Open Access Article
Open Access ArticleExploring the spectrum: an environmental examination of hydrogen's diverse colors
Hafsa Mehmoodab,
Haseeb Akbarab,
Pariyapat Nilsalabab and
Shabbir H. Gheewala *ab
*ab
aThe Joint Graduate School of Energy and Environment, King Mongkut's University of Technology Thonburi, Bangkok 10140, Thailand. E-mail: shabbir.ghe@kmutt.ac.th; shabbirg@hotmail.com
bCenter of Excellence on Energy Technology and Environment, Ministry of Higher Education, Science, Research and Innovation, Bangkok 10400, Thailand
First published on 17th December 2024
Abstract
Hydrogen is emerging as an immense source of energy having the potential to at least partly replace fossil fuels. It is an abundant element on earth, but does not mainly exist in free form. Hydrogen can be produced through different technologies and feedstocks, and based on these, it can be categorized into colors with different environmental impacts. This work aimed to review the environmental impacts of the production of gray (from natural gas without carbon capture and storage), brown (from coal gasification), blue (from fossil fuels with carbon capture and storage), green (from renewable energy or biological process), and turquoise (pyrolysis of natural gas) hydrogen and to identify sustainable hydrogen production pathways that minimize environmental impacts. Global warming, acidification, eutrophication, and resource depletion were considered as indicators to assess the environmental impacts. The results showed that brown hydrogen produced via coal gasification had the highest global warming, acidification, and resource depletion impacts among all the options considered. On the other hand, green hydrogen from electrolysis through wind energy had the lowest environmental impacts. However, adopting these hydrogen colors presents different challenges and opportunities. Success depends on effective policy frameworks, international cooperation, and technological readiness to ensure positive contributions to global sustainability goals.
Introduction
Energy is one of the main pillars of the development of a country. Presently, the major source of energy is fossil fuels, sharing about 76.5% of the global energy supply, followed by renewable energy at 19.8% and nuclear energy at 3.7%.1 Fossil fuels cannot continue to provide energy because they are finite, and their use contributes to environmental degradation, leading to global warming, extreme weather events, biodiversity loss, etc. It is estimated that by 2040, the energy demand will rise by 56%, and if this current reliance on fossil fuels persists to meet the increasing demand, greenhouse gas (GHG) emissions will escalate even further.2 The CO2 levels are already too high compared to the pre-industrial level, mainly due to the fossil fuel-driven energy sector. From 1990 to 2023, global CO2 emissions have increased by more than 75%, growing from 21.1 to 37.4 Gt.3 To reduce GHG emissions, various forms of renewable energy, such as wind, solar, hydro, thermal, biomass, etc., will have to provide for the bulk of future energy demands. However, the energy produced by many of the renewable systems fluctuates throughout the year. Hydrogen, in this regard, is highly versatile as a secondary energy carrier. It has the ability to address and mitigate the inherent variability of renewable sources and can help renewable energy sources contribute in a more efficient and significant way throughout the year and across geographical locations.4 Another important factor in the incorporation of hydrogen into the current energy mix is that it can help decarbonize the energy sector. It is gaining much attention because of its potential to address challenges like climate change and has the potential to reduce reliance on fossil fuels.5 Hydrogen has high energy intensity among various fuels and can be transferred over long distances without losing efficiency.6 Bringing hydrogen into the energy mix is a key to a clean way to store and transport energy, cutting down on fossil fuels, and making the primary energy supply more resilient to climate change. It is suitable for the planet, people, and pocket, and it can help to address several United Nations Sustainable Development Goals (SDGs) like SDG 7 (affordable and clean energy), SDG 13 (climate action), and SDG 17 (partnership for the goals).4Hydrogen is itself a colorless gas, but is now known by its different colors, which are derived from the technology and source of its production. The emissions generated during the production of hydrogen depend on the resources used for its production. Currently, approximately 95% of all the hydrogen is being produced from fossil fuels with adverse environmental impacts.7 Researchers are trying to find more promising and sustainable ways to produce hydrogen energy. Green hydrogen is increasingly popular because it has the potential to achieve a zero-carbon economy. It is produced from electricity generated by renewable energy sources like solar, wind, and hydro through the electrolysis of water and thus has less GHG emissions. The GHG emissions vary widely from technique to technique for hydrogen production. Thus, most of the studies focused only on GHG emissions linked with hydrogen production.8–10 Focusing solely on GHG emissions can mislead policymakers. For example, blue hydrogen, generated via steam methane reforming (SMR) of natural gas with carbon capture and storage (CCS), has the potential to achieve lower GHG emissions. The integration of CCS technology mitigates a significant portion of the CO2 emissions typically associated with SMR, making blue hydrogen a potentially favorable option in terms of reducing GHG emissions and addressing climate change concerns.11 However, when other environmental impacts such as acidification, eutrophication, and abiotic resource depletion are considered, the overall assessment could lead to a markedly different conclusion.
Most existing reviews have focused primarily on global warming, and some have also considered acidification.12,13 A few mentioned other impact categories, but did not analyze them any further.14 This study conducted a comprehensive review of the existing literature and employed metrics such as global warming, acidification, eutrophication, and resource depletion to analyze the environmental impacts across a comprehensive set of pathways of hydrogen production. In addition, this analysis was used to formulate a ranking of the pathways that were further qualified for short, medium, and long-term application using their technology readiness levels. Ultimately, this research aims to contribute to the global effort of transitioning to cleaner energy, reducing GHGs, and achieving carbon neutrality.
Methodology
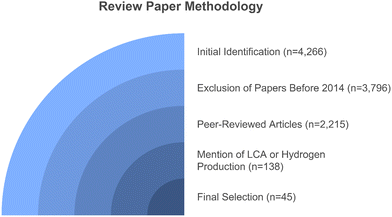
This work focuses on reviewing the environmental impacts of different production pathways of hydrogen represented popularly in the literature using different colors, viz., blue (from natural gas with carbon capture and storage), gray (from natural gas without carbon capture and storage), brown (from coal gasification), green (from renewable energy), and turquoise (pyrolysis of natural gas). The selection of hydrogen production pathways in this review reflects a strategic approach to encompass both established technologies, which are currently viable for near-term deployment, and emerging technologies with lower technological maturity but substantial potential to reduce environmental impacts. The inclusion of emerging pathways highlights their reliance on renewable feedstocks, making them attractive candidates for integration into long-term sustainable energy systems. Additionally, the selected pathways represent a balanced exploration of hydrogen production technologies derived from fossil fuels and those utilizing renewable energy sources, ensuring comprehensive coverage of current and future scenarios for hydrogen generation. This approach enables a thorough evaluation of the trade-offs between technological readiness, environmental performance, and alignment with decarbonization goals. For this study, articles were retrieved using Google Scholar and ScienceDirect databases as the search engines, employing the following search terms to ensure relevance to the scope of the review: “Hydrogen production” AND “Colors of hydrogen” AND “Environmental impact” (“Life Cycle Assessment” OR “LCA”). These terms were applied to search within the title, keywords, or abstract of articles to capture publications focused on the life cycle assessment of hydrogen production methods.A total of 4266 papers were initially identified in the databases. Papers published before 2014 were excluded (n = 3796), considered only peer-reviewed articles (n = 2215). During the screening process, studies that did not mention LCA or hydrogen production technologies in their abstracts were also excluded (n = 138). The final selection focused on papers discussing hydrogen production technologies with LCA integration, where only those that provided a comprehensive explanation of methodology and framework were included for further analysis (n = 45). The graphical representation of the article selection for the literature review is shown in Fig. 1.
To improve the quality further, highly cited studies were selected for review. The environmental impacts of hydrogen production of different colors by using the LCA methodology were investigated. LCA offers a systematic and analytical approach to assessing the environmental impact of products and services throughout their entire life cycle, from the extraction of raw materials to their disposal or recycling.15 Life cycle assessment provides a holistic approach that enables a thorough assessment of all environmental impacts, including resource depletion, greenhouse gas emissions, energy use, etc. The LCA is widely used in the scientific community. Notable hydrogen-related studies that have applied LCA for environmental assessment include those by Bhandari et al.,16 Osman et al.,14 and Salkuyeh et al.17 The LCA is particularly valuable as it identifies trade-offs between different impact categories and supports informed decision-making by comparing various hydrogen production methods. In contrast to other methodologies, such as the environmental impact assessment method, which focuses on specific projects. Life cycle assessment is superior to other methods, especially for comparative analysis.18 Other methodologies, such as carbon footprint analysis, which mainly assesses greenhouse gas emissions, LCA provides a broader, more detailed evaluation.
As replacement of fossil energy is the key issue of concern due to its non-renewability and emissions of greenhouse gases, global warming, acidification, eutrophication, and resource depletion were analyzed as the main environmental indicators of interest.19–21 Thus, the LCA methodology was chosen for comparing environmental performance of different colors of hydrogen. Further, these indicators were normalized to rank the hydrogen colors based on environmental impacts. These indicators were normalized by applying the minimum–maximum normalization technique,22 as shown in eqn (1).
