Bioactive surface-functionalized MXenes for biomedicine
Ting Lia,
Weipeng Qianga and
Bo Lei *abc
*abc
aFrontier Institute of Science and Technology, Xi'an Jiaotong University, Xi'an 710054, China. E-mail: rayboo@xjtu.edu.cn
bDepartment of Respiratory and Critical Care Medicine, The Second Affiliated Hospital of Xi’an Jiaotong University, Xi’an, 710004, China
cDepartment of Orthopedics, The First Affiliated Hospital of Xi'an Jiaotong University, Xi'an, P. R. 710061, China
First published on 2nd January 2025
Abstract
MXenes, with their good biocompatibility, excellent photovoltaic properties, excellent physicochemical properties, and desirable bioactivity, have broad application prospects in the field of tissue regeneration. MXenes have been used in a wide range of applications including biosensing, bioimaging, tumour/infection therapy, bone regeneration and wound repair. By applying bioactive materials to modify the surface of MXenes, a series of multifunctional MXene-based nanomaterials can be designed for different biomedical applications to achieve better therapeutic effects or more desirable biological functions. This paper reviews the existing studies on MXene-based bioactivities, surface modification strategies and biomedical applications. Finally, the challenges, trends and prospects of MXene nanomaterials are discussed. We expect that more and more well-designed MXene-based biomaterials will have a wider range of biomedical applications, thus providing favourable information for the clinical translation of nanomedicine.
1. Introduction
The rapid development of tissue engineering and regenerative medicine has placed higher demands on nanomaterials, requiring nanoparticles with excellent cytocompatibility, mechanical, electrical, optical and magnetic properties.1 Two-dimensional (2D) nanomaterials are a new class of nanomaterials with sheet-like structures with lateral dimensions greater than 100 nm and thicknesses typically less than 5 nm.2 With the vigorous development of nanoengineering materials and nanofabrication technology, various 2D nanomaterials such as graphene, graphene oxide, layered double hydroxide (LDH), and ultrathin black phosphorus (BP) nanosheets have emerged and have become a hot spot for research.3–6 2D nanomaterials are of interest in biomedical fields such as drug delivery, combination therapy, bioimaging, tissue engineering and biosensing due to their ultrathin structure, high specific surface area, unique optoelectronic properties and extraordinary mechanical properties.3,7,8MXene is a novel multifunctional 2D lamellar material developed by Naguib et al.9 It contains transition metal carbides, nitrides and carbon-nitrides with metallic conductivity and hydrophilicity, with the structural formula Mn+1XnTx (M = transition metal, X = carbons or nitrides, T = terminal group (e.g. –O, –OH or –F)).10 Compared with other 2D nanomaterials, MXenes’ rich functional groups and high reactivity, excellent mechanical properties, electronic properties, electrical conductivity and hydrophilicity, antioxidant properties, and biological properties make them fascinatingly attractive in biomedical fields; they have broadband absorption and light-harvesting properties in the near-infrared (NIR) region, covering both the first and second NIR biological windows, as well as strong photothermal conversion capabilities, with great potential for photothermal therapy (PTT) and photoacoustic imaging (PA).11,12 In addition, the high specific surface area of MXenes and the abundant functional groups on the surface can provide opportunities for surface modification of MXenes, which is very conducive to expanding the biological functions of MXenes.13,14 So far, the cutting-edge research on MXene-based biomaterials with various attractive physicochemical properties and biological functions has attracted increasing attention from the nanomedical science community.15,16
In this review, we will firstly introduce the properties possessed by MXene-based biomaterials in the field of regenerative medicine, including biocompatibility, antioxidant, anti-inflammatory, optical, and antimicrobial properties, and tissue regeneration-promoting properties. Then, we will discuss the existing studies on the bioactive surface-modification strategies of MXenes, which are specifically classified into small molecules, macromolecules, nanoparticles, and surface polymerisation modification strategies. Afterwards, we will present the latest research advances in MXene-based biomaterials for applications in bioimaging, cancer therapy, infection treatment, wound repair, bone regeneration and other biomedical fields. Finally, we will summarise the challenges and future prospects related to MXene-based biomaterials in the biomedical field. Overall, by gaining a deeper understanding of the bioactivities, surface modification strategies, application potentials, and problematic challenges associated with 2D MXene nanosheets, we will be able to continue to facilitate clinical translation for the benefit of human health.
2. Bioactive properties for regenerative medicine
MXene-related biomaterials have the advantages of large specific surface area and abundant surface functional groups, as well as excellent electronic, optical, mechanical, and physicochemical properties, which give them considerable potential for a variety of biomedical applications. In this section, the properties exhibited by MXenes, such as biocompatibility, antioxidant, anti-inflammatory, photothermal, antimicrobial, and tissue regeneration-promoting properties, which are mainly applied in the fields of tissue repair, tumor therapy, bio-imaging, and drug delivery, will be described in detail.2.1 Biocompatibility
Good biocompatibility is essential to ensure that MXenes can safely exert their therapeutic effects inside and on the surface of living organisms long term, so the biocompatibility of the designed MXene-based nanocomposites is crucial for their application in biomedical fields. Most MXenes exhibit concentration-dependent cytotoxicity, with good cytocompatibility at low concentrations and excessive concentrations leading to cytotoxic side effects. Our research team found that Ti3C2Tx MXene exhibited good cytocompatibility with normal cells such as adipose stem cells (ADSC), mouse myoblasts (C2C12), mouse macrophages (RAW264.7), mouse fibroblasts (L929), and human umbilical venous endothelial cells (HUVECs), and maintained 90% of the cellular activity at a concentration of up to 200 μg mL−1.17,18 Interestingly, in a comparative in vitro cytotoxicity assay using MTT assay to investigate the in vitro cytotoxicity of layered Ti3C2Tx MXene on two cancer cells (A549 and A375) and two normal cells (MRC-5 and HaCaT), MXenes were found to have a higher toxic effect on tumor cells than on normal cells.19 This may be attributed to the fact that the production of reactive oxygen species (ROS) exceeds the threshold level of oxidative stress in cancer cells, leading to apoptosis. The cytotoxicity of MXenes is mainly related to two mechanisms: ROS generation and direct exposure.20 The entry of MXenes into the cell results in the generation of ROS, which cause protein and DNA damage leading to cell death. Another mechanism of MXene toxicity is due to strong adhesion between MXenes and cell membranes, where MXenes and cell membranes interact directly through ionic interactions, hydrophobicity, van der Waals forces, and receptor–ligand binding, which leads to membrane instability and loss of cellular integrity. In addition, MXenes demonstrated superior blood compatibility of graphene oxide in whole-blood tests.21 Current research advances suggest that MXenes exhibit a relatively favorable safety profile in vivo, with toxicity in vivo dependent on dose, treatment duration, and mode of administration. For example, in an in vivo study in which a mouse model was injected with 2 mg kg−1 of modified MXene (Ti3C2-DOX nanosheets), no liver or kidney damage, inflammatory response, or tissue necrosis was observed in experimental animal sections.22 In a neurotoxicity and motility assay using a zebrafish embryo model, Ti3C2 was found to have almost negligible toxicological effects on muscle and neuronal activity in zebrafish embryos at a concentration of 50 μg mL−1.23 To further improve the biocompatibility and bioavailability of MXenes, researchers have modified the surface properties by coupling MXenes with biopolymers to reduce their toxicity. Studies have reported that modification of some biocompatible materials (e.g., PEG, PLGA, PVP, chitosan, poly-L-lysine, sericin protein, polydopamine, etc.) on the surface of MXenes can effectively improve the biocompatibility of MXenes.24 For example, after intravenous administration of 20 mg kg−1 of modified MXene (Nb2C-PVP nanosheets) to 4T1 ruffled tumor-bearing mice, no significant weight loss or tissue damage was detected in any of the major organs, demonstrating that the Nb2C-PVP nanosheets were safe and biocompatible at the doses tested in vivo.11 However, there are limited studies on the toxicity of MXenes in vivo, very few of which involve animal models, and to date, toxicity studies of MXenes in human models and clinical trials remain unknown. MXene materials exhibit good biocompatibility in biomedical applications but need to be surface modified to reduce their toxicity to ensure their safety and efficacy.2.2 Antioxidant property and biocatalysis
There exists an oxidative and antioxidant balance in biological organisms, but when subjected to adverse external stimuli this balance is disrupted in favor of an oxidative state, where excessive ROS are generated or accumulated within the organism, thereby exceeding the antioxidant capacity of the cells or tissues, resulting in a state of oxidative damage to biomolecules (e.g., proteins, lipids, DNA, etc.), which may lead to a series of more serious diseases. Therefore, the antioxidant properties of biomaterials can add another highlight to their applications in biomedical fields. Currently, several studies have reported the excellent antioxidant capacity of MXenes, which exhibit superior antioxidant capacity than most artificial materials and natural compounds. For example, Ti3C2Tx effectively scavenged O2˙−, ˙OH, 1,1-diphenyl-2-picrylhydrazyl radical (DPPH˙) and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) radicals (ABTS+).25 Another study showed that Ti3C2Tx effectively scavenged ROS in M1-type polarized macrophages.17 For the treatment of oxidative stress-related diseases, researchers have proposed a therapeutic strategy of applying nano-enzymes (a new generation of man-made enzymes), which have the advantages of high catalytic activity, stability, cost-effectiveness, and ease of synthesis, etc., and MXenes have also demonstrated their potential as nano-enzymes in a number of studies.26 Feng et al. reported a two-dimensional vanadium carbide (V2C) MXene nano-enzyme (MXenzyme) that mimics up to six naturally occurring enzymes, including superoxide dismutase (SOD), catalase (CAT), peroxidase (POD), glutathione peroxidase (GPx), thiol peroxidase (TPx), and halogen peroxidase (HPO).27 Based on these enzyme mimetic properties, the constructed V2C MXenzyme was not only highly biocompatible but also effective in alleviating ROS-mediated inflammation and neurodegenerative diseases. In another study, it was reported that two-dimensional V2C MXene could be used as a multi-enzyme mimetic for the treatment of ischemic stroke in vivo.28 The antioxidant properties of MXenes are closely related to their unique structure, and the process of their function in the organism actually utilizes MXenes to catalyze the occurrence of redox reactions. The abundance of functional groups on the surface of MXenes, such as –F, –OH, –O, etc. not only increases their reactivity, but also the presence of these functional groups provides MXenes with an active site for interacting with biomolecules, which is essential for the initiation and conduct of catalytic reactions.25 During biocatalysis, these functional groups can be used as intermediates of the reaction or be directly involved in the reaction, thus accelerating or modulating the reaction rate. The Mn+1Xn layered structure in MXenes, which is formed by alternating weak van der Waals forces, gives them a unique advantage in biocatalysis.25 This layered structure easily reacts with free radicals, similar to the interaction of the active center of an enzyme with a substrate, and can facilitate specific biochemical reactions. In biocatalysis, this structural property of MXenes can mimic the active site of enzymes in nature, thus effectively catalyzing specific biochemical reactions. In addition, MXene, as an electron-rich nanomaterial, can easily form pairs with electron-deficient free radicals, a property that is particularly important in biocatalysis. MXenes can react with free radicals through electron transfer, thus neutralizing the activity of free radicals and reducing the damage of biomolecules by oxidative stress. In summary, the biocatalytic mechanism of MXenes are closely related to their structural properties, which show great potential for mimicking enzyme activity, neutralizing free radicals and protecting biomolecules from oxidative damage in biocatalytic processes.MXenes exhibit excellent antioxidant capacity and biocatalytic potential due to their unique structure and abundant functional groups, which can effectively neutralize free radicals and protect biomolecules from oxidative damage, providing a new strategy for the treatment of oxidative stress-related diseases.
2.3 Anti-inflammatory property
The excellent antioxidant properties of MXenes lays the foundation for their anti-inflammatory properties. Fewer studies have been reported on the performance of MXenes, mainly focusing on the Ti3C2Tx type of MXene. Li et al. found that Ti3C2Tx could down-regulate the expression of M1-type markers such as IL-1β and TNF-α, and further quantitative flow cytometry analysis showed that the MXene could effectively reduce the expression of CD86 protein, an M1-type macrophage marker.17 Another study reported that MXene/HAP-coated surfaces with micro-fold morphology significantly affected macrophage polarization and promoted a shift in the macrophage to an anti-inflammatory, pro-healing M2 phenotype.29 To further improve the anti-inflammatory properties of MXenes, researchers managed to improve the anti-inflammatory properties of MXenes by modifying a number of molecules with anti-inflammatory activity on MXene nanosheets. For example, researchers coated epigallocatechin gallate (EGCG)/Fe metal polyphenol nanodots on small-sized Ti3C2Tx MXene nanosheets (MXene@EGCG); this is a naturally occurring polyphenol with anti-inflammatory properties, and the nanosheets showed good anti-inflammatory ability to reduce inflammation triggered by photothermal therapy (PTT).30 Another work reported a method of loading rhizopodophyllin (Phl) onto MXene nanosheets, and the anti-inflammatory effects of Phl and MXene were able to eliminate inflammation induced by PTT scavenging of ROS.31 In addition, a MXene@polydopamine-cryptotanshinone (MXene@PDA-CPT) nanosystem was developed.32 After incubation of macrophages with MXene@PDA-CPT nanosheets, it was able to significantly increase the expression level of IL-10 and down-regulate the cellular level of TNF-α, and the loadability of cryptotanshinone further extended the mitigating inflammation effect of this system. However, the current research on the anti-inflammatory properties of MXenes is still mainly focused on Ti3C2Tx, with less research on the anti-inflammatory properties of other classes of MXene, and the research on the related anti-inflammatory mechanism needs to be further deepened. MXenes, especially Ti3C2Tx, exhibited significant anti-inflammatory effects due to their excellent antioxidant properties, which were further enhanced by binding anti-inflammatory molecules, but the anti-inflammatory potential and mechanisms of other types of MXenes still need to be explored in depth.2.4 Optical property
As an emerging 2D material, MXenes have excellent optical properties (including light absorption, emission, and scattering) which play a critical role in their biomedical applications. MXenes exhibit strong absorption properties over a broad spectral range from UV-visible to near-infrared, allowing them to exhibit high absorption in the first (650–1000 nm) and second (1000–1350 nm) biological windows, which distinguishes them from conventional photothermal materials, and which is why they have unlimited potential for deep tissue photoacoustic imaging (PAI) and PTT.33 MXenes’ strong light absorption and localized surface plasmon resonance (LSPR) effect are the driving forces behind their photothermal conversion.34,35 MXenes exhibit better photothermal performance than most materials, e.g., gold nanorods have a photothermal efficiency of 21%,36 graphene oxide has a photothermal efficiency of 24.6%,37 and MXenes’ superior photothermal conversion efficiency can reach about 50% under certain conditions.22 As the first reported MXene-based photothermal agent, Ti3C2 nanosheets have shown considerable PTT efficacy in vivo. A study reported that the solution temperature of Ti3C2 can reach 57 °C after irradiation for 6 min at a relatively low concentration (72 ppm) with a photothermal conversion efficiency of 30.6%, which suggests that Ti3C2 nanosheets can efficiently and rapidly convert near-infrared light into thermal energy, and could be used as a durable photothermal agent for cancer PTT.38 The extinction coefficient of Ti3C2 nanosheets measured at 808 nm was 25.2 L g−1 cm−1, which was higher than that of GO nanosheets (3.6 L g−1 cm−1). Tantalum carbide (Ta4C3) is another MXene material with high photothermal conversion efficiency, which could also be used for PTT treatment of bacterial infected wounds. Ta4C3 nanosheets could be used for the treatment of diabetic wounds infected with drug-resistant bacteria as the temperature could be rapidly increased to 60–70 °C by irradiation at a concentration of 400 μg mL−1 for 10 min.39 In addition, niobium carbide (Nb2C), the latest MXene material, also has a very high photothermal conversion efficiency of 36.4% and 45.65% at 808 nm and 1064 nm, respectively, under near-infrared light.40 Attractively, MXene-based quantum dots (QDs) can achieve a certain amount of luminescence under specific wavelengths of excitation (currently mainly UV-blue excitation), which is achieved by MXene QDs utilizing surface defects and size effects to induce quantum confinement.41 Xue et al. prepared photoluminescent Ti3C2 MXene quantum dots (MQDs) by a simple hydrothermal method.41 The photoluminescence excitation (PLE) spectra of MQDs showed two sharp peaks at 250 nm and 320 nm, and the MQDs were found to be readily taken up by the cells through the process of endocytosis after co-incubation with macrophage cells for 4 h, and their cell confocal images showed bright blue and green and red colors at 405, 488 and 543 nm excitation, respectively, demonstrating that MQDs have great potential as polychromatic cell imaging reagents. More and more researchers have found that MXene QDs have luminescence properties, such as Ti2N, V2N, Nb2C, Ta4C3 and so on.42–45 However, further studies on the emission color, luminescence efficiency and mechanism of MXenes are still needed. In general, the optical properties of MXenes mainly depend on the structure and type of M and X sites as well as the stoichiometry of the surface termination groups.46 Therefore, we can prepare MXenes with different compositions, structures, and surface terminations as a means of modulating the optical properties, such as light absorption, emission, and scattering, and thus promote their diverse applications in biomedical fields.MXenes show great potential in the fields of photothermal therapy, photoacoustic imaging, and polychromatic cellular imaging due to their excellent optical properties, but their luminescence mechanism and performance optimization still need to be further investigated.
2.5 Antibacterial activity
Bacterial infections are a major cause of global health issues and are becoming the second leading cause of death worldwide (after ischemic heart disease). Bacterial infections can be caused by the release of harmful substances (toxins), direct invasion of tissues, or a combination of both, which can further impede wound healing and cause great suffering to the patient, and the global misuse of antibiotics has led to severe bacterial resistance. Therefore, effective antimicrobial activity without bacterial resistance is the ideal target for the application of bioactive materials in the antimicrobial direction. Due to their structural characteristics and excellent photothermal properties, MXenes have become a hot research topic in the antibacterial field. Currently, researchers have summarized the antibacterial mechanism of MXenes as physical damage, induced oxidative stress, as well as photothermal and photodynamic therapy.47 On the one hand, the hydrophilic and anionic nature of the MXene's surface enhances the interaction with the bacterial cell membrane, and the rough surface or sharp edges of MXenes cause physical damage to the bacterial membrane. In the study of Rasool et al. it was found that when E. coli and B. subtilis were treated with 100 μg mL−1 of Ti3C2Tx for 4 h, SEM observed severe membrane rupture and cytoplasmic leakage in both bacteria, and the antibacterial rate was 97.70 ± 2.87% and 97.04 ± 2.91%, respectively.48 Pandey et al. found that Nb2C2Tx and Nb4C3Tx nanosheets could kill more than 90% of E. coli and S. aureus at 120 μg mL−1.49 SEM and TEM images clearly showed that the nanosheets caused pore formation and partial disruption of the cell wall/cytoplasmic membrane upon entering the cells, which led to the efflux of the contents of the cells, resulting in the death of the bacteria. Interestingly, this antimicrobial capacity of MXenes shows a dependence on nanosheet size and treatment time, with smaller sized nanosheets tending to enter the bacteria more readily and disrupt cytoplasmic components by physical penetration or endocytosis.50 On the other hand, MXenes induce oxidative stress by inducing the production of ROS, which can cause damage to bacterial proteins, lipids, DNA and RNA. MXenes typically have a negative zeta potential, excellent electrical conductivity, and an abundance of electronic properties, which contributes to their affinity for cell surfaces and ease of formation of chemical reactions, and MXenes can form conductive bridges with lipid bilayers to cause ROS production, leading to bacterial death.48 Zheng et al. found that the MXene group produced 1.8-fold higher ROS than the control group, and further performed a lipid peroxidation assay and found that the MXene group was able to oxidize the bacterial membranes at a rate 1.3-fold higher than the control group.51 The results of this study suggested that MXene could induce oxidative stress through the production of ROS, which could cause damage to bacterial membranes. In most studies, MXenes tended to enhance their photocatalytic activity by preventing electron/hole recombination through the available surface-terminal groups and excellent electrical conductivity. For example, few-layer Ti3C2Tx generated much higher ROS compared with multi-layer Ti3C2Tx nanosheets.52 However, the most attractive strategies for MXene antimicrobials are PTT and photodynamic therapy (PDT), which are innovative approaches against drug-resistant bacteria and are highly dependent on the excellent photoactivation properties of MXenes. MXenes have excellent photothermal properties, converting light energy into heat and causing membrane rupture, protein denaturation and irreversible damage in bacteria. Qu et al. found that 50 μg mL−1 of MXene irradiated with 808 nm laser for 10 min (0.75 W cm−2) showed strong antibacterial activity against E. coli, S. aureus, and MRSA, with an antibacterial rate of more than 99%.18 On the other hand, MXenes also possess photodynamic antibacterial properties, which can transfer energy to the surrounding oxygen molecules after receiving light, generating ROS and thus destroying the internal structure of bacteria. In a study using 1,3-diphenylisobenzofuran (DPBF) as a ROS-trapping agent, after irradiation with a 532 nm laser (20 mW) for 30 min, a decrease in absorbance at 417 nm was found in the MXene group, demonstrating that it produced singlet oxygen (1O2).53 After irradiation with a xenon lamp (150 mW cm−2), it showed good antibacterial activity against E. coli and S. aureus. This good antibacterial activity was attributed to the synergistic effect of the MXene's photothermal and photodynamic forces.MXenes have shown strong potential in the antibacterial field due to their unique physical and chemical properties, providing a new strategy for antibacterial therapy by effectively combating bacteria, especially drug-resistant bacteria, through a variety of mechanisms such as physical damage, induced oxidative stress, as well as photothermal and photodynamic therapy.
2.6 Surface structure and promotion of tissue regeneration
MXenes have a large surface area and porosity, which can promote cell adhesion, proliferation and infiltration, which makes them also show infinite potential in promoting tissue regeneration, and more and more studies have reported that MXenes can promote the regeneration of tissues such as blood vessels, muscles, bones, skin and nerves.15,54 Compared with other 2D nanomaterials, the unique surface chemical groups –OH and –O of the MXene family endow them with high hydrophilicity as well as reactivity, providing more potential for surface functionalization. Compared with graphene's smoother surface, their rough surface microstructure and interlayer spacing promote adhesion and delivery of proteins and nutrients, properties that greatly increase the chances of cell adhesion and ultimately affect tissue repair and regeneration.55 In addition, the conductive properties of MXenes enable electrical stimulation, which shows promise in enhancing the soft tissue healing process.56 For example, Li et al. reported that MXene, which has electrical conductivity properties, promotes vascular and skeletal muscle regeneration through the upregulation of myosin heavy chain (MHC) and vascular endothelial growth factor (VEGF) expression.17 In another study, it was reported that the conductive MXene-PCL nerve-guiding catheter delivered physiological neuroelectric signals, induced angiogenesis, and stimulated nerve regeneration.57 MXene-based scaffold materials can provide a favorable microenvironment for osteoblast attachment, proliferation and differentiation. For example, it has been reported that photoactivation of MXene can also regulate the osteogenic differentiation of adipose-derived stem cells (ADSC) through the ERK signaling pathway by activating heat shock protein 70 (HSP70) and enhancing bone tissue repair.18 However, the current research on MXenes promoting tissue regeneration remains at a superficial level, and future work is expected to explore the mechanism of action of MXenes promoting tissue regeneration in depth.Due to their unique surface structure and physicochemical properties, MXenes have shown great potential in promoting the regeneration of various tissues such as blood vessels, muscles, bones, skin and nerves, but further in-depth studies are needed to reveal their specific mechanism of action in promoting tissue regeneration.
