Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Recent advances in carbon-based nanomaterials for multivalent-ion hybrid capacitors: a review
Xuan
Gao
ab,
Haoyu
Wu
c,
Chang
Su
d,
Chuanming
Lu
d,
Yuhang
Dai
 b,
Siyu
Zhao
b,
Xueying
Hu
a,
Fangjia
Zhao
a,
Wei
Zhang
b,
Siyu
Zhao
b,
Xueying
Hu
a,
Fangjia
Zhao
a,
Wei
Zhang
 a,
Ivan P.
Parkin
a,
Ivan P.
Parkin
 a,
Claire J.
Carmalt
a,
Claire J.
Carmalt
 *a and
Guanjie
He
*a and
Guanjie
He
 *ab
*ab
aChristopher Ingold Laboratory, Department of Chemistry, University College London, 20 Gordon Street, London, WC1H 0AJ, UK. E-mail: g.he@ucl.ac.uk; c.j.carmalt@ucl.ac.uk
bDepartment of Chemical Engineering, University College London, London, WC1E7JE, UK
cSchool of Physics, Xi’an Jiaotong University, No. 28, Xianning West Road, Xi’an, 710049, China
dNanyang Technopreneurship Center, Nanyang Technological University, 50 Nanyang Avenue, Singapore 639798, Singapore
First published on 27th February 2023
Abstract
Hybrid capacitors are emerging because of their ability to store large amounts of energy, cycle through charges quickly, and maintain stability even in harsh environments or at extreme temperatures. Hybrid capacitors with monovalent cations such as Li+, Na+, and K+ have been extensively studied. However, the flammable nature of organic electrolytes and the reactive alkali metallic electrodes have raised safety concerns. This has prompted the development of novel aqueous multivalent cation storage systems, which can provide several benefits, including high capacity and energy density, rapid charge transfer, and low cost. With these advantages and the energy storage properties, multivalent cations such as Zn2+, Mg2+, Ca2+, and Al3+ have been applied to multivalent-ion hybrid capacitors (MIHCs), and the latest developments and design ideas for these have been recently reviewed. However, an overview from the perspective of materials with unique advantages and experimental designs remains limited. Carbon-based nanomaterials are leading candidates for next-generation energy storage devices due to their outstanding properties in MIHCs. The use of carbon-based nanomaterials is attractive because these materials are inexpensive, scalable, safe, and non-toxic. They are also bioactive at the anode interface, allowing them to promote electrochemical reactions with redox species that would otherwise not take place. This paper reviews recent advances in MIHCs and related carbon-based materials and discusses the utilization of carbon materials in MIHCs and ideas for material design, electrochemical behavior, energy storage mechanisms, electrode design, and future research prospects. Based on the integration of related challenges and development, we aim to provide insights and commercialization reference for laboratory research. For the first time, combined with global intellectual property analysis, this paper summarizes the current main research institutions and enterprises of various hybrid capacitors, and provides important technical competition information and development trends for researchers and practitioners in the field of energy storage. Simultaneously, we provide a perspective for the development of MIHCs, a description of the existing research, and guidelines for the design, production, commercialization, and advancement of unique high-performance electrochemical energy storage devices.
Broader contextThis paper reviews recent advances in MIHCs and related carbon-based materials, discusses the utilization of carbon materials in MIHCs and ideas for material design, electrochemical behavior, energy storage mechanisms, electrode design, and future research prospects. Based on the integration of related challenges and development, we aim to provide insights and commercialization reference for laboratory research. In addition to summarizing the research progress in energy storage technology, materials science, etc., the global commercialization of the energy storage field is also discussed. For the first time, combined with global intellectual property analysis, this paper summarizes the current main research institutions and enterprises of various hybrid capacitors, and provides important technical competition information and development trends for researchers and practitioners in the field of energy storage. Simultaneously, we provide a perspective for the development of MIHCs, a description of the existing research, and guidelines for the design, production, commercialization, and advancement of unique high-performance electrochemical energy storage devices. |
1. Introduction
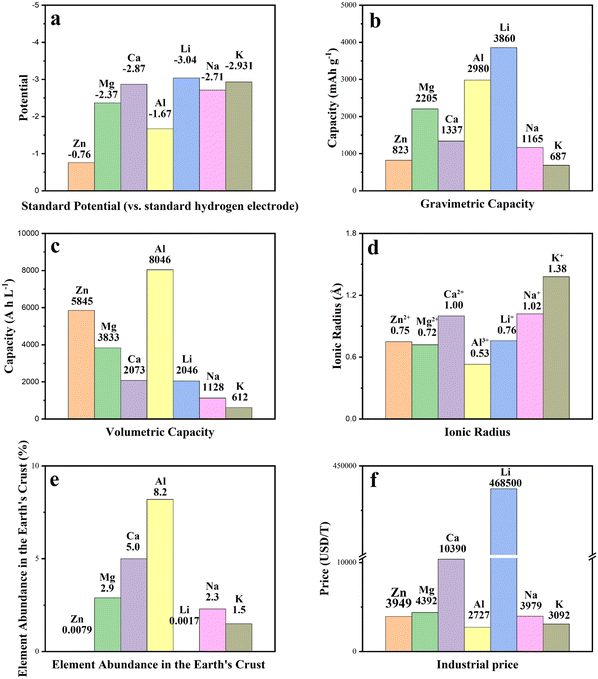
Due to the rapid emergence and increasing popularity of electric vehicles and portable electronics, energy storage devices have gained considerable attention. Over the last few decades, supercapacitors have emerged as one of the most exciting new battery technologies.1–3 Unlike conventional batteries, which store energy by means of chemical reactions, supercapacitors can be charged and discharged almost instantly, and can deliver sufficient power rapidly.4–7 Since supercapacitors are less expensive and lighter in weight than batteries, they have the potential to be employed in a wide range of applications. Their high charge–discharge rate, long service life, and broad application temperature range make them ideal for various electrical and electronic systems.8 However, poor energy density significantly restricts their scalability.By combining the advantages of batteries with supercapacitors, hybrid capacitors are suggested as alternative technologies. Hybrid capacitors are emerging because of their ability to store large amounts of energy, cycle through charges quickly, and maintain stability even in harsh environments or at extreme temperatures.9 Electrode materials with different features are used, one of which demonstrates electrostatic capacitance, while the other primarily demonstrates electrochemical capacitance. The electrolyte creates an ionically conductive medium between the two electrodes. Hybrid capacitors with monovalent cations such as Li+, Na+, and K+ have been extensively studied. However, the flammable nature of organic electrolytes and the reactive alkali metallic electrodes have raised safety concerns. This has prompted the development of novel aqueous multivalent cation storage systems which can provide several benefits, including high capacity and energy density, rapid charge transfer, and low cost.10
With these advantages, the energy storage mechanism of multivalent cations (Zn2+, Mg2+, Ca2+, and Al3+) has been applied to multivalent-ion hybrid capacitors (MIHCs), and the latest developments and design ideas for these have been recently reviewed.11–13 However, an overview from the perspective of materials with unique advantages and experimental designs remains limited. Carbon-based nanomaterials are leading candidates for next-generation energy storage devices due to their outstanding properties in MIHCs. The use of carbon-based nanomaterials is attractive because these materials are inexpensive, scalable, safe, and non-toxic while also being bioactive at the anode interface, which allows them to promote electrochemical reactions with redox species that would not otherwise take place.14
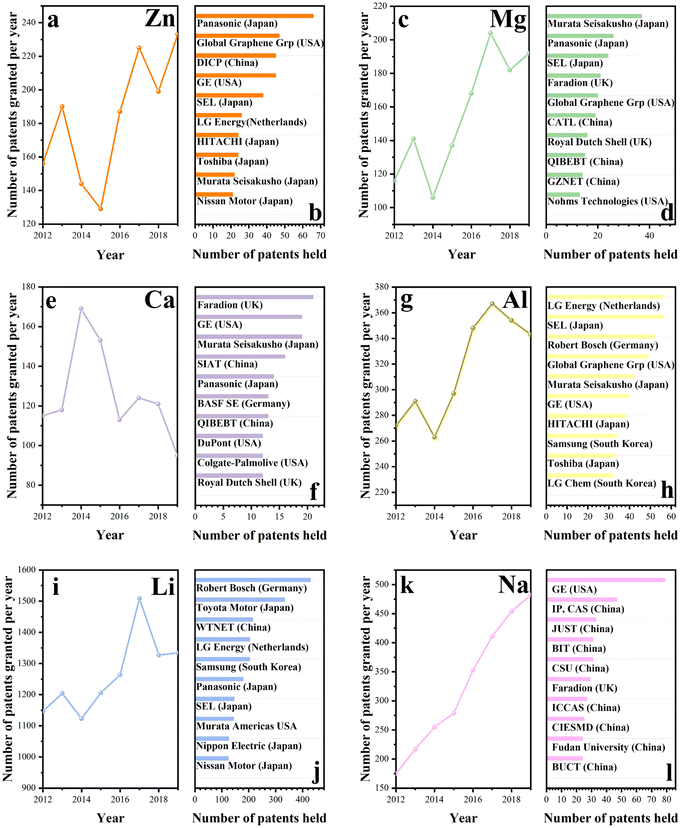
In fact, various energy storage technologies with metal cations as charge carriers are at different stages in the industry. Patents are legally protected against the copying of proprietary technology by competitors and provide a strong safeguard for commercialization. Hence, patents can reveal the current status of technological development effectively. Fig. 1 displays the patent analysis of different energy storage technologies with different charge carriers and the institutes or companies that currently hold the most patents for the corresponding technologies. As shown in Fig. 1, Li-ion energy storage techniques, which were on an upward trend from 2012, have gradually declined from 2017 onwards. Japanese companies (e.g., Toyota Motor Corporation, Panasonic Holdings Corporation, and Semiconductor Energy Laboratory) are the leaders in the industrial application of this technology. The Na-ion energy storage technology is the second most mature technology after lithium, with 3440 patents being recorded for the technology over the last decade. However, most of the patents are held by Chinese universities, reflecting the fact that Chinese companies have not yet commercialized sodium ion energy storage technology to a high degree. Similar to Li-ion energy storage technology, Japanese companies (e.g., Panasonic Co., Ltd, Semiconductor Energy Laboratory, and HITACHI) have the majority of patents in Zn-ion energy storage technology, as depicted in Fig. 1. Interestingly, this technology doubled in 2017 and 2019, with declining trends in the other years. China and the USA have mastered K-ion energy storage technology with comparable strength. However, while in the US, the technology is mainly held by companies, in China, it is still held by universities and research institutions. In contrast, Japanese companies keep leading the way in Mg-ion energy storage technologies, however, the available patents are not huge in number for this technology as a whole. Aluminum-ion energy storage technology is another developing area. The Netherlands mastery of the technology is impressive, while Japan and the USA also contributed to this technology. Finally, the development of Ca-ion energy storage technology is the slowest of all until now. Only 1200 technological patents have been granted in the last decade, which is in contrast to the number of patents for other cationic energy storage technologies. The competition for this technology is fierce, and various countries (e.g., UK, USA, Japan, China, and Germany) are actively involved.
