Interchain interactions raised the photo-induced [LS] → [HS*] transition temperature to 78 K in a cyanide-bridged [FeIII2CoII] chain†
Wen-Jing
Jiang
ab,
Yin-Shan
Meng
 a,
Han-Han
Lu
a,
Hai-Lang
Zhu
a,
Qiang
Liu
a,
Chunying
Duan
a,
Han-Han
Lu
a,
Hai-Lang
Zhu
a,
Qiang
Liu
a,
Chunying
Duan
 a,
Hiroki
Oshio
a,
Hiroki
Oshio
 *a and
Tao
Liu
*a and
Tao
Liu
 a
a
aDepartment State Key Laboratory of Fine Chemicals, Frontier Science Center for Smart Materials, Dalian University of Technology, Linggong Rd., 116024 Dalian, China. E-mail: liutao@dlut.edu.cn; oshio@chem.tsukuba.ac.jp
bFujian Science & Technology Innovation Laboratory for Optoelectronic Information of China, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou, Fujian 350108, P. R. China
First published on 20th November 2024
Abstract
Bistable mixed valence compounds have thermodynamically accessible phases at certain temperatures, and the electron transfer switches the electronic configurations by applying external stimuli like heat and light. Thermally induced phase transition temperatures range widely, while the photo-induced state conversions need irradiation at very low temperatures, such as below 30 K, and the photo-induced metastable state relaxes rapidly at low temperatures. We prepared new mixed-valence compounds of [Fe(bipy)(CN)4]2[CoL2] (L = 4-[(1E)-2-phenyldiazenyl]pyridine for 1-papy and 4-(2-phenylethynyl)pyridine for 1-pepy) in which cyanide-bridged squared cores form corner-shared chains with substantial interchain π–π contacts. Mössbauser spectra revealed that 1-papy and 1-pepy are in the high-spin (HS) state [(FeIIILS)2CoIIHS] at 300 K and the low-spin (LS) state [FeIILSFeIIILSCoIIILS] at 78 K, confirming the occurrence of the electron transfer coupled spin transition (ETCST). Magnetic susceptibility measurements suggested their Tc values of 231 and 260 K, respectively. Photoirradiation (808 nm) for 1-papy and 1-pepy at 10 K induced the state conversion from the [LS] to the [HS*] state, and the metastable [HS]* state relaxed to the thermodynamically stable [LS] states at temperatures (Trelax) of 130 and 90 K, respectively. Furthermore, the [LS] states in 1-papy and 1-pepy were fully converted to the [HS*] states by light irradiation at 78 and 50 K, respectively. The X-ray structural analyses showed characteristic coordination bond lengths for the metal ions in each electronic state before and after light irradiation, but shortened intrachain πL⋯πL contact distances, from 3.726(4) to 3.688(4) Å, were observed for 1-papy upon the state conversion from the [LS] to the [HS*] state, despite the swollen cell volumes from 2479 to 2566 Å3, respectively. Photomagneto and structural studies suggest that the intermolecular interactions increase the light-induced state conversion and relaxation temperatures.
10th anniversary statementNo one, except for the Editor-in-Chief, could have predicted the current status of ICF when the journal started in 2014. Since its inception, I have witnessed this journal's growth as an associate editor, reader, and author. In the early days, there were fewer submissions, and their quality wasn't as high as it is now. We recognize now that many papers are submitted worldwide, and ICF has become a well-known journal that delivers high-quality chemistry. How has this happened? I believe the enthusiasm of Chinese chemists has contributed to this journal's success, and their passion has spread among us. I hope some of the ICF papers will seed new chemistry in the future.Hiroki Oshio, Dalian University of Technology |
Introduction
Photo-responsive molecular systems have the intrinsic advantages of high spatial and temporal resolutions for future intelligent molecular devices. They have attracted intense research interests from the viewpoints of molecular energy conversion,1 photocatalysts,2,3 and switching materials.4–10 Precisely controlled molecular structure and function are critical for developing on-demand molecular devices. Among the photo-responsive systems, LIESST (= light-induced excited spin state trapping) in spin crossover (SCO) complexes and electron transfer coupled spin transition (ETCST) in mixed-valence compounds have been extensively focused on because of their photo-switchable properties.11–20 This is due to their ultrafast photoresponse on a femtosecond timescale with highly efficient state conversions and excellent recyclability in solids.21–24 Moreover, the photoswitches, accompanied by changes in the spin state, oxidation numbers, charge distribution, and structure, potentially possess fascinating magnetic and charge ordering properties, second-harmonic generation (SHG), and magnetization-induced SHG by mechanical and optical stimuli.25–30A family of cyanide-bridged metal complexes derived from Prussian Blue Analogues (PBAs), formulated as MIIIB[MIIA(CN)6], has been extensively investigated since the discovery of the first photomagnet of K0.2CoIII1.4[FeII(CN)6]·6.9H20.31 It exhibited reversible photo-induced conversions between diamagnetic ([FeIILS(μ-CN)CoIIILS]) and ferromagnetic ([FeIIILS(μ-CN)CoIIHS]) phases, which were accompanied by electron transfers (ET) between hetero-valent metal ions. In this conversion, the thermally stable high-spin [HS] ([FeIIILSCoIIHS]) state turns into the low-spin [LS] ([FeIILSCoIIILS]) state with decreasing temperatures (Tc). The light-irradiation to the [LS] state converts it into the photo-induced metastable [HS*] state, usually at low temperatures (Tirrad), which is followed by thermal relaxation to the [LS] state by raising the temperature (Trelax). Among the three-dimensional PBAs, Co3[Os(CN)6]2·6H2O and Co3[W(CN)8]2(pyrimidine)4·6H2O showed relatively high thermal stability of the [HS*] states up to Trelax = 120 and 150 K, respectively.32,33 In contrast, discrete and low-dimensional compounds showed thermal transitions at wide temperature ranges of Tc = 220–300K, but the [HS*] states relaxed thermally to the [LS] states below Trelax = 100 K except for {[(pzTp)FeIII(CN)3]4[CoII(pz)3-CCH2OH]4[ClO4]4} (PzTp: pyrazole derivative) at 200 K.34 It should also be noted that the photoconversion of the [LS] to the [HS*] state needs irradiation at quite low temperatures (Tirrad < 30 K). This prompts us to establish rational strategies to prepare the systems with higher photoconversion ([LS] → [HS*]) and thermal relaxation ([HS*] → [LS]) temperatures.
