Research progress of MXenes and layered double hydroxides for supercapacitors
Zhongtai
Lin
a,
Xue
Li
a,
Hao
Zhang
b,
Ben Bin
Xu
 c,
Priyanka
Wasnik
c,
Priyanka
Wasnik
 cd,
Handong
Li
ce,
Man Vir
Singh
cd,
Handong
Li
ce,
Man Vir
Singh
 fg,
Yong
Ma
fg,
Yong
Ma
 *a,
Tingxi
Li
*a and
Zhanhu
Guo
*a,
Tingxi
Li
*a and
Zhanhu
Guo
 *c
*c
aSchool of Material Science and Engineering, Shandong University of Science and Technology, Qingdao 266590, P. R. China. E-mail: mayong@sdust.edu.cn; litx@sdust.edu.cn
bTechnical Center, Xi'an Aerospace Sunvalor Chemical Co., Ltd, Xi'an 710086, P. R. China
cMechanical and Construction Engineering, Faculty of Engineering and Environment, Northumbria University, Newcastle Upon Tyne, NE1 8ST, UK. E-mail: zhanhu.guo@northumbria.ac.uk
dAdvanced Materials Division, Engineered Multifunctional Composites (EMC) Nanotech LLC, Knoxville, TN 37934, USA
eCollege of Materials Science and Engineering, Taiyuan University of Science and Technology, Taiyuan 030024, China
fDepartment of Chemistry, Dev Bhoomi Uttarakhand University, Dehradun 248007, India
gUniversity Centre for Research & Development, Chandigarh University, Mohali, Punjab 140413, India
First published on 21st June 2023
Abstract
As a new type of 2D materials, MXene has the features of good conductivity and high specific surface area. Layered double hydroxide (LDH) is often used in the field of supercapacitors owing to its special layered structure, strong adjustability, and large specific capacitance. In recent years, many researchers have combined MXenes and LDHs to prepare electrode materials with high capacitance performance and studied their preparation methods, morphology, and electrochemical properties. This study summarizes the fabrication methods of MXenes and LDHs, as well as the recent progress of MXene/LDH composites in the field of supercapacitors in recent years. Moreover, the corresponding study direction on supercapacitors has been prospected. This study aims to illustrate the application of MXene/LDH composites for supercapacitors and hopes to offer guidance for further research on the basis of these promising materials.
 Zhongtai Lin | Zhongtai Lin is pursuing his master's degree at the Shandong University of Science and Technology, majoring in materials and chemical engineering. |
 Xue Li | Xue Li is pursuing her master's degree at the Shandong University of Science and Technology, majoring in materials and chemical engineering. |
 Tingxi Li | Tingxi Li received his Ph.D. in Yamagata University in 2002 and is currently a professor at the Shandong University of Science and Technology in Qingdao, China. |
1. Introduction
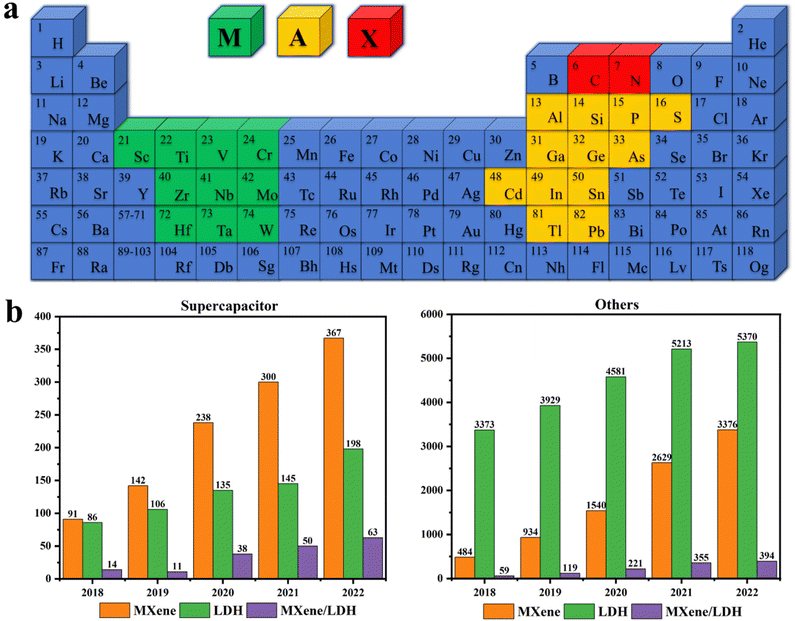
In recent years, supercapacitors have been favored because of their high power density, stable cycling performance, adjustable operating voltage, and wide operating temperature.1–6 One of the key technologies in supercapacitor research and development is the selection and use of electrode materials.7–11 Future supercapacitors possessing excellent energy and power density will depend on materials that store charges through fast redox reactions.12–15 Therefore, 2D materials are suitable candidates for advanced supercapacitor electrode materials.16–23Owing to their good electrical conductivity (104 S cm−1), mechanical properties (Young's modulus of 330 GPa), and specific capacitance (1500 F cm−3),24–29 MXenes have a very broad prospect in the application of electromagnetic shielding, microwave absorption, smart fabrics, and capacitive electrodes.30–35 The precursor for MXene is MAX.36–41Fig. 1a shows MAX elements, where M, A, and X are metal elements from a transition group, III or IV group, and a C or N, respectively.31,42–46 The general chemical formula of MXene is Mn+1XnTx, in which T denotes functional groups of –OH, O2−, or F− linked on its surface.47–51 Mashtalir et al.52 isolated monolayer MXene flakes by intercalating large organic molecules and layering agents, which opened the door to explore the true 2D properties of MXenes. Naguib et al.53 also found that ordered double transition metal carbides, such as Mo2TiC2Tx, Cr2TiC2Tx, and Mo2Ti2C3Tx, have added more than 25 members to the Mxene family.54–60 At present, more than 70 kinds of Mxene precursors have been explored, but the most widely studied is still the Ti3C2Tx material.40,61–68
MXenes are widely used as electrodes for supercapacitors owing to their excellent physicochemical properties.69–74 Since Lukatskaya et al.75 first proposed that cations of Na+, K+, NH4+, Mg2+, and Al3+ can spontaneously intercalate and desorb between Ti3C2Tx MXene layers, various supercapacitors based on MXene have been developed. Electrode materials, such as clay-like Ti3C2Tx MXene,76 macroporous Ti3C2Tx MXene,18,77 PPy/Ti3C2Tx,78 MXene/CNT,79 and MXene/LDH80 have also been extensively synthesized, and studied, showing huge application prospects in energy storage.81–88
Recently, layered bimetallic hydroxides (LDHs) have received an upsurge of study at around the globe owing to their adjustable composition, structure, and morphology.87,89–94 With their unique layered structure and anion exchange properties,17,95–98 LDHs have been widely used in catalysis, adsorption, pharmacy, photochemistry, electrochemical energy storage equipment, and other fields.99–103 LDHs have the general structural formula:104 M1−x2+Mx3+(OH)2Ax/nn−1·mH2O, where M2+ and M3+ represent divalent (e.g. M = Ni, Co, Cu, Mg, etc.) and trivalent (e.g. M = Al, Fe, Mn, Cr, etc.) metal ions, respectively.105–107 A represents the interlayer anion for separation (e.g. CO32−, NO3−, Cl−, etc.), which is used to balance the medium positive charge of LDHs main laminate.108–111 LDHs with different physicochemical properties are obtained by varying the molar ratios of M2+/M3+,112–114 the properties of metal cations, the type of interlayer anions, and other conditions.115–120
LDHs have attracted extensive and in-depth research attention in the field of electrochemistry.39,121–126 Sarfraz et al.127 summarized and discussed the preparation method of LDHs and their latest progress in supercapacitors. Zhao et al.128 focused on summarizing the relationship between various LDH composites and material structures and electrochemical properties, and the corresponding charge storage mechanism. Yan et al.129 Briefly introduced the preparation method, structural design, chemical modification, effective methods to improve the performance of supercapacitors, and future development of NiMn-LDH.
Although LDH materials have become an attractive candidate for ultra-high energy storage capacitor materials due to outstanding theoretical capacitance and low cost,130–136 the original LDH nanostructures have serious defects, including weak stability of the microstructure, poor conductivity, limited ion/electron transfer rate, surface re-stacking hinders the accessibility of the structure, and poor magnification performance and cycle stability in practical applications.39,137–140 Surprisingly, LDH materials can better interact with high-performance 2D materials through the –OH group on the surface to form solid nanocomposites.141–145 Through synergetic heterostructures, the structural characteristics of LDHs and MXenes are complementary, which has the hope to improve the shortcomings of LDHs and the relatively poor capacitance of MXene and realize the application of excellent electrode materials as supercapacitors.146–149Fig. 1b is a bar chart of the number of studies on MXene, LDH, and their composites in the field of supercapacitors and other fields published on the Web of Science. One can find that the number of studies published each year is on the rise, indicating that more and more people have begun to pay attention to these materials, as well as their superior properties and broad prospects in related fields. In recent years, Zhang et al.95 summarized the stripping methods of LDHs, Rohit et al.150 summarized the application of electrodeposited LDH in environment and energy, Kosnan et al.151 summarized the preparation of MXene and MoS2, and Luo et al.36 summarized the composite and research progress of MXene and conductive polymer, however, the review of MXene/LDH composites is very rare, therefore, here, various preparation methods of MXene and LDH materials and the research progress of their composites are reviewed in detail.
2. Preparation of MXenes
2.1 HF etching
The MAX phase can generally be exfoliated into the corresponding MXene by HF solution. Taking Ti3AlC2 as an example, in 2011 Naguib et al.152 placed the layered precursor Ti3AlC2 in HF for the first time to weaken the M–A metal bond and keep the M–X bond strong. Fig. 2a is a schematic diagram of the process of Ti3AlC2 being corroded by HF. HF preferentially selects weak Ti–Al bond, so that the Ti–C layer is gradually divided to generate MXenes phase materials. At the same time, the peeling of the Al atomic layer exposes the Ti element in the Ti–C layer, which combines with the functional groups of –OH and F− rich in the solution to form complexes. The prepared Ti3C2Tx presents an accordion-like layered stacking structure (as shown in Fig. 2b) and is hard to prepare single-layer layers of low-defect Ti3C2Tx. TEM images of exfoliated MXene nanosheets are shown in Fig. 2c and d. Reactions that may occur during the reaction include:153| Ti3AlC2 + 3HF = AlF3 + 3/2H2 + Ti3C2 | (1) |
| Ti3C2 + 2H2O = Ti3C2(OH)2 + H2 | (2) |
| Ti3C2 + 2HF = Ti3C2F2 + H2. | (3) |
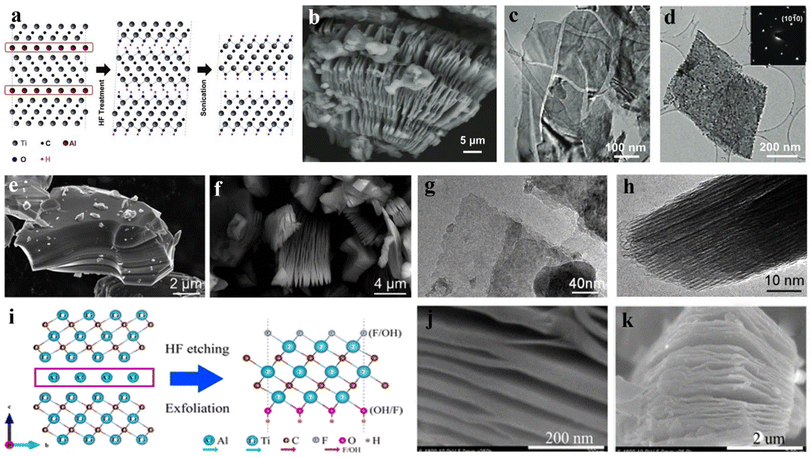 | ||
| Fig. 2 (a) Schematic diagram of corrosion behavior of Ti3AlC2; (b) SEM image of multi-layer Ti3C2Tx in the shape of the accordion; (c and d) TEM images of exfoliated MXene nanosheets.156 © 2011 WILEY-VCH Verlag GmbH & Co. KGaA, Weinhei. (e and f) SEM images of blocky Ti3AlC2 and layered Ti3C2Tx; TEM images of (g) exfoliated single-layer Ti3C2Tx MXene and (h) multi-layer Ti3C2 after ultrasonic treatment (side views).154 © 2013 Elsevier B.V. (i) Synthesis and structure diagram of Ti3C2Tx; (j and k) schematic diagram of multi-layer Ti3C2 after HF treatment.155 © 2015 American Chemical Society. | ||
Mashtalir et al.154 studied the kinetic control process of Al etching using 50 wt% HF, which shows that increasing the immersion temperature, increasing the reaction time, and reducing the initial maximum particle size is conducive to the rapid phase transition from bulk Ti3AlC2 to Ti3C2Tx (Fig. 2e and f). Besides, the TEM images shown in Fig. 2g and h confirm the successful preparation of exfoliated single-layer Ti3C2Tx MXene. Wang et al.155 synthesized Ti3C2Tx MXene by etching Ti3AlC2 powder in the presence of 50 wt% HF, resulting in the periphery of MXene with –OH or –F surface groups. After HF treatment, accordion-like morphology is obtained (Fig. 2j and k). For the surface structure of single-layer Ti3C2Tx, it is proved on the atomic scale that after the HF treatment, the functional groups would tend to be distributed on the surface of Ti atoms (Fig. 2i).
2.2 In situ generated HF etching
Etching with HF is a facile and versatile method to prepare MXenes, but HF is highly toxic and dangerous, and the synthesized MXenes may generate a large number of defects. In the later stage of preparation, macromolecular intercalation technology needs to be introduced, so the hydrolysis of hydrofluoride salt or the method of in situ etching of the precursor with fluoride salt and acid has emerged (as shown in Fig. 3a).157–159 Instead of adding HF directly, LiF and high-concentration HCl are used to generate HF to corrode the A layer through the metathesis reaction.84,160 It is found that HF synthesized in situ by applying the mixture of fluoride salts (LiF, NaF, CaF2, etc.) and HCl or H2SO4 is much milder than the pure HF added directly, and the obtained MXenes flakes have better etching properties including smaller lateral dimensions and fewer defects.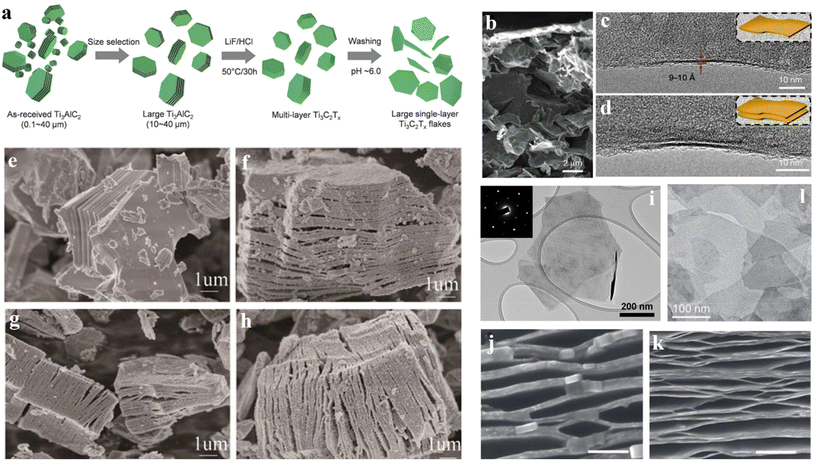 | ||
| Fig. 3 (a) Synthesis of Ti3C2Tx MXene flakes by etching Ti3AlC2 MAX particles with LiF and HCl.159 © 2020 WILEY-VCH Verlag GmbH & Co. KGaA, Weinhm. (b) SEM image of the internal thin film, showing the lamellar structure; (c and d) TEM images of single-layer and double-layer MXene edges.160 © 2014 Macmillan Publishers Limited. (e) SEM images of Ti3AlC2; SEM images of the products etched using (f) NaHF2, (g) KHF2, and (h) NH4HF2.157 © 2016 Elsevier Ltd. (i) TEM image of Ti3C2Tx MXene wafer, inset showing diffraction patterns for selected area.30 © 2020 WILEY-VCH Verlag GmbH & Co. KGaA, Weinhei. (j and k) SEM images of F-Ti3C2 intercalated by N2H4·H2O and DMF, respectively.52 © 2013 Macmillan Publishers Limited. (l) TEM image of d-Nb2CTx slice.163 © 2015 WILEY-VCH Verlag GmbH & Co. KGaA, Weinhm. | ||
In 2014, Ghidiu et al.160 used the mixture of HCl and LiF to etch Ti3AlC2 at 40 °C for 45 h, and successfully prepared a Ti3C2 2D layered structure material with excellent performance. Fig. 3b is an SEM image of the internal thin film, showing the lamellar structure. Fig. 3c and d are TEM images of monolayer and bilayer slices, respectively. The larger interlayer spacing has the potential to obtain more electrochemically active surfaces, providing faster electrolyte ion diffusion channels. The principle is shown below:
| 2Ti3AlC2 + 6LiF + 6HCl = 2Ti3C2 + Li3AlF6 + AlF3 + 3LiCl + 3H2. | (4) |
The obtained samples possess few defects, and the sheet size can reach several hundreds of nanometers (as shown in Fig. 3i). The single-layer or few-layer (thickness ≈ 3 μm) Ti3C2Tx can be obtained without ultrasonic and intercalation exfoliation. In addition, due to the entry of Li+, the interlayer spacing between Ti3C2Tx sheets is increased, making it hydrophilic.161 Although this method has a mild reaction, it is necessary to strictly control the relative ratio of LiF and HCl, etching time, and etching temperature during the preparation process, and it will contain many unetched MAX phases and surface groups such as –OH and –F.
Similarly, the mixtures of H2SO4 or HCl containing NaF, KF, CsF, [(C4H9)4NF], and CaF2 are used as etchants. Halim et al.162 used NH4HF2 to etch the MAX phase, and the corresponding reactions that occur are:
| 2Ti3AlC2 + 6NH4HF2 = 2(NH4)3AlF6 + 3H2 + 2Ti3C2 | (5) |
| Ti3C2 + aNH4HF2 + bH2O = (NH3)c(NH4)dTi3C2(OH)xFy. | (6) |
This process is slow, and the exfoliation and the intercalation proceed at the same time. Therefore, a more uniform atomic layer distribution of Ti3C2Tx was obtained. In 2017, Feng et al.157 etched the MAX phase with NaHF, KHF, and NH4HF2. At the same time, due to the insertion of ions into the middle of the MXene lamellae, the lamellar gap increased, and MXene with a larger interlayer gap was obtained. Fig. 3e, f, and h show the SEM images of Ti3AlC2 and MXene etched by NaHF2, KHF2, and NH4HF2, respectively. Compared with the HF corrosion method, this method is both safe and effective, and the prepared MXene is of better quality and has greater advantages in energy storage and electrical properties.
In addition, in 2013, Gogotsi et al.52 first used dimethyl sulfoxide (DMSO) as an intercalant to separate stacked Ti3C2 sheets, and then separated multilayer MXenes into monolayer or few-layer sheet structures by ultrasonic treatment. However, DMSO has a high boiling point, which may remain in the solution and be difficult to remove, replacing the active groups on the periphery of the MXene phase, and making the sheets stick together. After that, they proposed improved intercalating agents, tetrabutylammonium hydroxide (TBAOH), choline hydroxide, n-butylamine, etc. to separate Ti3CN, V2C, and Nb2C flakes.53,163Fig. 3j and k are the SEM images of F-Ti3C2 intercalated by N2H4·H2O (HM) and DMF, respectively, showing obvious macropores. Fig. 3l shows the TEM image of the d-Nb2CTx slice. However, these intercalators are commonly just suitable for particular MXene phases, and single-layer flake structures have not yet been obtained.
2.3 Alkaline etching
For avoiding the employment of hazardous fluoride salts and HF, new synthetic routes have been explored. Because of the strong binding between alkali and Al elements, it is theoretically feasible to prepare MXenes from the MAX phase as an etchant. Therefore, MXenes can be synthesized from the MAX phase using an alkali-based etching method. In Fig. 4a, Ti3AlC2 reacted in the presence of NaOH aqueous solution under various conditions is shown. Route a shows that aluminum hydroxide (oxide) hinders the aluminum extraction process at low temperatures. Route b exhibits that some aluminum hydroxide dissolves in NaOH at high temperatures and lower NaOH concentrations, but high water content causes MXene to oxidize and generate NTOs. Route c displays that high temperature and high NaOH concentration help to dissolve aluminum hydroxide in NaOH.164 Chemical reactions between Ti3AlC2 and NaOH aqueous solution are as follows:158| Ti3AlC2 + OH− + 5H2O = Ti3C2(OH)2 + Al(OH)4− + 5/2H2 | (7) |
| Ti3AlC2 + OH− + 5H2O = Ti3C2O2 + Al(OH)4− + 7/2H2. | (8) |
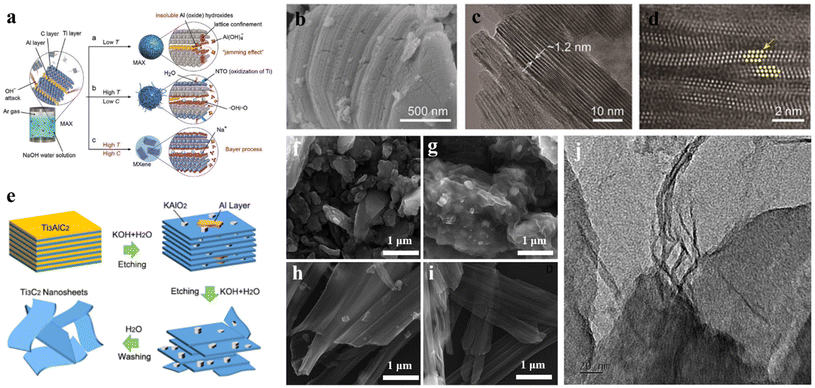 | ||
| Fig. 4 (a) Reaction of Ti3AlC2 and NaOH aqueous solution under different conditions; (b) SEM, (c) TEM, and (d) HAADF-STEM of Ti3C2Tx flakes.158 © 2018 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. (e) Schematic diagram of etching and peeling of Ti3AlC2 in KOH solution; SEM images of untreated Ti3AlC2 (f) and KOH treated Ti3AlC2 (g); (h and i) SEM and (j) TEM images of exfoliated nanosheets.166 © 2017 American Chemical Society. | ||
Thermochemical reactions of NaOH aqueous solution with Al oxide/hydroxide:158
| Al(OH)3 + OH− = Al(OH)4− | (9) |
| AlO(OH)(γ-AlO(OH)) + OH− + H2O = Al(OH)4− | (10) |
| AlO(OH) (α-AlO(OH)) + OH− + H2O = Al(OH)4−. | (11) |
Li et al. reported a hydrothermal process to produce Ti3C2Tx with the addition of NaOH.158 The outcomes indicate that at 270 °C, Al in Ti3AlC2 can be successfully and selectively removed in 27.5 M NaOH solution. SEM and TEM images (Fig. 4b–d) exhibit that the prepared Ti3C2Tx has a compact layered structure. In addition, Xie et al.165 successively treated Ti3AlC2 powder in 1 M NaOH solution and 1 M H2SO4 at 80 °C for 2 h to obtain Ti3C2Tx MXene. Li et al.166 treated Ti3AlC2 in high concentrations of KOH and found that –OH groups replaced the Al layer. Fully layered Ti3C2(OH)2 nanosheets were obtained by washing (Fig. 4e). After reacting with KOH solution for 6 h, the product was fanned out, but the particle profile of the precursor was basically maintained (Fig. 4f–i). Further etching of residual Al atoms could result in complete delamination, as shown in SEM images of Fig. 4h and i. One can observe the unorderly stacked nanosheets. Fig. 4j is a TEM image of exfoliated Ti3C2 nanosheets. Intercalation without fluorine functional groups and cations results in a larger interlayer distance, which showed higher capacitance and better rate capability of Ti3C2Tx in comparison with that prepared by HF etching.167
2.4 Electrochemical etching
The operating conditions of the above methods are relatively harsh, which are dangerous, and limited. Electrochemical etching is milder since it is carried out in fluorine-free electrolytes. Chloride ion (Cl–) has a strong binding ability with Al and can destroy the Ti–Al bond to produce Ti3C2Tx MXenes without any fluorine terminal. The subsequent opening of grain boundaries is conducive to further penetration of Cl- and the intercalation of other substances in the electrolyte. Yang et al.168 reported an anodic corrosion process to peel Ti3AlC2. The electrolyte is a mixture of 1.0 M NH4Cl and 0.2 M TMAOH (Fig. 5a). In the etching process, Cl- and Al have a strong binding ability, which breaks the Ti–Al bond. Moreover, the existence of NH4OH opens the edge etching of Ti3AlC2 and promotes deep etching under the surface. Fig. 5b shows SEM and HR-TEM images of Ti3AlC2 and Ti3C2Tx. Fig. 5c and d are SEM images of monolithic Ti3C2Tx. It can be seen that after electrochemical etching, the multilayer Ti3C2Tx powder remains tightly stacked, similar to the dense layered massive Ti3AlC2, showing no accordion-like structure. Nevertheless, due to the destruction of the Al–Ti bond, the etched powder can be separated into thin layers by ultrasound.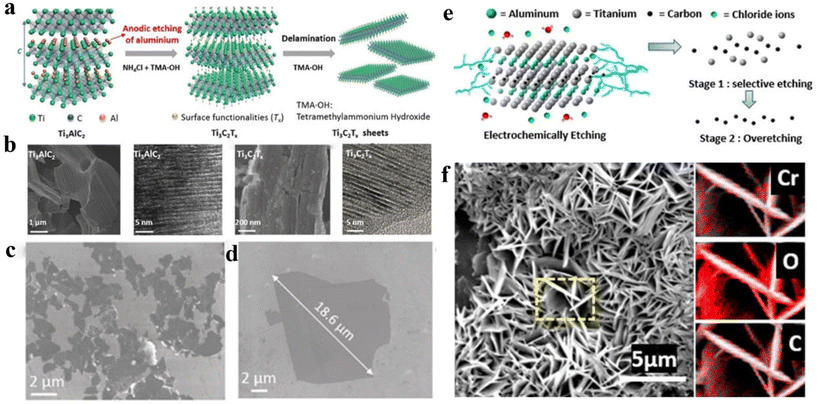 | ||
| Fig. 5 (a) Schematic of etching and layering process for Ti3C2Tx; (b) SEM and HR-TEM images of Ti3AlC2 and Ti3C2Tx, respectively; (c and d) SEM images of monolithic Ti3C2Tx.168 © 2018 Wiley-VCH Verlag GmbH & Co. KGaA, Weinhem. (e) Electroetching mechanism of Ti2AlC in HCl electrolyte; (f) SEM image of Cr2CTx with flower-like structure.169 © 2019 American Chemical Society. | ||
Pang et al.169 studied a universal method to synthesize Ti-based MXenes through a thermal-assisted electrochemical etching by adding dilute HCl solution, and extended the etching strategy of V2CTx and Cr2CTx. Fig. 5e exhibits the corresponding mechanism. Fig. 5f is an SEM image of Cr2CTx with a flower-like structure. After ultrasound-assisted liquid stripping and purification, the densely layered MXene is separated into a large number of sheets with transverse sizes greater than 1 μm and thickness of about 5–80 nm, which are assembled into flower structure Cr2CTx. The electrochemical etching method is an ideal method for preparing MXene. However, due to the lack of previous research, this method still has many shortcomings.