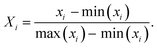 | (1) |
Overview of different hydrogen production techniques
In this section, a brief description of techniques, associated colors, technology readiness level, efficiency, and specific energy of different fuels are discussed.Steam methane reforming is a primary industrial process for producing hydrogen. It involves a chemical reaction where methane, typically from natural gas, reacts with steam under high temperatures (700 °C to 1000 °C) and pressure in the presence of a nickel-based catalyst.23 The principal reactions in the SMR process are shown in eqn (2) and (3).
| CH4 + H2O → CO + 3H2 | (2) |
| CO + H2O → CO2 + H2 | (3) |
• Coal gasification
Coal gasification is a process that converts coal into a synthesis gas (syngas) comprising primarily hydrogen, carbon monoxide, and often some carbon dioxide.24 The basic chemical reaction in coal gasification is typically represented as shown in eqn (4).
| C + H2O → CO + H2 | (4) |
• Biomass gasification
Biomass gasification is a thermochemical process that transforms organic materials into combustible gases at high temperatures with controlled oxygen or steam. This process starts with pyrolysis, where biomass is decomposed without oxygen to produce char, tar, and gases. The remaining biomass undergoes gasification reactions with steam and carbon dioxide, producing syngas rich in hydrogen, carbon monoxide, and methane. This syngas is cleaned to remove impurities like tar, particulates, and sulfur compounds. The hydrogen content is enhanced through the water–gas shift reaction, where carbon monoxide reacts with steam to produce additional hydrogen and carbon dioxide. Finally, the hydrogen is purified using techniques like pressure swing adsorption or membrane separation, yielding high-purity hydrogen for use in fuel cells, industrial processes, or as a clean energy carrier, providing a sustainable solution to manage biomass waste and decrease dependency on fossil fuels.
• Electrolysis
Hydrogen production via electrolysis involves using electricity to split water into hydrogen and oxygen, as shown in eqn (5). This method is eco-friendly when powered by renewable energy sources, producing “green hydrogen.” Key components include the electrodes (anode and cathode), electrolyte, and power source. Technologies vary in efficiency, including alkaline, PEM (proton exchange membrane), and SOEC (solid oxide electrolysis cells). Electrolysis is crucial for storing renewable energy and supplying clean fuel, particularly useful in heavy industry and transportation.25
| H2O + direct current electricity → H2 + 1/2O2 | (5) |
• Dark fermentation
Dark fermentation is a biological process used to produce hydrogen gas from organic materials without the need for light. It involves the anaerobic breakdown of organic substrates by microorganisms, typically bacteria, which results in the production of hydrogen along with other byproducts like carbon dioxide and organic acids.26 The basic chemical reaction in dark fermentation can be summarized by the conversion of glucose, a common substrate, into hydrogen, carbon dioxide, and other organic acids. The generalized chemical equation as shown in eqn (6).
| C6H12O6 → 2H2 + 2CO2 + 2CH3COOH | (6) |
• Photo fermentation
Photo fermentation is a process where photosynthetic bacteria use light to convert organic substrates into hydrogen. It primarily involves purple non-sulfur bacteria, which use sunlight to break down organic compounds like glucose into hydrogen and carbon dioxide, as shown in eqn (7) and (8). This method is valued for its use of renewable light energy, its ability to reduce waste by processing organic materials, and minimal carbon emissions. Commonly applied in waste treatment and renewable energy projects, photo fermentation offers a sustainable way to produce clean hydrogen fuel.27,28
| 6H2O + 6CO2 → C6H12O6 + 6O2 | (7) |
| C6H12O6 + 6H2O → 6CO2 + 12H | (8) |
• Thermochemical water splitting
Thermochemical water splitting cycles offer significant benefits, including the absence of catalysis requirements for individual chemical reactions. Water serves as the primary material for hydrogen production, and all other chemicals involved in the cycle are recyclable. Additional advantages of thermochemical water splitting cycles include: (i) no necessity for O2–H2 separation membranes, (ii) a moderate temperature range requirement of 600–1200 K, and (iii) minimal to no electrical energy requirements.29
Hydrogen colors
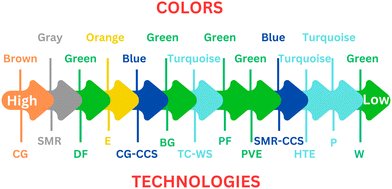
This section offers a comprehensive examination of the diverse hydrogen production techniques, each characterized by distinct colors, which are shown in Fig. 2. The use of color-coding serves as an intuitive means to categorize these methods, simplifying the understanding of their respective environmental footprints and the underlying technologies they employ. The color coding is not merely aesthetic but functional, providing a quick visual reference to gauge the environmental sustainability of each hydrogen production method. This simplifies understanding for stakeholders, enabling them to easily gauge the ecological impact of each method. Blue hydrogen comes from fossil fuels, but CO2 which comes as its byproduct is captured and stored. Gray hydrogen, made from natural gas through steam methane reforming without CCS technology, releases CO2 directly into the air. Brown hydrogen is coal-based, creating significant pollution and emitting CO2 and carbon monoxide. Lastly, methods using thermal energy for hydrogen production from fossil fuels are termed turquoise hydrogen.30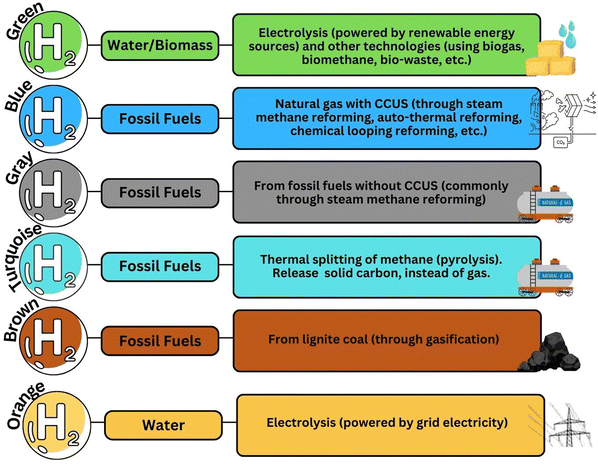 | ||
| Fig. 2 Different colors of hydrogen by the technologies and feedstocks.5,30,31 | ||
Technology readiness level and energy efficiency
The technology readiness levels (TRL) framework outlines the maturity of a technology from its inception to commercial deployment.32 It starts with the initial idea, where fundamental principles are defined, progressing to application formulated, which develops the concept and its practical applications.33 The next stages include concept needs validation, requiring prototyping; early prototype, demonstrating proof of concept; and large prototype, validating components in deployment-like conditions. Following this, full prototype at scale confirms functionality in real-world scenarios, while pre-commercial demonstration indicates operational success in expected conditions. First of a kind commercial denotes a successful commercial deployment, and commercial operation in relevant environment indicates market availability with potential for improvement. Finally, integration needed at scale highlights the necessity for further integration, and proof of stability reached signifies predictable growth. This framework captures the evolution of technologies from theoretical concepts to market-ready solutions. The TRLs along with the description are presented in Fig. 3.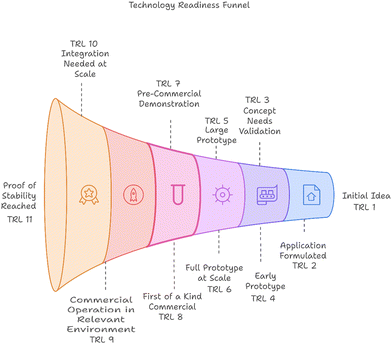 | ||
| Fig. 3 Description of technology readiness level (TRL) of hydrogen production technologies.32 | ||
Wilkinson et al.34 provided an overview of hydrogen production via different methods and various feedstocks, highlighting technology readiness levels. Electrolytic processes like alkaline electrolysis have the highest readiness at level 9, while newer methods like proton exchange membrane, solid oxide electrolysis, and anion exchange membrane range from levels 2 to 8. thermochemical methods like photovoltaic electrolysis and high-temperature electrolysis are at levels 5–7, while water-splitting cycles are lower at 3–4. Fossil fuel-based methods, particularly steam methane reforming with CCS, are mature at level 9, as are coal gasification and partial oxidation. Emerging technologies like methane cracking and syngas chemical looping are still developing, with readiness levels of 3–5. Biomass-based processes like SMR and gasification are at level 9, while biological methods such as dark fermentation and photo-fermentation are less advanced (levels 1–4). Table 1 covers hydrogen production pathways utilizing a range of energy feedstocks, each with varying technology readiness levels.