The thermal and photo-induced ETs are accompanied by intra- and intermolecular structural changes, charge redistributions, and charge reorganization, affecting the temperatures of thermal and photo-induced state conversions.35–42 The energy barrier for ET in a mixed-valence compound relates to the difference in free energy, which is also affected by the reorganization energy.43,44 The thermally stable [HS] and light-induced metastable [HS]* states relax to the thermodynamically stable state ([LS]) as the temperatures are lowered and raised, respectively. It is expected that the larger ΔG* value raises the thermal relaxation temperature (Trelax) from the [HS]/[HS]* to the [LS] states and allows a higher photoconversion temperature (Tirrad) from the [LS] to the [HS*] state (Scheme 1). There are two ways to increase the ΔG* value: (1) a greater nuclear coordinate difference between the two configurations and (2) stabilized [HS] and [HS*] configurations. Each metal ion has typical coordination bond lengths for its oxidation and spin states, which do not allow variation of the characteristic nuclear coordinate in each configuration, excluding strategy (1). Therefore, we chose the second one. We introduced intermolecular π⋯π interactions in an ETCST system to improve photo-induced transition temperatures (Tirrad) and the thermal stability (Trelax) of the photo-induced [HS*] state, in which component metal complexes were pre-designed to have a suitable redox potential for electron transfer.45
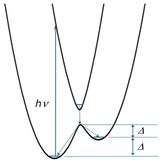 | ||
| Scheme 1 Proposed potential energy curves with the light-induced state conversion process for a class II mixed-valence system with [FeIILSCoIIILS] ([LS]) and [FeIIILSCoIIHS] ([HS]) configurations. | ||
Results and discussion
The reactions of Li[FeIII(bipy)(CN)4] (bipy = 2,2′-bipyridine) with Co(ClO4)2·6H2O and ligands L (papy = 4-[(1E)-2-phenyldiazenyl]-pyridine) or (pepy = 4-(2-phenylethynyl)pyridine) gave cyanide-bridged corner-shared chains of [Fe(bipy)III(CN)4]2[CoII(papy)2]·1.5H2O (1-papy) and [FeIII(bipy)(CN)4]2[CoII(pepy)2]·0.5H2O·CH3OH (1-pepy).
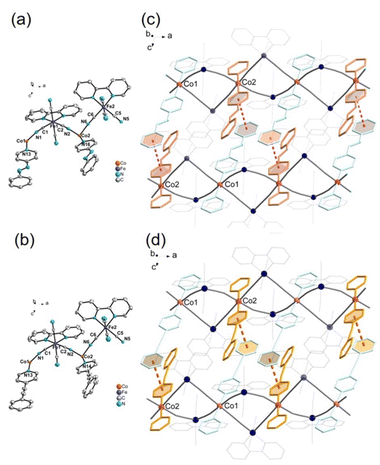
1-papy and 1-pepy crystallized in the triclinic space group of P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) . The crystallographic and detailed structural data are listed in Tables S1–S5.† In both complexes, the asymmetric unit contains two [FeIII(bipy)(CN)4]− complex anions (Fe1 and Fe2) and two [CoIIL2]2+ moieties (Co1 and Co2), where the Co1 and Co2 ions locate on the inversion center (Fig. 1a and b). The FeIII and CoII ions adopt six coordination environments, and the two FeIII and two CoII ions are alternately bridged by cyanide groups and form a slightly twisted square core. The square cores are further linked to form a corner-shared chain at the CoII ions (Fig. 1c and d). Six coordination sites of each FeIII ion are occupied by two nitrogen atoms from the bidentate ligand bipy and four cyanide carbon atoms. The CoII ions adopt elongated octahedral coordination geometries, and the equatorial sites are coordinated by four nitrogen atoms from the bridging cyanide groups. The axial positions are occupied by two nitrogen atoms of the pyridine derivatives of L. In the crystals, the phenyl and pyridine rings are nearly parallel in the Co1 site, while the dihedral angles of those rings in the Co2 site are 32.8° along the azo- and ethynyl-axes for 1-papy and 31.6° for 1-pepy. 1-papy and 1-pepy showed close π⋯π contacts between phenyl and pyridyl groups of ligands L coordinated to the Co ions (πL⋯πL) and between the bipyridine ligands coordinated to the Fe ions (πbipy⋯πbipy) in the adjacent chains (Fig. S1 and S2†).
