Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Cavity-containing supramolecular gels as a crystallization tool for hydrophobic pharmaceuticals†
Lena
Kaufmann
a,
Stuart R.
Kennedy
b,
Christopher D.
Jones
b and
Jonathan W.
Steed
*b
aFreie Universität Berlin, Takustr. 3, 14195 Berlin, Germany
bDurham University, South Road, DH1 3LE, UK. E-mail: jon.steed@durham.ac.uk
First published on 29th June 2016
Abstract
We present two approaches to low-molecular-weight supramolecular gels bearing hydrophobic cavities based on calixarene-containing building blocks. Gels are formed by a calixarene based tetrahydrazide gelator or a co-gel of a calixarene diammonium salt and a bis-crown ether. The calixarene hydrophobic cavity enables the complexation of hydrophobic drug molecules in a generic fashion thus providing an anchor site on the surface of the gel fibre to initiate drug crystal nucleation and growth. This technique potentially represents a route to growth of hard-to-nucleate polymorphic modifications. The co-gel comprising two components holding together by non-covalent ammonium-crown ether interaction can be easily switched back to the sol state by adding competitive binding cations.
Gels are a class of dynamic soft materials that are becoming increasingly important as they might open the way to completely new functional systems.1,2 The gel state is defined as “a two-component, colloidal dispersion with a continuous structure with macroscopic dimensions that is permanent on the time scale of the experiment and is solid-like in its rheological behaviour”.3,4 Compared with for example ceramics, metals or plastic, gels are not as long-lasting and stable, but instead provide interesting properties, e.g. the ability to be switched between gel and sol states by external stimuli and free fluid-like diffusion within their structure.5 Traditionally, cross-linked covalent polymers have been used as gelators.6–10 These polymers are notable for their ability to swell up by taking in solvent molecules, in amounts that can considerably outweigh their own mass.11 In the last two decades supramolecular gelation systems based on low-molecular-weight gelators (LMWGs) have received substantial attention owing to their straightforward preparation, interesting self-assembly properties and the reversibility of the inter-component interactions.12–20 In these systems small covalent building blocks with a defined chemical structure self-assemble through non-covalent, weak and reversible interactions like hydrogen bonding or π–π-stacking into one-dimensional fibres. By interconnection of these fibres a self-supporting three-dimensional network is formed and solvent molecules can be trapped within it by surface tension.13
One emerging potential application of such reversible and synthetically versatile gels is in the area of pharmaceutical crystallization.21–25 Control of polymorphic or solvate form is of key industrial importance.26–29 Gels based on LMWGs represent a highly tuneable medium adaptable to a range of solvents including those commonly used in pharmaceutical manufacture and they offer the possibility of specific gelator–solute interactions which may influence the solid form outcome and hence make LMWGs a useful part of a pharmaceutical solid form screening strategy.30,31 A supersaturated mixture of LMWG and solute represents a non-equilibrium, weakly coupled orthogonal self-assembling system in which gel and crystal assembly occurs in a self-sorting fashion but with the possibility of mutual influence on the emergent properties of the whole.22,32 Specifically binding of drug molecules to the locally ordered gel fibre surface offers a novel heterogeneous nucleation pathway in which the local periodicity of the gel may be imparted to the growing crystal nucleus, hence potentially subverting the normal polymorphic outcome of the crystallization process. In this work we report two approaches to the design of broadly applicable gels capable of binding, in a generic way, the hydrophobic residues of small molecule pharmaceuticals with this application in mind. Modern drugs are increasingly hydrophobic.33 By incorporating a series of hydrophobic cavities into the gel fibre18 it should be possible to bind a locally ordered layer of drug molecules to the gel fibres and, under supersaturation conditions, use these sites as the starting point for the crystallization of drug solid forms.
The basic structural element of the target gels incorporates a calix[4]arenes whose bowl shaped molecular structure offers a hydrophobic cavity suitable for the inclusion of hydrophobic residues of a range of potential solutes.34–36 These gelation systems should be widely applicable for all kind of drug molecules with a residue that can fit into the calixarene cavity. Calixarenes can be easily modified at either the upper and/or lower rims of the molecular skeleton.37,38 For calixarene incorporation into a gel we report two different concepts:
(1) A one-component approach, in which the calixarene monomers are functionalised with directional hydrogen bonding groups to enable them to act as LMWG.
