 2011, 385, 132–140 CrossRef.
- D. M. Reurink, J. D. Willott, H. D. W. Roesink and W. M. de Vos, ACS Appl. Polym. Mater., 2020, 2, 5278–5289 CrossRef CAS.
- M. A. Restrepo, S. Emonds, A. Zhao, F. Karakas, J. Kamp, H. Roth and M. Wessling, J. Membr. Sci., 2024, 689, 122157 CrossRef CAS.
- J. A. Asenjo and B. A. Andrews, J. Chromatogr. A, 2011, 1218, 8826–8835 CrossRef CAS PubMed.
- M. Iqbal, Y. Tao, S. Xie, Y. Zhu, D. Chen, X. Wang, L. Huang, D. Peng, A. Sattar, M. A. B. Shabbir, H. I. Hussain, S. Ahmed and Z. Yuan, Biol. Proced. Online, 2016, 18, 18 CrossRef PubMed.
- A. L. Grilo, M. Raquel Aires-Barros and A. M. Azevedo, Sep. Purif. Rev., 2016, 45, 68–80 CrossRef.
- S. Boeynaems, S. Alberti, N. L. Fawzi, T. Mittag, M. Polymenidou, F. Rousseau, J. Schymkowitz, J. Shorter, B. Wolozin, L. Van Den Bosch, P. Tompa and M. Fuxreiter, Trends Cell Biol., 2018, 28, 420–435 CrossRef CAS PubMed.
- E. Gomes and J. Shorter, J. Biol. Chem., 2019, 294, 7115–7127 CrossRef CAS PubMed.
- M. Chen, R. Xu, Y. Wu, J. Xiong, S. Z. Keleş and N. P. Hankins, J. Water Process Eng., 2024, 64, 105528 CrossRef.
- A. A. Tokmakov, A. Kurotani and K. I. Sato, Front. Mol. Biosci., 2021, 8, 775736 CrossRef CAS PubMed.
- I. Rayment, in Encyclopedia of Physical Science and Technology, ed. R. A. Meyers, Academic Press, New York, 3rd edn, 2003, pp. 191–218 DOI:10.1016/B0-12-227410-5/00616-5.
- S. B. Ebrahimi and D. Samanta, Nat. Commun., 2023, 14, 2411 CrossRef CAS PubMed.
- T. Tang, N. Wu, S. Tang, N. Xiao, Y. Jiang, Y. Tu and M. Xu, J. Agric. Food Chem., 2023, 71, 1788–1801 CrossRef CAS PubMed.
- M. Zhao, S. A. Eghtesadi, M. B. Dawadi, C. Wang, S. Huang, A. E. Seymore, B. D. Vogt, D. A. Modarelli, T. Liu and N. S. Zacharia, Macromolecules, 2017, 50, 3818–3830 CrossRef CAS.
- A. Shah and N. I. Malek, J. Ionic Liq., 2021, 1, 100006 CrossRef.
- L. Li, S. Srivastava, M. Andreev, A. B. Marciel, J. J. de Pablo and M. V. Tirrell, Macromolecules, 2018, 51, 2988–2995 CrossRef CAS.
- A. E. Neitzel, Y. N. Fang, B. Yu, A. M. Rumyantsev, J. J. de Pablo and M. V. Tirrell, Macromolecules, 2021, 54, 6878–6890 CrossRef CAS PubMed.
- R. Staňo, J. J. van Lente, S. Lindhoud and P. Košovan, Macromolecules, 2024, 57, 1383–1398 CrossRef PubMed.
- W. C. Blocher McTigue and S. L. Perry, Soft Matter, 2019, 15, 3089–3103 RSC.
- W. C. Blocher McTigue and S. L. Perry, Small, 2020, 16, 1907671 CrossRef CAS PubMed.
- K. A. Black, D. Priftis, S. L. Perry, J. Yip, W. Y. Byun and M. Tirrell, ACS Macro Lett., 2014, 3, 1088–1091 CrossRef CAS PubMed.
- S. Lindhoud and M. M. A. E. Claessens, Soft Matter, 2016, 12, 408–413 RSC.
- J. J. van Lente, M. M. A. E. Claessens and S. Lindhoud, Biomacromolecules, 2019, 20, 3696–3703 CrossRef CAS PubMed.
- J. J. van Lente and S. Lindhoud, Small, 2022, 18, 2105147 CrossRef CAS PubMed.
- J. van Lente, M. Pazos Urrea, T. Brouwer, B. Schuur and S. Lindhoud, Green Chem., 2021, 23, 5812–5824 RSC.
- X. Mi, W. C. Blocher McTigue, P. U. Joshi, M. K. Bunker, C. L. Heldt and S. L. Perry, Biomater. Sci., 2020, 8, 7082–7092 RSC.
