Dual-function applications of photochromic BiNbO4:Er3+ ceramics based on reversible upconversion luminescence modulation†
Asad
Ullah
 a,
Imran
Khan
a,
Yangke
Cun
a,
Yue
Liu
a,
Zhiguo
Song
a,
Imran
Khan
a,
Yangke
Cun
a,
Yue
Liu
a,
Zhiguo
Song
 ab,
Jianbei
Qiu
ab,
Jianbei
Qiu
 ab,
Cherkasova
Tatiana
c,
Anjun
Huang
*a,
Asif Ali
Haider
ab,
Cherkasova
Tatiana
c,
Anjun
Huang
*a,
Asif Ali
Haider
 *a and
Zhengwen
Yang
*a and
Zhengwen
Yang
 *ab
*ab
aCollege of Materials Science and Engineering, Kunming University of Science and Technology, Kunming, 650093, China. E-mail: aj.huang@outlook.com; 482631074@qq.com; yangzw@kust.edu.cn
bSouthwest United Graduate School, Kunming, 650093, China
cSchool of Chemistry and Oil and Gas Technology, Kuzbass State Technical University, Kemerovo, Russia
First published on 12th November 2024
Abstract
Photochromic luminescent phosphors have attracted considerable attention owing to their excellent optical properties, but they face the problem of limited application. Herein, the reversible photochromic and photo-/thermal bleaching phenomena of a BiNbO4 ceramic are reported, exhibiting a color change between ivory and grey upon alternating stimuli between 365/405 nm light illumination and 808 nm laser irradiation (or thermal treatment at 400 °C). Their potential coloration mechanisms are explained by the color center model, providing a comprehensive framework for understanding the original processes. Through Er3+ ion doping, the maximum coloration contrast decreases from 24% to 20%, while simultaneously enabling the observation of bright green upconversion luminescence. Relying on the combination of re-absorption and energy transfer processes, the upconversion luminescence intensity of the Er3+ ions can be effectively modulated, respectively, showing maximum regulation and recovery rates of 88.0% and 98.1%. The cycle measurements demonstrate the excellent anti-fatigue properties and reproducibility of BiNbO4:Er3+ ceramics, confirming their potential dual-functional applications in anti-counterfeiting and fingerprint acquisition.
Introduction
Photochromism involves reversible color changes upon exposure to light, offering distinct advantages in terms of fatigue resistance and responsiveness to various light wavelengths.1,2 Utilizing the discrepancy in light-harvesting capacity between two color states, the luminescence intensity of photochromic materials can be reversibly modulated,3,4 resulting in numerous uses in optical storage, anti-counterfeiting, and optical switching technologies.5,6 For example, Hu et al. proposed a three-dimensional optical storage application based on photochromism-induced Eu3+ luminescence modulation in tungsten–phosphate glass.7 Bai et al. reported multi-mode luminescence regulation in photochromic CaWO4:Bi/Yb/Er powders, which is promising in optical storage and anti-counterfeiting applications.8 Recently, Hu et al. reported utilizing reversible photochromic modifications, and this approach allows multi-functional anti-counterfeiting and optical storage applications with dynamic color and luminescence in the β-Ba2ScAlO5:Yb3+,Er3+ phosphor.9,10 Despite some achievements, current applications of inorganic photochromic materials remain predominantly concentrated in the optical storage and anti-counterfeiting fields.8,11 However, exploration of other novel and advanced applications of these materials is still relatively limited.The collection and identification of fingerprints is crucial in security and forensic applications, owing to their distinctiveness and permanence. Conventional techniques, such as optical and silicon chip-based methods, are constrained by limitations including poor image quality and durability challenges.12–14 Modern advancements aim to overcome these drawbacks by developing non-destructive and efficient methods for latent fingerprint acquisition.15,16 In earlier research, our group proposed a fingerprint acquisition method built on the laser-induced-thermal effect of lanthanide-doped WO3 and MoO3.15,17 The fingerprint recognition process for WO3 and MoO3 based photochromic materials generally requires significant time, taking approximately 30 minutes and 10 minutes, respectively. In 2022, Latha et al. reported a latent fingerprint detection method relying on photochromic upconversion luminescent BiOCl:Er3+ nanoparticles,18 exhibiting a fast coloration speed (1 min) with relatively low coloration contrast (4.25%). Thus, developing novel materials with high coloration contrast and fast photochromic speed is desirable for promoting the development of fingerprint acquisition.
Niobates offer several advantages as photochromic hosts, including superior optical properties, robust thermal/chemical stability, and environmental sustainability. A range of photochromic niobates has been reported in the literature, such as (K, Na)NbO3 ferroelectrics, NaNbO3:Sm/Er, LiNbO3, etc.19–21 Despite these promising attributes, applications of photochromic niobates in fingerprint acquisition have rarely been reported. In this work, the photochromic phenomenon of the BiNbO4 ceramic is reported, exhibiting a reversible color change between ivory and grey by means of flashing 365/405 nm light exposure and an 808 nm laser or thermal treatment. The potential photo-induced coloration mechanism of the pure BiNbO4 ceramic is systematically investigated. By cooperating with Er3+ ions, a bright green upconversion luminescence (UCL) can be observed, which can be reversibly modulated by coloration and bleaching processes. The influence of Er3+ ions on photochromic and luminescence properties is explored, developing dual-functional applications of anti-counterfeiting and fingerprint acquisition in BiNbO4:Er3+ ceramics.