(2) A two-component approach in which a supramolecular co-gelator is formed from the non-covalent interaction of two components that are non-gelators individually.
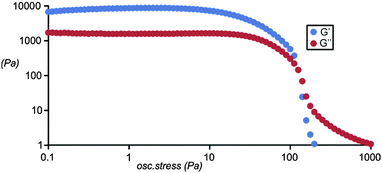
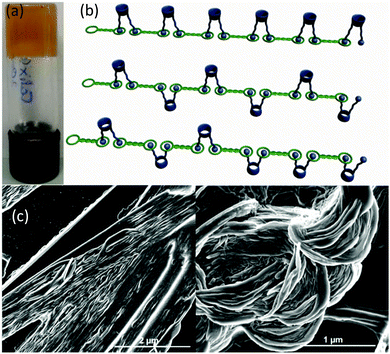
A single component gelator. Compound 1 (Scheme 1) was designed to produce gel fibres by mutual association of the hydrazide groups leaving the calixarene cavity exposed on the gel fibre surface. Compound 1 was prepared in moderate yield by functionalization of calix[4]arene with ethyl bromoacetate39 followed by reaction with hydrazine hydrate.40 The resulting hydrazide was then coupled with benzoylhydrazinyl oxobutanoic acid,41 leading to the desired product 1 with two diformylhydrazine groups (see ESI,† for details). The gelation ability of 1 was screened in a wide variety of organic solvents but proved to form strong reproducible gels only in 1,2-dibromoethane with a critical gelation concentration of 1% w/v as evidenced by the vial inversion test (Fig. 1b). The resulting gel was analysed by stress sweep rheometry (Fig. 2) which revealed a strong gel with a plateau G′ value of approximately 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Pa, around an order of magnitude higher than G′′ confirming the solid-like nature of the material.42,43 SEM images of the dried xerogel (Fig. 1c) revealed a fibrous structure typical of self-assembled fibrillar networks involving hydrogen bonded chains of gelators.42 We postulate that gelation occurs by the aggregation mode depicted in Fig. 1a. Placing 1 in a concentration of 2% w/v in a non-polar solvent like 1,2-dibromoethane leads to gel formation through multiple C
000 Pa, around an order of magnitude higher than G′′ confirming the solid-like nature of the material.42,43 SEM images of the dried xerogel (Fig. 1c) revealed a fibrous structure typical of self-assembled fibrillar networks involving hydrogen bonded chains of gelators.42 We postulate that gelation occurs by the aggregation mode depicted in Fig. 1a. Placing 1 in a concentration of 2% w/v in a non-polar solvent like 1,2-dibromoethane leads to gel formation through multiple C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯HN hydrogen bonding of the diformylhydrazine groups.19,44
O⋯HN hydrogen bonding of the diformylhydrazine groups.19,44
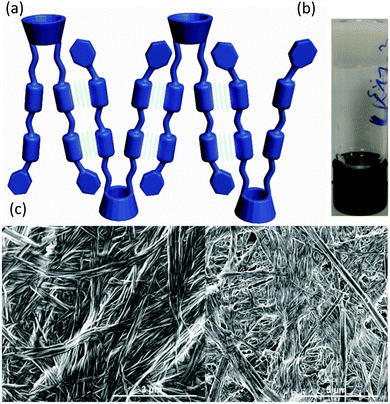 | ||
| Fig. 1 (a) Schematic of the proposed self-assembly of 1 to produce a gel fibre (b) vial inversion test of the 1,2-dibromoethane gel of 1 (2% w/v) (c) SEM images of the xerogel. | ||
A two-component co-gel. While suitable for proof of principle, compound 1 is not particularly versatile in its gelation properties and requires a number of steps to prepare. As a result we also investigated the preparation of multicomponent co-gels from simpler components. A bis(3-aminopropyl) calixarene is readily synthesised following the procedure reported by Durmaz45 which yields the tosylate salt 2 (as either the t-butyl form 2a or unsubstituted derivative 2b) on protonation with p-toluenesulfonic acid. Ammonium groups are well known to form host–guest complexes with crown ethers46,47 and we reasoned that a one dimensional hydrogen bonded polymer with potential gelation properties would result from the combination of 2 with bis(crown ether) 3 (Scheme 2).48 The proposed assembly mode is shown schematically in Fig. 3b. Synthetic details are given in the ESI.† The formation of compound 2b was also confirmed by single crystal X-ray crystallography as a chloroform solvate. The X-ray data is of poor quality and does not permit any quantitative description (see ESI†) but reveals the key gross structural details of the cone conformation and 1,3-disubstituted nature of the compound with the two ammonium groups situated on the same side of the calixarene cavity, Fig. 4, supporting the assembly mode proposed in Fig. 3b.
