Opportunities and critical factors of porous metal–organic frameworks for industrial light olefins separation
Tianhao
Lan
a,
Libo
Li
 *ab,
Yang
Chen
*ab,
Yang
Chen
 a,
Xiaoqing
Wang
a,
Jiangfeng
Yang
a,
Xiaoqing
Wang
a,
Jiangfeng
Yang
 a and
Jinping
Li
a and
Jinping
Li
 ab
ab
aCollege of Chemistry and Chemical Engineering, Shanxi Key Laboratory of Gas Energy Efficient and Clean Utilization, Taiyuan University of Technology, Taiyuan, 030024, Shanxi, P. R. China. E-mail: lilibo908@hotmail.com; Tel: +86 15635105908
bState Key Laboratory of Coal Conversion, Institute of Coal Chemistry, Chinese Academy of Sciences, Taiyuan 030024, Shanxi, P. R. China
First published on 7th May 2020
Abstract
Light olefins (ethylene, propylene, and 1,3-butadiene) are widely used as building blocks in the petrochemical industry and for the fabrication of everyday products. Developing energy cost-efficient porous materials for the separation of light olefins is fundamentally and industrially important. Metal–organic frameworks (MOFs), representing a novel class of porous materials, possess unique pore structures, endowing them with exceptional porosity, tunable pore sizes, and facile functionalization. These features permit distinct host–guest interactions and/or sieving effects, allowing the separation of molecules in an energy-efficient manner. However, some critical factors, such as large-scale production and fabrication into shaped forms need to be addressed for real industrial separations. To solve these issues, many novel approaches for synthesizing and shaping MOFs have recently been developed. In this review, we summarize and highlight recent advances in the realm of gas separation using MOFs as adsorbents, covering the progress made in improving the key features of MOFs that are necessary for their real-world applications, and opening a broader perspective on the current status of this field and its challenges.
1 Introduction
Light olefins (C2–C4) are important constituents of many products that we rely upon in our daily lives, and are currently produced from fossil-derived feedstocks such as naphtha and light paraffins. Nowadays, most of the machinery present in a generic industrial installation (e.g., oil refinery or polymer factory) consumes vast amounts of energy with the aim of purifying these products. Distillation remains the most commonly applied separation process. Specifically, this technology constitutes around 90–95% of all separations in the light-olefin and petroleum refining industries.1 However, the separation and purification process is also inefficient, and the aforementioned energy consumption accounts for around 40% of all energy input in the current chemical industry.2 The recent rise in energy security concerns and demands further emphasizes the relevance of this problem.In this regard, tremendous energy savings can be expected by applying advanced adsorptive separation and/or membrane-based processes by employing porous materials as separating agents.3 Notable research efforts have been devoted to the design and synthesis of efficient separation materials, such as carbon-based materials,4 zeolites,5 and metal–organic frameworks (MOFs)6 for the adsorptive separation of light olefins. Compared with classical adsorbents, such as zeolites and carbon-based materials, the new generation of MOFs (also called porous coordination polymers) offer great potential for addressing the above challenges by virtue of their customizable pore channels and propensity for fine-tuning of the pore system (pore aperture size, shape, and functionality).7–11 That's why MOFs can be applied to numerous fields such as gas storage,12–16 gas separations,17–20 chemical sensing,21–25 catalysis,26–31 molecular recognition32–35 and environmental remediation.36–38 For the chemistry and materials science communities, MOFs have been demonstrated to be promising for addressing some traditional technological problems (Scheme 1).39–42
Although a good deal of progress has been made, several steps are still required to scale-up MOF materials from studies of their basic science to the realm of applications. Looking forward, a future direction would be to focus on scaled-up syntheses,43–46 while still satisfying multiple criteria, such as long-term stability,47–53 high performance, and processability,54–56 all within a viable cost envelope.
Herein, we intend to give an overview on: (I) recent important scientific developments on MOFs for their potential industrial applications in the selected area of light-olefin separation, and correlations between their structural/chemical features and their adsorption or separation performances; (II) producing as well as shaping MOFs in a simple, easy, green, and low-cost way, and in sufficient amounts to meet the needs for such applications; (III) the state-of-the-art preparation of MOF-based devices for industrial implementation, including the many efforts of industry to patent and commercialize these compounds. This review aims to chart the path from the basic academia laboratory to the practical application of MOF materials in real separations, showing that these compounds can indeed be much more than intellectual exercises of an educated mind. The final goal, in any case, would be market implementation.
2 Pore chemistry and size control in MOFs for the separation of light olefins
Light olefins are among the most important feedstocks in the petrochemical industry, with a global production capacity of more than 420 million tons [ethylene (C2H4) 170 million, propylene (C3H6) 130 million, and 1,3-butadiene (C4H6) 120 million] in 2018. Today, due to increased worldwide demand for plastic products, there is tremendous interest in the development of new separation technologies and separation materials for the efficient isolation of light olefins from their mixtures. Moreover, some petrochemical industry purge gases or refinery gases are potentially significant sources of light olefins, with contents of up to 20–30%. About 6 million cubic meters of release gas is generated by oil refineries in China each year, and a high proportion of this gas resource is not well gathered, with some being directly burned and vented to the atmosphere. It is a great waste of energy and a source of significant CO2 emissions.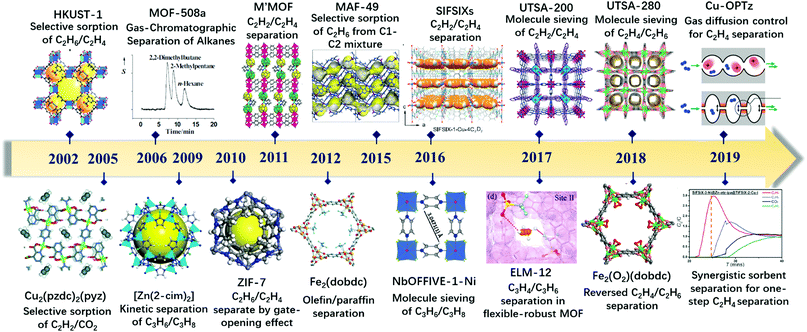 | ||
| Scheme 1 Timeline of important breakthroughs in the use of MOFs for the separation of light olefins. | ||
Porous materials can be used to separate olefins from these mixtures through pressure-swing adsorption or vacuum-swing adsorption. Qualitatively, the effectiveness of a particular porous material depends on how well it adsorbs olefins at the higher pressure and then how easily it releases them at the lower pressure.57–61
2.1 C2H4–C2H6 separation
The separation of olefin–paraffin mixtures, such as C2H4–C2H6, is a very important process because polymer-grade C2H4 products with purity ≥99.5% is the prime raw material in the chemical industry and the feedstock for 30% of all petrochemicals, such as polyethylene, polyvinyl chloride, cellulose acetate, and polyvinyl acetate polymer.1 Traditional olefin/paraffin separations have primarily relied on energy-intensive cryogenic distillation, which represents one of the most energy-intensive processes in industry.62 Adsorbent-based separations have been considered as being among the most energy-efficient alternatives. Studies of a number of MOFs have revealed that their excellent separations mainly rely on one or more of the following mechanisms: (1) an equilibrium-based mechanism; (2) a kinetic-based mechanism; (3) a gate-opening effect; and (4) a molecular sieving effect. Depending on whether C2H4 or C2H6 is captured, MOF materials can be categorized into C2H4- or C2H6-selective adsorbents, as detailed below.In the view of commercial adsorbent, researchers have made great efforts on zeolites for selective separation of C2H4 with molecule sieving effect. In 2012, Aguado et al. prepared a silver zeolite A (AgA) from NaX by ion-exchange.63 Compared with NaX, the pore size of AgA decreased from 4.24 and 4.84 Å to 4.35 and 4.36 Å, falling just between the kinetic diameters of ethane and ethylene. The gas mixture can be separated effectively by molecular sieving and π-complexation between Ag+ and ethylene. In 2017, Bereciartua et al. described the synthesis and structural determination of a flexible pure silica zeolite (ITQ-55).64 This material can dynamically separate ethylene from ethane with an unprecedented selectivity of close to 100.
The first example of an MOF material used to separate olefin/alkane mixtures was Cu-BTC65 (BTC = benzene-1,3,5-tricarboxylate). The double bond of the olefin can form a strong π-complex with the unsaturated Cu(II) ions in the MOF under low pressure, resulting in strong selective adsorption. The adsorption capacity of ethylene is thus larger than that of ethane, achieving the purpose of their separation. Wang et al.'s simulation results for single-component adsorption of ethylene yielded a value in excess of 6 mmol g−1.66 At 298 K and 100 kPa, Grand Canonical Monte Carlo (GCMC) calculations predict a separation selectivity for this material of more than 2.
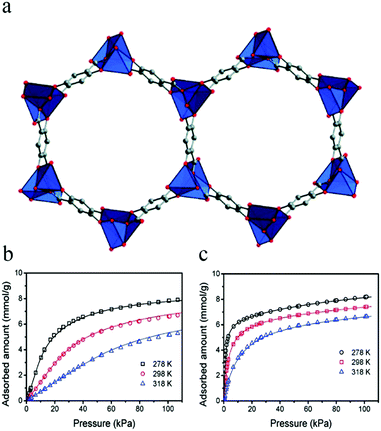
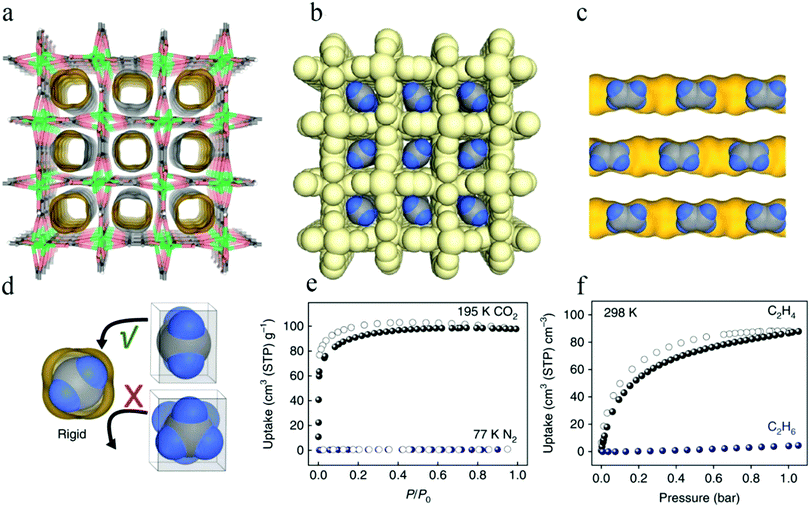
In 2011, Bao et al. reported Mg-MOF-74,67 which has a high density of unsaturated metal sites for the separation of olefin/alkane mixtures. Mg-MOF-74 has one-dimensional hexagonal channels with five-coordinate Mg(II) ions decorating their edges (Fig. 1a), and gas molecules are mainly adsorbed at the unsaturated metal sites. The results of gas adsorption experiments (Fig. 1b and c) showed that the material had good adsorption capacities for ethylene and ethane, and the amounts adsorbed at 298 K and 100 kPa in Mg-MOF-74 were measured as 6.9 and 7.2 mmol g−1, respectively. The ideal separation ability and high C2H4/C2H6 selectivity (13.9) of Mg-MOF-74 proves that it is feasible to separate such mixtures in a vacuum-swing adsorption process using this MOF as an adsorbent.
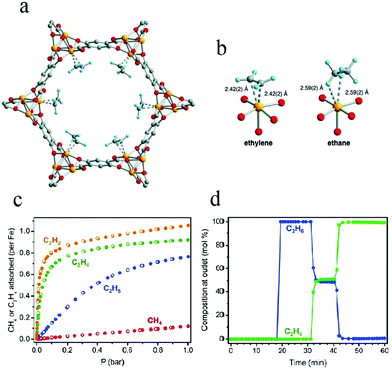
To enhance interactions between ethylene and unsaturated metal sites in an MOF adsorbent, in 2012, Bloch et al. applied Fe2(dobdc) (dobdc4− = 2,5-dioxido-benzenedicarboxylate) in the separation of C2H4/C2H6.68 Their adsorption experiments showed that Fe2(dobdc) exhibits a strong affinity for unsaturated light hydrocarbons, including C2H2, C2H4, and C3H6. Neutron powder diffraction studies indicated that the adsorption site of unsaturated light hydrocarbons lay within the Fe2(dobdc) channel, with Fe–C distances in the range 2.42–2.60 Å (Fig. 2). The interactions of C2H6 and CH4 with the metal cations in Fe2(dobdc) were seen to be weaker, as suggested by elongated Fe–C distances of around 3 Å. The C2H4/C2H6 selectivity was calculated to be 13–18 for an equimolar mixture at 318 K on the basis of ideal adsorbed solution theory (IAST), which is significantly higher than those of zeolite NaX or isostructural metal–organic framework Mg2(dobdc). Breakthrough experiments also proved that Fe2(dobdc) could separate an equimolar mixture of C2H4 and C2H6 into the pure component gases of 99.0–99.5% purity at 318 K and 100 kPa. This work represents a good example to illustrate the role of unsaturated metal sites in C2H4/C2H6 separation.
Subsequently, in 2014, Yang et al. adopted hydroxyl-functionalized porous MOF NOTT-300 (NOTT represent for University of Nottingham) to achieve high-capacity adsorption and separation of light hydrocarbons through multiple synergistic supramolecular interactions.69 The amounts of C2H4 and C2H6 adsorbed at 293 K and 1 bar in NOTT-300 were measured as 4.28 and 0.85 mmol g−1, respectively, giving rise to a notable uptake difference of 3.43 mmol g−1, which was greater than that of any MOF reported prior to this 2014 study. The selectivity for C2H4/C2H6 by IAST in NOTT-300 was calculated as 48.7, significantly higher than those of the previously reported materials, including Cu-BTC (4.0), Co-MOF-74 (6.0), Fe-MOF-74 (13.6), and PAF-1-SO3Ag (26.9). In situ synchrotron X-ray and neutron powder diffraction experiments revealed that the M–OH groups, aromatic –CH groups, and phenyl rings in NOTT-300 introduced weak, additive, supramolecular interactions with the adsorbed C2H4 molecules, with the C⋯HO distance between the C2H4 molecule and the hydroxyl group being about 4.62 Å. However, C2H6 was seen to reside at a much longer distance from the –OH group (C⋯O = 5.07 Å), indicative of weaker interactions with the NOTT-300 host. Consequently, the material showed excellent adsorption and separation performance.
In 2017, Bachman et al. built upon previous work by modifying the organic ligands of Fe2(dobdc), thereby synthesizing a series of MOFs of the type M2(m-dobdc) (M = Mg, Mn, Fe, Co, Ni; m-dobdc4− = 4,6-dioxido-1,3-benzenedicarboxylate) by employing a meta-substituted H4(m-dobdc) ligand.70 Fe2(m-dobdc) displayed the highest ethylene/ethane (>25) and propylene/propane (>55) selectivities at 298 K and 1 bar, together with olefin capacities exceeding 7 mmol g−1. In situ single-crystal X-ray diffraction analysis revealed that the vast improvement in selectivity arises from enhanced metal–olefin interactions induced by increased charge density at the metal sites. As a precondition of its selectivity, the material also shows fast adsorption kinetics because of its hexagonal channels of width about 12 Å, yielding highly pure olefins without a temperature swing. The excellent olefin/paraffin selectivity, high olefin capacity, rapid adsorption kinetics, and low raw material costs make Fe2(m-dobdc) a promising material for the separation of light hydrocarbons.
Another powerful strategy for achieving the separation of light hydrocarbons through the use of MOFs is to precisely tune their pore size to permit a molecular sieving effect. This strategy allows the complete separation of one component from others based on a molecular size or shape cut-off, avoids the co-adsorption of impurities without sacrificing the valuable uptake capacity of the porous medium, and enables infinite selectivity.
In 2018, Bao et al. synthesized a series of gallate-based MOF materials, M-gallate (M = Ni, Mg, Co),71 featuring three-dimensionally interconnected zigzag channels. The aperture sizes were ideally suited for molecular sieving of ethylene (3.28 × 4.18 × 4.84 Å) and ethane (3.81 × 4.08 × 4.82 Å). In breakthrough experiments with equimolar C2H4/C2H6 mixtures, members of the M-gallate series of MOFs all performed well, showing high selectivity for ethylene. In particular, Co-gallate at 298 K and 1 bar exhibited an unprecedented adsorption selectivity of 52.
In 2018, Lin et al. synthesized an ultramicroporous MOF [Ca(C4O4)(H2O)] (denoted as UTSA-280, UTSA represent for University of Texas USA) from calcium nitrate and squaric acid.72 UTSA-280 possesses rigid one-dimensional channels with cross-sectional areas of about 14.4 Å2 (Fig. 3), which falls between the sizes of C2H4 (13.7 Å2) and C2H6 (15.5 Å2), suggesting great size/shape sieving potential for their separation. At 298 K and 1 bar, the C2H4 uptake in UTSA-280 reaches 2.5 mmol g−1, whereas it adsorbs a negligible amount of C2H6 (0.098 mmol g−1). Breakthrough experiments validated the efficacy of this material for separating C2H4/C2H6 mixtures with high C2H4 productivity at 298 K and 1 bar. Furthermore, the material not only showed strong water stability, but could also be easily synthesized on a kilogram scale using environmentally friendly methods, making it possible to be applied in industry.
Although the molecular sieving strategy can achieve very high selectivity, small pore aperture will lead to lower adsorption capacity and kinetic diffusion rate, which will eventually affect the separation efficiency.
A large number of studies have indicated that transition metal cations, such as Cu(I), Ag(I), and Pt(I), show strong π-complexation with compounds containing double bonds, and exhibit a strong selective adsorption capacity. Therefore, introducing metal ions into MOFs is also a good strategy for improving selectivity and adsorption capacity for olefins.
In 2014, Li et al. introduced Ag(I) ions into PAF-1 for the first time and synthesized PAF-1-SO3Ag.73 Ag(I) ions can form π-complexes with the carbon–carbon double bonds of olefin molecules, thus increasing the adsorption affinity towards propylene and permitting the separation of propylene and alkanes. At 296 K and 1 bar, the amount of ethylene taken up by PAF-1-SO3Ag was about 4.1 mmol g−1. Although this is only a moderate level, those MOFs with higher adsorption capacities [such as MgMOF-74 (7.2 mmol g−1), Cu-BTC (7.2 mmol g−1), and NOTT-102 (5.8 mmol g−1)] are invariably characterized by poor stability. They commonly undergo partial framework degradation after exposure to moisture, inevitably leading to drastic decreases in ethylene uptake capacity upon reuse, but this is avoided by the high stability of PAF-1-SO3Ag. For an equimolar mixture of ethylene and ethane at 296 K, the adsorption selectivity obtained for PAF-1-SO3Ag at 100 kPa was evaluated as 27, higher than those of all MOF materials reported prior to that time. Breakthrough experiments confirmed that PAF-1-SO3Ag could effectively separate an equimolar mixture of ethylene and ethane into the pure component gases with purity greater than 99%.