Experimental section
BiNbO4:x mol% Er3+ (x = 0, 1, 3, 7, 10, and 12) ceramics were synthesized through a conventional high-temperature solid-state reaction technique using Bi2O3 (99.90%), Nb2O5 (99.99%), and Er2O3 (99.99%) as primary ingredients. In a typical synthesis procedure, the reactants were accurately weighed according to their molar ratios and transferred to an agate mortar. Adding a few drops of ethanol, the mixture was ground for 45 minutes to mix all raw materials thoroughly. Subsequently, the compound was transferred to a corundum crucible, which was sintered at 950 °C for 3 hours under an air atmosphere and then allowed to cool down to room temperature naturally. The BiNbO4:Er3+ phosphors were produced byre-grinding for 25 min. For the preparation of BiNbO4:Er3+ ceramics, the powders were pressed into discs with a diameter of 18 mm using an electrical machine (YLJ-60TAS) with two drops of polyvinyl alcohol (PVA) solution as adhesives. Then the discs were dried at 70 °C for 35 min to enhance hardness. To determine the photochromic properties of pure and Er3+ doped BiNbO4 ceramics, the 365 nm ultraviolet light lamp (12 W) and 405 nm LED light source (400 mW cm−2) were employed to implement coloration, and an 808 nm laser (3 W) with a 5 mm light spot was used to realize photobleaching. During the bleaching process, the sample was manipulated by an optical microscopic platform, in which the exposure duration could be accurately controlled by adjusting the laser scanning speed. Additionally, the color of the ceramics could also be faded by thermal treatment at 300 °C.The phase structure of the BiNbO4:Er3+ samples was determined using an X'Pert3 Powder X-ray diffractometer from Malvern Panalytical. Morphological and elemental characterization was conducted using scanning electron microscopy on a Hitachi SU8000 platform. The diffuse reflectance spectra were collected using a Hitachi U-4100 spectrophotometer. The upconversion luminescence spectra were measured with a Hitachi F-7000 spectrophotometer using a 980 nm laser as the excitation source. Upconversion luminescence of the fluorescence lifetime curves was recorded using an Edinburgh FLS980 spectrometer. The electron paramagnetic resonance signal (EPR) was acquired with a paramagnetic resonance spectrometer (from Bruker Bio Spin GmbH company), for which a 9.2 GHz frequency was used. X-ray photoelectron spectroscopy (XPS) analysis was achieved using a K-Alpha X-ray photoelectron spectrometer system from Thermo Scientific. The digital photos were taken using the extended exposure feature of a Nikon D7000 camera.
Results and discussion
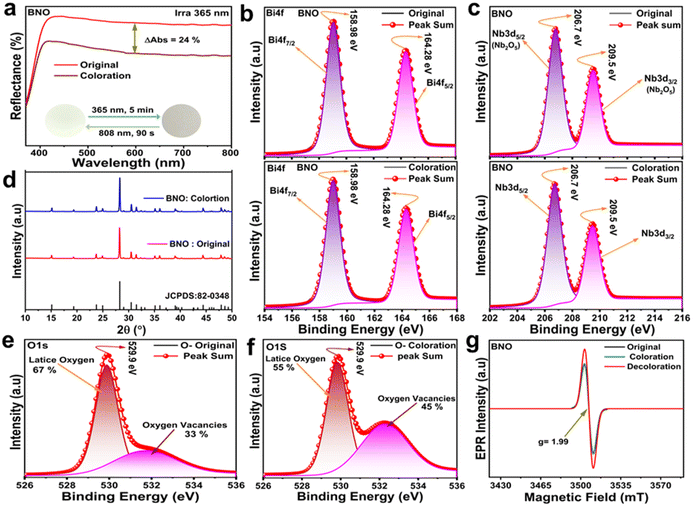
Undoped BiNbO4 ceramics were prepared using the conventional high-temperature solid-state method at 950 °C, which are labeled BNO. The photochromic phenomenon in the BNO ceramic can be obviously observed after 365 nm ultraviolet (UV) light exposure for 5 min, and its initial state can be restored by 808 nm laser stimulation for 90 s. Fig. 1a shows the diffuse reflection spectra of BNO, pre- and post-365 nm UV light irradiation for 5 min, exhibiting an intensity decrease in the whole visible spectral region. Coloration contrast (ΔAbs) is a subset of significant parameters to reflect the photochromic performance of a substance, which is defined as ΔAbs = (Ro − Rc)/Ro × 100%,22 where Ro and Rc refer to the reflectance of the original and photochromic samples, respectively. In our cases, the maximum ΔAbs at 600 nm is calculated to be 24%, corresponding to the color change from ivory to grey (inset photos in Fig. 1a). Generally, there are three potential mechanisms to explain inorganic photochromism: phase transition, ionic transformation, and the color centre model. To understand the root of photo-induced coloration in the BNO ceramic, the cationic valences in BNO are explored by the XPS technique. The Bi 4f and Nb 3d high-resolution XPS spectra of the original and photochromic BNO ceramics are described in Fig. 1b and c, respectively. Compared with the spectrum of the original sample, a chemical shift and a profile change are present in the spectrum of the photochromic sample, indicating that the cationic valence transformation is not responsible for the photo-induced coloration in the BNO ceramic. Following this, the XRD patterns of the BNO ceramic pre- and post-365 nm UV light stimulation are illustrated in Fig. 1d, in which the diffraction peaks in both the original and photochromic BNO ceramics are optimally aligned with the standard card of BNO (JCPDS: 82-0348),23 demonstrating that the photochromic mechanism of BNO is also irrelevant to the phase transition.Upon external stimulation, the electrons are pumped from the valence band (VB) to the conduction band (CB), which may be captured by the defects in the lattice during this transition process, resulting in the formation of color centers. Previously, numerous defect-induced inorganic photochromic materials have been developed and reported, such as BaMgSiO4 and lanthanide-doped TiO2,24,25 in which the oxygen vacancy plays a crucial role in their photo-induced coloration performance.26,27 From there, the O 1s high-resolution spectra of the original and photochromic BNO are displayed in Fig. 1e and f, which can be deconvoluted into two Gaussian peaks at 529.9 eV and 532.3 eV, corresponding to the lattice oxygen and oxygen vacancy.28,29 The proportion of the oxygen vacancy increased from 33% to 45% after 365 nm UV light irradiation for 5 min, implying that the photochromic properties of the BNO may be affected by the concentration of the oxygen vacancy. Except for the XPS technique, the EPR signal can also reflect the defects in a lattice. For example, the EPR signal at around g = 2 is usually ascribed to single-electron-trapped oxygen vacancies (VO˙). In terms of the BNO ceramic, the EPR signal at g = 1.99 can be detected (Fig. 1g), showing a stronger EPR intensity in the photochromic sample than that of the original sample. It is shown that the photo-induced color centers in the BNO ceramic should be responsible for its coloration phenomenon.
