Biosensing based on upconversion nanoparticles for food quality and safety applications
Riikka
Peltomaa
 a,
Elena
Benito-Peña
a,
Elena
Benito-Peña
 b,
Hans H.
Gorris
b,
Hans H.
Gorris
 *c and
María C.
Moreno-Bondi
*c and
María C.
Moreno-Bondi
 *b
*b
aDepartment of Biochemistry/Biotechnology, University of Turku, Kiinamyllynkatu 10, 20520, Turku, Finland
bDepartment of Analytical Chemistry, Faculty of Chemistry, Universidad Complutense de Madrid, 28040 Madrid, Spain. E-mail: mcmbondi@ucm.es
cInstitute of Analytical Chemistry, Chemo- and Biosensors, University of Regensburg, 93040 Regensburg, Germany. E-mail: hans-heiner.gorris@ur.de
First published on 12th November 2020
Abstract
Food safety and quality regulations inevitably call for sensitive and accurate analytical methods to detect harmful contaminants in food and to ensure safe food for the consumer. Both novel and well-established biorecognition elements, together with different transduction schemes, enable the simple and rapid analysis of various food contaminants. Upconversion nanoparticles (UCNPs) are inorganic nanocrystals that convert near-infrared light into shorter wavelength emission. This unique photophysical feature, along with narrow emission bandwidths and large anti-Stokes shift, render UCNPs excellent optical labels for biosensing because they can be detected without optical background interferences from the sample matrix. In this review, we show how this exciting technique has evolved into biosensing platforms for food quality and safety monitoring and highlight recent applications in the field.
Introduction
Safe food saves lives. Nutritious and safe food is vital to sustaining life and promoting good health. Since the beginning of humanity, however, foodborne diseases have been a significant cause of morbidity and mortality in all societies. According to the World Health Organization (WHO), more than 200 diseases, ranging from diarrhea to cancers, are related to unsafe food contaminated with natural toxins, harmful bacteria, viruses, parasites, or chemical substances.1 The global burden of foodborne diseases is considerable, and a total of some 600 million cases of foodborne illnesses are reported globally every year according to the WHO.2 It is also noteworthy that the most significant tragedies of foodborne diseases are played out in developing countries where children and poor people take the brunt of this burden. The absence of proper food storage infrastructure and trained human resources, together with poorly enforced regulations, contribute to the high risks. Beyond the individual level, unsafe food and foodborne diseases affect the economic development—the World Bank has estimated that 110 billion USD are lost annually in low- and middle-income economies due to lost productivity and medical expenses linked to unsafe food.1,3–5Significant risks for food safety include various food additives, drugs, pesticides and fertilizers, pathogenic microorganisms, including virus and bacteria, as well as other contaminants, such as dioxins, polychlorinated biphenyls (PCBs), polycyclic aromatic hydrocarbons (PAHs), heavy metals, and biotoxins (mycotoxins and marine toxins). On the other hand, for food quality monitoring the composition of food becomes important and various components, such as sugars, amino acids, alcohols, organic acids, cholesterol, polyphenol and fatty acids, and biogenic amines, are being monitored.6 The range of monitored analytes is vast, and food safety and quality assurance have to address numerous challenges and the interventions in every step of the food supply chain are of utmost importance to safeguard public health.7 In fact, the Food and Agriculture Organization of the United Nations (FAO) has established the equipment and infrastructure for a food control system as one of its foundations,4 and the European Food Safety Authority (EFSA) food controls check for hormones, chemical residues, bacterial and viral contamination as well as for overall hygiene, labelling and potential frauds. Consequently, there is a continuous need for new analytical methods for monitoring even low concentrations of various pathogens and chemicals that might contaminate food. For many circumstances, rapid and cost-effective methods are preferred, for example, for measurements in the field or on the production line. Ideally, such systems would enable analysis of foodstuffs with little or no sample pretreatment and, if possible, by individuals with scant scientific training. Biosensors and bioanalytical assays have become essential tools for such purposes.8–10
Biosensing is based on the specificity of biomolecular recognition and a simple measurement using different transduction methods, such as optical or electrochemical.11 Commonly used biorecognition elements include antibodies and aptamers, but also whole cells, or other bioinspired recognition elements.12 In their simplest format, bioassays, such as the widely used enzyme-linked immunosorbent assay (ELISA), are based on visual detection or colorimetric readers, but higher sensitivity can be achieved, for instance, with luminescent detection. The simplicity of such methods offers the possibility for home and field testing outside well-equipped laboratories. On the other hand, some biosensors can also offer real-time readout and even perform the entire process automatically.13,14 Significant research efforts in the field of biosensing, together with recent advances in material sciences and nanotechnology, have prompted the development of analytical tools, one more sophisticated than the next.11 For example, the potential for multiplexing is highly attractive for food surveillance and outbreak investigations as it may enable the detection of many targets simultaneously and overcome the costly and logistically challenging analysis of single targets one by one.15
Optical detection is particularly appealing for biosensing applications as it can be applied to multiplexed detection, and moreover, optics can be easily miniaturized at a relatively low cost.16 Moreover, in recent decades, biosensing based on nanomaterials with unique size-dependent physical and chemical properties has arisen as an exciting technology, which stems from the exceptional optical properties of these materials and the potential for simple yet sensitive detection.17–19 Nanoparticles, typically defined by their size of less than 100 nm in at least one direction, have gained significant attention in recent years. Their exceptional properties and tuneable morphology make them useful in many fields, including electronics,20 chemistry,21 biology,22 therapeutics,23 and biosensing.24,25 Consequently, nanoparticle-based biosensors have found their applications for fast, accurate, and low-cost analysis of various targets in many disciplines, including healthcare and food safety.7,17,26–28 In this review, we discuss biosensing for food safety and quality monitoring from the last few years based on upconversion nanoparticles (UCNPs), an interesting type of nanomaterial that bestows great potential owing to its unorthodox optical properties.
Background perspective on UCNPs
Fundamental concepts of upconversion
Photon-upconversion materials are capable of converting near-infrared (NIR) excitation light into shorter wavelength emission, typically at visible wavelengths. The upconversion emission provides numerous advantages including large anti-Stokes shift with sharp emission bands, the lack of interference from autofluorescence, high chemical and photostability.29 The upconversion luminescence process was first proposed by Bloembergen,30 and later demonstrated by Porter in 1961.31 Furthermore, in the 1960s,32,33 the pioneering work of François Auzel further confirmed that this unusual anti-Stokes emission is due to a sequential absorption of multiple photons and energy-transfer steps between lanthanide ions within an inorganic crystalline material.34 Later, the upconversion mechanisms have been ascribed to excited-state absorption (ESA), sequential absorption, energy-transfer upconversion (ETU), or photon avalanche (PA).35–37For the most widely employed ETU mode, an inorganic host matrix is doped with a sensitizer ion that absorbs NIR excitation light and an activator ion that emits light of shorter wavelengths.38 Since the first reports on the bottom-up synthesis of well-defined lanthanide-doped UCNPs about 15 years ago,39–41 their unique physiochemical properties have attracted increasing attention in analytical applications.42 The excellent photostability enables the detection of UCNPs down to a single nanoparticle under continuous high-power excitation.43,44 Moreover, inexpensive and compact laser diodes are efficient for the excitation of UCNPs, rendering potential instruments simple and affordable. UCNPs are also non-toxic, and for example, contrary to quantum dots they do not blink.45
The most efficient UCNPs consist of a host crystal lattice of low phonon energy, such as NaYF4, doped with lanthanide ions, Yb3+ as the sensitizer, and Er3+, Tm3+, or Ho3+ as the activator.38 These nanocrystals are typically synthesized by thermal decomposition in high-boiling organic solvents such as oleic acid, hydrothermal synthesis, or other approaches that yield highly uniform UCNPs of homogeneous size and shape.42 The crystal shape, size, and phase further depend on the type and concentration of dopant ions as well as the ligand-to-solvent ratio.46 In general, a pure crystal phase with minimal lattice defects and a well-defined interdopant distance are required for highly luminescent UCNPs. At synthesis temperatures above +300 °C, the hexagonal phase is obtained, which is about 10-fold more efficient compared to the cubic phase.45 By varying the dopant concentrations of the activator and sensitizer, the emission colours and their intensities can be adjusted,47 which is particularly important for multiplexed applications.48
A thorough characterization of these parameters is indispensable to improve their brightness, which is defined as the product of the absorption cross-section at the excitation wavelength and the emission quantum yield.49 Both the absorption cross-section of lanthanide ions and the quantum yields of UCNPs, however, are low compared to organic fluorophores.50 Furthermore, it is challenging to quantify the quantum yields accurately because they depend on the excitation power density. Standardized quantum yield measurements, e.g., by an integrating sphere setup, are therefore necessary to improve the luminescence efficiency of UCNPs.49 A round ribbon study was performed by Bonnet and colleagues to compare the upconversion efficiency of UCNPs synthesized by different groups.51
Despite many attractive properties, some inherent limitations have hampered the wide use of UCNPs. In particular, the luminescence of UCNPs is much weaker compared to the respective bulk materials because surface defects and the presence of high-energy vibrational modes—in particular from water—on the nanoparticle surface as well as inside the nanoparticle lead to luminescence quenching effects.42,52 The latter problem has been solved in a seminal study53 by preparing anhydrous rare-earth acetates for the synthesis of UCNPs, which resulted in UCNPs that were almost as bright as the bulk materials.
Surface quenching effects have been thoroughly addressed by the synthesis of core–shell nanoparticles, which can enhance the upconversion luminescence by a factor of 104 compared to UCNPs consisting only of the core.54 Simple passivating shells consist of the undoped core material (e.g., NaYF4) to separate the excited sensitizer and activator ions from the quenching centers at the nanoparticle surface and thus confine energy migration steps within the core.55 Active shells, by contrast, confine different types of dopant ions in separate shell layers to increase the dopant–dopant distance and avoid cross relaxation between them.56 This scheme has been employed, for example, for the separation of sensitizer (Yb3+) and activator (Er3+) ions.57 By separating the Yb3+ sensitizer ions in the core from the Er3+ activator ions in the shell, surface quenching effects of the sensitizer were strongly reduced. More commonly known, however, is the use of a Nd3+ layer to shift the excitation wavelength to 800 nm,58,59 which is less strongly absorbed by water than 980 nm light, leading to lower heating effects and thus better biocompatibility. In the Nd transfer cascade, the Nd3+ ions serve as the primary sensitizers that absorb 800 nm light, and Yb3+ bridges the energy transfer to the activator ions (Er3+, Tm3+, and Ho3+).
The synthesis of UCNPs,60 the understanding and enhancing53 of the upconversion process have been important research focuses and are a prerequisite for the development of novel biosensing schemes. Additionally, the preparation of water-dispersible UCNPs and the further bioconjugation is still challenging because the hydrophobic layer of oleic acid on the surface of UCNPs requires subsequent surface modification steps to yield a hydrophilic surface composition before these UCNPs can be employed in bioanalytical applications.61
Surface modification strategies for the generation of UCNP-labels
The best syntheses yielding high-quality and monodisperse UCNPs rely on high-boiling solvents. These synthesis strategies result in UCNPs capped with a hydrophobic layer of oleic acid. For analytical applications, however, it is necessary to render the UCNPs dispersible in aqueous buffers and further functionalize them with ligands, such as antibodies, to specifically target the analyte in the sample. Furthermore, these applications require stable UCNP dispersions that do not aggregate or precipitate over long time periods, avoid matrix effects and reduce non-specific interactions, for example, with plastic wells of microtiter plates or paper strips used for lateral flow assays. Since both the optical and instrumental background as well as the non-specific surface interactions contribute to the overall background signal, the need to avoid non-specific binding of the UCNP-label becomes the more pressing, the more optical background interferences are cleared by the unique photophysical features of UCNPs. Two routes of surface modification can be distinguished: (1) encapsulation techniques, where the original surface ligand remains in place and is covered by an additional layer of ligand, and (2) ligand exchange techniques, where oleic acid is replaced by a new ligand.Encapsulation strategies include coating by a silica shell,62 exploiting the hydrophobic interactions between oleic acid and amphiphilic polymers,63 layer-by-layer deposition of polyelectrolytes,64 as well as local photopolymerization of a polymer shell around.65 Additionally, oleic acid can be oxidized to obtain functional groups for further conjugation steps.66 In one case, a molecularly imprinted polymer (MIP) was directly prepared on dried UCNPs. The polymer with integrated UCNPs was subsequently ground into smaller fragments and used for the detection of food contamination.67
Ligand exchange is facilitated by a range of small molecules such as citric acid and cucurbit[7],68 or polymers such as poly(acrylic acid) (PAA) or DNA69 that coordinate to the positively charged lanthanide ions on the surface of UCNPs. A stronger coordination of the new ligand, cooperative interactions of multidentate ligands as well as mass action may all contribute to the ligand exchange reaction. However, most hydrophilic ligands—even when applied in large excess—cannot displace oleic acid completely from the surface of UCNPs. Therefore, oleic acid is typically removed first via an intermediate step. Either the solution is acidified (pH < 4) to protonate the oleic acid and thus reduce its ability to coordinate to the surface lanthanide ions,70 or nitrosonium tetrafluoroborate (NOBF4) is added to replace oleic acid.71 These so-called ligand-free UCNPs are actually covered by the weakly coordinating ligands H3O+ or BF4−, respectively, and are stable in polar solvents, such as water and dimethylformamide (DMF).
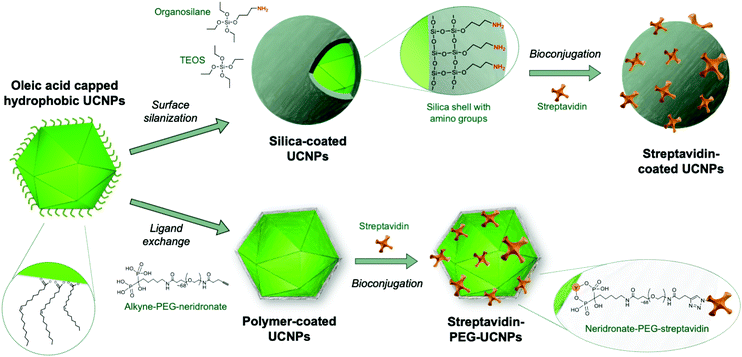
The large number of surface modification strategies investigated were comprehensively reviewed earlier,72 and the reader is referred to Andresen et al. (2019)38 for a more recent overview. Here, we focus on silica coating as an important example of encapsulation techniques as well as ligand exchange reactions involving bifunctional polymers (Fig. 1) because these techniques have shown to be most promising and are currently widely employed for generating labels in analytical applications. Both methods provide the ability to integrate functional groups that can be subsequently used for the attachment of antibodies or other analyte-specific ligands.
Silanol and other charged surface-exposed groups that are covalently anchored in the silica shell enable the separation of silica-coated UCNPs by agarose gel electrophoresis.62,74 In this way, monodisperse UCNPs can be distinguished and purified from distinct clusters of 2, 3 or more UCNPs (Fig. 2). However, in general, the silica shell on UCNPs is prone to non-specific binding to proteins or cells,75 which may interfere with possible applications in food analysis. It has also been described that a protein corona readily forms on the surface of silica nanoparticles.76 To preclude the random formation of a protein corona, a further layer of blocking proteins, such as bovine serum albumin (BSA), can be intentionally placed on top of the silica shell to achieve a repellent effect against non-specific binding of matrix proteins.74 Due to these limitations, there has been a shift from the use of silica encapsulation to ligand exchange reactions.
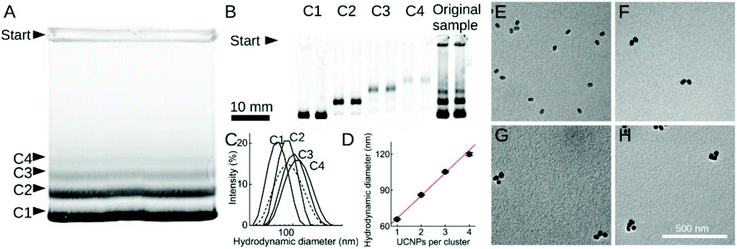 | ||
| Fig. 2 Size fractionation of silica-coated UCNP. (A and B) Agarose-gel electrophoresis of (A) non-purified sample and (B) samples C1–C4 isolated from the gel in (A). (C) DLS measurements of non-purified sample (dashed line) and of samples C1–C4. (D) Hydrodynamic diameter increasing linearly with the number of UCNPs per cluster. (E–H) TEM images of purified samples C1–C4. Reprinted with permission from Hlaváček et al. (2019).74 | ||
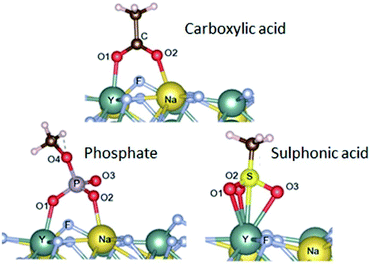 | ||
| Fig. 3 Adsorption configurations (and energies) calculated for carboxylate (−77.9 kcal mol−1), sulfonate (−80.0 kcal mol−1) and phosphate groups (−90.4 kcal mol−1). Reproduced from Duong et al. (2018)77 with permission from the Royal Society of Chemistry. | ||
In addition to high adsorption energies, it should be noted that the binding strength further increases with the number of coordination sites of the ligand. This has been exploited by using multidentate polymers.80 For example, PAA contains several carboxylic groups; some of these bind to the nanoparticle surface, while others are available for subsequent conjugation step with antibodies.81 Yet, another strategy uses the non-toxic (polyethylene) glycol (PEG) polymer to obtain stable colloids in aqueous buffers and low non-specific surface binding.82 In a series of experiments, PEG was equipped with one, two or four phosphate or phosphonate group(s) that bind specifically to the surface of UCNPs.83–85 The tetraphosphonate ligand anchored the PEG polymer most efficiently and prevented precipitation or aggregation of UCNPs even at high pH and in phosphate buffers over extended periods of time.
Kostiv et al. (2017)86 introduced a straightforward route to obtain multidentate phosphonate ligands by coupling the bisphosphonate neridronate to PEG. Our subsequent work showed that this approach is readily applicable to the design of heterobifunctional PEG linkers, where bisphosphonate binds to the UCNP surface while the other end carries a functional group amenable for subsequent conjugation steps.87 For example, an alkyne group enabled the conjugation of azide-modified antibody or streptavidin via a so-called click reaction.88 A direct conjugation of antibodies to UCNPs is typically required for multiplexed applications.48 By contrast, streptavidin-UCNP conjugates constitute generic labels that bind to of any type of biotinylated detection antibody, for example in immunoassays.89
In summary, the colloidal stability of UCNPSs—as well as other nanoparticles—is determined on the one hand by the binding strength of the ligand to the nanoparticle surface and on the other hand, by the realization of dispersibility, either through electrostatic repulsion between charged surface groups or through ligand solvability in aqueous buffers.90
Biosensing configurations using UCNPs
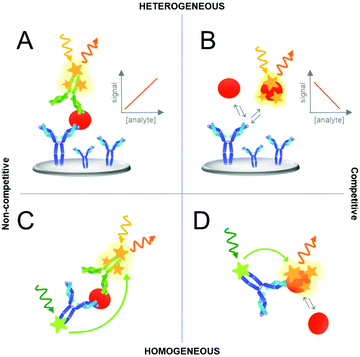
Various assay concepts or formats have been developed for analyte detection based on UCNPs or other labels. Fig. 4 represents a schematic of the most common assay formats used in immunoassays although the same concepts can be further applied to other biosensors or bioaffinity assays using different recognition elements, such as aptamers. Standard immunoassays can be categorized as “heterogeneous assays” because they involve a solid support where one of the assay components is immobilized. In heterogeneous assays, the washing steps reduce matrix effects, separate the unbound from bound detection reagents and thus the background signal of non-specific binding. By contrast, in “homogeneous assays” a change or modulation of the signal is observed as a result of analyte binding and no washing steps are required. In such simple “mix-and-measure” assay, the sample is added to a solution containing the detection reagents and a signal change directly indicates the presence of the analyte. Homogeneous assays are typically implemented using two different labels that interact only when in close proximity. The most important example of such interactions between two labels is Förster resonance energy transfer (FRET).91Another important distinction of immunoassays can be made based on a competitive and non-competitive analyte detection, both either in a heterogeneous or homogeneous assay format as illustrated in Fig. 4. A competitive, or reagent-limited, assay is based on the competition of the target analyte with a labeled analyte analog (tracer) for a limited number of binding sites. If the analyte is present, the signal decreases because the tracer is removed. By contrast, in non-competitive immunoassays, most importantly the sandwich immunoassay based on two antibodies, the detection label binds to the analyte. Therefore, the signal increases with the amount of analyte. It should be noted that these categories are generic and many subdivisions and mixed assay formats have been described.
Applications of UCNPs to food safety monitoring
Pathogenic bacteria
Microorganisms play a prominent role in the burden of foodborne illnesses despite the fact that many microbiological aspects of food safety have been studied for many decades. The most common pathogenic bacteria in food include, for example, Escherichia coli, Salmonella, Listeria monocytogenes, Campylobacter jejuni, and Clostridium perfringens. While common E. coli is harmless, pathogenic groups, such as enterohemorrhagic E. coli (EHEC), which produce Shiga toxins, are often involved in foodborne outbreaks and can cause serious health issues. Salmonella species are widely dispersed in nature and can cause gastrointestinal illness as well as typhoid fever, whereas C. jejuni is among the most important human bacterial enteric pathogens. Listeria is one of the main causes of death from foodborne illnesses owing to the severity of the disease it causes and a high case-fatality. Some species of Clostridium, mostly C. botulinum, are notorious producers of neurotoxins, which can cause a neuroparalytic disease known as botulism.15,92 Traditional culture-based methods for the detection of these pathogens are widely used as they are relatively cost-effective and have good sensitivity. Nevertheless, such techniques are severely restricted by lengthy analysis times—cell cultures and subsequent identification can take from several days up to a week, as in the case of L. monocytogenes.15,93 Thus, advances in biosensing and other technologies have made the identification and analysis of pathogens simpler, faster, and in many ways more convenient than traditional methods.94 ELISA, together with other assays and sensors, have become essential for the fast and simple screening of pathogenic bacteria, especially when costly and bulky equipment, expensive reagents, and trained personnel are not available. UCNPs present an intriguing alternative for such applications where their advantages, such as high chemical stability and practically infinite shelf-life, are of great value.In recent years, lateral flow assays (LFA) have been reported using antibodies as the recognition elements and UCNPs as the detection label for simple pathogen detection. LFAs for two different serotypes of Vibrio cholerae in one lateral flow strip resulted in a sensitivity between 104 and 105 cfu mL−1, although a preincubation step of 7 h was seen to improve the sensitivity down to 10 cfu mL−1.95 An example of a multiplex LFA was reported for a total of 10 foodborne pathogens using a system were 10 lateral flow strips were integrated. The strips overlapped at the sides of the sample pad and the liquid could be distributed synchronously and uniformly into the 10 channels. Detection limits between 104 and 105 cfu mL−1 were measured.96
For multiplex biosensing of bacterial pathogens, a combination of UCNPs and quantum dots (QDs) with a dual-excitation strategy has also been utilized. For example, Salmonella typhimurium and Staphylococcus aureus were detected using QDs and UCNPs functionalized with bacteria-specific aptamers. Furthermore, magnetic beads comprising short DNA sequences that were partially complementary to the aptamer sequences were used for separating the unbound label. The dual-excitation strategy could minimize the cross-talk between the luminescent signals for multiplexed detection, and detection limits of 16 and 28 cfu mL−1 for S. aureus, and S. typhimurium, respectively, were measured in the duplex assay.97 Further multiplexing of up to four targets revealed LODs between 12 and 28 cfu mL−1 for L. monocytogenes, S. aureus, S. typhimurium, and Pseudomonas aeruginosa using two UCNPs and two QDs in the same assay.98 The authors pointed out that further extending of the multiplex detection to five analytes in the system was hindered by significant signal crosstalk of the QDs due to the emission broadening upon oligonucleotide functionalization.
A different approach for the detection of multiple bacteria was established using a series of different functional UCNPs leading to electrostatic interactions between UCNPs and bacteria and subsequent upconversion luminescence enhancement.99 For example, seven pathogenic bacteria were detected simultaneously using guanidine-functionalized UCNPs (UCNP@GDN). In this approach, the strong positive charge of the guanidine group with two parallel hydrogen donor sites could be used as the recognition element for the detection of bacteria with a net negative surface charge. The electrostatic interaction or hydrogen bond interaction between the bacteria and the UCNP@GDN caused an enhancement of the emission, and detection of E. coli, Salmonella, Cronobacter sakazakii, Shigella flexneri, Vibrio parahaemolyticus, S. aureus, and L. monocytogenes could be established with a linear range from 103 to 108 cfu mL−1. In addition, tannic acid, which can be oxidized by hydrogen peroxide, could be used to stabilize and improve the sensitivity of the sensing system (Fig. 5).100
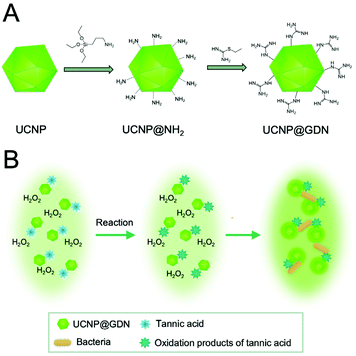 | ||
| Fig. 5 Detection of seven pathogenic bacteria was established using guanidine-functionalized UCNPs (UCNP@GDN). (A) Schematic representation of synthesis for UCNPs@GDN nanomaterials and (B) schematic of the detection principle based on the interaction between the bacteria and UCNPs. Figure adapted from Yin et al. (2019).100 | ||
Alternatively, different functional groups on the surface of the UCNPs have been utilized to differentiate pathogenic bacteria with different phenotypes. For the detection of seven common foodborne pathogenic bacteria, including two Gram-positive bacteria (S. aureus and L. monocytogenes) and five Gram-negative bacteria (E. coli, Salmonella, Cronobacter sakazakii, S. flexneri and V. parahaemolyticus), UCNPs were synthesized with three distinct surface chemistries. Bacterial cells were seen to enhance the emission intensity of the UCNPs due to the spotlight effect of the cell and the interaction. Since each UCNP could respond to the various species in a unique manner and generate corresponding features luminescence signal, the bacteria could be identified in real samples with 92.1% accuracy.101
Furthermore, a few homogeneous approaches have been established as a rapid and sensitive method for pathogen detection. For this purpose, UCNPs were combined with gold nanoparticles (AuNPs) and nanorods (AuNRs) as energy acceptors (luminescence quenchers). AuNPs or AuNRs were functionalized with aptamers and UCNPs with the corresponding complementary DNA. Thus, aptamer binding to the bacteria led to dissociation from the complementary DNA on the UCNPs, resulting in the recovery of upconversion emission (Fig. 6). The authors claim that the measuring principle is based on FRET, however, recent research confirms that energy transfer to AuNPs is better explained considering it is based on nanosurface energy transfer (NSET).102 The approach enabled detection of E. coli with a detection limit of 3 cfu mL−1 using AuNPs,103 and S. typhimurium with a detection limit of 11 cfu mL−1 using AuNRs.104
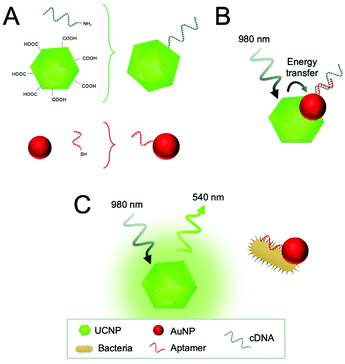 | ||
| Fig. 6 Detection of E. coli using an energy transfer sensing scheme. (A) Carboxyl-functionalized UCNPs are modified with the amino-modified complementary DNA of the aptamer (cDNA) in a condensation reaction and AuNPs with the thiol-modified aptamers through Au–S chemistry. (B) In the absence of bacteria, the cDNA hybridized to the aptamer and energy transfer occurs between the UCNPs and AuNPs. (C) In the presence of target bacteria, aptamers bind preferentially to the bacteria resulting in the dissociation of cDNA and recovery of the UCNP emission. Figure adapted from Jin et al. (2017).103 | ||
Mycotoxins
Mycotoxins are low-molecular weight toxic compounds produced as secondary metabolites by filamentous fungi. Close to 400 compounds, produced by some 350 fungal species, have been recognized as mycotoxins,105 although currently approximately 30 of them are considered a threat to human and animal health because of their toxic effects.106 Owing to the widespread of mycotoxigenic fungi worldwide and the natural origin of these toxins, their presence in foodstuffs is often inevitable107—according to recent surveys even up to 60–80% of crops are contaminated with mycotoxins.108 Aflatoxins are perhaps the most notorious of mycotoxins due to their carcinogenic properties109 and widespread human exposure to high toxin levels.110 Other major classes of mycotoxins include ochratoxins, fumonisins, trichothecenes, zearalenone, and ergot alkaloids.107National and international authorities have established regulatory limits for the most abundant and toxic mycotoxins, which has for its part prompted the development of new analytical methods for their detection. Conventional analytical methods for mycotoxin detection require lengthy extraction procedures, expensive chemical clean-up steps, and use of hazardous materials.107 Whilst chromatographic methods still continue their reign as the reference method of choice for accurate mycotoxin analysis, they have been complemented with bioanalytical methods which can offer a rapid analysis of mycotoxins even within a few minutes.111,112
Several ELISA-based test kits are commercially available for all regulated mycotoxins, and they can serve as an important tool for the first level screening and survey studies because of their simplicity and low cost.113 Furthermore, UCNP-based biosensing can offer interesting alternatives to the conventional ELISA also for mycotoxin detection. Table 1 presents a systematic comparison of selected examples of UCNP-based mycotoxin detection methods and their analytical performance.
