Research advances in the diagnosis of infectious disease by aptasensor technology
Hengxuan Li
ac,
Qiuxia Yangbc,
Xiaodong Lic,
Xiaoyi Fucd,
Jianhua Lie,
Yanjun Zhang
*e,
Weihong Tan
 *cf and
Peng Wang
*cf and
Peng Wang
 *c
*c
aMedical School, Faculty of Medicine, Tianjin University, Tianjin 300072, P. R. China
bTianjian Laboratory of Advanced Biomedical Sciences, Academy of Medical Sciences, Zhengzhou University, Zhengzhou 450001, P. R. China
cZhejiang Cancer Hospital, The Key Laboratory of Zhejiang Province for Nucleic Acids, Hangzhou Institute of Medicine (HIM), Chinese Academy of Sciences, Hangzhou 310022, P. R. China. E-mail: chemwangp@hnu.edu.cn; tan@him.cas.cn
dHangzhou Aptechone Biotechnology Company Limited, Hangzhou 310022, P. R. China
eZhejiang Key Laboratory of Public Health Detection and Pathogenesis Research, Zhejiang Provincial Center for Disease Control and Prevention, Hangzhou 310050, P. R. China. E-mail: yjzhang@cdc.zj.cn
fInstitute of Molecular Medicine (IMM), Renji Hospital, Shanghai Jiao Tong University School of Medicine and College of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, Shanghai 200240, P. R. China
First published on 18th April 2025
Abstract
Infectious diseases remain a major challenge to public health. The accurate and timely detection of pathogens responsible for these diseases is essential for controlling their spread, supporting clinical diagnosis, and enabling the application of appropriate therapies. Traditionally, the antibody-based assay has been the primary method for pathogen detection. However, recent advancements in aptamer-based technologies have initiated a transformative shift in diagnostic approaches. Aptamer-based sensors (aptasensors) are characterized by lower production costs and greater flexibility, making them compatible with various detection techniques. This broad applicability facilitates multifaceted, high-throughput applications, significantly improving the capacity to monitor and detect infectious diseases. In this review, we introduce the pathogenic mechanisms and characteristics of pathogens, provide an overview of recent advancements in the development of aptasensors for pathogen detection and highlight their versatility in identifying various infectious disease pathogens, including viruses, bacteria, parasites and other microorganisms. We systematically categorize aptasensors according to their detection mechanisms, including colorimetry, fluorescence, chemiluminescence, surface-enhanced Raman spectroscopy (SERS), surface plasmon resonance (SPR), electrochemistry and incorporation of field-effect transistors (FETs). We further demonstrate how these platforms leverage pathogen-specific biological features to achieve ultrasensitive and rapid diagnostics. Further optimization and validation of aptasensor platforms are anticipated to accelerate their clinical translation and industrialization. Advancing these innovative technologies will be crucial to meeting the growing demand for rapid, accurate and reliable pathogen detection across diverse clinical and environmental conditions, ultimately strengthening the ability to respond effectively to infectious disease threats.
1. Introduction
In today's interconnected world, infectious diseases constitute a major global threat to human health with their fast dissemination and elevated morbidity.1 Over the past few decades, outbreaks of viruses like coronavirus disease 2019 (COVID-19) and the Ebola virus have caused massive morbidity and mortality, but other infectious disease pathogens like Mycobacterium tuberculosis and Plasmodium have also brought persistent disease challenges, highlighting the need for advanced diagnostics. Rapid and precise detection of infectious disease pathogens is critical not only for timely treatment and management but also the control of disease outbreaks to prevent spread. For over five decades, antibodies have played a central role in the diagnosis of infectious disease pathogens.2 The intrinsic binding affinity of antibodies for their targets facilitates the capture of molecules in various immunoassays, such as enzyme-linked immunosorbent assay (ELISA), lateral flow immunoassay (LFIA) and other immunosensors.3–5 Despite their widespread application, many antibody-based methods often come with limitations, such as lengthy processing times, the need for complex infrastructure, and susceptibility to false positives or negatives.6–8 These challenges highlight the ongoing need for more efficient, reliable, and user-friendly diagnostics to detect infectious disease pathogens.Aptamers are short single-stranded DNA (ssDNA) or RNA molecules, typically ranging from 20 to 80 bases in length, developed from an oligonucleotide library through a process known as systematic evolution of ligands by exponential enrichment (SELEX). These molecules have emerged as a promising solution in the field of biosensing.9–12 The ability of aptamers to adopt unique secondary and tertiary structures enables them to bind efficiently to a broad range of targets, including small molecules, proteins, viruses and even larger pathogens, such as bacteria and parasites. Aptamers offer several advantages over traditional antibodies, including greater stability, lower immunogenicity, easier modification and reduced production costs.13 Research in the biomedical field has underscored their critical role in pathogen detection and infectious disease diagnosis, positioning aptamers as viable alternatives to antibody-based reagents.14,15
Owing to their unique advantages in diagnostics, aptamers have emerged as a standout component of biosensors for the detection of infectious disease pathogens. Novel detection technologies based on aptamers offer numerous benefits, including simplicity, rapidity, and high sensitivity, endowing them with vast potential for applications in infectious disease diagnostics. Significant advancements in aptamer-based sensor (aptasensor) technologies have further established them as a highly promising diagnostic strategy.16–19 Moreover, SELEX allows the rapid development of specific aptamers for pathogens during emerging outbreaks, as exemplified by the swift identification of aptamers for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in recent years. Once specific aptamers are identified, various detection schemes can be designed using different signal outputs, such as colorimetry, chemiluminescence, fluorescence, electrochemistry, and other methods.20–24 These approaches have driven significant breakthroughs in the sensitivity of pathogen detection. By acquiring specific aptamers and developing sensitive, stable aptasensors, it is possible to achieve rapid, accurate, and convenient diagnosis of infectious disease pathogens, even under adverse and challenging environmental conditions.
Recent reviews on aptasensor technology for infectious disease diagnostics have predominantly focused on summarizing advancements in SELEX strategies and categorizing aptamer-based biosensing methods according to target types, such as antigens, antibodies, or nucleic acids. These works systematically compile the latest progress in pathogen detection, focusing on the design and optimization of aptamers for specific biomarkers, alongside evaluations of analytical performance.25–27 In this review, we show how advancements in the development of aptasensors have revolutionized the sensitive detection of pathogens according to their characteristics and mechanisms of action. To accomplish this, we systematically categorize aptasensors according to their detection mechanisms, including colorimetry, fluorescence, chemiluminescence, surface-enhanced Raman spectroscopy (SERS), surface plasmon resonance (SPR), electrochemistry and incorporation of field-effect transistors (FETs) (Fig. 1). Finally, we explore future research directions in this vibrant and rapidly evolving field, emphasizing that these advancements will play a vital role in enhancing diagnostic capabilities and global health.
2. Infectious disease pathogens
Pathogens are diverse microorganisms that cause infectious diseases in their hosts. They are classified into non-cellular microorganisms, prokaryotic microorganisms, and eukaryotic microorganisms based on cellular structure and complexity.28–31 Understanding pathogenic mechanisms of these pathogens is critical for developing targeted prevention and detection measures. We summarize the various types of pathogens within these classifications and their pathogenic mechanisms of action in the context of aptasensor development.2.1 Non-cellular pathogens
Numerous maladies are triggered by viral infections, including influenza, hepatitis, AIDS, Ebola and COVID-19, causing severe illness and mortality, along with significant economic and societal repercussions. The swift and precise identification of viral infections is vital for curbing widespread transmission. Viral proteins are integral to various stages of the viral replicative cycle, including penetration into host cells, replication of viral genetic material, and eventual release of new viral particles. These proteins serve as targeted elements for the selection and refinement of aptamers. Nevertheless, the configurations of recombinant proteins do not consistently match those of their natural counterparts. Consequently, aptamers derived from recombinant proteins often show reduced binding affinity for their natural targets.40
Additionally, some aptamers have been selected using alternative methods, such as capturing whole viral particles on nitrocellulose membranes or attaching viral particles to microbeads for selection.41 Currently, multiple aptamers demonstrate considerable potential for diagnosing viral infections, including SARS-CoV-2, HIV, HPV and HBV.42–47 This is a reflection of the urgent need to employ aptamer technology for the development of more sensitive and specific detection methods to address the global challenges posed by viral infections.
Aptamers assume distinct three-dimensional (3D) configurations that allow them to attach to specific epitopes or structural features of the prion protein. Because prions have a distinct structure compared to normal proteins, aptamers can distinguish these subtle structural differences. It is SELEX that ensures the affinity of selected aptamers to prions by minimizing cross-reactivity with normal protein structure. However, the complexity and stability of prion structure may require extensive optimization and prolonged aptamer selection. Nonetheless, aptamers can bind to prion proteins (PrP) with high affinity and selectivity with the potential for development into diagnostics for the early identification and examination of prion-related diseases. Indeed, recent studies have successfully identified aptamers that specifically target prions, setting the stage for innovative diagnostic methods.50–52
2.2 Prokaryotic pathogens
Bacteria can be classified on the basis of characteristics observed through Gram staining. Gram-positive bacteria are identified by the presence of a thick layer of peptidoglycan in their cell wall, which effectively traps the crystal violet dye. As a result, these bacteria exhibit a distinct purple coloration under microscopic observation. This structural trait generally makes them more susceptible to antibiotics, such as penicillin, targeting cell wall synthesis. These bacteria produce pathogenic exotoxins. In contrast, Gram-negative bacteria possess an outer membrane rich in lipopolysaccharides and a thinner peptidoglycan layer. Therefore, Gram-negative bacteria appear pink, instead of purple, after counterstaining. The outer membrane is an effective barrier against numerous antibiotics and the immune system. Gram-negative bacteria frequently produce endotoxins, triggering severe inflammatory responses in the host. Bacteria are incredibly diverse, existing as microscopic, single-celled organisms in a multitude of environments. Bacterial diseases occur when these pathogenic organisms invade the body, proliferate and outnumber probiotics, or when they replicate in generally sterile tissues. Additionally, harmful bacteria may produce toxic substances that can cause further damage.57–60
Aptamers that exhibit specificity towards bacteria can be categorized into two main types. The first type includes those that are designed to target specific bacterial cell surface antigens or predetermined virulence factors. The second category encompasses aptamers that target entire bacterial cells, regardless of whether the molecular targets are already identified or remain unknown. Owing to their relatively large size, bacteria can be collected through centrifugation. Consequently, most aptamers intended for bacterial targets can effectively be selected through whole-cell-based SELEX.14 So far, bacterial aptamers are mainly designed to target Salmonella typhimurium (S. typhimurium), Escherichia coli (E. coli), Weissella viridescens, S. aureus and others.61–66
The necessity for mycoplasma monitoring has increased; accordingly, various techniques and protocols utilizing aptamers have been created to identify mycoplasma, supporting the integrity of healthy cell cultures and clinical research.71–74
Spirochetes are slender, spiral-shaped bacteria characterized by their unique axial filaments, which confer motility. This group includes such pathogens as Borrelia burgdorferi (Lyme disease) and Treponema pallidum (syphilis). Their spiral morphology facilitates movement through viscous environments like connective tissues. Spirochetes often evade the host immune system through antigenic variation, complicating diagnosis and treatment.76 Early detection is crucial, as these infections can lead to chronic health issues if left untreated.77
Rickettsiae are obligate intracellular bacteria transmitted primarily through arthropod vectors, such as ticks, lice, and fleas. These Gram-negative organisms invade endothelial cells, leading to diseases like Rocky Mountain spotted fever and typhus. Their pathogenicity is linked to their ability to escape phagosome-lysosome fusion, allowing intracellular survival and replication. The resulting vasculitis triggers systemic symptoms, including fever and rash, necessitating prompt diagnosis and treatment with tetracycline-class antibiotics.78
Most pathogens can be detected by polymerase chain reaction (PCR) technology. PCR is well known for its specificity and sensitivity, but it is also cumbersome and time-consuming.79 Antigen detection based on aptamers can detect these pathogens quickly and easily, but no convincing research has yet obtained apposite aptamers. However, as SELEX technology and aptamer modification advance, we could soon see the development of more aptamers with high affinity and specificity for pathogen detection.
