Open Access Article
Open Access ArticleRecent trends in gas sensing via carbon nanomaterials: outlook and challenges
Pallvi
Dariyal
ab,
Sushant
Sharma
bc,
Gaurav Singh
Chauhan
ab,
Bhanu Pratap
Singh
 *ab and
Sanjay R.
Dhakate
*ab and
Sanjay R.
Dhakate
 *ab
*ab
aAdvanced Carbon Products and Metrology, CSIR-National Physical Laboratory, Dr K. S. Krishnan Marg, New Delhi, 110012, India. E-mail: bps@nplindia.org; dhakate@nplindia.org
bAcademy of Scientific and Innovative Research (AcSIR), Ghaziabad-201002, India
cUniversity of Ulsan, Chemical Engineering Department, Ulsan, 44610, South Korea
First published on 28th October 2021
Abstract
The presence of harmful and poisonous gases in the environment can have dangerous effects on human health, and therefore portable, flexible, and highly sensitive gas sensors are in high demand for environmental monitoring, pollution control, and medical diagnosis. Currently, the commercialized sensors are based on metal oxides, which generally operate at high temperatures. Additionally, the desorption of chemisorbed gas molecules is also challenging. Hence, due to the large surface area, high flexibility, and good electrical properties of carbon nanomaterials (CNMs) such as carbon nanotubes, graphene and their derivatives (graphene oxide, reduced graphene oxide, and graphene quantum dots), they are considered to be the most promising chemiresistive sensing materials, where their electrical resistance is affected by their interaction with the analyte. Further, to increase their selectivity, nanocomposites of CNMs with metal oxides, metallic nanoparticles, chalcogenides, and polymers have been studied, which exhibit better sensing capabilities even at room temperature. This review summarizes the state-of-the-art progress in research related to CNMs-based sensors. Moreover, to better understand the analyte adsorption on the surface of CNMs, various sensing mechanisms and dependent sensing parameters are discussed. Further, several existing challenges related to CNMs-based gas sensors are elucidated herein, which can pave the way for future research in this area.
1. Introduction
The modern living standard is critically affecting the environment due to the continuous production of toxic gases, where their invisibility is a serious concern. Air pollutants such as NO2, COx, and CH4 are the major reason for the dangerous atmospheric changes, which are part of the environmental changes resulting in an increase in the Earth's temperature. Together with pollutant gases, there are several other hazardous gases in the surroundings such as CH4, NH3, H2, and H2S, which become explosive when mixed with air in a certain proportion. Besides these toxic gases, the vapours of numerous volatile organic compounds (VOCs) such as ethanol, toluene, triethylamine (TEA), formaldehyde, and acetone are another class of harmful gases that is dangerous to human health. Hence, it is essential to sense these gases for environmental analysis, industrial emission monitoring, medical diagnosis, agriculture, public safety and security purposes. Therefore, there is a strong demand for highly sensitive, portable, flexible, and cost-effective gas sensors. Depending on their sensing mechanism, there are various types of sensors, i.e., chemiresistive,1 field-effect transistor (FET),2 and micro-electromechanical system (MEMS)3 gas sensors, where chemiresistive sensors are highly explored.Currently, metal-oxide sensors (MOSs) are well commercialized for various practical applications such as hand-held ethanol sensors for drunk and driving cases, methane and hydrogen sensors for the safety of labours working in industries and mines, and acetone and toluene gas sensors for diabetic and lung cancer diagnosis.4 Thus far, SnO2, TiO2, WO3, ZnO, CuO, CdO and In2O3 have been widely considered for these real-life sensing applications. However, although these sensors show high sensitivity, their operating temperatures are fairly high, which increase their operational and maintenance cost. Furthermore, their sensitivity is affected by a change in their surface morphology. For instance, a hollow sphere (3-D) WO3-based sensor5 exhibited a higher sensing response than a 2-D thin film6 for the detection of 1 ppm NO2. Additionally, the recovery time is very high for these sensors given that the analytes are chemisorbed with a high binding energy. Although many phenomena, i.e., thermal treatment7 and UV irradiation,8 have been employed for chemical desorption, the recovery time is still very long.
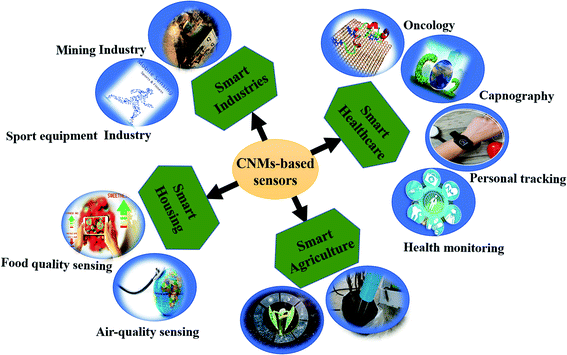
Therefore, to overcome the aforementioned issues, new alternatives, i.e., carbon nanomaterials (CNMs), have been explored as sensing materials in the past two decades.9–12 These CNMs offer a high surface area for absorption, which enables them to achieve high sensitivity. CNMs, i.e. CNTs, graphene, and their derivatives, possess high electron transportation properties with low noise. Moreover, CNMs gas sensors enable fast recovery via UV light. Additionally, their robustness is suitable for developing portable and flexible devices with high sensitivity to cost ratio. Thus, CNMs-based gas sensors are widely used in various fields, as shown in Fig. 1. The most recent application is to predict physiological conditions in the human body by detecting several VOCs exhaled during breathing.13–15
Despite the numerous advantages of pristine CNMs, they also have some serious drawbacks, such as low selectivity, low repeatability, and non-uniformity of the functional groups on graphene derivatives or number of CNT walls.16,17 Hence, without sacrificing their advantages, nanocomposites of CNMs with metal oxides, metallic nanoparticles, chalcogenides, and polymers, have been studied, and this new class of CNMs hybrid sensing materials has shown tremendous performances. Moreover, CNMs have good flexibility,18–20 and therefore can be employed to fabricate wearable sensors.
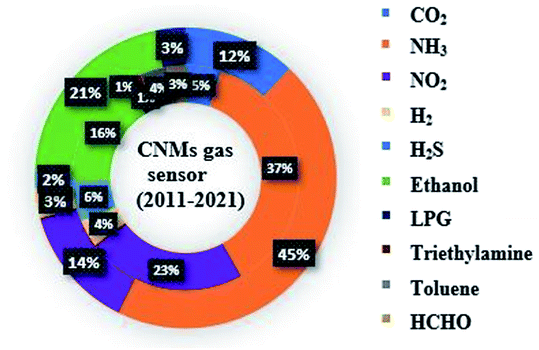
Numerous reviews have reported the recent progress on CNTs and graphene sensors. Recently, Sireesha et al. reported a broad review on CNT-based biosensors.21 Furthermore, Seesaard et al. described metal oxide-decorated or doped CNT-based hybrid nanocomposites as future sensors.22 In a similar direction, the current progress on CNT-based flexible sensors for CO2, H2S and NH3 was reviewed by Kumar.23 Another CNM (graphene) has also been explored and numerous articles have been published in the last decade (shown as the outer circle in Fig. 2). In 2018, Tian et al. proposed pristine, defected and functionalized graphene-based sensors.24 Further, a thorough review on graphene oxide as NO2, H2, NH3, H2S, acetone and humidity sensors was presented by Toda.12 Similarly, Martínez et al. presented a theoretical overview on graphene-based sensors for toxic gases.25 However, there are numerous reviews published on CNTs and graphene-based gas sensors individually, only a few reviews summarized both CNMs (CNTs and graphene)-based chemiresistive sensors in a comparative manner.10,26–28 Therefore, it is important to identify new trends in this area. The latest research on these two CNMs and their mechanism are explained briefly in this review. This review contains prominent parameters of CNMs as sensing materials. The significant research works on CNMs chemiresistive sensors towards various gases (greenhouse, explosive, and VOCs) are covered in this review. Moreover, the significant works done on CNM-based nanocomposites as gas sensors and their possible sensing mechanisms are also emphasized. Additionally, the selectivity and sensitivity enhancement factors of various dopants for CNMs and the responsible mechanisms are elaborated. Briefly, the latest achievements on CNMs in the sensing domain are discussed and their outlook for futuristic technologies is highlighted.
2. Properties of carbon nanostructures for air-quality sensing
Carbon nanostructures such as CNTs and graphene have many distinct properties, which have been exploited to develop next-generation sensors.29 The morphology, surface area, conductivity, chemical activity, and desorption ability of sensing materials are essential aspects for chemiresistive gas sensors, which are described in detail below.2.1 Morphology of sensing material
The efficiency of a sensor is highly influenced by its morphology.30 There are various morphologies of sensing materials, which can be classified as zero-dimensional (quantum dots),31,32 one-dimensional (nanowires and nanotubes),33,34 two-dimensional (sheets and belts),35 and three-dimensional (composed of 0-D, 1-D, and 2-D structures).36,37 These different morphologies have different advantages, including abundant surface active sites, high sensing rate, and enhanced gas diffusion. Among them, 3-D structures have the highest number of active sites, but their synthesis is complex. Therefore, the most preferred structures for high sensing performances are hollow 1-D and porous 2-D nanostructures given that they possess a large surface area for the adsorption of target gases. Accordingly, 1-D CNTs and 2-D graphene sheets are considered ideal sensing materials. In CNTs, their inner and outer surface can act as adsorption sites, while in graphene sheets, analytes can adsorb on their surface and penetrate their porous sheets (as shown in Fig. 3).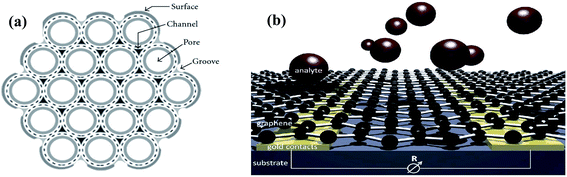 | ||
| Fig. 3 Available surface area on (a) CNTs11 (reprinted with permission from ref. 11. Copyright (2009), Hindawi Publisher, under Creative Commons Attribution 3.0 License) and (b) graphene38 (reprinted with permission from ref. 38. Copyright (2014), The Royal Society of Chemistry). | ||
2.2 High surface area and absorption capacity
For the adsorption of atmospheric oxygen and gas molecules, adsorption sites should be available. Therefore, a high surface area possessing abundant adsorption sites is essential. Graphene and CNTs both have a high surface area, which depends on their synthetic conditions, post-treatment, and available functional groups on their surface. The specific surface area of various CNMs, including graphene and its derivatives, CNTs, and CNFs, is presented in Table 1.| CNM structure | Sensing material | Specific surface area (m2 g−1) |
|---|---|---|
| 0-D | Graphene QDs | 0.066–2.57 (ref. 31) |
| 1-D | MWCNTs | 435 (ref. 39) |
| SWCNTs | 600 (ref. 40) | |
| MWCNTs | 91.223 (ref. 41) | |
| CNFs | 14.8 (ref. 42) | |
| Fluorinated CNFs having carbon black | 21 (ref. 43) | |
| 2-D | Monolayer graphene | 2630 (ref. 44) |
| Nanoporous graphene | 410.99 (ref. 45) | |
| Laser-induced graphene | 350 (ref. 46) | |
| Monolayer GO | 2391 (ref. 47) | |
| GO sheet | 37.24 (ref. 48) | |
| GOQDs | 324 (ref. 49) | |
| rGO | 64 (ref. 50) | |
| Superhydrophobic rGO | 850 (ref. 37) | |
| N doped rGO | 335.6 (ref. 51) | |
| N doped rGO | 247.7 (ref. 52) | |
| 3-D | 1-D MWCNT + 2-D Gr (50%) | 151.3 (ref. 53) |
| WS2-1-D CNFs | 6.44 (ref. 54) |
As mentioned in Table 1, CNFs have a very low specific surface area. Therefore, they have been explored the least as a gas sensing material. On the contrary, although graphene quantum dots (GrQDs) also have a low specific surface area, they have high charge mobility (due to quantum effects) and low toxicity.55 Therefore, nowadays, researchers are focusing on GrQD-based biosensors. Nevertheless, the other carbon allotropes such as CNTs and graphene have a high surface area, which makes them superior sensing materials.
Graphene has almost twice the surface area of CNTs as gas analytes can adsorb on both sides of the graphene sheets, but in CNTs, together with the surface of the nanotube walls, the interior pore of individual tubes, grooves, and interstitial channels formed between three adjacent tubes within the bundle can also act as adsorption sites given that they are generally found in bundles.11,56 The adsorption of analytes in these sites depends on the binding energy of the gas molecules. Additionally, these sites may not adsorb certain gases if the dimensions of the gas molecules are larger than the diameter of the site.
2.3. Electrical conductivity
For chemiresistive sensors, their responses are evaluated in terms of change in resistance. In zero bandgap graphene, the mobility of electrons is very high, which makes it easier to respond to gases. For the adsorption of gas molecules in 2-D graphene, the adsorption energy can be calculated using eqn (1).| Ea = E(sensing material+gas) − E(sensing material) + E(gas) | (1) |
Moreover, the bandgap of semiconducting CNTs can tuned via easy electron transfer phenomena. Furthermore, the oxygen groups of graphene derivatives, transition metal catalyst of CNTs, and their defective sites affect their bandgap, making them sensitive towards specific gases.
2.4 Chemical activity
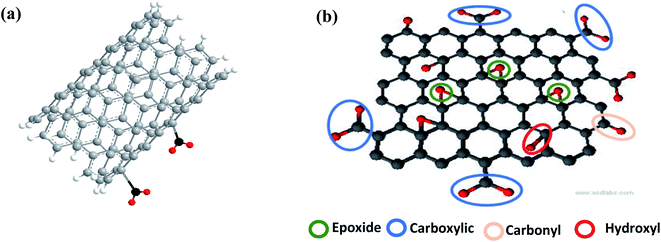
The intrinsic or pristine sensing materials show less interaction with analyte molecules due to the lack of defects or reactive sites on their surface.57 The presence of functional groups promotes gas adsorption.58 Moreover, these functional groups also help to improve the selectivity. Due to these favorable features, high chemical activity is crucial for improved sensing efficiencies, which contributes to the generation of more reactive sites (shown in Fig. 4). Both, CNTs and graphene have highly reactive surfaces, which are created by refluxing them.In terms of graphene derivatives, GO and rGO are preferable in comparison to pure graphene due to the presence of oxygen functional groups. Moreover, functionalized CNTs show a higher response than pristine CNTs,57,59 but their surface area depends on the refluxing process, i.e. time, temperature, and acid used.60 Due to this property, hybrid CNMs composites are easy to synthesize, which are the most recent researched sensing material.
2.5 Desorption of analyte
For long-term usability, the most important feature of sensors is the desorption of gas molecules with a fast recovery rate. In MOS sensors, thermal energy is essential for the desorption of gas molecules. Therefore, an in-built micro-heater should be present in the device, which makes it expensive and complex to design.7 Besides, it may also decrease the working life of sensors. Therefore, it is a challenge to recover the baseline resistance in MOS sensors within seconds at room temperature.However, in CNMs-based sensors, the recovery is accelerated with external energy, which can desorb the molecules attached to their surfaces. Accordingly, light activation is a tremendous technique, which contributes to reduce power consumption and miniaturization of sensing devices. Generally, UV and IR light are used for faster recovery in CNMs. UV light removes the oxygen atoms attached to the surface, and hence rapidly balances the baseline resistance of CNMs-based sensors. This happens due to the surface plasmon resonance occurring in the graphene layers or CNTs at very high frequency. The as-generated vibrations cause the analyte and oxygen to leave the CNMs surface. Conversely, IR light generates electron–hole (E–H) pairs in graphene, and when these E–H pairs recombine, photon radiation occurs. This radiation heat recovers the resistance of CNT. The other advantage of the light activation technique is its higher sensitivity given that UV light can affect the electronic properties by increasing the concentration of photocarriers, improving the interaction between the analyte molecules and sensing material.8 Hence, this technique is very beneficial for high sensitivity, stability, portability, and low power consumption in ideal chemiresistive gas sensors. Furthermore, in hybrid CNM sensing materials, besides UV light, visible, IR, and white light are also studied for the desorption of gas molecules.61,62
3. CNMs as gas sensing materials
Carbon nanostructures such as CNTs and graphene can detect very low concentrations of greenhouse and explosive gases. Therefore, employing the gas sensitivity of graphene and CNTs to build highly sensitive, low power-consuming gas sensors is not only of academic interest but also of great commercial interest.3.1 CNTs as gas sensors
CNT-based gas sensors have been the topic of research given that they are easy to synthesize, compact, and inexpensive. Furthermore, the high surface area, hollow geometry, and chemical activeness of carbon nanotubes (CNTs) make them attractive for gas sensing applications. Considering that the electronic properties of CNTs are highly sensitive towards any change in their chemical environment, several exciting works on pristine CNTs as chemiresistive sensors have been widely explored, where their sensitivity is affected by the number of walls, i.e., SWCNT-based sensors have higher sensitivity than MWCNTs for NO2 sensing even at a low operating temperature. In this case, Naje et al. (2016) investigated SWCNTs and MWCNTs for sensing 3% NO2 gas and observed 79.81% and 59.61% sensitivity, respectively.63Due to the lower sensitivity of pristine CNTs, functionalized CNTs have also been studied to enhance the selectivity towards specific analytes. In addition, various metallic nanoparticles and metal oxides have been incorporated into CNTs to achieve specificity to different analytes. In this case, ZnO-doped SWCNTs64 and ZnO-doped MWCNTs65 were investigated for NO2 sensing, which exhibited the highest sensitivity at an operating temperature of 150 °C and 300 °C, respectively. Nevertheless, the alignment of CNTs in the CNT network also affects the response of the sensor. Kumar et al. revealed that the highly aligned SWCNT network has seven times higher response than the randomly aligned SWCNT network towards 0.5 ppm NO2 gas molecules.66 Furthermore, alignment not only affects the sensitivity but also the detection limit, which can be reduced with an increase in alignment.
When CNTs come in contact with air, ionized oxygen is adsorbed on their surface. The accumulation of oxygen ions leads to an electron depletion region (EDR), and consequently the CNTs become p-type. The change in resistance of CNTs is due to the charge transfer between the analyte and CNT surface. This interaction mechanism can be classified as oxidation and reduction,70,71i.e., when the CNT interacts with electron-withdrawing or reducing gas/chemical (such as NH3), the resistance of the CNT increases due to electron transfer from the gas to the CNT. Consequently, the potential barrier energy decreases. On the contrary, when electron-accepting or oxidizing gas/chemical molecules (for example NO2) interact with the CNT surface, the gas molecules withdraw electrons from the CNTs. This increases the hole population, which decreases the resistance of the CNTs, increasing the barrier energy. Together with physisorption, chemisorption phenomena can also occur in CNTs.