- P. U. Joshi, C. Decker, X. Zeng, A. Sathyavageeswaran, S. L. Perry and C. L. Heldt, Biomacromolecules, 2024, 25, 741–753 CrossRef CAS PubMed.
- C. H. Porcel and J. B. Schlenoff, Biomacromolecules, 2009, 10, 2968–2975 CrossRef CAS PubMed.
- M. Müller, L. Wirth and B. Urban, Appl. Spectrosc., 2024, 78, 56–66 CrossRef PubMed.
- G. Ferrand-Drake del Castillo, R. L. N. Hailes and A. Dahlin, J. Phys. Chem. Lett., 2020, 11, 5212–5218 CrossRef CAS PubMed.
- S. M. Lalwani, P. Batys, M. Sammalkorpi and J. L. Lutkenhaus, Macromolecules, 2021, 54, 7765–7776 CrossRef CAS.
- Y. Zhang, F. Li, L. D. Valenzuela, M. Sammalkorpi and J. L. Lutkenhaus, Macromolecules, 2016, 49, 7563–7570 CrossRef CAS.
- Z. A. Digby, M. Yang, S. Lteif and J. B. Schlenoff, Macromolecules, 2022, 55, 978–988 CrossRef CAS.
- U. Scheler, Curr. Opin. Colloid Interface Sci., 2009, 14, 212–215 CrossRef CAS.
- S. Akoka, L. Barantin and M. Trierweiler, Anal. Chem., 1999, 71, 2554–2557 CrossRef CAS PubMed.
- G. Allegri, J. Huskens, R. P. Martinho and S. Lindhoud, J. Colloid Interface Sci., 2024, 672, 654–663 CrossRef CAS PubMed.
- L. Barantin, A. L. Pape and S. Akoka, Magn. Reson. Med., 1997, 38, 179–182 CrossRef CAS PubMed.
- R. P. Choudhury and M. Schönhoff, J. Chem. Phys., 2007, 127, 234702 CrossRef PubMed.
- L. Patel, O. Mansour, M. Crossman and P. Griffiths, Langmuir, 2019, 35, 9233–9238 CrossRef CAS PubMed.
- N. Proietti, M. E. Amato, G. Masci and A. L. Segre, Macromolecules, 2002, 35, 4365–4372 CrossRef CAS.
- A. B. Ruiz-Muelle, P. G. Moreno and I. Fernández, Talanta, 2021, 230, 122344 CrossRef CAS PubMed.
- S. M. Lalwani, K. Hellikson, P. Batys and J. L. Lutkenhaus, Macromolecules, 2024, 57, 4695–4705 CrossRef CAS PubMed.
- V. Guillou and T. Schönberger, J. Pharm. Biomed. Anal., 2022, 213, 114690 CrossRef CAS PubMed.
- T. Minato and M. Satoh, J. Polym. Sci., Part B: Polym. Phys., 2004, 42, 4412–4420 CrossRef CAS.
- S. A. Watson, A. J. Edwards and J. A. Parkinson, Anal. Lett., 2017, 50, 161–172 CrossRef CAS.
- J. L. Ochoa, S. Germann, B. Conklin, K. Kurita, D. J. Russell, C. Yang and J. G. Napolitano, Magn. Reson. Chem., 2024, 62, 4–10 CrossRef CAS PubMed.
- T. V. Chalikian, M. Totrov, R. Abagyan and K. J. Breslauer, J. Mol. Biol., 1996, 260, 588–603 CrossRef CAS PubMed.
- H. Jiang, P. Taranekar, J. R. Reynolds and K. S. Schanze, Angew. Chem., Int. Ed., 2009, 48, 4300–4316 CrossRef CAS PubMed.
- B. J. Olson and J. Markwell, Curr Protoc Protein Sci, 2007, ch. 3, Unit 3.4 Search PubMed.
- A. C. Obermeyer, C. E. Mills, X. H. Dong, R. J. Flores and B. D. Olsen, Soft Matter, 2016, 12, 3570–3581 RSC.
- C. S. Cummings and A. C. Obermeyer, Biochemistry, 2018, 57, 314–323 CrossRef CAS PubMed.
- V. Scandella, R. C. Paolicelli and M. Knobloch, Sci. Rep., 2020, 10, 14642 CrossRef CAS PubMed.
- M. R. Soboleski, J. Oaks and W. P. Halford, FASEB J., 2005, 19, 440–442 CrossRef PubMed.
- E. Spruijt, A. H. Westphal, J. W. Borst, M. A. Cohen Stuart and J. van der Gucht, Macromolecules, 2010, 43, 6476–6484 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |