Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Mass spectrometry reveals potential of β-lactams as SARS-CoV-2 Mpro inhibitors†
Tika R.
Malla
 a,
Anthony
Tumber
a,
Tobias
John
a,
Anthony
Tumber
a,
Tobias
John
 a,
Lennart
Brewitz
a,
Lennart
Brewitz
 a,
Claire
Strain-Damerell
a,
Claire
Strain-Damerell
 bc,
C David
Owen
bc,
C David
Owen
 bc,
Petra
Lukacik
bc,
Petra
Lukacik
 bc,
H. T. Henry
Chan
bc,
H. T. Henry
Chan
 a,
Pratheesh
Maheswaran
a,
Pratheesh
Maheswaran
 a,
Eidarus
Salah
a,
Fernanda
Duarte
a,
Eidarus
Salah
a,
Fernanda
Duarte
 a,
Haitao
Yang
d,
Zihe
Rao
d,
Martin A.
Walsh
a,
Haitao
Yang
d,
Zihe
Rao
d,
Martin A.
Walsh
 bc and
Christopher J.
Schofield
bc and
Christopher J.
Schofield
 *a
*a
aChemistry Research Laboratory, Department of Chemistry, 12 Mansfield Road, Oxford, OX1 3TA, UK. E-mail: christopher.schofield@chem.ox.ac.uk
bDiamond Light Source, Harwell Science & Innovation Campus, Didcot, Oxfordshire OX11 0DE, UK
cResearch Complex at Harwell, Harwell Science & Innovation Campus, Didcot, Oxfordshire OX11 0FA, UK
dShanghai Institute for Advanced Immunochemical Studies and School of Life Science and Technology, ShanghaiTech University, Shanghai, China
First published on 19th January 2021
Abstract
The main viral protease (Mpro) of SARS-CoV-2 is a nucleophilic cysteine hydrolase and a current target for anti-viral chemotherapy. We describe a high-throughput solid phase extraction coupled to mass spectrometry Mpro assay. The results reveal some β-lactams, including penicillin esters, are active site reacting Mpro inhibitors, thus highlighting the potential of acylating agents for Mpro inhibition.
The main viral protease (Mpro) of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)1 is a COVID-19 treatment target.2 Mpro along with the papain-like protease (PLpro), processes initially translated viral polyproteins to give cleaved proteins with biological functions essential for viral replication in cells.3 Following formation of a non-covalent enzyme-substrate complex, Mpro catalysis proceeds via His-41 enabled reaction of Cys-145 with a scissile peptide bond forming a hydrolytically labile thioester. Mpro cleaves after glutamine-residues with a preference for small-residues on the C-terminal side of the cleaved amide (Fig. 1A and B).4
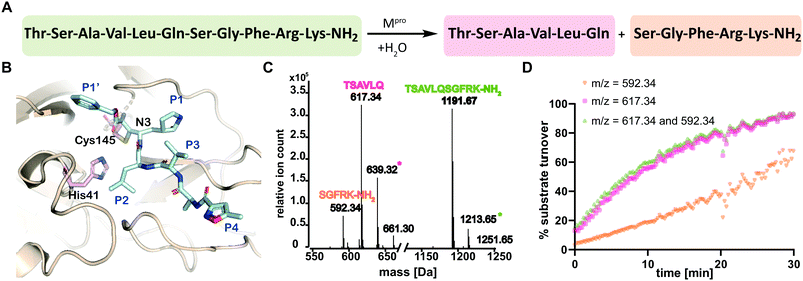 | ||
| Fig. 1 SPE-MS assay monitoring Mpro catalyzed cleavage of the TSAVLQ/SGFRK-NH2 substrate. (A) Mpro catalyzed hydrolysis of TSAVLQ/SGFRK-NH2. (B) View from a structure of Cys-145 linked Mpro-N3 complex (PDB ID: 6LU7);1 the Cys–His dyad is in pink; substrate binding sites are labelled in blue. (C) Deconvoluted mass spectrum of substrate/cleaved products after 10 minute incubation with Mpro. Note, the C-terminal product was not efficiently retained by the SPE cartridge resulting in a low abundance compared to the N-terminal cleavage product. Sodium ion adducts (+23 Da) for the TSAVLQ product (639 Da) and substrate (1214 Da) are labelled with magenta and green asterisks, respectively. (D) % substrate turnover based on integration of the total abundance of cleaved products (TSAVLQ or SGFRK-NH2) or the individual products. Conditions: 0.15 μM Mpro, 2.0 μM TSAVLQ/SGFRK-NH2 (1192 Da) (20 mM HEPES, pH 7.5, 50 mM NaCl). | ||
Most reported Mpro assays measure fluorescence, as precedented for other protease assays.1,2b,5 Whilst efficient, such methods do not simultaneously monitor substrate depletion/product formation and some compounds interfere with fluorescence.6 We were thus interested in establishing an alternative mass spectrometry (MS)-based high-throughput Mpro assay for identifying new inhibitors and testing known drugs.
