Self-assembly of a heterogeneous microreactor with carbon dots embedded in Ti-MOF derived ZnIn2S4/TiO2 microcapsules for efficient CO2 photoreduction†
Dongxue
Wu
a,
Qian
Liang
 *a,
Honglin
Si
b,
Xiong
Yan
b,
Hui
Huang
*a,
Honglin
Si
b,
Xiong
Yan
b,
Hui
Huang
 b,
Zhongyu
Li
b,
Zhongyu
Li
 *a and
Zhenhui
Kang
*a and
Zhenhui
Kang
 *bcd
*bcd
aJiangsu Key Laboratory of Advanced Catalytic Materials and Technology, School of Petrochemical Engineering, Changzhou University, Changzhou 213164, China. E-mail: qianliang@cczu.edu.cn; zhongyuli@mail.tsinghua.edu.cn
bInstitute of Functional Nano and Soft Materials Laboratory (FUNSOM), Jiangsu Key Laboratory for Carbon-Based Functional Materials & Devices, Soochow University, Suzhou 215123, China. E-mail: zhkang@suda.edu.cn
cMacao Institute of Materials Science and Engineering, Macau University of Science and Technology, Taipa, 999078, Macau SAR, China. E-mail: zhkang@must.edu.mo
dInstitute of Advanced Materials, Northeast Normal University, Changchun, 130024, China. E-mail: kangzh@nenu.edu.cn
First published on 10th November 2022
Abstract
The assembly of the heterogeneous microreactor is a promising approach for CO2 photoreduction attributed to its abundant microchannel, intimate contact, high exposed surface area, and favorable heat-mass transfer. Herein, we developed a metal–organic framework (MOF) derived in situ transformation strategy to construct a carbon dot (CD)-decorated ZnIn2S4/TiO2 (CDs/ZIS/TiO2) microreactor. Taking advantages of this hierarchical structure, the CDs/ZnIn2S4/TiO2 microreactor exhibits significantly enhanced photocatalytic CO2 reduction activity with a CH4 yield of 14.9 μmol g−1 h−1 and CH4 selectivity of 75.6% in the absence of a sacrificial agent, where the electron consumption rate (Relectron) of 157.6 μmol g−1 h−1 is 1.9 and 18.3 times higher than those of ZIS(60)/TiO2 and bare ZnIn2S4, respectively. The combination of transient photo-induced voltage (TPV), in situ Fourier transform infrared and electron spin resonance (ESR) spectra illustrate the photocatalytic mechanism and the effect of CDs on the electron transfer behavior. This work emphasizes a facile technique for developing a CD-based microreactor to achieve high-efficiency photocatalytic CO2 reduction performance.
1. Introduction
Solar-driven CO2 reduction is an attractive and efficient way to directly convert CO2 into fuels and high value-added products, such as CO, CH4, CH3OH, and C2H4.1–3 Among the C1 products, CH4 as a popular and clean energy source is one of the most important fuels, and so far, enormous efforts have been focused on the construction of semiconductor catalysts to improve the yield and selectivity of CH4.4–7 Ternary chalcogenide ZnIn2S4 with a layered structure can convert CO2 into useful fuels (e.g., CO, H2, CH4) with considerable performance due to their appropriate band gap structure and great visible-light absorption ability.8 Although ZnIn2S4-based catalysts present a high CO2-to-(CO + H2) conversion efficiency, the photocatalytic activity and selectivity of CH4 in their gas products are insufficient.9–11On account of the enhancement of CH4 production, the development of a ZnIn2S4-based heterogeneous microreactor can be considered an effective strategy due to its high surface-to-volume ratio, abundant microchannels, and favorable heat-mass transfer. This heterogeneous microreactor can strengthen the electron trapping ability, and thus requires more electrons and protons to generate CH4. Metal–organic frameworks (MOFs) with fascinating topology, large pore volume, and chemical adjustability provide an excellent platform to fabricate the semiconductors with a microcapsule structure12,13 including metal oxides,14 metal sulfides,15 layered double hydroxides (LDHs) by using ion-exchange or solvothermal method,16 which enables the encapsulation of numerous nanoparticles or nanosheets on the capsules. For instance, Bibi et al. found that thioacetamide (TAA) can decompose MIL-125 to form TiO2/CdS capsules after the post-solvothermal method, which exhibited enhanced photocatalytic activity.17 MOF-derived microcapsules as a crucial part of the microreactor can provide high porosity, large inner space, the enhanced spatial density of active site as well as an unimpeded electron transport channel.
Carbon dots (CDs) have both remarkable light-harvesting and electron-transfer/reservoir abilities, which may act as an important component of the microreactor.18–24 With CDs in a microreactor system, the transportation of photogenerated electrons in the encapsulation system will become faster and more efficient.25 CDs as electron storage containers may capture more electrons from the semiconductor catalyst and regulate the local charge distribution, thus acquiring more electrons to produce CH4.26 Also, CDs may facilitate the water oxidation reaction to provide more protons for CH4 instead of H2.27 It is predictable that rationally designing and assembling of the CD-modified ZnIn2S4 microreactor should be a promising approach to achieve the high activity and selectivity of CO2-to-CH4 conversion. While it is still a big challenge to assemble a productive heterogeneous microreactor by combining active components together through an effective and facile fabrication.
Herein, a carbon dot (CD)-modified ZnIn2S4/TiO2 (CDs/ZIS/TiO2) microreactor with hollow nanocages and a multi-shell structure was obtained by an ingenious one-step reaction strategy, in which the formed TiO2 microcapsule benefited from the corrosion of NH2-MIL-125 caused by thioacetamide (TAA). The as-prepared CDs/ZIS/TiO2 microreactors exhibit excellent CO2 photoreduction with CH4 yield (14.9 μmol g−1 h−1) without a sacrificial agent, which is much higher than that of pure ZnIn2S4. Besides, the CDs/ZIS/TiO2 microreactor presents a highly stable photocatalytic activity after six successive runs. The well-defined architecture with multi-shell structure, high surface area, and large inner space can improve the light absorption ability, shorten the diffusion pathway, and facilitate charge transfer. Importantly, CDs as electron “reservoirs” can effectively capture electrons and inhibit charge recombination. The proposed photocatalytic mechanism and charge transfer process were studied in detail using transient photo-induced voltage (TPV), in situ Fourier transform infrared and electron spin resonance (ESR) spectra.
