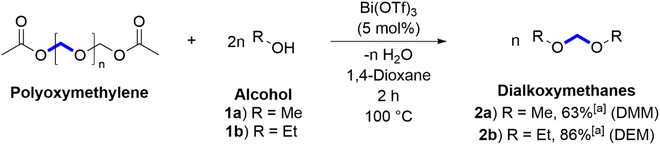Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Valorization of polyoxymethylene (POM) waste as a C1 synthon for industrially relevant dialkoxymethanes and cyclic aminals†
Matthew J.
Cullen
 ab,
Matthew G.
Davidson
ab,
Matthew G.
Davidson
 *ab,
Matthew D.
Jones
*ab,
Matthew D.
Jones
 *ab and
Jack A.
Stewart
*ab and
Jack A.
Stewart
 a
a
aDepartment of Chemistry, University of Bath, Claverton Down, Bath, BA2 7AY, UK. E-mail: mj205@bath.ac.uk
bInstitute of Sustainability and Climate Change, University of Bath, Claverton Down, Bath, BA2 7AY, UK. E-mail: chsmgd@bath.ac.uk
First published on 2nd December 2024
Abstract
Chemical recycling, either upcycling or returning to monomers, is a promising option for deriving value from plastic waste. Herein, we report two novel methods of upcycling waste polyoxymethylene (POM) to generate dialkoxymethanes (DAMs) by alcoholysis and cyclic aminals by aminolysis. A range of products are reported, including dimethoxymethane (DMM) and diethoxymethane (DEM), both of which are used industrially. Mixed plastic depolymerizations containing POM, both as a contaminant in other systems and as the intended substrate with other plastics as contaminants, were also investigated. The selectivity of four depolymerization reactions with respect to POM is reported alongside the selectivity of POM alcoholysis with respect to common contaminants. Finally, two mixed samples of post-consumer plastic waste containing POM, BPA-PC, and PET were sequentially and selectively degraded.
Sustainability spotlightConstructing a more sustainable plastics economy will play a key role in the transition to a carbon-neutral society, as well as contributing to the fight against the global plastics pollution crisis. This will require dramatically improved methods to recycle complex mixtures of plastic waste, including the development of effective chemical recycling – a promising technology for this transition. Research in this area utilising polyoxymethylene (POM) is sparse and we report new methods to valorize waste POM that are tolerant of mixed plastic feedstocks, with the aim of improving the sustainability of the material's end-of-life treatment. By developing new, sustainable and scalable industry relevant processes, valorizing waste plastic, and reducing the amount of plastic entering landfill, this work directly supports SDG-9 (Industry, Innovation, and Infrastructure), SDG-11 (Sustainable Cities and Communities), SDG-12 (Responsible Consumption and Production), SDG-14 (Life Below Water), and SDG-15 (Life on Land). |
Introduction
Plastics are ubiquitous in today's world. The combination of excellent mechanical properties, high chemical resistance, and low costs of production have made plastics the materials of choice for a wide range of applications.1 The global plastics economy, however, is linear rather than circular,2 and is unsustainable for two major reasons, commonly termed ‘the plastics problem’. First, the vast majority of plastics are produced from depleting, non-renewable fossil resources.3,4 Second, their current end-of-life treatment has led to a global plastic pollution crisis.2,5 In this context, continued reliance on plastics will require a global transition to a circular plastics economy, within which drastically improved mechanical and chemical recycling processes will be key.The utility and sustainability of mechanical recycling is limited by the unavoidable degradation of the recyclate's material properties.6,7 To minimise this, high purity feedstocks can be necessary; for example, even low amounts of poly(vinyl chloride) (PVC) as a contaminant in an otherwise pure stream of poly(ethylene terephthalate) (PET) can compromise the quality of the resulting material.6 Mechanical recycling can therefore require resource intensive sorting technologies. Chemical recycling, on the other hand, can produce value-added chemicals with no loss of quality.8–10 The products of chemical recycling can be used as monomers, returning to the original polymer and closing the loop, or can provide value in other applications through upcycling to useful, renewable chemical feedstocks. Chemical recycling methods can also be tolerant of mixed plastic feedstocks which is of obvious value in simplifying pretreatment and sorting strategies. The area has accordingly received increased attention in recent years,9,11 this research has mostly focused on demonstrating selectivity among carbonyl-containing polymers.12–19
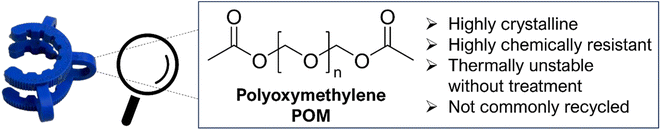
The application of chemical recycling on an industrial scale is challenged by high energy requirements and the need for expensive and precious catalysts.9,20 Recent advances have lowered these energy requirements and identified cheaper and milder catalysts to encourage the prospect of chemical recycling on larger scales. To date, the vast majority of this work has, understandably, focused on carbonyl containing polymers, particularly PET,12,21–23 bisphenol A-based polycarbonate (BPA-PC),24–29 and poly(lactic acid) (PLA).26,30–32 This focus has left the chemical recycling of other industrially relevant polymers comparatively under-studied. One such polymer is polyoxymethylene (POM) (Fig. 1).