Although the occurrence physisorption leads to low selectivity and sensitivity, low operating temperature, short response time, and fast recovery are advantages of physisorption, which are essential for an ideal sensor (not applied on well-commercialized MOS gas sensor). Therefore, several hazardous air pollutants such as NO2, CO, CO2, and CH4, explosive gases, and VOCs have been detected via CNT-based gas sensors with a shorter recovery time in comparison with MOS sensors.72 The effects of these gaseous agents have also been studied theoretically by using first-principal approximations and density calculations. In this case, Kumar et al. studied the adsorption mechanism theoretically via DFT implemented in the computational quantum chemical HF code of the 6-31G basis set on (1,1) and (2,2) CNTs.73 They concluded that after gas exposure there is less variation in the binding energy of (1,1) CNTs (7.92%) than (2,2) CNTs (17.32%), which indicates the lower sensitivity of (1,1) CNTs. Moreover, the band gap width of (2,2) CNTs decreased because a large number of electrons is free to move, which is directly related to the higher conductivity of (2,2) CNTs.
3.2 Graphene and its derivatives as gas sensors
Besides CNTs, a lot of research on graphene-based gas sensors has been performed. Due to the availability of a large surface area for the adsorption of gas molecules, graphene and its derivatives such as graphene oxide (GO), reduced graphene oxide (rGO), and graphene quantum dots (GrQDs) have been explored for the detection of various hazardous gases such as NO2, H2S, NH3, NO2, CO2, CH4 and CO gases.Due to the properties of graphene (as mentioned in Table 2), it can act as an advanced sensing material and even show a higher response than MWCNT-based sensors given that CNTs have a lower specific surface area than graphene. Moreover, its sensitivity depends on the number of graphene layers. In this case, Seekaew et al. examined the effect of the number of graphene layers for sensing NO2 and reported that bilayer graphene showed the highest response in comparison to monolayer and multi-layer graphene.76 Except for single-layer graphene, the parabolic-shaped bands of graphene have finite charge carriers (increase with an increase in the number of layers), resulting in a reduction in resistance. Conversely, for single-layer graphene (conical-shaped bands), the concentration of electrons transferred to NO2 gas molecules may be limited due to the lower availability of electronic states in the valence band (VB) compared with bilayer and multilayer graphene (as shown in Fig. 5a).
| Property | Graphene | Carbon nanotubes |
|---|---|---|
| Elastic limit | 20% | 16% |
| Specific surface area | 2360 m2 g−1 | 387 m2 g−1 |
| Electrical conductivity | 106 to 107 S m−1 | 108 S m−1 |
| Charge mobility | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm2 V−1 s−1 000 cm2 V−1 s−1 |
80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm2 V−1 s−1 000 cm2 V−1 s−1 |
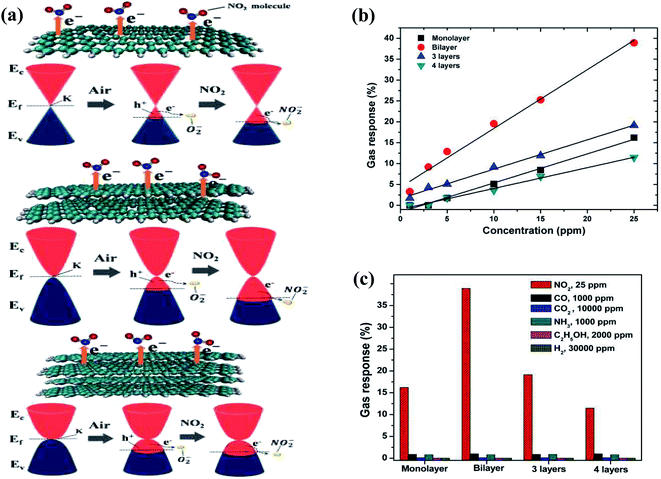 | ||
| Fig. 5 (a) Schematic and band diagrams of NO2 sensing mechanism of monolayer, bilayer and multilayer graphene gas sensors. (b) Gas response (ΔR/R) of layered graphene gas sensors as a function of NO2 concentration. (c) Selectivity plot of layered graphene-based sensor to various gases at room temperature76 (reprinted from ref. 76, Copyright (2017), with permission from Elsevier). | ||
Fig. 5b shows the gas response of monolayer, bilayer, 3-layered, and 4-layered graphene at different concentrations (0–25 ppm) of NO2. Additionally, the selectivity towards NO2 was also confirmed by testing them with various gases (as shown in Fig. 5c).
Although graphene has high sensitivity, intrinsically it has low selectivity. Therefore, several non-metals such as fluorine, boron, and nitrogen have also been investigated for improving the sensing performance of graphene. In 2015, Park et al. investigated the sensing of NH3 using fluorinated graphene oxide (F-GO),77 where the presence of fluorine (high electronegativity) lowered the Fermi energy level given that electrons migrated from the valence band to the LUMO. Thus, due to the electron migration, the sensitivity was enhanced. On the contrary, the sensing material having a high fluorine to carbon ratio decreased the response of the sensor because when NH3 gets adsorbed on F-GO, the electrons transfer to the LUMO owing to the enhanced Fermi level. Recently Srivastva et al. doped boron and nitrogen in a flexible graphene layer (FGL) for the detection of NH3.78 To achieve the strong adsorption of analyte molecules, the adsorption energy should be low. Thus, by doping boron in FGL, the adsorption energy (Eads) for NH3 decreased by two times (Eads = −0.5 eV), which directly affected the response of the sensor.
Furthermore, functionalized graphene (GO and rGO) has also been employed for sensing applications. Recently, Gao et al. (2020) studied the adsorption of H2S and CH4 on intrinsic, defected and doped graphene theoretically using the first-principals method.79 The required adsorption energies of intrinsic graphene, Ni-doped graphene, vacancy-defected graphene and graphene oxide for H2S are −0.038, −0.699, −2.934 and −1.258 eV, respectively. Conversely, the required adsorption energy towards CH4 increased in the order of intrinsic graphene (−0.022 eV), graphene oxide (−0.047 eV), Ni-doped graphene (−0.099 eV) and vacancy defeated graphene (−0.164 eV), which confirmed that intrinsic graphene requires a high Eads for H2S and CH4, resulting in weak physisorption. Among the three modified graphene, the vacancy-defected graphene is the best candidate for the adsorption of H2S and CH4. Hence, controlled defects in graphene have been widely studied for gas sensing applications.80 Furthermore, besides non-metals, various metallic particle- and metal oxide-doped graphene and their derivatives are also considered as good alternatives.81–85
3.3. CNM hybrid nanocomposite-based gas sensors
4. CNM hybrid nanocomposite-based gas sensors
4.1 NOx sensors
Nitrogen dioxide (toxic air-pollutant) is a by-product from the burning of gasoline, fuel, etc. in the exhaust of vehicles. According to the Immediately Dangerous to Life and Health (IDLH) values, more than 20 ppm of NO2 is fatal, given that this gas accelerates the risk of respiratory diseases in children and senior citizens.91 Furthermore, in daylight, NOx reacts with various VOCs and forms ozone, which can have several harmful effects on humans such as destruction to lung tissues and terrible effects on their functioning, mostly in asthmatic patients. Moreover, NOx gases can cause other environmental problems such as smog and acid rain. Due to the above-mentioned serious environmental threats, several governments have established many laws to limit NOx emissions. Therefore, the detection of NOx is necessary to employ some sort of feedback loop in the combustion process for its minimization.Furthermore, numerous experimental studies have been performed on CNT-based NO2 sensors. One of the pioneering works involved the detection of NO2 on MoS2 (chalcogenide)-decorated vertically aligned CNTs (VACNTs) grown on an Si substrate.34 When the as-designed sensor was exposed to an NO2 gas atmosphere, NO2 molecules were adsorbed on the edges and surface of the MoS2 hexagonal-shaped nanoplates (HNPs), where the electronic charge transferred from MoS2 to the gas molecules (illustrated in Fig. 6a), consequently decreasing the resistance of the sensor. In addition, NO2 gas molecules also adsorbed on the surface of the CNTs, which decreased the overall resistance of the sensor.
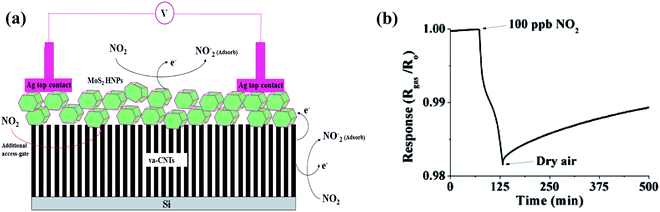 | ||
| Fig. 6 (a) Schematic explaining the sensing mechanism of MoS2 hexagonal nanoparticles (HNPs) on vertically aligned CNT array-based sensor. (b) Response of sensor at 100 ppb NO2 gas34 (reprinted with permission from ref. 34. Copyright (2017), John Wiley and Sons). | ||
The as-made sensor showed a sensitivity of 0.023% × ppb (shown in Fig. 6b).
Further, Su et al. detected NO2 gas on Au and Ag-decorated WO3-functionalized MWCNTs.8 Besides WO3 and CNTs, the gas molecules were also adsorbed on Au and Ag and due to charge transfer, enhancing the conductivity of the sensor. The results revealed that oxygen ions get adsorbed on the metals (Au and Ag), metal oxide (WO3) and CNT surface. When the sensor is exposed to NO2 gas, the molecules accept electrons to form gaseous ions and react with chemisorbed oxygen, leading to an increase in resistance, and the corresponding reaction is as follows:
| NO2(g) + O2−(ads) + 2e →NO2−(ads) + 2O−(ads) | (2) |
Besides the above-mentioned phenomenon, the formation of a Schottky barrier and p–p heterojunction between WO3 and p-type CNT can affect the width of the depletion layers (DL) and eventually change the resistance during sensing. For the recovery of the sensor, UV-LED is used, where the sensor prevents the immediate recombination process, resulting in an improvement in the photocatalytic reaction, and consequently an increase in the sensitivity of sensor compared to that without UV irradiation. The corresponding reaction is as follows:
| 2NO2(g) + e(hν) → 2NO(hν) + O2−(hν) | (3) |
The sensor showed 262% response at 500 ppb NO2 under UV irradiation and the calculated limit of detection (LOD) was 45 ppb.
Further, for bendable NO2 sensors, various CNT-polymer hybrids have been explored. In 2019, Kumar et al. reported NO2 sensing using a polyethyleneimine (PEI)-functionalized SWCNT sensor.93 The as-made sensor showed high sensitivity (37%) for 50 ppm NO2 at room temperature with quick recovery time (240 s).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 96 given that G-rGO was highly reduced compared to C-rGO. Due to its high reduction level, a large concentration of defects in terms of high adsorption sites was observed in G-rGO. Moreover, Park et al. (2020) synthesized semiconducting rGO, which showed a high response (∼32%) for 5 ppm NO2 with an extremely low LOD.97
96 given that G-rGO was highly reduced compared to C-rGO. Due to its high reduction level, a large concentration of defects in terms of high adsorption sites was observed in G-rGO. Moreover, Park et al. (2020) synthesized semiconducting rGO, which showed a high response (∼32%) for 5 ppm NO2 with an extremely low LOD.97
| Sensing material | Analyte | Operating temperature | Response | Response time | Recovery time | Limit of detection | References |
|---|---|---|---|---|---|---|---|
| a R a/Rg (oxidizing gas) or Rg/Ra (reducing gas). b ΔR/Ra. c ΔG/ΔG0. d ΔI/I. | |||||||
| ZnO-decorated MWCNTs | 10 ppm NO2 | 300 °C | 1.023a | 93.1 s | 285.2 s | — | Kwon et al. (2017)65 |
| Polypyrrole–nitrogen-doped MWCNTs | 5 ppm NO2 | RT | 24.82%b | 65 s | 668 s | <0.25 ppm | Liu et al. (2019)105 |
| Pd-MWCNTs | 1 ppm NO2 | 100 °C | 10%b | ∼220 s | ∼1700 s | — | Dilonardo et al. (2017)106 |
| Fe2O3-SWCNTs | 20 ppm NO2 | RT | 19%b | — | — | — | Hua et al. (2017)107 |
| SWCNT-PTFE | 0.75 ppm NO2 | RT | 21.58%b | 5 min | 15 min | <0.75 ppm | Agarwal et al. (2018)108 |
| 5 ppm NO2 | 167.7%b | ||||||
| f-SWCNTs with PEI | 50 ppm NO2 | RT | 37%b | 240 s | — | — | Kumar et al. (2020)93 |
| MWCNTs WO3 decorated with Au–Ag | 100 ppb NO2 | RT | 28%b | 267 s | — | 45 ppb | Su et al. (2020)8 |
| ZnO-SWCNTs | 1000 ppm NO2 | 150 °C | ∼900%b | 70 s | 100 s | — | Barthwal et al. (2018)64 |
| ZnO-rGO | 2.5 ppm NO2 | 110 °C | 33.11a | 182 s | 234 s | 1.3 ppb | Cao et al. (2020)99 |
| MoS2-rGO | 1 ppm NO2 | 25 °C | 6%b | 360 s | 720 s | 4.4 ppb | Yi et al. (2020)109 |
| SnS2-rGO | 8 ppm NO2 | RT | 49.8%c | 153 s | 76 s | 8.7 ppb | Wu et al. (2020)19 |
| Graphene on SiC substrate | 4 ppm NO2 | 105.1%b | — | — | 1 ppb | Yu et al. (2020)110 | |
| N-doped GrQDs-SnO2 | 100 ppb NO2 | 150 °C | 292a | 181 s | 81 s | 20 ppb | Purbia et al. (2020)104 |
| 50 °C | 4336a | 528 s | 384 s | ||||
| SnO2 incorporated CuO-rGO | 50 ppm NO2 | RT | ∼250%b | ∼90 s | ∼255 s | 150 ppb | Bo et al. (2020)111 |
| SnO2-rGO:Pd | 4 ppm NO2 | 200 °C | 185a | 8 s | 215 s | 0.5 ppm | Bhangere et al. (2020)101 |
| SnO2-rGO hydrogel | 5 ppm NO2 | RT | 32.1%c | 177 s | 260 s | 2.8 ppb | Wu et al. (2020)37 |
| 2.4 wt% rGO/Co3O4 | 5 ppm NO2 | RT | 26.8%b | 1.5 min | 40 min | 0.05 ppm | Zhang et al. (2018)83 |
| Bilayer Gr | 25 ppm NO2 | RT | 38.9%b | — | — | — | Seekaew et al. (2017)76 |
| Trilayer Gr | 25 ppm NO2 | ∼19%b | |||||
| 4 layers Gr | 25 ppm NO2 | ∼12%b | |||||
| Monolayer | 25 ppm NO2 | ∼16%b | |||||
| 3 wt% rGO-In2O3 | 1 ppm NO2 | 74 °C | 1337a | 208 s | 39 s | 10 ppb | Liu et al. (2017)100 |
| MoS2-Gr | 10 ppm NO2 | 200 °C | 69%b | 0.7 s | 0.9 s | 0.2 ppm | Hong et al. (2019)112 |
| TiO2–nitrogen-doped Gr QDs | 100 ppm NO2 | RT | 31.1%b | 235 s | 285 s | — | Murali et al. (2020)113 |
| 250 °C | 223%b | ||||||
| Defected Gr-pristine Gr | 100 ppm NO2 | 25 °C | ∼2.45d | 38 s | 238 s | Ma et al. (2019)80 | |
| ZnO-rGO | 40 ppm NO2 | RT | 48.4%b | — | — | 4 ppm | Jyoti et al. (2018)103 |
| SnO2-0.3% rGO | 10 ppm NO2 | RT | 2.021a | 171 s | 254 s | — | Gui et al. (2018)114 |
| 200 °C | 247.8a | 39 s | 15 s | ||||
| α-Fe2O3-12.2 wt% rGO | 5 ppm NO2 | RT | 8.2a | 2.1 min | 40 min | 0.05 ppm | Zhang et al. (2018)82 |
| Si-doped Gr | 50 ppm NO2 | RT | 21.5%b | 126 s | 378 s | 18 ppb | Niu et al. (2021)115 |
Although rGO has high sensitivity, it has irreversible recovery due to its cracked structure. Hence, to solve this issue, surface modification by decorating metal oxides such as ZnO, SnO2, Co3O4, In2O3, CuO, and Fe2O3 together with graphene, GO, and rGO has been explored for enhancing its sensing performance.82,83,99–102 In one of the works, Jyoti et al. synthesized a ZnO-decorated graphene-based sensor for NO2 gas detection.103 The as-made sensor showed 48.4% sensitivity for 40 ppm NO2 at room temperature. In another work, Zhang et al. synthesized an NO2 detector using SnO2/rGO as the sensing material.98 Furthermore, the sensor was exposed to four other gases (Cl2, NO, CO, and H2O) and showed selectivity towards NO2 gas (shown in Fig. 7b).
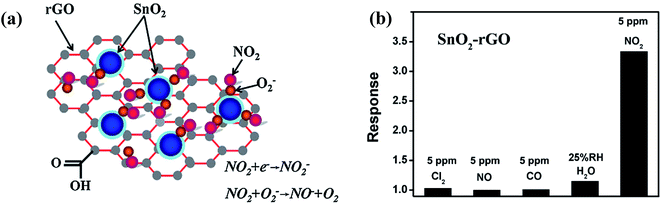 | ||
| Fig. 7 (a) Illustration of the sensing mechanism for the gas sensor based on SnO2/rGO nanohybrid. (b) Selectivity (calculated using Rg/Ra) histogram of SnO2/rGO gas sensor to various gases at room temperature.98 (reprinted from ref. 98, Copyright (2014), with permission from Elsevier). | ||
Similar to other graphene derivatives, graphene quantum dots have also been explored for NO2 sensing. In a recent study, Purbia et al. (2020) synthesized nitrogen-doped GrQD-functionalized SnO2via the wet chemical technique as a sensing material.104 Subsequently, a sensor film was fabricated via spin coating. The as-made sensor could sense a very low concentration (∼20 ppb) and showed an ultrahigh response (Rg/Ra = 4336) for 100 ppb NO2 at 50 °C.
As shown in Table 3, rGO-based composites are the most explored CNMs towards NO2 sensing given that they have easy synthesis procedures due to their good interaction with various MOs, MNPs, polymers and functional groups of rGO. However, although rGO is the most preferred, in terms of minimum detection limit, graphene (synthesized via CVD) is the most interesting CNM (1 ppb). Simultaneously, SWCNT- and MWCNT-based nanocomposites also achieved a low LOD (45 ppb). Additionally, most of these CNMs show good responses even at room temperature.