Solid phase extraction coupled with MS (SPE-MS) has been applied to high-throughput screens of enzymes.7 We envisaged it could simultaneously monitor both Mpro substrate depletion/product formation and covalent modification. The latter is of interest because many reported inhibitors of nucleophilic cysteine enzymes work by covalent reaction.8 Here we report how such an assay enabled identification of new Mpro inhibitors, including β-lactams, the most important antibacterial class.9
We developed conditions for an SPE-MS based SARS-CoV-2 Mpro assay (0.15 μM Mpro, 2.0 μM TSAVLQ/SGFRK-NH2, 20 mM HEPES, pH 7.5, 50 mM NaCl) using protein prepared as reported.1,10 Isolated Mpro was found to be active when monitoring turnover of peptide substrates, including TSAVLQ/SGFRK-NH2 which was cleaved to give TSAVLQ and SGFRK-NH2 fragments (Fig. 1C and D). Kinetic parameters were determined for the 11-mer substrate (Km = 14.4 μM; kcat = 2.7 min−1), both by monitoring substrate depletion and N-terminal product fragment formation (Fig. S1, ESI†). The efficiency (kcat/Km) of Mpro determined by SPE-MS (28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 M−1 s−1) is comparable to that observed for a similar substrate Mca–AVLQ/SGFRK(Dnp)K using a fluorescence resonance energy transfer (FRET) assay (27
500 M−1 s−1) is comparable to that observed for a similar substrate Mca–AVLQ/SGFRK(Dnp)K using a fluorescence resonance energy transfer (FRET) assay (27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 s−1, as reported and in our hands).1 Steady state kinetics for a 37-mer substrate were also investigated; a 2-fold increase in kcat/Km (60
000 M−1 s−1, as reported and in our hands).1 Steady state kinetics for a 37-mer substrate were also investigated; a 2-fold increase in kcat/Km (60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 026 M−1 s−1) was observed (Fig. S2, ESI†). Comparison of kinetic parameters for the SARS-CoV-2 Mpro and the related SARS-CoV Mpro reveal similar kcat/Km values (though the values for SARS-CoV were somewhat lower when using shorter substrates in an HPLC assay) (Table S1, ESI†). Note, the interconversion between monomeric/dimeric forms of Mpro has the potential to introduce complexity in kinetic analyses.11