2. Experimental section
2.1 Synthesis
3. Results and discussion
3.1 Material characterization
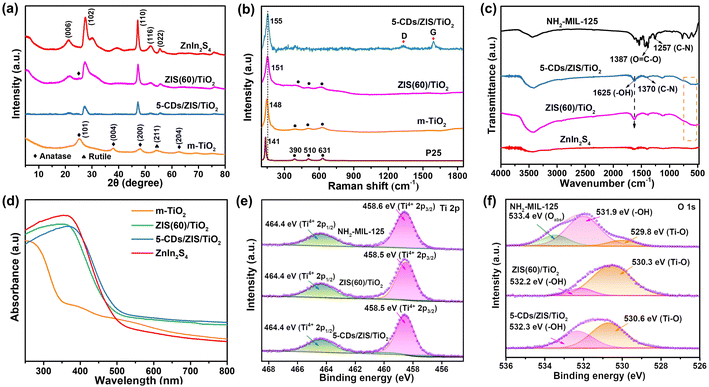
The synthetic procedure of the CDs/ZIS/TiO2 microreactor is schematically presented in Fig. 1a, in which this microreactor was prepared by a one-step in situ self-assembly method. The morphological characteristics of the ZIS/TiO2 microcapsule and CDs/ZIS/TiO2 microreactor were observed from SEM and TEM images. SEM images demonstrated that the pristine ZnIn2S4 presents flower-like microspheres including numerous nanosheets (Fig. S3a†), while NH2-MIL-125 has a uniform pill-like morphology with an average length of 600 nm (Fig. S3b†). When ZIS/TiO2 microcapsule was formed, its morphology was quite different from that of NH2-MIL-125 and ZnIn2S4 (Fig. S3c†). The microcapsule seems to be the expansion of NH2-MIL-125 caused by the effect of TAA and presents a round spindle shape. Besides, a few nanosheets on the surface belonged to the ZnIn2S4 layer. Few defects on the surface of the microcapsule can be seen from the SEM and TEM images of ZIS/TiO2, shown in Fig. S3f,† the hollow cavity can be clearly observed, in which the inner space can be found through the sharp contrast between the rough shell and central void space. Furthermore, due to the growth of the ZnIn2S4 nanosheet on the TiO2 microcapsule, the “double-shell”-like structure can be observed, and the average length of ZIS/TiO2 seems slightly greater than NH2-MIL-125, demonstrating that TAA affects not only the phase but also the shape of NH2-MIL-125 during the self-assembly process, thus the coexistence of ZnIn2S4 and TiO2 in the microcapsule shell. In order to further confirm the formation of TiO2 microcapsule, we investigated the morphology of m-TiO2, where only TAA was added to affect the morphology of NH2-MIL-125, without the Zn/In ions. From the SEM image, as displayed in Fig. S3g,† the as-prepared m-TiO2 still exhibits the microcapsule structure, and the HRTEM image of m-TiO2 showed the interplanar distance of 0.35 nm (Fig. S3h†), due to the (101) crystal plane of anatase TiO2, in agreement with the XRD results. The EDS pattern and elemental mapping (Fig. S3i and S4†) of m-TiO2 showed that the elements of Ti and O existed without other impurities.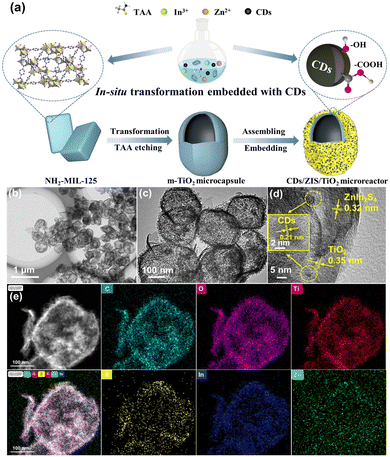 | ||
| Fig. 1 (a) Schematic illustration for the preparation of CDs/ZIS/TiO2 microreactor. (b and c) TEM images, (d) HRTEM image, and (e) EDS mapping of 5-CDs/ZIS/TiO2. | ||
From Fig. 1b, it can be seen that the CDs/ZIS/TiO2 microreactor maintained its microcapsule structure and the morphology of CDs/ZIS/TiO2 is similar to that of ZIS/TiO2, indicating that the microreactor was intact, indicating that CDs coupled with the ZIS/TiO2 microcapsule fabricated a multi-phase microreactor instead of destroying the original shape. From EDS patterns (Fig. S5†), Zn, In, S, Ti, O, and C elements can be observed in this microreactor. As shown in Fig. S2,† the pristine CDs exhibit well-dispersion with an average diameter of 3 nm, and after the addition of CDs in the in situ synthesis, the morphology of CDs/ZIS/TiO2 was evaluated from the HRTEM image (Fig. 1d). The HRTEM image of CDs/ZIS/TiO2 exhibits lattice fringes of 0.21, 0.32 and 0.35 nm that are ascribed to the (100), (102) and (101) crystal facets of CDs, ZnIn2S4 and TiO2, respectively, revealing the successful formation of the CDs/ZIS/TiO2 microreactor.29–31 From the EDX mapping analysis of CDs/ZIS/TiO2 (Fig. 1e), the uniform distribution of C, Zn, In, S, Ti, and O elements throughout the microreactor without agglomeration, further confirmed the formation of hierarchical structure.
The crystal structure information of the ZIS/TiO2 microcapsule and CDs/ZIS/TiO2 microreactor was investigated by XRD. As displayed in Fig. S6,† when only TAA reacted with NH2-MIL-125 without Zn and In source, the XRD patterns of NH-MIL-125 indicated the obvious phase change with the increasing TAA content. When TAA concentration was less than 25%, both the characteristic peaks of TiO2 and NH2-MIL-125 were observed, indicating the coexistence of TiO2 and NH2-MIL-125. When the TAA concentration was more than 35%, only the peaks of TiO2 at 25.2°, 37.9°, 48.0°, and 62.6° belonging to the (101), (004), (200) and (204) lattice planes of anatase phase (JCPDS 21-1272),32 and the peaks at 54.3° and 69.1° ascribed to (221) and (301) lattice planes of the rutile phase (JCPDS 21-1276),33 respectively, were observed, indicating that NH-MIL-125 was completely decomposed to form TiO2 in this case. When the Zn and In sources were added, ZnIn2S4 was formed on the TiO2 microcapsule. It can be seen from Fig. 2a and S7† that the main peaks of the composite belonged to the characteristic peaks of ZnIn2S4 at the (006), (102), (110), (116), and (022) lattice planes, and (101) lattice planes of anatase TiO2 were observed clearly, indicating the successful synthesis of ZIS/TiO2 microcapsules. The broad peaks of pristine CDs emerge at 23°, corresponding to the (002) crystal plane of graphite, which illustrates the amorphous phase (Fig. S1a†).34 As expected, CDs/ZIS/TiO2 exhibited similar diffraction peaks to ZIS/TiO2, and no characteristic peaks indexed to CDs were observed, probably caused by their low content, uniform distribution as well as small particle size, demonstrating that the microreactors were intact after the introduction of CDs through one-step self-assembly method.
To further investigate the structure of the CDs/ZIS/TiO2 microreactor, Raman spectra were collected and are displayed in Fig. 2b. Since ZnIn2S4 presents a weak Raman signal, we tested the Raman spectrum of m-TiO2, in which the peaks of m-TiO2 at 148, 394, 510, and 631 cm−1 are ascribed to the Eg, B1g, A1g, and Eg modes, respectively, confirming the successful synthesis of anatase TiO2.35 Compared with commercial P25, the obvious shift of m-TiO2 at 148 cm−1 is due to the formation of the microcapsule and the size effect of TiO2. For ZIS/TiO2 and CDs/ZIS/TiO2, the further shift at 151 and 155 cm−1 show that the introduction of ZnIn2S4 and CDs can enhance the interaction of the composite catalyst.36 Besides, two signals at 1335 and 1594 cm−1 resulting from the D and G bands of CDs, respectively, were observed, indicating that CDs are embedded in the ZIS/TiO2.37 The FTIR spectra of the ZIS/TiO2 microcapsule and the CDs/ZIS/TiO2 microreactor are displayed in Fig. 2c and S8,† respectively, and the functional groups of the as-prepared samples around 3480 cm−1 belonged to –OH bending vibration.38 The pristine NH2-MIL-125 exhibited the characteristic peaks around 500–800 cm−1 corresponding to the bending vibrations of the Ti–O–Ti group, the band at 1257 cm−1 is ascribed to the C–N group, and the bands in the range of 1350–1600 cm−1 could represent the –COOH group.39 Although bare ZnIn2S4 has no obvious characteristic peaks, after the formation of ZIS/TiO2 and CDs/ZIS/TiO2, the peaks in the range of 500–700 cm−1 and the band at 1625 cm−1 can be clearly observed, which represent the Ti–O group and –OH group, respectively, indicating that NH2-MIL-125 was indeed converted to TiO2 in the microreactor. Due to the small number of CDs, the characteristic peaks belonging to CDs were not observed in the microreactor. The above results verified the coexistence of the anatase TiO2 phase and CDs in the microreactor.