3. Bioactive surface strategies
As a new type of 2D nanomaterial, MXenes have many advantages over ordinary 2D nanomaterials, such as large specific surface area, extreme thinness, good biocompatibility, rich surface functional groups, and excellent electronic, mechanical, and physicochemical properties, etc. Numerous scientific researchers have carried out in-depth investigations on MXenes and realized the corresponding applications. However, MXenes still have some drawbacks in vivo, such as their slow degradation, poor dispersibility in water and the presence of some toxicity, and there are limitations to their excellent performance while meeting the requirements of various applications. Therefore, surface modification and functionalization of MXene-based materials are necessary to improve their performance and give them new functions.58 Fortunately, MXene-based materials are rich in surface end-group functionalities that impart hydrophilicity to MXenes. By controlling the surface end groups, surface active initiators, small molecules, and polymers as active sites for covalent binding, we can achieve flexible surface modification, functionalization, and scalable processing of MXenes to enable new functionalities such as drug loading.59 Regarding the chemical surface functionalization of MXenes, we next present aspects of bioactive small molecules, bioactive macromolecules, bioactive nanoparticles, and surface polymerization-induced bioactivity of modified MXenes and surface functionalization methods used to prepare them (Table 1).| Bioactive surface strategies | MXene composition | Functionalized components | Bioactive property | Applications | Ref. |
|---|---|---|---|---|---|
| Bioactive small molecules | Ti3C2Tx | [3-(2-Aminoethylamino)-propyl]trimethoxysilane (AEAPTMS) | Adsorbing or desorbing protons, pH responsive | Cross-linked conductive composites, dye adsorbents, membranes and drug carriers | 61 |
| Ti3C2Tx | C12E6 | Ion transport, thickness-independent electrochemical performances | Biosensing, catalysis, filtration | 62 | |
| Nb2CTx | Deionized water/ethanol-based solvothermal treatment | Electromagnetic wave (EMW) absorbing | EMW-absorbing material | 64 | |
| Ti3C2 | 3-Aminopropyltriethoxysilane (APTES) | Immobilized bio-receptor | Cancer biomarker, biosensor | 65 | |
| Ti3C2Tx | APTES | pH stability, thermal stability and reusability | Enzymes | 94 | |
| Ti3AlC2 | Ethylenediamine | Detecting and showing for cellular imaging | Biocompatibility nanoprobe | 66 | |
| Ti3C2Tx | APTES, thioketal, DOX, and polydopamine (PDA) | Antibacterial activity, photothermal properties, sensitive ROS/pH response | Drug delivery, antibacterial materials | 67 | |
| Ti3C2Tx | Au, polyethylene glycol (PEG) and DOX | pH-Responsive drug release, photothermal stability, biosafety | Drug delivery platform and cancer treatment | 14 | |
| Ti3C2Tx | GOX, DOX and PEG | Photothermal capability, CAT-like activity | Anti-cancer system | 95 | |
| Bioactive macromolecules | Nb2C | Polyvinyl pyrrolidone (PVP) | Photothermal conversion, enzyme-responsive, low phototoxicity | PTT and cancer treatment | 40 |
| Ti3C2Tx | Sodium alginate (SA) | High conductivity, easy processing, mechanical flexibility and high electromagnetic shielding performance | EMI shielding materials | 71 | |
| Ti3C2Tx | Soybean phospholipid (SP) | NIR absorption and high photothermal conversion efficiency | Tumor therapy | 38 | |
| Ti3C2Tx | Silk fibroin (SF) and polyacrylamide (PAM) | High tensile strength, excellent toughness, high electrical conductivity and self-adhesion | Flexible electronics | 72 | |
| Ti3C2Tx | Hyaluronic acid (HA) and hyperbranched polysiloxane (HSi) | Mechanical flexibility, good resistance to water/solvents and thermal camouflage | Reliable long-term infrared stealth | 73 | |
| Ti3AlC2 | Polyimide (PI) | Superelastic, mechanical flexibility and electrical conductivity | Microwave absorption coating, flexible strain sensor | 69 | |
| Ti3C2Tx | Polydiallyldimethylammonium chloride (PDDA)/PVA | Flexible and have electrical conductivities | Wearable electronics, electrochemical actuators | 70 | |
| 2D W1.33Ci-MXene | BSA | pH response, photothermal conversion, rapid biodegradation and biocompatibility | CT and PA imaging and cancer therapy | 74 | |
| Bioactive nanoparticles | Ta4C3 | IONPs and SP | Photothermal-conversion capability, imaging capability, biosafety | MR/CT imaging, PTT and tumor therapy | 76 |
| Ti3C2 | MnOx | MR imaging of tumors and photothermal-conversion performance | Tumor therapy, theranostic nanomedicine | 77 | |
| Ta4C3 | MnOx and SP | MR imaging capability, biosafety | PTT for tumor and PA imaging | 78 | |
| Ti3C2 | GdW10-based polyoxometalates (POMs) | Biocompatibility, diagnostic-imaging guidance, monitoring | Contrast agent, tumor hyperthermia nanotherapy | 85 | |
| Ti3C2Tx | AuNPs | Photothermal conversion efficiency, stability, antibacterial | Photothermal antibacterial | 79 | |
| Ti3C2 | Au, PEGylation | Stability, biocompatibility, photothermal effect and biosafety | Cancer therapy | 80 | |
| Ti3C2Tx | Plasmonic Au | Oxygen evolution performance | Oxygen evolution reaction (OER) | 81 | |
| Ti3C2 | Pt | POD-like activity | Glucose and glutathione assays | 83 | |
| Ti3C2 | Natural glucose oxidase (GOD) and IONPs | Catalyze, biocompatibility and photothermal performance | Nanocatalytic cancer therapy | 75 | |
| Ti3C2Tx | Bi2S3 | Photocatalytic activity, antibacterial infection and biocompatibility | Biocompatible photoelectrical devices | 96 | |
| Surface polymerization-induced bioactivity | Ti3AlC2 | Polydopamine (PDA) | Adsorption capacity, stability | Adsorbents | 89 |
| V2C | Poly(2-(dimethylamino)ethyl methacrylate) (PDMAEMA) | CO2 and temperature dual-responsive properties, conductivity | Smart responsive MXene systems | 93 | |
| Ti3C2Tx | N-Isopropylacrylamide (NIPAM) | Photothermal conversion, photothermal stability and thermal responsive | NIR-controlled “smart” windows, fluidic valves and photodetectors | 92 | |
| Ti3C2Tx | Poly(diallyldimethylammonium chloride) (PDDA) and PVA | Excellent conductivities, controlled thicknesses, and excellent flexibility | Wearable energy storage devices, fillers for radiofrequency shielding | 70 | |
| Ti3C2Tx | Poly(lactic-co-glycolic acid) (PLGA) and SP | Photothermal absorption and high photothermal conversion efficiency | Tumor therapy | 38 |
3.1 Bioactive small molecules
By modifying the surface of MXenes with various small molecules, this method can effectively enhance their performance characteristics such as mechanical stability, electrical properties and solution stability, which are easy to manipulate and inexpensive. Among them, the coupling agent is an important choice to prevent the structural degradation caused by spontaneous oxidation of MXenes on one hand, and improve their dispersion and interfacial bonding with polymer matrix on the other.60 Riazi et al. reported amine functionalization of the Ti3C2Tx MXene surface with [3-(2-aminoethylamino)-propyl]trimethoxysilane (AEAPTMS), and the functionalization changed the MXene surface charge from −35 mV to +25 mV at neutral pH, which made it possible to prepare self-assembled membranes in situ, and also confirmed the presence of mixtures of differently oriented AEAPTMS and the presence of both protonated and free amine groups on the Ti3C2Tx surface.61 The availability of free amine groups on the surface enables the fabrication of cross-linked conductive MXene/epoxy composites, dye adsorbents, high-performance membranes and drug carriers. In order to improve molecular interactions and thus enhance the filling symmetry, Xia et al. introduced nonionic surfactant hexaethylene glycol monododecyl ether (C12E6) on the surface of Ti3C2 MXenes (C12E6@Ti3C2). In the C12E6@Ti3C2 composite, strong hydrogen bonds were formed between the –O or –F groups on the surface of MXene and the –OH groups of C12E6.62 In addition, Lei et al. reported the one-step preparation of sulfonic acid-based functionalized MXene (named Ti3C2–SO3H) by the direct reaction of diazonium salts with Ti3C2 flakes (Fig. 1A).63 Due to the introduction of the anionic sulfonic acid group, this provides a new method for the preparation of functionalized MXenes and can be extended to the preparation of other functionalized MXenes with good design properties and performance. Nb2CTx MXene was further processed by a simple one-step solvothermal/hydrothermal method by Jin et al. Accordingly, the interlayer space and surface functional groups of Nb2CTx MXene were enhanced, which significantly improved the EM wave absorption performance.64 The solvothermal/hydrothermal treatment of Nb2CTx MXene was carried out using the low-toxicity solvents ethanol (Et) or deionized water (DI) instead of N,N-dimethylformamide (DMF). The results showed that Nb2CTx treated with Et as solvent absorbed EMW better.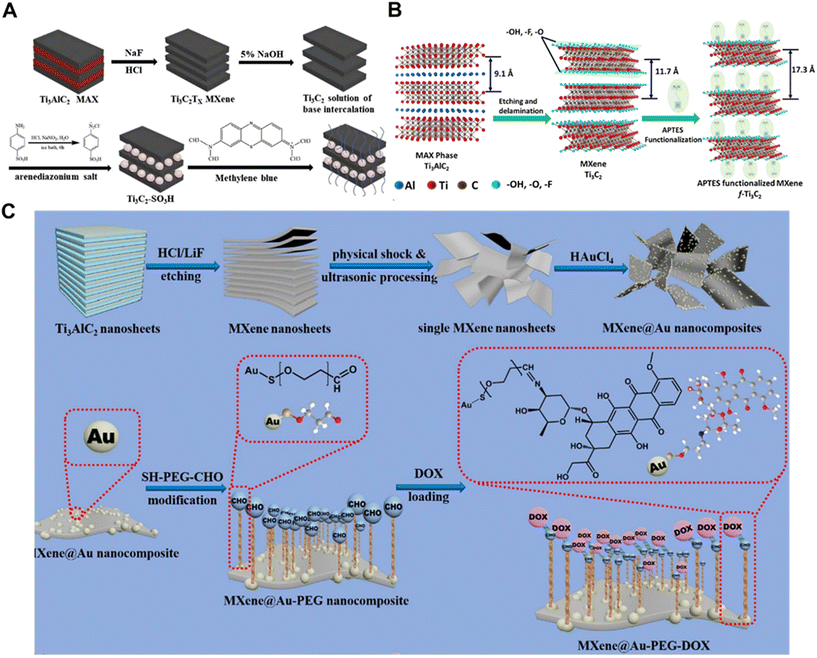 | ||
| Fig. 1 Strategies for functionalizing MXenes with bioactive small molecules. (A) The scheme for the preparation of pristine Ti3C2 and Ti3C2-SO3H.63 Reproduced from ref. 63 with permission from Elsevier, copyright 2019. (B) Schematic representation of the functionalization of the Ti3C2-MXene surface using APTES.65 Reproduced from ref. 65 with permission from Elsevier, copyright 2018. (C) Synthesis process for MXene and MXene@Au nanosheets and surface modification of MXene@Au using thiol polyethylene glycol aldehyde chains (SH-PEG-CHO), and the subsequent surface doxorubicin (DOX) loading.14 Reproduced from ref. 14 with permission from Elsevier, copyright 2022. | ||
Small molecules were chemically introduced on the surface of monolayer/multilayer MXene (Ti3C2) nanosheets with amino groups, and the resulting functionalized MXene nanosheets could be used as platforms for immobilization of different biomolecules. Kumar et al. synthesized ultrathin Ti3C2-MXene nanosheets by a minimally enhanced layer delamination method and homogeneously functionalized with aminosilane (F-Ti3C2-MXene) to provide covalent binding to immobilized bioreceptors (anti-CEA) for label-free, ultrasensitive cancer biomarker (carcinoembryonic antigen, CEA) detection (Fig. 1B).65 They investigated the effects of different redox probes on the electrochemical behavior of F-Ti3C2-MXene and found that ammonium hexamine-mineral ([Ru(NH3)6]3+) was a more ideal redox probe for biosensing. The prepared biofunctionalized Ti3C2-MXene opens a new window for the development of highly sensitive DNA, aptamer, enzyme, antibody, and cellular biosensors based on MXene, and can be further used for drug delivery applications. Meanwhile, MXenes have strong absorption of light in the wavelength range of 300–900 nm, which can burst the fluorescence of fluorescent groups by fluorescence resonance energy transfer (FRET) and can be used as highly efficient and broad-spectrum fluorescence bursting agents by combining with specific small molecules. Luo et al. investigated and prepared a biocompatible nanoprobe of N-Ti3C2 quantum dots (N-Ti3C2 QDs) based on MXene for the detection of intracellular glutathione.66 Based on non-radiative electron–hole annihilation, N-Ti3C2 quantum dots acted as fluorescence reporters and iron (Fe3+) acted as bursters. When N-Ti3C2 QDs/Fe3+ nanoprobes acted on cancer cells MCF-7, abundant GSH in the cancer cells could reduce Fe3+ to Fe2+, thus restoring the fluorescence of N-Ti3C2 QDs, and the N-Ti3C2 quantum dots/Fe3+ nanoprobes might provide a new pathway for imaging-guided accurate cancer diagnosis.
There have also been numerous studies on DOX-functionalized MXenes for tumor therapy. Zhang et al. used hydrofluoric acid etching and TBAOH intercalation to obtain well-dispersed MXene with small lateral dimensions.67 Functionalized MXene-TK-DOX@PDA nanoparticles have remarkable photothermal conversion properties and high photothermal stability, and exhibit sensitive ROS- and pH-responsive behaviors in the presence of H2O2 at pH = 5.5, which controls the local release of DOX and effectively reduces the premature release of the drug. The new MXene nanoparticles were designed for chemotherapeutic drug-targeted delivery with good antimicrobial activity, which provided a promising drug delivery strategy in the biomedical field and enriched the family of antimicrobial materials. In another study, Liu et al. reported the synthesis of an MXene@Au-PEG drug delivery platform for the highly loaded chemotherapeutic drug Adriamycin (DOX) with near-infrared (NIR) laser-triggered and pH-responsive drug release modes (Fig. 1C).14 In in vivo and in vitro experiments, the MXene@Au-PEG-DOX system exhibited good photostability, thermal stability and histocompatibility. In addition, based on the good photothermal conversion ability of Au particles and MXene, this system had a synergistic effect of photothermal ablation and chemotherapy in tumor treatment. The passive targeted release property of MXene@Au-PEG delivery platform also enhanced the cellular uptake of DOX at the tumor site, thus improving the efficiency of the drug.
By modifying the surface of MXenes with small molecules, it is possible to significantly enhance their performance in terms of mechanical, electrical, and solution stability, and to expand their applications in biomedical fields such as drug delivery, biosensing, and tumor therapy.
3.2 Bioactive macromolecules
In the past decades, macromolecular surface modification technology has rapidly developed into a key driver of biomaterials innovation, and polyphenolic components, vitamins, alkaloids, proteins, peptides, and so on, have attracted much attention as material surface modification components due to their excellent bioactivities. Therefore, many studies have also been conducted to modify the surface of MXenes with bioactive macromolecules to further enhance their biological properties.Some synthetic polymers, such as polyethylene glycol (PEG) and polyvinylpyrrolidone (PVP), are suitable for surface modification of MXenes.68 PVP is often used for surface modification of MXene materials to improve their stability. Lin et al. explored a new ultrathin 2D niobium carbide (Nb2C) MXene as a new type of phototherapeutic agent, and ultrathin transverse nano-Nb2C nanosheets had excellent near-infrared photothermal properties with very high photothermal conversion efficiency and ideal photothermal stability. The Nb2C nanosheets were further surface modified to prepare Nb2C-PVP colloidal solutions, which greatly improved the biocompatibility and physiological stability of the nanosheets, with no significant toxicity in vitro and in vivo.40 Liu et al. developed an interfacial enhancement strategy to construct versatile, superelastic and lightweight 3D MXene structures by bridging individual MXene sheets with polyimide macromolecules.69 The resulting lightweight aerogels were superelastic with large reversible compressibility, excellent fatigue resistance (1000 cycles at 50% strain), 20% reversible stretchability and high electrical conductivity of ≈4.0 S m−1. The excellent mechanical flexibility and electrical conductivity make the aerogel promising for applications in damping, microwave-absorbing coatings and flexible strain sensors. More interestingly, the material exhibited excellent microwave absorption properties at 9.59 GHz. Ling et al. prepared Ti3C2Tx/polymer composites by blending Ti3C2Tx MXene with charged poly(dienyldimethylammonium chloride) (PDDA) or electrically neutral PVA. The prepared composites were flexible with high electrical conductivity.70 The tensile strength of Ti3C2Tx/PVA composites was significantly improved compared with pure Ti3C2Tx or PVA films. This study is the first step in exploring the potential of MXene in polymer-based multifunctional nanobiocomposites for applications such as wearable electronics, electrochemical actuators, and radio frequency shielding.
In addition, many natural macromolecules can also be used for MXene surface modification. Sodium alginate (SA) is a linear polysaccharide copolymer derived from seaweed. As a natural biomaterial, SA is potentially an ideal candidate for polymer matrices, being abundant, environmentally friendly and having excellent mechanical properties, while SA possesses oxygen-containing functional groups (–OH, –COO, and ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), which can potentially facilitate the formation and termination of hydrogen bonding with MXenes. Shahzad et al. employed SA to perform a 45 μm-thick Ti3C2Tx thin film surface modification to show 92 dB EM shielding efficiency (>50 dB for 2.5 μm film), which is the highest EM shielding efficiency of any synthetic material of equivalent thickness produced to date.71 In addition, the natural polymer soybean phospholipid (SP) has also been widely used for surface modification of MXenes as an economical modifier to improve the cycling stability of MXenes and enhance the material penetration. Lin et al. reported that MAX-ceramic biomaterials exhibited unique functionalities for cancer photothermal ablation after being stripped into atom-thick (MXene) ultrathin nanosheets (Fig. 2A).38 The SP-modified Ti3C2 nanosheets showed good biocompatibility and may be of interest in exploring MXene-based ceramic biomaterials for biomedical applications. Silk fibroin (SF) is a natural fibrous protein composed of various amino acids such as tyrosine, glycine and alanine. It has both hydrophilic and hydrophobic blocks with abundant reactive groups and can form different secondary structures such as β-sheet and random curls through hydrogen bonding. Therefore, SF can form attractive interactions with MXenes through electrostatic interactions, hydrophobic interactions and hydrogen bonding. Li et al. prepared a novel electrically conductive MXene-based hydrogel by introducing SF-modified MXene (MXene-SF) into a polyacrylamide (PAM) network.72 It was shown that the SF coating on the surface of MXene could greatly improve its stability, and also induce the formation of various noncovalent interactions between MXene-SF and PAM chains to enhance its adhesion properties. The high conformal adhesion and low interfacial impedance enabled the hydrogel bioelectrode to precisely monitor weak electrophysiological signals. Hyaluronic acid (HA) is a linear polysaccharide chain containing N-acetylglucosamine and glucuronic acid that is sensitive to ROS such as hydrogen peroxide, superoxide radicals and hydroxyl radicals. Its oxidative instability can be solved by using HA-modified MXene. Guo et al. designed and fabricated an MXene-based hybrid network containing hyaluronic acid (HA) and hyperbranched polysiloxane (HSi) molecules (Fig. 2B).73 Notably, the presence of hyaluronic acid molecules limited the oxidation of MXene sheets without altering their infrared invisibility properties, which is superior to that of other water-soluble polymers, while HSi molecules could act as highly efficient cross-linking agents to produce strong interactions between MXene sheets and hyaluronic acid molecules. The optimized MXene/HA/HSi composites provided excellent mechanical flexibility, good water/solvent resistance and long-term stable thermal camouflage for reliable long-term infrared stealth. Another biocompatible natural macromolecule, bovine serum albumin (BSA), was attached to W1.33Ci-MXenes through van der Waals forces and hydrogen bonding interactions to improve the colloidal stability of the nanosheets, and this work provides an example of i-MXenes being tailored for biomedical applications through combinatorial and structural design on atoms (Fig. 2C).74
O), which can potentially facilitate the formation and termination of hydrogen bonding with MXenes. Shahzad et al. employed SA to perform a 45 μm-thick Ti3C2Tx thin film surface modification to show 92 dB EM shielding efficiency (>50 dB for 2.5 μm film), which is the highest EM shielding efficiency of any synthetic material of equivalent thickness produced to date.71 In addition, the natural polymer soybean phospholipid (SP) has also been widely used for surface modification of MXenes as an economical modifier to improve the cycling stability of MXenes and enhance the material penetration. Lin et al. reported that MAX-ceramic biomaterials exhibited unique functionalities for cancer photothermal ablation after being stripped into atom-thick (MXene) ultrathin nanosheets (Fig. 2A).38 The SP-modified Ti3C2 nanosheets showed good biocompatibility and may be of interest in exploring MXene-based ceramic biomaterials for biomedical applications. Silk fibroin (SF) is a natural fibrous protein composed of various amino acids such as tyrosine, glycine and alanine. It has both hydrophilic and hydrophobic blocks with abundant reactive groups and can form different secondary structures such as β-sheet and random curls through hydrogen bonding. Therefore, SF can form attractive interactions with MXenes through electrostatic interactions, hydrophobic interactions and hydrogen bonding. Li et al. prepared a novel electrically conductive MXene-based hydrogel by introducing SF-modified MXene (MXene-SF) into a polyacrylamide (PAM) network.72 It was shown that the SF coating on the surface of MXene could greatly improve its stability, and also induce the formation of various noncovalent interactions between MXene-SF and PAM chains to enhance its adhesion properties. The high conformal adhesion and low interfacial impedance enabled the hydrogel bioelectrode to precisely monitor weak electrophysiological signals. Hyaluronic acid (HA) is a linear polysaccharide chain containing N-acetylglucosamine and glucuronic acid that is sensitive to ROS such as hydrogen peroxide, superoxide radicals and hydroxyl radicals. Its oxidative instability can be solved by using HA-modified MXene. Guo et al. designed and fabricated an MXene-based hybrid network containing hyaluronic acid (HA) and hyperbranched polysiloxane (HSi) molecules (Fig. 2B).73 Notably, the presence of hyaluronic acid molecules limited the oxidation of MXene sheets without altering their infrared invisibility properties, which is superior to that of other water-soluble polymers, while HSi molecules could act as highly efficient cross-linking agents to produce strong interactions between MXene sheets and hyaluronic acid molecules. The optimized MXene/HA/HSi composites provided excellent mechanical flexibility, good water/solvent resistance and long-term stable thermal camouflage for reliable long-term infrared stealth. Another biocompatible natural macromolecule, bovine serum albumin (BSA), was attached to W1.33Ci-MXenes through van der Waals forces and hydrogen bonding interactions to improve the colloidal stability of the nanosheets, and this work provides an example of i-MXenes being tailored for biomedical applications through combinatorial and structural design on atoms (Fig. 2C).74
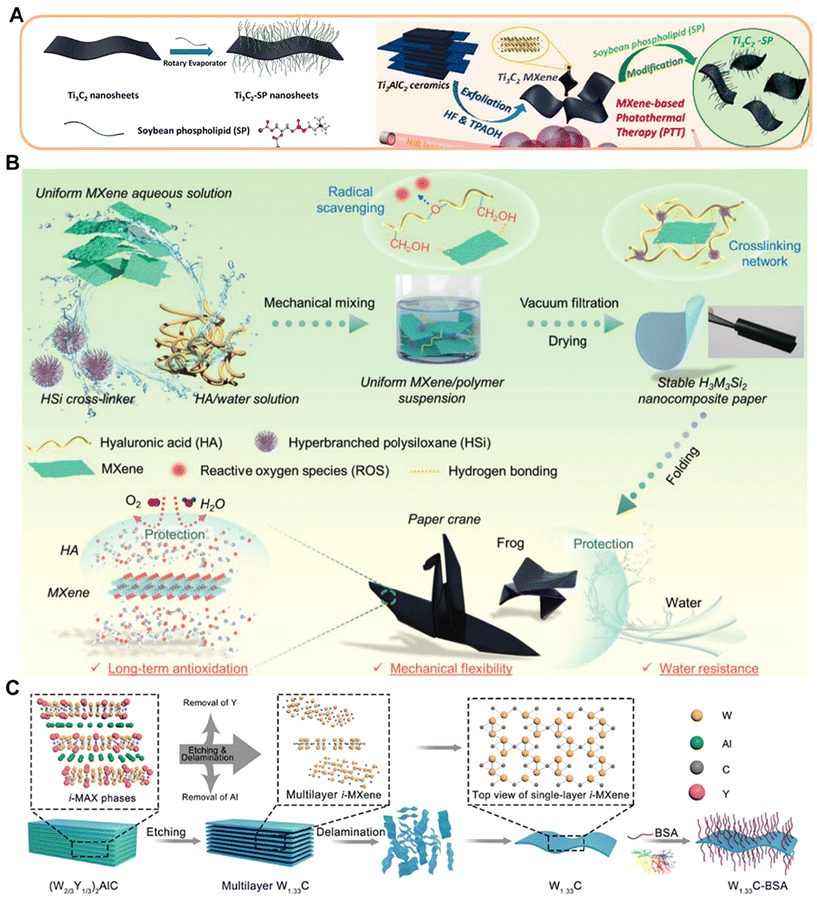 | ||
| Fig. 2 Strategies for functionalizing MXenes with bioactive macromolecules. (A) Schematics of surface modification of exfoliated Ti3C2 nanosheets modified with soybean phospholipid and Ti3C2-SP use for photothermal therapy.38 Reproduced from ref. 38 with permission from American Chemical Society, copyright 2016. (B) Schematic representation of the preparation process of polysiloxane-modified HA/MXene composite network and its application.73 Reproduced from ref. 73 with permission from Wiley-VCH, copyright 2024. (C) Schematic illustration on the construction of 2D W1.33Ci-BSA nanosheets as a 2D phototherapeutic agent.74 Reproduced from ref. 74 with permission from Wiley-VCH, copyright 2021. | ||
Modification of the MXene surface by bioactive macromolecules not only enhances its biocompatibility and stability, but also expands the applications of MXenes in biomedical fields, such as drug delivery, tissue engineering, antimicrobial activity and biosensors.
