From vanillin to biobased aromatic polymers
Hongru
Qiang†
 ,
Jiewen
Wang†
,
Hengxu
Liu†
,
Jiewen
Wang†
,
Hengxu
Liu†
 and
Yunqing
Zhu
and
Yunqing
Zhu
 *
*
Department of Polymeric Materials, School of Materials Science and Engineering, Tongji University, 4800 Caoan Road, Shanghai 201804, China. E-mail: 1019zhuyq@tongji.edu.cn
First published on 1st September 2023
Abstract
Aromatic compounds are platform chemicals used in the manufacture of commodity polymers. The development of bio-based aromatic compounds is expected to reduce the dependence on non-renewable resources. Lignin is the major source of bio-based aromatic chemicals and the development of lignin-based polymers is of broad interest. As one of the major commercialized aromatic compounds from lignin, vanillin offers limitless potential for the preparation of bio-based (semi)aromatic polymers owing to the presence of aldehyde and phenolic hydroxyl functional groups. This review highlights the recent advances in the construction of vanillin-based polymers, including thermosets (phenolic resins, epoxy resins, benzoxazine resins, etc.), thermoplastics (polyesters, polycarbonates, etc.), and covalent adaptable networks (CANs). An extensive analysis of the synthetic methods of vanillin-based monomers and the performance of the obtained vanillin-based (semi)aromatic polymers is provided, and future opportunities and challenges are also highlighted.
1. Introduction
Polymers play an important role in daily life, but the vast majority of them are derived from non-renewable petroleum-based chemicals.1 Faced with the pressure of petroleum shortage and environmental pollution caused by plastics, there is an ever-increasing concern about converting renewable biomass into a variety of valuable polymers.2,3 The development and utilization of bio-based polymers is considered an effective way to conserve petroleum resources and protect the environment.Renewable feedstocks are now widely used in the chemical industry for non-fuel applications, but most of them are derived from aliphatic or cycloaliphatic groups such as plant oils, cellulose and triglycerides. However, most of the essential chemicals are aromatic compounds ultimately derived from petroleum, for example, benzene, xylene and cumene. Aromatic compounds are key ingredients for building high-performance polymers, and thus there is tremendous interest in developing sustainable aromatic chemicals.
Some aromatic structures can be derived from natural compounds or occur naturally in limited quantities. For instance, cashew nut shell liquid (CNSL) is a substantial agricultural byproduct derived from cashew nut and cashew apple production, with global manufacturing reaching nearly one million tons annually. As a result, CNSL has emerged as a prominent and economically viable source of naturally occurring phenols.4 Derived from the cashew nut shell, cardanol is a highly promising renewable phenol source that offers a natural alternative to petrochemical-derived phenols for the production of bio-based thermosets.5 The utilization of furfural as a resource for bio-based polymers has been investigated, including its conversion into succinic acid and 1,4-butanediol, which serve as monomers for polybutylene succinate, as well as its conversion into bio-based terephthalic acid, one of the monomers for polyethylene terephthalate (PET).6
Different from CNSL, lignin is the second most abundant natural polymer after cellulose, accounting for approximately 15–30% of the dry weight of lignocellulosic biomass.7–12 It is the only scalable renewable raw material consisting of aromatic monomers, yet the vast majority of about 95% of lignin is burned as a low-value fuel.13 The direct use of lignin for the synthesis of polymers is limited because of its ill-defined structure depending on the origin, extraction, and pyrolysis processes, preventing them from being industrial raw materials.14 The depolymerization/pyrolysis of lignin has been extensively studied in recent years.15–24 In fact, the large amount of lignin produced each year has prompted many studies regarding its catalytic depolymerization.25,26
Among all of the lignin-derived fine chemicals, vanillin (4-hydroxy-3-methoxybenzaldehyde) is the only commercialized mono-aromatic compound obtained from lignin on a large scale. Vanillin is widely used as a flavoring and fragrance agent in food products and a fragrance agent in cosmetics as well as a starting material for the synthesis of several second-generation fine chemicals. Vanillin is derived from lignin, meaning that it is renewable and does not compete with food sources leading to food security issues.27 It can be obtained on an industrial scale from well-described and continuously improved processes with two chemically modifiable reactive groups (aldehyde and phenolic hydroxyl groups) to produce vanillin-derived monomers for preparing polymeric materials with diverse properties for tailored applications. Examples include coatings, adhesives, elastomers and optoelectronic materials. Different from p-hydroxybenzaldehyde, vanillin has an extra methoxy group. The presence of this methoxy group amplifies vanillin's reactivity with other compounds, offering substantial implications for monomer synthesis. In addition, it offers better decomposition properties,28 antimicrobial properties29 and enhanced mechanical properties through the formation of hydrogen bonding interactions.30 Through the lignin to vanillin route, the only commercial process is from lignin sulfonate, a by-product of the sulfite pulp and paper industry.27 However, the efficient extraction of vanillin from lignin remains a formidable challenge. In the fields of lignin hydrogenolysis, pyrolysis, and liquefaction, the complexity of the product composition introduces ambiguity in transformation mechanisms, posing challenges for effective separation and purification processes. Nowadays, vanillin is primarily derived from fossil hydrocarbons, which accounts for 85% of the worldwide supply. Furthermore, chemically synthesized vanillin costs only 1% of the naturally extracted vanillin (around USD 10 per kg).31,32
This review highlights some of the opportunities for creating sustainable polymers from vanillin. Vanillin feedstocks enable the production of polymeric materials with a wide range of properties to accommodate various application scenarios. Apart from traditional vanillin-based thermoplastic and thermoset polymeric materials, dynamic adaptable networks (CANs) are gradually emerging as a major development for vanillin-based polymers owing to advantages such as stimulus responsiveness, self-healing, reprocessability, recyclability, and/or degradability. This review aims to summarize in detail the synthesis and properties of thermoplastics, thermosets, and covalent adaptable networks (CANs) from vanillin as a starting material in the literature.
2. Vanillin-based thermoset polymers
2.1. Phenolic resins
Since 1911, phenolic resins have been the first industrially produced polymers for humans,33 and have been widely used in various industries, such as electrical laminates, carbon foams, adhesives, molding compounds, acid-resistant coatings and fibre-reinforced composites.34 Most phenolic resins are synthesized from non-renewable petroleum-based phenol and formaldehyde. To achieve green and sustainable production of phenolic resins, using renewable biomass resources is an effective way. There have been studies on phenol and formaldehyde substitution based on vanillin, and these studies have achieved the formaldehyde-free synthesis of phenolic resins.In the preparation of formaldehyde-free resols by vanillin and phenol, due to the fact that vanillin's phenolic hydroxyl group (pKa ∼7.4) is more acidic than that of phenol (pKa ∼10),35 these phenolic hydroxyl groups are preferentially deprotonated by alkaline catalysts instead of phenol's. Hence, the direct use of vanillin in the synthesis reaction of resol-type phenolic resins is unproductive. To solve this problem, Foyer et al. activated the reactivity of the vanillin aldehyde group by masking the hydroxyl group of vanillin.36 The author used a two-step method to convert the hydroxyl group into an aldehyde group to obtain the bifunctional vanillin precursor Van-n1Ald (Scheme 1a) and the phenol-based aldehyde Phenol-n1Ald for comparison. They can replace formaldehyde and react with phenol to synthesize phenolic resin. The results showed that Van-n1Ald phenolic resin derived from vanillin was cross-linked and rarely underwent aldol side reactions compared to Phenol-n1Ald, demonstrating higher thermal stability and char yield.
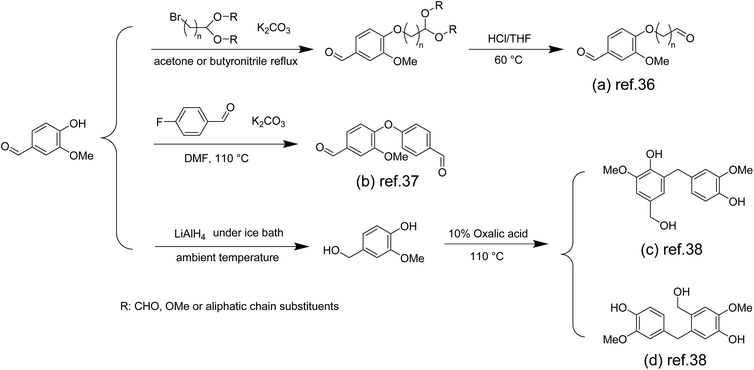 | ||
| Scheme 1 Overview of the preparation route for monomers used in the synthesis of phenolic resins: (a) Reproduced from ref. 36 with permission from Elsevier, copyright 2016. (b) Reproduced from ref. 37 with permission from Elsevier, copyright 2016. (c) Reproduced from ref. 38 with permission from Elsevier, copyright 2021. | ||
They also discovered another method of masking hydroxyl groups using 4-fluorobenzaldehyde as a raw material and synthesized new bifunctional and bio-based aromatic aldehyde precursors (Scheme 1b). The cured phenolic resins made from them exhibited extremely high thermal properties at 540 °C and 440 °C with a weight loss of 10% and char yields of 71% and 68%, respectively.37
For phenolic resin synthesis, these aromatic monomers are often characterized as the para site is substituted by alkyl groups, and partial or entire ortho sites are substituted by methoxy groups, making them particularly difficult to couple with other aldehydes. Yang et al. successfully introduced hydroxymethyl into the aromatic benzene ring of vanillin by reducing the aldehyde group at the para site of vanillin using lithium aluminum hydride.38 Using oxalic acid as a catalyst, the resulting vanillin derivative was polymerized directly into phenolic resins without the use of formaldehyde or other aldehydes and exhibited predominantly ortho–para condensation (Scheme 1c and d). Vanillin-based phenolic resins exhibited excellent adhesive strength (6.14 MPa), glass transition temperature (Tg = 107–115 °C) and thermal stability (temperature of 5% mass loss, Td5% = 213–231 °C). These properties are similar to those of commercial Novolak phenolic resins.