| Analyte | Assay format | Recognition element | UCNPs | Measurement | LOD (ng mL−1) | Sample | Ref. |
|---|---|---|---|---|---|---|---|
| Abbreviations: AFB1, aflatoxin B1; AuNR, gold nanorod; BHQ3, black hole quencher 3; cDNA, aptamer complementary DNA; FB1, fumonisins B1; GO, graphene oxide; LOD, limit of detection; MAb, monoclonal antibody; MNP, magnetic nanoparticle; PAA, poly(acrylic acid); OTA, ochratoxin A; SecAb, secondary antibody; UCN, upconversion nanocrystal; ZEA, zearalenone. | |||||||
| AFB1 | Competitive assay using MNPs | MAb | Improved UCNPs modified with the MAb | Photoluminescence spectrometer | 0.001 | Spiked oil | 114 |
| DON | |||||||
| ZEA | Competitive assay with cDNA using MNPs | Aptamer | Silica-coated UCNPs modified with the aptamer | Photoluminescence spectrometer | 0.007 (beer) | Spiked beer and corn | 118 |
| 0.126 (corn) | |||||||
| OTA | Competitive assay with cDNA on lateral flow strip | Aptamer | UCNPs modified with the aptamer | Visual and instrumental | 1.86 (*) | Spiked wheat and beer | 119 |
| AFB1 | Indirect competitive assay with MNPs | Mab | UCN-encoded microspheres modified with the toxin conjugate | Fluorescence microscope and flow cytometer | 9 (*) | Spiked corn powder | 115 |
| Labeled SecAb | |||||||
| OTA | Indirect competitive assay with MNPs | MAb | Blue and red UCN-encoded microspheres modified with the toxin-conjugates | Fluorescence microscope | 0.34 (OTA) | Spiked corn powder | 116 |
| ZEA | Labeled SecAb | 0.41 (ZEA) | |||||
| ZEA | Competitive with cDNA (homogeneous) | Aptamer | UCNP modified with the aptamer (AuNR as energy acceptor) | Photoluminescence spectrometer | 0.01 (ZEA) | Spiked corn | 122 |
| FB1 | 0.000003 (FB1) | ||||||
| OTA | Direct energy transfer (homogeneous) | Aptamer | Core–shell UCNPs modified with PAA and the aptamer (GO as energy acceptor) | Photoluminescence spectrometer | 0.001 | Spiked beer | 120 |
| OTA | Direct energy transfer (homogeneous) | Aptamer | PEG-coated UCNPs modified with the aptamer (BHQ3 as energy acceptor) | Photoluminescence spectrometer | 0.098 | Spiked red wine, grape juice, and beer | 121 |
For example, Chen et al. (2016) developed—what they called—improved UCNPs composed of NaYF4:Yb/Ho/Gd and NaYF4:Yb/Tm/Gd. They optimized the dopant ion concentrations and reaction temperature and modified the UCNPs with a silica coat. Under optimized conditions these particles showed stronger luminescence properties, broader biological applications, and better storage stabilities in comparison with traditional UCNPs. The competitive immunoassay based on magnetic nanoparticles functionalized with toxin–BSA-conjugates and antibody-functionalized improved UCNPs enabled detection of aflatoxin B1 (AFB1) and deoxynivalenol (DON) in a wide range (0.001–0.1 ng mL−1) with the limit of detection of 0.001 ng mL−1.114
Additionally, magnetic nanoparticles have been used in combination with UCNPs for AFB1 detection. A low detection limit of 9 pg mL−1 was achieved using magnetic nanoparticles and upconversion nanocrystals by simultaneous doping into the microspheres by the self-healing encapsulation method.115 Similar approaches with UCNP-encoded microspheres have been reported for simultaneous detection of two or three mycotoxins using multicolour UCNP-encoded microspheres.116,117 The indirect competitive immunoassays were based on carboxylated mesoporous polystyrene beads infused with UCNPs by means of the self-healing encapsulation strategy. Different UCNPs with distinguishable emission spectra were used as optical barcodes for simultaneous detection of ochratoxin A (OTA) and zearalenone (ZEA),116 or OTA, ZEA and AFB1.117 Duplex detection of OTA and ZEA was established using an epifluorescence imaging system with a CCD sensor,116 whereas also a portable device has been described based on a laser diode excitation, magnification with a pocket microscope, and a smartphone-based image analysis which offered reliable and accurate results in less than 1 min.117
While antibody-based methods are the most used in biosensing approaches, several examples of the use of aptamers in combination with UCNPs have been reported. For example, another application using magnetic nanoparticles as the solid support took advantage of aptamers for ZEA detection. In this competitive assay, a complementary strand for the aptamer was conjugated to silica-modified UCNPs, and ZEA detection was established in beer and corn samples with detection limits of 0.007 ng mL−1 and 0.126 ng mL−1, respectively.118
Another aptamer-based test was constructed on a lateral flow test strip for visual and instrumental determination of OTA using NaYF4:Yb,Er UCNPs. This assay relied on the competition between OTA and a complementary sequence for OTA-specific aptamer (Fig. 7). Different conjugation strategies for the aptamer immobilization were studied but the best performance in the assay was observed with the aptamer that was conjugated to carboxylated UCNP via an amino group in the 5′ position using carbodiimide crosslinking chemistry (with EDC/NHS). The lateral flow assay could be performed within 15 min and had no serious cross-reactivity with potential interferents. Visible green signals could be observed under the excitation of 980 nm, and OTA was detected in the range from 5 to 100 ng mL−1 with a limit of detection of 1.86 ng mL−1. The authors pointed out that currently the lack of a miniaturized and portable reader for the readout of their methods limits the commercial applications, but they were confident that better sensitivity can be achieved with a reader.119
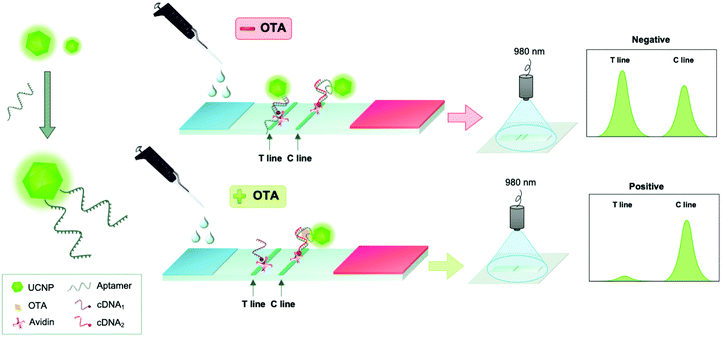 | ||
| Fig. 7 Detection of ochratoxin A (OTA) using an aptamer-based upconversion lateral flow assay. UCNPs were modified with OTA-specific aptamers (left). In the lateral flow assay, competition between OTA and its complementary sequence (cDNA) for OTA-specific aptamer was monitored. Figure adapted from Wu et al. (2018).119 | ||
Alternatively, detection methods based on energy transfer have been used as the basis for mycotoxin analysis using simple wash-free methods. For example, an aptasensor for OTA detection was established using core–shell UCNPs.120 In this work, the OTA aptamer was conjugated to UCNPs, which functioned as an energy donor and graphene oxide (GO) acted as energy acceptor. In close proximity of these two, the energy transfer process resulted in quenching of UCNP emission, and OTA was detected with an LOD on 0.001 ng mL−1. Another similar aptasensor used black hole quencher 3 (BHQ3) as the acceptor. In this case, energy transfer occurred between the red-emitting UCNPs and BHQ3 due to the overlap between the UCNP emission and the BHQ3 absorption (Fig. 8). An LOD of 0.098 ng mL−1 was achieved and the method was applied to the analysis of spiked coloured food samples, including red wine, grape juice, and beer with an assay time of only 10 min.121 Alternatively, quenching of UCNPs by gold nanorods—ascribed to the inner filter effect—was utilized for the detection of ZEA and fumonisins B1 (FB1).122
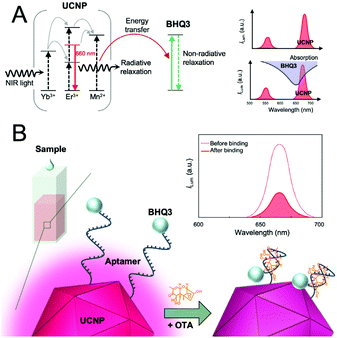 | ||
| Fig. 8 Detection of ochratoxin A (OTA) using a homogeneous aptasensor based on energy transfer. (A) Schematic energy level diagram of Mn2+-doped UCNPs (left) and BHQ3 (middle). Spectral overlap (lower right): the absorption spectrum of quencher (BHQ3) and the upconversion luminescence spectrum of the UCNPs. (B) Schematic illustration of the aptasensor using aptamers linked to UNCP and BHQ3. Strong upconversion luminescence is observed in the absence of OTA, however, weak luminescence is obtained in the presence of OTA that forms a G-quadruplex–OTA complex. Figure adapted from Jo et al. (2018).121 | ||
Pesticides
According to WHO, in 2018 more than 1000 pesticides were used worldwide to prevent pest damage or destruction of food. The toxicity of these chemicals depends, among other factors, on their function. For example, in humans the toxicity of herbicides is usually lower than that of insecticides. But the toxicity also depends on the dose and the route of exposure (skin, ingesta, etc.). Their adverse effects are observed above a certain level of exposure that needs to be strictly regulated and controlled. Therefore, in order to protect public health and comply with legislation worldwide, sensitive analytical methods are required to monitor the presence of pesticide residues in food and water.123 Literature on this subject is quite extensive and different techniques and sample preparation methods have been reported for the analysis of pesticides in food.124–129 Selected examples of the application of UCNPs in this area are discussed below.Organophosphorus (OP) pesticides, such as methylparathion, monocrotophos and dimethoate, were detected in fruits by monitoring the variation of the luminescence emission of a solution containing UCNPs mixed with AuNPs in the presence of acetylcholinesterase (AChE) and acethylcholine (ATC).130 The enzyme catalyzed the hydrolysis of ATC into thiocholine (TCh) that disrupted the electrostatic interactions between the UCNPs and the AuNPs, inducing their aggregation, with the corresponding luminescence recovery in the system. In the presence of the OP pesticide, the enzymatic activity of AChE was inhibited and the UCNP emission was quenched, obtaining a linear relationship between the inhibitory effect of the pesticide on the enzymatic activity and the logarithm of the pesticide concentration.
The luminescence of UCNPs coated with branched polyethylenimine (PEI) can be strongly quenched by energy transfer upon coordination of Cu(II) ions to the amino groups of PEI (Cu2+-PEI-UCNPs). This interaction has been exploited by Wang et al. (2019)131 for the development of AChE-based luminescence “off–on–off” biosensors for the analysis of OP pesticides in agricultural samples. In this approach TCh, the product of the enzymatic reaction, displaced the Cu(II) ions from the UCNPs–Cu2+ complex and the luminescence intensity was recovered. In the presence of diazinon, the enzymatic activity was inhibited and the signal was quenched. A detection limit of 0.05 ng mL−1 was obtained for the analysis of the OP with a linear detection range from 0.1 to 50 ng mL−1. Liu et al. (2019)132 also described the use of Cu2+-PEI-UCNPs to develop a luminescence “off–on” sensing approach for the colorimetric and fluorometric analysis of glyphosate with detection limits of 9.8 ng mL−1 and 1 μg mL−1, respectively. In the presence of H2O2 and as a result of the catalytic action of Cu(II) ions, the reagent 3,3′,5,5′-tetramethylbenzidine (TMB) was oxidized to a blue product, showing a spectral overlap with the emission peak of the UCNP at 660 nm and the luminescence was quenched by an inner filter effect. Glyphosate forms stable complexes with the Cu(II) ions inhibiting the catalytic activity of the Cu(II) ions and the luminescence intensity increased linearly with the pesticide concentration. These methods have the drawback that they require relatively long incubation times (30–60 min) at controlled temperatures.