In 2019, Mohamed et al. introduced Cu(I) ions into MFU-4l, replacing Zn(II) in the MOF by cation-exchange to synthesize Cu(I)-MFU-4l.74 These authors studied the amounts of olefins and alkanes adsorbed on Cu(I)-MFU-4l with different Cu/Zn ratios. Ethylene and ethane adsorption isotherms showed the ethylene adsorption capacities of all variants of Cu(I)-MFU-4l to be larger than that of MFU-4l. A steep increase in ethylene uptake was observed at very low pressures, which was attributed to its binding to the unsaturated Cu(I) active sites and subsequent uptake by physisorption. Continuous-wave electron paramagnetic resonance spectroscopy revealed the presence of partially saturated Cu(II) in the activated materials. As such sites would not coordinate olefins, their adsorption capacities were not as high as expected. The potential of these materials to separate olefins/paraffins at 296 K and 1 bar was investigated by breakthrough experiments with equimolar olefin/paraffin mixtures. High-grade propylene and ethylene (>99.999%) could be generated through temperature-concentration swing recycling from a Cu(I)-MFU-4l packed column with no measurable paraffin breakthrough.
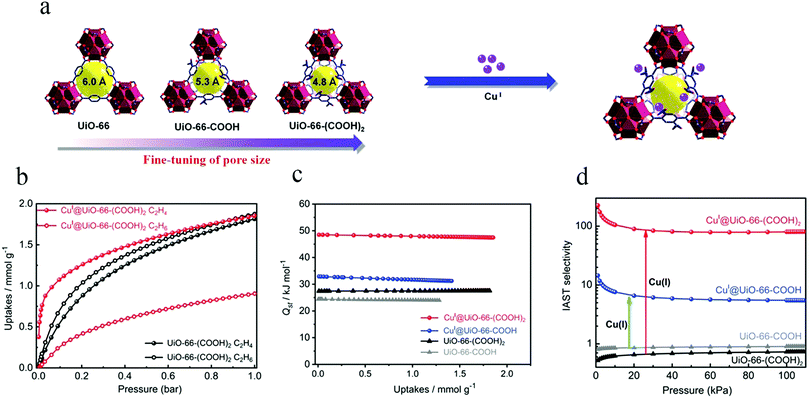
In 2019, Zhang et al. selected the UiO-66 (UiO represent for University of Oslo) family for studies of C2H4/C2H6 separation.75 They synthesized two different MOFs [denoted as UiO-66-COOH and UiO-66-(COOH)2] by using two organic linkers with different numbers of carboxyl groups, namely benzene-1,2,4-tricarboxylic acid (1,2,4-BTC) and benzene-1,2,4,5-tetracarboxylic acid (1,2,4,5-BTEC) (Fig. 4a). The pore window size could be systematically modulated through judicious choice of the organic linkers. These authors not only finely tuned the pore size, but also immobilized copper(I) ions on the framework. The obtained microporous CuI@UiO-66-(COOH)2 showed excellent performance for the separation of C2H4/C2H6, with a selectivity of 80.8, by virtue of its optimal pore size along with π-complexation ability (Fig. 4b and d). This type of material usually shows a strong interaction with ethylene, as manifested in a high heat of adsorption (Qst) (Fig. 4c), which will lead to a large desorption energy.
In 2019, Gu et al. proposed a strategy to achieve temperature-controlled regulation of guest molecule diffusion process based on the flipped molecular motion of ligand groups.76 They synthesized Cu-based porous coordination polymer Cu(OPTz) with butterfly-type ligand (Optz-ipa) containing isophthalic acid and phenothiazine-5,5-dioxide (OPTz). The OPTz group shows effective local motion with low rotation and flipping energies, therefore, the size of its cavity can be adjusted according to the variation of temperature. In gas adsorption experiment, the C2H4 adsorption of Cu(OPTz) was increased from 9 to 62 mL g−1 with the temperature changed from 180 to 320 K. In the temperature range of 200–370 K, C2H4 is more easily adsorbed than C2H6 and the maximum adsorption ratio of C2H4/C2H6 is 6.0 with the selectivity of C2H4/C2H6 about 75.
Some traditional porous materials (zeolite and porous carbon-based material) also exhibit preferential adsorption of C2H6, in general they are not satisfactory in separation processes due to low capacity and poor adsorption selectivity (normally lower than 3).77,78
As for MOFs, in 2010, Gücüyener et al. reported zeolite imidazolate framework ZIF-7 with unique gate-opening behavior, which was formed by connecting Zn metal clusters through benzimidazole linkers.79 The adsorption isotherms of several hydrocarbons were seen to increase rapidly with increasing pressure in the relevant range, but the adsorption capacity increased much more slowly in high and low pressure ranges. This type of adsorption isotherm is attributed to a specific mechanism involving gate opening, whereby specific threshold pressures control the uptake and release of individual molecules. This is a unique property of flexible MOFs. The main cavity of ZIF-7 can enlarge or contract depending on pressure variations because the linkers have some freedom to rotate through a certain angle. Because of the symmetry of the methyl structure, ZIF-7 has a lower opening pressure to C2H6. Consequently, there is a pressure range in which ZIF-7 can selectively adsorb C2H6 and not C2H4, suggesting that it might be possible to realize the reverse adsorption of C2H6/C2H4. Breakthrough experiments were carried out to evaluate the separation performance of ZIF-7 for C2H6/C2H4. The authors introduced a mixed gas of C2H6/C2H4/H2 (40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40
40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) into an adsorption column packed with ZIF-7. In breakthrough experiments, C2H4 passed through the adsorption bed first, and could thus be obtained in high purity. This was the first report of the selective adsorption of C2H6 from C2H6/C2H4 mixtures. However, the lack of C2H6 adsorption sites in ZIF-7 and the smaller pressure range between C2H6 and C2H4 led to lower separation efficiency.
20) into an adsorption column packed with ZIF-7. In breakthrough experiments, C2H4 passed through the adsorption bed first, and could thus be obtained in high purity. This was the first report of the selective adsorption of C2H6 from C2H6/C2H4 mixtures. However, the lack of C2H6 adsorption sites in ZIF-7 and the smaller pressure range between C2H6 and C2H4 led to lower separation efficiency.
In 2014, Pires et al. reported the MOF IRMOF-8 (IR represent for Isoreticular), which adsorbed greater amounts of ethane than of ethylene over a wide pressure range.80 Selectivity values between 3.4 and 1.6 were obtained, depending on the temperature and pressure. To explore the interaction of gas molecules with the surface of IRMOF-8, density functional theory (DFT) methods were applied to simulate the adsorption sites of the respective gases. Both experimental and computational results showed that, due to the difference in interaction energies of ethane and ethylene in IRMOF-8, this material preferentially adsorbs the former. The primary reason for this is that IRMOF-8 has neither unsaturated metal centers nor acid groups, so it does not have strong interactions with C2H4. The presence of two adjacent aromatic rings is an additional important factor affecting the separation performance, allowing for strong C–H⋯π interactions between the IRMOF-8 and C2H6.
Because the polarity of ethylene is greater than that of ethane, MOFs with active metal sites are not suitable for selective adsorption of the latter. Instead, adsorbents with inert pore surfaces (e.g., bearing aromatic or aliphatic moieties) should be selected. In 2018, Lin et al. proposed a feasible strategy for the design of MOF materials that preferentially adsorb C2H6.81 They found that increasing the effective contact area or the number of interactions between C2H6 molecules and the host framework can strengthen the binding affinity and lead to higher C2H6/C2H4 selectivities. They used Cu(Qc)2 (HQc = quinoline-5-carboxylic acid) as the adsorbent, the pore surface of which bears aromatic rings of low polarity, which can preferentially combine with C2H6, and the suitable pore size of Cu(Qc)2 provides a variety of interaction possibilities for the host and guest. Moreover, the larger quinoline moieties in Cu(Qc)2 dramatically lower its uptake capacity for C2H4. The calculated C2H6/C2H4 selectivity of Cu(Qc)2 for the corresponding binary equimolar mixture was up to 3.4 at 298 K and 100 kPa, which was higher than those of the other top-performing ethane-selective MOF materials at the time of this report. Neutron powder diffraction studies clearly revealed that Cu(Qc)2 displays self-adaptive sorption behavior towards C2H6, which enables continuous maintenance of close van der Waals contacts with C2H6 molecules in its optimized pore structure, and thus the preferential binding of C2H6 over C2H4. The same phenomenon can also be observed in NUM-7a which reported by Hu et al. in 2020.82
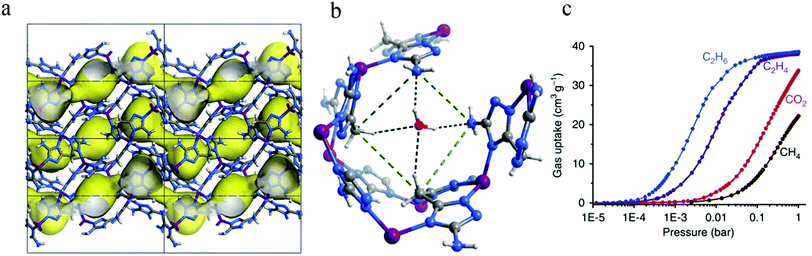
According to some previous research, the C2H6 molecule may interact more strongly with the framework of a material than C2H4 by virtue of the fact that it has more C–H bonds. If some polar functional groups are precisely introduced into an MOF, it may be possible to design a material with optimized pore size/shape and surface electrostatic distribution that can bind C2H6 much more strongly than C2H4. In 2015, Liao et al. reported the construction of a Zn-azolate MOF [Zn(batz)] [H2batz = bis(5-amino-1H-1,2,4-triazol-3-yl)methane],83 denoted as MAF-49, for rare selective adsorption of C2H6 over C2H4 (Fig. 5a and b). The pore surface of MAF-49 is rich with precisely arranged electronegative nitrogen atoms. Computational simulations indicated that the C2H6 molecule should form three strong C–H⋯N hydrogen bonds and three weak C–H⋯N electrostatic interactions with MAF-49. In contrast, for the C2H4 molecule, only two less strong C–H⋯N hydrogen bonds and two very weak C–H⋯N electrostatic interactions should be formed, because there is significant steric hindrance and electrostatic repulsion between the two C–H moieties of the two methylene groups and the neck of the host channel, which will push C2H4 into a position that is not conducive to the formation of strong hydrogen bonds. Gas adsorption isotherms showed that MAF-49 had a higher C2H6 uptake than that of C2H4 under low pressure at 316 K (Fig. 5c). Breakthrough experiments, injecting a 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 C2H4/C2H6 mixture, were carried out, which showed that C2H4 could be obtained with 99.995% purity.
1 C2H4/C2H6 mixture, were carried out, which showed that C2H4 could be obtained with 99.995% purity.
Li et al. also studied C2H6-selective adsorbents, and reported two kinds of MOFs, Ni(bdc)(ted)0.584 in 2016 and PCN-25085 in 2017. The surface area and total pore volume of Ni(bdc)(ted)0.5 were evaluated as 1701 m2 g−1 and 0.79 cm3 g−1, respectively, with an average pore diameter of 7.94 Å. The adsorption capacity of C2H6 at 100 kPa was measured as 6.93 mmol g−1, and the IAST C2H6/C2H4 selectivity was about 2 at 100 kPa. The main reason for selective adsorption of C2H6 over C2H4 in Ni(bdc)(ted)0.5 is the favored host–guest interactions between its –CH3 groups and the framework. PCN-250 (PCN represent for porous coordination network) is an Fe-based MOF material, which has excellent water and chemical stability. Its C2H6/C2H4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 and 1
15 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) selectivity was found to be in the range 1.9–4.0. At 100 kPa and 298 K, the adsorption capacities of PCN-250 for C2H6 and C2H4 were evaluated as 5.21 and 4.22 mmol g−1, respectively. It was found by a GCMC method that C2H6 and C2H4 have good adsorption positions around the four corners of benzene rings and the N atom of PCN-250. The influence of pore size and the difference in van der Waals adsorption energies are the reasons for the preferential adsorption of C2H6.
1) selectivity was found to be in the range 1.9–4.0. At 100 kPa and 298 K, the adsorption capacities of PCN-250 for C2H6 and C2H4 were evaluated as 5.21 and 4.22 mmol g−1, respectively. It was found by a GCMC method that C2H6 and C2H4 have good adsorption positions around the four corners of benzene rings and the N atom of PCN-250. The influence of pore size and the difference in van der Waals adsorption energies are the reasons for the preferential adsorption of C2H6.
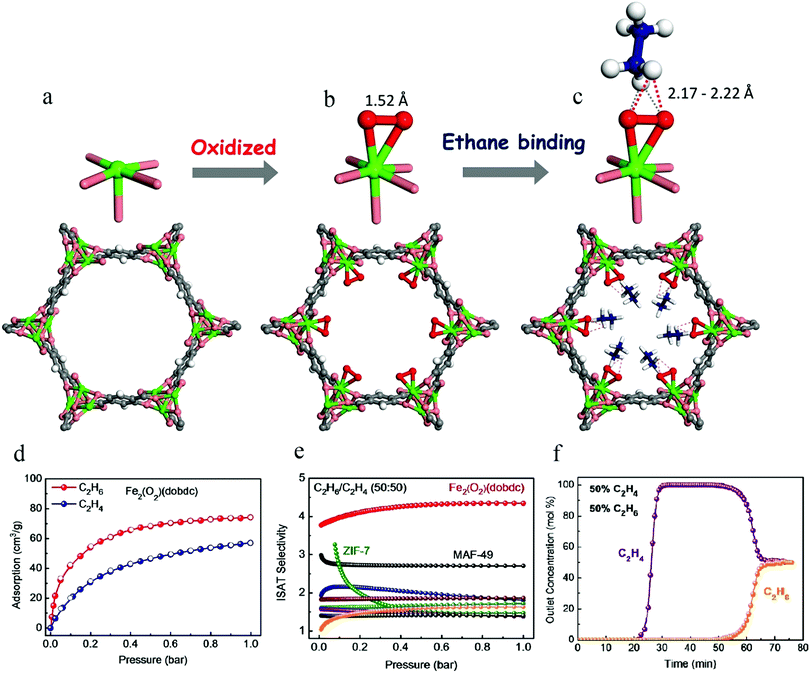
The reported MOFs capable of preferentially adsorbing C2H6 have typically lacked specific research objectives. Unsaturated metal sites are very common in MOFs. If the unsaturated metal sites in MOF materials can be modified, the resulting materials may exhibit stronger binding affinity with alkanes than alkenes, and might thus be utilized for the selective separation of C2H6 from C2H6/C2H4 mixtures. Inspired by natural metalloenzymes and some active catalytic intermediates for the synthesis of alkanes, Li et al. reported an MOF material with iron-peroxo sites, Fe2(O2)(dobdc), in 20186 (Fig. 6). Its C2H6 binding affinity was evident from single-component sorption isotherms at 298 K, which showed a much higher adsorption capacity for C2H6 (74.3 cm3 g−1) than for C2H4 (around 57 cm3 g−1) at 1 bar. High-resolution neutron powder diffraction experiments were conducted on C2D6-loaded and C2D4-loaded Fe2(O2)(dobdc) samples. The preferential binding of C2H6 at peroxo sites was established through C–D⋯O deuterium bonding (D⋯O 2.17–2.22 Å), with a D⋯O distance much shorter than the sum of the van der Waals radii of oxygen (1.52 Å) and deuterium (1.20 Å). Fe2(O2)(dobdc) exhibits an IAST selectivity of 4.4 for C2H6/C2H4 (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50), which sets a new benchmark. The clean and sharp separation in column breakthrough experiments performed with a C2H6/C2H4 (50
50), which sets a new benchmark. The clean and sharp separation in column breakthrough experiments performed with a C2H6/C2H4 (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) mixture further demonstrates that Fe2(O2)(dobdc) can readily produce high-purity C2H4 (≥99.99%) from C2H6/C2H4 mixtures.
50) mixture further demonstrates that Fe2(O2)(dobdc) can readily produce high-purity C2H4 (≥99.99%) from C2H6/C2H4 mixtures.
The same strategy can also be applied to other MOFs. Yang et al. reported “reversed C2H6/C2H4 adsorption” in Cr-BTC through the introduction of oxygen at its unsaturated metal sites in 2019.86 The oxidized Cr-BTC(O2) [Cr3(1,3,5-benzenetricarboxylate)2(O2)3] was found to bind C2H6 over C2H4 through its active Cr-superoxo sites. The C2H6/C2H4 (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, v/v) selectivity of Cr-BTC(O2) calculated by IAST was over 3 in the low-pressure region, suggesting a universality of this strategy. However, strong interaction between the C2H6 molecule and the active iron-peroxo site results in a high heat of adsorption (Qst = −66.8 kJ mol−1), which necessitates increased energy consumption during the regeneration process. Decoration of the C2H6 preferential binding sites with ligands would allow for a significant reduction in the associated heat of adsorption, but would require the construction of a favorable pore environment for the selective binding of C2H6.
50, v/v) selectivity of Cr-BTC(O2) calculated by IAST was over 3 in the low-pressure region, suggesting a universality of this strategy. However, strong interaction between the C2H6 molecule and the active iron-peroxo site results in a high heat of adsorption (Qst = −66.8 kJ mol−1), which necessitates increased energy consumption during the regeneration process. Decoration of the C2H6 preferential binding sites with ligands would allow for a significant reduction in the associated heat of adsorption, but would require the construction of a favorable pore environment for the selective binding of C2H6.
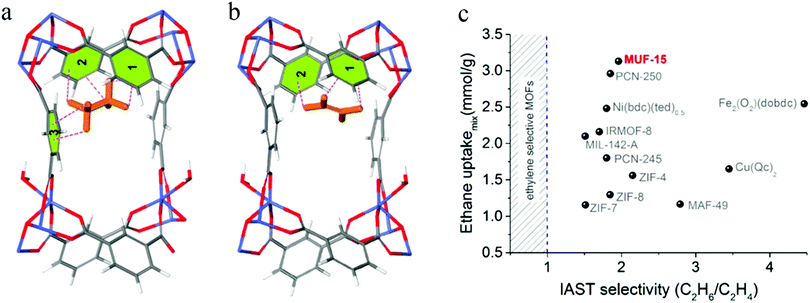
Large nanopores lead to a low selectivity, whereas high selectivity is invariably accompanied by very limited C2H6 uptake. In 2019, Qazvini et al. reported an MOF material showing a near-optimal trade-off in C2H6/C2H4 separations, named MUF-15 (MUF represent for Massey University Framework)87 (Fig. 7c). The C2H6 uptake capacity of MUF-15 at 1 bar (4.69 mmol g−1) is notably higher than those of some C2H6-selective adsorbents, namely Cu(Qc)2 (1.85 mmol g−1), ZIF-7 (1.85 mmol g−1), and MAF-49 (1.73 mmol g−1). This may be attributed to its large pore volume (0.51 cm3 g−1). The adsorption selectivity of C2H6/C2H4 mixtures was predicted on the basis of IAST using a range of starting compositions (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, 25
50, 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 75, and 10
75, and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90 C2H6/C2H4). MUF-15 exhibited a C2H6/C2H4 selectivity of around 2 for all three mixtures. Based on DFT calculations, the C2H6 molecules are bound in a pocket defined by four phenyl rings. There are C–H⋯π interactions between all six hydrogen atoms of C2H6 and three adjacent phenyl rings (Fig. 7a). In contrast, the C2H4 molecule only shows short contacts with two parallel edges of the cavity (Fig. 7b), which explains its high selectivity for C2H6. Furthermore, MUF-15 has excellent properties, such as low synthesis cost, robustness, and easy regeneration, which make it a promising MOF for industrial applications.