2.3 Eukaryotic pathogens
Aptamers have been widely developed for the detection of mycotoxins, which are toxic metabolites produced by fungi and pose serious health risks. Advancements in aptamer technology have resulted in the development of numerous promising aptasensors specifically designed to identify and quantify various mycotoxins. These aptasensors have been valuable tools for monitoring and managing fungal contamination in food and agricultural products.84–87
3. Aptasensors for pathogen detection
3.1 Colorimetric aptasensors
Widely used as a quantitative technique in chemistry, colorimetric analysis is predicated on measuring the intensity or concentration of compounds through the capacity to absorb specific light wavelengths. Colorimetry typically involves a color change that can be observed either by optical detectors or with the naked eye, and this change is proportional to the concentration of the analyte. The foundational principle of colorimetric analysis is the Beer–Lambert law, which describes a linear relationship between absorbance and concentration within a specified range.98 The TMB (3,3′,5,5′-tetramethylbenzidine) colorimetric assay is a conventional approach that relies on the enzymatic oxidation of TMB by specific enzymes, such as horseradish peroxidase (HRP). When hydrogen peroxide is present in the reaction mixture, HRP catalyzes the oxidation of TMB, resulting in a blue product. This reaction involves eliminating the 7-hydroxy group, forming a stable blue compound. The intensity of the color, measurable at 650 nm, is related to analyte concentration, making it a quantitative method applicable to various applications, including ELISA.99Traditional colorimetric methods employ chromogenic substrates or indicators, eliciting a visible color change upon reacting with the target analyte. Owing to their unique optical properties and ability to exhibit color variations in diverse environments, nanoparticles, particularly gold nanoparticles (AuNPs), have been the focus of extensive research as colorimetric probes for developing versatile biosensors over the past few decades. The underlying mechanism of this color change is primarily related to the plasmonic effect. When an analyte binds to AuNPs, it can lead to the aggregation of these particles. This aggregation results in interparticle surface plasmon coupling, which is responsible for the observed changes in color.100 Lateral flow immunoassay (LFIA) (immunochromatographic test) is a widely used diagnostic tool owing to its simplicity, convenience and rapid results, often employed in point-of-care testing (POCT) for various pathogens. AuNPs, as the color reagent, play a pivotal role in the functionality and effectiveness of LFIA.101
The advent of aptamers has ushered in a new era for colorimetric sensor technology, especially within the realm of pathogen detection. Owing to the higher stability, lower immunogenicity, and ease of modification, the incorporation of aptamers into colorimetric sensors has resulted in the development of highly selective and sensitive diagnostic tools.11
 | ||
| Fig. 2 (A) Illustration of colorimetric sandwich assay in which SARS-CoV-2 pseudoviruses are captured with biotinylated aptamers, using Pd–Ir nanocubes as peroxidase-mimicking nanozymes.39 Copyright 2022 American Chemical Society. (B) Schematic illustration of AUT for the detection of SARS-CoV-2 through a sandwich assay.100 Copyright 2024 Wiley. (C) Scheme for the binding mechanism of RPA70A and ssDNA within the signal amplification system.40 Copyright 2024 American Chemical Society. (D) Schematic illustration of the plasmonic nanoplatform for HCVcp detection.41 Copyright 2020 Elsevier. (E) Schematic illustration of the detection mechanism of microfluidic paper-based aptasensor.58 Copyright 2022 Elsevier. | ||
In the quest for rapid and sensitive diagnostic tools for infectious diseases, a recent study by Kang et al. introduced a groundbreaking methodology that utilizes aptamer-conjugated AuNPs in the LFIA platform designed for signal amplification. This innovative method enables the simultaneous detection of influenza A, influenza B, and COVID-19, as illustrated in Fig. 2C. These researchers employed a heterogeneous sandwich-type aptamer selection technique (H-SELEX) to identify specific aptamers that bind to the nucleocapsid protein (N protein) of each targeted virus. H-SELEX ensured that the aptamers effectively recognized and bound to their respective viral antigens. Additionally, the study assessed the sensitivity of each viral antigen within diluted nasopharyngeal fluid samples. The results demonstrated a remarkable sensitivity level with an LOD of 2.89 pg mL−1.43
Furthermore, isothermal amplification techniques have emerged as powerful tools for POCT, offering the advantage of operating at constant temperatures. As shown in Fig. 2D, Zhou et al. reported a plasmonic colorimetric nanoplatform for detecting the hepatitis C virus core protein (HCVcp). Aptamers were employed to transform the signal from the target protein into the nucleic acid signal, which was amplified through the catalytic hairpin assembly (CHA) amplification process. A specific short sequence (I) was engineered to hybridize with C7 (C7/I), ensuring that CHA amplification would initiate only in the presence of HCVcp. The color change induced by the interaction between HCVcp and the aptamer-based nanoplatform was utilized to enable visual detection at an extremely low concentration of 10−4 pg mL−1. After evaluation with serum samples from 30 donors displaying different viral loads, this nanoplatform exhibited greater sensitivity compared to standard ELISA kits, achieving a lower LOD and an increased positive detection rate.44
3.2 Fluorescent aptasensors
Fluorometry is a powerful and versatile technique employed in chemical analysis to detect and quantify various analytes. When the fluorescent target absorbs light at a specific wavelength, atoms in the target excite inner-shell electrons to higher energy states. These electrons then transition back to lower energy states, emitting characteristic fluorescence. The fluorescence was measured to determine concentration of the analyte. Previously, the fluorescence method had been applied in biomedical sensing, imaging and other fields.104–106 Fluorescent aptasensors operate by detecting variations in fluorescence polarization, wavelength, or intensity. These variations occur when target analytes interact with fluorescently-tagged aptamers. Moreover, small-molecule fluorescent dyes, quantum dots (QD) or upconversion nanoparticles conjugated with aptamers may serve as probes for an affordable, straightforward, quick, and user-friendly detection method. The significant correlation between signal intensity and analyte concentration (R2 = 0.98) enables quantitative determination of the target through calibration curve construction. The intensity strength of the emitted fluorescent signal is positively correlated with the content of the target, and the concentration of the analyte can be further calculated.107,108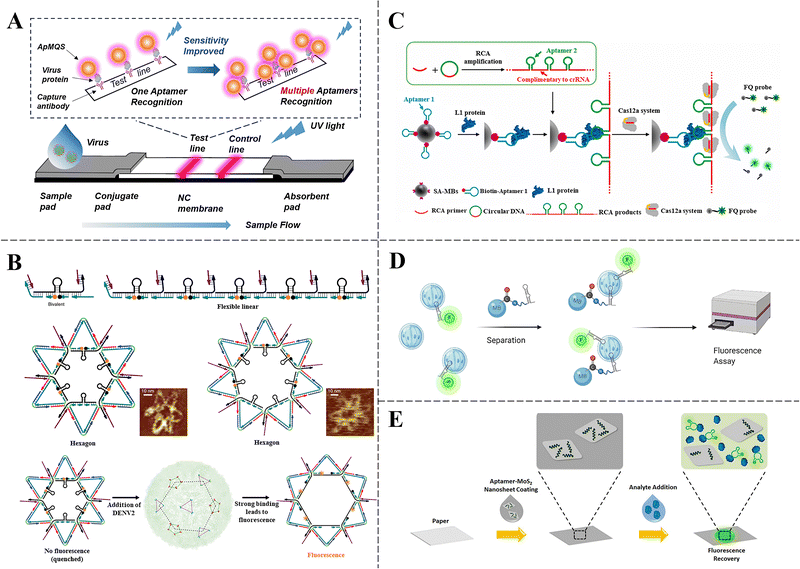 | ||
| Fig. 3 (A) The POCT platform was constructed by combining multiple-aptamer recognition with QDs fluorescence technology for the ultrasensitive detection of respiratory infectious diseases.107 Copyright 2024 Royal Society of Chemistry. (B) Schematic illustration of DNA star for the detection ED3.42 Copyright 2020 Springer Nature. (C) Scheme of the design of sandwich aptasensor assisted with RCA and CRISPR/Cas12a.43 Copyright 2024 Elsevier. (D) A sandwich assay using tagged Min_Crypto2 and Min_Crypto2 magnetic beads allows for fluorescence detection of oocysts in wastewater.90 Copyright 2021 Elsevier. (E) Paper-based MoS2 nanosheet-mediated FRET aptasensing process.91 Copyright 2017 Springer Nature. | ||
Furthermore, as shown in Fig. 3B, Wang et al. developed a star-shaped DNA structure featuring five motifs resembling molecular beacons to accurately align with the spatial configuration of envelope protein domain III (ED3) clusters present on the surface of the dengue virus (DENV). The resulting multivalent interactions strengthened the binding avidity to DENV. Strands containing 6-carboxylfluorescein (6-FAM, a fluorophore) and black hole quencher-1 (BHQ-1, a quencher) were hybridized to each inner edge surrounding the hairpin, transforming the DNA star-shaped aptamer complex into a probe for the virus. In the presence of DENV, the hairpins opened up and converted into single-stranded DNA (ssDNA) by the strong binding between aptamer and ED3 sites. The robust multivalent interactions caused the separation of FAM fluorophores from BHQ-1 quenchers, thereby generating a fluorescent signal. The DNA star-shaped aptamer sensor successfully detected DENV with remarkable sensitivity, achieving LODs of 102 pfu mL−1 and 103 pfu mL−1 in human serum and plasma, respectively. This innovative molecular platform could be extended for the detection and management of other pathogens.45 Huang et al. designed a sandwich detection assay by utilizing the high affinity and specificity of aptamers selected under stringent conditions and within complex environments to capture the HPV L1 protein, followed by rolling-circle amplification (RCA) and Cas12a-crRNA-mediated substrate cleavage for signal generation (Fig. 3C). The LOD in various matrices, including phosphate-buffered saline Tween 20 (PBST), cell-lysis buffer, urine, and cervical tissue lysates, was found to be 0.1 ng mL−1 in all cases. Several HPV subtypes in both urine and cervical tissue clinical samples were identified, showcasing the potential of this detection strategy for convenient HPV screening.46
Förster resonance energy transfer (FRET)- based fluorescent aptasensors are gaining prominence in various applications. FRET operates on the principle that two fluorophores possess specific electromagnetic characteristics. Notably, effective energy transfer between these fluorophores requires significant overlap with the donor's emission spectrum and the acceptor's excitation spectrum. This unique property allows researchers to monitor changes in distance between the pairs of fluorophores by analyzing the wavelength and intensity of the emitted light. Consequently, the FRET technique offers a reliable output for target molecule detection. FRET-based systems can be integrated into aptasensors through various forms. A common approach involves modifying aptamers with both donors and acceptors. In the absence of targets, the spatial arrangement of donors and acceptors keeps them distant from one another, preventing the FRET phenomenon. However, upon target binding, conformational change in the aptamer draws the FRET pair closer together, resulting in a measurable alteration in FRET signal.114 As shown in Fig. 3E, Lim et al. investigated the properties of different types of paper to develop a sensor based on aptamers and MoS2 nanosheets, employing FRET to indicate the presence of Plasmodium lactate dehydrogenase (pLDH) for rapid malaria diagnosis. The study systematically evaluated the hydrophilicity, surface morphology, and aptamer distribution across various paper types to optimize the sensor's performance. A test strip composed of aptamers and fluorescence-quenching MoS2 nanosheets was utilized to effectively and specifically identify the malarial biomarker pLDH. These findings highlighted the importance of material selection and sensor design in the development of aptamer-based diagnostic tools, paving the way for POCT in resource-limited conditions.94 The application of fluorescent aptasensors extends beyond viral detection, demonstrating broader applicability in the diagnostic landscape. This expansion of application areas highlights the adaptability and robustness of fluorescent aptasensors, positioning them as valuable assets in the arsenal against infectious diseases.