3.3 Bioactive nanoparticles
MXenes have a unique two-dimensional planar structure that provides a suitable substrate for simple decoration of various 0D functional nanoparticles, resulting in versatile 0D/2D composites. To extend the versatility of MXenes, anchoring magnetic nanoparticles (e.g., Fe3O4, MnOx) on the surface of MXenes enables the integration of diagnostics and combination therapy. Liang et al. reported the triggering of Fenton-based nanocatalytic reactions on 2D titanium carbide (Ti3C2) MXenes nanosheets and as a photothermal conversion nano-agent to further synergistically improve the efficiency of nanocatalytic cancer cell killing.75 The nano-enzymes Fe3O4 nanoparticles and natural glucose oxidase (GOD) were encapsulated on Ti3C2 MXenes as nanocarriers for composite nano-systems and as photothermal conversion nano-agents (Fig. 3A). The loaded GOD catalyzes the generation of a large number of hydrogen peroxide molecules from glucose phagocytosed by tumors, Fe3O4 nanoparticles further catalyze the generation of sufficient hydroxyl radicals to kill the cancer cells, and the Ti3C2 MXene substrate further enhances and accelerates this catalytic reaction under NIR-triggered photothermal conversion. Liu et al. reported the in situ growth of iron oxide nanoparticles (IONPs) on Ta4C3 MXenes by co-precipitation.76 The obtained composite Ta4C3-IONPs combines the enhanced MR imaging capability of IONPs and the photothermal effect of Ta4C3 MXenes for efficient cancer therapy. Although MXenes have excellent hydrophilicity, they have insufficient colloidal stability due to severe aggregation and precipitation in complex physiological media. Dai et al. developed for the first time a versatile but highly efficient “redox-induced growth” (RH-IG) method for in situ growth of MnOx nanosheets (MnOx/Ti3C2) on the two-dimensional Ti3C2 MXene surface, which can be used for effective tumor diagnosis and treatment.77 They also proposed a surface engineering strategy based on using a two-dimensional tantalum carbide (Ta4C3) MXene for in situ growth of manganese oxide nanoparticles on the Ta4C3 MXene surface by triggering a redox reaction between the reducing MXene surface and the strongly oxidizing MnO4-(MnOx/Ta4C3) (Fig. 3B).78 The integrated MnOx component endows the composite MXenes nanosheets with tumor microenvironment (TME)-responsive T1-weighted MR imaging capability.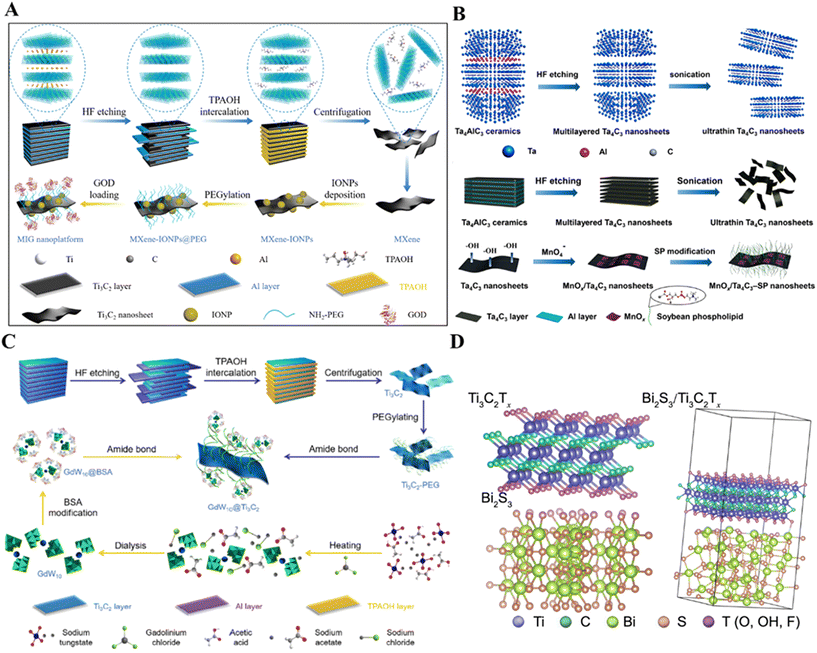 | ||
| Fig. 3 Strategies for functionalizing MXenes with bioactive nanoparticles. (A) Schematic illustration of the successive synthetic procedures of 2D Ti3C2 MXene and MIG nanoplatforms for in vivo synergistic photothermal-enhanced nanocatalytic cancer therapy.75 Reproduced from ref. 75 with permission from American Chemical Society, copyright 2019. (B) The scheme of the synthetic procedure for Ta4C3 and MnOx/Ta4C3-SP composite nanosheets, including HF etching, sonication exfoliation, in situ redox reaction between Ta4C3 nanosheets and postintroduced KMnO4, and subsequent surface SP modification.78 Reproduced from ref. 78 with permission from American Chemical Society, copyright 2017. (C) Schematic illustration for the synthesis of GdW10@Ti3C2 composite nanosheets, including chemical exfoliation of MAX-phase Ti3AlC2 ceramic to produce Ti3C2 MXene and further surface functionalization with GdW10 POM nanoclusters.85 Reproduced from ref. 85 with permission from Tsinghua University Press, copyright 2018. (D) Crystal structure of Ti3C2Tx, Bi2S3 and Ti3C2Tx/Bi2S3 after structure optimization.86 Reproduced from ref. 86 with permission from Springer Nature, copyright 2021. | ||
Various metal nanoparticle/MXene hybridizations have been reported many times, especially Au nanoparticles. Wen et al. reported a simple strategy to construct Au@Ti3C2Tx heterostructures with enhanced photothermal antimicrobial effect by utilizing the reducing activity of Ti3C2Tx NSs itself, and the in situ reduction of Au precursors on Ti3C2Tx substrates.79 A series of Au@Ti3C2Tx nanocomposites was obtained by adjusting the Au/Ti feed ratio without adding any surfactant. The photothermal conversion efficiency and stability of the composite were improved due to the LSPR effect of Au NPs’ interaction. Under 660 nm laser irradiation, more than 99% of Gram-positive S. aureus and Gram-negative E. coli were efficiently killed due to the synergistic effect of intrinsic physical damage and photothermal ablation. This work presents the potential of multifunctional Au@Ti3C2Tx heterostructures for fighting drug-resistant bacteria by photothermal treatment. Tang et al. synthesized multifunctional two-dimensional core–shell structures of Ti2C3@Au nanocomposites for imaging-guided cancer therapy via a seed-growth method.80 The stability and biocompatibility of Ti2C3@Au nanocomposites were significantly enhanced and their optical absorbance in both NIR-I and NIR-II bio-windows were greatly enhanced. The nanocomposites were successfully applied in photothermally enhanced RT through mild photothermal effects and good synergistic effects were realized. Wang et al. successfully prepared MXene Ti3C2Tx nanosheets mixed with Au nanoparticles (AuNPs@Ti3C2Tx) via electrostatic interactions and systematically investigated them for plasma-enhanced catalysis.81 The experimental results showed that the plasma MXene-based heterostructure could further enhance the catalytic activity of MXene effectively. Au–TiO2@Ti2C was synthesized by Wojciechowski et al. using isopropanol as a reducing agent and Ti(Oi-Pr)4 and Au3+ ions as precursors with stirring for 2 days.82 Geng et al. constructed Ti3C2/Pt hybrids with synergistically enhanced catalytic activity.83 TEM images of Ti3C2 nanosheets and Ti3C2/Pt with different Pt loadings showed that the lateral sizes of the prepared layers of Ti3C2 were between 100 nm and 300 nm, and the particles dispersed on the Ti3C2 sheets could be clearly seen after the deposition of Pt NPs. Although Ti3C2 does not possess oxidase and peroxidase-like activities, it can greatly and selectively enhance the peroxidase-like activity of Pt nano-enzymes. NIR irradiation can further specifically enhance the peroxidase-like activity of Ti3C2/Pt. This catalytic enhancement is due to the combined effect of the strong interfacial electronic effect and the unique photothermal effect of Ti3C2, which provides an effective means for the introduction of ideal carriers to improve the catalytic activity and selectivity of the nano-enzymes, and has a certain value of reference for the design of efficient nano-enzymes. However, these methods typically involve a multi-step process, are lengthy, and use surface stabilizers/reducers.84
Polyoxometalates (POMs) can be used for MXene surface modification. In a study, Ti3C2 MXenes were functionalized by GdW10-based POMs (Fig. 3C).85 The hybrid Ti3C2 MXenes had the enhanced CT/MR imaging capability of POM in multimodal imaging-guided PTT. Bi2S3 nanorods were grown in situ on the surface of Ti3C2Tx nanosheets.86 The results showed that the combination of Ti3C2Tx with n-type Bi2S3 promoted the separation and transfer of photogenerated charges, which accelerated the interfacial charge transfer in the photocatalytic process, increased the ROS yield, and was effective in eliminating bacterial infections (Fig. 3D). In addition, mesoporous silica nanoparticles (MSN), CeO2 nanoparticles, etc. can also be used for MXene modification.87,88
MXenes, as a unique two-dimensional material, form multifunctional 0D/2D composites by combining with 0D functional nanoparticles, and exhibit a wide range of potential applications in biomedical fields, especially in diagnostics, therapeutics, and photothermal conversion.
3.4 Surface polymerization-induced bioactivity
The hydroxyl (–OH), fluorine (–F) and many other reactive functional groups on the surface of MXenes allow the polymers to undergo non-in situ or in situ polymerization.The first is the in situ polymerization between polymers and MXenes with the help of hydrogen bonding, electrostatic interactions, ε-spin and in situ polymerization with monomers such as pyrroles, which effectively enhances their physicochemical properties and biological activities. Dopamine is formed by surface polymerization to form polydopamine (PDA), which is rich in amino and catechol groups in its main chain and is firmly bound to organic and inorganic surfaces through the formation of non-covalent and covalent interactions, and the surface bioactivity is significantly enhanced by it. For example, Qing et al. produced MXene/PDA composites by in situ polymerization of PDA first in MXene solution.89 In the work, it was found that PDA nanoparticles promoted the stripping of MXene by different adsorption measurements and had a strong synergistic effect in the removal of Cr(VI), and the stability was greatly improved, and the work suggested that MXene/PDA composites can be used as an alternative adsorbent (Fig. 4A). In addition, in the research of Lin et al., Ti3C2 was further modified using poly(lactic-co-glycolic acid) (PLGA) for intratumoral implantation.38 The high PTT efficiency in vivo on tumor xenografts successfully demonstrated the efficacy of PLGA/Ti3C2 for tumor therapy, expanding the possibilities of MXene nanosheets for biomedical applications, especially in anti-cancer applications. Ling et al. selected Ti3AlC2 as the MAX precursor and prepared Ti3C2Tx/PDDA composite films and Ti3C2Tx/PVA composite films by surface polymerization of two polymers: poly(dienyldimethylammonium chloride) (PDDA) and PVA, which have excellent flexibility, impressive electrical conductivity and hydrophilic surface (Fig. 4B).70 However, most of the MXene complexes synthesized by surface in situ polymerization are unstable and more complicated to operate, and thus can be limited in practical biomedical applications.
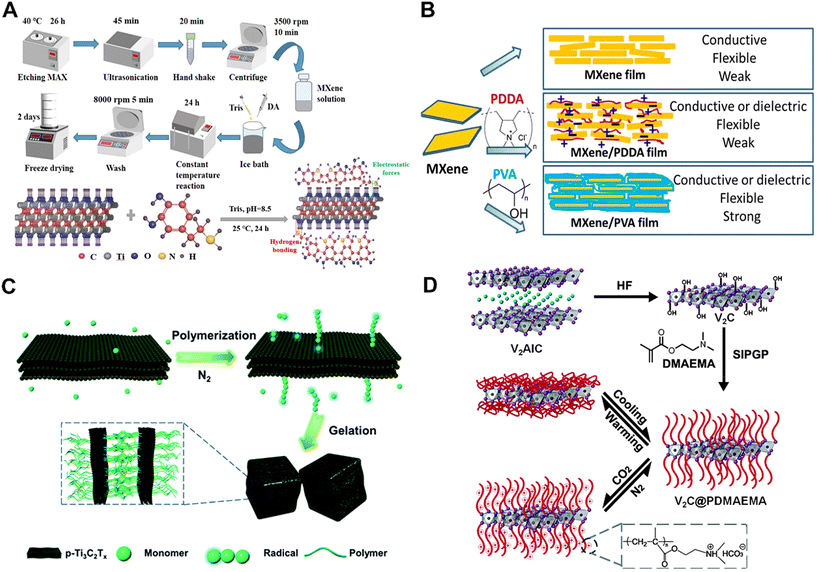 | ||
| Fig. 4 Strategies for functionalizing MXenes with surface polymerization-induced bioactivity. (A) Schematic illustrations showing the preparation processes of the MXene/PDA composite particles and stacking of PDA macromolecules on the nanosheet of MXene.89 Reproduced from ref. 89 with permission from American Chemical Society, copyright 2023. (B) A schematic illustration of MXene-based functional films with adjustable properties.70 Reproduced from ref. 70 with permission from National Academy of Sciences of America, copyright 2014. (C) Schematic illustration of p-Ti3C2Tx-initiated polymerization and subsequent gelation.92 Reproduced from ref. 92 with permission from the Royal Society of Chemistry, copyright 2019. (D) Schematic representation of preparing V2C@PDMAEMA smart hybrid systems. The C atoms are denoted by off-white color, the V atoms are denoted by purple color which occupy every other elemental atomic plane, the Al atoms are denoted by blue color, and occupy every third plane.93 Reproduced from ref. 93 with permission from the Royal Society of Chemistry, copyright 2015. | ||
Unlike common polymerization methods, self-initiated photografting and photopolymerization (SIPGP) eliminates the need for an anchoring layer, a self-assembled monolayer (SAM), and an initiator, and requires only a one-step reaction under UV irradiation at room temperature, which occurs directly on the surface of the material.90 SIPGP has been successfully applied to graphene, carbon nanotubes, etc., and is a simple method for polymerization.91 Recently, MXene prepared by Tao et al. could generate hydroxyl radicals and act as initiators for the free radical polymerization of a series of acrylic monomers.92 PNIPAM-based nanocomposite hydrogels were prepared by simply mixing N-isopropylacrylamide (NIPAM) with p-Ti3C2Tx nanosheets under deoxygenated conditions. The nanocomposite hydrogels had better photothermal stability than pristine Ti3C2Tx and were thermoresponsive (Fig. 4C). In addition, Chen et al. reported a powerful strategy by grafting poly(2-(dimethylamino)ethyl methacrylate) (PDMAEMA) brushes on the surface of MXenes. V2C MXene was prepared by soaking V2AlC powder in hydrofluoric acid at room temperature. Then, they utilized the hydroxyl group on the surface as a photoactive site for further growth and amplification of the polymer brushes by SIPGP. Finally, the CO2 and temperature dual-response properties of the V2C@PDMAEMA hybridized materials were further explored (Fig. 4D).93 SIPGP is a promising strategy to improve the raw performance of MXene, which could be tailored for biological applications.
The reactive functional groups on the surface of MXenes can be enhanced by a variety of polymerization methods to enhance their physicochemical properties and bioactivity, but stability and handling complexity remain major challenges for their biomedical applications.
MXenes will have more excellent physicochemical properties after surface modification and functionalization. Surface functionalization makes MXene materials suitable for electrochemical biosensors, catalysis, antimicrobial coatings, and drug delivery, etc., which have been widely explored in biomedical applications.
4. Regenerative medicine applications
The unique physicochemical and biological properties of 2D MXene materials offer new solutions for the diagnosis and treatment of clinically significant diseases. Novel 2D MXene materials have been extensively investigated in the biomedical field due to their diverse chemistry and structure as well as tunable physicochemical properties, including MXenes in bioimaging, cancer therapy, infection treatment, skin repair, bone regeneration, and other fields. This section highlights recent advances in MXene-based biomaterials for biomedical applications.4.1 Bioimaging
With the rapid development of nanotechnology in recent years, MXenes and MXene-based composites have attracted increasing attention from researchers due to their special structural, light- and heat-absorbing properties as well as surface multifunctionality; those that have the ability to satisfy the quantum size effect for fluorescence imaging, the intrinsic photothermal properties for photoacoustic imaging (PAI), the elemental enhancement of contrast for X-ray computerized tomography (CT) imaging, and the effective loading of functional contrast agents for magnetic resonance imaging (MRI) with payloads of functional contrast agents, have emerged as the most promising alternative candidates for bioimaging (Table 2).97| MXene composition | Synthesis strategy | Description | Applications | Ref. |
|---|---|---|---|---|
| Ti3C2 QDs | HF etching and hydrothermal treatment | Displays excitation-dependent photoluminescence spectra with quantum yields up to ≈10% | Fluorescent imaging and Zinc ion Sensor | 41 |
| Ti3C2Tx-derived GQDs | Dimethylformamide (DMF) heat treatment | Tunable photoluminescence, low cytotoxicity, good photostability and chemical inertness | Fluorescent inks, luminescent composites and fluorescent imaging | 98 |
| Ti3C2 QDs | HF etching and oleylamine heat treatment | Exhibits white photoluminescence and two-photon fluorescence properties | — | 99 |
| N-Ti3C2 QDs/Fe3+ | HF etching and hydrothermal treatment | Uniform size, excellent optical properties, biocompatible | Detection of glutathione in living cells and fluorescence imaging | 66 |
| Nb2C QDs | Pulsed ultrasound-assisted physicochemical stripping in TPAOH | Excellent chemical stability, biocompatibility, photobleaching resistance and enzyme responsive biodegradability | Metal ion sensing and fluorescence imaging | 110 |
| Nb2C QDs | Hydrothermal treatment | Exhibit excellent green fluorescence and exhibit excitation-dependent photoluminescence, anti-photobleaching and dispersion stability | Cu2+ sensing and cellular imaging | 44 |
| Ti3C2Tx-Pt-PEG | LiF and HCl etching methods | With POD-like activity, and shows desirable photothermal effects, generating high temperatures that enhance the catalytic activity of the nano-enzymes | PAI and the integration of diagnostic and therapeutic oncology | 111 |
| Ti3C2Tx@Dox | Layer less ionic liquid (IL) stripping technology | Exhibits high drug loading and pH/photosensitivity, good biosafety, excellent photoacoustic imaging | PAI and tumor PTT/PDT | 112 |
| Ti3C2/Cu2O | — | Generation of ˙OH via Fenton-like reactions for CDT in acidic tumor environments | PAI and tumor PTT/PDT | 113 |
| Ta4C3 | Combination of HF etching and probe sonication | Reasonable extinction coefficient and very high photothermal conversion efficiency (44.7%), and good photothermal stability | PAI, CT imaging and tumor therapy | 105 |
| V4C3 | HF etching and TPAOH intercalation and ultrasound-assisted processing | Capable of triggering Fenton-like reactions, photothermal ablation, and ROS generation to convert cold tumors to hot tumors (macrophages: M2 → M1) | PAI, CT imaging, and tumor PTT/PDT | 106 |
| Ti3C2@Au | HF etching and TPAOH stripping | Good biosafety, significantly enhanced absorbance in NIR-I and NIR-II windows, improves tumor oxygenation | PAI, CT imaging and tumor therapy | 80 |
| Ti3C2Tx-Gd | HF etching and sonication | Capture of Gd3+ ions leads to change of initial antimagnetic behavior to paramagnetic with high stability and cytocompatibility | MRI and PTT | 109 |
| MnOx/Ta4C3 composite nanosheets | HF etching and sonication | Highly biocompatible and biosafe, multiple imaging-guided photothermal thermotherapy significantly inhibits tumor growth | PAI, MRI, CT imaging and tumor therapy | 78 |
| Fe-Ti3C2 | LiF and HCI etching and sonication | NIR irradiation stores and releases ferrous ions and produces ROS through the synergistic action of the Fenton reaction and GSH reduction | MRI and tumor PTT/PDT | 114 |
| V2C | Using algal extraction to embed and layer V2AlC produces V2C | High absorption in the NIR region, with photothermal conversion efficiencies up to 48% | PAI, MRI, and PTT for Cancer | 115 |
Compared with conventional organic fluorophores, MXenes and their corresponding QDs show interesting fluorescence properties in bioimaging, such as tunable wavelength, high photostability, and desirable quantum yields. Xue and coworkers reported that photoluminescent Ti3C2 MXene quantum dots (MQDs) can be prepared by a facile hydrothermal method, and that they exhibit excitation-dependent PL behaviors with high quantum yields of as high as up to 9.9%.41 The application of MQDs as a multicolor cell imaging reagent was demonstrated by labeling the RAW264.7 cell line, which suggests that MXene-based QDs have great potential for applications in optical, biomedical and cellular imaging. As another example, Zhou et al. reported an unprecedented method to synthesize amphiphilic carbide-derived graphene quantum dots (GQDs) from layered Ti3C2Tx MXene by solvothermal treatment of the lamellar Ti3C2Tx MXene in dimethylformamide (DMF); the GQDs exhibited bright and tunable photoluminescence.98 Breast cancer cells (MCF-7) were incubated with GQD aqueous dispersions (20 μg mL−1) for 2 h, and strong green and blue fluorescence of MCF-7 cells was observed by laser excitation at 488 nm and 364 nm, respectively, which demonstrated that the GQDs could be internalized efficiently by MCF-7 cells, and the results indicated that GQDs could be used as fluorescent probes for cell imaging applications (Fig. 5A). Most of the MXene QDs (MQDs) reported so far show blue fluorescence emission, and Lu et al. have unprecedentedly devised a facile and high-yield method to prepare Ti3C2 MQDs with direct white photoluminescence (photoluminescence quantum yield, 9.36%) and two-photon fluorescence (TPFL).99
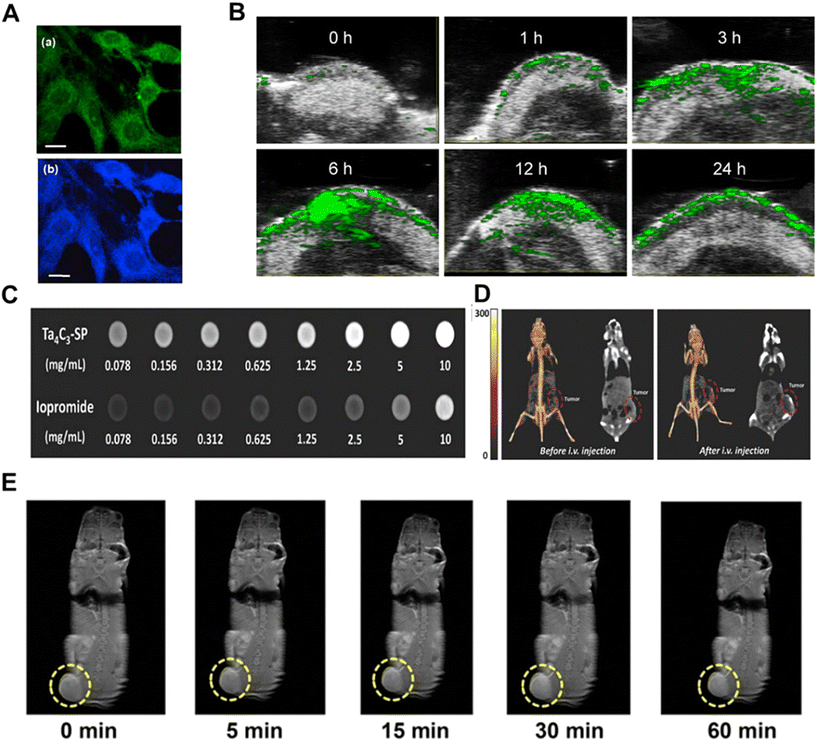 | ||
| Fig. 5 MXene-based bioimaging applications. (A) CLSM images of MCF-7 cells incubated with GQD for 2 h by excitation at 488 nm (a), 364 nm (b). The scale bar is 20 μm.98 Reproduced from ref. 98 with permission from Elsevier, copyright 2017. (B) In vivo PA images of tumor sites after intravenous injection of Ti3C2Tx-Pt-PEG at different time intervals.103 Reproduced from ref. 103 with permission from American Chemical Society, copyright 2022. (C) CT images of Ta4C3-SP nanosheet solution (top) and iodine bromide solution (bottom) at different concentrations.105 Reproduced from ref. 105 with permission from Wiley-VCH, copyright 2018. (D) In vivo CT contrast images (right panel) and 3D reconstructed CT images (left panel) of mice before and after intravenous injection of Ta4C3-SP nanosheet solution (10 mg mL−1, 200 μL) for 24 h.105 Reproduced from ref. 105 with permission from Wiley-VCH, copyright 2018. (J) T1-Weighted imaging of 4T1 tumor-bearing mice after prolonged intravenous injection of MnOx/Ta4C3-SP composite nanosheets.78 Reproduced from ref. 78 with permission from American Chemical Society, copyright 2017. | ||
PAI is a new non-invasive and non-ionizing biomedical imaging method developed in recent years that uses non-ionizing laser pulses to irradiate biological tissue. When the tissue absorbs light and converts it to heat, the released heat causes a localized temperature increase and thermal expansion of the tissue, which results in the emission of ultrasound waves (the photoacoustic effect).100 The photoacoustic signals generated by the photoacoustic effect at biological tissue sites carry information about the light-absorption properties of the tissue that can be further converted into light-absorption distribution images.101 The excellent photothermal conversion properties of MXene-based materials allow for potential PAI capabilities.102 For example, Zhu et al. reported a Ti3C2Tx-Pt-PEG nanocomposite with unique NIR-II absorbance and good photothermal conversion efficiency (31.78%), making it a good contrast-enhanced PA imaging nanomaterial.103 The PA images in vitro based on Ti3C2Tx-Pt-PEG nanocomposites showed unique PA signals, and the intensity of PA signals was concentration-dependent, with PA signals increasing significantly with the increase of sample concentration. After Ti3C2Tx-Pt-PEG (10 mg kg−1, 100 μL) was intravenously injected into 4T1 tumor-bearing female BALB/c mice, PA images were obtained at different time intervals, and the results showed that the PA signal intensity at the tumor site gradually increased with the increase of time and reached the maximum at 6 h due to the accumulation of Ti3C2Tx-Pt-PEG nanocomposites at the tumor site (Fig. 5B).