POM is a highly crystalline engineering thermoplastic produced from formaldehyde at a scale in excess of 1 Mtpa.33–35 It is commonly used in precisely engineered parts such as gears and clips and has found extensive use in, for example, the automotive industry. It is highly chemically resistant and insoluble in all common solvents.36 POM is typically end-capped post-polymerisation to increase its thermal stability, as non-stabilised POM chain-ends undergo endwise decomposition to formaldehyde under mild heating.37,38 This thermal instability has limited the mechanical recycling of POM due to the inevitable evolution of formaldehyde.39,40
There are few examples of the chemical recycling of POM in the literature. A process commercialised in the 1990s decomposed POM in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of water and concentrated sulfuric acid at temperatures up to 135 °C. This process produced a mixture of formaldehyde and trioxane that was then used to make virgin POM.41–43 Pyrolysis and solvothermal liquefication have been reported, although selectivities to valuable products were low.39,44 Moore et al. reported the electrochemical depolymerization of POM to produce a mixture of formaldehyde, oxydimethanol, trioxane, and formic acid.45 Milstein et al. recently reported new methods of upcycling the products of POM degradation following treatment in a solution of 1,4-dioxane and water at 150 °C using either 7 bar of H2 or 2 mol% of formic acid to produce a mixture of formaldehyde and methanediol. This mixture was activated by a Mn(I) pincer complex to produce methanol and CO2, or alternatively, to methylate various ketones and amines.46 Zhang et al. degraded POM in the presence of glutaric anhydride to produce a telechelic copolyester in one pot.47 In the most selective example in the literature, Beydoun and Klankermayer demonstrated bismuth triflate (Bi(OTf)3)-catalysed POM glycolysis using bio-derived diols to produce a number of cyclic acetals.33 This work highlighted the potential for the repeat unit of POM to be used as a C1 synthon and prompted us to view the upcycling of POM as a green alternative to formaldehyde.
1 mixture of water and concentrated sulfuric acid at temperatures up to 135 °C. This process produced a mixture of formaldehyde and trioxane that was then used to make virgin POM.41–43 Pyrolysis and solvothermal liquefication have been reported, although selectivities to valuable products were low.39,44 Moore et al. reported the electrochemical depolymerization of POM to produce a mixture of formaldehyde, oxydimethanol, trioxane, and formic acid.45 Milstein et al. recently reported new methods of upcycling the products of POM degradation following treatment in a solution of 1,4-dioxane and water at 150 °C using either 7 bar of H2 or 2 mol% of formic acid to produce a mixture of formaldehyde and methanediol. This mixture was activated by a Mn(I) pincer complex to produce methanol and CO2, or alternatively, to methylate various ketones and amines.46 Zhang et al. degraded POM in the presence of glutaric anhydride to produce a telechelic copolyester in one pot.47 In the most selective example in the literature, Beydoun and Klankermayer demonstrated bismuth triflate (Bi(OTf)3)-catalysed POM glycolysis using bio-derived diols to produce a number of cyclic acetals.33 This work highlighted the potential for the repeat unit of POM to be used as a C1 synthon and prompted us to view the upcycling of POM as a green alternative to formaldehyde.
Dialkoxymethanes (DAMs), a class of molecules characterised by two alkoxy groups connected by a methylene bridge, came to our attention as potential targets for POM upcycling as they have seen commercial relevance as fuel additives, solvents, and protecting groups, and are produced industrially through the reaction of formaldehyde and various alcohols.48–50 Many alternative routes to DAMs have been reported over the years, replacing formaldehyde as a methylene source with paraformaldehyde,51–53 dibromomethane,54–57 dichloromethane,58–60 dimethyl sulfoxide,61–67 or carbon dioxide,68 among others.69–71 Herein, we report, for the first time, the synthesis of DAMs using POM as a methylene source via Bi(OTf)3-catalysed POM alcoholysis. Having established the utility of this approach using pure POM feedstocks, we extend the potential usefulness of the reaction by demonstrating its applicability to feedstocks containing POM as either a major or minor component of mixed plastic waste.
Results and discussion
Alcoholysis
It was proposed that the acid-catalysed degradation of POM, using the conditions reported by Klankermayer and Beydoun,33 could be extended to the synthesis of simple DAMs (Scheme 1). Initially, we demonstrated methanolysis and ethanolysis to produce dimethoxymethane (DMM, Scheme 1, 2a) and diethoxymethane (DEM, Scheme 1, 2b). These reactions could be easily tracked using 1H, 13C{1H}, and 2D NMR spectroscopy to observe the readily identifiable resonances characteristic of the products' methylene bridges (spectra shown in ESI†). The characterisation of the POM used in these experiments is discussed in the ESI (Section 1.2).†72,73DMM and DEM were key target molecules in this investigation due to their established industrial applications. DMM, also known as methylal, has been used as a solvent, fuel additive, and a replacement for formaldehyde, but its most promising application has been in the synthesis of oxymethylene dimethyl ethers (OMEs). OMEs of certain chain lengths (n = 3 to 5) have very similar properties to diesel fuel and can be used as fuel additives to supress soot formation without significant engine modifications.48 DMM was successfully produced from the methanolysis of POM with a moderate conversion of 63% as determined by 1H NMR spectroscopy with an internal standard. Attempts to increase this conversion were unsuccessful; the product's high volatility (BP = 42 °C) may have resulted in losses post-reaction, which likely led to the conversion being underestimated.