4.2 COx sensors
COx (CO and CO2) are toxic, odorless, and colorless pollutants, which are dangerous to human beings. Low exposure may only cause nausea, vomiting and dizziness. However, higher consumption of these carbon gases can be very dangerous because CO is toxic, and CO2 is an asphyxiant at high concentrations, which can increase the risk of heart diseases. Hence, to reduce workplace injuries and accidents, gas detectors are required as safety measures. Additionally, CO2 is the biggest factor influencing global warming. Specifically, the radiative force by CO2 is increasing daily, which causes an imbalance in the received and radiated sunlight. Therefore, COx sensors are required for various applications, including environment monitoring and capnography.| O2(ads) + 3e → O2−(ads) + O− | (4) |
After the interaction of CO2 gas with ionized oxygen, a metastable compound CO3 was formed41 and released electrons, which further increased the potential barrier, and consequently the resistance of the CNT film decreased.
| CO2 + O2− → CO3 + 2e | (5) |
However, at high exposure, the metastable compound can interact with itself (represented by eqn (6)). Hence, in this situation, less change in the resistance was observed.
| CO3 + CO3 → 2CO2 + O2(gas) | (6) |
In one of the latest works, Ahmad et al. (2020) synthesized an MWCNT-alumina sensor via the sol–gel process (having various CNT concentrations of 0.6 wt%, 1.0 wt%, 1.5 wt%, 2.0 wt%, and 3.0 wt%) for the detection of CO2.116 The sensor having 2.0 wt% CNTs showed the highest response, and beyond this concentration, the sensing response gradually decreased due to the poor dispersion of the CNTs in the alumina matrix. As shown in Fig. 8, both physisorption and chemisorption phenomena occurred on the surface of the nanotubes. When CO2 was injected into the sensing chamber, the electrons from CO2 (reducing species) transferred to MWCNT (p-type). Due to charge delocalization, there was a shift in the energy band diagram. The calculated sensitivity (ΔR/Ra) was 7.3% at 450 ppm CO2 with a very short recovery time (14.15 s). For the desorption of the analyte molecules, external source UV light was used, decreasing the barrier height (ϕB) with a slight change from the original bandgap position (as illustrated in Fig. 8).
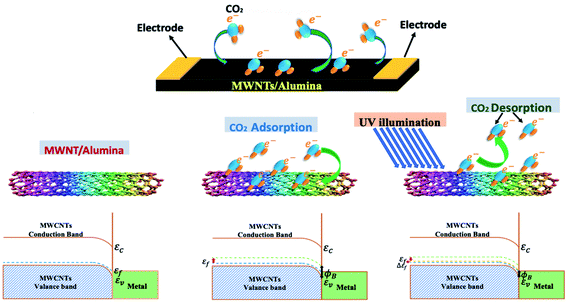 | ||
| Fig. 8 Schematic representation of electron transfer on CNT surface and energy band diagram116 (reprinted from ref. 116, Copyright (2017), with permission from Elsevier). | ||
Another exciting work presented the sensing mechanism of CO on poly(diallyl)dimethylammonium chloride (PDDA) solution-coated MWCNTs (2020).117 According to the described mechanism, there is a positive charge on the nitrogen atom in PDDA. When CO was introduced on the surface of the sensor, a charge transfer phenomenon occurred from CO to the quaternary NH4+ of PDDA. Due to this type of physisorption, the overall resistance of the sensor decreased and it eventually showed a high response. The highest sensitivity (11.51%) was achieved at 20 ppm CO.
| Sensing material | Analyte | Operating temperature | Response | Response time | Recovery time | Limit of detection | References |
|---|---|---|---|---|---|---|---|
| a ΔR/Ra. | |||||||
| ZnO-MWCNT | 25 ppm CO | 70 °C | 62%a | 2 s | 3 s | — | Özütok et al. (2019)129 |
| Polyaniline-MWCNTs | 500 ppm CO | RT | 6.83%a | 105 s | 210 s | — | Roy et al. (2018)130 |
| 1000 ppm CO | 26.73%a | 76 s | 227 s | ||||
| MWCNTs-PDDA | 1 ppm CO | RT | 5.25%a | 29 s | 33 s | 127 ppb | Roy et al. (2020)117 |
| 20 ppm CO | 11.51%a | 18 s | 45 s | ||||
| MWCNTs-alumina | 450 ppm CO2 | RT | 7.3%a | 53.7 s | 14.15 s | — | Ahmad et al. (2020)116 |
| MWCNTs | 5000 ppm CO2 | RT | 210%a | 30 s | 49 s | — | Kumar et al. (2019)73 |
| ZnO-rGO | 1000 ppm CO | 200 °C | 85.2%a | 7 s | 9 s | <10 ppm | Ha et al. (2018)50 |
| RT | 27.5%a | 14 s | 15 s | ||||
| rGO-Mn3O4 | 50 ppm CO | 25 °C | 70.8%a | 3 s | 6 s | — | John et al. (2020)131 |
| 4 ppm CO | 200 °C | 77.8%a | 2 s | 5 s | 4 ppm | ||
| 0.1 Pd-doped SnO2-Gr | 30 ppm CO | 250 °C | 99%a | 0.3 s | 0.3 s | — | Debataraja et al. (2019)132 |
It is observed that the research on CNMs-based COx detection sensors is less than that on other gases given that their pristine form shows a poor response towards COx gas. However, hybrid nanocomposites are studied comparatively more for CO sensing (observed from Table 4). Additionally, CNMs-based flexible sensors have attracted more attention given that the commercialized MO-based COx sensors have negligible flexibility.
4.3 CH4 sensors
Environmental methane (CH4) is a well-mixed ozone-depleting gas with the second biggest increment in radiative constraining after CO2.133 The major sources of CH4 gas are natural gas production and burning of agriculture biomass. According to the global monitoring system, the mole fraction of methane determined from marine surface sites reached up to 1876.3 ppb, which is 1.01 times higher than the previous year (1865.3 ppb).134 Furthermore, CH4 is an odourless and colourless gas, which can be explosive upon mixing with ambient oxygen. Therefore, its presence even in small concentrations is a major threat and needs to be detected.In another work, an SnO2–Pt/MWCNT-based sensor was synthesized and CH4 gas was sensed. The three major reasons for the high performance of the as-made sensor are p–n heterojunction formation, high surface area, and spill-over effect at the Pt nanoparticles.139 However, only few ab initio studies focusing on CNT-based methane sensors have been performed compared to other gases. Therefore, the understanding of the binding energy of CH4 gas on the CNT surface and doped CNT surface still needs to be addressed. Simultaneously, various doped graphene nanostructures have been explored for CH4 sensing either theoretically140 or experimentally.
| CH4 + 4O−(ads) → 2H2O + CO2 + 4e | (7) |
| CH4 + 2O2−(ads) → 2H2O + CO2 + 4e | (8) |
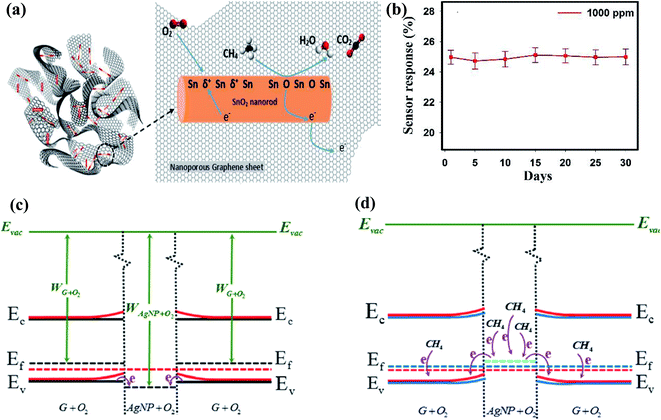 | ||
| Fig. 9 (a) Schematic representation of sensing mechanism for CH4 detection on SnO2-graphene-based sensor. (b) Stability graph of sensor (response calculated using ΔR/Ra)45 (reprinted from ref. 45, Copyright (2017), with permission from Elsevier). (c) Schematic illustration of the energy band diagrams for preabsorbed graphene and Ag NPs under ambient conditions. (d) Electron transfer upon exposure to CH4 (ref. 87) (reprinted with permission from ref. 87. Copyright (2019) American Chemical Society). | ||
The other explained sensing phenomenon is based on oxygen vacancies, which influence the sensor response141 for the detection of CH4 gas.
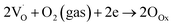 | (9) |
| CH4(gas) + 4OOx → CO2(gas) + 2H2O(gas) + 4VOx | (10) |
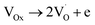 | (11) |
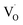 are shallow neutral oxygen vacancy, oxide anion healing of oxygen vacancy, and singly ionized oxygen vacancy, respectively. SnO2 shows in-plane oxygen vacancies,142 which promote the detection of CH4. The as-made sensor showed 24.9% response at 1000 ppm CH4 at 150 °C. Fig. 9b shows the stability of the as-made sensor even after 30 days.
are shallow neutral oxygen vacancy, oxide anion healing of oxygen vacancy, and singly ionized oxygen vacancy, respectively. SnO2 shows in-plane oxygen vacancies,142 which promote the detection of CH4. The as-made sensor showed 24.9% response at 1000 ppm CH4 at 150 °C. Fig. 9b shows the stability of the as-made sensor even after 30 days.
Further, Ag NP-decorated graphene-based smart sensing material was studied for CH4 sensing.87 The oxygen ions were chemisorbed on the Ag NPs and graphene and the WF of G + O2 was less than that of Ag NPs + O2 (as shown in Fig. 9c and d, respectively). During gas sensing, electrons move from Ag NPs + O2 to G + O2, which shifts the EF of Ag + O2 owing to the increase in the hole concentration of graphene and directly influences the response of the sensor. Some significant works on CH4 sensors using CNMs-based hybrid nanocomposites are shown in Table 5.
| Sensing material | Analyte | Operating temperature | Response | Response time | Recovery time | Limit of detection | References |
|---|---|---|---|---|---|---|---|
| a ΔR/Ra. | |||||||
| V2O5 filled in MWCNTs | 40 ppm CH4 | RT | 1.5% | ∼16 s | ∼120 s | — | Chimowa et al. (2017)143 |
| MWCNTs decorated with SnO2–Pt | 100 ppm CH4 | RT | 28.25%a | 176 s | 763 s | 490 ppb | Navazani et al. (2020)139 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ppm CH4 000 ppm CH4 |
94.26%a | 122 s | 1178 s | ||||
| Li ion doped CNT | 500 ppm CH4 | RT | 14.48%a | — | — | — | Chen et al. (2018)137 |
| TiO2 nanotubes-rGO | 800 ppm CH4 | 25 °C | 96.93%a | ∼18 s | ∼61 s | 10 ppm | Acharyya et al. (2016)144 |
| SnO2-rGO/PANI | 100 ppm CH4 | RT | 26.1%a | 360 s | 1150 s | — | Navazani et al. (2018)145 |
| PbS-3.5 wt% rGO | 100 ppm CH4 | RT | 45%a | 92 s | 65 s | — | Roshan et al. (2019)146 |
It has been reported that unfunctionalized and metal-decorated SWCNTs exhibit a very low response towards CH4 at room temperature.147 Therefore, MWCNT hybrid nanocomposites have attracted more attention than SWCNTs. Moreover, rGO has also been explored. Among all the mentioned sensors in Table 5, the TiO2-rGO-based sensors showed the highest response (96.93%) even at a low concentration (800 ppm CH4).
4.4 NH3 sensors
Ammonia (NH3) is a colorless gas with a strong smell, which has been used as a refrigeration gas for centuries. Although it has no global warming potential or impact on the ozone level, it is still essential to detect it given that a large number of people is exposed to ammonia gas by breathing its vapors from many cleaning products. The major drawback of ammonia is that it can be flammable at high concentrations. Besides, its low concentration inhalation leads to coughing and soreness to the nose given that it has a very suffocating smell, and beyond a certain concentration, it can cause burning sensation to the nose and throat. Furthermore, in extreme cases, it may damage the respiratory system completely. Besides the olfactory sense, skin, and eyes are affected, while NH3 gas interaction at low levels and higher levels it can cause burns and blindness, respectively. Vytenis documented a real incident of an NH3 explosion, which happened in West Texas in 2013, killing 15 people and injuring 200.148 Furthermore, NH3 gas reacts with water exothermically, and ammonia corrosion occurs in many metals such as Zn and Cu, and their alloys. Therefore, its detection is necessary.| O2(ads) + 2e → O2−(ads) | (12) |
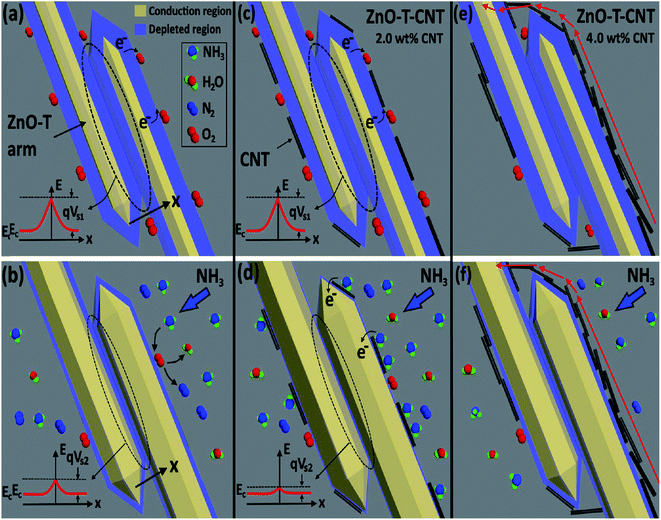 | ||
| Fig. 10 Illustration of sensor in air (a) ZnO-T, (c) ZnO-T-2 wt% CNT networks and (e) ZnO-T-4 wt% CNT networks and in NH3 atmosphere (b) ZnO-T, (d) ZnO-T-2 wt% CNT networks and (f) ZnO-T-4 wt% CNT networks155 (reprinted with permission from ref. 155. Copyright (2017) American Chemical Society). | ||
When NH3 gas came in contact with the sensing material, the chemisorbed oxygen ions acted as adsorption sites for the target gas and the released electrons tuned the bandgap.
| 2NH3 + 3/2O2− → N2 + 3H2O + 3e | (13) |
Consequently, the width of the depletion region decreased, leading to a decrease in ϕB (shown in left section of Fig. 10b). However, when 2 wt% and 4 wt% CNTs (seen in Fig. 10c and e, respectively) were coated on ZnO-T, electrons transferred from the reducing analyte to the CNTs very rapidly, and thus to ZnO-T (illustrated in Fig. 10d). Therefore, they helped to enhance the sensing response.
The sensing at a high concentration of CNTs (>2 wt%) had a negative effect on the sensitivity of ZnO-T given that the CNTs were accumulated on the ZnO arms, which decreased the number of adsorption sites (shown in Fig. 10f). Further, Guo et al. (2018) studied Fe3O4/CNTs as a sensing material for NH3 gas,156 where the explained sensing mechanism is based on magnetic catalysis and chemical bonding. Magnetite has ferrous (Fe2+) and ferric (Fe3+) atoms. When ammonia is exposed to the surface of the sensing material, the analyte molecules are adsorbed on it. The H atoms in NH3 bond chemically with the O atom of Fe3O4 (shown in Fig. 11). Moreover, the N atoms shared electrons with the Fe3+ atom. Therefore, together with chemical bonding, the magnetic effect of Fe3O4 was also beneficial for analyte adsorption. The effect of an external magnetic field on the sensitivity is described by the following equation:
| S = M × eλ×B2 | (14) |
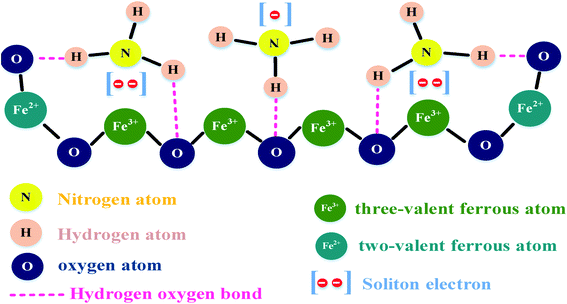 | ||
| Fig. 11 Model of the intermolecular binding force156 (reprinted with permission from ref. 156. Copyright (2018), MDPI Publisher, under Creative Commons Attribution 4.0 International License). | ||
However, besides Fe3O4, CNTs provide adsorption sites, resulting in high sensitivity. The overall sensitivity was enhanced because of the high adsorption capacity of Fe3O4.
Another CNT hybrid nanocomposite for NH3 sensing is the CNT–polymer matrix, where conducting polyaniline (PANI) is commonly used.157–159 Zhang et al. described the synergistic effect of PANI coated on an MoS2-functionalized MWCNT nanomaterial.160 The as-made sensor showed 49.66% sensitivity at 10 ppm NH3. According to the described model, when PANI is exposed to NH3 gas, electrons get transported from NH3 to PANI, forming NH4+. In addition, MWCNTs also react in the similar manner. Besides, the formation of a p–n heterojunction between p-type PANI and n-type MoS2 helped to improvise the sensing response of the as-made sensor. In another work with PANI-CNT for NH3 sensing, Ansari et al. synthesized a carboxyl-functionalized SWCNT-wrapped polyaniline nanofiber (PANI) composite via the in situ chemical oxidative polymerization technique90 and proposed the same sensing mechanism. When NH3 comes in contact with the reactive site of PANI and f-CNTs, the electrons transfer from the reducing gas to PANI and f-SWCNTs, resulting in an enhancement in the resistance of the sensor. The sensing response of the as-made PANI@f-SWCNT (24–25%) was higher than that of the pristine (5–6%) and functionalized SWCNTs (18–20%) towards 10 ppm NH3 due to the high availability of adsorption sites. Although the sensing performance of PANI@f-SWCNT was lower than that of PANI-MoS2@f-MWCNTs and its recovery time was much shorter. Moreover, less steps were involved in the synthesis of PANI@f-SWCNT compared to PANI-MoS2@f-MWCNTs, where MoS2 was synthesized via the hydrothermal route.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O…H–NH2 having an interaction energy of 46.0 kJ mol−1). Due to these interactions, the GO sensing device responded efficiently towards NH3 gas. Further, Srivastava et al. reported NH3 sensing on pure few-layer graphene (PFLGr) and boron-doped few-layer graphene (BFLGr).78 The adsorption energy for NH3 gas on BFLGr is higher than that on PFLGr given that the N atom of NH3 gets easily attached to B atoms (shown in Fig. 12a). Therefore, boron-doped graphene as a sensing material showed higher sensitivity (∼4 times higher than PFLGr) towards 32 ppm NH3. The BFLGr sensor showed high repeatability even after 25 days (shown in Fig. 12b).