CT imaging uses X-rays to penetrate different tissues of the body with different attenuation coefficients.104 It measures the intensity of X-rays through a certain thickness of the object to be measured and converts the X-ray intensity from black to white to grayscale. CT imaging has a high spatial resolution and deep tissue penetration and is used for 3D tomography of anatomical structures based on the difference in X-ray absorption between lesions and tissues, and is therefore widely used mainly for medical diagnostics. Since the X-ray attenuation coefficient increases with increasing atomic number, nanomaterials containing high atomic number (Z) elements are often explored as contrast agents (CAs) for CT imaging. In this regard, tantalum (Ta)-based MXenes (Ta4C3) can undoubtedly be used as CA for CT imaging because of the high atomic number of tantalum (Z = 73) and the large attenuation coefficient of X-rays.105 For example, Lin and his colleagues reported that CT images of different concentrations of biocompatible soybean phospholipid (SP)-modified Ta4C3 (Ta4C3-SP) in xanthan gum showed a dramatic enhancement of the signal as the concentration of Ta4C3-SP increased, and the signal intensity was stronger than that of commercial iodine-based CT CAs (Fig. 5C). Ta4C3-SP (20 mg kg−1) was intravenously injected into mice carrying 4T1 tumors for in vivo CT imaging, and the CT images obtained 24 hours after injection showed significant tumor contrast, which demonstrated that Ta4C3 MXene can be used as a promising CAs for CT imaging of tumors (Fig. 5D). In addition, Feng et al. reported a V4C3 MXene nanosheet with excellent photothermal and X-ray absorption properties for dual-modality PA/CT imaging.106
MRI has been widely used for clinical diagnostic imaging due to its high spatial resolution, excellent three-dimensional soft tissue contrast difference, and noninvasive properties.107 During MRI, the body is placed in a static magnetic field and certain radiofrequency (RF) pulses are used to excite the nucleus of a hydrogen atom and indicate the presence of protons (1H). When the RF pulses are stopped, the protons return to an equilibrium state in the magnetic field and produce weak energy (MRI signals) during the relaxation process. When these signals are received, magnetic resonance images can be obtained by processing them with spatial resolution and image reconstruction. The intensity of the MR signal is mainly dependent on the relaxation time of the hydrogen protons. The utilization of MXenes in MRI is limited by their usual antimagnetism; however, it is possible to functionalize the MXene with ferromagnetic or paramagnetic compounds and nanoparticles, which can be effective in increasing the difference in tissue MRI signals to improve imaging contrast and clarity, provide information about the directional location of tumors, and guide photothermal ablation and chemotherapy of tumors.108 In a study, Gd was utilized to chelate with Ti3C2Tx to obtain MXene-Gd, which can be used as a CA for MRI.109 The investigators found that the relaxation value of pristine MXene was close to zero due to the diamagnetic nature of the electronic degrees of freedom in the pristine flakes, and the relaxation (r1) increased significantly to 5.87 s−1/mM (at a 0.5 T magnetic field) after entrapment of Gd3+ ions. Dai et al. proposed another surface engineering strategy to grow manganese oxide nanoparticles in situ onto the surface of Ta4C3 MXene, and after further surface organic modification with soybean phospholipids (SP), the MnOx/Ta4C3-SP composite nanosheet system was obtained, which can be used for multiplexed imaging-guided photothermal tumor ablation.78 Among them, the Mn2+ released from the MnOx/Ta4C3-SP composite nanosheets could effectively enhance the relaxation of hydrogen protons, indicating its performance as an MRI CA in vivo. During the test period of 60 min after intravenous injection of MnOx/Ta4C3-SP composite nanosheets (20 mg kg−1, 100 μL) into 4T1 tumor-bearing mice in vivo, MRI signal enhancement of the tumor tissues and their corresponding enlargement of the T1-weighted MR images could be clearly observed (Fig. 5E).
Currently, most of the research on MXene bioimaging focuses on Ti3C2Tx, a class of MXene, and more luminescent nanomaterials based on other MXenes are expected to be developed for bioimaging applications in the future.
4.2 Cancer therapy
With excellent optical/electrical properties, biocompatibility, ease of functionalization and enzyme immobilization, MXenes can be used to enhance the biocompatibility, tumor-targeting ability, and anti-tumor effects of the resulting therapeutic platforms through elemental doping, surface oxidation, nanohybridization, or surface encapsulation to cope with the complex tumor microenvironment. MXenes and MXene-based nanomaterials are capable of inhibiting tumors through various therapeutic mechanisms, including PTT, chemodynamic therapy (CDT), PDT, and sonodynamic therapy (SDT), and have made significant advances in being used in various cancer models and therapeutic strategies (Table 3).| MXene composition | Synthesis strategy | Animal models | Treatment strategy | Ref. |
|---|---|---|---|---|
| Ti3C2-based anionic solid solutions | HF etch-assisted sonication | 4T1 tumor model in BALB/c mice | PTT | 116 |
| Ti3CN | HF etch-assisted sonication | The homograft 4 T1 tumor model | PTT | 117 |
| V4C3/ATO@BSA | HF/tetrapropylammonium hydroxide (TPAOH) method | 4T1 tumor-bearing mice model | Nanozyme catalytic/PTT | 118 |
| n-HA/g-C3N4/MXene scaffold (Ti3C2Tx) | HF etching and DMF ultrasonication | Saos-2 tumor-bearing mouse model | PTT/PDT | 124 |
| A wearable biological electrothermal patch based on Ti3C2Tx | HF etch-assisted sonication | B16F10 tumor-bearing C57BL/6J mice model | PTT | 125 |
| Mo2C-PVA | LiF/HCl and HF/TBAOH method | 4T1 tumor-bearing mice model | PTT | 11 |
| NMQDs-Ti3C2Tx | HF etching and micro-explosive methods | BALB/c nude mice bearing HeLa tumor xenograft model | CDT | 119 |
| FA@MXene/CuO2/GA (Ti3C2 MXene) | HF etch-assisted sonication | 4T1 tumor model in BALB/c mice | PTT/CDT | 120 |
| Cu2O/Ti3C2Tx | HF etching-assisted ultrasonic stripping method | The xenograft model of 4 T1 cells | PTT/CDT | 126 |
| Ti3C2Tx-GOx/DOX-PEG | LiF etching and TPAOH intercalation | 4T1 tumor-bearing BALB/c mice model | Chemo/starvation/photothermal combination therapy | 95 |
| CoPc-Mn/Ti3C2Tx | HF etching | B16-tumor-bearing mice model | PTT/CDT/PDT | 127 |
| BTO@Nb2C-PEG | HF etching and TPAOH intercalation | 4T1 tumor-bearing mice model | PTT/PDT | 121 |
| Ti3C2-DOX | LiF etching and TPAOH intercalation | HCT-116 tumor-bearing mice model | PTT/PDT/chemo therapy | 22 |
| H-Ti3C2 NSs | Chemical exfoliation and high-temperature treatment | 4T1 tumor-bearing mice model | PTT/SDT | 122 |
| Ti3C2/CuO2@BSA | HF etching and TPAOH intercalation | The U87 tumor-bearing BALB/c nude mice model | SDT/CDT | 123 |
As a typical non-invasive treatment method, PTT ablates tumors with the help of heat generated by light excitation, which has the advantages of high selectivity, non-invasiveness, low toxicity, low cost and strong targeting. However, the application of traditional anti-tumor PTT is often hampered by the limited depth of light penetration and the insufficient efficiency of material photothermal conversion. In recent years, MXenes have shown great potential in the treatment of deep-seated tumors due to their strong absorption in the NIR region and good photothermal conversion efficiency, and have become an excellent candidate for anti-tumor PTT under NIR-I and NIR-II windows. Tang et al. optimized the well-known electronic structure and NIR-II absorption of Ti3C2 using an anionic solid solution with the help of lattice N modulation and associated additional electron injection, which enhanced the response in the NIR-II region (Fig. 6A).116 Among them, Ti3C1.23N0.77 showed a high photothermal conversion efficiency of 12.0% at 1064 nm at a low concentration of 5 ppm, and exhibited efficient NIR-II PTT ablation of tumors both in vitro and in vivo. The results suggest that ideas for optimizing low-dose tumor photothermal therapy can be provided by modulating the anions in the MXene. Liu et al. successfully developed a unique metal–carbon nitride (d-Ti3CN) MXene with excellent photothermal properties for efficient photothermal therapy of tumors both in vitro and in vivo, and tumor-free treatment could be achieved at 1064 nm irradiation with minimal side effects.117 Although MXene exhibits better PTT performance than other photothermal nanomaterials in tumor therapy, researchers have found that surface modification of MXene can further enhance its photothermal effect. For example, by modifying the nanosystem (V4C3/ATO@BSA, VAB) obtained by modifying bovine albumin (BSA) and Atovaquone (ATO) on V4C3, Zhao et al. calculated that the photothermal conversion efficiency of VAB was as high as 61%, which was higher than that of V2C (48%), and the nanoplatform could exhibit significant anti-tumor effects by disrupting redox homeostasis as well as PTT in tumor cells.118
 | ||
| Fig. 6 MXene-based applications in cancer therapy. (A) Schematic illustration of Ti3C2-based anionic solid solutions for NIR-II photothermal therapy in vivo.116 Reproduced from ref. 116 with permission from Wiley-VCH, copyright 2023. (B) Schematic illustration of FA@MXene/CuO2/GA nanocomposite: synthesis and therapeutic mechanism.120 Reproduced from ref. 120 with permission from Wiley-VCH, copyright 2024. (C) Schematic illustration of the pyroelectric nanocatalysts BTO@Nb2C-PEG for PTT and PCT-augmented cancer therapy.121 Reproduced from ref. 121 with permission from Wiley-VCH, copyright 2023. (D) Schematic illustration of 2D MXene-originated in situ nanosonosensitizer generation for augmented and synergistic sonodynamic tumor nanotherapy.123 Reproduced from ref. 123 with permission from American Chemical Society, copyright 2022. | ||
CDT utilizes metal ions (e.g., Fe2+, Cu2+, Mn2+, Ti3+, and Nb2+) to catalyze a Fenton or Fenton-like reaction of H2O2 to produce cytotoxic ˙OH to kill tumors. However, there are challenges such as low catalytic efficiency, poor biocompatibility and even potential toxicity. Based on the excellent biocompatibility of MXene, it has become an emerging hot nanomaterial for researchers to implement effective and safe anti-tumor CDT. Li et al. proposed a Ti3C2Tx quantum dot with excellent biocompatibility that could generate a large amount of ˙OH through Fenton-like reaction when combined with H2O2, and it could inhibit tumors up to 91.9% without damaging normal tissues.119 To further enhance the application of MXene in tumor CDT, researchers have attempted to dope various metal ions (with CDT catalytic activity) into the structure of MXene nanosheets for synergistic PTT and CDT treatment of tumors. For example, Xiong et al. developed a near-infrared (NIR)-light-triggered MXene nanocomposite (FA@MXene/CuO2/GA), in which the photothermal effect of MXene significantly accelerated the CuO2-catalyzed conversion of ˙OH, resulting in an excellent synergistic PTT/CDT anticancer therapeutic effect (Fig. 6B).120
PDT has the advantage of being non-invasive and spatiotemporally specific to kill cancer cells with the help of localized ROS generation under light irradiation. However, the therapeutic efficacy of PDT is greatly reduced by the limited reactive oxygen species (ROS) generation due to the hypoxic tumor microenvironment and the rapid electron–hole pair complex mediated by light irradiation. MXene-based nanomaterials have excellent photothermal effects, and the heat generated by laser irradiation promotes electron–hole space separation, which reacts with the surrounding O2 and H2O molecules to produce toxic ROS, making them popular research materials for implementing PTT/PDT synergistic therapies. Zhang et al. rationally developed a multifunctional therapeutic nano-heterojunction in which two-dimensional niobium carbide (Nb2C) MXene was grown in situ with barium titanate (BTO) to generate a robust photogenerated pyroelectric catalyst called BTO@Nb2C nanosheets, which could enhance the ROS generation and achieve a synergistic effect of combining the materials’ PTT and PDT with tumor inhibition rate of 94.9% in vivo (Fig. 6C).121 As another example, Liu et al. synthesized a multifunctional nanoplatform (Ti3C2-DOX) that exhibited excellent photothermal conversion efficiency (∼58.3%) and effective single-linear oxygen generation (1O2) under 808 nm laser irradiation.22 In vitro and in vivo experiments demonstrated that Ti3C2-DOX showed enhanced biocompatibility, tumor-specific accumulation, and stimuli-responsive drug release behaviors, and achieved effective cancer cell killing and tumor tissue destruction via photothermal/photodynamic/chemotherapeutic synergistic therapy.
SDT has attracted wide interest in biomedicine for its novel noninvasive therapeutic approach triggered by ultrasound (US). Despite the advantages of high tissue penetration depth, absence of ionizing radiation and low cost, SDT still suffers from low ROS quantum yield, limited delivery efficiency and potential toxicity of acoustic sensitizers. Researchers have designed various MXene-based nanosystems to perform effective antitumor SDT. For example, Li et al. developed Ti3C2 MXene nanosheets (H-Ti3C2 NSs) with good acoustic sensitizer properties by a two-step method of chemical stripping and high-temperature treatment, and by high-temperature treatment, the oxygen defects of H-Ti3C2 NSs greatly increased.122 The electrons (e−) and holes (h) generated by US could be separated faster due to the increased oxidation, and then H-Ti3C2 NSs could take advantage of the abundant oxygen defects to prevent the recombination of e− and h under the irradiation of US, greatly improving the acoustic kinetic efficiency. The in vivo study further showed that the increased blood supply resulting from the mild photothermal effect significantly alleviated the hypoxic condition in the tumor microenvironment, showing that PTT enhanced the effect of SDT. In another study, a nanoacoustic sensitizer/nanocatalyst (Ti3C2/CuO2@BSA) was reported to achieve high-performance and synergistic acoustic/chemodynamic tumor therapy by generating nanoacoustic sensitizers in situ in response to the tumor microenvironment.123 Integration of CuO2 nanoparticles on 2D Ti3C2 MXene allowed in situ generation of H2O2 and oxidation of Ti3C2 to generate TiO2 nanosensitizers for acoustic sensitization in an acidic tumor microenvironment, while the oxidized carbon matrix enhanced the separation of e− and h, which further improved the efficacy of SDT (Fig. 6D). Ultrasound irradiation during the sonodynamic process also enhanced the copper-triggered Fenton-like reaction to generate more ROS, thus synergizing the SDT.
MXene-based nanomaterials exhibit multimodal therapeutic potential in cancer therapy, including PTT, CDT, PDT, and SDT, due to their excellent optical/electrical properties and biocompatibility, enhanced therapeutic efficacy and biocompatibility through different strategies and surface modifications, and due to responding effectively to the complex tumor microenvironment.
4.3 Infection therapy
Infectious diseases refer to a group of diseases caused by pathogens such as bacteria, fungi, viruses, and parasites that infect the human body, and are still the second leading cause of death in humans. When the body is attacked by an infection, it triggers intense oxidative stress and significant inflammatory responses that may impede the healing process of the disease. Especially for wounds damaged by multidrug-resistant bacteria, conventional antibiotics are powerless. In recent years, MXene-based materials have become an effective option for the treatment of infectious diseases due to their excellent antioxidant and anti-inflammatory properties, photothermal/photodynamic antimicrobial performance, and low bacterial resistance (Table 4).| MXene composition | Synthesis strategy | Description | Animal models | Ref. |
|---|---|---|---|---|
| Pt@V2C | HF etching and TPAOH intercalation | Effective in the removal of bacteria and bacterial biofilms and may modulate inflammation through the NOD-like receptor signaling pathway and the IL-17 signaling pathway | A subcutaneous abscess model in mice infected with MRSA, the MRSA-infected keratitis model | 128 |
| Ti3C2 MXene/Fe-MOFs composite (MXM) | LiF/HCl etching and ultrasonication | The photothermal conversion efficiency is close to 51.9% and exhibits NIR-enhanced POD-like activity | The MRSA infected burn wound model | 129 |
| HNTM/Ti3C2 hybrid nanomaterials (HN-Ti3C2) | HF etching and DFM heat treatment | Effective killing of MRSA by SDT and promotion of bone regeneration through activation of calcium, Wnt, and TGF-β signaling pathways | A MRSA-infected rat tibial osteomyelitis model | 130 |
| Nb2C@TP | HF etching and TPAOH intercalation | Significantly down-regulates energy metabolism-related pathways and activates Agr in bacteria, which fundamentally kills bacteria, inhibits biofilm formation and promotes biofilm separation | A mouse subcutaneous infection model | 131 |
| GA/OKGM/MT hydrogel (MT: Ti3C2@TiO2) | HF etch-assisted sonication | PTT effectively destroys bacteria and eliminates excess ROS, thereby facilitating the transition from the inflammatory to the proliferative phase | The S. aureus-infected wound model in SD rats | 133 |
| Monolayer high-entropy (HE) MXenes | Selectively etched through a high-entropy MAX phase (HE MAX) | NIR-II-enhanced endogenous oxidase mimetic activity, excellent photothermal conversion efficiency and very high biocatalytic activity | Bacterial keratitis (BK) and subcutaneous abscess infections model induced by MRSA | 134 |
| VS4/Ti3C2 (VSM) | LiF/HCl etching and ultrasonication | Schottky junction, POD-like activity and acoustic dynamics | A tibial plateau MRSA-infected bone defect model | 135 |
| CuS/Ti3C2Tx@VMP | HF etch-assisted sonication | Significantly promotes skin rejuvenation through pathogen elimination, angiogenesis promotion and collagen deposition | Full-thickness wounds infected by S. aureus | 136 |
| V2N | HF etching and TPAOH intercalation | Excellent OXD and POD catalytic activity, ideal photothermal conversion efficiency, abundant ROS generation | A mice subcutaneous abscess model in BALB/c mice | 137 |
| PLGA-Ti3C2Tx/Ag2S@LOx | LiF/HCl etch-assisted sonication | Promotes wound healing by killing bacteria, stopping bleeding, promoting epithelialization/collagen deposition in the wound bed and angiogenesis | An S. aureus-infected mouse skin wound model on the dorsal sites of mice | 138 |
| Ti3C2Tx, Ta4C3Tx, Mo2Ti2C3Tx and Nb4C3Tx | HF etching and TPAOH intercalation | Ti3C2Tx and Mo2Ti2C3Tx MXene showed antiviral activity against SARS-CoV-2 | — | 132 |
MXene-based nanomaterials with strong biocompatibility and photothermal conversion efficiency can be used for PTT antimicrobial treatment of bacterial infectious diseases. The laser irradiation range of the V2C MXene is limited to the near-infrared I region (NIR-I), which restricts its penetration into tissues and makes it difficult to achieve complete bacterial eradication with single-acting therapeutic strategies. To address this issue, He et al. attached platinum nanoparticles (Pt NPs) to V2C to form an artificial nanoplatform (Pt@V2C).128 Due to the localized surface plasmon resonance (LSPR) effect of Pt NPs and V2C, Pt@V2C exhibited enhanced photothermal conversion efficiency (59.6%) and longer irradiation lasing (NIR-II). Notably, Pt@V2C possessed CDT- and NIR-II-enhanced oxidase (OXD), POD-like activities. In an in vivo animal model, Pt@V2C effectively cleared MRSA from deep tissues in the setting of subcutaneous abscesses and bacterial keratitis through the synergistic effect of PTT/CDT, down-regulated the expression of CD86 and IL-6 in the infected tissues, accelerated abscess regression, and facilitated the healing of wounds and corneas (Fig. 7A). In another study, Zhao et al. proposed a strategy for NIR plasmonic enhancement of the catalytic and photothermal properties of nanoenzymes for effective anti-infective and antibacterial applications.129 A Ti3C2 MXene/Fe-MOFs composite (MXM) with NIR plasmonic-enhanced CDT and photothermal properties was successfully developed by loading metal–organic framework (MOF) nanoenzymes on Ti3C2 MXene. SEM images demonstrated the morphological changes of E. coli, MRSA, and C. albicans after treatment with NaAc buffer, H2O2, MXM, and MXM + H2O2 (100 μg mL−1) irradiation with or without NIR (Fig. 7B). The cell membranes of Control and H2O2 groups remained intact, and the treatment effect was negligible. However, some wrinkles in the bacterial membrane were observed in the MXM + H2O2 or MXM + NIR groups, indicating significant CDT activity and excellent photothermal properties of MXM, respectively. The results of in vivo experiments showed that the LSPR-induced MXM system has outstanding antibacterial properties and promotes rapid healing of MRSA-infected burn wounds. SDT is a novel strategy for overcoming the problem of bacterial resistance to antibiotic therapy. For example, Wang and his colleagues developed a HNTM/Ti3C2 hybridized nanomaterial (HN-Ti3C2) with Schottky heterojunction for efficient SDT for osteomyelitis and bone regeneration.130 Ti3C2 greatly improved the acoustic catalytic performance by rapidly transferring the carriers generated by HNTM under US irradiation and killed drug-resistant bacteria by generating a large amount of ROS, and it showed an excellent 99.75% antimicrobial effect against MRSA. In addition, the acoustic flow generated by HN-Ti3C2 at low intensity US could promote the proliferation of stem cells by regulating the cell cycle, DNA replication and apoptosis, thereby promoting the long-term repair of bone defects. In the MRSA-infected rat tibial osteomyelitis model, HN-Ti3C2 successfully eliminated the infection and significantly improved bone regeneration under US irradiation. The various synergistic effects generated based on MXenes hold great promise for the design and fabrication of novel and effective MXene nanocomposites to combat bacterial infections.
 | ||
| Fig. 7 MXene-based applications in infection therapy. (A) Schematic illustration of the construction, antibacterial, and anti-infective therapy of Pt@V2C nanoplatforms with photothermal and chemodynamic therapy.128 Reproduced from ref. 128 with permission from Wiley-VCH, copyright 2024. (B) SEM images of E. coli, MRSA and C. albicans exposed to different samples without and with NIR irradiation (808 nm laser, 1.25 W cm−2). MXM concentration is 200 μg mL−1. The concentration of E. coli, MRSA, and C. albicans is 1.0 × 108 CFU mL−1, respectively. H2O2 concentration is 100 μM.129 Reproduced from ref. 129 with permission from Wiley-VCH, copyright 2023. (C) (a) Preparation and characterization of Nb2C@TP. (b) Confocal 3D images of biofilm. Green indicates live bacteria, and red indicates dead bacteria.131 Reproduced from ref. 131 with permission from American Chemical Society, copyright 2020. (D) Overview of the mechanism of MXene-dependent viral inhibition.132 Reproduced from ref. 132 with permission from Elsevier, copyright 2021. | ||
With the proliferation of indwelling devices and implants, the associated infections caused by invasive bacterial adhesion and biofilm formation on medical implant surfaces are increasingly recognized as an intractable global public health problem. MXene nanosheets are promising safe candidates for implant surface modification, bringing biofilm-resistant properties to implantable devices. For example, researchers have combined two-dimensional Nb2C MXene nanosheets with medical titanium plates (TP) to craft a biofunctional Nb2C@TP (NTP) therapeutic platform for multimodal infection control with bacterial clearance and tissue regeneration (Fig. 7C).131 Confocal 3D images showed that the TP group was covered by live bacteria with dense stacked biofilm structures, while almost no dead and live bacteria biofilms were detected in the Nb2C@TP group (Fig. 7C). Nb2C@TP played a crucial role in inhibiting bacteria through multiple modes. In terms of biofilm elimination, Nb2C@TP activated an auxiliary gene regulator (Agr) that prevented bacterial adhesion and promoted biofilm separation when bacteria attempted to invade the implant surface. In addition, Nb2C@TP directly killed bacteria through metabolic pathways such as the downregulation of the tricarboxylic acid (TCA) cycle and the phosphotransferase system (PTS) pathway, leading to bacterial death. In terms of planktonic bacterial clearance, Nb2C@TP sensitized bacteria to thermotherapy and killed them via NIR-responsive thermotherapy. In addition to excellent bacterial clearance, Nb2C@TP attenuated excessive pro-inflammatory and oxidative stress in vivo and accelerated angiogenesis and tissue remodeling processes. Nb2C@TP holds promise for treating cases of implant-associated infections with bacterial/biofilm clearance and tissue remodeling capabilities.