The UV-vis DRS spectra of CDs/ZIS/TiO2 microreactors were collected to investigate their optical absorption ability and are shown in Fig. 2d. It was found that NH2-MIL-125 exhibited good visible-light absorption, attributable to the absorption of NH2-ligand, while the pristine ZnIn2S4 presents an absorption edge around 520 nm. For m-TiO2, the absorption edge was increased compared with P25, probably due to the change in morphology, as shown in Fig. S9.† When the ZIS/TiO2 microcapsule was formed, the absorption edge was slightly decreased in comparison with bare ZnIn2S4, probably due to the weak light absorption of TiO2. After the fabrication of CDs/ZIS/TiO2, the extended absorption edges in the visible-light range can be clearly observed, indicative of the strong absorption of CDs. Since the optical bandgap (Eg) of the photocatalyst is crucial to the determination of the photocatalytic mechanism, Eg values can be obtained from a Tauc plot on the basis of UV-vis DRS spectra (Fig. S10†). Eg values of NH2-MIL-125, ZnIn2S4, and m-TiO2 were calculated to be 2.48, 2.37, and 2.94 eV, respectively.
The specific surface area of the CDs/ZIS/TiO2 microreactor was obtained from N2 adsorption–desorption isotherms (Fig. S11†). The pristine NH2-MIL-125 exhibited a high BET surface area (1006 m2 g−1) and its N2 sorption isotherm belonged to type I, indicating the characteristics of the microporous material.40 In comparison, pure ZnIn2S4 exhibited a type-IV isotherm with an obvious hysteresis loop, and a BET surface area of 138 m2 g−1. After the formation of the microcapsule, some microporous structure emerges due to the TiO2 derived from NH2-MIL-125, and therefore, the BET surface area of ZIS(60)/TiO2 was up to 272 m2 g−1, much higher than that of ZnIn2S4. When CDs were added and the microreactor was constructed, the CDs/ZIS/TiO2 presented the BET surface area of 206 m2 g−1, slightly lower than that of ZIS/TiO2, demonstrating that CDs exist in the microreactor and occupy part of the channel. From the corresponding BJH diagrams (inset), the microcapsule and microreactor exhibit similar pore size distribution, and possessed the coexistence of micropore and mesopore with pore diameters of <20 nm.41 The rich porosity is conducive to offering more active sites and reducing the mass transfer resistance, which contributes to robust CO2 photoreduction.
The elemental composition and electron structure of the CDs/ZIS/TiO2 microreactor were analyzed by XPS spectra. From the XPS survey spectra (Fig. S12a†), the ZIS/TiO2 microcapsule and CDs/ZIS/TiO2 microreactor exhibited the expected presence of Ti, O, C, Zn, In, and S, and the peak intensity of the C element in CDs/ZIS/TiO2 was stronger than that in ZIS/TiO2, indicating that CDs were successfully embedded in ZIS/TiO2. Besides, the N element was observed in NH2-MIL-125 instead of ZIS/TiO2, suggesting that the N element was lost during the self-assembly process. As displayed in Fig. 2e, the Ti 2p peaks with binding energies of 458.6 and 464.4 eV were assigned to Ti 2p3/2 and Ti 2p1/2, respectively, indicative of the presence of Ti4+ in the pristine NH2-MIL-125, corresponding to the previous work.42 For ZIS/TiO2 and CDs/ZIS/TiO2, the peak slightly shifted to the lower binding energy of Ti 2p3/2, probably due to the phase change from the Ti–O cluster to TiO2.43 Notably, O 1s peak is of significance for investigating the surface unsaturated surrounding in Ti and further confirmed the formation of metal oxides (Fig. 2f). In the deconvoluted O 1s spectrum of NH2-MIL-125, the three signals at 529.8, 531.9, and 533.4 eV correspond to the Ti–O bond, –OH bond and adsorbed oxygen (Oabs), respectively.44 However, for ZIS/TiO2 and CDs/ZIS/TiO2, the area of the Ti–O band was significantly enhanced, demonstrating that a large amount of metal oxide was constructed.45 In addition, the C 1s spectra of NH2-MIL-125 presented four deconvoluted signals at 284.8, 285.3, 286.5, and 288.7 eV, which represent the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C–N, C–NH2, and C
C, C–N, C–NH2, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds, respectively, indicative of the presence of the C–N group (Fig. S12b†).46 For ZIS/TiO2 and CDs/ZIS/TiO2, the peaks of the amino group disappeared, and more C–C and C
O bonds, respectively, indicative of the presence of the C–N group (Fig. S12b†).46 For ZIS/TiO2 and CDs/ZIS/TiO2, the peaks of the amino group disappeared, and more C–C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups emerged, indicating the presence of surface functional groups such as hydroxyl and carboxyl around catalysts.47 From the high-resolution spectrum of N 1s (Fig. S12c†), the difference between NH2-MIL-125 and ZIS/TiO2 can be seen clearly. The N 1s spectrum of NH2-MIL-125 was deconvoluted into two signals at 399.2 and 402.6 eV, which belong to the amino group (C–N) and imine group (–NH–), respectively,48 while there were no observable N 1s signals in ZIS/TiO2 and CDs/ZIS/TiO2, suggesting that MOF-topological structure was completely converted into TiO2, successfully. Besides, as for Zn (Fig. S12d†), the binding energies of 1022.3 and 1045.4 eV correspond to Zn 2p3/2 and Zn 2p1/2, respectively, which is typical of Zn2+ in ZIS/TiO2 and CDs/ZIS/TiO2.49 For In 3d XPS spectra (Fig. S12e†), the peaks of ZIS/TiO2 centered at 445.3 and 452.8 eV are due to the In 3d5/2 and In 3d3/2, respectively.49 Compared with ZIS/TiO2, the binding energies of CDs/ZIS/TiO2 for In 3d region exhibited a slight positive shift, which resulted from the changed electron density caused by the effect of CDs. In Fig. S12f,† the binding energies of S 2p in ZIS/TiO2 at 162.0 and 163.3 eV are indicative of the presence of S2−, and also CDs/ZIS/TiO2 exhibited a larger positive shift compared with In 3d orbit, suggesting the effective electron transfer.50