026 M−1 s−1) was observed (Fig. S2, ESI†). Comparison of kinetic parameters for the SARS-CoV-2 Mpro and the related SARS-CoV Mpro reveal similar kcat/Km values (though the values for SARS-CoV were somewhat lower when using shorter substrates in an HPLC assay) (Table S1, ESI†). Note, the interconversion between monomeric/dimeric forms of Mpro has the potential to introduce complexity in kinetic analyses.11
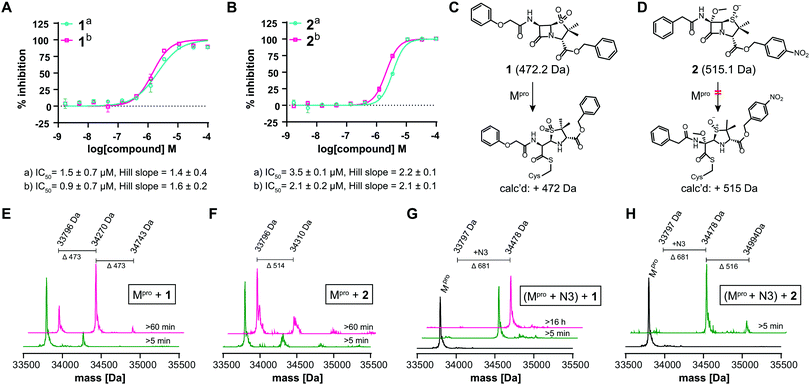
Next, the SPE-MS assay was validated for inhibition studies with ebselen,1 N3,1 disulfiram,1 and boceprevir12 using the 11-mer TSAVLQ/SGFRK-NH2 substrate (Table 1 and Fig. S3, ESI†). The ebselen IC50 was ∼0.09 μM under standard conditions (0.15 μM Mpro, 2.0 μM TSAVLQ/SGFRK-NH2i.e. [S] < Km, 20 mM HEPES, pH 7.5, 50 mM NaCl at ambient temperature) compared to an IC50 of ∼0.67 μM1 using a FRET assay (0.2 μM Mpro, 20 μM Mca–AVLQ/SGFRK(Dnp)K i.e. [S] ≈ Km, 50 mM Tris–HCl, pH 7.3, 1 mM EDTA, 30 °C)1 (Table 1, entry 1; Fig. S3D, ESI†).
| Inhibitor | IC50 (SPE-MS) [μM]ab | IC50 (SPE-MS)ac [μM] | IC50 (FRET) [μM] |
|---|---|---|---|
| a Mean of two independent replicates each performed in technical duplicate (n = 2 ± standard deviation, SD). Conditions: 0.15 μM Mpro and 2.0 μM TSAVLQ/SGFRK-NH2 substrate in 20 mM HEPES, pH 7.5, 50 mM NaCl. b 30 min inhibitor preincubation. c 60 min inhibitor preincubation. | |||
| Ebselen | 0.09 ± 0.07 | 0.09 ± 0.07 | 0.67 ± 0.091 |
| N3 | 0.04 ± 0.01 | 0.03 ± 0.01 | n.d. |
| Disulfiram | 0.60 ± 0.01 | 0.46 ± 0.02 | 9.35 ± 0.181 |
| Boceprevir | 11.0 ± 4.8 | 9.2 ± 5.5 | 2.70 ± 0.0512 |
We optimized the assay for studying covalent modifications with a higher Mpro concentration being used to enable robust analyses (1 μM Mpro), though IC50 and preliminary covalent modification data can be accumulated from the same experiment. SPE purification is denaturing, so monomer modification was observed. Assay validation used N3;1 predominantly (but not exclusively) a single N3 adduct was observed (Fig. 2A, B and Fig. S4, ESI†), consistent with structural work revealing Cys-145 reaction1 (Fig. 1B and Fig. S5, Table S2, ESI†). We exploited selective reaction of N3 to test selectivity of other inhibitors as exemplified with ebselen, comparing results for N3 treated/untreated Mpro (Fig. 2C, E and Fig, S6, ESI†). By contrast with N3, we saw time dependent modification of multiple residues with ebselen with or without N3 pre-treatment (Fig. 2C and E), implying reaction of some of the 11 non-active site cysteines (Fig. S5, ESI†). Ebselen was used as a readily available positive inhibition control in subsequent studies.