Using the photochromic phenomenon, the luminescence intensity of the inorganic phosphors can be reversibly modulated which can be widely applied in various advanced fields.30,31 Hence, the Er3+ ions are selected for doping into the BNO lattice as luminescence emitters. The sintering temperature may affect the photochromic properties of inorganic materials prepared by the solid-state method, so the Er3+-doped BNO ceramics were synthesized at sintering temperatures of 800 °C, 900 °C, 950 °C, and 1000 °C. It is found that the sample sintered at 950 °C exhibits the maximum coloration contrast (Fig. S1†). In addition, PVA solution was employed as an adhesive, so BNO:Er ceramics with and without PVA solution were prepared to investigate the influence of the PVA solution on the photochromism. The results show that the coloration contrast of the BNO:Er ceramics is not affected by adding the PVA solution (Fig. S2†). Fig. S3a† shows the XRD patterns of BNO:x mol% Er3+ (x = 0, 1, 3, 7, 10, 12) ceramics, measured in the 2θ range from 10° to 50°. All diffraction peaks align with JCPDS card 82-0348, with no impurity peaks detected, indicating that the doping of Er3+ does not change its lattice. Upon 980 nm laser excitation, bright UCL can be observed (Fig. S1b†), consisting of two green UCL peaks and a red UCL peak. The green UCL peaks at 535 nm and 555 nm are respectively associated with the 2H11/2 → 4I15/2 and 4S3/2 → 4I15/2 transitions of Er3+ ions, and the red UCL peaks at 658/674 nm are attributed to the 4F9/2 → 4I15/2 transition of Er3+. The UCL intensity initially increases and then decreases with increasing Er3+ concentration, showing an optimum Er3+ doping concentration of 10%. As a result, the BiNbO4:10 mol% Er3+ ceramic (marked as BNO:Er) is considered as an example to investigate its photochromic and UCL regulation behavior below. Fig. S3c† illustrates the 980 nm laser power-dependent UCL spectra of the BNO:Er ceramic, exhibiting steady decreases in the intensity of the green and red peaks as the laser power drops from 3.2 W to 0.2 W. The equation IUCL ∝ Pn is typically used to analyze the UCL mechanism, where IUCL represents the UCL intensity, P is the 980 nm laser power, and n is the number of photons.31–33 Fig. S3d† presents the plot of IUCL at 535, 555, 658, and 674 nm against the 980 nm laser power, in which the slopes of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (IUCL)/log
(IUCL)/log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (P) are respectively calculated to be 1.9, 1.65, and 1.60 for the peaks at 535, 555, and 674 nm, indicating that the green and red UCL of BNO:Er both originate from a two-photon process.
(P) are respectively calculated to be 1.9, 1.65, and 1.60 for the peaks at 535, 555, and 674 nm, indicating that the green and red UCL of BNO:Er both originate from a two-photon process.
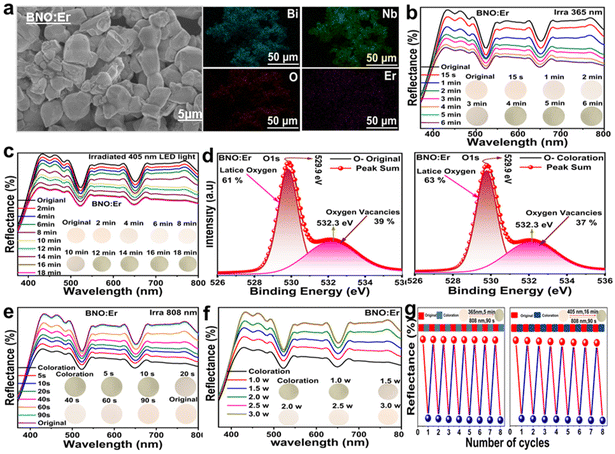
The particle size and morphology of the BNO:Er ceramic are confirmed from its SEM image (Fig. 2a), showing micro-scale particles with good crystallinity. The corresponding SEM-EDS mapping results demonstrate the uniform distribution of all elements, including Bi, Nb, O, and Er. Fig. 2b illustrates the reflection spectra and corresponding digital photos of BNO:Er as a function of 365 nm UV light irradiation durations, in which the color of BNO:Er gradually turns from light pink to dark grey. It is found that two characteristic absorption bands of Er3+ ions at 522 nm and 650 nm can be detected, causing the discrepancy between the initial and photochromic colors between BNO and BNO:Er. The reflectivity across the spectrum from 370 nm to 800 nm gradually reduces during the photoinduced coloration process, and the maximum ΔAbs at 600 nm is achieved after 365 nm UV light stimulation for 5 min, at around 20%. In addition to the 365 nm UV light, the photochromic phenomenon can also be observed with 405 nm LED stimulation (Fig. 2c), and the photochromic performance, such as color contrast and photochromic response time, is affected by the selection of the light source. Photochromism is saturated after 405 nm LED stimulation for 16 min, and the maximum ΔAbs at 600 nm is calculated to be 19%, slightly lower than that of BNO:Er by 365 nm UV light stimulation. Under 254 nm light and X-ray stimulation, the photochromic phenomenon was absent (Fig. S4†).
