Open Access Article
Open Access ArticleTowards circular carbo-chemicals – the metamorphosis of petrochemicals
J.-P.
Lange

Shell Global Solutions International B.V., Amsterdam, The Netherlands. E-mail: jean-paul.lange@shell.com; Tel: +31-20-630-3428
First published on 7th June 2021
Abstract
The petrochemical industry grew to become one of the world's largest industries during the 20th century. It is expected that it will continue to grow, as the world's population gets wealthier, social dynamics change and people demand more affordable and useful materials. The industry recognises that the Earth's carrying capacity is limited. It is adapting to seek to become a truly sustainable ‘carbo’-chemical industry. This paper will address the three main challenges of this transition: shifting hydrocarbon stock, climate change and circular economy. As the energy sector transitions from oil, coal and eventually natural gas, it is expected that the chemical industry will have access to abundant hydrocarbon stocks for which it can find valuable uses. But rising CO2 prices and increasing upgrading costs will likely encourage greater use of alternative, low-carbon feedstocks. In particular, there may be a development of biomass for manufacturing oxygenated chemical intermediates and bio-based materials. To help tackle climate change, the industry will need to reduce the CO2 emissions of its processes and utilities (energy sources). Ways to achieve this will include efficiency improvements, electrification of utilities and processes and switching to renewable H2; upgrading by-products to chemicals; and CO2 capture and storage or utilisation (CCS/CCU). The issue of plastic waste pollution is combining with the challenges discussed above to push society and governments towards a more circular economy. Customer demand for sustainable products is growing. New regulations (and technologies) are being rolled out for waste collection, sorting and recycling. In addition, the industry is making pledges to produce and use more sustainably. However, it is expected that fresh carbon will still have to enter the material cycle. It will be needed to feed the growth of the chemical industry and to compensate for inevitable recycling losses. For a truly circular industry, this fresh carbon would come from a renewable source, i.e. from atmospheric CO2, initially via biomass and later possibly from direct CO2 capture and utilisation (CCU).
Broader contextClimate change, plastic litter and reliance on fossil resources are three issues that deeply concern society today. They have also been recognized by the petrochemical industry and embraced in its ambition to keep serving humanity with valuable and affordable materials for everyday life, e.g. for health, energy, clothing, mobility, communication, household, leisure and many more. This perspective attempts to present an integral view of the challenges, opportunities and compromises that the petrochemical industry is facing. It describes how the industry could transform into a truly circular and sustainable carbo-chemical industry by relying on recycled and renewable carbon feedstock and energy sources. It concludes by discussing the pace of this metamorphosis and by warning us that it could take more than 50 years to reach completion. This metamorphosis will likely require the emerging carbo-chemical industry develop new alliances to properly balance the three Ps of sustainability – Prosperity, People and Planet. It will likely look for collaborating with sectors such as renewable electricity, agriculture and forestry, waste management and environmental protection as well as consumers and the public at large. |
1 Introduction
1.1 The multiple challenges
Humanity has reached an unprecedented level of wealth over the past century. But it has also disturbed the ecological system on which it depends, endangering its future. This dilemma is at the core of today's energy transition. Global energy consumption is expected to double during the first half of the 21st century, as population and prosperity increase in developing countries. By 2050, however, the world's population will need to have reduced its fossil CO2 emissions by more than 80% compared with today's level (i.e. >90% compared with ‘business as usual’) to limit global warming to less than 2 °C and restrict the damage from climate change. Consequently, many energy scenarios agree in expecting the consumption of coal, oil and natural gas to peak in the coming decades through impressive improvement in energy efficiency and growth in renewable energy, particularly renewable electricity, by 2050 (Fig. 1, top).1,7 The degree of electrification will likely vary for various energy sectors, e.g. from nearly 100% for building to some 50% for transport and industry.7 Significant pricing of CO2 emissions will be needed to incentivise the shift to a low-carbon economy.2 For instance, meeting the Paris Agreement could mean pricing CO2 at $100 per t by 2050 (Fig. 1, bottom),which would effectively increase the cost of oil by about $42 per bbl, the cost of gas by $5.5 per GJ and the cost of fossil H2 by $900 per t.†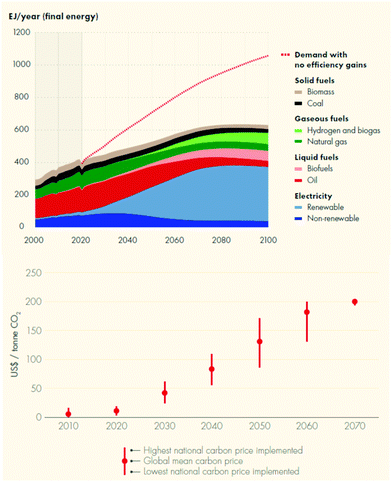 | ||
| Fig. 1 Shell Sky 1.5 scenario of final energy consumption (top)7 and the development of CO2 pricing (bottom)1 that is needed to meet the Paris agreement of <2 °C (NB: the energy consumption is based on the IEA 2020 report of primary energy supply corrected for the inefficiencies of electricity production). | ||
The energy transition will shake up the chemical industry, which for two centuries has developed by upgrading industrial by-products (from the coal industry until about 1950 and then from the oil industry). Firstly, in the short term the industry will have an abundance of fossil feedstock that has become available because of decreasing demand for transportation fuels. In the longer term, however, the chemical industry will need to pay an increasing portion of the cost of oil processing to prepare its feedstock. Secondly, the industry will also need to reduce its GHG emissions, which are a small fraction of today's global emissions but could represent a sizeable proportion in future decades.2–4 The chemical industry has also become aware of a third important development: the emergence of a circular economy. The issue of plastic waste in oceans, rivers and enormous open landfills has come to public attention, joining the other two factors in prompting calls for a circular economy.5 The industry wants to address the issue and has formed a global alliance to end plastic waste, involving the whole value chain, from producers to brand owners and waste collectors.6
This paper will briefly present today's petrochemical industry and then discuss various facets of the transformation into a circular carbo-chemical industry that is required for it to continue serving humanity while responding to the challenges outlined above. The three transformational forces – shifting fossil feedstock, CO2 emissions, and circular economy – interact with one another very significantly. They will nevertheless be discussed sequentially, and their interactions will be highlighted as we progress. These new developments will be illustrated by a range of actions taken by Shell. Initiatives are also being progressed by the other chemical majors, e.g. ExxonMobil, Total, BASF, Dow and Sabic, and the reader is invited to visit their websites to fully grasp the breath of this industry transition. Various aspects of the future of the petrochemical and plastic industries discussed here have been the subjects of valuable analysis and review papers that are highly recommended.8–12
1.2 Today's petrochemical industry
Today's petrochemical industry is reported to consume about 10% of fossil carbon used as feedstock and another 7% as energy to drive its chemical processes.13 The feedstock is primarily used to produce olefins and aromatics, also called base chemicals, at a scale of about 400 Mt a−1 globally (Fig. 2, top).10 The largest share of these base chemicals is used to produce hydrocarbon polymers such as polyolefins and polystyrene, while a smaller part is converted to intermediates by the insertion of oxygen, nitrogen or chlorine. Most of these intermediates are then used to make engineering plastics such as PVC, nylon, polyurethane and polycarbonates, while a minor share is used in solvents, detergents, plasticisers and multiple other applications in the fine chemical industry. Accordingly, some 90% of the output of the petrochemical industry consists of polymers.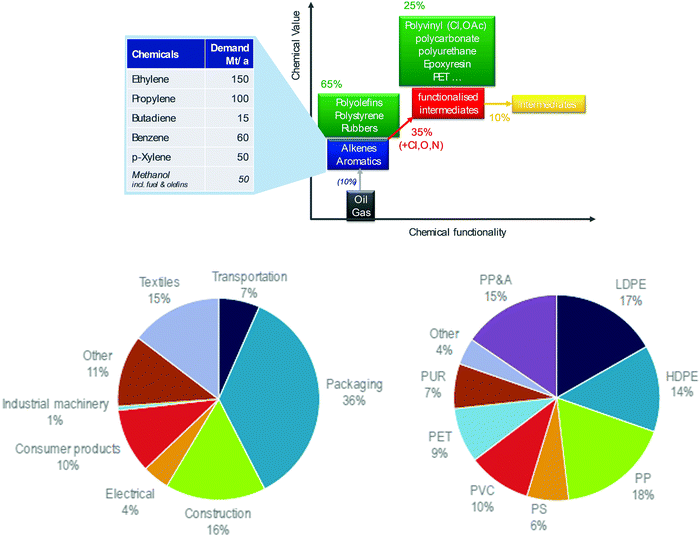 | ||
| Fig. 2 Structure of the global petrochemical industry (top) and types of polymers and global markets (bottom, from IEA10). | ||
Packaging accounts for the largest share (36% globally) (Fig. 2, bottom). Polymers are also used in construction (16%), automotive, electrical/electronic and many other markets.10 Petrochemicals are used in nearly all areas of modern life, from housing to health, clothing to sport, and transport to food. Through such pervasive use, the demand for chemicals is predicted to keep growing by ∼3% per year and thereby grow by a factor of 3–4 between 2000 and 2050. Chemical growth is expected to exceed the growth rates of global wealth (GDP) and energy demand.
Today, the petrochemical industry accounts for a small fraction (5%) of global CO2 emissions. On average, the CO2 emissions from producing base chemicals amount to about 1–2.5 kgCO2 kgproduct−1.9,12 This increases to ∼2.5 t/tplastic when considering plastic manufacture and ∼5 t/tplastic when including product use and disposal.9 If all these elements are included, plastics are estimated to account for 1.7 Gt a−1 of CO2eq. or ∼4% of global GHG emissions.14
The environmental footprint of the industry goes beyond CO2 because it also includes the co-production of organic and inorganic wastes such as salts or chlorinated by-products. These may amount to several kgwaste kgproduct−1 for chemical intermediates12,15,16 and up to 5–10 kgwaste kgpolymer−1 when considering the whole manufacturing chain for the production of polymers.15,16 The co-production of waste has been shown to correlate with and contribute to the cost of the main polymers.16 Hence, reducing waste production is likely to further help reduce manufacturing costs.