Different sensing schemes have also been described using aptamers as recognition elements in combination with UCNPs. Hu et al. (2016)133 reported a method for the detection of acetamiprid, a neonicotinoid insecticide, in which the aptamer interacted with AuNPs preventing their NaCl-induced aggregation and the luminescence intensity of UCNPs added to the solution was quenched by the AuNPs. Upon interaction with the pesticide, the aptamer changed its conformation from random coil to a hairpin structure and was released from the AuNPs that aggregated in the presence of NaCl, with the corresponding increment in the luminescence of the UCNPs. Variations in the aggregation extend of the AuNPs could also be followed by colorimetric measurements. The assay was simple and did not require complicated derivatization steps.
Alternatively, aptamers have been immobilized on the surface of AuNPs by hybridization to a complementary ssDNA covalently attached to the NP surface.134 The functionalized AuNPs (dsDNA-AuNPs) aggregated in the presence of and the colour of the solution changed from red to purple. In the presence of acetamiprid, the aptamer bound to the insecticide and dissociated from the AuNP increasing the stability against salt-induced aggregation and the colour turned red. A signal amplification was achieved by adding carboxylated silica-coated NaYF4:Yb,Er modified with random DNA (DNA-UCNPs). In the presence of acetamiprid, the luminescence ratio at 540 nm and 650 nm increased with the insecticide concentration due to an inner filter effect between the DNA-UCNPs and dsDNA-AuNPs, resulting in ten-fold decrease in the detection limit in comparison with the previous approach (0.36 nmol L−1vs. 3.2 nmol L−1). Acetamiprid has also been detected in food samples using a selective aptasensor and a complementary DNA probe attached to streptavidin-coated magnetic nanoparticles and UCNPs, respectively, with similar detection limits (0.65 μg L−1) but a simplified measuring protocol.135
Recently, an aptasensor for the detection of malathion has been reported using malathion-specific aptamers, a cationic polymer, poly dimethyl diallyl ammonium chloride (PDDA), AuNPs and carboxyl-modified UCNPs (NaYF4:Yb,Er).136 In the absence of the pesticide, the aptamer was bound by electrostatic interactions to PDAA and as the AuNPs were negatively charged the luminescence of the UCNPs was not affected. However, when malathion was added to the sample, the aptamer bound to the pesticide and free PDDA was adsorbed on the surface of the AuNPs, changing their surface charge and inducing an effective NSET with the UCNPs.
In a different approach, NaYF4:Yb3+,Tm3+@NaYF4 core–shell UCNPs were coated with Tween-20 to increase the water solubility and to interact with the long alkyl chains of an oxime-based pesticide probe (4-hydroxyl-3-oxime-N-dedocyl-1,8-naphthalimide, HODN).137 The luminescence of the UCNPs was almost completely quenched, but the signal at 803 nm remained unchanged and could be used as a reference for the ratiometric detection (I475 nm/I803 nm) of the pesticide. Upon addition of N,N-diisopropylethylamine (DIEA), the oximate groups of HODN were deprotonated and reacted with the pesticide mimic diethyl chlorophosphate (DCP) or the OP pesticide dimethoate, with the formation of a derivative that showed almost no absorbance at 475 nm, resulting in the suspension of the energy transfer effect between the Tween-20-UCNPs and HODN and to the luminescent recovery of the UCNPs. The method could be applied to the detection of dimethoate with a detection limit of 0.14 μmol L−1, although no real application was described in the work. The method did not show cross-reactivity with other pesticides such as chlorpyrifos or isoprocarb, unless high concentrations were tested (5 mmol L−1).
The blue and green emissions of white-light emitting upconversion nanoparticles (WL-UCNPs) can be effectively quenched, through an energy transfer mechanism, when they are functionalized with the coordination complex formed between Pb(II) ions and dithizone (DZ), while the red emission remains unchanged.138 In the presence of the pesticide thiram, this complex was easily dissociated due to the stronger coordination of the thiol groups in thiram than the nitrogen groups in dithizone. However, the new complex did not quench the luminescence of the WL-UCNPs and as the concentration of thiram in the sample increased a variation in the colour of the luminescence from red to cyan and then to white could be observed with bare eye. This measuring platform was transferred to a test paper format by immobilizing a solution of Pb (DZ)2-UCNPs on a filter paper and using a smartphone-based detection platform to monitor the colour changes achieving a detection limit of 0.4 μmol L−1 for thiram in aqueous samples.
Competitive immunoassays for the detection of pesticides, such as imidaclothiz139 have also been reported using amino-functionalized silica-coated UCNPs modified with the selective antibody and AuNPs bound to the coating antigen. The measuring principle was based on the inner filter effect, with the UCNP as the donor and the AuNP as the absorber. The method showed a 3-fold better sensitivity than that provided by ELISA or a fluorescent polarization immunoassay. Immunoassays based on UCNPs modified with the anti-pesticide antibody and magnetic NPs functionalized with the coating antigen have also been reported for atrazine.140
LFAs have also been implemented for the simultaneous detection of OPs, such as parathion, methyl-parathion and fenitrothion,141,142 or the herbicide 2,4-dichlorophenoxyacetic acid (2,4-D) and fenitrothion,142,143 replacing the classical colloidal gold nanoparticles by UCNPs coated with broad spectrum141 or selective monoclonal antibodies and immobilizing the antigen in the test zone. These approaches have enabled qualitative or semi-quantitative detection of these compounds within short analysis times.
Furthermore, an imprinted nanoprobe has been reported for the analysis of acetamiprid using encapsulated UCNPs, modified with tetraethoxysilane (TEOS) and [3-(methacryloyloxy)propyl] trimethoxysilan (MPS), as the signal readout.144 The polymer was prepared with the pesticide as template molecule, methacrylic acid as functional monomer, and ethylene glycol dimethacrylate (EGDMA) as the cross-linker. The luminescence intensity of the UCNPs@MIP at 542 nm was effectively quenched in the presence of the target compound. A similar approach has been reported for the analysis of antibiotics.145Table 2 summarizes examples of UCNP-based methods for pesticide detection.
| Analyte | Assay format | Recognition element | UCNPs | Measurement | LOD (ng mL−1) | Sample | Ref. |
|---|---|---|---|---|---|---|---|
| Abbreviations: AT, atrazine; AChE, acetylcholinesterase; ACM, acetamiprid; cDNA, complementary DNA; DZ, diazinon; 2,4-D, 2,4-dichlorophenoxyacetic acid; ET, energy transfer; FNT, fenitrothion; GPO, glyphosate; IFE, Inner filter effect; IMI, imidaclothiz, LOD, limit of detection; MAb, monoclonal antibody; MIP, Molecularly imprinted polymer; MNP, magnetic nanoparticle; MT, malathion; PAA-UCNPs, poly(acrylic acid) capped UCNPs; PDDA, polydimethyldiallyl ammonium chloride; Pb(DZ)2, Pb-dithizone complex; PRT; parathion; MPRT, methyl-parathion; PEI-UCNPs, polyethyleneimine capped UCNPs; PMAO, poly(maleic anhydride-alt-1-octadecene); PS-AT-OVA, polystyrene particles functionalized with atrazine-ovoalbumin.a nmol L−1.b IC50.c Pesticide concentration causing a complete invisibility of the test line. | |||||||
| DZ | Enzyme inhibition (ET) | AChE | PEI-NaGdF4:Yb/Tm UCNPs coordinated to Cu(II) ions | Photoluminescence spectrometer | 0.05 | Apples, pears, and green tea | 131 |
| GPO | Direct (ET + IFE) | Cu(II) | PEI-NaGdF4:Yb,Er UCNPs coordinated to Cu(II) ions | Photoluminescence spectrometer | 9.8 | Tea | 132 |
| ACM | Direct (IFE) | Aptamer | AuNPs with cDNA hybridized to the aptamer, silica coated COOH-NaYF4:Yb,Er UCNPs modified with a random DNA | Photoluminescence spectrometer (I654/I540 ratio) | 0.36a | Celery leaves, tea | 134 |
| ACM | Competitive with cDNA and aptamer-MNPs | Aptamer | Streptavidin coated UCNPs and MNPs modified with cDNA and the aptamer, respectively | Photoluminescence spectrometer | 0.65 | Pear, apple, wheat and cucumber | 135 |
| MT | Competitive with PDDA | Aptamer | COOH-NaYF4:Yb,Er UCNPs and AuNPs | Photoluminescence spectrometer | 1.42a | Matcha | 136 |
| Thiram | Direct (energy transfer) | Pb(DZ)2 | PMAO coated NaGdF4:Yb/Tm@NaGdF4:Tb/Eu@NaYF4 core/shell/shell UCNPs functionalized with Pb(DZ)2 | Photoluminescence spectrometer | 0.26a | Apple | 138 |
| IMI | Competitive assay | MAb | SiO2 coated NH2-NaYF4:Yb,Er UCNPs functionalized with the antibody, AuNPs coated with the antigen | Photoluminescence spectrometer | 2.1 | Pear, rice, apple, tomato, pakchoi, cabbage | 139 |
| AT | Competitive assay | MAb | PAA-UCNPs coated with the anti-atrazine Ab Mab and PS-AT-OVA | Photoluminescence spectrometer | 0.002 | Sugar cane juice | 140 |
| 0.02 | Corn and rice | ||||||
| PRT MPRT FNT | Competitive assay | MAb | Carboxylic UCNPs coated with the antibody via EDC/sulfo-NHS | Strip reader | 3.44 (PRT)b | Orange, cucumber, tomato | 141 |
| 3.98 (MPRT)b | |||||||
| 12.49 (FNT)b | |||||||
| 2,4-D | Competitive assay | MAb | Carboxyl-modified β-NaYF4:Yb,Er coated with the antibody via EDC/sulfo-NHS | — | 5 (2,4-D)c | Pear, apple, cucumber, tomatoes, rice, millet | 142 |
| FNT | 11 (FNT)c | ||||||
| 2,4-D | Competitive assay | MAb | Carboxyl-modified β-NaYF4:Yb,Er coated with the anti 2,4-D MAb via EDC/sulfo-NHS and PEI-modified β-NaY(Gd)F4:Yb,Er coated with the anti FNT MAb Ab via GA | — | 5 (2,4-D)c | — | 143 |
| FNT | 12 (FNT)c | ||||||
| ACM | Direct | MIP | SiO2 coated NaYF4:Yb,Er NPs as core and MIP as shell | Photoluminescence spectrometer | 8.3 | Apple, strawberry | 144 |
Antibiotics
Antibiotics have been broadly applied for the prevention and treatment of human and animal diseases since their discovery in 1928. In the 1950s, these pharmaceuticals began to be applied as feed supplements, due to their favourable effects on the growth and performance of farm animals. Consequently, the emergence of antibiotic resistant microbes prompted the European Union to ban their application as growth promoters in animal feed in 2006 and limit their use only for veterinary purposes.146 The presence of antibiotic residues in food can have a toxic effect on the consumer and they can also cause allergic reactions or disturb the digestive function. The prolonged intake of low concentrations of these drugs can lead to the development of resistance to the antibiotics commonly used in human or veterinary medicine.147–149 Nowadays, reduced investments in antibiotic research and development, the misuse of these pharmaceuticals in human and veterinary medicine, and the release of antibiotic resistant bacteria and antibiotic resistant genes in the environment are considered a major global threat for the public health. WHO and FAO have set the maximum residue limits (MRLs) for residues of drugs in foods,150 and there is a demand for sensitive analytical methods to monitor their presence in food and feed. Without a doubt, liquid chromatography coupled with triple quadrupole tandem mass spectrometry (LC-MS/MS) is the most widely used analytical technique for the qualitative and quantitative analysis of a large number of antibiotic residues simultaneously in food products.147,151 However, especially with the advent of nanotechnology, biosensors and bioassays have emerged as an alternative for more sophisticated analytical techniques to enable simple, low-cost, on-site, high-throughput, and sensitive screening analysis of antibiotics in food samples.152,153 Some selected examples of the application of UCNPs in this area are discussed below and summarized in Table 3.| Analyte | Assay format | Recognition element | UCNPs | Measurement | LOD (ng mL−1) | Sample | Ref. |
|---|---|---|---|---|---|---|---|
| Abbreviations: CAP, chloramphenicol; CLEN, clenbuterol; ENR, enrofloxacin; LOD, limit of detection; MAb, monoclonal antibody; MIP, molecularly imprinted polymer; MNP, magnetic nanoparticle; NOR, norfloxacin; OTC, oxytetracycline; PAA-capped UCNPs, poly(acrylic acid) capped UCNPs; PPs-NOR-OVA, polystyrene particles functionalized with coating antigen; SLF, sulfadimethoxine; SQX, sulfaquinoxaline; TET, tetracycline.a Lower antibiotic concentration leading to the appearance of a luminescent signal in the T-Line.b μg kg−1.c μmol L−1. | |||||||
| SQX | Competitive assay with UCNPs and AuNPs | MAb | PAA-caped UCNPs modified with SQX-OVA | Portable analyzer | 1 (buffer)a | Shrimp, chicken | 155 |
| 8 (food)a,b | |||||||
| NOR | Competitive assay | MAb | PAA-caped UCNPs coated with the MAb | Photoluminescence spectrometer | 2 (buffer)a | Milk | 156 |
| 10 (milk)a | |||||||
| NOR | Competitive assay | MAb | PAA-caped UCNPs modified with the antibody | Photoluminescence spectrometer | 0.010 | Spiked milk, honey, chicken, beef, pork, pork liver, and pork kidney, sea bass, squid, shrimp | 157 |
| SQX | Competitive assay with magnetic PPs-SQX-OVA | MAb | PAA-caped UCNPs modified with the antibody | Photoluminescence spectrometer | 0.1 (buffer) | Spiked chicken, beef, pork, milk, sea bass, shrimp | 158 |
| 0.5 (food)b | |||||||
| OTC | Direct energy transfer (homogeneous) | Aptamer | Silica coated UCNPs modified with GA and the aptamer (SYBR Green I as acceptor) | Photoluminescence spectrometer (I530/I477 ratio) | 0.054 | Spiked milk | 159 |
| ENR | Competitive with cDNA and aptamer-MNPs | Aptamer | Streptavidin coated UCNPs and MNPs modified with cDNA the aptamer, respectively | Photoluminescence spectrometer | 0.06 | Spiked fish | 160 |
| TET | Competitive with cDNA and aptamer-MNPs | Aptamer | GA-activated silica-coated UCNPs modified with cDNA, aptamer-functionalized MNPs | Photoluminescence spectrometer | 0.0062 | Spiked milk, pork | 161 |
| SLF | Direct energy transfer (homogeneous) | Aptamer | Streptavidin coated UCNPs and MNPs modified with cDNA the aptamer, respectively | Photoluminescence spectrometer | 0.11 | Spiked perch and catfish | 162 |
| CLEN | Polymer binding | MIP | MIP-coated UCNPs | Photoluminescence spectrometer | 0.12 | Pork and water | 145 |
| ENRO | Polymer binding | MIP | UCNPs and MNPs encapsulate in a silica core and coated with the MIP | Photoluminescence spectrometer | 2.50 × 10−7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
Fish | 163 |
Liu et al. (2014) proposed core–shell NaGdF4:Yb,Er@NaGdF4 NPs as an alternative to AuNPs for the development of LFA for the analysis of cephalexin (CEX).154 The luminescence of the NaGdF4:Yb,Er core was found to be greatly enhanced, as well as conserved better throughout the PEGylation process, in the presence of a NaGdF4 coating. A bifunctional ligand, PEG2000, bearing a maleimide group at one end and a diphosphate group at the other was selected for the functionalization of the nanoparticles and further coupling to the anti-cephalexin monoclonal antibody. The capture agent, CEX–BSA, was immobilized on the test line (T-line) and a bright luminescence was observed in the absence of the antibiotic which was weaker as the concentration of CEX increased. The LOD obtained with the UCNPs was comparable to that achieved with AuNPs. The authors claimed that the low background signal associated with upconversion luminescence would benefit sample analysis, although no real application is described in the work.