| Feedstock | Process type | Production technology | Technology readiness level |
|---|---|---|---|
| Water | Electrolytic | Alkaline electrolysis (AE) | 9 |
| Proton exchange membrane electrolysis (PEM) | 6–8 | ||
| Solid oxide electrolyser cell electrolysis (SOE) | 5 | ||
| Anion exchange membrane electrolysis (AEM) | 2–3 | ||
| Nanogap electrochemical cells | 1–3 | ||
| Thermochemical | Photovoltaic electrolysis (PVE) | 5–7 | |
| High-temperature electrolysis (HTE) | 5–7 | ||
| Thermochemical water-splitting cycles (TCC) | 3–4 | ||
| Fossil fuel | Photolytic | Photocatalytic water splitting | 1–3 |
| Thermochemical | Steam methane reforming (SMR) | 9 (8 with CCS) | |
| Partial oxidation – thermal or catalytic | 9 (8 with CCS) | ||
| Chemical looping reforming (CLR) | 8 (6 with CCS) | ||
| Coal gasification | 9 (7 with CCS) | ||
| Autothermal reforming (ATR) – dry or steam | 7 (5 with CCS) | ||
| Methane cracking (CRA) | 3–5 | ||
| Syngas chemical looping (SCL) | 3–5 | ||
| Biomass | Thermochemical | Steam methane reforming (SMR) | 9 (8 with CCS) |
| Biomass gasification | 9 (5 with CCS) | ||
| Autothermal reforming (ATR) – dry or steam | 7 (5 with CCS) | ||
| Biological | Dark fermentation | 2–4 | |
| Biological/photolytic | Photo-fermentation | 1–3 | |
| Other | Thermochemical | Co-product of industrial process (chlor-alkali) | 1–3 |
| Processing of non-organic waste products | 1–3 |
Goren et al.,38 presented energy efficiency of different hydrogen production technologies. The mean value energy efficiencies of different technologies with expected variations is presented in Fig. 4. Fossil fuel steam reforming is highly efficient at 72.5% (±10%), though its effectiveness can vary with feedstock and conditions. Fossil fuel gasification, less efficient at 55% (41–69%), is more inconsistent, making it less reliable for energy production. Biomass steam reforming has similar efficiency (71.5% ± 13%) but with slightly higher uncertainty, while biomass gasification is less efficient (45% ± 12%) and more sensitive to operational conditions. Dark fermentation offers moderate efficiency (60% ± 15%) but is highly variable, impacting its scalability. Photo-fermentation, with the lowest efficiency (16% ± 9%), is an emerging technology that needs further improvement due to significant energy losses. Microbial electrolysis cells (68% ± 9%) are more stable and promising for hydrogen production. Water electrolysis is also efficient (71.5% ± 10%) but depends on electricity sources, especially when using renewable energy.
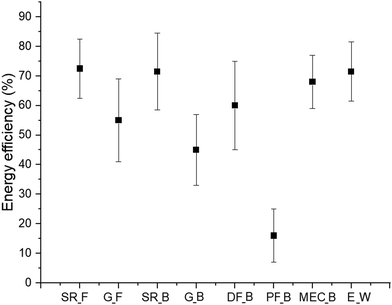 | ||
| Fig. 4 Average energy efficiency values of H2 production methods for different resources.38 SR_F is steam reforming using fossil, G_F is gasification using fossil, SR_B is steam reforming using biomass, G_B is gasification using biomass, DF_B is dark fermentation using biomass, PF_B is photo-fermentation, using biomass, MEC_B is microbial electrolysis cell using biomass, and E is electrolysis using water. | ||
Hydrogen production techniques with wider efficiency ranges, like dark fermentation and biomass gasification, suggest greater sensitivity to operational factors, such as feedstock quality and process conditions.39 This variability may affect their predictability and economic viability, requiring more precise control and technology optimization.40 Hydrogen production techniques like microbial electrolysis cells, with a high efficiency and lower uncertainty, indicate more stable option considering energy efficiency.
Specific energy of different fuels
Suleman et al.41 mentioned the specific energy content of various fuels, measured in kilojoules per gram as shown in Fig. 5. Wood, at the lower end, has a specific energy content of 14.9 kJ g−1. Ethanol and coal are slightly higher, at 29.7 and 30.2 kJ g−1, respectively. Biodiesel and diesel offer more energy per gram at 39.6 and 45.8 kJ g−1, followed closely by gasoline and oil at 46.5 and 47.9 kJ g−1. Propane and methane increase the energy content to 50.4 and 55.8 kJ g−1. Significantly, hydrogen stands out with a substantial 141.9 kJ g−1, highlighting its potential as a highly efficient fuel source with far greater energy density compared to traditional and other common fuels. This contrast underscores the advantages of hydrogen in energy applications, given its superior energy yield per mass unit.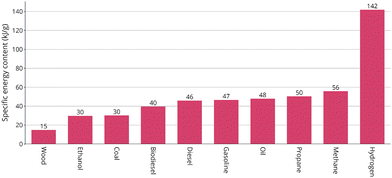 | ||
| Fig. 5 Specific energy of different fuels (kJ g−1).41 | ||
Environmental impacts of different colors of hydrogen
A comprehensive review was conducted involving forty-one highly cited peer-reviewed articles. These articles were selected based on their relevance and impact in the field to ensure a robust analysis of the environmental implications of hydrogen production. The review meticulously examines the technical scope of the hydrogen production methods discussed within these articles, delineating the specific processes and technologies that were evaluated. Additionally, the system boundaries for each study were clearly defined, outlining the limits of the assessments, such as the stages of production and the life cycle stages included. The evaluation also categorized the environmental impacts into various midpoint categories, which are crucial for understanding the direct effects associated with each stage of hydrogen production. These categories, along with the technical details and system boundaries, are detailed in Table 2, providing a structured overview of how environmental impacts are assessed in the context of hydrogen production across different studies. This structured approach allows for a thorough understanding of the methodologies used in assessing environmental impacts and facilitates a clear comparison across different hydrogen production technologies.| Authors | Scope of study | System boundary | Hydrogen production technology | Midpoint impact assessment |
|---|---|---|---|---|
| Global warming (GW), acidification (AC), eutrophication (EU), resource depletion (RD), land use (LU), water use (WU), ozone depletion (OD), human toxicity (HT), photochemical ozone formation (POF), cumulative energy demand (CED), coal gasification (CG), steam methane reforming (SMR), electrolysis via wind energy (W), biomass gasification (BG), photovoltaic electrolysis (PVE), autothermal reforming (ATR), thermal cracking (TC), high-temperature electrolysis (HTE), coal gasification with carbon capture (CG-CC), thermochemical water splitting (TC-WS), steam methane reforming with carbon capture (SMR-CC), electrolysis (E), photo fermentation (PF), dark fermentation (DF), chemical looping (CL), and pyrolysis (P). | ||||
| Acar & Dincer31 | Environment, social, & economic | NA | CG, ATR, DF, PF, BG, TC, & E | GW & AC |
| Sadeghi et al.42 | Environment | Gate-to-gate | SMR, CG, & TC | GW, AC, OD, EU, LU, & WU |
| Salkuyeh et al.17 | Environment | Cradle-to-gate | E, BG, CG, & SMR | GW, HT, AC, POFP, & EU |
| Burkhardt et al.43 | Economic, technical, environment, & thermodynamic | NA | CG, SMR, BG, PV-E, E, CG-CC, SMR-CC, TC, PVE, & PF | GW & AC |
| Zhang et al.44 | Environment | Cradle-to-grave | E | GW & CED |
| Chelvam et al.45 | Environment & economic | Cradle-to-gate | SMR & ATR | GW |
| Ji and Wang46 | Environment | Cradle-to-gate | SMR, BG, BG-CC, ATR, & E | GW, AC, & CED |
| Valente et al.47 | Environment & economic | NA | SMR, SMR-CC, CG, CG-CC, ATR, BG, & E, | GW & AC |
| Aydin et al.48 | Environment & economic | NA | E, PF, & DF | GW & AC |
| Dincer & Acar29 | Environment | Cradle-to-gate | E, PF, & DF | GW & AC |
| Martin-Gamboa et al.49 | Environment | Cradle-to-gate | BG | GW, AC, OD, RD, & EU |
| Susmozas et al.50 | Environment | Cradle-to-gate | BG | GW, AP, OD, & EU |