The emissions from chemical manufacturing are more than offset by the emissions saved by using the products made. For example, the International Council of Chemical Associations (ICCA) estimated an overall GHG saving of 4.4 Gt a−1 CO2eq. in 2005 (excluding fertilisers and crop protection) resulting from efficiency in insulation (2.4 Gt a−1), lighting (0.7 Gt a−1), packaging (0.2 Gt a−1), and transport (0.4 Gt a−1 through the reduction of weight or road/water/engine friction).11 But this does not imply that the energy productivity supported by chemical products offsets actual emissions; chemicals are not a carbon sink for emissions helping to reduce the actual carbon stock problem.
Let us now turn to the challenges that this important industry will face throughout the 21st century.
2 Shifts in fossil feedstock
Oil refineries and gas power plants continue to be built to meet growing energy demand. These plants are not expected to run out of fossil hydrocarbons, as often claimed in scientific papers, because the known resources of coal, oil and gas exceed by a factor of 10 the amounts consumed since the industrial revolution. Coal, oil and gas may, however, experience increasing competition from renewable energy, which is growing even faster and will increasingly challenge the utilization of existing fossil capacity. Since the energy transition is largely driven by government regulations, these shifts will proceed regionally. The present section will attempt to describe the impact this will have on the chemical industry, which today derives its feedstock from oil, gas and coal facilities.2.1 Crude oil
Oil refineries mainly produce fuels for road, air and water transportation. However, the demand for crude oil is expected to drop by 32%, which would lead to a rationalisation in the refining sector.17 Such reduction in fuel production corresponds to an ∼800 Mt a−1 capacity reduction or twice today's petrochemical output. The refining industry will therefore look at minimising margin loss by shifting more of its fuel production to making base chemicals.In the medium term (10–30 years), distillates (i.e. gasoline and diesel) will become an advantaged feed for chemicals because refineries will need to divert more product from transportation fuels to chemicals. Some complexes may select light paraffinic crude oils that are easily upgraded to chemicals. Light and sweet (low in sulphur) oils are becoming available from the US shale industry and are expected to drive global oil supply growth over the next five years.18 Others may prefer to divert the heavier oil fractions to chemicals. According to recent claims, adding processing units could stretch chemical output from today's 10% of refining output to 50%19 or even above 80%.20 Indeed, oil majors such as ExxonMobil and Shell have announced that they see petrochemicals as a growing business for decades ahead. Similarly, Gulf countries are seeking to diversify their economies beyond pumping raw crude oil. They have more than doubled their petrochemical output over the past decade, reaching 160 Mt a−1 in 2016, with Saudi Arabia leading with ∼70% of the area's capacity.21 Petrochemical capacity is also being ramped up in the other parts of the world, particularly in the US to exploit cheap shale gas, and in China to supply a growing market.22 According to Bloomberg, “As the age of the hydrocarbon enters its final era, the action increasingly moves to Asia and plastics take centre stage”.23 Oil-to-chemical facilities are now reaching scales of about 40–60 Mt a−1 in China (Rongsheng Petrochemical) and India (Indian Oil).
As for technology, two ways of shifting capacity from fuels to chemicals are being considered. In an evolutionary and indirect approach, the various cracking units are run at higher severity to crack the heavy oil fractions more thoroughly and increase the production of distillates. These will subsequently be upgraded to olefins and aromatics in steam cracker (for paraffin thermal cracking) and reformer (for paraffin dehydrogenation/cyclisation) units. In a more revolutionary and direct approach, a wider fraction of the crude is directly cracked to olefins in a steam cracker or a fluid catalytic cracking (FCC) unit. An example of the direct approach is ABB Lummus’ Heavy Oil Processing System (HOPS) technology that was made public in the early 1990s.24 Other developments include the Advanced Catalytic Olefins (ACO™) technology that has been developed by Kellogg Brown & Root and SK Innovation and the Indmax process, developed by Indian Oil.25 The hot flue gas from the cracking furnace is used to sequentially vaporise the various vaporisable fractions of crude oil and use them as feed for steam cracking. This technology is still available and applicable for light crudes. Alternatively, as elegantly reviewed by Corma et al.26 the FCC technology used today in manufacturing gasoline can be modified to process the largest portion of crude oil and convert it to olefins. The process and catalyst can be redesigned to crack the heavy fraction more thoroughly to olefins. Alternatively, a dedicated reactor can be added to crack the light paraffinic fractions under high severity, as applied in Shell's Milos process.27 Alternatively, this recalcitrant light fraction can be diverted to a steam cracker to produce olefins or a reformer to make aromatics. Irrespective of the approach adopted, the cost of upgrading crude oil to chemicals is likely to increase significantly because of the need for sizeable investment, increased demand for energy and hydrogen, and increased CO2 footprint. Moreover, the risk of retaining uncompetitive reserves of oil means the oil industry is reducing its investment in long-term mega-projects, which could eventually lead to oil shortages and price increases.28 All these developments could jeopardise the competitiveness of oil against alternative sources of chemicals such as gas or biomass, although there will likely be regional variations.
2.2 Coal
The use of abundant cheap coal as feedstock for making chemicals has been increasing in China since 2000.29 The chemicals share has gradually increased from 3% of coal production in 2010 to approximately 16% in 2018.30 Fertilisers aside, the main chemical product is methanol. Methanol capacity has grown to 15 Mt a−1 and has been increasingly used for producing polyethylene (PE) and polypropylene (PP) based on the Methanol-to-Olefin (MTO) technology developed at Dalian.31 Coal-based polyolefin reached a production capacity of 5 Mt a−1 in 2015.32 This production is still dwarfed by the 1.3 Gt a−1 of coal used for power generation in China.33 With air pollution in megacities forcing coal power plants to close, much coal remains available for chemical use. In the longer term, however, chemical use may also get regulated because of other environmental pressures, e.g. on CO2 emissions and water consumption. Coal may still be a viable chemical feedstock in the short term, but it faces an uncertain future in the longer term.302.3 Natural gas
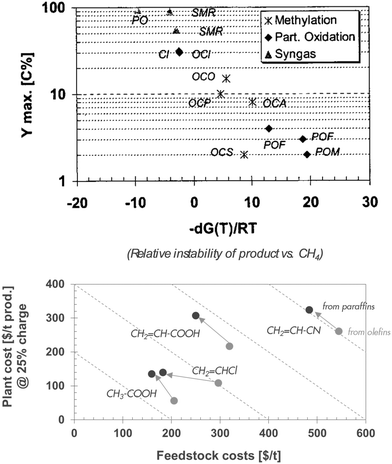 | ||
| Fig. 3 Challenges in using natural gas. Top: thermodynamic limitation to yields in direct conversion of CH4 (the acronyms are explained in the appendix).35 Bottom: economic trade-off between feed and plant costs in direct oxidation of alkanes to chemical intermediates (black) vs. conventional olefin conversion (grey) (the diagonal dotted lines of the bottom figure represent the sum of feed cost and plant cost, the lion's share of the total manufacturing cost).36 | ||
The exploitation of shale gas in the US has reinvigorated research on both reductive and oxidative routes over the past decade. Upon critical review,37 however, recent claims are not always convincing. Some claims of breaking through the yield barrier still need independent confirmation. Other developments on novel oxidants or novel engineering approaches (e.g. chemical looping) do not appear to be affordable. For the sake of conciseness, we will not review them here but refer the interested reader to other reviews.37
Eventually, considering the way thermodynamics is apparently limiting yields, we may have to accept proceeding via synthesis gas (for oxidation routes) or carbon and hydrogen (for reductive routes). The latter has recently been recognised as a promising route to CO2-free H2.38 As it produces 4 t of carbon per t of H2, it will have to find a large market for its carbon by-product.37
However, the research community has also spent decades looking for ways to bypass the olefin stage and directly convert light alkanes to more valuable chemical intermediates such as acetic acid (from ethane), acrylic acid and acrylonitrile (from propane), and methacrylic acid (from isobutane).42 The lack of industrial deployment so far suggests that the economic advantage is smaller than anticipated. Fig. 3 (bottom) shows indeed that the advantage of substituting olefins for a cheaper alkane as feedstock is largely offset by the additional processing cost of upgrading the more recalcitrant feedstock: all the processes appear to move towards lower feed cost and higher plant cost, roughly following the diagonal line of constant manufacturing cost.36
2.4 Key insight #1
The shift of transportation away from petrol and diesel and the shift of power from coal to natural gas provide the cheap fossil hydrocarbon feedstock for the petrochemical industry in the short term.In the longer term, however, the costs of oil processing will eventually be charged to the petrochemical industry and the demand for natural gas will eventually peak and then decrease, thereby weakening the competitiveness of fossil hydrocarbons.
Further weakening will come about by the increasing pressure on CO2 emissions and on plastic recycling, as will be discussed in the following sections.
3 CO2 emissions
The petrochemical industry might be a modest contributor (∼1.5 Gt a−1 or 5%) to anthropogenic CO2 emissions,2–4 but should be part of reducing emissions. Inaction could make its contribution to global CO2 emissions reach unacceptable levels by 2050, as the chemical industry grows by a factor of 3–4 and the energy sector succeeds in reducing its CO2 footprint by 80% (in line with the Paris Agreement). Inaction could also result in exorbitant increases in product prices of $200–1000 per t due to CO2 emission penalties, based on the emissions mentioned earlier and the evolution of CO2 pricing suggested in Fig. 1 (right).The petrochemical industry has been recognised as one of the sectors that are difficult to decarbonise because it needs carbon for its product and requires a lot of energy for its processes.9 We will review the options for reducing the industry's carbon footprint, starting with its utility sector, i.e. the generic provision of energy and hydrogen. We will then discuss conversion processes. We also need to look at the carbon footprint of the feedstock and will discuss this in Section 4 on circularity.