O…H–NH2 having an interaction energy of 46.0 kJ mol−1). Due to these interactions, the GO sensing device responded efficiently towards NH3 gas. Further, Srivastava et al. reported NH3 sensing on pure few-layer graphene (PFLGr) and boron-doped few-layer graphene (BFLGr).78 The adsorption energy for NH3 gas on BFLGr is higher than that on PFLGr given that the N atom of NH3 gets easily attached to B atoms (shown in Fig. 12a). Therefore, boron-doped graphene as a sensing material showed higher sensitivity (∼4 times higher than PFLGr) towards 32 ppm NH3. The BFLGr sensor showed high repeatability even after 25 days (shown in Fig. 12b).
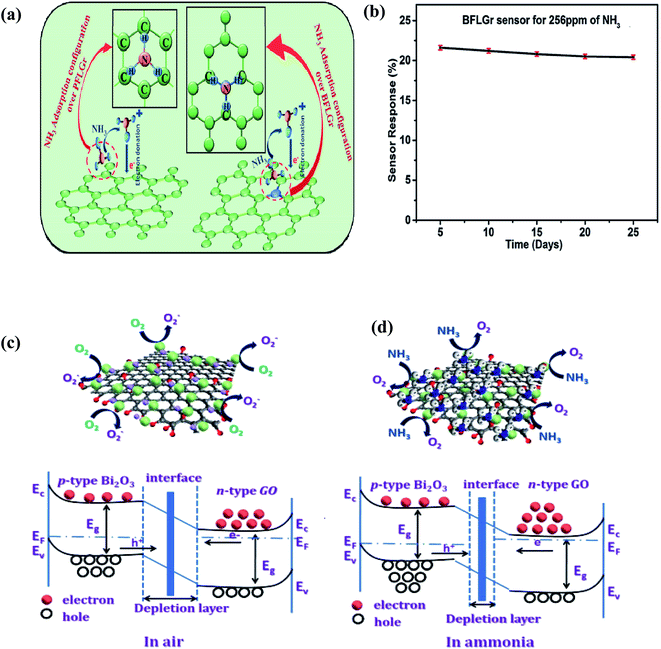 | ||
| Fig. 12 (a) Schematic illustration of NH3 adsorption and proposed gas sensing mechanism of the PFLGr and BFLGr sensor. (b) Plot of sensor response for a period of 25 days78 (reprinted with permission from ref. 78. Copyright (2020), The Royal Society of Chemistry). Representation and band diagram of Bi2O3-doped graphene oxide in (c) air and (d) ammonia86 (reprinted from ref. 86, Copyright (2021), with permission from Elsevier). | ||
In a recent work, Ghule et al. (2021) studied various metal oxide (NiO, ZnO, and Bi2O3)-doped GO sensors and concluded that the Bi2O3-GO (81.23%) sensor exhibits the maximum response at 50 ppm NH3 in comparison with ZnO-GO (60%), NiO-GO (20%) and pristine GO (∼3–4%).86 The sensing mechanism is based on the reduction of the DL width, as explained in Fig. 12d.
Another work employed zeolite imidazole framework-rGO (ZIF-67-rGO) as a sensing material for NH3 gas, which was synthesized via the hydrothermal process.164 When the reducing gas (NH3) passed through the sensing chamber, the charge transferred from ZIF-67 (WF = 1.98 eV) to rGO (WF = 1–1.69 eV). Therefore, the DL width was reduced, and consequently the resistance decreased. On the contrary, the sensing mechanism is different to that of p-type CNTs given that the resistance should increase after NH3 adsorption because ZIF-67-rGO acts as an n-type semiconductor. Table 6 presents the significant works performed on NH3 sensors synthesized using CNM hybrid nanocomposites.
| Sensing material | Analyte | Operating temperature | Response | Response time | Recovery time | Limit of detection | Reference |
|---|---|---|---|---|---|---|---|
| a ΔR/Ra. | |||||||
| WO3 nanobricks-1 wt% CNT | 30 ppm NH3 | 50 °C | 12.5%a | 210 s | 330 s | 150 ppm | Le et al. (2019)165 |
| 10 ppm NH3 | RT | 6.8%a | |||||
| Pd-MWCNTs | 1000 ppm NH3 | 100 °C | 32%a | — | — | — | Dilonardo et al. (2017)106 |
| PANI-CNT | 10 ppm NH3 | RT | ∼610%a | 85 s | 20 s | <200 ppb | Xue et al. (2017)88 |
| f-SWCNTs-PANI | 10 ppm NH3 | RT | 24–25%a | 1–4 s | 8–10 s | — | Ansari et al. (2020)90 |
| f-SWCNTs with HNO3 | 18–20%a | 9–10 s | 30–32 s | ||||
| p-SWCNTs | 5–6%a | 12–15 min | 40–42 min | ||||
| f-MWCNTs with red-phenol | 100 ppm NH3 | — | 18–23.2%a | 6–8 s | 30–50 s | — | Saxena et al. (2020)166 |
| Polypyrrole – f-CNTs with NH2 | 0.1 ppm NH3 | RT | 525%a | 138 s | 465 s | 0.04 ppb | Hamouma et al. (2018)167 |
| Graphene oxide | 100 ppm NH3 | RT | 45%a | 24 s | 18 s | — | Khurshid et al. (2020)48 |
| CrO3 intercalated multilayer Gr | 50 ppm NH3 | 180 °C | 54%a | 10 s | 20 s | — | Jaiswal et al. (2020)162 |
| AgNPs-rGO | 0.1 ppm NH3 | RT | — | 5 s | 6 s | 1.2 ppb | Karaduman et al. (2017)168 |
| 1 ppm NH3 | 6.52%a | ||||||
| PtNPs-rGO | 0.1 ppm NH3 | RT | — | 7 s | 8 s | 16 ppb | Karaduman et al. (2017)168 |
| 1 ppm NH3 | 2.87%a | ||||||
| AuNPs-rGO | 0.1 ppm NH3 | RT | — | 13 s | 17 s | 1.6 ppb | Karaduman et al. (2017)168 |
| 1 ppm NH3 | 0.5%a | ||||||
| Au GNRs | 25 ppm NH3 | RT | 34%a | 224 s | 178 s | — | Seifaddini et al. (2019)169 |
| 75 mM meta toluic acid functionalized GO | 100 ppm NH3 | RT | 12.2%a | 60 s | 80 s | — | Kumar et al. (2020)170 |
| WS2-rGO | 10 ppm NH3 | 33.5 °C | 121%a | 60 s | 300 s | — | Wang et al. (2018)171 |
| TiO2-rGO | 10 ppm NH3 | RT | 170a | 114 s | 304 s | — | Ye et al. (2017)172 |
| MWCNT-Gr hybrid film | 300 ppm NH3 | RT | — | 40 s | 96 s | — | Bisht et al. (2014)173 |
According to the information in Table 6, it can be concluded that the LOD value of CNMs-based composite for NH3 gas sensors is generally very low (in the ppb range). In addition, most of the ammonia gas sensors having CNMs as the sensing material show good sensitivity at ambient temperature. The sensor made with TiO2-decorated rGO has the highest response (ΔR/Ra = 170).
4.5 H2 sensors
Hydrogen (H2) is a non-toxic, non-radioactive, and non-polluting gas, producing no hazardous combustion products, but under certain conditions such as 18–59% mixing in air, it becomes highly explosive given that it requires a low ignition energy, and thus can be extremely dangerous. Moreover, H2 is a colourless and odourless gas, and therefore it is impossible to detect it without any detection device given that free hydrogen is highly reactive. It is ten times more flammable and even twenty times more explosive than gasoline. Also, the flames of fires caused by H2 are invisible, which makes it a serious hazard to work with. Moreover, the H2 explosion limit is confinement dependent, which can create a blast wave that can destroy nearby buildings and injure people. Therefore, a significant number of investigations on hydrogen stations have focused on its ignition, deflagration, and detonation. Recently, in 2019, a hydrogen explosion occurred in a US silicone plant in Waukegan, IIlinois.174 According to the US Chemical Safety and Hazard Investigation Board, the plant lacked was hydrogen detectors. Therefore, precise hydrogen detectors are highly required.Moreover, it has also been reported that for the enhancement of the sensing response towards H2 gas, the presence of functionalized groups on the surface of CNTs such as COOH and OH plays a crucial role.177 Furthermore, together with CNTs, Pd is generally used due to its high catalytic activity towards H2 gas.84,178,179 In 2018, Xiao et al. performed H2 sensing on Pd nanoparticle (Pd NP)-decorated SWCNTs.180 When H2 is exposed to the Pd-decorated CNTs, it would interact in two ways, either as H2 dissolved in Pd to decrease the WF of Pd (electron transfer from Pd to CNTs) or H2 dissociated on the Pd NPs to initiate the spill-over of H atoms (Fig. 13a). The spill-over H atoms diffuse on the surface of the CNTs and directly donate electrons to the CNTs, inducing a delocalized EDR, and thus increasing the resistance. The reactions occurring during exposure to H2 are as follows:
| H2 → 2Hatom on surface → Hatom in Pd | (15) |
| O2 + 2Hatom on surface → 2OH, OH →H2O | (16) |
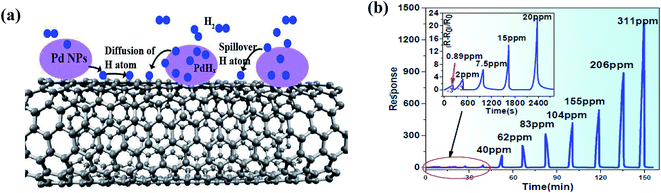 | ||
| Fig. 13 (a) Schematic of H2-Pd-CNT interaction on CNT surface. (b) Response to different H2 concentrations at ambient temperature180 (adopted with permission from ref. 180. Copyright (2018) American Chemical Society). | ||
Fig. 13b shows the response at different concentrations (0.89–311 ppm) of H2 gas, where the sensor showed a much higher response (>1200) at 311 ppm.
Similar to Pd, Pt is also very encouraging due to similar phenomena.181 One of the early research works was on Pt-doped TiO2@F-CNTs for H2 gas sensing.182 Besides the catalytic effect of functionalized CNTs, the Schottky barrier formed at the boundary of Pt and TiO2 also leads to electron transfer from Pt to TiO2, and then from TiO2 to CNTs, and these electrons produce E–H pairs in the CNTs, which consequently increase the overall resistance. Moreover, when H2 gas molecules interact with Pt, they dissociate into hydrogen ions (H+ and H−), and then diffuse into Pt. The sensitivity of the f-MWCNTs-TiO2-Pt based sensor was 1.35, 2.53, 4.75, and 19 times higher than that of f-MWCNTs-TiO2-Pt, f-MWCNTs-Pt, f-MWCNTs and pristine MWCNTs, respectively.
Another existing work explains the sensing mechanism of graphene decorated with Pd–Ag NPs.188 Physisorption and chemisorption occur during H2 sensing, where chemisorption occurs due to the formation of strong metal hydrides (Pd–H) having covalent bonds.189 Recently, Achary et al. proposed ZnFe2O4–Pd decorated rGO as an H2 sensing material.190 The as-fabricated sensor showed a high response of 11.43% towards 200 ppm H2 at room temperature. Fig. 14 shows the possible sensing mechanism of the sensor.
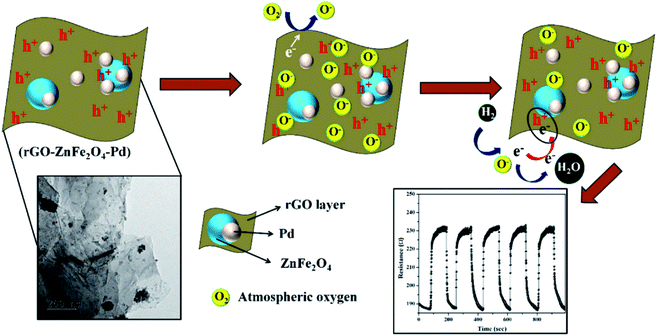 | ||
| Fig. 14 Plausible sensing mechanism of ZnFe2O4–Pd decorated rGO towards H2 gas190 (reprinted from ref. 190. Copyright (2020), with permission from Elsevier). | ||
Due to the high surface availability and high charge mobility of rGO, the sensor showed a high response. Table 7 shows the various CNM hybrid nanocomposite-based sensors for H2 gas.
| Sensing material | Analyte | Operating temperature | Response | Response time | Recovery time | Limit of detection | Reference |
|---|---|---|---|---|---|---|---|
| a ΔR/Ra. | |||||||
| MWCNT decorated with Pd | 4% H2 | — | 35.30%a | — | — | — | Yan et al. (2019)191 |
| Pd-CNTs | 311 ppm H2 | RT | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000%a 000%a |
7 s | 89 s | 0.89 ppm | Xiao et al. (2018)180 |
| f-CNT with COOH and OH | 10% H2 | RT | 5.7%a | 35 s | 55 s | Han et al. (2019)192 | |
| Acidic-MWCNTs-TiO2-Pt | 0.05% H2 | RT | 3.9%a | 20 s | — | Dhall et al. (2017)182 | |
| Pt–Gr like carbon wrapped CNTs | 4% H2 in air | RT | 42.8%a | 120 s | — | <0.1% | Baro et al. (2018)193 |
| MWCNTs | 10% H2 | — | 0.4%a | — | — | 7100 ppm | Park et al. (2020)176 |
| Crumped MWCNTs | 10% H2 | 1.3%a | 2700 ppm | ||||
| Au-Gr | 500 ppm H2 | — | 5.46%a | 16 s | 274 s | — | Kim et al. (2019)194 |
| Pt-rGO | 0.5% H2 | 50 | 8%a | 63 s | 104 s | — | Lu et al. (2018)195 |
| Pt decorated ZnO-rGO | 400 ppm H2 | 100 | 99a | 12 s | 412 s | — | Drmosh et al. (2019)196 |
| CuO-rGO | 1500 ppm H2 | RT | ∼11%a | < 80 s | < 60 s | 10 ppm | Zhang et al. (2017)197 |
As observed, Pd- and Pt-doped CNMs show high sensitivity towards H2 gas given that these MPs show chemical sensitization based on the spill-over mechanism. To the best of our knowledge, Pd-CNTs show an ultrahigh response for H2 sensing (∼100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000%).
000%).
4.6 H2S sensors
Dihydrogen sulphide (H2S) is a colourless, flammable gas that smells like rotten eggs. Hence, it can affect the eyes, smelling sense and respiratory system. Long-duration exposure to H2S can even paralyse the nervous system. Therefore, detectors are needed for H2S. A level of H2S gas at or above 100 ppm is lethal according to the IDLH values. Additionally, this gas is a silent threat, often invisible to the body's senses.| 2H2S(g) + 3O2−(ads) → 2H2O(g) + 2SO2(g) + 3e | (17) |
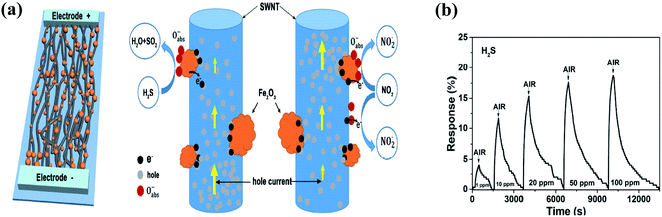 | ||
| Fig. 15 (a) Schematic of the gas sensor based on SWNT-Fe2O3 composite film and schematic explaining the H2S sensing mechanism. (b) Response (ΔR/Ra) and recovery curves of sensor upon exposure to H2S (1, 10, 20, 50, and 100 ppm) with complete recovery107 (reprinted from ref. 107, Copyright (2017), with permission from Elsevier). | ||
The released electrons are adsorbed on the surface of the Fe2O3 NPs, and then transferred to the SWCNT film, where the E–H recombine, leading to a decrease in the concentration of hole carriers in the SWCNTs, which increases the resistance of the gas sensor.
Fig. 15b represents the response of the sensor towards 1 ppm to 100 ppm H2S.
Srivastva et al. (2019) analysed zigzag pristine, boron and nitrogen-doped (10,0) SWCNTs using the Atomistix Toolkit-Virtual NanoLab (ATK-VNL) simulation software.198 The computational results of H2S adsorption on the pristine SWCNTs (80.16%) showed the highest sensitivity and lowest recovery time compared with B-doped (60.79%) and N-doped CNT (78.76%). Further, Nobari et al. studied amide-functionalized SWCNTs as an H2S sensor computationally using the AVAGADRO software199 and reported the maximum sensitivity of 89.3% at 40 mV.
Together with SWCNTs, MWCNTs have also been explored for H2S sensing applications. Ibrahim et al. dissolved different concentrations of MWCNTs (0.01 and 0.1 mg mL−1) in poly(2-methoxy-5-(2′-ethythexyloxy)-1,4-phenylenevinylene) films, which were studied for H2S sensing.200 The sample having a higher content of MWCNTs showed higher sensitivity (104.45%) in comparison with the sample having less content of CNTs (11.70%) given that it provides more adsorption sites.