O groups emerged, indicating the presence of surface functional groups such as hydroxyl and carboxyl around catalysts.47 From the high-resolution spectrum of N 1s (Fig. S12c†), the difference between NH2-MIL-125 and ZIS/TiO2 can be seen clearly. The N 1s spectrum of NH2-MIL-125 was deconvoluted into two signals at 399.2 and 402.6 eV, which belong to the amino group (C–N) and imine group (–NH–), respectively,48 while there were no observable N 1s signals in ZIS/TiO2 and CDs/ZIS/TiO2, suggesting that MOF-topological structure was completely converted into TiO2, successfully. Besides, as for Zn (Fig. S12d†), the binding energies of 1022.3 and 1045.4 eV correspond to Zn 2p3/2 and Zn 2p1/2, respectively, which is typical of Zn2+ in ZIS/TiO2 and CDs/ZIS/TiO2.49 For In 3d XPS spectra (Fig. S12e†), the peaks of ZIS/TiO2 centered at 445.3 and 452.8 eV are due to the In 3d5/2 and In 3d3/2, respectively.49 Compared with ZIS/TiO2, the binding energies of CDs/ZIS/TiO2 for In 3d region exhibited a slight positive shift, which resulted from the changed electron density caused by the effect of CDs. In Fig. S12f,† the binding energies of S 2p in ZIS/TiO2 at 162.0 and 163.3 eV are indicative of the presence of S2−, and also CDs/ZIS/TiO2 exhibited a larger positive shift compared with In 3d orbit, suggesting the effective electron transfer.50
3.2 Photocatalytic CO2 reduction
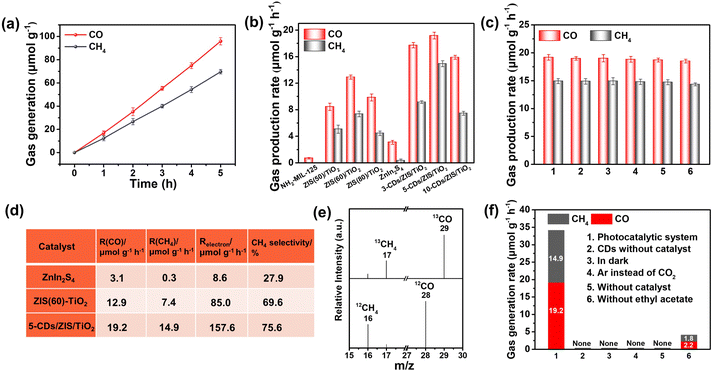
With the CDs/ZIS/TiO2 microreactor as a photocatalyst, the photocatalytic performances for CO2 reduction were conducted in H2O/EA solution without any photosensitizer or sacrificial agent, in which EA could enhance the solubility of CO2 gas. During these experiments, CO and CH4 were detected as main products in the as-prepared catalysts under simulated solar light irradiation, as displayed in Fig. 3. Only a small amount of CO product (0.7 μmol g−1 h−1) over pristine NH2-MIL-125 was detected, while the bare ZnIn2S4 presented low CO (3.1 μmol g−1 h−1) and CH4 (0.3 μmol g−1 h−1) yields. After the formation of the ZIS/TiO2 microcapsule, CO2 photoreduction was remarkably enhanced compared with the precursors. The different weight ratios of ZnIn2S4 and TiO2 were considered (Fig. 3b), and the optimal ZIS(60)/TiO2 exhibited CO and CH4 yields of up to 12.9 and 7.4 μmol g−1 h−1, respectively. Notably, the introduction of CDs on ZIS(60)/TiO2 further boosted the photocatalytic performances, 5-CDs/ZIS/TiO2 showed the maximum CO (19.2 μmol g−1 h−1) and CH4 (14.9 μmol g−1 h−1) yields, in which, the concentration of products revealed a linearly enhancing trend with duration time. Such outstanding activity originated from the CDs/ZIS/TiO2 as a productive heterogeneous microreactor. The yield of CH4 generation over the as-prepared CDs/ZIS/TiO2 is regarded as one of the most competitive performances for ZnIn2S4-based catalysts after an extensive literature search in the field of similar photocatalysts as illustrated in Table S5.†Based on electron consumption rates (Relectron, CH4: 8e−; CO: 2e−), CH4 selectivity was evaluated by the following equation: CH4 selectivity (%) = [8R(CH4)]/[2R(CO) + 8R(CH4)] × 100%, where R(CO) and R(CH4) are the conversion rates of CO and CH4, respectively. As displayed in Fig. 3d and S14,† the 5-CDs/ZIS/TiO2 microreactor exhibited the highest CH4 selectivity (75.6%), and Relectron was up to 157.6 μmol g−1 h−1, which were 1.9 and 18.3 times higher those of ZIS(60)/TiO2 and bare ZnIn2S4, respectively, indicating that e more protons formed in the microreactor can facilitate the CH4 production from CO2 reduction. Importantly, O2 as the main oxidation product was observed, as displayed in Fig. S16,† verifying the overall photocatalytic CO2 reduction. The O2 yield of 5-CDs/ZIS/TiO2 (11.3 μmol h−1 g−1) was higher than that of ZIS(60)/TiO2 (7.1 μmol h−1 g−1), indicating that CDs have a positive effect on H2O oxidation reaction. Additionally, the stoichiometric ratio of the consumed electrons and holes for ZIS(60)/TiO2 and 5-CDs/ZIS/TiO2 was 1.1 and 1.2, respectively, which illustrated that the two values are close to 1. To further evaluate the oxidation capacity of the CDs/ZIS/TiO2 microreactor, oxygen evolution reaction (OER) was revealed by the linear sweep voltammetry (LSV) performances (Fig. S17†). The increased current density of ZIS(60)/TiO2 was observed compared with the pristine ZnIn2S4, indicating that the microcapsule presented an excellent OER activity. Obviously, after the formation of the CDs-modified microreactor, all the CDs/ZIS/TiO2 samples possessed further enhanced current density, meaning that the microreactor with higher OER performance drove the complete CO2 reduction reaction.
The long-term durability of CDs/ZIS/TiO2 was investigated by cycle tests as displayed in Fig. 3c. After six successive cycles over 30 h, a negligible decrease in the photocatalytic performance of 5-CDs/ZIS/TiO2 was detected, with only ca. 3.6% and 4.0% deactivation of CO and CH4 yields, respectively. The 13C labeled isotope experiments confirmed that the generated CO and CH4 indeed originated from catalysts instead of other carbon sources, as illustrated in Fig. 3e. 13CO (m/z = 29) and 13CH4 (m/z = 17) were observed, revealing that the products are from the CO2 photoreduction. Furthermore, the controlled tests were performed to determine the origin of the products (Fig. 3f). Negligible amounts of carbon-containing products were observed when the reaction system was tested without the photocatalyst, absence of light irradiation, and in Ar atmosphere instead of high-purity CO2, demonstrating that the photo-excited process was indispensable for this reaction, and the other carbon impurities existed in the photocatalyst and the reaction system could not afford any CO2 reduction products.