2.2. Epoxy resins
Epoxy resins, as one of the three most important thermosetting polymers, have been widely employed in a multitude of fields such as coatings, adhesives, laminated circuit boards, electronic component encapsulations, and advanced composites because of their excellent adhesion, chemical resistance, mechanical properties, and dielectric properties.39–43 Nowadays, almost all of the epoxy resins are produced from fossil resources, and 90% of the commercially available epoxy resins are diglycidyl ether of bisphenol A (DGEBA) from the reaction of bisphenol A with epichlorohydrin.44 Bisphenol A, fully dependent on fossil resources, accounts for >67% of the molar mass of DGEBA.45 Researchers have been exploring and developing epoxy resins based on renewable raw materials, such as vanillin.46Although the reaction between epichlorohydrin and alcohol is the most common approach to introduce an epoxide group, vanillin has only one phenol group, leading to failures in producing diepoxides. The oxidation or reduction of the formyl group of vanillin,47,48 or the utilization of pentaerythritol to react with the formyl group and “couple” two vanillin molecules to obtain a difunctional phenol,49 has been explored to introduce difunctional epoxy groups. Wang et al. proposed a method in which a Schiff base intermediate (produced by the coupling reaction of vanillin and diamine) was formed first and further reacted with diethyl phosphite to obtain phosphorus-containing difunctional phenols.50 These difunctional phenols were subsequently reacted with epichlorohydrin to obtain flame-retardant epoxy resins EP1 and EP2 (Scheme 2a and b). The authors found that the vanillin-based epoxy resin cured by this method had a very high Tg of ∼214 °C, a tensile strength of ∼80.3 MPa and a tensile modulus of ∼2709 MPa, which are much higher than those of the commonly used epoxy resin DGEBA with a Tg value of 166 °C, a tensile strength of 76.4 MPa and a tensile modulus of 1893 MPa. Shibata et al. also prepared a new biobased diepoxide (DGEDVCP) via the crossed aldol condensation of vanillin and cyclopentanone and the subsequent glycidylation (Scheme 2c).51
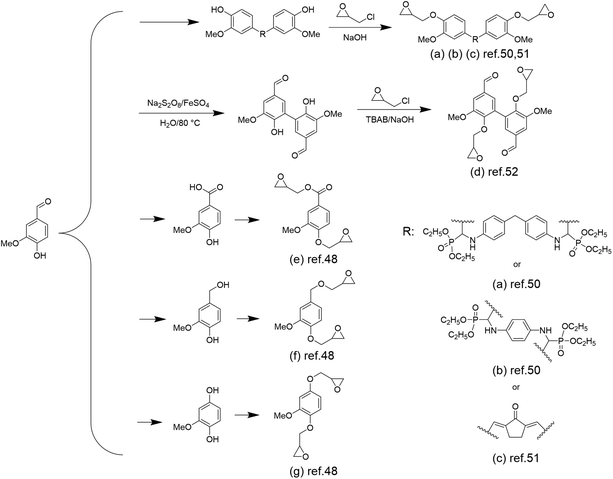 | ||
| Scheme 2 Overview of the synthetic route of vanillin-based epoxy monomers: (a) and (b) Reproduced from ref. 50 with permission from the American Chemical Society, copyright 2017. (c) Reproduced from ref. 51 with permission from Elsevier, copyright 2017. (d) Reproduced from ref. 52 with permission from the American Chemical Society, copyright 2020. (e), (f) and (g) Reproduced from ref. 48 with permission from Elsevier, copyright 2015. | ||
Besides traditional epoxy resins, degradable epoxy resins have also been developed by introducing ester and amide bonds into a cross-linked network. Fang et al. prepared a diepoxide compound via the oxidative coupling of vanillin followed by epichlorohydrin glycidylation (Scheme 2d).52 Epoxidized-divanillin (EDV) was cured with the petroleum-based, commercially available hardener isophorone diamine (IPDA) and a biobased-diamine (GX-3090). This epoxy has a storage modulus (1.7–2.3 GPa) and a glass transition temperature similar to those of commercial resins, as well as good solvent resistance. Most importantly, it showed good degradation properties, with almost 40% of the segments being solubilized because of the cleavage of the network cured in acetone after being treated with 1 M HCl for 24 h at room temperature.
In addition to coupling reactions, the oxidation or reduction of the aldehyde group of vanillin into either acid or alcohol has also been explored. By converting to acid or alcohol, epichlorohydrin glycidylation could occur simultaneously at the phenol and acid/alcohol sites, resulting in diepoxides. Fache et al. constructed three vanillin derivatives (methoxyhydroquinone, vanillic acid and vanillyl alcohol) starting from vanillin and then glycidylated them to obtain the bio-based aromatic diepoxy monomers diglycidyl ether of methoxyhydroquinone (DGEMHY) (Scheme 2g), diglycidyl ether of vanillic acid (DGEVAC) (Scheme 2e) and diglycidyl ether of vanillyl alcohol (DGEVA) (Scheme 2f). These monomers were then copolymerized with the amine hardener isophorone diamine (IPDA) to afford materials with properties comparable to the commercially available DGEBA/IPDA resins.48
2.3. Vinyl ester resins
Vinyl ester resins (VERs), made from the esterification of epoxy resins, are unsaturated thermosetting resins used to formulate composites for a variety of commercial applications in the transportation and construction industries.53,54 VERs consist of vinyl ester monomers and also contain reactive diluents (typically styrene) to reduce viscosity and improve processability.Styrene is a non-renewable commodity chemical derived from fossil sources, possessing volatility and posing health hazards to humans.55 To address these issues, Yu et al. prepared renewable styrene substitutes from biomass-derived vanillin, expecting to change the properties without sacrificing the desired properties (such as viscosity, reactivity, and tensile strength).56 The authors synthesized 3-allyl-5-vinylveratrol (AVV) (Scheme 3a) from vanillin and demonstrated that AVV could be an alternative to styrene in the formation of a thermosetting resin prepared using dimethacrylated-epoxidized-sucrose soyate (DMESS). The thermomechanical and tensile properties were very close to those of resins prepared from styrene. Vinyl ester resins derived from the renewable resource vanillin are also being used in additive manufacturing applications. Bassett et al. first prepared methacrylated vanillin (MV) (Scheme 3b) using methacrylic anhydride and vanillin via a solvent-free technique, and then obtained a low-viscous vanillin-based resin with 100% atom efficiency, which was additively manufactured using stereolithography (SLA).57 It was found that postprocessing with Formlabs Form Cure (FC) resulted in a 23% increase in total cure and improved final cured polymer properties. Additive manufacturing with postprocessing increased the degree of cure by 10% compared to the same material with thermal and postcuring, but the thermogravimetric and thermomechanical properties were minimally affected. The vanillin-based resin prepared by SLA and postprocessing had a Tg value of 153 °C and a Young's modulus of 4900 MPa.
 | ||
| Scheme 3 Synthesis route to vinyl ester monomers from biomass-derived vanillin: (a) Reproduced from ref. 56 with permission from the American Chemical Society, copyright 2018. (b) Reproduced from ref. 57 with permission from the American Chemical Society, copyright 2020. | ||
2.4. Benzoxazine resins
Compared with other thermosetting resins, polybenzoxazine has been widely studied for its superior properties, such as high flexibility in molecular design, low water absorption, flame retardancy, good mechanical and thermal properties, near-zero shrinkage, excellent chemical resistance and even self-healing properties.58–61 Benzoxazine monomers can be synthesized by judicious selection of phenolic (e.g. vanillin) and amine precursors to yield the desired structure for various application scenarios.Furfurylamine containing a furan ring was used as a precursor to construct benzoxazines. Amornkitbamrung et al. introduced benzoxazine thermosetting resins in the construction of self-healing materials to improve the mechanical properties and thermal stability of the materials (Scheme 4a). They prepared bio-based benzoxazine/epoxy copolymers using vanillin, furfurylamine and epoxidized castor oil, and functionalized them with glutaric anhydride (GA) to make copolymers with self-healing properties.62
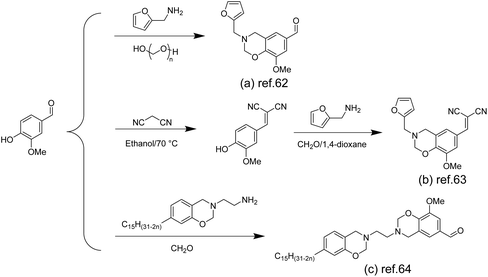 | ||
| Scheme 4 The synthesis of benzoxazines from vanillin: (a) Reproduced from ref. 62 with permission from Elsevier, copyright 2022. (b) Reproduced from ref. 63 with permission from Elsevier, copyright 2020. (c) Reproduced from ref. 64 with permission from the Royal Society of Chemistry, copyright 2016. | ||
Samy et al. also prepared similar structures using furfurylamine and formaldehyde for capacitor applications (Scheme 4b), but they additionally introduced cyanide groups into the aldehyde group of vanillin to increase the N content and thus improve the electrochemical properties.63
Aliphatic amines as precursors were used to construct benzoxazines. Benzoxazines can be polymerized under heating conditions, but the melting temperature (Tm) of vanillin-based benzoxazines is close to the polymerization temperature (Tp), so melt processing (molding) between these two temperatures becomes difficult. To solve this problem, Puchot et al. prepared bifunctional benzoxazines (Scheme 4c) based on biophenols with large differences between Tm and Tp.64 Specifically, monosubstituted cardanol-based benzoxazine monomers were prepared by exploiting the low reactivity of cardanol and further coupled with vanillin to obtain vanillin-cardanol dibenzoxazines.
2.5. Cyanate ester resins
Cyanate resins are a new class of high performance resins emerging in recent years, and have been widely used in electronics, aerospace, coatings and optical instruments due to their excellent mechanical and dielectric properties.65,66 Harvey et al. synthesized bisphenol precursors from vanillin via the combination of the McMurry coupling reaction and cyanate reaction.67 The obtained 1,2-bis(4-cyanato-3-methoxyphenyl)ethane could be thermally cured under moderate conditions to produce a thermoset material with Tg up to 202 °C, which is comparable to some of the petroleum-derived epoxy resins. It is worth noting that cyanate exhibits excellent decomposition properties benefitting from the methoxy group in the ortho positions to the hydroxyl group of vanillin. Although the extent to which the ortho methoxy groups contribute to the decomposition of these renewable resins is unclear, it is speculated that the donation of the electron density into the ring likely lowers the decomposition temperature, making the generation of phenolic products more favorable than simpler decomposition products such as CO2.282.6. Polythioethers
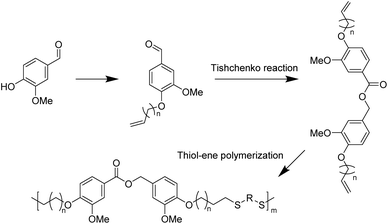
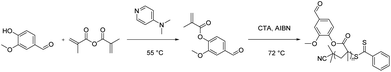
With good chemical stability and biocompatibility, polythioether has attracted great interest in the fields of coatings,68,69 optics,70 electronic devices,71 3D printing,72 and biomedical implants.73 In recent years, thiol–ene click polymerization has been widely used for the preparation of bio-based polythioether. Thiol–ene polymerization has the advantages of a high reaction rate, simple procedure and formation of homogeneous cross-linked structures compared with conventional polymerization.74The Tishchenko reaction is a disproportionation/dimerization reaction occurring between two aldehyde groups to afford a carboxyl ester bond, which has 100% atom efficiency but has rarely been applied to polyester chemistry. In 2020, Ren et al. presented a toolbox combining the Williamson and Tishchenko reactions to prepare a series of α,ω-diene-functionalized carboxylic ester monomers.75 Vanillin-based polythioethers were then prepared via the thiol–ene reaction between vanillin diene and different thiols. The structural diversity of α,ω-diene-functionalized carboxylic ester monomers allowed for the preparation of a series of polythioethers with diverse properties.