. The crystallographic and detailed structural data are listed in Tables S1–S5.† In both complexes, the asymmetric unit contains two [FeIII(bipy)(CN)4]− complex anions (Fe1 and Fe2) and two [CoIIL2]2+ moieties (Co1 and Co2), where the Co1 and Co2 ions locate on the inversion center (Fig. 1a and b). The FeIII and CoII ions adopt six coordination environments, and the two FeIII and two CoII ions are alternately bridged by cyanide groups and form a slightly twisted square core. The square cores are further linked to form a corner-shared chain at the CoII ions (Fig. 1c and d). Six coordination sites of each FeIII ion are occupied by two nitrogen atoms from the bidentate ligand bipy and four cyanide carbon atoms. The CoII ions adopt elongated octahedral coordination geometries, and the equatorial sites are coordinated by four nitrogen atoms from the bridging cyanide groups. The axial positions are occupied by two nitrogen atoms of the pyridine derivatives of L. In the crystals, the phenyl and pyridine rings are nearly parallel in the Co1 site, while the dihedral angles of those rings in the Co2 site are 32.8° along the azo- and ethynyl-axes for 1-papy and 31.6° for 1-pepy. 1-papy and 1-pepy showed close π⋯π contacts between phenyl and pyridyl groups of ligands L coordinated to the Co ions (πL⋯πL) and between the bipyridine ligands coordinated to the Fe ions (πbipy⋯πbipy) in the adjacent chains (Fig. S1 and S2†).
57Fe Mössbauer spectra of 1-papy and 1-pepy were measured to characterize the electronic states of the complexes at 300 and 78 K (Fig. S3†), where the Mössbauer parameters of the isomer shift (δ) and quadrupole splitting (ΔEQ) were calculated relative to stainless steel. The Mössbauer spectra of 1-papy and 1-pepy at 300 K are composed of one quadrupole doublet with the parameters δ = −0.10 mm s−1 and ΔEQ = 1.54 mm s−1 and δ = −0.12 mm s−1 and ΔEQ = 1.53 mm s−1, respectively, assignable to an FeIIILS ion. Each Mössbauer spectrum of the complexes at 78 K showed two quadrupole doublets with the parameters δ = −0.037 mm s−1 and ΔEQ = 1.62 mm s−1 and δ = 0.070 mm s−1 and ΔEQ = 0.81 mm s−1 for 1-papy and δ = 0.032 mm s−1 and ΔEQ = 0.82 mm s−1 and δ = −0.073 mm s−1 and ΔEQ = 1.63 mm s−1 for 1-pepy, characteristic of FeIIILS and FeIILS ions, respectively. The peak area ratios of FeIILS/FeIIILS were, respectively, 0.47/0.53 and 0.48/0.52, confirming the complete conversions from the [HS] ([(FeIIILS)2CoIIHS]) to [LS] ([FeIILSFeIIILSCoIIILS]) states by thermally induced ETCST. UV-vis-NIR spectra of the solid samples of 1-papy and 1-pepy were measured in the temperature range of 300–78 K (Fig. S4†). As the temperature was lowered from 300 K, both compounds exhibited new broad absorption bands with the peak maxima at ca. 900 nm, which can be assigned to the intervalence charge transfer (IVCT) band from the FeIILS to CoIIILS ions,37,38 confirming the occurrence of thermal ETCST.
The temperature-dependences of magnetic susceptibilities for 1-papy and 1-pepy were measured from 2 to 300 K in the heating (↑) and cooling (↓) modes (Fig. 2). The results suggest reversible ETCST between the [HS] and [LS] states. The transition temperatures (Tc) are 231 K for 1-papy and 260 K for 1-pepy. Note that 1-papy showed a small thermal hysteresis with transition temperatures of 234 K and 228 K in the heating and cooling mode, respectively, but 1-pepy did not show the hysteresis. The χmT values at 300 K are 4.34 and 4.36 cm3 mol−1 K for 1-papy and 1-pepy, respectively, which are close to the value expected for the magnetically isolated two FeIIILS (S = 1/2) and one CoIIHS (S = 3/2) ions in the [HS] state, with significant orbital contributions. As the temperatures were lowered, the χmT values gradually decreased to reach plateau values of 0.93 cm3 mol−1 K for 1-papy and 0.90 cm3 mol−1 K for 1-pepy at ca.120 K. These χmT values are slightly higher than the values for one paramagnetic FeIIILS (S = 1/2) ion in the [LS] state, which might be due to paramagnetic impurities. As the temperatures were further decreased, gradual increases in the χmT values were observed below 30 K, and the results indicate the occurrence of intrachain ferromagnetic interactions, of which the exchange coupling constant is stronger for 1-papy.
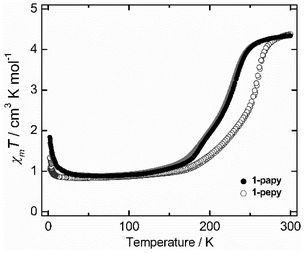 | ||
| Fig. 2 Temperature dependences of χmT values for 1-papy (●) and 1-pepy (○) under a magnetic field of 1000 Oe from 2 to 300 K in the heating (↑) and cooling (↓) modes. | ||
Light irradiation experiments were conducted for 1-papy and 1-pepy in the [LS] state using a laser light (808 nm), which corresponds to the MMCT band, at 10 K (Fig. 3). The light irradiation resulted in sudden increases of the χmT values, confirming the conversions from the thermally stable [LS] to the light-induced metastable [HS*], [(FeIIILS)2CoIIHS], states. It should be noted that single crystal X-ray analyses for 1-papy and 1-pepy before and after photoirradiation supported the substantial conversion from the [LS] to the [HS*] state, respectively, at 78 and 50 K (see below). Temperature dependences of the magnetic susceptibility data for the [HS*] state were measured from 2 to 150 K for both compounds. The χmT values exhibited sharp increases from 2 K to 20 K, reaching the maximum values of 40.26 cm3 mol−1 K at 4.8 K for 1-papy and 10.46 cm3 mol−1 K at 4.0 K for 1-pepy. The smaller maximum value for 1-pepy can be understood by the weaker intrachain ferromagnetic interactions than that for 1-papy. Upon further increasing the temperature, the χmT values gradually decreased and merged with the ones for the unirradiated sample at Trelax = 130 and 90 K, respectively, for 1-papy and 1-pepy. Due to the remarkable thermal stabilities of the photo-induced [HS*] states for the complexes, we explored the photomagnetic performances at 78 and 50 K, respectively (Fig. 3, inset). The laser light irradiation (808 nm) of 1-papy at 78 K showed a sudden increase of the χmT values, reaching the saturation value (2.60 cm3 mol−1 K) within ca. 3 min, which is close to the theoretical one (2.625 cm3 mol−1 K, g = 2.0) for the magnetically isolated two FeIIILS ions and one CoIIHS ion. Note that the irradiation to 1-papy at 80 K took ten min. to reach the saturation value. In contrast, 1-pepy showed a subtle increase of the χmT values upon photoirradiation at 78 K. However, the X-ray structure analyses (see below) before and after the photoirradiation suggested photoconversion from the [LS] to the [HS*] state at 50 K for 1-pepy. The result demonstrates the occurrence of photomagnetic conversion from the [LS] to the [HS*] state even at 78 K for 1-papy, and the origin of the thermal stability of the [HS*] states will be discussed below.