3.1 Utilities
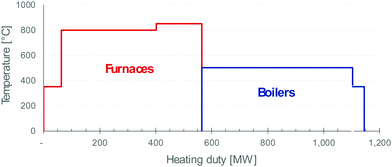 | ||
| Fig. 4 Typical profile of energy demand of petrochemical plants (furnaces in red and boilers in blue). | ||
The steam cracker is a key unit in the energy balance of a petrochemical complex.45 For instance, a large-scale naphtha cracker (e.g. with 3 Mt a−1 intake) converts 20–25% of its feed into process fuel (CH4 and fuel) that is fired to supply the ∼900 MW needed to run the cracker. The heat of hot off-gas is then used to raise steam to run other units. The H2 produced together with CH4 is generally recovered for use in hydrogenation reactions.
The second measure consists of CCS, i.e. capturing the CO2 produced by boilers and furnaces and sequestering it underground. For example, refineries and petrochemical complexes at the port of Rotterdam are working together on building a 50-km-long pipeline to collect their CO2 emissions and sequester them 3.5 km below sea level in an old gas reservoir with a capacity of 40 Mt CO2.49 According to the Global CCS institute,50 there are currently 21 plants worldwide that capture and sequester CO2. The total CCS capacity amounts to 37 Mt a−1, which corresponds to 0.1% of today's anthropogenic CO2 emissions. CO2 sequestration is generally used to enhance oil recovery, i.e. pumping CO2 into oil fields in order to push oil out. But dedicated storage has recently started to appear. For instance, Shell has so far sequestered 4 Mt of CO2 by its Quest project51 in Canada and is a partner in the Porthos project52 development to sequester 2.5 Mt of CO2 offshore of Rotterdam, the Netherlands. The cost of CCS depends on numerous factors but a major one is the concentration of CO2 that needs to be captured. The cost of CCS in the power generation sector has been claimed to have decreased, e.g. from $100 per t CO2 in 2005 to $60 per t in 2017, and may reach $40 per t in 2020–2025.50 However, public perceptions could impact its potential. CCS is obviously not limited to utilities. It has the potential to reduce process emissions, e.g. by capturing and sequestering CO2 co-produced in oxidation processes. All current credible studies to reach 1.5 degrees require large-scale CCS deployment. This is the subject of a later section.
CO2 can also be captured (from flue/off-gas) for conversion to chemical products, an approach that is often called carbon capture and utilisation (CCU) – N.B.: CO2capture from air will be discussed in Section 4.3. To deliver a net reduction in GHG emissions, CCU typically requires extensive reduction of CO2 using renewable hydrogen. Today, CCU appears to be very inefficient and expensive, particularly when compared with the available alternatives for utilities discussed in this section.53 However, CCU may be an alternative to CCS for managing CO2 released as a by-product of chemical manufacturing processes. We will therefore defer its discussion until Section 3.0, which focuses on process by-products.
Nature can also capture CO2 from the air and store it in biomass such as trees, crops and algae. Protecting existing forests and supporting reforestation programmes are therefore part of tackling climate change. The Earth is said to be able to carry 30% more forest canopy than it does today, which could raise the amounts of greenhouse gases stored by around 200 Gt of carbon (or ∼730 Gt of CO2).54 This would be the equivalent of around 18 years of global CO2 emissions at today's levels. The energy and petrochemical sectors can help conservation organisations here. For example, Shell is participating in reforestation programmes in the Netherlands, Spain and Scotland.55
Some processing equipment is easily converted to electricity. This applies to shaft power, e.g. for pumps and compressors. It also applies to low-temperature boilers that provide steam at ∼150 °C and low pressure (a few atmospheres). Similarly, distillation trains are already equipped with heat pumps when separating components with close boiling points. Other approaches to electrification are more challenging, however. For example, the electrification of high-temperature applications (350–800 °C) is practised in laboratories and other small-scale units, typically at the kW scale. Deployment at a scale of 100 MW (Fig. 4) remains unproven. Large-scale application will present new challenges in terms of equipment design, construction materials, process control, and system integration. Nevertheless, Shell and Dow Chemicals have joined forces to develop an electric furnace for steam crackers that will contribute to reducing carbon emissions from the manufacture of chemicals.57
Another challenging task is to switch from steam reforming to water electrolysis for H2 manufacture. The technology was proven at a large scale some decades ago in Norway, with a total capacity of more than 100 MW (multiple electrolysers with a total capacity of 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 N m3 h−1 or 20 kt a−1 of H2).58 Today, large-scale electrolyser units (∼5 MW) are said to cost >$500 kW−1 investment or $1800 per N m3 h−1 of H2.58 This means that electricity makes up 90% of the cost of producing the H2. Electrolysers are still at a relatively small scale compared to world-scale steam reformers, which are operating at 100
000 N m3 h−1 or 20 kt a−1 of H2).58 Today, large-scale electrolyser units (∼5 MW) are said to cost >$500 kW−1 investment or $1800 per N m3 h−1 of H2.58 This means that electricity makes up 90% of the cost of producing the H2. Electrolysers are still at a relatively small scale compared to world-scale steam reformers, which are operating at 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–300
000–300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 N m3 h−1 or 100–200 kt a−1.59 Larger-scale electrolysers have entered the refining industry. For example, Shell is part of the European consortium Refhyne, which is piloting a 10 MW PEM electrolyser to produce 1.3 kt a−1 of renewable hydrogen for Shell's Rheinland refinery. Today, renewable hydrogen (also cold green hydrogen) is said to be produced for €4–8 kg−1, depending on the region,60 but within a decade this could drop to €2.5 kg−1.61 However, the economic premises need to be reviewed before using the numbers seriously.
000 N m3 h−1 or 100–200 kt a−1.59 Larger-scale electrolysers have entered the refining industry. For example, Shell is part of the European consortium Refhyne, which is piloting a 10 MW PEM electrolyser to produce 1.3 kt a−1 of renewable hydrogen for Shell's Rheinland refinery. Today, renewable hydrogen (also cold green hydrogen) is said to be produced for €4–8 kg−1, depending on the region,60 but within a decade this could drop to €2.5 kg−1.61 However, the economic premises need to be reviewed before using the numbers seriously.
The challenges of electrification stretch beyond equipment design. Deep electrification requires a profound review of the complete heat integration of the whole chemical complex to provide or remove the right amount of energy for chemical processes. The complex also needs to operate at the right temperature level and be able to manage trips and process changes in a resilient and dynamic manner. Deep electrification also requires a careful analysis of the grid infrastructure and how the grid interacts with the industrial complex, e.g. to ensure that a shut-down at the complex cannot destabilise the grid. Finally, society will have to prioritize the access to green electrons, especially in regions without plentiful renewable capacity. It may be better to use green electrons for transport and buildings than for industry.
The cost and intermittency of renewable electricity warrant further consideration (see below). An equally important challenge, the fate of the fuel gas of the cracker, will be discussed later, in Section 3.2.
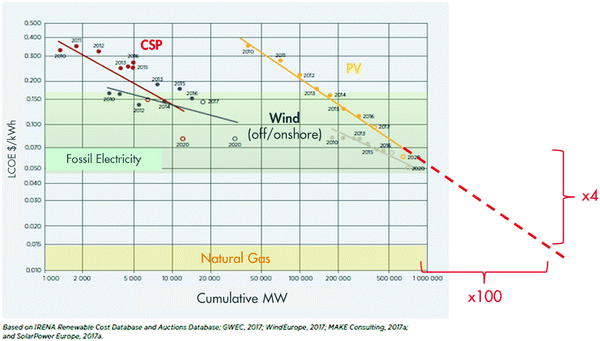 | ||
| Fig. 5 Falling cost of renewable electricity and remaining cost gap with natural gas used as energy in industry (adapted from IRENA Renewable Power Generation Costs in 201765). | ||
Another major barrier to the electrification of utilities is the intermittency of renewable electricity. In ideal subtropical locations, photovoltaic (PV) facilities are operating at daily cycle with ∼30% capacity utilisation.62 Other locations will be subject to additional seasonal cycles and might operate at a lower capacity. As for wind, temperate offshore locations probably provide the highest capacity utilisation, at around 40%.62 Chemical complexes, however, are designed to run 24/7, and accommodating the intermittency of wind and solar power is costly. One can partly smoothen the production of renewable electricity by combining sources, for example, combining PV and wind. But more measures are likely needed to secure 24/7 delivery. One additional measure is to oversize the generation facility and store the excess energy for delivery when needed. Energy can then be stored as electricity (batteries), as heat (molten salt), as potential-energy (water pumped to higher lakes or gas pressurised in caves), or as fuel (water electrolysis and H2 storage).63,64 Another measure is to design hybrid utilities that operate with renewable electricity when available, and switch to fossil electricity when necessary. But all these options require additional investment that needs to be added to the cost of renewable electricity reported in Fig. 5.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 66,67) is competitive with that of wind and solar, with the advantage of continuous supply. However, the levelized cost does not seem to consider the cost of recycling nuclear waste, e.g. through closed or breeder fuel cycles, an omission that should be corrected to avoid substituting a short-term CO2-waste problem by a long-term radioactive-waste problem.
66,67) is competitive with that of wind and solar, with the advantage of continuous supply. However, the levelized cost does not seem to consider the cost of recycling nuclear waste, e.g. through closed or breeder fuel cycles, an omission that should be corrected to avoid substituting a short-term CO2-waste problem by a long-term radioactive-waste problem.
3.2 Process and by-products
A complementary approach to reducing the carbon footprint of petrochemical processes is to keep improving them, as the industry has been doing from its very beginning.47,48 Improvements have been realised in catalyst performance, process design and operation, energy and chemical consumption, and many more. Examples taken from Shell processes show clear reductions in resource consumption, waste production, hazard and cost.48 However, the industry has also been developing new and cleaner routes for its least efficient processes, e.g. the manufacture of diisocyanates, methylmethacrylate, nylon intermediates (adipic acid, hexamethylene diamine and caprolactam) and carbonates.15,16 Such developments will continue in the future.The industry will also have to look at where it can switch from carbon-rich to hydrogen-rich feedstock to reduce its CO2 emissions. Carbon-rich feedstocks are known to lead to high carbon emissions, as illustrated in Fig. 6 for methanol and ammonia synthesis.