2.5 Lewis acid molten salt etching
Whether it is prepared by HF direct etching method or fluorine salt plus acid in situ etching method, it is more or less harmful to the environment and experimenters. The preparation of MXene via etching of the precursor in the presence of molten salt gradually ranks among the mainstream preparation methods because it can reduce the damage of layered MXene in the experimental process and is friendly to the environment. Based on the principle of Lewis alkali reaction, the molten salt method uses the cations of the molten salt to capture the A layer of the MAX phase, so that the A layer is oxidized and removed to obtain the MXene lamellar structure. In this method, the solid salt must be heated to the molten state before the reaction can be carried out, so the requirement of the reaction temperature is very high. However, above 800 °C, MXene can phase change to form a 3D crystalline structure,170 so the reaction temperature of this method is generally controlled below 800 °C.Urbankowski et al.171 synthesized Ti4N3/MXene by etching Al atoms in the Ti4AlN3 precursor with molten fluoride salts (KF, NaF, and LiF) in an argon environment at 550 °C, and then used (C4H9)4NOH (TBAOH) as the intercalation agent to obtain the stratification of MXene (Fig. 6a). SEM images also show the successful preparation of Ti4N3 with a typical accordion-like structure (Fig. 6b). Li et al.172 proposed a Lewis acid molten salt preparation method to prepare MXene. At 750 °C, taking the synthesis of Ti3C2Tx by Ti3SiC2 and CuCl2 as an example (Fig. 6c), the principles are as follows:
| Ti3SiC2 + 2CuCl2 = Ti3C2 + SiCl4 + 2Cu | (12) |
| Ti3C2 + CuCl2 = Ti3C2Cl2 + Cu. | (13) |
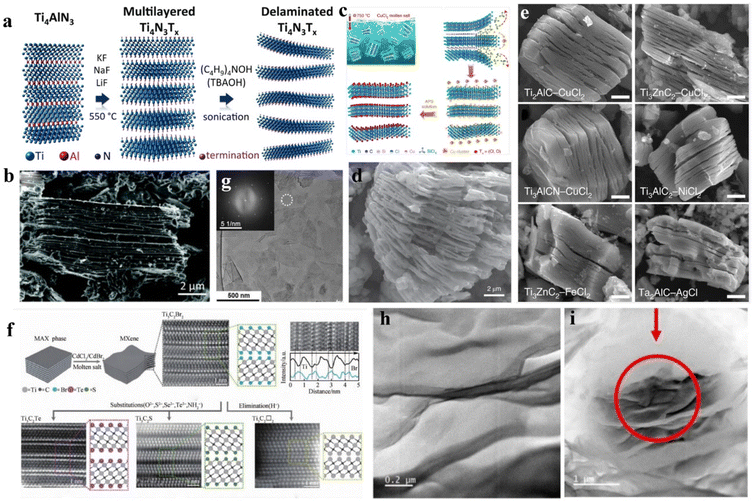 | ||
| Fig. 6 (a) Schematic of preparation of Ti4N3Tx with adding molten salt at 550 °C, and TBAOH as intercalator; (b) SEM image of the processed Ti4N3Tx.171 © 2016 The Royal Society of Chemistry. (c) Schematic of preparation of Ti3C2Tx MXene; (d) SEM image of MS-Ti3C2Tx MXene; (e) SEM image of MXene materials prepared with different molten salts.172 © 2021 Springer Nature Limited. (f) The surface reaction of MXene in molten inorganic salt; (g) image after multi-layer Ti3C2Tn MXene layering.173 © 2020 The American Association for the Advancement of Science. The formation of nanolaminates after ion irradiation analysed by HRTEM: (h) stacked nanolaminates and (i) localized cluster of nanolaminates.174 © 2020 Elsevier B.V. | ||
They also found that etching the Ti3AlC2 MAX phase with the addition of molten ZnCl2 and other Lewis acid salts above 500 °C can produce Ti3C2Cl2 MXenes containing Cl surface terminals.172Fig. 6d shows an SEM image of the prepared MS-Ti3C2Tx MXene. By increasing the ratio of MAX![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ZnCl2, Ti3ZnC2 is further changed into Ti3C2Cl2 MXene, which eliminates unnecessary oxidation and hydrolysis in the presence of etching molten salts. Moreover, a general way to synthesize MXene in the presence of Lewis acid molten salt is proposed. Fig. 6e shows SEM images of the MXene material finally obtained from the stripping system determined according to the mapping map. Vladislav Kamysbayev et al.173 fabricated a variety of MXenes containing Cl or Br surface terminals such as Ti3C2Cl2 in CdCl2 and Ti3C2Br2 in CdBr2. Fig. 6f exhibits the surface reaction of MXenes in molten inorganic salts. Fig. 6g displays an SEM image of Ti3C2Tn MXene after delamination.
ZnCl2, Ti3ZnC2 is further changed into Ti3C2Cl2 MXene, which eliminates unnecessary oxidation and hydrolysis in the presence of etching molten salts. Moreover, a general way to synthesize MXene in the presence of Lewis acid molten salt is proposed. Fig. 6e shows SEM images of the MXene material finally obtained from the stripping system determined according to the mapping map. Vladislav Kamysbayev et al.173 fabricated a variety of MXenes containing Cl or Br surface terminals such as Ti3C2Cl2 in CdCl2 and Ti3C2Br2 in CdBr2. Fig. 6f exhibits the surface reaction of MXenes in molten inorganic salts. Fig. 6g displays an SEM image of Ti3C2Tn MXene after delamination.
The preparation of MXene materials through the Lewis acid molten salt stripping route obviously increases security and lowers the difficulty to treat waste liquid. However, it has not been widely used because of the harsh reaction conditions of high reaction temperature and low etching rate. Recently, Horak et al.174 prepared MXene thin films by low-energy ion beam sputtering, annealing, and strong ion beam irradiation. Fig. 6h shows the HAADF-STEM image, presenting the layered structure composed of stacked crystalline flakes with smooth surfaces. In addition, argon ion irradiation produces defects in MXene materials, such as nanoplate precipitation (Fig. 6i). However, at present, it is only a preliminary preparation process, and there are still existing problems such as high equipment requirements and difficult processes.
2.6 Chemical vapor deposition
Compared with the above strategy involving chemical etching to extract the “A” layer from MAX, researchers also studied the preparation of MXenes by chemical vapor deposition (CVD), which is usually used to prepare ultra-thin materials with a thickness of nano level.175 The specific step of the CVD method is to gasify various raw materials into the system for chemical reaction, and then deposit them on the crystal surface preset in the reaction chamber to obtain a film, or the gaseous raw materials react directly with the crystal and deposit them on the surface to form a film. When preparing MXene by the CVD method, the suitable C source or N source is gasified at high temperature and reacted on the surface of transition metal foil to obtain ultra-thin layers with nano thickness. The obtained MXene crystal structure and surface group distribution are highly controllable, with few crystal defects and excellent electrical conductivity.176,177Halim et al.162 prepared sputter-deposited epitaxial Ti3AlC2 films in ultra-high vacuum systems by DC magnetron sputtering. Epitaxial Ti3C2Tx films can be prepared by etching epitaxial Ti3AlC2 films at room temperature in HF or NH4HF2 solutions. As shown in Fig. 7a–c, STEM images of Ti3AlC2, Ti3C2Tx after HF etching, and Ti3C2Tx after NH4HF2 etching are separately shown. The results show that due to the intercalation of NH3 and NH4+, the lattice parameter c (∼25 Å) of Ti3C2Tx thin films etched with NH4HF2 is 25% larger than that etched with HF, and has higher transparency and resistivity. Furkan Turker et al.178 used methane as the C source and carried out CVD with Mo foil placed on copper foil at 1090 °C to obtain 2D ultra-thin Mo2C material. In Fig. 7d and e, SEM images of Mo2C are shown. Thin crystals with small planes are observed, which proved the successful growth of Mo2C. Thirumal et al.179 used C2H4 as a carbon source to grow MXene on carbon nanotubes (CNTs) at a fixed temperature of 700 °C. Fig. 7f is the preparation flow chart. SEM images of Fig. 7g–i display the grown Ti3C2Tx CNTs hybrid composite. However, the MXene material prepared by the CVD method is a large-size single layer, which is not easy to stack into a loose structure. Its own lattice gap is small, and ions cannot be embedded, so it is difficult to obtain intercalation capacitance.177 Moreover, the periphery groups of MXene materials prepared via this method are mainly –CH3 or –N, the electric double layer effect is not obvious, the contribution to ion adsorption is low, and the electrochemical performance is not prominent.176
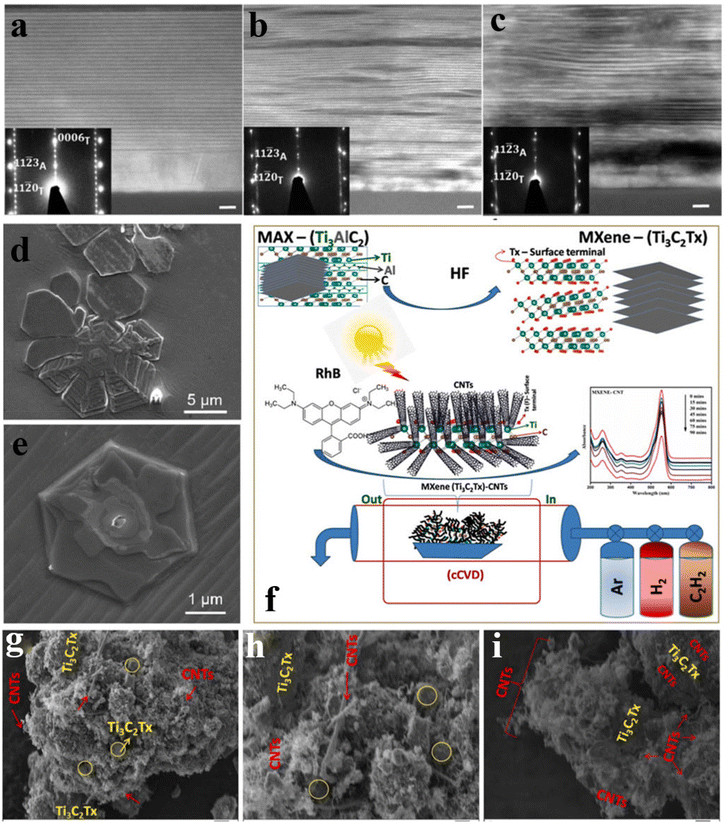 | ||
| Fig. 7 STEM images of the cross-section of (a) Ti3AlC2, (b) Ti3C2Tx, and (c) Ti3C2Tx IC films grown on the substrate. The illustration shows the SAED images of the substrate and film. (d and e) SEM images of Mo2C crystal grown on copper foil.178 © 2020 The American Ceramic Society. (f) Flow chart of synthesis of Ti3C2Tx carbon nanotube hybrid by CVD technology; (g–i) SEM images of Ti3C2Tx grown on carbon nanotubes.179 © 2021 Elsevier Ltd. | ||
Moreover, the electrochemical performances of MXene prepared using different preparation methods have been summarized in recent years in the form of a table. Table 1 shows the performance of different MXene materials in the three-electrode test, and Table 2 shows the two-electrode electrochemical performance of the assembled devices.
| Electrode | Electrolyte | Potential window/V | Condition | Capacitance | Cyclic performance | Ref. |
|---|---|---|---|---|---|---|
| Ti3C2Tx-10 | 1 M H2SO4 | −0.4–0.1 | 1 A g−1 | 372 F g−1 | 4 A g−1, 5000, 95% | 180 |
| Mo2CTx | 1 M H2SO4 | −0.3–0.3 | 0.3 A g−1 | 79.14 F g−1 | 5 A g−1, 5000, 98% | 181 |
| C@Mxene | 3 M H2SO4 | −0.4–0.6 | 1 A g−1 | 447.67 F g−1 | — | 182 |
| N, O co-doped C@Ti3C2 | 6 M KOH | −1.0 to −0.2 | 1 A g−1 | 250.6 F g−1 | 5 A g−1, 5000, 94% | 183 |
| Ti3C2Tx-DC | 1 M KCl | −0.8–0 | 0.5 A g−1 | 353 F g−1 | 1 A g−1, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 99% 000, 99% |
184 |
| Mo1.33CTz MXene | 1 M H2SO4 | −0.3–0.3 | 0.5 A g−1 | 436 F g−1 | 10 A g−1, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 98% 000, 98% |
185 |
| MXene hydrogel | — | −1.1 to −0.1 | 5 A g−1 | 370 F g−1 | 1000 mV s−1, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 98% 000, 98% |
186 |
| MXene-NPO | 1 M KOH | 0.1–0.5 | 0.5 A g−1 | 639 C g−1 | 10 A g−1, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 85% 000, 85% |
187 |
| V2CTx | 1 M H2SO4 | −0.8 to −0.3 | 0.5 A g−1 | 171.4 F g−1 | 2 A g−1, 5000, 89.1% | 188 |
| MXene films | 3 M H2SO4 | −0.5–0.3 | 0.5 A g−1 | 351 F g−1 | 5 A g−1, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 90.5% 000, 90.5% |
189 |
| Ti3C2Tx/N-doped OMC | 3 M KOH | −1.0–0 | 1 A g−1 | 329 F g−1 | 100 mV s−1, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 97% 000, 97% |
190 |
| V4C3Tx | 1 M H2SO4 | −0.25–0.2 | 2 mV s−1 | 209 F g−1 | 10 A g−1, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 97.23% 000, 97.23% |
191 |
| MXene/C | 1 M H2SO4 | −0.6–0.2 | 2 mV s−1 | 403 mF cm−2 | 2500 cycles, 90% | 192 |
| V4C3 | 1 M H2SO4 | −0.4–0.4 | 5 mV s−1 | 330 F g−1 | 5 mV s−1, 3000, 90% | 193 |
| MXene film | 1 M Na2SO4 | −0.7 to −0.3 | 5 mV s−1 | 1225 μF cm−2 | 60 μA cm−2, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 85% 000, 85% |
194 |
| MoS2–Ti3C2Tx | 1 M H2SO4 | 0–0.5 | 5 mV s−1 | 405.7 F g−1 | 10 A g−1, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 82% 000, 82% |
195 |
| CNF/MXene@SnS2 | 1 M H2SO4 | −0.2–0.4 | 10 mV s−1 | 190 F g−1 | 5 A g−1, 5000, 87.4% | 196 |
| d-Ti3C2 | 1 M KOH | −1.0 to −0.4 | 20 mV s−1 | 134 F g−1 | 100 mV s−1, 5000, 94% | 197 |
| MXene/CNF | 1 M H2SO4 | 0–0.7 | 300 mV s−1 | 90 F g−1 | 100 mV s−1, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 98% 000, 98% |
198 |
| NH3–V4C3Tx | 1 M H2SO4 | −0.3–0.3 | 10 mV s−1 | 210 F g−1 | 10 A g−1, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 96.3% 000, 96.3% |
199 |
| Devices | Electrolyte | Potential window/V | Capacitance | Energy density | Power density | Cyclic performance | Ref. |
|---|---|---|---|---|---|---|---|
| Ti3C2Tx-10//Ti3C2Tx-10 | PVA/H2SO4 | 0–1.0 | 0.5 A g−1, 151 F g−1 | 4.7 W h kg−1 | 242 W kg−1 | 2 A g−1, 4000, 85% | 180 |
| Mo2CTx//Mo2CTx | 1 M H2SO4 | 0–0.6 | 0.2 A g−1, 64.74 F g−1 | 16 W h L−1 | 1449.1 W L−1 | 1 A g−1, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 89.2% 000, 89.2% |
181 |
| C@MXene//C@MXene | 3 M H2SO4 | −0.8–0.8 | 1 A g−1, 90.8 F g−1 | 303.91 W h g−1 | 11.92 W g−1 | 10 A g−1, 4000, 80.1% | 182 |
| AT2-NH3//AT2-NH3 | 6 M KOH | 0–1.2 | 1 A g−1, 53.8 F g−1 | 10.8 W h kg−1 | 600 W kg−1 | 2 A g−1, 5000, 90% | 183 |
| MXene-NPO//PPD-rGO | PVA/H2SO4 | 0–1.5 | 1 A g−1, 187 F g−1 | 72.6 W h kg−1 | 932 W kg−1 | 5 A g−1, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 94% 000, 94% |
187 |
| MoS2–Ti3C2Tx//MoS2–Ti3C2Tx | — | 0–0.6 | 0.5 A g−1, 115.2 F g−1 | 5.1 W h kg−1 | 298 W kg−1 | 5 A g−1, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 72.3% 000, 72.3% |
195 |
| CNF/MXene@SnS2//CNF/MXene@SnS2 | PVA/H2SO4 | 0–0.6 | 1 mA cm−2, 205 mF cm−2 | 6.7 μW h cm−2 | 1206 μW cm−2 | 10 mA cm−2, 4000, 91.5% | 196 |
3. Preparation of LDHs
3.1 Coprecipitation method
Coprecipitation is the most traditional way to fabricate LDHs. The universal method is to mix the solution of metal ions (M2+/M3+) with a precipitant (such as OH−, CO32−) at an appropriate pH,200 and then conduct hydrothermal treatment. LDHs with different particle sizes, morphology, and crystallinity are synthesized by controlling the reaction time, concentration, temperature, and reactants.201,202Woo et al.203 synthesized a new phase of ZnCo-LDHs for the first time by coprecipitation reaction with hydrogen peroxide as the oxidant. SEM images of Fig. 8a and b show a sheet shape, and the primary particle size is hundreds of nanometers. A stable colloidal suspension of ZnCo-LDH was successfully prepared by dispersing ZnCo-LDH-NO3− powder in formamide. A very thin ZnCo-LDH sheet is displayed in the HR-TEM image of Fig. 8c. The corresponding SAED showed an obvious hexagonal pattern, meaning that the pattern of LDH after peeling does not change. Djebbi et al.204 prepared MgAl-LDH and ZnAl-LDH nanosheets using the same method, and the morphologies of the product are shown in Fig. 8d and e. The particle sizes of them are about 50–200 nm and 100–500 nm, respectively. After testing, it was found that the former had better electrochemical properties than the later, which proves that the change of divalent metal cations plays a key decisive in the resulting electrochemical performances. Zhang et al.205 deposited NiCo LDH on rGO sheet by the deposition method. With the progress of the reaction, staggered NiCo LDH nanosheets gradually appeared (Fig. 8f and g). The TEM image in Fig. 8h shows that the entire surface of rGO is uniformly coated with staggered NiCo-LDH of about 2.4 nm thickness. In the EDS mapping shown in Fig. 8i, the uniform distribution of Ni, Co, C and O was found, demonstrating the successful preparation.
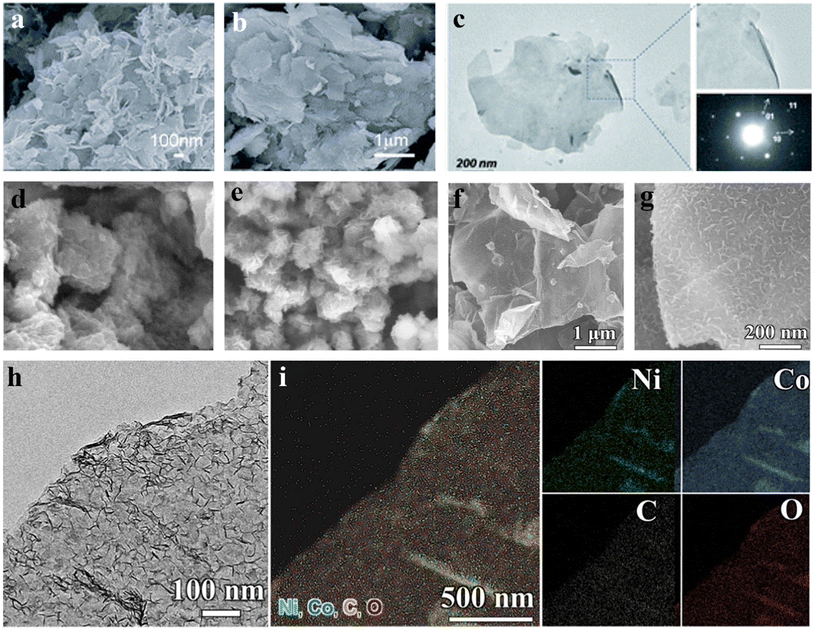 | ||
| Fig. 8 (a and b) SEM images of ZnCo-LDH; (c) TEM image and SAED of ZnCo-LDH nanosheets.203 © 2011 The Royal Society of Chemistry. (d and e) SEM images of MgAl-LDH and ZnAl-LDH.204 © 2016 Elsevier B.V. (f and g) SEM images of NiCo-LDH/rGO; (h and i) TEM image and EDS mapping of NiCo-LDH/rGO.205 © 2020 Elsevier Inc. | ||
3.2. Solvent/hydrothermal method
The solvent/hydrothermal method is the most common synthesis method of LDHs. It has the advantages of simple and easy operation, environmental protection, and cleaning in the synthesis process. Because of the easy regulation of the reaction time and temperature, LDHs fabricated by solvent/hydrothermal method commonly possess the features of good crystallinity, homogeneous size, and uniform morphology.206–208 Rao et al.209 prepared MgAl LDH by a simple method of urea hydrolysis and hydrothermal synthesis, as shown in Fig. 9a. The sample is hexagonal and its size is less than 1 mm. Compared with the conventional coprecipitation method, the product did not need repeated washing and alkali removal.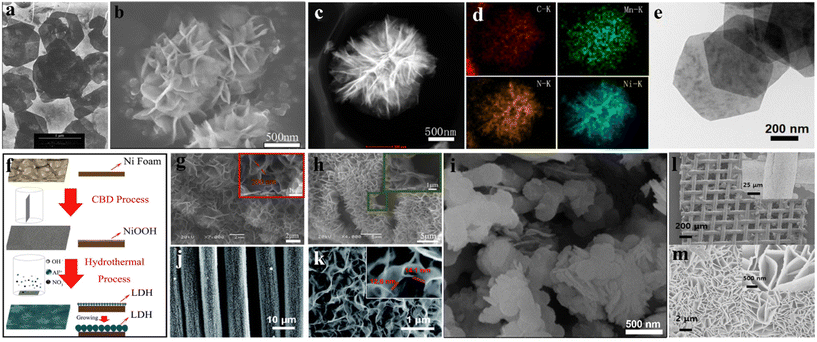 | ||
| Fig. 9 (a) SEM image of MgAl LDH.209 © 2004 Elsevier Ltd. (b) SEM image of NiMn LDHs; (c) HAADF-STEM image of NiMn LDHs; (d) corresponding EDS mapping analysis image.208 © 2018 Elsevier Ltd. (e) SEM image of Ni4Yb(OH)10NO3·3H2O nanosheets.210 © 2018 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. (f) Preparation mechanism of NiAl-LDH; (g) SEM image of NiAl-LDH; (h) side view of NiAl-LDH.211 © 2013 Elsevier B.V. (i) SEM image of LDH/CSs composite.212 © 2017 The Nonferrous Metals Society of China. Published by Elsevier Ltd. (j) SEM images of NiCu-LDH material grown uniformly on carbon fibers; (k) locally enlarged FESEM image.213 © 2017 Science China Press and Springer-Verlag GmbH Germany; (l and m) SEM images of NiCo LDH samples on the surface of textile.214 © 2018 Elsevier B.V. | ||
Wang et al.208 fabricated NiMn LDHs via a simple one-step hydrothermal method, and adjusted their interlayer space by keeping the mole number of urea less than the stoichiometric ratio and controlling the reaction time. Fig. 9b shows an SEM image of NiMn LDHs, which have a flower-like morphology with layered nanosheets and slight aggregation. The HAADF-STEM image and the corresponding EDS spectra (Fig. 9c and d) clearly confirm the well-defined morphology of NiMn LDHs, in which Mn, Ni, C, and N are uniformly distributed throughout the LDHs. The electrochemical tests indicated the specific capacitance of NiMn LDHs at 1 A g−1 of 846.5 C g−1. Duan et al. synthesized thin-layer hexagonal structure Ni4Yb(OH)10NO3·3H2O nanosheets by the solvothermal method.210 The product morphology is shown in Fig. 9e, which is hexagonal nanosheets with a diagonal length of about 1.5 μm. Moreover, it was found that the sample possesses remarkable energy storage properties and excellent cycle stability. At 1 A g−1, it can show 1482.1 F g−1 specific capacitance, and can still maintain 71.8% capacitance after 2000 cycles.
3.3 In situ growth method
The in situ growth method involves placing the conductive base material as a template into the metal salt solution, and these metal ions grow LDHs on their surface. The most commonly used conductive substrates are carbon cloth or nickel foam, which can increase the conductivity of LDHs. Wang et al.211 successfully produced NiAl-LDH onto nickel foam via the in situ method (Fig. 9f). The periphery of nickel foam is covered by petal-like NiAl-LDH with 200 nm thickness (Fig. 9g and h). Concurrently, these NiAl-LDH sheets are arranged perpendicular to the substrate, forming a porous nanowall structure and leaving huge pores.Huang et al.212 fabricated CoAl-LDH on the periphery of carbon spheres (CSs), as shown in Fig. 9i. It is proved that the prepared composites having large specific surface areas increase the contact between active materials and electrolytes. Through electrochemical tests, it was found that at 1 A g−1 and 10 A g−1, the composites separately showed specific capacitances of 1198 F g−1 and 920 F g−1. Wang et al.213 synthesized NiCu LDH by applying a one-step solvothermal method on carbon fiber cloth (CFC). After the reaction, the CFC was uniformly coated with an active material porous layer (Fig. 9j). SEM image demonstrates the hierarchical porous layer composed of ultrathin nanosheets (Fig. 9k). Through the tests, one can view that the sample displays excellent electrochemical properties. Jeong et al.214 prepared NiCo-LDHs by a solvothermal method on nickel-coated fabric. It was found that the entire surface was decorated with vertical NiCo-LDH (Fig. 9l). Fig. 9m proves that NiCo-LDH nanosheets are uniformly arrayed on the surface of the textile. Since the nanosheets form a rich pore structure, it obviously reduces the ion diffusion distance and enables the electrolyte to easily penetrate into the internal electrode.
3.4 Electrochemical deposition method
The electrode prepared by electrochemical deposition has the advantage of directly fabricating the electrode in one step, which simplifies the electrode preparation process. At the same time, the material prepared by electrochemical deposition has a high specific capacitance. Therefore, this method is one of the most active ways to fabricate materials as electrodes at present. During the preparation process, by selecting appropriate electrode materials and electrolytes, and adjusting the electrode potential and current to control the direction and speed of the reaction, the required substances are produced on the electrode. Han et al.215 prepared a 3D NiCo-LDH network on activated carbon cloth (ACC) having high-quality loading through electrochemical deposition. In the SEM image shown in Fig. 10a, the nanosheets form a 3D network structure with a uniform distribution on the carbon fiber surface. The SEM image of Fig. 10b presents that these nanosheets are uniformly covered on ACC. The asymmetric supercapacitor (ASC) based on NiCo-LDH@ACC serving as a positive electrode exhibits power density and energy density of 2.24 mW h cm−2 and 3.71 mW cm−2. Fig. 10c exhibits the illustration of assembling NiCo-LDH@ACC.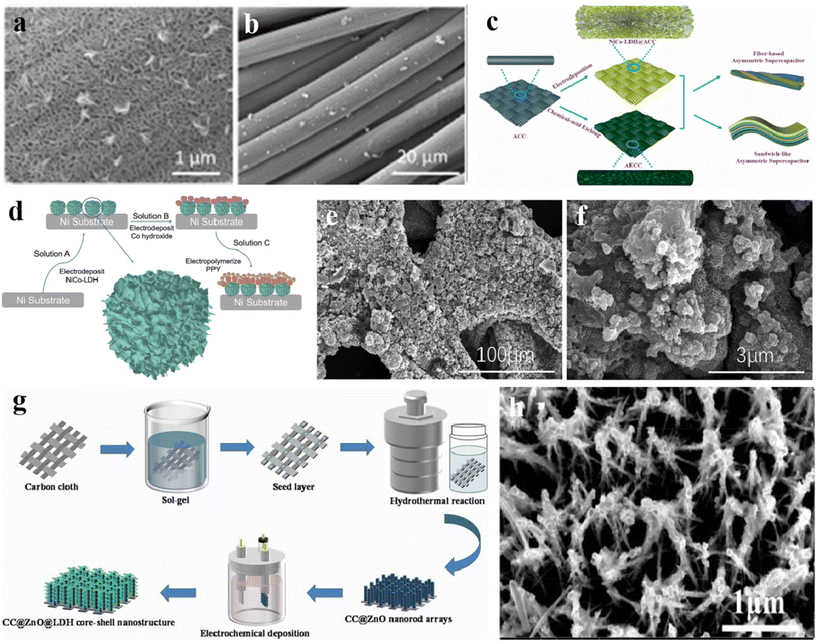 | ||
| Fig. 10 (a and b) SEM images of NiCo(NA)-LDH@ACC; (c) synthesis and assembly of NiCo-LDH@ACC ASC.215 © 2021 Elsevier Ltd. (d) Schematic of the preparation of NiCo-LDH/Co(OH)2@PPY and corresponding (e and f) SEM images.216 © 2021 Elsevier B.V. (g) The synthesis route of ZnO@NiCo-LDH; (h) FESEM image of ZnO@NiCo-LDH300s.217 © 2021 Informa UK Limited, trading as Taylor & Francis Group. | ||
Yao et al.216 prepared NiCo LDH/Co(OH)2@PPY electrode by −2.0 V electrochemical deposition. The formation process is displayed in Fig. 10d. Through the adjustment of time and voltage, the obtained material presents a cauliflower-like nanostructure (Fig. 10e and f), which is conducive to charge transfer and electrolyte penetration. Chen et al.217 synthesized ZnO@NiCo-LDH composites on the surface of carbon cloth (CC) through the hydrothermal method and electrochemical deposition (Fig. 10g). The influences of the reaction time on the resulting electrochemical performances are researched in detail. The outcomes indicate that the NiCo-LDH film with a deposition time of 300 s can form an interconnected network morphology (Fig. 10h). This electrode possesses good area capacitance (667.3 mF cm−2 at 1 mA cm−2) and cycle stability (86.78% capacitance retention after 2000 cycles). In addition, the ASC based on this electrode displays the energy density of 0.044 mW h cm−2 at the power density of 1.17 mW cm−2.
Furthermore, the electrochemical performances of LDH prepared via different methods are summarized in recent years in the form of a table. Table 3 shows the performance of different LDH materials in the three-electrode test, and Table 4 shows the two-electrode electrochemical properties of the assembled device.
| Electrode | Electrolyte | Potential window/V | Condition | Capacitance | Cyclic performance | Ref. |
|---|---|---|---|---|---|---|
| Mn–Ni LDO-C | 1 M KOH | 0–0.6 | 1 mA cm−2 | 2.36 C g−1 | 2 mA cm−2, 5000, 92.1% | 218 |
| ZnNiFe-LDH@Cu(OH)2/CF | 6 M KOH | 0.15–0.45 | 3 mA cm−2 | 6100 mF cm−1 | 30 mA cm−2, 5000, 88.1% | 219 |
| P-Ni(OH)2@Co(OH)2/NF | 1 M LiOH | 0–0.5 | 1 mA cm−2 | 2.36 C cm−2 | 20 mA cm−2, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 91% 000, 91% |
220 |
| ZIF-8-C@NiAl-LDH | — | 0–0.5 | 5 mV s−1 | 1403 F g−1 | 4 A g−1, 1000, 77% | 221 |
| Ni-foam@Cu–Al LDH/g-C3N4 | 6 M KOH | −0.1–0.5 | 0.4 A g−1 | 838.8 F g−1 | 50 mV s−1, 5000, 92.71% | 222 |
| NiCoZn-LDH@PANI | 3 M KOH | −0.1–0.4 | 1 A g−1 | 1749 F g−1 | 10 A g−1, 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 89% 000, 89% |
223 |
| NiCo2Al-LDH/N-GO | 2 M KOH | 0–0.6 | 1 A g−1 | 1136.6 F g−1 | 10 A g−1, 5000, 90.1% | 224 |
| CoMn LDHs/NF | 1 M LiOH | −0.2–0.45 | 1 A g−1 | 1409 F g−1 | 1 A g−1, 3000, 93.2% | 225 |
| Co, Mn-LDH@NF | 3 M KOH | 0–0.4 | 1 A g−1 | 1140 F g−1 | 1 A g−1, 3000, 86.5% | 226 |
| NiMnCr-LDHs@CS/NF | 2 M KOH | 0–0.5 | 3 A g−1 | 569 C g−1 | 20 A g−1, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 76% 000, 76% |
227 |
| NiCo2S4/Ni–Co LDH | 3 M KOH | 0–0.5 | 1 A g−1 | 1765 F g−1 | 5 A g−1, 2000, 56% | 228 |
| CoFe-ONP/LDH/MLG | 2 M KOH | 0–0.4 | 1 A g−1 | 882.5 F g−1 | 1 A g−1, 5000, 67.1% | 229 |
| NiCo/NiMn-LDH | 6 M KOH | 0–0.4 | 1 A g−1 | 2950 F g−1 | 10 A g−1, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 79% 000, 79% |
230 |
| Co-LDH/G2 | 1 M KOH | 0–0.45 | 2 A g−1 | 400 F g−1 | 2 A g−1, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 90.4% 000, 90.4% |
231 |
| NiCo2O4@Co–Fe LDH | 2 M KOH | 0–0.4 | 1 A g−1 | 1557.5 F g−1 | — | 232 |
| Ni–Al LDH/NNDG | 6 M KOH | 0–0.5 | 1 A g−1 | 975 F g−1 | 5 A g−1, 5000, 86% | 233 |
| Co3O4@CoNi-LDH | 2 M KOH | 0–0.5 | 0.5 A g−1 | 2676.9 F g−1 | 30 A g−1, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 67% 000, 67% |
234 |
| Ni–Co LDH/CFC | 1 M KOH | 0–0.55 | 1 A g−1 | 1540 F g−1 | 10 A g−1, 5000, 84.6% | 235 |
| NiCo-LDH/NF | 2 M KOH | 0–0.5 | 1 A g−1 | 1198 F g−1 | 5 A g−1, 2000, 81.6% | 236 |
| NiFe-LDH/NF | 1 M KOH | 0–0.4 | 5 A g−1 | 2708 F g−1 | 10 A g−1, 500, 42.6% | 237 |
| Device | Electrolyte | Potential window/V | Capacitance | Energy density | Power density | Cyclic performance | Ref. |
|---|---|---|---|---|---|---|---|
| Mn–Ni LDO-C//AC | 1 M KOH | 0–2.0 | 1 A g−1, 563.2 C g−1 | 78.2 W h kg−1 | 499.7 W kg−1 | 2 A g−1, 5000, 85.3% | 218 |
| NiCoZn-LDH@PANI//AC | KOH | 0–1.6 | 1 A g−1, 97.0 F g−1 | 37.2 W h kg−1 | 362 W kg−1 | 5 A g−1, 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 90% 000, 90% |
223 |
| NiCo2Al-LDH/N-GO//AC | PVA/KOH | 0–1.6 | 1 A g−1, 80.2 F g−1 | 42.25 W h k−1 | 800 W kg−1 | 10 A g−1, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 88.9% 000, 88.9% |
224 |
| CoMn LDHs/NF//AC | 1 M LiOH | 0–1.5 | 1 A g−1, 118 F g−1 | 34.5 W h kg−1 | 362.3 W kg−1 | 5 A g−1, 3000, 83.7% | 225 |
| ZnNiFe-LDH@Cu(OH)2/CF//AC | PVA/KOH | 0–1.0 | 0.4 A g−1, 88 F g−1 | 44 W h kg−1 | 720 W kg−1 | 5 A g−1, 5000, 83.4% | 219 |
| P-Ni(OH)2@Co(OH)2/NF//Fe2O3/CC | 1 M LiOH | 0–1.6 | 1 mA cm−2, 4.4 C cm−2 | 0.21 mW h cm−2 | 16 mW cm−2 | 20 mA cm−2, 5000, 81% | 220 |
| NiMnCr LDHs@CS/NF//FeOOH/NF | 2 M KOH | 0–1.7 | 1 A g−1, 167.4 F g−1 | 48 W h kg−1 | 402.7 W kg−1 | 5 A g−1, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 83% 000, 83% |
227 |
| NiCo/NiMn-LDH//AC | — | 0–1.6 | 1 A g−1, 185.1 F g−1 | 45.16 W h kg−1 | 1600 W kg−1 | 10 A g−1, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 82.2% 000, 82.2% |
230 |
| NiCo2O4@Co-Fe LDH//AC | 2 M KOH | 0–1.7 | 1 A g−1, 64.76 F g−1 | 28.94 W h kg−1 | 950 W kg−1 | 2 A g−1, 5000, 76.9% | 232 |
| Ni–Al LDH/NNDG//AC | PVA/KOH | 0–1.6 | 2.5 A g−1, 166 F g−1 | 46 W h kg−1 | 1991 W kg−1 | 10 A g−1, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 95% 000, 95% |
233 |
| Co3O4@CoNi-LDH//AC | PVA/KOH | 0–1.6 | 1 A g−1, 195.9 F g−1 | 61.23 W h kg−1 | 750 W kg−1 | 10 A g−1, 5000, 99% | 234 |
| Ni–Co LDH/CFC//rGO/NF | 1 M KOH | 0–1.6 | 1 A g−1, 104.1 F g−1 | 37 W h kg−1 | 800 W kg−1 | 10 A g−1, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 84.6% 000, 84.6% |
235 |
| NiCo-LDH/NF//AC | 2 M KOH | 0–1.6 | 1 A g−1, 81.8 F g−1 | 29.1 W h kg−1 | 903.1 W kg−1 | 2 A g−1, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 88.3% 000, 88.3% |
236 |
| NiFe-LDH//AC | 1 M KOH | 0–1.6 | 1 A g−1, 141.3 F g−1 | 50.2 W h kg−1 | 800 W kg−1 | 4 A g−1, 2000, 65% | 237 |
| CoSx/Ni–Co LDH//AC | 6 M KOH | 0–1.6 | 1 A g−1, 100 F g−1 | 35.8 W h kg−1 | 800 W kg−1 | 5 A g−1, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 94.5% 000, 94.5% |
238 |
4. Research progress of MXenes and LDHs composites for supercapacitors
MXenes have typical 2D layered structure characteristics, high specific surface area, good electrical conductivity as well as abundant surface groups, which can be used as matrix materials.239 LDHs with high aspect area are potentially excellent materials for supercapacitors, but their low conductivity and agglomeration behavior seriously influence the improvement of electrochemical performance.240 Therefore, the fabrication of LDHs on the surface of MXenes is hoped to prepare electrode materials with high performance. The following describes the recent progress of LDH/MXene in the field of supercapacitors, mainly including the preparation and performances of the electrode materials and the assembled devices.Li et al.241 prepared NiFe LDH/Ti3C2 MXene composite by a hydrothermal method, as shown in Fig. 11a, in which NiFe LDH nanosheets were regularly and vertically arrayed on the surface of MXene nanosheets (Fig. 11b and c). Besides, the element mapping certifies the uniform distribution of Ti, Ni, and Fe (Fig. 11d). The CV tests of the prepared electrode were performed (Fig. 11e), and the peaks are related to the reduction of Ni2+, Fe3+ (ref. 242 and 243) and the oxidation of Ni, Fe.244–246 All curves remained unchanged, manifesting excellent reversibility. Fig. 11f exhibits GCD curves, and discharge and charge potential platforms are in line with those of CV curves. Moreover, the initial discharge capacitance at 0.1 A g−1 is 1376.4 mA h g−1. Fig. 11g shows the corresponding cycle stability for 200 cycles at 0.1 A g−1. In the case of 1 A g−1, the composite shows 726.1 mA h g−1 capacitance (Fig. 11h). The excellent properties benefit from MXene providing a conductive framework as well as NiFe LDH exposing the active surface. Moreover, the assembled device verifies the practical application of the electrode (Fig. 11i). It can be found that CV curves do not possess a perfect rectangle owing to the existence of two energy storage mechanisms (Fig. 11j). GCD curves present almost the same linear shapes and there is no IR drop (Fig. 11k), meaning remarkable reversibility. At a power density of 1158 W kg−1, the device has an energy density of 53 W h kg−1. In addition, after 500 cycles, the capacitance is 46.7 mA h g−1, and the coulombic efficiency at 1 A g−1 is 96% (Fig. 11l), which implies good cycle stability.
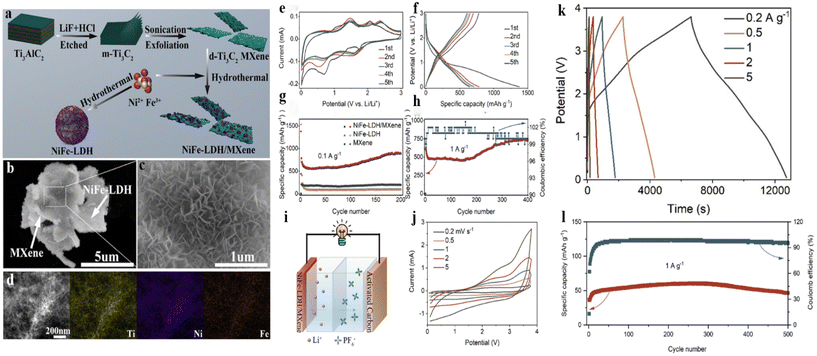 | ||
| Fig. 11 (a) Schematic of fabrication of NiFe-LDH/MXene composite; (b and c) SEM images and (d) elemental mapping; (e and f) CV and GCD curves. Cycling capacitance at (g) 0.1 A g−1 and (h) 1 A g−1; (i) schematic of the NiFe-LDH/MXene//AC and corresponding (j) CV curves, (k) GCD profiles and (l) cycle stability.241 © 2021, American Chemical Society. | ||
Wang et al.247 synthesized intercalated LDH MXene LDH (LM-4) by coprecipitation. NiMn-LDH nanosheets are grown on the periphery of the Ti3C2 sheet (Fig. 12a). The TEM image also confirms the successful preparation of NiMn-LDH nanosheets (Fig. 12b). The existence of MXene increases the conductivity (Fig. 12c), and LDH uniformly covering the surface of MXene exhibits more available active sites. The electron coupling between different components increases the cycling stability (Fig. 12d). All-solid-state asymmetric supercapacitor based on LM-4 working as the positive electrode was constructed (Fig. 12h). Fig. 12e shows the CV curves of the device with varied voltage windows in the range of 1.3–1.7 V. The typical symmetric GCD curves illustrate the excellent electrochemical reversibility at 1 to 10 A g−1 (Fig. 12f). The cyclic stability of the device was further studied (Fig. 12g). The device exhibits 90.3% capacitance retention after 5000 cycles and an energy density of 44.7 W h kg−1 at a power density of 800 W kg−1.
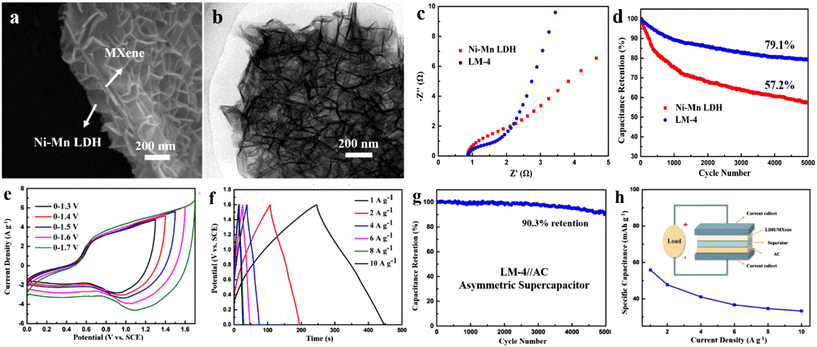 | ||
| Fig. 12 (a) SEM and (b) TEM images of MXene@NiMn-LDH; (c) Nyquist plots and (d) cyclic performance at 6 A g−1 of NiMn-LDH and LM-4; (e) CV curves at 50 mV s−1, (f) GCD curve and (g) cycle performance at 6 A g−1 of LM-4//AC; (h) specific capacitance curves at different current densities, illustration is a schematic diagram of an asymmetric device.247 © 2020 Elsevier B.V. | ||
Guo et al.248 used 3D foam nickel (NF) and Ti3C2Tx layers as substrates and prepared NiAl-LDH composites by the immersion hydrothermal method (Fig. 13a–c). The NiAl-LDH array on the substrate has stronger electrochemical performance because of the synergy effect of different parts. CV and GCD tests were performed by the employment of a three-electrode system (Fig. 13d and e). There is little change in shape for CV curves, suggesting good reversibility of the redox reaction. There are two platforms for GCD curves ascribed to the oxidation–reduction reaction of the composite, illustrating the pseudo-capacitance characteristics. Besides, the ASC device is assembled with NiAl-LDH/MXene and AC, whose voltage window reached 1.7 V (Fig. 13f). Fig. 13g and h show the corresponding CV and GCD curves. The almost unchanged shape for CV curves suggests the device possesses impressive fast charge and discharge characteristics. On the basis of GCD curves, the specific capacitance of NiAl-LDH/MXene//AC at 1 A g−1 is about 50 F g−1. After 5000 cycles, the retention rate can reach 89.1% (Fig. 13i). It is calculated that the device has an energy density of 27.6 W h kg−1 at a power density of 255 W kg−1.