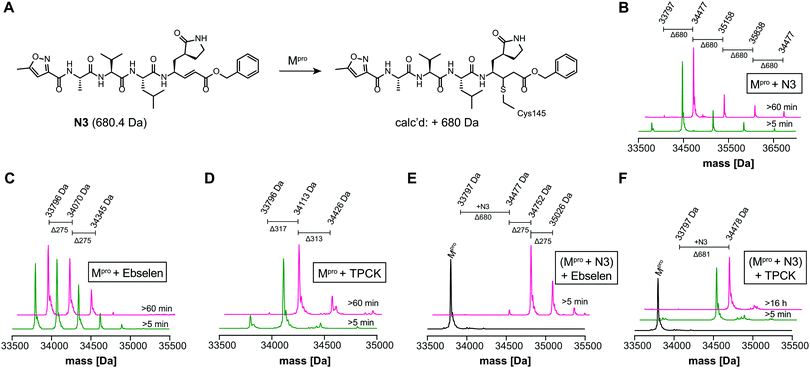 | ||
| Fig. 2 N3 dependent reaction monitoring the active site selectivity of inhibitors. (A) Reaction of N3 with the Mpro active site Cys-145.1 (B) N3, (C) ebselen and (D) TPCK modifies Mpro in a covalent manner. (E) Ebselen covalently modifies multiple Mpro cysteine residues in the presence of N3. (F) TPCK does not covalently modify Mpro in the presence of N3, suggesting that it selectively reacts with active site Cys-145. Black spectra: wild-type Mpro (33796 Da). | ||
The assay was used to screen the Library of Pharmacologically Active Compounds (LOPAC) and a library of 1600 small-molecule active pharmaceutical ingredients (API) at 20 μM compound (Fig. S7, ESI†). Excellent Z′-factors13 and signal to noise ratios reveal the assay robustness (Fig. S7, ESI†). In addition to ebselen (identification of which validates the method), diverse inhibitors (≥80% at a fixed 20 μM inhibitor concentration) were identified, some (related to) known inhibitors,1,14 including auranofin, cisplatin, IPA-3, bismuth subsalicylate, thioguanine, carmustine, and disulfiram (Tables S3 and S4, ESI†).
IC50s were determined for compounds with ≥80% inhibition at 20 μM, excluding known interference compounds.15 Auranofin (IC50 ∼ 1.5 μM; reported IC50 ∼ 0.5 μM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14), an α-chloroketone (TPCK) (IC50 ∼ 0.8 μM), IPA-3 (IC50 ∼ 0.1 μM), and 5-thioguanine (IC50 ∼ 13.5 μM) are some of the more potent inhibitors (Fig. S8, ESI†). Some of these covalently modified Mpro, sometimes with more than one reaction being observed (Fig. S9–S15, ESI†). Active site selectivity was investigated using N3 treated and untreated Mpro. Following N3 treatment, in some cases, e.g. TPCK and Nα-p-toluenesulfonyl-L-lysine chloromethyl ketone, substantial covalent modification was no longer observed, implying selective Cys-145 reaction (Fig. 2D, F and Fig. S12, S13, ESI†). Although further validation is required, with BAY 11-7082 and IPA-3 the multiple adducts observed with unmodified Mpro were diminished when the active site was N3 blocked, suggesting reaction with Cys-145 might alter the Mpro conformation (Fig. S9 and S11, ESI†).
14), an α-chloroketone (TPCK) (IC50 ∼ 0.8 μM), IPA-3 (IC50 ∼ 0.1 μM), and 5-thioguanine (IC50 ∼ 13.5 μM) are some of the more potent inhibitors (Fig. S8, ESI†). Some of these covalently modified Mpro, sometimes with more than one reaction being observed (Fig. S9–S15, ESI†). Active site selectivity was investigated using N3 treated and untreated Mpro. Following N3 treatment, in some cases, e.g. TPCK and Nα-p-toluenesulfonyl-L-lysine chloromethyl ketone, substantial covalent modification was no longer observed, implying selective Cys-145 reaction (Fig. 2D, F and Fig. S12, S13, ESI†). Although further validation is required, with BAY 11-7082 and IPA-3 the multiple adducts observed with unmodified Mpro were diminished when the active site was N3 blocked, suggesting reaction with Cys-145 might alter the Mpro conformation (Fig. S9 and S11, ESI†).