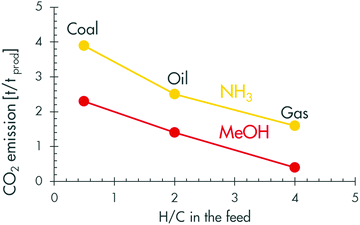 | ||
| Fig. 6 Carbon intensity of fossil feedstocks for producing methanol and ammonia (adapted from Neelis68). | ||
But all the improvements will unlikely deliver 100% efficiency. Hence, chemical processes will still waste some valuable feedstock in the form of undesirable and unavoidable by-products. Examples include CO2 co-produced in oxidation processes, e.g. for producing ethylene oxide, maleic and phthalic anhydride, and acrylic and methacrylic acid. Unavoidable by-products also include CH4 produced during steam cracking or aromatic dealkylation. In the future, these by-products should not be released into the atmosphere (CO2) or used as fuel gas (CH4). Instead, they should be recovered and used as valuable feedstock for more chemicals.
Molecular separation is responsible for about half of the US industrial energy use and 10–15% of the total US energy use.69 Half of the energy used for molecular separation is consumed in distillation, with the main contribution coming from crude distillation, ethene/propene purification and BTX purification. CO2 and CH4 recovery from off-gas is also a significant energy consumer. Energy-lean alternative technologies are needed, e.g. based on affinity or membrane separation.
As mentioned earlier while discussing utilities (Section 3.1.2), CO2 can be captured from off-gases and sequestered to avoid release into the atmosphere. Alternatively, CO2 can be used as feedstock (CCU) in two main ways. Firstly, CO2 can react with high-energy reactants such as epoxides to make cyclic or acyclic carbonates, or with ammonia to make urea. Secondly, CO2 can be converted to valuable products through reduction either electrolytically or with hydrogen. Importantly, the reduction agent needs to be ‘decarbonised’ to contribute to a net CO2 saving. However, the reduction of CO2 to chemical products will probably remain an economic challenge for decades to come, as illustrated by the following consideration. Let us consider hypothetical CO2 conversion to a variety of products and assume that the price of the final product needs to cover only the cost of the stoichiometric hydrogen consumption. For the sake of argument and quite unrealistically, we will ignore all other costs – of investment, catalyst, chemicals and energy. We will also assume that CO2 is free or, even better, has a heavy negative price of $100 per t that penalises CO2 emission. Under such very optimistic assumptions, only a few commodity products could afford costly low-carbon H2, namely formic acid and ethylene glycol (Fig. 7). Most other chemical intermediates cannot afford H2 at ∼$4 kg−1. Renewable hydrogen is more expensive than this in most parts of the world60,61 but will probably drop to this level in a few decades as PV and electrolyser technologies improve (Fig. 7). One should also realize the burden CCU could pose on the production of green electricity: the production of 1 Mt a−1 of methanol will require 1.25 GW of low-carbon electricity or 4 GW of installed capacity for intermittent generation (see Section 4.4).
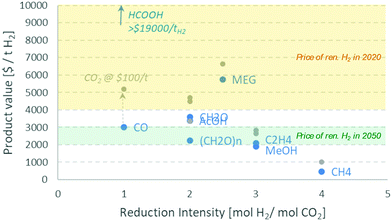 | ||
| Fig. 7 Affordable price of renewable H2 for hypothetical CO2 conversion assuming that the full product value is used to pay for the stoichiometric H2 consumption, without placeholder for investment and operating costs (cost of renewable H2 from ref. 60,61; HCOOH: formic acid, MEG: ethylene glycol, CO: carbon monoxide, CH2O: formaldehyde, AcOH: acetic acid, C2H4: ethene, MeOH: methanol, CH4: methane, (CH2O)n: sugar). | ||
In a low-carbon world, the CH4 by-product of chemical processes, e.g. of steam crackers, may no longer be a desirable process fuel. It may be valued more as a chemical feedstock. So far, the most valuable feedstock use is likely to be as synthesis gas, e.g. for carbonylation or hydroformylation reactions. Classic examples include the hydroformylation of propene of higher olefins to n-butanol or detergent alcohols. Such processes are likely to be too small to make full use of the ∼500 kt a−1 of CH4 that is produced by a naphtha cracking plant of 1 Mt a−1 ethylene capacity. Direct upgrading to chemicals is a very challenging task that will be briefly discussed later.
3.3 Key insight #2
Cutting the CO2 emission of petrochemical plants to a net zero level will be very challenging. Efficiency improvements will reduce emissions at modest cost but also at modest volumes. Electrification of utilities and processes and switch to renewable H2 promise larger CO2 reduction but will be expensive: it will require major investments for electricity production, storage and utilisation and will require new technologies and investment to divert the unavoidable by-products (e.g. CH4) from process fuels to chemical products. Beyond a few niche applications, CCU will require massive amounts of electricity and thereby massive investments. In fact, CCS is probably the most promising option for CO2 abatement for the short term.Moreover, these measures are insufficient, for none of them address the intrinsic carbon intensity of the fossil feedstock of the industry. This will be discussed below.
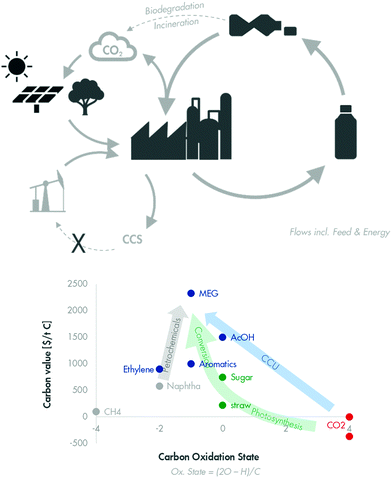
4 Circular economy
The forces of the energy transition discussed above combine with a broader societal aspiration, namely the development of a circular economy throughout all sectors.5 For the petrochemical industry, the circular economy calls for closing carbon cycles (Fig. 8, top). It starts by collecting and recycling our used carbon, with waste plastic being the most visible example. Options for recycling used carbon and, more specifically, waste plastic will be discussed in Section 4.1.However, recycling is inevitably accompanied by losses and therefore requires a significant amount of fresh input. This fresh carbon input will come from fossil carbon, e.g. from oil or gas, for some time (Fig. 8, bottom left). Applying CCS to the CO2 produced during recycling will help reduce the environmental footprint of plastic but will not make the industry truly circular. A circular economy will require feeding renewable carbon into the fresh carbon input stream. Renewable carbon is derived from atmospheric CO2. As discussed in Section 3.2, the reduction of ‘free’ or even ‘penalised’ CO2 to chemicals is unlikely to be affordable except in a few cases. Adding the expense of capturing CO2 from the atmosphere will make the cost of CCU more prohibitive.
The use of biomass as renewable carbon could be more attractive. Biomass has already captured CO2 from the atmosphere and has also performed much of the costly reduction duty needed (Fig. 8 – bottom right). This will be the subject of a later section (Section 4.3).
4.1 Recycled carbon – plastic recycling
Efficient recycling of the used polymer should ensure efficient recycling of carbon and aim at minimising energy consumption and waste production over the whole life of the product. This implies operating the shortest possible recycle loop. Depending on the quality and purity of the waste, priority should therefore be given to reuse, then reprocessing (mechanical recycling), then depolymerisation, then conversion of a general hydrocarbon feedstock and, as a last resort, energy recovery (Fig. 9, top). This priority list is often presented as the priority pyramid in waste management, which really starts with reducing the use of polymers, e.g. by avoiding superfluous packaging or using more advanced polymers in lower amounts.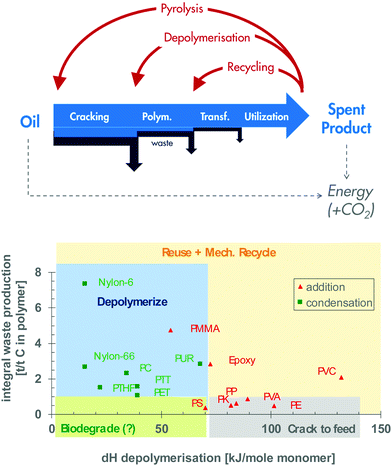 | ||
| Fig. 9 Options for plastic recycling (adapted from Lange15). | ||
Globally, about 12% of used plastic is recycled mechanically, while <1% is recycled back to its monomer.70 The rest is either incinerated (25%) or ends up in landfill or unmanaged dumps. Accordingly, mechanical recycling has developed well over the years by using the pure and clean waste fractions, often coming from industrial waste. Minor polymer contaminants that are present in such streams are generally stabilised by additives prior to blending recycled resins with virgin ones.71,72
While very efficient, mechanical recycling is bound to be limited to a few cycles, using the minor fraction of the purest and cleanest waste stream.73,74 But even these purest streams are slightly contaminated (e.g. with additives and foreign polymers) and may have been slightly degraded upon use or processing (e.g. oxidation and/or minor depolymerisation). Blending them in virgin polymers requires the use of compatibilizers, which further increases the degree of contamination of the final product. In fact, mechanical recycling seems to mainly consist of downcycling, e.g. from PET bottles to PET fibers or from PP textile to playground equipment.73,74 Moreover, mechanical recycling is unsafe for food packaging since it is difficult to guarantee the purity required. Hence, there is a need for complementary recycling options, particularly chemical recycling. So, let us review the limitations of mechanical recycling.
In these circumstances, chemical recycling is needed.71,72 Some polymers can and should be depolymerised back to their monomers. Others can only be cracked to a general feedstock, while still others have no attractive chemical recycling options. The basis for these recommendations is illustrated in Fig. 9 (bottom).15 The horizontal axis helps identify the polymers that are easily depolymerised back to their monomer (dH < 70 kJ mol−1, typically condensation polymers) and those that are only cracked to a general hydrocarbon through more severe pyrolysis (dH >70 kJ mol−1, typically addition polymers). The y-axis represents the resources wasted when producing the polymer. It thereby represents the ‘incentive’ to recover the monomer rather than degrade it back to hydrocarbon. This simple mapping suggests the benefits of cracking polyolefins back to general feedstocks (lower right quadrants) while depolymerising PET and polyamides back to their monomers (upper left quadrants).