| Mehmeti et al.51 | Environment | Cradle-to-gate | SMR, CG, BG, E & DF | GW, AC, OD, RD, & EU |
| Hamedani et al.52 | Environment | Cradle-to-gate | BG | GW, AC, & EU |
| Siddiqui & Dincer53 | Environment | Cradle-to-gate | E, BG & CG | GW |
| Valente et al.9 | Environment | Cradle-to-gate | — | GW |
| Palmer et al.54 | Environment | Gate-to-gate | PVE | GW |
| Parkinson et al.10 | Environment & economic | Cradle-to-gate | PVE | GW |
| Patel et al.55 | Environment | Gate-to-gate | SMR & SMR-CCS | GW |
| Singh et al.56 | Environment | Gate-to-gate | SMR | GW |
| Kerscher et al.57 | Environment | Gate-to-gate | SMR | GW |
| Burchart et al.58 | Environment | Gate-to-gate | CG | GW |
| Al-Qahtani et al.59 | Environment | Gate-to-gate | CG, CG, SMR, BG, & PVE | GW |
| Suleman et al.41 | Environment | Gate-to-gate | CG, SMR, BG, PVE, & W | GW, AC, RD, & EU |
| Ozturk & Dincer60 | Environment | Gate-to-gate | SMR | GW, AC, RD, & EU |
| Cortés et al.61 | Environment | Gate-to-gate | SMR | GW, AC, RD, & EU |
| Reaño62 | Environment | Gate-to-gate | CG, BG, E, & DF | GW, AC, RD, & EU |
| Delpierre et al.63 | Environment | Gate-to-gate | E | GW |
| Sadeghi and Ghandehariun64 | Environment | Gate-to-gate | PVE | GW, AC, AD, EU, & OD |
| Mio et al.65 | Environment | Gate-to-gate | PEV, E, & SMR | GW, AC, AD, EU, FPMF, ME, WU, & OD |
| Iyer et al.66 | Environment | Cradle-to-gate | PEV & W | GW |
| Okeke et al.67 | Environment & economic | Cradle to gate | P, P-CCS | GW & AC |
| Hren et al.68 | Environment | Gate-to-gate | SMR, BG, ATR, E, DF, & CL | GW, AC, EU |
| Lin et al.69 | Environment | Gate-to-gate | P | GW |
| Ganeshan et al.70 | Environment & economic | Gate-to-gate | PF, BG, & E | GW |
| Batgi & Dincer71 | Environment | Gate-to-gate | SMR | GW, AD, OD, HT, & AC |
| Wu et al.72 | Environment & economic | Gate-to-gate | BG | GW, OD, AC, & EU |
| Zheng et al.73 | Environment | Gate-to-gate | BG, SMR, & E | GW |
| Zang et al.74 | Environment & economic | Gate-to-gate | SMR, SMR-CCS, & ATR | GW |
| Gu et al.75 | Environment | Gate-to-gate | PVE | GW |
| Weidner et al.76 | Environment & economic | Cradle to gate | PEV, SMR, SMR-CCS, & W | GW |
| Ajeeb et al.77 | Environment | Cradle to gate | E | GW, AC, AD, EU, FPMF, ME, WU, & OD |
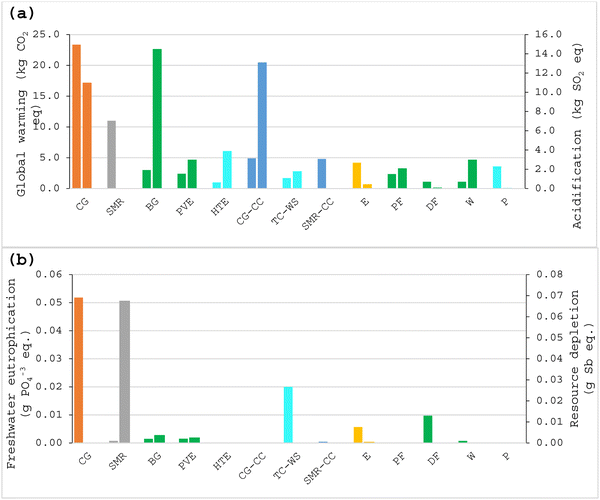
A detailed comparative analysis of various hydrogen colors based on their environmental impacts was carried out, which includes global warming, acidification, eutrophication, and resource depletion, and their median values for each color of hydrogen are shown in Fig. 6. Moreover, the mean values and the standard error of these are given in Table 3. Coal gasification exhibits the highest environmental impact among the methods analyzed, with a mean global warming impact of 20.80 kg CO2 eq. per kg H2, acidification 29.44 kg SO2 eq. per kg H2, resource depletion 1.4 × 10−5 kg Sb eq. per kg H2, and the eutrophication is 0.04 kg PO43− eq. per kg H2 which is the second highest in the case of coal gasification. These high values indicate that coal gasification contributes significantly to degrade the environment. Steam methane reforming has a moderate environmental impact with a global warming impact of 9.43 kg CO2 eq. per kg H2, acidification 7.49 kg SO2 eq. per kg H2, and resource depletion 0.37 kg Sb eq. per kg H2. The eutrophication impact at 0.0037 kg PO43− eq. per kg H2 is quite low compared to the other colors of hydrogen. Biomass gasification (BG) and photovoltaic electrolysis (PVE) offer a balance between lower global warming and manageable acidification and resource depletion values. These methods provide moderate environmental impacts, making them more sustainable compared to fossil-based methods. High-temperature electrolysis (HTE) stands out with the lowest global warming impact of 1.25 kg CO2 eq. per kg H2; its acidification is also relatively low at of 3.88 kg SO2 eq. per kg H2. Coal gasification with carbon capture (CG-CC) and steam methane reforming with carbon capture (SMR-CC) show reduced global warming almost by half compared to their non-capture counterparts. However, these methods still present significant acidification and resource depletion. Electrolysis (E) has moderate global warming and acidification impacts but stands out with the highest eutrophication of 0.011 kg PO43− eq. per kg H2, indicating a significant impact on nutrient pollution. Photo fermentation (PF) and dark fermentation (DF) show relatively low environmental impacts. Electrolysis via wind energy (W) exhibits a low global warming impact of 1.1 kg CO2 eq. per kg H2 and acidification 2.98 kg SO2 eq. per kg H2, resource depletion 1.7 × 10−4 kg Sb eq. per kg H2, making it one of the most environmentally friendly methods.
| Technologies | Global warming (kg CO2 eq. per kg H2) | Acidification (kg SO2 eq. per kg H2) | Eutrophication (kg PO43− eq. per kg H2) | Resource depletion (kg Sb eq. per kg H2) |
|---|---|---|---|---|
| Coal gasification (CG), steam methane reforming (SMR), biomass gasification (BG), photovoltaic electrolysis (PVE), high-temperature electrolysis (HTE), coal gasification with carbon capture (CG-CC), thermochemical water splitting (TC-WS), steam methane reforming with carbon capture (SMR-CC), electrolysis (E), photo fermentation (PF), dark fermentation (DF), and pyrolysis (P), and electrolysis via wind energy (W). | ||||
| CG | 2.1 × 101 ± 1.9 × 100 | 2.9 × 101 ± 1.4 × 101 | 4.0 × 10−2 ± 1.6 × 10−2 | 1.4 × 10−5 ± 1.5 × 10−6 |
| SMR | 9.4 × 100 ± 7.5 × 10−1 | 7.5 × 100 ± 2.1 × 100 | 3.7 × 10−3 ± 2.1 × 10−3 | 3.7 × 10−1 ± 3.1 × 10−1 |
| BG | 3.9 × 100 ± 6.4 × 10−1 | 1.5 × 101 ± 5.1 × 100 | 3.8 × 10−3 ± 2.3 × 10−3 | 3.8 × 10−3 ± 0.0 × 100 |
| PVE | 2.6 × 100 ± 3.2 × 10−1 | 6.1 × 100 ± 2.9 × 100 | 3.3 × 10−3 ± 2.0 × 10−3 | 1.8 × 10−3 ± 7.9 × 10−4 |
| HTE | 1.3 × 100 ± 3.4 × 10−1 | 3.9 × 100 ± 4.1 × 10−1 | — | — |
| CG-CC | 5.6 × 100 ± 2.3 × 100 | 1.3 × 101 ± 0.0 × 100 | — | — |
| TC-WS | 4.0 × 100 ± 2.1 × 100 | 1.3 × 100 ± 6.7 × 10−1 | 2.0 × 10−2 ± 0.0 × 100 | — |
| SMR-CC | 5.6 × 100 ± 7.5 × 10−1 | 1.2 × 10−2 ± 3.1 × 10−3 | 4.5 × 10−4 ± 1.4 × 10−4 | — |
| E | 1.3 × 101 ± 3.4 × 100 | 1.9 × 101 ± 1.1 × 101 | 1.1 × 10−2 ± 3.6 × 10−3 | 3.2 × 10−3 ± 1.4 × 10−3 |
| PF | 3.1 × 100 ± 9.2 × 10−1 | 2.1 × 100 ± 9.0 × 10−1 | — | — |
| DF | 5.7 × 100 ± 1.9 × 100 | 5.8 × 10−1 ± 3.3 × 10−1 | 7.7 × 10−2 ± 7.1 × 10−2 | — |
| W | 1.1 × 100 ± 1.5 × 10−1 | 3.0 × 100 ± 1.0 × 100 | 7.5 × 10−4 ± 6.5 × 10−4 | 1.7 × 10−4 ± 3.3 × 10−5 |
| P | 5.1 × 100 ± 1.1 × 100 | 1.1 × 10−2 ± 1.6 × 10−3 | 1.2 × 10−5 ± 0.0 × 100 | — |
Discussion
Based on the mean normalized score, the comparison of hydrogen production methods reveals a spectrum of environmental impacts. Coal gasification (Brown) stands out with the highest burden at 0.63, indicating significant environmental challenges. It is closely followed by steam methane reforming (Gray) and dark fermentation (Green), which have burdens of 0.43 and 0.42, respectively. These conventional methods contribute heavily to global warming, acidification, freshwater eutrophication, and resource depletion.Environmental impacts are moderate to high particularly due to the highest freshwater eutrophication compared to the other technologies. Dark fermentation typically utilizes organic waste, agricultural residues, or biomass as substrates, which often contain elevated levels of phosphorus (such as phosphates). If not properly controlled, the resulting wastewater or byproducts can release these nutrients into freshwater systems, contributing to eutrophication. Additionally, dark fermentation generates volatile fatty acids (VFAs) as byproducts. Inadequate handling of effluents containing VFAs can further drive nutrient cycling in aquatic environments, intensifying the eutrophication process.