The most recent developments in MIHCs and related carbon-based materials are reviewed herein. The integrated characteristics of hybrid capacitors, related to the structures and mechanisms of MIHCs are covered first. Then the application of carbon materials in MIHCs is explained, including the types of carbon materials, material design concepts, electrochemical behaviors, energy storage mechanisms, electrode designs, and future research directions. In addition to a summary of existing research, we present an outlook for the development of MIHCs and give the direction for the design, manufacture, and development of high-performance electrochemical energy storage devices.
2. Design mechanism of MIHCs
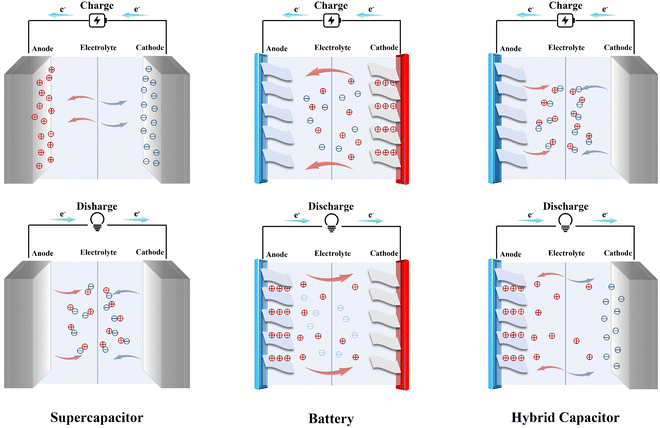
Since MIHCs are designed based on the mechanism of supercapacitors and multivalent cation batteries, as shown in Fig. 2, the structures and mechanisms of supercapacitors and multivalent cation batteries are discussed first.2.1. Concepts of supercapacitors and multivalent cation batteries
| Item | EDLCs | Pseudo-capacitors |
|---|---|---|
| Electrode material | Carbon materials | Transition metal oxides, conducting polymers, and hydroxides, etc. |
| Electrode with chemical activity | No | Yes |
| Energy storage mechanism | Physical absorption | Redox reaction |
| Advantages | Stable performance in long-term cycle | High specific capacitance (>1000 F g−1) |
| Fast charge and discharge | High energy density (>35 W h kg−1) | |
| High power density | ||
| Wide operating temperature window | ||
| Disadvantages | Low energy density (<15 W h kg−1) | Lack of stability during cycle |
2.2. Concept of multivalent-ion hybrid capacitors
Supercapacitors have excellent characteristics, such as fast charging rates and long service life, however, their power density is low, which limits the industrial application of supercapacitors. Multivalent cation batteries have a high energy density, but their output power and cycle stability have also defined their application range. MIHCs combine the advantages of both and circumvents the disadvantages of supercapacitors and multivalent cation batteries through the combination of battery-type electrodes and capacitor-type electrodes. When the capacitor-type electrode acts as the cathode of MIHCs and the battery-type electrode acts as the anode of MIHCs, the cathode acquires an electric double layer (EDL) or Faradaic intercalation occurs during charging, while anions and high-valent metal cations diffuse back into the electrolyte during discharge. When the capacitor-type electrode is used as the anode of MIHCs and the battery-type electrode is used as the cathode of MIHCs, the adsorption of high-valent cations on the battery-type cathode enables the MIHCs to have a high power density.MIHCs has several advantages over conventional supercapacitors and multivalent cation batteries. First, MIHCs exhibit high energy densities, similar to batteries, and high power densities, similar to supercapacitors. This combination makes them suitable for applications that require both high energy and high power delivery, such as electric vehicles and renewable energy storage. Second, MIHCs use multivalent ions, such as zinc, magnesium or aluminum, in the electrolyte, which results in faster charging and discharging compared to traditional supercapacitors that use only monovalent ions. MIHCs also have longer cycle lives than traditional supercapacitors, as they are not limited by the same capacitance fading mechanisms. Furthermore, MIHCs can be made safer than batteries due to the use of non-flammable electrolytes and the absence of high voltage, which reduces the risk of thermal runaway and short-circuit events. Additionally, MIHCs can be made more environmentally friendly compared to batteries by using biodegradable materials and reducing the use of heavy metals.
2.3. Electrolytes
Electrolytes are usually divided into aqueous and nonaqueous, as described below, and play an important role in improving electrode reaction efficiency and increasing the reaction rate. Improving the ionic and electronic conductivity of electrolytes is one of the current research focuses of researchers, which affects the reversible capacity and cycle stability of MIHCs.In 2021, a novel redox bromide-ion additive aqueous Mg-ion hybrid capacitor (MHC) was proposed by inserting the Br3−/Br− redox additive into 1 M MgSO4 electrolyte in an effort to increase its energy density.16 The designed hybrid capacitor has a maximum specific capacity of 268.1 mA h g−1 at a current density of 2 A g−1 across a broad voltage range of 2.6 V. (0–2.6 V). In addition, a maximum energy density of 262.3 W h kg−1 may be attained at a power density of 1956.8 W kg−1. In the field of Zn-ion hybrid capacitors (ZHCs), Wu et al. studied the electrochemical characteristics of aqueous Zn-ion energy storage technology related to the electrolyte type and concentration.17 The average Coulombic efficiency (CE) of Zn stripping/plating was determined to be as follows: Zn(CF3SO3)2 → Zn(CH3COO)2 → ZnCl2 → ZnSO4 → Zn(NO3)2, as shown in Fig. 4. Additionally, the electrolyte concentration had a noticeable influence on the CE of ZHCs, with the CE rising as the electrolyte concentration rises. The highest CE of Zn stripping/plating was achieved with Zn(CF3SO3)2 electrolyte at concentrations of 3 to 4 M. This is due to the higher interaction between H2O and CF3SO3− anions than between Zn2+ cations and H2O, which limits the generation of by-products during zinc plating procedures.
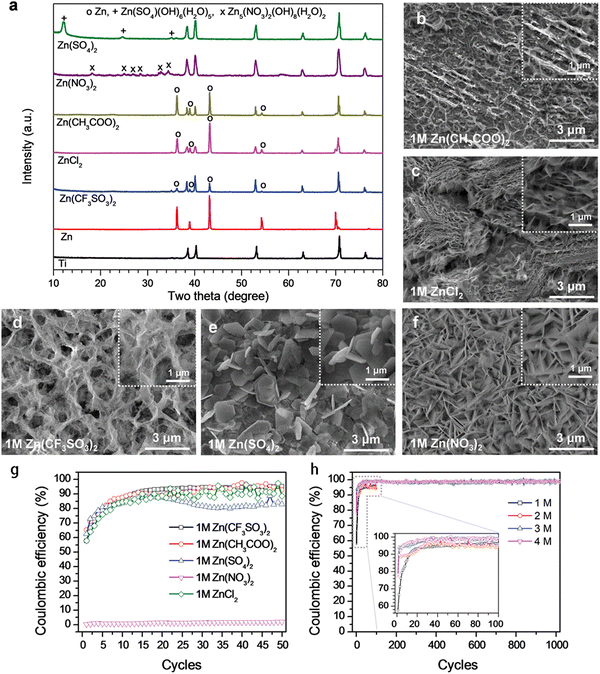 | ||
| Fig. 4 Characterization of the Zn plating behavior in electrolytes containing different Zn salts. (a) XRD patterns and (b–f) SEM images of pristine Zn and Ti foils, and Ti foils with Zn layer plated in different 1 M Zn salts. (g) CEs of Zn stripping/plating in electrolytes containing different 1 M zinc salts. (h) CEs in Zn(CF3SO3)2 electrolyte of different concentrations, inset: magnified view of the first 100 cycles denoted by grey dashed lines.17 Reused with permission from Wiley. | ||
2.3.2.1. Organic electrolyte. In 2021, Wang et al. proposed ZHCs with a nonaqueous electrolyte which achieved high cathode mass loading and high anode zinc utilization rates while maintaining a high capacity.18 The proposed ZHCs consist of a porous carbon cathode generated from a metal organic framework (MOF), a metallic zinc anode, and N,N-dimethylformamide (DMF) electrolyte containing Zn2+. The cathode's charge storage occurs mainly in macropores, demonstrating good rate performance under high mass loading. The DMF-based electrolyte may help in smooth deposition, permitting dendrite-free Zn plating/stripping, while its aprotic nature avoids unwanted H2 evolution. It was determined that the majority of the charge storage of the MOF-derived porous carbon cathode was due to anion and cation adsorption/exchange in macropores, as shown by in situ attenuated total reflection-Fourier transform infrared spectroscopy (ATR-FTIR). As a result, the capacitor exhibits stability after 9000 cycles and a zinc utilization rate of 2.2%. With a high mass loading of 40 mg cm−2, the energy density approached 25.9 W h kg−1. DMF-based electrolytes provide a feasible solution for capacitors operating at both high and low temperatures (−65 to 100 °C).
2.3.2.2. Ionic liquid electrolytes. Recently, trifluoromethanesulfonyl (TFSI−) has been commonly used in ionic liquid electrolytes. Tobias et al. proposed the reversible (de)intercalation of TFSI anions from a Mg-based ionic liquid electrolyte, Mg(TFSI)2 in Pyr14TFSI, within graphite//activated carbon hybrid dual-ion capacitors (DICs).19 The Mg-based DICs were compared to cells with pure Pyr14TFSI and LiTFSI-Pyr14TFSI electrolytes (Pry14: N-butyl-N-methylpyrrolidinium), as well as graphite//Li metal dual-ion cells with LiTFSI-Pyr14TFSI electrolytes. At 5.2 V vs. Li/Li+, Mg-containing DIC cells outperform Li-based cells, exhibiting a greater capacity (87 vs. 85 mA h g−1) and better CE (98 vs. 96%). These findings may pave the way for more research on and performance enhancements of Mg-based energy storage technology, as well as hybrid DIC chemistry with other cations, particularly multivalent cations.
With TFSI anions, Li et al. developed a new non-aqueous electrolyte (Zn(TFSI)2/Pyr14TFSI/AN) based on acetonitrile (AN) combined with ionic liquids (Pyr14TFSI) and Zn(TFSI)2.20 According to the study, the Raman spectral peaks changed dramatically with addition of 4 and 6 vt% AN. In the electrolyte containing 4 vt% AN, the free AN fraction predominates in comparison to the quantity of Zn2+-bound AN, while the electrolyte containing 6 vt% AN exhibits a reverse relationship. Additionally, the findings illustrate the development of a Zn2+ solvated structure. The coordination number of Zn–TFSI fell from 3.897 to 3.593 as the AN amount increased, but the coordination number of Zn–AN grew from 0.1 to 0.403. Simultaneously, the frequency of AN molecules in the initial solvation shell of Zn2+ increased steadily as the quantity of AN increased. As a result, the coordination number of Zn–AN increased as the quantity of AN increased. This indicates a competition between TFSI− and AN molecules, as well as a significant interaction between the AN molecules and Zn2+ in the hybrid electrolyte.