90 C2H6/C2H4). MUF-15 exhibited a C2H6/C2H4 selectivity of around 2 for all three mixtures. Based on DFT calculations, the C2H6 molecules are bound in a pocket defined by four phenyl rings. There are C–H⋯π interactions between all six hydrogen atoms of C2H6 and three adjacent phenyl rings (Fig. 7a). In contrast, the C2H4 molecule only shows short contacts with two parallel edges of the cavity (Fig. 7b), which explains its high selectivity for C2H6. Furthermore, MUF-15 has excellent properties, such as low synthesis cost, robustness, and easy regeneration, which make it a promising MOF for industrial applications.
A similar pore surface decoration strategy has recently reported by Yang et al.88 They reported a series of MOF materials, namely M-PNMI (M = Mn, Zn, Cd), which were synthesized using N,N′-di(4-pyridyl)-1,4,5,8-naphthalenetetracarboxydiimide. In this work, ligands with polar binding sites were introduced on the pore surfaces of the investigated MOFs, which was shown to effectively facilitate the binding of C2H6 molecules. The pore structure in these materials can provide a suitable preferential binding environment for C2H6, resulting in a higher binding affinity for C2H6 over C2H4. Based on this mechanism, M-PNMI can efficiently remove C2H6 from C2H6/C2H4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 and 1
9 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15, v/v) mixtures to directly produce highly pure C2H4 (>99.99%) under ambient conditions.
15, v/v) mixtures to directly produce highly pure C2H4 (>99.99%) under ambient conditions.
It is worth mentioning that to separate ethylene from industrial gases, apart from ethane, trace amounts impurities of acetylene and CO2 also should be considered. In 2019, Chen et al. solved this challenge perfectly by developing synergistic sorbent separation technology.89 They put three physisorbents in a packed-bed geometry. Among them, TIFSIX-2-Cu-i (TIFSIX = TiF62−, 2 = 4,4′-dipyridylacetylene, i = interpenetrated) is used to remove acetylene, Zn-atz-ipa (atz = 3-amino-1,2,4-triazolate; ipa = isophthalate) is for capture of C2H6 and CO2 can be selective removed by SIFSIX-3-Ni (SIFSIX = SiF62−, 3 = pyrazine). By using the packing sequence of SIFSIX-3-Ni@Zn-atz-ipa@TIFSIX-2-Cu-i, directly separation of quaternary gas mixture (C2H2–C2H4–C2H6–CO2) to product high-purity ethylene is achieved in one step.
2.2 C3H6–C3H8 separation
Adsorptive separation of C3H6/C3H8 based on porous materials holds great promise for enabling a possible revolution from traditional energy-intensive cryogenic distillation to energy-efficient adsorbent-based separation. Like C2H4/C2H6 separation, C3H6/C3H8 separation is also dominated by one or more of the four mechanisms (equilibrium-based mechanism, kinetic-based mechanism, gate-opening effect, and molecular sieving effect) in MOFs. A high purity C3H6 with 99.5% minimum is required as the raw material of polypropylene. Although it is a daunting challenge to design ideal porous materials for the target separation task, immense effort from both academia and industry has been incessant over the past two decades.As mentioned above, M2(m-dobdc) is a structural isomer of M-MOF-74. However, its ability to separate C3H6/C3H8 is better than that of M-MOF-74 due to an increase in charge density at its metal sites. In 2017, Bachman et al. evaluated the C3H6/C3H8 separation ability of M2(m-dobdc) (M = Mn, Fe, Co, Ni; m-dobdc4− = 4,6-dioxido-1,3-benzenedicarboxylate).70 Among these systems, Fe2(m-dobdc) showed the best selectivity (>55) for C3H6/C3H8 (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) under ambient conditions, and the adsorption capacity for C3H6 exceeded 7 mmol g−1.
50) under ambient conditions, and the adsorption capacity for C3H6 exceeded 7 mmol g−1.
While equilibrium-based separations are achieved by the preferential equilibrated uptake of one component over the other, kinetic separations are accomplished by a difference in diffusion rates of adsorbates in and out of the adsorbent. Consequently, kinetic separation can efficiently prevent the generation of high heat of adsorption during the separation process.
In 2009, Li et al. realized the first study illustrating the kinetic separation of C3H6/C3H8 on ZIFs [Zn(2-cim)2, Zn(2-bim)2, ZIF-8].93 Under equilibrium conditions, C3H6 and C3H8 adsorption measurements on these three ZIFs revealed similar adsorption capacities for both molecules, implying that thermodynamic separation would be impractical. However, C3H6 and C3H8 exhibit remarkably different diffusion rates in ZIF-8 (Fig. 8a and b), suggesting that this material has great potential for the separation of these two very similar molecules.
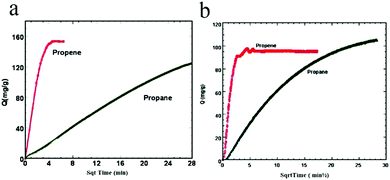 | ||
| Fig. 8 Propene and propane uptakes by ZIF-8 (a) and Zn(2-cim)2 (b) as a function of the square root of time. | ||
In 2011, Lee et al. reported a series of isostructural, non-catenated, zinc-pillared, paddlewheel MOF materials, including materials designated as DTO, TO, DBTO, and BTO.94 Compared with DTO and TO, the pore sizes of DBTO and BTO are made suitably smaller by the introduction of Br atoms. The results of gas separation experiments showed that DBTO and BTO bearing Br atoms display higher kinetic selectivity for propylene and propane than DTO and TO with larger pore size.
In 2017, Peng et al. reported another example of the kinetic separation of C3H6 and C3H8 by employing the MOF Zn(ox)0.5(trz) (ox = oxalate, trz = 1,2,4-triazole).95 Because of its zigzag-shaped ultramicroporous 1D channels with matching pore apertures, Zn(ox)0.5(trz) showed good C3H6 uptake, and very high kinetic selectivity values were achieved for C3H6/C3H8 mixtures over a wide temperature range between 303 and 363 K. At 323 K, the kinetic separation coefficient of Zn(ox)0.5(trz) reached the highest value at the time of this report (1565).
Similarly, ELM-12, reported by Li et al. in 2018, with appropriate channels (Fig. 9a) also achieved the desired kinetic separation performance.96 The kinetic C3H6/C3H8 selectivity was up to 204 at 298 K and 971 at 308 K (Fig. 9b). This high kinetic selectivity was manifested in real separation performance; a C3H6/C3H8 (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, v/v) mixture could be efficiently separated by passage through a column loaded with ELM-12, and concentrated C3H6 (>82%) could be quickly recovered during the regeneration step. Moreover, ELM-12 can be easily regenerated and synthesized on a large scale. Gas adsorption and powder X-ray diffraction (PXRD) measurements showed that ELM-12 maintained its structural integrity and gas uptake capacity over 3 years of storage under ambient conditions, suggesting that it should be favorable for practical industrial applications.
50, v/v) mixture could be efficiently separated by passage through a column loaded with ELM-12, and concentrated C3H6 (>82%) could be quickly recovered during the regeneration step. Moreover, ELM-12 can be easily regenerated and synthesized on a large scale. Gas adsorption and powder X-ray diffraction (PXRD) measurements showed that ELM-12 maintained its structural integrity and gas uptake capacity over 3 years of storage under ambient conditions, suggesting that it should be favorable for practical industrial applications.
Although the above materials exhibit excellent separation performances, it is inevitable that a small amount of propane will be co-adsorbed during the separation process. However, the molecular sieving strategy can effectively block the co-adsorption of propane so as to obtain highly pure propylene. In 2016, Cadiau et al. made important progress in the separation of propylene and propane.97 Through precise control of the pore size of MOF materials, complete exclusion of propane was achieved, so as to effectively separate propylene/propane gas mixtures based on molecular sieving (Fig. 10). NbOFFIVE-1-Ni is isostructural to SIFSIX-3-Ni, and is built from Ni(II)-pyrazine square-grid layers and NbOF52− pillars. The rational use of NbOF52− rather than SiF62− gives rise to a longer metal–fluorine distance (1.95 Å) and a concomitant tilting of the pyrazine moieties. Consequently, NbOFFIVE-1-Ni shows a smaller pore aperture size (3.0471 Å) than SIFSIX-3-Ni (4.965 Å). Molecular-sieving-based C3H6/C3H8 separation was further confirmed by breakthrough experiments with a 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 C3H6/C3H8 mixture, resulting in a C3H6 productivity of 0.6 mmol g−1 for a given cycle.
50 C3H6/C3H8 mixture, resulting in a C3H6 productivity of 0.6 mmol g−1 for a given cycle.
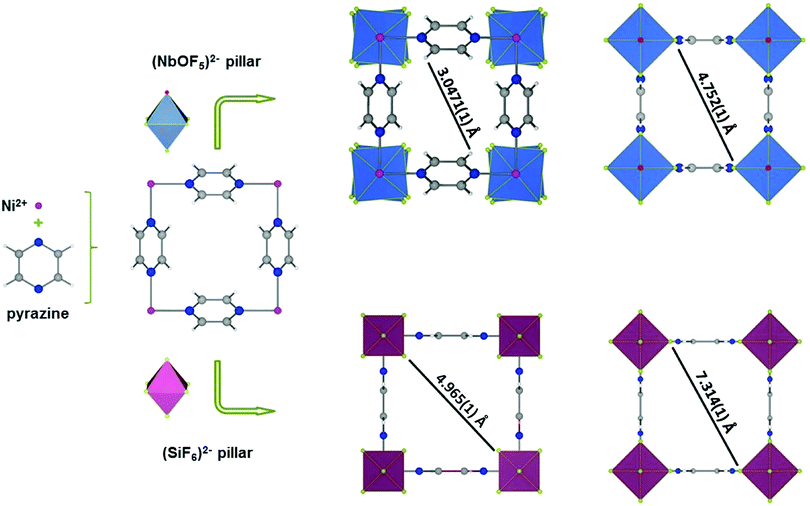 | ||
| Fig. 10 Structural description of NbOFFIVE-1-Ni highlighting the arrangement of building blocks, and comparison with the parent SIFSIX-3-Ni. | ||
In 2018, similar molecular sieving of propane/propylene was achieved in a tailor-made rare-earth-based MOF.98 Wang et al. reported the synthesis of Y6(OH)8(abtc)3(H2O)6(DMA)2 (denoted as Y-abtc; abtc = 3,3,5,5-azobenzene-tetracarboxylate; DMA = dimethylammonium). This MOF was rationally designed through topology-guided replacement of inorganic building units, which involved precise tuning of its pore apertures by judicious selection of structure topology, inorganic nodes, and organic linkers. The resultant Y-abtc has optimal pore window size (4.72 Å), which falls between the kinetic diameters of C3H6 (4.68 Å) and C3H8 (5.1 Å). Therefore, this material can effectively separate propane and propylene through a molecular sieving mechanism. Multicomponent breakthrough experiments indicated that polymer-grade C3H6 (99.5%) could be obtained from a typical cracking product mixture composition. Moreover, Y-abtc exhibits high thermal and hydrothermal stability due to its hexanuclear Y6 clusters, which indicates that this MOF has potential for efficient separation of C3H8/C3H6 mixtures.
As a unique phenomenon in flexible MOFs, gate-opening effect also plays a positive role in gas separation. In 2018, Wang et al. utilized NJU-bai8 (constructed by Cu and 5-(pyrimidin-5-yl)isophthalate acid) for separation of C3H6/C3H8 mixture.99 Single-component adsorption isotherms of C3H6 and C3H8 shows that this material has an obvious gate-opening effect on both gases at 298 K. When the pressure rises to a certain value, their adsorption capacity rises sharply. In 348 K, this material can only adsorb C3H6 (gate-opening pressure as 49.0 kPa) below 1 bar, thus exhibits extremely high selectivity for C3H6/C3H8. According to the breakthrough experiments, NJU-bai8 shows good separation performance for C3H6/C3H8 mixtures in a wide temperature rage (298–348 K). The difference in adsorption capacity of NJU-bai8 toward the two gases can be attributed to the flat pore size and strong acid–base interaction between C3H6 and the framework.
In 2019, Yu et al. synthesized a flexible MOF {Cu-(FPBDC)·DMF}n (NKU-FlexMOF-1) (H2FPBDC = 5-(5-fluoropyridin-3-yl)-1,3-benzenedicarboxylic acid).100 This material can be induced structural transformation by different guest molecules, thus showing unique adsorption behavior for C2H6, C3H6 and C3H8. In different temperature ranges, the adsorption capacity increases with the increase of temperature. Based on this characteristic, NKU-FlexMOF-1 shows excellent ability to separate C3H6/C3H8 at 273 and 298 K. In addition, the thermal stability of NKU-FlexMOF-1 reaches approximately 613 K, and this material also remains stable in solutions with a pH of 3–11, demonstrating its potential for industrial separation.
In 2013, Böhme et al.101 found that although the pore size of ZIF-8 is only 3.4 Å, under certain conditions it can adsorb molecules larger than this, such as n-alkanes (4.3 Å), branched alkanes (>5.4 Å), and 2,3-dimethylbutane (5.8 Å). From this, it can be inferred that ZIF-8 has a flexible structure like that of ZIF-7. Gas adsorption experiments showed that ZIF-8 adsorbed larger amounts of C3H8 than of C3H6 at lower pressures. This phenomenon was attributed to the gate-opening mechanism of ZIF-8.
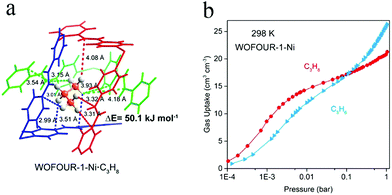
In 2020, Yang et al. reported the MOF material Ni(bpe)2(WO4) [denoted as WOFOUR-1-Ni; bpe = 1,2-bis(4-pyridyl)ethylene].102 Its cavity has a cubic shape with a size of 5.6 Å, which is close to the kinetic diameter of C3H8. The aromatic-ring-appended surface of the cage provides more electronegative binding sites relative to the channels (Fig. 11a), so as to realize multiple interactions with the adsorbed molecules. Therefore, its cage may plausibly show preferential adsorption of C3H8 because of the abundance of binding sites (C–H) and large van der Waals surface of C3H8. Meanwhile, it exhibits shape-selectivity for C3H8 because of its better matched shape than for that of C3H6 with a relatively planar configuration. Based on the above two factors, WOFOUR-1-Ni shows good performance in separating propane/propylene (Fig. 11b), with recorded C3H8/C3H6 selectivities of 1.62–2.75. Through column breakthrough experiments, propylene with a purity of 99.6% could be obtained without adsorption–desorption cycles, further improving the separation efficiency. This work reveals a novel strategy for designing propane-selective materials. To date, however, the adsorption capacities and selectivities for C3H8 of C3H8-selective MOF materials have still been very low, and much effort is needed to develop more efficient materials.
2.3 Separation of C4 olefins
C4 olefins, including 1,3-C4H6, n-C4H8, and i-C4H8, are very important feedstocks for the production of synthetic rubbers and other chemical products. Typically, C4 olefins are produced by fluidized catalytic cracking and steam cracking processes, but the C4 olefins obtained from these techniques generally consist of 30–60% 1,3-C4H6, 10–20% n-C4H8, and 10–30% i-C4H8, along with 3–10% of saturated C4H10. Due to the similar structures of the C4 olefins, their separation presents one of the great challenges among hydrocarbon purifications.In 2017, Zhang et al. reported a series of SIFSIX materials [GeFSIX-2-Cu-i (ZU-32), NbFSIX-2-Cu-i (ZU-52), GeFSIX-14-Cu-i (ZU-33)] capable of selectively recognizing different C4 olefins.103 They fine-tuned the cavity size and functional site disposition in the MOFs in increments of 0.2 Å by rationally altering the anion pillars and organic linkers. GeFSIX-14-Cu-i has a pore size of 4.2 Å, which is larger than the smallest cross-section of C4H6 but narrower than those of n-C4H8 and i-C4H8, permitting the size exclusion of these specific C4 olefins. Dispersion-corrected density functional theory (DFT-D) results showed that after C4H6 enters into GeFSIX-14-Cu-i, its acidic hydrogen atoms should become strongly bonded with two electronegative F atoms of the GeF62− pillars, with C-H⋯F distances of 2.09, 2.90, 2.18, and 2.30 Å, respectively. Based on the molecular sieving effect and the strong interactions between the gas and the MOF surface, GeFSIX-14-Cu-i shows excellent selectivity for C4H6, n-C4H8, and i-C4H8.
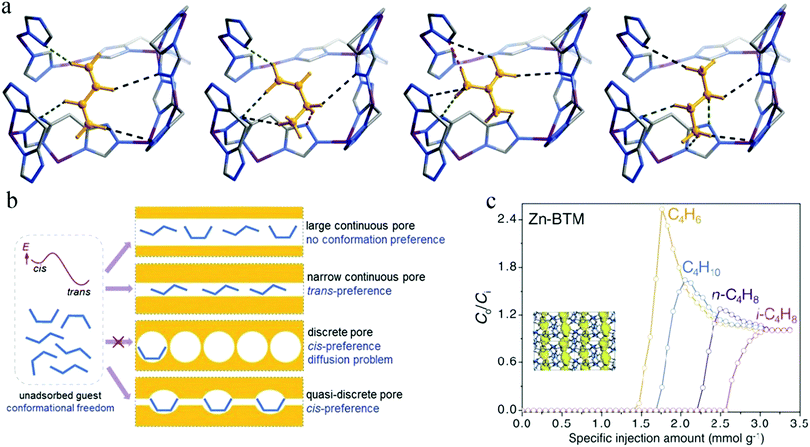
MOFs can often selectively bind C4H6 at their unsaturated metal sites. However, it is difficult to recover products from the adsorbents because heating is necessary, which induces polymerization. In 2017, Liao et al. reported a hydrophilic MOF [Zn2(btm)2], also denoted as Zn-BTM [H2btm = bis(5-methyl-1H-1,2,4-triazol-3-yl)methane], the quasi-discrete pores of which can induce conformational changes in flexible guest molecules104 (Fig. 12a). Such a conformational change would be difficult in C4H6 due to its high binding energy, thus disfavoring its adsorption and realizing the preferential adsorption of n-C4H8, i-C4H8, and C4H10 (Fig. 12b). As shown in the mixture breakthrough curves for C4 hydrocarbons, Zn-BTM afforded a distinctive elution sequence with quite different breakthrough times in the sequence C4H6 < C4H10 < n-C4H8 < i-C4H8 (Fig. 12c), which proves the preferential adsorption of the latter three. It was further demonstrated that Zn-BTM can efficiently purify C4H6 to the required level (≥99.5%).