The XRD patterns, Bi 4f/Nb 3d/Er 4d high-resolution XPS spectra, and EPR signals of BNO:Er before and after 365 nm UV light irradiation for 5 min are shown in Fig. S5.† The phase transition and cationic valence transformation are absent in BNO:Er ceramics, so the photochromic behaviour should resemble that of BNO ceramic, which is driven by color centre generation. Noticeably, different from the BNO ceramic, the proportion of oxygen vacancies decreases after 365 nm stimulation (Fig. 2d), indicating the absence of photogenerated oxygen vacancies in the BNO:Er ceramic. Additionally, the photogenerated electrons may be captured by oxygen vacancies, leading to the formation of color centers related to VO˙ and a decrease of the oxygen vacancy concentration. To further explore the reason for the difference in maximum coloration contrasts (ΔAbs at 600 nm) between BNO and BNO:Er, the SEM image of the BNO ceramic is presented in Fig. S6† Compared with BNO:Er, the BNO ceramic exhibits a smaller particle size, more voids, worse crystallinity, and higher aggregation. The void structure may facilitate the formation of photoinduced oxygen vacancies and color centers, improving the coloration performance of the BNO ceramic. On the other hand, the incorporation of dopants may affect the formation energy of oxygen vacancies and photoinduced oxygen vacancies are hard to generate in BNO:Er, resulting in fewer color centers and worse coloration performance than that of BNO ceramics.34,35
The color of the photochromic BNO:Er ceramic gradually faded upon 808 nm laser irradiation or thermal treatment, showing an increase in reflectivity ranging from 370 to 800 nm (Fig. 2e and Fig. S7†). After 808 nm laser (3 W) irradiation for 2 min, the surface temperature of the BNO:Er ceramic is estimated by using an IR camera, around 98 °C, which is much lower than the thermo-bleaching temperature of 300 °C (Fig. S8†). This reveals that the bleaching process after 808 nm irradiation should not be due to the laser-induced thermal effect. The color of photochromic-saturated BNO:Er can be returned to its initial state upon 808 nm laser irradiation for 90 s or heat treatment at 300 °C for 4 min, and the reflectivity at 600 nm could be respectively recovered to 98.9% and 96.3% of the initial state, suggesting that the photochromic and bleaching processes in the BNO:Er ceramic are fully reversible. The influence of 808 nm laser power and heating temperature on the bleaching speed of BNO:Er is investigated, as shown in Fig. 2f and Fig. S7,† verifying that a high laser power or heating temperature can facilitate the release of confined photoinduced electrons and accelerate the decoloration process. Regarding photochromic materials, the anti-fatigue is a significant parameter in evaluating their performance. The above results prove that the reversible photochromic-bleaching properties of BNO:Er ceramics could be realized through alternating light–light or light–heat treatment. Alternating between 365 nm UV light irradiation for 5 min (or 405 nm LED for 16 min) and 808 nm laser stimulation for 90 s (or 300 °C for 4 min), the variations in reflectivity at 600 nm are plotted in Fig. 2g and Fig. S9.† This reveals that the reflectivity at 600 nm for photochromic BNO:Er fluctuates within a small range in eight cycles, demonstrating remarkable reversibility and excellent reproducibility.
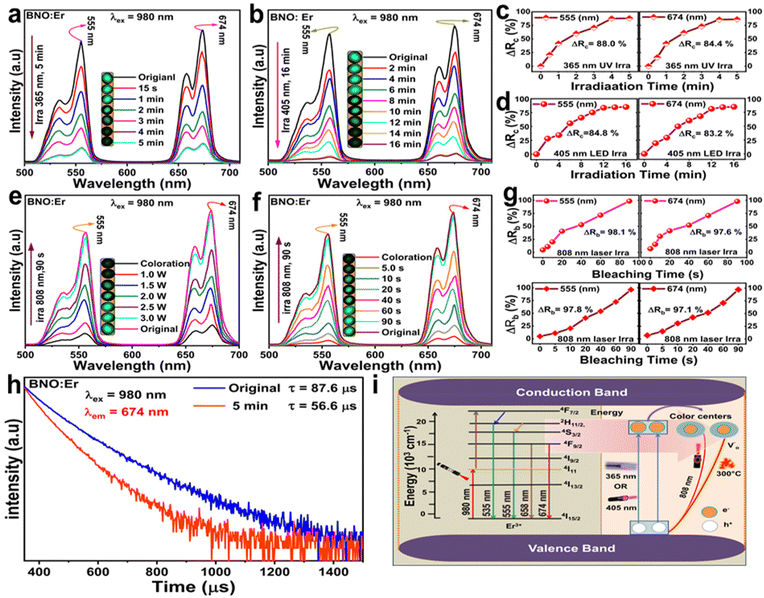
Relying on the overlap between diffuse reflection spectra and UCL spectra of the BNO:Er ceramic, the red and green UCL peak intensities exhibit reversible changes due to re-absorption effects. Under 980 nm laser excitation, the UCL spectra as a function of 365 nm UV light (or 405 nm LED) irradiation durations are illustrated in Fig. 3a and b, showing UCL quenching with increasing stimuli time. The UCL modulation behaviour can be quantitatively reflected by the UCL modulation rate (ΔRc), which can be defined by the equation ΔRc = (Io − Ic)/Ic × 100%,36 where Io and Ic represent the UCL integral peak area of the original and colored BNO:Er ceramics. The ΔRc at 555 nm and 674 nm gradually increases with prolonging of the 365 nm UV light stimulation (Fig. 3c), reaching a maximum at 5 min, respectively up to 88.0% (555 nm) and 84.4% (674 nm). The difference in the maximum ΔRc of the green UCL peak (555 nm) and the red UCL peak (674 nm) should be due to the discrepancy between the maximum ΔAbs at 555 nm (19.6%) and 674 nm (18.1%), resulting in the change in UCL re-absorption efficiencies at various wavelengths. Compared to 365 nm irradiation, BNO:Er stimulated by a 405 nm LED exhibits a slightly lower ΔRc value for both green (555 nm, 84.8%) and red (674 nm, 83.2%) UCL peaks.