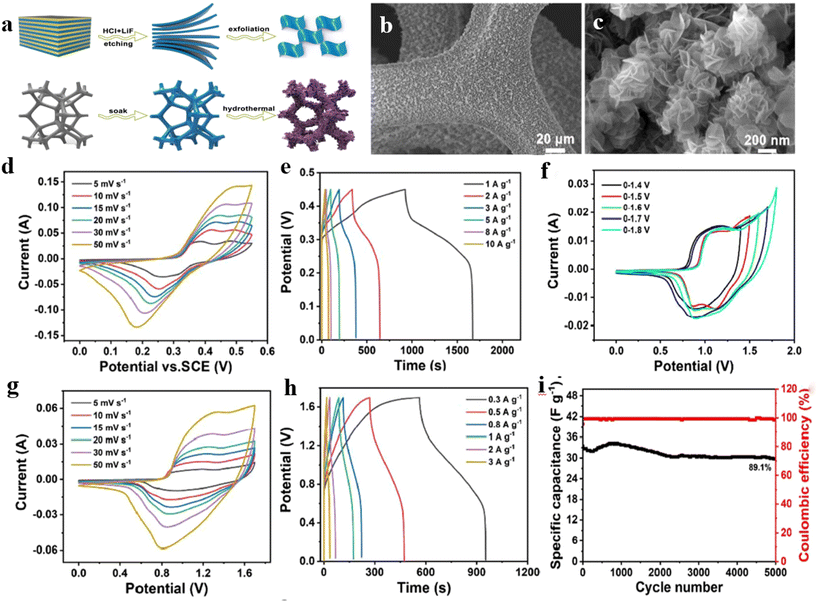 | ||
| Fig. 13 (a) Roadmap for preparing NiAl LDH/MXene on foam nickel; (b and c) SEM images of NiAl-LDH/MXene composite; (d) CV curves at different scanning rates; (e) GCD curves at different current densities; CV curves of NiAl-LDH/MXene//AC (f) under different voltage windows and (g) at different scanning rates; (h) GCD curves of NiAl-LDH/MXene//AC at different current densities; (i) cycle performance and coulombic efficiency of NiAl-LDH/MXene//AC at 3 A g−1.248 © 2022 The author(s), under exclusive licence to Springer-Verlag GmbH Germany, part of Springer Nature. | ||
Chen et al.249 prepared intercalated MXene/GO/NiMn-LDH (MGL) by coprecipitation (Fig. 14a–c). The sheet-like LDH could increase the specific capacitance. In addition, Go acted as a thin layer, covering the periphery of the complex to accelerate charge transfer. The electrochemical properties of the samples were investigated in detail in a 6 M KOH electrolyte. Under the same scanning rate (Fig. 14d), the current density of MGL is the highest, declaring that the introduction of Go and MXene greatly improves the electrochemical properties of LDH. GCD curves of MGL exhibit a much longer time than that of other materials at the same current density (Fig. 14e). From the EIS results shown in Fig. 14f, it is noteworthy that the excellent capacitance retention of the composite is attributed to LDH with good conductivity and MXene with low resistance. The cyclic stability further proves the improvement of electrochemical performance (Fig. 14g). An asymmetric device is prepared with MGL as the positive electrode. CV curves of MGL//AC in the range of 1.4–2.0 V are shown (Fig. 14h). Further measurements proceeded at 0–1.8 V (Fig. 14i), and CV curves with olive shape are shown from 10 mV s−1 to 100 mV s−1. The symmetric GCD diagram shows that the device has excellent reversibility at 1–20 A g−1 (Fig. 14j). In Fig. 14k, the device still has a 94.7% capacitance retention rate after 4000 cycles and has an energy density of 55.3 W h kg−1 at a power density of 800 W kg−1.
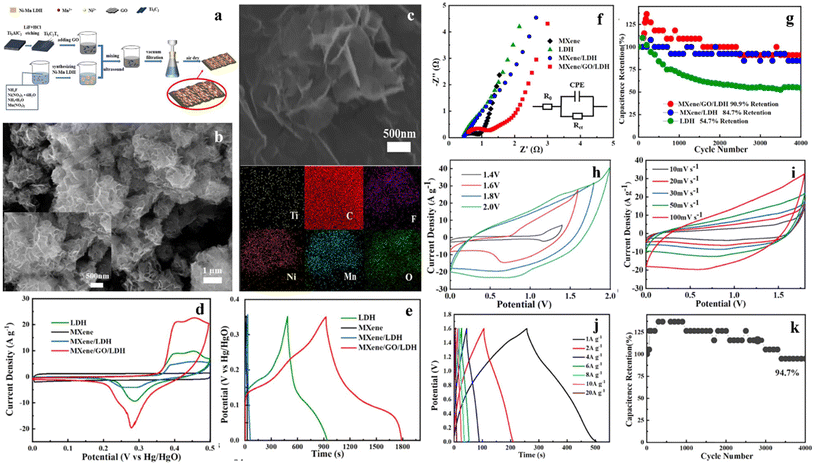 | ||
| Fig. 14 (a) Schematic diagram of preparing MGL composite; (b) SEM image of MXene/GO/NiMn-LDH; (c) element mapping of Ti, C, F, Ni, Mn, and O; (d) CV curves at 20 mV s−1; (e) GCD curves at 1 A g−1; (f) Nyquist plots of different electrodes; (g) cycle test at 5 A g−1 for 4000 cycles; (h) CV curves of MGL//AC in different scanning potential windows at 100 mV s−1; (i) CV curves of MGL//AC at different scanning rates; (j) GCD curves of MGL//AC at different current densities; (k) cycle test of MGL//AC at 5 A g−1.249 © 2021 Elsevier Ltd. | ||
Wang et al.250 uniformly grown NiCoAl-LDH nanosheets on MXene sheets by the hydrothermal method to form NiCoAl-LDH/V4C3Tx heterostructures (Fig. 15a and b). In SEM and TEM images of Fig. 15b and e, it can be readily viewed that the NiCoAl-LDH nanosheets are regularly grown on MXene sheets forming a porous network. CV measurements were performed on different electrodes at 10 mV s−1 (Fig. 15f). For all three samples, two obvious Faraday redox peaks having relatively large potential separation can be seen, showing typical cell type behavior.251 At 10 mV s−1, the CV area of the NiCoAl-LDH/V4C3Tx heterostructure is the largest, illustrating the best specific capacitance. GCD tests were carried out at 2 A g−1 (Fig. 15g). The platform also proved the battery type behavior of the composite. In order to prove the practical application of the heterostructure, the supercapacitor was produced (Fig. 15h). The voltage window of the device was investigated by CV curves (Fig. 15i), and it can be seen that the appropriate voltage window was 0–1.6 V. The GCD curves of the NiCoAl-LDH/V4C3Tx//AC hybrid device were collected under different current densities (Fig. 15j). Fig. 15k displays the capacitance performance of the device, which presents a high specific capacitance of 194 F g−1 at 1 A g−1 and 102 F g−1 at 20 A g−1. Fig. 15l exhibits the cycle test of the device for 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles within 0–1.6 V at 20 A g−1, showing 98% capacitance retention rate after 10
000 cycles within 0–1.6 V at 20 A g−1, showing 98% capacitance retention rate after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, and 96% coulombic efficiency (Fig. 15l).
000 cycles, and 96% coulombic efficiency (Fig. 15l).
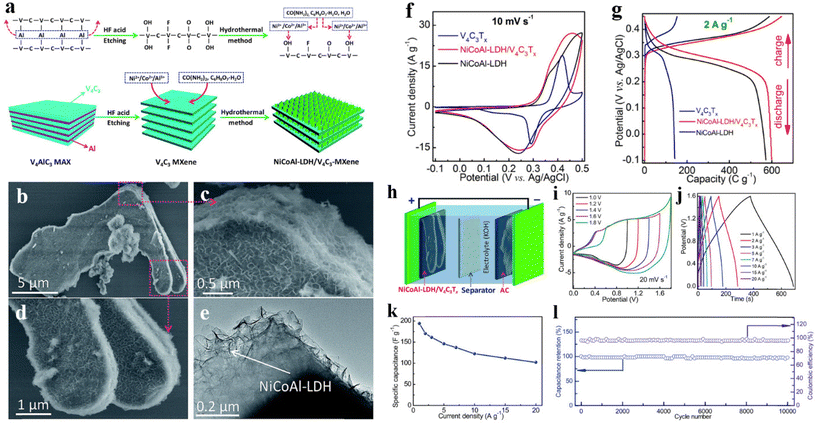 | ||
| Fig. 15 (a) Schematic of synthesis of NiCoAl-LDH/V4C3Tx composite; (b–d) FE-SEM image of NiCoAl-LDH/V4C3Tx; (e) TEM image of NiCoAl-LDH/V4C3Tx; (f) CV curves of different samples at 10 mV s−1; (g) GCD testing of different samples at 2 A g−1; (h) schematic diagram of NiCoAl-LDH/V4C3Tx//AC hybrid supercapacitor; (i) CV curves of the device under different operating voltage windows; (j) GCD curves of the device at various current densities; (k) specific capacitance of the device under different discharge current densities; (l) cycle performance of the device at 20 A g−1.250 © 2019 The Royal Society of Chemistry. | ||
Zhou et al.240 fabricated low-layer MXene with dimethyl sulfoxide, and then NiFe-LDH was deposited on its surface using a hydrothermal method to obtain the NiF-LDH/MXene composite (Fig. 16a and b).237,252 The Al element in Ti3AlC2 was selectively etched by HF to obtain a multilayer MXene (Fig. 16c). Fig. 16d shows a TEM image of MXene. Fig. 16e and f shows SEM images of NiFe-LDH/MXene composites. Adding MXene to LDH solves the problem of LDH agglomeration. In addition, LDH grown on MXene suppresses the aggregation and self-overlap between MXene sheets caused by the van der Waals force, further increasing the layer spacing of MXene. The three-electrode tests were performed in 1 M KOH solution. Compared with MXene and LDH, NiFe-LDH/MXene composite showed higher peak redox current (Fig. 16g), indicating better capacitance characteristics. The nonlinear discharge curves of the composite showed typical pseudo capacitance behavior (Fig. 16h), in accordance with the CV results. Compared with MXene and LDH, NiFe LDH/MXene had a longer discharge time and has optimal energy storage properties. Fig. 16i exhibits Nyquist curves of different samples. It was found that the internal resistance of the prepared composite is less than that of pure LDH, which proves the addition of MXene provides more conductive transmission paths for electron transmission. Besides, it can be seen that the slope of the composite is larger than that of LDH, showing ideal capacitance characteristics. Fig. 16j shows the CV curves of the NiFe-LDH/MXene//AC device. Fig. 16k shows the GCD curves of the device under various current densities. When the current density was 1 A g−1, the specific capacitance of the device reached 135.7 F g−1. It can also be seen that the power density was 758.27 W kg−1 when the energy density was 42.4 W h kg−1. In Fig. 16l, the electrochemical property of NiFe-LDH/MXene composites improved during the first 400 cycles, which may be due to sufficient electrolyte penetration and electrode activation.253 Furthermore, 84% specific capacitance remained after 1000 cycles, proving that this 2D interconnected porous structure effectively prevented structural variation and collapse of LDH during the electrochemical reaction, which is important for the stability of the electrode.
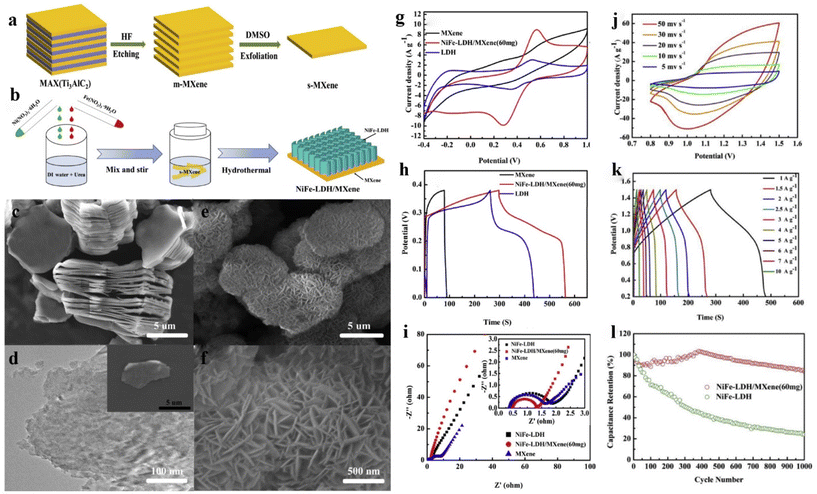 | ||
| Fig. 16 (a) Schematic of s-MXene synthesis; (b) schematic of preparation of NiFe-LDH/MXene composite; (c) SEM image of m-MXene; (d) TEM image of s-MXene; (e and f) SEM images of NiFe-LDH/MXene composites; (g) CV curves at 5 mV s−1, (h) GCD curves at 1 A g−1 and (i) Nyquist curves of different samples; (j) CV curves of NiFe-LDH/MXene based asymmetric SC (ASC); (k) GCD curves of ACS; (l) cycle performances of NiFe-LDH/MXene//AC.240 © 2020 Hydrogen Energy Publications LLC. Published by Elsevier Ltd. | ||
NiMn-LDH nanosheets were deposited on single-layer MXene through in situ synthesis (Fig. 17a and b).254 The existence of chemical bonding between LDH and MXene favors accelerated ion diffusion and electron transfer of the composite. Fig. 17c and d shows the EIS curves and GCD curves of different samples at 2 A g−1, it can be seen that the NiMn-LDH/MXene (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) composite exhibits the smallest impedance and the longest charge and discharge time, whose electrochemical performance is superior to other materials. Fig. 17e and f displays the CV and GCD curves of the best sample. Through calculation, the specific capacitance at 0.5 A g−1 was found to be 1575 F g−1. Fig. 17g presents 10
1) composite exhibits the smallest impedance and the longest charge and discharge time, whose electrochemical performance is superior to other materials. Fig. 17e and f displays the CV and GCD curves of the best sample. Through calculation, the specific capacitance at 0.5 A g−1 was found to be 1575 F g−1. Fig. 17g presents 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 charge–discharge cycles at 5 A g−1, and a 90.3% capacitance retention rate was achieved. Fig. 17h is a voltage window diagram of the ASC device. One can see that the voltage can be expanded to 1.5 V. Fig. 17i displays GCD curves under 1.5 V. Fig. 17j is a cycle performance test chart, and in the case of 2 A g−1, 91.8% capacitance retention rate was obtained after 10
000 charge–discharge cycles at 5 A g−1, and a 90.3% capacitance retention rate was achieved. Fig. 17h is a voltage window diagram of the ASC device. One can see that the voltage can be expanded to 1.5 V. Fig. 17i displays GCD curves under 1.5 V. Fig. 17j is a cycle performance test chart, and in the case of 2 A g−1, 91.8% capacitance retention rate was obtained after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. At the same time, AC//NiMn-LDH/MXene (2
000 cycles. At the same time, AC//NiMn-LDH/MXene (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) ASC devices show an energy density of 126 W h kg−1 at a power density of 0.74 kW kg−1 and a lower energy density of 97 W h kg−1 at a power density of 3.3 kW kg−1.
1) ASC devices show an energy density of 126 W h kg−1 at a power density of 0.74 kW kg−1 and a lower energy density of 97 W h kg−1 at a power density of 3.3 kW kg−1.
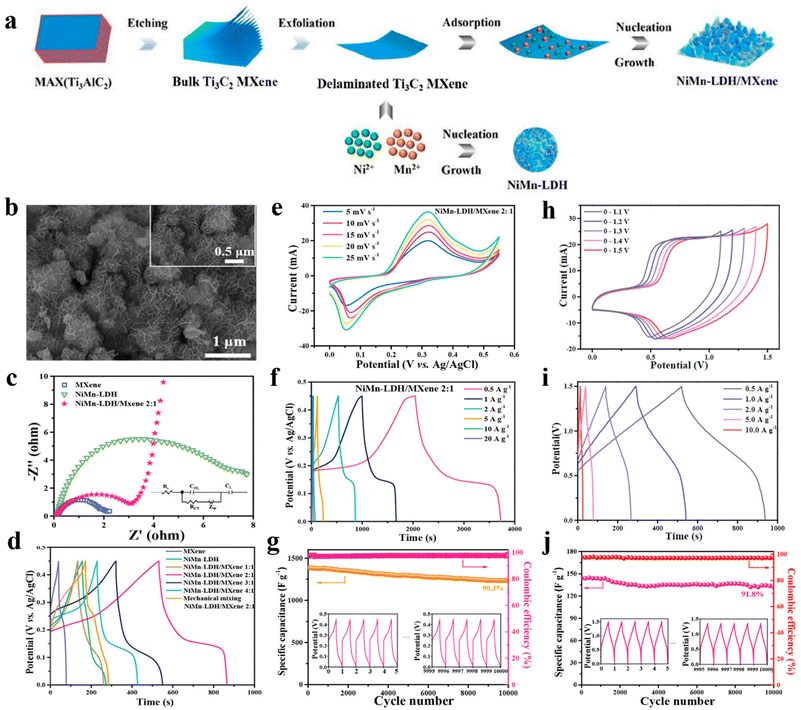 | ||
Fig. 17 (a) Schematic of preparing NiMn-LDH/MXene; (b) SEM images of NiMn-LDH/MXene (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); (c) Nyquist plots of different samples and (d) GCD curves at 2 A g−1 of different samples; (e) CV curves at 5–25 mV s−1, (f) GCD curves at 0.5–20 A g−1 and (g) cling performance over 10 1); (c) Nyquist plots of different samples and (d) GCD curves at 2 A g−1 of different samples; (e) CV curves at 5–25 mV s−1, (f) GCD curves at 0.5–20 A g−1 and (g) cling performance over 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 5 A g−1 of NiMn-LDH/MXene (2 000 cycles at 5 A g−1 of NiMn-LDH/MXene (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); (h) CV curves in different voltage windows at 5 mV s−1, (i) GCD curves at 1.5 V and (j) cycling stability of AC//NiMn-LDH/MXene (2 1); (h) CV curves in different voltage windows at 5 mV s−1, (i) GCD curves at 1.5 V and (j) cycling stability of AC//NiMn-LDH/MXene (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) ASC device at 2 A g−1.254 © 2020 American Chemical Society. 1) ASC device at 2 A g−1.254 © 2020 American Chemical Society. | ||
Zhang et al.255 synthesized CoNiMn-LDH/V2CTx composite through the electrostatic cooperative assembly by a hydrothermal method, as exhibited in Fig. 18a. From Fig. 18b, one can find that a nanoflower-like morphology was obtained in which LDH nanoflowers are tightly coated on the surface of MXene nanosheets. TEM images in Fig. 18c and d testify that the inter-plane spacing is very consistent with the unique plane of LDH, also proving the successful preparation of the composite. From Fig. 18e, it can be viewed that the composite exhibits a large specific surface area characteristic with uniform distribution of V, C, Ni, Mn, and Co elements. In the 6 M KOH solution, the electrochemical performances are evaluated with three-electrode configurations. In Fig. 18f, two redox peaks of CV curves are seen at 10 mV s−1, indicating that the reversible Faraday reaction happens. Of particular attention is that the composite electrode exhibits the largest CV integration area because of the metal ion doping effect, which suggests that the Faraday reaction speed is fast and the specific capacitance is high. In Fig. 18g, the discharge time of the composite electrode is the longest, indicating that its specific capacitance is the highest. The specific capacitances of V2CTx, NiMn-LDH/V2CTx, and CoNiMn-LDH/V2CTx at 1 A g−1 are 38, 591, and 1005 F g−1 (Fig. 18h). In the CV curves of Fig. 18i, the anode and cathode peaks separately move towards more anode and more cathode directions with the increase of scanning rate, implying the improvement of ion and electron transfer rates. An ASC device was fabricated in 6 M KOH electrolyte using CoNiMn-LDH/V2CTx as the cathode and V2CTx MXene as the anode (Fig. 18j). In Fig. 18k, because of the obvious polarization behavior in 1.6 V voltage window, 1.4 V was chosen as the optimal value. In Fig. 18l and m, CV and the GCD curves of the ASC device show a combined shape of an electric double-layer capacitance and pseudocapacitance. At 2 A g−1, the specific capacitance was 112 F g−1 after 3000 cycles, meaning that the device had excellent cycle stability (Fig. 18n).
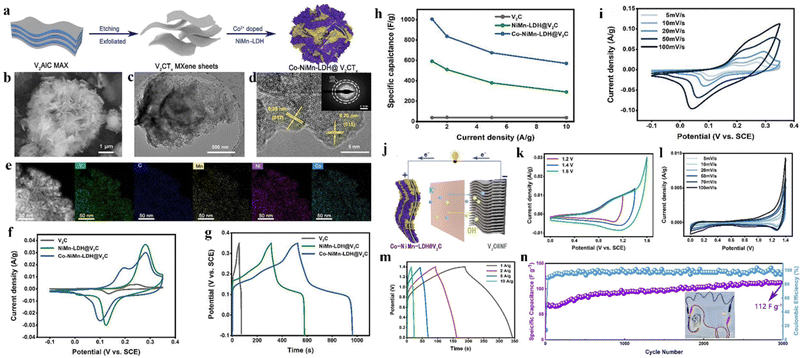 | ||
| Fig. 18 (a) Schematic of preparation of CoNiMn-LDH/V2CTx composite; (b) SEM and (c and d) TEM images of CoNiMn-LDH/V2CTx; (e) elemental mapping of V, C, Mn, Ni, and Co; (f) CV curves at 10 mV s−1; (g) GCD curves at 1 A g−1 and (h) rate capability of different composites; (i) CV curves of the CoNiMn-LDH/V2CTx electrode; (j) schematic of preparation of CoNiMn-LDH/V2CTx//V2C ASC device; (k) CV curves of the ASC at 50 mV s−1; (l) CV curves of ASC at different scan rates; (m) GCD curves of the ASC at various scan rates; (n) cycling performances of ASC at 2 A g−1 after 3000 cycles.255 © 2021 Elsevier B.V. | ||
Niu et al.256 prepared CoM (M = Fe, Mn)/MXene layered composite via the hydrothermal method (Fig. 19a), demonstrated that the properties of the single metal compound are adjusted by the introduction of other metal ions, as well as studying the influence of various metal ions. It was found that the presentation of Fe element greatly increases the specific capacitance (Fig. 19f), while the introduction of Mn ions reduces the ion diffusion resistance (Fig. 19g). The specific capacitance of the prepared CoFe-LDH/MXene (CF-MX10) was 664 F g−1 at 1 A g−1, which is higher than that of CoMn-LDH/MXene (CM-MX10, 524 F g−1) and CO(OH)2/MXene (C-MX10, 226 F g−1). The high specific capacitance corresponds to the strong cooperation effect of different components, which greatly improves the conductivity and electrochemical stability. Fig. 19b–d shows SEM images of CF-MX10 composites. It can be observed that a large number of CoFe-LDH nanosheets are coated on the periphery of the MXene sheet, forming a layered structure (Fig. 19c), giving rise to excellent ionic/electronic conductivity and high energy storage properties. SEM image (Fig. 19d) presents that the structure of CoFe-LDH nanosheets is almost the same as a triangle with a needle-like structure at the top. Therefore, the layered morphology has lots of edges and points, resulting in forming a large number of active sites. Fig. 19h displays CV curves of the electrode, which has two pairs of obvious peaks, suggesting that the capacitance is dominated by the Faraday redox reaction. In addition, the almost symmetrical shape of the GCD curves (Fig. 19i) indicates that the electrode has significant reversibility. Fig. 19j exhibits CV curves under various voltage windows. Nevertheless, in the case of 2.0 V potential, the curve is obviously polarized, meaning that the oxygen evolution behavior is serious. Hence, 0–1.8 V was chosen as the voltage window for the asymmetric device. Fig. 19k displays CV curves of CF-MX10//AC ASC in 0–1.8 V. Fig. 19l exhibits GCD curves of CF-MX10//AC. When the power density was 450 W kg−1, the energy density of ASC was 18.5 W h kg−1. When the energy density was 4.5 W h kg−1, the power density of ASC was 9000 W kg−1. In addition, Fig. 19m proves the cycle test of the device. One can see at 10 A g−1, after 8000 cycles, a 95% specific capacitance retention rate was achieved. These results indicated that the device had excellent stability.