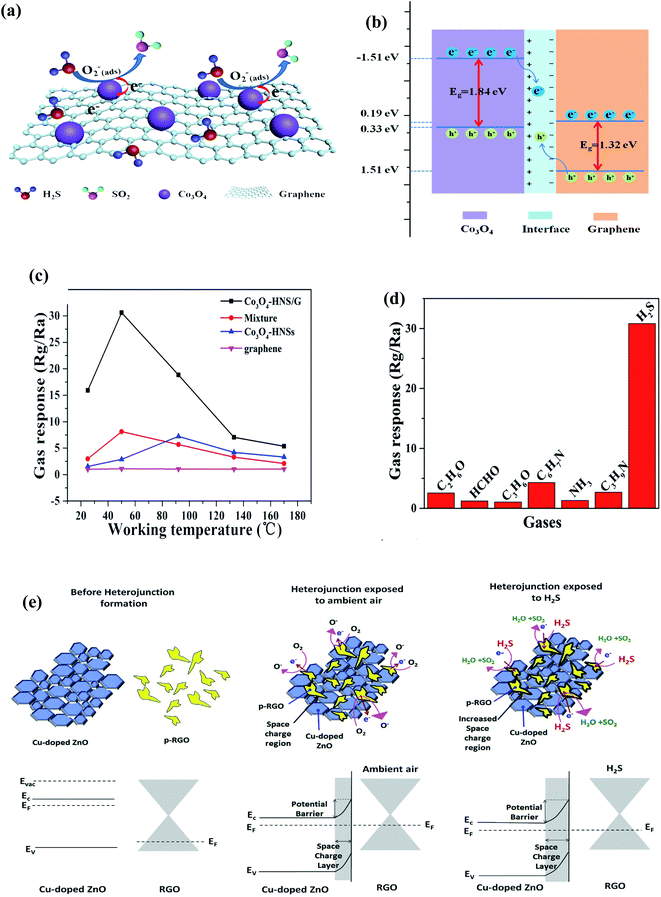 | ||
| Fig. 16 (a) Schematic depiction of the sensing mechanism of the sensor, (b) energy band structure for the heterojunction of Co3O4 and graphene, (c) responses of Co3O4-HNS/G, Co3O4-HNSs, and graphene versus various operating temperatures to 50 ppm of H2S and (d) plot of sensor constructed by Co3O4-2HNS/G to different gases with a concentration of 50 ppm at 50 °C (ref. 205) (adopted with permission from ref. 205. Copyright (2019) American Chemical Society). (e) Energy band diagram of Cu-doped ZnO/rGO nanocomposite206 (reprinted from ref. 206, Copyright (2020), with permission from Elsevier). | ||
In another work, Yang et al. used NiO–nitrogen-doped rGO as a sensing material for H2S sensing.52 After exposure, the analyte gas attached to the pre-adsorbed oxygen, which was confirmed via XPS, where the concentration of pre-adsorbed oxygen decreased by up to ∼84% due to the redox reaction between the oxygen ions and H2S gas.
| H2S(gas) → H2S(ads) | (18) |
| 2H2S(ads) + 3O2−(ads) → 2SO2(ads) + 2H2O + 3e | (19) |
The as-made sensor showed high sensitivity (54.6%) even at 50 °C. Further, NiO–boron–nitrogen-doped rGO was synthesized chemically and used as an H2S detector.207 By doping boron and nitrogen, the absorption of oxygen anions increased, which increased the number of adsorption sites for the gas. Moreover, the electronegative boron and electropositive nitrogen were attributed to the localized electrostatic potential given that the B-active sites are beneficial for the easy capture of oxygen anions, whereas the N-active sites are beneficial for converting the surface-adsorbed oxygen into oxygen radicals. Although the sensitivity was not affected much, the LOD was five-times lower than that of the NiO–nitrogen-doped rGO-based sensor.
Recently, Shewale et al. fabricated an H2S gas sensor based on Cu-doped ZnO decorated with rGO nanosheets at ambient temperature.206 The response of the as-made sensor depends on the defected sites, SCR, and formation of a p–n junction between the metallic rGO and semiconducting Cu-doped ZnO, where the electrons transfer from rGO to Cu-doped ZnO. Upon exposure to the analyte gas, the H2S molecules get adsorbed on the surface of the sensor, and the interaction occurs between the pre-chemisorbed oxygen molecules and H2S gas-discharged free electrons, which neutralize the holes in rGO, and therefore reduce the size of the charge conduction channels, leading to an increase in the width of the SCR, further increasing the response of the sensor. Additionally, sensing affects the Schottky barrier height and contributes to the performance of the sensor. This sensor detected H2S molecules (150 ppm) within 12 s, which decreased with an increase in concentration. The noise of the sensor (0.0208) and detection limit (136 ppb) were calculated as follows:
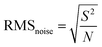 | (20) |
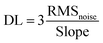 | (21) |
A very small change in the response of the sensor (5%) was observed after 35 days. Moreover, the selectivity of the sensor was confirmed by its lower sensitivity towards H2 gas. Table 8 summarizes a few recent works on CNM hybrid nanocomposites as H2S sensors.
| Sensing material | Analyte | Operating temperature | Response | Response time | Recovery time | Limit of detection | Reference |
|---|---|---|---|---|---|---|---|
| a R a/Rg (oxidizing gas) or Rg/Ra (reducing gas). b ΔR/Ra. c ΔR/ΔC. | |||||||
| Fe2O3-SWCNTs | 100 ppm H2S | RT | 18.3%b | — | — | — | Hua et al. (2017)107 |
| CNTs-SnO2-CuO | 40 ppm H2S | RT | 19%c | 240 s | 600 s | 10 ppm | Zhao et al. (2020)81 |
| 3 wt% CNTs-doped CuO–SnO2 | 0.1 ppm H2S | 40 °C | 4.441a | 8.3 s | 11.5 s | — | Fan et al. (2019)208 |
| SnO2-rGO | 4 ppm NO2 | 200 °C | 185a | 8 s | 215 s | 0.5 ppm | Bhangere et al. (2020)101 |
| 40 ppm H2S | 3.7a | 240 s | 1240 s | 2 ppm | |||
| Co3O4-4.6 wt% rGO | 50 ppm H2S | 50 °C | 30.6a | — | 170 s | 0.1 ppm | Liu et al. (2019)205 |
| 100 ppm H2S | 62.13a | ||||||
| NiO–(boron–nitrogen-doped rGO) | 20 ppm H2S | 150 °C | 16.6a | 38 s | 44 s | 24 ppb | Shanmugasundaram et al. (2019)207 |
| 50 ppm H2S | 35 °C | 1.85a | 28 s | 75 s | |||
| 100 ppm H2S | 50 °C | 5.84a | 29 s | 78 s | |||
| 100 ppm H2S | 100 °C | ∼82a | |||||
| Cu-doped ZnO-rGO | 100 ppm H2S | 24 °C | 0.87%b | 14 s | 32 s | 136 ppb | Shewale et al. (2020)206 |
| CuO-rGO | 5 ppm H2S | 100 °C | ∼28a | 20 s | 920 s | — | Yin et al. (2019)209 |
| Cu2O-rGO | 1 ppm H2S | 40 °C | 20%b | — | — | — | Zhou et al. (2019)210 |
| Au–SnO2-rGO | 50 ppm SOF2 | 110 °C | 15.9%b | 41 s | 68 s | — | Zhang et al. (2019)211 |
| 50 ppm H2S | −14.8%b | 26 s | 35 s | ||||
| GrQD–SnO2/ZnO | 0.1 ppm H2S | RT | 15.9a | 14 s | 13 s | — | Shao et al. (2020)212 |
| 1.0 wt% rGO-loaded ZnFe2O4 NFs | 1 ppm H2S | 350 °C | 147a | <10 s | ∼500 s | 0.14 ppb | Hoang et al. (2019)213 |
| 450 °C | <10 s | ∼130 s | |||||
| SnO2-rGO | 100 ppm H2S | 125 °C | 33.025%b | 209 s | 900 s | 42 ppb | Chu et al. (2018)214 |
| 10 ppm SOF2 | −0.324%b | 255 s | 330 s | 510 ppb | |||
| NiO–nitrogen-doped rGO | 50 ppm H2S | 50 °C | 24.96a | 12 s | 100 ppb | Yang et al. (2017)52 | |
| 100 ppm H2S | 92 °C | 31.95a | 36 s | ||||
| 10 ppm H2S | 133 °C | 8.42a | 197 s | ||||
| 0.1 ppm H2S | 92 °C | 54.06a | |||||
| 92 °C | ∼10.5a | ||||||
| 92 °C | 1.6a | ||||||
According to Table 8, it can be seen that CNTs are less explored than graphene derivatives (rGO) for H2S sensing. The reason for this may be that together with high sensitivity, rGO hybrid composites show very small LOD values.
4.7 Volatile organic compound sensors
The vapours of numerous volatile organic compounds (VOCs) such as ethanol (C2H5OH), toluene (C7H8), liquid petroleum gas (LPG), triethylamine (TEA), formaldehyde (HCHO), and acetone are another class of gases that should be sensed given that their presence can be harmful. Moreover, various VOCs are exhaled during breathing. Thus, the detection of these exhaled VOCs can help in the prediction of several physiological conditions in the human body.215 Consequently, it may be possible to diagnose cancer and other diseases.Recently, Guo et al. fabricated an ethanol sensor having functionalized CNT-decorated ZnSnO3 (hollow box), which was synthesized via the hydrothermal technique.216 While sensing ethanol vapors, CO2 and H2O gases are produced after the interaction of the analyte molecules with the pre-adsorbed oxygen ions, and the corresponding series of reactions occur as follows:
| e + O2(abs) → O2− | (22) |
| 2e + O2(abs) → 2O− | (23) |
| C2H5OH + 3O2− → 2CO2 + 3H2O + 3e | (24) |
| C2H5OH + 6O− → 2CO2 + 3H2O + 6e | (25) |
As shown in Fig. 17a, the released electrons are delivered to ZnSnO3, and consequently decrease the DL thickness. Together with ZnSnO3, CNTs also have a great impact on the performance of the sensor given that in air, a p(CNT)–n(ZnSnO3) heterojunction is formed, where the electrons transfer from ZnSnO3 to the CNTs due to their different Fermi levels, and after exposure to ethanol, the electrons released back to ZnSnO3. Moreover, the BET surface area of CNT@ZnSnO3 is higher (45.73 m2 g−1) by up to seven-folds that of ZnSnO3. Besides, the dipole–dipole interaction between the COOH groups of the functionalized CNTs and OH group of ethanol enhanced the sensing behaviour of the as-made sensors. However, to check the selectivity, the sensor was exposed to 100 ppm ethanol, acetone, benzene, methylbenzene, formaldehyde, and ammonia at 240 °C and it showed the highest sensitivity towards ethanol (shown in Fig. 17b).
 | ||
| Fig. 17 (a) Ethanol sensing mechanism of ZnSnO3 hollow particles. (b) Sensing response (Ra/Rg) of CNT@ZnSnO3 exposed to different types of gases, i.e. ethanol, acetone, benzene, methyl-1-benzene, formaldehyde and ammonia (from left to right)216 (reprinted from ref. 216, Copyright (2020), with permission from Elsevier). | ||
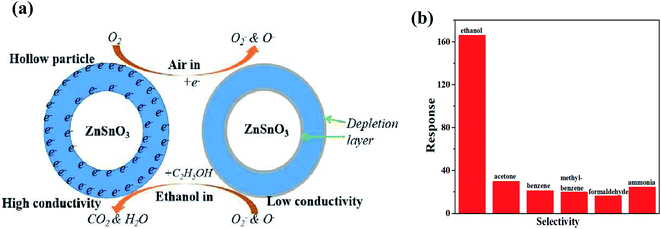
Likewise, CNT-based acetone sensors were also fabricated. In one of the works, Dai et al. studied α-Fe2O3 nanowires wrapped on MWCNTs (shown in Fig. 18a) for sensing acetone.224 The basic phenomenon of the absorption of ambient oxygen on the surface of Fe2O3 was attributed to the increase in electron concentration. The released electrons from α-Fe2O3 were captured by the CNTs, leading to a change in resistance. In addition, when α-Fe2O3 and MWCNTs come in contact, p–n heterojunction is formed (having different bandgaps) at the interface of the CNTs and iron oxide (illustrated in Fig. 18b), which promotes the performance of the sensor. Nonetheless, the surface area of α-Fe2O3-CNT was higher than that of α-Fe2O3. For the investigation of selectivity, the as-made sensor was studied using various types of gases such as methanol, formaldehyde, ethanol, acetone, toluene, and benzene (shown in Fig. 18d). Moreover, Jia et al. studied the same sensing material for acetone having a flower-type morphology of iron oxide.219 The proposed sensing mechanism model is same as described above (illustrated in Fig. 18e), but due to the metal oxide having different structures, the sensing response was affected.
 | ||
| Fig. 18 (a) Qualitative band diagrams of α-Fe2O3 and CNTs, (b) energy band structure of the CNTs@α-Fe2O3 heterostructure in air, (c) resistance curves of pure α-Fe2O3 and α-Fe2O3-MWCNTs towards varying concentrations of acetone (30–100 ppm) at 225°, (d) response curve towards various organic compounds224 (adapted with permission from ref. 224. Copyright (2017) American Chemical Society), and (e) Schematic of the gas sensing mechanism of α-Fe2O3-MWCNT nanocomposites. (f) response curve of pure α-Fe2O3 and α-Fe2O3-MWCNTs towards 50 ppm acetone at 220 °C.219 (Reprinted from ref. 219, Copyright (2017), with permission from Elsevier). | ||
Fig. 18f shows the response curve indicating the response and recovery time of the α-Fe2O3 (3.4 s/10.6 s) and α-Fe2O3-MWCNTs (2.3 s/10.6 s) sensors. Further for LPG sensing, a PANI-CNT-V2O5 hybrid nanocomposite was studied as a sensing material.225 When the sensing material is exposed to LPG, the analyte molecules are adsorbed on CNT-V2O5 and the adsorbed molecules are oxidized due to the transfer of electrons from PANI owing to the increase in resistance. The sensor showed a small LOD value (10 ppm). Recently, Reddy et al. studied a CeO2 nano-hexagon-decorated rGO/CNT heterostructure for LPG sensing.226 After exposure, the chemisorbed oxygen ions oxidized the LPG reducing gas molecules and released electrons to CeO2-rGO/CNT and increased the conductivity. Moreover, rGO and CNTs provided abundant adsorption sites for atmospheric oxygen, thus resulting in a high sensing response. During LPG sensing, a series of reactions occurs as follows:
| CnH2n+2 + O2− ↔ CnH2n: O(gas) + e + H2O → CO2(gas) + H2O | (26) |
| C4H10 + 13/2O2− ↔ 4CO2(gas) + 5H2O +13/2e | (27) |
| C3H8 + 5O2− ↔ 3CO2(gas) + 4H2O + 5e | (28) |
In another work, Septiani et al. sensed toluene vapours using an MWCNT-ZnO-based sensor.227 During sensing, the analyte gas came in contact with oxygen ions and released electrons, which reduced the resistance of the sensor. Moreover, when the analyte gas contacted the sensor, the barrier height decreased and the resistance of the sensor decreased. Also, the formation of a heterojunction also helped to improve the sensing performance.
Recently, Yuan et al. sensed TEA using a double-layer Co3O4 coated on rGO (D-Co3O4/rGO)-based sensor.228 The adsorption of oxygen anions on Co3O4 (p-type) results in the formation of a hole accumulation layer (HAL). When the reducing gas TEA interacts with these chemisorbed oxygen ions, the corresponding reaction takes place, as follows:
| 2N(CH2CH3) + 13O− → 4CO2 + N2 + 5H2O + 13e | (29) |
The released electrons lead to a decrease in the HAL thickness. Moreover, a p–p isotherm junction is formed between rGO and Co3O4, also contributing to the electron transfer, and thus affects the response of the sensor. Besides, the double-layered structure provides a high surface area for the adsorption of more analyte molecules (illustrated in Fig. 19a).
 | ||
| Fig. 19 (a) Schematic of the sensing mechanism of D-Co3O4/rGO composite228 (reprinted from ref. 228.,Copyright (2019), with permission from Elsevier). (b) Energy band diagram of the B-rGO/SnO2@Au heterostructure sensor235 (reprinted from ref. 235, Copyright (2020), with permission from Elsevier). (c) Schematic diagram of the possible gas sensing mechanisms of rGO/α-Fe2O3 nanocomposite. (d) Dynamic response–recovery curve (100 ppm TEA) of pure α-Fe2O3 spindles and rGO/α-Fe2O3 nanocomposite at 280 °C (ref. 236) (reprinted from ref. 236, Copyright (2017), with permission from Elsevier). | ||
Likewise, Peng et al. synthesized boron-doped graphene coated with Au@SnO2 composite for TEA sensing.235 The presence of Au NPs promotes the adsorption of oxygen ions, resulting in a wider EDR at the Au@SnO2 interface, which influences the overall sensor response (illustrated in Fig. 19b). In addition, more adsorption sites are provided by Au NPs. The as-designed sensor showed a high response (∼69%) for 1 ppm TEA.
Wei et al. doped 1 wt% rGO in α-Fe2O3 to improve the sensing performance for TEA.236 When exposed to air, α-Fe2O3 donates electrons to oxygen, forming an EDL, whose width decreases via gas adsorption. In addition, rGO is another crucial factor for improved sensing via the formation of a p–n heterojunction at the interface of α-Fe2O3 and rGO. Fig. 19c describes the mechanism for TEA sensing. The hybrid sensor showed 2.7-times higher sensitivity at 280 °C. The response–recovery curves of the pure α-Fe2O3 spindles and rGO/α-Fe2O3 nanocomposite at 280 °C are illustrated in Fig. 19d.
Moreover, Seekaew et al. (2019) fabricated a toluene (C7H8) gas sensor based on a graphene–CNT hybrid nanostructure decorated with TiO2,237 where a Schottky metal-semiconductor junction is formed between G/CNT NS-TiO2. Upon exposure, the C7H8 vapors interact with the pre-absorbed oxygen ions according to the following reaction:
| C7H8(gas) + 9O−(ads) → 7CO2(gas) + 4H2O(gas) + 9e | (30) |
The released electrons move to TiO2, which further increase the barrier height (illustrated in Fig. 20b), and consequently increase the resistance of the sensor. The as-made sensor showed a higher response than CNT-decorated TiO2 given that the TiO2 NPs were well dispersed on the CNTs grown on graphene because they were agglomerated.
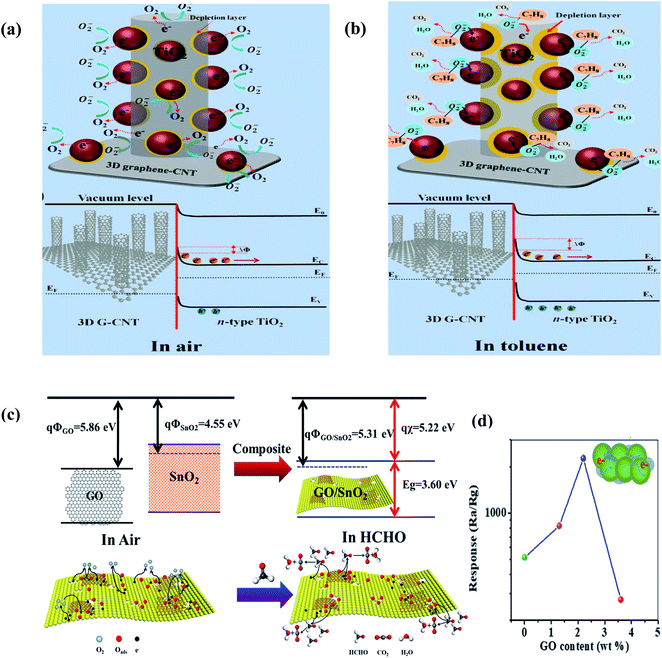 | ||
| Fig. 20 Schematic and energy band diagrams of 3D TiO2/G-CNT gas sensors in (a) air and (b) toluene237 (reprinted from ref. 237, Copyright (2019), with permission from Elsevier). (c) Schematic description of formaldehyde sensing mechanism on GO/SnO2. (d) Relation between response vs. GO content for GO/SnO2 NS-T (T = 450 °C, 475 °C, 500 °C, and 525 °C) operated at 60 °C (ref. 238) (reprinted with permission from ref. 238. Copyright (2017) American Chemical Society). | ||
Similar to other organic compounds, LPG vapors are also detected using graphene-based sensors.239 In one study, Goutham and coworkers synthesized a CdO-doped graphene nanocomposite for LPG sensing.240 In LPG sensing, the gas molecules interact with the pre-absorbed oxygen ions on the surface of the sensor, producing CO2, H2O, and electrons.