In 2023, the same group subsequently performed UV thermally induced thiol–ene click polymerization between the α,ω-diene monomers and thiols with various functional groups to prepare a range of thiol–ene networks with tunable properties (Scheme 5).76 Notably, the thermal stability and mechanical properties of the cross-linked networks obtained from 1,2-ethanedithiol and 2,4,6,8-tetramethyl-2,4,6,8-tetravinylcyclotetrasiloxane as copolymer monomers were further enhanced. All poly(thioether) networks exhibited good transparency and were susceptible to degradation in weakly alkaline aqueous solutions due to their inherent ester groups. This demonstrates the great potential of α,ω-diene monomers derived from novel vanillin for the preparation of degradable thermosetting materials.
3. Vanillin-based thermoplastic polymers
Thermosetting and thermoplastic polymers are the two primary categories of commodity polymers. Thermoplastic polymers offer several advantages over thermosetting polymers, such as recyclability and the ability to be reshaped, making them more sustainable. As a result, extensive research efforts are being devoted to exploring vanillin-based thermoplastic polymers. Prominent categories currently under investigation include polyvanillin, vanillin-derived polyesters, polycarbonates, unsaturated polyolefins, and polyacrylates.3.1. Polyvanillin
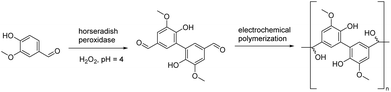
A rather straightforward electrochemical polymerization of vanillin to afford polyvanillin has been reported by Amarasekara and colleagues.77 The desired product was obtained from vanillin dimers through an electrochemically mediated coupling polymerization technique (Scheme 6). The dimer was synthesized through a peroxidase enzyme-catalyzed dimerization reaction with a high yield (91%). The resulting polyvanillin material displayed exceptional thermal stability, as evidenced by its high decomposition onset temperature of 300 °C. Additionally, the material exhibited a 50% decomposition temperature (T50%) value of 440 °C, further highlighting its excellent thermal stability. The efficient and environmentally friendly aqueous electrochemical synthesis method of this study offers a convenient pathway towards producing functionalized polymers based on renewable resources.3.2. Polyesters
Vanillin, as an aromatic monomer, plays a pivotal role in imparting exceptional thermal and mechanical properties to aromatic polyesters. There are generally two approaches to synthesizing vanillin-based polyesters: one involves the modification of the phenolic hydroxyl and aldehyde groups of vanillin to produce vanillin-based monomers, such as diols, diacids/diesters, or hydroxy acids/hydroxy esters. The other method involves the polymerization synthesis of vanillin-based polyesters.78The synthesis of vanillin-based polymers through transesterification polymerization between vanillin derivatives and conventional diols was reported by Llevot et al. in 2015.79 Two symmetrical biphenyl monomers derived from vanillin, methylated divanillyl diol and the methylated dimethylvanillate dimer, were successfully synthesized and employed as (co)monomers for the design of renewable (semi)aromatic polyesters. Notably, the polyesters with a molar mass of 65 kg mol−1 formed from the compound (Scheme 7a) exhibited a Tg value of 38 °C, a storage modulus of 8.1 GPa, and a thermal decomposition temperature (Td5%) of 319 °C. The same conditions were applied for the polymerization of methylated divanillyl diol with a range of biologically sourced diesters. By varying the structure of the diesters, a series of polyesters were synthesized. The resulting polyesters exhibited an amorphous morphology and displayed a Tg value ranging widely from −5 to 139 °C, which is beneficial for accommodating various application scenarios.
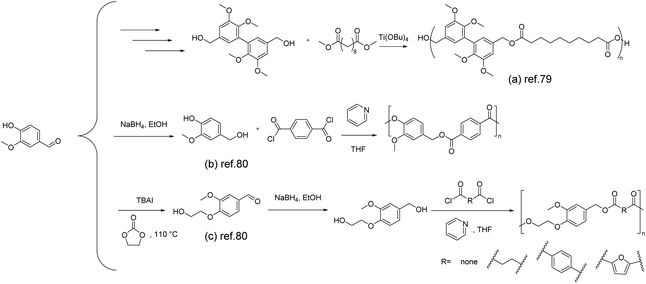 | ||
| Scheme 7 Schematic of the synthesis route of vanillin-derived polyesters: (a) Reproduced from ref. 79 with permission from the Royal Society of Chemistry, copyright 2015. (b) and (c) Reproduced from ref. 80 with permission from MDPI, copyright 2020. | ||
Zhao and co-workers reported a green and sustainable approach to producing bio-based polyesters from vanillin and ethylene carbonate.80 They prepared two aromatic diols, 4-(hydroxymethyl)-2-methoxyphenol (Scheme 7b) and 2-(4-(hydroxymethyl)-2-methoxyphenoxy)ethan-1-ol (Scheme 7c), by reducing vanillin and hydroxyethylated vanillin using NaBH4, respectively. The diols were then subjected to traditional condensation with acyl chlorides, resulting in the synthesis of a range of new polyesters. It is noteworthy that the polyesters obtained from vanillyl alcohol and terephthaloyl chloride with a molar mass of 21 kg mol−1 exhibit higher thermal stability, with a 5% mass loss temperature reaching 293 °C. Furthermore, due to the increased rigidity of the diol, these polyesters demonstrate comparable or even higher glass transition temperatures than PBT and PET (38 °C and 67 °C, respectively).
Apart from copolymerization between vanillin-based diesters with additional diols, Xanthopoulou and co-workers reported a new semi-aromatic polyester, poly(hexylene vanillate) (PHV). PHV was obtained from vanillin-based 4-(6-hydroxyethoxy)-3-methoxybenzoic acid via homopolymerization.81 PHV is a polyester with two distinct functional groups (ester and ether) in its backbone, leading to a complex degradation pathway. Due to its similarity to PET in structure, a similar degradation pathway is expected for PHV. Two different thermal degradation processes were reported: polyester with β-hydrogen atoms primarily undergoes scission by β-cleavage, while random scission pathways have also been detected.
Additionally, based on the presence of vanillin analogues, a degradation pathway similar to lignocellulosic biomass is expected, where C–O scission pathways dominate. Therefore, a complex mechanism involving both scission and random cleavage pathways is anticipated during the thermal decomposition of PHV.
3.3. Polycarbonates
Polycarbonates represent a class of materials that fall between commodity plastics and engineering plastics. They are usually synthesized through reactions between bisphenols and phosgene or carbonate esters.82As discussed in the above sections, there have been many approaches toward the synthesis of vanillin-based bisphenols. Therefore, copolymerization of vanillin-based bisphenols with carbonate esters is considered as the most feasible way to afford vanillin-based polycarbonates. For example, once the vanillin-based bisphenol was synthesized by Harvey et al. via the McMurry coupling reaction,67 diphenyl carbonate was used to react with the obtained bisphenol to give polycarbonates (Scheme 8a). The thermal stability of the polycarbonate was assessed using thermogravimetric analysis, revealing a 5% mass loss at 290 °C and a 10% mass loss at 318 °C, indicating good heat resistance. In this study, the degradation behavior was not specifically investigated. However, considering the similarity in chemical structures, the degradation process of the material may be similar to commercially available polycarbonates. Potential pathways could involve the chain scission of isopropylidene linkages and hydrolysis/alcoholysis of carbonate linkages.83
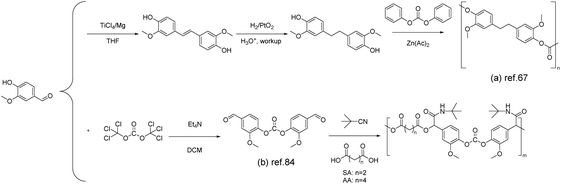 | ||
| Scheme 8 The synthesis of polycarbonates from vanillin: (a) Reproduced from ref. 67 with permission from the Royal Society of Chemistry, copyright 2015. (b) Reproduced from ref. 84 with permission from the Royal Society of Chemistry, copyright 2018. | ||
Alternatively, vanillin-based polycarbonates were also synthesized via the multicomponent polymerization of bis(4-formyl-2-methoxyphenyl) carbonate (BFMC) (Scheme 8b), diacids and tert-butyl isonitrile. BFMC was prepared through the reaction of vanillin with triphosgene.84 The BFMC monomer was employed to prepare a series of polycarbonate oligomers with side-chain amide groups (PCEA). The stability of PCEAs can reach temperatures as high as 235 °C (Td5%), depending on their molar mass (between 3.4 kg mol−1 and 5.3 kg mol−1), beyond which gradual decomposition takes place. The onset decomposition temperature of the PCEA increased with an increase in molecular weight. The decomposition process occurred in two stages, potentially attributed to the breakdown of ester and carbonate linkages. PCEA exhibited an amorphous nature, with a glass transition temperature range from 70 to 106 °C and no detectable melting temperature. PCEAs exhibit adjustable Tg values, owing to variations in monomer combinations and molecular weights. In addition, the utilization of isonitrile derivatives offers a facile route to introduce functional side groups for certain application scenarios. The presence of both carbonate (–O–CO–O–) and amide (–CO–NH–) linkages along the polymer chain also suggest potential degradability. Although PCEA primarily stems from renewable sources, the monomer synthesis process still involves the use of triphosgene, a highly toxic chemical. Replacing it with alternatives could enhance its environmental friendliness.
3.4. Unsaturated polyolefins
Unsaturated, linear, high-molecular-weight polyolefins can be successfully synthesized through the method of acyclic diene metathesis (ADMET), allowing the incorporation of various functional groups and enabling the synthesis of renewable polymers. Followed by the dimerization of vanillin, a type of vanillin-based α,ω-diene was further obtained using the Wittig reagent by A. Llevot et al.85 The corresponding unsaturated polyolefins (Scheme 9a) were synthesized by using the Grubbs 2nd and Hoveyda–Grubbs 2nd catalysts with molar masses of 16 kg mol−1 and 29 kg mol−1, respectively. It is noteworthy that the vanillin-based unsaturated polyolefin exhibited a high glass transition temperature of ∼160 °C, which was attributed to its rigid chemical structure and full trans configuration. Additionally, the polymer showed excellent thermal stability with 5% weight loss occurring at 380 °C.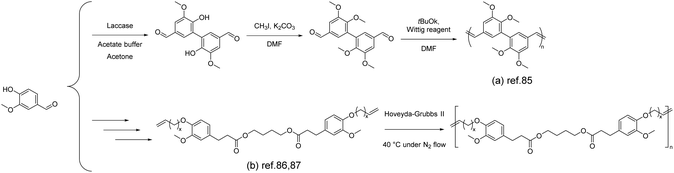 | ||
| Scheme 9 The synthesis of vanillin-derived unsaturated polyolefins by ADMET polymerization: (a) Reproduced from ref. 85 with permission from the Royal Society of Chemistry, copyright 2015. (b) Reproduced from ref. 86 with permission from Elsevier, copyright 2015 and reproduced from ref. 87 with permission from the Royal Society of Chemistry, copyright 2013. | ||
A similar approach was also adopted by Barbara et al.86 In the mentioned work, vanillin was dimerized using laccase from Trametes versicolor as the catalyst. Then divanillin was methylated to yield methylated divanillin. A Wittig reaction was performed on methylated divanillin, employing methyltriphosphonium iodide as the Wittig reagent, yielding the diene monomer. The reported diene monomer synthesis method presented here is based on the group's previous work.87 Starting from vanillin, ethyl dihydroferulate was synthesized and subsequently subjected to ester exchange reactions with several polyols to obtain the bisphenol. The obtained bisphenol was then further reacted with bromo-alkenes through a Williamson reaction to yield the diene monomer (Scheme 9b). Depending on the structure of the vanillin-based α,ω-diene monomers, the corresponding Tg values can be tuned (−28 to 18.2 °C). Due to the presence of aromatic moieties and the rigidity imparted by the vanillin component, all polymers exhibited thermal stability (Td5%) within the range of 257–360 °C. Decreasing the length of the alkene chain (from decene to butene) increased in Tg (C10 < C6 < C2), elevating the glass transition temperature in the range of −28 to 18.2 °C.