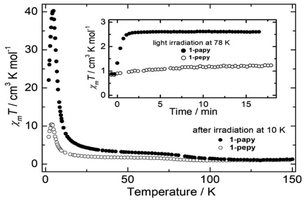 | ||
| Fig. 3 Temperature dependence of χmT values for 1-papy (●) and 1-pepy (○) after photoirradiation (808 nm) at 10 K and time courses under irradiation at 78 K (inset). | ||
Considering a large magnetic anisotropy of CoIIHS ions and the intrachain ferromagnetic interactions in the [HS*] state, the chains in the [HS*] state would show single-chain magnetic properties. AC magnetic susceptibility measurements for the [HS*] state of 1-papy showed frequency-dependent in-phase (χ′) and out-of-phase (χ′′) signals of which the peak maxima shifted to higher temperatures as the frequency was increased (Fig. S5a†). The Mydosh parameter, φ = (ΔTp/Tp)/Δ(logf) (Tp: peak temperature of χ′′ and f: ac frequency), was obtained to be 0.11, suggesting that the [HS*] state is a single-chain magnet and excluding possible spin-glass or long-range magnetic ordering. The relaxation times (τ) were extracted based on the Arrhenius equation, τ = τ0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(Δ/kBT) (τ = 1/2πf), and the fitted pre-exponential factor (τ0) and the relaxation energy barrier (Δ/kB) for 1-papy were 9.86 × 10−11 s and 40.08 K, respectively, which are in the typical range of single-chain magnets (Fig. S5b†). The [HS*] state for 1-pepy also showed the slow relaxation of magnetization with frequency-dependent AC signals (Fig. S6†). The φ value (0.16) fell in the range for single-chain magnets. The Arrhenius plot for the out-of-phase AC signals gave the parameters τ0 = 5.16 × 10−10 s and Δ/kB = 27.61 K for 1-pepy. The correlation lengths (n)46,47 and the number of [(FeIIILS)2CoIIHS] units in the photo-induced SCMs were estimated as 35 and 23 for 1-papy and 1-pepy, respectively (Fig. S7†).
exp(Δ/kBT) (τ = 1/2πf), and the fitted pre-exponential factor (τ0) and the relaxation energy barrier (Δ/kB) for 1-papy were 9.86 × 10−11 s and 40.08 K, respectively, which are in the typical range of single-chain magnets (Fig. S5b†). The [HS*] state for 1-pepy also showed the slow relaxation of magnetization with frequency-dependent AC signals (Fig. S6†). The φ value (0.16) fell in the range for single-chain magnets. The Arrhenius plot for the out-of-phase AC signals gave the parameters τ0 = 5.16 × 10−10 s and Δ/kB = 27.61 K for 1-pepy. The correlation lengths (n)46,47 and the number of [(FeIIILS)2CoIIHS] units in the photo-induced SCMs were estimated as 35 and 23 for 1-papy and 1-pepy, respectively (Fig. S7†).