Chemical recycling becomes particularly critical in valorising the 10% of plastic waste present in municipal solid waste (MSW).75 After preliminary sorting, around 60% of the plastic component consists of polyolefins (HDPE, LDPE, LLDPE and PP), with the rest being PET, PVC, PS and other minor polymers. Further and careful sorting may deliver a modest quantity of relatively pure polymers that can be considered for mechanical recycling. However, it also produces mixed fractions that, at best, allow mechanical ‘downcycling’ for applications with low-quality requirements. More often, though, the residual plastic stream cannot be reprocessed because of contamination, for example, and it cannot be depolymerised, for example, because of its high polyolefin content (lower right quadrant in Fig. 9, bottom). It therefore needs to be pyrolysed back to a hydrocarbon stream. This technology produces paraffinic/olefinic waxes under mild conditions, aromatic products under more severe conditions and gas and char at the highest severity.72,76 The hydrocarbon product can eventually, after proper treatment, be processed into a synthetic fuel or cracked further into lower olefins. Steam cracking typically delivers olefins and aromatic base chemicals with an ∼65 w% yield, with co-production of ∼10% of gasoline and 25% of fuel gas and fuel oil. A similar product slate is expected from processing the pyrolysis oil obtained from polyolefins. At the end of 2019, Shell announced it had used its cracker at the Norco Refinery, in Louisiana, USA, to process pyrolysis oil which had been made from waste plastic by Nexus Fuels.77 It also announced its ambition to process 1 Mt a−1 of plastic waste by 2025.
But one should not underestimate the challenge of waste collection. A small amount of plastic waste, such as small-sized single-use packaging, could remain uncollected and end up in the environment. They could be made of biodegradable material to stop them from accumulating in the environment (Fig. 9, bottom).
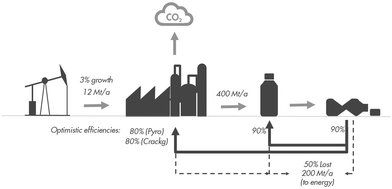
However, recycle loops inevitably have losses. The case study presented in Box 1 shows that these can be quite significant, up to 50%. These losses will need to be compensated for by fresh input. Fresh input will also be needed to feed the growth of the chemical industry. Hence, fresh input could still be quite significant, e.g. 400–550 Mt a−1 by 2050 (see Box 1).
Box 1. Inefficiency of carbon recycling.Plastic recycling will be accompanied by material losses at every step of the cycle, from collection to sorting to high/low grade material, mechanical recycling of the high-grade fraction and chemical recycling of the low-grade fraction. Assuming optimistic efficiencies of 90% for collection, 80% for sorting (20% to high-grade, 60% to low-grade), and realistic efficiencies of 90% for mechanical recycling and 80% + 75% for the pyrolysis and steam cracking steps of mechanical recycling, we arrive at an overall efficiency of 50%. Hence, ∼50% of the material is lost. Ideally, the lost material will avoid ending up in the environment as uncontrolled litter or controlled landfill, and will instead be valorised as energy or fuel.The lost material and an expected 3% demand growth need to be covered by fresh carbon input. For today's scale of ∼350 Mt a−1, this implies a need for 185 Mt a−1 of fresh input. By 2050, the required fresh input could have grown to 400–450 Mt a−1. |
4.2 Key insight #3
The deployment of collection, sorting and recycling technologies will lead to a cascade of solutions that vary from recycling the cleanest stream with the highest efficiency to destroying the dirtiest ones with minimum health and environmental impact. But complete recycling will remain a utopia, for material losses will be unavoidable throughout the cycle. Fresh carbon input will be needed to compensate for recycling losses and business growth.In the short to mid-term, the fresh carbon input may contain fossil carbon that is ideally accompanied by CCS to avoid net CO2 emissions. But all the factors discussed so far will eventually erode the competitiveness of fossil carbon and open the field to renewable carbon, i.e. biomass and atmospheric CO2.
4.3 Renewable carbon – biomass
Biomass is a convenient way to feed atmospheric CO2 into the petrochemical value chain, because it has captured CO2 from the atmosphere and has also done >2/3 of the reduction duty required (Fig. 8 – bottom right). The true promise of bio-based chemicals will depend on factors including feedstock selection, availability and sustainability, and product and process selection.But are these feedstocks available in enough volume to supply the 400–550 Mt a−1 needed for the chemical cycle by 2050? Today's grain and sugar production for food and feed amounts to 3–4 Gt a−1. This volume is expected to keep growing in the future as agricultural knowledge spreads throughout the world. Hence, some 60–80 Mt a−1 (i.e. 2%) could be diverted to chemicals without disturbing the food/feed supply. Such production volume could take some 20–25 years to develop, considering today's volume of 7 Mt a−1 and growth of 10%.79 By then, one can expect lignocellulosic technologies to have been deployed and improved to the point of being competitive for chemical production. They could then contribute to the further growth of bio-based chemicals, catching up on sugar/starch-based chemicals in 20 years and then growing to ∼400–550 Mt a−1 over the following 20 years, i.e. by about 2080 (still assuming the annual growth of 10%). Once lignocellulose is competitive, the potential stock of biomass becomes more than enough, with conservative estimates of 6–18 Gt a−1 (100–300 EJ) being available after deducting for world food supply and allowing for sustainable water and land management.80
One also needs to consider the environmental footprint of biomass. Since biomass stores atmospheric carbon and solar energy, bio-based chemicals are generally produced with lower CO2 emissions and lower non-renewable energy demand than fossil-based chemicals (Fig. 10, bottom).81,82 However, the production of biomass may affect air and water quality, water and land usage or biodiversity.81 These factors are determined by the specifics of the project (e.g. location, climate, agricultural practice). A proper balancing of advantages and disadvantages must therefore be done on a project-by-project basis.
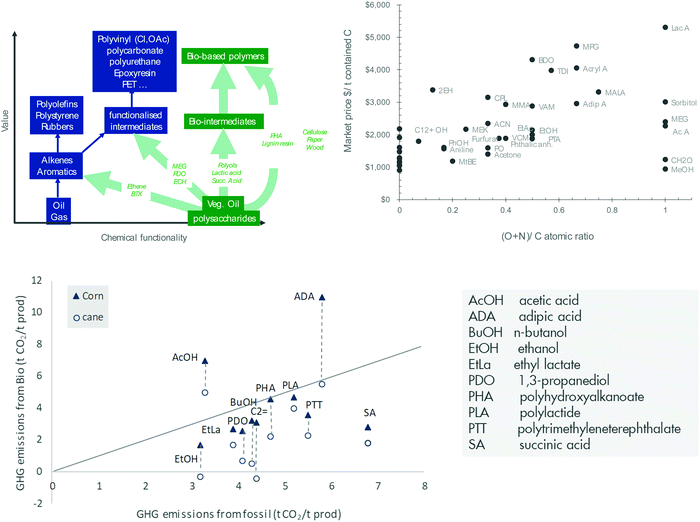 | ||
| Fig. 10 Bio-based chemicals: strategies for product selection (top left). Market prices of drop-in base and intermediate chemicals (top right, acronyms are clarified in the appendix, updated for $70 per bbl crude from ref. 78). Lifecycle GHG emissions of chemicals are generally lower when derived from sugars than fossil feedstock (bottom, adapted from ref. 82). | ||
An alternative strategy that is also widely encountered in the industry is the production of novel intermediates or materials (Fig. 10, left). Novel intermediates include lactic acid, succinic acid, furandicarboxylic acid, levulinic acid, propane diol and isosorbide. Novel materials can also be derived from biomass through simple modification of biopolymers without the need for extensive depolymerisation. Typical examples include nanocellulose and lignin materials. Novel intermediates and materials might be more readily and efficiently manufactured from biomass. They might therefore come at a lower cost. However, the market does not yet exist and needs to be created. The pace of deployment may therefore be limited by the creation of the market. The history of the petrochemical industry has shown that it takes some 20 years for a new polymer to break through and an additional 20 years to mature its market.
Since it is renewable, biomass is intrinsically circular. This raises the possibility that bio-based chemicals can also be largely circular, if the manufacturing process involves the use of small amounts of energy or renewable energy and chemicals. This potential for circularity does not release bio-based chemicals from the need to be recycled through the smallest possible recycle loop. Biodegradation and incineration remain options of last resort, e.g. for products that are difficult to collect and therefore liable to end up in the environment.
The valorisation of biomass will also require the development of alternative separation technologies with low-energy demand.84,85 Intermediates are often produced in water and therefore costly to recover, particularly when they have a higher boiling point than water.86 Moreover, bio-based intermediates and bio-based products are generally polar and therefore prone to the formation of azeotropes that are challenging to break using distillation.
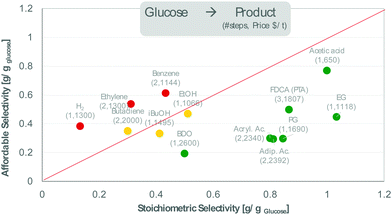
Box 2. Valorising the oxygen of biomass.The economic potential of biomass conversion routes can be readily assessed by checking that the stoichiometric selectivity of a process exceeds the affordable selectivity, e.g. as defined by eqn (1) [a].
A similar analysis can be applied to the direct valorization of lignocellulose. With feedstock and product prices of $80 per t and $700 per t, the production of mixed mono/diaromatics would require a selectivity of 40 wt%, which corresponds to <10% of the carbon being lost to gas and char. For the production of sugars, priced at $300 per t, the combination of pretreatment and hydrolysis requires a sugar selectivity of 160 wt%, which is about three times the sugar content of lignocellulose. ----- Economic premises: glucose: $300 per t; conversion cost $200 per t per step; product prices from [b] References: [a] J.-P. Lange; Catal. Sci. Technol., 2016, 6, 4759 – 4767; [b] A.J.J. Straathof, A. Bampouli; BioFPR 2017, 11, 798–810 |
4.4 Renewable carbon – atmospheric CO2
Atmospheric CO2 could also be used directly as a renewable carbon source. There has to be CO2 capture from the air and deep CO2 reduction with renewable electricity, either directly via CO2/H2O co-electrolysis or indirectly via water electrolysis and subsequent CO2 hydrogenation. Such approach will require much electricity and high investment.The conversion of CO2 to methanol using electrolytic H2 is reported to require about 10 MWh renewable electricity to produce 1 ton of methanol.87 To match a world-scale methanol of 1 Mt a−1 capacity such process will require about 1.25 GW of electricity or nearly 4 GW of installed wind or solar capacity, when considering a production factor of ∼30% due to intermittency. Further conversion of methanol, e.g. using MTO technology, can deliver about 400 kt a−1, about a quarter of the production of a world-scale steam cracker.