DEM has seen industrial use as a solvent for organometallic reactions due to its superior hydrophobicity compared to THF and has been proposed as a replacement for CH2Cl2 in phase transfer reactions.49,50,74 DEM was successfully produced from the ethanolysis of POM with a good conversion of 86% as determined by 1H NMR spectroscopy with an internal standard.
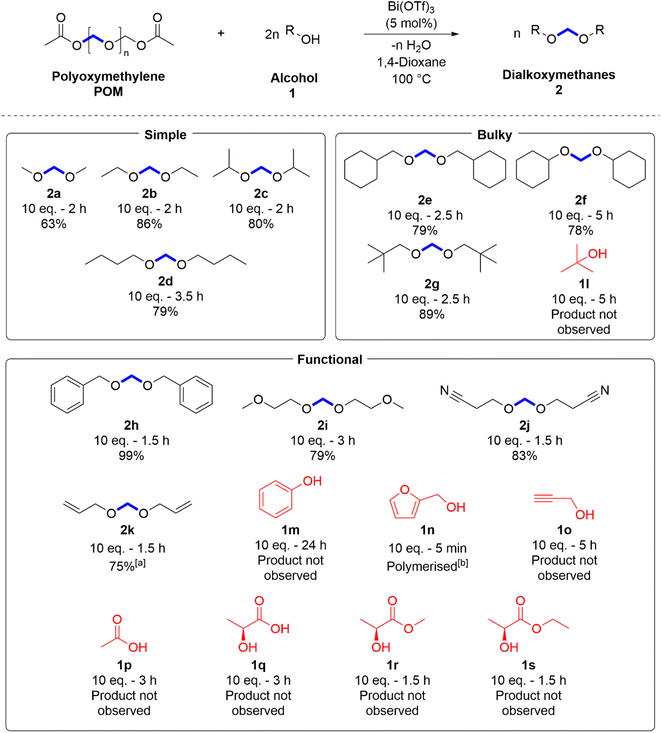
After the successful demonstration of POM methanolysis and ethanolysis, the next step was to broaden the substrate scope. POM alcoholysis was attempted with nineteen substrates selected to test the system's functional group tolerance (Scheme 2).
Eleven substrates successfully produced DAMs (2a–k), confirmed by the presence of the characteristic methylene bridge protons by 1H, 13C{1H} and 2D NMR spectroscopy with conversions between 75% and 99%. No side products were observed in these reactions, although as previously observed by Klankermayer et al., trioxane and POM oligomers were observed as intermediate species.33 No conversion to the intended product was observed for the remaining eight substrates (1l–s).
Conversion did not significantly change for either secondary alcohols (2c and 2f) or bulky primary alcohols (2e and 2g), showing that the reaction is tolerant to a moderate level of steric hindrance. 2f took twice as long as 2e to fully consume the solid polymer, which is likely due to steric effects of the alcohol. The tertiary alcohol substrate (1l) did not form product, presumably due to steric effects.
The reaction was shown to be tolerant of ethers (2i), nitriles (2j), and alkenes (2k), but no conversion was observed with the acid (1p). The alkyne substrate (1o) appeared to degrade under the hot, acidic reaction conditions, as the clear solution rapidly went black upon heating. Benzyl alcohol (2h) gave the highest observed conversion, although phenol (1m) showed no conversion at 24 h. Lactic acid (1q) and two lactate esters (1r and 1s) formed a wide array of side products, including both lactide and PLA oligomers, without forming the intended products. Attention is drawn to furfuryl alcohol (1n), which solidified and began to swell only minutes after heating began, yielding an insoluble black solid. Furfuryl alcohol has been shown to rapidly auto-polymerize and uncontrollably cross-link under acidic conditions,75 which explained the observed behaviour. Some of the intended products that were not formed in this work have been successfully synthesised by other methods (1l–o),52,59,60,67 but publications reporting production of the lighter DAMs from simple alcohols (2a–c) are rare.60,70
A range of factors were investigated for ethanolysis in order to optimise reaction conditions. A small screen of co-solvents deemed likely to solubilise POM oligomers was conducted (Table S2†). No significant difference was observed between reactions in 2-MeTHF, THF, and without the addition of a co-solvent, although all were notably slower than in 1,4-dioxane. The effect of catalyst loading was investigated (Table S3†). A ten-fold drop in catalyst loading, from 5 mol% to 0.5 mol%, led to an increase in reaction time from two to five hours, and at 0.1 mol%, the reaction took 22 hours. The effect of alcohol excess relative to the polymer repeat unit was investigated (Table S4†). Loadings from 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 up to 20
1 up to 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 showed no significant difference in reaction performance, but below 3
1 showed no significant difference in reaction performance, but below 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, conversion dropped noticeably. This observation suggested that a lower excess of alcohol could be used without affecting the reaction.
1, conversion dropped noticeably. This observation suggested that a lower excess of alcohol could be used without affecting the reaction.
