Electrochemical electrophilic bromination/spirocyclization of N-benzyl-acrylamides to brominated 2-azaspiro[4.5]decanes†
Zhongyi
Zhang
ab,
Zhong-Wei
Hou
*a,
Hao
Chen
a,
Pinhua
Li
 b and
Lei
Wang
b and
Lei
Wang
 *abc
*abc
aAdvanced Research Institute and College of Pharmaceutical Sciences, Taizhou University, Jiaojiang, Zhejiang 318000, P. R. China. E-mail: zhongwei.hou@tzc.edu.cn; leiwang88@hotmail.com
bDepartment of Chemistry, Huaibei Normal University, Huaibei, Anhui 235000, P. R. China
cState Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Shanghai 200032, P. R. China
First published on 30th March 2023
Abstract
An electrochemical electrophilic bromination/spirocyclization of N-benzyl-acrylamides with 2-bromoethan-1-ol as the brominating reagent has been developed. The paired electrolysis employs low concentrations of bromine produced from both cathodic reduction and anodic oxidation as an electrophile, and realizes an H2O-involved electrophilic spirocyclization. A number of unexplored brominated 2-azaspiro[4.5]decanes are obtained with satisfactory yields under mild conditions, and the reaction exhibits good efficiency at the gram-scale synthesis. In addition, this approach is further highlighted by late-stage transformations and synthetic applications in constructing cyclohepta[c]pyrrole-1,6-diones via debromination tandem cyclization of brominated 2-azaspiro[4.5]decanes.
Introduction
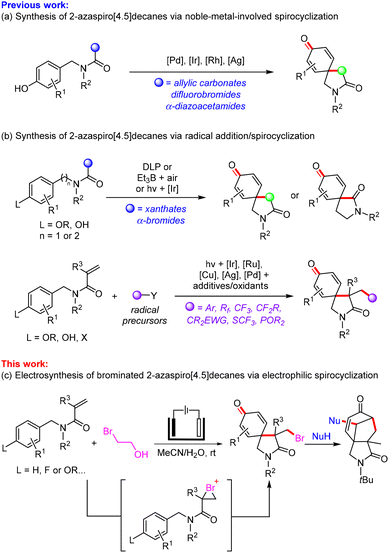
Spiro compounds are common scaffolds in natural products and bioactive molecules, and also can be used as pivotal synthetic intermediates in organic synthesis.1 Among them, azaspiro compounds have attracted much attention as unique building blocks.2 In recent years, noble-metal-involved spirocyclization of phenol derivatives containing allylic carbonates, difluorobromides or α-diazoacetamides is an efficient route to 2-azaspiro[4.5]decanes (Scheme 1a).3 The intramolecular radical dearomatizing spirocyclization could be accomplished under dilauroyl peroxide-mediated, Et3B/air-mediated or visible-light-induced iridium-catalysis conditions (Scheme 1b, top).4 In addition, variously functionalized 2-azaspiro[4.5]decanes were also synthesized through photo-catalyzed and metal-mediated intermolecular radical addition/spirocyclization of N-benzyl acrylamides (Scheme 1b, bottom).5 However, these reported methods often require the use of extra-oxidants and metal reagents, and anisoles or phenols as activated substrates. Moreover, hypervalent iodine-mediated intramolecular spirocyclization reactions have been explored by Zhang, Nevado, Chabaud and Guillou, providing a straightforward way to construct 2-azaspiro[4.5]decanes.6Organic electrochemistry realizes redox reactions through anodic oxidation and cathodic reduction without the need for traditional chemical reagents.7 Due to its safety, controllability and environmental friendliness, organic electrosynthesis is widely applied in organic chemistry.8 Recently, electrochemical dearomatizing spirocyclization could be achieved for the synthesis of spiro compounds under oxidant-free conditions.9 Guo and coworkers described an electrochemical selenation/spirocyclization of N-aryl alkynamides to give selenated 1-azaspiro[4.5]trienones.10 Chen and Xu developed an electrochemical oxidative halospirocyclization of N-aryl alkynamides to synthesize 1-azaspiro[4.5]trienones.11 However, a metal-free and oxidant-free method for the electrophilic functionalized spirocyclization of alkenes is still lacking.12 Using 1,3-dibromo-5,5-dimethylhydantoin (DBDMH) as an electrophilic reagent, You and co-workers reported electrophilic bromination initiated spirocyclization at −20 °C for the preparation of azaspiro[5.5]decanes.13
Based on the above progress and our previous reports on electrochemical bromination of (hetero)arenes,14 we herein developed a metal- and oxidant-free approach for the electrochemical electrophilic bromination/spirocyclization of N-benzyl acrylamides with 2-bromoethan-1-ol as the brominating reagent for the synthesis of unexplored brominated 2-azaspiro[4.5]decanes, which could react with nucleophilic reagents to form cyclohepta[c]pyrrole-1,6-diones via a debromination tandem cyclization under simple basic conditions (Scheme 1c).