3.5. Polymethacrylate
Holmberg et al. reported the synthesis of renewable homopolymers and block copolymers through the functionalization of vanillin followed by reversible addition–fragmentation chain transfer (RAFT) polymerization (Scheme 10).88 The methacrylate moiety was introduced onto vanillin via esterification between phenol and methacrylic anhydride to yield vanillyl methacrylate. The final homopolymer with molar masses between 9.8 kg mol−1 and 27 kg mol−1 exhibited a Tg value and degradation temperature comparable to those of polystyrene (120 °C and ≤300 °C, respectively). This work suggests the potential of using vanillin as a renewable replacement for styrene.4. Vanillin-based covalent adaptable networks (CANs)
Vanillin is widely used as a monomer in the synthesis of thermoplastics and thermosets. Compared to thermoplastics, thermosets offer good mechanical properties, chemical resistance, and dimensional stability. However, thermosets are difficult to reprocess as well as degrade for recycling due to the formation of cross-linked structures, weakening the sustainability of vanillin-based materials. Covalent Adaptable Networks (CANs) appeared as an innovative polymer family that lies between thermoplastics and thermosets. CANs rely on the use of dynamic covalent bonds applied to cross-linked polymer networks. CANs are generally divided into two categories, dissociative CANs and associative CANs, based on the type of bond exchange mechanism. Independent dissociation and reorganization of dynamic covalent bonds occur in dissociative CANs.89 In contrast, associative CANs, also known as vitrimers, have dynamic covalent bond breakage and reformation occurring simultaneously.90 Dynamic covalent bonds (DCBs) can be reversibly broken or reorganized in the presence of light, heat, and certain solvents. Several types of dynamic covalent bonds have been explored and used to develop CANs, including ester bonds,91,92 disulfide bonds,93–95 imine bonds,96–99 boron ester bonds,100,101 acetal bonds,102 and vinylogous urethane bonds.103 The introduction of dynamic covalent bonds can induce network rearrangements, emerging as a sustainable alternative for developing recyclable thermosets.104 This also provides crosslinked polymers with desirable functionalities, such as self-healing, weldability, and reconfigurability, thus prolonging the service life of the materials they are used in. Vanillin offers additional possibilities because its aldehyde group can form dynamic imine linkages by condensation with amines or dynamic acetal linkages by condensation with polyols.4.1. Schiff base polymers
Yu et al. prepared vanillin-based epoxy resins (Van-Ep/IPDA) by curing vanillin-based monoepoxide with isophorone diamine (IPDA).105 The vitrimers showed comparable Young's modulus, elongation at break, and thermal stability to the cured bisphenol A epoxy resin (E51). Van-Ep/IPDA reached a glass transition temperature of 121 °C and a Td5% of 222 °C. Stress relaxation experiments showed that CANs demonstrated a fast stress relaxation behavior, with a relaxation time of only 50 s at 150 °C. This suggests that the dynamic imine bonding provides the material with excellent reprocessability. Even after three reprocessing cycles, all samples exhibit almost the same glass transition temperature as the original materials. In addition, these CANs can undergo degradation under acidic conditions.
To further improve the thermal/mechanical properties, Wang et al. used a rigid 4,4′-methylenebiscyclohexanamine (PACM) to cure vanillin-based monoepoxide instead. The obtained materials, MB-PACMs, have a higher glass transition temperature (Tg) of 172 °C and a higher tensile length of 81 MPa due to the high π-conjugated Schiff base structure and its associated hydrogen bonding.106 MB-PACM can be hydrolyzed at room temperature under mildly acidic conditions first to a Schiff base containing oligomer, which is then further hydrolyzed until the Schiff base bond has completely vanished. In addition, carbon fibre reinforced composites prepared with MB-PACM as the matrix can also degrade at room temperature under mildly acidic conditions and the recovered carbon fiber retains the original textile structure, chemical structure, surface morphology, and mechanical properties.
A simultaneous reaction between primary amines and aldehydes/epoxides usually leads to a less controlled chemical structure. Therefore, Xu et al. synthesized a bifunctional epoxide by direct coupling of vanillin with hydrazone followed by a nucleophilic substitution reaction with epichlorohydrin.29 In this case, an imine bond was preformed within diepoxide monomers. The subsequent polymerization only involved the reaction between epoxides and amines, leading to a well-defined network. In addition to good ductility and reprocessability (Fig. 1a), one of the obtained materials has a high initial creep temperature of ∼105 °C, which is mainly attributed to the good stability of the hydrazine bonds at 100 °C and good hydrazine interchangeability at elevated temperatures. Meanwhile, the degradation of dihydrazone CANs exhibited temperature, solvent and acidity dependence. The dihydrazone structure followed a two-step degradation mechanism consisting of acid-catalyzed hydrolysis to a monohydrazone followed by further hydrolysis of the monohydrazone (Fig. 1b). Furthermore, the CANs exhibited a high killing rate (95.8%) against Gram-negative bacteria (E. coli) owing to the high antibacterial feature of the hydrazone bond and methoxy group in vanillin.
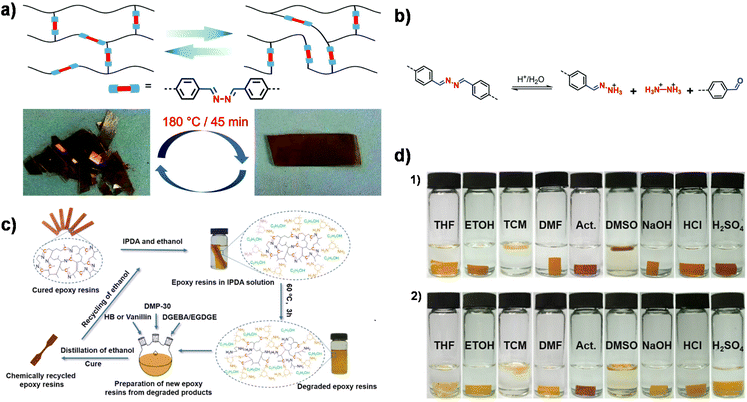 | ||
| Fig. 1 (a) Schematic diagram of dynamic exchange of imine bonds and reprocessing of dihydrazone CANs at a pressure of 10 MPa. (b) Degradation mechanism of dihydrazone CANs. Reproduced from ref. 29 with permission from the Royal Society of Chemistry, copyright 2020. (c) Chemical recycling process of cured epoxy/IH-VAN and epoxy/IH-HB resins. (d) Photographs of epoxy resins cured by IH-VAN (1) and IH-HB (2) in different solvents for 96 h. Reproduced from ref. 109 with permission from the American Chemical Society, copyright 2020. | ||
In the previous presentation, epoxides were typically introduced by the reaction of epichlorohydrin with the hydroxyl group of vanillin phenol. This significantly limits the types of epoxides available, especially the renewable epoxy compounds. In recent years, an increasing number of vegetable oil-based epoxy molecules have been reported. Therefore, it is also worthwhile to investigate how such renewable epoxides can be copolymerized with vanillin to construct CANs with a higher bio-content. To address this issue, instead of preparing imine-containing epoxides, Zeng's group synthesized a vanillin-based imine-containing bisphenol (VA) and then reacted it with epoxidized soybean oil (ESO) to obtain a bio-based epoxy CAN (ESO-VA) with a tensile strength of 7.7 MPa, a Young's modulus of 41.4 MPa and a Tg value of 27.6 °C.107 To further improve the thermomechanical properties, vanillin-based imine-containing bisphenols with higher rigidity (VSBs) were synthesized to afford bio-based epoxy CANs with controlled mechanical properties, reprocessability, reconfigurability, and weldability.108
Memon et al. synthesized two imine-containing curing agents (IH-VAN and IH-HB) from petroleum-based p-hydroxy benzaldehyde (HB) and vanillin (VAN).109 The IH-VAN epoxy resin exhibited superior performance with Tg >120 °C, tensile strength >60 MPa, Young's modulus >2500 MPa, and good solvent resistance (Fig. 1d). Bio-based IH-VAN-cured epoxy resin has comparable thermal and mechanical properties compared to petroleum-based IH-HB-cured epoxy resin. Notably, chemical degradation products can be reused to prepare new epoxy resins, thus achieving a closed-loop recycling process (Fig. 1c).
Early in 2012, Amarasekara used 3% hydrogen peroxide as an oxidant to catalyze the oxidation of vanillin by horseradish peroxidase to give a dimer (Scheme 11a).110 Subsequent condensation with alkyl diamines of different chain lengths gave Schiff base polymers with degrees of polymerization (DPs) of 25–32.