3. Classification of MIHCs
Hybrid capacitors are designed to have the most significant qualities of supercapacitors, namely, high-power capability and extended cycle life, while having a better specific energy density than symmetric supercapacitors, which have solely electrostatic interactions between two electrodes. Typically, this is accomplished by building an asymmetric cell with an EDLC electrode and a redox battery electrode. The ideal case is that the specific capacitance of a battery electrode (CBATT) is semi-infinite. As a result, the EDLC electrode dominates the full-cell specific capacitance (Ctot) of a hybrid capacitor. EDLC electrodes are often constructed from high surface area activated carbon (AC), and hence their capacitance may be approximated from the specific capacitance of the activated carbon (CAC) from which they are constructed. As a result, the hybrid, asymmetric supercapacitor's specific capacitance, Ctot, is comparable to that of AC. This follows the formula:| 1/(mtotCtot) = 1/(mACCAC) + 1/(mBATTCBATT) |
For symmetrical supercapacitors, on the other hand, Ctot is restricted to 0.25CAC according to the following formula: 1/(mtotCtot) = 2/(mACCAC), where mtot equals 2 times mAC in this instance. As a result, hybrid capacitors have a higher Ctot than symmetrical supercapacitors.
3.1. Classification by multivalent ion
MHCs are still in their infancy, due to the issue of passivation coatings forming on the surface of the metallic magnesium anode in electrolytes. This issue precludes the long-term reversible deposition/dissolution of Mg2+ ions and so limits the practical use of MHCs. Due to the fact that the passivation films created on magnesium anodes are impermeable to Mg2+ ions, reversible magnesium deposition/dissolution requires solutions based on ether solvents and magnesium organo-haloaluminate complex electrolytes without forming passivation films on magnesium electrodes. Compatibility concerns make it difficult to match these complicated electrolyte solutions with cathodes; the magnesium organo-haloaluminate electrolytes are often nucleophilic and chemically reactive with electrophilic oxide electrodes. Additionally, due to the large ionic size of the magnesium ionic complexes, cathodes made usinhg porous carbon cannot be used in the magnesium organo-haloaluminate electrolytes. As a result, proper electrode materials and electrolytes, as well as thorough investigation, are critical.
In 2022, Pan et al. developed an MHC with high capacity and stability based on expanded layer spacing MoS2 (E-MoS2) nanosheet anodes.22 The authors demonstrated that expanded layer spacing reduced ion diffusion resistance and offered more active sites so that MoS2 with expanded interlayer spacing was a prospective negative electrode material for rechargeable MHCs. The E-MoS2-based MHC exhibited an advantageous specific capacitance of 274.2 F g−1 at 0.5 A g−1 and a high energy density of 192.8 W h kg−1 at a power density of 1682.7 W kg−1. Notably, the proposed MHC demonstrated superior long-term capacity retention, retaining 93.8% of its capacitance after 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. The enhanced capacity and stability were attributed to the increased number of active sites for Mg2+ storage and the decreased ion diffusion resistance provided by the expansion of the MoS2 layer spacing.
000 cycles. The enhanced capacity and stability were attributed to the increased number of active sites for Mg2+ storage and the decreased ion diffusion resistance provided by the expansion of the MoS2 layer spacing.
It has been challenging to create Ca-based energy storage devices based on the standard rocking-chair mechanism due to the lack of an ideal mix of compatible electrode materials and electrolytes. Direct application of metallic calcium anodes falls short of achieving the criteria of commercialization, especially showing a poor CE in low-power state. The reversibility of metallic calcium anodes is limited because of the creation of a passivation layer during the oxidation of Ca2+. Sn is utilized as the battery electrode in the majority of Ca energy storage systems. Sn combines with Ca2+ during the process to generate a Ca–Sn alloy (Ca7Sn6). When the alloy interacts, the electrons exhibit fast transmission.
Tang et al. deconstructed the basic challenge-confronting calcium-based energy storage devices and established a multi-ion reaction method necessary to develop a full device.23 To develop a comprehensive Ca-ion energy storage device, a multi-ion reaction method was devised, and a capacitor–battery hybrid mechanism was purposefully chosen. To achieve a longer life span, quicker kinetics, increased capacity, and higher voltage, a capacitor–battery hybrid mechanism was used to build a hybrid Ca-ion energy storage device at ambient temperature using typical organic electrolytes. Due to the intricate design, it has a good reversible capacity of 92 mA h g−1 after 1000 cycles.
Meanwhile, AHCs have long been constrained in their kinetics and reversibility by the high electrostatic field around the bare Al3+. While using hydrated Al3+ as charge carriers may alleviate this problem, their huge size puts stringent restrictions on the pore architecture of electrode materials. Qu et al. devised an adaptive pore-structure remolding technique for capacitive electrode materials in order to achieve very compact and orderly storage of hydrated Al3+.24 The cathode was made by graphene-based remolding of highly ordered and compact porous carbon (RHOPC) and the anode was made by remolding alkali-treated Ti3C2Tx (RAT-Ti3C2Tx). The proposed AHC demonstrated a high operating voltage of up to 2.0 V, an energy density of 112 W h L−1, and a power density of 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 W L−1 after 10
000 W L−1 after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles.
000 cycles.
3.2. Classification by configuration
From the configuration point of view, MIHCs consist of battery-type electrodes, electrolyte, and capacitor-type electrodes. They are classified into two types: (1) capacitor-type cathodes (carbon materials or pseudocapacitive materials) versus battery-type anodes (metallic electrodes) and (2) battery-type cathodes (transition metal oxides) versus capacitor-type anodes (carbon materials or pseudocapacitive materials). The energy storage process in capacitive electrodes is associated with the adsorption/desorption or intercalation/de-intercalation of ions at the cathode or anode. The energy storage process in the battery-type electrode differs significantly. The former is due to metal ion deposition/stripping at anodes, whereas the latter is due to metal ion insertion/extraction at cathodes.| Electrode material | Electrolyte | Voltage (V) | Energy density | Power density | Performance | Application | Ref. |
|---|---|---|---|---|---|---|---|
| Zn-doped δ-MnO2 | 2 M ZnSO4 | 0–2 | 157.2 W h kg−1 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 W kg−1 000 W kg−1 |
80.2% after 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 2 A g−1 000 cycles at 2 A g−1 |
Zn-based | 25 |
| AMX-Zn | 2 M ZnSO4 | 0.3–1.9 | 60.2 W h kg−1 | 7.04 kW kg−1 | 92.5% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 3.3 A g−1 000 cycles at 3.3 A g−1 |
Zn-based | 26 |
| V2O5 | 2 M ZnSO4 | −1 to 1.5 | 34.6 W h kg−1 | 1.3 kW kg−1 | 97.3% after 6000 cycles at 0.5 A g−1 | Zn-based | 27 |
| V2O5-activated carbon | 3 M Zn(CF3SO3)2 | 0–2 | 53.13 W h kg−1 | 1384.6 W kg−1 | ∼99% after 4000 cycles at 0.1 mA cm−2 | Zn-based | 28 |
| MnO2-CNTs | 2.69 M ZnSO4 and 0.135 M MnSO4 | 0–2 | 98.6 W h kg−1 | 2480.6 W kg−1 | 83.6% after 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 1 A g−1 000 cycles at 1 A g−1 |
Zn-based | 29 |
| PDA@3DVAG | 2 M ZnSO4 | 0.5–2 | 46.14 W h kg−1 | 2183 W kg−1 | 61.8% after 3000 cycles at 1 A g−1 | Zn-based | 30 |
| δ-MnO2@CAC | 2 M ZnSO4 | 0–1.9 | 90 W h kg−1 | 3838 W kg−1 | 80.7% after 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 10 A g−1 000 cycles at 10 A g−1 |
Zn-based | 31 |
| V2CTx | 1 M LiSO4 | 0.1–1.85 | 386.2 W h kg−1 | — | 100% after 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 10 A g−1 000 cycles at 10 A g−1 |
Zn-based | 32 |
| 2 M ZnSO4 | |||||||
| MnO2/graphite hybrids | 2 M ZnSO4 | 0.8–1.8 | 247 W h kg−1 | 11.78 kW kg−1 | 80.8% after 1000 cycles at 1 A g−1 | Zn-based | 33 |
| 0.5 M MnSO4 | |||||||
| K–MnO2 | 1 M MgSO4 | 0–1.8 | 85.2 W h kg−1 | 360 W kg−1 | 96.7% after 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 5 A g−1 000 cycles at 5 A g−1 |
Mg-based | 34 |
| E-MoS2 | 1 M MgSO4 | 0–1.8 | 192.8 W h kg−1 | 1627 W kg−1 | 93.8% after 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 5 A g−1 000 cycles at 5 A g−1 |
Mg-based | 35 |
| AlxMnO2-z/AC | 0.5 M MgSO4 | 0–2 | 104.86 W h kg−1 | 69.44 W kg−1 | 86% after 2000 cycles at 1 A g−1 | Mg-based | 36 |
| Mg-OMS-2/graphene | 0.5 M Mg(NO3)2 | 0–2 | 46.9 Wh kg−1 | 70 W kg−1 | 93% after 300 cycles at 0.1 A g−1 | Mg-based | 37 |
| Mg-OMS-2/graphene | 0.5 M MgCl2 | 0–2 | — | — | 75.7% after 300 cycles at 0.1 A g−1 | Mg-based | 37 |
| Mg-OMS-2/graphene | 0.5 M MgSO4 | 0–2 | — | — | 57.5% after 300 cycles at 0.1 A g−1 | Mg-based | 37 |
| RAT-Ti3C2Tx | 1 M Al2(SO4)3 | 0–2 | 112 W h L−1 | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 W L−1 000 W L−1 |
91.8% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 5 A g−1 000 cycles at 5 A g−1 |
Al-based | 24 |
| CuFe-PBA | 1 M Al(NO3)3 | 0–2 | 13 W h kg−1 | — | ∼80% after 1000 cycles at 0.25 A g−1 | Al-based | 38 |
| MCM/V2O5 | 1 M Al2(SO4)3 | 0–1.6 | 18.0 W h kg−1 | 147 W kg−1 | 88% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 0.5 A g−1 000 cycles at 0.5 A g−1 |
Al-based | 39 |
| Sn | Ca(PF6)2 | 1.5–4.8 | — | — | 84% after 1000 cycles at 0.2 A g−1 | Ca-based | 23 |
4. Carbon-based nanomaterials in MIHCs
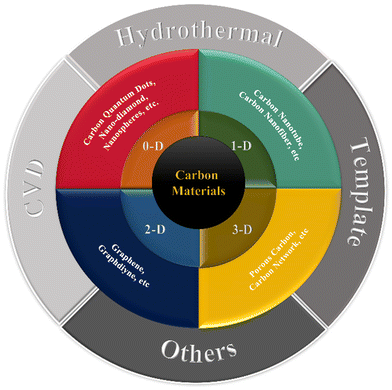
Carbon materials occur in a variety of forms. Due to their high SSA, plentiful pores, chemical inertness, and good conductivity, porous carbon (PC), graphene, carbon aerogels (CAs), and carbon nanotubes (CNTs) are intriguing research topics for application in MIHCs. When carbon materials are reduced to the nanoscale, their characteristics significantly alter. The varying dimensions of the carbon nanostructure impart diverse qualities to carbon-based materials. As a result, this section will explore numerous carbon nanostructures with varying dimensions. The current classification of different carbon nanomaterials is shown in Fig. 5.Currently, for carbon nanomaterials, effective ways to increase performance include doping, hybridization, surface modification and specific structure construction. The carbon-based nanomaterials used for different MIHCs and their performance with potentials intervals are shown in Table 3. The research on carbon materials used in ZHCs dominates the development of carbon materials for MIHCs.