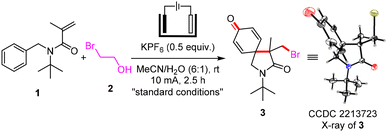
At the beginning of the study, the electrolysis reaction conditions of N-benzyl-N-(tert-butyl)methacrylamide (1) with 2-bromoethan-1-ol (2) were screened. The electrochemical electrophilic bromination/spirocyclization was performed in a simple undivided cell with reticulated vitreous carbon (RVC) as the anode and a platinum plate as the cathode (Table 1). 4-(Bromomethyl)-2-(tert-butyl)-4-methyl-2-azaspiro[4.5]deca-6,9-diene-3,8-dione (3) was obtained in 71% isolated yield in a solution of KPF6 in MeCN/H2O (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at 10 mA and room temperature for 2.5 h (Table 1, entry 1). The structure of 3 was determined by X-ray single crystal diffraction analysis (see the ESI for details†). Various bromides, such as BnBr (entry 2), CH2BrCN (entry 3), CH2BrNO2 (entry 4), CH2Br2 (entry 5), CBr4 (entry 6) and CHBr(COOEt)2 (entry 7), were investigated as the source of bromide under simple conditions, and 26–66% yields of 3 were obtained. On reducing the amount of 2-bromoethan-1-ol (2) to 2.0 equivalents, brominated 2-azaspiro[4.5]decane (3) was generated in 64% yield (entry 8). Applying other supporting electrolytes including LiClO4 (entry 9), Me4NBF4 (entry 10), nBu4NBF4 (entry 11) and nBu4NClO4 (entry 12) did not afford better results. The proportion of water in the mixed solvent has a great influence on the reaction efficiency (entries 13 and 14). We did not find the generation of product 3 under electricity-free conditions (entry 15). The electrochemical electrophilic bromination/spirocyclization occurred smoothly under a nitrogen atmosphere, providing the desired product 3 in 70% yield (entry 16).
1) at 10 mA and room temperature for 2.5 h (Table 1, entry 1). The structure of 3 was determined by X-ray single crystal diffraction analysis (see the ESI for details†). Various bromides, such as BnBr (entry 2), CH2BrCN (entry 3), CH2BrNO2 (entry 4), CH2Br2 (entry 5), CBr4 (entry 6) and CHBr(COOEt)2 (entry 7), were investigated as the source of bromide under simple conditions, and 26–66% yields of 3 were obtained. On reducing the amount of 2-bromoethan-1-ol (2) to 2.0 equivalents, brominated 2-azaspiro[4.5]decane (3) was generated in 64% yield (entry 8). Applying other supporting electrolytes including LiClO4 (entry 9), Me4NBF4 (entry 10), nBu4NBF4 (entry 11) and nBu4NClO4 (entry 12) did not afford better results. The proportion of water in the mixed solvent has a great influence on the reaction efficiency (entries 13 and 14). We did not find the generation of product 3 under electricity-free conditions (entry 15). The electrochemical electrophilic bromination/spirocyclization occurred smoothly under a nitrogen atmosphere, providing the desired product 3 in 70% yield (entry 16).