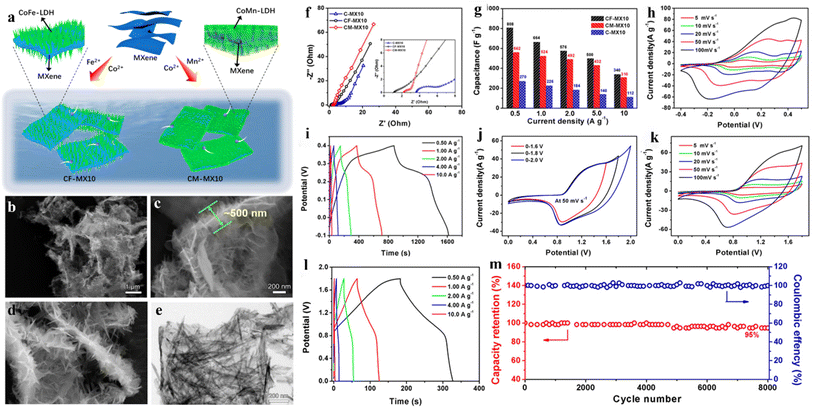 | ||
| Fig. 19 (a) Schematic diagram of fabricating CoFe-MXene and CoMn-MXene layered composites; (b–d) SEM and (e) TEM images of CF-MX10; (f) EIS curves of CF-MX10, CM-MX10, and C-MX10; (g) specific capacitance bar chart; (h and i) CV and GCD curves of CF-MX10 electrode; (j) CV curves under various voltage windows; (k) CV curves of ASC at various scan rates; (l) GCD curves of ASC at various current densities; (m) cyclic stability test at 10 A g−1.256 © 2021 Elsevier B.V. | ||
Fig. 20a exhibits a process for preparing a positive electrode of Ti3C2Tx@LDH-Ag. Fig. 20b and c are SEM images of Ti3C2Tx and NiCoAl-LDH, respectively. Fig. 20d is an SEM image of Ti3C2Tx MXene/NiCoAl-LDH, in which the NiCoAl-LDH nanosheets are evenly covered on Ti3C2Tx. To demonstrate the effectiveness of multicomponent composites, electrochemical performances are measured at the same conditions including CV, GCD, and rate plots (Fig. 20e–g). For improving the energy density, Zhang et al.257 proposed an in-plane hybrid supercapacitor (IHSC) using a special positive electrode on the fabric (Fig. 20h). By combining NiCoAl-LDH with high capacitance, Ti3C2Tx MXene with good conductivity as the skeleton and Ag nanowires, the battery type electrode achieved an excellent capacitance of 592 C g−1 at 1 A g−1, having a cycle life of more than 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 times. Fig. 20i exhibits GCD curves of the IHSC device based on KOH gel on the fabric, which can still maintain a substantially triangular shape. The surface capacitances calculated from the discharge curves are separately 64, 55, 43, 33, 24, and 17 mF cm−2 at 0.5, 1, 1.5, 2, 3, and 4 mA cm−2 (Fig. 20j). After 10
000 times. Fig. 20i exhibits GCD curves of the IHSC device based on KOH gel on the fabric, which can still maintain a substantially triangular shape. The surface capacitances calculated from the discharge curves are separately 64, 55, 43, 33, 24, and 17 mF cm−2 at 0.5, 1, 1.5, 2, 3, and 4 mA cm−2 (Fig. 20j). After 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 2 mA cm−2, 80% of the initial capacitance was maintained (Fig. 20k). A bending test was carried out to prove the flexibility, and Fig. 20l shows photos of the devices encapsulated by transparent tape in different bending cases. The bending angle is from 0° to 150°. Fig. 20m shows that bending deformation does not influence the shape and the area of the CV curve. In order to further evaluate the flexibility of the manufactured device, fatigue resistance tests were performed, and the obtained GCD curves under different bending cycles in 1000 cycles are shown in Fig. 20o. With 1000 bending cycles, the capacitance retention rate remained at 83%, although the capacitance retention rate rapidly decreased in the first 100 bending cycles.
000 cycles at 2 mA cm−2, 80% of the initial capacitance was maintained (Fig. 20k). A bending test was carried out to prove the flexibility, and Fig. 20l shows photos of the devices encapsulated by transparent tape in different bending cases. The bending angle is from 0° to 150°. Fig. 20m shows that bending deformation does not influence the shape and the area of the CV curve. In order to further evaluate the flexibility of the manufactured device, fatigue resistance tests were performed, and the obtained GCD curves under different bending cycles in 1000 cycles are shown in Fig. 20o. With 1000 bending cycles, the capacitance retention rate remained at 83%, although the capacitance retention rate rapidly decreased in the first 100 bending cycles.
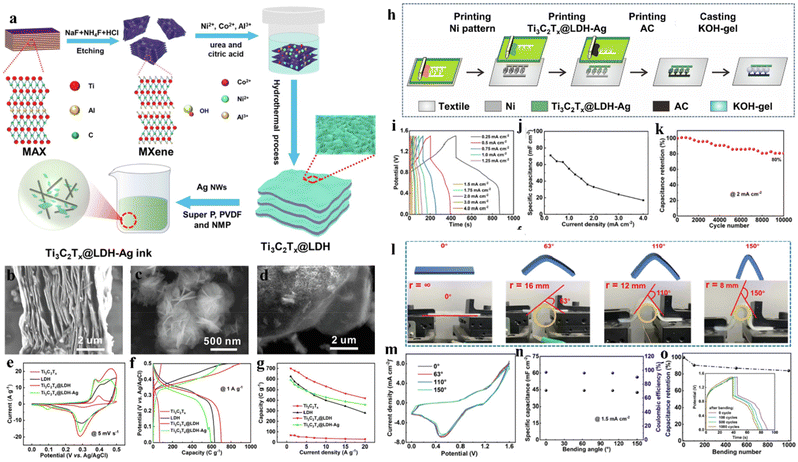 | ||
| Fig. 20 (a) Schematic of preparing Ti3C2Tx@LDH and Ti3C2Tx@LDH-Ag composite ink; (b) SEM image of multilayer Ti3C2Tx and (c) SEM image of NiCoAl-LDH; (d) SEM image of Ti3C2Tx@LDH; (e) CV curve at 5 mV s−1; (f) GCD curve at 1 A g−1; (g) capacitance performance of different materials; (h) manufacturing scheme of IHSC devices; (i) GCD curve of IHSC at 0.25 to 4.0 mA cm−2; (j) the change of area capacitance with discharge current; (k) cycling property of IHSC at 2 mA cm−2; (l) images of IHSC in different bending states; (m) CV curves of IHSC under various bending states at 100 mV s−1; (n) surface capacitance and coulombic efficiency under different bending states; (o) the relationship between the capacitance retention and the number of bends of IHSC devices.257 © 2022 Elsevier B.V. | ||
Moreover, the specific capacitance and the resistance of MXene, LDH, and their composite materials at the same current density were compared, and the results are presented as bar graphs, as shown in Fig. 21. The left figure shows that the specific capacitance of composite materials is higher than that of pure MXene and LDH materials, indicating that the synergistic effect of the two can improve the specific capacitance. The right figure displays that the resistance of the composite material is between that of MXene and LDH, proving that the combination of MXene and LDH can reduce the resistance of LDH.
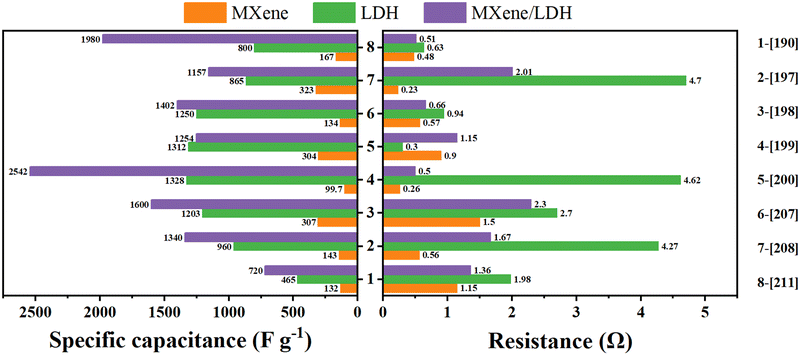 | ||
| Fig. 21 Bar graphs of the comparison for the resistance and a specific capacitance of MXene, LDH, and their composites at the same current density. | ||
Furthermore, the electrochemical performances of MXene/LDH prepared by different preparation methods are summarized in recent years in the form of a table. Table 5 shows the performance of different MXene/LDH materials in the three-electrode test, and Table 6 shows the two-electrode electrochemical properties of the assembled device.
| Electrode | Electrolyte | Potential window/V | Condition | Capacitance | Cyclic performance | Ref. |
|---|---|---|---|---|---|---|
| NiAl-LDH/MXene | 6 M KOH | 0–0.55 | 1 A g−1 | 1061 F g−1 | 4 A g−1, 4000, 70% | 80 |
| NiFe-LDH/MXene | 1 M KOH | 0–0.37 | 1 A g−1 | 720.2 F g−1 | — | 240 |
| Ni–Mn LDH@MXene | 6 M KOH | 0–0.5 | 1 A g−1 | 179 mA h g−1 | 6 A g−1, 5000, 79.1% | 247 |
| NiAl-LDH/MXene | 2 M KOH | 0–0.45 | 1 A g−1 | 1600 F g−1 | 10A g−1, 3000, 78% | 248 |
| MXene/GO/LDH | 6 M KOH | 0–0.35 | 1 A g−1 | 241.9 mA h g−1 | 5 A g−1, 4000, 90.9% | 249 |
| NiCoAl-LDH/V4C3Tx | 1 M KOH | −0.1–0.45 | 1 A g−1 | 627 C g−1 | 20 A g−1, 3000, 82.6% | 250 |
| Co-NiMn-LDH/V4C3Tx | 6 M KOH | −0.1–0.35 | 1 A g−1 | 1005 F g−1 | — | 255 |
| CoFe-LDH/MXene | 1 M KOH | −0.1–0.4 | 0.5 A g−1 | 808 F cm−2 | 10 A g−1, 8000, 80% | 256 |
| Ti3C2Tx@NiCoAl LDH-Ag | 1 M KOH | 0–0.5 | 1 A g−1 | 592 C g−1 | 10 A g−1, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 88% 000, 88% |
257 |
| NiCo-LDHs/MXene | 1 M (NH4)2SO4 | −0.2–0.6 | 0.5 A g−1 | 1207 F g−1 | 0.5 A g−1, 5000, 93% | 258 |
| NiCo-LDH/Ti3C2 | 6 M KOH | 0–0.6 | 2 A g−1 | 984 F g−1 | 30 A g−1, 5000, 76% | 259 |
| NiMn-LDH/MXene | 6 M KOH | 0–0.45 | 0.5 A g−1 | 1575 F g−1 | 5 A g−1, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 90.3% 000, 90.3% |
254 |
| Ti3C2/Ni–Co–Al-LDH | 1 M KOH | 0–0.6 | 1 A g−1 | 748.2 F g−1 | 2 A g−1, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 78.7% 000, 78.7% |
260 |
| NiCoFe-LDH/Ti3C2 | 1 M KOH | 0–0.3 | 1 A g−1 | 1990 F g−1 | 10 A g−1, 5000, 84.8% | 261 |
| NiCo2-LDHs@MXene/rGO | 2 M KOH | 0–0.45 | 1 A g−1 | 332.3 mA h g−1 | 5 A g−1, 5000, 87.5% | 262 |
| MXene/CoAl-LDH-80% | 3 M KOH | 0–0.5 | 1 A g−1 | 1930 F g−1 | — | 263 |
| Device | Electrolyte | Potential window/V | Capacitance | Energy density | Power density | Cycling stability | Ref. |
|---|---|---|---|---|---|---|---|
| NiFe-LDH/MXene//AC | — | 0.2–1.5 | 1 A g−1, 135.7 F g−1 | 42.4 W h kg−1 | 758.2 W kg−1 | 1 A g−1, 1000, 84% | 240 |
| MXene@Ni–Mn LDH//AC | PVA/KOH | 0–1.6 | 1 A g−1, 56 mA h g−1 | 44.7 W h kg−1 | 800 W kg−1 | 6 A g−1, 5000, 90.3% | 247 |
| NiAl-LDH/MXene//AC | 2 M KOH | 0–1.7 | 3 A g−1, 37 F g−1 | 27.6 W h kg−1 | 255 W kg−1 | 3 A g−1, 5000, 89.1% | 248 |
| MXene/GO/LDH//AC | PVA/KOH | 0–1.6 | 1 A g−1, 69.1 mA h g−1 | 55.3 W h kg−1 | 800 W kg−1 | 5 A g−1, 4000, 94.7% | 249 |
| NiCoAl-LDH/V4C3Tx//AC | 1 M KOH | 0–1.6 | 1 A g−1, 194 F g−1 | 71.7 W h kg−1 | 830 W kg−1 | 20 A g−1, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 98% 000, 98% |
250 |
| CoNiMn/V4C3Tx//V4C3Tx | 6 M KOH | 0–1.4 | 1 A g−1, 110 F g−1 | 30.16 W h kg−1 | 700 W kg−1 | 2 A g−1, 3000, 95.7% | 255 |
| CoFe-LDH/MXene//AC | 1 M KOH | 0–1.8 | 0.5 A g−1, 295 F g−1 | 18.5 W h kg−1 | 450 W kg−1 | 10 A g−1, 8000, 95% | 256 |
| Ti3C2Tx@NiCoAl LDH-Ag//AC | PVA/KOH | 0–1.5 | 0.5 mA cm−2, 64 mF cm−2 | 22.18 μW h cm−2 | 3.0 mW cm−2 | 2 mA cm−2, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 80% 000, 80% |
257 |
| MXene-LDH@NF//MWCNT@NF | PVA/KOH | 0–1.6 | — | 36.7 W h kg−1 | 1440 W kg−1 | — | 259 |
| NiMn-LDH/MXene//AC | 6 M KOH | 0–1.5 | 0.5 A g−1, 169.8 F g−1 | 126 W h kg−1 | 740 W kg−1 | 2 A g−1, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 91.8% 000, 91.8% |
254 |
| Ti3C2/Ni–Co–Al-LDH//AC | PVA/KOH | 0–1.6 | 0.5 A g−1, 128.89 F g−1 | 45.8 W h kg−1 | 346 W kg−1 | 5 A g−1, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 97.8% 000, 97.8% |
260 |
| NiCoFe-LDH/Ti3C2//RGO | 1 M KOH | 0–1.55 | — | 54.4 W h kg−1 | 895.1 W kg−1 | 5 A g−1, 5000, 80.2% | 261 |
| NiCo2-LDHs@MXene/rGO//MXene/rGO | 2 M KOH | 0–1.4 | 1 A g−1, 240 F g−1 | 65.3 W h kg−1 | 700 W kg−1 | 5 A g−1, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 92.8% 000, 92.8% |
262 |
| MXene/CoAl-LDH-80%//MXene/GO-5% | 3 M KOH | 0.4–1.6 | 1 A g−1, 112 C g−1 | 30.9 W h kg−1 | 992.6 W kg−1 | 30 A g−1, 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 94.4% 000, 94.4% |
263 |
5. Summary
Here, the fabrication methods of MXene and LDH and their composites, as well as the latest progress and achievements in the application frontier of supercapacitors are reviewed. MXene has become an inevitable hotspot in the field of energy storage, especially in the field of supercapacitors in the past decade. Its simple synthesis strategy, unique combination, and adjustable properties of specific applications provide support for it. However, the urgent need for the enhancement of structure and morphology has realized the idea of the introduction of complementary materials. LDHs provide enhanced electrochemical activity and high specific surface area, avoid the problem of re-blockage, and enhance conductivity. In addition, the structures of these heterostructures play a synergistic interface function and induce a separate energy storage contribution while improving the overall interface structure and electrochemical stability. In addition, each MXene nanocomposite has different 2D heterostructures and special advantages. Its composition needs to be optimized to achieve strong supercapacitor performance.At last, the real potential coming from 2D materials and heterostructures is large enough, which provides a huge base to understand the interaction dynamics and the energy storage of other 2D materials such as H-BN, MOFs, and covalent organic skeletons. MXene is used to produce heterostructures for driving towards one of the optimal electrode materials. The MXene/LDH heterostructures can be transformed into supercapacitor devices, for instance, rugged wires, flexible and micro-supercapacitors for portable and wearable electronic devices, with excellent device compatibility Hence, MXene/LDH heterostructures are expected to become electrode materials for the next generation of advanced energy storage applications.
Author contributions
Zhongtai Lin: conception and designed the work, writing-original draft, and editing. Xue Li: methodology, data curation. Hao Zhang, Ben Bin Xu: formal analysis, investigation. Priyanka Wasnik, Handong Li, Man Vir Singh: software, supervision. Yong Ma, Tingxi Li, Zhanhu Guo: reviewing and editing.Conflicts of interest
The authors declare that they have no conflicts of interest.Acknowledgements
We gratefully appreciate the support of the Natural Science Foundation of Shandong (ZR2019BB063).References
- C. Hwang, T.-L. Chung and E. A. Sanders, Attitudes and Purchase Intentions for Smart Clothing, Cloth. Text. Res. J., 2016, 34, 207–222 CrossRef
.
- Q. Huang, D. Wang and Z. Zheng, Textile-Based Electrochemical Energy Storage Devices, Adv. Energy Mater., 2016, 6, 1600783 CrossRef
.
- L.-C. Jia, Y.-K. Li and D.-X. Yan, Flexible and efficient electromagnetic interference shielding materials from ground tire rubber, Carbon, 2017, 121, 267–273 CrossRef CAS
.
- F. Ahmed, A. Umar, S. Kumar, N. M. Shaalan, N. Arshi, M. G. Alam, P. M. Z. Hasan, S. M. Ramay, R. Khan, A. Aljaafari and A. Alshoaibi, Manganese dioxide nanoparticles/reduced graphene oxide nanocomposites for hybrid capacitive desalination, Adv. Compos. Hybrid Mater., 2023, 6, 19 CrossRef CAS
.
- M. Xu, Y. Huang, R. Chen, Q. Huang, Y. Yang, L. Zhong, J. Ren and X. Wang, Green conversion of Ganoderma lucidum residues to electrode materials for supercapacitors, Adv. Compos. Hybrid Mater., 2021, 4, 1270–1280 CrossRef CAS
.
- X. L. Hao, Y. Y. Ma, H. Y. Zang, Y. H. Wang, Y. G. Li and E. B. Wang, A polyoxometalate-encapsulating cationic metal-organic framework as a heterogeneous catalyst for desulfurization, Chemistry, 2015, 21, 3778–3784 CrossRef CAS PubMed
.
- L. Chen, C. Lan, B. Xu and K. Bi, Progress on material characterization methods under big data environment, Adv. Compos. Hybrid Mater., 2021, 4, 235–247 CrossRef
.
- H. Liu, T. Xu, Q. Liang, Q. Zhao, D. Zhao and C. Si, Compressible cellulose nanofibrils/reduced graphene oxide composite carbon aerogel for solid-state supercapacitor, Adv. Compos. Hybrid Mater., 2022, 5, 1168–1179 CrossRef CAS
.
- S. Liu, H. Du, K. Liu, M.-G. Ma, Y.-E. Kwon, C. Si, X.-X. Ji, S.-E. Choi and X. Zhang, Flexible and porous Co3O4-carbon nanofibers as binder-free electrodes for supercapacitors, Adv. Compos. Hybrid Mater., 2021, 4, 1367–1383 CrossRef CAS
.
- Y. Ma, X. Xie, W. Yang, Z. Yu, X. Sun, Y. Zhang, X. Yang, H. Kimura, C. Hou, Z. Guo and W. Du, Recent advances in transition metal oxides with different dimensions as electrodes for high-performance supercapacitors, Adv. Compos. Hybrid Mater., 2021, 4, 906–924 CrossRef CAS
.
- R. Pan, M. Jia, Y. Li, X. Li and T. Dou, In Situ Delamination of Ferrierite Zeolite and its Performance in the Catalytic Cracking of C4 Hydrocarbons, Chin. J. Chem. Eng., 2014, 22, 1237–1242 CrossRef CAS
.
- Y.-P. Zhang, Y. Li, G.-C. Xu, J.-Y. Li, H.-Y. Luo, J.-Y. Li, L. Zhang and D.-Z. Jia, Synthesis, crystal structure, DNA/bovine serum albumin binding and antitumor activity of two transition metal complexes with 4-acylpyrazolone derivative, Appl. Organomet. Chem., 2019, 33, e4668 CrossRef
.
- H.-Y. Sun, X.-Y. Wang, H.-X. Dai, G.-P. Zhang and F.-B. Wu, Effect of Exogenous Glutathione and Selenium on Cadmium-Induced Changes in Cadmium and Mineral Concentrations and Antioxidative Metabolism in Maize Seedlings, Asian J. Chem., 2013, 25, 2970–2976 CrossRef CAS
.
- X. Wang, Z. Liu, X. Wei, F. Guo, P. Li and S. Guo, Synthesis of 2,6-Dimethylnaphthalene over Sapo-11, Sapo-5 and Mordenite Molecular Sieves, Braz. J. Chem. Eng., 2017, 34, 295–306 CrossRef CAS
.
- L. Zhi, J. Li, X. Li, Y. Chen, Y. Song, J. Yu and Q. Zhang, Enhancing water solubility of N-dodecyl-d-gluconamide
surfactant using borax, Chem. Phys. Lett., 2019, 725, 87–91 CrossRef CAS
.
- K. S. Novoselov, A. Mishchenko, A. Carvalho and A. H. Castro Neto, 2D materials and van der Waals heterostructures, Science, 2016, 353, aac9439 CrossRef CAS PubMed
.
- Y. Wei, W. Luo, Z. Zhuang, B. Dai, J. Ding, T. Li, M. Ma, X. Yin and Y. Ma, Fabrication of ternary MXene/MnO2/polyaniline nanostructure with good electrochemical performances, Adv. Compos. Hybrid Mater., 2021, 4, 1082–1091 CrossRef CAS
.
- W. Luo, Y. Wei, Z. Zhuang, Z. Lin, X. Li, C. Hou, T. Li and Y. Ma, Fabrication of Ti3C2Tx MXene/polyaniline composite films with adjustable thickness for high-performance flexible all-solid-state symmetric supercapacitors, Electrochim. Acta, 2022, 406, 139871 CrossRef CAS
.
- F. B. M. Ahmed, D. Khalafallah, M. Zhi and Z. Hong, Porous nanoframes of sulfurized NiAl layered double hydroxides and ternary bismuth cerium sulfide for supercapacitor electrodes, Adv. Compos. Hybrid Mater., 2022, 5, 2500–2514 CrossRef CAS
.
- G. Li, L. Wang, X. Lei, Z. Peng, T. Wan, S. Maganti, M. Huang, V. Murugadoss, I. Seok, Q. Jiang, D. Cui, A. Alhadhrami, M. M. Ibrahim and H. Wei, Flexible, yet robust polyaniline coated foamed polylactic acid composite electrodes for high-performance supercapacitors, Adv. Compos. Hybrid Mater., 2022, 5, 853–863 CrossRef CAS
.
- D. K. Maurya, R. Dhanusuraman, Z. Guo and S. Angaiah, Composite polymer electrolytes: progress, challenges, and future outlook for sodium-ion batteries, Adv. Compos. Hybrid Mater., 2022, 5, 2651–2674 CrossRef CAS
.
- A. Osman, A. Elhakeem, S. Kaytbay and A. Ahmed, A comprehensive review on the thermal, electrical, and mechanical properties of graphene-based multi-functional epoxy composites, Adv. Compos. Hybrid Mater., 2022, 5, 547–605 CrossRef
.
- D. Wang, A. Mukhtar, M. Humayun, K. Wu, Z. Du, S. Wang and Y. Zhang, A Critical Review on Nanowire-Motors: Design, Mechanism and Applications, Chem. Rec., 2022, 22, e202200016 CAS
.
- L. Pu, J. Zhang, N. K. L. Jiresse, Y. Gao, H. Zhou, N. Naik, P. Gao and Z. Guo, N-doped MXene derived from chitosan for the highly effective electrochemical properties as supercapacitor, Adv. Compos. Hybrid Mater., 2022, 5, 356–369 CrossRef CAS
.
- Y. Shao, Y. Zhu, R. Zheng, P. Wang, Z. Zhao and J. An, Highly sensitive and selective surface molecularly imprinted polymer electrochemical sensor prepared by Au and MXene modified glassy carbon electrode for efficient detection of tetrabromobisphenol A in water, Adv. Compos. Hybrid Mater., 2022, 5, 3104–3116 CrossRef CAS
.
- W. Luo, Y. Sun, Y. Han, J. Ding, T. Li, C. Hou and Y. Ma, Flexible Ti3C2Tx MXene/polypyrrole composite films for high-performance all-solid asymmetric supercapacitors, Electrochim. Acta, 2023, 441, 141818 CrossRef CAS
.
- J. Ren, C. Wang, J. Ding, T. Li and Y. Ma, Magnetic Core–Shell Fe3O4@polypyrrole@4-vinylpyridine Composites for the Removal of Multiple Dyes, ACS Appl. Polym. Mater., 2022, 4, 9449–9462 CrossRef CAS
.
- Y. Wen, X. Ge, W. Gao, W. Wei, Y. Qiao and H. Chang, Synthesis and aggregation properties of ethylene glycol ester-based cationic Gemini surfactants, Colloid Interface Sci., 2020, 37, 100274 CrossRef CAS
.
- L. Zhi, X. Shi, E. Zhang, Y. Pan, X. Li, H. Wang and W. Liu, Synthesis and properties of stellate lactosamide quaternary ammonium
surfactants, Colloids Surf., A, 2021, 628, 127317 CrossRef CAS
.
- S. Seyedin, S. Uzun, A. Levitt, B. Anasori, G. Dion, Y. Gogotsi and J. M. Razal, MXene Composite and Coaxial Fibers with High Stretchability and Conductivity for Wearable Strain Sensing Textiles, Adv. Funct. Mater., 2020, 30, 1910504 CrossRef CAS
.
- B. Dai, Y. Ma, F. Dong, J. Yu, M. Ma, H. K. Thabet, S. M. El-Bahy, M. M. Ibrahim, M. Huang, I. Seok, G. Roymahapatra, N. Naik, B. B. Xu, J. Ding and T. Li, Overview of MXene and conducting polymer matrix composites for electromagnetic wave absorption, Adv. Compos. Hybrid Mater., 2022, 5, 704–754 CrossRef
.