In addition to other compounds, HCHO vapors are also detected using graphene-based sensors.241 In this field, Wang et al. used graphene oxide in situ SnO2 sheets as a sensing material for the detection of formaldehyde (shown in Fig. 20c). A Schottky junction is formed between GO and SnO2, and the electrons transfer from SnO2 to GO, thus decreasing the resistance of the sensor. Moreover, GO helps to decrease the agglomeration of the SnO2 NPs. Accordingly, it increases the available adsorption sites for a high sensor response. When HCHO (electron donor) interacts with the sensing material, the following reaction takes place:
| HCHO(g) + O−/O2−(ads) → HCOOH(g) + e | (31) |
| HCHO(g) + O−/O2−(ads) → CO2(g) + H2O(g) + e | (32) |
Beyond 2.2 wt% GO in the sensor, the response of the sensor started to decrease (shown in Fig. 20b) due to the poor dispersion of the sheets.
Besides the above-mentioned volatile compounds, several other VOCs such as DMMP242 and ethanol243 have also been sensed using graphene hybrid nanocomposite sensors. Table 9 summarizes the recent works on CNM hybrid nanocomposites towards various VOCs.
| Sensing material | Analyte | Operating temperature | Response | Response time | Recovery time | Limit of detection | Reference |
|---|---|---|---|---|---|---|---|
| a R a/Rg (oxidizing gas) or Rg/Ra (reducing gas). b ΔR/Ra. | |||||||
| α-Fe2O3-MWCNTs | 50 ppm C3H6O | 220 °C | 20.32a | 2.3 s | 10.6 s | — | Jia et al. (2019)219 |
| CNTs-ZnSnO3 | 100 ppm C2H5OH | 240 °C | 166a | 6 s | — | — | Guo et al. (2020)216 |
| CNT-rGO decorated with Co3O4 | 50 ppm C2H5OH | RT | 1.36%b | — | — | — | Morsy et al. (2019)244 |
| CNT-V2O5 polymerized with PANI | 50 ppm LPG | 30 °C | 300%b | 20 s | 15 s | — | Albaris et al. (2019)225 |
| CeO2-decorated rGO-CNT | 400 ppm LPG | RT | 42%b | 26 s | 98 s | — | Reddy et al. (2020)226 |
| CNTs decorated via Fe2O3 | 5 vol% LPG | RT | — | 10 s | 59 s | — | Chaitongrat et al. (2019)72 |
| ZnO NRs-MWCNTs | 100 ppm C2H5OH | 370 °C | 26.1a | 2 s | 16 s | — | Cao et al. (2018)245 |
| CNTs coated via Au NPs | 800 ppm propanone | RT | 2.98%b | — | — | — | Lam et al. (2019)246 |
| Boron-doped Gr coated Au–SnO2 | 1 ppm TEA | 100 °C | 69.1%b | 27 s | 100 ppb | Peng et al. (2020)235 | |
| Fe2O3-rGO | 50 ppm TEA | 280 °C | 24%a | 2 s | 7 s | — | Wei et al. (2020)236 |
| Double layer Co3O4/rGO | 100 ppm TEA | 200 °C | ∼25a | 30 s | 32 s | — | Yuan et al. (2019)228 |
| 12 layered r (GO/rGO) | 50 ppm DMMP | RT | 8.95%b | 4 min | 3 min | — | Wang et al. (2019)242 |
| TiO2-Gr-CNT hybrid | 500 ppm toluene vapors | RT | 42.9%a | 9 s | 11 s | — | Seekaew et al. (2019)237 |
| rGO-Au | 50 ppm LPG | RT | 22.5a | ∼5 s | ∼35 s | 50 ppm | Taheri et al. (2018)239 |
| SnO2-GO | 100 ppm HCHO | 60 °C | 2275.7a | 81.3 s | 33.7 s | — | Wang et al. (2019)238 |
| HA-HCl-rGO | 16 ppm HCHO | RT | 75%b | 0.023 ppm | Zhou et al. (2020)241 | ||
| Pd–SnO2-Gr | 2% C2H5OH | 200 °C | 14.8%b | ∼15 s | ∼12.5 s | — | Dhall et al. (2018)243 |
As shown in Table 9, various VOCs were detected using CNM hybrid nanocomposite-based sensors. However, there are diverse compounds (>400) exhaled during breathing, which can help to predict typhoid, lung cancer, breast cancer, asthma, kidney malfunctioning, etc. Therefore, more research should be performed on CNM hybrid nanocomposites for the detection of VOCs.
As mentioned in Section 4, CNMs-based composites show high sensitivity towards various gases but atmospheric moisture is also one of the major concern which is described in next section.
5. Effect of atmospheric moisture
It has been reported that environmental humidity can limit the real-life applications of CNMs-based gas sensors, where it has positive or negative effects, depending on various factors such as the type of gas that should be sensed, its concentration and the operating temperature of the sensor. When moisture interacts with CNT-based sensors, electrons migrate from H2O to CNT, which can convert p-type CNTs to n-type CNTs. In this case, Zhang et al. synthesized an SWCNT-based composite as a gas sensor, which had a negligible effect from moisture.247 According to the reported mechanism, PANI (negative response to relative humidity (RH)) and SWCNTs (positive response to RH) balanced the humidity affect.Moreover, in terms of pristine graphene (hydrophilic), moisture or water molecules adsorb on its surface, and consequently block its active sites. The other class of graphene, GO, has many functional groups on its surface, which can form hydrogen bonds between moisture molecules and its oxygenated functional groups, and therefore can endure the sensing performance towards humidity. Specifically, due to the hydrophilic nature of GO, H2O molecules get adsorbed and can form a molecularly thin layer on its surface, which is attributed to the high adsorption of gas, where the layer thickness can be enhanced with RH. Wu et al. concluded that a GO-based sensor had a three times larger value at 70% RH towards 1 ppm NO2.37 Further Khurshid et al. reported similar humidity effects for NH3 gas. Upon the interaction of NH3 with moisture, the water molecules act as an electron acceptor.48
Although humidity has a positive effect on GO-based sensors, the response of the sensor declines with respect to moisture. Accordingly, rGO has better immunity to RH given that it is hydrophobic in nature, and thus rGO-based sensors are less impaired by humidity. Due to this unique feature, rGO-based composites has little impact from RH.
In addition, the effects of humidity on CNMs-based gas sensors is affected by a variation in temperature. Wu et al. showed that by increasing the temperature, the humidity effect can be nullified.37 Further, the concentration of gas analytes can also influence the moisture effect. In this context, Tang et al. fabricated an NH3 sensor using ppy/rGO as the sensing material and studied the effect of moisture towards 1 ppm to 4 ppm NH3.248 Although CNMs-based sensors have the ability to avoid moisture, still a lot of work is required for their applications in the field.
Besides humidity effects, many other problems of CNMs-based sensors are discussed in Section 6.
6. Current challenges
Although nanocarbon-based sensors have high sensitivity, more efforts are required for their profit-oriented establishment given that they have many challenges such as low reproducibility,16 cross-sensitivity,249 non-uniform dispersion,12 irreversible recovery,95 low stability of functional groups, and defects.250 Furthermore, besides the expensive and complex synthesis of CNMs, they require sophisticated handling during device fabrication and sensing,251 for example, it is difficult to control the layers of graphene, its degree of functionality and walls of CNTs with a particular chiral family. In the case of graphene synthesized via CVD, transferring the graphene sheet is also a challenge.252 Together with these challenges, the other dire challenge is cross-sensitivity given that the sensing environment having a mixture of gases with similar structures and belonging to the same family may interfere with the response of the sensor. Therefore, the major challenge is that pristine CNMs show low sensitivity and selectivity,181,253 which can be resolved by functionalizing them either with metal oxides or functional groups, i.e. carboxylic and ketone groups. Furthermore, for an ideal sensor, its LOD should be low to sense a very small concentration of gases. By using aligned sensing structures, the LOD can be reduced. Moreover, flexible sensors are in a high demand, which researchers are working on nowadays.20 In this case, various polymers incorporated with CNTs and graphene, GO and rGO have been explored without ruining their sensitivity. Moreover, for medical diagnosis applications, less toxic sensors are of great importance, but CNMs have a high toxic effect on the human body,20,254 and thus researchers are working on introducing the less toxic GrQDs (0-D) as a new sensing material, but a lot of work has to be performed. To date, most of the research on CNM chemiresistive sensors is based on lab-scale tests. Hence, for industrialization, the large-scale production of CNMs such as graphene and CNTs is also a major concern.7. Conclusion
In the past two decades, the progress and application of carbon nanomaterials (CNMs)-based chemiresistive sensor technology have been increasing at a considerable rate due to the inherent morphology and properties of CNMs. CNMs such as CNTs, graphene and their derivatives have abundant adsorption sites, tunable electrical properties, low density, high carrier mobility, low operating temperature, long lifetime, and easy recovery, which make them suitable alternatives to the standard MOS chemiresistive sensors towards various toxic pollutant gases, explosive gases, and volatile compounds. Theoretically and experimentally, it has been established that the electrical resistance and local density of states of CNMs can be reversibly changed upon exposure to certain vapours.According to the latest trend in chemiresistive sensor application, it was found that numerous studies related to CNMs and their hybrid nanocomposites are considered ideal sensing materials. Due to the presence of a higher surface area, planar structure, better bending ability, low electrical noise, easy functionalization, and high availability of adsorption sites, graphene is the most preferable candidate as a gas sensing material. However, pristine graphene has some drawbacks such as lack of functional groups, difficult synthesis and handling process, and thus its derivatives have also been studied as sensing materials. Among the graphene derivatives, rGO has the highest sensitivity due to its high charge mobility and presence of vacancies, which are created via the removal of oxygen groups. Similarly, other CNMs such as CNTs have also been explored for gas sensing applications. Although graphene-based sensors show better sensitivity, CNTs still have some advantages over them. In the case of CNTs, MWCNTs are favoured due to their easy synthesis, whereas SWCNTs possess high repeatability. Nevertheless, for a wide range of gas sensing, higher selectivity, higher response and good flexibility, various nanostructures such as chalcogenides, metallic nanoparticles, metal oxides and polymers are incorporated in these CNMs. These composite-based smart sensors are highly active towards several harmful and toxic gases, which are greatly adopted in the research field for industrial applications and summarized herein. The future of CNMs-based hybrid sensors is undoubtedly very bright given that these sensors outperform the commercialized MOS sensors because the commercialized sensors are bulky and have high operating temperatures due to the chemisorption of analytes molecules. This new class of sensors can show superior sensitivity and selectivity, which can be used in a plethora of applications.
Conflicts of interest
The author assures that this manuscript has not been submitted elsewhere for publication and all authors have been informed. We declare no conflict of interest.Acknowledgements
Authors are highly grateful to Director, CSIR-NPL for his kind permission to write a review article and publish the same. The author would like to acknowledge the Department of Science and Technology for SRF fellowship.References
- H. Li, J. Zhang, G. Li, F. Tan, R. Liu, R. Li, T. Zhang, H. Jin and Q. Li, Carbon, 2014, 66, 369–376 CrossRef CAS.
- L. Sacco, S. Forel, I. Florea and C.-S. Cojocaru, Carbon, 2020, 157, 631–639 CrossRef CAS.
- Z. Hou, J. Wu, W. Zhou, X. Wei, D. Xu, Y. Zhang and B. Cai, IEEE Trans. Electron Devices, 2007, 54, 1545–1548 CAS.
- R. Malik, V. K. Tomer, Y. K. Mishra and L. Lin, Appl. Phys. Rev., 2020, 7, 021301 CAS.
- C. Wang, C. Feng, M. Wang, X. Li, P. Cheng, H. Zhang, Y. Sun, P. Sun and G. Lu, RSC Adv., 2015, 5, 29698–29703 RSC.
- A. Maity and S. Majumder, Sens. Actuators, B, 2015, 206, 423–429 CrossRef CAS.
- R. Kumar, X. Liu, J. Zhang and M. Kumar, Nano-Micro Lett., 2020, 12, 1–37 CrossRef PubMed.
- P.-G. Su and J.-H. Yu, Sens. Actuators, A, 2020, 303, 111718 CrossRef CAS.
- S. W. Lee, W. Lee, Y. Hong, G. Lee and D. S. Yoon, Sens. Actuators, B, 2018, 255, 1788–1804 CrossRef CAS.
- K. Xu, C. Fu, Z. Gao, F. Wei, Y. Ying, C. Xu and G. Fu, Instrum. Sci. Technol., 2018, 46, 115–145 CrossRef CAS.
- Y. Wang and J. T. Yeow, J. Sens., 2009, 2009, 1–24 CrossRef.
- K. Toda, R. Furue and S. Hayami, Anal. Chim. Acta, 2015, 878, 43–53 CrossRef CAS PubMed.
- K. M. Tripathi, T. Kim, D. Losic and T. T. Tung, Carbon, 2016, 110, 97–129 CrossRef CAS.
- J. E. Ellis and A. Star, ChemPlusChem, 2016, 81, 1248 CrossRef CAS PubMed.
- T. T. Tung, M. T. Tran, J.-F. Feller, M. Castro, T. Van Ngo, K. Hassan, M. J. Nine and D. Losic, Carbon, 2020, 159, 333–344 CrossRef CAS.
- T. Han, A. Nag, S. C. Mukhopadhyay and Y. Xu, Sens. Actuators, A, 2019, 291, 107–143 CrossRef CAS.
- S. Z. N. Demon, A. I. Kamisan, N. Abdullah, S. A. M. Noor, O. K. Khim, N. A. M. Kasim, M. Z. A. Yahya, N. A. A. Manaf, A. F. M. Azmi and N. A. Halim, Sens. Mater., 2020, 32, 759–777 CAS.
- U. Yaqoob, D.-T. Phan, A. I. Uddin and G.-S. Chung, Sens. Actuators, B, 2015, 221, 760–768 CrossRef CAS.
- J. Wu, Z. Wu, H. Ding, Y. Wei, W. Huang, X. Yang, Z. Li, L. Qiu and X. Wang, Sens. Actuators, B, 2020, 305, 127445 CrossRef CAS.
- E. Singh, M. Meyyappan and H. S. Nalwa, ACS Appl. Mater. Interfaces, 2017, 9, 34544–34586 CrossRef CAS PubMed.
- M. Sireesha, V. Jagadeesh Babu, A. S. Kranthi Kiran and S. Ramakrishna, Nanocomposites, 2018, 4, 36–57 CrossRef CAS.
- T. Seesaard, T. Kerdcharoen and C. Wongchoosuk, in Semiconductor Gas Sensors, Elsevier, 2020, pp. 185–222 Search PubMed.
- S. Kumar, V. Pavelyev, N. Tripathi, V. Platonov, P. Sharma, R. Ahmad, P. Mishra and A. Khosla, J. Electrochem. Soc., 2020, 167, 047506 CrossRef CAS.
- W. Tian, X. Liu and W. Yu, Appl. Sci., 2018, 8, 1118 CrossRef.
- H. Cruz-Martínez, H. Rojas-Chávez, F. Montejo-Alvaro, Y. A. Peña-Castañeda, P. T. Matadamas-Ortiz and D. I. Medina, Sensors, 2021, 21, 1992 CrossRef PubMed.
- X. Vilanova, Sensors, 2020, 20(5), 1373 CrossRef PubMed.
- E. Llobet, Sens. Actuators, B, 2013, 179, 32–45 CrossRef CAS.
- S. Mao, G. Lu and J. Chen, J. Mater. Chem. A, 2014, 2, 5573–5579 RSC.
- R. B. Mathur, B. P. Singh and S. Pande, Carbon nanomaterials: synthesis, structure, properties and applications, CRC Press, 2016 Search PubMed.
- T. Lin, X. Lv, S. Li and Q. Wang, Sensors, 2017, 17, 2779 CrossRef PubMed.
- B. Mehrdad-Vahdati, S. Pourhashem, M. Sedghi, Z. Vaezi, B. Shojaedin-Givi, A. Rashidi and H. Naderi-Manesh, Toxicol. in Vitro, 2019, 61, 104649 CrossRef CAS PubMed.
- M. Li, T. Chen, J. J. Gooding and J. Liu, ACS Sens., 2019, 4, 1732–1748 CrossRef CAS PubMed.
- W. Li, L.-S. Zhang, Q. Wang, Y. Yu, Z. Chen, C.-Y. Cao and W.-G. Song, J. Mater. Chem., 2012, 22, 15342–15347 RSC.
- G. Deokar, P. Vancsó, R. Arenal, F. Ravaux, J. Casanova-Cháfer, E. Llobet, A. Makarova, D. Vyalikh, C. Struzzi and P. Lambin, Adv. Mater. Interfaces, 2017, 4, 1700801 CrossRef.
- N. Zheng, S. Yang, H. Xu, Z. Lan, Z. Wang and H. Gu, Vacuum, 2020, 171, 109011 CrossRef CAS.
- J. Wu, K. Tao, J. Miao and L. K. Norford, IEEE MEMS, 2018, 901–904 CAS.
- J. Wu, Z. Wu, H. Ding, Y. Wei, W. Huang, X. Yang, Z. Li, L. Qiu and X. Wang, ACS Appl. Mater. Interfaces, 2020, 12, 2634–2643 CrossRef CAS PubMed.
- A. Zöpfl, M.-M. Lemberger, M. König, G. Ruhl, F.-M. Matysik and T. Hirsch, Faraday Discuss., 2014, 173, 403–414 RSC.
- C. Lu and H. Chiu, Chem. Eng. J., 2008, 139, 462–468 CrossRef CAS.
- M. E. Birch, T. A. Ruda-Eberenz, M. Chai, R. Andrews and R. L. Hatfield, Ann. Occup. Hyg., 2013, 57, 1148–1166 CAS.
- U. Kumar and B. C. Yadav, J. Taiwan Inst. Chem. Eng., 2019, 96, 652–663 CrossRef CAS.
- S. Dash and A. Patnaik, Microw. Opt. Technol. Lett., 2018, 60, 1183–1187 CrossRef.
- J. S. Im, S. C. Kang, S.-H. Lee and Y.-S. Lee, Carbon, 2010, 48, 2573–2581 CrossRef CAS.
- U. K. Sur, Int. J. Electrochem., 2012, 2012, 237689 Search PubMed.