In addition to antimicrobial activity, several studies have demonstrated the potential application of 2D materials as antiviral agents. In one study, MXenes were found to possess antiviral activity, and have the potential to be used as therapeutic agents for viral infectious diseases in the future.132 Using SARS-CoV-2 as a model, the researchers explored the antiviral properties and immune profiles of a large group of four highly stable and well-characterized MXenes (Ti3C2Tx, Ta4C3Tx, Mo2Ti2C3Tx and Nb4C3Tx). It was found that Ti3C2Tx and Mo2Ti2C3Tx showed antiviral activity against SARS-CoV-2, with Ti3C2Tx being the most effective material tested against SARS-CoV-2 particles, and did not produce cytotoxicity in any of the peripheral blood mononuclear cell populations. The authors described the mechanism of MXene-dependent antiviral activity by docking analysis, proteomics data, and comparison with SARS-CoV-2 protein interaction profiles (Fig. 7D). Ti3C2Tx exerts its viral inhibitory activity not only on the cell surface but also through different signaling mechanisms including membrane trafficking, GPCR signaling, mitochondrial function, metabolic pathways and viral replication. The results indicated that host proteins such as GNG5, GRPEL1 and NUTF2 are important regulators of Ti3C2Tx-dependent antiviral activity. These host proteins interact with viral NSP7, NSP10 and NSP15 proteins, respectively. During SARS-CoV-2 infection, NSP7 plays an important role in membrane transport and G protein-coupled receptor (GPCR) signaling, and NSP15 is involved in vesicular and nuclear transport mechanisms. NSP7 and NSP10 are among the several proteins that can alter the endosomal membrane compartment to facilitate viral entry and replication. Thus, MXenes are able to exert their antiviral activity by modulating these viral proteins and pathways that are important for viral transmission. This study provides guidance for future research into the implementation of MXene nanomaterials in tools targeting viral infectious diseases as well as any immune system-related syndromes.
MXene-based nanomaterials show great potential in the field of infectious disease therapy due to their unique physicochemical properties. These materials not only possess excellent biocompatibility and photothermal conversion efficiency for effective PTT antimicrobial activity, but also synergize antimicrobial activity through multiple mechanisms such as CDT, PDT, and SDT. The surface functional groups of MXenes allow for easy functionalization, enhancing their prospects for application in antimicrobial and antiviral therapies, especially in combating infections with multidrug-resistant bacteria. In addition, the antiviral activity of MXenes provides a new direction for the treatment of viral infectious diseases in the future. In summary, MXene-based nanomaterials have a wide range of applications in the treatment of infectious diseases and provide a powerful tool for the development of novel antibacterial and antiviral therapeutic strategies.
4.4 Wound healing
Wounds are breaks in the skin caused by various external injuries. The presence of a wound greatly increases the risk of infection, which often leads to deterioration of the wound condition, prolonged wound healing, a complicated healing process, and even death.139 Wound healing is a complex process involving multiple cell types within the epidermis, dermis, and subcutaneous layers and consists of four highly integrated and overlapping phases: hemostasis, inflammation, proliferation, and tissue remodeling.140 All of these steps must be performed precisely to successfully heal wounds. MXenes have antimicrobial, anti-inflammatory and antioxidant properties, promoting cell adhesion and proliferation as well as tissue regeneration, and the integration of MXenes with other bioactive materials to form multifunctional composites has emerged as a promising strategy for wound healing (Table 5).| MXene composition | Synthesis strategy | Characteristic | Animal models | Ref. |
|---|---|---|---|---|
| FPSa@Ti3C2Tx | LiF/HCl etch-assisted sonication | Injectable, rapid gelation, conductive, photothermal, antioxidant, anti-inflammatory and promotes vascular regeneration | A mouse full-thickness skin wound MRSA infection model and a burn wound model | 142 |
| F127-Ti3C2Tx-Exo (FM-Exo) | LiF/HCl etch-assisted sonication | Photothermal antimicrobial, promotes fibroblast proliferation, migration and angiogenic capacity of endothelial cells and overcomes high glucose-derived immunosuppression | The full-thickness cutaneous wound model | 143 |
| MNPs@MXene-hydrogel | LiF/HCl etch-assisted sonication | Antimicrobial resistance, photothermal properties, multi-stimulus responsiveness and controlled drug delivery capabilities | The full-thickness cutaneous wound and subcutaneous infected wound of the rat model | 144 |
| HA-DA/Ti3C2@PDA | HF etching and TPAOH intercalation | Hemostasis, O2 release, scavenging of ROS as well as reactive nitrogen, promotion of proliferation and migration of HUVECs and modulation of macrophage phenotype | The full-thickness cutaneous wound model in infected diabetic mice | 145 |
| Ti3C2@TA/Fe-hydrogel | HF etching and TPAOH intercalation | POD/CAT-like activity, antioxidant, resistance to protein adsorption and bacterial adhesion, antimicrobial, promotion of granulation tissue regeneration, epithelial formation and collagen deposition | Burn wound model in SD rats | 148 |
| FGDN hydrogel (Nb2C@PDA) | HF etching and TPAOH intercalation | Hemostatic, eradication of bacterial infections, anti-inflammatory and antioxidant, angiogenesis, promotion of granulation tissue production, collagen deposition and re-epithelialization | A mouse full-thickness cutaneous MRSA-infected wound model | 149 |
| Ti3C2Tx composite fibers | — | Unidirectional thermal conductivity, photothermal properties, and the ability to effectively kill drug-resistant bacteria and residual cancer cells after surgery | Tumor recurrence models in NVSG mice and full-thickness skin wound in SD rats | 150 |
| MN-PGA-Ti3C2-GOx | — | Reduces local glucose and pH, antioxidant, induces mild hyperthermia, enhances catalytic activity of GOx, promotes cell proliferation, migration, angiogenesis and tissue remodeling | A diabetic mouse dorsal wound model | 151 |
| Fe2O3/Ti3C2-MXene@GOx (FMG) | HF etching and sonication | Excellent photothermal, photodynamic, and chemodynamic properties, induces bacterial iron death | A full-thickness skin defect model in diabetic SD rats with S. aureus infection | 146 |
| AgNPs/Ti3C2Tx/GG/Alg-PBA | — | Mechanical properties, excellent healing ability, reliable injectability, easy degradability, biocompatibility, antimicrobial capacity, ability to monitor body signals | A murine full-thickness wound model | 147 |
| Ti3C2Tx-based conductive composite ionogel patch | LiF/HCl etch-assisted sonication | Good photothermal effect to kill bacteria and prevent wound infection, on-demand electrical stimulation to promote wound healing by increasing the expression of growth factors, good transmittance for visualization and real-time monitoring of treatment effect | The infected skin wound model | 152 |
Bacterial infections (persistent bacterial colonization, high oxidative stress and severe inflammation) are the first serious challenge for wound healing. MXenes have excellent antimicrobial properties; however, MXenes alone cannot meet the requirements for treating the complexity of bacterial-infected wounds. Biomedical hydrogels with good hydrophilicity, biocompatibility, extracellular matrix-like properties and customizable properties have attracted much attention from researchers in inducing skin wound repair.141 Researchers have proposed improved strategies for introducing MXenes into hydrogel systems, both to enhance the performance of the MXene and to stimulate new functionalities. For example, Ma et al. designed a hydrogel (FPSa@M) self-assembled from Ti3C2Tx MXene and poly (salicylic acid)-Pluronic F127-poly (salicylic acid) (Fig. 8A).142 FPSa@M exhibits injectability, rapid gelation, electrical conductivity, as well as beneficial antioxidant and photothermal effects. The photothermal temperature-tunable FPSa@M hydrogel effectively achieved complete photothermal eradication of high concentrations of multidrug-resistant bacteria and significantly promoted healing of complex wounds caused by MRSA infections or burns by remodeling the oxidative stress and inflammatory microenvironment of wound healing through the activation of heat shock protein 90 (HSP90), scavenging of ROS, and angiogenesis. In another study, a simple and effective nano-hydrogel composite (FM-Exo) of F127-MXene (FM) composite with M2 macrophage exosomes (Exo) was reported.143 The effect of macrophage-conditioned medium obtained from the FM-Exo group on the behavior of HUVECs in a high-glucose microenvironment was investigated, and the results showed that high-glucose stimulation impaired the migratory and tube-forming abilities of HUVECs compared with those of the normal-glucose group, and that HUVECs treated with the FM-Exo-conditioned medium exhibited optimal migratory as well as tube-forming abilities. Yang et al. designed an MXene-based hydrogel based on multiple stimulus responses.144 When exposed to NIR or AMF, the temperature of the MXene increased significantly, leading to the contraction of the hydrogel system, thus precisely controlling the release of therapeutic drug AgNPs and eliminating bacteria adhering to the wound depth. Wound images also demonstrated that this MXene-based hydrogel exhibited the best wound closure under NIR irradiation. In addition, another study found that the MXenes-based hydrogel treatment group could effectively reduce ROS levels in wounds, up-regulate the expression of epidermal growth factor (EGF) and vascular endothelial growth factor (VEGF), and down-regulate the expression of hypoxia-inducible factor-1 α (HIF-1α), which suggests that the MXene-based hydrogel could accelerate diabetic wound healing by advancing the formation of the epidermis, alleviating local hypoxia and neovascularization.145
 | ||
| Fig. 8 MXene-based applications in wound healing. (A) Schematic diagram illustrating single platform of FPSa@M hydrogel integrated diverse capabilities to accelerate complete regeneration of MDR bacterial infected wounds and burn wounds.142 Reproduced from ref. 142 with permission from Elsevier, copyright 2024. (B) Schematic diagram of ferroptosis bio-heterojunction (F-bio-HJs). Flowchart of the preparation steps and procedure for FMG bio-HJ and the mechanism of extra-intracellular bacteria-targeted ferroptosis and hunger-triggered cell protection induced by F-bio-HJ.146 Reproduced from ref. 146 with permission from Wiley-VCH, copyright 2023. | ||
Diabetes mellitus (DM) is an autoimmune metabolic disorder characterized by persistent high blood glucose levels, elevated ROS, and excessive inflammation that poses a serious threat to human health. The diabetic hyperglycemic microenvironment prevents collagen deposition, granulation tissue formation and vascular maturation, leading to chronic diabetic wound formation. In response to the high complication and failure rate of intracellular bacterial infections in diabetic wounds, Dai et al. conceptualized and fabricated an innovative bacterial-targeted ferrite bio-hybrid junction (F-bio-HJ) composed of Fe2O3/Ti3C2-MXene and GOx (Fig. 8B).146 Under NIR irradiation, Fe2O3/Ti3C2-MXene@GOx (FMG) catalyzed the production of large amounts of ROS, which attacked the extracellular bacterial membranes, facilitated the penetration of synchronously produced Fe2+/Fe3+ into the bacteria, and led to the death of the planktonic bacteria through iron-leaping, Fe2+ overloading and lipid peroxidation. In addition, FMG promoted ferroptosis in intracellular bacteria by transporting Fe2+ to them via inward ferritin (FPN). When GOx is depleted of glucose, FMG exerts a starvation-protective effect, helping macrophages to escape from ferroptosis by activating the 5′-monophosphate (AMP) activated protein kinase (AMPK) pathway. The in vivo results demonstrated that FMG could induce extracellular bacterial-targeted iron oxidation and promote regeneration of infected diabetic skin without triggering ferroptosis in normal cells. This study provides a promising MXene-based approach for the treatment of diabetic infected wounds.
In recent years, various conductive hydrogel-based flexible epidermal sensors have been well developed for human healthcare monitoring but lack the comprehensive and efficient therapeutic capabilities for reliable and sensitive diagnostic healthcare sensing and timely treatment. MXene nanosheets are considered as attractive candidates for the preparation of conductive hydrogel epidermal sensors that can simultaneously achieve the ability to have reliable self-healing capabilities and significant sensing performance for wearable human–computer interaction and accelerated wound healing for subsequent medical treatment. For example, Li et al. introduced antimicrobial silver nanoparticle-coated MXene (AgNPs/MXene) nanosheet networks into guar gum (GG) and phenylboronic acid grafted sodium alginate (Alg-PBA) polymer networks, resulting in the facile preparation of healing, injectable, and antimicrobial MXene hydrogels (AgNPs/MXene/GG/Alg-PBA).147 This MXene hydrogel can be used for wearable human–computer interaction and high-performance human health monitoring for potential rehabilitation and diagnosis of cardiovascular and muscle-related diseases, as well as highly effective wound therapies that significantly accelerate wound healing. Epidermal sensors based on MXene's flexible conductive hydrogels show unlimited potential, with the promise of integrating diagnosis, detection and therapy in the future.
4.5 Bone regeneration
Due to the poor self-healing ability of bone defects, the repair of bone defects remains one of the most challenging problems in clinical practice. Currently, the application of biomaterials and bio-scaffolds implanted into the defect site to promote the healing of bone defects is the main therapeutic approach for bone defect repair. In recent years, the emergence of two-dimensional materials has brought new opportunities for biomaterials. As a ceramic-based two-dimensional nanomaterial with unique physicochemical properties, such as electrical conductivity, hydrophilicity, antimicrobial, and photothermal effects, MXenes have a very broad application prospect in bone defect biomaterials (Table 6).153| MXene composition | Application form | Bioactive property | Animal models | Ref. |
|---|---|---|---|---|
| (Ti2AlN)/polycaprolactone | Scaffold | Hydrophilicity, cytocompatibility, mechanical properties, cell adhesion ability and osteogenic differentiation ability | Rat tibial bone defect model and rabbit maxillofacial bone defect model | 164 |
| Nb2C, bioactive glass scaffolds (BGS) | Scaffold | Photothermal properties, inhibit tumor cell proliferation, promote vascular regeneration as well as bone regeneration | Bone malignancy model and bone defect model of SD rats | 165 |
| Ti3C2Tx, β-TCP, Zn and Sr ions | 3D-printed scaffold | ROS scavenging ability, NIR responsivity, enhanced mechanical properties, supports new bone mineralization and bone regeneration | Rat cranial defect model | 156 |
| Ti3C2Tx, polycaprolactone (PCL) | Electrospinning PCL membranes scaffold | Conductivity, cell adhesion, antibacterial | — | 166 |
| Ti3C2, self-assembled silk fibroin methacrylate (SF-MA) | 3D-printed theragenerative scaffolds | Photothermal anti-osteosarcoma ablation and bone regeneration | — | 158 |
| Nano-hydroxyapatite particles (n-HA), MXene nanosheets and g-C3N4 | Scaffold | Photodynamic and photothermal effects, osteogenic differentiation and inhibits the proliferation of bone tumor cells | Saos-2 tumor-bearing mouse model | 167 |
| Ti3C2, bioactive glass (BG) | 3D-printing scaffold | Photothermal conversion, bone-tumor killing and bone-tissue regeneration | Saos-2 bone tumor model in BALB/c nude mice | 168 |
| Ti3C2, hydroxyapatite and sodium alginate | Scaffold | High mechanical strength, biocompatibility, promoted mineralization, bone regeneration | Rat calvarial defect model | 169 |
| Mo2Ti2C3 MXene, acrylamidemonomer, deacetylated chitosan, glacial acetic acid and gelatin | Hydrogel | Biocompatibility, electrical conductivity, viscosity enhancement and promotion of osteogenic differentiation | Calvarial bone defects mode of mice | 155 |
| Ti3AlC2, Regenerated silk fibroin (RSF) | Hydrogel | Re-establishment of the electrical microenvironment for bone regeneration | Calvarial defect model of SD rats | 56 |
| Ti3C2Tx, dexamethasone (Dex), poly(N-isopropylacrylamide)-co-N-(hydroxymethyl) acrylamide hydrogel | Hydrogel | Photothermal effect, drug release and anti-apoptosis of bone-derived mesenchymal stem cells | Calvarial defects of SD rats | 160 |
| GelMA/β-TCP/sodium alginate (Sr2+)/MXene (Ti3C2) | Hydrogel scaffolds | Photothermal antibacterial, bone regeneration | S. aureus-infected mandible defects of rats | 170 |
| Ti3C2 nanosheets, gelatin-based nanocomposite hydrogels | Hydrogel | Biodegradable, mechanical, ROS scavenging, and osteogenic inducing properties | Critical-sized calvarial defect repair of SD rats | 171 |
| Ti3C2Tx Nanosheets | Nanosheet | Thermal effect, antibacterial activity, inhibit the expression of inflammation factors, induce the expression of pro-angiogenic factors | Skull defect model of rats | 18 |
| Poly(lactic acid) (PLA), Ti3C2Tz | Nanocomposite membranes | High mechanical strength, enhance the adhesion, proliferation, and osteogenic differentiation | — | 161 |
| Multilayered Ti3C2Tx | Nanosheets | Cytocompatible, osteoinductivity and bone regeneration activity | Calvarial defect model of rats | 162 |
| Ti3C2Tx, ultralong hydroxyapatite nanowires (UHAPNWs) | Nanocomposite membranes | Mechanical properties, biocompatibility and osteoinductivity | Calvarial defect mode of rat | 163 |
The repair and regeneration of bone defects is a complex and continuous process involving various internal and external environmental factors. The work of Qu et al. revealed that photoactivated MXene (Ti3C2Tx) nanosheets had positive effects on promoting both bone and soft tissue regeneration, and further investigated the detailed effects and potential mechanisms of photoactivated MXene on tissue regeneration.18 The photoactivated MXene exhibited good thermal effect and strong antibacterial activity, inhibited the expression of inflammatory factors and methicillin-resistant Staphylococcus aureus (MRSA) infection, and induced the expression of pro-angiogenic factors and soft tissue wound repair. Photoactivated MXene can also regulate osteogenic differentiation of adipose-derived stem cells (ADSCs) by activating heat shock protein 70 (HSP70) through the ERK signaling pathway to promote bone tissue repair. This work revealed the development of bioactive MXene photothermal activation as an effective strategy for the simultaneous regeneration of bone and soft tissue (Fig. 9A). Based on the above findings, a new idea based on MXene was provided for bone tissue repair. In order to further optimize the performance of MXene to enhance its osteogenic activity, the researchers sought to endow MXene with different functions through different modification strategies to cope with the complex pathological environment.
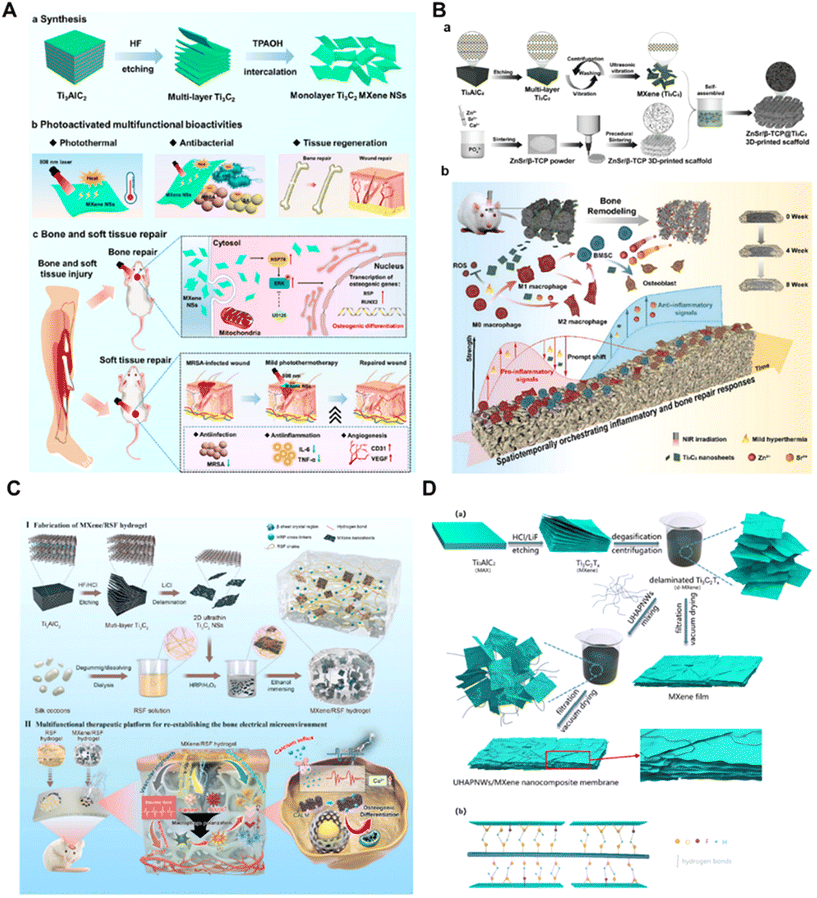 | ||
Fig. 9 MXene-based applications in bone regeneration. (A) Synthesis process, multifunctional bioactivities, and the application of photoactivated MXene nanosheets in bone and soft tissue regeneration.18 Reproduced from ref. 18 with permission from American Chemical Society, copyright 2023. (B) Schematic illustration of the fabrication process (a) and their applications (enhance osteogenesis by spatiotemporally orchestrating inflammatory and bone repair responses) (b) of Ti3C2Tx MXene-decorated 3D-printed ceramic scaffold.156 Reproduced from ref. 156 with permission from Wiley-VCH, copyright 2024. (C) Schematic showing the fabrication and application of multifunctional electroactive hydrogels for the diversified treatment of bone defects.56 Reproduced from ref. 56 with permission from KeAi, copyright 2022. (D) (a) Schematic illustration of the fabrication of the UHAPNWs/MXene nanocomposite membrane. (b) Schematic diagram of the interaction between UHAPNWs and MXene, involving the hydrogen bonds formed by terminated surface groups of MXene (–F, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O or –OH) and hydroxyl in UHAPNWs.163 Reproduced from ref. 163 with permission from Elsevier, copyright 2021. O or –OH) and hydroxyl in UHAPNWs.163 Reproduced from ref. 163 with permission from Elsevier, copyright 2021. | ||
For larger bone defects, the application of MXene-based materials as biomaterial scaffolds in defect repair and bone regeneration has attracted much attention.154 MXene (Ti2AlN)/PCL composite bone repair scaffolds were successfully prepared using 3D printing technology for in situ maxillofacial bone defect repair.155 The results of rat tibial bone defect repair experiments and rabbit maxillofacial bone defect repair experiments showed that the PCL@Ti2AlN scaffolds promoted new bone formation, and the scaffolds could induce new bone formation and in situ regeneration of bone defects in vivo, with good clinical applications. Wang et al. constructed a multifunctional 3D mimetic bone scaffold by rationally integrating photon-responsive 2D ultrathin niobium carbide (Nb2C) MXene nanosheets (NSs) into a 3d-printed bone mimetic scaffold (NBGS) for osteosarcoma treatment.155 The integrated 2D Nb2C-MXene NSs had a specific photon response in the NIR-II biological window with high tissue penetration depth to efficiently kill bone cancer cells, and calcium and phosphate released during scaffold degradation could promote the mineralization of new bone tissue. Huang et al. modified conventional β-tricalcium phosphate-based scaffolds by ultrathin MXene-Ti3C2 modification and Zn2+/Sr2+ ion substitution, which resulted in excellent reactive oxygen species scavenging, near-infrared responsiveness, enhanced mechanical properties, and precise temporal immunomodulation of the bone healing process by the smart 3D scaffold-enhanced bone regeneration in the rat model of cranial bone defects (Fig. 9B).156 In the work of Diedkova et al. biocompatible and biodegradable nanofibrous scaffolds were prepared from polycaprolactone (PCL) by electrostatic spinning, and the MXene coatings formed a suitable environment for cell attachment and proliferation, with a mild antimicrobial effect, and possessed good structural, chemical, electrical and biological properties.157 A bifunctional thermally regenerative 3D aerogel composite scaffold was developed by hybridization of photocrosslinked silk fibroin (SF) biopolymers with MXene (Ti3C2) 2D nanosheets by Pektas et al. This scaffold could mediate the growth and proliferation of preosteogenic cell lines in vitro, and had a strong photothermal effect under remote irradiation with near-infrared laser light, while significantly stimulating bone mineral deposition on the surface of the scaffold.158 By constructing specific biomaterial scaffolds according to the cause of bone defects and endowing them with more comprehensive biological properties, these scaffolds could reproduce the important features of bone and mimic the function of natural bone tissues, thus further accelerating the repair process of bone defects and regenerating damaged tissues.159
Various physical and chemical properties of MXenes, such as the osteogenic differentiation ability which promotes cell growth and bone regeneration in the damaged area after bone defects, and the good antimicrobial ability of MXene led by Ti3C2, combined with the unique near-infrared thermal effect, can be introduced into the design of composites for the repair of bone defects as additive materials represented by the form of hydrogels. Hu et al. prepared an electroactive hydrogel based on regenerated silk fibroin (RSF) and bioencapsulated MXene to promote effective bone regeneration.56 This MXene/RSF hydrogel could also be used as a piezoresistive pressure sensor, which activated Ca2+/CALM signaling and promoted direct osteogenesis under electrical stimulation, modulating the immune microenvironment and neovascularization, thus re-establishing the electrical microenvironment for bone regeneration (Fig. 9C). Wang et al. added Mo2Ti2C3 MXene to hydrogels to improve mechanical strength and electrical conductivity.155 Mo2Ti2C3 MXene hydrogels have properties such as biocompatibility, electrical conductivity, surface functionalization, chemical cross-linking, elastic modulus, and viscosity, and good osteogenic capacity, which promotes the osteogenic genes and osteogenic proteins in vitro. Animal models of cranial bone defects show that Mo2Ti2C3 MXene hydrogel promotes osteogenesis and neurogenesis in bone defects in vivo. Chen et al. demonstrated superior osteogenic properties using a biocompatible dexamethasone (Dex)-loaded MXene-poly(N-isopropylacrylamide)-co-N-(hydroxymethyl) acrylamide hydrogel, which was capable of supersensitive release of Dex under NIR irradiation at ≈42 °C.160 Mild heat and supersensitive release of Dex synergistically counteracted apoptosis of bone-derived mesenchymal stem cells and osteogenic differentiation. MXene-based hydrogels are incredible candidates for bone repair applications that hold significant promise for biomedical applications due to their unique properties, including large surface area, tunable chemistry, and compatibility with living organisms.