| Entry | Variation from the standard conditions | Yieldb (%) |
|---|---|---|
| a Reaction conditions: RVC anode, Pt cathode, undivided cell, 1 (0.20 mmol), 2-bromoethan-1-ol (2, 0.60 mmol), KPF6 (0.10 mmol), MeCN/H2O (6.0 mL/1.0 mL), rt, 10 mA, 2.5 h (4.6 F mol−1). b Yield determined by the 1H NMR analysis of the crude reaction mixture using 1,3,5-trimethoxybenzene as the internal standard. c Isolated yield. | ||
| 1 | None | 73 (71)c |
| 2 | BnBr instead of 2 | 40 |
| 3 | CH2BrCN instead of 2 | 66 |
| 4 | CH2BrNO2 instead of 2 | 55 |
| 5 | CH2Br2 instead of 2 | 56 |
| 6 | CBr4 instead of 2 | 39 |
| 7 | CHBr(COOEt)2 instead of 2 | 26 |
| 8 | 2 (2.0 equiv.) | 64 |
| 9 | LiClO4 (0.5 equiv.) as electrolyte | 58 |
| 10 | Me4NBF4 (0.5 equiv.) as electrolyte | 39 |
| 11 | n Bu4NBF4 (0.5 equiv.) as electrolyte | 68 |
| 12 | n Bu4NClO4 (0.5 equiv.) as electrolyte | 48 |
| 13 | MeCN/H2O (13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
65 |
| 14 | MeCN/H2O (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
17 |
| 15 | No electricity | 0 |
| 16 | With N2 | 70 |
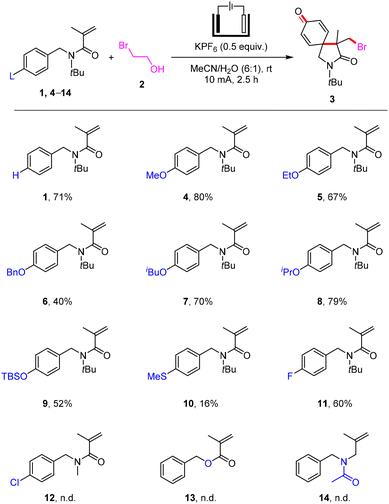
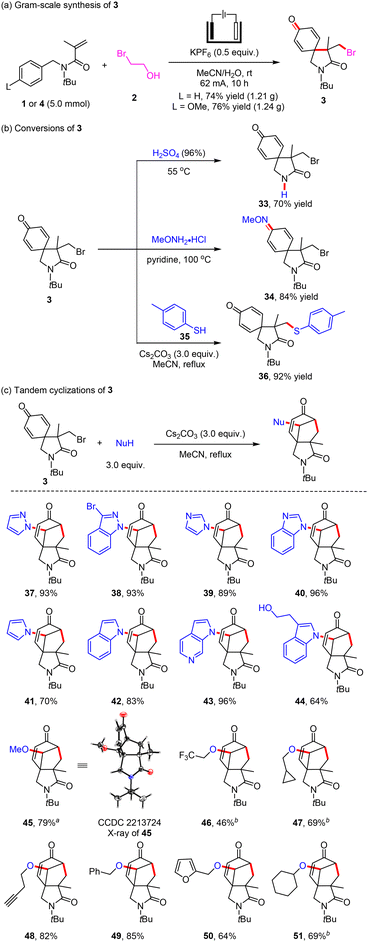
After determining the conditions, we first explored the scope of para-substituents on the benzyl moiety for the electrochemical bromination/spirocyclization (Scheme 2). N-Benzyl- (1, 71% yield), N-(4-alkoxybenzyl)- (4–8, 40–80% yields) and N-(4-((tert-butyldimethylsilyl)oxy)benzyl)-acrylamide (9, 52% yield) all could successfully react with 2-bromoethan-1-ol (2) to give brominated 2-azaspiro[4.5]decane (3) in accepted yields. The formation of 3 was inhibited when using N-(4-(methylthio)benzyl)acrylamide (10, 16% yield) as the substrate. N-(4-Fluorobenzyl)acrylamide (11) could also be applied to this paired electrolysis reaction, providing the corresponding product 3 in 60% isolated yield. Regrettably, the electrochemical electrophilic bromination/spirocyclization was unsuccessful when employing N-(4-chlorobenzyl)acrylamide (12), benzyl methacrylate (13) and N-benzyl-N-(2-methylallyl)acetamide (14) as substrates.