The screen identified β-lactam drugs as potential Mpro inhibitors, including penicillins and cephalosporins (Table S5, ESI†). This was of interest, as in preliminary work we observed some β-lactams react covalently (data not shown). β-Lactam antibiotics form stable acyl–enzyme complexes with bacterial nucleophilic serine enzymes; they inhibit other nucleophilic serine enzymes including proteases and β-lactamases16 and nucleophilic cysteine enzymes.17
Studies on cephalosporins identified as potential inhibitors from the screen revealed no substantial covalent Mpro modification, though cephalosporin C Zn(II) salt and cephalosporin C Na(I) salt inhibited. However, the IC50s for cephalosporin C Zn(II) salt and ZnCl2 were similar, indicating much of the inhibition is due to Zn(II) ions (Fig. S16, ESI†), as observed for cephalosporin C Zn(II) salt inhibition of other enzymes.7b
We further investigated β-lactam reactions with Mpro using a diverse set of β-lactams (Fig. S17, ESI†). Though most β-lactams were inactive (IC50 > 100 μM), two penicillin esters manifested IC50s < 5 μM, i.e.1: a penicillin V sulfone C3 benzyl ester (IC50 ∼ 1.5 μM), and 2: a derivative of penicillin G sulfoxide C3 p-nitrobenzyl ester (IC50 ∼ 3.5 μM), both with similar potency with either 30 or 60 min preincubation (Fig. 3A and B). Other β-lactams inhibited, though more weakly (Fig. S17, ESI†). The inhibition by the penicillin benzyl esters may, in part, reflect binding of the N3 benzyl ester, likely binding in the P1′ or P2 pocket (Fig. 1B).1 Structures of Mpro complexed with a β-lactam were not obtained; however, docking studies reveal potential of 1 and 2 to bind favourably at the active site (Fig. S18, ESI†), in the case of 1 in a manner enabling Cys-145 reaction.
Evidence for covalent reaction was observed with representatives of the penem, carbapenem prodrug, penicillin, penicillin sulfone, clavam, cephem, and monobactam β-lactam sub-families (Fig. S19–S21, ESI†). In some cases, e.g. clavulanate (Fig. S19H, ESI†) and moxalactam (Fig. S21F, ESI†), (partial) inhibitor fragmentation was observed. There was no clear correlation between a propensity to react covalently and Mpro inhibition; in some cases evidence for partial covalent modification, but no inhibition was observed (Fig. S17, ESI†). Covalent modification was observed with 1, but only to a small (<10%) extent with 2, suggesting the latter likely inhibits principally by a non-covalent interaction (Fig. 3C–H). After Cys-145 blocking with N3, no reaction with 1 was observed (Fig. 3G). Minor further modification of Cys-145 reacted Mpro was observed with 2 (Fig. 3H), suggesting the low levels of covalent modification by 2 do not solely involve Cys-145.
In summary, SPE-MS is a useful method for Mpro assays enabling analysis of inhibition by both substrate depletion/product formation. The method complements reported in vitro Mpro assays and compares favourably to those in terms of its robustness and ability to enable efficient high-throughput screening/repurposing efforts. The SPE-MS assay also enables ready analysis of covalent Mpro modification and use of Mpro reacted with a selective inhibitor such as N3/TPCK informs on whether covalent reaction of a test inhibitor occur at the active site or not.
Although the available evidence implies that β-lactams can inhibit Mpro non-covalently, the observation that some react with and inhibit Mpro by covalent active site modification should promote interest in the development of inhibitors for Mpro and other thiol proteases working via cysteinyl S-acylation. By contrast with S-alkylating inhibitors, which can have toxicity issues, S-acylation has not been widely explored for nucleophilic cysteine proteases.
The identification of β-lactam containing Mpro inhibitors with structures closely related to drugs should promote work on the development of related compounds for progression towards clinical use for treatment of COVID-19 and viral diseases.
We thank the Oxford COVID fund and its generous donors, the BBSRC, MRC, the Wellcome Trust, Cancer Research UK and GSK for funding, and D. Ebner (TDI, Oxford) for supplying the compound libraries. We thank present and past group members including T. Suits and J. Brem and support staff including A. Hardy, Diamond and RCaH support staff who have supported our COVID-19 work, including those who made compounds for initial screening. T. R. M. was supported by the BBSRC (BB/M011224/1). T. J. thanks the EPSRC Oxford-GSK-Crick Program (EP/R512060/1) and GSK. H. T. H. C. thanks the EPSRC SBM CDT (EP/L015838/1) and the Clarendon Fund. We thank J. E. Baldwin, R. M. Adlington and R.D.G. Cooper for encouragement and inspiration.