- Y. Cao, M. Weng, M. H. H. Mahmoud, A. Y. Elnaggar, L. Zhang, I. H. El Azab, Y. Chen, M. Huang, J. Huang and X. Sheng, Flame-retardant and leakage-proof phase change composites based on MXene/polyimide aerogels toward solar thermal energy harvesting, Adv. Compos. Hybrid Mater., 2022, 5, 1253–1267 CrossRef CAS
.
- H. Cheng, L. Xing, Y. Zuo, Y. Pan, M. Huang, A. Alhadhrami, M. M. Ibrahim, Z. M. El-Bahy, C. Liu, C. Shen and X. Liu, Constructing nickel chain/MXene networks in melamine foam towards phase change materials for thermal energy management and absorption-dominated electromagnetic interference shielding, Adv. Compos. Hybrid Mater., 2022, 5, 755–765 CrossRef CAS
.
- X. Zhao, M. Zheng, X. Gao, J. Zhang, E. Wang and Z. Gao, The application of MOFs-based materials for antibacterials adsorption, Coord. Chem. Rev., 2021, 440, 213970 CrossRef CAS
.
- X. Li, C. Yang, C. He, L. Zhi, J. Bai, J. Guo and W. Li, The sintering behavior of Fe-based oxygen carrier with straw ash and sawdust ash by thermodynamic and thermomechanical analysis, Fuel Process. Technol., 2022, 235, 107346 CrossRef CAS
.
- W. Luo, Y. Ma, T. Li, H. K. Thabet, C. Hou, M. M. Ibrahim, S. M. El-Bahy, B. B. Xu and Z. Guo, Overview of MXene/conducting polymer composites for supercapacitors, J. Energy Storage, 2022, 52, 105008 CrossRef
.
- H. Cheng, Y. Pan, Q. Chen, R. Che, G. Zheng, C. Liu, C. Shen and X. Liu, Ultrathin flexible poly(vinylidene fluoride)/MXene/silver nanowire film with outstanding specific EMI shielding and high heat dissipation, Adv. Compos. Hybrid Mater., 2021, 4, 505–513 CrossRef CAS
.
- D. Kong, Z. M. El-Bahy, H. Algadi, T. Li, S. M. El-Bahy, M. A. Nassan, J. Li, A. A. Faheim, A. Li, C. Xu, M. Huang, D. Cui and H. Wei, Highly sensitive strain sensors with wide operation range from strong MXene-composited polyvinyl alcohol/sodium carboxymethylcellulose double network hydrogel, Adv. Compos. Hybrid Mater., 2022, 5, 1976–1987 CrossRef CAS
.
- H. Wang, H. Zhang, K. Zhao, A. Nie, S. Alharthi, M. A. Amin, Z. M. El-Bahy, H. Li, L. Chen, B. B. Xu, Y. Ma and T. Li, Research progress on electromagnetic wave absorption based on magnetic metal oxides and their composites, Adv. Compos. Hybrid Mater., 2023, 6, 120 CrossRef
.
- C. Wang, X. Liu, T. Yang, D. Sridhar, H. Algadi, B. B. Xu, Z. M. El-Bahy, H. Li, Y. Ma, T. Li and Z. Guo, An overview of metal-organic frameworks and their magnetic composites for the removal of
pollutants, Sep. Purif. Technol., 2023, 320, 124144 CrossRef CAS
.
- K. Sun, Z. Liu, S. Song, W. Liu, P. Wang, T. Zhang, Y. Xue, Y. Wang and Y. Tan, Effect of Hydroxyl Groups on CuCoMg Nanosheets for Ethanol and Higher Alcohol Synthesis from Syngas, Ind. Eng. Chem. Res., 2021, 60, 2388–2399 CrossRef CAS
.
- X. Xiao, H. Wang, P. Urbankowski and Y. Gogotsi, Topochemical synthesis of 2D materials, Chem. Soc. Rev., 2018, 47, 8744–8765 RSC
.
- Q. Gao, Y. Pan, G. Zheng, C. Liu, C. Shen and X. Liu, Flexible multilayered MXene/thermoplastic polyurethane films with excellent electromagnetic interference shielding, thermal conductivity, and management performances, Adv. Compos. Hybrid Mater., 2021, 4, 274–285 CrossRef CAS
.
- L. Ouyang, W. Huang, M. Huang and B. Qiu, Polyaniline improves granulation and stability of aerobic granular sludge, Adv. Compos. Hybrid Mater., 2022, 5, 1126–1136 CrossRef CAS
.
- M. Zheng, X. Zhao, K. Wang, Y. She and Z. Gao, Highly Efficient Removal of Cr(VI) on a Stable Metal–Organic Framework Based on Enhanced H-Bond Interaction, Ind. Eng. Chem. Res., 2019, 58, 23330–23337 CrossRef CAS
.
- X. Zhao, Y. Wei, H. Zhao, Z. Gao, Y. Zhang, L. Zhi, Y. Wang and H. Huang, Functionalized metal-organic frameworks for effective removal of rocephin in aqueous solutions, J. Colloid Interface Sci., 2018, 514, 234–239 CrossRef CAS PubMed
.
- P. Eklund, M. Beckers, U. Jansson, H. Högberg and L. Hultman, The Mn+1AXn phases: Materials science and thin-film processing, Thin Solid Films, 2010, 518, 1851–1878 CrossRef CAS
.
- X. He, S. Li, R. Shen, Y. Ma, L. Zhang, X. Sheng, Y. Chen, D. Xie and J. Huang, A high-performance waterborne polymeric composite coating with long-term anti-corrosive property based on phosphorylation of chitosan-functionalized Ti3C2Tx MXene, Adv. Compos. Hybrid Mater., 2022, 5, 1699–1711 CrossRef CAS
.
- D. Wang, R. Wang, K. Huang, M. Lei and H. Tang, Si-P-Ti stabilized Si-P/Ti3C2Tx nanohybrids for enhanced lithium-ion storage, Adv. Compos. Hybrid Mater., 2022, 5, 1362–1375 CrossRef CAS
.
- X.-L. Hao, S.-F. Jia, Y.-Y. Ma, H.-Y. Wang and Y.-G. Li, Two new Keggin-type polyoxometalate-based entangled coordination networks constructed from metal-organic chains with dangling arms, Inorg. Chem. Commun., 2016, 72, 132–137 CrossRef CAS
.
- X. Zhao, X. Gao, Y. N. Zhang, M. Wang, X. Gao and B. Liu, Construction of dual sulfur sites in metal-organic framework for enhanced mercury(II) removal, J. Colloid Interface Sci., 2023, 631, 191–201 CrossRef CAS PubMed
.
- O. Mashtalir, M. Naguib, V. N. Mochalin, Y. Dall'Agnese, M. Heon, M. W. Barsoum and Y. Gogotsi, Intercalation and delamination of layered carbides and carbonitrides, Nat. Commun., 2013, 4, 1716 CrossRef PubMed
.
- M. Naguib, R. R. Unocic, B. L. Armstrong and J. Nanda, Large-scale delamination of multi-layers transition metal carbides and carbonitrides “MXenes”, Dalton Trans., 2015, 44, 9353–9358 RSC
.
- I. M. Chirica, A. G. Mirea, Ş. Neaţu, M. Florea, M. W. Barsoum and F. Neaţu, Applications of MAX phases and MXenes as catalysts, J. Mater. Chem. A, 2021, 9, 19589–19612 RSC
.
- Z. Wang, K. Yin, Y. Zhang, K. Sun, L. Xie, M. Cong, S. Cao, Y. Lei, X. Li and R. Fan, Two-dimensional Ti3C2Tx/carbonized wood metacomposites with weakly negative permittivity, Adv. Compos. Hybrid Mater., 2022, 5, 2369–2377 CrossRef CAS
.
- Y. Ma, C. Hou, H. Zhang, Q. Zhang, H. Liu, S. Wu and Z. Guo, Three-dimensional core-shell Fe3O4/Polyaniline coaxial heterogeneous nanonets: Preparation and high performance supercapacitor electrodes, Electrochim. Acta, 2019, 315, 114–123 CrossRef CAS
.
- X. Zhao, Y. Zhao, M. Zheng, S. Liu, W. Xue, G. Du, T. Wang, X. Gao, K. Wang, J. Hu, Z. Gao and H. Huang, Efficient separation of vitamins mixture in aqueous solution using a stable zirconium-based metal-organic framework, J. Colloid Interface Sci., 2019, 555, 714–721 CrossRef CAS PubMed
.
- S. Song, C. Liu, R. Ding, X. Gao, M. Wang, Z. Li and X. Zhao, Isomerous Al-BDC-NH2 metal-organic frameworks for metronidazole removal: Effect of topology structure, J. Solid State Chem., 2022, 314, 123376 CrossRef CAS
.
- Y. Wang, Z. Shi, C. Fan, X. Wang, X. Hao and Y. Chi, Synthesis, characterization, and photocatalytic properties of BiOBr catalyst, J. Solid State Chem., 2013, 199, 224–229 CrossRef CAS
.
- X. Zhao, L. Pei, H. Fan, Y. Zhang, B. Liu, X. Gao and Y. Wei, Synergic coordination and precipitation effects induced by free carboxyl for separation of iron(III) and nickel(II) in zirconium-metal-organic framework, J. Solid State Chem., 2021, 302, 122460 CrossRef CAS
.
- P. Lin, J. Xie, Y. He, X. Lu, W. Li, J. Fang, S. Yan, L. Zhang, X. Sheng and Y. Chen, MXene aerogel-based phase change materials toward solar energy conversion, Sol. Energy Mater. Sol. Cells, 2020, 206, 110229 CrossRef CAS
.
- D. Wang, Y. Fang, W. Yu, L. Wang, H. Xie and Y. Yue, Significant solar energy absorption of MXene Ti3C2Tx nanofluids via localized surface plasmon resonance, Sol. Energy Mater. Sol. Cells, 2021, 220, 110850 CrossRef CAS
.
- R. Wang, Z. Zhang, P. Du, Z. Fu, K. Huang, K. Xu, Y. Du, D. Fan, R. Zhang and M. Lei, Efficient synthesis of sulfur-modified cobalt hydroxide self-supported electrocatalysts for enhanced oxygen evolution, Adv. Compos. Hybrid Mater., 2022, 5, 2491–2499 CrossRef CAS
.
- J. Zhang, J. Yang, L. Cheng, Y. Wang and G. Feng, Adsorption of acetylene on Sn-doped Ni(111) surfaces: a density functional study, J. Mol. Model, 2020, 26, 310 CrossRef CAS PubMed
.
- X. Zhao, T. Wang, G. Du, M. Zheng, S. Liu, Z. Zhang, Y. Zhang, X. Gao and Z. Gao, Effective Removal of Humic Acid from Aqueous Solution in an Al-Based Metal–Organic Framework, J. Chem. Eng. Data, 2019, 64, 3624–3631 CrossRef CAS
.
- X. Zhao, Y. Wang, Y. Li, W. Xue, J. Li, H. Wu, Y. Zhang, B. Li, W. Liu, Z. Gao and H. Huang, Synergy Effect of Pore Structure and Amount of Carboxyl Site for Effective Removal of Pb2+ in Metal–Organic Frameworks, J. Chem. Eng. Data, 2019, 64, 2728–2735 CrossRef CAS
.
- Z.-Q. Gao, H.-J. Li, J.-Z. Gu, Q.-H. Zhang and A. M. Kirillov, Metal-organic and supramolecular networks driven by 5-chloronicotinic acid: Hydrothermal self-assembly synthesis, structural diversity, luminescent and magnetic properties, J. Solid State Chem., 2016, 241, 121–130 CrossRef CAS
.
- C. Zhi, R. Zhang and B. Wang, Comparative studies about CO methanation over Ni(211) and Zr-modified Ni(211) surfaces: Qualitative insight into the effect of surface structure and composition, Mol. Catal., 2017, 438, 1–14 CrossRef CAS
.
- N. Wu, B. Zhao, X. Chen, C. Hou, M. Huang, A. Alhadhrami, G. A. M. Mersal, M. M. Ibrahim and J. Tian, Dielectric properties and electromagnetic simulation of molybdenum disulfide and ferric oxide-modified Ti3C2Tx MXene hetero-structure for potential microwave absorption, Adv. Compos. Hybrid Mater., 2022, 5, 1548–1556 CrossRef CAS
.
- W. Wang, J. Ren, C. Wang, M. Zheng, Y. Ma, X. Yin, J. Ding, C. Hou and T. Li, Magnetic Fe3O4/polypyrrole–salicylaldehyde composite for efficient removal of Mn(VII) from aqueous solution by double–layer adsorption, J. Appl. Polym. Sci., 2022, 139, e52515 CAS
.
- W. Wang, M. Zheng, J. Ren, M. Ma, X. Yin, T. Li and Y. Ma, Fabrication of magnetic Fe3O4/MnO2/TiO2/polypyrrole heterostructure for efficient adsorption of Mn7+ from aqueous solution, J. Appl. Polym. Sci., 2022, 139, 52199 CrossRef CAS
.
- B. Dai, F. Dong, H. Wang, Y. Qu, J. Ding, Y. Ma, M. Ma and T. Li, Fabrication of CuS/Fe3O4@polypyrrole flower-like composites for excellent electromagnetic wave absorption, J. Colloid Interface Sci., 2023, 634, 481–494 CrossRef CAS PubMed
.
- Y.-Z. Wen, Y.-Q. Xue, Z.-X. Cui and Y. Wang, Thermodynamics of nanoadsorption from solution: Theoretical and experimental research, J. Chem. Thermodyn., 2015, 80, 112–118 CrossRef CAS
.
- H. Lu, B. Zhao, D. Zhang, Y. Lv, B. Shi, X. Shi, J. Wen, J. Yao and Z. Zhu, Room temperature aqueous solution synthesis of pinacol (C6) by photocatalytic C-C coupling of isopropanol, J. Photochem. Photobiol., A, 2013, 272, 1–5 CrossRef CAS
.
- M. R. Lukatskaya, O. Mashtalir, C. E. Ren, Y. Dall'Agnese, P. Rozier, P. L. Taberna, M. Naguib, P. Simon, M. W. Barsoum and Y. Gogotsi, Cation intercalation and high volumetric capacitance of two-dimensional titanium carbide, Science, 2013, 341, 1502–1505 CrossRef CAS PubMed
.
- G. Sharma, E. Muthuswamy, M. Naguib, Y. Gogotsi, A. Navrotsky and D. Wu, Calorimetric Study of Alkali Metal Ion (K+, Na+, Li+) Exchange in a Clay-Like MXene, J. Phys. Chem. C, 2017, 121, 15145–15153 CrossRef CAS
.
- M. R. Lukatskaya, S. Kota, Z. Lin, M.-Q. Zhao, N. Shpigel, M. D. Levi, J. Halim, P.-L. Taberna, M. W. Barsoum, P. Simon and Y. Gogotsi, Ultra-high-rate pseudocapacitive energy storage in two-dimensional transition metal carbides, Nat. Energy, 2017, 2, 17105 CrossRef CAS
.
- W. Luo, Y. Sun, Z. Lin, X. Li, Y. Han, J. Ding, T. Li, C. Hou and Y. Ma, Flexible Ti3C2Tx MXene/V2O5 composite films for high-performance all-solid supercapacitors, J. Energy Storage, 2023, 62, 106807 CrossRef
.
- X. Xie, M.-Q. Zhao, B. Anasori, K. Maleski, C. E. Ren, J. Li, B. W. Byles, E. Pomerantseva, G. Wang and Y. Gogotsi, Porous heterostructured MXene/carbon nanotube composite paper with high volumetric capacity for sodium-based energy storage devices, Nano Energy, 2016, 26, 513–523 CrossRef CAS
.
- Y. Wang, H. Dou, J. Wang, B. Ding, Y. Xu, Z. Chang and X. Hao, Three-dimensional porous MXene/layered
double hydroxide composite for high performance supercapacitors, J. Power Sources, 2016, 327, 221–228 CrossRef CAS
.
- C. Lai, Y. Guo, H. Zhao, H. Song, X. Qu, M. Huang, S. W. Hong and K. Lee, High-performance double “ion-buffering reservoirs” of asymmetric supercapacitors enabled by battery-type hierarchical porous sandwich-like Co3O4 and 3D graphene aerogels, Adv. Compos. Hybrid Mater., 2022, 5, 2557–2574 CrossRef CAS
.
- C. Lai, Y. Wang, L. Fu, H. Song, B. Liu, D. Pan, Z. Guo, I. Seok, K. Li, H. Zhang and M. Dong, Aqueous flexible all-solid-state NiCo-Zn batteries with high capacity based on advanced ion-buffering reservoirs of NiCo2O4, Adv. Compos. Hybrid Mater., 2022, 5, 536–546 CrossRef CAS
.
- Z. Yan, J. Li, Q. Chen, S. Chen, L. Luo and Y. Chen, Synthesis of CoSe2/Mxene composites using as high-performance anode materials for lithium-ion batteries, Adv. Compos. Hybrid Mater., 2022, 5, 2977–2987 CrossRef CAS
.
- Y. Wei, M. Zheng, W. Luo, B. Dai, J. Ren, M. Ma, T. Li and Y. Ma, All pseudocapacitive MXene-MnO2 flexible asymmetric supercapacitor, J. Energy Storage, 2022, 45, 103715 CrossRef
.
- J. Zhang, Y. Wang, Y. Wang and M. Zhang, Catalytic Activity for Oxygen Reduction Reaction on CoN2 Embedded Graphene: A Density Functional Theory Study, J. Electrochem. Soc., 2017, 164, F1122–F1129 CrossRef CAS
.
- L. Zhi, X. Shi, E. Zhang, C. Gao, H. Gai, H. Wang, Z. Liu and T. Zhang, Synthesis and Performance of Double-Chain Quaternary Ammonium Salt Glucosamide Surfactants, Molecules, 2022, 27, 2149 CrossRef CAS PubMed
.
- S. D. Mao, F. H. Lin, Y. Y. Zhao, X. Y. Li, Y. L. Zhang, Y. P. Dong, J. L. Zhao, H. Zhang and B. Wang, Preparation of the polyvinyl alcohol thermal energy storage film containing the waste fly ash based on the phase change material, Polym. Eng. Sci., 2022, 62, 3433–3440 CrossRef CAS
.
- B. Wang, F. H. Lin, Y. Y. Zhao, X. Y. Li, Y. C. Liu, J. B. Li, X. J. Han, S. X. Liu, X. R. Ji, J. Luo and Y. H. Wei, Isotactic Polybutene-1/Bamboo Powder Composites with Excellent Properties at Initial Stage of Molding, Polymers, 2019, 11, 1981 CrossRef CAS PubMed
.
- K. Qu, Z. Sun, C. Shi, W. Wang, L. Xiao, J. Tian, Z. Huang and Z. Guo, Dual-acting cellulose nanocomposites filled with carbon nanotubes and zeolitic imidazolate framework-67 (ZIF-67)-derived polyhedral porous Co3O4 for symmetric supercapacitors, Adv. Compos. Hybrid Mater., 2021, 4, 670–683 CrossRef CAS
.
- S. Sarwar, M.-C. Lin, M. R. Ahasan, Y. Wang, R. Wang and X. Zhang, Direct growth of cobalt-doped molybdenum disulfide on graphene nanohybrids through microwave irradiation with enhanced electrocatalytic properties for hydrogen evolution reaction, Adv. Compos. Hybrid Mater., 2022, 5, 2339–2352 CrossRef CAS
.
- Z. Sun, K. Qu, J. Li, S. Yang, B. Yuan, Z. Huang and Z. Guo, Self-template biomass-derived nitrogen and oxygen co-doped porous carbon for symmetrical supercapacitor and dye adsorption, Adv. Compos. Hybrid Mater., 2021, 4, 1413–1424 CrossRef CAS
.
- R. Xue, H. Guo, W. Yang, S.-L. Huang and G.-Y. Yang, Cooperation between covalent organic frameworks (COFs) and metal organic frameworks (MOFs): application of COFs-MOFs hybrids, Adv. Compos. Hybrid Mater., 2022, 5, 1595–1611 CrossRef CAS
.
- S. D. Mao, M. Zhang, F. H. Lin, X. Y. Li, Y. Y. Zhao, Y. L. Zhang, Y. F. Gao, J. Luo, X. D. Chen and B. Wang, Attapulgite Structure Reset to Accelerate the Crystal Transformation of Isotactic Polybutene, Polymers, 2022, 14, 3820 CrossRef CAS PubMed
.
- B. Wang, F. H. Lin, X. Y. Li, X. R. Ji, S. X. Liu, X. J. Han, Z. Q. Yuan and J. Luo, Transcrystallization of Isotactic Polypropylene/Bacterial Cellulose Hamburger Composite, Polymers, 2019, 11, 508 CrossRef PubMed
.
- Y. P. Zhang, H. F. Xu and S. Lu, Preparation and application of layered double hydroxide nanosheets, RSC Adv., 2021, 11, 24254–24281 RSC
.
- X. Cao, Z. Jia, D. Hu and G. Wu, Synergistic construction of three-dimensional conductive network and double heterointerface polarization via magnetic FeNi for broadband microwave absorption, Adv. Compos. Hybrid Mater., 2022, 5, 1030–1043 CrossRef CAS
.
- R. Ge and L. Chen, Ultra-small RuO2 nanoparticles supported on carbon cloth as a high-performance pseudocapacitive electrode, Adv. Compos. Hybrid Mater., 2022, 5, 696–703 CrossRef CAS
.
- X. Li, Z. Lin, Y. Wei, W. Luo, J. Ding, T. Li and Y. Ma, MXene-MnO2-CoNi layered double hydroxides//activated carbon flexible asymmetric supercapacitor, J. Energy Storage, 2022, 22, 105668 CrossRef
.
- Z. Shan, P. S. Archana, G. Shen, A. Gupta, M. G. Bakker and S. Pan, NanoCOT: Low-Cost Nanostructured Electrode Containing Carbon, Oxygen, and Titanium for Efficient Oxygen Evolution Reaction, J. Am. Chem. Soc., 2015, 137, 11996–12005 CrossRef CAS PubMed
.
- Z. Gu, J. J. Atherton and Z. P. Xu, Hierarchical layered double hydroxide nanocomposites: structure, synthesis and applications, Chem. Commun., 2015, 51, 3024–3036 RSC
.
- P. Hu, S. Dong, F. Yuan, X. Li and C. Hong, Hollow carbon microspheres modified with NiCo2S4 nanosheets as a high-performance microwave absorber, Adv. Compos. Hybrid Mater., 2022, 5, 469–480 CrossRef CAS
.
- B. Wang, K. Nie, X. R. Xue, F. H. Lin, X. Y. Li, Y. B. Xue and J. Luo, Preparation of Maleic Anhydride Grafted Polybutene and Its Application in Isotactic Polybutene-1/Microcrystalline Cellulose Composites, Polymers, 2018, 10, 393 CrossRef PubMed
.
- H. Sun, X. Wang, H. Li, J. Bi, J. Yu, X. Liu, H. Zhou and Z. Rong, Selenium modulates cadmium-induced ultrastructural and metabolic changes in cucumber seedlings, RSC Adv., 2020, 10, 17892–17905 RSC
.
- G. M. Tomboc, J. Kim, Y. Wang, Y. Son, J. Li, J. Y. Kim and K. Lee, Hybrid layered double hydroxides as multifunctional nanomaterials for overall water splitting and supercapacitor applications, J. Mater. Chem. A, 2021, 9, 4528–4557 RSC
.
- L. Xiao, H. Qi, K. Qu, C. Shi, Y. Cheng, Z. Sun, B. Yuan, Z. Huang, D. Pan and Z. Guo, Layer-by-layer assembled free-standing and flexible nanocellulose/porous Co3O4 polyhedron hybrid film as supercapacitor electrodes, Adv. Compos. Hybrid Mater., 2021, 4, 306–316 CrossRef CAS
.
- P. Xie, Y. Liu, M. Feng, M. Niu, C. Liu, N. Wu, K. Sui, R. R. Patil, D. Pan, Z. Guo and R. Fan, Hierarchically porous Co/C nanocomposites for ultralight high-performance microwave absorption, Adv. Compos. Hybrid Mater., 2021, 4, 173–185 CrossRef CAS
.
- B. Dai, F. Dong, J. Yu, S. Feng, H. Wang, T. Li, M. Ma, J. Ding and Y. Ma, Construction of Ni@polypyrrole nanochains/Ti3C2Tx ternary composites
with excellent microwave absorption properties, Mater. Chem. Front., 2022, 6, 3179–3192 RSC
.