During the bleaching process, the UCL intensity of the photochromic BNO:Er ceramic is gradually restored owing to the weakening of the re-absorption effect. As the 808 nm laser power increases from 1.0 W to 3.0 W, the green and red UCL intensities increase (Fig. 3e), nearly returning to their initial state after 808 nm (3 W) laser irradiation for 4 min. Fig. 3f and Fig. S10† show the UCL spectra varying with 808 nm laser illumination duration or thermal treatment temperature, showing an enhancement of the green and red UCL intensities. The effectiveness of UCL modulation during bleaching can be measured using the bleaching degree (ΔRb), expressed by ΔRb = Ib/Io × 100%,37 where Ib represents the UCL intensities of the bleached intensity of BNO:Er ceramics. After light stimulation (808 nm laser for 90 s or 405 nm LED for 16 min) or thermal treatment (300 °C for 4 min), the maximum ΔRb values for green and red UCL peaks are higher than 96.3% (Fig. 3g and Fig. S11†), verifying the reversible UCL regulation in the BNO:Er ceramic. The UCL modulation stability by photochromism and photo- or thermal-bleaching was assessed using cycle measurements. Under alternating exposure to 365 nm UV light (or 405 nm LED light) for 5 min (or 16 min) and subsequent 808 nm laser stimulation for 90 s (or heat treatment at 300 °C for 4 min), no UCL degradation is observed in eight cycles (Fig. S12†), highlighting the strength and reliability of UCL modulation in BNO:Er ceramics for potential practical applications.
In addition to the re-absorption effect, the energy transfer from luminescence emitters to the defects or/and color center may lead to luminescence quenching of photochromic materials. Therefore, the UCL decay curves (λex = 980 nm, λem = 674 nm) of the pristine and photochromic-saturated BNO:Er ceramics are shown in Fig. 3h, which can be well fitted by the mono-exponential equation. The fitted lifetimes are respectively calculated to be 87.6 μs and 56.6 μs for the pristine and photochromic-saturated BNO:Er ceramics, demonstrating that the combination of the re-absorption effect and the energy transfer process is co-responsible for the UCL quenching during coloration of BNO:Er. The photochromic-bleaching and UCL regulation mechanisms are summarized in Fig. 3i. Upon exposure to 365 nm UV light (or 405 nm LED), electrons are pumped from the VB to the CB in BNO:Er ceramics, which are trapped by oxygen vacancies, generating color centers and resulting in a color change. In this process, the re-absorption and energy transfer from Er3+ ions to defects or/and color centers cause the UCL quenching of Er3+ ions.38,39 During the bleaching process, the captured electrons within the oxygen vacancies are released by the 808 nm laser stimulation (or thermal treatment), resulting in the reversion of the color and UCL intensity of the photochromic BNO:Er ceramic to its original state.
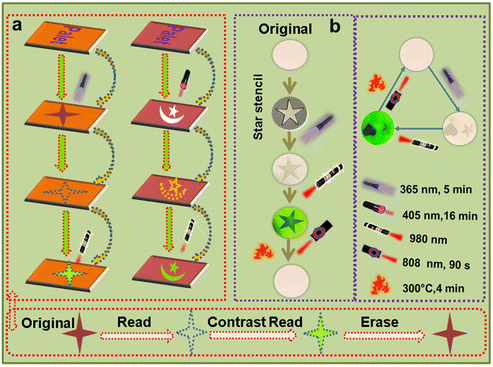
Lanthanide-doped UCL materials have gained attention in the security field owing to their anti-Stokes shift characteristics. For example, Xie et al. prepared a kind of multimodal nanocomposite composed of lanthanide-doped upconverting nanoparticles and EuSe semiconductors, exhibiting potential applications in advanced optical anti-counterfeiting and information storage.40 Utilizing the reversible photochromic and UCL modulation behaviors, the anti-counterfeiting application of the BNO:Er ceramic has been developed. Fig. 4a illustrates the schematic graphic diagram of the input, readout, and erase processes of the photochromic BNO:Er ceramic for security applications. Using a mask covering the surface of the BNO:Er ceramic, the light-brown security patterns can be written on the surface of the ceramic by UV/visible light irradiation. The high signal–noise ratio is beneficial for an accurate readout, so the UCL mode is employed to realize the readout of the anti-counterfeiting pattern owing to a higher UCL modulation rate than the coloration contrast in the BNO:Er ceramic. Finally, the input photochromic patterns can be erased by photo-bleaching or thermal-bleaching processes, implementing the recycling of resources. Fig. 4b shows practical digital photos of the BNO:Er ceramic used as an anti-counterfeiting agent. Upon 365 nm UV light irradiation for 5 min or 405 nm LED illumination for 16 min, the patterns of a star and a heart can be written on the surface of the BNO:Er ceramic. Upon 980 nm excitation, the patterns become more and more clear with the laser power increasing from 1.0 W to 3.0 W. Relying on photo-bleaching using 808 nm laser stimulation for 90 s or thermal-bleaching at 300 °C for 4 min, the photochromic patterns can be fully cleaned. This reveals that the BNO:Er ceramic is a promising candidate as an anti-counterfeiting agent.