Conflicts of interest
There are no conflicts to declare.Notes and references
- Z. Jin, X. Du, Y. Xu, Y. Deng and M. Liu, et al. , Nature, 2020, 582, 289 CrossRef CAS.
- (a) K. Anand, G. J. Palm, J. R. Mesters, S. G. Siddell and J. Ziebuhr, et al. , EMBO J., 2002, 21, 3213 CrossRef CAS; (b) H. Yang, M. Yang, Y. Ding, Y. Liu and Z. Lou, et al. , Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 13190 CrossRef CAS; (c) A. K. Gosh, M. Brindisi, D. Shahabi, M. E. Chapman and A. D. Mesecar, ChemMedChem, 2020, 15, 907 CrossRef.
- J. Ziebuhr, E. J. Snijder and A. E. Gorbalenya, J. Gen. Virol., 2000, 81, 853 CrossRef CAS.
- L. Zhang, D. Lin, X. Sun, U. Curth and C. Drosten, et al. , Science, 2020, 368, 409 CrossRef CAS.
- K. Fan, P. Wei and Q. Feng, et al. , J. Biol. Chem., 2004, 279, 1637 CrossRef CAS.
- (a) A. Simeonov and M. I. Davis, Interference with Fluorescence and Absorbance, 2004 Search PubMed; (b) D. S. Auld, N. T. Southall, A. Jadhav, R. L. Johnson and D. J. Diller, et al. , J. Med. Chem., 2008, 51, 2372 CrossRef CAS; (c) A. Simeonov, A. Jadhav, C. J. Thomas, Y. Wang and R. Huang, et al. , J. Med. Chem., 2008, 51, 2363 CrossRef CAS.
- (a) J. Meng, M. T. Lai, V. Munshi, J. Grobler and J. McCauley, et al. , J. Biomol. Screen., 2015, 20, 606 CrossRef CAS; (b) L. Brewitz, A. Tumber, I. Pfeffer, M. A. McDonough and C. J. Schofield, Sci. Rep., 2020, 10, 8650 CrossRef CAS; (c) L. Brewitz, A. Tumber, X. Zhang and C. J. Schofield, Bioorg. Med. Chem., 2020, 28, 115675 CrossRef CAS.
- A. K. Ghosh, I. Samanta, A. Mondal and W. R. Liu, ChemMedChem, 2019, 14, 889 CrossRef CAS.
- E. Y. Klein, T. P. Van Boeckel, E. M. Martinez, S. Pant and S. Gandra, et al. , Proc. Natl. Acad. Sci. U. S. A., 2018, 115, E3463 CrossRef CAS.
- A. Douangamath, D. Fearon, P. Gehrtz, T. Krojer and P. Lukacik, et al. , Nat. Commun., 2020, 11, 5047 CrossRef CAS.
- T. J. El-Baba, C. A. Lutomski, A. L. Kantsadi, T. R. Malla and T. John, et al. , Angew. Chem., Int. Ed., 2020 DOI:10.1002/anie.202010316.
- C. Ma, M. D. Sacco, B. Hurst, J. A. Townsend and Y. Hu, et al. , Cell Res., 2020, 30, 678 CrossRef CAS.
- J. H. Zhang, T. D. Chung and K. R. Oldenburg, J. Biomol. Screen., 1999, 4, 67 CrossRef.
- Z. He, W. Zhao, W. Niu, X. Gao and X. Gao, et al. , bioRxiv, 2020 DOI:10.1101/2020.05.28.120642.
- J. B. Baell and G. A. Holloway, J. Med. Chem., 2010, 53, 2719 CrossRef CAS.
- (a) C. L. Tooke, P. Hinchliffe, E. C. Bragginton, C. K. Colenso and V. H. A. Hirvonen, et al. , J. Mol. Biol., 2019, 431, 3472 CrossRef CAS; (b) W. B. Knight, A. L. Maycock and B. G. Green, et al. , Biochemistry, 1992, 31, 4980 CrossRef CAS.
- M. A. Gun, B. Bozdogan and A. Y. Coban, Future Microbiol., 2020, 15, 937 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and inhibition data. See DOI: 10.1039/d0cc06870e |
| This journal is © The Royal Society of Chemistry 2021 |