3.3 Mechanism for the CO2 photoreduction
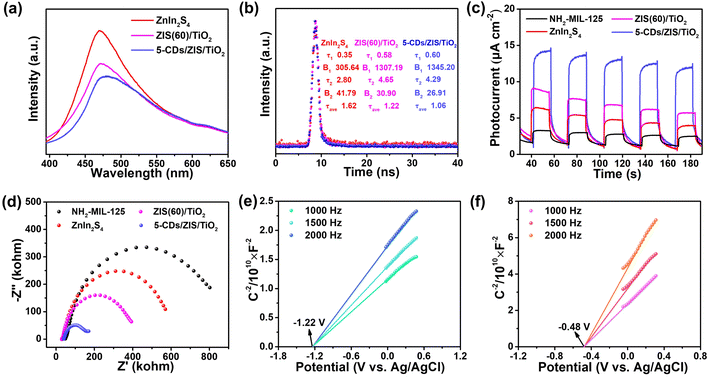
Steady-state and time-resolved PL spectra were used as powerful tools to investigate the electron transfer of the CDs/ZIS/TiO2 microreactor. As displayed in Fig. 4a, the PL intensity of ZIS/TiO2 decreased in comparison with the pure ZnIn2S4, which indicated that the formed hierarchical hollow morphology with a shorter charge transfer distance could reduce the charge recombination. After the introduction of CDs, the interfacial interaction between ZnIn2S4 and TiO2 became stronger, which can remarkably improve the charge transfer efficiency, leading to CDs/ZIS/TiO2 with the lowest PL intensity.51 The average PL lifetimes (τave) of the representative catalysts were further investigated using TR-PL spectra with values of 1.62, 1.22, 1.06 ns for ZnIn2S4, ZIS(60)/TiO2 and 5-CDs/ZIS/TiO2, respectively, as shown in Fig. 4b. The PL lifetime of ZIS(60)/TiO2 presents a shorter τave than ZnIn2S4, attributable to fast electron transfer on the surface of the microcapsule, resulting from intimate contact. The faster PL decay and shorter τave in 5-CDs/ZIS/TiO2 indicate that CDs indeed act as charge mediators in a microreactor.52 The observations of decay in both steady-state and time-resolved PL results demonstrate that microcapsule and multi-phase microreactors can effectively suppress the charge carrier recombination.The electrochemical performances were further studied to analyze the effect of the multi-phase microreactor on the photoinduced charge separation efficiency. The photocurrent of ZIS(60)/TiO2 was much higher than that of ZnIn2S4 and unconverted NH2-MIL-125 under simulated sunlight irradiation (Fig. 4c). Also, 5-CDs/ZIS/TiO2 indicates that the further enhanced current density, which implies that CDs in the microreactor can accelerate the electron transfer rate.34
Corresponding to the photocurrent results, ZIS(60)/TiO2 and 5-CDs/ZIS/TiO2 showed smaller radii in the EIS Nyquist diagram (Fig. 4d), which represented the decreasing charge transfer resistance, facilitating the charge transport and boosting reaction kinetics in photocatalytic performance.53 Furthermore, the flat-band potentials (EFB) of ZnIn2S4 and TiO2 were obtained from Mott–Schottky (M–S) plots at frequencies of 1000, 1500, and 2000 Hz (Fig. 4e and f).54 The plots of ZnIn2S4 and TiO2 display positive slopes, indicative of typical n-type semiconductors. The EFB values of ZnIn2S4 (−1.22 V vs. Ag/AgCl) and m-TiO2 (−0.48 V vs. Ag/AgCl) were determined by extrapolating the M–S plots, and correspondingly, EFB values are −1.02 and −0.28 V (vs. NHE) for ZnIn2S4 and m-TiO2 according to the ENHE = EAg/AgCl + 0.197,55 respectively, where EFB values are close to the conduction band (ECB) potentials of n-type semiconductor.56 Consequently, ECB values of ZnIn2S4 and m-TiO2 are −1.02 and −0.28 V (vs. NHE), respectively, and together with band gaps (Eg) from the Tauc plots, the corresponding valence band values (EVB) of ZnIn2S4 and m-TiO2 are 1.35 V and 2.66 V (vs. NHE), respectively.
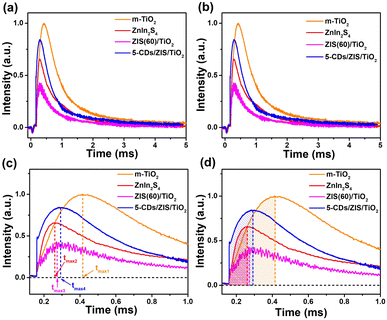
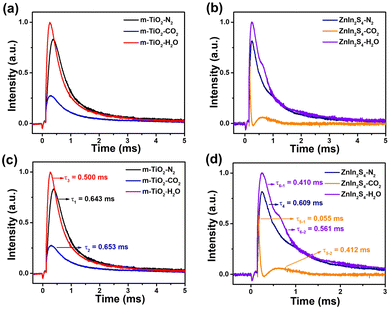
Transient photovoltage (TPV) tests were performed to analyze the charge transfer kinetics on the interfaces of the photocatalysts. The TPV relaxation curves of m-TiO2, ZnIn2S4, ZIS/TiO2, and CDs/ZIS/TiO2 are shown in Fig. 5a. Furthermore, as shown in Fig. 5b, the electron recombination rates existing in the photocatalysts were investigated by the use of the attenuation constants (τ). The τs of m-TiO2 and ZnIn2S4 were 0.682 and 0.609 ms, respectively, while the τ of ZIS/TiO2 was 0.544 ms. The reason for the smaller τ of ZIS/TiO2 is that the formation of the heterojunction between m-TiO2 and ZnIn2S4 causes a part of the charges to recombine before being collected by the working electrode. In addition, the formation of the heterojunction is beneficial for charge transfer. The τ of CDs/ZIS/TiO2 is 0.421 ms, revealing that the addition of CDs further facilitates electron transport. As shown in Fig. 5c, tmax was used to estimate the rate of the charge extraction process. There is a great difference between the tmax of m-TiO2 (tmax1 = 0.256 ms) and ZnIn2S4 (tmax2 = 0.101 ms). However, compared with that of m-TiO2, tmax3 (0.114 ms) becomes much smaller, indicating that the integration of m-TiO2 and ZnIn2S4 promotes the charge extraction process. After the addition of CDs, tmax4 (0.133 ms) is still much smaller than that of m-TiO2, proving that the addition of CDs also accelerates the charge extraction process. Fig. 5d shows the area of the shadow part (A) of m-TiO2, ZnIn2S4, ZIS/TiO2, and CDs/ZIS/TiO2, which correspond to the maximum charge extraction of the catalysts. It is worth noting that the A of m-TiO2 (A1 = 0.192) and ZnIn2S4 (A2 = 0.0511) are larger than that of ZIS/TiO2 (A3 = 0.0341), which is attributed to the recombination of the charge on heterojunction interfaces after being excited by the laser, resulting in the smaller amounts of charges collected by the working electrode. Furthermore, the A of CDs/ZIS/TiO2 (A4 = 0.0934) is larger than that of ZIS/TiO2, demonstrating that CDs can enhance the electron extraction ability of the photocatalyst. The surface effective charge (ne) is used to further determine the three eigenvalues of TPV (τ, tmax, A), which can be calculated from the equation of ne = (A × τ)/tmax. For photocatalysts, the value of ne represents the amount of the charge that is involved in the photocatalytic redox reaction.57 The ne of m-TiO2, ZnIn2S4, ZIS/TiO2, and CDs/ZIS/TiO2 are 0.510, 0.307, 0.162, and 0.295, respectively. Similarly, the ne of ZIS/TiO2 becomes smaller than those of m-TiO2 and ZnIn2S4, which was also caused by the heterojunction formed between the two components. The ne of CDs/ZIS/TiO2 increases by ca. 1.82 times compared with that of ZIS/TiO2, suggesting that the introduction of CDs is beneficial for the photocatalytic reaction. In summary, the heterojunction formed between m-TiO2 and ZnIn2S4 facilitated the charge transfer process. In addition, CDs not only play the role of regulating the charge transfer process but also improve the ability of the photocatalyst to extract electrons for the photocatalytic reaction.