In addition, MXenes can exist in various forms such as thin films and nanocomposites, and the rich functional group structure on their surface gives them the potential to carry drugs, growth factors, further expanding their application prospects in bone defect repair.153 In another work, Chen et al. prepared biocompatible Ti3C2Tz-reinforced polylactic acid (PLA) nanocomposite membranes with high biocompatibility, and the optimized nanocomposite membranes showed a 33% increase in ultimate tensile strength compared with the pure PLA membranes.161 The incorporation of Ti3C2Tz enhanced the biological properties of the membranes, and facilitated the adherence, proliferation, and osteogenic differentiation of mouse preosteoblasts in vitro. Zhang et al. used flexible freestanding Ti3C2Tx MXene films to evaluate the cytocompatibility and osteoinductive capacity of Ti3C2Tx MXene in vitro and investigated the host tissue response and guided bone regeneration capacity of the material in vivo.162 In addition, Fu et al. prepared free-standing UHAPNWs/MXene nanocomposite membranes by introducing different weight ratios of ultra-long hydroxyapatite nanowires (UHAPNWs) into MXene to explore their potential in bone regeneration (Fig. 9D).163 The incorporation of UHAPNWs improved mechanical properties and hydrophilicity, and enhanced cell adhesion, proliferation and osteogenic differentiation. Furthermore, UHAPNWs/MXene nanocomposite membrane quantitatively and qualitatively effectively enhanced bone tissue formation in rat cranial bone defects, a novel inorganic composite material for bone tissue regeneration.
In order to fully utilize MXene-based capabilities in the field of bone repair, further research is necessary to thoroughly evaluate its compatibility with biological systems, refine its manufacturing methods, design more sophisticated methods to enhance its functionality, and encourage collaboration between different disciplines to promote its practical application.
4.6 Other applications
Numerous studies have shown that MXene-based materials possess a range of favorable biological, physical and chemical properties. These properties include biocompatibility, antimicrobial properties, drug loading and antioxidant capacity, large specific surface area, tunable size, NIR absorption, metal conductivity and abundant surface functional groups. Based on these properties, MXenes also have potential applications in muscle tissue engineering, nerve regeneration, drug delivery, and biosensors, and related studies have emerged (Table 7).| MXene composition | Synthesis strategy | Application | Animal models | Ref. |
|---|---|---|---|---|
| Ti3C2Tx | LiF/HCl etch-assisted sonication | Promoting angiogenesis and skeletal muscle regeneration | The tibial anterior muscle defects model in SD rats | 17 |
| Nb2C MXene-functionalized hydrogel (OPTN) | HF etching and TPAOH intercalation | Promote skeletal muscle regeneration and functional restoration | A rat tibialis anterior muscle defect model | 172 |
| Sweat-stable MXene epidermal electrode (Ti3C2Tx) | LiF/HCl etch-assisted sonication | An all-in-one muscle theranostic platform | — | 173 |
| MOF-Ti3C2Tx | LiF/HCl etch-assisted sonication | Artificial muscles | — | 174 |
| ECM-Ti3C2 hydrogel | LiF/HCl etch-assisted sonication | Improve myocardial infarction and promote heart tissue regeneration | Mouse myocardial infarction model | 175 |
| Ti2C-cryogel | 10% HF etching | Repair of myocardial infarction | Myocardial infarction model in SD rats | 186 |
| MXene-functionalized collagen biomaterials (Ti3C2Tx) | LiF/HCl etch-assisted sonication | Cardiac tissue engineering | — | 187 |
| Ti3C2Tx-based artificial nerve guidance (MXPLT) | LiF/HCl etch-assisted sonication | Enhanced regeneration of peripheral nerve injury | A SD rat model with a 10 mm sciatic nerve defect | 178 |
| Ti3C2Tx-PLGA | LiF/HCl etch-assisted sonication | Neural stem cell engineering | — | 179 |
| Ti3C2-SP | HF etching and TPAOH intercalation | Drug delivery and tumor therapy | 4T1 breast tumor-bearing mice model | 180 |
| DOX-Ti3C2Tx-DNA hydrogel | LiF/HCl etch-assisted sonication | Drug delivery and tumor therapy | The HeLa tumor-bearing animal model with BALB/c nude mice | 181 |
| G-MXene@F/C | — | Drug delivery and colon cancer treatment | CT26 colon tumor-bearing mice model | 188 |
| C-MOF/Ti3C2Tx | LiF/HCl etch-assisted sonication | Biosensor (uric acid (UA) and glucose (Glu) monitoring, muscle therapy) | — | 184 |
| Ti3C2Tx-based screen-printed electrode | HF etching, stirring and layering at 50 °C | Biosensor (continuous multi-component monitoring in whole blood) | — | 185 |
Muscles are soft, elastic tissues attached to bones or internal organs that possess the ability to contract and play an important role in generating voluntary movement. Regeneration of muscle tissue following severe mechanical injuries and muscle-related diseases remains a challenge. It has been demonstrated that conductive nanocomposites can effectively modulate the formation of skeletal muscle tissues in vivo and promote their regeneration. MXenes have excellent electrical conductivity and have the potential to be applied in skeletal muscle regeneration. Li et al. found that Ti3C2Tx could regulate the cellular microenvironment through anti-inflammatory and antioxidant activities, promoting angiogenesis, muscle fiber formation and skeletal muscle regeneration (Fig. 10A).17 Muscle pictures and immunohistochemical staining results of myosin heavy chain (MHC) from animal experiments of rat tibialis anterior muscle defects showed the superior ability of MXene to promote skeletal muscle regeneration. It has also been found that Nb2C MXene-functionalized hydrogel can restore skeletal muscle regeneration function by reducing ROS levels, attenuating inflammation, decreasing fibrosis, and promoting angiogenesis, neo-myotube formation and neurogenesis.172 Song et al. designed an integrated Ti3C2Tx-based epidermal electrode for diagnostic muscle therapy, which could comfortably record electromyography (EMG) signals as well as enable electrical stimulation and electrothermal therapy for diagnostic muscle therapy.173 In addition, based on MXene's excellent mechanical properties and metal conductivity, MXene can be engineered as an artificial muscle.174 Myocardial infarction (MI) is the deadliest of all cardiovascular diseases and is usually caused by coronary artery blockage, which results in damage to heart muscle cells due to ischemia. This process usually results in the release of excess ROS, leading to excessive oxidative stress. One of the promising strategies for the treatment of MI is the design and fabrication of electrically conductive cardiac patches to facilitate the electrophysiological coupling of the cardiac patch to the host tissue. MXenes, with their excellent electrical conductivity, biocompatibility, antioxidant properties and hydrophilicity, are a promising candidate for the preparation of myocardial patches. Ren et al. developed an injectable antioxidant/conductive hydrogel (E-MXene) by combining MXene (Ti3C2) with cardiomyocyte extracellular matrix (ECM) hydrogels (Fig. 10B).175 E-MXene hydrogel has high moisture and porosity, which enabled MXene to be uniformly distributed in the ECM to build a suitable conductive network that could fully utilize the antioxidant and conductive properties of MXene. After E-MXene was injected into the infarcted area of the mouse heart, the cardiac function of MI mice was improved by decreasing the excessive production of ROS to improve the tissue microenvironment, and enhancing the electrical signal conduction between the fibrotic scar tissues and the normal cardiac tissues.
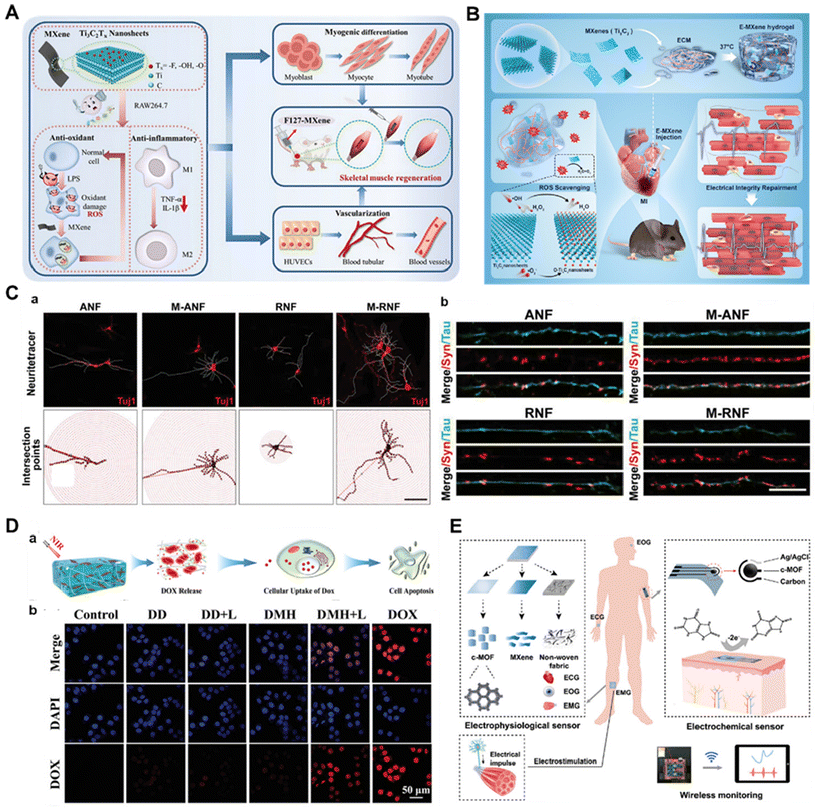 | ||
| Fig. 10 MXene-based materials in other applications. (A) Schematic diagram of MXene regulating the myogenic differentiation and skeletal muscle regeneration.17 Reproduced from ref. 17 with permission from Wiley-VCH, copyright 2023. (B) Schematic illustration of the preparation and application of E-MXene hydrogel in mice MI.175 Reproduced from ref. 175 with permission from Elsevier, copyright 2024. (C) (a) The contours of 2D structures (top) and concentric rings for Sholl analysis (bottom) of NSC-derived neurons cultured on ANF, M-ANF, RNF, and M-RNF in differentiation medium for 7 days (scale bar, 100 μm) and (b) representative high-magnification fluorescence images of synaptophysin (red) and Tau (green) labeling after differentiation of NSCs for 14 days on the four substrates (scale bar, 10 μm).179 Reproduced from ref. 179 with permission from Wiley-VCH, copyright 2023. (D) (a) Schematic diagram of the NIR irradiation-triggered DOX release from the DMH hydrogel to induce cancer cell apoptosis and (b) CLSM images of HeLa cells incubated with different buffer solution.181 Reproduced from ref. 181 with permission from Wiley-VCH, copyright 2022. (E) Schematic diagram of an integrated biosensor for electrophysiological and electrochemical sensing. Data are transmitted wirelessly.184 Reproduced from ref. 184 with permission from Wiley-VCH, copyright 2023. | ||
Neuronal cells are electrically active and bioelectrical in nature, and adhesion-mediated signaling pathways are critical for regulating neuronal cell function and determining their fate, especially cell spreading, proliferation, and differentiation.176 Therefore, the development of bioactive materials with both electrical conductivity and cell adhesion properties has great potential for promoting neural tissue regeneration. MXenes have excellent electrical conductivity, which will facilitate neurite growth and enhance neuronal development. Secondly, MXene's high hydrophilicity and rich surface functional groups facilitate the optimization of the bioadhesive properties and surface functionalization of the substrate, which can promote the adhesion of neural cells as well as modulate their behavior.177 The above properties of MXene also mean it has unlimited potential in the field of neural tissue engineering. Wang et al. created a novel neural conduit with microchannel guidance (MXPLT) by 4D printing, which consisted of a monolayer of MXene Ti3C2Tx nanosheets and poly(L-lactide-co-trimethylene carbonate) (PLATMC).178 The printed nerve conduit had shape memory and automatically rolled into a tubular structure at 37 °C, firmly wrapping around the severed ends of the defective nerves. Its high ductility and elasticity could withstand nerve deformation caused by muscle movement. Micro-channels constructed through 4D printing accelerated cell migration during the nerve repair process, resulting in faster and better repair of long-distance nerve defects. The addition of MXenes improved the electrical conductivity of the nerve conduit, which created a complete electrical pathway during the nerve repair process and ultimately repaired peripheral nerve damage. In another study, Ti3C2Tx MXene was used as a coating nanomaterial for aligned/randomly aligned poly (lactic acid) (PLLA) nanofibers, named M-ANF or M-RNF, respectively, and it was found that the addition of MXene promoted an increase in the number of neuromasts, their lengths, branching complexity, and synaptic densities (Fig. 10C).179
Drug delivery platforms can be used to improve the stability and specific targeting ability of drugs in biological environments, as well as to improve the stability of chemotherapy in vivo, by polymers or targeting functional agents. MXene-based biomaterials offer numerous advantages in building drug delivery platforms. First, due to their unique lamellar structure, very large specific surface area, and nano-size, MXene-based biomaterials provide abundant anchor sites for efficient loading and delivery of therapeutic molecules and allow for easy distribution and accumulation of drugs at the therapeutic site. In addition, utilizing the physicochemical, electronic, and optical properties of MXene-based biomaterials will effectively integrate the drug delivery platforms with other bioimaging and therapeutic modalities (e.g., PTT, PDT, CDT, SDT, etc.), which will facilitate the development of smart drug delivery platforms. Han et al. developed Ti3C2 MXene nanosheets with soybean phospholipid (SP) modification as effective drug carriers for loading and delivery of DOX and as synergistic therapeutic agents against cancer.180 These Ti3C2 MXenes not only possessed a drug loading capacity of up to 211.8%, but also exhibited pH-responsive and NIR-triggered on-demand drug release properties. To improve therapeutic efficacy and reduce drug dosage, delivery efficiency can be further enhanced by using DNA, peptides or antibodies for surface functionalization or hydrogel encapsulation. He et al. established a promising and efficient photothermal-chemotherapeutic synergistic cancer treatment platform by integrating DNA hydrogels with Ti3C2Tx-based MXene as a photothermal agent and DOX as a loaded chemotherapeutic drug.181 Under NIR irradiation, the temperature increase induced by the photothermal MXene nanosheets triggered a reversible transformation of the DOX-loaded MXene-DNA hydrogel from gel to solution, during which the DNA double-stranded cross-linking structure released DOX, resulting in highly efficient localized cancer therapy (Fig. 10D). Upon removal of NIR irradiation, the DNA double-stranded structure and hydrogel matrix were reformed, and recombination of free DOX and adaptive transformation of the hydrogel could be achieved. From the CLSM images of HeLa cells, it was observed that there was DOX release and uptake only in the cells of the material-treated group under NIR irradiation (Fig. 10D).
The lamellar structure of MXenes facilitates modification by various surface terminations, providing a rich variety of surface-active sites that can be used as a biosensing platform with high responsiveness to various external stimuli. In addition, MXenes have excellent conductivity, which facilitates low noise and high selectivity in biosensing reactions. Therefore, these unique properties make MXene a highly promising biosensor material. MXene-based wearable sensors with flexibility, gas sensors, glucose sensors and fluidic biosensors have been well-studied and demonstrated to have good specificity, selectivity, and sensitivity.182,183 For example, Lin et al. designed an electrophysiological MOF/MXene electrode using highly conductive MXene and a porous conductive metal–organic framework (c-MOF, Ni3(HITP)2), which could realize a multifunctional biosensor integrating the electrochemical detection of uric acid (UA) and glucose (Glu) in sweat, electrophysiological signal acquisition, and electrical stimulation for treatment (Fig. 10E).184 MOF/MXene electrodes enabled muscle therapy through electrical stimulation with lower electrode/skin interface impedance, higher stability and signal-to-noise ratio (SNR), and high charge storage capacity (CSC). In another study, an electrochemical microfluidic biosensor based on Ti3C2Tx MXene was designed that can be applied to hemodialysis, enabling continuous detection of urea, uric acid and creatinine levels in human blood.185 These efforts pave new paths for MXene applications in biomedical and sensing fields.
5. Conclusion and perspective
MXenes have attracted much attention in biomedicine and include many transition metal carbides and nitrides. Novel 2D MXene materials have been widely studied in the biomedical field due to their diverse chemical structures, tunable physicochemical properties, and desirable bioactivities. Despite the achievements of MXene-based biomaterials in biomedicine, research in this field is still in its infancy and there are many critical issues that need to be addressed.MXene-based biomaterials used in the biomedical field face higher demands, requiring both good biosafety and corresponding biological functions in vivo, and at the same time their degradation efficiency in vivo needs to be considered to avoid negative impacts on the organism. Although many preliminary studies have demonstrated the generally low cytotoxicity of MXenes currently used in biomedicine, most of these studies have been conducted at in vitro levels or in small animals such as mice at low doses, and there is a lack of systematic evaluations of the long-term biosafety of MXenes in large animal models (e.g., rabbits, dogs, pigs, and primates). In addition, there are fewer studies on the degradability of MXene-based biomaterials, and only some MXenes have been reported to be degradable. For biodegradable MXenes, the short-term toxicity in vivo and effects of their degradation products should be evaluated. For non-biodegradable MXenes, the long-term toxicity in vivo should be evaluated to understand their biodistribution and clearance rate in the body. More data on the safety and degradability of MXenes in vivo are needed in the future.
Putting aside the safety and degradability of MXenes in vivo, there are still many limitations for their translation from the laboratory to the clinic. On the one hand, the cellular uptake behavior, biological interactions, and the specific mechanism of action of MXenes on tissue regeneration are not yet clear. On the other hand, in order to cope with more complex physiological environments in the human body, it is necessary to establish different large-scale animal models or even clinical trials to evaluate the role played by MXene-based bioactive materials in the organism. In the future, more research is needed to investigate the mechanism of action of MXenes on cells and tissues by establishing more animal models to lay the foundation for clinical conversion. In addition, surface modification strategies can further enrich the biological functions of MXenes, and the current surface modification strategies for MXenes are classified as small-molecule modification, macromolecule modification, bioactive nanoparticle modification, and surface polymerization modification. The research on small-molecule modification of MXenes mainly focuses on the modification by silane agents, and more modification strategies of MXenes by other bioactive small molecules are expected to be explored in the future.
MXene-based biomaterials offer a new lease of life for tumor ablation as well as infection treatment, overcoming the limited therapeutic efficacy of certain therapies alone and the development of bacterial resistance. In addition, the fabrication of ultrasmall MXene-based QDs and their applications in therapy and regeneration require further attention. MXene QDs can be modified or coated on their surface with different materials or targeting tools to prolong the circulation and accumulate at the site of interest for imaging, enabling the diagnosis of diseases. The most promising future direction is to build multifunctional MXene-based therapeutic systems, using multiple therapeutic approaches to achieve diagnostic, combined therapeutic integration.
The unstable and highly oxidizable characteristics of MXenes limit their use in biomedical applications. The surface of MXenes is usually composed of –OH, –O or –F, making the surface hydrophilic, which together with the high surface charge leads to rapid aggregation and precipitation of few-layer MXenes in biological media, thus affecting their physicochemical properties and biological functions. In addition, although MXenes disperse very well in water, they are easily oxidized by oxygen in water and are very unfavorable for storage. Therefore, surface chemical modification of MXenes and surface engineering strategies could address the issues of stability, storage and sterilization, which are very important for clinical translation. Macromolecular modification for MXenes is mainly based on non-covalent interactions; e.g., surface modification with macromolecules such as PEG, BSA, and HA can modulate the hydrophilicity and biocompatibility of MXene solutions but suffers from poor stability. In the modification of MXenes with bioactive nanoparticles (e.g., Fe3O4, MnOx, Au, Pt, etc.), although this can broaden their potential for therapeutic (PTT, PDT, CDT, etc.) and diagnostic imaging applications, the cytotoxicity of the metal ions needs to be considered. Surface polymerization modification methods (e.g., modification by PDA, PLGA, Ppy, etc.) have the problems of difficult operation and complicated steps. In the future, optimization or development of new modification methods of MXenes in various ways to give them more stable properties and more comprehensive functions is expected.
Currently, the research on MXenes in the biomedical field is still focused on tumor therapy and antibacterial therapy, which mainly rely on PTT and PDT of MXenes, but most of the research on the photothermal performance of MXenes is focused on NIR-I. To cope with the treatment of deeper tissues, there is an urgent need for the development of MXene composites that can respond to NIR-II light. For the research of MXenes in tissue regeneration, there are more studies in the field of skin regeneration as well as bone regeneration, while there are fewer related reports in the field of muscle tissue engineering and nerve regeneration. In addition, most of the MXenes used in biomedical research are Ti/Nb-based carbons, mainly focusing on Ti3C2Tx, while there are fewer reports on other types of MXenes (e.g., Ta4C3 and V2C, etc.). In the future, the development of various types of different MXenes with broader applications in the biomedical field is expected.
MXene-based biomaterials offer new perspectives for biomedicine, and in the future, inexpensive mass production methods and new strategies for easy surface modification should be developed. We believe that as the scientific community continues to explore MXenes, these challenges will soon be solved, and the field of MXene composites will thrive in biomedicine with even more rational technological innovations.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was jointly supported by the National Natural Science Foundation of China (grant No. 52172288, 52302354), Funds for International Cooperation and Exchange of the National Natural Science Foundation of China (grant No. 52411540232), Natural Science Basic Research Project of Shaanxi Province of China (grant No. 2020JQ-564), Young Talent Support Plan of Xi'an Jiaotong University of China (grant No. QY6J003), and the Fundamental Research Funds for the Central Universities.References
- N. Zahin, R. Anwar and D. Tewari, et al., Nanoparticles and its biomedical applications in health and diseases: special focus on drug delivery, Environ. Sci. Pollut. Res., 2020, 27(16), 19151–19168 CrossRef CAS PubMed.