As for the cost, the chemical hydrogenation of CO2 is arguably the cheapest of all process steps. Catalytic technologies are already available to convert CO2/H2 to methanol or hydrocarbons.88,89 All other steps of the manufacturing chain are much more expensive and account together for the lion's share of the investment and production costs of CO2-derived fuels and chemicals.90 Working backwards, this applies to water electrolysis to get H2 (see Section 3.1.3) and even more for the co-electrolysis of CO2 and H2O, which is at an early stage of development.91 The generation of renewable electricity (see Section 3.1.4, Fig. 5) and the capture of CO2 from the atmosphere can also be costly.92 Furthermore, all these emerging technologies need to be proven at scale. Overall, CO2-derived fuels and chemicals, being methanol or Fischer–Tropsch hydrocarbons, are estimated to cost €1200–1700 per t at 220 kt a−1 scale,93 which is very expensive when compared to methanol ($300 per t) and naphtha (∼$500 per t) and even sugars ($300 per t). However, methanol and naphtha might not be the best product targets. Like biomass, CO2 valorisation will offer better opportunities when targeting less reduced oxygenated products such as formic acid and ethylene glycol (Section 3.1.3). The cost structure might be more promising when targeting less reduced products, as discussed and illustrated in Fig. 7 and confirmed for CO2 electroreduction.43
Although more expensive than biomass today, the valorisation of atmospheric CO2 should not be discarded. Technology is improving rapidly and the manufacturing route may have a much lower environmental footprint in terms of GHG emissions, and land and water use than bio-based routes.93
4.5 Key insight #4
Eventually, the chemical industry would have to switch to plastic waste and biomass (ultimately CO2) as feedstock to reduce the CO2 intensity of plastics and achieve full circularity.The switch of feedstock, from reduced to oxidised carbon, may lead to a different product slate, one that has more oxidised materials. In the long term, hydrocarbon polymers such as polyolefins may be superseded by oxygenated polymers such as polyesters or bio-based materials (e.g. based on wood, straw or paper) in various applications.
The whole sorting and recycling chain that is being built now will have to evolve to accommodate more bio-based plastics and materials.
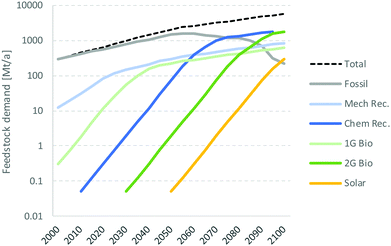
4.6 Pace of the transition
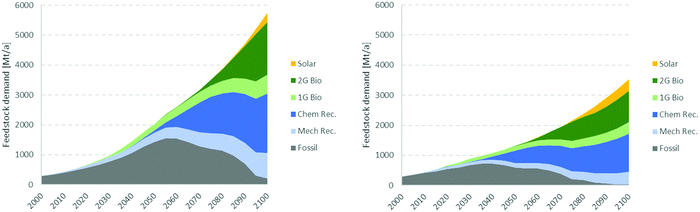
Inspired by the historical emergence of energy technologies reported by Kramer and Haigh,94 a model can be developed to illustrate the potential emergence of new technologies and their respective feedstock for the carbo-chemical industry during the 21st century. Based on the premises described in Box 3, the new technologies could remain modest around 30% (20% for recycling and 10% for bio) until ∼2050 but could overtake fossil feedstock by ∼2070 (Fig. 11). Such development would see fossil-based chemicals peaking in about 2050 and ending the century at ∼0.2 Gt a−1, slightly below today's level and 4% of the total demand of ∼5.7 Gt a−1. It should be stressed that the present scenario is describing the growth of demand, not that of chemical production. Part of the demand might indeed shift from today's plastics to natural light-weight materials such as wood, paper, cardboard, natural fibers, etc.This scenario represents an aggressive feedstock diversification. Low oil prices or technological setbacks could cause diversification to develop more slowly. For example, reducing the early growth rate from 20 to 15% would shift the peak in fossil feedstock back to ∼2100. But it also underlines the challenge that the chemical industry is facing in decarbonizing utilities and feedstock to meet the Paris agreement. How can it become carbon neutral by 2050 if it needs to use three times more fossil hydrocarbon as feedstock than today? Can it all be compensated by CCS or NBS? This not very likely. The industry must transition much faster than modelled here. So, it is important to understand the basis of the premises applied.
The model was derived from the deployment of energy technologies over the past 50 years.94 This development was driven by balancing convenience and energy price for the consumer, and balancing profitability and economic risks for the industry, all in a period of high economic growth. But these conditions do not apply today anymore. The slower economic growth and the drive are shifting to societal and governmental concerns about the state of the environment. Let us assume that these conditions could accelerate the development of new technologies and also accelerate the deployment of non-technical enablers such as infrastructure, governance, education of future installers and operators. Let us also assume that the public accepts new facilities in its own backyard. If it all works out, the transition to recycled and renewable feedstock could materialise a bit earlier. If, for instance, the new technologies are deployed with 30% annual growth instead of 20%, the peak in fossil feedstock could shift by 5 years to around 2050 and from a maximum demand of 1.5 Gt a−1 to 1.2 Gt a−1.
But such a scenario is not yet complete: the economic burden of such accelerated transition could also reduce the growth of the global economy and of the demand for chemicals. If we now assume that the accelerated deployment of new technologies discussed above also reduces the growth of the global economy to 2% beyond 2025, the demand for chemicals could be limited to 3.5 Gt a−1 by 2100 and the demand for fossil chemicals could peak around 2040 and 0.7 Gt a−1 (Fig. 11, right), i.e. 10 years earlier than estimated for the market-driven scenario and with 50% peak demand for fossil feedstock.
But such a scenario may still need more refinements. It could bring social tensions, as part of the people recognize that they cannot reach the wealth they aspire to and another part needs to give in what it has enjoyed so far. This would further affect the economic development and the demand for chemicals. Such refinements are better left to social and modelling scientists and will not be considered further here.
Box 3. Illustrative scenario of potential emergence of new feedstock for chemicals.The market-driven model assumes that the overall demand for petrochemicals amounts to 300 Mt a−1 in 2000 and will grow by 4% annually until 2050 and then by 2% a year until 2100. During this period plastic recyclate, first and second generation (1G and 2G) bio-feedstock, and solar chemicals gradually displace fossil feedstock. Novel technologies are assumed to start at 50 kt a−1 scale and grow by 20% annually until they reach about 10% of their “mature share” of petrochemicals defined in the table below, typically after ∼30 years. Their growth rate decreases gradually to the total growth (4% or 2%) within 20 years and stays there until 2100. The starting date and mature share assumed for the various technologies were defined arbitrarily, though sensibly, as reported in the table below. The remaining demand is then satisfied by traditional fossil feedstock, which is assumed to eventually drop to zero when all alternative technologies have fully matured. The resulting development of new technologies is illustrated in the figure below.
|
4.7 Key insight #5
The transition from fossil to recycled and renewable carbon as feedstock for chemicals is likely to take most of the 21st century, even when accelerated beyond the pace expected for market-driven development. The demand for fossil feedstock could peak around 2030–2050 and reach 0.7–1.5 Gt a−1, i.e. 2–5 times the demand of 2000.5 Conclusion
During the energy transition, the petrochemical industry will initially have an abundance of hydrocarbon feedstock because light-duty transport will have less demand for distillate fractions of crude oil, and C2+ hydrocarbons will be produced with natural gas. But the growing demand for chemicals will be unable to compensate for the fall in demand for transportation fuels. The combination of decreasing fuel revenues, increasing investment intensity and increasing CO2 pricing could eventually challenge the industry on several fronts, namely CO2 emissions and alternative low-carbon feedstock (recycled and renewable carbon).Like all sectors, the chemical industry is being urged to minimise its contribution to climate change. Managing the chemical industry's CO2 emissions will require redefining its utilities and processes. For example, redefining the industry's utilities will probably require a combination of improved efficiency, a shift to low-carbon fuel, electrification, CCS, and water electrolysis to produce H2. Emissions from chemical processes will probably be tackled by improving efficiency, switching to hydrogen-rich feedstock (e.g. from coal to oil and ultimately from gas to bio-feedstocks) and converting unavoidable process by-products such as CH4 and CO2 to chemicals. All these measures will require very large investment and will take time to be deployed. Furthermore, they do not address the intrinsic carbon intensity of the fossil feedstock, which will eventually force the industry to switch to renewable carbon as feedstock, i.e. first to biomass and, possibly, to CO2 in the longer term.
Society and industry are also being urged to solve the problem of plastic pollution. Proper management will start with strong governance to stimulate people to collect their used plastic responsibly. However, it will also require a resilient approach that recycles or disposes of waste according to its quality and follows a responsible hierarchy of waste management. Such an approach will comprise a combination of mechanical recycling, chemical recycling (e.g. depolymerisation and cracking to feedstock) and, ultimately, energy valorisation (e.g. by conversion to fuel or co-incineration with organic waste).