Electrolysis through the grid (Orange) and coal gasification with carbon capture (Blue) show moderate environmental impacts, with normalized scores around 0.35, suggesting that carbon capture can somewhat mitigate the burden associated with coal-based processes. Biomass gasification (Green) and thermochemical water splitting (Turquoise) are among the lower-impact methods, with scores of 0.18 and 0.15, respectively, reflecting their potential for more sustainable hydrogen production.
At the lower end of the impact spectrum are several advanced and renewable-based technologies. Methods such as photo-fermentation (Green), photovoltaic electrolysis (Green), steam methane reforming with carbon capture (Blue), high-temperature electrolysis (Turquoise), and pyrolysis (Turquoise) all exhibit normalized scores below 0.1, indicating relatively minimal environmental impact. Among these, wind power-based electrolysis (Green) emerges as the most environmentally friendly option, with the lowest burden of 0.03.
This ranking highlights a clear progression from high-impact, fossil fuel-based methods to more sustainable, low-impact renewable, and advanced thermal processes. The spectrum of environmental impacts reveals how methods like wind-powered electrolysis and biomass gasification can significantly reduce environmental harm in areas such as global warming, acidification, freshwater eutrophication, and resource depletion potential, paving the way for greener hydrogen production technologies.
Based on the normalized scores, different colors of hydrogen and techniques were ranked from high to low environmental impacts, as shown in Fig. 7. Brown hydrogen, produced via coal gasification, exhibits the highest environmental impacts among the hydrogen production methods under consideration. This process is characterized by significant contributions to global warming, acidification, and resource depletion. These high values indicate that coal gasification significantly exacerbates climate change, contributes to the formation of acid rain, and depletes non-renewable resources at a substantial rate, making it the least sustainable option for hydrogen production.
In contrast, hydrogen production through electrolysis using wind energy, often referred to as green hydrogen, has the lowest environmental impacts among the methods analyzed. This process involves splitting water into hydrogen and oxygen using electricity generated from wind turbines, a renewable energy source.
Bolz et al.78 identified five key barriers to adopting new technologies: regulation, technology, costs, availability, and acceptance. Jeje et al.79 discussed four key challenges in hydrogen production: technology, economy, regulation, and infrastructure. Technological hurdles include efficiency and scalability, while high costs limit economic feasibility. Inconsistent policies and underdeveloped infrastructure further hinder widespread adoption. Addressing these is vital for advancing hydrogen as an energy source.
In this prospect, hydrogen production technologies face challenges related to TRL, feedstock, and methods. Low TRL technologies like photocatalytic water splitting struggle with feasibility, efficiency, and high R&D costs. Addressing these issues requires more R&D funding, innovation, collaboration, and pilot projects. Medium TRL technologies like biolysis and PEM face barriers such as the need for technical optimization, high production costs, and lack of clear policies. Overcoming these challenges requires research to improve efficiency and reduce costs, along with supportive policies and public–private partnerships to foster adoption and innovation. High TRL technologies like alkaline electrolysis and gasification face challenges in infrastructure and scale. Despite being commercially viable, they require extensive infrastructure, such as pipelines and storage, and high capital costs, especially for renewable-powered electrolysis. Additionally, technologies like coal and natural gas gasification raise environmental concerns unless combined with carbon capture and storage (CCS). Addressing these issues requires major investments in infrastructure, renewable energy integration, and CCS to meet decarburization goals.
In summary, different feedstocks and production methods face unique challenges. For example, water-based technologies like alkaline electrolysis are efficient but have high energy demands.80 Biomass technologies, such as gasification, are viable but raise environmental concerns (land use change), competition with other commodities that use biomass as raw material, and a lack of biomass availability.81 Natural gas and hydrocarbon methods, like steam methane reforming, are efficient and cost-effective but rely on fossil fuels, leading to carbon emission issues.12
Several possible solutions can be implemented to address the challenges of hydrogen production methods. For water-based technologies like alkaline electrolysis, integrating renewable energy sources and improving system efficiency can reduce high energy demands. Biomass technologies, such as gasification, can benefit from sustainable sourcing practices, enhanced biomass availability, and carbon capture to mitigate environmental concerns. For natural gas and hydrocarbon methods like steam methane reforming, adopting low-carbon technologies, incorporating carbon capture and storage, and exploring green hydrogen alternatives can help reduce carbon emissions and reliance on fossil fuels.
Comparing different studies
By comparing different studies, it becomes evident that the variability in results often stems from differences in system boundaries, technological assumptions, and selected impact metrics. For instance, Bhandari et al.,16 Busch et al.,82 Wilkinson et al.,34 and Ji and Wang46 report divergent outcomes for global warming potential (GWP) and acidification potential because of their distinct methodological approaches and scope of assessments.Technology-specific impacts further illustrate these variations. For example, steam methane reforming (SMR) and biomass gasification (BG) both exhibit significant environmental effects, but in different ways. SMR is typically associated with higher GWP due to its dependence on fossil fuels, leading to greater greenhouse gas emissions. In contrast, BG's environmental impact fluctuates based on the type of biomass used and the processing methods applied, suggesting that BG can either mitigate or exacerbate environmental harm depending on these factors.
Studies with broader scopes, such as Acar & Dincer,31 provide a more comprehensive overview of hydrogen production pathways but may lack in-depth analysis of specific technologies. Conversely, more focused studies, such as Cetinkaya et al.,83 deliver detailed insights into particular technologies, offering a granular perspective on their environmental impacts, though they might overlook broader, system-level implications. Additionally, Nikolaidis & Poullikkas84 examine greenhouse gas emissions and the economic efficiency of hydrogen production technologies, further highlighting how both environmental and economic factors shape the sustainability of different hydrogen pathways. Overall, the wide variation in study findings emphasizes the need for standardized methods and transparent assumptions when comparing the environmental impacts of hydrogen production technologies.
Bhandari et al.16 conducted a life cycle assessment of hydrogen production via electrolysis. Their study found that the GWP for hydrogen production through biomass gasification is higher than that of steam methane reforming, contrary to the findings of most other studies, which typically indicate that GWP is greater for SMR than biomass gasification. Ji and Wang46 compared various hydrogen production methods and found that the acidification potential is higher for biomass gasification compared to coal gasification. Conversely, Valente et al.47 reported that coal gasification has a higher acidification potential than biomass gasification for hydrogen production. These differing findings highlight the variability in environmental impacts depending on the study and the specific conditions or assumptions used in each assessment.
Overall, these insights suggest that while current studies provide valuable information, there is no one-size-fits-all answer to the environmental impacts of hydrogen production methods. A nuanced approach that considers specific conditions, technologies, and contexts is essential for a comprehensive understanding.
Gaps that need to be addressed
Existing studies often fall short in providing comprehensive lifecycle analyses that encompass all stages from feedstock extraction through to production. Most studies currently use a gate-to-gate approach, which overlooks the significant environmental impacts associated with feedstock. Additionally, many studies consider only one or two impact categories, limiting the depth of their insights. Hydrogen production methods, including electrolysis, require substantial water resources. Future research should focus on water consumption and its potential effects on local water supplies, particularly in regions facing water scarcity. The environmental impacts of byproducts and waste from hydrogen production, such as carbon dioxide from steam methane reforming, also need greater scrutiny. Research should explore effective strategies for managing and mitigating these byproducts. Furthermore, the land use changes resulting from hydrogen production, especially in the case of biohydrogen or large-scale renewable energy projects, should be evaluated for their impacts on ecosystems and biodiversity. Future studies should also investigate how new hydrogen production technologies scale up and integrate into existing infrastructure, assessing their long-term sustainability and environmental effects.Reasons behind varying results
The environmental impact of hydrogen production can vary depending on the regional energy mix and the availability of resources such as water or biomass. Variations in data sources, assumptions, and accuracy can significantly influence LCA outcomes, as factors like resource consumption, emissions, and energy use differ. The inclusion or exclusion of certain life cycle stages, such as transportation, can also alter the results. Additionally, how environmental burdens are allocated in multi-output processes, such as by-products from hydrogen production, can lead to discrepancies, with different allocation methods (e.g., mass-based, economic, or energy-based) producing varied results. Even when using the same technology, differences in operational efficiency or feedstock quality can affect energy consumption and emissions, leading to divergent LCA conclusions. Studies may emphasize different impact categories, such as global warming potential or resource depletion, and the weighting of these categories further contributes to varying conclusions. The selected time horizon, choice of simulation models (Simapro, OpenLCA, GaBi), and assessment methods (ReCiPe, CML) also play crucial roles in influencing LCA outcomes, especially for emerging technologies.Potential future research directions
In hydrogen production and LCA, there needs to be a more consistent application of midpoint and endpoint impact categories, particularly for crucial environmental metrics like global warming potential (GWP), acidification potential, and resource depletion. Harmonizing functional units, such as per kilogram of hydrogen produced, and appropriately allocating emissions in complex processes, like those involving co-products, are essential steps to achieving more reliable assessments.A significant gap in the current understanding of hydrogen production's environmental impacts is the reliance on outdated or regionally specific data. This lack of transparency in energy mix assumptions, emission factors, and technological efficiencies often undermines the credibility of many studies. Future research should focus on creating open-source databases that provide transparent and up-to-date life cycle inventory (LCI) data. Such databases would improve the accuracy of environmental assessments, particularly if they incorporate region-specific variables, such as local energy mixes, regulatory frameworks, and resource availability. This would facilitate more precise global assessments of hydrogen production technologies.