In situ TPV experiments were performed to understand the photocatalytic reaction over the catalysts. Fig. 6 displays the in situ TPV results of m-TiO2 and ZnIn2S4 under an atmosphere of N2-saturated MeCN, CO2-saturated MeCN, and 0.5 vol% H2O/MeCN (v/v), respectively. Compared with m-TiO2, the TPV intensity of ZnIn2S4 exhibits a sharper decrease when the atmosphere changes from N2-saturated MeCN to CO2-saturated MeCN, indicating that ZnIn2S4 provides active sites for the CO2 reduction reaction, which consumes electrons. Similarly, the H2O oxidation reaction consumes holes, which will lead to an increase in TPV intensity. However, the increase of TPV intensity of m-TiO2 and ZnIn2S4 are close when the atmosphere changes from N2-saturated MeCN to 0.5 vol% H2O/MeCN (v/v). Therefore, in order to further analyze the active sites of H2O oxidation, the attenuation constants (τ) of the in situ TPV curves were calculated, as shown in Fig. 6c and d. It is worth noting that, for ZnIn2S4, there exist two attenuation processes both in CO2-saturated and 0.5 vol% H2O/MeCN (v/v). Therefore, after calculating the τ of each attenuation process (τ5-1 = 0.055 ms, τ5-2 = 0.412 ms, τ6-1 = 0.410 ms, τ6-1 = 0.561 ms), the average τs of ZnIn2S4 under CO2-saturated and 0.5 vol% H2O/MeCN (v/v) (τ5avg = 0.190 ms, τ6avg = 0.490 ms) were calculated using the formula (x) provided in the ESI.† The changing percentage of τ (Δτ) was calculated using the following formula (Δτ = (τN2 − τH2O/CO2)/τN2 × 100%) to study the influence of CO2 or H2O on charge recombination process. For m-TiO2, Δτ(H2O) (22.2%) is much higher than Δτ(CO2) (1.56%), which proves that the introduction of H2O makes great effect on its charge recombination process, suggesting that m-TiO2 provides active sites for the H2O oxidation reaction.31,34 Similarly, for ZnIn2S4, Δτ(CO2) (68.8%) is much higher than Δτ(H2O) (19.5%), indicating that ZnIn2S4 provided active sites for the CO2 reduction reaction.34,58
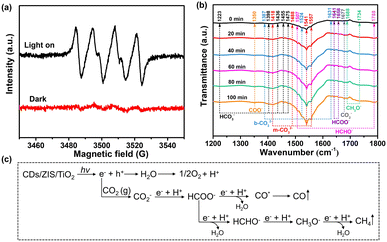
Electron spin resonance (ESR) spectra of CDs/ZIS/TiO2 were used to further ascertain the photocatalytic mechanism as displayed in Fig. 7a. The obvious DMPO-˙O2− signals exhibit that the generated electrons on CDs/ZIS/TiO2 can effectively produce ˙O2− species, which means that the position of the electrons on the CB is more negative than the potential of superoxide radical (O2/˙O2−, −0.33 eV).59 Thus, the above results indicate that the electrons are accumulated on the CB of ZnIn2S4 instead of m-TiO2, which is consistent with the TPV results, both confirming the formation of the Z-scheme mechanism.60 Besides, we conducted in situ FTIR spectroscopy to illustrate the reaction pathway in the CO2 photoreduction over the CDs/ZIS/TiO2 microreactor, as displayed in Fig. 7b. It is seen that some peaks of multiple intermediate products emerge, which gradually become stronger with the extension of the irradiation time. After CO2 and H2O gas were adsorbed on CDs/ZIS/TiO2 in the dark for 30 min, bicarbonate species (HCO3−, 1223, 1398, 1436, 1455 and 1475 cm−1),61 monodentate carbonate species (m-CO32−, 1418, 1488, 1541 and 1557 cm−1) and bidentate carbonate species (b-CO32−, 1387, 1524 and 1631 cm−1) were observed in the reaction process,62,63 revealing that the absorbed CO2 and dissociative H2O molecules exist on the surface of CDs/ZIS/TiO2. Besides, new peaks emerge and the active CO2− peaks at 1677 cm−1 can be observed. The peaks of formaldehyde (HCHO−, 1507 and 1788 cm−1), methoxy groups (CH3O−, 1688 and 1734 cm−1), formic acid species (HCOO−, 1641 and 1658 cm−1), and carboxylate species (COO−, 1350 cm−1) are detected, indicating that they are primary intermediates during CO2 photoreduction.64–66 Besides, there are no CH4 peaks, probably due to its nonpolar as well as low affinity.
According to the in situ FTIR results, HCHO−, CH3O−, HCOO− and COO− groups are the significant intermediates, and coupled with the TPV and ESR results, the formed Z-scheme over CDs/ZIS/TiO2 was deduced, as shown in Fig. S18.† Under sunlight irradiation, the photoinduced electrons in the CB of m-TiO2 recombine with the holes in the VB of ZnIn2S4, and meanwhile, the accumulation of electrons on the CB of ZnIn2S4 and holes on the VB of m-TiO2 possesses strong redox ability for CO2 reduction and O2 oxidation.67 Besides, the CDs in this heterostructure act as an electron conductor and reservoir, in which electrons transported to the surface are captured by CDs, further reducing the charge recombination to promote the redox reaction. As for the formed microreactor, the numerous ultrathin nanosheets as the outer layer are beneficial to CD implantation and CO2 adsorption. Furthermore, the multi-shell structure, high surface area, and large inner space can promote light utilization by multiple reflections, and facilitate the fast diffusion of gaseous products.
4. Conclusions
In summary, we have demonstrated a novel CDs/ZnIn2S4/TiO2 microreactor prepared using a MOF-mediated strategy. During the synthetic process, the thioacetamide (TAA) can facilitate the dissolution of the inner core for NH2-MIL-125 to form TiO2 microcapsules, and with the precipitation of Zn2+/In3+ ions and CDs, a novel productive heterogeneous microreactor with multi-shell structure was obtained. The obtained CDs/ZIS/TiO2 exhibited high photocatalytic performance and selectivity for CO2 photoreduction into CH4 without any sacrificial agent. The yield of CH4 over optimal CDs/ZIS/TiO2 was up to 14.9 μmol g−1 h−1 with CH4 selectivity of 75.6%, and the Relectron reaches 157.6 μmol g−1 h−1. The in situ TPV measurements indicated that m-TiO2 provided active sites for the H2O oxidation reaction, and ZnIn2S4 provided active sites for the CO2 reduction reaction. Coupled with ESR, photoelectrochemical and in situ Fourier transform infrared spectra, a Z-scheme mechanism for CDs/ZIS/TiO2 is proposed. Furthermore, the CDs in this microreactor as an electron acceptor play a significant role in the improvement of charge separation efficiency. This work not only provides an effective strategy to construct MOF-derived productive heterogeneous microreactor for photocatalytic CO2 conversion but is also an inspiration for developing the integrated catalytic system in the field of artificial photosynthesis.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by the National Key Basic Research Program of China (2020YFA0406104/2020YFA0406101), the National MCF Energy R&D Program (2018YFE0306105), the Innovative Research Group Project of the National Natural Science Foundation of China (51821002), National Natural Science Foundation of China (51725204, 51972216, 21876015, 52272043, 52271223, 52202107, 52201269), Natural Science Foundation of Jiangsu Province (BK20220028, BK20190041), Natural Science Foundation of Jiangsu Province-Excellent Youth Foundation (BK20190102), Key-Area Research and Development Program of GuangDong Province (2019B010933001), Collaborative Innovation Center of Suzhou Nano Science & Technology, Qinglan Project Foundation of Jiangsu Province, the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), and the 111 Project.Notes and references
- S. F. Ji, Y. Qu, T. Wang, Y. J. Chen, G. F. Wang, X. Li, J. C. Dong, Q. Y. Chen, W. Y. Zhang, Z. D. Zhang, S. Y. Liang, R. Yu, Y. Wang, D. S. Wang and Y. D. Li, Angew. Chem., Int. Ed., 2020, 59, 10651–10657 CrossRef CAS PubMed
.
- C. L. Wang, Z. X. Sun, Y. Zheng and Y. H. Hu, J. Mater. Chem. A, 2019, 7, 865–887 RSC
.