1-papy and 1-pepy have identical {Fe2Co} skeletons. However, they exhibited different photomagnetic behaviors: the irradiation temperatures for the complete conversion of ETCST at Tirrad = 78 and 50 K, and the thermal relaxation temperatures of Trelax = 130 and 90 K, respectively (Fig. 3 and Fig. S8†). It is, therefore, expected that the interchain interactions would be responsible for their photomagnetic behavior, and we performed single crystal X-ray analyses for 1-papy and 1-pepy before and after photoirradiation at 78 and 50 K, respectively (Tables S6–S10†). The results revealed that upon ETCST no distinct coordination bond length changes on the iron sites were observed, with the average bond lengths of FeIILS–CCN, FeIILS–N, FeIIILS–CCN, and FeIIILS–N being 1.922, 1.950, 1.934, and 1.956 Å for 1-papy and 1.914, 1.928, 1.936, and 1.928 Å for 1-pepy, respectively. In contrast, the bond distances around cobalt ions showed substantial changes of CoIIILS–NCN, CoIIILS–N, CoIIHS–NCN, and CoIIHS–N to 1.902, 1.978, 2.093, and 2.151 Å for 1-papy and to 1.890, 1.965, 2.096, and 2.151 Å)for 1-pepy, respectively. Distortion of the octahedron about metal ions can be determined using the parameters 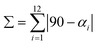 and
and 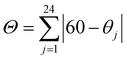 , where αi is the cis N–Co–N angle and θj is the unique N–Co–N angle measured on the projection of two triangular faces of the octahedron along their common pseudo-threefold axis.48,49 Σ is a measure of the deviation from an ideal octahedral coordination geometry and Θ represents the extent of the distortion from the perfect octahedron (Oh, Θ = 60°) toward a trigonal prismatic structure (D3h, Θ = 0°). Upon photoirradiation, the Θ values for the Co1 and Co2 ions changed, respectively, from 20.67 and 25.19 to 20.85 and 30.36 for 1-papy and from 20.85 and 33.56 to 21.71 and 49.70 for 1-pepy (Table S11†). Given the substantial changes in the coordination bond lengths and the octahedral distortions (ΔΣCo2 = 6.96–12.76° and ΔΘCo2 = 5.17–15.51°) on the Co sites before and after the photoirradiation, such significant changes in the vicinity of the Co ions would be propagated to the whole crystals through the interchain π⋯π contacts.50
, where αi is the cis N–Co–N angle and θj is the unique N–Co–N angle measured on the projection of two triangular faces of the octahedron along their common pseudo-threefold axis.48,49 Σ is a measure of the deviation from an ideal octahedral coordination geometry and Θ represents the extent of the distortion from the perfect octahedron (Oh, Θ = 60°) toward a trigonal prismatic structure (D3h, Θ = 0°). Upon photoirradiation, the Θ values for the Co1 and Co2 ions changed, respectively, from 20.67 and 25.19 to 20.85 and 30.36 for 1-papy and from 20.85 and 33.56 to 21.71 and 49.70 for 1-pepy (Table S11†). Given the substantial changes in the coordination bond lengths and the octahedral distortions (ΔΣCo2 = 6.96–12.76° and ΔΘCo2 = 5.17–15.51°) on the Co sites before and after the photoirradiation, such significant changes in the vicinity of the Co ions would be propagated to the whole crystals through the interchain π⋯π contacts.50
It is worth mentioning that some interchain contact distances show substantial shortening upon photo-induced ETCST, even though the cell volume was swollen upon conversion from the [LS] to the [HS*] state (Table S11†). For 1-papy, the interchain πL⋯πL and πbipy⋯πbipy contact distances were, respectively, shortened from 3.726(4) to 3.688(4) Å (Δdis = −0.038 Å) and from 3.838(4) to 3.771(4) Å (Δdis = −0.067 Å) upon the conversion from the [LS] to the [HS*] state. At the same time, the cell volume expanded from 2479.5(5) to 2566.4(4) Å3 (Δvol = +86.9 Å3). The corresponding contact distances for 1-pepy did not change much: the corresponding distances are πL⋯πL = 3.776(1) and 3.766(1) Å (Δdis = −0.01 Å) and πbipy⋯πbipy = 3.887(1) and 3.827(1) Å (Δdis = −0.06 Å), and the cell volumes are 2545 and 2655 Å3 (Δvol = +110 Å3) upon the state conversion. The results suggest that the shortened interchain π⋯π contact distances upon light irradiation stabilize the [HS*] state, leading to the substantially increased ΔG*HS value, stabilizing the [HS*] configuration, and higher Tirrad and Trelax temperatures for ETCST. On the other hand, it was proposed in SCO systems that the cooperativity contributes to the LIESST effect, such as stabilizing the photo-induced [HS*] state and raising the thermal relaxation temperature.51–56 In the ET and ETCST processes, particularly in non zero dimensional PBAs, the electron transfers cause redistributions of charges on the whole solids, in which the intermolecular, interchain, and interlayer interactions could play crucial roles in stabilizing the photo-induced [HS*] states towards high temperatures.
Conclusion
We prepared cyanide-bridged [Fe2Co]-based chain compounds of 1-papy and 1-pepy. The complexes showed thermal and photo-induced ETCST behavior, and the photo-induced [HS*], [(FeIIILS)2CoIIHS], states were confirmed to be single-chain magnets. Photomagnetic, structural, and spectroscopic studies suggested that interchain interactions are crucial to the thermal stabilization of the photo-induced [HS*] state. Among the cyanide bridged molecular systems showing the intermetallic electron transfer, 1-papy exhibited very high photo-induced and relaxation temperatures at Tirrad = 78 and Trelax = 130 K, respectively, which is due to the unique interchain π⋯π stacks. The present study proves the importance of intermolecular interactions to stabilize photo-induced ETCST performances. In the ET and ETCST processes, the electron transfers cause redistributions of charges on the whole solids, in which the intermolecular, interchain, and interlayer interactions could play crucial roles in stabilizing the photo-induced [HS*] states towards high temperatures. Introductions of intermolecular π⋯π interaction networks and maybe hydrogen bonds into crystalline solids provide a controllable approach for increasing the thermal stabilization of the photo-induced [HS*] state. The present results would help rational designs of molecular architectures with photo-switchable and multifunctional materials for practical applications.Author contributions
W. J. Jiang: conceptualization, data curation, investigation, formal analysis, and writing – original draft; Y. S. Meng and H. Oshio: formal analysis and writing – review and editing; Q. Liu: investigation (57Fe Mössbauer spectra); H. H. Lu and H. L. Zhu: validation (reproducibility of experiments); C. Y. Duan: project administration; T. Liu: conceptualization, writing – review and editing, and funding acquisition.Data availability
The data supporting this article have been included as part of the ESI.† Crystallographic data for 1-papy and 1-pepy have been deposited at the CCDC for 1-papy under 2247067 at 110 K, 2247068 at 290 K, 2247069 at 78 K, and 2247070 after irradiation at 78 K; and for 1-pepy under 2247071 at 110 K, 2247073 at 300 K, 2247074 at 50 K, and 2247075 after irradiation at 50 K.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grants, 22025101, 22222103, 22173015, 21871039, 91961114, 22071017, 22103009 and 22203013), and the Fundamental Research Funds for the Central Universities, China (DUT22LAB606).References
- J. K. McCusker, Electronic structure in the transition metal block and its implications for light harvesting, Science, 2019, 363, 484–488 CrossRef CAS.