2.4 Removal of alkyne from alkene
The separation of acetylene (C2H2) from C2H2/C2H4 and of propyne (C3H4) from C3H4/C3H6 mixtures are important but challenging industrial-scale gas separation tasks. Current commercial technologies include partial hydrogenation and solvent extraction, but these are high-cost and energy-intensive. Adsorption-based porous materials offer great promise for developing cost-effective and energy-efficient separation technologies.In 2011, Xiang et al. reported the first study on MOFs for C2H2/C2H4 separation.105 They reported two tunable microporous enantiopure mixed MOFs, M′MOF-2 (Zn3(BDC)3[Cu(SalPycy)]·(G)x) and M′MOF-3 (Zn3(CDC)3[Cu(SalPycy)]·(G)x). They found that M′MOF-2a could only slightly differentiate C2H2 from C2H4, with a low selectivity of 1.6, whereas M′MOF-3a displayed a significantly higher selectivity of 25.5. This can be attributed to the smaller micropores within M′MOF-3a, which favor its higher size-specific C2H2/C2H4 separation effect. The narrower molecular form of C2H2 (3.32 × 3.34 × 5.70 Å3) allows its free entry into the micropores in M′MOF-3a, whereas C2H4 molecules (3.28 × 4.18 × 4.84 Å3) are basically blocked or the kinetics of their uptake is much slower. Even at room temperature, the C2H2/C2H4 selectivity of M′MOF-3a is 5.2, which means it has great potential for this separation.
Many new materials have subsequently been developed for the separation of C2H2/C2H4. However, most of these materials are subject to a trade-off between C2H2 adsorption capacity and C2H2/C2H4 selectivity. Because of the narrow pore space of M′MOF-3, the adsorption capacity of acetylene is only 1.88 mol kg−1 at 295 K and 100 kPa. In contrast, although the unsaturated metal sites in Fe-MOF-74 notably enhance its C2H2 uptake capacity, the large nanopores lead to a low selectivity of 2.08.
In 2015, Hu et al. synthesized another MOF (UTSA-100) for C2H2/C2H4 separation.106 Its single-crystal X-ray structure reveals that it has a three-dimensional framework with rhombic open zigzag nanochannels with amino- and tetrazole-functionalized walls, with narrow windows of 3.3 Å and suitable cages of dimensions 4.0 Å. The C2H2/C2H4 selectivity is 10.72 because of these structural features. Moreover, the –NH2 groups in the channels can preferentially combine with C2H2, giving rise to a moderately high C2H2 uptake of 95.6 cm3 g−1 at 296 K and 1 bar.
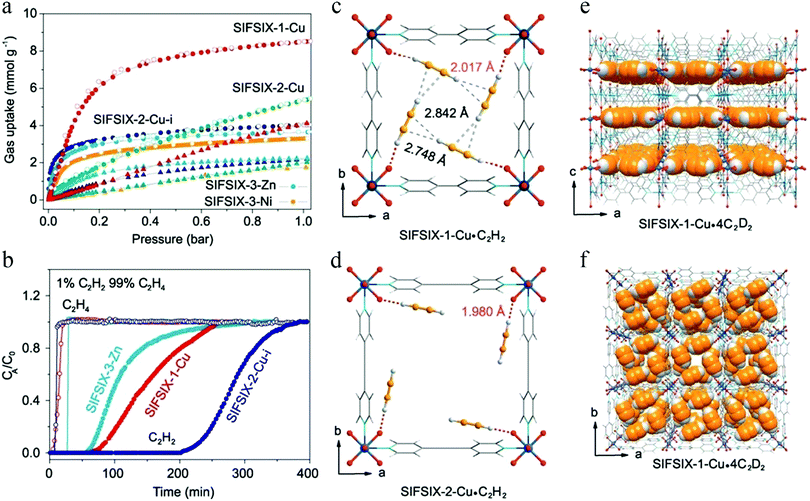
In 2016, the abovementioned trade-off in C2H2/C2H4 separation was significantly overcome in a series of SIFSIX materials reported by Cui et al.107 The channels of SIFSIX series materials are composed of the inorganic anion SiF62−, and the pore size can be adjusted by changing the length of the organic ligands. The weakly basic nature of the SiF62− sites and their geometric disposition enables strong binding with weakly acidic C2H2 molecules. Therefore, all of these SIFSIX materials display distinctly higher C2H2 uptake than C2H4 uptake (Fig. 13a). Among them, SIFSIX-2-Cu-i exhibits a remarkably high C2H2 adsorption capacity of 2.1 mmol g−1 at 298 K and 0.025 bar, with a C2H2/C2H4 selectivity as high as 39.7–44.8. DFT calculations (Fig. 13c and d) and neutron powder diffraction studies (Fig. 13e and f) predict each C2H2 molecule to be simultaneously bound by two F atoms from different nets through cooperative C–H⋯F hydrogen bonding, giving rise to strong interactions with the framework, thus leading to exceptionally high C2H2 uptake and selectivity. The amount of C2H2 captured from a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 mixture by SIFSIX-2-Cu-i was as high as 780 mmol L−1, such that this material can efficiently purify C2H4 to the desired level (>99.998%) (Fig. 13b).
99 mixture by SIFSIX-2-Cu-i was as high as 780 mmol L−1, such that this material can efficiently purify C2H4 to the desired level (>99.998%) (Fig. 13b).
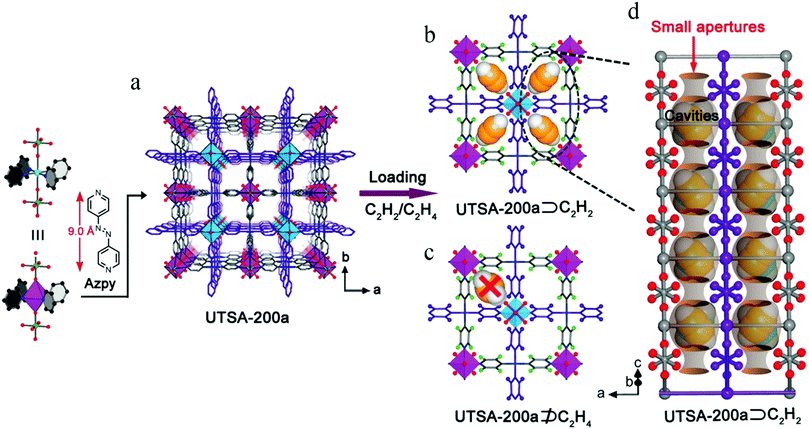
In spite of the above results, the pore size of SIFSIX-2-Cu-i is about 4.4 Å, larger than the kinetic diameter of C2H4 (4.2 Å), which can therefore not be entirely excluded. In 2018, Li et al. synthesized SIFSIX-14-Cu-i (UTSA-200) by replacing 4,4′-dipyridylacetylene (dpa, 9.6 Å) in SIFSIX-2-Cu-i with a shorter organic ligand 4,4′-azopyridine (azpy, 9.0 Å).108 The pore size of SIFSIX-14-Cu-i (UTSA-200a) decreased to 3.4 Å, between the dimensions of C2H4 (4.2 Å) and C2H2 (3.2 Å) (Fig. 14). As shown by the adsorption isotherms for C2H2 and C2H4, UTSA-200a exhibits a steep and high C2H2 uptake of 116 cm3 cm−3 at 298 K and 1 bar. In contrast, the smaller static pore size of UTSA-200a can completely prevent the entry of C2H4 molecules below 0.2 bar, leading to very little uptake (ca. 0.25 mmol g−1) at up to 0.7 bar at 298 K, which is dramatically lower than that of SIFSIX-2-Cu-i. Based on neutron powder diffraction studies, the ultra-strong C2H2 adsorption in UTSA-200 mainly originates from stronger C–D⋯F deuterium bonding (1.921 Å) compared to that in SIFSIX-2-Cu-i (2.015 Å). UTSA-200a exhibits the record selectivity of over 6000 at 298 K and 1 bar for a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 C2H2/C2H4 mixture. In breakthrough experiments, UTSA-200a demonstrated a record purification capacity for the removal of trace C2H2 from C2H4, with a C2H2 uptake capacity of 1.18 mmol g−1 from a 1
99 C2H2/C2H4 mixture. In breakthrough experiments, UTSA-200a demonstrated a record purification capacity for the removal of trace C2H2 from C2H4, with a C2H2 uptake capacity of 1.18 mmol g−1 from a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 C2H2/C2H4 mixture, thereby producing C2H4 with a purity of 99.9999%. UTSA-200a thus successfully overcomes the trade-off in C2H2/C2H4 separation and sets a new benchmark in terms of both capacity and selectivity.
99 C2H2/C2H4 mixture, thereby producing C2H4 with a purity of 99.9999%. UTSA-200a thus successfully overcomes the trade-off in C2H2/C2H4 separation and sets a new benchmark in terms of both capacity and selectivity.
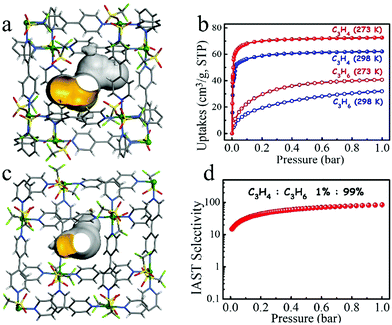
In 2017, Li et al. reported the first example of the use of a flexible, robust MOF [Cu(bpy)2(OTf)2] (ELM-12; bpy = 4,4-bipyridine, OTf− = trifluoromethanesulfonate) to remove the low concentration of C3H4 from a C3H4/C3H6 mixture (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99, v/v).109 ELM-12 consists of a rigid square-grid copper bipyridine scaffold with dynamic dangling OTf− groups. It has two kinds of cavities, type I cavities are dumbbell-shaped with small pockets (6.1 Å × 4.3 Å × 4.3 Å) at each end, which are interconnected through a small aperture (3.2 Å × 4.3 Å). Type II cavities are ellipsoid-shaped with a size of 6.8 Å × 4.0 Å × 4.2 Å and are separated from type I cavities by dynamic OTf− groups (Fig. 15a and b). The size and shape of these cavities matches well with C3H4 (6.2 Å × 3.8 Å × 3.8 Å), as opposed to C3H6 (6.5 Å × 4.0 Å × 3.8 Å), so that the adsorption capacity of the former (1.83 mmol g−1) is much higher than that of the latter (0.67 mmol g−1) at 298 K and 0.01 bar. Breakthrough experiments revealed that ELM-12 can effectively remove the trace C3H4 from a C3H4/C3H6 (1
99, v/v).109 ELM-12 consists of a rigid square-grid copper bipyridine scaffold with dynamic dangling OTf− groups. It has two kinds of cavities, type I cavities are dumbbell-shaped with small pockets (6.1 Å × 4.3 Å × 4.3 Å) at each end, which are interconnected through a small aperture (3.2 Å × 4.3 Å). Type II cavities are ellipsoid-shaped with a size of 6.8 Å × 4.0 Å × 4.2 Å and are separated from type I cavities by dynamic OTf− groups (Fig. 15a and b). The size and shape of these cavities matches well with C3H4 (6.2 Å × 3.8 Å × 3.8 Å), as opposed to C3H6 (6.5 Å × 4.0 Å × 3.8 Å), so that the adsorption capacity of the former (1.83 mmol g−1) is much higher than that of the latter (0.67 mmol g−1) at 298 K and 0.01 bar. Breakthrough experiments revealed that ELM-12 can effectively remove the trace C3H4 from a C3H4/C3H6 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99) mixture under ambient conditions to produce C3H6 with a purity of 99.9998% (Fig. 15c and d). As expected, ELM-12 exhibits a very high C3H4/C3H6 selectivity of up to 84 for such a C3H4/C3H6 (1
99) mixture under ambient conditions to produce C3H6 with a purity of 99.9998% (Fig. 15c and d). As expected, ELM-12 exhibits a very high C3H4/C3H6 selectivity of up to 84 for such a C3H4/C3H6 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99) both of which C3D4 molecules interact with OTf− groups through C–D⋯O deuterium bonds. Because of its strong binding affinity to C3H4 and molecular sieving mechanism, ELM-12 offers excellent performance for the separation of C3H4/C3H6.
99) both of which C3D4 molecules interact with OTf− groups through C–D⋯O deuterium bonds. Because of its strong binding affinity to C3H4 and molecular sieving mechanism, ELM-12 offers excellent performance for the separation of C3H4/C3H6.
In order to increase the binding affinity of C3H4, so as to produce C3H6 of higher purity, in 2018, Yang et al. investigated a series of hybrid ultramicroporous materials with pillared inorganic anions, including SIFSIX-1-Cu, SIFSIX-2-Cu-i, and SIFSIX-3-Ni,110 for C3H4 removal. All three of these SIFSIX materials displayed steep C3H4 uptakes in the low-pressure region, and larger adsorption amounts of C3H4 than of C3H6 at 1 bar. In particular, SIFSIX-3-Ni shows a near-saturation C3H4 uptake of 2.65 mmol g−1 at an extremely low pressure (0.003 bar), with a C3H4/C3H6 selectivity of over 250. PXRD experimental results showed that one unit cell of SIFSIX-3-Ni traps one C3H4 molecule, and that all of the hydrogen atoms of C3H4 are engaged in strong interactions with SiF62− anions through multiple hydrogen-bonding or dipole–dipole interactions (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Hδ+⋯Fδ−, C–Hδ+⋯Fδ−). Breakthrough experiments further confirmed that SIFSIX-3-Ni can remove trace C3H4 from C3H6 with a purification capacity of 1187 mL g−1 at an ultralow concentration of 1000 ppm.
Hδ+⋯Fδ−, C–Hδ+⋯Fδ−). Breakthrough experiments further confirmed that SIFSIX-3-Ni can remove trace C3H4 from C3H6 with a purification capacity of 1187 mL g−1 at an ultralow concentration of 1000 ppm.
In 2018, Yang et al. further reported the one-step purification of C3H6 from multicomponent gases (propyne and propadiene) using a single material NbOFFIVE-2-Cu-i (also termed as ZU-62, NbOFFIVE = NbOF52−, i = interpenetrated).111 The adoption of the asymmetric anion pillar NbOF52− as well as the interpenetrated structure successfully customized the separate energy favorable binding sites/space for the propyne and propadiene molecules within ZU-62 so that it can simultaneously adsorb them. ZU-62 features asymmetric O/F node coordination, which leads to the formation of three types of nanopores bearing aperture sizes of 6.75 Å (Site I), 6.94 Å (Site II), 7.20 Å (Site III), respectively. First-principles DFT-D calculated results show that propyne will be preferentially adsorbed on site III, while propadiene is more inclined to site I, leading to the introduced propyne and propadiene are able to target the corresponding suitable residence without mutual interference, revealing an interesting “classified storage” phenomenon. The propylene uptake at 298 K under 5000 ppm was only 0.05 mmol g−1, almost negligible and the selectivity via the IAST calculation of the binary gases, propyne/propylene and propadiene/propylene (0.5/99.5) was up to 48 and 34. This work provides some clues for the one-step purification from multicomponent mixtures.
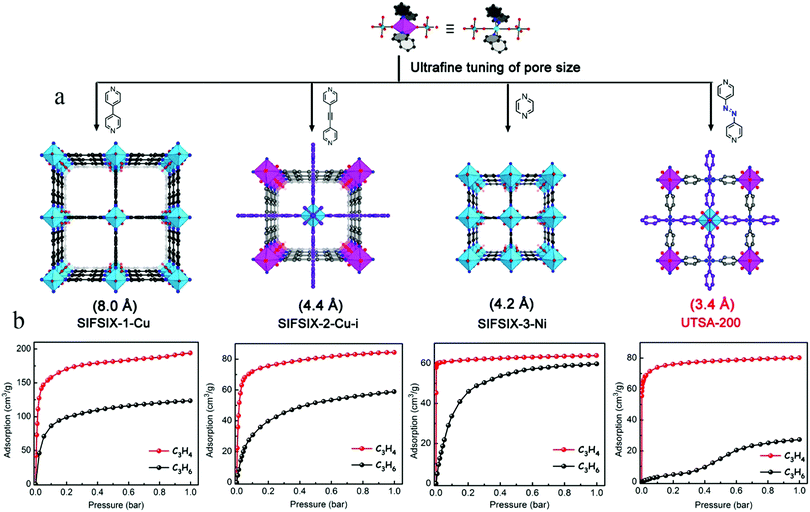
Although the three above-described materials exhibit high C3H4 adsorption capacities, they only exhibit moderately high gas-separation performances because of their comparatively large pores capable of including both C3H4 and C3H6 molecules, thus limiting the throughput of the desired C3H6 product. In 2018, Li et al. conducted a comprehensive screening of a series of MOFs with a broad range of structures, pore sizes, and functionalities (Fig. 16a). Among them, the ultramicroporous UTSA-200 ([Cu(azpy)2(SiF6)]n; azpy = 4,4′-azopyridine) was identified as the best-performing material to remove trace amounts of C3H4 from C3H4/C3H6 mixtures.112 The static pore size of UTSA-200 is about 3.4 Å, which is much smaller than the dimensions of C3H4 (4.2 Å) and C3H6 (4.6 Å). A first-principles dispersion-corrected DFT study of UTSA-200 indicated that, after the adsorption of C3H4 molecules, the N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond and pyridine rings of azpy exhibit obvious rotation and distortion, which enlarges the pores to about 4.2 Å, thereby allowing the passage of a C3H4 molecule, but blocking C3H6 in the low-pressure region (<0.2 bar). In addition, each adsorbed C3H4 molecule is bound by two SiF62− sites from different nets through cooperative C–H⋯F and C
N bond and pyridine rings of azpy exhibit obvious rotation and distortion, which enlarges the pores to about 4.2 Å, thereby allowing the passage of a C3H4 molecule, but blocking C3H6 in the low-pressure region (<0.2 bar). In addition, each adsorbed C3H4 molecule is bound by two SiF62− sites from different nets through cooperative C–H⋯F and C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) H⋯F hydrogen bonding. UTSA-200 shows unprecedentedly high C3H4 adsorption capacity (95 cm3 cm−3 at 0.01 bar and 298 K) and C3H4/C3H6 selectivity (over 20
H⋯F hydrogen bonding. UTSA-200 shows unprecedentedly high C3H4 adsorption capacity (95 cm3 cm−3 at 0.01 bar and 298 K) and C3H4/C3H6 selectivity (over 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) (Fig. 16b). Breakthrough experiments showed that UTSA-200 can remove trace C3H4 from mixtures of compositions 1
000) (Fig. 16b). Breakthrough experiments showed that UTSA-200 can remove trace C3H4 from mixtures of compositions 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 and 0.1
99 and 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99.9, producing C3H6 with a purity of 99.9999%, in yields of 62.0 and 142.8 mmol g−1, respectively.
99.9, producing C3H6 with a purity of 99.9999%, in yields of 62.0 and 142.8 mmol g−1, respectively.
3 Critical factors for industrial applications
A wide range of promising porous MOF materials have been reported to date, and their potential use in the separation of light olefins was reviewed in the previous subsections. However, before they can be used in real industrial applications, there are some critical factors that need to be addressed. Fortunately, in recent years, more academic research groups and commercial companies have stepped up their efforts towards producing MOFs that meet the requirements, even though this can be a challenging task.3.1 Stability
MOFs are connected by organic ligands and inorganic metal ions, and hence show low stability compared with traditional adsorbents (zeolites, activated carbon, or silica). This has been recognized as a major barrier that limits their practical applications.In 2011, Yang et al. found that the water stability of MOFs can be improved by methyl modification.113 The authors used 2-methylterephthalic acid and 2,5-dimethylterephthalic acid to synthesize CH3MOF-5 and DiCH3MOF-5, respectively. PXRD experiments showed that the structures of these two materials did not collapse after they were exposed to ambient air with humidity 32–37% for 4 days (the PXRD pattern of pure MOF-5 is lost after exposure to air for 45 min). These results strongly imply that introducing hydrophobic methyl groups enhances the structural stability of the MOF-5-type framework. For the adsorption of H2 (1 bar, 77 K), CH3MOF-5 (1.42 wt%) was basically as effective as MOF-5 (1.44 wt%), whereas DiCH3MOF-5 (1.29 wt%) showed only a slightly lower uptake. After exposure to air for 4 days, the adsorption capacity of DiCH3MOF-5 remained unchanged, whereas that of CH3MOF-5 decreased due to partial decomposition. This work provides a good strategy for enhancing the water stability of MOF materials, but the introduction of a large number of methyl groups into the pores will inevitably lead to a reduction in porosity.