To improve the sustainability of the process, ‘reuse’ of the Bi(OTf)3 catalyst was investigated. Bi(OTf)3 is an extremely hygroscopic solid, which makes recycling by isolation and subsequent reuse challenging, particularly on the lab scale. A semi-continuous process, in which the reaction vessel is reloaded with reagents has shown promise in a previous study.33 A large scale POM ethanolysis reaction was reloaded twice with POM and ethanol (see ESI Section 1.7, Table S5†) over the course of three days without additional catalyst. Conversions were low (52% initially, falling to 33% upon second reloading, 41% overall), but this may be due to poor stirring and mass transfer. Future work will build on these findings and seek to further improve the sustainability of POM alcoholysis through catalyst recycling.
While isolation of products 2a–k in high yield is possible, due to the products' general lack of functionality and tendency to form azeotropes, most would require distillation procedures beyond the scope of this preliminary investigation.48,74,76 To exemplify product isolation, 2k was produced on a five-gram of POM scale and isolated via a silica plug. In this example, a conversion of 42%, resulted in an isolated yield of 40% demonstrating the general feasibility of this approach.
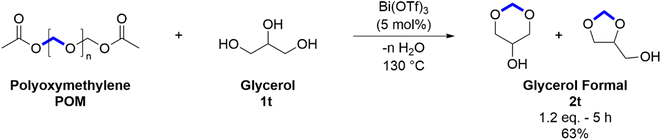
To further test the scope of the reaction, POM degradation using glycerol was demonstrated (Scheme 3). This reaction produces a mixture of two isomers known together as glycerol formal (2t) with a conversion of 63%. Glycerol formal is a commercially available mixture, the properties and broad industrial applications of which have been well described elsewhere.77–79 A 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio of the six-membered ring isomer to the five-membered ring isomer was observed which is consistent with other studies in the literature.78,80
2 molar ratio of the six-membered ring isomer to the five-membered ring isomer was observed which is consistent with other studies in the literature.78,80
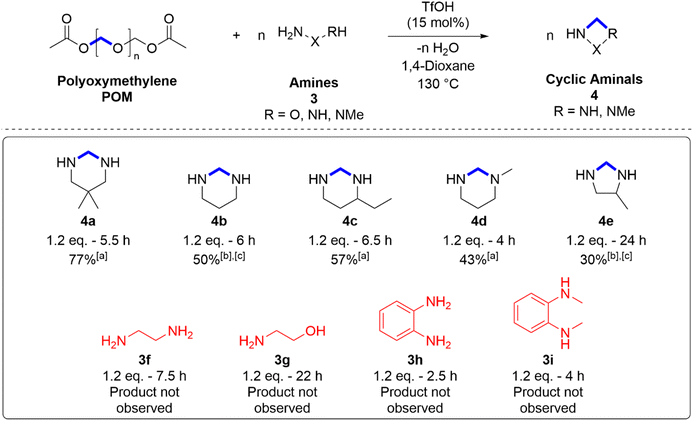
Aminolysis
To further explore the utility of acid-catalysed POM degradation, aminolysis to produce cyclic aminals was also attempted (Scheme 4). Cyclic aminals are rarely discussed in the literature but have been described as biologically important and have seen use as pharmaceutical intermediates and as starting materials for herbicide additives.81,82 Initial attempts at POM aminolysis used optimised alcoholysis conditions (5 mol% Bi(OTf)3, 100 °C) and were unsuccessful. Typically, a rapid colour change to dark brown was observed upon addition of the diamines to solutions containing Bi(OTf)3, and none of the expected products were identified. It was found, however, that POM aminolysis proceeded at 130 °C using TfOH as a catalyst (15 mol% relative to polymer repeat unit) in place of Bi(OTf)3.POM aminolysis was attempted with nine substrates. Five cyclic aminals were successfully produced (4a–e) with moderate to low conversions. Four substrates showed no conversion to product (3f–i). Cyclic aminals have been shown to be unstable in acidic media.83 This instability likely contributes to the low conversions observed. The characteristic methylene bridge signals were found in the region of ∼3.6 ppm in the 1H NMR spectrum and were strongly overlapped by the peak corresponding to the reaction solvent, 1,4-dioxane. Clearer detection of some of the products was achieved by substituting 1,4-dioxane for DMSO, although this change resulted in lower conversions. Notable is the success of a secondary amine substrate to form an asymmetric aminal product (4d) containing a tertiary amine. As predicted, due to the Thorpe–Ingold effect, the bulkier diamine substrates were able to cyclise more easily with higher conversions. The lack of observed conversion to product for substrates 3f and 3g suggested that six-membered products were more easily formed than five-membered. This effect has also been observed in the formation of cyclic carbonates via glycolysis of BPA-PC.84
POM alcoholysis of mixed plastic feedstocks