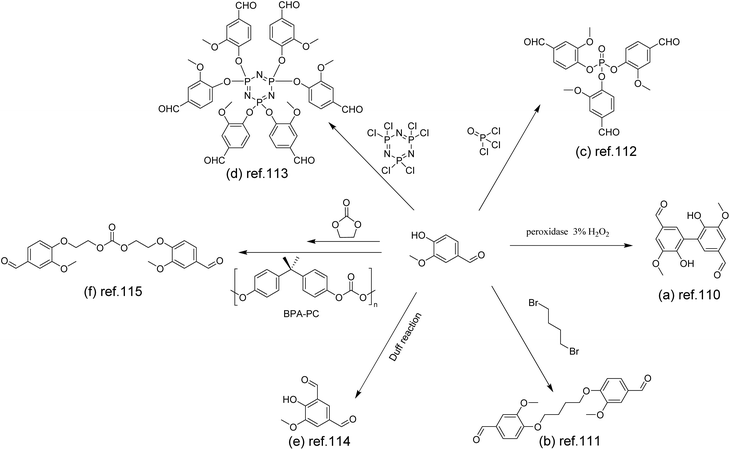 | ||
| Scheme 11 Synthetic route to polyimines via the functionalization of vanillin: (a) Reproduced from ref. 110 with permission from Hindawi, copyright 2012. (b) Reproduced from ref. 111 with permission from the American Chemical Society, copyright 2018. (c) Reproduced from ref. 112 with permission from the American Chemical Society, copyright 2020. (d) Reproduced from ref. 113 with permission from Elsevier, copyright 2021. (e) Reproduced from ref. 114 with permission from the American Chemical Society, copyright 2022. (f) Reproduced from ref. 115 with permission from John Wiley & Sons, copyright 2022. | ||
Alternatively, coupling reactions utilizing the phenolic hydroxyl can also afford di-, tri-, and even hexafunctional aldehydes. For instance, Geng et al. used 1,4-dibromoalkanes to couple vanillin (Scheme 11b), which were then polymerized with conventional diamines and triamines to obtain vanillin-based polyimine vitrimers.111 These vitrimers could be reprocessed at elevated temperatures due to the thermal reversibility of imine bonds. After reprocessing, the mechanical properties were maintained at the same level as those of the original samples (Fig. 2). The vitrimers were completely degraded under mild conditions (24 h at pH 2 & 50 °C). The reformed aldehydes and amines could then be recycled.
 | ||
| Fig. 2 Chemical recycling of cross-linked polyimine films (conditions: 50 °C, pH = 2, 24 h). Reproduced from ref. 111 with permission from the American Chemical Society, copyright 2018. | ||
To reach a higher bio-content, Zhou et al. designed di- and tri-aldehyde compounds (Scheme 11c) to polymerize with a commercial bio-based aliphatic amine.112 The mechanical and thermal properties of the obtained vitrimers with different cross-linking degrees were studied. The glass transition temperature (Tg) of vitrimers increased with the increase of the cross-linking density from 8.3 °C to 32.2 °C. The vitrimers underwent rapid degradation in 0.1 M HCl aqueous solution. After degradation, the aldehyde monomer was collected and reused to re-prepare vitrimers in a closed-loop.
The hexa-aldehyde monomer HVP (Scheme 11d) obtained by coupling vanillin with hexachlorocyclotriphosphazene was first reported by Liu et al.113 Applied as the matrix of carbon fibres, the high crosslinking density and high P/N content of HVP-based vitrimers demonstrated excellent flame retardancy and mechanical properties of the resulting composites. In addition, the non-destructive closed-loop recycling of carbon fibres and HVP/D230 monomers was also achieved under mild conditions. It is noteworthy that the recycled HVP/D230-CF showed almost the same mechanical properties as the original HVP/D230-CF.
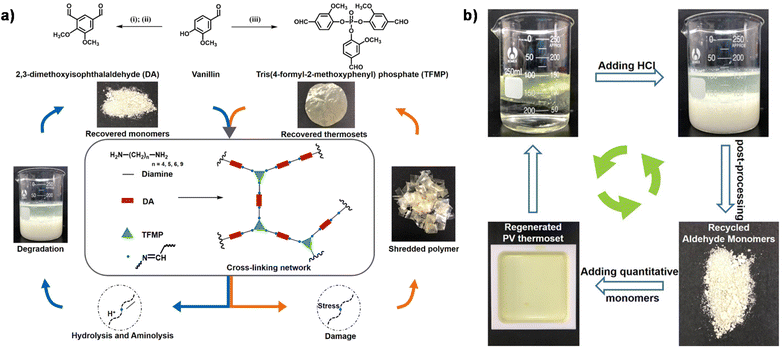
In addition to the coupling method, our group recently developed a new approach to introducing another aldehyde group directly onto the benzene ring of vanillin via the Duff reaction (Scheme 11e),114 followed by condensation with renewable diamines of different carbon chain lengths [1,4-butanediamine (BDA), 1,5-pentanediamine (PDA), 1,6-hexanediamine (HDA)]. By subsequent polymerization with biorenewable diamines and trialdehydes, a series of fully biobased vitrimers with a high aromatic content (59.2–61.3 wt%) were prepared. These fully biobased vitrimers achieved high tensile strength values (58.0 MPa) and storage modulus (3.07 GPa), which are essentially comparable to conventional thermosets. The Tg values of these vitrimers were tunable over a wide range (from 27 °C to 78 °C) to accommodate different application scenarios. Furthermore, these vitrimers could undergo a closed-loop recycle through both thermomechanical and chemical pathways (Fig. 3).114 Saito et al. combined for the first time the upcycling of polycarbonates and the benefits of reversible covalent bonds to create new high-performance materials (Fig. 4).115 BPA-PC waste particles were quantitatively converted to bisphenol A and di-vanillin ethoxy carbonate (DVEC) (Scheme 11f) via a vanillin derivative under solvent-free conditions and successfully applied it to the preparation of polyimine vitrimers (CL-P(ImC)-TREN). Furthermore, the cross-linked poly(imine-carbonate)s were subjected to efficient selective closed-loop recycling and showed selective separation of mixed plastic waste streams. The study pioneered the incorporation of sustainability and circularity, that is the utilization of bio-based resources, upcycling of commodity plastics, and closed-loop recycling of new polymers, providing new ideas for building a circular economy. In terms of utilization of polycarbonate waste particles, Reddy et al. also synthesized WPC-derived aldehydes (WPCCHO) from waste polycarbonate (WPC) and vanillin derivatives, which were then condensed with two aromatic diamines to produce polycarbonate imide vitrimers (PCIs).116 Unexpectedly, PCIs exhibited excellent resistance to acid hydrolysis and remained stable for 6 months even when immersed in 5 M H2SO4 THF/H2O solution (Fig. 5). The highly crosslinked network prevents cleavage of the imine bond, and this exceptional resistance to acid hydrolysis extends the application of PCIs in acidic environments. However, under mild conditions in the presence of hexylamine, it showed excellent degradation properties with rapid and complete depolymerization within 4 hours.
 | ||
| Fig. 3 (a) Synthetic route and reprocessing of the fully bio-based vitrimers. (b) Digital photographs of the chemical recycling process of PV-NDA-25 through the recovered monomer. Reproduced from ref. 114 with permission from the American Chemical Society, copyright 2022. | ||
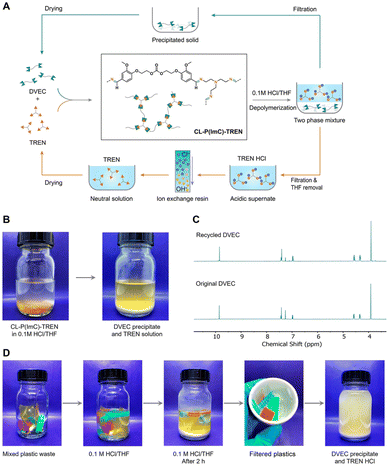 | ||
| Fig. 4 (A) Schematic representation of the closed-loop recycling scheme demonstrated with crosslinked poly(imine-carbonate)s (CL-P(ImC)-TREN). (B) Photographs showing the chemical depolymerization of CL-P(ImC)-TREN. The two monomers DVEC and TREN were isolated. (C) 1H NMR spectra of the original DVEC (bottom) and recycled DVEC (top). (D) Photographs showing the selective chemical depolymerization process of CL-P(ImC)-TREN (yellow) from a plastic waste mixture. DVEC and TREN HCl were able to be isolated and purified under acidic conditions for 2 h. Reproduced from ref. 115 with permission from John Wiley & Sons, copyright 2022. | ||
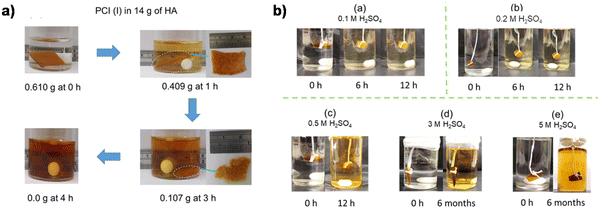 | ||
| Fig. 5 (a) Digital photographs of degradation of PCI (I) by HA. (b) Digital photographs of acid hydrolysis experiments of PCI (I) at different concentrations: (a) 0.1 M, (b) 0.2 M, (c) 0.5 M, (d) 3 M, and (e) 5 M H2SO4 in 30 mL of THF/H2O (v/v, 8/2) at 50 °C. Reproduced from ref. 116 with permission from the American Chemical Society, copyright 2023. | ||
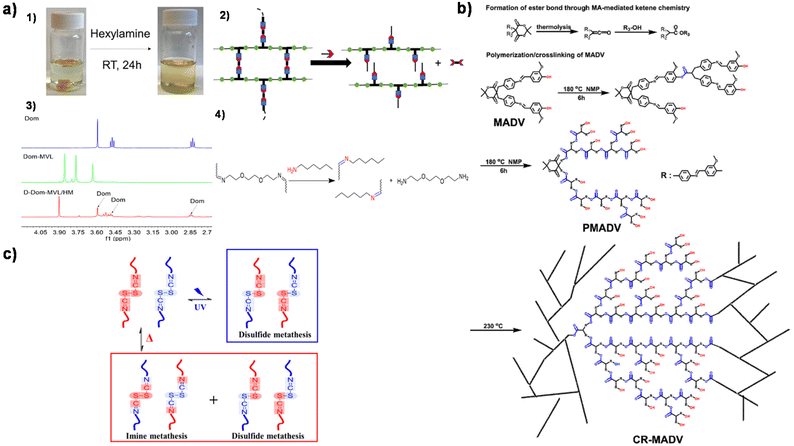 | ||
| Fig. 6 (a) Degradation of X-Dom-MVL. (1) X-Dom-MVL could be dissolved in hexylamine/THF solution. (2) The proposed reaction mechanism for chemical recycling using hexylamine/THF. (3) 1H NMR spectra of Dom, Dom-MVL, and the degraded product of X-Dom-MVL in hexylamine. (4) Schematic representation of imine bond exchange in hexylamine/THF. Reproduced from ref. 118 with permission from the American Chemical Society, copyright 2020. (b) The formation of ester bonds through MA-mediated ketene and the schematic representation of cross-linking reactions. Reproduced from ref. 120 with permission from the Royal Society of Chemistry, copyright 2023. (c) Schematic illustration of metatheses in the Schiff base according to different external stimuli. Reproduced from ref. 121 with permission from Elsevier, copyright 2019. | ||
In addition to being the primary reactive monomer, vanillin-based compounds can be used as functional co-monomers to improve the performance of conventional polymers. For example, Coates et al. introduced a vanillin-based epoxide monomer in the ring-opening copolymerization (ROCOP) of epoxides and anhydrides to give aldehyde-containing polyesters.119 The aldehyde-containing polyesters were then cross-linked with linear diamines to provide reprocessable thermosets with high tensile strength and solvent resistance. This ROCOP approach provides a flexible platform to probe the relationship between the CAN architecture and the observed dynamics.