| Electrode material | Electrolyte | Voltage [V] | Energy density | Power density | Cycling stability | Dimension | Application | Ref. |
|---|---|---|---|---|---|---|---|---|
| Commercial AC | 2 M ZnSO4 | 0.2–1.8 | 104.8 W h kg−1 | 383.5 W kg−1 | 95.1% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 4 A g−1 000 cycles at 4 A g−1 |
0-D | Zn-based | 40 |
| AC treated by gradient acid | Glutaraldehyde crosslinked gelatin | 0.2–1.8 | — | — | 92.1% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 1 A g−1 000 cycles at 1 A g−1 |
0-D | Zn-based | 41 |
| AC-PHC | 1 M ZnSO4 | 0.2–1.8 | 117 W h kg−1 | 160 W kg−1 | 95% after 5000 cycles at 5 A g−1 | 0-D | Zn-based | 42 |
| AC | Mg(TFSI)2-Pyr14TFSI | 0.6–2.0 | — | — | >100% after 50 cycles at 50 mA g−1 | 0-D | Mg-based | 19 |
| Mg-OMS-2/graphene | 0.5 M Mg(NO3)2 | 0–2 | 46.9 W h kg−1 | 70 W kg−1 | 93% after 300 cycles at 0.1 A g−1 | 0-D | Mg-based | 37 |
| Mg-OMS-2/graphene | 0.5 M MgCl2 | 0–2 | — | — | 75.7% after 300 cycles at 0.1 A g−1 | 0-D | Mg-based | 37 |
| Mg-OMS-2/graphene | 0.5 M MgSO4 | 0–2 | — | — | 57.5% after 300 cycles at 0.1 A g−1 | 0-D | Mg-based | 37 |
| MEC | 0.5 M Mg(TFSI)2 | −2 to 2 | 106 W h kg−1 | 11.87 kW kg−1 | >90% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 1 A g−1 000 cycles at 1 A g−1 |
0-D | Mg-based | 43 |
| AC | 0.5 M MgCl2 | −0.8 to 0.8 | 103.2 W h kg−1 | 2 kW kg−1 | 92% after 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 10 A g−1 000 cycles at 10 A g−1 |
0-D | Mg-based | 44 |
| AC | Ca(PF6)2 | 1.5–4.8 | — | — | 84% after 1000 cycles at 0.2 A g−1 | 0-D | Ca-based | 23 |
| MEC | 0.5 M Ca(TFSI)2 | −2 to 2 | — | — | — | 0-D | Ca-based | 43 |
| AC | [EMIm]Cl & AlCl3 | 0–2 | 51 W h kg−1 | 9.285 kW kg−1 | 97.9% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 5 A g−1 000 cycles at 5 A g−1 |
0-D | Al-based | 45 |
| MCM/V2O5 | 1 M Al2(SO4)3 | 0–1.6 | 18.0 W h kg−1 | 147 W kg−1 | 88% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 0.5 A g−1 000 cycles at 0.5 A g−1 |
0-D | Al-based | 39 |
| NCN/carbon nanotube | 2 M ZnSO4 | 0.2–1.6 | 124.1 W h kg−1 | 34.7 W kg−1 | 100% after 1000 cycles at 1 A g−1 | 0-D + 1-D | Zn-based | 46 |
| CNT/PANI | 2 M ZnSO4 | 0.5–1.5 | 104 W h kg−1 | 8.3 kW kg−1 | Nearly 100% after 1000 cycles at 1 A g−1 | 1-D | Zn-based | 47 |
| CNT/paper | 2 M ZnSO4 | 0.2–1.8 | — | — | Nearly 100% after 7000 cycles at 2 A g−1 | 1-D | Zn-based | 48 |
| CNT/paper | 2 M ZnSO4 | 0.2–1.8 | — | — | Nearly 100% after 7000 cycles at 2 A g−1 | 1-D | Zn-based | 48 |
| CNPK | 1 M ZnSO4 | 0–1.9 | 81.1 W h kg−1 | 13.366 kW kg−1 | 101.8% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 5 A kg−1 000 cycles at 5 A kg−1 |
2-D | Zn-based | 49 |
| NPCNs | 2 M ZnSO4 | 0–1.7 | 64.8 W h kg−1 | 1099 W kg−1 | Nearly 100% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 5 A g−1 000 cycles at 5 A g−1 |
2-D | Zn-based | 50 |
| MLCNs | 1 M ZnSO4 | 0.2–1.8 | 102.1 W h kg−1 | 16.9 kW kg−1 | 98.7% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 20 A g−1 000 cycles at 20 A g−1 |
2-D | Zn-based | 51 |
| OPCNF-20 | 1 M ZnSO4 | 0–3.6 | 97.7 W h kg−1 | 9.9 kW kg−1 | 81% after 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 1 A g−1 000 cycles at 1 A g−1 |
2-D | Zn-based | 52 |
| rGO + B90/FTO | 2 M ZnSO4 | 0.2–1 | 668 mW h kg−1 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 mW kg−1 250 mW kg−1 |
∼90% after 1000 cycles at 20 mA g−1 | 2-D | Zn-based | 53 |
| In situ pillared Mxene | 0.1 M ZnSO4 | 0.01–1 | — | — | Over 96% after 1000 cycles at 0.2 A g−1 | 2-D | Zn-based | 54 |
| Graphene | 2 M ZnSO4 | 0.1–1.9 | 78.32 W h kg−1 | 8010 W kg−1 | 90.8% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 1 A g−1 000 cycles at 1 A g−1 |
2-D | Zn-based | 55 |
| WC-6ZnN-12U | 1 M ZnSO4 | 0.2–1.8 | 109.5 W h kg−1 | 225 W kg−1 | 92.7% after 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 2 A g−1 000 cycles at 2 A g−1 |
2-D | Zn-based | 56 |
| PPy/N-rGO | 2 M ZnSO4 | 0–1.6 | 232.5 W h kg−1 | 160 W kg−1 | 85% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 7 A g−1 000 cycles at 7 A g−1 |
2-D | Zn-based | 57 |
| Graphene | 6% KI & MgSO4 | 0–1.3 | 69.3 W h kg−1 | 2.5 kW kg−1 | 89% after 5000 cycles at 5 A g−1 | 2-D | Mg-based | 58 |
| AC | Mg(TFSI)2-Pyr14TFSI | 0.6–2.0 | — | — | >100% after 50 cycles at 50 mA g−1 | 2-D | Mg-based | 19 |
| RHOPC | 1 M Al2(SO4)3 | 0–2 | 112 W h L−1 | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 W L−1 000 W L−1 |
91.8% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 5 A g−1 000 cycles at 5 A g−1 |
0-D | Al-based | 24 |
| 3D porous carbon | Glutaraldehyde crosslinked gelatin/ZnSO4 | 0.2–1.8 | 82.36 W h kg−1 | 3760 W kg−1 | 87.6% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 1 A g−1 000 cycles at 1 A g−1 |
3-D | Zn-based | 59 |
| ACTS-800 | 3 M ZnSO4 | 0–2 | 127 W h kg−1 | 7920 W kg−1 | 100% after 1000 cycles at 10 A g−1 | 3-D | Zn-based | 60 |
| HHPC6 | 2 M ZnSO4 | 0.2–1.8 | 117.6 W h kg−1 | 40 kW kg−1 | 88% after 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 5 A g−1 000 cycles at 5 A g−1 |
3-D | Zn-based | 61 |
| PZC-A750 | 1 M Zn(CF3SO3)2 | 0.2–1.8 | 107.3 W h kg−1 | 24.9 kW kg−1 | Nearly 100% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 10 A g−1 000 cycles at 10 A g−1 |
3-D | Zn-based | 62 |
| MOF derived carbon (MDC) | 1 M ZnSO4 | 0.1–1.7 | 7.5 W h kg−1 | 85.5 kW kg−1 | 99% after 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 1 A g−1 000 cycles at 1 A g−1 |
3-D | Zn-based | 63 |
| BSCs | 1 M ZnSO4 | 0–1.0 | 197.7 W h kg−1 | 15.221 kW kg−1 | 106.1% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 0.1 A g−1 000 cycles at 0.1 A g−1 |
3-D | Zn-based | 64 |
| CDC | 2 M ZnSO4 | 0–2.0 | 243 W h kg−1 | 492 W kg−1 | 85% after 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 0.5 A g−1 000 cycles at 0.5 A g−1 |
3-D | Zn-based | 65 |
| 3D printed rGO | 1 M ZnSO4 | 0.01–1.8 | 266 μW h cm−2 | ∼100 μW cm−2 | 75% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 10 A g−1 000 cycles at 10 A g−1 |
3-D | Zn-based | 66 |
| 3D AC | Zn(BF)4 with [EMIN]BF4 | −0.7 to 2.4 | 220 W h kg−1 | 9.5 kW kg−1 | 95% after 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 2 A g−1 000 cycles at 2 A g−1 |
3-D | Zn-based | 67 |
4.1. Zero-dimensional carbon materials
The term “zero-dimensional (0-D) carbon materials” refers to sphere-shaped carbon materials with an aspect ratio of ∼1. Activated carbon (AC), carbon quantum dots (CQD), nanodiamonds, and carbon nanospheres are the most common 0-D carbon materials. 0-D carbon materials usually show high SSAs (hundreds to thousands of m2 g−1) and customizable pore size and distribution, which are crucial parameters affecting the supercapacitor's performance.0-D carbon materials are extensively employed as electrode materials, with electrons provided either by hopping between neighboring nanoparticles’ trap states or via diffusive movement within the extended states, which is hindered by (de)trapping processes.68,69 The absence of continuity between carbon nanoparticles is unfavorable for enhancing the electrical conductivity and, as a result, the power density reduces. Typically, 0-D carbon compounds are synthesized from carbon-rich precursors by thermal activation at high temperatures (700–1200 °C) with H2O, CO2, and air, or chemical activation at lower temperatures (600–800 °C) with H3PO4, KOH, ZnCl2, and others.70 Previously published data indicate that activated carbon could show a specific capacitance of 100–300 F g−1.71–73 Song et al. effectively prepared activated carbon cathode material for ZHCs by direct KOH activation utilizing pitch coke (PHC) and petroleum coke (PMC) as precursors.42 The results exhibited excellent electrochemical performances with an exceptional specific capacitance of 146.4 mA h g−1 at 0.1 A g−1 after 5000 cycles.
0-D carbon nanospheres have a multitude of applications in MIHCs, where they may facilitate the conduction of cations in electrodes with an optimal pore size distribution. Chen et al. presented an electrode strategy for dual ion adsorption and manufactured nitrogen-doped mesoporous carbon nanospheres for ZHCs, as shown in Fig. 6.74 The study illustrates the usage of nitrogen-doped mesoporous carbon nanospheres (NMCSs) with hierarchically distributed pores and improved Zn2+ storage capacity. The as-prepared aqueous ZHCs had a notable specific capacity of 157.8 mA h g−1, a maximum energy density of 126.2 W h kg−1 at 0.2 A g−1, and an ultra-high power density of 39.9 kW kg−1 with a rapid charging time of 5.5 s.