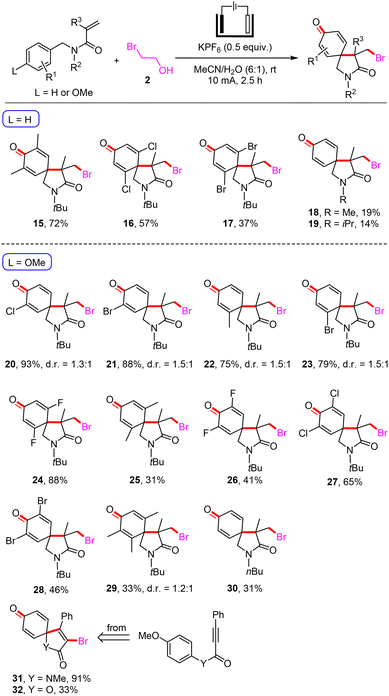
We then investigated the compatibility of the electrochemical electrophilic bromination/spirocyclization by electrolyzing N-(4-unsubstituted-benzyl)-acrylamides and 2 with H2O as the oxygen source under metal-free and oxidant-free conditions (Scheme 3). Substrates with 3,5-dimethyl, 2,6-dichloro and 2,6-dibromo groups on the benzene rings were well tolerated, and the corresponding brominated 2-azaspiro[4.5]decanes (15–17) were obtained in 37–72% yields. When the tert-butyl group on the nitrogen-atom was replaced with the methyl (18, 19% yield) or iso-propyl (19, 14% yield) group, the reaction efficiency greatly reduced. The linking group on the nitrogen atom has a great influence on this electrophilic spirocyclization reaction, which may be due to the obvious steric hindrance effect. Despite our many attempts, the electrochemical electrophilic bromination/spirocyclization of N-(4-unsubstituted-benzyl)-acrylamides was not as good as expected. In addition, the reactions showed good compatibility with N-(4-methoxybenzyl)-acrylamides, including mono-substituted groups (20–23, 75–93% yields), di-substituted groups (24–28, 31–88% yields), and a tri-substituted group (29, 33% yield) in the benzyl moiety. A series of highly functionalized brominated 2-azaspiro[4.5]decanes were also synthesized with satisfactory efficiency under simple and mild conditions. When the nitrogen atom of the substrate was attached with one n-butyl group, the spiro product 30 was obtained in 31% yield. Furthermore, this approach was also applicable to the electrochemical electrophilic bromination/spirocyclization of N-(4-methoxyphenyl)-N-methyl-3-phenylpropiolamide and 4-methoxyphenyl 3-phenylpropiolate with 2 to construct brominated 1-azaspiro[4.5]deca-3,6,9-triene-2,8-dione (31) in 91% yield and 1-oxaspiro[4.5]deca-3,6,9-triene-2,8-dione (32) in 33% yield. Some unsuccessful substrates such as N-benzyl-acrylamides containing a thiophene or indole structure in the benzyl moiety are listed in the ESI.†
Furthermore, gram-scale synthesis and a variety of further transformation reactions of 3 were performed (Scheme 4). The gram-scale brominated spiroproduct 3 was obtained in 74% yield or 76% yield, respectively, by the electrolysis of 1 or 4 with 2 at 62 mA and room temperature for 10 h (Scheme 4a). The tert-butyl moiety in 3 could be removed to obtain 33 in 70% yield under acidic conditions. The brominated spiroproduct 3 could undergo dehydration condensation with MeONH2·HCl to give imine 34 in 84% yield, and could also react with 4-methylbenzenethiol (35) to produce a nucleophilic substitution product 36 in 92% yield (Scheme 4b). In addition, when nitrogen-containing heterocycles, such as pyrazole (37, 93% yield), indazole (38, 93% yield), imidazole (39, 89% yield), benzoimidazole (40, 96% yield), pyrrole (41, 70% yield), indole (42, 83% yield), 1H-pyrrolo[2,3-c]pyridine (43, 96% yield) and tryptophol (44, 64% yield), were used as nucleophiles, the corresponding cyclohepta[c]pyrrole-1,6-diones were obtained in good yields via debromination tandem cyclization under simple basic conditions (Scheme 4c). Alkoxy cyclohepta[c]pyrrole-1,6-diones (45–51, 46–85% yields) could also be prepared efficiently by using sodium alkoxide and alcohols as nucleophiles. The structure of 45 was determined by X-ray single crystal diffraction analysis (see the ESI for details†). These results demonstrated the potential value of the obtained products in synthetic chemistry (Scheme 4).