Established technologies like steam methane reforming have been extensively studied, but emerging technologies, including electrolysis methods like anion exchange membrane, proton exchange membrane, and solid oxide electrolyzer, as well as advanced biomass gasification, lack comprehensive impact assessments. These technologies, particularly those still in development, need detailed assessments to understand their scalability and potential environmental impacts. Future research should focus on conducting targeted LCAs for emerging hydrogen technologies like microbial electrolysis cells, photo-fermentation, and thermochemical water-splitting cycles. Comparative studies are also essential to evaluate how these new technologies fare against traditional fossil fuel-based hydrogen pathways in terms of environmental sustainability.
While LCA studies on hydrogen production focus solely on environmental impacts, the social and economic aspects are often neglected. Job creation, resource availability, and geopolitical factors play a crucial role in the overall sustainability of hydrogen technologies. Future research should integrate social life cycle assessment and techno-economic analyses to evaluate trade-offs between environmental performance and socio-economic benefits. Case studies exploring the socio-economic impacts of hydrogen production in different regions, particularly in developing countries, would provide valuable insights into local impacts and opportunities.
Many studies do not consider how region-specific policies, regulatory incentives, and resource availability impact hydrogen production's environmental outcomes. These factors can greatly influence the overall viability of hydrogen technologies. Future research should focus on comparative studies that assess how regional policies, such as carbon taxes or renewable energy subsidies, affect both the environmental and economic performance of hydrogen production pathways. Moreover, it is crucial to explore hydrogen production in resource-constrained regions, such as water-scarce areas, and to assess the environmental trade-offs, including water usage and land degradation.
Challenges and opportunities
Considering the environmental perspective, green hydrogen generated from electrolysis using wind energy, followed by high-temperature electrolysis and photo fermentation, are the most favorable. These pathways align well with several of the Sustainable Development Goals (SDGs), such as SDG 7 (affordable and clean energy) and SDG 13 (climate action), due to their low emissions and reliance on renewable energy sources. However, these techniques currently suffer from low efficiency percentages and technology readiness levels, as outlined in Table 1. Practical implementation of these technologies at a commercial level in the near future remains challenging, especially for developing countries where feasibility is a significant concern.From a policy standpoint, implementing these environmentally friendly technologies requires robust support through government incentives, subsidies, or regulations that encourage investment in renewable technologies. For developing nations, international cooperation and funding might be necessary to build the infrastructure needed to adopt these advanced technologies. Green hydrogen produced from biomass presents a viable alternative for nations rich in biomass due to the renewable nature of the feedstock. It achieves nearly 50% efficiency, and its technology readiness level of 9 indicates that it is well-developed. This aligns with SDG 12 (responsible consumption and production) by promoting the efficient use of natural resources. Moreover, it could help to mitigate energy poverty as outlined in SDG 7, particularly in rural areas of developing countries.
Additionally, countries could consider blue hydrogen (with carbon capture and storage) and gray hydrogen (SMR) as alternatives. These methods, which utilize natural gas as a feedstock, offer a pragmatic step towards transitioning from fossil fuels to more sustainable energy sources. This approach could serve as an interim solution that supports SDG 9 (Industry, Innovation, and Infrastructure) by developing new technologies and infrastructure for hydrogen production. While these methods are neither significantly environmentally friendly nor particularly harmful, they do offer high-efficiency percentages and are technologically advanced, as given in Table 1. Policy-wise, blue and gray hydrogen production might require regulations that ensure the carbon capture and storage component effectively reduces CO2 emissions, thus contributing positively to SDG 13 (climate action). Additionally, creating a regulatory framework that supports fair and sustainable natural gas extraction practices could help achieve SDG 15 (life on land).
In summary, while the adoption of these hydrogen technologies poses certain challenges, they offer considerable opportunities to advance multiple SDGs. Effective policy frameworks, international cooperation, and technological readiness are crucial for their successful implementation and to ensure they contribute positively to global sustainability goals.
Recommendations
Based on the comparative analysis of the environmental impacts of various hydrogen colors based on production methods, it is recommended to prioritize green hydrogen production, particularly through wind-powered, high-temperature electrolysis, and photo fermentation, due to their minimal contributions to global warming, acidification, and resource depletion.• Enhancing and expanding the use of carbon capture technologies for coal gasification and steam methane reforming can mitigate some of their environmental drawbacks, although a gradual phase-out of coal gasification is advised due to its high environmental impact.
• Supporting research and development in innovative hydrogen production methods, such as high-temperature electrolysis and thermochemical water splitting, is crucial for further reducing environmental impacts.
• Policymakers should implement subsidies, tax incentives, and financial support for green hydrogen projects, along with developing regulations and standards to limit environmental impacts.
• Increasing public awareness and engaging stakeholders are essential for garnering support and collaboratively transitioning to greener hydrogen production methods.
• It is also recommended to consider all significant impact categories while conducting life cycle assessments, as focusing solely on global warming can overlook other critical environmental impacts.
Conclusion
Hydrogen is emerging as a significant energy source with the potential to replace fossil fuels partially. Hydrogen can be produced through various technologies and feedstocks, and based on these, it is categorized into different colors, each with distinct environmental impacts. Indicators such as global warming, acidification, freshwater eutrophication, and resource depletion were considered to assess these impacts. The results indicated that brown hydrogen produced via coal gasification generally had the highest impact among all the options considered. Conversely, green hydrogen (biomass gasification) and green hydrogen (photovoltaic electrolysis) offer moderate environmental impacts, presenting a viable balance between sustainability and technology readiness levels. Photo-fermentation hydrogen production has relatively low environmental impacts; however, its technology readiness level is only 1 to 3, making it unsuitable for immediate use. Policymakers should still consider this technique as a long-term option for hydrogen production, as it could contribute to achieving various Sustainable Development Goals and net-zero emissions in the future. Steam methane reforming with carbon capture and storage has relatively low environmental impacts as well and a Technology Readiness Level of 8, making it a viable option for hydrogen production while minimizing environmental harm. This technology can support policymakers in achieving SDG 7 (affordable and clean energy), SDG 13 (climate action), and net-zero emissions in the near future. Overall, the adoption of these hydrogen production methods presents some challenges and opportunities as well. Success depends on effective policy frameworks, international cooperation, and technological readiness to ensure positive contributions to global sustainability goals.Author contributions
Ms Hafsa Mehmood and Dr Haseeb Akbar reviewed and analyzed the work and wrote the manuscript. Dr Pariyapat Nilsalab improved the idea, while Prof. Shabbir H. Gheewala conceptualized and supervised the study.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge the Joint Graduate School of Energy and Environment, King Mongkut’s University of Technology Thonburi, Bangkok, Thailand, and the Center of Excellence on Energy Technology and Environment, Ministry of Higher Education, Science, Research and Innovation, Thailand, for their support.References
- OurWorldindata, 2024. Global primary energy consumption by source. https://ourworldindata.org/grapher/global-energy-substitution? stackMode = relative, (accessed July 15, 2024).
- EIA projects world energy consumption will increase 56% by 2040. https://www.eia.gov/todayinenergy/detail.php?id=12251 (accessed July 15, 2024).
- CO2 Emissions in 2023, A new record high, but is there light at the end of the tunnel? https://iea.blob.core.windows.net/assets/33e2badc-b839-4c18-84ce-f6387b3c008f/CO2Emissionsin2023.pdf (accessed July 15, 2024).
- B. S. Zainal, P. J. Ker, H. Mohamed, H. C. Ong, I. M. R. Fattah, S. A. Rahman and T. I. Mahlia, Recent advancement and assessment of green hydrogen production technologies, Renewable Sustainable Energy Rev., 2024, 189, 113941 Search PubMed.
- J. Incer-Valverde, A. Korayem, G. Tsatsaronis and T. Morosuk, “Colors” of hydrogen: definitions and carbon intensity, Energy Convers. Manage., 2023, 291, 117294 Search PubMed.
- P. Saha, F. A. Akash, S. M. Shovon, M. U. Monir, M. T. Ahmed, M. F. H. Khan and R. Akter, Grey, blue, and green hydrogen: a comprehensive review of production methods and prospects for zero-emission energy, Int. J. Green Energy, 2023, 1–15 Search PubMed.
- J. A. Riera, R. M. Lima and O. M. Knio, A review of hydrogen production and supply chain modeling and optimization, Int. J. Hydrogen Energy, 2023, 48(37), 13731–13755 Search PubMed.