3.3 Chemiluminescent aptasensors
The energy transition between molecular orbitals results in the emission of light. Luminescence occurs when electrons within a molecule transition from higher energy orbitals to lower energy ones, releasing excess energy in the form of photons. When this luminescent phenomenon is utilized in a chemical reaction, it is termed chemiluminescence. In analytical chemistry, chemiluminescence is employed for the detection and quantification of various analytes owing to its high sensitivity and low background signal. Notably, this process does not require an external light source for excitation, which makes it particularly advantageous for detecting and quantifying targets with minimal interference from other substances. The simplicity and sensitivity of chemiluminescent assays make them valuable tools in research and diagnostics.115–118 Recently, the application of aptamers in chemiluminescent sensors has broadened the scope of this technology. Aptamers act as recognition elements that selectively bind to target analytes and initiate a chemiluminescent reaction. The incorporation of chemiluminescent aptasensors has resulted in increased sensitivity and specificity, along with the capacity for label-free detection of infectious disease pathogens.Guanine chemiluminescence has emerged as a highly sensitive optical sensing method based on the unique properties of DNA aptamers.119 Lee et al. developed a convenient and cost-effective chemiluminescent aptasensor aimed at the precise and rapid detection of norovirus GII in both tap water and artificial urine. The incorporation of additional guanines into the modified aptamer significantly boosted sensitivity through intra-chemiluminescent resonance transfer (intra-CRET). Furthermore, the reaction between the extra guanines and 3,4,5-trimethoxylphenylglyoxal (TMPG) generated high-energy intermediates that efficiently transferred energy to the fluorescent dye 6-FAM. The energy transfer process resulted in the emission of bright chemiluminescence and improved the signal of norovirus GII. Notably, the aptasensor functioned without requiring labor-intensive or time-consuming procedures, enabling the accurate quantification of trace amounts of the norovirus GII capsid and achieving an LOD as low as 80 ng mL−1. The promising technology developed in this biosensor study holds significant potential for broader applications. It is anticipated that this chemiluminescent aptasensor promoted the development of biosensors adept at rapidly quantifying a range of food-borne pathogens in various sample types and ultimately improved food safety and public health monitoring efforts.47
Rapid magnetic separation is commonly used in chemiluminescent assays. Deng et al. obtained DNA aptamers that specifically bound to hepatitis B surface antigen (HBsAg) after 13 selection rounds. The obtained aptamers against HBsAg were immobilized on the surface of magnetic nanoparticles (MNPs) and combined with an alkaline phosphatase (ALP)-AMPPD chemiluminescence system. Finally, a chemiluminescence aptasensor based on magnetic separation was constructed to diagnose HBV infection. The aptasensor functioned effectively, despite the presence of interfering substances, and exhibited high specificity for detecting HBsAg in serum samples with an LOD of 0.1 ng mL−1, which is much lower than the 0.5 ng mL−1 limit of a typical ELISA used in hospitals.120 Furthermore, in order to achieve a higher level of sensitivity, Zheng et al. developed an ALP-AMPPD chemiluminescent aptasensor based on double-functionalized gold magnetic composite nanoparticles (Au@Fe3O4@SiO2 NPs). The nanoparticles allowed the attachment of aptamer-targeted HBsAg, facilitating fast magnetic separation and improving the detection signal. The LOD of this chemiluminescent aptasensor was as low as 0.05 ng mL−1. This method simplified HBV detection and enhanced sensitivity, underscoring the potential of aptasensors in infectious disease diagnostics.121
3.4 Surface-enhanced Raman spectroscopy (SERS) aptasensors
Raman spectroscopy is a scattering technique that delivers the “distinct chemical fingerprint” of various molecules. SERS is an effective analytical method that utilizes roughened metal surfaces or metal nanoparticles to intensify Raman scattering signals. This intensification is primarily attributed to two mechanisms to amplify the Raman response of molecules: electromagnetic field enhancement and chemical enhancement. Electromagnetic field enhancement is the result of localized surface plasmon resonances (LSPRs) that focus and amplify the electromagnetic field found in metal nanostructures at the nanoscale. Chemical enhancement involves charge transfer between the adsorbed molecules and the metal surface, increasing the Raman signal.122–124 SERS is particularly sensitive for detecting trace amounts of molecules; even single molecules can be detected under optimal conditions. The technique is widely applied in numerous fields, including biomedical diagnostics, chemical analysis and environmental monitoring based on its high sensitivity, specificity, and ability to provide molecular fingerprinting information.125–127Aptamers present a more attractive choice for SERS applications because they can be easily modified in a sequence-specific way, incorporating an anchor for attachment to the metallic surface.128 Moreover, typical aptamers are significantly smaller than antibodies, which substantially reduces the distance between the reporters and the SERS substrate. SERS-based sensors have been applied to create diagnostic tools for various medical applications.129,130 In the SERS aptasensor, Raman reporter-modified aptamers are generally affixed to the nanostructured metal-dielectric platform to identify and seize target molecules. When exposed to laser light, the energy of photons is altered by the interactions between aptamers and pathogens, thereby enabling the quantification of targets.131
 | ||
| Fig. 4 (A) Schematic illustration of the SERS imaging-based assay using a 3D nano-popcorn plasmonic aptasensor for the quantitative evaluation of A/H1N1 virus.130 Copyright 2020 Elsevier. (B) Scheme of sandwich-like aptasensor for influenza virus detection.131 Copyright 2019 Public Library of Science. (C) Schematic illustration of the detection of E. coli O157:H7 by the developed GNBs aptasensor.60 Copyright 2020 American Chemical Society. (D) Schematic representation of the SERS aptasensor based on AuNPs-functionalized PDMS film for detection of S. aureus.61 Copyright 2021 Elsevier. | ||
The Zavyalova group also utilized highly sensitive and fast SERS aptasensors for detecting influenza virus H3N2. SERS offered signal amplification on the order of 106 to 109, while aptamers targeting hemagglutinin (HA) ensured specific recognition of influenza virus. As shown in Fig. 4B, this group presented a highly sensitive SERS aptasensor for the detection of the influenza virus. The aptasensor utilizes immobilized primary aptamers to capture the virus and Cy3-labeled secondary aptamers for specific detection. The optimized SERS substrates, combined with the excitation of a 532 nm laser, contributed to the high sensitivity, achieving an LOD of 10−4 hemagglutination units per probe.134
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cfu mL−1, with an LOD of 3 cfu mL−1.63
000 cfu mL−1, with an LOD of 3 cfu mL−1.63Currently, most SERS sensing techniques are predominantly implemented in liquid systems. These methods typically require multiple detection steps and are often costly, limiting their practicality and widespread application across various fields. In contrast, polydimethylsiloxane (PDMS) emerges as a promising solid material that offers a range of advantageous properties, including excellent transparency, impressive heat resistance and remarkable stability. The integration of PDMS with nanomaterials is expected to be applied as a solid-state SERS substrate.135 Chen et al. developed a novel SERS aptasensor for the selective and sensitive detection of S. aureus, as shown in Fig. 4D. The detection platform is based on AuNPs-functionalized PDMS film which captures the bacteria by using specific aptamers through gold–sulfur bonding. The signal probes, Au@Ag nanoflowers (NFs), were synthesized via a galvanic replacement process and conjugated with 4-mercaptobenzoic acid (4-MBA) as the Raman reporter molecule. This SERS aptasensor offers a promising approach for the rapid and specific detection of S. aureus, with potential implications for diagnosing and managing staphylococcal infections. A linear relationship was observed between the signal of 4-MBA at 1085 cm−1 and the concentration of S. aureus, ranging from 4.3 × 10 cfu mL−1 to 4.3 × 107 cfu mL−1 with an LOD of 13 cfu mL−1. This method was effectively utilized on spiked samples, resulting in a recovery rate between 92.5% and 110%.64
3.5 Surface plasmon resonance (SPR) aptasensors
SPR is an advanced optical detection technique that operates through the observation of shifts in evanescent wave signals which result from molecular alterations on a gold surface. The SPR sensor relies on the sensitivity of surface plasmons for the precise measurement of refractive index changes which correspond to the concentration of targets, making the SPR sensor an effective tool for real-time monitoring. SPR technology has emerged as a highly sensitive detection system without requiring labels, making it particularly advantageous for various applications.136,137Aptamers, which are specific types of nucleic acids, present significant advantages over traditional protein-based sensing approaches, such as those utilizing antibodies or enzymes. Compared to antibodies, aptamers have a longer shelf life and exhibit less steric hindrance, enhancing their performance as recognition elements in SPR biosensors. Within a typical SPR aptasensor configuration, aptamers are immobilized onto the gold substrate, serving as the key recognition probes. The interaction between aptamers and their targets leads to measurable changes in SPR angle.138
Localized surface plasmon resonance (LSPR) offers advantages over SPR by its ability to provide more substantial and more localized electromagnetic field enhancements, which, in turn, can lead to higher sensitivity and better spatial resolution in sensing applications. Min et al. developed a label-free biosensor to detect the avian influenza virus (AIV H5N1), employing a multi-functional DNA 3-way junction (3WJ) integrated with a hollow Au spike-like nanoparticle (hAuSN) via LSPR (Fig. 5A). Each component of the DNA 3WJ was respectively designed to combine with an HA-binding aptamer, a FAM dye and a thiol group. The assembly of each functional fragment was verified using TBM-native polyacrylamide gel electrophoresis (PAGE). Furthermore, the hAuSN was immobilized on an indium-tin-oxide (ITO) substrate for LSPR evaluation. The DNA 3WJ was affixed to the hAuSN electrode through the thiol group. This advanced biosensor could detect HA protein in both phosphate-buffered saline (PBS) and diluted chicken serum, achieving an LOD as low as 1 pM.141 Nevertheless, traditional SPR sensors face limitations in selectivity and sensitivity compared to fluorescence detection methods.
