Expression of hyperconjugative stereoelectronic interactions in borazines†
Abstract
This paper discusses hyperconjugative stereoelectronic effects in borazines. A series of alkyl-substituted borazines were synthesized and analysed by NMR spectroscopy and X-ray diffraction. Supported by NBO analyses, the significant decreases in 1JCH coupling constant for the CH groups adjacent to the boron atoms are consistent with the presence of 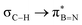 and
and 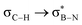 interactions. These interactions lower the electrophilicity of boron atoms, enhancing moisture stability and establishing these molecules as valuable scaffolds in synthetic chemistry and materials science.
interactions. These interactions lower the electrophilicity of boron atoms, enhancing moisture stability and establishing these molecules as valuable scaffolds in synthetic chemistry and materials science.

- This article is part of the themed collection: ChemComm 60th Anniversary Board Member Collection


 Please wait while we load your content...
Please wait while we load your content...