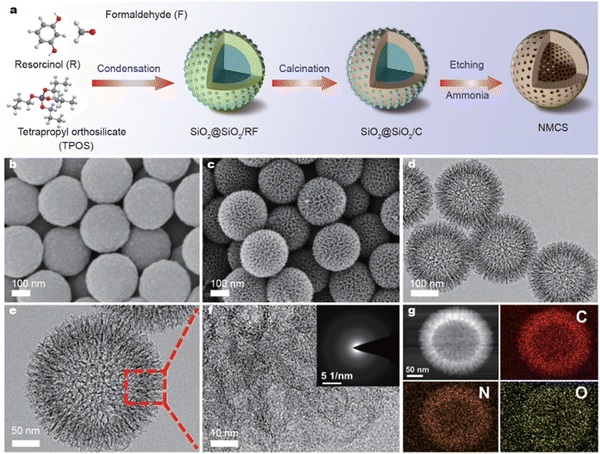 | ||
| Fig. 6 (a) Schematic illustration of the fabrication of NMCSs. SEM images of (b) SiO2@SiO2/C and (c) NMCSs. (d and e) TEM and (f) high-resolution (g) HAADF-STEM image and elemental mappings of NMCSs.74 Reused with permission from Springer Nature. | ||
4.2. One-dimensional carbon materials
One-dimensional (1-D) carbon materials are attractive candidates for MIHCs electrodes owing to their lengthy 1-D nanostructure, which facilitates the formation of a sequential network for charge transfer.75Aqueous ZHCs have been widely explored in the literature. Dong et al. have indicated that functionalized carbon nanotubes outperform pristine CNTs in terms of electrochemical performance taking advantage of better chemical adsorption ability and hydrophilicity of Zn2+, and the functionalized carbon nanotube (f-CNT)/polyaniline (PANI) nanocomposites successfully integrated capacitive energy storage mechanism of f-CNTs with the redox reaction energy storage mechanism of PANI, therefore exhibiting better comprehensive electrochemical properties, such as good rate performance, high capacity, and long cycle life.47 In addition, with the prepared nanomaterial, flexible and high-performance zinc-based devices have been generated. Micromorphologies of pure PANI and hydroxylated carbon nanotube (h-CNT)/PANI nanocomposites are shown in Fig. 7a–c.47 Pure PANI samples generated by oxidation polymerization were nanorods with a diameter of less than 50 nm. In h-CNT/PANI nanocomposites, the particles showed similar nanorod-like morphology, albeit their width and length were much reduced. The TEM in Fig. 7c demonstrates the PANI coating on the surface of h-CNTs in the h-CNT/PANI nanocomposite. In such a situation, electrons may travel via highly conductive h-CNTs to PANI, hence facilitating PANI's involvement in redox processes. The presence of h-CNT contributes to the enhancement of PANI's hydrophilicity.
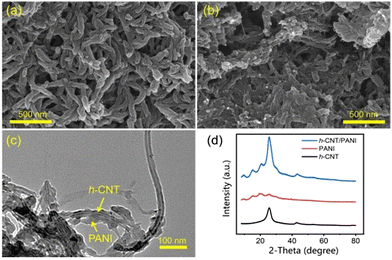 | ||
| Fig. 7 SEM images of (a) pure PANI and (b) h-CNT/PANI nanomaterial. (c) TEM image of h-CNT/PANI nanomaterial. (d) XRD patterns of h-CNTs, pure PANI and h-CNT/PANI nanomaterial.47 Reused with permission from Elsevier. | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 40 A g−1 under the condition of high loading (14.45 mg cm−2).
000 cycles at 40 A g−1 under the condition of high loading (14.45 mg cm−2).
4.3. Two-dimensional carbon materials
Two-dimensional (2-D) carbon materials, such as graphene, have garnered significant interest for their use in hybrid capacitor electrodes due to several unique properties. First of all, 2-D carbon materials possess high mechanical stability and flexibility, making them ideal for use in flexible electronic devices and energy storage systems. The mechanical stability of 2-D carbon materials enables them to withstand the repeated charge–discharge cycles and electrochemical reactions that occur in hybrid capacitors, while their flexibility makes them well-suited for use in flexible and bendable electronics. On the other hand, 2-D carbon materials have high electrical conductivity and thermal conductivity, making them ideal for use as electrodes in hybrid capacitors. The high electrical conductivity enables fast and efficient flow of charge in the capacitor, reducing the internal resistance and improving the power density of the device. The high thermal conductivity helps to dissipate heat during high-power operation, reducing the risk of thermal damage to the device. In addition, 2-D carbon materials have a high surface area and chemical stability, making them highly resistant to degradation in aqueous and non-aqueous electrolytes. The high surface area provides ample room for electrochemical reactions to occur, resulting in a higher capacitance compared to that of traditional 3-D electrodes. The chemical stability of 2-D carbon materials helps to extend the life of the capacitor, increasing its reliability and durability.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm2 V−1 s−1). Graphene possesses a number of inherent chemical and physical properties, including high mechanical strength (1 TPa), excellent mass and heat transfer capability, extremely high light transmittance (97%), and a large SSA (2675 m2 g−1).
000 cm2 V−1 s−1). Graphene possesses a number of inherent chemical and physical properties, including high mechanical strength (1 TPa), excellent mass and heat transfer capability, extremely high light transmittance (97%), and a large SSA (2675 m2 g−1).
Recently, Dou et al. have found that it is currently challenging to create cathode materials that are compatible with Zn-ion hybrid capacitors due to the poor knowledge of charge storage behavior,88 as shown in Fig. 8. However, the bulk of previous research has concentrated on understanding the influence of oxygen-containing groups without taking the graphitic structure into account. The surface properties of reduced graphene oxide (rGO) nanosheets are used to optimize their charge storage capacity and electrochemical kinetics. In addition to the contribution of oxygen-containing groups, the reversible adsorption/desorption of H+ on carbon atoms of rGO sheets has been shown to contribute. Electrochemical studies and density functional theory calculations show that H+ causes cloud rupture in the aromatic domain, as well as graphitic structural distortion/restoration and C sp2–sp3 re-hybridization. The rGO thermally treated at 200 °C achieves the optimum electrochemical performance with a specific capacitance of 245 F g−1 at 0.5 A g−1 and a retention of 53% at 20 A g−1. The mentioned study has pushed the frontiers of proton adsorption chemistry and provided additional information on the development of novel electrode materials.
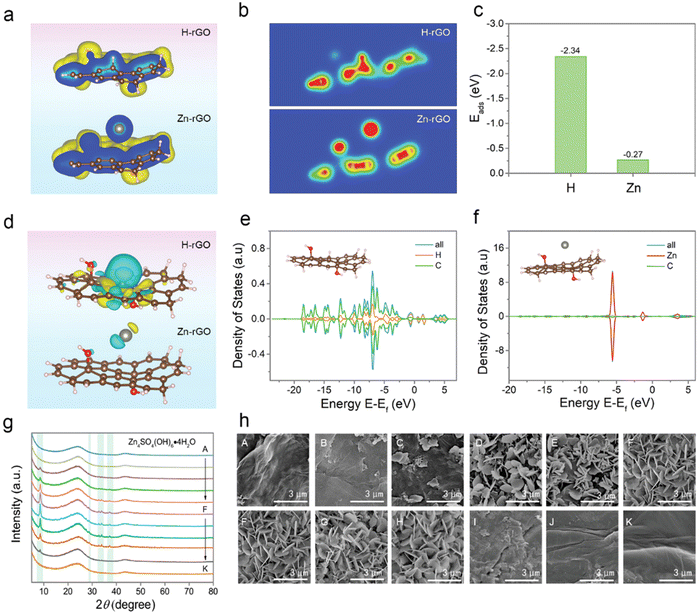 | ||
| Fig. 8 (a) Structural energy, (b) charge distribution and (c) calculated adsorption energy, (d) optimized charge-density-difference patterns, and (e and f) density of the states of rGO with the H+ and Zn2+ adsorption.88 Reused with permission from Wiley. | ||
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C bond may enhance the chemical characteristics of carbon compounds when stimulated externally (light, magnet, and electricity).
C bond may enhance the chemical characteristics of carbon compounds when stimulated externally (light, magnet, and electricity).
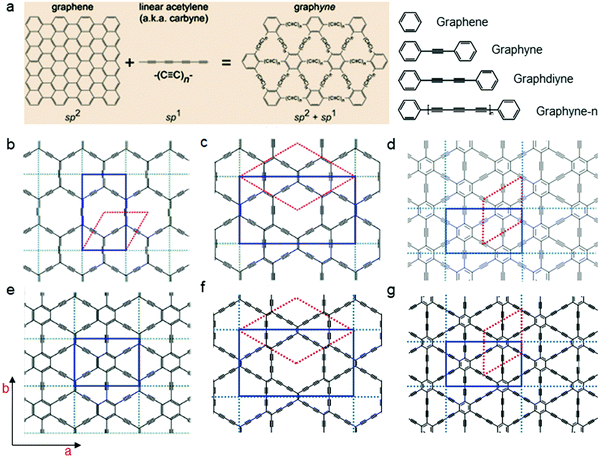 | ||
| Fig. 9 Chemical structures and nomenclature. (a) Structure schematics of graphene and graphynes. (b) α-graphyne; (c) β-graphyne; (d) γ-graphyne; (e) 6,6,12-graphyne; (f) β-graphdiyne; and (g) GDY.89 Reused with permission from Royal Society of Chemistry. | ||
Additionally, enriched-conjugated systems exhibit excellent theoretical conductivity and charge transfer rates. Due to these qualities, GDY has the potential to significantly enhance the manufacture of high-performance electrical products. The processes used to synthesize GDY are classified as dry or wet methods.90–92 Li et al. utilized a 2D all-carbon GDY with superior 2D strength and strong mixed conductivities for both electrons and ions to shield multidimensional nickel cobalt oxide nanostructures and got an electrode for hybrid supercapacitors. The in situ formed GDY forms 3D interpenetrating networks with nanostructures, resulting in a considerable increase in conductivity and prevention of structural deterioration. The built hybrid asymmetric supercapacitor exhibited an excellent capacitance of 200.9 F g−1 at 1 A g−1, with an energy density of 62.8 W h kg−1 and a power density of 747.9 W kg−1. In a long-term 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000-cycle test at a current density of 20 A g−1, the capacity retention can reach 97.7%.
000-cycle test at a current density of 20 A g−1, the capacity retention can reach 97.7%.