In addition to vitrimers with only a single type of dynamic bond, the introduction of multiple dynamic interactions could promote faster stress relaxation behaviour in the vitrimers. Hung et al. used MA-mediated ketone chemistry to construct CANs with dynamic polyester backbones, while vanillin was involved in monomer synthesis to introduce imine bonds (Fig. 6b).120 This synthetic route allows greater flexibility in molecular design. The relatively low activation energy of imine exchange compared to ester bond exchange reduces the total activation energy of CAN exchange to 73 kJ mol−1. CANs have good thermal properties with a high Tg value of 213 °C and a thermal stability of 345 °C. In addition, they are reprocessable and recyclable. Lee et al. proposed a type of vitrimer featuring both an imine bond and a disulfide bond, by simply combining biorenewable cystine and vanillin (Fig. 6c).121 Disulfide bonds generally allow self-healing via metathesis under ambient conditions, and imine bonds also allow self-healing and reprocessing via rapid metathesis reactions with few side reactions. The crosslinker contains both imine and disulfide bonds, showing excellent self-healing and recyclability with a low Ea value for relaxation behavior (77 ± 1.5 kJ mol−1).
4.2. Polyacetals
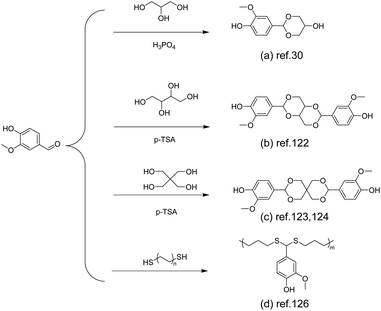 | ||
| Scheme 12 Schematic of the synthesis route to vanillin-derived polyacetals: (a) Reproduced from ref. 30 with permission from the Royal Society of Chemistry, copyright 2020. (b) Reproduced from ref. 122 with permission from the American Chemical Society, copyright 2023. (c) Reproduced from ref. 123 with permission from the American Chemical Society, copyright 2019 and reproduced from ref. 124 with permission from the American Chemical Society, copyright 2023. (d) Reproduced from ref. 126 with permission from John Wiley & Sons, copyright 2023. | ||
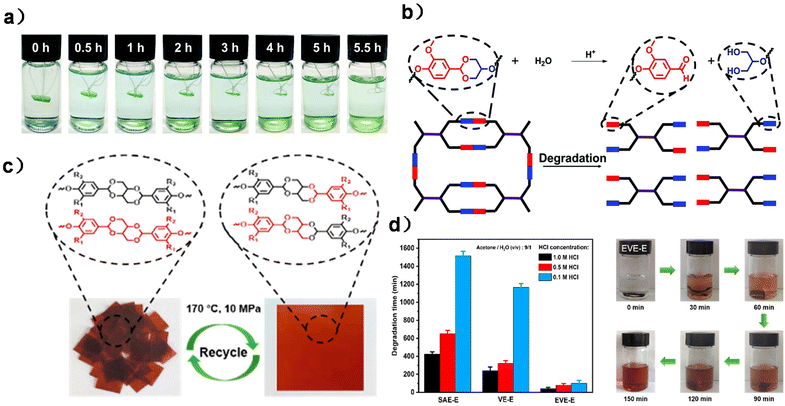 | ||
| Fig. 7 (a) Degradation of DGHMDO-DDM in 0.1 M HCl acetone/water (9/1, v/v) solution at 50 °C and at different times. (b) Degradation mechanism of DGHMDO-DDM. Reproduced from ref. 30 with permission from the Royal Society of Chemistry, copyright 2020. (c) Images of EVE-E before and after reprocessing. (d) Degradation times of three CANs under different acidic conditions and the hydrolysis process of the EVE-E film in 0.5 M HCl solution (acetone/H2O = 9/1) at 70 °C. Reproduced from ref. 122 with permission from the American Chemical Society, copyright 2023. | ||
Based on the formation of monoacetal, Lin and co-workers synthesized a bicyclic bis-acetal monomer (Scheme 12b) via the reaction between vanillin and erythritol. The obtained bisphenol was then applied to form a fully bio-based CAN by polyaddition with epoxidized soybean oil (ESO) as the comonomer and cross-linking agent. Due to the dynamic and degradable properties of acetal moieties, the CAN material has good recyclability and degradability and the degraded products can be reused, indicating closed-loop recyclability (Fig. 7c and d).122
In another recent work, Zhang et al. modified the structure by introducing NPG (neopentyl glycol) instead of 1,6-hexanediol, copolymerized with a spirocyclic acetal structure and DMT. The glass transition temperature of the obtained polyester (PNVT) was up to 103 °C. The degradation under acidic conditions yielded telechelic polymers consisting entirely of aldehyde groups as end groups, which were confirmed by MALDI-TOF-MS.124
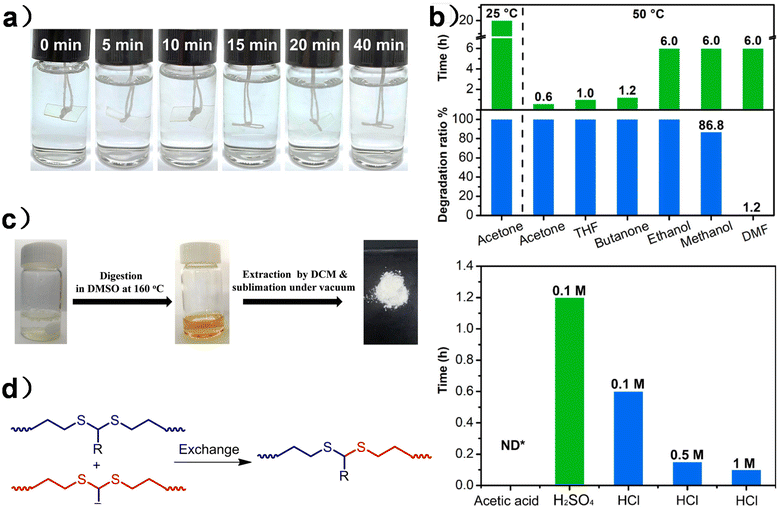 | ||
| Fig. 8 (a) Degradation process of PU-HMDO at different times in a 0.1 M HCl H2O/acetone (1/9, v/v) solution. (b) Degradation of PU-HMDO under different conditions. Reproduced from ref. 125 with permission from the American Chemical Society, copyright 2020. (c) Polythioacetals in DMSO before and after thermal treatment at 160 °C for 12 hours. (d) Schematic illustration of dynamic exchanges between dithioacetals. Reproduced from ref. 126 with permission from John Wiley & Sons, copyright 2023. | ||
5. Summary and outlook
The exploration of bio-based renewable resources is of great importance in today's context. Among such resources, lignin stands as the second largest biomass resource, surpassed only by cellulose, while vanillin stands as the most established commercial product derived from lignin. Consequently, the study of vanillin is of considerable importance. This review highlights the growing interest among researchers in the synthesis of vanillin-based polymers, as evidenced by the majority of recent studies discussed. The rigid benzene ring structure of vanillin could contribute excellent mechanical properties, facilitating the construction of diverse polymers. With its aldehyde and phenolic hydroxyl groups, vanillin offers limitless potential for the preparation of bio-based derivatives. As the demand for high-performance, environmentally friendly materials derived from renewable resources continues to rise, vanillin emerges as a promising and abundant resource. A comprehensive examination of vanillin-derived polymers across a range of categories, encompassing versatile materials, recyclable and degradable options, and thermoset polymers like phenolic resins, epoxy resins, and benzoxazole resins, among others, was conducted. Furthermore, thermoplastic polymers such as polyesters and polycarbonates, as well as polymers incorporating dynamic covalent bonds like Schiff base and acetal, are included, presenting a holistic perspective on vanillin-based derivatives. A thorough survey of the synthesis methods employed in the preparation of these renewable polymers, derived from vanillin-based monomers, is provided, ensuring an extensive understanding. Additionally, an insightful comparative analysis of the performance characteristics exhibited by these vanillin-based polymers is presented, enhancing the comprehension of their respective merits and capabilities.However, the utilization of vanillin-based compounds is still facing challenges. Firstly, the complicated synthesis approaches of many vanillin derivatives pose limitations on manufacturing with high yields. Despite the availability of biomass feedstocks like lignin, which serve as the source of vanillin, their complex chemical structures present challenges in achieving economically efficient approaches for obtaining uniform and controllable bio-based compounds. Moreover, the relatively high-cost and limited production scale of vanillin impede its widespread applications. In the case of vanillin-based vitrimers, current dynamic covalent bonds primarily consist of amine bonds and (thio)acetal bonds that have demonstrated degradability via hydrolysis or aminolysis. The incorporation of dynamic covalent bonds has also enhanced the self-healing, recyclability, and reprocessability of vanillin-based polymers. Nevertheless, there remain unresolved issues concerning the design of thermoset materials (preferably vitrimers) that can maintain long-term stability under harsh conditions, while simultaneously exhibiting chemical recyclability and thermomechanical reprocessability. In addition, the current reprocessing of vanillin-based vitrimers requires high temperatures as the dynamic covalent bonds involved are usually thermo-sensitive. Therefore, introducing dynamic covalent bonds with other types of sensitivities (e.g. light-sensitive) might largely expand the available reprocessing methods and reduce the corresponding energy consumption. Despite the remaining issues, the examples discussed in this review show the potential of vanillin-based polymers to replace petro-based analogues. As lignin refining and vanillin modification technologies continue to advance, vanillin-based polymers will certainly become an ideal alternative for the preparation of high-performance, functional polymeric materials that could eventually be widely commercialized. This advancement holds tremendous promise in the field of materials science, paving the way for bio-based material manufacturing.
Conflicts of interest
The authors declare no competing financial interests.Acknowledgements
This work was supported by the National Natural Science Foundation of China (51903190, 22175131) and the Shanghai Pujiang Program (19PJ1409600).References
- Y. Zhu, C. Romain and C. K. Williams, Nature, 2016, 540, 354–362 CrossRef CAS PubMed.
- A. Rahimi and J. M. García, Nat. Rev. Chem., 2017, 1, 0046 CrossRef.
- M. MacLeod, H. P. H. Arp, M. B. Tekman and A. Jahnke, Science, 2021, 373, 61–65 CrossRef CAS PubMed.
- N. Naik, B. Shivamurthy, B. H. S. Thimmappa, Z. H. Guo and R. Bhat, J. Compos. Sci., 2022, 6, 294 CrossRef CAS.
- C. Lu, X. Wang, Y. Shen, J. Wang, Q. Yong and F. Chu, J. Polym. Sci., 2022, 60, 2866–2874 CrossRef CAS.
- Y. Tachibana, M. Yamahata, H. Ichihara and K.-I. Kasuya, Polym. Degrad. Stab., 2017, 146, 121–125 CrossRef CAS.
- L. B. Brenelli, F. Mandelli, A. Z. Mercadante, G. J. d. M. Rocha, S. A. Rocco, A. F. Craievich, A. R. Gonçalves, D. d. C. Centeno, M. de Oliveira Neto and F. M. Squina, Ind. Crops Prod., 2016, 83, 94–103 CrossRef CAS.
- W. Boerjan, J. Ralph and M. Baucher, Annu. Rev. Plant Biol., 2003, 54, 519–546 CrossRef CAS PubMed.