- Y. Wang, Y. Zhang, H. Lu, Y. Chen, Z. Liu, S. Su, Y. Xue, J. Yao and H. Zeng, Novel N-doped ZrO2 with enhanced visible-light photocatalytic activity for hydrogen production and degradation of organic dyes, RSC Adv., 2018, 8, 6752–6758 RSC
.
- H. Lu, B. Zhao, R. Pan, J. Yao, J. Qiu, L. Luo and Y. Liu, Safe and facile hydrogenation of commercial Degussa P25 at room temperature with enhanced photocatalytic activity, RSC Adv., 2014, 4, 1128–1132 RSC
.
- H. Lu, Y. Wang, Y. Wang, W. Liang and J. Yao, Adjusting phase transition of titania-based nanotubes via hydrothermal and post treatment, RSC Adv., 2015, 5, 89777–89782 RSC
.
- S. Song, Z. Hao, L. Dong, J. Li and Y. Fang, A bubble-based EMMS model for pressurized fluidization and its validation with data from a jetting fluidized bed, RSC Adv., 2016, 6, 111041–111051 RSC
.
- A. Guzman-Vargas, J. Vazquez-Samperio, M. A. Oliver-Tolentino, G. Ramos-Sanchez, J. L. Flores-Moreno and E. Reguera, Influence on the Electrocatalytic Water Oxidation of M2+/M3+ Cation Arrangement in NiFe LDH: Experimental and Theoretical DFT Evidences, Electrocatalysis, 2017, 8, 383–391 CrossRef CAS
.
- Y. Guo, D. Wang, T. Bai, H. Liu, Y. Zheng, C. Liu and C. Shen, Electrostatic self-assembled NiFe2O4/Ti3C2Tx MXene nanocomposites for efficient electromagnetic wave absorption at ultralow loading level, Adv. Compos. Hybrid Mater., 2021, 4, 602–613 CrossRef CAS
.
- S. Feng, F. Zhai, H. Su, D. Sridhar, H. Algadi, B. B. Xu, R. A. Pashameah, E. Alzahrani, H. M. Abo-Diefi, Y. Ma, T. Li and Z. Guo, Progress of metal organic frameworks-based composites in electromagnetic wave absorption, Mater. Today Phys., 2023, 30, 100950 CrossRef
.
- W. Cheng, Y. Wang, S. Ge, X. Ding, Z. Cui and Q. Shao, One-step microwave hydrothermal preparation of Cd/Zr-bimetallic metal-organic frameworks for enhanced photochemical properties, Adv. Compos. Hybrid Mater., 2021, 4, 150–161 CrossRef CAS
.
- L. Guo, Y. Zhang, J. Zheng, L. Shang, Y. Shi, Q. Wu, X. Liu, Y. Wang, L. Shi and Q. Shao, Synthesis and characterization of ZnNiCr-layered double hydroxides with high adsorption activities for Cr(VI), Adv. Compos. Hybrid Mater., 2021, 4, 819–829 CrossRef CAS
.
- Z. Jia, X. Zhang, Z. Gu and G. Wu, MOF-derived Ni-Co bimetal/porous carbon composites as electromagnetic wave absorber, Adv. Compos. Hybrid Mater., 2023, 6, 28 CrossRef CAS
.
- B. Wang, F. H. Lin, X. Y. Li, Z. W. Zhang, X. R. Xue, S. X. Liu, X. R. Ji, Q. Yu, Z. Q. Yuan, X. D. Chen and J. Luo, Isothermal Crystallization and Rheology Properties of Isotactic Polypropylene/Bacterial Cellulose Composite, Polymers, 2018, 10, 1284 CrossRef PubMed
.
- B. Wang, S. Mao, F. Lin, M. Zhang, Y. Zhao, X. Zheng, H. Wang and J. Luo, Interfacial Compatibility on the Crystal Transformation of Isotactic Poly (1-Butene)/Herb Residue Composite, Polymers, 2021, 13, 1654 CrossRef CAS PubMed
.
- X. Li, J. He, M. Liu, J. Bai, Z. Bai and W. Li, Interaction between Coal and Biomass during Co-Gasification: A Perspective Based on the Separation of Blended Char, Processes, 2022, 10, 286 CrossRef CAS
.
- M. Pathak and C. S. Rout, Hierarchical NiCo2S4 nanostructures anchored on nanocarbons and Ti3C2Tx MXene for high-performance flexible solid-state asymmetric supercapacitors, Adv. Compos. Hybrid Mater., 2022, 5, 1404–1422 CrossRef CAS
.
- Z. Yan, Z. Sun, A. Li, H. Liu, Z. Guo and L. Qian, Three-dimensional porous flower-like S-doped Fe2O3 for superior lithium storage, Adv. Compos. Hybrid Mater., 2021, 4, 716–724 CrossRef CAS
.
- M. Zheng, J. Ren, C. Wang, Y. Ma, J. Ding, T. Li, A. Y. Elnaggar, I. H. E. Azab, M. H. H. Mahmoud, S. M. El-Bahy, I. Seok, N. Naik, G. Roymahapatra, V. Murugadoss, M. Huang, B. B. Xu and Z. Guo, Magnetite@poly(p-phenylenediamine) core–shell composite modified with salicylaldehyde for adsorption and separation of Mn(VII) from polluted water, J. Nanostruct. Chem., 2022, 139, e52515 Search PubMed
.
- D. S. Wang, A. Mukhtar, K. M. Wu, L. Gu and X. Cao, Multi-Segmented Nanowires: A High Tech Bright Future, Materials, 2019, 12, 3908 CrossRef CAS PubMed
.
- B. Wang, H.-R. Zhang, C. Huang, L. Xiong, J. Luo and X.-D. Chen, Study on non-isothermal crystallization behavior of isotactic polypropylene/bacterial cellulose composites, RSC Adv., 2017, 7, 42113–42122 RSC
.
- Y. Wang, H. Lu, Y. Wang, J. Qiu, J. Wen, K. Zhou, L. Chen, G. Song and J. Yao, Facile synthesis of TaOxNy photocatalysts with enhanced visible photocatalytic activity, RSC Adv., 2016, 6, 1860–1864 RSC
.
- M. Sarfraz and I. Shakir, Recent advances in layered double hydroxides as electrode materials for high-performance electrochemical energy storage devices, J. Energy Storage, 2017, 13, 103–122 CrossRef
.
- M. Zhao, Q. Zhao, B. Li, H. Xue, H. Pang and C. Chen, Recent progress in layered double hydroxide based materials for electrochemical capacitors: design, synthesis and performance, Nanoscale, 2017, 9, 15206–15225 RSC
.
- A. L. Yan, X. C. Wang and J. P. Cheng, Research Progress of NiMn Layered Double Hydroxides for Supercapacitors: A Review, Nanomaterials, 2018, 8, 747 CrossRef PubMed
.
- W. Yang, D. Peng, H. Kimura, X. Zhang, X. Sun, R. A. Pashameah, E. Alzahrani, B. Wang, Z. Guo, W. Du and C. Hou, Honeycomb-like nitrogen-doped porous carbon decorated with Co3O4 nanoparticles for superior electrochemical performance pseudo-capacitive lithium storage and supercapacitors, Adv. Compos. Hybrid Mater., 2022, 5, 3146–3157 CrossRef CAS
.
- Z. Yang, L. Han, X. Fu, Y. Wang, H. Huang and M. Xu, Double-safety flexible supercapacitor basing on zwitterionic hydrogel: over-heat alarm and flame-retardant electrolyte, Adv. Compos. Hybrid Mater., 2022, 5, 1876–1887 CrossRef CAS
.
- M. M. Zheng, Y. D. Wei, J. J. Ren, B. Dai, W. L. Luo, M. L. Ma, T. X. Li and Y. Ma, 2-aminopyridine functionalized magnetic core-shell Fe3O4@polypyrrole composite for removal of Mn(VII) from aqueous solution by double-layer adsorption, Sep. Purif. Technol., 2021, 277, 1383–5866 CrossRef
.
- Y. Ma, Z. Zhuang, M. Ma, Y. Yang, W. Li, J. Lin, M. Dong, S. Wu, T. Ding and Z. Guo, Solid polyaniline dendrites consisting of high aspect ratio branches self-assembled using sodium lauryl sulfonate as soft templates: Synthesis and electrochemical performance, Polymer, 2019, 182, 0032–3861 CrossRef
.
- Z. Du, D. Wang, X. Zhang, Z. Yi, J. Tang, P. Yang, R. Cai, S. Yi, J. Rao and Y. Zhang, Core-Shell Structured SiO2@NiFe LDH Composite for Broadband Electromagnetic Wave Absorption, Int. J. Mol. Sci., 2022, 24, 504 CrossRef PubMed
.
- C. Zhi, Q. Wang, B. Wang, D. Li and R. Zhang, Insight into the mechanism of methane synthesis from syngas on a Ni(111) surface: a theoretical study, RSC Adv., 2015, 5, 66742–66756 RSC
.
- X. Li, J. Ren, D. Sridhar, B. B. Xu, H. Algadi, Z. M. El-Bahy, Y. Ma, T. Li and Z. Guo, Progress of layered double hydroxide-based materials for supercapacitors, Mater. Chem. Front., 2023, 7, 1520–1561 RSC
.
- C. Yin, C. Wang and Q. Hu, Selective removal of As(V) from wastewater with high efficiency by glycine-modified Fe/Zn-layered double hydroxides, Adv. Compos. Hybrid Mater., 2021, 4, 360–370 CrossRef CAS
.
- Y. Zhang, L. Liu, L. Zhao, C. Hou, M. Huang, H. Algadi, D. Li, Q. Xia, J. Wang, Z. Zhou, X. Han, Y. Long, Y. Li, Z. Zhang and Y. Liu, Sandwich-like CoMoP2/MoP heterostructures coupling N, P co-doped carbon nanosheets as advanced anodes for high-performance lithium-ion batteries, Adv. Compos. Hybrid Mater., 2022, 5, 2601–2610 CrossRef CAS
.
- J. Zhao, K. Bao, M. Xie, D. Wei, K. Yang, X. Zhang, C. Zhang, Z. Wang and X. Yang, Two-dimensional ultrathin networked CoP derived from Co(OH)2 as efficient electrocatalyst for hydrogen evolution, Adv. Compos. Hybrid Mater., 2022, 5, 2421–2428 CrossRef CAS
.
- C. Zhang, D. Wang, L. Dong, K. Li, Y. Zhang, P. Yang, S. Yi, X. Dai, C. Yin, Z. Du, X. Zhang, Q. Zhou, Z. Yi, J. Rao and Y. Zhang, Microwave Absorption of α-Fe2O3@diatomite Composites, Int. J. Mol. Sci., 2022, 23, 9362 CrossRef CAS PubMed
.
- Y. Zhao, F. Liu, Z. Zhao, P. Bai, Y. Ma, A. Alhadhrami, G. A. M. Mersal, Z. Lin, M. M. Ibrahim and Z. M. El-Bahy, Direct ink printing reduced graphene oxide/KCu7S4 electrodes for high-performance supercapacitors, Adv. Compos. Hybrid Mater., 2022, 5, 1516–1526 CrossRef CAS
.
- Y. Zhao, F. Liu, K. Zhu, S. Maganti, Z. Zhao and P. Bai, Three-dimensional printing of the copper sulfate hybrid composites for supercapacitor electrodes with ultra-high areal and volumetric capacitances, Adv. Compos. Hybrid Mater., 2022, 5, 1537–1547 CrossRef CAS
.
- Z. Zhuang, W. Wang, Y. Wei, T. Li, M. Ma and Y. Ma, Preparation of polyaniline nanorods/manganese dioxide nanoflowers core/shell nanostructure and investigation of electrochemical performances, Adv. Compos. Hybrid Mater., 2021, 4, 938–945 CrossRef CAS
.
- D. Wang, Y. Hu, Z. Cui, P. Yang, Z. Du, Y. Hou, P. Yang, J. Rao, C. Wang and Y. Zhang, Sulfur vacancy regulation and multipolarization of NixCo1S nanowires-decorated biotemplated structures to promote microwave absorption, J. Colloid Interface Sci., 2023, 646, 991–1001 CrossRef CAS PubMed
.
- Y. Zhang, H. Wang, X. Wang, B. Liu and Y. Wei, An anti-oil-fouling and robust superhydrophilic MnCo2O4 coated stainless steel mesh for ultrafast oil/water mixtures separation, Sep. Purif. Technol., 2021, 264, 118435 CrossRef CAS
.
- K. Nasrin, V. Sudharshan, K. Subramani and M. Sathish, Insights into 2D/2D MXene Heterostructures for Improved Synergy in Structure toward Next-Generation Supercapacitors: A Review, Adv. Funct. Mater., 2022, 32, 2110267 CrossRef CAS
.
- X. Zhao, Y. Zhang, Y. Hou, Z. Zhao, Y. Gong, Y. Wang, H. Wei and V. G. Pol, Synergistic effect of tailored 3D/2D Ti3C2Tx/CoS2/C nanostructured composite anode for significantly enhanced Li-ion storage, Adv. Compos. Hybrid Mater., 2022, 5, 2988–3001 CrossRef CAS
.
- C. X. Shi, Y. Z. Li, Q. Liu, Z. P. Chen, S. S. Li and R. Q. Yu, Label-free microRNA detection through analyzing the length distribution pattern of the residual fragments of probe DNA produced during exonuclease III assisted signal amplification by mass spectrometry, Talanta, 2021, 231, 122414 CrossRef CAS PubMed
.
- G. Yuan, T. Wan, A. BaQais, Y. Mu, D. Cui, M. A. Amin, X. Li, B. B. Xu, X. Zhu, H. Algadi, H. Li, P. Wasnik, N. Lu, Z. Guo, H. Wei and B. Cheng, Boron and fluorine Co-doped laser-induced graphene towards high-performance micro-supercapacitors, Carbon, 2023, 212, 118101 CrossRef CAS
.
- R. C. Rohit, A. D. Jagadale, S. K. Shinde and D. Y. Kim, A review on electrodeposited layered double hydroxides for energy and environmental applications, Mater. Today Commun., 2021, 27, 102275 CrossRef CAS
.
- M. A. Kosnan, M. A. Azam, N. E. Safie, R. F. Munawar and A. Takasaki, Recent Progress of Electrode Architecture for MXene/MoS2 Supercapacitor: Preparation Methods and Characterizations, Micromachines, 2022, 13, 1837 CrossRef PubMed
.
- M. Naguib, M. Kurtoglu, V. Presser, J. Lu, J. Niu, M. Heon, L. Hultman, Y. Gogotsi and M. W. Barsoum, Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2, Adv. Mater., 2011, 23, 4248–4253 CrossRef CAS PubMed
.
- M. Naguib, O. Mashtalir, J. Carle, V. Presser, J. Lu, L. Hultman, Y. Gogotsi and M. W. Barsoum, Two-Dimensional Transition Metal Carbides, ACS Nano, 2012, 6, 1322–1331 CrossRef CAS PubMed
.
- O. Mashtalir, M. Naguib, B. Dyatkin, Y. Gogotsi and M. W. Barsoum, Kinetics of aluminum extraction from Ti3AlC2 in hydrofluoric acid, Mater. Chem. Phys., 2013, 139, 147–152 CrossRef CAS
.
- X. Wang, X. Shen, Y. Gao, Z. Wang, R. C. Yu and L. Chen, Atomic-Scale Recognition of Surface Structure and Intercalation Mechanism of Ti3C2X, J. Am. Chem. Soc., 2015, 137, 2715–2721 CrossRef CAS PubMed
.
- M. Naguib, M. Kurtoglu, V. Presser, J. Lu, J. Niu, M. Heon, L. Hultman, Y. Gogotsi and M. W. Barsoum, Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2, Adv. Mater., 2011, 23, 4248–4253 CrossRef CAS PubMed
.
- A. Feng, Y. Yu, Y. Wang, F. Jiang, Y. Yu, L. Mi and L. Song, Two-dimensional MXene Ti3C2 produced by exfoliation of Ti3AlC2, Mater. Des., 2017, 114, 161–166 CrossRef CAS
.
- T. Li, L. Yao, Q. Liu, J. Gu, R. Luo, J. Li, X. Yan, W. Wang, P. Liu, B. Chen, W. Zhang, W. Abbas, R. Naz and D. Zhang, Fluorine-Free Synthesis of High-Purity Ti3C2Tx (T=OH, O) via Alkali Treatment, Angew. Chem., Int. Ed., 2018, 57, 6115–6119 CrossRef CAS PubMed
.
- J. Zhang, N. Kong, S. Uzun, A. Levitt, S. Seyedin, P. A. Lynch, S. Qin, M. Han, W. Yang, J. Liu, X. Wang, Y. Gogotsi and J. M. Razal, Scalable Manufacturing of Free-Standing, Strong Ti3C2Tx MXene Films with Outstanding Conductivity, Adv. Mater., 2020, 32, 2001093 CrossRef CAS PubMed
.
- M. Ghidiu, M. R. Lukatskaya, M.-Q. Zhao, Y. Gogotsi and M. W. Barsoum, Conductive two-dimensional titanium carbide ‘clay’ with high volumetric capacitance, Nature, 2014, 516, 78–81 CrossRef CAS PubMed
.
- Z. Zhang, Z. Cai, Y. Zhang, Y. Peng, Z. Wang, L. Xia, S. Ma, Z. Yin, R. Wang, Y. Cao, Z. Li and Y. Huang, The recent progress of MXene-Based microwave absorption materials, Carbon, 2021, 174, 484–499 CrossRef CAS
.
- J. Halim, M. R. Lukatskaya, K. M. Cook, J. Lu, C. R. Smith, L.-A. Naslund, S. J. May, L. Hultman, Y. Gogotsi, P. Eklund and M. W. Barsoum, Transparent Conductive Two-Dimensional Titanium Carbide Epitaxial Thin Films, Chem. Mater., 2014, 26, 2374–2381 CrossRef CAS PubMed
.
- O. Mashtalir, M. R. Lukatskaya, M.-Q. Zhao, M. W. Barsoum and Y. Gogotsi, Amine-Assisted Delamination of Nb2C MXene for Li-Ion Energy Storage Devices, Adv. Mater., 2015, 27, 3501–3506 CrossRef CAS PubMed
.
-
A. N. Adamson, E. J. Bloore and A. R. Carr, Basic Principles of Bayer Process Design, in Essential Readings in Light Metals, 2013, 100–117 Search PubMed
.
- X. Xie, Y. Xue, L. Li, S. Chen, Y. Nie, W. Ding and Z. Wei, Surface Al leached Ti3AlC2 as a substitute for carbon for use as a catalyst support in a harsh corrosive electrochemical system, Nanoscale, 2014, 6, 11035–11040 RSC
.
- G. Li, L. Tan, Y. Zhang, B. Wu and L. Li, Highly Efficiently Delaminated Single-Layered MXene Nanosheets with Large Lateral Size, Langmuir, 2017, 33, 9000–9006 CrossRef CAS PubMed
.
- L. Huang, T. Li, Q. Liu and J. Gu, Fluorine-free Ti3C2Tx as anode materials for Li-ion batteries, Electrochem. Commun., 2019, 104, 106472 CrossRef CAS
.
- S. Yang, P. Zhang, F. Wang, A. G. Ricciardulli, M. R. Lohe, P. W. M. Blom and X. Feng, Fluoride-Free Synthesis of Two-Dimensional Titanium Carbide (MXene) Using A Binary Aqueous System, Angew. Chem., Int. Ed., 2018, 57, 15491–15495 CrossRef CAS PubMed
.
- S.-Y. Pang, Y.-T. Wong, S. Yuan, Y. Liu, M.-K. Tsang, Z. Yang, H. Huang, W.-T. Wong and J. Hao, Universal Strategy for HF-Free Facile and Rapid Synthesis of Two-dimensional MXenes as Multifunctional Energy Materials, J. Am. Chem. Soc., 2019, 141, 9610–9616 CrossRef CAS PubMed
.
- M. Naguib, V. Presser, D. Tallman, J. Lu, L. Hultman, Y. Gogotsi and M. W. Barsoum, On the Topotactic Transformation of Ti2AlC into a Ti-C-O-F Cubic Phase by Heating in Molten Lithium Fluoride in Air, J. Am. Ceram. Soc., 2011, 94, 4556–4561 CrossRef CAS
.
- P. Urbankowski, B. Anasori, T. Makaryan, D. Er, S. Kota, P. L. Walsh, M. Zhao, V. B. Shenoy, M. W. Barsoum and Y. Gogotsi, Synthesis of two-dimensional titanium nitride Ti4N3 (MXene), Nanoscale, 2016, 8, 11385–11391 RSC
.
- Y. Li, H. Shao, Z. Lin, J. Lu, L. Liu, B. Duployer, P. O. A. Persson, P. Eklund, L. Hultman, M. Li, K. Chen, X.-H. Zha, S. Du, P. Rozier, Z. Chai, E. Raymundo-Pinero, P.-L. Taberna, P. Simon and Q. Huang, A general Lewis acidic etching route for preparing MXenes with enhanced electrochemical performance in non-aqueous electrolyte, Nat. Mater., 2021, 20, 571–571 CrossRef CAS PubMed
.
- V. Kamysbayev, A. S. Filatov, H. Hu, X. Rui, F. Lagunas, D. Wang, R. F. Klie and D. V. Talapin, Covalent surface modifications and superconductivity of two-dimensional metal carbide MXenes, Science, 2020, 369, 979–983 CrossRef CAS PubMed
.
- P. Horak, S. Bakardjieva, J. Vacik, X. Rui and R. Klie, Preparation of Ti2C MXene phase by ion beam sputtering and ion irradiation, Nucl. Instrum. Methods Phys. Res., Sect. B, 2020, 469, 49–51 CrossRef CAS
.
- Y. Gogotsi, Chemical vapour deposition: Transition metal carbides go 2D, Nat. Mater., 2015, 14, 1079–1080 CrossRef CAS PubMed
.
- C. Xu, L. Wang, Z. Liu, L. Chen, J. Guo, N. Kang, X.-L. Ma, H.-M. Cheng and W. Ren, Large-area high-quality 2D ultrathin Mo2C superconducting crystals, Nat. Mater., 2015, 14, 1135–1141 CrossRef CAS PubMed
.
- P. R. Whelan, B. S. Jessen, R. Wang, B. Luo, A. C. Stoot, D. M. A. Mackenzie, P. Braeuninger-Weimer, A. Jouvray, L. Prager, L. Camilli, S. Hofmann, P. Boggild and T. J. Booth, Raman spectral indicators of catalyst decoupling for transfer of CVD grown 2D materials, Carbon, 2017, 117, 75–81 CrossRef CAS
.
- F. Turker, O. R. Caylan, N. Mehmood, T. S. Kasirga, C. Sevik and G. C. Buke, CVD synthesis and characterization of thin Mo2C crystals, J. Am. Ceram. Soc., 2020, 103, 5586–5593 CrossRef CAS
.
- V. Thirumal, R. Yuvakkumar, P. S. Kumar, G. Ravi, S. P. Keerthana and D. Velauthapillai, Facile single-step synthesis of MXene@CNTs hybrid nanocomposite by CVD method to remove hazardous pollutants, Chemosphere, 2022, 286, 131733 CrossRef CAS PubMed
.
- X. Zhang, Y. Liu, S. Dong, J. Yang and X. Liu, Flexible electrode based on multi-scaled MXene (Ti3C2Tx) for supercapacitors, J. Alloys Compd., 2019, 790, 517–523 CrossRef CAS
.
- H. He, J. Wang, Q. Xia, L. Wang, Q. Hu and A. Zhou, Effect of electrolyte on supercapacitor performance of two-dimensional molybdenum carbide (Mo2CTx) MXene prepared by hydrothermal etching, Appl. Surf. Sci., 2021, 568, 150791 Search PubMed
.
- T. Bai, W. G. Wang, G. F. Xue, S. Y. Li, W. X. Guo, M. D. Ye and C. X. Wu, Free-Standing, Flexible Carbon@MXene Films with Cross-Linked Mesoporous Structures toward Supercapacitors and Pressure Sensors, ACS Appl. Mater. Interfaces, 2021, 13, 57576–57587 CrossRef CAS PubMed
.
- Z. H. Pan and X. H. Ji, Facile synthesis of nitrogen and oxygen co-doped C@Ti3C2 MXene for high performance symmetric supercapacitors, J. Power Sources, 2019, 439, 227068 CrossRef CAS
.
- H. Wang and X. M. Wu, High capacitance of dipicolinic acid-intercalated MXene in neutral water-based electrolyte, Chem. Eng. J., 2020, 399, 125850 CrossRef CAS
.
- J. Halim, A. S. Etman, A. Elsukova, P. Polcik, J. Palisaitis, M. W. Barsoum, P. O. A. Persson and J. Rosen, Tailored synthesis approach of (Mo2/3Y1/3)2AlC i-MAX and its two-dimensional derivative Mo1.33CTz MXene: enhancing the yield, quality, and performance in supercapacitor applications, Nanoscale, 2021, 13, 311–319 RSC
.