- A. V. Akimov, A. J. Neukirch and O. V. Prezhdo, Theoretical insights into photoinduced charge transfer and catalysis at oxide interfaces, Chem. Rev., 2013, 113, 4496–4565 CrossRef CAS PubMed.
- S. E. Canton, K. S. Kjær, G. Vankó, T. B. Van Driel, S.-I. Adachi, A. Bordage, C. Bressler, P. Chabera, M. Christensen and A. O. Dohn, Visualizing the non-equilibrium dynamics of photoinduced intramolecular electron transfer with femtosecond X-ray pulses, Nat. Commun., 2015, 6, 6359 CrossRef CAS PubMed.
- E. Collet, M.-H. Lemée-Cailleau, M. Buron-Le Cointe, H. Cailleau, M. Wulff, T. Luty, S.-Y. Koshihara, M. Meyer, L. Toupet and P. Rabiller, Laser-induced ferroelectric structural order in an organic charge-transfer crystal, Science, 2003, 300, 612–615 CrossRef CAS PubMed.
- M. Chollet, L. Guerin, N. Uchida, S. Fukaya, H. Shimoda, T. Ishikawa, K. Matsuda, T. Hasegawa, A. Ota and H. Yamochi, Gigantic, photoresponse in ¼-filled-band organic salt (EDO-TTF)2PF6, Science, 2005, 307, 86–89 CrossRef CAS PubMed.
- T. Naito, T. Karasudani, S. Mori, K. Ohara, K. Konishi, T. Takano, Y. Takahashi, T. Inabe, S. Nishihara and K. Inoue, Molecular photoconductor with simultaneously photocontrollable localized spins, J. Am. Chem. Soc., 2012, 134, 18656–18666 CrossRef CAS.
- M. M. Paquette, D. Plaul, A. Kurimoto, B. O. Patrick and N. L. Frank, Opto-spintronics: Photoisomerization-induced spin state switching at 300 K in photochrome cobalt-dioxolene thin films, J. Am. Chem. Soc., 2018, 140, 14990–15000 CrossRef CAS PubMed.
- O. Sato, Dynamic molecular crystals with switchable physical properties, Nat. Chem., 2016, 8, 644–656 CrossRef CAS.
- L. Wang and Q. Li, Photochromism into nanosystems: Towards lighting up the future nanoworld, Chem. Soc. Rev., 2018, 47, 1044–1097 RSC.
- E. Coronado, Molecular magnetism: From chemical design to spin control in molecules, materials and devices, Nat. Rev. Mater., 2020, 5, 87–104 CrossRef.
- Y.-S. Meng, O. Sato and T. Liu, Manipulating metal-to-metal charge transfer for materials with switchable functionality, Angew. Chem., Int. Ed., 2018, 57, 12216–12226 ( Angew. Chem. , 2018 , 130 , 12394–12405 ) CrossRef CAS.
- Y.-S. Meng and T. Liu, Manipulating spin transition to achieve switchable multifunctions, Acc. Chem. Res., 2019, 52, 1369–1379 CrossRef CAS.
- K. S. Kumar and M. Ruben, Sublimable spin-crossover complexes: From spin-state switching to molecular devices, Angew. Chem., Int. Ed., 2021, 60, 7502–7521 CrossRef CAS.
- N.-T. Yao, L. Zhao, C. Yi, Q. Liu, Y.-M. Li, Y.-S. Meng, H. Oshio and T. Liu, Manipulating selective metal-to-metal electron transfer to achieve multi-phase transitions in an asymmetric [Fe2Co]-assembled mixed-valence chain, Angew. Chem., Int. Ed., 2022, 61, e202115367 ( Angew. Chem. , 2022 , 134 , e202115367 ) CrossRef CAS PubMed.
- J.-X. Hu, Q. Li, H.-L. Zhu, Z.-N. Gao, Q. Zhang, T. Liu and G.-M. Wang, Achieving large thermal hysteresis in an anthracene-based manganese(II) complex via photo-induced electron transfer, Nat. Commun., 2022, 13, 2646 CrossRef CAS PubMed.
- C. Mathonière, Metal-to-metal electron transfer: A powerful tool for the design of switchable coordination compounds, Eur. J. Inorg. Chem., 2018, 3–4, 248–258 CrossRef.
- D. Aguilà, Y. Prado, E. S. Koumousi, C. Mathonière and R. Clérac, Switchable Fe/Co Prussian Blue networks and molecular analogues, Chem. Soc. Rev., 2016, 45, 203–224 RSC.
- A. Bousseksou, G. Molnár, L. Salmon and W. Nicolazzi, Molecular spin crossover phenomenon: Recent achievements and prospects, Chem. Soc. Rev., 2011, 40, 3313–3335 RSC.
- M. Arczyński, J. Stanek, B. Sieklucka, K. R. Dunbar and D. Pinkowicz, Site-selective photoswitching of two distinct magnetic chromophores in a propeller-like molecule to achieve four different magnetic states, J. Am. Chem. Soc., 2019, 141, 19067–19077 CrossRef PubMed.
- S. Ohkoshi, K. Nakagawa, K. Imoto, H. Tokoro, Y. Shibata, K. Okamoto, Y. Miyamoto, M. Komine, M. Yoshikiyo and A. Namai, A photoswitchable polar crystal that exhibits superionic conduction, Nat. Chem., 2020, 12, 338–344 CrossRef CAS PubMed.