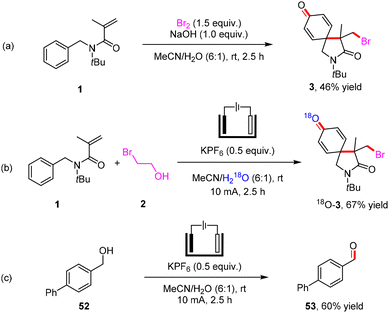
In order to understand the reaction process, we further carried out control experiments (Scheme 5). When Br2 was added to the solution of N-benzyl-N-(tert-butyl)methacrylamide (1) in MeCN/H2O (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the electrophilic bromination/spirocyclization could be achieved in 46% yield at room temperature for 2.5 h. 18O-3 was formed by electrolyzing 1 and 2 with MeCN/H218O (6
1), the electrophilic bromination/spirocyclization could be achieved in 46% yield at room temperature for 2.5 h. 18O-3 was formed by electrolyzing 1 and 2 with MeCN/H218O (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as the solvent, indicating that the oxygen atom in product 3 was derived from H2O. Benzyl alcohol 52 was oxidized at the anode to form an aldehyde (53) under electrochemical conditions. In addition, electrochemical C–H bromination of (hetero)arenes with 2-bromoethan-1-ol as the brominating reagent through ethylene oxide and H2 evolution has been reported by our group.14
1) as the solvent, indicating that the oxygen atom in product 3 was derived from H2O. Benzyl alcohol 52 was oxidized at the anode to form an aldehyde (53) under electrochemical conditions. In addition, electrochemical C–H bromination of (hetero)arenes with 2-bromoethan-1-ol as the brominating reagent through ethylene oxide and H2 evolution has been reported by our group.14
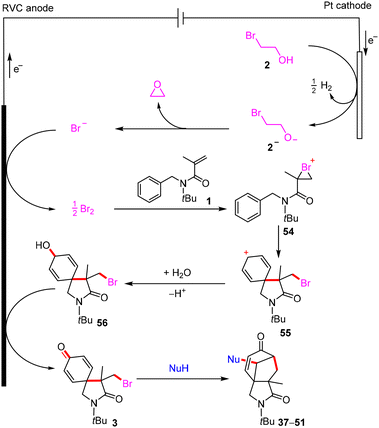
Based on the above experimental results, mechanism studies, and previous reports,14,15 a possible mechanism for the electrochemical electrophilic bromination/spirocyclization is outlined in Scheme 6 with the electrolysis of 1 and 2 as an example. Initially, 2-bromoethan-1-ol (2) is reduced to yield H2 and an alkoxy anion [2]−. The Br− produced by the intramolecular cyclization of [2]− by releasing ethylene oxide is preferentially oxidized at the anode to afford Br2, because the oxidation potential of Br− (Ep/2 = 1.05 V vs. Ag/AgCl) is significantly lower than that of 1 (Ep/2 = 1.99 V vs. Ag/AgCl) and 4 (Ep/2 = 1.72 V vs. Ag/AgCl). The electrophilic addition of 1 and Br2 gives a bromonium ion intermediate 54, followed by an H2O-involved spirocyclization to produce 56. Finally, 56 is further oxidized at the anode to give the final product 3. The brominated 2-azaspiro[4.5]decanes can react with nucleophiles to construct cyclohepta[c]pyrrole-1,6-diones via debromination tandem cyclization.