Despite all possible efforts, one should not expect to achieve full carbon recycling. Fresh input will be needed to compensate for the carbon losses in the cycle. Initially, this will come from fossil hydrocarbons whose use will be mitigated by CCS. But fossil hydrocarbons will gradually be replaced by renewable carbon, particularly biomass for supplying high-value oxygenated intermediates. As technology matures, first generation (1G) biomass feedstock will be complemented by second generation (2G) biomass, and products will gradually move towards lower-value commodity chemicals. In the longer term, some renewable carbon may come from direct capture of CO2 from the atmosphere, followed by partial reduction with renewable electricity and water. However, the technology needs to develop dramatically before it can compete with biomass. The underlying technology development to this longer-term shift to renewable carbon as feedstock is not being solely driven by chemicals, but by the broader energy transition, e.g. to provide renewable carbon-based fuels. They will become part of the transition of the chemical industry.
Overall, the transition of the chemical industry from fossil to recycled and renewable carbon could take most of the 21st century. The demand for fossil carbon may still increase until 2030–2050 and only then decrease to near zero by 2100.
Conflicts of interest
There are no conflicts to declare.Appendix – list of acronyms
| 2EH | 2-Ethyl hexanol |
| ACN | Acetonitrile |
| AcOH | Acetic acid |
| ADA | Adipic acid |
| BDO | 1,4-Butanediol |
| BTX | Benzene, toluene, xylenes |
| BuOH | n-Butanol |
| Cl | Methane chlorination |
| CPL | Caprolactam |
| EtOH | Ethanol |
| EtLa | Ethyl lactate |
| MALA | Maleic anhydride |
| MEG | Monoethylene glycol |
| MEK | Methyl ethyl ketone (butanone) |
| MMA | Methylmethacrylate |
| MPG | Monopropylene glycol |
| MTBE | Methyl t-butyl ether |
| OCl | Methane oxychlorination |
| OCA | Oxidative coupling of methane to aromatics |
| OCO | Oxidative coupling of methane to olefins |
| OCP | Oxidative coupling of methane to paraffin |
| OCS | Oxidative coupling of methane/toluene to styrene |
| PC | Polycarbonate |
| PDO | 1,3-Propane diol |
| PE | Polyethylene |
| PHA | Polyhydroxyalkanoate |
| PK | Polyketone |
| PLA | Polylactide |
| PMMA | Polymethylmethacrylate |
| PO | Propylene oxide (Fig. 10) or partial oxidation to syngas (Fig. 3) |
| POF | Partial oxidation of methane to formaldehyde |
| POM | Partial oxidation of methane to methanol |
| PP | Polypropylene |
| PS | Polystyrene |
| PTA | Purified terephthalic acid |
| PTHF | Polytetrahydrofuran |
| PTT | Polytrimethyleneterephthalate |
| PUR | Polyurethane |
| PVA | Polyvinyl alcohol |
| PVC | Polyvinylchloride |
| SA | Succinic acid |
| SMR | Steam methane reforming |
| TDI | Toluene diisocyanate |
| VAM | Vinyl acetate monomer |
| VCM | Vinyl chloride monomer |
Acknowledgements
The author is grateful to his colleagues Piet Huizenga and Marc Klokkenburg for their valuable contribution to the sections on crude oil (Section 2.1) and utilities (Section 3.1), respectively, and for reviewing the whole manuscript.References
- Shell Scenarios – SKY – meeting the goals of the Paris agreement; http://www.shell.com/skyscenario (last visited on April 2021).
- J. M. Allwood and J. M. Cullen, Sustainable Materials with both eyes open, UIT, Cambridge, 2012 Search PubMed.
- https://www.iea.org/reports/the-future-of-petrochemicals (last visited on April 2021).
- https://www.ipcc.ch/site/assets/uploads/2018/02/ipcc_wg3_ar5_chapter10.pdf (last visited on April 2021).
- McKinsey – circular economy (2016) http://www.mckinsey.com/~/media/McKinsey/Business%20Functions/Sustainability/Our%20Insights/The%20circular%20economy%20Moving%20from%20theory%20to%20practice/The%20circular%20economy%20Moving%20from%20theory%20to%20practice.ashx (last visited on April 2021).
- Alliance to End Plastic Waste; https://endplasticwaste.org/?gclid=CjwKCAjwlbr8BRA0EiwAnt4MTuz9BFjWZD24Jue8u8qJXYB8o4BlzFKw5osbO3aN63tKZohVO3jiERoCPqwQAvD_BwE (last visited on April 2021).
- The Energy Transformation Scenarios – Waves, Island and sky 1.5 (2021) – https://www.shell.com/promos/energy-and-innovation/download-full-report/_jcr_content.stream/1612814283728/d14d37b7dd060d78b65bfee3c7654520e10381aa/shell-energy-transformation-scenarios-report.pdf (last visited on April 2021).
- Ellen McArthur foundations – the new plastic economy, rethinking the future of plastics, 2016.
- Energy Transition Commission, Mission Possible: Reaching net-zero carbon emissions from harder-to-abate sectors, 2018, https://www.energy-transitions.org/publications/mission-possible/ (last visited on April 2021).
- Int. Energy Agency (IEA), The future of petrochemicals, towards more sustainable plastics and fertilizers, 2018.
- ICCA, Innovations for Greenhouse Gas Reductions – a life cycle quantification of carbon abatement solutions enabled by the chemical industry, 2009.
- ICCA/IEA/DECHEMA Roadmap Catalysis; Petrochemical Industry Energy & GHG Savings via Catalysis –Still a Large Opportunity, 2013, http://www.americanchemistry.com/Catalysis-Roadmap/ (last visited on April 2021).
- R. C. Valencia, The future of the chemical industry by 2050, Wiley-VCH, Weinheim, 2013 Search PubMed.
- J. Zheng and S. Suh, Nat. Clim. Change, 2019, 9, 374–378 CrossRef.
- J.-P. Lange, Green Chem., 2002, 4(6), 546–550 RSC.
- J.-P. Lange, in Sustainable Development in the Process Industry – cases and impacts, ed. P. Harmsen, Wiley, 2010, pp. 23–38 Search PubMed.
- https://www.iea.org/fuels-and-technologies/oil (last visited on April 2021).
- International Energy Agency (IEA), World Energy Outlook, 2018, https://www.iea.org/reports/world-energy-outlook-2018 (last visited on April 2021).
- D. Moore, Hydrocarbon Processing, “Viewpoint: The opportunity for greater growth and value—Considerations for crude-to-chemicals projects”, Hydrocarbon Processing, December 201.
- D. McCarthy, Crude to Chemicals – Opportunities and Challenges of n Industry Game-Changer, MERTC, Bahrein, 2017 Search PubMed.
- M. Sergie, Bloomberg, June 4. 2018 http://www.bloomberg.com/news/articles/2018-06-04/mideast-bets-on-100-billion-industry-as-oil-use-outlook-dims.
- A. H. Tullo, Chem. Eng. News, 2019, 978, https://cen.acs.org/business/petrochemicals/future-oil-chemicals-fuels/97/i8 Search PubMed.
- Blomberg, Global Oil Refining Faces Shake-Up From Asian Plastics Boom (2) (2020) https://news.bloomberglaw.com/environment-and-energy/global-oil-refining-faces-shake-up-from-asian-plastics-boom-2 (last visited on April 2021).
- ABBLummus Crest Inc., (presentation) HOPS, “Heavy Oil Processing System”, Jun. 15, 1992 TCC PEW Meeting, pp. 1–18.
- M. De Falco, New Catalytic Process for Production of Olefins: http://www.oil-gasportal.com/new-catalytic-process-for-production-of-olefins/ (last visited on April 2021).
- A. Corma, E. Corresa, Y. Mathieu, L. Sauvanaud, S. Al-Bogami, M. S. Al-Ghrami and A. Bourane, Crude oil to chemicals: light olefins from crude oil, Catal. Sci. Technol., 2017, 7, 12 RSC.
- Y.-M. Chen, “Evolution of FCC – Past Present and Future and The Challenges of Operating a High Temperature CFB System” in 10th International Conference on Circulating Fluidized Beds and Fluidization Technology – CFB-10, T. Knowlton, PSRI Eds, ECI Symposium Series, (2013). http://dc.engconfintl.org/cfb10/72.
- G. Sachs, Re-imagining Big Oil: How Energy Companies can successfully adapt to climate change, Equity Search, November 1, 2018, http://www.goldmansachs.com/insights/pages/reports/re-imagining-big-oils-f/re-imagining-big-oils-report-pdf.pdf (last visited on April 2021).
- J. Li and S. Hu, Resources, Conserv. Recycl., 2017, 124, 13 CrossRef.
- O'Reilly, CTO and MTO projects in China may decelerate, Hydrocarbon Engineering, 2019, https://www.hydrocarbonengineering.com/petrochemicals/29052019/cto-and-mto-projects-in-china-may-decelerate/ (last visited on April 2021).
- M. Yang, D. Fan, Y. Wei, P. Tian and Z. Liu, Adv. Mater., 2019, 31, 1902181 CrossRef CAS PubMed.
- A. M. Houri, Chem. Eng. News, 2015, 3031 Search PubMed.
- https://en.wikipedia.org/wiki/Coal_in_China (last visited on April 2021).
- J.-P. Lange, Catal. Today, 2001, 64, 3–8 CrossRef CAS.
- J.-P. Lange, K. P. de Jong, J. Ansorge and P. J. A. Tijm, Stud. Surf. Sci. Catal., 1997, 107, 81–86 CrossRef CAS.
- J.-P. Lange, in Sustainable strategies for the upgrading of natural gas: fundamentals, challenges and opportunities, ed. E. G. Derouane, et al., Kluwer 2005, pp. 51–83 Search PubMed.
- C. Mesters, Annu. Rev. Chem. Biomol. Eng., 2016, 7, 223–238 CrossRef PubMed.
- U. P. M. Ashik, W. M. A. Wan Daud and H. F. Abbas, Renewable Sustainable Energy Rev., 2015, 44, 221–256 CrossRef CAS.
- Z. Nawaz, Rev. Chem. Eng., 2015, 31, 413–436 CAS.
- J.-P. Lange, R. J. Schoonebeek, P. G. D. L. Mercera and F. W. van Breukelen, Appl. Catal., A, 2005, 283, 243–253 CrossRef CAS.
- E. E. Stangland, Annu. Rev. Chem. Biomol. Eng., 2018, 9, 341–364 CrossRef CAS PubMed.