In 2012, Decoste et al. introduced a plasma-enhanced chemical vapor deposition (PECVD) method to improve the water stability of MOFs.114 Depositing perfluorohexane (PFH) on the surface of Cu-BTC by PECVD was found to successfully prevent the formation of water clusters and hydrolysis. The PXRD results confirmed that the structure of Cu-BTC-plasma showed no obvious changes compared with that of Cu-BTC (Fig. 17a). After immersion in water at room temperature for 24 h, the structure of Cu-BTC-plasma remained intact, demonstrating its excellent water stability. It has been found that water molecules first coordinate at copper atoms of site I in Cu-BTC. Secondary binding of water occurs at sites I′ (the large cage interior), which occurs through hydrogen-bonding with water molecules adsorbed at sites I (Fig. 17b). According to GCMC and thermogravimetric analyses, PFH preferentially adsorbs at sites I′. The binding of PFH away from site I allows water molecules to still coordinate to the Cu atoms in Cu-BTC, but the hydrophobic nature of PFH in the pores prevents the formation of water clusters at higher humidity levels. This work provides a new way of enhancing the water stability of MOFs. However, the method is rather complex, only suitable for specific MOFs, and the porosity and specific surface area are decreased by about 30% after modification.
Around the same time, Yang et al. proposed a new method for improving the water stability of MOFs without using additional solvents or chemicals.115 They placed IRMOF-1 in a tube furnace, and carbonized it at a target temperature under nitrogen so as to transform the organic ligands on its surface into an amorphous carbon coating. Through precisely controlling the temperature and gas flow, they basically maintained the crystal structure and pore size distribution of the material. Due to the hydrophobicity of carbon, this method effectively avoids direct exposure of the MOF to moisture, thereby preventing hydrolysis. PXRD analysis showed that the structure of the carbon coating on IRMOF-1 remained intact after being exposed to air of 34% humidity for 14 days. After heat treatment at 510 °C, the crystal structure of the material was still maintained after soaking in water for 2 h. This work demonstrated that the amorphous carbon surface formed during heat treatment hindered hydrolysis of the MOF, even upon submersion in water.
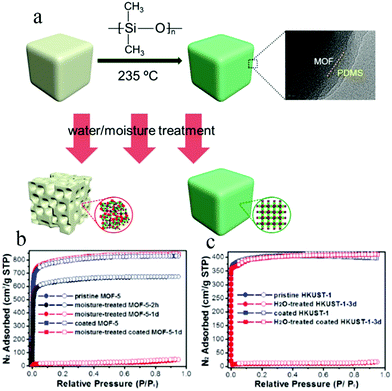
To develop a more facile method, in 2014 Zhang et al. also used a vapor deposition technique to apply hydrophobic polydimethylsiloxane (PDMS) on the surface of MOF materials116 (Fig. 18a). MOF-5, HKUST-1, and ZnBT were successfully coated with PDMS and their stabilities were tested. MOF-5 and coated MOF-5 were placed in air of 55% humidity for 2 days, whereupon the coated MOF-5 maintained the same morphology and smooth surface before and after the treatment, and its structure and porosity were largely preserved, as verified by powder XRD patterns and N2 adsorption isotherms (Fig. 18b and c). In the case of coated HKUST-1, its BET surface area was unchanged after soaking in water for three months. This method is simple and easy to operate, and the MOFs coated with PDMS not only show superhydrophobicity and excellent water stability, but also retain their inherent porosities and high specific surface areas.
Optical sensing and adsorption-based methods are considered as very promising separation methods for the removal of organic pollutants from wastewater. However, a precondition of applying MOFs for the detection and removal of pollutants is water stability of their frameworks. To synthesize MOFs with highly stable structures, in 2016, Wang et al. reported the use of zirconium ions for the preparation of a variety of MOF materials for the purification of wastewater, including BUT-12 [Zr6O4(OH)8(H2O)4(CTTA)8/3; H3CTTA = 5′-(4-carboxyphenyl)-2′,4′,6′-trimethyl-[1,1′:3′,1′′-terphenyl]-4,4′′-dicarboxylic acid] and BUT-13 [Zr6O4(OH)8(H2O)4(TTNA)8/3; H3TTNA = 6,6′,6′′-(2,4,6-trimethylbenzene-1,3,5-triyl)tris(2-naphthoic acid)].117 In BUT-12, the carboxylate ligand CTTA3− is functionalized with three methyl groups. These three methyl groups increase the hydrophobicity of the framework, so that the material exhibits excellent water stability. The material was soaked in water, HCl solutions, and NaOH solution (pH = 10) both at room temperature and boiling temperature. After 24 h, the PXRD patterns showed that the crystallinity and structure of the material had not changed, which confirmed its excellent stability.
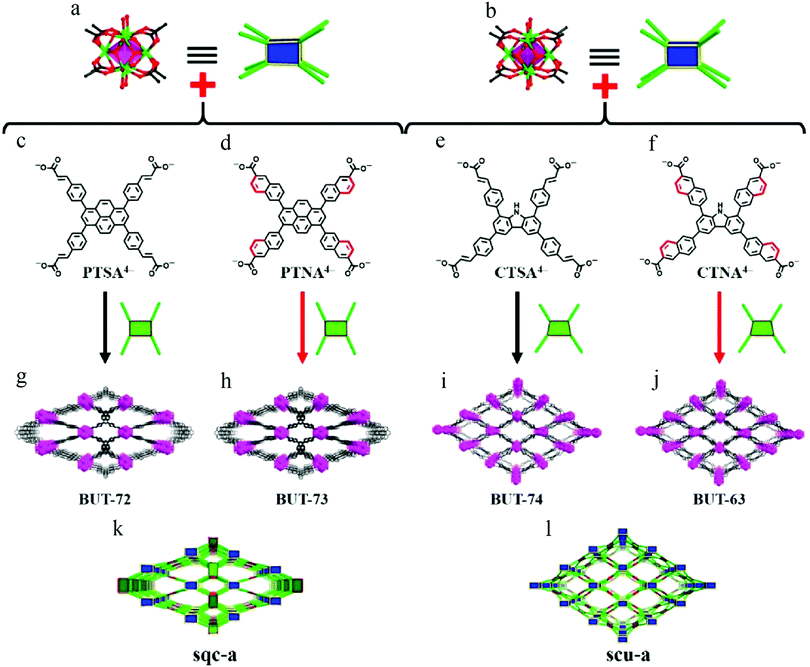
To further enhance the coordination bond between the metal and the ligand, modification of the configuration of the ligand would seem to be a good choice. In 2019, Wang et al. demonstrated that fixing the flexible arms of core-shared ligands enhanced the stability of their Zr(IV)-MOFs118 by blocking the rotation of ligand rotamers. Four kinds of MOFs were synthesized (Fig. 19), two of which had the same pyrene core (BUT-72, BUT-73), while the other two had the same carbazole core (BUT-74, BUT-63). The difference between each pair lies in whether the side arm is flexible styrene or rigid naphthalene. After soaking in HCl, NaOH, or water for 24 h, the PXRD peaks of all treated BUT-72 and BUT-74 samples had disappeared, indicating collapse of the framework, but the structures of BUT-73 and BUT-63 were still intact after the same treatment. This was because the destruction of MOFs is usually induced by disrupting the coordination moieties of ligands, and rigid ligands usually require a higher activation energy, such that the original configuration of ligands with rigid arms is easier to maintain.
In 2008, Cavka et al. reported the rigid MOF UiO-66, which contains highly stable secondary structural units.47 It is composed of six Zr(I) ions forming an octahedral cage structure, and each Zr(I) ion is connected with 12 H2BDC ligands, which is the highest coordination number reported for an MOF. In addition, Zr has a high oxygen affinity, and thus forms strong Zr–O bonds. The high affinity of Zr for O and its compact structure endow UiO-66 with outstanding thermal stability. Its crystal structure is stable at 500 °C, and its framework can withstand a mechanical pressure of 1 MPa. Its powder XRD pattern remained virtually unaltered after soaking samples in solvents such as water, DMF, benzene, and acetone for 24 h.
The concept of MIXMOF provides us with an effective method for adjusting the structure and characteristics of pores. In 2010, Marx et al. applied this strategy in the synthesis of MIL-53(Al).120 They used benzene-1,4-dicarboxylate (BDC) and 2-aminobenzene-1,4-dicarboxylate (ABDC) to synthesize a series of MOFs of the general formula Al(OH)(BDC)1−x(ABDC)x (x = 0.1, 0.5, 0.9) with mixed ligands, whereby the content of amine groups was controlled by adjusting the ratio of the two ligands. PXRD patterns proved that both ligands were incorporated into the framework. The thermal stability of the material was tested by thermogravimetry and differential thermogravimetry. The temperature of maximum decomposition rate was shifted from 593 °C for the MIXMOF with 10% ABDC to 537 °C for that with 50% ABDC and 494 °C for that with 90% ABDC. The results show that adjusting the ratio of the two ligands can significantly change the thermal stability of the MIXMOF.
In 2011, Colombo et al. first proposed that the pKa value of a coordinating atom on a ligand can be regarded as an approximate indicator of the bond strength between a metal and the ligand.121 This is because the assembly of MOFs is controlled by Lewis acid–base theory. From this perspective, these authors synthesized a series of MOFs with high stability by searching for ligands with high pKa values, as exemplified by pyrazolate [1,3,5-tris(1H-pyrazol-4-yl)benzene] (H3BTP), with pKa 19.8. Results showed M3(BTP)2 systems (M = Co, Ni, Cu, Zn) to have high thermal and chemical stability, especially Ni3(BTP)2, which maintained its structure whether heated to 410 °C in air or soaked with boiling aqueous solutions of pH 2–14 for two weeks. Thus, this stability parallels, or even surpasses, that of zeolites.
In 2011, Kang et al.122 carried out an extensive investigation of the stability differences of isotypic MOFs with different central metal ions. They selected an M-BDC (M = Al, Cr, V) series of MOFs and tested their stabilities. Firstly, the three respective materials were placed in a basic solution (7.0 × 10−2 M NaOH) at room temperature. It was found that the XRD peaks and BET surface area of Al-BDC and V-BDC decreased sharply with the contact time. However, Cr-BDC proved to be very stable in the basic solution and showed no change in BET surface area or XRD pattern. Similar results were observed in acidic solution (7.0 × 10−2 M HCl) and deionized water (pH 6.7). Therefore, the chemical and moisture stabilities of these three materials follow the order Cr-BDC > Al-BDC > V-BDC. The results show that the chemical and moisture stabilities of the materials are related to the inertness or lability of the metal ions. In other words, the chemical stability of isotypic MOFs increases with increasing inertness (or decreasing lability) of the central metal ions. The thermal stabilities of these MOFs were also tested by TGA, and follow the order Al-BDC > Cr-BDC > V-BDC, mirroring the strengths of the metal–oxygen bonds in the common oxides (Al2O3, 514 kJ mol−1, Cr2O3, 447 kJ mol−1, and V2O5, 383 kJ mol−1). Finally, we note that the stability of a purified MOF may be lower than that of an MOF filled with an uncoordinated linker.
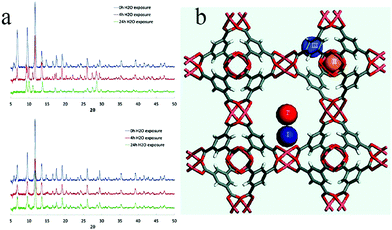
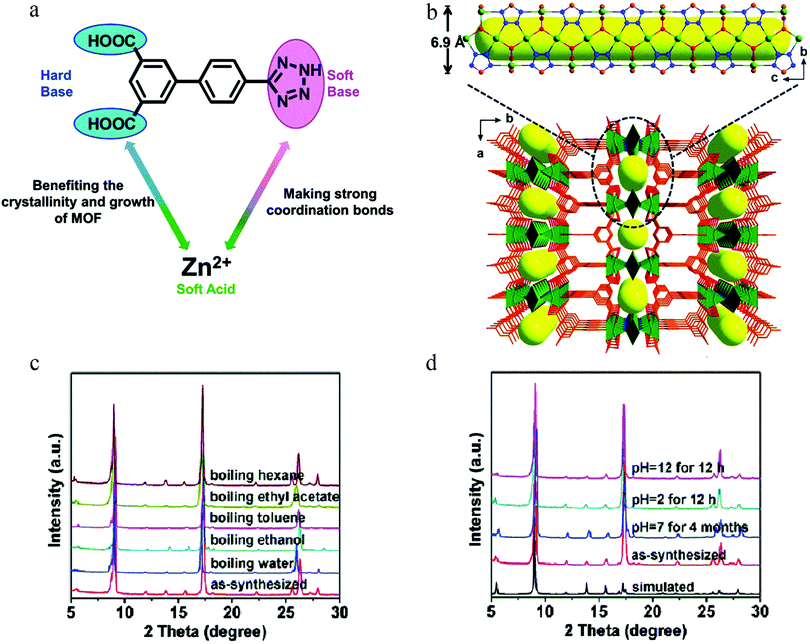
According to the hard–soft-acid–base theory (HSAB), soft-acid metal cations (such as Zn(II) and Cu(II) ions) can form a strong coordination bond with soft-base N donor ligands, which is conducive to enhancing the stability of an MOF, whereas weak bonding with a hard-base (acid ligand) is conducive to improving the crystallinity of a material so as to facilitate structural analysis. Based on this, in 2016, Hu et al. used 4′-(1H-tetrazol-5-yl)-[1,1′-biphenyl]-3,5-dicarboxylic acid (H3TZBPDC), which contains both soft-base (nitrogen) and hard-base (carboxylic acid) coordination sites, to synthesize the ultrastable Zn(II)–organic framework USTC-7 with soft acid (Zn(II) ions)123 (Fig. 20a and b). Stability tests showed that USTC-7 could not only retain its crystallinity in diverse boiling solvents (ethanol, ethyl acetate, toluene, hexane) for 12 h, but also in aqueous solutions at pH 2–12 (Fig. 20c and d).
3.2 Balance of diffusion rate and selectivity
Generally, the trade-off between physical adsorption selectivity and diffusion dynamics of porous materials is a major barrier for efficient gas separation and purification through physisorption. Some ultramicropore MOFs exhibits very high adsorption selectivity for the certain gas molecule with its tailor-made pore cavity, but its separation performance usually restricted by the low diffusion rate. While, the construction of hierarchically-porous MOFs (HP-MOF) can solve this problem ideally. On the other hand, if the MOF materials have large pore size, it may not be suitable for effectively recognize for some small molecules, because a large pore space usually leads to low adsorption selectivity. For small-molecule gases, separation performance can be improved by adjusting the density of host–guest interactions and adsorption sites in the small pore space. In this context, the concept of pore space partition (PSP) has been introduced.44,124–128In 2015, after considering the disadvantages of the traditional method for preparing large porous MOFs such as expensive ligands and difficulty in accurate control, Huang et al. proposed a new strategy for preparation of stable hierarchical-pore MOFs by in situ self-assembled template method with low cost, simple and general use (Fig. 21).130 Different from the traditional hard template and soft template methods, acid-sensitive metal–organic assemblies (MOAs) were used as a template to synthesize stable HP-MOF. The MOAs not only have a good interaction and affinity with the reaction precursor, but also can be easily removed, so as to maintain the structural integrity of the target porous materials. In this study, 19 kinds of stable HP-MOFs were successfully prepared, and the mesoporous size could be controlled by the dosage of template, which provided a solid foundation for adsorption/separation of large molecules, heterogeneous catalysis and drug release on MOFs.
In 2018, Feng et al. reported a common synthesis strategy called linker thermolysis for constructing ultra-stable hierarchical-pore MOFs using thermal-sensitive linkers that are easily cracked.131 After thermal decomposition, the linkers of the multivariate MOF crystal breaks to generate a crystal defect, and the mesoporous are produced while the high crystallinity and stability of the original MOF are still retained. Its pore size distribution can be precisely controlled by adjusting the proportion of thermal-sensitive ligands, heating time and heating temperature. The synthetic ZrO2@HP-UiO-66 (UiO-66-NH2-26%–350 °C–2 h) by linker thermolysis in this work not only possesses 5.5 nm mesoporous, but also promotes the formation of ultrasmall metal oxide nanoparticles and exposure of more functional sites, showing high catalytic activity in Lewis acid-catalysed reactions.
In 2019, Ye et al. successfully modified FJU-88 with a triangular ligand [Tripp = 2,4,6-tris(4-pyridyl)pyridine] by the PSP method to realize a novel porous MOF (FJU-90) with dual functionalities for the challenging C2H2/CO2 separation.133 The pore dimensions of FJU-90 decreased from 12.0 × 9.4 Å2 to 5.4 × 5.1 Å2, which greatly improved the separation performance for C2H2/CO2. The optimized pore size and the hydrogen bond between C2H2 and an O atom on the pore surface raise the adsorption capacity of C2H2 to 180 cm3 g−1, while that of CO2 is reduced to 103 cm3 g−1 at 298 K and 1 bar. Although the IAST selectivity (4.3) of FJU-90 is lower than that of UTSA-74a (8.2) and those of other excellent materials, due to the combination of its high absorption capacity and moderate selectivity for C2H2/CO2, FJU-90 exhibits the highest gravimetric productivity with a value of 5.10 mol kg−1, which is much higher than that for UTSA-74a. It is worth noting that the stability of FJU-90 is much higher than that of FJU-88, which can be ascribed to the introduction of the Tripp ligand. This work represents an outstanding example of application of the PSP strategy for rationally designing microporous MOF materials for challenging gas separations.
3.3 Scale-up synthesis
Central to the translation of these new materials into industrial separation processes is the ability to manufacture MOFs at the required scale, purity, and price for implementation. Depending on the final purpose, diverse MOFs may be prepared by employing several distinct synthetic methodologies. Processes that are faster, with lower energy consumption, are clearly more attractive for industrial purposes.134–137 Often, the feasibility of preparing the desired materials on a large scale, a difficult but important task, is the factor that determines the method that is ultimately selected.In 2005, BASF Aktiengesellschaft first synthesized HKUST-1 by an electrochemical approach.138 The method involved immersion of a copper plate in a solution containing the organic linker 1,3,5-benzenetricarboxylic acid (BTC) and an electrolyte. When a certain potential was applied, the copper electrode released Cu(II) ions into the solution, which could react with the dissolved linker. During a period of 150 min at a potential of 12–19 V, the current was 1.3 A, and a greenish-blue precipitate was formed. This product had a specific surface area of 1820 m2 g−1, which was higher than that of the corresponding material synthesized by a solvothermal method.