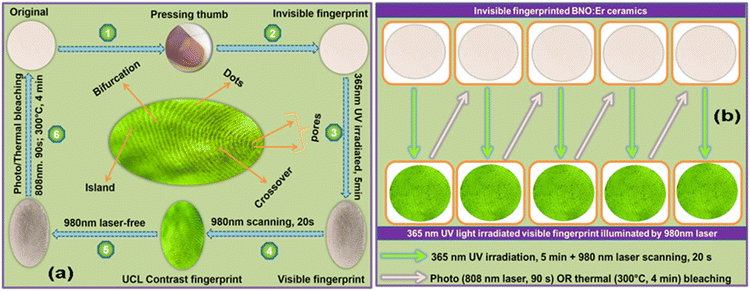
In addition to the anti-counterfeiting application, the fingerprint acquisition and recognition application of the BNO:Er ceramic is proposed and developed. Fig. 5a and Fig. S13† illustrate the procedure to acquire, identify, and erase fingerprint patterns based on the BNO:Er ceramic. In the first step, the thumb presses on the surface of the ceramic, leaving an invisible fingerprint on the surface of the BNO:Er ceramic. Then, a vague fingerprint pattern gradually appears with increasing 365 nm UV light (or 405 nm LED) irradiation duration. After exposing to 365 nm UV (12 W) light for 4 min or 405 nm LED (400 mW cm−2) for 16 min, the external light stimuli are stopped, and a BNO:Er ceramic with a fingerprint pattern is obtained. To achieve a clearer fingerprint pattern, a 980 nm laser scan with an integration time of 20 s is used to realize fingerprint recognition in the UCL mode. In the final step, the fingerprint can be erased using an 808 nm laser (3.0 W) for 90 s or by heat treatment at 300 °C for 4 min.
Previously, photonic barcodes and fingerprint detection applications of lanthanide luminescence have been proposed.41 Combining the properties of photochromism and lanthanide luminescence, a fingerprint acquisition and recognition application of lanthanide-doped MoO3 and WO3 has also been reported.15,17 However, the acquisition and recognition speeds are usually slow, several tens of minutes, restricting their practical application. The fast acquisition of fingerprint patterns in the photochromic BNO:Er ceramic, about 4 min, exhibits its advantages in fingerprint acquisition and recognition applications. Fig. 5b shows the fatigue resistance of the BNO:Er ceramic in fingerprint recognition, in which the fingerprint can be accurately identified in five cycles by alternating stimulation between 365 nm UV light (12 W) for 5 min (or 405 nm LED for 16 min) and an 808 nm laser (3.0 W) for 90 s (or thermal treatment at 300 °C for 4 min), indicating its good repeatability and excellent reproducibility. Additionally, an aging experiment was carried out to test the stability of fingerprint acquisition in the BNO:Er ceramic (Fig. S14†), revealing that the fingerprint patterns can last for more than 10 days and fade completely in about a month. Therefore, the above results prove that the BNO:Er ceramic is a kind of dual-functional material, which is promising in the anti-counterfeiting and fingerprint acquisition fields.
Conclusions
Undoped and lanthanide-doped BiNbO4 ceramics were fabricated by the traditional high-temperature solid-state method, exhibiting an obvious photochromic phenomenon under 365 nm UV light or 405 nm LED irradiation. The color of the undoped BiNbO4 ceramic reversibly changes between ivory and grey during photochromic and photo-/thermal-bleaching processes, which is associated with the formation and disappearance of the color centers. Owing to the hindering effect of Er3+ doping on the formation of photo-induced oxygen vacancies, the maximum coloration contrast of the ceramic decreases after Er3+ ion doping. Relying on the re-absorption and energy transfer from Er3+ ions to defects/color centers, photochromism-induced reversible UCL modulation is realized, achieving a maximum UCL modulation of 88.2%. The cycle measurements confirm that the photochromic and UCL regulation of the Er3+-doped BiNbO4 ceramics has good reversibility and reproducibility. Based on their excellent photochromic and UCL properties, dual-functional applications (e.g. anti-counterfeiting and fingerprint acquisition) of Er3+-doped BiNbO4 ceramics are developed, showing promising prospects.Author contributions
Asad Ullah: synthesis, formal analysis, data curation, and original draft writing. Imran Khan and Yangke Cun: data analysis. Yue Liu, Zhiguo Song, and Jianbei Qiu: visualization and validation. Cherkasova Tatiana: visualization. Anjun Huang and Asif Ali Haider: formal analysis, data curation, and review & editing. Zhengwen Yang: supervision, formal analysis, and review & editing.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China-Yunnan Joint Fund (U2102215), the National Natural Science Foundation of High-end Foreign Experts Introduction Plan (G2022039008L), the Academician Expert Workstation of Cherkasova Tatiana in Yunnan Province (202305AF150099), and the Yunnan Province Major Science and Technology Special Plan (202302AB080005).References
- Q. Dong, X. Feng, J. Qiu and S. Zhou, Main group elements activated near-infrared photonic materials, Laser Photonics Rev., 2024, 2301053 CrossRef
.
- Q. Zhang, H. Sun, H. Li, X. Wang, X. Hao, J. Song and S. An, Reversible photoresponsive switching in Bi2.5Na0.5Nb2O9-based luminescent ferroelectrics, Chem. Commun., 2015, 51, 16316–16319 RSC
.
- Q. Zhang, H. Sun, X. Wang, X. Hao and S. An, Reversible luminescence modulation upon photochromic reactions in rare-earth doped ferroelectric oxides by in situ photoluminescence spectroscopy, ACS Appl. Mater. Interfaces, 2015, 7, 25289–25297 CrossRef CAS
.
- J. Zhou, Q. Liu, W. Feng, Y. Sun and F. Li, Upconversion luminescent materials: Advances and applications, Chem. Rev., 2015, 115, 395–465 CrossRef CAS PubMed
.
- X. Bai, Z. Yang, Y. Zhan, Z. Hu, Y. Ren, M. Li, Z. Xu, A. Ullah, I. Khan, J. Qiu, Z. Song, B. Liu and Y. Wang, Novel strategy for designing photochromic ceramic: Reversible upconversion luminescence modification and optical information storage application in the PbWO4:Yb3+, Er3+ photochromic ceramic, ACS Appl. Mater. Interfaces, 2020, 12, 21936–21943 CrossRef CAS PubMed
.
- F. Yang, B. Jia, T. Wei, C. Zhao, Q. Zhou, Z. Li, M. Du, M. Wang, Y. Liu and C. Xie, Reversible regulation of upconversion luminescence in new photochromic ferroelectric materials: Bi4−xErxTi3O12 ceramics, Inorg. Chem. Front., 2019, 6, 2756–2766 RSC
.