4.4. Three-dimensional carbon materials
Increased dimensionality means that a greater proportion of the active surface is in contact with the electrolyte, which significantly improves the electrochemical characteristics of electrode materials.93 A three-dimensional (3-D) structure with well-connected holes, from this vantage point, not only provides continuous channels for optimal electrolyte contact, but also speeds up the charge transfer by minimizing diffusion paths.94 3-D carbon materials garnered significant interest for their use in hybrid capacitors due to several unique properties. First, 3-D carbon materials possess high surface area and conductivity, making them ideal for use as electrodes. The high surface area provides ample room for electrochemical reactions to occur, resulting in a higher capacitance compared to 2-D electrodes. The high conductivity of 3-D carbon materials ensures that the flow of charge is fast and efficient, reducing the internal resistance and improving the power density of the hybrid capacitors. Second, 3-D carbon materials are flexible and lightweight, making them suitable for use in energy storage systems. Their mechanical stability and durability also make them ideal for use in demanding applications where high-performance energy storage is required. Third, 3-D carbon materials have a high chemical stability and corrosion resistance, making them highly resistant to degradation in aqueous and non-aqueous electrolytes. This property is particularly important in hybrid capacitors, where the electrode materials are subjected to repeated charge–discharge cycles and electrochemical reactions. The high chemical stability of 3-D carbon materials helps to extend the life of the capacitor, increasing its reliability and durability. Fourth, 3-D carbon materials are environmentally friendly, as they are made from renewable resources and do not contain harmful or toxic chemicals. This property makes them suitable for use in environmentally sensitive applications, such as in the automotive and aerospace industries, where the use of hazardous materials is strictly regulated.Typically, 3-D carbon materials are generated using CVD, hydrothermal, or template methods. Due to sustainable development requirements, researchers have adopted some bio-residue-derived biochars for 3-D carbon material construction. Milica et al. suggested using a porous activated carbon produced from biochar to obtain Al-ion supercapacitors. As shown in Fig. 10, carbonized vine shoots (VS) were tested as electrode material for supercapacitors with aqueous electrolyte. Biochar prepared by pre-carbonization of VS (at 300 °C) was impregnated with ZnCl2 at 600–700 °C to produce carbon with large micropore and mesopore volumes and a large SSA of close to 1500 m2 g−1. A high specific capacitance was achieved in an Al-based electrolyte, allowing a working voltage of 1.8 V and delivering an energy density of 24 W h kg−1 at 1 A g−1.95
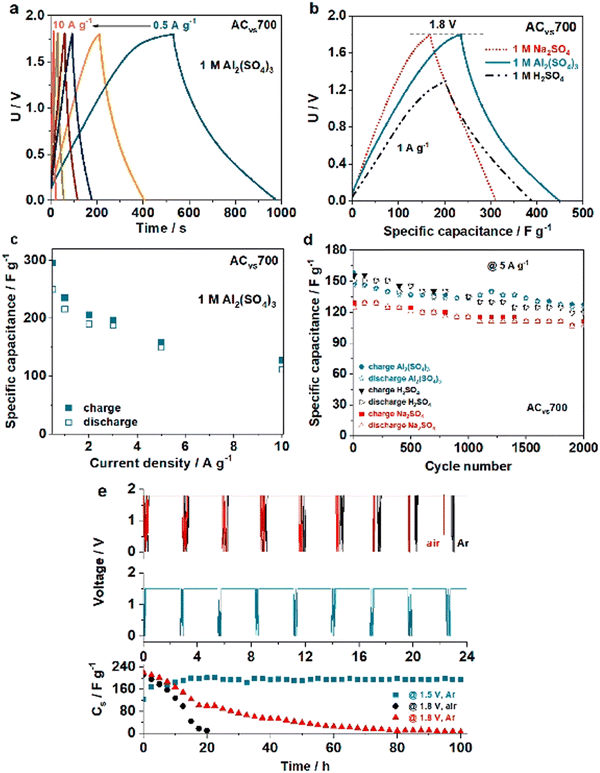 | ||
| Fig. 10 (a) Charge/discharge curves at different current rates of Al2(SO4)3 electrolyte in a VS-based capacitor. (b) Na2SO4 and H2SO4 as comparison. (c) Specific capacitance, (d) number of cycles (e) floating test at 1.8 V of Al2(SO4)3 electrolyte in a VS-based capacitor in comparison with Na2SO4 and H2SO4.95 Reused with permission from Elsevier. | ||
4.5. Carbon-based cathode design
The mechanism of adsorption/desorption or embedding/de-embedding of multivalent metal cations on carbon-based electrodes relies on the study of the solvation properties of various metals, mostly thought to correspond to the ion diameter. Understanding the solvation environment of each metal cation system and the energy required for desolvation may facilitate the study of carbon-based cathodes. Multivalent cations have a high charge density and strong interaction with the electrolyte and cathode material, making dissociation and solid-state diffusion kinetically unfavorable, which is one of the biggest challenges in developing multivalent metal cation batteries and other energy storage devices.96 Typically, researchers look to low current densities and high temperatures to promote the embedding of metal cations, but under these conditions, side reactions tend to contribute more to capacity and are detrimental to cycling stability. In recent studies, proton embedding has been the dominant reaction in many multivalent cationic energy storage devices, largely due to the aqueous electrolyte being employed.97 In fact, however, protons can still be generated by solvent decomposition even in anhydrous systems. Cathode design usually takes the storage of solvated molecules or complex ions (e.g., MgCl+, AlCl4−, etc. in chlorine-containing systems) into consideration rather than the bare metal cations after stripping.96 For example, much of the work in ZHC research has focused on studying aqueous solutions, such as ZnSO4 or Zn(CF3SO4)2, as electrolyte systems. The presence of zinc salts in the electrolyte system inevitably produces zinc ions and solvated Zn([Zn(H2O)6]2+) ions. Their diameters differ greatly between 1.48 and 8.60 Å, respectively, and this increased size limits their embedding in the micro-pores of the carbon material, thus limiting the capacity.98The weak bonding of multivalent metal cations to the surface of the carbon material is also a limitation for the capacity of MIHCs, especially for Mg2+. William et al. illustrated through the energy band structure that when Mg2+ are adsorbed onto graphene, the Fermi energy level remains at the Dirac point and thus no charge transfer occurs.99 Adjusting the layer spacing of the carbon material to adjust the Fermi energy level is a possible strategy, which can be inspired by the design of monovalent cationic capacitors.100 These challenges and realities have led researchers to focus on the design of cathode structures. It has been shown that the insertion of surface functional groups, heteroatom doping, and the introduction of surface defects are key methods for enhancing the electrochemical characteristics of carbon-based materials. Moreover, researchers have expended effort in optimizing the SSA and pore size distribution of carbon-based electrode materials. Nevertheless, the link between the pore characteristics of carbon materials and their energy storage capacity is ambiguous and needs further experimental and theoretical support.
The physical adsorption/desorption of ions on the surface of carbon materials is thought to be strongly influenced by the SSA. Kang et al. found that when activated carbon particles with an SSA of 142 m−2 g−1 were used as a cathode, the discharge capacity of the assembled ZHCs was only 9 mA h g−1 at a current density of 0.1 A g−1; when a carbon black particle with a higher SSA of 1408 m−2 g−1 was used as the cathode material, it achieved a discharge capacity of 78 mA h g−1 at 0.1 A g−1.101 Notably, the cathode is involved in a series of side reactions in addition to the physical adsorption/desorption of cations. In the case of ZHCs, for example, the deposition/dissolution of Zn4SO4(OH)6–5H2O is involved in the cathode reaction. This deposition/dissolution process is irreversible and may lead to a rise in pH throughout the charge/discharge cycle of the ZHC, resulting in the creation of zinc oxide and the development of undesirable Zn dendrites.
Some researchers have argued that the SSA is not the only factor that affects MIHCs capacity. First, not all micropores on the electrode material are accessible to the electrolyte ions. A cathode material design that considers both a suitable pore size distribution and a high specific surface area is an effective strategy to increase the capacity of MIHCs. An ideal pore structure should have a hierarchical structure with macropores (>50 nm) for electrolyte penetration, mesopores (2–50 nm) for ion transport, and micropores (2 nm) for charge storage.102 Ion migration in micropores is proportional to the size of the solvated molecules and the diameter of the pore.103 In other words, ions have trouble crossing the energy barrier and entering pores when the size of the solvent molecules and solvated ions is larger than the size of the pores. Thus, although increasing the number of micropores increases the SSA, this is not necessarily accompanied by an increase in capacitance. Due to their pore size, mesoporous materials facilitate the fast transport of ions, hence improving electrochemical characteristics.104 Additionally, a porous structure with a restricted distribution might shorten the ion transit length, improving the electrode kinetics.105
5. Conclusion and outlook
Compared to conventional metal ion batteries and supercapacitors, MIHCs offer high energy density and high-power density, ultra-long cycle life, safety, and more available polyvalent metal resources, making them very competitive for use in the energy storage industry. To fulfill the urgent need for high-performance energy storage and conversion, it is desired to develop innovative electrode materials for MIHCs. In particular, cathode development remains a major general challenge. As the multivalent metal cation energy storage technologies of interest have covered zinc, magnesium, calcium, aluminum, etc., new members will be validated by drawing on the test procedures of mature energy storage technologies, such as monovalent ion supercapacitors, etc. It is worth noting that various research groups have invested efforts in improving the overall performance of MIHCs with the help of carbon nanomaterials of different dimensions (0, 1, 2, and 3) and asymmetrically designed pseudocapacitive materials, which has improved the specific capacitance of MIHCs devices. In this review, we comprehensively summarise the recent progress of multivalent cation hybrid capacitors and carbon-based electrodes, and from the existing representative results and comparisons, we draw the challenges that still need to be solved and the scope of future work.(1) More battery-type electrode active materials should be developed, especially “beyond metal” type electrodes, and their reaction mechanisms should be studied in more detail. Due to the constraint of electrode materials, the majority of studies focus on ZHCs, whereas MHCs, CHCs, and AHCs get relatively little attention. The “beyond metal” material is crucial to the development of the anodes for these three hybrid capacitors, allowing for more stable cycling and increased efficiency. The future development of MIHCs anodes will focus on making them lightweight, highly stable, and cost-efficient. Moreover, the electrochemical reaction mechanism of multivalent cations in MIHCs electrodes remains obscure. In contrast to monovalent cations, the ionic diameter, charge number, reactivity, ionic diffusion coefficient, and binding energy between ions and active materials of multivalent cations result in a distinct insertion–extraction kinetic energy storage mechanism in battery-type electrodes. The complexity of energy storage research is increasing. Other energy storage processes should exist in battery-type electrodes of MIHCs than ion adsorption/desorption, necessitating the adoption of more efficient and improved material characterization methodologies and electrochemical tests. With the advancement of technology, in situ infrared spectroscopy, in situ X-ray diffraction, and X-ray photoelectron spectroscopy may be used to monitor the surface groups, phases, and chemical states of electrodes in different charge–discharge states.
(2) Optimizing the structure of carbon-based electrode materials is an area that needs further attention. The cycling behaviour and power density of electrodes for all pseudocapacitive energy storage techniques must be enhanced since their performance is limited by the slow redox kinetics associated with intrinsic ion/electron transport. It is necessary to expand the potential window of carbon-based electrode materials in order to increase their working potential and energy density. Controlling the porosity of carbon-based materials is another crucial area of study. The optimal pore shape and size, as well as the relationship between carbonization precursors and carbonization procedures should be examined, in order to discover the optimum kinetics of carbon-based materials in the cycling behaviour of MIHCs. 3-D carbon compounds are structurally more appropriate for use as electrodes. Considering the recent research progress and the properties of carbon materials, the 3-D structure is more suitable for MIHC energy storage than 0-D, 1-D, and 2-D.