In 2016, Campagnol et al. studied the mechanism of HKUST-1 synthesis by anodic deposition.139 They divided the electrochemical synthesis of this MOF into four stages: (I) initial nucleation; (II) growth of HKUST-1 islands; (III) intergrowth; and (IV) crystal detachment. In the initial stage, the metal substrate starts dissolving anodically in the solution containing the linker, and the first crystals nucleate on the electrode surface. After the first nuclei are formed, new crystals tend to nucleate next to them, forming islands of intergrown MOF. At the same time, those already nucleated grow to dimensions of several microns, eventually forming a compact layer. In the last stage, the crystals detach from the surface and expose empty spots of bare metal.
To date, MOFs have mainly been synthesized under hydrothermal/solvothermal conditions. This method requires long reaction times and incurs heavy energy consumption. In recent years, microwave irradiation has been used to synthesize various inorganic nanomaterials, such as zeolites and MOFs. The method is based on the interaction of electromagnetic waves with any material containing mobile electric charges, which can greatly shorten the reaction time and improve the product yields.
In 2005, Jhung et al. carried out pioneering work on the microwave-assisted synthesis of MOFs. A water-based synthesis of MIL-100 in the presence of hydrofluoric acid was reported.140 Materials were synthesized in a microwave oven at 220 °C for 1, 2, or 4 h, respectively, in a sealed Teflon autoclave. The results showed that unreacted chromium was consumed in about 2 h. After 4 h, the obtained yield of the crystals was 44%, akin to the 45% achieved by conventional synthesis after 4 days. Thus, it can be seen that the microwave-assisted synthesis is about 20 times faster than the conventional synthesis.
In 2015, in a study by Bag et al., nine isostructural microporous lanthanide MOFs were synthesized on a large scale by microwave-assisted solvothermal reaction within 5 min.141 At the same temperature, conventional solvothermal reactions required a longer reaction time (2 days) and slow evaporation (5 days) to produce these compounds in similar yields. Moreover, only 10 mg of high-quality material could be obtained by the solvothermal method, whereas up to 2 g of the MOF could be produced by the microwave-assisted synthesis. This work not only demonstrated a great reduction in reaction time, but also a successful large-scale laboratory synthesis. It highlights that microwave-assisted synthesis is of great significance in the production of MOFs.
For most chemical syntheses, reactions are carried out in solution, whereas mechanochemical synthesis relies on mechanical forces and essentially does not require solvents or only a small amount of solvent. It has the advantages of low energy consumption, fast throughput, and the ability to quantitatively synthesize the targeted materials. In 2015, Crawford et al. proposed the use of twin-screw extrusion (TSE) and single-screw extrusion (SSE) for the continuous synthesis of various metal complexes, and to provide high yields of high purity products at a specific temperature and shear force without a solvent or with only a small amount of solvent142 (Fig. 23). Using this method, the commercially important MOFs HKUST-1, ZIF-8, and MAF-4 were successfully prepared. These authors synthesized Cu3(BTC)2 from Cu(II) hydroxide and benzene-1,3,5-tricarboxylic acid at the appropriate 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio by solvent-assisted TSE employing screw speeds in the range 55–250 rpm. The space time yield was 144
2 molar ratio by solvent-assisted TSE employing screw speeds in the range 55–250 rpm. The space time yield was 144![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 kg per m3 per day. The high yield can be attributed to the near absence of solvent in the synthetic process, which usually occupies most of the reactor volume, together with the high reaction rates possible between such highly concentrated (or even neat) reactants under these conditions.
000 kg per m3 per day. The high yield can be attributed to the near absence of solvent in the synthetic process, which usually occupies most of the reactor volume, together with the high reaction rates possible between such highly concentrated (or even neat) reactants under these conditions.
 | ||
| Fig. 23 Twin-screw extruder with key parts highlighted. The two screws which convey and knead the reactants are housed in the barrel. | ||
3.4 Shaping
For industrial separation processes, adsorbents often need to be shaped or packed into given morphologies, such as granules,143–146 pellets,143,147,148 thin films,149–153 and so on,154–157 so as to reduce the pressure drop across the adsorbent bed as well as to provide gas diffusion channels and to enhance the mechanical stability. However, mechanical issues (e.g., stability and phase integrity) related to the use of MOFs remain largely unexplored in the literature. Typically, these compounds are obtained as loose powders (composed of small crystallites) with low packing densities. For this reason, MOFs should be shaped into suitable geometries before loading into the adsorption bed, in order to resist attrition and avoid disintegration into a powder, which could contaminate tanks, pipes, or other apparatus.Fortunately, both companies (mainly BASF) and some academic research groups have already realized this high industrial importance, and hundreds of patents relating to the industrial properties of MOFs have been submitted to the European Patent Office.
In 2016, Chen et al. used a continuous-phase transformation processing strategy to convert MOFs into processable fluids, shaped bodies, and even MOF foams that could be reversibly interconverted between these states158 (Fig. 24). They introduced HKUST-1@Fe3O4 core–shell nanoparticles into carboxymethylcellulose solution to obtain a high particle content (25.0–45.4 wt%) magnetic fluid (HKUST-1@Fe3O4-MF). A cup reactor was manufactured by a solvent-induced hardening process. The excellent catalytic ability (conversion 76% and selectivity 93%) was also verified by catalytic tests. In order to further improve the catalytic efficiency and develop a more practical catalyst form for industrial application, a light yet robust foam with hierarchical porosity was obtained by freeze-drying of HKUST-1@Fe3O4-MF. Based on the adsorption isotherm of N2 at 77 K, the surface area of the foam was 196 m2 g−1, and its pore volume was 0.65 m3 g−1. Robustness is one of the key prerequisites for practical application. Weight pressing, tape tests, and collision experiments proved that the foam had extraordinary mechanical stability. The loading capacity of this MOF could even reach 80 wt% without reducing its mechanical stability. Moreover, this MOF foam presented high permeability with a low pressure drop (<20 Pa, 500 mL min−1) because of its hierarchical porous structure and uniform distribution, making it potentially useful as a membrane reactor for various important catalytic and separation processes.
The bottleneck that prevents natural gas from becoming a transportation fuel is the development of materials that can store it in a sufficiently compact form at ambient temperature. In 2017, Tian et al. used a sol–gel method to synthesize a porous monolithic MOF without using additional binders or high pressure.159 The capacity of their product was 259 cm3 cm−3 after successful packing and densification at 65 bar. This is the highest value for methane storage in a porous solid reported to date, representing an increase of 50% on any previously reported experimental value. The article also pointed out that the drying conditions of the MOF play a key role in its shaping (Fig. 25a). Only a dense monolith was obtained when the dense gel was dried under mild conditions, whereas a powder was obtained when the dense gel was dried at high temperature. Nanoindentation tests on the monolithic MOF (Fig. 25b) showed robust mechanical properties, and its hardness was found to be at least 130% greater than those of previously studied conventional MOF counterparts. This discovery represents a substantial step forward in the use of MOFs in high-capacity energy storage and other industrial applications.
At present, the most common and simple shaping method is pelletization and extrusion. However, this method always causes a significant reduction in the surface area of MOFs. An ideal shaping strategy would enhance the stability of an MOF without significantly changing its internal structure. Another common technique is to add a binder to an MOF. In 2018, Mallick et al. successfully improved the mechanical properties of an MOF after shaping it using a polymer as a binder, and minimized the impact of the shaping process on the structure and adsorption performance of the MOF.160 They selected two distinct polymers, namely a rubbery polymer (polyethylene glycol; PEG) and a glassy polymer (poly(methyl methacrylate); PMMA), as binders for NbOFFIVE-1-Ni shaping, and compared the shaping effects. Through some simple processing, the MOF powder was shaped into cubic beads with a side length of about 1 cm. The amount of binder could be adjusted according to the target hardness of the MOF. Various tests showed that PMMA as a binder performs better than PEG in terms of material hardness, surface area, and adsorption performance. The hardness and adsorption performance of NbOFFIVE-1-Ni beads with 10% PMMA proved to be the best. In addition, this shaping strategy has been successfully applied to ZIF-8, UiO-66, and HKUST-1, whereby the reduction of surface area was less than 15%, and the mechanical strength was greatly improved. It can be concluded that the PMMA binder technique offers great promise for the requisite shaping on a large industrial scale.
4 Summary and prospects
Light olefins are fundamental chemical industry feedstocks. As they are obtained through traditional steam cracking or thermal decomposition of paraffins, they are obtained as mixtures, but must be separated or highly purified for further use. Due to their very similar physical properties, their separation processes are difficult, as can be seen from the various rectification columns in petrochemical industry factories all around the world. Indeed, 10–15% of global energy production has hitherto been used for the separation and purification of such industrial commodities through cryogenic distillation. Adsorptive separation based on porous materials is a promising technology to lower energy expenditure. Compared to conventional porous materials (activated carbon and zeolites), MOFs represent a unique class of materials, with exceptional porosity and diverse functionalities, which allow the establishment of different relationships between such structures and their final properties, thus showing great promise in addressing the important issue of the separation of light olefins. In this progress report, we have summarized recent advances in exploiting MOF materials for the efficient separation and purification of several typical light-olefin mixtures. It is noted that, although extensive research has been carried out, the development of MOFs as a class of new absorbing materials for the separation and purification of light olefins is still at an early stage, and some underdeveloped issues still need to be considered and addressed.Fortunately, and as clearly shown in the present review, much progress has been made, and several visionary companies have already brought some MOFs from the laboratory to the real commodity market in recent years. Although we are still not sure as to whether MOFs can replace all of today's well-known industrial compounds (such as zeolites, silicas, or active carbons), we are rather close to answering this question. It is time for researchers engaged in MOF studies to prove that MOF materials are vital to our modern lifestyle and, therefore, can establish their technological and industrial importance. This shift in direction has already started, with a large number of patents having been filed to date. Future challenges concern the preparation of MOFs as well as MOF-based devices in a simple, easy, green, and low-cost way, and in large amounts capable of meeting the relevant demands.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 21922810, 21908153, 21908155 and 21878205), Coal Bed Methane Joint Foundation of Shanxi (2016012006), and program of Innovative Talents of Higher Education Institutions of Shanxi.Notes and references
- S. Chu, Y. Cui and N. Liu, The path towards sustainable energy, Nat. Mater., 2016, 16, 16–22 CrossRef PubMed
.
- D. S. Sholl and R. P. Lively, Seven chemical separations to change the world, Nature, 2016, 532, 435–437 CrossRef PubMed
.
- T. Ren, M. Patel and K. Blok, Olefins from conventional and heavy feedstocks: Energy use in steam cracking and alternative processes, Energy, 2006, 31, 425–451 CrossRef CAS
.
- M. Rungta, L. Xu and W. J. Koros, Carbon molecular sieve dense film membranes derived from Matrimid® for ethylene/ethane separation, Carbon, 2012, 50, 1488–1502 CrossRef CAS
.
- P. J. Bereciartua, Á. Cantín, A. Corma, J. L. Jordá, M. Palomino, F. Rey, S. Valencia, E. W. Corcoran Jr., P. Kortunov and P. I. Ravikovitch,
et al., Control of zeolite framework flexibility and pore topology for separation of ethane and ethylene, Science, 2017, 358, 1068–1071 CrossRef CAS PubMed
.
- L. Li, R. B. Lin, R. Krishna, H. Li, S. Xiang, H. Wu, J. Li, W. Zhou and B. Chen, Ethane/ethylene separation in a metal–organic framework with iron-peroxo sites, Science, 2018, 362, 443–446 CrossRef CAS PubMed
.
- S. Kitagawa, R. Kitaura and S.-i. Noro, Functional Porous Coordination Polymers, Angew. Chem., Int. Ed., 2004, 43, 2334–2375 CrossRef CAS PubMed
.
- H. Furukawa, K. E. Cordova, M. O'Keeffe and O. M. Yaghi, The Chemistry and Applications of Metal–Organic Frameworks, Science, 2013, 341, 1230444 CrossRef PubMed
.
- H. C. Zhou, J. R. Long and O. M. Yaghi, Introduction to Metal–Organic Frameworks, Chem. Rev., 2012, 112, 673–674 CrossRef CAS PubMed
.
- G. Maurin, C. Serre, A. Cooper and G. Ferey, The new age of MOFs and of their porous–related solids, Chem. Soc. Rev., 2017, 46, 3104–3107 RSC
.
- M. Peplow, Materials science: The hole story, Nature, 2015, 520, 148–150 CrossRef CAS PubMed
.
- Y. He, W. Zhou, G. Qian and B. Chen, Methane storage in metal–organic frameworks, Chem. Soc. Rev., 2014, 43, 5657–5678 RSC
.
- B. Li, H.-M. Wen, H. Wang, H. Wu, M. Tyagi, T. Yildirim, W. Zhou and B. Chen, A porous metal–organic framework with dynamic pyrimidine groups exhibiting record high methane storage working capacity, J. Am. Chem. Soc., 2014, 136, 6207–6210 CrossRef CAS PubMed
.
- B. Li, H.-M. Wen, W. Zhou, J. Q. Xu and B. Chen, Porous metal–organic frameworks: promising materials for methane storage, Chem, 2016, 1, 557–580 CAS
.
- M. K. Taylor, T. e. Runčevski, J. Oktawiec, M. I. Gonzalez, R. L. Siegelman, J. A. Mason, J. Ye, C. M. Brown and J. R. Long, Tuning the Adsorption–Induced Phase Change in the Flexible Metal− Organic Framework Co(bdp), J. Am. Chem. Soc., 2016, 138, 15019–15026 CrossRef CAS PubMed
.
- R. B. Getman, Y.-S. Bae, C. E. Wilmer and R. Q. Snurr, Review and analysis of molecular simulations of methane, hydrogen, and acetylene storage in metal–organic frameworks, Chem. Rev., 2012, 112, 703–723 CrossRef CAS PubMed
.
- R. Vaidhyanathan, S. S. Iremonger, G. K. Shimizu, P. G. Boyd, S. Alavi and T. K. Woo, Direct observation and quantification of CO2 binding within an amine-functionalized nanoporous solid, Science, 2010, 330, 650–653 CrossRef CAS PubMed
.
- R. Banerjee, A. Phan, B. Wang, C. Knobler, H. Furukawa, M. O'Keeffe and O. M. Yaghi, High–Throughput Synthesis of Zeolitic Imidazolate Frameworks and Application to CO2 Capture, Science, 2008, 319, 939–943 CrossRef CAS PubMed
.
- P. Nugent, Y. Belmabkhout, S. D. Burd, A. J. Cairns, R. Luebke, K. Forrest, T. Pham and S. Ma, B. Space and L. Wojtas, Porous materials with optimal adsorption thermodynamics and kinetics for CO2 separation, Nature, 2013, 495, 80–84 CrossRef CAS PubMed
.
- Y. Peng, Y. Li, Y. Ban, H. Jin, W. Jiao, X. Liu and W. Yang, Metal–corganic framework nanosheets as building blocks for molecular sieving membranes, Science, 2014, 346, 1356–1359 CrossRef CAS PubMed
.
- J. An, C. M. Shade, D. A. Chengelis-Czegan, S. Petoud and N. L. Rosi, Zinc–adeninate metal–organic framework for aqueous encapsulation and sensitization of near–infrared and visible emitting lanthanide cations, J. Am. Chem. Soc., 2011, 133, 1220–1223 CrossRef CAS PubMed
.
- R. B. Lin, F. Li, S. Y. Liu, X. L. Qi, J. P. Zhang and X. M. Chen, A Noble–Metal–Free Porous Coordination Framework with Exceptional Sensing Efficiency for Oxygen, Angew. Chem., Int. Ed., 2013, 52, 13429–13433 CrossRef CAS PubMed
.
- Y. Cui, B. Chen and G. Qian, Lanthanide metal–organic frameworks for luminescent sensing and light-emitting applications, Coord. Chem. Rev., 2014, 273, 76–86 CrossRef
.
- Z. Hu, B. J. Deibert and J. Li, Luminescent metal–organic frameworks for chemical sensing and explosive detection, Chem. Soc. Rev., 2014, 43, 5815–5840 RSC
.
- R. B. Lin, S. Y. Liu, J. W. Ye, X. Y. Li and J. P. Zhang, Photoluminescent metal–organic frameworks for gas sensing, Adv. Sci., 2016, 3, 1500434 CrossRef PubMed
.
- M. Zhao, K. Yuan, Y. Wang, G. Li, J. Guo, L. Gu, W. Hu, H. Zhao and Z. Tang, Metal–organic frameworks as selectivity regulators for hydrogenation reactions, Nature, 2016, 539, 76–80 CrossRef CAS PubMed
.
- Q.-L. Zhu, J. Li and Q. Xu, Immobilizing metal nanoparticles to metal–organic frameworks with size and location control for optimizing catalytic performance, J. Am. Chem. Soc., 2013, 135, 10210–10213 CrossRef CAS PubMed
.
- B. Li, K. Leng, Y. Zhang, J. J. Dynes, J. Wang, Y. Hu, D. Ma, Z. Shi, L. Zhu, D. Zhang, Y. Sun, M. Chrzanowski and S. Ma, Metal–organic framework based upon the synergy of a Brønsted acid framework and Lewis acid centers as a highly efficient heterogeneous catalyst for fixed-bed reactions, J. Am. Chem. Soc., 2015, 137, 4243–4248 CrossRef CAS PubMed
.
- H. Noh, Y. Cui, A. W. Peters, D. R. Pahls, M. A. Ortuño, N. A. Vermeulen, C. J. Cramer, L. Gagliardi, J. T. Hupp and O. K. Farha, An exceptionally stable metal–organic framework supported molybdenum (VI) oxide catalyst for cyclohexene epoxidation, J. Am. Chem. Soc., 2016, 138, 14720–14726 CrossRef CAS PubMed
.
- Y.-T. Xu, X. Xiao, Z.-M. Ye, S. Zhao, R. Shen, C.-T. He, J.-P. Zhang, Y. Li and X.-M. Chen, Cage-confinement pyrolysis route to ultrasmall tungsten carbide nanoparticles for efficient electrocatalytic hydrogen evolution, J. Am. Chem. Soc., 2017, 139, 5285–5288 CrossRef CAS PubMed
.
- Q. Yang, Q. Xu and H.-L. Jiang, Metal–organic frameworks meet metal nanoparticles: synergistic effect for enhanced catalysis, Chem. Soc. Rev., 2017, 46, 4774–4808 RSC
.
- M. C. Das, Q. Guo, Y. He, J. Kim, C.-G. Zhao, K. Hong, S. Xiang, Z. Zhang, K. M. Thomas and R. Krishna, Interplay of metalloligand and organic ligand to tune micropores within isostructural mixed–metal organic frameworks (M′MOFs) for their highly selective separation of chiral and achiral small molecules, J. Am. Chem. Soc., 2012, 134, 8703–8710 CrossRef CAS PubMed
.
- M. M. Wanderley, C. Wang, C.-D. Wu and W. Lin, A chiral porous metal–organic framework for highly sensitive and enantioselective fluorescence sensing of amino alcohols, J. Am. Chem. Soc., 2012, 134, 9050–9053 CrossRef CAS PubMed
.
- Y. Inokuma, S. Yoshioka, J. Ariyoshi, T. Arai, Y. Hitora, K. Takada, S. Matsunaga, K. Rissanen and M. Fujita, X-ray analysis on the nanogram to microgram scale using porous complexes, Nature, 2013, 495, 461–466 CrossRef CAS PubMed
.