 | ||
| Fig. 3 (a) Vial inversion test of the 2a·3 co-gel (2% w/v in 1,2,4-trichlorobenzene) (b) schematic view of the interaction of the proposed self-assembly mode. (c) SEM images of the xerogel. | ||
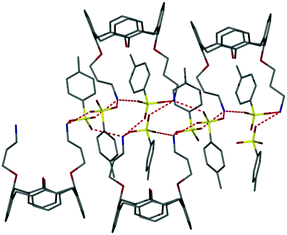 | ||
| Fig. 4 X-ray crystal structure of the ammonium terminated calixarene 2b showing hydrogen bonding to the tosylate counter ions. | ||
Neither 2 nor 3 alone are gelators individually, however, after screening 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixtures of the compounds in a variety of solvents we established that a 1
1 mixtures of the compounds in a variety of solvents we established that a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of either 2a or 2b with 3 in 1,2,4-trichlorobenzene at 6% w/v results in gel formation (Fig. 3a). Gel formation was recognised by a simple vial inversion test and confirmed by stress sweep rheology and SEM. The solid like nature of the gel was confirmed by the much greater plateau value of G′ compared to G′′ (see ESI,† Fig. S1) while the SEM images confirm the fibrillar nature of the xerogel. Interestingly the 2·3 co-gel formed only with tosylate counter ions. The analogous hexafluorophosphate or trifluoromethanesulfonate salts precipitated instead of forming gels in 1,2,4-trichlorobenzene. This anion dependence suggests that the gelation properties may be anion controlled allowing selective gel turn-off, for example to recover gel grown crystals.21 In addition, even the tosylate salt of the gel can be ‘switched off’ in the presence of potassium ions. An aliquot of 10 μl of a saturated solution of KPF6 in acetonitrile was carefully layered onto the gel. After 3 hours the whole gel collapsed and did not re-form on heating and cooling (Fig. 5). The gel was unaffected by acetonitrile alone. This experiment suggests that K+ can competitively bind with the crown ether and break up the hydrogen bonded polymer structure, with the strong binding of K+ displacing the ammonium ions.49
1 mixture of either 2a or 2b with 3 in 1,2,4-trichlorobenzene at 6% w/v results in gel formation (Fig. 3a). Gel formation was recognised by a simple vial inversion test and confirmed by stress sweep rheology and SEM. The solid like nature of the gel was confirmed by the much greater plateau value of G′ compared to G′′ (see ESI,† Fig. S1) while the SEM images confirm the fibrillar nature of the xerogel. Interestingly the 2·3 co-gel formed only with tosylate counter ions. The analogous hexafluorophosphate or trifluoromethanesulfonate salts precipitated instead of forming gels in 1,2,4-trichlorobenzene. This anion dependence suggests that the gelation properties may be anion controlled allowing selective gel turn-off, for example to recover gel grown crystals.21 In addition, even the tosylate salt of the gel can be ‘switched off’ in the presence of potassium ions. An aliquot of 10 μl of a saturated solution of KPF6 in acetonitrile was carefully layered onto the gel. After 3 hours the whole gel collapsed and did not re-form on heating and cooling (Fig. 5). The gel was unaffected by acetonitrile alone. This experiment suggests that K+ can competitively bind with the crown ether and break up the hydrogen bonded polymer structure, with the strong binding of K+ displacing the ammonium ions.49
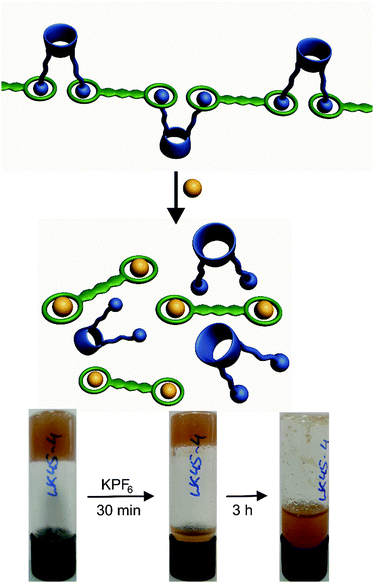 | ||