- C. L. Tan, X. H. Cao and X. J. Wu, et al., Recent Advances in Ultrathin Two-Dimensional Nanomaterials, Chem. Rev., 2017, 117(9), 6225–6331 CrossRef CAS.
- T. T. Hu, X. Mei and Y. J. Wang, et al., Two-dimensional nanomaterials: fascinating materials in biomedical field, Sci. Bull., 2019, 64(22), 1707–1727 CrossRef CAS.
- M. Qiu, W. X. Ren and T. Jeong, et al., Omnipotent phosphorene: a next-generation, two-dimensional nanoplatform for multidisciplinary biomedical applications, Chem. Soc. Rev., 2018, 47(15), 5588–5601 RSC.
- Q. Wang and D. O'Hare, Recent Advances in the Synthesis and Application of Layered Double Hydroxide (LDH) Nanosheets, Chem. Rev., 2012, 112(7), 4124–4155 CrossRef CAS.
- C. H. Lui, L. Liu and K. F. Mak, et al., Ultraflat graphene, Nature, 2009, 462(7271), 339–341 CrossRef CAS PubMed.
- S. Liu, X. T. Pan and H. Y. Liu, Two-Dimensional Nanomaterials for Photothermal Therapy, Angew. Chem., Int. Ed., 2020, 59(15), 5890–5900 CrossRef CAS PubMed.
- Y. J. Chen, Y. K. Wu and B. B. Sun, et al., Two-Dimensional Nanomaterials for Cancer Nanotheranostics, Small, 2017, 13(10), 1603446 CrossRef.
- M. Naguib, M. Kurtoglu and V. Presser, et al., Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2, Adv. Mater., 2011, 23(37), 4248–4253 CrossRef CAS.
- B. Soundiraraju and B. K. George, Two-Dimensional Titanium Nitride (Ti2N) MXene: Synthesis, Characterization, and Potential Application as Surface-Enhanced Raman Scattering Substrate, ACS Nano, 2017, 11(9), 8892–8900 CrossRef CAS PubMed.
- W. Feng, R. Y. Wang and Y. D. Zhou, et al., Ultrathin Molybdenum Carbide MXene with Fast Biodegradability for Highly Efficient Theory-Oriented Photonic Tumor Hyperthermia, Adv. Funct. Mater., 2019, 29(22), 1901942 CrossRef.
- H. Huang, C. H. Dong and W. Feng, et al., Biomedical engineering of two-dimensional MXenes, Adv. Drug Delivery Rev., 2022, 184, 114178 CrossRef CAS PubMed.
- D. Saha, J. Dalmieda and V. Patel, Surface-Modified MXenes: Simulation to Potential Applications, ACS Appl. Electron. Mater., 2023, 5(6), 2933–2955 CrossRef CAS.
- A. P. Liu, Y. Liu and G. J. Liu, et al., Engineering of surface modified Ti3C2Tx MXene based dually controlled drug release system for synergistic multitherapies of cancer, Chem. Eng. J., 2022, 448, 137691 CrossRef CAS.
- J. P. Ma, L. Zhang and B. Lei, Multifunctional MXene-Based Bioactive Materials for Integrated Regeneration Therapy, ACS Nano, 2023, 17(20), 19526–19549 CrossRef CAS.
- H. Lin, Y. Chen and J. L. Shi, Insights into 2D MXenes for Versatile Biomedical Applications: Current Advances and Challenges Ahead, Adv. Sci., 2018, 5(10), 1500518 Search PubMed.
- T. Li, J. P. Ma and W. S. Wang, et al., Bioactive MXene Promoting Angiogenesis and Skeletal Muscle Regeneration through Regulating M2 Polarization and Oxidation Stress, Adv. Healthcare Mater., 2023, 12(4), 2201862 CrossRef CAS.
- X. Qu, Y. Guo and C. Xie, et al., Photoactivated MXene Nanosheets for Integrated Bone–Soft Tissue Therapy: Effect and Potential Mechanism, ACS Nano, 2023, 17(8), 7229–7240 CrossRef CAS PubMed.
- A. M. Jastrzebska, A. Szuplewska and T. Wojciechowski, et al., Studies on cytotoxicity of delaminated Ti3C2 MXene, J. Hazard. Mater., 2017, 339, 1–8 CrossRef CAS PubMed.
- P. Ganguly, A. Breen and S. C. Pillai, Toxicity of Nanomaterials: Exposure, Pathways, Assessment, and Recent Advances, ACS Biomater. Sci. Eng., 2018, 4(7), 2237–2275 CrossRef CAS PubMed.
- T. Y. Wang, X. Y. Sun and X. Guo, et al., Ultraefficiently Calming Cytokine Storm Using Ti3C2Tx MXene, Small Methods, 2021, 5(5), 2001108 CrossRef CAS.
- G. Y. Liu, J. H. Zou and Q. Y. Tang, et al., Surface Modified Ti3C2 MXene Nanosheets for Tumor Targeting Photothermal/Photodynamic/Chemo Synergistic Therapy, ACS Appl. Mater. Interfaces, 2017, 9(46), 40077–40086 CrossRef CAS.
- G. K. Nasrallah, M. Al-Asmakh and K. Rasool, et al., Ecotoxicological assessment of Ti3C2Tx (MXene) using a zebrafish embryo model, Environ. Sci.: Nano, 2018, 5(4), 1002–1011 RSC.
- H. Y. Huang, R. M. Jiang and Y. L. Feng, et al., Recent development and prospects of surface modification and biomedical applications of MXenes, Nanoscale, 2020, 12(3), 1325–1338 RSC.
- J. Liu, W. Lu and X. F. Lu, et al., Versatile Ti3C2Tx MXene for free-radical scavenging, Nano Res., 2022, 15(3), 2558–2566 CrossRef PubMed.
- S. Iravani and R. S. Varma, MXene-Based Composites as Nanozymes in Biomedicine: A Perspective, Nano-Micro Lett., 2022, 14, 213 CrossRef CAS.
- W. Feng, X. G. Han and H. Hu, et al., 2D vanadium carbide MXenzyme to alleviate ROS-mediated inflammatory and neurodegenerative diseases, Nat. Commun., 2021, 12(1), 2203 CrossRef CAS PubMed.
- H. Hu, H. Huang and L. L. Xia, et al., Engineering vanadium carbide MXene as multienzyme mimetics for efficient in ischemic stroke treatment, Chem. Eng. J., 2022, 440, 135810 CrossRef CAS.
- M. A. Tokmedash and J. H. Min, Designer Micro-/Nanocrumpled MXene Multilayer Coatings Accelerate Osteogenesis and Regulate Macrophage Polarization, ACS Appl. Mater. Interfaces, 2024, 16(17), 21415–21426 CrossRef.
- T. Liao, Z. Chen and Y. Kuang, et al., Small-size Ti3C2Tx MXene nanosheets coated with metal-polyphenol nanodots for enhanced cancer photothermal therapy and anti-inflammation, Acta Biomater., 2023, 159, 312–323 CrossRef CAS.
- Q. Q. Qiao, J. Y. Wang and B. J. Li, et al., Ti3C2Tx MXene nanosheet-based drug delivery/cascaded enzyme system for combination cancer therapy and anti-inflammation, Appl. Mater. Today, 2024, 38, 102215 CrossRef.
- Z. J. Li, W. Wei and M. M. Zhang, et al., Cryptotanshinone-Doped Photothermal Synergistic MXene@PDA Nanosheets with Antibacterial and Anti-Inflammatory Properties for Wound Healing, Adv. Healthcare Mater., 2023, 12(28), 2301060 CrossRef CAS.
- D. X. Xu, Z. D. Li and L. S. Li, et al., Insights into the Photothermal Conversion of 2D MXene Nanomaterials: Synthesis, Mechanism, and Applications, Adv. Funct. Mater., 2020, 30(47), 2000712 CrossRef CAS.
- K. Chaudhuri, M. Alhabeb and Z. X. Wang, et al., Highly Broadband Absorber Using Plasmonic Titanium Carbide (MXene), ACS Photonics, 2018, 5(3), 1115–1122 CrossRef CAS.
- X. T. Jiang, A. V. Kuklin and A. Baev, et al., Two-dimensional MXenes: From morphological to optical, electric, and magnetic properties and applications, Phys. Rep., 2020, 848, 1–58 CrossRef CAS.
- J. Zeng, D. Goldfeld and Y. N. Xia, A Plasmon-Assisted Optofluidic (PAOF) System for Measuring the Photothermal Conversion Efficiencies of Gold Nanostructures and Controlling an Electrical Switch, Angew. Chem., Int. Ed., 2013, 52(15), 4169–4173 CrossRef CAS.
- J. T. Robinson, S. M. Tabakman and Y. Y. Liang, et al., Ultrasmall Reduced Graphene Oxide with High Near-Infrared Absorbance for Photothermal Therapy, J. Am. Chem. Soc., 2011, 133(17), 6825–6831 CrossRef CAS.
- H. Lin, X. G. Wang and L. D. Yu, et al., Two-Dimensional Ultrathin MXene Ceramic Nanosheets for Photothermal Conversion, Nano Lett., 2017, 17(1), 384–391 CrossRef CAS PubMed.
- Y. P. Wang, J. Yang and Y. H. Ma, et al., Ta3C4 Nanosheets as a Novel Therapeutic Platform for Photothermal-Driven ROS Scavenging and Immune Activation against Antibiotic-Resistant Infections in Diabetic Wounds, Small, 2024, 20(36), 2400741 CrossRef CAS.
- H. Lin, S. S. Gao and C. Dai, et al., A Two-Dimensional Biodegradable Niobium Carbide (MXene) for Photothermal Tumor Eradication in NIR-I and NIR-II Biowindows, J. Am. Chem. Soc., 2020, 142(23), 10567–10567 CrossRef CAS PubMed.
- Q. Xue, H. J. Zhang and M. S. Zhu, et al., Photoluminescent Ti3C2 MXene Quantum Dots for Multicolor Cellular Imaging, Adv. Mater., 2017, 29(15), 1604847 CrossRef.
- A. S. Sharbirin, S. Roy and T. T. Tran, et al., Light-emitting Ti2N (MXene) quantum dots: synthesis, characterization and theoretical calculations, J. Mater. Chem. C, 2022, 10(16), 6508–6514 RSC.
- S. Akhtar, J. Singh and T. T. Tran, et al., Synthesis and optical properties of light-emitting V2N MXene quantum dots, Opt. Mater., 2023, 138, 113660 CrossRef CAS.
- X. Yan, J. F. Ma and K. X. Yu, et al., Highly green fluorescent Nb2C MXene quantum dots for Cu2+ ion sensing and cell imaging, Chin. Chem. Lett., 2020, 31(12), 3173–3177 CrossRef CAS.
- S. Z. Li, J. F. Ma and X. L. Zhao, et al., Highly fluorescence Ta4C3 MXene quantum dots as fluorescent nanoprobe for heavy ion detection and stress monitoring of fluorescent hydrogels, Chin. Chem. Lett., 2022, 33(4), 1850–1854 CrossRef CAS.
- A. V. Mohammadi, J. Rosen and Y. Gogotsi, The world of two-dimensional carbides and nitrides (MXenes), Science, 2021, 372(6547), 1165–1179 Search PubMed.
- S. R. Ye, H. C. Zhang and H. Y. Lai, et al., MXene: A wonderful nanomaterial in antibacterial, Front. Bioeng. Biotechnol., 2024, 12, 1338549 Search PubMed.
- K. Rasool, M. Helal and A. Ali, et al., Antibacterial Activity of Ti3C2Tx MXene, ACS Nano, 2016, 10(3), 3674–3684 CrossRef CAS.
- R. P. Pandey, P. A. Rasheed and T. Gomez, et al., Effect of Sheet Size and Atomic Structure on the Antibacterial Activity of Nb-MXene Nanosheets, ACS Appl. Nano Mater., 2020, 3(11), 11372–11382 CrossRef CAS.
- A. A. Shamsabadi, M. S. Gh and B. Anasori, et al., Antimicrobial Mode-of-Action of Colloidal Ti3C2Tx MXene Nanosheets, ACS Sustainable Chem. Eng., 2018, 6(12), 16586–16596 CrossRef.
- K. Y. Zheng, S. Li and L. Jing, et al., Synergistic Antimicrobial Titanium Carbide (MXene) Conjugated with Gold Nanoclusters, Adv. Healthcare Mater., 2020, 9(19), 2001007 CrossRef CAS.
- M. Rosales, A. Garcia and V. M. Fuenzalida, et al., Unprecedented arsenic photo-oxidation behavior of few- and multi-layer Ti3C2Tx nano-sheets, Appl. Mater. Today, 2020, 20, 100769 CrossRef.
- X. F. Liu, H. C. Xie and S. Zhuo, et al., Ru(II) Complex Grafted Ti3C2Tx MXene Nano Sheet with Photothermal/Photodynamic Synergistic Antibacterial Activity, Nanomaterials, 2023, 13(6), 0958 CrossRef CAS PubMed.
- S. Iravani, E. N. Zare and P. Makvandi, Multifunctional MXene-Based Platforms for Soft and Bone Tissue Regeneration and Engineering, ACS Biomater. Sci. Eng., 2024, 10(4), 1892–1909 CrossRef CAS PubMed.
- Y. Fu, J. Zhang and H. Lin, et al., 2D titanium carbide(MXene) nanosheets and 1D hydroxyapatite nanowires into free standing nanocomposite membrane: in vitro and in vivo evaluations for bone regeneration, Mater. Sci. Eng., C, 2021, 118, 111367 CrossRef CAS.
- Z. C. Hu, J. Q. Lu and T. W. Zhang, et al., Piezoresistive MXene/Silk fibroin nanocomposite hydrogel for accelerating bone regeneration by Re-establishing electrical microenvironment, Bioact. Mater., 2023, 22, 1–17 CAS.
- L. P. Nan, Z. Lin and F. Wang, et al., Ti3C2Tx MXene-Coated Electrospun PCL Conduits for Enhancing Neurite Regeneration and Angiogenesis, Front. Bioeng. Biotechnol., 2022, 10, 850650 CrossRef PubMed.
- M. Mozafari and M. Soroush, Surface functionalization of MXenes, Mater. Adv., 2021, 2(22), 7277–7307 RSC.
- M. M. Ashebo, N. N. Liu and F. Yu, et al., Surface functional modification of Nb2CTx MXene for high performance capacitive deionization, Sep. Purif. Technol., 2024, 343, 127125 CrossRef.
- J. J. Ji, L. F. Zhao and Y. F. Shen, et al., Covalent stabilization and functionalization of MXene via silylation reactions with improved surface properties, FlatChem, 2019, 17, 100128 CrossRef CAS.
- H. Riazi, M. Anayee and K. Hantanasirisakul, et al., Surface Modification of a MXene by an Aminosilane Coupling Agent, Adv. Mater. Interfaces, 2020, 7(6), 1902008 CrossRef CAS.
- Y. Xia, T. Mathis and M. Q. Zhao, et al., Thickness independent capacitance of vertically aligned liquid crystalline MXenes, Abstr. Pap. Am. Chem. Soc., 2018, 256, 409–412 Search PubMed.
- Y. Lei, Y. Cui and Q. Huang, et al., Facile preparation of sulfonic groups functionalized Mxenes for efficient removal of methylene blue, Ceram. Int., 2019, 45(14), 17653–17661 CrossRef CAS.
- Z. Y. Jin, Y. F. Fang and X. X. Wang, et al., Ultra-efficient electromagnetic wave absorption with ethanol-thermally treated two-dimensional Nb2CTx nanosheets, J. Colloid Interface Sci., 2019, 537, 306–315 CrossRef CAS PubMed.
- S. Kumar, Y. J. Lei and N. H. Alshareef, et al., Biofunctionalized two-dimensional Ti3C2 MXenes for ultrasensitive detection of cancer biomarker, Biosens. Bioelectron., 2018, 121, 243–249 CrossRef CAS PubMed.
- W. Luo, H. X. Liu and X. Liu, et al., Biocompatibility nanoprobe of MXene N-Ti3C2 quantum dot/Fe3+ for detection and fluorescence imaging of glutathione in living cells, Colloids Surf., B, 2021, 201, 111631 CrossRef CAS.
- W. J. Zhang, S. W. Li and V. Vijayan, et al., ROS- and pH-Responsive Polydopamine Functionalized Ti3C2Tx MXene-Based Nanoparticles as Drug Delivery Nanocarriers with High Antibacterial Activity, Nanomaterials, 2022, 12(24), 4392 CrossRef CAS PubMed.
- L. Chen, X. Y. Dai and W. Feng, et al., Biomedical Applications of MXenes: From Nanomedicine to Biomaterials, Acc. Mater. Res., 2022, 3(8), 785–798 CrossRef CAS.
- J. Liu, H. B. Zhang and X. Xie, et al., Multifunctional, Superelastic, and Lightweight MXene/Polyimide Aerogels, Small, 2018, 14(45), 1802479 CrossRef.
- Z. Ling, C. E. Ren and M. Q. Zhao, et al., Flexible and conductive MXene films and nanocomposites with high capacitance, Proc. Natl. Acad. Sci. U. S. A., 2014, 111(47), 16676–16681 CrossRef CAS.
- F. Shahzad, M. Alhabeb and C. B. Hatter, et al., Electromagnetic interference shielding with 2D transition metal carbides (MXenes), Science, 2016, 353(6304), 1137–1140 CrossRef CAS PubMed.
- N. Li, X. L. Wang and Y. Liu, et al., Ultrastretchable, Self-Adhesive and conductive MXene nanocomposite hydrogel for body-surface temperature distinguishing and electrophysiological signal monitoring, Chem. Eng. J., 2024, 483, 149303 CrossRef CAS.
- B. F. Guo, Y. J. Wang and C. F. Cao, et al., Large-Scale, Mechanically Robust, Solvent-Resistant, and Antioxidant MXene-Based Composites for Reliable Long-Term Infrared Stealth, Adv. Sci., 2024, 11(17), 2309392 CrossRef CAS.
- B. Zhou, H. Yin and C. Dong, et al., Biodegradable and Excretable 2D W1.33Ci-MXene with Vacancy Ordering for Theory-Oriented Cancer Nanotheranostics in Near-Infrared Biowindow, Adv. Sci., 2021, 8(24), 2101043 CrossRef CAS PubMed.
- R. J. Liang, Y. S. Li and M. F. Huo, et al., Triggering Sequential Catalytic Fenton Reaction on 2D MXenes for Hyperthermia-Augmented Synergistic Nanocatalytic Cancer Therapy, ACS Appl. Mater. Interfaces, 2019, 11(46), 42917–42931 CrossRef CAS.
- Z. Liu, H. Lin and M. L. Zhao, et al., 2D Superparamagnetic Tantalum Carbide Composite MXenes for Efficient Breast-Cancer Theranostics, Theranostics, 2018, 8(6), 1648–1664 CrossRef CAS PubMed.
- C. Dai, H. Lin and G. Xu, et al., Biocompatible 2D Titanium Carbide (MXenes) Composite Nanosheets for pH-Responsive MRI-Guided Tumor Hyperthermia, Chem. Mater., 2017, 29(20), 8637–8652 CrossRef CAS.
- C. Dai, Y. Chen and X. X. Jing, et al., Two-Dimensional Tantalum Carbide (MXenes) Composite Nanosheets for Multiple Imaging-Guided Photothermal Tumor Ablation, ACS Nano, 2017, 11(12), 12696–12712 CrossRef CAS.
- S. Q. Wen, Y. L. Xiong and S. F. Cai, et al., Plasmon-enhanced photothermal properties of Au@Ti3C2Tx nanosheets for antibacterial applications, Nanoscale, 2022, 14(44), 16572–16580 RSC.
- W. Tang, Z. Dong and R. Zhang, et al., Multifunctional Two-Dimensional Core–Shell MXene@Gold Nanocomposites for Enhanced Photo–Radio Combined Therapy in the Second Biological Window, ACS Nano, 2019, 13(1), 284–294 CrossRef CAS PubMed.
- J. Wang, X. Q. Wei and X. Y. Wang, et al., Plasmonic Au Nanoparticle@Ti3C2Tx Heterostructures for Improved Oxygen Evolution Performance, Inorg. Chem., 2021, 60(8), 5890–5897 CrossRef CAS PubMed.
- T. Wojciechowski, A. Rozmyslowska-Wojciechowska and G. Matyszczak, et al., Ti3C2 MXene Modified with Ceramic Oxide and Noble Metal Nanoparticles: Synthesis, Morphostructural Properties, and High Photocatalytic Activity, Inorg. Chem., 2019, 58(11), 7602–7614 CrossRef CAS.
- H. Q. Geng, Z. J. Li and Q. Liu, et al., Boosting the peroxidase-like activity of Pt nanozymes by a synergistic effect of Ti3C2 nanosheets for dual mechanism detection, Dalton Trans., 2022, 51(31), 11693–11702 RSC.
- R. Yang, S. Q. Wen and S. F. Cai, et al., MXene-based nanomaterials with enzyme-like properties for biomedical applications, Nanoscale Horiz., 2023, 8(10), 1333–1344 RSC.
- L. Y. Zong, H. X. Wu and H. Lin, et al., A polyoxometalate-functionalized two-dimensional titanium carbide composite MXene for effective cancer theranostics, Nano Res., 2018, 11(8), 4149–4168 CrossRef CAS.
- J. Li, Z. Li and X. Liu, et al., Interfacial engineering of Bi2S3/Ti3C2Tx MXene based on work function for rapid photo-excited bacteria-killing, Nat. Commun., 2021, 12(1), 1224 CrossRef CAS.
- H. Zheng, S. Q. Wang and F. Cheng, et al., Bioactive anti-inflammatory, antibacterial, conductive multifunctional scaffold based on MXene@CeO2 nanocomposites for infection-impaired skin multimodal therapy, Chem. Eng. J., 2021, 424, 130148 CrossRef CAS.
- Q. H. Yang, H. H. Yin and T. M. Xu, et al., Engineering 2D Mesoporous Silica@MXene-Integrated 3D-Printing Scaffolds for Combinatory Osteosarcoma Therapy and NO-Augmented Bone Regeneration, Small, 2020, 16(14), 1906814 CrossRef CAS PubMed.
- Q. Qing, X. Y. Shi and S. Z. Hu, et al., Synchronously Enhanced Removal Ability and Stability of MXene through Biomimetic Modification, Langmuir, 2023, 39(27), 9453–9467 CrossRef CAS PubMed.
- M. Steenackers, A. Küller and S. Stoycheva, et al., Structured and Gradient Polymer Brushes from Biphenylthiol Self-Assembled Monolayers by Self-Initiated Photografting and Photopolymerization (SIPGP), Langmuir, 2009, 25(4), 2225–2231 CrossRef CAS.
- P. Xiao, C. J. Wan and J. C. Gu, et al., 2D Janus Hybrid Materials of Polymer-Grafted Carbon Nanotube/Graphene Oxide Thin Film as Flexible, Miniature Electric Carpet, Adv. Funct. Mater., 2015, 25(16), 2428–2435 CrossRef CAS.
- N. Tao, D. P. Zhang and X. L. Li, et al., Near-infrared light-responsive hydrogels peroxide-decorated MXene-initiated polymerization, Chem. Sci., 2019, 10(46), 10765–10771 RSC.
- J. Chen, K. Chen and D. Tong, et al., CO2 and temperature dual responsive “Smart” MXene phases, Chem. Commun., 2015, 51(2), 314–317 RSC.
- C. Y. Ding, J. M. Liang and Z. Zhou, et al., Photothermal enhanced enzymatic activity of lipase covalently immobilized on functionalized Ti3C2Tx nanosheets, Chem. Eng. J., 2019, 378, 122205 CrossRef CAS.
- Q. Q. Qiao, J. Y. Wang and K. Long, et al., A cascaded enzyme system based on the catalase-like activity of Ti3C2Tx MXene nanosheets for the efficient combination cancer therapy, Nano Today, 2024, 54, 102059 CrossRef CAS.
- J. F. Li, Z. Y. Li and X. M. Liu, et al., Interfacial engineering of Bi2S3/Ti3C2Tx MXene based on work function for rapid photo-excited bacteria-killing, Nat. Commun., 2021, 12(1), 1224 CrossRef CAS PubMed.