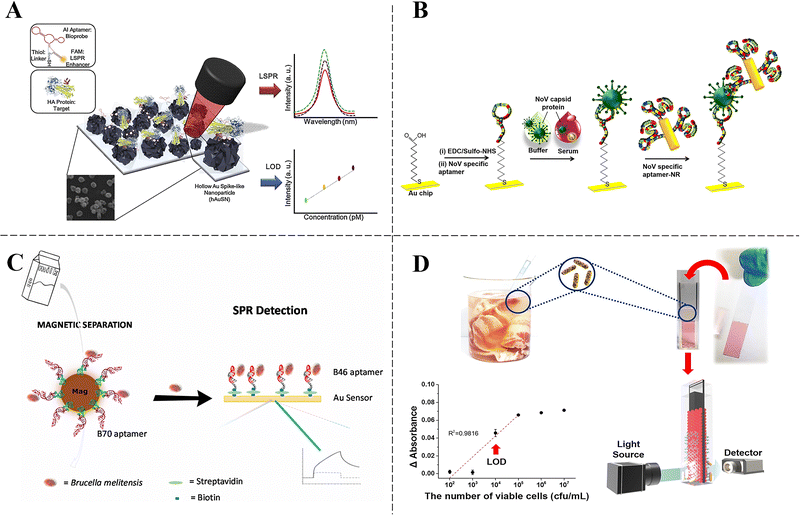 | ||
| Fig. 5 (A) Schematic image of the fabricated AIV detection biosensor based on the LSPR method.138 Copyright 2019 Elsevier. (B) Schematic illustration showing the aptamer–aptamer sandwich assay strategy for norovirus capsid protein.139 Copyright 2018 Elsevier. (C) Overall strategy for SPR detection of Brucella cells in milk samples.140 Copyright 2022 Elsevier. (D) Detection of S. typhimurium in pork using the aptamer-based localized surface plasmon resonance (LSPR) sensing chip.62 Copyright 2017 Springer Nature. | ||
An accurate and efficient detection method is crucial for diagnosing infections and preventing their spread. As illustrated in Fig. 5B, the Lee group developed an innovative sandwich assay based on SPR enhanced with nanorods (NRs) to detect norovirus capsid protein at attomolar concentrations. This assay employs a unique pair of aptamers that demonstrate the highest binding affinity. Aptamer II was covalently bonded to a chemically treated thin gold chip surface, while aptamer I was linked to the surface of the Au NR. The surface sandwich assembly was accomplished through the sequential adsorption of norovirus capsid protein and aptamer I-coated Au NR onto the aptamer II surface. This sensor enabled the detection of norovirus protein concentrations as low as 70 aM in buffer solutions. Furthermore, the aptamer-NR-coated sandwich assay assessed norovirus capsid protein concentrations in human serum samples.142
Nonetheless, numerous LSPR biosensors face challenges owing to low reproducibility, primarily from difficulties in immobilizing large areas of plasmonic-active nanoparticles, while aiming to lower LODs. To solve these issues, Huh et al. developed an innovative, label-free and portable plasmonic biosensor device specifically for the ultra-sensitive and selective detection of S. typhimurium in pork samples (Fig. 5D). The self-assembly of AuNPs was used to achieve a precisely controlled diameter of 20 nm for the AuNP monolayers, which was essential for generating longitudinal wavelength extinction shifts via the LSPR signal. By integrating aptamers with the LSPR chips, the biosensor achieved an exceptional upper LOD of approximately 104 cfu mL−1 for S. typhimurium without the demand for any pre-enrichment step in pure culture conditions and the artificially contaminated pork.65
3.6 Electrochemical aptasensors
Electrochemical detection is a robust analytical technique that focuses on measuring electrical signals generated by redox reactions at the interface between the electrode and the electrolyte solution. The principle of electrochemical detection is based on the transfer of electrons between a species of interest and an electrode, which results in a current or potential change that can be quantified. This method is sensitive, selective, and offers a broad detection range, spanning from small ions to large biomolecules.144 The technique encompasses various modes, including amperometry, voltammetry, and potentiometry. In amperometry, a constant potential is applied, and the resulting current response, which is directly proportional to the concentration of the analyte, is recorded. Voltammetry, in contrast, involves varying the potential and measuring the current response, providing information into redox mechanisms and enabling both qualitative and quantitative analyses. Potentiometry measures the potential difference between a working electrode and a reference electrode, correlating to the concentration of analytes.145,146 Advances in nanomaterials and microelectrode fabrication have further increased the sensitivity and selectivity of electrochemical sensors, making them indispensable tools in modern analytical chemistry.147Owing to high sensitivity and specificity, electrochemical aptasensors have emerged as a focal point in biosensing research. Compared to other sensing technologies, electrochemical aptasensors are cost-effective and offer rapid response times.148 A variety of aptasensors have been developed, employing different transduction methods, such as voltammetric, amperometric, impedimetric, and potentiometric techniques, which detect target analytes through distinct electrochemical signals.149,150 Typically, electrochemical aptasensors feature an electrode surface, commonly made from gold or carbon-based materials, which serves as the platform for immobilizing a redox probe-labeled aptamer. By measuring changes in current or resistivity resulting from redox reactions between targets and aptamers on the electrode, the electrochemical aptasensor accurately determines the concentration of analytes, making it an invaluable tool for both analytical and clinical applications.151,152
Nanopore sensing technology is an emerging analytical technique that utilizes nanopores to detect and characterize individual molecules, such as DNA, RNA, proteins, and other biopolymers. Nanopore sensing technology operates on the principle of measuring changes in ionic current as targets pass through the nanopore under an applied electric field. As shown in Fig. 6A, Edel et al. applied a multiplexed nanopore sensing approach using encoded molecular probes to detect both viral antigens and RNA. They focused on the D614G mutation in the spike protein of SARS-CoV-2, which is associated with increased infectivity and transmission. The method leverages nanopore technology coupled with molecular probes designed to identify specific mutations, allowing for the discrimination of SARS-CoV-2 variants of concern (VOC), including Alpha, Delta, and Omicron. The results demonstrated high specificity and sensitivity with the ability to detect even single-nucleotide mutations.154 Lu et al. reported an innovative aptamer-nanopore system to detect and distinguish infectious human adenoviruses (HAdV), SARS-CoV-2 and other types of viruses, with remarkable sensitivity and selectivity (Fig. 6B). They employed a solid-state nanopore coated with aptamers to capture the viruses and measured changes in ion transport properties. The system demonstrated remarkable sensitivity and selectivity, quantifying HAdV across a broad range from 6 pfu mL−1 to 6 × 104 pfu mL−1, with the LOD reaching as low as 1 pfu mL−1 for HAdV and 104 copies per mL for SARS-CoV-2. The aptamer-nanopore sensor has been effectively applied for the detection of both enveloped and non-enveloped viruses in diverse samples, including water, saliva, and serum.155
 | ||
| Fig. 6 (A) Schematic illustration of multiplexed detection of viral proteins and RNAs from patients’ samples.151 Copyright 2023 Springer Nature. (B) Scheme depicting modification of the nanopore and the interaction between the aptamer and infectious HAdV samples.152 Copyright 2021 The American Association for the Advancement of Science. (C) Representative diagram for the fabrication of the molecularly imprinted (MIP)- based aptasensor and its use for the determination of S. aureus.154 Copyright 2022 Springer Nature. (D) Schematic illustration of the detection of Mycobacterium tuberculosis secreted immunogenic protein MPT64.63 Copyright 2019 Springer Nature. (E) Representation of stepwise aptasensor preparation and PfLDH responses in two different pH environments.156 Copyright 2018 Elsevier. | ||
Tuberculosis is an infectious disease that poses a significant threat to global health, leading to considerable rates of illness and mortality among affected populations.158,159 Kanayeva et al. offered an innovative detection method for the identification of the Mycobacterium tuberculosis secreted protein MPT64. They employed an interdigitated electrode (IDE) as a platform for capturing this immunogenic protein and then utilized electrochemical impedance spectroscopy (EIS) as the primary detection technique (Fig. 6D). The successful immobilization of aptamers onto the IDE was achieved through a co-adsorbent method, optimizing the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 aptamer to 6-mercaptohexanol. The sensor demonstrated remarkable sensitivity with an LOD of 4.1 fM in buffer. Clinical validation revealed 100% specificity and 76.47% sensitivity for sputum samples, as well as 88.24% sensitivity for serum samples.66
100 aptamer to 6-mercaptohexanol. The sensor demonstrated remarkable sensitivity with an LOD of 4.1 fM in buffer. Clinical validation revealed 100% specificity and 76.47% sensitivity for sputum samples, as well as 88.24% sensitivity for serum samples.66
3.7 Field-effect transistor (FET) aptasensors
FET-based detection is a sensitive and label-free technique that leverages the principle of semiconductor physics to transduce biological or chemical signals into electrical signals. FET is a voltage-controlled semiconductor device composed of a source, drain, and gate. The gate modulates the current flow between the source and drain. When a target analyte binds to the gate surface, it modulates the local electric field, affecting channel conductivity. This change in conductivity is proportional to the concentration of the analyte, allowing for real-time, quantitative analysis.161,162 The sensitivity of FET detection is improved by the use of nanomaterials or specific surface modifications that increase the surface area and provide more binding sites for the analyte. Additionally, incorporating functionalized layers, or molecular recognition elements, can improve selectivity. FETs are versatile and have been employed in various configurations, including label-free DNA, protein, and small- molecule detection, as well as in environmental and clinical diagnostics. The technique's real-time monitoring capability, low cost, and potential for miniaturization make it a promising tool in biosensing.163–165The FET aptasensor integrates aptamers onto the gate electrode surface whereon the applied voltage modulates the intensity of the drain current. The interaction between aptamers and targets at the gate electrode induces changes in the drain current. The exceptional performance of the FET aptasensor stems from its highly sensitive detection capabilities, portability, cost-effectiveness, and rapid operational efficiency. Moreover, the sensor shows considerable potential for detecting infectious disease pathogens, marking a significant advancement in diagnostic technology.
Electrochemical deposition is an efficient technique that offers precise control over the shape and size of products in a short time. Electrochemical deposition produces nanostructures rapidly and ensures a stable connection with the substrate through direct deposition.168 Graphene stands out as an excellent substrate in electropolymerization because it is chemically inert, thus providing stability and exhibiting flexibility for FET applications. Moreover, the high electrical conductivity of graphene improves efficiency of the process, and its ability to be transferred onto diverse substrates enhances versatility, making it highly valuable for advanced electrochemical applications. Lee et al. described a multidimensional conductive nanofilm (MCNF)-based FET aptasensor to monitor serum HBsAg level, incorporating vertically oriented carboxylic polypyrrole nanowires and a graphene layer. The aptasensor demonstrated exceptional sensitivity, detecting HBsAg at an ultralow concentration of 10 aM amidst various interfering biomolecules. Furthermore, given its optimized sensing ability in human serum and artificial saliva, the MCNF-based aptasensor showed promise for noninvasive, real-time diagnosis.169
4. Perspectives and conclusions
In this study, we introduced the pathogenic mechanisms and characteristics of pathogens, together with a systematic review of recent research achievements on various aptasensors in the field of infectious disease pathogen detection. We reported and summarized the applications of aptamers in biosensors, including those based on colorimetry, fluorescence, chemiluminescence, SERS, SPR, electrochemistry and FET platforms, with different signal conversion methods and amplification strategies. While antibodies are extensively utilized in clinical diagnostics for the rapid detection of pathogens, their application is significantly hindered by high immunogenicity and elevated production costs. In contrast, aptamers can be mass-produced using solid-phase chemical synthesis, significantly improving research and development efficiency. Nevertheless, only a limited number of mature aptamer-based products have been developed for application in clinical diagnostics. Many obstacles hinder the development and application of aptamers. First, the conformational diversity of nucleic acids is more constrained in comparison to antibodies, limiting their binding versatility. Aptamers are single-stranded nucleic acid molecules, which also faces the conformational lability, affecting the precise detection of pathogens under complex environments. The enhanced binding capacity of aptamers can be achieved through some strategies: stabilization through divalent cation supplementation (e.g., Mg2+), or structural reinforcement via targeted modifications such as 8-methoxypsoralen-based photochemically covalent lock.171–173 Second, aptamers possess high negative charges, complicating their interaction with negatively charged targets. Third, aptamers synthesized in vitro often demonstrate a range of bioavailability and binding properties that lead to unpredictable performance in vivo environments. Therefore, additional and numerous clinical data are required to validate aptamers’ efficacy and safety in actual applications.Consequently, numerous challenges related to the feasibility of aptamer applications in the diagnosis of infectious disease pathogens need to be addressed. Apart from the inherent limitations of their properties, the industrialization of aptasensors in the field of infectious disease pathogen detection also faces several challenges. On the one hand, many pathogenic microorganisms, such as Bordetella pertussis and Treponema pallidum, currently lack clear high-affinity aptamers. On the other hand, as a novel biological detection tool, aptasensors need to go through a strict regulatory approval process, involving complex clinical trials and safety assessments, both of which increase the time and cost associated with industrialization. Despite the many advantages of aptamer technology, it takes time for the market to accept and trust the new technology. Therefore, educational and promotional efforts are crucial for improving market acceptance of aptamer technology.