| Fig. 5 Schematic representation of the collapse of gel 2·3 and photographs of the gel samples upon addition of upon addition of KPF6 in acetonitrile solution. | ||
Crystallization experiments. Preliminary crystallization experiments with model drug substances were carried out in order to demonstrate the utility of the new calixarene-containing gels in a pharmaceutical crystallization context. Compounds tested were paracetamol, the NSAID fenbufen (4-(4-biphenyl)-4-oxobutyric acid), the depigmentation drug monobenzone (4-(benzyloxy)phenol) and the antifungal agent chlorphenesin (3-(p-chlorophenoxy)propane-1,2-diol). The drug substances were dissolved in a hot sol containing either gelator 1 in 1,2-dibromoethane or mixed gelator 2·3 in 1,2,4-trichlorobenzene. Upon cooling gels formed in a similar way to the gels obtained in the absence of the drug solutes. Crystals were recovered manually and characterised by X-ray crystallography. The solid forms isolated corresponded to the known polymorphs obtained via non-gel methods in each case with the exception of chlorphenesin, for which the X-ray crystal structure is not reported in the CSD and was obtained from a sample produced in a gel of 2b·3. We were subsequently also able to obtain diffraction quality crystals of the same solid form of chlorphenesin via solution methods. Interestingly, the structure exhibits Z′ = 2 and is based on an extensive network of hydrogen bonded rings and chains. It thus fits with Brock's analysis that dialcohol structures should exhibit a tendency towards Z′ > 1.50 Full structural details are given in the ESI.†
These preliminary crystal growth experiments establish the feasibility of designing gels with controllable properties bearing hydrophobic cavity-containing units and establish proof-of-principle for their application as generally applicable pharmaceutical crystallization media for hydrophobic drugs.
Two new low-molecular-weight supramolecular gels containing hydrophobic cavities have been prepared based on either a unimolecular or two-component co-gel system. While these first generation LMWG are not particularly versatile gelators they establish proof-of-principle that calixarene based gelators may be used to crystallise a variety of drug substances containing hydrophobic residues. The formation of organic crystals within a self-assembled gel medium represents weakly coupled orthogonal self-assembly under non-equilibrium conditions.22,32,51,52 Further work aims to expand the range of solvent systems gelled with a view to the discovery of novel polymorphic forms and integration of ‘off the shelf’ hydrophobic gelators within a polymorph screening method.
L. K. is very grateful to the DFG (German Research Foundation) for a research fellowship. We thank the Engineering and Physical Sciences Research Council for funding (project EP/J013021/1).
Notes and references
-
L. Kaufmann and C. A. Schalley, Analytical Methods in Supramolecular Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, 2012, ch. 1, p. 1 DOI:10.1002/9783527644131
.
- A. R. Hirst, B. Escuder, J. F. Miravet and D. K. Smith, Angew. Chem., Int. Ed., 2008, 47, 8002 CrossRef CAS PubMed
.
- P. J. Flory, Faraday Discuss. Chem. Soc., 1974, 57, 7 RSC
.
- L. A. Estroff and A. D. Hamilton, Chem. Rev., 2004, 104, 1201 CrossRef CAS PubMed
.
- T. Kato, N. Mizoshita and K. Kishimoto, Angew. Chem., Int. Ed., 2006, 45, 38 CrossRef CAS PubMed
.