Hu et al. (2017)155 compared the performance of two LFAs based on luminescence quenching for the analysis of sulfaquinoxaline (SQX) using alternatively QDs (λexc = 370 nm; λem = 532 nm) or UCNPs (λexc = 980 nm; λem = 542 nm) as the luminescence donors and AuNPs as the acceptors for the energy transfer. In the absence of the antibiotic, the antibody-coated AuNPs bound to the donor particles coated with the SQX-conjugate and the luminescence of the T-line was completely quenched. In the presence of SQX, the luminescence intensity in the T-line increased as less AuNP-labelled antibody was bound to the coating antigen with the corresponding increase in the signal. The visual detection limit in standard solutions, calculated as the lowest antibiotic concentration leading to the appearance of a signal in the T-line, was the same using UCNPs and QDs (1 ng mL−1). However, the use of only AuNPs as labels provided a value ten times higher (10 ng mL−1). Therefore, the use of UCNPs significantly improved the performance of traditional colloidal gold-based LFIAs. A similar comparison was made for norfloxacin (NOR) analysis.156 In this approach, PAA-capped UCNPs were functionalized with the anti-NOR monoclonal antibody and the NOR-conjugate was deposited on the T-line. However, in this application, the visual detection limit obtained using UCNPs was lower (milk samples, 2.5 μL L−1) than with the QDs (10 μL L−1) or the conventional AuNPs (20 μL L−1). The authors attributed this behavior to the unique luminescent properties of these materials that avoid any autofluorescence from the matrix components. Moreover, UCNPs are also more stable in laboratory conditions than the other NPs, and the fact that lower amount of antibody is required for the development of the test strips leads to lower costs.
In another approach, NOR detection in foodstuffs was carried out using PAA-capped UCNPs functionalized with an anti-NOR monoclonal antibody and polystyrene NPs functionalized with the NOR-conjugate.157 The competitive assay was not selective to NOR and showed cross-reactivity to other fluoroquinolones, especially ciprofloxacin, nalidixic acid, and enrofloxacin, due to the broad-spectrum characteristics of the antibody applied in the assay. Sample treatment was relatively simple, milk samples could be analyzed directly without sample treatment and the results showed a good correlation with those obtained by an ELISA kit. The similar approach using polystyrene magnetic microspheres modified with the coating antigen, was applied to the analysis of SQX with LODs of 0.1 μg L−1 in buffer and 0.5 μg kg−1 in food samples.158
As an alternative to antibodies, aptamers have also been used for the analysis of antibiotics in food samples. For example, oxytetracycline (OTC) was detected in spiked milk samples using a biosensing device based on energy transfer between silica-coated UCNPs and SYBR Green I as an acceptor.159 The UCNPs derivatized with glutaraldehyde were functionalized with the OTC selective aptamer and hybridized with the complementary DNA (cDNA) before the addition of the SYBR Green I. The fluorescence of SYBR Green I increases strongly when intercalated in double-stranded DNA. Upon intercalation, the emission of the UCNPs at 477 nm was quenched by SYBR Green I that emits at 530 nm. In the presence of the antibiotic, SYBR Green I was released due to DNA dehybridization, and the I530/I477 ratio decreases with increasing OTC concentrations with an LOD of 0.054 ng mL−1. The aptamer showed no cross-reactivity in the presence of tetracycline and doxycycline, and the results were successfully compared with those obtained by an ELISA kit.
In a different approach, the aptamer was immobilized on the surface of magnetic nanoparticles (MNPs) and the cDNA attached to silica-coated UCNPs derivatized with glutaraldehyde.164 In the presence of chloramphenicol, the target analyte, the complex MNP-aptamer-cDNA-UCNP dissociated and the emission at 542 nm (λexc = 980 nm) decreased with increasing antibiotic concentrations. The authors pointed out that the use of MNPs enabled the antibiotic capture and preconcentration, as well as an amplification of the luminescent signal. Other antibiotics, such as enrofloxacin, tetracycline or sulfadimethoxine, have been detected in foodstuff using the same sensing scheme and the corresponding selective aptamer.160–162
MIPs have also been used as recognition elements for antibiotic analysis in combination with UCNPs.145,163 Tang et al. (2018)163 reported the synthesis of a composite material encapsulating the UCNPs together with MNPs in a sol–gel matrix (MUCPs) followed by photopolymerization of the MIP using the visible light emitted by the UCNPs when excited at 980 nm. The polymer was prepared with benzophenone as initiator, enrofloxacin as template and methacrylic acid as functional monomer (MUCPs@MIP). The average thickness of the MIP layer was tuned by adjusting the polymerization time and the luminescence emission of the (MUCPs@MIP) was quenched in the presence of the antibiotic. The authors hypothesized that the sensing mechanism was based on photo-induced electron transfer. However, the polymer was not selective to enrofloxacin but presented an important cross-reactivity with other fluroquinolone antibiotics, such as fleroxacin, levofloxacin, enoxacin, and ciprofloxacin.
Metal ions
Metals ions and metalloids play an essential role in many biological systems. The main source of these elements in food, including crops, livestock, seafood, and groundwater is natural or because of anthropogenic activities. Thus, human exposure to such inorganic elements through food intake is inevitable and, in the case of the bulk metals (e.g., sodium, potassium, calcium) and some trace metals (e.g., iron, cobalt, selenium), they are of nutritional significance. However, some other metals, such as lead, nickel or mercury, may have toxic effects and are associated with diseases, including cancer or neurological pathologies.165,166The amount of metals which may be present in foodstuff are well established by worldwide legislations, such as EU Regulation 1881/2006 which sets out the maximum levels for certain metals (lead, tin, cadmium, arsenic and mercury) in human food and beverages at low levels, for example 0.01 mg kg−1 for lead and cadmium in some infant formulae.167 Other relevant ions, such as nitrate, nitrite and halogenated anions, such as bromate or fluoride, are also of interest in foodstuff quality control and their natural and exogenous occurrence is also under control.1,150,168 For these reasons, improved analytical techniques and alternative technologies are necessary to guarantee sensitive and reliable ion detection in food. Although inductively coupled plasma-atomic emission spectrometry (ICP-AES) and mass spectrometry (ICP-MS), atomic absorption spectrometry (AAS), graphite furnace atomic absorption spectrometry (GFAAS), and ion chromatography (IC) are broadly used for analysing these metals in food, ion-selective electrodes, and some voltamperometric techniques are commonly used with comparable sensitivity and selectivity.169 UCNP-based sensors have been confirmed as a reliable alternative for the detection of a variety of cations and anions in biosystems and environmental samples;170 however, only a few UCNPs-based assays have been developed for their detection in foodstuff (Table 4).
| Analyte | Assay format | Recognition element | UCNPs | Measurement | LOD | Sample | Ref. |
|---|---|---|---|---|---|---|---|
| Abbreviations: PEI, polyethylenimine; OPD, o-phenylenediamine; AuNR, gold nanorod; NHM, nanohybrid materials; cDNA, aptamer complementary DNA; GO, graphene oxide; LOD, limit of detection; MNP, magnetic nanoparticle; PEI, polyethylenimine. | |||||||
| Ag(I) | Direct energy transfer (homogeneous) | — | PEI-coated UCNPs (OPD as quencher) | Photoluminescence spectrometer | 33 nM | Spiked tap water | 180 |
| Pb(II) | Competitive with cDNA (homogeneous) | ssDNA | UCNP modified with the ssDNA (MNPs-AuNPs as energy acceptor) | Photoluminescence spectrometer | 5.7 nM | Spiked tea and wastewater | 181 |
| Zn(II) | ZIP mRNAs as markers of Zn deficiency | ssDNA | UCNPs modified with the ssDNA(GO as quencher) | Photoluminescence spectrometer and smartphone | — | Barley | 182 |
| Direct assay (homogeneous) | |||||||
| Hg(II) | Competitive with cDNA (homogeneous) | Aptamer | UCNP modified with the aptamer (AuNP as energy acceptor) | Photoluminescence spectrometer | 60 nM | Spiked tap water and milk | 172 |
| F− | Direct energy transfer (homogeneous) | Curcumin | Oleic acid capped UCNPs | Absorbance and luminescence measured with adapted spectrometer | 25 μM (absorbance) | Spiked tap water and milk | 173 |
| 5 μM (luminescence) | |||||||
Homogeneous assays based on UCNPs for the detection of metals and ions in foodstuff and other sample types mostly rely on the exceptionally efficient luminescence quenchers, such as gold, silver or graphene oxide nanomaterials.170,171 For example, Liu et al. (2018) presented a turn-on aptasensor for Hg2+ based UCNPs and AuNPs.172 As shown in Fig. 9, the assay used short-stranded aptamer-coated AuNPs that quenched the emission of light upon hybridization with the corresponding bases of a long-stranded aptamer linked to the UCNPs. In the presence of Hg2+, the long-stranded aptamer folds into a hairpin structure, which is attributed to the T–T mispairs in the aptamer that can selectively capture Hg2+ ions and form T–Hg2+–T base pairs. Thus, folding of the long-stranded aptamer releases the AuNP-modified strands and the luminescence of UCNPs is recovered as the distance between the two nanomaterials increases. Under optimized conditions, tap water and milk samples were analysed with good selectivity and sensitivity, achieving a low detection limit of 60 nmol L−1.
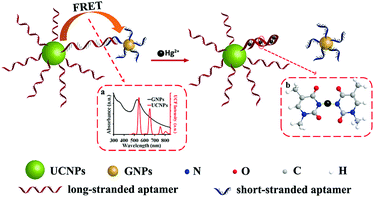 | ||
| Fig. 9 Schematic description of the UCNPs-aptamers-GNPs sensor for Hg2+. Reprinted (adapted) with permission from Liu et al.172 Copyright (2018) American Chemical Society. GNP: gold nanoparticle. | ||
Although recognition elements, such as antibodies or enzymes, are the receptor-of-choice in most UCNP-based sensors for ion detection, aptamers or organic small molecules are the most commonly used. For example, Chen and coworkers173 reported a curcumin-UCNP system consisting of NaYF4:Yb/Er/Tm nanoprobes mixed with the natural herbal supplement to make a dual colorimetric and luminescent approach for the detection of fluoride ion in tap water and milk. The assay principle was ascribed to the effective luminescence quenching of UCNPs by the curcumin through an inner filter effect. When F− reacted with curcumin, a shift of the maximum absorption peak of the dye occurred from 417 nm to 560 nm and the UCNPs emission at 546 nm and 657 nm was attenuated (Fig. 10). Under optimized conditions, the UCNP-curcumin system enabled the quantification of F− in the linear range of 25–200 μmol L−1 (colorimetric) and 5–200 μmol L−1 (ratiometric luminescence) with limits of detection as low as 25 μmol L−1 and 5 μmol L−1, respectively. The selectivity against other common anions (e.g., Cl−, Br−, CN−) was remarkable, although the authors pointed out that the presence of Fe2+ should be avoided since it prevents the formation of the curcumin-F− complex. Finally, the approach was validated through the analysis of real samples such as tap water and milk, with acceptable recoveries and reproducibility (RSD 0.94–22%).