- M. R. Giraldi, J. L. François and C. Martin-del-Campo, Life cycle assessment of hydrogen production from a high-temperature electrolysis process coupled to a high-temperature gas nuclear reactor, Int. J. Hydrogen Energy, 2015, 40(10), 4019–4033 Search PubMed.
- A. Valente, D. Iribarren and J. Dufour, Life cycle sustainability assessment of hydrogen from biomass gasification: a comparison with conventional hydrogen, Int. J. Hydrogen Energy, 2019, 44(38), 21193–21203 Search PubMed.
- B. Parkinson, P. Balcombe, J. F. Speirs, A. D. Hawkes and K. Hellgardt, Levelized cost of CO2 mitigation from hydrogen production routes, Energy Environ. Sci., 2019, 12(1), 19–40 Search PubMed.
- C. Bauer, K. Treyer, C. Antonini, J. Bergerson, M. Gazzani, E. Gencer and M. Van der Spek, On the climate impacts of blue hydrogen production, Sustain, Energy Fuels, 2022, 6(1), 66–75 Search PubMed.
- P. J. Megía, A. J. Vizcaíno, J. A. Calles and A. Carrero, Hydrogen production technologies: from fossil fuels toward renewable sources. A mini review, Energy Fuels, 2021, 35(20), 16403–16415 Search PubMed.
- M. P. Maniscalco, S. Longo, M. Cellura, G. Miccichè and M. Ferraro, Critical review of life cycle assessment of hydrogen production pathways, Environments, 2024, 11(6), 108 Search PubMed.
- A. I. Osman, N. Mehta, A. M. Elgarahy, M. Hefny, A. Al-Hinai, A. A. H. Al-Muhtaseb and D. W. Rooney, Hydrogen production, storage, utilisation and environmental impacts: a review, Environ. Chem. Lett., 2022, 1–36 Search PubMed.
- A. Tukker, Life cycle assessment as a tool in environmental impact assessment, Environ. Impact Assess. Rev., 2000, 20(4), 435–456 Search PubMed.
- R. Bhandari, C. A. Trudewind and P. Zapp, Life cycle assessment of hydrogen production via electrolysis – A review, J. Cleaner Prod., 2014, 85, 151–163 Search PubMed.
- Y. K. Salkuyeh, B. A. Saville and H. L. MacLean, Techno-economic analysis and life cycle assessment of hydrogen production from natural gas using current and emerging technologies, Int. J. Hydrogen Energy, 2017, 42(30), 18894–18909 Search PubMed.
- B. Morero, M. B. Rodriguez and E. A. Campanella, Environmental impact assessment as a complement of life cycle assessment. Case study: Upgrading of biogas, Bioresour. Technol., 2015, 190, 402–410 Search PubMed.
- W. F. Lamb, T. Wiedmann, J. Pongratz, R. Andrew, M. Crippa, J. G. Olivier and J. Minx, A review of trends and drivers of greenhouse gas emissions by sector from 1990 to 2018, Environ. Res. Lett., 2021, 16(7), 073005 Search PubMed.
- D. Adams, D. H. Oh, D. W. Kim, C. H. Lee and M. Oh, Prediction of SOx–NOx emission from a coal-fired CFB power plant with machine learning: plant data learned by deep neural network and least square support vector machine, J. Cleaner Prod., 2020, 270, 122310 Search PubMed.
- W. Sun and G. Yao, Impact of mineral resource depletion on energy use: role of energy extraction, CO2 intensity, and natural resource sustainability, Resour. Policy, 2023, 86, 104175 Search PubMed.
- H. Benhar, A. Idri and J. L. Fernández-Alemán, Data preprocessing for heart disease classification: a systematic literature review, Comput. Methods Programs Biomed., 2020, 195, 105635 Search PubMed.
- E. Meloni, M. Martino and V. Palma, A short review on Ni-based catalysts and related engineering issues for methane steam reforming, Catalysts, 2020, 10(3), 352 Search PubMed.
- P. A. Le, V. D. Trung, P. L. Nguyen, T. V. B. Phung, J. Natsuki and T. Natsuki, The current status of hydrogen energy: an overview, RSC Adv., 2023, 13(40), 28262–28287 Search PubMed.
- S. Bhandari, D. K. Poudel, R. Marahatha, S. Dawadi, K. Khadayat, S. Phuyal and N. Parajuli, Microbial enzymes used in bioremediation, J. Cheminf., 2021, 2021(1), 8849512 Search PubMed.
- X. Qu, H. Zeng, Y. Gao, T. Mo and Y. Li, Bio-hydrogen production by dark anaerobic fermentation of organic wastewater, Front. Chem., 2022, 10, 978907 Search PubMed.
- S. M. Kotay and D. Das, Biohydrogen as a renewable energy resource—prospects and potentials, Int. J. Hydrogen Energy, 2008, 33(1), 258–263 Search PubMed.
- B. Uyar, G. Kars, İ. Eroğlu, M. Yücel and U. Gündüz, Hydrogen production via photofermentation, State of the art and progress in production of biohydrogen, Bentham Science Publishers, 2012, pp. 54–77 Search PubMed.
- I. Dincer and C. Acar, Review and evaluation of hydrogen production methods for better sustainability, Int. J. Hydrogen Energy, 2015, 40(34), 11094–11111 Search PubMed.
- Hydrogen Europe, 2024. Colors of Hydrogen. https://hydrogeneurope.eu/in-a-nutshell/(accessed July 22, 2024).
- C. Acar and I. Dincer, Selection criteria and ranking for sustainable hydrogen production options, Int. J. Hydrogen Energy, 2022, 47(95), 40118–40137 Search PubMed.
- ETP Clean Energy Technology Guide – Data Tools – IEA. https://www.iea.org/data-and-statistics/data-tools/etp-clean-energytechnology-guide (accessed July 15, 2024).
- F. R. Malik, H. B. Yuan, J. C. Moran and N. Tippayawong, Overview of hydrogen production technologies for fuel cell utilization, Eng. Sci. Technol. Int. J., 2023, 43, 101452 Search PubMed.
- J. Wilkinson, T. Mays and M. McManus, Review and meta-analysis of recent life cycle assessments of hydrogen production, Clean. Environ. Syst., 2023, 9, 100116 Search PubMed.
- F. Dawood, M. Anda and G. M. Shafiullah, Hydrogen production for energy: an overview, Int. J. Hydrogen Energy, 2020, 45(7), 3847–3869 Search PubMed.
- H. Thomas, Options for producing low-carbon hydrogen at scale, 2018, https://www.h2knowledgecentre.com/content/policypaper1839 (accessed July 16, 2024).
- The Future of Hydrogen: Seizing Today's Opportunities. International Energy Agency, Paris. https://www.iea.org/reports/the-future-of-hydrogen (accessed July 12, 2024).
- A. Y. Goren, I. Dincer and A. Khalvati, A comprehensive review on environmental and economic impacts of hydrogen production from traditional and cleaner resources, J. Environ. Chem. Eng., 2023, 111187 Search PubMed.
- A. Abuadala, I. Dincer and G. F. Naterer, Exergy analysis of hydrogen production from biomass gasification, Int. J. Hydrogen Energy, 2010, 35(10), 4981–4990 Search PubMed.
- H. Song, G. Yang, P. Xue, Y. Li, J. Zou, S. Wang, H. Yang and H. Chen, Recent development of biomass gasification for H2 rich gas production, Appl. Energy Combust. Sci., 2022, 10, 100059 Search PubMed.
- F. Suleman, I. Dincer and M. Agelin-Chaab, Environmental impact assessment and comparison of some hydrogen production options, Int. J. Hydrogen Energy, 2015, 40(21), 6976–6987 Search PubMed.
- S. Sadeghi, S. Ghandehariun and M. A. Rosen, Comparative economic and life cycle assessment of solar-based hydrogen production for oil and gas industries, Energy, 2020, 208, 118347 Search PubMed.
- J. Burkhardt, A. Patyk, P. Tanguy and C. Retzke, Hydrogen mobility from wind energy – A life cycle assessment focusing on the fuel supply, Appl. Energy, 2016, 181, 54–64 Search PubMed.
- J. Zhang, B. Ling, Y. He, Y. Zhu and Z. Wang, Life cycle assessment of three types of hydrogen production methods using solar energy, Int. J. Hydrogen Energy, 2022, 47(30), 14158–14168 Search PubMed.
- K. Chelvam, M. M. Hanafiah, K. Al Ali and A. Al Blooshi, Gate-to-gate life cycle analysis of a pilot-scale solar driven two-step thermochemical hydrogen sulfide decomposition for hydrogen production, J. Cleaner Prod., 2023, 428, 139369 Search PubMed.
- M. Ji and J. Wang, Review and comparison of various hydrogen production methods based on costs and life cycle impact assessment indicators, Int. J. Hydrogen Energy, 2021, 46(78), 38612–38635 Search PubMed.
- A. Valente, D. Iribarren and J. Dufour, Harmonising the cumulative energy demand of renewable hydrogen for robust comparative life-cycle studies, J. Cleaner Prod., 2018, 175, 384–393 Search PubMed.