-
Polymer Gels and Networks, ed. Y. Osada, A. R. Khokhlov and O. Osada, Marcel Dekker Inc., New York, 2001 Search PubMed
.
- K. Almdal, J. Dyre, S. Hvidt and O. Kramer, Polym. Gels Networks, 1993, 1, 5 CrossRef CAS
.
- A. C. Balazs, Nature, 2013, 493, 172 CrossRef CAS PubMed
.
- Y. Amamoto, J. Kamada, H. Otsuka, A. Takahara and K. Matyjaszewski, Angew. Chem., Int. Ed., 2011, 50, 1660 CrossRef CAS PubMed
.
- D. Steinhilber, T. Rossow, S. Wedepohl, F. Paulus, S. Seiffert and R. Haag, Angew. Chem., Int. Ed., 2013, 52, 13538 CrossRef CAS PubMed
.
- H. J. Schneider and R. M. Strongin, Acc. Chem. Res., 2009, 42, 1489 CrossRef CAS PubMed
.
- J. W. Steed, Chem. Commun., 2011, 47, 1379 RSC
.
- P. Terech and R. G. Weiss, Chem. Rev., 1997, 97, 3133 CrossRef CAS PubMed
.
- D. J. Abdallah and R. G. Weiss, Adv. Mater., 2000, 12, 1237 CrossRef CAS
.
- N. M. Sangeetha and U. Maitra, Chem. Soc. Rev., 2005, 34, 821 RSC
.
- P. Sahoo, R. Sankolli, H.-Y. Lee, S. R. Raghavan and P. Dastidar, Chem. – Eur. J., 2012, 18, 8057 CrossRef CAS PubMed
.
- X. Cai, K. Liu, J. Yan, H. Zhang, X. Hou, Z. Liu and Y. Fang, Soft Matter, 2012, 8, 3756 RSC
.
- J. A. Foster and J. W. Steed, Angew. Chem., Int. Ed., 2010, 49, 6718 CrossRef CAS PubMed
.
- F. Fages, F. Vögtle and M.
![[Z with combining breve]](https://www.rsc.org/images/entities/char_005a_0306.gif) inic, Top. Curr. Chem., 2005, 256, 77 CrossRef CAS PubMed
inic, Top. Curr. Chem., 2005, 256, 77 CrossRef CAS PubMed .
- M.
![[Z with combining breve]](https://www.rsc.org/images/entities/char_005a_0306.gif) inic, F. Vögtle and F. Fages, Top. Curr. Chem., 2005, 256, 39 CrossRef
inic, F. Vögtle and F. Fages, Top. Curr. Chem., 2005, 256, 39 CrossRef .
- J. A. Foster, M.-O. M. Piepenbrock, G. O. Lloyd, N. Clarke, J. A. K. Howard and J. W. Steed, Nat. Chem., 2010, 2, 1037 CrossRef CAS PubMed
.
- D. K. Kumar and J. W. Steed, Chem. Soc. Rev., 2014, 43, 2080 RSC
.
- F. Aparicio, E. Matesanz and L. Sánchez, Chem. Commun., 2012, 48, 5757 RSC
.
- M. Pauchet, T. Morelli, S. Coste, J.-J. Malandain and G. Coquerel, Cryst. Growth Des., 2006, 6, 1881 CAS
.
- Y. Diao, K. E. Whaley, M. E. Helgeson, M. A. Woldeyes, P. S. Doyle, A. S. Myerson, T. A. Hatton and B. L. Trout, J. Am. Chem. Soc., 2012, 134, 673 CrossRef CAS PubMed
.
-
R. Hilfiker, Polymorphism: In the Pharmaceutical Industry, Wiley-VCH, Weinheim, 2006 Search PubMed
.
-
J. Bernstein, Polymorphism in Molecular Crystals, Clarendon Press, Oxford, 2002 Search PubMed
.
- J. Bauer, S. Spanton, R. Henry, J. Quick, W. Dziki, W. Porter and J. Morris, Pharm. Res., 2001, 18, 859 CrossRef CAS
.