- Z. Hu, W. P. Lustig, J. Zhang, C. Zheng, H. Wang, S. J. Teat, Q. Gong, N. D. Rudd and J. Li, Effective detection of mycotoxins by a highly luminescent metal–organic framework, J. Am. Chem. Soc., 2015, 137, 16209–16215 CrossRef CAS PubMed
.
- Z. Hasan and S. H. Jhung, Removal of hazardous organics from water using metal–organic frameworks (MOFs): plausible mechanisms for selective adsorptions, J. Hazard. Mater., 2015, 283, 329–339 CrossRef CAS PubMed
.
- S. Y. Moon, Y. Liu, J. T. Hupp and O. K. Farha, Instantaneous Hydrolysis of Nerve–Agent Simulants with a Six–Connected Zirconium–Based Metal–Organic Framework, Angew. Chem., Int. Ed., 2015, 54, 6795–6799 CrossRef CAS PubMed
.
- J. E. Mondloch, M. J. Katz, W. C. Isley III, P. Ghosh, P. Liao, W. Bury, G. W. Wagner, M. G. Hall, J. B. DeCoste and G. W. Peterson, Destruction of chemical warfare agents using metal–organic frameworks, Nat. Mater., 2015, 14, 512–516 CrossRef CAS PubMed
.
- A. Schoedel, Z. Ji and O. M. Yaghi, The role of metal–organic frameworks in a carbon-neutral energy cycle, Nat. Energy, 2016, 1, 1–13 Search PubMed
.
- H. Li, K. Wang, Y. Sun, C. T. Lollar, J. Li and H.-C. Zhou, Recent advances in gas storage and separation using metal–organic, Mater. Today, 2018, 21, 108–121 CrossRef CAS
.
- B. Li, H. M. Wen, Y. Cui, W. Zhou, G. Qian and B. Chen, Emerging Multifunctional Metal–Organic Framework Materials, Adv. Mater., 2016, 28, 8819–8860 CrossRef CAS PubMed
.
- W. G. Cui, T. L. Hu and X. H. Bu, Metal–Organic Framework Materials for the Separation and Purification of Light Hydrocarbons, Adv. Mater., 2020, 32, 1806445 CrossRef CAS PubMed
.
- A. U. Czaja, N. Trukhan and U. Müller, Industrial applications of metal–organic frameworks, Chem. Soc. Rev., 2009, 38, 1284–1293 RSC
.
- M. Gaab, N. Trukhan, S. Maurer, R. Gummaraju and U. Müller, The progression of Al-based metal–organic frameworks – From academic research to industrial production and applications, Microporous Mesoporous Mater., 2012, 157, 131–136 CrossRef CAS
.
- M. Rubio-Martinez, C. Avci-Camur, A. W. Thornton, I. Imaz, D. Maspoch and M. R. Hill, New synthetic routes towards MOF production at scale, Chem. Soc. Rev., 2017, 46, 3453–3480 RSC
.
- B. Yilmaz, N. Trukhan and U. Müller, Industrial Outlook on Zeolites and Metal Organic Frameworks, Chin. J. Catal., 2012, 33, 3–10 CrossRef CAS
.
- J. H. Cavka, S. Jakobsen, U. Olsbye, N. Guillou, C. Lamberti, S. Bordiga and K. P. Lillerud, A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability, J. Am. Chem. Soc., 2008, 130, 13850–13851 CrossRef PubMed
.
- K. S. Park, Z. Ni, A. P. Cote, J. Y. Choi, R. Huang, F. J. Uribe-Romo, H. K. Chae, M. O'Keeffe and O. M. Yaghi, Exceptional chemical and thermal stability of zeolitic imidazolate frameworks, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 10186–10191 CrossRef CAS PubMed
.
- M. Bosch, M. Zhang and H.-C. Zhou, Increasing the Stability of Metal–Organic Frameworks, Adv. Chem., 2014, 2014, 1–8 CrossRef
.
- N. C. Burtch, H. Jasuja and K. S. Walton, Water stability and adsorption in metal–organic frameworks, Chem. Rev., 2014, 114, 10575–10612 CrossRef CAS PubMed
.
- J. Canivet, A. Fateeva, Y. Guo, B. Coasne and D. Farrusseng, Water adsorption in MOFs: fundamentals and applications, Chem. Soc. Rev., 2014, 43, 5594–5617 RSC
.
- M. Kim, J. F. Cahill, H. Fei, K. A. Prather and S. M. Cohen, Postsynthetic ligand and cation exchange in robust metal–organic frameworks, J. Am. Chem. Soc., 2012, 134, 18082–18088 CrossRef CAS PubMed
.
- T. C. Wang, W. Bury, D. A. Gomez-Gualdron, N. A. Vermeulen, J. E. Mondloch, P. Deria, K. Zhang, P. Z. Moghadam, A. A. Sarjeant, R. Q. Snurr, J. F. Stoddart, J. T. Hupp and O. K. Farha, Ultrahigh surface area zirconium MOFs and insights into the applicability of the BET theory, J. Am. Chem. Soc., 2015, 137, 3585–3591 CrossRef CAS PubMed
.
- S. Hermes, F. Schröder, R. Chelmowski, C. Wöll and R. A. Fischer, Selective Nucleation and Growth of Metal–Organic Open Framework Thin Films on Patterned COOH/CF3-Terminated Self-Assembled Monolayers on Au(111), J. Am. Chem. Soc., 2005, 127, 13744–13745 CrossRef CAS PubMed
.
- M. Rubio-Martinez, C. Avci-Camur, A. W. Thornton, I. Imaz, D. Maspoch and M. R. Hill, New synthetic routes towards MOF production at scale, Chem. Soc. Rev., 2017, 46, 3453–3480 RSC
.
- D. Zacher, O. Shekhah, C. Wöll and R. A. Fischer, Thin films of metal–organic frameworks, Chem. Soc. Rev., 2009, 38, 1418–1429 RSC
.
- Z. Bao, G. Chang, H. Xing, R. Krishna, Q. Ren and B. Chen, Potential of microporous metal–organic frameworks for separation of hydrocarbon mixtures, Energy Environ. Sci., 2016, 9, 3612–3641 RSC
.
- Z. R. Herm, E. D. Bloch and J. R. Long, Hydrocarbon Separations in Metal–Organic Frameworks, Chem. Mater., 2013, 26, 323–338 CrossRef
.
- J. R. Li, R. J. Kuppler and H. C. Zhou, Selective gas adsorption and separation in metal–organic frameworks, Chem. Soc. Rev., 2009, 38, 1477–1504 RSC
.
- R. B. Lin, S. Xiang, H. Xing, W. Zhou and B. Chen, Exploration of porous metal–organic frameworks for gas separation and purification, Coord. Chem. Rev., 2019, 378, 87–103 CrossRef CAS
.
- S. Mukherjee, A. V. Desai and S. K. Ghosh, Potential of metal–organic frameworks for adsorptive separation of industrially and environmentally relevant liquid mixtures, Coord. Chem. Rev., 2018, 367, 82–126 CrossRef CAS
.
- S. Seifzadeh Haghighi, M. R. Rahimpour, S. Raeissi and O. Dehghani, Investigation of ethylene production in naphtha thermal cracking plant in presence of steam and carbon dioxide, Chem. Eng. J., 2013, 228, 1158–1167 CrossRef CAS
.
- S. Aguado, G. Bergeret, C. Daniel and D. Farrusseng, Absolute Molecular Sieve Separation of Ethylene/Ethane Mixtures with Silver Zeolite A, J. Am. Chem. Soc., 2012, 134, 14635–14637 CrossRef CAS PubMed
.
- P. J. Bereciartua, A. Cantin, A. Corma, J. L. Jorda, M. Palomino, F. Rey, S. Valencia, E. W. Corcoran, P. Kortunov, P. I. Ravikovitch, A. Burton, C. Yoon, Y. Wang, C. Paur, J. Guzman, A. R. Bishop and G. L. Casty, Control of zeolite framework flexibility and pore topology for separation of ethane and ethylene, Science, 2017, 358, 1068–1071 CrossRef CAS PubMed
.
- Q. M. Wang, D. Shen, M. Bülow, M. L. Lau, S. Deng, F. R. Fitch, N. O. Lemcoff and J. Semanscin, Metallo–organic molecular sieve for gas separation and purification, Microporous Mesoporous Mater., 2002, 55, 217–230 CrossRef CAS
.
- S. Wang, Q. Yang and C. Zhong, Adsorption and separation of binary mixtures in a metal–organic framework Cu-BTC: A computational study, Sep. Purif. Technol., 2008, 60, 30–35 CrossRef CAS
.
- Z. Bao, S. Alnemrat, L. Yu, I. Vasiliev, Q. Ren, X. Lu and S. Deng, Adsorption of ethane, ethylene, propane, and propylene on a magnesium-based metal–organic framework, Langmuir, 2011, 27, 13554–13562 CrossRef CAS PubMed
.
- E. D. Bloch, W. L. Queen, R. Krishna, J. M. Zadrozny, C. M. Brown and J. R. Long, Hydrocarbon separations in a metal–organic framework with open iron(II) coordination sites, Science, 2012, 335, 1606–1610 CrossRef CAS PubMed
.
- S. Yang, A. J. Ramirez-Cuesta, R. Newby, V. Garcia-Sakai, P. Manuel, S. K. Callear, S. I. Campbell, C. C. Tang and M. Schroder, Supramolecular binding and separation of hydrocarbons within a functionalized porous metal–organic framework, Nat. Chem., 2014, 7, 121–129 CrossRef PubMed
.
- J. E. Bachman, M. T. Kapelewski, D. A. Reed, M. I. Gonzalez and J. R. Long, M2(m-dobdc) (M = Mn, Fe, Co, Ni) Metal–Organic Frameworks as Highly Selective, High–Capacity Adsorbents for Olefin/Paraffin Separations, J. Am. Chem. Soc., 2017, 139, 15363–15370 CrossRef CAS PubMed
.
- Z. Bao, J. Wang, Z. Zhang, H. Xing, Q. Yang, Y. Yang, H. Wu, R. Krishna, W. Zhou, B. Chen and Q. Ren, Molecular Sieving of Ethane from Ethylene through the Molecular Cross–Section Size Differentiation in Gallate-based Metal–Organic Frameworks, Angew. Chem., Int. Ed., 2018, 57, 16020–16025 CrossRef CAS PubMed
.
- R. B. Lin, L. Li, H. L. Zhou, H. Wu, C. He, S. Li, R. Krishna, J. Li, W. Zhou and B. Chen, Molecular sieving of ethylene from ethane using a rigid metal–organic framework, Nat. Mater., 2018, 17, 1128–1133 CrossRef CAS PubMed
.
- B. Li, Y. Zhang, R. Krishna, K. Yao, Y. Han, Z. Wu, D. Ma, Z. Shi, T. Pham, B. Space, J. Liu, P. K. Thallapally, J. Liu, M. Chrzanowski and S. Ma, Introduction of pi-complexation into porous aromatic framework for highly selective adsorption of ethylene over ethane, J. Am. Chem. Soc., 2014, 136, 8654–8660 CrossRef CAS PubMed
.
- M. H. Mohamed, Y. Yang, L. Li, S. Zhang, J. P. Ruffley, A. G. Jarvi, S. Saxena, G. Veser, J. K. Johnson and N. L. Rosi, Designing Open Metal Sites in Metal–Organic Frameworks for Paraffin/Olefin Separations, J. Am. Chem. Soc., 2019, 141, 13003–13007 CrossRef CAS PubMed
.
- L. Zhang, L. Li, E. Hu, L. Yang, K. Shao, L. Yao, K. Jiang, Y. Cui, Y. Yang, B. Li, B. Chen and G. Qian, Boosting Ethylene/Ethane Separation within Copper(I)–Chelated Metal–Organic Frameworks through Tailor–Made Aperture and Specific pi-Complexation, Adv. Sci., 2020, 7, 1901918 CrossRef CAS PubMed
.
- C. Gu, N. Hosono, J. J. Zheng, Y. Sato, S. Kusaka, S. Sakaki and S. Kitagawa, Design and control of gas diffusion process in a nanoporous soft crystal, Science, 2019, 363, 387–391 CrossRef CAS PubMed
.
- J. Kim, L. C. Lin, R. L. Martin, J. A. Swisher, M. Haranczyk and B. Smit, Large-Scale Computational Screening of Zeolites for Ethane/Ethene Separation, Langmuir, 2012, 28, 11914–11919 CrossRef CAS PubMed
.
- X. Wang, Y. Wu, J. Peng, Y. Wu, J. Xiao, Q. Xia and Z. Li, Novel glucosamine-based carbon adsorbents with high capacity and its enhanced mechanism of preferential adsorption of C2H6 over C2H4, Chem. Eng. J., 2019, 358, 1114–1125 CrossRef CAS
.
- C. Gucuyener, J. van den Bergh, J. Gascon and F. Kapteijn, Ethane/Ethene Separation Turned on Its Head: Selective Ethane Adsorption on the Metal–Organic Framework ZIF-7 through a Gate-Opening Mechanism, J. Am. Chem. Soc., 2010, 132, 17704–17706 CrossRef PubMed
.
- J. Pires, M. L. Pinto and V. K. Saini, Ethane selective IRMOF-8 and its significance in ethane–ethylene separation by adsorption, ACS Appl. Mater. Interfaces, 2014, 6, 12093–12099 CrossRef CAS PubMed
.
- R. B. Lin, H. Wu, L. Li, X. L. Tang, Z. Li, J. Gao, H. Cui, W. Zhou and B. Chen, Boosting Ethane/Ethylene Separation within Isoreticular Ultramicroporous Metal–Organic Frameworks, J. Am. Chem. Soc., 2018, 140, 12940–12946 CrossRef CAS
.
- F. Z. Sun, S. Q. Yang, R. Krishna, Y. H. Zhang, Y. P. Xia and T. L. Hu, Microporous Metal−Organic Framework with a Completely Reversed Adsorption Relationship for C2 Hydrocarbons at Room Temperature, ACS Appl. Mater. Interfaces, 2020, 12, 6105–6111 CrossRef CAS PubMed
.
- P. Q. Liao, W. X. Zhang, J. P. Zhang and X. M. Chen, Efficient purification of ethene by an ethane-trapping metal–organic framework, Nat. Commun., 2015, 6, 8697 CrossRef PubMed
.
- W. Liang, F. Xu, X. Zhou, J. Xiao, Q. Xia, Y. Li and Z. Li, Ethane selective adsorbent Ni(bdc)(ted)0.5 with high uptake and its significance in adsorption separation of ethane and ethylene, Chem. Eng. Sci., 2016, 148, 275–281 CrossRef CAS
.
- Y. Chen, Z. Qiao, H. Wu, D. Lv, R. Shi, Q. Xia, J. Zhou and Z. Li, An ethane-trapping MOF PCN-250 for highly selective adsorption of ethane over ethylene, Chem. Eng. Sci., 2018, 175, 110–117 CrossRef CAS
.
- L. Yang, W. Zhou, H. Li, A. Alsalme, L. Jia, J. Yang, J. Li, L. Li and B. Chen, Reversed ethane/ethylene adsorption in a metal–organic framework via introduction of oxygen, Chin. J. Chem. Eng., 2020, 28, 593–597 CrossRef
.
- O. T. Qazvini, R. Babarao, Z. L. Shi, Y. B. Zhang and S. G. Telfer, A Robust Ethane–Trapping Metal–Organic Framework with a High Capacity for Ethylene Purification, J. Am. Chem. Soc., 2019, 141, 5014–5020 CrossRef CAS PubMed
.
- L. Yang, Y. Wang, Y. Chen, J. Yang, X. Wang, L. Li and J. Li, Microporous metal–organic framework with specific functional sites for efficient removal of ethane from ethane/ethylene mixtures, Chem. Eng. J., 2020, 387, 124137 CrossRef CAS
.
- K. J. Chen, D. G. Madden, S. Mukherjee, T. Pham, K. A. Forrest, A. Kumar, B. Space, J. Kong, Q. Y. Zhang and M. J. Zaworotko, Synergistic sorbent separation for one-step ethylene purification from a four-component mixture, Science, 2019, 366, 241–246 CrossRef CAS PubMed
.
- N. Lamia, M. Jorge, M. A. Granato, F. A. Almeida Paz, H. Chevreau and A. E. Rodrigues, Adsorption of propane, propylene and isobutane on a metal–organic framework: Molecular simulation and experiment, Chem. Eng. Sci., 2009, 64, 3246–3259 CrossRef CAS
.
- J. W. Yoon, Y. K. Seo, Y. K. Hwang, J. S. Chang, H. Leclerc, S. Wuttke, P. Bazin, A. Vimont, M. Daturi, E. Bloch, P. L. Llewellyn, C. Serre, P. Horcajada, J. M. Greneche, A. E. Rodrigues and G. Ferey, Controlled reducibility of a metal–organic framework with coordinatively unsaturated sites for preferential gas sorption, Angew. Chem., Int. Ed., 2010, 49, 5949–5952 CrossRef CAS PubMed
.
- Y. S. Bae, C. Y. Lee, K. C. Kim, O. K. Farha, P. Nickias, J. T. Hupp, S. T. Nguyen and R. Q. Snurr, High propene/propane selectivity in isostructural metal–organic frameworks with high densities of open metal sites, Angew. Chem., Int. Ed., 2012, 51, 1857–1860 CrossRef CAS PubMed
.
- K. Li, D. H. Olson, J. Seidel, T. J. Emge, H. Gong, H. Zeng and J. Li, Zeolitic imidazolate frameworks for kinetic separation of propane and propene, J. Am. Chem. Soc., 2009, 131, 10368–10369 CrossRef CAS PubMed
.
- C. Y. Lee, Y. S. Bae, N. C. Jeong, O. K. Farha, A. A. Sarjeant, C. L. Stern, P. Nickias, R. Q. Snurr, J. T. Hupp and S. T. Nguyen, Kinetic separation of propene and propane in metal–organic frameworks: controlling diffusion rates in plate–shaped crystals via tuning of pore apertures and crystallite aspect ratios, J. Am. Chem. Soc., 2011, 133, 5228–5231 CrossRef CAS PubMed
.
- J. Peng, H. Wang, D. H. Olson, Z. Li and J. Li, Efficient kinetic separation of propene and propane using two microporous metal organic frameworks, Chem. Commun., 2017, 53, 9332–9335 RSC
.
- L. Li, R.-B. Lin, X. Wang, W. Zhou, L. Jia, J. Li and B. Chen, Kinetic separation of propylene over propane in a microporous metal–organic framework, Chem. Eng. J., 2018, 354, 977–982 CrossRef CAS
.
- A. Cadiau, K. Adil, P. Bhatt, Y. Belmabkhout and M. Eddaoudi, A metal–organic framework–based splitter for separating propylene from propane, Science, 2016, 353, 137–140 CrossRef CAS PubMed
.
- H. Wang, X. Dong, V. Colombo, Q. Wang, Y. Liu, W. Liu, X. L. Wang, X. Y. Huang, D. M. Proserpio, A. Sironi, Y. Han and J. Li, Tailor–Made Microporous Metal–Organic Frameworks for the Full Separation of Propane from Propylene Through Selective Size Exclusion, Adv. Mater., 2018, 30, e1805088 CrossRef PubMed
.
- X. Wang, R. Krishna, L. Li, B. Wang, T. He, Y.-Z. Zhang, J.-R. Li and J. Li, Guest-dependent pressure induced gate-opening effect enables effective separation of propene and propane in a flexible MOF, Chem. Eng. J., 2018, 346, 489–496 CrossRef CAS
.