- S. F. Jafri, E. S. Koumousi, M.-A. Arrio, A. Juhin, D. Mitcov, M. Rouzieres, P. Dechambenoit, D. Li, E. Otero, F. Wilhelm, A. Rogalev, L. Joly, J.-P. Kappler, C. C. D. Moulin, C. Mathonière, R. Clérac and P. Sainctavit, Atomic scale evidence of the switching mechanism in a photomagnetic CoFe dinuclear Prussian Blue analogue, J. Am. Chem. Soc., 2019, 141, 3470–3479 CrossRef CAS PubMed.
- W. Zhang and K. J. Gaffney, Mechanistic studies of photoinduced spin crossover and electron transfer in inorganic complexes, Acc. Chem. Res., 2015, 48, 1140–1148 CrossRef CAS PubMed.
- W. Zhang, R. Alonso-Mori, U. Bergmann, C. Bressler, M. Chollet, A. Galler, W. Gawelda, R. G. Hadt, R. W. Hartsock, T. Kroll, K. S. Kjær, K. Kubiček, H. T. Lemke, H. W. Liang, D. A. Meyer, M. M. Nielsen, C. Purser, J. S. Robinson, E. I. Solomon, Z. Sun, D. Sokaras, T. B. van Driel, G. Vankó, T.-C. Weng, D. Zhu and K. J. Gaffney, Tracking excited-state charge and spin dynamics in iron coordination complexes, Nature, 2014, 509, 345–348 CrossRef CAS.
- A. Mondal, Y. Li, M. Seuleiman, M. Julve, L. Toupet, M. Buron-Le Cointe and R. Lescouëzec, On/off photoswitching in a cyanide-bridged {Fe2Co2} magnetic molecular square, J. Am. Chem. Soc., 2013, 135, 1653–1656 CrossRef CAS PubMed.
- N. Hoshino, F. Iijima, G. N. Newton, N. Yoshida, T. Shiga, H. Nojiri, A. Nakao, R. Kumai, Y. Murakami and H. Oshio, Three-way switching in a cyanide-bridged [CoFe] chain, Nat. Chem., 2012, 4, 921–926 CrossRef CAS PubMed.
- S.-I. Ohkoshi, S. Takano, K. Imoto, M. Yoshikiyo, A. Namai and H. Tokoro, 90-Degree optical switching of output second-harmonic light in chiral photomagnet, Nat. Photonics, 2014, 8, 65–71 CrossRef CAS.
- W. Huang, X. Ma, O. Sato and D. Wu, Controlling dynamic magnetic properties of coordination clusters via, switchable electronic configuration, Chem. Soc. Rev, 2021, 50, 6832–6870 RSC.
- L. Zhao, Y.-S. Meng, Q. Liu, O. Sato, Q. Shi, H. Oshio and T. Liu, Switching the magnetic hysteresis of an [FeII–NC–WV]-based coordination polymer by photoinduced reversible spin crossover, Nat. Chem., 2021, 13, 698–705 CrossRef CAS PubMed.
- M. Gavara-Edo, R. Córdoba, F. Javier Valverde-Muñoz, J. Herrero-Martín, J. Antonio Real and E. Coronado, Electrical sensing of the thermal and light-induced spin transition in robust contactless spin-crossover/graphene hybrid devices, Adv. Mater., 2022, 34, 2202551 CrossRef CAS.
- V. Rubio-Giménez, S. Tatay and C. Martí-Gastaldo, Electrical conductivity and magnetic bistability in metal–organic frameworks and coordination polymers: Charge transport and spin crossover at the nanoscale, Chem. Soc. Rev., 2020, 49, 5601–5638 RSC.
- O. Sato, T. Iyoda, A. Fujishima and K. Hashimoto, Photoinduced magnetization of a cobalt–iron cyanide, Science, 1996, 272, 704 CrossRef CAS PubMed.
- C. Avendano, M. G. Hilfiger, A. Prosvirin, C. Sanders, D. Stepien and K. R. Dunbar, Temperature and light induced bistability in a Co3[Os(CN)6]2·6H2O Prussian Blue analog, J. Am. Chem. Soc., 2010, 132, 13123–13125 CrossRef CAS PubMed.
- S. Ohkoshi, S. Ikeda, T. Hozumi, T. Kashiwagi and K. Hashimoto, Photoinduced magnetization with a high curie temperature and a large coercive field in a cyano-bridged cobalt–tungstate bimetallic assembly, J. Am. Chem. Soc., 2006, 128, 5320–5321 CrossRef CAS.
- D. Li, R. Clérac, O. Roubeau, E. Harté, C. Mathonière, R. Le Bris and S. M. Holmes, Magnetic and optical bistability driven by thermally and photoinduced intramolecular electron transfer in a molecular cobalt–iron Prussian Blue analogue, J. Am. Chem. Soc., 2008, 130, 252–258 CrossRef CAS PubMed.
- V. Escax, G. Champion, M.-A. Arrio, M. Zacchigna, C. Cartier dit Moulin and A. Bleuzen, The Co ligand field: A key parameter in photomagnetic CoFe Prussian Blue derivatives, Angew. Chem., Int. Ed., 2005, 44, 4798–4801 ( Angew. Chem. , 2005 , 117 , 4876–4879 ) CrossRef CAS.
- X.-Y. Wang, C. Avendano and K. R. Dunbar, Molecular magnetic materials based on 4d and 5d transition metals, Chem. Soc. Rev., 2011, 40, 3213–3238 RSC.
- M. Nihei, Y. Sekine, N. Suganami, K. Nakazawa, A. Nakao, H. Nakao, Y. Murakami and H. Oshio, Controlled intramolecular electron transfers in cyanide-bridged molecular squares by chemical modifications and external stimuli, J. Am. Chem. Soc., 2011, 133, 3592–3600 CrossRef CAS PubMed.