-
Polymorphism in Pharmaceutical Solids, ed. H. G. Brittain, Marcel Dekker Inc., New York, 1999 Search PubMed
.
- S. Byrn, R. Pfeiffer, M. Gany, C. Hoiberg and G. Poochikian, Pharm. Res., 1995, 12, 945 CrossRef CAS
.
- E. H. Lee, Asian J. Pharm. Sci., 2014, 9, 163 CrossRef
.
- A. Brizard, M. Stuart, K. van Bommel, A. Friggeri, M. de Jong and J. van Esch, Angew. Chem., Int. Ed., 2008, 47, 2063 CrossRef CAS PubMed
.
- C. Wischke and S. P. Schwendeman, Int. J. Pharm., 2008, 364, 298 CrossRef CAS PubMed
.
- R. Joseph and C. P. Rao, Chem. Rev., 2011, 111, 4658 CrossRef CAS PubMed
.
-
C. D. Gutsche, Calixarenes Revisited, Royal Society of Chemistry, Cambridge, 1998 Search PubMed
.
- A. Ikeda and S. Shinkai, Chem. Rev., 1997, 97, 1713 CrossRef CAS PubMed
.
- S. Eymur, E. Akceylan, O. Sahin, A. Uyanik and M. Yilmaz, Tetrahedron, 2014, 70, 4471 CrossRef CAS
.
- A. Uyanik, M. Bayrakci, S. Eymur and M. Yilmaz, Tetrahedron, 2014, 70, 9307 CrossRef CAS
.
- D. M. Rudkevich, W. Verboom and D. N. Reinhoudt, J. Org. Chem., 1994, 59, 3683 CrossRef CAS
.
- E. Quinlan, S. E. Matthews and T. Gunnlaugsson, Tetrahedron Lett., 2006, 47, 9333 CrossRef CAS
.
- X. Zhang, C.-H. Liu, L.-H. Liu, F.-Y. Wu, L. Guo, X.-Y. Sun, C.-J. Wang and Y.-B. Jiang, Org. Biomol. Chem., 2003, 1, 728 CAS
.
- G. Yu, X. Yan, C. Han and F. Huang, Chem. Soc. Rev., 2013, 42, 6697 RSC
.
-
C. Chassenieux and L. Bouteiller, in Supramolecular Chemistry from Molecules to Nanomaterials, ed. P. A. Gale and J. W. Steed, John Wiley & Sons, Chichester, 2012, vol. 2, p. 517 Search PubMed
.
- R. Santra, K. Banerjee and K. Biradha, Chem. Commun., 2011, 47, 10740 RSC
.
- M. Durmaz, J. Inclusion Phenom. Macrocyclic Chem., 2012, 74, 361 CrossRef CAS
.
- J. D. Badjic, V. Balzani, A. Credi, S. Silvi and J. F. Stoddart, Science, 2004, 303, 1845 CrossRef CAS PubMed
.
- M. Lohse, K. Nowosinski, N. L. Traulsen, A. J. Achazi, L. K. S. von Krbek, B. Paulus, C. A. Schalley and S. Hecht, Chem. Commun., 2015, 51, 9777 RSC
.
- W. Jiang and C. A. Schalley, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 10425 CrossRef CAS PubMed
.
- R. M. Izatt, K. Pawlak, J. S. Bradshaw and R. L. Bruening, Chem. Rev., 1991, 91, 1721 CrossRef CAS
.
- C. P. Brock and L. L. Duncan, Chem. Mater., 1994, 6, 1307 CrossRef CAS
.
- A. Heeres, C. van der Pol, M. Stuart, A. Friggeri, B. L. Feringa and J. van Esch, J. Am. Chem. Soc., 2003, 125, 14252 CrossRef CAS PubMed
.
- M. Laupheimer, K. Jovic, F. E. Antunes, M. da Graca Martins Miguel and C. Stubenrauch, Soft Matter, 2013, 9, 3661 RSC
.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis of new compounds, experimental details for crystallization and gelation studies. CCDC 1478493. Underlying research data for this paper is available in accordance with EPSRC open data policy from DOI: 10.15128/37720c73c. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6cc04037c |
| This journal is © The Royal Society of Chemistry 2016 |