- M. H. Yu, B. Space, D. Franz, W. Zhou, C. He, L. Li, R. Krishna, Z. Chang, W. Li, T. L. Hu and X. H. Bu, Enhanced Gas Uptake in a Microporous Metal−Organic Framework via a Sorbate Induced-Fit Mechanism, J. Am. Chem. Soc., 2019, 141, 17703–17712 CrossRef CAS PubMed
.
- U. Bohme, B. Barth, C. Paula, A. Kuhnt, W. Schwieger, A. Mundstock, J. Caro and M. Hartmann, Ethene/ethane and propene/propane separation via the olefin and paraffin selective metal–organic framework adsorbents CPO-27 and ZIF-8, Langmuir, 2013, 29, 8592–8600 CrossRef PubMed
.
- L. Yang, X. Cui, Q. Ding, Q. Wang, A. Jin, L. Ge and H. Xing, Polycatenated Molecular Cage-Based Propane Trap for Propylene Purification with Recorded Selectivity, ACS Appl. Mater. Interfaces, 2020, 12, 2525–2530 CrossRef CAS PubMed
.
- Z. Zhang, Q. Yang, X. Cui, L. Yang, Z. Bao, Q. Ren and H. Xing, Sorting of C4 Olefins with Interpenetrated Hybrid Ultramicroporous Materials by Combining Molecular Recognition and Size-Sieving, Angew. Chem., Int. Ed., 2017, 56, 16282–16287 CrossRef CAS PubMed
.
- P. Q. Liao, N. Y. Huang, W. X. Zhang, J. P. Zhang and X. M. Chen, Controlling guest conformation for efficient purification of butadiene, Science, 2017, 356, 1193–1196 CrossRef CAS
.
- S. C. Xiang, Z. Zhang, C. G. Zhao, K. Hong, X. Zhao, D. R. Ding, M. H. Xie, C. D. Wu, M. C. Das, R. Gill, K. M. Thomas and B. Chen, Rationally tuned micropores within enantiopure metal–organic frameworks for highly selective separation of acetylene and ethylene, Nat. Commun., 2011, 2, 204 CrossRef PubMed
.
- T. L. Hu, H. Wang, B. Li, R. Krishna, H. Wu, W. Zhou, Y. Zhao, Y. Han, X. Wang, W. Zhu, Z. Yao, S. Xiang and B. Chen, Microporous metal–organic framework with dual functionalities for highly efficient removal of acetylene from ethylene/acetylene mixtures, Nat. Commun., 2015, 6, 7328 CrossRef CAS PubMed
.
- X. Cui, K. Chen, H. Xing, Q. Yang, R. Krishna, Z. Bao, H. Wu, W. Zhou, X. Dong, Y. Han, B. Li, Q. Ren, M. J. Zaworotko and B. Chen, Pore chemistry and size control in hybrid porous materials for acetylene capture from ethylene, Science, 2016, 353, 141–144 CrossRef CAS PubMed
.
- B. Li, X. Cui, D. O'Nolan, H. M. Wen, M. Jiang, R. Krishna, H. Wu, R. B. Lin, Y. S. Chen, D. Yuan, H. Xing, W. Zhou, Q. Ren, G. Qian, M. J. Zaworotko and B. Chen, An Ideal Molecular Sieve for Acetylene Removal from Ethylene with Record Selectivity and Productivity, Adv. Mater., 2017, 29, 1704210 CrossRef PubMed
.
- L. Li, R. B. Lin, R. Krishna, X. Wang, B. Li, H. Wu, J. Li, W. Zhou and B. Chen, Flexible–Robust Metal–Organic Framework for Efficient Removal of Propyne from Propylene, J. Am. Chem. Soc., 2017, 139, 7733–7736 CrossRef CAS PubMed
.
- L. Yang, X. Cui, Q. Yang, S. Qian, H. Wu, Z. Bao, Z. Zhang, Q. Ren, W. Zhou, B. Chen and H. Xing, A Single-Molecule Propyne Trap: Highly Efficient Removal of Propyne from Propylene with Anion-Pillared Ultramicroporous Materials, Adv. Mater., 2018, 30, 1705374 CrossRef PubMed
.
- L. Yang, X. Cui, Z. Zhang, Q. Yang, Z. Bao, Q. Ren and H. Xing, An Asymmetric Anion–Pillared Metal–Organic Framework as a Multisite Adsorbent Enables Simultaneous Removal of Propyne and Propadiene from Propylene, Angew. Chem., Int. Ed., 2018, 57, 13145–13149 CrossRef CAS PubMed
.
- L. Li, H. M. Wen, C. He, R. B. Lin, R. Krishna, H. Wu, W. Zhou, J. Li, B. Li and B. Chen, A Metal–Organic Framework with Suitable Pore Size and Specific Functional Sites for the Removal of Trace Propyne from Propylene, Angew. Chem., Int. Ed., 2018, 57, 15183–15188 CrossRef CAS PubMed
.
- J. Yang, A. Grzech, F. M. Mulder and T. J. Dingemans, Methyl modified MOF-5: a water stable hydrogen storage material, Chem. Commun., 2011, 47, 5244–5246 RSC
.
- J. B. Decoste, G. W. Peterson, M. W. Smith, C. A. Stone and C. R. Willis, Enhanced stability of Cu-BTC MOF via perfluorohexane plasma-enhanced chemical vapor deposition, J. Am. Chem. Soc., 2012, 134, 1486–1489 CrossRef CAS PubMed
.
- S. J. Yang and C. R. Park, Preparation of highly moisture-resistant black-colored metal organic frameworks, Adv. Mater., 2012, 24, 4010–4013 CrossRef CAS PubMed
.
- W. Zhang, Y. Hu, J. Ge, H. L. Jiang and S. H. Yu, A facile and general coating approach to moisture/water–resistant metal–organic frameworks with intact porosity, J. Am. Chem. Soc., 2014, 136, 16978–16981 CrossRef CAS PubMed
.
- B. Wang, X. L. Lv, D. Feng, L. H. Xie, J. Zhang, M. Li, Y. Xie, J. R. Li and H. C. Zhou, Highly Stable Zr(IV)–Based Metal–Organic Frameworks for the Detection and Removal of Antibiotics and Organic Explosives in Water, J. Am. Chem. Soc., 2016, 138, 6204–6216 CrossRef CAS PubMed
.
- C. Dong, J. Bai, X. L. Lv, W. Wu, J. Lv and J. R. Li, Fixing Flexible Arms of Core–Shared Ligands to Enhance the Stability of Metal–Organic Frameworks, Inorg. Chem., 2019, 58, 15909–15916 CrossRef CAS PubMed
.
- N. Li, J. Xu, R. Feng, T. L. Hu and X. H. Bu, Governing metal–organic frameworks towards high stability, Chem. Commun., 2016, 52, 8501–8513 RSC
.
- S. Marx, W. Kleist, J. Huang, M. Maciejewski and A. Baiker, Tuning functional sites and thermal stability of mixed-linker MOFs based on MIL-53(Al), Dalton Trans., 2010, 39, 3795–3798 RSC
.
- V. Colombo, S. Galli, H. J. Choi, G. D. Han, A. Maspero, G. Palmisano, N. Masciocchi and J. R. Long, High thermal and chemical stability in pyrazolate-bridged metal–organic frameworks with exposed metal sites, Chem. Sci., 2011, 2, 1311–1319 RSC
.
- I. J. Kang, N. A. Khan, E. Haque and S. H. Jhung, Chemical and thermal stability of isotypic metal–organic frameworks: effect of metal ions, Chemistry, 2011, 17, 6437–6442 CrossRef CAS PubMed
.
- Y. Hu, M. Ding, X. Q. Liu, L. B. Sun and H. L. Jiang, Rational synthesis of an exceptionally stable Zn(II) metal–organic framework for the highly selective and sensitive detection of picric acid, Chem. Commun., 2016, 52, 5734–5737 RSC
.
- S. Chen, J. Zhang, T. Wu, P. Feng and X. Bu, Multiroute synthesis of porous anionic frameworks and size-tunable extraframework organic cation-controlled gas sorption properties, J. Am. Chem. Soc., 2009, 131, 16027–16029 CrossRef CAS PubMed
.
- S. T. Zheng, J. T. Bu, Y. Li, T. Wu, F. Zuo, P. Feng and X. Bu, Pore space partition and charge separation in cage-within-cage indium-organic frameworks with high CO2 uptake, J. Am. Chem. Soc., 2010, 132, 17062–17064 CrossRef CAS PubMed
.
- X. Zhao, X. Bu, Q. G. Zhai, H. Tran and P. Feng, Pore space partition by symmetry-matching regulated ligand insertion and dramatic tuning on carbon dioxide uptake, J. Am. Chem. Soc., 2015, 137, 1396–1399 CrossRef CAS PubMed
.
- S. T. Zheng, T. Wu, B. Irfanoglu, F. Zuo, P. Feng and X. Bu, Multicomponent self-assembly of a nested Co24@Co48 metal–organic polyhedral framework, Angew. Chem., Int. Ed., 2011, 50, 8034–8037 CrossRef CAS PubMed
.
- S. T. Zheng, T. Wu, F. Zuo, C. Chou, P. Feng and X. Bu, Mimicking zeolite to its core: porous sodalite cages as hangers for pendant trimeric M3(OH) clusters (M = Mg, Mn, Co, Ni, Cd), J. Am. Chem. Soc., 2012, 134, 1934–1937 CrossRef CAS PubMed
.
- D. Feng, Z. Y. Gu, J. R. Li, H. L. Jiang, Z. Wei and H. C. Zhou, Zirconium-Metalloporphyrin PCN-222: Mesoporous Metal–Organic Frameworks with Ultrahigh Stability as Biomimetic Catalysts, Angew. Chem., Int. Ed., 2012, 51, 10307–10310 CrossRef CAS PubMed
.
- H. Huang, J. R. Li, K. Wang, T. Han, M. Tong, L. Li, Y. Xie, Q. Yang, D. Liu and C. Zhong, An in situ self-assembly template strategy for the preparation of hierarchical-pore metal–organic frameworks, Nat. Commun., 2015, 6, 8847 CrossRef CAS PubMed
.
- L. Feng, S. Yuan, L. L. Zhang, K. Tan, J. L. Li, A. Kirchon, L. M. Liu, P. Zhang, Y. Han, Y. J. Chabal and H. C. Zhou, Creating Hierarchical Pores by Controlled Linker Thermolysis in Multivariate Metal−Organic Frameworks, J. Am. Chem. Soc., 2018, 140, 2363–2372 CrossRef CAS PubMed
.
- X. J. Hong, Q. Wei, Y. P. Cai, B. B. Wu, H. X. Feng, Y. Yu and R. F. Dong, Pillar-Layered Metal–Organic Framework with Sieving Effect and Pore Space Partition for Effective Separation of Mixed Gas C2H2/C2H4, ACS Appl. Mater. Interfaces, 2017, 9, 29374–29379 CrossRef CAS PubMed
.
- Y. Ye, Z. Ma, R. B. Lin, R. Krishna, W. Zhou, Q. Lin, Z. Zhang, S. Xiang and B. Chen, Pore Space Partition within a Metal–Organic Framework for Highly Efficient C2H2/CO2 Separation, J. Am. Chem. Soc., 2019, 141, 4130–4136 CrossRef CAS PubMed
.
- M. D. Allendorf, C. A. Bauer, R. K. Bhakta and R. J. Houk, Luminescent metal–organic frameworks, Chem. Soc. Rev., 2009, 38, 1330–1352 RSC
.
- D. Farrusseng, S. Aguado and C. Pinel, Metal–organic frameworks: opportunities for catalysis, Angew. Chem., Int. Ed., 2009, 48, 7502–7513 CrossRef CAS PubMed
.
- P. Horcajada, R. Gref, T. Baati, P. K. Allan, G. Maurin, P. Couvreur, G. Ferey, R. E. Morris and C. Serre, Metal–organic frameworks in biomedicine, Chem. Rev., 2012, 112, 1232–1268 CrossRef CAS PubMed
.
- K. Sumida, D. L. Rogow, J. A. Mason, T. M. McDonald, E. D. Bloch, Z. R. Herm, T. H. Bae and J. R. Long, Carbon dioxide capture in metal–organic frameworks, Chem. Rev., 2012, 112, 724–781 CrossRef CAS
.
- U. Mueller, M. Schubert, F. Teich, H. Puetter, K. Schierle-Arndt and J. Pastré, Metal–organic frameworks—prospective industrial applications, J. Mater. Chem., 2006, 16, 626–636 RSC
.
- N. Campagnol, T. R. C. Van Assche, M. Li, L. Stappers, M. Dincă, J. F. M. Denayer, K. Binnemans, D. E. De Vos and J. Fransaer, On the electrochemical deposition of metal–organic frameworks, J. Mater. Chem. A, 2016, 4, 3914–3925 RSC
.
- S. H. Jhung, J. H. Lee and J. S. Chang, Microwave synthesis of a nanoporous hybrid material, chromium trimesate, Bull. Korean Chem. Soc., 2005, 26, 880–881 CrossRef CAS
.
- P. P. Bag, X. S. Wang and R. Cao, Microwave-assisted large scale synthesis of lanthanide metal–organic frameworks (Ln-MOFs), having a preferred conformation and photoluminescence properties, Dalton Trans., 2015, 44, 11954–11962 RSC
.
- D. Crawford, J. Casaban, R. Haydon, N. Giri, T. McNally and S. L. James, Synthesis by extrusion: continuous, large-scale preparation of MOFs using little or no solvent, Chem. Sci., 2015, 6, 1645–1649 RSC
.
- D. Bazer-Bachi, L. Assié, V. Lecocq, B. Harbuzaru and V. Falk, Towards industrial use of metal–organic framework: Impact of shaping on the MOF properties, Powder Technol., 2014, 255, 52–59 CrossRef CAS
.
- J. Kim, S.-H. Kim, S.-T. Yang and W.-S. Ahn, Bench-scale preparation of Cu3(BTC)2 by ethanol reflux: Synthesis optimization and adsorption/catalytic applications, Microporous Mesoporous Mater., 2012, 161, 48–55 CrossRef CAS
.
- P.-J. Kim, Y.-W. You, H. Park, J.-S. Chang, Y.-S. Bae, C.-H. Lee and J.-K. Suh, Separation of SF6 from SF6/N2 mixture using metal–organic framework MIL-100(Fe) granule, Chem. Eng. J., 2015, 262, 683–690 CrossRef CAS
.
- M. A. Moreira, J. C. Santos, A. F. Ferreira, J. M. Loureiro, F. Ragon, P. Horcajada, K. E. Shim, Y. K. Hwang, U. H. Lee, J. S. Chang, C. Serre and A. E. Rodrigues, Reverse shape selectivity in the liquid-phase adsorption of xylene isomers in zirconium terephthalate MOF UiO-66, Langmuir, 2012, 28, 5715–5723 CrossRef CAS
.
- D. Liu, J. J. Purewal, J. Yang, A. Sudik, S. Maurer, U. Mueller, J. Ni and D. J. Siegel, MOF-5 composites exhibiting improved thermal conductivity, Int. J. Hydrogen Energy, 2012, 37, 6109–6117 CrossRef CAS
.
- G. W. Peterson, J. B. DeCoste, T. G. Glover, Y. Huang, H. Jasuja and K. S. Walton, Effects of pelletization pressure on the physical and chemical properties of the metal–organic frameworks Cu3(BTC)2 and UiO-66, Microporous Mesoporous Mater., 2013, 179, 48–53 CrossRef CAS
.
- K. Eum, A. Rownaghi, D. Choi, R. R. Bhave, C. W. Jones and S. Nair, Fluidic Processing of High-Performance ZIF-8 Membranes on Polymeric Hollow Fibers: Mechanistic Insights and Microstructure Control, Adv. Funct. Mater., 2016, 26, 5011–5018 CrossRef CAS
.
- H. Ji, S. Hwang, K. Kim, C. Kim and N. C. Jeong, Direct in Situ Conversion of Metals into Metal–Organic Frameworks: A Strategy for the Rapid Growth of MOF Films on Metal Substrates, ACS Appl. Mater. Interfaces, 2016, 8, 32414–32420 CrossRef CAS PubMed
.
- W.-J. Li, J. Lü, S.-Y. Gao, Q.-H. Li and R. Cao, Electrochemical preparation of metal–organic framework films for fast detection of nitro explosives, J. Mater. Chem. A, 2014, 2, 19473–19478 RSC
.
- C. Scherb, A. Schodel and T. Bein, Directing the structure of metal–organic frameworks by oriented surface growth on an organic monolayer, Angew. Chem., Int. Ed., 2008, 47, 5777–5779 CrossRef CAS
.
- E. Biemmi, C. Scherb and T. Bein, Oriented Growth of the Metal Organic Framework Cu3(BTC)2(H2O)3·xH2O Tunable with Functionalized Self-Assembled Monolayers, J. Am. Chem. Soc., 2007, 129, 8054–8055 CrossRef CAS PubMed
.
- A. de la Pena Ruigomez, D. Rodriguez-San-Miguel, K. C. Stylianou, M. Cavallini, D. Gentili, F. Liscio, S. Milita and O. M. Roscioni,
et al., Direct On-Surface Patterning of a Crystalline Laminar Covalent Organic Framework Synthesized at Room Temperature, Chemistry, 2015, 21, 10666–10670 CrossRef CAS PubMed
.
- B. Garai, A. Mallick and R. Banerjee, Photochromic metal–organic frameworks for inkless and erasable printing, Chem. Sci., 2016, 7, 2195–2200 RSC
.
- H. Jiang, Q. Wang, H. Wang, Y. Chen and M. Zhang, MOF-74 as an Efficient Catalyst for the Low-Temperature Selective Catalytic Reduction of NOx with NH3, ACS Appl. Mater. Interfaces, 2016, 8, 26817–26826 CrossRef CAS PubMed
.
- J. Park and M. Oh, Construction of flexible metal–organic framework (MOF) papers through MOF growth on filter paper and their selective dye capture, Nanoscale, 2017, 9, 12850–12854 RSC
.
- Y. Chen, X. Huang, S. Zhang, S. Li, S. Cao, X. Pei, J. Zhou, X. Feng and B. Wang, Shaping of Metal–Organic Frameworks: From Fluid to Shaped Bodies and Robust Foams, J. Am. Chem. Soc., 2016, 138, 10810–10813 CrossRef CAS PubMed
.
- T. Tian, Z. Zeng, D. Vulpe, M. E. Casco, G. Divitini, P. A. Midgley, J. Silvestre-Albero, J. C. Tan, P. Z. Moghadam and D. Fairen-Jimenez, A sol–gel monolithic metal–organic framework with enhanced methane uptake, Nat. Mater., 2018, 17, 174–179 CrossRef CAS PubMed
.
- A. Mallick, G. Mouchaham, P. M. Bhatt, W. Liang, Y. Belmabkhout, K. Adil, A. Jamal and M. Eddaoudi, Advances in Shaping of Metal–Organic Frameworks for CO2 Capture: Understanding the Effect of Rubbery and Glassy Polymeric Binders, Ind. Eng. Chem. Res., 2018, 57, 16897–16902 CrossRef CAS
.
| This journal is © the Partner Organisations 2020 |