(3) Developing ecologically responsible, low-cost, and energy-efficient industrial manufacturing techniques for carbon nanomaterials. One obstacle preventing the industrialization of carbon-based materials is the high cost and high energy consumption of the carbonization and activation process. Therefore, it is vital to establish new manufacturing methods that are more streamlined. Notably, despite the fact that fine carbon material structures have been validated in the laboratory and have shown outstanding electrochemical performance, obstacles still exist in the synthesis of carbon nanomaterials on an industrial scale. Due to the mismatch between industrial test standards and laboratory test standards, a gap arises between scientific study and practical application that can only be bridged by the adoption of unified test standards. Understanding the compatibility of carbon materials with the development of electrode coating processes is essential, and special configurations of MIHCs can be achieved using screen printing, 3-D printing and other methods.
(4) The commercialization of carbon materials for use in MIHCs has been driven by the growing demand for high-power energy storage devices in a variety of industries, including consumer electronics, renewable energy, and electric vehicles. In recent years, the production of carbon-based materials for hybrid capacitors has become more industrialized, with companies investing in large-scale production facilities and expanding their product offerings to meet the growing demand. Various energy storage technologies using metal cations as charge carriers are at different industrial stages. Japanese companies have succesfully applied lithium-ion energy storage, sodium-ion energy storage and magnesium-ion energy storage technologies to industrial production, while Chinese companies are making efforts to commercialize sodium-ion energy storage technologies to a higher degree and the market size of sodium-ion energy storage is predicted to be over USD 6 billion in China by 2025. Dutch companies’ mastery of aluminum ion energy storage technology is impressive, while calcium ion energy storage technology has been the slowest to develop in recent years. In the future, the commercialization of energy storage technology should be the focus of the development of enterprises in various countries. Despite the rapid growth and industrialization of carbon materials in hybrid capacitors, there are still some challenges that must be overcome to fully realize the potential of these materials. These include issues related to increasing mass loading in electrodes, scaling up production, improving performance, and reducing cost. Nevertheless, the continuous development and commercialization of carbon materials for hybrid capacitors has the potential to be greatly improved in the next decade.
Author contributions
X. G. and G. H. conceived the project; C. J. C., I. P. P. and G. H. supervised the students; and all authors contributed to writing and revising the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The work was supported by Dean's prize-China Scholarship Council and Engineering and Physical Sciences Research Council (EPRSC; EP/V027433/1; EP/Y008707/1 and EP/V027433/2).References
- W. Zhang, Y. Wu, Z. Xu, H. Li, M. Xu, J. Li, Y. Dai, W. Zong, R. Chen and L. He, Adv. Energy Mater., 2022, 12, 2201065 CrossRef CAS.
- W. Zuo, R. Li, C. Zhou, Y. Li, J. Xia and J. Liu, Adv. Sci., 2017, 4, 1600539 CrossRef PubMed.
- G. Z. Chen, Int. Mater. Rev., 2017, 62, 173–202 CrossRef CAS.
- A. Riaz, M. R. Sarker, M. H. M. Saad and R. Mohamed, Sensors, 2021, 21, 5041 CrossRef CAS PubMed.
- X. Gao, X. Sun, J. Liu, N. Gao and H. Li, J. Energy Storage, 2019, 25, 100901 CrossRef.
- X. Sun, J. Gao, C. Wang, X. Gao, J. Liu, N. Gao, H. Li, Y. Wang and K. Yu, J. Chem. Eng., 2020, 383, 123198 CrossRef CAS.
- R. Chen, H. Ling, Q. Huang, Y. Yang and X. Wang, Small, 2022, 18, 2106356 CrossRef CAS PubMed.
- H. Xu and M. Shen, Int. J. Energy Res., 2021, 45, 20524–20544 CrossRef.
- R. Thangavel, B. Moorthy, D. K. Kim and Y. S. Lee, Adv. Energy Mater., 2017, 7, 1602654 CrossRef.
- H. Li, W. Zhang, K. Sun, J. Guo, K. Yuan, J. Fu, T. Zhang, X. Zhang, H. Long and Z. Zhang, Adv. Energy Mater., 2021, 11, 2100867 CrossRef CAS.
- S. Nagamuthu, Y. Zhang, Y. Xu, J. Sun, F. Uz Zaman, D. K. Denis, L. Hou and C. Yuan, J. Mater. Chem. A, 2021, 10, 357–378 RSC.
- A. D. Jagadale, R. C. Rohit, S. K. Shinde and D. Y. Kim, ChemNanoMat, 2021, 7, 1082–1098 CrossRef CAS.
- S. J. Patil, N. R. Chodankar, S.-K. Hwang, G. S. R. Raju, K. S. Ranjith, Y. S. Huh and Y.-K. Han, Energy Storage Mater., 2022, 45, 1040–1051 CrossRef.
- X. Gao, C. Zhang, Y. Dai, S. Zhao, X. Hu, F. Zhao, W. Zhang, R. Chen, W. Zong and Z. Du, Small Struct., 2022, 2200316 Search PubMed.
- X. Gao, X. Sun, Z. Jiang, Q. Wang, N. Gao, H. Li, H. Zhang, K. Yu and C. Su, New J. Chem., 2019, 43, 3907–3912 RSC.
- L. Han, J. Li, X. Zhang, H. Huang, Z. Yang, G. Zhu, M. Xu and L. Pan, ACS Sustainable Chem. Eng., 2021, 9, 9165–9176 CrossRef CAS.
- S. Wu, Y. Chen, T. Jiao, J. Zhou, J. Cheng, B. Liu, S. Yang, K. Zhang and W. Zhang, Adv. Energy Mater., 2019, 9, 1902915 CrossRef CAS.
- X. Qiu, N. Wang, Z. Wang, F. Wang and Y. Wang, Angew. Chem., Int. Ed., 2021, 60, 9610–9617 CrossRef CAS PubMed.
- P. Meister, V. Küpers, M. Kolek, J. Kasnatscheew, S. Pohlmann, M. Winter and T. Placke, Batteries Supercaps, 2021, 4, 504–512 CrossRef CAS.
- L. Zhang, Z. Liu, G. Wang, J. Feng and Q. Ma, Nanoscale, 2021, 13, 17068–17076 RSC.
- T. Mandai, ACS Appl. Mater. Interfaces, 2020, 12, 39135–39144 CrossRef CAS PubMed.
- G. Pan, J. Li, L. Han, W. Peng, X. Xu, T. Lu, M. A. Amin, Y. Yamauchi, M. Xu and L. Pan, Inorg. Chem. Front., 2022, 9, 1666–1673 RSC.
- N. Wu, W. Yao, X. Song, G. Zhang, B. Chen, J. Yang and Y. Tang, Adv. Energy Mater., 2019, 9, 1803865 CrossRef.
- H. Ma, H. Chen, Y. Hu, B. Yang, J. Feng, Y. Xu, Y. Sun, H. Cheng, C. Li and X. Yan, Energy Environ. Sci., 2022, 15, 1131–1143 RSC.
- S. He, Z. Mo, C. Shuai, W. Liu, R. Yue, G. Liu, H. Pei, Y. Chen, N. Liu and R. Guo, Appl. Surf. Sci., 2022, 577, 151904 CrossRef CAS.
- Z. Li, D. Guo, D. Wang, M. Sun and H. Sun, J. Power Sources, 2021, 506, 230197 CrossRef CAS.
- X. Ma, J. Wang, X. Wang, L. Zhao and C. Xu, J. Mater. Sci.: Mater. Electron., 2019, 30, 5478–5486 CrossRef CAS.
- B. D. Boruah, B. Wen, S. Nagane, X. Zhang, S. D. Stranks, A. Boies and M. De Volder, ACS Energy Lett., 2020, 5, 3132–3139 CrossRef CAS.
- S. Wang, Q. Wang, W. Zeng, M. Wang, L. Ruan and Y. Ma, Nano-Micro Lett., 2019, 11, 1–12 CrossRef CAS PubMed.
- R. Cui, Z. Zhang, H. Zhang, Z. Tang, Y. Xue and G. Yang, Nanomaterials, 2022, 12, 386 CrossRef CAS PubMed.
- J. Shi, S. Wang, Q. Wang, X. Chen, X. Du, M. Wang, Y. Zhao, C. Dong, L. Ruan and W. Zeng, J. Power Sources, 2020, 446, 227345 CrossRef CAS.
- X. Li, M. Li, Q. Yang, H. Li, H. Xu, Z. Chai, K. Chen, Z. Liu, Z. Tang and L. Ma, ACS Nano, 2020, 14, 541–551 CrossRef CAS PubMed.
- J. Cao, D. Zhang, X. Zhang, S. Wang, J. Han, Y. Zhao, Y. Huang and J. Qin, Appl. Surf. Sci., 2020, 534, 147630 CrossRef CAS.
- L. Xu, G. Pan, J. Wang, J. Li, Z. Gong, T. Lu and L. Pan, Sustainable Energy Fuels, 2022, 6, 5290–5299 RSC.
- G. Pan, J. Li, L. Han, W. Peng, X. Xu, T. Lu, M. A. Amin, Y. Yamauchi, M. Xu and L. Pan, Inorg. Chem. Front., 2022, 9, 1666–1673 RSC.
- Y. Ding, S. Zhang, J. Li, Y. Sun, B. Yin, H. Li, Y. Ma, Z. Wang, H. Ge and D. Su, Adv. Funct. Mater., 2022, 2210519 Search PubMed.
- H. Zhang, K. Ye, K. Zhu, R. Cang, X. Wang, G. Wang and D. Cao, ACS Sustainable Chem. Eng., 2017, 5, 6727–6735 CrossRef CAS.
- Z. Li, K. Xiang, W. Xing, W. C. Carter and Y. M. Chiang, Adv. Energy Mater., 2015, 5, 1401410 CrossRef.
- M. Tian, R. Li, C. Liu, D. Long and G. Cao, ACS Appl. Mater. Interfaces, 2019, 11, 15573–15580 CrossRef CAS PubMed.
- J. Yang, M. A. Bissett and R. A. Dryfe, ChemSusChem, 2021, 14, 1700–1709 CrossRef CAS PubMed.
- J. Wu, R. Liu, M. Li, X. Luo, W. Lai, X. Zhang, F. Yu and Y. Chen, J. Energy Storage, 2022, 48, 103996 CrossRef.
- X. Zhang, X. Tian, Y. Song, J. Wu, T. Yang and Z. Liu, Fuel, 2022, 310, 122485 CrossRef CAS.
- S. Moon, S. M. Lee, H. K. Lim, H. J. Jin and Y. S. Yun, Adv. Energy Mater., 2021, 11, 2101054 CrossRef CAS.
- X. F. Ma, H. Y. Li, X. Zhu, W. Ren, X. Zhang, J. Diao, B. Xie, G. Huang, J. Wang and F. Pan, Small, 2022, 18, 2202250 CrossRef CAS PubMed.