There is currently little understanding in the literature of the behaviour of POM in mixed plastic feedstock degradations. However, in developing industrially relevant processes, it is important to understand both the effect of contamination from other plastics in POM valorization and the effect of POM as a contaminant in the chemical recycling of other plastics. The compatibility of POM with various other plastics and degradation conditions was investigated, the results of which are shown in Fig. 2.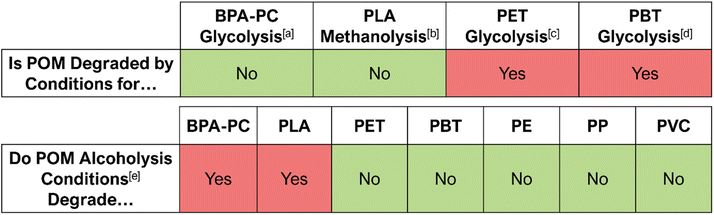 | ||
| Fig. 2 Results of depolymerizations with POM as a contaminant (top) and POM depolymerizations with other contaminants (bottom). ‘Yes’, in red, signifies undesired degradation of the contaminant, and that the reaction is not selective. ‘No’, in green, signifies that the contaminant was not degraded, and that the reaction is selective. The conditions and catalyst used for BPA-PC glycolysis, PLA methanolysis, and PET glycolysis are from previous work.26 [a] – BPA-PC glycolysis conditions: 1.3 mol% Zn half-salan complex (Zn(BAP)2),‡ 1.2 equiv. rac-1,3-butanediol, 5 mL 2-MeTHF, 75 °C.26 [b] – PLA methanolysis conditions: 0.72 mol% Zn half-salan complex (Zn(BAP)2),‡ 7 equiv. MeOH, 4 mL THF, 50 °C.26 [c] – PET glycolysis conditions: 1.3 mol% Zn half-salan complex (Zn(BAP)2),‡ 20.6 equiv. ethylene glycol, 180 °C.26 [d] – PBT glycolysis conditions: 1.3 mol% Zn half-salan complex (Zn(BAP)2),‡ 20.6 equiv. 1,4-butanediol, 180 °C. [e] – POM alcoholysis conditions: 5 mol% Bi(OTf)3, 10 equiv. EtOH, 5 mL 1,4-dioxane, 100 °C. | ||
POM was found to be stable under the conditions chosen (see Fig. 2 caption) for BPA-PC glycolysis and PLA methanolysis with no mass loss being observed. No POM was recovered after reacting under PET or poly(butylene terephthalate) (PBT) glycolysis conditions, and traces of 1,3-dioxolane were observed by 1H NMR spectroscopy. It was therefore concluded that POM is unstable under PET and PBT glycolysis conditions. End-capped POM is reported to be stable up to only approximately 160 °C,36 and given that the conditions for PET and PBT glycolysis require 180 °C, it is likely that the majority of the POM degraded to formaldehyde.
The conditions for POM alcoholysis were not found to be selective with respect to BPA-PC or PLA and partially degraded both polymers. The conditions were selective with respect to PET, PBT, PE, PP, and PVC, as no mass loss of any contaminant was observed.
To demonstrate the utility of the selectivities described in Fig. 2, two mixed samples of post-consumer plastic waste containing POM were sequentially degraded. The first was a sample containing BPA-PC from a broken pair of safety glasses and POM from a broken glassware clip (Fig. 3, top). The BPA-PC was depolymerized with a conversion to BPA of 96%, and no mass loss of POM was observed. The remaining POM was washed, dried, and underwent ethanolysis to produce DEM with a conversion of 75%. The second was a sample containing POM from a broken glassware clip and PET from a soft drink bottle (Fig. 3, bottom). The POM underwent ethanolysis to produce DEM with a conversion of 85%, and no mass loss of PET was observed. The remaining PET was washed, dried, and depolymerized by glycolysis to produce bis(2-hydroxyethyl) terephthalate (BHET) with a conversion of 82%. These experiments demonstrate the value of establishing relative selectivities in order to design sequential chemical processes for chemical valorization and potentially simplify pretreatment and sorting strategies for mixed plastic waste.
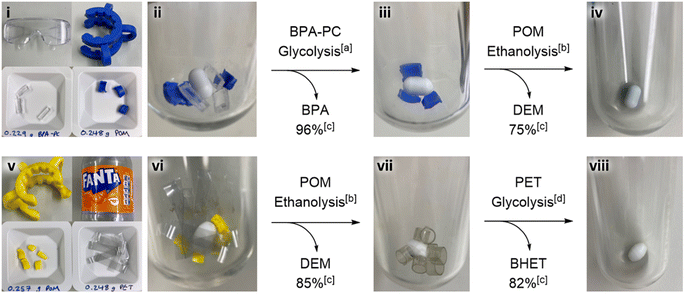 | ||
| Fig. 3 Sequential chemical recycling of two mixed samples of post-consumer plastic waste containing POM. The first (upper) shows: (i) sources of BPA-PC and POM, (ii) plastic pre-reaction, (iii) untouched POM after full consumption of the BPA-PC to produce BPA, and (iv) empty flask after full consumption of the POM to produce DEM. The second (lower) shows: (v) sources of POM and PET, (vi) plastic pre-reaction, (vii) untouched PET after full consumption of the POM to produce DEM, and (viii) the empty flask after full consumption of PET to produce bis(2-hydroxyethyl) terephthalate (BHET). [a] – BPA-PC glycolysis conditions: 1.3 mol% Zn half-salan complex (Zn(BAP)2),‡ 1.2 equiv. rac-1,3-butanediol, 5 mL 2-MeTHF, 75 °C.26 [b] – Optimised POM alcoholysis conditions: 1 mol% Bi(OTf)3, 3 equiv. EtOH, 5 mL 1,4-dioxane, 100 °C. [c] – Conversion determined by 1H NMR spectroscopy using trimethoxybenzene as an internal standard. [d] – PET glycolysis conditions: 1.3 mol% Zn half-salan complex (Zn(BAP)2),‡ 20.6 equiv. ethylene glycol, 180 °C.26 | ||