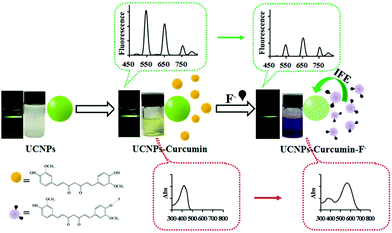 | ||
| Fig. 10 Schematic Illustration of UCNPs–curcumin system response to F−. Reprinted (adapted) with permission from Liu et al.173 Copyright (2017) American Chemical Society. | ||
In the case of these hazardous compounds, a variety of recognition elements used in combination with upconversion technology is quite diverse, highlighting MIPs, aptamers or even covalent organic frameworks (COFs). For example, BPA, 17-b-estradiol, or MCLR has been detected using UCNPs and, all of them, based on the ability of specific nanomaterials to function as quenchers.174,175 An interesting example is the assay reported by Ren et al. (2019) who simultaneously detected BPA and E2 in water, food, and biological samples. The aptasensor relied on a competitive assay between each analyte and its corresponding tetrahedron complementary DNA (T-cDNA) probe, immobilized onto 2D nanohybrids of black phosphorous (BP) and AuNPs, for the specific aptamers. In this case, the BP-Au nanohybrids acted as energy acceptor, allowing sensitive detection of BPA (LOD 7.8 pg mL−1) and E2 (LOD 92 pg mL−1). The authors also claimed that, compared with other sensor approaches, such as QDs or AuNPs, their strategy showed a wider linear range (BPA 0.01–100 ng mL−1 and E2 0.1–100 ng mL−1), faster and better response in complex matrices, although sensitivity is not as good as electrochemical methods.
Other food contaminants
In addition to all the above mentioned contaminants, other hazardous substances may be present in food intentionally added or not. They can be introduced at any stage of the food chain (production, packaging, transport, etc.) or also because of environmental contamination (soil, air, water). These compounds have different origins and they include, for example, anabolic agents (clenbuterol, steroids), adulterants like melamine, additives of packaging materials, such as bisphenol A (BPA), cyanotoxins, global pollutants as explosives, among others. Some of them are controlled by rigorous regulations, for example, anabolic and adulterant compounds, as well as certain pollutants, should comply with the maximum levels set out in food safety regulations and legislation worldwide, such as EU regulations.167,176–178Chromatographic methods, typically based on GC- and LC-MS/MS, are routinely used to quantify these compounds in food samples with highly accurate results. Nevertheless, screening methods, such as ELISAs, have been developed to assess food safety in the early stages, and many have been commercially available for more than a decade. In this context, UCNP-based screening tests are also a new analytical alternative for this kind of substances and some competitive approaches have been reported in recent years (Table 5). For example, Zhou and coworkers (2016)179 developed an LFA for the detection of the natural plant toxin, abrin. In this study, they prepared nine monoclonal antibodies against abrin and identified the best MAbs pairs to obtain a sandwich-based LFA with a remarkable Test/Control signal ratio and good positive response at a concentration of 10 ng mL−1 for the toxin. The performance of the LFA was comprehensively evaluated using both commercially available UCNPs (NaYF4:Yb3+,Er3+) and LFA cassettes, and the results showed that the assay was as rapid and as specific as other conventional gold-based LFAs but very sensitive (0.1 ng mL−1). Even more, very robust responses were observed when analysing real samples, such as cookies, sausages, milk and other complex food matrices.
| Analyte | Assay format | Recognition element | UCNPs | Measurement | LOD | Sample | Ref. |
|---|---|---|---|---|---|---|---|
| Abbreviations: BPA, bisphenol A; E2, 17-β-estradiol; BP, black phosphorous; Au, gold; mAb, monoclonal antibody; UPT, upconverting phosphor technology; MCLR, microcystis LR; PFOS, perfluoroctane sulfonate; COFs, covalent organic frameworks; CLB, clenbuterol; ECL, electrochemiluminescence; EIS, electrochemical impedance spectroscopy; GCE, fluorescence spectrometer; ChOx, choline oxidase; PAA, poly(acrylic acid); TNP, 2,4,6-trinitrophenol; GDN, guanidine; DES, diethylstilbestrol; MIP, molecularly imprinted polymer; LOD, limit of detection; MAb, monoclonal antibody; UCN, upconversion nanocrystal. | |||||||
| BPA | Displacement assay (homogeneous) | Aptamers | UCNPs modified with the aptamer (BP-Au@T-cDNA as energy acceptor) | Photoluminescence spectrometer | BPA: 7.8 pg mL−1 | Spiked water, milk and biological samples | 174 |
| E2 | E2: 92 pg mL−1 | ||||||
| Abrin | Sandwich immunoassay on lateral flow strip | MAb pairs | UCNPs decorated with the MAbs | UPT biosensor lumineter (handmade) | Solid-food samples: 0.5–10 ng mL−1 | Spiked milk powder, flour, white sugar, soft drink juice, beer, water, cookie, soybean, cashew, sausage | 179 |
| Beverages:0.3–0.43 ng mL−1 | |||||||
| MCLR | Competitive assay with cDNA on lateral flow strip | Aptamer | Avidin-coated UCNP@PAA with biotinylated aptamer (MoS2 with cDNA as quencher) | Photoluminescence spectrometer | 0.002 ng mL−1 | Spiked tap and lake water | 175 |
| PFOS | Direct energy transfer (homogeneous) | COFs | Aminated UCNPs core–shell structured with COFs | Photoluminescence spectrometer | 0.15 pM | Spiked tap water, food packaging | 183 |
| Choline | Direct energy transfer (homogeneous) | Choline: ChOx | UCNP/PAA (Fe3+ as energy acceptor) | H2O2 oxidizes Fe2+ to Fe3+ which caused UCNP quenching | Choline: 0.5 μM | Spiked infant formula milk powder | 184 |
| H2O2 | H2O2: — | H2O2: 0.1 μM | |||||
| TNP | Direct energy transfer (homogeneous) | GDN | Silica-capped UCNPs coated with GDN | Photoluminescence spectrometer | 0.78 pM | Spiked beverages | 185 |
| DES | Displacement assay (homogeneous) | MAb | UCNP/PAA coated with BSA-DES conjugate (PDA/BP/MAb-DES as quencher) | Photoluminescence spectrometer | 83 ng mL−1 | Spiked milk, beef, puffer fish | 186 |
Conclusions and future perspectives
The unique optical properties of UCNPs, in particular the ability for background-free optical detection, bestow great potential for food safety and quality monitoring. We have followed the route from the synthesis of bright UCNPs over surface modification strategies to the conjugation of biorecognition elements on the nanoparticle surface. A careful design of these steps is essential for the development of upconversion-based biosensing schemes ranging from microtiter plate assays over lateral flow assays to homogeneous assays. There are examples of various assay schemes that enable the detection of all important food contaminants, such as pathogenic bacteria, mycotoxins, pesticides, antibiotics, metal ions as well as other food contaminants.While new synthesis approaches and core–shell architectures have continuously improved the quantum yield of UCNPs, it is still lower compared to quantum dots or fluorescent dyes. The resulting lower brightness, however, is compensated by the ability to excite UCNPs with high power densities and their detection without optical background interference. With the background approaching zero, UCNPs feature an excellent signal-to-background ratio (S/B), which provides the opportunity to push the limit of detection in food analysis lower and lower—even single analyte molecule detection (digital assays) has become feasible.44 Additional advantages are a rapid assay time, low costs and that little or no sample pretreatment is required. Consequently, such assays are well suited for applications in field or at the food production site. Further contributing to their rapid evolution is the relative ease of implementing such biosensing schemes and the availability of commercial instruments for the upconversion readout that are entering the market. While not all of these new developments will make it into real-world applications and in many cases their reproducibility and accuracy need to be improved, UCNPs have become highly attractive and versatile optical labels that in the long term have the potential to replace conventional detection systems not only in food analysis but also in other fields of analytical and diagnostic applications.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work has been funded by the Spanish Ministerio de Ciencia, Innovación y Universidades (RTI2018-096410-B-C21). H. H. G. acknowledges funding from the Heisenberg Program of the German Research Foundation (DFG, GO 1968/7-1).References
- World Health Organization (WHO), Food Safety and Zoonoses, http://www.who.int/foodsafety, (accessed August 2020).
- World Health Organization (WHO), Foodborne diseases burden epidemiology reference group 2007–2015, WHO estimates of the global burden of foodborne diseases, 2015 Search PubMed.
- S. Jaffee, S. Henson, L. Unnevehr, D. Grace and E. Cassou, The Safe Food Imperative: Accelerating Progress in Low- and Middle-Income Countries, Agriculture and Food Series, World Bank, Washington, DC, 2019, (Available at: https://openknowledge.worldbank.org/handle/10986/30568, accessed September 2020) Search PubMed.
- Food and Agriculture Organization of the United Nations (FAO), Food safety and quality, http://www.fao.org/food-safety, (accessed August 2020).
- Food and Agriculture Organization of the United Nations (FAO), The Future of Food and Agriculture: Trends and Challenges, Food and Agriculture Organization of the United Nations, Rome, 2017 Search PubMed.
- State of the Art in Biosensors – Environmental and Medical Applications, ed. T. Rinken, InTech, 2013 Search PubMed.
- M. Eleftheriadou, G. Pyrgiotakis and P. Demokritou, Curr. Opin. Biotechnol., 2017, 44, 87–93 CrossRef CAS.
- E. B. Bahadır and M. K. Sezgintürk, Anal. Biochem., 2015, 478, 107–120 CrossRef.
- H. Sharma and R. Mutharasan, Sens. Actuators, B, 2013, 183, 535–549 CrossRef CAS.
- B. Van Dorst, J. Mehta, K. Bekaert, E. Rouah-Martin, W. De Coen, P. Dubruel, R. Blust and J. Robbens, Biosens. Bioelectron., 2010, 26, 1178–1194 CrossRef CAS.
- A. Sadana and N. Sadana, Handbook of Biosensors and Biosensor Kinetics, Elsevier, Amsterdam, 2011 Search PubMed.
- R. Peltomaa, E. Benito-Peña and M. C. Moreno-Bondi, Anal. Bioanal. Chem., 2018, 410, 747–771 CrossRef CAS.
- R. Köppen, M. Koch, D. Siegel, S. Merkel, R. Maul and I. Nehls, Appl. Microbiol. Biotechnol., 2010, 86, 1595–1612 CrossRef.
- I. Y. Goryacheva, S. D. Saeger, S. A. Eremin and C. V. Peteghem, Food Addit. Contam., 2007, 24, 1169–1183 CrossRef CAS.
- Food Microbiology: Fundamentals and Frontiers, ed. M. P. Doyle and L. R. Beuchat, ASM Press, Washington, D.C., 3rd edn, 2007 Search PubMed.
- Biosensing for the 21st Century, ed. R. Renneberg and D. Andresen, Springer, Berlin, 2008 Search PubMed.
- H. Kang, L. Wang, M. O'Donoghue, Y. C. Cao and W. Tan, in Optical Biosensors, ed. F. S. Ligler and C. R. Taitt, Elsevier, Amsterdam, 2nd edn, 2008, pp. 583–621 Search PubMed.
- M. R. Willner and P. J. Vikesland, J. Nanobiotechnol., 2018, 16, 95 CrossRef CAS.
- Y. Wang and T. V. Duncan, Curr. Opin. Biotechnol., 2017, 44, 74–86 CrossRef CAS.
- M. G. Panthani and B. A. Korgel, Annu. Rev. Chem. Biomol. Eng., 2012, 3, 287–311 CrossRef CAS.
- S. Handa, Y. Wang, F. Gallou and B. H. Lipshutz, Science, 2015, 349, 1087–1091 CrossRef CAS.
- M. De, P. S. Ghosh and V. M. Rotello, Adv. Mater., 2008, 20, 4225–4241 CrossRef CAS.
- R. A. Petros and J. M. DeSimone, Nat. Rev. Drug Discovery, 2010, 9, 615–627 CrossRef CAS.
- M. Holzinger, A. Le Goff and S. Cosnier, Front. Chem., 2014, 2, 63 Search PubMed.
- P. D. Howes, R. Chandrawati and M. M. Stevens, Science, 2014, 346, 1247390–1247390 CrossRef.
- Z. Farka, T. Juřík, D. Kovář, L. Trnková and P. Skládal, Chem. Rev., 2017, 117, 9973–10042 CrossRef CAS.
- D. Tang, Y. Cui and G. Chen, Analyst, 2013, 138, 981 RSC.
- Z. Zhang, S. Shikha, J. Liu, J. Zhang, Q. Mei and Y. Zhang, Anal. Chem., 2019, 91, 548–568 CrossRef CAS.
- C. Chen, C. Li and Z. Shi, Adv. Sci., 2016, 3, 1600029 CrossRef.
- N. Bloembergen, Phys. Rev. Lett., 1959, 2, 84–85 CrossRef CAS.
- J. F. Porter, Phys. Rev. Lett., 1961, 7, 414–415 CrossRef CAS.
- F. M. Auzel, C. R. Acad. Sci. Paris, 1966, 819 CAS.
- F. Auzel, C. R. Acad. Sci. Paris, 1966, 262, 1016–1019 Search PubMed.
- F. Auzel, Chem. Rev., 2004, 104, 139–173 CrossRef CAS.
- G. Liu, Chem. Soc. Rev., 2015, 44, 1635–1652 RSC.
- F. Wang, R. Deng, J. Wang, Q. Wang, Y. Han, H. Zhu, X. Chen and X. Liu, Nat. Mater., 2011, 10, 968–973 CrossRef CAS.