- I. Shown, S. Samireddi, Y. C. Chang, R. Putikam, P. Chang, A. Sabbah, F. Fu, W. Chen, C. Wu, T. Yu, P. Chung, M. C. Lin, L. Chen and K. Chen, Nat. Commun., 2018, 9, 169 CrossRef PubMed
.
- J. Li, H. L. Huang, W. J. Xue, K. Sun, X. H. Song, C. R. Wu, L. Nie, Y. Li, C. Y. Liu, Y. Pan, H. L. Jiang, D. H. Mei and C. L. Zhong, Nat. Catal., 2021, 4, 719–729 CrossRef CAS
.
- Y. J. Ma, X. X. Yi, S. L. Wang, T. Li, B. Tan, C. C. Chen, T. Majima, E. R. Waclawik, H. Y. Zhu and J. Y. Wang, Nat. Commun., 2022, 13, 1400 CrossRef CAS PubMed
.
- H. Wu, X. Y. Kong, X. M. Wen, S. Chai, E. C. Lovell, J. W. Tang and Y. H. Ng, Angew. Chem., Int. Ed., 2021, 60, 8455–8459 CrossRef CAS PubMed
.
- S. L. Wang, M. Xu, T. Y. Peng, C. X. Zhang, T. Li, I. Hussain, J. Y. Wang and B. Tan, Nat. Commun., 2019, 10, 676 CrossRef CAS PubMed
.
- Y. Zhang, Y. X. Wu, L. Wan, H. J. Ding, H. X. Li, X. Y. Wang and W. H. Zhang, Appl. Catal., B, 2022, 311, 121255 CrossRef CAS
.
- S. B. Wang, B. Y. Guan and X. W. Lou, J. Am. Chem. Soc., 2018, 140, 5037–5040 CrossRef CAS PubMed
.
- K. Zhu, J. O. Yang, Q. Zeng, S. G. Meng, W. Teng, Y. H. Song, S. Tang and Y. J. Cui, Chin. J. Catal., 2020, 41, 454–463 CrossRef CAS
.
- P. She, B. Y. Guan, J. Y. Sheng, Y. Y. Qi, G. Y. Qiao, H. B Rui, G. Y. Lu, J. S. Qin and H. Rao, Catal. Sci. Technol., 2022, 12, 1092–1099 RSC
.
- S. T. Wu, Z. Xin, S. C. Zhao and S. T. Sun, Nano Res., 2019, 12, 2736–2742 CrossRef CAS
.
- T. He, X. B. Xu, B. Ni, H. F. Lin, C. Z. Li, W. P. Hu and X. Wang, Angew. Chem., Int. Ed., 2018, 57, 10148–10152 CrossRef CAS PubMed
.
- Y. Liu, X. F Zhou, Z. R. Jia, H. J. Wu and G. L. Wu, Adv. Funct. Mater., 2022, 32, 2204499 CrossRef CAS
.
- H. T. Fan, Y. J. Jin, K. C. Liu and W. S. Liu, Adv. Sci., 2022, 9, 2104579 CrossRef CAS PubMed
.
- H. J. Xu, C. F. Shan, X. X. Wu, M. Z. Sun, B. Huang, Y. Tang and C. H. Yan, Energy Environ. Sci., 2020, 13, 2949–2956 RSC
.
- R. Bibi, H. L. Huang, M. Kalulu, Q. H. Shen, L. F. Wei, O. Oderinde, N. X. Li and J. C. Zhou, ACS Sustainable Chem. Eng., 2019, 7, 4868–4877 CrossRef CAS
.
- Q. Y. Wu, J. J. Cao, X. Wang, Y. Liu, Y. J. Zhao, H. Wang, Y. Liu, H. Huang, F. Liao, M. W. Shao and Z. H. Kang, Nat. Commun., 2021, 12, 483 CrossRef CAS PubMed
.
- H. Q. Song, J. K. Yu, Z. Y. Tang, B. Yang and S. Y. Lu, Adv. Energy Mater., 2022, 12, 2102573 CrossRef CAS
.
- J. Wu, Y. D. Han, Y. C. Bai, X. T. Wang, Y. J. Zhou, W. X. Zhu, T. W. He, Y. M. Wang, H. Huang, Y. Liu and Z. H. Kang, Adv. Funct. Mater., 2022, 32, 2203647 CrossRef CAS
.
- Y. J. Dong, Q. Han, Q. Y. Hu, C. J. Xu, C. Z. Dong, Y. Peng, Y. Ding and Y. Q. Lan, Appl. Catal., B, 2021, 293, 120214 CrossRef CAS
.
- B. Li, W. Peng, J. Zhang, J. C. Lian, T. Huang, N. Cheng, Z. Y. Luo, W. Q. Huang, W. Y. Hu, A. L. Pan, L. Jiang and G. F. Huang, Adv. Funct. Mater., 2021, 31, 2100816 CrossRef CAS
.
- Y. J. Zhao, L. L. Xu, X. Wang, Z. Z. Wang, Y. Liu, Y. Wang, Q. L. Wang, Z. T. Wang, H. Huang, Y. Liu, W. Y. Wong and Z. H. Kang, Nano Today, 2022, 43, 101428 CrossRef CAS
.
- Y. Liu, X. Wang, Y. J. Zhao, Q. Y. Wu, H. D. Nie, H. L. Si, H. Huang, Y. Liu, M. W. Shao and Z. H. Kang, Nano Res., 2022, 15, 4000–4007 CrossRef CAS
.
- S. S. Mondal, S. R. Das, L. Sahoo, S. Dutta and U. Gautam, J. Am. Chem. Soc., 2022, 144, 2580–2589 CrossRef CAS PubMed
.
- Q. Li, S. C Wang, Z. X. Sun, Q. J. Tang, Y. Q. Liu, L. Z. Wang, H. Q. Wang and Z. B. Wu, Nano Res., 2019, 12, 2749–2759 CrossRef CAS
.
- X. Q. Gu, Z. M. Chen, Y. Li, J. Wu, X. Wang, H. Huang, Y. Liu, B. Dong, M. W. Shao and Z. H. Kang, ACS Appl. Mater. Interfaces, 2021, 13, 24814–24823 CrossRef CAS PubMed
.
- Z. Z. Su, B. X. Zhang, J. B. Shi, D. X. Tan, F. Y. Zhang, L. F. Liu, X. N. Tan, D. Shao, G. Y. Yang and J. L. Zhang, Sustain. Energy Fuels, 2019, 3, 1233–1238 RSC
.
- X. W. Shi, C. Dai, X. Wang, J. Y. Hu, J. Y. Zhang, L. X. Zheng, L. Mao, H. J. Zheng and M. S. Zhu, Nat. Commun., 2022, 13, 1287 CrossRef CAS PubMed
.
- Q. Cheng, Y. J. Yuan, R. Tang, Q. Y. Liu, L. Bao, P. Wang, J. S. Zhong, Z. Y. Zhao, Z. T. Yu and Z. G. Zou, ACS Catal., 2022, 12, 2118–2125 CrossRef CAS
.
- Q. Liang, X. T. Yan, Z. Y. Li, Z. Y. Wu, H. Shi, H. Huang and Z. H. Kang, J. Mater. Chem. A, 2022, 10, 4279–4287 RSC
.
- Y. Lu, W. J. Yin, K. L. Peng, K. Wang, Q. Hu, A. Selloni, F. R. Chen, L. M. Liu and M. L. Sui, Nat. Commun., 2018, 9, 2752 CrossRef PubMed
.