- M. Soleymaniha, M. A. Shahbazi and A. R. Rafieerad, et al., Promoting Role of MXene Nanosheets in Biomedical Sciences: Therapeutic and Biosensing Innovations, Adv. Healthcare Mater., 2019, 8(1), 1801137 CrossRef.
- L. Zhou, F. M. Wu and J. H. Yu, et al., Titanium carbide (Ti3C2Tx) MXene: A novel precursor to amphiphilic carbide-derived graphene quantum dots for fluorescent ink, light-emitting composite and bioimaging, Carbon, 2017, 118, 50–57 CrossRef CAS.
- S. Y. Lu, L. Z. Sui and Y. Liu, et al., White Photoluminescent Ti3C2 MXene Quantum Dots with Two-Photon Fluorescence, Adv. Sci., 2019, 6(9), 1801470 CrossRef PubMed.
- Q. R. Fu, R. Zhu and J. B. Song, et al., Photoacoustic Imaging: Contrast Agents and Their Biomedical Applications, Adv. Mater., 2019, 31(6), 1805875 CrossRef.
- L. H. V. Wang and S. Hu, Photoacoustic Tomography: In Vivo Imaging from Organelles to Organs, Science, 2012, 335(6075), 1458–1462 CrossRef CAS.
- V. K. Deb and U. Jain, Ti3C2 (MXene), an advanced carrier system: role in photothermal, photoacoustic, enhanced drugs delivery and biological activity in cancer therapy, Drug Delivery Transl. Res., 2024, 14, 3009–3031 CrossRef.
- Y. L. Zhu, Z. Wang and R. X. Zhao, et al., Pt Decorated Ti3C2Tx MXene with NIR-II Light Amplified Nanozyme Catalytic Activity for Efficient Phototheranostics, ACS Nano, 2022, 16(2), 3105–3118 CrossRef CAS.
- W. A. Kalender, X-ray computed tomography, Phys. Med. Biol., 2006, 51(13), R29–R43 CrossRef.
- H. Lin, Y. W. Wang and S. S. Gao, et al., Theranostic 2D Tantalum Carbide (MXene), Adv. Mater., 2018, 30(4), 1703284 CrossRef.
- K. K. Feng, C. L. Li and R. Xiong, et al., Multifunctional Theranostic 2D Vanadium Carbidel for Enhanced Cancer Immunotherapy, Adv. Funct. Mater., 2024, 2406529 CrossRef CAS.
- E. Terreno, D. Delli Castelli and A. Viale, et al., Challenges for Molecular Magnetic Resonance Imaging, Chem. Rev., 2010, 110(5), 3019–3042 CrossRef CAS.
- A. Sundaram, J. S. Ponraj and C. Wang, et al., Engineering of 2D transition metal carbides and nitrides MXenes for cancer therapeutics and diagnostics, J. Mater. Chem. B, 2020, 8(23), 4990–5013 RSC.
- V. Neubertova, O. Guselnikova and Y. Yamauchi, et al., Covalent functionalization of Ti3C2Tx MXene flakes with Gd-DTPA complex for stable and biocompatible MRI contrast agent, Chem. Eng. J., 2022, 446, 136939 CrossRef CAS.
- G. H. Yang, J. L. Zhao and S. Z. Yi, et al., Biodegradable and photostable Nb2C MXene quantum dots as promising nanofluorophores for metal ions sensing and fluorescence imaging, Sens. Actuators, B, 2020, 309, 127735 CrossRef CAS.
- Y. Zhu, Z. Wang and R. Zhao, et al., Pt Decorated Ti3C2Tx MXene with NIR-II Light Amplified Nanozyme Catalytic Activity for Efficient Phototheranostics, ACS Nano, 2022, 16(2), 3105–3118 CrossRef CAS.
- B. Lu, S. Hu and D. Wu, et al., Ionic liquid exfoliated Ti3C2Tx MXene nanosheets for photoacoustic imaging and synergistic photothermal/chemotherapy of cancer, J. Mater. Chem. B, 2022, 10(8), 1226–1235 RSC.
- Z. C. Wang, H. M. Li and W. Y. She, et al., 3-Bromopyruvate-Loaded Ti3C2 MXene/Cu2O Nanosheets for Photoacoustic Imaging-Guided and Hypoxia-Relieving Enhanced Photothermal/Chemodynamic Therapy, Anal. Chem., 2023, 95(2), 1710–1720 CAS.
- Y. Z. Wu, X. R. Song and W. Xu, et al., NIR-Activated Multimodal Photothermal/Chemodynamic/Magnetic Resonance Imaging Nanoplatform for Anticancer Therapy by Fe(II) Ions Doped MXenes (Fe-Ti3C2), Small, 2021, 17(33), 2101705 CrossRef CAS.
- S. Zada, W. H. Dai and Z. Kai, et al., Algae Extraction Controllable Delamination of Vanadium Carbide Nanosheets with Enhanced Near-Infrared Photothermal Performance, Angew. Chem., Int. Ed., 2020, 59(16), 6601–6606 CrossRef CAS PubMed.
- X. F. Tang, Y. H. Zhu and D. L. Duan, et al., Anionic Solid Solution MXene for Low-Dosage NIR-II Tumor Photothermal Therapy, Adv. Funct. Mater., 2023, 33(42), 2305965 CrossRef CAS.
- Y. H. Zhu, X. F. Tang and Q. Liu, et al., Metallic Carbonitride MXene Based Photonic Hyperthermia for Tumor Therapy, Small, 2022, 18(22), 2200646 CrossRef CAS.
- R. X. Zhao, Y. L. Zhu and L. L. Feng, et al., Architecture of Vanadium-Based MXene Dysregulating Tumor Redox Homeostasis for Amplified Nanozyme Catalytic/Photothermal Therapy, Adv. Mater., 2024, 36(2), 2307115 CrossRef CAS.
- X. S. Li, F. Liu and D. P. Huang, et al., Nonoxidized MXene Quantum Dots Prepared by Microexplosion Method for Cancer Catalytic Therapy, Adv. Funct. Mater., 2020, 30(24), 2000308 CrossRef CAS.
- P. Z. Xiong, X. D. Wei and L. Zhou, et al., Near-Infrared Light-Triggered MXene Nanocomposite for Tumor-Specific Mild Photothermal-Enhanced Chemodynamic Therapy, Adv. Funct. Mater., 2024, 2405124 CrossRef CAS.
- S. Zhang, L. N. Wu and W. Q. Shi, et al., Nanoheterojunction Engineering Enables NIR-II-Triggered Photonic Hyperthermia and Pyroelectric Catalysis for Tumor-Synergistic Therapy, Adv. Funct. Mater., 2023, 33(29), 2302360 CrossRef CAS.
- G. Q. Li, X. Y. Zhong and X. W. Wang, et al., Titanium carbide nanosheets with defect structure for photothermal-enhanced sonodynamic therapy, Bioact. Mater., 2022, 8, 409–419 CAS.
- M. Zhang, D. Y. Yang and C. H. Dong, et al., Two-Dimensional MXene-Originated Nanosonosensitizer Generation for Augmented and Synergistic Sonodynamic Tumor Nanotherapy, ACS Nano, 2022, 16(6), 9938–9952 CrossRef CAS.
- G. Zhang, Y. Lu and J. Song, et al., A multifunctional nano-hydroxyapatite/MXene scaffold for the photothermal/dynamic treatment of bone tumours and simultaneous tissue regeneration, J. Colloid Interface Sci., 2023, 652, 1673–1684 CrossRef CAS.
- X. K. Ju, J. Kong and G. H. Qi, et al., A wearable electrostimulation-augmented ionic-gel photothermal patch doped with MXene for skin tumor treatment, Nat. Commun., 2024, 15(1), 762 CrossRef CAS.
- H.-Y. Xia, B.-Y. Li and Y.-T. Ye, et al., Transition Metal Oxide-Decorated MXenes as Drugless Nanoarchitectonics for Enriched Nanocatalytic Chemodynamic Treatment, Adv. Healthcare Mater., 2024, 13(10), 2303582 CrossRef CAS.
- F. Duan, Q. Jia and G. Liang, et al., Schottky Junction Nanozyme Based on Mn-Bridged Co-Phthalocyanines and Ti3C2Tx Nanosheets Boosts Integrative Type I and II Photosensitization for Multimodal Cancer Therapy, ACS Nano, 2023, 17(12), 11290–11308 CrossRef CAS.
- X. J. He, Y. Lv and Y. L. Lin, et al., Platinum Nanoparticles Regulated V2C MXene Nanoplatforms with NIR-II Enhanced Nanozyme Effect for Photothermal and Chemodynamic Anti-Infective Therapy, Adv. Mater., 2024, 36(25), 2400366 CrossRef CAS PubMed.
- X. P. Zhao, Y. Chen and R. X. Niu, et al., NIR Plasmonic Nanozymes: Synergistic Enhancement Mechanism and Multi-Modal Anti-Infection Applications of MXene/MOFs, Adv. Mater., 2024, 36(8), 2307839 CrossRef CAS PubMed.
- H. C. Wang, N. Mu and Y. Q. He, et al., Ultrasound-controlled MXene-based Schottky heterojunction improves anti-infection and osteogenesis properties, Theranostics, 2023, 13(5), 1669–1683 CrossRef CAS.
- C. Yang, Y. Luo and H. Lin, et al., Niobium Carbide MXene Augmented Medical Implant Elicits Bacterial Infection Elimination and Tissue Regeneration, ACS Nano, 2021, 15(1), 1086–1099 CrossRef CAS.
- M. A. Unal, F. Bayrakdar and L. Fusco, et al., 2D MXenes with antiviral and immunomodulatory properties: A pilot study against SARS-CoV-2, Nano Today, 2021, 38, 101136 CrossRef CAS PubMed.
- X. A. Qiu, L. X. Nie and P. Liu, et al., From hemostasis to proliferation: Accelerating the infected wound healing through a comprehensive repair strategy based on GA/OKGM hydrogel loaded with MXene@TiO2 nanosheets, Biomaterials, 2024, 308, 122548 CrossRef CAS.
- X. J. He, Y. A. Qian and C. L. Wu, et al., Entropy-Mediated High-Entropy MXenes Nanotherapeutics: NIR-II-Enhanced Intrinsic Oxidase Mimic Activity to Combat Methicillin-Resistant Infection, Adv. Mater., 2023, 35(26), 2211432 CrossRef CAS.
- Y. Q. He, X. Liu and J. Lei, et al., Bioactive VS4-based sonosensitizer for robust chemodynamic, sonodynamic and osteogenic therapy of infected bone defects, J. Nanobiotechnol., 2024, 22, 31 CrossRef CAS PubMed.
- B. Li, W. Yang and R. Shu, et al., Antibacterial and Angiogenic (2A) Bio-Heterojunctions Facilitate Infectious Ischemic Wound Regeneration via an Endogenous–Exogenous Bistimulatory Strategy, Adv. Mater., 2024, 36(6), 2307613 CrossRef CAS.
- X. S. Sun, X. J. He and Y. Zhu, et al., Valence-switchable and biocatalytic vanadium-based MXene nanoplatform with photothermal-enhanced dual enzyme-like activities for anti-infective therapy, Chem. Eng. J., 2023, 451, 138985 CrossRef CAS.
- X. Zhou, Z. Y. Wang and Y. K. Chan, et al., Infection Micromilieu-Activated Nanocatalytic Membrane for Orchestrating Rapid Sterilization and Stalled Chronic Wound Regeneration, Adv. Funct. Mater., 2022, 32(7), 2109469 CrossRef CAS.
- M. Kharaziha, A. Baidya and N. Annabi, Rational Design of Immunomodulatory Hydrogels for Chronic Wound Healing, Adv. Mater., 2021, 33(39), 2100176 CrossRef CAS.
- G. C. Gurtner, S. Werner and Y. Barrandon, et al., Wound repair and regeneration, Nature, 2008, 453(7193), 314–321 CrossRef CAS.
- Y. Li, J. Wang and Y. Wang, et al., Advanced electrospun hydrogel fibers for wound healing, Composites, Part B, 2021, 223, 109101 CrossRef CAS.
- J. Ma, S. Li and L. Zhang, et al., Oxidativestress-scavenging thermo-activated MXene hydrogel for rapid repair of MRSA impaired wounds and burn wounds, Mater. Today, 2024, 80, 139–155 CrossRef CAS.
- X. Q. Jiang, J. P. Ma and K. K. Xue, et al., Highly Bioactive MXene-M2-Exosome Nanocomposites Promote Angiogenic Diabetic Wound Repair through Reconstructing High Glucose-Derived Immune Inhibition, ACS Nano, 2024, 18(5), 4269–4286 CrossRef CAS PubMed.
- X. Yang, C. Q. Zhang and D. W. Deng, et al., Multiple Stimuli-Responsive MXene-Based Hydrogel as Intelligent Drug Delivery Carriers for Deep Chronic Wound Healing, Small, 2022, 18(5), 2104368 CrossRef CAS.
- Y. Li, R. Z. Fu and Z. G. Duan, et al., Artificial Nonenzymatic Antioxidant MXene Nanosheet-Anchored Injectable Hydrogel as a Mild Photothermal-Controlled Oxygen Release Platform for Diabetic Wound Healing, ACS Nano, 2022, 16(5), 7486–7502 CrossRef CAS PubMed.
- W. Y. Dai, R. Shu and F. Yang, et al., Engineered Bio-Heterojunction Confers Extra- and Intracellular Bacterial Ferroptosis and Hunger-Triggered Cell Protection for Diabetic Wound Repair, Adv. Mater., 2024, 36(9), 2305277 CrossRef CAS.
- M. K. Li, Y. F. Zhang and L. S. Y. Lian, et al., Flexible Accelerated-Wound-Healing Antibacterial MXene-Based Epidermic Sensor for Intelligent Wearable Human-Machine Interaction, Adv. Funct. Mater., 2022, 32(47), 2208141 CrossRef CAS.
- X. R. Kang, Y. Li and Z. G. Duan, et al., A Mxene@TA/Fe dual-nanozyme composited antifouling hydrogel for burn wound repair, Chem. Eng. J., 2023, 476, 146420 CrossRef CAS.
- Z. T. Yang, H. Zheng and H. Y. Yin, et al., Niobium carbide doped ROS/temperature dual-responsive multifunctional hydrogel for facilitating MRSA-infected wound healing, Chem. Eng. J., 2023, 471, 144634 CrossRef CAS.
- Y. N. Ding, L. Xu and S. N. Chen, et al., Mxene composite fibers with advanced thermal management for inhibiting tumor recurrence and accelerating wound healing, Chem. Eng. J., 2023, 459, 141529 CrossRef CAS.
- Y. C. Gan, B. Liang and Y. Gong, et al., Mxene-based mild hyperthemia microneedle patch for diabetic wound healing, Chem. Eng. J., 2024, 481, 148592 CrossRef CAS.
- X. K. Ju, J. Kong and G. H. Qi, et al., Photoelectric-driven conductive composite ionogel patch for effective wound healing, eScience, 2024, 4(2), 100223 CrossRef.
- X. P. Chen, Y. P. Cheng and H. Wu, Recent trends in bone defect repair and bone tissue regeneration of the two-dimensional material MXene, Ceram. Int., 2023, 49(12), 19578–19594 CrossRef CAS.
- M. A. Khabisi, F. Shirini and K. Shirini, et al., Additively manufactured MAX- and MXene-composite scaffolds for bone regeneration-recent advances and future perspectives, Colloids Surf., B, 2023, 225, 113282 CrossRef CAS.
- H. Y. Wang, Y. C. Hsu and C. E. Wang, et al., Conductive and Enhanced Mechanical Strength of Mo2Ti2C3 MXene-Based Hydrogel Promotes Neurogenesis and Bone Regeneration in Bone Defect Repair, ACS Appl. Mater. Interfaces, 2024, 16(14), 17208–17218 CrossRef CAS.
- B. Z. Huang, S. S. Li and S. M. Dai, et al., Ti3C2Tx MXene-Decorated 3D-Printed Ceramic Scaffolds for Enhancing Osteogenesis by Spatiotemporally Orchestrating Inflammatory and Bone Repair Responses, Adv. Sci., 2024, 2400229 CrossRef CAS.
- K. Diedkova, A. D. Pogrebnjak and S. Kyrylenko, et al., Polycaprolactone-MXene Nanofibrous Scaffolds for Tissue Engineering, ACS Appl. Mater. Interfaces, 2023, 15(11), 14033–14047 CAS.
- H. K. Pektas, Y. Demidov and A. Ahvan, et al., MXene-Integrated Silk Fibroin-Based Self-Assembly-Driven 3D-Printed Theragenerative Scaffolds for Remotely Photothermal Anti-Osteosarcoma Ablation and Bone Regeneration, ACS Mater. Au, 2023, 3(6), 711–726 CrossRef CAS PubMed.
- A. Maleki, M. Ghomi and N. Nikfarjam, et al., Biomedical Applications of MXene-Integrated Composites: Regenerative Medicine, Infection Therapy, Cancer Treatment, and Biosensing, Adv. Funct. Mater., 2022, 32(34), 2203430 CrossRef CAS.
- Y. Chen, W. W. Liu and S. J. Wan, et al., Superior Synergistic Osteogenesis of MXene-Based Hydrogel through Supersensitive Drug Release at Mild Heat, Adv. Funct. Mater., 2024, 34(2), 2309191 CrossRef CAS.
- K. Chen, Y. H. Chen and Q. H. Deng, et al., Strong and biocompatible poly(lactic acid) membrane enhanced by Ti3C2Tx (MXene) nanosheets for Guided bone regeneration, Mater. Lett., 2018, 229, 114–117 CrossRef CAS.
- J. B. Zhang, Y. Fu and A. C. Mo, Multilayered Titanium Carbide MXene Film for Guided Bone Regeneration, Int. J. Nanomed., 2019, 14, 10091–10102 CrossRef CAS.
- Y. Fu, J. B. Zhang and H. Lin, et al., 2D titanium carbide(MXene) nanosheets and 1D hydroxyapatite nanowires into free standing nanocomposite membrane: in vitro and in vivo, evaluations for bone regeneration, Mater. Sci. Eng., C, 2021, 118, 111367 CrossRef CAS PubMed.
- Z. M. Xu, Y. X. Zhang and H. Y. Dai, et al., 3D printed MXene (Ti2AlN)/polycaprolactone composite scaffolds for maxillofacial bone defect repair, J. Ind. Eng. Chem., 2022, 114, 536–548 CrossRef CAS.
- J. H. Yin, S. S. Pan and X. Guo, et al., Nb2C MXene-Functionalized Scaffolds Enables Osteosarcoma Phototherapy and Angiogenesis/Osteogenesis of Bone Defects (vol 13, 30, 2021), Nano-Micro Lett., 2022, 14, 30 CrossRef PubMed.
- K. Diedkova, A. D. Pogrebnjak and S. Kyrylenko, et al., Polycaprolactone–MXene Nanofibrous Scaffolds for Tissue Engineering, ACS Appl. Mater. Interfaces, 2023, 15(11), 14033–14047 CAS.
- G. N. Zhang, Y. Lu and J. B. Song, et al., A multifunctional nano-hydroxyapatite/MXene scaffold for the photothermal/dynamic treatment of bone tumours and simultaneous tissue regeneration, J. Colloid Interface Sci., 2023, 652, 1673–1684 CrossRef CAS.
- S. S. Pan, J. H. Yin and L. D. Yu, et al., 2D MXene-Integrated 3D-Printing Scaffolds for Augmented Osteosarcoma Phototherapy and Accelerated Tissue Reconstruction, Adv. Sci., 2020, 7(2), 1901511 CrossRef CAS PubMed.
- X. Mi, Z. Y. Su and Y. Fu, et al., 3D printing of Ti3C2-MXene-incorporated composite scaffolds for accelerated bone regeneration, Biomed. Mater., 2022, 17(3), 035002 CrossRef CAS.
- R. Nie, Y. Sun and H. X. Lv, et al., 3D printing of MXene composite hydrogel scaffolds for photothermal antibacterial activity and bone regeneration in infected bone defect models, Nanoscale, 2022, 14(22), 8112–8129 RSC.
- J. Zhao, T. H. Wang and Y. C. Zhu, et al., Enhanced osteogenic and ROS-scavenging MXene nanosheets incorporated gelatin-based nanocomposite hydrogels for critical-sized calvarial defect repair, Int. J. Biol. Macromol., 2024, 269, 131914 CrossRef CAS.
- H. Zheng, Z. T. Yang and L. Zhou, et al., Bioactive Nb2C MXene-Functionalized Hydrogel with Microenvironment Remodeling and Enhanced Neurogenesis to Promote Skeletal Muscle Regeneration and Functional Restoration, Small, 2024, 2310483 CrossRef CAS.
- D. K. Song, G. Ye and Y. Zhao, et al., An All-in-One, Bioderived, Air-Permeable, and Sweat-Stable MXene Epidermal Electrode for Muscle Theranostics, ACS Nano, 2022, 16(10), 17168–17178 CrossRef CAS PubMed.
- M. Garai, M. Mahato and S. Nam, et al., Metal Organic Framework-MXene Nanoarchitecture for Fast Responsive and Ultra-Stable Electro-Ionic Artificial Muscles, Adv. Funct. Mater., 2023, 33(10), 2212252 CrossRef CAS.
- X. Y. Ren, M. Xiao and J. Yang, et al., Injectable MXene conductive hydrogel improves myocardial infarction through scavenging ROS and repairing myocardium electrical integrity, Chem. Eng. J., 2024, 481, 148791 CrossRef CAS.
- W. H. Chooi and S. Y. Chew, Modulation of cell-cell interactions for neural tissue engineering: Potential therapeutic applications of cell adhesion molecules in nerve regeneration, Biomaterials, 2019, 197, 327–344 CrossRef CAS PubMed.
- J. Zou, J. Wu and Y. Z. Wang, et al., Additive-mediated intercalation and surface modification of MXene, Chem. Soc. Rev., 2022, 51(8), 3314–3314 RSC.
- Z. L. Wang, Y. Zheng and L. Qiao, et al., 4D-Printed MXene-Based Artificial Nerve Guidance Conduit for Enhanced Regeneration of Peripheral Nerve Injuries, Adv. Healthcare Mater., 2024, 2401093 CrossRef CAS.
- Y. D. Zhu, X. Y. Ma and L. P. Li, et al., Surface Functional Modification by Ti3C2Tx MXene on PLLA Nanofibers for Optimizing Neural Stem Cell Engineering, Adv. Healthcare Mater., 2023, 12(25), 2370149 CrossRef.
- X. X. Han, J. Huang and H. Lin, et al., 2D Ultrathin MXene-Based Drug-Delivery Nanoplatform for Synergistic Photothermal Ablation and Chemotherapy of Cancer, Adv. Healthcare Mater., 2018, 7(9), 1701394 CrossRef PubMed.
- P. P. He, X. X. Du and Y. Cheng, et al., Thermal-Responsive MXene-DNA Hydrogel for Near-Infrared Light Triggered Localized Photothermal-Chemo Synergistic Cancer Therapy, Small, 2022, 18(40), 2200263 CrossRef CAS.
- Q. U. Zahra, S. Ullah and F. Shahzad, et al., MXene-based aptasensors: Advances, challenges, and prospects, Prog. Mater. Sci., 2022, 129, 100967 CrossRef CAS.
- U. Amara, I. Hussain and M. Ahmad, et al., 2D MXene-Based Biosensing: A Review, Small, 2023, 19(2), 2205249 CrossRef CAS PubMed.
- X. Lin, D. Song and T. Shao, et al., A Multifunctional Biosensor via MXene Assisted by Conductive Metal–Organic Framework for Healthcare Monitoring, Adv. Funct. Mater., 2024, 34(11), 2311637 CrossRef CAS.
- J. Liu, X. T. Jiang and R. Y. Zhang, et al., MXene-Enabled Electrochemical Microfluidic Biosensor: Applications toward Multicomponent Continuous Monitoring in Whole Blood, Adv. Funct. Mater., 2019, 29(6), 1807326 CrossRef.
- G. L. Ye, Z. B. Wen and F. Wen, et al., Mussel-inspired conductive Ti2C-cryogel promotes functional maturation of cardiomyocytes and enhances repair of myocardial infarction, Theranostics, 2020, 10(5), 2047–2066 CrossRef CAS.
- G. A. Asaro, M. Solazzo and M. Suku, et al., MXene functionalized collagen biomaterials for cardiac tissue engineering driving iPSC-derived cardiomyocyte maturation, npj 2D Mater. Appl., 2023, 7, 44 CrossRef CAS PubMed.
- W. Yang, L. Wang and L. Fan, et al., Photothermal Responsive Microcarriers Encapsulated With Cangrelor and 5-Fu for Colorectal Cancer Treatment, Small Methods, 2024, 8(7), 2301002 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |
