Is the single-ion conductor cubic Li7La3Zr2O12 a binary ionic electrolyte?
Abstract
Garnet-type cubic Li7La3Zr2O12 (c-LLZO) is a single-ion conductor, but the dynamics of lithium dendrite initiation during one-way plating in Li|c-LLZO|Li symmetric cells demonstrate several trends that all resemble the dendrite initiation mechanisms found in binary liquid electrolytes. This Opinion article provides an analysis of the possible charge carriers and discusses the four species that coexist in c-LLZO when continued electrochemical reactions take place at the opposite interfaces of the ceramic electrolyte. The new understanding explains the possibility of significant concentration polarization before the onset of dendrites in c-LLZO, without violating commonly accepted rules and physical laws. We conclude that under Faradaic reaction conditions, c-LLZO is a binary ionic electrolyte with 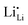 as the positive charge carrier and
as the positive charge carrier and  the negative charge carrier, among native but neutral Li×Li and V×Li. Li ions are still the sole conducting ion, but the electrochemically generated Li vacancies significantly alter the long-range transport behavior, rendering the single-ion conductor a binary electrolyte.
the negative charge carrier, among native but neutral Li×Li and V×Li. Li ions are still the sole conducting ion, but the electrochemically generated Li vacancies significantly alter the long-range transport behavior, rendering the single-ion conductor a binary electrolyte.

- This article is part of the themed collection: Recent Review Articles


 Please wait while we load your content...
Please wait while we load your content...