Conclusion
In summary, two novel methods of chemically recycling POM that harness the polymer's repeat unit as a C1 synthon have been reported. The first produced dialkoxymethanes, an industrially relevant class of compounds that have not previously been synthesised from plastic waste. The second produced cyclic aminals which have seen modest application to date but have not been previously synthesised from plastic waste. The behaviour of POM in mixed plastic chemical recycling systems was established for the first time, with the selectivity of POM alcoholysis with respect to a number of contaminants reported. These relative selectivities were exploited to demonstrate the sequential chemical recycling of two mixed samples of post-consumer plastic waste containing POM, illustrating the promise of chemical recycling strategies as a potential solution to help alleviate ‘the plastics problem’.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank Abbott Diabetes Care and the Institute of Sustainability and Climate Change at the University of Bath for funding (PhD studentship for MJC). We thank Dr Carlo Di Iulio and Dr Hana Postulkova of Abbot Diabetes Care for useful discussions. The support of the Core Research Facilities at the University of Bath is also gratefully acknowledged, particularly Dr Tim Woodman. JAS, MDJ and MGD acknowledge support from the EPSRC (UK Catalysis Hub EP/R027129/1).References
- H. Sardon and A. P. Dove, Science, 2018, 360, 380–381 CrossRef CAS.
- World Economic Forum, Ellen MacArthur Foundation and McKinsey & Company, The New Plastics Economy: Rethinking the Future of Plastics, 2016 Search PubMed.
- S. J. Davis, K. Caldeira and H. D. Matthews, Science, 2010, 329, 1330–1333 CrossRef CAS.
- Plastics – the Facts 2022, Plastics Europe, 2022, https://plasticseurope.org/knowledge-hub/plastics-the-facts-2022/, accessed January 2024 Search PubMed.
- R. Geyer, J. R. Jambeck and K. L. Law, Sci. Adv., 2017, 3, e1700782 CrossRef PubMed.
- Z. O. G. Schyns and M. P. Shaver, Macromol. Rapid Commun., 2021, 42, 2000415 CrossRef CAS PubMed.
- J. M. Garcia and M. L. Robertson, Science, 2017, 358, 870–872 CrossRef CAS PubMed.
- J. Payne, P. McKeown and M. D. Jones, Polym. Degrad. Stab., 2019, 165, 170–181 CrossRef CAS.
- A. Rahimi and J. M. García, Nat. Rev. Chem, 2017, 1, 0046 CrossRef.
- T. Thiounn and R. C. Smith, J. Polym. Sci., 2020, 58, 1347–1364 CrossRef CAS.
- J. Payne and M. D. Jones, ChemSusChem, 2021, 14, 4041–4070 CrossRef CAS PubMed.
- E. Feghali and T. Cantat, ChemSusChem, 2015, 8, 980–984 CrossRef CAS PubMed.
- R. Yang, G. Xu, B. Dong, X. Guo and Q. Wang, ACS Sustain. Chem. Eng., 2022, 10, 9860–9871 CrossRef CAS.
- A. J. Spicer, A. Brandolese and A. P. Dove, ACS Macro Lett., 2024, 189–194, DOI:10.1021/acsmacrolett.3c00751.
- S. Westhues, J. Idel and J. Klankermayer, Sci. Adv., 2018, 4, eaat9669 CrossRef CAS PubMed.
- A. Carné Sánchez and S. R. Collinson, Eur. Polym. J., 2011, 47, 1970–1976 CrossRef.
- C. Jehanno, J. Demarteau, D. Mantione, M. C. Arno, F. Ruipérez, J. L. Hedrick, A. P. Dove and H. Sardon, Angew. Chem., Int. Ed., 2021, 60, 6710–6717 CrossRef CAS PubMed.
- M. Arifuzzaman, B. G. Sumpter, Z. Demchuk, C. Do, M. A. Arnould, M. A. Rahman, P.-F. Cao, I. Popovs, R. J. Davis, S. Dai and T. Saito, Mater. Horiz., 2023, 10, 3360–3368 RSC.
- J. A. Stewart, J. I. Pearce, M. J. Cullen, G. Kociok-Köhn, B. D. Ward, M. G. Davidson and M. D. Jones, Catal. Today, 2024, 115037, DOI:10.1016/j.cattod.2024.115037.
- T. Uekert, A. Singh, J. S. DesVeaux, T. Ghosh, A. Bhatt, G. Yadav, S. Afzal, J. Walzberg, K. M. Knauer, S. R. Nicholson, G. T. Beckham and A. C. Carpenter, ACS Sustain. Chem. Eng., 2023, 11, 965–978 CrossRef CAS.
- C. Jehanno, I. Flores, A. P. Dove, A. J. Müller, F. Ruipérez and H. Sardon, Green Chem., 2018, 20, 1205–1212 RSC.