Currently, researchers are focused on developing functional aptamers with improved affinity and specificity for clinical infectious disease pathogens, with the goal of increasing detection sensitivity and enabling precise diagnosis of drug-resistant and highly virulent strains. Expanding the development of functional aptamers through SELEX for the specific recognition of clinical pathogens, optimizing existing aptamers through chemical modifications and nucleic acid cyclization to enhance stability, and establishing preprocessing methods for clinical samples are all essential steps. Concurrently, the development of more advanced and diverse functional nucleic acid-based biosensors, along with the commercialization of portable high-sensitivity detection devices, holds significant importance for advancing the application of functional nucleic acids in the detection of pathogenic microorganisms. At present, some companies, such as NOXXON Pharma, AptaTargets and Aptechone, have focused on advancing the industrialization and commercialization of aptamer-based technology. Notably, in 2022, our research group obtained two Conformité Européenne (CE) markings for aptamer-based antigen detection reagents involving respiratory diseases.
The future development of aptasensor technology in infectious disease diagnosis lies in a synergistic integration of intelligent molecular diagnosis and biomaterial innovation. Current efforts focus on refining functional aptamers through enhanced SELEX protocols, chemical modifications, and nucleic acid cyclization to improve affinity, specificity, and stability against clinical pathogens, artificial intelligence (AI) and machine learning will further accelerate aptamer discovery. Furthermore, the development of portable platforms for automated sample processing and wireless connectivity for real-time data sharing will revolutionize point-of-care diagnostics, such as emerging smartphone-integrated microfluidic technology.174 Material science innovations, such as graphene-based FETs and quantum dot technology, will enhance sensitivity and optimize signal-to-noise ratios. Aptamer based biofunctionalized nanomaterials are expected to achieve dual-mode pathogen capture and signal amplification. We look forward to collaborating with researchers worldwide to promote the development of the pathogen detection field and make a contribution to global public health (Table 1).
| Aptasensor type | Target type | Target pathogens | Target protein | Detection method | LOD | Ref. |
|---|---|---|---|---|---|---|
| Colorimetric aptasensors | Virus | SARS-CoV-2 | Spike protein | ELABA | 6.3 × 103 copies per mL | 42 |
| SARS-CoV-2 | Spike protein | Colorimetric sandwich assay | 2.18 × 102 copies per mL | 103 | ||
| Influenza Influenza B SARS-CoV-2 | Nucleoprotein Nucleocapsid protein | LFIA | 2.89 pg mL−1 | 43 | ||
| Hepatitis C virus | Core protein | Plasmonic colorimetric nanoplatform | 10−4 pg mL−1 | 44 | ||
| Bacteria | E. coli O157:H7 S. typhimurium | — | Paper-based microfluidic assay | 103 CFU mL−1 E. coli 102 CFU mL−1 S. typhimurium | 61 | |
| Mycoplasma | Mycoplasma hyorhinis | — | LFIA | 103 CCU mL−1. | 74 | |
| Fluorescent aptasensors | Virus | SARS-CoV-2 | Nucleocapsid protein | Dual structure-switching aptamer-mediated signal amplification | 5.87 fg | 109 |
| SARS-CoV-2 | Nucleocapsid protein | Multiple- aptamer recognition strategy combined with QD lateral flow immunoassay | 1.427 pg mL−1 | 110 | ||
| SARS-CoV-2 | Nucleocapsid protein | Activatable TSA strategy using HRP-conjugated DNA aptamers | 48.9 ng mL−1 | 111 | ||
| Dengue | Envelope protein domain III | Star-shaped DNA architecture | 102 pfu mL−1 in human serum; 103 pfu mL−1 in human plasma | 45 | ||
| Human papillomavirus (HPV) | L1 protein | Sandwich detection assay based on RCA and Cas12a-crRNA | 0.1 ng mL−1 | 46 | ||
| Bacteria | Weissella viridescens | — | Fluorescence polarization sensor | 102 CFU mL−1 | 62 | |
| Prion | Prion | PrP | Dual-aptamer strategy and sandwich structure of MMPs and QDs | 0.01% brain homogenate | 112 | |
| Mycoplasma | Mycoplasma hyorhinis | — | Flow cytometer, fluorescence microscope, or microplate reader | — | 113 | |
| Parasites | Cryptosporidium parvum (C. parvum) | Oocysts | Fluorescence microplate-based assay | 5 C. parvum oocysts in 300 μL of wastewater | 93 | |
| Plasmodium | pLDH | MoS2 nanosheet-based sensor relying on FRET | — | 94 | ||
| Chemiluminescent aptasensors | Virus | Norovirus GII | Capsid protein | Intra chemiluminescent resonance transfer (intra-CRET) | 80 ng mL−1 | 47 |
| Hepatitis B | Surface antigen (HBsAg) | Rapid magnetic separation chemiluminescent assay | 0.1 ng mL−1 | 120 | ||
| Hepatitis B | HBsAg | Double-functionalized gold nanoparticles based aptasensor | 0.05 ng mL−1 | 121 | ||
| SERS aptasensors | Virus | A/H1N1 | Nucleoprotein | 3D nano-popcorn plasmonic substrate-based SERS | 97 pfu mL−1 | 133 |
| Influenza virus | HA | Sandwich SERS assay | 10−4 hemagglutination units per probe | 134 | ||
| Bacteria | E. coli O157:H7 | — | GNBs modified with aptamers and RhB | 3 cfu mL−1 | 63 | |
| Staphylococcus aureus (S. aureus) | — | AuNPs PDMS film | 13 cfu mL−1 | 64 | ||
| SPR aptasensors | Virus | HIV type I | Tat protein | SPReTIRE and SE | 1 pM (1.5 pg mL−1) | 140 |
| AIV H5N1 HA | HA | LSPR | 1 pM | 141 | ||
| Norovirus | Capsid protein | Gold NR enhanced surface sandwich assay | 70 aM | 142 | ||
| Bacteria | B. melitensis | — | Efficient magnetic isolation SPR | 27 ± 11 cells | 143 | |
| S. typhimurium | — | Self-assembly of AuNPs monolayers LSPR | 104 cfu mL−1 | 65 | ||
| Electrochemical aptasensors | Virus | Norovirus | — | 3D electrochemical aptasensor | 0.28 ng mL−1 | 153 |
| SARS-CoV-2 | D614G mutation in the spike protein | Nanopore sensing | — | 154 | ||
| Human adenoviruses (HAdV) and SARS-CoV-2 | — | Nanopore sensing | 1 pfu mL−1 (HAdV) 102 copies per mL (SARS-CoV-2) | 155 | ||
| Bacteria | S. aureus | — | MIT | 1 CFU mL−1 | 156 | |
| Mycobacterium tuberculosis | Secreted protein MPT64 | EIS | 4.1 fM | 66 | ||
| Prion | Prion | PrP | PEC | 50.9 fM | 50 | |
| Parasites | Plasmodium falciparum | PfLDH | Impedance spectroscopy | 0.84 pM | 160 | |
| Fungi | Ochratoxin | OTA | EIS | 0.167 ng mL−1 | 85 | |
| FET aptasensors | Virus | SARS-CoV-2 | Spike protein | Intrinsic silicon thin film transistor | 10 fM | 166 |
| AIV H5N1 | HA | FET aptasensor | 5.9 pM | 167 | ||
| Hepatitis B | HBsAg | Multidimensional conductive nanofilm (MCNF)-based FET aptasensor | 10 aM | 169 | ||
| Parasites | Plasmodium falciparum | PfGDH | IDμE FET | 16.7 pM in spiked buffer | 170 | |
| 48.6 pM in serum samples |
Data availability
The data that support the findings of this review study are openly available and collected from the published research articles, review papers with permission of publishers.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (22304160), China Postdoctoral Science Foundation (2024M763326), Hangzhou Institute of Medicine, Chinese Academy of Sciences (2024ZZBS12), the Zhejiang Leading Innovation and Entrepreneurship Team (2022R01006), the Pioneer Research and Development Program of Zhejiang Province, China (2024SDYXS0003), Joint Funds for the Innovation of Science and Technology, Fujian Province (2023Y9267), Natural Science Foundation of Henan Province (242300421125), and Key Project of the Medical and Health Science and Technology Plan of Zhejiang Province (WKJ-ZJ-2547).References
- J. Lessler, N. G. Reich, R. Brookmeyer, T. M. Perl, K. E. Nelson and D. A. Cummings, Lancet Infect. Dis., 2009, 9, 291–300 CrossRef PubMed.
- A. H. Peruski and L. F. Peruski, Clin. Diagn. Lab. Immunol., 2003, 10, 506–513 CAS.
- G. Zhang, J. Guo and X. Wang, Methods Mol. Biol., 2009, 504, 169–183 CrossRef CAS PubMed.
- P. Peng, C. Liu, Z. D. Li, Z. R. Xue, P. Mao, J. Hu, F. Xu, C. Y. Yao and M. L. You, TrAC, Trends Anal. Chem., 2022, 152, 116605 CrossRef CAS.
- Y. Y. Xia, J. Si and Z. Y. Li, Biosens. Bioelectron., 2016, 77, 774–789 CrossRef CAS PubMed.
- M. Tomita and K. Tsumoto, Immunotherapy, 2011, 3, 371–380 CrossRef CAS PubMed.
- X. Zhao, C. W. Lin, J. Wang and D. H. Oh, J. Microbiol. Biotechnol., 2014, 24, 297–312 CrossRef CAS PubMed.
- H. Li, P. Er Saw and E. Song, Cell. Mol. Immunol., 2020, 17, 451–461 CrossRef CAS PubMed.
- L. Li, S. Xu, H. Yan, X. Li, H. S. Yazd, X. Li, T. Huang, C. Cui, J. Jiang and W. Tan, Angew. Chem., Int. Ed., 2021, 60, 2221–2231 CrossRef CAS PubMed.
- J. Hoinka, A. Berezhnoy, P. Dao, Z. E. Sauna, E. Gilboa and T. M. Przytycka, Nucleic Acids Res., 2015, 43, 5699–5707 CrossRef CAS PubMed.
- C. Ducrot and M. Piffoux, Mol. Ther.–Nucleic Acids, 2023, 33, 254–256 CrossRef CAS PubMed.
- K. H. Cole and A. Luptak, Methods Enzymol., 2019, 621, 329–346 CAS.
- S. L. Auwardt, Y. J. Seo, M. Ilgu, J. Ray, R. R. Feldges, S. Shubham, L. Bendickson, H. A. Levine and M. Nilsen-Hamilton, Sci. Rep., 2018, 8, 15712 CrossRef PubMed.