- E. M. Chan, E. S. Levy and B. E. Cohen, Adv. Mater., 2015, 27, 5753–5761 CrossRef CAS.
- E. Andresen, U. Resch-Genger and M. Schäferling, Langmuir, 2019, 35, 5093–5113 CrossRef CAS.
- S. Heer, O. Lehmann, M. Haase and H.-U. Güdel, Angew. Chem., Int. Ed., 2003, 42, 3179–3182 CrossRef CAS.
- S. Heer, K. Kömpe, H.-U. Güdel and M. Haase, Adv. Mater., 2004, 16, 2102–2105 CrossRef CAS.
- J.-C. Boyer, F. Vetrone, L. A. Cuccia and J. A. Capobianco, J. Am. Chem. Soc., 2006, 128, 7444–7445 CrossRef CAS.
- X. Zhu, J. Zhang, J. Liu and Y. Zhang, Adv. Sci., 2019, 6, 1901358 CrossRef CAS.
- D. Mendez-Gonzalez, E. López-Cabarcos, J. Rubio-Retama and M. Laurenti, Adv. Colloid Interface Sci., 2017, 249, 66–87 CrossRef CAS.
- Z. Farka, M. J. Mickert, M. Pastucha, Z. Mikušová, P. Skládal and H. H. Gorris, Angew. Chem., Int. Ed., 2020, 59, 10746–10773 CrossRef CAS.
- M. Haase and H. Schäfer, Angew. Chem., Int. Ed., 2011, 50, 5808–5829 CrossRef CAS.
- A. Noculak, A. Podhorodecki, G. Pawlik, M. Banski and J. Misiewicz, Nanoscale, 2015, 7, 13784–13792 RSC.
- A. Gulzar, J. Xu, P. Yang, F. He and L. Xu, Nanoscale, 2017, 9, 12248–12282 RSC.
- H. H. Gorris and O. S. Wolfbeis, Angew. Chem., Int. Ed., 2013, 52, 3584–3600 CrossRef CAS.
- U. Resch-Genger and H. H. Gorris, Anal. Bioanal. Chem., 2017, 409, 5855–5874 CrossRef CAS.
- J.-C. Boyer and F. C. J. M. van Veggel, Nanoscale, 2010, 2, 1417–1419 RSC.
- M. S. Meijer, P. A. Rojas-Gutierrez, D. Busko, I. A. Howard, F. Frenzel, C. Würth, U. Resch-Genger, B. S. Richards, A. Turshatov, J. A. Capobianco and S. Bonnet, Phys. Chem. Chem. Phys., 2018, 20, 22556–22562 RSC.
- R. Arppe, I. Hyppänen, N. Perälä, R. Peltomaa, M. Kaiser, C. Würth, S. Christ, U. Resch-Genger, M. Schäferling and T. Soukka, Nanoscale, 2015, 7, 11746–11757 RSC.
- C. Homann, L. Krukewitt, F. Frenzel, B. Grauel, C. Würth, U. Resch-Genger and M. Haase, Angew. Chem., Int. Ed., 2018, 57, 8765–8769 CrossRef CAS.
- S. Fischer, N. D. Bronstein, J. K. Swabeck, E. M. Chan and A. P. Alivisatos, Nano Lett., 2016, 16, 7241–7247 CrossRef CAS.
- N. J. J. Johnson, S. He, S. Diao, E. M. Chan, H. Dai and A. Almutairi, J. Am. Chem. Soc., 2017, 139, 3275–3282 CrossRef CAS.
- G. Chen, H. Ågren, T. Y. Ohulchanskyy and P. N. Prasad, Chem. Soc. Rev., 2015, 44, 1680–1713 RSC.
- K. Huang, H. Liu, M. Kraft, S. Shikha, X. Zheng, H. Ågren, C. Würth, U. Resch-Genger and Y. Zhang, Nanoscale, 2018, 10, 250–259 RSC.
- X. Xie, N. Gao, R. Deng, Q. Sun, Q.-H. Xu and X. Liu, J. Am. Chem. Soc., 2013, 135, 12608–12611 CrossRef CAS.
- J. Shen, G. Chen, A.-M. Vu, W. Fan, O. S. Bilsel, C.-C. Chang and G. Han, Adv. Opt. Mater., 2013, 1, 644–650 CrossRef.
- L. Sun, R. Wei, J. Feng and H. Zhang, Coord. Chem. Rev., 2018, 364, 10–32 CrossRef CAS.
- X. Chen, D. Peng, Q. Ju and F. Wang, Chem. Soc. Rev., 2015, 44, 1318–1330 RSC.
- A. Hlaváček, A. Sedlmeier, P. Skládal and H. H. Gorris, ACS Appl. Mater. Interfaces, 2014, 6, 6930–6935 CrossRef.
- L.-L. Li, R. Zhang, L. Yin, K. Zheng, W. Qin, P. R. Selvin and Y. Lu, Angew. Chem., Int. Ed., 2012, 51, 6121–6125 CrossRef CAS.
- E. Palo, S. Lahtinen, H. Päkkilä, M. Salomäki, T. Soukka and M. Lastusaari, Langmuir, 2018, 34, 7759–7766 CrossRef CAS.
- S. Beyazit, S. Ambrosini, N. Marchyk, E. Palo, V. Kale, T. Soukka, B. Tse Sum Bui and K. Haupt, Angew. Chem., Int. Ed., 2014, 53, 8919–8923 CrossRef CAS.
- Y. Wu, D. Li, F. Zhou, H. Liang, Y. Liu, W. Hou, Q. Yuan, X. Zhang and W. Tan, Chem. Sci., 2018, 9, 5427–5434 RSC.
- Y. Tang, M. Li, Z. Gao, X. Liu, X. Gao, T. Ma, X. Lu and J. Li, Food Anal. Methods, 2017, 10, 2964–2973 CrossRef.
- Y. Sun, W. Zhang, B. Wang, X. Xu, J. Chou, O. Shimoni, A. T. Ung and D. Jin, Chem. Commun., 2018, 54, 3851–3854 RSC.
- L.-L. Li, P. Wu, K. Hwang and Y. Lu, J. Am. Chem. Soc., 2013, 135, 2411–2414 CrossRef CAS.
- N. Bogdan, F. Vetrone, G. A. Ozin and J. A. Capobianco, Nano Lett., 2011, 11, 835–840 CrossRef CAS.
- A. Dong, X. Ye, J. Chen, Y. Kang, T. Gordon, J. M. Kikkawa and C. B. Murray, J. Am. Chem. Soc., 2011, 133, 998–1006 CrossRef CAS.
- A. Sedlmeier and H. H. Gorris, Chem. Soc. Rev., 2015, 44, 1526–1560 RSC.
- R. Arppe, L. Mattsson, K. Korpi, S. Blom, Q. Wang, T. Riuttamäki and T. Soukka, Anal. Chem., 2015, 87, 1782–1788 CrossRef CAS.
- A. Hlaváček, M. J. Mickert, T. Soukka, S. Lahtinen, T. Tallgren, N. Pizúrová, A. Król and H. H. Gorris, Anal. Chem., 2019, 91, 1241–1246 CrossRef.
- R. P. Bagwe, L. R. Hilliard and W. Tan, Langmuir, 2006, 22, 4357–4362 CrossRef CAS.
- C. Pisani, J. C. Gaillard, C. Dorandeu, C. Charnay, Y. Guari, J. Chopineau, J. M. Devoisselle, J. Armengaud and O. Prat, Nanoscale, 2017, 9, 5769–5772 RSC.
- H. T. T. Duong, Y. Chen, S. A. Tawfik, S. Wen, M. Parviz, O. Shimoni and D. Jin, RSC Adv., 2018, 8, 4842–4849 RSC.
- R. B. Liebherr, T. Soukka, O. S. Wolfbeis and H. H. Gorris, Nanotechnology, 2012, 23, 485103 CrossRef.
- Y. Cao, Y. Yang, Y. Shan and Z. Huang, ACS Appl. Mater. Interfaces, 2016, 8, 5998–6003 CrossRef CAS.
- S. F. Himmelstoß and T. Hirsch, Part. Part. Syst. Charact., 2019, 36, 1900235 CrossRef.
- S. Lahtinen, A. Lyytikäinen, N. Sirkka, H. Päkkilä and T. Soukka, Microchim. Acta, 2018, 185, 220 CrossRef.
- J. M. Harris and R. B. Chess, Nat. Rev. Drug Discovery, 2003, 2, 214–221 CrossRef CAS.
- J.-C. Boyer, M.-P. Manseau, J. I. Murray and F. C. J. M. van Veggel, Langmuir, 2010, 26, 1157–1164 CrossRef CAS.
- P. Cao, L. Tong, Y. Hou, G. Zhao, G. Guerin, M. A. Winnik and M. Nitz, Langmuir, 2012, 28, 12861–12870 CrossRef CAS.
- G. Zhao, L. Tong, P. Cao, M. Nitz and M. A. Winnik, Langmuir, 2014, 30, 6980–6989 CrossRef CAS.
- U. Kostiv, V. Lobaz, J. Kučka, P. Švec, O. Sedláček, M. Hrubý, O. Janoušková, P. Francová, V. Kolářová, L. Šefc and D. Horák, Nanoscale, 2017, 9, 16680–16688 RSC.
- U. Kostiv, Z. Farka, M. J. Mickert, H. H. Gorris, N. Velychkivska, O. Pop-Georgievski, M. Pastucha, E. Odstrčilíková, P. Skládal and D. Horák, Biomacromolecules, 2020, 21, 4502–4513 CrossRef CAS.
- Z. Farka, M. J. Mickert, Z. Mikušová, A. Hlaváček, P. Bouchalová, W. Xu, P. Bouchal, P. Skládal and H. H. Gorris, Nanoscale, 2020, 12, 8303–8313 RSC.
- Z. Farka, M. J. Mickert, A. Hlaváček, P. Skládal and H. H. Gorris, Anal. Chem., 2017, 89, 11825–11830 CrossRef CAS.
- M. H. Stewart, K. Susumu, B. C. Mei, I. L. Medintz, J. B. Delehanty, J. B. Blanco-Canosa, P. E. Dawson and H. Mattoussi, J. Am. Chem. Soc., 2010, 132, 9804–9813 CrossRef CAS.
- FRET – Förster Resonance Energy Transfer, ed. I. L. Medintz and N. Hildebrandt, Wiley-VCH Verlag GmbH & Co, Weinheim, Germany, 2014 Search PubMed.
- D. G. Newell, M. Koopmans, L. Verhoef, E. Duizer, A. Aidara-Kane, H. Sprong, M. Opsteegh, M. Langelaar, J. Threfall, F. Scheutz, J. van der Giessen and H. Kruse, Int. J. Food Microbiol., 2010, 139(Suppl. 1), S3–S15 CrossRef.
- High Throughput Screening for Food Safety Assessment, ed. A. K. Bhunia, M. S. Kim and C. R. Taitt, Elsevier, UK, 2015 Search PubMed.
- G. López-Campos, J. V. Martínez-Suárez, M. Aguado-Urda and V. López-Alonso, in Microarray Detection and Characterization of Bacterial Foodborne Pathogens, Springer US, Boston, MA, 2012, pp. 13–32 Search PubMed.
- M. Hao, P. Zhang, B. Li, X. Liu, Y. Zhao, H. Tan, C. Sun, X. Wang, X. Wang, H. Qiu, D. Wang, B. Diao, H. Jing, R. Yang, B. Kan and L. Zhou, PLoS One, 2017, 12, e0179937 CrossRef.
- Y. Zhao, H. Wang, P. Zhang, C. Sun, X. Wang, X. Wang, R. Yang, C. Wang and L. Zhou, Sci. Rep., 2016, 6, 21342 CrossRef CAS.
- H. Kurt, M. Yüce, B. Hussain and H. Budak, Biosens. Bioelectron., 2016, 81, 280–286 CrossRef CAS.
- M. Yüce, H. Kurt, B. Hussain, C. W. Ow-Yang and H. Budak, ChemistrySelect, 2018, 3, 5814–5823 CrossRef.
- Y. Li, X. Liu, X. Yang, H. Lei, Y. Zhang and B. Li, ACS Nano, 2017, 11, 10672–10680 CrossRef CAS.
- M. Yin, C. Wu, H. Li, Z. Jia, Q. Deng, S. Wang and Y. Zhang, ACS Omega, 2019, 4, 8953–8959 CrossRef CAS.
- M. Yin, C. Jing, H. Li, Q. Deng and S. Wang, J. Nanobiotechnol., 2020, 18, 41 CrossRef CAS.
- C. Chen and N. Hildebrandt, Trends Anal. Chem., 2020, 123, 115748 CrossRef CAS.
- B. Jin, S. Wang, M. Lin, Y. Jin, S. Zhang, X. Cui, Y. Gong, A. Li, F. Xu and T. J. Lu, Biosens. Bioelectron., 2017, 90, 525–533 CrossRef CAS.
- K. Cheng, J. Zhang, L. Zhang, L. Wang and H. Chen, Spectrochim. Acta, Part A, 2017, 171, 168–173 CrossRef CAS.
- S. Bräse, A. Encinas, J. Keck and C. F. Nising, Chem. Rev., 2009, 109, 3903–3990 CrossRef.
- J. W. Bennett and M. Klich, Clin. Microbiol. Rev., 2003, 16, 497–516 CrossRef CAS.
- R. Bhat, R. V. Rai and A. A. Karim, Compr. Rev. Food Sci. Food Saf., 2010, 9, 57–81 CrossRef CAS.
- M. Eskola, G. Kos, C. T. Elliott, J. Hajšlová, S. Mayar and R. Krska, Crit. Rev. Food Sci. Nutr., 2020, 60, 2773–2789 CrossRef CAS.