- V. Sinha, M. R. Patel and J. V. Patel, J. Polym. Environ., 2010, 18, 8–25 CrossRef CAS.
- E. Barnard, J. J. Rubio Arias and W. Thielemans, Green Chem., 2021, 23, 3765–3789 RSC.
- E. Quaranta, D. Sgherza and G. Tartaro, Green Chem., 2017, 19, 5422–5434 RSC.
- R. Piñero, J. García and M. J. Cocero, Green Chem., 2005, 7, 380–387 RSC.
- J. M. Payne, M. Kamran, M. G. Davidson and M. D. Jones, ChemSusChem, 2022, 15, e202200255 CrossRef CAS PubMed.
- K. Saito, C. Jehanno, L. Meabe, J. L. Olmedo-Martínez, D. Mecerreyes, K. Fukushima and H. Sardon, J. Mater. Chem. A, 2020, 8, 13921–13926 RSC.
- J. G. Kim, Polym. Chem., 2020, 11, 4830–4849 RSC.
- Y. Liu and X.-B. Lu, J. Polym. Sci., 2022, 60, 3256–3268 CrossRef CAS.
- J. M. Payne, G. Kociok-Köhn, E. A. C. Emanuelsson and M. D. Jones, Macromolecules, 2021, 54, 8453–8469 CrossRef CAS.
- J. Stewart, M. Fuchs, J. Payne, O. Driscoll, G. Kociok-Köhn, B. D. Ward, S. Herres-Pawlis and M. D. Jones, RSC Adv., 2022, 12, 1416–1424 RSC.
- P. McKeown and M. D. Jones, Sustainable Chem., 2020, 1, 1–22 CrossRef.
- K. Beydoun and J. Klankermayer, ChemSusChem, 2020, 13, 488–492 CrossRef CAS PubMed.
- ChemAnalyst, Decode the Future of Polyoxymethylene (POM), https://www.chemanalyst.com/industry-report/polyoxymethylene-market-2903#:%7E:text=TheglobalPolyoxymethylene(POM)market,theforecastperioduntil2032, (accessed March 12, 2024).
- D. L. Jarvis, in Brydson's Plastics Materials, ed. M. Gilbert and M. Deans, 2016, 8th edn, p. 513 Search PubMed.
- V. W. Kern and H. Cherdron, Die Makromolekulare Chem., 1960, 40, 101–117 CrossRef.
- White Paper – DuPont Delrin acetal homopolymer, https://www.emcoplastics.com/assets/pdf/acetal/DelrinHomopolymerAdvantagesOverAcetalCopolymerDupont.pdf.
- M. Haubs, K. Kurz and G. Sextro, in Ullmann's Encyclopedia of Industrial Chemistry, 2012, DOI:10.1002/14356007.a21_591.pub2.
- G. Berkowicz, T. M. Majka and W. Żukowski, Energy Convers. Manage., 2020, 214, 112888 CrossRef CAS.
- V. M. Archodoulaki, S. Lüftl, T. Koch and S. Seidler, Polym. Degrad. Stab., 2007, 92, 2181–2189 CrossRef CAS.
- Hoechst Ag Starts Up Pom Recycling Plant, https://www.plasticsnews.com/article/19961209/NEWS/312099971/hoechst-ag-starts-up-pom-recycling-plant, (accessed January 18, 2024).
- HOECHST – Polyacetal recycling plant starts operation, https://www.plasteurope.com/news/HOECHST_t19656/, (accessed January 18, 2024).
- M. Karl-Friedrich, R. Gerhard and F. Dietrich, CA Pat., 2055131A1, 1992 Search PubMed.
- V. Ghalandari, H. Smith, A. Scannell and T. Reza, Waste Manage., 2024, 174, 126–139 CrossRef CAS.
- Y. Zhou, J. Rodríguez-López and J. S. Moore, Nat. Commun., 2023, 14, 4847 CrossRef CAS.
- L. Lu, J. Luo, M. Montag, Y. Diskin-Posner and D. Milstein, J. Am. Chem. Soc., 2024, 146, 22017–22026 CrossRef.
- S. Chen, X. Zhang, C. Zhang and X. Zhang, Macromolecules, 2024, 57, 7841–7846 CAS.
- R. Sun, I. Delidovich and R. Palkovits, ACS Catal., 2019, 9, 1298–1318 CrossRef CAS.
- M. Coleman, Chim. Oggi, 2009, 27, 43–45 CAS.
- N. W. Boaz and B. Venepalli, Org. Process Res. Dev., 2001, 5, 127–131 CrossRef CAS.
- A. R. A. S. Deshmukh, V. K. Gumaste and B. M. Bhawal, Synth. Commun., 1995, 25, 3939–3944 CrossRef CAS.
- D. D. Pathak and J. J. Gerald, Synth. Commun., 2003, 33, 1557–1561 CrossRef CAS.
- M. Subaramanian, A. Bera, B. L. V. Prasad and E. Balaraman, J. Chem. Sci., 2017, 129, 1153–1159 CrossRef CAS.
- A. Kh. Nazaretyan, G. O. Torosyan and A. T. Babayan, J. Appl. Chem. USSR, 1986, 58, 2580–2583 Search PubMed.
