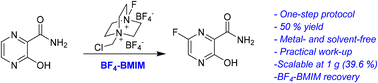
Favipiravir is an important selective antiviral that emerged as an alternative against COVID-19 during the pandemic. Its synthesis has gained great interest and the conventional strategies proceed through multiple-step protocols (6–7 reaction steps), which involve, in addition, several drawbacks with global yields, lower than 34%. Herein, a simple, economical, eco-friendly and scalable (1 g) one-step protocol for the synthesis of favipiravir from the direct fluorination of the available 3-hydroxy-2-pyrazinecarboxamide with Selectfluor® is reported. The reaction proceeds easily in BF4-BMIM through a simple operational work-up, affording the favipiravir with a yield of 50% without the need of a special catalyst/additive. The key point of the present strategy was the use of the ionic liquid of BF4-BMIM, which helps to minimize the several chemical limitations derived from 3-hydroxy-2-pyrazinecarboxamide as a substrate for the direct Selectfluor-mediated fluorination. All these chemical reactivity aspects are also discussed in detail.