- C. Peng, T. Zhou, P. Wei, H. Q. Ai, B. P. Zhou, H. Pan, W. K. Xu, J. B. Jia, K. Zhang, H. J. Wang and H. Yu, Chem. Eng. J., 2022, 439, 135685 CrossRef CAS
.
- Q. Liang, S. Zhao, Z. Y. , Li, Z. Y. Wu, H. Shi, H. Huang and Z. H. Kang, ACS Appl. Mater. Interfaces, 2021, 13, 40754–40765 CrossRef PubMed
.
- Y. X. Wang, Y. Y. Zhang, X. J. Zhu, Y. Liu and Z. B. Wu, Appl. Catal., B, 2022, 316, 121610 CrossRef CAS
.
- X. X. Zhang, Y. G. Xiao, S. S. Cao, Z. L. Yin and Z. Q. Liu, J. Clean. Prod., 2022, 352, 131560 CrossRef CAS
.
- Y. J. Bai, X. B. Yi, B. Li, S. W. Chen and Z. J. Fan, Appl. Surf. Sci., 2022, 578, 151993 CrossRef CAS
.
- Z. Man, Y. Meng, X. C. Lin, X. R. Dai, L. P. Wang and D. Z. Liu, Chem. Eng. J., 2022, 431, 133952 CrossRef CAS
.
- G. L. Mo, L. X. Wang and J. H. Luo, Sep. Purif. Technol., 2021, 277, 119643 CrossRef CAS
.
- Z. G. Yin, T. T. Song, W. T. Zhou, Z. L. Wang and Y. Ma, J. Catal., 2021, 402, 289–299 CrossRef CAS
.
- K. M. Kamal, R. Narayan, N. Chandran, S. Popović, M. A. Nazrulla, J. Kovač, N. Vrtovec, M. Bele, N. Hodnik, M. M. Kržmanc and B. Likozar, Appl. Catal., B, 2022, 307, 121181 CrossRef CAS
.
- X. M. Cheng, X. Y. Dao, S. Q. Wang, J. Zhao and W. Y. Sun, ACS Catal., 2021, 11, 650–658 CrossRef CAS
.
- Y. Chen, G. B. Mao, Y. W. Tang, H. Wu, G. Wang, L. Zhang and Q. Liu, Chin. J. Catal., 2021, 42, 225–234 CrossRef CAS
.
- X. D. Zhang, K. Yue, R. Z. Rao, J. F. Chen, Q. Liu, Y. Yang, F. K. Bi, Y. X. Wang, J. C. Xu and N. Liu, Appl. Catal., B, 2022, 310, 121300 CrossRef CAS
.
- L. B. Wang, B. Chen, L. Y. Zhang and J. G. Yu, Small, 2021, 17, 2103447 CrossRef CAS PubMed
.
- S. H. Wu, X. F Xing, D. Wang, J. Z. Zhang, J. M. Chu, C. C. Yu, Z. T. Wei, M. L. Hu, X. Zhang and Z. X. Li, ACS Sustain. Chem. Eng., 2020, 8, 148–153 CrossRef CAS
.
- X. J. Yang, H. W. Sun, G. Y. Li, T. C. An and W. Choi, Appl. Catal., B, 2021, 294, 120252 CrossRef CAS
.
- X. J. Li, K. Y. Zhang, X. B. Huang, Z. Y. Wu, D. F. Zhao and G. Wang, Nanoscale, 2021, 13, 19671–19681 RSC
.
- S. Q. Zhang, X. Liu, C. B Liu, S. L. Luo, L. L. Wang, T. Ca, Y. X. Zeng, J. L. Yuan, W. Y. Dong, Y. Pei and Y. T. Liu, ACS Nano, 2018, 12, 751–758 CrossRef CAS PubMed
.
- K. Wang, X. H. Li, N. Wang, Q. H. Shen, M. C. Liu, J. C. Zhou and N. X. Li, Ind. Eng. Chem. Res., 2021, 60, 8720–8732 CrossRef CAS
.
- P. She, B. Y. Guan, J. Y. Sheng, Y. Y. Qi, G. Y. Qiao, H. B. Rui, G. Y. Lu, J.-S. Qin and H. Rao, Catal. Sci. Technol., 2022, 12, 1092–1099 RSC
.
- H. H. Li, F. Zhang, H. F. Wang, J. R. Xue, Y. M. Guo, Q. Z. Qian and G. Q. Zhang, Energy Environ. Sci., 2021, 14, 5339–5346 RSC
.
- C. H. Liu, Y. H. Yao, L. Sun, L. L. Luo, W. C. Wang and Z. D. Chen, Chem. Commun., 2021, 57, 9846–9849 RSC
.
- A. R. Amani-Ghadim, F. Khodam and M. S. Dorraji, J. Mater. Chem. A, 2019, 7, 11408–11422 RSC
.
- T. Wang, Q. Y. Men, X. Q. Liu, H. Q. Zhan and Y. Q. Wang, Sep. Purif. Technol., 2022, 294, 121215 CrossRef CAS
.
- X. H. Ma, D. Y. Li, Y. H. Jiang, H. C. Jin, L. Y. Bai, J. Qi, F. F. You and F. L. Yuan, J. Colloid Interface Sci., 2022, 628, 768–776 CrossRef CAS PubMed
.
- Y. Li, Y. Zhao, J. Wu, Y. D. Han, H. Huang, Y. Liu and Z. H. Kang, J. Mater. Chem. A, 2021, 9, 25453–25462 RSC
.
- Y. X. Li, Y. X. Liu, X. Liu, Y. L. Liu, Y. Y. Cheng, P. Zhang, P. J. Deng, J. J. Deng, Z. H. Kang and H. T. Li, Nano Res., 2022, 15, 6026–6035 CrossRef CAS
.
- J. Z. Zhao, M. X. Ji, H. L. Chen, Y.-X. Weng, J. Zhong, Y. J. Li, S. Y. Wang, Z. R. Chen, J. X. Xia and H. M. Li, Appl. Catal., B, 2022, 307, 121162 CrossRef CAS
.
- Y. Ke, Q. Liang, S. Zhao, Z. H. Zhang, X. Z. Li and Z. Y. Li, Inorg. Chem., 2022, 61, 2652–2661 CrossRef CAS PubMed
.
- J. X. Xu, Y. F. Chen, M. Chen, J. Wang and L. Wang, Chem. Eng. J., 2022, 442, 136208 CrossRef CAS
.
- P. Verma, F. A. Rahimi, D. Samanta, A. Kundu, J. Dasgupta and T. K. Maji, Angew. Chem., Int. Ed., 2022, 61, e202116094 CrossRef CAS PubMed
.
- G. M. Ren, S. T. Liu, Z. Z. Li, H. C. Bai, X. D. Hu and X. C. Meng, Sol. RRL, 2022, 6, 2200154 CrossRef CAS
.
- L. B. Wang, B. Cheng, L. Y. Zhang and J. G. Yu, Small, 2021, 17, 2103447 CrossRef CAS PubMed
.
- B. X. Ni, H. Jiang, W. Y. Guo, Q. J. Xu and Y. L. Min, Appl. Catal., B, 2022, 307, 121141 CrossRef CAS
.
- Z. R. Miao, Q. L. Wang, Y. F. Zhang, L. P. Meng and X. X. Wang, Appl. Catal., B, 2022, 301, 120802 CrossRef CAS
.
- Y. J. Ma, Q. Tang, W. Y. Sun, Z. Y. Yao, W. H. Zhu, T. Li and J. Y. Wang, Appl. Catal., B, 2020, 270, 118856 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ta07217c |
| This journal is © The Royal Society of Chemistry 2022 |