- T. X. Shang, Z. F. Lin, C. S. Qi, X. C. Liu, P. Li, Y. Tao, Z. T. Wu, D. W. Li, P. Simon and Q. H. Yang, 3D Macroscopic Architectures from Self-Assembled MXene Hydrogels, Adv. Funct. Mater., 2019, 29, 1903096 CrossRef
.
- H. B. Zhang, Z. Y. Li, Z. Y. Hou, H. Mei, Y. Feng, B. Xu and D. F. Sun, Self-assembly of MOF on MXene nanosheets and in-situ conversion into superior nickel phosphates/MXene battery-type electrode, Chem. Eng. J., 2021, 425, 130602 CrossRef CAS
.
- H. He, Q. Xia, B. Wang, L. Wang, Q. Hu and A. Zhou, Two-dimensional vanadium carbide (V2CTx) MXene as supercapacitor electrode in seawater electrolyte, Chin. Chem. Lett., 2020, 31, 984–987 CrossRef CAS
.
- Z. Fan, Y. Wang, Z. Xie, X. Xu, Y. Yuan, Z. Cheng and Y. Liu, A nanoporous MXene film enables flexible supercapacitors with high energy storage, Nanoscale, 2018, 10, 9642–9652 RSC
.
- A. E. Allah, J. Wang, Y. V. Kaneti, T. Li, A. A. Farghali, M. H. Khedr, A. K. Nanjundan, B. Ding, H. Dou, X. Zhang, B. Yoshio and Y. Yamauchi, Auto-programmed heteroarchitecturing: Self-assembling ordered mesoporous carbon between two-dimensional Ti3C2Tx MXene layers, Nano Energy, 2019, 65, 103991 CrossRef CAS
.
- X. Wang, S. Lin, H. Tong, Y. Huang, P. Tong, B. Zhao, J. Dai, C. Liang, H. Wang, X. Zhu, Y. Sun and S. Dou, Two-dimensional V4C3 MXene as high performance electrode materials for supercapacitors, Electrochim. Acta, 2019, 307, 414–421 CrossRef CAS
.
- T.-Y. Zhang, C. Cui, R.-F. Cheng, M.-M. Hu and X.-H. Wang, Fabrication of Planar Porous MXene/Carbon Composite Electrodes by Simultaneous Am monization/Carbonization, J. Inorg. Mater., 2020, 35, 112–118 Search PubMed
.
- R. Syamsai and A. N. Grace, Synthesis, properties and performance evaluation of vanadium carbide MXene as supercapacitor electrodes, Ceram. Int., 2020, 46, 5323–5330 CrossRef
.
- D. Wen, X. Wang, L. Liu, C. Hu, C. Sun, Y. R. Wu, Y. L. Zhao, J. X. Zhang, X. D. Liu and G. B. Ying, Inkjet Printing Transparent and Conductive MXene (Ti3C2Tx) Films: A Strategy for Flexible Energy Storage Devices, ACS Appl. Mater. Interfaces, 2021, 13, 17766–17780 CrossRef CAS PubMed
.
- W. Hou, Y. Sun, Y. Zhang, T. Wang, L. Wu, Y. Du and W. Zhong, Mixed-dimensional heterostructure of few-layer MXene based vertical aligned MoS2 nanosheets for enhanced supercapacitor performance, J. Alloys Compd., 2021, 859, 157797 CrossRef CAS
.
- C. Y. Cai, W. B. Zhou and Y. Fu, Bioinspired MXene nacre with mechanical robustness for highly flexible all-solid-state photothermo-supercapacitor, Chem. Eng. J., 2021, 418, 129275 CrossRef CAS
.
- H. Wang, J. Zhang, Y. Wu, H. Huang and Q. Jiang, Achieving high-rate capacitance of multi-layer titanium carbide (MXene) by liquid-phase exfoliation through Li-intercalation, Electrochem. Commun., 2017, 81, 48–51 CrossRef CAS
.
- H. Hwang, S. Byun, S. Yuk, S. Kim, S. H. Song and D. Lee, High-rate electrospun Ti3C2Tx MXene/carbon nanofiber electrodes for flexible supercapacitors, Appl. Surf. Sci., 2021, 556, 149710 CrossRef CAS
.
- H. Li, X. Wang, H. Li, S. Lin, B. Zhao, J. Dai, W. Song, X. Zhu and Y. Sun, Capacitance improvements of V4C3Tx by NH3 annealing, J. Alloys Compd., 2019, 784, 923–930 CrossRef CAS
.
- S. Kulandaivalu, N. H. N. Azman and Y. Sulaiman, Advances in Layered Double Hydroxide/Carbon Nanocomposites Containing Ni2+ and Co2+/3+ for Supercapacitors, Front. Mater., 2020, 7, 147 CrossRef
.
- D. G. Evans and X. Duan, Preparation of layered double hydroxides and their applications as additives in polymers, as precursors to magnetic materials and in biology and medicine, Chem. Commun., 2006, 485–496 RSC
.
- H. Yi, S. Liu, C. Lai, G. Zeng, M. Li, X. Liu, B. Li, X. Huo, L. Qin, L. Li, M. Zhang, Y. Fu, Z. An and L. Chen, Recent Advance of Transition-Metal-Based Layered Double Hydroxide Nanosheets: Synthesis, Properties, Modification, and Electrocatalytic Applications, Adv. Energy Mater., 2021, 11, 2002863 CrossRef CAS
.
- M. A. Woo, M.-S. Song, T. W. Kim, I. Y. Kim, J.-Y. Ju, Y. S. Lee, S. J. Kim, J.-H. Choy and S.-J. Hwang, Mixed valence Zn–Co-layered double hydroxides and their exfoliated nanosheets with electrode functionality, J. Mater. Chem., 2011, 21, 4286 RSC
.
- M. A. Djebbi, M. Braiek, P. Namour, A. B. H. Amara and N. Jaffrezic-Renault, Layered double hydroxide materials coated carbon electrode: New challenge to future electrochemical power devices, Appl. Surf. Sci., 2016, 386, 352–363 CrossRef CAS
.
- L. Zhang, P. Cai, Z. Wei, T. Liu, J. Yu, A. A. Al-Ghamdi and S. Wageh, Synthesis of reduced graphene oxide supported nickel-cobalt-layered double hydroxide nanosheets for supercapacitors, J. Colloid Interface Sci., 2021, 588, 637–645 CrossRef CAS PubMed
.
- H. Li, G. Zhu, Z.-H. Liu, Z. Yang and Z. Wang, Fabrication of a hybrid graphene/layered double hydroxide material, Carbon, 2010, 48, 4391–4396 CrossRef CAS
.
- L. Zhang, H. Xia, S. Liu, Y. Zhou, Y. Zhao and W. Xie, Nickel-Cobalt Hydroxides with Tunable Thin-Layer Nanosheets for High-Performance Supercapacitor Electrode, Nanoscale Res. Lett., 2021, 16, 83 CrossRef CAS PubMed
.
- X. Wang, J. Zhang, S. Yang, H. Yan, X. Hong, W. Dong, Y. Liu, B. Zhang and Z. Wen, Interlayer space regulating of NiMn layered double hydroxides for supercapacitors by controlling hydrothermal reaction time, Electrochim. Acta, 2019, 295, 1–6 CrossRef CAS
.
- M. M. Rao, B. R. Reddy, M. Jayalakshmi, V. S. Jaya and B. Sridhar, Hydrothermal synthesis of Mg–Al hydrotalcites by urea hydrolysis, Mater. Res. Bull., 2005, 40, 347–359 CrossRef CAS
.
- H. Duan, S. Zheng, J. Zhuang, T. Wang, K. Chen, S. Liu, C. Chen and H. Pang, Synthesis of Ni4Yb(OH)10NO3·3H2O Nanosheets for Electrode Materials in Electrochemical Energy Storage, ChemElectroChem, 2018, 5, 3150–3154 CrossRef CAS
.
- B. Wang, Q. Liu, Z. Qian, X. Zhang, J. Wang, Z. Li, H. Yan, Z. Gao, F. Zhao and L. Liu, Two steps in situ structure fabrication of Ni-Al layered double hydroxide on Ni foam and its electrochemical performance for supercapacitors, J. Power Sources, 2014, 246, 747–753 CrossRef CAS
.
- Q. Huang, K.-Y. Liu, F. He, S.-R. Zhang, Q.-L. Xie and C. Chen, Fabrication of cobalt aluminum-layered double hydroxide nanosheets/carbon spheres composite as novel electrode material for supercapacitors, Trans. Nonferrous Met. Soc. China, 2017, 27, 1804–1814 CrossRef
.
- T. Wang, S. Zhang and H. Wang, Binary NiCu layered double hydroxide nanosheets for enhanced energy storage performance as supercapacitor electrode, Sci. China Mater., 2018, 61, 296–302 CrossRef CAS
.
- Y.-M. Jeong, I. Son and S.-H. Baek, Binder-free of NiCo-layered double hydroxides on Ni-coated textile for wearable and flexible supercapacitors, Appl. Surf. Sci., 2019, 467, 963–967 CrossRef
.
- X. Han, J. Li, J. Lu, S. Luo, J. Wan, B. Li, C. Hu and X. Cheng, High mass-loading NiCo-LDH nanosheet arrays grown on carbon cloth by electrodeposition for excellent electrochemical energy storage, Nano Energy, 2021, 86, 106079 CrossRef CAS
.
- P. Yao, Z. Li, J. Zhu, X. Ran, Z. Shi and J. Zhu, Controllable synthesis of NiCo-LDH/Co(OH)2@PPY composite via electrodeposition at high deposition voltages for high-performance supercapacitors, J. Alloys Compd., 2021, 875, 160042 CrossRef CAS
.
- Y. B. Chen, L. A. Ma, X. Zhang, L. K. Huang, H. X. Chen and Q. T. Wang, ZnO@NiCo-LDH core-shell nanostructures grown on carbon cloth for high-performance flexible supercapacitor electrodes, Mater. Technol., 2021, 37, 1146–1155 CrossRef
.
- S. Jiang, Y. Qiao, T. Fu, W. Peng, T. Yu, B. Yang, R. Xia and M. Gao, Integrated Battery–Capacitor Electrodes: Pyridinic N-Doped Porous Carbon-Coated Abundant Oxygen Vacancy Mn–Ni-Layered Double Oxide for Hybrid Supercapacitors, ACS Appl. Mater. Interfaces, 2021, 13, 34374–34384 CrossRef CAS PubMed
.
- H. Fu, A. Zhang, F. Jin, H. Guo, W. Huang, W. Cheng and J. Liu, Origami and layered-shaped ZnNiFe-LDH synthesized on Cu(OH)2 nanorods array to enhance the energy storage capability, J. Colloid Interface Sci., 2022, 607, 1269–1279 CrossRef CAS PubMed
.
- K. Li, B. Zhao, J. Bai, H. Ma, Z. Fang, X. Zhu and Y. Sun, A High-Energy-Density Hybrid Supercapacitor with P-Ni(OH)2@Co(OH)2 Core-Shell Heterostructure and Fe2O3 Nanoneedle Arrays as Advanced Integrated Electrodes, Small, 2020, 16, e2001974 CrossRef PubMed
.
- B. Han, G. Cheng, E. Zhang, L. Zhang and X. Wang, Three dimensional hierarchically porous ZIF-8 derived carbon/LDH core-shell composite for high performance supercapacitors, Electrochim. Acta, 2018, 263, 391–399 CrossRef CAS
.
- S. P. Adhikari, G. P. Awasthi, K. S. Kim, C. H. Park and C. S. Kim, Synthesis of three-dimensional mesoporous Cu-Al layered double hydroxide/g-C3N4 nanocomposites on Ni-foam for enhanced supercapacitors with excellent long-term cycling stability, Dalton Trans., 2018, 47, 4455–4466 RSC
.
- W. Hu, L. Chen, X. Wu, M. Du, Y. Song, Z. Wu and Q. Zheng, Slight Zinc Doping by an Ultrafast Electrodeposition Process Boosts the Cycling Performance of Layered Double Hydroxides for Ultralong-Life-Span Supercapacitors, ACS Appl. Mater. Interfaces, 2021, 13, 38346–38357 CrossRef CAS PubMed
.
- X. Wang, X. Li, C. Huang, C. Hao, C. Ge and Y. Guo, Fabrication of NiCoAl-layered double hydroxide/N-GO for high energy all-solid-state asymmetric supercapacitors, Appl. Surf. Sci., 2020, 527, 146891 CrossRef CAS
.
- D. Chen, H. Chen, X. Chang, P. Liu, Z. Zhao, J. Zhou, G. Xu, H. Lin and S. Han, Hierarchical CoMn-layered double hydroxide nanowires on nickel foam as electrode material for high-capacitance supercapacitor, J. Alloys Compd., 2017, 729, 866–873 CrossRef CAS
.
- D. Su, Z. Tang, J. Xie, Z. Bian, J. Zhang, D. Yang, D. Zhang, J. Wang, Y. Liu, A. Yuan and Q. Kong, Co, Mn-LDH nanoneedle arrays grown on Ni foam for high performance supercapacitors, Appl. Surf. Sci., 2019, 469, 487–494 CrossRef CAS
.
- M. Li, A. Addad, M. Dolci, P. Roussel, M. Naushad, S. Szunerits and R. Boukherroub, NiMnCr layered double hydroxide-carbon spheres modified Ni foam: An efficient positive electrode for hybrid supercapacitors, Chem. Eng. J., 2020, 396, 125370 CrossRef CAS
.
- L. Luo, B. He, W. Kong and Z. Wang, NiCo2S4/Ni–Co layered double hydroxide nanocomposite prepared by a vapor-phase hydrothermal method for electrochemical capacitor application, J. Alloys Compd., 2017, 705, 349–355 CrossRef CAS
.
- J. Xu, K. Liao, K. Song, J. Wu, X. Hu, H. Gao, F. Hu and J. P. Cheng, Fast in situ synthesis of CoFe layered double hydroxide onto multi-layer graphene for electrochemical capacitors, J. Solid State Electrochem., 2017, 22, 1037–1045 CrossRef
.
- X. Chen, M. He, Y. Zhou, G. He, C. Meng, Q. Cheng and F. Li, Design of hierarchical double-layer NiCo/NiMn-layered double hydroxide nanosheet arrays on Ni foam as electrodes for supercapacitors, Mater. Today Chem., 2021, 21, 100507 CrossRef CAS
.
- X. Chu, T. Deng, W. Zhang, D. Wang, X. Liu, C. Zhang, T. Qin, L. Zhang, B. Zhang, C. Chen and W. Zheng, Architecture of Co-layered double hydroxide nanocages/graphene composite electrode with high electrochemical performance for supercapacitor, J. Energy Chem., 2018, 27, 507–512 CrossRef
.
- W. Chen, J. Wang, K. Y. Ma, M. Li, S. H. Guo, F. Liu and J. P. Cheng, Hierarchical NiCo2O4@Co-Fe LDH core-shell nanowire arrays for high-performance supercapacitor, Appl. Surf. Sci., 2018, 451, 280–288 CrossRef CAS
.
- H. Tian, W. Bao, Y. Jiang, L. Wang, L. Zhang, O. Sha, C. Wu and F. Gao, Fabrication of Ni-Al LDH/nitramine-N-doped graphene hybrid composites via a novel self-assembly process for hybrid supercapacitors, Chem. Eng. J., 2018, 354, 1132–1140 CrossRef CAS
.
- J. J. Zhou, Q. Li, C. Chen, Y. L. Li, K. Tao and L. Han, Co3O4@CoNi-LDHcore/shell nanosheet arrays for high-performance battery-type supercapacitors, Chem. Eng. J., 2018, 350, 551–558 CrossRef CAS
.
- Y. Li, L. Shan, Y. Sui, J. Qi, F. Wei, Y. He, Q. Meng, Y. Ren and J. Liu, Ultrathin Ni–Co LDH nanosheets grown on carbon fiber cloth via electrodeposition for high-performance supercapacitors, J. Mater. Sci.: Mater. Electron., 2019, 30, 13360–13371 CrossRef CAS
.
- Y. Wang, Z. Yin, G. Yan, Z. Wang, X. Li, H. Guo and J. Wang, New insight into the electrodeposition of NiCo layered double hydroxide and its capacitive evaluation, Electrochim. Acta, 2020, 336, 135734 CrossRef CAS
.
- Y. Lu, B. Jiang, L. Fang, F. Ling, F. Wu, B. Hu, F. Meng, K. Niu, F. Lin and H. Zheng, An investigation of ultrathin nickel-iron layered double hydroxide nanosheets grown on nickel foam for high-performance supercapacitor electrodes, J. Alloys Compd., 2017, 714, 63–70 CrossRef CAS
.
- X. Guan, M. Huang, L. Yang, G. Wang and X. Guan, Facial design and synthesis of CoSx/Ni-Co LDH nanocages with rhombic dodecahedral structure for high-performance asymmetric supercapacitors, Chem. Eng. J., 2019, 372, 151–162 CrossRef CAS
.
- V. S. Sivasankarapillai, T. S. K. Sharma, K.-Y. Hwa, S. M. Wabaidur, S. Angaiah and R. Dhanusuraman, MXene Based Sensing Materials: Current Status and Future Perspectives, ES Energy Environ., 2022, 15, 4–14 CAS
.
- H. Zhou, F. Wu, L. Fang, J. Hu, H. Luo, T. Guan, B. Hu and M. Zhou, Layered NiFe-LDH/MXene nanocomposite electrode for high-performance
supercapacitor, Int. J. Hydrogen Energy, 2020, 45, 13080–13089 CrossRef CAS
.
- C. Li, D. Zhang, J. Cao, P. Yu, M. Okhawilai, J. Yi, J. Qin and X. Zhang, Ti3C2 MXene-Encapsulated NiFe-LDH Hybrid Anode for High-Performance Lithium-Ion Batteries and Capacitors, ACS Appl. Energy Mater., 2021, 4, 7821–7828 CrossRef CAS
.
- M. Tian, C. Liu, Z. G. Neale, J. Zheng, D. Long and G. Cao, Chemically Bonding NiFe-LDH Nanosheets on rGO for Superior Lithium-Ion Capacitors, ACS Appl. Mater. Interfaces, 2019, 11, 35977–35986 CrossRef CAS PubMed
.
- X. Zhu, Y. Zhong, H. Zhai, Z. Yan and D. Li, Nanoflake nickel hydroxide and reduced graphene oxide composite as anode materials for high capacity lithium ion batteries, Electrochim. Acta, 2014, 132, 364–369 CrossRef CAS
.
- C. Li, Z. Xue, J. Qin, M. Sawangphruk, P. Yu, X. Zhang and R. Liu, Synthesis of nickel hydroxide/delaminated-Ti3C2 MXene nanosheets as promising anode material for high performance lithium ion battery, J. Alloys Compd., 2020, 842, 155812 CrossRef CAS
.
- M. Zhang, X. Chen and X. Yang, Electrochemical energy storage of NiO/NiFe2O4 nanosheets derived from Ni-Fe layered double hydroxides, J. Nanopart. Res., 2020, 22, 96 CrossRef CAS
.
- Y. Xiao, J. Zai, B. Tian and X. Qian, Formation of NiFe2O4/Expanded Graphite Nanocomposites with Superior Lithium Storage Properties, Nano-Micro Lett., 2017, 9, 34 CrossRef PubMed
.
- W. Wang, D. Jiang, X. Chen, K. Xie, Y. Jiang and Y. Wang, A sandwich-like nano-micro LDH-MXene-LDH for high-performance supercapacitors, Appl. Surf. Sci., 2020, 515, 145982 CrossRef CAS
.
- J. Guo, Z. Bian, L. Ye, Y. Shang, F. Guo, Y. Zhang and J. Xu, Double layers combined with MXene and in situ grown NiAl-LDH arrays on nickel foam for enhanced asymmetric supercapacitors, Ionics, 2022, 28, 2967–2977 CrossRef CAS
.
- M. Chen, J. Chen, X. Tan, W. Yang, H. Zou and S. Chen, Facile self-assembly of sandwich-like MXene/graphene oxide/nickel–manganese layered double hydroxide nanocomposite for high performance supercapacitor, J. Energy Storage, 2021, 44, 103456 CrossRef
.
- X. Wang, H. Li, H. Li, S. Lin, J. Bai, J. Dai, C. Liang, X. Zhu, Y. Sun and S. Dou, Heterostructures of Ni–Co–Al layered double hydroxide assembled on V4C3 MXene for high-energy hybrid supercapacitors, J. Mater. Chem. A, 2019, 7, 2291–2300 RSC
.
- Y. Gogotsi and R. M. Penner, Energy Storage in Nanomaterials - Capacitive Pseudocapacitive, or Battery-like?, ACS Nano, 2018, 12, 2081–2083 CrossRef CAS PubMed
.
- L. Yu, L. Hu, B. Anasori, Y.-T. Liu, Q. Zhu, P. Zhang, Y. Gogotsi and B. Xu, MXene-Bonded Activated Carbon as a Flexible Electrode for High-Performance Supercapacitors, ACS Energy Lett., 2018, 3, 1597–1603 CrossRef CAS
.
- X. Ge, C. Gu, Z. Yin, X. Wang, J. Tu and J. Li, Periodic stacking of 2D charged sheets: Self-assembled superlattice of Ni-Al layered double hydroxide (LDH) and reduced graphene oxide, Nano Energy, 2016, 20, 185–193 CrossRef CAS
.
- D. Zhang, J. Cao, X. Zhang, N. Insin, R. Liu and J. Qin, NiMn Layered Double Hydroxide Nanosheets In-situ Anchored on Ti3C2 MXene via Chemical Bonds for Superior Supercapacitors, ACS Appl. Energy Mater., 2020, 3, 5949–5964 CrossRef CAS
.
- Y. Zhang, J. Cao, J. Li, Z. Yuan, D. Li, L. Wang and W. Han, Self-assembled Cobalt-doped NiMn-layered double hydroxide (LDH)/V2CTx MXene hybrids for advanced aqueous electrochemical energy storage properties, Chem. Eng. J., 2022, 430, 132992 CrossRef CAS
.
- R. Niu, R. Han, Y. Huang, L. Dai, H. Zhao, Y. Wang, J. Zhu, S. Tang and J. Sun, Hydrothermal ion exchange synthesis of CoM(M=Fe or Mn)/MXene 2D/2D hierarchal architectures for enhanced energy storage, J. Alloys Compd., 2022, 894, 162385 CrossRef CAS
.
- C. Zhang, R. Guo, H. Wang, X. Xie and C. Du, Composite electrodes with NiCoAl-LDH coated Ti3C2Tx MXene and incorporated Ag nanowires for screen-printable in-plane hybrid supercapacitors on textiles, Appl. Surf. Sci., 2022, 598, 153796 CrossRef CAS
.
- X. Wu, B. Huang, Q. Wang and Y. Wang, High energy density of two-dimensional MXene/NiCo-LDHs interstratification assembly electrode: Understanding the role of interlayer ions and hydration, Chem. Eng. J., 2020, 380, 122456 CrossRef CAS
.
- H. Li, F. Musharavati, E. Zalenezhad, X. Chen, K. N. Hui and K. S. Hui, Electrodeposited Ni-Co layered double hydroxides on titanium carbide as a binder-free electrode for supercapacitors, Electrochim. Acta, 2018, 261, 178–187 CrossRef CAS
.
- R. Zhao, M. Wang, D. Zhao, H. Li, C. Wang and L. Yin, Molecular-Level Heterostructures Assembled from Titanium Carbide MXene and Ni–Co–Al Layered Double-Hydroxide Nanosheets for All-Solid-State Flexible Asymmetric High-Energy Supercapacitors, ACS Energy Lett., 2017, 3, 132–140 CrossRef
.
- J. Chen, Y. Ren, H. Zhang, J. Qi, Y. Sui and F. Wei, Ni-Co-Fe layered double hydroxide coated on Ti3C2 MXene for high-performance asymmetric supercapacitor, Appl. Surf. Sci., 2021, 562, 150116 CrossRef CAS
.
- J. Zheng, X. Pan, X. Huang, D. Xiong, Y. Shang, X. Li, N. Wang, W.-M. Lau and H. Y. Yang, Integrated NiCo2-LDHs@MXene/rGO aerogel: Componential and structural engineering towards enhanced performance stability of hybrid supercapacitor, Chem. Eng. J., 2020, 396, 125197 CrossRef CAS
.
- H. Niu, X. Yang, Q. Wang, X. Jing, K. Cheng, K. Zhu, K. Ye, G. Wang, D. Cao and J. Yan, Electrostatic self-assembly of MXene and edge-rich CoAl layered double hydroxide on molecular-scale with superhigh volumetric performances, J. Energy Chem., 2020, 46, 105–113 CrossRef
.
| This journal is © the Partner Organisations 2023 |