- M. G. Kulkarni, S. I. Davawala, A. K. Doke and A. V. Doke, Indian J. Chem., 2003, 42B, 2121–2123 CAS.
- M.-L. Wang, S.-L. Hsu and Y.-M. Hsieh, Chem. Eng. Commun., 2006, 193, 1368–1383 CrossRef CAS.
- Y. M. Loksha, E. B. Pedersen, R. Loddo, G. Sanna, G. Collu, G. Giliberti and P. L. Colla, J. Med. Chem., 2014, 57, 5169–5178 CrossRef CAS PubMed.
- P. L. André Cornélis, Synthesis, 1982, 2, 162–163 CrossRef.
- L. Zhan, R. Pan, P. Xing and B. Jiang, Tetrahedron Lett., 2016, 57, 4036–4038 CrossRef CAS.
- D. Sánchez-Roa, M. E. G. Mosquera and J. Cámpora, J. Org. Chem., 2021, 86, 16725–16735 CrossRef PubMed.
- T. Sato, Y. Saito, M. Kainosho and K. Hata, Bull. Chem. Soc. Jpn., 1967, 40, 391–394 CrossRef.
- S. Hanessian, G. Yang-Chung, P. Lavallee and A. G. Pernet, J. Am. Chem. Soc., 1972, 94, 8929–8931 CrossRef CAS.
- B. S. Bal and H. W. Pinnick, J. Org. Chem., 1979, 44, 3727–3728 CrossRef CAS.
- R. M. Munavu, J. Org. Chem., 1980, 45, 3341–3343 CrossRef CAS.
- M. Guiso, C. Procaccio, M. R. Fizzano and F. Piccioni, Tetrahedron Lett., 1997, 38, 4291–4294 CrossRef CAS.
- Y. Z. Guobiao Chu, C. Li and Y. Zhang, Synthesis, 2009, 22, 3828–3832 Search PubMed.
- R. Talukdar, New J. Chem., 2019, 43, 13334–13338 RSC.
- B. G. Schieweck and J. Klankermayer, Angew. Chem., Int. Ed., 2017, 56, 10854–10857 CrossRef CAS PubMed.
- B. C. Barot and H. W. Pinnick, J. Org. Chem., 1981, 46, 2981–2983 CrossRef CAS.
- K. Beydoun, K. Thenert, J. Wiesenthal, C. Hoppe and J. Klankermayer, ChemCatChem, 2020, 12, 1944–1947 CrossRef CAS.
- B. Miao, M. Xue, S. Ji, Z. Wang and Y. Zhang, J. Org. Chem., 2023, 88, 1128–1134 CrossRef CAS.
- M. Berer, G. Pinter and M. Feuchter, J. Appl. Polym. Sci., 2014, 131, 40831 CrossRef.
- O. Tooley, W. Pointer, R. Radmall, M. Hall, V. Beyer, K. Stakem, T. Swift, J. Town, T. Junkers, P. Wilson, D. Lester and D. Haddleton, Macromol. Rapid Commun., 2024, 45, 2300692 CrossRef CAS.
- D. L. Martin and P. W. Reynolds, US Pat., 4740273A, 1987 Search PubMed.
- G. Tondi, N. Cefarin, T. Sepperer, F. D’Amico, R. J. F. Berger, M. Musso, G. Birarda, A. Reyer, T. Schnabel and L. Vaccari, Polymers, 2019, 11, 2126 CrossRef CAS PubMed.
- X. Zhang, S. Zhang and C. Jian, Chem. Eng. Res. Des., 2011, 89, 573–580 CrossRef CAS.
- D. M. Sanderson, J. Pharm. Pharmacol., 2011, 11, 150–156 CrossRef.
- J. Deutsch, A. Martin and H. Lieske, J. Catal., 2007, 245, 428–435 CrossRef CAS.
- V. R. Ruiz, A. Velty, L. L. Santos, A. Leyva-Pérez, M. J. Sabater, S. Iborra and A. Corma, J. Catal., 2010, 271, 351–357 CrossRef CAS.
- A. C. da Silva Ferreira, J.-C. Barbe and A. Bertrand, J. Agric. Food Chem., 2002, 50, 2560–2564 CrossRef CAS.
- A. R. Katritzky, S. K. Singh and H.-Y. He, J. Org. Chem., 2002, 67, 3115–3117 CrossRef CAS PubMed.
- T. Kang, S. Gao, L.-X. Zhao, Y. Zhai, F. Ye and Y. Fu, J. Agric. Food Chem., 2021, 69, 45–54 CrossRef CAS PubMed.
- E. Sawatzky, A. Drakopoulos, M. Rölz, C. Sotriffer, B. Engels and M. Decker, Beilstein J. Org. Chem., 2016, 12, 2280–2292 CrossRef CAS PubMed.
- C. Jehanno, J. Demarteau, D. Mantione, M. C. Arno, F. Ruipérez, J. L. Hedrick, A. P. Dove and H. Sardon, ACS Macro Lett., 2020, 9, 443–447 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4su00547c |
| ‡ Zn(BAP)2 refers to a Zn half-salan complex reported by Jones et al.,26 see Fig. S1 (ESI).† |
| This journal is © The Royal Society of Chemistry 2025 |