- Y.-Z. Zhang, P. Ferko, D. Siretanu, R. Ababei, N. P. Rath, M. J. Shaw, R. Clérac, C. Mathonière and S. M. Holmes, Thermochromic and photoresponsive cyanometalate Fe/Co Squares: Toward control of the electron transfer temperature, J. Am. Chem. Soc., 2014, 136, 16854–16864 CrossRef CAS PubMed.
- C. Y. Zheng, J. P. Xu, Z. X. Yang, J. Tao and D. F. Li, Factors impacting electron transfer in cyano-bridged {Fe2Co2} clusters, Inorg. Chem., 2015, 54, 9687–9689 CrossRef CAS PubMed.
- R. Ohtani and S. Hayami, Frontispiece: Guest-dependent spin-transition behavior of porous coordination polymers, Chem. – Eur. J., 2017, 23, 2236–2248 CrossRef CAS PubMed.
- E. S. Koumousi, I. R. Jeon, Q. Gao, P. Dechambenoit, D. N. Woodruff, P. Merzeau, L. Buisson, X. L. Jia, D. F. Li, F. Volatron, C. Mathonière and R. Clérac, Metal-to-metal electron transfer in Co/Fe Prussian Blue molecular analogues: The ultimate miniaturization, J. Am. Chem. Soc., 2014, 136, 15461–15464 CrossRef CAS.
- J. Glatz, J.-R. Jiménez, L. Godeffroy, H. J. Bardeleben, L. Fillaud, E. Maisonhaute, Y. Li, L.-M. Chamoreau and R. Lescouëzec, Enlightening the alkali ion role in the photomagnetic effect of FeCo Prussian Blue analogues, J. Am. Chem. Soc., 2022, 144, 10888–10901 CrossRef CAS PubMed.
- N. S. Hush, Adiabatic theory of outer sphere electron-transfer reactions in solution, Trans. Faraday Soc., 1961, 57, 557–580 RSC.
- R. A. Marcus, Chemical and electrochemical electron-transfer theory, Annu. Rev. Phys. Chem., 1964, 15, 155–196 CrossRef CAS.
- M. Cammarata, S. Zerdane, L. Balducci, G. Azzolina, S. Mazerat, C. Exertier, M. Trabuco, M. Levantino, R. Alonso-Mori, J. M. Glownia, S. Song, L. Catala, T. Mallah, S. F. Matar and E. Collet, Charge transfer driven by ultrafast spin transition in a CoFe Prussian Blue analogue, Nat. Chem., 2021, 13, 10–14 CrossRef CAS.
- H. Miyasaka, M. Julve, M. Yamashita and R. Clérac, Slow dynamics of the magnetization in one-dimensional coordination polymers: Single-chain magnets, Inorg. Chem., 2009, 48, 3420–3437 CrossRef CAS PubMed.
- H.-L. Sun, Z.-M. Wang and S. Gao, Strategies towards single-chain magnets, Coord. Chem. Rev., 2010, 254, 1081–1100 CrossRef CAS.
- M. A. Halcrow, Structure:function relationships in molecular spin-crossover complexes, Chem. Soc. Rev., 2011, 40, 4119 RSC.
- J. K. McCusker, A. L. Rheingold and D. N. Hendrickson, Empirical correlations between activation parameters and ligand structure in a series of polypyridyl ferrous complexes, Inorg. Chem., 1996, 35, 2100–2112 CrossRef CAS.
- M. Itoi, I. Maurin, F. Varret, F. A. Frye, D. R. Talham, D. Chernyshov and K. Boukheddaden, When local deformations trigger lattice instability: Flow diagram investigations for photoinduced and quenched metastable states in a Prussian Blue analog, Phys. Rev. B: Condens. Matter Mater. Phys., 2013, 88, 094104 CrossRef.
- M. Nadeem, J. Cruddas, G. Ruzzi and B. J. Powell, Toward high-temperature light-induced spin-state trapping in spin-crossover materials: The interplay of collective and molecular effects, J. Am. Chem. Soc., 2022, 144, 9138–9148 CrossRef CAS.
- J. F. Letard, P. Guionneau, O. Nguyen, J. S. Costa, S. Marcen, G. Chastanet, M. Marchivie and L. Goux-Capes, A guideline to the design of molecular-based materials with long-lived photomagnetic lifetimes, Chem. – Eur. J., 2005, 11, 4582–4589 CrossRef CAS PubMed.
- G. Chastanet, C. Desplanches, C. Baldé, P. Rosa, M. Marchivie and P. Guionneau, A critical review of the T(LIESST) temperature in spin crossover materials − What it is and what it is not, Chem. Sq., 2018, 2, 2 Search PubMed.
- A. Hauser, Light-induced spin crossover and the high-spin→low-spin relaxation, Top. Curr. Chem., 2004, 234, 155–198 CrossRef CAS.
- M. Seredyuk, K. Znovjyak, F. Javier Valverde-Muñoz, I. da Silva, M. Carmen Muñoz, Y. S. Moroz and J. Antonio Real, 105 K Wide room temperature spin transition memory due to a supramolecular latch mechanism, J. Am. Chem. Soc., 2022, 144, 14297–14309 CrossRef CAS PubMed.
- S. Hayami, Z.-Z. Gu, M. Shiro, Y. Einaga, A. Fujishima and O. Sato, First observation of light-induced excited spin state trapping for an iron(III) complex, J. Am. Chem. Soc., 2000, 122, 7126–7127 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2247067–2247071 and 2247073–2247075. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4qi02428a |
| This journal is © the Partner Organisations 2025 |