- Y. Su, L. J. Zhu, Y. F. Wu, Z. H. Liu and W. T. Xu, TrAC, Trends Anal. Chem., 2022, 157, 116731 CrossRef CAS.
- Y. F. He, J. L. Yuan, I. M. Khan, L. L. Zhang, P. F. Ma and Z. P. Wang, Food Control, 2023, 153, 109891 CrossRef CAS.
- Y. Huang, X. Yan, L. Zhao, X. Qi, S. Wang and X. Liang, Microchem. J., 2019, 150, 104179 CrossRef CAS.
- Y. S. Kim, J. Chung, M. Y. Song, J. Jurng and B. C. Kim, Biosens. Bioelectron., 2014, 54, 195–198 CrossRef CAS PubMed.
- X. Chang, C. Zhang, C. Lv, Y. Sun, M. Zhang, Y. Zhao, L. Yang, D. Han and W. Tan, J. Am. Chem. Soc., 2019, 141, 12738–12743 CrossRef CAS PubMed.
- M. Sun, S. Liu, X. Wei, S. Wan, M. Huang, T. Song, Y. Lu, X. Weng, Z. Lin, H. Chen, Y. Song and C. Yang, Angew. Chem., Int. Ed., 2021, 60, 10266–10272 CrossRef CAS PubMed.
- H. X. Xu, H. Tian, J. S. Deng, Q. M. Zhuo, J. H. Cui, J. Z. Wang, Y. A. Yin and P. Yu, Miner. Eng., 2023, 203, 108304 CrossRef CAS.
- D. J. Sarkar, B. K. Behera, P. K. Parida, V. K. Aralappanavar, S. Mondal, J. Dei, B. K. Das, S. Mukherjee, S. Pal, P. Weerathunge, R. Ramanathan and V. Bansal, Biosens. Bioelectron., 2023, 219, 114771 CrossRef CAS PubMed.
- A. Nourizad, S. Golmohammadi, A. Aghanejad and M. R. Tohidkia, Environ. Res., 2023, 236, 116726 CrossRef CAS PubMed.
- H. C. Maltezou, A. Papanikolopoulou, S. Vassiliu, K. Theodoridou, G. Nikolopoulou and N. V. Sipsas, Viruses, 2023, 15, 865 CrossRef CAS PubMed.
- E. J. Chow, T. M. Uyeki and H. Y. Chu, Nat. Rev. Microbiol., 2023, 21, 195–210 CAS.
- Y. Su, L. J. Zhu, Y. F. Wu, Z. H. Liu and W. T. Xu, TrAC, Trends Anal. Chem., 2022, 157, 116731 CrossRef CAS.
- K. S. Park, Biosens. Bioelectron., 2018, 102, 179–188 CrossRef CAS PubMed.
- B. B. Lou, Y. F. Liu, M. L. Shi, J. Chen, K. Li, Y. F. Tan, L. W. Chen, Y. W. Wu, T. Wang, X. Q. Liu, T. Jiang, D. M. Peng and Z. B. Liu, TrAC, Trends Anal. Chem., 2022, 157, 116738 CrossRef CAS PubMed.
- S. B. Johnson and M. Parker, Nat. Rev. Genet., 2019, 20, 313–315 CrossRef CAS PubMed.
- A. Hiebl, A. Auer, G. Bagrinovschi, M. Stejskal, R. Hirt, H. T. Rumenapf, A. Tichy and F. Kunzel, J. Small Anim. Pract., 2019, 60, 594–600 CrossRef CAS PubMed.
- B. A. Han and J. M. Drake, EMBO Rep., 2016, 17, 785–789 CrossRef CAS PubMed.
- A. S. Hancock, P. J. Younis, D. S. Beggs, P. D. Mansell and M. F. Pyman, Aust. Vet. J., 2015, 93, 349–353 CrossRef CAS PubMed.
- A. Luchicchi, T. Pattij, J. N. M. Viaña, S. de Kloet and N. Marchant, J. Neurosci. Methods, 2021, 348, 109004 CrossRef CAS PubMed.
- C. Romeo, C. J. McInnes, T. D. Dale, C. Shuttleworth, S. Bertolino, L. A. Wauters and N. Ferrari, Anim. Conserv., 2019, 22, 14–23 CrossRef.
- S. J. Vincent, B. A. Coutts and R. A. C. Jones, PLoS One, 2014, 9, e91224 CrossRef PubMed.
- D. S. Burke and R. R. Redfield, Ann. Intern. Med., 1986, 105, 968 CrossRef CAS PubMed.
- A. E. Gorbalenya, M. Krupovic, A. Mushegian, A. M. Kropinski, S. G. Siddell, A. Varsani, M. J. Adams, A. J. Davison, B. E. Dutilh, B. Harrach, R. L. Harrison, S. Junglen, A. M. Q. King, N. J. Knowles, E. J. Lefkowitz, M. L. Nibert, L. Rubino, S. Sabanadzovic, H. Sanfacon, P. Simmonds, P. J. Walker, F. M. Zerbini, J. H. Kuhn and V. E. Comm, Nat. Microbiol., 2020, 5, 668–674 CrossRef PubMed.
- S. F. Elena, Proc. Natl. Acad. Sci. U. S. A., 2023, 120, e2310785120 CrossRef CAS PubMed.
- P. Palese, H. Y. Zheng, O. G. Engelhardt, S. Pleschka and A. GarciaSastre, Proc. Natl. Acad. Sci. U. S. A., 1996, 93, 11354–11358 CrossRef CAS PubMed.
- P. Griffiths and M. Reeves, Nat. Rev. Microbiol., 2021, 19, 759–773 CrossRef CAS PubMed.
- C. Narayan, J. Kwon, C. Kim, S. J. Kim and S. K. Jang, Analyst, 2020, 145, 1473–1482 RSC.
- J. W. Park, S. Jin Lee, E. J. Choi, J. Kim, J. Y. Song and M. Bock Gu, Biosens. Bioelectron., 2014, 51, 324–329 CrossRef CAS PubMed.
- J. X. Li, Z. J. Zhang, J. Gu, R. Amini, A. G. Mansfield, J. R. Xia, D. White, H. D. Stacey, J. C. Ang, G. Panesar, A. Capretta, C. D. M. Filipe, K. Mossman, B. J. Salena, J. B. Gubbay, C. Balion, L. Soleymani, M. S. Miller, D. Yamamura, J. D. Brennan and Y. F. Li, J. Am. Chem. Soc., 2022, 144, 23465–23473 CrossRef CAS PubMed.
- J. Kim, S. Baek, J. Nam, J. Park, K. Kim, J. Kang and G. Yeom, Anal. Chem., 2024, 96, 1725–1732 CrossRef CAS PubMed.
- X. Li, C. Yin, Y. Wu, Z. Zhang, D. Jiang, D. Xiao, X. Fang and C. Zhou, Biosens. Bioelectron., 2020, 147, 111488 CrossRef CAS PubMed.
- P. S. Kwon, S. Ren, S. J. Kwon, M. E. Kizer, L. Kuo, M. Xie, D. Zhu, F. Zhou, F. M. Zhang, D. Kim, K. Fraser, L. D. Kramer, N. C. Seeman, J. S. Dordick, R. J. Linhardt, J. Chao and X. Wang, Nat. Chem., 2020, 12, 26–35 CrossRef CAS PubMed.
- G. T. Yang, W. Li, S. Zhang, B. Hu and Z. Huang, Talanta, 2024, 266, 125039 CrossRef CAS PubMed.
- B. Kim, K. W. Chung and J. H. Lee, J. Pharm. Biomed. Anal., 2018, 152, 315–321 CrossRef CAS PubMed.
- M. F. Tuite and T. R. Serio, Nat. Rev. Mol. Cell Biol., 2010, 11, 823–833 CrossRef CAS PubMed.
- K. Giles, A. L. Woerman, D. B. Berry and S. B. Prusiner, Cold Spring Harbor Perspect. Biol., 2017, 9, a023499 CrossRef PubMed.
- X. X. Yan, J. J. Li, R. Y. Yang, Y. M. Li, X. H. Zhang and J. H. Chen, Sens. Actuators, B, 2018, 255, 2187–2193 CrossRef CAS.
- S. J. Xiao, P. P. Hu, X. D. Wu, Y. L. Zou, L. Q. Chen, L. Peng, J. A. Ling, S. J. Zhen, L. Zhan, Y. F. Li and C. Z. Huang, Anal. Chem., 2010, 82, 9736–9742 CrossRef CAS PubMed.
- H. J. Zhang, Y. H. Lu, Y. J. Long, Q. L. Wang, X. X. Huang, R. Zhu, X. L. Wang, L. P. Liang, P. Teng and H. Z. Zheng, Anal. Methods, 2014, 6, 2982–2987 RSC.
- A. Dance, Nature, 2023, 619, 424–426 CrossRef CAS PubMed.
- B. X. Wang, D. Leshchiner, L. J. Luo, M. Tuncel, K. Hokamp, J. C. D. Hinton and D. M. Monack, Nat. Genet., 2024, 56, 1–12 CrossRef PubMed.
- B. P. Howden, S. G. Giulieri, T. W. F. Lung, S. L. Baines, L. K. Sharkey, J. Y. H. Lee, A. Hachani, I. R. Monk and T. P. Stinear, Nat. Rev. Microbiol., 2023, 21, 380–395 CrossRef CAS PubMed.
- L. E. Spoor, E. Richardson, A. C. Richards, G. J. Wilson, C. Mendonca, R. K. Gupta, P. R. McAdam, S. Nutbeam-Tuffs, N. S. Black, J. P. O'Gara, C. Y. Lee, J. Corander and J. R. Fitzgerald, Microb. Genomics, 2015, 1, e000036 Search PubMed.
- N. Davati and A. Ghorbani, PLoS One, 2024, 19, e0311434 CrossRef CAS PubMed.
- A. Tang, Y. Shi, Q. Q. Dong, S. H. Wang, Y. Ge, C. Y. Wang, Z. M. Gong, W. Z. Zhang and W. Chen, Crit. Care, 2023, 27, 467 CrossRef PubMed.
- S. Tripathi, D. Purchase, M. Govarthanan, R. Chandra and S. Yadav, Environ. Monit. Assess., 2023, 195, 75 CrossRef PubMed.
- J. W. Wilson, M. J. Schurr, C. L. LeBlanc, R. Ramamurthy, K. L. Buchanan and C. A. Nickerson, Postgrad. Med. J., 2002, 78, 216–224 CrossRef CAS PubMed.
- S. B. Somvanshi, A. M. Ulloa, M. Zhao, Q. Liang, A. K. Barui, A. Lucas, K. M. Jadhav, J. P. Allebach and L. A. Stanciu, Biosens. Bioelectron., 2022, 207, 114214 CrossRef CAS PubMed.
- P. F. Ma, N. Duan, H. Ye, Y. Xia, Z. Y. Ding and Z. P. Wang, Talanta, 2022, 246, 123499 CrossRef CAS PubMed.
- S. Zhou, C. Lu, Y. Li, L. Xue, C. Zhao, G. Tian, Y. Bao, L. Tang, J. Lin and J. Zheng, ACS Sens., 2020, 5, 588–596 CrossRef CAS PubMed.
- A. Zhu, S. Ali, Y. Xu, Q. Ouyang and Q. Chen, Biosens. Bioelectron., 2021, 172, 112806 CrossRef CAS PubMed.