Conclusions
In conclusion, we have developed an electrochemical electrophilic bromination/spirocyclization of N-benzyl-acrylamides with 2-bromoethan-1-ol as the brominating reagent. This method utilizes the bromine produced by cathodic reduction and anodic oxidation as an electrophile, avoids the need for metal and oxidizing reagents, and can be easily performed on a gram scale under mild conditions. A variety of late-stage transformations of brominated 2-azaspiro[4.5]decanes further confirm the applicability of this electrochemical bromination/spirocyclization.Author contributions
Z. Zhang performed the experiments. Z. Zhang and Z.-W. Hou analyzed the data. H. Chen analyzed the X-ray crystallography data. Z.-W. Hou, P. Li and L. Wang designed and supervised the project. Z.-W. Hou and L. Wang wrote the manuscript. All of the authors discussed the results and contributed to the preparation of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge the National Natural Science Foundation of China (22101201, 22071171) and the Natural Science Foundation of Zhejiang Province (LQ22B020005, LZ22B020003) for the financial support of this work.References
- (a) H. Sorek, A. Rudi, I. Goldberg, M. Aknin and Y. Kashman, J. Nat. Prod., 2009, 72, 784–786 CrossRef CAS PubMed; (b) S. P. Roche and J. A. Porco, Angew. Chem., Int. Ed., 2011, 50, 4068–4093 CrossRef CAS PubMed; (c) A. R. Diaz-Marrero, G. Porras, Z. Aragon, J. M. de la Rosa, E. Dorta, M. Cueto, L. D'Croz, J. Mate and J. Darias, J. Nat. Prod., 2011, 74, 292–295 CrossRef CAS PubMed.
- (a) L. Yu, A. Dai, W. Zhang, A. Liao, S. Guo and J. Wu, J. Agric. Food Chem., 2022, 70, 10693–10707 CrossRef CAS PubMed; (b) R. Westphal, E. V. Filho, F. Medici, M. Benaglia and S. J. Greco, Synthesis, 2022, 2927–2975 CAS; (c) A. Ding, M. Meazza, H. Guo, J. W. Yang and R. Rios, Chem. Soc. Rev., 2018, 47, 5946–5996 RSC; (d) X. Xie, L. Wang, Q. Zhou, Y. Ma, Z.-M. Wang and P. Li, Chin. Chem. Lett., 2022, 33, 5069–5073 CrossRef CAS.
- (a) T. Nemoto, Y. Ishige, M. Yoshida, Y. Kohno, M. Kanematsu and Y. Hamada, Org. Lett., 2010, 12, 5020–5023 CrossRef CAS PubMed; (b) Q.-F. Wu, W.-B. Liu, C.-X. Zhuo, Z.-Q. Rong, K.-Y. Ye and S.-L. You, Angew. Chem., Int. Ed., 2011, 50, 4455–4458 CrossRef CAS PubMed; (c) Y. Li, L. Zhang, L. Zhang, Y. Wu and Y. Gong, Eur. J. Org. Chem., 2013, 8039–8047 CrossRef CAS; (d) H. Nakayama, S. Harada, M. Kono and T. Nemoto, J. Am. Chem. Soc., 2017, 139, 10188–10191 CrossRef CAS PubMed; (e) Z. Yuan, W. Wei, A. Lin and H. Yao, Org. Lett., 2016, 18, 3370–3373 CrossRef CAS PubMed.
- (a) T. R. Ibarra-Rivera, R. Gamez-Montano and L. D. Miranda, Chem. Commun., 2007, 43, 3485–3487 RSC; (b) A. Millán-Ortiz, G. López-Valdez, F. Cortez-Guzmán and L. D. Miranda, Chem. Commun., 2015, 51, 8345–8348 RSC; (c) B. Hu, Y. Li, W. Dong, K. Ren, X. Xie, J. Wan and Z. Zhang, Chem. Commun., 2016, 52, 3709–3712 RSC.