- IARC, Monographs on the evaluation of carcinogenic risks to humans, some naturally occurring substances: food items and constituents, heterocyclic aromatic amines and mycotoxins, International Agency for Research on Cancer, Lyon, France, 1993, vol. 56 Search PubMed.
- S. Goyal, K. G. Ramawat and J. M. Mérillon, in Fungal Metabolites, ed. J.-M. Merillon and K. G. Ramawat, Springer International Publishing, Cham, Switzerland, 2016, pp. 1–29 Search PubMed.
- S. A. Tittlemier, B. Cramer, C. Dall'Asta, M. H. Iha, V. M. T. Lattanzio, R. J. Malone, C. Maragos, M. Solfrizzo, M. Stranska-Zachariasova and J. Stroka, World Mycotoxin J., 2019, 12, 3–29 CrossRef CAS.
- F. Berthiller, C. Brera, C. Crews, M. H. Iha, R. Krska, V. M. T. Lattanzio, S. MacDonald, R. J. Malone, C. Maragos, M. Solfrizzo, J. Stroka and T. B. Whitaker, World Mycotoxin J., 2016, 9, 5–30 CrossRef CAS.
- L. Anfossi, C. Giovannoli and C. Baggiani, Curr. Opin. Biotechnol., 2016, 37, 120–126 CrossRef CAS.
- Q. Chen, W. Hu, C. Sun, H. Li and Q. Ouyang, Anal. Chim. Acta, 2016, 938, 137–145 CrossRef CAS.
- Y. Zhang, Z. Liao, Y. Liu, Y. Wan, J. Chang and H. Wang, Microchim. Acta, 2017, 184, 1471–1479 CrossRef CAS.
- M. Yang, M. Cui, W. Wang, Y. Yang, J. Chang, J. Hao and H. Wang, Anal. Bioanal. Chem., 2020, 412, 81–91 CrossRef CAS.
- M. Yang, Y. Zhang, M. Cui, Y. Tian, S. Zhang, K. Peng, H. Xu, Z. Liao, H. Wang and J. Chang, Nanoscale, 2018, 10, 15865–15874 RSC.
- Z. Wu, E. Xu, M. F. J. Chughtai, Z. Jin and J. Irudayaraj, Food Chem., 2017, 230, 673–680 CrossRef CAS.
- S. Wu, L. Liu, N. Duan, W. Wang, Q. Yu and Z. Wang, Microchim. Acta, 2018, 185, 497 CrossRef.
- S. Dai, S. Wu, N. Duan, J. Chen, Z. Zheng and Z. Wang, Biosens. Bioelectron., 2017, 91, 538–544 CrossRef CAS.
- E.-J. Jo, J.-Y. Byun, H. Mun, D. Bang, J. H. Son, J. Y. Lee, L. P. Lee and M.-G. Kim, Anal. Chem., 2018, 90, 716–722 CrossRef CAS.
- D. He, Z. Wu, B. Cui, Z. Jin and E. Xu, Microchim. Acta, 2020, 187, 254 CrossRef CAS.
- World Health Organization (WHO), Pesticide residues in food, http://www.who.int/news-room/fact-sheets/detail/pesticide-residues-in-food, (accessed August 2020).
- L. Fang, X. Liao, B. Jia, L. Shi, L. Kang, L. Zhou and W. Kong, Biosens. Bioelectron., 2020, 164, 112255 CrossRef CAS.
- S. T. Narenderan, S. N. Meyyanathan and B. Babu, Food Res. Int., 2020, 133, 109141 CrossRef CAS.
- Y. Tang, C. B. Craven, N. J. P. Wawryk, J. Qiu, F. Li and X.-F. Li, Trends Anal. Chem., 2020, 128, 115918 CrossRef CAS.
- Z. Guo, Z. Zhu, S. Huang and J. Wang, Food Addit. Contam., Part A, 2020, 37, 1180–1201 CrossRef CAS.
- K. Mejía-Carmona, E. V. S. Maciel and F. M. Lanças, Electrophoresis, 2020, 41, 1680–1693 CrossRef.
- S. Knoll, T. Rösch and C. Huhn, Anal. Bioanal. Chem., 2020, 412, 6149–6165 CrossRef CAS.
- Q. Long, H. Li, Y. Zhang and S. Yao, Biosens. Bioelectron., 2015, 68, 168–174 CrossRef CAS.
- P. Wang, H. Li, M. M. Hassan, Z. Guo, Z.-Z. Zhang and Q. Chen, J. Agric. Food Chem., 2019, 67, 4071–4079 CrossRef CAS.
- Z. Liu, L. Yang, A. S. Sharma, M. Chen and Q. Chen, Microchim. Acta, 2019, 186, 835 CrossRef CAS.
- W. Hu, Q. Chen, H. Li, Q. Ouyang and J. Zhao, Biosens. Bioelectron., 2016, 80, 398–404 CrossRef CAS.
- L. Yang, H. Sun, X. Wang, W. Yao, W. Zhang and L. Jiang, Microchim. Acta, 2019, 186, 308 CrossRef.
- N. Sun, Y. Ding, Z. Tao, H. You, X. Hua and M. Wang, Food Chem., 2018, 257, 289–294 CrossRef CAS.
- Q. Chen, R. Sheng, P. Wang, Q. Ouyang, A. Wang, S. Ali, M. Zareef and M. M. Hassan, Spectrochim. Acta, Part A, 2020, 241, 118654 CrossRef CAS.
- S. Wang, X. Wang, X. Chen, X. Cao, J. Cao, X. Xiong and W. Zeng, RSC Adv., 2016, 6, 46317–46324 RSC.
- H. Sun, Q. Mei, S. Shikha, J. Liu, J. Zhang and Y. Zhang, Microchim. Acta, 2019, 186, 106 CrossRef.
- H. You, X. Hua, L. Feng, N. Sun, Q. Rui, L. Wang and M. Wang, Microchim. Acta, 2017, 184, 1085–1092 CrossRef CAS.
- W. Sheng, Y. Shi, J. Ma, L. Wang, B. Zhang, Q. Chang, W. Duan and S. Wang, Microchim. Acta, 2019, 186, 564 CrossRef.
- R. Zou, Y. Chang, T. Zhang, F. Si, Y. Liu, Y. Zhao, Y. Liu, M. Zhang, X. Yu, X. Qiao, G. Zhu and Y. Guo, Front. Chem., 2019, 7, 18 CrossRef CAS.
- J. Yu, T. Guo, W. Zhang, B. Li, L. Liu and R. Hua, J. Alloys Compd., 2019, 771, 187–194 CrossRef CAS.
- J. Yu, T. Guo, W. Zhang, Y. Zhu, B. Li and R. Hua, Mater. Res. Bull., 2019, 111, 133–139 CrossRef CAS.
- Q. Yu, C. He, Q. Li, Y. Zhou, N. Duan and S. Wu, Microchim. Acta, 2020, 187, 222 CrossRef CAS.
- Y. Tang, Z. Gao, S. Wang, X. Gao, J. Gao, Y. Ma, X. Liu and J. Li, Biosens. Bioelectron., 2015, 71, 44–50 CrossRef CAS.
- C. Kirchhelle, Palgrave Commun., 2018, 4, 96 CrossRef.
- J. Chen, G.-G. Ying and W.-J. Deng, J. Agric. Food Chem., 2019, 67, 7569–7586 CrossRef CAS.
- T. T. H. Van, Z. Yidana, P. M. Smooker and P. J. Coloe, J. Glob. Antimicrob. Resist., 2020, 20, 170–177 CrossRef.
- C. D. Iwu, L. Korsten and A. I. Okoh, MicrobiologyOpen, 2020, e1035 CAS.
- Food and Agriculture Organization of the United Nations (FAO), Codex Alimentarius International Food Standards, http://www.fao.org/fao-who-codexalimentarius, (accessed August 2020).
- S. Bogialli and A. Di Corcia, Anal. Bioanal. Chem., 2009, 395, 947–966 CrossRef CAS.
- V. Gaudin, Biosens. Bioelectron., 2017, 90, 363–377 CrossRef CAS.
- M. Majdinasab, R. K. Mishra, X. Tang and J. L. Marty, Trends Anal. Chem., 2020, 127, 115883 CrossRef CAS.
- C. Liu, W. Ma, Z. Gao, J. Huang, Y. Hou, C. Xu, W. Yang and M. Gao, J. Mater. Chem. C, 2014, 2, 9637–9642 RSC.
- G. Hu, W. Sheng, J. Li, Y. Zhang, J. Wang and S. Wang, Anal. Chim. Acta, 2017, 982, 185–192 CrossRef CAS.
- G. Hu, S. Gao, X. Han and L. Yang, Food Anal. Methods, 2020, 13, 1069–1077 CrossRef.
- G. Hu, W. Sheng, Y. Zhang, X. Wu and S. Wang, Anal. Bioanal. Chem., 2015, 407, 8487–8496 CrossRef CAS.
- G. Hu, W. Sheng, Y. Zhang, J. Wang, X. Wu and S. Wang, J. Agric. Food Chem., 2016, 64, 3908–3915 CrossRef CAS.
- H. Zhang, C. Fang, S. Wu, N. Duan and Z. Wang, Anal. Biochem., 2015, 489, 44–49 CrossRef CAS.
- X. Liu, L. Su, L. Zhu, X. Gao, Y. Wang, F. Bai, Y. Tang and J. Li, Sens. Actuators, B, 2016, 233, 394–401 CrossRef CAS.
- Q. Ouyang, Y. Liu, Q. Chen, Z. Guo, J. Zhao, H. Li and W. Hu, Food Control, 2017, 81, 156–163 CrossRef CAS.
- X. Liu, T. Gao, X. Gao, T. Ma, Y. Tang, L. Zhu and J. Li, Microchim. Acta, 2017, 184, 3557–3563 CrossRef CAS.
- Y. Tang, H. Liu, J. Gao, X. Liu, X. Gao, X. Lu, G. Fang, J. Wang and J. Li, Talanta, 2018, 181, 95–103 CrossRef CAS.
- S. Wu, H. Zhang, Z. Shi, N. Duan, C. Fang, S. Dai and Z. Wang, Food Control, 2015, 50, 597–604 CrossRef CAS.
- M. I. Castro-González and M. Méndez-Armenta, Environ. Toxicol. Pharmacol., 2008, 26, 263–271 CrossRef.
- A. Khan, S. Khan, M. A. Khan, Z. Qamar and M. Waqas, Environ. Sci. Pollut. Res. Int., 2015, 22, 13772–13799 CrossRef CAS.
- European Commission, Off. J. Eur. Union, 2006, L364, 5–24 Search PubMed.
- M. Parvizishad, A. Dalvand, A. H. Mahvi and F. Goodarzi, Health Scope, 2017, 6, e14164 Search PubMed.
- S. Otles and V. H. Ozyurt, in Handbook of Food Chemistry, ed. P. C. K. Cheung and B. M. Mehta, Springer, Berlin, Heidelberg, 2015, pp. 133–149 Search PubMed.
- B. Gu and Q. Zhang, Adv. Sci., 2018, 5, 1700609 CrossRef.
- S. Wilhelm, ACS Nano, 2017, 11, 10644–10653 CrossRef CAS.
- Y. Liu, Q. Ouyang, H. Li, M. Chen, Z. Zhang and Q. Chen, J. Agric. Food Chem., 2018, 66, 6188–6195 CrossRef CAS.
- Y. Liu, Q. Ouyang, H. Li, Z. Zhang and Q. Chen, ACS Appl. Mater. Interfaces, 2017, 9, 18314–18321 CrossRef CAS.
- S. Ren, Q. Li, Y. Li, S. Li, T. Han, J. Wang, Y. Peng, J. Bai, B. Ning and Z. Gao, Microchim. Acta, 2019, 186, 151 CrossRef.
- J. Lv, S. Zhao, S. Wu and Z. Wang, Biosens. Bioelectron., 2017, 90, 203–209 CrossRef CAS.
- European Commission, Off. J. Eur. Union, 2010, L15, 1–72 Search PubMed.
- European Commission, Off. J. Eur. Union, 2011, L12, 1–89 Search PubMed.
- European Commission, Off. J. Eur. Union, 2010, L12, 1–87 Search PubMed.
- X. Liu, Y. Zhao, C. Sun, X. Wang, X. Wang, P. Zhang, J. Qiu, R. Yang and L. Zhou, Sci. Rep., 2016, 6, 34926 CrossRef CAS.
- Q. Long, Y. Wen, H. Li, Y. Zhang and S. Yao, J. Fluoresc., 2017, 27, 205–211 CrossRef CAS.
- M. Chen, M. Hassan, H. Li and Q. Chen, Microchim. Acta, 2020, 187, 85 CrossRef CAS.
- D. Giust, M. I. Lucío, A. H. El-Sagheer, T. Brown, L. E. Williams, O. L. Muskens and A. G. Kanaras, ACS Nano, 2018, 12, 6273–6279 CrossRef CAS.
- J. Li, C. Zhang, M. Yin, Z. Zhang, Y. Chen, Q. Deng and S. Wang, ACS Omega, 2019, 4, 15947–15955 CrossRef CAS.
- L. Zhang, S. Yin, J. Hou, W. Zhang, H. Huang, Y. Li and C. Yu, Food Chem., 2019, 270, 415–419 CrossRef CAS.
- W. Wang, H. Li, M. Yin, K. Wang, Q. Deng, S. Wang and Y. Zhang, Sens. Actuators, B, 2018, 255, 1422–1429 CrossRef CAS.
- S. Ren, Y. Li, Q. Guo, Y. Peng, J. Bai, B. Ning and Z. Gao, Microchim. Acta, 2018, 185, 429 CrossRef.
| This journal is © The Royal Society of Chemistry 2021 |