- M. P. Pandey and C. S. Kim, Chem. Eng. Technol., 2011, 34, 29–41 CrossRef CAS.
- H. Lange, S. Decina and C. Crestini, Eur. Polym. J., 2013, 49, 1151–1173 CrossRef CAS.
- D. W. Wei, H. Wei, A. C. Gauthier, J. Song, Y. Jin and H. Xiao, J. Bioresour. Bioprod., 2020, 5, 1–15 CrossRef CAS.
- A. J. Ragauskas, G. T. Beckham, M. J. Biddy, R. Chandra, F. Chen, M. F. Davis, B. H. Davison, R. A. Dixon, P. Gilna, M. Keller, P. Langan, A. K. Naskar, J. N. Saddler, T. J. Tschaplinski, G. A. Tuskan and C. E. Wyman, Science, 2014, 344, 1246843 CrossRef PubMed.
- D. Stewart, Ind. Crops Prod., 2008, 27, 202–207 CrossRef CAS.
- S. K. Singh and P. L. Dhepe, Bioresour. Technol., 2016, 221, 310–317 CrossRef CAS PubMed.
- Z. H. Sun, B. Fridrich, A. de Santi, S. Elangovan and K. Barta, Chem. Rev., 2018, 118, 614–678 CrossRef CAS PubMed.
- C. F. Zhang and F. Wang, Acc. Chem. Res., 2020, 53, 470–484 CrossRef CAS PubMed.
- G. Velvizhi, K. Balakumar, N. P. Shetti, E. Ahmad, K. K. Pant and T. M. Aminabhavi, Bioresour. Technol., 2022, 343, 126151 CrossRef CAS PubMed.
- L. A. Z. Torres, A. L. Woiciechowski, V. O. D. Tanobe, S. G. Karp, L. C. G. Lorenci, C. Faulds and C. R. Soccol, J. Cleaner Prod., 2020, 263, 121499 CrossRef.
- S. Gillet, M. Aguedo, L. Petitjean, A. R. C. Morais, A. M. D. Lopes, R. M. Lukasik and P. T. Anastas, Green Chem., 2017, 19, 4200–4233 RSC.
- C. L. Chio, M. Sain and W. S. Qin, Renewable Sustainable Energy Rev., 2019, 107, 232–249 CrossRef CAS.
- J. R. Banu, S. Kavitha, R. Y. Kannah, T. P. Devi, M. Gunasekaran, S. H. Kim and G. Kumar, Bioresour. Technol., 2019, 290, 121790 CrossRef PubMed.
- K. Lee, Y. X. Jing, Y. Q. Wang and N. Yan, Nat. Rev. Chem., 2022, 6, 635–652 CrossRef PubMed.
- J. Zakzeski, P. C. Bruijnincx, A. L. Jongerius and B. M. Weckhuysen, Chem. Rev., 2010, 110, 3552–3599 CrossRef CAS PubMed.
- C. Li, X. Zhao, A. Wang, G. W. Huber and T. Zhang, Chem. Rev., 2015, 115, 11559–11624 CrossRef CAS PubMed.
- H.-R. Bjørsvik and F. Minisci, Org. Process Res. Dev., 1999, 3, 330–340 CrossRef.
- Y. V. Erofeev, V. L. Afanas'eva and R. G. Glushkov, Pharm. Chem. J., 1990, 24, 501–510 CrossRef.
- M. Fache, B. Boutevin and S. Caillol, ACS Sustainable Chem. Eng., 2015, 4, 35–46 CrossRef.
- B. G. Harvey, C. M. Sahagun, A. J. Guenthner, T. J. Groshens, L. R. Cambrea, J. T. Reams and J. M. Mabry, ChemSusChem, 2014, 7, 1964–1969 CrossRef CAS PubMed.
- X. Xu, S. Ma, S. Wang, J. Wu, Q. Li, N. Lu, Y. Liu, J. Yang, J. Feng and J. Zhu, J. Mater. Chem. A, 2020, 8, 11261–11274 RSC.
- B. Wang, S. Ma, Q. Li, H. Zhang, J. Liu, R. Wang, Z. Chen, X. Xu, S. Wang, N. Lu, Y. Liu, S. Yan and J. Zhu, Green Chem., 2020, 22, 1275–1290 RSC.
- Y. Liu, L. Sun, Y.-X. Huo and S. Guo, Microb. Cell Fact., 2023, 22, 147 CrossRef CAS PubMed.
- W. Jiang, X. Chen, Y. Feng, J. Sun, Y. Jiang, W. Zhang, F. Xin and M. Jiang, Fermentation, 2023, 9, 389 CrossRef CAS.
- K. Hirano and M. Asami, React. Funct. Polym., 2013, 73, 256–269 CrossRef CAS.
- T. Takeichi and N. Furukawa, in Polymer Science: A Comprehensive Reference, 2012, vol. 5, pp. 723–751 Search PubMed.
- M. Ragnar, C. T. Lindgren and N.-O. Nilvebrant, J. Wood Chem. Technol., 2000, 20, 277–305 CrossRef CAS.
- G. Foyer, B. H. Chanfi, B. Boutevin, S. Caillol and G. David, Eur. Polym. J., 2016, 74, 296–309 CrossRef CAS.
- G. Foyer, B.-H. Chanfi, D. Virieux, G. David and S. Caillol, Eur. Polym. J., 2016, 77, 65–74 CrossRef CAS.
- W. Yang, L. Jiao, X. Wang, W. Wu, H. Lian and H. Dai, Int. J. Biol. Macromol., 2021, 166, 1312–1319 CrossRef CAS PubMed.
- Q. Zhang, M. Molenda and T. M. Reineke, Macromolecules, 2016, 49, 8397–8406 CrossRef CAS.
- T. Vidil, F. Tournilhac, S. Musso, A. Robisson and L. Leibler, Prog. Polym. Sci., 2016, 62, 126–179 CrossRef CAS.
- R. Auvergne, S. Caillol, G. David, B. Boutevin and J. P. Pascault, Chem. Rev., 2014, 114, 1082–1115 CrossRef CAS PubMed.
- S. H. Song, K. H. Park, B. H. Kim, Y. W. Choi, G. H. Jun, D. J. Lee, B. S. Kong, K. W. Paik and S. Jeon, Adv. Mater., 2013, 25, 732–737 CrossRef CAS PubMed.
- S. Ma, X. Liu, Y. Jiang, Z. Tang, C. Zhang and J. Zhu, Green Chem., 2013, 15, 245–254 RSC.
- J. M. Raquez, M. Deléglise, M. F. Lacrampe and P. Krawczak, Prog. Polym. Sci., 2010, 35, 487–509 CrossRef CAS.
- S. Ma, X. Liu, L. Fan, Y. Jiang, L. Cao, Z. Tang and J. Zhu, ChemSusChem, 2014, 7, 555–562 CrossRef CAS PubMed.
- C. O. Tuck, E. Perez, I. T. Horvath, R. A. Sheldon and M. Poliakoff, Science, 2012, 337, 695–699 CrossRef CAS PubMed.
- M. Fache, A. Viola, R. Auvergne, B. Boutevin and S. Caillol, Eur. Polym. J., 2015, 68, 526–535 CrossRef CAS.
- M. Fache, R. Auvergne, B. Boutevin and S. Caillol, Eur. Polym. J., 2015, 67, 527–538 CrossRef CAS.
- T. Koike, Polym. Eng. Sci., 2012, 52, 701–717 CrossRef CAS.
- S. Wang, S. Ma, C. Xu, Y. Liu, J. Dai, Z. Wang, X. Liu, J. Chen, X. Shen, J. Wei and J. Zhu, Macromolecules, 2017, 50, 1892–1901 CrossRef CAS.
- M. Shibata and T. Ohkita, Eur. Polym. J., 2017, 92, 165–173 CrossRef CAS.
- Z. Fang, S. Nikafshar, E. L. Hegg and M. Nejad, ACS Sustainable Chem. Eng., 2020, 8, 9095–9103 CrossRef CAS.
- P. N. Shah, N. Kim, Z. Huang, M. Jayamanna, A. Kokil, A. Pine, J. Kaltsas, E. Jahngen, D. K. Ryan, S. Yoon, R. F. Kovar and Y. Lee, RSC Adv., 2015, 5, 38673–38679 RSC.
- A. W. Bassett, C. M. Breyta, A. E. Honnig, J. H. Reilly, K. R. Sweet, J. J. La Scala and J. F. Stanzione, Eur. Polym. J., 2019, 111, 95–103 CrossRef CAS.
- J. T. Cohen, G. Carlson, G. Charnley, D. Coggon, E. Delzell, J. D. Graham, H. Greim, D. Krewski, M. Medinsky, R. Monson, D. Paustenbach, B. Petersen, S. Rappaport, L. Rhomberg, P. B. Ryan and K. Thompson, J. Toxicol. Environ. Health, Part B, 2002, 5, 1–265 CAS.
- A. Z. Yu, E. M. Serum, A. C. Renner, J. M. Sahouani, M. P. Sibi and D. C. Webster, ACS Sustainable Chem. Eng., 2018, 6, 12586–12592 CrossRef CAS.
- A. W. Bassett, A. E. Honnig, C. M. Breyta, I. C. Dunn, J. J. La Scala and J. F. Stanzione, ACS Sustainable Chem. Eng., 2020, 8, 5626–5635 CrossRef CAS.
- M. Arslan, B. Kiskan and Y. Yagci, Macromolecules, 2015, 48, 1329–1334 CrossRef CAS.
- H. Ishida and Y. Rodriguez, Polymer, 1995, 36, 3151–3158 CrossRef CAS.
- O. S. Taskin, B. Kiskan and Y. Yagci, Macromolecules, 2013, 46, 8773–8778 CrossRef CAS.
- B. Kiskan and Y. Yagci, J. Polym. Sci., Part A: Polym. Chem., 2014, 52, 2911–2918 CrossRef CAS.
- L. Amornkitbamrung, S. Leungpuangkaew, T. Panklang, C. Jubsilp, S. Ekgasit, S. H. Um and S. Rimdusit, J. Sci.: Adv. Mater. Devices, 2022, 7, 100446 CAS.
- M. M. Samy, M. G. Mohamed and S.-W. Kuo, Eur. Polym. J., 2020, 138, 109954 CrossRef CAS.
- L. Puchot, P. Verge, T. Fouquet, C. Vancaeyzeele, F. Vidal and Y. Habibi, Green Chem., 2016, 18, 3346–3353 RSC.
- C. P. R. Nair, D. Mathew and K. N. Ninan, in New Polymerization Techniques and Synthetic Methodologies, 2001, vol. 155, pp. 1–99 Search PubMed.
- T. Fang and D. A. Shimp, Prog. Polym. Sci., 1995, 20, 61–118 CrossRef CAS.