- S. Y. Oh, N. S. Heo, S. Shukla, H. J. Cho, A. T. E. Vilian, J. Kim, S. Y. Lee, Y. K. Han, S. M. Yoo and Y. S. Huh, Sci. Rep., 2017, 7, 10130 CrossRef PubMed.
- M. Sypabekova, K. Dukenbayev, A. Tsepke, A. Akisheva, N. Oralbayev and D. Kanayeva, Sci. Rep., 2019, 9, 16273 CrossRef PubMed.
- S. Razin, D. Yogev and Y. Naot, Microbiol. Mol. Biol. Rev., 1998, 62, 1094–1156 CrossRef CAS PubMed.
- O. A. Chernova, E. S. Medvedeva, A. A. Mouzykantov’, N. B. Baranova and V. M. Chernov, Acta Nat., 2016, 8, 24–34 CrossRef CAS.
- T. Li and N. Lee, N. Engl. J. Med., 2018, 379, 1262 CrossRef PubMed.
- R. J. Geraghty, A. Capes-Davis, J. M. Davis, J. Downward, R. I. Freshney, I. Knezevic, R. Lovell-Badge, J. R. W. Masters, J. Meredith, G. N. Stacey, P. Thraves and M. Vias, Br. J. Cancer, 2014, 111, 1021–1046 CrossRef PubMed.
- Y. Liu, W. Jiang, S. Yang, J. Hu, H. Lu, W. Han, J. Wen, Z. Zeng, J. Qi, L. Xu, H. Zhou, H. Sun and Y. Zu, ACS Sens., 2019, 4, 2028–2038 CrossRef CAS PubMed.
- Q. Wan, X. Liu, Z. Zeng, Z. Chen, Y. Liu and Y. Zu, Int. J. Mol. Sci., 2020, 21, 3784 CrossRef CAS PubMed.
- P. Fu, Z. Sun, Z. Yu, Y. Zhang, J. Shen, H. Zhang, W. Xu, F. Jiang, H. Chen and W. Wu, Anal. Chem., 2014, 86, 1701–1709 CrossRef CAS PubMed.
- Y. B. Zhang, J. Dai, Y. Yang, J. X. Guo, L. Q. Cao and M. Ye, J. Biomed. Nanotechnol., 2022, 18, 166–174 CrossRef CAS PubMed.
- A. Ressler and S. Kurz, JAMA, J. Am. Med. Assoc., 2023, 330, 1398 CrossRef PubMed.
- J. D. Scott, Lancet Infect. Dis., 2016, 16, 637 CrossRef PubMed.
- W. L. Kong and R. J. Machida, Mol. Ecol. Resour., 2022, 22, 2627–2639 CrossRef CAS PubMed.
- J. McGinn and R. L. Lamason, Pathog. Dis., 2021, 79, ftab019 CrossRef CAS PubMed.
- M. Teymouri, S. Mollazadeh, H. Mortazavi, Z. N. Ghale-Noie, V. Keyvani, F. Aghababaei, M. R. Hamblin, G. Abbaszadeh-Goudarzi, H. Pourghadamyari, S. M. R. Hashemian and H. Mirzaei, Pathol., Res. Pract., 2021, 221, 153443 CrossRef CAS PubMed.
- A. A. Q. A. Al-Shaarani and L. Pecoraro, Front. Microbiol., 2024, 15, 1428415 CrossRef PubMed.
- R. Lucking, M. C. Aime, B. Robbertse, A. N. Miller, T. Aoki, H. A. Ariyawansa, G. Cardinali, P. W. Crous, I. S. Druzhinina, D. M. Geiser, D. L. Hawksworth, K. D. Hyde, L. Irinyi, R. Jeewon, P. R. Johnston, P. M. Kirk, E. Malosso, T. W. May, W. Meyer, H. R. Nilsson, M. Opik, V. Robert, M. Stadler, M. Thines, D. Vu, A. M. Yurkov, N. Zhang and C. L. Schoch, Nat. Microbiol., 2021, 6, 540–548 CrossRef PubMed.
- J. P. Richardson, Pathogens, 2022, 11, 459 CrossRef PubMed.
- T. R. T. Dagenais and N. P. Keller, Clin. Microbiol. Rev., 2009, 22, 447–465 CrossRef CAS PubMed.
- X. D. Guo, F. Wen, N. Zheng, M. Saive, M. L. Fauconnier and J. Q. Wang, Front. Chem., 2020, 8, 195 CrossRef CAS PubMed.
- X. J. Xia, M. Li, M. Wang, M. Q. Gu, K. N. Chi, Y. H. Yang and R. Hu, J. Biomed. Nanotechnol., 2020, 16, 1296–1303 CrossRef CAS PubMed.
- C. Wang, L. L. Sun and Q. Zhao, Chin. Chem. Lett., 2019, 30, 1017–1020 CrossRef CAS.
- X. L. Tang, Y. Hua, Q. Guan and C. H. Yuan, Eur. J. Clin. Microbiol. Infect. Dis., 2016, 35, 587–595 CrossRef CAS PubMed.
- H. C. Leggett, C. K. Cornwallis and S. A. West, PLoS Pathog., 2012, 8, e1002512 CrossRef CAS PubMed.
- K. Venugopal, F. Hentzschel, G. Valkiunas and M. Marti, Nat. Rev. Microbiol., 2020, 18, 177–189 CrossRef CAS PubMed.
- A. K. C. Leung, A. A. M. Leung, A. H. C. Wong and K. L. Hon, Recent Pat. Inflammation Allergy Drug Discovery, 2020, 14, 133–145 CrossRef CAS.
- Z. Lu, G. Sankaranarayanan, K. A. Rawlinson, V. Offord, P. J. Brindley, M. Berriman and G. Rinaldi, Front. Trop. Dis., 2021, 2, 713123 CrossRef PubMed.
- C. A. Lippi, S. J. Mundis, R. Sippy, J. M. Flenniken, A. Chaudhary, G. Hecht, C. J. Carlson and S. J. Ryan, Parasites Vectors, 2023, 16, 302 CrossRef PubMed.
- E. M. Hassan, B. R. Dixon, S. A. Sattar, A. Stalker, B. Örmeci and M. C. DeRosa, Talanta, 2021, 222, 121618 CrossRef CAS PubMed.
- A. Geldert, Kenry and C. T. Lim, Sci. Rep., 2017, 7, 17510 CrossRef PubMed.
- J. D. Ospina-Villa, C. Lopez-Camarillo, C. A. Castanon-Sanchez, J. Soto-Sanchez, E. Ramirez-Moreno and L. A. Marchat, Genes, 2018, 9, 584 CrossRef PubMed.
- N. K. Singh, P. Jain, S. Das and P. Goswami, Anal. Chem., 2019, 91, 4213–4221 CrossRef CAS PubMed.
- N. K. Singh, P. D. Thungon, P. Estrela and P. Goswami, Biosens. Bioelectron., 2019, 123, 30–35 CrossRef CAS PubMed.
- F. Q. Yang and L. Ge, Sensors, 2023, 23, 9887 CrossRef PubMed.
- C. E. Kidd, M. R. Kidd and H. A. Hofmann, Integr. Comp. Biol., 2009, 49, E254 CrossRef PubMed.
- T. T. Bezuneh, T. H. Fereja, S. A. Kitte, H. J. Li and Y. D. Jin, Talanta, 2022, 248, 123611 CrossRef CAS PubMed.
- K. Wang, M. Wang, T. Ma, W. Li and H. Zhang, Biosensors, 2022, 13, 39 CrossRef PubMed.
- Z. J. Zhang, J. X. Li, J. Gu, R. Amini, H. D. Stacey, J. C. Ang, D. White, C. D. M. Filipe, K. Mossman, M. S. Miller, B. J. Salena, D. Yamamura, P. Sen, L. Soleymani, J. D. Brennan and Y. F. Li, Chem. – Eur. J., 2022, 28, e202200078 CrossRef CAS PubMed.
- Q. Wang, J. Li, Z. Zhang, R. Amini, A. Derdall, J. Gu, J. Xia, B. J. Salena, D. Yamamura, L. Soleymani and Y. Li, Angew. Chem., Int. Ed., 2025, 137, e202415226 CrossRef.
- K. Li, Y. Lyu, Y. Huang, S. Xu, H. W. Liu, L. Chen, T. B. Ren, M. Xiong, S. Huan, L. Yuan, X. B. Zhang and W. Tan, Proc. Natl. Acad. Sci. U. S. A., 2021, 118, e2018033118 CrossRef CAS PubMed.
- T. B. Ren, Z. Y. Wang, Z. Xiang, P. Lu, H. H. Lai, L. Yuan, X. B. Zhang and W. Tan, Angew. Chem., Int. Ed., 2021, 60, 800–805 CrossRef CAS PubMed.
- L. Du, Y. N. Hou, D. D. Fu, J. Li, J. Ao, A. X. Ma, Q. Q. Wan, Z. G. Wang, S. L. Liu and L. J. Zhang, ACS Nano, 2024, 23090–23103 CrossRef CAS PubMed.
- X. Xu, A.-a Liu and D. Pang, Chem. Res. Chin. Univ., 2024, 40, 162–172 CrossRef CAS.
- Z. Y. Yuwen, Q. Zeng, Q. Z. Ye, Y. X. Zhao, J. X. Zhu, K. Chen, H. W. Liu and R. H. Yang, Angew. Chem., Int. Ed., 2023, 62, e202302957 CrossRef CAS PubMed.
- J. Lim, S. U. Son, J. Ki, S. Kim, J. Lee, S. Jang, S. B. Seo, H. Jang, T. Kang, J. Jung, E. Kim and E. K. Lim, Biosens. Bioelectron., 2024, 259, 116375 CrossRef CAS PubMed.
- H. X. Li, X. Y. Fu, Q. M. You, D. W. Shi, L. X. Su, M. H. Song, R. Z. Peng, T. Fu, P. Wang and W. H. Tan, J. Mater. Chem. B, 2025, 13, 1681–1689 RSC.
- Z. Y. Huang, Z. Y. Du, J. Li, D. Han, J. X. He, Y. B. Yang, D. Wang, Y. Liang, Y. S. Yang, R. Z. Peng and W. H. Tan, Anal. Chem., 2024, 97, 328–336 CrossRef PubMed.
- Y. Wang, G. Song, S. Liao, Q. Qin, Y. Zhao, L. Shi, K. Guan, X. Gong, P. Wang, X. Yin, Q. Chen and X. B. Zhang, Angew. Chem., Int. Ed., 2021, 60, 19779–19789 CrossRef CAS PubMed.
- Q. Y. Wan, X. H. Liu, Z. H. Zeng, Z. H. Chen, Y. T. Liu and Y. L. Zu, Int. J. Mol. Sci., 2020, 21, 3784 CrossRef CAS PubMed.
- Z. Y. Yuwen, T. L. Zou, Z. H. He, Z. L. Su, Y. J. Gong, H. W. Liu and R. H. Yang, Anal. Chem., 2024, 96, 20318–20329 CrossRef CAS PubMed.
- M. Yang, J. Huang, J. Fan, J. Du, K. Pu and X. Peng, Chem. Soc. Rev., 2020, 49, 6800–6815 RSC.
- V. R. Viviani, G. F. Pelentir and V. R. Bevilaqua, Biosensors, 2022, 12, 400 CrossRef CAS PubMed.
- Y. Li, X. He, W. Zhu, H. Li and W. Wang, Anal. Bioanal. Chem., 2022, 414, 75–83 CrossRef CAS PubMed.