- (a) G. Han, Y. Liu and Q. Wang, Org. Lett., 2014, 16, 3188–3191 CrossRef CAS PubMed; (b) Z. Zhang, X.-J. Tang and W. R. Dolbier Jr., Org. Lett., 2016, 18, 1048–1051 CrossRef CAS PubMed; (c) S. Tang, L. Yuan, Z.-Z. Li, Z.-Y. Peng, Y.-L. Deng, L.-N. Wang, G.-X. Huang and R.-L. Sheng, Tetrahedron Lett., 2017, 58, 2127–2130 CrossRef CAS; (d) L. Yuan, S.-M. Jiang, Z.-Z. Li, Y. Zhu, J. Yu, L. Li, M.-Z. Li, S. Tang and R.-R. Sheng, Org. Biomol. Chem., 2018, 16, 2406–2410 RSC; (e) J. Wu, D. Ma, G. Tang and Y. Zhao, Org. Lett., 2019, 21, 7674–7678 CrossRef CAS PubMed; (f) C. Li, Y. Zhao, J. Zhou, X. Wang, J. Hou, Y. Song, W. Liu and G. Han, Org. Biomol. Chem., 2020, 18, 8376–8380 RSC; (g) C.-C. Peng, L.-J. Wu and S.-F. Pi, Org. Biomol. Chem., 2021, 19, 7602–7606 RSC; (h) C.-C. Peng, F. Long, Y.-C. Hu, Z.-R. Zhou, K.-Y. Zhang, R. Wang, M.-H. Ye, H.-B. Xiao and L.-J. Wu, J. Org. Chem., 2022, 87, 2740–2747 CrossRef CAS PubMed; (i) W. Kong, E. Merino and C. Nevado, Angew. Chem., Int. Ed., 2014, 53, 5078–5082 CrossRef CAS PubMed; (j) G. Dagousset, C. Simon, E. Anselmi, B. Tuccio, T. Billard and E. Magnier, Chem. – Eur. J., 2017, 23, 4282–4286 CrossRef CAS PubMed.
- (a) J. Liang, J. Chen, F. Du, X. Zeng, L. Li and H. Zhang, Org. Lett., 2009, 11, 2820–2823 CrossRef CAS PubMed; (b) Z. Xu, K. Huang, T. Liu, M. Xie and H. Zhang, Chem. Commun., 2011, 47, 4923–4925 RSC; (c) W. Kong, M. Casimiro, N. Fuentes, E. Merino and C. Nevado, Angew. Chem., Int. Ed., 2013, 52, 13086–13090 CrossRef CAS PubMed; (d) L. Chabaud, T. Hromjakova, M. Rambla, P. Retailleau and C. Guillou, Chem. Commun., 2013, 49, 11542–11544 RSC.
- (a) S. D. Minteer and P. S. Baran, Acc. Chem. Res., 2020, 53, 545–546 CrossRef CAS PubMed; (b) L. F. T. Novaes, J. Liu, Y. Shen, L. Lu, J. M. Meinhardt and S. Lin, Chem. Soc. Rev., 2021, 50, 7941–8002 RSC; (c) P. Xiong and H.-C. Xu, Acc. Chem. Res., 2019, 52, 3339–3350 CrossRef CAS PubMed; (d) Y. Jiang, K. Xu and C. Zeng, Chem. Rev., 2018, 118, 4485–4540 CrossRef CAS PubMed; (e) H. Wang, X. Gao, Z. Lv, T. Abdelilah and A. Lei, Chem. Rev., 2019, 119, 6769–6787 CrossRef CAS PubMed; (f) Y. Yuan and A. Lei, Acc. Chem. Res., 2019, 52, 3309–3324 CrossRef CAS PubMed; (g) L. Ackermann, Acc. Chem. Res., 2020, 53, 84–104 CrossRef CAS PubMed; (h) J. E. Nutting, M. Rafiee and S. S. Stahl, Chem. Rev., 2018, 118, 4834–4885 CrossRef CAS PubMed; (i) A. Wiebe, T. Gieshoff, S. Möhle, E. Rodrigo, M. Zirbes and S. R. Waldvogel, Angew. Chem., Int. Ed., 2018, 57, 5594–5619 CrossRef CAS PubMed; (j) R. Francke and R. D. Little, Chem. Soc. Rev., 2014, 43, 2492–2521 RSC; (k) M. D. Kärkäs, Chem. Soc. Rev., 2018, 47, 5786–5865 RSC; (l) K. D. Moeller, Chem. Rev., 2018, 118, 4817–4833 CrossRef CAS PubMed; (m) J. E. Nutting, M. Rafiee and S. S. Stahl, Chem. Rev., 2018, 118, 4834–4885 CrossRef CAS PubMed; (n) J. Yoshida, A. Shimizu and R. Hayashi, Chem. Rev., 2018, 118, 4702–4730 CrossRef CAS PubMed; (o) Y. Okada and K. Chiba, Chem. Rev., 2018, 118, 4592–4630 CrossRef CAS PubMed; (p) S.-H. Shi, Y. Liang and N. Jiao, Chem. Rev., 2021, 121, 485–505 CrossRef CAS PubMed; (q) M.-J. Luo, Q. Xiao and J.-H. Li, Chem. Soc. Rev., 2022, 51, 7206–7237 RSC; (r) Z.-W. Hou, H.-C. Xu and L. Wang, Curr. Opin. Electrochem., 2022, 34, 100988 CrossRef CAS; (s) X. Cheng, A. Lei, T.-S. Mei, H.-C. Xu, K. Xu and C. Zeng, CCS Chem., 2022, 4, 1120–1152 CrossRef CAS.