- M. Kooti, S. Keshtkar, M. Askarieh and A. Rashidi, Sens. Actuators, B, 2019, 281, 96–106 CrossRef CAS.
- M. G. Stanford, K. Yang, Y. Chyan, C. Kittrell and J. M. Tour, ACS Nano, 2019, 13, 3474–3482 CrossRef CAS PubMed.
- S. Zhang, H. Wang, J. Liu and C. Bao, Mater. Lett., 2020, 261, 127098 CrossRef CAS.
- F. Khurshid, M. Jeyavelan, T. Hussain, M. S. L. Hudson and S. Nagarajan, Mater. Chem. Phys., 2020, 242, 122485 CrossRef CAS.
- A. Mikhraliieva, V. Zaitsev, O. Tkachenko, M. Nazarkovsky and E. V. Benvenutti, 2020, arXiv preprint arXiv:2006.05634.
- N. H. Ha, D. D. Thinh, N. T. Huong, N. H. Phuong, P. D. Thach and H. S. Hong, Appl. Surf. Sci., 2018, 434, 1048–1054 CrossRef.
- D. Liu, C. Fu, N. Zhang, H. Zhou and Y. Kuang, Electrochim. Acta, 2016, 213, 291–297 CrossRef CAS.
- M. Yang, X. Zhang, X. Cheng, Y. Xu, S. Gao, H. Zhao and L. Huo, ACS Appl. Mater. Interfaces, 2017, 9, 26293–26303 CrossRef CAS PubMed.
- J. Huang, X. Yang, S.-C. Her and Y.-M. Liang, Sensors, 2019, 19, 317 CrossRef PubMed.
- J.-H. Cha, S.-J. Choi, S. Yu and I.-D. Kim, J. Mater. Chem. A, 2017, 5, 8725–8732 RSC.
- S. Fasbender, L. Zimmermann, R.-P. Cadeddu, M. Luysberg, B. Moll, C. Janiak, T. Heinzel and R. Haas, Sci. Rep., 2019, 9, 1–13 CAS.
- S. Kumar, M. Mittal, I. Kaur, K. Dharamvir, B. D. Pant and L. M. Bharadwaj, Eur. Phys. J.: Appl. Phys., 2013, 64, 20401 CrossRef.
- N. Janudin, N. Abdullah, W. M. Z. Wan Yunus, F. M. Yasin, M. H. Yaacob, N. Mohamad Saidi and N. A. Mohd Kasim, J. Nanotechnol., 2018, 2018, 2107898 Search PubMed.
- P. Slobodian, P. Riha, A. Lengalova, P. Svoboda and P. Saha, Carbon, 2011, 49, 2499–2507 CrossRef CAS.
- C. Cao, C. Hu, L. Fang, S. Wang, Y. Tian and C. Pan, J. Nanomater., 2011, 2011 Search PubMed.
- K. Jirakittidul, N. Vittayakorn, R. Manrean, N. Pornteeranawapat and S. Neamyooyong, Mater. Res. Express, 2019, 6, 115003 CrossRef CAS.
- M. Zhao, L. Yan, X. Zhang, L. Xu, Z. Song, P. Chen, F. Dong and W. Chu, J. Mater. Chem. C, 2017, 5, 1113–1120 RSC.
- M. Chen, L. Zou, Z. Zhang, J. Shen, D. Li, Q. Zong, G. Gao, G. Wu and Z. Zhang, Carbon, 2018, 130, 281–287 CrossRef CAS.
- A. N. Naje, R. R. Ibraheem and F. T. Ibrahim, Photonic Sens., 2016, 6, 153–157 CrossRef CAS.
- S. Barthwal, B. Singh and N. B. Singh, Mater. Today: Proc., 2018, 5, 15439–15444 CAS.
- Y. J. Kwon, A. Mirzaei, S. Y. Kang, M. S. Choi, J. H. Bang, S. S. Kim and H. W. Kim, Appl. Surf. Sci., 2017, 413, 242–252 CrossRef CAS.
- D. Kumar, P. Chaturvedi, P. Saho, P. Jha, A. Chouksey, M. Lal, J. Rawat, R. Tandon and P. Chaudhury, Sens. Actuators, B, 2017, 240, 1134–1140 CrossRef CAS.
- A. K. Sharma, A. Mahajan, R. Bedi, S. Kumar, A. Debnath and D. Aswal, Appl. Surf. Sci., 2018, 427, 202–209 CrossRef CAS.
- A. K. Sharma, A. Mahajan, R. Saini, R. Bedi, S. Kumar, A. Debnath and D. Aswal, Sens. Actuators, B, 2018, 255, 87–99 CrossRef CAS.
- N. D. Hoang, V. Van Cat, M. H. Nam, V. N. Phan, A. T. Le and N. Van Quy, Sens. Actuators, A, 2019, 295, 696–702 CrossRef CAS.
- N. Donato, M. Latino and G. Neri, Carbon Nanotubes: Res. Appl., 2011, 14, 229–242 Search PubMed.
- P. Dariyal, A. K. Arya, B. Singh and S. Dhakate, J. Mater. Sci., 2020, 1–29 Search PubMed.
- B. Chaitongrat and S. Chaisitsak, Mater. Sci. Forum, 2019, 947, 47–51 Search PubMed.
- U. Kumar and B. Yadav, J. Taiwan Inst. Chem. Eng., 2019, 96, 652–663 CrossRef CAS.
- S. S. Varghese, S. Lonkar, K. Singh, S. Swaminathan and A. Abdala, Sens. Actuators, B, 2015, 218, 160–183 CrossRef CAS.
- J. E. Proctor, D. M. Armada and A. Vijayaraghavan, An introduction to graphene and carbon nanotubes, CRC Press, 2017 Search PubMed.
- Y. Seekaew, D. Phokharatkul, A. Wisitsoraat and C. Wongchoosuk, Appl. Surf. Sci., 2017, 404, 357–363 CrossRef CAS.
- M.-S. Park, K. H. Kim, M.-J. Kim and Y.-S. Lee, Colloids Surf., A, 2016, 490, 104–109 CrossRef CAS.
- S. Srivastava, S. K. Jain, G. Gupta, T. Senguttuvan and B. K. Gupta, RSC Adv., 2020, 10, 1007–1014 RSC.
- X. Gao, Q. Zhou, J. Wang, L. Xu and W. Zeng, Nanomaterials, 2020, 10, 299 CrossRef CAS PubMed.
- J. Ma, M. Zhang, L. Dong, Y. Sun, Y. Su, Z. Xue and Z. Di, AIP Adv., 2019, 9, 075207 CrossRef.
- Y. Zhao, J. Zhang, Y. Wang and Z. Chen, Nanoscale Res. Lett., 2020, 15, 1–8 CrossRef PubMed.
- B. Zhang, G. Liu, M. Cheng, Y. Gao, L. Zhao, S. Li, F. Liu, X. Yan, T. Zhang and P. Sun, Sens. Actuators, B, 2018, 261, 252–263 CrossRef CAS.
- B. Zhang, M. Cheng, G. Liu, Y. Gao, L. Zhao, S. Li, Y. Wang, F. Liu, X. Liang and T. Zhang, Sens. Actuators, B, 2018, 263, 387–399 CrossRef CAS.
- S. Tang, W. Chen, H. Zhang, Z. Song, Y. Li and Y. Wang, Front. Chem., 2020, 8, 174 CrossRef CAS PubMed.
- R. Peng, Y. Li, T. Liu, P. Si, J. Feng, J. Suhr and L. Ci, Mater. Chem. Phys., 2020, 239, 121961 CrossRef CAS.
- B. G. Ghule, N. M. Shinde, S. D. Raut, S. F. Shaikh, A. M. Al-Enizi, K. H. Kim and R. S. Mane, J. Colloid Interface Sci., 2021, 589, 401–410 CrossRef CAS PubMed.
- R. Ghanbari, R. Safaiee, M. H. Sheikhi, M. M. Golshan and Z. K. Horastani, ACS Appl. Mater. Interfaces, 2019, 11, 21795–21806 CrossRef CAS PubMed.
- L. Xue, W. Wang, Y. Guo, G. Liu and P. Wan, Sens. Actuators, B, 2017, 244, 47–53 CrossRef CAS.
- L. Cai and C. Wang, Flexible and Stretchable Medical Devices, 2018, pp. 7–51 Search PubMed.
- N. Ansari, M. Y. Lone, Shumaila, J. Ali, M. Zulfequar, M. Husain, S. Islam and S. Husain, J. Appl. Phys., 2020, 127, 044902 CrossRef CAS.
- H. R. Ludwig, S. G. Cairelli and J. J. Whalen, Documentation for immediately dangerous to life or health concentrations (IDLHS), US Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, Division of Standards Development and Technology Transfer, 1994 Search PubMed.
- I. M. Sharafeldin and N. K. Allam, New J. Chem., 2017, 41, 14936–14944 RSC.
- S. Kumar, V. Pavelyev, P. Mishra and N. Tripathi, Bull. Mater. Sci., 2020, 43, 61 CrossRef CAS.
- T. Han, S. Gao, Z. Wang, T. Fei, S. Liu and T. Zhang, J. Alloys Compd., 2019, 801, 142–150 CrossRef CAS.
- Y. R. Choi, Y.-G. Yoon, K. S. Choi, J. H. Kang, Y.-S. Shim, Y. H. Kim, H. J. Chang, J.-H. Lee, C. R. Park, S. Y. Kim and H. W. Jang, Carbon, 2015, 91, 178–187 CrossRef CAS.
- N. Sharma, R. Vyas, V. Sharma, H. Rahman, S. Sharma and K. Sachdev, Appl. Nanosci., 2020, 10, 517–528 CrossRef CAS.
- J. Park, Y. Kim, S. Y. Park, S. J. Sung, H. W. Jang and C. R. Park, Carbon, 2020, 159, 175–184 CrossRef CAS.
- H. Zhang, J. Feng, T. Fei, S. Liu and T. Zhang, Sens. Actuators, B, 2014, 190, 472–478 CrossRef CAS.
- P. Cao, Y. Cai, D. Pawar, S. Navale, C. N. Rao, S. Han, W. Xu, M. Fang, X. Liu and Y. Zeng, Chem. Eng. J., 2020, 125491 CrossRef CAS.
- J. Liu, S. Li, B. Zhang, Y. Wang, Y. Gao, X. Liang, Y. Wang and G. Lu, J. Colloid Interface Sci., 2017, 504, 206–213 CrossRef CAS PubMed.
- B. Bhangare, N. S. Ramgir, A. Pathak, K. Sinju, A. Debnath, S. Jagtap, N. Suzuki, K. Muthe, C. Terashima and D. Aswal, Mater. Sci. Semicond. Process., 2020, 105, 104726 CrossRef CAS.
- Z. Li, Y. Liu, D. Guo, J. Guo and Y. Su, Sens. Actuators, B, 2018, 271, 306–310 CrossRef CAS.
- Jyoti, N. Kanaujiya and G. D. Varma, AIP Conf. Proc., 2018, 1953, 030039 CrossRef.
- R. Purbia, Y. M. Kwon, H.-D. Kim, Y. S. Lee, H. Shin and J. M. Baik, J. Mater. Chem. A, 2020, 8, 11734–11742 RSC.
- B. Liu, X. Liu, Z. Yuan, Y. Jiang, Y. Su, J. Ma and H. Tai, Sens. Actuators, B, 2019, 295, 86–92 CrossRef CAS.
- E. Dilonardo, M. Penza, M. Alvisi, R. Rossi, G. Cassano, C. Di Franco, F. Palmisano, L. Torsi and N. Cioffi, Beilstein J. Nanotechnol., 2017, 8, 592–603 CrossRef CAS PubMed.
- C. Hua, Y. Shang, Y. Wang, J. Xu, Y. Zhang, X. Li and A. Cao, Appl. Surf. Sci., 2017, 405, 405–411 CrossRef CAS.
- P. B. Agarwal, B. Alam, D. S. Sharma, S. Sharma, S. Mandal and A. Agarwal, Flexible Printed Electron., 2018, 3, 035001 CrossRef.
- N. Yi, Z. Cheng, H. Li, L. Yang, J. Zhu, X. Zheng, Y. Chen, Z. Liu, H. Zhu and H. Cheng, Mater. Today Phys., 2020, 15, 100265 CrossRef.
- C. Yu, Q. Liu, Z. He, X. Gao, E. Wu, J. Guo, C. Zhou and Z. Feng, J. Semicond., 2020, 41, 032101 CrossRef CAS.
- Z. Bo, X. Wei, X. Guo, H. Yang, S. Mao, J. Yan and K. Cen, Chem. Phys. Lett., 2020, 137485 CrossRef CAS.
- H. S. Hong, N. H. Phuong, N. T. Huong, N. H. Nam and N. T. Hue, Appl. Surf. Sci., 2019, 492, 449–454 CrossRef CAS.
- G. Murali, M. Reddeppa, C. Seshendra Reddy, S. Park, T. Chandrakalavathi and M.-D. Kim, ACS Appl. Mater. Interfaces, 2020, 12, 13428–13436 CrossRef CAS PubMed.
- Y.-h. Gui, H.-y. Wang, K. Tian, H.-s. Guo, H.-z. Zhang, S.-m. Fang and Y. Wang, Ceram. Int., 2018, 44, 4900–4907 CrossRef CAS.
- F. Niu, Z.-W. Shao, H. Gao, L.-M. Tao and Y. Ding, Sens. Actuators, B, 2021, 328, 129005 CrossRef CAS.
- Z. Ahmad, S. Manzoor, M. Talib, S. Islam and P. Mishra, Mater. Sci. Eng., B, 2020, 255, 114528 CrossRef CAS.
- N. Roy, R. Sinha, T. T. Daniel, H. B. Nemade and T. K. Mandal, IEEE Sens. J., 2020, 13245–13252 CAS.
- K. K. Paulla and A. A. Farajian, J. Phys. Chem. C, 2013, 117, 12815–12825 CrossRef CAS.
- M. Noei, Vacuum, 2016, 131, 194–200 CrossRef CAS.
- A. Hosseingholipourasl, S. Hafizah Syed Ariffin, M. T. Ahmadi, S. S. Rahimian Koloor, M. Petrů and A. Hamzah, Sensors, 2020, 20, 357 CrossRef CAS PubMed.
- P. Karthik, P. Gowthaman, M. Venkatachalam and A. Rajamanickam, J. Mater. Sci.: Mater. Electron., 2020, 31, 3695–3705 CrossRef CAS.
- N. Osouleddini and S. F. Rastegar, J. Electron Spectrosc. Relat. Phenom., 2019, 232, 105–110 CrossRef CAS.
- N. Tit, K. Said, N. M. Mahmoud, S. Kouser and Z. H. Yamani, Appl. Surf. Sci., 2017, 394, 219–230 CrossRef CAS.
- Z. Zheng and H. Wang, Chem. Phys. Lett., 2019, 721, 33–37 CrossRef CAS.
- S. Impeng, A. Junkaew, P. Maitarad, N. Kungwan, D. Zhang, L. Shi and S. Namuangruk, Appl. Surf. Sci., 2019, 473, 820–827 CrossRef CAS.
- D. Cortés-Arriagada, N. Villegas-Escobar and D. E. Ortega, Appl. Surf. Sci., 2018, 427, 227–236 CrossRef.
- X. Fan, K. Elgammal, A. D. Smith, M. Östling, A. Delin, M. C. Lemme and F. Niklaus, Carbon, 2018, 127, 576–587 CrossRef CAS.
- E. Salih and A. I. Ayesh, Phys. E, 2020, 125, 114418 CrossRef.
- F. Özütok, I. K. Er, S. Acar and S. Demiri, J. Mater. Sci.: Mater. Electron., 2019, 30, 259–265 CrossRef.
- A. Roy, A. Ray, P. Sadhukhan, K. Naskar, G. Lal, R. Bhar, C. Sinha and S. Das, Synth. Met., 2018, 245, 182–189 CrossRef CAS.
- N. John and K. Abraham, Sens. Actuators, B, 2020, 325, 128749 CrossRef CAS.
- A. Debataraja, N. L. W. Septiani, B. Yuliarto, B. Sunendar and H. Abdullah, Ionics, 2019, 25, 4459–4468 CrossRef CAS.
- J. H. Butler and S. A. Montzka, NOAA Earth System Research Laboratory, 2016, vol. 58 Search PubMed.
- E. Dlugokencky, NOAA/ESRL, available at: www.esrl.noaa.gov/gmd/ccgg/trends_ch4/, accessed November, 12, 2018 Search PubMed.
- J. Kathirvelan and R. Vijayaraghavan, Bull. Mater. Sci., 2015, 38, 909–913 CrossRef CAS.
- M. T. Humayun, R. Divan, L. Stan, D. Rosenmann, D. Gosztola, L. Gundel, P. A. Solomon and I. Paprotny, IEEE Sens. J., 2016, 16, 8692–8699 CAS.
- X. Chen, Z. Huang, J. Li, C. Wu, Z. Wang and Y. Cui, Vacuum, 2018, 154, 120–128 CrossRef CAS.
- C. Wu, J. Li, Z. Guo, T. Zhang, X. Chen and M. Jiao, J. Electrochem. Soc., 2020, 167, 145501 CrossRef CAS.
- S. Navazani, M. Hassanisadi, M. Eskandari and Z. Talaei, Synth. Met., 2020, 260, 116267 CrossRef CAS.
- S. Nikmanesh, R. Safaiee and M. H. Sheikhi, Mater. Chem. Phys., 2021, 257, 123808 CrossRef CAS.
- Z. Wang, T. Zhang, T. Han, T. Fei, S. Liu and G. Lu, Sens. Actuators, B, 2018, 266, 812–822 CrossRef.
- J. Tawale, A. Kumar, S. Dhakate and A. Srivastava, Mater. Chem. Phys., 2017, 201, 372–383 CrossRef CAS.
- G. Chimowa, Z. P. Tshabalala, A. A. Akande, G. Bepete, B. Mwakikunga, S. S. Ray and E. M. Benecha, Sens. Actuators, B, 2017, 247, 11–18 CrossRef CAS.
- D. Acharyya and P. Bhattacharyya, IEEE Electron Device Lett., 2016, 37, 656–659 CAS.
- S. Navazani, A. Shokuhfar, M. Hassanisadi, M. Askarieh, A. Di Carlo and A. Agresti, Talanta, 2018, 181, 422–430 CrossRef CAS PubMed.
- H. Roshan, M. H. Sheikhi, M. K. F. Haghighi and P. Padidar, IEEE Sens. J., 2020, 20, 2526–2532 CAS.