- Z. Hu, X. Huang, Z. Yang, J. Qiu, Z. Song, J. Zhang and G. Dong, Reversible 3D optical data storage and information encryption in photo-modulated transparent glass medium, Light: Sci. Appl., 2021, 10, 140 CrossRef CAS
.
- X. Bai, Y. Cun, Z. Xu, Y. Zi, A. A. Haider, A. Ullah, I. Khan, J. Qiu, Z. Song and Z. Yang, Multiple anti-counterfeiting and optical storage of reversible dual-mode luminescence modification in photochromic CaWO4:Yb3+, Er3+, Bi3+ phosphor, Chem. Eng. J., 2022, 429, 132333 CrossRef CAS
.
- J. Hu, X. Bian, R. Wang, L. Liu, N. Ilyas, F. Wang, Z. Song and H. Fu, Giant enhancement in upconversion luminescence of β-Ba2ScAlO5:Yb3+/Er3+ phosphor by the intermediate band through Ca2+ doping, Chem. Mater., 2022, 34, 3089–3098 CrossRef CAS
.
- J. Hu, Y. Wu, B. Duan, Y. Li, F. Wang, T. Liu, W. Jin and C. Ding, Novel strategy for designing photochromic ceramic: Reversible upconversion luminescence modification of the α-Ba2ScAlO5:Yb3+/Er3+ for optical information storage and multiple anti-counterfeiting, Ceram. Int., 2024, 50, 19873–19880 CrossRef CAS
.
- X. Bai, Z. Yang, Z. Xu, Y. Cun, Y. Zi, H. Zhao, Y. Song, Y. He, A. A. Haider, J. Qiu, Z. Song, A. Huang, C. Tatiana and Z. Yang, Optical memory and anti-counterfeiting application based on luminescence reversible modification and color contrast of photochromic phosphor, Sci. China Mater., 2023, 66, 2408–2417 CrossRef CAS
.
- H. Chen, R.-L. Ma, Y. Chen and L. J. Fan, Fluorescence development of latent fingerprint with conjugated polymer nanoparticles in aqueous colloidal solution, ACS Appl. Mater. Interfaces, 2017, 9, 4908–4915 CrossRef CAS
.
- M. Wang, M. Li, A. Yu, J. Wu and C. Mao, Rare earth fluorescent nanomaterials for enhanced development of latent fingerprints, ACS Appl. Mater. Interfaces, 2015, 7, 28110–28115 CrossRef CAS
.
- Y. Zhang, L. Luo, K. Li, W. Li and Y. Hou, Reversible up-conversion luminescence modulation based on UV-vis light-controlled photochromism in Er3+ doped Sr2SnO4, J. Mater. Chem. C, 2018, 6, 13148–13156 RSC
.
- M. Li, Z. Yang, Y. Wen, J. Ruan, Y. Ren, J. Qiu, Z. Song, Y. Wang and B. Liu, Fingerprint acquisition: Fingerprint acquisition based on photo-thermal coloration of MoO3 ceramic upon the irradiation of multiband light outside the bandgap, Adv. Mater. Technol., 2020, 5, 2070069 CrossRef CAS
.
- Z. Zhang and D. Peng, Recent advances in enhancement techniques for blood fingerprints, CRC Crit. Rev. Anal. Chem., 2023, 53, 442–461 CrossRef CAS PubMed
.
- J. Ruan, Z. Yang, Y. Wen, M. Li, Y. Ren, J. Qiu, Z. Song and Y. Wang, Laser induced thermochromism and reversible upconversion emission modulation of a novel WO3:Yb3+, Er3+ ceramic: dual-modal fingerprint acquisition application, Chem. Eng. J., 2020, 383, 123180 CrossRef CAS
.
- N. Latha, B. Daruka Prasad, D. R. Lavanya, B. R. Radhakrushna, H. B. Premkumar, S. C. Sharma, P. Lalitha and H. Nagabhushana, Photochromic, down-conversion nano bismuth chloride layered material: Latent fingerprint visualization data security applications, J. Lumin., 2022, 252, 119328 CrossRef CAS
.
- J. Liu, Y. Zhang, H. Sun, Q. Zhang, X. Wang and X. Hao, Reversible up-conversion emission and photo-switching properties in Er doped (K, Na)NbO3 ferroelectrics, J. Lumin., 2019, 207, 85–92 CrossRef CAS
.
- P. Wang, J. Lin, X. Wu, C. Zhao, M. Gao, C. Lin and Q. J. C. I. Zhang, Trap-induced self-recoverable photochromism of rare-earth doped sodium niobate translucent ceramics, Ceram. Int., 2021, 47, 31702–31712 CrossRef CAS
.
- D. J. Lee and Y. S. Lee, Bi3+-driven defect formation and related optical multimode in luminescent ferroelectrics Sm3+-doped LiNbO3, J. Lumin., 2024, 120879 CrossRef CAS
.
- A. A. Haider, Y. Cun, X. Bai, Z. Xu, Y. Zi, J. Qiu, Z. Song, A. Huang and Z. Yang, Anti-counterfeiting applications by photochromism induced modulation of reversible upconversion luminescence in TiO2:Yb3+, Er3+ ceramic, J. Mater. Chem. C, 2022, 10, 6243–6251 RSC
.
- S. Xiong, Y. Liu, T. Li, F. Li and W. Cao, A facile hydrothermal preparation of O-deficient BiNbO4 nanorods for effective sonocatalytic decontamination, Ceram. Int., 2020, 46, 21790–21793 CrossRef CAS
.
- Y. Ren, Z. Yang, M. Li, J. Ruan, J. Zhao, J. Qiu, Z. Song and D. Zhou, Reversible upconversion luminescence modification based on photochromism in BaMgSiO4:Yb3+, Tb3+ ceramics for anti-counterfeiting applications, Adv. Opt. Mater., 2019, 7, 1900213 CrossRef
.