- M. I. Aydin, I. Dincer and H. Ha, Development of Oshawa hydrogen hub in Canada: a case study, Int. J. Hydrogen Energy, 2021, 46(47), 23997–24010 Search PubMed.
- M. Martín-Gamboa, D. Iribarren, A. Susmozas and J. Dufour, Delving into sensible measures to enhance the environmental performance of biohydrogen: a quantitative approach based on process simulation, life cycle assessment and data envelopment analysis, Bioresour. Technol., 2016, 214, 376–385 Search PubMed.
- A. Susmozas, D. Iribarren, P. Zapp, J. Linßen and J. Dufour, Life-cycle performance of hydrogen production via indirect biomass gasification with CO2 capture, Int. J. Hydrogen Energy, 2016, 41(42), 19484–19491 Search PubMed.
- A. Mehmeti, A. Angelis-Dimakis, G. Arampatzis, S. J. McPhail and S. Ulgiati, Life cycle assessment and water footprint of hydrogen production methods: from conventional to emerging technologies, Environments, 2018, 5(2), 24 Search PubMed.
- R. S. Hamedani, M. Villarini, A. Colantoni, M. Moretti and E. Bocci, Life cycle performance of hydrogen production via agro-industrial residue gasification—A small scale power plant study, Energies, 2018, 11(3), 675 Search PubMed.
- O. Siddiqui and I. Dincer, A well-to-pump life cycle environmental impact assessment of some hydrogen production routes, Int. J. Hydrogen Energy, 2019, 44(12), 5773–5786 Search PubMed.
- G. Palmer, A. Roberts, A. Hoadley, R. Dargaville and D. Honnery, Life-cycle greenhouse gas emissions and net energy assessment of large-scale hydrogen production via electrolysis and solar PV, Energy Environ. Sci., 2021, 14(10), 5113–5131 Search PubMed.
- G. H. Patel, J. Havukainen, M. Horttanainen, R. Soukka and M. Tuomaala, Climate change performance of hydrogen production based on life cycle assessment, Green Chem., 2024, 26(2), 992–1006 Search PubMed.
- S. Singh, G. Pandey, G. K. Rath, H. P. Veluswamy and N. Molokitina, Life cycle assessment of biomass-based hydrogen production technologies: a review, Int. J. Green Energy, 2023, 1–16 Search PubMed.
- F. Kerscher, A. Stary, S. Gleis, A. Ulrich, H. Klein and H. Spliethoff, Low-carbon hydrogen production via electron beam plasma methane pyrolysis: techno-economic analysis and carbon footprint assessment, Int. J. Hydrogen Energy, 2021, 46(38), 19897–19912 Search PubMed.
- D. Burchart, M. Gazda-Grzywacz, P. Grzywacz, P. Burmistrz and K. Zarębska, Life cycle assessment of hydrogen production from coal gasification as an alternative transport fuel, Energies, 2022, 16(1), 383 Search PubMed.
- A. Al-Qahtani, B. Parkinson, K. Hellgardt, N. Shah and G. Guillen-Gosalbez, Uncovering the true cost of hydrogen production routes using life cycle monetisation, Appl. Energy, 2021, 281, 115958 Search PubMed.
- M. Ozturk and I. Dincer, Comparative environmental impact assessment of various fuels and solar heat for a combined cycle, Int. J. Hydrogen Energy, 2019, 44(10), 5043–5053 Search PubMed.
- A. Cortés, G. Feijoo, A. Chica, J. F. Da Costa-Serra and M. T. Moreira, Environmental implications of biohydrogen-based energy production from steam reforming of alcoholic waste, Ind. Crops Prod., 2019, 138, 111465 Search PubMed.
- R. L. Reaño, Assessment of environmental impact and energy performance of rice husk utilization in various biohydrogen production pathways, Bioresour. Technol., 2020, 299, 122590 Search PubMed.
- M. Delpierre, J. Quist, J. Mertens, A. Prieur-Vernat and S. Cucurachi, Assessing the environmental impacts of wind-based hydrogen production in the Netherlands using ex-ante LCA and scenarios analysis, J. Cleaner Prod., 2021, 299, 126866 Search PubMed.
- S. Sadeghi and S. Ghandehariun, Environmental impacts of a standalone solar water splitting system for sustainable hydrogen production: a life cycle assessment, Int. J. Hydrogen Energy, 2023, 48(50), 19326–19339 Search PubMed.
- A. Mio, E. Barbera, A. M. Pavan, A. Bertucco and M. Fermeglia, Sustainability analysis of hydrogen production processes, Int. J. Hydrogen Energy, 2024, 54, 540–553 Search PubMed.
- R. K. Iyer, J. H. Prosser, J. C. Kelly, B. D. James and A. Elgowainy, Life-cycle analysis of hydrogen production from water electrolyzers, Int. J. Hydrogen Energy, 2024, 81, 1467–1478 Search PubMed.
- I. J. Okeke, B. A. Saville and H. L. MacLean, Low carbon hydrogen production in Canada via natural gas pyrolysis, Int. J. Hydrogen Energy, 2023, 48(34), 12581–12599 Search PubMed.
- R. Hren, A. Vujanović, Y. Van Fan, J. J. Klemeš, D. Krajnc and L. Čuček, Hydrogen production, storage and transport for renewable energy and chemicals: an environmental footprint assessment, Renewable Sustainable Energy Rev., 2023, 173, 113113 Search PubMed.
- J. Lin, S. Liu, Z. Han, R. Ma, C. Cui and S. Sun, Scaled-up microwave pyrolysis of sludge for hydrogen-rich biogas and life cycle assessment: parameters synergistic optimization, carbon footprint analysis and technology upgrade, Chem. Eng. J., 2023, 452, 139551 Search PubMed.
- P. Ganeshan, V. S. Vigneswaran, S. C. Gowd, D. Kondusamy, N. Krishnamoorthy and D. Kumar, et al., How does techno-economic analysis and lifecycle assessment help in commercializing the biohydrogen supply chain?, Fuel, 2023, 341, 127601 Search PubMed.
- S. U. Batgi and I. Dincer, A study on comparative environmental impact assessment of thermochemical cycles and steam methane reforming processes for hydrogen production processes, Comput. Chem. Eng., 2024, 180, 108514 Search PubMed.
- N. Wu, K. Lan and Y. Yao, An integrated techno-economic and environmental assessment for carbon capture in hydrogen production by biomass gasification, Resour., Conserv. Recycl., 2023, 188, 106693 Search PubMed.
- X. Zheng, J. Wang, J. Huang, X. Xu, J. Tang and P. Hou, et al., Environmental impact assessment of a combined bioprocess for hydrogen production from food waste, Waste Manage., 2024, 173, 152–159 Search PubMed.
- G. Zang, E. J. Graham and D. Mallapragada, H2 production through natural gas reforming and carbon capture: a techno-economic and life cycle analysis comparison, Int. J. Hydrogen Energy, 2024, 49, 1288–1303 Search PubMed.
- Y. Gu, Q. Chen, D. Wang and Z. Tang, Strategy-based optimization and life cycle environmental impact assessment of a stable photovoltaic power-to-hydrogen system, Energy Convers. Manage., 2024, 315, 118747 Search PubMed.
- T. Weidner, V. Tulus and G. Guillén-Gosálbez, Environmental sustainability assessment of large-scale hydrogen production using prospective life cycle analysis, Int. J. Hydrogen Energy, 2023, 48(22), 8310–8317 Search PubMed.
- W. Ajeeb, P. Baptista and R. C. Neto, Life cycle analysis of hydrogen production by different alkaline electrolyser technologies sourced with renewable energy, Energy Convers. Manage., 2024, 316, 118840 Search PubMed.
- S. Bolz, J. Thiele and T. Wendler, Regional capabilities and hydrogen adoption barriers, Energy Policy, 2024, 185, 113934 Search PubMed.
- S. O. Jeje, T. Marazani, J. O. Obiko and M. B. Shongwe, Advancing the hydrogen production economy: a comprehensive review of technologies, sustainability, and future prospects, Int. J. Hydrogen Energy, 2024, 78, 642–661 Search PubMed.
- M. El-Shafie, Hydrogen production by water electrolysis technologies: a review, Results Eng., 2023, 101426 Search PubMed.
- How bioenergy contributes to a sustainable future. Bioenergy Review. IEA BIOENERGY REPORT 2023. https://www.ieabioenergyreview.org/wp-content/uploads/2022/12/IEA_BIOENERGY_REPORT.pdf (accessed July 25, 2024).
- P. Busch, A. Kendall and T. Lipman, A systematic review of life cycle greenhouse gas intensity values for hydrogen production pathways, Renewable Sustainable Energy Rev., 2023, 184, 113588 Search PubMed.
- E. Cetinkaya, I. Dincer and G. F. Naterer, Life cycle assessment of various hydrogen production methods, Int. J. Hydrogen Energy, 2012, 37(3), 2071–2080 Search PubMed.
- P. Nikolaidis and A. Poullikkas, A comparative overview of hydrogen production processes, Renewable Sustainable Energy Rev., 2017, 67, 597–611 Search PubMed.
| This journal is © The Royal Society of Chemistry 2025 |