- M. J. Bezdek, S.-X. L. Luo, K. H. Ku and T. M. Swager, Proc. Natl. Acad. Sci. U. S. A., 2021, 118 Search PubMed.
- V. Babrauskas, J. Fire Sci., 2017, 35, 396–414 CrossRef CAS.
- D. V. Truong, B. T. Linh, N. M. Kien, L. T. L. Anh, N. C. Tu, N. D. Chien and N. H. Lam, Mater. Trans., 2020, 61, 1540–1543 CrossRef CAS.
- V. Le, T. Duong, L. T. Luu, T. Pham, L. Nguyen and T. Nguyen, J. Met., Mater. Miner., 2019, 29, 61–68 Search PubMed.
- T. Wu, D. Lv, W. Shen, W. Song and R. Tan, Sens. Actuators, B, 2020, 316, 128198 CrossRef CAS.
- S. Abdulla and B. Pullithadathil, Langmuir, 2020, 36, 11618–11628 CrossRef CAS PubMed.
- N. Ansari, M. Y. Lone, J. Ali, M. Husain and S. Husain, AIP Conf. Proc., 2020, 020033 CrossRef.
- R. Ridhi, S. Gautam, G. Saini, S. Tripathi, J. Rawat and P. Jha, Mater. Today: Proc., 2020, 28, 1759–1763 CAS.
- F. Schütt, V. Postica, R. Adelung and O. Lupan, ACS Appl. Mater. Interfaces, 2017, 9, 23107–23118 CrossRef PubMed.
- T. Guo, T. Zhou, Q. Tan, Q. Guo, F. Lu and J. Xiong, Sensors, 2018, 18, 3542 CrossRef PubMed.
- H.-Y. Du, J. Wang, P.-J. Yao, Y.-W. Hao and X.-G. Li, J. Mater. Sci., 2013, 48, 3597–3604 CrossRef CAS.
- S. Abdulla, T. L. Mathew and B. Pullithadathil, Sens. Actuators, B, 2015, 221, 1523–1534 CrossRef CAS.
- J.-H. Lim, N. Phiboolsirichit, S. Mubeen, M. A. Deshusses, A. Mulchandani and N. V. Myung, Nanotechnology, 2010, 21, 075502 CrossRef PubMed.
- D. Zhang, Z. Wu, P. Li, X. Zong, G. Dong and Y. Zhang, Sens. Actuators, B, 2018, 258, 895–905 CrossRef CAS.
- T. Liang, R. Liu, C. Lei, K. Wang, Z. Li and Y. Li, Micromachines, 2020, 11, 965 CrossRef PubMed.
- M. Jaiswal, R. Kumar, J. Mittal and P. Jha, Sens. Actuators, B, 2020, 310, 127826 CrossRef CAS.
- V. Haridas, A. Sukhananazerin, J. Mary Sneha, B. Pullithadathil and B. Narayanan, Appl. Surf. Sci., 2020, 517, 146158 CrossRef CAS.
- N. Garg, M. Kumar, N. Kumari, A. Deep and A. L. Sharma, ACS Omega, 2020, 5, 27492–27501 CrossRef CAS PubMed.
- X. V. Le, T. L. A. Luu, H. L. Nguyen and C. T. Nguyen, Vacuum, 2019, 168, 108861 CrossRef CAS.
- S. Saxena and A. K. Srivastava, AIP Conf. Proc., 2020, 020032 CrossRef CAS.
- O. Hamouma, N. Kaur, D. Oukil, A. Mahajan and M. M. Chehimi, Synth. Met., 2019, 258, 116223 CrossRef CAS.
- I. Karaduman, E. Er, H. Çelikkan, N. Erk and S. Acar, J. Alloys Compd., 2017, 722, 569–578 CrossRef CAS.
- P. Seifaddini, R. Ghasempour, M. Ramezannezhad and A. Nikfarjam, Mater. Res. Express, 2019, 6, 045054 CrossRef.
- R. Kumar, A. Kumar, R. Singh, R. Kumar, D. Kumar, S. K. Sharma and M. Kumar, Mater. Chem. Phys., 2020, 240, 121922 CrossRef CAS.
- X. Wang, D. Gu, X. Li, S. Lin, S. Zhao, M. N. Rumyantseva and A. M. Gaskov, Sens. Actuators, B, 2019, 282, 290–299 CrossRef CAS.
- Z. Ye, H. Tai, R. Guo, Z. Yuan, C. Liu, Y. Su, Z. Chen and Y. Jiang, Appl. Surf. Sci., 2017, 419, 84–90 CrossRef CAS.
- A. Bisht, S. Chockalingam, O. Panwar, A. Kesarwani, B. Singh and V. Singh, Sci. Adv. Mater., 2015, 7, 1424–1434 CrossRef CAS.
- J. Johnson, Chem. Eng. News, 2020, 98(1), 17 CrossRef.
- S.-Y. Guo, P.-X. Hou, H.-X. Wang, C. Shi, H.-T. Fang and C. Liu, Carbon, 2019, 151, 156–159 CrossRef CAS.
- J. Park, I. R. Rang, K Lee and H. J. Kim, Sensors, 2019, 19(18), 3878 CrossRef CAS PubMed.
- M. Han, J. K. Kim, J. Lee, H. K. An, J. P. Yun, S.-W. Kang and D. Jung, J. Nanosci. Nanotechnol., 2020, 20, 4011–4014 CrossRef CAS PubMed.
- J. H. Kim, J. G. Jeon, R. Ovalle-Robles and T. J. Kang, Int. J. Hydrogen Energy, 2018, 43, 6456–6461 CrossRef CAS.
- C. McConnell, S. N. Kanakaraj, J. Dugre, R. Malik, G. Zhang, M. R. Haase, Y.-Y. Hsieh, Y. Fang, D. Mast and V. Shanov, ACS Omega, 2020, 5, 487–497 CrossRef CAS PubMed.
- M. Xiao, S. Liang, J. Han, D. Zhong, J. Liu, Z. Zhang and L. Peng, ACS Sens., 2018, 3, 749–756 CrossRef CAS PubMed.
- J. K. Kim, J. Lee, S. H. Kong and D. Jung, J. Sens. Technol., 2018, 27, 132–136 Search PubMed.
- S. Dhall, K. Sood and R. Nathawat, Int. J. Hydrogen Energy, 2017, 42, 8392–8398 CrossRef CAS.
- G. Tabares, A. Redondo-Cubero, L. Vazquez, M. Revenga, S. Cortijo-Campos, E. Lorenzo, A. De Andrés, E. Ruiz and J. Pau, Sens. Actuators, A, 2020, 304, 111884 CrossRef CAS.
- D. Kathiravan, B.-R. Huang and A. Saravanan, ACS Appl. Mater. Interfaces, 2017, 9, 12064–12072 CrossRef CAS PubMed.
- D. Zhang, H. Chang and Y. Zhang, J. Mater. Sci.: Mater. Electron., 2017, 28, 1667–1673 CrossRef.
- M. Chen, L. Zou, Z. Zhang, J. Shen, D. Li, Q. Zong, G. Gao, G. Wu, J. Shen and Z. Zhang, Carbon, 2018, 130, 281–287 CrossRef CAS.
- T. Kamal, J. Alloys Compd., 2017, 729, 1058–1063 CrossRef CAS.
- B. Sharma and J.-S. Kim, Int. J. Hydrogen Energy, 2018, 43, 11397–11402 CrossRef CAS.
- X. Tang, P.-A. Haddad, N. Mager, X. Geng, N. Reckinger, S. Hermans, M. Debliquy and J.-P. Raskin, Sci. Rep., 2019, 9, 3653 CrossRef PubMed.
- L. S. K. Achary, B. Maji, A. Kumar, S. P. Ghosh, J. P. Kar and P. Dash, Int. J. Hydrogen Energy, 2020, 45, 5073–5085 CrossRef CAS.
- K. Yan, Y. Toku and Y. Ju, Int. J. Hydrogen Energy, 2019, 44, 6344–6352 CrossRef CAS.
- M. Han, J. K. Kim, S.-W. Kang and D. Jung, Appl. Surf. Sci., 2019, 481, 597–603 CrossRef CAS.
- M. Baro and S. Ramaprabhu, Int. J. Hydrogen Energy, 2018, 43, 16421–16429 CrossRef.
- Y. Kim, Y. S. Choi, S. Y. Park, T. Kim, S.-P. Hong, T. H. Lee, C. W. Moon, J.-H. Lee, D. Lee and B. H. Hong, Nanoscale, 2019, 11, 2966–2973 RSC.
- X. Lu, X. Song, C. Gu, H. Ren, Y. Sun and J. Huang, J. Phys. Chem. Solids, 2018, 116, 324–330 CrossRef CAS.
- Q. A. Drmosh, Z. H. Yamani, A. H. Hendi, M. A. Gondal, R. A. Moqbel, T. A. Saleh and M. Y. Khan, Appl. Surf. Sci., 2019, 464, 616 CrossRef CAS.
- D. Zhang, N. Yin, C. Jiang and B. Xia, J. Mater. Sci.: Mater. Electron., 2017, 28, 2763–2768 CrossRef CAS.
- R. Srivastava, H. Suman, S. Shrivastava and A. Srivastava, Chem. Phys. Lett., 2019, 731, 136575 CrossRef CAS.
- S. Bagherzadeh-Nobari, K. H. Istadeh and R. Kalantarinejad, Phys. E, 2020, 115, 113691 CrossRef CAS.
- I. Ibrahim, A. Khalid and M. A. Wahid, J. Phys.: Conf. Ser., 2018, 1032 Search PubMed.
- E. Salih and A. I. Ayesh, Mater. Today Commun., 2020, 101823 Search PubMed.
- Z. Song, Z. Wei, B. Wang, Z. Luo, S. Xu, W. Zhang, H. Yu, M. Li, Z. Huang and J. Zang, Chem. Mater., 2016, 28, 1205–1212 CrossRef CAS.
- Z. Khodadadi, Phys. E, 2018, 99, 261–268 CrossRef CAS.
- A. Reshak and S. Auluck, J. Appl. Phys., 2014, 116, 103702 CrossRef.
- L. Liu, M. Yang, S. Gao, X. Zhang, X. Cheng, Y. Xu, H. Zhao, L. Huo and Z. Major, ACS Appl. Nano Mater., 2019, 2, 5409–5419 CrossRef CAS.
- P. S. Shewale and K.-S. Yun, J. Alloys Compd., 2020, 155527 CrossRef CAS.
- A. Shanmugasundaram, N. D. Chinh, Y.-J. Jeong, T. F. Hou, D.-S. Kim, D. Kim, Y.-B. Kim and D.-W. Lee, J. Mater. Chem. A, 2019, 7, 9263–9278 RSC.
- J. Fan, P. Liu, X. Chen, H. Zhou, S. Fu and W. Wu, Nanotechnology, 2019, 30, 475501 CrossRef CAS PubMed.
- L. Yin, H. Wang, L. Li, H. Li, D. Chen and R. Zhang, Appl. Surf. Sci., 2019, 476, 107–114 CrossRef CAS.
- Y. Zhou, Y. Wang and Y. Guo, Mater. Lett., 2019, 254, 336–339 CrossRef CAS.
- X. X. Zhang, M. S. Pi, H. Cui, C. D. Chen, Z. G. Zhang and J. Tang, Front. Chem., 2019, 7, 476 CrossRef PubMed.
- S. Shao, X. Chen, Y. Chen, L. Zhang, H. W. Kim and S. S. Kim, ACS Appl. Nano Mater., 2020, 5220–5230 CrossRef CAS.
- N. Van Hoang, C. M. Hung, N. D. Hoa, N. Van Duy, I. Park and N. Van Hieu, Sens. Actuators, B, 2019, 282, 876–884 CrossRef CAS.
- J. Chu, X. Wang, D. Wang, A. Yang, P. Lv, Y. Wu, M. Rong and L. Gao, Carbon, 2018, 135, 95–103 CrossRef CAS.
- A. Rydosz, Sensors, 2018, 18, 2298 CrossRef PubMed.
- R. Guo, H. Wang, R. Tian, D. Shi, H. Li, Y. Li and H. Liu, Ceram. Int., 2020, 46, 7065–7073 CrossRef CAS.
- S. Young and Z. Lin, Microsyst. Technol., 2018, 24, 55–58 CrossRef CAS.
- B. Chaitongrat and S. Chaisitsak, J. Nanomater., 2018, 1–11 Search PubMed.
- X. Jia, C. Cheng, S. Yu, J. Yang, Y. Li and H. Song, Sens. Actuators, B, 2019, 300, 127012 CrossRef CAS.
- N. Kohli, A. Hastir, M. Kumari and R. C. Singh, Sens. Actuators, A, 2020, 314, 112240 CrossRef CAS.
- M. Yoosefian, H. Raissi and A. Mola, Sens. Actuators, B, 2015, 212, 55–62 CrossRef CAS.
- X. Zhou, C. Zhao, C. Chen, J. Chen and Y. Li, Appl. Surf. Sci., 2020, 146595 CrossRef CAS.
- J. Wang, M. Tanaka and M. Okochi, IEEE Sens. J., 2020, 1–3 Search PubMed.
- M. Dai, L. Zhao, H. Gao, P. Sun, F. Liu, S. Zhang, K. Shimanoe, N. Yamazoe and G. Lu, ACS Appl. Mater. Interfaces, 2017, 9, 8919–8928 CrossRef CAS PubMed.
- H. Albaris and G. Karuppasamy, Appl. Nanosci., 2019, 9, 1719–1729 CrossRef CAS.
- M. S. B. Reddy, S. Kailasa, B. G. Rani, P. Munindra, K. Bikshalu and K. V. Rao, SN Appl. Sci., 2020, 2, 1–13 Search PubMed.
- N. L. W. Septiani, B. Yuliarto and H. K. Dipojono, Appl. Phys. A: Mater. Sci. Process., 2017, 123, 166 CrossRef.
- Z. Yuan, J. Zhao, F. Meng, W. Qin, Y. Chen, M. Yang, M. Ibrahim and Y. Zhao, J. Alloys Compd., 2019, 793, 24–30 CrossRef CAS.
- R. Peng, Y. Li, J. Chen, P. Si, J. Feng, L. Zhang and L. Ci, Sens. Actuators, A, 2018, 283, 128–133 CrossRef CAS.
- M. Hassan, Z.-H. Wang, W.-R. Huang, M.-Q. Li, J.-W. Liu and J.-F. Chen, Sensors, 2017, 17, 2245 CrossRef PubMed.
- V. Kumar, K. Vikrant and K.-H. Kim, TrAC, Trends Anal. Chem., 2019, 121, 115694 CrossRef CAS.
- Z. Bo, M. Yuan, S. Mao, X. Chen, J. Yan and K. Cen, Sens. Actuators, B, 2018, 256, 1011–1020 CrossRef CAS.
- T. Alizadeh and L. H. Soltani, J. Hazard. Mater., 2013, 248–249, 401–406 CrossRef CAS PubMed.
- V. Munusami, K. Arutselvan and S. Vadivel, Diamond Relat. Mater., 2020, 108167 Search PubMed.
- R. Peng, Y. Li, T. Liu, P. Si, J. Feng, J. Suhr and L. Ci, Mater. Chem. Phys., 2020, 239, 121961 CrossRef CAS.
- Q. Wei, J. Sun, P. Song, J. Li, Z. Yang and Q. Wang, Sens. Actuators, B, 2020, 304, 127306 CrossRef CAS.
- Y. Seekaew, A. Wisitsoraat, D. Phokharatkul and C. Wongchoosuk, Sens. Actuators, B, 2019, 279, 69–78 CrossRef CAS.
- D. Wang, L. Tian, H. Li, K. Wan, X. Yu, P. Wang, A. Chen, X. Wang and J. Yang, ACS Appl. Mater. Interfaces, 2019, 11, 12808–12818 CrossRef CAS PubMed.
- M. Taheri, Z. Feizabadi, S. Jafari and N. Mansour, J. Electron. Mater., 2018, 47, 7232–7239 CrossRef CAS.
- S. Goutham, N. Jayarambabu, C. Sandeep, K. K. Sadasivuni, D. S. Kumar and K. V. Rao, Microchim. Acta, 2019, 186, 62 CrossRef PubMed.
- L. Zhou, R. Qian, S. Zhuo, Q. Chen, Z. Wen and G. Li, J. Saudi Chem. Soc., 2020, 364–373 CrossRef CAS.
- Y. Wang, M. Yang, W. Liu, L. Dong, D. Chen and C. Peng, J. Mater. Chem. C, 2019, 7, 9248–9256 RSC.
- S. Dhall, M. Kumar, M. Bhatnagar and B. Mehta, Int. J. Hydrogen Energy, 2018, 43, 17921–17927 CrossRef CAS.
- M. Morsy, I. Yahia, H. Zahran and M. Ibrahim, J. Inorg. Organomet. Polym. Mater., 2019, 29, 416–422 CrossRef CAS.
- F. Cao, C. Li, M. Li, H. Li and B. Yang, Micro Nano Lett., 2018, 13, 779–783 CrossRef CAS.
- A. D. K.-T. Lam, Z.-D. Lin, H.-Y. Lu and S.-J. Young, Microsyst. Technol., 2019, 1–4 Search PubMed.
- T. Zhang, S. Mubeen, B. Yoo, N. V. Myung and M. A. Deshusses, Nanotechnology, 2009, 20, 255501 CrossRef PubMed.
- X. Tang, J.-P. Raskin, N. Kryvutsa, S. Hermans, O. Slobodian, A. N. Nazarov and M. Debliquy, Sens. Actuators, B, 2020, 305, 127423 CrossRef CAS.
- C. Cantalini, L. Valentini, I. Armentano, L. Lozzi, J. M. Kenny and S. Santucci, Sens. Actuators, B, 2003, 95, 195–202 CrossRef CAS.
- S. Prezioso, F. Perrozzi, L. Giancaterini, C. Cantalini, E. Treossi, V. Palermo, M. Nardone, S. Santucci and L. Ottaviano, J. Phys. Chem. C, 2013, 117, 10683–10690 CrossRef CAS.
- X. Liu, S. Cheng, H. Liu, S. Hu, D. Zhang and H. Ning, Sensors, 2012, 12, 9635–9665 CrossRef PubMed.
- J. Leclercq and P. Sveshtarov, Bulg. J. Phys., 2016, 43, 121–147 Search PubMed.
- S. Mao, S. Cui, G. Lu, K. Yu, Z. Wen and J. Chen, J. Mater. Chem., 2012, 22, 11009–11013 RSC.
- N. Vallabani, S. Mittal, R. K. Shukla, A. K. Pandey, S. R. Dhakate, R. Pasricha and A. Dhawan, J. Biomed. Nanotechnol., 2011, 7, 106–107 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2021 |