- S. Gopal and M. H. Al-Hazmi, in Petrochemical Catalyst Materials, Processes, and Emerging Technologies, ed. H. Al-Megren and T. Xiao, IGI Global, ProQuest Ebook Central, 2016, pp. 22–52 Search PubMed.
- Shell, Linde and Shell team up to commercialise lower-carbon technology for ethylene (2020) https://www.shell.com/business-customers/chemicals/media-releases/2020-media-releases/linde-and-shell-team-up-to-commercialise-lower-carbon-technology.html (last visited on April 2021).
- P. Meriaudeau and C. Naccache, Catal. Rev., Sci. Eng., 1997, 39(1–2), 5–48 CrossRef CAS.
- H. Zimmermann and R. Walzl, “Ethylene”, Ullmann's Encyclopedia of Industrial Chemicals, Wiley-VCH, 2009, vol. 13, pp. 465–529 Search PubMed.
- McKinsey, Energy Transition – mission (im)possible for industry? (2016); McKinsey, Decarbonization of industrial sectors: the next frontiers (2018).
- J.-P. Lange, CATTECH, 2001, 5, 82–95 CrossRef CAS.
- J.-P. Lange, ChemSusChem, 2009, 2, 587–592 CrossRef CAS PubMed.
- Port of Rotterdam, 102 million euros in funding on the horizon for Porthos carbon storage project (2020) https://www.portofrotterdam.com/en/news-and-press-releases/102-million-euros-in-funding-on-the-horizon-for-porthos-project (last visited on April 2021).
- Global CCS Institute, The Global Status of CCS, Australia, 2017.
- Carbon capture: the quest for cleaner energy (last visited on April 2021).
- Porthos Carbon Capture and Storage (CCS) Project; https://www.nsenergybusiness.com/projects/porthos-carbon-capture-and-storage-ccs-project/ (last visited on April 2021).
- A. Kätelhön, R. Meys, S. Deutz, S. Suh and A. Bardow, Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 11187–11194 CrossRef PubMed.
- J.-F. Bastin, Y. Finegold, C. Garcia, D. Mollicone, M. Rezende, D. Routh, C. M. Zohner and T. W. Crowther, Science, 2019, 365, 76–79 CrossRef CAS PubMed.
- Shell, Nature-based solutions https://www.shell.com/energy-and-innovation/new-energies/nature-based-solutions.html (last visited on April 2021).
- van Gastel, Solar Magazine (7 dec. 2018), Shell rondt bouw zonnepark Moerdijk af (2018) https://solarmagazine.nl/nieuws-zonne-energie/i17341/shell-rondt-bouw-zonnepark-moerdijk-af (last visited on April 2021).
- Shell, Dow and Shell team up to develop electric cracking technology (2020) https://www.shell.com/business-customers/chemicals/media-releases/2020-media-releases/dow-and-shell-team-up-to-develop-electric-cracking-technology.html#:~:text=Dow%2C%20Inc.,making%20them%20CO2%20intensive. (last visited on April 2021).
- G. Henning, Langås (NEL Hydrogen), “Renewable Energy and Hydrogen Export”, Trondheim, Norway – March 24th 2015 http://www.sintef.no/contentassets/9b9c7b67d0dc4fbf9442143f1c52393c/9-hydrogen-production-in-large-scale-henning-g.-langas-nel-hydrogen.pdf (last visited on April 2021).
- K. Overwater, Z. Abdin and V. Khurana, TechnipFMC Hydrogen technology, Consistent market leadership 2018 http://www.technipfmc.com/-/media/technipfmc/Downloads/phase-3/onoff/on_hydrogen_lr.pdf?la=en&hash=91689EF9117F58FF03545F6794CA8935C3739E28 (last visited on April 2021).
- Zero Emission platform, “Commercial Scale Feasibility of Clean Hydrogen” report http://www.zeroemissionsplatform.eu/news/news/1669-launch-of-zep-report-commercial-scale-feasibility-of-clean-hydrogen.html (last visited on April 2021).
- G. Glenk and S. Reichelstein, Nat. Energy, 2019, 4, 216–222 CrossRef CAS.
- A. Tremel, Electricity-based Fuels, Springer, Cham, Switzerland, 2018 Search PubMed.
- Lazard, “Lazard's levelized cost of storage” version 2.0, 2016, (https://www.lazard.com/media/438042/lazard-levelized-cost-of-storage-v20.pdf) (last visited on April 2021).
- O. Schmidt, S. Melchior, A. Hawkes and I. Staffell, Joule, 2019, 3, 81–100 CrossRef.
- IRENA, Renewable Power Generation Costs in 2017, International Renewable Energy Agency, Abu Dhabi, 2018 Search PubMed.
- N. Boccard, Energy Policy, 2014, 66, 450–461 CrossRef.
- IEA/NEA, Projected Costs of Generating Electricity – 2020 Edition; https://www.oecd-nea.org/jcms/pl_51110/projected-costs-of-generating-electricity-2020-edition?details=true (last visited on April 2021).
- M. L. Neelis, PhD thesis, Univ. Utrecht, The Netherlands, 2008.
- D. S. Sholl and R. P. Lively, Nature, 2016, 532, 435–437 CrossRef PubMed.
- T. Hundertmark, M. Mayer, C. McNally, T. J. Simons and C. Witte, “How plastics-waste recycling could transform the chemical industry”, December 2018, McKinsey on Chemicals http://www.mckinsey.com/industries/chemicals/our-insights/how-plastics-waste-recycling-could-transform-the-chemical-industry (last visited on April 2021).
- I. A. Ignatyev, W. Thielemans and B. Vander Beke, ChemSusChem, 2014, 7, 1579–1593 CrossRef CAS PubMed.
- K. Ragaert, L. Delva and K. Van Geem, Waste Manage., 2017, 69, 24–58 CrossRef CAS PubMed.
- https://earth911.com/business-policy/how-many-times-recycled/ (last visited on April 2021).
- G. P. Thomas, “Recycling of Polypropylene (PP)“ AZO cleantech 2012, https://www.azocleantech.com/article.aspx? ArticleID=240#∼:text=This%20varies%20with%20the%20use,as%20clothes%20or%20playground%20equipment. (last visited on April 2021).
- E. Villanueva, End-of-waste criteria for waste plastic for conversion, JRC Technical reports, European Commission, 2014, http://publications.jrc.ec.europa.eu/repository/bitstream/JRC91637/2014-jrc91637%20.pdf (last visited on April 2021).
- G. Lopez, M. Artetxe, M. Amutio, J. Bilbao and M. Olazar, Renewable Sustainble Energy Rev., 2017, 73, 346 CrossRef CAS.
- Shell, “Shell uses plastic waste to produce chemicals“, 2019, https://www.shell.com/business-customers/chemicals/media-releases/2019-media-releases/shell-uses-plastic-waste-to-produce-chemicals.html (last visited on April 2021).
- J.-P. Lange, in Catalysis for Renewables: from feedstock to energy production, ed. G. Centi and R. A. van Santen, Wiley-VCH, Weinheim, 2007, vol. 238(1), pp. 21–51 Search PubMed.
- Nova Institute, Bio-based Building Blocks and Polymers, 2016 (http://www.bio-based.eu/markets).
- R. Slade, A. Bauen and R. Gross, Nat. Clim. Change, 2014, 4, 99–105 CrossRef.
- M. Weiss, J. Haufe, M. Carus, M. Brandao, S. Bringezu, B. Hermann and M. K. Patel, J. Ind. Ecol., 2012, S169–S181 CrossRef CAS.
- J. P. Lange, Nat. Catal., 2021, 4, 186–192 CrossRef.
- M. Dusselier, M. Mascal and B. F. Sels, in Selective Catalysis for Renewable Feedstocks and Chemicals, ed. K. Nicholas, Topics in Current Chemistry, Springer, Cham, 2014, vol. 353 Search PubMed.
- S. Ramaswamy, H.-J. Huang and B. V. Ramarao, Separation and purification technologies in biorefineries, Wiley, Chichester, 2013 Search PubMed.
- A. A. Kiss, J.-P. Lange, B. Schuur, D. W. F. Brilman, A. G. J. van der Ham and S. R. A. Kersten, Biomass Bioenergy, 2016, 95, 296–309 CrossRef CAS.
- J.-P. Lange, ChemSusChem, 2017, 10, 245–252 CrossRef CAS PubMed.
- P. Gabrielli, M. Gazzani and M. Mazzotti, Ind. Eng. Chem. Res., 2020, 59, 7033–7045 CrossRef CAS.
- C. Song, ACS Symposium Series, American Chemical Society, Washington, DC, 2002, pp. 1–30 Search PubMed.
- J. A. Martens, A. Bogaerts, N. De Kimpe, P. A. Jacobs, G. B. Marin, K. Rabaey, M. Saeys and S. Verhelst, ChemSusChem, 2017, 10, 1039–1055 CrossRef CAS PubMed.
- A. González-Garay, M. S. Frei, A. Al-Qahtani, C. Mondelli, G. Guillén-Gosálbez and J. Pérez-Ramírez, Energy Environ. Sci., 2019, 12, 3425–3436 RSC.
- S. Verma, B. Kim, H.-R. Jhong, S. Ma and P. J. A. Kenis, ChemSusChem, 2016, 9, 1972–1979 CrossRef CAS PubMed.
- D. W. Keith, G. Holmes, D. St. Angelo and K. Heidel, Joule, 2018, 2, 1–22 CrossRef.
- P. Schmidt, V. Batteiger, A. Roth, W. Weindorf and T. Raksha, Chem. Ing. Tech., 2018, 90, 127–140 CrossRef CAS.
- G. J. Kramer and M. Haigh, Nature, 2009, 462, 568–569 CrossRef CAS PubMed.
Footnotes |
| † The penalties were determined by applying a penalty of $366 per t, the price of the carbon contained in CO2, to the carbon contained in oil or natural gas; H2 was assumed to emit 9 tons of CO2 per ton of H2. |
| ‡ The average net present cost of electricity generation for a generating plant over its lifetime. |
| This journal is © The Royal Society of Chemistry 2021 |