- B. G. Harvey, A. J. Guenthner, H. A. Meylemans, S. R. L. Haines, K. R. Lamison, T. J. Groshens, L. R. Cambrea, M. C. Davis and W. W. Lai, Green Chem., 2015, 17, 1249–1258 RSC.
- L. Pezzana and M. Sangermano, Prog. Org. Coat., 2021, 157, 106295 CrossRef CAS.
- J. Dai, S. Ma, L. Zhu, S. Wang, L. Yang, Z. Song, X. Liu and J. Zhu, Polymer, 2017, 108, 215–222 CrossRef CAS.
- J. Zuo, Z. Liu, C. Luo and F. Lin, Prog. Org. Coat., 2021, 151, 106048 CrossRef CAS.
- C. L. Frewin, M. Ecker, A. Joshi-Imre, J. Kamgue, J. Waddell, V. R. Danda, A. M. Stiller, W. E. Voit and J. J. Pancrazio, Polymers, 2019, 11, 902 CrossRef PubMed.
- L. Chen, Q. Wu, G. Wei, R. Liu and Z. Li, J. Mater. Chem. C, 2018, 6, 11561–11568 RSC.
- T. O. Machado, C. Sayer and P. H. H. Araujo, Eur. Polym. J., 2017, 86, 200–215 CrossRef CAS.
- C. E. Hoyle and C. N. Bowman, Angew. Chem., Int. Ed., 2010, 49, 1540–1573 CrossRef CAS PubMed.
- T. Ren, Q. Chen, C. Zhao, Q. Zheng, H. Xie and M. North, Green Chem., 2020, 22, 1542–1547 RSC.
- Z. Gao, Y. You, Q. Chen, M. North and H. Xie, Green Chem., 2023, 25, 172–182 RSC.
- A. S. Amarasekara, B. Wiredu and A. Razzaq, Green Chem., 2012, 14, 2395–2397 RSC.
- Y. Liu and X. B. Lu, J. Polym. Sci., 2022, 60, 3256–3268 CrossRef CAS.
- A. Llevot, E. Grau, S. Carlotti, S. Grelier and H. Cramail, Polym. Chem., 2015, 6, 6058–6066 RSC.
- C. Zhao, C. Huang, Q. Chen, I. D. V. Ingram, X. Zeng, T. Ren and H. Xie, Polymers, 2020, 12, 586 CrossRef CAS PubMed.
- E. Xanthopoulou, Z. Terzopoulou, A. Zamboulis, S. Koltsakidis, D. Tzetzis, K. Peponaki, D. Vlassopoulos, N. Guigo, D. N. Bikiaris and G. Z. Papageorgiou, ACS Sustainable Chem. Eng., 2023, 11, 1569–1580 CrossRef CAS.
- Y. Liu and X. B. Lu, J. Polym. Sci., 2022, 60, 3256–3268 CrossRef CAS.
- B. N. Jang and C. A. Wilkie, Polym. Degrad. Stab., 2004, 86, 419–430 CrossRef CAS.
- D. Bai, Q. Chen, Y. Chai, T. Ren, C. Huang, I. D. V. Ingram, M. North, Q. Zheng and H. Xie, RSC Adv., 2018, 8, 34297–34303 RSC.
- A. Llevot, E. Grau, S. Carlotti, S. Grelier and H. Cramail, Polym. Chem., 2015, 6, 7693–7700 RSC.
- I. Barbara, A. L. Flourat and F. Allais, Eur. Polym. J., 2015, 62, 236–243 CrossRef CAS.
- F. Pion, A. F. Reano, P.-H. Ducrot and F. Allais, RSC Adv., 2013, 3, 8988–8997 RSC.
- A. L. Holmberg, J. F. Stanzione, R. P. Wool and T. H. Epps, ACS Sustainable Chem. Eng., 2014, 2, 569–573 CrossRef CAS.
- W. Denissen, J. M. Winne and F. E. Du Prez, Chem. Sci., 2016, 7, 30–38 RSC.
- D. Montarnal, M. Capelot, F. Tournilhac and L. Leibler, Science, 2011, 334, 965–968 CrossRef CAS PubMed.
- F. I. Altuna, C. E. Hoppe and R. J. J. Williams, Eur. Polym. J., 2019, 113, 297–304 CrossRef CAS.
- T. Liu, C. Hao, S. Zhang, X. Yang, L. Wang, J. Han, Y. Li, J. Xin and J. Zhang, Macromolecules, 2018, 51, 5577–5585 CrossRef CAS.
- A. Ruiz de Luzuriaga, R. Martin, N. Markaide, A. Rekondo, G. Cabañero, J. Rodríguez and I. Odriozola, Mater. Horiz., 2016, 3, 241–247 RSC.
- Z. Huang, Y. Wang, J. Zhu, J. Yu and Z. Hu, Compos. Sci. Technol., 2018, 154, 18–27 CrossRef CAS.
- S. Guggari, F. Magliozzi, S. Malburet, A. Graillot, M. Destarac and M. Guerre, ACS Sustainable Chem. Eng., 2023, 11, 6021–6031 CrossRef CAS PubMed.
- H. Zheng, Q. Liu, X. Lei, Y. Chen, B. Zhang and Q. Zhang, J. Polym. Sci., Part A: Polym. Chem., 2018, 56, 2531–2538 CrossRef CAS.
- R. Mo, J. Hu, H. Huang, X. Sheng and X. Zhang, J. Mater. Chem. A, 2019, 7, 3031–3038 RSC.
- J. Stouten, M. K. N. de Roy and K. V. Bernaerts, Mater. Today Sustainability, 2023, 22, 100396 CrossRef.
- P.-X. Tian, Y.-D. Li, Y. Weng, Z. Hu and J.-B. Zeng, Eur. Polym. J., 2023, 193, 112078 CrossRef CAS.
- Y. Chen, Z. Tang, Y. Liu, S. Wu and B. Guo, Macromolecules, 2019, 52, 3805–3812 CrossRef CAS.
- F. Dong, X. Yang, L. Guo, Y. Qian, P. Sun, Z. Huang, X. Xu and H. Liu, J. Colloid Interface Sci., 2023, 631, 239–248 CrossRef CAS PubMed.
- N. Lu, Q. Li, S. Ma, B. Wang, X. Xu, S. Wang, J. Ye, J. Qiu and J. Zhu, Eur. Polym. J., 2021, 147, 110291 CrossRef CAS.
- S. Engelen, A. A. Wróblewska, K. De Bruycker, R. Aksakal, V. Ladmiral, S. Caillol and F. E. Du Prez, Polym. Chem., 2022, 13, 2665–2673 RSC.
- M. A. Rashid, M. N. Hasan, M. A. R. Dayan, M. S. Ibna Jamal and M. K. Patoary, Reactions, 2023, 4, 66–91 CrossRef CAS.
- Q. Yu, X. Peng, Y. Wang, H. Geng, A. Xu, X. Zhang, W. Xu and D. Ye, Eur. Polym. J., 2019, 117, 55–63 CrossRef CAS.
- S. Wang, S. Ma, Q. Li, X. Xu, B. Wang, W. Yuan, S. Zhou, S. You and J. Zhu, Green Chem., 2019, 21, 1484–1497 RSC.
- X.-L. Zhao, Y.-Y. Liu, Y. Weng, Y.-D. Li and J.-B. Zeng, ACS Sustainable Chem. Eng., 2020, 8, 15020–15029 CrossRef CAS.
- Y.-Y. Liu, J. He, Y.-D. Li, X.-L. Zhao and J.-B. Zeng, Composants Commun., 2020, 22, 100445 CrossRef.
- H. Memon, H. Liu, M. A. Rashid, L. Chen, Q. Jiang, L. Zhang, Y. Wei, W. Liu and Y. Qiu, Macromolecules, 2020, 53, 621–630 CrossRef CAS.
- A. S. Amarasekara and A. Razzaq, ISRN Polym. Sci., 2012, 2012, 1–5 CrossRef.
- H. Geng, Y. Wang, Q. Yu, S. Gu, Y. Zhou, W. Xu, X. Zhang and D. Ye, ACS Sustainable Chem. Eng., 2018, 6, 15463–15470 CrossRef CAS.
- Z. Zhou, X. Su, J. Liu and R. Liu, ACS Appl. Polym. Mater., 2020, 2, 5716–5725 CrossRef CAS.
- T. Liu, J. Peng, J. Liu, X. Hao, C. Guo, R. Ou, Z. Liu and Q. Wang, Composites, Part B, 2021, 224, 109188 CrossRef CAS.
- K. Hong, Q. Sun, X. Zhang, L. Fan, T. Wu, J. Du and Y. Zhu, ACS Sustainable Chem. Eng., 2022, 10, 1036–1046 CrossRef CAS.
- K. Saito, F. Eisenreich, T. Turel and Z. Tomovic, Angew. Chem., Int. Ed., 2022, 61, e202211806 CrossRef CAS PubMed.
- K. S. K. Reddy, Y.-C. Chen, R.-J. Jeng, M. M. Abu-Omar and C.-H. Lin, ACS Sustainable Chem. Eng., 2023, 11, 8580–8591 CrossRef CAS.
- F. Masson, C. Decker, S. Andre and X. Andrieu, Prog. Org. Coat., 2004, 49, 1–12 CrossRef CAS.
- Y. Xu, K. Odelius and M. Hakkarainen, ACS Sustainable Chem. Eng., 2020, 8, 17272–17279 CrossRef CAS.
- R. L. Snyder, C. A. L. Lidston, G. X. De Hoe, M. J. S. Parvulescu, M. A. Hillmyer and G. W. Coates, Polym. Chem., 2020, 11, 5346–5355 RSC.
- D.-Y. Hung and Y.-L. Liu, Polym. Chem., 2023, 14, 1339–1349 RSC.
- S.-H. Lee, S.-R. Shin and D.-S. Lee, Mater. Des., 2019, 172, 107774 CrossRef CAS.
- W. Zhang, F. Gao, X. Chen, L. Shen, Y. Chen and Y. Lin, ACS Sustainable Chem. Eng., 2023, 11, 3065–3073 CrossRef CAS.
- S. V. Mankar, M. N. Garcia Gonzalez, N. Warlin, N. G. Valsange, N. Rehnberg, S. Lundmark, P. Jannasch and B. Zhang, ACS Sustainable Chem. Eng., 2019, 7, 19090–19103 CrossRef CAS.
- S. V. Mankar, J. Wahlberg, N. Warlin, N. G. Valsange, N. Rehnberg, S. Lundmark, P. Jannasch and B. Zhang, ACS Sustainable Chem. Eng., 2023, 11, 5135–5146 CrossRef CAS.
- B. Wang, S. Ma, X. Xu, Q. Li, T. Yu, S. Wang, S. Yan, Y. Liu and J. Zhu, ACS Sustainable Chem. Eng., 2020, 8, 11162–11170 CrossRef CAS.
- Y. Jin, C. Hu, J. Wang, Y. Ding, J. Shi, Z. Wang, S. Xu and L. Yuan, Angew. Chem., Int. Ed., 2023, e202305677 CAS.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2023 |