- Z. Lin, C. Zuo, J. Zheng, F. Chen, C. Ma, C. Chang, J. Wan, X. Yuan, W. Tang, Z. Wen and Y. Cao, High-contrast photochromic properties with reversible luminescence modulation behaviors in Ba3MgSi2O8:Sm3+ ceramics, J. Lumin., 2023, 258, 119800 CrossRef CAS
.
- D. Kim, U. Jung, W. Heo, N. Kumar and J. Park, Arrays of TiO2 nanosphere monolayers on GaN-based LEDs for the improvement of light extraction, Appl. Sci., 2023, 13, 3042 CrossRef CAS
.
- Y. Zhang, Y. Li, H. Yu, K. Yu and H. Yu, Interfacial defective Ti3+ on Ti/TiO2 as visible-light
responsive sites with promoted charge transfer and photocatalytic performance, J. Mater. Sci. Technol., 2022, 106, 139–146 CrossRef CAS
.
- J. Yao, C. Huang, C. Liu and M. Yang, Upconversion luminescence nanomaterials: A versatile platform for imaging, sensing, and therapy, Talanta, 2020, 208, 120157 CrossRef CAS
.
- R. Feng, X. Bai, Y. Zi, Y. Song, H. Zhao, Y. Cun, Y. Liu, J. Qiu, Z. Song, A. Huang, Z. Xu and Z. Yang, Upconversion luminescence reversible modulation of photochromic Ba2YTaO6 double perovskite phosphors toward anticounterfeiting application, Adv. Opt. Mater., 2024, 12, 2301293 CrossRef CAS
.
- H. Wang, J. Lin, B. Deng, T. Lin, C. Lin, Y. Cheng, X. Wu, X. Zheng and X. Yu, Reversible multi-mode modulations of optical behavior in photochromic-translucent Nd-doped K0.5Na0.5NbO3 ceramics, J. Mater. Chem. C, 2020, 8, 2343–2352 RSC
.
- Y. Zhan, Z. Yang, Z. Xu, Z. Hu, X. Bai, Y. Ren, M. Li, A. Ullah, I. Khan, J. Qiu, Z. Song, B. Liu and Y. Wang, , Electrochromism induced reversible upconversion luminescence modulation of WO3:Yb3+, Er3+ inverse opals for optical storage application, Chem. Eng. J., 2020, 394, 124967 CrossRef CAS
.
- Y. Wu, S. Lin, J. Liu, Y. Ji, J. Xu, L. Xu and K. Chen, Efficient up-conversion red emission from TiO2:Yb, Er nanocrystals, Opt. Express, 2017, 25, 22648–22657 CrossRef CAS
.
- K. Saidi, M. Yangui, C. H. Álvarez, M. Dammak, I. R. M. Benenzuela and M. Runowski, Multifunctional optical sensing with lanthanide-doped upconverting nanomaterials: Improving detection performance of temperature and pressure in the visible and NIR ranges, ACS Appl. Mater. Interfaces, 2024, 16, 19137–19149 CrossRef CAS
.
- J. Bi, T. Wei, L. Shen, F. Yang, C. Zhao, M. Wang, Q. Yang and Y. Lin, Tunable upconversion luminescence in new Ho3+/Yb3+-doped SrBi4Ti4O15 photochromic ceramics for switching application, J. Am. Ceram. Soc., 2021, 104, 1785–1796 CrossRef CAS
.
- A. A. Haider, H. Zhao, Y. Zi, Z. Xu, X. Bai, Y. Cun, Y. Liu, H. Babeker, A. Saeed, Z. Song, J. Qiu, A. Huang, J. Liao and Z. Yang, Advances in reversible luminescence modification and applications of inorganic phosphors based on chromism reaction, Adv. Opt. Mater., 2024, 12, 2302265 CrossRef CAS
.
- H. Zhao, Y. Li, C. Mi, Y. Zi, X. Bai, A. A. Haider, Y. Cun, A. Huang, Y. Liu, J. Qiu, Z. Song, J. Liao, J. Zhou and Z. Yang, NIR regeneration and visible luminescence modification in photochromic glass: A novel encryption and 3D optical storage medium, InfoMat, 2024, e12546 CrossRef CAS
.
- J. Andres, R. D. Hersch, J. E. Moser and A. S. Chauvin, A new anti-counterfeiting feature relying on invisible luminescent full color images printed with lanthanide-based inks, Adv. Funct. Mater., 2014, 24, 5029–5036 CrossRef CAS
.
- P. Gerner and H. U. Güdel, Absorption and upconversion light emission properties of Er3+ and Yb3+/Er3+ codoped NaYS2, Chem. Phys. Lett., 2005, 413, 105–109 CrossRef CAS
.
- Y. Zi, Z. Yang, Z. Xu, X. Bai, A. Ullah, I. Khan, A. A. Haider, J. Qiu, Z. Song, Y. Wang and Y. Cun, A novel upconversion luminescence temperature sensing material: Negative thermal expansion Y2Mo3O12:Yb3+, Er3+ and positive thermal expansion Y2Ti2O7:Yb3+, Er3+ mixed phosphor, J. Alloys Compd., 2021, 880, 160156 CrossRef CAS
.
- Y. Xie, Y. Song, G. Sun, P. Hu, A. Bednarkiewicz and L. Sun, Lanthanide-doped heterostructured nanocomposites toward advanced optical anti-counterfeiting and information storage, Light: Sci. Appl., 2022, 11, 150 CrossRef CAS PubMed
.
- J. Chen, M. Li, R. Sun, Y. Xie, J. R. Reimers and L. Sun, Enhancement of luminescence from lanthanide metal–organic frameworks by ytterbium and calcium doping: Application to photonic barcodes and fingerprint detection, Adv. Funct. Mater., 2024, 34, 2315276 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4qi02440k |
| This journal is © the Partner Organisations 2025 |