- (a) P. R. D. Murray, J. H. Cox, N. D. Chiappini, C. B. Roos, E. A. McLoughlin, B. G. Hejna, S. T. Nguyen, H. H. Ripberger, J. M. Ganley, E. Tsui, N. Y. Shin, B. Koronkiewicz, G. Qiu and R. R. Knowles, Chem. Rev., 2022, 122, 2017–2291 CrossRef CAS PubMed; (b) N. E. S. Tay, D. Lehnherr and T. Rovis, Chem. Rev., 2022, 122, 2487–2649 CrossRef CAS PubMed; (c) Z. Zou, W. Zhang, Y. Wang and Y. Pan, Org. Chem. Front., 2021, 8, 2786–2798 RSC; (d) Y. Yu, J.-S. Zhong, K. Xu, Y. Yuan and K.-Y. Ye, Adv. Synth. Catal., 2020, 362, 2102–2119 CrossRef; (e) Z. Wang, Y. Sun, L.-Y. Shen, W.-C. Yang, F. Meng and P. Li, Org. Chem. Front., 2022, 9, 853–873 RSC.
- (a) M. Estruch-Blasco, I. Bosque, D. Guijarro and J. C. Gonzalez-Gomez, Org. Chem. Front., 2021, 8, 5130–5138 RSC; (b) C. Zhang, F. Bu, C. Zeng, D. Wang, L. Lu, H. Zhang and A. Lei, CCS Chem., 2021, 3, 1404–1412 Search PubMed; (c) W.-C. Yang, M.-M. Zhang, Y. Sun, C.-Y. Chen and L. Wang, Org. Lett., 2021, 23, 6691–6696 CrossRef CAS PubMed; (d) Y. Zhang, C. Ma, J. Struwe, J. Feng, G. Zhu and L. Ackermann, Chem. Sci., 2021, 12, 10092–10096 RSC; (e) C. R. Reddy and D. H. Kolgave, J. Org. Chem., 2021, 86, 17071–17081 CrossRef PubMed; (f) Z. Shi, W.-Z. Wang, N. Li, Y. Yuan and K.-Y. Ye, Org. Lett., 2022, 24, 6321–6325 CrossRef CAS PubMed; (g) K. Mo, X. Zhou, J. Wu and Y. Zhao, Org. Lett., 2022, 24, 2788–2792 CrossRef CAS PubMed.
- J. Hua, Z. Fang, M. Bian, T. Ma, M. Yang, J. Xu, C. Liu, W. He, N. Zhu, Z. Yang and K. Guo, ChemSusChem, 2020, 13, 2053–2059 CrossRef CAS PubMed.
- K. Yu, X. Kong, J. Yang, G. Li, B. Xu and Q. Chen, J. Org. Chem., 2021, 86, 917–928 CrossRef CAS PubMed.
- C.-Y. Wu, G.-B. Deng, M. Hu, W.-T. Wei and J.-H. Li, Synthesis, 2014, 2585–2590 CAS.
- Q. Yin and S.-L. You, Org. Lett., 2012, 14, 3526–3529 CrossRef CAS PubMed.
- Y. Lv, Z.-W. Hou, P. Li and L. Wang, Org. Chem. Front., 2023, 10, 990–995 RSC.
- (a) Z. Zhou, Y. Yuan, Y. Cao, J. Qiao, A. Yao, J. Zhao, W. Zuo, W. Chen and A. Lei, Chin. J. Chem., 2019, 37, 611–615 CrossRef CAS; (b) X. Yan, S. Wang, Z. Liu, Y. Luo, P. Wang, W. Shi, X. Qi, Z. Huang and A. Lei, Sci. China: Chem., 2022, 65, 762–770 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2213723 and 2213724. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3gc00728f |
| This journal is © The Royal Society of Chemistry 2023 |