- Y. I. Kim, B. Kim, J. Baek, J.-H. Kim and J. Yoo, J. Electrochem. Soc., 2022, 169, 120521 CrossRef.
- W. Han, G. Liu, W. Seo, H. Lee, H. Chu and W. Yang, Carbon, 2021, 184, 534–543 CrossRef CAS.
- X. Li, Y. Li, S. Xie, Y. Zhou, J. Rong and L. Dong, J. Chem. Eng., 2022, 427, 131799 CrossRef CAS.
- L. Dong, W. Yang, W. Yang, H. Tian, Y. Huang, X. Wang, C. Xu, C. Wang, F. Kang and G. Wang, J. Chem. Eng., 2020, 384, 123355 CrossRef CAS.
- H. Zhang, Z. Chen, Y. Zhang, Z. Ma, Y. Zhang, L. Bai and L. Sun, J. Mater. Chem. A, 2021, 9, 16565–16574 RSC.
- P. Shang, M. Liu, Y. Mei, Y. Liu, L. Wu, Y. Dong, Z. Zhao and J. Qiu, Small, 2022, 18, 2108057 CrossRef CAS PubMed.
- F. Wei, P. Xu, C. Xu, M. Han, S. Ran and Y. Lv, Solid State Ionics, 2022, 28, 1419–1426 CAS.
- H. He, J. Lian, C. Chen, Q. Xiong and M. Zhang, J. Chem. Eng., 2021, 421, 129786 CrossRef CAS.
- B. D. Boruah, A. Mathieson, B. Wen, C. Jo, F. Deschler and M. De Volder, Nano Lett., 2020, 20, 5967–5974 CrossRef CAS PubMed.
- P. A. Maughan, N. Tapia-Ruiz and N. Bimbo, Electrochim. Acta, 2020, 341, 136061 CrossRef CAS.
- X. Zhang, C. Chen, S. Gao, X. Luo, Y. Mo, B. Cao and Y. Chen, J. Energy Storage, 2021, 42, 103037 CrossRef.
- G. Lou, G. Pei, Y. Wu, Y. Lu, Y. Wu, X. Zhu, Y. Pang, Z. Shen, Q. Wu and S. Fu, J. Chem. Eng., 2021, 413, 127502 CrossRef CAS.
- P. Pattananuwat, R. Pornprasertsuk, J. Qin and S. Prasertkaew, RSC Adv., 2021, 11, 35205–35214 RSC.
- N. S. Shaikh, N. S. Padalkar, V. C. Lokhande, T. Ji, S. P. Patil, S. R. Sabale, H. M. Shaikh, J. S. Shaikh, S. Praserthdam and P. Kanjanaboos, Energy Fuels, 2022, 36, 7186–7193 CrossRef CAS.
- K. Shang, Y. Liu, P. Cai, K. Li and Z. Wen, J. Mater. Chem. A, 2022, 10, 6489–6498 RSC.
- J. N. Ramavath, M. Raja, K. Balakumar and R. Kothandaraman, J. Electrochem. Soc., 2021, 168, 010538 CrossRef CAS.
- G. Chen, Z. Hu, Z. Pan and D. Wang, J. Energy Storage, 2021, 38, 102534 CrossRef.
- X. Zhu, F. Guo, Q. Yang, H. Mi, C. Yang and J. Qiu, J. Power Sources, 2021, 506, 230224 CrossRef CAS.
- T. Xiong, Y. Shen, W. S. V. Lee and J. Xue, Nano Mater. Sci., 2020, 2, 159–163 CrossRef.
- X. Zhang, Y. Zhang, H. Zhang, Y. Zhang, Z. Ma and L. Sun, ChemistrySelect, 2021, 6, 6937–6943 CrossRef CAS.
- L. Yang, J. Li, Y. Zhou and J. Yao, J. Energy Storage, 2022, 50, 104252 CrossRef.
- H. Xu, W. He, Z. Li, J. Chi, J. Jiang, K. Huang, S. Li, G. Sun, H. Dou and X. Zhang, Adv. Funct. Mater., 2022, 32, 2111131 CrossRef CAS.
- L. Zhang, G. Wang, J. Feng, Q. Ma, Z. Liu and X. Yan, ChemElectroChem, 2021, 8, 1289–1297 CrossRef CAS.
- Y. Wang, L. Zhang, H. Hou, W. Xu, G. Duan, S. He, K. Liu and S. Jiang, J. Mater. Sci., 2021, 56, 173–200 CrossRef CAS.
- K. D. Benkstein, N. Kopidakis, J. Van de Lagemaat and A. J. Frank, J. Phys. Chem. B, 2003, 107, 7759–7767 CrossRef CAS.
- M. Sevilla and R. Mokaya, Energy Environ. Sci., 2014, 7, 1250–1280 RSC.
- W. Zhang, Y. Song, Y. Wang, S. He, L. Shang, R. Ma, L. Jia and H. Wang, J. Mater. Chem. B, 2020, 8, 3676–3682 RSC.
- L. Wei, M. Sevilla, A. B. Fuertes, R. Mokaya and G. Yushin, Adv. Funct. Mater., 2012, 22, 827–834 CrossRef CAS.
- V. V. Obreja, Phys. E, 2008, 40, 2596–2605 CrossRef CAS.
- Z. Peng, J. Guo, Q. He, S. Li, L. Tan and Y. Chen, Sci. China Mater., 2022, 65, 1–11 Search PubMed.
- M. Zhi, C. Xiang, J. Li, M. Li and N. Wu, Nanoscale, 2013, 5, 72–88 RSC.
- C. T. Kingston and B. Simard, Anal. Lett., 2003, 36, 3119–3145 CrossRef CAS.
- R. Peng, Y. Wang, W. Tang, Y. Yang and X. Xie, Polymers, 2013, 5, 847–872 CrossRef.
- T. Yan, Y. Wu, W. Yi and Z. Pan, Sens. Actuators, A, 2021, 327, 112755 CrossRef CAS.
- H. Zhang, G. Cao, Y. Yang and Z. Gu, J. Electrochem. Soc., 2007, 155, K19 CrossRef.
- D.-W. Jung, C.-S. Lee, S. Park and E.-S. Oh, Korean J. Met. Mater., 2011, 49, 419–424 CAS.
- K. H. An, K. K. Jeon, J. K. Heo, S. C. Lim, D. J. Bae and Y. H. Lee, J. Electrochem. Soc., 2002, 149, A1058 CrossRef CAS.
- M. Hughes, G. Z. Chen, M. S. Shaffer, D. J. Fray and A. H. Windle, Chem. Mater., 2002, 14, 1610–1613 CrossRef CAS.
- Y. Zhang, J. Sun, J. Tan, C. Ma, S. Luo, W. Li and S. Liu, Fuel, 2021, 305, 121622 CrossRef CAS.
- J. Tian, Y. Shi, W. Fan and T. Liu, Compos. Commun., 2019, 12, 21–25 CrossRef.
- S. Sharma, S. Basu, N. P. Shetti, K. Mondal, A. Sharma and T. M. Aminabhavi, ACS Sustainable Chem. Eng., 2022, 10, 1334–1360 CrossRef CAS.
- H. Aydın, U. Kurtan, M. Demir and S. Karakuş, Energy Fuels, 2022, 36(4), 2212–2219 CrossRef.
- H. He, J. Lian, C. Chen, Q. Xiong, C. C. Li and M. Zhang, Nano-Micro Lett., 2022, 14, 1–15 CrossRef PubMed.
- H. Xu, W. He, Z. Li, J. Chi, J. Jiang, K. Huang, S. Li, G. Sun, H. Dou and X. Zhang, Adv. Funct. Mater., 2022, 2111131 CrossRef CAS.
- X. Gao, H. Liu, D. Wang and J. Zhang, Chem. Soc. Rev., 2019, 48, 908–936 RSC.
- N. Yang, Y. Liu, H. Wen, Z. Tang, H. Zhao, Y. Li and D. Wang, ACS Nano, 2013, 7, 1504–1512 CrossRef CAS PubMed.
- X. Gao, J. Zhou, R. Du, Z. Xie, S. Deng, R. Liu, Z. Liu and J. Zhang, Adv. Mater., 2016, 28, 168–173 CrossRef CAS PubMed.
- N. Parvin, Q. Jin, Y. Wei, R. Yu, B. Zheng, L. Huang, Y. Zhang, L. Wang, H. Zhang and M. Gao, Adv. Mater., 2017, 29, 1606755 CrossRef PubMed.
- I. Hussain, S. Sahoo, M. S. Sayed, M. Ahmad, M. S. Javed, C. Lamiel, Y. Li, J.-J. Shim, X. Ma and K. Zhang, Coord. Chem. Rev., 2022, 458, 214429 CrossRef CAS.
- M. Yu, W. Qiu, F. Wang, T. Zhai, P. Fang, X. Lu and Y. Tong, J. Mater. Chem. A, 2015, 3, 15792–15823 RSC.
- A. Gezović, J. Mišurović, B. Milovanović, M. Etinski, J. Krstić, V. Grudić, R. Dominko, S. Mentus and M. J. Vujković, J. Power Sources, 2022, 538, 231561 CrossRef.
- Y. Liang, H. Dong, D. Aurbach and Y. Yao, Nat. Energy, 2020, 5, 646–656 CrossRef CAS.
- Z. Pan, X. Liu, J. Yang, X. Li, Z. Liu, X. J. Loh and J. Wang, Adv. Energy Mater., 2021, 11, 2100608 CrossRef CAS.
- N. T. Aristote, X. Deng, K. Zou, X. Gao, R. Momen, F. Li, W. Deng, H. Hou, G. Zou and X. Ji, J. Alloys Compd., 2022, 913, 165216 CrossRef CAS.
- Y. Liu, B. V. Merinov and W. A. Goddard III, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, 3735–3739 CrossRef CAS PubMed.
- P. Yu, W. Tang, F.-F. Wu, C. Zhang, H.-Y. Luo, H. Liu and Z.-G. Wang, Rare Met., 2020, 39, 1019–1033 CrossRef CAS.
- L. Dong, X. Ma, Y. Li, L. Zhao, W. Liu, J. Cheng, C. Xu, B. Li, Q.-H. Yang and F. Kang, Energy Storage Mater., 2018, 13, 96–102 CrossRef.
- K. Zhao, L. Zhao, W. Zhou, L. Rao, S. Wen, Y. Xiao, B. Cheng and S. Lei, J. Energy Storage, 2022, 52, 104910 CrossRef.
- H. Takamatsu, M. S. Khan, T. Araki, C. Urita, K. Urita and T. Ohba, Sustainable Energy Fuels, 2022, 6, 2001–2009 RSC.
- R. B. Ambade, H. Lee, K. H. Lee, H. Lee, G. K. Veerasubramani, Y.-B. Kim and T. H. Han, J. Chem. Eng., 2022, 436, 135041 CrossRef CAS.
- H. Huang, Y. Zhao, T. Cong, C. Li, N. Wen, X. Zuo, Y. Guo, H. Zhang, Z. Fan and L. Pan, Adv. Funct. Mater., 2022, 32, 2110777 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2023 |