- C. Lu, C. Zhang, P. Wang, Y. Zhao, Y. Yang, Y. Wang, H. Yuan, S. Qu, X. Zhang and G. Song, Chem, 2020, 2314–2334 CAS.
- S. Cho, L. Park, R. Chong, Y. T. Kim and J. H. Lee, Biosens. Bioelectron., 2014, 52, 310–316 CrossRef CAS PubMed.
- Z. J. Xi, R. R. Huang, Z. Y. Li, N. Y. He, T. Wang, E. B. Su and Y. Deng, ACS Appl. Mater. Interfaces, 2015, 7, 11215–11223 CrossRef CAS PubMed.
- Z. J. Xi, Q. Gong, C. Wang and B. Zheng, Sci. Rep., 2018, 8, 9444 CrossRef PubMed.
- M. Usman, J. W. Tang, F. Li, J. X. Lai, Q. H. Liu, W. Liu and L. Wang, J. Adv. Res., 2023, 51, 91–107 CrossRef CAS PubMed.
- D. Li, K. Aubertin, D. Onidas, P. Nizard, N. Felidj, F. Gazeau, C. Mangeney and Y. Luo, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2022, 14, e1795 CAS.
- R. Chen, S. Li, S. Ren, D. Han, K. Qin, X. Jia, H. Zhou and Z. Gao, Adv. Colloid Interface Sci., 2024, 331, 103235 CrossRef CAS PubMed.
- X. Qiao, Z. Xue, L. Liu, K. Liu and T. Wang, Adv. Mater., 2019, 31, e1804275 CrossRef PubMed.
- X. Qiao, B. Su, C. Liu, Q. Song, D. Luo, G. Mo and T. Wang, Adv. Mater., 2018, 30, 1702275 CrossRef PubMed.
- Z. Zhang, W. Yu, J. Wang, D. Luo, X. Qiao, X. Qin and T. Wang, Anal. Chem., 2017, 89, 1416–1420 CrossRef CAS PubMed.
- X. Meshik, S. Farid, M. Choi, Y. Lan, S. Mukherjee, D. Datta, M. Dutta and M. A. Stroscio, Crit. Rev. Biomed. Eng., 2015, 43, 277–296 CrossRef PubMed.
- J. H. Granger, N. E. Schlotter, A. C. Crawford and M. D. Porter, Chem. Soc. Rev., 2016, 45, 3865–3882 RSC.
- N. N. Durmanov, R. R. Guliev, A. V. Eremenko, I. A. Boginskaya, I. A. Ryzhikov, E. A. Trifonova, E. V. Putlyaev, A. N. Mukhin, S. L. Kalnov, M. V. Balandina, A. P. Tkachuk, V. A. Gushchin, A. K. Sarychev, A. N. Lagarkov, I. A. Rodionov, A. R. Gabidullin and I. N. Kurochkin, Sens. Actuators, B, 2018, 257, 37–47 CrossRef CAS.
- R. R. Yuan, H. K. Li and H. M. He, Dalton Trans., 2021, 50, 14091–14104 RSC.
- Y. Zhang, B. S. Lai and M. Juhas, Molecules, 2019, 24, 941 CrossRef PubMed.
- H. Chen, S. G. Park, N. Choi, J. I. Moon, H. Dang, A. Das, S. Lee, D. G. Kim, L. Chen and J. Choo, Biosens. Bioelectron., 2020, 167, 112496 CrossRef CAS PubMed.
- V. I. Kukushkin, N. M. Ivanov, A. A. Novoseltseva, A. S. Gambaryan, I. V. Yaminsky, A. M. Kopylov and E. G. Zavyalova, PLoS One, 2019, 14, e0216247 CrossRef CAS PubMed.
- B. Fortuni, T. Inose, S. Uezono, S. Toyouchi, K. Umemoto, S. Sekine, Y. Fujita, M. Ricci, G. Lu, A. Masuhara, J. A. Hutchison, L. Latterini and H. Uji-i, Chem. Commun., 2017, 53, 11298–11301 RSC.
- M. Pirzada and Z. Altintas, Micromachines, 2020, 11, 356 CrossRef PubMed.
- L. H. Guo, X. D. Zhou and D. H. Kim, Biosens. Bioelectron., 2011, 26, 2246–2251 CrossRef CAS PubMed.
- S. M. Yoo, D. K. Kim and S. Y. Lee, Talanta, 2015, 132, 112–117 CrossRef CAS PubMed.
- M. O. Çaglayan, F. Sayar, G. Demirel, B. Garipcan, B. Otman, B. Çelen and E. Piskin, Nanomedicine, 2009, 5, 152–161 CrossRef PubMed.
- M. O. Caglayan and Z. Ustundag, Spectrochim. Acta, Part A, 2020, 227, 117748 CrossRef CAS PubMed.
- T. Lee, G. H. Kim, S. M. Kim, K. Hong, Y. Kim, C. Park, H. Sohn and J. Min, Colloids Surf., B, 2019, 182, 110341 CrossRef CAS PubMed.
- S. Kim, S. Lee and H. J. Lee, Sens. Actuators, B, 2018, 273, 1029–1036 CrossRef CAS.
- A. D. Dursun, B. A. Borsa, G. Bayramoglu, M. Y. Arica and V. C. Ozalp, Talanta, 2022, 239, 123074 CrossRef CAS PubMed.
- L. P. Guo, Y. N. Zhao, Q. Huang, J. Huang, Y. B. Tao, J. J. Chen, H. Y. Li and H. Liu, Microsyst. Nanoeng., 2024, 10, 65 CrossRef CAS PubMed.
- J. Baranwal, B. Barse, G. Gatto, G. Broncova and A. Kumar, Chemosensors, 2022, 10, 363 CrossRef CAS.
- A. C. Power, B. Gorey, S. Chandra and J. Chapman, Nanotechnol. Rev., 2018, 7, 19–41 CrossRef CAS.
- J. W. Ding and W. Qin, TrAC, Trends Anal. Chem., 2020, 124, 115803 CrossRef CAS.
- Y. Xu, G. F. Cheng, P. G. He and Y. Z. Fang, Electroanalysis, 2009, 21, 1251–1259 CrossRef CAS.
- R. Abd-Ellatief and M. R. Abd-Ellatief, Diagnostics, 2021, 11, 104 CrossRef PubMed.
- A. Villalonga, B. Mayol, R. Villalonga and D. Vilela, Sens. Actuators, B, 2022, 369, 132318 CrossRef CAS.
- R. Q. Yuan, J. Cai, H. J. Ma, Y. Luo, L. H. Wang and S. Su, Chemosensors, 2023, 11, 488 CrossRef CAS.
- A. Radi and M. R. Abd-Ellatief, Diagnostics, 2021, 11, 104 CrossRef CAS PubMed.
- H. Jiang, Z. K. Sun, C. Zhang and X. Weng, Sens. Actuators, B, 2022, 354, 131232 CrossRef CAS.
- R. Ren, S. Cai, X. Fang, X. Wang, Z. Zhang, M. Damiani, C. Hudlerova, A. Rosa, J. Hope, N. J. Cook, P. Gorelkin, A. Erofeev, P. Novak, A. Badhan, M. Crone, P. Freemont, G. P. Taylor, L. Tang, C. Edwards, A. Shevchuk, P. Cherepanov, Z. Luo, W. Tan, Y. Korchev, A. P. Ivanov and J. B. Edel, Nat. Commun., 2023, 14, 7362 CrossRef CAS PubMed.
- A. S. Peinetti, R. J. Lake, W. Cong, L. Cooper, Y. Wu, Y. Ma, G. T. Pawel, M. E. Toimil-Molares, C. Trautmann, L. Rong, B. Marinas, O. Azzaroni and Y. Lu, Sci. Adv., 2021, 7, eabh2848 CrossRef CAS PubMed.
- M. M. El-Wekil, H. M. Halby, M. Darweesh, M. E. Ali and R. Ali, Sci. Rep., 2022, 12, 12502 CrossRef CAS PubMed.
- M. H. Mahnashi, A. M. Mahmoud, K. Alhazzani, A. AZ, M. M. Algahtani, A. M. Alaseem, Y. S. A. Alqahtani and M. M. El-Wekil, Microchem. J., 2021, 168, 106439 CrossRef CAS.
- D. Goletti, G. Meintjes, B. B. Andrade, A. Zumla and S. Shan Lee, Int. J. Infect. Dis., 2025, 150, 107325 CrossRef PubMed.
- A. Tamura, T. Fukami, A. Hebisawa and F. Takahashi, J. Infect. Chemother., 2020, 26, 315–317 CrossRef PubMed.
- G. Figueroa-Miranda, L. Y. Feng, S. C. C. Shiu, R. M. Dirkzwager, Y. W. Cheung, J. A. Tanner, M. J. Schöning, A. Offenhäusser and D. Mayer, Sens. Actuators, B, 2018, 255, 235–243 CrossRef CAS.
- C. A. Vu and W. Y. Chen, Sensors, 2019, 19, 4214 CrossRef CAS PubMed.
- T. Sakata, Commun. Chem., 2024, 7, 35 CrossRef CAS PubMed.
- N. Alnaji, A. Wasfi and F. Awwad, Sci. Rep., 2023, 13, 4485 CrossRef CAS PubMed.
- D. Sadighbayan, M. Hasanzadeh and E. Ghafar-Zadeh, TrAC, Trends Anal. Chem., 2020, 133, 116067 CrossRef CAS PubMed.
- R. S. Hao, L. Liu, J. Y. Yuan, L. L. Wu and S. B. Lei, Biosensors, 2023, 13, 426 CrossRef CAS PubMed.
- G. Seo, G. Lee, M. J. Kim, S. H. Baek, M. Choi, K. B. Ku, C. S. Lee, S. Jun, D. Park, H. G. Kim, S. J. Kim, J. O. Lee, B. T. Kim, E. C. Park and S. I. Kim, ACS Nano, 2020, 14, 5135–5142 CrossRef CAS PubMed.
- J. Kwon, Y. Lee, T. Lee and J. H. Ahn, Anal. Chem., 2020, 92, 5524–5531 CrossRef CAS PubMed.
- E. Song and J. W. Choi, Nanomaterials, 2013, 3, 498–523 CrossRef CAS PubMed.
- K. H. Cho, D. H. Shin, J. Oh, J. H. An, J. S. Lee and J. Jang, ACS Appl. Mater. Interfaces, 2018, 10, 28412–28419 CrossRef CAS PubMed.
- N. K. Singh, P. D. Thungon, P. Estrela and P. Goswami, Biosens. Bioelectron., 2019, 123, 30–35 CrossRef CAS PubMed.
- F. Zhou, P. Wang, J. H. Chen, Z. J. Zhu, Y. S. Li, S. J. Wang, S. C. Wu, Y. Y. Sima, T. Fu, W. H. Tan and Z. L. Zhao, Nucleic Acids Res., 2022, 50, 9039–9050 CrossRef CAS PubMed.
- M. R. Dunn, R. M. Jimenez and J. C. Chaput, Nat. Rev. Chem., 2017, 1, 76 CrossRef CAS.
- J. Buck, J. Noeske, J. Wöhnert and H. Schwalbe, Nucleic Acids Res., 2010, 38, 4143–4153 CrossRef CAS PubMed.
- B. F. Wang, Y. W. Li, M. F. Zhou, Y. L. Han, M. Y. Zhang, Z. L. Gao, Z. T. Liu, P. Chen, W. Du, X. C. Zhang, X. J. Feng and B. F. Liu, Nat. Commun., 2023, 14, 1341 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |